User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Cutaneous Manifestations of Nutritional Excess: Pathophysiologic Effects of Hyperglycemia and Hyperinsulinemia on the Skin
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
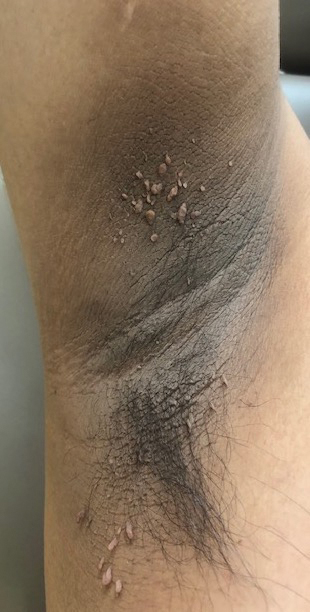
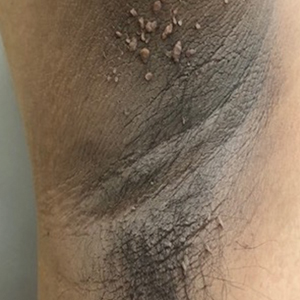
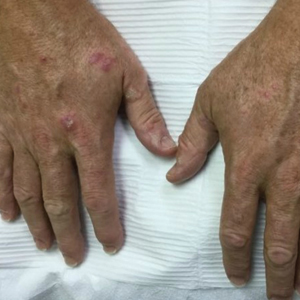
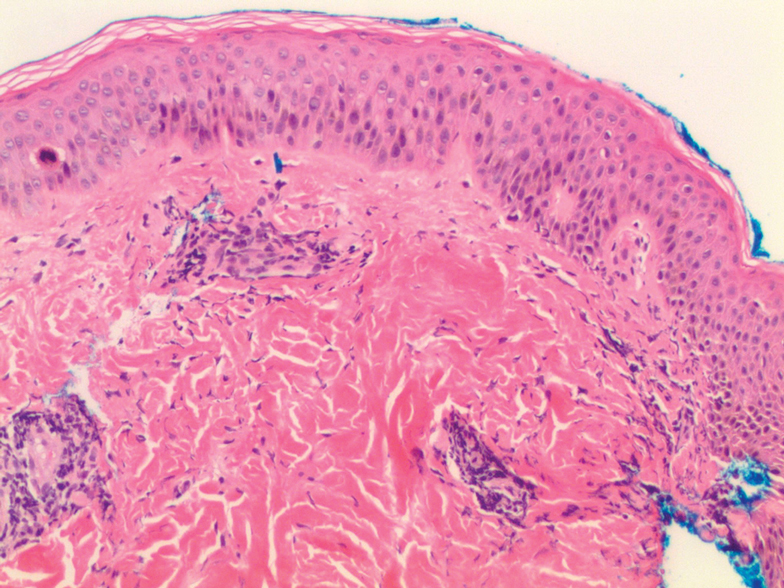
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10
Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
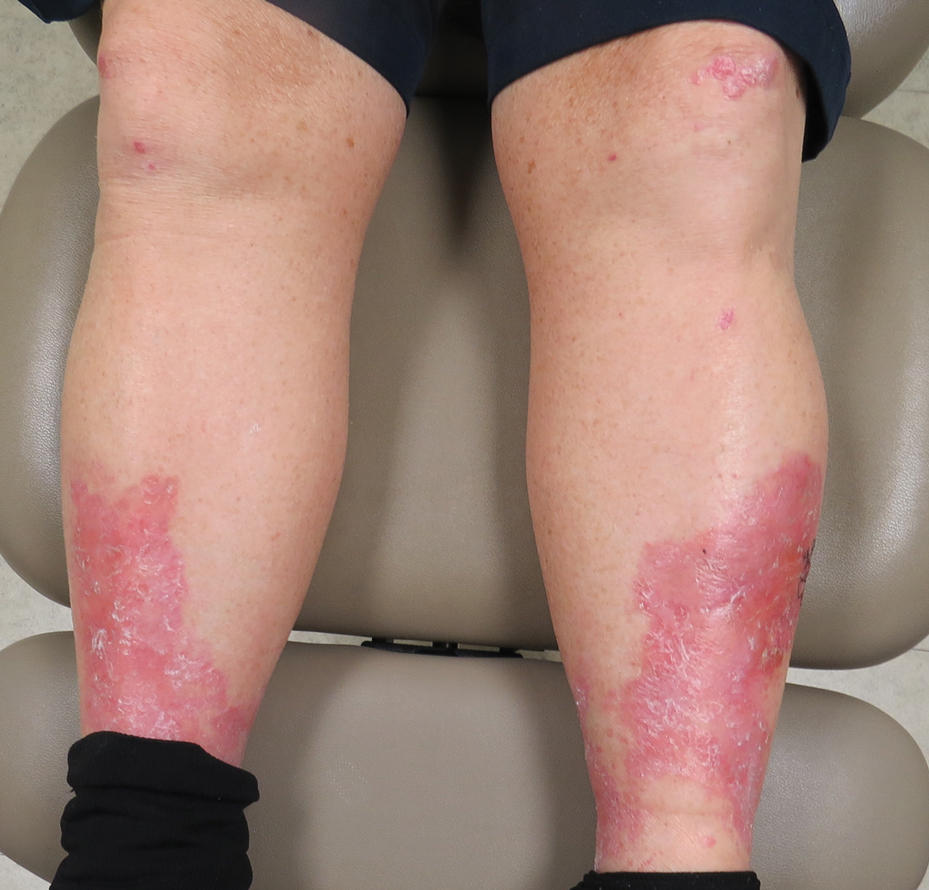
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
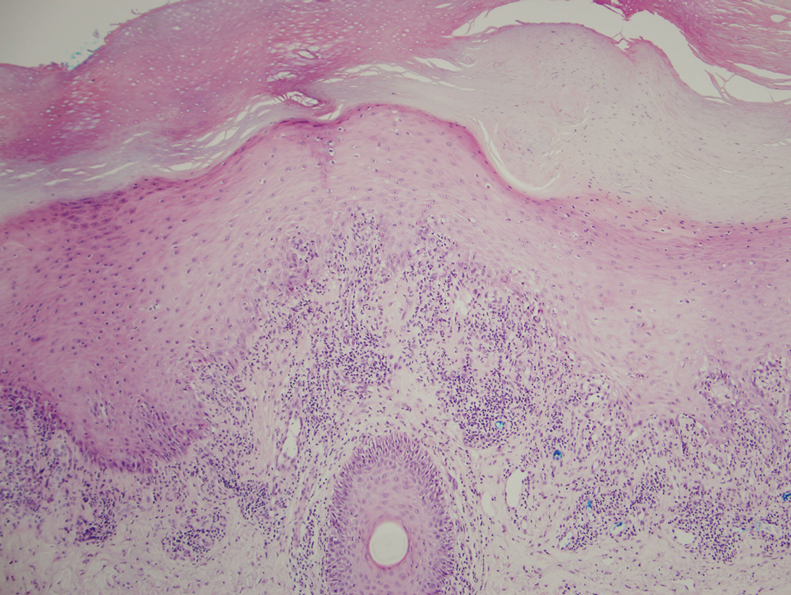
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
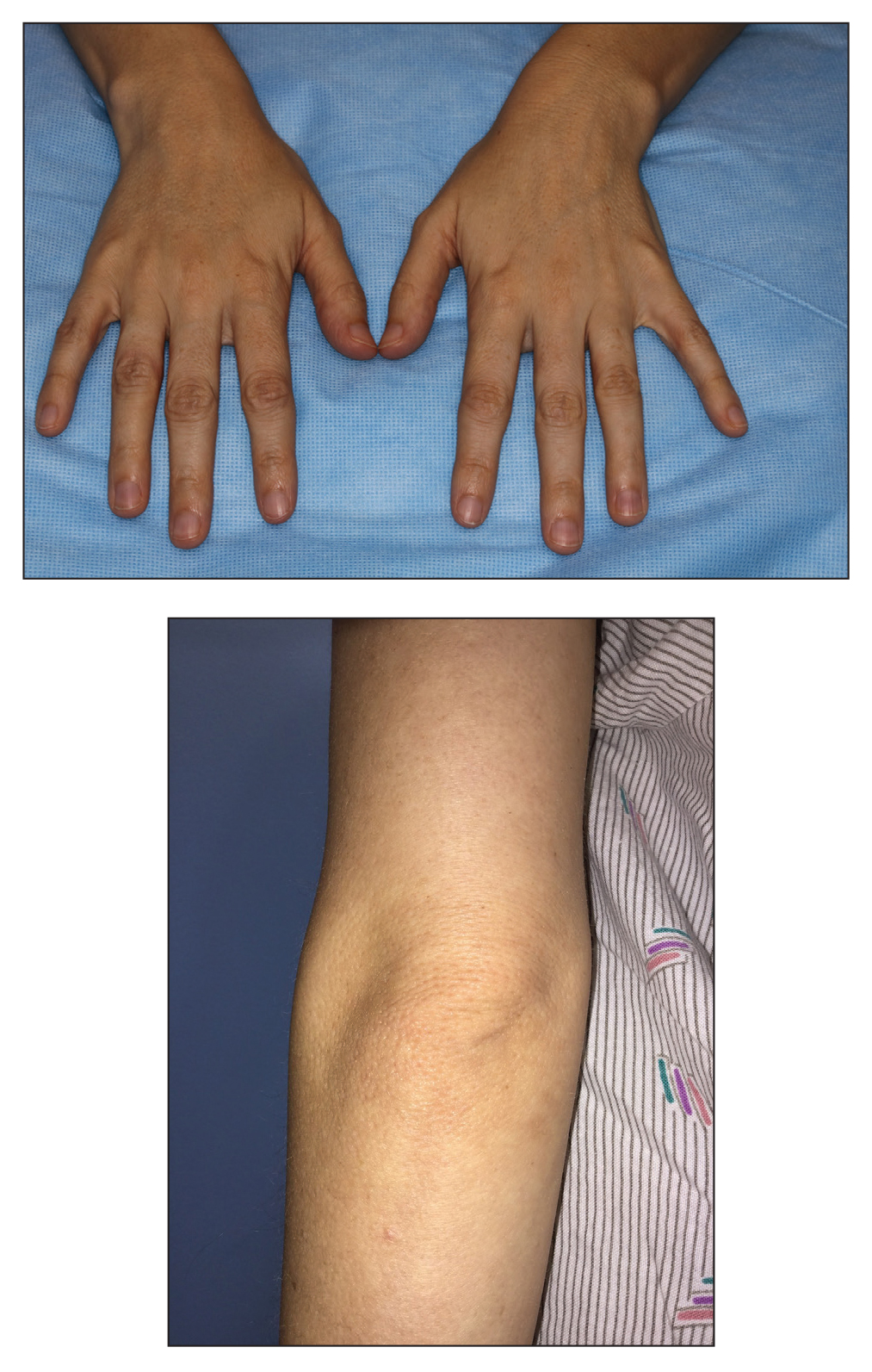
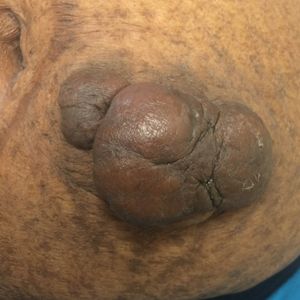
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
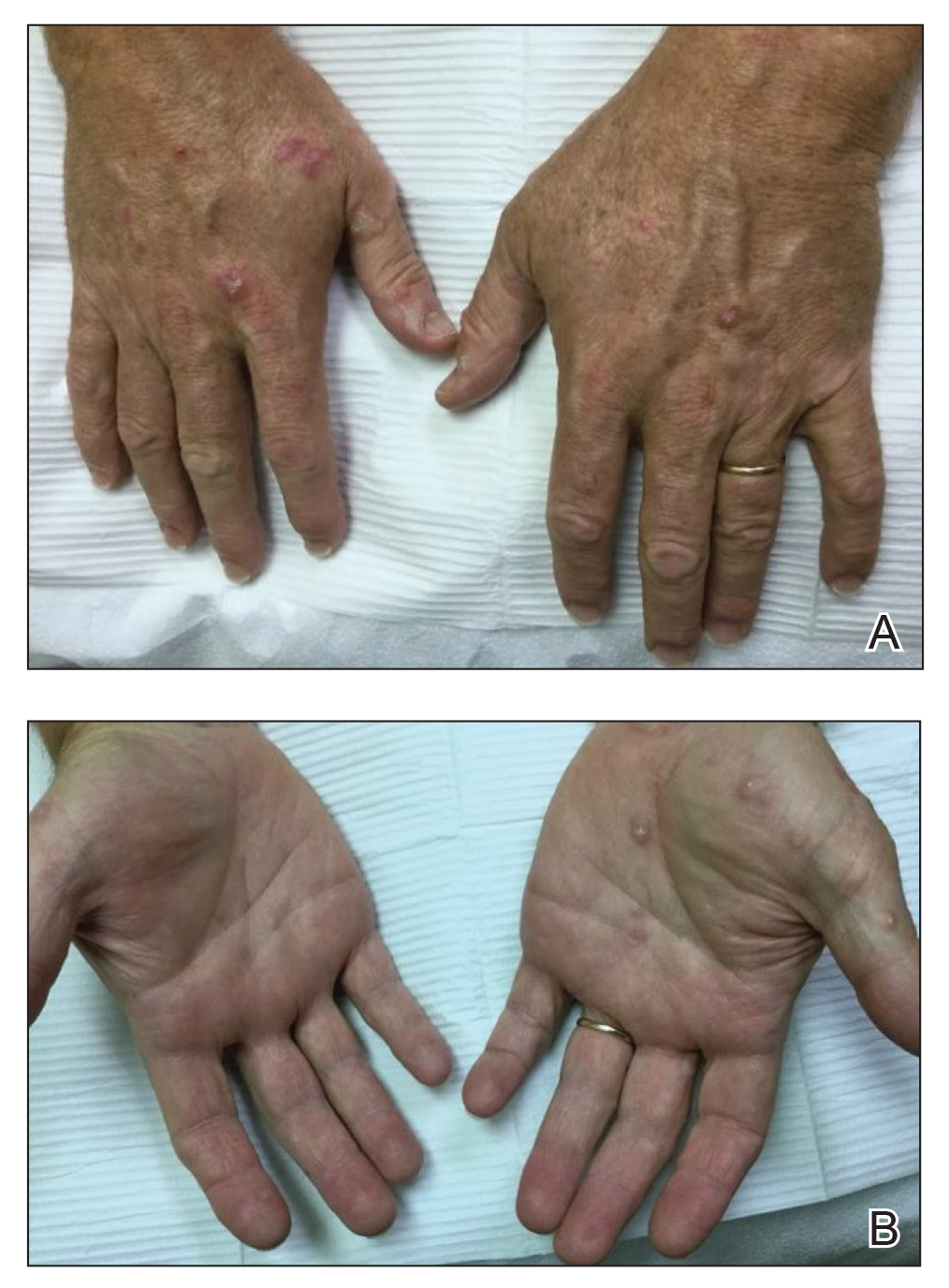
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
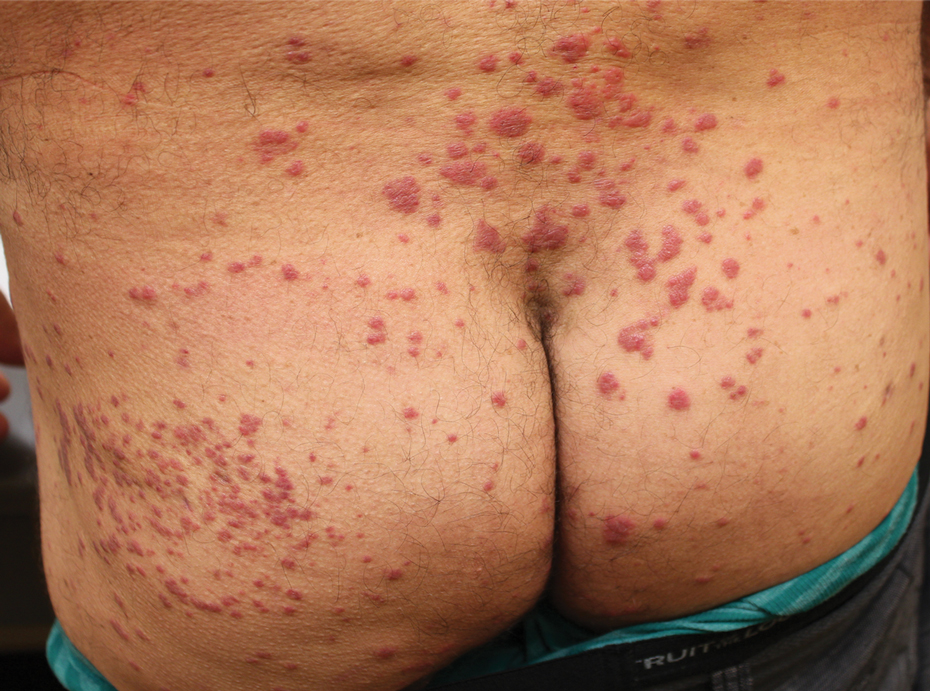
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10
Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
Nutritional dermatoses are classically associated with dietary nutrient deficiencies; however, cutaneous disease as a consequence of nutrient excess often is overlooked. Chronic hyperglycemia and hyperinsulinemia resulting from excess carbohydrate intake may be implicated in a number of cutaneous pathologies, of which every dermatologist should be aware.1-3
Although diabetic patients exhibit many cutaneous manifestations of excess carbohydrate consumption, the absence of a diagnosis of type 2 diabetes mellitus (T2DM) does not necessarily preclude them.4-6 Emerging evidence now highlights the development of insulin resistance well before a patient ever meets the diagnostic criteria for T2DM.7,8 Cutaneous disease can provide early insight into a patient’s glucose tolerance and may be the first sign of metabolic derangement. Prompt recognition of these cutaneous alterations and management of the patient’s underlying systemic disease can improve their quality of life and help prevent severe systemic complications associated with insulin resistance and impaired glucose tolerance.
