User login
Clinical Psychiatry News is the online destination and multimedia properties of Clinica Psychiatry News, the independent news publication for psychiatrists. Since 1971, Clinical Psychiatry News has been the leading source of news and commentary about clinical developments in psychiatry as well as health care policy and regulations that affect the physician's practice.
Dear Drupal User: You're seeing this because you're logged in to Drupal, and not redirected to MDedge.com/psychiatry.
Depression
adolescent depression
adolescent major depressive disorder
adolescent schizophrenia
adolescent with major depressive disorder
animals
autism
baby
brexpiprazole
child
child bipolar
child depression
child schizophrenia
children with bipolar disorder
children with depression
children with major depressive disorder
compulsive behaviors
cure
elderly bipolar
elderly depression
elderly major depressive disorder
elderly schizophrenia
elderly with dementia
first break
first episode
gambling
gaming
geriatric depression
geriatric major depressive disorder
geriatric schizophrenia
infant
ketamine
kid
major depressive disorder
major depressive disorder in adolescents
major depressive disorder in children
parenting
pediatric
pediatric bipolar
pediatric depression
pediatric major depressive disorder
pediatric schizophrenia
pregnancy
pregnant
rexulti
skin care
suicide
teen
wine
section[contains(@class, 'nav-hidden')]
footer[@id='footer']
div[contains(@class, 'pane-pub-article-cpn')]
div[contains(@class, 'pane-pub-home-cpn')]
div[contains(@class, 'pane-pub-topic-cpn')]
div[contains(@class, 'panel-panel-inner')]
div[contains(@class, 'pane-node-field-article-topics')]
section[contains(@class, 'footer-nav-section-wrapper')]
Not always implemented or enforced: Harassment policies at work
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Many companies, government agencies, and organizations have implemented policies and procedures to shield employees from sexual and other forms of harassment. The U.S. Department of Health & Human Services and the American Medical Association are just two examples.
Employers can tap a rich lode of guidance and resources to craft these antiharassment policies. The National Institutes of Health’s resource page is a good site for hospitals to check out.
But how effective have official policies proved in deterring harassment in medical workplaces? After all, in a study by the American Association of Medical Colleges, 34% of female faculty said they had experienced sexual harassment irrespective of such policies. And in a recent Medscape survey of more than 3,000 physicians, 27% reported that they had either witnessed or been subjected to sexual harassment or misconduct at work during the past 4 years.
When policies are absent or unenforced
She believes employer rules and policies generally are helpful in establishing who fields harassment complaints and in creating at least some accountability.
On the other hand, policies that don’t recognize anonymous complaints effectively discourage harassment victims from coming forward, Dr. Rohr-Kirchgraber argues. Even those policies that do allow anonymous complaints may have limitations.
For example, the NIH policy on reporting harassment acknowledges that “officials must follow up on all allegations of harassment and cannot guarantee that your identity will not become apparent during the process. Please note that if you remain anonymous, key details about the allegation or concern [may] be omitted. This will limit the NIH’s ability to conduct an inquiry and take corrective action as warranted.”
Risks in pressing a harassment case
A complainant whose name becomes public risks getting a reputation as a problem employee or suffering workplace retaliation, according to Dr. Rohr-Kirchgraber. She recalls a colleague who was on a clinical education track until she lodged a harassment complaint. Abruptly, she was told she was needed on a service with fewer teaching opportunities.
With such risks in mind, respondents to the Medscape survey advised employees in medical workplaces to familiarize themselves with policies and procedures before pressing a case.
“Document everything,” an ophthalmologist urged, including time, place, offender, and witnesses. Present that information to your supervisor, and if nothing is done, hire a lawyer, a gastroenterologist suggested.
But taking the situation to the Equal Employment Opportunity Commission can be complicated, Roberta Gebhard, DO, past AMWA president and founder of its Gender Equity Task Force, told this news organization.
“They talk to the employer and get the employer’s side of the story and eventually render a decision about whether you have a case you can put through and file a lawsuit,” she said. “I don’t know of any other situation in which you need ‘permission’ to file a lawsuit.”
Nevertheless, an attorney can be helpful with cases, and when someone is terminated, a lawyer can possibly have it overturned or converted to a resignation, Dr. Gebhard said.
“And always have a lawyer review your contract before you take the job,” she advised. The lawyer might adjust the contract’s verbiage in ways that can protect one down the road in the event of a potential termination. “It’s money very well spent.”
More education needed
Dr. Rohr-Kirchgraber said that protection against harassment goes beyond the employer’s policies and procedures. Building an overall consciousness of what harassment is should begin with employee onboarding, she said.
“The harasser may not even recognize that what they’re doing or saying is a form of harassment, so we need better education,” Dr. Rohr-Kirchgraber emphasized.
A version of this article originally appeared on Medscape.com.
Immunodeficiencies tied to psychiatric disorders in offspring
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.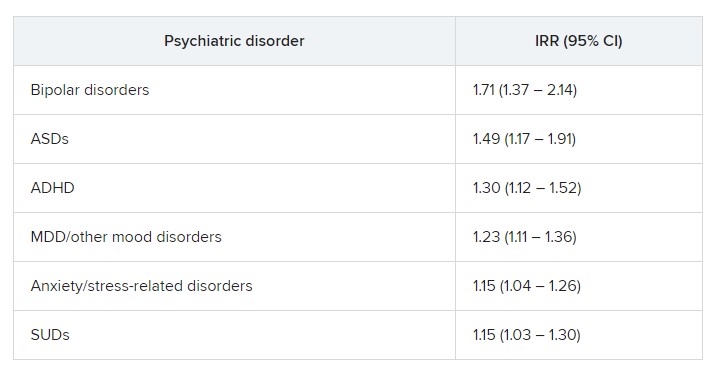
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
new research suggests.
Results from a cohort study of more than 4.2 million individuals showed that offspring of mothers with PIDs had a 17% increased risk for a psychiatric disorder and a 20% increased risk for suicidal behavior, compared with their peers with mothers who did not have PIDs.
The risk was more pronounced in offspring of mothers with both PIDs and autoimmune diseases. These risks remained after strictly controlling for different covariates, such as the parents’ psychiatric history, offspring PIDs, and offspring autoimmune diseases.
The investigators, led by Josef Isung, MD, PhD, Centre for Psychiatry Research, department of clinical neuroscience, Karolinska Institutet, Stockholm, noted that they could not “pinpoint a precise causal mechanism” underlying these findings.
Still, “the results add to the existing literature suggesting that the intrauterine immune environment may have implications for fetal neurodevelopment and that a compromised maternal immune system during pregnancy may be a risk factor for psychiatric disorders and suicidal behavior in their offspring in the long term,” they wrote.
The findings were published online in JAMA Psychiatry.
‘Natural experiment’
Maternal immune activation (MIA) is “an overarching term for aberrant and disrupted immune activity in the mother during gestation [and] has long been of interest in relation to adverse health outcomes in the offspring,” Dr. Isung noted.
