User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
HHS proposes pathways for drug importation
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Officials at the U.S. Department of Health and Human Services have announced a new plan that they say would lay the foundation for safe importation of certain medications, with the aim of expanding drug access and lowering prescription costs for patients.
The action plan, unveiled July 31, outlines two pathways for drug importation from foreign markets. The first route would authorize states, wholesalers, or pharmacists to propose pilot demonstrations on how they would import drugs from Canada into the United States, provided these are versions of drugs already approved by the Food and Drug Administration. Similarly, a second pathway would allow manufacturers that sell in foreign countries the opportunity to import drugs that are versions of FDA-approved medications.
HHS Secretary Alex M. Azar II said the action plan is part of President Trump’s drug-pricing blueprint and is intended to combat the sky-high price tags on many prescription medications.
“President Trump has been clear: For too long American patients have been paying exorbitantly high prices for prescription drugs that are made available to other countries at lower prices,” Mr. Azar said in a statement. “[The] announcement outlines the pathways the administration intends to explore to allow safe importation of certain prescription drugs to lower prices and reduce out of pocket costs for American patients. This is the next important step in the administration’s work to end foreign freeloading and put American patients first.”
Under the first pathway, HHS would review plans submitted by states, pharmacists, or drugmakers that outline how the entities would import Health Canada–approved drugs that are in compliance with the federal Food, Drug, and Cosmetic Act. The importation would occur in a manner that assures the drug’s validity and meets the cost requirements of federal rule making, according to an HHS fact sheet.
Demonstration projects would be time-limited and require regular reporting to ensure safety and cost conditions are being met.
Under the second pathway, manufacturers of FDA-approved drug products would be able to import versions of those drugs that they sell in foreign countries through a special process to be outlined by the agency. As part of the process, drugmakers would need to establish that the foreign version is the same as the U.S. version. The FDA would then allow the drug to be labeled for sale in the U.S. and imported, according to the fact sheet. HHS officials said they believe that manufacturers would use this pathway to offer U.S. patients lower-cost versions of their drugs and the medications affected could potentially include those used to treat diabetes, rheumatoid arthritis, cardiovascular disorders, and cancer.
“In recent years, multiple manufacturers have stated (either publicly or in statements to the Administration) that they wanted to offer lower cost versions but could not readily do so because they were locked into contracts with other parties in the supply chain,” HHS officials stated in the fact sheet. “This pathway would highlight an opportunity for manufacturers to use importation to offer lower-cost versions of their drugs.”
HHS plans to introduce its action plan through a formal notice of proposed rulemaking, which has not yet been finalized. Some elements of the final proposal may differ from its initial descriptions to reflect further consideration of the relevant issues, the agency noted.
Acting FDA Commissioner Ned Sharpless, MD, said the agency has a unique role to play in promoting competition that can help reduce drug prices and improve access to medicine for Americans.
“Driving down drug prices requires a comprehensive approach and we must continue to look at all innovative solutions to this challenge,” Dr. Sharpless said in a statement. “[The] proposal is the result of the hard work by the dedicated staff of the FDA, in close collaboration with HHS and the White House, to identify potential pathways we can pursue to support the safe importation of certain prescription drugs.”
Sen. Lamar Alexander (R-Tenn.), chair of the Health, Education, Labor and Pensions committee, said the administration’s proposal sounds promising as long as the plan ensures the safety and efficacy of imported medications.
“This is the first administration to take concrete steps to allow importation of prescription drugs to reduce their cost and I welcome it,” Sen. Alexander said in a statement. “The key for me is whether this plan preserves the Food and Drug Administration’s gold standard for safety and effectiveness. Millions of Americans every day buy prescription drugs relying on the FDA’s guarantee of quality.”
Critics say hospital price transparency proposal ‘misses the mark’
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
A proposal by the Centers for Medicare & Medicaid Services to require full price transparency, including the disclosure of both list prices and payer-negotiated prices, is already receiving pushback.
Rick Pollack, president and CEO of the American Hospital Association, said in a statement that “mandating disclosure of negotiated rates between insurers and hospitals is the wrong approach,” adding that it “could seriously limit the choices available to patients in the private market and fuel anticompetitive behavior among commercial health insurers in an already highly concentrated insurance industry.”
The requirement for hospital price transparency was posted online July 29 as part of the proposed annual update to the hospital outpatient prospective payment system (OPPS) for 2020. It is scheduled for publication in the Federal Register on Aug. 9.
