User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
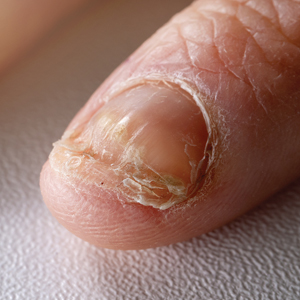
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.
Results
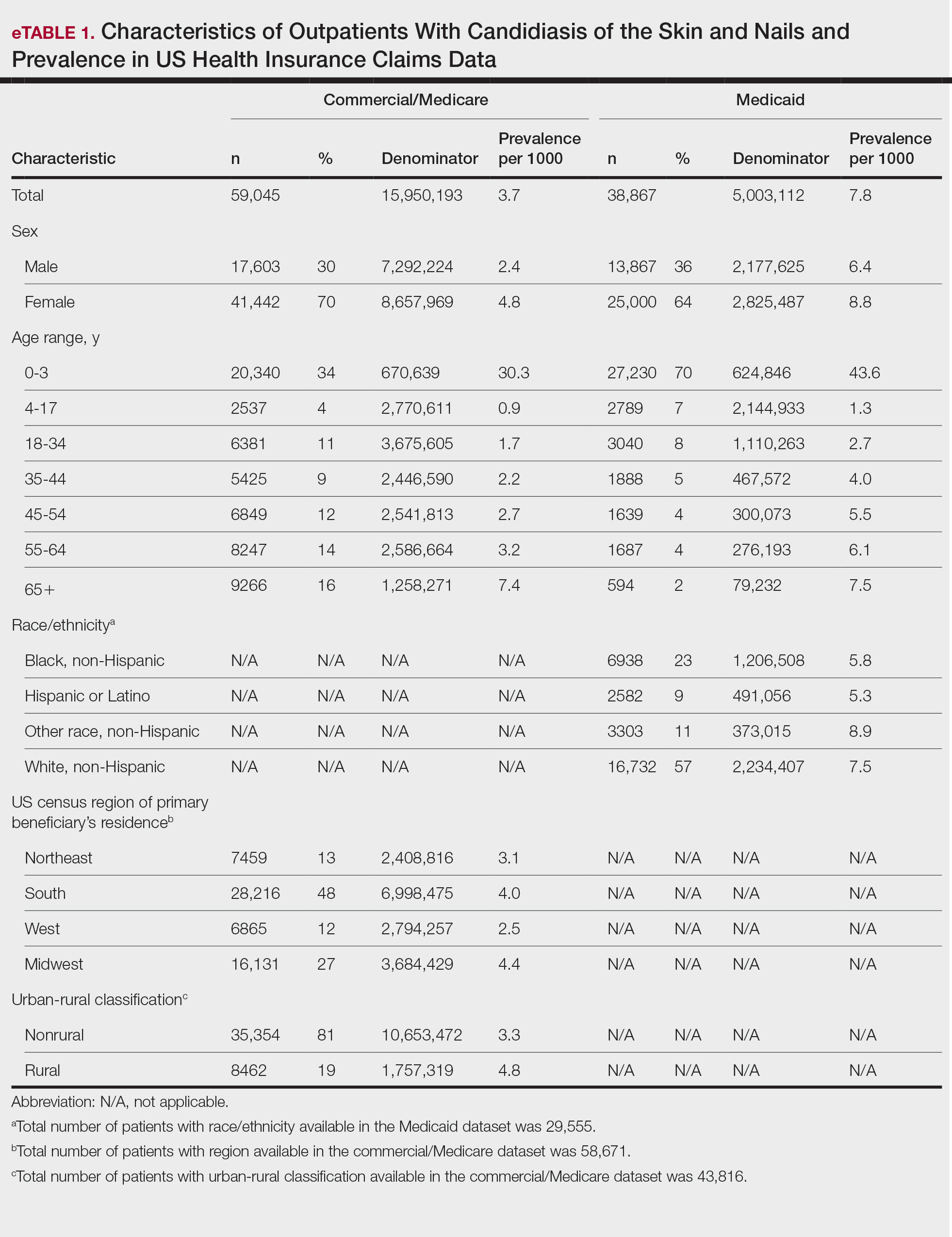
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).
Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).

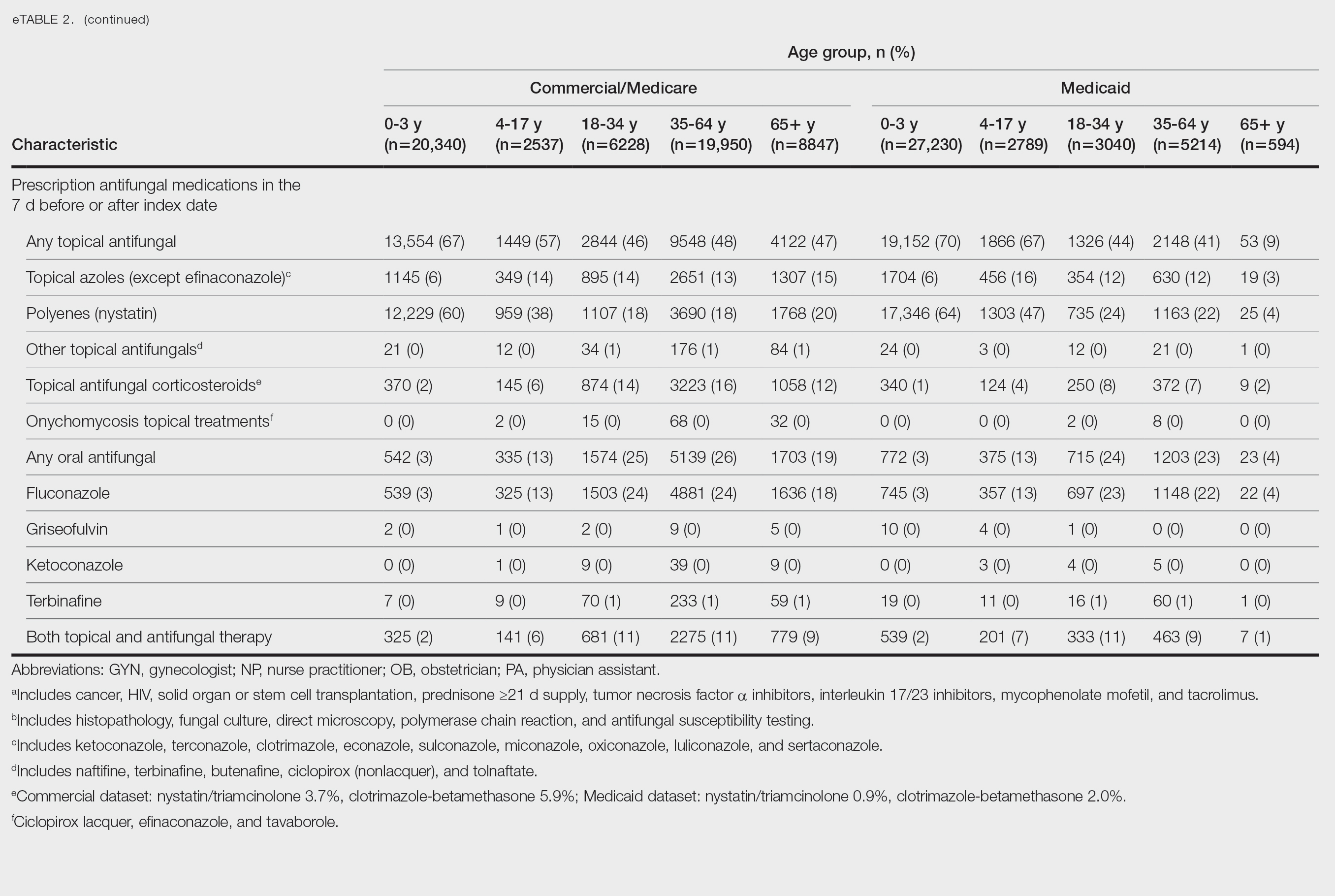
Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Practice Points
- Candidiasis of the skin or nails is a common outpatient condition that is most frequently diagnosed in infants, toddlers, and adults aged 65 years or older.
- Most cases are diagnosed clinically without diagnostic testing and treated with topical antifungals, but increased attention to formal diagnosis and treatment may be warranted given the emergence of antifungal-resistant Candida species.
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).


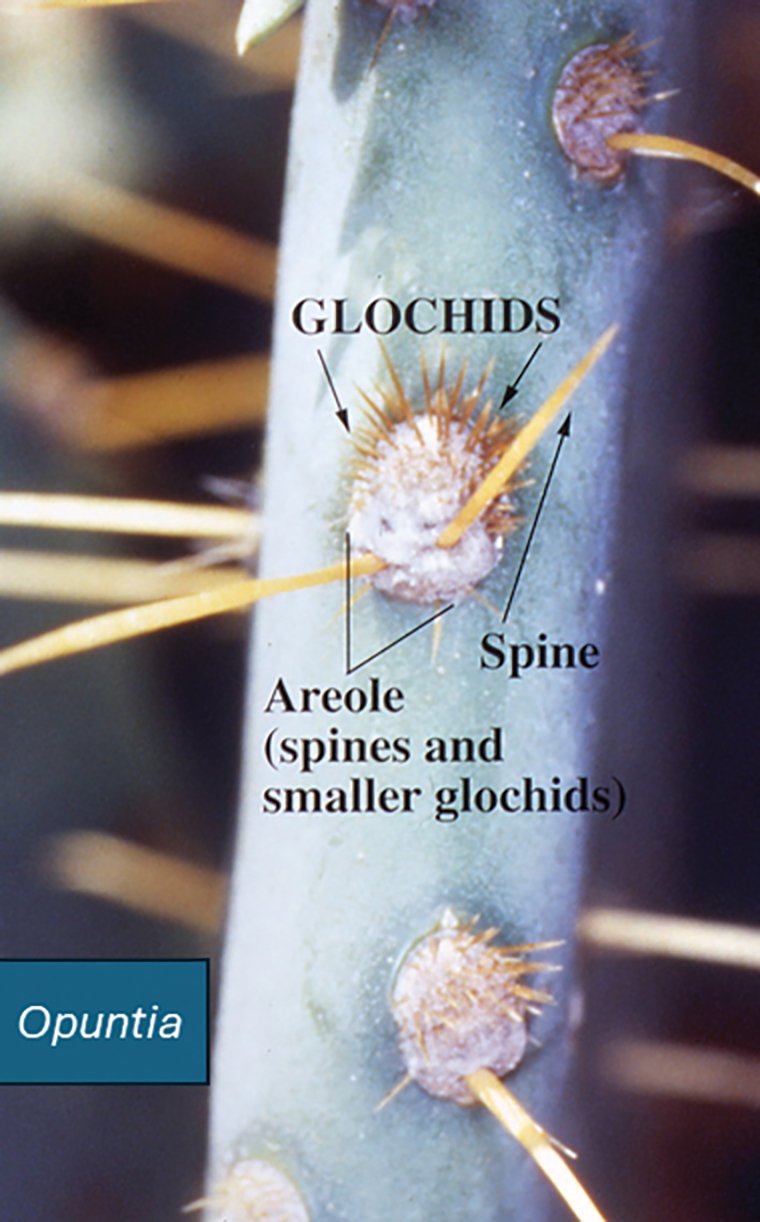
Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
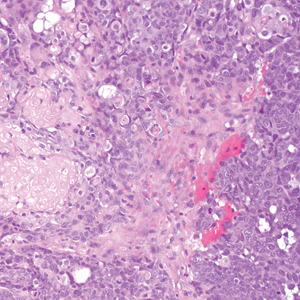
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
The genus of flowering plants commonly known as prickly pear cacti (Opuntia) or sabra are native to the Americas but are naturalized in many parts of the world, particularly southwest Asia and Sicily, Italy, where they are grown commercially and commonly are seen growing on rocky hillsides. (Figure 1). A prickly pear cactus has paddles that represent modified stems, and the spines are modified leaves (Figure 2). Its bright red or yellow flowers, dark-red fruit, low water requirement, and adaptability to poor-quality soil make it an attractive plant for landscaping and an important agricultural crop in many parts of the world, including the United States, Mexico, and Southern Europe. The prickly pear fruit is tasty but loaded with seeds and often is eaten fresh or used to make jam. The paddles are sometimes cut into strips, breaded or battered, and fried. The spines are easily embedded in skin and are an important cause of dermatitis.


Identifying Features
Opuntia species are found in both warm and temperate zones and grow well in arid climates. Like other cacti, they are distinguished by their water-hoarding stems and glochids (needlelike modified leaves). In prickly pears, the stems flatten to leaflike paddles that alternate in direction. Photosynthesis occurs in the stem tissues, while modified leaves (spines) are purely for defense against predators and unsuspecting humans. Opuntia species are easily identified by their broad flattened stems and dark-red fruits, both of which bear glochids (Figures 3-5).