“In relation to negative psychiatric outcomes, there is an abundance of preclinical evidence that has shown a negative impact on offspring secondary to MIA. And in humans, there are several observational studies supporting this link,” he said in an interview.
Dr. Isung added that PIDs are “rare conditions” known to be associated with repeated infections and high rates of autoimmune diseases, causing substantial disability.
“PIDs represent an interesting ‘natural experiment’ for researchers to understand more about the association between immune system dysfunctions and mental health,” he said.
Dr. Isung’s group previously showed that individuals with PIDs have increased odds of psychiatric disorders and suicidal behavior. The link was more pronounced in women with PIDs – and was even more pronounced in those with both PIDs and autoimmune diseases.
In the current study, “we wanted to see whether offspring of individuals were differentially at risk of psychiatric disorders and suicidal behavior, depending on being offspring of mothers or fathers with PIDs,” Dr. Isung said.
“Our hypothesis was that mothers with PIDs would have an increased risk of having offspring with neuropsychiatric outcomes, and that this risk could be due to MIA,” he added.
The researchers turned to Swedish nationwide health and administrative registers. They analyzed data on all individuals with diagnoses of PIDs identified between 1973 and 2013. Offspring born prior to 2003 were included, and parent-offspring pairs in which both parents had a history of PIDs were excluded.
The final study sample consisted of 4,294,169 offspring (51.4% boys). Of these participants, 7,270 (0.17%) had a parent with PIDs.
The researchers identified lifetime records of 10 psychiatric disorders: obsessive-compulsive disorder, ADHD, autism spectrum disorders, schizophrenia and other psychotic disorders, bipolar disorders, major depressive disorder and other mood disorders, anxiety and stress-related disorders, eating disorders, substance use disorders, and Tourette syndrome and chronic tic disorders.
The investigators included parental birth year, psychopathology, suicide attempts, suicide deaths, and autoimmune diseases as covariates, as well as offsprings’ birth year and gender.
Elucidation needed
Results showed that, of the 4,676 offspring of mothers with PID, 17.1% had a psychiatric disorder versus 12.7% of offspring of mothers without PIDs. This translated “into a 17% increased risk for offspring of mothers with PIDs in the fully adjusted model,” the investigators reported.
The risk was even higher for offspring of mothers who had not only PIDs but also one of six of the individual psychiatric disorders, with incident rate ratios ranging from 1.15 to 1.71.
“In fully adjusted models, offspring of mothers with PIDs had an increased risk of any psychiatric disorder, while no such risks were observed in offspring of fathers with PIDs” (IRR, 1.17 vs. 1.03; P < .001), the researchers reported.
A higher risk for suicidal behavior was also observed among offspring of mothers with PIDS, in contrast to those of fathers with PIDs (IRR, 1.2 vs. 1.1; P = .01).
The greatest risk for any psychiatric disorder, as well as suicidal behavior, was found in offspring of mothers who had both PIDs and autoimmune diseases (IRRs, 1.24 and 1.44, respectively).
“The results could be seen as substantiating the hypothesis that immune disruption may be important in the pathophysiology of psychiatric disorders and suicidal behavior,” Dr. Isung said.
“Furthermore, the fact that only offspring of mothers and not offspring of fathers with PIDs had this association would align with our hypothesis that MIA is of importance,” he added.
However, he noted that “the specific mechanisms are most likely multifactorial and remain to be elucidated.”
Important piece of the puzzle?
In a comment, Michael Eriksen Benros, MD, PhD, professor of immunopsychiatry, department of immunology and microbiology, health, and medical sciences, University of Copenhagen, said this was a “high-quality study” that used a “rich data source.”
Dr. Benros, who is also head of research (biological and precision psychiatry) at the Copenhagen Research Centre for Mental Health, Copenhagen University Hospital, was not involved with the current study.
He noted that prior studies, including some conducted by his own group, have shown that maternal infections overall did not seem to be “specifically linked to mental disorders in the offspring.”
However, “specific maternal infections or specific brain-reactive antibodies during the pregnancy period have been shown to be associated with neurodevelopmental outcomes among the children,” such as intellectual disability, he said.
Regarding direct clinical implications of the study, “it is important to note that the increased risk of psychiatric disorders and suicidality in the offspring of mothers with PID were small,” Dr. Benros said.
“However, it adds an important part to the scientific puzzle regarding the role of maternal immune activation during pregnancy and the risk of mental disorders,” he added.
The study was funded by the Söderström König Foundation and the Fredrik and Ingrid Thuring Foundation. Neither Dr. Isung nor Dr. Benros reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Slowing, not stopping, Alzheimer’s a better goal for clinical trials?
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
and may be a more realistic goal for clinical AD drug trials, a new report suggests.
The report is a yearlong undertaking by an expert work group convened by the Alzheimer’s Association and was prompted, in part, by the fallout from the U.S. Food and Drug Administration’s controversial decision to grant aducanumab (Aduhelm) accelerated approval, which came over the objection of an advisory panel that found the drug was ineffective.
The report’s authors call for a “reframing” of how researchers define “clinically meaningful” in randomized controlled trials (RCTs), noting that it’s time to adjust expectations of outcomes from relatively short clinical trials.
“Without lowering the bar, are we expecting too much from a clinical trial by expecting that unless the disease is halted in its tracks and there’s no progression, we failed at treatment?” the report’s lead author and group leader Ronald C. Petersen, MD, PhD, lead author, chair of the work group, and professor of neurology at the Mayo Clinic, Rochester, Minn., told this news organization.
Interpretations of clinical meaningfulness are used in the drug approval process and in decisions about whether an insurer will cover the cost of treatment, the authors note.
While the report doesn’t provide a consensus definition of clinically meaningful benefit, it does offer a starting point for a conversation about how the phrase should be defined in the context of RCTs for disease-modifying therapies (DMTs) in AD, Dr. Petersen said.
“What we tried to do was to put it into some kind of perspective and at least have people reflect on this: If you’re going to design the perfect drug trial in Alzheimer’s disease, what would it be? We wanted to get people to think about it without digging in their heels for or against,” he added.
The report was published online in Alzheimer’s and Dementia: The Journal of the Alzheimer’s Association.
A proactive measure
The expert group began its work in January 2022, less than a year after the FDA approved aducanumab. Since the panel began its work, the FDA has approved a second AD drug, lecanemab (Leqembi), and denied accelerated approval of a third medication, donanemab.
“At the time we started this group, we had one approved treatment, and we just knew that there were others on the way, and we needed to be prepared to have this conversation and be more proactive than reactive,” Christopher Weber, PhD, director of global science initiatives for the Alzheimer’s Association and co-author of the report, said in an interview.
The work group suggests that simply slowing disease progression might be a desired goal for drug trials, especially early on, before cognition and memory are affected.
They also note that a benefit identified during an 18-month clinical trial may ultimately lead to even more meaningful changes over coming years, well beyond the trial’s end.
