User login
2019-nCoV outbreak: A few lessons learned for pediatric practices
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
In late January, signs were posted in all of the offices in our faculty medical practice building.
Combined with current worldwide health concerns and flu season, we are now asking all patients two questions:
1. Do you have a fever, cough or shortness of breath?
2. Have you traveled to China in the last 2 weeks, or have you had contact with someone who has and who now is sick?
Similar signs appeared in medical offices and EDs across the city. Truth be told, when the signs first went up, some thought it was an overreaction. I practice in a city in the Southeast that is not a port of entry and has no scheduled international passenger flights. Wuhan City, China and the threat of 2019 novel coronavirus (2019-nCoV) seemed very far away.
As the international tally of cases has grown, so have local concerns.
Hopefully, proactive public health measures to care for the few individuals currently infected in the United States and appropriately assessing individuals arriving from mainland China will prevent widespread circulation of 2019-nCoV here. If this is the case, most of us likely will never see a case of the virus. Still, there are important lessons to be learned from current preparedness efforts.
A travel history is important. Several years ago, during the height of concern over the spread of Ebola, the health care systems in which I practice asked everyone about travel to West Africa as soon as they approached the registration desk. In the intervening years, asking about a travel history largely was delegated to providers, and I suspect it largely was driven by patient presentation. Child presenting with 10 days of fever? The clinician likely took a travel history. Child presenting for runny nose, ear ache, or rash? Maybe not. With more consistent screening, we are learning how frequently our patients and their families do travel, and that is helping us expand our differential diagnosis.
We need to practice cough etiquette. Patients who endorse respiratory symptoms as part of 2019 n-CoV screening are handed a mask. Those who have traveled to China in the last 14 days are promptly escorted to an exam room. In truth, we should be following cough etiquette and offering all patients with respiratory symptoms a mask. Heightened awareness of this practice may help prevent the spread of much more common viruses such as influenza. Reliable processes to recognize and rapidly triage patients with an infectious illness are critically important in ambulatory settings, and now we have an opportunity to trial and improve these processes. No one wants a child with measles or chicken pox to sit in the waiting room!
Offices must stock personal protective equipment to comply with standard precautions. The recommended PPE when caring for a patient with 2019 n-CoV includes a gown, gloves, mask (n95 or PAPR if available), and eye protection, such as a face shield or goggles. An initial survey of PPE supplies locally revealed of shortage of PPE for eye protection in some offices. Eye protection should be readily available in pediatric and other primary care offices because it must be used as part of standard precautions during procedures likely to generate droplets of blood or body fluids. Examples of common procedures that require eye protection include swabbing the nasopharynx to obtain a specimen for respiratory virus testing or swabbing the throat to test for group A streptococcus.
We should use diagnostic testing judiciously. Over the last couple of weeks, we’ve had a couple of patients who wanted to be tested for 2019 n-CoV but did not meet person under investigation (PUI) criteria. Public health authorities, who must approve all 2019 n-CoV testing, said no. This is enforced diagnostic stewardship, but it is a reminder that, when a diagnostic test is performed in a person with a low likelihood of disease, there is a risk of a false-positive result. What if we applied this principle to tests we send routinely? We would send fewer urine cultures in patients with normal urinalyses and stop testing infants for Clostridioides difficile.
Frontline providers must partner with public health colleagues during outbreaks. Providers have been instructed to immediately notify local or state health departments when a patient is suspected of having 2019 n-CoV specifically because the PUI criteria are met. This notification was crucial in diagnosing the first cases of 2019 n-CoV in the United States. Nine of the first 11 U.S. cases were in travelers from Wuhan, and according to the Centers for Disease Control and Prevention, eight of these “were identified as a result of patients seeking clinical care for symptoms and clinicians connecting with the appropriate public health systems.” Locally, daytime and after hours phone numbers for the health department have been posted in offices across our health care system. The state health department is hosting well-attended webinars to provide updates and answer questions from clinicians. We may never have a case of 2019 n-CoV in Kentucky, but activities like these build relationships between providers and our colleagues in public health, strengthening infrastructure and the capacity to respond to future outbreaks. I suspect the same is true in many other communities.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
ID Consult: It’s not necessarily over when measles infection clears
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
As I write, I imagine readers groaning at yet another measles story. But in early November 2019, in Portland, Oregon, Judy Guzman-Cottrill, DO, recently was groaning at yet another measles case.
Dr. Guzman-Cottrill, a pediatric infectious diseases specialist at Doernbecher Children’s Hospital, recently shared details provided by the local health department:
An unimmunized child developed measles while traveling outside the county. The child may have exposed others at Portland International Airport, a medical center in Vancouver, and potentially at another children’s hospital in the area.
As of Nov. 7, 2019, 1,261 cases of measles from 31 states had been reported to the Centers for Disease Control and Prevention – more cases in a single year since 1992. The case in Portland added at least one to that total, although public officials warned that additional cases could occur Nov. 18th through Dec. 9 (given the incubation period). Like the child in Oregon, most of the individuals who developed measles nationwide in 2019 were unimmunized. At press time, from Jan. 1 to Dec. 5, 2019, 1,276 individual cases of measles have been confirmed in 31 states; CDC released measles reports monthly.
The reasons for refusal of measles vaccine vary, but historically, some parents have made a calculated risk. Measles is rare. Most children are vaccinated. My child will be protected by herd immunity. In some communities, that is no longer true, as we have seen in 2019.
Other parents have decided – erroneously – that measles infection is less risky than measles vaccine. We need to be able to tell them the facts. Thirty percent of individuals who contract measles will develop at least one complication, according to the Centers for Disease Control and Prevention. One in four will be hospitalized. While death from acute measles infection is uncommon, children remain at risk for sequelae months or years after the initial infection.
For example, measles is known to suppress the immune system, an effect that lasts for months or years after the initial infection. Practically, this means that once a child recovers from acute measles infection, he or she has an increased susceptibility to other infections that may last for years. Two studies published late in 2019 described the immune “amnesia” that occurs following measles infection. Essentially, the immune system forgets how to fight other pathogens, leaving children vulnerable to potentially life-threatening infections.
Michael Mina, MD, of the Harvard T.H. Chan School of Public Health, Boston, and colleagues measured the effects of measles infection on the immune system by studying blood samples taken from 77 unimmunized children in the Netherlands before and after measles infection.1 Two months after recovery from mild measles, children had lost a median of 33 % (range, 12%-73%) of preexisting antibodies against a range of common viruses and bacteria. The median loss was 40% after severe measles (range 11% to 62%). Similar changes were not observed after measles vaccine.
