User login
Mitchel is a reporter for MDedge based in the Philadelphia area. He started with the company in 1992, when it was International Medical News Group (IMNG), and has since covered a range of medical specialties. Mitchel trained as a virologist at Roswell Park Memorial Institute in Buffalo, and then worked briefly as a researcher at Boston Children's Hospital before pivoting to journalism as a AAAS Mass Media Fellow in 1980. His first reporting job was with Science Digest magazine, and from the mid-1980s to early-1990s he was a reporter with Medical World News. @mitchelzoler
ILC: Direct Antivirals Safely Clear HCV Despite ESRD
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
AT THE INTERNATIONAL LIVER CONGRESS 2015
ILC: Direct antivirals safely clear HCV despite ESRD
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
VIENNA – A 12-week, fixed-dose regimen safely and effectively eradicated chronic hepatitis C infection from the first 10 patients with advanced chronic kidney disease in a multicenter U.S. series.
The regimen of three direct antiviral agents is already on the U.S. market. So far, 20 HCV patients with advanced chronic kidney disease have been treated and there have been no cases of virologic failure. All 10 patients who have been followed for at least 4 weeks after completing treatment sustained their virologic response, Dr. Paul J. Pockros said at the meeting sponsored by the European Association for the Study of the Liver.
The series has exclusively enrolled patients with genotype 1 hepatitis C virus (HCV) infection. The seven enrolled patients infected with genotype 1b HCV received the three direct antiviral agents only, the combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir, which together are marketed as Viekira Pak. The 13 patients with a genotype 1a infection received treatment with both the three-drug regimen plus ribavirin, which was effective but resulted in significant hemoglobin reduction in eight patients that required dosage interruptions. All patients were then able to restart and continue treatment, said Dr. Pockros, a gastroenterologist at the Scripps Clinic in La Jolla, Calif.
The efficacy and safety of these three direct antivirals in patients with advanced chronic kidney disease – an estimated glomerular filtration rate (eGFR) of no greater than 30 mL/min/1.73 m2 – contrasts with the caution that exists for another major, direct antiviral agent for hepatitis C eradication, sofosbuvir, which is marketed as Sovaldi as an individual drug and as Harvoni when formulated with ledipavir. The labels for both forms of sofosbuvir say that the drug’s safety and efficacy “has not been established in patients with severe renal impairment (eGFR <30 mL/min/1.73m2) or end stage renal disease (ESRD) requiring hemodialysis. No dose recommendation can be given for patients with severe renal impairment or ESRD.”
A new phase of the trial starting soon will test whether patients infected with genotype 1a HCV can be effectively treated with the three direct antiviral agents alone without ribavirin, Dr. Pockros said. Another soon-to-start aspect of the trial will test the regimen in patients with cirrhosis. The current series has so far enrolled only treatment naive patients without cirrhosis.
The RUBY-1 trial has been run at nine U.S. centers, where researchers enrolled seven patients with stage 4 chronic kidney disease (an eGFR of 15-29 ml/min/ 1.73m2), and 13 patients on hemodialysis and with stage 5 chronic kidney disease, defined as an eGFR of less than 15 mL/min/1.73m2. Fourteen of the 20 enrolled patients (70%) were African American, and 15% were Hispanic, a demographic pattern that is “a fair representation” of U.S. patients with both hepatitis C infection and end-stage renal disease, Dr. Prockros said.
The three direct antivirals tested in the current study are all metabolized in the liver and require no dose modification when used in patients with renal dysfunction. Pharmacokinetic studies done as part of the study showed no differences in blood levels of these drugs in the patients with advanced chronic kidney disease compared with historical patients with better renal function. Several reports published in 2014 documented the efficacy of ombitasvir, paritaprevir plus ritonavir, and dasabuvir for eradicating chronic HCV infection in patients with more normal renal function (N. Engl. J. Med. 2014;370:1594-1603, 1604-14, 1973-82, 1983-92).
RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: The trio of direct antiviral agents marketed as Viekira Pak safely eradicated chronic genotype 1 hepatitis C infection in patients with chronic kidney disease.
Major finding: All 10 patients followed so far to at least 4 weeks after completing treatment maintained a sustained virologic response.
Data source: RUBY-1, an open-label series of 20 patients with chronic HCV and advanced chronic kidney disease.
Disclosures: RUBY-1 was sponsored by AbbVie, which markets Viekira Pak. Dr. Pockros disclosed ties with AbbVie, Janssen, Bristol-Myers Squibb, Gilead, Merck, Conatus, and Roche Molecular.
Europe Issues Hepatitis C Treatment Priority List
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
EXPERT ANALYSIS FROM THE INTERNATIONAL LIVER CONGRESS 2015
ILC: Europe issues hepatitis C treatment priority list
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.
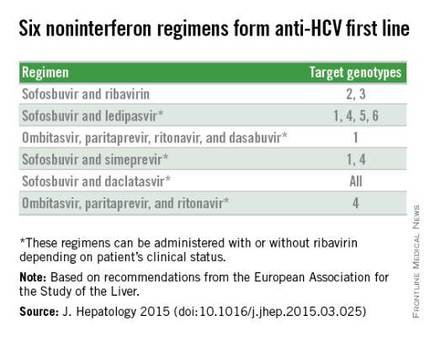
With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
VIENNA – The new European recommendations for diagnosing and treating hepatitis C infection highlight the paradox gripping the field: Safe and potent antiviral drugs are available to cure most patients after 12 weeks of treatment, but cure is not broadly available because the agents are prohibitively expensive.
“Virtually everyone infected by hepatitis C virus [HCV] has the right to be treated,“ Dr. Jean-Michel Pawlotsky said during a session at the meeting sponsored by the European Association for the Study of the Liver (EASL) that introduced the association’s new hepatitis C treatment recommendations.
The recommendations, released online around the time of the meeting, say: “Because of the approval of highly efficacious new HCV treatment regimens, access to therapy must be broadened.” In addition, they call for expanded screening to find more of the many unidentified cases of chronic hepatitis C infection (J. Hepatology 2015 [doi:10.1016/j.jhep.2015.03.025]). “However, we also have to acknowledge the reality that these drugs are currently too expensive, and the huge number of patients with HCV infection makes it impossible that we could treat all infected patients over the next couple of years,” said Dr. Pawlotsky, head of the department of virology at the Henri Mondor University Hospital in Créteil, France.
