User login
Dermatologic surgeons debut adverse event reporting database
The
CAPER is a voluntary reporting system designed to collect reports of patients’ adverse events encountered during dermatologic surgery procedures, both cosmetic and those related to skin cancer. The goals of the CAPER registry are to provide safety monitoring, identify practice and/or education gaps associated with adverse events, and identify potential adverse event risk factors.
“CAPER is a registry overseen by a group of board-certified dermatologists, clinicians, and researchers with more than 20 years of experience in patient care and physician advocacy who are committed to improving safety outcomes,” according to an ASDSA press release. “The collaboration between Northwestern University and ASDSA will ensure that CAPER becomes the common place for dermatologic surgeons and their staff to report adverse events from devices, drugs or biologics.”
The launch of the database is important because it fills a gap in adverse event reporting, Murad Alam, MD, professor of dermatology and chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, said in an interview.
There has been no central registry specifically for reporting adverse events associated with dermatologic surgical procedures, including cosmetic and injectable treatments, he said. “While minimally invasive cosmetic and skin procedures have been proven to be exceedingly safe, this registry will provide an early warning system to identify any problems that do occur, so these can be addressed promptly. This registry will allow dermatologists, patients, and industry scientists to work together to further improve the safety of dermatologic procedures,” added Dr. Alam, the past ASDSA president, and current chair of the ASDSA’s Federal Affairs Work Group.
In addition, “recent reports of the possible interaction between some filler injections and certain COVID vaccines confirms the timeliness of redoubling our emphasis on safety. Dermatologists have always been at the forefront of maximizing the patient experience while minimizing risk; this registry is further evidence of that ongoing commitment,” he emphasized.
The CAPER database will gather information on a variety of dermatologic and cosmetic procedures, including those involving topicals and injectables (such as botulinum toxin, fillers, and chemical peels), devices (such as lasers and microneedling devices), cellular-based therapies (such as platelet-rich plasma and stem cell treatments), and surgical treatments (such as liposuction and hair transplantation), Dr. Alam said.
“Novel procedures, and those yet to be devised, as long as they relate to skin surgery or cosmetic improvement, will also be able to be reported. We encourage the reporting of all associated adverse events, even if it is not clear what caused the event. No dermatologic or cosmetic procedures will be excluded from reporting,” he added.
The purpose of the CAPER registry is “to help patients, physicians, and industry work collaboratively to ensure the highest levels of patient safety,” Dr. Alam continued. Data entered into the registry will be deidentified and will remain confidential, and as data on particular topics accumulate, the data “may be analyzed to better understand the patient experience and, secondly, to develop strategies to further improve safety,” he noted.
“One unique element of this registry is that it is focused on dermatologic and cosmetic procedures,” Dr. Alam added. “As a result, those managing and analyzing the data collected will be attuned to the particular concerns associated with such procedures and the patients receiving them.”
For more information and to report dermatologic surgery-related adverse events, go to caper.net.
The
CAPER is a voluntary reporting system designed to collect reports of patients’ adverse events encountered during dermatologic surgery procedures, both cosmetic and those related to skin cancer. The goals of the CAPER registry are to provide safety monitoring, identify practice and/or education gaps associated with adverse events, and identify potential adverse event risk factors.
“CAPER is a registry overseen by a group of board-certified dermatologists, clinicians, and researchers with more than 20 years of experience in patient care and physician advocacy who are committed to improving safety outcomes,” according to an ASDSA press release. “The collaboration between Northwestern University and ASDSA will ensure that CAPER becomes the common place for dermatologic surgeons and their staff to report adverse events from devices, drugs or biologics.”
The launch of the database is important because it fills a gap in adverse event reporting, Murad Alam, MD, professor of dermatology and chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, said in an interview.
There has been no central registry specifically for reporting adverse events associated with dermatologic surgical procedures, including cosmetic and injectable treatments, he said. “While minimally invasive cosmetic and skin procedures have been proven to be exceedingly safe, this registry will provide an early warning system to identify any problems that do occur, so these can be addressed promptly. This registry will allow dermatologists, patients, and industry scientists to work together to further improve the safety of dermatologic procedures,” added Dr. Alam, the past ASDSA president, and current chair of the ASDSA’s Federal Affairs Work Group.
In addition, “recent reports of the possible interaction between some filler injections and certain COVID vaccines confirms the timeliness of redoubling our emphasis on safety. Dermatologists have always been at the forefront of maximizing the patient experience while minimizing risk; this registry is further evidence of that ongoing commitment,” he emphasized.
The CAPER database will gather information on a variety of dermatologic and cosmetic procedures, including those involving topicals and injectables (such as botulinum toxin, fillers, and chemical peels), devices (such as lasers and microneedling devices), cellular-based therapies (such as platelet-rich plasma and stem cell treatments), and surgical treatments (such as liposuction and hair transplantation), Dr. Alam said.
“Novel procedures, and those yet to be devised, as long as they relate to skin surgery or cosmetic improvement, will also be able to be reported. We encourage the reporting of all associated adverse events, even if it is not clear what caused the event. No dermatologic or cosmetic procedures will be excluded from reporting,” he added.
The purpose of the CAPER registry is “to help patients, physicians, and industry work collaboratively to ensure the highest levels of patient safety,” Dr. Alam continued. Data entered into the registry will be deidentified and will remain confidential, and as data on particular topics accumulate, the data “may be analyzed to better understand the patient experience and, secondly, to develop strategies to further improve safety,” he noted.
“One unique element of this registry is that it is focused on dermatologic and cosmetic procedures,” Dr. Alam added. “As a result, those managing and analyzing the data collected will be attuned to the particular concerns associated with such procedures and the patients receiving them.”
For more information and to report dermatologic surgery-related adverse events, go to caper.net.
The
CAPER is a voluntary reporting system designed to collect reports of patients’ adverse events encountered during dermatologic surgery procedures, both cosmetic and those related to skin cancer. The goals of the CAPER registry are to provide safety monitoring, identify practice and/or education gaps associated with adverse events, and identify potential adverse event risk factors.
“CAPER is a registry overseen by a group of board-certified dermatologists, clinicians, and researchers with more than 20 years of experience in patient care and physician advocacy who are committed to improving safety outcomes,” according to an ASDSA press release. “The collaboration between Northwestern University and ASDSA will ensure that CAPER becomes the common place for dermatologic surgeons and their staff to report adverse events from devices, drugs or biologics.”
The launch of the database is important because it fills a gap in adverse event reporting, Murad Alam, MD, professor of dermatology and chief of cutaneous and aesthetic surgery in the department of dermatology at Northwestern University, said in an interview.
There has been no central registry specifically for reporting adverse events associated with dermatologic surgical procedures, including cosmetic and injectable treatments, he said. “While minimally invasive cosmetic and skin procedures have been proven to be exceedingly safe, this registry will provide an early warning system to identify any problems that do occur, so these can be addressed promptly. This registry will allow dermatologists, patients, and industry scientists to work together to further improve the safety of dermatologic procedures,” added Dr. Alam, the past ASDSA president, and current chair of the ASDSA’s Federal Affairs Work Group.
