User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Treatment Delay in Melanoma: A Risk Factor Analysis of an Impending Crisis
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
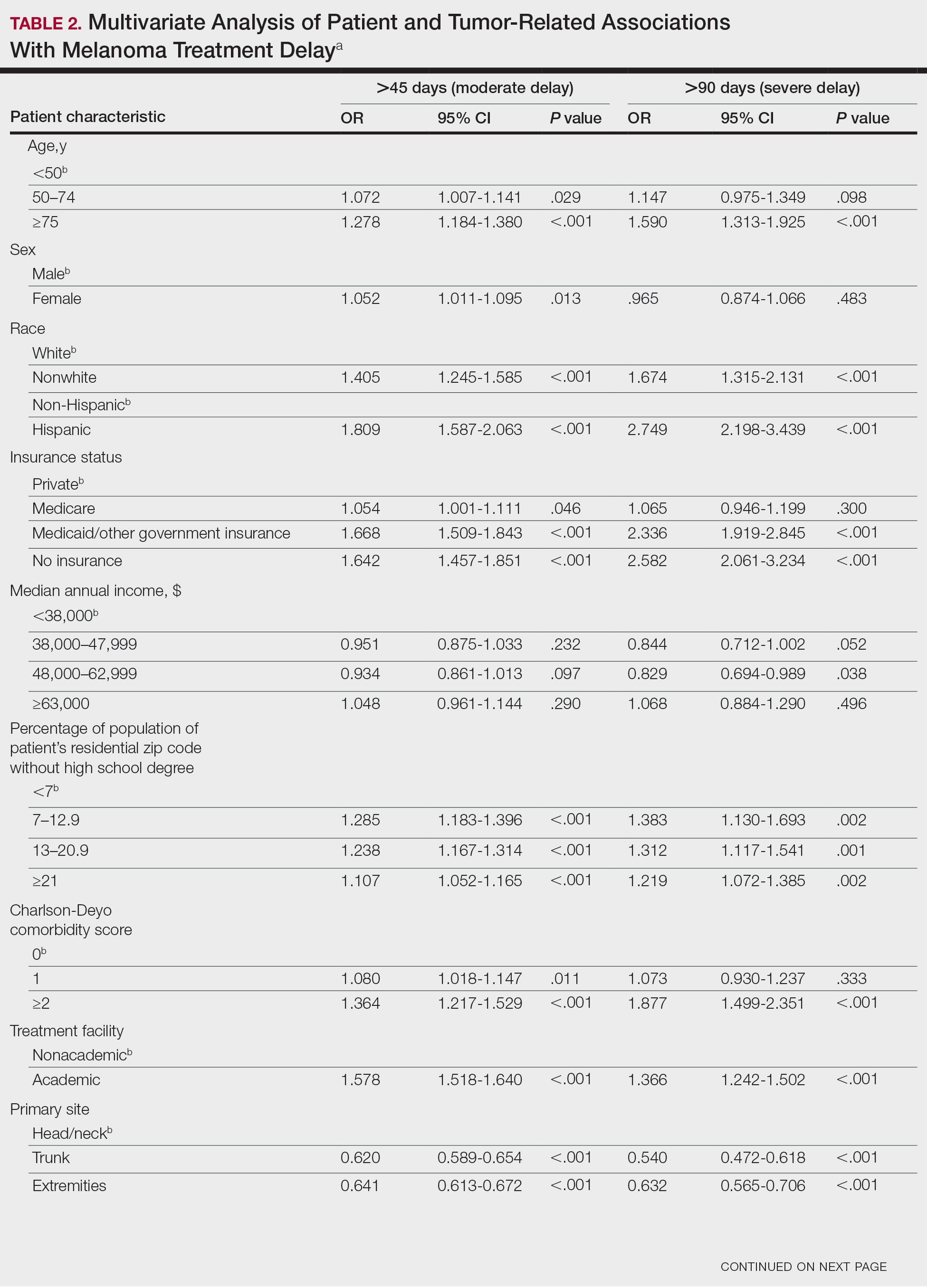
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
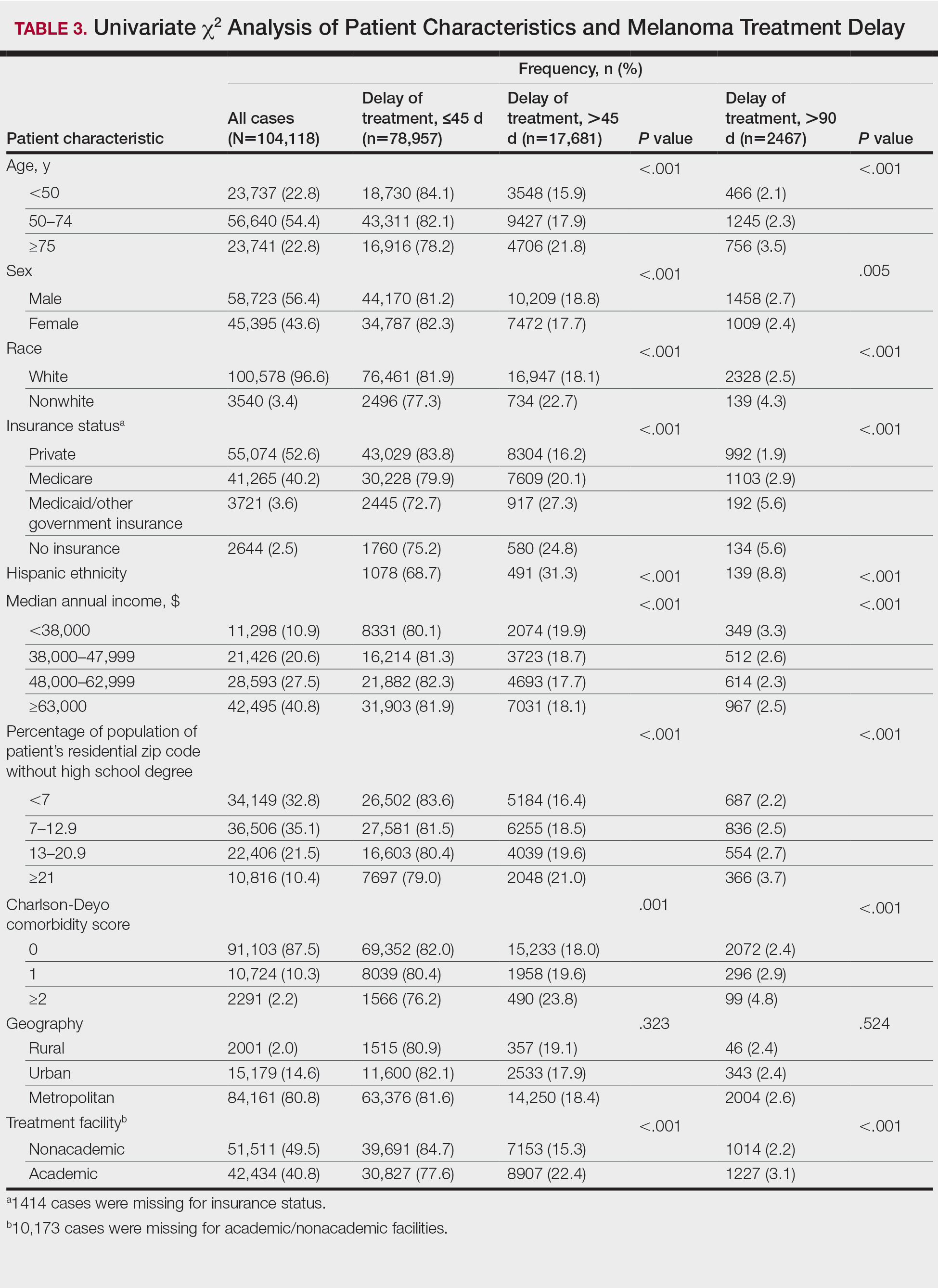
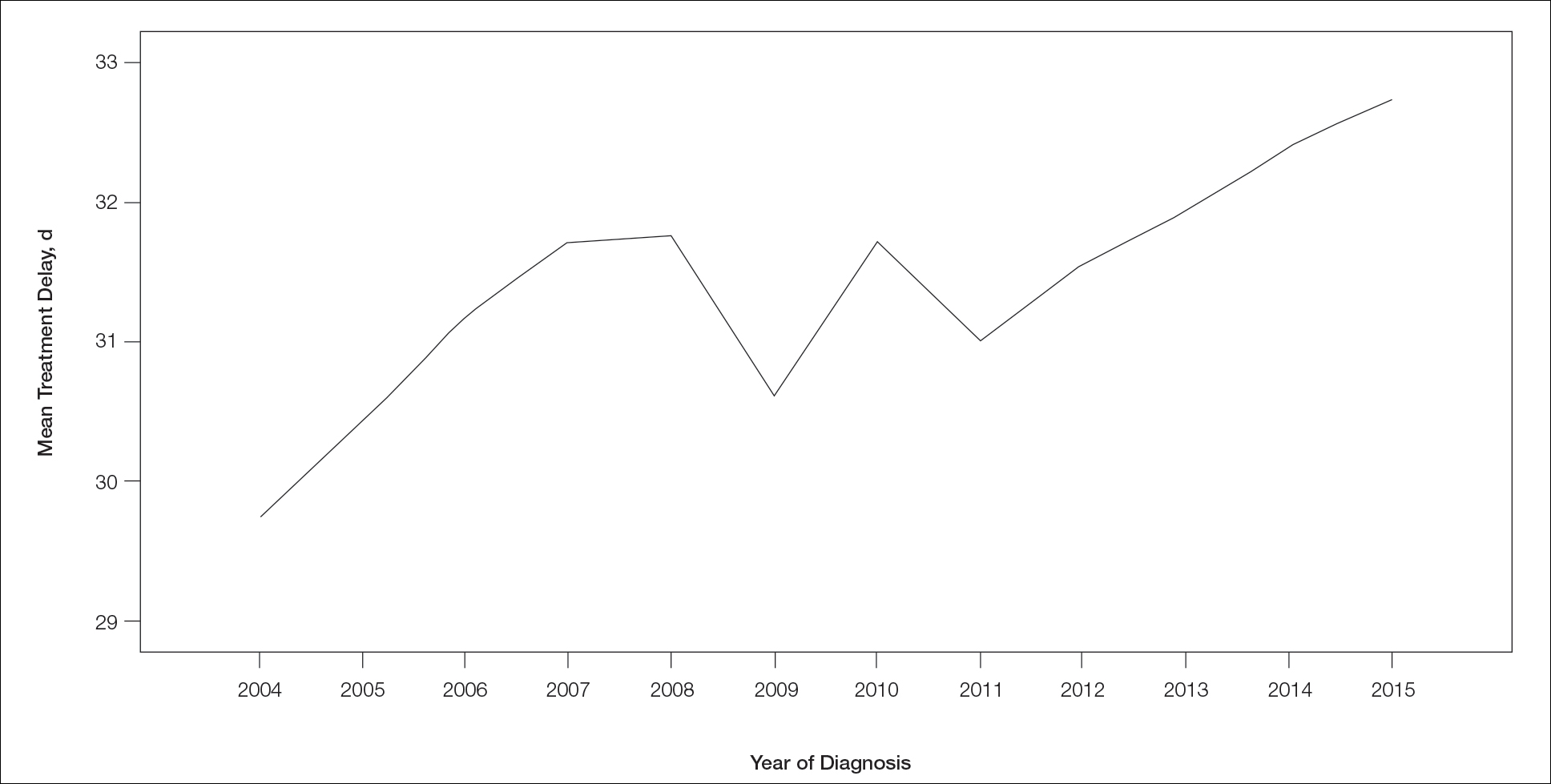
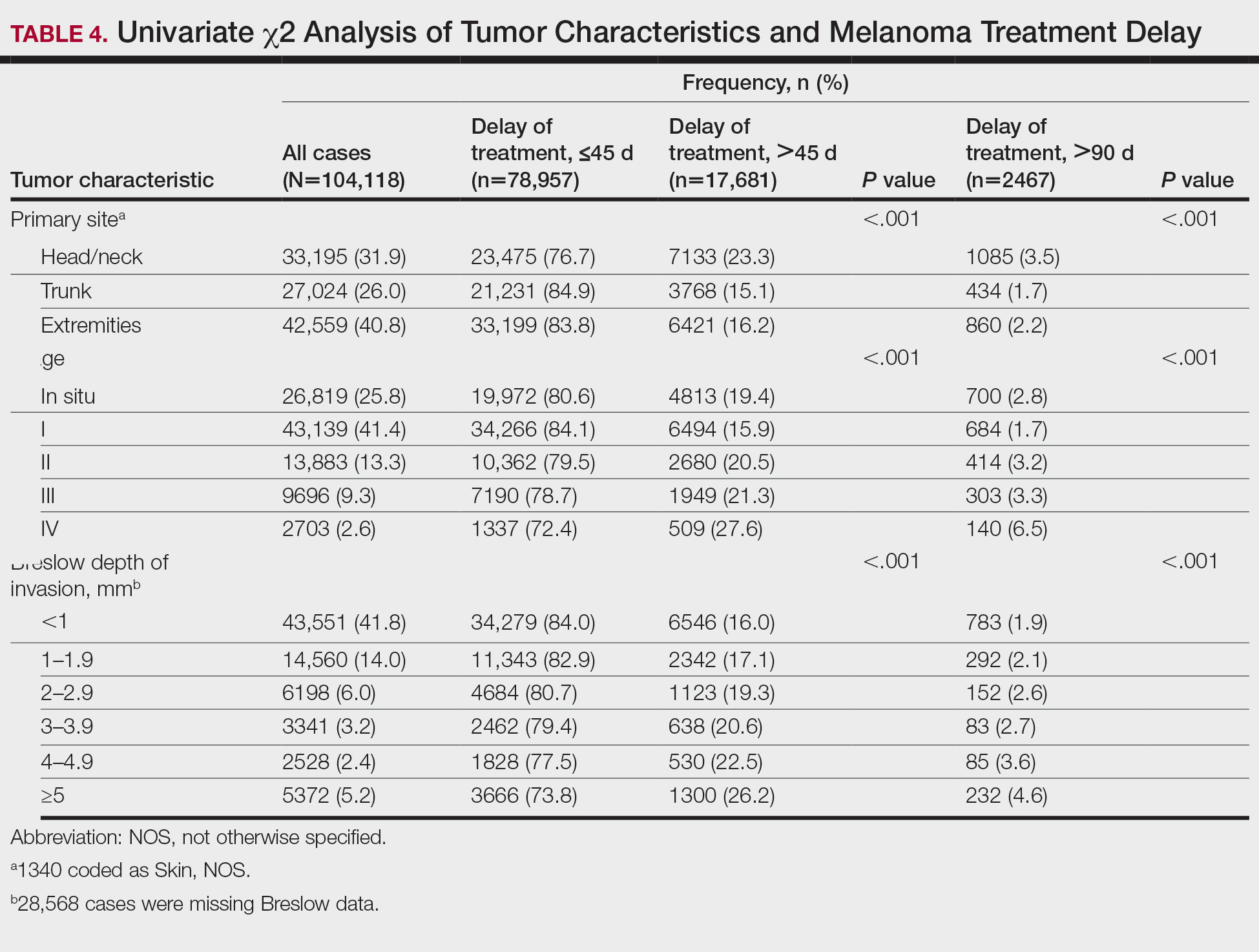
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
Melanoma is the most lethal skin cancer and is the second most common cancer in adolescents and young adults.1 It is the fifth most common cancer in the United States based on incidence, which has steadily risen for the last 2 decades.2,3 For melanoma management, delayed initial diagnosis has been associated with more advanced lesions at presentation and poorer outcomes.4 However, the prognostic implications of delaying melanoma management after diagnosis merits further scrutiny.
This study investigates the associations between melanoma treatment delay (MTD) and patient and tumor characteristics. Although most cases undergo surgical treatment first, more advanced stages may require initiating chemotherapy, radiation therapy, or immunotherapy. In addition, patients who are poor surgical candidates may opt for topical field therapy, such as imiquimod for superficial lesions, prior to more definitive treatment.5 In the Medicaid population, patients who are older than 85 years, married, and previously diagnosed with another melanoma and who also have an increased comorbidity burden have a higher likelihood of MTD.6 For nonmelanoma skin cancers, patient denial is the most common patient-specific factor accounting for treatment delay.7 For this study, our aim was to further evaluate the independent risk factors associated with MTD.
Methods
Case Selection
The National Cancer Database (NCDB) was queried for all cutaneous melanoma cases from 2004 to 2015 (N=525,271). The NCDB is an oncology database sourced from more than 1500 accredited cancer facilities in the United States and Puerto Rico. It receives cases from academic hospitals, Veterans Health Administration hospitals, and community centers.8 Annually, the database collects approximately 70% of cancer diagnoses and 48% of melanoma diagnoses in the United States.9,10 Per institutional guidelines, this analysis was determined to be exempt from institutional review board approval due to the deidentified nature of the dataset.
The selection scheme is illustrated in Table 1. International Statistical Classification of Diseases and Related Health Problems histology codes 8720/3 through 8780/3 combined with the site and morphology primary codes C44.0 through C44.9 identified all patients with a diagnosis of cutaneous melanoma. Primary site was established with the histology codes in the following manner: C44.0 through C44.4 for head/neck primary, C44.5 for trunk primary, C44.6 through C44.7 for extremity primary, and C44.8 through C44.9 for not otherwise specified. Because the NCDB does not specify cause of death, any cases in which the melanoma diagnosis was not the patient’s primary (or first) cancer diagnosis were excluded because of potential ambiguity. Cases lacking histologic confirmation of the diagnosis after primary site biopsy or cases diagnosed from autopsy reports also were excluded. Reports missing staging data or undergoing palliative management were removed. In total, 104,118 cases met the inclusion criteria.
