User login
Acupuncture failed to reduce chronic knee pain
Neither traditional nor laser acupuncture is more effective than sham acupuncture at lessening pain or improving function in patients older than 50 years who have moderate to severe chronic knee pain, according to a report published online September 30 in JAMA.
In a randomized, partially blinded clinical trial, 282 participants aged 50 and older were randomly assigned to receive traditional needle acupuncture (70 patients), laser acupuncture (71 patients), sham laser acupuncture (70 patients), or no acupuncture (71 control subjects) in 20-minute sessions delivered once or twice weekly for 12 weeks.
At baseline, all the participants reported having moderate to severe knee pain and morning stiffness lasting less than 30 minutes on most days. They completed detailed questionnaires at baseline, 12 weeks, and 1 year measuring pain on walking or standing, physical function, activity restriction, health-related quality of life, and global change in knee pain and function over time, said Rana S. Hinman, Ph.D., of the Centre for Health, Exercise, and Sports Medicine, University of Melbourne, and her associates.
At 12 weeks and at 1 year, there were no significant differences in any of these outcomes between active acupuncture and sham acupuncture. Both needle and laser acupuncture improved pain at 12 weeks when compared with the control group, but this improvement was “of a clinically unimportant magnitude” and did not persist, the investigators said (JAMA 2014 September 30 [doi:10.1001/jama.2014.12660]).
Clinical guidelines vary with regard to acupuncture for knee osteoarthritis. The American College of Rheumatology “conditionally recommends” the treatment in patients who are candidates for arthroplasty, the Osteoarthritis Research Society International is “uncertain” about its efficacy, the European League Against Rheumatism failed to reach consensus on the treatment and doesn’t address it in final recommendations, the American Academy of Orthopedic Surgeons “cannot recommend” it, and the National Institute for Health and Care Excellence’s guidelines recommend against it. “The findings from our trial support these latter recommendations,” Dr. Hinman and her associates said.
Neither traditional nor laser acupuncture is more effective than sham acupuncture at lessening pain or improving function in patients older than 50 years who have moderate to severe chronic knee pain, according to a report published online September 30 in JAMA.
In a randomized, partially blinded clinical trial, 282 participants aged 50 and older were randomly assigned to receive traditional needle acupuncture (70 patients), laser acupuncture (71 patients), sham laser acupuncture (70 patients), or no acupuncture (71 control subjects) in 20-minute sessions delivered once or twice weekly for 12 weeks.
At baseline, all the participants reported having moderate to severe knee pain and morning stiffness lasting less than 30 minutes on most days. They completed detailed questionnaires at baseline, 12 weeks, and 1 year measuring pain on walking or standing, physical function, activity restriction, health-related quality of life, and global change in knee pain and function over time, said Rana S. Hinman, Ph.D., of the Centre for Health, Exercise, and Sports Medicine, University of Melbourne, and her associates.
At 12 weeks and at 1 year, there were no significant differences in any of these outcomes between active acupuncture and sham acupuncture. Both needle and laser acupuncture improved pain at 12 weeks when compared with the control group, but this improvement was “of a clinically unimportant magnitude” and did not persist, the investigators said (JAMA 2014 September 30 [doi:10.1001/jama.2014.12660]).
Clinical guidelines vary with regard to acupuncture for knee osteoarthritis. The American College of Rheumatology “conditionally recommends” the treatment in patients who are candidates for arthroplasty, the Osteoarthritis Research Society International is “uncertain” about its efficacy, the European League Against Rheumatism failed to reach consensus on the treatment and doesn’t address it in final recommendations, the American Academy of Orthopedic Surgeons “cannot recommend” it, and the National Institute for Health and Care Excellence’s guidelines recommend against it. “The findings from our trial support these latter recommendations,” Dr. Hinman and her associates said.
Neither traditional nor laser acupuncture is more effective than sham acupuncture at lessening pain or improving function in patients older than 50 years who have moderate to severe chronic knee pain, according to a report published online September 30 in JAMA.
In a randomized, partially blinded clinical trial, 282 participants aged 50 and older were randomly assigned to receive traditional needle acupuncture (70 patients), laser acupuncture (71 patients), sham laser acupuncture (70 patients), or no acupuncture (71 control subjects) in 20-minute sessions delivered once or twice weekly for 12 weeks.
At baseline, all the participants reported having moderate to severe knee pain and morning stiffness lasting less than 30 minutes on most days. They completed detailed questionnaires at baseline, 12 weeks, and 1 year measuring pain on walking or standing, physical function, activity restriction, health-related quality of life, and global change in knee pain and function over time, said Rana S. Hinman, Ph.D., of the Centre for Health, Exercise, and Sports Medicine, University of Melbourne, and her associates.
At 12 weeks and at 1 year, there were no significant differences in any of these outcomes between active acupuncture and sham acupuncture. Both needle and laser acupuncture improved pain at 12 weeks when compared with the control group, but this improvement was “of a clinically unimportant magnitude” and did not persist, the investigators said (JAMA 2014 September 30 [doi:10.1001/jama.2014.12660]).
Clinical guidelines vary with regard to acupuncture for knee osteoarthritis. The American College of Rheumatology “conditionally recommends” the treatment in patients who are candidates for arthroplasty, the Osteoarthritis Research Society International is “uncertain” about its efficacy, the European League Against Rheumatism failed to reach consensus on the treatment and doesn’t address it in final recommendations, the American Academy of Orthopedic Surgeons “cannot recommend” it, and the National Institute for Health and Care Excellence’s guidelines recommend against it. “The findings from our trial support these latter recommendations,” Dr. Hinman and her associates said.
Key clinical point: Acupuncture did not decrease chronic knee pain in patients over 50.
Major finding: No pain or function outcomes were significantly different between active and sham acupuncture at 12 weeks or 1 year.
Data source: A randomized, partially blinded Zelen-design clinical trial involving 282 older patients with moderate to severe knee pain who were treated for 12 weeks and followed for 1 year.
Disclosures: This study was funded by the National Health and Medical Research Council of Australia and the Australian Research Council. Dr. Hinman reported receiving royalties from the sale of the Gel Melbourne OA shoe (Asics), and her associates reported numerous ties to industry sources.
Esophageal cancer screen rarely incites legal claims
Physicians’ failure to screen patients for esophageal cancer rarely incites legal claims, according to a Research Letter published online September 30 in JAMA. Endoscopic screening for esophageal adenocarcinoma is recommended for patients with chronic symptoms of gastroesophageal reflux disease only if they have additional risk factors. But physician surveys indicate that many clinicians order or perform upper-GI endoscopy in symptomatic patients with no additional risk factors, out of fear of litigation for missing a cancer, said Dr. Megan A. Adams of the division of gastroenterology, University of Michigan, Ann Arbor, and her associates.
To assess the actual risk of such litigation, the investigators analyzed information from “the largest U.S. medical professional liability claims database,” which includes insurance companies that collectively cover more than two-thirds of private-practice physicians across the country. They identified 278,220 claims filed against physicians in 1985-2012, of which 761 were related to upper-GI endoscopy. Of the 268 claims that involved esophageal malignancies, 19 were filed for failure to screen a low-risk patient for esophageal cancer, and only 4 of those were paid to the claimants.
In comparison, 17 claims were filed and 8 were paid for complications arising from upper-GI endoscopies done for “questionable” indications. Thus, clinicians who perform the procedure because of fear of litigation for missing an esophageal cancer are just as likely to be sued for complications of an unnecessary endoscopy, noted Dr. Adams and her associates (JAMA 2014;312:1348-9).
