User login
Pandemic necessitates new strategies to treat migraine
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
Patients with migraine who are unable to continue preventive procedures such as onabotulinumtoxinA injections during the COVID-19 pandemic may be at risk of worsening migraine, according to an article published March 30 in Headache. To address this scenario, including monoclonal antibodies against calcitonin gene-related peptide (CGRP) or the CGRP receptor, the authors said.
“This is a particularly vulnerable time for individuals with migraine and other disabling headache disorders, with many physical and mental stressors, increased anxiety, and changes in daily routine which may serve as triggering factors for worsening headache,” said lead author Christina L. Szperka, MD, director of the pediatric headache program at Children’s Hospital of Philadelphia, and colleagues.
Acute treatment
The authors described potential treatment regimens based on their experience as headache specialists and the experiences of their colleagues. For acute therapy options, NSAIDs, triptans, and neuroleptics may be used in combination when needed. Medications within the same drug category should not be combined, however, and triptans, dihydroergotamine, and lasmiditan should not be coadministered within 24 hours. Since the 2015 American Headache Society guideline for the acute treatment of migraine, the Food and Drug Administration has approved additional acute migraine medications, including ubrogepant, rimegepant, and lasmiditan, the authors said. The agency also cleared several neuromodulation devices for the acute treatment of migraine.
Although few drugs have been studied as treatments for unusually prolonged severe headaches, headache doctors often recommend NSAIDs before patients seek care at an emergency department or infusion center, the authors said. NSAID options include indomethacin, ketorolac, naproxen, nabumetone, diclofenac, and mefenamic acid. Neuroleptics also may be used. “Long-acting triptan medications can be used as bridge therapies, as is often done in the treatment of menstrually related migraine or in the treatment of medication overuse headache,” they said. “We propose a similar strategy can be trialed as a therapeutic option for refractory or persistent migraine.”
The authors also described the use of antiepileptics and corticosteroids, as well as drugs that may treat specific symptoms, such as difficulty sleeping (hydroxyzine or amitriptyline), neck or muscle pain (tizanidine), and aura with migraine (magnesium). Clinicians should avoid the use of opioids and butalbital, they said.
Preventive treatment
“While the injection of onabotulinumtoxinA is an effective treatment for chronic migraine, the procedure can put the patient and the provider at higher risk of COVID-19 given the close contact encounter,” wrote Dr. Szperka and colleagues. “We believe that other migraine preventive treatments should be utilized first when possible.” Since the publication of a guideline on preventive migraine therapies in 2012, the FDA has approved additional preventive therapies, including the anti-CGRP monoclonal antibodies erenumab‐aooe, galcanezumab‐gnlm, fremanezumab‐vfrm, and eptinezumab‐jjmr. “The first three are intended for self‐injection at home, with detailed instructions available for each product on its website,” they said.
Among angiotensin‐converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs), candesartan has evidence of efficacy and tolerability in migraine prevention. Lisinopril has been considered possibly effective. “There has been recent concern in the media about the possibility of these medications interfering with the body’s response to COVID‐19,” the authors said, although this theoretical concern was not based on experimental or clinical data. “For patients in need of a new preventive therapy, the potential for benefit with an ACE/ARB must be weighed against the theoretical increased risk of infection.”
In addition, studies indicate that melatonin may prevent migraine with few side effects and that zonisamide may be effective in patients who have an inadequate response to or experience side effects with topiramate.
Policy changes and telehealth options
Effectively treating patients with migraine during the pandemic requires policy changes, according to the authors. “Migraine preventive prior authorization restrictions need to be lifted for evidence‐based, FDA‐approved therapies; patients need to be able to access these medications quickly and easily. Patients should not be required to fail older medications,” they said. “Similarly, in order to permit the transition of patients from onabotulinumtoxinA to anti‐CGRP [monoclonal antibodies], insurers should remove the prohibition against simultaneous coverage of these drug classes.” Insurers also should loosen restrictions on the off-label use of acute and preventive medication for adolescents, Dr. Szperka and coauthors suggest.
“In the era of COVID‐19, telehealth has become an essential modality for most headache specialists, given the need for providers to take significant precautions for both their patients and themselves, limiting touch or close contact,” they said. Patients with headache may warrant additional screening for COVID-19 as well. “As headache has been reported as an early symptom of COVID‐19, patients with worsening or new onset severe headache should be reviewed for exposure risk and any other symptoms which may be consistent with COVID‐19 infection,” the authors said.
There was no direct funding for the report. Dr. Szperka and a coauthor receive salary support from the National Institutes of Health. Dr. Szperka also has received grant support from Pfizer, and her institution has received compensation for her consulting work for Allergan. Several coauthors disclosed consulting and serving on speakers’ bureaus for and receiving research support from various pharmaceutical companies.
SOURCE: Szperka CL et al. Headache. 2020 Mar 30. doi: 10.1111/head.13810.
FROM HEADACHE
When to treat, delay, or omit breast cancer therapy in the face of COVID-19
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Nothing is business as usual during the COVID-19 pandemic, and that includes breast cancer therapy. That’s why two groups have released guidance documents on treating breast cancer patients during the pandemic.
A guidance on surgery, drug therapy, and radiotherapy was created by the COVID-19 Pandemic Breast Cancer Consortium. This guidance is set to be published in Breast Cancer Research and Treatment and can be downloaded from the American College of Surgeons website.
A group from Memorial Sloan Kettering Cancer Center (MSKCC) created a guidance document on radiotherapy for breast cancer patients, and that guidance was recently published in Advances in Radiation Oncology.
Prioritizing certain patients and treatments
As hospital beds and clinics fill with coronavirus-infected patients, oncologists must balance the need for timely therapy for their patients with the imperative to protect vulnerable, immunosuppressed patients from exposure and keep clinical resources as free as possible.
“As we’re taking care of breast cancer patients during this unprecedented pandemic, what we’re all trying to do is balance the most effective treatments for our patients against the risk of additional exposures, either from other patients [or] from being outside, and considerations about the safety of our staff,” said Steven Isakoff, MD, PhD, of Massachusetts General Hospital Cancer Center in Boston, who is an author of the COVID-19 Pandemic Breast Cancer Consortium guidance.
The consortium’s guidance recommends prioritizing treatment according to patient needs and the disease type and stage. The three basic categories for considering when to treat are:
- Priority A: Patients who have immediately life-threatening conditions, are clinically unstable, or would experience a significant change in prognosis with even a short delay in treatment.
- Priority B: Deferring treatment for a short time (6-12 weeks) would not impact overall outcomes in these patients.
- Priority C: These patients are stable enough that treatment can be delayed for the duration of the COVID-19 pandemic.
“The consortium highly recommends multidisciplinary discussion regarding priority for elective surgery and adjuvant treatments for your breast cancer patients,” the guidance authors wrote. “The COVID-19 pandemic may vary in severity over time, and these recommendations are subject to change with changing COVID-19 pandemic severity.”
For example, depending on local circumstances, the guidance recommends limiting immediate outpatient visits to patients with potentially unstable conditions such as infection or hematoma. Established patients with new problems or patients with a new diagnosis of noninvasive cancer might be managed with telemedicine visits, and patients who are on follow-up with no new issues or who have benign lesions might have their visits safely postponed.
Surgery and drug recommendations
High-priority surgical procedures include operative drainage of a breast abscess in a septic patient and evacuation of expanding hematoma in a hemodynamically unstable patient, according to the consortium guidance.