Variables of Interest
The NCDB database codes for a variable “Treatment Started, Days from Dx” are defined as the number of days between the date of diagnosis and the date on which treatment—surgery, radiation, systemic, or other therapy—of the patient began at any facility.11 Treatment delays were classified as more than 45 days or more than 90 days. These thresholds were chosen based on previous studies citing a 45-day recommendation as the timeframe in which primary site excision of melanoma should occur for improved outcomes.1,6,12 Additionally, the postponement cutoffs were aligned with prior studies on surgical delay in melanoma for the Medicaid population.6 Delays of 45 days were labeled as moderate MTD (mMTD), whereas postponements more than 90 days were designated as severe MTD (sMTD).
Patient and tumor characteristics were analyzed for associations with MTD (Table 2). Covariates included age, sex, race (white vs nonwhite), Hispanic ethnicity, insurance status (private; Medicare, Medicaid or other government insurance; and no insurance), median annual income of the patient’s residential zip code (based on 2008-2012 census data), percentage of the population of the patient’s residential zip code without a high school degree (based on 2008-2012 census data), Charlson-Deyo (CD) comorbidity score (a weighted score derived from the sum scores for comorbid conditions), geographic location (rural, urban, and metropolitan), and treatment facility (academic vs nonacademic). Tumor characteristics included primary site (head/neck, trunk, and extremities), stage, and Breslow depth of invasion. Tumor stage was determined using the American Joint Committee on Cancer 6th and 7th editions, depending on the patient’s year of diagnosis.
Statistical Methods
χ2 and Fisher exact tests were used to analyze categorical variables involving patient demographics and tumor characteristics by bivariate analysis (Tables 3 and 4). Multivariate analysis determined the relative impact on MTD by including variables that significantly differed on bivariate χ2 analysis (Table 2). Multivariate modeling determined odds ratio (OR) and corresponding 95% CI for the risk-adjusted associations of the variables with MTD. All statistical analyses were performed using SPSS Statistics version 23 (IBM). P<.05 was considered statistically significant, and all statistical tests were 2-tailed. Line graph figures by year of diagnosis were modeled by SPSS using the mean days of delay per year. Independent sample t tests assessed for differences in mean values.
Results
The final study population included 104,118 patients, most of whom were male (56.4%), white (96.6%), and aged 50 to 74 years (54.4%). Most patients were privately insured (52.6%), had no CD comorbidities (87.5%), and lived in metropolitan cities (80.4%)(Table 3). A large majority (95,473 [91.7%]) of patients received surgery as the first means of treatment, with a smaller portion (863 [0.8%]) having unspecified systemic therapy first. The remaining cases were first treated with chemotherapy (1738 [1.7%]), immunotherapy (382 [0.4%]), or radiation (490 [0.5%]), and the rest did not specify treatment sequence. The tumors were most commonly located on the extremities (40.7%), were stage I (41.2%), and had a Breslow depth of less than 1 mm (41.6%).
Treatment delay averaged 31.55 days, with a median of 27 days. Overall mean MTD increased significantly from 29.74 days in 2004 to 32.55 days in 2015 (2-tailed t test; P<.001)(Figure). A total of 78,957 cases (75.8%) received treatment within 45 days, whereas 2467 cases (2.5%) were postponed past 90 days. On bivariate analysis, age, sex, race, insurance status, Hispanic ethnicity, median annual income of residential zip code, percentage of the population of the patient’s residential zip code with high school degrees, CD score, and academic treatment facility held significant associations with mMTD and sMTD (P<.05)(Table 3). Analyzing bivariate associations with pertinent tumor characteristics—primary site, stage, and Breslow depth—also held significant associations with mMTD and sMTD (P<.001)(Table 4).
On multivariate analysis, controlling for the variables significant on bivariate analysis, multiple factors showed independent associations with MTD (Table 2). Patients aged 50 to 74 years were more likely to have mMTD (reference: <50 years; P=.029; OR=1.072). Patients 75 years and older showed greater rates of mMTD (reference: <50 years; P<.001; OR=1.278) and sMTD (P<.001; OR=1.590). Women had more mMTD (P=.013; OR=1.052). Nonwhite patients had greater rates of both mMTD (reference: white; P<.001; OR=1.405) and sMTD (P<.001; OR=1.674). Hispanic patients also had greater mMTD (reference: non-Hispanic: P<.001; OR=1.809) and sMTD (P<.001; OR=2.749). Compared to patients with private insurance, those with Medicare were more likely to have mMTD (P=.046; OR=1.054). Patients with no insurance or Medicaid/other government insurance showed more mMTD (no insurance: P<.001, OR=1.642; Medicaid/other: P<.001, OR=1.668) and sMTD (no insurance: P<.001, OR=2.582; Medicaid/other: P<.001, OR=2.336).
With respect to the median annual income of the patient’s residential zip code, patients residing in areas with a median income of $48,000 to $62,999 were less likely to have an sMTD (reference: <$38,000; P=.038; OR=0.829). Compared with patients residing in zip codes where a high percentage of the population had high school degrees, areas with higher nongraduate rates had greater overall rates of MTD (P<.001). Patients with more CD comorbidities also held an association with mMTD (CD1 with reference: CD0; P=.011; OR=1.080)(CD2 with reference: CD0; P<.001; OR=1.364) and sMTD (CD2 with reference: CD0; P<.001; OR=1.877). Academic facilities had greater rates of mMTD (reference: nonacademic facilities; P<.001; OR=1.578) and sMTD (P<.001; OR=1.366). In reference to head/neck primaries, primary sites on the trunk and extremities showed fewer mMTD (trunk: P<.001, OR=0.620; extremities: P<.001, OR=0.641) and sMTD (trunk: P<.001, OR=0.540; extremities: P<.001, OR=0.632). Compared with in situ disease, stage I melanomas were less likely to have treatment delay (mMTD: P<.001, OR=0.902; sMTD: P<.001, OR=0.690), whereas stages II (mMTD: P<.001, OR=1.130), III (mMTD: P<.001, OR=1.196; sMTD: P=.023, OR=1.204), and IV (mMTD: P<.001, OR=1.690; sMTD: P<.001, OR=2.240) were more highly associated with treatments delays.
Comment
The path to successful melanoma management involves 2 timeframes. One is time to diagnosis and the other is time to treatment. With 24.2% of patients receiving treatment later than 45 days after diagnosis, MTD is common and, according to our results, has increased on average from 2004 to 2015. This delay may be partially explained by a shortage of dermatologists, leading to longer wait times and follow-up.13,14 Melanoma treatment delay also varied based on insurance status. Unsurprisingly, those with private insurance showed the lowest rates of MTD. Those with no insurance, Medicare, or Medicaid/other government insurance likely faced greater socioeconomic barriers to health care, such as coverage issues.15 Transportation, low health literacy, and limited work schedule flexibility have been described as additional hurdles to health care that could contribute to this finding.16,17 Similarly, nonwhite patients, Hispanic patients, and those from zip codes with low high school graduation rates had more MTD. Although these findings may be explained by socioeconomic barriers and heightened distrust of the health care system, it also is important to consider physician accessibility.18,19
Considering the 2011 Affordable Care Act along with the 2014 Medicaid expansion, our study holds implications on the impact of these legislations on melanoma treatment. Studies have supported expected rises in Medicaid coverage.20,21 The overall uninsured rate in the United States declined from 16% in 2010 to 9.1% in 2015.22 In our study, the uninsured population showed the highest average MTD rates, though those with Medicaid also had significant MTD. Another treacherous hurdle for patients is the coordination of care among dermatologists, oncologists, general surgeons, plastic surgeons, and Mohs surgeons as a multidisciplinary team. Lott et al6 found that patients who received both biopsy and excision from a dermatologist had the shortest treatment delays, whereas those who had a dermatologist biopsy the site and a different surgeon—including Mohs surgeons—excise it experienced significantly greater MTDs (probablility of MTD >45 days was 31% [95% CI, 24%-37%]. This discordant care and referrals could explain the surprising finding that treatment at an academic facility was independently associated with more MTD, possibly due to the care transitions and referrals that disproportionately affect academic centers and multidisciplinary teams, as mentioned above, regarding the transition of care to other physicians (eg, plastic surgeon). A total of 70.1% of our cases treated at academic facilities reported a prior diagnosis at another facility. These results should not dissuade the pursuit of multidisciplinary treatment teams but should raise caution to untimely referrals.
Age, sex, and race were all associated with more MTD. Patients older than 50 years likely face more complex decisions regarding treatment burden, quality of life, and functional outcomes of more aggressive treatments. High rates of surgical refusal for a number of malignancies have been documented in the elderly population,23-25 which is of particular concern for the high surgery burden of head and neck melanomas,26 as further supported by the findings of more MTD for head and neck primaries. As with elderly patients, patients with higher comorbidity scores and more advanced tumors face similar family–patient care discussions to guide treatment. Additionally, women were more likely to experience MTD, which may be connected to a greater concern for cosmesis27 and necessitate more complex management options, such as Mohs micrographic surgery (a procedure that has gained some support for melanoma excision with the help of immunostaining).28
There are several limitations to this study. Accurate data rely on precise record keeping, reporting, and coding by the contributing institutions. The NCDB case diagnosis is derived from data entry without a centralized review process by experienced dermatopathologists. We could not assess the effects of tumor diameter, as these data were inadequately recorded within the dataset. The NCDB also does not provide details on specific immunotherapy or chemotherapy agents. The NCDB also is a facility-based data source, potentially biasing the melanoma data toward thicker advanced tumors more readily managed at such institutions. Lastly, it is impossible to distinguish between patient-related (ie, difficult decision-making) and health care–related (ie, health care accessibility) delays. Nonetheless, we maintain that minimizing MTD is important for survival outcomes and for limiting the progression of melanomas, regardless of the underlying rationale. We believe that our study expands on conclusions previously limited to a Medicare population.
Conclusion
According to the NCDB, mean MTD has increased significantly from 2004 to 2015. Our results suggest that MTD is relatively common in the United States, thereby increasing the risk for metastases. Higher MTD rates are independently associated with being older than 50 years, female, nonwhite, not privately insured, Hispanic, and treated at an academic facility; having a positive comorbidity history and stage II to IV tumors; and residing in a zip code with a low high school graduation rate. Stage I tumors, primaries not located on the head or neck, and residing in a zip code with a higher median income are associated with lower MTD rates. Policymakers, patients, and dermatologists should better recognize these risk factors to facilitate patient guidance and health equity.
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
- Huff LS, Chang CA, Thomas JF, et al. Defining an acceptable period of time from melanoma biopsy to excision. Dermatol Reports. 2012;4:E2.
- Matthews NH, Li WQ, Qureshi AA, et al. Epidemiology of Melanoma. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30.
- Nelson BR, Hamlet KR, Gillard M, et al. Sebaceous carcinoma. J Am Acad Dermatol. 1995;33:1-15.
- Fan Q, Cohen S, John B, et al. Melanoma in situ treated with topical imiquimod for management of persistently positive margins: a review of treatment methods. Ochsner J. 2015;15:443-447.
- Lott JP, Narayan D, Soulos PR, et al. Delay of surgery for melanoma among Medicare beneficiaries. JAMA Dermatol. 2015;151:731-741.
- Renzi C, Mastroeni S, Mannooranparampil TJ, et al. Delay in diagnosis and treatment of squamous cell carcinoma of the skin. Acta Derm Venereol. 2010;90:595-601.
- Winchester DP, Stewart AK, Phillips JL, et al. The National Cancer Database: past, present, and future. Ann Surg Oncol. 2010;17:4-7.
- Raval MV, Bilimoria KY, Stewart AK, et al. Using the NCDB for cancer care improvement: an introduction to available quality assessment tools. J Surg Oncol. 2009;99:488-490.
- Turkeltaub AE, Pezzi TA, Pezzi CM, et al. Characteristics, treatment, and survival of invasive malignant melanoma (MM) in giant pigmented nevi (GPN) in adults: 976 cases from the National Cancer Data Base (NCDB). J Am Acad Dermatol. 2016;74:1128-1134.
- Boffa DJ, Rosen JE, Mallin K, et al. Using the National Cancer Database for outcomes research: a review. JAMA Oncol. 2017;3:1722-1728.
- Riker AI, Glass F, Perez I, et al. Cutaneous melanoma: methods of biopsy and definitive surgical excision. Dermatol Ther. 2005;18:387-393.
- Kimball AB, Resneck JS Jr. The US dermatology workforce: a specialty remains in shortage. J Am Acad Dermatol. 2008;59:741-745.
- Glazer AM, Farberg AS, Winkelmann RR, et al. Analysis of trends in geographic distribution and density of US dermatologists. JAMA Dermatol. 2017;153:322-325.
- Okoro CA, Zhao G, Dhingra SS, et al. Peer reviewed: lack of health insurance among adults aged 18 to 64 years: findings from the 2013 Behavioral Risk Factor Surveillance System. Prev Chronic Dis. 2015;12:E231.
- Syed ST, Gerber BS, Sharp LK. Traveling towards disease: transportation barriers to health care access. J Community Health. 2013;38:976-993.
- Valerio M, Cabana MD, White DF, et al. Understanding of asthma management: Medicaid parents’ perspectives. Chest. 2006;129:594-601.
- Kaplan CP, Nápoles A, Davis S, et al. Latinos and cancer information: perspectives of patients, health professionals and telephone cancer information specialists. J Health Dispar Res Pract. 2016;9:154-167.
- Armstrong K, Ravenell KL, McMurphy S, et al. Racial/ethnic differences in physician distrust in the United States. Am J Public Health. 2007;97:1283-1289.
- Moss HA, Havrilesky LJ, Chino J. Insurance coverage among women diagnosed with a gynecologic malignancy before and after implementation of the Affordable Care Act. Gynecol Oncol. 2017;146:457-464.
- Moss HA, Havrilesky LJ, Zafar SY, et al. Trends in insurance status among patients diagnosed with cancer before and after implementation of the Affordable Care Act. J Oncol Pract. 2018;14:E92-E102.
- Obama B. United States health care reform: progress to date and next steps. JAMA. 2016;316:525-532.
- Crippen MM, Brady JS, Mozeika AM, et al. Impact of body mass index on operative outcomes in head and neck free flap surgery. Otolaryngol Head Neck Surg. 2018;159:817-823.
- Verkooijen HM, Fioretta GM, Rapiti E, et al. Patients’ refusal of surgery strongly impairs breast cancer survival. Ann Surg. 2005;242:276-280.
- Wang J, Wang FW. Refusal of cancer-directed surgery strongly impairs survival of patients with localized hepatocellular carcinoma. Int J Surg Oncol. 2010;2010:381795.
- Zito PM, Scharf R. Cancer, melanoma, head and neck. StatPearls. StatPearls Publishing; 2018.
- Al-Dujaili Z, Henry M, Dorizas A, et al. Skin cancer concerns particular to women. Int J Womens Dermatol. 2017;3:S49-S51.
- Etzkorn JR, Jew OS, Shin TM, et al. Mohs micrographic surgery with melanoma antigen recognized by T cells 1 (MART-1) immunostaining for atypical intraepidermal melanocytic proliferation. J Am Acad Dermatol. 2018;79:1109-1116.e1
Practice Points
- Melanoma treatment delays (MTDs) have been linked to poor outcomes.
- Based on the National Cancer Database, the mean MTD has increased significantly from 2004 to 2015 (P11<.001).
- More delays are seen in patients who are older than 50 years, female, nonwhite, not privately insured, and treated at an academic facility and who have more advanced tumor stage and head/neck primaries.