“There may be legitimate reasons to screen for esophageal cancer in some [low-risk] patients, but our findings suggest that the risk of a medical professional liability claim for failing to screen is not one of them,” they noted.
Physicians’ failure to screen patients for esophageal cancer rarely incites legal claims, according to a Research Letter published online September 30 in JAMA. Endoscopic screening for esophageal adenocarcinoma is recommended for patients with chronic symptoms of gastroesophageal reflux disease only if they have additional risk factors. But physician surveys indicate that many clinicians order or perform upper-GI endoscopy in symptomatic patients with no additional risk factors, out of fear of litigation for missing a cancer, said Dr. Megan A. Adams of the division of gastroenterology, University of Michigan, Ann Arbor, and her associates.
To assess the actual risk of such litigation, the investigators analyzed information from “the largest U.S. medical professional liability claims database,” which includes insurance companies that collectively cover more than two-thirds of private-practice physicians across the country. They identified 278,220 claims filed against physicians in 1985-2012, of which 761 were related to upper-GI endoscopy. Of the 268 claims that involved esophageal malignancies, 19 were filed for failure to screen a low-risk patient for esophageal cancer, and only 4 of those were paid to the claimants.
In comparison, 17 claims were filed and 8 were paid for complications arising from upper-GI endoscopies done for “questionable” indications. Thus, clinicians who perform the procedure because of fear of litigation for missing an esophageal cancer are just as likely to be sued for complications of an unnecessary endoscopy, noted Dr. Adams and her associates (JAMA 2014;312:1348-9).
“There may be legitimate reasons to screen for esophageal cancer in some [low-risk] patients, but our findings suggest that the risk of a medical professional liability claim for failing to screen is not one of them,” they noted.
Physicians’ failure to screen patients for esophageal cancer rarely incites legal claims, according to a Research Letter published online September 30 in JAMA. Endoscopic screening for esophageal adenocarcinoma is recommended for patients with chronic symptoms of gastroesophageal reflux disease only if they have additional risk factors. But physician surveys indicate that many clinicians order or perform upper-GI endoscopy in symptomatic patients with no additional risk factors, out of fear of litigation for missing a cancer, said Dr. Megan A. Adams of the division of gastroenterology, University of Michigan, Ann Arbor, and her associates.
To assess the actual risk of such litigation, the investigators analyzed information from “the largest U.S. medical professional liability claims database,” which includes insurance companies that collectively cover more than two-thirds of private-practice physicians across the country. They identified 278,220 claims filed against physicians in 1985-2012, of which 761 were related to upper-GI endoscopy. Of the 268 claims that involved esophageal malignancies, 19 were filed for failure to screen a low-risk patient for esophageal cancer, and only 4 of those were paid to the claimants.
In comparison, 17 claims were filed and 8 were paid for complications arising from upper-GI endoscopies done for “questionable” indications. Thus, clinicians who perform the procedure because of fear of litigation for missing an esophageal cancer are just as likely to be sued for complications of an unnecessary endoscopy, noted Dr. Adams and her associates (JAMA 2014;312:1348-9).
“There may be legitimate reasons to screen for esophageal cancer in some [low-risk] patients, but our findings suggest that the risk of a medical professional liability claim for failing to screen is not one of them,” they noted.
Key clinical point: Physicians are rarely sued for failure to screen for esophageal cancer.
Major finding: Among 278,220 legal claims against physicians in 1985-2012, 19 of the 268 that involved esophageal malignancies were filed for failure to screen.
Data source: A medical professional liability claims database.
Disclosures: Dr. Adams reported having no financial disclosures.
Combination therapy superior for elderly with COPD
Combination therapy with long-acting beta-agonists plus inhaled corticosteroids is superior to long-acting beta-agonists alone in older patients with COPD, especially those who also have asthma, according to a report published online Sept. 16 in JAMA.
The combination therapy has proved superior in clinical trials, but those studies excluded older patients, patients with comorbidities (especially asthma), and patients taking long-acting anticholinergic medications. To compare the two treatment approaches in a real world setting, researchers performed a retrospective observational study involving 38,266 Ontario residents (aged 66 and older) who were diagnosed as having COPD and who initiated therapy during an 8-year period.
A total of 8,712 of these adults who were new users of combination long-acting beta-agonists plus corticosteroids were propensity matched with 3,160 who were new users of long-acting beta-agonists alone. The mean age of these participants was 77 years, and they had a median of seven comorbid diseases; 28% had comorbid asthma. These study participants were followed for up to 5 years, with a median follow-up of 2.6 years, said Dr. Andrea S. Gershon of the Institute for Clinical Evaluative Sciences, Toronto, and her associates.
The primary study outcome, a composite of all-cause mortality and COPD-related hospitalization, occurred in 64% of patients in the combination-therapy group, which was a “modest but significantly lower” rate than in the single-therapy group (67%). This small but significant difference persisted when mortality and hospitalizations were analyzed separately. Two subgroups of patients showed even greater benefit from the combination therapy: those with concomitant asthma, and those who were not taking long-acting anticholinergics for their COPD, the investigators reported (JAMA 2014 Sept. 16 [doi:10.1001/jama.2014.11432]).
This study was supported by Physicians’ Services Incorporated Foundation; the Canadian Institutes of Health Research Institute of Nutrition, Metabolism, and Diabetes; the University of Toronto; the Institute for Clinical Evaluative Sciences; and the Ontario Ministry of Health and Long-Term Care. Dr. Gershon and her associates reported no relevant financial conflicts.
Perhaps the most noteworthy finding of Dr. Gershon and her associates is that the combination therapy proved to be more effective, even though the study participants were much different from those in whom this treatment was validated in randomized clinical trials.
Clinical outcomes in these real world patients were better than might have been expected, even though they were older, much more diverse, and often sicker than the patients in the clinical trials.
Dr. Peter M.A. Calverley is at the Institute of Ageing and Chronic Disease at the University of Liverpool (England). He made these remarks in an editorial accompanying Dr. Gershon’s report (JAMA 2014;312:1101-2). Dr. Calverley reported ties to GlaxoSmithKline, Boehringer Ingelheim, Takeda, Novartis, and Astra Zeneca.
Perhaps the most noteworthy finding of Dr. Gershon and her associates is that the combination therapy proved to be more effective, even though the study participants were much different from those in whom this treatment was validated in randomized clinical trials.
Clinical outcomes in these real world patients were better than might have been expected, even though they were older, much more diverse, and often sicker than the patients in the clinical trials.
Dr. Peter M.A. Calverley is at the Institute of Ageing and Chronic Disease at the University of Liverpool (England). He made these remarks in an editorial accompanying Dr. Gershon’s report (JAMA 2014;312:1101-2). Dr. Calverley reported ties to GlaxoSmithKline, Boehringer Ingelheim, Takeda, Novartis, and Astra Zeneca.
Perhaps the most noteworthy finding of Dr. Gershon and her associates is that the combination therapy proved to be more effective, even though the study participants were much different from those in whom this treatment was validated in randomized clinical trials.
Clinical outcomes in these real world patients were better than might have been expected, even though they were older, much more diverse, and often sicker than the patients in the clinical trials.
