User login
AAD announces diversity initiatives
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
not only within the academy itself, but also in the profession of dermatology overall.
“Last year’s events surrounding social justice issues and the disproportionate impact of COVID-19 on minority communities underscored an urgent need for the academy to outline a strategy to address gaps in diversity, equity, and inclusion across the academy’s programs, provide better access to dermatologic care, and expand the pipeline for prospective dermatologists,” according to an AAD statement introducing the plan.
“The AAD has long recognized the importance of fostering diversity in the dermatology specialty and increasing dermatologic services to underserved populations as a key strategic goal,” Kanya Ferguson, MD, chair of the AAD’s diversity committee, said in an interview.
“The importance and urgency of furthering these goals have been underscored by the social justice events of 2020 and the disproportionate impact that COVID-19 has had, specifically on Black and Latino communities,” added Dr. Ferguson, of the department of dermatology, at the University of Iowa, Iowa City. “The 3-year plan comprehensively expands current diversity, equity, and inclusion initiatives in an effort to accelerate the Academy’s progress toward its strategic goals.”
“Numerous barriers persist that contribute to the narrowing pipeline in medicine and ultimately in dermatology,” Dr. Ferguson noted. “The AAD’s diversity, equity, and inclusion initiatives, toolkits, and resources aim to address some of these barriers through early exposure, pipeline programming, and mentorship.”
As for the next steps, “the diversity committee will be working hard over the next few years to coordinate the integration and adoption of initiatives throughout the Academy’s activities,” she added. “This work will take a significant amount of collaboration and the committee is excited to move this forward in a meaningful and sustainable way.”
The AAD’s diversity committee headed the development of the plan, unanimously approved by the AAD’s board of directors, which outlines four key goals for the next 3 years, presented in the Diversity in Dermatology plan as follows:
“Promote and facilitate diversity, equity, and inclusion within the AAD.” Steps toward this goal include facilitating diverse representation on AAD committees, councils, and task forces, increasing representation of skin of color session speakers and lecture topics at Academy meetings, and ensuring equity in the selection process for awards including the Leadership Forum, Academic Dermatology Leadership Program, Advanced Leadership Forum, Journal of the AAD Editorial Mentorship Program, and other leadership activities.
- “Ensure dermatologic education and research encompasses health disparities and skin of color, and advocate for Black and Latino patient representation in research.” Steps toward this goal include increasing use of images reflecting the full spectrum of skin types, ensuring that skin of color populations receive information about dermatologic diseases, and supporting underrepresented minority (URM) dermatology physician scientists in leadership and professional development.
- “Expand Academy’s Advocacy Priorities to prioritize addressing health inequities.” Steps toward this goal include prioritizing issues that affect minority and marginalized populations, establishing relationships with relevant congressional leadership, and advocating for patient support groups for diseases that disproportionately impact skin of color patients.
- “Increase the number of practicing dermatologists who are underrepresented minorities and provide leadership and professional development programming.” Steps toward this goal include expanding the AAD mentorship program to include physician scientists, expanding diversity champion programs, expanding outreach to URM college students in STEM majors, and launching an AAD Summer Diversity & Inclusion camp for younger students to promote interest in a medical career.
The AAD diversity committee also has assembled a toolkit of resources designed to help its members learn how to talk about race, be an effective ally, and achieve cultural competency. Additional updated resources include guidelines on mentorship and outreach.
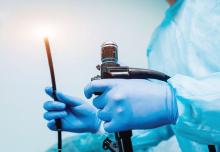
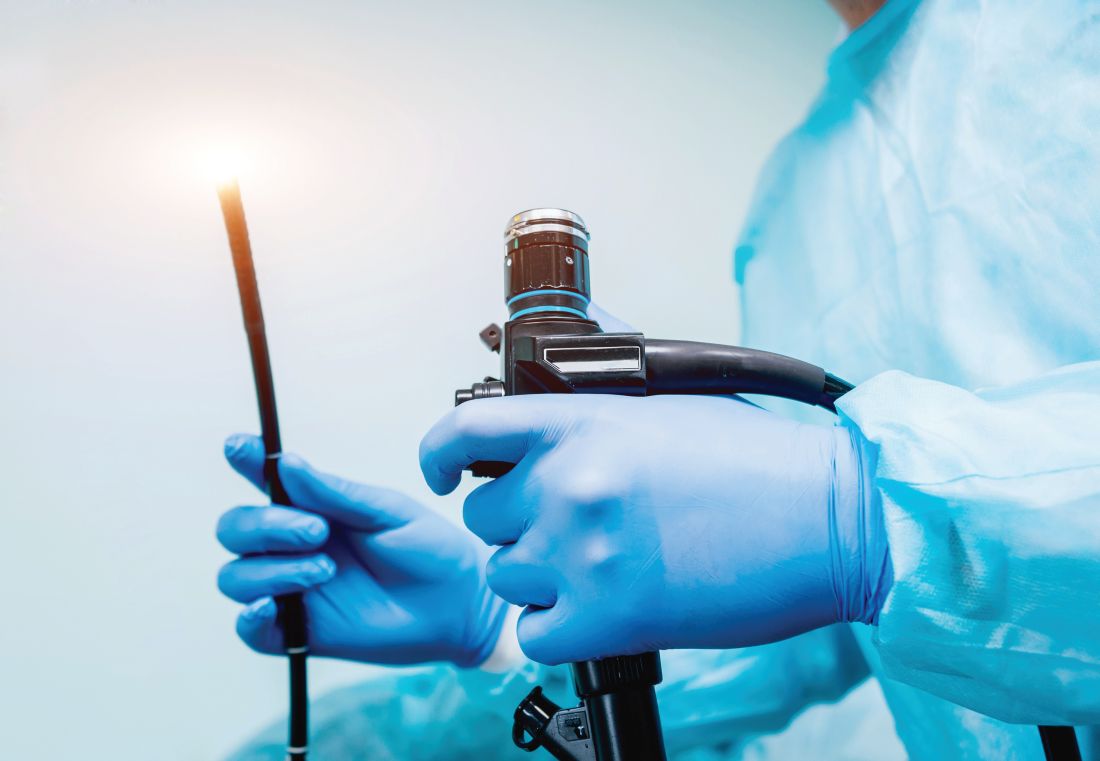
Ergonomic consultation spares endoscopists a pain in the neck
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
Assessment of position and posture by a physical therapist can help reduce and prevent injury in endoscopists, based on data from a pilot study of eight individuals.