CMS is proposing, beginning in calendar year 2020, that hospitals make publicly available their “standard charges,” defined as the gross – or list – price of for all services provided by the hospital, as well as payer-specific negotiated prices. To allow for price comparisons, prices would be posted on the Internet in a machine-readable file that includes common billing or accounting codes and a description of the item of service being delivered.
Additionally, hospitals must make payer-specific negotiated prices for “shoppable” services, defined as services that can be scheduled in advance – such as x-rays, outpatient visits, imaging and laboratory tests, or bundled services like a cesarean delivery with pre- and postdelivery care – in a consumer-friendly manner.
“As deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal,” CMS Administrator Seema Verma said during a July 29 press conference. “In fact, a recent poll showed that the majority of Americans have tried to get pricing information before getting care, but have found it challenging to find that information.”
She noted that patients may see prices that range from 150% of Medicare rates to more than 400% for the same service.
Hospitals will need to display at least 300 shoppable services, including 70 that are CMS selected and 230 that are hospital selected, according to a fact sheet outlining this and other proposed OPPS updates for 2020.
“If a hospital does not provide one or more of the 70 CMS selected shoppable services, the hospital must select additional shoppable services such that the total number of shoppable services is at least 300,” the fact sheet states.
Information on pricing will be required to be updated at least annually.
CMS is including enforcement tools as part of the proposal, including fines to hospitals for noncompliance.
“Price transparency creates a marketplace where providers compete on the basis of cost and quality that will lower cost,” Ms. Verma said.
However, that notion has been challenged by America’s Health Insurance Plans (AHIP).
Matt Eyles, president and CEO of AHIP said in a statement that “multiple experts, including the Federal Trade Commission, agree that disclosing privately negotiated rates will make it harder to bargain for lower rates, creating a floor, not a ceiling, for the prices that hospitals would be willing to accept. Publicly disclosing competitively negotiated, proprietary rates will push prices and premiums higher, not lower, for consumers, patients, and taxpayers.”
Mr. Pollack of the American Hospital Association agreed. “While we support transparency, [this] proposal misses the mark, exceeds the Administration’s legal authority, and should be abandoned.”
Ms. Verma said she believed the agency had legal authority to impose this requirement and is not worried about possible lawsuits that could challenge this provision.
“This administration is not afraid of those things,” she said. “We are not about protecting the status quo when it doesn’t work for patients.”
Key clinical point: CMS proposes complete transparency in hospital prices.
Major finding: Hospitals would be required to make public the list prices, as well as all payer-negotiated prices.
Study details: CMS asserts that the disclosure of pricing data will lead to reduced prices through market competition.
Disclosures: CMS, as issuer of the proposed rule, makes no disclosures.
Source: Proposed rule updating the hospital outpatient prospective payment system for 2020.
Hemoglobin levels are associated with long-term dementia risk
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
This U-shaped association “may relate to differences in white matter integrity and cerebral perfusion,” the researchers wrote in Neurology.
“With around 10% of people over age 65 having anemia in the Americas and Europe and up to 45% in African and southeast Asian countries, these results could have important implications for the burden of dementia,” said study author M. Arfan Ikram, MD, PhD, in a news release. Dr. Ikram is a professor of epidemiology at Erasmus Medical Center in Rotterdam, the Netherlands.
Prior studies have found that low hemoglobin levels are associated with adverse health outcomes, such as coronary heart disease, stroke, and mortality, but data about the relationship between hemoglobin levels and dementia risk have been limited.
A population-based cohort study
To examine the long-term association of hemoglobin levels and anemia with risk of dementia, Dr. Ikram and coauthors analyzed data from the Rotterdam Study, an ongoing population-based cohort study in the Netherlands that started in 1990. Their analysis included data from 12,305 participants without dementia who had serum hemoglobin measured at baseline (mean age, 64.6 years; 57.7% women).
During a mean follow-up of 12.1 years, 1,520 participants developed dementia, 1,194 of whom had Alzheimer’s disease.
“Both low and high hemoglobin levels were associated with increased dementia risk,” the authors wrote. Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
About 6% of the participants had anemia – that is, a hemoglobin level of less than 8.1 mmol/L for men and less than 7.5 mmol/L for women. Anemia was associated with a 34% increased risk of dementia and a 41% increased risk of Alzheimer’s disease.
Of the 745 people with anemia, 128 developed dementia, compared with 1,392 of the 11,560 people who did not have anemia (17% vs. 12%).
A U-shaped association
The researchers also examined hemoglobin in relation to vascular brain disease, structural connectivity, and global cerebral perfusion among 5,267 participants without dementia who had brain MRI. White matter hyperintensity volume and hemoglobin had a U-shaped association, similar to that for dementia and hemoglobin. In addition, hemoglobin inversely correlated to cerebral perfusion.
