User login
Many EM docs have treated COVID-19 patients without proper PPE: Survey
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
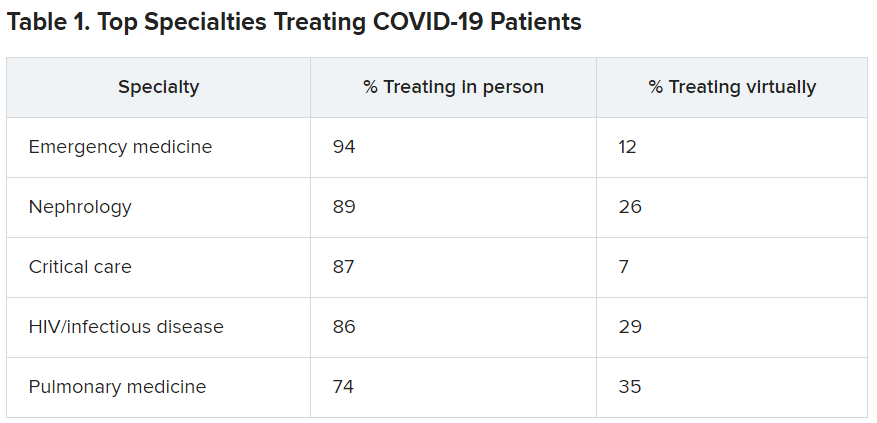
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
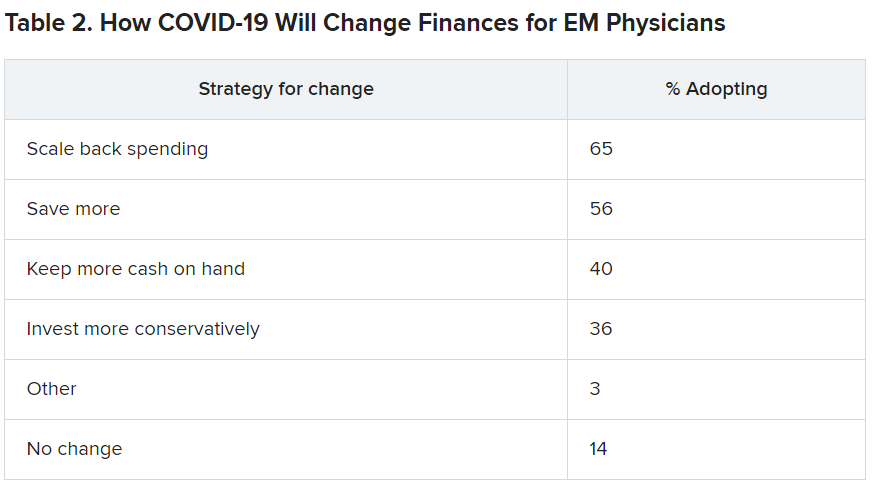
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
Many emergency medicine (EM) physicians who responded to a Medscape survey said they have treated COVID-19 patients without appropriate personal protective equipment (PPE).
In the Medscape Emergency Medicine Physicians’ COVID-19 Experience Report, 21% of respondents said that that was sometimes the case; 7% said that it was often the case; and 1% said they always treat patients without appropriate PPE.
EM physicians were the physicians most likely to treat COVID-19 patients in person.
For comparison, among family medicine physicians, 58% said that they have treated COVID-19 patients in person, and 45% said they were treating them via telemedicine.
Data for the report were gathered from June 9 to July 20 as part of Medscape’s COVID-19 experience survey for all physicians. That survey drew more than 5,000 responses.
Nearly all (98%) of EM physicians who have treated COVID-19 patients said that they have done so since the beginning, when the World Health Organization declared a pandemic on March 11, 2020. For all U.S. physicians, the percentage was much higher than that – 73% said they had treated COVID-19 patients from the start.
EM physicians have often found themselves sacrificing their own safety for the sake of patients. More than half of EM physicians (54%) said that they had knowingly taken personal safety risks to treat a COVID-19 emergency, a percentage far higher than the 30% of all physicians who said they had done so.
