User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
HHS declares coronavirus emergency, orders quarantine
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”
The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”
The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
The federal government declared a formal public health emergency on Jan. 31 to aid in the response to the 2019 Novel Coronavirus (2019-nCoV). The declaration, issued by Health and Human Services Secretary Alex. M. Azar II gives state, tribal, and local health departments additional flexibility to request assistance from the federal government in responding to the coronavirus.
"While this virus poses a serious public health threat, the risk to the American public remains low at this time, and we are working to keep this risk low."*
2019-nCoV—the first such action taken by the Centers for Disease Control and Prevention in more than 50 years.
“This decision is based on the current scientific facts,” Nancy Messonnier, MD, director of the National Center for Immunization and Respiratory Diseases, said during a press briefing Jan. 31. “While we understand the action seems drastic, our goal today, tomorrow, and always continues to be the safety of the American public. We would rather be remembered for over-reacting than under-reacting.”
These actions come on the heels of the World Health Organization’s Jan. 30 declaration of 2019-nCoV as a public health emergency of international concern, and from a recent spike in cases reported by Chinese health officials. “Every day this week China has reported additional cases,” Dr. Messonnier said. “Today’s numbers are a 26% increase since yesterday. Over the course of the last week, there have been nearly 7,000 new cases reported. This tells us the virus is continuing to spread rapidly in China. The reported deaths have continued to rise as well. In addition, locations outside China have continued to report cases. There have been an increasing number of reports of person-to-person spread, and now, most recently, a report in the New England Journal of Medicine of asymptomatic spread.”
The quarantine of passengers will last 14 days from when the plane left Wuhan, China. Martin Cetron, MD, who directs the CDC’s Division of Global Migration and Quarantine, said that the quarantine order “offers the greatest level of protection for the American public in preventing introduction and spread. That is our primary concern. Prior epidemics suggest that when people are properly informed, they’re usually very compliant with this request to restrict their movement. This allows someone who would become symptomatic to be rapidly identified. Offering early, rapid diagnosis of their illness could alleviate a lot of anxiety and uncertainty. In addition, this is a protective effect on family members. No individual wants to be the source of introducing or exposing a family member or a loved one to their virus. Additionally, this is part of their civic responsibility to protect their communities.”
* This story was updated on 01/31/2020.
CDC: Opioid prescribing and use rates down since 2010
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
Trends in opioid prescribing and use from 2010 to 2016 offer some encouragement, but opioid-attributable deaths continued to increase over that period, according to the Centers for Disease Control and Prevention.

Prescribing rates dropped during that period, as did daily opioid dosage rates and the percentage of patients with high daily opioid dosages, Gail K. Strickler, PhD, of the Institute for Behavioral Health at Brandeis University in Waltham, Mass., and associates wrote in MMWR Surveillance Summaries.
Their analysis involved 11 of the 12 states (Washington was unable to provide data for the analysis) participating in the CDC’s Prescription Behavior Surveillance System, which uses data from the states’ prescription drug monitoring programs. The 11 states represented about 38% of the U.S. population in 2016.
The opioid prescribing rate fell in 10 of those 11 states, with declines varying from 3.4% in Idaho to 33.0% in Ohio. Prescribing went up in Texas by 11.3%, but the state only had data available for 2015 and 2016. Three other states – Delaware, Florida, and Idaho – were limited to data from 2012 to 2016, the investigators noted.
As for the other measures, all states showed declines for the mean daily opioid dosage. Texas had the smallest drop at 2.9% and Florida saw the largest, at 27.4%. All states also had reductions in the percentage of patients with high daily opioid dosage, with decreases varying from 5.7% in Idaho to 43.9% in Louisiana, Dr. Strickler and associates reported. A high daily dosage was defined as at least 90 morphine milligram equivalents for all class II-V opioid drugs.
“Despite these favorable trends ... opioid overdose deaths attributable to the most commonly prescribed opioids, the natural and semisynthetics (e.g., morphine and oxycodone), increased during 2010-2016,” they said.
It is possible that a change in mortality is lagging “behind changes in prescribing behaviors” or that “the trend in deaths related to these types of opioids has been driven by factors other than prescription opioid misuse rates, such as increasing mortality from heroin, which is frequently classified as morphine or found concomitantly with morphine postmortem, and a spike in deaths involving illicitly manufactured fentanyl combined with heroin and prescribed opioids since 2013,” the investigators suggested.
SOURCE: Strickler GK et al. MMWR Surveill Summ. 2020 Jan 31;69(1):1-14.
FROM MMWR SURVEILLANCE SUMMARIES
Get familiar with evidence on these supplements
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
NEW ORLEANS – With more than 10% of children receiving complementary or alternative medicine (CAM), you should be familiar with what does and doesn’t work when it comes to using supplements for various medical issues, said Cora Breuner, MD, a professor of pediatrics at the University of Washington, Seattle, and attending physician at Seattle Children’s Hospital.
Dr. Breuner presented an overview of more than a dozen popular supplements with their uses and evidence at the American Academy of Pediatrics annual meeting. Most of the evidence comes from studies in adults, not children, and the evidence overall is sometimes scant, but it can guide physicians in discussing options with parents interested in CAM.
Butterbur
This root primarily is used to treat migraines via anti-inflammatory effects. The ideal dose is 50-75 mg daily in 2-3 divided doses for children aged 8-9 years and 100-150 mg daily in 2-3 divided doses for those aged 10 and older (Headache. 2005 Mar;45:196-203; Eur J Pain. 2008;12:301-13; Neurology. 2012 Apr 24;78[17]:1346-53).
Adverse effects are mostly gastrointestinal, such as diarrhea and stomach upset, and dermal/allergic reactions, such as itchy eyes, asthma, and itching.