The prioritization scheme the EASL panel published gives highest treatment priority to patients with F3 or F4 liver fibrosis on the Metavir scoring system, HIV- or hepatitis B virus–coinfected patients, liver transplant candidates or recent recipients, and patients with significant extrahepatic manifestations, debilitating fatigue, or a high risk for transmitting infection. The list puts patients with moderate liver fibrosis, with a Metavir score of F2, a step down in priority but still rates them candidates for treatment. But the prioritization table rates patients with F1 or F0 fibrosis and none of the other stated complications as reasonable for deferred treatment.
This approach, as well as the general scheme recommended for drug selection, is “remarkably similar” to the guidance first issued and then revised last year by experts assembled by the American Association for the Study of Liver Diseases and the Infectious Diseases Society of America, commented Dr. Donald M. Jensen, a hepatologist in Oak Park, Ill., who helped draft the U.S. guidance. “The more these two guidelines are consistent, the more powerful they are,” Dr. Jensen said as an invited discussant at the session.
Perhaps the biggest difference between the HCV treatment recommendations from the European and American groups is that the EASL document included daclatasvir (Daklinza), a direct-acting antiviral for HCV that became available for use in Europe in 2014, but which remains under Food and Drug Administration review for U.S. use as of May 2015.

With daclatasvir as an option, the EASL panel highlighted six HCV regimens as first-line HCV options that all avoid treatment with interferon. The recommendations further fine-tune the options from this list of six regimens depending on the infecting HCV genotype, as well as special clinical situations such as compensated or decompensated cirrhosis, recent liver transplant, or end-stage renal disease. The EASL panel also firmly relegated interferon-containing regimens to second-line status.
Another significant issue in choosing among HCV treatments are drug-drug interactions. The EASL panel endorsed a website maintained by the University of Liverpool (England) as an excellent source for researching drug-interaction issues, Dr. Pawlotsky said.
On Twitter @mitchelzoler
EXPERT ANALYSIS FROM THE INTERNATIONAL LIVER CONGRESS 2015
ILC: Liraglutide shows NASH benefit in small trial
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
VIENNA – The oral hypoglycemic liraglutide produced significant histologic resolution of nonalcoholic steatohepatitis after nearly a year of treatment in a phase II randomized trial of 52 patients.
Although liraglutide (Victoza) is already available in the United States and Europe for treating diabetes, its use specifically for treating nonalcoholic steatohepatitis (NASH), advanced fatty liver disease with currently no approved treatment, should await results from a larger, phase III trial, Dr. Matthew J. Armstrong said at the meeting sponsored by the European Association for the Study of the Liver.
“Oral diabetes drugs have been extensively investigated for NASH, but this is the first time a GLP [glucagon-like peptide]-1 analog has been looked at for NASH in a randomized trial with placebo control,” said Dr. Armstrong, a researcher at the Centre for Liver Research of the University of Birmingham, England. “We feel that GLP-1 analogs like liraglutide give you the whole package” of improving lipid levels, weight, and glycemic control while also addressing liver disease.
“One of the biggest killers in patients with NASH is cardiovascular disease. The weight reduction and improved glycemic control and lipids should improve overall cardiovascular outcomes,” Dr. Armstrong said during a press conference at the meeting.
The issue with a drug like liraglutide is “will it have an effect independent of weight loss,” said Dr. Markus Peck-Radosavljevic, vice-chairman at the department of gastroenterology and hepatology at the University of Vienna. “We know that weight loss will help patients with nonalcoholic fatty-liver disease, but the problem is that patients often do not lose weight. What we would like is a drug with a beneficial effect that is independent of weight loss.”
Liraglutide, an analog of the gut satiety hormone GLP-1 but with extended physiologic half-life, “seems to have an effect independent of weight loss,” Dr. Armstrong said. In addition to its documented efficacy for producing weight loss and decreasing glycosylated hemoglobin A1c, liraglutide showed activity in animal and in vitro models for improving liver-enzyme levels, oxidative stress, and hepatic steatosis.
The Liraglutide Efficacy and Action in Nonalcoholic steatohepatitis (LEAN) trial randomized 52 patients aged 18-60 years with biopsy-confirmed NASH to daily liraglutide treatment or placebo for 48 weeks. Researchers at four U.K. centers uptitrated patients on liraglutide to 1.8 mg/day over the study’s first 2 weeks and then kept patents at that dosage. The average age of the patients was 51 years, a majority were men, a third had type 2 diabetes, the average hemoglobin A1c was 6.0%, and the average body mass index was more than 35 kg/m2.
After 48 weeks on treatment, follow-up biopsies on 23 of the patients on liraglutide and 22 on placebo showed 9 liraglutide patients (39%) had resolution of their NASH without worsening fibrosis, the study’s primary endpoint, compared with 2 NASH resolutions (9%) in the control arm, Dr. Armstrong reported.
The 39% efficacy rate for resolutions surpassed the study’s prespecified threshold of 38% to define effective treatment.
Liraglutide-treated patients showed improvements in several other clinical and metabolic parameters including fibrosis and steatosis, as well as an average 5-kg greater weight loss compared with placebo and a 0.45% drop in hemoglobin A1c compared with placebo that fell short of statistical significance.
The safety analysis showed that liraglutide was “well tolerated with an acceptable safety profile in NASH patients,” Dr. Armstrong said. The phase II study was “as much about safety as efficacy,” as the feasibility of treating NASH patients with liraglutide had been uncertain until now.
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Daily liraglutide injections for 48 weeks safely increased resolution of nonalcoholic steatohepatitis.
Major finding: NASH resolved in 39% of liraglutide-treated patients and 9% of controls.
Data source: LEAN, a randomized trial of 52 patients with biopsy-proven NASH enrolled at four U.K. centers.
Disclosures: LEAN and Dr. Armstrong received educational grants and free study medication from Novo Nordisk, the company that markets liraglutide (Victoza).