Dr. Peter M.A. Calverley is at the Institute of Ageing and Chronic Disease at the University of Liverpool (England). He made these remarks in an editorial accompanying Dr. Gershon’s report (JAMA 2014;312:1101-2). Dr. Calverley reported ties to GlaxoSmithKline, Boehringer Ingelheim, Takeda, Novartis, and Astra Zeneca.
Combination therapy with long-acting beta-agonists plus inhaled corticosteroids is superior to long-acting beta-agonists alone in older patients with COPD, especially those who also have asthma, according to a report published online Sept. 16 in JAMA.
The combination therapy has proved superior in clinical trials, but those studies excluded older patients, patients with comorbidities (especially asthma), and patients taking long-acting anticholinergic medications. To compare the two treatment approaches in a real world setting, researchers performed a retrospective observational study involving 38,266 Ontario residents (aged 66 and older) who were diagnosed as having COPD and who initiated therapy during an 8-year period.
A total of 8,712 of these adults who were new users of combination long-acting beta-agonists plus corticosteroids were propensity matched with 3,160 who were new users of long-acting beta-agonists alone. The mean age of these participants was 77 years, and they had a median of seven comorbid diseases; 28% had comorbid asthma. These study participants were followed for up to 5 years, with a median follow-up of 2.6 years, said Dr. Andrea S. Gershon of the Institute for Clinical Evaluative Sciences, Toronto, and her associates.
The primary study outcome, a composite of all-cause mortality and COPD-related hospitalization, occurred in 64% of patients in the combination-therapy group, which was a “modest but significantly lower” rate than in the single-therapy group (67%). This small but significant difference persisted when mortality and hospitalizations were analyzed separately. Two subgroups of patients showed even greater benefit from the combination therapy: those with concomitant asthma, and those who were not taking long-acting anticholinergics for their COPD, the investigators reported (JAMA 2014 Sept. 16 [doi:10.1001/jama.2014.11432]).
This study was supported by Physicians’ Services Incorporated Foundation; the Canadian Institutes of Health Research Institute of Nutrition, Metabolism, and Diabetes; the University of Toronto; the Institute for Clinical Evaluative Sciences; and the Ontario Ministry of Health and Long-Term Care. Dr. Gershon and her associates reported no relevant financial conflicts.
Combination therapy with long-acting beta-agonists plus inhaled corticosteroids is superior to long-acting beta-agonists alone in older patients with COPD, especially those who also have asthma, according to a report published online Sept. 16 in JAMA.
The combination therapy has proved superior in clinical trials, but those studies excluded older patients, patients with comorbidities (especially asthma), and patients taking long-acting anticholinergic medications. To compare the two treatment approaches in a real world setting, researchers performed a retrospective observational study involving 38,266 Ontario residents (aged 66 and older) who were diagnosed as having COPD and who initiated therapy during an 8-year period.
A total of 8,712 of these adults who were new users of combination long-acting beta-agonists plus corticosteroids were propensity matched with 3,160 who were new users of long-acting beta-agonists alone. The mean age of these participants was 77 years, and they had a median of seven comorbid diseases; 28% had comorbid asthma. These study participants were followed for up to 5 years, with a median follow-up of 2.6 years, said Dr. Andrea S. Gershon of the Institute for Clinical Evaluative Sciences, Toronto, and her associates.
The primary study outcome, a composite of all-cause mortality and COPD-related hospitalization, occurred in 64% of patients in the combination-therapy group, which was a “modest but significantly lower” rate than in the single-therapy group (67%). This small but significant difference persisted when mortality and hospitalizations were analyzed separately. Two subgroups of patients showed even greater benefit from the combination therapy: those with concomitant asthma, and those who were not taking long-acting anticholinergics for their COPD, the investigators reported (JAMA 2014 Sept. 16 [doi:10.1001/jama.2014.11432]).
This study was supported by Physicians’ Services Incorporated Foundation; the Canadian Institutes of Health Research Institute of Nutrition, Metabolism, and Diabetes; the University of Toronto; the Institute for Clinical Evaluative Sciences; and the Ontario Ministry of Health and Long-Term Care. Dr. Gershon and her associates reported no relevant financial conflicts.
FROM JAMA
Key clinical point: Combination therapy with long-acting beta-agonists plus inhaled corticosteroids should be considered for older patients with COPD.Major finding: The primary study outcome, a composite of all-cause mortality and COPD-related hospitalization, occurred in 64% of patients in the combination-therapy group, which was a “modest but significantly lower” rate than in the single-therapy group (67%).
Data source: A retrospective observational cohort study of elderly COPD patients comparing outcomes between 8,712 who took combination therapy and 3,160 who took single therapy, who were followed for up to 5 years.
Disclosures: This study was supported by Physicians’ Services Incorporated Foundation; the Canadian Institutes of Health Research Institute of Nutrition, Metabolism, and Diabetes; the University of Toronto; the Institute for Clinical Evaluative Sciences; and the Ontario Ministry of Health and Long-Term Care. Dr. Gershon and her associates reported no relevant financial conflicts.
Most acute VTE therapies yield similar outcomes
For patients with acute venous thromboembolism, both clinical and safety outcomes were similar among seven of eight possible treatment strategies assessed in a network meta-analysis published online Sept. 16 in JAMA.
Clinicians have several treatment options but little guidance for choosing among them when managing acute VTE. Many strategies have shown promising results when assessed in single studies, but there have been few direct comparison studies. So investigators performed a network meta-analysis of 45 articles involving 44,989 patients, which enabled them to compare the safety and efficacy of eight possible approaches. The sample sizes of these studies ranged from 60 to 8,240 participants, with a median of 298. The median follow-up period was 3 months, with a range of 3-8 months.
The currently accepted standard treatment for acute VTE is the use of parenteral low-molecular-weight heparin (LMWH) for a minimum of 5 days, followed by transition to a vitamin K antagonist. This approach was compared against parenteral unfractionated heparin followed by a vitamin K antagonist; parenteral fondaparinux followed by a vitamin K antagonist; parenteral LMWH combined with dabigatran; parenteral LMWH combined with edoxaban; oral rivaroxaban; oral apixaban; and parenteral LMWH alone, said Dr. Lana A. Castellucci of the Ottawa Hospital Research Institute, University of Ottawa, and her associates.
Compared with standard parenteral LMWH plus a vitamin K antagonist, six of these approaches yielded comparable reductions in recurrent VTE and induced comparable rates of major bleeding, the investigators said (JAMA 2014 September 16 [doi:10.1001/jama.2014.10538]).
The only strategy that was less effective at reducing the rate of recurrent VTE was parenteral unfractionated heparin plus a vitamin K antagonist. However, “there are clinical circumstances that necessitate the use of unfractionated heparin, including for patients with severe renal insufficiency and those with massive or submassive pulmonary embolism who are potential candidates for thrombolysis or thrombectomy,” Dr. Castellucci and her associates noted.
Oral rivaroxaban and oral apixaban appeared to be associated with the lowest risk of major bleeding. “Future direct comparison trials, patient-level network meta-analyses, or high-quality nonrandomized studies are required to confirm our findings,” they added.
This study was supported by the Heart and Stroke Foundation of Canada, the University of Ottawa, the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, and the Heart and Stroke Foundation of Ontario. Dr. Castellucci reported no financial conflicts of interest; some of her associates reported ties to Bayer, Biomerieux, Boehringer Ingelheim, Bristol-Myers Squibb, Leo Pharma, Pfizer, and Sanofi.
*Correction, 9/17/2014: An earlier version of this article misstated the Key Clinical Point in the Vitals section.