A second group of researchers led by Velislava N. Petrova, PhD, of the Wellcome Sanger Institute in Cambridge, England, investigated genetic changes in 26 unvaccinated children from the Netherlands who previously had measles. They found that measles infection reduced the diversity of immune cells available to recognize and fight infections and depleted memory B cells, essentially returning the immune to a more immature state.2
Parents also need to know that children who develop measles are at risk for noninfectious complications.
Yes, SSPE is a rare, but it is not as rare as we once thought. In 2017, investigators in California described 17 cases of SSPE identified in that state between 1998 and 2005.3 The incidence of SSPE was 1 in 1,367 for children less than 5 years at the time of measles infection and 1 in 609 for children less than 12 months when they contracted the virus.
Dr. Guzman-Cottrill has seen a case of SSPE, and she hopes to never see another one. “He had been a healthy 11-year-old boy,” she recalled. “He played soccer and basketball and did well in school.” In the beginning, his symptoms were insidious and nonspecific, Dr. Guzman-Cottrill and colleagues wrote in a 2016 issue of Morbidity and Mortality Weekly Report.4 He started to struggle in school. He dozed off in the middle of meals. He started to drop things. Over a 4-month period, the boy developed progressive spasticity, became unable to eat or drink, and could no longer recognize or communicate with his family. “That’s when I met him,” Dr. Guzman-Cottrill said. “It was heartbreaking, and there was very little we could do for him except give the family a diagnosis. He eventually died in hospice care, nearly 4 years after his symptoms began.”
The boy had been infected with measles at 1 year of age while living in the Philippines. Dr. Guzman-Cottrill emphasized that this family had not refused measles immunization. The child had received a measles vaccine at 8 months of age, but a single vaccine at such a young age wasn’t enough to protect him.
We can hope for change in 2020, including improved immunization rates and a decline in measles cases. If that happens, measles will no longer be a hot topic in the news. We’ll likely never know what happens to the children infected in 2019, those who are facing the current cold and flu season with impaired immune systems. A decade or more will pass before we’ll know if anyone develops SSPE. For now, all we can do is wait … and worry.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Norton Children’s Hospital, also in Louisville. Dr. Bryant had no relevant financial disclosures. Email her at pdnews@mdedge.com.
References
1. Science. 2019 Nov 1;366:599-606.
2. Science Immunology. 2019 Nov 1;4:eaay6125.
Is your office ready for a case of measles?
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
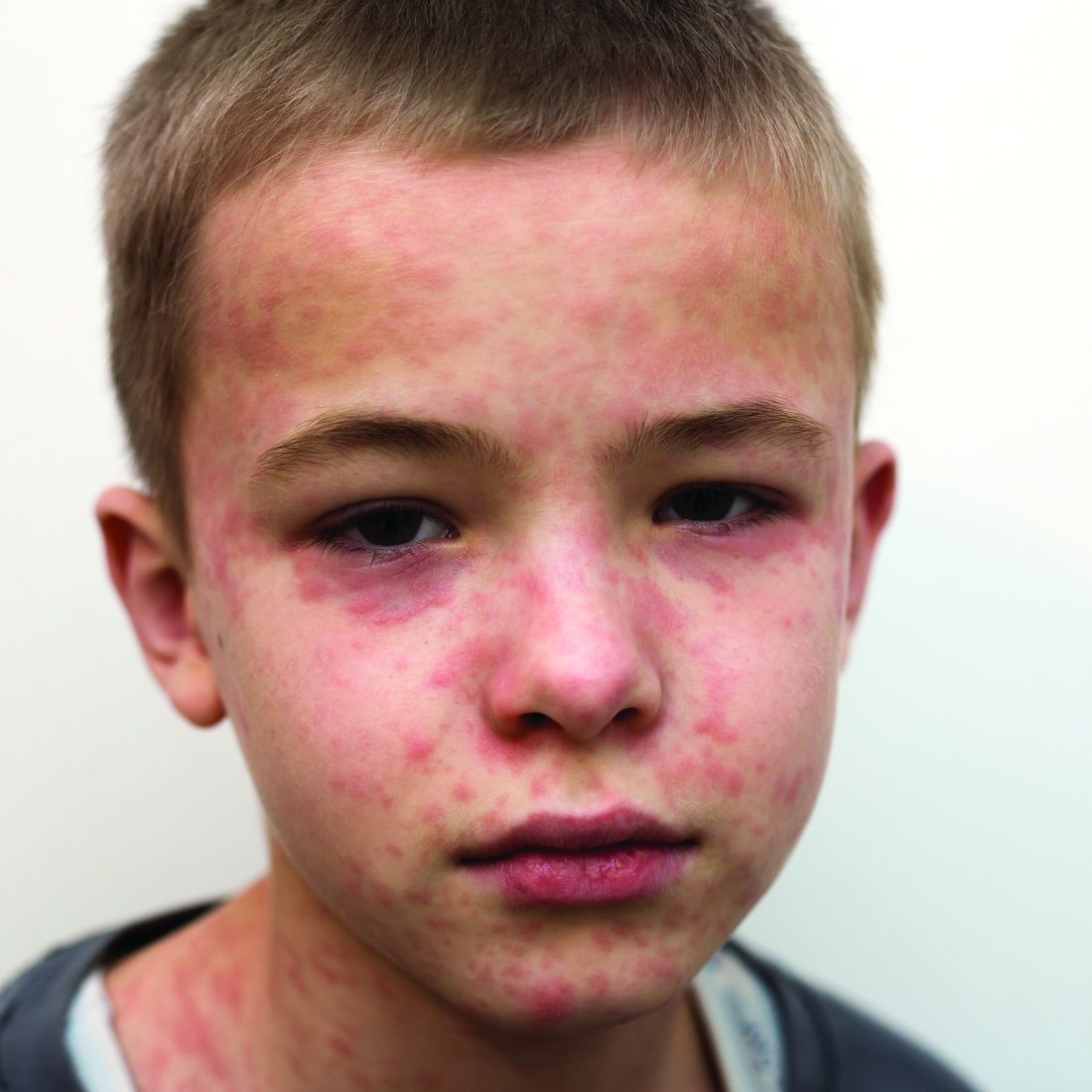
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
It’s a typically busy Friday and the doctor is running 20 minutes behind schedule. He enters the next exam room and the sight of the patient makes him forget the apology he had prepared.
The 10 month old looks miserable. Red eyes. Snot dripping from his nose. A red rash that extends from his face and involves most of the chest, arms, and upper thighs.
“When did this start?” he asks the mother as he searches for a surgical mask in the cabinet next to the exam table.
“Two days after we returned from our vacation in France,” the worried young woman replies. “Do you think it could be measles?”
