User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
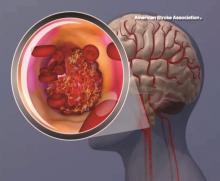
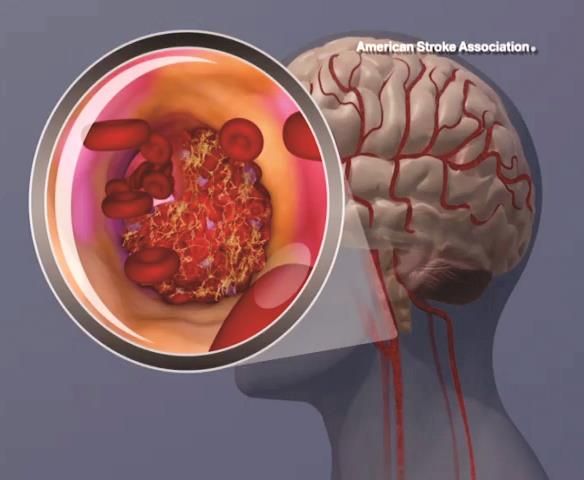
BP levels during endovascular stroke therapy affect neurologic outcomes
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
For patients with acute ischemic stroke, prolonged durations of blood pressure above or below certain thresholds during endovascular therapy may be linked to poor functional outcome, results of a retrospective study suggest.
Mean arterial blood pressure (MABP) lower than 70 mm Hg for 10 minutes or more, or higher than 90 mm Hg for 45 minutes or more, represented “critical thresholds” associated with worse neurologic outcomes, the study authors wrote in JAMA Neurology.
“These results suggest MABP may be a modifiable therapeutic target to prevent or reduce poor functional outcome in patients undergoing endovascular therapy for acute ischemic stroke, and that MABP should possibly be maintained within such narrow limits, wrote the authors, led by Mads Rasmussen, MD, PhD, of the department of anesthesia at Aarhus (Denmark) University Hospital.
The findings come from an analysis of BP data from 365 patients with acute ischemic stroke enrolled in three randomized trials evaluating different strategies for anesthesia. Among those patients, the mean age was approximately 71 years, and about 45% were women.
The investigators looked at a variety of BP-related variables during endovascular therapy to assess their impact on functional outcome, based on modified Rankin Scale (mRS) scores at 90 days.
Having an MABP below 70 mm Hg for a cumulative time of at least 10 minutes substantially increased odds of higher 90-day mRS scores (odds ratio, 1.51; 95% confidence interval, 1.02-2.22), according to Dr. Rasmussen and colleagues. The number needed to harm (NNH) at this threshold was 10; in other words, to harm 1 patient, 10 patients are needed with procedural MABP below 70 mm Hg for at least 10 minutes.
Likewise, having an MABP above 90 mm Hg for a cumulated time of at least 45 minutes significantly increased odds of higher 90-day mRS scores, with an OR of 1.49 (95% CI, 1.11-2.02) and a number needed to harm of 10.
Odds of shifting toward a worse neurologic outcome increased by 62% for every continuous 10 minutes of MABP below 70 mm Hg, and by 8% for every continuous 10 minutes above 90 mm Hg.
The maximum MABP during the procedure was significantly associated with neurologic outcomes in the study, while by contrast, maximum procedural systolic BP was not, according to the investigators.
In general, the study findings suggest that MABP is “more sensitive” than systolic BP when assessing hypotension and hypertension in these patients. However, these findings are subject to a number of limitations, the investigators wrote, including the retrospective nature of the analysis and the selected group of patients enrolled in studies designed to evaluate anesthesia strategies, not hemodynamic management.
“Randomized studies are needed to determine the optimal blood pressure management strategy during endovascular therapy,” the investigators wrote.
Dr. Rasmussen reported grant support from the Health Research Foundation of Central Denmark Region and the National Helicopter Emergency Medical Service Foundation. Coauthors reported receiving grant support from the Novo Nordisk Foundation; a research award from the Patient-Centered Outcomes Research Institute; and personal fees from Abbott Medical Sweden, I4L Innovation for Life, Boehringer Ingelheim, Medtronic, and Zoll.
SOURCE: Rasmussen M et al. JAMA Neurol. 2020 Jan 27. doi: 10.1001/jamaneurol.2019.4838.
FROM JAMA NEUROLOGY
Opioid deaths boost donor heart supply
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
SNOWMASS, COLO. – The tragic opioid epidemic has “one small bright spot”: an expanding pool of eligible donor hearts for transplantation, Akshay S. Desai, MD, said at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
For decades, the annual volume of heart transplantations performed in the U.S. was static because of the huge mismatch between donor organ supply and demand. But heart transplant volume has increased steadily in the last few years – a result of the opioid epidemic.
Data from the U.S. Organ Procurement and Transplantation Network show that the proportion of donor hearts obtained from individuals who died from drug intoxication climbed from a mere 1.5% in 1999 to 17.6% in 2017, the most recent year for which data are available. Meanwhile, the size of the heart transplant waiting list, which rose year after year in 2009-2015, has since declined (N Engl J Med. 2019 Feb 7;380[6]:597-9).
