User login
Young women with insomnia at higher risk for car accidents
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
and reported daytime sleepiness represent a subpopulation at specific risk, according to an analysis of a 5-year population sample. The new research was published online in Sleep and led by Charles Morin, PhD, of Laval University, Quebec City.
The risks of daytime sleepiness and MVA are generally thought of in the context of obstructive sleep apnea (OSA) or men, but the results of the new work suggest that insomnia should not be overlooked, according to Krishna Sundar, MD, clinical professor of pulmonary, critical care, and sleep medicine, and medical director of the Sleep-Wake Center, at the University of Utah, Salt Lake City.
“The notion has been that it may keep them more hypervigilant and less prone to motor vehicle accidents because they are less able to fall asleep even if they want to during the daytime, as compared to other conditions like sleep apnea where there is a higher tendency to doze off,” Dr. Sundar said in an interview.
It should also be remembered that patients aren’t always completely reliable when it comes to self-assessment, according to Brandon M. Seay, MD, a pediatric pulmonologist and sleep specialist at Children’s Healthcare of Atlanta. “Most people with insomnia won’t say they are sleepy in the daytime, but when you objectively look, you do see an element of daytime sleepiness even if it’s not perceived that well by insomnia patients,” said Dr. Seay.
The heightened risks in young women with insomnia is notable, according to Dr. Sundar. Insomnia is more common in women, and they may also be more susceptible to unintended consequences of sleep medications because they metabolize them more slowly. “Especially for younger women, if they are insomniac and on prescription medicines, and if they have excess daytime sleepiness, this [risk of MVA] needs to be factored in,” said Dr. Sundar.
Insomnia is a condition that waxes and wanes over time, and can vary in its presentation across age groups, which is why the authors chose to conduct a prospective longitudinal study in a Canadian sample. They recruited 3,413 adults with insomnia (median age, 49.0 years; range, 18-96; 61.5% female). After 5 years, the retention rate was 68.7%.
After filling out baseline information, participants were asked every 6 months about MVAs and what role they believed daytime consequences of insomnia played if an accident occurred. Prescription and over-the-counter medication use were also self-reported.
In the first 2 years of the study, 8.2% of women aged 18-29 reported MVAs, which was the highest of any demographic (range, 2.3%-4.3%). By the third year, the frequency in this group overlapped that of men in the same age group, and both remained higher than older age groups.
Participants judged that insomnia consequences played a role in 39.4% of reported MVA. In 17.2% of accidents, participants said insomnia consequences contributed at least 50% of the cause.
MVA risk was associated individually with presence of insomnia symptoms (hazard ratio [HR], 1.20; 95% confidence interval, 1.00-1.45) and daytime fatigue (HR, 1.21; 95% CI, 1.01-1.47), but there were only trends toward associations with sleeping fewer than 6 hours (P = .16) and excessive daytime sleepiness (P = .06). MVAs were associated with reported past-year use of prescribed sleep medications (HR, 1.50; 95% CI, 1.17-1.91) and reported use of OTC medications (HR, 1.42; 95% CI, 1.02-1.98).
In women aged 18-29, MVAs were associated with insomnia symptoms (HR, 1.83; 95% CI, 1.13-2.98) and excessive daytime sleepiness (HR, 2.42; 95% CI, 1.11-5.24).
The study was limited by its reliance on self-reporting and lack of data on specific medications used.
The study was funded by the Canadian Institutes of Health.
SOURCE: Morin C et al. Sleep. 2020 Feb 29. DOI: 10.1093/sleep/zsaa032.
FROM SLEEP
Largest meeting on cancer research canceled: AACR
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
The biggest cancer research meeting of the year has been canceled as a reaction to the novel coronavirus (COVID-19) outbreak, which has also led to many other medical conferences being canceled or postponed.
The annual meeting of the American Association for Cancer Research (AACR) was due to take place April 24-29 in San Diego, California. More than 24,000 delegates from 80 countries and more than 500 exhibitors were expected to attend.
There are plans to reschedule it for later this year.
This has been a “difficult decision,” said the AACR board of directors, but “we believe that the decision to postpone the meeting is absolutely the correct one to safeguard our meeting participants from further potential exposure to the coronavirus.”
The board goes on to explain that “this evidence-based decision was made after a thorough review and discussion of all factors impacting the annual meeting, including the US government’s enforcement of restrictions on international travelers to enter the US; the imposition of travel restrictions issued by US government agencies, cancer centers, academic institutions, and pharmaceutical and biotech companies; and the counsel of infectious disease experts. It is clear that all of these elements significantly affect the ability of delegates, speakers, presenters of proffered papers, and exhibitors to participate fully in the annual meeting.”
Other cancer conferences that were planned for March and that have been canceled include the following:
- European Breast Cancer Conference (EBCC), Barcelona, Spain, which was to have taken place March 18-20. This conference has been postponed and will now take place September 30 to October 2 at the same venue. Abstracts that have been accepted for the initial conference will remain in the program, and organizers will reopen abstract submissions in May.
- National Comprehensive Cancer Network (NCCN), Orlando, Florida, was scheduled for March 19-22. This conference has been postponed. No new dates have been provided, but the society notes that “NCCN staff is working as quickly as possible to notify all conference registrants about the postponement and further information regarding the refund process.”
- European Association of Urology (EAU), Amsterdam, the Netherlands, at which there is always new research presented on prostate, kidney, and bladder cancer, was due to take place March 20-24. This conference has been postponed to July 2020.
- Society of Gynecologic Oncology (SGO), in Toronto, Canada, which was scheduled for March 28-31. SGO is “exploring alternatives for delivering the science and education.”