For patients with acute venous thromboembolism, both clinical and safety outcomes were similar among seven of eight possible treatment strategies assessed in a network meta-analysis published online Sept. 16 in JAMA.
Clinicians have several treatment options but little guidance for choosing among them when managing acute VTE. Many strategies have shown promising results when assessed in single studies, but there have been few direct comparison studies. So investigators performed a network meta-analysis of 45 articles involving 44,989 patients, which enabled them to compare the safety and efficacy of eight possible approaches. The sample sizes of these studies ranged from 60 to 8,240 participants, with a median of 298. The median follow-up period was 3 months, with a range of 3-8 months.
The currently accepted standard treatment for acute VTE is the use of parenteral low-molecular-weight heparin (LMWH) for a minimum of 5 days, followed by transition to a vitamin K antagonist. This approach was compared against parenteral unfractionated heparin followed by a vitamin K antagonist; parenteral fondaparinux followed by a vitamin K antagonist; parenteral LMWH combined with dabigatran; parenteral LMWH combined with edoxaban; oral rivaroxaban; oral apixaban; and parenteral LMWH alone, said Dr. Lana A. Castellucci of the Ottawa Hospital Research Institute, University of Ottawa, and her associates.
Compared with standard parenteral LMWH plus a vitamin K antagonist, six of these approaches yielded comparable reductions in recurrent VTE and induced comparable rates of major bleeding, the investigators said (JAMA 2014 September 16 [doi:10.1001/jama.2014.10538]).
The only strategy that was less effective at reducing the rate of recurrent VTE was parenteral unfractionated heparin plus a vitamin K antagonist. However, “there are clinical circumstances that necessitate the use of unfractionated heparin, including for patients with severe renal insufficiency and those with massive or submassive pulmonary embolism who are potential candidates for thrombolysis or thrombectomy,” Dr. Castellucci and her associates noted.
Oral rivaroxaban and oral apixaban appeared to be associated with the lowest risk of major bleeding. “Future direct comparison trials, patient-level network meta-analyses, or high-quality nonrandomized studies are required to confirm our findings,” they added.
This study was supported by the Heart and Stroke Foundation of Canada, the University of Ottawa, the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, and the Heart and Stroke Foundation of Ontario. Dr. Castellucci reported no financial conflicts of interest; some of her associates reported ties to Bayer, Biomerieux, Boehringer Ingelheim, Bristol-Myers Squibb, Leo Pharma, Pfizer, and Sanofi.
*Correction, 9/17/2014: An earlier version of this article misstated the Key Clinical Point in the Vitals section.
For patients with acute venous thromboembolism, both clinical and safety outcomes were similar among seven of eight possible treatment strategies assessed in a network meta-analysis published online Sept. 16 in JAMA.
Clinicians have several treatment options but little guidance for choosing among them when managing acute VTE. Many strategies have shown promising results when assessed in single studies, but there have been few direct comparison studies. So investigators performed a network meta-analysis of 45 articles involving 44,989 patients, which enabled them to compare the safety and efficacy of eight possible approaches. The sample sizes of these studies ranged from 60 to 8,240 participants, with a median of 298. The median follow-up period was 3 months, with a range of 3-8 months.
The currently accepted standard treatment for acute VTE is the use of parenteral low-molecular-weight heparin (LMWH) for a minimum of 5 days, followed by transition to a vitamin K antagonist. This approach was compared against parenteral unfractionated heparin followed by a vitamin K antagonist; parenteral fondaparinux followed by a vitamin K antagonist; parenteral LMWH combined with dabigatran; parenteral LMWH combined with edoxaban; oral rivaroxaban; oral apixaban; and parenteral LMWH alone, said Dr. Lana A. Castellucci of the Ottawa Hospital Research Institute, University of Ottawa, and her associates.
Compared with standard parenteral LMWH plus a vitamin K antagonist, six of these approaches yielded comparable reductions in recurrent VTE and induced comparable rates of major bleeding, the investigators said (JAMA 2014 September 16 [doi:10.1001/jama.2014.10538]).
The only strategy that was less effective at reducing the rate of recurrent VTE was parenteral unfractionated heparin plus a vitamin K antagonist. However, “there are clinical circumstances that necessitate the use of unfractionated heparin, including for patients with severe renal insufficiency and those with massive or submassive pulmonary embolism who are potential candidates for thrombolysis or thrombectomy,” Dr. Castellucci and her associates noted.
Oral rivaroxaban and oral apixaban appeared to be associated with the lowest risk of major bleeding. “Future direct comparison trials, patient-level network meta-analyses, or high-quality nonrandomized studies are required to confirm our findings,” they added.
This study was supported by the Heart and Stroke Foundation of Canada, the University of Ottawa, the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, and the Heart and Stroke Foundation of Ontario. Dr. Castellucci reported no financial conflicts of interest; some of her associates reported ties to Bayer, Biomerieux, Boehringer Ingelheim, Bristol-Myers Squibb, Leo Pharma, Pfizer, and Sanofi.
*Correction, 9/17/2014: An earlier version of this article misstated the Key Clinical Point in the Vitals section.
FROM JAMA
Key clinical point: Seven of the eight available VTE therapies are equally safe and effective.*
Major finding: Compared with standard parenteral LMWH plus a vitamin K antagonist, six treatment approaches yielded comparable reductions in recurrent VTE and induced comparable rates of major bleeding; the only approach that was less effective at reducing the rate of recurrent VTE was parenteral unfractionated heparin plus a vitamin K antagonist.
Data source: A network meta-analysis of 45 articles on studies involving 44,989 patients with acute VTE who were treated using any of eight strategies and followed for a median of 3 months.
Disclosures: This study was supported by the Heart and Stroke Foundation of Canada, the University of Ottawa, the Canadian Institutes of Health Research, the Canadian Network and Centre for Trials Internationally, and the Heart and Stroke Foundation of Ontario. Dr. Castellucci reported no financial conflicts of interest; some of her associates reported ties to Bayer, Biomerieux, Boehringer Ingelheim, Bristol-Myers Squibb, Leo Pharma, Pfizer, and Sanofi.
AHA/ACC: No to universal ECG screen in healthy young people
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
Twelve-lead ECG should not be used to screen healthy young people in the general population for occult cardiovascular abnormalities, according to a scientific statement jointly released Sept. 15 by the American Heart Association and the American College of Cardiology
Such screening has been advocated as a way to identify young people at risk for sudden death. Those at risk then could avoid participating in sports that could trigger a fatal CV event, and cardioverter-defibrillators could be implanted in young patients who would benefit.
The issue is ontroversial, however. Opponents argue that existing screening technology produces too many false-positive and false-negative results to be useful on a large scale. In addition, such screening would be exhorbitantly expensive, diverting scarce health care resources away from other, more practical programs that would be more beneficial to the approximately 60 million young people in this patient population.
“[We] acknowledge the tragic nature of sudden deaths in the young, but do not believe the available data support a significant public health benefit from using the 12-lead ECG as a universal screening tool,” said Dr. Barry J. Maron, chair, and Dr. Richard A. Friedman, cochair, of the AHA/ACC writing committee that presented the scientific statement published online in Circulation and the Journal of the American College of Cardiology (Circulation 2014 Sept. 15 [doi: 10.1161/CIR.0000000000000025]).
The Pediatric and Congenital Electrophysiology Society and the American College of Sports Medicine also endorsed the statement.