Other surgical situations are more nuanced. For example, for patients with triple-negative breast cancer (TNBC) or HER2-positive disease, the guidance recommends neoadjuvant chemotherapy or HER2-targeted chemotherapy in some cases. In other cases, institutions may proceed with surgery before chemotherapy, but “these decisions will depend on institutional resources and patient factors,” according to the authors.
The guidance states that chemotherapy and other drug treatments should not be delayed in patients with oncologic emergencies, such as febrile neutropenia, hypercalcemia, intolerable pain, symptomatic pleural effusions, or brain metastases.
In addition, patients with inflammatory breast cancer, TNBC, or HER2-positive breast cancer should receive neoadjuvant/adjuvant chemotherapy. Patients with metastatic disease that is likely to benefit from therapy should start chemotherapy, endocrine therapy, or targeted therapy. And patients who have already started neoadjuvant/adjuvant chemotherapy or oral adjuvant endocrine therapy should continue on these treatments.
Radiation therapy recommendations
The consortium guidance recommends administering radiation to patients with bleeding or painful inoperable locoregional disease, those with symptomatic metastatic disease, and patients who progress on neoadjuvant chemotherapy.
In contrast, older patients (aged 65-70 years) with lower-risk, stage I, hormone receptor–positive, HER2-negative cancers who are on adjuvant endocrine therapy can safely defer or omit radiation without affecting their overall survival, according to the guidance. Patients with ductal carcinoma in situ, especially those with estrogen receptor–positive disease on endocrine therapy, can safely omit radiation.
“There are clearly conditions where radiation might reduce the risk of recurrence but not improve overall survival, where a delay in treatment really will have minimal or no impact,” Dr. Isakoff said.
The MSKCC guidance recommends omitting radiation for some patients with favorable-risk disease and truncating or accelerating regimens using hypofractionation for others who require whole-breast radiation or post-mastectomy treatment.
The MSKCC guidance also contains recommendations for prioritization of patients according to disease state and the urgency of care. It divides cases into high, intermediate, and low priority for breast radiotherapy, as follows:
- Tier 1 (high priority): Patients with inflammatory breast cancer, residual node-positive disease after neoadjuvant chemotherapy, four or more positive nodes (N2), recurrent disease, node-positive TNBC, or extensive lymphovascular invasion.
- Tier 2 (intermediate priority): Patients with estrogen receptor–positive disease with one to three positive nodes (N1a), pathologic stage N0 after neoadjuvant chemotherapy, lymphovascular invasion not otherwise specified, or node-negative TNBC.
- Tier 3 (low priority): Patients with early-stage estrogen receptor-positive breast cancer (especially patients of advanced age), patients with ductal carcinoma in situ, or those who otherwise do not meet the criteria for tiers 1 or 2.
The MSKCC guidance also contains recommended hypofractionated or accelerated radiotherapy regimens for partial and whole-breast irradiation, post-mastectomy treatment, and breast and regional node irradiation, including recommended techniques (for example, 3-D conformal or intensity modulated approaches).
The authors of the MSKCC guidance disclosed relationships with eContour, Volastra Therapeutics, Sanofi, the Prostate Cancer Foundation, and Cancer Research UK. The authors of the COVID-19 Pandemic Breast Cancer Consortium guidance did not disclose any conflicts and said there was no funding source for the guidance.
SOURCES: Braunstein LZ et al. Adv Radiat Oncol. 2020 Apr 1. doi:10.1016/j.adro.2020.03.013; Dietz JR et al. 2020 Apr. Recommendations for prioritization, treatment and triage of breast cancer patients during the COVID-19 pandemic. Accepted for publication in Breast Cancer Research and Treatment.
Home-based chemo skyrockets at one U.S. center
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Major organization opposes concept
Major organization opposes concept
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
In the fall of 2019, the University of Pennsylvania in Philadelphia started a pilot program of home-based chemotherapy for two treatment regimens (one via infusion and one via injection). Six months later, the Cancer Care at Home program had treated 40 patients.
The uptake within the university’s large regional health system was acceptable but not rapid, admitted Amy Laughlin, MD, a hematology-oncology fellow involved with the program.
Then COVID-19 arrived, along with related travel restrictions.
Suddenly, in a 5-week period (March to April 7), 175 patients had been treated – a 300% increase from the first half year. Program staff jumped from 12 to 80 employees. The list of chemotherapies delivered went from two to seven, with more coming.
“We’re not the pilot anymore – we’re the standard of care,” Laughlin told Medscape Medical News.
“The impact [on patients] is amazing,” she said. “As long as you are selecting the right patients and right therapy, it is feasible and even preferable for a lot of patients.”
For example, patients with hormone-positive breast cancer who receive leuprolide (to shut down the ovaries and suppress estrogen production) ordinarily would have to visit a Penn facility for an injection every month, potentially for years. Now, a nurse can meet patients at home (or before the COVID-19 pandemic, even at their place of work) and administer the injection, saving the patient travel time and associated costs.
This home-based chemotherapy service does not appear to be offered elsewhere in the United States, and a major oncology organization – the Community Oncology Alliance – is opposed to the practice because of patient safety concerns.
The service is not offered at a sample of cancer centers queried by Medscape Medical News, including the Dana-Farber Cancer Institute in Boston, the Moffitt Cancer Center in Tampa, the Huntsman Cancer Institute in Salt Lake City, Utah, and Moores Cancer Center, the University of California, San Diego.
Opposition because of safety concerns
On April 9, the Community Oncology Alliance (COA) issued a statement saying it “fundamentally opposes home infusion of chemotherapy, cancer immunotherapy, and cancer treatment supportive drugs because of serious patient safety concerns.”
The COA warned that “many of the side effects caused by cancer treatment can have a rapid, unpredictable onset that places patients in incredible jeopardy and can even be life-threatening.”
In contrast, in a recent communication related to COVID-19, the National Comprehensive Cancer Network tacitly endorsed the concept, stating that a number of chemotherapies may potentially be administered at home, but it did not include guidelines for doing so.
The American Society of Clinical Oncology said that chemotherapy at home is “an issue [we] are monitoring closely,” according to a spokesperson.
What’s involved
Criteria for home-based chemotherapy at Penn include use of anticancer therapies that a patient has previously tolerated and low toxicity (that can be readily managed in the home setting). In addition, patients must be capable of following a med chart.
The chemotherapy is reconstituted at a Penn facility in a Philadelphia suburb. A courier then delivers the drug to the patient’s home, where it is administered by an oncology-trained nurse. Drugs must be stable for at least a few hours to qualify for the program.
The Penn program started with two regimens: EPOCH (etoposide, vincristine, doxorubicin, cyclophosphamide, and prednisone) for lymphoma, and leuprolide acetate injections for either breast or prostate cancer.
The two treatments are polar opposites in terms of complexity, common usage, and time required, which was intended, said Laughlin.
Time to deliver the chemo varies from a matter of minutes with leuprolide to more than 2 hours for rituximab, a lymphoma drug that may be added to EPOCH.
The current list of at-home chemo agents in the Penn program also includes bortezomib, lanreotide, zoledronic acid, and denosumab. Soon to come are rituximab and pembrolizumab for lung cancer and head and neck cancer.
Already practiced in some European countries
Home-based chemotherapy dates from at least the 1980s in the medical literature and is practiced in some European countries.