Musculoskeletal injuries among endoscopists are gaining more attention: One technical review indicated that the “prevalence of musculoskeletal pain or injuries ranged from 29% to 89% of gastroenterologists.” While data on avoiding musculoskeletal injury related to endoscopy are limited, recognition of the role of ergonomics is increasing, Stacy A. Markwell, a physical therapist in Chapel Hill, N.C., and colleagues, wrote in a study published in Gastrointestinal Endoscopy.
The mental concentration required along with the physical demands on manipulating the scope have been shown to negatively impact posture, the researchers noted.
The researchers reviewed data from eight endoscopists who were aged 32-71 years; they had a range of clinical experience and were performing 6-30 colonoscopies and 3-21 upper endoscopies per week.
These endoscopists volunteered for an ergonomic intervention involving use of an individualized wellness plan. They completed the Nordic Musculoskeletal Questionnaire to evaluate musculoskeletal complaints during the past 12 months and the past 7 days. Three of the eight participants reported pain at work at initial assessment, which often worsened over the course of the day, and five mentioned fatigue while working. They specified 22 pain sites, mainly in the neck and back. In addition, participants were photographed to evaluate posture in a static position and self-selected “tired” positions.
“When frequent or consistent posturing resulted in suboptimal joint alignment, muscle length, loading at end range of muscle or joints, and/or prolonged static active positioning, participants were photographed to provide personalized feedback for wellness education,” the researchers wrote.
The physical therapist used information from the evaluation and photographs to develop individual plans to improve the ergonomics of the endoscopic suite with adjustments to the location of the bed and positioning of chairs, standing surfaces, and monitors and keyboards. In addition to adjusting the endoscopic suite, the physical therapist developed individual wellness plans including exercises to relieve pain and improve posture, as well as pain education to help clinicians recognize and manage pain and fatigue.
By the end of the study, in a follow-up 6-12 months after the wellness intervention, 63% of pain sites (14 of 22) reported by participants were reduced in intensity or resolved, 32% were unchanged (7 of 22), and 4% increased (1 of 22).
Overall, seven of the eight participants said that the pictures of their posture along with the movement analysis was helpful, and three participants asked for reassessment by the physical therapist. In this study, the average cost of the wellness program was $500.
“All endoscopists reported that the wellness plan was helpful, with procedure suite and posture recommendations being the most beneficial,” the researchers reported. “Upon gaining insight with visualization of their posture and movement during endoscopy, participants’ understanding and motivation to make corrections was intensified.”
The study findings were limited by several factors including the small size, use of a single physical therapist, short follow-up, lack of controls, and use of a single site, the researchers noted. However, “our study provides a detailed, pragmatic, and reproducible framework for performing an individualized physical therapist–directed comprehensive assessment and personalized wellness plan in the workplace to help meet the challenges of ergonomics in endoscopy.”
Recognition of the value of ergonomics is rising
“Endoscopy related injury and disability is a known hazard of our profession,” said Gyanprakash A. Ketwaroo, MD, of Baylor College of Medicine, Houston, in an interview. “Any studies to assess and, more importantly, offer ways to prevent such injury are immediately relevant. In this context, ergonomics for endoscopy is an increasing area of research.”
Dr. Ketwaroo said that the study results were not surprising. “I agree with authors that there is a paucity of general ergonomic training and assessment. Specific individualized wellness plans are rare. Developing an individual plan based on observation by physical therapists, and taking into account baseline injury or predisposition to injury would be expected to be more high yield for preventing injury and improving performance
“I believe the main take-home message from the study is that an individualized ergonomic plan based on assessment and feedback by physical therapists appears promising for optimizing endoscopic performance to minimize injury and reduce fatigue,” Dr. Ketwaroo said. However, “long-term studies in much larger samples will be needed to document objective findings of reduced injury or fatigue.”
The study received no outside funding. The researchers had no financial conflicts to disclose. Dr. Ketwaroo serves on the GI & Hepatology News editorial advisory board.
FROM GASTROINTESTINAL ENDOSCOPY
Zika vaccine candidate shows promise in phase 1 trial
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
in a phase 1 study.
Although Zika cases have declined in recent years, “geographic expansion of the Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” wrote Nadine C. Salisch, PhD, of Janssen Vaccines and Prevention, Leiden, the Netherlands, and colleagues in a paper published in Annals of Internal Medicine.
No vaccine against Zika is yet available, although more than 10 candidates have been studied in preclinical trials to date, they said.
The researchers randomized 100 healthy adult volunteers to an experimental Zika vaccine candidate known as Ad26.ZIKV.001 in either one-dose or two-dose regimens of 5x1010 viral particles (low dose) or 1x1011 viral particles (high dose) or placebo. Approximately half (55%) of the participants were women, and 72% were White.
Approximately 80% of patients in both two-dose groups showed antibody responses for a year after vaccination. Geometric mean titers (GMTs) reached peak of 823.4 in the low-dose/low-dose group and 961.5 in the high-dose/high-dose group. At day 365, the GMTs for these groups were 68.7 and 87.0, respectively.
A single high-dose vaccine achieved a similar level of neutralizing antibody titers, but lower peak neutralizing responses than the two-dose strategies, the researchers noted.
Most of the reported adverse events were mild to moderate, and short lived; the most common were injection site pain or tenderness, headache, and fatigue, the researchers said. After the first vaccination, 75% of participants in the low-dose groups, 88% of participants in high-dose groups, and 45% of participants receiving placebo reported local adverse events. In addition, 73%, 83%, and 40% of the participants in the low-dose, high-dose, and placebo groups, respectively, reported systemic adverse events. Reports were similar after the second vaccination. Two serious adverse events not related to vaccination were reported; one case of right lower lobe pneumonia and one case of incomplete spontaneous abortion.
The researchers also explored protective efficacy through a nonlethal mouse challenge model. “Transfer of 6 mg of IgG from Ad26.ZIKV.001 vaccines conferred complete protection from viremia in most recipient animals, with statistically significantly decreased breakthrough rates and cumulative viral loads per group compared with placebo,” they said.
The study findings were limited by the inability to assess safety and immunogenicity in an endemic area, the researchers noted. However, “Ad26.ZIKV.001 induces potent ZIKV-specific neutralizing responses with durability of at least 1 year, which supports further clinical development if an unmet medical need reemerges,” they said. “In addition, these data underscore the performance of the Ad26 vaccine platform, which Janssen is using for different infectious diseases, including COVID-19,” they noted.