ILC: NAFLD a stealth trigger of hepatocellular carcinoma
VIENNA – Nonalcoholic fatty liver disease often goes overlooked as a cause of liver cancer despite its increasing role as a major driver of hepatocellular carcinoma.
During 2004-2009, nonalcoholic fatty liver disease (NAFLD) appeared to be the primary cause for 26% of the hepatocellular carcinoma (HCC) diagnosed among U.S. patients, according to data collected by the National Cancer Institute, Dr. Zobair M. Younossi reported at the meeting sponsored by the European Association for the Study of the Liver.
That placed NAFLD second only to infection by hepatitis C virus, which linked with 48% of HCC cases. Third and fourth places went to alcoholic liver disease, causing 14% of cases, and hepatitis B virus infection, the trigger for 8% of HCC cases.
“There is a huge gap in understanding and recognition of NAFLD, and especially progressive fatty-liver disease – nonalcoholic steatohepatitis (NASH), as important diseases and that some of these patients will develop cirrhosis and HCC,” Dr. Younossi said in an interview. The aggressiveness with which physicians follow NAFLD and specifically NASH patients for development of HCC “is not as well implemented as it is for patients with viral hepatitis,” said Dr. Younossi, head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Patients with NASH “don’t get screened enough, and, when they are screened, the strategy is not done well” as it usually relies on ultrasound, which is problematic in obese patients. Obesity is a common comorbidity in patients with NAFLD or NASH, he said. Dr. Younossi highlighted a recommendation made last year by the American Association for the Study of Liver Diseases that suggested consideration of regular HCC screening in patients with NASH and cirrhosis (Hepatology 2012:55:2005-23). He recommended routine screening particularly for NAFLD patients who have developed NASH and also cirrhosis or advanced liver fibrosis, a complication that can be estimated without a liver biopsy by way of the NAFLD fibrosis score calculator.
Another striking finding of the new analysis was that HCC associated with NAFLD was deadlier than were cases linked with other causes, with a 21% relative increase in 1-year mortality following initial HCC diagnosis compared with liver cancers caused by viral hepatitis or alcohol use. “Patients with HCC and NAFLD have a worse prognosis than any other type of HCC,” Dr. Younossi said.
The analysis used linked data from the Surveillance Epidemiology and End Results (SEER) program run by the National Cancer Institute, and the Centers for Medicare & Medicaid Services, and included 5,748 HCC cases and 17,244 matched control individuals without HCC. The SEER data showed that total U.S. HCC cases rose by roughly a third during the period 2004-2009, Dr. Younossi reported.
Patients with HCC linked with NAFLD were significantly older than were other HCC patients, at an average age of 72 years for the NAFLD group compared with 66 years for everyone else, and mortality came an average of 4 months faster in the NAFLD subgroup. By 1 year after HCC diagnosis, mortality was 62% among the NAFLD patients and 50% among everyone else.
Although results from prior reports had linked HCC and NAFLD, the new findings “strengthen the evidence” using a population-based registry, Dr. Younossi said. The new results also show for the first time the increased first-year mortality from HCC in NAFLD patients. He speculated that the frequent coexistence of diabetes in NAFLD patients might contribute to the more aggressive course, as well as later diagnosis because of unsuspected disease and also the challenge of using ultrasound to screen obese patients. Dr. Younossi recommended using CT imaging as an alternative for noninvasively finding potential cases of HCC.
On Twitter @mitchelzoler
VIENNA – Nonalcoholic fatty liver disease often goes overlooked as a cause of liver cancer despite its increasing role as a major driver of hepatocellular carcinoma.
During 2004-2009, nonalcoholic fatty liver disease (NAFLD) appeared to be the primary cause for 26% of the hepatocellular carcinoma (HCC) diagnosed among U.S. patients, according to data collected by the National Cancer Institute, Dr. Zobair M. Younossi reported at the meeting sponsored by the European Association for the Study of the Liver.
That placed NAFLD second only to infection by hepatitis C virus, which linked with 48% of HCC cases. Third and fourth places went to alcoholic liver disease, causing 14% of cases, and hepatitis B virus infection, the trigger for 8% of HCC cases.
“There is a huge gap in understanding and recognition of NAFLD, and especially progressive fatty-liver disease – nonalcoholic steatohepatitis (NASH), as important diseases and that some of these patients will develop cirrhosis and HCC,” Dr. Younossi said in an interview. The aggressiveness with which physicians follow NAFLD and specifically NASH patients for development of HCC “is not as well implemented as it is for patients with viral hepatitis,” said Dr. Younossi, head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Patients with NASH “don’t get screened enough, and, when they are screened, the strategy is not done well” as it usually relies on ultrasound, which is problematic in obese patients. Obesity is a common comorbidity in patients with NAFLD or NASH, he said. Dr. Younossi highlighted a recommendation made last year by the American Association for the Study of Liver Diseases that suggested consideration of regular HCC screening in patients with NASH and cirrhosis (Hepatology 2012:55:2005-23). He recommended routine screening particularly for NAFLD patients who have developed NASH and also cirrhosis or advanced liver fibrosis, a complication that can be estimated without a liver biopsy by way of the NAFLD fibrosis score calculator.
Another striking finding of the new analysis was that HCC associated with NAFLD was deadlier than were cases linked with other causes, with a 21% relative increase in 1-year mortality following initial HCC diagnosis compared with liver cancers caused by viral hepatitis or alcohol use. “Patients with HCC and NAFLD have a worse prognosis than any other type of HCC,” Dr. Younossi said.
The analysis used linked data from the Surveillance Epidemiology and End Results (SEER) program run by the National Cancer Institute, and the Centers for Medicare & Medicaid Services, and included 5,748 HCC cases and 17,244 matched control individuals without HCC. The SEER data showed that total U.S. HCC cases rose by roughly a third during the period 2004-2009, Dr. Younossi reported.
Patients with HCC linked with NAFLD were significantly older than were other HCC patients, at an average age of 72 years for the NAFLD group compared with 66 years for everyone else, and mortality came an average of 4 months faster in the NAFLD subgroup. By 1 year after HCC diagnosis, mortality was 62% among the NAFLD patients and 50% among everyone else.