A 2018 randomized study of adjuvant treatment with capecitabine and oxaliplatin for stage II/III colon cancer in Denmark, where home-based care has been practiced for the past 2 years and is growing in use, concluded that “it might be a valuable alternative to treatment at an outpatient clinic.”
However, in the study, there was no difference in quality of life between the home and outpatient settings, which is somewhat surprising, inasmuch as a major appeal to receiving chemotherapy at home is that it is less disruptive compared to receiving it in a hospital or clinic, which requires travel.
Also, chemo at home “may be resource intensive” and have a “lower throughput of patients due to transportation time,” cautioned the Danish investigators, who were from Herlev and Gentofte Hospital.
A 2015 review called home chemo “a safe and patient‐centered alternative to hospital‐ and outpatient‐based service.” Jenna Evans, PhD, McMaster University, Toronto, Canada, and lead author of that review, says there are two major barriers to infusion chemotherapy in homes.
One is inadequate resources in the community, such as oncology-trained nurses to deliver treatment, and the other is perceptions of safety and quality, including among healthcare providers.
COVID-19 might prompt more chemo at home, said Evans, a health policy expert, in an email to Medscape Medical News. “It is not unusual for change of this type and scale to require a seismic event to become more mainstream,” she argued.
Reimbursement for home-based chemo is usually the same as for chemo in a free-standing infusion suite, says Cassandra Redmond, PharmD, MBA, director of pharmacy, Penn Home Infusion Therapy.
Private insurers and Medicare cover a subset of infused medications at home, but coverage is limited. “The opportunity now is to expand these initiatives ... to include other cancer therapies,” she said about coverage.
This article first appeared on Medscape.com.
Amid coronavirus concerns, researchers urge mental health interventions for patients with dementia
according to a letter published online ahead of print March 30 in Lancet. Consistent with recommendations from Alzheimer’s Disease International and other dementia experts, teams that include mental health professionals, social workers, nursing home administrators, and volunteers should collaborate to provide mental health care for people with dementia. Experts in dementia should lead each team and support team members from other disciplines, wrote Huali Wang, MD, chair of clinical research at Peking University Institute of Mental Health in Beijing, and colleagues.
Interventions could be administered through telehealth, said the authors. Teams led by mental health professionals could use electronic media to provide self-help guidance for reducing stress, such as relaxation or meditation exercise. These teams also could use telephone hotlines to support behavioral management, and psychological counselors could provide online consultations for caregivers in nursing homes or in the community. “We encourage people who have a parent with dementia to have more frequent contact or spend more time with their parent, or to take on some of the caregiving duties so as to give the carer some respite time,” wrote Dr. Wang and colleagues.
Many local authorities are banning visits to nursing home residents to reduce the latter’s risk of COVID-19 infection. As a consequence, these elderly people are becoming more isolated, and anxiety is increasing among nursing home staffs.
In China, five organizations, including the Chinese Society of Geriatric Psychiatry and Alzheimer’s Disease Chinese, responded to the COVID-19 outbreak by publishing recommendations for providing mental health and psychosocial support. Groups of providers from various disciplines offered free counseling services for people with dementia and their caregivers. “These approaches minimized the complex impact of both COVID-19 outbreak and dementia,” wrote the authors.
“China has contained the epidemic, and business is starting to return to normal,” they continued. “We believe that learning lessons from China would empower the world to tackle the COVID-19 pandemic, with little risk of compromising the quality of life of people living with dementia and their carers.”
Dr. Wang has received lecture fees from Eisai China and Lundbeck China. She owns the copyright for the neuropsychiatric symptoms individualized management system. Her coauthors reported serving as advisory board members and receiving fees from companies such as Biogen, Novartis, and Genentech.
SOURCE: Wang H et al. Lancet. 2020 Mar 30. doi: 10.1016/S0140-6736(20)30755-8.
according to a letter published online ahead of print March 30 in Lancet. Consistent with recommendations from Alzheimer’s Disease International and other dementia experts, teams that include mental health professionals, social workers, nursing home administrators, and volunteers should collaborate to provide mental health care for people with dementia. Experts in dementia should lead each team and support team members from other disciplines, wrote Huali Wang, MD, chair of clinical research at Peking University Institute of Mental Health in Beijing, and colleagues.
Interventions could be administered through telehealth, said the authors. Teams led by mental health professionals could use electronic media to provide self-help guidance for reducing stress, such as relaxation or meditation exercise. These teams also could use telephone hotlines to support behavioral management, and psychological counselors could provide online consultations for caregivers in nursing homes or in the community. “We encourage people who have a parent with dementia to have more frequent contact or spend more time with their parent, or to take on some of the caregiving duties so as to give the carer some respite time,” wrote Dr. Wang and colleagues.
Many local authorities are banning visits to nursing home residents to reduce the latter’s risk of COVID-19 infection. As a consequence, these elderly people are becoming more isolated, and anxiety is increasing among nursing home staffs.
In China, five organizations, including the Chinese Society of Geriatric Psychiatry and Alzheimer’s Disease Chinese, responded to the COVID-19 outbreak by publishing recommendations for providing mental health and psychosocial support. Groups of providers from various disciplines offered free counseling services for people with dementia and their caregivers. “These approaches minimized the complex impact of both COVID-19 outbreak and dementia,” wrote the authors.
“China has contained the epidemic, and business is starting to return to normal,” they continued. “We believe that learning lessons from China would empower the world to tackle the COVID-19 pandemic, with little risk of compromising the quality of life of people living with dementia and their carers.”
Dr. Wang has received lecture fees from Eisai China and Lundbeck China. She owns the copyright for the neuropsychiatric symptoms individualized management system. Her coauthors reported serving as advisory board members and receiving fees from companies such as Biogen, Novartis, and Genentech.
SOURCE: Wang H et al. Lancet. 2020 Mar 30. doi: 10.1016/S0140-6736(20)30755-8.
according to a letter published online ahead of print March 30 in Lancet. Consistent with recommendations from Alzheimer’s Disease International and other dementia experts, teams that include mental health professionals, social workers, nursing home administrators, and volunteers should collaborate to provide mental health care for people with dementia. Experts in dementia should lead each team and support team members from other disciplines, wrote Huali Wang, MD, chair of clinical research at Peking University Institute of Mental Health in Beijing, and colleagues.
Interventions could be administered through telehealth, said the authors. Teams led by mental health professionals could use electronic media to provide self-help guidance for reducing stress, such as relaxation or meditation exercise. These teams also could use telephone hotlines to support behavioral management, and psychological counselors could provide online consultations for caregivers in nursing homes or in the community. “We encourage people who have a parent with dementia to have more frequent contact or spend more time with their parent, or to take on some of the caregiving duties so as to give the carer some respite time,” wrote Dr. Wang and colleagues.
Many local authorities are banning visits to nursing home residents to reduce the latter’s risk of COVID-19 infection. As a consequence, these elderly people are becoming more isolated, and anxiety is increasing among nursing home staffs.
In China, five organizations, including the Chinese Society of Geriatric Psychiatry and Alzheimer’s Disease Chinese, responded to the COVID-19 outbreak by publishing recommendations for providing mental health and psychosocial support. Groups of providers from various disciplines offered free counseling services for people with dementia and their caregivers. “These approaches minimized the complex impact of both COVID-19 outbreak and dementia,” wrote the authors.