Caffeine
Caffeine is the most popular drug of choice for reducing drowsiness and increasing alertness and has the strongest evidence base, including for improving sports and work performance (J Strength Cond Res. 2010 Jan;24[1]:257-65). Regular caffeine use can lead to dependence, however, and it can cause anxiety, nervousness, irritability, insomnia, peptic ulcers, palpitations, gastroesophageal reflux disease (GERD), and tremors. Withdrawal can involve headaches, irritability, and anxiety.
Cannabidiol
Marijuana has more than 80 cannabinoids, and a nonpsychoactive one, cannabidiol, makes up about 40% of cannabis extracts, Dr. Breuner said. It’s been used as an anticonvulsant and to combat anxiety, psychosis, nausea and rheumatoid arthritis pain. In a study using a rat model for arthritis, inflammation and pain-related behaviors decreased in rats that received cannabidiol (Eur J Pain. 2016 Jul;20[6]:936-48).
A human dose would be about 160-300 mg daily, but side effects can include dry mouth, hypotension, lightheadedness, psychomotor slowing, sedation, and sleepiness.
Coenzyme Q10
This antioxidant is fat-soluble and has a chemical structure similar to vitamin K. It has been used in people with autism, chronic fatigue syndrome, fatigue from chemotherapy, Lyme disease, and muscular dystrophy, but the evidence focuses on fibromyalgia. One study of patients with fibromyalgia found that a 300-mg daily dose for 40 days reduced pain by 52%-56%, fatigue by 47%, morning tiredness by 56%, and tender points by 44%, compared with baseline (Antioxid Redox Signal. 2013;19[12]:1356-61.)
In another, 200 mg of coenzyme Q10 with 200 mg ginkgo daily for 3 months resulted in improvement of quality of life measures, including physical fitness levels, emotional feelings, social activities, overall health, and pain (J Int Med Res. 2002;30:195-9).
Potential adverse effects of coenzyme Q10 include nausea, vomiting, diarrhea, appetite suppression, and heartburn, albeit typically in less than 1% of patients.
Echinacea
Echinacea actually is approved in Germany for supportive therapy in treating upper respiratory tract infections, urogenital infections, and wound healing, Dr. Breuner said. Hypothesized mechanisms of action include stimulation of the alternate complement pathway, immune-modulating effects, activating nonspecific T cells, inhibiting viral replication, and enhancing phagocytosis.
However, in clinical studies, echinacea did not reduce the duration or severity of upper respiratory tract infections or the occurrence or severity of infection, compared with placebo (JAMA. 2003 Dec 3;290[21]:2824-30; N Engl J Med. 2005 Jul 28;353[4]:341-8); this was tested in children aged 2-11 years in the first study, and the mean age of the subjects in the second study was 21 years. A 2014 Cochrane review found no overall benefits for treating common colds but noted the possibility of “a weak benefit from some echinacea products” based on individual trials with consistently positive, yet nonsignificant, trends, albeit with “questionable clinical relevance” (Cochrane Database Syst Rev. 2014 Feb 20;[2]:CD000530).
People with autoimmune conditions or who are immunocompromised should not use echinacea.
Magnesium
Magnesium also is used to treat migraines with a dose of 300-500 mg daily, although also it can be consumed in food, such as soy beans, black beans, tofu, seeds, nuts, whole grains, and shellfish (Expert Rev Neurother. 2009 Mar;9[3]:369-79; Neurology. 2012 Apr 24;78[17]:1346-53).
Side effects can include diarrhea and interactions with bisphosphonates, antibiotics] and diuretics. Taking proton pump inhibitors also may reduce magnesium levels.
Melatonin
Melatonin, a synthetic version of the hormone produced in humans to signal the onset of nighttime, has been studied extensively for jet lag, insomnia, shift-work disorder, circadian rhythm disorders, and withdrawal from benzodiazepine and nicotine.
Research shows that melatonin can improve sleep onset, duration, and quality. Some research has shown increased total sleep time (PLoS One. 2013 May 17;8(5):e63773).
Some evidence suggests it has endocrine-disrupting adverse effects, such as inhibiting ovulation and impairing glucose utilization.
N-acetyl cysteine (NAC)
Although it’s primarily an antidote for acetaminophen and carbon monoxide poisoning, NAC has been used for a wide range of conditions, including reducing lipoprotein levels with hyperlipidemia and reducing risk of cardiovascular events in people with end-stage renal disease and other conditions. It also has been used in people with bipolar disorder, schizophrenia, PTSD, substance disorders, and Tourette syndrome.
“Some clinical research shows that taking NAC 900 mg daily for 4 weeks, followed by 900 mg twice daily for 4 weeks and then 900 mg three times daily for 4 weeks improves symptoms of irritability in children with autism,” Dr. Breuner said. Other research showed reduced irritability in children with autism when they took 1,200 mg of NAC daily with risperidone, compared with risperidone alone. One study also has found “that NAC adds to the effect of citalopram in improving resistance/control to compulsions in OCD children and adolescents” (Iran J Psychiatry. 2017 Apr;12[2]:134-141).
Side effects can include diarrhea, nausea, and heartburn.
Omega-3 fatty acids: DHA and EHA
Docosahexanoic acid (DHA) and eicosapentanoic acid (EHA) have been used to treat ADHD, depression, heart disease, and also to lower the risk of macular degeneration.
A systematic review of 25 randomized controlled trials of more than 3,600 subjects found that “omega-3 supplementation generally correlated with improvements in blood biomarkers” (Nutrients. 2018 Aug 15;10[8]. pii: E1094). A small study in children with Tourette syndrome found that omega-3 fatty acids did not reduce tic scores, but “may be beneficial in reduction of tic-related impairment” for some children and teens (Pediatrics. 2012 Jun;129[6]:e1493-500).
Possible adverse effects include fishy taste, belching, nosebleeds, nausea, loose stools, and – at higher doses – decreased blood coagulation.