The aim of this review is to highlight both common and rare cutaneous manifestations associated with the persistent consumption of high glycemic load diets, resultant hyperglycemic and hyperinsulinemic states, and the pathophysiologic mechanisms that underlie them.
Acanthosis Nigricans
Acanthosis nigricans (AN) is a highly prevalent cutaneous finding in individuals with insulin resistance that clinically presents as thickened, hyperpigmented, velvety plaques on the intertriginous and flexural surfaces. The most frequently involved sites include the neck, axillae (Figure), and inframammary and inguinal folds. Black and Hispanic patients most commonly are affected. Although classically associated with T2DM, AN also can be observed in normoglycemic individuals.7-9 One recent study reported the rate of AN to be 36% in a cohort of middle-aged patients (N=320) with normal fasting blood glucose levels, while the rate of AN in matched patients with hyperglycemia (prediabetes and T2DM) was approximately 50%.7 Quantification of insulin resistance was performed using the homeostatic model assessment of insulin resistance index. Interestingly, the specificity for insulin resistance in normoglycemic and hyperglycemic subjects with AN was 85% and 90%, respectively.7 These findings suggest that AN may serve as a convenient surrogate marker for subclinical insulin resistance, a conclusion that has been reported in a series of previous studies.8-10
Although the pathogenesis of AN has not been fully elucidated, it is known that persistently elevated blood glucose triggers continual secretion of insulin and insulinlike growth factor 1 (IGF-1), which results in the overstimulation of insulin and IGF-1 receptors on keratinocytes and dermal fibroblasts through direct and indirect pathways.11,12 The resultant cellular proliferation can be observed histologically in the forms of orthokeratotic hyperkeratosis and papillomatosis, as occurs in AN.11,13 Further supporting the association between elevated insulin and AN are reports of AN developing at sites of repeated insulin injection as well as genetic mutations in the insulin receptor resulting in severe AN in children.14-16
The treatment of AN ultimately focuses on improving glycemic control and reducing insulin resistance through lifestyle modification and pharmacotherapy with agents such as metformin.11,13 Dermatologic treatment with oral and topical keratolytic agents such as isotretinoin and other retinoids, salicylic acid, urea, or ammonium lactate may be used, but their efficacy generally has been limited.11,13,17,18
Diabetic Dermopathy
Diabetic dermopathy (DD), commonly known as shin spots, refers to the red-brown, atrophic, circinate macules and patches that often appear on the lower extremities in patients with T2DM. Although the pretibial area is the most frequently involved site, other areas of bony prominence such as the forearms can be affected. The prevalence of DD in the diabetic population can be exceedingly high, with some studies reporting incidence rates greater than 50%, particularly in those with poorly controlled T2DM.19-21 Interestingly, DD also has been documented in patients without T2DM and has been postulated to be an early sign of insulin resistance.20,22
The pathogenesis of DD remains uncertain, but one proposed mechanism is through microvascular damage caused by hyperglycemia-induced, nonenzymatic glycation, possibly in conjunction with mild trauma, that leads to the deposition of hemosiderin and melanin in the skin.20,23 A recent study identified increased vascularization of dermopathy lesions when compared with surrounding tissue.24 Subcutaneous nerve ischemia and degeneration secondary to diabetic neuropathy also have been postulated as causative.20,23 Given the lack of effective therapies and the asymptomatic nature of DD, treatment typically is not pursued. However, DD is associated with other diabetic microvascular complications, including diabetic nephropathy, retinopathy, and neuropathy. For this reason, identification of DD warrants further characterization and management of a patient’s underlying diabetes.19,20
Scleredema Diabeticorum
Scleredema diabeticorum (SD) refers to the slowly progressive, painless thickening and woody induration of the neck, shoulders, and upper back in individuals with long-standing, poorly controlled diabetes. The condition is almost exclusively seen in the diabetic population, with prevalence rates reported to be as high as 14%.25-27 Although SD generally is asymptomatic, some individuals may experience restricted mobility and decreased sensation in affected areas.25,27,28 The diagnosis of SD frequently is missed or ignored clinically. Biopsy can provide diagnostic confirmation of this entity, as histopathology reveals a thickened reticular dermis with an accumulation of collagen and adjacent mucinous infiltrate with no edema or sclerosis.28,29
Although the pathogenesis of SD is not well established, it is theorized that the binding of advanced glycation end products (AGEs) to collagen fibers impairs proper cross-linking and degradation by collagenase.29-31 It is well known that hyperglycemic conditions can promote endogenous formation of AGEs, which occur when reducing sugar molecules become glycated through a nonenzymatic reaction.30-32 The Western diet also is high in preformed AGEs, which are created primarily through certain high-heat cooking methods such as frying and grilling.31,32 Hyperglycemia-induced stimulation of fibroblasts also has been proposed as a driver of increased collagen deposition observed histologically in SD.28,29,33 Treatment of SD can be difficult, as there are no consistently reported therapies, and even improvement in glycemic control does not appear to reverse this condition.29 Case reports have demonstrated some efficacy with various phototherapeutic modalities, including psoralen plus UVA and narrowband UVB phototherapy.34-36
Ichthyosiform Skin Changes
Ichthyosiform skin changes refer to areas of xerosis and scaling that classically present on the anterior distal lower extremities. Although ichthyosiform alterations have been associated with numerous systemic diseases, they often represent an early finding in diabetic patients.27,37 The development of ichthyosiform skin changes has been linked to the formation and accumulation of AGEs, which can cause defective cell adhesion in the stratum corneum.37,38 Treatment with topical emollients and keratolytics may prove beneficial for the skin but do not improve the underlying systemic condition.39
Acrochordons
Acrochordons (skin tags) are common benign fibroepithelial polyps that classically present on the face, neck, and trunk. The underlying mechanism responsible for the development of acrochordons is uncertain, but the association with insulin resistance and impaired carbohydrate metabolism is well validated.40-46 Several large cross-sectional and case-control studies have reported rates of T2DM ranging from 23% to 72% in patients with acrochordons.41,42,47 The pathophysiology may involve an increase in tissue and epidermal growth factors driven by elevated serum insulin levels, stimulation of IGF-1 receptors, and a localized proliferation of cutaneous tissue in elastin-poor areas.45,48,49 Interestingly, the quantity of acrochordons has been positively correlated with fasting blood glucose levels. Additionally, the presence of 30 or more acrochordons was found to increase the risk of developing T2DM.41 Therefore, the presence and number of acrochordons may serve as a convenient indicator of systemic glycemic control and insulin resistance. Screening for T2DM is warranted in individuals without a prior diagnosis who present with multiple acrochordons.
Keratosis Pilaris
Keratosis pilaris (KP) is a benign skin condition characterized by pink-red, erythematous, monomorphic, follicular papules often seen on the extensor arms, thighs, buttocks, and cheeks. Keratosis pilaris is exceedingly common in the general population but occurs more frequently and with more extensive involvement in those with atopic dermatitis and T2DM.27,50,51 The mechanism underlying the hyperkeratosis and inflammatory change observed in KP is not well understood and is likely multifactorial.52,53 Hyperandrogenism, as a consequence of hyperinsulinemia, may play an important role in KP, as elevated circulating androgens are known drivers of keratinocyte proliferation of the pilosebaceous unit of hair follicles.52,54 Support for this theory includes the clinical exaggeration of KP frequently encountered around puberty when androgen levels peak.55,56 Moreover, one study found a higher incidence of KP among adolescent patients with type 1 diabetes mellitus than among healthy age-matched controls.27 The most effective treatment of KP appears to be laser therapy, particularly the Q-switched Nd:YAG laser. Numerous topical modalities have been employed to treat KP but exhibit limited efficacy, including mineral oil, tacrolimus, azelaic acid, and salicylic acid, among others.57
Necrobiosis Lipoidica
Necrobiosis lipoidica (NL) is a chronic granulomatous skin condition of unknown origin that presents with well-demarcated, yellow-brown, atrophic patches and plaques often found exclusively on the shins. There is a strong association with type 1 diabetes mellitus, with reported rates ranging from 11% to 65% in patients with NL.58-60 In a recent retrospective study of 236 patients with NL, 58.5% of patients had diabetes.61 Nevertheless, NL is a rare entity that affects less than 1% of the diabetic population.60 Given its correlation with diabetes, it has been postulated that the pathogenesis of NL is due to microvascular ischemic changes resulting from prolonged hyperglycemia.60 However, studies revealing an increase in blood flow to NL lesions suggest that the condition may instead be attributed to an inflammatory process.62 Despite the disfiguring appearance, the lesions of NL often are asymptomatic. Pain or pruritus may develop secondary to ulceration, which occurs in approximately one-third of patients. Although many treatment options have been attempted—including topical and intralesional corticosteroids, immunomodulators, platelet inhibitors, and phototherapy—efficacy is limited.60
Bullosis Diabeticorum
Bullosis diabeticorum (BD) is the abrupt onset of noninflammatory vesicles and bullae developing in the setting of diabetes. The prevalence of BD in the diabetic population ranges from 0.16% to 0.5%.63-66 Bullosis diabeticorum occasionally has been reported to occur prior to the onset of diabetes, warranting screening hemoglobin A1c in patients without an established diagnosis of diabetes.67 Bullae most commonly present over the acral surfaces, but the lower extremities also are routinely affected. Bullae typically are large and painless, contain clear fluid, and may progress from tense to flaccid over the course of several days. Although histologic analysis reveals nonspecific findings, biopsy may be useful in excluding other bullous disorders. Because BD is a benign condition that spontaneously resolves over several weeks, treatment rarely is pursued.63,64
Generalized Granuloma Annulare
Generalized granuloma annulare (GA) is an idiopathic inflammatory cutaneous disorder characterized by pink-red, arciform and annular, nonscaly, beaded papules and plaques. Granuloma annulare can be localized or generalized with perforating, patch, and palmoplantar variants. Although the pathogenesis is poorly understood, some studies have demonstrated a correlation between GA and type 1 diabetes mellitus.68-71 Generalized GA appears to be most strongly associated with diabetes, and approximately 10% to 15% of cases occur in this population.70,72 Because GA has been reported to precede the diagnosis of diabetes, patients with generalized or recurrent localized GA should be screened for persistent hyperglycemia with a hemoglobin A1c test.71,73 Although some GA is self-resolving, treatment options for persevering GA include topical and intralesional steroids, isotretinoin, dapsone, tacrolimus, antimalarials, biologic medications, and psoralen plus UVA therapy.74
Final Thoughts
Mechanistic links between common cutaneous conditions and persistent hyperglycemic and hyperinsulinemic states are slowly emerging. Hyperglycemia promotes nonenzymatic glycation of the vascular endothelium as well as formation of AGEs that impair cross-linking of collagen in the skin. The consequent microangiopathic damage may lead to cutaneous conditions such as DD, NL, and BD. In addition to microvascular compromise, impaired collagen cross-linking may result in ichthyosiform skin changes and SD. Hyperinsulinemia causes increased circulating levels of IGF-1, which leads to the overactivation of IGF-1 receptors present on fibroblasts and keratinocytes. This aberrant IGF-1 signaling drives cellular hyperproliferation and differentiation, which may be responsible for cutaneous findings such as AN, KP, and/or acrochordons. An insulin-dependent increase in IGF-1 and androgenic signaling may have implications for hormonally driven inflammatory skin disorders such as acne vulgaris and hidradenitis suppurativa, warranting further investigation.
Physicians should be aware of these dermatologic manifestations and their proposed underlying pathophysiologic mechanisms related to impaired glucose tolerance and insulin resistance. A diagnosis of T2DM is not a prerequisite for metabolic disturbance, and the skin may serve as the first clue to underlying systemic disease. Early identification of these cutaneous conditions may lead to timely patient counseling, lifestyle modification, and/or medical management, preventing the long-term sequelae associated with metabolic disorders.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
- Kolb H, Kempf K, Röhling M, et al. Insulin: too much of a good thing is bad. BMC Med. 2020;18:224.
- Thomas DD, Corkey BE, Istfan NW, et al. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3:1727-1747.
- Saklayen MG. The global epidemic of the metabolic syndrome. Curr Hypertens Rep. 2018;20:12.
- Holzer G, Straßegger B, Volc-Platzer B. Cutaneous manifestations of metabolic syndrome. Hautarzt. 2016;67:982-988.
- Lause M, Kamboj A, Fernandez Faith E. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Duff M, Demidova O, Blackburn S, et al. Cutaneous manifestations of diabetes mellitus. Clin Diabetes. 2015;33:40-48.
- Álvarez-Villalobos NA, Rodríguez-Gutiérrez R, González-Saldivar G, et al. Acanthosis nigricans in middle-age adults: a highly prevalent and specific clinical sign of insulin resistance. Int J Clin Pract. 2020;74:E13453.
- Bhagyanathan M, Dhayanithy D, Parambath VA, et al. Acanthosis nigricans: a screening test for insulin resistance--an important risk factor for diabetes mellitus type-2. J Family Med Prim Care. 2017;6:43-46.
- Stuart CA, Gilkison CR, Smith MM, et al. Acanthosis nigricans as a risk factor for non-insulin dependent diabetes mellitus. Clin Pediatr (Phila). 1998;37:73-79.
- Hud JA Jr, Cohen JB, Wagner JM, et al. Prevalence and significance of acanthosis nigricans in an adult obese population. Arch Dermatol. 1992;128:941-944.
- Hermanns-Lê T, Scheen A, Piérard GE. Acanthosis nigricans associated with insulin resistance: pathophysiology and management. Am J Clin Dermatol. 2004;5:199-203.
- Cruz PD Jr, Hud JA Jr. Excess insulin binding to insulin-like growth factor receptors: proposed mechanism for acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):82S-85S.
- Higgins SP, Freemark M, Prose NS. Acanthosis nigricans: a practical approach to evaluation and management. Dermatol Online J. 2008;14:2.
- Buzási K, Sápi Z, Jermendy G. Acanthosis nigricans as a local cutaneous side effect of repeated human insulin injections. Diabetes Res Clin Pract. 2011;94:E34-E36.
Tuhan H, Ceylaner S, Nalbantoǧlu Ö, et al. A mutation in INSR in a child presenting with severe acanthosis nigricans. J Clin Res Pediatr Endocrinol. 2017;9:371-374.
- Accili D, Barbetti F, Cama A, et al. Mutations in the insulin receptor gene in patients with genetic syndromes of insulin resistance and acanthosis nigricans. J Invest Dermatol. 1992;98(6 suppl):S77-S81.
- Romo A, Benavides S. Treatment options in insulin resistance obesity-related acanthosis nigricans. Ann Pharmacother. 2008;42:1090-1094.
- Treesirichod A, Chaithirayanon S, Chaikul T, et al. The randomized trials of 10% urea cream and 0.025% tretinoin cream in the treatment of acanthosis nigricans [published online January 3, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2019.1708855
- Ragunatha S, Anitha B, Inamadar AC, et al. Cutaneous disorders in 500 diabetic patients attending diabetic clinic. Indian J Dermatol. 2011;56:160-164.
- Morgan AJ, Schwartz RA. Diabetic dermopathy: a subtle sign with grave implications. J Am Acad Dermatol. 2008;58:447-451.
- George SM, Walton S. Diabetic dermopathy. Br J Diabetes. 2014;14:95-97.
- Bustan RS, Wasim D, Yderstræde KB, et al. Specific skin signs as a cutaneous marker of diabetes mellitus and the prediabetic state--a systematic review. Dan Med J. 2017;64:A5316.
- McCash S, Emanuel PO. Defining diabetic dermopathy. J Dermatol. 2011;38:988-992.
- Brugler A, Thompson S, Turner S, et al. Skin blood flow abnormalities in diabetic dermopathy. J Am Acad Dermatol. 2011;65:559-563.
- Sattar MA, Diab S, Sugathan TN, et al. Scleroedema diabeticorum: a minor but often unrecognized complication of diabetes mellitus. Diabet Med. 1988;5:465-468.
- Venencie PY, Powell FC, Su WP, et al. Scleredema: a review of thirty-three cases. J Am Acad Dermatol. 1984;11:128-134.
- Yosipovitch G, Hodak E, Vardi P, et al. The prevalence of cutaneous manifestations in IDDM patients and their association with diabetes risk factors and microvascular complications. Diabetes Care. 1998;21:506-509.