Ad26 vector platform shows consistency
“Development of the investigational Janssen Zika vaccine candidate was initiated in 2015, and while the incidence of Zika virus has declined since the 2015-2016 outbreak, spread of the ‘carrier’ Aedes aegypti mosquito to areas where population-level immunity is low poses a substantial risk for future epidemics,” lead author Dr. Salisch said in an interview. For this reason, researchers say the vaccine warrants further development should the need reemerge, she said.
“Our research has found that while a single higher-dose regimen had lower peak neutralizing responses than a two-dose regimen, it achieved a similar level of neutralizing antibody responses at 1 year, an encouraging finding that shows our vaccine may be a useful tool to curb Zika epidemics,” Dr. Salisch noted. “Previous experience with the Ad26 vector platform across our investigational vaccine programs have yielded similarly promising results, most recently with our investigational Janssen COVID-19 vaccine program, for which phase 3 data show a single-dose vaccine met all primary and key secondary endpoints,” she said.
“The biggest barrier [to further development of the candidate vaccine] is one that we actually consider ourselves fortunate to have: The very low incidence of reported Zika cases currently reported worldwide,” Dr. Salisch said. “However, the current Zika case rate can change at any time, and in the event the situation demands it, we are open to alternative regulatory pathways to help us glean the necessary insights on vaccine safety and efficacy to further advance the development of this candidate,” she emphasized.
As for additional research, “there are still questions surrounding Zika transmission and the pathomechanism of congenital Zika syndrome,” said Dr. Salisch. “Our hope is that a correlate of protection against Zika disease, and in particular against congenital Zika syndrome, can be identified,” she said.
Consider pregnant women in next phase of research
“A major hurdle in ZIKV vaccine development is the inability to conduct large efficacy studies in the absence of a current outbreak,” Ann Chahroudi, MD, of Emory University, Atlanta, and Sallie Permar, MD, of Weill Cornell Medicine, New York, wrote in an accompanying editorial.
The current study provided some efficacy data using a mouse model, but “these data are obviously not conclusive for human protection,” they said.
“A further challenge for ZIKV vaccine efficacy trials will be to demonstrate fetal protection from [congenital Zika syndrome] after adult immunization. There should be a clear plan to readily deploy phase 3 trials for the most promising vaccines to emerge from phase 1 and 2 in the event of an outbreak, as was implemented for Ebola, including infant follow-up,” they emphasized.
The editorialists noted that the study did not include pregnant women, who represent a major target for immunization, but they said that vaccination of pregnant women against other neonatal pathogens such as influenza and tetanus has been effective. “Candidate ZIKV vaccines proven safe in phase 1 trials should immediately be assessed for safety and efficacy in pregnant women,” they said. Although Zika infections are not at epidemic levels currently, resurgence remains a possibility and the coronavirus pandemic “has taught us that preparedness for emerging infections is crucial,” they said.
Zika vaccine research is a challenge worth pursuing
“It is important to continue Zika vaccine research because of the unpredictable nature of that infection,” Kevin Ault, MD, of the University of Kansas, Kansas City, said in an interview. “Several times Zika has gained a foothold in unexposed and vulnerable populations,” Dr. Ault said. “Additionally, there are some data about using this vector during pregnancy, and eventually this vaccine may prevent the birth defects associated with Zika infections during pregnancy, he noted.
Dr. Ault said he was not surprised by the study findings. “This is a promising early phase vaccine candidate, and this adenovirus vector has been used in other similar trials,” he said. Potential barriers to vaccine development include the challenge of conducting late phase clinical trials in pregnant women, he noted. “The relevant endpoint is going to be clinical disease, and one of the most critical populations is pregnant women,” he said. In addition, “later phase 3 trials would be conducted in a population where there is an ongoing Zika outbreak,” Dr. Ault emphasized.
The study was supported by Janssen Vaccines and Infectious Diseases.
Dr. Chahroudi had no financial conflicts to disclose. Dr. Permar disclosed grants from Merck and Moderna unrelated to the current study. Dr. Ault had no relevant financial conflicts to disclose; he has served as an adviser to the Centers for Disease Control and Prevention, the World Medical Association, the National Vaccine Program Office, and the National Institute for Allergy and Infectious Diseases. He is a fellow of the Infectious Disease Society of American and a fellow of ACOG.
FROM ANNALS OF INTERNAL MEDICINE
Menopause transition affects heart health risks
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
Menopause is a key time to monitor women for the development or increase of cardiovascular risk factors, according to a new consensus statement developed by the Task Force on Gender of the European Society of Cardiology and a multidisciplinary ESC working group on Women’s Health in Menopause.
“After menopause, traditional cardiovascular risk factors are adversely affected – particularly hypertension,” wrote Angela H.E.M. Maas, MD, of Radboud University Medical Center, Nijmegen, Netherlands, and colleagues.
“Since the first ESC consensus paper on the management of cardiovascular risk in perimenopausal women was published in 2007, we have a greater understanding on the role of female-specific risk factors for cardiovascular disease (CVD),” they said.
In a consensus statement published in the European Heart Journal, the authors presented clinical guidance for diagnosis and management of cardiovascular risk factors during the menopause transition. The transition to menopause increases a woman’s risk for developing several CVD risk factors, including central adiposity, increased insulin resistance, a proatherogenic lipid profile, and autonomic dysfunction that can contribute to increased heart rate variability, according to the statement.
Estrogen changes may affect ischemic disease
In general, obstructive coronary artery disease (CAD) strikes women later than men, but coronary vasomotor conditions are a common cause of ischemic heart disease in women with or without CAD, the authors noted.
“Lower estrogen levels after menopause are related to altered vascular function, enhanced inflammation, and up-regulation of other hormonal systems such as the renin–angiotensin–aldosterone system, the sympathetic nervous system, and reduced nitric oxide–dependent vasodilation,” they wrote. They recommended use of the coronary artery calcium score for screening middle-aged women who are symptomatic or at intermediate cardiovascular risk.
The transition to menopause causes changes in lipid profiles, and a rise in blood pressure in particular “may be both a direct effect of hormonal changes on the vasculature and metabolic changes with aging,” but hypertension in early post menopause is “often poorly managed,” the authors noted.
Compared with asymptomatic women, women who suffer from severe menopausal symptoms often have increased cardiovascular disease risk factors. For example, the Women’s Health Initiative (WHI) study showed a 48% increased risk of incident diabetes at follow-up in women with severe symptoms of hot flashes and night sweats, the authors wrote. Clinicians should also be aware of the increased immune reactivity that occurs during and after menopause and the increased CVD risk associated with autoimmune and endocrine disorders, they said.