“China has contained the epidemic, and business is starting to return to normal,” they continued. “We believe that learning lessons from China would empower the world to tackle the COVID-19 pandemic, with little risk of compromising the quality of life of people living with dementia and their carers.”
Dr. Wang has received lecture fees from Eisai China and Lundbeck China. She owns the copyright for the neuropsychiatric symptoms individualized management system. Her coauthors reported serving as advisory board members and receiving fees from companies such as Biogen, Novartis, and Genentech.
SOURCE: Wang H et al. Lancet. 2020 Mar 30. doi: 10.1016/S0140-6736(20)30755-8.
REPORTING FROM THE LANCET
Conducting cancer trials amid the COVID-19 pandemic
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
More than three-quarters of cancer clinical research programs have experienced operational changes during the COVID-19 pandemic, according to a survey conducted by the Association of Community Cancer Centers (ACCC) during a recent webinar.
The webinar included insights into how some cancer research programs have adapted to the pandemic, a review of guidance for conducting cancer trials during this time, and a discussion of how the cancer research landscape may be affected by COVID-19 going forward.
The webinar was led by Randall A. Oyer, MD, president of the ACCC and medical director of the oncology program at Penn Medicine Lancaster General Health in Pennsylvania.
The impact of COVID-19 on cancer research
Dr. Oyer observed that planning and implementation for COVID-19–related illness at U.S. health care institutions has had a predictable effect of limiting patient access and staff availability for nonessential services.
Coronavirus-related exposure and/or illness has relegated cancer research to a lower-level priority. As a result, ACCC institutions have made adjustments in their cancer research programs, including moving clinical research coordinators off-campus and deploying them in clinical areas.
New clinical trials have not been opened. In some cases, new accruals have been halted, particularly for registry, prevention, and symptom control trials.
Standards that have changed and those that have not
Guidance documents for conducting clinical trials during the pandemic have been developed by the Food and Drug Administration, the National Cancer Institute’s Cancer Therapy Evaluation Program and Central Institutional Review Board, and the National Institutes of Health’s Office of Extramural Research. Industry sponsors and parent institutions of research programs have also disseminated guidance.
Among other topics, guidance documents have addressed:
- How COVID-19-related protocol deviations will be judged at monitoring visits and audits
- Missed office visits and endpoint evaluations
- Providing investigational oral medications to patients via mail and potential issues of medication unavailability
- Processes for patients to have interim visits with providers at external institutions, including providers who may not be personally engaged in or credentialed for the research trial
- Potential delays in submitting protocol amendments for institutional review board (IRB) review
- Recommendations for patients confirmed or suspected of having a coronavirus infection.
Dr. Oyer emphasized that patient safety must remain the highest priority for patient management, on or off study. He advised continuing investigational therapy when potential benefit from treatment is anticipated and identifying alternative methods to face-to-face visits for monitoring and access to treatment.
Dr. Oyer urged programs to:
- Maintain good clinical practice standards
- Consult with sponsors and IRBs when questions arise but implement changes that affect patient safety prior to IRB review if necessary
- Document all deviations and COVID-19 related adaptations in a log or spreadsheet in anticipation of future questions from sponsors, monitors, and other entities.
New questions and considerations
In the short-term, Dr. Oyer predicts fewer available trials and a decreased rate of accrual to existing studies. This may result in delays in trial completion and the possibility of redesign for some trials.
He predicts the emergence of COVID-19-focused research questions, including those assessing the course of coronavirus infection in various malignant settings and the impact of cancer-directed treatments and supportive care interventions (e.g., treatment for graft-versus-host disease) on response to COVID-19.
To facilitate developing a clinically and research-relevant database, Dr. Oyer stressed the importance of documentation in the research record, reporting infections as serious adverse events. Documentation should specify whether the infection was confirmed or suspected coronavirus or related to another organism.
In general, when coronavirus infection is strongly suspected, Dr. Oyer said investigational treatments should be interrupted, but study-specific criteria will be forthcoming on that issue.
Looking to the future
For patients with advanced cancers, clinical trials provide an important option for hope and clinical benefit. Disrupting the conduct of clinical trials could endanger the lives of participants and delay the emergence of promising treatments and diagnostic tests.
When the coronavirus pandemic recedes, advancing knowledge and treatments for cancer will demand renewed commitment across the oncology care community.
Going forward, Dr. Oyer advised that clinical research staff protect their own health and the safety of trial participants. He encouraged programs to work with sponsors and IRBs to solve logistical problems and clarify individual issues.
He was optimistic that resumption of more normal conduct of studies will enable the successful completion of ongoing trials, enhanced by the creative solutions that were devised during the crisis and by additional prospective, clinically annotated, carefully recorded data from academic and community research sites.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
Evaluating and managing the patient with nipple discharge
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4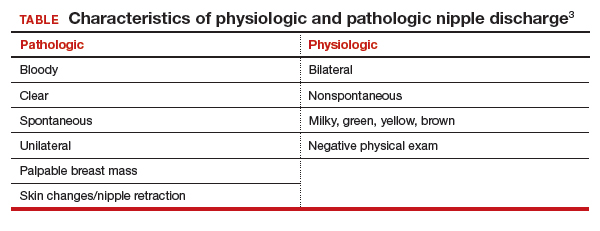
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.
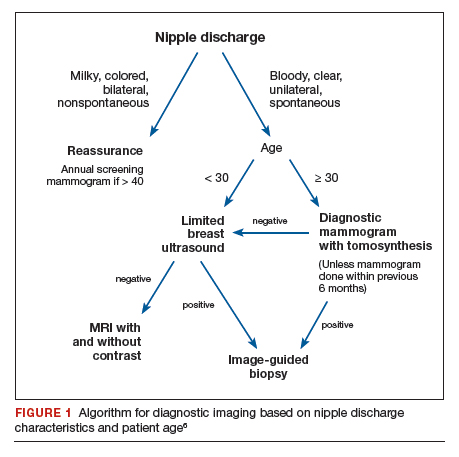
Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.

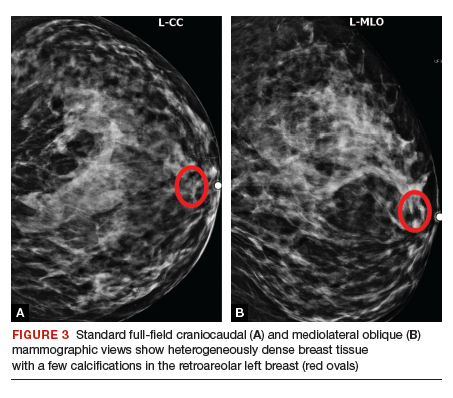
Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.

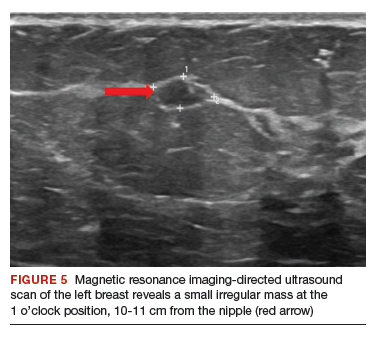
Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
CASE Young woman with discharge from one nipple
A 26-year-old African American woman presents with a 10-month history of left nipple discharge. The patient describes the discharge as spontaneous, colored dark brown to yellow, and occurring from a single opening in the nipple. The discharge is associated with left breast pain and fullness, without a palpable lump. The patient has no family or personal history of breast cancer.