St. John’s wort
This herb has long been used to treat depression and appears to work by inhibiting serotonin reuptake, monoamine oxidase (MAO), 5-hydroxytryptamine (5-HT), dopamine, noradrenaline, gamma aminobutyric acid (GABA), and glutamate. A 2005 Cochrane review found St. John’s wort to work better than placebo with similar effectiveness as standard antidepressants for mild to moderate depression, but its benefit for major depression is questionable (Cochrane Database Syst Rev. 2005 Apr 18;[2]:CD000448).
An ideal dose is 300 mg daily, but physicians should be aware of the herb’s potential for certain drug interactions. It may increase metabolism of warfarin, cyclosporin, HIV protease inhibitors, theophylline, digoxin, and oral contraceptives (Expert Opin Drug Metab Toxicol. 2012 Jun;8[6]:691-708). Other potential side effects include decreased platelet aggregation, serotonin syndrome, and photosensitivity.
Turmeric (curcumin)
Turmeric is an anti-inflammatory agent used for a wide range of complaints, but research primarily has focused on its use for pain. No studies exist in children, but a handful of studies have found reduction in joint pain and rheumatoid arthritis symptoms in adults with 500-mg doses twice daily (Phytother Res. 2012 Nov;26[11]:1719-25; J Med Food. 2017 Oct;20[10]:1022-30). One of these studies focused on a specific product, Instaflex, that contained turmeric among multiple other active ingredients (Nutr J. 2013 Nov 25;12[1]:154).
Potential adverse effects of turmeric/curcumin include constipation, dyspepsia, diarrhea, dissension, reflux, nausea, vomiting, itching, and hives.
Zinc
Like echinacea, zinc is commonly used to treat the common cold. A 2013 Cochrane review of randomized, controlled trials found that taking zinc “within 24 hours of onset of symptoms reduces the duration of common cold symptoms in healthy people, but some caution is needed due to the heterogeneity of the data” (Cochrane Database Syst Rev. 2013 Jun 18;[6]:CD001364). The dose is 75 mg a day, and potential adverse effects include bad taste, nausea, and anosmia.
Dr. Breuner said she had no relevant financial disclosures.
EXPERT ANALYSIS FROM AAP 19
Dietary flavonol intake linked to reduced risk of Alzheimer’s
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
Onset of Alzheimer’s disease (AD) was inversely associated with intake of flavonols, a subclass of flavonoids with antioxidant and anti-inflammatory properties, according to the study authors.
The rate of developing AD was reduced by 50% among individuals reporting high intake of kaempferol, a flavonol plentiful in leafy green vegetables, and by 38% for high intake of the flavonols myricetin and isorhamnetin, researchers said in a report published in Neurology.
The findings are from the Rush Memory and Aging Project (MAP), a large, prospective study of older individuals in retirement communities and public housing in the Chicago area that has been ongoing since 1997.
“Although there is more work to be done, the associations that we observed are promising and deserve further study,” said Thomas M. Holland, MD, of the Rush Institute for Healthy Aging in Chicago, and coauthors.
Those associations between flavonol intake and AD help set the stage for U.S. POINTER and other randomized, controlled trials that seek to evaluate the effects of dietary interventions in a more rigorous way, according to Laura D. Baker, PhD, associate professor of internal medicine at Wake Forest University, Winston-Salem, N.C.
“This kind of data helps us feel like we are looking in the right direction in the randomized, controlled trials,” Dr. Baker said in an interview.
Dr. Baker is an investigator in the U.S. POINTER study, which will in part evaluate the impact of the MIND diet, which has been shown to slow cognitive decline with age in a previously published MAP study.
However, in the absence of randomized, controlled trial data, Dr. Baker cautioned against “prematurely advocating” for specific dietary approaches when speaking to patients and caregivers now.
“What I say is, we know for sure that the standard American Heart Association diet has been shown in clinical trials to reduce the risk of heart disease, and in terms of brain health, if you can reduce risk of heart disease, you are protecting your brain,” she said in the interview.
The present MAP study linking a reduced rate of AD to flavonol consumption is believed to be the first of its kind, though two previous studies from the early 2000s did find inverse associations between incident AD and intake of flavonoids, of which flavonoids are just one subclass, said Dr. Holland and coinvestigators in their report.
Moreover, in a MAP study published in 2018, Martha Clare Morris, ScD, and coauthors concluded that consuming about a serving per day of green leafy vegetables and foods rich in kaempferol, among other nutrients and bioactive compounds, may help slow cognitive decline associated with aging.
To more specifically study the relationship between kaempferol and other flavonols and the development of AD, Dr. Holland and colleagues evaluated data for MAP participants who had completed a comprehensive food frequency questionnaire and underwent at least two evaluations to assess incidence of disease.
The mean age of the 921 individuals in the present analysis was 81 years, three-quarters were female, and over approximately 6 years of follow-up, 220 developed AD.
The rate of developing AD was 48% lower among participants reporting the highest total dietary intake of flavonols, compared with those reporting the lowest intake, Dr. Holland and coauthors reported.
Intake of the specific flavonols kaempferol, myricetin, and isorhamnetin were associated with incident AD reductions of 50%, 38%, and 38%, respectively. Another flavonol, quercetin, was by contrast not inversely associated with incident AD, according to the report.
Kaempferol was independently associated with AD in subsequent analyses, while there was no such independent association for myricetin, isorhamnetin, or quercetin, according to Dr. Holland and coinvestigators.
Further analyses of the data suggested the linkages between flavonols and AD were independent of lifestyle factors, dietary intakes, or cardiovascular conditions, they said in their report.
“Confirmation of these findings is warranted through other longitudinal epidemiologic studies and clinical trials, in addition to further elucidation of the biologic mechanisms,” they concluded.
The study was funded by grants from the National Institutes of Health and the USDA Agricultural Research Service. Dr. Holland and coauthors said that they had no disclosures relevant to their report.