Overall, the move to cancel medical conferences over the next few months is a good idea, commented F. Perry Wilson, MD, MSCE, associate professor of medicine and director of Yale’s Program of Applied Translational Research, in a Medscape Medical News commentary.
“There’s a pretty straightforward case here,” he argued. “Medical professionals are at higher risk for exposure to coronavirus because we come into contact with lots and lots of patients. Gathering a large group of medical professionals in a single place increases the risk for exposure further. Factor in airplane flights to and from the conferences, and the chance that infection is spread is significant.”
This article first appeared on Medscape.com.
HRQOL deteriorates after disease progression in metastatic cancer
, results of an observational study suggest.
The findings highlight the importance of patient-relevant outcomes when evaluating novel therapies for patients with metastatic cancers, according to Norbert Marschner, MD, of Praxis für interdisziplinäre onkologie und hämatologie in Freiburg, Germany, and colleagues. The researchers reported the findings in JAMA Network Open.
They used four nationwide German registries to evaluate the association of disease progression with HRQOL in patients receiving systemic therapy for metastatic colorectal, lung, pancreatic, or breast cancer.
The analysis included 2,314 adults with documented disease progression across 203 institutions in Germany. Data collection occurred during routine follow-up visits at participating centers during 2011-2018.
Various patient-reported outcome questionnaires were used to measure HRQOL and symptom severity among participants. For the present study, the team enrolled patients at the start of any systemic palliative treatment, defined as targeted therapy, chemotherapy, or endocrine therapy.
Mixed-model analyses of more than 8,000 questionnaires showed that the first disease progression was associated with significant deterioration in 37 of 45 HRQOL scales overall, 17 of which were considered clinically meaningful.
With respect to cancer type, significant worsening after the first progression occurred in 12 of 14 colorectal cancer HRQOL scales, 11 of 14 lung cancer scales, 10 of 10 pancreatic cancer scales, and 4 of 7 breast cancer scales.
The deterioration in global HRQOL associated with the first progression was of greatest magnitude in lung cancer (6.7 points; P < .001), followed by pancreatic cancer (5.4 points; P < .001), colorectal cancer (3.5 points; P = .002), and breast cancer (2.4 points; P = .001).
The researchers also found that 38 of 45 HRQOL scales showed a greater degree of worsening after the second disease progression than after the first. They observed significant worsening after the second disease progression in 32 of 45 HRQOL scales, and all 32 were considered clinically meaningful.
The researchers acknowledged that a key limitation of this study was the observational design. As a result, the study did not include specifications related to tumor assessment, such as frequency, timing, or criteria.
“We suggest that progression-related endpoints in metastatic breast, colorectal, lung, or pancreatic cancer should be considered when evaluating the benefit of novel treatments, in addition to survival, morbidity, and HRQOL outcomes,” the researchers concluded.
The registries used in this study are funded by iOMEDICO and industry sponsors. The authors disclosed relationships with iOMEDICO and several pharmaceutical companies.
SOURCE: Marschner N et al. JAMA Netw Open. 2020 Mar 10. doi: 10.1001/jamanetworkopen.2020.0643.
, results of an observational study suggest.
The findings highlight the importance of patient-relevant outcomes when evaluating novel therapies for patients with metastatic cancers, according to Norbert Marschner, MD, of Praxis für interdisziplinäre onkologie und hämatologie in Freiburg, Germany, and colleagues. The researchers reported the findings in JAMA Network Open.
They used four nationwide German registries to evaluate the association of disease progression with HRQOL in patients receiving systemic therapy for metastatic colorectal, lung, pancreatic, or breast cancer.
The analysis included 2,314 adults with documented disease progression across 203 institutions in Germany. Data collection occurred during routine follow-up visits at participating centers during 2011-2018.
Various patient-reported outcome questionnaires were used to measure HRQOL and symptom severity among participants. For the present study, the team enrolled patients at the start of any systemic palliative treatment, defined as targeted therapy, chemotherapy, or endocrine therapy.
Mixed-model analyses of more than 8,000 questionnaires showed that the first disease progression was associated with significant deterioration in 37 of 45 HRQOL scales overall, 17 of which were considered clinically meaningful.
With respect to cancer type, significant worsening after the first progression occurred in 12 of 14 colorectal cancer HRQOL scales, 11 of 14 lung cancer scales, 10 of 10 pancreatic cancer scales, and 4 of 7 breast cancer scales.
The deterioration in global HRQOL associated with the first progression was of greatest magnitude in lung cancer (6.7 points; P < .001), followed by pancreatic cancer (5.4 points; P < .001), colorectal cancer (3.5 points; P = .002), and breast cancer (2.4 points; P = .001).
The researchers also found that 38 of 45 HRQOL scales showed a greater degree of worsening after the second disease progression than after the first. They observed significant worsening after the second disease progression in 32 of 45 HRQOL scales, and all 32 were considered clinically meaningful.
The researchers acknowledged that a key limitation of this study was the observational design. As a result, the study did not include specifications related to tumor assessment, such as frequency, timing, or criteria.
“We suggest that progression-related endpoints in metastatic breast, colorectal, lung, or pancreatic cancer should be considered when evaluating the benefit of novel treatments, in addition to survival, morbidity, and HRQOL outcomes,” the researchers concluded.
The registries used in this study are funded by iOMEDICO and industry sponsors. The authors disclosed relationships with iOMEDICO and several pharmaceutical companies.
SOURCE: Marschner N et al. JAMA Netw Open. 2020 Mar 10. doi: 10.1001/jamanetworkopen.2020.0643.