Nipple discharge is the third most common breast-related symptom (after palpable masses and breast pain), with an estimated prevalence of 5% to 8% among premenopausal women.1 While most causes of nipple discharge reflect benign issues, approximately 5% to 12% of breast cancers have nipple discharge as the only symptom.2 Not surprisingly, nipple discharge creates anxiety for both patients and clinicians.
In this article, we—a breast imaging radiologist, gynecologist, and breast surgeon—outline key steps for evaluating and managing patients with nipple discharge.
Two types of nipple discharge
Nipple discharge can be characterized as physiologic or pathologic. The distinction is based on the patient’s history in conjunction with the clinical breast exam.
Physiologic nipple discharge often is bilateral, nonspontaneous, and white, yellow, green, or brown (TABLE).3 It often is due to nipple stimulation, and the patient can elicit discharge by manually manipulating the breast. Usually, multiple ducts are involved. Galactorrhea refers specifically to milky discharge and occurs most commonly during pregnancy or lactation.2 Galactorrhea that is not associated with pregnancy or lactation often is related to elevated prolactin or thyroid-stimulating hormone levels or to medications. One study reported that no cancers were found when discharge was nonspontaneous and colored or milky.4
Pathologic nipple discharge is defined as a spontaneous, bloody, clear, or single-duct discharge. A palpable mass in the same breast automatically increases the suspicion of the discharge, regardless of its color or spontaneity.2 The most common cause of pathologic nipple discharge is an intraductal papilloma, a benign epithelial tumor, which accounts for approximately 57% of cases.5
Although the risk of malignancy is low for all patients with nipple discharge, increasing age is associated with increased risk of breast cancer. One study demonstrated that among women aged 40 to 60 years presenting with nipple discharge, the prevalence of invasive cancer is 10%, and the percentage jumps to 32% among women older than 60.6
Breast exam. For any patient with nonlactational nipple discharge, we recommend a thorough breast examination. Deep palpation of all quadrants of the symptomatic breast, especially near the nipple areolar complex, should elicit nipple discharge without any direct squeezing of the nipple. If the patient’s history and physical exam are consistent with physiologic discharge, no further workup is needed. Reassure the patient and recommend appropriate breast cancer screening. Encourage the patient to decrease stimulation or manual manipulation of the nipples if the discharge bothers her.
Continue to: CASE Continued: Workup...
CASE Continued: Workup
On physical exam, the patient’s breasts are noted to be cup size DDD and asymmetric, with the left breast larger than the right; there is no contour deformity. There is no skin or nipple retraction, skin rash, swelling, or nipple changes bilaterally. No dominant masses are appreciated bilaterally. Manual compression elicits no nipple discharge.
Although the discharge is nonbloody, its spontaneity, unilaterality, and single-duct/orifice origin suggest a pathologic cause. The patient is referred for breast imaging.
Imaging workup for pathologic discharge
The American College of Radiology (ACR) Appropriateness Criteria is a useful tool that provides an evidence-based, easy-to-use algorithm for breast imaging in the patient with pathologic nipple discharge (FIGURE 1).6 The algorithm is categorized by patient age, with diagnostic mammography recommended for women aged 30 and older.6 Diagnostic mammography is recommended if the patient has not had a mammogram study in the last 6 months.6 For patients with no prior mammograms, we recommend bilateral diagnostic mammography to compare symmetry of the breasts.

Currently, no studies show that digital breast tomosynthesis (3-D mammography) has a benefit compared with standard 2-D mammography in women with pathologic nipple discharge.6 Given the increased sensitivity of digital breast tomosynthesis for cancer detection, however, in our practice it is standard to use tomosynthesis in the diagnostic evaluation of most patients.
Mammography
On mammography, ductal carcinoma in situ (DCIS) usually presents as calcifications. Both the morphology and distribution of calcifications are used to characterize them as suspicious or, typically, benign. DCIS usually presents as fine pleomorphic or fine linear branching calcifications in a segmental or linear distribution. In patients with pathologic nipple discharge and no other symptoms, the radiologist must closely examine the retroareolar region of the breast to assess for faint calcifications. Magnification views also can be performed to better characterize calcifications.
The sensitivity of mammography for nipple discharge varies in the literature, ranging from approximately 15% to 68%, with a specificity range of 38% to 98%.6 This results in a relatively low positive predictive value but a high negative predictive value of 90%.7 Mammographic sensitivity largely is limited by increased breast density. As more data emerge on the utility of digital breast tomosynthesis in dense breasts, mammographic sensitivity for nipple discharge will likely increase.
Ultrasonography
As an adjunct to mammography, the ACR Appropriateness Criteria recommends targeted (or “limited”) ultrasonography of the retroareolar region of the affected breast for patients aged 30 and older. Ultrasonography is useful to assess for intraductal masses and architectural distortion, and it has higher sensitivity but lower specificity than mammography. The sensitivity of ultrasonography for detecting breast cancer in patients presenting with nipple discharge is reported to be 56% to 80%.6 Ultrasonography can identify lesions not visible mammographically in 63% to 69% of cases.8 Although DCIS usually presents as calcifications, it also can present as an intraductal mass on ultrasonography.
The ACR recommends targeted ultrasonography for patients with nipple discharge and a negative mammogram, or to evaluate a suspicious mammographic abnormality such as architectural distortion, focal asymmetry, or a mass.6 For patient comfort, ultrasonography is the preferred modality for image-guided biopsy.
For women younger than 30 years, targeted ultrasonography is the initial imaging study of choice, according to the ACR criteria.6 Women younger than 30 years with pathologic nipple discharge have a very low risk of breast cancer and tend to have higher breast density, making mammography less useful. Although the radiation dose from mammography is negligible given technological improvements and dose-reduction techniques, ultrasonography remains the preferred initial imaging modality in young women, not only for nipple discharge but also for palpable lumps and focal breast pain.
Mammography is used as an adjunct to ultrasonography in women younger than 30 years when a suspicious abnormality is detected on ultrasonography, such as an intraductal mass or architectural distortion. In these cases, mammography can be used to assess for extent of disease or to visualize suspicious calcifications not well seen on ultrasonography.
For practical purposes regarding which imaging study to order for a patient, it is most efficient to order both a diagnostic mammogram (with tomosynthesis, if possible) and a targeted ultrasound scan of the affected breast. Even if both orders are not needed, having them available increases efficiency for both the radiologist and the ordering physician.
Continue to: CASE Continued: Imaging findings...
CASE Continued: Imaging findings
Given her age, the patient initially undergoes targeted ultrasonography. The grayscale image (FIGURE 2) demonstrates multiple mildly dilated ducts (white arrows) with surrounding hyperechogenicity of the fat (red arrows), indicating soft tissue edema. No intraductal mass is imaged. Given that the ultrasonography findings are not completely negative and are equivocal for malignancy, bilateral diagnostic mammography (FIGURE 3, left breast only) is performed. Standard full-field craniocaudal (FIGURE 3A) and mediolateral oblique (FIGURE 3B) mammographic views demonstrate a heterogeneously dense breast with a few calcifications in the retroareolar left breast (red ovals). No associated mass or architectural distortions are noted. The mammographic and sonographic findings do not reveal a definitive biopsy target.


Ductography
When a suspicious abnormality is visualized on either mammography or ultrasonography, the standard of care is to perform an image-guided biopsy of the abnormality. When the standard workup is negative or equivocal, the standard of care historically was to perform ductography.
