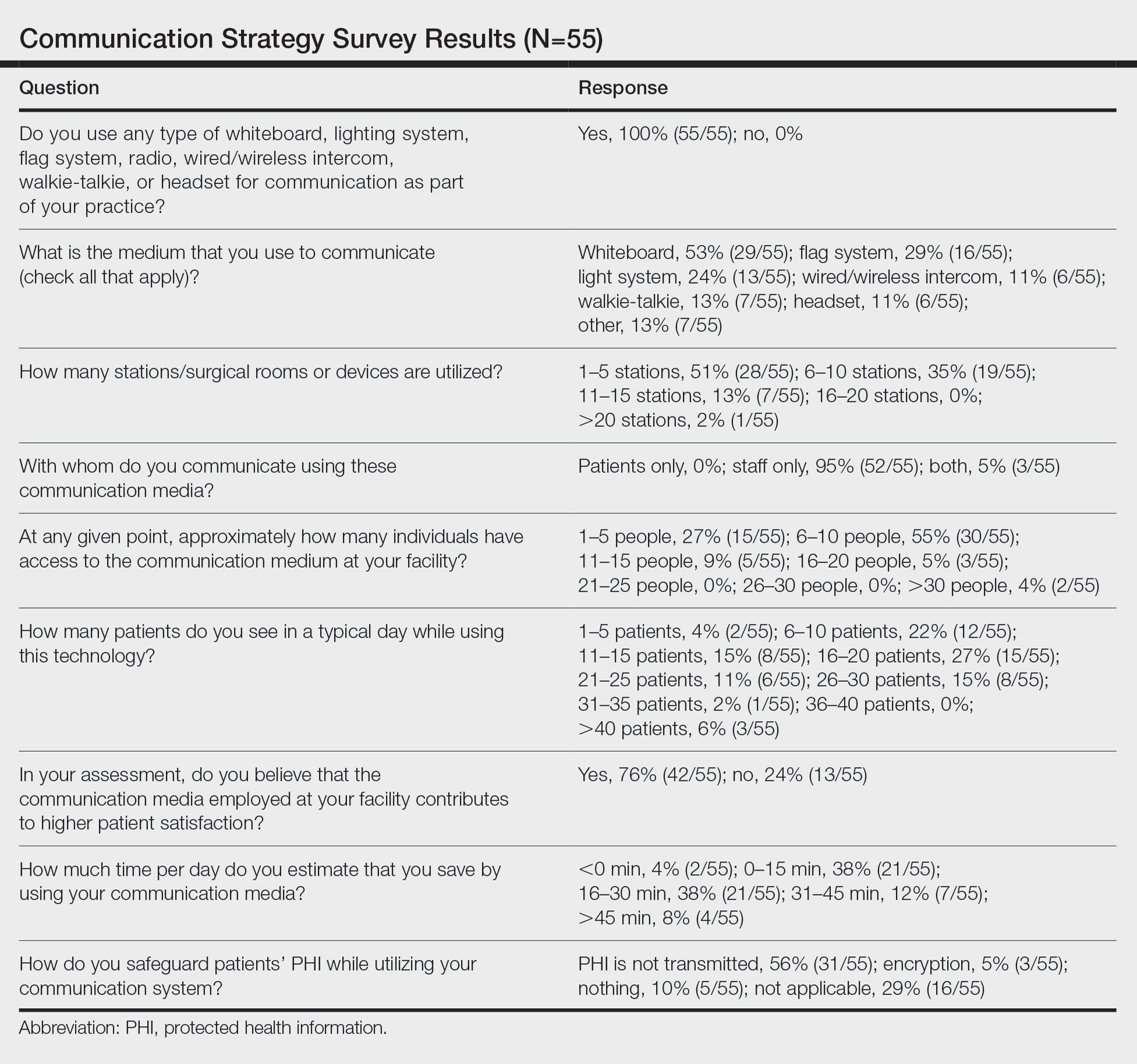
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
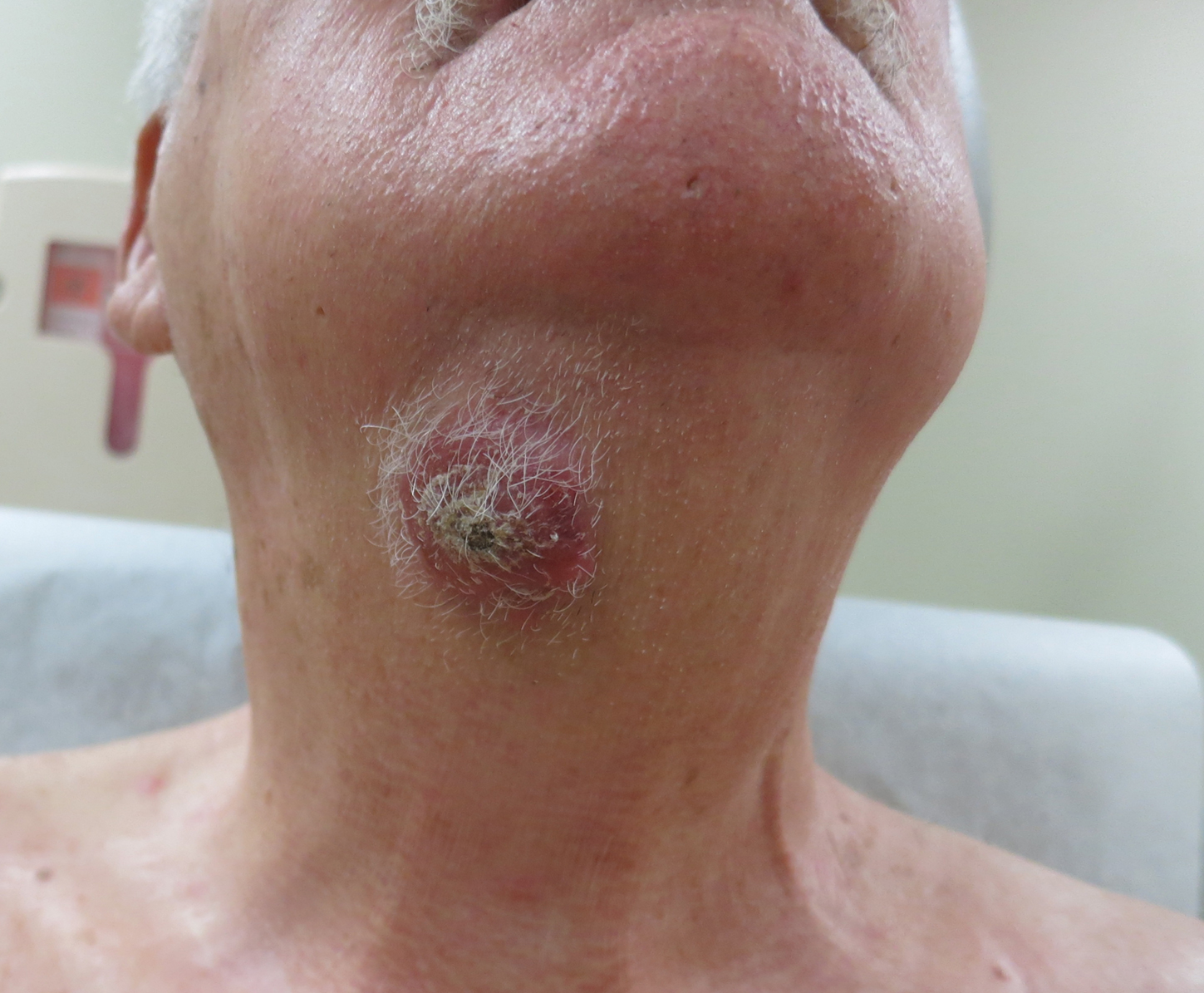
Nodule on the Neck
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
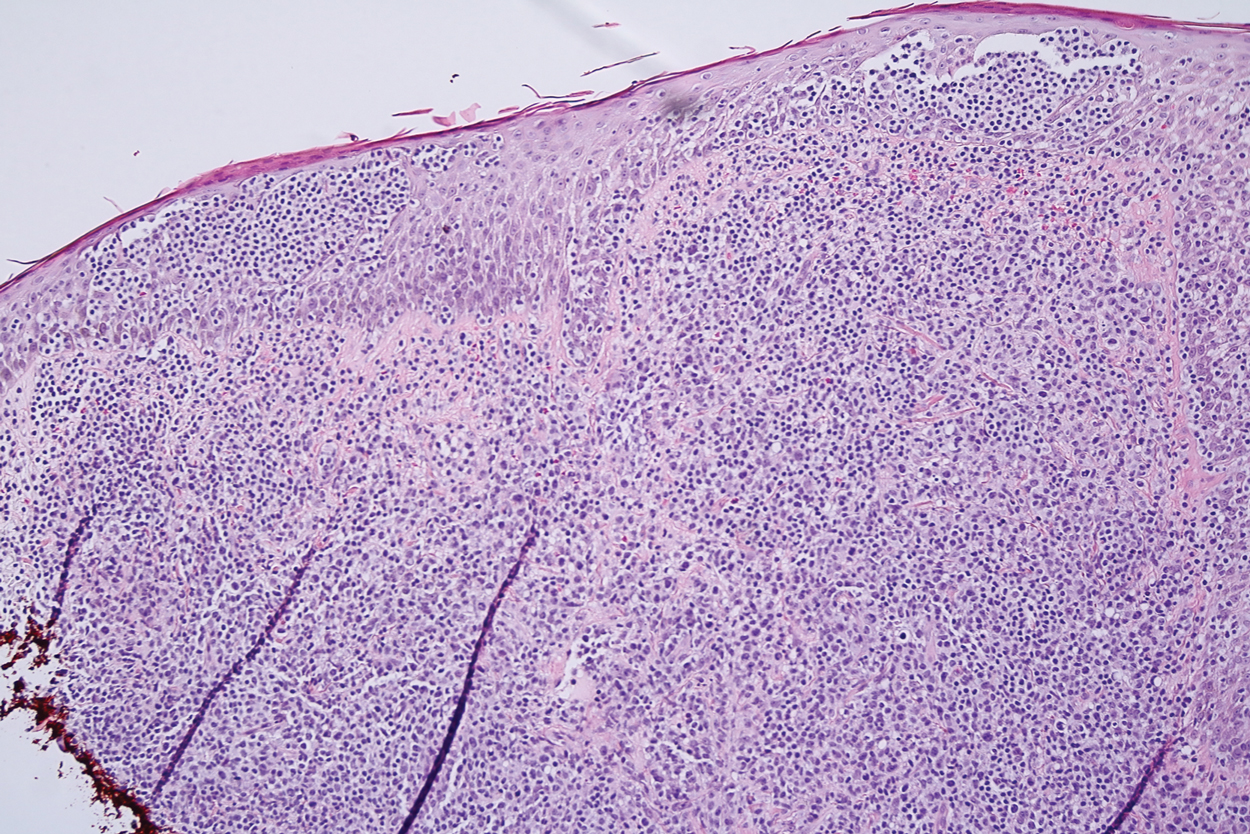
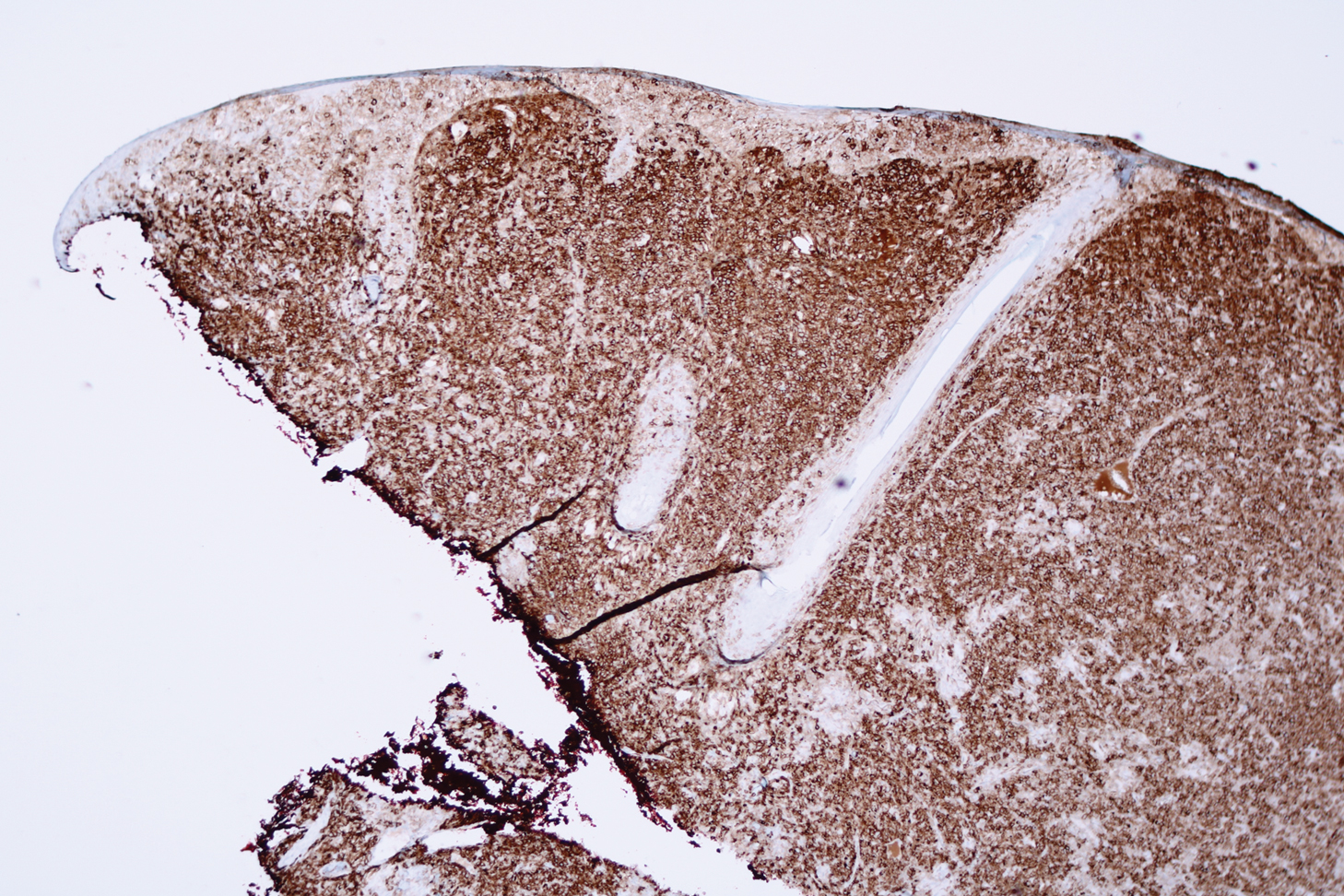
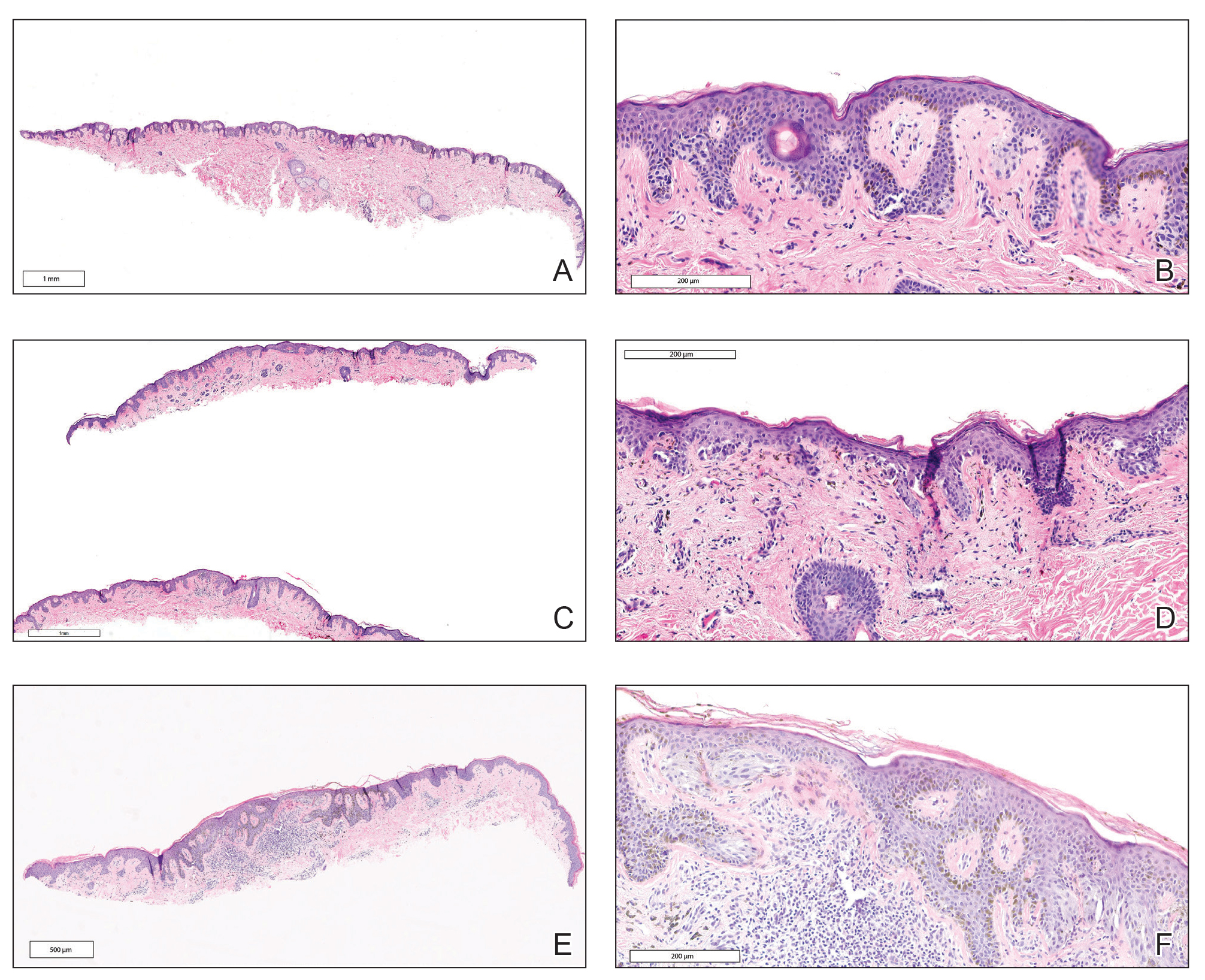
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
The Diagnosis: Primary Cutaneous Anaplastic Large Cell Lymphoma
Microscopic analysis showed a dense proliferation of mononuclear cells filling and expanding the dermis with focal epidermotropism (Figure 1). Immunohistochemistry demonstrated strong and diffuse staining for CD3, CD4, and CD30 (Figure 2) and lack of staining for anaplastic lymphoma kinase (ALK). Workup to exclude systemic disease was initiated and included unremarkable computed tomography (CT) of the neck, chest, abdomen, and pelvis along with no abnormal cells on bone marrow biopsy. Complete blood cell count, basic metabolic panel, and lactate dehydrogenase were within reference range. Given the lack of evidence for systemic involvement, a diagnosis of primary cutaneous anaplastic large cell lymphoma (PC-ALCL) was made. The treatment plan for our patient with a solitary lesion was localized radiation therapy.
Primary cutaneous CD30+ lymphoproliferative disorders encompass a spectrum of conditions, with premalignant lymphomatoid papulosis (LyP) at one extreme and the malignant PC-ALCL on the other.1 The diagnosis of PC-ALCL is made by clinicopathologic correlation, and lesions typically present abruptly as solitary or grouped nodules with a tendency to ulcerate over time. Spontaneous regression has been reported, but relapse in the skin is frequent.2
A representative, typically excisional, biopsy should be performed if the clinician suspects PC-ALCL. Histologic criteria include a dense dermal infiltrate of large pleomorphic cells and the expression of CD30 in at least 75% of tumor cells.3 Primary cutaneous anaplastic large cell lymphoma typically lacks the ALK gene translocation with the nucleophosmin gene, NPM, that is common in systemic disease; however, a small subset of PC-ALCL may be ALK positive and indicate a higher chance of transformation into systemic disease.2
The extent of the lymphoma should be staged to exclude the possibility of systemic disease. This assessment includes a complete physical examination; laboratory investigation, including complete blood cell count with differential and blood chemistries; and radiography. A positron emission tomography-CT scan of the neck, chest, abdomen, and pelvis, or a whole-body integrated positron emission tomography-CT are sufficient for the radiographic examination.3
The initial choice of treatment for solitary or localized PC-ALCL is localized radiation therapy or low-dose methotrexate. Targeted therapy such as brentuximab has been shown to be effective for those with multifocal systemic involvement or refractory disease.2 Cure rates from radiation therapy alone approach 95%.3 It is important to highlight radiation therapy as the initial management plan to increase awareness and to avoid inappropriate treatment of PC-ALCL with traditional chemotherapy.
Large lesions of LyP may appear similar to PC-ALCL on histopathology, making the two entities difficult to distinguish. However, in contrast to PC-ALCL, LyP classically has a different clinical course characterized by waxing and waning crops of lesions that typically are smaller (<1 cm) than those of PC-ALCL.2 Large cell transformation of mycosis fungoides is another entity to consider, but these patients usually have a known history of mycosis fungoides.4
Keratoacanthomas, considered to be a variant of a well-differentiated squamous cell carcinoma, present as rapidly enlarging crateriform nodules with a keratotic core. They usually are found on the head and neck or sun-exposed areas of the extremities and may regress spontaneously.5 Histology will show atypical, highly differentiated squamous epithelia. Merkel cell carcinoma also has a predilection for the head and neck in older patients and may present as a rapidly growing nodule. However, histology will show an aggressive tumor with small round blue cells, and immunohistochemistry will show the characteristic paranuclear dot staining for CK20 along with staining for various neuroendocrine markers. Similarly, atypical fibroxanthoma is a low-grade sarcoma that also presents on the head and neck of elderly sun-damaged patients.5 Histology will show dermal proliferation of spindle cells that often extend up against the epidermis along with pleomorphism and atypical mitoses. Basal cell carcinoma is a common tumor that can present on the head and neck in sun-damaged patients. Nodular basal cell carcinomas can enlarge and ulcerate, but growth is seen over years rather than weeks.5 Histology characteristically will show tumor islands composed of basaloid cells with peripheral palisading and clefting between the tumor islands and the stroma.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
- Swerdlow SH, Campo E, Pileri SA, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127:2375-2390.
- Brown RA, Fernandez-Pol S, Kim J. Primary cutaneous anaplastic large cell lymphoma. J Cutan Pathol. 2017;44:570-577.
- Kempf W, Pfaltz K, Vermeer MH, et al. EORTC, ISCL, and USCLC consensus recommendations for the treatment of primary cutaneous CD30-positive lymphoproliferative disorders: lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Blood. 2011;118:4024-4035.
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part II. prognosis, management, and future directions. J Am Acad Dermatol. 2014;70:223.e1-17.
- Bolognia JL, Jorizzo JL, Schaffer JV. Dermatology. 3rd ed. Saunders Elsevier; 2015:475-489.
An 80-year-old man presented to our clinic with a large lesion on the right upper neck of approximately 4 weeks’ duration. He reported that it was rapidly increasing in size and had bled on several occasions. No treatments were attempted prior to the initial visit. He denied any constitutional symptoms. The patient had a history of nonmelanoma skin cancers but no other chronic medical problems. Physical examination revealed a large, 35×40-mm, erythematous nodule with central ulceration and overlying hyperkeratosis on the right upper neck. No palpable cervical, supraclavicular, or axillary lymphadenopathy was observed. An excisional biopsy of the lesion was obtained.