Between Jan. 1 and Aug. 8, 2019, 1,182 cases of measles had been confirmed in the United States. That’s more than three times the number of cases reported in all of 2018, and the highest number of cases reported in a single year in more than a quarter century. While 75% of the cases this year have been linked to outbreaks in New York, individuals from 30 states have been affected.
Given the widespread nature of the outbreak, With measles in particular, time is limited to deliver effective postexposure prophylaxis and prevent the spread of measles in the community, making it difficult to develop a plan on the fly.
Schedule strategically. You don’t want a patient with measles hanging out in your waiting room. According to the American Academy of Pediatrics, measures to prevent the transmission of contagious infectious agents in ambulatory facilities begin at the time the visit is scheduled. When there is measles transmission in the community, consider using a standardized script when scheduling patients that includes questions about fever, rash, other symptoms typical for measles, and possible exposures. Some offices will have procedures in place that can be adapted to care for patients with suspected measles. When a patient presents for suspected chicken pox, do you advise them to come at the end of the day to minimize exposures? Enter through a side door? Perform a car visit?
Triage promptly. Mask patients with fever and rash, move to a private room, and close the door.
Once measles is suspected, only health care personnel who are immune to measles should enter the exam room. According to the Centers for Disease Control and Prevention, presumptive evidence of measles immunity in health care providers is written documentation of vaccination with two doses of live measles or MMR vaccine administered at least 28 days apart, laboratory evidence of immunity (that is, positive measles IgG), laboratory confirmation of disease, or birth before 1957.
Even though health care providers born before 1957 are presumed to have had the disease at some point and have traditionally been considered immune, the CDC suggests that health care facilities consider giving these individuals two doses of MMR vaccine unless they have prior laboratory confirmation of disease immunity. Do you know who in your office is immune or would you need to scramble if you had an exposure?
When measles is suspected, health care personnel should wear an N-95 if they have been fit tested and the appropriate mask is available. Practically, most ambulatory offices do not stock N-95 masks and the next best choice is a regular surgical mask.
Order the recommended tests to confirm the diagnosis, but do not wait for the results to confirm the diagnosis. The CDC recommends testing serum for IgM antibodies and sending a throat or nasopharyngeal swab to look for the virus by polymerase chain reaction testing. Measles virus also is shed in the urine so collecting a urine specimen for testing may increase the chances of finding the virus. Depending on where you practice, the tests may take 3 days or more to result. Contact your local health department as soon as you consider a measles diagnosis.
Discharge patients home or transferred to a higher level of care if this is necessary as quickly as possible. Fortunately, most patients with measles do not require hospitalization. Do not send patients to the hospital simply for the purpose of laboratory testing if this can be accomplished quickly in your office or for evaluation by other providers. This just creates the potential for more exposures. If a patient does require higher-level care, provider-to-provider communication about the suspected diagnosis and the need for airborne isolation should take place.
Keep the door closed. Once a patient with suspected measles is discharged from a regular exam room, the door should remain closed, and it should not be used for at least 1 hour. Remember that infectious virus can remain in the air for 1-2 hours after a patient leaves an area. The same is true for the waiting room.
Develop the exposure list. In general, patients and family members who were in the waiting room at the same time as the index patient and up to 1-2 hours after the index patient left are considered exposed. Measles is highly contagious and 9 out of 10 susceptible people who are exposed will develop disease. How many infants aged less than 1 year might be in your waiting room at any given time? How many immunocompromised patients or family members? Public health authorities can help determine who needs prophylaxis.
Don’t get anxious and start testing everyone for measles, especially patients who lack typical signs and symptoms or exposures. Ordering a test in a patient who has a low likelihood of measles is more likely to result in a false-positive test than a true-positive test. False-positive measles IgM tests can be seen with some viral infections, including parvovirus and Epstein-Barr. Some rheumatologic disorders also can contribute to false-positive tests.
Review your office procedure for vaccine counseling. The 10 month old with measles in the opening vignette should have been given an MMR vaccine before travel. The vaccine is recommended for infants aged 6-11 months who are traveling outside the United States, but it doesn’t count toward the vaccine series. Reimmunize young travelers at 12-15 months and again at 4-6 years. The CDC has developed a toolkit that contains resources for taking to parents about vaccines. It is available at https://www.cdc.gov/measles/toolkit/healthcare-providers.html.
What infectious disease should parents be most worried about?
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
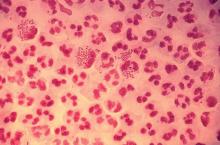
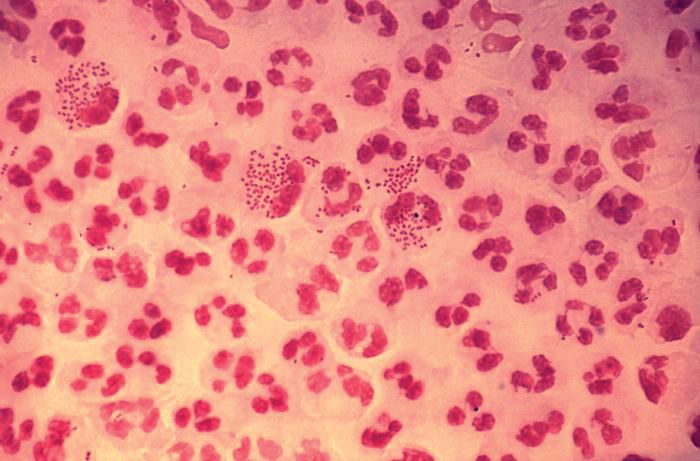
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
I think the question was intended as polite, dinner party chit chat ... maybe an attempt by a gracious hostess to make sure everyone was engaged in conversation.
“So what pediatric infectious disease should parents be most worried about?” she asked me.
I’ll admit that a couple of perfectly respectable and noncontroversial possibilities crossed my mind before I answered.
Acute flaccid myelitis? Measles?
When I replied, “gonorrhea,” conversation at the table pretty much stopped.
Let me explain. Acute flaccid myelitis is a polio-like neurologic condition that has been grabbing headlines. Yes, it is concerning that most cases have occurred in children and some affected children are left with long-term deficits. Technically though, AFM is a neurologic rather than an infectious disease. When cases occur, we suspect a viral infection but according to the Centers for Disease Control and Prevention, no pathogen has been consistently identified from the spinal fluid of infected patients. From August 2014 to September 2018, the CDC received information on 368 confirmed cases, so AFM fortunately is still rare.
News reports describe measles outbreaks raging in Europe – more than 41,000 cases so far this year, and 40 deaths – and warn that the United States could be next. But let’s be honest: We have a safe and effective vaccine for measles and outbreaks like this don’t happen when individuals are appropriately immunized. Parents, immunize your children. If you are lucky enough to be traveling to Europe with your baby, remember that MMR vaccine is indicated for 6- to 11-month olds, but it doesn’t count in the 2-dose series.