In addition, the report authors call for the development of better research tools to more accurately assess meaningful change. The Clinical Dementia Rating (CDR) scale is currently the key instrument used as a primary outcome measure in RCTs. However, the report’s authors note that it may not be adequate to measure meaningful change in early-stage disease.
“Developing better tools certainly should be on the radar screen for all of us, because I think we can do better,” Dr. Petersen said. “The CDR, as good as it is and as long as it’s been used in the field, is a pretty blunt instrument, and it’s the result of subjective ratings.”
‘Quality of mind’
Jason Karlawish, MD, professor of medicine, medical ethics, health policy, and neurology at the University of Pennsylvania, Philadelphia, said measuring the actual impact of a drug on a patient’s disease and quality of life has been a hot topic in the AD field for some time, but settling on a definition of “clinically meaningful” that everyone agrees upon will be a challenge.
“I think the idea of ‘clinically meaningful’ is truly a socially constructed idea,” said Dr. Karlawish, co-director of Penn’s Memory Center, who did not work on the report.
“You can come up with objective measures of cognition, but a measure to call something ‘clinically meaningful’ ultimately requires some sort of negotiated social order among clinicians and patients and others who have immediate interest in the health and well-being of the patient.”
Dr. Karlawish added that he’s interested in the conversations the report might prompt and the challenges it could highlight, especially when it comes to how meaningful clinical benefit can be measured, regardless of how it’s defined.
“Hidden in this conversation about clinically meaningful treatments in Alzheimer’s disease is, frankly, not quality of life, but quality of mind,” said Dr. Karlawish. “No measure captures acceptably the very thing that everyone actually cares a lot about and why we view this disease as so dreadful, which is damage to our mind.”
More evidence needed
The development of such tools will take time. What does that mean for drugs already in the pipeline? Members of the work group argue that those trials must move forward at the same time new tools are being created.
“We need to continue to refine, develop better instruments, [and] develop tools that are going to assess the disease in its more subtle features early on, even in the so-called ‘pre-symptomatic’ stage of the disease,” said lead author Dr. Petersen. “We shouldn’t wait for the development of that before intervening if we have a drug that seems to work.”
However, not everyone who agrees with the premise of the report agrees with this position, including Joel S. Perlmutter, MD, professor of neurology, Washington University School of Medicine, St. Louis, who also commented on the report.
As reported by this news organization, Dr. Perlmutter was one of three physicians who resigned from the FDA advisory panel that voted against approving aducanumab after the agency moved forward anyway.
“We have to be careful not to recommend DMTs that we hope will help without strong evidence, especially when potential side effects are not trivial,” Dr. Perlmutter said. “We have to have evidence before making these recommendations so we don’t end up harming people more than helping them.”
The report received no specific funding. Dr. Petersen received consulting fees from Roche, Nestle, Merck, Biogen, Eisai, and Genentech. Full disclosures are included in the original article. Dr. Perlmutter and Dr. Karlawish report no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM ALZHEIMER’S AND DEMENTIA
What’s new in brain health?
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
This transcript has been edited for clarity.
Dear colleagues, I am Christoph Diener from the medical faculty of the University of Duisburg-Essen in Germany.
Treatment of tension-type headache
I would like to start with headache. You are all aware that we have several new studies regarding the prevention of migraine, but very few studies involving nondrug treatments for tension-type headache.
A working group in Göttingen, Germany, conducted a study in people with frequent episodic and chronic tension-type headache. The first of the four randomized groups received traditional Chinese acupuncture for 3 months. The second group received physical therapy and exercise for 1 hour per week for 12 weeks. The third group received a combination of acupuncture and exercise. The last was a control group that received only standard care.
The outcome parameters of tension-type headache were evaluated after 6 months and again after 12 months. Previously, these same researchers published that the intensity but not the frequency of tension-type headache was reduced by active therapy.
In Cephalalgia, they published the outcome for the endpoints of depression, anxiety, and quality of life. Acupuncture, exercise, and the combination of the two improved depression, anxiety, and quality of life. This shows that nonmedical treatment is effective in people with frequent episodic and chronic tension-type headache.
Headache after COVID-19
The next study was published in Headache and discusses headache after COVID-19. In this review of published studies, more than 50% of people with COVID-19 develop headache. It is more frequent in young patients and people with preexisting primary headaches, such as migraine and tension-type headache. Prognosis is usually good, but some patients develop new, daily persistent headache, which is a major problem because treatment is unclear. We desperately need studies investigating how to treat this new, daily persistent headache after COVID-19.
SSRIs during COVID-19 infection
The next study also focuses on COVID-19. We have conflicting results from several studies suggesting that selective serotonin reuptake inhibitors might be effective in people with mild COVID-19 infection. This hypothesis was tested in a study in Brazil and was published in JAMA, The study included 1,288 outpatients with mild COVID-19 who either received 50 mg of fluvoxamine twice daily for 10 days or placebo. There was no benefit of the treatment for any outcome.
Preventing dementia with antihypertensive treatment
The next study was published in the European Heart Journal and addresses the question of whether effective antihypertensive treatment in elderly persons can prevent dementia. This is a meta-analysis of five placebo-controlled trials with more than 28,000 patients. The meta-analysis clearly shows that treating hypertension in elderly patients does prevent dementia. The benefit is higher if the blood pressure is lowered by a larger amount which also stays true for elderly patients. There is no negative impact of lowering blood pressure in this population.
Antiplatelet therapy
The next study was published in Stroke and reexamines whether resumption of antiplatelet therapy should be early or late in people who had an intracerebral hemorrhage while on antiplatelet therapy. In the Taiwanese Health Registry, this was studied in 1,584 patients. The researchers divided participants into groups based on whether antiplatelet therapy was resumed within 30 days or after 30 days. In 1 year, the rate of recurrent intracerebral hemorrhage was 3.2%. There was no difference whether antiplatelet therapy was resumed early or late.
Regular exercise in Parkinson’s disease
The final study is a review of nonmedical therapy. This meta-analysis of 19 randomized trials looked at the benefit of regular exercise in patients with Parkinson’s disease and depression. The analysis clearly showed that rigorous and moderate exercise improved depression in patients with Parkinson’s disease. This is very important because exercise improves not only the symptoms of Parkinson’s disease but also comorbid depression while presenting no serious adverse events or side effects.
