User login
IBD, especially Crohn’s disease, linked to cervical neoplasia
Women who have inflammatory bowel disease, particularly those with Crohn’s disease, are at increased risk for developing cervical neoplasia, according to a report published online in Clinical Gastroenterology and Hepatology.
In a population-based nationwide cohort study involving 27,408 Danish women with newly diagnosed IBD and 1,508,334 control subjects matched for age and area of residence, a significant excess in cervical neoplasia also was observed for up to 10 years before the GI diagnosis. This bidirectional association indicates that an as-yet-unknown factor underlies susceptibility to both disorders, said Dr. Christine Rungoe of the department of epidemiology research, Statens Serum Institut, Copenhagen, and her associates.
“Patients with IBD should be encouraged to follow the screening program for cervical neoplasia, and clinicians should be aware of the slightly increased risk of HPV-related cervical lesions in IBD patients,” they noted.
Some previous studies have shown an increased risk of cervical neoplasia among IBD patients, but others have found no association. Some experts have proposed that either the underlying immunologic changes in IBD or the treatment of IBD with immunosuppressive drugs may impair patients’ ability to clear HPV infections, thus raising the risk of cervical neoplasia.
Dr. Rungoe and her colleagues examined the issue using data from Danish national registries that covered approximately 4 million women residing there during the past 32 years. They identified 18,691 women newly diagnosed with ulcerative colitis and 8,717 newly diagnosed as having Crohn’s disease and compared their rates of cervical neoplasia against those of control subjects during a median of 8 years of follow-up.
Women with ulcerative colitis showed a slightly but significantly increased risk of developing both low-grade (RR, 1.15) and high-grade (RR, 1.12) squamous intraepithelial lesions but no increased risk of cervical cancer. Women with Crohn’s disease showed a significantly increased risk of developing both low-grade (RR, 1.26) and high-grade (RR, 1.28) squamous intraepithelial lesions, as well as an even greater risk (RR, 1.53) of cervical cancer.
These associations were not the result of higher rates of cervical screening among the women with IBD than among the control subjects, since the two study groups had comparable screening rates. The associations also were not related to IBD therapy, since the use of mesalamine, azathioprine, and corticosteroids appeared to have no influence on rates of cervical neoplasia. The one exception to this finding was that women with Crohn’s disease who used TNF-alpha antagonists were at increased risk only for high-grade intraepithelial lesions of the cervix, the investigators said (Clin. Gastroenterol. Hepatol. 2014 July 30 [doi:10.1016/j.cgh.2014.07.036]).
An additional notable finding was that the odds of having a history of both high-grade intraepithelial and cancerous lesions of the cervix were markedly higher in the women with IBD than in the control subjects. “This is a novel finding that may suggest a yet unexplored common susceptibility to IBD and cervical neoplasia rather than an etiologic role of IBD … in the development of cervical neoplasia,” Dr. Rungoe and her associates wrote.
This study was funded in part by the Danish Council of Independent Research. The investigators reported having no relevant financial disclosures.
Women who have inflammatory bowel disease, particularly those with Crohn’s disease, are at increased risk for developing cervical neoplasia, according to a report published online in Clinical Gastroenterology and Hepatology.
In a population-based nationwide cohort study involving 27,408 Danish women with newly diagnosed IBD and 1,508,334 control subjects matched for age and area of residence, a significant excess in cervical neoplasia also was observed for up to 10 years before the GI diagnosis. This bidirectional association indicates that an as-yet-unknown factor underlies susceptibility to both disorders, said Dr. Christine Rungoe of the department of epidemiology research, Statens Serum Institut, Copenhagen, and her associates.
“Patients with IBD should be encouraged to follow the screening program for cervical neoplasia, and clinicians should be aware of the slightly increased risk of HPV-related cervical lesions in IBD patients,” they noted.
Some previous studies have shown an increased risk of cervical neoplasia among IBD patients, but others have found no association. Some experts have proposed that either the underlying immunologic changes in IBD or the treatment of IBD with immunosuppressive drugs may impair patients’ ability to clear HPV infections, thus raising the risk of cervical neoplasia.
Dr. Rungoe and her colleagues examined the issue using data from Danish national registries that covered approximately 4 million women residing there during the past 32 years. They identified 18,691 women newly diagnosed with ulcerative colitis and 8,717 newly diagnosed as having Crohn’s disease and compared their rates of cervical neoplasia against those of control subjects during a median of 8 years of follow-up.
Women with ulcerative colitis showed a slightly but significantly increased risk of developing both low-grade (RR, 1.15) and high-grade (RR, 1.12) squamous intraepithelial lesions but no increased risk of cervical cancer. Women with Crohn’s disease showed a significantly increased risk of developing both low-grade (RR, 1.26) and high-grade (RR, 1.28) squamous intraepithelial lesions, as well as an even greater risk (RR, 1.53) of cervical cancer.
These associations were not the result of higher rates of cervical screening among the women with IBD than among the control subjects, since the two study groups had comparable screening rates. The associations also were not related to IBD therapy, since the use of mesalamine, azathioprine, and corticosteroids appeared to have no influence on rates of cervical neoplasia. The one exception to this finding was that women with Crohn’s disease who used TNF-alpha antagonists were at increased risk only for high-grade intraepithelial lesions of the cervix, the investigators said (Clin. Gastroenterol. Hepatol. 2014 July 30 [doi:10.1016/j.cgh.2014.07.036]).
An additional notable finding was that the odds of having a history of both high-grade intraepithelial and cancerous lesions of the cervix were markedly higher in the women with IBD than in the control subjects. “This is a novel finding that may suggest a yet unexplored common susceptibility to IBD and cervical neoplasia rather than an etiologic role of IBD … in the development of cervical neoplasia,” Dr. Rungoe and her associates wrote.
This study was funded in part by the Danish Council of Independent Research. The investigators reported having no relevant financial disclosures.
Women who have inflammatory bowel disease, particularly those with Crohn’s disease, are at increased risk for developing cervical neoplasia, according to a report published online in Clinical Gastroenterology and Hepatology.
In a population-based nationwide cohort study involving 27,408 Danish women with newly diagnosed IBD and 1,508,334 control subjects matched for age and area of residence, a significant excess in cervical neoplasia also was observed for up to 10 years before the GI diagnosis. This bidirectional association indicates that an as-yet-unknown factor underlies susceptibility to both disorders, said Dr. Christine Rungoe of the department of epidemiology research, Statens Serum Institut, Copenhagen, and her associates.
“Patients with IBD should be encouraged to follow the screening program for cervical neoplasia, and clinicians should be aware of the slightly increased risk of HPV-related cervical lesions in IBD patients,” they noted.
Some previous studies have shown an increased risk of cervical neoplasia among IBD patients, but others have found no association. Some experts have proposed that either the underlying immunologic changes in IBD or the treatment of IBD with immunosuppressive drugs may impair patients’ ability to clear HPV infections, thus raising the risk of cervical neoplasia.
Dr. Rungoe and her colleagues examined the issue using data from Danish national registries that covered approximately 4 million women residing there during the past 32 years. They identified 18,691 women newly diagnosed with ulcerative colitis and 8,717 newly diagnosed as having Crohn’s disease and compared their rates of cervical neoplasia against those of control subjects during a median of 8 years of follow-up.
Women with ulcerative colitis showed a slightly but significantly increased risk of developing both low-grade (RR, 1.15) and high-grade (RR, 1.12) squamous intraepithelial lesions but no increased risk of cervical cancer. Women with Crohn’s disease showed a significantly increased risk of developing both low-grade (RR, 1.26) and high-grade (RR, 1.28) squamous intraepithelial lesions, as well as an even greater risk (RR, 1.53) of cervical cancer.
These associations were not the result of higher rates of cervical screening among the women with IBD than among the control subjects, since the two study groups had comparable screening rates. The associations also were not related to IBD therapy, since the use of mesalamine, azathioprine, and corticosteroids appeared to have no influence on rates of cervical neoplasia. The one exception to this finding was that women with Crohn’s disease who used TNF-alpha antagonists were at increased risk only for high-grade intraepithelial lesions of the cervix, the investigators said (Clin. Gastroenterol. Hepatol. 2014 July 30 [doi:10.1016/j.cgh.2014.07.036]).
An additional notable finding was that the odds of having a history of both high-grade intraepithelial and cancerous lesions of the cervix were markedly higher in the women with IBD than in the control subjects. “This is a novel finding that may suggest a yet unexplored common susceptibility to IBD and cervical neoplasia rather than an etiologic role of IBD … in the development of cervical neoplasia,” Dr. Rungoe and her associates wrote.
This study was funded in part by the Danish Council of Independent Research. The investigators reported having no relevant financial disclosures.
Key clinical point: Women with IBD, especially Crohn’s disease, are at increased risk for developing cervical neoplasia.