Dermatologic Implications of Prickly Pear Injury
Prickly pear spines are very small, sharp, and difficult to see. They embed in the skin in great numbers when the plant or its fruit are handled by unsuspecting humans and have a tendency to burrow into soft tissue and underlying structures. It is very difficult to remove prickly pear spines with forceps, and attempts to do so often drive them deeper into the skin.1 Better results are obtained by tape stripping or using water-activated cosmetic pore strips.
Cactus spine injuries may lead to mucoceles of the oral mucosa and sinuses, especially in individuals who attempt to bite into the fruit without first scorching the spines with a blow torch.2 Inflammatory responses to the embedded spines are common and often result in prolonged erythematous inflammatory papules at sites of injury. Recalcitrant dermatitis and edema of underlying tissues typically occur near the point of entry of a prickly pear spine and extend to areas where the spine migrates.3,4 Individuals who casually brush up against the plant may not be aware that they have been inoculated with the spines and may not relate the prior accidental contact with the onset of erythematous papules and edema that occurs days later. Biopsy may reveal the prickly pear spines or a granulomatous reaction pattern within the dermis. Linear patterns of necrosis surrounded by palisading histiocytes may be noted, representing the tract of the inoculation injury.
If identified in tissue, glochids are variably refractile and measure 40 to 70 µm in diameter. Glochids initiate a delayed-type hypersensitivity and foreign body response. A T-helper 1 cytokine signal is typical, and there may be a secondary influx of neutrophils, but tissue eosinophilia is uncommon. Systemic inflammation also has been reported, including eosinophilic cholangitis without biliary stricture5 and septic and aseptic arthritis near the site of leaf puncture and at distant sites.6,7 Allergic contact dermatitis has been reported due to contact with the fruit of the plant and can be confirmed by patch testing.8,9
Potential Medicinal Benefits
Prickly pear cacti have shown potential medicinal properties. While the spines may produce intense inflammation when embedded in the skin, extracts of the fruit and leaf juices have shown anti-inflammatory properties. Various vesicle and polysaccharide extracts of Opuntia cacti have been shown to reduce environmental and chemical stressors associated with open wounds.10-12 Preclinical studies also have suggested that they could be helpful in speeding the wound-healing process when applied topically. Opuntia species also have shown promise in reducing hyperpigmentation after topical application.13 Preliminary data in animals also have suggested that oral administration of the fruit may slow kidney deterioration in patients with diabetes.14 Following tissue penetration by the spines, Opuntia extracts have demonstrated the ability to prevent calcium deposition in soft tissue.15 Similar preliminary data also have suggested that Opuntia extracts may reduce toxicity from cadmium, chromium, methotrexate, and acetaminophen.16-19 Extracts from the peel of the red pitaya (Hylocereus polyrhizus), a closely related cactus, have been studied for their potential to prevent the advance of alcohol-associated liver disease, suggesting that studies evaluating the benefits of prickly pear cacti and related species may be worth pursuing.20
Final Thoughts
Prickly pear cacti have the potential to act as both friend and foe. The flowers and fruit are beautiful, and the plant is well adapted to xeriscape gardens in areas under perpetual water restriction. The fruit and flesh are edible if handled properly, and prickly pear jam is delicious. While the spines are capable of inflicting local injury and migrating to internal sites, causing arthritis and other deep tissue injury, extracts of the fruit and stems have potential uses for their anti-inflammatory effects and ability to protect against toxic injury. Further studies are needed to evaluate the therapeutic potential of Opuntia and related species.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
- Ford AM, Haywood ST, Gallo DR. Novel method for removing embedded cactus spines in the emergency department. Case Rep Emerg Med. 2019;2019:6062531.
- Patel D, Clarkson J, Amirapu S. Frontal sinus post-traumatic mucocele secondary to a cactus spine. N Z Med J. 2020;133:112-115.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Ruini C, von Braunmühl T, Ruzicka T, et al. Granulomatous reaction after cholla cactus spine injury. Cutis. 2020;105:143-145;E2.
- Kitagawa S, Okamura K, Ichihara S, et al. Eosinophilic cholangitis without biliary stricture after cactus spine injury. Am J Gastroenterol. 2022;117:1731.
- Ontiveros ST, Minns AB. Accidental arthrotomy causing aseptic monoarthritis due to agave sap: a case report. Clin Pract Cases Emerg Med. 2021;5:246-248.
- Kim S, Baradia H, Sambasivan A. The use of ultrasonography in expediting septic joint identification and treatment: a case report. Am J Phys Med Rehabil. 2020;99:449-451.
- Yoon HJ, Won CH, Moon SE. Allergic contact dermatitis due to Opuntia ficus-indica var. saboten. Contact Dermatitis. 2004;51:311-312.
- Bonamonte D, Foti C, Gullo G, et al. Plant contact dermatitis. In: Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis. 2021; Springer, Cham. doi:10.1007/978-3-030-49332-5_16
- Valentino A, Conte R, Bousta D, et al. Extracellular vesicles derived from Opuntia ficus-indica fruit (OFI-EVs) speed up the normal wound healing processes by modulating cellular responses. Int J Mol Sci. 2024;25:7103.
- Das IJ, Bal T. Evaluation of Opuntia-carrageenan superporous hydrogel (OPM-CRG SPH) as an effective biomaterial for drug release and tissue scaffold. Int J Biol Macromol. 2024;256(Pt 2):128503.
- Adjafre BL, Lima IC, Alves APNN, et al. Anti-inflammatory and healing effect of the polysaccharidic extract of Opuntia ficus-indica cladodes in cutaneous excisional wounds in rats. Int J Exp Pathol. 2024;105:33-44.
- Chiu CS, Cheng YT, Chan YJ, et al. Mechanism and inhibitory effects of cactus (Opuntia dillenii) extract on melanocytes and its potential application for whitening cosmetics. Sci Rep. 2023;13:501.
- Sutariya B, Saraf M. Betanin, isolated from fruits of Opuntia elatior Mill attenuates renal fibrosis in diabetic rats through regulating oxidative stress and TGF-β pathway. J Ethnopharmacol. 2017;198:432-443.
- Partovi N, Ebadzadeh MR, Fatemi SJ, et al. Effect of fruit extract on renal stone formation and kidney injury in rats. Nat Prod Res. 2018;32:1180-1183.
- Zhu X, Athmouni K. HPLC analysis and the antioxidant and preventive actions of Opuntia stricta juice extract against hepato-nephrotoxicity and testicular injury induced by cadmium exposure. Molecules. 2022;27:4972.
- Akacha A, Badraoui R, Rebai T, et al. Effect of Opuntia ficus indica extract on methotrexate-induced testicular injury: a biochemical, docking and histological study. J Biomol Struct Dyn. 2022;40:4341-4351.
- González-Ponce HA, Martínez-Saldaña MC, Tepper PG, et al. Betacyanins, major components in Opuntia red-purple fruits, protect against acetaminophen-induced acute liver failure. Food Res Int. 2020;137:109461.
- Akacha A, Rebai T, Zourgui L, et al. Preventive effect of ethanolic extract of cactus (Opuntia ficus-indica) cladodes on methotrexate-induced oxidative damage of the small intestine in Wistar rats. J Cancer Res Ther. 2018;14(Suppl):S779-S784.
- Yeh WJ, Tsai CC, Ko J, et al. Hylocereus polyrhizus peel extract retards alcoholic liver disease progression by modulating oxidative stress and inflammatory responses in C57BL/6 mice. Nutrients. 2020;12:3884.
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Dermatologic Implications of Prickly Pear Cacti (Opuntia)
Practice Points
- Prickly pear cacti have fine spines that must be removed via scorching or mechanical means before the fruit can be handled safely.
- Prickly pear spines that become embedded in the skin are associated with local and systemic inflammatory conditions as well as allergic contact dermatitis.
- Preclinical studies have suggested that extracts of the prickly pear cactus could be used in medicine for their anti-inflammatory effects.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Environmental and Lifestyle Triggers of Rosacea
Environmental and Lifestyle Triggers of Rosacea
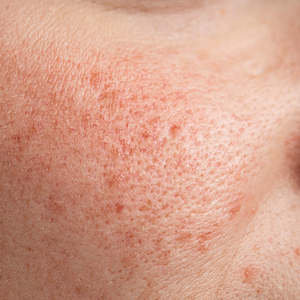
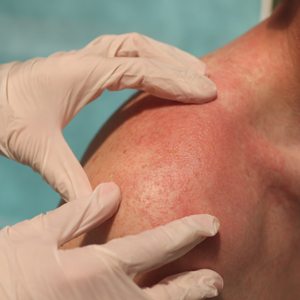
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.
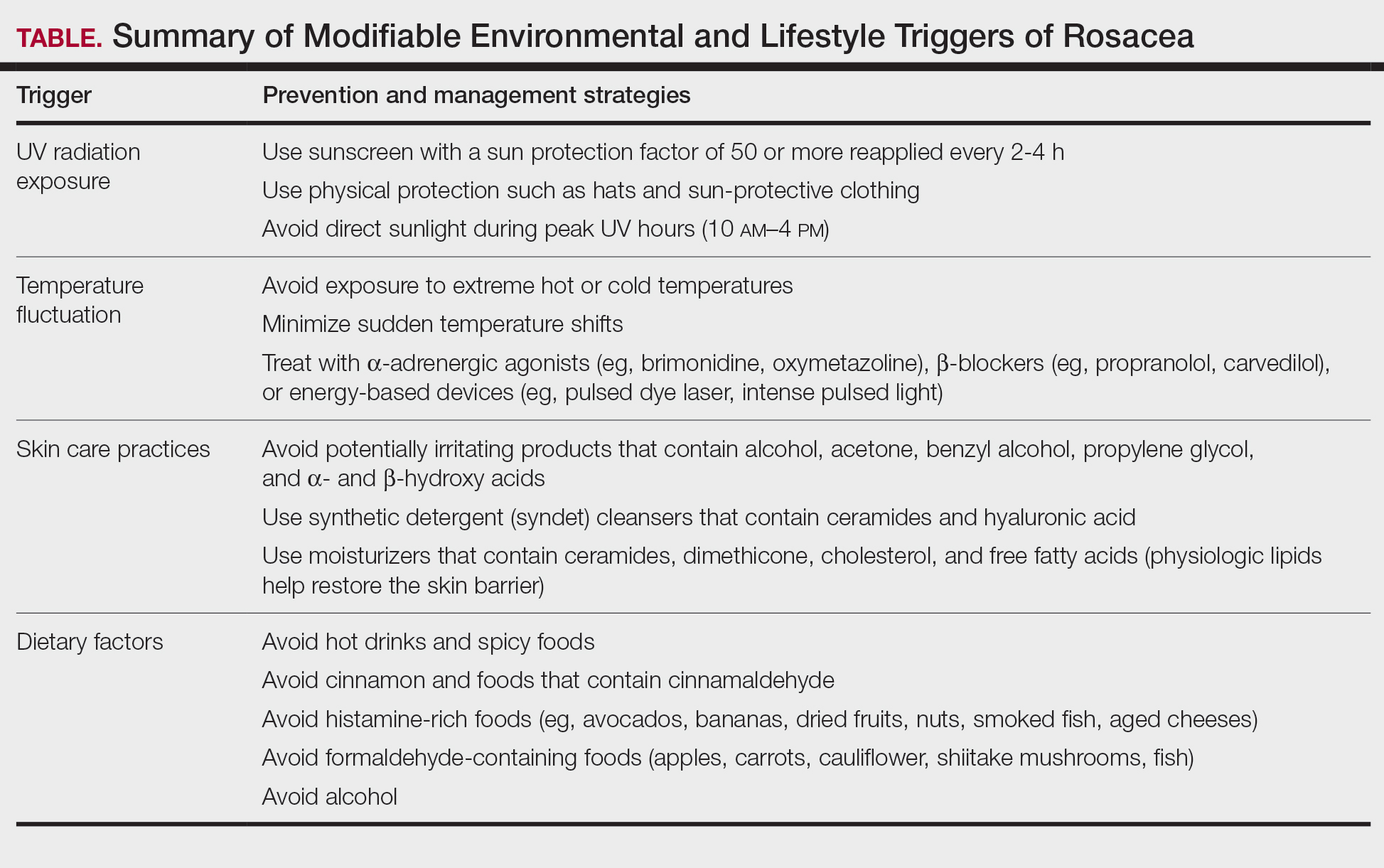
UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.

UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
Rosacea is a chronic inflammatory skin disease characterized by erythema, flushing, telangiectasias, papules, pustules, and rarely, phymatous changes that primarily manifest in a centrofacial distribution.1,2 Although establishing the true prevalence of rosacea may be challenging due to a wide spectrum of clinical manifestations, current studies estimate that it is between 5% to 6% of the global adult population and that rosacea most commonly is diagnosed in patients aged 30 and 60 years, though it occasionally can affect adolescents and children.3,4 Although the origin and pathophysiology of rosacea remain incompletely understood, the condition arises from a complex interplay of genetic, environmental, immune, microbial, and neurovascular factors; this interplay ultimately leads to excessive production of inflammatory and vasoactive peptides, chronic inflammation, and neurovascular hyperreactivity.1,5-7
Identifying triggers can be valuable in managing rosacea, as avoidance of these exposures may lead to disease improvement. In this review, we highlight 4 major environmental triggers of rosacea—UV radiation exposure, temperature fluctuation, skin care practices, and diet—and their roles in its pathogenesis and management. A high-level summary of recommendations can be found in the Table.