In addition, “recent reports of the possible interaction between some filler injections and certain COVID vaccines confirms the timeliness of redoubling our emphasis on safety. Dermatologists have always been at the forefront of maximizing the patient experience while minimizing risk; this registry is further evidence of that ongoing commitment,” he emphasized.
The CAPER database will gather information on a variety of dermatologic and cosmetic procedures, including those involving topicals and injectables (such as botulinum toxin, fillers, and chemical peels), devices (such as lasers and microneedling devices), cellular-based therapies (such as platelet-rich plasma and stem cell treatments), and surgical treatments (such as liposuction and hair transplantation), Dr. Alam said.
“Novel procedures, and those yet to be devised, as long as they relate to skin surgery or cosmetic improvement, will also be able to be reported. We encourage the reporting of all associated adverse events, even if it is not clear what caused the event. No dermatologic or cosmetic procedures will be excluded from reporting,” he added.
The purpose of the CAPER registry is “to help patients, physicians, and industry work collaboratively to ensure the highest levels of patient safety,” Dr. Alam continued. Data entered into the registry will be deidentified and will remain confidential, and as data on particular topics accumulate, the data “may be analyzed to better understand the patient experience and, secondly, to develop strategies to further improve safety,” he noted.
“One unique element of this registry is that it is focused on dermatologic and cosmetic procedures,” Dr. Alam added. “As a result, those managing and analyzing the data collected will be attuned to the particular concerns associated with such procedures and the patients receiving them.”
For more information and to report dermatologic surgery-related adverse events, go to caper.net.
Adalimumab earns FDA approval for ulcerative colitis in children
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
Adalimumab has received approval from the Food and Drug Administration for use in pediatric patients aged 5 years and older with moderately to severely active ulcerative colitis (UC), according to a press release from manufacturer AbbVie.
Approval was based on data from the phase 3 ENVISION 1 randomized, double-blind study (NCT02065557) in which 60% of patients receiving the higher induction dose showed clinical remission according to Partial May Score (PMS) at 8 weeks, 45% of whom were in remission according to Full Mayo Score (FMS) at 1 year. The approval means that children with UC and their families have the option of a subcutaneous biologic that can be administered at home.
In the ENVISION 1 study, clinical remission was defined as a PMS (based on stool frequency, rectal bleeding, and physician’s global assessment) ranging from 0 to 3 at the end of the 8-week induction period or FMS (which adds endoscopy) at the end of 52 weeks as less than or equal to 2 points total, with no individual subscore greater than 1. The study included children aged 4-17 years with active UC who were randomized to a low-dose or high-dose group.
“Through week 8, patients in both dosage groups received 2.4 mg/kg (maximum of 160 mg) at week 0, 1.2 mg/kg (maximum of 80 mg) at week 2, and 0.6 mg/kg (maximum of 40 mg) at weeks 4 and 6. The higher-dosage group also received an additional dosage of 2.4 mg/kg (maximum of 160 mg) at week 1,” according to the company press release. Between weeks 8 and 52, patients received double-blind placebo or 0.6 mg/kg adalimumab (maximum of 40 mg) every other week (maintenance standard dose) or every week (maintenance high dose).
No new adalimumab safety signals were noted in the study; headache and worsening UC were the most frequently reported treatment-emergent adverse events, and 22.6% of patients experienced a serious adverse event. “No deaths, malignancies, active tuberculosis or demyelinating disease were observed in this study,” according to the company. Full prescribing information can be found on the FDA website.
Approval expands options
“The number of FDA-approved biologics to treat pediatric UC has been limited,” said Atsushi Sakuraba, MD, PhD, an inflammatory bowel disease specialist at the University of Chicago, in an interview. “Infliximab is approved for pediatric UC and CD [Crohn’s disease], but adalimumab was only approved for CD in pediatric patients, and vedolizumab and ustekinumab are approved for neither UC nor CD in pediatric patients,” he said. “Thus, the addition of adalimumab as a treatment option for pediatric UC is of great benefit for pediatricians and patients.”
The approval will impact clinical practice in several ways, Dr. Sakuraba said. “Adalimumab may be used for those who lose response to infliximab, but may also be used as a first-line biologic because it is self-injectable,” he emphasized. Given that adalimumab is already in use for pediatric patients with Crohn’s disease, Dr. Sakuraba said he does not see any barriers to implementation of its use for children with UC. In addition, “the potential for better disease control, reduction of disease-related complications, and improved quality of life outweighs the risk of adverse reactions,” Dr. Sakuraba said. “The news of no new safety concern in the 52-week study period further supports the safety of biologic treatment in pediatric patients,” he added.
As for additional research: “It remains to be determined whether combination therapy with an immunomodulator would be more effective than monotherapy, and also whether there are any additional risks of adverse events with the addition of immunomodulators such as long-term risk of malignancy, especially lymphoproliferative disorders,” Dr. Sakuraba noted. “Therapeutic options for pediatric [inflammatory bowel disease] are limited and lagging, compared to adult patients, so studies of non-TNF [tumor necrosis factor] biologics such vedolizumab and ustekinumab are also awaited,” he said.
Dr. Sakuraba had no financial conflicts to disclose.
This article was updated March 3, 2021.
ACR, AAD, AAO, RDS issue joint statement on safe use of hydroxychloroquine
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
Hydroxychloroquine can be used safely and effectively with attention to dosing, risk factors, and screening, but communication among physicians, patients, and eye care specialists is key to optimizing outcomes and preventing complications, according to a joint statement from four medical societies.
The American College of Rheumatology, American Academy of Dermatology, Rheumatologic Dermatology Society, and the American Academy of Ophthalmology have produced a statement, published in Arthritis & Rheumatology, “to emphasize points of agreement that should be recognized by practitioners in all specialties,” lead author James T. Rosenbaum, MD, of Oregon Health & Science University, Portland, and colleagues wrote.
The statement was developed by a working group that included rheumatologists, ophthalmologists, and dermatologists with records of published studies on the use of hydroxychloroquine (HCQ) and its toxicity. The statement updated elements of the 2016 American Academy of Ophthalmology guidelines for monitoring patients for retinal toxicity when using HCQ.
“The need for collaborative management has triggered this joint statement, which applies only to managing the risk of HCQ retinopathy and does not include consideration of cardiac, muscle, dermatologic, or other toxicities,” the authors noted.
The authors emphasized that HCQ plays a valuable role in controlling many rheumatic diseases, and should not be abandoned out of fear of retinopathy. However, proper dosing, recognition of risk factors, and screening strategies are essential.
Dosing data
Data on HCQ dosing and retinopathy are limited, but the authors cited a study of 2,361 rheumatic disease patients with an average HCQ dosing regimen of 5.0 mg/kg per day or less in which the toxicity risk was less than 2% for up to 10 years of use. Although data show some increase in risk with duration of use, “for a patient with a normal screening exam in a given year, the risk of developing retinopathy in the ensuing year is low (e.g., less than 5%), even after 20 years of use,” the authors said.