, results of an observational study suggest.
The findings highlight the importance of patient-relevant outcomes when evaluating novel therapies for patients with metastatic cancers, according to Norbert Marschner, MD, of Praxis für interdisziplinäre onkologie und hämatologie in Freiburg, Germany, and colleagues. The researchers reported the findings in JAMA Network Open.
They used four nationwide German registries to evaluate the association of disease progression with HRQOL in patients receiving systemic therapy for metastatic colorectal, lung, pancreatic, or breast cancer.
The analysis included 2,314 adults with documented disease progression across 203 institutions in Germany. Data collection occurred during routine follow-up visits at participating centers during 2011-2018.
Various patient-reported outcome questionnaires were used to measure HRQOL and symptom severity among participants. For the present study, the team enrolled patients at the start of any systemic palliative treatment, defined as targeted therapy, chemotherapy, or endocrine therapy.
Mixed-model analyses of more than 8,000 questionnaires showed that the first disease progression was associated with significant deterioration in 37 of 45 HRQOL scales overall, 17 of which were considered clinically meaningful.
With respect to cancer type, significant worsening after the first progression occurred in 12 of 14 colorectal cancer HRQOL scales, 11 of 14 lung cancer scales, 10 of 10 pancreatic cancer scales, and 4 of 7 breast cancer scales.
The deterioration in global HRQOL associated with the first progression was of greatest magnitude in lung cancer (6.7 points; P < .001), followed by pancreatic cancer (5.4 points; P < .001), colorectal cancer (3.5 points; P = .002), and breast cancer (2.4 points; P = .001).
The researchers also found that 38 of 45 HRQOL scales showed a greater degree of worsening after the second disease progression than after the first. They observed significant worsening after the second disease progression in 32 of 45 HRQOL scales, and all 32 were considered clinically meaningful.
The researchers acknowledged that a key limitation of this study was the observational design. As a result, the study did not include specifications related to tumor assessment, such as frequency, timing, or criteria.
“We suggest that progression-related endpoints in metastatic breast, colorectal, lung, or pancreatic cancer should be considered when evaluating the benefit of novel treatments, in addition to survival, morbidity, and HRQOL outcomes,” the researchers concluded.
The registries used in this study are funded by iOMEDICO and industry sponsors. The authors disclosed relationships with iOMEDICO and several pharmaceutical companies.
SOURCE: Marschner N et al. JAMA Netw Open. 2020 Mar 10. doi: 10.1001/jamanetworkopen.2020.0643.
FROM JAMA NETWORK OPEN
American Headache Society updates guideline on neuroimaging for migraine
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
Migraine with atypical features may require neuroimaging, according to the guideline. These include an unusual aura; change in clinical features; a first or worst migraine; a migraine that presents with brainstem aura, confusion, or motor manifestation; migraine accompaniments in later life; headaches that are side-locked or posttraumatic; and aura that presents without headache.
Assessing the evidence
The recommendation to avoid MRI or CT in otherwise neurologically normal patients with migraine carried a grade A recommendation from the American Headache Society, while the specific considerations for neuroimaging was based on consensus and carried a grade C recommendation, according to lead author Randolph W. Evans, MD, of the department of neurology at Baylor College of Medicine in Houston, and colleagues.
The recommendations, published in the journal Headache (2020 Feb;60(2):318-36), came from a systematic review of 23 studies of adults at least 18 years old who underwent MRI or CT during outpatient treatment for migraine between 1973 and 2018. Ten studies looked at CT neuroimaging in patients with migraine, nine studies examined MRI neuroimaging alone in patients with migraine, and four studies contained adults with headache or migraine who underwent either MRI or CT. The majority of studies analyzed were retrospective or cross-sectional in nature, while four studies were prospective observational studies.
Dr. Evans and colleagues noted that neuroimaging for patients with suspected migraine is ordered for a variety of reasons, such as excluding conditions that aren’t migraine, diagnostic certainty, cognitive bias, practice workflow, medicolegal concerns, addressing patient and family anxiety, and addressing clinician anxiety. Neuroimaging also can be costly, they said, adding up to an estimated $1 billion annually according to one study, and can lead to additional testing from findings that may not be clinically significant.
Good advice, with caveats
In an interview, Alan M. Rapoport, MD, editor-in-chief of Neurology Reviews, said that while he generally does not like broad guideline recommendations, the recommendation made by the American Headache Society to avoid neuroimaging in patients with a normal neurological examination without any atypical features and red flags “takes most of the important factors into consideration and will work almost all the time.” The recommendation made by consensus for specific considerations of neuroimaging was issued by top headache specialists in the United States who reviewed the data, and it is unlikely a patient with a migraine as diagnosed by the International Classification of Headache Disorders with a normal neurological examination would have a significant abnormality that would appear with imaging, Dr. Rapoport said.
“If everyone caring for migraine patients knew these recommendations, and used them unless the patients fit the exclusions mentioned, we would have more efficient clinical practice and save lots of money on unnecessary scanning,” he said.
However, Dr. Rapoport, clinical professor of neurology at the University of California, Los Angeles, founder of the New England Center for Headache, and past president of The International Headache Society, said that not all clinicians will be convinced by the American Headache Society’s recommendations.
“Various third parties often jump on society recommendations or guidelines and prevent smart clinicians from doing what they need to do when they want to disregard the recommendation or guideline,” he explained. “More importantly, if a physician feels the need to think out of the box and image a patient without a clear reason, and the patient cannot pay for the scan when a medical insurance company refuses to authorize it, there can be a bad result if the patient does not get the study.”