Four percent of EM physicians have received a positive diagnosis of COVID-19 via testing. An additional 2% have been confirmed as having COVID on the basis of symptoms.
Steep income drops
Survey authors wrote that two-thirds of EM physicians have experienced income loss during the pandemic. Most (71%) saw their income drop by between 11% and 50%; 11% saw a decrease of more than 50%. Among other specialties, the percentages of those who have experienced a drop of more than 50% are far higher. Among ophthalmologists, 51% said they had experienced such a drop; among allergists, 46%; plastic surgeons, 46%; and otolaryngologists, 45%.
Asked whether their burnout levels have increased in the wake of COVID-19, 74% of EM physicians said burnout had intensified; 23% reported no change; and 3% said burnout had lessened.
Reports of loneliness have been widespread during the pandemic, owing to stay-at-home orders and social distancing. More EM physicians than physicians in general said feelings of loneliness had increased for them in the past year.
More than half of EM doctors (55%) said they are experiencing more loneliness in the pandemic, compared with 46% of all physicians who felt that way; 42% said those feelings have not changed; and 3% said they have been less lonely.
Grief and stress relief
Fewer than half (42%) of the respondents reported that their workplace offers clinician activities to help with grief and stress; 39% said their workplace didn’t offer such help; and 19% said they were unsure.
The percentages were nearly identical to the percentages of physicians overall who answered whether their workplace offered help for grief and stress.
Along with insecurity regarding physical and mental health, COVID-19 has introduced more questions about financial health. Here’s a look at how emergency physicians said they would change the way they save and spend.
Challenges to daily practice
By the time this survey was taken, a large percentage of patients had delayed or avoided urgent or routine medical care for reasons related to COVID-19, so survey authors asked whether EM physicians’ patient population had changed.
Survey authors wrote that “most EM physicians (82%) are seeing patients with non-COVID diseases, such as cardiovascular problems or diabetes, who otherwise probably would have sought treatment earlier.”
COVID-19 has also thrown a major obstacle into most EM physicians’ careers by preventing them from doing the job to the best of their ability. That loss is one of the three primary components of burnout.
More than two-thirds (67%) said COVID-19 has hampered their ability to be as good a doctor as they would like.
A version of this article first appeared on Medscape.com.
IDSA panel updates guidelines on COVID molecular diagnostic tests
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Saliva spit tests stack up well against the gold standard for molecular COVID-19 tests – the back-of-the-nose deep swab – without the discomfort and induced coughing or sneezing of the test taker, updated guidelines indicate.
In a press briefing on Jan. 6, the Infectious Diseases Society of America explained the findings of an expert panel that reviewed the literature since the IDSA released its first guidelines in May.
The panel found that saliva tests were especially effective if the test included instructions to cough or clear the throat before spitting into the tube, said panel chair Kimberly E. Hanson, MD, MHS, of University of Utah Health, Salt Lake City.
Throat swab alone less effective
Using a throat swab alone was less effective and missed more cases than the other methods, she said.
The IDSA has updated its recommendation: A saliva test or swabs from either the middle or front of the nose front are preferred to a throat swab alone.
A combination of saliva and swabs from the front and middle of the nose and throat together “looked pretty much equivalent” to the gold-standard deep swab, the panel found.
She acknowledged, however, that multiple swabs exacerbate already challenging supply issues.
Saliva samples do come with challenges, Dr. Hanson noted. A laboratory must validate that its systems can handle the stickier material. And asking a patient to cough necessitates more personal protective equipment for the health care professional.
Each center will have to tailor the specimen type it chooses, based on what resources it has available and the setting – whether in a hospital or a drive-through operation, for instance, she said.
Rapid testing vs. standard
Panel member Angela M. Caliendo, MD, PhD, of Brown University, Providence, R.I., said the panel preferred rapid polymerase chain reaction tests and standard, laboratory-based PCR tests over a rapid isothermal test.