Fatal Case of Levamisole-Induced Vasculopathy in a Cocaine User
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
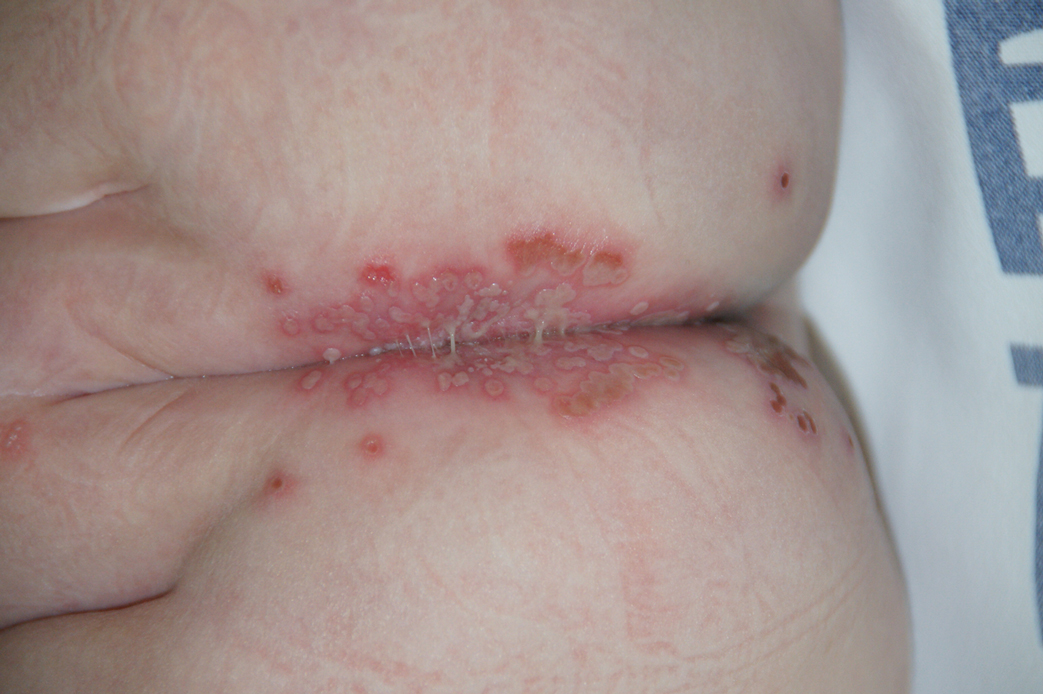
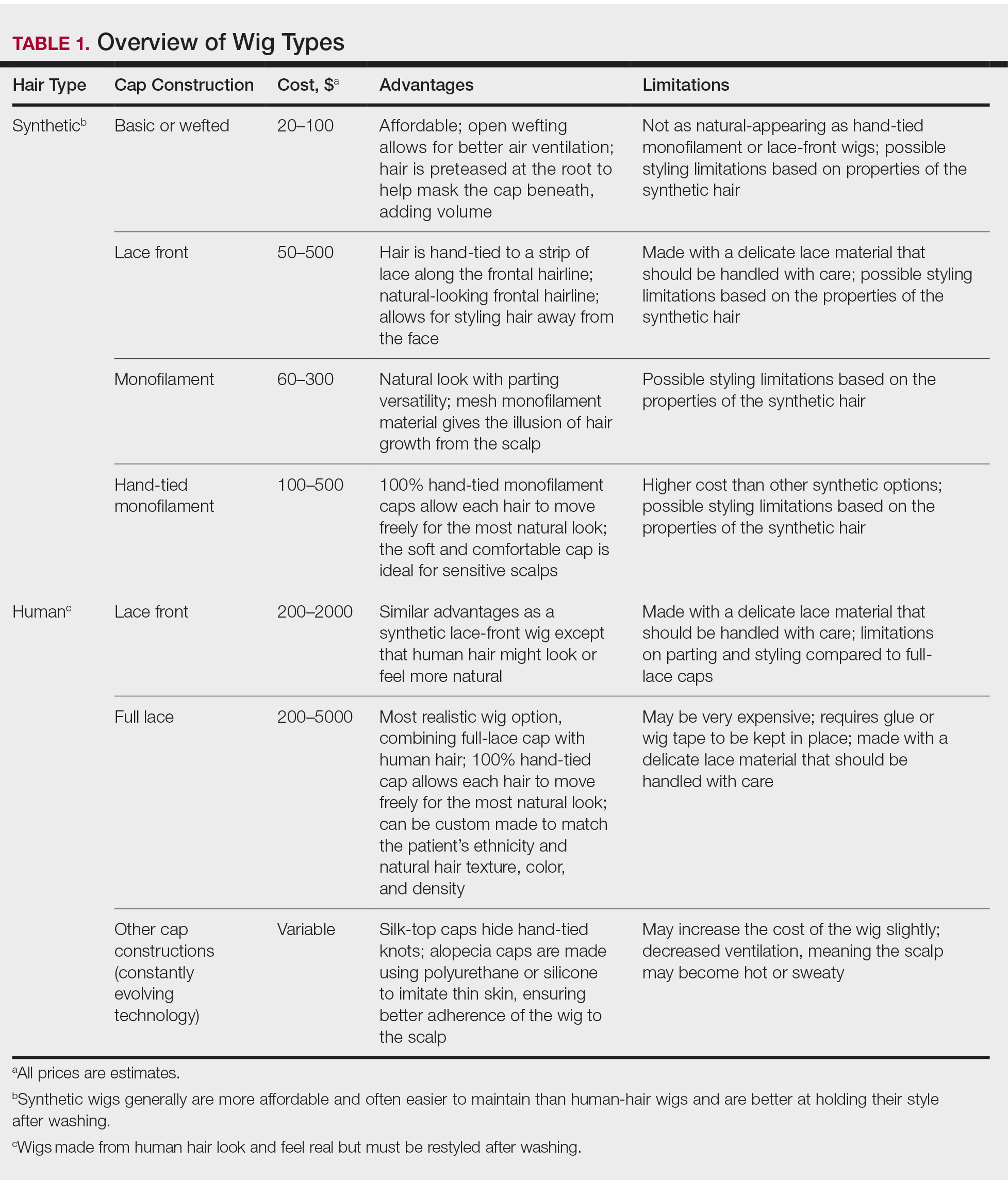
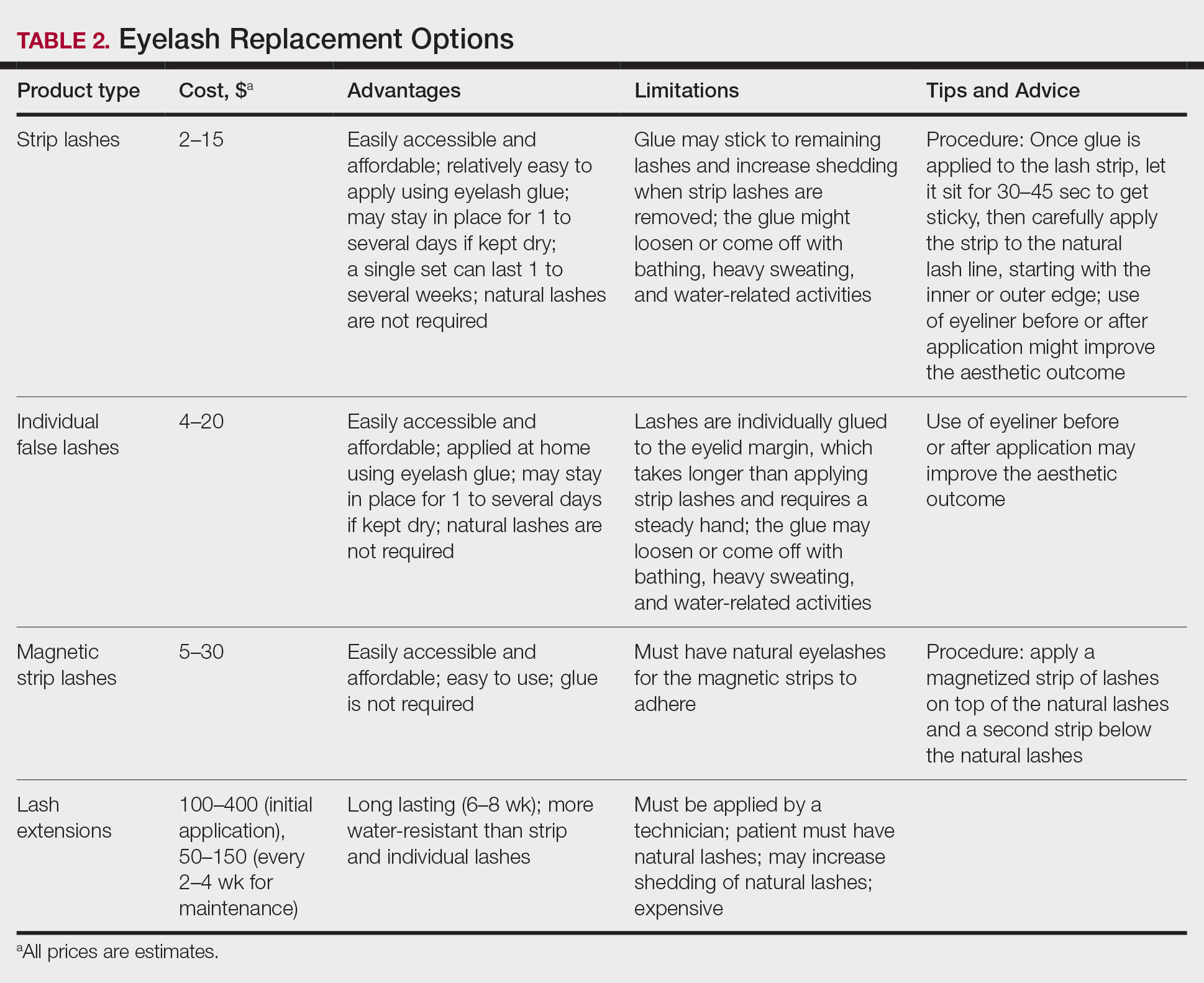
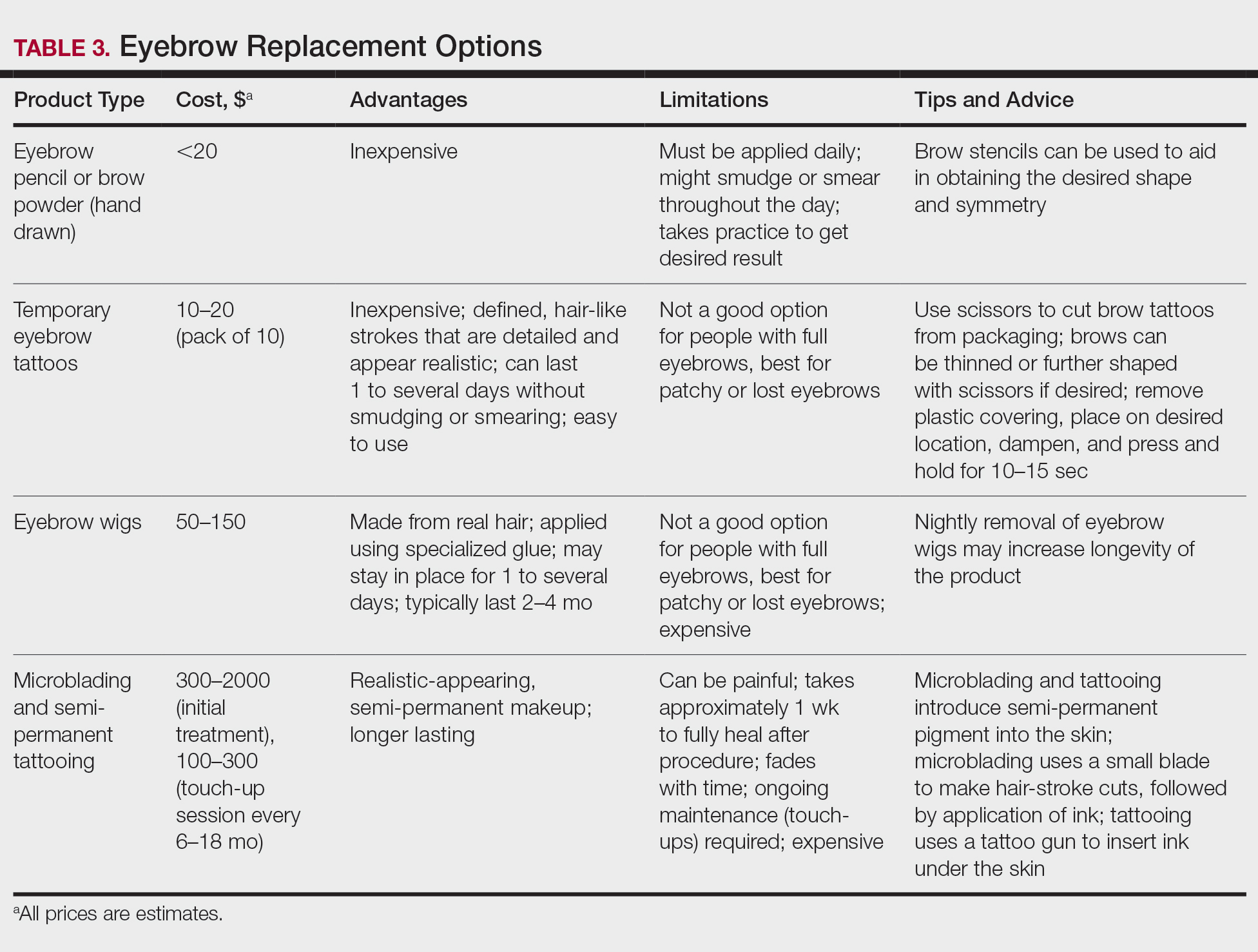
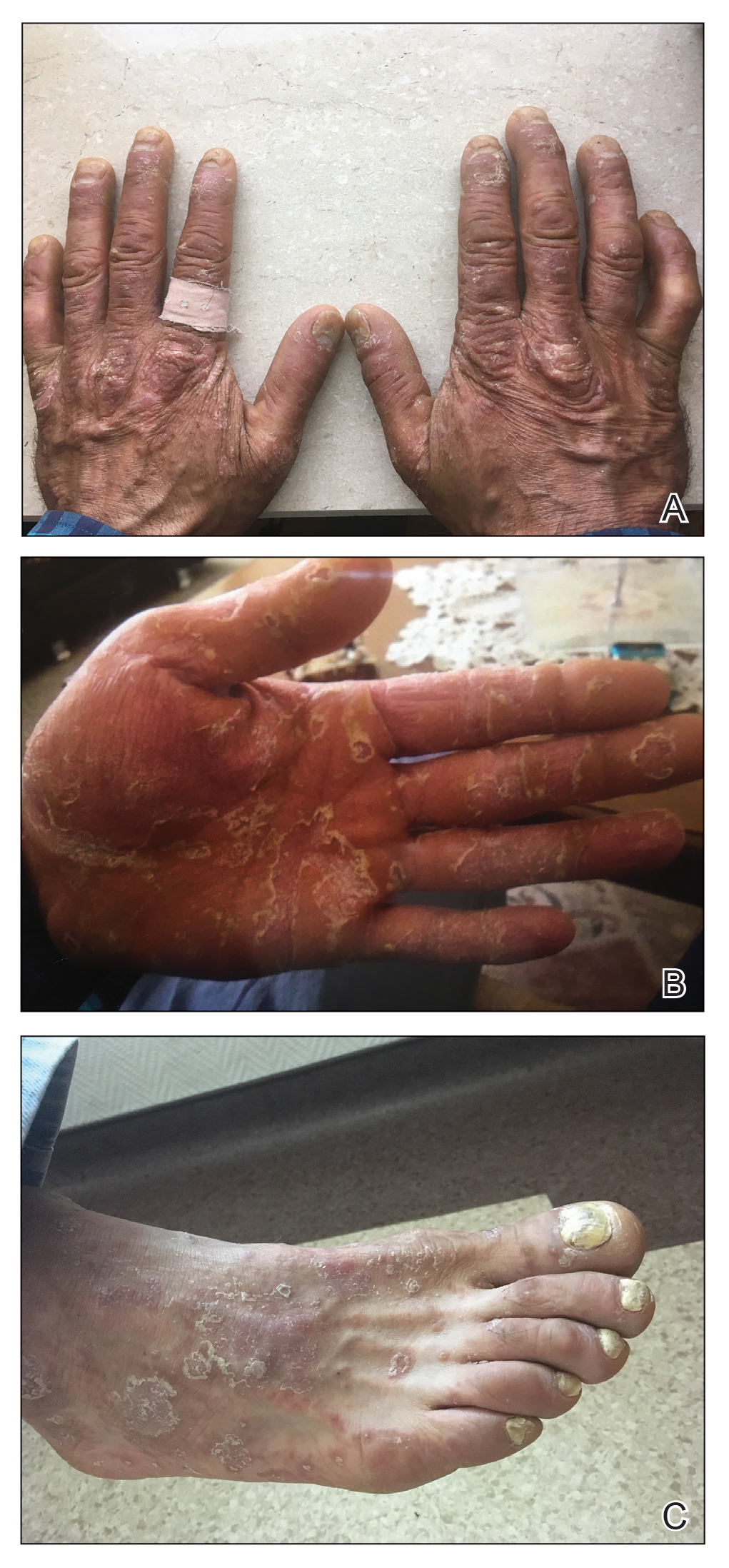
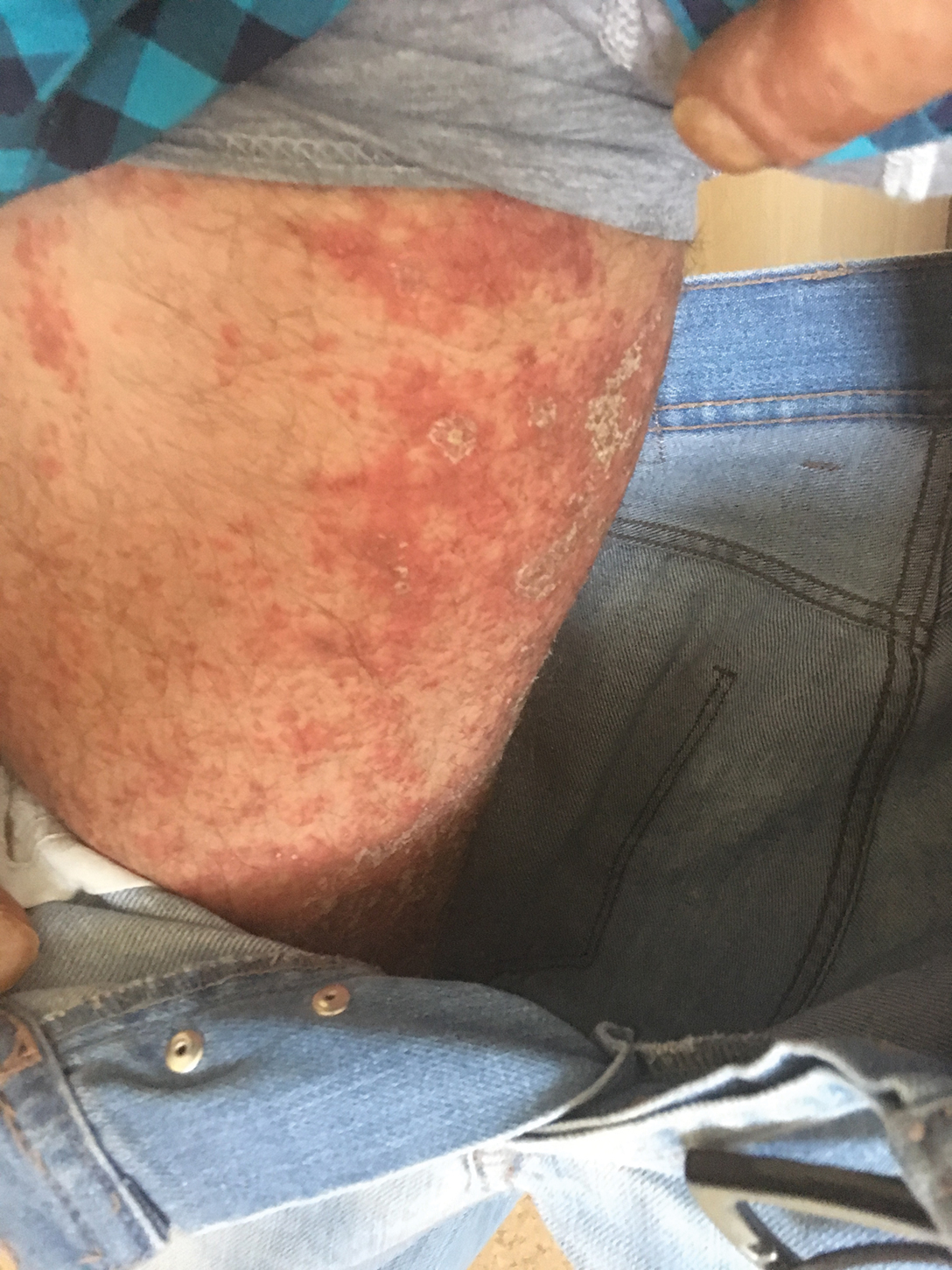
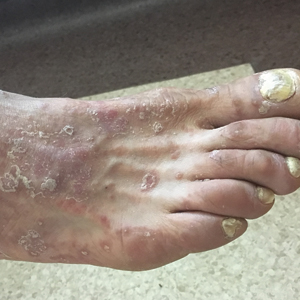
