User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Tattoo Hypersensitivity Reactions: Inky Business
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
Sometimes regrettable yet increasingly common, tattoos are an ancient art form used in modern times as a mark of artistic and cultural expression. Allergic contact dermatitis (ACD) to tattoo ink is rare, but the popularity of tattoos makes ACD an increasingly recognized occurrence. In a retrospective study of 38,543 patch-tested patients, only 29 (0.08%) had tattoo-related ACD, with the majority of patients being female and young adults. The most common contact allergy was to paraphenylenediamine (PPD), which occurred in 22 (76%) patients.1 In this article, we will walk you through the rainbow of tattoo ACD, covering hypersensitivity reactions to both temporary and permanent tattoo inks.
Temporary Tattoo Inks
Henna is the most common temporary tattoo ink. Derived from the plant Lawsonia inermis, henna is an orange dye that has been used in many parts of the world, particularly in Islamic and Hindu cultures, to dye skin, hair, and fabrics. Application of henna tattoos is common for weddings and other celebrations, and brides may wear elaborate henna patterns. To create these tattoos, henna powder is mixed with water and sometimes essential oils and is then applied to the skin for several hours. After application, the henna pigment lawsone (2-hydroxy-1,4-naphthoquinone) interacts with keratin and leaves a red-orange stain on the skin2; longer application time leads to a deeper color. Most traditional cutaneous henna designs fade in 2 to 6 weeks, but some last longer. Red henna generally is considered safe with low incidence of contact allergy. What is referred to as black henna usually is red henna mixed with PPD, a black dye, which is added to deepen the color. Paraphenylenediamine is highly sensitizing; patients can become sensitized to the PPD in the tattoo itself.2 One study confirmed the presence of PPD in black henna tattoos, with chemical analysis of common preparations revealing concentrations ranging from less than 1% to 30%.2 Patients who undergo patch testing for tattoo reactions often are strongly positive to PPD and have concomitant reactions to azo dyes, black rubber, and anesthetics. Other aromatic amines including aminophenols have been identified in black henna tattoo ink, and these chemicals also may contribute to ACD.3 Less common sources of contact allergy from temporary black henna tattoos include resorcinol,4 para-tertiary butylphenol formaldehyde resin,5 and fragrance.6
Clinically, ACD to PPD in temporary tattoos presents 1 to 3 days after application if the patient is already sensitized or 4 to 14 days if the patient is sensitized by the tattoo ink.2 Most patients notice erythema, edema, vesicles, papules, and/or bullae, but other less common reactions including generalized dermatitis, systemic symptoms, urticaria, and pustules have been described.2 Postinflammatory hypopigmentation or hyperpigmentation also can occur.
Because of the sensitizing nature of black henna tattoos, consumers are turning to natural temporary tattoos. Jagua temporary tattoos, with pigment derived from the sap of fruit from the Genipa americana tree, have been associated with ACD.7 This black dye is applied and washed off in a similar fashion to henna tattoos. Importantly, a recent analysis of jagua dye identified no PPD. In one case, a patient who developed ACD to a jagua tattoo was patch tested to components of the dye and had a positive reaction to genipin, a component of the fruit extract.7 Thus, jagua tattoos often are marketed as safe but are an emerging source of contact dermatitis to temporary tattoos.
Permanent Tattoo Inks
Permanent tattoos are created by injecting small amounts of ink into the dermis. As the name suggests, these tattoos are permanent. Tattoos are common; nearly one-third of Americans have at least 1 tattoo.1 Historically, tattoos were created using black pigment composed of amorphous carbon or black iron oxides.8,9 Metallic pigments (eg, mercury, chromium, cobalt, cadmium) were once used to add color to tattoos, but these metals are now only rarely used; in fact, a 2019 study of tattoo ink components identified 44 distinct pigments in 1416 permanent inks, with an average of 3 pigments per ink.8 Of the 44 pigments, 10 had metallic components including iron, barium, zinc, copper, molybdenum, and titanium. The remaining 34 pigments contained carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes. The authors noted that nearly one-quarter of the tattoo pigments identified in their study had been reported as contact allergens.8
Typically, reactions to permanent tattoo inks manifest as an eczematous dermatitis occurring weeks to years after tattoo application.9,10 The dermatitis usually is locally confined to the tattoo and may be limited to particular colors; occasionally, a new tattoo reaction may trigger concurrent inflammation in older tattoos. Many tattoo reactions occur as a response to red pigment but also have occurred with other tattoo ink components.9 Many researchers have speculated as to whether the reaction is related to the ink component itself or from the photochemical breakdown of the ink by exposure to UV radiation and/or laser therapy.9
Red Pigment
Red ink is the most common color reported to cause tattoo hypersensitivity reactions. Historically, red tattoo pigments include mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal,11 but today’s tattoo inks primarily are composed of other pigments, such as quinacridone and azo dyes.12 Several cases of red tattoo ink hypersensitivity reactions exist in the literature, many without completion of patch tests or without positive patch tests to relevant red pigments.11-15
Black Pigment
In general, reactions to permanent black tattoo ink are rare; however, a few case reports exist. Black pigment can be created with India ink (carbon), logwood (chrome), iron oxide, and titanium.16,17 Shellac can be used as a binding agent in tattoo ink; there is at least one report of a reaction to black tattoo ink with a positive patch test to shellac and the original black ink.18
Metals
When utilized in tattoos, metals can create a variety of colors; several have been reported to cause ACD. There has been at least one reported case of a tattoo hypersensitivity reaction to a gold tattoo, with positive patch testing for gold sodium thiosulfate.19 Green tattoo inks also have been confirmed to contain metal. One case of nickel allergy from a green tattoo has been reported, with a positive patch test for nickel sulfate and tissue confirmation of the presence of nickel with micro X-ray fluorescence and laser ablation inductively coupled plasma mass spectrometry.20 Another case series described 3 patients with pruritus and chronic dermatitis associated with green tattoos who had positive patch tests to potassium dichromate, and the green tattoo pigment flared during patch testing. Chromium oxide was presumed to be present in the green tattoo pigment, and potassium dichromate avoidance in products and food improved both the pruritus and dermatitis.21
Azo Pigments
Azo pigments frequently are used in modern tattoos due to their vibrant colors. One case of hypersensitivity to azo pigment involved an eczematous ulcerated plaque overlying yellow, red, and green ink in a recently applied tattoo. Patch testing with the inks originally used in the tattoo was negative. The authors noted that the 3 problematic ink colors all contained pigment yellow 65—an azo pigment—and attributed the reaction to this dye.22 In another azo reaction, a patient had erythema and pruritus overlying a tattoo applied 1 month prior. Patch testing was positive for aminoazobenzene, an azo pigment that was present in the orange ink of the tattoo.23
Management of Tattoo Hypersensitivity Reactions
Hypersensitivity reactions to temporary tattoos are just that—temporary. Topical steroids and time generally will allow these reactions to resolve. In the setting of vigorous reactions, patients may develop postinflammatory hypopigmentation or hyperpigmentation that may last for months. Unfortunately, bullous tattoo reactions can lead to scarring and keloid formation, requiring more aggressive therapy.
Management of reactions to permanent tattoos is more challenging. High-potency topical steroids under occlusion or intralesional corticosteroid injections may aid in treating pruritus or discomfort. For severe reactions, oral corticosteroids may be required. Patients also may consider laser tattoo removal; however, providers should be aware that there have been rare reports of systemic urticarial reactions from this procedure.24,25 Obviously limited by location and size, excision also may be offered.
Patch Testing for Tattoo Ink Contact Allergy
When patients present for evaluation and management of tattoo ACD, it is important to also consider other causes, including granulomatous tattoo reaction, pseudolymphoma, and lichenoid tattoo reaction. A biopsy can be helpful if the diagnosis is in question.
Patch testing for contact allergy to temporary tattoo inks should include PPD, fragrance, aminophenols, resorcinol, para-tertiary butylphenol formaldehyde, and essential oils. Jagua currently is not available for commercial purchase but also should be considered if the patient has the original product or in research settings. If the individual tattoo ingredients can be identified, they also should be tested. In this scenario, recall reactions may occur; testing with the tattoo paste should be avoided if the prior reaction was severe. Importantly, patients with a PPD allergy should be counseled to avoid hair dyes that contain PPD. Many patients who are sensitized to PPD have strong reactions on patch testing and are at risk for severe reactions if PPD or PPD-related compounds are encountered in hair dye.
Patch testing for ACD to permanent tattoos is complex. In most cases, patch testing is of limited utility because many of the chemicals that have been reported to cause ACD are not commercially available. Additionally, a 2014 study of 90 patients with chronic tattoo reactions found that the majority had negative patch testing to the European baseline series (66%), disperse dyes (87%), and tattoo inks (87%–92%). The investigators theorized that the allergens causing tattoo reactions are formed by haptenization of “parent” chemicals in the dermis, meaning application of chemicals present in the original tattoo ink may not identify the relevant allergen.26 If patch testing is performed, it is most ideal if individual pigment ingredients can be identified. Allergens to be considered for testing include azo dyes, aromatic amines, iron oxide, barium, zinc, copper, molybdenum, titanium, gold sodium thiosulfate, nickel sulfate, carbon, shellac, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), quinophthalone (yellow) dyes, mercuric sulfide (vermilion, cinnabar), scarlet lake, cadmium red, carmine, and cochineal, many of which are not commercially available for purchase.
Final Interpretation
As tattoos become increasingly trendy, tattoo ACD should be recognized by the astute dermatologist. The most common allergen associated with tattoo ACD is PPD, but other potential allergens include azo dyes and newer pigments. Unlike tattoos of the past, today’s inks are unlikely to contain toxic metals. Diagnosing ACD caused by permanent tattoo inks requires a high degree of suspicion, as patch testing may be of limited utility.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
- Warshaw EM, Schlarbaum JP, Taylor JS, et al. Allergic reactions to tattoos: retrospective analysis of North American Contact Dermatitis Group data, 2001-2016. J Am Acad Dermatol. 2020;82:E61-E62.
- de Groot AC. Side-effects of henna and semi-permanent ‘black henna’ tattoos: a full review. Contact Dermatitis. 2013;69:1-25.
- Romita P, Foti C, Mascia P, et al. Eyebrow allergic contact dermatitis caused by m-aminophenol and toluene-2,5-diamine secondary to a temporary black henna tattoo. Contact Dermatitis. 2018;79:51-52.
- Ormerod E, Hughes TM, Stone N. Allergic contact dermatitis caused by resorcinol following a temporary black henna tattoo. Contact Dermatitis. 2017;77:187-188.
- Rodrigo-Nicolás B, de la Cuadra J, Sierra C, et al. Contact dermatitis from a temporary tattoo in a boy with contact allergy to p-tert butyl phenol formaldehyde resin. Dermatitis. 2014;25:37-38.
- Temesvári E, Podányi B, Pónyai G, et al. Fragrance sensitization caused by temporary henna tattoo. Contact Dermatitis. 2002;47:240.
- Bircher AJ, Scherer Hofmeier K, Schlegel U, et al. Genipin in temporary jagua tattoos—black dye causing severe allergic dermatitis. Dermatitis. 2019;30:375-376.