Risk factor recognition
“High daily [HCQ] dosage relative to body weight and cumulative dose are the primary risk factors for retinopathy,” the authors noted. Reduced renal function is an additional risk factor, and patients with renal insufficiency should be monitored and may need lower doses.
In addition, patients with a phenotype of initial parafoveal toxicity may be at increased risk for advanced disease evidenced by damage to the foveal center. “The phenotype of initial parafoveal toxicity is not universal, and in many patients (East Asians particularly) the retinal changes may appear initially along the pericentral vascular arcades,” so these patients should be screened with additional tests beyond the central macula, they emphasized.
Screening strategies
Patients should receive a baseline retinal exam within a few months of starting HCQ to rule out underlying retinal disease, according to the statement. The goal of screening is “to detect early retinopathy before a bullseye becomes visible on ophthalmoscopy, since at that severe stage the damage tends to progress even after discontinuing the medication and may eventually threaten central vision,” the authors said.
In the absence of risk factors, patients can defer screening for 5 years, but should be screened annually from 5 years and forward, they said. Examples of underlying retinal disease include “significant macular degeneration, severe diabetic retinopathy, or hereditary disorders of retinal function, but these are judgments best made by the ophthalmologist since mild and stable abnormalities that do not interfere with interpretation of critical diagnostic tests may not be a contraindication” to use of HCQ.
The consensus opinion statement has limitations, notably the shortage of data on optimum HCQ dosage and the lack of prospective studies of toxicity, including the need for studies of the impact of blood levels on toxicity and studies of pharmacogenomics to stratify risk, the authors noted.
“It is important that the drug is not stopped prematurely, but also that it is not continued in the face of definitive evidence of retinal toxicity except in some situations with unusual medical need,” they said.
“Suggestive or uncertain findings should be discussed with the patient and prescribing physician to justify further examinations, but the drug need not be stopped until evidence for retinopathy is definitive, in particular for patients with active rheumatic or cutaneous disease,” and the overall risk of retinopathy remains low if the principles described in the statement are followed, they concluded.
First author Dr. Rosenbaum disclosed financial relationships with AbbVie, UCB, Gilead, Novartis, Horizon, Roche, Eyevensys, Santen, Corvus, Affibody, Kyverna, Pfizer, Horizon, and UpToDate. Another 5 of the study’s 11 authors also disclosed relationships with multiple companies.
FROM ARTHRITIS & rHEUMATOLOGY
ACOG advises on care for transgender patients
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Transgender patients have unique needs regarding obstetric and gynecologic care as well as preventive care, and ob.gyns. can help by providing support, education, and understanding, according to new guidance from the American College of Obstetricians and Gynecologists.
“The American College of Obstetricians and Gynecologists opposes discrimination on the basis of gender identity, urges public and private health insurance plans to cover necessary services for individuals with gender dysphoria, and advocates for inclusive, thoughtful, and affirming care for transgender individuals,” according to the committee opinion, published in the March issue of Obstetrics & Gynecology. The opinion was developed jointly by ACOG’s Committee on Gynecologic Practice and Committee on Health Care for Underserved Women, led by Beth Cronin, MD, of Brown University, Providence, R.I., and Colleen K, Stockdale, MD, of the University of Iowa, Iowa City.
“Lack of awareness, knowledge, and sensitivity, as well as bias from health care professionals leads to inadequate access to, underuse of, and inequities within the health care system for transgender patients,” the authors wrote.
The committee opinion provides guidance for ob.gyns. on topics including inclusivity, routine screening, fertility and reproductive issues, hormone therapy, medication use, and surgery.
“One of the most incredible things about being an ob.gyn. is that this field is a hybrid of primary care and surgical practice,” said K. Ashley Brandt, DO, in an interview. “Many patients seek out care from ob.gyns. for routine screening such as a Pap test, for initiation of hormone therapy, or for postoperative management,” said Dr. Brandt, an ob.gyn. and a plastic surgeon at Reading Hospital/Tower Health System in West Reading, Pa. “Many of my colleagues are starting to see an increase in transgender and gender-nonconforming individuals and do not know where to access resources or information on basic care needs. I think ACOG issuing this guidance is a great first step in providing an overview for the ob.gyn., who otherwise haven’t had formal training in transgender medicine,” she emphasized.
Dr. Brandt said she was not surprised by any of the recommendations. “These recommendations, while evolving and updating as new data emerge, have been in place by WPATH (the World Professional Association for Transgender Health) and the Endocrine Society for quite some time,” she noted. “However, this updated committee opinion is a summary of recommendations that are relevant to the clinical practice of an ob.gyn.”
“Since the publication of Care for Transgender Adolescents (2017) and Healthcare for Transgender Individuals (2011), there has been an exponential increase in data that have helped to improve and guide best practices for this patient population including better defining risks, needs, therapy, and follow-up,” said Nancy Sokkary, MD, a specialist in pediatric and adolescent gynecology in Macon, Ga., in an interview. “This document also served as an opportunity for ACOG to educate ob.gyns. about health inequities and emphasize need for gender-affirming and inclusive care,” she said.
“These recommendations are consistent with literature that has been published over the last several years,” she added. “It is certainly important for ob.gyns. to have a document unequivocally supporting hysterectomies and bilateral salpingo-oophorectomy as medically necessary for transgender patients that desire these procedures for their transition.”
Inclusive environment
Approximately 1.4 million adults and 150,000 youth aged 13-17 years in the United States identify as transgender, but these individuals are often marginalized socially and economically, which can lead to worse health outcomes, according to the committee. “Creating a safe and affirming health care environment for all patients, including transgender individuals, is essential,” the authors said. Steps to create a supportive office setting include educating staff to avoid assumptions about sex and gender, and ask appropriately about choice of pronouns and orientation. Use patient forms that reflect a full range of options and places for patients to write in a response. Also, use electronic medical records to track information on use of names other than legal names. “Ob.gyns. play an important role in caring for gender-nonconforming people,” said Dr. Sokkary. “Ob.gyn. providers may have varying levels of participation in gender-affirming hormone or surgery provision, but they can universally conduct routine health maintenance, contraceptive and fertility counseling, and obstetric care in a respectful and inclusive environment,” she said.
Track transition issues
The opinion notes that many gender-transition medications can be prescribed not only by ob.gyns., but by a range of health care professionals with training and education. When it comes to medication and surgery, neither medication nor surgery is required for legally changing one’s name or gender, but patient desires vary from those seeking only letters of support for such legal changes to those who want to pursue hormone therapy or procedures such as chest surgery, hysterectomy, or phalloplasty.
Transgender patients seeking care from ob.gyns. include transmasculine and transfeminine individuals who are seeking various degrees of masculinizing or feminizing therapies.