Dr. Rapoport noted that the guideline does not address situations where neuroimaging may not pick up conditions that lead to migraine, such as a subarachnoid or subdural hemorrhage, reversible cerebral vasoconstriction syndrome, or early aspects of low cerebrospinal fluid pressure syndrome. Anxiety on the part of the patient or the clinician is another area that can be addressed by future research, he said.
“If the clinician does a good job of explaining the odds of anything significant being found with a typical migraine history and normal examination, and the patient says [they] need an MRI with contrast to be sure, it will be difficult to dissuade them,” said Dr. Rapoport. “If you don’t order one, they will find a way to get one. If it is abnormal, you could be in trouble. Also, if the clinician has no good reason to do a scan but has anxiety about what is being missed, it will probably get done.”
There was no funding source for the guidelines. The authors reported personal and institutional relationships in the form of advisory board memberships, investigator appointments, speakers bureau positions, research support, and consultancies for a variety of pharmaceutical companies, agencies, institutions, publishers, and other organizations.
FROM HEADACHE
TBI deaths from falls on the rise
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.
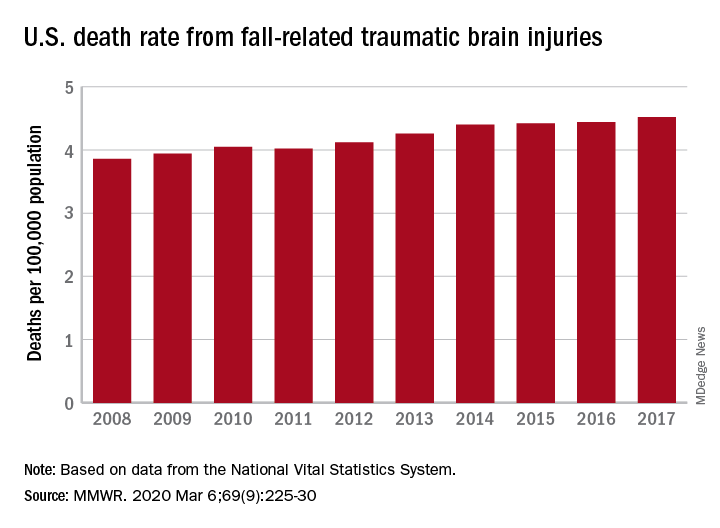
Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
A 17% surge in mortality from fall-related traumatic brain injuries from 2008 to 2017 was driven largely by increases among those aged 75 years and older, according to investigators from the Centers for Disease Control and Prevention.

Nationally, the rate of deaths from traumatic brain injuries (TBIs) caused by unintentional falls rose from 3.86 per 100,000 population in 2008 to 4.52 per 100,000 in 2017, as the number of deaths went from 12,311 to 17,408, said Alexis B. Peterson, PhD, and Scott R. Kegler, PhD, of the CDC’s National Center for Injury Prevention and Control in Atlanta.
“This increase might be explained by longer survival following the onset of common diseases such as stroke, cancer, and heart disease or be attributable to the increasing population of older adults in the United States,” they suggested in the Mortality and Morbidity Weekly Report.
The rate of fall-related TBI among Americans aged 75 years and older increased by an average of 2.6% per year from 2008 to 2017, compared with 1.8% in those aged 55-74. Over that same time, death rates dropped for those aged 35-44 (–0.3%), 18-34 (–1.1%), and 0-17 (–4.3%), they said, based on data from the National Vital Statistics System’s multiple cause-of-death database.
The death rate increased fastest in residents of rural areas (2.9% per year), but deaths from fall-related TBI were up at all levels of urbanization. The largest central cities and fringe metro areas were up by 1.4% a year, with larger annual increases seen in medium-size cities (2.1%), small cities (2.2%), and small towns (2.1%), Dr. Peterson and Dr. Kegler said.
Rates of TBI-related mortality in general are higher in rural areas, they noted, and “heterogeneity in the availability and accessibility of resources (e.g., access to high-level trauma centers and rehabilitative services) can result in disparities in postinjury outcomes.”
State-specific rates increased in 45 states, although Alaska was excluded from the analysis because of its small number of cases (less than 20). Increases were significant in 29 states, but none of the changes were significant in the 4 states with lower rates at the end of the study period, the investigators reported.
“In older adults, evidence-based fall prevention strategies can prevent falls and avert costly medical expenditures,” Dr. Peterson and Dr. Kegler said, suggesting that health care providers “consider prescribing exercises that incorporate balance, strength and gait activities, such as tai chi, and reviewing and managing medications linked to falls.”
SOURCE: Peterson AB, Kegler SR. MMWR. 2019 Mar 6;69(9):225-30.
FROM MMWR
Researchers honored by ACS, IASLC
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.
The International Association for the Study of Lung Cancer (IASLC) is naming the Translational Research Lectureship Award after Fred R. Hirsch, MD, PhD, of the Tisch Cancer Institute and Icahn School of Medicine at Mount Sinai, New York.
Dr. Hirsch was a longtime member of the IASLC and served as chief executive officer of the association from 2013 through October 2018. During this time, Dr. Hirsch grew the IASLC staff from 5 to 23 people and doubled the organization’s membership. The IASLC World Conference on Lung Cancer became an annual meeting under Dr. Hirsch’s direction and reported record attendance, according to their website.
The recipient of the Fred R. Hirsch Lectureship Award for Translational Research will be recognized at the IASLC 2020 World Conference on Lung Cancer, which is set to take place in Singapore on August 9-12, 2020.
In other news, the American Cancer Society (ACS) announced that it has awarded the 2020 Medal of Honor to three researchers. The recipients will be recognized at a black-tie ceremony in New York on Nov. 11, 2020.