SOURCE: Holland TM et al. Neurology. 2020 Jan 29. doi: 10.1212/WNL.0000000000008981.
FROM NEUROLOGY
Docs weigh pulling out of MIPS over paltry payments
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
If you’ve knocked yourself out to earn a Merit-Based Incentive Payment System (MIPS) bonus payment, it’s pretty safe to say that getting a 1.68% payment boost probably didn’t feel like a “win” that was worth the effort.
And although it saved you from having a negative 5% payment adjustment, many physicians don’t feel that it was worth the effort.
On Jan. 6, the Centers for Medicare & Medicaid Services announced the 2020 payouts for MIPS.
Based on 2018 participation, the bonus for those who scored a perfect 100 is only a 1.68% boost in Medicare reimbursement, slightly lower than last year’s 1.88%. This decline comes as no surprise as the agency leader admits: “As the program matures, we expect that the increases in the performance thresholds in future program years will create a smaller distribution of positive payment adjustments.” Overall, more than 97% of participants avoided having a negative 5% payment adjustment.
Indeed, these bonus monies are based on a short-term appropriation of extra funds from Congress. After these temporary funds are no longer available, there will be little, if any, monies to distribute as the program is based on a “losers-feed-the-winners” construct.
It may be very tempting for many physicians to decide to ignore MIPS, with the rationale that 1.68% is not worth the effort. But don’t let your foot off the gas pedal yet, since the penalty for not participating in 2020 is a substantial 9%.
However, it is certainly time to reconsider efforts to participate at the highest level.
Should you or shouldn’t you bother with MIPS?
Let’s say you have $75,000 in revenue from Medicare Part B per year. Depending on the services you offer in your practice, that equates to 500-750 encounters with Medicare beneficiaries per year. (A reminder that MIPS affects only Part B; Medicare Advantage plans do not partake in the program.)
The recent announcement reveals that perfection would equate to an additional $1,260 per year. That’s only if you received the full 100 points; if you were simply an “exceptional performer,” the government will allot an additional $157. That’s less than you get paid for a single office visit.
The difference between perfection and compliance is approximately $1,000. Failure to participate, however, knocks $6,750 off your bottom line. Clearly, that’s a substantial financial loss that would affect most practices. Obviously, the numbers change if you have higher – or lower – Medicare revenue, but it’s important to do the math.
Why? Physicians are spending a significant amount of money to comply with the program requirements. This includes substantial payments to registries – typically $200 to >$1,000 per year – to report the quality measures for the program; electronic health record (EHR) systems, many of which require additional funding for the “upgrade” to a MIPS-compatible system, are also a sizable investment.
These hard costs pale in comparison with the time spent on understanding the ever-changing requirements of the program and the process by which your practice will implement them. Take, for example, something as innocuous as the required “Support Electronic Referral Loops by Receiving and Incorporating Health Information.”
You first must understand the elements of the measure: What is a “referral loop?” When do we need to generate one? To whom shall it be sent? What needs to be included in “health information?” What is the electronic address to which we should route the information? How do we obtain that address? Then you must determine how your EHR system captures and reports it.
Only then comes the hard part: How are we going to implement this? That’s only one of more than a dozen required elements: six quality measures, two (to four) improvement activities, and four promoting interoperability requirements. Each one of these elements has a host of requirements, all listed on multipage specification sheets.
The government does not seem to be listening. John Cullen, MD, president of the American Academy of Family Physicians, testified at the Senate Finance Committee in May 2019 that MIPS “has created a burdensome and extremely complex program that has increased practice costs ... ” Yet, later that year, CMS issued another hefty ruling that outlines significant changes to the program, despite the fact that it’s in its fourth performance year.
Turning frustration into action
Frustration or even anger may be one reaction, but now is an opportune time to determine your investment in the program. At a minimum, it’s vital to understand and meet the threshold to avoid the penalty. It’s been shifting to date, but it’s now set at 9% for perpetuity.
First, it’s crucial to check on your participation status. CMS revealed that the participation database was recently corrected for so-called inconsistencies, so it pays to double-check. It only takes seconds: Insert your NPI in the QPP Participation Status Tool to determine your eligibility for 2020.
In 2020, the threshold to avoid the penalty is 45 points. To get the 45 points, practices must participate in two improvement activities, which is not difficult as there are 118 options. That will garner 15 points. Then there are 45 points available from the quality category; you need at least 30 to reach the 45-point threshold for penalty avoidance.
Smart MIPS hacks that can help you
To obtain the additional 30 points, turn your attention to the quality category. There are 268 quality measures; choose at least six to measure. If you report directly from your EHR system, you’ll get a bonus point for each reported measure, plus one just for trying. (There are a few other opportunities for bonus points, such as improving your scores over last year.) Those bonus points give you a base with which to work, but getting to 45 will require effort to report successfully on at least a couple of the measures.
The quality category has a total of 100 points available, which are converted to 45 toward your composite score. Since you need 30 to reach that magical 45 (if 15 were attained from improvement activities), that means you must come up with 75 points in the quality category. Between the bonus points and measuring a handful of measures successfully through the year, you’ll achieve this threshold.
There are two other categories in the program: promoting interoperability (PI) and cost. The PI category mirrors the old “meaningful use” program; however, it has become increasingly difficult over the years. If you think that you can meet the required elements, you can pick up 25 more points toward your composite score.
Cost is a bit of an unknown, as the scoring is based on a retrospective review of your claims. You’ll likely pick up a few more points on this 15-point category, but there’s no method to determine performance until after the reporting period. Therefore, be cautious about relying on this category.
The best MIPS hack, however, is if you are a small practice. CMS – remarkably – defines a “small practice” as 15 or fewer eligible professionals. If you qualify under this paradigm, you have multiple options to ease compliance:
Apply for a “hardship exemption” simply on the basis of being small; the exemption relates to the promoting operability category, shifting those points to the quality category.