Although results from prior reports had linked HCC and NAFLD, the new findings “strengthen the evidence” using a population-based registry, Dr. Younossi said. The new results also show for the first time the increased first-year mortality from HCC in NAFLD patients. He speculated that the frequent coexistence of diabetes in NAFLD patients might contribute to the more aggressive course, as well as later diagnosis because of unsuspected disease and also the challenge of using ultrasound to screen obese patients. Dr. Younossi recommended using CT imaging as an alternative for noninvasively finding potential cases of HCC.
On Twitter @mitchelzoler
VIENNA – Nonalcoholic fatty liver disease often goes overlooked as a cause of liver cancer despite its increasing role as a major driver of hepatocellular carcinoma.
During 2004-2009, nonalcoholic fatty liver disease (NAFLD) appeared to be the primary cause for 26% of the hepatocellular carcinoma (HCC) diagnosed among U.S. patients, according to data collected by the National Cancer Institute, Dr. Zobair M. Younossi reported at the meeting sponsored by the European Association for the Study of the Liver.
That placed NAFLD second only to infection by hepatitis C virus, which linked with 48% of HCC cases. Third and fourth places went to alcoholic liver disease, causing 14% of cases, and hepatitis B virus infection, the trigger for 8% of HCC cases.
“There is a huge gap in understanding and recognition of NAFLD, and especially progressive fatty-liver disease – nonalcoholic steatohepatitis (NASH), as important diseases and that some of these patients will develop cirrhosis and HCC,” Dr. Younossi said in an interview. The aggressiveness with which physicians follow NAFLD and specifically NASH patients for development of HCC “is not as well implemented as it is for patients with viral hepatitis,” said Dr. Younossi, head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Patients with NASH “don’t get screened enough, and, when they are screened, the strategy is not done well” as it usually relies on ultrasound, which is problematic in obese patients. Obesity is a common comorbidity in patients with NAFLD or NASH, he said. Dr. Younossi highlighted a recommendation made last year by the American Association for the Study of Liver Diseases that suggested consideration of regular HCC screening in patients with NASH and cirrhosis (Hepatology 2012:55:2005-23). He recommended routine screening particularly for NAFLD patients who have developed NASH and also cirrhosis or advanced liver fibrosis, a complication that can be estimated without a liver biopsy by way of the NAFLD fibrosis score calculator.
Another striking finding of the new analysis was that HCC associated with NAFLD was deadlier than were cases linked with other causes, with a 21% relative increase in 1-year mortality following initial HCC diagnosis compared with liver cancers caused by viral hepatitis or alcohol use. “Patients with HCC and NAFLD have a worse prognosis than any other type of HCC,” Dr. Younossi said.
The analysis used linked data from the Surveillance Epidemiology and End Results (SEER) program run by the National Cancer Institute, and the Centers for Medicare & Medicaid Services, and included 5,748 HCC cases and 17,244 matched control individuals without HCC. The SEER data showed that total U.S. HCC cases rose by roughly a third during the period 2004-2009, Dr. Younossi reported.
Patients with HCC linked with NAFLD were significantly older than were other HCC patients, at an average age of 72 years for the NAFLD group compared with 66 years for everyone else, and mortality came an average of 4 months faster in the NAFLD subgroup. By 1 year after HCC diagnosis, mortality was 62% among the NAFLD patients and 50% among everyone else.
Although results from prior reports had linked HCC and NAFLD, the new findings “strengthen the evidence” using a population-based registry, Dr. Younossi said. The new results also show for the first time the increased first-year mortality from HCC in NAFLD patients. He speculated that the frequent coexistence of diabetes in NAFLD patients might contribute to the more aggressive course, as well as later diagnosis because of unsuspected disease and also the challenge of using ultrasound to screen obese patients. Dr. Younossi recommended using CT imaging as an alternative for noninvasively finding potential cases of HCC.
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: U.S. epidemiologic data link nonalcoholic fatty liver disease to a quarter of hepatocellular carcinoma cases.
Major finding: NAFLD linked with 26% of U.S. hepatocellular carcinoma cases during 2004-2009, the second leading cause of liver cancer.
Data source: National Cancer Institute registry of 5,748 U.S. hepatocellular carcinoma cases and 17,244 matched, cancer-free controls.
Disclosures: Dr. Younossi has been a consultant to Gilead, Abbvie, Bristol-Myers Squibb, GlaxoSmithKline, Intercept, and Salix.
ILC: Statins linked to better outcomes in hepatitis C cirrhosis
VIENNA – Patients infected with hepatitis C virus who developed cirrhosis and received statin treatment had significantly lower rates of both death and cirrhosis decompensation, compared with cirrhosis patients who did not receive a statin in a confounder-adjusted analysis of data from more than 2,700 patients in a U.S. Department of Veterans Affairs database.
While this suggestive evidence is not strong enough to warrant routinely prescribing statins to cirrhosis patients, it does highlight the need to prescribe a statin to any cirrhosis patient who qualifies for the drug by standard criteria because of established cardiovascular disease or as part of primary prevention when there is elevated cardiovascular risk, Dr. Arpan Mohanty said at the meeting sponsored by the European Association for the Study of the Liver.
Conventional wisdom has often led to withholding statins from patients with liver disease out of concern for risk of statin-induced hepatotoxicity, said Dr. Mohanty, a gastroenterology researcher at Yale University in New Haven, Conn. But the new findings suggesting such overwhelming benefit from statin treatment in these patients indicates that “statin use should not be avoided” when patients with liver disease would otherwise qualify for statin treatment. “Statins should be prescribed when required for atherosclerosis,” she said, adding that in New Haven her program has run sessions to educate primary care physicians on this.
The study used data collected during 1996-2009 by the Hepatitis C Virus Clinical Case Registry of the U.S. Department of Veterans Affairs, which includes more than 340,000 veterans, of whom more than 45,000 had been diagnosed with cirrhosis. Further analysis identified 1,323 eligible veterans from this group on statin treatment, and 12,522 not on statin treatment. Propensity score matching narrowed the study group down to 685 hepatitis C virus–infected veterans with cirrhosis who were on statin treatment, and 2,062 closely matched veterans infected with HCV and with cirrhosis but not receiving statin therapy.