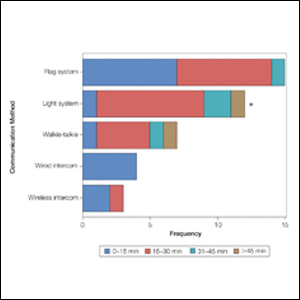
Mohs Micrographic Surgery During the COVID-19 Pandemic: Considering the Patient Perspective
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
Guidelines on Skin Cancer Surgeries During the COVID-19 Pandemic
At the start of the COVID-19 pandemic, the Centers for Disease Control and Prevention issued recommendations to decrease the spread of SARS-CoV-2 and optimize the use of personal protective equipment (PPE) for frontline workers.1 In the field of dermatologic surgery, the American College of Mohs Surgery, the National Comprehensive Cancer Network, the American Society for Dermatologic Surgery, and the American Academy of Dermatology made recommendations to postpone nonessential and nonurgent procedures.2-4 The initial guidelines of the American College of Mohs Surgery advised cancellation of all elective surgeries and deferred treatment of most cases of basal cell carcinoma for as long as 3 months; low-risk squamous cell carcinoma (SCC) and melanoma in situ treatment was deferred for as long as 2 or 3 months.3 Additional recommendations were made to reserve inpatient visits for suspicious lesions and high-risk cancers, postpone other nonessential and nonurgent appointments, and utilize telemedicine whenever possible.5
These recommendations led to great uncertainty and stress for patients and providers. Although numerous important variables, such as patient risk factors, severity of disease, availability of PPE and staff, and patient-to-provider transmission were considered when creating these guidelines, the patient’s experience likely was not a contributing factor.
COVID-19 Transmission During Mohs Surgery
There have been concerns that surgeons performing Mohs micrographic surgery (MMS) might be at an increased risk for COVID-19, given their close contact with high-risk sites (ie, nose, mouth) and cautery-generated aerosols; most of the estimated transmission risk associated with MMS has been based on head and neck surgery experience and publications.6-8 Tee and colleagues9 recently published their institution’s MMS COVID-19 preventive measures, which, to their knowledge, have prevented all intraoperative transmission of SARS-CoV-2, even in disease-positive patients. Currently, evidence is lacking to support a high risk for SARS-CoV-2 transmission during MMS when proper PPE and personal hygiene measures as well as strict infection control protocols—presurgical COVID-19 testing in high-risk cases, COVID-19 screening optimization, visitor restrictions, and appropriate disinfection between patients—are in place.
The Impact of Postponing Treatment on Patients
Although studies have focused on the effects of the COVID-19 pandemic on physicians practicing MMS,10 little is known about the effects of delays in skin cancer treatment on patients. A survey conducted in the United Kingdom investigating the patient’s perspective found that patients expressed worry and concern about the possibility that their MMS would be postponed and greatly appreciated continuation of treatment during the pandemic.11
Other medical specialties have reported their patient experiences during the pandemic. In a study examining patient perception of postponed surgical treatment of pelvic floor disorders due to COVID-19, nearly half of survey respondents were unhappy with the delay in receiving care. Furthermore, patients who reported being unhappy were more likely to report feelings of isolation and anxiety because their surgery was postponed.12 In another study involving patients with lung cancer, 9.1% (N=15) of patients postponed their treatment during the COVID-19 pandemic because of pandemic-related anxiety.13
With the goal of improving care at our institution, we conducted a brief institutional review board–approved survey to evaluate how postponing MMS treatment due to the COVID-19 pandemic affected patients. All MMS patients undergoing surgery in June 2020 and July 2020 (N=99) were asked to complete our voluntary and anonymous 23-question survey in person during their procedure. We obtained 88 responses (response rate, 89%). Twenty percent of surveyed patients (n=18) reported that their MMS had been postponed; 78% of those whose MMS was postponed (n=14) indicated some level of anxiety during the waiting period. It was unclear which patients had their treatment postponed based on national guidelines and which ones elected to postpone surgery.
Tips for Health Care Providers
Patient-provider communication highlighting specific skin cancer risk and the risk vs benefit of postponing treatment might reduce anxiety and stress during the waiting period.14 A study found that COVID-19 posed a bigger threat than most noninvasive skin cancers; therefore, the authors of that study concluded that treatment for most skin cancers could be safely postponed.15 Specifically, those authors recommended prioritizing treatment for Merkel cell carcinoma, invasive SCC, and melanoma with positive margins or macroscopic residual disease. They proposed that all other skin cancers, including basal cell carcinoma, SCC in situ, and melanoma with negative margins and no macroscopic residual disease, could be safely delayed for as long as 3 months.15
For patients with multiple risk factors for COVID-19–related morbidity or mortality, delaying skin cancer treatment likely has less risk than contracting the virus.15 This information should be communicated with patients. Investigation of specific patient concerns is warranted, and case-by-case evaluation of patients’ risk factors and skin cancer risk should be considered.
Based on the current, though limited, literature, delaying medical treatment can have a negative impact on the patient experience. Furthermore, proper precautions have been shown to limit intraoperative transmission of SARS-CoV-2 during MMS, but research is lacking. Practitioners should utilize shared decision-making and evaluate a given patient’s risk factors and concerns when deciding whether to postpone treatment. We encourage other institutions to evaluate the effects that delaying MMS has had on their patients, as further studies would improve understanding of patients’ experiences during a pandemic and potentially influence future dermatology guidelines.