Dr. Diener is a professor in the department of neurology at Stroke Center–Headache Center, University Duisburg-Essen, Germany. He disclosed ties with Abbott, Addex Pharma, Alder, Allergan, Almirall, Amgen, Autonomic Technology, AstraZeneca, Bayer Vital, Berlin Chemie, Bristol-Myers Squibb, Boehringer Ingelheim, Chordate, CoAxia, Corimmun, Covidien, Coherex, CoLucid, Daiichi Sankyo, D-Pharm, Electrocore, Fresenius, GlaxoSmithKline, Grunenthal, Janssen-Cilag, Labrys Biologics Lilly, La Roche, Lundbeck, 3M Medica, MSD, Medtronic, Menarini, MindFrame, Minster, Neuroscore, Neurobiological Technologies, Novartis, Novo Nordisk, Johnson & Johnson, Knoll, Paion, Parke-Davis, Pierre Fabre, Pfizer Inc, Schaper and Brummer, Sanofi-Aventis, Schering-Plough, Servier, Solvay, St. Jude, Talecris, Thrombogenics, WebMD Global, Weber and Weber, Wyeth, and Yamanouchi. Dr. Diener has served as editor of Aktuelle Neurologie, Arzneimitteltherapie, Kopfschmerz News, Stroke News, and the Treatment Guidelines of the German Neurological Society; as co-editor of Cephalalgia; and on the editorial board of The Lancet Neurology, Stroke, European Neurology, and Cerebrovascular Disorders. The department of neurology in Essen is supported by the German Research Council, the German Ministry of Education and Research, European Union, National Institutes of Health, Bertelsmann Foundation, and Heinz Nixdorf Foundation. Dr. Diener has no ownership interest and does not own stocks in any pharmaceutical company. A version of this article originally appeared on Medscape.com.
No benefit of long-acting antipsychotics in schizophrenia?
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
In a multicountry, randomized, open-label study of more than 500 adults with schizophrenia, participants received either LAI paliperidone, LAI aripiprazole, or the respective oral formulation of these antipsychotics.
Results showed no significant difference between the combined oral and combined LAI treatment groups in time to all-cause discontinuation.
“We found no substantial advantage for LAI antipsychotic treatment over oral treatment, regarding time to discontinuation in patients with early-phase schizophrenia,” write investigators, led by Inge Winter-van Rossum, PhD, assistant visiting professor at Mount Sinai, New York, and affiliated with King’s College London and UMC Utrecht (the Netherlands).
This indicates that “there is no reason to prescribe LAIs instead of oral antipsychotics if the goal is to prevent discontinuation of antipsychotic medication in daily clinical practice,” they add.
The findings were published online in The Lancet Psychiatry.
Previous conflicting results
Maintenance treatment with antipsychotic medication reduces risk for relapse considerably, with treatment discontinuation being “by far the most important reason for relapse,” the investigators write.
LAIs “seem theoretically to be a way to enhance medication continuation and thereby reduce the risk for relapse,” they add. This is because LAIs enable a rapid response to nonadherence and remove the need for patients to remember to take their medications on a daily basis.
However, previous research has “provided conflicting results,” regarding the effectiveness of LAIs in accomplishing this. Moreover, the subject has not been thoroughly investigated in early-stage schizophrenia, the researchers note.
Therefore, they decided to conduct the EULAST study to compare LAI and oral formulations in terms of all-cause discontinuation.
The trial was conducted at 50 general hospitals and psychiatric specialty clinics located in 15 European countries and Israel and included 511 participants in the intention-to-treat sample (67% men; mean age, 30.5 years).
All were randomly assigned 1:1:1 to receive either LAI paliperidone, LAI aripiprazole, or their respective oral formulations.
The combined OA treatment group consisted of 247 patients; the combined LAI group consisted of 264 patients.
Randomization was stratified by country and illness duration (5 months to 3 years vs. 4-7 years). Participants were followed up to 19 months, with all-cause discontinuation during that time serving as the primary endpoint.
All-cause discontinuation was defined as the allocated treatment was stopped or used at doses outside the allowed range, medication was switched or augmented with another antipsychotic after visit four, the patient missed a monthly visit and did not show up after being reminded, the patient withdrew consent for the study, or the clinician withdrew the patient from the study.
After the baseline visit, patients already taking antipsychotics were also randomly assigned. The next 4 weeks were then used to cross-taper between the prestudy antipsychotic and the agent they would be treated with during the study.
LAIs not superior
Results showed the LAI group did not have lower rates of hospitalization.
In addition, the discontinuation rates between the two combined groups were very similar at 71% for the oral antipsychotics group versus 64% in the LAIs group (hazard ratio, 1.6; 95% confidence interval, 0.94-1.43; P = .18).
Moreover, “no significant difference was found in the time to all-cause discontinuation between the combined oral and combined LAI treatment groups (P = .17),” the researchers report.
Reasons for discontinuation also did not differ significantly between the groups: 12% of patients in the OA group discontinued treatment because of efficacy vs. 17% of patients in the combined LAI group. The difference was not significant and the time to discontinuation also did not differ.
The main reason for discontinuation in both groups was safety concerns, affecting 10% and 13% of the combined OA and LAI groups, respectively, which was not a significant between-group difference.
Illness duration had a significant effect on time to all-cause discontinuation, with patients who had longer illness duration showing a poorer response, compared with those who had shorter duration (HR, 1.26; 95% CI, 1.01-1.56; P = .038).
However, stratifying participants by illness duration showed no significant difference between the subgroups (P = .25 and .34, respectively).
There was a significant between-group difference in discontinuation due to “other reasons,” with 49% vs. 34% of patients in the OA and LAI groups, respectively, discontinuing (HR, 1.51; 95% CI, 1.15-1.98; P = .0034). Moreover, the LAI group showed significantly longer continued use of medication vs the OA group (P = .0029).
“After separating the reasons for discontinuation into no efficacy, safety reasons, and other reasons, we only found a significant difference in favor of LAI for the ‘other reasons’ category; although the number of patients discontinuing medication for this reason over the follow-up period did not differ, patients on LAI continued treatment for a longer time,” the investigators write.
They acknowledge that this finding is “difficult to interpret, given the wide variety of reasons for discontinuation captured in this category,” which prevented an “informative subgroup analysis.”
Nevertheless, since there is “no consistent evidence supporting the use of LAI over oral antipsychotics” in patients with early-phase schizophrenia, their use should be “carefully considered on an individual risk-benefit basis,” they conclude.
No ‘real-world’ implications?
John M. Kane, MD, codirector and professor, Institute of Behavioral Science, Feinstein Institutes for Medical Research, Manhasset, N.Y., said that overall, this was a “large, potentially valuable study.” However, he raised several concerns.
“I think the investigators made a much too emphatic statement about the lack of value of LAIs in early-phase patients when discontinuation is the primary outcome,” he said, noting that other studies have come to the opposite conclusion.
Dr. Kane, who is also a professor of psychiatry at Hofstra/Northwell, New York, was not involved with the current research.
“RCTs [randomized controlled trials] in general are not necessarily the best way to evaluate the impact of LAIs [which] usually represent a small percentage of potentially eligible patients and are likely to include patients who are more adherent than those who would not agree to participate in an RCT,” he said. He added that the investigators “did not report on how many patients were screened and refused to be considered.”
Also, Dr. Kane noted that half of the participants were recruited from inpatient services, and so may have been “more unstable” at baseline. “Patients with residual positive symptoms are more likely to relapse on LAIs than patients who are in remission. This could potentially reduce the advantage of the LAI,” he said.
In addition, he took issue with the definition of all-cause discontinuation, which included the need for augmentation with another antipsychotic or use outside the normal range.