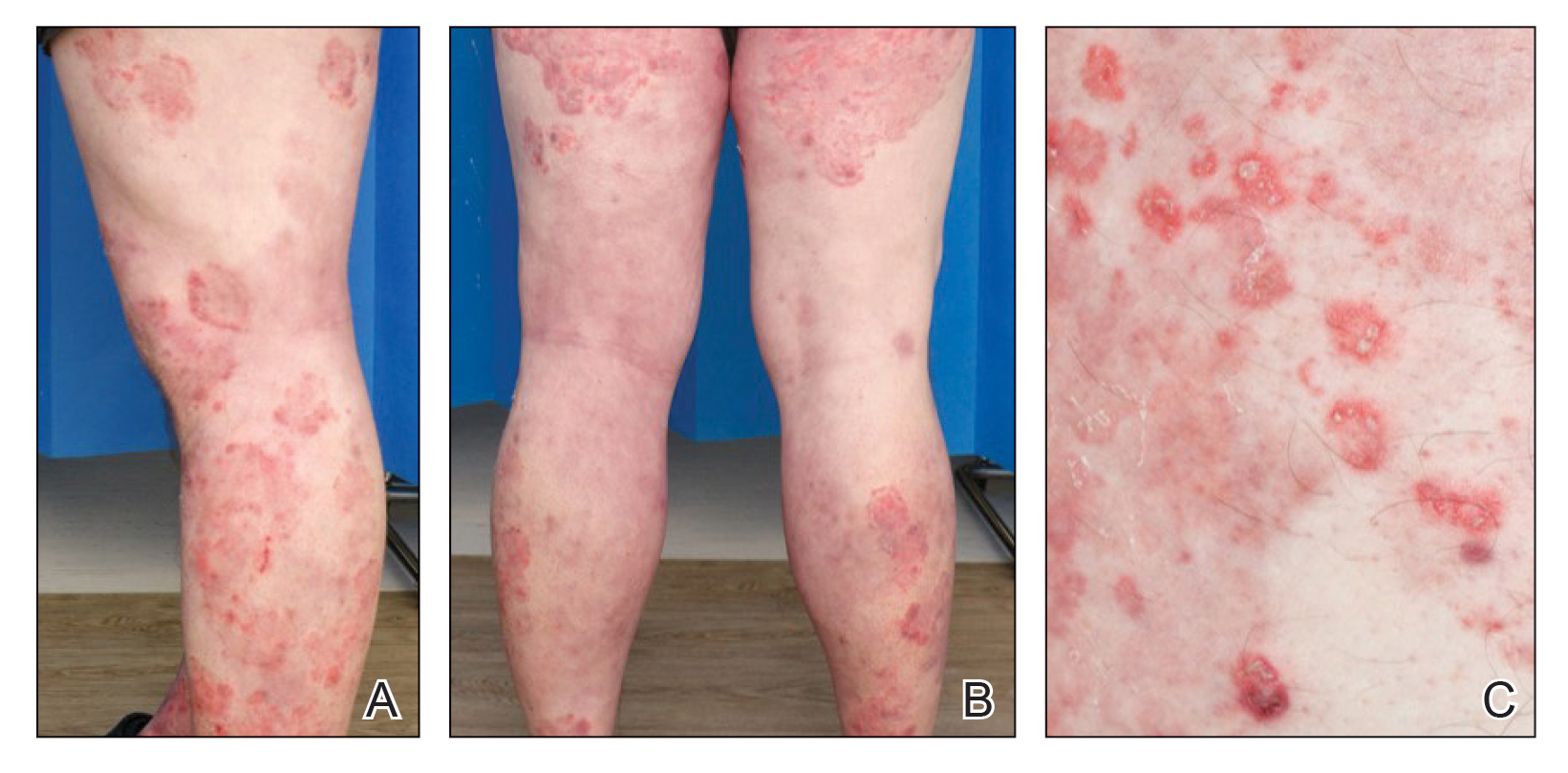
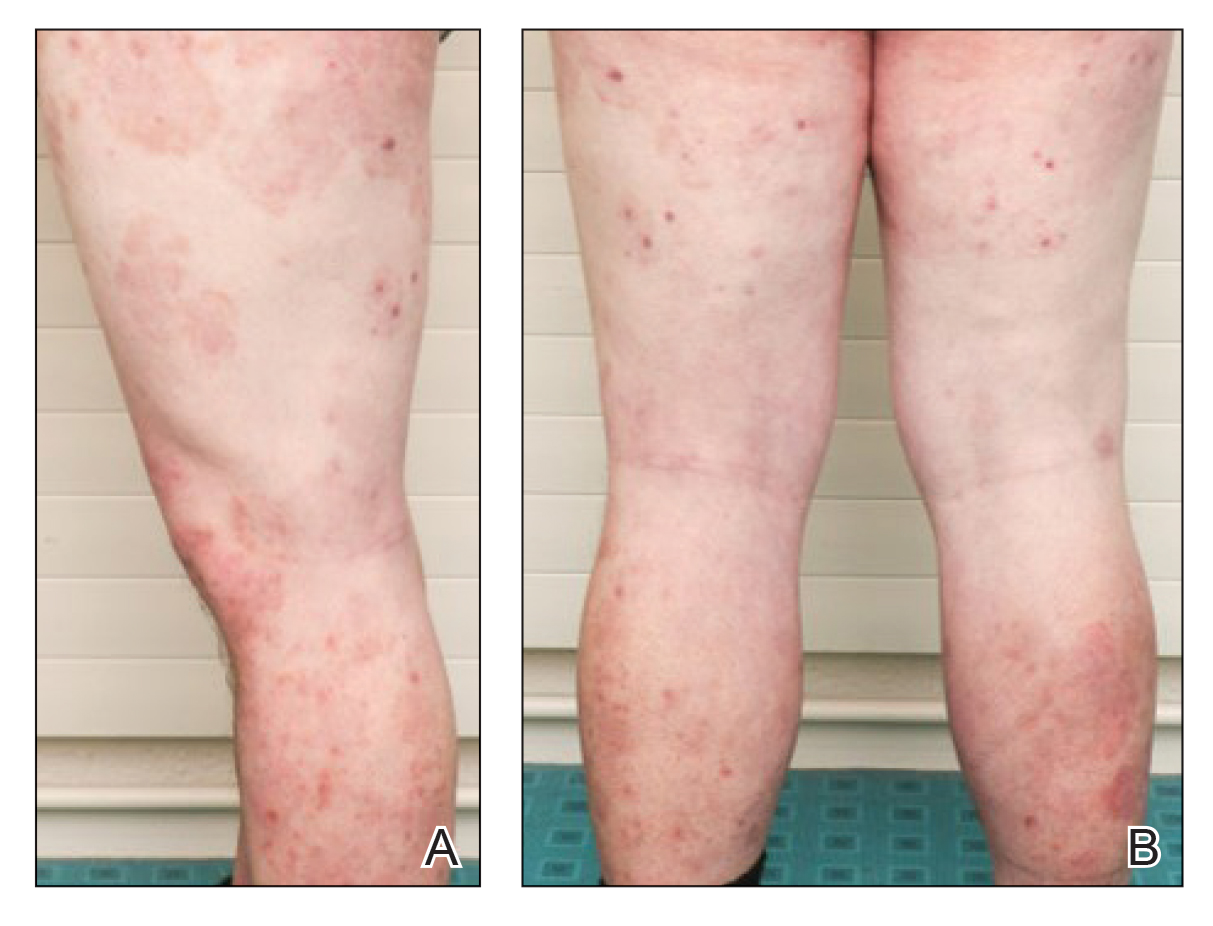
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.
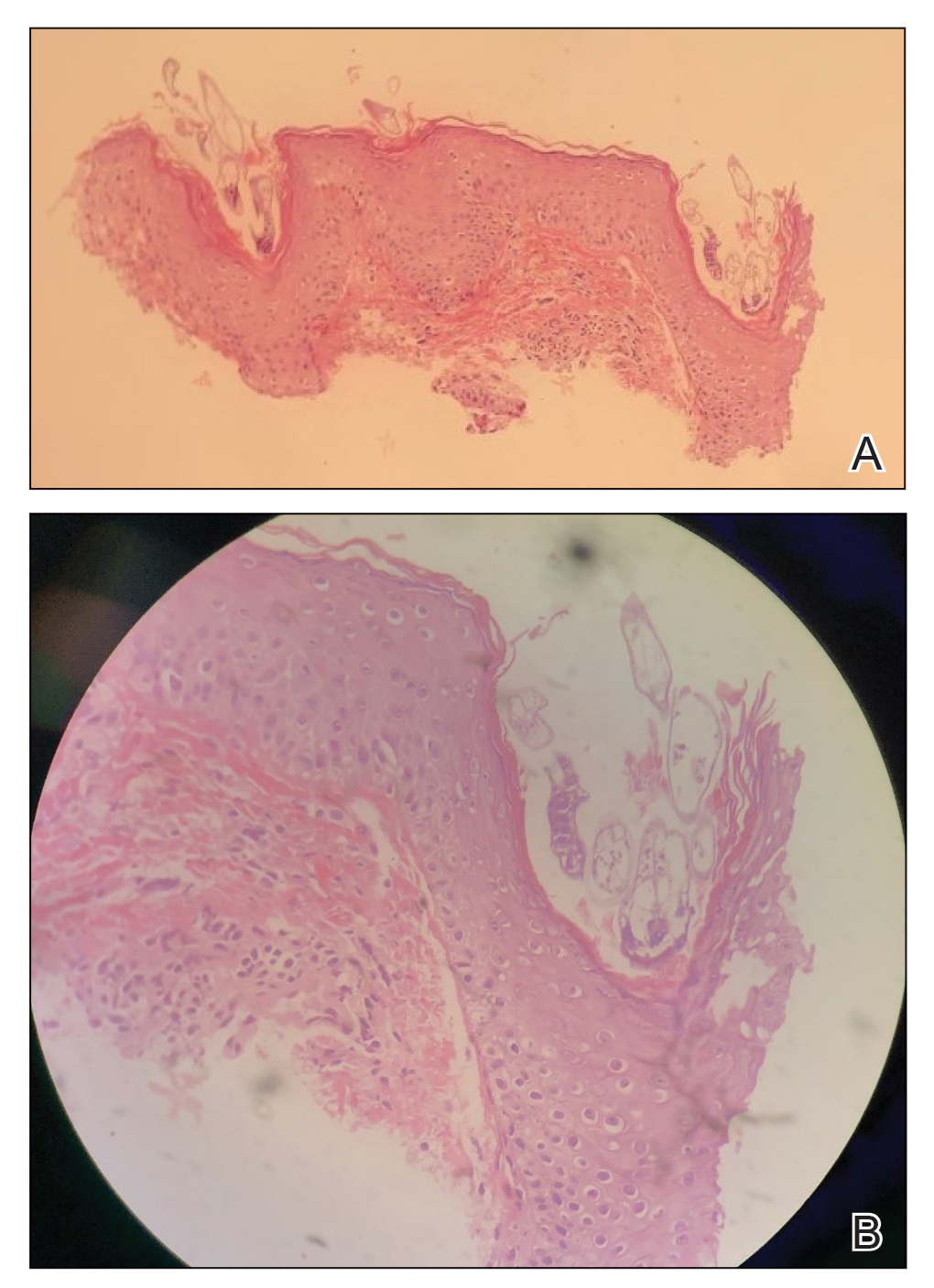
Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.
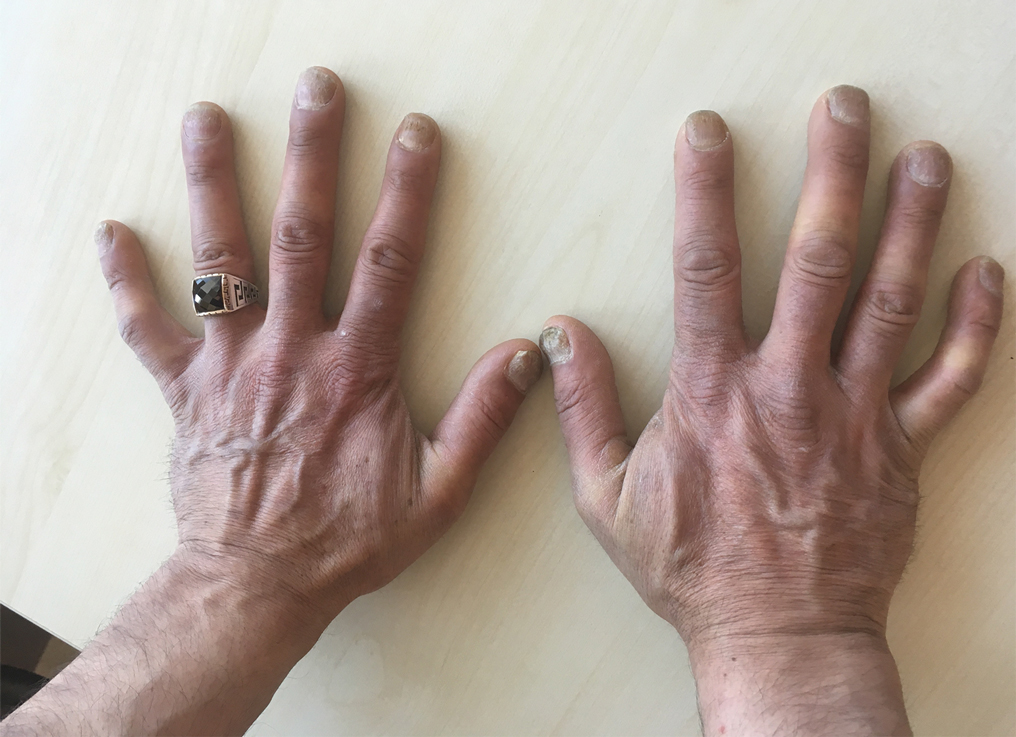
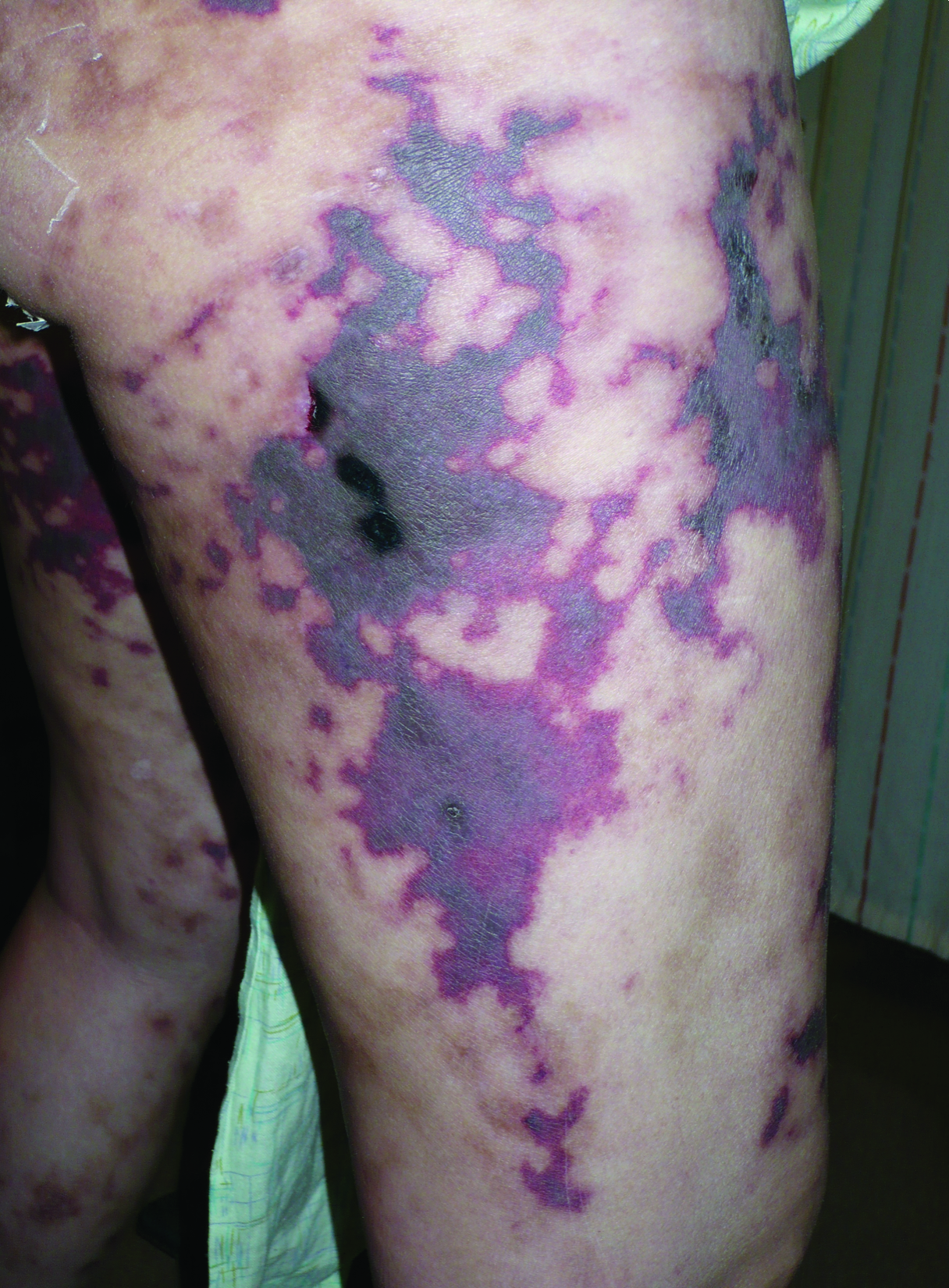
Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.
Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
To the Editor:
Levamisole is a veterinary anthelmintic drug with immunomodulating properties that was once approved by the US Food and Drug Administration for the treatment of various conditions, including autoimmune diseases, cancer, pediatric kidney disease, and chronic infections.1-4 Levamisole was banned in 2000 after reports of associated agranulocytosis and a characteristic painful purpuric vasculitis.4,5 Despite the ban, its use persists due to its increasing incorporation as an adulterant in cocaine, presumably for its dopaminergic properties that potentiate psychotropic effects.6 In 2009, the Drug Enforcement Administration reported that 69% of seized cocaine in the United States contains this chemical, with an average concentration of 10%.5 Levamisole-induced vasculopathy (LIV) typically resolves following the cessation of cocaine without further treatment necessary. We present a fatal case of LIV to emphasize that early recognition and discontinuation of the offending agent could be lifesaving.
A 40-year-old woman with a history of cocaine abuse was admitted with tender, reticular, purpuric, and erythematous patches and plaques on the lower extremities with areas of necrosis (Figure 1). The lesions had been present intermittently for 6 months. She tried topical mupirocin and oral amoxicillin clavulanate without improvement. She also described polyarthralgia in the hands, but the remainder of the review of symptoms and physical examination was negative.

Coagulation studies and white blood cell counts were within reference range. A urine toxicology screen was positive for cocaine; however, urine testing for levamisole was not performed given the short half-life of levamisole in vivo. A biopsy of one of the skin lesions on the right thigh showed pauci-inflammatory superficial and deep vein thrombosis with recanalization (Figure 2). A rheumatology workup revealed an elevated C-reactive protein level, low C3, positive antinuclear antibody, positive anti–double-stranded DNA, positive anticardiolipin antibody, positive lupus anticoagulant, and positive perinuclear antineutrophil cytoplasmic antibody (ANCA). Tests for HIV, hepatitis B and C, cryoglobulinemia, and cytomegalovirus were negative. Given the clinical picture and laboratory findings, levamisole-induced vasculitis was deemed likely. The patient was treated with appropriate skin and wound care. She was discharged with a prednisone taper and oral cephalexin and was counseled on cocaine cessation.
Five months later, the patient was readmitted for lower extremity edema and worsening painful lesions that had progressed to involve the legs, thighs, buttocks, flanks, and the tip of her nose. A deep vein thrombosis workup was negative. She admitted to ongoing cocaine use that was confirmed with urine toxicology. Coagulation studies and white blood cell counts remained within reference range. Repeat skin biopsy was consistent with prior findings, demonstrating thrombosis of superficial and deep vessels with recanalization. In addition, it showed focal epidermal necrosis and a perivascular infiltrate of lymphocytes, histiocytes, and rare neutrophils. She was placed on high-dose methylprednisolone. Over the course of the next month, her urine continued to test positive for cocaine, and she developed necrotizing fasciitis necessitating lower extremity amputation, abdominal washout, and debridement. She quickly deteriorated, developing multiorgan failure with sepsis, leading to death. Of note, the patient was never found to have neutropenia or agranulocytosis throughout the disease course.
Because levamisole is no longer in clinical use, reports of its adverse effects come exclusively from users of cocaine, whether via smoking or snorting. Levamisole-induced vasculopathy typically is painful and purpuric, with or without necrosis, in a retiform or stellate pattern and commonly involves the extremities, trunk, face, and external ears.7 The average age of presentation is 43 years and it more commonly is seen in women.8
Levamisole-induced vasculopathy remains a diagnosis of exclusion, so it is important to rule out other treatable causes. The differential diagnosis for purpura associated with vasculitis also includes other antineutrophilic cytoplasmic–associated vasculitides (eg, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis), infectious purpura fulminans, antiphospholipid syndrome, cryoglobulinemia, and disseminated intravascular coagulation.9 In LIV patients, perinuclear ANCAs are present in up to 90% of cases, and cytoplasmic ANCAs in 19% to 59% of cases.10,11 Although leukopenia and neutropenia complicate approximately 60% of LIV cases, they are not required to make the diagnosis.11,12 Elevated erythrocyte sedimentation rate, normal coagulation studies, and positive antineutrophil antibodies and lupus anticoagulant further aid in the diagnosis.8 Urine should be tested for cocaine in suspected patients. Urine also can be tested for levamisole, which is challenging because of the short half-life of 5.6 hours. Only 2% to 5% of levamisole is excreted unchanged in the urine, and testing requires gas chromatography and mass spectrometry that was not readily available to perform on our patient.7 In addition to laboratory and urine studies, hair strand testing,10 skin biopsy, and histologic findings also can be used to support the diagnosis.
The pathogenesis of LIV is not completely understood, but it is thought to be an immune complex–mediated process based on immunofluorescence studies in the skin.13,14 Classic pathologic findings include multiple fibrin thrombi within small vessels in the superficial and deep dermis, leukocytoclastic vasculitis of small vessels consisting of fibrinoid necrosis of the vessel wall, extravasated erythrocytes, karyorrhectic debris, and angiocentric inflammation.14 Direct immunofluorescence is not routinely performed but most commonly demonstrates deposition of IgA, IgM, and C3.14,15
Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae. Steroids have been used as treatment of prominent vasculitis with variable success; however, immunosuppressive effects should be closely monitored, especially with inpatients with concurrent granulocytopenia. Broad-spectrum antibiotics have been used in cases with fever and agranulocytosis. Cutaneous lesions typically disappear within 2 to 3 weeks, and serologic markers resolve within 2 to 10 months. Recurrent use of cocaine generally results in recurrent neutropenia and skin eruptions, supporting the causal role. Our patient’s recurrent prolonged cocaine use with vasculopathy was assumed to be the source of the necrotizing fasciitis that led to a cascade of sepsis, rapidly progressing multiorgan failure, and ultimate demise.
Presentation of a purpuric vasculopathy, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Although the patient may initially deny cocaine use, it is important to keep this diagnosis in mind when the clinical picture fits, and urine toxicology screen should be ordered when there is question. Physicians and patients should be wary of potential complications, even death. Early recognition and discontinuation of the offending agent could be lifesaving.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
- Menni S, Pistritto G, Gianotti R, et al. Ear lobe necrosis by levamisole-induced occlusive vasculitis in a pediatric patient. Pediatr Dermatol. 1997;14:477-479.
- Symoens J, Veys E, Mielants M, et al. Adverse reactions to levamisole. Cancer Treat Rep. 1978;62:1721-1730.
- Vogel CL, Silverman MA, Mansell PW, et al. Mechanism of levamisole-induced granulocytopenia in breast cancer patients. Am J Hematol. 1980;9:171-183.
- Rongioletti F, Ghio L, Ginevri F, et al. Purpura of the ears: a distinctive vasculopathy with circulating autoantibodies complicating long-term treatment with levamisole in children. Br J Dermatol. 1999;140:948-951.
- Centers for Disease Control and Prevention (CDC). Agranulocytosis associated with cocaine use—four states, March 2008–November 2009. MMWR Morb Mortal Wkly Rep. 2009;58:1381-1385.
- Zhu NY, Legatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med. 2009;150:287-289.
- Gross RL, Brucker J, Bahce-Altuntas A, et al. A novel cutaneous vasculitis syndrome induced by levamisole-contaminated cocaine. Clin Rheumatol. 2011;30:1385-1392.
- Trehy ML, Brown DJ, Woodruff JT, et al. Determination of levamisole in urine by gas chromatography-mass spectrometry. J Anal Toxicol. 2011;35:545-550.
- Waller JM, Feramisco JD, Alberta-Wszolek L, et al. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit? J Am Acad Dermatol. 2010;63:530-535.
- Pearson T, Bremmer M, Cohen J, et al. Vasculopathy related to cocaine adulterated with levamisole: a review of the literature. Dermatol Online J. 2012;18:1.
- Arora NP. Cutaneous vasculopathy and neutropenia associated with levamisole-adulterated cocaine. Am J Med Sci. 2013;345:45-51.
- Chai PR, Bastan W, Machan J, et al. Levamisole exposure and hematologic indices in cocaine users. Acad Emerg Med. 2011;18:1141-1147.
- Lazareth H, Peytavin G, Polivka L, et al. The hairy-print for levamisole-induced vasculitis. BMJ Case Rep. 2012;2012:bcr2012006602.
- Chung C, Tumeh PC, Birnbaum R, et al. Characteristic purpura of the ears, vasculitis, and neutropenia—a potential public health epidemic associated with levamisole-adulterated cocaine. J Am Acad Dermatol. 2011;65:722-725.
- Jenkins J, Babu K, Hsu-Hung E, et al. ANCA-positive necrotizing vasculitis and thrombotic vasculopathy induced by levamisole-adulterated cocaine: a distinctive clinicopathologic presentation. J Am Acad Dermatol. 2011;65:E14-E16.
Practice Points
- Levamisole-induced vasculopathy usually resolves upon cessation of cocaine use without long-term sequelae.
- Presentation of a purpuric vasculitis, with or without associated neutropenia and positive autoantibodies, should prompt the consideration of levamisole-contaminated cocaine use in the clinician’s differential. Early recognition and discontinuation of the offending agent could be lifesaving.
Genital Primary Herpetic Infection With Concurrent Hepatitis in an Infant
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
To the Editor:
Cutaneous herpes simplex virus (HSV) infection generally involves mucocutaneous junctions, but virtually any area of the skin can be affected.1 When the genital area of adult patients is affected, the disease usually is sexually transmitted and mainly caused by HSV-2. In infants, genital primary herpetic infection is rare and more commonly is caused by HSV-1 than by HSV-2. We report a rare case of genital primary herpetic infection with concurrent hepatitis in an infant.
An 8-month-old infant with no underlying medical problems, including atopic dermatitis, was referred for erythematous grouped vesicles with erosions on the perianal area of 4 days’ duration (Figure). The skin color appeared normal, not icterus. She also had a fever (temperature, 37.9 °C), and her urination pattern had changed from normal to frequent leakage, possibly owing to pain related to the eroded lesions. Physical examination did not reveal palpable inguinal lymph nodes. The oral mucosa was not involved. The patient’s father had a history of recurrent herpetic infection on both the perioral and perianal areas.
A Tzanck smear revealed giant multinucleated cells with multiple inflammatory cells. Laboratory tests revealed marked leukocytosis, elevated liver enzymes (aspartate aminotransferase, 141 IU/L [reference range, 15 IU/L–60 IU/L]; alanine aminotransferase, 422 IU/L [reference range, 13 IU/L–45 IU/L]), and was positive for herpes simplex viral IgM but negative for herpes simplex viral IgG. A viral culture also demonstrated the growth of HSV. An abdominal ultrasound was normal. Based on the cutaneous and laboratory findings, genital primary herpetic infection with concurrent hepatitis was diagnosed. Intravenous acyclovir 50 mg was administered 3 times daily for 7 days, and a wet dressing with topical mupirocin was employed daily until the skin lesions healed. The fever subsided soon after starting treatment. The liver enzyme counts decreased gradually in serial follow-up (aspartate aminotransferase, 75 IU/L; alanine aminotransferase, 70 IU/L).
Primary herpetic infection usually is asymptomatic, but when symptoms do occur, it is characterized by the sudden onset of painful vesicle clusters over erythematous edematous skin. Lesions can be associated with fever and malaise and may involve the perineum. Urinary symptoms may occur. The average age of onset ranges from 6 months to 4 years. The virus commonly is transmitted by asymptomatic carriers. Autoinoculation from concomitant oral primary herpetic infection or individuals with active herpetic infection is one possible route of transmission. In our patient, we assumed that she acquired the virus from her father during close contact. A diagnosis can be made clinically using direct methods including culture, Tzanck smear, or polymerase chain reaction, or indirect methods such as serologic tests.2
Hepatitis secondary to HSV infection is rare, especially in immunocompetent patients. It occurs during primary infection and rarely during recurrent infection with or without concomitant skin lesions.3 Symptoms include fever, anorexia, nausea, vomiting, abdominal pain, leukopenia, coagulopathy, and marked elevation of serum transaminase levels without jaundice. Based on our patient’s elevated liver enzyme levels and virological evidence of acute primary HSV infection, a lack of evidence of other hepatic viral infections, and the presence of herpes simplex viremia, we concluded that this infant had viral hepatitis as a part of the clinical presentation of primary HSV infection. We did not perform a direct liver biopsy considering her age and accompanying risks.4
Primary herpetic infection usually has a benign course and a short duration. In children, the prognosis depends on underlying immunologic status, not a particular type of HSV. In children with atopic dermatitis, primary herpetic infection tends to occur earlier and is more severe. Early treatment with acyclovir is effective; intravenous treatment is not required unless local complications or systemic involvement are present. Long-term follow-up is recommended because of the possibility of recurrence.
Although the possibility of systemic involvement including hepatitis due to HSV infection is low, awareness among dermatologists about primary herpetic infection and its possible complications would be helpful in the diagnosis and treatment, especially for atypical or extensive cases.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
- Jenson HB, Shapiro ED. Primary herpes simplex virus infection of a diaper rash. Pediatr Infect Dis J. 1987;6:1136-1138.
- Batalla A, Flórez A, Dávila P, et al. Genital primary herpes simplexinfection in a 5-month-old infant. Dermatol Online J. 2011;17:8.
- Norvell JP, Blei AT, Jovanovic BD, et al. Herpes simplex virus hepatitis: an analysis of the published literature and institutional cases. Liver Transpl. 2007;13:1428-1434.
- Chen CK, Wu SH, Huang YC. Herpetic gingivostomatitis with severe hepatitis in a previously healthy child. J Microbiol Immunol Infect. 2012;45:324-325.
Practice Points
- Parents with a history of herpes simplex virus (HSV) need to be educated before the baby is born to be careful about direct skin contact with the child to prevent the spread of HSV infection.
- Although systemic involvement is not typical, additional tests to rule out internal organ involvement may be required, especially in children.
Pruritic and Pustular Eruption on the Face
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).
Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).
Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
The Diagnosis: Demodicosis
A clinical diagnosis of facial demodicosis triggered by topical corticosteroid therapy was suspected in our patient. A 3-mm punch biopsy of the right forehead demonstrated a follicular infundibulum rich in Demodex folliculorum with a discrete mononuclear infiltrate in the dermis (Figure 1), confirming the diagnosis. The patient was prescribed metronidazole gel 2% twice daily. Complete clearance of the facial rash was observed at 1-week follow-up (Figure 2).