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
- Center for Disease Control and Prevention. COVID-19. Accessed April 20, 2021. https://www.cdc.gov/coronavirus/2019-ncov/index.html
- American College of Mohs Surgery. Mohs surgery ambulatory protocol during COVID pandemic (version 6-3-20). June 4, 2020. Accessed April 20, 2021. http://staging.mohscollege.org/UserFiles/AM20/Member%20Alert/MohsSurgeryAmbulatoryProtocolDuringCOVIDPandemicFinal.pdf
- COVID-19 resources. National Comprehensive Cancer Network website. Accessed April 20, 2021. https://www.nccn.org/covid-19
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed April 10, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- Geskin LJ, Trager MH, Aasi SZ, et al. Perspectives on the recommendations for skin cancer management during the COVID-19 pandemic.J Am Acad Dermatol. 2020;83:295-296. doi:10.1016/j.jaad.2020.05.002
- Yuan JT, Jiang SIB. Urgent safety considerations for dermatologic surgeons in the COVID-19 pandemic. Dermatol Online J. 2020;26:1. Accessed April 20, 2021. http://escholarship.org/uc/item/2qr3w771
- Otolaryngologists may contract COVID-19 during surgery. ENTtoday. March 20, 2020. Accessed April 20, 2021. https://www.enttoday.org/article/otolaryngologists-may-contract-covid-19-during-surgery/
- Howard BE. High-risk aerosol-generating procedures in COVID-19: respiratory protective equipment considerations. Otolaryngol Head Neck Surg. 2020;163:98-103. doi:10.1177/0194599820927335
- Tee MW, Stewart C, Aliessa S, et al. Dermatological surgery during the COVID-19 pandemic: experience of a large academic center. J Am Acad Dermatol. 2021;84:1094-1096. doi:10.1016/j.jaad.2020.12.003
- Hooper J, Feng H. The impact of COVID-19 on micrographic surgery and dermatologic oncology fellows. Dermatol Surg. 2020;46:1762-1763. doi:10.1097/DSS.0000000000002766
- Nicholson P, Ali FR, Patalay R, et al. Patient perceptions of Mohs micrographic surgery during the COVID-19 pandemic and lessons for the next outbreak. Clin Exp Dermatol. 2021;46:179-180. doi:10.1111/ced.14423
- Mou T, Brown O, Gillingham A, et al. Patients’ perceptions on surgical care suspension for pelvic floor disorders during the COVID-19 pandemic. Female Pelvic Med Reconstr Surg. 2020;26:477-482. doi:10.1097/SPV.0000000000000918
- Fujita K, Ito T, Saito Z, et al. Impact of COVID-19 pandemic on lung cancer treatment scheduling. Thorac Cancer. 2020;11:2983-2986. doi:10.1111/1759-7714.13615
- Nikumb VB, Banerjee A, Kaur G, et al. Impact of doctor-patient communication on preoperative anxiety: study at industrial township, Pimpri, Pune. Ind Psychiatry J. 2009;18:19-21. doi:10.4103/0972-6748.57852
- Baumann BC, MacArthur KM, Brewer JD, et al. Management of primary skin cancer during a pandemic: multidisciplinary recommendations. Cancer. 2020;126:3900-3906. doi:10.1002/cncr.32969