- Liszewski W, Warshaw EM. Pigments in American tattoo inks and their propensity to elicit allergic contact dermatitis. J Am Acad Dermatol. 2019;81:379-385.
- Serup J, Hutton Carlsen K, Dommershausen N, et al. Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermatitis. 2020;82:73-82.
- Bjerre RD, Ulrich NH, Linneberg A, et al. Adverse reactions to tattoos in the general population of Denmark. J Am Acad Dermatol. 2018;79:770-772.
- Bhardwaj SS, Brodell RT, Taylor JS. Red tattoo reactions. Contact Dermatitis. 2003;48:236-237.
- Gaudron S, Ferrier-Le Bouëdec MC, Franck F, et al. Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermatitis. 2015;72:97-105.
- de Winter RW, van der Bent SAS, van Esch M, et al. Allergic reaction to red cosmetic lip tattoo treated with hydroxychloroquine. Dermatitis. 2019;30:82-83.
- Greve B, Chytry R, Raulin C. Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermatitis. 2003;49:265-266.
- Ruiz-Villaverde R, Fernandez-Crehuet P, Aguayo-Carreras P, et al. Inflammatory reactions to red tattoo inks: three cases highlighting an emerging problem. Sultan Qaboos Univ Med J. 2018;18:E215-E218.
- Gallo R, Parodi A, Cozzani E, et al. Allergic reaction to India ink in a black tattoo. Contact Dermatitis. 1998;38:346-347.
- de Cuyper C, Lodewick E, Schreiver I, et al. Are metals involved in tattoo-related hypersensitivity reactions? a case report. Contact Dermatitis. 2017;77:397-405.
- González-Villanueva I, Hispán Ocete P, Silvestre Salvador JF. Allergic contact dermatitis caused by a black tattoo ink in a patient allergic to shellac. Contact Dermatitis. 2016;75:247-248.
- Tammaro A, Tuchinda P, Persechino S, et al. Contact allergic dermatitis to gold in a tattoo: a case report. Int J Immunopathol Pharmacol. 2011;24:1111-1113.
- van der Bent SAS, Berg T, Karst U, et al. Allergic reaction to a green tattoo with nickel as a possible allergen. Contact Dermatitis. 2019;81:64-66.
- Jacob SE, Castanedo-Tardan MP, Blyumin ML. Inflammation in green (chromium) tattoos during patch testing. Dermatitis. 2008;19:E33-E34.
- González-Villanueva I, Álvarez-Chinchilla P, Silvestre JF. Allergic reaction to 3 tattoo inks containing pigment yellow 65. Contact Dermatitis. 2018;79:107-108.
- Tammaro A, De Marco G, D’Arino A, et al. Aminoazobenzene in tattoo: another case of allergic contact dermatitis. Int J Dermatol. 2017;56:E79-E81.
- Willardson HB, Kobayashi TT, Arnold JG, et al. Diffuse urticarial reaction associated with titanium dioxide following laser tattoo removal treatments. Photomed Laser Surg. 2017;35:176‐180.
- England RW, Vogel P, Hagan L. Immediate cutaneous hypersensitivity after treatment of tattoo with Nd:YAG laser: a case report and review of the literature. Ann Allergy Asthma Immunol. 2002;89:215‐217.
- Serup J, Carlsen KH. Patch test study of 90 patients with tattoo reactions: negative outcome of allergy patch test to baseline batteries and culprit inks suggests allergen(s) are generated in the skin through haptenization. Contact Dermatitis. 2014;71:255-263.
Practice Points
- Temporary tattoo pigments include red henna, black henna, and jagua.
- Black henna tattoos contain paraphenylenediamine, the most common allergen in temporary tattoos.
- Modern permanent tattoo ink components include metals, carbon, azo, diketopyrrolopyrrole, quinacridone, anthraquinone, dioxazine (purple), or quinophthalone (yellow) dyes.
- Patch testing for tattoo contact allergy is complex and challenging.
Are You Up-to-date on Dermal Fillers?
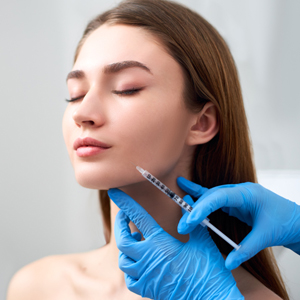
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
The popularity of injectable fillers for aesthetic use continues to rise, and cosmetic injectors must select from an increasing range of options to achieve optimal outcomes. In addition to formulating a treatment plan and having an intimate knowledge of the facial anatomy, filler selection is critical along with an appreciation of both approved and off-label indications for these products. Appropriate patient selection and treatment technique can minimize adverse events (AEs) and allow for the best outcomes.
The US Food and Drug Administration (FDA) approved the first injectable hyaluronic acid (HA) filler in 2003, the first addition since the approval of bovine collagen in 1981. To date, there are now 4 groups of approved fillers: (1) HA (Belotero Balance [Merz North America, Inc], Juvèderm products [Allergan], Restylane products [Galderma Laboratories, LP], Resilient HA products [Revance Therapeutics Inc and Teoxane SA]), (2) calcium hydroxylapatite (Radiesse [Merz North America, Inc]), (3) poly-L-lactic acid (Sculptra Aesthetic [Galderma Laboratories, LP]), and (4) polymethylmethacrylate (Bellafill [Suneva Medical, Inc]).1-3 Given the versatility of this wide portfolio of products, which often are used in combination with one another, we have advanced from the early goals of filling isolated lines or wrinkles on the face to the 3-dimensional restructuring of an entire treatment area. The increasing diversity of products, particularly the range of rheologic properties of HA fillers, allows the injector to strategically select the type of filler and depth of injection to achieve the desired treatment outcome. The duration of the treatment effects also is related to the properties of the filler.4,5
Advancements in injectable fillers also have led to new applications both on and off the face. Many pivotal clinical trials of fillers were performed in isolated anatomic areas, most commonly the nasolabial folds, leading to FDA approval of this indication. Other FDA-approved indications for fillers include lip augmentation (Juvèderm Ultra, Juvèderm Volbella, Restylane, Restylane Silk, Restylane Kysse), human immunodeficiency virus–associated lipoatrophy (Sculptra Aesthetic, Radiesse), volumization of the dorsal hands (Radiesse, Restylane Lyft), acne scarring (Bellafill), and age-related volume loss of the midface (Juvèderm Voluma, Restylane Lyft). Although it is considered off label, treatment of the temples, brows, tear troughs, jawline, horizontal neck lines, and etched-in radial cheek lines has been reported.6-9 It is legal to use fillers to treat these areas, but data have not yet been evaluated by the FDA to officially grant their approval, which likely will change with the conclusion of many ongoing industry-sponsored trials.
Adverse events from filler injections range from the anticipated transient tenderness, swelling, and bruising, which are likely to resolve in a matter of days, to the most severe complications—intravascular occlusion with permanent sequelae, namely tissue necrosis, blindness or visual compromise, and stroke. It is critical to obtain written informed consent prior to proceeding with dermal filler injections. Masterful knowledge of the facial anatomy, in particular the location and depth of key vascular structures, is critical in minimizing these severe AEs. Injection technique, including use of a microcannula, can reduce the risk, in addition to administration of small volumes of filler at a time, aspiration prior to injection, and use of a retrograde injection technique. There also are variations in the predicted courses of vascular structures, as demonstrated in a cadaveric study showing 4 variants of the course of the angular artery.10
Hyaluronic acid fillers are the most commonly used of the available products, and hyaluronidase, which can dissolve the filler, can be utilized to manage emergent and nonemergent AEs.11 Physical examination findings related to impending necrosis include blanching of the skin in the distribution of a key vessel with a mottled or reticulated purple discoloration. Hyaluronidase, on the order of hundreds of units, may be injected into the area of vascular compromise until reperfusion is achieved, in addition to administering aspirin and applying warm compresses to the area.11,12 The most severe AEs are blindness and/or stroke, associated with findings such as immediate vision loss, pain, nausea, vomiting, and neurologic compromise. Although the glabella, nose, nasolabial folds, and forehead are the most common anatomic areas associated with these AEs (in order of frequency), injections in all areas of the face have been associated with blindness.13,14 Retrobulbar and/or peribulbar injection of hyaluronidase for management of vision changes has been reported, but in most cases vision loss associated with dermal filler injections is not reversible.14,15
Nonemergent uses of enzyme reversal of filler placement include correcting undesirable aesthetic outcomes, such as asymmetry, misplaced filler, or even delayed granulomatous reactions. Hyaluronidase dosage should be determined by the amount and type of filler that was delivered to the patient. All HA fillers are not created equally, and evidence from dosing studies indicates that higher cross-linked and more cohesive fillers require higher doses of hyaluronidase.11 For example, Juvèderm Voluma, created as a mixture of low- and high-molecular-weight HA, has a higher cross-linking ratio. Approximately 30 U of hyaluronidase are suggested to dissolve 0.1 cc of Juvèderm Voluma as compared to 10 U of hyaluronidase for 0.1 cc of Juvèderm Ultra and 5 U for 0.1 cc of Restylane.11
Treatment with dermal fillers generally is safe and effective, and as new fillers come to the market, we must identify how they will help further our goal of improving patient outcomes. The effects of coronavirus disease 19 on aesthetic medicine are still unclear, yet this uncertainty should not deflect treating clinicians from overlooking the fundamentals of dermal fillers. In addition to considering the appropriate use of each filler based on its unique characteristics and indications, we must be sure that we are prepared with the tools to manage any and all possible complications.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.
- Jiang B, Ramirez M, Ranjit-Reeves R, et al. Noncollagen dermal fillers: a summary of the clinical trials used for their FDA approval. Dermatol Surg. 2019;45:1585-1596.
- Monheit G, Kaufman-Janette J, Joseph J, et al. Efficacy and safety of two resilient hyaluronic acid fillers in the treatment of moderate-to-severe nasolabial folds [published online March 24, 2020]. Dermatol Surg. doi:10.1097/DSS0000000000002391.
- Kaufman-Janette J, Taylor SC, Cox SE, et al. Efficacy and safety of a new resilient hyaluronic acid dermal filler, in the correction of moderate-to-severe nasolabial folds: a 64-week, prospective, multicenter, controlled, randomized, double-blind and within-subject study. J Cosmet Dermatol. 2019;18:1244-1253.
- Jones D, Murphy D. Volumizing hyaluronic acid filler for midface volume deficit: 2 year results from a pivotal single-blind randomized controlled study. Dermatol Surg. 2013;39:1602-1611.
- Hausauer AK, Jones DH. Long-term correction of iatrogenic lipoatrophy with volumizing hyaluronic acid filler. Dermatol Surg. 2018;44(suppl 1):S60-S62.
- Black J, Jones D. Cohesive polydensified matrix hyaluronic acid for the treatment of etched-in fine facial lines: a 6-month, open-label clinical trial. Dermatol Surg. 2018;44:1002-1011.
- Breithaupt A, Jones D, Braz A, et al. Anatomic basis for safe and effective volumization of the temple. Dermatol Surg. 2015;41:S278-S283.