Demodex folliculorum is a mite that parasitizes the pilosebaceous follicles of human skin, becoming pathogenic with excessive colonization.1-3 Facial demodicosis presents as pruritic papules and pustules in a pilosebaceous distribution on the face.2,3 The diagnosis of facial demodicosis can be difficult due to similarities with many other common facial rashes such as acne, rosacea, contact dermatitis, and folliculitis.
Similar to facial demodicosis, rosacea can present with erythema, papules, and pustules on the face. Patients with rosacea also have a higher prevalence and degree of Demodex mite infestation.4 However, these facial rashes can be differentiated when symptom acuity and personal and family histories are considered. In patients with a long-standing personal and/or family history of erythema, papules, and pustules, the chronicity of disease implies a diagnosis of rosacea. The acute onset of symptomatology and absence of personal or family history of rosacea in our patient favored a diagnosis of demodicosis.
Pityrosporum folliculitis is an eruption caused by Malassezia furfur, the organism implicated in tinea versicolor. It can present similarly to Demodex folliculitis with follicular papules and pustules on the forehead and back. Features that help to differentiate Pityrosporum folliculitis from Demodex folliculitis include involvement of the upper trunk and onset after antibiotic usage.5 Diagnosis of Pityrosporum folliculitis can be confirmed by potassium hydroxide preparation, which demonstrates spaghetti-and-meatball-like hyphae and spores.
Eosinophilic folliculitis is a chronic sterile folliculitis that usually presents as intensely pruritic papules and pustules on the face with an eruptive onset. There are 3 variants of disease: the classic form (also known as Ofuji disease), immunosuppression-associated disease, and infancy-associated disease.6 Eosinophilic folliculitis also may present with annular plaques and peripheral blood eosinophilia, and it is more prevalent in patients of Japanese descent. Differentiation from Demodex folliculitis can be done by histologic examination, which demonstrates spongiosis with exocytosis of eosinophils into the epithelium and a clear deficiency of infiltrating mites.6
Miliaria, also known as sweat rash, is a common condition that occurs due to occlusion of eccrine sweat glands.7 Clinically, miliaria is characterized by erythematous papules ranging from 2 to 4 mm in size that may be vesicular or pustular. Miliaria and demodicosis may have similar clinical presentations; however, several characteristic differences can be noted. Based on the pathophysiology, miliaria will not be folliculocentric, which differs from demodicosis. Additionally, miliaria most commonly occurs in areas of occlusion, such as in skin folds or on the trunk under tight clothing. Miliaria rarely can appear confluent and sunburnlike.8 Furthermore, miliaria is less common on the face, while demodicosis almost exclusively is found on the face.
We present the case of an otherwise healthy patient with acute onset of pruritic papules and pustules involving the superior face following topical corticosteroid use. Similar cases frequently are encountered by dermatologists and require broad differential diagnoses.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
- Aylesworth R, Vance JC. Demodex folliculorum and Demodex brevis in cutaneous biopsies. J Am Acad Dermatol. 1982;7:583-589.
- Kligman AM, Christensen MS. Demodex folliculorum: requirements for understanding its role in human skin disease. J Invest Dermatol. 2011;131:8-10.
- Zomorodian K, Geramishoar M, Saadat F, et al. Facial demodicosis. Eur J Dermatol. 2004;14:121-122.
- Chang YS, Huang YC. Role of Demodex mite infestation in rosacea: a systematic review and meta-analysis. J Am Acad Dermatol. 2017;77:441-447.
- Prindaville B, Belazarian L, Levin NA, et al. Pityrosporum folliculitis: a retrospective review of 110 cases. J Am Acad Dermatol. 2018;78:511-514.
- Lankerani L, Thompson R. Eosinophilic pustular folliculitis: case report and review of the literature. Cutis. 2010;86:190-194.
- Hölzle ER, Kligman AM. The pathogenesis of miliaria rubra. role of the resident microflora. Br J Dermatol. 1978;99:117-137.
- Al-Hilo MM, Al-Saedy SJ, Alwan AI. Atypical presentation of miliaria in Iraqi patients attending Al-Kindy Teaching Hospital in Baghdad: a clinical descriptive study. Am J Dermatol Venereol. 2012;1:41-46.
A 37-year-old man presented with a progressively pruritic and pustular eruption on the face of 2 weeks’ duration. Twenty days prior to the rash onset, he began treatment for scalp psoriasis with a mixture of salicylic acid 20 mg/mL and betamethasone dipropionate 0.5 mg/mL, which inadvertently extended to the facial area. One week after rash onset, he presented to the emergency department at a local hospital, where he was given intravenous hydrocortisone 100 mg with no improvement of the rash. He had no history of skin cancer, and his family history was negative for dermatologic disease. At the time of examination, he had an erythematous eruption of follicular micropustules on the forehead, upper and lower eyelids, temples, cheeks, and mandible.
Innovations in Dermatology Spring Abstract Compendium
Head to Toe: Recommendations for Physician Head and Shoe Coverings to Limit COVID-19 Transmission
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).
Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).
Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.
Personal protective equipment (PPE) is an important component in limiting transmission of SARS-CoV-2. The World Health Organization and Centers for Disease Control and Prevention issued guidelines for appropriate PPE use, but recommendations for head and shoe coverings are lacking. In this article, we analyze the literature on pathogen transmission via hair and shoes and make evidence-based recommendations for PPE selection during the COVID-19 pandemic.
Pathogens on Shoes and Hair
Hair and shoes may act as vehicles for pathogen transmission. In a study that simulated contamination of uncovered skin in health care workers after intubating manikins in respiratory distress, 8 (100%) had fluorescent markers on the hair, 6 (75%) on the neck, and 4 (50%) on the shoes.1 In another study of postsurgical operating room (OR) surfaces (517 cultures), uncovered shoe tops and reusable hair coverings had 10-times more bacterial colony–forming units compared to other surfaces. On average, disposable shoe covers/head coverings had less than one-third bacterial colony–forming units compared with uncovered shoes/reusable hair coverings.2
Hair characteristics and coverings may affect pathogen transmission. Exposed hair may collect bacteria, as Staphylococcus aureus and Staphylococcus epidermidis attach to both scalp and facial hair. In one case, β-hemolytic streptococci cultured from the scalp of a perioperative nurse was linked to postsurgical infections in 20 patients.3 Hair coverings include bouffant caps and skullcaps. The bouffant cap is similar to a shower cap; it is relatively loose and secured around the head with elastic. The skullcap, or scrub cap, is tighter but leaves the neck nape and sideburns exposed. In a study comparing disposable bouffant caps, disposable skullcaps, and home-laundered cloth skullcaps worn by 2 teams of 5 surgeons, the disposable bouffant caps had the highest permeability, penetration, and microbial shed of airborne particles.4
Physicians’ shoes may act as fomites for transmission of pathogens to patients. In a study of 41 physicians and nurses in an acute care hospital, shoe soles were positive for at least one pathogen in 12 (29.3%) participants; methicillin-resistant Staphylococcus aureus was most common. Additionally, 98% (49/50) of shoes worn outdoors showed positive bacterial cultures compared to 56% (28/50) of shoes reserved for the OR only.5 In a study examining ventilation effects on airborne pathogens in the OR, 15% of OR airborne bacteria originated from OR floors, and higher bacterial counts correlated with a higher number of steps in the OR.2 In another study designed to evaluate SARS-CoV-2 distribution on hospital floors, 70% (7/10) of quantitative polymerase chain reaction assays performed on floor samples from intensive care units were positive. In addition, 100% (3/3) of swabs taken from hospital pharmacy floors with no COVID-19 patients were positive for SARS-CoV-2, meaning contaminated shoes likely served as vectors.6 Middle East respiratory syndrome, SARS-CoV-2, and influenza viruses may survive on porous and nonporous materials for hours to days.7Enterococcus, Candida, and Aspergillus may survive on textiles for up to 90 days.3
Recommendations for Hair and Shoe Coverings
We recommend that physicians utilize disposable skullcaps to cover the hair and consider a hooded gown or coverall for neck/ear coverage. We also recommend that physicians designate shoes that remain in the workplace and can be easily washed or disinfected at least weekly; physicians may choose to wash or disinfect shoes more often if they frequently are performing procedures that generate aerosols. Additionally, physicians should always wear shoe coverings when caring for patients (Table 1).
Our hair and shoe covering recommendations may serve to protect dermatologists when caring for patients. These protocols may be particularly important for dermatologists performing high-risk procedures, including facial surgery, intraoral/intranasal procedures, and treatment with ablative lasers and facial injectables, especially when the patient is unmasked. These recommendations may limit viral transmission to dermatologists and also protect individuals living in their households. Additional established guidelines by the American Academy of Dermatology, American Society for Dermatologic Surgery, and World Health Organization are listed in Table 2.8-10
Current PPE recommendations that do not include hair and shoe coverings may be inadequate for limiting SARS-CoV-2 exposure between and among physicians and patients. Adherence to head covering and shoe recommendations may aid in reducing unwanted SARS-CoV-2 transmission in the health care setting, even as the pandemic continues.
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
- Feldman O, Meir M, Shavit D, et al. Exposure to a surrogate measure of contamination from simulated patients by emergency department personnel wearing personal protective equipment. JAMA. 2020;323:2091-2093. doi:10.1001/jama.2020.6633
- Alexander JW, Van Sweringen H, Vanoss K, et al. Surveillance of bacterial colonization in operating rooms. Surg Infect (Larchmt). 2013;14:345-351. doi:10.1089/sur.2012.134
- Blanchard J. Clinical issues—August 2010. AORN Journal. 2010;92:228-232. doi:10.1016/j.aorn.2010.06.001
- Markel TA, Gormley T, Greeley D, et al. Hats off: a study of different operating room headgear assessed by environmental quality indicators. J Am Coll Surg. 2017;225:573-581. doi:10.1016/j.jamcollsurg.2017.08.014
- Kanwar A, Thakur M, Wazzan M, et al. Clothing and shoes of personnel as potential vectors for transfer of health care-associated pathogens to the community. Am J Infect Control. 2019;47:577-579. doi:10.1016/j.ajic.2019.01.028
- Guo ZD, Wang ZY, Zhang SF, et al. Aerosol and surface distribution of severe acute respiratory syndrome coronavirus 2 in hospital wards, Wuhan, China, 2020. Emerg Infect Dis. 2020;26:1583-1591. doi:10.3201/eid2607.200885
- Otter JA, Donskey C, Yezli S, et al. Transmission of SARS and MERS coronaviruses and influenza virus in healthcare settings: the possible role of dry surface contamination. J Hosp Infect. 2016;92:235-250. doi:10.1016/j.jhin.2015.08.027
- Centers for Disease Control and Prevention. Science Brief: SARS-CoV-2 and Surface (Fomite) Transmission for Indoor Community Environments. https://www.cdc.gov/coronavirus/2019-ncov/more/science-and-research/surface-transmission.html#ref10
- American Academy of Dermatology. Clinical guidance for COVID-19. Accessed March 15, 2021. https://www.aad.org/member/practice/coronavirus/clinical-guidance
- Narla S, Alam M, Ozog DM, et al. American Society of Dermatologic Surgery Association (ASDSA) and American Society for Laser Medicine & Surgery (ASLMS) guidance for cosmetic dermatology practices during COVID-19. Updated January 11, 2021. Accessed March 15, 2021. https://www.asds.net/Portals/0/PDF/asdsa/asdsa-aslms-cosmetic-reopening-guidance.pdf
- World Health Organization. Country & technical guidance—coronavirus disease (COVID-19). Accessed March 15, 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance-publications
Practice Points
- Consistent use of personal protective equipment, including masks, face shields, goggles, and gloves, may limit transmission of SARS-CoV-2.
- Hair and shoes also may transmit SARS-CoV-2, but recommendations for hair and shoe coverings to prevent SARS-CoV-2 are lacking.
Long-Distance Dermatology: Lessons From an Interview on Remote Practice During a Pandemic and Beyond
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
For the US health care system, the year 2020 was one of great change as well as extreme pain and hardship: some physical, but much emotional and financial. Dermatologists nationwide have not been sheltered from the winds of change. Yet as with most great challenges, one also can discern great change for the better if you look for it. One area of major growth in the wake of the COVID-19 pandemic is the expansion of telehealth, specifically teledermatology.
Prior to the pandemic, teledermatology was in a phase of modest expansion.1 Since the start of the pandemic, however, the adoption of telemedicine services in the United States has been beyond exponential. Before the pandemic, an estimated 15,000 Medicare recipients received telehealth services on a weekly basis. Yet by the end of April 2020, only 3 months after the first reported case of COVID-19 in the United States, nearly 1.3 million Medicare beneficiaries were utilizing telehealth services on a weekly basis.2 The Centers for Medicare & Medicaid Services has recognized the vast increase in need and responded with the addition of 144 new telehealth services covered by Medicare in the last year. In December 2020, the Centers for Medicare & Medicaid Services moved to make many of the previously provisional policies permanent, expanding long-term coverage for telehealth services,2 and use of teledermatology has expanded in parallel. Although the impetus for this change was simple necessity, the benefits of expanded teledermatology are likely to drive its continued incorporation into our daily practices.
Kevin Wright, MD, is a staff dermatologist at the Naval Medical Center San Diego (San Diego, California) and an Associate Professor of Dermatology at the Uniformed Services University of the Health Sciences (Bethesda, Maryland). In this interview, we discussed his experience incorporating a teledermatology component into his postresidency practice, the pros and cons of teledermatology practice, and ways that residents can prepare for a future in teledermatology.
Would you start by briefly describing your work model now?
My primary job is a Monday-through-Friday classic dermatology clinic job. On the weekends or days off, I see asynchronous and synchronous teledermatology through a specialized platform. On weekends, I tend to see anywhere between 20 and 40 patients in about a 6-hour period with breaks in between.
What does a typical “weekend” day of work look like?
In general, I’ll wake up early before my family and spend maybe an hour working. Oftentimes, that will be in my truck parked down by the beach, where I will go for a run or surf before logging on. If I have 40 visits scheduled that day, I can spend a few hours, message most of them, clarify some aspects of the visit, then go and have breakfast with my family before logging back on and completing the encounters.
Is most of your interaction with patients asynchronous, messaging back and forth to take history?
A few states require a phone call, so those are synchronous, and every Medicaid patient requires a video call. I do synchronous visits with all of my isotretinoin patients at first. It’s a mixed bag, but a lot of my visits are done entirely asynchronously.
What attracted you to this model?
During residency, I always felt that many of the ways we saw patients seemed extraordinarily inefficient. My best example of this is isotretinoin follow-ups. Before this year, most of my colleagues were uncomfortable with virtual isotretinoin follow-ups or thought it was a ridiculous idea. Frankly, I never shared this sentiment. Once I had my own board certification, I knew I was going to pursue teledermatology, because seeing kids take a half day off of school to come in for a 10-minute isotretinoin appointment (that’s mainly just a conversation about sports) just didn’t make sense to me. So I knew I wanted to pursue this idea, I just didn’t know exactly how. One day I was approached by a close friend and mentor of mine who had just purchased a teledermatology platform. She asked me if I would like to moonlight once I graduated and I jumped at the opportunity.
What steps did you take prior to graduating to help prepare you to practice teledermatology?
The most important thing I did—and the most important thing I think for third-year residents to do—is to set myself up for success by starting the US Drug Enforcement Administration (DEA) licensure and certification process. Once you have a DEA number, you can apply for Medicare and Medicaid. The nice thing about Medicare is you can start billing immediately after you apply, which is important. The reimbursement isn’t as high, but they pay faster, which allows you to start seeing patients through teledermatology right away. In a pinch, you could see all Medicare patients and make a living until you’ve completed the rest of the process. Once you have a Medicare and Medicaid number, you can apply for credentialing through private payers. However, the Medicare process takes 3 months, and private-payers credentialing takes about 90 days as well. That’s a lot of time! Before finishing residency, I recommend you make sure you have an unrestricted DEA license and you apply for Medicare/Medicaid credentials. Then, when you’re looking at future employment, you can start getting state licenses almost immediately in whatever states you anticipate needing them.
What are the top 3 benefits of incorporating teledermatology into your practice?
Accessibility is one huge benefit. If you’re practicing in a rural area, you’re basically giving [patients] back their time. Teledermatology takes patients much less time, and they get the same level of care. That’s a big selling point. Your patients will be very happy and loyal because of that.
The other thing I never would have foreseen before starting teledermatology is the amazing follow-up you can get. I think many dermatology residents will agree that there are those patients where you think, “Wow, I wish I could see them back. I wonder how they did,” but you never see them again. That’s not the case with teledermatology. I have a running list of all my interesting cases, and I’ll just shoot them a message 2 or 4 weeks later and at their convenience, they can submit a quick photo. I get that excellent feedback, and that’s huge to me for my own personal education and growth.
The third would be experience. I have 24 state medical licenses, and I see patients of all varieties: all socioeconomic backgrounds and skin types and many with severe skin conditions never managed before by a specialist. That, frankly, has increased my comfort level for seeing patients of all types. It forces me to expand my utilization of certain therapies because some people can’t afford 95% of medications we prescribe commonly. I find that challenge very rewarding. It’s something I’m not sure you can achieve by just practicing within your bubble. Inevitably you are going to see a certain type of patient that your hospital or practice attracts by merit of its geography or catchment area. Teledermatology allows you to see the full spectrum of dermatology.
What are the biggest cons to incorporating teledermatology into your practice?