Multiple strategies to reduce risk
Strategies to address the cardiovascular risk in menopause include assessing glucose, lipid levels, and blood pressure during the transition to menopause, according to the statement.
In addition, they recommended increasing employer awareness of menopause, as changes may interfere with working ability. A healthy lifestyle including healthy diet and regular exercise can help reduce cardiovascular risks and relieve symptoms. Menopausal hormone therapy (MHT) may be indicated to relieve symptoms, including symptoms of depression, and provide cardioprotection for younger women around the time of menopause, according to the statement.
However, “MHT is not recommended in women at high CV risk and after a previous CVD event,” and all women should be assessed for cardiovascular risk factors before starting MHT, they emphasized.
Results raise awareness of cardiovascular health and menopause link
“Over the past 20 years, our knowledge of how menopause might contribute to cardiovascular disease has dramatically evolved,” said Samar El Khoudary, MD, of the University of Pittsburg, in an interview.
“We have accumulated data that consistently point to the menopause transition as a time of change in cardiovascular health. As such, there is a compelling need to discuss the implications of the accumulating body of literature on this topic,” she said. “The goal is to raise awareness for both health care providers and women of the significant adverse cardiovascular health changes accompanying the menopause transition and to point out the importance of adopting prevention strategies early during this stage,” she explained.
The impact of the hormonal changes of menopause on CVD risk “is very complex,” Dr. El Khoudary said. “Until now, we could not prove that using estrogen therapy is cardioprotective,” she emphasized. “Studies point to the need to consider the timing of hormone use, as well as types and route of administration,” she noted. “The truth is that, although the menopause transition is associated with an acceleration in CVD risk, the exact mechanism still is not completely clear. Hormone changes contribute, but they are not the ultimate contributor,” she added.
Research gaps include data on lifestyle and behavioral interventions
“Irrespective of the accumulating findings showing adverse changes in multiple cardiovascular health parameters, as women transition through menopause, we do not have data documenting current status of ideal cardiovascular health components during the menopause transition among women,” said Dr. El Khoudary. “The limited data we have [suggest] that a very small proportion of women transitioning through menopause eat a healthy diet (less than 20%) or practice physical activity (about7.2%) at a level that matches the current recommendations,” she noted.
“Lifestyle and behavioral interventions are critical to maintain a healthy heart and reduce heart disease; we do not have adequate randomized clinical trials testing these interventions specifically during the menopause transition,” she said.
“Similarly, we are in need of randomized clinical trials of therapeutic interventions such as lipid-lowering medications and menopause hormone therapy in women transitioning through menopause,” said Dr. El Khoudary. “This high-risk population has not been the focus of previous clinical trials, leaving us with questions of how the results from these studies might apply to women during the menopause transition,” she said.
Consensus invites collaboration
“I commend the group for putting together a statement that crosses practice and specialty boundaries,” said Lubna Pal, MD, of Yale School of Medicine, New Haven, Conn., in an interview. Although the statement does not present novel information, it “has the power of unifying the various providers by bringing focus on the individual elements spanning a woman’s life that cumulatively determine her lifetime health risk,” she said. Preeclampsia may be a risk factor for cardiovascular disease later in life, and events in reproductive age may determine a woman’s trajectory during the transition to menopause and beyond, Dr. Pal noted.
“The consensus statement will likely be read by internists and family medicine providers as well as ob.gyns.; it encourages all those involved in caring for female patients to take on the responsibility of ‘passing on the baton,’ such that all women who are deemed at an enhanced risk for cardiovascular disease are assured due diligence in care through stringent surveillance and timely interventions,” said Dr. Pal. “It is a call for the various providers who care for women at distinct stages of life to work together toward a shared goal of optimizing every woman’s health across her lifespan,” she said.
“More research is needed for us to better understand the mechanisms at play” in the development of cardiovascular risk and in understanding the continuity of changes across women’s lifespans, Dr. Pal said. “We have associations, but not much information about causation,” she emphasized. However, the statement promotes the dissemination of information about women’s health and sensitizes providers to the potential and the power of preventive care. “We should be much more liberal and loud in holding conversations about risk quantification and risk reduction, and this statement is a resounding effort toward identifying and mitigating long-term cardiovascular risk, even if only through promoting a healthier lifestyle in those deemed at risk,” she added.
The statement received no outside funding. Lead author Dr. Maas had no financial conflicts to disclose. Dr. El Khoudary had no financial conflicts to disclose. Dr. Pal had no relevant financial conflicts to disclose.
FROM THE EUROPEAN HEART JOURNAL
Updated WIC in pregnancy boosts infant outcomes
Developmental outcomes in the first 2 years of life improved in children whose mothers received the revised Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) while pregnant, based on data from approximately 1,200 women.
Maternal nutrition is essential to healthy fetal development, and the WIC was revised in 2009 to align with current dietary guidelines and to support the health of women and children in low-income households, wrote Alice Guan, MPH, of the University of California, San Francisco, and colleagues.
“However, no researchers, to our knowledge, have evaluated effects of this revision on downstream child health or development,” they said.
In a study published in Pediatrics, the researchers reviewed data from mothers and their children who participated in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) longitudinal cohort study conducted in Tennessee between 2006 and 2011. Their quasi-experimental analysis included 700 women who received WIC during pregnancy and 525 women who did not.
The researchers considered core developmental outcomes of child growth, cognitive development, and socioemotional development at age 12 months and 24 months, and age 4-6 years.
Overall, infants of women who received the WIC food package showed significant increases in length-for-age z scores at 12 months of age (.33, representing approximately one-fifth of a standard deviation), compared to infants of women who did not receive the revised WIC package.
In addition, the Bayley Scales of Infant Development cognitive composite score showed a 4.3-point increase at 24 months of age (approximately one-third of a standard deviation) compared to infants of women who did not receive the revised WIC package.
No effects on growth at age 24 months or on cognitive development at age 4-6 years were noted, which suggests that the impact of the WIC program during pregnancy may fade over time, the researchers said.
“The magnitude of the findings in this study represents clinically relevant effect sizes and provides evidence that one of the largest U.S. safety net policies improves developmental outcomes among low-income and marginalized children,” they noted.
The study findings were limited by several factors including the statistical, quasi-experimental design; the reliance on self-reports for information on income, receipt of WIC, and other variables; and a potential lack of generalizability to other states, the researchers noted. However, the results support findings from previous studies and were strengthened by the review of multiple outcomes and use of a longitudinal database, they said.