The patients averaged 56 years old, 98% were men, and comorbidities were common; a third had a history of coronary artery disease, more than 80% had hypertension, more than half had diabetes, and more than half had alcohol dependency. Among patients with a serum cholesterol level greater than 200 mg/dL, 57% were not on a statin; among those with a serum low-density cholesterol level of about 160 mg/dL, 35% were not receiving a statin. “Statin use is low in patients with cirrhosis, even in those with high cardiovascular risk,” Dr. Mohanty said.
She and her associates tracked the incidence of death for a median of more than 2 years in these patients, and they followed new episodes of cirrhosis decompensation for nearly 2 years. With adjustment for age, body mass index, serum albumin, and fibrosis-4 and MELD (Model for End-Stage Liver Disease) scores, the rates of both death and cirrhosis decompensation were each a statistically significant 45% lower among the patients on statins, compared with those not on a statin, they reported.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
VIENNA – Patients infected with hepatitis C virus who developed cirrhosis and received statin treatment had significantly lower rates of both death and cirrhosis decompensation, compared with cirrhosis patients who did not receive a statin in a confounder-adjusted analysis of data from more than 2,700 patients in a U.S. Department of Veterans Affairs database.
While this suggestive evidence is not strong enough to warrant routinely prescribing statins to cirrhosis patients, it does highlight the need to prescribe a statin to any cirrhosis patient who qualifies for the drug by standard criteria because of established cardiovascular disease or as part of primary prevention when there is elevated cardiovascular risk, Dr. Arpan Mohanty said at the meeting sponsored by the European Association for the Study of the Liver.
Conventional wisdom has often led to withholding statins from patients with liver disease out of concern for risk of statin-induced hepatotoxicity, said Dr. Mohanty, a gastroenterology researcher at Yale University in New Haven, Conn. But the new findings suggesting such overwhelming benefit from statin treatment in these patients indicates that “statin use should not be avoided” when patients with liver disease would otherwise qualify for statin treatment. “Statins should be prescribed when required for atherosclerosis,” she said, adding that in New Haven her program has run sessions to educate primary care physicians on this.
The study used data collected during 1996-2009 by the Hepatitis C Virus Clinical Case Registry of the U.S. Department of Veterans Affairs, which includes more than 340,000 veterans, of whom more than 45,000 had been diagnosed with cirrhosis. Further analysis identified 1,323 eligible veterans from this group on statin treatment, and 12,522 not on statin treatment. Propensity score matching narrowed the study group down to 685 hepatitis C virus–infected veterans with cirrhosis who were on statin treatment, and 2,062 closely matched veterans infected with HCV and with cirrhosis but not receiving statin therapy.
The patients averaged 56 years old, 98% were men, and comorbidities were common; a third had a history of coronary artery disease, more than 80% had hypertension, more than half had diabetes, and more than half had alcohol dependency. Among patients with a serum cholesterol level greater than 200 mg/dL, 57% were not on a statin; among those with a serum low-density cholesterol level of about 160 mg/dL, 35% were not receiving a statin. “Statin use is low in patients with cirrhosis, even in those with high cardiovascular risk,” Dr. Mohanty said.
She and her associates tracked the incidence of death for a median of more than 2 years in these patients, and they followed new episodes of cirrhosis decompensation for nearly 2 years. With adjustment for age, body mass index, serum albumin, and fibrosis-4 and MELD (Model for End-Stage Liver Disease) scores, the rates of both death and cirrhosis decompensation were each a statistically significant 45% lower among the patients on statins, compared with those not on a statin, they reported.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
VIENNA – Patients infected with hepatitis C virus who developed cirrhosis and received statin treatment had significantly lower rates of both death and cirrhosis decompensation, compared with cirrhosis patients who did not receive a statin in a confounder-adjusted analysis of data from more than 2,700 patients in a U.S. Department of Veterans Affairs database.
While this suggestive evidence is not strong enough to warrant routinely prescribing statins to cirrhosis patients, it does highlight the need to prescribe a statin to any cirrhosis patient who qualifies for the drug by standard criteria because of established cardiovascular disease or as part of primary prevention when there is elevated cardiovascular risk, Dr. Arpan Mohanty said at the meeting sponsored by the European Association for the Study of the Liver.
Conventional wisdom has often led to withholding statins from patients with liver disease out of concern for risk of statin-induced hepatotoxicity, said Dr. Mohanty, a gastroenterology researcher at Yale University in New Haven, Conn. But the new findings suggesting such overwhelming benefit from statin treatment in these patients indicates that “statin use should not be avoided” when patients with liver disease would otherwise qualify for statin treatment. “Statins should be prescribed when required for atherosclerosis,” she said, adding that in New Haven her program has run sessions to educate primary care physicians on this.
The study used data collected during 1996-2009 by the Hepatitis C Virus Clinical Case Registry of the U.S. Department of Veterans Affairs, which includes more than 340,000 veterans, of whom more than 45,000 had been diagnosed with cirrhosis. Further analysis identified 1,323 eligible veterans from this group on statin treatment, and 12,522 not on statin treatment. Propensity score matching narrowed the study group down to 685 hepatitis C virus–infected veterans with cirrhosis who were on statin treatment, and 2,062 closely matched veterans infected with HCV and with cirrhosis but not receiving statin therapy.
The patients averaged 56 years old, 98% were men, and comorbidities were common; a third had a history of coronary artery disease, more than 80% had hypertension, more than half had diabetes, and more than half had alcohol dependency. Among patients with a serum cholesterol level greater than 200 mg/dL, 57% were not on a statin; among those with a serum low-density cholesterol level of about 160 mg/dL, 35% were not receiving a statin. “Statin use is low in patients with cirrhosis, even in those with high cardiovascular risk,” Dr. Mohanty said.