- Ferreli C, Gasparini G, Parodi A, et al. Cutaneous manifestations of scleroderma and scleroderma-like disorders: a comprehensive review. Clin Rev Allergy Immunol. 2017;53:306-336.
- Martín C, Requena L, Manrique K, et al. Scleredema diabeticorum in a patient with type 2 diabetes mellitus. Case Rep Endocrinol. 2011;2011:560273.
- Gkogkolou P, Böhm M. Advanced glycation end products: key players in skin aging? Dermatoendocrinol. 2012;4:259-270.
- Nguyen HP, Katta R. Sugar sag: glycation and the role of diet in aging skin. Skin Therapy Lett. 2015;20:1-5.
- Uribarri J, Woodruff S, Goodman S, et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J Am Diet Assoc. 2010;110:911-916.e912.
- Tran K, Boyd KP, Robinson MR, et al. Scleredema diabeticorum. Dermatol Online J. 2013;19:20718.
- Nakajima K, Iwagaki M, Ikeda M, et al. Two cases of diabetic scleredema that responded to PUVA therapy. J Dermatol. 2006;33:820-822.
- Xiao T, Yang Z-H, He C-D, et al. Scleredema adultorum treated with narrow-band ultraviolet B phototherapy. J Dermatol. 2007;34:270-272.
- Kokpol C, Rajatanavin N, Rattanakemakorn P. Successful treatment of scleredema diabeticorum by combining local PUVA and colchicine: a case report. Case Rep Dermatol. 2012;4:265-268.
- Sanli H, Akay BN, Sen BB, et al. Acquired ichthyosis associated with type 1 diabetes mellitus. Dermatoendocrinol. 2009;1:34-36.
- Patel N, Spencer LA, English JC 3rd, et al. Acquired ichthyosis. J Am Acad Dermatol. 2006;55:647-656.
- Oji V, Traupe H. Ichthyosis: clinical manifestations and practical treatment options. Am J Clin Dermatol. 2009;10:351-364.
- Shah R, Jindal A, Patel N. Acrochordons as a cutaneous sign of metabolic syndrome: a case-control study. Ann Med Health Sci Res. 2014;4:202-205.
- Rasi A, Soltani-Arabshahi R, Shahbazi N. Skin tag as a cutaneous marker for impaired carbohydrate metabolism: a case-control study. Int J Dermatol. 2007;46:1155-1159.
- Kahana M, Grossman E, Feinstein A, et al. Skin tags: a cutaneous marker for diabetes mellitus. Acta Derm Venereol. 1987;67:175-177.
- Tamega Ade A, Aranha AM, Guiotoku MM, et al. Association between skin tags and insulin resistance. An Bras Dermatol. 2010;85:25-31.
- Senel E, Salmanoǧlu M, Solmazgül E, et al. Acrochordons as a cutaneous sign of impaired carbohydrate metabolism, hyperlipidemia, liver enzyme abnormalities and hypertension: a case-control study [published online December 21, 2011]. J Eur Acad Dermatol Venereol. doi:10.1111/j.1468-3083.2011.04396.x
- Köseoǧlu HG, Bozca BC, Basşorgun C, et al. The role of insulin-like growth factor in acrochordon etiopathology. BMC Dermatol. 2020;20:14.
- Singh SK, Agrawal NK, Vishwakarma AK. Association of acanthosis nigricans and acrochordon with insulin resistance: a cross-sectional hospital-based study from North India. Indian J Dermatol. 2020;65:112-117.
- Margolis J, Margolis LS. Letter: skin tags--a frequent sign of diabetes mellitus. N Engl J Med. 1976;294:1184.
- González-Saldivar G, Rodríguez-Gutiérrez R, Ocampo-Candiani J, et al. Skin manifestations of insulin resistance: from a biochemical stance to a clinical diagnosis and management. Dermatol Ther (Heidelb). 2017;7:37-51.
- Ellis DL, Nanney LB, King LE Jr. Increased epidermal growth factor receptors in seborrheic keratoses and acrochordons of patients with the dysplastic nevus syndrome. J Am Acad Dermatol. 1990;23(6 pt 1):1070-1077.
- Hirt PA, Castillo DE, Yosipovitch G, et al. Skin changes in the obese patient. J Am Acad Dermatol. 2019;81:1037-1057.
- Yosipovitch G, Mevorah B, Mashiach J, et al. High body mass index, dry scaly leg skin and atopic conditions are highly associated with keratosis pilaris. Dermatology. 2000;201:34-36.
- Thomas M, Khopkar US. Keratosis pilaris revisited: is it more than just a follicular keratosis? Int J Trichology. 2012;4:255-258.
- Gruber R, Sugarman JL, Crumrine D, et al. Sebaceous gland, hair shaft, and epidermal barrier abnormalities in keratosis pilaris with and without filaggrin deficiency. Am J Pathol. 2015;185:1012-1021.
- Barth JH, Wojnarowska F, Dawber RP. Is keratosis pilaris another androgen-dependent dermatosis? Clin Exp Dermatol. 1988;13:240-241.
- Hwang S, Schwartz RA. Keratosis pilaris: a common follicular hyperkeratosis. Cutis. 2008;82:177-180.
- Poskitt L, Wilkinson JD. Natural history of keratosis pilaris. Br J Dermatol. 1994;130:711-713.
- Maghfour J, Ly S, Haidari W, et al. Treatment of keratosis pilaris and its variants: a systematic review [published online September 14, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1818678
- O'Toole EA, Kennedy U, Nolan JJ, et al. Necrobiosis lipoidica: only a minority of patients have diabetes mellitus. Br J Dermatol. 1999;140:283-286.
- Muller SA, Winkelmann RK. Necrobiosis lipoidica diabeticorum. a clinical and pathological investigation of 171 cases. Arch Dermatol. 1966;93:272-281.
- Reid SD, Ladizinski B, Lee K, et al. Update on necrobiosis lipoidica: a review of etiology, diagnosis, and treatment options. J Am Acad Dermatol. 2013;69:783-791.
- Hashemi DA, Brown-Joel ZO, Tkachenko E, et al. Clinical features and comorbidities of patients with necrobiosis lipoidica with or without diabetes. JAMA Dermatology. 2019;155:455-459.
- Ngo B, Wigington G, Hayes K, et al. Skin blood flow in necrobiosis lipoidica diabeticorum. Int J Dermatol. 2008;47:354-358.
- Zhang AJ, Garret M, Miller S. Bullosis diabeticorum: case report and review. N Z Med J. 2013;126:91-94.
- Larsen K, Jensen T, Karlsmark T, et al. Incidence of bullosis diabeticorum--a controversial cause of chronic foot ulceration. Int Wound J. 2008;5:591-596.
- El Fekih N, Zéglaoui F, Sioud A, et al. Bullosis diabeticorum: report of ten cases. Tunis Med. 2009;87:747-749.
- Lipsky BA, Baker PD, Ahroni JH. Diabetic bullae: 12 cases of a purportedly rare cutaneous disorder. Int J Dermatol. 2000;39:196-200.
- Lopez PR, Leicht S, Sigmon JR, et al. Bullosis diabeticorum associated with a prediabetic state. South Med J. 2009;102:643-644.
- Muhlemann MF, Williams DR. Localized granuloma annulare is associated with insulin-dependent diabetes mellitus. Br J Dermatol. 1984;111:325-329.
- Haim S, Friedman-Birnbaum R, Haim N, et al. Carbohydrate tolerance in patients with granuloma annulare. Br J Dermatol. 1973;88:447-451.
- Dabski K, Winkelmann RK. Generalized granuloma annulare: clinical and laboratory findings in 100 patients. J Am Acad Dermatol. 1989;20:39-47.
- Agrawal P, Pursnani N, Jose R, et al. Granuloma annulare: a rare dermatological manifestation of diabetes mellitus. J Family Med Prim Care. 2019;8:3419-3421.
- Studer EM, Calza AM, Saurat JH. Precipitating factors and associated diseases in 84 patients with granuloma annulare: a retrospective study. Dermatology. 1996;193:364-368.
- Spicuzza L, Salafia S, Capizzi A, et al. Granuloma annulare as first clinical manifestation of diabetes mellitus in children: a case report. Diabetes Res Clin Pract. 2012;95:E55-E57.
- Wang J, Khachemoune A. Granuloma annulare: a focused review of therapeutic options. Am J Clin Dermatol. 2018;19:333-344.
Practice Points
- Dermatologists should be aware of common cutaneous conditions associated with chronic hyperglycemia and hyperinsulinemia, such as acanthosis nigricans, diabetic dermopathy, scleredema diabeticorum, ichthyosiform skin changes, acrochordons, and keratosis pilaris.
- More rare cutaneous pathologies related to chronically elevated blood glucose and/or insulin levels include necrobiosis lipoidica, bullosis diabeticorum, and generalized granuloma annulare.
- The cutaneous manifestations of persistent hyperglycemia and hyperinsulinemia may precede a formal diagnosis of diabetes mellitus and may be the first signs of metabolic derangement.
- Early recognition and management of these cutaneous conditions can help maximize patient quality of life and avoid long-term sequelae associated with insulin resistance and prolonged hyperglycemia.
Reimbursement for Teledermatology During the COVID-19 Public Health Emergency: Change Has Come, But Will It Stay?
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
The world of telemedicine—especially teledermatology—had been a sleepy underutilized afterthought for most physicians until we were faced with a global pandemic the likes of which none of us had seen in our lifetimes. And just like that, teledermatology went from an afterthought to part of the “new normal.” Although those of us already practicing telemedicine knew of potential pitfalls and concerns, this great social experiment of throwing everyone into unexplored territory led to a great deal of frustration with technology and workflows that were not optimized for dermatology visits. The process is still changing, and the technical aspects of conducting teledermatology visits will no doubt improve, but what about the bigger question of reimbursement? Without adequate payments and financial models, the long-term future of telemedicine is uncertain, so an understanding of the current and likely future landscape of telemedicine reimbursement is critical.
Waivers During the Public Health Emergency
The declaration of a public health emergency (PHE)allowed for significant flexibility by the Centers for Medicare & Medicaid Services (CMS) during the coronavirus disease 2019 (COVID-19) pandemic. Importantly, the CMS was permitted to act quickly to allow telehealth to flourish during the worst of the pandemic and throughout the declared PHE, which has been extended several times already. Currently, the PHE is set to expire on April 20, 2021, but may be extended again if the pandemic is ongoing. The most important of these waivers was probably the removal of both the originating site and geographic requirements for telehealth services.1 Prior to the COVID-19 PHE, a patient would have to travel to a doctor’s office, hospital, or skilled nursing facility to receive telehealth care (originating site requirement), and even then this was only allowed in defined rural areas of the country (geographic requirement). Both of these requirements were waived, allowing for any patient to receive telehealth services within their own homes. Concurrently, the requirement that patients must have an established relationship with the provider (ie, telehealth could not be used to provide care to new patients) also was waived.1
In the spirit of expanding access to care and providing reasonable reimbursement for medical services, other changes were made for which the CMS should be commended. In acknowledging that many Medicare/Medicaid beneficiaries may not have access to devices that permit real-time, 2-way audio/video communication, which previously were necessary to qualify for a telehealth encounter, the CMS decided to cover telephone visits and provide reimbursement at the level of an established visit.1 They also changed the billing structure to remove the place of service (POS) designation for telehealth (POS 02) and replace it with the normal physician’s office POS designation (usually POS 11), bringing back a telehealth modifier (modifier -95) in the process. The benefit of this change is solely to increase reimbursement for these services, as telehealth POS services generally are covered at lower facility rates, whereas POS 11 codes are reimbursed at the full level of a nonfacility physician’s office rate.
Finally, other waivers such as the Office of Civil Rights’ decision to waive HIPAA (Health Insurance Portability and Accountability Act) violations for telehealth platforms during the PHE allowed offices to take on telemedicine quickly without having to implement a new infrastructure.2 Numerous codes were added to the list of covered services for telehealth, but these generally are not relevant for dermatologists. The CMS also allowed physicians’ offices to waive the patient responsibility/co-pay during the COVID-19 PHE, which previously was not allowed due to concerns about the anti-kickback statute.1 These co-pay waivers were intended to remove another barrier to care for patients who were hesitant to participate in virtual visits. For the most part, the waiver of state licensing requirements is a bit less useful. As part of the CMS waiver, providers technically are allowed to see out-of-state Medicare/Medicaid beneficiaries, but state licensing laws are still in effect; thus, in the absence of a blanket state-level waiver (which some states enacted, modeled after the Uniform Emergency Volunteer Health Practitioner Act of 20063), providers still cannot see most out-of-state patients from a legal and malpractice coverage standpoint.
An important flexibility during the COVID-19 PHE is one that often is underrecognized. The CMS has been clear about the ability to provide direct supervision for advanced practice providers (APPs) and residents via telehealth during the PHE, which allows for incident-to billing for APPs at remote sites given that the supervising physician is immediately available via an interactive, 2-way, live audio/video telecommunications method. It also allows for direct supervision of APPs and residents using such technology. For dermatology, which does not have a primary care waiver, an attending must still directly supervise each patient and see the patient via a live audio/video modality but does not have to be on-site to do so. This is a very interesting concept that, if extended, could truly impact practice management for the long-term.
Response From Commercial Insurance Carriers
Tracking along with the CMS waivers and flexibilities during the PHE, most commercial carriers quickly adopted similar policies to cover telehealth services. It should be noted that for most commercial insurance carriers, the coverage was already broader than Medicare/Medicaid coverage for telehealth prior to the PHE, so in many ways it is an extension of that concept and acceptance of telemedicine as a whole. What is sometimes confusing, though, is that various policies and requirements around billing exist; for example, while most carriers emulated the POS requirements that the CMS adopted, some carriers still stuck with the telemedicine POS but paid full in-office visit rates for those codes. Some carriers adopted higher reimbursement rates for telephone visits, similar to the CMS, while others instructed providers to just bill for the established office visit codes and allowed for telephone-only visits to qualify for these billing codes. Some carriers also waived co-pays for telehealth visits for their members (whether related to COVID-19 or not). It is beyond the scope of this article to delve into the specifics, which may vary not only by carrier but by region and plan. However, it is important to stay on top of one’s insurance carriers to find out what their latest directives are for billing for telehealth.
Postpandemic Teledermatology
What about the future of teledermatology? Although many dermatologists have adopted telehealth services out of necessity during the COVID-19 PHE, the jury is still out on the long-term forecast for telemedicine in dermatology. Concerns about liability/malpractice and technology issues abound, and for many, the headaches of teledermatology—such as trying to focus on a blurry photograph of a nevus that the patient is concerned about—make it unappealing. Some of these issues will be addressed by better technology, but the reimbursement structure must continue for teledermatology to remain in widespread use.
Currently, the biggest question facing telehealth is whether the waivers for originating site and geographic requirements will be able to continue. The CMS itself does not have the statutory authority to make these changes permanent and was only allowed to act due to a waiver under section 1135 of the Social Security Act during a PHE. It would take an act of Congress to change the law to allow for this specific expansion of telehealth services. A number of federal bills, including S 2741 (Creating Opportunities Now for Necessary and Effective Care Technologies [CONNECT] for Health Act of 2019) and S 4796 (Fair Care Act of 2020) from the Senate, contain such provisions, but none have been passed at the time of writing. There does seem to be broad support of the concept of expanding telemedicine access, such as noted by New York State Governor Andrew Cuomo in his 2021 State of the State address,4 but it remains to be seen when action will come.
Some regulations, such as the HIPAA waiver and the ability to waive co-pays, are not slated to continue after the pandemic. The ability to supervise residents via telehealth (real-time audio/video) has been made permanent, but only in rural areas. Direct supervision of APPs via telehealth will continue through the end of the calendar year of the PHE or the end of 2021, whichever comes later, but it remains to be seen whether remote supervision will continue. The CMS has stated in its comments that it is looking at this issue closely and may establish certain guardrails to ensure quality of care is maintained.1 Telephone/audio-only visits also may come under further scrutiny, but research has supported the concept that patients who are more likely to gain access through audio-only modalities are older, Medicare/Medicaid (vs commercial), and Black (vs White) patients,5 so it would indeed introduce an unfair barrier to access if such coverage was rolled back.