Practice Points
- There is little evidence that supports a high risk for SARS-CoV-2 transmission during Mohs micrographic surgery when proper personal protective equipment and strict infection control protocols are in place.
- The effects of treatment delays due to COVID-19 on the patient experience have not been well studied, but the limited literature suggests a negative association.
- Shared decision-making and evaluation of individual patient risk factors and concerns should be considered when deciding whether to postpone skin cancer treatment.
Clinical Use of a Diagnostic Gene Expression Signature for Melanocytic Neoplasms
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
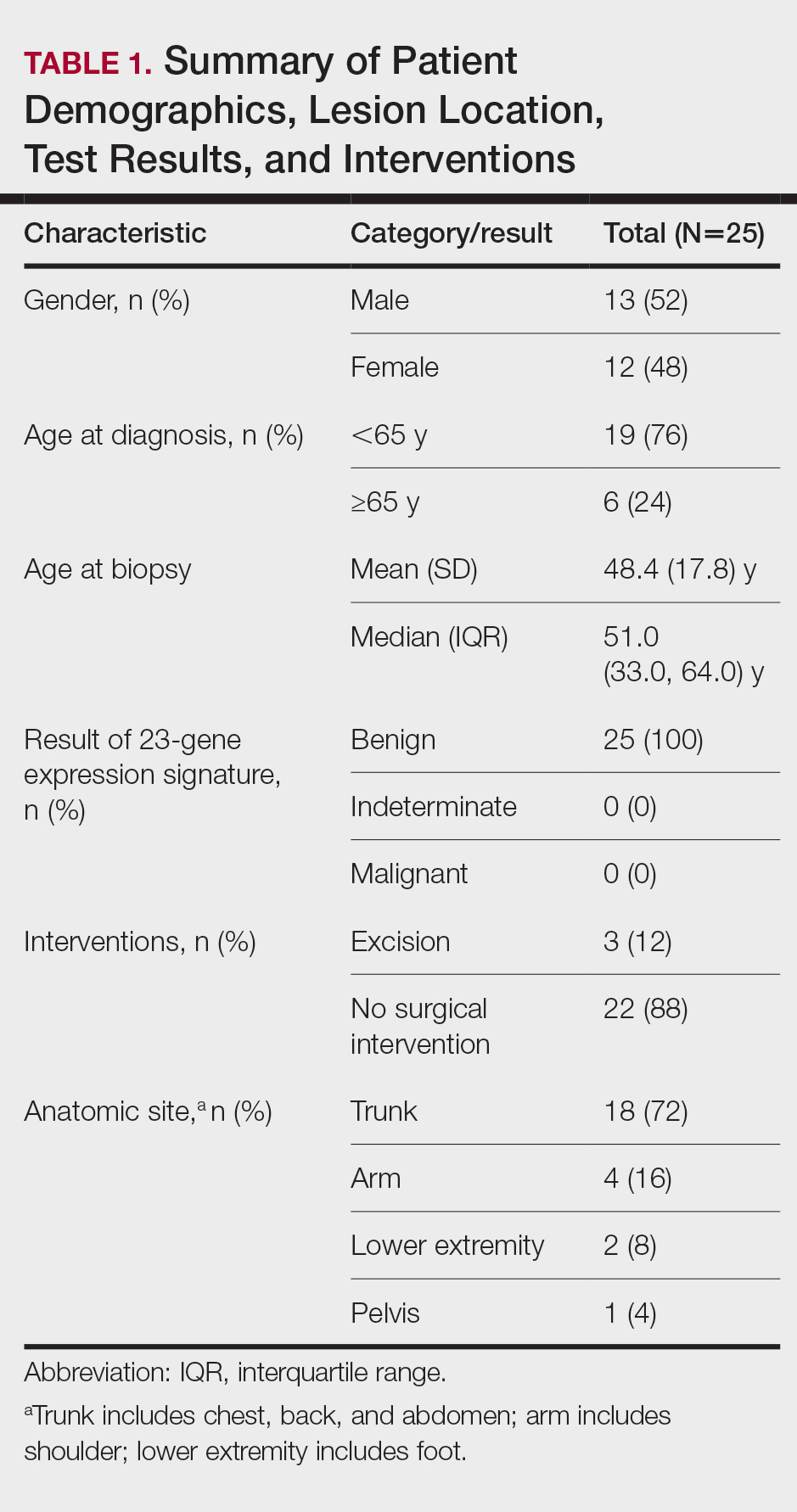
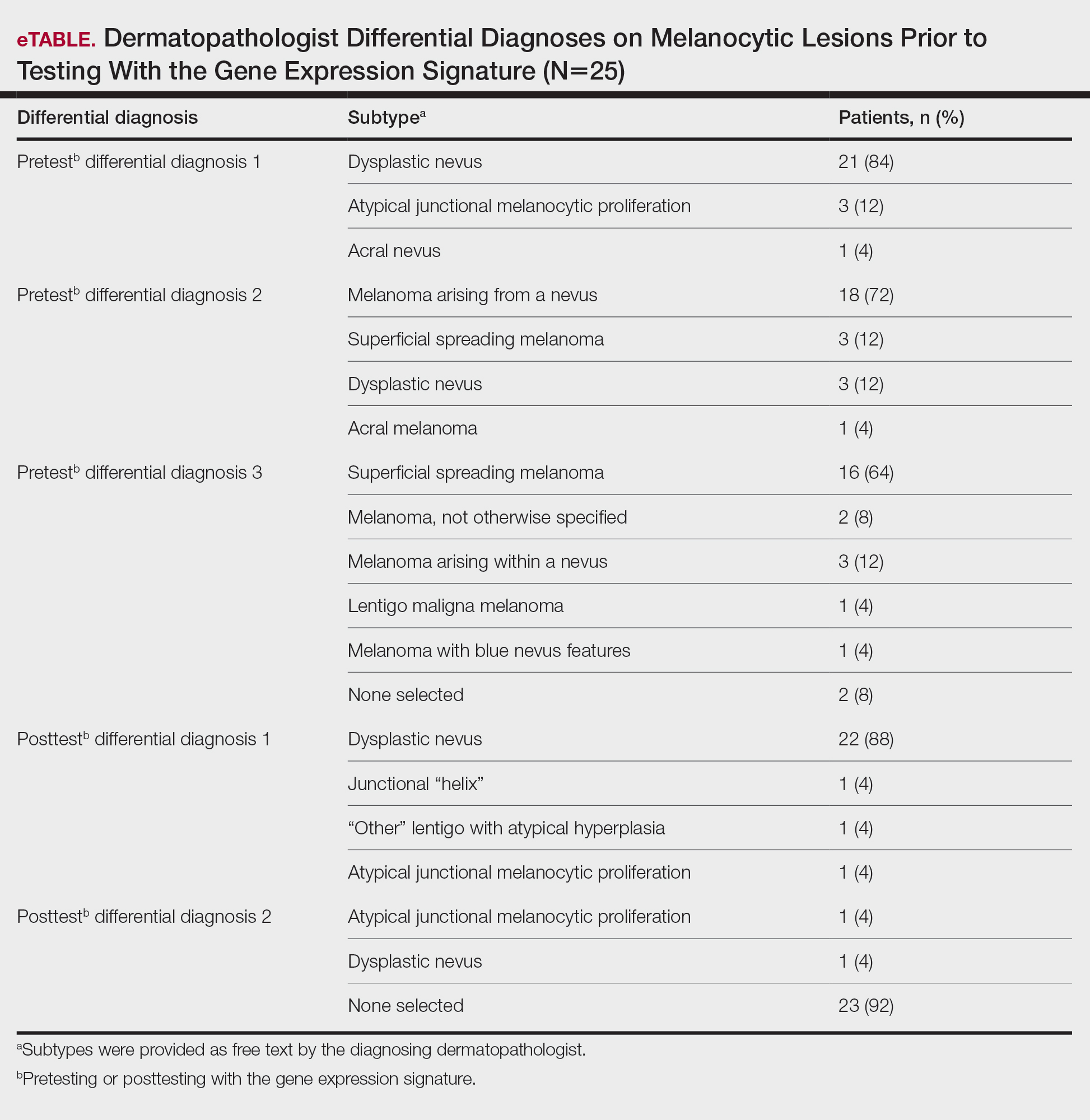
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
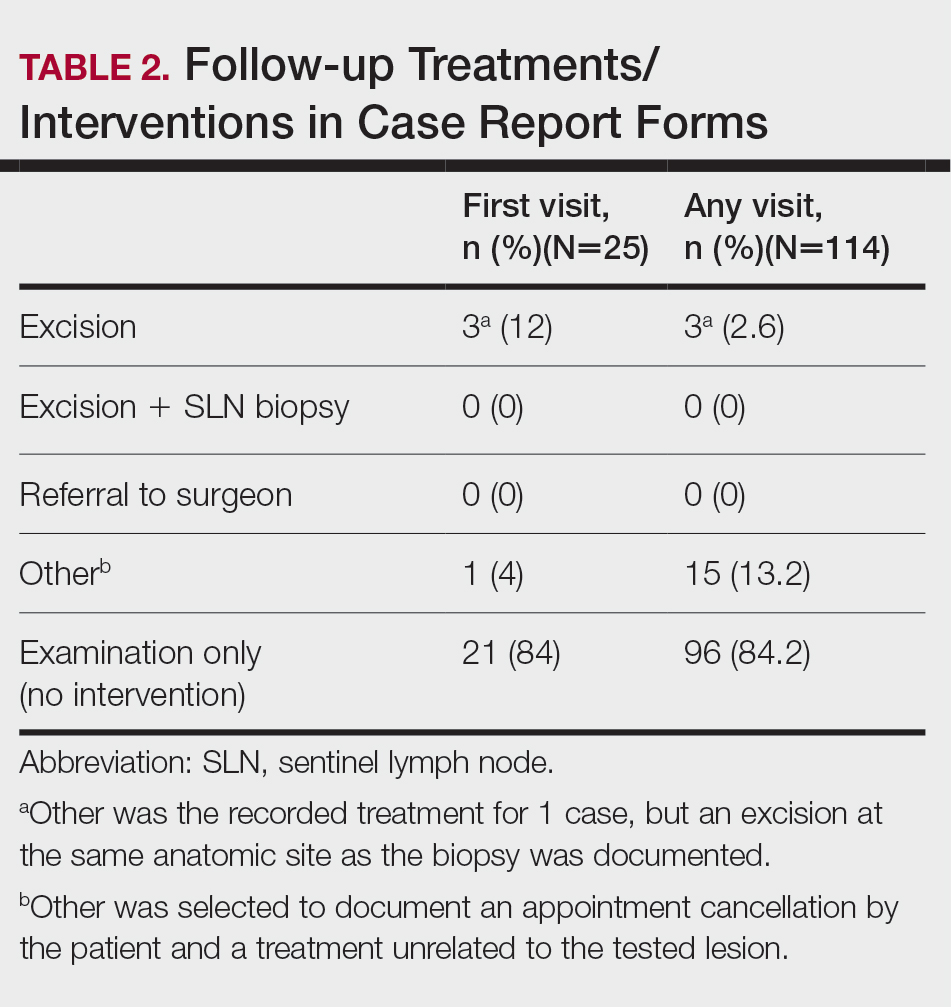
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
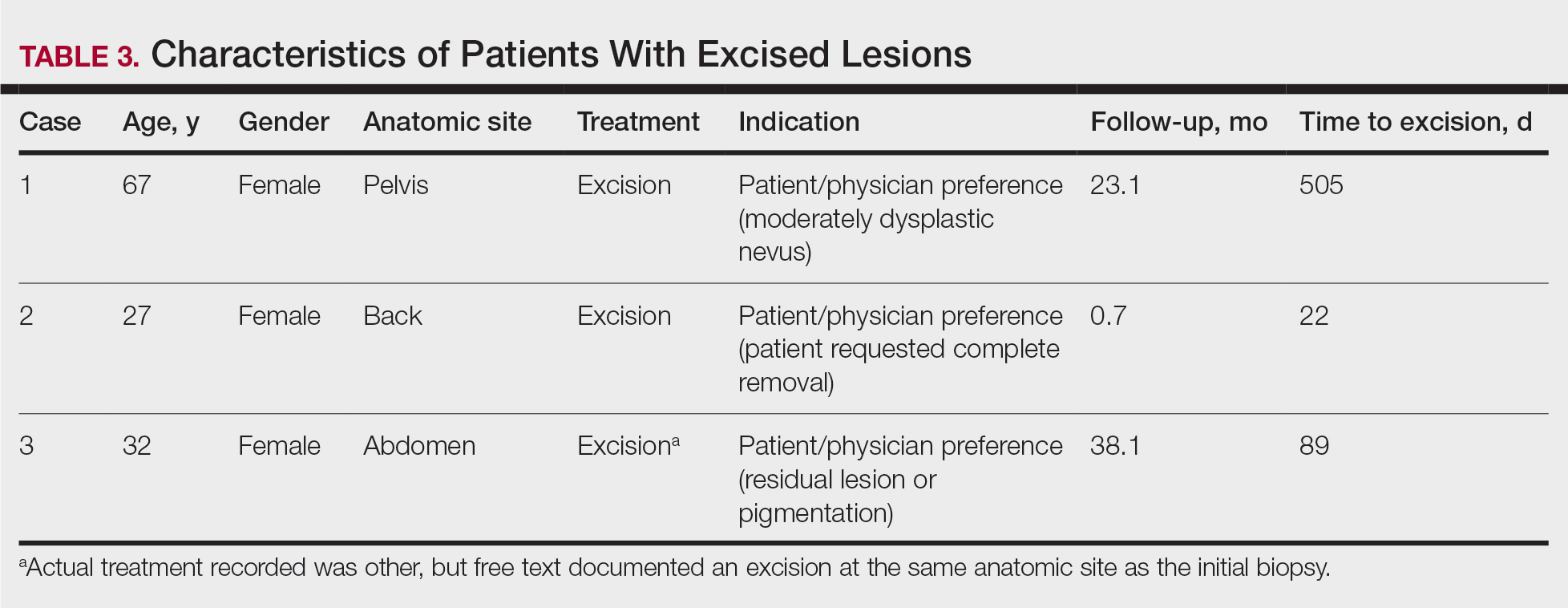
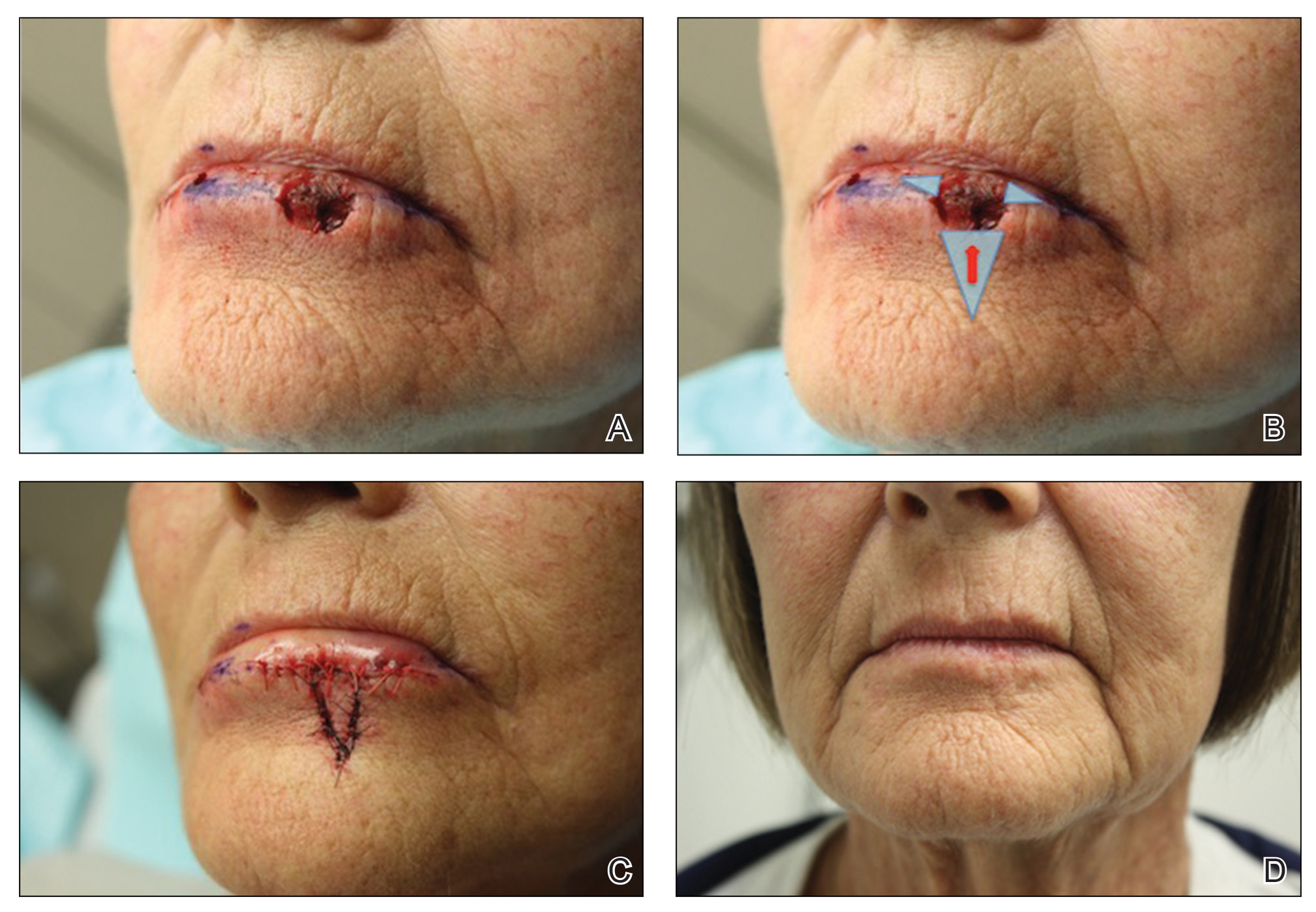
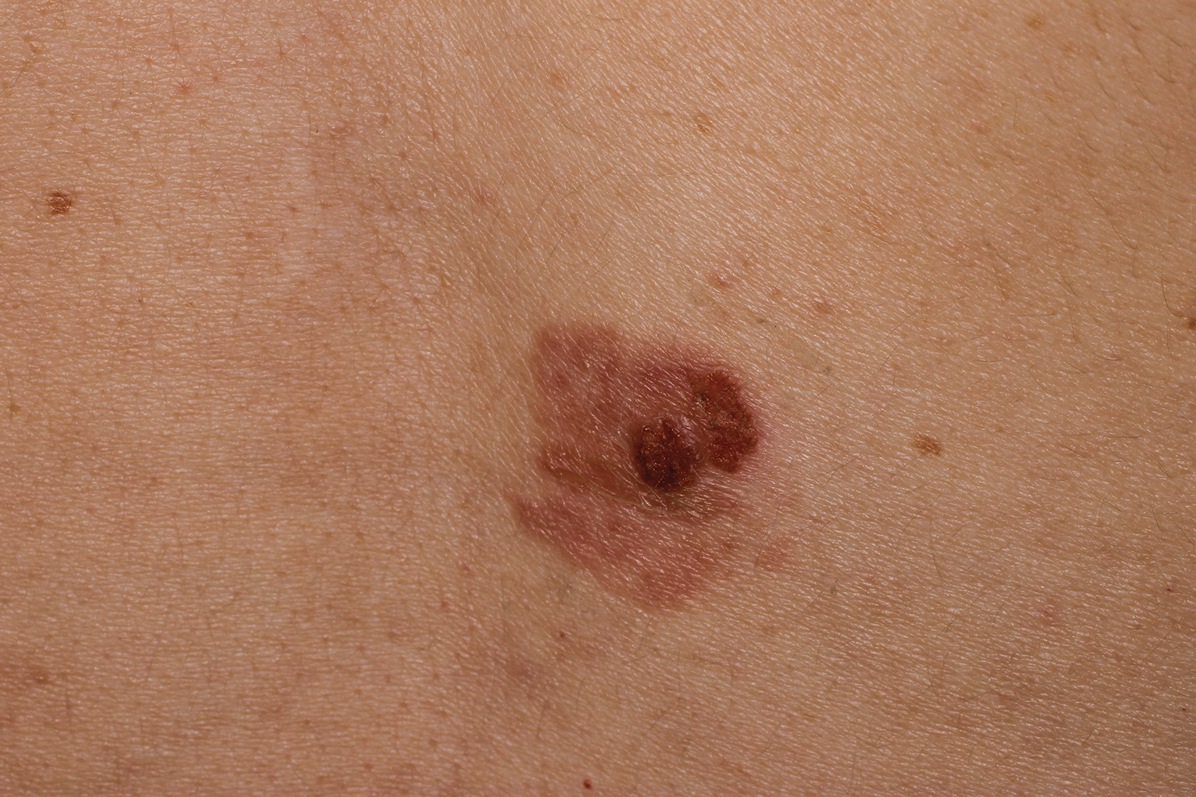
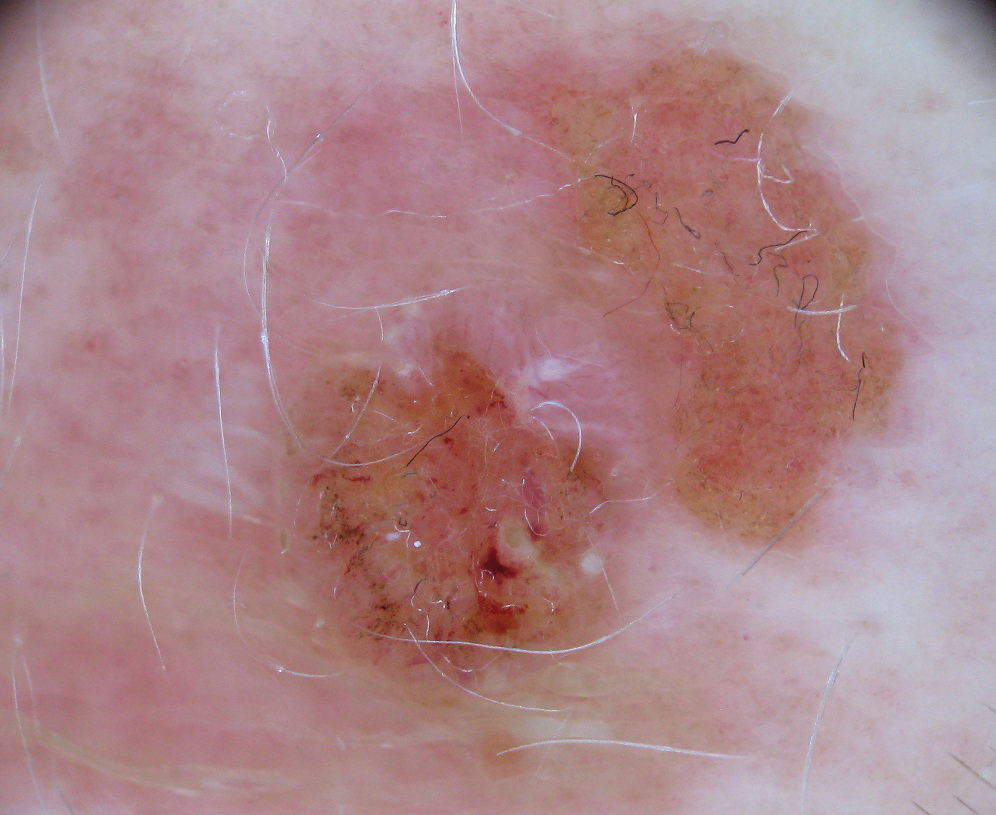
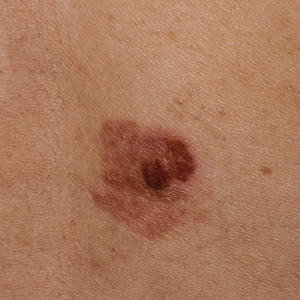
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
According to National Institutes of Health estimates, more than 90,000 new cases of melanoma were diagnosed in 2018.1 Overall 5-year survival for patients with melanoma exceeds 90%, but individual survival estimates are highly dependent on stage at diagnosis, and survival decreases markedly with metastasis. Therefore, early and accurate diagnosis is critical.
Diagnosis of melanocytic neoplasms usually is performed by dermatopathologists through microscopic examination of stained tissue biopsy sections, a technically simple and effective method that enables a definitive diagnosis of benign nevus or malignant melanoma to be made in most cases. However, approximately 15% of all biopsied melanocytic lesions will exhibit some degree of histopathologic ambiguity,2-4 meaning that some of their microscopic features will be characteristic of a benign nevus while others will suggest the possibility of malignant melanoma. Diagnostic interpretations often vary in these cases, even among experts, and a definitive diagnosis of benign or malignant may be difficult to achieve by microscopy alone.2-4 Because of the marked reduction in survival once a melanoma has metastasized, these diagnostically ambiguous lesions often are treated as possible malignant melanomas with complete surgical excision (or re-excision). However, some experts suggest that many histopathologically ambiguous melanocytic neoplasms are, in fact, benign,5 a notion supported by epidemiologic evidence.6,7 Therefore, excision of many ambiguous melanocytic neoplasms might be avoided if definitive diagnosis could be achieved.