Gain three points per quality measure, regardless of data completeness; this compares to just one point for other physicians.
Capture all of the points available from the Improvement Activities category by confirming participation with just a single activity. (This also applies to all physicians in rural or Health Professional Shortage Areas.)
In the event that you don’t qualify as a “small practice” or you’re still falling short of the requirements, CMS allows for the ultimate “out”: You can apply for exemption on the basis of an “extreme and uncontrollable circumstance.” The applications for these exceptions open this summer.
Unless you qualify for the program exemption, it’s important to keep pace with the program to ensure that you reach the 45-point threshold. It may not, however, be worthwhile to gear up for all 100 points unless your estimate of the potential return – and what it costs you to get there – reveals otherwise. MIPS is not going anywhere; the program is written into the law.
But that doesn’t mean that CMS can’t make tweaks and updates. Hopefully, the revisions won’t create even more administrative burden as the program is quickly turning into a big stick with only a small carrot at the end.
Elizabeth Woodcock is president of Woodcock & Associates in Atlanta. She has disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Costs are keeping Americans out of the doctor’s office
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.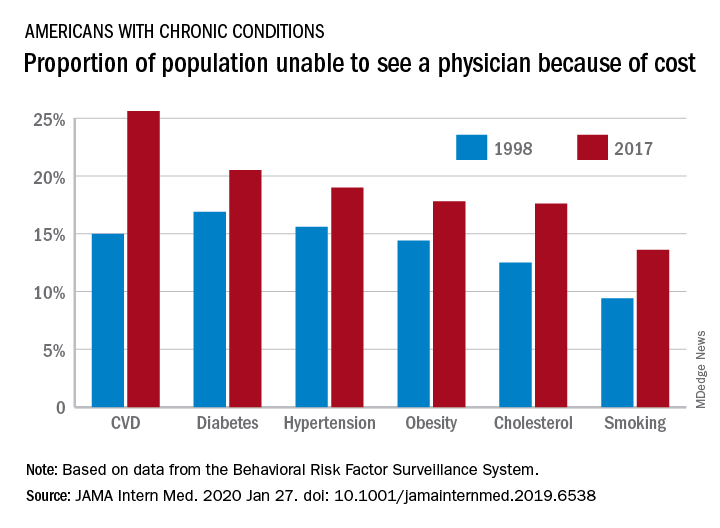
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
The cost of health care is keeping more Americans from seeing a doctor, even as the number of individuals with insurance coverage increases, according to a new study.
“Despite short-term gains owing to the [Affordable Care Act], over the past 20 years the portion of adults aged 18-64 years unable to see a physician owing to the cost increased, mostly because of an increase among persons with insurance,” Laura Hawks, MD, of Cambridge (Mass.) Health Alliance and Harvard Medical School in Boston and colleagues wrote in a new research report published in JAMA Internal Medicine.
“In 2017, nearly one-fifth of individuals with any chronic condition (diabetes, obesity, or cardiovascular disease) said they were unable to see a physician owing to cost,” they continued.
Researchers examined 20 years of data (January 1998 through December 2017) from the Centers for Disease Control and Prevention’s Behavioral Risk Factor Surveillance System to identify trends in unmet need for physician and preventive services.
Among adults aged 18-64 years who responded to the survey in 1998 and 2017, uninsurance decreased by 2.1 percentage points, falling from 16.9% to 14.8%. But at the same time, the portion of adults who were unable to see a physician because of cost rose by 2.7 percentage points, from 11.4% to 15.7%. Looking specifically at adults who had insurance coverage, the researchers found that cost was a barrier for 11.5% of them in 2017, up from 7.1% in 1998.
These results come against a backdrop of growing medical costs, increasing deductibles and copayments, an increasing use of cost containment measures like prior authorization, and narrow provider networks in the wake of the transition to value-based payment structures, the authors noted.
“Our finding that financial access to physician care worsened is concerning,” Dr. Hawks and her colleagues wrote. “Persons with conditions such as diabetes, hypertension, cardiovascular disease, and poor health status risk substantial harms if they forgo physician care. Financial barriers to care have been associated with increased hospitalizations and worse health outcomes in patients with cardiovascular disease and hypertension and increased morbidity among patients with diabetes.”
One of the trends highlighted by the study authors is the growing number of employers offering plans with a high deductible.
“Enrollment in a high-deductible health plan, which has become increasingly common in the last decade, a trend uninterrupted by the ACA, is associated with forgoing needed care, especially among those of lower socioeconomic status,” the authors wrote. “Other changes in insurance benefit design, such as imposing tiered copayments and coinsurance obligations, eliminating coverage for some services (e.g., eyeglasses) and narrowing provider networks (which can force some patients to go out-of-network for care) may also have undermined the affordability of care.”
There was some positive news among the findings, however.
“The main encouraging finding from our analysis is the increase in the proportion of persons – both insured and uninsured – receiving cholesterol checks and flu shots,” Dr. Hawk and her colleagues wrote, adding that this increase “may be attributable to the increasing implementation of quality metrics, financial incentives, and improved systems for the delivery of these services.”
However, not all preventive services that had cost barriers eliminated under the ACA saw improvement, such as cancer screening. They note that the proportion of women who did not receive mammography increased during the study period and then plateaued, but did not improve following the implementation of the ACA. The authors described the reasons for this as “unclear.”
Dr. Hawks received funding support from an Institutional National Research Service award and from Cambridge Health Alliance, her employer. Other authors reported membership in Physicians for a National Health Program.
SOURCE: Hawks L et al. JAMA Intern Med. 2020 Jan 27. doi: 10.1001/jamainternmed.2019.6538.
FROM JAMA INTERNAL MEDICINE
Celecoxib oral solution treats migraine effectively in randomized trial
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
, according to trial results published in the January issue of Headache.