Masculinizing therapies may result in development of facial hair, deepening voice, and changes in muscle mass, but patients undergoing masculinizing therapies should be reminded of the potential for continued ovulation, according to the opinion. “The only absolute contraindications to masculinizing hormone therapy are current pregnancy, unstable coronary artery disease, and polycythemia (hematocrit greater than 55%),” the authors wrote.
Feminizing therapies have no absolute contraindications, but “risks include venous thromboembolism (VTE), hypertriglyceridemia, development of gallstones, and elevated liver enzymes,” they noted.
Talk about sex and fertility
Clinicians treating transgender patients should discuss fertility and parenting early in the process of any gender transition, ideally before the patient undergoes hormone therapy or surgery, according to the opinion. Fertility preservation options for transgender patients are the same as for cisgender patients who wish to preserve fertility for various reasons, and include “sperm banking, oocyte preservation, embryo preservation, and in some cases, ovarian or testicular tissue cryopreservation,” the authors noted.
However, patients who do not desire pregnancy but may have the potential to become pregnant or impregnate others should be counseled on contraceptive options and reminded that gender-affirming hormone therapy alone does not provide effective contraception, they emphasized. In addition, “all patients should be counseled on barrier use for prevention of sexually transmitted diseases,” they said.
Consistent routine screening and preventive care
The committee opinion also states that transgender patients should undergo routine screening for any anatomical structures that are present, such as breast cancer screening for transmasculine individuals with breast tissue, and cervical cancer screening for those with a cervix. Transfeminine individuals should undergo prostate cancer screening in accordance with the recommendations for cisgender men, the authors said.
“As for all patients, transgender individuals should be counseled about the importance of routine preventive health care,” according to the opinion. “All individuals should be routinely screened for intimate partner violence, depression, substance use, cancer, and other health care needs and should be screened for sexually transmitted infections and counseled about appropriate immunizations based on age and risk factors, including HPV vaccination,” the authors said.
“We continue to see patient discrimination and discomfort with the medical system as a barrier to preventive care among gender-nonconforming individuals,” said Dr. Sokkary. “[Ensuring] that your clinic is a safe, inclusive place is a good start. Also, having providers such as ob.gyns. and family medicine physicians provide gender-affirming care in addition to routine screening and testing is helpful,” she said.
One of the ongoing challenges of counseling transgender patients across a range of age groups, from youth through menopause, is a lack of data on the long-term effects of hormone therapy or surgical intervention, Dr. Brandt noted. “Since there is a paucity of this information, many of the screening recommendations fall in line with that of cisgender patients; however, this is not always the case as screening is determined by hormonal usage, risk factors, and surgical state. It is important for clinicians to be aware of evolutions in screening that will continue to occur as more evidence becomes available,” she emphasized.
In addition, “This document did not include specific guidance for transgender and gender-diverse adolescents, and there are many factors and recommendations that are unique to this population,” Dr. Sokkary said.
Barriers and overcoming them
The main barrier to care with transgender and gender-nonconfirming patients is access to care and finding providers who are competent in gender-affirming health, Dr. Brandt noted. “Another significant barrier involves caring for transgender male patients in a traditionally ‘women’s health’ specialty,” she said. “While the office of an ob.gyn. can be very affirming for transgender women, it has the potential to exacerbate discomfort in transgender male patients,” she noted. “Having gender-affirming posters and pamphlets in the waiting area are ways to make patients feel more at ease. Another of the ways to overcome this barrier is education of the staff and health care providers,” added Dr. Brandt. “Fortunately, this is starting to occur at medical school and residency levels. For ob.gyns. already in practice, articles such as this committee opinion can serve as a resource for providers seeking to understand health care needs of this community,” she said.
“Cost and insurance coverage continue to be barriers, but this has improved immensely: There are now several local and national resources that can help with this depending on the issue,” said Dr. Sokkary. “Additionally, we still lack robust data that define cancer risk among transgender individuals, and until we have more evidence-based recommendations providers should follow screening outlined in this document,” she said.
Use the ACOG opinion as a starting point
“This committee opinion is a great introduction and summary for ob.gyns. seeking to understand basic care needs for gender-nonconforming individuals,” said Dr. Brandt. “However, I strongly encourage ob.gyns. who wish to truly incorporate gender-affirming care as part of their routine clinical practice to participate in continuing education, read the WPATH standards of care among many of the resources provided in the committee opinion, and attend conferences that are specific to transgender health and medicine,” she said.
The opinion received no outside funding. The authors were vetted by ACOG and had no relevant financial conflicts to disclose. Dr. Brandt had no financial conflicts to disclose. Dr. Sokkary had no financial conflicts to disclose.
Heavier girls hit hormonal puberty earlier, but develop breasts later
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
Girls with more body fat experienced earlier menarche and hormone changes, but later full breast development, compared with those with normal weight, according to longitudinal data from 90 girls aged 8-15 years.
A link between obesity and early puberty has been observed among U.S. girls for decades, but more recent studies suggest that “girls with greater childhood adiposity have earlier thelarche and progress through puberty at a faster rate than normal weight girls,” wrote Madison T. Ortega, MD, of the National Institute of Environmental Health Sciences, Durham, N.C., and colleagues. However, studies involving hormone levels have yielded mixed results, they said.
In a study published in the Journal of Clinical Endocrinology & Metabolism , the researchers followed 36 girls with overweight or obesity and 54 girls with normal weight for 4 years; normal weight was defined as body mass index in the 5th to 85th percentile, overweight was defined as BMI in the 85th to 95th percentile, and obese was defined as greater than 95th percentile. Overweight and obese were combined into one category for comparison with normal weight girls.
Participants had an average of 2.8 study visits during this period and provided additional information by phone and online. Visits included measurement of total body fat using dual-energy x-ray absorptiometry (DXA), Tanner staging, breast ultrasound for morphological staging (BMORPH; A-E), pelvic ultrasound, hormone tests, and menarchal status assessment.
Overall, girls with overweight/obesity (OW/OB) had significantly more advanced breast development at baseline than did those with normal weight (NW), but these girls progressed through BMORPH stage D later than did NW girls. Early-stage breast development was not affected by total body fat. However, “an increase of 5 percentage points in mean total body fat, for example, was associated with a 26% decrease in the transition rate out of stage D,” the researchers noted.
Hormone levels were similar at baseline for follicle-stimulating hormone, inhibin B, estrone (E1), total and free testosterone, and androstenedione. However, these levels increased more quickly after 1 year for girls with OW/OB, while they plateaued in girls with NW and dropped among girls with lower total body fat. Total body fat had no apparent effect on other reproductive hormones including luteinizing hormone, modified vaginal maturation index, and estradiol 2.
The average age of menarche was 12.4 years across all participants, but girls with higher total body fat at baseline were more likely to reach menarche at a younger age. “For every 1-unit increase in visit one total body fat, the chance of achieving menarche at any given time point was 3% higher,” the researchers said. No interaction appeared between race and total body fat with regard to menarche.