A gene expression signature was developed and validated for use as an adjunct to traditional methods of differentiating malignant melanocytic neoplasms from their benign counterparts.8-11 This test quantifies the RNA transcripts produced by 14 genes known to be overexpressed in malignant melanomas by comparison to benign nevi. These values are then combined algorithmically with measurements of 9 reference genes to produce an objective numerical score that is classified as benign, malignant, or indeterminate. When used by board-certified dermatopathologists and dermatologists confronting ambiguous melanocytic lesions, the test produces substantial increases in definitive diagnoses and prompts changes in treatment recommendations.12,13 However, the long-term consequences of foregoing surgical excision of melanocytic neoplasms that are diagnostically ambiguous but classified as benign by this test have not yet been formally assessed. In the current study, prospectively tested patients whose ambiguous melanocytic neoplasms were classified as benign by the gene expression signature were followed for up to 4.5 years to evaluate the long-term safety of treatment decisions aligned with benign test results.
Methods
Study Population
As part of a prior study,12 US-based dermatopathologists submitted tissue sections from biopsied melanocytic neoplasms determined to be diagnostically ambiguous by histopathology for analysis with the gene expression signature (Myriad Genetics, Inc). Diagnostically ambiguous lesions were those lesions that were described as ambiguous, uncertain, equivocal, indeterminate, or other synonymous terms by the submitting dermatopathologist and therefore lacked a confident diagnosis of benign or malignant prior to testing. Patients initially were tested between May 2014 and August 2014, with samples submitted through a prospective clinical experience study designed to assess the impact of the test on diagnosis and treatment decisions. This study was performed under an institutional review board waiver of consent (Quorum #33403/1).
Patients were eligible for inclusion in the current study if their biopsy specimens (1) had an uncertain preliminary diagnosis according to the submitting dermatopathologist (pretest diagnosis of indeterminate); (2) received a negative (benign) score from the gene expression test; (3) were treated as benign by the dermatologist(s) involved in follow-up care; and (4) were submitted by a single site (St. Joseph Medical Center, Houston, Texas). Although a single dermatopathology site was used for this study, multiple dermatologists were involved in the final treatment of these patients. Patients with benign scores who received additional intervention were excluded, as they may have a lower rate of adverse events (ie, metastasis) than those who did not receive intervention and would therefore skew the analysis population. A total of 25 patients from the prior study met these inclusion criteria. The previously collected12 pretest and posttest de-identified data were compiled from the commercial laboratory databases, and the patients were followed from the time of testing via medical record review performed by the dermatology providers at participating sites. Clinical follow-up data were collected using study-specific case report forms (CRFs) that captured the following: (1) the dates and results of clinical follow-up visits; (2) the type(s) of treatment and interventions (if any) performed at those visits; (3) the specific indication for any intervention performed; (4) any evidence of persistent, locally recurrent, and/or distant melanocytic neoplasia (whether definitively attributable to the tested lesion or not); and (5) death from any cause. The CRF assigned interventions to 1 of 5 categories: excision, excision with sentinel lymph node biopsy, referral to dermatologic or other surgeon, examination only (without surgical intervention), and other. Selection of other required a free-text description of the treatment and indications. Pertinent information not otherwise captured by the CRF also was recordable as free text.
Gene Expression Testing
Gene expression testing was carried out at the time of specimen submission in the prior study12 as described previously.14 Briefly, formalin-fixed, paraffin-embedded, unstained tissue sections and/or tissue blocks were submitted for testing along with a single hematoxylin and eosin–stained slide used to identify and designate the representative portion(s) of the lesion to be tested. These areas were macrodissected from unstained tissue sections and pooled for RNA extraction. Expression of 14 biomarker genes and 9 reference genes was measured via
Statistical Analysis
Demographic and other baseline characteristics of the patient population were summarized. Follow-up time was calculated as the interval between the date a patient’s gene expression test result was first issued to the provider and the date of the patient’s last recorded visit during the study period. All patient dermatology office visits within the designated follow-up period were documented, with a nonstandard number of visits and follow-up time across all study patients. Statistical analyses were conducted using SAS software (SAS Institute Inc), R software version 3.5.0 (R Foundation for Statistical Computing), and IBM SPSS Statistics software (IBM SPSS Statistics for Windows, Version 25).
Results
Patient Sample
A total of 25 ambiguous melanocytic neoplasms from 25 patients met the study inclusion criteria of a benign gene expression result with subsequent treatment as a benign neoplasm during follow-up. The patient sample statistics are summarized in Table 1. Most patients were younger than 65 years, with an average age at the time of biopsy of 48.4 years. All 25 neoplasms produced negative (benign) gene expression signature scores, all were diagnosed as benign nevi posttest by the submitting dermatopathologist, and all patients were initially treated in accordance with the benign diagnosis by the dermatologist(s) involved in clinical follow-up care. Prior to testing with the gene expression signature, most of these histopathologically indeterminate lesions received differential diagnoses, the most common of which were dysplastic nevus (84%), melanoma arising from a nevus (72%), and superficial spreading melanoma (64%; eTable). After testing with the gene expression signature and receiving a benign score, most lesions received a single differential diagnosis of dysplastic nevus (88%).
Follow-up and Survival
Clinical follow-up time ranged from 0.6 to 53.3 months, with a mean duration (SD) of 38.5 (16.6) months, and patients attended an average of 4 postbiopsy dermatology appointments (mean [SD], 4.6 [3.6]). According to the participating dermatology care providers, none of the 25 patients developed any indication during follow-up that the diagnosis of benign nevus was inaccurate. No patient had evidence of locally recurrent or metastatic melanoma, and none died during the study period.
Treatment/Interventions
The treatment recorded in the CRF was examination only for 21 of 25 patients, excision for 3, and other for 1 (Table 2). Because the explanation for the selection of other in this case described an excision performed at the same anatomic location as the biopsy, this treatment also was considered an excision for purposes of the study analyses. The 3 excisions all occurred at the first postbiopsy dermatology encounter. Across all follow-up visits, no additional surgical interventions occurred (Table 2).
The first excision (case 1) involved a 67-year-old woman with a lesion on the mid pubic region described clinically as an atypical nevus that generated a pretest histopathologic differential diagnosis including dysplastic nevus, superficial spreading melanoma, and melanoma arising within a nevus (Table 3; Figure, A and B). The gene expression test result was benign (score, −5.4), and the final pathology report diagnosis was nevus with junctional dysplasia, moderate. Surgical excision was performed at the patient’s first return visit, 505 days after initial diagnosis, with moderately dysplastic nevus as the recorded indication for removal. No repigmentation or other evidence of local recurrence or progression was detected, and the treating dermatologist indicated no suspicion that the original diagnosis of benign nevus was incorrect during the 23-month follow-up period.
The second excision (case 2) involved a 27-year-old woman with a pigmented neoplasm on the mid upper back (Figure, C and D) biopsied to rule out dysplastic nevus that resulted in a pretest histopathologic differential diagnosis of dysplastic nevus vs superficial spreading melanoma or melanoma arising within a nevus. The gene expression test result classified the lesion as benign (score, −2.9), and the final pathology diagnosis was nevus, compound, with moderate dysplasia. Despite the benign diagnosis, residual neoplasm (or pigmentation) at the biopsy site prompted the patient to request excision at her first postbiopsy visit, 22 days after testing (Table 3). The CRF completed by the dermatologist reported no indication that the benign diagnosis was inaccurate, but the patient was subsequently lost to follow-up.
The third excision (case 3) involved a 32-year-old woman with a pigmented lesion on the abdomen (Table 3; Figure, E and F). The clinical description was irregular-appearing black papule, nevus with atypia, and the histopathologic differential diagnosis again included dysplastic nevus, superficial spreading melanoma, and melanoma arising within a preexisting nevus. The gene expression signature result was benign (score, −7.2), and the final diagnosis issued within the accompanying pathology report was nevus with moderate junctional dysplasia. Despite the benign diagnosis, excision was performed 89 days after test result availability, with apparent residual pigmentation as the specified indication. As with the other 2 cases, the treating dermatologist confirmed that neither clinical features nor follow-up events suggested malignancy.
Comment
This study followed a cohort of 25 patients with histopathologically ambiguous melanocytic neoplasms that were classified as benign by a diagnostic gene expression test with the intent of determining the outcomes of patients whose treatment aligned with their benign test result. All patients initially were managed according to their test result. During an average posttest clinical follow-up time of more than 3 years (38.5 months), the 25 biopsied lesions, most of which received a differential diagnosis of dysplastic nevus, were regarded as benign nevi by their dermatologists, and the vast majority (88%) received no further surgical intervention. Three patients underwent subsequent excision of the biopsied lesion, with patient or physician preference as the indication in each instance. None of the 25 patients developed evidence of local recurrence, metastasis, or other findings that prompted doubt of the benign diagnosis. The absence of adverse events during clinical follow-up, particularly given that most lesions were not subjected to further intervention, supports use of the gene expression test as a safe and effective adjunct to the diagnosis and treatment of ambiguous melanocytic neoplasms by dermatologists and dermatopathologists.
Ambiguous melanocytic neoplasms evaluated without the aid of molecular adjuncts often result in equivocal or less-than-definitive diagnoses, and further surgical intervention is commonly undertaken to mitigate against the possibility of a missed melanoma.13 In this study, treatment that was aligned with the benign test result allowed most patients to avoid further surgical intervention, which suggests that adjunctive use of the gene signature can contribute to reductions in the physical and economic burdens imposed by unnecessary surgical interventions.15,16 Moreover, any means of increasing accurate and definitive diagnoses may produce an immediate impact on health outcomes by reducing the anxiety that uncertainty often provokes in patients and health care providers alike.
Study Limitations
This study must be interpreted within the context of its limitations. Obtaining meaningful patient outcome data is a common challenge in health care research due to the requisite length of follow-up and sometimes the lack of definitive evidence of adverse events. This is particularly difficult for melanocytic neoplasms because of an apparent inclination for patients with benign diagnoses to abandon follow-up and an increasing tendency for even minimal diagnostic uncertainty to prompt complete excision. Additionally, the only definitive clinical outcome for melanocytic neoplasms is distant metastasis, which (fortunately for patients) is relatively rare. Not surprisingly, studies documenting clinical outcomes of patients with ambiguous melanocytic neoplasms tested prospectively with diagnostic adjuncts are scarce, and this study’s sample size and clinical follow-up compare favorably with the few that exist.17,18 Although most melanomas declare themselves through recurrence or metastasis within several years of initial biopsy,1,19 some are clinically dormant for as long as 10 years after initial detection.20,21 This may be particularly true for the small or early-stage lesions that now comprise the majority of biopsied neoplasms, and such events would go undetected by this study and many others. It also must be recognized that uneventful follow-up, regardless of duration, cannot prove that a biopsied melanocytic neoplasm was benign. Although only 5 patients had a follow-up time of less than 2 years (the time frame in which most recurrence or metastasis will occur), it cannot be definitively proven that a minimum of 2 years recurrence- or metastasis-free survival indicates a benign lesion. Many early-stage malignant melanomas are eradicated by complete excision or even by the initial biopsy if margins are uninvolved.
Because these limitations are intrinsic to melanocytic neoplasms and current management strategies, they pertain to all investigations seeking insights into biological potential through clinical outcomes. Similarly, all current diagnostic tools and procedures have the potential for sampling error, including histopathology. The rarity of adverse outcomes (recurrence and metastasis) in patients with benign test results within this cohort indicates that false-negative results are uncommon, which is further evidenced by a similar rarity of adverse events in prior studies of the gene expression signature.8-10,22 A particular strength of this study is that most of the ambiguous melanocytic neoplasms followed did not undergo excision after the initial biopsy, an increasingly uncommon situation that may increase their likelihood to be informative.
It must be emphasized that the gene expression test, similar to other diagnostic adjuncts, is neither a replacement for histopathologic interpretation nor a substitute for judgment. As with all tests, it can produce false-positive and false-negative results. Therefore, it should always be interpreted within the constellation of the many other data points that must be considered when making a distinction between benign nevus and malignant melanoma, including but not limited to patient age, family and personal history of melanoma, anatomic location, clinical features, and histopathologic findings. As is the case for many diseases, careful consideration of all relevant input is necessary to minimize the risk of misdiagnosis that might occur should any single data point prove inaccurate, including the results of adjunctive molecular tests.
Conclusion
Ancillary methods are emerging as useful tools for the diagnostic evaluation of melanocytic neoplasms that cannot be assigned definitive diagnoses using traditional techniques alone. This study suggests that patients with ambiguous melanocytic neoplasms may benefit from diagnoses and treatment decisions aligned with the results of a gene expression test, and that for those with a benign result, simple observation may be a safe alternative to surgical excision. This expands upon prior observations of the test’s influence on diagnoses and treatment decisions and supports its role as part of dermatopathologists’ and dermatologists’ decision-making process for histopathologically ambiguous melanocytic lesions.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
- Noone AM, Howlander N, Krapcho M, et al, eds. SEER Cancer Statistics Review, 1975-2015. National Cancer Institute website. Updated September 10, 2018. Accessed April 21, 2021. https://seer.cancer.gov/archive/csr/1975_2015/
- Shoo BA, Sagebiel RW, Kashani-Sabet M. Discordance in the histopathologic diagnosis of melanoma at a melanoma referral center. J Am Acad Dermatol. 2010;62:751-756.
- Veenhuizen KC, De Wit PE, Mooi WJ, et al. Quality assessment by expert opinion in melanoma pathology: experience of the pathology panel of the Dutch Melanoma Working Party. J Pathol. 1997;182:266-272.
- Elmore JG, Barnhill RL, Elder DE, et al. Pathologists’ diagnosis of invasive melanoma and melanocytic proliferations: observer accuracy and reproducibility study. BMJ. 2017;357:j2813. doi:10.1136/bmj.j2813
- Glusac EJ. The melanoma ‘epidemic’, a dermatopathologist’s perspective. J Cutan Pathol. 2011;38:264-267.
- Welch HG, Woloshin S, Schwartz LM. Skin biopsy rates and incidence of melanoma: population based ecological study. BMJ. 2005;331:481.
- Swerlick RA, Chen S. The melanoma epidemic. Is increased surveillance the solution or the problem? Arch Dermatol. 1996;132:881-884.
- Ko JS, Matharoo-Ball B, Billings SD, et al. Diagnostic distinction of malignant melanoma and benign nevi by a gene expression signature and correlation to clinical outcomes. Cancer Epidemiol Biomarkers Prev. 2017;26:1107-1113.
- Clarke LE, Flake DD 2nd, Busam K, et al. An independent validation of a gene expression signature to differentiate malignant melanoma from benign melanocytic nevi. Cancer. 2017;123:617-628.