Two hours after treatment, a significantly greater proportion of patients who received the liquid solution, known as DFN-15, had freedom from pain and freedom from their most bothersome accompanying symptom – nausea, photophobia, or phonophobia – compared with patients who received placebo. The pain freedom rates were 35.6% with celecoxib oral solution and 21.7% with placebo. The rates of freedom from the most bothersome symptom were 57.8% with celecoxib oral solution and 44.8% with placebo.
About 9% of patients who received celecoxib oral solution had treatment-emergent adverse events related to the study drug, the most common of which were dysgeusia (4.2%) and nausea (3.2%). In comparison, about 6% of patients who received placebo had treatment-emergent adverse events. There were no serious treatment-emergent adverse events.
“DFN‐15 has the potential to become a reliable and convenient acute therapeutic option for patients with migraine,” said lead author Richard B. Lipton, MD, and colleagues. Dr. Lipton is affiliated with the Albert Einstein College of Medicine in New York.
Assessing celecoxib in migraineurs
Evidence-based guidelines recommend nonsteroidal anti-inflammatory drugs (NSAIDs), including aspirin, diclofenac, ibuprofen, and naproxen, as effective acute migraine treatments, but these medications may increase the risk of adverse gastrointestinal events, the authors said. Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is indicated for the treatment of acute pain in patients with ankylosing spondylitis, osteoarthritis, primary dysmenorrhea, and rheumatoid arthritis. Although it produces analgesia similar to other NSAIDs, among patients with osteoarthritis and rheumatoid arthritis, celecoxib is associated with significantly lower risk of gastrointestinal events, compared with naproxen and ibuprofen, and significantly lower risk of renal events, compared with ibuprofen.
Researchers have studied an oral capsule form of celecoxib (Celebrex, Pfizer) as an acute treatment for migraine in an open-label study that compared celecoxib with naproxen sodium. “While preliminary results suggest comparable efficacy but better tolerability than widely used and guideline-recommended NSAIDs, celecoxib is not currently approved for migraine,” the authors said.
Compared with the oral capsule formulation, the oral liquid solution DFN-15 has a faster median time to peak concentration under fasting conditions (within 1 hour vs. 2.5 hours), which “could translate into more rapid onset of pain relief,” the authors said. In addition, DFN-15 may have greater bioavailability, which could lower dose requirements and improve safety and tolerability. To compare the efficacy, tolerability, and safety of 120-mg DFN-15 with placebo for the acute treatment of migraine, researchers conducted a randomized, double-blind, placebo-controlled study.
Participants used single-dose bottles
Researchers randomized 622 patients 1:1 to DFN-15 or placebo, and 567 treated a migraine during the trial. Patients had a mean age of 40 years, and 87% were female. Patients had episodic migraine with or without aura, no signs of medication overuse, and two-eight migraine attacks per month. For the trial, patients treated a single migraine attack of moderate to severe intensity within 1 hour of onset. “Each subject was given a single‐dose bottle of DFN‐15 120 mg or matching placebo containing 4.8 mL liquid,” Dr. Lipton and colleagues said. “They were instructed to drink the entire contents of the bottle to ensure complete consumption of study medication.”
Freedom from pain and freedom from the most bothersome symptom at 2 hours were the coprimary endpoints. “DFN‐15 was also significantly superior to placebo on multiple secondary 2‐hour endpoints, including freedom from photophobia, pain relief, change in functional disability from baseline, overall and 24‐hour satisfaction with treatment, and use of rescue medication,” they reported.
“A new COX‐2 inhibitor that is effective and rapidly absorbed could provide an important new option for a wide range of patients,” the authors said. “Though cross‐study comparisons are problematic, the current results for DFN‐15 indicate that its efficacy is similar to that of NSAIDs and small‐molecule calcitonin gene‐related peptide receptor antagonists (gepants), based on placebo‐subtracted rates pain freedom in acute treatment trials (14%‐21%). DFN‐15 may also be useful among triptan users, who are at elevated risk of medication‐overuse headache and for whom TEAEs within 24 hours postdose are common. ... The form and delivery system of DFN‐15 – a ready‐to‐use solution in a 4.8‐mL single‐use bottle – may support patient adherence.”
The trial had robust placebo response rates, which may have been influenced by “the novelty of a ready‐made oral solution, which has not been previously tested for the acute treatment of migraine,” the authors noted. In addition, the trial does not address the treatment of mild pain or treatment across multiple attacks.
The trial was supported by Dr. Reddy’s Laboratories, manufacturer of DFN-15. Two authors are employed by and own stock in Dr. Reddy’s. Dr. Lipton and a coauthor disclosed research support from and consulting for Dr. Reddy’s.
SOURCE: Lipton RB et al. Headache. 2020;60(1):58-70. doi: 10.1111/head.13663.
FROM HEADACHE
Genetic factor linked to impaired memory after heading many soccer balls
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
according to authors of a recent longitudinal study. Worse verbal memory was linked to high levels of ball heading among those players who were APOE e4–positive, compared with those who were APOE e4–negative, according to the authors, led by Liane E. Hunter, PhD, of the Gruss Magnetic Resonance Imaging Center at Albert Einstein College of Medicine, New York.
These findings, while preliminary, do raise the possibility that “safe levels for soccer heading” could be proposed to protect players from harm or that APOE e4-positive players might be advised to limit their exposure to head impacts, Dr. Hunter and coauthors wrote in a report in JAMA Neurology.
However, the findings should “in no way” be used to justify APOE testing to make clinical decisions regarding the safety of playing soccer, said Sarah J. Banks, PhD, of the University of California, San Diego, and Jesse Mez, MD, of Boston University in a related editorial (doi: 10.1001/jamaneurol.2019.4451). “Like most good science, the study provides an important, but incremental, step to understanding gene-environment interactions in sports,” Dr. Banks and Dr. Mez wrote in their editorial.