“These findings provide timely and critical evidence for the role that WIC plays in improving the health of the nation’s most vulnerable populations, suggesting meaningful impacts of the revised WIC food package on child development,” the researchers said. In addition, “considering the relatively modest scope of the 2009 revision, more substantial updates to the program based on up-to-date nutritional guidance may have substantial effects on improving the health of WIC recipients,” they concluded.
Findings support program’s value
“Pediatrics has always had a commitment to reducing disparities in health care, and we are the main clinicians to see many Medicaid patients on a regular basis,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“We all know that pregnant women eating nutritiously ought to help child outcomes, but the current study provides an evidence base for something that seems like common sense,” he noted.
Having such an evidence base is helpful to reinforce the value of the WIC program for its intended recipients, especially in the wake of the COVID-19 pandemic when many funding sources are stretched thin, Dr. Lessin said.
The WIC is intended to try to reduce racial and socioeconomic disparities in the most basic form possible, by helping people who are disadvantaged get enough high-quality food to eat, but results of the program’s impact have not been well studied, he said.
“Outcomes are fiendishly difficult to measure,” and the study is subject to the limitations of its statistical nature, he said. But the large sample size adds support to the findings, which are encouraging, Dr. Lessin noted.
Other potential areas for research include comparing the quality of WIC programs in different states, but such research is very difficult, Dr. Lessin noted. However, the findings might encourage states with less robust WIC programs to consider increasing support, he said.
The study was funded by the National Institutes of Health (National Heart, Lung, and Blood Institute); the National Institute on Aging; the University of California, San Francisco, National Center of Excellence in Women’s Health; and the Urban Child Institute. The researchers had no financial conflicts to disclose. Dr. Lessin serves on the editorial advisory board of Pediatric News and had no relevant financial conflicts to disclose.
Developmental outcomes in the first 2 years of life improved in children whose mothers received the revised Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) while pregnant, based on data from approximately 1,200 women.
Maternal nutrition is essential to healthy fetal development, and the WIC was revised in 2009 to align with current dietary guidelines and to support the health of women and children in low-income households, wrote Alice Guan, MPH, of the University of California, San Francisco, and colleagues.
“However, no researchers, to our knowledge, have evaluated effects of this revision on downstream child health or development,” they said.
In a study published in Pediatrics, the researchers reviewed data from mothers and their children who participated in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) longitudinal cohort study conducted in Tennessee between 2006 and 2011. Their quasi-experimental analysis included 700 women who received WIC during pregnancy and 525 women who did not.
The researchers considered core developmental outcomes of child growth, cognitive development, and socioemotional development at age 12 months and 24 months, and age 4-6 years.
Overall, infants of women who received the WIC food package showed significant increases in length-for-age z scores at 12 months of age (.33, representing approximately one-fifth of a standard deviation), compared to infants of women who did not receive the revised WIC package.
In addition, the Bayley Scales of Infant Development cognitive composite score showed a 4.3-point increase at 24 months of age (approximately one-third of a standard deviation) compared to infants of women who did not receive the revised WIC package.
No effects on growth at age 24 months or on cognitive development at age 4-6 years were noted, which suggests that the impact of the WIC program during pregnancy may fade over time, the researchers said.
“The magnitude of the findings in this study represents clinically relevant effect sizes and provides evidence that one of the largest U.S. safety net policies improves developmental outcomes among low-income and marginalized children,” they noted.
The study findings were limited by several factors including the statistical, quasi-experimental design; the reliance on self-reports for information on income, receipt of WIC, and other variables; and a potential lack of generalizability to other states, the researchers noted. However, the results support findings from previous studies and were strengthened by the review of multiple outcomes and use of a longitudinal database, they said.
“These findings provide timely and critical evidence for the role that WIC plays in improving the health of the nation’s most vulnerable populations, suggesting meaningful impacts of the revised WIC food package on child development,” the researchers said. In addition, “considering the relatively modest scope of the 2009 revision, more substantial updates to the program based on up-to-date nutritional guidance may have substantial effects on improving the health of WIC recipients,” they concluded.
Findings support program’s value
“Pediatrics has always had a commitment to reducing disparities in health care, and we are the main clinicians to see many Medicaid patients on a regular basis,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“We all know that pregnant women eating nutritiously ought to help child outcomes, but the current study provides an evidence base for something that seems like common sense,” he noted.
Having such an evidence base is helpful to reinforce the value of the WIC program for its intended recipients, especially in the wake of the COVID-19 pandemic when many funding sources are stretched thin, Dr. Lessin said.
The WIC is intended to try to reduce racial and socioeconomic disparities in the most basic form possible, by helping people who are disadvantaged get enough high-quality food to eat, but results of the program’s impact have not been well studied, he said.
“Outcomes are fiendishly difficult to measure,” and the study is subject to the limitations of its statistical nature, he said. But the large sample size adds support to the findings, which are encouraging, Dr. Lessin noted.
Other potential areas for research include comparing the quality of WIC programs in different states, but such research is very difficult, Dr. Lessin noted. However, the findings might encourage states with less robust WIC programs to consider increasing support, he said.
The study was funded by the National Institutes of Health (National Heart, Lung, and Blood Institute); the National Institute on Aging; the University of California, San Francisco, National Center of Excellence in Women’s Health; and the Urban Child Institute. The researchers had no financial conflicts to disclose. Dr. Lessin serves on the editorial advisory board of Pediatric News and had no relevant financial conflicts to disclose.
Developmental outcomes in the first 2 years of life improved in children whose mothers received the revised Special Supplemental Nutrition Program for Women, Infants, and Children (WIC) while pregnant, based on data from approximately 1,200 women.
Maternal nutrition is essential to healthy fetal development, and the WIC was revised in 2009 to align with current dietary guidelines and to support the health of women and children in low-income households, wrote Alice Guan, MPH, of the University of California, San Francisco, and colleagues.
“However, no researchers, to our knowledge, have evaluated effects of this revision on downstream child health or development,” they said.
In a study published in Pediatrics, the researchers reviewed data from mothers and their children who participated in the Conditions Affecting Neurocognitive Development and Learning in Early Childhood (CANDLE) longitudinal cohort study conducted in Tennessee between 2006 and 2011. Their quasi-experimental analysis included 700 women who received WIC during pregnancy and 525 women who did not.
The researchers considered core developmental outcomes of child growth, cognitive development, and socioemotional development at age 12 months and 24 months, and age 4-6 years.