Several surprising findings
The study is important because “there have been no longitudinal studies in U.S. girls to examine how total body fat affects serum reproductive hormones or the development of the breast and ovaries using ultrasound imaging,” corresponding author Natalie Shaw, MD, of the National Institute of Environmental Health Sciences, said in an interview.
Dr. Shaw said she was surprised by several of the study findings. “Others have reported increased male-like hormones (androgens) in overweight/obese girls in cross-sectional studies; however, we were surprised to find that FSH and inhibin B were also elevated in girls with excess body fat,” she said. “We also found, unexpectedly, that even though the breast bud appears earlier in overweight/obese girls (thelarche), which signals the onset of puberty, the breast matured more slowly during the course of puberty in overweight/obese girls compared with normal weight girls,” she noted.
“The main take-home message is that puberty looks different in girls with excess body fat; they develop breast tissue earlier, yet take longer to achieve a fully mature breast, and they undergo menarche earlier,” Dr. Shaw said. Clinicians should be aware of the hormonal differences based on body fat, Dr. Shaw emphasized. “Girls with greater body fat had higher levels of FSH (a pituitary hormone), inhibin B (an ovarian hormone), and male-like reproductive hormones (e.g., testosterone) that are made by the adrenal glands and the ovaries in the late stages of puberty,” she said.
Potential implications for adulthood
“The findings in this study contribute to better understanding how total body fat impacts hormonal findings of puberty,” M. Susan Jay, MD, of the Medical College of Wisconsin and Children’s Hospital of Wisconsin, Milwaukee, said in an interview. “Prior studies have linked weight gain as a factor that contributes to pubertal development, but this study is attempting to longitudinally investigate how body weight may affect clinical and biochemical pubertal markers in girls,” she noted.
“The take-home message is that this study and other earlier studies have illustrated that puberty is not a fixed pattern in all individual girls,” Dr. Jay emphasized. “Rather, there are environmental factors which can impact pubertal course,” she said. “In effect, there are pathways through puberty in individual adolescents that require greater ongoing studies to further identify the arc of puberty and the impact of how the length in various stages may affect exposure to estrogen and other neurohormonal factors,” she explained. These factors impact not only adolescence but also future health in adulthood, she said.
“Ongoing prospective studies are needed to identify how factors such as body weight can affect adolescent pubertal development and the possible impact long after adolescence for health issues such as breast cancer,” Dr. Jay added.
The study findings were limited by several factors including the available data from only two completed study visits for most participants, as well as the racial differences among body weight groups and lack of standardized timing for blood draws, the researchers noted.
The study was supported in part by the National Institute of Environmental Health Sciences, and corresponding author Dr. Shaw disclosed support as a Lasker Clinical Research Scholar. The other researchers, as well as Dr. Jay, had no disclosures.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY & METABOLISM
Ob.gyns. report high burnout prior to pandemic
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.
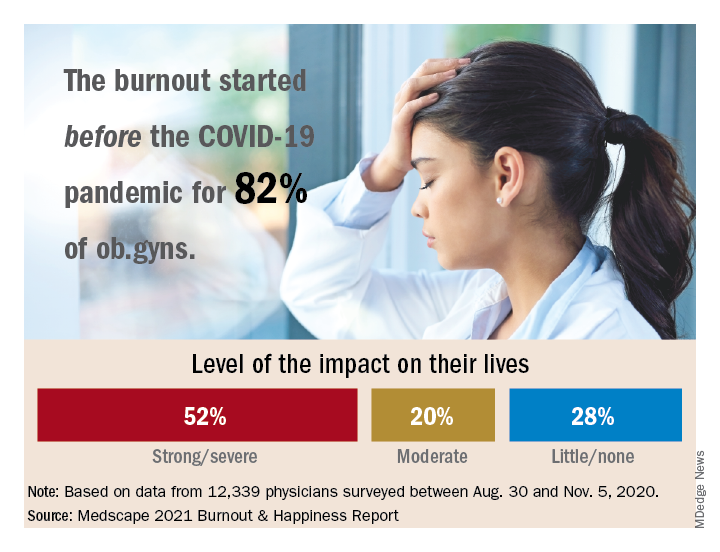
More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”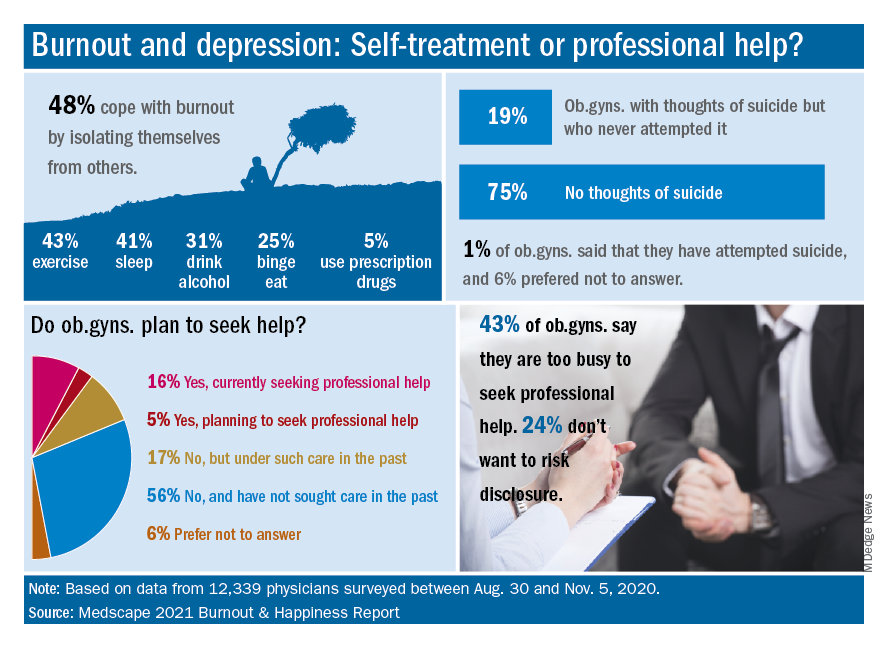
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.

More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
Among ob.gyns. who reported burnout in the past year, 82% say they felt burned out before the advent of the coronavirus pandemic, according the Medscape Obstetrician & Gynecologist Lifestyle, Happiness, & Burnout Report.
The past year brought unusual challenges to physicians in all specialties in different ways.
“Whether on the front lines of treating COVID-19 patients, pivoting from in-person to virtual care, or even having to shutter their practices, physicians faced an onslaught of crises, while political tensions, social unrest, and environmental concerns probably affected their lives outside of medicine,” wrote Keith L. Martin and Mary Lyn Koval, both of Medscape Business of Medicine, in the introduction to the report.
Although more physicians said their burnout began prior to the pandemic, 81% of ob.gyns. reported that they were happy outside of work prior to the pandemic. However, those reporting happiness outside of work dropped to 57% after the pandemic started.