The results remained consistent after adjustment for factors such as smoking, high blood pressure, high cholesterol, and alcohol use.
A limitation of the study is that the participants lived in the Netherlands and were primarily of European descent, so the results may not apply to other populations, the authors wrote.
Dr. Ikram noted that the study does not prove that low or high hemoglobin levels cause dementia. “More research is needed to determine whether hemoglobin levels play a direct role in this increased risk or whether these associations can be explained by underlying issues or other vascular or metabolic changes.”
The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
SOURCE: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
FROM NEUROLOGY
Key clinical point: Adults with low levels of hemoglobin and adults with high levels of hemoglobin may have an increased risk of dementia.
Major finding: Compared with participants in the middle quintile of hemoglobin levels (8.57-8.99 mmol/L), participants in the lowest quintile (less than 8.11 mmol/L) had a hazard ratio of dementia of 1.29, and participants in the highest quintile (greater than 9.40 mmol/L) had an HR of 1.20.
Study details: An analysis of data from 12,305 participants in the Rotterdam Study, a population-based cohort study in the Netherlands, who were followed up for an average of 12 years.
Disclosures: The study was supported by the Netherlands Cardiovascular Research Initiative; Erasmus Medical Centre; Erasmus University Rotterdam; Netherlands Organization for Scientific Research; Netherlands Organization for Health Research and Development; Research Institute for Diseases in the Elderly; Netherlands Genomic Initiative; Dutch Ministry of Education, Culture, and Science; Dutch Ministry of Health, Welfare, and Sports; European Commission; Municipality of Rotterdam; Netherlands Consortium for Healthy Aging; and Dutch Heart Foundation. The authors reported no relevant disclosures.
Source: Ikram MA et al. Neurology. 2019 Jul 31. doi: 10.1212/WNL.0000000000008003.
Exposure to patients with migraine increases likelihood of stigmatizing attitudes
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
Philadelphia – , according to an analysis presented at the annual meeting of the American Headache Society.
“We need to understand why this is true,” said Robert Shapiro, MD, PhD, professor of neurological sciences at the University of Vermont in Burlington. The finding also raises questions about which measures could successfully mitigate these stigmatizing attitudes.
An examination of data from OVERCOME
Stigma is a social process by which people are excluded from society because of particular traits that they have. The process encompasses stereotypes, prejudice, and discrimination. Data suggest that the level of stigma that people with migraine experience is similar to that experienced by people with epilepsy. Other data indicate that people without migraine are equally likely to hold stigmatizing attitudes toward people with migraine and people with epilepsy.
Dr. Shapiro and colleagues examined data from the Observational Survey of the Epidemiology, Treatment, and Care of Migraine (OVERCOME) study to better understand the attitudes that people without migraine have toward those who have the disorder. The data were gathered in fall 2018 through a web-based survey of a representative U.S. sample population. The researchers focused on a random sample of 2,000 people without migraine who responded to 11 questions about their attitudes toward patients with migraine. Responses described the frequency of holding attitudes and were scored on a 5-point Likert scale. The researchers categorized the responses “don’t know,” “never,” and “rarely” as “no” answers, and “sometimes,” “often,” and “very often” as “yes” answers. In addition, Dr. Shapiro and colleagues characterized each responder’s proximity to migraine according to the number of people with migraine that he or she knew (0, 1, or 2 or more) and the type of relationship (none, coworker, friend, or family member).
Sample was demographically representative
The demographic and socioeconomic characteristics of the study sample were similar to those of the most recent U.S. census data. The population’s mean age was 48, and 51% were female. Approximately 65% of respondents were non-Hispanic white, 14% were Hispanic, 11% were non-Hispanic black, 5% were Asian, and 5% were “other.” Approximately 45% of respondents reported that they had never known anyone with migraine. “Given the prevalence of migraine, it’s extraordinary that only 13% acknowledged that they had known two or more people with migraine,” said Dr. Shapiro. The finding raises questions about whether people with migraine have received adequate diagnoses and are aware of their disorder, he added. About 5% of the sample reported knowing only a coworker with migraine, and 37% reported knowing only one person with migraine.
About 31% of respondents thought that people with migraine use the disorder to avoid school or work commitments, and 33% thought that patients used migraine to avoid family or social commitments. Approximately 27% of respondents thought that people with migraine used it to get attention. About 45% of respondents thought that migraine should be treated easily, and 36% thought that people have migraine because of their own unhealthy behavior.