Lewis C. Cantley, PhD, of Weill Cornell Medicine, New York, won the Medal of Honor for Basic Research. This award honors researchers whose work will have a “lasting impact on the cancer field” or who have made important discoveries or inventions within the field, according to the ACS.
Dr. Cantley won the award for research that has improved our understanding of cancer metabolism. He is known for his contributions to the discovery and study of phosphoinositide 3-kinase, which plays a role in many cancers and has become a target for therapies.
Leslie Bernstein, PhD, of City of Hope National Medical Center in Duarte, Calif., has won the Medal of Honor in Cancer Control. This award honors individuals who have made strides in public health, public communication, or public policy that have had an impact on cancer control.
Dr. Bernstein won the award for her work linking physical activity to a reduced risk of breast cancer. She is currently investigating links between hormone exposures, physical activity, obesity, and cancer, as well as examining how breast cancer impacts patients’ lives after treatment.
Ching-Hon Pui, MD, of St. Jude Children’s Research Hospital in Memphis, Tenn., has won the Medal of Honor in Clinical Research. This award honors researchers whose work has significantly improved cancer patients’ outcomes.
Dr. Pui won the award for his work in childhood acute lymphoblastic leukemia. Dr. Pui’s work has led to increased global treatment access, improved survival rates, and better quality of life for patients with childhood acute lymphoblastic leukemia.
Stress-related disorders linked to later neurodegenerative diseases
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
Individuals with posttraumatic stress disorder (PTSD), acute stress reaction, adjustment disorder, or other stress reactions had an 80% increased risk of vascular neurodegenerative diseases, according to results of the study, which was based on Swedish population registry data.
Risk of primary neurodegenerative diseases was increased as well in people with those conditions, but only by 31%, according to lead author Huan Song, MD, PhD, of Sichuan University in Chengdu, China.
“The stronger association observed for neurodegenerative diseases with a vascular component, compared with primary neurodegenerative diseases, suggested a considerable role of a possible cerebrovascular pathway,” Dr. Song and coauthors said in a report on the study appearing in JAMA Neurology.
While some previous studies have linked stress-related disorders to neurodegenerative diseases – particularly PTSD and dementia – this is believed to be the first, according to the investigators, to comprehensively evaluate all stress-related disorders in relation to the most common neurodegenerative conditions.
When considering neurodegenerative conditions separately, they found a statistically significant association between stress-related disorders and Alzheimer’s disease, while linkages with Parkinson’s disease and amyotrophic lateral sclerosis (ALS) were “comparable” but associations did not reach statistical significance, according to investigators.
Based on these findings, stress reduction should be recommended in addition to daily physical activity, mental activity, and a heart-healthy diet to potentially reduce risk of onset or worsening of cognitive decline, according to Chun Lim, MD, PhD, medical director of the cognitive neurology unit at Beth Israel Deaconess Medical Center in Boston.
“We don’t really have great evidence that anything slows down the progression of Alzheimer’s disease, but there are some suggestions that for people who lead heart-healthy lifestyles or adhere to a Mediterranean diet, fewer develop cognitive issues over 5-10 years,” Dr. Lim said in an interview. “Because of this paper, stress reduction may be one additional way to hopefully help these patients these patients that have or are concerned about cognitive issues.”
The population-matched cohort of the study included 61,748 individuals with stress-related disorders and 595,335 matched individuals without those disorders, while the sibling-matched cohort included 44,839 individuals with those disorders and 78,482 without. The median age at the start of follow-up was 47 years and 39.4% of those with stress-related disorders were male.
During follow-up, the incidence of neurodegenerative diseases per 1,000 person-years was 1.50 for individuals with stress-related disorders, versus 0.82 for those without stress-related disorders, according to the report. Risk of primary neurodegenerative diseases was increased among those with stress-related disorders, compared with those without, with a hazard ratio of 1.31 (95% confidence interval, 1.15-1.48). However, the risk of vascular neurodegenerative diseases was significantly higher, with an HR of 1.80 (95% CI, 1.40-2.31; P = .03 for the difference between hazard ratios).
Results of the matched sibling cohort supported results of the population-matched cohort, though the elevated risk of vascular neurodegenerative diseases among those with stress-related disorders was “slightly lower” than in the population-based cohort, Dr. Song and coauthors wrote in their report.
Beyond causing a host of hormonal and medical issues, stress can lead to sleep issues that may have long-term consequences, Dr. Lim noted in the interview.
“There’s some thought that quality sleep is important for memory formation, and if people are under a fair amount of stress and they have really poor sleep, that can also lead to cognitive issues including memory impairment,” he said.
“There are these multiple avenues that may be contributing to the accelerated development of these kinds of issues,” he added, “so I think this paper suggests more ways to counsel the patients about using lifestyle modifications to slow down the development of these cognitive impairments.”
Funding for the study came from the Swedish Research Council, Icelandic Research Fund; ,European Research Council the Karolinska Institutet, Swedish Research Council, and West China Hospital. Authors of the study provided disclosures related to those organizations as well as Shire/Takeda and Evolan.
SOURCE: Song H et al. JAMA Neurol. 2020 Mar 9. doi: 10.1001/jamaneurol.2020.0117.
FROM JAMA NEUROLOGY
The possibilities of pembrolizumab plus chemo in breast cancer treatment
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
In this edition of “Applying research to practice,” I highlight I-SPY2 and other studies of pembrolizumab plus chemotherapy in breast cancer patients.