“This happens often in clinical practice. If someone’s symptoms aren’t sufficiently controlled by an LAI alone, for example, they often receive more of that antipsychotic or another drug. This perhaps makes the EULAST study somewhat less ‘real-world’,” Dr. Kane said.
More information needed
In an accompanying editorial, Martina Hahn, PharmD, PhD, department of psychiatry, psychosomatics, and psychotherapy, University Hospital-Goethe University, Frankfurt, Germany, and Sibylle Christine Roll, MD, PHD, department of mental health, Varisano Hospital in Frankfurt, note that comedications were neither documented nor analyzed by the researchers.
“Drug-drug interactions could be responsible for relapse or poor tolerability,” they write.
Moreover, pharmacogenetic information was not available nor were serum concentrations that could have been used for dose optimization after switching antipsychotic formulations, they note.
This information would have provided “a deeper understanding of why some patients do not respond or show side effects,” the editorialists write. “The use of therapeutic drug monitoring, drug interaction checks, and pharmacogenetic testing could improve treatment outcomes in both study settings and clinical practice.”
Financial support and study medication was provided by Lundbeck and Otsuka. Dr. Winter-van Rossum reports no relevant financial relationships. Disclosures for the other investigators are fully listed in the original paper. Dr. Kane is or has been a consultant to or received honoraria for lectures from Alkermes , Biogen, Boehringer Ingelheim, Cerevel, Dainippon Sumitomo, H. Lundbeck, HLS, Intracellular Therapies, Janssen, Karuna, Merck, Newron, Otsuka, Roche, Saladax, Sunovion, and TEVA. He is also a shareholder in The Vanguard Research Group, LB Pharma, Health Rhythms, North Shore Therapeutics, and Medincell. Dr. Hahn reports having received honoraria for lecture from Otsuka and advisory board participation for Rovi. Dr. Roll reports advisory board participation for Recordati, Otsuka, and Janssen.
A version of this article first appeared on Medscape.com.
FROM THE LANCET PSYCHIATRY
‘Sighing’ tops mindfulness for reduced stress, improved mood
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a randomized controlled study, daily breathwork – especially cyclic breathing, which emphasizes shorter inhalations and prolonged exhalations – was associated with greater improvement in mood and a slower respiratory rate than mindfulness meditation.
“We were pleased that just 5 minutes a day of the breathing exercises positively affected mood and resulted in slower respiratory rate, indicating reduced arousal,” coinvestigator David Spiegel, MD, who directs the Center for Stress and Health at Stanford (Calif.) University, told this news organization.
The findings were published online in Cell Reports Medicine.
Intentional breath control
Controlled breathwork has emerged as a potential tool to manage stress and boost well-being.
In the new study, researchers compared three different daily 5-minute breathwork exercises to an equal amount of mindfulness meditation over 1 month in 108 healthy adults recruited mostly from an undergraduate psychology class at Stanford: 33 participants practiced cyclic hyperventilation, which emphasizes robust inhalation, short retention and rapid exhalation, 30 did exhale-focused cyclic sighing, 21 performed box breathing, which emphasizes equal duration of inhalation, breath retention, and exhalation, and 24 practiced mindfulness meditation (the control group).
The primary endpoints were improvement in mood and anxiety, as well as reduced physiologic arousal (respiratory rate, heart rate, and heart rate variability). Physiological data was collected using a wearable WHOOP strap.
All four groups showed significant daily improvement in mood, as well as reduction in anxiety and negative mood, but there were significant differences between mindfulness meditation and breathwork.
Using a mixed-effects model, the researchers showed that breathwork, especially the exhale-focused cyclic sighing, produced greater improvement in mood (P < .05) and reduction in respiratory rate (P < .05), compared with mindfulness meditation.
Specific patterns vs. passive attention
The finding supports the team’s hypothesis that intentional control over breath with specific breathing patterns produces more benefit to mood than passive attention to one’s breath, as in mindfulness meditation practice.
“It turned out that the cyclic sighing was indeed most soothing,” Dr. Spiegel noted.
“We expected that because of respiratory sinus arrhythmia. Exhaling is accomplished by increasing pressure in the chest, which increases venous return to the heart, triggering parasympathetic slowing of heart rate via the sinoatrial node,” he said.
Dr. Spiegel added that, conversely, inspiration reduces venous return, triggering sympathetic activity and increased heart rate.
“The magnitude of this heart rate variability is associated with better health, including recovery from myocardial infarction and even cancer survival. So self-soothing is a good thing, and we expected an advantage for cyclic sighing,” he said.
“If you’re looking to improve sleep and reduce daytime stress, recover from intense work, life, and/or training, then interventions that facilitate autonomic control (and indeed you can control it), brief (5 minutes) structured breathwork is among the more powerful (and zero cost) tools,” tweeted senior investigator Andrew Huberman, PhD, professor of neurobiology at Stanford.
Immediate application?
Sara Lazar, PhD, Massachusetts General Hospital and Harvard Medical School, Boston, said the findings are “interesting” but cautioned that this is “just one study with a pretty small sample size,” and it only enrolled healthy college students.
Dr. Lazar, who also runs the Lazar Lab for Meditation Research at Mass General, noted that she would want to see a future study “done with working-age adults and with clinical populations.”
“It should also be noted that mindfulness had a bigger effect on negative affect, which could have implications for conditions such as depression or trauma,” said Dr. Lazar, who was not involved with the current research.
Also weighing in, Steven R. Thorp, PhD, professor at California School of Professional Psychology, Alliant International University, San Diego, said in an interview the study is “really interesting and well done.”
“Although breathing exercises and breathing retraining are commonly found in psychosocial interventions, especially for anxiety disorders, there have been few empirical studies comparing different breathing protocols,” Dr. Thorp said.
In this study, the passive observation of breaths (mindfulness) and specific breathwork interventions “all worked to decrease state anxiety; but the breathwork, particularly the cyclic sighing protocol, produced a greater overall reduction in respiratory rate and increase in positive mood,” he noted.
“These techniques can be recommended by all clinicians because all clients have access to their breath at all times – and only 5 minutes of daily practice can yield the benefits. Moreover, as the authors note, the immediate benefits may encourage clients to engage with the breathwork and potentially other aspects of treatment,” Dr. Thorp said.
The study was funded by Victor and Winnie Koo and Tianren Culture and a Stanford School of Medicine Discovery Innovation Award. WHOOP donated the wrist straps used in the study, but was not involved in the study’s design or analysis. Dr. Huberman is an advisor to WHOOP. Dr. Lazar and Dr. Thorp have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
Medicare ‘offers’ cancer patient a choice: Less life or more debt
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
We’re gonna need a bigger meth lab
In case you’ve been living under a rock for the past 15 years, the TV show “Breaking Bad” details the spiraling rise and downfall of a high school chemistry teacher who, after developing a case of terminal lung cancer, starts producing methamphetamine to provide for his family in response to the steep cost of treatment for his cancer.