- Clarke LE, Warf BM, Flake DD 2nd, et al. Clinical validation of a gene expression signature that differentiates benign nevi from malignant melanoma. J Cutan Pathol. 2015;42:244-252.
- Minca EC, Al-Rohil RN, Wang M, et al. Comparison between melanoma gene expression score and fluorescence in situ hybridization for the classification of melanocytic lesions. Mod Pathol. 2016;29:832-843.
- Cockerell CJ, Tschen J, Evans B, et al. The influence of a gene expression signature on the diagnosis and recommended treatment of melanocytic tumors by dermatopathologists. Medicine (Baltimore). 2016;95:e4887. doi:10.1097/MD.0000000000004887
- Cockerell C, Tschen J, Billings SD, et al. The influence of a gene-expression signature on the treatment of diagnostically challenging melanocytic lesions. Per Med. 2017;14:123-130.
- Warf MB, Flake DD 2nd, Adams D, et al. Analytical validation of a melanoma diagnostic gene signature using formalin-fixed paraffin-embedded melanocytic lesions. Biomark Med. 2015;9:407-416.
- Guy GP Jr, Ekwueme DU, Tangka FK, et al. Melanoma treatment costs: a systematic review of the literature, 1990-2011. Am J Prev Med. 2012;43:537-545.
- Guy GP Jr, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Egnatios GL, Ferringer TC. Clinical follow-up of atypical spitzoid tumors analyzed by fluorescence in situ hybridization. Am J Dermatopathol. 2016;38:289-296.
- Fischer AS, High WA. The difficulty in interpreting gene expression profiling in BAP-negative melanocytic tumors. J Cutan Pathol. 2018;45:659-666. doi:10.1111/cup.13277
- Vollmer RT. The dynamics of death in melanoma. J Cutan Pathol. 2012;39:1075-1082.
- Osella-Abate S, Ribero S, Sanlorenzo M, et al. Risk factors related to late metastases in 1,372 melanoma patients disease free more than 10 years. Int J Cancer. 2015;136:2453-2457.
- Faries MB, Steen S, Ye X, et al. Late recurrence in melanoma: clinical implications of lost dormancy. J Am Coll Surg. 2013;217:27-34.
- Ko JS, Clarke LE, Minca EC, et al. Correlation of melanoma gene expression score with clinical outcomes on a series of melanocytic lesions. Hum Pathol. 2019;86:213-221.
Practice Point
- Implementation of a gene expression signature in the diagnosis of histopathologically ambiguous lesions can safely increase diagnostic accuracy and optimize treatment.
Mycosis Fungoides in Black Patients: Time for a Better Look
Recent advances in the immunopathogenesis and therapy of cutaneous T-cell lymphoma (CTCL) have shown great promise for the care of patients with mycosis fungoides (MF) and Sézary syndrome (SS).1-3 Research into the tumor microenvironment, microbiome, and molecular genetics may yield further information as we strive to develop MF/SS therapy from the bench to the bedside.3 Although progress has been made on multiple fronts in MF, some important—particularly epidemiologic and clinical—questions remain unanswered.
Racial disparities are well known to exist in CTCLs, particularly MF and SS.4-7 The incidence of MF and SS in the United States is higher in African American/Black patients than in White patients4; in addition, MF has an earlier age at onset in Black patients compared with White patients.4,5 Gender disparities also exist, with relatively more Black females than males affected with MF4-6; in particular, early-onset MF (ie, <40 years of age) is more common in Black females than Black males.6,7 According to Surveillance, Epidemiology, and End Results (SEER) data4 and the US National Cancer Database,5 African American/Black patients with MF have worse outcomes compared with other races (shorter overall survival and higher mortality) and also exhibit higher stages of disease at presentation (stage IIb or higher).5 Black race also was found to be a predictor of poor overall survival after accounting for disease characteristics, socioeconomicfactors, and types of treatment. The factors responsible for these racial disparities remain unclear.
A fortuitous collision of interests and technology may have helped to shed light on some of the reasons for these racial disparities in MF. Nearly 2 decades ago, high-quality, whole-body digital cutaneous photography was implemented by the Dermatology Service at Memorial Sloan Kettering Cancer Center Dermatology Service (New York, New York).8 Although the standardized 20-pose positioning images initially were used for the follow-up evaluation of patients with multiple nevi and melanomas, we incorporated the same photography technique into our multidisciplinary Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center. The multiplicity and clinical heterogeneity of MF lesions is well known, as is the fact that individual MF lesions may develop, respond to therapy, or change independently of other lesions in a given patient. We regularly reviewed these digital images with patients during their visits to assess treatment responses, discussed the need for changes in therapy in the face of progressive disease, and provided encouragement and positive reinforcement for those who improved with time-consuming regimens (eg, phototherapy).
Ultimately, as we became more familiar with looking at images in skin of color, we recognized different clinical features among our Black patients. In the literature, hypopigmented MF is a variant that typically is characterized by CD8+-predominant T cells and is seen more frequently in dark-skinned patients.9 In contrast, hyperpigmented MF has been considered a relatively rare presentation of MF.10 However, using only clinical and demographic information, we were able to identify 2 very different prognostic groups: those with hypopigmented lesions and those with only hyperpigmented and/or erythematous skin lesions.11 In our retrospective review of 157 African American/Black MF patients at our institution—122 with early-stage and 35 with late-stage MF—45% of patients had hypopigmented lesions vs 52% with hyperpigmented and/or erythematous lesions but no hypopigmentation. Those with hypopigmentation had superior outcomes, with better overall survival (P=.002) and progression-free survival (P=.014). In addition, more than 80% of patients who progressed or died from disease had hyperpigmented and/or erythematous lesions without hypopigmentation.11
Sometimes we have to go backward to go forward. Going from the bedside to the bench in our Black MF/SS patients—initially through the clinical recognition of prognostically different lesions, and then through clinicopathologic correlation with immunophenotyping and molecular studies—should provide important clues. Further investigation of Black patients who share similar pigmentary phenotypes of MF also may shed light on the pathogenetic mechanisms responsible for these prognostically significant skin findings. Through these efforts, we hope to identify higher-risk patients, which ultimately will lead to earlier intervention, more effective therapeutic regimens, and improved outcomes.
- Durgin JS, Weiner DM, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: pathways and targets for immune restoration and tumor eradication. J Am Acad Dermatol. 2021;84:587-595.
- Weiner DM, Durgin JS, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: current and future approaches. J Am Acad Dermatol. 2021;84:597-604.
- Quaglino P, Fava P, Pileri A, et al. Phenotypical markers, molecular mutations, and immune microenvironment as targets for new treatments in patients with mycosis fungoides and/or Sézary syndrome. J Invest Dermatol. 2021;141:484-495.
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423.
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.
- Balagula Y, Dusza SW, Zampella J, et al. Early-onset mycosis fungoides among African American women: a single-institution study. J Am Acad Dermatol. 2014;71:597-598.
- Virmani P, Levin L, Myskowski PL, et al. Clinical outcome and prognosis of young patients with mycosis fungoides. Pediatr Dermatol. 2017;34:547-553.
- Halpern AC, Marghoob AA, Bialoglow TW, et al. Standardized positioning of patients (poses) for whole body cutaneous photography. J Am Acad Dermatol. 2003;49:593-598.
- Rodney IJ, Kindred C, Angra K, et al. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808-814.
- Kondo M, Igawa K, Munetsugu T, et al. Increasing numbers of mast cells in skin lesions of hyperpigmented mycosis fungoides with large-cell transformation. Ann Dermatol. 2016;28:115-116.
- Geller S, Lebowitz E, Pulitzer MP, et al. Outcomes and prognostic factors in African American and Black patients with mycosis fungoides/Sézary syndrome: retrospective analysis of 157 patients from a referral cancer center. J Am Acad Dermatol. 2020;83:430-439.
Recent advances in the immunopathogenesis and therapy of cutaneous T-cell lymphoma (CTCL) have shown great promise for the care of patients with mycosis fungoides (MF) and Sézary syndrome (SS).1-3 Research into the tumor microenvironment, microbiome, and molecular genetics may yield further information as we strive to develop MF/SS therapy from the bench to the bedside.3 Although progress has been made on multiple fronts in MF, some important—particularly epidemiologic and clinical—questions remain unanswered.
Racial disparities are well known to exist in CTCLs, particularly MF and SS.4-7 The incidence of MF and SS in the United States is higher in African American/Black patients than in White patients4; in addition, MF has an earlier age at onset in Black patients compared with White patients.4,5 Gender disparities also exist, with relatively more Black females than males affected with MF4-6; in particular, early-onset MF (ie, <40 years of age) is more common in Black females than Black males.6,7 According to Surveillance, Epidemiology, and End Results (SEER) data4 and the US National Cancer Database,5 African American/Black patients with MF have worse outcomes compared with other races (shorter overall survival and higher mortality) and also exhibit higher stages of disease at presentation (stage IIb or higher).5 Black race also was found to be a predictor of poor overall survival after accounting for disease characteristics, socioeconomicfactors, and types of treatment. The factors responsible for these racial disparities remain unclear.
A fortuitous collision of interests and technology may have helped to shed light on some of the reasons for these racial disparities in MF. Nearly 2 decades ago, high-quality, whole-body digital cutaneous photography was implemented by the Dermatology Service at Memorial Sloan Kettering Cancer Center Dermatology Service (New York, New York).8 Although the standardized 20-pose positioning images initially were used for the follow-up evaluation of patients with multiple nevi and melanomas, we incorporated the same photography technique into our multidisciplinary Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center. The multiplicity and clinical heterogeneity of MF lesions is well known, as is the fact that individual MF lesions may develop, respond to therapy, or change independently of other lesions in a given patient. We regularly reviewed these digital images with patients during their visits to assess treatment responses, discussed the need for changes in therapy in the face of progressive disease, and provided encouragement and positive reinforcement for those who improved with time-consuming regimens (eg, phototherapy).
Ultimately, as we became more familiar with looking at images in skin of color, we recognized different clinical features among our Black patients. In the literature, hypopigmented MF is a variant that typically is characterized by CD8+-predominant T cells and is seen more frequently in dark-skinned patients.9 In contrast, hyperpigmented MF has been considered a relatively rare presentation of MF.10 However, using only clinical and demographic information, we were able to identify 2 very different prognostic groups: those with hypopigmented lesions and those with only hyperpigmented and/or erythematous skin lesions.11 In our retrospective review of 157 African American/Black MF patients at our institution—122 with early-stage and 35 with late-stage MF—45% of patients had hypopigmented lesions vs 52% with hyperpigmented and/or erythematous lesions but no hypopigmentation. Those with hypopigmentation had superior outcomes, with better overall survival (P=.002) and progression-free survival (P=.014). In addition, more than 80% of patients who progressed or died from disease had hyperpigmented and/or erythematous lesions without hypopigmentation.11
Sometimes we have to go backward to go forward. Going from the bedside to the bench in our Black MF/SS patients—initially through the clinical recognition of prognostically different lesions, and then through clinicopathologic correlation with immunophenotyping and molecular studies—should provide important clues. Further investigation of Black patients who share similar pigmentary phenotypes of MF also may shed light on the pathogenetic mechanisms responsible for these prognostically significant skin findings. Through these efforts, we hope to identify higher-risk patients, which ultimately will lead to earlier intervention, more effective therapeutic regimens, and improved outcomes.
Recent advances in the immunopathogenesis and therapy of cutaneous T-cell lymphoma (CTCL) have shown great promise for the care of patients with mycosis fungoides (MF) and Sézary syndrome (SS).1-3 Research into the tumor microenvironment, microbiome, and molecular genetics may yield further information as we strive to develop MF/SS therapy from the bench to the bedside.3 Although progress has been made on multiple fronts in MF, some important—particularly epidemiologic and clinical—questions remain unanswered.
Racial disparities are well known to exist in CTCLs, particularly MF and SS.4-7 The incidence of MF and SS in the United States is higher in African American/Black patients than in White patients4; in addition, MF has an earlier age at onset in Black patients compared with White patients.4,5 Gender disparities also exist, with relatively more Black females than males affected with MF4-6; in particular, early-onset MF (ie, <40 years of age) is more common in Black females than Black males.6,7 According to Surveillance, Epidemiology, and End Results (SEER) data4 and the US National Cancer Database,5 African American/Black patients with MF have worse outcomes compared with other races (shorter overall survival and higher mortality) and also exhibit higher stages of disease at presentation (stage IIb or higher).5 Black race also was found to be a predictor of poor overall survival after accounting for disease characteristics, socioeconomicfactors, and types of treatment. The factors responsible for these racial disparities remain unclear.
A fortuitous collision of interests and technology may have helped to shed light on some of the reasons for these racial disparities in MF. Nearly 2 decades ago, high-quality, whole-body digital cutaneous photography was implemented by the Dermatology Service at Memorial Sloan Kettering Cancer Center Dermatology Service (New York, New York).8 Although the standardized 20-pose positioning images initially were used for the follow-up evaluation of patients with multiple nevi and melanomas, we incorporated the same photography technique into our multidisciplinary Cutaneous Lymphoma Clinic at Memorial Sloan Kettering Cancer Center. The multiplicity and clinical heterogeneity of MF lesions is well known, as is the fact that individual MF lesions may develop, respond to therapy, or change independently of other lesions in a given patient. We regularly reviewed these digital images with patients during their visits to assess treatment responses, discussed the need for changes in therapy in the face of progressive disease, and provided encouragement and positive reinforcement for those who improved with time-consuming regimens (eg, phototherapy).
Ultimately, as we became more familiar with looking at images in skin of color, we recognized different clinical features among our Black patients. In the literature, hypopigmented MF is a variant that typically is characterized by CD8+-predominant T cells and is seen more frequently in dark-skinned patients.9 In contrast, hyperpigmented MF has been considered a relatively rare presentation of MF.10 However, using only clinical and demographic information, we were able to identify 2 very different prognostic groups: those with hypopigmented lesions and those with only hyperpigmented and/or erythematous skin lesions.11 In our retrospective review of 157 African American/Black MF patients at our institution—122 with early-stage and 35 with late-stage MF—45% of patients had hypopigmented lesions vs 52% with hyperpigmented and/or erythematous lesions but no hypopigmentation. Those with hypopigmentation had superior outcomes, with better overall survival (P=.002) and progression-free survival (P=.014). In addition, more than 80% of patients who progressed or died from disease had hyperpigmented and/or erythematous lesions without hypopigmentation.11
Sometimes we have to go backward to go forward. Going from the bedside to the bench in our Black MF/SS patients—initially through the clinical recognition of prognostically different lesions, and then through clinicopathologic correlation with immunophenotyping and molecular studies—should provide important clues. Further investigation of Black patients who share similar pigmentary phenotypes of MF also may shed light on the pathogenetic mechanisms responsible for these prognostically significant skin findings. Through these efforts, we hope to identify higher-risk patients, which ultimately will lead to earlier intervention, more effective therapeutic regimens, and improved outcomes.