The investigators reviewed the evidence both for and against ECG screening for all young people and for the subgroup of young athletes, who number an estimated 10 million in the United States. They found that universal screening using ECGs “would be an undertaking of enormous magnitude, with massive resource demands.” Moreover, the net benefit would be “trivial,” given the very low prevalence of abnormalities that cause sudden death in youths and the extremely low risk of sudden death even in these at-risk people.
The nationwide registry of sudden deaths among athletes documents approximately 75 cardiovascular deaths per year – a frequency that is much lower than that for virtually every other cause of death in this age group. By comparison, motor vehicle accidents cause approximately 2,500-fold more deaths per year than cardiovascular events during sports, they noted.
In addition, 12-lead ECG would make an “imperfect” screening test, especially “in a real-world mass screening setting [when] readers and technicians [who have] vastly different expertise and efficiency are confronted with large numbers of studies to perform and interpret rapidly,” the authors noted.
The overlap between normal and abnormal ECG measurements is “a major obstacle,” with readings from people with high-risk cardiovascular disease sometimes indistinguishable from those of healthy patients. Misplacement of electrodes, selection of inadequate bandwidth, inadvertent lead reversal, and imprecise measurement of the QT interval are common operator-related difficulties.
At present, the rates of both false-positive and false-negative ECG results are unacceptably high for large-scale screening, the committee said. False-positive results lead to unnecessary and expensive further testing; unwarranted restriction from sports and other activities; anxiety and other adverse psychological consequences; and impediments to insurability or employment.
Even if the false-positive rate could be reduced to only 5% of all ECGs, the authors added, in a population of 10 million athletes, this would disqualify 500,000 healthy people from sports until they underwent further testing to exclude heart abnormalities.
Restricting ECG screening only to athletes would lessen these problems but would introduce others. For example, confining mass screening to a certain segment of the population necessarily excludes other segments. In the case of restricting ECG screening to college athletes, it could appear that this process moves from being exclusionary to being discriminatory and even elitist.
Overall, the statement’s authors concluded that “currently there is insufficient information available to support the view that universal screening ECGs in asymptomatic young people for CVD is appropriate or possible on a national basis for the United States, in competitive athletes or in the general youthful population, and practical issues essentially exclude either strategy from any realistic consideration.”
The statement is available from the AHA at http://my.americanheart.org and from the ACC at http://cardiosource.org.
This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
FROM CIRCULATION
Key clinical point: Don’t use 12-lead ECG to screen healthy young people for cardiovascular abnormalities.
Major finding: Insufficient information is available to support universal screening ECGs in asymptomatic young people for cardiovascular abnormalities, either competitive athletes or the general youthful population – and practical issues essentially exclude ECG screening from any realistic consideration.
Data source: A scientific statement based on a review of the available evidence regarding the use of 12-lead ECG to screen healthy people in the general population aged 12-25 years for occult CVD.
Disclosures: This scientific statement was supported by the American Heart Association and the American College of Cardiology. The authors’ financial disclosures were not available.
Fitness attenuates systolic BP’s age-related rise
Systolic blood pressure begins a steady rise among men in their mid-40s, but that increase can be attenuated by physical fitness, to the extent that very fit men can stave off high systolic BP by a decade, compared with their unfit age-mates, according to a report published online Sept. 15 in the Journal of the American College of Cardiology.
Investigators performed a secondary analysis of data from the Aerobics Center Longitudinal Study to assess the trajectory of blood pressure over time and determine whether physical fitness, as measured by maximal treadmill exercise testing, influenced that trajectory. The ACLS was a large cohort study of healthy adults who underwent serial preventive medical assessments between 1970 and 2006, said Dr. Junxiu Liu of the department of epidemiology and biostatistics, University of South Carolina, Columbia, and her associates.
For their study, Dr. Liu and her colleagues focused on 13,953 men aged 20-90 years at baseline who participated in the ACLS, almost all of whom were white and 95% of whom were college-educated professionals. Overall, systolic blood pressure showed a linear increase with aging, while diastolic blood pressure did not. For each increase of 1 year in age, systolic BP increased by 0.30 mm Hg.
Systolic BP typically began to exceed 120 mm Hg at roughly age 46 years vin men with the lowest level of physical fitness but did not do so until age 54 years in those with the highest level of physical fitness. This suggests that engaging in physical activity reduces the duration of elevated systolic BP, which in turn may reduce the risk of developing cardiovascular disease, other hypertension-related chronic diseases, medical costs, major morbidity, and mortality, the investigators said (J. Am. Coll. Cardiol. 2014;64:1245-53 [doi:10.1016/j.jacc.2014.06.1184]).
In an analysis that accounted for numerous factors, age, body fat percent, waist circumference, resting heart rate, fasting glucose level, triglyceride level, cholesterol level, alcohol consumption status, and parental history of hypertension were all positively associated with systolic BP.
The major new finding from this study is that men who were in the highest fitness category demonstrated lower systolic blood pressure throughout the adult life span, compared with men who had moderate and low fitness levels. This difference could not be explained by differences in body fatness, waist circumference, lipid profiles, smoking status, alcohol intake, or other factors related to blood pressure, which were statistically controlled for. Indeed, habitual aerobic exercise/physical activity may even counteract the burden of cardiometabolic abnormalities that accelerate artery stiffening and therefore slow the onset and severity of isolated systolic hypertension. Dr. Stanley S. Franklin is in the heart disease prevention program at the University of California, Irvine. Gary L. Pierce, Ph.D., is in the department of health and human physiology at the University of Iowa, Iowa City. They made these remarks in an editorial accompanying Dr. Liu’s report (J. Am. Coll. Cardiol. 2014;1254-6 [doi:10.1016;j.jacc.2014.06.1183]). Dr. Franklin and Dr. Pierce reported no financial conflicts of interest.
The major new finding from this study is that men who were in the highest fitness category demonstrated lower systolic blood pressure throughout the adult life span, compared with men who had moderate and low fitness levels. This difference could not be explained by differences in body fatness, waist circumference, lipid profiles, smoking status, alcohol intake, or other factors related to blood pressure, which were statistically controlled for. Indeed, habitual aerobic exercise/physical activity may even counteract the burden of cardiometabolic abnormalities that accelerate artery stiffening and therefore slow the onset and severity of isolated systolic hypertension. Dr. Stanley S. Franklin is in the heart disease prevention program at the University of California, Irvine. Gary L. Pierce, Ph.D., is in the department of health and human physiology at the University of Iowa, Iowa City. They made these remarks in an editorial accompanying Dr. Liu’s report (J. Am. Coll. Cardiol. 2014;1254-6 [doi:10.1016;j.jacc.2014.06.1183]). Dr. Franklin and Dr. Pierce reported no financial conflicts of interest.
The major new finding from this study is that men who were in the highest fitness category demonstrated lower systolic blood pressure throughout the adult life span, compared with men who had moderate and low fitness levels. This difference could not be explained by differences in body fatness, waist circumference, lipid profiles, smoking status, alcohol intake, or other factors related to blood pressure, which were statistically controlled for. Indeed, habitual aerobic exercise/physical activity may even counteract the burden of cardiometabolic abnormalities that accelerate artery stiffening and therefore slow the onset and severity of isolated systolic hypertension. Dr. Stanley S. Franklin is in the heart disease prevention program at the University of California, Irvine. Gary L. Pierce, Ph.D., is in the department of health and human physiology at the University of Iowa, Iowa City. They made these remarks in an editorial accompanying Dr. Liu’s report (J. Am. Coll. Cardiol. 2014;1254-6 [doi:10.1016;j.jacc.2014.06.1183]). Dr. Franklin and Dr. Pierce reported no financial conflicts of interest.