While there are some studies tying APOE e4 to poorer neuropsychiatric performance in boxers and U.S. football players, there are no such studies looking at the role of APOE e4 in soccer players exposed to repetitive “subconcussive” ball heading, according to Dr. Hunter and coresearchers. Accordingly, they sought to analyze APOE e4 and neuropsychological performance in relation to ball heading in 352 adult amateur soccer players enrolled in the Einstein Soccer Study between November 2013 and January 2018. About three-quarters of the players were male, and the median age at enrollment was 23 years.
The players completed a computer-based questionnaire designed to estimate their exposure to soccer heading at enrollment and at follow-up visits every 3-6 months. To test verbal memory at each visit, players were asked to memorize a 12-item grocery list, and then asked to recall the items 20 minutes later.
High levels of heading were linked to poorer performance on the verbal memory task, similar to one previously reported study, investigators said.
There was no association overall of APOE e4 and heading with performance on the shopping list task, according to investigators. By contrast, there was a 4.1-fold increased deficit in verbal memory for APOE e4–positive players with high heading exposure, compared with those with low exposure, investigators reported. Likewise, there was an 8.5-fold increased deficit in verbal memory for APOE e4–positive players with high versus moderate heading exposure.
That said, the absolute difference in performance was “subtle” and difficult to interpret in the context of a cross-sectional study, Dr. Banks and Dr. Mez said in their editorial.
In absolute terms, the mean decrease in scores on the 13-point shopping list task between the high and low heading exposure was 1.13 points greater for the APOE e4–positive group, compared with the APOE e4–negative group, and the decrease between the high and moderate heading exposure groups was 0.98 points greater, according to the report.
“The effect size of our interaction is relatively small,” Dr. Hunter and colleagues acknowledged in their report. “However, similar to the widely cited model of disease evolution in Alzheimer disease, our findings may be evidence of early subclinical effects, which could accumulate in APOE e4–positive players over a protracted time frame and ultimately be associated with overt clinical dysfunction.”
Several study authors said they had received grants from the National Institutes of Health and affiliated institutes, the Migraine Research Foundation, and the National Headache Foundation. They reported disclosures related to Amgen, Avanir, Biohaven Holdings, Biovision, Boston Scientific, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, and Pfizer, among others.
SOURCE: Hunter LE et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4828.
FROM JAMA Neurology
Journal editors seek more complete disclosure from authors
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
A group of leading medical journal editors is seeking to improve the completeness and transparency of financial disclosure reporting with a proposed new disclosure form that puts more onus on readers to decide whether relationships and activities should influence how they view published papers.
The proposed changes are described in an editorial published simultaneously today in the Annals of Internal Medicine, British Medical Journal, Journal of the American Medical Association, The Lancet, New England Journal of Medicine, and several other journals whose editors are members of the International Committee of Medical Journal Editors (ICMJE).
“While no approach to disclosure will be perfect or foolproof, we hope the changes we propose will help promote transparency and trust,” the editorial stated (Ann Intern Med. 2020 Jan 27. doi: 10.7326/M19-3933).
The ICMJE adopted its currently used electronic form – the “ICMJE Form for the Disclosure of Potential Conflicts of Interest” – 10 years ago in an effort to create some uniformity amidst a patchwork of differing disclosure requirements for authors.
It’s not known how many journals outside of the ICMJE’s member journals routinely use the disclosure form, but the organization’s website houses an extensive list of journals whose editors or publishers have requested to be listed as following the ICMJE’s recommendations for editing, reporting, and publishing, including those concerning disclosures. The ICMJE does not “certify” journals. The full set of recommendations was updated in December 2019.
Most authors are committed to transparent reporting, but “opinions differ over which relationships or activities to report,” the editorial stated.
An author might choose to omit an item that others deem important because of a difference in opinion regarding “relevance,” confusion over definitions, or a simple oversight. Some authors may be “concerned that readers will interpret the listing of any item as a ‘potential conflict of interest’ as indicative of problematic influence and wrongdoing,” the editorial stated.
The revised form, like the current one, asks authors to disclose relationships and activities that are directly related to the reported work, as well as those that are topically related (within the broadly defined field addressed in the work). But unlike the current form, the new version provides a checklist of relationships and activities and asks authors to check ‘yes’ or ‘no’ for each one (and to name them when the answer is ‘yes’).
Items in the checklist include grants, payments/honoraria for lectures, patents issued or planned, stock/stock options, and leadership or fiduciary roles in committees, boards, or societies.
The proposed new form makes no mention of “potential conflicts of interest” or “relevancy,” per say. Authors aren’t asked to determine what might be interpreted as a potential conflict of interest, but instead are asked for a “complete listing” of what readers may find “pertinent” to their work.
“We’re trying to move away from calling everything a [potential] ‘conflict,’ ” Darren B. Taichman, MD, PhD, secretary of ICMJE and executive editor of the Annals of Internal Medicine, said in an interview. “We want to remove for authors the concern or stigma, if you will, that anything listed on a form implies that there is something wrong, because that’s just not true. … We want readers to decide what relationships are important as they interpret the work.”
Dr. Taichman said in the interview that the ICMJE’s updating of the form was more a function of “good housekeeping” and continuous appreciation of disclosure as an important issue, rather than any one specific issue, such as concern over a “relevancy” approach to disclosures.
The ICMJE is seeking feedback about its proposed form, which is available with a link for providing comments, at www.icmje.org.
Broader national efforts
Editors and others have been increasingly moving, however, toward asking for more complete disclosures where authors aren’t asked to judge “relevancy” and where readers can make decisions on their own. The American Society of Clinical Oncology, which produces the Journal of Clinical Oncology (JCO) as well as practice guidelines and continuing medical education programs, moved about 5 years ago to a system of general disclosure that asks physicians and others to disclose all financial interests and industry relationships, with no qualifiers.