Individuals who knew people with migraine consistently held more negative attitudes toward those people, compared with those who did not know anyone with migraine. “These data are a little alarming,” said Dr. Shapiro. “They point to the difficulties that people with disabling migraine often encounter in having their experiences with the disease receive validation and understanding.”
Among the study’s strengths is the fact that it examined a large, population-based sample. The survey was conducted before many of the newer medications for migraine were available, and respondents were not likely to have been influenced by commercials that raised awareness of migraine, said Dr. Shapiro. The sample was not random, however, and the survey questions were based on the investigators’ interests, rather than on objective data. The generalizability of the results is in question, he added.
Dr. Shapiro consults for Eli Lilly, which sponsored the OVERCOME study.
SOURCE: Shapiro R et al. AHS 2019. Abstract OR15.
REPORTING FROM AHS 2019
CMS plans to give MIPS an overhaul
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Changes are coming to the Merit-based Incentive Payment System track of the Quality Payment Program and officials at the Centers for Medicare & Medicaid Services say these revisions are aimed at making the transition to value-based care easier for physicians.
The new framework for the Merit-based Incentive Payment System (MIPS) program was included as part of a proposed rule that updated both the physician fee schedule and the Quality Payment Program (QPP) for 2020. The proposed rule was posted online July 29, 2019, and is scheduled for publication in the Federal Register on Aug. 14. Comments on the rule are due on Sept. 27.
“We are overhauling the Merit-based Incentive Payment System to reduce reporting burden, making sure the measures relevant to clinicians as they move toward value-based care,” CMS Administrator Seema Verma said during a July 29 press conference. “Clinicians will now report on fewer, more meaningful measures that are aligned to their specialty or practice area, making it easier to participate in MIPS. We are looking for the public’s input on this new framework so that we can build a better program together.”
CMS is proposing a new conceptual framework called MIPS Value Pathways (MVPs), which would apply to future proposals beginning in the 2021 performance year.
“The goal is to move away from siloed activities and measures and more towards an aligned set of measure options more relevant to a clinician’s scope of practice that is meaningful to patient care,” the CMS said in a fact sheet highlighting the changes.
The framework would align and connect measures across the four performance categories (quality, cost, promoting interoperability, and improvement activities) and there would be MVP measures for different specialties.
“A clinician or group would be in one MVP associated with their specialty or with a condition, reporting on the same measures and activities as other clinicians and groups in that MVP,” according to the fact sheet.
As part of the proposed framework, the CMS aims to provide “enhanced data and feedback to clinicians.”
In the meantime, the agency is proposing other updates to the program, including adjustments to the weighting of the performance category in 2020. The quality category would drop from 45% to 40%, while the cost category would rise from 15% to 20%. No changes in the weighting of the interoperability (25%) and improvement activities (15%) are proposed.
A number of measures are altered in each of the performance categories, such as increasing the data completeness requirement in the quality category from reporting on 60% of Medicare Part B patients to 70%, changes to patient-centered medical home criteria in the improvement activities performance category, and requiring a yes/no response to the query of the Prescription Drug Monitoring Program measure in the promoting interoperability category.
The range of adjustment, by statute for the 2020 performance year, will go up to 9% (plus or minus) depending on the MIPS scoring, expanding from the 7% (plus or minus) range in the 2019 performance year.
A number of provisions of the Quality Payment Program program are proposed to have no change, including the low-volume threshold and opt-in policy, the MIPS performance period, and EHR certification requirements. No quality measures were changed based on changes to clinical guidelines.
The American Medical Association voiced support for the proposal.
“The AMA commends CMS for requesting input on a simplified option that would give physicians the choice to focus on episodes of care rather than following the current, more fragmented approach,” AMA President Patrice Harris, MD, said in a statement. “Making MIPS more clinically relevant and less burdensome is a top priority for the AMA and we believe CMS is taking an important step toward this goal.”
However, AMGA had a different take, expressing concern that MIPS is not becoming a pathway to value-based care.
The group, which represents multispecialty medical groups and integrated health systems, noted that, while the statutory range for bonus payments may be expanding, CMS is estimating that overall payment adjustment will be only 1.4%.
“In light of this significantly reduced adjustment, AMGA is concerned that MIPS is no longer a transition tool to value-based care, but instead represents a regulatory burden that does not support physician group practices and integrated systems of care that are investing in delivery models based on care coordination and improving population health,” AMGA said in a statement. “In addition, this adjustment undermines the intent of Congress to use MACRA [Medicare Access and CHIP Reauthorization Act] to move the health care system to value-based payment.”
Key clinical point: The Centers for Medicare & Medicaid Services proposes an overhaul to the Merit-based Incentive Payment System track of the Quality Payment Program.