Systolic blood pressure begins a steady rise among men in their mid-40s, but that increase can be attenuated by physical fitness, to the extent that very fit men can stave off high systolic BP by a decade, compared with their unfit age-mates, according to a report published online Sept. 15 in the Journal of the American College of Cardiology.
Investigators performed a secondary analysis of data from the Aerobics Center Longitudinal Study to assess the trajectory of blood pressure over time and determine whether physical fitness, as measured by maximal treadmill exercise testing, influenced that trajectory. The ACLS was a large cohort study of healthy adults who underwent serial preventive medical assessments between 1970 and 2006, said Dr. Junxiu Liu of the department of epidemiology and biostatistics, University of South Carolina, Columbia, and her associates.
For their study, Dr. Liu and her colleagues focused on 13,953 men aged 20-90 years at baseline who participated in the ACLS, almost all of whom were white and 95% of whom were college-educated professionals. Overall, systolic blood pressure showed a linear increase with aging, while diastolic blood pressure did not. For each increase of 1 year in age, systolic BP increased by 0.30 mm Hg.
Systolic BP typically began to exceed 120 mm Hg at roughly age 46 years vin men with the lowest level of physical fitness but did not do so until age 54 years in those with the highest level of physical fitness. This suggests that engaging in physical activity reduces the duration of elevated systolic BP, which in turn may reduce the risk of developing cardiovascular disease, other hypertension-related chronic diseases, medical costs, major morbidity, and mortality, the investigators said (J. Am. Coll. Cardiol. 2014;64:1245-53 [doi:10.1016/j.jacc.2014.06.1184]).
In an analysis that accounted for numerous factors, age, body fat percent, waist circumference, resting heart rate, fasting glucose level, triglyceride level, cholesterol level, alcohol consumption status, and parental history of hypertension were all positively associated with systolic BP.
Systolic blood pressure begins a steady rise among men in their mid-40s, but that increase can be attenuated by physical fitness, to the extent that very fit men can stave off high systolic BP by a decade, compared with their unfit age-mates, according to a report published online Sept. 15 in the Journal of the American College of Cardiology.
Investigators performed a secondary analysis of data from the Aerobics Center Longitudinal Study to assess the trajectory of blood pressure over time and determine whether physical fitness, as measured by maximal treadmill exercise testing, influenced that trajectory. The ACLS was a large cohort study of healthy adults who underwent serial preventive medical assessments between 1970 and 2006, said Dr. Junxiu Liu of the department of epidemiology and biostatistics, University of South Carolina, Columbia, and her associates.
For their study, Dr. Liu and her colleagues focused on 13,953 men aged 20-90 years at baseline who participated in the ACLS, almost all of whom were white and 95% of whom were college-educated professionals. Overall, systolic blood pressure showed a linear increase with aging, while diastolic blood pressure did not. For each increase of 1 year in age, systolic BP increased by 0.30 mm Hg.
Systolic BP typically began to exceed 120 mm Hg at roughly age 46 years vin men with the lowest level of physical fitness but did not do so until age 54 years in those with the highest level of physical fitness. This suggests that engaging in physical activity reduces the duration of elevated systolic BP, which in turn may reduce the risk of developing cardiovascular disease, other hypertension-related chronic diseases, medical costs, major morbidity, and mortality, the investigators said (J. Am. Coll. Cardiol. 2014;64:1245-53 [doi:10.1016/j.jacc.2014.06.1184]).
In an analysis that accounted for numerous factors, age, body fat percent, waist circumference, resting heart rate, fasting glucose level, triglyceride level, cholesterol level, alcohol consumption status, and parental history of hypertension were all positively associated with systolic BP.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Key clinical point: Physically fit men may postpone the inevitable rise of systolic blood pressure by 10 years.
Major finding: Systolic BP typically began to exceed 120 mm Hg at approximately age 46 years among men with the lowest level of physical fitness but did not do so until age 54 years among men with the highest level of physical fitness.
Data source: A secondary analysis of data from a longitudinal cohort study, involving 13,953 men aged 20-90 years at baseline who underwent serial medical assessments between 1970 and 2006.
Disclosures: This study was supported by the National Institutes of Health. Dr. Liu reported no financial conflicts of interest; an associate reported ties to Technogym, Santech, Clarity, Coca-Cola, and BodyMedia.
Benralizumab fails to decrease COPD exacerbations
Benralizumab, a monoclonal antibody to an interleukin-5 receptor that reduces eosinophil counts in the sputum and the blood and has been used to treat asthma, failed to decrease acute exacerbations of chronic obstructive pulmonary disease in an industry-sponsored phase II clinical trial reported at the European Respiratory Society International Congress 2014.
In what they described as the first study of a biologic treatment for eosinophilic COPD, researchers found that benralizumab rapidly depleted both sputum and blood eosinophils "to a much greater extent than did inhaled or oral corticosteroids in other studies," but nonetheless failed to fulfill the primary endpoint of reducing the rate of acute exacerbations, Dr. Christopher E. Brightling said in a paper simultaneously presented at the Congress and published online in Lancet (2014 [doi:10.1016/S2213-2600(14)70187-0]).
During a 3-year period, the double-blind trial enrolled 101 adults aged 45-80 years who were current or past smokers and had moderate to severe COPD. They were treated at 26 medical centers in the United Kingdom, Poland, Germany, Canada, the United States, Denmark, and Spain. These participants were randomly assigned to receive a total of eight subcutaneous injections of 100 mg benralizumab (51 patients) or a matching placebo (50 patients) at intervals over the course of 48 weeks, said Dr. Brightling of the National Institute for Health Research Respiratory Biomedical Research Unit, University of Leicester, England.
The primary endpoint, the annualized rate of acute COPD exacerbations at week 56, was 0.95 with benralizumab and 0.92 with placebo, a nonsignificant difference. There also was no significant difference between the two study groups in the need for oral corticosteroids or intravenous antibiotics, nor in the rate of hospitalization.
Benralizumab did deplete blood and sputum eosinophils at the first assessment of these outcomes, an effect that persisted throughout the treatment period and well beyond. It also produced clinically significant improvements in both prebronchodilator and postbronchodilator forced expiratory volume in 1 second, from the first assessment of these outcomes until week 80 –32 weeks after the final dose was administered.
The benralizumab group had a higher rate of serious adverse events than did the placebo group, but none of those events were considered to be drug related. No hypersensitivity reactions or immune-complex disorders occurred.
This study was funded by MedImmune, maker of benralizumab, which also participated in study design, data analysis, and manuscript preparation. Dr. Brightling reported receiving grants from MedImmune, GlaxoSmithKline, Roche, Novartis, and Chiesi; his associates reported ties to numerous industry sources.
The utilization of specific targeted therapies is rapidly expanding and holds great promise in pulmonary medicine to improve outcomes while exposing our patients to less systemic side effects. In this case, the anti–IL-5 receptor antibody performed as expected in reducing eosinophilic inflammation, but it was unsuccessful in reducing exacerbation rates, suggesting that if one therapeutic focus exists in this specific population of patients, it may not be solely part of the eosinophilic inflammatory pathway. The biologic factors leading to COPD exacerbations are quite numerous and present a complicated “target,” and it may prove difficult to find one drug that fits all patients – and as we learn more about the factors that affect different types of patients, we will be able to tailor therapies to fit a given individual.