Final Thoughts
Overall, we have made much progress in teledermatology. Once utilized by a small fraction of dermatologists, the vast majority of us turned to teledermatology to sustain our practices during the COVID-19 pandemic. Moving forward, there are 2 critical factors to consider: continued technological innovation and permanent coverage for telehealth reimbursement at in-office visit levels. With these challenges resolved, we can move forward and consider novel models that may be able to deliver dermatologic care to a broader patient population, thereby solving the critical issue of access to care for so many patients in need in our country.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
- Medicare Program; CY 2021 Payment Policies Under the Physician Fee Schedule and Other Changes to Part B Payment Policies; Medicare Shared Savings Program Requirements; Medicaid Promoting Interoperability Program Requirements for Eligible Professionals; Quality Payment Program; Coverage of Opioid Use Disorder Services Furnished by Opioid Treatment Programs; Medicare Enrollment of Opioid Treatment Programs; Electronic Prescribing for Controlled Substances for a Covered Part D Drug; Payment for Office/Outpatient Evaluation and Management Services; Hospital IQR Program; Establish New Code Categories; Medicare Diabetes Prevention Program (MDPP) Expanded Model Emergency Policy; Coding and Payment for Virtual Check-in Services Interim Final Rule Policy; Coding and Payment for Personal Protective Equipment (PPE) Interim Final Rule Policy; Regulatory Revisions in Response to the Public Health Emergency (PHE) for COVID-19; and Finalization of Certain Provisions from the March 31st, May 8th and September 2nd Interim Final Rules in Response to the PHE for COVID-19. Fed Registr. 2020;85:84472-85377. To be codified at 42 CFR §400, 410, 414, 415, 423, 424, and 425. https://www.federalregister.gov/documents/2020/12/28/2020-26815/medicare-program-cy-2021-payment-policies-under-the-physician-fee-schedule-and-other-changes-to-part
- Office for Civil Rights. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. Reviewed January 20, 2021. Accessed January 25, 2021. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html
- Hoffman DA. Increasing access to care: telehealth during COVID-19 [published online June 16, 2020]. J Law Biosci. doi:10.1093/jlb/lsaa043
- Governor Cuomo announces proposal to expand access to telehealth for all as part of 2021 State of the State. New York State website. Published January 10, 2021. Accessed January 25, 021. https://www.governor.ny.gov/news/governor-cuomo-announces-proposal-expand-access-telehealth-all-part-2021-state-state#:~:text=and%20Rural%20Communities-,Governor%20Andrew%20M.,2021%20State%20of%20the%20State.&text=New%20Yorkers%20have%20adapted%20throughout,into%20our%20existing%20healthcare%20system
- Gilson SF, Umscheid CA, Laiteerapong N, et al. Growth of ambulatory virtual visit and differential use by patient sociodemographics at one urban academic medical center during the COVID-19 pandemic: retrospective analysis. JMIR Med Inform. 2020;8:E24544.
An Unusual Presentation of Cutaneous Metastatic Lobular Breast Carcinoma
In women, breast cancer is the leading cancer diagnosis and the second leading cause of cancer-related death,1 as well as the most common malignancy to metastasize to the skin.2 Cutaneous breast carcinoma may present as cutaneous metastasis or can occur secondary to direct tumor extension. Five percent to 10% of women with breast cancer will present clinically with metastatic cutaneous disease, most commonly as a recurrence of early-stage breast carcinoma.2
In a published meta-analysis that investigated the incidence of tumors most commonly found to metastasize to the skin, Krathen et al3 found that cutaneous metastases occurred in 24% of patients with breast cancer (N=1903). In 2 large retrospective studies from tumor registry data, breast cancer was found to be the most common tumor involving metastasis to the skin, and 3.5% of the breast cancer cases identified in the registry had cutaneous metastasis as the presenting sign (n=35) at time of diagnosis.4
We report an unusual presentation of cutaneous metastatic lobular breast carcinoma that involved diffuse cutaneous lesions and rapid progression from onset of the breast mass to development of clinically apparent metastatic skin lesions.
Case Report
A 59-year-old woman with an unremarkable medical history presented to our dermatology clinic for evaluation of new widespread lesions that developed over a period of months. The eruption was asymptomatic and consisted of numerous bumpy lesions that reportedly started on the patient’s neck and progressively spread to involve the trunk. Physical examination revealed multiple flesh-colored, firm nodules scattered across the upper back, neck, and chest (Figure 1). Bilateral cervical and axillary lymphadenopathy also was noted. Upon questioning regarding family history of malignancy, the patient reported that her brother had been diagnosed with colon cancer. Although she was not up to date on age-appropriate malignancy screenings, she did report having a diagnostic mammogram 1 year prior that revealed a suspicious lesion on the left breast. A repeat mammogram of the left breast 6 months later was read as unremarkable.
Two 3-mm representative punch biopsies were performed. Hematoxylin and eosin staining revealed a basket-weave stratum corneum with underlying epidermal atrophy. A relatively monomorphic epithelioid cell infiltrate extending from the superficial reticular dermis into the deep dermis and displaying an open chromatin pattern and pink cytoplasm was observed, as well as dermal collagen thickening. Linear, single-filing cells along with focal irregular nests and scattered cells were observed (Figure 2). Immunohistochemical staining was positive for cytokeratin 7 (Figure 3A), epithelial membrane antigen, and estrogen receptor (Figure 3B) along with gross cystic disease fluid protein 15; focal progesterone receptor positivity also was present. Cytokeratin 20, cytokeratin 5/6, carcinoembryonic antigen, p63, CDX2, paired box gene 8, thyroid transcription factor 1, and human epidermal growth factor receptor 2/neu stains were negative. Findings identified in both biopsies were consistent with metastatic cutaneous lobular breast carcinoma.
A complete blood cell count and complete metabolic panels were within normal limits, aside from a mildly elevated alkaline phosphatase level. Breast ultrasonography was unremarkable. Stereotactic breast magnetic resonance imaging (MRI) revealed a 9.4-cm mass in the upper outer quadrant of the right breast as well as enlarged lymph nodes 2.2 cm from the left axilla. A subsequent bone scan demonstrated focal activity in the left lateral fourth rib, left costochondral junction, and right anterolateral fifth rib—it was unclear whether these lesions were metastatic or secondary to trauma from a fall the patient reportedly had sustained 2 weeks prior. Lumbar MRI without gadolinium contrast revealed extensive abnormal heterogeneous signal intensity of osseous structures consistent with osseous metastasis.
Subsequent diagnostic bilateral breast ultrasonography and percutaneous left lymph node biopsy revealed pathology consistent with metastatic lobular breast carcinoma with near total effacement of the lymph node and extracapsular extension concordant with previous MRI findings. The mass in the upper outer quadrant of the right breast that previously was observed on MRI was not identifiable on this ultrasound. It was recommended that the patient pursue MRI-guided breast biopsy to have the breast lesion further characterized. She was referred to surgical oncology at a tertiary center for management; however, the patient was lost to follow-up, and there are no records available indicating the patient pursued any treatment. Although we were unable to confirm the patient’s breast lesion that previously was seen on MRI was the cause of the metastatic disease, the overall clinical picture supported metastatic lobular breast carcinoma.
Comment
Tumor metastasis to the skin accounts for approximately 2% of all skin cancers5 and typically is observed in advanced stages of cancer. In women, breast carcinoma is the most common type of cancer to exhibit this behavior.2 Invasive ductal carcinoma represents the most common histologic subtype of breast cancer overall,6,7 and breast adenocarcinomas, including lobular and ductal breast carcinomas, are the most common histologic subtypes to exhibit metastatic cutaneous lesions.8
Invasive lobular breast carcinoma represents approximately 10% of invasive breast cancer cases. Compared to invasive ductal carcinoma, there tends to be a delay in diagnosis often leading to larger tumor sizes relative to the former upon detection and with lymph node invasion. These findings may be explained by the greater difficulty of detecting invasive lobular carcinomas by mammography and clinical breast examination compared to invasive ductal carcinomas.9-11 Additionally, invasive lobular carcinomas are more likely to be positive for estrogen and progesterone receptors compared to invasive ductal carcinomas,12 which also was consistent in our case.
Cutaneous metastases of breast cancer most commonly are found on the anterior chest wall and can present as a wide spectrum of lesions, with nodules as the most common primary dermatologic manifestation.13 Cutaneous metastatic lesions commonly have been described as firm, mobile, round or oval, solitary or grouped nodules. The color of the nodules varies and may be flesh-colored, brown, blue, black, pink, and/or red-brown. The lesions often are asymptomatic but may ulcerate.2
In our case, the distribution of lesions was a unique aspect that is not typical of most cases of metastatic cutaneous breast carcinoma. The nodules appeared more scattered and involved multiple body regions, including the back, neck, and chest. Although cutaneous breast cancer metastases have been documented to extend to these body regions, a review of PubMed articles indexed for MEDLINE using the terms cutaneous metastatic lobular breast carcinoma, breast carcinoma, and metastatic breast cancer suggested that it is uncommon for these multiple areas to be simultaneously affected.4,14 Rather, the more common clinical presentation of cutaneous metastatic breast carcinoma is as a solitary nodule or group of nodules localized to a single anatomic region.14
Another notable feature of our case was the rapid development of the cutaneous lesions relative to the primary tumor. This patient developed diffuse lesions over a period of several months, and given that her mammogram performed the previous year was negative for any abnormalities, one could suggest that the metastatic lesions developed less than a year from onset of the primary tumor. A previous study involving 41 patients with a known clinical primary visceral malignancy (ie, breast, lung, colon, esophageal, gastric, pancreatic, kidney, thyroid, prostate, or ovarian origin) found that it takes approximately 3 years on average for cutaneous metastases to develop from the onset of cancer diagnosis (range, 1–177 months).14 In the aforementioned study, 94% of patients had stage III or IV disease at time of skin metastasis, with the majority of those demonstrating stage IV disease. However, it also is possible that these breast tumors evaded detection or were too small to be identified on prior imaging.14 A review of our patient’s medical records did not indicate documentation of any visual or palpable breast changes prior to the onset of the clinically detected metastatic nodules.
Conclusion
Biopsy with immunohistochemical staining ultimately yielded the diagnosis of metastatic lobular breast carcinoma in our patient. Providers should be aware of the varying clinical presentations that may arise in the setting of cutaneous metastasis. When faced with lesions suspicious for cutaneous metastasis, biopsy is warranted to determine the correct diagnosis and ensure appropriate management. Upon diagnosis of cutaneous metastasis, prompt coordination with the primary care provider and appropriate referral to multidisciplinary teams is necessary. Clinical providers also should maintain a high index of suspicion when evaluating patients with cutaneous metastasis who have a history of normal malignancy screenings.
- American Cancer Society. Cancer facts & figures 2015. Accessed January 7, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421-1424.
- Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773-2780.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Dixon J, Anderson R, Page D, et al. Infiltrating lobular carcioma of the breast. Histopathology. 1982;6:149-161.
- Yeatman T, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma: implications for management. Ann Surg. 1995;222:549-559.
- Silverstein M, Lewinski BS, Waisman JR, et al. Infiltrating lobular carcinoma: is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673-1677.
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046-1052.
- Mordenti C, Peris K, Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerol. 2000;9:143-148.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
In women, breast cancer is the leading cancer diagnosis and the second leading cause of cancer-related death,1 as well as the most common malignancy to metastasize to the skin.2 Cutaneous breast carcinoma may present as cutaneous metastasis or can occur secondary to direct tumor extension. Five percent to 10% of women with breast cancer will present clinically with metastatic cutaneous disease, most commonly as a recurrence of early-stage breast carcinoma.2
In a published meta-analysis that investigated the incidence of tumors most commonly found to metastasize to the skin, Krathen et al3 found that cutaneous metastases occurred in 24% of patients with breast cancer (N=1903). In 2 large retrospective studies from tumor registry data, breast cancer was found to be the most common tumor involving metastasis to the skin, and 3.5% of the breast cancer cases identified in the registry had cutaneous metastasis as the presenting sign (n=35) at time of diagnosis.4
We report an unusual presentation of cutaneous metastatic lobular breast carcinoma that involved diffuse cutaneous lesions and rapid progression from onset of the breast mass to development of clinically apparent metastatic skin lesions.
Case Report
A 59-year-old woman with an unremarkable medical history presented to our dermatology clinic for evaluation of new widespread lesions that developed over a period of months. The eruption was asymptomatic and consisted of numerous bumpy lesions that reportedly started on the patient’s neck and progressively spread to involve the trunk. Physical examination revealed multiple flesh-colored, firm nodules scattered across the upper back, neck, and chest (Figure 1). Bilateral cervical and axillary lymphadenopathy also was noted. Upon questioning regarding family history of malignancy, the patient reported that her brother had been diagnosed with colon cancer. Although she was not up to date on age-appropriate malignancy screenings, she did report having a diagnostic mammogram 1 year prior that revealed a suspicious lesion on the left breast. A repeat mammogram of the left breast 6 months later was read as unremarkable.
Two 3-mm representative punch biopsies were performed. Hematoxylin and eosin staining revealed a basket-weave stratum corneum with underlying epidermal atrophy. A relatively monomorphic epithelioid cell infiltrate extending from the superficial reticular dermis into the deep dermis and displaying an open chromatin pattern and pink cytoplasm was observed, as well as dermal collagen thickening. Linear, single-filing cells along with focal irregular nests and scattered cells were observed (Figure 2). Immunohistochemical staining was positive for cytokeratin 7 (Figure 3A), epithelial membrane antigen, and estrogen receptor (Figure 3B) along with gross cystic disease fluid protein 15; focal progesterone receptor positivity also was present. Cytokeratin 20, cytokeratin 5/6, carcinoembryonic antigen, p63, CDX2, paired box gene 8, thyroid transcription factor 1, and human epidermal growth factor receptor 2/neu stains were negative. Findings identified in both biopsies were consistent with metastatic cutaneous lobular breast carcinoma.
A complete blood cell count and complete metabolic panels were within normal limits, aside from a mildly elevated alkaline phosphatase level. Breast ultrasonography was unremarkable. Stereotactic breast magnetic resonance imaging (MRI) revealed a 9.4-cm mass in the upper outer quadrant of the right breast as well as enlarged lymph nodes 2.2 cm from the left axilla. A subsequent bone scan demonstrated focal activity in the left lateral fourth rib, left costochondral junction, and right anterolateral fifth rib—it was unclear whether these lesions were metastatic or secondary to trauma from a fall the patient reportedly had sustained 2 weeks prior. Lumbar MRI without gadolinium contrast revealed extensive abnormal heterogeneous signal intensity of osseous structures consistent with osseous metastasis.
Subsequent diagnostic bilateral breast ultrasonography and percutaneous left lymph node biopsy revealed pathology consistent with metastatic lobular breast carcinoma with near total effacement of the lymph node and extracapsular extension concordant with previous MRI findings. The mass in the upper outer quadrant of the right breast that previously was observed on MRI was not identifiable on this ultrasound. It was recommended that the patient pursue MRI-guided breast biopsy to have the breast lesion further characterized. She was referred to surgical oncology at a tertiary center for management; however, the patient was lost to follow-up, and there are no records available indicating the patient pursued any treatment. Although we were unable to confirm the patient’s breast lesion that previously was seen on MRI was the cause of the metastatic disease, the overall clinical picture supported metastatic lobular breast carcinoma.
Comment
Tumor metastasis to the skin accounts for approximately 2% of all skin cancers5 and typically is observed in advanced stages of cancer. In women, breast carcinoma is the most common type of cancer to exhibit this behavior.2 Invasive ductal carcinoma represents the most common histologic subtype of breast cancer overall,6,7 and breast adenocarcinomas, including lobular and ductal breast carcinomas, are the most common histologic subtypes to exhibit metastatic cutaneous lesions.8
Invasive lobular breast carcinoma represents approximately 10% of invasive breast cancer cases. Compared to invasive ductal carcinoma, there tends to be a delay in diagnosis often leading to larger tumor sizes relative to the former upon detection and with lymph node invasion. These findings may be explained by the greater difficulty of detecting invasive lobular carcinomas by mammography and clinical breast examination compared to invasive ductal carcinomas.9-11 Additionally, invasive lobular carcinomas are more likely to be positive for estrogen and progesterone receptors compared to invasive ductal carcinomas,12 which also was consistent in our case.
Cutaneous metastases of breast cancer most commonly are found on the anterior chest wall and can present as a wide spectrum of lesions, with nodules as the most common primary dermatologic manifestation.13 Cutaneous metastatic lesions commonly have been described as firm, mobile, round or oval, solitary or grouped nodules. The color of the nodules varies and may be flesh-colored, brown, blue, black, pink, and/or red-brown. The lesions often are asymptomatic but may ulcerate.2
In our case, the distribution of lesions was a unique aspect that is not typical of most cases of metastatic cutaneous breast carcinoma. The nodules appeared more scattered and involved multiple body regions, including the back, neck, and chest. Although cutaneous breast cancer metastases have been documented to extend to these body regions, a review of PubMed articles indexed for MEDLINE using the terms cutaneous metastatic lobular breast carcinoma, breast carcinoma, and metastatic breast cancer suggested that it is uncommon for these multiple areas to be simultaneously affected.4,14 Rather, the more common clinical presentation of cutaneous metastatic breast carcinoma is as a solitary nodule or group of nodules localized to a single anatomic region.14
Another notable feature of our case was the rapid development of the cutaneous lesions relative to the primary tumor. This patient developed diffuse lesions over a period of several months, and given that her mammogram performed the previous year was negative for any abnormalities, one could suggest that the metastatic lesions developed less than a year from onset of the primary tumor. A previous study involving 41 patients with a known clinical primary visceral malignancy (ie, breast, lung, colon, esophageal, gastric, pancreatic, kidney, thyroid, prostate, or ovarian origin) found that it takes approximately 3 years on average for cutaneous metastases to develop from the onset of cancer diagnosis (range, 1–177 months).14 In the aforementioned study, 94% of patients had stage III or IV disease at time of skin metastasis, with the majority of those demonstrating stage IV disease. However, it also is possible that these breast tumors evaded detection or were too small to be identified on prior imaging.14 A review of our patient’s medical records did not indicate documentation of any visual or palpable breast changes prior to the onset of the clinically detected metastatic nodules.