Major finding: The move is intended to make measures more meaningful to clinicians.
Study details: Measures would be more focused to specialties through Merit-based Incentive Payment System Value Pathways, with all those reporting on a specialty or condition reporting on more streamlined measures.
Disclosures: CMS, as the issuer of the rules, makes no disclosures.
CMS is proposing higher payments for E/M visits
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
Physicians could be getting more money for evaluation and management (E/M) visits under a new proposal from the Centers for Medicare & Medicaid Services.
The increased funding is part of a proposed rule that provides the annual update to the Medicare physician fee schedule for 2020, as well as updates for the Quality Payment Program. The proposed rule was posted online July 29 and is scheduled to be published in the Federal Register on Aug. 14. Comments are due to CMS on Sept. 27.
CMS officials are seeking to increase Medicare payments to physicians starting in 2021 for E/M visits, based on recommendations from the American Medical Association’s Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be “rewarding the time that doctors spend with patients,” CMS Administrator Seema Verma said during a July 29 teleconference with reporters.
A fact sheet highlighting changes in the proposed physician fee schedule update for 2020 notes that the agency also is looking to add a new CPT code for prolonged services time, also to commence in 2021.
“The RUC recommendations reflect a robust survey approach by the AMA, including surveying over 50 specialty types [that] demonstrate that office/outpatient E/M visits are generally more complex and require additional resources for most clinicians,” the fact sheet states.
Physicians would also get paid for care management services related to patients with a single chronic condition, rather than only patients with multiple chronic conditions, as current regulations state. There’s also a proposal to increase payments for transitional care management services provided after a Medicare patient is discharged from an inpatient stay or certain outpatient stays.
The proposed update to the physician fee schedule also puts into regulation a new benefit for opioid use disorder treatment that was authorized under the SUPPORT (Substance Use-Disorder Prevention that Promotes Opioid Recovery and Treatment for Patients and Communities) Act. To meet the requirements of the law, CMS has included in the proposal definitions for opioid treatment programs and opioid use disorder treatment services; enrollment policies for programs; bundled payment rates for treatment programs, with adjusters for geography and annual updates; flexibility for telehealth services; and zero beneficiary copays for a time-limited duration.
The American Medical Association praised proposed changes to documentation requirements that are included in the rule.
Patrice Harris, MD, the AMA president, said in a statement that the proposed rule “will streamline reporting requirements, reduce note bloat, improve workflow, and contribute to a better environment for health care professionals and their Medicare patients.”
The proposal also includes a modification to the physician supervision requirements for physician assistants that would give PAs “greater flexibility to practice more broadly in the current health system in accordance with state law and state scope of practice,” the fact sheet notes.
Overall, Dr. Harris appeared to have a good first impression of the proposed update, noting that the AMA is “pleased to see important policy revisions that will bring us closer to a more patient-centered health care system that promotes the key principles of affordability, accessibility, quality, and innovation.”
Mayo Clinic takes honors as top hospital
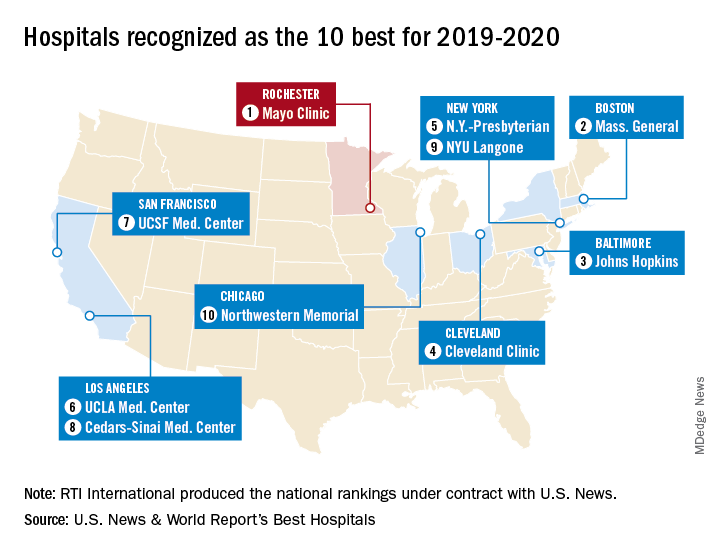
The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.

The Rochester, Minn., hospital has now been No. 1 on the U.S. News Honor Roll for 4 consecutive years. The last institution to head the list before that streak, Massachusetts General Hospital in Boston, was second this year, followed by Johns Hopkins Hospital in Baltimore, the Cleveland Clinic, and New York-Presbyterian Hospital–Columbia and Cornell in New York, U.S. News reported July 30.