The utilization of specific targeted therapies is rapidly expanding and holds great promise in pulmonary medicine to improve outcomes while exposing our patients to less systemic side effects. In this case, the anti–IL-5 receptor antibody performed as expected in reducing eosinophilic inflammation, but it was unsuccessful in reducing exacerbation rates, suggesting that if one therapeutic focus exists in this specific population of patients, it may not be solely part of the eosinophilic inflammatory pathway. The biologic factors leading to COPD exacerbations are quite numerous and present a complicated “target,” and it may prove difficult to find one drug that fits all patients – and as we learn more about the factors that affect different types of patients, we will be able to tailor therapies to fit a given individual.
The utilization of specific targeted therapies is rapidly expanding and holds great promise in pulmonary medicine to improve outcomes while exposing our patients to less systemic side effects. In this case, the anti–IL-5 receptor antibody performed as expected in reducing eosinophilic inflammation, but it was unsuccessful in reducing exacerbation rates, suggesting that if one therapeutic focus exists in this specific population of patients, it may not be solely part of the eosinophilic inflammatory pathway. The biologic factors leading to COPD exacerbations are quite numerous and present a complicated “target,” and it may prove difficult to find one drug that fits all patients – and as we learn more about the factors that affect different types of patients, we will be able to tailor therapies to fit a given individual.
Benralizumab, a monoclonal antibody to an interleukin-5 receptor that reduces eosinophil counts in the sputum and the blood and has been used to treat asthma, failed to decrease acute exacerbations of chronic obstructive pulmonary disease in an industry-sponsored phase II clinical trial reported at the European Respiratory Society International Congress 2014.
In what they described as the first study of a biologic treatment for eosinophilic COPD, researchers found that benralizumab rapidly depleted both sputum and blood eosinophils "to a much greater extent than did inhaled or oral corticosteroids in other studies," but nonetheless failed to fulfill the primary endpoint of reducing the rate of acute exacerbations, Dr. Christopher E. Brightling said in a paper simultaneously presented at the Congress and published online in Lancet (2014 [doi:10.1016/S2213-2600(14)70187-0]).
During a 3-year period, the double-blind trial enrolled 101 adults aged 45-80 years who were current or past smokers and had moderate to severe COPD. They were treated at 26 medical centers in the United Kingdom, Poland, Germany, Canada, the United States, Denmark, and Spain. These participants were randomly assigned to receive a total of eight subcutaneous injections of 100 mg benralizumab (51 patients) or a matching placebo (50 patients) at intervals over the course of 48 weeks, said Dr. Brightling of the National Institute for Health Research Respiratory Biomedical Research Unit, University of Leicester, England.
The primary endpoint, the annualized rate of acute COPD exacerbations at week 56, was 0.95 with benralizumab and 0.92 with placebo, a nonsignificant difference. There also was no significant difference between the two study groups in the need for oral corticosteroids or intravenous antibiotics, nor in the rate of hospitalization.
Benralizumab did deplete blood and sputum eosinophils at the first assessment of these outcomes, an effect that persisted throughout the treatment period and well beyond. It also produced clinically significant improvements in both prebronchodilator and postbronchodilator forced expiratory volume in 1 second, from the first assessment of these outcomes until week 80 –32 weeks after the final dose was administered.
The benralizumab group had a higher rate of serious adverse events than did the placebo group, but none of those events were considered to be drug related. No hypersensitivity reactions or immune-complex disorders occurred.
This study was funded by MedImmune, maker of benralizumab, which also participated in study design, data analysis, and manuscript preparation. Dr. Brightling reported receiving grants from MedImmune, GlaxoSmithKline, Roche, Novartis, and Chiesi; his associates reported ties to numerous industry sources.
Benralizumab, a monoclonal antibody to an interleukin-5 receptor that reduces eosinophil counts in the sputum and the blood and has been used to treat asthma, failed to decrease acute exacerbations of chronic obstructive pulmonary disease in an industry-sponsored phase II clinical trial reported at the European Respiratory Society International Congress 2014.
In what they described as the first study of a biologic treatment for eosinophilic COPD, researchers found that benralizumab rapidly depleted both sputum and blood eosinophils "to a much greater extent than did inhaled or oral corticosteroids in other studies," but nonetheless failed to fulfill the primary endpoint of reducing the rate of acute exacerbations, Dr. Christopher E. Brightling said in a paper simultaneously presented at the Congress and published online in Lancet (2014 [doi:10.1016/S2213-2600(14)70187-0]).
During a 3-year period, the double-blind trial enrolled 101 adults aged 45-80 years who were current or past smokers and had moderate to severe COPD. They were treated at 26 medical centers in the United Kingdom, Poland, Germany, Canada, the United States, Denmark, and Spain. These participants were randomly assigned to receive a total of eight subcutaneous injections of 100 mg benralizumab (51 patients) or a matching placebo (50 patients) at intervals over the course of 48 weeks, said Dr. Brightling of the National Institute for Health Research Respiratory Biomedical Research Unit, University of Leicester, England.
The primary endpoint, the annualized rate of acute COPD exacerbations at week 56, was 0.95 with benralizumab and 0.92 with placebo, a nonsignificant difference. There also was no significant difference between the two study groups in the need for oral corticosteroids or intravenous antibiotics, nor in the rate of hospitalization.
Benralizumab did deplete blood and sputum eosinophils at the first assessment of these outcomes, an effect that persisted throughout the treatment period and well beyond. It also produced clinically significant improvements in both prebronchodilator and postbronchodilator forced expiratory volume in 1 second, from the first assessment of these outcomes until week 80 –32 weeks after the final dose was administered.
The benralizumab group had a higher rate of serious adverse events than did the placebo group, but none of those events were considered to be drug related. No hypersensitivity reactions or immune-complex disorders occurred.
This study was funded by MedImmune, maker of benralizumab, which also participated in study design, data analysis, and manuscript preparation. Dr. Brightling reported receiving grants from MedImmune, GlaxoSmithKline, Roche, Novartis, and Chiesi; his associates reported ties to numerous industry sources.
FROM THE ERS INTERNATIONAL CONGRESS 2014/THE LANCET
Key clinical point: Benralizumab was no better than oral steroids or intravenous antibiotics for preventing COPD exacerbations.
Major finding: The annualized rate of acute COPD exacerbations at week 56 was 0.95 with benralizumab and 0.92 with placebo, and there was no significant difference between the two study groups in the need for oral corticosteroids or intravenous antibiotics, nor in the rate of hospitalization.
Data source: A randomized double-blind international phase II clinical trial involving 101 adults with moderate to severe COPD who received either benralizumab or placebo injections over the course of 48 weeks and were followed for up to 80 weeks.
Disclosures: This study was funded and designed by MedImmune, maker of benralizumab. Dr. Brightling reported receiving grants from MedImmune, GlaxoSmithKline, Roche, Novartis, and Chiesi; his associates reported ties to numerous industry sources.