“One does not have to do a ‘deep dive’ to understand the top reasons reported for burnout,” said Mark P. Trolice, MD, director of Fertility CARE: The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando, in an interview. “Many conversations I have with colleagues are about the frustration of learning and managing electronic health records, insurance reimbursements, and a work-life balance. In addition, more physician practices are being purchased by hospitals or private-equity networks [that are] reducing and/or eliminating the autonomy of physicians.
“While all [respondents] exhibited a dramatic decline in ‘happiness’ prepandemic, compared with our current situation, ob.gyns. were no exception,” he added.
Burnout and suicidal thoughts
Overall, 26% of ob.gyn. survey respondents reported being burned out, 6% reported being depressed, and 18% reported being both burned out and depressed. Of those who reported burnout, 52% said burnout had “a strong or severe impact on my life,” while 20% reported a moderate impact and 28% reported little or no impact.

More than half (56%) of ob.gyns. who reported either depression or burnout said they had not sought professional help, although 17% reported receiving professional care in the past.
The main reason given for not seeking professional help was that burnout and depressive symptoms were not severe enough to merit it, according to 50% of respondents who reported burnout or depression but were not seeking help. In addition, 43% said they were too busy to seek help, 36% said they could deal with their symptoms without professional help, and 24% said they did not want to risk disclosure of their symptoms.
The most common cause of burnout was an overload of bureaucratic tasks, reported by 52% of respondents, followed by “lack of respect from administrators/employers, colleagues, or staff” (43%), and insufficient compensation or reimbursement (39%).
Notably, 19% of ob.gyns. reported suicidal thoughts, and 1% said they had attempted suicide.
“The most concerning statistic from this survey was in reference to suicidal ideation,” said Dr. Trolice. “Approximately one in five ob.gyns. have contemplated suicide, compared with 4.8% of adults age 18 and older in the U.S. reporting in 2019.”
Dr. Trolice said he was not surprised that relatively few ob.gyns. sought help for mental health issues. “Physicians are very private and usually do not seek help from colleagues, presumably from hubris. While this is unfortunate, all hospitals and health care organization should implement regular assessments of physicians’ health to ensure optimal performance from a professional and personal basis.”
Balance and self-care
The top workplace concern, by a large margin, was for work-life balance, reported by 44% of respondents, followed by compensation (19%), combining work and parenting (18%), and relationships with staff and colleagues (8%).
Approximately one-third (36%) of the ob.gyn. respondents said they made time to focus on personal well-being, compared with 35% of physicians overall. Although only 13% reported exercising every day, a total of 69% exercised at least twice a week, similar to the 70% of physicians overall who reported exercising at least twice a week.
“Work-life balance is high on the list of concerns, but physicians are split 50/50 on whether they would accept a salary reduction to improve this aspect of their lives,” Dr. Trolice said.
“Social relationships are a proven value to mental health, yet nearly 50% of ob.gyns. who reported feeling burnout use isolationism as their coping skill, citing a lack of severity to require treatment,” he noted. Nevertheless, more than 80% of responders were married and described their relationship as “good or very good.”
Address burnout at individual and organizational levels
“Sadly, the findings are not surprising,” said Iris Krisha, MD, of Emory University, Atlanta, in an interview. “Burnout rates have been steadily increasing among physicians across all specialties.” Barriers to reducing burnout exist at the organizational and individual level, therefore strategies to reduce burnout should address individual and organizational solutions, Dr. Krishna emphasized. “At the organizational level, solutions may include developing manageable workloads, creating fair productivity targets, encouraging physician engagement in work structure, supporting flexible work schedules, and allowing for protected time for education and exercise. On the individual level, physicians can work to develop stress management strategies, engage in mindfulness and self-care.”
To reduce the burden of bureaucratic tasks, “health care organizations can work toward optimizing electronic medical records and hire staff to offload clerical work, and physicians can seek training in efficiency,” said Dr. Krishna. In addition, “health care organizations can reduce the stigma that may surround burnout or mental health issues, as well as promote a culture of wellness and resilience,” to help reduce and prevent burnout.
Find positivity and purpose
Improving the workplace experience so physicians feel engaged and in control as they navigate their many responsibilities may help reduce burnout, said Dr. Trolice. On the individual level, “finding your purpose to give you more meaning at work, discovering the power of hope to embrace optimism, and building friendships at work for greater engagement with others,” can help as well.
“In the face of adversity and setbacks, people in happier workplaces tend to be better at coping with and recovering from work pressure and at reconciling conflict,” Dr. Trolice emphasized. “The practice of medicine has dramatically changed for many physicians compared with the original expectations when they applied to medical school. Nevertheless, it behooves physician to adapt to 21st century medical care as they remind themselves of their purpose.”
The report included responses from 12,339 physicians across 29 specialties who completed a 10-minute online survey between Aug. 30 and Nov. 5, 2020. Participants were required to be practicing U.S. physicians.
Frozen sections can guide biopsies for giant cell arteritis, but are they feasible?
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
Positive findings from frozen sections of a first temporal artery biopsy can effectively identify giant cell arteritis, ruling out in those cases the need to perform a second biopsy on the contralateral side and arguing against the use of simultaneous bilateral biopsies, according to results from a retrospective study of nearly 800 patients who underwent the procedure at the Mayo Clinic during 2010-2018.
Although temporal artery biopsy (TAB) remains the standard diagnostic test for giant cell arteritis (GCA), second TAB procedures are often performed in patients with a high level of suspicion for GCA, which may result in unnecessary treatments and complications, Devon A. Cohen, MD, of the Mayo Clinic, Rochester, Minn., and colleagues wrote. (Dr. Cohen is now a clinical fellow in ophthalmology at the Massachusetts Eye and Ear Infirmary.)
At the Mayo Clinic, TAB specimens are first examined with frozen sections at the time of the biopsy; this process, followed within days by formalin-fixed tissue permanent sections, is unique to Mayo. “A frozen section–guided sequential TAB is commonly performed, with the results of the first biopsy obtained within minutes, which determines the need for evaluation of the contralateral side,” the researchers said. However, the use of frozen sections to evaluate patients with GCA has not been well studied.
In a retrospective cohort study published in JAMA Ophthalmology, the researchers identified TAB patients aged 40 years and older who underwent TAB procedures between Jan. 1, 2010, and Dec. 1, 2018, at the Mayo Clinic. The average age of the patients was 72 years, and 41% were men.
Strong positive predictions from frozen sections
The researchers analyzed 1,162 TABs from 795 patients using frozen and permanent histologic sections.
Overall, 119 patients (15.0%) and 138 TABs had positive permanent section findings, and 103 (86.6%) of these patients also had positive frozen section findings, including 4 false positives and 20 false negatives. The frozen section specificity and sensitivity was 99.4% and 83.2%, respectively, for detecting inflammation suggestive of GCA, and the positive and negative predictive values were 96.1% and 96.6%, respectively. Positive and negative likelihood ratios for frozen section were 140.6 and 0.17, respectively.