To start off, some patients have boundary issues. Every 200 patients or so, I’ll have someone who submits a visit at 11:30
The second is reimbursement. In other practice models I can bill more in half the time by seeing a patient in person, doing a skin screening and a few biopsies. I believe there’s always a role for teledermatology in any practice, but ultimately dermatologists are pragmatic people who need to be smart about time management. At some point, it becomes difficult to pay the bills if reimbursement is lacking. That’s one of the bigger downsides to teledermatology. We still need to figure out how to reimburse to incentivize what’s best for the patient.
Could you talk more about the effect on work-life balance?
I think the things that make teledermatology appealing are the same things that could end up disrupting your work-life balance. On the positive side, you can vacation in Hawaii, work for 2 hours each morning, and pay for the whole thing. That’s very appealing to me! The downside is that there are always patients in the queue. In some sense, your waiting room is always half-full, 24/7. Mentally, you have to become comfortable with that, and you have to develop boundaries. I have very specific times I do teledermatology and then I log off. This helps me establish boundaries and creates balance.
You touched on it earlier regarding isotretinoin visits, but what other facets of practice do you think are particularly well-suited to teledermatology?
There are a few that I’ve incorporated into my practice quite aggressively. Almost all acne is going to go to a teledermatology visit. That’s in large part due to payer parity. For the most part, you make the same doing an acne visit online as you will doing it in person. Your patients will be getting the same level of care, better follow-up, and you’ll make the same amount of money. Another thing I do as a patient courtesy is wound checks postsurgery or post-Mohs [micrographic surgery]. There is a huge benefit there to seeing your patients because you can identify infections early, answer simple questions, and reduce in-person clinic visits. That’s a win.
What are visit types you feel are not well-suited to teledermatology or that you approach with more caution?
This will be different for everyone to some degree. I think practitioners need to be alert and use their best judgement when approaching any new patient or new concern. Pigmented lesions certainly give me pause. Although the technology is getting better every day, I believe there is still a gap between seeing a photo of a lesion and seeing a pigmented lesion in person, being able to get up close and examine it dermoscopically. Teledermoscopy, however, is an emerging business model as well, and it will be interesting to see what role this can play as it gets incorporated.
You mentioned having medical licenses in several states. Can you describe the process you went through to obtain these licenses?
It’s a painful process. I started realizing this was something I wanted to incorporate after residency, so I started looking into applying for medical licenses early. Teledermatology companies often will reimburse you and help you to get licenses. I was lucky enough to get assistance, which was essential because it is an onerous process. If you can work that into your contract during negotiations that would be ideal. Not everyone will be as lucky as I was, though. If that doesn’t pertain to you, pick a few states that have larger populations around you, where you know that they have a lot of need and start applying there. Be aware that medical licensure takes about 6 months. Having this started around mid–third year is important.
Employers want someone they can use right away, so I found it very beneficial to approach an employer and be able to explain to them tangibly where you are in the process. For example, “I’ve got my DEA license, Medicare, Medicaid number, and I have licensure in your state and all the surrounding states.” You then have a leg to stand on with your negotiating. If you do the legwork and can then negotiate a higher percentage, you’ll make up the licensure fees in a half day of work. It’s an investment toward your professional career.
Any final thoughts?
I think that insurers are very interested in teledermatology because there’s a potential for huge cost savings. As the dust settles with COVID-19 and we see how telemedicine has changed medicine in general, I really think that payers are going to be more aggressive about requiring teledermatology from their dermatologists. I think residents need to anticipate that teledermatology will be some part of their practice in the future and should start planning now to be prepared for this brave new world going forward.
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
- Yim KM, Florek AG, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era. Telemed J E Health. 2018;24:691-697.
- Shatzkes MM, Borha EL. Permanent expansion of Medicare telehealth services. The National Law Review website. Published December 7, 2020. Accessed April 13, 2021. https://www.natlawreview.com/article/permanent-expansion-medicare-telehealth-services
Resident Pearl
- One result of the COVID-19 pandemic is the aggressive adoption of teledermatology across the United States. Graduating residents should be preparing for a scope of practice that incorporates teledermatology.
A Practical Guide to Treatment of Hair Loss Beyond Standard Therapy
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.
Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.
Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).
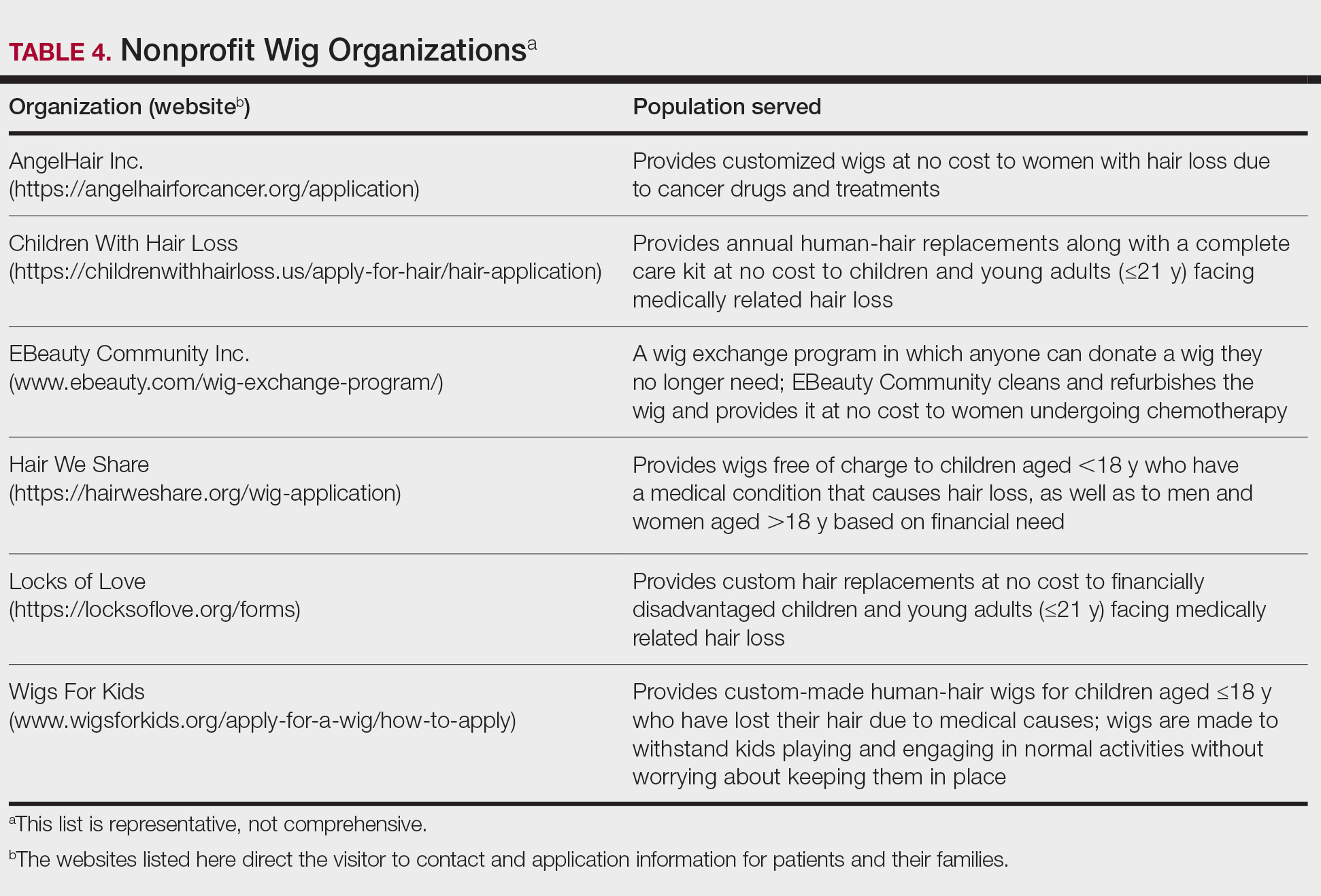
Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.
Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.
Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
When I was a medical student rotating in dermatology, a patient with extensive alopecia looked at my long thick hair and said tearfully, “I just wish I could have hair like yours.”
I smiled, removed my wig, and replied, “You can have hair like mine.”
Determination and Perseverance
I was 2 years old when I was given a diagnosis of alopecia areata. Bald spots on my scalp would come and go for years but were not overly burdensome until I turned 12. At that point, my hair loss escalated despite frequent intralesional injections of triamcinolone; every 2 steps forward were followed by 3 steps backward.
As a freshman in high school, I finally took control of my condition and emotions, shaved my head, and purchased a wig—actions that confronted my hair loss and awoke a determination and perseverance that I did not think I would ever gain while living with this condition. As McGettigan1 wrote in the Journal of the American Academy of Dermatology in 2004, “Being diagnosed with [alopecia areata] does not mean one cannot have a full and meaningful life. By choosing to confront the condition and turn its negative aspects into positive actions, one can succeed in life.”1
As a Provider, Another Perspective
Now, as a dermatology resident, I have the distinct perspective of being patient and provider. Patients often want to know, “Why is this happening?”, “Is my hair going to grow back?”, and “What treatments are available?”
They want to feel supported, understood, and heard.
As health care providers, we must understand that hair loss can result in overwhelming fear, hopelessness, and loss of self-esteem. Although we can give good news and offer helpful treatment options to some patients, there are those for whom medical treatment fails, and we can offer no more than a supportive hand and warm smile.
But can we do even more than that? The answer is: “Yes.”
Management Options
I recommend that all patients with hair loss should receive a copy of the aforementioned McGettigan1 article, “Ahead With No Hair,” which is geared toward patients with alopecia areata but offers inspiring words to any patient struggling to cope with hair loss. Dermatologists also can offer management options for patients with hair loss, including camouflage, wigs, and cosmetic replacement of eyelashes and eyebrows. Of note, several companies offer wigs and brow replacement options for men and children.
Camouflage
We can offer creative and readily available camouflage options for patients with hair loss. For small bald spots and thinning hair on the scalp, keratin hair-building fibers can be extremely useful. This over-the-counter product comes in a variety of natural hair colors, conceals the underlying skin, and adds fullness to hair. The keratin fibers have an innate static charge that allows them to adhere to the hair shaft. Daily application typically is necessary; duration can be maximized if hair spray or other brand-specific bonding spray is used following application of the fibers. A simple online search using the term keratin hair building fibers will reveal many online and in-store options with 4- or 5-star reviews. Most negative reviews pertain to sweating or moisture that causes clumping, but overall this is an easy and affordable option for mild hair loss.
Wigs
For patients hoping to mask moderate or severe hair loss, I recommend wigs, which can be made from synthetic fibers or human hair. In order to effectively guide patients, it is helpful for providers to have some knowledge about the 2 types of wigs. Synthetic wigs are of variable quality, ranging from costume-grade to top-quality products that look and feel like human hair. They are more affordable and often are easier to maintain than human-hair wigs, and hairstyles hold up better after washing. Many synthetic wigs cannot withstand heat from a hot iron and have a slightly shorter lifespan (6–12 months) than human-hair wigs (1–2 years).
Human hair wigs are made of real human hair, so they look and feel natural. These wigs can be made from European, African, Indian, Malaysian, Chinese, or other ethnic hair. Patients can choose the texture of the hair, including silky (smooth), kinky (mimicking natural blow-dried Black hair), and yaki (mimicking relaxed Black hair), as well as the curl pattern (straight, wavy, or curly), length, color, density, and cap construction.
The cap of a wig is what the hair is tied to. The construction of wig caps varies to allow for realistic hair lines as well as security for active use or up-dos. Among the many cap-construction options, the most realistic-appearing are hand-tied monofilament, lace-front, and full-lace wigs, all of which may require tape or glue to keep them in place. Some wig companies offer nonslip so-called “alopecia caps” for patients with no scalp hair. Patients who find their wig irritating to the scalp should consider wearing a nylon wig cap or liner.
Wigs can be purchased in store or online and can be pre-made or custom-built to be tailored to the patient’s specific desires and expectations. The cost depends on the type and quality of hair, cap construction, and length; prices can range from less than $100 to more than $5000.
When choosing a wig, which option—synthetic or human hair—is better for a given patient? Synthetic wigs are rather inexpensive and easy to care for, making them great for new users and those who want to try different styles and colors. Human-hair wigs can be custom-made to match the patient’s natural hair; however, they require extra care to maintain their longevity. Both types of wigs have pros and cons depending on the patient’s budget, time required for maintenance and styling, and needs (Table 1). I encourage patients to have fun with all wig options: Now is the time, I tell them, to try out the cute or daring hair style they have always wanted. The great thing is that if the patient does not like their wig, they can readily change it.
Good-quality wigs are expensive but sometimes are necessary to regain self-confidence and improve one’s quality of life. Advise patients to call their health insurance company to find out if a cranial or scalp prosthesis is covered by their policy. Coverage might require a written prescription for a cranial prosthesis, listing the diagnosis, diagnosis code, and letter of medical necessity. Patients can then purchase the wig online or through a certified distributor depending on their insurance requirements and obtain reimbursement (partial or full coverage). If a wig is not covered by insurance, a cranial prosthesis might be a flexible spending account–eligible expense. For guidance on the reimbursability of wigs, visit the National Alopecia Areata Foundation (NAAF) website (www.naaf.org/AccessHealthcare).
Eyelashes and Eyebrows
Cosmetic replacement of eyelashes (Table 2) and eyebrows (Table 3) is another treatment option that physicians can offer to hair-loss patients. For patients who desire false eyelashes, strip lashes that are glued to the eyelid margin are easiest to apply (but with caution—do not get glue in the eyes!). There are magnetic lashes, but these require natural lashes on which to adhere them. Eyebrows can be hand-drawn using brow pencils or powders with or without a stencil to maintain symmetry. There are even brow wigs and temporary brow tattoos that can last 1 to several days. Semi-permanent tattooing, including microblading, is an option that has amazing results but can be painful and expensive, often requiring touch-ups every 6 to 18 months.
Resources Abound
Experiencing and treating hair loss can be overwhelming, but there are countless resources available for patients. The NAAF has utility beyond the concerns of alopecia areata patients; there also is useful information on YouTube and social media, and support groups exist for hair-loss patients. I recommend starting with the NAAF website, which offers many helpful resources and support groups for patients and their families, including tips on applying for insurance reimbursement and drafting an appeal letter. Lastly, several nonprofit organizations serve the hair-replacement needs of children and adults with hair loss (Table 4).

Final Thoughts
My experience as a patient with alopecia has been long and initially was challenging; however, I found the silver lining after choosing to confront my literal and figurative “losses” and move forward—to grow, so to speak. With the use of custom-made human-hair wigs, false strip eyelashes, and a mix of eyebrow replacement options, I have been able to regain my confidence and self-esteem. Now, my goal as a physician—a goal that I hope you will share—is to be knowledgeable about hair-replacement options and provide information and resources to patients to help them feel empowered, brave, and beautiful.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
- McGettigan ML. Ahead with no hair. J Am Acad Dermatol. 2004;51(1 suppl):18-19.
Practice Points
- Keratin hair-building fibers can help thinning hair appear thick and full.
- Wigs are useful in masking moderate to severe hair loss.
- False eyelashes, eyebrow wigs, temporary eyebrow tattoos, microblading, and other semipermanent makeup can disguise the loss of eyelashes and eyebrows.
Erythema Multiforme–like Dermatitis Due to Isoniazid Hypersensitivity in a Patient With Psoriasis
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
To the Editor:
Psoriasis vulgaris is a chronic autoimmune inflammatory disease and biologic agents, such as anti–tumor necrosis factor α (TNF-α), are alternative drugs in case of resistance or adverse events to conventional ones.1 The limitation of these agents is immunosuppression that may cause infections such as tuberculosis (TB). Prophylaxis is indicated to latent TB diseases if the purified protein derivative (tuberculin) skin test is higher than 5 mm before starting these treatments. The challenge in TB treatment is adverse drug reactions (ADRs) that are reported in 4% to 6% of cases.2,3
Erythema multiforme–like dermatitis is a rare skin rash that develops due to isoniazid (INH). The clinical presentation includes erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH. Skin biopsy and patch tests are the supportive diagnostic methods. Isoniazid-associated skin rashes rarely are reported and generally are not severe enough to terminate the drug. We present a patient with psoriasis who received TB prophylaxis before anti–TNF-α use. He presented with erythema multiforme–like dermatitis due to INH. Withdrawal of the drug and treatment of the lesions were the first steps of intolerance, followed by a patch test with the culprit drug after recovery. We discuss the diagnostic drug allergy evaluation and treatment approach.
A 37-year-old man presented with a 15-year history of severe psoriasis with frequent flares. He was treated with various topical and systemic agents including acitretin and methotrexate at 4-year intervals. Despite the addition of phototherapy, he underwent a new treatment with anti–TNF-α, as the disease control with other treatments was insufficient. Before starting anti–TNF-α, preventive treatment against TB with INH (300 mg/d) was indicated with 20 mm of purified protein derivative. On approximately the 20th day of treatment, he developed pruritic erythema with desquamation and exfoliation localized to the hands and feet (Figure 1). Isoniazid was discontinued and a topical steroid was initiated. After 3 weeks, the skin lesions were completely improved and INH was reinitiated at the same dose with antihistamine prophylaxis (oral levocetirizine 5 mg/d). Seven days later, similar skin lesions presented that were more extensive on the arms and legs (Figure 2). Complete blood cell counts, renal and hepatic function tests, and hepatitis markers were within reference range in consultation with the allergy division. To distinguish the lesions from a psoriasis attack, a punch biopsy of the eruptive dermatitis showed erythema multiforme–like dermatitis including dermal edema and perivascular lymphocytic infiltration with no relation to psoriasis but consistent with a drug eruption. Isoniazid was discontinued, and the skin lesions resolved after 4 weeks of topical steroid and oral antihistamine use (Figure 3). There was no other drug use except INH, and a skin patch test with INH was positive at 72 hours (Figure 4). Skin tests with INH were done to 5 healthy lesions that were negative. Finally, TB prophylaxis was performed with rifampicin (10 mg/kg/d [600 mg/d]) for 4 months with no ADRs. The patient’s psoriasis lesions improved with anti–TNF-α that was initiated 1 month after starting TB prevention with rifampicin.
