User login
Myeloproliferative neoplasms increase risk for arterial and venous thrombosis
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Clinical question: What are the risks for arterial and venous thrombosis in patients with myeloproliferative neoplasms (MPNs)?
Background: Myeloproliferative neoplasms include polycythemia vera, essential thrombocythemia, and primary myelofibrosis. Prior studies have investigated the incidence of arterial and venous thrombosis in patients with myeloproliferative neoplasms, but the actual magnitude of thrombosis risk relative to the general population is unknown.
Study design: Retrospective matched-cohort study.
Setting: Sweden, using the Swedish Inpatient and Cancer Registers.
Synopsis: Using data from 1987 to 2009, 9,429 patients with MPNs were compared with 35,820 control participants to determine hazard ratios for arterial thrombosis, venous thrombosis, and any thrombosis. The highest hazard ratios were seen within 3 months of MPN diagnosis, with hazard ratios of 4.0 (95% confidence interval, 3.6-4.4) for any thrombosis, 3.0 (95% CI, 2.7-3.4) for arterial thrombosis, and 9.7 (95% CI, 7.8-12.0) for venous thrombosis. Risk decreased but remained significantly elevated through follow-up out to 20 years after diagnosis. This decrease was thought to be caused by effective thromboprophylactic and cytoreductive treatment of the MPN.
This study demonstrates significantly elevated risk for thrombosis in patients with MPNs, highest shortly after diagnosis. It suggests the importance of timely diagnosis and treatment of MPNs to decrease early thrombosis risk.
Bottom line: Patients with MPNs have increased rates of arterial and venous thrombosis, with the highest rates within 3 months of diagnosis.
Citation: Hultcrantz M et al. Risk for arterial and venous thrombosis in patients with myeloproliferative neoplasms. Ann Intern Med. 2018 Mar 6;168(5):317-25.
Dr. Komsoukaniants is a hospitalist at UC San Diego Health and an assistant clinical professor at the University of California, San Diego.
Steroids do not reduce mortality in patients with septic shock
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Among patients with septic shock undergoing mechanical ventilation, does hydrocortisone reduce 90-day mortality?
Background: Septic shock is associated with a significant mortality risk, and there is no proven pharmacologic treatment other than fluids, vasopressors, and antimicrobials. Prior randomized, controlled trials have resulted in mixed outcomes, and meta-analyses and clinical practice guidelines also have not provided consistent guidance.
Study design: Randomized, controlled, double-blinded trial.
Setting: Medical centers in Australia, Denmark, New Zealand, Saudi Arabia, and the United Kingdom.
Synopsis: Over a 4-year period from 2013 to 2017, 3,658 patients with septic shock undergoing mechanical ventilation were randomized to receive either a continuous infusion of 200 mg/day of hydrocortisone for 7 days or placebo. The primary outcome, death within 90 days, occurred in 511 patients (27.9%) in the hydrocortisone group and in 526 patients (28.8%) in the placebo group (P = .50).
In secondary outcome analyses, patients in the hydrocortisone group had faster resolution of shock (3 vs. 4 days; P less than .001) and a shorter duration of initial mechanical ventilation (6 vs. 7 days; P less than .001), and fewer patients received blood transfusions (37.0% vs. 41.7%; P = .004). There was no difference in mortality at 28 days, recurrence of shock, number of days alive out of the ICU and hospital, recurrence of mechanical ventilation, rate of renal replacement therapy, and incidence of new-onset bacteremia or fungemia.
Bottom line: Administering hydrocortisone in patients with septic shock who are undergoing mechanical ventilation does not reduce 90-day mortality.
Citation: Venkatesh B et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. 2018 Jan 19. doi: 10.1056/NEJMoa1705835.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Prompting during rounds decreases lab utilization in patients nearing discharge
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Clinical question: Does prompting hospitalists during interdisciplinary rounds to discontinue lab orders on patients nearing discharge result in a decrease in lab testing?
Background: The Society of Hospital Medicine, as part of the Choosing Wisely campaign, has recommended against “repetitive complete blood count and chemistry testing in the face of clinical and lab stability.” Repeated phlebotomy has been shown to increase iatrogenic anemia and patient discomfort. While past interventions have been effective in decreasing lab testing, this study focused on identifying and intervening on patients who were clinically stable and nearing discharge.
Study design: Prospective, observational study.
Setting: Tertiary care teaching hospital in New York.
Synopsis: As part of structured, bedside, interdisciplinary rounds, over the course of a year, this study incorporated an inquiry to identify patients who were likely to be discharged in the next 24-48 hours; the unit medical director or nurse manager then prompted staff to discontinue labs for these patients when appropriate. This was supplemented by education of clinicians and regular review of lab utilization data with hospitalists.
The percentage of patients with labs ordered in the 24 hours prior to discharge decreased from 50.1% in the preintervention period to 34.5% in the postintervention period (P = .004). The number of labs ordered per patient-day dropped from 1.96 to 1.83 (P = .01).
Bottom line: An intervention with prompting during structured interdisciplinary rounds decreased the frequency of labs ordered for patients nearing hospital discharge.
Citation: Tsega S et al. Bedside assessment of the necessity of daily lab testing for patients nearing discharge. J Hosp Med. 2018 Jan 1;13(1):38-40.
Dr. Huang is associate chief of the division of hospital medicine at UC San Diego Health and an associate professor of medicine at the University of California, San Diego.
Strategies to Improve Hepatocellular Carcinoma Surveillance in Veterans With Hepatitis B Infection (FULL)
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
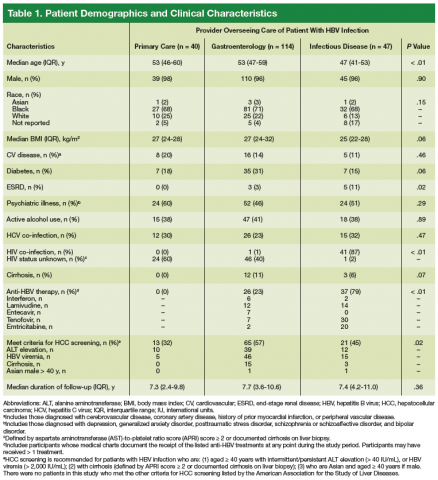
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
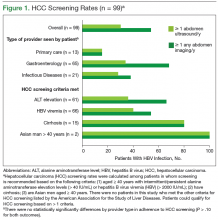
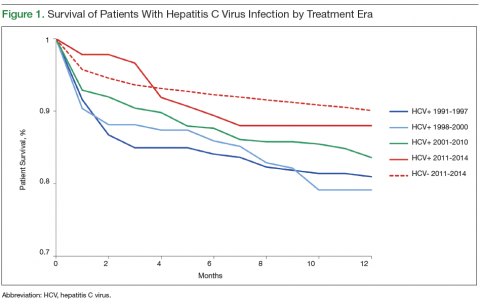
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
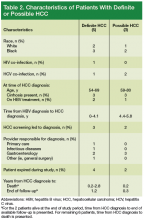
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
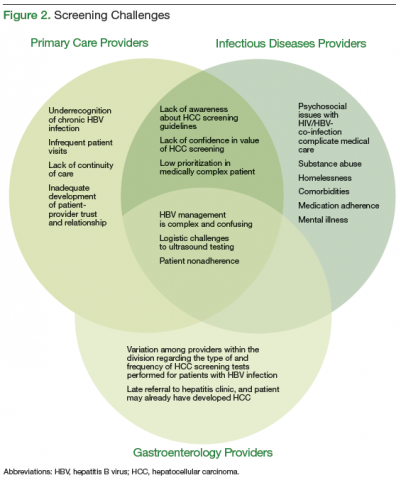
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
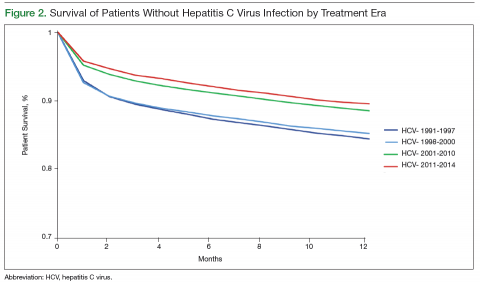
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
The incidence of hepatocellular carcinoma (HCC) is rising in the U.S., with an estimated 8,500 to 11,500 new cases occurring annually, representing the ninth leading cause of U.S. cancer deaths.1,2 An important risk factor for HCC is infection with hepatitis B virus (HBV), an oncogenic virus. Patients with HBV infection have an associated 5- to 15-fold increased risk of HCC, compared with that of the general population.3 Despite clinician awareness of major risk factors for HCC, the disease is often diagnosed at an advanced stage when patients have developed a high tumor burden or metastatic disease and have few treatment options.4
It is well recognized that U.S. veterans are disproportionately affected by hepatitis C virus (HCV) infection, which also places them at risk for HCC. In contrast, the prevalence of HBV infection, which has shared routes of transmission with HCV, and its associated complications among U.S. veterans has not been fully characterized. A recent national study showed that 1% of > 2 million veterans tested for HBV infection had a positive hepatitis B surface antigen (HBsAg), indicating active HBV infection.5
Routine surveillance for HCC among high-risk patients, such as those with chronic HBV infection, can lead to HCC detection at earlier stages, allowing curative treatments to be pursued more successfully.6-9 Furthermore, HBV infection can promote development of HCC even in the absence of cirrhosis.10,11 Therefore, according to the American Association for the Study of Liver Diseases (AASLD) guidelines, HCC screening with abdominal ultrasound is recommended every 6 to 12 months for patients with chronic HBV infection who have additional risk factors for HCC, including those aged ≥ 40 years, and patients with cirrhosis or elevated alanine aminotransferase levels (ALTs).10
Overall adherence to HCC screening recommendations in the U.S. has been low, although rates have varied depending on the underlying risk factor for HCC, provider type, patient characteristics, and practice setting.12-20 In a 2012 systematic review, the pooled HCC surveillance rate was 18.4%, but nonwhite race, low socioeconomic status, and follow-up in primary care (rather than in subspecialty clinics) were all associated with lower surveillance rates.18 Low rates of HCC screening also have been seen among veterans with cirrhosis and chronic HCV infection, and a national survey of VHA providers suggested that provider- and facility-specific factors likely contribute to variation in HCC surveillance rates.14
There are few data on HCC incidence and surveillance practices specifically among veterans with chronic HBV infection. Furthermore, the reasons for low HCC surveillance rates or potential interventions to improve adherence have not been previously explored, although recent research using national VA data showed that HCC surveillance rates did not differ significantly between patients with HBV infection and patients with HCV infection.14
Considering that veterans may be at increased risk for chronic HBV infection and subsequently for HCC and that early HCC detection can improve survival, there is a need to assess adherence to HCC screening in VA settings and to identify modifiable factors associated with the failure to pursue HCC surveillance. Understanding barriers to HCC surveillance at the patient, provider, and facility level can enable VA health care providers (HCPs) to develop strategies to improve HCC screening rates in the veteran population.