The Mayo Clinic finished first in five of the 16 specialties used in the evaluation process – diabetes and endocrinology; ear, nose, and throat; gastroenterology and GI surgery; urology; and nephrology – and had a total of 13 top-five rankings. Massachusetts General had one first place in psychiatry and six total top fives, while Johns Hopkins, despite its lower overall ranking, had three top rankings – geriatrics, neurology and neurosurgery, and rheumatology – and nine top fives, the U.S. News data show.
This year’s 30th edition of the rankings involved 4,653 community inpatient hospitals, of which 1,447 received a “High Performing” rating in at least one of the nine procedures and conditions assessed and 165 were nationally ranked (top 50) in at least one of the 16 specialties. Twelve specialties are ranked largely on objective data, with the three most recent years of an annual expert opinion survey of specialized physicians also factored in. The other four specialties are ranked based entirely on the survey of expert opinion.
The research organization RTI International conducted the physician survey and produced the Best Hospitals methodology and national rankings under contract with U.S. News.
CMS proposes improved E/M payments, additional price transparency for hospitals
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
gtwachtman@mdedge.com
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
gtwachtman@mdedge.com
The Centers for Medicare & Medicaid Services is proposing improvements to physician payments and an overhaul of the Merit-based Incentive Payment System (MIPS) track of the Quality Payment Program.
In a separate proposal also released on July 29, the agency proposed that hospitals be required to make more pricing information publicly available.
The Medicare Outpatient Prospective Payment System proposed rule for the 2020 annual update would require hospitals to not only publish their gross charges, but also the negotiated price by specific payer for select services that can be scheduled by a patient in advance.
The proposal states "that hospitals make public their standard changes (both gross charges and payer-specific negotiated charges) for all items and services online in a machine-readable format" which would allow them to be included in price transparency tools and electronic health records.
"Hospitals would be required to post all their payer-specific negotiated rates, which are the prices actually paid by insurers," CMS Administrator Seema Verma said during a July 29 conference call with reporters.
As "deductibles rise and with 29 million uninsured, patients have the right to know the price of health care services so they can shop around for the best deal," she said.
The rule also comes with new enforcement tools so that CMS can ensure hospitals are complying with the rule, should it be finalized.
Hospitals would need to start publishing list prices and payer-specific negotiated prices beginning Jan. 1, 2020.
In a separate proposal to update the physician fee schedule for 2020, CMS is looking to increase Medicare payments in 2021 for evaluation and management (E/M) visits based on recommendations from the American Medical Association's Relative Value Scale Update Committee (AMA-RUC).
With this update, the agency will be "rewarding the time that doctors spend with patients," Administrator Verma said.
The fact sheet on the proposed update to the physician fee schedule also highlights improvements to case management payments, allowing physicians to get paid for case management services if the patient only has one high-risk condition.
"For 2021, we are overhauling the Merit-based Incentive Payment System, or MIPS, to reduce reporting burden, making sure the measures are relevant to clinicians as they move toward value-based care," she said, noting that clinicians would be reporting on fewer, more meaningful measures that are aligned to their specialty or practice area, "making it easier to participate in MIPS."
Look for in depth analysis of both proposals shortly on this website.
gtwachtman@mdedge.com
Dr. Alexa will see you now: Is Amazon primed to come to your rescue?
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Now that it’s upending the way you play music, cook, shop, hear the news, and check the weather, the friendly voice emanating from your Amazon Alexa–enabled smart speaker is poised to wriggle its way into all things health care.
Amazon has big ambitions for its devices. It thinks Alexa, the virtual assistant inside them, could help doctors diagnose mental illness, autism, concussion, and Parkinson’s disease. It even hopes Alexa will detect when you’re having a heart attack.
At present, Alexa can perform a handful of health care–related tasks: “She” can track blood glucose levels, describe symptoms, access postsurgical care instructions, monitor home prescription deliveries, and make same-day appointments at the nearest urgent care center.
Amazon has partnered with numerous health care companies, including several in California, to let consumers and employees use Alexa for health care purposes. Workers at Cigna Corp. can manage their health improvement goals and earn wellness incentives with Alexa. And Alexa helps people who use Omron Healthcare’s blood pressure monitor, HeartGuide, track their readings.
But a flood of new opportunities is emerging since Alexa won permission to use protected patient health records controlled under the U.S. privacy law known as HIPAA.