She and her associates tracked the incidence of death for a median of more than 2 years in these patients, and they followed new episodes of cirrhosis decompensation for nearly 2 years. With adjustment for age, body mass index, serum albumin, and fibrosis-4 and MELD (Model for End-Stage Liver Disease) scores, the rates of both death and cirrhosis decompensation were each a statistically significant 45% lower among the patients on statins, compared with those not on a statin, they reported.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE INTERNATIONAL LIVER CONGRESS 2015
Key clinical point: Hepatitis C–infected patients with associated cirrhosis had fewer deaths and decompensations when receiving statin treatment.
Major finding: Statin treatment linked with 45% reductions in both death and decompensation after a roughly 2-year follow-up.
Data source: A retrospective analysis of data collected by the U.S. Department of Veterans Affairs on about 2,700 veterans with cirrhosis and infected with hepatitis C virus.
Disclosures: Dr. Mohanty had no relevant financial disclosures.
ELCC: Urine tumor DNA shows high testing promise
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
GENEVA – Blood sampling provides a less invasive alternative to tumor biopsy for collecting cancer DNA for mutation testing, but retrieving tumor DNA from a patient’s urine is least invasive of all.
In an early phase of clinical investigation, researchers assessed the feasibility of using urine to collect cell-free tumor DNA to detect mutations in 34 patients with advanced non–small cell lung cancer. The results suggested that testing circulating tumor (ct) DNA isolated from patients’ urine was sensitive compared with testing DNA from biopsied specimens of the primary tumor, and it was able to flag tumor changes early, before clinically-identifiable effects appeared, Dr. Hatim Husain said at the European Lung Cancer Conference.
He used ctDNA isolated from patients’ urine to test for the presence of three different resistance mutations within the epidermal growth factor receptor (EGFR) gene. Genetic testing of biopsy specimens showed 10 patients carried the T790M mutation, 18 carried an exon 19 deletion, and eight carried an exon 21 L858R mutation. Of these 36 mutations in 34 patients, testing ctDNA isolated from urine identified 35 as positive, a 97% overall concordance rate.
In addition, testing with ctDNA from urine also picked up three additional T790M mutations not seen in the three corresponding tumor-biopsy specimens, but in patients with high clinical suspicion for carrying an EGFR mutation, Dr. Husain reported.
Further evidence for the utility of urinary ctDNA came from following 22 patients on treatment with erlotinib (Tarceva) and monitored for their acquisition of an EGFR-gene mutation making the tumor erlotinib resistant. Dr. Husain and his associates ran a DNA test every 3-6 weeks and tracked the time until patients developed radiographic progression. Using urinary ctDNA, they found four patients who developed EGFR mutations 29-111 days before clinical progression of the tumor became radiographically apparent.
The ctDNA that ends up in a patient’s urine starts out circulating in the blood; urine works as the main elimination route. Urine ctDNA is more concentrated than in blood, and ctDNA remains stable in urine at ambient temperature for 2 weeks, said Dr. Husain, an oncology researcher at the University of California San Diego, La Jolla.
“These interim results suggest that use of urinary ctDNA has potential to detect EGFR T790M status in a higher number of study subjects and may make some patients eligible for therapy who would by tissue biopsy be falsely classified as negative,” Dr. Husain said in a written statement. “Detecting the emergence of EGFR T790M mutations before progression has the potential to enable physicians to better align therapeutic selection and inform early therapeutic decision making,” he said.
Testing ctDNA in patients’ urine is a “novel way to do noninvasive testing,” said Dr. Egbert F. Smit, professor of pulmonary medicine at VU University Medical Center in Amsterdam and the meeting’s designated discussant for Dr. Husain’s report. “It’s attractive for collecting ctDNA because you get a high concentration, and it has potential for a high level of sensitivity. It may have potential for showing how a tumor reacts to treatment. The method seems robust, but we still need data on reproducibility and cost effectiveness,” Dr. Smit cautioned.
On Twitter @mitchelzoler
AT ELCC 2015
Key clinical point: Urine showed potential as a fully noninvasive source for circulating tumor DNA with high testing concordance compared with tumor-biopsy DNA in initial clinical experience with 34 lung cancer patients.
Major finding: Urine circulating tumor DNA had overall concordance of 97% compared with tumor-biopsy DNA for detecting treatment-altering mutations.
Data source: Single-center study of 34 patients with advanced non-small cell lung cancer.
Disclosures: The study was sponsored by Trovagene, the company developing the tests used in the study. Several coauthors on the study are Trovagene employees. Dr. Husain had no personal disclosures.
VIDEO: NAFLD increasingly causing U.S. hepatocellular carcinomas
VIENNA – Nonalcoholic fatty liver disease (NAFLD) now stands as the second most common cause of U.S. cases of hepatocellular carcinoma, and with highly effective drug regimens now sharply dropping the prevalence of hepatitis C virus infection, NAFLD – a complication of obesity – is poised to snag the top spot, Dr. Zobair Younossi said in a video interview at the meeting sponsored by the European Association for the Study of the Liver.
In an analysis that combined U.S. national cancer registry data collected by the National Cancer Institute and morbidity diagnostic codes collected by Medicare, Dr. Younossi calculated that, during 2004-2009 among U.S. adults covered by Medicare, 24% of patients newly diagnosed with hepatocellular carcinoma (HCC) had NAFLD as their pre-existing chronic liver disease, compared with 48% who had hepatitis C virus infection as their trigger. The third most common cause of HCC was alcoholic liver disease (14%), followed by hepatitis B virus infection (8%).
Dr. Younossi’s analysis also included survival data for each HCC case in the first year following diagnosis, which showed that NAFLD-associated cases also were deadlier, linking with a statistically significant 20% increase in mortality compared with HCC associated with other causes. Quicker lethality of NAFLD-linked HCC is probably due to the more advanced stage at diagnosis, he said.
The findings highlight the need for improved surveillance in these patients, a process complicated by the challenge of imaging the liver in obese patients, said Dr. Younossi, chairman of medicine and head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Dr. Younossi has been a consultant to Gilead, Abbvie, Bristol-Myers Squibb, GlaxoSmithKline, Intercept, and Salix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIENNA – Nonalcoholic fatty liver disease (NAFLD) now stands as the second most common cause of U.S. cases of hepatocellular carcinoma, and with highly effective drug regimens now sharply dropping the prevalence of hepatitis C virus infection, NAFLD – a complication of obesity – is poised to snag the top spot, Dr. Zobair Younossi said in a video interview at the meeting sponsored by the European Association for the Study of the Liver.