Major finding: Women with Crohn’s disease showed a significantly increased risk of developing low-grade (RR, 1.26) and high-grade (RR, 1.28) squamous intraepithelial lesions and cervical cancer (RR, 1.53), while those with ulcerative colitis showed a slightly increased risk of developing both low-grade (RR, 1.15) and high-grade (RR, 1.12) squamous intraepithelial lesions but no increased risk of cervical cancer.
Data source: A population-based Danish cohort study involving 18,691 women with ulcerative colitis, 8,717 with Crohn’s disease, and 1,508,334 control subjects matched for age and area of residence.
Disclosures: This study was funded in part by the Danish Council of Independent Research. Dr. Rungoe and her associates reported having no relevant financial disclosures.
Adding abiraterone improves suppression of intraprostate androgens
Adding abiraterone to standard neoadjuvant therapy using luteinizing hormone-releasing hormone agonists induces markedly more effective androgen suppression within the prostate tissue in men who have high-risk localized prostate cancer, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
Patients with localized high-risk prostate cancer show the highest recurrence rates after prostatectomy, with PSA relapse ranging from 40% to 65%. Abiraterone intensifies androgen deprivation therapy because it inhibits all sources of androgens: testicular, adrenal, prostate, and (presumably) tumor, said Dr. Mary-Ellen Taplin of the Dana-Farber Cancer Institute, Boston, and her associates.
In an open-label phase II clinical trial, Dr. Taplin and colleagues assessed 58 men who had localized prostate cancer plus PSA levels above 10 ng/ml, a PSA velocity greater than 2 ng/ml during the preceding year, and/or a Gleason score of 7 or higher. These participants were randomly assigned to receive 12 weeks of either LHRH agonists alone (28 men) or LHRH agonists plus abiraterone (30 men) in the randomized portion of the study. Then participants who had not received the abiraterone crossed over, and all the participants received another 12 weeks of abiraterone before undergoing prostatectomy (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2013.53.4578]).
In biopsies taken at 12 weeks, the addition of abiraterone reduced median levels of testosterone within the prostate by 37% and reduced median levels of dihydrotestosterone by 86%. Further study is needed to determine whether this laboratory response indicating reduced tumor burden translates into a clinical response of improved survival or quality of life, Dr. Taplin and her associates said.
The study by Taplin et al demonstrates that targeting androgen receptors in the preprostatectomy setting with abiraterone has a clear biologic effect, decreasing tissue levels of androgens. But this effect hasn’t yet been shown to translate into clinical benefit, and cannot be considered a standard of care.
One of the most striking findings is what this neoadjuvant therapy did not accomplish: It didn’t eradicate the cancer. Many patients showed residual cancer in specimens taken at subsequent prostatectomy: 54% had a pathologic stage of T3 or higher, 18% had positive lymph nodes, 14% had positive margins, and the pathologic complete response rate was only 7%.
Eric J. Small, M.D., is at the University of California, San Francisco. He has received research funding from Janssen Oncology and Dendreon and has served as a consultant to Kite Pharma. Dr. Small made these remarks in an editorial accompanying Dr. Taplin’s report (J. Clin. Oncol. 2014 [doi:10.1200/JCO.2014.57.8534]).
The study by Taplin et al demonstrates that targeting androgen receptors in the preprostatectomy setting with abiraterone has a clear biologic effect, decreasing tissue levels of androgens. But this effect hasn’t yet been shown to translate into clinical benefit, and cannot be considered a standard of care.
One of the most striking findings is what this neoadjuvant therapy did not accomplish: It didn’t eradicate the cancer. Many patients showed residual cancer in specimens taken at subsequent prostatectomy: 54% had a pathologic stage of T3 or higher, 18% had positive lymph nodes, 14% had positive margins, and the pathologic complete response rate was only 7%.
Eric J. Small, M.D., is at the University of California, San Francisco. He has received research funding from Janssen Oncology and Dendreon and has served as a consultant to Kite Pharma. Dr. Small made these remarks in an editorial accompanying Dr. Taplin’s report (J. Clin. Oncol. 2014 [doi:10.1200/JCO.2014.57.8534]).
The study by Taplin et al demonstrates that targeting androgen receptors in the preprostatectomy setting with abiraterone has a clear biologic effect, decreasing tissue levels of androgens. But this effect hasn’t yet been shown to translate into clinical benefit, and cannot be considered a standard of care.
One of the most striking findings is what this neoadjuvant therapy did not accomplish: It didn’t eradicate the cancer. Many patients showed residual cancer in specimens taken at subsequent prostatectomy: 54% had a pathologic stage of T3 or higher, 18% had positive lymph nodes, 14% had positive margins, and the pathologic complete response rate was only 7%.
Eric J. Small, M.D., is at the University of California, San Francisco. He has received research funding from Janssen Oncology and Dendreon and has served as a consultant to Kite Pharma. Dr. Small made these remarks in an editorial accompanying Dr. Taplin’s report (J. Clin. Oncol. 2014 [doi:10.1200/JCO.2014.57.8534]).
Adding abiraterone to standard neoadjuvant therapy using luteinizing hormone-releasing hormone agonists induces markedly more effective androgen suppression within the prostate tissue in men who have high-risk localized prostate cancer, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
Patients with localized high-risk prostate cancer show the highest recurrence rates after prostatectomy, with PSA relapse ranging from 40% to 65%. Abiraterone intensifies androgen deprivation therapy because it inhibits all sources of androgens: testicular, adrenal, prostate, and (presumably) tumor, said Dr. Mary-Ellen Taplin of the Dana-Farber Cancer Institute, Boston, and her associates.
In an open-label phase II clinical trial, Dr. Taplin and colleagues assessed 58 men who had localized prostate cancer plus PSA levels above 10 ng/ml, a PSA velocity greater than 2 ng/ml during the preceding year, and/or a Gleason score of 7 or higher. These participants were randomly assigned to receive 12 weeks of either LHRH agonists alone (28 men) or LHRH agonists plus abiraterone (30 men) in the randomized portion of the study. Then participants who had not received the abiraterone crossed over, and all the participants received another 12 weeks of abiraterone before undergoing prostatectomy (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2013.53.4578]).
In biopsies taken at 12 weeks, the addition of abiraterone reduced median levels of testosterone within the prostate by 37% and reduced median levels of dihydrotestosterone by 86%. Further study is needed to determine whether this laboratory response indicating reduced tumor burden translates into a clinical response of improved survival or quality of life, Dr. Taplin and her associates said.
Adding abiraterone to standard neoadjuvant therapy using luteinizing hormone-releasing hormone agonists induces markedly more effective androgen suppression within the prostate tissue in men who have high-risk localized prostate cancer, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
Patients with localized high-risk prostate cancer show the highest recurrence rates after prostatectomy, with PSA relapse ranging from 40% to 65%. Abiraterone intensifies androgen deprivation therapy because it inhibits all sources of androgens: testicular, adrenal, prostate, and (presumably) tumor, said Dr. Mary-Ellen Taplin of the Dana-Farber Cancer Institute, Boston, and her associates.
In an open-label phase II clinical trial, Dr. Taplin and colleagues assessed 58 men who had localized prostate cancer plus PSA levels above 10 ng/ml, a PSA velocity greater than 2 ng/ml during the preceding year, and/or a Gleason score of 7 or higher. These participants were randomly assigned to receive 12 weeks of either LHRH agonists alone (28 men) or LHRH agonists plus abiraterone (30 men) in the randomized portion of the study. Then participants who had not received the abiraterone crossed over, and all the participants received another 12 weeks of abiraterone before undergoing prostatectomy (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2013.53.4578]).
In biopsies taken at 12 weeks, the addition of abiraterone reduced median levels of testosterone within the prostate by 37% and reduced median levels of dihydrotestosterone by 86%. Further study is needed to determine whether this laboratory response indicating reduced tumor burden translates into a clinical response of improved survival or quality of life, Dr. Taplin and her associates said.
Key clinical point: Adding abiraterone to standard hormone therapy before prostatectomy suppresses androgens within the tumor tissue more effectively.
Major finding: Abiraterone cut median intraprostate levels of testosterone by 37% and of dihydrotestosterone by 86%.
Data source: A randomized phase II clinical trial assessing 12-24 weeks of neoadjuvant androgen suppression in 58 men who had high-risk prostate cancer.
Disclosures: This trial was supported by Ortho Biotech Oncology Research and Development (now Janssen R&D), a number of academic institutions, as well as several government agencies. Dr. Taplin reported serving as a consultant to and receiving research funding from Janssen Pharmaceuticals; her associates reported ties to numerous industry sources.