UV Radiation Exposure
Exposure to UV radiation is a known trigger of rosacea and may worsen symptoms through several mechanisms.8,9 It increases the production of inflammatory cytokines, which enhance the release of vascular endothelial growth factor, promoting angiogenesis and vasodilation.10 Exposure to UV radiation also contributes to tissue inflammation through the production of reactive oxygen species, further mediating inflammatory cascades and leading to immune dysregulation.11,12 Interestingly, though the mechanisms by which UV radiation may contribute to the pathophysiology of rosacea are well described, it remains unclear whether chronic UV exposure plays a major role in the pathogenesis or disease progression of rosacea.1 Studies have observed that increased exposure to sunlight seems to be correlated with increased severity of redness but not of papules and pustules.13,14
Despite some uncertainty regarding the relationship between rosacea and chronic UV exposure, sun protection is a prudent recommendation in this patient population, particularly given other risks of exposure to UV radiation, such as photoaging and skin cancer.9,15,16 Sun protection can be accomplished using broad-spectrum sunscreen (sun protection factor 50 or higher, reapplied every 2 to 4 hours) or by wearing physical protection (eg, hats, sun-protective clothing) along with avoidance of sun exposure during peak UV hours (ie, 10 am-
Temperature Fluctuation
Both heat and cold exposure have been suggested as triggers for rosacea, thought to be mediated through dysregulations in neurovascular and thermal pathways, resulting in increased flushing and erythema.6 Skin affected by rosacea exhibits a lower threshold for temperature and pain stimuli, resulting in heightened hypersensitivity compared to normal skin.18 Exposure to heat activates thermosensitive receptors found in neuronal and nonneuronal tissues, triggering the release of vasoactive neuropeptides.1 Among these, transient receptor potential (TRP) channels seem to play a crucial role in neurovascular reactivity and have been studied in the pathophysiology of rosacea.1,8 Overexpression or excessive stimulation of TRPs by various environmental triggers, such as heat or cold, leads to increased neuropeptide production, ultimately contributing to persistent erythema and vascular dysfunction, as well as a burning or stinging sensation.1,2 Moreover, rapid temperature changes, such as moving from freezing outdoor conditions into a heated environment, may also trigger flushing due to sudden vasodilation.2
Adopting behavioral strategies such as preventing overheating, minimizing sudden temperature shifts, and protecting the skin from cold can help reduce rosacea flare-ups, particularly flushing. For patients who do not achieve sufficient relief through lifestyle modifications alone, targeted pharmacologic treatments are available to help manage these symptoms. Topical α-adrenergic agonists (eg, brimonidine, oxymetazoline) are effective in reducing erythema and flushing by causing vasoconstriction.15,19 For persistent erythema and telangiectasias, pulsed dye laser and intense pulsed light therapies can be effective treatments, as they target hemoglobin in blood vessels, leading to their destruction and a subsequent reduction in erythema.20 Other medications such as topical metronidazole, azelaic acid, calcitonin-gene related peptide inhibitors, and systemic ß-blockers also can be used to treat flushing and redness.15,21
Skin Care Practices
Due to the increased tissue inflammation and potential skin barrier dysfunction, rosacea-affected skin is highly sensitive, and skin care practices or products that disrupt the already compromised skin barrier can contribute to flare-ups. General recommendations should include use of gentle cleansers and moisturizers to prevent dry skin and improve skin barrier function22 as well as avoidance of ingredients that are common irritants and inducers of allergic contact dermatitis (eg, fragrances).9
Cleansing the face should be limited to 1 to 2 times daily, as excessive cleansing and use of harsh formulations with exfoliative ingredients can lead to skin irritation and worsening of symptoms.9 Overcleansing can lead to alterations in cutaneous pH and strip the stratum corneum of healthy components such as lipids and natural moisturizing factors. Common ingredients in cleansers that should be avoided due to their irritant nature include alcohol, acetone, benzyl alcohol, propylene glycol, and α- and ß-hydroxy acids. Instead, syndet (synthetic detergent) cleansers that contain ceramides, hyaluronic acid, or other hydrating agents with a near-physiological pH can be helpful for dry and sensitive skin.23 Toners with high alcohol content and astringent-based products also should be avoided.
Optimal moisturizers for rosacea-affected skin should contain physiologic lipids that help replace a healthy skin barrier as well as relieve dryness and seal in moisture. Beneficial barrier-restoring ingredients include ceramides, dimethicone, cholesterol, and free fatty acids as well as humectants such as glycerin and hyaluronic acid.9,23,24 Applying moisturizer immediately after cleansing and prior to the application of any topical treatments also can help decrease irritation.
As mentioned previously, sun protection is a cornerstone in the management of rosacea and can help reduce redness and skin irritation. Using combination formulas, such as moisturizers with a sun protection factor of at least 50, can be effective.25 Additionally, products with antioxidant or anti-inflammatory ingredients such as niacinamide and allantoin can further support skin health. Lastly, formulations containing green pigments may also be beneficial, as they provide cosmetic camouflage to neutralize redness.26
Dietary Factors
Several dietary factors have been proposed as triggers for rosacea, but conclusive evidence remains limited.27 Foods and beverages that generate heat (eg, hot drinks, spicy foods) may exacerbate rosacea by causing vasodilation and stimulating TRP channels, resulting in flushing.18 While capsaicin, found in spicy foods, may lead to flushing through similar activation of TRP channels, current evidence has not proved a specific and consistent role in the pathogenesis of rosacea.18,27 Similarly, cinnamaldehyde, found in cinnamon and many commercial cinnamon-containing foods as well as various fruits and vegetables, activates thermosensitive receptors that may worsen rosacea symptoms.28 Other potential triggers include histamine-rich foods (eg, avocados, bananas, dried fruits, nuts, smoked fish, aged cheeses), which can lead to skin hypersensitivity and flushing, and formaldehyde-containing foods (eg, apples, carrots, cauliflower, shiitake mushrooms, fish), though the role these types of foods play in rosacea remains unclear.1,29-31
The relationship between caffeine and rosacea is complex. While caffeine commonly is found in coffee, tea, and soda, some studies have suggested that coffee consumption may reduce rosacea risk due to its vasoconstrictive and anti-inflammatory effects.28,32 In contrast, alcohol—particularly white wine and liquor—has been associated with increased rosacea risk due to its effect on vasodilation, inflammation, and oxidative stress.33 Despite anecdotal reports, the role of dairy products in rosacea remains unclear, with conflicting studies suggesting dairy consumption may exacerbate or protect against rosacea.27,28 Given the variability in dietary triggers, patients with rosacea may benefit from using a dietary journal to identify and avoid foods that exacerbate their symptoms, though more research is needed to establish clear recommendations.
Conclusion
Rosacea is a complex condition influenced by genetic, immune, microbial, and environmental factors. Triggers such as UV exposure, temperature fluctuations, alterations in the skin microbiome, and diet contribute to disease exacerbation through mechanisms like vasodilation, neurogenic inflammation, and immune dysregulation. These triggers often interact, compounding their effects and making symptom management more challenging and multifaceted.
Successful rosacea treatment relies on identifying and minimizing patient-specific triggers, as lifestyle modifications can reduce flare-ups and improve outcomes. When combined with interventional, oral, and topical therapies, these adjustments enhance treatment effectiveness and contribute to better long-term disease control. Clinicians should adopt a personalized holistic approach by educating patients on common triggers, recommending lifestyle changes, and integrating medical treatments as necessary. Future research should continue exploring the relationships between rosacea and environmental factors to develop more targeted and evidence-based recommendations.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
- Steinhoff M, Schauber J, Leyden JJ. New insights into rosacea pathophysiology: a review of recent findings. J Am Acad Dermatol. 2013;69(6 suppl 1):S15-S26.
- Buddenkotte J, Steinhoff M. Recent advances in understanding and managing rosacea. F1000Res. 2018;7:F1000 Faculty Rev-1885.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289. doi:10.1111/bjd.16481
- Chamaillard M, Mortemousque B, Boralevi F, et al. Cutaneous and ocular signs of childhood rosacea. Arch Dermatol. 2008;144:167-171.
- Abram K, Silm H, Maaroos H, et al. Risk factors associated with rosacea. J Eur Acad Dermatol Venereol. 2010;24:565-571.
- Gerber PA, Buhren BA, Steinhoff M, et al. Rosacea: the cytokine and chemokine network. J Investig Dermatol Symp Proc. 2011;15:40-47.
- Steinhoff M, Buddenkotte J, Aubert J, et al. Clinical, cellular, and molecular aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:2-11.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:749-758.
- Morgado‐Carrasco D, Granger C, Trullas C, et al. Impact of ultraviolet radiation and exposome on rosacea: key role of photoprotection in optimizing treatment. J Cosmet Dermatol. 2021;20:3415-3421.
- Suhng E, Kim BH, Choi YW, et al. Increased expression of IL‐33 in rosacea skin and UVB‐irradiated and LL‐37‐treated HaCaT cells. Exp Dermatol. 2018;27:1023-1029.
- Tisma VS, Basta-Juzbasic A, Jaganjac M, et al. Oxidative stress and ferritin expression in the skin of patients with rosacea. J Am Acad Dermatol. 2009;60:270-276.
- Kulkarni NN, Takahashi T, Sanford JA, et al. Innate immune dysfunction in rosacea promotes photosensitivity and vascular adhesion molecule expression. J Invest Dermatol. 2020;140:645-655.E6.
- Bae YI, Yun SJ, Lee JB, et al. Clinical evaluation of 168 Korean patients with rosacea: the sun exposure correlates with the erythematotelangiectatic subtype. Ann Dermatol. 2009;21:243-249.
- McAleer, MA, Fitzpatrick P, Powell FC. Papulopustular rosacea: prevalence and relationship to photodamage. J Am Acad Dermatol. 2010;63:33-39.
- Van Zuuren EJ. Rosacea. N Engl J Med. 2017;377:1754-1764.
- Two AM, Wu W, Gallo RL, et al. Rosacea. J Am Acad Dermatol. 2015;72:761-770.
- Nichols K, Desai N, Lebwohl MG. Effective sunscreen ingredients and cutaneous irritation in patients with rosacea. Cutis. 1998;61:344-346.
- Guzman-Sanchez DA, Ishiuji Y, Patel T, et al. Enhanced skin blood flow and sensitivity to noxious heat stimuli in papulopustular rosacea. J Am Acad Dermatol. 2007;57:800-805.
- Fowler J Jr, Jackson M, Moore A, et al. Efficacy and safety of once-daily topical brimonidine tartrate gel 0.5% for the treatment of moderate to severe facial erythema of rosacea: results of two randomized, double-blind, and vehicle-controlled pivotal studies. J Drugs Dermatol. 2013;12:650-656.
- van Zuuren EJ, Fedorowicz Z, Tan J, et al. Interventions for rosacea based on the phenotype approach: an updated systematic review including GRADE assessments. Br J Dermatol.2019;181:65-79.
- Wienholtz NKF, Christensen CE, Do TP, et al. Erenumab for treatment of persistent erythema and flushing in rosacea: a nonrandomized controlled trial. JAMA Dermatol.2024;160:612-619.
- Del Rosso JQ, Thiboutot D, Gallo R, et al. Consensus recommendations from the American Acne & Rosacea Society on the management of rosacea, part 1: a status report on the disease state, general measures, and adjunctive skin care. Cutis. 2013;92:234-240.
- Baldwin H, Alexis AF, Andriessen A, et al. Evidence of barrier deficiency in rosacea and the importance of integrating OTC skincare products into treatment regimens. J Drugs Dermatol. 2021;20:384-392.
- Schlesinger TE, Powell CR. Efficacy and tolerability of low molecular weight hyaluronic acid sodium salt 0.2% cream in rosacea. J Drugs Dermatol. 2013;12:664-667.
- Williams JD, Maitra P, Atillasoy E, et al. SPF 100+ sunscreen is more protective against sunburn than SPF 50+ in actual use: results of a randomized, double-blind, split-face, natural sunlight exposure clinical trial. J Am Acad Dermatol. 2018;78:902-910.E2.
- Draelos ZD. Cosmeceuticals for rosacea. Clin Dermatol. 2017;35:213-217.
- Yuan X, Huang X, Wang B, et al. Relationship between rosacea and dietary factors: a multicenter retrospective case–control survey. J Dermatol. 2019;46:219-225.
- Alia E, Feng H. Rosacea pathogenesis, common triggers, and dietary role: the cause, the trigger, and the positive effects of different foods. Clin Dermatol. 2022;40:122-127.
- Branco ACCC, Yoshikawa FSY, Pietrobon AJ, et al. Role of histamine in modulating the immune response and inflammation. Mediators Inflamm. 2018;2018:1-10.
- Darrigade A, Dendooven E, Aerts O. Contact allergy to fragrances and formaldehyde contributing to papulopustular rosacea. Contact Dermatitis. 2019;81:395-397.
- Linauskiene K, Isaksson M. Allergic contact dermatitis from formaldehyde mimicking impetigo and initiating rosacea. Contact Dermatitis. 2018;78:359-361.
- Al Reef T, Ghanem E. Caffeine: well-known as psychotropic substance, but little as immunomodulator. Immunobiology. 2018;223:818-825.
- Drago F, Ciccarese G, Herzum A, et al. Rosacea and alcohol intake. J Am Acad Dermatol. 2018;78:E25.
Environmental and Lifestyle Triggers of Rosacea
Environmental and Lifestyle Triggers of Rosacea
PRACTICE POINTS
- It is important to routinely assess and individualize rosacea management by encouraging use of symptom and trigger diaries to guide lifestyle modifications.
- Patients with rosacea should be encouraged to use mild, fragrance-free cleansers, barrier-supporting moisturizers, and daily broad-spectrum sunscreen and to avoid common irritants.
- Address flushing and erythema with behavioral and medical strategies; counsel patients on minimizing abrupt temperature shifts and consider topical Symbolα-adrenergic agonists, anti-inflammatory agents, or laser therapies when lifestyle measures alone are insufficient.
- Lifestyle recommendations (eg, optimal skin care practices, avoidance of dietary triggers) should be incorporated in treatment plans.
Verrucous Nodule on the Cheek
Verrucous Nodule on the Cheek
THE DIAGNOSIS: Pilomatrix Carcinoma
Histopathology revealed poorly circumscribed dermal nodules composed of large pleomorphic and highly atypical basaloid cells as well as increased mitoses. Foci of central necrosis admixed with keratinized cells containing pale eosinophilic cytoplasm and faint nuclear outlines without nuclei also were present. Immunohistochemistry for p63 was positive, while adipophilin, BerEP4, cytokeratin 20, and carcinoembryonic antigen were negative. Tumor cells also demonstrated strong and diffuse nuclear and cytoplasmic β-catenin staining, leading to a diagnosis of pilomatrix carcinoma (PC). The tumor was treated with Mohs micrographic surgery, and the patient was subsequently lost to follow-up.
Pilomatrix carcinoma, historically known as calcifying epitheliocarcinoma of Malherbe, is a rare, locally aggressive, low-grade adnexal tumor of germinative hair follicle matrix cell origin. Similar to its benign pilomatrixoma counterpart, malignant PC manifests as a firm, nontender, asymptomatic nodule most commonly (but not exclusively) manifesting in the head and neck region; however, in contrast to benign pilomatrixoma, PC is a rapidly growing tumor with a high rate of local recurrence after surgical excision and has the potential to become metastatic.1
Pilomatrix carcinoma occurs most often in the fifth through seventh decades of life, with a male-to-female ratio of approximately 1.3:1.1 Due to its rarity, PC management guidelines are not well defined. Histologically, PC will show asymmetry, poor circumscription, and an infiltrative growth pattern at low power. Pilomatrix carcinoma is further characterized by the presence of nodules of atypical basaloid cells demonstrating pleomorphism and nuclear hyperchromatism, increased mitotic index, and the presence of ghost cells (Figure 1).2 Ghost cells are evidence of matrical differentiation. The transition from basaloid to ghost cells may be abrupt. Intralesional calcification is possible but less common.2,3 The tumor nodules can be surrounded by a dense desmoplastic stroma with a predominantly lymphohistiocytic infiltrate.2 Immunohistochemical stains that support a PC diagnosis include lymphoid enhancer-binding factor 1 (LEF1), Ki-67, β-catenin, and p53. Although not specific for malignancy, nuclear LEF1 helps confirm matrical (hair matrix) differentiation.4 Pilomatrix carcinomas show a markedly elevated Ki-67 proliferation marker, reflecting high mitotic activity.5 While benign pilomatricoma may show patchy or minimal p53 staining, PC can demonstrate diffuse strong p53 positivity, consistent with the p53 pathway dysregulation seen in malignant matrical neoplasms.6 Most classically, PC stains strongly positive for nuclear and cytoplasmic β-catenin. Aberrant β-catenin disrupting normal Wnt/β-catenin/Tcf-Lef pathway regulation, which ultimately promotes cellular differentiation and division, is proposed to play a role in tumorigenesis.6,7
The differential diagnoses for PC include basal cell carcinoma (BCC), Merkel cell carcinoma, moderately differentiated squamous cell carcinoma, and porocarcinoma. Basal cell carcinoma is a common tumor occurring on the head and neck regions that typically manifests as a slow-growing, flesh-colored, pink or pigmented papule, plaque, or nodule. Spontaneous bleeding or ulceration can sometimes occur. Basal cell carcinoma has various histologic subtypes, with tumors potentially exhibiting more than one histologic pattern. Common features of BCC include basaloid nodules arising from the epidermis, peripheral palisading, clefting artifacts, and a myxoid stroma (Figure 2).8 These features help distinguish BCC from PC histologically, although there is a rare matrical BCC subtype with a handful of reported cases expressing features of both.9 Staining can be a helpful differentiator as pancellular staining for LEF1, and β-catenin is exclusively observed in the pilomatrixoma and PC, in contrast to BCC, which shows staining confined to focal germinative matrix cell nests.10
Squamous cell carcinoma (SCC) also commonly manifests clinically in the head and neck region and is associated with sun damage. Squamous cell carcinoma can be histologically graded based on cellular differentiation, from well differentiated to poorly differentiated subtypes. Moderately differentiated SCC is characterized histologically by reduced keratinization, frequent loss of intercellular bridges, and enlarged pleomorphic cells demonstrating a high degree of atypia and frequent abnormal mitoses (Figure 3).11 Similar to PC, moderately differentiated SCC also may comprise basaloid cells but lacks shadow cells. Further distinction from PC can be made through immunohistochemistry. Expression of p63, p40, MNF116, and CK903 expression help identify the squamous origin of the tumor and are useful in the diagnosis of less-differentiated SCC.12 In addition, SCC does not show matrical differentiation (ghost cells).
Merkel cell carcinoma is a rare and aggressive skin cancer that manifests as a rapidly growing, sometimes ulcerating nodule or plaque with a predilection for sun‐exposed areas of the skin. Merkel cell carcinoma is characterized by neuroendocrine differentiation. The gold standard diagnostic modalities are histopathology and immunohistochemistry. Characteristic histopathologic findings include diffuse atypical blue cells with large nuclei, minimal cytoplasm, and frequent mitoses (Figure 4).13,14 Staining with cytokeratin 20 and neuroendocrine markers such as synaptophysin and chromogranin A on immunohistochemistry supports the diagnosis, as does positive AE1/3; neuron-specific enolase and epithelial membrane antigen; and negative S100, carcinoembryonic antigen, and leukocyte common antigen staining.13,14
Porocarcinoma is a rare malignant growth arising from the cutaneous intraepidermal ducts of the sweat glands. Porocarcinomas may originate from benign eccrine poromas, but the etiology remains poorly understood. Clinically, porocarcinoma manifests as a flesh-colored, erythematous, or violaceous firm, single, dome-shaped papule or nodule that can ulcerate and may be asymptomatic, itchy, or painful.15 Porocarcinoma poses a diagnostic challenge due to the variability of both its clinical presentation and histopathologic findings. The histology often resembles that of cutaneous squamous cell carcinoma or poroma. On hematoxylin and eosin staining, porocarcinoma is characterized by poromatous basaloid cells with cytologic atypia and ductal differentiation. Common histopathologic features include formation of mature ducts lined with cuboidal epithelial cells, foci of necrosis, intracytoplasmic lumina, and squamous differentiation (Figure 5).15 Carcinoembryonic antigen and epithelial membrane antigen immunohistochemical staining to identify ductal structures may help to distinguish porocarcinoma from other tumors. Cluster of Differentiation 117/c-KIT, cytokeratin 19, and BerEP4 positivity also have been shown to be useful in diagnosing porocarcinoma. CD117/c-KIT highlights eccrine ductal differentiation16; CK19 supports adnexal ductal differentiation and often is increased in malignant poroid neoplasms17; and BerEP4, although classically used for BCC diagnosis, also may be positive in porocarcinoma, particularly in ductal areas, and can support the diagnosis.18
- Toffoli L, Bazzacco G, Conforti C, et al. Pilomatrix carcinoma: report of two cases of the head and review of the literature. Curr Oncol. 2023;30:1426-1438. doi:10.3390/curroncol30020109
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.E2. doi:10.1016/j.jaad.2014.02.042
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38. doi:10.1111/cup.13046
- Reymundo-Jiménez A, Martos-Cabrera L, Muñoz-Hernández P, et al. Usefulness of LEF-1 immunostaining for the diagnosis of matricoma. Actas Dermosifiliogr. 2022;113:T907-T910. doi:10.1016/j.ad.2022.08.003
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498. doi:10.1002/1097-0142(19930415)71:8<2491 ::aid-cncr2820710811>3.0.co;2-i
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding β-catenin. J Cutan Pathol. 2005;32:148-157. doi:10.1111/j.0303-6987.2005.00267.x
- Abula A, Ma SQ, Wang S, et al. Case report: Pilomatrix carcinoma with PDL1 expression and CDKN2A aberrant. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1337400
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
- Kanitakis J, Ducroux E, Hoelt P, et al. Basal-cell carcinoma with matrical differentiation: report of a new case in a renal-transplant recipient and literature review. Am J Dermatopathol. 2018;40:E115-E118. doi:10.1097 /DAD.0000000000001146
- White C, Farsi M, Esguerra D, et al. Not your average skin cancer: a rare case of pilomatrix carcinoma. J Clin Aesthet Dermatol. 2020; 13:40-42.
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2010;2011:210813. doi:10.1155/2011/210813
- Balas¸escu E, Gheorghe AC, Moroianu A, et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamouscell carcinomas (review). Exp Ther Med. 2022;23:383. doi:10.3892 /etm.2022.11308
- Zhang Z, Shi W, Zhang R. Facial Merkel cell carcinoma in a 92-year-old man: a case report. Clin Case Rep. 2024;12:E9523. doi:10.1002/ccr3.9523
- Rapini R. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Miyamoto K, Yanagi T, Maeda T, et al. Diagnosis and management of porocarcinoma. Cancers. 2022;14:5232. doi:10.3390 /cancers14215232
- Goto K. Immunohistochemistry for CD117 (KIT) is effective in distinguishing cutaneous adnexal tumors with apocrine/eccrine or sebaceous differentiation from other epithelial tumors of the skin. J Cutan Pathol. 2015;42:480-488. doi:10.1111/cup.12492
- Requena L, Sangüeza O. General principles for the histopathologic diagnosis of neoplasms with eccrine and apocrine differentiation. Classification and histopathologic criteria for eccrine and apocrine differentiation. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:19-24. doi:10.1007/978- 3-319-45704-8_2
- Huet P, Dandurand M, Pignodel C, et al. Metastasizing eccrine porocarcinoma: report of a case and review of the literature. J Am Acad Dermatol. 1996;35:860-864. doi:10.1016/s0190-9622(96)90105-x
THE DIAGNOSIS: Pilomatrix Carcinoma
Histopathology revealed poorly circumscribed dermal nodules composed of large pleomorphic and highly atypical basaloid cells as well as increased mitoses. Foci of central necrosis admixed with keratinized cells containing pale eosinophilic cytoplasm and faint nuclear outlines without nuclei also were present. Immunohistochemistry for p63 was positive, while adipophilin, BerEP4, cytokeratin 20, and carcinoembryonic antigen were negative. Tumor cells also demonstrated strong and diffuse nuclear and cytoplasmic β-catenin staining, leading to a diagnosis of pilomatrix carcinoma (PC). The tumor was treated with Mohs micrographic surgery, and the patient was subsequently lost to follow-up.
Pilomatrix carcinoma, historically known as calcifying epitheliocarcinoma of Malherbe, is a rare, locally aggressive, low-grade adnexal tumor of germinative hair follicle matrix cell origin. Similar to its benign pilomatrixoma counterpart, malignant PC manifests as a firm, nontender, asymptomatic nodule most commonly (but not exclusively) manifesting in the head and neck region; however, in contrast to benign pilomatrixoma, PC is a rapidly growing tumor with a high rate of local recurrence after surgical excision and has the potential to become metastatic.1
Pilomatrix carcinoma occurs most often in the fifth through seventh decades of life, with a male-to-female ratio of approximately 1.3:1.1 Due to its rarity, PC management guidelines are not well defined. Histologically, PC will show asymmetry, poor circumscription, and an infiltrative growth pattern at low power. Pilomatrix carcinoma is further characterized by the presence of nodules of atypical basaloid cells demonstrating pleomorphism and nuclear hyperchromatism, increased mitotic index, and the presence of ghost cells (Figure 1).2 Ghost cells are evidence of matrical differentiation. The transition from basaloid to ghost cells may be abrupt. Intralesional calcification is possible but less common.2,3 The tumor nodules can be surrounded by a dense desmoplastic stroma with a predominantly lymphohistiocytic infiltrate.2 Immunohistochemical stains that support a PC diagnosis include lymphoid enhancer-binding factor 1 (LEF1), Ki-67, β-catenin, and p53. Although not specific for malignancy, nuclear LEF1 helps confirm matrical (hair matrix) differentiation.4 Pilomatrix carcinomas show a markedly elevated Ki-67 proliferation marker, reflecting high mitotic activity.5 While benign pilomatricoma may show patchy or minimal p53 staining, PC can demonstrate diffuse strong p53 positivity, consistent with the p53 pathway dysregulation seen in malignant matrical neoplasms.6 Most classically, PC stains strongly positive for nuclear and cytoplasmic β-catenin. Aberrant β-catenin disrupting normal Wnt/β-catenin/Tcf-Lef pathway regulation, which ultimately promotes cellular differentiation and division, is proposed to play a role in tumorigenesis.6,7

The differential diagnoses for PC include basal cell carcinoma (BCC), Merkel cell carcinoma, moderately differentiated squamous cell carcinoma, and porocarcinoma. Basal cell carcinoma is a common tumor occurring on the head and neck regions that typically manifests as a slow-growing, flesh-colored, pink or pigmented papule, plaque, or nodule. Spontaneous bleeding or ulceration can sometimes occur. Basal cell carcinoma has various histologic subtypes, with tumors potentially exhibiting more than one histologic pattern. Common features of BCC include basaloid nodules arising from the epidermis, peripheral palisading, clefting artifacts, and a myxoid stroma (Figure 2).8 These features help distinguish BCC from PC histologically, although there is a rare matrical BCC subtype with a handful of reported cases expressing features of both.9 Staining can be a helpful differentiator as pancellular staining for LEF1, and β-catenin is exclusively observed in the pilomatrixoma and PC, in contrast to BCC, which shows staining confined to focal germinative matrix cell nests.10

Squamous cell carcinoma (SCC) also commonly manifests clinically in the head and neck region and is associated with sun damage. Squamous cell carcinoma can be histologically graded based on cellular differentiation, from well differentiated to poorly differentiated subtypes. Moderately differentiated SCC is characterized histologically by reduced keratinization, frequent loss of intercellular bridges, and enlarged pleomorphic cells demonstrating a high degree of atypia and frequent abnormal mitoses (Figure 3).11 Similar to PC, moderately differentiated SCC also may comprise basaloid cells but lacks shadow cells. Further distinction from PC can be made through immunohistochemistry. Expression of p63, p40, MNF116, and CK903 expression help identify the squamous origin of the tumor and are useful in the diagnosis of less-differentiated SCC.12 In addition, SCC does not show matrical differentiation (ghost cells).