In an analysis that combined U.S. national cancer registry data collected by the National Cancer Institute and morbidity diagnostic codes collected by Medicare, Dr. Younossi calculated that, during 2004-2009 among U.S. adults covered by Medicare, 24% of patients newly diagnosed with hepatocellular carcinoma (HCC) had NAFLD as their pre-existing chronic liver disease, compared with 48% who had hepatitis C virus infection as their trigger. The third most common cause of HCC was alcoholic liver disease (14%), followed by hepatitis B virus infection (8%).
Dr. Younossi’s analysis also included survival data for each HCC case in the first year following diagnosis, which showed that NAFLD-associated cases also were deadlier, linking with a statistically significant 20% increase in mortality compared with HCC associated with other causes. Quicker lethality of NAFLD-linked HCC is probably due to the more advanced stage at diagnosis, he said.
The findings highlight the need for improved surveillance in these patients, a process complicated by the challenge of imaging the liver in obese patients, said Dr. Younossi, chairman of medicine and head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Dr. Younossi has been a consultant to Gilead, Abbvie, Bristol-Myers Squibb, GlaxoSmithKline, Intercept, and Salix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
VIENNA – Nonalcoholic fatty liver disease (NAFLD) now stands as the second most common cause of U.S. cases of hepatocellular carcinoma, and with highly effective drug regimens now sharply dropping the prevalence of hepatitis C virus infection, NAFLD – a complication of obesity – is poised to snag the top spot, Dr. Zobair Younossi said in a video interview at the meeting sponsored by the European Association for the Study of the Liver.
In an analysis that combined U.S. national cancer registry data collected by the National Cancer Institute and morbidity diagnostic codes collected by Medicare, Dr. Younossi calculated that, during 2004-2009 among U.S. adults covered by Medicare, 24% of patients newly diagnosed with hepatocellular carcinoma (HCC) had NAFLD as their pre-existing chronic liver disease, compared with 48% who had hepatitis C virus infection as their trigger. The third most common cause of HCC was alcoholic liver disease (14%), followed by hepatitis B virus infection (8%).
Dr. Younossi’s analysis also included survival data for each HCC case in the first year following diagnosis, which showed that NAFLD-associated cases also were deadlier, linking with a statistically significant 20% increase in mortality compared with HCC associated with other causes. Quicker lethality of NAFLD-linked HCC is probably due to the more advanced stage at diagnosis, he said.
The findings highlight the need for improved surveillance in these patients, a process complicated by the challenge of imaging the liver in obese patients, said Dr. Younossi, chairman of medicine and head of the Center for Liver Diseases at Inova Fairfax Hospital in Falls Church, Va.
Dr. Younossi has been a consultant to Gilead, Abbvie, Bristol-Myers Squibb, GlaxoSmithKline, Intercept, and Salix.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE INTERNATIONAL LIVER CONGRESS 2015
Unrecognized Hepatitis C Linked With Advanced Hepatic Fibrosis
VIENNA – Roughly half of American adults with chronic hepatitis C infection are unaware of their infection, and about one-fifth of these people with unsuspected infection likely have advanced liver fibrosis, according to a new analysis of U.S. data.
These findings “strengthen the recommendation for hepatitis C virus (HCV) screening in asymptomatic individuals,” Dr. Prowpanga Udompap said at the meeting sponsored by the European Association for the Study of the Liver.
People infected by HCV with advanced liver fibrosis have top priority for receiving curative drug treatment, according to recommendations by the American Association for the Study of the Liver and the Infectious Diseases Society of America.
People who have HCV-associated liver fibrosis that goes untreated also risk having their infection become more refractory to cure over time, they risk progressive hepatic deterioration that will eventually become symptomatic, and they face increasing risk for developing liver cancer, noted Dr. W. Ray Kim, senior author of the study and professor of medicine and chief of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Kim said he was surprised that such a large percentage of Americans who have unrecognized HCV infection also probably have substantial hepatic damage.
“To me it’s alarming that 20% of people who are not aware of their HCV infection are treatment candidates. These people are out there, but not getting treated,” he said in an interview.
Current U.S. HCV screening recommendations from the Centers for Disease Control and Prevention call for screening all Americans born during 1945-1965, “but there is no incentive to screen” and many U.S. primary care physicians don’t have HCV screening on their radar, he said.
The analysis conducted by Dr. Udompap and Dr. Kim used data collected by the National Health and Nutrition Examination Survey during 2001-2012, when the National Center for Health Statistics administered HCV testing to 45,000 of the 62,000 individuals who participated in the survey during this period.
Of the 45,000 people tested, 420 (0.9%) screened antibody positive and had infection confirmed by a second, RNA-based test. The HCV positive patients then received a survey that included a question of whether they were aware of their HCV status before their current test result notification. One hundred sixty-three people (39%) completed and returned the survey: Eighty-three said they had previously been unaware they were HCV positive, and 80 said that they had known about their infection. The 50% rate of awareness of HCV chronic infection is consistent with a previously reported rate (Hepatology 2012;55:1652-61), said Dr. Udompap, a gastroenterology researcher at Stanford.
Individuals who were aware of their infection and those who were not had very similar demographic and clinical parameters. The average age was 53 years, and about two-thirds were men.
Dr. Udompap ran estimates of each respondent’s liver fibrosis and cirrhosis severity using the FIB-4 score and APRI score and data collected during the survey on age, liver enzyme levels, and platelet counts. These calculations showed that 22% of those ignorant of their HCV-positive status had a high probability of having advanced fibrosis, and 11% had a high probability of having cirrhosis, Dr. Udompap reported.
These rates tracked close to those of the people who knew about their HCV-positive status, of whom 15% had a high probability of having advanced liver fibrosis and 11% were highly likely to have cirrhosis.
Dr. Udompap reported having no financial disclosures. Dr. Kim has been a consultant to several drug companies that market, or are developing drugs, used to eradicate hepatitis C infections.