Before, Alexa had been limited to providing generic responses about medical conditions. Now that it can transmit private patient information, Amazon has extended its Alexa Skills Kit, the software development tools used to add functions. Soon, the virtual assistant will be able to send and receive individualized patient records, allowing health care companies to create services for consumers to use at home.
Amazon’s efforts in this domain are important because, with its 100 million smart devices in use worldwide, it could radically change the way consumers get health information and even treatment – and not just tech-savvy consumers. Analysts expect that 55% of U.S. households will have smart speakers by 2022.
Some of Alexa’s new skills depend on a little-understood feature of the devices: They listen to every sound around them. They have to in order to be ready to respond to a request, like “Alexa, how many tablespoons in a half-pint?” or “Put carrots on the shopping list.”
University of Washington researchers recently published a study in which they taught Alexa and two other devices – an iPhone 5s and a Samsung Galaxy S4 – to listen for so-called agonal breathing, the distinct gasping sounds that are an early-warning sign in about half of all cardiac arrests. These devices correctly identified agonal breathing in 97% of instances, while registering a false positive only 0.2% of the time.
Earlier research had shown that a machine learning system could recognize cardiac arrest during 911 emergency calls more accurately and far faster than human dispatchers could.
Amazon, which declined to comment for this article, holds a patent on an acoustic technology that recognizes and could act on significant audio interruptions. Combined with patented technology from the University of Washington that differentiates coughs and sneezes from other background noises, for example, Alexa could discern when someone is ill and suggest solutions.
Because Amazon also holds patents on monitoring blood flow and heart rate through an Alexa-enabled camera, Alexa could send vitals to a doctor’s office before you head to your appointment and continue to monitor your condition after you get home.
“It opens possibilities to deliver care at a distance,” said Sandhya Pruthi, MD, lead investigator for several breast cancer prevention trials at the Mayo Clinic, which has been on the front lines of using voice assistants in health care. “Think about people living in small towns who aren’t always getting access to care and knowing when to get health care,” she said. “Could this be an opportunity, if someone had symptoms, to say, ‘It’s time for this to get checked out’?”
A growing number of clinics, hospitals, home health care providers and insurers have begun experimenting with products using Alexa:
- Livongo, a Mountain View, Calif.–based startup focused on managing chronic diseases, sells an Alexa-connected blood glucose monitor that can help diabetes patients track their condition.
- Home health care provider Libertana Home Health, based in Sherman Oaks, Calif., created an Alexa skill that lets elderly or frail residents connect with caregivers, sets up reminders about medications, reports their weight and blood pressure, and schedules appointments.
- Cedars-Sinai Medical Center in Los Angeles put Amazon devices loaded with a plug-in called Aiva into more than 100 rooms to connect patients with staff and to provide hands-free television controls. Unlike a static call button, the voice-controlled device can tell nurses why a patient needs help and can then tell the patient the status of their request.
- Boston Children’s Hospital, which offered the first Alexa health care software with an educational tool called Kids MD, now uses Alexa to share postsurgical recovery data between a patient’s home and the hospital.
Many medical technology companies are tantalized by the possibilities offered by Alexa and similar technologies for an aging population. A wearable device could transmit information about falls or an uneven gait. Alexa could potentially combat loneliness. It is learning how to make conversation.
“Alexa can couple a practical interaction around health care with an interaction that can engage the patient, even delight the patient,” said elder care advocate Laurie M. Orlov.
It and other voice assistants also might help bring some relief to doctors and other medical practitioners who commonly complain that entering medical information into EHRs is too time consuming and detracts from effective interactions with patients.
This technology could work in the background to take notes on doctor-patient meetings, even suggesting possible treatments. Several startup companies are working on such applications.
One such company is Suki, based in Redwood City, Calif., which bills itself as “Alexa for doctors.” Its artificial intelligence software listens in on interactions between doctors and patients to write up medical notes automatically.
Amazon devices will need to excel at conversational artificial intelligence, capable of relating an earlier phrase to a subsequent one, if it is to remain dominant in homes.
In a 2018 interview on Amazon’s corporate blog, Rohit Prasad, a company vice president who is head scientist for Amazon Alexa, described Alexa’s anticipated evolution using “federated learning” that lets algorithms make themselves smarter by incorporating input from a wide variety of sources.
“With these advances, we will see Alexa become more contextually aware in how she recognizes, understands and responds to requests from users,” Prasad said.
This Kaiser Health News story first published on California Healthline, a service of the California Health Care Foundation. KHN is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Does endovascular thrombectomy benefit stroke patients with large infarcts?
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Patients who had thrombectomies had improved outcomes in an unadjusted statistical analysis, but these differences did not remain significant after adjustment for baseline age, clinical severity, and other key prognostic variables. However, the analysis was underpowered.