20-year follow-up supports adjuvant radiotherapy for DCIS
Adjuvant radiotherapy continues to protect against recurrences – albeit “modestly” – for a full 20 years after women undergo breast-conserving surgery for DCIS, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
In an extended follow-up study involving 1,046 of the 1,067 original participants in the Swedish Ductal Carcinoma in Situ (SweDCIS) clinical trial in 1987-1999, there were 258 recurrences: 129 cases of DCIS and 129 cases of invasive cancer in the ipsilateral breast. Another 115 women developed contralateral DCIS or invasive cancer. A total of 232 women died, including 41 who died from breast cancer, said Dr. Fredrik Warnberg of the department of surgical sciences, Uppsala (Sweden) Academic Hospital, and his associates.
There were 93 recurrences among women who had been randomized to adjuvant radiotherapy, compared with 165 recurrences in the control group treated with breast-conserving surgery only. This corresponds to an absolute risk reduction of 12% and a relative risk reduction of 37.5% for adjuvant radiotherapy. The absolute risk reduction was more pronounced for recurrent DCIS (10%) than for invasive cancer (2%), and it occurred mainly among women in the older age groups – those aged 52 years and older at diagnosis, the investigators said (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2014.56.2595]).
Contralateral breast cancer developed more often in the radiotherapy group (67 cases) than in the control group (48 cases), but this difference was not statistically significant, said Dr. Warnberg and his associates.
“The balance between protection against local recurrences and the downsides of radiotherapy currently speaks in favor of adjuvant breast irradiation. However, the more modest protective effect specifically for invasive recurrences and the possible increase in risk of contralateral cancer still call for the need to find groups of patients for whom radiotherapy could be avoided,” they noted.
One could reasonably ask whether these findings are still relevant today, given that so many aspects of patient evaluation and selection for breast-conserving therapy are very different now than they were when SweDCIS was begun in 1987-1999.
The results are relevant in that they demonstrate that, even though radiotherapy substantially reduced local failure rates, it didn’t change the risk of metastases or breast cancer death for DCIS patients as a whole. They are, however, no longer relevant in that radiotherapy’s ability to reduce the risk of local recurrence is much smaller for many, if not most, of today’s patients, compared with the trial participants.
Dr. Abram Recht is deputy chief of radiation oncology at Beth Israel Deaconess Medical Center and professor of radiation oncology at Harvard Medical School, Boston. He made these remarks in an editorial accompanying Dr. Warnberg’s report (J. Clin. Oncol. 2014 Oct. 13 [doi:10.1200/JCO.2014.58.1066]).
One could reasonably ask whether these findings are still relevant today, given that so many aspects of patient evaluation and selection for breast-conserving therapy are very different now than they were when SweDCIS was begun in 1987-1999.
The results are relevant in that they demonstrate that, even though radiotherapy substantially reduced local failure rates, it didn’t change the risk of metastases or breast cancer death for DCIS patients as a whole. They are, however, no longer relevant in that radiotherapy’s ability to reduce the risk of local recurrence is much smaller for many, if not most, of today’s patients, compared with the trial participants.
Dr. Abram Recht is deputy chief of radiation oncology at Beth Israel Deaconess Medical Center and professor of radiation oncology at Harvard Medical School, Boston. He made these remarks in an editorial accompanying Dr. Warnberg’s report (J. Clin. Oncol. 2014 Oct. 13 [doi:10.1200/JCO.2014.58.1066]).
One could reasonably ask whether these findings are still relevant today, given that so many aspects of patient evaluation and selection for breast-conserving therapy are very different now than they were when SweDCIS was begun in 1987-1999.
The results are relevant in that they demonstrate that, even though radiotherapy substantially reduced local failure rates, it didn’t change the risk of metastases or breast cancer death for DCIS patients as a whole. They are, however, no longer relevant in that radiotherapy’s ability to reduce the risk of local recurrence is much smaller for many, if not most, of today’s patients, compared with the trial participants.
Dr. Abram Recht is deputy chief of radiation oncology at Beth Israel Deaconess Medical Center and professor of radiation oncology at Harvard Medical School, Boston. He made these remarks in an editorial accompanying Dr. Warnberg’s report (J. Clin. Oncol. 2014 Oct. 13 [doi:10.1200/JCO.2014.58.1066]).
Adjuvant radiotherapy continues to protect against recurrences – albeit “modestly” – for a full 20 years after women undergo breast-conserving surgery for DCIS, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
In an extended follow-up study involving 1,046 of the 1,067 original participants in the Swedish Ductal Carcinoma in Situ (SweDCIS) clinical trial in 1987-1999, there were 258 recurrences: 129 cases of DCIS and 129 cases of invasive cancer in the ipsilateral breast. Another 115 women developed contralateral DCIS or invasive cancer. A total of 232 women died, including 41 who died from breast cancer, said Dr. Fredrik Warnberg of the department of surgical sciences, Uppsala (Sweden) Academic Hospital, and his associates.
There were 93 recurrences among women who had been randomized to adjuvant radiotherapy, compared with 165 recurrences in the control group treated with breast-conserving surgery only. This corresponds to an absolute risk reduction of 12% and a relative risk reduction of 37.5% for adjuvant radiotherapy. The absolute risk reduction was more pronounced for recurrent DCIS (10%) than for invasive cancer (2%), and it occurred mainly among women in the older age groups – those aged 52 years and older at diagnosis, the investigators said (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2014.56.2595]).
Contralateral breast cancer developed more often in the radiotherapy group (67 cases) than in the control group (48 cases), but this difference was not statistically significant, said Dr. Warnberg and his associates.
“The balance between protection against local recurrences and the downsides of radiotherapy currently speaks in favor of adjuvant breast irradiation. However, the more modest protective effect specifically for invasive recurrences and the possible increase in risk of contralateral cancer still call for the need to find groups of patients for whom radiotherapy could be avoided,” they noted.
Adjuvant radiotherapy continues to protect against recurrences – albeit “modestly” – for a full 20 years after women undergo breast-conserving surgery for DCIS, according to a report published online Oct. 13 in the Journal of Clinical Oncology.
In an extended follow-up study involving 1,046 of the 1,067 original participants in the Swedish Ductal Carcinoma in Situ (SweDCIS) clinical trial in 1987-1999, there were 258 recurrences: 129 cases of DCIS and 129 cases of invasive cancer in the ipsilateral breast. Another 115 women developed contralateral DCIS or invasive cancer. A total of 232 women died, including 41 who died from breast cancer, said Dr. Fredrik Warnberg of the department of surgical sciences, Uppsala (Sweden) Academic Hospital, and his associates.
There were 93 recurrences among women who had been randomized to adjuvant radiotherapy, compared with 165 recurrences in the control group treated with breast-conserving surgery only. This corresponds to an absolute risk reduction of 12% and a relative risk reduction of 37.5% for adjuvant radiotherapy. The absolute risk reduction was more pronounced for recurrent DCIS (10%) than for invasive cancer (2%), and it occurred mainly among women in the older age groups – those aged 52 years and older at diagnosis, the investigators said (J. Clin. Oncol. 2014 October 13 [doi:10.1200/JCO.2014.56.2595]).
Contralateral breast cancer developed more often in the radiotherapy group (67 cases) than in the control group (48 cases), but this difference was not statistically significant, said Dr. Warnberg and his associates.
“The balance between protection against local recurrences and the downsides of radiotherapy currently speaks in favor of adjuvant breast irradiation. However, the more modest protective effect specifically for invasive recurrences and the possible increase in risk of contralateral cancer still call for the need to find groups of patients for whom radiotherapy could be avoided,” they noted.
Key clinical point: 20-year follow-up data from the SweDCIS trial show that adjuvant radiotherapy continues to protect “modestly” against invasive recurrences.
Major finding: Adjuvant radiotherapy conferred an absolute risk reduction of 12% and a relative risk reduction of 37.5% against recurrent DCIS and invasive breast cancer at 20 years.
Data source: Extended follow-up of 1,046 participants in the SweDCIS randomized prospective clinical trial assessing the usefulness of adjuvant radiotherapy after breast-conserving surgery for DCIS.
Disclosures: The SweDCIS trial was supported by the Swedish Breast Cancer Association. Dr. Warnberg reported receiving research funding from Prelude.
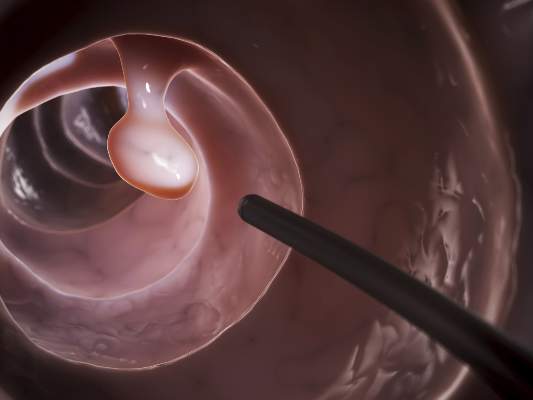
Efficacy, Not Tolerability, of Bowel Prep Is Primary
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
FROM GASTROENTEROLOGY
Efficacy, not tolerability, of bowel prep is primary
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
The efficacy, not the tolerability, of bowel cleansing is the primary concern in patients undergoing colonoscopy, because of the substantial adverse consequences of inadequate bowel preparation, according to a consensus report published simultaneously in Gastroenterology, the American Journal of Gastroenterology, and Gastrointestinal Endoscopy.