Ductography is an invasive procedure that involves cannulating the suspicious duct with a small catheter and injecting radiopaque dye into the duct under mammographic guidance. While the sensitivity of ductography is higher than that of both mammography and ultrasonography, its specificity is lower than that of either modality.
Most cases of pathologic discharge are spontaneous and are not reproducible on the day of the procedure. If the procedural radiologist cannot visualize the duct that is producing the discharge, the procedure cannot be performed. Although most patients tolerate the procedure well, ductography produces patient discomfort from cannulation of the duct and injection of contrast.
Magnetic resonance imaging
Dynamic contrast-enhanced magnetic resonance imaging (MRI) is the most sensitive imaging study for evaluating pathologic nipple discharge, and it has largely replaced ductography as an adjunct to mammography and ultrasonography. MRI’s sensitivity for detecting breast cancer ranges from 93% to 100%.6 In addition, MRI allows visualization of the entire breast and areas peripheral to the field of view of a standard ductogram or ultrasound scan.9
Clinicians commonly ask, “Why not skip the mammogram and ultrasound scan and go straight to MRI, since it is so much more sensitive?” Breast MRI has several limitations, including relatively low specificity, cost, use of intravenous contrast, and patient discomfort (that is, claustrophobia, prone positioning). MRI should be utilized for pathologic discharge only when the mammogram and/or targeted ultrasound scans are negative or equivocal.
CASE Continued: Additional imaging
A contrast-enhanced MRI of the breasts (FIGURE 4) demonstrates a large area of non-mass enhancement (red oval) in the left breast, which involves most of the upper breast extending from the nipple to the posterior breast tissue; it measures approximately 7.3 x 14 x 9.1 cm in transverse, anteroposterior, and craniocaudal dimensions, respectively. There is no evidence of left pectoralis muscle involvement. An MRI-directed second look left breast ultrasonography (FIGURE 5) is performed, revealing a small irregular mass in the left breast 1 o’clock position, 10 to 11 cm from the nipple (red arrow). This area had not been imaged in the prior ultrasound scan due to its posterior location far from the nipple. Ultrasound-guided core needle biopsy is performed; moderately differentiated invasive ductal carcinoma (IDC) with high-grade DCIS is found.


Continue to: When to refer for surgery...
When to refer for surgery
No surgical evaluation or intervention is needed for physiologic nipple discharge. As mentioned previously, reassure the patient and recommend appropriate breast cancer screening. In the setting of pathologic discharge, however, referral to a breast surgeon may be indicated after appropriate imaging workup has been done.
Since abnormal imaging almost always results in a recommendation for image-guided biopsy, typically the biopsy is performed prior to the surgical consultation. Once the pathology report from the biopsy is available, the radiologist makes a radiologic-pathologic concordance statement and recommends surgical consultation. This process allows the surgeon to have all the necessary information at the initial visit.
However, in the setting of pathologic nipple discharge with normal breast imaging, the surgeon and patient may opt for close observation or surgery for definitive diagnosis. Surgical options include single-duct excision when nipple discharge is localized to one duct or central duct excision when nipple discharge cannot be localized to one duct.
CASE Continued: Follow-up
The patient was referred to a breast surgeon. Given the extent of disease in the left breast, breast conservation was not possible. The patient underwent left breast simple mastectomy with sentinel lymph node biopsy and prophylactic right simple mastectomy. Final pathology results revealed stage IA IDC with DCIS. Sentinel lymph nodes were negative for malignancy. The patient underwent adjuvant left chest wall radiation, endocrine therapy with tamoxifen, and implant reconstruction. After 2 years of follow-up, she is disease free.
In summary
Nipple discharge can be classified as physiologic or pathologic. For pathologic discharge, a thorough physical examination should be performed with subsequent imaging evaluation. First-line tools, based on patient age, include diagnostic mammography and targeted ultrasonography. Contrast-enhanced MRI is then recommended for negative or equivocal cases. All patients with pathologic nipple discharge should be referred to a breast surgeon following appropriate imaging evaluation. ●
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
- Alcock C, Layer GT. Predicting occult malignancy in nipple discharge. ANZ J Surg. 2010;80:646-649.
- Patel BK, Falcon S, Drukteinis J. Management of nipple discharge and the associated imaging findings. Am J Med. 2015;128:353-360.
- Mazzarello S, Arnaout A. Five things to know about nipple discharge. CMAJ. 2015;187:599.
- Goksel HA, Yagmurdur MC, Demirhan B, et al. Management strategies for patients with nipple discharge. Langenbecks Arch Surg. 2005;390:52-58.
- Vargas HI, Vargas MP, Eldrageely K, et al. Outcomes of clinical and surgical assessment of women with pathological nipple discharge. Am Surg. 2006;72:124-128.
- Expert Panel on Breast Imaging; Lee S, Tikha S, Moy L, et al. American College of Radiology Appropriateness Criteria: Evaluation of nipple discharge. https://acsearch.acr.org /docs/3099312/Narrative/. Accessed February 2, 2020.
- Cabioglu N, Hunt KK, Singletary SE, et al. Surgical decision making and factors determining a diagnosis of breast carcinoma in women presenting with nipple discharge. J Am Coll Surg. 2003;196:354-364.
- Morrogh M, Park A, Elkin EB, et al. Lessons learned from 416 cases of nipple discharge of the breast. Am J Surg. 2010;200:73-80.
- Morrogh M, Morris EA, Liberman L, et al. The predictive value of ductography and magnetic resonance imaging in the management of nipple discharge. Ann Surg Oncol. 2007;14:3369-3377.
‘Brutal’ plan to restrict palliative radiation during pandemic
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
A major comprehensive cancer center at the epicenter of the New York City COVID-19 storm is preparing to scale back palliative radiation therapy (RT), anticipating a focus on only oncologic emergencies.
“We’re not there yet, but we’re anticipating when the time comes in the next few weeks that we will have a system in place so we are able to handle it,” Jonathan Yang, MD, PhD, of Memorial Sloan Kettering Cancer Center (MSKCC) in New York City, told Medscape Medical News.
Yang and an expert panel of colleagues reviewed high-impact evidence, prior systematic reviews, and national guidelines to compile a set of recommendations for triage and shortened palliative rRT at their center, should the need arise.
The recommendations on palliative radiotherapy for oncologic emergencies in the setting of COVID-19 appear in a preprint version in Advances in Radiation Oncology, released by the American Society of Radiation Oncology.
Yang says the recommendations are a careful balance between the risk of COVID-19 exposure of staff and patients with the potential morbidity of delaying treatment.
“Everyone is conscious of decisions about whether patients need treatment now or can wait,” he told Medscape Medical News. “It’s a juggling act every single day, but by having this guideline in place, when we face the situation where we do have to make decisions, is helpful.”
The document aims to enable swift decisions based on best practice, including a three-tiered system prioritizing only “clinically urgent cases, in which delaying treatment would result in compromised outcomes or serious morbidity.”
“It’s brutal, that’s the only word for it. Not that I disagree with it,” commented Padraig Warde, MB BCh, professor, Department of Radiation Oncology, University of Toronto, and radiation oncologist, Princess Margaret Cancer Centre, Toronto, Ontario, Canada.