But gonorrhea?
In 2017, the World Health Organization included Neisseria gonorrhoeae on its list of bacteria that pose the greatest threat to human health and for which new antibiotics are urgently needed. The popular media are calling N. gonorrhoeae one of the new “superbugs.” Globally, patients are being diagnosed with strains of gonorrhea that are resistant to all commonly used antibiotics. As reported during IDWeek 2018 this October, patients also are being diagnosed in the United States.
Sancta St. Cyr, MD, of the Centers for Disease Control and Prevention, and her colleagues reported data from the Gonococcal Isolate Surveillance Project (GISP) and trends in multidrug resistant (MDR) and extensively-drug resistant (XDR) gonorrhea in the United States. A gonococcal isolate with resistance or elevated minimum inhibitory concentrations (MIC) to greater than or equal to two classes of antimicrobials is classified as MDR and an isolate with elevated MICs to greater than or equal to three classes of antimicrobials is classified as XDR. The MIC is the lowest antimicrobial concentration that inhibits growth of bacteria in the laboratory and rising MICs – evidence that higher levels of an antibiotic are needed to stop bacterial growth – can be an early indicator that resistance is emerging.
More than 150,000 gonococcal isolates were tested between 1987 and 2016. The first isolates with elevated MICs to cephalosporins and macrolides were identified in 1998, and since 2011, MDR resistance rates have hovered around 1%. In 2016, the rate was 1.1%, down from 1.3% in 2011. A single XDR isolate with resistance to fluoroquinolones with elevated MICs to both cephalosporins and macrolides was identified in 2011.
One could look at these data and ask if this is a “glass half full or half empty” situation, but I propose that clinicians and public health officials should not look at these data and be reassured that rates of MDR-gonorrhea remained stable between 2010 and 2016. According to a recent surveillance report released by the CDC, the absolute number of cases of gonorrhea has continued to rise. In 2017, there were 555,608 cases reported in the United States, a 67% increase since 2013. If we assume that rates of resistance in 2017 were similar to those in 2016, that’s more than 5,000 cases of MDR-gonorrhea in a single year.
“That’s bad,” one of my dining companions agreed. “But is gonorrhea really a pediatric issue?”
To answer that question, we just have to look at the numbers. According to the 2017 Youth Risk Behavior Survey, the percentage of high school students who had ever had sex was approximately 40% and about 10% of students had four or more lifetime partners. More than 45% of sexually active students denied the use of a condom during the last sexual intercourse. Certainly, that puts many teenagers at risk for sexually transmitted infections (STIs). Perhaps it shouldn’t be surprising that public health authorities report that half of all new STIs occur in individuals aged 15-24 years. Moreover, 25% of sexually active adolescent girls contract at least one STI.
Gonorrhea is the second most commonly reported notifiable disease in the United States, and according to the CDC, rates of disease in 2017 were highest among adolescents and young adults. In females specifically, the highest rates of gonorrhea were observed among those aged 20-24 years (684.8 cases per 100,000 females) and 15-19 years (557.4 cases per 100,000 females).
It makes sense that pediatricians and parents advocate for making the reduction of gonorrhea transmission rates a public health priority. We also need to recognize that prompt diagnosis and appropriate treatment are critical. Since 2015, dual therapy with ceftriaxone and azithromycin is the only CDC-recommended treatment for gonorrhea.
At that dinner party, my closest friend, who also happens to be a pediatrician, rolled her eyes and shot me look that I’m sure meant, “Nobody really wants to talk about gonorrhea over dessert.” Still, because she is a good friend she said, “So basically you’re saying that and if this keeps up, we may see kids with untreatable infection. Now that is scary.”
I kept quiet after that but I wanted to mention that in 2017, less than 85% of patients diagnosed with gonorrhea at selected surveillance sites received the recommended treatment with two antibiotics. Patients with inadequately treated gonorrhea are at risk for a host of sequelae. Women can develop pelvic inflammatory disease, abscesses, chronic pelvic pain, and damage of the fallopian tubes that can lead to infertility. Men can develop epididymitis, which occasionally results in infertility. Rarely, N. gonorrhoeae can spread to the blood and cause life-threatening infection. Of course, patients who aren’t treated appropriately may continue to spread the bacteria. Scary? You bet.
For pediatricians who need a refresher course in the treatment of STIs, there are free resources available. The CDC’s 2015 STD Treatment Guidelines are available in a free app; the app contains a nice refresher on taking a sexual history. There also is a print version, wall chart, and pocket guide. Providers also may want to check out the National STD Curriculum offered by the University of Washington STD Prevention Training Center and the University of Washington. Visit https://www.std.uw.edu/.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@mdedge.com.
Don’t give up on influenza vaccine
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
I suspect most health care providers have heard the complaint, “The vaccine doesn’t work. One year I got the vaccine, and I still came down with the flu.”
Over the years, I’ve polished my responses to vaccine naysayers.
Influenza vaccine doesn’t protect you against every virus that can cause cold and flu symptoms. It only prevents influenza. It’s possible you had a different virus, such as adenovirus, coronavirus, parainfluenza virus, or respiratory syncytial virus.
Some years, the vaccine works better than others because there is a mismatch between the viruses chosen for the vaccine, and the viruses that end up circulating. Even when it doesn’t prevent flu, the vaccine can potentially reduce the severity of illness.
The discussion became a little more complicated in 2016 when the Centers for Disease Control and Prevention Advisory Committee on Immunization Practices withdrew its support for the live attenuated influenza virus vaccine (LAIV4) because of concerns about effectiveness. During the 2015-2016 influenza season, LAIV4 demonstrated no statistically significant effectiveness in children 2-17 years of age against H1N1pdm09, the predominant influenza strain. Fortunately, inactivated injectable vaccine did offer protection. An estimated 41.8 million children aged 6 months to 17 years ultimately received this vaccine during the 2016-2017 influenza season.
Now with the 2017-2018 influenza season in full swing, some media reports are proclaiming the influenza vaccine is only 10% effective this year. This claim is based on an interim analysis of data from the most recent flu season in Australia and the effectiveness of the vaccine against the circulating H3N2 virus strain. News from the U.S. CDC is more encouraging. The H3N2 virus contained in this year’s vaccine is the same as that used last year, and so far, circulating H3N2 viruses in the United States are similar to the vaccine virus. Public health officials suggest that we can hope that the vaccine works as well as it did last year, when overall vaccine effectiveness against all circulating flu viruses was 39%, and effectiveness against the H3N2 virus specifically was 32%.