“What’s amazing is that, even though these patients might have historically been considered high risk in general, the organs recovered from these patients – and particularly the hearts – don’t seem to be any worse in terms of allograft survival than the organs recovered from patients who died from other causes, which are the traditional sources, like blunt head trauma, gunshot wounds, or stroke, that lead to brain death. In general, these organs are useful and do quite well,” according to Dr. Desai, medical director of the cardiomyopathy and heart failure program at Brigham and Women’s Hospital, Boston.
He highlighted several other recent developments in the field of cardiac transplantation that promise to further expand the donor heart pool, including acceptance of hepatitis C–infected donors and organ donation after circulatory rather than brain death. Dr. Desai also drew attention to the unintended perverse consequences of a recent redesign of the U.S. donor heart allocation system and discussed the impressive improvement in clinical outcomes with mechanical circulatory support. He noted that, while relatively few cardiologists practice in the highly specialized centers where heart transplants take place, virtually all cardiologists are affected by advances in heart transplantation since hundreds of thousands of the estimated 7 million Americans with heart failure have advanced disease.
Heart transplantation, he emphasized, is becoming increasingly complex. Recipients are on average older, sicker, and have more comorbidities than in times past. As a result, there is greater need for dual organ transplants: heart/lung, heart/liver, or heart/kidney. Plus, more patients come to transplantation after prior cardiac surgery for implantation of a ventricular assist device, so sensitization to blood products is a growing issue. And, of course, the pool of transplant candidates has expanded.
“We’re now forced to take patients previously considered to have contraindications to transplant; for example, diabetes was a contraindication to transplant in the early years, but now it’s the rule in 35%-40% of our patients who present with advanced heart failure,” the cardiologist noted.
Transplants from HCV-infected donors to uninfected recipients
Hearts and lungs from donors with hepatitis C viremia were traditionally deemed unsuitable for transplant. That’s all changed in the current era of highly effective direct-acting antiviral agents for the treatment of HCV infection.
In the DONATE HCV trial, Dr. Desai’s colleagues at Brigham and Women’s Hospital showed that giving HCV-uninfected recipients of hearts or lungs from HCV-viremic donors a shortened 4-week course of treatment with sofosbuvir-velpatasvir (Epclusa) beginning within a few hours after transplantation uniformly blocked viral replication. Six months after transplantation, none of the study participants had a detectable HCV viral load, and all had excellent graft function (N Engl J Med. 2019 Apr 25;380[17]:1606-17).
“This is effective prevention of HCV infection by aggressive upfront therapy,” Dr. Desai explained. “We can now take organs from HCV-viremic patients and use them in solid organ transplantation. This has led to a skyrocketing increase in donors with HCV infection, and those donations have helped us clear the waiting list.”
Donation after circulatory death
Australian transplant physicians have pioneered the use of donor hearts obtained after circulatory death in individuals with devastating neurologic injury who didn’t quite meet the criteria for brain death, which is the traditional prerequisite. In the new scenario, withdrawal of life-supporting therapy is followed by circulatory death, then the donor heart is procured and preserved via extracorporeal perfusion until transplantation.
The Australians report excellent outcomes, with rates of overall survival and rejection episodes similar to outcomes from brain-dead donors (J Am Coll Cardiol. 2019 Apr 2;73[12]:1447-59). The first U.S. heart transplant involving donation after circulatory death took place at Duke University in Durham, North Carolina. A multicenter U.S. clinical trial of this practice is underway.
If the results are positive and the practice of donation after circulatory death becomes widely implemented, the U.S. heart donor pool could increase by 30%.
Recent overhaul of donor heart allocation system may have backfired
The U.S. donor heart allocation system was redesigned in the fall of 2018 in an effort to reduce waiting times. One of the biggest changes involved breaking down the category with the highest urgency status into three new subcategories based upon sickness. Now, the highest-urgency category is for patients in cardiogenic shock who are supported by extracorporeal membrane oxygenation (ECMO) or other temporary mechanical circulatory support devices.
But an analysis of United Network for Organ Sharing (UNOS) data suggests this change has unintended adverse consequences for clinical outcomes.
Indeed, the investigators reported that the use of ECMO support is fourfold greater in the new system, the use of durable left ventricular assist devices (LVADs) as a bridge to transplant is down, and outcomes are worse. The 180-day rate of freedom from death or retransplantation was 77.9%, down significantly from 93.4% in the former system. In a multivariate analysis, patients transplanted in the new system had an adjusted 2.1-fold increased risk of death or retransplantation (J Heart Lung Transplant. 2020 Jan;39[1]:1-4).
“When you create a new listing system, you create new incentives, and people start to manage patients differently,” Dr. Desai observed. “Increasingly now, the path direct to transplant is through temporary mechanical circulatory support rather than durable mechanical circulatory support. Is that a good idea? We don’t know, but if you look at the best data, those on ECMO or percutaneous VADs have the worst outcomes. So the question of whether we should take the sickest of sick patients directly to transplant as a standard strategy has come under scrutiny.”