Meanwhile, here in 2023 in the real world, we have Paul Davis, a retired physician in Ohio, who’s being forced to choose between an expensive cancer treatment and bankrupting his family, since Medicare’s decided it doesn’t want to cover the cost. Hey, we’ve seen this one before!
A bit of backstory: In November 2019, Dr. Davis was diagnosed with uveal melanoma, a very rare type of cancer that affects eye tissue. The news got worse in 2022 when the cancer spread to his liver, a move which typically proves fatal within a year. However, in a stroke of great news, the Food and Drug Administration approved the drug Kimmtrak earlier that year, which could be used to treat his cancer. Not cure, of course, but it would give him more time.
His initial treatments with the drug went fine and were covered, but when he transferred his care from a hospital in Columbus to one closer to home, big problem. Medicare decided it didn’t like that hospital and abruptly cut off coverage, denying the local hospital’s claims. That leaves Dr. Davis on the hook for his cancer treatment, and it’s what you might call expensive. Expensive to the tune of $50,000.
A week.
Apparently the coding the local hospital submitted was wrong, indicating that Dr. Davis was receiving Kimmtrak for a type of cancer that the FDA hadn’t approved the drug for. So until the government bureaucracy works itself out, his treatment is on hold, leaving all his faith in Medicare working quickly to rectify its mistake. If it can rectify its mistake. We’re not hopeful.
And in case you were wondering, if Dr. Davis wanted to go full Walter White, the average street price of meth is about $20-$60 per gram, so to pay for his treatment, he’d need to make at least a kilogram of meth every week. That’s, uh, quite a lot of illegal drug, or what we here at the LOTME office would call a fun Saturday night.
When you give a mouse a movie
Researchers have been successfully testing Alzheimer drugs on mice for years, but none of the drugs has proved successful in humans. Recent work, however, might have found the missing link, and it’s a combination no one ever thought of before: mice and movies.
Turns out that Orson Welles’ 1958 film noir classic “Touch of Evil” tapped a part of the mouse brain that has been overlooked: the hippocampus, which is crucial for learning and memory. Previous researchers thought it was just used as a kind of GPS system, but that’s only partially true.
Not only did the mice choose to pay attention to the movie clip, but the hippocampus responded to the visual stimuli only when the rodents saw the scenes from the clip later in the order that they were presented and not in a scrambled order. These findings represent a “major paradigm shift” in studying mouse recall, Mayank Mehta, PhD, of the University of California, Los Angeles, said in a statement from the school.
This breakthrough could run parallel to Alzheimer’s patients struggling with similar defects. “Selective and episodic activation of the mouse hippocampus using a human movie opens up the possibility of directly testing human episodic memory disorders and therapies using mouse neurons, a major step forward,” said coauthor Chinmay Purandare, PhD, who is now at the University of California, San Francisco.
Who would have thought that a classic film would help advance Alzheimer research?
A less human way to study mosquitoes
We here at LOTME have a history with mosquitoes. We know they don’t like us, and they know that we don’t like them. Trust us, they know. So when humans gain a little ground in the war against the buzzy little bloodsuckers, we want to share the joy.
To know the enemy, scientists have to study the enemy, but there is a problem. “Many mosquito experiments still rely on human volunteers and animal subjects,” bioengineering graduate student Kevin Janson, said in a statement from Rice University. Most people don’t like being bitten by mosquitoes, so that kind of testing can be expensive.
Is there a way to automate the collection and processing of mosquito behavior data using inexpensive cameras and machine-learning software? We’re glad you asked, because Mr. Janson and the research team, which includes bioengineers from Rice and tropical medicine experts from Tulane University, have managed to eliminate the need for live volunteers by using patches of synthetic skin made with a 3D printer.
“Each patch of gelatin-like hydrogel comes complete with tiny passageways that can be filled with flowing blood” from a chicken, sheep, or cow, they explained, and proof-of-concept testing showed that mosquitoes would feed on hydrogels without any repellent and stay away from those treated with a repellent.
To conduct the feeding tests, the blood-infused hydrogels are placed in a clear plastic box that is surrounded by cameras.
A bunch of mosquitoes are then tossed in the box and the cameras record all their insect activities: how often they land at each location, how long they stay, whether or not they bite, how long they feed, etc. Humans don’t have to watch and don’t have to be food sources.
Humans don’t have to be food sources, and we just pictured the future of mosquito control. Imagine a dozen Arnold Schwarzenegger–style Terminators, covered in 3D-printed skin, walking through your neighborhood in the summer while wearing sweat-soaked, brightly colored clothing. The mosquitoes wouldn’t be able to stay away, but guess what? They’re feeding off robots with nonhuman skin and nonhuman blood, so we win. It’s good to have a cerebral cortex.
Getting medieval on brain surgery
Let’s get one thing clear: The so-called “Dark Ages” were not nearly as dark as they’re made out to be. For one thing, there’s a world beyond Western Europe. The Roman Empire didn’t collapse everywhere. But even in Western Europe, the centuries between the fall of Rome and the Renaissance were hardly lacking in cultural development.
That said, we wouldn’t want to be in the position of the seventh-century noblewoman whose remains were recently uncovered in a Byzantine fortress in central Italy with multiple cross-shaped incisions in her skull. Yes, this unfortunate woman underwent at least two brain surgeries.
Then again, maybe not. Nothing like it had been discovered at the site, and while the markings – signs of a procedure called trepanation – can be surgical in nature, there are other explanations. For example, the Avar people practiced ritual trepanation during the same time period, but they were hundreds of miles away in the Carpathian mountains, and there was no evidence to support that a different form of ritualistic trepanation ever took place in Byzantine-era Italy.
The investigators then moved on to a form of judicial punishment called decalvatio, which involves mutilation by scalping. Look, the Dark Ages weren’t dark, but no one said they were fun. Anyway, this was discarded, since decalvatio was only meted out to soldiers who deserted the battlefield.
That brings us back to surgery. While one of the trepanations was fully engraved into her skull, indicating that the woman died soon after the surgery, she also bore indications of a healed trepanation. A 50% success rate isn’t terrible for our medieval surgeon. Sure, the Incas managed 80%, but even during the Civil War brain surgery only had a 50% success rate. And that’s the end of the story, nothing more to say about our medieval Italian woman.
Nope. Nothing at all.
Fine. While a surgical procedure was deemed most likely, the study investigators found no direct evidence of a medical condition. No trauma, no tumor, nothing. Just a couple of suggestions of “a systemic pathological condition,” they said. Okay, we swear, it really wasn’t that bad in the Middle [Editor’s note: Approximately 5,000 more words on medieval culture not included. This is a medical column, thank you very much.]
Time for a national ketamine registry, experts say
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
The number of ketamine clinics has risen dramatically, with little to no oversight. Prescriptions are being written by providers who lack training in safe ketamine use and online startups are selling the drug for at-home use, taking advantage of a temporary federal regulation that makes it easier to prescribe controlled substances without an in-person patient assessment.