I’m upping my game when talking to parents about flu vaccine. I mention one study conducted between 2010 and 2012 in which influenza immunization reduced a child’s risk of being admitted to an intensive care unit with flu by 74% (J Infect Dis. 2014 Sep 1;210[5]:674-83). I emphasize that flu vaccine reduces the chance that a child will die from flu. According to a study published in 2017, influenza vaccine reduced the risk of death from flu by 65% in healthy children and 51% in children with high-risk medical conditions (Pediatrics. 2017 May. doi: 10.1542/peds.2016-4244).
When I’m talking to trainees, I no longer just focus on the match between circulating strains of flu and vaccine strains. I mention that viruses used to produce most seasonal flu vaccines are grown in eggs, a process that can result in minor antigenic changes in the hemagglutinin protein, especially in H3N2 viruses. These “egg-adapted changes” may result in a vaccine that stimulates a less effective immune response, even with a good match between circulating strains and vaccine strains. For example, Zost et al. found that the H3N2 virus that emerged during the 2014-2015 season possessed a new hemagglutinin-associated glycosylation site (Proc Natl Acad Sci U S A. 2017 Nov 21;114[47]:12578-83). Although this virus was represented in the 2016-2017 influenza vaccine, the egg-adapted version lost the glycosylation site, resulting in decreased vaccine immunogenicity and less protection against H3N2 viruses circulating in the community.
The real take-home message here is that we need better flu vaccines. In the short term, cell-based flu vaccines that use virus grown in animal cells are a potential alternative to egg-based vaccines. In the long term, we need a universal flu vaccine. The National Institute of Allergy and Infectious Diseases is prioritizing work on a vaccine that could provide long-lasting protection against multiple subtypes of the virus. According to a report on the National Institutes of Health website, such a vaccine could “eliminate the need to update and administer the seasonal flu vaccine each year and could provide protection against newly emerging flu strains,” including those with the potential to cause a pandemic. The NIH researchers acknowledge, however, that achieving this goal will require “a broad range of expertise and substantial resources.”
Until new vaccines are available, we need to do a better job of using available, albeit imperfect, flu vaccines. During the 2016-2017 season, only 59% of children 6 months to 17 years were immunized, and there were 110 influenza-associated deaths in children, according to the CDC. It’s likely that some of these were preventable.
The total magnitude of suffering associated with flu is more difficult to quantify, but anecdotes can be illuminating. A friend recently diagnosed with influenza shared her experience via Facebook. “Rough night. I’m seconds away from a meltdown. My body aches so bad that I can’t get comfortable on the couch or my bed. Can’t breathe, and I cough until I vomit. My head is about to burst along with my ears. Just took a hot bath hoping that would help. I don’t know what else to do. The flu really sucks.”
Indeed. Even a 1 in 10 chance of preventing the flu is better than no chance at all.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Group B streptococcus
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
It once was a very a common scenario. A baby born at term looks fine for the first 24 hours of life. Without much warning, the infant develops grunting, tachypnea, and tachycardia. Sepsis is suspected, and within a few hours, group B streptococcus (GBS) is isolated from a blood culture.
According to the CDC, a woman colonized with Group B strep at the time of delivery has a 1 in 200 chance of delivering a baby who will develop GBS disease. Antibiotics during labor drop that risk to 1 in 4,000. It’s not perfect – there are still about 1,000 cases annually in the United States – but is has been a major step forward. In recent years, the incidence of early-onset GBS disease has fallen to just under 0.3 cases per 1,000 live births, and some experts think rates could go even lower with improved adherence to current guidelines.
Reducing late-onset GBS disease requires a different strategy. Efforts to develop a GBS vaccine that could be given to pregnant women continue, and recent phase 2 trials of a trivalent polysaccharide-protein conjugate vaccine looked promising. Fingers crossed that we won’t have to wait until we celebrate the 75th anniversary of Pediatric News to tout the impact of maternal immunization on reducing GBS disease in infants.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Hepatitis C is a pediatric disease now
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
The baby looked perfect: healthy term male, weight at the 60th percentile, normal exam. The mother, a 26-year-old diagnosed with hepatitis C virus (HCV) infection during her pregnancy, looked alternately hopeful and horrified as I explained what implications her infection could have for her baby.
“Most babies will be fine,” I explained. “Of all mothers with hepatitis C infection, just under 6% will pass the infection on to their babies.” Transmission rates are twice as high in infants born to women with high HCV viral loads or those coinfected with HIV. The risk of transmission from women with undetectable HCV RNA is almost zero. Unfortunately, this mother did not fall into that category.
At that moment, however, I didn’t have time to be concerned about the numbers. My focus was one mother and her newborn baby.
“What if my baby is one of the unlucky ones who gets infected?” the mother asked, cuddling her infant. “What then?”
We know a lot about the course of hepatitis C in adults. An estimated 75%-86% of those infected will go on to develop chronic infection. Long-term sequelae include cirrhosis, liver failure, and hepatocellular carcinoma.
The course of HCV in children appears to be different. Twenty-five percent to 40% of vertically infected children will spontaneously clear their infection, most by 2 years of age. Occasionally, that might not happen until 7 years of age. Most who are chronically infected experience few symptoms, and fortunately cirrhosis and liver failure rarely present in childhood. In a large cohort of Italian children, half of whom were thought to be infected perinatally, less than 2% progressed to decompensated cirrhosis after 10 years of infection. According to the CDC, most children infected at birth “do well during childhood,” but more research is needed to understand the long-term effects of perinatal hepatitis C in children.
New antivirals have revolutionized the care of HCV-infected adults and now offer the hope of cure for up to 90%. None of these drugs are currently approved for use in children younger than 12 years, although clinical trials are underway. Because most cases of HCV in children are indolent, some children may not require treatment until adulthood.
July 28th was World Hepatitis Day and this year’s theme was Eliminate Hepatitis. To eliminate the problem of hepatitis C in children, pediatricians and others involved in the care of children need to get involved.
We need to know the scope of the problem
Since 2015, Kentucky has mandated reporting of all HCV-infected pregnant women and children through age 60 months, as well as all infants born to all HCV-infected women. At present though, there is substantial variability in state reporting requirements. We likely need a standardized case definition for perinatal HCV and national reporting criteria.
We need some clear guidance about testing during pregnancy
This should come from public health authorities, the American Academy of Pediatrics and the American College of Obstetricians and Gynecologists.
Jonathan Mermin, MD, director of CDC’s National Center for HIV/AIDS, Viral Hepatitis, STD, and TB Prevention, has said, “Women are screened throughout pregnancy for many conditions that threaten their health. An expectant mother at risk for hepatitis C deserves to be tested. Knowing her status is the only way she can access the best hepatitis care and treatment – both for herself and her baby.” Yet, routine hepatitis C testing is not recommended during pregnancy, in part because there are no established interventions to prevent mother-to-child transmission of HCV. Instead, women are to be screened for risk factors and tested if they are present. As we learned with hepatitis B and HIV, risk factor screening is hard and misses individuals who are infected.