Methods
The authors conducted a mixed-methods study at the Corporal Michael J. Crescenz VAMC (CMCVAMC) in Philadelphia, Pennsylvania. Both quantitative and qualitative data were collected to evaluate current HCC screening practices for patients with HBV infection and to identify barriers to adherence to nationally recommended screening guidelines. The CMCVAMC Institutional Review Board approved all study activities.
Inclusion Criteria
Patients were included in the quantitative study if they had ≥ 1 positive HBsAg test documented between September 1, 2003 and August 31, 2008; and ≥ 2 visits to a CMCVAMC provider within 6 months during the study period. Patients who had negative results on repeat HBsAg testing in the absence of antiviral therapy were excluded. From September 1, 2003 to December 31, 2014, the authors reviewed the Computerized Patient Record System (CPRS) medical records of eligible patients. Patients were assigned to a HCP group (ie, infectious disease [ID], gastroenterology [GI], or primary care) identified as being primarily responsible for management of their HBV infection.
Focus Group Implementation
Separate focus group discussions were held for primary care (2 focus groups), ID (1 focus group), and GI (1 focus group) providers, for a total of 4 focus groups. The focus group discussions were facilitated by 1 study team member (who previously had worked but had no affiliation with CMCVAMC at the time of the study). All CMCVAMC HCPs involved in the care of patients with chronic HBV infection were sent a letter that outlined the study goals and requested interested HCPs to contact the study team. The authors developed a focus group interview guide that was used to prompt discussion on specific topics, including awareness of HCC screening guidelines, self-reported practice, reasons behind nonadherence to screening, and potential interventions to improve adherence. No incentives were given to HCPs for their participation.
HCC Screening Guidelines
The main study endpoint was adherence to HCC screening guidelines for patients with HBV infection, as recommended by the AASLD.9 Specifically, AASLD guidelines recommend that patients with HBV infection at high risk for HCC should be screened using abdominal or liver ultrasound examination every 6 to 12 months. High risk for HCC was defined as: (1) presence of cirrhosis; (2) aged > 40 years and ALT elevation and/or high HBV DNA level > 2,000 IU/mL; (3) family history of HCC; (4) African Americans aged > 20 years; or (5) Asian men aged > 40 years and Asian women aged > 50 years.10
Cirrhosis was defined by documented cirrhosis diagnosis on liver biopsy or by aspartate aminotransferase-to-platelet ratio index (APRI) ≥ 2, which accurately identifies cirrhosis (METAVIR stage F4) in patients with chronic HBV.21 For each patient qualifying for HCC screening, the annual number of abdominal ultrasounds performed during the study period was determined, and adherence was defined as having an annual testing frequency of ≥ 1 ultrasound per year.
Providers may not have obtained a screening ultrasound if another type of abdominal imaging (eg, computed tomography [CT] or magnetic resonance imaging [MRI]) had been performed for a separate indication and could be reviewed to evaluate for possible HCC. Therefore, the annual number of all abdominal imaging tests, including ultrasound, CT, and MRI, also was determined. Adherence, in this case defined as having ≥ 1 abdominal imaging test per year, was evaluated as a secondary endpoint.
To evaluate whether providers were recommending HCC screening, CPRS records were reviewed using the following search terms: “HCC,” “ultrasound,”
“u/s,” “hepatitis B,” and “HBV.” Patients whose CPRS records did not document their HBV infection status or mention HCC screening were identified.
HCC Diagnoses
Incident HCC diagnoses were identified during the study period, and the diagnostic evaluation was further characterized. An HCC diagnosis was considered definite if the study participant had an ICD-9 code recorded for HCC (ICD-9 155.0) or histologic diagnosis of HCC by liver biopsy. The use of an ICD-9 code for HCC diagnosis had been validated previously in a retrospective chart review of VA data.22 An HCC diagnosis was considered possible if the participant did not meet the aforementioned definition but had radiographic and clinical findings suggestive of HCC.
Statistical Analyses
Differences in the demographic and clinical characteristics of patients with HBV infection seen by primary care, GI, and ID providers were assessed using chi-square or Fisher exact tests for categoric data and analysis of variance or Kruskal-Wallis tests, as appropriate, for continuous data. The
proportion and 95% confidence interval (CI) of patients with adherence to HCC screening guidelines were determined by provider type. Differences in outcomes by provider group were evaluated using chi-square tests. The proportions of patients whose CPRS records did not mention their HBV infection status or address HCC screening were determined. Last, HCC incidence (diagnoses/person-years) was determined by dividing the number of definite or possible HCC cases by the total follow-up time in person-years among those with and without cirrhosis (defined earlier) as well as in those who met criteria for HCC surveillance.
For the qualitative work, all focus group discussions were recorded, and transcripts were reviewed by 3 members of the study team to categorize responses into themes, using an iterative process. Discrepancies in coding of themes were resolved by mutual agreement among the reviewers. Analysis focused on highlighting the similarities and differences among the different specialties and identifying strategies to improve provider adherence to HCC screening guidelines.
Results
Among 215 patients with a positive HBsAg test between September 1, 2003 and August 31, 2008, 14 patients were excluded because they had either a negative HBsAg test on follow-up without antiviral treatment or were not retained in care. The final study population included 201 patients with a median follow-up of 7.5 years. Forty (20%) had their HBV infection managed by primary care, while 114 (57%) had GI, and 47 (23%) had ID providers. There were 15 patients who had no documentation in the CPRS of being chronically infected with HBV despite having a positive HBsAg test during the study period.
Patients with HBV infection seen by the different provider groups were fairly similar with respect to sex, race, and some medical comorbidities (Table 1). All but 1 of the patients co-infected with HIV/HBV was seen by ID providers and were younger and more likely to receive anti-HBV therapy than were patients who were HBV mono-infected. Patients with cirrhosis or other risk factors that placed them at increased risk for HCC were more likely to be followed by GI providers.
According to AASLD recommendations, 99/201 (49.3%) of the cohort qualified for HCC screening (Figure 1). Overall adherence to HCC screening was low, with only 15/99 (15%) having ≥ 1 annual abdomen ultrasound. Twenty-seven patients (27%) had ≥ 1 type of abdominal imaging test (including ultrasound, CT, and MRI scans) performed annually. Although primary care HCPs had lower adherence rates compared with that of the other provider groups, these differences were not statistically significant (P > .1 for all comparisons).