The panel defined rapid tests as those for which results are available within an hour after a test provider has the specimen in hand. They excluded home tests for this category.
The only rapid isothermal test that had enough data on which to issue a recommendation was the ID NOW test (Abbott Labs), she noted.
Rapid PCR tests performed just as well as the standard laboratory-based tests, she said, with a high sensitivity of “97% on average and a very high specificity.”
But the rapid isothermal test had an average sensitivity of only about 80%, compared with the lab-based PCR test, Dr. Caliendo said, yielding a substantial number of false-negative results.
Testing centers will have to weigh the considerable advantages of having results in 15 minutes with a rapid isothermal test and being able to educate positive patients about immediate isolation against the potential for false negatives, which could send positive patients home thinking they don’t have the virus – and thus potentially spreading the disease.
And if a clinician gets a negative result with the rapid isothermal test, but has a strong suspicion the person has COVID or lives in an area with high prevalence, a backup test with a rapid PCR or laboratory-based test should be administered.
“You will miss a certain percentage of people using this rapid isothermal test,” she said.
However, Dr. Caliendo said, if the only available option is the isothermal test, “you should definitely use it because it’s certainly better than not testing at all.”
On a positive note, she said, all the varieties of tests have high specificity, so “you’re not going to see a lot of false-positive results.”
The guidelines back in May didn’t make recommendations on rapid tests, she said, because there weren’t enough data in the literature.
Dr. Caliendo noted that most of the available data were for symptomatic patients, but there are some data that show the amount of virus in the respiratory tract is similar for people with and without symptoms. The panel, therefore, expects that the performance of the various assays would be similar whether or not a person had symptoms.
Testing the immunocompromised
Dr. Hanson said the original recommendation in May was to do molecular testing for asymptomatic people who were awaiting a transplant or were waiting to start immunosuppressive therapy for cancer or an autoimmune disease. Now the current guidelines “make no recommendation for or against screening” in those cases.
Dr. Hanson added that the panel feels that patients awaiting bone marrow and solid organ transplants should have the testing because of the high risks that will result if patients have contracted the virus.
But for those with cancer or an autoimmune disease, the panel decided to leave it up to each physician to assess individual risk and determine whether the patient should be tested.
Home testing
The IDSA guidelines didn’t weigh in on home testing because the products are so new and studies so far have included fewer than 200 patients. But Dr. Caliendo said they clearly perform better earlier in the disease phase – the first 5-7 days – when the amount of the virus is higher.
Dr. Hanson and Dr. Caliendo also fielded a question about what the new virus variant, first discovered in the United Kingdom and now spreading to other countries (including the United States) means for diagnostic testing.
“So far we think with the majority of tests that are [emergency use] authorized, it doesn’t look like this new variant should really affect test performance,” Dr. Hanson said.
The variant has differences in the spike gene, and many of the current tests detect and identify SARS-CoV-2 without the spike gene so they wouldn’t be affected, she added.
Dr. Caliendo agreed: “I think the vast majority of our tests should be in good shape.”
Dr. Hanson and Dr. Caliendo disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Partnering with dietitians can bridge gaps in IBD care
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
Partnering with dietitians can bridge gaps in IBD care
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Working with a registered dietitian (RD) can help ensure that changing the way patients with inflammatory bowel disease (IBD) eat won’t deprive them of the nutrients they need.
Depending on the location and resources of a medical practice, calling in a dietitian may seem like a luxury. But making those connections can be more accessible during the COVID-19 pandemic as more dietitians are working virtually.
Kelly Issokson, MS, RD, clinical nutrition coordinator for IBD at Cedars Sinai Medical Center in Los Angeles, suggested two websites that allow users to search for RDs by ZIP code or by those working virtually: the International Foundation for Gastrointestinal Disorders and eatright.org, the website for the professional body for the dietetics community, which also has a searchable database.
Ashwin N. Ananthakrishnan, MD, MPH, director of the Crohn’s and colitis center at Massachusetts General Hospital in Boston, said it’s key for gastroenterologists to communicate what exactly they want the dietitian to address and not merely refer the patient.