- Dallara JM, Baspeyras M, Bui P, et al. Calcium hydroxylapatite for jawline rejuvenation: consensus recommendations. J Cosmet Dermatol. 2014;13:3-14.
- Minokadeh A, Black J, Jones D. Effacement of transverse neck lines with VYC-15L and a cohesive polydensified matrix hyaluronic acid. Dermatol Surg. 2019;45:941-948.
- Kim YS, Choi DY, Gil YC, et al. The anatomical origin and course of the angular artery regarding its clinical implications. Dermatol Surg. 2014;40:1070-1076.
- Jones DH. Update on emergency and nonemergency use of hyaluronidase in aesthetic dermatology. JAMA Dermatol. 2018;154:763-764.
- Cohen JL, Biesman BS, Dayan SH, et al. Treatment of hyaluronic acid filler-induced impending necrosis with hyaluronidase: consensus recommendations. Aesthet Surg J. 2015;35:844-849.
- Beleznay K, Carruthers J, Humphrey S, et al. Avoiding and treating blindness from fillers: a review of the world literature. Dermatol Surg. 2015;41:1097-1117.
- Beleznay K, Carruthers J, Humphrey S, et al. Update on avoiding and treating blindness from fillers: a recent review of the world literature. Aesthet Surg J. 2019;39:662-674.
- Chestnut C. Restoration of visual loss with retrobulblar hyaluronidase injection after hyaluronic acid filler. Dermatol Surg. 2018;44:435-437.
Wellness for the Dermatology Resident
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
Resident wellness is a topic that has become increasingly important in recent years due to physician burnout. A prior Cutis Resident Corner column discussed the prevalence of physician burnout and how it can affect dermatologists.1 When discussing resident burnout, dermatology may not be a specialty that immediately comes to mind, considering that dermatology is mostly outpatient based, with few emergencies and critically ill patients. In a JAMA study assessing levels of burnout by specialty, dermatology residents were the lowest at approximately 30%.2 However, this still means that 3 out of every 10 dermatology residents feel burnt out.
Burnout in Dermatology
In 2017, results from a survey of 112 dermatology residents in Canada about burnout was published in the British Journal of Dermatology.3 The numbers were staggering; the results showed that more than 50% of dermatology residents experienced high levels of emotional exhaustion and depersonalization, and 40% had low levels of personal accomplishment. Additionally, 52% experienced low or depressed mood, 20% reported feelings of hurting themselves within the last year, and more than 25% had high anxiety levels.3
Dermatology requires a high level of daily studying, which is a major source of stress for many dermatology residents. The survey of dermatology residents in Canada showed that the top stressor for 61% of survey respondents was studying, specifically for the board examination.3 Dermatology is an academically rigorous specialty. We are responsible for recognizing every disease process affecting the skin, including hundreds that are extremely uncommon. We must understand these disease processes at a molecular level from a basic science standpoint and at a microscopic level through our knowledge of dermatopathology. Much of what we see in clinic are bread-and-butter dermatologic conditions that do not necessarily correlate with the rare diseases that we study. This differs from other specialties in which residents learn much of their specialty knowledge through their clinical work.
Current Challenges
We are training in a uniquely challenging time, providing care for our patients amid the coronavirus disease 2019 pandemic. Many of us are dealing with constant levels of stress and worry about the health and safety of ourselves, along with our friends, families, and patients. Some residents have been redeployed to work in unfamiliar roles in the emergency department or hospital wards, while others adjust to new roles in teledermatology. I also cannot talk about resident wellness without recognizing the challenges faced by physicians who are racial and religious minorities. This is especially true for black physicians, as they face unconscious biases and microaggressions daily derived from implicit racism; this leads to discrimination in every area of life and ultimately harms their emotional and psychological well-being.4 Additionally, black physicians are underrepresented in dermatology, making up only 4.3% of dermatology residents in the 2013-2014 academic year.5,6 Underrepresentation can serve as a major stressor for racial and religious minorities and should be considered when addressing resident wellness to ensure their voices are heard and validated.
Focusing on Wellness
What can we do to improve wellness? A viewpoint published in JAMA Surgery in 2015 by Salles et al7 from the Stanford University Department of Surgery (Stanford, California) discussed their Balance in Life (BIL) program, which was established after one of their residency graduates tragically died by suicide shortly after graduating from residency. The BIL program addresses 4 different facets of well-being—professional, physical, psychological, and social—and lists the specific actions taken to improve these areas of well-being.7
I completed my transitional year residency at St. Vincent Hospital (Indianapolis, Indiana). The program emphasizes the importance of resident wellness. They established a department-sponsored well-being program to improve resident wellness,8 with its objectives aligning with the 4 areas of well-being that were outlined in the Stanford viewpoint.7 A short Q&A with me was published in the supplemental material as a way of highlighting their residents.9 I will outline the 4 areas of well-being, with suggestions based on the Stanford BIL program, the well-being innovation program at St. Vincent, and initiatives at my current dermatology residency program at the University of Wisconsin (UW) in Madison.
The 4 Areas of Well-being
Stanford BIL Program
One of the changes implemented was starting a resident mentorship program. Each junior resident selects a senior resident as a mentor with department-sponsored quarterly lunch meetings.7 Another initiative is a leadership training program, which includes an outdoor rope course each year focusing on leadership and team building.7
UW Dermatology
Monthly meetings are held with our program director and coordinator so that we can address any concerns or issues as they arise and brainstorm solutions together. During the coronavirus disease 2019 pandemic, we had weekly resident town halls with department leadership with transparency about our institution’s current status.
Physical Well-being
Stanford BIL Program
One method of improving physical well-being included stocking healthy snacks for residents and providing incoming residents with a guide of physicians, dentists, and fitness venues to promote regular health care. We have adopted the same at UW with healthy snacks available in our resident workroom.
St. Vincent Internal Medicine Wellness
There are monthly fitness challenges for a variety of physical wellness activities such as sleep, mindfulness minutes, nutrition, and step challenges.8
UW Dermatology
In addition to healthy snacks in our workroom, we also have various discounted fitness classes available for employees, along with discounts on gym memberships, kayak rentals, and city bike-share programs.
Psychological Well-Being
Stanford BIL Program
They enlisted a clinical psychologist available for residents to talk to regularly about any issues they face and to help manage stress in their lives.7
St. Vincent Internal Medicine Wellness
Faculty and coordinators provide S.A.F.E.—secure, affirming, friendly, and empathetic—zones to provide confidential and judgment-free support for residents.8 They also host photography competitions; residents submit photographs of nature, and the winning photographs are printed and displayed throughout the work area.
UW Dermatology
We have made changes to beautify our resident workroom with photograph collages of residents and other assorted décor to make it a more work-friendly space.
Social Well-being
Common themes highlighted by all 3 programs include the importance of socializing outside of the workplace, team-building activities, and resident retreats. Social media accounts on Instagram at St. Vincent (@stvimresidency) and at UW (@uwderm) highlight resident accomplishments and promote interconnectedness when residents are not together in clinics or hospitals.
Final Thoughts
Resident wellness will continue to be an important topic for discussion in the future, especially given the uncertain times right now during our training. Focusing on the 4 areas of well-being can help to prevent burnout and improve resident wellness.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
- Croley JAA. #Dermlife and the burned-out resident. Cutis. 2019;104:E32-E33.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among US resident physicians. JAMA. 2018;320:1114-1130.
- Shoimer I, Patten S, Mydlarski PR. Burnout in dermatology residents: a Canadian perspective [published online November 1, 2017]. Br J Dermatol. 2018;178:270-271.
- Grills CN, Aird EG, Rowe D. Breathe, baby, breathe: clearing the way for the emotional emancipation of black people. Cultural Studies & Critical Methodologies. 2016;16:333-343.
- Imadojemu S, James WD. Increasing African American representation in dermatology. JAMA Dermatol. 2016;152:15-16.
- Brotherton SE, Etzel SI. Graduate medical education, 2013-2014. JAMA. 2014;312:2427-2445.
- Salles A, Liebert CA, Greco RS. Promoting balance in the lives of resident physicians: a call to action. JAMA Surg. 2015;150:607-608.
- Fick L, Axon K, Potini Y, et al. Improving overall resident and faculty wellbeing through program-sponsored innovations. MedEdPublish. Published September 27, 2019. doi:10.15694/mep.2019.000184.1.
- St. Vincent Internal Medicine Residency Wellness Bulletin. https://www.mededpublish.org/manuscriptfiles/2586/Supplementary%20File%203_Wellness%20Bulletin.pdf. Published April 2018. Accessed August 5, 2020.
Resident Pearls
- Resident wellness is an important issue affecting resident physicians of all specialties, including dermatology.
- To improve wellness, changes can be made by targeting the following 4 areas of well-being: professional, physical, psychological, and social.
Apremilast and Systemic Retinoid Combination Treatment for Moderate to Severe Palmoplantar Psoriasis
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).

Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
To the Editor:
Psoriasis is a chronic inflammatory papulosquamous skin disease affecting 2% to 3% of the population.1 Its pathogenesis is multifactorial, consisting of a disrupted skin barrier and dysregulated immune activation.2
A wide armamentarium of topical and systemic treatments targeting different aspects of the disease pathogenesis have been developed over the years.3,4 Psoriasis was once considered a skin disease exclusively, but accumulating evidence suggests that it is accompanied by a multitude of systemic inflammatory comorbidities.5 This insight supports the concept of systemic treatment for patients with moderate to severe psoriasis. As a chronic disease, psoriasis requires continuous therapy. The treatment approach should focus on achieving efficacy and minimizing side effects. These goals can be achieved by combination, rotational, and sequential treatment approaches.6 Many therapeutic combinations have proven effective, using beneficially different mechanisms of action (MOAs) and toxicity profiles.7 We present a patient with moderate to severe recalcitrant palmoplantar psoriasis who demonstrated improvement with combination therapy.
A 50-year-old man presented with palmoplantar psoriasis of 7 years’ duration. His medical history included mild hyperlipidemia treated with atorvastatin. Prior topical treatments including calcipotriene, betamethasone dipropionate, and tacrolimus ointment did not result in improvement. Persistent acral involvement required further intervention, and the excimer laser was added to the therapeutic regimen with a minor additive therapeutic value. Acitretin (25 mg/d) was initiated; however, the disease flared up soon after. Acitretin was discontinued, and the patient was treated with apremilast (30 mg twice daily) for 9 months with a slight improvement. Physical examination revealed erythematous, fissured, scaly plaques involving both the palms and soles. Acitretin (25 mg/d) was reintroduced to the therapeutic regimen, and the acitretin-apremilast combination was used for 2 months. With this regimen, the patient experienced 90% improvement (Figures 1 and 2).