- Durgin JS, Weiner DM, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: pathways and targets for immune restoration and tumor eradication. J Am Acad Dermatol. 2021;84:587-595.
- Weiner DM, Durgin JS, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: current and future approaches. J Am Acad Dermatol. 2021;84:597-604.
- Quaglino P, Fava P, Pileri A, et al. Phenotypical markers, molecular mutations, and immune microenvironment as targets for new treatments in patients with mycosis fungoides and/or Sézary syndrome. J Invest Dermatol. 2021;141:484-495.
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423.
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.
- Balagula Y, Dusza SW, Zampella J, et al. Early-onset mycosis fungoides among African American women: a single-institution study. J Am Acad Dermatol. 2014;71:597-598.
- Virmani P, Levin L, Myskowski PL, et al. Clinical outcome and prognosis of young patients with mycosis fungoides. Pediatr Dermatol. 2017;34:547-553.
- Halpern AC, Marghoob AA, Bialoglow TW, et al. Standardized positioning of patients (poses) for whole body cutaneous photography. J Am Acad Dermatol. 2003;49:593-598.
- Rodney IJ, Kindred C, Angra K, et al. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808-814.
- Kondo M, Igawa K, Munetsugu T, et al. Increasing numbers of mast cells in skin lesions of hyperpigmented mycosis fungoides with large-cell transformation. Ann Dermatol. 2016;28:115-116.
- Geller S, Lebowitz E, Pulitzer MP, et al. Outcomes and prognostic factors in African American and Black patients with mycosis fungoides/Sézary syndrome: retrospective analysis of 157 patients from a referral cancer center. J Am Acad Dermatol. 2020;83:430-439.
- Durgin JS, Weiner DM, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: pathways and targets for immune restoration and tumor eradication. J Am Acad Dermatol. 2021;84:587-595.
- Weiner DM, Durgin JS, Wysocka M, et al. The immunopathogenesis and immunotherapy of cutaneous T cell lymphoma: current and future approaches. J Am Acad Dermatol. 2021;84:597-604.
- Quaglino P, Fava P, Pileri A, et al. Phenotypical markers, molecular mutations, and immune microenvironment as targets for new treatments in patients with mycosis fungoides and/or Sézary syndrome. J Invest Dermatol. 2021;141:484-495.
- Nath SK, Yu JB, Wilson LD. Poorer prognosis of African-American patients with mycosis fungoides: an analysis of the SEER dataset, 1988 to 2008. Clin Lymphoma Myeloma Leuk. 2014;14:419-423.
- Su C, Nguyen KA, Bai HX, et al. Racial disparity in mycosis fungoides: an analysis of 4495 cases from the US National Cancer Database. J Am Acad Dermatol. 2017;77:497-502.
- Balagula Y, Dusza SW, Zampella J, et al. Early-onset mycosis fungoides among African American women: a single-institution study. J Am Acad Dermatol. 2014;71:597-598.
- Virmani P, Levin L, Myskowski PL, et al. Clinical outcome and prognosis of young patients with mycosis fungoides. Pediatr Dermatol. 2017;34:547-553.
- Halpern AC, Marghoob AA, Bialoglow TW, et al. Standardized positioning of patients (poses) for whole body cutaneous photography. J Am Acad Dermatol. 2003;49:593-598.
- Rodney IJ, Kindred C, Angra K, et al. Hypopigmented mycosis fungoides: a retrospective clinicohistopathologic study. J Eur Acad Dermatol Venereol. 2017;31:808-814.
- Kondo M, Igawa K, Munetsugu T, et al. Increasing numbers of mast cells in skin lesions of hyperpigmented mycosis fungoides with large-cell transformation. Ann Dermatol. 2016;28:115-116.
- Geller S, Lebowitz E, Pulitzer MP, et al. Outcomes and prognostic factors in African American and Black patients with mycosis fungoides/Sézary syndrome: retrospective analysis of 157 patients from a referral cancer center. J Am Acad Dermatol. 2020;83:430-439.
Reconstruction Technique for Defects of the Cutaneous and Mucosal Lip: V-to-flying-Y Closure
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?
Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?
Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
Practice Gap
Reconstruction of a lip defect poses challenges to the dermatologic surgeon. The lip is a free margin, where excess tension can cause noticeable distortion in facial aesthetics. Distortion of that free margin might not only disrupt the appearance of the lip but affect function by impairing oral competency and mobility; therefore, when choosing a method of reconstruction, the surgeon must take free margin distortion into account. Misalignment of the vermilion border upon reconstruction will cause a poor aesthetic result in the absence of free margin distortion. When a surgical defect involves more than one cosmetic subunit of the lip, great care must be taken to repair each subunit individually to achieve the best cosmetic and functional results.
The suitability of traditional approaches to reconstruction of a defect that crosses the vermilion border—healing by secondary intention, primary linear repair, full-thickness wedge repair, partial-thickness wedge repair, and combined cutaneous and mucosal advancement1—depends on the depth of the lesion.
Clinical Presentation
A 66-year-old woman with a 4×6-mm, invasive, well-differentiated squamous cell carcinoma of the left lower lip was referred for Mohs micrographic surgery. Removal of the tumor required 2 stages to obtain clear margins, leaving a 1.0×1.2-cm defect that crossed the vermilion border (Figure, A). How would you repair this defect?
Selecting a Technique to Close the Surgical Defect
For this patient, we had several options to consider in approaching closure, including several that we rejected. Because the defect crossed cosmetic subunit boundaries, healing by secondary intention was avoided, as it would cause contraction, obliterate the vermilion border, and result in poor functional and cosmetic results. We decided against primary closure, even with careful attention to reapproximation of the vermilion border, because the width of the defect would have required a large Burow triangle that extended into the oral cavity. For defects less than one-third the width of the lip, full-thickness wedge repair can yield excellent cosmetic results but, in this case, would decrease the oral aperture and was deemed too extensive a procedure for a relatively shallow defect.2
Instead, we chose to perform repair with V to Y advancement of skin below the cutaneous defect, up to the location of the absent vermilion border, combined with small, horizontal, linear closure of the mucosal portion of the defect. This approach is a variation of a repair described by Jin et al,3 who described using 2 opposing V-Y advancement flaps to repair defects of the lip. This repair has provided excellent cosmetic results for a small series of our patients, preserving the oral aperture and maintaining the important aesthetic location of the vermilion border. In addition, the technique makes it unnecessary for the patient to undergo a much larger repair, such as a full- or partial-thickness wedge when the initial defect is relatively shallow.
Closure Technique
It is essential to properly outline the vermilion border of the lip before initiating the repair, ideally before any infiltration of local anesthesia if the surgeon anticipates that tumor extirpation might cross the vermilion border.
Repair
Closing then proceeds as follows:
• The cutaneous portion of the defect is drawn out in standard V to Y fashion, carrying the incision through the dermis and into subcutaneous tissue. The pedicle of the flap is maintained at the base of the island, serving as the blood supply to the flap.
• The periphery of the flap and surrounding tissue is undermined to facilitate movement superiorly into the cutaneous portion of the defect.
• A single buried vertical mattress suture can be placed at the advancing border of the island, holding it in place at the anticipated location of the vermilion border. The secondary defect created by the advancing V is closed to help push the island into place and prevent downward tension on the free margin of the lip.
• The remaining defect of the vermilion lip is closed by removing Burow triangles at the horizontal edges on each side of the remaining defect (Figure, B). The triangles are removed completely within the mucosal lip, with the inferior edge of the triangle placed at the vermilion border.
• The defect is closed in a primary linear horizontal fashion, using buried vertical mattress sutures and cutaneous approximation.
The final appearance of the sutured defect yields a small lateral extension at the superior edge of the V to Y closure, giving the appearance of wings on the Y, prompting us to term the closure V-to-flying-Y (Figure, C).
Although the limited portion of the mucosal lip that is closed in this fashion might appear thinner than the remaining lip, it generally yields a cosmetically acceptable result in the properly selected patient. Our experience also has shown an improvement in this difference in the months following repair. A full mucosal advancement flap might result in a more uniform appearance of the lower lip, but it is a larger and more difficult procedure for the patient to endure. Additionally, a full mucosal advancement flap risks uniformly creating a much thinner lip.
Postoperative Course
Sutures were removed 1 week postoperatively. Proper location of the vermilion border, without distortion of the free margin, was demonstrated. At 11-month follow-up, excellent cosmetic and functional results were noted (Figure, D).
Practice Implications
This repair (1) demonstrates an elegant method of closing a relatively shallow defect that crosses the vermilion border and (2) allows the surgeon to address each cosmetic subunit individually. We have found that this repair provides excellent cosmetic and functional results, with little morbidity.
The lip is a common site of nonmelanoma squamous cell carcinoma. Poorly planned closing after excision of the tumor risks notable impairment of function or cosmetic distortion. When a defect of the lip crosses cosmetic subunits, it is helpful to repair each subunit individually. V-to-flying-Y closure is an effective method to close defects that cross the vermilion border, resulting in well-preserved cosmetic appearance and function.
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
- Ishii LE, Byrne PJ. Lip reconstruction. Facial Plast Surg Clin North Am. 2009;17:445-453. doi:10.1016/j.fsc.2009.05.007
- Sebben JE. Wedge resection of the lip: minimizing problems. J Dermatol Surg Oncol. 1985;11:60-64. doi:10.1111/j.1524-4725.1985.tb02892.x
- Jin X, Teng L, Zhang C, et al. Reconstruction of partial-thickness vermilion defects with a mucosal V-Y advancement flap based on the orbicularis oris muscle. J Plast Reconstr Aesthet Surg. 2011;64:472-476. doi:10.1016/j.bjps.2010.07.017
Tanning Attitudes and Behaviors in Adolescents and Young Adults
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
Intentional tanning—through sun exposure and tanning beds—is an easily avoidable contributor to skin cancer development and an important area for public education. Since the advent of social media, a correlation between social media use and increased indoor tanning behaviors has been reported.1 In 2010, 11.3% of US adults aged 18 to 29 years reported using a tanning bed in the last 12 months.2 The American Academy of Dermatology first published their “Position Statement on Indoor Tanning” in 1998, endorsing a ban on the sale of indoor tanning equipment for nonmedical purposes.3
Although there has been no outright ban on indoor tanning, regulations have been put in place in many states—including Texas, where (as of 2013) a person younger than 18 years must have written consent from their parent(s) to use a tanning bed. Despite efforts of organizations including the American Academy of Dermatology and the government to educate the public on skin cancer prevention and sun safety, the skin cancer rate has been steadily increasing over the last 20 years.
There is a constant campaign among dermatologists to educate their patients on how to reduce or avoid the risk for skin cancer, including the use of sunscreen and avoidance of tanning. Adolescents and young adults are an especially important demographic to reach and educate because increased UV light exposure during these years leads to a greatly increased risk for skin cancer later in life.4 Data on the overall prevalence of tanning and the demographics of participation in tanning activities are important to capture and can be used to efficiently target higher-risk populations.
In this study, we aimed to investigate the attitudes and behaviors of adolescents and young adults regarding sun protection and tanning. We also aimed to determine which avenues, including social media, would be most effective at educating about skin cancer awareness and sun protection to the higher-risk younger population.
Materials and Methods
We developed an institutional review board–approved protocol for the prospective collection of data from registered patients at the dermatology clinic of the Mays Cancer Center at the University of Texas Health at San Antonio. A paper survey containing 15 rating-scale questions was administered to 60 patients aged 13 to 27 years. Surveys were administered during intake, prior to the patients’ visit with a dermatologist; all visits were of a functional (not cosmetic) nature. Data collection spanned June to August 2018. Survey results were entered into Research Electronic Data Capture (REDCap) software for qualitative analysis.
Results
Sixty patients responded to the survey. The mean age of respondents was 19.5 years. No surveys were excluded from the data set. Table 1 provides baseline characteristics of respondents. Some respondents left questions unanswered, resulting in questions with fewer than 60 responses.
Among respondents to the survey, 70% (42/60) reported it is very important to protect their skin from sun exposure, and 30% (18/60) reported it is somewhat important. Regarding sunscreen use, 70% (42/60) indicated they use sunscreen only before outdoor activities, 12% (7/60) use sunscreen daily, and 17% (10/60) never use sunscreen. Of those who use sunscreen, 52% (28/54) do so to prevent skin damage and aging and 44% (24/54) to prevent skin cancer. Twenty-three percent (13/56) of respondents reported finding tanned skin attractive; 26% (14/55) reported wanting to be tan. Looking at race, 28% (10/36) of Whites, 25% (5/20) of Spanish/Hispanic/Latinos, and 22% (2/9) of Asians found tanned skin attractive; no Black respondents found tanned skin attractive.
Regarding tanning, 12% (7/57) reported using a tanning bed in their lifetime and 4% (2/57) in the last year; 34% (19/56) reported deliberately tanning outdoors; and 9% (5/56) reported using sunless or spray-on tanning. Dermatologists (75% [42/56]), primary care physicians (69.6% [39/56]), and parents (46.4% [26/56]) were perceived as more effective sources of skin care education; among media modalities, television (33.9% [19/56]), Instagram (30.4% [17/60]), and YouTube (23.2% [13/60]) were perceived as more effective sources of skin care education (Table 2).
Comment
Perceptions of Tanning
Almost one-quarter of respondents found tanned skin attractive, which might reflect a shift from prior generations. Compared to the 11% of respondents in the 2010 survey,2 only 3.5% (2/57) of our respondents reported using a tanning bed in the last year, which could reflect the results of recent Texas legislation restricting the use of tanning beds by adolescents.
An alarming number of respondents reported going outdoors with the intention of tanning; although it appears that indoor tanning education has been successful, this finding shows that there is still a need for sun protection education because outdoor tanning is not a suitable alternative. A small number of respondents reported getting a sunless or spray-on tan, which is a risk-free alternative to indoor tanning.