We need to ensure that HCV-exposed infants are identified and followed appropriately.
In a study of HCV-exposed infants born to women in Philadelphia, 84% did not receive adequate testing for HCV infection. In human terms, 537 children were born to HCV-positive mothers during the study period and 4 of 84 (5%) children tested were found to be infected. Assuming that 5% of HCV-exposed infants will develop chronic infection, 23 additional children were undiagnosed and, therefore, were not being followed for potential sequelae.
HCV-infected mothers in this study were more likely than non-infected mothers to be socioeconomically disadvantaged – specifically, unmarried, less educated, and publicly insured – suggesting that access to care may have played a role. When you add in drug use as a common risk factor for HCV infection, it is easy to understand why some at-risk infants are lost to follow-up.
Investigators in the Philadelphia study suggested that there might be more to the story. They proposed that pediatricians might be unaware of the need for testing because they had not been alerted to the mother’s HCV status by the obstetrician, the birthing hospital, or the mother herself. Finally, they theorized that many pediatricians “may be unaware or skeptical of the guidelines for testing children exposed to HCV.” This is a problem that we can solve.
I finished the visit with this mother by reassuring her that she could breastfeed her infant as planned as long as she did not have cracked or bleeding nipples. I also explained the schedule for testing. A 2002 National Institutes of Health consensus statement recommends that infants perinatally exposed to HCV have two HCV RNA tests between 2 and 6 months of age and/or be tested for HCV antibodies after 15 months. North American Society for Pediatric Gastroenterology, Hepatology and Nutrition (NASPGHAN) Practice Guidelines for Diagnosis and Management of Hepatitis C Infection in Infants, Children, and Adolescents recommend testing for HCV antibodies at 18 months of age (J Pediatr Gastroenterol Nutr. 2012 Jun;54[6]:838-55). If a family requests earlier testing, a serum HCV RNA test can be done as early as 2 months of age. If positive, NASPGHAN recommends testing after 12 months of age to evaluate for chronic infection.
My practice has adopted the National Institutes of Health consensus statement approach because many of the families we see experience significant anxiety about the diagnosis, and this mother was no exception. As noted in the expert guidelines, this was a situation in which “early exclusion of HCV infection is reassuring and may be worth the added expense.”
“So first test at 2 months?” she asked. “Until then, we can’t do anything but wait?”
It is estimated that there are 23,000 to 46,000 U.S. children living with HCV. The wait for pediatricians is over. , and we need to educate ourselves about diagnosis and management. A first step might be to begin asking expectant mothers and the mothers of newborns if they know their HCV status.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Norton Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
It isn’t over until it’s over
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Pediatricians take heart.
Yes, I know it is discouraging when families occasionally ignore our advice and refuse vaccines for their children. It is even worse when political leaders who ought to know better question the safety and value of vaccines.
But let’s not lose perspective. Let me share a quick reminder of why vaccines are (almost) universally considered one of the greatest public health achievements of the 20th century.
Not long ago, I reviewed a clinical case with students as part of a medical microbiology course. A 6-year-old girl presented with fever, headache, and flaccid paralysis of the right arm with areflexia. With little prompting, the students generated a short differential diagnosis. Enterovirus. West Nile virus. “I guess we should include polio,” one student offered. “But who gets that anymore?”
A mere 120 years changes everything. At the dawn of the 20th century, we didn’t even know with certainty what caused polio, although infection was suspected.
On Sept. 9, 1954, the Courier-Journal, a newspaper in my hometown of Louisville, Ky., carried a story about the annual number of polio cases in Jefferson County, noting that they had reached 198 and General Hospital had opened a polio ward usually reserved for epidemics. Concerns about the infection were rippling throughout the state, and the paper reported that at least one high school marching band had elected to withdraw from annual Kentucky State Fair competition because of concerns about infection.
My mom was 10 years old in the summer of 1954, and she recalls that it was a “scary” time. Swimming pools closed. Parents refused to allow their children to go to movie theaters or the local amusement park because of fear that they might come into contact with the virus. My mom said, “Then one of my friends was diagnosed with polio. We had played together the week before she got sick. We worried that we were going to get sick, too. And once you got sick, you didn’t necessarily get better.”
I probably don’t need to remind you that both Dr. Sabin and Dr. Salk did develop successful poliovirus vaccines. Dr. Enders, along with junior colleagues Fred C. Robbins, MD, and Thomas H. Weller, MD, developed the techniques to grow poliovirus and other viruses in culture, making the work of Dr. Sabin and Dr. Salk possible. For this, Dr. Enders, Dr. Robbins, and Dr. Weller received the Nobel Prize in 1954.
Regarding the prediction of long-term protection, I’d say we’re there. According to the Centers for Disease Control and Prevention, wild poliovirus cases have declined more than 99.9% since 1988. According to the Global Polio Eradication Initiative, that means that there are approximately 10 million people walking today who would have otherwise been paralyzed by the disease.
In 2015, there were only 74 cases identified in the world, and these were localized to two countries. Even better, a global commission announced that wild poliovirus type 2 had been eradicated from the world. Eradicated. The last known transmission occurred in India in 1999.
Type 3 poliovirus may not be far behind. The last known case of wildtype poliovirus 3 was detected in 2012.
The complete story of poliovirus eradication efforts could read like a suspense novel: There have been twists and turns, some missed deadlines, and now a bit of irony. Success, in large part, has hinged on the use of trivalent, live attenuated oral poliovirus vaccine (tOPV) throughout much of the world. Now eradication of all polio disease is going to require withdrawal of OPV in countries that still use it.
Rarely, the live attenuated vaccine viruses contained in OPV can cause polio, and since 2012, vaccine-derived cases have exceeded wild poliovirus cases. Vaccine-derived cases include vaccine-associated paralytic polio (VAPP) – paralysis occurs in a vaccine recipient or a close contact – as well as cases of circulating vaccine-derived polioviruses (cVDPVs). Remember that vaccine viruses are shed in the stool, and in communities with low immunization rates, they circulate and acquire mutations that confer the transmissibility and neurovirulence properties of wild viruses. Ultimately, cVDPVs lead to outbreaks.