Like many places, Toronto is not yet experiencing the COVID-19 burden of New York City, but Warde says the MSKCC guideline is useful for everyone. “Other centers should review it and see how they could deal with resource limitations,” he said. “It’s sobering and sad, but if you don’t have the staff to treat all patients, which particular patients do you choose to treat?”
In a nutshell, the MSKCC recommendations defines Tier 1 patients as having oncologic emergencies that require palliative RT, including “cord compression, symptomatic brain metastases requiring whole-brain radiotherapy, life-threatening tumor bleeding, and malignant airway obstruction.”
According to the decision-making guideline, patients in Tiers 2 and 3 would have their palliative RT delayed. This would include Tier 2 patients whose needs are not classified as emergencies, but who have either symptomatic disease for which RT is usually the standard of care or asymptomatic disease for which RT is recommended “to prevent imminent functional deficits.” Tier 3 would be symptomatic or asymptomatic patients for whom RT is “one of the effective treatment options.”
“Rationing is always very difficult because as physicians you always want to do everything you can for your patients but we really have to strike the balance on when to do what, said Yang. The plan that he authored anticipates both reduced availability of radiation therapists as well as aggressive attempts to limit patients’ infection exposure.
“If a patient’s radiation is being considered for delay due to COVID-19, other means are utilized to achieve the goal of palliation in the interim, and in addition to the tier system, this decision is also made on a case-by-case basis with departmental discussion on the risks and benefits,” he explained.
“There are layers of checks and balances for these decisions...Obviously for oncologic emergencies, radiation will be implemented. However for less urgent situations, bringing them into the hospital when there are other ways to achieve the same goal, potential risk of exposure to COVID-19 is higher than the benefit we would be able to provide.”
The document also recommends shorter courses of RT when radiation is deemed appropriate.
“We have good evidence showing shorter courses of radiation can effectively treat the goal of palliation compared to longer courses of radiation,” he explained. “Going through this pandemic actually forces radiation oncologists in the United States to put that evidence into practice. It’s not suboptimal care in the sense that we are achieving the same goal — palliation. This paper is to remind people there are equally effective courses of palliation we can be using.”
“[There’s] nothing like a crisis to get people to do the right thing,” commented Louis Potters, MD, professor and chair of radiation medicine at the Feinstein Institutes, the research arm of Northwell Health, New York’s largest healthcare provider.
Northwell Health has been at the epicenter of the New York outbreak of COVID-19. Potters writes on an ASTRO blog that, as of March 26, Northwell Health “has diagnosed 4399 positive COVID-19 patients, which is about 20% of New York state and 1.2% of all cases in the world. All cancer surgery was discontinued as of March 20 and all of our 23 hospitals are seeing COVID-19 admissions, and ICU care became the primary focus of the entire system. As of today, we have reserved one floor in two hospitals for non-COVID care such as trauma. That’s it.”
Before the crisis, radiation medicine at Northwell consisted of eight separate locations treating on average 280 EBRT cases a day, not including SBRT/SRS and brachytherapy cases. “That of course was 3 weeks ago,” he notes.
Commenting on the recommendations from the MSKCC group, Potters told Medscape Medical News that the primary goal “was to document what are acceptable alternatives for accelerated care.”
“Ironically, these guidelines represent best practices with evidence that — in a non–COVID-19 world — make sense for the majority of patients requiring palliative radiotherapy,” he said.
Potters said there has been hesitance to transition to shorter radiation treatments for several reasons.
“Historically, palliative radiotherapy has been delivered over 2 to 4 weeks with good results. And, as is typical in medicine, the transition to shorter course care is slowed by financial incentives to protract care,” he explained.
“In a value-based future where payment is based on outcomes, this transition to shorter care will evolve very quickly. But given the current COVID-19 crisis, and the risk to patients and staff, the incentive for shorter treatment courses has been thrust upon us and the MSKCC outline helps to define how to do this safely and with evidence-based expected efficacy.”
This article first appeared on Medscape.com.
CBT by phone reduces depression in Parkinson’s disease
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
, according to trial results published in Neurology. The treatment’s effect on depression is “moderated by the reduction of negative thoughts,” the target of the intervention, the researchers said.
Telephone-based CBT may be a convenient option for patients, said lead study author Roseanne D. Dobkin, PhD, of the department of psychiatry at Rutgers Robert Wood Johnson Medical School in Piscataway, N.J., and the VA New Jersey Health Care System in Lyons. “A notable proportion of people with Parkinson’s [disease] do not receive the much needed mental health treatment to facilitate proactive coping with the daily challenges superimposed by their medical condition,” Dr. Dobkin said in a news release. “This study suggests that the effects of the [CBT] last long beyond when the treatment stopped and can be used alongside standard neurological care.”
An undertreated problem
Although depression affects about half of patients with Parkinson’s disease and is associated with physical and cognitive decline, it often goes overlooked and undertreated, the study authors said. Data about the efficacy and tolerability of antidepressants are mixed. CBT holds promise for reducing depression in Parkinson’s disease, prior research suggests, but patients may have limited access to in-person sessions because of physical and geographic barriers.
To assess the efficacy of telephone-based CBT for depression in Parkinson’s disease, compared with community-based treatment as usual, Dr. Dobkin and colleagues conducted a randomized controlled trial. Their study included 72 patients with Parkinson’s disease at an academic medical center. Participants had a depressive disorder, were between aged 35 and 85 years, had stable Parkinson’s disease and mental health treatment for at least 6 weeks, and had a family member or friend willing to participate in the study. The investigators excluded patients with possible dementia or marked cognitive impairment and active suicidal plans or intent.
Participants were randomly assigned to receive usual care plus telephone-based CBT or usual care only. Patients taking antidepressants were evenly divided between the groups.
Telephone-based CBT consisted of weekly 1-hour sessions for 10 weeks. During 6 months of follow-up, patients could receive one session per month if desired. The CBT “targeted negative thoughts (e.g., ‘I have no control’; ‘I am helpless’) and behaviors (e.g., avoidance, excessive worry, lack of exercise),” the investigators said. In addition, therapists trained patients’ care partners by telephone to help patients between sessions. Treatment as usual was defined by patients’ health care teams. For most participants in both groups, treatment as usual included taking antidepressant medication or receiving psychotherapy in the community.
Change in Hamilton Depression Rating Scale (HAM-D) score was the primary outcome. Secondary outcomes included whether patients considered their depression much improved and improvements in depression severity (as measured by the Beck Depression Inventory [BDI]), anxiety (as measured by the Hamilton Anxiety Rating Scale [HAM-A]), and quality of life. The researchers also assessed negative thinking using the Inference Questionnaire. Blinded raters assessed outcomes.
Sustained improvements
Thirty-seven patients were randomized to receive telephone-based CBT, and 35 were randomized to treatment as usual. Overall, 70% were taking antidepressants, and 14% continued receiving psychotherapy from community providers of their choice during the trial. Participants’ average age was 65 years, and 51% were female.
Post treatment, mean improvement in HAM-D score from baseline was 6.53 points in the telephone-based CBT group, compared with −0.27 points in the control group. “Effects at the end of treatment were maintained at 6-month follow-up,” the researchers reported.
About 40% of patients in the CBT group reported that their depression was much improved or very much improved, compared with none of the patients in the control group. Responders had mild to minimal symptomatology on the HAM-D, which indicates that the changes were clinically significant, the authors said.
Secondary outcomes also favored telephone-based CBT. “The intervention was feasible and highly acceptable, yielding an 88% retention rate over the 9-month trial,” Dr. Dobkin and colleagues said.