As many as 20%-25% of all colonoscopies reportedly have inadequate bowel cleansing, which is associated with lower rates of lesion detection, longer procedure times, and increased electrocautery risks, in addition to the excess costs and risks of repeat procedures. “Efficacy should be a higher priority than tolerability, [so] the choice of a bowel-cleansing regimen should be based on cleansing efficacy first and patient tolerability second,” said Dr. David A. Johnson of Eastern Veterans Administration Medical School, Norfolk (Va.) and his associates on the U.S. Multi-Society Task Force on Colorectal Cancer.
The USMSTF comprised representatives from the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, who compiled recommendations and wrote this report based on a systematic review and meta-analysis of the literature concerning bowel preparation from 1980 to the present.
When selecting a bowel-cleansing regimen for patients, physicians should consider the patient’s medical history, medications, and, if applicable, the adequacy of bowel preparation for previous colonoscopies. High-quality evidence shows that a split-dose regimen of 4 liters of polyethylene glycol–electrolyte lavage solution (PEG-ELS) provides superior bowel cleansing to other doses and solutions. The second dose ideally should begin 4-6 hours and conclude at least 2 hours before the procedure time. However, same-day regimens are acceptable, especially for patients scheduled for an afternoon colonoscopy.
Using a split-dose regimen allows some flexibility with the diet on the day before the procedure. Instead of ingesting only clear liquids, patients can consume either a full liquid or a low-residue diet for part or all of the day preceding colonoscopy, which improves their tolerance of the bowel preparation.
Over-the-counter, nonapproved (by the FDA) bowel-cleansing agents have widely varying efficacy, ranging from adequate to excellent. They generally are safe, but “caution is required when using these agents in certain populations” such as patients with chronic kidney disease. The routine use of adjunctive agents intended to enhance purgation or visualization of the mucosa is not recommended, Dr. Johnson and his associates said (Gastroenterology 2014;147:903-24).
At present, the evidence is insufficient to allow recommendation of specific bowel-preparation regimens for special patient populations such as the elderly, children and adolescents, people with inflammatory bowel disease, patients who have undergone bariatric surgery, and people with spinal cord injury. However, sodium phosphate preparations should be avoided in the elderly, children, and people with IBD. Additional bowel purgatives can be considered for patients at risk for inadequate preparation, such as those with a history of constipation, those using opioids or other constipating medications, those who have undergone previous colon resection, and those with spinal cord injury.
The report also includes recommendations concerning patient education, the risk factors for inadequate bowel preparation, assessing the adequacy of bowel preparation before and during the procedure, and salvage options for cases in which preparation is inadequate. It is available at http://dx.doi.org/10.1053/j.gastro.2014.07.002.
FROM GASTROENTEROLOGY
Key clinical point: Efficacy, not tolerability, of bowel preparation is the primary concern before colonoscopy.
Major finding: Up to 20%-25% of all colonoscopies have inadequate bowel preparation, which lowers detection rates, lengthens procedure time, raises electrocautery risks, and raises overall costs and risks by requiring repeat procedures.
Data source: A consensus statement based on a systematic review and meta-analysis of the literature concerning bowel preparation for colonoscopy.
Disclosures: This report was supported by the American College of Gastroenterology, the American Gastroenterological Association, and the American Society for Gastrointestinal Endoscopy, with further support provided by the U.S. Veterans Health Administration. Dr. Johnson reported serving as a consultant and clinical investigator for Epigenomics, Given Imaging, and Exact Sciences; his associates reported numerous ties to industry sources.
Myocardial geometry, function altered in obese children
Obese children and adolescents show significant adverse alterations in myocardial geometry and function on echocardiography, compared with normal-weight children, according to a report published online Oct. 8 in JACC: Cardiovascular Imaging.
In a prospective, cross-sectional cohort study in Germany, researchers performed two-dimensional echocardiography in 61 obese and 40 nonobese participants aged 8-21 years. All the children were white, free of known disease, and not taking any medications, said Dr. Norman Mangner of the University of Leipzig and Heart Center Leipzig and his associates.
Global ejection fraction was normal in both study groups. However, the obese participants showed thickened LV walls and larger chamber dimensions; as a result, their calculated LV mass and LV mass index were roughly 40% higher than those of nonobese children. Left atrial volume, LA volume index, right atrial area, and right ventricular diameter all were also increased, compared with those of nonobese children. In addition, z scores for LA diameter were above the 95th percentile in a significantly higher percentage of the obese participants (33.3%) than nonobese participants (10%), the investigators said (J. Am. Coll. Cardiol. Img. 2014 Oct. 8 [doi: 10.1016/j.jcmg.2014.08.006]).
Regarding myocardial function, both tissue Doppler-derived peak systolic velocity and deformation in the basoseptal region were reduced in the obese children. Average longitudinal LV strain, strain rate, and displacement – all measures of longitudinal function – were significantly reduced. Average LV circumferential strain was significantly blunted in the obese participants, but the average LV circumferential strain rate and radial function were not significantly different between the two study groups. And diastolic function was decreased in the obese children, as evidenced by their reduced mitral E- to mitral A-wave peak velocity (E/A ratio), reduced mitral annulus tissue Doppler imaging (TDI) peak E-wave velocity, and increased E/E’ ratios.
“It is important to note that [these changes] do not necessarily translate into clinically relevant functional impairment,” Dr. Mangner and his associates said.
A longitudinal study is needed to clarify the clinical significance of these alterations and to explore whether weight loss will reverse them, they added.
This study was supported in part by grants from the German Research Foundation and the Clinical Research Group. One of Dr. Mangner’s associates reported ties to Medtronic, St. Jude Medical, Claret Medical, Boston Scientific, and Edwards; Dr. Mangner and his other associates reported having no financial disclosures.
Obese children and adolescents show significant adverse alterations in myocardial geometry and function on echocardiography, compared with normal-weight children, according to a report published online Oct. 8 in JACC: Cardiovascular Imaging.
In a prospective, cross-sectional cohort study in Germany, researchers performed two-dimensional echocardiography in 61 obese and 40 nonobese participants aged 8-21 years. All the children were white, free of known disease, and not taking any medications, said Dr. Norman Mangner of the University of Leipzig and Heart Center Leipzig and his associates.
Global ejection fraction was normal in both study groups. However, the obese participants showed thickened LV walls and larger chamber dimensions; as a result, their calculated LV mass and LV mass index were roughly 40% higher than those of nonobese children. Left atrial volume, LA volume index, right atrial area, and right ventricular diameter all were also increased, compared with those of nonobese children. In addition, z scores for LA diameter were above the 95th percentile in a significantly higher percentage of the obese participants (33.3%) than nonobese participants (10%), the investigators said (J. Am. Coll. Cardiol. Img. 2014 Oct. 8 [doi: 10.1016/j.jcmg.2014.08.006]).
Regarding myocardial function, both tissue Doppler-derived peak systolic velocity and deformation in the basoseptal region were reduced in the obese children. Average longitudinal LV strain, strain rate, and displacement – all measures of longitudinal function – were significantly reduced. Average LV circumferential strain was significantly blunted in the obese participants, but the average LV circumferential strain rate and radial function were not significantly different between the two study groups. And diastolic function was decreased in the obese children, as evidenced by their reduced mitral E- to mitral A-wave peak velocity (E/A ratio), reduced mitral annulus tissue Doppler imaging (TDI) peak E-wave velocity, and increased E/E’ ratios.
“It is important to note that [these changes] do not necessarily translate into clinically relevant functional impairment,” Dr. Mangner and his associates said.
A longitudinal study is needed to clarify the clinical significance of these alterations and to explore whether weight loss will reverse them, they added.
This study was supported in part by grants from the German Research Foundation and the Clinical Research Group. One of Dr. Mangner’s associates reported ties to Medtronic, St. Jude Medical, Claret Medical, Boston Scientific, and Edwards; Dr. Mangner and his other associates reported having no financial disclosures.
Obese children and adolescents show significant adverse alterations in myocardial geometry and function on echocardiography, compared with normal-weight children, according to a report published online Oct. 8 in JACC: Cardiovascular Imaging.
In a prospective, cross-sectional cohort study in Germany, researchers performed two-dimensional echocardiography in 61 obese and 40 nonobese participants aged 8-21 years. All the children were white, free of known disease, and not taking any medications, said Dr. Norman Mangner of the University of Leipzig and Heart Center Leipzig and his associates.