Earlier in January 2020, the Accreditation Council for Continuing Medical Education issued proposed revisions to its Standards for Integrity and Independence in Accredited Continuing Education. These revisions, which are open for comment, require CME providers to collect disclosure information about all financial relationships of speakers and presenters. It’s up to the CME provider to then determine which relationships are relevant, according to the proposed document.
More change is on the way, as disclosure issues are being deliberated nationally in the wake of a highly publicized disclosure failure at Memorial Sloan Kettering Cancer Center in 2018. Chief medical officer José Baselga, MD, PhD, failed to report millions of dollars of industry payments and ownership interests in journal articles he wrote or cowrote over several years.
In February 2019, leaders from journals, academia, medical societies, and other institutions gathered in Washington for a closed-door meeting to hash out various disclosure related issues.
Hosted by the Association of American Medical Colleges and cosponsored by Memorial Sloan Kettering Cancer Center, ASCO, JAMA, and the Council of Medical Specialty Societies, the meeting led to a series of working groups that are creating additional recommendations “due out soon in 2020,” Heather Pierce, senior director of science policy and regulatory counsel for the AAMC, said in an interview.
Among the questions being discussed: What disclosures should be verified and who should do so? How can disclosures be made more complete and easier for researchers? And, “most importantly,” said Ms. Pierce, how can policy requirements across each of these sectors be aligned so that there’s more coordination and oversight – and with it, public trust?
Some critics of current disclosure policies have called for more reporting of compensation amounts, and Ms. Pierce said that this has been part of cross-sector discussions.
The ICMJE’s proposed form invites, but does not require, authors to indicate what payments were made to them or their institutions. “Part of this is due to the fact that it’s hard to define, let alone agree on, what’s an important amount,” Dr. Taichman said.
A push for registries
The ICMJE is also aiming to make the disclosure process more efficient for authors – and to eliminate inconsistent and incomplete disclosures – by accepting disclosures from web-based repositories, according to the editorial. Repositories allow authors to maintain an inventory of their relationships and activities and then create electronic disclosures that are tailored to the requirements of the ICMJE, medical societies, and other entities.
The AAMC-run repository, called Convey, is consistent with ICMJE reporting requirements and other criteria (e.g., there are no fees for individuals to enter, store, or export their data), but the development of other repositories may be helpful “for meeting regional, linguistic, and regulatory needs” of authors across the world, the editorial stated.
The Annals of Internal Medicine and the New England Journal of Medicine are both currently collecting disclosures through Convey. The platform was born from discussions that followed a 2009 Institute of Medicine report on conflicts of interest.
Signers of the ICMJE editorial include representatives of the National Library of Medicine and the World Association of Medical Editors, in addition to editors in chief and other leaders of the ICMJE member journals.
FROM ANNALS OF INTERNAL MEDICINE
Modafinil use in pregnancy tied to congenital malformations
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
Modafinil exposure during pregnancy was associated with an approximately tripled risk of congenital malformations in a large Danish registry-based study.
Modafinil (Provigil) is commonly prescribed to address daytime sleepiness in narcolepsy and multiple sclerosis. An interim postmarketing safety analysis showed increased rates of major malformation in modafinil-exposed pregnancies, so the manufacturer issued an alert advising health care professionals of this safety signal in June 2019, wrote Per Damkier, MD, PhD, corresponding author of a JAMA research letter reporting the Danish study results. The postmarketing study had shown a major malformation rate of about 15% in modafinil-exposed pregnancies, much higher than the 3% background rate.
Dr. Damkier and Anne Broe, MD, PhD, both of the department of clinical biochemistry and pharmacology at Odense (Denmark) University Hospital, compared outcomes for pregnant women who were prescribed modafinil at any point during the first trimester of pregnancy with those who were prescribed an active comparator, methylphenidate, as well as with those who had neither exposure. Methylphenidate is not associated with congenital malformations and is used for indications similar to modafinil.
Looking at all pregnancies for whom complete records existed in Danish health registries between 2004 and 2017, the investigators found 49 modafinil-exposed pregnancies, 963 methylphenidate-exposed pregnancies, and 828,644 pregnancies with neither exposure.
Six major congenital malformations occurred in the modafinil-exposed group for an absolute risk of 12%. Major malformations occurred in 43 (4.5%) of the methylphenidate-exposed group and 32,466 (3.9%) of the unexposed group.
Using the extensive data available in public registries, the authors were able to perform logistic regression to adjust for concomitant use of other psychotropic medication; comorbidities such as diabetes and hypertension; and demographic and anthropometric measures such as maternal age, smoking status, and body mass index.
After this statistical adjustment, the researchers found that modafinil exposure during the first trimester of pregnancy was associated with an odds ratio of 3.4 (95% confidence interval, 1.2-9.7) for major congenital malformation, compared with first-trimester methylphenidate exposure. Compared with the unexposed cohort, modafinil-exposed pregnancies had an adjusted odds ratio of 2.7 (95% CI, 1.1-6.9) for major congenital malformation.
A total of 13 (27%) women who took modafinil had multiple sclerosis, but the authors excluded women who’d received a prescription for the multiple sclerosis drug teriflunomide (Aubagio), a known teratogen. Sleep disorders were reported for 39% of modafinil users, compared with 4.5% of methylphenidate users. Rates of psychoactive drug use were 41% for the modafinil group and 30% for the methylphenidate group.
The authors acknowledged the possibility of residual confounders affecting their results, and of the statistical problems with the very small sample size of modafinil-exposed pregnancies. Also, actual medication use – rather than prescription redemption – wasn’t captured in the study.
The study was partially funded by the Novo Nordisk Foundation. The authors reported no conflicts of interest.
SOURCE: Damkier P, Broe A. JAMA. 2020;323(4):374-6.
FROM JAMA