This case of erythema multiforme–like dermatitis was diagnosed with acral involvement, a positive patch test to INH, and lymphocytic inflammation in a skin biopsy. It was a drug-induced reaction, as skin lesions developed during INH intake and improved after drug withdrawal.
Isoniazid, also known as isonicotinylhydrazide, is an oral antibiotic used for the treatment of TB and other mycobacteria. Protective treatment against latent TB primarily is done with daily INH for 6 or 9 months; alternatively, INH may be taken weekly with rifapentine for 3 months or daily with rifampicin for 4 months. Daily rifampicin alone for 4 months also is an option. In general, these regimens have similar efficacy; however, in terms of safety, the rifampicin and rifapentine combination regimens have fewer hepatotoxicity events compared to the INH alone regimen, but there are more cutaneous and flulike reactions and gastrointestinal intolerance.4 Cutaneous ADRs to TB treatment such as mild itchiness and cutaneous eruptions usually are observed within 2 months of drug initiation. Pyrazinamide was reported as the most common drug associated with cutaneous ADRs, and INH was the rarest offending drug.5
The frequency of ADRs to INH is approximately 5.4%, and the most prevalent ADRs include asymptomatic elevation of serum liver enzyme concentrations, peripheral neuropathy, and hepatotoxicity, and skin lesions are less common.2 Our patient’s laboratory test results excluded vitamin B deficiency, hepatic and renal dysfunction, and neuropathy.
Previously reported skin reactions related to INH were late-type reactions such as maculopapular rash, dermatitis, erythema multiforme, drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome, Stevens-Johnson syndrome, and toxic epidermal necrolysis.5,6 The concerning prediagnosis of psoriatic exacerbation in our patient was ruled out by the absence of typical skin lesions such as well-defined, erythematous plaques and pustules and atypical localization such as the dorsal hands and feet rather than the knees, elbows, lumbosacral region, scalp, and abdomen, which is typical of psoriasis. DRESS syndrome was unlikely with the absence of fever, lymphadenopathy, hypereosinophilia, leukocytosis, and renal and hepatic dysfunction.7 There were no widespread blisters, epidermal detachment, or mucosal involvement on the trunk or face typically associated with Stevens-Johnson syndrome and toxic epidermal necrolysis.7,8 A possible diagnosis of contact dermatitis was suspected with likely skin lesions as exfoliation and chapping, typical localization on the hands and feet, and positive patch test that supported sensitization to the drug. However, the patient’s skin lesions were not eczematous (characterized by erythema, vesiculation, exudation, or bullous edema in the acute phase), and were not localized to areas of irritant exposure.3 In our patient, erythematoedematous lesions in an acral distribution with no mucosal involvement and systemic exposure to INH was compatible with erythema multiforme, whereas the absence of target appearance, positive patch test, and late appearance were incompatible with erythema multiforme.8
Because the clinical picture did not fit contact dermatitis or erythema multiforme, a diagnosis of erythema multiforme–like noneczematous dermatitis was suggested. Noneczematous dermatitis has subtypes that include purpuric, lichenoid, pustular, lymphomatoid, dyshidrosiform, and pigmented, as well as erythema multiforme–like contact eruptions.9 These clinical entities are not associated with contact exposure, but are related to systemic exposure, as seen in our patient.10 The patch test positivity and skin biopsy report also supported the diagnosis of erythema multiforme–like dermatitis. Erythema multiforme–like dermatitis is thought to be caused by medications or infections inducing immunocomplexes and lymphocytic infiltration in the dermis and subepidermis. Nevertheless, the prognosis was self-limiting in both.8 The clinical polymorphism caused by INH in this patient was suggested to be related with individual susceptibility, variability of contact-activating modalities, and the targeted cutaneous structures. Furthermore, among the risk factors for cutaneous ADRs—HIV, polypharmacy, older age, and preexisting renal and liver impairment—the only notable factor in this patient was psoriasis as an autoimmune disorder.
Patients with skin diseases such as psoriasis should be followed up by closer monitoring during INH use. Withdrawal of the drug and symptomatic treatment of the lesions with corticosteroid and antihistamine are the first steps of drug intolerance. After complete recovery and termination of antiallergic drugs, diagnostic tests are recommended if the drug reaction was not life-threatening. Skin prick and intradermal tests are useful in early-type drug reactions, whereas patch testing and late evaluation of an intradermal test may be helpful in the diagnosis of delayed-type reactions. The full dose of INH is avoided in an intradermal test against irritation. A patch test with INH was performed by diluting a 100-mg tablet with 1 mL of distilled water, and used as 1/100, 1/10, and 1/1 dilutions.8 Patch testing with INH also was done in 5 healthy control patients to exclude the irritation effect in this case. The rechallenge of INH was done in a controlled manner in our patient to rule out psoriasis activation since it was a localized skin reaction with no serious ADR. An oral provocation test with the culprit drug is the gold standard of drug allergy diagnosis that should be done in a tertiary hospital with an intensive care unit.
This case of erythema multiforme–like dermatitis due to INH is interesting due to systemic intake of INH, which resulted in dermatitis with localized involvement similar to erythema multiforme but with no immunologic processes or prior sensitization. With the increasing use of anti–TNF-α treatment, INH use will be more prevalent than in the past for the treatment of latent TB. Even though the skin-restricted ADRs of INH are rare and minor, particular attention should be paid to patients with dermatologic diseases. In our case, diagnostic drug allergy evaluation was performed to optimize the second-line treatment of TB infection, in addition to early withdrawal of the culprit drug.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
- Vide J, Magina S. Moderate to severe psoriasis treatment challenges through the era of biological drugs.An Bras Dermatol. 2017;92:668-674.
- Gülbay BE, Gürkan OU, Yildiz OA, et al. Side effects due to primary antituberculosis drugs during the initial phase of therapy in 1149 hospitalized patients for tuberculosis. Respir Med. 2006;100:1834-1842.
- Holdiness MR. Contact dermatitis to antituberculosis drugs. Contact Dermatitis. 1986;15:282-288.
- Getahun H, Matteelli A, Abubakar I, et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J. 2015;46:1563-1576.
- Tan WC, Ong CK, Kang SC, et al. Two years review of cutaneous adverse drug reaction from first line anti-tuberculous drugs. Med J Malaysia. 2007;62:143-146.
- Özkaya E.Eczematous-type multiple drug allergy from isoniazid and ethambutol with positive patch test results. Cutis. 2013;92:121-124.
- Fernando SL. Drug-reaction eosinophilia and systemic symptoms and drug-induced hypersensitivity syndrome. Australas J Dermatol. 2014;55:15-23.
- Rebollo S, Sanchez P, Vega JM, et al. Hypersensitivity syndrome from isoniazid with positive patch test. Contact Dermatitis. 2001;45:306.
- Sokumbi O, Wetter DA. Clinical features, diagnosis, and treatment of erythema multiforme: a review for the practicing dermatologist. Int J Dermatol. 2012;51:889-902.
- Bonamonte D, Foti C, Vestita M, et al. Nummular eczema and contact allergy: a retrospective study. Dermatitis. 2012;23:153-157.
Practice Points
- Hypersensitivity skin reactions to antituberculosis (TB) drugs are on the rise due to the increasing use of anti–tumor necrosis factor α. Isoniazid (INH) use will be more prevalent than in the past for the treatment of latent TB.
- Even though the skin-restricted adverse events to INH are rare and minor, particular attention should be paid to patients with dermatologic diseases such as psoriasis.
Tinea Incognito Mimicking Pustular Psoriasis in a Patient With Psoriasis and Cushing Syndrome
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
To the Editor:
The term tinea incognito was introduced by Ive and Marks1 in 1968 and refers to unusual clinical presentations of tinea due to the application of topical corticosteroids. Tinea incognito, which does not feature the classical clinical characteristics of tinea corporis such as well-defined, erythematous, scaly patches and elevated borders, is regularly misdiagnosed as inflammatory dermatosis.2 Immunosuppression caused by topical and/or systemic steroids predisposes patients to the development of tinea.3 Herein, a case of widespread pustular tinea incognito mimicking pustular psoriasis along with failure of tumor necrosis factor (TNF) inhibitor treatment is reported in a patient with chronic plaque psoriasis and steroid-induced Cushing syndrome.
A 46-year-old man with a 25-year history of psoriasis was referred to the dermatologic outpatient clinic with a severe flare-up of chronic plaque psoriasis. Prior treatments included methotrexate and acitretin without response. Narrowband UVB treatment was discontinued due to claustrophobia. Topical treatment with calcipotriol 0.005%–betamethasone dipropionate 0.05% gel was reported to be ineffective. The patient was administered prednisone over several months in a primary care setting at a dosage of 35 mg daily when he presented to the dermatology clinic. Physical examination revealed widespread chronic plaque psoriasis of the trunk and extremities, and a psoriasis area and severity index score of 15 was calculated. The patient had onychodystrophy with subungual hyperkeratosis of all toenails. Signs of prednisone-induced Cushing syndrome, including central obesity, lipodystrophy, and red striae, were noted.
Treatment was started by dermatology with the TNF inhibitor adalimumab at an initial dose of 80 mg, followed by subsequent 40-mg doses every other week; prednisone was tapered off. Topical treatment with a 4-week course of clobetasol propionate cream 0.05% daily for psoriatic lesions was initiated.
Six weeks after the initial consultation, the patient presented to the hospital’s emergency department with worsening symptoms of itchy, burning, and painful skin after good initial improvement. The patient’s skin started to burn upon application of clobetasol and the rash worsened. The patient did not use emollients. At that point, the patient was on a daily dose of 15 mg of prednisone. On dermatologic review, multiple partially annular lesions with subtle scaling and multiple pustules on the arms and legs as well as the buttocks and groin were noticed. These lesions were confined to sites of prior psoriasis as marked by postinflammatory hyperpigmentation (Figure 1). Widespread tinea was assumed, and treatment with fluconazole 50 mg daily was administered for 4 weeks. Direct examination of skin scrapings from the patient’s thigh showed hyphae, and fungal culture was positive for Trichophyton rubrum. Scrapings from the patient’s hallux nail remained inconclusive due to bacterial overgrowth. At 4-week follow-up, the patient’s skin had cleared entirely and showed only postinflammatory changes (Figure 2). Healthy proximal nail growth was observed. Fluconazole was continued at a once-weekly dose of 150 mg together with adalimumab at a dose of 40 mg every 2 weeks and a prednisone tapering schedule.
This case describes pustular tinea incognito in a patient with chronic plaque psoriasis. As the name indicates, tinea incognito can mimic other skin conditions and classically is linked to topical application of corticosteroids.1 Tinea incognito can be a diagnostic challenge. Kim et al4 reported a diagnostic delay of 15 months and the frequent requirement for the involvement of a second physician or dermatologist. Treatment with topical or systemic corticosteroids is a risk factor for dermatophyte infections because of their immunosuppressive action.3,5 Although recommended by current guidelines, a large number of psoriatic patients are treated with systemic steroids, predominantly prescribed in primary care, that can lead to iatrogenic Cushing syndrome, as demonstrated in this patient.6
In addition to systemic and topical steroids, the reported patient was started on the TNF inhibitor adalimumab prior to the onset of the tinea. Cases of patients on TNF inhibitors with widespread tinea are scarce. Bardazzi et al7 reported 2 cases of widespread nonpustular tinea in patients with psoriasis on TNF inhibitor treatment without further immunomodulating treatment. They hypothesized that TNF-α could be an important cytokine in the defense against dermatophytes.7
Whether psoriasis itself is a risk factor for tinea is still under debate, but tinea pedum and onychomycosis seem to have higher prevalence among psoriatic patients.8,9 As in this patient, bacterial overgrowth of hyperkeratotic nail samples can confound the culture’s clinical significance, thereby hindering the diagnosis of onychomycosis in patients with psoriasis.10 Alteras et al8 hypothesized that autoinoculation from preexisting onychomycosis or tinea pedum was the underlying mechanism of tinea incognito.
This patient’s hyperkeratotic nails showed healthy regrowth after initiation of both fluconazole and adalimumab, though it remained unclear whether preexisting onychomycosis was a possible source of tinea incognito. The finding that the patient’s tinea was almost exclusively limited to the sites of prior psoriatic lesions argues for autoinoculation and spreading accelerated by application of topical steroids triggered by the immunosuppressive effects of both topical and systemic steroids. The TNF inhibitor treatment may have helped to unmask the dermatophyte infection rather than contributing to it, as it cleared the psoriatic plaques.
Apart from psoriasis, tinea incognito most commonly is mistaken for other inflammatory conditions such as eczema, folliculitis, rosacea, granuloma annulare, and discoid lupus erythematosus.2 Inflammatory tinea can present with pustules due to the increased occurrence of neutrophil invasion.11This patient’s symptoms worsened 4 weeks after the initiation of TNF inhibitor treatment, which suggested treatment failure. However, clearance of the preexisting psoriatic lesions with remnant hyperpigmentation only argued for good response to TNF inhibitor treatment. The main differential diagnosis of this case of tinea incognito was generalized pustular psoriasis. The patient also was being treated with systemic and topical steroids, both known for their potential to trigger pustular psoriasis.12,13 Furthermore, TNF inhibitors have been described as a trigger for predominantly palmoplantar pustulosis but also are additionally associated with generalized pustular psoriasis.14
This case aims to raise awareness that tinea incognito can imitate both pustular psoriasis and TNF inhibitor treatment failure. Furthermore, the presented findings highlight risks associated with the treatment of psoriasis with systemic steroids. Pustular tinea incognito should be considered in the differential diagnosis of pustular psoriasis, especially in the setting of immunosuppression. After initial improvement, worsening of symptoms such as itching and burning as well as extension of the lesions upon application of topical steroids are regularly described in tinea incognito and can be present in addition to the more typical annular presentation of lesions as a clue to the diagnosis.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
- Ive FA, Marks R. Tinea incognito. Br Med J. 1968;3:149-152.
- Arenas R, Moreno-Coutiño G, Vera L, et al. Tinea incognito. Clin Dermatol. 2010;28:137-139.
- Rouzaud C, Chosidow O, Brocard A, et al. Severe dermatophytosis in solid organ transplant recipients: a French retrospective series and literature review [published online January 25, 2018]. Transpl Infect Dis. doi:10.1111/tid.12799
- Kim WJ, Kim TW, Mun JH, et al. Tinea incognito in Korea and its risk factors: nine-year multicenter survey. J Korean Med Sci. 2013;28:145-151.
- Ohta Y, Saitoh N, Tanuma H, et al. Local cytokine expression in steroid-modified tinea faciei. J Dermatol. 1998;25:362-366.
- Augustin M, Schäfer I, Reich K, et al. Systemic treatment with corticosteroids in psoriasis-health care provision far beyond the S3-guidelines. J Dtsch Dermatol Ges. 2011;9:833-838.
- Bardazzi F, Balestri R, Rech G, et al. Dermatophytosis during anti-TNF-α monoclonal antibody therapy. Mycoses. 2011;54:E619-E620.
- Alteras I, Ingberg A, Segal R, et al. The incidence of skin manifestations by dermatophytes in patients with psoriasis. Mycopathologia. 1986;95:37-39.
- Leibovici V, Ramot Y, Siam R, et al. Prevalence of tinea pedis in psoriasis, compared to atopic dermatitis and normal controls—a prospective study. Mycoses. 2014;57:754-758.
- Tsentemeidou A, Vyzantiadis TA, Kyriakou A, et al. Prevalence of onychomycosis amongst patients with nail psoriasis who are not receiving immunosuppressive agents: results of a pilot study. Mycoses. 2017;60:830-835.
- Hirschmann JV, Raugi GJ. Pustular tinea pedis. J Am Acad Dermatol. 2000;42:132-133.
- Brenner M, Molin S, Ruebsam K, et al. Generalized pustular psoriasis induced by systemic glucocorticosteroids: four cases and recommendations for treatment. Br J Dermatol. 2009;161:964-966.
- Boxley JD, Dawber RP, Summerly R. Generalized pustular psoriasis on withdrawal of clobetasol propionate ointment. Br Med J. 1975;2:255-256.
- Kucharekova M, Winnepenninckx V, Frank J, et al. Generalized pustulosis induced by adalimumab in a patient with rheumatoid arthritis—a therapeutic challenge. Int J Dermatol. 2008;47:25-28.
Practice Points
- Tinea incognito and its altered clinical presentation can provide clinical challenges and often is diagnosed with delay.
- Immunosuppression, such as iatrogenic Cushing syndrome, is a risk factor for tinea incognito.
- Pustular tinea incognito is a differential diagnosis of pustular psoriasis that can mimic tumor necrosis factor inhibitor treatment failure in patients with psoriasis.