During the study period, 5 definite and 3 possible HCC cases were identified (Table 2). Routine screening for HCC led to 5 diagnoses, and the remaining 3 cases were identified during a workup for abnormal examination findings or another suspected malignancy. Among the 8 patients with a definite or a possible diagnosis, 5 were managed by GI providers and 6 had cirrhosis by the time of HCC diagnosis. All but 2 of these patients died during the study period from HCC or related complications. Incidence of HCC was 2.8 and 0.45 cases per 100 person-years in those with and without cirrhosis, respectively. Among those meeting criteria for HCC surveillance, the incidence of HCC was 0.88 cases per 100 person-years overall.
Barriers to Guideline Adherence
Nineteen providers participated in the focus group discussions (9 primary care, 5 GI, and 5 ID). Physicians and nurse practitioners (n = 18; 95%) comprised the majority of participants. Health care providers had varying years of clinical experience at the CMCVAMC, ranging from < 1 year to > 20 years.
The authors identified 3 categories of major barriers contributing to nonadherence to HCC screening guidelines: (1) knowledge barriers, including
underrecognition of chronic HBV infection and lack of awareness about HCC screening guidelines; (2) motivational barriers to recommending HCC screening; and (3) technical/logistic challenges. Additional time was spent in the focus groups devising strategies to address identified barriers. An overlap in barriers to screening adherence was identified by the different HCPs (Figure 2).
Underrecognition of Chronic HBV Infection
For patients to receive appropriate HCC screening, HCPs first must be aware of their patients’ HBV infection status. However, in all the focus groups, providers indicated that chronic HBV infection likely is underdiagnosed in the veteran population because veterans at risk for HBV acquisition might not be tested, HBV serologic tests may be misinterpreted, and there may be failure to communicate positive test results during provider transitions, such as from the inpatient to outpatient setting. Typically, new HBV diagnoses are identified by CMCVAMC primary care and ID physicians, the latter serving as primary care providers (PCPs) for patients with HIV infection. All primary care and ID providers routinely obtained viral hepatitis screening in patients new to their practice, but they stated that they may be less likely to pursue HCC screening for at-risk patients.
Providers suggested implementing HBV-specific educational campaigns throughout the year to highlight the need for ongoing screening and to provide refreshers on interpretation of HBV screening serologies. They advised that, to increase appeal across providers, education should be made available in different formats, including seminars, clinic handouts, or online training modules.
An important gap in test result communication was identified during the focus group discussions. Veterans hospitalized in the psychiatric ward undergo HBV and HCV screenings (ie, testing for HBsAg, hepatitis B surface antibody, and HCV antibody) on admission, but no clear protocol ensured that positive screening tests were followed up in the outpatient setting. The majority of providers indicated that all newly identified diagnoses of HBV infection should receive at least an initial evaluation by a GI provider. Therefore, during discussion with the GI providers, it was proposed that the laboratory automatically notify the viral hepatitis clinic about all positive test results and the clinic designate a triage nurse to coordinate appropriate follow-up and GI referral as needed.
Unaware of HCC Screening Guidelines
Both primary care and ID providers reported that a lack of familiarity with HCC screening guidelines likely contributed to low screening rates at the CMCVAMC. Most discussants were aware that patients with HBV infection should be screened for HCC, but they did not know which test to perform, which patients to screen, and how often. Further, providers reported that chronic HBV infection was seen less frequently than was chronic HCV infection, contributing to reduced familiarity and comfort level with managing patients with HBV infection. Several participants from both primary care and ID provider groups stated they extrapolated guidelines from chronic HCV management in which HCC screening is recommended only for patients with cirrhosis and applied them to patients with HBV infection.23 In contrast, GI providers reported that they were knowledgeable about HCC screening recommendations and routinely incorporated AASLD guidelines into their practice.
To address this varying lack of awareness, all providers reiterated their support for the development of educational campaigns to be made available in different formats about HBV-related topics, including ongoing screening and interpretation of HBV screening serologies. In addition, primary care and GI providers agreed that all newly identified cases of HBV infection should receive an initial assessment by a GI provider who could outline an appropriate management strategy and determine whether GI or primary care follow-up was appropriate. In contrast, the ID providers did not endorse automatic referral to the GI clinic of new HBV diagnoses in their patients with HIV infection. Instead, ID providers stated that they were confident they could manage chronic HBV infection in their patients with HIV infection independently and refer patients as needed.
Motivational Barriers
Lack of confidence in the value of HCC screening for patients with chronic HBV infection was prevalent among primary care and ID physicians and led to reduced motivation to pursue screening tests. One provider noted that HCC is a “rare enough event that the utility of screening for this in our patient population is unclear.” Both sets of providers contrasted their different approaches to colon cancer and HCC screening: Colon cancer screening “has become more normalized and [we] have good data that early detection improves survival.” Another provider said, “There is lack of awareness about the potential benefit of HCC screening.”
Acknowledging that most patients have multiple comorbidities and often require several tests or interventions, providers in both primary care and the ID focus groups reported that it was difficult to prioritize HCC screening. Among ID physicians who primarily see patients who are co-infected with HIV/HBV, adherence to antiretroviral therapy (along with social issues, including homelessness and active substance use) often predominates clinical visits. Consequently, one participant stated, “Cancer screening goes down on the list of priorities.”
Technical Challenges
All providers identified health system and patientspecific factors that prevent successful adherence to HCC screening guidelines. At the study site, to obtain an ultrasound, the provider completes a requisition that goes directly to the radiology department, which is then responsible for contacting the patient and scheduling the ultrasound test. Ultrasound requisitions can go uncompleted for various reasons, including (1) inability to contact patients because of inaccurate contact information in the medical records; (2) long delays in test scheduling, leading to forgotten or missed appointments; and (3) lack of protocol for rescheduling missed appointments.
All providers agreed that difficulty in getting their patients to follow through on ordered tests is a major impediment to successful HCC surveillance. All providers described patient-specific factors that contribute to low HCC surveillance rates, poor medication adherence, and challenges to the overall care of these patients. These factors included active substance use, economic difficulties, and comorbidities. In addition, providers reported that alternative screening tests that could be administered at the time of the clinic visit, such as blood draws or fecal occult blood test cards, were more likely to be completed successfully in their individual practices.
Furthermore, there was variation in the way providers described the test rationale to patients, which they agreed may influence a patient’s likelihood of obtaining the test. Some providers informed their patients that the ultrasound test was intended to screen specifically for liver cancer, and they believed that concern about possible malignancy motivated patients to follow through with this testing. One of the GI providers noted that his
patients obtained recommended HCC screening because they had faced other serious consequences of HBV infection and were motivated to avoid further complications. However, other providers expressed concern that mentioning cancer might generate undue patient anxiety and instead described the test to patients as a way of evaluating general liver health. They acknowledged that placing less importance on the ultrasound test may lead to lower patient adherence.
Primary care and ID providers suggested that educational campaigns developed especially for patients may help address some of these patient specific factors. Referring to the success of public service announcements about colon cancer screening or direct-to-consumer advertising of medications, providers felt that similar approaches would be valuable for educating high-risk patients about the potential benefits of HCC surveillance and early detection.
Discussion
In this study, an extremely low HCC surveillance rate was observed among veterans with chronic HBV infection, despite HCC incidence rates that were comparable with those observed among patients in Europe and North America.24 Importantly, the incidence rate among those who met HCC surveillance criteria in this study was 0.88 cases per 100 person-years, which exceeded the 0.2 theoretical threshold incidence for efficacy of surveillance.4 This study adds to the growing body of literature demonstrating poor adherence to HCC surveillance among high-risk groups, including those with cirrhosis and chronic HCV and HBV infections.5,14,25 Because of the missed opportunities for HCC surveillance in veterans with HBV infection, the authors explored important barriers and potential strategies to improve adherence to HCC screening. Through focus groups with an open-ended discussion format, the authors were able to more comprehensively assess barriers to screening and discuss possible interventions, which had not been possible in prior studies that relied primarily on surveys.
Barriers to Screening
Underrecognition of HBV infection was recognized as a major barrier to HCC screening and likely contributed to the low HCC surveillance rates seen in this study, particularly among PCPs, who generally represent a patient’s initial encounter with the health care system. Among veterans with positive HBsAg testing during the study period, 7% had no chart documentation of being chronically infected with HBV. Through focus group discussions, it became clear that these missed cases were most frequently due to misinterpretation of HBV serologies or incomplete handoff of test results.
To prevent these errors, an automated notification process was proposed and is being developed at the CMCVAMC, whereby GI providers evaluate all positive HBsAg tests received by the laboratory to determine the appropriate follow-up. Another approach previously shown to be successful in increasing disease recognition and follow-up is the integration of hepatitis care services into other clinics (eg, substance use disorder) that serve veterans who have a high prevalence of viral hepatitis and/or risk factors.26 Proper identification of all chronic HBV patients who may need screening for HCC is the first step toward improving HCC surveillance rates.
Lack of information about HCC screening guidelines and evidence supporting screening recommendations was a recurring theme in all the focus groups and may help explain varying rates of screening adherence among the providers. Despite acknowledging the lack of awareness about screening guidelines, ID specialists were less likely than were PCPs to endorse a need for GI referral for all patients with HBV infection.