The provider should know what therapies exist and then have the dietitian walk the patient through the plan, he said.
Mark Mattar, MD, with MedStar Georgetown University Hospital in Washington, said that, in addition to connecting patients with dietitians, “I always refer my patient to the Crohn’s and Colitis Foundation for the most recently updated patient education materials on nutrition.”
Panelists at the Advances in Inflammatory Bowel Diseases 2020 annual meeting on Wednesday weighed in on dietary considerations for two patient scenarios posed by Maria Abreu, MD, director of the Crohn’s & Colitis Center at the University of Miami.
The first scenario involved a 54-year-old man with long-standing fibrostenotic Crohn’s disease, recently hospitalized for obstruction and discharged with a prescription for prednisone 40 mg daily. The patient had been on infliximab (Remicade), and now is taking now adalimumab (Humira) weekly. He will undergo surgery to remove an ileal stricture. Dr. Abreu asked what dietary changes the panelists would make to ensure adequate nutrition prior to surgery and prevent another obstruction.
Dr. Ananthakrishnan said he would check vitamin B₁₂, vitamin D, iron, and albumin levels to see if any micronutrients need to be replaced.
He said that, although he thinks low-fiber diets are used too often as the default for Crohn’s and ulcerative colitis, in this case he would recommend low fiber and urge the patient to avoid raw fruits, vegetables, nuts, and seeds.
The patient can remove the skins and still have shakes and smoothies to get the benefits of fiber-containing foods without the fiber component, he said.
Discussing a pediatric version of that scenario, Andrew Grossman, MD, a pediatric gastroenterologist at the Children’s Hospital of Philadelphia, said he would turn to enteral nutrition therapy.
“We would strongly encourage using a formula to try to improve nutritional status, which we know can improve surgical outcomes,” he said.
The second case was a 15-year-old girl with growth stunting. She was diagnosed at age 10 with Crohn’s disease, currently has moderate disease, and continues to have five to seven liquid bowel movements daily, along with abdominal pain after meals. She is starting adalimumab induction.
Dr. Grossman said, “first, I would not be managing this alone. I would be managing this with a dietitian and working together to improve outcomes. We need to consider aggressive therapy, and to me that would include consideration of biological therapy but also possible dietary therapy – the Crohn’s Disease Exclusion Diet or enteral nutrition therapy as possibilities.”
He pointed out that in pediatrics there must be consideration both for what the parent wants the child to do and what the child is willing to do.
“My primary focus would be on improving caloric intake, working with the dietitian to avoid foods that bother the most,” he said.
Dr. Issokson said she would recommend either exclusive enteral nutrition or a specific carbohydrate diet (SCD) for the teen.
“We see [SCD] doesn’t impair growth in our patients as long as they are being followed by a dietitian, and we’re making sure they are getting adequate nutrient intake,” she said.
Dr. Abreu said in an interview that “diet is important in patients with IBD; it is a complement to the therapies that we use and a potential opportunity to solidify a long-lived remission.”
“Although studies of diet are only now being done,” she said, “we already have some good foundational ideas about diet and its role in reducing inflammation and reducing symptoms.” And she added that treating gastroenterologists should certainly avoid telling patients that “diet does not matter.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Ananthakrishnan, Dr. Grossman, and Dr. Issokson have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
GI physicians urge COVID-19 vaccines for all IBD patients
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
A version of this article originally appeared on Medscape.com.
GI physicians urge COVID-19 vaccines for all IBD patients
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Gastroenterologists at the Advances in Inflammatory Bowel Disease 2020 annual meeting said they will strongly advise their patients to take the COVID-19 vaccines as they become available.
Announcement that the first vaccine, Pfizer’s, was recommended for emergency use authorization came in the middle of AIBD’s Thursday evening COVID-19 session.
Miguel Regueiro, MD, chair of the department of gastroenterology, hepatology, and nutrition at Cleveland Clinic in Ohio, said, “We’re uniformly recommending this to all our patients.”