A key finding was that favorable outcomes in patients with large core volumes was strongly time dependent, which was consistent with previous data from the Highly Effective Reperfusion Using Multiple Endovascular Devices (HERMES) collaboration.
Faster treatment is the key to maximizing benefit for patients with poor collateral blood flow and a large ischemic core at baseline. As treatment work flow improves and more patients are transported directly to a thrombectomy-capable center, the number who benefit from reperfusion, despite a large ischemic core, is likely to further increase.
Ongoing randomized clinical trials are assessing the practical question of who to treat with thrombectomy when the estimated ischemic core volume is large.
Bruce C. V. Campbell, MBBS, PhD , of the University of Melbourne made these comments in an accompanying editorial. He reported research support from the several Australian research foundations. He also reported unrestricted grant funding for the Extending the Time for Thrombolysis in Emergency Neurological Deficits–Intra-Arterial (EXTEND-IA) trial to the Florey Institute of Neuroscience and Mental Health in Parkville, Australia, from Covidien (Medtronic).
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
Endovascular thrombectomy may benefit patients with stroke with large infarcts, an analysis suggests. The intervention may be more likely to benefit patients who “are treated early and have a core volume less than 100 cm3,” researchers reported in JAMA Neurology.
Clinical trials evaluating thrombectomy have largely excluded patients with large ischemic cores. To examine whether thrombectomy produces reasonable functional and safety outcomes in patients with stroke with large infarcts, compared with medical management alone, the investigators conducted a prespecified secondary analysis of data from the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
A nonrandomized study
Amrou Sarraj, MD, of the University of Texas, Houston, and his coauthors analyzed data from 105 patients in the prospective, multicenter cohort study, which enrolled patients between January 2016 and February 2018. Their analysis included data from patients who had large ischemic cores on CT (Alberta Stroke Program Early CT Score, 0-5) or on CT perfusion images (an ischemic core volume of at least 50 cm3). The SELECT study included patients with moderate to severe stroke and anterior circulation large-vessel occlusion who presented up to 24 hours from the time they last were known to be well. In the SELECT study, local investigators decided whether patients received endovascular thrombectomy or medical management alone in a nonrandomized fashion.
The 105 patients had a median age of 66 years, and 43% were female. Of the patients with large infarcts, 62 (59%) received endovascular thrombectomy plus medical management, and the rest received medical management alone.
At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27). In addition, endovascular thrombectomy was associated with better functional outcome, less infarct growth (44 vs. 98 mL), and smaller final infarct volume (97 vs. 190 mL).
The rates of neurologic worsening and symptomatic intracerebral hemorrhage were similar in both treatment groups, while mortality was lower among patients who received thrombectomy (29% vs. 42%). The likelihood of functional independence with endovascular thrombectomy decreased by 40% with each 1-hour delay in treatment and by 42% with each 10-cm3 increase in stroke volume.
Of 10 patients with core volumes greater than 100 cm3 who received endovascular thrombectomy, none had a favorable outcome.
“Although the odds of good outcomes for patients with large cores who received [endovascular thrombectomy] markedly decline with increasing core size and time to treatment, these data suggest potential benefits,” Dr. Sarraj and colleagues concluded. “Randomized clinical trials are needed.”
The authors noted that the results “did not reach significance after adjusting for baseline imbalances” and that “the small sample size limits the power of this analysis.”
The study was funded by an unrestricted grant from Stryker Neurovascular to the University of Texas. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. In addition, he is a site principal investigator for the TREVO Registry and DEFUSE 3 trials. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
SOURCE: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.
FROM JAMA NEUROLOGY
Key clinical point: and who have a core volume less than 100 mL.
Major finding: At 90 days, 31% of the patients who received endovascular thrombectomy achieved functional independence (modified Rankin Scale score of 0-2), compared with 14% of patients who received medical management alone (odds ratio, 3.27).
Study details: A prespecified secondary analysis of nonrandomized data from 105 patients in the Optimizing Patient Selection for Endovascular Treatment in Acute Ischemic Stroke (SELECT) study.
Disclosures: The study was funded by an unrestricted grant from Stryker Neurovascular. Dr. Sarraj is a consultant, speaker bureau member, and advisory board member for Stryker and is the principal investigator for a planned randomized, controlled trial (SELECT 2) funded by an unrestricted grant from Stryker to his institution. Coauthors reported financial ties with Stryker and various device and pharmaceutical companies.
Source: Sarraj A et al. JAMA Neurol. 2019 Jul 29. doi: 10.1001/jamaneurol.2019.2109.