Improved durable LVAD technology brings impressive clinical outcomes
Results of the landmark MOMENTUM 3 randomized trial showed that 2-year clinical outcomes with the magnetically levitated centrifugal-flow HeartMate 3 LVAD now rival those of percutaneous mitral valve repair using the MitraClip device. Two-year all-cause mortality in the LVAD recipients was 22% versus 29.1% with the MitraClip in the COAPT trial and 34.9% in the MITRA-FR trial. The HeartMate 3 reduces the hemocompatibility issues that plagued earlier-generation durable LVADs, with resultant lower rates of pump thrombosis, stroke, and GI bleeding. Indeed, the outcomes in MOMENTUM 3 were so good – and so similar – with the HeartMate 3, regardless of whether the intended treatment goal was as a bridge to transplant or as lifelong destination therapy, that the investigators have recently proposed doing away with those distinctions.
“It is possible that use of arbitrary categorizations based on current or future transplant eligibility should be clinically abandoned in favor of a single preimplant strategy: to extend the survival and improve the quality of life of patients with medically refractory heart failure,” according to the investigators (JAMA Cardiol. 2020 Jan 15. doi: 10.1001/jamacardio.2019.5323).
The next step forward in LVAD technology is already on the horizon: a fully implantable device that eliminates the transcutaneous drive-line for the power supply, which is prone to infection and diminishes overall quality of life. This investigational device utilizes wireless coplanar energy transfer, with a coil ring placed around the lung and fixed to the chest wall. The implanted battery provides more than 6 hours of power without a recharge (J Heart Lung Transplant. 2019 Apr;38[4]:339-43).
“The first LVAD patient has gone swimming in Kazakhstan,” according to Dr. Desai.
Myocardial recovery in LVAD recipients remains elusive
The initial hope for LVADs was that they would not only be able to serve as a bridge to transplantation or as lifetime therapy, but that the prolonged unloading of the ventricle would enable potent medical therapy to rescue myocardial function so that the device could eventually be explanted. That does happen, but only rarely. In a large registry study, myocardial recovery occurred in only about 1% of patients on mechanical circulatory support. Attempts to enhance the process by add-on stem cell therapy have thus far been ineffective.
“For the moment, recovery is still a hope, not a reality,” the cardiologist said.
He reported serving as a consultant to more than a dozen pharmaceutical or medical device companies and receiving research grants from Alnylam, AstraZeneca, Bayer Healthcare, MyoKardia, and Novartis.
bjancin@mdedge.com
EXPERT ANALYSIS FROM ACC SNOWMASS 2020
Vigilance safely keeps AFib patients off anticoagulants post ablation
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
NATIONAL HARBOR, MD. – A pilot program of daily arrhythmia self-vigilance has allowed selected patients with no atrial fibrillation following a catheter ablation procedure to safely come off a regimen of daily oral anticoagulation despite having residual risk factors for ischemic stroke.
This program, which started several years ago at the University of Pennsylvania in Philadelphia, has now managed 190 patients and followed them for a median of just over 3 years, and during 576 patient-years of follow-up, just a single patient had an ischemic cerebrovascular event that occurred with no atrial fibrillation (AFib) recurrence and appeared to be caused by an atherosclerotic embolism, Francis E. Marchlinski, MD, said at the annual International AF Symposium.
Although this strategy has not yet been tested in a prospective, randomized trial, this anecdotal, single-center experience suggests that the approach is “safe and effective” for selected patients who are eager to come off of their anticoagulation regimen when they remain arrhythmia free following catheter ablation of their AFib, said Dr. Marchlinski, professor of medicine and director of electrophysiology at the University of Pennsylvania. He and his associates developed this strategy as a way to more safely allow these patients to stop taking a daily oral anticoagulant because he found that many patients were stopping on their own, with no safety strategy in place.
“Patients tell me they don’t want to be on an oral anticoagulant because a parent had a hemorrhagic stroke, and they say they’re willing to accept the risk” of having an ischemic stroke by coming off anticoagulation. “This is a way for them to do it safely,” Dr. Marchlinski said in an interview. He stressed that he only allows his patients to go this route if they understand the risk and accept their shared responsibility for vigilant, twice-daily pulse monitoring to detect resumption of an irregular heart beat.
Since 2011, Dr. Marchlinski’s program ablated 1,216 patients with AFib who then remained arrhythmia free during 3 weeks of continuous ECG monitoring following their procedure. Among these patients, 443 had a CHA2DS2-VAScscore of either 0 (men) or 1 (women) that indicated no ongoing need for oral anticoagulation according to current guidelines. Of the remaining 773 patients with a CHA2DS2-VASc score of at least 1 in men and 2 in women, the clinicians determined 583 to be ineligible for the program because of their unwillingness to accept the risk, unwillingness to comply with daily pulse checks, a history of asymptomatic AFib, a CHA2DS2-VASc score greater than 4, or a resting pulse above 90 beats per minute, leaving 190 patients eligible to participate. Among these patients, 105 (55%) had a CHA2DS2-VASc score of 2-4, which should prompt anticoagulation according to current guidelines.Participating patients committed to check their resting pulse by palpation at least twice daily and to contacting the program immediately if their resting rate spiked by more than 20 beats per minutes or in another way seemed irregular. Patients were also instructed to restart their oral anticoagulation immediately if they experienced AFib symptoms that persisted for more than 5 minutes. Many patients in the program also use a wearable device (usually a watch) to monitor their resting pulse and to generate a 30-second ECG recording that they can send as an electronic file to the University of Pennsylvania staff. “We embrace wearables,” Dr. Marchlinski said. Those without a wearable can undergo transtelephonic EEG monitoring to document a suspected arrhythmia recurrence, and all patients undergo annual monitoring by continuous ECG for at least 2 weeks.During follow-up, in addition to the 1 patient free from recurrent AFib who had an atherosclerotic embolism, 34 patients resumed anticoagulant treatment because of AFib recurrence; 12 withdrew from the program because of noncompliance or preference, or because an exclusion appeared; 29 resumed oral anticoagulation transiently but then discontinued the drug a second time when their AFib recurrence resolved; and 114 patients (60% of the starting cohort of 190) remained completely off anticoagulation during a median of 37 months. These data updated a published report from Dr. Marchlinski and his associates on their first 99 patients followed for a median of 30 months (J Cardiovasc Electrophysiol. 2019 May;30[5]:631-8).