“The [vaccines] leading the pack do not have any replicating virus and thus can be used in immunocompromised people,” Maria Abreu, MD, director of the Crohn’s & colitis center at the University of Miami, told this news organization. “Although it is true that we don’t know – and won’t know for a while – whether the high levels of efficacy seen with the mRNA vaccines so far will be achieved in patients who are immunocompromised, there is every reason to believe that [the vaccine] will still be enough to protect them from complications of COVID-19.”
The bottom line, she said, is that “it’s much safer to get a vaccine than it is to take your chances of getting COVID-19.”
David T. Rubin, MD, chief of gastroenterology, hepatology, and nutrition at UChicago Medicine, said in a session earlier in the day, “Emerging information about the messenger RNA looks like it’s going to be safe for our population, but of course we want to see more. Messenger RNA degrades within days of giving it, so it’s not expected to linger or generate any other problems we can think of.”
Dr. Abreu said there’s no evidence that inflammatory bowel disease (IBD) patients are more susceptible to COVID-19 infection even though the entry molecules are expressed in the GI tract. “They are really not differentially expressed in IBD and, if anything, some of our more potent therapies reduce the expression of these molecules in the GI tract,” she said.
Regarding how IBD medications affect outcomes if patients are infected with COVID-19, Dr. Abreu pointed out that corticosteroids seem to be associated with worse outcomes. “I would posit that it has to do with initially allowing there to be a lot of very rapid viral replication,” she said.
And she also noted that any of the mainstay drugs for IBD – the anti–tumor necrosis factor (TNF) therapies – are showing promise as treatments for COVID-19.
Updates from the IBD-COVID-19 registry
Michael Kappelman, MD, MPH, from the University of North Carolina at Chapel Hill said information from the Secure-IBD registry, which collects real-time global information on how COVID-19 affects IBD patients, suggests that these patients “may have a more severe course than the general population, but not by much.”
He reported the registry had logged more than 3,300 reported COVID-19 cases among IBD patients from 62 countries.
Registry outcomes through the end of November have found a mean age of reported cases of 40 years, and that 21% of patients were hospitalized with an average length of stay of 10.2 days, 4% required intensive care unit admission, and 2% died.
The majority of the deaths reported to Secure-IBD occurred in patients older than 60 years, Dr. Kappelman said, adding that the hospitalizations and death rates in IBD patients with no comorbidities were relatively low.
“My belief is that available data are actually more reassuring than alarming,” he said.
Dr. Kappelman and other investigators found that combination therapy that includes thiopurines and thiopurine monotherapy are “associated with about a fourfold risk of the requirement for intensive care or mortality from COVID,” compared with anti-TNF monotherapy.
In cases reported to Secure-IBD, about 25% of IBD patients with COVID-19 developed new GI symptoms, primarily diarrhea and abdominal pain, he said.
In his practice, Dr. Kappelman said, he minimizes use of steroids and has found that COVID-19 adds a reason to favor anti-TNF over 6-mercaptopurine (6-MP) plus azathioprine.
He also advises “a high alert for COVID-19 in patients with new GI symptoms.”
Dr. Abreu has relationships with Boehringer Ingelheim, Cosmo Biopharma, Eli Lilly, Gilead, Janssen, Landos Biopharma, Prometheus Bioscience, Takeda, UCB Biopharma, Pfizer, and Prometheus Laboratories. Dr. Rubin has served as a director, officer, partner, employee, adviser, consultant, or trustee for AbbVie, Abgenomics, Allergan, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Dizal Pharmaceuticals, GalenPharma/Atlantica, Genentech/Roche, Gilead Sciences, Ichnos Sciences, GlaxoSmithKline, Janssen, Eli Lilly, Pfizer, Prometheus Laboratories, Reistone, Shire, Takeda Pharmaceutical, and Techlab. In addition, he has received research grants from AbbVie, Genentech/Roche, Janssen Pharmaceuticals, Prometheus Laboratories, Shire, and Takeda Pharmaceutical Company; and holds stock options in Abgenomics and Biomica. Regueiro and Kappelman have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
IBD: Fecal calprotectin’s role in guiding treatment debated
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
Help your patients better understand their IBD treatment options by sharing AGA’s patient education, “Living with IBD,” in the AGA GI Patient Center at www.gastro.org/IBD.