Palmoplantar psoriasis is a debilitating dermatosis that is extremely challenging to treat and is unresponsive to many modalities.8 Increased understanding of psoriasis mechanisms paved the path for the development of highly targeted biologic therapies9 with fewer side effects than drugs such as cyclosporine that indiscriminately neutralize multiple components of the immune system. Although highly specific, these targeted approaches are not without side effects10 and lead to diverse therapeutic outcomes, particularly when prescribed for palmoplantar psoriasis.11,12
The small-molecule inhibitor of phosphodiesterase 4—apremilast—was approved for plaque psoriasis treatment in late 2014. Although not fully elucidated, its MOA involves interfering with intracellular signaling, leading to increased intracellular cyclic adenosine monophosphate levels in inflammatory cells and keratinocytes.13 Proximal interruption of the pathologic cascade leads to the reduction of multiple proinflammatory cytokines with a simultaneous increase in anti-inflammatory mediators.13 Its efficacy and safety in the treatment of psoriasis have been shown in phase 2 and 3 clinical trials.14,15 In contrast to traditional oral therapies for psoriasis (ie, methotrexate, cyclosporine, acitretin), no laboratory test monitoring is needed and the safety profile is notably better.16
Acitretin, the active metabolite of etretinate, modulates epidermal differentiation and has immunomodulating activities.17 It commonly is used for treating palmoplantar psoriasis.8 Until recently, it was the only nonimmunosuppressive systemic treatment for psoriasis, and its combination with other systemic treatments, particularly biologics, has been advocated.18 Prior reports showed remarkable disease improvement when combining acitretin with alefacept, etanercept, infliximab, adalimumab, and ustekinumab.19 The optimal combination should include modalities with different MOAs without overlapping toxicities.19 Apremilast and acitretin have different MOAs and side-effect profiles, but another theoretical advantage is that they both interfere with intracellular signaling on the transcription level rather than affecting extracellular targets.13
Our patient with moderate to severe recalcitrant palmoplantar psoriasis demonstrated approximately 90% improvement following apremilast and acitretin combination therapy. This treatment regimen should be considered in cases of persistent acral disease resistant to other therapeutic efforts.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Nograles KE, Davidovici B, Krueger JG. New insights in the immunologic basis of psoriasis. Semin Cutan Med Surg. 2010;29:3-9.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 4. guidelines of care for the management and treatment of psoriasis with traditional systemic agents. J Am Acad Dermatol. 2009;61:451-485.
- Menter A, Korman NJ, Elmets CA, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 3. guidelines of care for the management and treatment of psoriasis with topical therapies. J Am Acad Dermatol. 2009;60:643-659.
- Ryan C, Kirby B. Psoriasis is a systemic disease with multiple cardiovascular and metabolic comorbidities. Dermatol Clin. 2015;33:41-44.
- Lebwohl M, Menter A, Koo J, et al. Combination therapy to treat moderate to severe psoriasis. J Am Acad Dermatol. 2004;50:416-430.
- Cather JC, Menter A. Combining traditional agents and biologics for the treatment of psoriasis. Semin Cutan Med Surg. 2005;24:37-45.
- Janagond AB, Kanwar AJ, Handa S. Efficacy and safety of systemic methotrexate vs. acitretin in psoriasis patients with significant palmoplantar involvement: a prospective, randomized study. J Eur Acad Dermatol Venereol. 2013;27:E384-E389.
- Campa M, Mansouri B, Warren R, et al. A review of biologic therapies targeting IL-23 and IL-17 for use in moderate-to-severe plaque psoriasis [published online December 29, 2015]. Dermatol Ther (Heidelb). 2015;6:1-12.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Jacobi A, Schuler G, Hertl M. Differential clinical response to alefacept in combination with methotrexate in two patients with refractory palmar psoriasis. Br J Dermatol. 2007;156:178-180.
- Meyer V, Goerge T, Luger TA, et al. Successful treatment of palmoplantar hyperkeratotic psoriasis with a combination of etanercept and alitretinoin. J Clin Aesthet Dermatol. 2011;4:45-46.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Papp K, Reich K, Leonardi CL, et al. Apremilast, an oral phosphodiesterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1). J Am Acad Dermatol. 2015;73:37-49.
- Paul C, Cather J, Gooderham M, et al. Efficacy and safety of apremilast, an oral phosphodiesterase 4 inhibitor, in patients with moderate-to-severe plaque psoriasis over 52 weeks: a phase III, randomized controlled trial (ESTEEM 2). Br J Dermatol. 2015;173:1387-1399.
- Zerilli T, Ocheretyaner E. Apremilast (Otezla): a new oral treatment for adults with psoriasis and psoriatic arthritis. P T. 2015;40:495-500.
- Pilkington T, Brogden RN. Acitretin—a review of its pharmacology and therapeutic use. Drugs. 1992;43:597-627.
- Lebwohl M. Combining the new biologic agents with our current psoriasis armamentarium. J Am Acad Dermatol. 2003;49:S118-S124.
- Heinecke GM, Luber AJ, Levitt JO, et al. Combination use of ustekinumab with other systemic therapies: a retrospective study in a tertiary referral center. J Drugs Dermatol. 2013;12:1098-1102.
Practice Points
- Palmoplantar psoriasis is challenging to treat and is unresponsive to many modalities.
- Combination, rotational, and sequential treatment approaches may minimize side effects and loss of efficacy as well as enhance treatment responses.
- Apremilast and acitretin combination therapy led to 90% skin improvement in a case of severe recalcitrant palmoplantar psoriasis.
Extensive Purpura and Necrosis of the Leg
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.
Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
The Diagnosis: Disseminated Mucormycosis
Histopathologic examination of a 6-mm punch biopsy of the edge of the lesion revealed numerous intravascular, broad, nonseptate hyphae in the deep vessels and perivascular dermis that stained bright red with periodic acid-Schiff (Figure). Acid-fast bacilli and Gram stains were negative. Tissue culture grew Rhizopus species. Given the patient's overall poor prognosis, her family decided to pursue hospice care following this diagnosis.

Mucormycosis (formerly zygomycosis) refers to infections from a variety of genera of fungi, most commonly Mucor and Rhizopus, that cause infections primarily in immunocompromised individuals.1 Mucormycosis infections are characterized by tissue necrosis that results from invasion of the vasculature and subsequent thrombosis. The typical presentation of cutaneous mucormycosis is a necrotic eschar accompanied by surrounding erythema and induration.2 Diagnosis is based on clinical suspicion, requiring additional testing with skin biopsy and tissue cultures for confirmation.
Cutaneous infection is the third most common presentation of mucormycosis, following rhinocerebral and pulmonary involvement.1 Although rhinocerebral and pulmonary infections normally are caused by inhalation of spores, cutaneous mucormycosis typically is caused by local inoculation, often following skin trauma.2 The skin is the most common location of iatrogenic mucormycosis, often from skin injury related to surgery, catheters, and adhesive tape.3 Most patients with cutaneous mucormycosis have underlying conditions such as hematologic malignancies, diabetes mellitus, or immunosuppression.1 However, outbreaks have occurred in immunocompetent patients following natural disasters.4 Cutaneous mucormycosis disseminates in 13% to 20% of cases in which mortality rates typically exceed 90%.1
Treatment consists of prompt surgical debridement and antifungal agents such as amphotericin B, posaconazole, and isavuconazonium sulfate.1 Our patient had multiple risk factors for infection, including hematopoietic stem cell transplantation, prolonged neutropenia, and treatment with eculizumab, a monoclonal antibody against C5 that blocks the terminal complement cascade. Eculizumab has been associated with increased risk for meningococcemia,5 but the association with mucormycosis is rare. We highlight the importance of recognizing and promptly diagnosing cutaneous mucormycosis given the difficulty of treating this disease and its poor prognosis.
Disseminated aspergillosis demonstrates septate rather than nonseptate hyphae on biopsy. Disseminated intravascular coagulation and purpura fulminans may be associated with thrombocytopenia but demonstrate thrombotic microangiopathy on biopsy. Pyoderma gangrenosum demonstrates neutrophilic infiltrate on biopsy.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
- Roden MM, Zaoutis TE, Buchanan WL, et al. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41:634-653.
- Petrikkos G, Skiada A, Lortholary O, et al. Epidemiology and clinical manifestations of mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S23-S34.
- Rammaert B, Lanternier F, Zahar JR, et al. Healthcare-associated mucormycosis. Clin Infect Dis. 2012;54(suppl 1):S44-S54.
- Neblett Fanfair R, Benedict K, Bos J, et al. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012;367:2214-2225.
- McNamara LA, Topaz N, Wang X, et al. High risk for invasive meningococcal disease among patients receiving eculizumab (Soliris) despite receipt of meningococcal vaccine. MMWR Morb Mortal Wkly Rep. 2017;66:734-737.
A 57-year-old woman presented with expanding purpura on the left leg of 2 weeks’ duration following a recent hematopoietic stem cell transplant for refractory diffuse large B-cell lymphoma. Prior to dermatologic consultation, the patient had been hospitalized for 2 months following the transplant due to Clostridium difficile colitis, Enterococcus faecium bacteremia, cardiac arrest, delayed engraftment with pancytopenia, and atypical hemolytic uremic syndrome with acute renal failure requiring hemodialysis and treatment with eculizumab. Her care team in the hospital initially noticed a small purpuric lesion on the posterior aspect of the left knee. The patient subsequently developed persistent fevers and expansion of the lesion, which prompted consultation of the dermatology service. Physical examination revealed a 22×10-cm, rectangular, indurated, purpuric plaque with central dusky, violaceous to black necrosis with superficial skin sloughing and peripheral dusky erythema extending from the inner thigh to the lower leg. The left distal leg felt cool, and both dorsalis pedis and posterior tibial pulses were absent. Laboratory test results revealed neutropenia and thrombocytopenia (white blood cell count, 0.2×103 /mm3 [reference range, 5–10×103 /mm3 ]; hematocrit, 23.2% [reference range, 41%–50%]; platelet count, 105×103 /µL [reference range, 150–350×103 /µL]). A punch biopsy was performed.
Risk Factors and Management of Skin Cancer Among Active-Duty Servicemembers and Veterans
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
Melanoma Risk for Servicemembers
Dr. Dunn: Active-duty jobs are quite diverse. We have had almost every civilian occupation category—everything from clerical to food service to outdoor construction workers. Federal service and active-duty military service could lead to assignments that involve high sunlight exposure and subsequently higher risk for melanoma and nonmelanoma skin cancer.
Dr. Miller: I found 2 articles on the topic. The first published in June 2018 reviewed melanoma and nonmelanoma skin cancers in the military.1 Riemenschneider and colleagues1 looked at 9 studies. Statistically, there was increased risk of melanoma associated with service and/or prisoner-of-war status. In World War II, they found tropical environments had the highest risk. And the highest rates were in the US Air Force.
The other article provided US Department of Defense data on skin cancer incidence rates, incidence rates of malignant melanoma in relation to years of military service overall, and the rates for differing military occupational groups.2 The researchers demonstrated that fixed-wing pilots and crew members had the highest rates of developing melanoma. The general trend was that the incidence rate was exponentially higher with more missions flown in relation to years of active service, which I thought was rather interesting.
For other occupational categories, the rate increase was not as great as those involved in aviation. Yes, it’s probably related to exposure. Flying at 40,000 feet on a transcontinental airplane trip is equivalent to the radiation dosage of a chest X-ray. Given all the training time and operational flying for the Air Force, it is anticipated that that mutagenic radiation would increase rates. An aircraft does not offer a lot of protection, especially in the cockpit.