This experience underscored the need for ongoing rhythm monitoring even in the absence of AFib symptoms, as six patients developed asymptomatic AFib detected by monitoring, including one patient whose recurrence occurred 30 months after the ablation procedure.
Dr. Marchlinski stressed the stringent selection process he applies to limit this approach to patients who are willing to faithfully monitor their pulse and symptoms daily, and who accept the risk that this approach may pose and their responsibility to stay in contact with the clinical team. The program calls patients at the 6-month mark between annual monitoring to remind them of their need for daily attention.
“Being off anticoagulants is very important to these patients,” he explained, and he highlighted the added workload this strategy places on his staff. “I think this has legs” for adoption by other cardiac arrhythmia programs, “but it depends on the time the staff is willing to spend” monitoring and following these patients, some of whom regularly send in ECG traces from their wearable devices for assessment. “It takes a village” to make this program work, he said.
Dr. Marchlinski has been a consultant to or has received honoraria from Abbott EP/St. Jude, Biosense Webster, Biotronik, Boston Scientific, and Medtronic.
REPORTING FROM THE AF SYMPOSIUM 2020
Zika virus: Birth defects rose fourfold in U.S. hardest-hit areas
according to the Centers for Disease Control and Prevention.
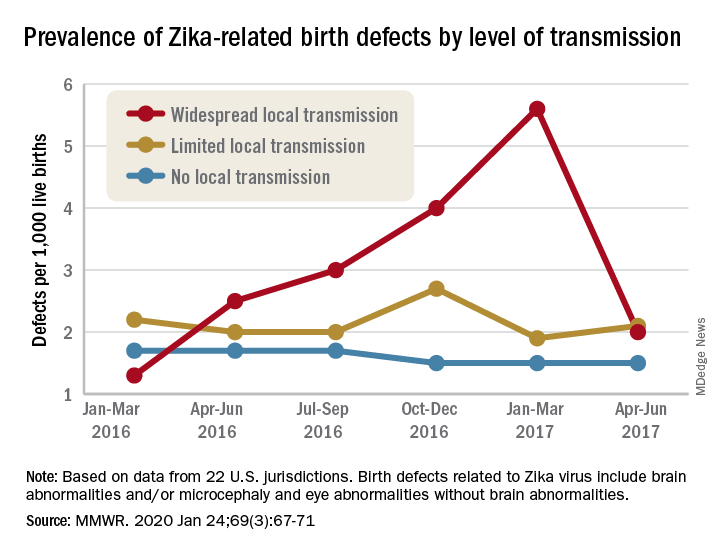
That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
according to the Centers for Disease Control and Prevention.

That spike in the prevalence of brain abnormalities and/or microcephaly or eye abnormalities without brain abnormalities came during January through March 2017, about 6 months after the Zika outbreak’s reported peak in the jurisdictions with widespread local transmission, Puerto Rico and the U.S. Virgin Islands, wrote Ashley N. Smoots, MPH, of the CDC’s National Center on Birth Defects and Developmental Disabilities and associates in the Morbidity and Mortality Weekly Report.
In those two territories, the prevalence of birth defects potentially related to Zika virus infection was 5.6 per 1,000 live births during January through March 2017, compared with 1.3 per 1,000 in January through March 2016, they reported.
In the southern areas of Florida and Texas, where there was limited local Zika transmission, the highest prevalence of birth defects, 2.7 per 1,000, occurred during October through December 2016, and was only slightly greater than the baseline rate of 2.2 per 1,000 in January through March 2016, the investigators reported.
Among the other 19 jurisdictions (including Illinois, Louisiana, New Jersey, South Carolina, and Virginia) involved in the analysis, the rate of Zika virus–related birth defects never reached any higher than the 1.7 per 1,000 recorded at the start of the study period in January through March 2016, they said.
“Population-based birth defects surveillance is critical for identifying infants and fetuses with birth defects potentially related to Zika virus regardless of whether Zika virus testing was conducted, especially given the high prevalence of asymptomatic disease. These data can be used to inform follow-up care and services as well as strengthen surveillance,” the investigators wrote.
SOURCE: Smoots AN et al. MMWR. 2020 Jan 24;69(3):67-71.
FROM MMWR
Cannabis for sleep: Short-term benefit, long-term disruption?
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
, new research shows.
Investigators found whole-plant medical cannabis use was associated with fewer problems with respect to waking up at night, but they also found that frequent medical cannabis use was associated with more problems initiating and maintaining sleep.