A version of this article originally appeared on Medscape.com.
IBD: Fecal calprotectin’s role in guiding treatment debated
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Questions on fecal calprotectin’s usefulness as a measure of intestinal inflammation in inflammatory bowel disease (IBD) dominated the viewer chat after the opening session of Advances in Inflammatory Bowel Diseases 2020 Annual Meeting.
The measure is often used to differentiate irritable bowel syndrome (IBS) from IBD.
Panelists differed on how predictive fecal calprotectin is for disease status and what information the stool concentration of calprotectin imparts. Several experts discussed calprotectin cutoffs for when disease would be considered in remission or when a colonoscopy is needed for evaluation.
Bruce E. Sands, MD, of the Icahn School of Medicine at Mount Sinai, New York, said about the noninvasive test: “It can be very tricky to use.”
Variation by time of day, by person
He explained that there can be individual differences, and that the concentration may be different in the first stool of the day compared with the last.
“There’s a lot of variation, which makes the cutoffs good on average for populations but a little bit more difficult to apply to individuals,” he said.
Dr. Sands said the marker has more merit for people with large-bowel inflammation but is not quite as accurate a marker for patients with exclusively small-bowel inflammation.
Moderator Steven Hanauer, MD, professor of medicine, gastroenterology, and hepatology at Northwestern University, Chicago, asked Dr. Sands what his next move would be if a patient had a concentration of 160 mcg/mg.
Sands called concentrations between 150 and 250 mcg/mg “a gray zone.”
“That usually indicates for me a need to evaluate with a colonoscopy,” he said.
“If we’re talking about using fecal calprotectin to rule out IBS, the cutoff there is more like 50, 55. But that isn’t how we’re generally using it as IBD practitioners.”
Sunanda V. Kane, MD, MSPH, a gastroenterologist with the Mayo Clinic in Rochester, Minn., said in an interview that 160 mcg/mg in a patient with IBD “means to me likely some minimal disease but not enough for me to make drastic changes to a medical regimen.”
She said about the measure, “We need to understand its limitations as well as strengths. Right now, insurance companies consider it ‘experimental’ and a lot of companies will not cover it. Ironically, they will cover the cost of a colonoscopy but not a stool test.”
Use as a benchmark
Dr. Sands said if he’s doing a colonoscopy to establish that the patient is in remission and knows what the fecal calprotectin level is at the time, he uses it as a benchmark for the future to judge whether the patient is deviating from remission.
He added that the negative predictive value of fecal calprotectin with a cutoff of 100 mcg/mg is “actually pretty good so you can avoid a number of unnecessary colonoscopies to look for recurrence.”
William J. Sandborn, MD, of the University of California, San Diego, said about the marker, “We use it some, but a cutoff of 50 is very specific. You can think of that as equivalent to a Mayo endoscopy score of 0 in ulcerative colitis and probably histologic remission.”
Cutoffs above 50 mcg/mg are “not very clear,” he said.
He said given the lack of consensus on the panel, “others might take some pause about that discomfort.”
Dr. Sandborn pointed out that little is known about elevated calprotectin in ulcerative proctitis and whether it is elevated in Crohn’s ileitis.
Dr. Kane said other factors will affect fecal calprotectin levels.
“We have some data to say that if you are on a proton pump inhibitor that that changes fecal calprotectin levels. Patients who have inflamed pseudopolyps may have quiescent disease around the pseudopolyps that may elevate the fecal calprotectin.”
But it can have particular benefit in some patient populations, she said.
She pointed to a study that concluded calprotectin levels can be used in pregnant ulcerative colitis patients to gauge disease activity noninvasively.