We just had the anniversary of the Apollo 11 mission. Those astronauts received the equivalent of about 40 chest X-rays going to the moon and back. Exposure to UV and at higher altitudes cosmic radiation explains why we would see that more in Air Force personnel.
Dr. Bandino: At high altitude there is less ozone protecting you, although the shielding in a cockpit is better in modern aircraft. As an Air Force member, that was one of the first things I thought about was that an aviator has increased skin cancer risk. But it’s apt to think of military service in general as an occupational risk because there are so many contingency operations and deployments. Regarding sun exposure, sunscreen is provided nowadays and there is more sun awareness, but there is still a stigma and reluctance to apply the sunscreen. It leaves people’s skin feeling greasy, which is not ideal when one has to handle a firearm. It can also get in someone’s eyes and affect vision and performance during combat operations. In other words, there are many reasons that would reduce the desire to wear sunscreen and therefore increase exposure to the elements.
A great current example is coronavirus disease 2019 (COVID-19) operations. Although I’m a dermatologist and typically work inside, I’ve been tasked to run a COVID-19 screening tent in the middle of a field in San Antonio, and thus I’ve got to make sure I take my sunscreen out there every day. The general population may not have that variability in their work cycle and sudden change in occupational UV exposure.
Dr. Miller: I was deployed in a combat zone for operations Desert Shield and Desert Storm. I was with the 2nd Armored Division of the US Army deployed to the desert. There really wasn’t an emphasis on photoprotection. It’s just the logistics. The commanders have a lot more important things to think about, and that’s something, usually, that doesn’t get a high priority. The US military is deploying to more places near the equator, so from an operational sense, there’s probably something to brief the commanders about in terms of the long-term consequences of radiation exposure for military servicemembers.
Dr. Dunn: If you look at deployments over the past 2 decades, we have been putting tens of thousands of individuals in high UV exposure regions. Then you have to look at the long-term consequence of the increased incidence of skin cancer in those individuals. What is the cost of that when it comes to treatment of precancerous lesions and skin cancer throughout a life expectancy of 80-plus years?
Dr. Bandino: With most skin cancers there is such long lag time between exposures and development. I wish there were some better data and research out there that really showed whether military service truly is an independent risk factor or if it’s just specific occupation types within the military. I have family members who both work in contracting services and had served in the military. Would their skin cancer risk be the same as others who are doing similar jobs without the military service?
Dr. Dunn: I have had county employees present for skin cancer surgery and with them comes a form that relates to disability. For groundskeepers or police, we assumed that skin cancer is occupation related due to the patient’s increased sun exposure. Their cancers may be unrelated to their actual years of service, but it seems that many light-skinned individuals in the military are going to develop basal cell and squamous cell skin cancer in the coming decades, which likely is going to be attributed to their years of federal service, even though they may have had other significant recreational exposure outside of work. So, my gut feeling is that we are going to see skin cancer as a disability tied to federal service, which is going to cost us.
Dr. Logemann: Yes, I think there are always going to be confounders—what if the servicemembers used tanning beds, or they were avid surfers? It’s going to be difficult to always parse that out.
Dr. Miller: In talking about melanoma, you really have to parse out the subsets. Is it melanoma in situ, is it superficial, is it acral, is it nodular? They all have different initiation events.
Nodular melanomas probably don’t need UV light to initiate a tumor. Another risk factor is having more than 100 moles or many atypical moles, which puts that person in a higher risk category. Perhaps when soldiers, airmen, and navy personnel get inducted, they should be screened for their mole population because that is a risk factor for developing melanoma, and then we can intervene a little bit and have them watch their UV exposure.
Dr. Jarell: You can’t overstate the importance of how heterogeneous melanoma is as a disease. While there are clearly some types of melanoma that are caused by UV radiation, there are also many types that aren’t. We don’t understand why someone gets melanoma on the inner thigh, bottom of the foot, top of the sole, inside the mouth, or in the genital region—these aren’t places of high sun exposure.
Lentigo maligna, as an example, is clearly caused by UV radiation in most cases. But there are so many other different types of melanoma that you can’t just attribute to UV radiation, and so you get into this whole other discussion as to why people are getting melanoma—military or not.
Dr. Bandino: When volunteering for military service, there’s the DoDMERB (Department of Defense Medical Examination Review Board) system that screens individuals for medical issues incompatible with military service such as severe psoriasis or atopic dermatitis. But to my knowledge, the DoDMERB process focuses more on current or past issues and does little to investigate for future risk of disease. A cutaneous example would be assessing quantity of dysplastic nevi, Fitzpatrick scale 1 phenotype, and family history of melanoma to determine risk of developing melanoma in someone who may have more UV exposure during their military service than a civilian. This dermatological future risk assessment was certainly not something I was trained to do as a flight surgeon when performing basic trainee flight physicals prior to becoming a dermatologist.
Dr. Jarell: I am a little bit hard-pressed to generalize the military as high occupational risk for melanoma. There are clearly other professions—landscapers, fishermen—that are probably at much higher risk than, say, your general military all-comers. Us physicians in the military were probably not at increased risk compared to other physicians in the United States. We have to be careful not to go down a slippery slope and designate all MOSs (military occupational specialties) as at increased risk for skin cancer, in particular melanoma. Nonmelanoma skin cancer, such as basal cell and squamous cell carcinoma, is clearly related to the proportional amount of UV exposure. But melanoma is quite a diverse cancer that has many, many disparate etiologies.
Dr. Dunn: The entry physical into the military is an opportunity to make an impact on the number of nonmelanoma skin cancers that would arise in that population. There is an educational opportunity to tell inductees that nonmelanoma skin cancer is going to occur on convex surfaces of the sun-exposed skin—nose, ears, forehead, chin, tops of the shoulders. If offered sun protection for those areas and you stretch the potential impact of that information over tens of thousands of military members over decades, you might actually come up with a big number of people that not only decreases their morbidity but also dramatically decreased the cost to the system as a whole.
Dr. Jarell: You also have to factor in ethnicity and the role it plays in someone’s likelihood to get skin cancer—melanoma or nonmelanoma skin cancer. Darker-skinned people are at certainly decreased risk for different types of skin cancers.
Dr. Dunn: Yes, that would have to be part of the education and should be. If you have light skin and freckles, then you’re at much higher risk for nonmelanoma skin cancer and need to know the high-risk areas that can be protected by sunblock and clothing.
Dr. Logemann: One thing that might be a little bit unique in the military is that you’re living in San Antonio one minute, and then the next minute you’re over in Afghanistan with a different climate and different environment. When you’re deployed overseas, you might have a little bit less control over your situation; you might not have a lot of sunscreen in a field hospital in Afghanistan. Whereas if you were just living in San Antonio, you could go down to the store and buy it.
Dr. Miller: Is sunblock now encouraged or available to individuals in deployment situations or training situations where they’re going to have prolonged sun exposure every day? Is it part of the regimen, just like carrying extra water because of the risk for dehydration?
Dr. Logemann: To the best of my knowledge, it is not always included in your normal rations or uniform and it may be up to the servicemember to procure sunscreen.
Dr. Bandino: There have been improvements, and usually you at least have access to sunscreen. In many deployed locations, for example, you have the equivalent of a small PX (post exchange) or BX (base exchange), where they have a variety of products for sale from toothbrushes to flip-flops, and now also sunscreen. Of course, the type and quality of the sunscreen may not be that great. It’s likely going to be basic SPF (sun protection factor) 15 or 30 in small tubes. As a recent example, I participated in a humanitarian medical exercise in South America last summer and was actually issued sunscreen combined with DEET, which is great but it was only SPF 30. The combination product is a good idea for tropical locations, but in addition to people just not wanting to wear it, the DEET combination tends to burn and sting a little bit more; you can get a heat sensation from the DEET; and the DEET can damage plastic surfaces, which may not be ideal for deployed equipment.
The other problem is quantity. We all learned in residency the appropriate sunscreen quantity of at least 1 fl oz for the average adult body, and that’s what we counsel our patients on, but what they issued me was 1 small 2- to 3-fl oz tube. It fit in the palm of my hand, and that was my sunscreen for the trip.
So, I do think, even though there have been some improvements, much of sun protection will still fall on the individual servicemember. And, as mentioned, depending on your ethnicity, some people may need it more than others. But it is an area where there probably could be continued improvements.
Dr. Logemann: In addition to sunscreen, I think that maybe we should be taking into consideration some simple measures. For example, is it necessary for people to stand out in formation at 2
Dr. Dunn: I think we all kind of agree that the military service is diverse and that many of the subcategories of occupations within the military lead to increased sun exposure by mandate. We advise sun protection by physical barriers and sunblock.
Diagnosis of Skin Cancer Via Telemedicine
Dr. Dunn: I have friends who remain in the VA (US Department of Veterans Affairs) system, and they are involved with telemedicine in dermatology, which can reduce waiting time and increase the number of patients seen by the dermatologist. In-person and teledermatology visits now are available to servicemembers on active duty and retirees.
Dr. Bandino: At our residency program (San Antonio Uniformed Services Health Education Consortium), we’ve had asynchronous teledermatology for over a decade, even before I was a resident. We provide it primarily as a service for patients at small bases without access to dermatology. Some bases also use it as part of their prescreening process prior to authorizing an in-person dermatology consultation.
Certainly, with the coronavirus pandemic, civilian dermatology is seeing a boom in the teledermatology world that had been slowly increasing in popularity for the last few years. In our residency program, teledermatology has traditionally been just for active-duty servicemembers or their dependents, but now due to the coronavirus pandemic, our teledermatology services have significantly expanded to include adding synchronous capability. We have patients take pictures before their virtual appointment and/or FaceTime during the appointment. Even after the pandemic, there will likely be more integration of synchronous teledermatology going forward as we’re seeing some of the value. Of course, I’m sure we would all agree that accurate diagnosis of pigmented lesions can be very challenging with teledermatology, not to mention other diagnostic limitations. But I think there is still utility and it should only get better with time as technology improves. So, I’m hopeful that we can incorporate more of it in the military.
Dr. Logemann: I’m definitely aware that we have different telehealth opportunities available, even using some newer modalities that are command approved in recent weeks. My experience has been for more complicated dermatology, so people are in remote locations, and they’re being seen by a nondermatologist, and they have questions about how to approach management. But I’m not aware of telemedicine as a screening tool for skin cancer in the military or among my civilian colleagues. I would hope that it could be someday because we’re developing these total-body photography machines as well. It could be a way for a nondermatologist who identifies a lesion to have it triaged by a dermatologist. To say, “Oh yeah, that looks like a melanoma. They need to get in sooner vs later,” but not on a large-scale sort of screening modality.
Dr. Bandino: In my recent experience, it has definitely been a helpful triage tool. In the military, this form of triage can be particularly helpful if someone is overseas to determine whether he/she needs to evacuated and evaluated in-person right away.
Dr. Jarell: It’s been useful in looking at benign things. People have shown me in the past few weeks a lot of seborrheic keratoses and a lot of benign dermal nevus-type things, and I say, “Don’t worry about that.” And you can tell if the resolution is good enough. But a lot of people have shown me things in the past few weeks that have clearly been basal cell carcinoma, which we can probably let that ride out for a few more weeks, but I’m not sure if maybe somebody has an amelanotic melanoma. Maybe you need to come in and get that biopsied ASAP. Or something that looks like a melanoma. The patient should probably come in and get that biopsied.