In 2013, the Global Polio Eradication Initiative published a new “endgame plan” for polio that outlined a stepwise approach for removing OPV from immunization programs. First, it called on all countries to introduce at least one dose of inactivated poliovirus vaccine by the third quarter of 2015, immunizing infants at 14 weeks or at first contact thereafter. Second, it called for all countries to replace tOPV with a bivalent vaccine containing only types 1 and 3 by 2016. Given the eradication of wild poliovirus type 2, keeping type 2 in the oral vaccine just creates risk. An estimated 40% of VAPP cases and 98% of cVDPVs detected since 2012 were caused by poliovirus type 2. The type 2 component of tOPV also interferes with the immune response to the other types. Once poliovirus eradication has been achieved and certified, hopefully no later than 2019, all OPV will be withdrawn.
What’s the role of pediatricians in the United States in polio eradication? For now, our job is to continue to protect all children in the United States against all three types of poliovirus. Current Advisory Committee on Immunization Practices (ACIP) recommendations specify 4 doses of trivalent inactivated poliovirus vaccine (IPV) at ages 2 months, 4 months, 6-18 months, and 4-6 years. Children vaccinated outside the United States with bivalent vaccine, including immigrants and refugees, will need to be revaccinated. Those without appropriate documentation of vaccine (written, dated records that specify trivalent vaccine) also should be revaccinated.
Serologic testing for immunity is no longer recommended. In the past, children without documentation of vaccines could be tested for neutralizing antibodies to poliovirus types 1, 2, and 3. Moving forward, serologic testing for antibodies to poliovirus type 2 won’t be available because it requires live virus, and in accordance with World Health Organization recommendations, laboratories have been destroying supplies of poliovirus type 2.
We also need to make sure that our patients who are traveling internationally receive all recommended vaccines, including a dose of IPV when appropriate. Specific recommendations can be found on the CDC’s pages for travelers.
A 2015 statement from the American Academy of Pediatrics called on pediatricians to consider polio as a potential diagnosis of any child presenting with fever and acute flaccid paralysis (Pediatrics. 2015 Jan;135[1]:196-202). When polio is suspected, public health authorities should be notified and two stool samples collected 24 hours apart, and within 14 days of the onset of paralysis, sent for testing. According to lead author Walter A. Orenstein, MD, “because most polio infections are silent, a case of paralytic polio in the United States may have been acquired from an asymptomatic individual, so a history of travel to a polio-infected area may be absent in the case of paralysis.”
I’ll second what my mom said. Scary.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She said she had no relevant financial disclosures. Email her at pdnews@frontlinemedcom.com.
Make HIV testing of adolescents routine
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at pdnews@frontlinemedcom.com.
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at pdnews@frontlinemedcom.com.
Nearly 2 decades ago, I was a pediatric infectious diseases fellow fielding a call from a community pediatrician seeking advice on patient management. The patient in question was a 15-year-old male with fever, rash, and cervical adenopathy – a good clinical story for Epstein-Barr virus infection. A heterophile antibody test was negative, however, as were EBV titers.
We talked for a couple of minutes about the vagaries of EBV testing, as well as other organisms that could cause a mononucleosis-like illness. “Cytomegalovirus is a possibility, along with toxoplasmosis,” I told him. “I’d also test for HIV.”
There was a moment of silence and little throat-clearing. “I don’t think we need to that,” he finally responded. “I’ve known this boy since he was a baby, and I’m sure HIV’s not an issue. He’s not that kind of kid.”
Bear in mind that we lived in a Midwestern city with low rates of HIV, and I suspect this seasoned pediatrician had never seen a case. I argued (as only an impassioned trainee can) that every kid is the kind that could be at risk for HIV, and testing was ultimately done (and was negative).
A lot has changed in the intervening years. HIV infection, at least in adolescents and adults, can be controlled with a single pill taken once a day. Children infected perinatally can grow up and have (uninfected) children of their own. We have reasonably effective pre- and postexposure prophylaxis.
One thing that hasn’t changed, however, is the reluctance of some of us to test our patients for HIV. So what’s up with that?
It’s not because the virus has gone away. On Oct. 14, 2016, amid little fanfare, the Centers for Disease Control and Prevention released the United States Summary of Notifiable Infectious Diseases and Conditions for 2014. A total of 35,606 cases of HIV infection were diagnosed in the United States and reported to the CDC, and 7,723 were in individuals aged 15-24 years.
It is possible that the number of cases in adolescents is even higher. The CDC estimates as many as 60% of youth with HIV don’t know that they are infected, likely because they’ve never been tested. According to the 2015 Youth Risk Behavior Survey (YRBS), only 10% of United States high school students had ever been tested for HIV, and the number of teens tested has been dropping over time. In 2013, for example, the prevalence of having ever been tested for HIV was 13%.
It’s not because today’s teenagers lack risk factors, including sexual activity and drug use. Just over 30% of the U.S. students surveyed for the YRBS reported sexual intercourse with at least one person in the preceding 3 months, and more than 11% had had four or more lifetime partners. Among sexually active teenagers in the United States, only 57% reported that they or their partner used a condom during last sexual intercourse. Overall, 2% of those surveyed admitted a history of injecting an illegal drug.
It’s not because public health experts haven’t deemed testing a priority. The CDC recommends that everyone aged 13-64 years should get tested at least once. Annual testing is recommended for some individuals, including sexually active gay and bisexual males, those who have had more than one sexual partner since their last HIV test, and those who have another sexually transmitted disease. A 2011 American Academy of Pediatrics policy statement affirms the need for routine testing, calling for all adolescents living in geographic areas with an HIV prevalence greater than 0.1% to be offered routine HIV screening at least once by age 16-18 years. In communities with a lower prevalence, the AAP recommends routine HIV testing for sexually active adolescents as well as those with other risk factors, including substance use. Annual HIV testing is recommended for high-risk teenagers, and whenever testing for other sexually transmitted infections (STIs) is performed.
It’s probably not that most teenagers are being offered HIV tests and they’re declining. In 2008, the emergency department at Le Bonheur Children’s Hospital in Memphis, Tenn., implemented a protocol for routine, opt-out HIV screening for medically stable patients aged 13-18 years (Pediatrics. 2009 Oct;124:1076-84). Of the 2,002 patients approached for screening over an approximately 7-month period, only 267 (13%) opted out and of those, 73 had already been tested.
Yet many of us still are not testing. More recently, investigators in Philadelphia performed a retrospective, cross-sectional study of 1,000 randomly selected 13- to 19-year-old patients attending routine well visits conducted at 29 pediatric primary care practices to assess clinician documentation of sexual history and screening for STIs and HIV (J Pediatr. 2014 Aug;165[2]:343-7). Only 212 visits (21.2%) had a documented sexual history, and only 16 patients were tested for HIV (1.6%). HIV testing was more likely to be performed on older adolescents, those of non-Hispanic black race/ethnicity, and those with nonprivate insurance. Study authors called the results “concerning” and advocated for standardized protocols, documentation templates, and electronic decision support to facilitate improved sexual health assessments and screening.