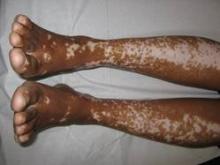
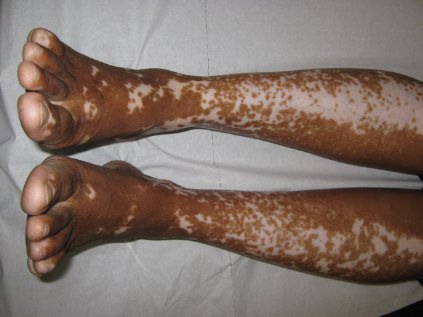
Vitiligo, alopecia more likely in GVHD when donor is female and recipient is male
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
The development of vitiligo or alopecia areata is not common in patients with chronic graft-versus-host disease, but it is significantly more likely to occur when the donor was female, especially when the recipient was male, according to a report published online Sept. 10 in JAMA Dermatology.
Fifteen case reports and small series in the literature have reported the development of vitiligo or alopecia areata after allogeneic hematopoietic stem cell transplantation, most often among patients who first developed chronic graft-versus-host disease (GVHD) after the procedure. To further explore the frequency of these two skin autoimmune manifestations in GVHD and to identify associated risk factors, researchers performed a retrospective cross-sectional analysis involving 282 adult and pediatric patients referred to the National Institutes of Health Clinical Center for GVHD in 2004-2013.
They identified 15 GVHD patients with vitiligo (4.9%) and 2 with alopecia areata (0.7%); one of these patients had both skin disorders. Most of the 15 patients had undergone stem-cell transplantation to treat chronic myelogenous leukemia (CML) (5 patients) or acute leukemia or myelodysplastic syndrome (5 patients), and most had had a human leukocyte antigen–identical donor. Twelve of the 15 developed the skin disorder after having GVHD for more than 1 year, said Rena C. Zuo of the National Cancer Institute’s dermatology branch and her associates.
"Notably, CML accounts for only about 300 of 7,892 (3.8%) allogeneic hematopoietic stem-cell transplantations per year in the United States, a relative minority among indicated diseases," they said.
In what they described as the first study to identify an association between donor/recipient sex mismatch and the development of concomitant autoimmunity in patients with chronic GVHD, the investigators found that 14 of the 15 patients who developed vitiligo or alopecia areata had female donors, 2 of whom had previously given birth; the gender of the donor in the 15th case was unknown. Nine of these 14 recipients were male, "resulting in a female-to-male sex mismatch in [at least] 64% of cases," Ms. Zuo and her colleagues said (JAMA Dermatol. 2014 Sept. 10 [doi:10.1001/jamadermatol.2014.1550]).
Both parous female donors and donor-recipient sex mismatch are known risk factors for GVHD. The risk of autoimmunity in female-to-male transplants "may reflect the activity of skin-homing donor T cells specific for recipient minor histocompatibility antigens encoded by Y-chromosome genes, a mechanism previously implicated in both GVHD and graft-versus-tumor responses," the investigators added.
This study was supported by the National Institutes of Health and the National Cancer Institute. Ms. Zuo and her associates reported no financial conflicts of interest.
FROM JAMA DERMATOLOGY
Key clinical point: Female-to-male stem cell donation ups the risk for vitiligo in GVHD.
Major finding: 14 of the 15 patients who developed vitiligo or alopecia areata had female donors (the gender of the 15th donor was unknown), and 9 of these 14 recipients were male.
Data source: A retrospective cross-sectional analysis involving 282 adults and children with chronic GVHD, 15 of whom developed vitiligo or alopecia areata.
Disclosures: This study was supported by the National Institutes of Health and the National Cancer Institute. Dr. Zuo and her associates reported no financial conflicts of interest.
NHLBI Expert Panel Issues Guideline on Sickle Cell Disease
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
FROM JAMA
NHLBI expert panel issues guideline on sickle cell disease
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
Yawn et al. have made a monumental effort to produce practical, evidence-based guidelines, but they were hampered at every turn by a pervasive lack of good quality evidence on which to base their recommendations. Still missing from this guideline are suggestions for how often and when to screen for kidney disease, how to screen for and treat the common clinical problem of asthma-like symptoms (when standard therapies are contraindicated in SCD), how to advocate for patients with the common sequelae of silent cerebral infarcts, or when to consider hematopoietic stem-cell transplantation.
The expert panel also failed to include representatives from the people most affected by SCD: patients and their families. Failure to listen to the perspective of the families, understand which of these recommendations are important to them, and deal with the obstacles families face in implementing the recommendations is a critically important omission.
Dr. Michael R. DeBaun is in the department of pediatrics at the Vanderbilt-Meharry Center of Excellence in Sickle Cell Disease, Nashville. He made his remarks in an editorial accompanying Dr. Yawn’s report (JAMA 2014:312;1004-5). Dr. DeBaun reported no financial conflicts of interest.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
The "much anticipated" guideline to help primary care and emergency clinicians improve the management of sickle cell disease includes a consensus treatment protocol for implementing hydroxyurea therapy and more detailed guidance regarding long-term transfusion therapy, according to a summary report published online September 9 in Journal of the American Medical Association.
Sickle cell disease (SCD), a life-threatening genetically transmitted disorder affecting 70,000-100,000 Americans, is associated with a wide array of complex acute and chronic complications that require immediate medical attention. But high-quality data on which to base management decisions are sorely lacking, and clinicians get little in the way of guidance from existing recommendations. One result is that "the two most widely available disease-modifying therapies, hydroxyurea and long-term transfusions, are underused, and hematopoietic stem cell transplantation, the only curative approach, has been used in only a small proportion of affected individuals," said Dr. Barbara P. Yawn and her associates on the National Heart, Lung, and Blood Institute expert panel that issued the summary report.
Even this guideline is somewhat rudimentary due to the dearth of good data "in virtually every area related to SCD management," and cannot help but leave "many uncertainties for health professionals caring for individuals with SCD." But it is hoped that this guideline will furnish a critical foundation for future research and will now begin "to facilitate improved and more accessible care for all affected individuals," said Dr. Yawn, director of research at Olmsted Medical Center, Rochester, Minn., and her associates.
The guideline is based on an extensive literature review of more than 13,000 abstracts and articles, which was winnowed to 1,583 original studies regarding SCD. From this, a team of health care professionals in family medicine, internal medicine, pediatric and adult hematology, psychiatry and mental health, transfusion medicine, obstetrics and gynecology, maternal/fetal medicine, and emergency department nursing compiled the guideline as well as the summary, entitled Evidence-Based Management of Sickle Cell Disease: Expert Panel Report 2014 (JAMA 2014 September 9 [doi:10.1001/jama.2014.10517]).
In addition to establishing a protocol for implementing hydroxyurea therapy, the guideline addresses changes in pneumococcal vaccination recommendations for adults and children; annual transcranial Doppler screening coupled with long-term transfusion therapy when necessary to prevent stroke in children aged 2-16 years; rapid initiation of opioids for severe pain during vasoocclusive crises; analgesics and physical therapy for avascular necrosis; ACE inhibitor treatment for adults with microalbuminuria; referral to specialists for screening and treatment of proliferative retinopathy; echocardiography to assess signs of pulmonary hypertension; and monitoring for iron overload in patients receiving transfusion therapy.
Both the summary report and the full guideline are available at http://www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines/.
FROM JAMA
Major finding: The two most widely available disease-modifying therapies for SCD, hydroxyurea and long-term transfusions, are underused, in large part because no evidence-based treatment protocols have been devised until now.
Data source: A review of the literature and compilation of management guidelines "to assist health care professionals in the management of common issues of sickle cell disease."
Disclosures: The National Heart, Lung, and Blood Institute sponsored the development of this guideline. All expert panel members served voluntarily. Many reported numerous ties to industry sources.