“Cannabis may improve overall sleep in the short term,” study investigator Sharon Sznitman, PhD, University of Haifa (Israel) Faculty of Social Welfare and Health Sciences, said in an interview. “But it’s also very interesting that when we looked at frequency of use in the group that used medical cannabis, individuals who had more frequent use also had poorer sleep in the long term.
“This suggests that while cannabis may improve overall sleep, it’s also possible that there is a tolerance that develops with either very frequent or long-term use,” she added.
The study was published online Jan. 20 in BMJ Supportive and Palliative Care.
A common problem
Estimates suggest chronic pain affects up to 37% of adults in the developed world. Individuals who suffer chronic pain often experience comorbid insomnia, which includes difficulty initiating sleep, sleep disruption, and early morning wakening.
For its part, medical cannabis to treat chronic pain symptoms and manage sleep problems has been widely reported as a prime motivation for medical cannabis use. Indeed, previous studies have concluded that the endocannabinoid system plays a role in sleep regulation, including sleep promotion and maintenance.
In recent years, investigators have reported the beneficial effects of medical cannabis for sleep. Nevertheless, some preclinical research has also concluded that chronic administration of tetrahydrocannabinol may result in tolerance to the sleep-enhancing effects of cannabis.
With that in mind, the researchers set out to examine the potential impact of whole-plant medicinal cannabis on sleep problems experienced by middle-aged patients suffering from chronic pain.
“People are self-reporting that they’re using cannabis for sleep and that it helps, but as we know, just because people are reporting that it works doesn’t mean that it will hold up in research,” Dr. Sznitman said.
The study included 128 individuals (mean age, 61±6 years; 51% females) with chronic neuropathic pain: 66 were medical cannabis users and 62 were not.
Three indicators of insomnia were measured using the 7-point Likert scale to assess issues with sleep initiation and maintenance.
In addition, investigators collected sociodemographic information, as well as data on daily consumption of tobacco, frequency of alcohol use, and pain severity. Finally, they collected patient data on the use of sleep-aid medications during the past month as well as tricyclic antidepressant use.
Frequent use, more sleep problems?
On average, medical cannabis users were 3 years younger than their nonusing counterparts (mean age, 60±6 vs. 63±6 years, respectively, P = .003) and more likely to be male (58% vs 40%, respectively, P = .038). Otherwise, the two groups were comparable.
Medical cannabis users reported taking the drug for an average of 4 years, at an average quantity of 31 g per month. The primary mode of administration was smoking (68.6%), followed by oil extracts (21.4%) and vaporization (20%).
Results showed that, of the total sample, 24.1% reported always waking up early and not falling back to sleep, 20.2% reported always having difficulty falling asleep, and 27.2% reported always waking up during the night.
After adjusting for patient age, sex, pain level, and use of sleep medications and antidepressants, medical cannabis use was associated with fewer problems with waking up at night, compared with nonmedical cannabis use. No differences were found between groups with respect to problems falling asleep or waking up early without being able to fall back to sleep, Dr. Sznitman and associates reported.
The final analysis of a subsample of patients that only included medical cannabis users showed frequency of medical cannabis use was associated with sleep problems, they said.
Specifically, more frequent cannabis use was associated with more problems related to waking up at night, as well as problems falling asleep.
Sleep problems associated with frequent medical cannabis use may signal the development of tolerance to the agent. However, frequent users of medical cannabis also maybsuffer pain or other comorbidities, which, in turn, may be linked to more sleep problems.
Either way, Dr. Sznitman said the study might open the door to another treatment option for patients suffering from chronic pain who struggle with sleep.
“If future research shows that the effect of medical cannabis on sleep is a consistent one, then we may be adding a new therapy for sleep problems, which are huge in society and especially in chronic pain patients,” she said.
Early days
Commenting on the findings in an interview, Ryan G. Vandrey, PhD, who was not involved in the study, said the findings are in line with previous research.
“I think the results make sense with respect to the data I’ve collected and from what I’ve seen,” said Dr. Vandrey, associate professor of psychiatry and behavioral sciences at Johns Hopkins Medicine in Baltimore.
“We typically only want to use sleep medications for short periods of time,” he continued. “When you think about recommended prescribing practices for any hypnotic medication, it’s usually short term, 2 weeks or less. Longer-term use often leads to tolerance, dependence, and withdrawal symptoms when the medication is stopped, which leads to an exacerbation of disordered sleep,” Dr. Vandrey said.
Nevertheless, he urged caution when interpreting the results.
“I think the study warrants caution about long-term daily use of cannabinoids with respect to sleep,” he said. “But we need more detailed evaluations, as the trial wasn’t testing a defined product, specific dose, or dose regimen.
“In addition, this was all done in the context of people with chronic pain and not treating disordered sleep or insomnia, but the study highlights the importance of recognizing that long-term chronic use of cannabis is not likely to fully resolve sleep problems.”
Dr. Sznitman agreed that the research is still in its very early stages.
“We’re still far from saying we have the evidence to support the use of medical cannabis for sleep,” she said. “For in the end it was just a cross-sectional, observational study, so we cannot say anything about cause and effect. But if these results pan out, they could be far-reaching and exciting.”
The study was funded by the University of Haifa and Rambam Hospital in Israel, and by the Evelyn Lipper Foundation. Dr. Sznitman and Dr. Vandrey have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
FROM BMJ SUPPORTIVE AND PALLIATIVE CARE
Wuhan virus: What clinicians need to know
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
As the Wuhan coronavirus story unfolds, , according to infectious disease experts.