Compared with other control conditions, treatment-as-usual controls may enhance the effect size of an intervention, the authors noted. In addition, factors such as therapeutic relationship, time, and attention likely contribute to psychotherapy outcomes.
Success may hinge on cognitive ability
“The success of this trial highlights the need for further efficacy studies targeting neuropsychiatric manifestations of [Parkinson’s disease] and adds urgency to the discussion over policies regarding access to tele–mental health, especially for vulnerable populations with limited access to in-person mental health services,” Gregory M. Pontone, MD, and Kelly A. Mills, MD, wrote in an accompanying editorial. Dr. Pontone and Dr. Mills are affiliated with Johns Hopkins University in Baltimore.
“Only rudimentary evidence” exists to guide the treatment of depression in patients with Parkinson’s disease, the editorialists said. “Patient preference and tolerability suggest that nonpharmacologic therapies, such as CBT, are preferred as first-line treatment. Yet access to qualified CBT practitioners, especially those with a clinical knowledge of [Parkinson’s disease], is limited.”
Despite its advantages and the encouraging results, CBT may have important limitations as well, they said. Patients require a certain degree of cognitive ability to benefit from CBT, and the prevalence of dementia among patients with Parkinson’s disease is about 30%.
Nevertheless, the trial provided evidence of target engagement. “Though caveats include the single-blind design and potential confounding by time spent with patient and caregiver, the authors demonstrated that improvement was mediated by the mechanism of CBT – a reduction in negative thinking.”
The trial was funded by the Michael J. Fox Foundation for Parkinson’s Research and the Parkinson’s Alliance (Parkinson’s Unity Walk). Dr. Mills disclosed a patent pending for a system for phase-dependent cortical brain stimulation, National Institutes of Health funding, pending funding from the Michael J. Fox Foundation, and commercial research support from Global Kinetics Corporation. Dr. Pontone is a consultant for Acadia Pharmaceuticals.
SOURCE: Dobkin RD et al. Neurology. 2020 Apr 1. doi: 10.1212/WNL.0000000000009292.
FROM NEUROLOGY
Advice from the front lines: How cancer centers can cope with COVID-19
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
according to the medical director of a cancer care alliance in the first U.S. epicenter of the coronavirus outbreak.
Jennie R. Crews, MD, the medical director of the Seattle Cancer Care Alliance (SCCA), discussed the SCCA experience and offered advice for other cancer centers in a webinar hosted by the Association of Community Cancer Centers.
Dr. Crews highlighted the SCCA’s use of algorithms to predict which patients can be managed via telehealth and which require face-to-face visits, human resource issues that arose at SCCA, screening and testing procedures, and the importance of communication with patients, caregivers, and staff.
Communication
Dr. Crews stressed the value of clear, regular, and internally consistent staff communication in a variety of formats. SCCA sends daily email blasts to their personnel regarding policies and procedures, which are archived on the SCCA intranet site.
SCCA also holds weekly town hall meetings at which leaders respond to staff questions regarding practical matters they have encountered and future plans. Providers’ up-to-the-minute familiarity with policies and procedures enables all team members to uniformly and clearly communicate to patients and caregivers.
Dr. Crews emphasized the value of consistency and “over-communication” in projecting confidence and preparedness to patients and caregivers during an unsettling time. SCCA has developed fact sheets, posted current information on the SCCA website, and provided education during doorway screenings.
Screening and testing
All SCCA staff members are screened daily at the practice entrance so they have personal experience with the process utilized for patients. Because symptoms associated with coronavirus infection may overlap with cancer treatment–related complaints, SCCA clinicians have expanded the typical coronavirus screening questionnaire for patients on cancer treatment.
Patients with ambiguous symptoms are masked, taken to a physically separate area of the SCCA clinics, and screened further by an advanced practice provider. The patients are then triaged to either the clinic for treatment or to the emergency department for further triage and care.
Although testing processes and procedures have been modified, Dr. Crews advised codifying those policies and procedures, including notification of results and follow-up for both patients and staff. Dr. Crews also stressed the importance of clearly articulated return-to-work policies for staff who have potential exposure and/or positive test results.
At the University of Washington’s virology laboratory, they have a test turnaround time of less than 12 hours.
Planning ahead
Dr. Crews highlighted the importance of community-based surge planning, utilizing predictive models to assess inpatient capacity requirements and potential repurposing of providers.
The SCCA is prepared to close selected community sites and shift personnel to other locations if personnel needs cannot be met because of illness or quarantine. Contingency plans include specialized pharmacy services for patients requiring chemotherapy.
The SCCA has not yet experienced shortages of personal protective equipment (PPE). However, Dr. Crews said staff require detailed education regarding the use of PPE in order to safeguard the supply while providing maximal staff protection.
Helping the helpers
During the pandemic, SCCA has dealt with a variety of challenging human resource issues, including:
- Extending sick time beyond what was previously “stored” in staff members’ earned time off.
- Childcare during an extended hiatus in school and daycare schedules.
- Programs to maintain and/or restore employee wellness (including staff-centered support services, spiritual care, mindfulness exercises, and town halls).
Dr. Crews also discussed recruitment of community resources to provide meals for staff from local restaurants with restricted hours and transportation resources for staff and patients, as visitors are restricted (currently one per patient).
Managing care
Dr. Crews noted that the University of Washington had a foundational structure for a telehealth program prior to the pandemic. Their telehealth committee enabled SCCA to scale up the service quickly with their academic partners, including training modules for and certification of providers, outfitting off-site personnel with dedicated lines and hardware, and provision of personal Zoom accounts.
SCCA also devised algorithms for determining when face-to-face visits, remote management, or deferred visits are appropriate in various scenarios. The algorithms were developed by disease-specialized teams.
As a general rule, routine chemotherapy and radiation are administered on schedule. On-treatment and follow-up office visits are conducted via telehealth if possible. In some cases, initiation of chemotherapy and radiation has been delayed, and screening services have been suspended.
In response to questions about palliative care during the pandemic, Dr. Crews said SCCA has encouraged their patients to complete, review, or update their advance directives. The SCCA has not had the need to resuscitate a coronavirus-infected outpatient but has instituted policies for utilizing full PPE on any patient requiring resuscitation.
In her closing remarks, Dr. Crews stressed that the response to COVID-19 in Washington state has required an intense collaboration among colleagues, the community, and government leaders, as the actions required extended far beyond medical decision makers alone.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations. He is based in St. Louis. He has no conflicts of interest.
FDA removes pregnancy category C warning from certain MS medications
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.
The FDA based the decision on data from more than 1,000 real-world pregnancies, including pregnancies from a large epidemiologic study and published studies over several decades, which found no connection between use of interferon-beta products during early pregnancy and an increased risk of major birth defects, according to the FDA.
As a result, the labels for both medications will no longer have the pregnancy category C designation; however, patients should continue to notify their health care provider if they are pregnant or plan to become pregnant.
The FDA decision to remove the warning follows a similar decision by the European Medicines Agency last year.
“Many women with MS are diagnosed during their childbearing years. With this important update for Plegridy and Avonex, healthcare providers have more data to inform appropriate treatment paths for patients who may be pregnant or planning for pregnancy,” said Bernd Kieseier, MD, MHBA, executive director and head of global MS at Worldwide Medical, Biogen, in a press release.