All of this comes at a time when recreational use of ketamine, known on the street as “Special K,” is rising, and reports to poison control centers and drug seizures by the U.S. Drug Enforcement Agency (DEA) are climbing.
In a scenario where enthusiasm for the drug is larger than the body of evidence supporting its clinical use, support is growing for the creation of a ketamine registry to collect data on dosage, treatment frequency, adverse events, and long-term outcomes in patients receiving the therapy for depression and other mental health conditions.
“In the past, there was this question of whether a registry was even needed,” said Gerard Sanacora, MD, PhD, a professor of psychiatry at Yale University, New Haven, Conn., who has pushed for a registry for more than 5 years.
“Now, not only are people being treated with this in large numbers, but it’s also started to push the envelope with at-home dosing,” Dr. Sanacora said in an interview. “It’s come to the point that everybody agrees we do need some way to track it.”
An idea whose time has come
Interest in ketamine’s antidepressant effects has grown since 2000, when a small study suggested the drug rapidly improved depressive symptoms. Research now suggests ketamine reduces symptoms in patients with treatment-resistant depression (TRD).
Studies linking ketamine to relief of depressive symptoms are small and mostly retrospective, and none has offered longitudinal information on long-term outcomes, including side effects and the risk of addiction.
Still, clinicians desperate to help the one-third of patients with major depression who fail to respond to first-line treatments often prescribe the drug anyway.
In 2017, Dr. Sanacora, who also is director of the Yale Depression Research Program at the Yale School of Medicine, was the lead author of a consensus statement that sought to help physicians administer ketamine safely and appropriately in patients with severe depression and other mood disorders.
In that paper, Dr. Sanacora and his coauthors advocated for the creation of a ketamine registry. Such a database, they argued, would provide much-needed data for large, long-term studies, which could be used to develop treatment guidelines, certification programs, and possibly even accreditation standards for providers. Meanwhile, researchers and clinicians in the United Kingdom were also calling for a ketamine registry.
While there seemed to be wide consensus that such a registry was needed, there was no clear path to creating one and no clear line to an agency that would take responsibility for maintaining it.
Because the registry wouldn’t be tied to a drug indication, Dr. Sanacora was told the U.S. Food and Drug Administration wouldn’t take it on. The project also fell outside the purview of the U.S. Department of Health & Human Services, the National Institute of Mental Health (NIMH), and the DEA.
“I haven’t met anybody who has said this is a terrible idea, but nobody seems to have a clear mechanism of doing it, and it doesn’t seem to fall directly under anybody’s jurisdiction,” Dr. Sanacora said.
Dr. Sanacora and other ketamine registry advocates were met with an endless stream of questions. Who would pay for it? How would they get providers to participate? Who would run it and how would the data be shared? The barriers to implementation seemed insurmountable.
A changing landscape
Five years later, these barriers remain. However, advocates note support for a registry is growing, due in large part to a series of developments over the past 6 years that they believe have altered the ketamine landscape.
Chief among these was the 2019 FDA approval of esketamine, a nasal formulation of ketamine, for the treatment of resistant depression. The drug’s indication was expanded in 2020 to include major depressive disorder and acute suicidal ideation or behavior. The drug is only available through a restricted distribution system – the Spravato Risk Evaluation and Mitigation Strategy (REMS) – because of the risk for serious adverse events, including sedation and dissociation, as well as the potential for abuse or misuse.
A sharp increase in the number of ketamine prescribers and clinics has also heightened interest in a ketamine registry. In the last year alone, membership in the American Society of Ketamine Physicians, Psychotherapists, and Practitioners (ASKP) – a nonprofit trade organization for clinicians who prescribe ketamine for mental health disorders and pain conditions – swelled from 300 individual providers to more than 500.
The number of ketamine clinics in the United States has also grown exponentially and is estimated to be anywhere from 500 to 750. A spokesperson with HHS said such clinics are not regulated by the department or any other federal agency but instead are subject to oversight by individual states.
Although recreational use of ketamine remains low overall, there are signs that illicit use is rising, including an increase in DEA seizures of illicit ketamine and reports of ketamine-related poisonings to the nation’s poison control centers. Data on recreational use is spotty, at best. The Centers for Disease Control and Prevention National Vital Statistics System – the primary source of information on drug-related mortality in the United States – does not report on ketamine.
At-home ketamine use soars
Perhaps the most significant development came in March 2020 in the early days of the pandemic. To ease access to therapeutic schedule II-V controlled substances, the DEA issued a waiver that relaxed restrictions in the Ryan Haight Act, legislation that requires that patients be seen at least once in person before receiving a prescription for this class of drugs.
Under the waiver, DEA-registered practitioners are allowed to prescribe these substances – including ketamine, a schedule III substance – via telemedicine, without an in-person exam.
Startup companies cropped up almost overnight to prescribe oral ketamine online for at-home use, with almost no oversight. A spokesperson with the DEA told this news organization that the agency is working to make these “temporary” regulations permanent.
Under the relaxed DEA guidelines, a prescriber only needs to have a DEA license to dispense a ketamine prescription. An alarming number of clinics and online startups are staffed by individuals with no training in ketamine use and, in some cases, no formal mental health training at all, said Lisa Harding, MD, vice president of ASKP and a clinical instructor of psychiatry at Yale School of Medicine.
“The biggest problem is not the ketamine itself, it’s that the majority of practitioners are not psychiatrists, so they don’t have mental health training,” Dr. Harding said. “The fact that an untrained person, any practitioner with no mental health training, can administer this treatment once they have a state license to give ketamine ... then how are you protecting the patients?”
That question prompted ASKP to create the first known program to train psychiatrists, and other qualified mental health practitioners who prescribe ketamine, how to use the drug safely and effectively. The program, scheduled for June, will also include discussion by leaders in the field about how a ketamine registry might address these and other patient safety concerns.
“Nobody is really investigating the standard to which these clinics and online companies should be held, and I think a registry would help with that,” she said in an interview.
The path forward
While ASKP leadership supports the idea of a ketamine registry, Dr. Harding said the organization would need assurances the effort would not create a barrier to treatment.
“It will take somebody bringing all of us to the table and figuring that out,” Dr. Harding said.
Conversations like that with stakeholders would be one of the first steps toward creating a registry, Dr. Sanacora said.
“The more complicated we make this registry, the less compliance we’re going to get,” Dr. Sanacora said. “Our first step is to understand the major impediments and figure out how we can make this easier for people.”
Ideally, the registry would take advantage of existing data-collection tools, such as electronic health records (EHR), and include some sort of patient data entry mechanism, Dr. Sanacora said. The effort will also require skilled biostatisticians and a database system that is easy to manage.
And, of course, the registry will need a large number of patients to gather sufficient data to conduct high-quality research to develop treatment guidelines, training, and accreditation standards. A good target would be about 10,000 patients, Dr. Sanacora said.
All of this requires funding, which is the first hurdle registry advocates must clear. Dr. Sanacora is working on identifying funding sources and said that after working on this for years, he is hopeful that progress can be made.