“We are asking that of everyone with fever and respiratory symptoms who comes to our clinics, hospital, or emergency room. It’s a powerful screening tool,” said William Schaffner, MD, professor of preventive medicine and infectious diseases at Vanderbilt University Medical Center, Nashville, Tenn.
In addition to fever, common signs of infection include cough, shortness of breath, and breathing difficulties. Some patients have had diarrhea, vomiting, and other gastrointestinal symptoms. In more severe cases, infection can cause pneumonia, severe acute respiratory syndrome, kidney failure, and death. The incubation period appears to be up to 2 weeks, according to the World Health Organization (WHO).
If patients exhibit symptoms and either they or a close contact has returned from China recently, take standard airborne precautions and send specimens – a serum sample, oral and nasal pharyngeal swabs, and lower respiratory tract specimens if available – to the local health department, which will forward them to the Centers for Disease Control and Prevention (CDC) for testing. Turnaround time is 24-48 hours.
The 2019 Novel Coronavirus (2019-nCoV), identified as the cause of an outbreak of respiratory illness first detected in December in association with a live animal market in Wuhan, China, has been implicated in almost 2,000 cases and 56 deaths in that country. Cases have been reported in 13 countries besides China. Five cases of 2019-nCoV infection have been confirmed in the United States, all in people recently returned from Wuhan. As the virus spreads in China, however, it’s almost certain more cases will show up in the United States. Travel history is key, Dr. Schaffner and others said.
Plan and rehearse
The first step to prepare is to use the CDC’s Interim Guidance for Healthcare Professionals to make a written plan specific to your practice to respond to a potential case. The plan must include notifying the local health department, the CDC liaison for testing, and tracking down patient contacts.
“It’s not good enough to just download CDC’s guidance; use it to make your own local plan and know what to do 24/7,” said Daniel Lucey, MD, an infectious disease expert at Georgetown University Medical Center, Washington, D.C.
“Know who is on call at the health department on weekends and nights,” he said. Know where the patient is going to be isolated; figure out what to do if there’s more than one, and tests come back positive. Have masks on hand, and rehearse the response. “Make a coronavirus team, and absolutely have the nurses involved,” as well as other providers who may come into contact with a case, he added.
“You want to be able to do as well as your counterparts in Washington state and Chicago,” where the first two U.S. cases emerged. “They were prepared. They knew what to do,” Dr. Lucey said.
Those first two U.S. patients – a man in Everett, Wash., and a Chicago woman – developed symptoms after returning from Wuhan, a city of 11 million just over 400 miles inland from the port city of Shanghai. On Jan. 26 three more cases were confirmed by the CDC, two in California and one in Arizona, and each had recently traveled to Wuhan. All five patients remain hospitalized, and there’s no evidence they spread the infection further. There is also no evidence of human-to-human transmission of other cases exported from China to any other countries, according to the WHO.
WHO declined to declare a global health emergency – a Public Health Emergency of International Concern, in its parlance – on Jan. 23. The step would have triggered travel and trade restrictions in member states, including the United States. For now, at least, the group said it wasn’t warranted at this point.
Fatality rates
The focus right now is China. The outbreak has spread beyond Wuhan to other parts of the country, and there’s evidence of fourth-generation spread.
Transportation into and out of Wuhan and other cities has been curtailed, Lunar New Year festivals have been canceled, and the Shanghai Disneyland has been closed, among other measures taken by Chinese officials.
The government could be taking drastic measures in part to prevent the public criticism it took in the early 2000’s for the delayed response and lack of transparency during the global outbreak of another wildlife market coronavirus epidemic, severe acute respiratory syndrome (SARS). In a press conference Jan. 22, WHO officials commended the government’s containment efforts but did not say they recommended them.
According to WHO, serious cases in China have mostly been in people over 40 years old with significant comorbidities and have skewed towards men. Spread seems to be limited to family members, health care providers, and other close contacts, probably by respiratory droplets. If that pattern holds, WHO officials said, the outbreak is containable.
The fatality rate appears to be around 3%, a good deal lower than the 10% reported for SARS and much lower than the nearly 40% reported for Middle East respiratory syndrome (MERS), another recent coronavirus mutation from the animal trade.
The Wuhan virus fatality rate might drop as milder cases are detected and added to the denominator. “It definitely appears to be less severe than SARS and MERS,” said Amesh Adalja, MD, an infectious disease physician in Pittsburgh and emerging infectious disease researcher at Johns Hopkins University, Baltimore.
SARS: Lessons learned
In general, the world is much better equipped for coronavirus outbreaks than when SARS, in particular, emerged in 2003.
WHO officials in their press conference lauded China for it openness with the current outbreak, and for isolating and sequencing the virus immediately, which gave the world a diagnostic test in the first days of the outbreak, something that wasn’t available for SARS. China and other countries also are cooperating and working closely to contain the Wuhan virus.
“What we know today might change tomorrow, so we have to keep tuned in to new information, but we learned a lot from SARS,” Dr. Shaffner said. Overall, it’s likely “the impact on the United States of this new coronavirus is going to be trivial,” he predicted.