I suspect we all can do better. I’m not a primary care provider, but I do see adolescents with a variety of complaints. I’m pretty diligent about testing teenagers admitted with unexplained fever, vague constitutional symptoms, and those with symptoms that suggest another STI. I’m less effective at discussing HIV testing with those being treated for a postop wound infection, or a routine community-acquired pneumonia.
December is a good time to reflect on practice and make resolutions for the new year. I resolve to talk to more of my adolescent patients about HIV. Who’s with me?
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky., and Kosair Children’s Hospital, also in Louisville. Email her at pdnews@frontlinemedcom.com.
Summer flu? Think variant swine influenza virus infection
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.
Two children presented with influenza, and both recovered without the need for hospitalization. This scenario would fail to pique the interest of any pediatrician in January. But what about when it happens in July?
In early August, public health authorities in Ohio announced that two children had tested positive for the variant swine influenza virus H3N2v. Both children had direct contact with pigs at the Clark County Fair in late July. Along with a handful of cases diagnosed in Michigan, these represent the first H3N2v cases in the United States in 2016.
Influenza viruses that normally circulate in swine are designated as “variant” when they infect humans. According to the Centers for Disease Control and Prevention (CDC), human infections with H1N1v, H1N2v, and H3N2v have been identified in the United States. Influenza A H3N2v viruses carrying the matrix gene from the 2009 H1N1 pandemic virus were first detected in pigs in 2010, and in people in the summer of 2011. Since that time, 357 human cases have been reported from 14 states, with nearly 75% occurring in Indiana and Ohio. Most infections occurred after prolonged exposure to pigs at agricultural fairs.
Fortunately, most H3N2v infections have been mild: Since July 2012, only 21 individuals have required hospitalization and a single case resulted in death. Notably, though, many of the hospitalizations involved children.
On Aug. 15, the Centers for Disease Control and Prevention released Interim Guidance for Clinicians on Human Infections with Variant Influenza Viruses.
Because variant virus infection is indistinguishable from seasonal influenza or any other virus that cause influenzalike illness (think fever, cough, sore throat), physicians and other frontline providers need to maintain an index of suspicion. The key is eliciting a history of swine exposure in the week before illness onset. Practically, this means asking about direct contact with pigs, indirect contact with pigs, or close contact with an ill person who has had contact with pigs. Kudos to the astute clinicians in Ohio who thought to send the appropriate influenza testing in July.
When variant influenza virus is suspected, a nasopharyngeal swab or aspirate should be obtained for testing at a state public health lab or the CDC. Rapid antigen tests for influenza may be falsely negative in the setting of H3N2v infection, just as they may be with seasonal influenza infection. Molecular tests such as reverse transcription polymerase chain reaction (RT-PCR) are likely more sensitive, but cannot distinguish variant influenza A viruses from seasonal influenza A viruses.
The Kentucky State Fair opened on Aug. 18, making the CDC guidance especially timely for health care providers in my area. I called a friend who is a pediatric emergency medicine physician to ask if she and her colleagues were routinely screening patients for encounters of the porcine kind.
“For example, are you asking, ‘Have you been showing, raising or feeding swine? Have you been to the pig barn at the fair?’ ”
When my friend quit laughing, I confessed to her that I had not been doing that routinely either. The exposure history is often the most interesting part of the infectious disease evaluation and in the last month, I’ve asked about exposure to sheep (a risk factor for Q fever), exposure to chickens (a risk factor for Salmonella infections), and exposure to beaver dams (a risk factor for blastomycosis). But I’ve not asked about exposure to pigs.
“The emergency medicine approach is to avoid a lot of viral diagnostic testing unless it is going to impact management,” she said. “Tell me how this changes management of my patient.”
From the patient perspective, making a presumptive diagnosis of H3N2v infection would open the door to empiric treatment with antivirals, at least for individuals who are hospitalized, have severe or progressive disease, or who at high risk for complications of influenza. Neuraminidase inhibitors, including oral oseltamivir, inhaled zanamivir, and intravenous peramivir, can be used for treatment of H3N2v infections.
From a societal perspective, making the diagnosis gives public health experts the opportunity to investigate and potentially prevent further infections by isolating sick pigs. Human to human transmission of H3N2v is rare, but has occasionally occurred in households and in one instance, a child care setting. Careful surveillance of each swine flu case in a human is important to exclude the possibility that the virus has developed the ability to spread efficiently from person to person, creating the risk for an epidemic.
Seasonal influenza vaccine does not prevent infection with variant viruses, so avoidance is key. Those at high risk for complications from influenza infection, including children younger than 5 years of age and those with asthma, diabetes, heart disease, immunocompromised conditions, and neurologic or neurodevelopmental disorders, are urged to avoid pigs and swine barns when visiting fairs where the animals are present. Everyone else needs to follow common sense measures to prevent the spread of infection.
• Don’t take food or drink into pig areas; don’t eat, drink or put anything in your mouth in pig areas.
• Don’t take toys, pacifiers, cups, baby bottles, strollers, or similar items into pig areas.
• Wash your hands often with soap and running water before and after exposure to pigs. If soap and water are not available, use an alcohol-based hand rub.
• Avoid close contact with pigs that look or act ill.
• Take protective measures if you must come in contact with pigs that are known or suspected to be sick. This includes wearing personal protective equipment like protective clothing, gloves, and masks that cover your mouth and nose when contact is required.
• To further reduce the risk of infection, minimize contact with pigs in the pig barn and arenas.
It shouldn’t be surprising that flu viruses spread from pigs to people in the same way that regular seasonal influenza spread from person to person. An infected pig coughs or sneezes influenza-containing droplets, and these droplets are inhaled or swallowed by a susceptible human. That makes avoiding contact with pigs that look or act ill especially important. For the record, a pig with flu might have fever, depression, cough, nasal or eye discharge, eye redness, or a poor appetite.
On the bright side, you can’t get H3N2v or any other variant virus from eating properly prepared pork meat. Fairgoers can feel free to indulge in a deep-fried pork chop or one of this year’s featured food items: a basket of French fries topped with smoked pork, cheddar cheese sauce, red onions, jalapeño peppers and barbecue sauce.
Or maybe not. The CDC has a web page devoted to food safety at fairs and festivals. It notes that cases of foodborne illness increase during summer months, and usual safety controls “like monitoring of food temperatures, refrigeration, workers trained in food safety and washing facilities, may not be available when cooking and dining at fairs and festivals.”
The public is urged to seek out “healthy options” from fair vendors first. If healthy options aren’t available, we’re advised to consider bringing food from home to save money and calories.
Sigh. I remember when summer used to be more fun.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville, Ky. and Kosair Children’s Hospital, also in Louisville.