Infectious disease providers emphasized motivational barriers to HCC surveillance, which were driven by their lack of confidence in the sensitivity of the screening test and lack of awareness of improved survival with earlier HCC diagnosis. Within the past few years, studies have challenged the quality of existing evidence to support routine HCC surveillance, which possibly fueled these providers’ uncertainty about its relevance for their patients with HBV infection.27,28 Nonetheless, there seems to be limited feasibility for obtaining additional high-quality data to clarify this issue, possibly through randomized controlled trials, because of sufficient existing patient and provider preference for conducting HCC surveillance.29
The GI providers who routinely treat HCC are likely to have a different perspective from PCPs about the frequency of HCC occurrence in chronic HBV infection and the demonstrable survival benefit with early detection and thus may have greater motivation to pursue screening. Similarly, providers observed that patients who understood that the abdominal ultrasound was for the early detection of liver cancer seemed to be more likely to be adherent with providers’ ultrasound recommendations. In the absence of a clear understanding of the potential benefits of HCC screening tests, providers may be more reluctant to recommend the tests and patients may be less likely to complete them.
Education
To address these knowledge and motivational barriers, providers emphasized the need for educational opportunities designed to close these knowledge gaps and provide resources for additional information. Given the differing levels of training and experience among providers, educational programs should be multifaceted and encompass different modalities, such as in-person seminars, online training modules, and clinic-based reminders, to reach all HCPs.
Additionally, providers advocated implementing educational efforts aimed at high-risk patients to raise awareness about liver cancer. Because such programs can provide more information than can be conveyed during a brief clinic visit, they may help quell patient anxiety that is induced by the idea of liver cancer screening—an important concern expressed by various providers.
Adherence to any recommended test or medication regimen has been shown to be inversely linked to the technical or logistic complexity of the recommendation.30 At CMCVAMC an unwieldy process for obtaining abdominal or liver ultrasounds—the recommended HCC screening test—contributed to low rates of HCC surveillance. Providers noted anecdotally that screening tests that could be given during the clinic visit, such as blood draws or even fecal occult blood test cards, were more likely to be successfully completed than tests that required additional outside visits. There is no standard approach for scheduling screening sonography across the VA system, but studying screening adherence at various facilities could help identify best practices that warrant national implementation. Proposing changes to the process for ordering and obtaining an ultrasound were outside the scope of this study, given that it did not involve additional relevant staff such as radiologists and ultrasound technicians. However, this area represents future investigation that is needed to achieve substantial improvements to HCC surveillance rates within the VA health system.
Limitations
This study should be interpreted in the context of several potential limitations. The retrospective study design limited the authors to the existing CPRS data. However, chart review primarily focused on abstracting objective data, such as the number of abdominal imaging studies performed, to arrive at a quantitative measure of HCC surveillance that likely was subject to less bias. The findings of the study, conducted at a single VA facility in Philadelphia, may not be generalizable beyond a veteran population in an urban setting. In addition, providers in the focus groups were self-motivated to participate and might not represent the experiences of other providers. Last, the relatively small number of patients seen by the different HCPs in this study may have precluded having sufficient power to detect differences in adherence rates at the provider level.
Conclusion
An extremely low HCC surveillance rate was observed among veterans with chronic HBV infection in this study. Health care providers at the CMCVAMC identified multiple challenges to ensuring routine HCC surveillance in high-risk HBV-infected patients that likely have contributed to the extremely low rates of HCC observed over the past decade.
In this qualitative study, although broad themes and areas of agreement emerged across the different HCP groups involved in caring for patients with HBV infection, there were notable differences between groups in their approaches to HCC surveillance. Engaging with HCPs about proposed interventions based on the challenges identified in the study focus groups resulted in a better understanding of their relative importance and the development of interventions more likely to be successful.
Click here to read the digital edition.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
1. Centers for Disease Control and Prevention. Hepatocellular carcinoma—United States 2001—2006. Morb Mortal Wkly Rep. 2010;59(17):517-520. Updated May 7, 2010. Accessed April 4, 2017.
2. El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127(5)(suppl 1):S27-S34.
3. El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557-2576.
4. Bruix J, Sherman M; American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020-1022.
5. Serper M, Choi G, Forde KA, Kaplan DE. Care delivery and outcomes among US
veterans with hepatitis B: a national cohort study. Hepatology. 2016;63(6):1774-1782.
6. El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Therap Adv Gastroenterol. 2011;4(1):5-10.
7. Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30(1):37-47.
8. Stravitz RT, Heuman DM, Chand N, et al. Surveillance for hepatocellular carcinoma in patients with cirrhosis improves outcome. Am J Med. 2008;121(12):119-126.
9. Tong MJ, Blatt LM, Kao VW. Surveillance for hepatocellular carcinoma in patients with chronic viral hepatitis in the United States of America. J Gastroenterol Hepatol. 2001;16(5):553-559.
10. Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661-662.
11. Trépo C, Chan HL-Y, Lok A. Hepatitis B virus infection. The Lancet. 2014;384(9959):2053-2063.
12. Bharadwaj S, Gohel TD. Perspectives of physicians regarding screening patients at risk of hepatocellular carcinoma. Gastroenterol Rep. 2016;4(3):237-240.
13. Chalasani N, Said A, Ness R, Hoen H, Lumeng L. Screening for hepatocellular carcinoma in patients with cirrhosis in the United States: results of a national survey. Am J Gastroenterol. 1999;94(8):2224-2229.
14. El-Serag HB, Alsarraj A, Richardson P, et al. Hepatocellular carcinoma screening practices in the Department of Veterans Affairs: findings from a national facility survey. Dig Dis Sci. 2013;58(11):3117-3126.
15. Khalili M, Guy J, Yu A, et al. Hepatitis B and hepatocellular carcinoma screening among Asian Americans: survey of safety net healthcare providers. Dig Dis Sci. 2011;56(5):1516-1523.
16. Leake I. Hepatitis B: AASLD guidelines not being followed. Nat Rev Gastroenterol Hepatol. 2014;11(6):331.
17. Patwardhan V, Paul S, Corey KE, et al. Hepatocellular carcinoma screening rates vary by etiology of cirrhosis and involvement of gastrointestinal sub-specialist. Dig Dis Sci. 2011;56(11):3316-3322.
18. Singal AG, Yopp A, Skinner SC, Packer M, Lee WM, Tiro JA. Utilization of hepatocellular carcinoma surveillance among American patients: a systematic review. J Gen Intern Med. 2012;27(7):861-867.
19. Wong CR, Garcia RT, Trinh HN, et al. Adherence to screening for hepatocellular carcinoma among patients with cirrhosis or chronic hepatitis B in a community setting. Dig Dis Sci. 2009;54(12):2712-2721.
20. Wu Y, Johnson KB, Roccaro G, et al. Poor adherence to AASLD guidelines for chronic hepatitis B management and treatment in a large academic medical center. Am J Gastroenterol. 2014;109(6):867-875.
21. Kim BK, Kim DY, Park JY, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int. 2010;30(4):546-553.
22. Kramer JR, Giordano TP, Souchek J, Richardson P, Hwang LY, El-Serag HB. The effect of HIV coinfection on the risk of cirrhosis and hepatocellular carcinoma in U.S. veterans with hepatitis C. Am J Gastroenterol. 2005;100(1):56-63.
23. Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
24. El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142(6):1264-1273.e1.
25. Davila JA, Henderson L, Kramer JR, et al. Utilization of surveillance for hepatocellular carcinoma among hepatitis C virus-infected veterans in the United States. Ann Intern Med. 2011;154(2):85-93.
26. Hagedorn H, Dieperink E, Dingmann D, et al. Integrating hepatitis prevention services into a substance use disorder clinic. J Subst Abuse Treat. 2007;32(4):391-398.
27. Kansagara D, Papak J, Pasha AS, et al. Screening for hepatocellular carcinoma in chronic liver disease: a systematic review. Ann Intern Med. 2014;161(4):261-269.
28. Lederle FA, Pocha C. Screening for liver cancer: the rush to judgment. Ann Intern Med. 2012;156(5):387-389.
29. Poustchi H, Farrell GC, Strasser SI, Lee AU, McCaughan GW, George J. Feasibility of conducting a randomized control trial for liver cancer screening: is a randomized controlled trial for liver cancer screening feasible or still needed? Hepatology. 2011;54(6):1998-2004.
30. Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence.
Ther Clin Risk Manag. 2005;1(3):189-199.
Sharpen your ax
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Recently, I had a trauma call at my scenic little hospital in Maine. “Bleeding leg wound, Dr, Crosslin. We’ve got pressure on it. Come soon.” During my jog across the parking lot to the ER, I drifted into my residency mantra and started reciting the ABCs of trauma care:
Airway, Breathing, CT scan.