Dr. Lucey, however, recalled that the SARS outbreak in Toronto in 2003 started with one missed case. A woman returned asymptomatic from Hong Kong and spread the infection to her family members before she died. Her cause of death wasn’t immediately recognized, nor was the reason her family members were sick, since they hadn’t been to Hong Kong recently.
The infection ultimately spread to more than 200 people, about half of them health care workers. A few people died.
If a virus is sufficiently contagious, “it just takes one. You don’t want to be the one who misses that first patient,” Dr. Lucey said.
Currently, there are no antivirals or vaccines for coronaviruses; researchers are working on both, but for now, care is supportive.
This article was updated with new case numbers on 1/26/20.
FDA: Cybersecurity vulnerabilities identified in GE Healthcare monitoring devices
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
The Food and Drug Administration has issued a warning that certain GE Healthcare Clinical Information Central Stations and Telemetry Servers have cybersecurity vulnerabilities that may introduce risk to monitored patients.
silence alarms, generate false alarms, and interfere with alarms of patient monitors connected to these devices, according to an “Urgent Medical Device Correction” letter issued by GE Healthcare in November 2019.
The affected devices are the ApexPro Telemetry Server and CARESCAPE Telemetry Server, the CARESCAPE Central Station (CSCS) version 1, and the CIC Pro Clinical Information Center Central Station version 1. These devices are used in health care facilities for displaying information, such as the patient’s physiological parameters, and for monitoring patient status from a central location in a facility.
No adverse events related to the vulnerabilities have been reported to the FDA. Health care facility staff should update their devices when GE Healthcare issues a software patch that addresses the vulnerability, separate the network connecting patient monitors using affected devices from the rest of the hospital, and use firewalls and other means to minimize the risk of remote or local network attacks.
“The FDA takes reports of cybersecurity vulnerabilities in medical devices seriously and will continue to work with GE Healthcare as the firm develops software patches to correct these vulnerabilities as soon as possible. The FDA will continue to assess new information concerning the vulnerabilities and will keep the public informed if significant new information becomes available,” the FDA said in the Safety Communication.
Neurologic disease doesn’t discriminate against anyone
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
In 1982, I went to my first concert. It was Rush, on their “Signals” tour, and I loved it. In fact, I went back and saw them again about a year later. I bought a concert T-shirt at the first one. I still have it somewhere, though I am pretty sure it hasn’t fit me in years.
I loved their music before the concert, enjoyed it even more afterwards, and still do. Their albums are all on my computer and phone, and part of the daily soundtrack of my life when working at my desk, driving, and walking (I’m trying to fit back in the shirt).
On Jan. 7, 2020, Neil Peart, the trio’s remarkably gifted drummer, died of a neurologic disease.
According to the news, he had a glioblastoma multiforme, a tumor terrifying for its aggressiveness, difficulty of treatment, and lack of preventable risk factors.
The cost of neurologic disease is terrible. Glioblastoma multiforme, unfortunately, is far from rare, nor is it the only one. In recent times, entertainers afflicted with neurologic disease have included Neil Diamond, Linda Ronstadt, Peter Falk, Glen Campbell, Charlton Heston, Gene Siskel, Michael J. Fox, Stephen Hillenburg, Teri Garr, Annette Funicello, Robin Williams, Dudley Moore, and most recently Ozzy Osbourne.
That’s a pretty short list, too, far from all-encompassing. The majority of people with these disorders won’t be in the news. Their everyday struggles, stories, and losses are known only to family, friends, and the medical team doing its best to help.
Medical technology advances every year. In the 22 years since I began practicing, we’ve made remarkable strides in some areas – multiple sclerosis, for example. But our work in so many other areas is nowhere close. The increasing knowledge as to the mechanisms and causes of Alzheimer’s disease have, to date, failed to translate into treatment success.
That’s not to say we should give up. Far from it. Our species has gotten where we are by always wanting to get over the next hill. Initial failures will always outnumber successes. But when you’re a doctor dealing with the very real human cost of neurologic disease, that’s not much consolation. And it’s far less so for the patients and families affected who come to us for help.
We use terms like “burden” or “cost” to discuss the financial aspects of illness, but they often don’t seem adequate to describe the real effects. The emotional damages. The gifted musicians and loved family members lost. Family members struggling with the difficult role of being care givers.
Neurologic disease doesn’t discriminate against anyone, regardless of age, fame, or talent. I’ll stay here and do my best for all of them who come to me. I’m certainly not on the front line of research. That’s incredibly important, but I’ll leave it to others. My work is where the patients are every day.
Thank you for the music, Neil.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
Actor Alan Alda discusses using empathy as an antidote to burnout
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – Physicians and other medical professionals who routinely foster empathic connections with patients may be helping themselves steer clear of burnout.
That’s what iconic actor Alan Alda suggested during a media briefing at Scripps Research on Jan. 16, 2020.

“There’s a tremendous pressure on doctors now to have shorter and shorter visits with their patients,” said the 83-year-old Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016 for his work as a champion of science. “A lot of that time is taken up with recording on a computer, which can only put pressure on the doctor.”
Practicing empathy, he continued, “kind of opens people up to one another, which inspirits them.”
Mr. Alda appeared on the research campus to announce that Scripps Research will serve as the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at Stony Brook (N.Y.) University, a nonprofit organization that Mr. Alda helped found in 2009.