Merkel cell carcinoma is a rare and aggressive skin cancer that manifests as a rapidly growing, sometimes ulcerating nodule or plaque with a predilection for sun‐exposed areas of the skin. Merkel cell carcinoma is characterized by neuroendocrine differentiation. The gold standard diagnostic modalities are histopathology and immunohistochemistry. Characteristic histopathologic findings include diffuse atypical blue cells with large nuclei, minimal cytoplasm, and frequent mitoses (Figure 4).13,14 Staining with cytokeratin 20 and neuroendocrine markers such as synaptophysin and chromogranin A on immunohistochemistry supports the diagnosis, as does positive AE1/3; neuron-specific enolase and epithelial membrane antigen; and negative S100, carcinoembryonic antigen, and leukocyte common antigen staining.13,14

Porocarcinoma is a rare malignant growth arising from the cutaneous intraepidermal ducts of the sweat glands. Porocarcinomas may originate from benign eccrine poromas, but the etiology remains poorly understood. Clinically, porocarcinoma manifests as a flesh-colored, erythematous, or violaceous firm, single, dome-shaped papule or nodule that can ulcerate and may be asymptomatic, itchy, or painful.15 Porocarcinoma poses a diagnostic challenge due to the variability of both its clinical presentation and histopathologic findings. The histology often resembles that of cutaneous squamous cell carcinoma or poroma. On hematoxylin and eosin staining, porocarcinoma is characterized by poromatous basaloid cells with cytologic atypia and ductal differentiation. Common histopathologic features include formation of mature ducts lined with cuboidal epithelial cells, foci of necrosis, intracytoplasmic lumina, and squamous differentiation (Figure 5).15 Carcinoembryonic antigen and epithelial membrane antigen immunohistochemical staining to identify ductal structures may help to distinguish porocarcinoma from other tumors. Cluster of Differentiation 117/c-KIT, cytokeratin 19, and BerEP4 positivity also have been shown to be useful in diagnosing porocarcinoma. CD117/c-KIT highlights eccrine ductal differentiation16; CK19 supports adnexal ductal differentiation and often is increased in malignant poroid neoplasms17; and BerEP4, although classically used for BCC diagnosis, also may be positive in porocarcinoma, particularly in ductal areas, and can support the diagnosis.18

THE DIAGNOSIS: Pilomatrix Carcinoma
Histopathology revealed poorly circumscribed dermal nodules composed of large pleomorphic and highly atypical basaloid cells as well as increased mitoses. Foci of central necrosis admixed with keratinized cells containing pale eosinophilic cytoplasm and faint nuclear outlines without nuclei also were present. Immunohistochemistry for p63 was positive, while adipophilin, BerEP4, cytokeratin 20, and carcinoembryonic antigen were negative. Tumor cells also demonstrated strong and diffuse nuclear and cytoplasmic β-catenin staining, leading to a diagnosis of pilomatrix carcinoma (PC). The tumor was treated with Mohs micrographic surgery, and the patient was subsequently lost to follow-up.
Pilomatrix carcinoma, historically known as calcifying epitheliocarcinoma of Malherbe, is a rare, locally aggressive, low-grade adnexal tumor of germinative hair follicle matrix cell origin. Similar to its benign pilomatrixoma counterpart, malignant PC manifests as a firm, nontender, asymptomatic nodule most commonly (but not exclusively) manifesting in the head and neck region; however, in contrast to benign pilomatrixoma, PC is a rapidly growing tumor with a high rate of local recurrence after surgical excision and has the potential to become metastatic.1
Pilomatrix carcinoma occurs most often in the fifth through seventh decades of life, with a male-to-female ratio of approximately 1.3:1.1 Due to its rarity, PC management guidelines are not well defined. Histologically, PC will show asymmetry, poor circumscription, and an infiltrative growth pattern at low power. Pilomatrix carcinoma is further characterized by the presence of nodules of atypical basaloid cells demonstrating pleomorphism and nuclear hyperchromatism, increased mitotic index, and the presence of ghost cells (Figure 1).2 Ghost cells are evidence of matrical differentiation. The transition from basaloid to ghost cells may be abrupt. Intralesional calcification is possible but less common.2,3 The tumor nodules can be surrounded by a dense desmoplastic stroma with a predominantly lymphohistiocytic infiltrate.2 Immunohistochemical stains that support a PC diagnosis include lymphoid enhancer-binding factor 1 (LEF1), Ki-67, β-catenin, and p53. Although not specific for malignancy, nuclear LEF1 helps confirm matrical (hair matrix) differentiation.4 Pilomatrix carcinomas show a markedly elevated Ki-67 proliferation marker, reflecting high mitotic activity.5 While benign pilomatricoma may show patchy or minimal p53 staining, PC can demonstrate diffuse strong p53 positivity, consistent with the p53 pathway dysregulation seen in malignant matrical neoplasms.6 Most classically, PC stains strongly positive for nuclear and cytoplasmic β-catenin. Aberrant β-catenin disrupting normal Wnt/β-catenin/Tcf-Lef pathway regulation, which ultimately promotes cellular differentiation and division, is proposed to play a role in tumorigenesis.6,7

The differential diagnoses for PC include basal cell carcinoma (BCC), Merkel cell carcinoma, moderately differentiated squamous cell carcinoma, and porocarcinoma. Basal cell carcinoma is a common tumor occurring on the head and neck regions that typically manifests as a slow-growing, flesh-colored, pink or pigmented papule, plaque, or nodule. Spontaneous bleeding or ulceration can sometimes occur. Basal cell carcinoma has various histologic subtypes, with tumors potentially exhibiting more than one histologic pattern. Common features of BCC include basaloid nodules arising from the epidermis, peripheral palisading, clefting artifacts, and a myxoid stroma (Figure 2).8 These features help distinguish BCC from PC histologically, although there is a rare matrical BCC subtype with a handful of reported cases expressing features of both.9 Staining can be a helpful differentiator as pancellular staining for LEF1, and β-catenin is exclusively observed in the pilomatrixoma and PC, in contrast to BCC, which shows staining confined to focal germinative matrix cell nests.10

Squamous cell carcinoma (SCC) also commonly manifests clinically in the head and neck region and is associated with sun damage. Squamous cell carcinoma can be histologically graded based on cellular differentiation, from well differentiated to poorly differentiated subtypes. Moderately differentiated SCC is characterized histologically by reduced keratinization, frequent loss of intercellular bridges, and enlarged pleomorphic cells demonstrating a high degree of atypia and frequent abnormal mitoses (Figure 3).11 Similar to PC, moderately differentiated SCC also may comprise basaloid cells but lacks shadow cells. Further distinction from PC can be made through immunohistochemistry. Expression of p63, p40, MNF116, and CK903 expression help identify the squamous origin of the tumor and are useful in the diagnosis of less-differentiated SCC.12 In addition, SCC does not show matrical differentiation (ghost cells).

Merkel cell carcinoma is a rare and aggressive skin cancer that manifests as a rapidly growing, sometimes ulcerating nodule or plaque with a predilection for sun‐exposed areas of the skin. Merkel cell carcinoma is characterized by neuroendocrine differentiation. The gold standard diagnostic modalities are histopathology and immunohistochemistry. Characteristic histopathologic findings include diffuse atypical blue cells with large nuclei, minimal cytoplasm, and frequent mitoses (Figure 4).13,14 Staining with cytokeratin 20 and neuroendocrine markers such as synaptophysin and chromogranin A on immunohistochemistry supports the diagnosis, as does positive AE1/3; neuron-specific enolase and epithelial membrane antigen; and negative S100, carcinoembryonic antigen, and leukocyte common antigen staining.13,14

Porocarcinoma is a rare malignant growth arising from the cutaneous intraepidermal ducts of the sweat glands. Porocarcinomas may originate from benign eccrine poromas, but the etiology remains poorly understood. Clinically, porocarcinoma manifests as a flesh-colored, erythematous, or violaceous firm, single, dome-shaped papule or nodule that can ulcerate and may be asymptomatic, itchy, or painful.15 Porocarcinoma poses a diagnostic challenge due to the variability of both its clinical presentation and histopathologic findings. The histology often resembles that of cutaneous squamous cell carcinoma or poroma. On hematoxylin and eosin staining, porocarcinoma is characterized by poromatous basaloid cells with cytologic atypia and ductal differentiation. Common histopathologic features include formation of mature ducts lined with cuboidal epithelial cells, foci of necrosis, intracytoplasmic lumina, and squamous differentiation (Figure 5).15 Carcinoembryonic antigen and epithelial membrane antigen immunohistochemical staining to identify ductal structures may help to distinguish porocarcinoma from other tumors. Cluster of Differentiation 117/c-KIT, cytokeratin 19, and BerEP4 positivity also have been shown to be useful in diagnosing porocarcinoma. CD117/c-KIT highlights eccrine ductal differentiation16; CK19 supports adnexal ductal differentiation and often is increased in malignant poroid neoplasms17; and BerEP4, although classically used for BCC diagnosis, also may be positive in porocarcinoma, particularly in ductal areas, and can support the diagnosis.18

- Toffoli L, Bazzacco G, Conforti C, et al. Pilomatrix carcinoma: report of two cases of the head and review of the literature. Curr Oncol. 2023;30:1426-1438. doi:10.3390/curroncol30020109
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.E2. doi:10.1016/j.jaad.2014.02.042
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38. doi:10.1111/cup.13046
- Reymundo-Jiménez A, Martos-Cabrera L, Muñoz-Hernández P, et al. Usefulness of LEF-1 immunostaining for the diagnosis of matricoma. Actas Dermosifiliogr. 2022;113:T907-T910. doi:10.1016/j.ad.2022.08.003
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498. doi:10.1002/1097-0142(19930415)71:8<2491 ::aid-cncr2820710811>3.0.co;2-i
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding β-catenin. J Cutan Pathol. 2005;32:148-157. doi:10.1111/j.0303-6987.2005.00267.x
- Abula A, Ma SQ, Wang S, et al. Case report: Pilomatrix carcinoma with PDL1 expression and CDKN2A aberrant. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1337400
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
- Kanitakis J, Ducroux E, Hoelt P, et al. Basal-cell carcinoma with matrical differentiation: report of a new case in a renal-transplant recipient and literature review. Am J Dermatopathol. 2018;40:E115-E118. doi:10.1097 /DAD.0000000000001146
- White C, Farsi M, Esguerra D, et al. Not your average skin cancer: a rare case of pilomatrix carcinoma. J Clin Aesthet Dermatol. 2020; 13:40-42.
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2010;2011:210813. doi:10.1155/2011/210813
- Balas¸escu E, Gheorghe AC, Moroianu A, et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamouscell carcinomas (review). Exp Ther Med. 2022;23:383. doi:10.3892 /etm.2022.11308
- Zhang Z, Shi W, Zhang R. Facial Merkel cell carcinoma in a 92-year-old man: a case report. Clin Case Rep. 2024;12:E9523. doi:10.1002/ccr3.9523
- Rapini R. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Miyamoto K, Yanagi T, Maeda T, et al. Diagnosis and management of porocarcinoma. Cancers. 2022;14:5232. doi:10.3390 /cancers14215232
- Goto K. Immunohistochemistry for CD117 (KIT) is effective in distinguishing cutaneous adnexal tumors with apocrine/eccrine or sebaceous differentiation from other epithelial tumors of the skin. J Cutan Pathol. 2015;42:480-488. doi:10.1111/cup.12492
- Requena L, Sangüeza O. General principles for the histopathologic diagnosis of neoplasms with eccrine and apocrine differentiation. Classification and histopathologic criteria for eccrine and apocrine differentiation. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:19-24. doi:10.1007/978- 3-319-45704-8_2
- Huet P, Dandurand M, Pignodel C, et al. Metastasizing eccrine porocarcinoma: report of a case and review of the literature. J Am Acad Dermatol. 1996;35:860-864. doi:10.1016/s0190-9622(96)90105-x
- Toffoli L, Bazzacco G, Conforti C, et al. Pilomatrix carcinoma: report of two cases of the head and review of the literature. Curr Oncol. 2023;30:1426-1438. doi:10.3390/curroncol30020109
- Herrmann JL, Allan A, Trapp KM, et al. Pilomatrix carcinoma: 13 new cases and review of the literature with emphasis on predictors of metastasis. J Am Acad Dermatol. 2014;71:38-43.E2. doi:10.1016/j.jaad.2014.02.042
- Jones C, Twoon M, Ho W, et al. Pilomatrix carcinoma: 12-year experience and review of the literature. J Cutan Pathol. 2018;45:33-38. doi:10.1111/cup.13046
- Reymundo-Jiménez A, Martos-Cabrera L, Muñoz-Hernández P, et al. Usefulness of LEF-1 immunostaining for the diagnosis of matricoma. Actas Dermosifiliogr. 2022;113:T907-T910. doi:10.1016/j.ad.2022.08.003
- Sau P, Lupton GP, Graham JH. Pilomatrix carcinoma. Cancer. 1993;71:2491-2498. doi:10.1002/1097-0142(19930415)71:8<2491 ::aid-cncr2820710811>3.0.co;2-i
- Lazar AJF, Calonje E, Grayson W, et al. Pilomatrix carcinomas contain mutations in CTNNB1, the gene encoding β-catenin. J Cutan Pathol. 2005;32:148-157. doi:10.1111/j.0303-6987.2005.00267.x
- Abula A, Ma SQ, Wang S, et al. Case report: Pilomatrix carcinoma with PDL1 expression and CDKN2A aberrant. Front Immunol. 2024;15. doi:10.3389/fimmu.2024.1337400
- Cameron MC, Lee E, Hibler BP, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol. 2019;80:303-317. doi:10.1016/j.jaad.2018.03.060
- Kanitakis J, Ducroux E, Hoelt P, et al. Basal-cell carcinoma with matrical differentiation: report of a new case in a renal-transplant recipient and literature review. Am J Dermatopathol. 2018;40:E115-E118. doi:10.1097 /DAD.0000000000001146
- White C, Farsi M, Esguerra D, et al. Not your average skin cancer: a rare case of pilomatrix carcinoma. J Clin Aesthet Dermatol. 2020; 13:40-42.
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2010;2011:210813. doi:10.1155/2011/210813
- Balas¸escu E, Gheorghe AC, Moroianu A, et al. Role of immunohistochemistry in the diagnosis and staging of cutaneous squamouscell carcinomas (review). Exp Ther Med. 2022;23:383. doi:10.3892 /etm.2022.11308
- Zhang Z, Shi W, Zhang R. Facial Merkel cell carcinoma in a 92-year-old man: a case report. Clin Case Rep. 2024;12:E9523. doi:10.1002/ccr3.9523
- Rapini R. Practical Dermatopathology. 3rd ed. Elsevier; 2021.
- Miyamoto K, Yanagi T, Maeda T, et al. Diagnosis and management of porocarcinoma. Cancers. 2022;14:5232. doi:10.3390 /cancers14215232
- Goto K. Immunohistochemistry for CD117 (KIT) is effective in distinguishing cutaneous adnexal tumors with apocrine/eccrine or sebaceous differentiation from other epithelial tumors of the skin. J Cutan Pathol. 2015;42:480-488. doi:10.1111/cup.12492
- Requena L, Sangüeza O. General principles for the histopathologic diagnosis of neoplasms with eccrine and apocrine differentiation. Classification and histopathologic criteria for eccrine and apocrine differentiation. In: Requena L, Sangüeza O, eds. Cutaneous Adnexal Neoplasms. Springer International Publishing; 2017:19-24. doi:10.1007/978- 3-319-45704-8_2
- Huet P, Dandurand M, Pignodel C, et al. Metastasizing eccrine porocarcinoma: report of a case and review of the literature. J Am Acad Dermatol. 1996;35:860-864. doi:10.1016/s0190-9622(96)90105-x
Verrucous Nodule on the Cheek
Verrucous Nodule on the Cheek
A 73-year-old man presented to the dermatology department for evaluation of an asymptomatic verrucous brown nodule on the right superior malar cheek of a few months’ duration. The patient reported a history of hyperlipidemia and hypertension and no prior treatment at the site of the nodule. A biopsy of the lesion was performed.
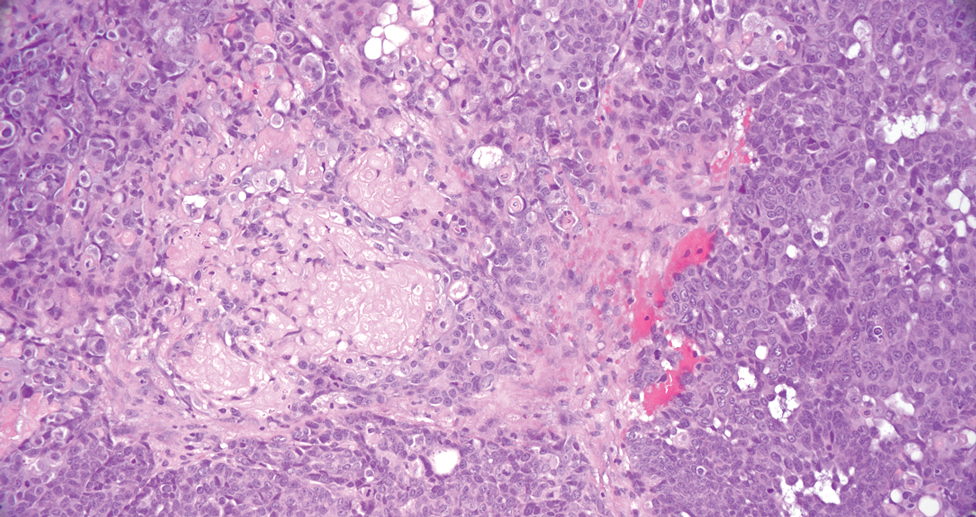
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.
Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.


Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
Dermatology trainees are no strangers to standardized examinations that assess basic science and medical knowledge, from the Medical College Admission Test and the National Board of Medical Examiners Subject Examinations to the United States Medical Licensing Examination series (I know, cue the collective flashbacks!). As a dermatology resident, you will complete a series of 6 examinations, culminating with the final APPLIED Exam, which assesses a trainee's ability to apply therapeutic knowledge and clinical reasoning in scenarios relevant to the practice of general dermatology.1 This article features high-yield tips and study resources alongside test-day strategies to help you perform at your best.
The Path to Board Certification for Dermatology Trainees
After years of dedicated study in medical school, navigating the demanding match process, and completing your intern year, you have finally made it to dermatology! With the USMLE Step 3 out of the way, you are now officially able to trade in electrocardiograms for Kodachromes and dermoscopy. As a dermatology trainee, you will complete the American Board of Dermatology (ABD) Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, which covers dermatology fundamentals and is proctored at your home institution.1 This exam is solely for informational purposes, and ultimately no minimum score is required for certification purposes. Subsequently, second- and third-year residents sit for 4 CORE Exam modules assessing advanced knowledge of the major clinical areas of the specialty: medical dermatology, surgical dermatology, pediatric dermatology, and dermatopathology. These exams consist of 75 to 100 multiple-choice questions per each 2-hour module and are administered either online in a private setting, via a secure online proctoring system, or at an approved testing center. The APPLIED Exam is the final component of the pathway and prioritizes clinical acumen and judgement. This 8-hour, 200-question exam is offered exclusively in person at approved testing centers to residents who have passed all 4 compulsory CORE modules and completed residency training. There is a 20-minute break between sections 1 and 2, a 60-minute break between sections 2 and 3, and a 20-minute break between sections 3 and 4.1 Following successful completion of the ABD Certification Pathway, dermatologists maintain board certification through quarterly CertLink questions, which you must complete at least 3 quarters of each year, and regular completion of focused practice improvement modules every 5 years. Additionally, one must maintain a full and unrestricted medical license in the United States or Canada and pay an annual fee of $150.
High-Yield Study Resources and Exam Preparation Strategies
Growing up, I was taught that proper preparation prevents poor performance. This principle holds particularly true when approaching the ABD Certification Pathway. Before diving into high-yield study resources and comprehensive exam preparation strategies, here are some big-picture essentials you need to know:
- Your residency program covers the fee for the BASIC Exam, but the CORE and APPLIED Exams are out-of-pocket expenses. As of 2026, you should plan to budget $2450 ($200 for 4 CORE module attempts and $2250 for the APPLIED Exam) for all 5 exams.2
- Testing center space is limited for each test date. While the ABD offers CORE Exams 3 times annually in 2-week windows (Winter [February], Summer [July], and Fall [October/November]), the APPLIED Exam is only given once per year. For the best chance of getting your preferred date, be sure to register as early as possible (especially if you live and train in a city with limited testing sites).
- After you have successfully passed your first CORE Exam module, you may take up to 3 in one sitting. When taking multiple modules consecutively on the same day, a 15-minute break is configured between each module.
Study Resources
When it comes to studying, there are more resources available than you will have time to explore; therefore, it is crucial to prioritize the ones that best match your learning style. Whether you retain information through visuals, audio, reading comprehension, practice questions, or spaced repetition, there are complimentary and paid high-yield tools designed to support how you learn and make the most of your valuable time outside of clinical responsibilities (Table). Furthermore, there are numerous discipline-specific textbooks and resources encompassing dermatopathology, dermoscopy, trichology, pediatric dermatology, surgical dermatology, cosmetic dermatology, and skin of color.11-13 As a trainee, you also have access to the American Academy of Dermatology’s Learning Center (https://learning.aad.org/Catalogue/AAD-Learning-Center) featuring the Question of the Week series, Board Prep Plus question bank, Dialogues in Dermatology podcast, and continuing medical education articles. Additionally, board review sessions occur at many local, regional, and national dermatology conferences annually.