Airway, Breathing, C-spine collar.
Airway, Breathing, Consult with ortho.
Okay, so it’s been a while. Four years doesn’t seem like a long time, but that little span serves up a lot of change. You settle into a routine in your isolated, bucolic New England coastal town, where most trauma is related to hauling up lobster crates and having Massachusetts drivers scare the moxie out of locals in the crosswalks, and you forget about the hundreds of Level 1 traumas you managed over 5 years in Boston. The drilled-down, rapid sequence of the primary and secondary surveys gets lost, if just for a moment. Your confident swagger is replaced with a measured, humble shuffle into Trauma Bay 1. Do I scan the leg now? Did I feel for pulses in the foot? Wait, where do the major vessels branch again?
After addressing the issue at hand (or in this case, at foot), I kept thinking about the woodsman’s statement. I reflected on how I felt when I entered the trauma bay. Had I been doing enough to keep my own mental tools sharp? Well, actually, no. When did things slip just enough to allow hesitation and a bit of doubt to creep in? Probably sooner than I would care to admit. I certainly don’t think it took all of these 4 years for it to happen.
There has been some discussion of late surrounding the changes to maintenance of certification requirements from the American Board of Surgery. As with anything in surgery, we all need a chance to grumble about how things were better in the good old days. But then we grudgingly have to acknowledge that maybe – just maybe – the new approach makes some sense.
Did anyone really enjoy reporting on a 3-year cycle and taking a high-stakes, nausea-inducing exam every 10 years? I certainly wasn’t looking forward to reporting in this year about my “progress,” especially given how dull I seem to have become in so many subcategories just 4 years after graduation. But reporting every 5 years? That appeals to my inner slacker. Having a more-frequent-but-way-less-stressful examination that can be tailored to my practice? Yes, I’ll give that a shot.
It’s no secret we all are driven to care more about the things we enjoy doing, and educational science has established, quite firmly, the increased likelihood of concrete learning in higher numbers of loosely related fields when the primary subject is of particular interest to the learner. Elementary school teachers implemented that particular tidbit a long time ago. For me, the drive to excel leads me to the oncology, endocrine, and complex hernia reconstruction arenas. I do not pretend to be the world’s authority on trauma surgery, or anorectal surgery, or vascular surgery. I leave that expertise to others I secretly have judged to be far more pathological than myself. But I would be willing to glean more from reviewing those particular subjects if the overall focus is geared toward improving my knowledge and skill in cancer surgery.
In this ultramodern era, when the compendium of medical and surgical knowledge infinitely outpaces our ability to provide “one-stop shopping” services, perhaps it is time we accept the limitations of our interests and our abilities as part of the natural, beneficial evolution of good medical practice. The College’s willingness to work with the ABS to address the hot-button issue of continuing education in an interactive, relevant, timely manner should be a major point of pride. Rather than clinging to the dull ways of the past, I think we all are going to benefit from carrying a collectively sharper ax.
Dr. Crosslin is a general surgeon practicing in Rockport, Maine.
Heart Transplantation Outcomes in Patients With Hepatitis C Virus Infection: Potential Impact of Newer Antiviral Treatments After Transplantation (FULL)
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
More than 185 million people worldwide, including more than 4 million in the U.S., are infected with the hepatitis C virus (HCV).1 Because of the indolent nature of the disease, actual prevalence is underestimated.2,3 Detection of HCV in people already infected is estimated to continue to increase over the next decade.4 Although primary manifestations of the disease are the result of liver damage, HCV infection is a systemic illness. In a study of more than 19,000 patients, HCV infection was identified as an independent risk factor for development of heart failure.5 In the U.S., prevalence of HCV infection in patients with heart failure is reported to be as high as 15%, much higher than the general population prevalence of 1.8%.6 When first identified in 1989, HCV infection was considered incurable. Clinical trials have since found a steady improvement in outcome, and now the disease is considered curable in up to 90% of cases.7
Clinical outcomes of heart transplantation (HTx) historically have been inferior in patients with HCV infection.8,9 The authors hypothesized that the literature on HTx outcomes has not accounted for the improvements in HCV infection treatment options that have occurred since the 1990s. In the study reported here, United Network of Organ Sharing (UNOS) data on adult HTx was used to evaluate clinical outcomes of HCV infection over 4 treatment eras.
Material and Methods
The authors analyzed UNOS data on adult HTx from January 1991 to March 2014. Two groups were created: patients with HCV infection (HC+) and noninfected patients (HC–). Eligible patients were aged > 18 years. Hepatitis C virus status was defined with antibody testing at time of HTx. Patients with multiorgan transplantation or with hepatitis B virus or HIV infection were excluded. For comparison of post-HTx survival, the 23-year study period was divided into 4 eras reflecting the evolution in HCV infection treatment options in the U.S. (Table 1). The first medication was interferon α (IFN-α), which was used alone (first era, 1991-1997) and then with the newly introduced ribavirin (second era, 1998-2000). The combination of IFN-α and ribavirin increased sustained virologic response rates, but the rates of adverse effects (AEs), such as cytopenia and depression, were high, and many patients could not tolerate the extended (48-week) regimen.10,11
Peginterferon, a long-acting IFN introduced in 2001, significantly increased adherence to 2-drug treatment for HCV infection, and its use in combination with ribavirin marked the third era (2001-2010). The fourth era (2011-2014) began with the introduction of direct-acting antiviral agents and their remarkable results. Since 2014, direct protease inhibitors without IFN found a dramatic impact on HCV treatment: fewer AEs, shorter treatment (24 weeks), and high (> 90%) sustained virologic response.7,12,13
Statistical Analysis
Categoric variables were analyzed with the χ2 test or the Fisher exact test and are reported as percentages. Continuous variables were analyzed with the Student t test or the Wilcoxon rank sum test and are reported as means, medians, and SDs. Statistical significance was set at P < .05. Survival curves were plotted with the Kaplan-Meier method, and comparisons made with log-rank tests. Analysis was performed with SAS Version 9.3 (Cary, NC).
Results
Between January 1991 and March 2014, adult HTx was performed 36,589 times, including 778 times (2.1%) in patients with HCV infection. There was no significant difference in percentage of HC+ patients who underwent HTx over the 4 treatment eras (first, 2.1%; second, 2.9%; third, 2.1%; fourth, 1.6%) (Table 2). Mean patient age for the HC+ and HC– groups was comparable. Percentage of African American patients was higher in the HC+ group than in the HC– group (18.9% vs 15.0%), as was percentage of patients of other race (11.2% vs 9.2%; P = .0008).
Regarding indications for HTx, ischemic (and nonischemic) cardiomyopathy was similar in prevalence between the 2 groups, but the “other” heart failure etiologies (congenital heart disease, valvular heart disease, postpartum cardiomyopathy, restrictive heart disease) were more prevalent in the HC+ group (12.9% vs 9.7%; P = .013). The HC+ group also had higher rates of tobacco use history (42.2% vs 40.1%; P = .002) and hypertension (23.1% vs 20.9%; P = .014). Mean (SD) bilirubin level at time of transplantation for the HC+ and HC– groups was comparable: 1.12 (1) mg/dL and 1.11 (1) mg/dL, respectively (P = .707). Of the heart donor variables (Table 3), only tobacco use history was significantly higher in the HC+ group (23.5% vs 19.8%; P = .008).
Survival Data
Mean (SD) overall follow-up was 6.2 (5.3) years (median, 5 years; range, 0-23.3 years) for all patients; 5.6 (4.3) years (median, 5.05 years; range, 0-23.2 years) for HC+ patients; and 6.2 (5.3) years (median, 6.1 years; range, 0-23.2 years) for HC– patients.
HC+ patients’ survival rates were 82.5% (1 year), 64.4% (5 years), and 42.1% (10 years), and HC– patients’ rates were 87.2% (1 year), 73.4% (5 years), and 54.7% (10 years). The HC+ group’s inferior survival at 1, 5, and 10 years was statistically significant (P < .0002) (Table 4).
During the first era (1991-1997), HC+ patients’ survival rates were 81.0% (1 year), 73.3% (2 years), and 61.4% (5 years), and HC– patients’ rates were 85.0% (1 year), 80.6% (2 years), and 70.3% (5 years) (P < .05). During the second era (1998-2000), HC+ patients’ rates were 79.1% (1 year), 74.6% (2 years), and 62.0% (5 years), and HC– patients’ rates were 85.4% (1 year), 81.7% (2 years), and 72.0% (5 years) (P < .05). During the third era (2001-2010), HC+ patients’ rates were 83.6% (1 year), 78.6% (2 years), and 66.8% (5 years), and HC– patients’ rates were 88.4% (1 year), 84.6% (2 years), and 75.4% (5 years) (P < .05).