Pathologic complete response (pCR) rates up to 60% were reported for patients with high-risk, stage II/III breast cancer who received pembrolizumab plus standard neoadjuvant chemotherapy (NAC) in I-SPY2, an ongoing platform trial designed to screen multiple agents and pinpoint those with a high probability of success (JAMA Oncol. 2020 Feb 13. doi: 10.1001/jamaoncol.2019.6650).
The addition of pembrolizumab to NAC doubled pCR rates in all three biomarker signatures studied, including ERBB2 (HER2)-negative, hormone receptor (HR)-positive/ERBB2-negative, and triple-negative breast cancer (TNBC).
As a result, pembrolizumab “graduated” from I-SPY2, with a more than 99% predictive probability that the pembrolizumab-plus-NAC approach would be superior to NAC alone in a phase 3 trial. In the HR-positive/ERBB2-negative signature, pembrolizumab is the first agent to graduate among the 10 agents studied since I-SPY2 opened in 2010.
The control arm in I-SPY2 had 181 patients randomized to standard NAC (paclitaxel followed by doxorubicin plus cyclophosphamide). The pembrolizumab arm included 69 patients who received the same NAC regimen plus pembrolizumab, given concurrently with paclitaxel.
The estimated pCR rates in all ERBB2-negative patients were 44% in the pembrolizumab arm and 17% in the control arm. Among the 40 HR-positive/ERBB2-negative patients, the estimated pCR rates were 30% and 13%, respectively. In the 29 TNBC patients, the estimated pCR rates were 60% and 22%, respectively.
Extensive residual cancer burden was less often seen in the pembrolizumab-treated patients than in the comparison group. At a median follow-up of 2.8 years in the pembrolizumab arm and 3.5 years in the NAC arm, 3-year event-free survival was similar between the arms. However, the investigators cautioned against drawing conclusions from this exploratory analysis in a small number of patients. Testifying to the importance of the primary endpoint of pCR rate, patients who achieved pCR had excellent outcomes regardless of their assigned study arms.
Immune-related adverse events in the pembrolizumab-treated patients were generally grade 1 or 2 and were managed with dose interruption or corticosteroid therapy. Most commonly seen was thyroid dysfunction in 13% of patients, as in previously published reports. Adrenal insufficiency occurred more often than expected (8.7%), for unclear reasons, with five of the six reported cases occurring more than 30 days after the last dose of pembrolizumab.
The bigger picture: Putting I-SPY2 results into context
It is well known that responses to pembrolizumab monotherapy in patients with advanced, refractory breast cancer are infrequent. In contrast, in previously untreated patients with PD-L1 positive TNBC, pembrolizumab monotherapy produced a response rate of 21% in KEYNOTE-086 (Ann Oncol. 2019 Mar 1;30(3):405-11). This response rate is similar to that observed with standard chemotherapy, but responses with pembrolizumab were more durable.
In the phase 3 KEYNOTE-355 trial (NCT02819518), researchers are comparing pembrolizumab plus chemotherapy to placebo plus chemotherapy in patients with previously untreated, stage IV TNBC with high PD-L1 expression. Researchers saw a significant and clinically meaningful improvement in progression-free survival in the pembrolizumab arm, according to a recent announcement from Merck. These results lend credence to the I-SPY2 authors’ hypothesis that immune-targeted agents would show their greatest benefit in early-stage breast cancer patients.
In fact, results from I-SPY2 have been confirmed by results from the phase 3 KEYNOTE-522 trial, which were recently published (N Engl J Med 2020;382:810-21) and presented at the San Antonio Breast Cancer Symposium. I-SPY2 predicted that pembrolizumab would be superior to standard NAC in TNBC patients in a phase 3 trial, and it was.
In KEYNOTE-522, the pCR rate was significantly higher in early-stage TNBC patients who received pembrolizumab plus NAC than in early-stage TNBC patients who received placebo plus NAC. The pCR rate was 64.8% in the pembrolizumab-NAC arm and 51.2% in the placebo–NAC arm (estimated treatment difference, 13.6 percentage points; 95% CI, 5.4 to 21.8; P less than .001).
These results are exciting. Results from I-SPY2 and KEYNOTE-522 whet the appetite for results of KEYNOTE-756, an ongoing trial of pembrolizumab plus NAC in HR-positive/ERBB2-negative patients (NCT03725059). Hopefully, the efficacy and toxicity results of KEYNOTE-756 will be as exciting as the I-SPY2 results predict they will be. Among patients with early stage breast cancer whose tumor characteristics are adverse enough to require NAC, better regimens are needed to attain pCR, a validated surrogate for long-term freedom from recurrence.
Dr. Lyss was a community-based medical oncologist and clinical researcher for more than 35 years before his recent retirement. His clinical and research interests were focused on breast and lung cancers as well as expanding clinical trial access to medically underserved populations.
Mammography does not reduce breast cancer deaths in women 75 and older
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
While more than half of women aged 75 years and older receive annual mammograms, they do not see a reduced risk of death from breast cancer, compared with women who have stopped regular screening, according to a study published in Annals of Internal Medicine.
The lack of benefit is not because older women’s cancer risk is low; a third of breast cancer deaths occur in women diagnosed at or after age 70 years, according to study author Xabier García-Albéniz, MD, PhD, of Harvard University in Boston, and colleagues.
The lack of benefit is not because mammography is less effective in women older than 75 years; indeed, it becomes a better diagnostic tool as women age, said Otis Brawley, MD, of Johns Hopkins University, Baltimore, the author of an editorial related to the study. Rather, the lack of benefit is because breast cancer treatment in older women is less successful, he clarified.