Conclusion
Biopsy with immunohistochemical staining ultimately yielded the diagnosis of metastatic lobular breast carcinoma in our patient. Providers should be aware of the varying clinical presentations that may arise in the setting of cutaneous metastasis. When faced with lesions suspicious for cutaneous metastasis, biopsy is warranted to determine the correct diagnosis and ensure appropriate management. Upon diagnosis of cutaneous metastasis, prompt coordination with the primary care provider and appropriate referral to multidisciplinary teams is necessary. Clinical providers also should maintain a high index of suspicion when evaluating patients with cutaneous metastasis who have a history of normal malignancy screenings.
In women, breast cancer is the leading cancer diagnosis and the second leading cause of cancer-related death,1 as well as the most common malignancy to metastasize to the skin.2 Cutaneous breast carcinoma may present as cutaneous metastasis or can occur secondary to direct tumor extension. Five percent to 10% of women with breast cancer will present clinically with metastatic cutaneous disease, most commonly as a recurrence of early-stage breast carcinoma.2
In a published meta-analysis that investigated the incidence of tumors most commonly found to metastasize to the skin, Krathen et al3 found that cutaneous metastases occurred in 24% of patients with breast cancer (N=1903). In 2 large retrospective studies from tumor registry data, breast cancer was found to be the most common tumor involving metastasis to the skin, and 3.5% of the breast cancer cases identified in the registry had cutaneous metastasis as the presenting sign (n=35) at time of diagnosis.4
We report an unusual presentation of cutaneous metastatic lobular breast carcinoma that involved diffuse cutaneous lesions and rapid progression from onset of the breast mass to development of clinically apparent metastatic skin lesions.
Case Report
A 59-year-old woman with an unremarkable medical history presented to our dermatology clinic for evaluation of new widespread lesions that developed over a period of months. The eruption was asymptomatic and consisted of numerous bumpy lesions that reportedly started on the patient’s neck and progressively spread to involve the trunk. Physical examination revealed multiple flesh-colored, firm nodules scattered across the upper back, neck, and chest (Figure 1). Bilateral cervical and axillary lymphadenopathy also was noted. Upon questioning regarding family history of malignancy, the patient reported that her brother had been diagnosed with colon cancer. Although she was not up to date on age-appropriate malignancy screenings, she did report having a diagnostic mammogram 1 year prior that revealed a suspicious lesion on the left breast. A repeat mammogram of the left breast 6 months later was read as unremarkable.
Two 3-mm representative punch biopsies were performed. Hematoxylin and eosin staining revealed a basket-weave stratum corneum with underlying epidermal atrophy. A relatively monomorphic epithelioid cell infiltrate extending from the superficial reticular dermis into the deep dermis and displaying an open chromatin pattern and pink cytoplasm was observed, as well as dermal collagen thickening. Linear, single-filing cells along with focal irregular nests and scattered cells were observed (Figure 2). Immunohistochemical staining was positive for cytokeratin 7 (Figure 3A), epithelial membrane antigen, and estrogen receptor (Figure 3B) along with gross cystic disease fluid protein 15; focal progesterone receptor positivity also was present. Cytokeratin 20, cytokeratin 5/6, carcinoembryonic antigen, p63, CDX2, paired box gene 8, thyroid transcription factor 1, and human epidermal growth factor receptor 2/neu stains were negative. Findings identified in both biopsies were consistent with metastatic cutaneous lobular breast carcinoma.
A complete blood cell count and complete metabolic panels were within normal limits, aside from a mildly elevated alkaline phosphatase level. Breast ultrasonography was unremarkable. Stereotactic breast magnetic resonance imaging (MRI) revealed a 9.4-cm mass in the upper outer quadrant of the right breast as well as enlarged lymph nodes 2.2 cm from the left axilla. A subsequent bone scan demonstrated focal activity in the left lateral fourth rib, left costochondral junction, and right anterolateral fifth rib—it was unclear whether these lesions were metastatic or secondary to trauma from a fall the patient reportedly had sustained 2 weeks prior. Lumbar MRI without gadolinium contrast revealed extensive abnormal heterogeneous signal intensity of osseous structures consistent with osseous metastasis.
Subsequent diagnostic bilateral breast ultrasonography and percutaneous left lymph node biopsy revealed pathology consistent with metastatic lobular breast carcinoma with near total effacement of the lymph node and extracapsular extension concordant with previous MRI findings. The mass in the upper outer quadrant of the right breast that previously was observed on MRI was not identifiable on this ultrasound. It was recommended that the patient pursue MRI-guided breast biopsy to have the breast lesion further characterized. She was referred to surgical oncology at a tertiary center for management; however, the patient was lost to follow-up, and there are no records available indicating the patient pursued any treatment. Although we were unable to confirm the patient’s breast lesion that previously was seen on MRI was the cause of the metastatic disease, the overall clinical picture supported metastatic lobular breast carcinoma.
Comment
Tumor metastasis to the skin accounts for approximately 2% of all skin cancers5 and typically is observed in advanced stages of cancer. In women, breast carcinoma is the most common type of cancer to exhibit this behavior.2 Invasive ductal carcinoma represents the most common histologic subtype of breast cancer overall,6,7 and breast adenocarcinomas, including lobular and ductal breast carcinomas, are the most common histologic subtypes to exhibit metastatic cutaneous lesions.8
Invasive lobular breast carcinoma represents approximately 10% of invasive breast cancer cases. Compared to invasive ductal carcinoma, there tends to be a delay in diagnosis often leading to larger tumor sizes relative to the former upon detection and with lymph node invasion. These findings may be explained by the greater difficulty of detecting invasive lobular carcinomas by mammography and clinical breast examination compared to invasive ductal carcinomas.9-11 Additionally, invasive lobular carcinomas are more likely to be positive for estrogen and progesterone receptors compared to invasive ductal carcinomas,12 which also was consistent in our case.
Cutaneous metastases of breast cancer most commonly are found on the anterior chest wall and can present as a wide spectrum of lesions, with nodules as the most common primary dermatologic manifestation.13 Cutaneous metastatic lesions commonly have been described as firm, mobile, round or oval, solitary or grouped nodules. The color of the nodules varies and may be flesh-colored, brown, blue, black, pink, and/or red-brown. The lesions often are asymptomatic but may ulcerate.2
In our case, the distribution of lesions was a unique aspect that is not typical of most cases of metastatic cutaneous breast carcinoma. The nodules appeared more scattered and involved multiple body regions, including the back, neck, and chest. Although cutaneous breast cancer metastases have been documented to extend to these body regions, a review of PubMed articles indexed for MEDLINE using the terms cutaneous metastatic lobular breast carcinoma, breast carcinoma, and metastatic breast cancer suggested that it is uncommon for these multiple areas to be simultaneously affected.4,14 Rather, the more common clinical presentation of cutaneous metastatic breast carcinoma is as a solitary nodule or group of nodules localized to a single anatomic region.14
Another notable feature of our case was the rapid development of the cutaneous lesions relative to the primary tumor. This patient developed diffuse lesions over a period of several months, and given that her mammogram performed the previous year was negative for any abnormalities, one could suggest that the metastatic lesions developed less than a year from onset of the primary tumor. A previous study involving 41 patients with a known clinical primary visceral malignancy (ie, breast, lung, colon, esophageal, gastric, pancreatic, kidney, thyroid, prostate, or ovarian origin) found that it takes approximately 3 years on average for cutaneous metastases to develop from the onset of cancer diagnosis (range, 1–177 months).14 In the aforementioned study, 94% of patients had stage III or IV disease at time of skin metastasis, with the majority of those demonstrating stage IV disease. However, it also is possible that these breast tumors evaded detection or were too small to be identified on prior imaging.14 A review of our patient’s medical records did not indicate documentation of any visual or palpable breast changes prior to the onset of the clinically detected metastatic nodules.
Conclusion
Biopsy with immunohistochemical staining ultimately yielded the diagnosis of metastatic lobular breast carcinoma in our patient. Providers should be aware of the varying clinical presentations that may arise in the setting of cutaneous metastasis. When faced with lesions suspicious for cutaneous metastasis, biopsy is warranted to determine the correct diagnosis and ensure appropriate management. Upon diagnosis of cutaneous metastasis, prompt coordination with the primary care provider and appropriate referral to multidisciplinary teams is necessary. Clinical providers also should maintain a high index of suspicion when evaluating patients with cutaneous metastasis who have a history of normal malignancy screenings.
- American Cancer Society. Cancer facts & figures 2015. Accessed January 7, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421-1424.
- Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773-2780.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Dixon J, Anderson R, Page D, et al. Infiltrating lobular carcioma of the breast. Histopathology. 1982;6:149-161.
- Yeatman T, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma: implications for management. Ann Surg. 1995;222:549-559.
- Silverstein M, Lewinski BS, Waisman JR, et al. Infiltrating lobular carcinoma: is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673-1677.
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046-1052.
- Mordenti C, Peris K, Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerol. 2000;9:143-148.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
- American Cancer Society. Cancer facts & figures 2015. Accessed January 7, 2021. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2015/cancer-facts-and-figures-2015.pdf
- Tan AR. Cutaneous manifestations of breast cancer. Semin Oncol. 2016;43:331-334.
- Krathen RA, Orengo IF, Rosen T. Cutaneous metastasis: a meta-analysis of data. South Med J. 2003;96:164-167.
- Lookingbill DP, Spangler N, Sexton FM. Skin involvement as the presenting sign of internal carcinoma. a retrospective study of 7316 cancer patients. J Am Acad Dermatol. 1990;22:19-26.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Li CI, Anderson BO, Daling JR, et al. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289:1421-1424.
- Li CI, Daling JR. Changes in breast cancer incidence rates in the United States by histologic subtype and race/ethnicity, 1995 to 2004. Cancer Epidemiol Biomarkers Prev. 2007;16:2773-2780.
- Lookingbill DP, Spangler N, Helm KF. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29:228-236.
- Dixon J, Anderson R, Page D, et al. Infiltrating lobular carcioma of the breast. Histopathology. 1982;6:149-161.
- Yeatman T, Cantor AB, Smith TJ, et al. Tumor biology of infiltrating lobular carcinoma: implications for management. Ann Surg. 1995;222:549-559.
- Silverstein M, Lewinski BS, Waisman JR, et al. Infiltrating lobular carcinoma: is it different from infiltrating duct carcinoma? Cancer. 1994;73:1673-1677.
- Li CI, Uribe DJ, Daling JR. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93:1046-1052.
- Mordenti C, Peris K, Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerol. 2000;9:143-148.
- Sariya D, Ruth K, Adams-McDonnell R, et al. Clinicopathologic correlation of cutaneous metastases: experience from a cancer center. Arch Dermatol. 2007;143:613-620.
Practice Points
- Clinical providers should be aware of the varying presentations of metastatic cutaneous breast carcinomas.
- Clinicians should remain alert to the possibility of breast cancer as a cause of cutaneous metastases, even in patients with recent negative breast cancer screening.
Pitted Depressions on the Hands and Elbows
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.
Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.
Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
The Diagnosis: Bazex‐Dupré‐Christol Syndrome
Bazex‐Dupré‐Christol syndrome (BDCS) is a rare X-linked dominant genodermatosis characterized by a triad of hypotrichosis, follicular atrophoderma, and multiple basal cell carcinomas (BCCs). Since first being described in 1964,1 there have been fewer than 200 reported cases of BDCS.2 Although a causative gene has not yet been identified, the mutation has been mapped to an 11.4-Mb interval in the Xq25-27.1 region of the X chromosome.3
Classically, congenital hypotrichosis is the first observed symptom and can present shortly after birth.4 It typically is widespread, though sometimes it may be confined to the eyebrows, eyelashes, and scalp. Follicular atrophoderma, which occurs due to a laxation and deepening of the follicular ostia, is seen in 80% of cases and typically presents in early childhood as depressions lacking hair.2 It commonly is found on the face, extensor surfaces of the elbows and knees, and dorsal aspects of the hands and feet. Physical examination of our patient revealed follicular atrophoderma on both the dorsal surfaces of the hands and the extensor surfaces of the elbows. Hair shaft anomalies including pili torti, pili bifurcati, and trichorrhexis nodosa are infrequently observed symptoms of BDCS.2
Basal cell carcinoma often manifests in the second or third decades of life, though there are reports of BCC developing in BDCS patients as young as 3 years. Basal cell carcinoma typically arises on sun-exposed areas, especially the face, neck, and chest. These lesions can be pigmented or nonpigmented and range from 2 to 20 mm in diameter.4 Our patient presented with a BCC on the forehead (Figure 1). Histopathologic evaluation showed a proliferation of basaloid cells with peripheral palisading (Figure 2), confirming the diagnosis of BCC.
Milia, which are not considered part of the classic BDCS triad, are seen in 70% of cases.2 They commonly are found on the face and often diminish with age. Milia may precede the formation of follicular atrophoderma and BCC. Hypohidrosis most commonly occurs on the forehead but can be widespread.2 Other less commonly observed features include epidermal cysts, hyperpigmentation of the face, and trichoepitheliomas.4 The management of BDCS involves frequent clinical examinations, BCC treatment, genetic counseling, and photoprotection.2,4
Nevoid BCC syndrome (NBCCS), also known as Gorlin-Goltz syndrome, is an autosomal-dominant disease characterized by multiple nevoid BCCs, macrocephaly with a large forehead, cleft lip or palate, jaw keratocysts, palmar and plantar pits, and calcification of the falx cerebri.5 Nevoid BCC syndrome is caused by a mutation in the PTCH1 gene in the hedgehog signaling pathway.6 The absence of common symptoms of NBCCS including macrocephaly, palmar or plantar pits, and cleft lip or palate, as well as negative genetic testing, suggested that our patient did not have NBCCS.
Rombo syndrome shares features with BDCS. Similar to BDCS, symptoms of Rombo syndrome include follicular atrophy, milialike papules, and BCC. Patients with Rombo syndrome typically present with atrophoderma vermiculatum on the cheeks and forehead in childhood.7 This atrophoderma presents with a pitted atrophic appearance in a reticular pattern on sun-exposed areas. Other distinguishing features from BDCS include cyanotic redness of sun-exposed skin and telangiectatic vessels.8
Multiple hereditary infundibulocystic BCC is another rare genodermatosis that is characterized by the presence of multiple infundibulocystic BCCs on the face and genitals. Infundibulocystic BCC is a well-differentiated subtype of BCC characterized by buds and cords of basaloid cells with scant stroma. Multiple hereditary infundibulocystic BCC is inherited in an autosomal-dominant fashion and has been linked to SUFU mutation in the sonic hedgehog pathway.9
Rothmund-Thomson syndrome is an autosomalrecessive disorder characterized by sparse hair, skeletal and dental abnormalities, and a high risk for developing keratinocyte carcinomas. It is differentiated from BDCS clinically by the presence of erythema, edema, and blistering, resulting in poikiloderma, plantar hyperkeratotic lesions, and bone defects.10
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
- Bazex A. Génodermatose complexe de type indéterminé associant une hypotrichose, un état atrophodermique généralisé et des dégénérescences cutanées multiples (épitheliomas baso-cellulaires). Bull Soc Fr Derm Syphiligr. 1964;71:206.
- Al Sabbagh MM, Baqi MA. Bazex-Dupre-Christol syndrome: review of clinical and molecular aspects. Int J Dermatol. 2018;57:1102-1106.
- Parren LJ, Abuzahra F, Wagenvoort T, et al. Linkage refinement of Bazex-Dupre-Christol syndrome to an 11.4-Mb interval on chromosome Xq25-27.1. Br J Dermatol. 2011;165:201-203.
- Abuzahra F, Parren LJ, Frank J. Multiple familial and pigmented basal cell carcinomas in early childhood--Bazex-Dupre-Christol syndrome. J Eur Acad Dermatol Venereol. 2012;26:117-121.
- Shevchenko A, Durkin JR, Moon AT. Generalized basaloid follicular hamartoma syndrome versus Gorlin syndrome: a diagnostic challenge. Pediatr Dermatol. 2018;35:E396-E397.
- Fujii K, Miyashita T. Gorlin syndrome (nevoid basal cell carcinoma syndrome): update and literature review. Pediatr Int. 2014;56:667-674.
- van Steensel MA, Jaspers NG, Steijlen PM. A case of Rombo syndrome. Br J Dermatol. 2001;144:1215-1218.
- Lee YC, Son SJ, Han TY, et al. A case of atrophoderma vermiculatum showing a good response to topical tretinoin. Ann Dermatol. 2018;30:116-118.
- Schulman JM, Oh DH, Sanborn JZ, et al. Multiple hereditary infundibulocystic basal cell carcinoma syndrome associated with a germline SUFU mutation. JAMA Dermatol. 2016;152:323-327.
- Larizza L, Roversi G, Volpi L. Rothmund-Thomson syndrome. Orphanet J Rare Dis. 2010;5:2.
A 28-year-old woman presented for evaluation of a pearly papule on the forehead of several months’ duration that was concerning for basal cell carcinoma (BCC). She had a history of numerous BCCs starting at the age of 17 years. She denied radiation or other carcinogenic exposures and had no other notable medical history. The patient’s mother and grandmother also had numerous BCCs. Physical examination revealed hypotrichosis; numerous 3- to 5-mm white cystic papules on the face, chest, and upper arms; and 1- to 5-mm pitted depressions on the dorsal aspects of the hands (top) and extensor surfaces of the elbows (bottom). A proliferation of basaloid cells with peripheral palisading was seen on histopathologic evaluation. Genetic testing revealed no protein patched homolog 1, PTCH1, or suppressor of fused homolog, SUFU, gene mutations.