Survival data for the fourth treatment era (2011-2014) were available only for 1 and 2 years. HC+ patients’ survival rates were 89.01% (1 year) and 81.89% (2 years), and HC– patients’ rates were 91.00% (1 year) and 86.00% (2 years). The inferior survival found for the HC+ group during the 3 preceding eras was not found this era, during which HC+ and HC– patients had comparable rates of survival at 1 year (Figures 1 and 2).
Survival 1 year after HTx was compared between the HC+ and HC– groups over the 4 treatment eras (Figures 3 and 4). The HC+ patients’ survival after HTx improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91% (P = .9).
Hepatic decompensation leading to death was uncommon, but the rate was significantly higher (P = .0001) in the HC+ group (2.8%) than in the HC– group (0.6%).
Discussion
HCV infection is a risk factor for the development of cardiovascular illness and advanced heart failure. Given the worldwide prevalence of HCV infection, more HC+ patients will be evaluated for HTx in the future.2-4 Significant progress has been made in HCV infection treatment since the virus was first described. What was once incurable now has up to a 90% cure rate with newer treatment options.7,12,13 The present study findings showed consistent improvement in HC+ patients’ post-HTx survival during each treatment era. During the latest era, HC+ and HC– patients’ post-HTx survival was statistically similar.
It is possible that HC+ patients’ improvement in post-HTx survival could have resulted from improvement in overall post-HTx survival.14 Over the 23-year study period, the survival rates of both groups (HC+, HC–) improved, likely secondary to improved immunosuppression and perioperative care, but the magnitude of improvement was more pronounced in the HC+ group. HC+ patients’ post-HTx survival improved from 81% during the earliest era (1991-1997) to 89% during the latest era (2011-2014), whereas HC– patients’ survival improved from 85% to 91%. The improvement in HC+ patients’ short-term survival over the study period was substantial but did not reach statistical significance (P = .9).
Over the study period, the percentage of HC+ patients who underwent HTx remained low, ranging from 2.9% during the second era (1998-2000) to 1.7% during the fourth era (2011-2014). Overall, only 2.2% of study patients were HC+ at time of transplantation—a rate similar to previously reported rates.8,15 This rate likely represents a selection bias for HTx listing, in which patients with nearly normal liver function were selected for HTx, as evident by normal bilirubin levels in both groups. In the present study, a high proportion of HC+ patients were not white. This distribution also was noted in epidemiologic studies of HCV infection by ethnicity.6 In the U.S., the highest prevalence of HCV infection was noted in African Americans and the lowest in whites. According to the U.S. census report, African Americans constitute 12% of the total U.S. population,16 whereas 22% of HC+ patients are African American.1
It has been postulated that the immunosuppression that accompanies the post-HTx state accelerates HCV disease progression and shortens HC+ patients’ survival.17,18 These concerns were not validated in the most recent studies of kidney, liver, and heart transplantation in HC+ patients.15,19,20 Furthermore, where post-HTx cause of death was examined in HC+ patients, death was attributed primarily to post-HTx malignancy and bacterial sepsis but seldom directly to hepatic failure. In a study in which liver function was serially monitored after HTx in 11 HC+ patients, immunosuppression did not affect HCV disease progression, and there was no liver function impairment.15 In the same study, the 3 more recent HTx patients (of the 11) had their serial HCV viral load monitored. Viral load remained steady in 2 of the patients and decreased in the third. Similarly, there has been a theoretical concern that heightened immunologic status in HC+ patients might lead to more frequent rejection episodes. However, this concern has not been substantiated in reported studies.21,22 In the present study, the rate of graft failure as the cause of death was 11.2% in HC+ patients and 13% in HC– patients, though the difference was not statistically significant. Thus, concerns about HCV reactivation during immunocompromise or during increased allograft rejection were not substantiated.
Vasculopathy is a leading cause of morbidity and mortality after HTx and seems to be influenced by HC+. In a study of HC+ donor hearts transplanted to HC– patients, the prevalence of HCV infection in the recipients was 75% at a mean follow-up of 4.2 years.23 Serial angiogram showed coronary vasculopathy in 46% of HC+ patients and 24% of HC– patients 3.2 years after HTx.23 Similar concerns were raised in another small study, in which 2 of 4 HC+ patient deaths at 3.7-year follow-up were attributed to cardiovascular causes with features similar to those of transplantation vasculopathy.24 Those findings contrast with the present findings of cardiovascular deaths in 17.6% of HC+ patients and 17.2% of HC– patients. Outcomes similar to the present outcomes were reported by Lee and colleagues: Post-HTx cardiovascular deaths occurred in 16.4% of HC+ patients and 15.2% of HC– patients.8
Survival data on HC+ patients who undergo HTx are mixed, with some studies finding similar shortterm and midterm post-HTx survival21,22 and others finding decreased survival.8,9 It is difficult to interpret survival results from these studies, as some have included HCV infection that developed after HTx,9 and others have excluded early postoperative deaths from analysis.21 In addition, in the larger of these studies, which spanned 15 years, propensity matching was used for survival analysis. 8
It is possible that the selection and treatment of HC+ patients who were awaiting or underwent HTx changed over the study period. Thus, it might not be accurate to compare HTx outcomes of patients without considering the significant progress that has been made in the management of HCV infection. Although the present study’s aggregate (23-year) post-HTx survival results at 1, 5, and 10 years were similar to those reported in large series of HC+ patients who underwent HTx, the present early and intermediate survival results showed consistent improvements over time.8,9 This improvement in survival of HC+ patients was not examined in previous studies.
In the present study, the distribution of causes of post-HTx deaths was diverse (Table 5). Although the leading causes of death were cardiovascular or were related to sepsis or multi-organ failure, deaths attributed to liver failure were uncommon. Only 2.8% of deaths in the HC+ group and 0.6% of deaths in the HC– group were attributed to liver failure (P ≤ .001). These findings are similar to the cause-specific mortality reported in the literature.8,9,15,22 It is possible that these mortality results may be secondary to selection of patients with preserved liver function.
Future of HCV Infection and Heart Transplantation
Modern diagnostic methods will be used to accurately assess HC+ patients for HCV disease burden, and treatment will be provided before HTx is performed. Historically, the major limitations to treating HC+ patients awaiting HTx have been the long (48 week) duration of therapy and the anemia that can exacerbate heart failure symptoms and shorten the safe HTx waiting time.25 Most of the HCV treatment AEs have been attributed to use of IFN-α. Newer HCV treatments (direct-acting protease inhibitors without IFN) are of shorter duration (24 weeks) and have fewer AEs and higher cure rates. Most important, these treatments obtain similar cure rates irrespective of viral load, viral genotype, patient race, and previous HCV response status.7,12,13
Authors researching other solid-organ transplantation (eg, liver, kidney) have studied HCV pretreatment and found sustained virologic response before and after transplantation.26,27 Although these studies were conducted before the advent of direct-acting protease inhibitors, the feasibility of treating HCV before transplantation has been demonstrated.
Limitations
The limitations of retrospective data analysis are applicable to the present findings. Although HTx is infrequently performed in HC+ patients, the authors used a large national database and a 23-year study period and thus were able to gather a significant number of patients and perform meaningful statistical analysis. HCV disease burden, which influences disease progression, is much better quantified with recent quantitative viral loads, but these were not available in the UNOS database. It should be noted that UNOS does not gather information on HCV genotypes or forms of treatment received, both of which influence treatment response and prognosis. It is therefore not possible to elucidate, from the UNOS database, the influences of virus genotype and treatment response on post-HTx outcomes.
Conclusion
The treatment of HCV infection has significantly evolved since the virus was identified in 1989. At first the disease was considered incurable, but now a > 90% cure rate is possible with newer treatment regimens. This study found significant improvements in post-HTx outcomes in HC+ patients between 1991 and 2014. Both HC+ and HC– patients have had similar post-HTx clinical outcomes in recent years. The noted improvements in post-HTx survival in HC+ patients may be secondary to better patient selection or more effective antiviral treatments. Future studies will provide the answers.
Click here to read the digital edition.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
1. Razavi H, Waked I, Sarrazin C, et al. The present and future disease burden of hepatitis C virus (HCV) infection with today’s treatment paradigm. J Viral Hepat. 2014;21(suppl 1):34-59.
2. Di Bisceglie AM. Natural history of hepatitis C: its impact on clinical management. Hepatology. 2000;31(4):1014-1018.
3. Tong MJ, el-Farra NS, Reikes AR, Co RL. Clinical outcomes after transfusionassociated hepatitis C. N Engl J Med. 1995;332(22):1463-1466.
4. Davis GL, Alter MJ, El-Serag H, Poynard T, Jennings LW. Aging of hepatitis C virus (HCV)-infected persons in the United States: a multiple cohort model of HCV prevalence and disease progression. Gastroenterology. 2010;138(2):513-521.