Study details
Dr. García-Albéniz and colleagues looked at data from 1,058,013 women enrolled in Medicare across the United States during 2000-2008. All subjects were aged 70-84 years and had a life expectancy of at least 10 years, at least one recent mammogram, and no history of breast cancer.
There are little randomized trial data available on mammography and breast cancer deaths for women in their early 70s and none for women older than 75 years. To compensate for this, the researchers aimed to emulate a prospective trial by looking at deaths over an 8-year period for women aged 70 and older who either continued annual screening or stopped it. The investigators conducted separate analyses for women aged 70-74 years and those 75-84 years of age.
Diagnoses of breast cancer were, not surprisingly, higher in the continued-screening group, but this did not translate to serious reductions in death.
In the continued-screening group, the estimated 8-year risk for breast cancer was 5.5% in women aged 70-74 and 5.8% in women aged 75-84 years. Among women who stopped screening, the estimated 8-year risk for breast cancer was 3.9% in both age groups.
Among women aged 70-74 years, the estimated 8-year risk for breast cancer death was slightly reduced with continued screening: 2.7 deaths per 1,000 women, compared with 3.7 deaths per 1,000 women for those who stopped screening. The risk difference was –1.0 deaths per 1,000 women, and the hazard ratio was 0.78.
Among women aged 75-84 years, there was no difference in estimated 8-year risk for breast cancer death. Women treated under a continued screening protocol had 3.8 deaths per 1,000, while the stop-screening group had 3.7 deaths per 1,000. The risk difference was 0.07 deaths per 1,000 women, and the hazard ratio was 1.00.
Interpreting the results
In the editorial accompanying this study, Dr. Brawley praised its design as “especially useful in breast cancer screening,” as “prospective randomized studies of mammography are not feasible and are perhaps no longer ethical in older women … because mammography is so widely accepted.”
In an interview, Dr. Brawley stressed that the findings do not argue for denying women aged 75 years and older mammography screening. Decisions about screening require a value judgment tailored to each individual patient’s perceived risks and benefits, he said.
In the absence of randomized trial evidence, “the jury will always be out” on the benefits of regular mammography for women 75 and older, Dr. Brawley said. “A clinical trial or a modeling study always tells you about an average person who doesn’t exist,” he added. “I predict that, in the future, we will have more parameters to tell us, ‘this is a person who’s 80 years old who is likely to benefit from screening; this is a person who is 75 years old who is unlikely to benefit.’ ”
And focusing too much on screening, he said, can divert attention from a key driver of breast cancer mortality in older women: inadequate treatment.
In the United States, Dr. Brawley said, “There’s a lot of emphasis on screening but fewer people writing about the fact that nearly 40% of American women get less than optimal treatment once they’re diagnosed.”
Dr. Brawley cited a 2013 modeling study showing that improvements in delivering current treatments would save more women even if screening rates remained unaltered (Cancer. 2013 Jul 15;119[14]:2541-8).
Among women in their 70s and 80s, Dr. Brawley said, some of the barriers to effective breast cancer care aren’t related to treatment efficacy but to travel and other logistical issues that can become more pronounced with age. “Unfortunately, there’s very little research on why, for women in their 70s and 80s, the treatments don’t work as well as they work in women 20 years younger,” he said.
Dr. García-Albéniz and colleagues’ study was funded by the National Institutes of Health. One coauthor reported financial ties to industry. Dr. Brawley discloses no conflicts of interest related to his editorial.
SOURCE: García-Albéniz X et al. Ann Intern Med 2020. doi: 10.7326/M18-1199.
FROM ANNALS OF INTERNAL MEDICINE
New study suggests milk could increase breast cancer risk
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Hot on the heels of a review from top nutrition scientists that cautioned against drinking cow’s milk comes another study with another caution: Drinking milk increases the risk of developing breast cancer, say the researchers. But this finding comes from an observational study, and there may be confounders that are not accounted for, says an expert not involved with the study.
The latest research was based on data from the long-running larger study called Adventist Health Study-2 (AHS-2), which is looking at diet and health among Seventh Day Adventists in North America. Past results from this study have suggested that Seventh Day Adventists have longer life spans and lower rates of some cancers, perhaps because of healthier lifestyles.
The latest analysis suggests that milk raises breast cancer risk, and the more you drink the higher your risk may be.
“Consuming as little as 1/4 to 1/3 cup of dairy milk per day was associated with an increased risk of breast cancer of 30%,” first author Gary E. Fraser, MBChB, PhD, said in a press statement. Fraser is affiliated with the School of Public Health at Loma Linda University, California.
“By drinking up to 1 cup per day, the associated risk went up to 50%, and for those drinking 2 to 3 cups per day, the risk increased further to 70% to 80%,” he added.
The findings were published February 25 in the International Journal of Epidemiology.
“The AHS study is provocative, but it’s not enough to warrant a change in guidelines. The caution being espoused by the authors is not warranted given the observational nature of this study,” commented Don Dizon, MD, director of Women’s Cancers, Lifespan Cancer Institute at Brown University in Providence, Rhode Island. He was not involved with the study and was approached by Medscape Medical News for comment.
Because of its observational design, the study cannot prove that cow’s milk causes breast cancer, Dizon emphasized.
“I’d want to see if the findings are replicated [by others]. Outside of a randomized trial of [cow’s] milk vs no milk or even soy, and incident breast cancers, there will never be undisputable data,” he said.
“Probably the biggest point [about this study] is not to overinflate the data,” Dizon added.
He noted that the results were significant only for postmenopausal women, and not for premenopausal women. Moreover, analyses showed significant associations only for hormone receptor–positive cancers.