“I had reached a point where it felt like there was no path forward,” Dr. Sanacora said. “But now I have renewed optimism that something can be done. And something does need to be done, largely for public health reasons but also to optimize the treatment.”
A version of this article first appeared on Medscape.com.
Saying goodbye: How to transition teens to adult medical care
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
However, many clinicians feel insufficiently prepared to provide comprehensive transition services. This can result in the actual handoff or transfer into adult care being abrupt, incomplete, or outright unsuccessful. By following the recommended best practices of transitions, providers of pediatric care can ensure that this challenging goodbye prepares everyone for the next steps ahead.
Using a structured transition process
In 2011, a health care transition clinical report based on expert opinion and practice consensus and endorsed by the American Academy of Pediatrics, American Academy of Family Physicians, and American College of Physicians – Society of Internal Medicine was released. This report provided a decision-making algorithm for “practice-based implementation of transition for all youth beginning in early adolescence.”
The Got Transition organization, funded by the Maternal Child Health Bureau and Health Resources and Services Administration, provides web-based information and materials for health care providers and families to establish a smooth and successful transition. At the center of these recommendations are the Six Core Elements of Health Care Transition – the essential components of a structured transition process: 1) transition policy/guide; 2) tracking and monitoring; 3) readiness; 4) planning; 5) transfer of care, and 6) transition completion.
This transition process should start early in adolescence, preferably by age 12-14 years, to give adequate time to progress successfully through these elements and improve the likelihood of a smooth, final transfer into the care of an adult clinician.
Preparing your patients for transfer
Despite the availability of these recommendations, national surveys show that the overwhelming majority of adolescents with and without special health care needs report not receiving transition services. Lack of time, resources, interest, and patients being lost to care during adolescence all contribute to this deficit in care. Without transition preparation, the actual handoff or transfer to adult care can be difficult for adolescents, caregivers, and clinicians alike. Adolescents and caregivers may feel a sense of abandonment or have inadequate health knowledge/literacy, pediatric clinicians may fear that the patient is not ready for the expected independence, and adult clinicians face numerous challenges integrating these young patients into their practice.
A structured transition process can help the family and clinicians know what to expect during the transfer of care. Pediatric clinicians can gradually move from a pediatric model of care, in which the caregiver is the center of communication, to an adult model, putting the patient at the center. By encouraging the adolescent to be the direct communicator, the pediatric clinician can promote independence and assess health knowledge, allowing for education where gaps exist.
Assisting the patient in identifying and even meeting the adult clinician well ahead of the final transfer date can also make the process less daunting for the adolescent.
Adult clinicians should consider allowing more time for the first visit with a new young adult patient and welcome caregiver input early in the transfer process, particularly for patients with a chronic disease. By engaging patients and families in an intentional, gradual transition process with an expected outcome, all those involved will be more prepared for the final handoff.
Utilizing transition tools and engaging the adolescent
Numerous tools can assist in the preparation for transfer to adult care. These include transition summaries and emergency plans, which contain essential information such as current medical problems, allergies, medications, prior procedures and treatments, and sick day plans. Such tools can also be built into electronic medical records for easy modification and updating. They can be used as methods to engage and teach adolescents about their disease history and current regimen and can contain essential components for information handoff at the time of transfer to adult care. If the patient carries a rare diagnosis, or one that has historically been associated with lower survival to adulthood, these transfer documents can also include summary information about disease states and contact information for pediatric specialty clinicians.
Adolescent engagement in their health care during the time of transition can also be prompted through the use of patient portals within an electronic health record. Such portals put health information directly at the adolescent’s fingertips, provide them with an outlet for communication with their clinicians, and give reminders regarding health maintenance.
Completing the transfer: The final handoff
The best and most recommended means of relaying information at the time of transfer to adult care is a direct, verbal handoff between clinicians. This direct handoff has several goals:
(1) To ensure the patient has scheduled or attended the first appointment with the adult clinician
(2) To ensure record transfer has occurred successfully
(3) To answer any questions the receiving clinician may have about prior or ongoing care.
(4) To offer the adult clinician ongoing access to the pediatric clinician as an “expert” resource for additional questions.
By remaining available as a resource, the pediatric clinician can alleviate concerns for both the patient and caregiver as well as the receiving adult clinician.
As valuable as verbal handoffs can be, they are not always possible due to patients not having selected an adult clinician prior to leaving the pediatric clinician, an inability to reach the receiving clinician, and/or time limitations. Many of these barriers can be alleviated by early discussions of transitions of care as well as utilization of structured documentation tools as noted above.
It is also recommended that the pediatric clinician follows up with the patient and/or caregiver several months after the transfer is complete. This allows for the adolescent and/or the caregiver to reflect on the transition process and provide feedback to the pediatric clinicians and their practice for ongoing process improvement.
Reflection as a pediatrician
Ideally, all transition steps occur for the adolescent; in our opinion, a crucial component is to prepare the adolescent patient for the change from a pediatric to adult model of care, in which they are independent in their health communication and decision-making. By engaging adolescents to understand their health, how to maintain it, and when to seek care, we empower them to advocate for their own health as young adults. With appropriate health knowledge and literacy, adolescents are more likely to actively engage with their health care providers and make healthy lifestyle choices. So though saying goodbye may still be difficult, it can be done with the confidence that the patients will continue to get the care they need as they transition into adulthood.
Dr. Kim is assistant clinical professor, department of pediatrics, University of California, San Diego. Dr. Mennito is associate professor of pediatrics and internal medicine, Medical University of South Carolina, Charleston, S.C. Dr. Kim and Dr. Mennito have disclosed no relevant financial relationships. A version of this article originally appeared on Medscape.com.
Using devices to calm children can backfire long term
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.
according to developmental behavioral pediatricians at University of Michigan Health C. S. Mott Children’s Hospital, Ann Arbor.
What to know
- Using a mobile device to distract children from how they are feeling may displace opportunities for them to develop independent, alternative methods to self-regulate, especially in early childhood.
- Signs of increased dysregulation could include rapid shifts between sadness and excitement, a sudden change in mood or feelings, and heightened impulsivity.
- The association between device-calming and emotional consequences may be particularly high among young boys and children who are already experiencing hyperactivity, impulsiveness, and a strong temperament that makes them more likely to react intensely to feelings such as anger, frustration, and sadness.
- While occasional use of media to occupy children is expected and understandable, it is important that it not become a primary or regular soothing tool, and children should be given clear expectations of when and where devices can be used.
- The preschool-to-kindergarten period is a developmental stage in which children may be more likely to exhibit difficult behaviors, such as tantrums, defiance, and intense emotions, but parents should resist using devices as a parenting strategy.
This is a summary of the article, “Longitudinal Association Between Use of Mobile Devices for Calming and Emotional Reactivity and Executive Functioning in Children Aged 3 to 5 Years,” published in JAMA Pediatrics on Dec. 20, 2022. The full article can be found on jamanetwork.com. A version of this article originally appeared on Medscape.com.