Despite all respondents stating that protecting skin from the sun is important, most respondents surveyed do not use sunscreen daily. More respondents use sunscreen to prevent damage and aging than to prevent skin cancer. Young people might be more alarmed by the threat of early aging and losing their “youthful appearance” than by the possibility of developing skin cancer in the distant future. This discrepancy might indicate a lack of knowledge and be an important focus for future education efforts.
Perceptions of Trustworthiness of Education Sources
Our findings show dermatologists and primary care physicians are important educators on skin protection. Primary care physicians should remain vigilant to recognize at-risk patients who would benefit from skin protection education, especially those who do not see a dermatologist. Education of young people focusing on their concern over maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Although education provided by a physician is effective, using media—particularly social media—might be more efficient. Television, Instagram, and YouTube were listed by respondents as the 3 most preferred media outlets for skin health education, which shows important areas of focus for future advertising. Facebook was listed at a surprisingly low level, possibly showing the change in use of certain social media websites among this age group. According to the Pew Research Center, the most widely used social media apps among young adults aged 18 to 29 years are YouTube (91%), Facebook (63%), Instagram (67%), and Snapchat (62%). More than half of the same demographic visit Facebook (74%), Instagram (63%), Snapchat (61%), and YouTube (51%) daily.5 Although respondents to our survey were not specifically asked about the frequency of their use of social media and our data set includes patients younger than 18 years, we know that social media use has been increasing over the last decade among adolescents.1 Therefore, we assume that more than one-half of respondents to our survey use their reported social media platforms daily.
Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist. Unlike printed pamphlets and posters, advertisements through social media can use metrics such as age, race, gender, and interests to target high-risk individuals.
Study Limitations
This was a single-site study of currently enrolled dermatology patients who might be more aware of skin protection than the general population because they are being treated by a dermatologist. Survey questions regarding demographics, required by our institution, could not effectively differentiate Hispanic and White patients. Respondents could have been subject to the Hawthorne effect—awareness that their behavior is being observed—when responding to the survey because it was administered in the office prior to being seen by a dermatologist.
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
- Falzone AE, Brindis CD, Chren M-M, et al. Teens, tweets, and tanning beds: rethinking the use of social media for skin cancer prevention. Am J Prev Med. 2017;53(3 suppl 1):S86-S94.
- Centers for Disease Control and Prevention. Use of indoor tanning devices by adults—United States, 2010. MMWR Morb Mortal Wkly Rep. 2012;61:323-326.
- American Academy of Dermatology. Position statement on indoor tanning. Amended November 14, 2009. Accessed January 10, 2021. https://server.aad.org/Forms/Policies/Uploads/PS/PS-Indoor%20Tanning%2011-16-09.pdf?
- American Academy of Dermatology. Indoor tanning. Accessed January 10, 2020. https://www.aad.org/media/stats-indoor-tanning
- Perrin A, Anderson M. Share of U.S. adults using social media, including Facebook, is mostly unchanged since 2018. Pew Research Center; April 10, 2019. Accessed April 16, 2021. https://www.pewresearch.org/fact-tank/2019/04/10/share-of-u-s-adults-using-social-media-including-facebook-is-mostly-unchanged-since-2018/
PRACTICE POINTS
- Dermatologists are the preferred educators of skin care for adolescents and young adults.
- Social media is an underused medium for skin cancer prevention education and can reach those who do not regularly see a dermatologist.
- Education of young people focusing on their concerns about maintaining a youthful appearance instead of the possibility of developing skin cancer in the future might be more effective.
Communication Strategies in Mohs Micrographic Surgery: A Survey of Methods, Time Savings, and Perceived Patient Satisfaction
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
Mohs micrographic surgery (MMS) entails multiple time-consuming surgical and histological examinations for each patient. As surgical stages are performed and histological sections are processed, an efficient communication method among providers, medical assistants, histotechnologists, and patients is necessary to avoid delays. To address these and other communication issues, providers have focused on ways to increase clinic efficiency and improve patient-reported outcomes by utilizing new or repurposed communication technologies in their Mohs practice.
Prior reports have highlighted the utility of hands-free headsets that allow real-time communication among staff members as a means of increasing clinic efficiency and decreasing patient wait times.1-4 These systems may mediate a more rapid turnover between stages by mitigating the need for surgeons and support staff to assemble within a designated workspace.1,3,4 However, there is no single or standardized communication method that best suits all surgical suites and MMS practices. Our study aimed to identify the current communication strategies employed by Mohs surgeons and thereby ascertain which method(s) portend(s) the highest benefit in average daily time savings and provider-perceived patient satisfaction.
Materials and Methods
Survey Instrument
A new 10-question electronic survey was published on the SurveyMonkey website, and a link to the survey was provided in a quarterly email that originated from the American College of Mohs Surgery and was distributed to all 1735 active members. Responses were obtained from January 2019 to February 2019.
Statistical Analysis
A statistical analysis was done to determine any significant associations among the providers’ responses. P<.05 was used to determine statistical significance. A Cochran-Armitage test for trend was used to identify significant associations between the number of rooms and the communication systems that were used. Thus, 7 total tests—1 for each device (whiteboard, light system, flag system, wired intercom, wireless intercom, walkie-talkie, or headset)—were conducted. The Cochran-Armitage test also was used to determine whether the probability of using the device was affected by the number of stations/surgical rooms that were attended by the Mohs surgeons. To determine whether the communication devices used were associated with higher patient satisfaction, a χ2 test was conducted for each device (7 total tests), testing the categories of using that device (yes/no) and patient satisfaction (yes/no). A Fisher exact test of independence was used in any case where the proportion for the device and patient satisfaction was 25% or higher. To determine whether the communication method was associated with increased time savings, 7 total Cochran-Armitage tests were conducted, 1 for each device. A logistic regression model was used to determine whether there was a significant association between the number of stations and the likelihood of reporting patient satisfaction.
Results
Eighty-eight surgeons responded to the survey, with a response rate of 5% (88/1735). A total of 55 surgeons completed the survey in its entirety and were included in the data analysis. The most commonly used communication mediums were whiteboards (29/55 [53%]), followed by a flag system (16/55 [29%]) and a light system (13/55 [24%]). Most Mohs surgeons (52/55 [95%]) used the communication media to communicate with their staff only, and 76% (42/55) of Mohs surgeons believed that their communication media contributed to higher patient satisfaction. Overall, 58% (32/55) of Mohs surgeons stated that their communication media saved more than 15 minutes (on average) per day. The use of a whiteboard and/or flag system was reported as the least efficient method, with average daily time savings of 13 minutes. With the introduction of newer technology (wired or wireless intercoms, headsets, walkie-talkies, or internal messaging systems such as Skype) to the whiteboard and/or flag system, the time savings increased by 10 minutes per day. Nearly 25% (14/55) of surgeons utilized more than 1 communication system.
As the number of stations in an MMS suite increased, the probability of using a whiteboard to track the progress of the cases decreased. There were no statistically significant associations identified between the number of stations and the use of other communication devices (ie, flag system, light system, wireless intercom, wired intercom, walkie-talkie, headset). The stratified percentages of the amount of time savings for each communication modality are presented in the Figure (whiteboards and headsets were excluded because they did not increase time savings). The use of a light system was the only communication modality found to be statistically associated with an increase in provider-reported time savings (P=.0482; Figure). In addition, our analysis did not show an improvement in provider-reported patient satisfaction with any of the current systems used in MMS clinics.
Comment
The process of transmitting information among the medical team during MMS is a complex interplay involving the relay of crucial information, with many opportunities for the introduction of distraction and error. Despite numerous improvements in the efficiency of the preparation of histological specimens and implementation of various time-saving and tissue-saving surgical interventions, relatively little attention has been given to address the sometimes chaotic and challenging process of organizing results from each stage of multiple patients in an MMS surgical suite.5
As demonstrated by our survey, incorporation of a light-based system into an MMS clinic may improve workplace efficiency by decreasing the redundant use of support staff and allowing Mohs surgeons to transition from one station to the next seamlessly. Light-based communication systems provide an immediate notification for support staff via color-coded and/or numerically coded indicators on input switches located outside and inside the examination/surgery rooms. The switch indicators can be depressed with minimal disruption from station to station, thereby foregoing the need to interrupt an ongoing excision or closure to convey the status of the case. These systems may then permit enhanced clinic and workflow efficiency, which may help to shorten patient wait times.
Study Limitation
Although all members of the American College of Mohs Surgery were invited to participate in this online survey, only a small number (N=55) completed it in its entirety. Moreover, sample sizes for some of the communication devices were small. As a result, many of the tests might be lacking sufficient power to detect possible relationships, which might be identified in future larger-scale studies.
Conclusion
Our study supports the use of light-based communication systems in MMS suites to improve efficiency in the clinic. Based on our analysis, light-based communication methods were significantly associated with improved time savings (P=.0482). Our study did not show an improvement in provider-reported satisfaction with any of the current systems used in MMS clinics. We hope that this information will help guide providers in implementing new communication techniques to improve clinic efficiency.
Acknowledgments
The authors would like to thank Ms. Kathy Kyler (Oklahoma City, Oklahoma) for her assistance in preparing this manuscript. Support for Dr. Chen and Mr. Stubblefield was provided through National Institutes of Health, National Institute of General Medical Sciences [Grant 2U54GM104938-06, PI Judith James].
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
- Chen T, Vines L, Wanitphakdeedecha R, et al. Electronically linked: wireless, discrete, hands-free communication to improve surgical workflow in Mohs and dermasurgery clinic. Dermatol Surg. 2009;35:248-252.
- Lanto AB, Yano EM, Fink A, et al. Anatomy of an outpatient visit. An evaluation of clinic efficiency in general and subspecialty clinics. Med Group Manage J. 1995;42:18-25.
- Kantor J. Application of Google Glass to Mohs micrographic surgery: a pilot study in 120 patients. Dermatol Surg. 2015;41:288-289.
- Spurk PA, Mohr ML, Seroka AM, et al. The impact of a wireless telecommunication system on efficiency. J Nurs Admin. 1995;25:21-26.
- Dietert JB, MacFarlane DF. A survey of Mohs tissue tracking practices. Dermatol Surg. 2019;45:514-518.
Practice Points
- There are limited studies evaluating the efficacy of different communication methods in Mohs micrographic surgery (MMS) clinics.
- This study suggests that incorporation of a light-based system into an MMS clinic improves workplace efficiency.
Home Treatment of Presumed Melanocytic Nevus With Frankincense
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
To the Editor:
Melanocytic nevi are ubiquitous, and although they are benign, patients often desire to have them removed. We report a patient who presented to our clinic after attempting home removal of a concerning mole on the back with frankincense, a remedy that she found online.
A 43-year-old woman presented with a worrisome mole on the back. She had no personal history of skin cancer, but her father had a history of melanoma in situ in his 60s. The patient reported that she had the mole for years, but approximately 1 month prior to her visit she noticed that it began to bleed and crust, causing concern for melanoma. She read online that the lesion could be removed with topical application of the essential oil frankincense; she applied it directly to the lesion on the back. Within hours she developed a burn where it was applied with associated blistering.
Clinically, the lesion appeared as a darkly pigmented, well-circumscribed papule with hemorrhagic crust overlying a well-demarcated pink plaque (Figure 1). Dermatoscopically, the lesion lacked a pigment network and demonstrated 2 distinct pink papules with peripheral telangiectasia and a pink background with white streaks (Figure 2). A shave biopsy of the lesion demonstrated a nodular basal cell carcinoma extending to the base and margin.
Frankincense is the common name given to oleo-gum-resins of Boswellia species.1 It has been studied extensively for anti-inflammatory and antitumoral properties. It has been demonstrated that high concentrations of its active component, boswellic acid, can have a cytotoxic or cytostatic effect on certain malignant cell lines, such as melanoma, in vitro.2,3 It also has been shown to be antitumoral in mouse models.4 There are limited in vivo studies in the literature assessing the effects of boswellic acid or frankincense on cutaneous melanocytic lesions or other cutaneous malignancies, such as basal cell carcinoma.
A Google search of home remedy mole removal yielded more than 1,000,000 results. At the time of submission, the top 5 results all listed frankincense as a potential treatment along with garlic, iodine, castor oil, onion juice, pineapple juice, banana peels, honey, and aloe vera. None of the results cited evidence for their treatments. Although all recommended dilution of the frankincense prior to application, none warned of potential risks or side effects of its use.
Natural methods of home mole removal have long been sought after. Escharotics are most commonly utilized, including bloodroot (Sanguinaria canadensis), zinc chloride, Chelidonium majus, and Solanum sodomaeum. Many formulations are commercially available online, despite the fact that they can be mutilating and potentially dangerous when used without appropriate supervision.5 This case and an online search demonstrated that these agents are not only potentially harmful home remedies but also are currently falsely advertised as effective therapeutic management for melanocytic nevi.
Approximately 6 million individuals in the United States search the internet for health information daily, and as many as 41% of those do so to learn about alternative medicine.5,6 Although information gleaned from search engines can be useful, it is unregulated and often can be inaccurate. Clinicians generally are unaware of the erroneous material presented online and, therefore, cannot appropriately combat patient misinformation. Our case demonstrates the need to maintain an awareness of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
- Du Z, Liu Z, Ning Z, et al. Prospects of boswellic acids as potential pharmaceutics. Planta Med. 2015;81:259-271.
- Eichhorn T, Greten HJ, Efferth T. Molecular determinants of the response of tumor cells to boswellic acids. Pharmaceuticals (Basel). 2011;4:1171-1182.
- Zhao W, Entschladen F, Liu H, et al. Boswellic acid acetate induces differentiation and apoptosis in highly metastatic melanoma and fibrosarcoma cell. Cancer Detect Prev. 2003;27:67-75.
- Huang MT, Badmaev V, Ding Y, et al. Anti-tumor and anti-carcinogenic activities of triterpenoid, beta-boswellic acid. Biofactors. 2000;13:225-230.
- Adler BL, Friedman AJ. Safety & efficacy of agents used for home mole removal and skin cancer treatment in the internet age, and analysis of cases. J Drugs Dermatol. 2013;12:1058-1063.
- Kanthawala S, Vermeesch A, Given B, et al. Answers to health questions: internet search results versus online health community responses. J Med Internet Res. 2016;18:E95.
Practice Points
- Many patients seek natural methods of home mole removal online, including topical application of essential oils such as frankincense.
- These agents often are unregulated and can be potentially harmful when used without appropriate supervision.
- Dermatologists should be aware of common online fallacies to better answer patient questions and guide them to more accurate sources of dermatologic information and appropriate treatment.