“We know that breast cancer increases in incidence with age, so this tracks with that particular trend. It suggests there may be confounders not accounted for in this study,” he said.
Research so far has been inconclusive on a possible link between dairy and increased risk for breast cancer. Dairy has even been tied to decreased risk for breast cancer, according to the World Cancer Research Fund.
Study Details
The current study included 52,795 Seventh Day Adventist women from North America who did not have cancer at the start of the study. Women had a mean age of 57.1 years, and 29.7% were black. At baseline, women reported their dietary patterns for the past year using food frequency questionnaires. For 1011 women, researchers double-checked food intake with 24-hour diet questionnaires, and verified soy intake by analyzing urine levels of soy isoflavones.
Data on invasive breast cancer diagnoses came from national registries in the US and Canada. Over the course of 7.9 years, 1057 women developed invasive breast cancer. Results were adjusted for a range of factors related to breast cancer risk, including diet, lifestyle, and family history of breast cancer.
Overall, women who consumed the most calories from dairy per day had 22% increased risk for breast cancer, compared with women with the fewest calories from dairy (hazard ratio, 1.22; 95% confidence interval, 1.05-1.40; P = .008). Women who drank the most cow›s milk per day had 50% increased risk for breast cancer compared with women who drank the least (HR, 1.50; 95% CI, 1.22 - 1.84; P less than .001).
Drinking full or reduced fat cow’s milk did not change the findings (P for trend = .002 and P for trend less than .0001, respectively).
No significant association was found between breast cancer risk and cheese or yogurt consumption (P = .35 and P = .80, respectively).
Need for Change?
US dietary guidelines are under review. A new version, which will cover pregnant women and children under age 2 for the first time, is expected later this year.
Current guidelines recommend that adults and children aged 9 and over drink three 8 oz glasses of milk per day, or equivalent portions of yogurt, cheese, and other dairy products.
“Evidence from this study suggests that people should view that recommendation with caution,” Fraser said.
Milk Is Complex Topic
A top nutrition scientist agrees. Walter Willett, MD, DrPH, professor of epidemiology and nutrition at Harvard T.H. Chan School of Public Health in Boston, Massachusetts, told Medscape Medical News: “There is little scientific justification for the recommendation of 3 cups of milk per day. This new study adds a further reason for caution.”
“This was a high-quality study conducted by experienced investigators,” Willett said. Strengths of the study include the high soy intake and low consumption of foods from animal sources, factors that are hard to study in other populations.
Willett was a coauthor, along with David Ludwig, MD, PhD, also from Harvard, of the recent review published in the New England Journal of Medicine that questioned the science behind milk-drinking recommendations. An article about this review on Medscape Medical News has attracted a huge number of comments from our readers.
Milk is a complex topic, Willett explained. As a good source of essential nutrients, especially calcium and vitamin D, cow’s milk has been touted to have several health benefits, especially decreased fracture risk. But Willett said calcium recommendations have probably been overstated, and current evidence does not support high milk intake for fracture prevention.
Other benefits include improved nutrition in low-income settings, taller stature, and decreased colorectal cancer risk. But cow’s milk has also been linked to increased risk for some cancers, including prostate and endometrial cancer. Many of the benefits derived from nutrients found in milk may be obtained from other sources without these risks, according to Willett.
“Given the risks and benefits, we suggest a possible range from zero to two servings per day of dairy foods, including milk, cheese, and yogurt. If intake is zero or one serving, taking a calcium/vitamin D supplement would be good to consider,” he said.
However, Fraser and Willett also suggested another option: replacing cow’s milk with soy milk. Analyses from the current study showed no significant association between consumption of soy and breast cancer, independent of dairy (P for trend = .22).
In addition, substituting average amounts of soy milk for cow’s milk was linked to a 32% drop in risk for breast cancer among postmenopausal women (HR, 0.68; 95% CI, 0.55-0.85, P = .002). However, these results were not significant among premenopausal women (HR, 0.70; 95% CI, 0.36-1.38; P = .31).
“The suggestion that replacing some or all of [cow’s] milk with soy milk may reduce risk of breast cancer is consistent with other studies supporting a benefit of soy milk for risk of breast cancer,” Willett said.
“If someone does choose soy milk, picking one with minimal amounts of added sugar is desirable,” he added.
Drinking Milk, or Some Related Factor?
Fraser, the lead author of the current study, said in a statement that the results provide “fairly strong evidence that either dairy milk or some other factor closely related to drinking dairy milk is a cause of breast cancer in women.”
That ‘other’ factor is probably complicated, but may be related to what humans have done to cows. To increase milk production, humans have bred cows to have higher levels of insulin-like growth factor, which in turn has been linked to some cancers, including breast cancer.
Sex hormones in cow’s milk may also be involved. About 75% of a dairy herd is pregnant and the cows are by definition lactating. So the milk they produce may have higher levels of progestins and estrogens, which may play a role in hormone-responsive breast cancer.
Other factors that researchers did not measure in this study, such as poverty and the income of participants, may be at play.
But to know what’s really going on, all agree that more research is needed.
“The overall evidence so far has not shown a clear increase or decrease in risk of breast cancer with higher [cow’s] milk intake. Thus, this topic needs further examination,” Willett said.
The study was funded by the National Cancer Institute at the National Institutes of Health, and the World Cancer Research Fund in the UK. Three of the authors report following largely vegetarian diets. All authors report regular and free use of dairy products without religious or other restrictions. No authors report associations with the soy product or dairy industries. Willett reports being a consultant during the design and early years of the Adventist study, but has not been involved with it for at least 8 years. Dizon has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.