Dr. Miller: I think we can rely on teledermatology. It’s all predicated on the resolution because we’re all trained in pattern recognition. I think it’s very useful to screen for things that look clinically benign. We have to understand that most dermatology is practiced by nondermatologists in the United States, and many studies show that their diagnostic accuracy is 20%, at best maybe 50%. So, they do need to reach out to a dermatologist and perhaps get some guidance on what to do. I think it could be a very useful tool if used appropriately.
Dr. Dunn: If used appropriately, teledermatology could function in a couple of ways. One, it could allow us to declare lesions to be wholly benign, and only should a lesion change would it need attention. The second is that it would allow us to accelerate the process of getting a patient to us—physically in front of us—for a biopsy if a suspicious lesion is seen. A by-product of that process would be that if patients who have wholly benign, nonworrisome lesions could be screened by telemedicine, then physical appointments where a patient is in front of the doctor would be more open. In other words, let’s say if 25% of all lesional visits could be declared benign via telemedicine that would allow dermatology to preserve its face-to-face appointments for patients who are more likely to have cancer and require procedures like skin biopsy.
Love it or hate it, I think we’re getting it no matter what now. Telemedicine creeped along forever and within 6 weeks it’s become ubiquitous. It’s phenomenal how fast we had to adapt to a system or perish in private practice. Sometimes these episodes that we go through have good consequences as well as bad consequences. Telemedicine probably has been needed for a long time and the insurers were not covering it very well, but suddenly a stay-at-home mandate has unveiled valuable technology—something that we probably should have been able to use more and be adequately reimbursed.
Surgical Treatment of Skin Cancer
Dr. Dunn: Treatment historically has been surgical for nonmelanoma and melanoma skin cancers. Some radiation devices have gained popularity again in the past decade or so, but excisional surgery remains the standard treatment for skin cancer. Nonmelanoma skin cancers almost all are probably treated surgically still, with a small percentage treated with superficial radiation.
Access to care is important to discuss. Are Mohs surgeons readily available, or are plastic surgeons, general surgeons, or vascular surgeons in the federal system contributing to the care of skin cancer? Are they doing excisional surgery after biopsies are done? Are they doing excisional biopsies with the intent of cure?
Dr. Logemann: For active duty, I don’t see any issues getting access to the medical center for Mohs micrographic surgery. Sometimes, if we have a lot of volume, some patients may get deferred to the network, but in my experience, it would not typically be an active-duty servicemember. An active-duty servicemember would get care rendered at one of the medical centers for Mohs surgery. Typically the active-duty–aged population isn’t getting much skin cancer. It certainly does happen, but most of the skin cancers frequently that are treated at medical centers are not infrequently retirees.
Dr. Bandino: Because of our residency program, we are required to have Mohs surgery capability to be ACGME (Accreditation Council for Graduate Medical Education) accredited. We typically have 3 Mohs surgeons, so we never have a problem with access.
In the military, I just refer cases to our Mohs surgeons and everything is taken care of in-house. In fact, this is an area where we may even have better access than the civilian world because there are no insurance hurdles or significant delay in care since our Mohs surgeons aren’t typically booked up for 3 to 4 months like many civilian Mohs surgeons. This is especially true for complex cases since we provide hospital-based care with all specialty services under the same umbrella. So, for example, if the Mohs surgeons have an extensive and complex case requiring multidisciplinary care such as ENT (ear, nose, and throat), facial plastics, or radiation-oncology, they’re all in-house with no insurance issues to navigate. This of course is not usual for most military bases and is only capable at bases attached to a large medical center. There are some similar scenarios in the civilian world with university medical centers and managed care organizations, but we may still have a slight advantage in accessibility and cost.
Dr. Dunn: There are guidelines from the National Comprehensive Cancer Network as to how to treat nonmelanoma and melanoma skin cancer. Almost all of them are surgical and almost all of them are safe, outpatient, local anesthetic procedures with a high cure rate. The vast majority of melanoma and nonmelanoma skin cancers can be handled safely and effectively with minimal morbidity and almost no known mortalities from the treatments themselves. Some of the cancers have been identified as high risk for metastasis and mortality, but they’re relatively uncommon still. The good news about skin cancer is that the risk of death remains very small.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
- Riemenschneider K, Liu J, Powers JG. Skin cancer in the military: a systematic review of melanoma and nonmelanoma skin cancer incidence, prevention, and screening among active duty and veteran personnel.J Am Acad Dermatol. 2018;78:1185-1192.
- Brundage JF, Williams VF, Stahlman S, et al. Incidence rates of malignant melanoma in relation to years of military service, overall and in selected military occupational groups, active component, U.S. Armed Forces, 2001-2015. MSMR. 2017;24:8-14.
Painful Hemorrhagic Erosions
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.
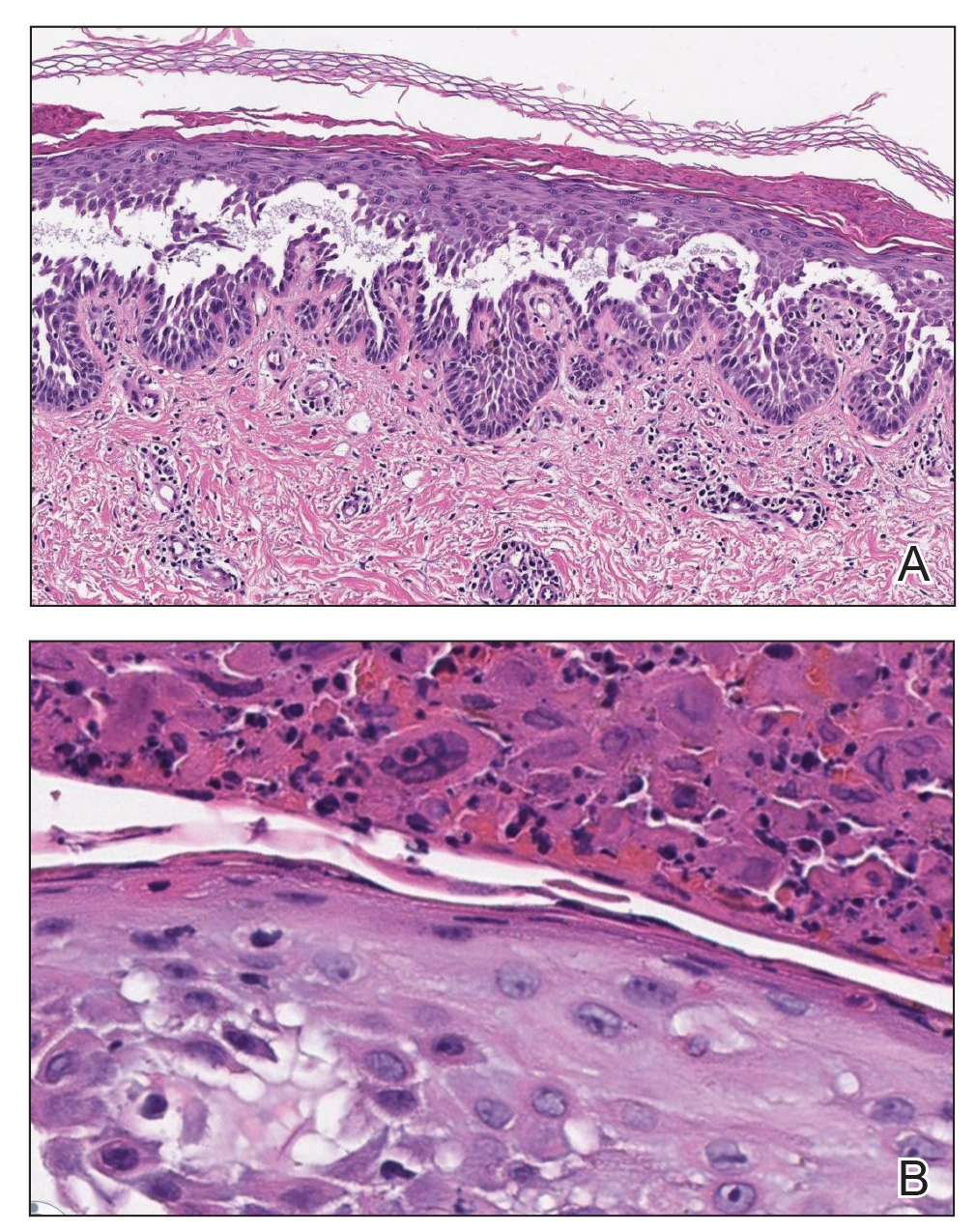
Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).
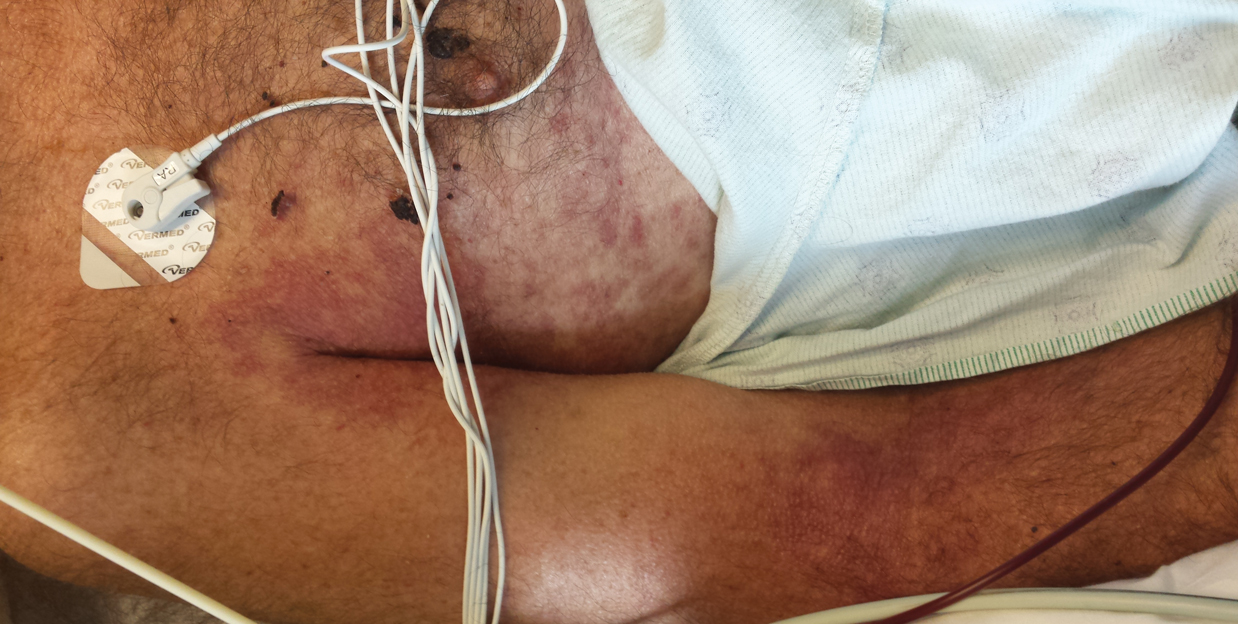
Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
The Diagnosis: Kaposi Varicelliform Eruption (Eczema Herpeticum)
Polymerase chain reaction confirmed presence of herpes simplex virus (HSV) type 1, and the patient was started on intravenous acyclovir (10 mg/kg every 8 hours). Diagnosis was further supported by histopathologic examination with confirmatory immunohistochemistry (Figure 1). The patient's anemia and thrombocytopenia also were attributed to widespread HSV infection.