5. Younossi ZM, Stepanova M, Nader F, Younossi Z, Elsheikh E. Associations of chronic hepatitis C with metabolic and cardiac outcomes. Aliment Pharmacol Ther. 2013;37(6):647-652.
6. Alter MJ, Kruszon-Moran D, Nainan OV, et al. The prevalence of hepatitis C virus infection in the United States, 1988 through 1994. N Engl J Med. 1999;341(8):556-562.
7. Ferenci P, Bernstein D, Lalezari J, et al; PEARL-III Study; PEARL-IV Study. ABT-450/r-ombitasvir and dasabuvir with or without ribavirin for HCV. N Engl J Med. 2014;370(21):1983-1992.
8. Lee I, Localio R, Brensinger CM, et al. Decreased post-transplant survival among heart transplant recipients with pre-transplant hepatitis C virus positivity. J Heart Lung Transplant. 2011;30(11):1266-1274.
9. Fong TL, Hou L, Hutchinson IV, Cicciarelli JC, Cho YW. Impact of hepatitis C infection on outcomes after heart transplantation. Transplantation. 2009;88(9):1137-1141.
10. Fried MW, Shiffman ML, Reddy KR, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975-982.
11. Manns MP, McHutchison JG, Gordon SC, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958-965.
12. Poordad F, Hezode C, Trinh R, et al. ABT-450/r-ombitasvir and dasabuvir with ribavirin for hepatitis C with cirrhosis. N Engl J Med. 2014;370(21):1973-1982.
13. Zeuzem S, Dusheiko GM, Salupere R, et al. Sofosbuvir and ribavirin in HCV genotypes 2 and 3. N Engl J Med. 2014;370(21):1993-2001.
14. Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirtieth official adult heart transplant report—2013; focus theme: age. J Heart Lung Transplant. 2013;32(10):951-964.
15. Cano O, Almenar L, Martínez-Dolz L, et al. Course of patients with chronic hepatitis C virus infection undergoing heart transplantation. Transplant Proc. 2007;39(7):2353-2354.
16. Hobbs F, Stoops N. Demographic Trends in the 20th Century. Washington, DC: U.S. Government Printing Office; 2002. U.S. Census Bureau, Census 2000 Special Reports, Series CENSR-4. https://www.census.gov/prod/2002pubs/censr-4.pdf. Issued November 2002. Accessed February 7, 2017.
17. Pol S, Samuel D, Cadranel J, et al. Hepatitis and solid organ transplantation. Transplant Proc. 2000;32(2):454-457.
18. Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med. 2011;364(25):2429-2438.
19. Roth D, Zucker K, Cirocco R, et al. The impact of hepatitis C virus infection on renal allograft recipients. Kidney Int. 1994;45(1):238-244.
20. Garcia-Saenz-de-Sicilia M, Olivera-Martinez MA, Grant WJ, et al. Impact of anti-thymocyte globulin during immunosuppression induction in patients with hepatitis C after liver transplantation. Dig Dis Sci. 2014;59(11): 2804-2812.
21. Lake KD, Smith CI, Milfred-La Forest SK, Pritzker MR, Emery RW. Outcomes of hepatitis C positive (HCV+) heart transplant recipients. Transplant Proc. 1997;29(1-2):581-582.
22. Shafii AE, Su JW, Smedira NG, et al. The effect of recipient hepatitis C virus infection on outcomes following heart transplantation. Transplant Proc. 2010;42(5):1784-1787.
23. Haji SA, Starling RC, Avery RK, et al. Donor hepatitis-C seropositivity is an independent risk factor for the development of accelerated coronary vasculopathy and predicts outcome after cardiac transplantation. J Heart Lung Transplant. 2004;23(3):277-283.
24. Castella M, Tenderich G, Koerner MM, et al. Outcome of heart transplantation in patients previously infected with hepatitis C virus. J Heart Lung Transplant. 2001;20(5):595-598.
25. Hoofnagle JH, Seeff LB. Peginterferon and ribavirin for chronic hepatitis C. N Engl J Med. 2006;355(23):2444-2451.
26. Thomas RM, Brems JJ, Guzman-Hartman G, Yong S, Cavaliere P, Van Thiel DH. Infection with chronic hepatitis C virus and liver transplantation: a role for interferon therapy before transplantation. Liver Transpl. 2003;9(9):905-915.
27. Everson GT, Terrault NA, Lok AS, et al. A randomized controlled trial of pretransplant antiviral therapy to prevent recurrence of hepatitis C after liver transplantation. Hepatology. 2013;57(5):1752-1762.
The hospitalist as intensivist
Clinical question: What roles, training, and support do hospitalists have and perceive in the intensive care unit?
Background: There is a well-documented shortage of intensivists in the United States, which has left hospitalists to help fill the gap of care. Hospitalists, however, have varied levels of critical care knowledge and skills. In some regions, more than 80% of hospitalists deliver care in the ICU. It is unknown how much support hospitalized patients receive from board-certified critical care physicians.
Study design: Multistage cross-section survey.
Setting: Web-based survey initially sent through the Critical Care Task Force professional networks and later sent to 4,000 hospitalists randomly selected from Society of Hospital Medicine’s national electronic mailing list of 12,000 hospitalists.
Synopsis: This study includes 425 responses, approximately 10% of those solicited. Compared with the annual SHM survey, this included more hospitalists from academic hospitals (24% vs. 14.8%) and fewer from nonteaching hospitals (41% vs. 58.7%). A total of 77% of responders provide care in the ICU, with 66% serving as the attending physician.
Rural and nonacademic hospitalists are more prevalent in the ICU (96% rural vs. 73% nonrural; 90% nonacademic vs. 67% academic), are more likely to serve as the primary physician for all or most ICU patients (85% rural vs. 62% nonrural; 81% nonacademic vs. 44% academic), and provide all critical care services (55% rural vs. 10% nonrural; 64% nonacademic vs. 25% academic).
Many rural (43%) and nonacademic (42%) hospitalists feel that they are expected to practice beyond their perceived scope of expertise at least some of the time, which was correlated with perceived difficulty in transferring patients to higher levels of care. About 90% of rural hospitalists report at least insufficient support from board-certified intensivists. Of all responders in the study, 85% indicated interest in additional critical care training in some form, short of fellowship training.
Bottom line: Most hospitalists provide care in the ICU, however hospitalists provide critical care at significantly higher rates in rural and nonacademic hospital settings. This care is being provided with a perceived lack of intensivist support and training.
Citation: Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: What roles, training, and support do hospitalists have and perceive in the intensive care unit?
Background: There is a well-documented shortage of intensivists in the United States, which has left hospitalists to help fill the gap of care. Hospitalists, however, have varied levels of critical care knowledge and skills. In some regions, more than 80% of hospitalists deliver care in the ICU. It is unknown how much support hospitalized patients receive from board-certified critical care physicians.
Study design: Multistage cross-section survey.
Setting: Web-based survey initially sent through the Critical Care Task Force professional networks and later sent to 4,000 hospitalists randomly selected from Society of Hospital Medicine’s national electronic mailing list of 12,000 hospitalists.
Synopsis: This study includes 425 responses, approximately 10% of those solicited. Compared with the annual SHM survey, this included more hospitalists from academic hospitals (24% vs. 14.8%) and fewer from nonteaching hospitals (41% vs. 58.7%). A total of 77% of responders provide care in the ICU, with 66% serving as the attending physician.
Rural and nonacademic hospitalists are more prevalent in the ICU (96% rural vs. 73% nonrural; 90% nonacademic vs. 67% academic), are more likely to serve as the primary physician for all or most ICU patients (85% rural vs. 62% nonrural; 81% nonacademic vs. 44% academic), and provide all critical care services (55% rural vs. 10% nonrural; 64% nonacademic vs. 25% academic).
Many rural (43%) and nonacademic (42%) hospitalists feel that they are expected to practice beyond their perceived scope of expertise at least some of the time, which was correlated with perceived difficulty in transferring patients to higher levels of care. About 90% of rural hospitalists report at least insufficient support from board-certified intensivists. Of all responders in the study, 85% indicated interest in additional critical care training in some form, short of fellowship training.
Bottom line: Most hospitalists provide care in the ICU, however hospitalists provide critical care at significantly higher rates in rural and nonacademic hospital settings. This care is being provided with a perceived lack of intensivist support and training.
Citation: Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: What roles, training, and support do hospitalists have and perceive in the intensive care unit?
Background: There is a well-documented shortage of intensivists in the United States, which has left hospitalists to help fill the gap of care. Hospitalists, however, have varied levels of critical care knowledge and skills. In some regions, more than 80% of hospitalists deliver care in the ICU. It is unknown how much support hospitalized patients receive from board-certified critical care physicians.
Study design: Multistage cross-section survey.