Global ejection fraction was normal in both study groups. However, the obese participants showed thickened LV walls and larger chamber dimensions; as a result, their calculated LV mass and LV mass index were roughly 40% higher than those of nonobese children. Left atrial volume, LA volume index, right atrial area, and right ventricular diameter all were also increased, compared with those of nonobese children. In addition, z scores for LA diameter were above the 95th percentile in a significantly higher percentage of the obese participants (33.3%) than nonobese participants (10%), the investigators said (J. Am. Coll. Cardiol. Img. 2014 Oct. 8 [doi: 10.1016/j.jcmg.2014.08.006]).
Regarding myocardial function, both tissue Doppler-derived peak systolic velocity and deformation in the basoseptal region were reduced in the obese children. Average longitudinal LV strain, strain rate, and displacement – all measures of longitudinal function – were significantly reduced. Average LV circumferential strain was significantly blunted in the obese participants, but the average LV circumferential strain rate and radial function were not significantly different between the two study groups. And diastolic function was decreased in the obese children, as evidenced by their reduced mitral E- to mitral A-wave peak velocity (E/A ratio), reduced mitral annulus tissue Doppler imaging (TDI) peak E-wave velocity, and increased E/E’ ratios.
“It is important to note that [these changes] do not necessarily translate into clinically relevant functional impairment,” Dr. Mangner and his associates said.
A longitudinal study is needed to clarify the clinical significance of these alterations and to explore whether weight loss will reverse them, they added.
This study was supported in part by grants from the German Research Foundation and the Clinical Research Group. One of Dr. Mangner’s associates reported ties to Medtronic, St. Jude Medical, Claret Medical, Boston Scientific, and Edwards; Dr. Mangner and his other associates reported having no financial disclosures.
Key clinical point: Obese children and adolescents have increased left ventricular mass, decreased LV diastolic function, and other adverse myocardial changes.
Major finding: Obese children and adolescents showed thickened LV walls and larger chamber dimensions, so their calculated LV mass and LV mass index were about 40% higher than those of nonobese children.
Data source: A prospective cross-sectional cohort study involving 61 obese and 40 nonobese participants aged 8-21 years who underwent cardiac echocardiography.
Disclosures: This study was supported in part by grants from the German Research Foundation and the Clinical Research Group. One of Dr. Mangner’s associates reported ties to Medtronic, St. Jude Medical, Claret Medical, Boston Scientific, and Edwards; Dr. Mangner and his other associates reported having no financial disclosures.
Duodenoscopes transmitted resistant E. coli to 39 patients
Contaminated duodenoscopes triggered a 2013 outbreak of a rare strain of Escherichia coli infections at a single hospital in northeastern Illinois, according to a report published online Oct. 7 in JAMA.
Unlike previous clusters of bacterial transmission related to these devices, this one could not be attributed to lapses in infection-control practices, defects in the duodenoscopes, or device malfunction. Instead, the carbapenem-resistant bacteria in this outbreak — NDM-producing (New Delhi metallo-beta-lactamase) E. coli — were recovered from the enclosed elevator channel of side-viewing duodenoscopes that had been cleaned, maintained, and used according to manufacturer’s instructions. “It appears that these devices have the potential to remain contaminated with pathogenic bacteria even after the recommended reprocessing is performed,” said Dr. Lauren Epstein of the division of healthcare quality promotion, Centers for Disease Control and Prevention, and her associates.
“Facilities should be aware of the potential for transmission of antimicrobial-resistant organisms via this route and should conduct regular reviews of their duodenoscope reprocessing procedures to ensure optimal manual cleaning and disinfection,” they noted.
The outbreak was discovered by a laboratory that routinely screens carbapenem-resistant Enterobacteriaceae isolates for NDM production and notifies the CDC of positive results, to ensure quick control of these dangerous organisms. The first case was identified from results of a urine culture, and six more patients who had been treated at the same hospital during a 4-month period also were found to have positive clinical cultures. Screening cultures were then performed on these patients’ roommates and on ward mates of the index case, turning up another two cases of carbapenem-resistant NDM-producing E. coli infection.
A review of the medical records showed that six of these patients had undergone endoscopic retrograde cholangiopancreatography (ERCP) at this hospital. The facility then notified all 226 patients who had undergone a procedure with any of the hospital’s three duodenoscopes during the preceding 9 months of their potential exposure, and offered them testing and treatment. A total of 27 additional cases were found, plus 1 additional case in the roommate of a patient who had been transferred to a long-term care facility.
In all, 35 of the 39 cases in this outbreak had been exposed to the hospital’s duodenoscopes.
A case-control analysis involving nine case patients and 27 control subjects who had been hospitalized during the study period showed that the infection was indeed strongly related to ERCP. Environmental cultures were collected from numerous areas of the hospital, and the only positive results were obtained from the elevator channels of two duodenoscopes. But no breaches were found in the reprocessing of the equipment.
Nevertheless, the hospital switched from automated high-level disinfection of the devices using an enzymatic cleaner to gas sterilization using ethylene oxide. Subsequent culturing showed no further contamination of the equipment, and no further cases of infection have occurred, the investigators said (JAMA 2014 [doi:10.1001/jama.2014.12720]).
Endoscopic facilities typically use high-level disinfection, as opposed to sterilization, for duodenoscopes. Although the switch to sterilization at this hospital may have been instrumental in containing the outbreak, the evidence from this single incident is not compelling enough to recommend that all such facilities make this change. Moreover, sterilization’s long processing and aeration time, the toxicity of some sterilizing agents to staff and patients, and its incompatibility with some devices make widespread adoption unlikely, Dr. Epstein and her associates said.
Unlike other endoscopes, duodenoscopes have an elevator channel “that allows for the use and manipulation of a guide wire. At the terminal end of the elevator channel is a mechanical piece containing a cantilevered elevator mechanism; the intricate design surrounding the elevator mechanism makes accessing all surfaces during manual cleaning difficult,” they noted.
The key concern raised by this outbreak is whether current endoscope reprocessing guidelines are adequate to ensure patient safety, or whether endoscopes with their long, narrow channels, right-angle turns, difficult to clean components, and heavy microbial contamination are impossible to reliably disinfect.
The ethylene oxide sterilization used to terminate this outbreak is not a satisfactory long-term solution. The FDA hasn’t cleared this technique for sterilizing endoscopes, many hospitals no longer carry ethylene oxide, and the 12- to 15-hour processing time is unwieldy.
William A. Rutala, Ph.D., and Dr. David J. Weber are in the department of hospital epidemiology at University of North Carolina Health Care and the division of infectious diseases at the University of North Carolina, Chapel Hill. Dr. Rutala reported ties to Clorox, Advanced Sterilization Products, and 3M, and Dr. Weber reported ties to Clorox, Merck, and Pfizer. These remarks were taken from their editorial accompanying Dr. Epstein’s report (JAMA 2014;312:1405-6).
The key concern raised by this outbreak is whether current endoscope reprocessing guidelines are adequate to ensure patient safety, or whether endoscopes with their long, narrow channels, right-angle turns, difficult to clean components, and heavy microbial contamination are impossible to reliably disinfect.
The ethylene oxide sterilization used to terminate this outbreak is not a satisfactory long-term solution. The FDA hasn’t cleared this technique for sterilizing endoscopes, many hospitals no longer carry ethylene oxide, and the 12- to 15-hour processing time is unwieldy.
William A. Rutala, Ph.D., and Dr. David J. Weber are in the department of hospital epidemiology at University of North Carolina Health Care and the division of infectious diseases at the University of North Carolina, Chapel Hill. Dr. Rutala reported ties to Clorox, Advanced Sterilization Products, and 3M, and Dr. Weber reported ties to Clorox, Merck, and Pfizer. These remarks were taken from their editorial accompanying Dr. Epstein’s report (JAMA 2014;312:1405-6).
The key concern raised by this outbreak is whether current endoscope reprocessing guidelines are adequate to ensure patient safety, or whether endoscopes with their long, narrow channels, right-angle turns, difficult to clean components, and heavy microbial contamination are impossible to reliably disinfect.
The ethylene oxide sterilization used to terminate this outbreak is not a satisfactory long-term solution. The FDA hasn’t cleared this technique for sterilizing endoscopes, many hospitals no longer carry ethylene oxide, and the 12- to 15-hour processing time is unwieldy.
William A. Rutala, Ph.D., and Dr. David J. Weber are in the department of hospital epidemiology at University of North Carolina Health Care and the division of infectious diseases at the University of North Carolina, Chapel Hill. Dr. Rutala reported ties to Clorox, Advanced Sterilization Products, and 3M, and Dr. Weber reported ties to Clorox, Merck, and Pfizer. These remarks were taken from their editorial accompanying Dr. Epstein’s report (JAMA 2014;312:1405-6).
Contaminated duodenoscopes triggered a 2013 outbreak of a rare strain of Escherichia coli infections at a single hospital in northeastern Illinois, according to a report published online Oct. 7 in JAMA.