Overall, infants of women who received the WIC food package showed significant increases in length-for-age z scores at 12 months of age (.33, representing approximately one-fifth of a standard deviation), compared to infants of women who did not receive the revised WIC package.
In addition, the Bayley Scales of Infant Development cognitive composite score showed a 4.3-point increase at 24 months of age (approximately one-third of a standard deviation) compared to infants of women who did not receive the revised WIC package.
No effects on growth at age 24 months or on cognitive development at age 4-6 years were noted, which suggests that the impact of the WIC program during pregnancy may fade over time, the researchers said.
“The magnitude of the findings in this study represents clinically relevant effect sizes and provides evidence that one of the largest U.S. safety net policies improves developmental outcomes among low-income and marginalized children,” they noted.
The study findings were limited by several factors including the statistical, quasi-experimental design; the reliance on self-reports for information on income, receipt of WIC, and other variables; and a potential lack of generalizability to other states, the researchers noted. However, the results support findings from previous studies and were strengthened by the review of multiple outcomes and use of a longitudinal database, they said.
“These findings provide timely and critical evidence for the role that WIC plays in improving the health of the nation’s most vulnerable populations, suggesting meaningful impacts of the revised WIC food package on child development,” the researchers said. In addition, “considering the relatively modest scope of the 2009 revision, more substantial updates to the program based on up-to-date nutritional guidance may have substantial effects on improving the health of WIC recipients,” they concluded.
Findings support program’s value
“Pediatrics has always had a commitment to reducing disparities in health care, and we are the main clinicians to see many Medicaid patients on a regular basis,” Herschel Lessin, MD, of Children’s Medical Group, Poughkeepsie, N.Y., said in an interview.
“We all know that pregnant women eating nutritiously ought to help child outcomes, but the current study provides an evidence base for something that seems like common sense,” he noted.
Having such an evidence base is helpful to reinforce the value of the WIC program for its intended recipients, especially in the wake of the COVID-19 pandemic when many funding sources are stretched thin, Dr. Lessin said.
The WIC is intended to try to reduce racial and socioeconomic disparities in the most basic form possible, by helping people who are disadvantaged get enough high-quality food to eat, but results of the program’s impact have not been well studied, he said.
“Outcomes are fiendishly difficult to measure,” and the study is subject to the limitations of its statistical nature, he said. But the large sample size adds support to the findings, which are encouraging, Dr. Lessin noted.
Other potential areas for research include comparing the quality of WIC programs in different states, but such research is very difficult, Dr. Lessin noted. However, the findings might encourage states with less robust WIC programs to consider increasing support, he said.
The study was funded by the National Institutes of Health (National Heart, Lung, and Blood Institute); the National Institute on Aging; the University of California, San Francisco, National Center of Excellence in Women’s Health; and the Urban Child Institute. The researchers had no financial conflicts to disclose. Dr. Lessin serves on the editorial advisory board of Pediatric News and had no relevant financial conflicts to disclose.
FROM PEDIATRICS
TNF inhibitors may slow spinal progression in axial spondyloarthritis
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
Patients with axial spondyloarthritis showed reduced spinal radiographic progression after treatment with tumor necrosis factor (TNF) inhibitors, based on data from 314 adults in a prospective cohort study.
Evidence of a link between inflammation and axial damage in patients with axial spondyloarthritis (axSpA) has been reported, and these patients are routinely treated with NSAIDs and TNF inhibitors (TNFi), wrote Alexandre Sepriano, MD, PhD, of Leiden (the Netherlands) University Medical Center, and colleagues.
“However, and despite significant efforts, it remains to be clarified whether there is also an effect of these drugs on axial damage accrual,” they noted.
In a study published in Arthritis & Rheumatology, the researchers recruited consecutive patients from rheumatology practices in Northern Alberta to enroll in the Follow Up Research Cohort in Ankylosing Spondylitis Treatment (ALBERTA FORCAST) observational cohort study. The average age of the patients was 41 years, 74% were men, 83% were HLA-B27 positive, and the average duration of symptoms was 18 years.
Progression was measured via spine radiographs every 2 years for up to 10 years; the radiographs were scored using the modified Stoke Ankylosing Spondylitis Spine Score (mSASSS). In addition, the researchers assessed the interaction between TNFi exposure and clinical disease activity using the Ankylosing Spondylitis Disease Activity Score (ASDAS) and the impact on mSASSS every 2 years. The analysis included 442 2-year intervals.
Overall, the researchers found a significant interaction between ASDAS and TNFi at the start of the interval, followed by gradient effect of ASDAS at the start of the interval on mSASSS 2 years later, which was more than twice as high in patients never treated with TNFi (beta = 0.41), compared with patients who were continuously treated with a TNFi (beta = 0.16).
“Similarly, patients treated with TNFi were 30% less likely to develop a new syndesmophyte 2 years later compared to those not treated,” the researchers said.
TNFi also directly slowed progression, as treated patients averaged 0.85 mSASSS units less 2 years later, compared with untreated patients.
Of note, “treatment with NSAIDs during follow-up was neither associated with the outcome nor did it modify or confound the association between TNFi and mSASSS,” the researchers said. In addition, “the direct effect of TNFi on mSASSS was still present after adjusting for a propensity score,” they wrote.
The study results were limited by several factors including the observational design, lack of data on long-term treatment effects, and inability to assess individual TNFi drugs separately, the researchers noted.
However, “the present study informs the rheumatology community by addressing the question as to whether or not TNFi inhibit radiographic progression in axSpA and if this effect is mediated solely by their effects on inflammation, as measured by the ASDAS, or whether additional mechanisms may be relevant,” they emphasized.
“A better understanding of these mechanisms might open avenues to further treatment strategies that might finally lead to effective disease modification in axial SpA,” they concluded.
The ALBERTA FORCAST study was supported by AbbVie. Several authors disclosed financial relationships with AbbVie and other manufacturers of TNFi.
FROM ARTHRITIS & RHEUMATOLOGY
FDA alert confirms heart and cancer risks with tofacitinib (Xeljanz)
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
FDA alert confirms heart and cancer risks with tofacitinib (Xeljanz)
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
The Food and Drug Administration has alerted the public to an increased risk of serious heart-related problems and cancer risk associated with the Janus kinase inhibitor tofacitinib (Xeljanz, Xeljanz XR), based on early results from a safety clinical trial comparing tofacitinib and tumor necrosis factor inhibitors in patients with rheumatoid arthritis (RA).