In a multivariate analysis, the odds of a positive permanent section TAB significantly increased with age (odds ratio, 1.04), vision loss (OR, 2.72), diplopia (OR, 3.33), headache (OR, 2.32), weight loss (OR, 2.37), and anorexia (OR, 5.65).
A total of 60 patients underwent bilateral TABs, and 307 patients underwent bilateral frozen section–guided sequential TABs; the discordance rates based on permanent sections were 5.0% and 5.5%, respectively.
Those discordance rates are “an important result applying to everyone working with patients suspected for GCA,” Patricia Chévez-Barrios, MD, of Houston Methodist Hospital, wrote in an accompanying editorial. “This is on the low end of what was previously published (3%-40%) and supports the relative low need for bilateral synchronous TAB for the diagnosis of GCA.”
A key issue in GCA diagnosis is the need to confirm inflammation, Dr. Chévez-Barrios said. “The surgeon must obtain a significant portion of the artery, and the pathologist should review several sections and levels of the tissue to confidently say whether there is inflammation or no.”
Frozen sections can spare patients from second procedures
The findings suggest a role for frozen section to help to determine whether a unilateral or bilateral simultaneous TAB should be performed, the study authors noted.
“If the frozen section is positive on the first TAB, a contralateral TAB is deferred, given the very low false-positive rate (0.6%). However, if the frozen section does not align with the permanent section result, in particular if the frozen section is positive but permanent section is negative, the patient returns for a TAB on the contralateral side if the GCA suspicion remains high,” they said.
The use of frozen sections requires ideal conditions in order to be effective, Dr. Chévez-Barrios said. The Mayo Clinic approach “is only possible because of their appropriate hospital setting, the training of the histotechnologists, and the experience of the pathologists interpreting the stains and sections. For most pathology laboratories outside of the Mayo Clinic, frozen sections on arteries are the exception and are used only in specific scenarios.”
In addition, the American College of Rheumatology recommends that patients with a high suspicion of GCA should begin corticosteroids as soon as laboratory studies are obtained; “As a result, if a TAB is performed after treatment begins, the typical active pattern of inflammation in the artery changes,” Dr. Chévez-Barrios said. “This further challenges the diagnosis in a frozen section setting because of the need for immunohistochemistry.” Although frozen sections are feasible in specialized settings such as the Mayo Clinic, most patients receive adequate diagnosis and treatment based on permanent sections.
The study findings were limited by several factors including the use of data from patients at a single center and the unique setup of the Mayo Clinic to perform rapid processing of frozen sections, the researchers noted.
“Additionally, we acknowledge that there is controversy regarding the clinical interpretation of healed arteritis. At our institution, healed arteritis is interpreted in the context of patient clinical characteristics and radiographic findings, which may differ from other institutions and may impact the results of this study,” they said.
Overall, the results support the potential of frozen sections in guiding TAB, although “more studies with a comparative analysis of laboratory results, clinical symptoms, and patient demographic characteristics between positive and negative frozen and permanent TAB results are needed to confirm our findings,” they concluded.
The study received no outside funding. One author reported receiving grants from Eli Lilly and Kiniksa Pharmaceuticals as well as personal fees from Genentech-Roche and Sanofi. Dr. Chévez-Barrios had no financial conflicts to disclose.
FROM JAMA OPHTHALMOLOGY
ASDSA warns of rogue insulin pen use for DIY fillers
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
.
In the safety warning, issued on Feb. 18, the ASDSA reported that ASDSA members, all board-certified dermatologists, have seen evidence online of young people using so-called “hyaluron pens” to inject hyaluronic acid filler in the epidermal and upper dermal skin.
The pens being used and promoted in social media for do-it-yourself filler injections are medical devices originally developed for insulin injections. “The use of air pressure technology causes these pens to deliver the hyaluronic acid to insert nanoscale molecules of the filler through the skin,” according to the ASDSA statement. Marketing materials state that the pens can be used to create volume and shape in the lips, and to improve the appearance of nasolabial lines, marionette lines, brow lines known as “elevens,” and forehead wrinkles. Claims that the hyaluronic acid only reaches the papillary layer of the dermis, and is therefore safe, do not alleviate the risk of injury in inexperienced hands, the ASDSA statement points out.
“We are concerned about California children falling prey to products that are not appropriate and safe for them to use,” Elan Newland, MD, member of the ASDSA and the California Society for Dermatology and Dermatological Surgery (CalDerm), said in the statement. “The power of social media is very strong, especially for impressionable teenagers. CalDerm supports alerting consumers and regulators of the dangers of these pens,” he said.
“TikTok is proving to be an extremely powerful platform to communicate, entertain, and even educate, which is why many physicians are getting involved and finding success there. Unfortunately, just like the World Wide Web, there is misinformation there and even dangerous lies,” Sandra Lee, MD, who practices in Upland, Calif. (and is also known as “Dr. Pimple Popper”), said in the statement.
“It’s very concerning to see young people posting a How To on injecting their own lips with hyaluronic acid serum using an ‘airgun’ pen, which acts much like a BB gun to push with force the product under the skin,” she added. “So many things can go wrong.”
The ASDSA has contacted the Food and Drug Administration to report these safety concerns. “In addition, the ASDSA is alerting state medical and estheticians’ boards regarding these patient safety concerns and alerting consumers directly about the risks through social media and other education materials,” according to the statement.
Short sleep predicts incident dementia and all-cause mortality
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).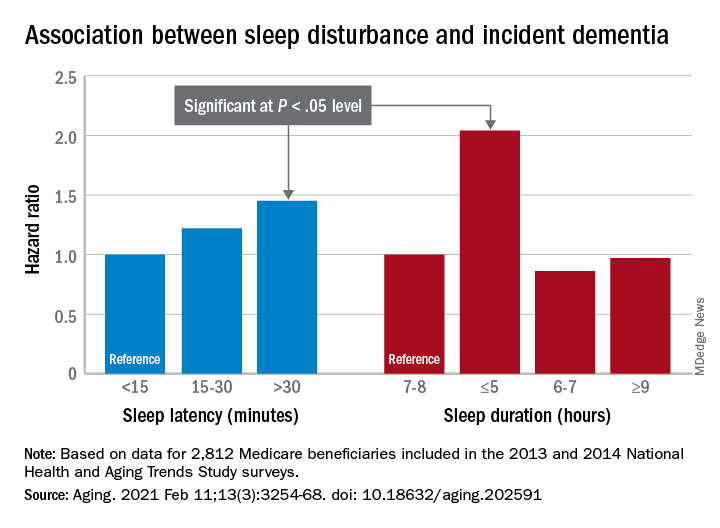
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
More evidence has emerged linking sleep deficiency, dementia, and mortality.
“Sleep disturbance and insufficiency have been shown to be associated with both the development and progression of Alzheimer’s disease and with all-cause mortality,” wrote Rebecca S. Robbins, PhD, of Brigham and Women’s Hospital, Boston, and colleagues. However, research on this topic has yielded conflicting results, and “few studies have included a comprehensive set of sleep characteristics in a single examination of incident dementia and all-cause mortality.”