VIENNA – Roughly half of American adults with chronic hepatitis C infection are unaware of their infection, and about one-fifth of these people with unsuspected infection likely have advanced liver fibrosis, according to a new analysis of U.S. data.
These findings “strengthen the recommendation for hepatitis C virus (HCV) screening in asymptomatic individuals,” Dr. Prowpanga Udompap said at the meeting sponsored by the European Association for the Study of the Liver.
People infected by HCV with advanced liver fibrosis have top priority for receiving curative drug treatment, according to recommendations by the American Association for the Study of the Liver and the Infectious Diseases Society of America.
People who have HCV-associated liver fibrosis that goes untreated also risk having their infection become more refractory to cure over time, they risk progressive hepatic deterioration that will eventually become symptomatic, and they face increasing risk for developing liver cancer, noted Dr. W. Ray Kim, senior author of the study and professor of medicine and chief of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Kim said he was surprised that such a large percentage of Americans who have unrecognized HCV infection also probably have substantial hepatic damage.
“To me it’s alarming that 20% of people who are not aware of their HCV infection are treatment candidates. These people are out there, but not getting treated,” he said in an interview.
Current U.S. HCV screening recommendations from the Centers for Disease Control and Prevention call for screening all Americans born during 1945-1965, “but there is no incentive to screen” and many U.S. primary care physicians don’t have HCV screening on their radar, he said.
The analysis conducted by Dr. Udompap and Dr. Kim used data collected by the National Health and Nutrition Examination Survey during 2001-2012, when the National Center for Health Statistics administered HCV testing to 45,000 of the 62,000 individuals who participated in the survey during this period.
Of the 45,000 people tested, 420 (0.9%) screened antibody positive and had infection confirmed by a second, RNA-based test. The HCV positive patients then received a survey that included a question of whether they were aware of their HCV status before their current test result notification. One hundred sixty-three people (39%) completed and returned the survey: Eighty-three said they had previously been unaware they were HCV positive, and 80 said that they had known about their infection. The 50% rate of awareness of HCV chronic infection is consistent with a previously reported rate (Hepatology 2012;55:1652-61), said Dr. Udompap, a gastroenterology researcher at Stanford.
Individuals who were aware of their infection and those who were not had very similar demographic and clinical parameters. The average age was 53 years, and about two-thirds were men.
Dr. Udompap ran estimates of each respondent’s liver fibrosis and cirrhosis severity using the FIB-4 score and APRI score and data collected during the survey on age, liver enzyme levels, and platelet counts. These calculations showed that 22% of those ignorant of their HCV-positive status had a high probability of having advanced fibrosis, and 11% had a high probability of having cirrhosis, Dr. Udompap reported.
These rates tracked close to those of the people who knew about their HCV-positive status, of whom 15% had a high probability of having advanced liver fibrosis and 11% were highly likely to have cirrhosis.
Dr. Udompap reported having no financial disclosures. Dr. Kim has been a consultant to several drug companies that market, or are developing drugs, used to eradicate hepatitis C infections.
VIENNA – Roughly half of American adults with chronic hepatitis C infection are unaware of their infection, and about one-fifth of these people with unsuspected infection likely have advanced liver fibrosis, according to a new analysis of U.S. data.
These findings “strengthen the recommendation for hepatitis C virus (HCV) screening in asymptomatic individuals,” Dr. Prowpanga Udompap said at the meeting sponsored by the European Association for the Study of the Liver.
People infected by HCV with advanced liver fibrosis have top priority for receiving curative drug treatment, according to recommendations by the American Association for the Study of the Liver and the Infectious Diseases Society of America.
People who have HCV-associated liver fibrosis that goes untreated also risk having their infection become more refractory to cure over time, they risk progressive hepatic deterioration that will eventually become symptomatic, and they face increasing risk for developing liver cancer, noted Dr. W. Ray Kim, senior author of the study and professor of medicine and chief of gastroenterology and hepatology at Stanford (Calif.) University.
Dr. Kim said he was surprised that such a large percentage of Americans who have unrecognized HCV infection also probably have substantial hepatic damage.
“To me it’s alarming that 20% of people who are not aware of their HCV infection are treatment candidates. These people are out there, but not getting treated,” he said in an interview.
Current U.S. HCV screening recommendations from the Centers for Disease Control and Prevention call for screening all Americans born during 1945-1965, “but there is no incentive to screen” and many U.S. primary care physicians don’t have HCV screening on their radar, he said.
The analysis conducted by Dr. Udompap and Dr. Kim used data collected by the National Health and Nutrition Examination Survey during 2001-2012, when the National Center for Health Statistics administered HCV testing to 45,000 of the 62,000 individuals who participated in the survey during this period.
Of the 45,000 people tested, 420 (0.9%) screened antibody positive and had infection confirmed by a second, RNA-based test. The HCV positive patients then received a survey that included a question of whether they were aware of their HCV status before their current test result notification. One hundred sixty-three people (39%) completed and returned the survey: Eighty-three said they had previously been unaware they were HCV positive, and 80 said that they had known about their infection. The 50% rate of awareness of HCV chronic infection is consistent with a previously reported rate (Hepatology 2012;55:1652-61), said Dr. Udompap, a gastroenterology researcher at Stanford.
Individuals who were aware of their infection and those who were not had very similar demographic and clinical parameters. The average age was 53 years, and about two-thirds were men.
Dr. Udompap ran estimates of each respondent’s liver fibrosis and cirrhosis severity using the FIB-4 score and APRI score and data collected during the survey on age, liver enzyme levels, and platelet counts. These calculations showed that 22% of those ignorant of their HCV-positive status had a high probability of having advanced fibrosis, and 11% had a high probability of having cirrhosis, Dr. Udompap reported.
These rates tracked close to those of the people who knew about their HCV-positive status, of whom 15% had a high probability of having advanced liver fibrosis and 11% were highly likely to have cirrhosis.
Dr. Udompap reported having no financial disclosures. Dr. Kim has been a consultant to several drug companies that market, or are developing drugs, used to eradicate hepatitis C infections.
AT THE INTERNATIONAL LIVER CONGRESS 2015