Exam Preparation Strategy
A comprehensive preparation strategy should begin during your first year of residency and appropriately intensify in the months leading up to the BASIC, CORE, and APPLIED Exams. Ultimately, active learning is ongoing, and your daily clinical work combined with program-sanctioned didactics, journal reading, and conference attendance comprise your framework. I often found it helpful to spend 30 to 60 minutes after clinic each evening reviewing high-yield or interesting cases from the day, as our patients are our greatest teachers. To reinforce key concepts, I used a combination of premade Anki decks14 and custom flashcards for topics that required rote memorization and spaced repetition. Podcasts such as Cutaneous Miscellaneous, The Grenz Zone, and Dermasphere became valuable learning tools that I incorporated into my commutes and long runs. I also enjoyed listening to the Derm In-Review audio study guide.19 Early in residency, I also created a digital notebook on OneNote (https://onenote.cloud.microsoft/en-us/)—organized by postgraduate year and subject—to consolidate notes and procedural pearls. As a fellow, I still use this note-taking system to organize notes from laser and energy-based device trainings and catalogue high-yield conference takeaways. Finally, task management applications can further help you achieve your study goals by organizing assignments, setting deadlines, and breaking larger objectives into manageable steps, making it easier to stay focused and on track.
Test Day Strategies
After sitting for many standardized examinations on the journey to dermatology residency, I am certain that you have cultivated your own reliable test day rituals and strategies; however, if you are looking for additional ones to add to your toolbox, here are a few that helped me stay calm, focused, and in the zone throughout my time in residency.
The Day Before the Test
- Secure your test-day snacks and preferred form of hydration. I am a fan of cheese sticks for protein and fruit for vitamins and antioxidants. Additionally, I always bring something salty and something sweet (usually chocolate or sour gummy snacks) just in case I happen to get a specific craving on test day.
- Make sure you have valid forms of identification in accordance with the test center policy.16
- Confirm your exam location and time. Testing center details can be found on the Pearson Vue portal,16 which is easily accessed via the “ABD Tools” tab on the official ABD website (https://www.abderm.org/). Additionally, the exam location, time, and directions to the test center are located in your Pearson Vue confirmation email.
- Trust that you are prepared. Try your best to avoid last-minute cramming and prioritize a good night’s sleep.
The Day of the Test
- Center yourself before the exam. I prefer to start my morning with a run to clear my mind; however, you can also consider other mindfulness exercises such as deep breathing or positive grounding affirmations.
- Arrive early and dress in layers. You never know if the testing location will run warm or cold.
- Pace yourself, trust your gut instincts, and do not be afraid to mark and move on if you get stuck on a particular question. Ultimately, make sure you answer every question, as you will not have points deducted for guessing.
- Make sure to plan something you are excited about for after the exam! That may mean celebrating with co-residents, spending time with loved ones, or just relaxing on the couch and finally catching up on that show you have been meaning to watch for weeks but have not had time for because you have been focused on studying (yes, we all have that one show).
Final Thoughts
While this article is not comprehensive of all ABD Certification Pathway preparation materials and resources, I hope that you will find it helpful along your residency journey. Starting dermatology residency can feel like drinking from a firehose: there is an overwhelming volume of new information, unfamiliar terminology, and a demanding workflow that varies considerably from that of intern year.17 As a resident, it is vital to prioritize your mental health and well-being, as the journey is a marathon rather than a sprint.18
Never forget that you have already come this far; trust in your journey and remember what is meant for you will not miss you. Juggling 6 exams during residency alongside clinical and personal responsibilities is no small feat. With a strong study plan and smart test-day strategies, I have no doubt you will become a board-certified dermatologist!
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
- ABD certification pathway info center. Accessed October 1, 2025. https://www.abderm.org/residents-and-fellows/abd-certification-pathway/abd-certification-pathway-info-center
- American Board of Dermatology. General exam information. Accessed January 13, 2026. https://www.abderm.org/exams/general-exam-information
- James WD, Elston DM, Treat JR, et al, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2020.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Nelson KC, Cerroni L, Schaffer JV, eds. Dermatology: Comprehensive Board Review and Practice Examinations. 2nd ed. Elsevier; 2019.
- Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology Essentials. 2nd ed. Elsevier; 2023.
- Saavedra AP, Kang S, Amagai M, et al, eds. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 9th ed. McGraw Hill; 2023.
- Kang S, Amagai M, Bruckner AL, et al, eds. Fitzpatrick’s Dermatology. 9th ed. McGraw Hill; 2019.
- Alikhan A, Hocker TL, eds. Review of Dermatology. Elsevier; 2017.
- Leventhal JS, Levy LL. Self-Assessment in Dermatology: Questions and Answers. 2nd ed. Elsevier; 2024.
- Association of Academic Cosmetic Dermatology. Resources for dermatology residents. Accessed October 15, 2025. https://theaacd.org/resident-resources/
- Mukosera GT, Ibraheim MK, Lee MP, et al. From scope to screen: a collection of online dermatopathology resources for residents and fellows. JAAD Int. 2023;12:12-14. doi:10.1016/j.jdin.2022.12.007
- Shabeeb N. Dermatology resident education for skin of color. Cutis. 2020;106:E18-E20. doi:10.12788/cutis.0099
- Azhar AF. Review of 3 comprehensive Anki flash card decks for dermatology residents. Cutis. 2023;112:E10-E12. doi:10.12788/cutis.0813
- ODAC Dermatology. Derm In-Review. Accessed October 22, 2025. https://dermatologyinreview.com/odac/
- American Board of Dermatology (ABD) certification testing with Pearson VUE. Accessed October 19, 2025. https://www.pearsonvue.com/us/en/abd.html
- Lim YH. Transitioning from an intern to a dermatology resident. Cutis. 2022;110:E14-E16. doi:10.12788/cutis.0638
- Lim YH. Prioritizing mental health in residency. Cutis. 2022;109:E36-E38. doi:10.12788/cutis.0551
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Dermatology Boards Demystified: Conquer the BASIC, CORE, and APPLIED Exams
Practice Points
- To become a board-certified dermatologist, one must complete the American Board of Dermatology Certification Pathway—a staged evaluation beginning with a BASIC Exam for first-year residents, followed by 4 CORE Exam modules and a final APPLIED Exam following residency completion.
- When it comes to studying, there are more resources available than you will have time to explore fully. With so many options available, it is crucial to prioritize the ones that best match your learning style.
- A comprehensive study strategy begins during your first year of residency and appropriately intensifies in the months leading up to the exams. Make sure to cultivate test day strategies to help you stay calm, focused, and in the zone.
Comprehensive Patch Testing: An Essential Tool for Care of Allergic Contact Dermatitis
Comprehensive Patch Testing: An Essential Tool for Care of Allergic Contact Dermatitis
Allergic contact dermatitis (ACD) is a common skin condition affecting approximately 20% of the general population in the United States.1 Allergic contact dermatitis is a unique disease in that there is an opportunity for complete cure through allergen avoidance; however, this requires proper identification of the offending allergen. When the culprit allergen is not identified or removed from the patient’s environment, chronic ACD can develop, leading to persistent inflammation and related symptoms, reduced quality of life, and greater economic burden for patients and the health care system.2,3
Patch testing (PT) is the only available diagnostic test for ACD, allowing for identification and subsequent avoidance of contact allergens. Patch testing involves applying allergens—typically chemicals that can be found in personal care products—onto the skin for 48 hours. Delayed readings are completed 72 to 168 hours after application. Interpretation of relevance and patient counseling, with resultant allergen avoidance, are required for a successful patient experience. Patch testing is considered safe in tested populations; rare risks associated with PT include active sensitization and anaphylaxis.4
There are many screening series available, with the number of screening allergens ranging from 35 (T.R.U.E. [Thin-Layer Rapid Use Epicutaneous] test) to 90 (American Contact Dermatitis Society [ACDS] Core series). Comprehensive PT generally refers to the completion of PT for all potentially relevant and testable allergens for a given patient, which typically involves testing beyond a screening series. Currently in the United States, comprehensive PT typically includes testing for 80 to 90 allergens and any additional potentially relevant allergens based on the clinical history and patient exposures. A 2018 survey noted that, of 149 ACDS members, 82% always used a baseline screening series for PT, with 62% of these routinely testing 80 allergens and 18% routinely testing 70 allergens.5 Additionally, nearly 70% always or sometimes tested with supplemental or additional series. In other words, advanced patch testers were routinely testing 70 to 80 allergens in their screening series, and most were testing additional allergens to ensure the best care for their patients.
To account for emerging allergens, accommodate changes in allergen test concentrations recommended by ACDS and the North American Contact Dermatitis Group (NACDG), and address the need for comprehensive PT for most patients, recommended screening series are regularly updated by patch test societies and expert panels such as the ACDS and the NACDG. When the ACDS Core series6 was introduced in 2013, it consisted of 80 recommended allergens.7 The panel was updated in 20178 and again in 2020,6 most recently with 90 allergens. The NACDG has collected patch test data since at least 19929 and revisits their recommended screening series on a 2-year cycle, evaluating test concentrations and adding and removing allergens based on allergen trends, allergen performance, patient need, and emergence of new allergens; the current NACDG series consists of 80 allergens. This article illustrates the clinical and public health value of comprehensive PT and the vital role of allergen access in the comprehensive patch test process, with the ultimate goal of optimizing care for patients with ACD.
Value of Comprehensive Patch Testing for ACD
Early PT represents the most cost-effective approach to the diagnosis and management of ACD. Lack of access to PT can lead to delayed diagnosis, resulting in continued exposure to the offending allergen, disease chronicity, and ultimately worse quality-of-life scores compared with patients who are diagnosed early.10 Earlier diagnosis also can minimize costs by avoiding unnecessary treatments. Without access to comprehensive PT, patients could potentially be erroneously diagnosed with atopic dermatitis and subsequently treated with expensive biologic therapies (eg, dupilumab, which costs approximately $4000 per dose or $104,000 per year11), when allergen avoidance would have been curative with minimal cost. The continued value of comprehensive PT, especially in the era of the atopic dermatitis therapeutic revolution, cannot be more strongly emphasized.
Among 140 patients with ACD, 87% found PT useful, 91% were able to avoid allergens, and 57% noted improvement or resolution of their dermatitis after avoidance of identified allergens.12 A multicenter prospective observational study demonstrated that PT improved dermatology-specific quality of life and reduced resources used for patients with ACD compared to non–patch tested individuals.13 Another study found that patients with ACD who underwent PT and were confirmed as having relevant positive contact allergens showed improvement in both perceived eczema severity and Dermatology Life Quality Index scores just 2 months after testing.14 This effect is attributed to the identification and subsequent avoidance of clinically relevant contact allergens. In a study of 519 patients with dermatitis, Dermatology Life Quality Index scores improved significantly after PT regardless of whether the results were positive or negative, indicating benefits for the care and treatment of dermatitis, even in the setting of negative patch test results (P< .001).15 This could because they were still counseled on gentle skin care and management of their dermatitis at the PT visit. Improvements in disease severity also have been observed in adults and children after PT, with most patients having partial to complete clearance of their dermatitis.16,17 This is not surprising, as comprehensive PT allows clinicians to diagnose the cause of ACD by finding the exact allergen triggering the eruption and then guide patients through avoidance of these allergens to eventually clear their dermatitis.
Comprehensive Patch Testing Captures Allergen Trends
Dermatologists who perform PT in the United States currently have access to a diverse array of allergens, with more than 500 different allergens available. Access to and utilization of these allergens are essential for the comprehensive evaluation needed for our patients.
Comprehensive PT has uncovered emerging allergens such as dimethyl fumarate, the potent cause of sofa dermatitis18; isobornyl acrylate, which is found in wearable diabetic monitors19; and acetophenone azine, which can cause shin guard ACD in athletes.20 Increasing prevalence of ACD to these allergens would not have been identified without provider access to PT. Patch testing also has identified emerging allergen trends, such as the methylisothiazolinone allergy epidemic.21 All of these emerging allergens, identified through PT, have been named Contact Allergen of the Year by the ACDS due to their newfound relevance.18-20
In contrast, allergen prevalence can decrease over time, leading to removal from screening panels; examples include methyldibromo glutaronitrile, which is no longer widely present in consumer products, and thimerosal, which has frequent positive results but low relevance due to its infrequent use in personal care products. In response to comprehensive PT studies, allergen concentrations may be modified, as in the case of formaldehyde, which has notable irritant potential at higher tested concentrations but remains on the ACDS Core Allergen Series with a test concentration that optimizes the number of true positive reactions while decreasing irritant reactions.6 Likewise, nickel sulfate test concentrations were increased in the NACDG screening series due to evidence that testing at 5% identifies more nickel contact allergy than testing at 2.5% without considerably increasing irritant reactions.22
Allergen Choice and Flexibility are Key to Optimal Screening
Dermatologists who perform PT usually choose their screening series based on expert consensus and recommendations.6,23 Additional test allergens for comprehensive PT typically are chosen based on patient exposures, regional trends, and clinical expertise. This flexibility traditionally has allowed for the opportunity to identify culprit allergens that are relevant for the individual patient; for example, a hairdresser may have daily exposure to resorcinol, whereas a massage therapist may have regular exposure to essential oils. Testing only a standard screening series may miss the culprit allergen for both patients. For optimal patient outcomes, allergen choice and flexibility are key.
Currently, the 35-allergen T.R.U.E. test is the only US Food and Drug Administration–approved patch test; however, multiple studies have shown that comprehensive PT, including supplemental allergens, considerably improves the diagnostic yield and clinical outcomes in ACD. A 6-year retrospective study found that using an extended screening series identified an additional 10.8% of patients (n=585) with positive tests who were negative to the T.R.U.E. test.24 Patch testing with the T.R.U.E. test alone would miss almost half of the positive reactions detected by the NACDG 80-panel screening series. Furthermore, an additional 21.1% of 3056 tested patients had at least one relevant reaction to a supplemental allergen that was not present in the NACDG screening series.23 In a retrospective study of 791 patients patch tested with the NACDG screening series and 2 supplemental series, 19.5% and 12.1% of patients, respectively, had positive reactions to supplemental allergens.25 This reinforces the importance of comprehensive PT beyond a more limited screening series. Testing more allergens identifies more causative allergens for patients.
Changes in Utilization May Affect Patient Care
Recent data have shown a shift in patch test utilization. An analysis of Medicare Part B fee-for-service claims for PT between 2010 and 2018 demonstrated that an increase in patch test utilization during this period was driven mainly by nonphysician providers and allergists.26 From 2012 to 2017, the number of patients patch tested by allergists grew by 20.3% compared to only 1.84% for dermatologists.27 Since dupilumab was approved in 2017 for the management of moderate to severe atopic dermatitis, claims data from 2017 to 2022 showed an exponential increase in its utilization, while patch test utilization has markedly decreased.28
Dermatologists are the predominant experts in ACD, but these concerning trends suggest decreasing utilization of PT by dermatologists, possibly due to lack of required residency training in PT, cost of patch test allergens and supplies with corresponding static reimbursement rates, staff time and training required for an excellent PT experience, comparative ease of biologic prescription vs the time-intensive process of comprehensive PT, and perceived high barrier of entry into PT. This may limit patient access to high-quality comprehensive PT and more importantly, a chance for our patients to experience resolution of their skin disease.
Final Thoughts
Comprehensive PT is safe, effective, and readily available. Unfettered access to a wide range of allergens improves diagnostic accuracy and quality of life and reduces economic burden from sick leave, job loss, and treatment costs. Patch testing remains the one and only way to identify causative allergens for patients with ACD, and comprehensive PT is the most ideal approach for excellent patient care.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.
- Weidinger S, Novak N. Hand eczema. Lancet. 2024;404:2476-2486.
- Garg V, Brod B, Gaspari AA. Patch testing: uses, systems, risks/benefits, and its role in managing the patient with contact dermatitis. Clin Dermatol. 2021;39:580-590.
- Rodriguez-Homs LG, Taylor J, Liu B, et al. Patch test practice patterns of members of the American Contact Dermatitis Society. Dermatitis. 2020;31:272-275.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series. Dermatitis. 2013;24:7-9.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 Update. Dermatitis. 2017;28:141-143.
- Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group standard tray patch test results (1992 to 1994). Am J Contact Dermat. 1995;6:160-165.
- Kadyk DL, McCarter K, Achen F, et al. Quality of life in patients with allergic contact dermatitis. J Am Acad Dermatol. 2003;49:1037-1048.
- Dupixent® (dupilumab): pricing and insurance. Sanofi US. Updated June 2025. Accessed January 9, 2026. https://www.dupixent.com/support-savings/cost-insurance
- Woo PN, Hay IC, Ormerod AD. An audit of the value of patch testing and its effect on quality of life. Contact Dermatitis. 2003;48:244-247.
- Rajagopalan R, Anderson R. Impact of patch testing on dermatology-specific quality of life in patients with allergic contact dermatitis. Am J Contact Dermat. 1997;8:215-221.
- Thomson KF, Wilkinson SM, Sommer S, et al. Eczema: quality of life by body site and the effect of patch testing. Br J Dermatol. 2002;146:627-630.
- Boonchai W, Charoenpipatsin N, Winayanuwattikun W, et al. Assessment of the quality of life (QoL) of patients with dermatitis and the impact of patch testing on QoL: a study of 519 patients diagnosed with dermatitis. Contact Dermatitis. 2020;83:182-188.
- Johnson H, Rao M, Yu J. Improved or not improved, that is the question: patch testing outcomes from the Massachusetts General Hospital. Contact Dermatitis. 2024;90:324-327.
- George SE, Yu J. Patch testing outcomes in children at the Massachusetts General Hospital. J Am Acad Dermatol. 2024;91:354-356.
- McNamara D. Dimethyl fumarate named 2011 allergen of the year.Int Med News. February 3, 2011. Accessed January 9, 2026. https://www.mdedge.com/internalmedicine/article/20401/dermatology/dimethyl-fumarate-named-2011-allergen-year
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Societyallergen of the year. Cutis. 2020;105:283-285.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Svedman C, Ale I, Goh CL, et al. Patch testing with nickel sulfate 5.0% traces significantly more contact allergy than 2.5%: a prospective study within the International Contact Dermatitis Research Group. Dermatitis. 2022;33:417-420.
- Houle MC, DeKoven JG, Atwater AR, et al. North American Contact Dermatitis Group Patch Test Results: 2021-2022. Dermatitis. 2025;36:464-476.
- Sundquist BK, Yang B, Pasha MA. Experience in patch testing: a 6-year retrospective review from a single academic allergy practice. Ann Allergy Asthma Immunol. 2019;122:502-507.
- Atwater AR, Liu B, Walsh R, et al. Supplemental patch testing identifies allergens missed by standard screening series. Dermatitis. 2024;35:366-372.
- Ravishankar A, Freese RL, Parsons HM, et al. Trends in patch testing in the Medicare Part B fee-for-service population. Dermatitis. 2022;33:129-134.
- Cheraghlou S, Watsky KL, Cohen JM. Utilization, cost, and provider trends in patch testing among Medicare beneficiaries in the United States from 2012 to 2017. J Am Acad Dermatol. 2021;85:1218-1226.
- Santiago Mangual KP, Rau A, Grant-Kels JM, et al. Increasing use of dupilumab and decreasing use of patch testing in medicare patients from 2017 to 2022: a claims database study. Dermatitis. 2025;36:538-540.
Allergic contact dermatitis (ACD) is a common skin condition affecting approximately 20% of the general population in the United States.1 Allergic contact dermatitis is a unique disease in that there is an opportunity for complete cure through allergen avoidance; however, this requires proper identification of the offending allergen. When the culprit allergen is not identified or removed from the patient’s environment, chronic ACD can develop, leading to persistent inflammation and related symptoms, reduced quality of life, and greater economic burden for patients and the health care system.2,3
Patch testing (PT) is the only available diagnostic test for ACD, allowing for identification and subsequent avoidance of contact allergens. Patch testing involves applying allergens—typically chemicals that can be found in personal care products—onto the skin for 48 hours. Delayed readings are completed 72 to 168 hours after application. Interpretation of relevance and patient counseling, with resultant allergen avoidance, are required for a successful patient experience. Patch testing is considered safe in tested populations; rare risks associated with PT include active sensitization and anaphylaxis.4
There are many screening series available, with the number of screening allergens ranging from 35 (T.R.U.E. [Thin-Layer Rapid Use Epicutaneous] test) to 90 (American Contact Dermatitis Society [ACDS] Core series). Comprehensive PT generally refers to the completion of PT for all potentially relevant and testable allergens for a given patient, which typically involves testing beyond a screening series. Currently in the United States, comprehensive PT typically includes testing for 80 to 90 allergens and any additional potentially relevant allergens based on the clinical history and patient exposures. A 2018 survey noted that, of 149 ACDS members, 82% always used a baseline screening series for PT, with 62% of these routinely testing 80 allergens and 18% routinely testing 70 allergens.5 Additionally, nearly 70% always or sometimes tested with supplemental or additional series. In other words, advanced patch testers were routinely testing 70 to 80 allergens in their screening series, and most were testing additional allergens to ensure the best care for their patients.
To account for emerging allergens, accommodate changes in allergen test concentrations recommended by ACDS and the North American Contact Dermatitis Group (NACDG), and address the need for comprehensive PT for most patients, recommended screening series are regularly updated by patch test societies and expert panels such as the ACDS and the NACDG. When the ACDS Core series6 was introduced in 2013, it consisted of 80 recommended allergens.7 The panel was updated in 20178 and again in 2020,6 most recently with 90 allergens. The NACDG has collected patch test data since at least 19929 and revisits their recommended screening series on a 2-year cycle, evaluating test concentrations and adding and removing allergens based on allergen trends, allergen performance, patient need, and emergence of new allergens; the current NACDG series consists of 80 allergens. This article illustrates the clinical and public health value of comprehensive PT and the vital role of allergen access in the comprehensive patch test process, with the ultimate goal of optimizing care for patients with ACD.
Value of Comprehensive Patch Testing for ACD
Early PT represents the most cost-effective approach to the diagnosis and management of ACD. Lack of access to PT can lead to delayed diagnosis, resulting in continued exposure to the offending allergen, disease chronicity, and ultimately worse quality-of-life scores compared with patients who are diagnosed early.10 Earlier diagnosis also can minimize costs by avoiding unnecessary treatments. Without access to comprehensive PT, patients could potentially be erroneously diagnosed with atopic dermatitis and subsequently treated with expensive biologic therapies (eg, dupilumab, which costs approximately $4000 per dose or $104,000 per year11), when allergen avoidance would have been curative with minimal cost. The continued value of comprehensive PT, especially in the era of the atopic dermatitis therapeutic revolution, cannot be more strongly emphasized.
Among 140 patients with ACD, 87% found PT useful, 91% were able to avoid allergens, and 57% noted improvement or resolution of their dermatitis after avoidance of identified allergens.12 A multicenter prospective observational study demonstrated that PT improved dermatology-specific quality of life and reduced resources used for patients with ACD compared to non–patch tested individuals.13 Another study found that patients with ACD who underwent PT and were confirmed as having relevant positive contact allergens showed improvement in both perceived eczema severity and Dermatology Life Quality Index scores just 2 months after testing.14 This effect is attributed to the identification and subsequent avoidance of clinically relevant contact allergens. In a study of 519 patients with dermatitis, Dermatology Life Quality Index scores improved significantly after PT regardless of whether the results were positive or negative, indicating benefits for the care and treatment of dermatitis, even in the setting of negative patch test results (P< .001).15 This could because they were still counseled on gentle skin care and management of their dermatitis at the PT visit. Improvements in disease severity also have been observed in adults and children after PT, with most patients having partial to complete clearance of their dermatitis.16,17 This is not surprising, as comprehensive PT allows clinicians to diagnose the cause of ACD by finding the exact allergen triggering the eruption and then guide patients through avoidance of these allergens to eventually clear their dermatitis.
Comprehensive Patch Testing Captures Allergen Trends
Dermatologists who perform PT in the United States currently have access to a diverse array of allergens, with more than 500 different allergens available. Access to and utilization of these allergens are essential for the comprehensive evaluation needed for our patients.
Comprehensive PT has uncovered emerging allergens such as dimethyl fumarate, the potent cause of sofa dermatitis18; isobornyl acrylate, which is found in wearable diabetic monitors19; and acetophenone azine, which can cause shin guard ACD in athletes.20 Increasing prevalence of ACD to these allergens would not have been identified without provider access to PT. Patch testing also has identified emerging allergen trends, such as the methylisothiazolinone allergy epidemic.21 All of these emerging allergens, identified through PT, have been named Contact Allergen of the Year by the ACDS due to their newfound relevance.18-20
In contrast, allergen prevalence can decrease over time, leading to removal from screening panels; examples include methyldibromo glutaronitrile, which is no longer widely present in consumer products, and thimerosal, which has frequent positive results but low relevance due to its infrequent use in personal care products. In response to comprehensive PT studies, allergen concentrations may be modified, as in the case of formaldehyde, which has notable irritant potential at higher tested concentrations but remains on the ACDS Core Allergen Series with a test concentration that optimizes the number of true positive reactions while decreasing irritant reactions.6 Likewise, nickel sulfate test concentrations were increased in the NACDG screening series due to evidence that testing at 5% identifies more nickel contact allergy than testing at 2.5% without considerably increasing irritant reactions.22
Allergen Choice and Flexibility are Key to Optimal Screening
Dermatologists who perform PT usually choose their screening series based on expert consensus and recommendations.6,23 Additional test allergens for comprehensive PT typically are chosen based on patient exposures, regional trends, and clinical expertise. This flexibility traditionally has allowed for the opportunity to identify culprit allergens that are relevant for the individual patient; for example, a hairdresser may have daily exposure to resorcinol, whereas a massage therapist may have regular exposure to essential oils. Testing only a standard screening series may miss the culprit allergen for both patients. For optimal patient outcomes, allergen choice and flexibility are key.
Currently, the 35-allergen T.R.U.E. test is the only US Food and Drug Administration–approved patch test; however, multiple studies have shown that comprehensive PT, including supplemental allergens, considerably improves the diagnostic yield and clinical outcomes in ACD. A 6-year retrospective study found that using an extended screening series identified an additional 10.8% of patients (n=585) with positive tests who were negative to the T.R.U.E. test.24 Patch testing with the T.R.U.E. test alone would miss almost half of the positive reactions detected by the NACDG 80-panel screening series. Furthermore, an additional 21.1% of 3056 tested patients had at least one relevant reaction to a supplemental allergen that was not present in the NACDG screening series.23 In a retrospective study of 791 patients patch tested with the NACDG screening series and 2 supplemental series, 19.5% and 12.1% of patients, respectively, had positive reactions to supplemental allergens.25 This reinforces the importance of comprehensive PT beyond a more limited screening series. Testing more allergens identifies more causative allergens for patients.
Changes in Utilization May Affect Patient Care
Recent data have shown a shift in patch test utilization. An analysis of Medicare Part B fee-for-service claims for PT between 2010 and 2018 demonstrated that an increase in patch test utilization during this period was driven mainly by nonphysician providers and allergists.26 From 2012 to 2017, the number of patients patch tested by allergists grew by 20.3% compared to only 1.84% for dermatologists.27 Since dupilumab was approved in 2017 for the management of moderate to severe atopic dermatitis, claims data from 2017 to 2022 showed an exponential increase in its utilization, while patch test utilization has markedly decreased.28
Dermatologists are the predominant experts in ACD, but these concerning trends suggest decreasing utilization of PT by dermatologists, possibly due to lack of required residency training in PT, cost of patch test allergens and supplies with corresponding static reimbursement rates, staff time and training required for an excellent PT experience, comparative ease of biologic prescription vs the time-intensive process of comprehensive PT, and perceived high barrier of entry into PT. This may limit patient access to high-quality comprehensive PT and more importantly, a chance for our patients to experience resolution of their skin disease.
Final Thoughts
Comprehensive PT is safe, effective, and readily available. Unfettered access to a wide range of allergens improves diagnostic accuracy and quality of life and reduces economic burden from sick leave, job loss, and treatment costs. Patch testing remains the one and only way to identify causative allergens for patients with ACD, and comprehensive PT is the most ideal approach for excellent patient care.
Allergic contact dermatitis (ACD) is a common skin condition affecting approximately 20% of the general population in the United States.1 Allergic contact dermatitis is a unique disease in that there is an opportunity for complete cure through allergen avoidance; however, this requires proper identification of the offending allergen. When the culprit allergen is not identified or removed from the patient’s environment, chronic ACD can develop, leading to persistent inflammation and related symptoms, reduced quality of life, and greater economic burden for patients and the health care system.2,3
Patch testing (PT) is the only available diagnostic test for ACD, allowing for identification and subsequent avoidance of contact allergens. Patch testing involves applying allergens—typically chemicals that can be found in personal care products—onto the skin for 48 hours. Delayed readings are completed 72 to 168 hours after application. Interpretation of relevance and patient counseling, with resultant allergen avoidance, are required for a successful patient experience. Patch testing is considered safe in tested populations; rare risks associated with PT include active sensitization and anaphylaxis.4
There are many screening series available, with the number of screening allergens ranging from 35 (T.R.U.E. [Thin-Layer Rapid Use Epicutaneous] test) to 90 (American Contact Dermatitis Society [ACDS] Core series). Comprehensive PT generally refers to the completion of PT for all potentially relevant and testable allergens for a given patient, which typically involves testing beyond a screening series. Currently in the United States, comprehensive PT typically includes testing for 80 to 90 allergens and any additional potentially relevant allergens based on the clinical history and patient exposures. A 2018 survey noted that, of 149 ACDS members, 82% always used a baseline screening series for PT, with 62% of these routinely testing 80 allergens and 18% routinely testing 70 allergens.5 Additionally, nearly 70% always or sometimes tested with supplemental or additional series. In other words, advanced patch testers were routinely testing 70 to 80 allergens in their screening series, and most were testing additional allergens to ensure the best care for their patients.
To account for emerging allergens, accommodate changes in allergen test concentrations recommended by ACDS and the North American Contact Dermatitis Group (NACDG), and address the need for comprehensive PT for most patients, recommended screening series are regularly updated by patch test societies and expert panels such as the ACDS and the NACDG. When the ACDS Core series6 was introduced in 2013, it consisted of 80 recommended allergens.7 The panel was updated in 20178 and again in 2020,6 most recently with 90 allergens. The NACDG has collected patch test data since at least 19929 and revisits their recommended screening series on a 2-year cycle, evaluating test concentrations and adding and removing allergens based on allergen trends, allergen performance, patient need, and emergence of new allergens; the current NACDG series consists of 80 allergens. This article illustrates the clinical and public health value of comprehensive PT and the vital role of allergen access in the comprehensive patch test process, with the ultimate goal of optimizing care for patients with ACD.
Value of Comprehensive Patch Testing for ACD
Early PT represents the most cost-effective approach to the diagnosis and management of ACD. Lack of access to PT can lead to delayed diagnosis, resulting in continued exposure to the offending allergen, disease chronicity, and ultimately worse quality-of-life scores compared with patients who are diagnosed early.10 Earlier diagnosis also can minimize costs by avoiding unnecessary treatments. Without access to comprehensive PT, patients could potentially be erroneously diagnosed with atopic dermatitis and subsequently treated with expensive biologic therapies (eg, dupilumab, which costs approximately $4000 per dose or $104,000 per year11), when allergen avoidance would have been curative with minimal cost. The continued value of comprehensive PT, especially in the era of the atopic dermatitis therapeutic revolution, cannot be more strongly emphasized.
Among 140 patients with ACD, 87% found PT useful, 91% were able to avoid allergens, and 57% noted improvement or resolution of their dermatitis after avoidance of identified allergens.12 A multicenter prospective observational study demonstrated that PT improved dermatology-specific quality of life and reduced resources used for patients with ACD compared to non–patch tested individuals.13 Another study found that patients with ACD who underwent PT and were confirmed as having relevant positive contact allergens showed improvement in both perceived eczema severity and Dermatology Life Quality Index scores just 2 months after testing.14 This effect is attributed to the identification and subsequent avoidance of clinically relevant contact allergens. In a study of 519 patients with dermatitis, Dermatology Life Quality Index scores improved significantly after PT regardless of whether the results were positive or negative, indicating benefits for the care and treatment of dermatitis, even in the setting of negative patch test results (P< .001).15 This could because they were still counseled on gentle skin care and management of their dermatitis at the PT visit. Improvements in disease severity also have been observed in adults and children after PT, with most patients having partial to complete clearance of their dermatitis.16,17 This is not surprising, as comprehensive PT allows clinicians to diagnose the cause of ACD by finding the exact allergen triggering the eruption and then guide patients through avoidance of these allergens to eventually clear their dermatitis.
Comprehensive Patch Testing Captures Allergen Trends
Dermatologists who perform PT in the United States currently have access to a diverse array of allergens, with more than 500 different allergens available. Access to and utilization of these allergens are essential for the comprehensive evaluation needed for our patients.
Comprehensive PT has uncovered emerging allergens such as dimethyl fumarate, the potent cause of sofa dermatitis18; isobornyl acrylate, which is found in wearable diabetic monitors19; and acetophenone azine, which can cause shin guard ACD in athletes.20 Increasing prevalence of ACD to these allergens would not have been identified without provider access to PT. Patch testing also has identified emerging allergen trends, such as the methylisothiazolinone allergy epidemic.21 All of these emerging allergens, identified through PT, have been named Contact Allergen of the Year by the ACDS due to their newfound relevance.18-20
In contrast, allergen prevalence can decrease over time, leading to removal from screening panels; examples include methyldibromo glutaronitrile, which is no longer widely present in consumer products, and thimerosal, which has frequent positive results but low relevance due to its infrequent use in personal care products. In response to comprehensive PT studies, allergen concentrations may be modified, as in the case of formaldehyde, which has notable irritant potential at higher tested concentrations but remains on the ACDS Core Allergen Series with a test concentration that optimizes the number of true positive reactions while decreasing irritant reactions.6 Likewise, nickel sulfate test concentrations were increased in the NACDG screening series due to evidence that testing at 5% identifies more nickel contact allergy than testing at 2.5% without considerably increasing irritant reactions.22
Allergen Choice and Flexibility are Key to Optimal Screening
Dermatologists who perform PT usually choose their screening series based on expert consensus and recommendations.6,23 Additional test allergens for comprehensive PT typically are chosen based on patient exposures, regional trends, and clinical expertise. This flexibility traditionally has allowed for the opportunity to identify culprit allergens that are relevant for the individual patient; for example, a hairdresser may have daily exposure to resorcinol, whereas a massage therapist may have regular exposure to essential oils. Testing only a standard screening series may miss the culprit allergen for both patients. For optimal patient outcomes, allergen choice and flexibility are key.
Currently, the 35-allergen T.R.U.E. test is the only US Food and Drug Administration–approved patch test; however, multiple studies have shown that comprehensive PT, including supplemental allergens, considerably improves the diagnostic yield and clinical outcomes in ACD. A 6-year retrospective study found that using an extended screening series identified an additional 10.8% of patients (n=585) with positive tests who were negative to the T.R.U.E. test.24 Patch testing with the T.R.U.E. test alone would miss almost half of the positive reactions detected by the NACDG 80-panel screening series. Furthermore, an additional 21.1% of 3056 tested patients had at least one relevant reaction to a supplemental allergen that was not present in the NACDG screening series.23 In a retrospective study of 791 patients patch tested with the NACDG screening series and 2 supplemental series, 19.5% and 12.1% of patients, respectively, had positive reactions to supplemental allergens.25 This reinforces the importance of comprehensive PT beyond a more limited screening series. Testing more allergens identifies more causative allergens for patients.
Changes in Utilization May Affect Patient Care
Recent data have shown a shift in patch test utilization. An analysis of Medicare Part B fee-for-service claims for PT between 2010 and 2018 demonstrated that an increase in patch test utilization during this period was driven mainly by nonphysician providers and allergists.26 From 2012 to 2017, the number of patients patch tested by allergists grew by 20.3% compared to only 1.84% for dermatologists.27 Since dupilumab was approved in 2017 for the management of moderate to severe atopic dermatitis, claims data from 2017 to 2022 showed an exponential increase in its utilization, while patch test utilization has markedly decreased.28
Dermatologists are the predominant experts in ACD, but these concerning trends suggest decreasing utilization of PT by dermatologists, possibly due to lack of required residency training in PT, cost of patch test allergens and supplies with corresponding static reimbursement rates, staff time and training required for an excellent PT experience, comparative ease of biologic prescription vs the time-intensive process of comprehensive PT, and perceived high barrier of entry into PT. This may limit patient access to high-quality comprehensive PT and more importantly, a chance for our patients to experience resolution of their skin disease.
Final Thoughts
Comprehensive PT is safe, effective, and readily available. Unfettered access to a wide range of allergens improves diagnostic accuracy and quality of life and reduces economic burden from sick leave, job loss, and treatment costs. Patch testing remains the one and only way to identify causative allergens for patients with ACD, and comprehensive PT is the most ideal approach for excellent patient care.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.
- Weidinger S, Novak N. Hand eczema. Lancet. 2024;404:2476-2486.
- Garg V, Brod B, Gaspari AA. Patch testing: uses, systems, risks/benefits, and its role in managing the patient with contact dermatitis. Clin Dermatol. 2021;39:580-590.
- Rodriguez-Homs LG, Taylor J, Liu B, et al. Patch test practice patterns of members of the American Contact Dermatitis Society. Dermatitis. 2020;31:272-275.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series. Dermatitis. 2013;24:7-9.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 Update. Dermatitis. 2017;28:141-143.
- Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group standard tray patch test results (1992 to 1994). Am J Contact Dermat. 1995;6:160-165.
- Kadyk DL, McCarter K, Achen F, et al. Quality of life in patients with allergic contact dermatitis. J Am Acad Dermatol. 2003;49:1037-1048.
- Dupixent® (dupilumab): pricing and insurance. Sanofi US. Updated June 2025. Accessed January 9, 2026. https://www.dupixent.com/support-savings/cost-insurance
- Woo PN, Hay IC, Ormerod AD. An audit of the value of patch testing and its effect on quality of life. Contact Dermatitis. 2003;48:244-247.
- Rajagopalan R, Anderson R. Impact of patch testing on dermatology-specific quality of life in patients with allergic contact dermatitis. Am J Contact Dermat. 1997;8:215-221.
- Thomson KF, Wilkinson SM, Sommer S, et al. Eczema: quality of life by body site and the effect of patch testing. Br J Dermatol. 2002;146:627-630.
- Boonchai W, Charoenpipatsin N, Winayanuwattikun W, et al. Assessment of the quality of life (QoL) of patients with dermatitis and the impact of patch testing on QoL: a study of 519 patients diagnosed with dermatitis. Contact Dermatitis. 2020;83:182-188.
- Johnson H, Rao M, Yu J. Improved or not improved, that is the question: patch testing outcomes from the Massachusetts General Hospital. Contact Dermatitis. 2024;90:324-327.
- George SE, Yu J. Patch testing outcomes in children at the Massachusetts General Hospital. J Am Acad Dermatol. 2024;91:354-356.
- McNamara D. Dimethyl fumarate named 2011 allergen of the year.Int Med News. February 3, 2011. Accessed January 9, 2026. https://www.mdedge.com/internalmedicine/article/20401/dermatology/dimethyl-fumarate-named-2011-allergen-year
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Societyallergen of the year. Cutis. 2020;105:283-285.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Svedman C, Ale I, Goh CL, et al. Patch testing with nickel sulfate 5.0% traces significantly more contact allergy than 2.5%: a prospective study within the International Contact Dermatitis Research Group. Dermatitis. 2022;33:417-420.
- Houle MC, DeKoven JG, Atwater AR, et al. North American Contact Dermatitis Group Patch Test Results: 2021-2022. Dermatitis. 2025;36:464-476.
- Sundquist BK, Yang B, Pasha MA. Experience in patch testing: a 6-year retrospective review from a single academic allergy practice. Ann Allergy Asthma Immunol. 2019;122:502-507.
- Atwater AR, Liu B, Walsh R, et al. Supplemental patch testing identifies allergens missed by standard screening series. Dermatitis. 2024;35:366-372.
- Ravishankar A, Freese RL, Parsons HM, et al. Trends in patch testing in the Medicare Part B fee-for-service population. Dermatitis. 2022;33:129-134.
- Cheraghlou S, Watsky KL, Cohen JM. Utilization, cost, and provider trends in patch testing among Medicare beneficiaries in the United States from 2012 to 2017. J Am Acad Dermatol. 2021;85:1218-1226.
- Santiago Mangual KP, Rau A, Grant-Kels JM, et al. Increasing use of dupilumab and decreasing use of patch testing in medicare patients from 2017 to 2022: a claims database study. Dermatitis. 2025;36:538-540.
- Alinaghi F, Bennike NH, Egeberg A, et al. Prevalence of contact allergy in the general population: a systematic review and meta-analysis. Contact Dermatitis. 2019;80:77-85.
- Lim HW, Collins SAB, Resneck JS, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.
- Weidinger S, Novak N. Hand eczema. Lancet. 2024;404:2476-2486.
- Garg V, Brod B, Gaspari AA. Patch testing: uses, systems, risks/benefits, and its role in managing the patient with contact dermatitis. Clin Dermatol. 2021;39:580-590.
- Rodriguez-Homs LG, Taylor J, Liu B, et al. Patch test practice patterns of members of the American Contact Dermatitis Society. Dermatitis. 2020;31:272-275.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2020 Update. Dermatitis. 2020;31:279-282.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series. Dermatitis. 2013;24:7-9.
- Schalock PC, Dunnick CA, Nedorost S, et al. American Contact Dermatitis Society Core Allergen Series: 2017 Update. Dermatitis. 2017;28:141-143.
- Marks JG, Belsito DV, DeLeo VA, et al. North American Contact Dermatitis Group standard tray patch test results (1992 to 1994). Am J Contact Dermat. 1995;6:160-165.
- Kadyk DL, McCarter K, Achen F, et al. Quality of life in patients with allergic contact dermatitis. J Am Acad Dermatol. 2003;49:1037-1048.
- Dupixent® (dupilumab): pricing and insurance. Sanofi US. Updated June 2025. Accessed January 9, 2026. https://www.dupixent.com/support-savings/cost-insurance
- Woo PN, Hay IC, Ormerod AD. An audit of the value of patch testing and its effect on quality of life. Contact Dermatitis. 2003;48:244-247.
- Rajagopalan R, Anderson R. Impact of patch testing on dermatology-specific quality of life in patients with allergic contact dermatitis. Am J Contact Dermat. 1997;8:215-221.
- Thomson KF, Wilkinson SM, Sommer S, et al. Eczema: quality of life by body site and the effect of patch testing. Br J Dermatol. 2002;146:627-630.
- Boonchai W, Charoenpipatsin N, Winayanuwattikun W, et al. Assessment of the quality of life (QoL) of patients with dermatitis and the impact of patch testing on QoL: a study of 519 patients diagnosed with dermatitis. Contact Dermatitis. 2020;83:182-188.
- Johnson H, Rao M, Yu J. Improved or not improved, that is the question: patch testing outcomes from the Massachusetts General Hospital. Contact Dermatitis. 2024;90:324-327.
- George SE, Yu J. Patch testing outcomes in children at the Massachusetts General Hospital. J Am Acad Dermatol. 2024;91:354-356.
- McNamara D. Dimethyl fumarate named 2011 allergen of the year.Int Med News. February 3, 2011. Accessed January 9, 2026. https://www.mdedge.com/internalmedicine/article/20401/dermatology/dimethyl-fumarate-named-2011-allergen-year
- Nath N, Reeder M, Atwater AR. Isobornyl acrylate and diabetic devices steal the show for the 2020 American Contact Dermatitis Societyallergen of the year. Cutis. 2020;105:283-285.
- Raison-Peyron N, Sasseville D. Acetophenone azine. Dermatitis. 2021;32:5-9.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Svedman C, Ale I, Goh CL, et al. Patch testing with nickel sulfate 5.0% traces significantly more contact allergy than 2.5%: a prospective study within the International Contact Dermatitis Research Group. Dermatitis. 2022;33:417-420.
- Houle MC, DeKoven JG, Atwater AR, et al. North American Contact Dermatitis Group Patch Test Results: 2021-2022. Dermatitis. 2025;36:464-476.
- Sundquist BK, Yang B, Pasha MA. Experience in patch testing: a 6-year retrospective review from a single academic allergy practice. Ann Allergy Asthma Immunol. 2019;122:502-507.
- Atwater AR, Liu B, Walsh R, et al. Supplemental patch testing identifies allergens missed by standard screening series. Dermatitis. 2024;35:366-372.
- Ravishankar A, Freese RL, Parsons HM, et al. Trends in patch testing in the Medicare Part B fee-for-service population. Dermatitis. 2022;33:129-134.
- Cheraghlou S, Watsky KL, Cohen JM. Utilization, cost, and provider trends in patch testing among Medicare beneficiaries in the United States from 2012 to 2017. J Am Acad Dermatol. 2021;85:1218-1226.
- Santiago Mangual KP, Rau A, Grant-Kels JM, et al. Increasing use of dupilumab and decreasing use of patch testing in medicare patients from 2017 to 2022: a claims database study. Dermatitis. 2025;36:538-540.
Comprehensive Patch Testing: An Essential Tool for Care of Allergic Contact Dermatitis
Comprehensive Patch Testing: An Essential Tool for Care of Allergic Contact Dermatitis
Practice Points
- Comprehensive patch testing refers to patch testing beyond a screening series to capture allergens that otherwise would be missed using a limited panel.
- Comprehensive patch testing can identify emerging allergens and shifting allergen trends.
- Recent changes in patch test utilization have the potential to negatively affect patient care.
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
When Skin Cancer Awareness Month rolls around every May, my social media feed is inundated with posts extolling the benefits of total body skin examinations and the life-saving potential of skin cancer screenings; however, time and again the US Preventive Services Task Force (USPSTF)—the leading authority on evidence-based public health recommendations in the United States—has found the evidence supporting skin cancer screenings to be insufficient. The USPSTF has cited a lack of high-quality studies and inadequate data to recommend screening for the general population, excluding those at elevated risk due to personal, family, or occupational history.1 A 2019 Cochrane review went further, concluding that current evidence refutes the utility of population-based screening for melanoma.2
Despite these findings, skin cancer screenings and total body skin examinations remain popular among patients both with and without a personal or family history of cutaneous malignancy. Indeed, the anecdotal experience of dermatologists worldwide suggests an intangible benefit to screening that persists, even if robust data to support it remain elusive.
Putting aside studies that suggest these screenings help identify melanomas at earlier stages and with reduced Breslow thicknesses,3 there is a crucial benefit from face-to-face interaction between medical professionals and the public during skin cancer screening events or health fairs. This interaction has become especially important in an era when misinformation thrives online and so-called skin care “experts” with no formal training can amass tens of thousands—or even millions—of followers on social media.
So, what are the intangible benefits of the face-to-face interactions that occur naturally during skin cancer screenings? The most obvious is education. While the USPSTF may not recommend routine screening for skin cancer in the general population, it does endorse education for children, adolescents, and adults on the importance of minimizing exposure to UV radiation, particularly those with lighter skin tones.4 Publicly advertised skin cancer screenings at health fairs or other community events may offer an opportunity to raise awareness about sun safety and protection, including the value of peak UV avoidance, sun-protective clothing, and proper sunscreen use; these settings also serve as platforms for health care providers to counter misinformation, including concerns about sunscreen safety both for the patient and the environment, overhyped risks for vitamin D deficiency from sun avoidance, and myths about low skin cancer risk in patients with skin of color.
While the benefits of skin self-examination (SSE) remain uncertain, especially in low-risk populations, screening events provide an opportunity to educate patients on who is most likely to benefit from SSE and in whom the practice may cause more harm than good.5 For higher-risk individuals such as melanoma survivors or those with a strong family history, screening fairs can serve as meaningful touchpoints that reinforce the importance of sun protection and regular examinations with a health care provider. For those eager to perform SSEs, these events offer the chance to teach best practices—how to conduct SSEs effectively, what features to look for (eg, the ABCDE method or the ugly duckling sign), and when to seek professional care.
Finally (and importantly), skin cancer screening events provide peace of mind for patients. Reassurance from a professional about a benign skin lesion can alleviate anxiety that might otherwise lead to emergency or urgent care visits. While cellulitis and other skin infections are the most common dermatologic conditions seen in emergency settings, benign neoplasms and similar nonurgent conditions still contribute a substantial burden to urgent care systems in the United States.6 Outside emergency care, systems-level data support what many of us observe in practice: two of the most common reasons for referral to dermatology are benign neoplasms and epidermoid cysts, accounting for millions of visits annually.7 In fact, recent claims data suggest that the most common diagnosis made in US dermatology clinics in 2023 was (you guessed it!) seborrheic keratosis.8
What if instead of requiring a patient to wait weeks for a primary care appointment and months for a dermatology referral—all while worrying about a rapidly growing pigmented lesion and incurring costs in copays, travel, lost wages, and time away from work—we offered a fast, trustworthy, and free evaluation that meets the patient where they live, work, or socialize? An evaluation that not only eases their fears but also provides meaningful education about skin cancer prevention and screening guidelines? While precautions must of course be taken to ensure that the quality and completeness of such an examination equals that of an in-clinic evaluation, if services of this quality can be provided, public screening events may offer a simple, accessible, and valuable solution that delivers peace of mind and helps reduce unnecessary strain on emergency, primary, and specialty care networks.
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
- US Preventive Services Task Force; Mangione CM, Barry MJ, Nicholson WK, et al. Screening for skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2023;329:1290-1295. doi:10.1001/jama.2023.4342
- Johansson M, Brodersen J, Gøtzsche PC. Screening for reducing morbidity and mortality in malignant melanoma. Cochrane Database Syst Rev. 2019;6:CD012352. doi:10.1002/14651858.CD012352.pub2
- Matsumoto M, Wack S, Weinstock MA, et al. Five-year outcomes of a melanoma screening initiative in a large health care system. JAMA Dermatol. 2022;158:504-512. doi:10.1001/jamadermatol.2022.0253
- Grossman DC, Curry SJ, Owens DK, et al. Behavioral counseling to prevent skin cancer: US Preventive Services Task Force recommendation statement. JAMA. 2018;319:1134-1142.
- Ersser SJ, Effah A, Dyson J, et al. Effectiveness of interventions to support the early detection of skin cancer through skin self‐examination: a systematic review and meta‐analysis. Br J Dermatol. 2019;180:1339-1347. doi:10.1111/bjd.17529
- Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1266. doi:10.1016/j.jaad.2016.07.054
- Grada A, Muddasani S, Fleischer AB Jr. Trends in office visits for the five most common skin diseases in the United States. J Clin Aesthet Dermatol. 2022;15:E82-E86.
- Definitive Healthcare. What are the most common diagnoses by dermatologists? Published January 31, 2024. Accessed May 5, 2025. https://www.definitivehc.com/resources/healthcare-insights/top-dermatologist-diagnoses
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Screening for Meaning: Do Skin Cancer Screening Events Accomplish Anything?
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2
In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.
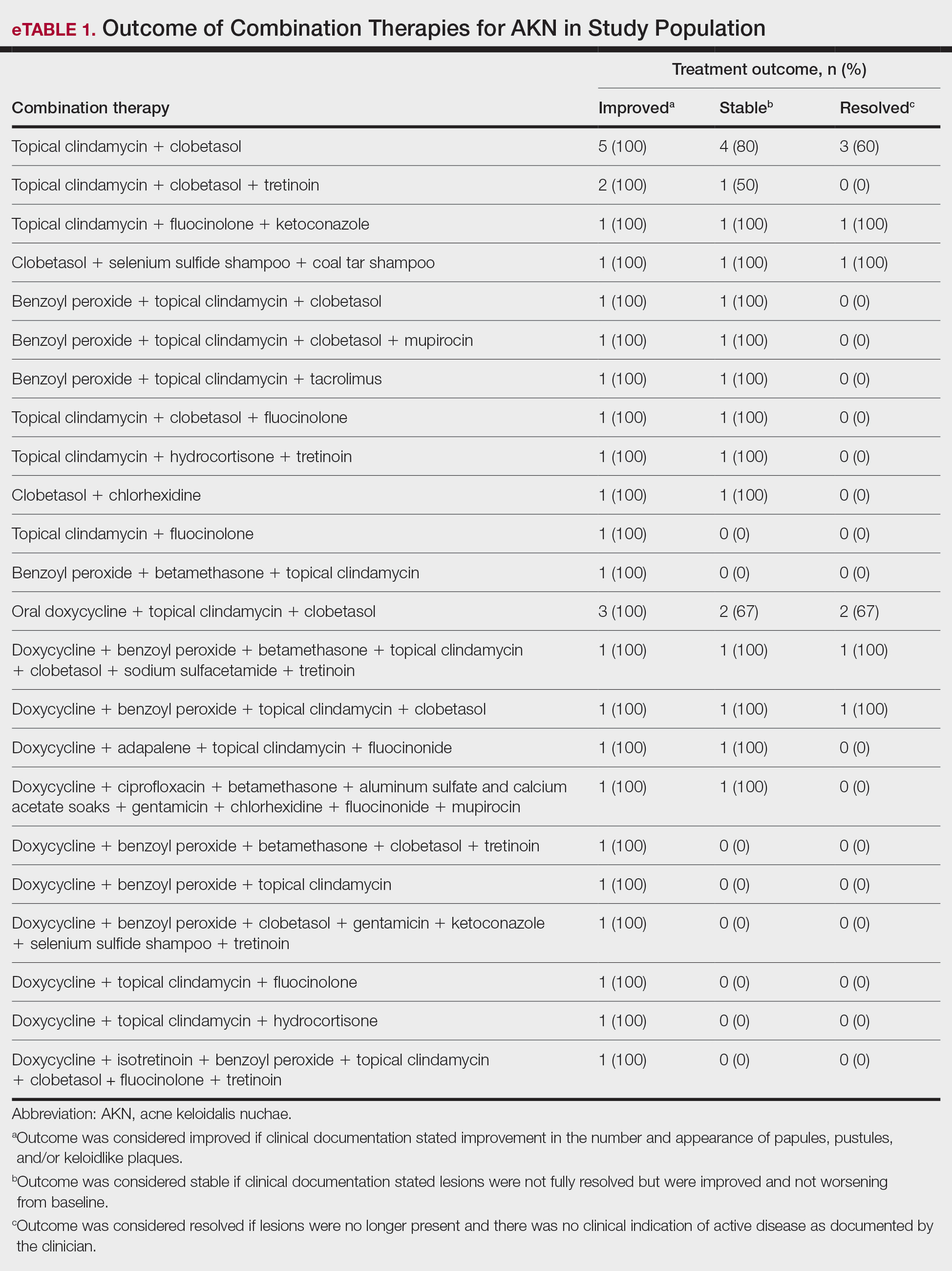
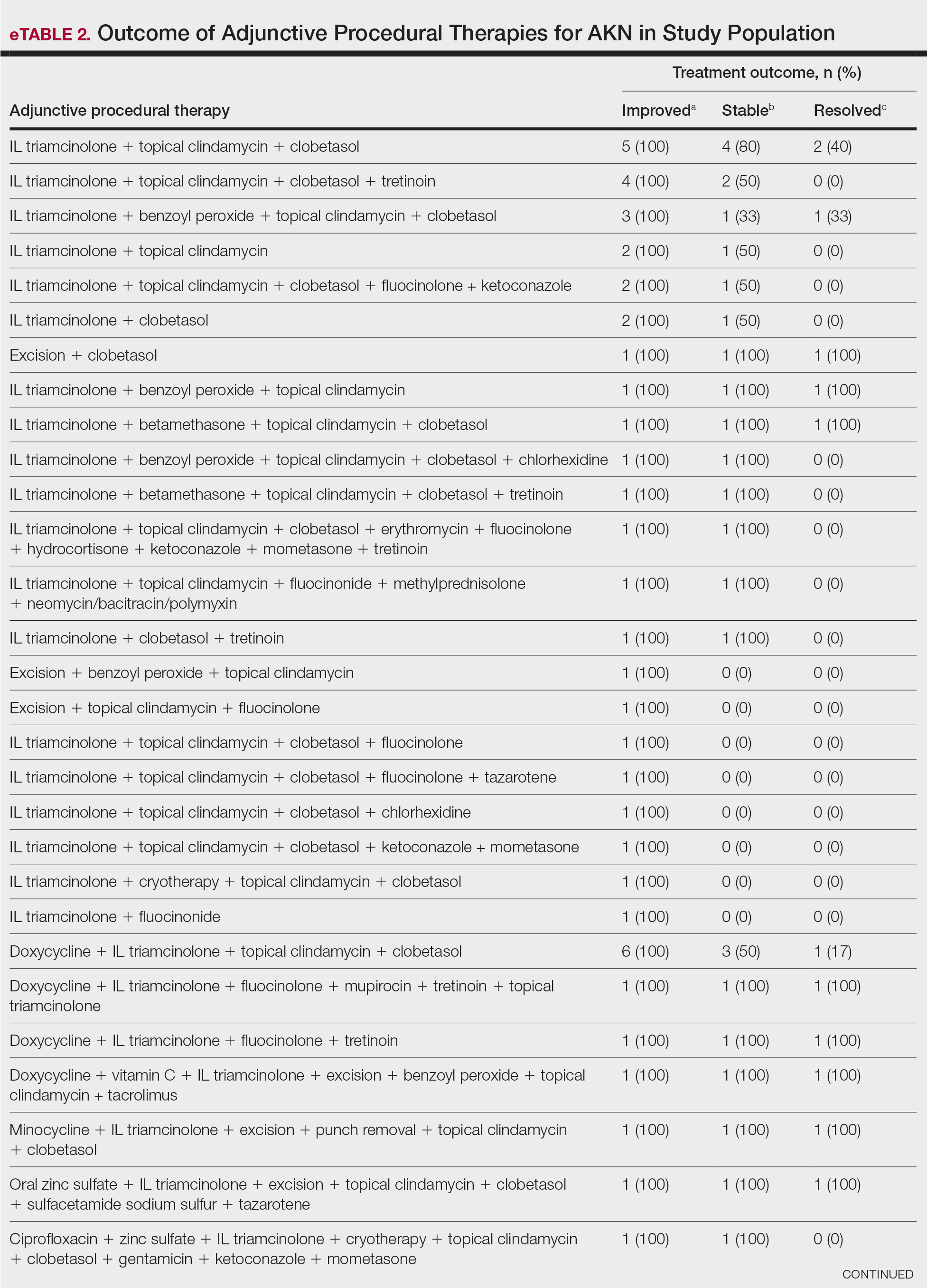
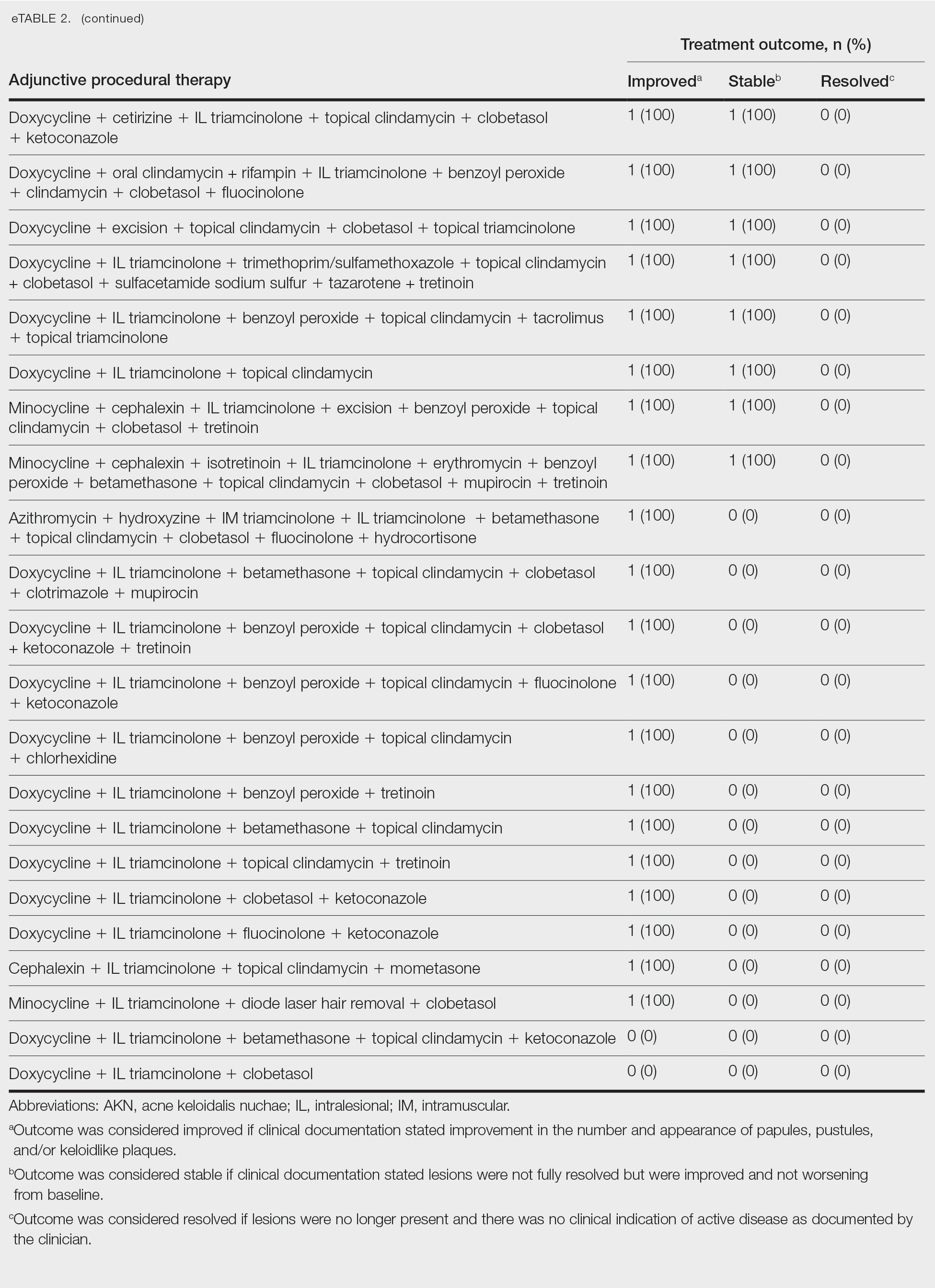
Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
Acne keloidalis nuchae (AKN) classically presents as chronic inflammation of the hair follicles on the occipital scalp/nape of the neck manifesting as papules and pustules that may progress to keloidlike scarring.1 Photographs depicting the typical clinical presentation of AKN are shown in the Figure. In the literature, AKN has been described as primarily occurring in postpubertal males of African descent.2 Despite its similar name, AKN is not related to acne vulgaris.3 The underlying cause of AKN is hypothesized to be multifactorial, including inflammation, infection, and trauma.2 Acne keloidalis nuchae is most common in males aged 14 to 50 years, which may indicate that increased androgens contribute to its development.3 In some cases, patients have reported developing AKN lesions after receiving a haircut or shaving, suggesting a potential role of trauma to the hair follicles and secondary infection.2 Histopathology typically shows a perifollicular inflammatory infiltrate that obscures the hair follicles with associated proximal fibrosis.4 On physical examination, dermoscopy can be used to visualize perifollicular pustules and fibrosis, which appears white, in the early stages of AKN. Patients may present with tufted hairs in more advanced stages.5 Patients with AKN often describe the lesions as pruritic and painful.2