In a study published in Aging, the researchers identified 2,812 adults aged 65 years and older from the National Health and Aging Trends Study (NHATS), a nationally representative longitudinal study of Medicare beneficiaries aged 65 years and older in the United States.
Participants completed surveys about sleep disturbance and duration in 2013 (1,575 individuals) and in 2014 (1,237 individuals), and the researchers examined the relationship between sleep disturbance and deficiency and incident dementia and all-cause mortality over the next 5 years. The average age of the study participants was 76.9 years, 60% were women, and 72% were White.
Overall, approximately 60% of the participants reported never or rarely having problems with alertness, approximately half said that they rarely or never napped, and more than half said they fell asleep in 15 minutes or less. Approximately 70% rated their sleep quality as good or very good, and more than 90% said they rarely or never snored.
The researchers examined the relationships between sleep characteristics and the development of incident dementia over 5 years. In a fully adjusted Cox multivariate analysis, individuals who slept 5 hours or less per night had approximately twice the risk for incident dementia as those who slept longer (hazard ratio, 2.04); risk of dementia also was higher among those who took 30 minutes or longer to fall asleep (HR, 1.45).
In addition, the risk of all-cause mortality was significantly higher among individuals who reported difficulty maintaining alertness some days or most days/every day (HR, 1.49 and HR, 1.65, respectively), routinely napping some days or most days/every day (HR, 1.38 and HR, 1.73, respectively), poor or very poor sleep quality (HR, 1.75), and sleeping 5 hours or less each night (HR, 2.38).
The study findings were limited by several factors including a population representing only one-quarter of the NHATS cohort, which prevented nationally representative estimates, the availability of only 2 years of sleep data, and small sample size for certain response categories, the researchers noted.
However, “our study offers a contribution to the literature on sleep among aging populations in its assessment of incident dementia and all-cause mortality and a range of sleep characteristics among older adults,” they said. In particular, “short sleep duration was a strong predictor of both incident dementia and all-cause mortality, suggesting this may be a sleep characteristic that is important – over and above the other predictors – of adverse outcomes among older adults,” and future areas for research include the development of novel behavioral interventions to improve sleep in this population.
The study was supported in part by the National Institute for Occupational Safety and Health; the National Heart, Lung, and Blood Institute; the National Institute on Aging; and the Brigham Research Institute Fund to Sustain Research Excellence. Lead author Dr. Robbins disclosed fees from Denihan Hospitality, Rituals Cosmetics, Dagmejan, Asystem, and SleepCycle. Several coauthors disclosed relationships with multiple pharmaceutical companies, and support from various philanthropic organizations.
FROM AGING
Dried blood spot tests show sensitivity as cCMV screen
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
Dried blood spot testing showed sensitivity comparable to saliva as a screening method for congenital cytomegalovirus infection in newborns, based on data from more than 12,000 newborns.
Congenital cytomegalovirus (cCMV) is a common congenital virus in the United States, but remains underrecognized, wrote Sheila C. Dollard, PhD, of the Centers for Disease Control and Prevention in Atlanta, and colleagues.
“Given the burden associated with cCMV and the proven benefits of treatment and early intervention for some affected infants, there has been growing interest in universal newborn screening,” but an ideal screening strategy has yet to be determined, they said.
In a population-based cohort study published in JAMA Pediatrics, the researchers screened 12,554 newborns in Minnesota, including 56 with confirmed CMV infection. The newborns were screened for cCMV via dried blood spots (DBS) and saliva collected 1-2 days after birth. The DBS were tested for CMV DNA via polymerase chain reaction (PCR) at the University of Minnesota (UMN) and the CDC.
The overall sensitivity rate was 85.7% for a combination of laboratory results from the UMN and the CDC, which had separate sensitivities of 73.2% and 76.8%, respectively.
The specificity of the combined results was 100.0% (100% from both UMN and CDC), the combined positive predictive value was 98.0% (100.0% from UMN, 97.7% from CDC), and the combined negative predictive value was 99.9% (99.9% from both UMN and CDC).
By comparison, saliva swab test results showed sensitivity of 92.9%, specificity of 99.9%, positive predictive value of 86.7%, and negative predictive value of 100.0%.
The study findings were limited by several factors including the false-positive and false-negative results from saliva screening. Overall, the false-positive rate was 0.06%, which is comparable to rates from other screening techniques, the researchers said. “The recent Food and Drug Administration approval of a point-of-care neonatal saliva CMV test (Meridian Bioscience), underscores the importance of further clarifying the role of false-positive saliva CMV test results and underscores the requirement for urine confirmation for diagnosis of cCMV,” they added.
However, the study findings support the acceptability and feasibility of cCMV screening, as parents reported generally positive attitudes about the process, the researchers said.
The study is ongoing, and designed to follow infants with confirmed cCMV for up to age 4 years to assess clinical outcomes, they added. “Diagnostic methods are always improving, and therefore, our results show the potential of DBS to provide low-cost CMV screening with smooth integration of sample collection, laboratory testing, and follow-up,” they concluded.
Findings lay foundation for widespread use
“By using enhanced PCR methods, Dollard et al. have rekindled the hope that NBDBS [newborn dried blood spots] testing may be a viable method for large-scale, universal newborn screening for congenital CMV,” Gail J. Demmler-Harrison, MD, of Texas Children’s Hospital, Houston, wrote in an accompanying editorial. Congenital CMV is a common infection, but accurate prevalence remains uncertain because not all newborns are tested, she noted. Detection of CMV currently may involve urine, saliva, and blood, but challenges to the use of these methods include “a variety of constantly evolving DNA detection methods,” she said.
Although urine and saliva samples have been proposed for universal screening, they would require the creation of new sample collection and testing programs. “The routine of collecting the NBDBS samples on all newborns and the logistics of routing them to central laboratories and then reporting results to caregivers is already in place and are strengths of NBDBS samples for universal newborn screening,” but had been limited by a less sensitive platform than urine or saliva, said Dr. Demmler-Harrison.
“The results in the study by Dollard et al. may be a total game changer for the NBDBS proponents,” she emphasized. “Furthermore, scientists who have adapted even more sensitive DNA detection assays, such as the loop-mediated isothermal assay for detection of DNA in clinical samples from newborns, may be able to adapt loop-mediated isothermal assay methodology to detect CMV DNA in NBDBS,” she added.
“By adapting the collection methods, by using optimal filter paper to enhance DNA adherence, by improving DNA elution procedures, and by developing novel amplification and detection methods, NBDBS may soon meet the challenge and reach the sensitivity and specificity necessary for universal screening for congenital CMV,” she concluded.
The study was supported by the CDC, the Minnesota Department of Health, the National Vaccine Program Office (U.S. federal government), and the University of South Carolina Disability Research and Dissemination Center.
Dr. Dollard and Dr. Demmler-Harrison had no financial conflicts to disclose.
FROM JAMA PEDIATRICS