The FDA is awaiting further results from the trial, but in a safety communication issued on Feb. 4, the agency advised patients not to discontinue tofacitinib without consulting their health care providers and advised health care professionals to weigh the risks and benefits when prescribing the drug and continue to follow the current prescribing information.
Tofacitinib was approved for treatment of RA in 2012 at a 5-mg dose. After this approval, the FDA required drug manufacturer Pfizer to conduct a safety clinical trial that included the 5-mg twice-daily dose and a 10-mg twice-daily dose that is currently approved only for ulcerative colitis. In addition to RA and ulcerative colitis, tofacitinib is approved for adults with active psoriatic arthritis and patients aged 2 years or older with active polyarticular course juvenile idiopathic arthritis.
Pfizer announced partial results of the study, known as the ORAL Surveillance trial, in a press release on Jan. 27. The randomized trial included 4,362 RA patients aged 50 years and older who received either 5-mg or 10-mg doses of tofacitinib or a TNF inhibitor (adalimumab or etanercept).
The full results have yet to be released, but based on data from approximately 10,000 person-years for the combined tofacitinib groups and approximately 5,000 person-years for the TNF inhibitor group, the rate of major cardiovascular adverse events was significantly higher in the combined tofacitinib group, compared with the TNF inhibitor group (0.98 vs. 0.73 per 100 person-years; hazard ratio, 1.33). In addition, the rate of adjudicated malignancies was significantly higher in the tofacitinib group, compared with the TNF inhibitor group (1.13 vs. 0.77 per 100 person-years; HR, 1.48).
In February 2019, the FDA issued a warning stating an increased risk of pulmonary embolism and death associated with the 10-mg twice-daily dose of tofacitinib, following interims results from the safety study.
In July 2019, the FDA added a boxed warning to tofacitinib advising of the increased risk for pulmonary embolism and death associated with the 10-mg twice-daily dose.
The FDA encouraged health care professionals and patients to report any side effects from tofacitinib or other medications through the FDA MedWatch program online or by phone at 1-800-332-1088.
Until nuances revealed, no change in practice
The preliminary study findings contain some nuances that are a bit complicated from a statistical standpoint, according to Daniel Furst, MD, professor emeritus of medicine at the University of California, Los Angeles; adjunct professor at the University of Washington, Seattle; and research professor at the University of Florence (Italy).
This is supposed to be a noninferiority study, so something might not be noninferior, “but that doesn’t mean it is inferior,” explained Dr. Furst, who is also a member of the MDedge Rheumatology Editorial Advisory Board.
Dr. Furst said he was surprised by the study findings, because “I didn’t expect there to be any differences, and in fact it is not clear how great the differences are” among the groups in the study, he said.
When the complete findings are released, in one of the instances, “the statistics may show a very small statistical difference that indicates we may have to be more careful in this particularly high-risk group,” Dr. Furst noted.
“When we understand the data more closely, we may find that there are some nuances we need to be careful about,” he said. However, “until those data are out, I would not make any changes in my practice.”
Whether the current study findings represent a class effect is “impossible to say,” since tofacitinib affects three enzymes, while other JAK inhibitors affect only one or two, he noted.
Dr. Furst disclosed receiving grant/research support from and/or consulting for AbbVie, Actelion, Amgen, Bristol-Myers Squibb, Corbus, the National Institutes of Health, Novartis, Pfizer, and Roche/Genentech.
Updated on 2/8/2021.
Survey finds practice gaps in counseling women with hidradenitis suppurativa about pregnancy
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
that surveyed 59 women with HS.
Previous studies have shown the potential for adverse pregnancy outcomes associated with inflammatory conditions such as systemic vasculitis and lupus, but such data on HS and pregnancy are limited, which makes patient counseling a challenge, Ademide A. Adelekun, MD, of the University of Pennsylvania, Philadelphia, and colleagues wrote.
In a research letter published in JAMA Dermatology, they reported their findings from an email survey of female patients at two academic dermatology departments. A total of 59 women responded to the survey; their average age was 32 years, the majority (76%) had Hurley stage II disease, and 29 (49%) reported having ever been pregnant.
Two of the 29 women (7%) were pregnant at the time of the study survey; 20 of the other 27 pregnant women (74%) said they had full-term births, 4 (15%) reported miscarriages, and 3 (11%) had undergone an abortion.
A total of five patients (9%) reported difficulty getting pregnant after 1 year, and seven (12%) reported undergoing fertility treatments.
Nearly three-quarters of the women (73%) reported that HS had a negative impact on their sexual health, and 54% said they wished their doctors provided more counseling on HS and pregnancy.
A total of 14 patients (24%) said they believed HS affected their ability to become pregnant because of either decreased sexual activity or decreased fertility caused by HS medications, and nearly half (49%) said they believed that discontinuing all HS medications during pregnancy was necessary for safety reasons.
Patients also expressed concern about the possible heritability of HS: 80% said that physicians had not counseled them about HS heritability and 68% expressed concern that their child would have HS.
In addition, 83% said they had not received information about the potential impact of HS on pregnancy, and 22%, or 13 women, were concerned that childbirth would be more difficult; 11 of these 13 women (85%) had HS that affected the vulva and groin, and 4 of the 8 women who reported concerns about difficulty breastfeeding had HS that involved the breast.
Of the 59 patients surveyed, 12 (20%) said they believed HS poses risks to the child, including through transmission of HS in 8 (67%) or through an infection during a vaginal delivery in 7 women (58%).
The prevalence of HS patients’ concerns about pregnancy “may have unfavorable implications for family planning and mental health and may play a role in the inadequate treatment of HS in patients who are pregnant or planning to become pregnant,” the authors noted. “Family planning and prenatal counseling are particularly critical for those with HS given that clinicians weigh the risks of medication use against the benefits of disease control, which is associated with improved pregnancy outcomes for those with inflammatory conditions.”
The study findings were limited by several factors including “recall bias, low response rate, use of a nonvalidated survey, and generalizability to nonacademic settings,” the researchers noted. However, the results emphasize the often-underrecognized concerns of women with HS and the need for improvements in pregnancy-related counseling and systematic evaluation of outcomes.
The researchers had no financial conflicts to disclose. This study was funded by a FOCUS Medical Student Fellowship in Women’s Health grant.
FROM JAMA DERMATOLOGY
Algorithm trims time to treatment of acute hypertension in pregnancy
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
Use of a semiautonomous algorithm to initiate treatment for hypertension emergencies in pregnancy significantly increased the number of individuals treated promptly, based on data from 959 obstetric patients.