Setting: Web-based survey initially sent through the Critical Care Task Force professional networks and later sent to 4,000 hospitalists randomly selected from Society of Hospital Medicine’s national electronic mailing list of 12,000 hospitalists.
Synopsis: This study includes 425 responses, approximately 10% of those solicited. Compared with the annual SHM survey, this included more hospitalists from academic hospitals (24% vs. 14.8%) and fewer from nonteaching hospitals (41% vs. 58.7%). A total of 77% of responders provide care in the ICU, with 66% serving as the attending physician.
Rural and nonacademic hospitalists are more prevalent in the ICU (96% rural vs. 73% nonrural; 90% nonacademic vs. 67% academic), are more likely to serve as the primary physician for all or most ICU patients (85% rural vs. 62% nonrural; 81% nonacademic vs. 44% academic), and provide all critical care services (55% rural vs. 10% nonrural; 64% nonacademic vs. 25% academic).
Many rural (43%) and nonacademic (42%) hospitalists feel that they are expected to practice beyond their perceived scope of expertise at least some of the time, which was correlated with perceived difficulty in transferring patients to higher levels of care. About 90% of rural hospitalists report at least insufficient support from board-certified intensivists. Of all responders in the study, 85% indicated interest in additional critical care training in some form, short of fellowship training.
Bottom line: Most hospitalists provide care in the ICU, however hospitalists provide critical care at significantly higher rates in rural and nonacademic hospital settings. This care is being provided with a perceived lack of intensivist support and training.
Citation: Sweigart JR et al. Characterizing hospitalist practice and perceptions of critical care delivery. J Hosp Med. 2018 Jan 1;13(1):6-12.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Guidelines to optimize treatment of reduced ejection fraction heart failure
Clinical question: What guidance is there for clinical care of complex heart failure patients?
Background: The prevalence of heart failure (HF)is escalating and consumes significant health care resources, inflicts significant morbidity and mortality, and greatly affects quality of life. There is a plethora of research, multiple medical therapies, devices, and care strategies that have been shown to improve outcomes in heart failure patients. Previous publications have reviewed evidence-based literature but left a gap in knowledge for those more-complex areas or lacked practical clinical guidance. This policy document was created to guide physicians in informed decision making in a directed decision pathway form.
Study design: Expert consensus guidelines.
Setting: American College of Cardiology Task Force on Expert Consensus Decision Pathways.
Synopsis: A multidisciplinary group of specialties including physicians, nurses, pharmacists, epidemiologists, and patient advocacy groups addressed 10 pivotal issues in heart failure through literature review, expert consensus, and round table discussion.
Ten principles were identified and addressed:
- Initiating, adding, or switching to new evidenced-based guideline-directed therapy.
- Achieving optimal therapy using multiple HF drugs and therapies.
- Knowing when to refer a patient to an HF specialist.
- Addressing the challenges of care coordination for team-based HF treatment.
- Improving patient adherence.
- Managing specific patient cohorts, such as African Americans, older adults, and the frail.
- Managing your patients’ cost of care for HF.
- Reducing costs and managing the increased complexity of HF.
- Managing the most common cardiac and noncardiac comorbidities.
- Integrating palliative care and transitioning patients to hospice care
Bottom line: Structured guidelines to provide practical and actionable recommendations to improve heart failure outcomes, integrate evidence-based medicine when available, and utilize expert conscious when evidence-based medicine is not available.
Citation: Yancy CW et al. 2017 ACC expert consensus on decision pathway for optimizing of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018 Jan 16;71(2):201-30.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: What guidance is there for clinical care of complex heart failure patients?
Background: The prevalence of heart failure (HF)is escalating and consumes significant health care resources, inflicts significant morbidity and mortality, and greatly affects quality of life. There is a plethora of research, multiple medical therapies, devices, and care strategies that have been shown to improve outcomes in heart failure patients. Previous publications have reviewed evidence-based literature but left a gap in knowledge for those more-complex areas or lacked practical clinical guidance. This policy document was created to guide physicians in informed decision making in a directed decision pathway form.
Study design: Expert consensus guidelines.
Setting: American College of Cardiology Task Force on Expert Consensus Decision Pathways.
Synopsis: A multidisciplinary group of specialties including physicians, nurses, pharmacists, epidemiologists, and patient advocacy groups addressed 10 pivotal issues in heart failure through literature review, expert consensus, and round table discussion.
Ten principles were identified and addressed:
- Initiating, adding, or switching to new evidenced-based guideline-directed therapy.
- Achieving optimal therapy using multiple HF drugs and therapies.
- Knowing when to refer a patient to an HF specialist.
- Addressing the challenges of care coordination for team-based HF treatment.
- Improving patient adherence.
- Managing specific patient cohorts, such as African Americans, older adults, and the frail.
- Managing your patients’ cost of care for HF.
- Reducing costs and managing the increased complexity of HF.
- Managing the most common cardiac and noncardiac comorbidities.
- Integrating palliative care and transitioning patients to hospice care
Bottom line: Structured guidelines to provide practical and actionable recommendations to improve heart failure outcomes, integrate evidence-based medicine when available, and utilize expert conscious when evidence-based medicine is not available.
Citation: Yancy CW et al. 2017 ACC expert consensus on decision pathway for optimizing of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018 Jan 16;71(2):201-30.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Clinical question: What guidance is there for clinical care of complex heart failure patients?
Background: The prevalence of heart failure (HF)is escalating and consumes significant health care resources, inflicts significant morbidity and mortality, and greatly affects quality of life. There is a plethora of research, multiple medical therapies, devices, and care strategies that have been shown to improve outcomes in heart failure patients. Previous publications have reviewed evidence-based literature but left a gap in knowledge for those more-complex areas or lacked practical clinical guidance. This policy document was created to guide physicians in informed decision making in a directed decision pathway form.
Study design: Expert consensus guidelines.
Setting: American College of Cardiology Task Force on Expert Consensus Decision Pathways.
Synopsis: A multidisciplinary group of specialties including physicians, nurses, pharmacists, epidemiologists, and patient advocacy groups addressed 10 pivotal issues in heart failure through literature review, expert consensus, and round table discussion.
Ten principles were identified and addressed:
- Initiating, adding, or switching to new evidenced-based guideline-directed therapy.
- Achieving optimal therapy using multiple HF drugs and therapies.
- Knowing when to refer a patient to an HF specialist.
- Addressing the challenges of care coordination for team-based HF treatment.
- Improving patient adherence.
- Managing specific patient cohorts, such as African Americans, older adults, and the frail.
- Managing your patients’ cost of care for HF.
- Reducing costs and managing the increased complexity of HF.
- Managing the most common cardiac and noncardiac comorbidities.
- Integrating palliative care and transitioning patients to hospice care
Bottom line: Structured guidelines to provide practical and actionable recommendations to improve heart failure outcomes, integrate evidence-based medicine when available, and utilize expert conscious when evidence-based medicine is not available.
Citation: Yancy CW et al. 2017 ACC expert consensus on decision pathway for optimizing of heart failure treatment: Answers to 10 pivotal issues about heart failure with reduced ejection fraction. J Am Coll Cardiol. 2018 Jan 16;71(2):201-30.
Dr. Muñoa is a hospitalist at Denver Health Medical Center and an assistant professor of medicine at the University of Colorado at Denver, Aurora.
Emergency Imaging: Femoral Pseudoaneurysm
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Don’t delay hip-fracture surgery
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.
Background: Guidelines from the American College of Surgeons and Canadian Institute for Health recommend hip fracture surgery within 48 hours. However, a time-to-surgery threshold after which mortality and complications are increased has not been determined. This study aims to determine a time to surgery threshold for hip-fracture surgery.
Study design: Retrospective cohort trial.
Setting: 72 hospitals in Ontario, Ca., during April 1, 2009-March 31, 2014.
Synopsis: Of the 42,230 adult patients in this study, 14,174 (33.6%) received hip-fracture surgery within 24 hours of emergency department arrival. A matched patient analysis of early surgery (within 24 hours of ED arrival) vs. delayed surgery determined that patients undergoing early operation experienced lower 30-day mortality (5.8% vs 6.5%) and fewer complications (myocardial infarction, deep vein thrombosis, pulmonary embolism, and pneumonia). Major bleeding was not assessed as a complication. Also omitted from analysis were patients undergoing nonoperative hip-fracture management.
These findings suggest a time to surgery of 24 hours may represent a threshold defining higher risk. Two-thirds of patients in this study surpassed this threshold. Hospitalists seeing patients with hip fracture should balance time delay risks with the need for medical optimization.
Bottom line: Wait time greater than 24 hours for adults undergoing hip fracture surgery is associated with an increased risk of 30-day mortality and complications.
Citation: Pincus D et al. Association between wait time and 30-day mortality in adults undergoing hip fracture surgery. JAMA. 2017 Nov 28;318(20):1994-2003.
Dr. Moulder is assistant professor, University of Virginia Health System.