Advice for Applying to Dermatology as an Applicant of Color: Keep Going
As the dermatology admissions cycle restarts, I reflect back on my journey as a Black woman applying to dermatology. Before deciding, I internally questioned, “Is dermatology right for me?” There were not many faces that looked like mine within the field. After committing to dermatology, I asked dermatologists—almost any who would spare a few minutes to talk to me—how to get into this specialty and be successful when applying. I spoke to advisors and friends at my home department, emailed dermatologists far and wide, approached conference lecturers after their presentations, sought out advice from current residents, and asked prior applicants what they thought was important to match into dermatology. There had been too many unmatched students before me who had achieved good grades and aced US Medical Licensing Examination Step 1. The equation for success was missing a variable.
Mentorship
One weekend, I attended a conference for patients with skin of color. I talked to a student who had taken a year off (retroactively after not matching in prior years). She told me that the biggest key to matching was mentorship; forming a strong relationship with a clinician or investigator who had seen how well you perform in clinic or during research was paramount. Having a collaborator or instructor write you a letter of recommendation and make calls on your behalf could be the difference between matching or taking another year off. More often than any other aspect of the application, it is a lack of mentorship that many students of color do not have access to when pursuing a highly competitive specialty such as dermatology.1,2 In such a small field, applicants need someone to vouch for them—to speak on their behalf compassionately, invite them to collaborate on research projects, and inform them of conference opportunities to present their work.
Representation in Dermatology
We are told that you can accomplish anything with hard work and grit; however, without the platform to show how effectively you have worked, your efforts may never be seen. The diversity statistics for dermatology are clear and disheartening. Although 13% of Americans are Black, only 3% of all dermatologists are Black.2,3 Just over 4% of dermatologists are Hispanic compared with 16% of the general population. The Association of American Medical Colleges reported that the overall 2015 medical school acceptance rate was 41%.4 White (44%), Asian (42%), and Hispanic or Latino (42%) applicants all had similar acceptance rates; however, only one-third of Black applicants were accepted. At graduation in 2015, White individuals were 51% of matriculants. Medical graduates were only 6% Black.4 What percentage of these 6% Black graduates thought about applying into dermatology? How many had someone to encourage them to pursue the specialty or a mentor who they could ask about the nuances and strategy to be a competitive applicant?
In addition to discrimination, social psychologists have described stereotype threat, a risk for minorities that occurs when negative stereotypes associated with an individual’s group status become relevant after perceived cues.5 Therefore, some students of color might avoid competitive specialties such as dermatology because of this internalized lack of confidence in their own abilities and performance thinking, “I’ll never be good enough to match into dermatology.” I have seen this discouraging perception when classmates doubt their own talent and achievements, which is a variation of imposter syndrome—when an individual doubts their abilities and may have an internal fear of eventually being exposed as a fraud.
After several publications received press coverage on the lack of diversity in dermatology applicant selection,3,6,7 I looked around at my interview group composed of 25 to 40 interviewees and on average saw 2 to 3 Black applicants around the room. We always found a way during the packed interview day to find time to introduce ourselves. I almost always left with a new friend who shared feelings of anxiety, uncertainty, hope, and gratefulness from being the few Black people in the room. Bootstrapping might have helped us to make it into medical school, pass shelf examinations, and even get a great Step 1 score. However, the addition of mentorship—or better yet, sponsorship—helped to get us an interview in this competitive field. The impact of mentorship has been especially true for research, which has shown that students often gravitate toward mentors who look like them.8 However, the reality is that many Black and Hispanic students may be at a disadvantage for finding mentors in this way given that there are less than 10% of dermatologists who identify as individuals with skin of color. During the process of applying to dermatology, my greatest advocates were ethnically and racially diverse. The proverb is that it takes a village to raise a child; this reality extends to the medical student’s ability to thrive, not only in residency but also in the residency application process. My sponsors have been as different as their advice and perspectives, which helped me to think about the varied ways I viewed myself as an applicant and shaped what I looked for in residency.
Final Thoughts
Now that I have been a resident in the Department of Dermatology at the Warren Alpert Medical School of Brown University, I excitedly look for opportunities to mentor medical students and help create equity in the application process. Dermatology needs to increase the representation of minority applicants. Efforts to encourage minority medical students include joining the National Medical Association dermatology section through the Student National Medical Association, membership in the Skin of Color Society, getting involved with the Dermatology Interest Group at more medical schools, and awareness of medical student–friendly dermatology conferences. In addition, I was able to establish lifelong mentorship through the American Academy of Dermatology’s Minority Diversity Mentorship Program. One important component is an enhanced effort to increase the number of financial scholarships for away rotations (post–coronavirus disease 2019 pandemic) or application expenses geared to help underrepresented minorities. To truly increase diversity in dermatology, perhaps we need more physicians and residents willing to encourage students of color that dermatology is achievable.
- Brunsma DL, Embrick DG, Shin JH. Graduate students of color: race, racism, and mentoring in the white waters of academia. Sociology of Race and Ethnicity. 2017;3:1-13.
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Current trends in medical education. American Association of Medical Colleges. Accessed January 20, 2021. http://www.aamcdiversityfactsandfigures2016.org/report-section/section-3/
- Spencer SJ, Logel C, Davies PG. Stereotype threat [published online September 10, 2015]. Annu Rev Psychol. 2016;67:415-437.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Blake-Beard S, Bayne ML, Crosby FJ, et al. Matching by race and gender in mentoring relationships: keeping our eyes on the prize. J Social Issues. 2011;67:622-643.
As the dermatology admissions cycle restarts, I reflect back on my journey as a Black woman applying to dermatology. Before deciding, I internally questioned, “Is dermatology right for me?” There were not many faces that looked like mine within the field. After committing to dermatology, I asked dermatologists—almost any who would spare a few minutes to talk to me—how to get into this specialty and be successful when applying. I spoke to advisors and friends at my home department, emailed dermatologists far and wide, approached conference lecturers after their presentations, sought out advice from current residents, and asked prior applicants what they thought was important to match into dermatology. There had been too many unmatched students before me who had achieved good grades and aced US Medical Licensing Examination Step 1. The equation for success was missing a variable.
Mentorship
One weekend, I attended a conference for patients with skin of color. I talked to a student who had taken a year off (retroactively after not matching in prior years). She told me that the biggest key to matching was mentorship; forming a strong relationship with a clinician or investigator who had seen how well you perform in clinic or during research was paramount. Having a collaborator or instructor write you a letter of recommendation and make calls on your behalf could be the difference between matching or taking another year off. More often than any other aspect of the application, it is a lack of mentorship that many students of color do not have access to when pursuing a highly competitive specialty such as dermatology.1,2 In such a small field, applicants need someone to vouch for them—to speak on their behalf compassionately, invite them to collaborate on research projects, and inform them of conference opportunities to present their work.
Representation in Dermatology
We are told that you can accomplish anything with hard work and grit; however, without the platform to show how effectively you have worked, your efforts may never be seen. The diversity statistics for dermatology are clear and disheartening. Although 13% of Americans are Black, only 3% of all dermatologists are Black.2,3 Just over 4% of dermatologists are Hispanic compared with 16% of the general population. The Association of American Medical Colleges reported that the overall 2015 medical school acceptance rate was 41%.4 White (44%), Asian (42%), and Hispanic or Latino (42%) applicants all had similar acceptance rates; however, only one-third of Black applicants were accepted. At graduation in 2015, White individuals were 51% of matriculants. Medical graduates were only 6% Black.4 What percentage of these 6% Black graduates thought about applying into dermatology? How many had someone to encourage them to pursue the specialty or a mentor who they could ask about the nuances and strategy to be a competitive applicant?
In addition to discrimination, social psychologists have described stereotype threat, a risk for minorities that occurs when negative stereotypes associated with an individual’s group status become relevant after perceived cues.5 Therefore, some students of color might avoid competitive specialties such as dermatology because of this internalized lack of confidence in their own abilities and performance thinking, “I’ll never be good enough to match into dermatology.” I have seen this discouraging perception when classmates doubt their own talent and achievements, which is a variation of imposter syndrome—when an individual doubts their abilities and may have an internal fear of eventually being exposed as a fraud.
After several publications received press coverage on the lack of diversity in dermatology applicant selection,3,6,7 I looked around at my interview group composed of 25 to 40 interviewees and on average saw 2 to 3 Black applicants around the room. We always found a way during the packed interview day to find time to introduce ourselves. I almost always left with a new friend who shared feelings of anxiety, uncertainty, hope, and gratefulness from being the few Black people in the room. Bootstrapping might have helped us to make it into medical school, pass shelf examinations, and even get a great Step 1 score. However, the addition of mentorship—or better yet, sponsorship—helped to get us an interview in this competitive field. The impact of mentorship has been especially true for research, which has shown that students often gravitate toward mentors who look like them.8 However, the reality is that many Black and Hispanic students may be at a disadvantage for finding mentors in this way given that there are less than 10% of dermatologists who identify as individuals with skin of color. During the process of applying to dermatology, my greatest advocates were ethnically and racially diverse. The proverb is that it takes a village to raise a child; this reality extends to the medical student’s ability to thrive, not only in residency but also in the residency application process. My sponsors have been as different as their advice and perspectives, which helped me to think about the varied ways I viewed myself as an applicant and shaped what I looked for in residency.
Final Thoughts
Now that I have been a resident in the Department of Dermatology at the Warren Alpert Medical School of Brown University, I excitedly look for opportunities to mentor medical students and help create equity in the application process. Dermatology needs to increase the representation of minority applicants. Efforts to encourage minority medical students include joining the National Medical Association dermatology section through the Student National Medical Association, membership in the Skin of Color Society, getting involved with the Dermatology Interest Group at more medical schools, and awareness of medical student–friendly dermatology conferences. In addition, I was able to establish lifelong mentorship through the American Academy of Dermatology’s Minority Diversity Mentorship Program. One important component is an enhanced effort to increase the number of financial scholarships for away rotations (post–coronavirus disease 2019 pandemic) or application expenses geared to help underrepresented minorities. To truly increase diversity in dermatology, perhaps we need more physicians and residents willing to encourage students of color that dermatology is achievable.
As the dermatology admissions cycle restarts, I reflect back on my journey as a Black woman applying to dermatology. Before deciding, I internally questioned, “Is dermatology right for me?” There were not many faces that looked like mine within the field. After committing to dermatology, I asked dermatologists—almost any who would spare a few minutes to talk to me—how to get into this specialty and be successful when applying. I spoke to advisors and friends at my home department, emailed dermatologists far and wide, approached conference lecturers after their presentations, sought out advice from current residents, and asked prior applicants what they thought was important to match into dermatology. There had been too many unmatched students before me who had achieved good grades and aced US Medical Licensing Examination Step 1. The equation for success was missing a variable.
Mentorship
One weekend, I attended a conference for patients with skin of color. I talked to a student who had taken a year off (retroactively after not matching in prior years). She told me that the biggest key to matching was mentorship; forming a strong relationship with a clinician or investigator who had seen how well you perform in clinic or during research was paramount. Having a collaborator or instructor write you a letter of recommendation and make calls on your behalf could be the difference between matching or taking another year off. More often than any other aspect of the application, it is a lack of mentorship that many students of color do not have access to when pursuing a highly competitive specialty such as dermatology.1,2 In such a small field, applicants need someone to vouch for them—to speak on their behalf compassionately, invite them to collaborate on research projects, and inform them of conference opportunities to present their work.
Representation in Dermatology
We are told that you can accomplish anything with hard work and grit; however, without the platform to show how effectively you have worked, your efforts may never be seen. The diversity statistics for dermatology are clear and disheartening. Although 13% of Americans are Black, only 3% of all dermatologists are Black.2,3 Just over 4% of dermatologists are Hispanic compared with 16% of the general population. The Association of American Medical Colleges reported that the overall 2015 medical school acceptance rate was 41%.4 White (44%), Asian (42%), and Hispanic or Latino (42%) applicants all had similar acceptance rates; however, only one-third of Black applicants were accepted. At graduation in 2015, White individuals were 51% of matriculants. Medical graduates were only 6% Black.4 What percentage of these 6% Black graduates thought about applying into dermatology? How many had someone to encourage them to pursue the specialty or a mentor who they could ask about the nuances and strategy to be a competitive applicant?
In addition to discrimination, social psychologists have described stereotype threat, a risk for minorities that occurs when negative stereotypes associated with an individual’s group status become relevant after perceived cues.5 Therefore, some students of color might avoid competitive specialties such as dermatology because of this internalized lack of confidence in their own abilities and performance thinking, “I’ll never be good enough to match into dermatology.” I have seen this discouraging perception when classmates doubt their own talent and achievements, which is a variation of imposter syndrome—when an individual doubts their abilities and may have an internal fear of eventually being exposed as a fraud.
After several publications received press coverage on the lack of diversity in dermatology applicant selection,3,6,7 I looked around at my interview group composed of 25 to 40 interviewees and on average saw 2 to 3 Black applicants around the room. We always found a way during the packed interview day to find time to introduce ourselves. I almost always left with a new friend who shared feelings of anxiety, uncertainty, hope, and gratefulness from being the few Black people in the room. Bootstrapping might have helped us to make it into medical school, pass shelf examinations, and even get a great Step 1 score. However, the addition of mentorship—or better yet, sponsorship—helped to get us an interview in this competitive field. The impact of mentorship has been especially true for research, which has shown that students often gravitate toward mentors who look like them.8 However, the reality is that many Black and Hispanic students may be at a disadvantage for finding mentors in this way given that there are less than 10% of dermatologists who identify as individuals with skin of color. During the process of applying to dermatology, my greatest advocates were ethnically and racially diverse. The proverb is that it takes a village to raise a child; this reality extends to the medical student’s ability to thrive, not only in residency but also in the residency application process. My sponsors have been as different as their advice and perspectives, which helped me to think about the varied ways I viewed myself as an applicant and shaped what I looked for in residency.
Final Thoughts
Now that I have been a resident in the Department of Dermatology at the Warren Alpert Medical School of Brown University, I excitedly look for opportunities to mentor medical students and help create equity in the application process. Dermatology needs to increase the representation of minority applicants. Efforts to encourage minority medical students include joining the National Medical Association dermatology section through the Student National Medical Association, membership in the Skin of Color Society, getting involved with the Dermatology Interest Group at more medical schools, and awareness of medical student–friendly dermatology conferences. In addition, I was able to establish lifelong mentorship through the American Academy of Dermatology’s Minority Diversity Mentorship Program. One important component is an enhanced effort to increase the number of financial scholarships for away rotations (post–coronavirus disease 2019 pandemic) or application expenses geared to help underrepresented minorities. To truly increase diversity in dermatology, perhaps we need more physicians and residents willing to encourage students of color that dermatology is achievable.
- Brunsma DL, Embrick DG, Shin JH. Graduate students of color: race, racism, and mentoring in the white waters of academia. Sociology of Race and Ethnicity. 2017;3:1-13.
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Current trends in medical education. American Association of Medical Colleges. Accessed January 20, 2021. http://www.aamcdiversityfactsandfigures2016.org/report-section/section-3/
- Spencer SJ, Logel C, Davies PG. Stereotype threat [published online September 10, 2015]. Annu Rev Psychol. 2016;67:415-437.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Blake-Beard S, Bayne ML, Crosby FJ, et al. Matching by race and gender in mentoring relationships: keeping our eyes on the prize. J Social Issues. 2011;67:622-643.
- Brunsma DL, Embrick DG, Shin JH. Graduate students of color: race, racism, and mentoring in the white waters of academia. Sociology of Race and Ethnicity. 2017;3:1-13.
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Current trends in medical education. American Association of Medical Colleges. Accessed January 20, 2021. http://www.aamcdiversityfactsandfigures2016.org/report-section/section-3/
- Spencer SJ, Logel C, Davies PG. Stereotype threat [published online September 10, 2015]. Annu Rev Psychol. 2016;67:415-437.
- Granstein RD, Cornelius L, Shinkai K. Diversity in dermatology—a call for action. JAMA Dermatol. 2017;153:499-500.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Blake-Beard S, Bayne ML, Crosby FJ, et al. Matching by race and gender in mentoring relationships: keeping our eyes on the prize. J Social Issues. 2011;67:622-643.
Resident Pearl
- Finding a strong mentor who can both advocate for and help guide a student of color through the admissions process is integral to matching into dermatology
Male Genital Examinations: Special Considerations and Pearls for Dermatologists
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
Men have unique dermatologic needs yet are significantly less likely than women to visit a dermatologist’s office.1 Male patients might have preconceived notions about the nature of dermatology visits and necessary areas of the body to be examined: For example, male patients might associate the genital examination with a urologist and not expect a dermatologist to complete such a seemingly private examination.2
Genital examinations are currently underperformed: Only one-quarter of dermatologists report examining a male patient’s genitals at most or all visits.3 In this commentary, we discuss the importance of genital examinations in men’s dermatology, specific issues that can arise, and strategies to enhance the quality and frequency of genital examinations in male patients.