Dr. Sands, Dr. Sandborn, Dr. Kane, and Dr. Hanauer have disclosed having no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Medical societies waive fees, weigh other options during pandemic
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
COVID-19’s toll on member facilities pushed the American Academy of Sleep Medicine (AASM) recently to take a sizable gamble.
AASM announced in September that it would waive facility fees at all 2,648 AASM-accredited sleep facilities for 2021.
At $1,800-$2,600 for each facility, that will mean lost revenue of between $4.8 million and $6.9 million, but it’s a risk the academy felt it had to take.
AASM President Kannan Ramar, MBBS, MD, said in an interview that they are betting on the future of the field.
An internal survey of members, he said, found that nearly half (46%) of the 551 respondents thought they might have to close by the end of the year.
In addition, 66% reported a lower patient volume in the past month, and 36% reported that their practice or facility had to apply for loans or other financial assistance because of COVID-19, AASM said in its press release.
“We are hoping that if we help our members through this, they will be there for our patients,” Dr. Ramar said.
Other medical societies also are weighing options, straddling the line between needing income to provide resources for members but being acutely aware of the financial toll the pandemic is taking, according to one sampling.
As previously reported, primary care practices are projected to lose more than $68,000 in revenue per full-time physician in 2020, after steep drops in office visits and the collection of fees from March to May, according to a study led by researchers in the Blavatnik Institute at Harvard Medical School, Boston.
Those losses were calculated without considering a potential second wave of COVID-19 this year, the authors noted.
‘We can survive this’
Although AASM waived fees for its member facilities, individual physician fees have not been reduced so far. But the group is looking for more ways to help lower the economic burden on members, Dr. Ramar said.
“I don’t think we’ve ever been in this situation in the 45 years of the academy. This is a once-in-a-lifetime event for challenges we’re going through,” he said. “The board and the leadership realized that, if we’re going to do something, this is the time to do it.”
In addition to waiving the fees, AASM and the AASM Foundation are offering relief funding to state and regional sleep societies and research award recipients through programs created in response to COVID-19.
Some societies said they are not making changes to their dues or fees, some are forgoing cost-of-living fee increases, and some are waiving registration fees for annual meetings.
The American College of Allergy, Asthma and Immunology (ACAAI) waived most members’ registration fees for its annual meeting in November. Typically, that fee would be $500-$800 per member, plus charges for some premium sessions, Michael Blaiss, MD, ACAAI executive medical director, said.
Dr. Blaiss said in an interview that the college thought offering its 6,000 members essentially 25 free hours of CME would benefit them more than waiving annual membership dues, which are about $425 for physicians in the United States.
If the pandemic stretches through 2021, Dr. Blaiss said, “We can survive this. I’m not worried about that at all.”
But he acknowledged the painful effect on medical societies.
“I don’t think any organization would tell you it’s not having an effect on their income,” he said. “I know it is for us and for virtually any medical organization. A high percentage of income comes from the annual meeting.”
Waiving dues has not been a high priority among members in communications so far, Blaiss said.
American Academy of Dermatology President Bruce H. Thiers, MD, said in an interview that there will be no cost-of-living increase for 2021 dues, and AAD members can request a reduction in dues, which will be considered on a case-by-case basis.
“We understand that many members will have to make tough financial decisions,” he said.
In addition, AAD, which has more than 20,000 members, is exploring payment options to help members spread out the cost of membership.
ACP extends membership
The American College of Physicians, whose membership cycle starts in July, did not reduce dues but extended membership at no cost for 3 months through September to its 163,000 members, Phil Masters, MD, ACP’s vice president of membership, said in an interview.
It also expanded its educational offerings related to the pandemic, including webinars on physician wellness and issues regarding telemedicine.
He said expanding educational resources rather than waiving dues was an intentional decision after much discussion because “we’re primarily a services resource organization.”
Membership data are still being calculated, but early indications are that membership is not increasing this year, after seeing annual growth of about 2%-2.5%, Dr. Masters said. He noted that income is down “by several percent.” Annual membership dues average about $500 for physicians who have been practicing for 10 years.