In this study, we evaluated the most common treatment regimens used over a 6-year period by patients in the Los Angeles County hospital system in California and their efficacy on AKN lesions. Our study includes one of the largest cohorts of patients reported to date and as such demonstrates the real-world effects that current treatment regimens for AKN have on patient outcomes nationwide.
Methods
We performed a retrospective cross-sectional analysis of patient medical records from the Los Angeles County hospital system i2b2 (i2b2 tranSMART Foundation) clinical data warehouse over a 6-year period (January 2017–January 2023). We used the International Statistical Classification of Diseases, Tenth Revision codes L73.0 (acne keloid) and L73.1 (pseudofolliculitis barbae) to conduct our search in order to identify as many patients with follicular disorders as possible to include in the study. Of the 478 total medical records we reviewed, 183 patients were included based on a diagnosis of AKN by a dermatologist.
We then collected data on patient demographics and treatments received, including whether patients had received monotherapy or combination therapy. Of the 183 patients we initially identified, 4 were excluded from the study because they had not received any treatment, and 78 were excluded because no treatment outcomes were documented. The 101 patients who were included had received either monotherapy or a combination of treatments. Treatment outcomes were categorized as either improvement in the number and appearance of papules and/or keloidlike plaques, maintenance of stable lesions (ie, well controlled), and/or resolution of lesions as documented by the treating physician. No patients had overall worsening of their disease.
Results
Of the 101 patients included in the study, 34 (33.7%) received a combination of topical, systemic, and procedural treatments; 34 (33.7%) received a combination of topical and procedural treatments; 17 (16.8%) were treated with topicals only; 13 (12.9%) were treated with a combination of topical and systemic treatments; and 3 (3.0%) were treated with monotherapy of either a topical, systemic, or procedural therapy. Systemic and/or procedural therapy combined with topicals was provided as a first-line treatment for 63 (62.4%) patients. Treatment escalation to systemic or procedural therapy for those who did not respond to topical treatment was observed in 23 (22.8%) patients. The average number of unique treatments received per patient was 3.67.
Clindamycin and clobetasol were the most prescribed topical treatments, doxycycline was the most prescribed systemic therapy, and intralesional (IL) triamcinolone was the most performed procedural therapy. The most common treatment regimens were topical clindamycin and clobetasol, topical clindamycin and clobetasol with IL triamcinolone, and topical clindamycin and clobetasol with both IL triamcinolone and doxycycline.
Improvement in AKN lesions was reported for the majority of patients with known treatment outcomes across all types of regimens. Ninety-eight percent (99/101) of patients had improvement in lesions, 55.5% (56/101) had well-controlled lesions, and 20.8% (21/101) achieved resolution of disease. The treatment outcomes are outlined in eTables 1 and 2.