“This will be a center where people can come to get training in effective communication,” Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards, told an audience of scientists and medical professionals prior to the media briefing.
“It’s an experiential kind of training,” he explained. “We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that, when you get a diagnosis, your life is over,” said Mr. Alda, who played army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H.”
“Under the burden of that belief, some people won’t tell their family or workplace colleagues,” he said. “There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
Race, ethnicity may influence outcomes after supratentorial intracerebral hemorrhage
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
researchers reported Jan. 22 in Neurology.
“There has been considerable research on stroke in older people, but there is still much to be learned about stroke in younger people and how it affects people of different races and ethnicities,” study author Daniel Woo, MD, professor of neurology at the University of Cincinnati, said in a news release. “Our study found that, even when you account for factors that affect outcomes, such as how big the stroke is, race and ethnicity were still independent predictors of how well people would recover.”
A subset of ERICH participants
To examine predictors of functional outcome in young patients with ICH, researchers analyzed data from a subset of patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study. ERICH enrolled patients with nontraumatic ICHs at 42 sites in the United States. It included 1,000 non-Hispanic black patients, 1,000 non-Hispanic white patients, and 1,000 Hispanic patients. Participants self-reported race and ethnicity.
Lead author Laura C. Miyares from Yale School of Medicine in New Haven, Conn., and colleagues analyzed data from 418 patients in ERICH who were aged 18-49 years and had supratentorial ICH. The cohort had an average age of 43 years, and 69% were male. In this subset, 41% were black, 12% were white, and 47% were Hispanic.
The primary outcome was modified Rankin Scale (mRS) score 3 months after the ICH, and the investigators defined a poor outcome as a score of 4 or greater. At 3 months, 35% had a poor functional outcome. Approximately 18% were unable to walk without assistance and attend to their bodily needs (mRS 4); 8% were bedridden, incontinent, and required nursing care (mRS 5); and 10% had died (mRS 6).
The percentage of patients with a poor functional outcome was 52% among white patients, 35% among black patients, and 31% among Hispanic patients. In a univariable analysis, black patients had a 51% reduction in odds of a poor outcome, compared with white patients, and Hispanic patients had a 59% reduction.
“The association between race/ethnicity and 3-month post-ICH functional outcome remained significant after adjusting for age, sex, premorbid disability, ICH location, ICH volume, [intraventricular hemorrhage] extension, systolic blood pressure, and [Glasgow Coma Scale] score on admission,” the researchers said. “In multivariable analysis, using white patients as the reference category, black patients had a 58% reduction in the odds of poor functional outcome at 3 months and Hispanic patients had a 66% reduction in the same odds.”
Their analysis identified the importance of other risk factors as well. “The volume of the hematoma, the most powerful predictor of outcome in older patients with ICH, was also found to be the most significant predictor of poor outcome in young patients,” they said.
Vascular risks and oral anticoagulants
About 80% of the young adults with ICH had a history of diagnosed hypertension. In nearly half, the condition was untreated. “After hypertension, the most common stroke risk factors in the young were diabetes, high cholesterol, smoking, and alcohol abuse,” the authors said. “In combination, these results indicate that vascular risk factors, especially untreated, could explain a large proportion of cases of ICH in the young.”
“Our results also point to treatment with oral anticoagulants before hospitalization as a potential mediator of the effect of race/ethnicity on short-term functional outcomes,” they said. About 8% of the white patients used oral anticoagulants, compared with 4% of the black patients and 1% of the Hispanic patients. Oral anticoagulant treatment “is a known risk factor for ICH and an established predictor of poor outcome in this condition. However, because only a small proportion of enrolled young patients with ICH were on [oral anticoagulants] prior to presentation, these results should be further validated by future studies.”
The study’s limitations include the broad categorization of racial and ethnic groups, the fact that younger patients with supratentorial ICH were more likely to be black or Hispanic and less likely to be white, and the exclusion of a significant proportion of cases of young white patients with smaller ICH volumes because of missing data, the researchers noted. Although the cohort was large, researchers may need to study more patients to capture differences among racial and ethnic groups, the investigators said.
The association between race/ethnicity and functional outcome could relate to “distinct pathophysiologies of the initial bleed or unique mechanisms of secondary injury,” the researchers suggested. “Future studies are necessary to probe the potential biological and social mediators of these findings to elucidate the role of race/ethnicity in ICH severity and functional recovery, and to develop improved prognostication for a racially varied population.”
ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
SOURCE: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.
FROM NEUROLOGY
Key clinical point: Among young adults with supratentorial intracerebral hemorrhage (ICH), black race and Hispanic ethnicity are associated with better functional outcomes, compared with white race.
Major finding: In multivariable analysis, black patients had a 58% reduction in the odds of poor functional outcome at 3 months, compared with white patients, and Hispanic patients had a 66% reduction.
Study details: An analysis of data from a subset of 418 patients in the Ethnic/Racial Variations in Intracerebral Hemorrhage (ERICH) study.
Disclosures: ERICH is supported by the National Institute of Neurological Disorders and Stroke. Authors disclosed grants from the government, professional societies, and a university.
Source: Miyares LC et al. Neurology. 2020 Jan 22. doi: 10.1212/WNL.0000000000008930.