“We’re well positioned to tolerate the ups and downs,” he said, but he acknowledged that “there’s no question the financial impact has been devastating on some practices.”
Like some other associations, ACP decided to cancel this year’s annual meeting, which had been planned for April. The 2021 annual meeting will be conducted online from April 29 to May 1.
Smaller organizations that rely heavily on income from the annual meeting will be severely challenged the longer the pandemic continues, Dr. Masters said.
The decision is not as simple as whether to reduce or eliminate dues, he noted. Organizations will have to reexamine their missions and structure their fees and offerings according to the needs of members.
“It’s a balance in doing things for the community at large and balancing the need to be sensitive to financial implications,” Dr. Masters said.
This article first appeared on Medscape.com.
FDA authorizes baricitinib combo for COVID-19
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.
The US Food and Drug Administration (FDA) Nov. 19 issued an emergency use authorization (EUA) for the Janus kinase inhibitor baricitinib (Olumiant, Eli Lilly) in combination with remdesivir (Veklury, Gilead) for treating hospitalized adults and children at least 2 years old with suspected or confirmed COVID-19.
The combination treatment is meant for patients who need supplemental oxygen, mechanical ventilation, or extracorporeal membrane oxygenation (ECMO).
Baricitinib/remdesivir was shown in a clinical trial to reduce time to recovery within 29 days of starting the treatment compared with a control group who received placebo/remdesivir, according to the FDA press release.
The median time to recovery from COVID-19 was 7 days for the combination group vs. 8 days for those in the placebo/remdesivir group. Recovery was defined as either discharge from the hospital or “being hospitalized but not requiring supplemental oxygen and no longer requiring ongoing medical care,” the agency explained in the press release.
The odds of a patient dying or being ventilated at day 29 was lower in the combination group compared with those taking placebo/remdesivir, the press release said without providing specific data. “For all of these endpoints, the effects were statistically significant,” the agency stated.
The safety and efficacy continues to be evaluated. Baricitinib alone is not approved as a treatment for COVID-19.
“The FDA’s emergency authorization of this combination therapy represents an incremental step forward in the treatment of COVID-19 in hospitalized patients, and FDA’s first authorization of a drug that acts on the inflammation pathway,” said Patrizia Cavazzoni, MD, acting director of the FDA’s Center for Drug Evaluation and Research.
“Despite advances in the management of COVID-19 infection since the onset of the pandemic, we need more therapies to accelerate recovery and additional clinical research will be essential to identifying therapies that slow disease progression and lower mortality in the sicker patients,” she said.
As a JAK inhibitor, baricitinib interferes with a pathway that leads to inflammation. Baricitinib is already prescribed as an oral medication and is FDA-approved for treating moderate to severe rheumatoid arthritis.
The data supporting the EUA for the combination treatment are based on a randomized, double-blind, placebo-controlled clinical trial (ACTT-2), conducted by the National Institute of Allergy and Infectious Diseases (NIAID).
The trial followed patients for 29 days and included 1,033 patients with moderate to severe COVID-19; 515 patients received baricitinib/remdesivir, and 518 patients received placebo/remdesivir.
The FDA emphasizes that an EUA is not a full FDA approval.
In reviewing the combination, the FDA “determined that it is reasonable to believe that baricitinib, in combination with remdesivir, may be effective in treating COVID-19 for the authorized population” and the known benefits outweigh the known and potential risks. Additionally, there are no adequate, approved, and available alternatives for the treatment population.
“Today’s action demonstrates the FDA’s steadfast efforts to make potential COVID-19 treatments available in a timely manner, where appropriate, while continuing to support research to further evaluate whether they are safe and effective,” said FDA Commissioner Stephen M. Hahn, MD. “As part of our Coronavirus Treatment Acceleration Program, the FDA continues to use every possible avenue to facilitate new treatments for patients as quickly as possible to combat COVID-19.”
This article first appeared on Medscape.com.