Unlike previous clusters of bacterial transmission related to these devices, this one could not be attributed to lapses in infection-control practices, defects in the duodenoscopes, or device malfunction. Instead, the carbapenem-resistant bacteria in this outbreak — NDM-producing (New Delhi metallo-beta-lactamase) E. coli — were recovered from the enclosed elevator channel of side-viewing duodenoscopes that had been cleaned, maintained, and used according to manufacturer’s instructions. “It appears that these devices have the potential to remain contaminated with pathogenic bacteria even after the recommended reprocessing is performed,” said Dr. Lauren Epstein of the division of healthcare quality promotion, Centers for Disease Control and Prevention, and her associates.
“Facilities should be aware of the potential for transmission of antimicrobial-resistant organisms via this route and should conduct regular reviews of their duodenoscope reprocessing procedures to ensure optimal manual cleaning and disinfection,” they noted.
The outbreak was discovered by a laboratory that routinely screens carbapenem-resistant Enterobacteriaceae isolates for NDM production and notifies the CDC of positive results, to ensure quick control of these dangerous organisms. The first case was identified from results of a urine culture, and six more patients who had been treated at the same hospital during a 4-month period also were found to have positive clinical cultures. Screening cultures were then performed on these patients’ roommates and on ward mates of the index case, turning up another two cases of carbapenem-resistant NDM-producing E. coli infection.
A review of the medical records showed that six of these patients had undergone endoscopic retrograde cholangiopancreatography (ERCP) at this hospital. The facility then notified all 226 patients who had undergone a procedure with any of the hospital’s three duodenoscopes during the preceding 9 months of their potential exposure, and offered them testing and treatment. A total of 27 additional cases were found, plus 1 additional case in the roommate of a patient who had been transferred to a long-term care facility.
In all, 35 of the 39 cases in this outbreak had been exposed to the hospital’s duodenoscopes.
A case-control analysis involving nine case patients and 27 control subjects who had been hospitalized during the study period showed that the infection was indeed strongly related to ERCP. Environmental cultures were collected from numerous areas of the hospital, and the only positive results were obtained from the elevator channels of two duodenoscopes. But no breaches were found in the reprocessing of the equipment.
Nevertheless, the hospital switched from automated high-level disinfection of the devices using an enzymatic cleaner to gas sterilization using ethylene oxide. Subsequent culturing showed no further contamination of the equipment, and no further cases of infection have occurred, the investigators said (JAMA 2014 [doi:10.1001/jama.2014.12720]).
Endoscopic facilities typically use high-level disinfection, as opposed to sterilization, for duodenoscopes. Although the switch to sterilization at this hospital may have been instrumental in containing the outbreak, the evidence from this single incident is not compelling enough to recommend that all such facilities make this change. Moreover, sterilization’s long processing and aeration time, the toxicity of some sterilizing agents to staff and patients, and its incompatibility with some devices make widespread adoption unlikely, Dr. Epstein and her associates said.
Unlike other endoscopes, duodenoscopes have an elevator channel “that allows for the use and manipulation of a guide wire. At the terminal end of the elevator channel is a mechanical piece containing a cantilevered elevator mechanism; the intricate design surrounding the elevator mechanism makes accessing all surfaces during manual cleaning difficult,” they noted.
Contaminated duodenoscopes triggered a 2013 outbreak of a rare strain of Escherichia coli infections at a single hospital in northeastern Illinois, according to a report published online Oct. 7 in JAMA.
Unlike previous clusters of bacterial transmission related to these devices, this one could not be attributed to lapses in infection-control practices, defects in the duodenoscopes, or device malfunction. Instead, the carbapenem-resistant bacteria in this outbreak — NDM-producing (New Delhi metallo-beta-lactamase) E. coli — were recovered from the enclosed elevator channel of side-viewing duodenoscopes that had been cleaned, maintained, and used according to manufacturer’s instructions. “It appears that these devices have the potential to remain contaminated with pathogenic bacteria even after the recommended reprocessing is performed,” said Dr. Lauren Epstein of the division of healthcare quality promotion, Centers for Disease Control and Prevention, and her associates.
“Facilities should be aware of the potential for transmission of antimicrobial-resistant organisms via this route and should conduct regular reviews of their duodenoscope reprocessing procedures to ensure optimal manual cleaning and disinfection,” they noted.
The outbreak was discovered by a laboratory that routinely screens carbapenem-resistant Enterobacteriaceae isolates for NDM production and notifies the CDC of positive results, to ensure quick control of these dangerous organisms. The first case was identified from results of a urine culture, and six more patients who had been treated at the same hospital during a 4-month period also were found to have positive clinical cultures. Screening cultures were then performed on these patients’ roommates and on ward mates of the index case, turning up another two cases of carbapenem-resistant NDM-producing E. coli infection.
A review of the medical records showed that six of these patients had undergone endoscopic retrograde cholangiopancreatography (ERCP) at this hospital. The facility then notified all 226 patients who had undergone a procedure with any of the hospital’s three duodenoscopes during the preceding 9 months of their potential exposure, and offered them testing and treatment. A total of 27 additional cases were found, plus 1 additional case in the roommate of a patient who had been transferred to a long-term care facility.
In all, 35 of the 39 cases in this outbreak had been exposed to the hospital’s duodenoscopes.
A case-control analysis involving nine case patients and 27 control subjects who had been hospitalized during the study period showed that the infection was indeed strongly related to ERCP. Environmental cultures were collected from numerous areas of the hospital, and the only positive results were obtained from the elevator channels of two duodenoscopes. But no breaches were found in the reprocessing of the equipment.
Nevertheless, the hospital switched from automated high-level disinfection of the devices using an enzymatic cleaner to gas sterilization using ethylene oxide. Subsequent culturing showed no further contamination of the equipment, and no further cases of infection have occurred, the investigators said (JAMA 2014 [doi:10.1001/jama.2014.12720]).
Endoscopic facilities typically use high-level disinfection, as opposed to sterilization, for duodenoscopes. Although the switch to sterilization at this hospital may have been instrumental in containing the outbreak, the evidence from this single incident is not compelling enough to recommend that all such facilities make this change. Moreover, sterilization’s long processing and aeration time, the toxicity of some sterilizing agents to staff and patients, and its incompatibility with some devices make widespread adoption unlikely, Dr. Epstein and her associates said.
Unlike other endoscopes, duodenoscopes have an elevator channel “that allows for the use and manipulation of a guide wire. At the terminal end of the elevator channel is a mechanical piece containing a cantilevered elevator mechanism; the intricate design surrounding the elevator mechanism makes accessing all surfaces during manual cleaning difficult,” they noted.
Key clinical point: 39 cases of NDM-producing carbapenem-resistant E. coli were linked to contaminated duodenoscopes at one hospital.
Major finding: 35 of the 39 cases in this outbreak had been exposed to the hospital’s duodenoscopes.
Data source: An epidemiologic investigation into a cluster of rare E. coli infections at a hospital in Illinois, which included a case-control study involving 35 patients.
Disclosures: This study was supported by the Centers for Disease Control and Prevention. Dr. Epstein and associates reported having no financial disclosures.
Authors’ industry ties may bias neuraminidase inhibitors reviews
In systematic reviews of research on neuraminidase inhibitors for either prevention or treatment of influenza, authors who had ties to drug manufacturers were much more likely to present the evidence as favorable and to recommend the drugs than were authors who had no such conflicts of interest, according to a report published online Oct. 6 in Annals of Internal Medicine.
“Reviewers with such conflicts of interest were also less likely to address issues with the underlying primary clinical evidence, such as publication bias and the lack of access to comprehensive study data,” said Adam G. Dunn, Ph.D., of the Centre for Health Informatics, University of New South Wales, Sydney, and his associates (Ann. Intern. Med. 2014 Oct.6 [doi:10.7326/M14-0933]).
The findings suggest that “further measures may be necessary to ensure that industry collaborations do not compromise the scientific evidence,” the investigators noted.
“If the benefits of neuraminidase inhibitors are eventually found to have been inflated, millions of patients will have been unnecessarily exposed to drugs that may be of little or no benefit,” the researchers added.
Dr. Dunn and his colleagues observed that reviews of neuraminidase inhibitors had widely divergent conclusions, with some strongly endorsing the agents as influenza prophylaxis or treatment, while others questioned the drugs’ safety and efficacy.
To examine whether industry influence may have contributed to these inconsistent conclusions, the investigators analyzed 26 systematic reviews. Thirteen assessed prophylaxis, and 24 assessed treatment; so, there were 37 distinct assessments. Seven of those were Cochrane analyses.
Two of the investigators read redacted copies of the reviews and rated them as favorable or unfavorable toward the use of neuraminidase inhibitors. Those copies showed no identifying information for the authors and no journal name or formatting clues. The agreement between the two investigators was “strong,” at 86%.