Invaluable Aspect of Care
Thorough inspection of a male patient’s genital region is an important part of conducting a total-body skin examination (TBSE) for routine surveillance and evaluation of genital dermatoses. Sexually transmitted infections, warts, and other common lesions can be missed in diagnosis without careful inspection of the genital region. Additionally, scrotal malignancies, such as primary and metastatic melanoma and basal cell carcinoma, though rare, might be overlooked until symptoms become severe.4,5
There is no substitute for a physical examination but, in certain circumstances, it might be appropriate for a dermatologist to ask a patient if he has concerning lesions on his genitals. However, patients often are unsure of worrisome signs, and areas of the perineum might not be easily visible to a patient. Genital inspection during the physical examination allows for a teachable moment, during which the dermatologist can educate the patient about benign lesions and variants, such as pearly penile papules, seborrheic keratoses, and sebaceous cysts.6 These lesions might not require intervention but should be monitored for atypical features or infection.6
Also, the dermatologist might incidentally discover transmissible lesions, such as condylomata caused by human papillomavirus, which has been shown to be present in approximately 50% of men in the United States7—many of whom are unaware. Inflammatory dermatoses, such as psoriasis, often affect the genitals and go unnoticed; prompt intervention can decrease the likelihood of complications.6
Protocol for Genital Examinations
To examine the genitals, all surfaces of the penis, scrotum, and perineum should be evaluated, with anatomic and pathologic variants noted. The patient or physician should stretch the penis, maneuvering it in multiple directions so that all aspects can be examined. In uncircumcised men, the foreskin should be retracted so that the head of the penis can be examined, followed by replacement of the foreskin by the patient.8 The scrotum also should be examined and lifted to fully view the perineum.
Providers should not grasp the penis with the whole hand but use the thumb and first finger to hold the head of the penis to maneuver it.8 Similarly, using the back of the hand and fingers to manipulate the genitals establishes boundaries and sets a clinical tone for the examination.
Unintentional Erection
Unique to the male dermatologic examination is the unintentional patient erection; a physician might be unsure of how to approach such a potentially awkward situation. An erection is not always an indication of sexual arousal; rather, it can reflect an autonomic reflex in response to physical stimulation. Erections occur commonly in health care settings, especially if the genitals are being manipulated.9
Generally, the course of action here depends on the patient’s response.10 For patients who appear unbothered, it might be appropriate to ignore the erection and proceed with the examination, especially if the physician is not actively examining the genital region. If the patient appears embarrassed, the physician can say “This is completely normal” or “Random erections are common” to normalize the situation. Joking or laughing should be avoided. For a patient who appears upset, the physician can step outside the room for a brief period to give the patient privacy, then re-enter and ask him if he is comfortable continuing with the examination.
When a patient develops an erection, the physician might become uncomfortable and, consciously or subconsciously, increase the pace of the examination, which is a natural tendency, but expediency at the expense of comprehensive care is inappropriate.
Examiner’s Body Language and Tone
Throughout the genital examination, the physician should be mindful of their comments and body language to avoid exacerbating patient vulnerability. Using anatomic terms, rather than colloquial ones, to describe the genitalia is advised to prevent misunderstanding and maintain a professional clinical environment. Providers should be prepared to explain anatomic terms because some patients are not familiar with medical terminology.
Presence of a Chaperone
Involving a chaperone, as recommended by the American Medical Association, might make a patient more comfortable and alleviate potential misunderstanding. Still, physicians should be aware that some patients might feel uncomfortable with a chaperone, interpreting their presence as an expectation of impropriety.11 Universal offering of a chaperone to all patients, regardless of the gender of the physician, as well as appropriate signage in the clinical environment, normalizes chaperone invitation and use.
Other Helpful Considerations
Various strategies in the male genital examination can increase patient and physician comfort and improve care:
- The patient should be offered a gown before a TBSE or any skin examination during which the genitals will be examined.
- The patient should be allowed to keep his shorts or underwear on to avoid the feeling of being naked, which can provoke anxiety. Prior to beginning the examination, disclose that it will include “under the covered areas.”
- Ask the patient for permission to conduct the examination, enumerate the steps, and provide a rationale for a genital examination. These steps help gain cooperation, alleviate anticipation, and prevent surprise.
- To increase the patient’s comfort level, he can be asked whether he prefers to be examined supine or standing.
- Consider allowing the patient, himself, to expose and manipulate his genitals during the examination to increase his involvement and sense of autonomy.
- For genital examinations, patients often prefer that the examiner be a physician of the same gender. Accommodating a patient’s request regarding the examiner’s gender might not always be possible, but the medical practice should make an honest attempt to oblige.
Lastly, providers should be cognizant of the needs of male sexual and gender minority populations (ie, gay, bisexual, transgender/gender diverse, queer or questioning, intersex, and asexual persons). For example, transgender women might retain male anatomy or have surgical alteration of the genital region that also requires evaluation. In such patient populations, the genital examination is equally important to evaluate for dermatologic conditions that require treatment.
Final Thoughts
The male genital examination is an important component of the TBSE, as dermatologists can recognize lesions before symptoms present. Robust educational methods for trainees and practitioners in dermatology are lacking, and development of curricula might be beneficial to increase comfort in performing the genital examination. Still, use of the procedures described in this commentary can normalize the men’s genital examination, optimize the physical examination, and improve men’s overall dermatologic health.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Brezinski EA, Harskamp CT, Ledo L, et al. Public perception of dermatologists and comparison with other medical specialties: results from a national survey. J Am Acad Dermatol. 2014;71:875-881.
- Rieder EA, Mu EW, Wang J, et al. Dermatologist practices during total body skin examinations: a survey study. J Drugs Dermatol. 2018;17:516-520.
- Gonzalez CD, Hawkes JE, Bowles TL. Malignant melanoma scrotal metastasis: the importance of the genital examination. JAAD Case Rep. 2017;3:10-12.
- Solimani F, Juratli H, Hoch M, et al. Basal cell carcinoma of the scrotum: an important but easily overlooked entity. J Eur Acad Dermatol Venereol. 2018;32:E254-E255.
- Gabrielson AT, Le TV, Fontenot C, et al. Male genital dermatology: a primer for the sexual medicine physician. Sex Med Rev. 2019;7:71-83.
- Han JJ, Beltran TH, Song JW, et al. Prevalence of genital human papillomavirus infection and human papillomavirus vaccination rates among US adult men: National Health and Nutrition Examination Survey (NHANES) 2013-2014. JAMA Oncol. 2017;3:810-816.
- Albaugh JA, Kellogg-Spadt S. Genital and dermatologic examination. part II: the male patient. Urol Nurs. 2003;23:366-367.
- Dean RC, Lue TF. Physiology of penile erection and pathophysiology of erectile dysfunction. Urol Clin North Am. 2005;32:379-395.
- Norwick P, Weston GK, Grant-Kels JM. Erection ethics. J Am Acad Dermatol. 2019;81:1225.
- Vogel L. Chaperones: friend or foe, and to whom? CMAJ. 2012;184:642-643.
Practice Points
- Genital examinations are an important aspect of comprehensive dermatologic care for male patients.
- Unintentional patient erections are unique to male patients and should be addressed professionally, depending on the patient’s reaction.
- In addition to being mindful of body language and tone, dermatologists may consider involving a chaperone when performing genital examinations to optimize patient experience.
Hypertrophic Lichen Planus–like Eruption Following Pembrolizumab
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
To the Editor:
Pembrolizumab, a humanized monoclonal anti–programmed cell death protein 1 (PD-1) antibody, acts by blocking negative immune regulators such as PD-1.1 Since its approval by the US Food and Drug Administration in 2014, the use of PD-1 inhibitors such as pembrolizumab has dramatically increased, and they are now the standard of care for cancers such as melanoma, lung cancer, and renal cell carcinoma.2,3 With increased use comes a better understanding of the cutaneous adverse effects that may occur. To date, almost 50% of patients treated with PD-1 inhibitors will develop an adverse cutaneous reaction.4 Thus far, cases of patients developing vitiligo, bullous pemphigoid, psoriasis, granulomatous skin reactions, severe cutaneous reactions (ie, toxic epidermal necrolysis), lupus erythematosus, and lichenoid reactions have been described.3,5,6 There are fewer than 30 documented cases of lichenoid reactions due to anti–PD-1 treatment described in the literature, increasing the importance of case reports to demonstrate a full range of cutaneous findings.3 We present a case of a reaction to pembrolizumab with an eruption of lichenoid papules predominantly involving the hands and feet as well as nail changes.
A 60-year-old man with ocular melanoma metastatic to the right lung, transverse colon, and right axillary lymph nodes presented with a chief concern of growing skin lesions present for 6 weeks on the hands and feet. The lesions were tender to the touch and occasionally drained a clear fluid. He also reported nail fragility. Of note, the patient was being treated for metastatic melanoma with pembrolizumab infusions every 3 weeks, which started 6 weeks prior to the onset of the eruption.
Physical examination demonstrated lichenoid papules on the dorsal and ventral aspects of the hands and feet (Figure 1), as well as longitudinal ridging on numerous fingernails and mild koilonychia. A punch biopsy revealed lichenoid interface dermatitis with irregular epidermal hyperplasia (Figure 2). A diagnosis of hypertrophic lichen planus–like drug eruption in response to pembrolizumab was made and clobetasol cream was prescribed.
At 1-month follow-up, the patient reported notable improvement with clobetasol, and he was transitioned to tacrolimus ointment 0.1%. He continued to improve until a month later when he reported new lesions arising a week after a pembrolizumab infusion. He continued to use clobetasol cream for flares and tacrolimus ointment for maintenance.
Almost 3 months after the initial visit, the patient presented with inflammation around his right third fingernail of 1 week’s duration, with more notable fragility than his other nails. No trauma was described, and the nail abnormality was attributed to pembrolizumab. Clobetasol cream and biotin 3 mg daily resulted in improvement, and no other nails were affected in a similar way.
Programmed cell death protein 1 blockers are associated with a variety of adverse events including hypothyroidism, gastrointestinal abnormalities, fatigue, and skin disorders.7 In one study (N=83), cutaneous adverse drug events were found to occur in 42% (35/83) of patients following pembrolizumab therapy, with the most common cutaneous lesions being maculopapular eruptions (29% [24/83]), pruritus (12% [10/83]), and hypopigmentation (8% [7/83]).5
A total of 29 cases of lichenoid dermatitis following anti–PD-1 therapy have been described in the literature.3 Cases range from an eruption of photodistributed hyperkeratotic papules and plaques to hypertrophic vesiculobullous lesions.3,6 Suggested pathophysiology involves blocking the interaction of programmed death ligand 1 on keratinocytes with PD-1 on T cells.3 Management typically includes topical or systemic steroids. Cyclosporine and acitretin also have been successful in a small number of patients. Most patients continue anti–PD-1 treatment with systemic therapy.3
Our patient represents a similar lichenoid eruption; however, the distribution on the dorsal and ventral aspects of the hands and feet as well as nail dystrophy make the presentation unique. Anticancer drugs that increase the T-cell immune response by altering the complex signaling among T cells, antigen-presenting cells, and tumor cells have been associated with cutaneous eruptions. Although the exact mechanism is still not fully understood, clinical suspicion of a pembrolizumab reaction should remain high on the differential in the setting of hyperkeratotic papules in association with anti–PD-1 therapy.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
- Homet Moreno B, Ribas A. Anti-programmed cell death protein-1/ligand-1 therapy in different cancers. Br J Cancer. 2015;112:1421-1427.
- Robert C, Ribas A, Wolchok JD, et al. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384:1109-1117.
- Simonsen AB, Kaae J, Elleback E, et al. Cutaneous adverse reactions to anti-PD-1 treatment: a systematic review. J Am Acad Dermatol. 2020;83:1415-1424.
- Hwang SJ, Carlos G, Wakade D, et al. Cutaneous adverse events (AEs) of anti-programmed cell death (PD)-1 therapy in patients with metastatic melanoma: a single-institution cohort. J Am Acad Dermatol. 2016;74:455-461.
- Sanlorenzo M, Vujic I, Daud A, et al. Pembrolizumab cutaneous adverse events and their association with disease progression. JAMA Dermatol. 2015;151:1206-1212.
- Joseph RW, Cappel M, Goedjen B, et al. Lichenoid dermatitis in three patients with metastatic melanoma treated with anti-PD-1 therapy. Cancer Immunol Res. 2015;3:18-22.
- Hamid O, Robert C, Daud A, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369:134-144.
Practice Points
- With an increased use of immunotherapy medications such as pembrolizumab for various cancers, it is important that dermatologists are aware of the wide range of adverse cutaneous reactions that can occur, including lichenoid reactions.
- Hypertrophic lichen planus should be considered in the differential diagnosis of patients with cutaneous lesions in addition to nail findings developing after starting programmed cell death protein 1 inhibitor therapy.
Erythema, Blisters, and Scars on the Elbows, Knees, and Legs
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
The Diagnosis: Epidermolysis Bullosa Acquisita
The diagnosis of epidermolysis bullosa acquisita (EBA) was made based on the clinical and pathologic findings. A blistering disorder that resolves with milia is characteristic of EBA. Hematoxylin and eosin staining demonstrated a pauci-inflammatory separation between the epidermis and dermis (Figure 1). Direct immunofluorescence studies showed linear IgG deposition along the basement membrane zone while C3 was negative (Figure 2). Salt-split skin was essential, as it revealed IgG deposition to the floor of the split (Figure 3), a pattern seen in EBA and not bullous pemphigoid (BP).1
Epidermolysis bullosa acquisita is an acquired autoimmune bullous disorder that results from antibodies to type VII collagen, an anchoring fibril that attaches the lamina densa to the dermis. The epidemiology and etiology of the trigger that leads to antibody production are not well known, but an association between EBA and inflammatory bowel disease has been described.2 Although this disease may present in childhood, EBA most commonly is a disorder seen in adults and the elderly. A classic noninflammatory mechanobullous form as well as an inflammatory BP-like form are the most commonly encountered presentations. Light microscopy demonstrates subepidermal cleavage without acantholysis. In the inflammatory BP-like subtype, an inflammatory infiltrate may be present. Direct immunofluorescence is remarkable for a linear band of IgG deposits along the basement membrane zone, with or without C3 deposition in a similar pattern.1
Bullous pemphigoid is within the differential of EBA. It can be difficult to differentiate clinically, especially when a patient has the BP-like variant of EBA because, as the name implies, it mimics BP. Patients with BP often will report a pruritic patch that will then develop into an urticarial plaque. Scarring and milia rarely are seen in BP but can be observed in the multiple presentations of EBA. Hematoxylin and eosin staining and direct immunofluorescence may be almost identical, and differentiating between the 2 disorders can be a challenge. Immunodeposition in EBA occurs in a U-shaped, serrated pattern, while the pattern in BP is N-shaped and serrated.3 Although the U-shaped, serrated pattern is relatively specific, it is not always easy to interpret and requires a high-quality biopsy specimen, which can be difficult to discern with certainty in suboptimal preparations. Another way to differentiate between the 2 entities is to utilize the salt-split skin technique, as performed in our patient. With salt-split skin, the biopsy is placed into a solution of 1 mol/L sodium chloride and incubated at 4 °C (39 °F) for 18 to 24 hours. A blister is then produced at the level of the lamina lucida, which allows for the staining of immunoreactants to occur either above or below that split (commonly referred to as staining on the roof or floor of the blister cavity). With EBA, there is immunoreactant deposition on the floor of the blister, while the opposite occurs in BP.4
Epidermolysis bullosa simplex is the most common type of epidermolysis bullosa, with keratin genes KRT5 and KRT14 as frequent mutations. Patients develop blisters, vesicles, bullae, and milia on traumatized areas of the body such as the hands, elbows, knees, and feet. This disease presents early in childhood. Histology exhibits a cell-poor subepidermal blister.5 With porphyria cutanea tarda, reduced activity of uroporphyrinogen decarboxylase, a major enzyme in the heme synthesis pathway, leads to blisters with erosions and milia on sun-exposed areas of the body. Histologic evaluation reveals a subepidermal pauci-inflammatory vesicle with festooning of the dermal papillae and amphophilic basement membrane within the epidermis. Direct immunofluorescence of porphyria cutanea tarda demonstrates IgM and C3 in the vessels.6 Sweet syndrome is a neutrophilic dermatosis that presents as erythematous, edematous, hot, and tender plaques along with fever and leukocytosis. It is associated with myeloproliferative disorders. Biopsy demonstrates papillary dermal edema along with diffuse neutrophilic infiltrate.7
Numerous medications have been recommended for the treatment of EBA, ranging from steroids to steroid-sparing drugs such as colchicine and dapsone.8,9 Our patient was educated on physical precautions and was started on dapsone alone due to comorbid diabetes mellitus and renal disease. Within a few weeks of initiating dapsone, he observed a reduction in erythema, and within months he experienced a decrease in blister eruption frequency.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
- Vorobyev A, Ludwig RJ, Schmidt E. Clinical features and diagnosis of epidermolysis bullosa acquisita. Expert Rev Clin Immunol. 2017;13:157-169.
- Reddy H, Shipman AR, Wojnarowska F. Epidermolysis bullosa acquisita and inflammatory bowel disease: a review of the literature. Clin Exp Dermatol. 2013;38:225-230.