Comment
Most clinicians opted for a multitherapy treatment regimen, and improvement was noted in most patients regardless of which regimen was chosen. As expected, patients who had mild or early disease generally received topical agents first, including most commonly a mid- to high-potency steroid, antibiotic, retinoid, and/or antifungal; specifically, clindamycin, clobetasol, and fluocinolone were the most common agents chosen. Patients with severe disease were more likely to receive systemic and/or procedural treatments, including oral antibiotics or IL steroid injections most commonly. Improvement was documented in the majority of patients using these treatment regimens, and some patients did achieve full resolution of disease.
Our data cannot be used to determine which treatment alone is most effective for patients with AKN, as the patients in our study had varying levels of disease activity and types of lesions, and most received combination therapy. What our data do show is that combination therapies often work well to control or improve disease, but also that current therapeutic options only rarely lead to full resolution of disease.
Limitations of our study included an inability to stratify disease, an inability to rigorously analyze specific treatment outcomes since most patients did not receive monotherapy. The strength of our study is its size, which allows us to show that many different treatment regimens currently are being employed by dermatologists to treat AKN, and most of these seem to be somewhat effective.
Conclusion
Acne keloidalis nuchae is difficult to treat due to a lack of understanding of which pathophysiologic mechanisms dominate in any given patient, a lack of good data on treatment outcomes, and the variability of ways that the disease manifests. Thus far, as shown by the patients described in this study, the most efficacious treatment regimens seem to be combination therapies that target the multifactorial causes of this disease. Physicians should continue to choose treatments based on disease severity and cutaneous manifestations, tailor their approach by accounting for patient preferences, and consider a multimodal approach to treatment.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systematic review of the literature. Dermatol Ther. 2016;6:363-378. doi:10.1007/s13555-016-0134-5<
- Ogunbiyi A, Adedokun B. Perceived aetiological factors of folliculitis keloidalis nuchae (acne keloidalis) and treatment options among Nigerian men. Br J Dermatol. 2015;173(Suppl 2):22-25. doi:10.1111/bjd.13422
- East-Innis ADC, Stylianou K, Paolino A, et al. Acne keloidalis nuchae: risk factors and associated disorders – a retrospective study. Int J Dermatol. 2017;56:828-832. doi:10.1111/ijd.13678
- Goette DK, Berger TG. Acne keloidalis nuchae. A transepithelial elimination disorder. Int J Dermatol. 1987;26:442-444. doi:10.1111/j.1365-4362.1987.tb00587.x
- Chouk C, Litaiem N, Jones M, et al. Acne keloidalis nuchae: clinical and dermoscopic features. BMJ Case Rep. 2017;2017:bcr2017222222. doi:10.1136/bcr-2017-222222
Treatment of Acne Keloidalis Nuchae in a Southern California Population
Treatment of Acne Keloidalis Nuchae in a Southern California Population
PRACTICE POINTS
- Acne keloidalis nuchae (AKN) is a rare inflammatory skin disease that manifests with papules, pustules, and plaques on the occipital scalp.
- Initial treatment for patients with mild to moderate AKN disease most commonly is topical clindamycin and clobetasol; patients with moderate to severe AKN disease may require adjunctive treatment with oral doxycycline and/or intralesional triamcinolone.
- Combination therapy that targets the multifactorial pathophysiology of AKN (inflammatory, infectious, and traumatic) is most efficacious overall.
- The majority of patients experience improvement of AKN with treatment, but full resolution is less common.