At the same time, Dr. Dunn and his associates searched for all the financial conflicts of interest for all the authors of these reviews, paying special attention to ties to the pharmaceutical companies that made the neuraminidase inhibitors under review. To do so, they went beyond the affiliations and funding listed in the systematic reviews, examining the authors’ personal and institutional websites, as well as disclosure lists provided by GlaxoSmithKline and Roche. They also performed Web searches combining the authors’ names, drug names, and manufacturers’ names.
Reviewers who had ties to the pharmaceutical industry rated neuraminidase inhibitors favorably in 88% (seven of eight) systematic reviews. In contrast, reviewers who had no such ties rated the drugs favorably in only 17% (5 of 29) of systematic reviews. That pattern persisted when prophylaxis and treatment were assessed separately.
Regarding prophylaxis, 100% (2 of 2) of the reviews with financial conflicts of interest were favorable, compared with only 9% (1 of 11) of those without conflicts of interest. Regarding treatment, 83% (5 of 6) of the reviews with financial conflicts of interest were favorable, compared with only 22% (4 of 18) of those without conflicts of interest.
In addition, reviewers who had ties to the pharmaceutical industry were less likely to include information about publication bias in their reports (14%, or only 1 of 7), compared with reviewers who had no such ties (79%, or 15 of 19), Dr. Dunn and his associates said.
The study findings indicate that financial conflicts of interest “are associated with product assessments favorable to the sponsors involved,” the authors asserted.
Factors that may contribute to biased conclusions include “the design of the review, the patient populations and outcomes assessed, the selective inclusion of primary evidence, [and] the critical appraisal of evidence quality and provenance,” the investigators noted. In addition, “the tone, emphasis, and interpretation provided by the authors may also influence the message that is conveyed,” they said.
The Australian National Health and Medical Research Council funded the study. Dr. Dunn and his associates reported having no financial conflicts of interest.
Data and opinions are always colored by a variety of external factors, including financial relationships. Specialists who care for a specific condition are also at risk of wanting to believe a new therapy will make a difference in all patients and not parsing the data for limitations.
Good stewardship and astute clinical care require understanding what is effective. Unfortunately, published data usually reflect efficacy (the drug works in highly specified conditions) and not effectiveness (its impact when in generalized use). As a Medicaid medical director, I am engaged in a continuous search for effectiveness assessments and have to sift through a lot of assessments of uncertain validity.
Dr. William Golden is professor of medicine and public health at University of Arkansas, Little Rock, and medical director of Arkansas DHS/Medicaid.
Data and opinions are always colored by a variety of external factors, including financial relationships. Specialists who care for a specific condition are also at risk of wanting to believe a new therapy will make a difference in all patients and not parsing the data for limitations.
Good stewardship and astute clinical care require understanding what is effective. Unfortunately, published data usually reflect efficacy (the drug works in highly specified conditions) and not effectiveness (its impact when in generalized use). As a Medicaid medical director, I am engaged in a continuous search for effectiveness assessments and have to sift through a lot of assessments of uncertain validity.
Dr. William Golden is professor of medicine and public health at University of Arkansas, Little Rock, and medical director of Arkansas DHS/Medicaid.
Data and opinions are always colored by a variety of external factors, including financial relationships. Specialists who care for a specific condition are also at risk of wanting to believe a new therapy will make a difference in all patients and not parsing the data for limitations.
Good stewardship and astute clinical care require understanding what is effective. Unfortunately, published data usually reflect efficacy (the drug works in highly specified conditions) and not effectiveness (its impact when in generalized use). As a Medicaid medical director, I am engaged in a continuous search for effectiveness assessments and have to sift through a lot of assessments of uncertain validity.
Dr. William Golden is professor of medicine and public health at University of Arkansas, Little Rock, and medical director of Arkansas DHS/Medicaid.
In systematic reviews of research on neuraminidase inhibitors for either prevention or treatment of influenza, authors who had ties to drug manufacturers were much more likely to present the evidence as favorable and to recommend the drugs than were authors who had no such conflicts of interest, according to a report published online Oct. 6 in Annals of Internal Medicine.
“Reviewers with such conflicts of interest were also less likely to address issues with the underlying primary clinical evidence, such as publication bias and the lack of access to comprehensive study data,” said Adam G. Dunn, Ph.D., of the Centre for Health Informatics, University of New South Wales, Sydney, and his associates (Ann. Intern. Med. 2014 Oct.6 [doi:10.7326/M14-0933]).
The findings suggest that “further measures may be necessary to ensure that industry collaborations do not compromise the scientific evidence,” the investigators noted.
“If the benefits of neuraminidase inhibitors are eventually found to have been inflated, millions of patients will have been unnecessarily exposed to drugs that may be of little or no benefit,” the researchers added.
Dr. Dunn and his colleagues observed that reviews of neuraminidase inhibitors had widely divergent conclusions, with some strongly endorsing the agents as influenza prophylaxis or treatment, while others questioned the drugs’ safety and efficacy.
To examine whether industry influence may have contributed to these inconsistent conclusions, the investigators analyzed 26 systematic reviews. Thirteen assessed prophylaxis, and 24 assessed treatment; so, there were 37 distinct assessments. Seven of those were Cochrane analyses.
Two of the investigators read redacted copies of the reviews and rated them as favorable or unfavorable toward the use of neuraminidase inhibitors. Those copies showed no identifying information for the authors and no journal name or formatting clues. The agreement between the two investigators was “strong,” at 86%.
At the same time, Dr. Dunn and his associates searched for all the financial conflicts of interest for all the authors of these reviews, paying special attention to ties to the pharmaceutical companies that made the neuraminidase inhibitors under review. To do so, they went beyond the affiliations and funding listed in the systematic reviews, examining the authors’ personal and institutional websites, as well as disclosure lists provided by GlaxoSmithKline and Roche. They also performed Web searches combining the authors’ names, drug names, and manufacturers’ names.
Reviewers who had ties to the pharmaceutical industry rated neuraminidase inhibitors favorably in 88% (seven of eight) systematic reviews. In contrast, reviewers who had no such ties rated the drugs favorably in only 17% (5 of 29) of systematic reviews. That pattern persisted when prophylaxis and treatment were assessed separately.
Regarding prophylaxis, 100% (2 of 2) of the reviews with financial conflicts of interest were favorable, compared with only 9% (1 of 11) of those without conflicts of interest. Regarding treatment, 83% (5 of 6) of the reviews with financial conflicts of interest were favorable, compared with only 22% (4 of 18) of those without conflicts of interest.
In addition, reviewers who had ties to the pharmaceutical industry were less likely to include information about publication bias in their reports (14%, or only 1 of 7), compared with reviewers who had no such ties (79%, or 15 of 19), Dr. Dunn and his associates said.
The study findings indicate that financial conflicts of interest “are associated with product assessments favorable to the sponsors involved,” the authors asserted.
Factors that may contribute to biased conclusions include “the design of the review, the patient populations and outcomes assessed, the selective inclusion of primary evidence, [and] the critical appraisal of evidence quality and provenance,” the investigators noted. In addition, “the tone, emphasis, and interpretation provided by the authors may also influence the message that is conveyed,” they said.
The Australian National Health and Medical Research Council funded the study. Dr. Dunn and his associates reported having no financial conflicts of interest.
In systematic reviews of research on neuraminidase inhibitors for either prevention or treatment of influenza, authors who had ties to drug manufacturers were much more likely to present the evidence as favorable and to recommend the drugs than were authors who had no such conflicts of interest, according to a report published online Oct. 6 in Annals of Internal Medicine.
“Reviewers with such conflicts of interest were also less likely to address issues with the underlying primary clinical evidence, such as publication bias and the lack of access to comprehensive study data,” said Adam G. Dunn, Ph.D., of the Centre for Health Informatics, University of New South Wales, Sydney, and his associates (Ann. Intern. Med. 2014 Oct.6 [doi:10.7326/M14-0933]).
The findings suggest that “further measures may be necessary to ensure that industry collaborations do not compromise the scientific evidence,” the investigators noted.
“If the benefits of neuraminidase inhibitors are eventually found to have been inflated, millions of patients will have been unnecessarily exposed to drugs that may be of little or no benefit,” the researchers added.
Dr. Dunn and his colleagues observed that reviews of neuraminidase inhibitors had widely divergent conclusions, with some strongly endorsing the agents as influenza prophylaxis or treatment, while others questioned the drugs’ safety and efficacy.
To examine whether industry influence may have contributed to these inconsistent conclusions, the investigators analyzed 26 systematic reviews. Thirteen assessed prophylaxis, and 24 assessed treatment; so, there were 37 distinct assessments. Seven of those were Cochrane analyses.
Two of the investigators read redacted copies of the reviews and rated them as favorable or unfavorable toward the use of neuraminidase inhibitors. Those copies showed no identifying information for the authors and no journal name or formatting clues. The agreement between the two investigators was “strong,” at 86%.