- Vodegel RM, Jonkman MF, Pas HH, et al. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol. 2004;151:112-118.
- Gardner KM, Crawford RI. Distinguishing epidermolysis bullosa acquisita from bullous pemphigoid without direct immunofluorescence. J Cutan Med Surg. 2018;22:22-24.
- Sprecher E. Epidermolysis bullosa simplex. Dermatol Clin. 2010;28:23-32.
- Maynard B, Peters MS. Histologic and immunofluorescence study of cutaneous porphyrias. J Cutan Pathol. 1992;19:40-47.
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018:79:987-1006.
- Kirtschig G, Murrell D, Wojnarowska F, et al. Interventions for mucous membrane pemphigoid and epidermolysis bullosa acquisita. Cochrane Database Syst Rev. 2003;1:CD004056
- Gürcan HM, Ahmed AR. Current concepts in the treatment of epidermolysis bullosa acquisita. Expert Opin Pharmacother. 2011;12:1259-1268.
A 69-year-old man presented with an asymptomatic rash on the extensor surfaces of 2 years' duration. He reported recurrent blisters that would then scar over. The lesions did not occur in relation to any known trauma. The patient's medical history revealed dialysis-dependent end-stage renal disease secondary to type 2 diabetes mellitus. His medications were noncontributory, and there was no family history of blistering disorders. He had tried triamcinolone cream without any improvement. Physical examination was remarkable for erythematous blisters and bullae with scales and milia on the elbows, knees, and lower legs. The oral mucosa was unremarkable. Shave biopsies of the skin for direct immunofluorescence and salt-split skin studies were obtained.
Cutaneous Insulin-Derived Amyloidosis Presenting as Hyperkeratotic Nodules
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.
Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.
Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).
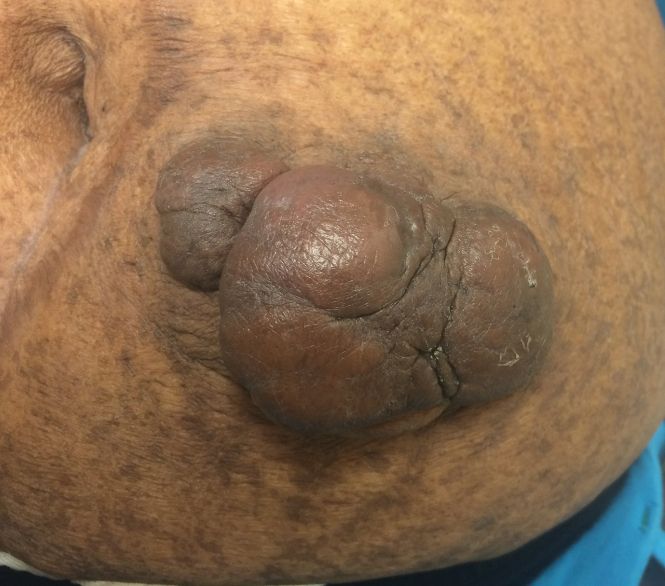
Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.
Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
Amyloidosis consists of approximately 30 protein-folding disorders sharing the common feature of abnormal extracellular amyloid deposition. In each condition, a specific soluble precursor protein aggregates to form the insoluble fibrils of amyloid, characterized by the beta-pleated sheet structure.1 Amyloidosis occurs as either a systemic or localized process. Insulin-derived (AIns) amyloidosis, a localized process occurring at insulin injection sites, was first reported in 1983.2 There were fewer than 20 reported cases until 2014, when 57 additional cases were reported by just 2 institutions,3,4 indicating that AIns amyloidosis may be more common than previously thought.3,5
Despite the increasing prevalence of diabetes mellitus and insulin use, there is a paucity of published cases of AIns amyloidosis. The lack of awareness of this condition among both dermatologists and general practitioners may be in part due to its variable clinical manifestations. We describe 2 patients with unique presentations of localized amyloidosis at repeated insulin injection sites.
Case Reports
Patient 1
A 39-year-old man with a history of type 1 diabetes mellitus presented with 4 asymptomatic nodules on the lateral thighs in areas of previous insulin injection. He first noticed the lesions 9 months prior to presentation and subsequently switched the injection site to the abdomen without development of new nodules. Despite being compliant with his insulin regimen, he had a long history of irregular glucose control, including frequent hypoglycemic episodes. The patient was using regular and neutral protamine hagedorn insulin.
On physical examination, 2 soft, nontender, exophytic nodules were noted on each upper thigh with surrounding hyperpigmented and hyperkeratotic collarettes (Figure 1). The nodules ranged in size from 2 to 3.5 cm in diameter.

Remarkable laboratory data included a fasting glucose level of 207 mg/dL (reference range, 70–110 mg/dL) and a glycohemoglobin of 8.8% (reference range, <5.7%). Serum protein electrophoresis and immunofixation were normal. Histopathology of the lesions demonstrated diffuse deposition of pink amorphous material associated with prominent papillomatosis, hyperkeratosis, and acanthosis (Figure 2). Congo red staining was positive with green birefringence under polarized light, indicative of amyloid deposits (Figure 3). Liquid chromatography–tandem mass spectrometry of the specimens was consistent with deposition of AIns amyloidosis.


Due to the size and persistent nature of the lesions, the nodules were removed by tangential excision. In addition, the patient was advised to continue rotating injection sites frequently. His blood glucose levels are now well controlled, and he has not developed any new nodules.
Patient 2
A 53-year-old woman with a history of type 2 diabetes mellitus presented with painful subcutaneous nodules on the lower abdomen at sites of previous insulin injections. The nodules developed approximately 1 month after she started treatment with neutral protamine hagedorn insulin and had been slowly enlarging over the past year. She tried switching injection sites after noticing the lesions, but the nodules persisted. The patient had a long history of poor glucose control with chronically elevated glycohemoglobin and blood glucose levels.
On physical examination, 2 hyperpigmented, exophytic, smooth nodules were noted on the right and left lower abdomen, ranging in size from 2.5 to 5.5 cm in diameter (Figure 4).

Relevant laboratory data included a fasting glucose level of 197 mg/dL and a glycohemoglobin of 9.3%. A biopsy of the lesion on the left lower abdomen revealed eosinophilic amorphous deposits with fissuring in the dermis (Figure 5). Congo red stain was positive with green birefringence under polarized light. Liquid chromatography–tandem mass spectrometry of the specimen showed deposition of AIns amyloid. The patient began injecting away from the amyloid nodules without development of any new lesions. The original nodules have persisted, and surgical excision is planned.

Comment
Insulin is the suspected precursor protein in AIns amyloidosis, but the exact pathogenesis is unknown. The protein that is derived from insulin in these tumors is now identified as AIns amyloidosis.5,6 It is hypothesized that insulin accumulates locally and is converted to amyloid by an unknown mechanism.7 Other potential contributory factors include chronic inflammation and foreign body reactions developing around amyloid deposits, as well as repeated trauma from injections into a single site.4,5 It appears that lesions may derive from a wide range of insulin types and occur after variable time periods.
A majority of cases of iatrogenic amyloid have been described as single, firm, subcutaneous masses at an injection site that commonly are misdiagnosed as lipomas or lipohypertrophy.7-11 To our knowledge, none of the reported cases resembled the multiple, discrete, exophytic nodules seen in our patients.3,4 The surrounding hyperkeratosis noted in patient 1 is another uncommon feature of AIns amyloidosis (Figures 1 and 2). Only 3 AIns amyloidosis cases described lesions with acanthosis nigricans–like changes, only 1 of which provided a clinical image.6,7,12The mechanism for the acanthosis nigricans–like changes may have been due to the high levels of insulin at the injection site. It has been suggested that the activation of insulinlike growth factor receptor by insulin leads to the proliferation of keratinocytes and fibroblasts.6 Histologic examination of AIns amyloidosis lesions generally demonstrates deposition of homogenous eosinophilic material consistent with amyloid, as well as positive Congo red staining with green birefringence by polarization. Immunohistologic staining with insulin antibody with or without proteomic analysis of the amyloid deposits can confirm the diagnosis. In both of our patients’ specimens, liquid chromatography–tandem mass spectrometry was performed for proteomic analysis, and results were consistent with AIns amyloidosis.
Reports in the literature have suggested that the deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.4,11,13 Hence, injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis. In their study of 4 patients, Nagase et al4 compared serum insulin levels after insulin injection into amyloid nodules vs insulin levels after injection into normal skin. Insulin absorption at the amyloid sites was 34% of that at normal sites. Given these results, patients should be instructed to inject away from the amyloid deposit once it is identified.6 Glucose levels should be monitored closely when patients first inject away from the amyloid mass, as injection of the same dosage to an area of normal skin can lead to increased insulin absorption and hypoglycemia.4,6 It is possible that the frequent hypoglycemic episodes noted in patient 1 were due to increased insulin sensitivity after switching to injection sites away from amyloid lesions.
Conclusion
Our patients demonstrate unique presentations of localized cutaneous amyloidosis at repeated insulin injection sites. We report these cases to complement the current data of iatrogenic amyloidosis and provide insight into this likely underreported phenomenon.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
- Hazenberg BPC. Amyloidosis: a clinical overview. Rheum Dis Clin North Am. 2013;39:323-345.
- Storkel S, Schneider HM, Muntefering H, et al. Iatrogenic, insulin-dependent, local amyloidosis. Lab Invest. 1983;48:108-111.
- D’souza A, Theis JD, Vrana JA, et al. Pharmaceutical amyloidosis associated with subcutaneous insulin and enfuvirtide administration. Amyloid. 2014;21:71-75.
- Nagase T, Iwaya K, Iwaki Y, et al. Insulin-derived amyloidosis and poor glycemic control: a case series. Am J Med. 2014;127:450-454.
- Gupta Y, Singla G, Singla R. Insulin-derived amyloidosis. Indian J Endocrinol Metab. 2015;19:174-177.
- Kudo-Watanuki S, Kurihara E, Yamamoto K, et al. Coexistence of insulin-derived amyloidosis and an overlying acanthosis nigricans-like lesion at the site of insulin injection. Clin Exp Dermatol. 2013;38:25-29.
- Yumlu S, Barany R, Eriksson M, et al. Localized insulin-derived amyloidosis in patients with diabetes mellitus: a case report. Hum Pathol. 2009;40:1655-1660.
- Okamura S, Hayashino Y, Kore-Eda S, et al. Localized amyloidosis at the site of repeated insulin injection in a patient with type 2 diabetes. Diabetes Care. 2013;36:E200.
- Dische FE, Wernstedt C, Westermark GT, et al. Insulin as an amyloid-fibril protein at sites of repeated insulin injections in a diabetic patient. Diabetologia. 1988;31:158-161.
- Swift B, Hawkins PN, Richards C, et al. Examination of insulin injection sites: an unexpected finding of localized amyloidosis. Diabetic Med. 2002;19:881-882.
- Albert SG, Obadiah J, Parseghian SA, et al. Severe insulin resistance associated with subcutaneous amyloid deposition. Diabetes Res Clin Pract. 2007;75:374-376.
- Nandeesh BN, Rajalakshmi T, Shubha B. Cutaneous amyloidosis and insulin with coexistence of acanthosis nigricans. Indian J Pathol Microbiol. 2014;57:127-129.
- Endo JO, Rocken C, Lamb S, et al. Nodular amyloidosis in a diabetic patient with frequent hypoglycemia: sequelae of repeatedly injecting insulin without site rotation. J Am Acad Dermatol. 2010;63:E113-E114.
Practice Points
- Deposition of amyloid at insulin injection sites has the potential to interfere with insulin absorption, leading to poor glucose control.
- Patients with insulin-derived (AIns) amyloidosis may initially present after noticing nodular deposits.
- Insulin injection site rotation is a crucial aspect of treatment and prevention of AIns amyloidosis.
Testosterone Pellet–Induced Generalized Drug Eruption
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
To the Editor:
Testosterone-replacement therapy (TRT) is indicated for hypogonadism. The benefits of TRT are well documented, with multiple options available for delivery. Testosterone pellet implantation (TPI) is an effective treatment option for hypogonadism with minimal adverse reactions. Availability of TRT is increasing, as facilities are offering off-label applications. Although TPI generally is well tolerated, cutaneous reactions have been documented. We present a patient with drug-induced dermatitis following TPI.
A 51-year-old man with hypogonadism presented with an extremely pruritic rash that began on the left buttock 3 days after receiving his fourth TPI. The patient had received subcutaneous insertions of 8 testosterone pellets (75 mg per pellet every 6 months) to the left buttock. He denied any history of a similar rash. His medical history was remarkable for hyperlipidemia, which was controlled with niacin and omega-3 fatty acids (fish oil). Other medications included glucosamine. Before presenting to our clinic, he was given a 40-mg intramuscular injection of triamcinolone acetonide and trimethoprim-sulfamethoxazole twice daily for 7 days, a methylprednisolone dose pack, and triamcinolone ointment 0.1% twice daily by his primary care physician, all without improvement of the rash.
Physical examination revealed multiple well-circumscribed, coalescing clusters of darkly erythematous papules and dermal plaques of varying size on the buttocks with extension to the lower back, abdomen, and thighs (Figure 1). The differential diagnosis included lichenoid eruption, pseudolymphoma, sarcoidosis, and granuloma annulare.
Histologic examination of a punch biopsy revealed an epidermis with a normal stratum corneum and subtle cell-poor vacuolar interface dermatitis with rare necrotic keratinocytes. There was a mild perivascular lymphocytic infiltrate with slight edema within the dermis without notable eosinophils or findings indicative of a vasculitic process (Figure 2).
Oral prednisone 60 mg daily and betamethasone ointment 0.05% applied twice daily were started, with notable improvement of the rash in 1 week (Figure 3). Given the temporal relationship of the TPI, histologic findings suggestive of drug eruption, and resolution of symptoms shortly after treatment, a diagnosis of testosterone pellet–induced generalized dermatitis was established.
Testosterone-replacement therapy is the principal treatment of male pathologic hypoandrogenism, but off-label prescription frequently occurs for age-related hypogonadism and hypoactive sexual desire disorder.1 Testosterone-replacement therapy also can enhance sexual desire and function and improve mood in premenopausal and postmenopausal women with testosterone deficiency.2 Delivery options include topicals, intramuscular injections, oral formulations, transdermal patches and gels, and subcutaneous placement of testosterone pellets (TPI).Cutaneous reactions to TPI are rare. Hirsutism, male-pattern hair loss, and acne are possible cutaneous adverse reactions.3 In addition, a localized erythematous pruritic eruption at the implantation site and an immunologic foreign-body reaction to testosterone pellets have been reported.4
In one case report, a man developed recurrent ill-defined, erythematous, scaly plaques and patches over the buttocks and thighs, consistent with testosterone-induced eczematous dermatitis, subsequent to his second TPI. The patient presented with the eruption within 4 weeks after the most recent implantation, similar to our case, but differed temporally in initial presentation, presenting after the second implantation.5 Our case differed in morphologic presentation (dermal plaques as opposed to eczematous change) and refractoriness to triamcinolone injection.
Testosterone-replacement therapy is becoming more widely available. Lack of regulation of proper marketing by such facilities as medical spas that offer TPI for off-label applications has led to a rampant increase in TRT prescribing, possibly foreshadowing an increase in adverse cutaneous reactions to TRT.6
Our case of histologically consistent testosterone pellet–induced dermatitis highlights a rare cutaneous adverse reaction that can occur subsequent to TPI and illustrates the efficacy of high-dose oral steroids as a treatment option. With increased use of TRT, physicians should be cognizant of the potential adverse cutaneous effects related to this treatment and counsel patients appropriately prior to initiating treatment.
Acknowledgment
We thank the patient for granting permission to publish this case.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
- Clayton AH, Kingsberg SA, Goldstein I. Evaluation and management of hypoactive sexual desire disorder. Sex Med. 2018;6:59-74.
- Glaser R, Dimitrakakis C. Testosterone therapy in women: myths and misconceptions. Maturitas. 2013;74:230-234.
- Testopel (testosterone pellet) [package insert]. Endo Pharmaceuticals, Inc; 2016. Accessed December 16, 2020. https://dailymed.nlm.nih.gov/dailymed/fda/fdaDrugXsl.cfm?setid=a1741a0b-3d4c-42dc-880d-a06e96cce9ef&type=display
- Cavender RK, Fairall M. Subcutaneous testosterone pellet implant (Testopel) therapy for men with testosterone deficiency syndrome: a single-site retrospective safety analysis. J Sex Med. 2009;6:3177-3192.
- Heldt Manica LA, Cohen PR. Testosterone pellet associated dermatitis: report and review of Testopel-related cutaneous adverse effects. Cureus. 2017;9:e1560.
- Mintzes B. The marketing of testosterone treatments for age-related low testosterone or ‘Low T’. Curr Opin Endocrinol Diabetes Obes. 2018;25:224-230.
Practice Points
- Dermatologists should be aware that testosterone pellet implantation can cause dermatitis overlying the implantation site, which can generalize and differ in morphologic presentation.
- For patients presenting with a suspected case of testosterone pellet–induced dermatitis, a high-dose oral corticosteroid can be deployed as an effective therapy.