Data show poor compliance with the current American College of Obstetricians and Gynecologists recommendations for treatment of acute severe hypertension with no more than 30-60 minutes’ delay; low compliance may be caused by “multiple factors including lack of intravenous access, inadequate health care practitioner or nursing availability, and implicit racial biases,” wrote Courtney Martin, DO, of Loma Linda (Calif.) University School of Medicine and colleagues.
Semiautomated treatment algorithms have been used to improve timely treatment of conditions including myocardial infarction, heart failure, acute stroke, and asthma, but their use in obstetrics to date has been limited, the researchers noted.
In a retrospective cohort study published in Obstetrics & Gynecology, the researchers identified pregnant and postpartum women treated for severe hypertension at a single center between January 2017 and March 2020. A semiautonomous treatment algorithm was implemented between May 2018 and March 2019. The algorithm included vital sign monitoring, blood pressure thresholds for diagnosis of severe hypertension, and automated order sets for recommended first-line antihypertensive therapy. The primary outcomes were treatment with antihypertensive therapy within 15, 30, and 60 minutes of diagnosis. “Severe hypertension was defined as systolic blood pressure 160 mm Hg or higher or diastolic blood pressure 110 mm Hg or higher,” the researchers said.
The study population was divided into three groups; a preimplementation group (373 patients) managed between January 2017 and April 2018, a during-implementation group (334 patients) managed between May 2018 and March 2019, and a postimplementation group (252 patients) managed between April 2019 and March 2020. Patient demographics were similar among all three groups.
Timely treatment improves with algorithm
Overall, treatment of severe hypertension within 15 minutes of diagnosis was 36.5% preimplementation, 45.8% during implementation, and 55.6% postimplementation. Severe hypertension treatment within 30 minutes of diagnosis was 65.9% preimplementation, 77.8% during implementation, and 79.0% post implementation. Differences were significant between pre- and post implementation for 15 minutes and 30 minutes, but no significant differences occurred in the patients treated within 60 minutes before and after implementation of the algorithm.
The study findings were limited by several factors, including the inability to separate peer-to-peer education and other training from the impact of the algorithm, as well as a lack of data on the effect of the algorithm on maternal or neonatal outcomes, the researchers noted.
However, the results support the potential of a semiautonomous algorithm to significantly improve adherence to the recommended treatment guidelines for severe hypertension in pregnancy and post partum, they said. Given the expected increase in hypertensive disorders in pregnancy because of the trends in older age and higher obesity rates in pregnant women, “Integration of semiautonomous treatment algorithms similar to ours into routine obstetric practices could help reduce the health care burden and improve clinical outcomes, especially in areas with limited health care resources,” they concluded.
Algorithm may reduce disparities
The overall rise in maternal mortality in the United States remains a concern, but “Even more concerning are the disturbing racial disparities that persist across socioeconomic strata,” wrote Alisse Hauspurg, MD, of the University of Pittsburgh in an accompanying editorial. “There is clear evidence that expeditious treatment of obstetric hypertensive emergency reduces the risk of severe morbidities including stroke, eclampsia, and maternal death,” she emphasized, but compliance with the ACOG recommendations to treat severe hypertension within 30-60 minutes of confirmation remains low, she said.
In this study, not only did use of the algorithm reduce time to antihypertensive therapy, but more than 50% of patients were treated for severe hypertension within 15 minutes, and more than 90% within 60 minutes, “which was sustained after the implementation phase,” and aligns with the ACOG recommendations, Dr. Hauspurg said. “Although Martin et al.’s algorithm was limited to the initial management of obstetric hypertensive emergency, it could readily be expanded to follow the full ACOG algorithm for management of hypertension in pregnancy,” she noted.
In addition, Black women are more frequently diagnosed with hypertensive disorders of pregnancy, including severe hypertension, and the algorithm might improve disparities, she said.
“It is plausible that widespread implementation of such a semiautonomous algorithm at hospitals across the country could reduce delays in treatment and prevent hypertension-related morbidities,” said Dr. Hauspurg. “The use of innovative approaches to management of severe hypertension and other obstetric emergencies has the potential to allow provision of more equitable care by overcoming health care practitioner and system biases, which could meaningfully reduce disparities in care and change the trajectory of maternal morbidity and mortality in the United States,” she emphasized.
Need to create culture of safety
“Maternal mortality in the United States is the highest among developed nations, and shocking disparities exist in outcomes for non-Hispanic Black and American Indian/Alaskan Native women,” said Lisa Hollier, MD, of Texas Children’s Health Plan in Bellaire. “In a California review of maternal deaths, the greatest quality improvement opportunities were missed diagnosis and ineffective treatment of preeclampsia and related diseases, which occurred in 65% of the cases where women died of preeclampsia/eclampsia,” she said.
The current study “is very timely as more and more states across the nation are participating in the AIM (Alliance for Innovation on Maternal Health) programs to prevent pregnancy-related mortality,” Dr. Hollier noted.
“This study demonstrated a significant association between implementation of the algorithm and an increased percentage of treatment of severe hypertension within 30 minutes,” Dr. Hollier said. “With the implementation of a comprehensive program that included treatment algorithms, the Illinois Perinatal Quality Collaborative improved timely treatment for women with severe high blood pressure, increasing the percentage of patients treated within 60 minutes from 41% at baseline to 79% in the first year of the project.”
The take-home message is that “implementation of the semiautonomous treatment algorithm can address important clinical variation, including delays in appropriate treatment of severe hypertension,” said Dr. Hollier. However, “One of the potential barriers [to use of an algorithm] is the need for accurate, real-time clinical assessment. Resources must be available to ensure appropriate monitoring,” Dr. Hollier noted. “Collaboration and support of implementation of these treatment algorithms must extend through the nursing staff, the physicians, and advanced-practice providers. Medical staff and administrative leaders are essential in creating a culture of safety and continuous process improvement,” she said.
In addition, “long-term follow-up on the implementation of broader quality improvement programs is essential,” Dr. Hollier said. “While implementation of an algorithm can, and did, result in process improvements, assessment of broader implementation of evidence-based bundles, combined with a systematic approach to redesign of multiple related processes needs to occur and include outcomes of severe maternal morbidity and mortality,” she explained.
The study received no outside funding. The researchers had no financial conflicts to disclose.
Neither Dr. Hauspurg nor Dr. Hollier had financial conflicts to disclose.
FROM OBSTETRICS & GYNECOLOGY