At the same time, Dr. Dunn and his associates searched for all the financial conflicts of interest for all the authors of these reviews, paying special attention to ties to the pharmaceutical companies that made the neuraminidase inhibitors under review. To do so, they went beyond the affiliations and funding listed in the systematic reviews, examining the authors’ personal and institutional websites, as well as disclosure lists provided by GlaxoSmithKline and Roche. They also performed Web searches combining the authors’ names, drug names, and manufacturers’ names.
Reviewers who had ties to the pharmaceutical industry rated neuraminidase inhibitors favorably in 88% (seven of eight) systematic reviews. In contrast, reviewers who had no such ties rated the drugs favorably in only 17% (5 of 29) of systematic reviews. That pattern persisted when prophylaxis and treatment were assessed separately.
Regarding prophylaxis, 100% (2 of 2) of the reviews with financial conflicts of interest were favorable, compared with only 9% (1 of 11) of those without conflicts of interest. Regarding treatment, 83% (5 of 6) of the reviews with financial conflicts of interest were favorable, compared with only 22% (4 of 18) of those without conflicts of interest.
In addition, reviewers who had ties to the pharmaceutical industry were less likely to include information about publication bias in their reports (14%, or only 1 of 7), compared with reviewers who had no such ties (79%, or 15 of 19), Dr. Dunn and his associates said.
The study findings indicate that financial conflicts of interest “are associated with product assessments favorable to the sponsors involved,” the authors asserted.
Factors that may contribute to biased conclusions include “the design of the review, the patient populations and outcomes assessed, the selective inclusion of primary evidence, [and] the critical appraisal of evidence quality and provenance,” the investigators noted. In addition, “the tone, emphasis, and interpretation provided by the authors may also influence the message that is conveyed,” they said.
The Australian National Health and Medical Research Council funded the study. Dr. Dunn and his associates reported having no financial conflicts of interest.
Key clinical point: Literature reviewers with ties to drug manufacturers are more likely to recommend neuraminidase inhibitors (for influenza) made by those manufacturers than are other reviewers.
Major finding: Authors who had ties to the pharmaceutical industry rated neuraminidase inhibitors favorably in 88% of systematic reviews, while authors who had no such ties rated the drugs favorably in only 17% of systematic reviews.
Data source: A retrospective analysis of 26 systematic reviews of the literature regarding neuraminidase inhibitors for prevention and treatment of influenza, plus a search for the authors’ ties to the pharmaceutical industry.
Disclosures: This study was funded by the Australian National Health and Medical Research Council. Dr. Dunn and his associates reported having no financial conflicts of interest.
Timing of Gluten Introduction in Infancy Doesn’t Matter
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Timing of gluten introduction in infancy doesn’t matter
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The findings of these two multicenter clinical trials will change the conceptual landscape of celiac disease.
From now on, it will be difficult for anyone to continue to recommend the introduction of gluten specifically at the age of 4-6 months, because this will not reduce the risk of developing celiac disease. And although breastfeeding is important to overall health, it doesn’t appear to protect against celiac disease.
Jonas F. Ludvigsson, M.D., Ph.D., is in the department of medical epidemiology and biostatistics at the Karolinska Institute, Stockholm, and the department of pediatrics at Orebro (Sweden) University Hospital. Peter H. R. Green, M.D., is at the Celiac Disease Center at Columbia University, New York. These remarks were taken from their editorial accompanying Dr. Lionetti’s and Dr. Vriezinga’s reports (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMe1408011]).
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
The timing of introducing gluten into babies’ diets doesn’t alter their risk for developing celiac disease later, according to two separate reports published online Oct. 1 in the New England Journal of Medicine.
Currently, many clinicians recommend introducing small amounts of gluten during a proposed “window of opportunity” at 4-6 months of age – preferably while maintaining breast-feeding, which is considered protective – as a way of preventing the later development of celiac disease, especially in at-risk children. But this advice is based on the findings of a few small observational studies and remains controversial. To better examine whether the timing of gluten introduction influences the risk for celiac disease, separate groups of investigators performed prospective, randomized clinical trials assessing different dietary interventions in at-risk children.
They found that neither introducing small amounts of gluten at 4-6 months of age nor delaying that introduction until 12 months of age reduced the risk of later celiac disease, and that breastfeeding status also did not significantly affect that risk.
In the Risk of Celiac Disease and Age at Gluten Introduction (CELIPREV) trial, 707 newborns who had at least one affected first-degree relative were enrolled at 20 medical centers across Italy during a 5-year period. They were randomly assigned to gluten introduction (in the form of pasta, semolina, or biscuits) at age 6 months (379 babies) or delayed introduction at age 12 months (328 babies) and were followed for 5-11 years.
Overall, at age 10 years the prevalence of celiac disease autoimmunity was 16.5%, and the prevalence of overt celiac disease was 13.2%, said Dr. Elena Lionetti of the department of pediatrics at the University of Catania, Italy, and her associates.
At 2 years of age, the proportion of children with overt celiac disease was significantly higher in the group in which gluten was introduced at 6 months (12%) than in the delayed-introduction group (5%). However, that difference quickly resolved, so that by age 5 and persisting through age 10, the proportions of children with overt celiac disease were the same (16%) in both study groups. This pattern was the same for the proportions of children with celiac disease autoimmunity.
In addition, “We did not detect an effect of breastfeeding on the development of celiac disease: the mean duration of breastfeeding was very similar for at-risk children among whom celiac disease developed and at-risk children in whom the disorder did not develop (5.6 and 5.8 months, respectively),” the investigators said.
The only factor that was significantly associated with the development of both celiac autoimmunity and overt celiac disease was carrying a high-risk HLA genotype. The type of relative with celiac disease, the number of affected relatives, the child’s dietary pattern, and the presence or absence of early intestinal infection all showed no association.
“Our findings do not support the ‘window of tolerance’ hypothesis” and instead indicate that early dietary factors seem to play a minor role in celiac disease risk, Dr. Lionetti and her associates noted (N. Engl. J. Med. 2014 Oct. 2 [doi:10.1056/NEJMoa1400697]).
In the European multicenter project Prevent Coeliac Disease (PreventCD), 963 high-risk infants aged 0-3 months were enrolled through celiac disease organizations in Croatia, Germany, Hungary, Israel, Italy, the Netherlands, Poland, and Spain. They were randomly assigned to receive either a small amount of wheat gluten (483 babies) or a matching placebo (480 babies) every day from 4 months through 6 months of age. The amount of gluten was equivalent to 2%-3% of that contained in a slice of bread.
At the conclusion of this intervention, parents were advised to introduce gluten gradually, in accordance with standardized recommendations. Parents also were allowed to breastfeed according to their own preferences, which permitted the researchers to assess several subgroups according to the duration and exclusivity of breastfeeding, said Dr. Sabine L. Vriezinga of the department of pediatrics at Leiden (the Netherlands) University Medical Center, and her associates.
The primary outcome of the trial – the frequency of celiac disease at age 3 years – was not significantly different between babies who received gluten (5.9%) and those who did not (4.5%). It also was no different among babies who were not breastfed, were breastfed for 3 or fewer months, were breastfed for 4-5 months, or were breastfed for 6 or more months. And it was not influenced by the country of origin; the number and type of affected relatives; the presence or absence of rotavirus vaccination; the presence or absence of early gastrointestinal or respiratory infections; or the mean dietary intake of gluten, the investigators said (N. Engl. J. Med. 2014 October 2 [doi:10.1056/NEJMoa1404172]).
These findings do not support guidelines that advocate early exposure to small amounts of gluten or maintenance of breastfeeding at the time of gluten introduction, “or any specific feeding recommendation with respect to the timing of gluten introduction for infants at risk for celiac disease,” Dr. Vriezinga and her associates said.
The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: No age “window” exists for introducing gluten to avert celiac disease.
Major finding: At ages 3-10 years, similar percentages of all study groups had overt celiac disease, no matter when they were exposed to gluten in the diet.
Data source: A prospective, randomized, controlled trial involving 832 babies followed for 5-11 years for the development of celiac disease, and a prospective, randomized, double-blind trial involving 963 babies followed for 3-6 years.
Disclosures: The PreventCD trial was supported by grants from the European Commission, the Azrieli Foundation, Deutsche Zöliakie Gesellschaft, Eurospital, Fondazione Celiachia, Fria Bröd, Instituto de Salud Carlos III, Spanish Society for Pediatric Gastroenterology, Hepatology, and Nutrition, Komitet Badan Naukowych, Fundacja Nutricia, Hungarian Scientific Research Funds, Stichting Coeliakie Onderzoek Nederland, Thermo Fisher Scientific, and the European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. The CELIPREV trial was supported by the Celiac Foundation of the Italian Celiac Society. A few coauthors from Dr. Lionetti’s study were scientific consultants or received grant support from diagnostics companies, but most had no relevant financial disclosures. A few coauthors from Dr. Vriezinga’s study received speaker’s fees or research support from pharmaceutical companies, but the majority had no relevant financial disclosures.