Approximately 8 hours after the patient was started on acyclovir, he developed increasing tremors, confusion, and impaired speech. Lumbar puncture confirmed the presence of HSV-1 in the cerebrospinal fluid. Despite ongoing intravenous antiviral therapy, he required intubation 6 days after hospitalization due to impaired mental status and myoclonic jerking. He remained intubated, unresponsive, and in critical condition for 9 days before he gradually began to demonstrate cognitive recovery. He subsequently was weaned off the ventilator, his mental status returned to normal, and his skin rash slowly resolved (Figure 2).

Hailey-Hailey disease (HHD), also known as familial benign chronic pemphigus, is a rare autosomal-dominant condition first described by Howard and Hugh Hailey in 1939.1 It is a chronic blistering process characterized by epidermal fragility, often manifesting as macerated fissured erosions in areas exposed to heat and friction (eg, axillae, groin). Hailey-Hailey disease results from a defective calcium transporter (ATP2C1 gene), leading to impaired keratinocyte adhesion.2
Eczema herpeticum refers to the dissemination of herpes infection to areas of compromised skin barrier. Although originally used to describe HSV infection in patients with atopic dermatitis, eczema herpeticum has been described in various conditions that affect the skin barrier function, including Darier disease, ichthyosis vulgaris, pemphigus foliaceus, pemphigus vulgaris, and mycosis fungoides, among others.3 When applied to skin conditions other than atopic dermatitis, it sometimes is referred to as Kaposi varicelliform eruption.2
Hailey-Hailey disease commonly is complicated by a bacterial or fungal infection, including impetigo, tinea, or candidiasis. The first case of HHD complicated by HSV infection was reported in 1973.4 A PubMed search of articles indexed for MEDLINE using the terms benign familial pemphigus AND herpes, Hailey-Hailey AND herpes, Hailey-Hailey AND eczema herpeticum, Hailey-Hailey AND Kaposi varicelliform eruption, and Hailey-Hailey herpeticum revealed 15 cases of HHD complicated by eczema herpeticum.4-6 Herpes simplex virus encephalitis is a rare and life-threatening complication of eczema herpeticum.7,8 We report a case of HSV encephalitis resulting from eczema herpeticum in a patient with HHD.
The clinical differential includes a flare of the patient's known HHD, secondary bacterial or fungal infection, or a superimposed viral infection (eg, HSV, zoster). Histologic evidence of herpetic infection would be absent in an uncomplicated flare of HHD. Impetigo is a superficial bacterial infection that can present in 2 clinical forms: a vesiculopustular type and less commonly a bullous type. It is caused by Staphylococcus aureus in most cases. In multiple myeloma with cutaneous dissemination, a monoclonal proliferation of plasma cells would be evident. Lastly, tinea corporis is caused by dermatophytes that can be seen on hematoxylin and eosin or periodic acid-Schiff staining.
The diagnosis of eczema herpeticum in a patient with HHD should be considered in patients who present with grouped vesicles or hemorrhagic or punched-out erosions in areas of pre-existing HHD. The diagnosis can be confirmed by Tzanck smear, viral culture, polymerase chain reaction, or histopathology (with or without immunohistochemistry).1,2,6 When eczema herpeticum is suspected, prompt antiviral administration is imperative to limit life-threatening systemic spread.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
- Hailey J, Hailey H. Familial benign chronic pemphigus. Arch Dermatol. 1939;39:679-685.
- de Aquino Paulo Filho T, deFreitas YK, da Nóbrega MT, et al. Hailey-Hailey disease associated with herpetic eczema-the value of the Tzanck smear test. Dermatol Pract Concept. 2014;4:29-31.
- Flint ID, Spencer DM, Wilkin JK. Eczema herpeticum in association with familial benign chronic pemphigus. J Am Acad Dermatol. 1993;28(2, pt 1):257-259.
- Leppard B, Delaney TJ, Sanderson KV. Chronic benign familial pemphigus. induction of lesions by Herpesvirus hominis. Br J Dermatol. 1973;88:609-613.
- Lee GH, Kim YM, Lee SY, et al. A case of eczema herpeticum with Hailey-Hailey disease. Ann Dermatol. 2009;21:311-314.
- Zamperetti M, Pichler M, Perino F, et al. Ein fall von morbus Hailey-Hailey in verbindung mit einem eczema herpeticatum. J Dtsch Dermatol Ges. 2016;14:1035-1038.
- Ingrand D, Briquet I, Babinet JM, et al. Eczema herpeticum of the child. an unusual manifestation of herpes simplex virus infection. Clin Pediatr (Phila). 1985;24:660-663.
- Finlow C, Thomas J. Disseminated herpes simplex virus: a case of eczema herpeticum causing viral encephalitis. J R Coll Physicians Edinb. 2018;48:36-39.
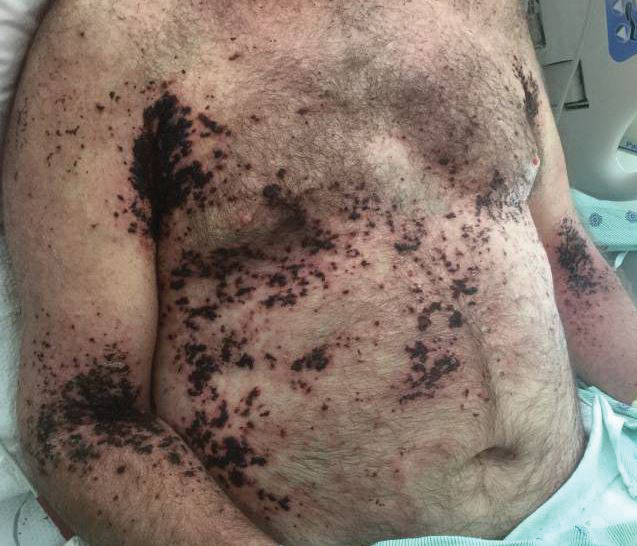
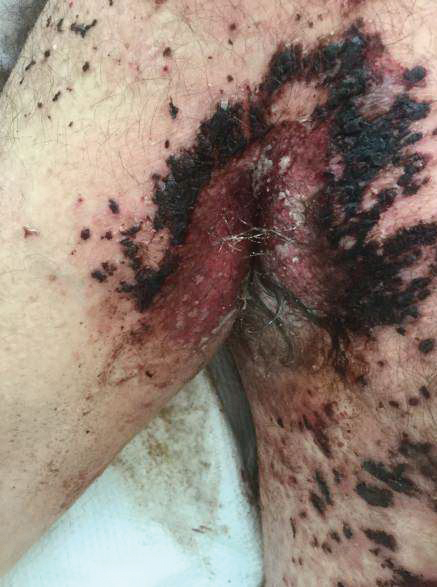
A 62-year-old man with a long-standing history (>40 years) of Hailey-Hailey disease was admitted from an outside hospital due to anemia (hemoglobin, 8.6 g/dL [reference range, 14.0–17.5 g/dL]), thrombocytopenia (platelets, 7×103 /µL [reference range, 150–350×103 /µL]), and worsening skin rash. The patient reported that his Hailey-Hailey disease worsened abruptly 1 month prior to admission and had progressed steadily since then. He described the rash as painful, especially with movement. Over the preceding month, he had been treated with topical triamcinolone, topical diphenhydramine, oral prednisone, fluconazole, and oral clindamycin, all without improvement. The skin lesions continued to worsen and persistently bled; he then presented to our institution for further care.
Physical examination demonstrated widespread shallow erosions with hemorrhagic drainage and crusting located on the lower back, chest, abdomen (top), axillae (bottom), groin, arms, and legs. No vesicles or pustules were noted. The patient had no cognitive dysfunction or focal neurologic deficits. A punch biopsy was performed.
Atretic Cephalocele With Hypertrichosis
To the Editor:
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.
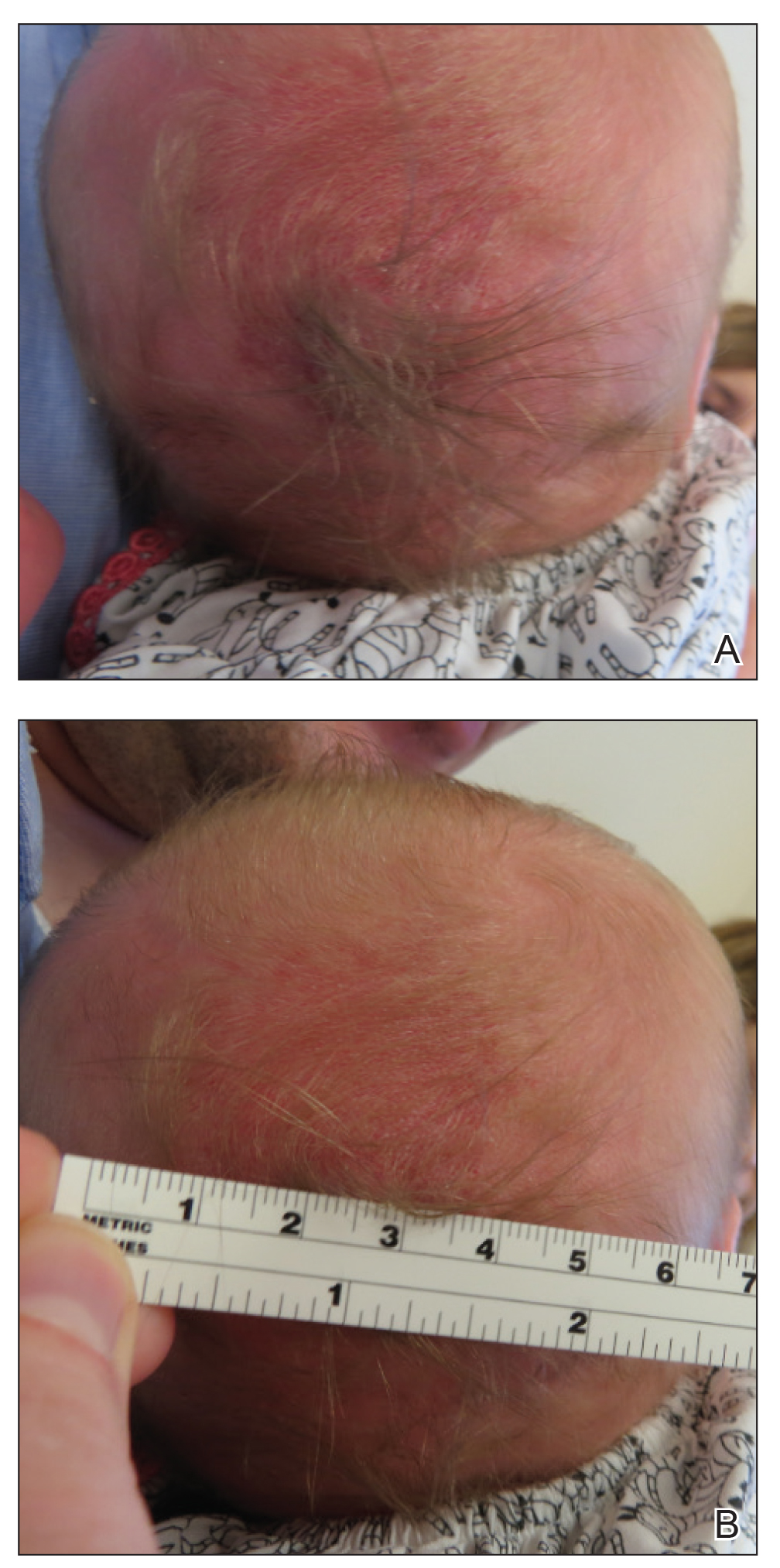
Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
To the Editor:
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.

Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
To the Editor:
A 2-week-old female infant presented to our dermatology clinic for evaluation of a 4.0×4.5-cm pink-red patch with a 1-cm central nodule and an overlying tuft of hair on the midline occipital region (Figure). The patient was born at 39 weeks’ gestation to nonconsanguineous parents via a normal spontaneous vaginal delivery and had an unremarkable prenatal course with no complications since birth. The red patch and tuft of hair were noted at birth, and the parents reported that the redness varied somewhat in size throughout the day and from day to day. An initial neurologic workup revealed no gross neurologic abnormalities. A head ultrasound revealed a soft-tissue hypervascular nodule that appeared separate from bony structures but showed evidence of a necklike extension from the nodule to the underlying soft tissues. The ultrasound could not definitively rule out intracranial extension; gross brain structures appeared normal. The initial differential diagnosis consisted of a congenital hemangioma (either a rapidly involuting or noninvoluting subtype), meningioma, or cephalocele.

Consultation with the pediatric neurosurgery service was sought, and magnetic resonance imaging of the head was performed, which demonstrated a cystic lesion within the subcutaneous soft tissue in the midline posterior scalp approximately 2 cm above the torcula. There also was a thin stalk extending from the cyst and going through an osseous defect within the occipital bone and attaching to the falx cerebri. There was no evidence of any venous communication with the cerebral sinus tracts or intraparenchymal extension. No intracranial abnormalities were noted. Given the radiographic evidence, a presumptive diagnosis of an atretic cephalocele was made with the plan for surgical repair.
The patient was re-evaluated at 3 and 4 months of age; there were no changes in the size or appearance of the lesion, and she continued to meet all developmental milestones. At 9 months of age the patient underwent uncomplicated neurosurgery to repair the cephalocele. Histopathologic examination of the resected lesion was consistent with an atretic cephalocele and showed positive staining for epithelial membrane antigen, which further confirmed a meningothelial origin; no glial elements were identified. The postoperative course was uncomplicated, and the patient was healing well at a follow-up examination 2 weeks after the procedure.
This case highlights the importance of an extensive workup when a patient presents with a midline lesion and hypertrichosis. The patient’s red patch, excluding the hair tuft, was reminiscent of a vascular malformation or hemangioma precursor lesion given the hypervascularity, the history of the lesion being present since birth, the lack of neurologic symptomatology, and the history of meeting all developmental milestones. The differential diagnosis for this patient was extensive, as many neurologic conditions present with cutaneous findings. Having central nervous system (CNS) and cutaneous comorbidities coincide underscores their common neuroectodermal origin during embryogenesis.1,2
Atretic cephalocele is a rare diagnosis, with the prevalence of cephaloceles estimated to be 0.8 to 3.0 per 10,000 births.3 It typically occurs in either the parietal or occipital scalp as a skin nodule with a hair tuft or alopecic lesion with or without a hair collar. A cephalocele is defined as a skin-covered protrusion of intracranial contents through a bony defect. Central nervous system tissue, meninges, or cerebrospinal fluid can protrude outside the skull with this condition. An atretic cephalocele refers to a cephalocele that arrested in development and represents approximately 40% to 50% of all cephaloceles.4 Various hypotheses have explained the development of atretic cephaloceles: it represents a neural crest remnant, regression of a meningocele in utero, injury of multipotential mesenchymal cells, and either failure of the neural tube to close or reopening of the neural tube after closure.4-6 There is evidence of developmental defects in skin appendages including sweat and sebaceous glands, arrector pili muscles, and hair follicles in and around the skin overlying the cephalocele, suggesting that there is a developmental abnormality of not only the CNS but also the cutaneous tissue.5 Typical radiographic findings include a cystic lesion with underlying defect in the skull. A vertical positioning of the straight sinus also has been demonstrated to be a consistent finding that can aid in diagnosis.4
Imaging is of utmost importance when a patient presents with a tuft of hair on the scalp to rule out intracranial extension and associated abnormalities such as gray matter heterotopia, hypogenesis of the corpus callosum, hydrocephalus, and Dandy-Walker and Walker-Warburg syndromes, which have all been associated with atretic cephaloceles.4,7 The impact of location of the intracranial abnormality on prognosis has been contested, with some reporting a better prognosis with occipital cephalocele vs parietal cephalocele while others have found the opposite to be true.6,7
Cutaneous abnormalities presenting with hypertrichosis (ie, hair tuft, hair collar) and/or capillary malformations increase the likelihood of a cranial dysraphism, especially when these findings present together and occur in and around the midline. Clinical examination cannot rule out an underlying connection to the CNS; these findings require appropriate radiographic imaging assessment prior to any procedural intervention.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
- Drolet BA, Clowry L, McTigue K, et al. The hair collar sign: marker for cranial dysraphism. Pediatrics. 1995;96(2, pt 1):309-313.
- Sewell MJ, Chiu YE, Drolet BA. Neural tube dysraphism: review of cutaneous markers and imaging. Pediatr Dermatol. 2015;32:161-170.
- Carvalho DR, Giuliani LR, Simão GN, et al. Autosomal dominant atretic cephalocele with phenotype variability: report of a Brazilian family with six affected in four generation. Am J Med Genet A. 2006;140:1458-1462.
- Bick DS, Brockland JJ, Scott AR. A scalp lesion with intracranial extension. atretic cephalocele. JAMA Otolaryngol Head Neck Surg. 2015;141:289-290.
- Fukuyama M, Tanese K, Yasuda F, et al. Two cases of atretic cephalocele, and histological evaluation of skin appendages in the surrounding skin. Clin Exp Dermatol. 2016;41:48-52.
- Martinez-Lage JF, Sola J, Casas C, et al. Atretic cephalocele: the tip of the iceberg. J Neurosurg. 1992;77:230-235.
- Yakota A, Kajiwara H, Kohchi M, et al. Parietal cephalocele: clinical importance of its atretic form and associated malformation. J Neurosurg. 1988;69:545-551.
Practice Points
- Atretic cephalocele is a rare diagnosis occurring on the scalp as a nodule with an overlying hair tuft or alopecia with or without a hair collar.
- Imaging is of utmost importance when presented with a tuft of hair on the midline to rule out intracranial extension and associated abnormalities.
Erythematous Plaque on the Scalp With Alopecia
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.
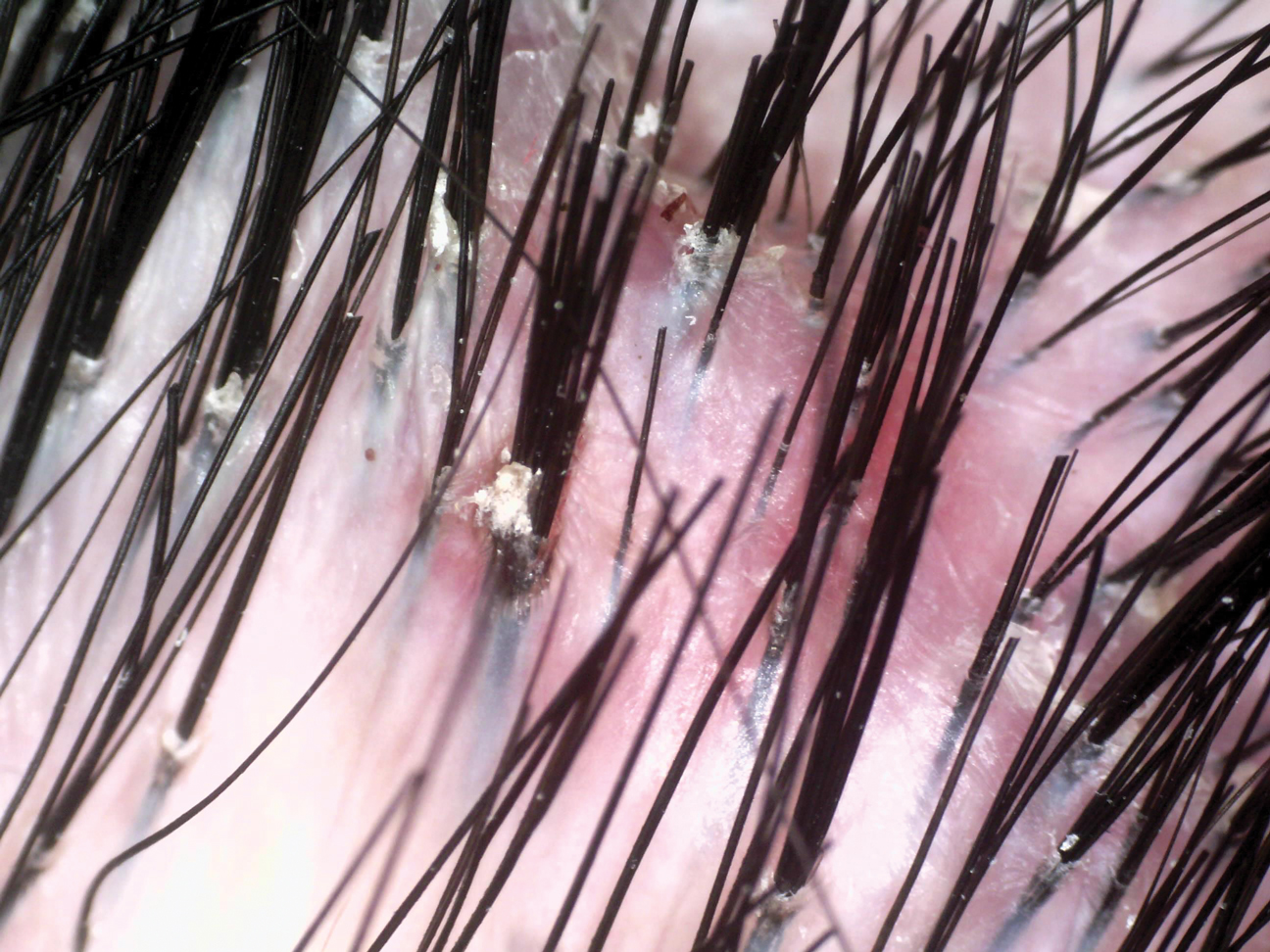
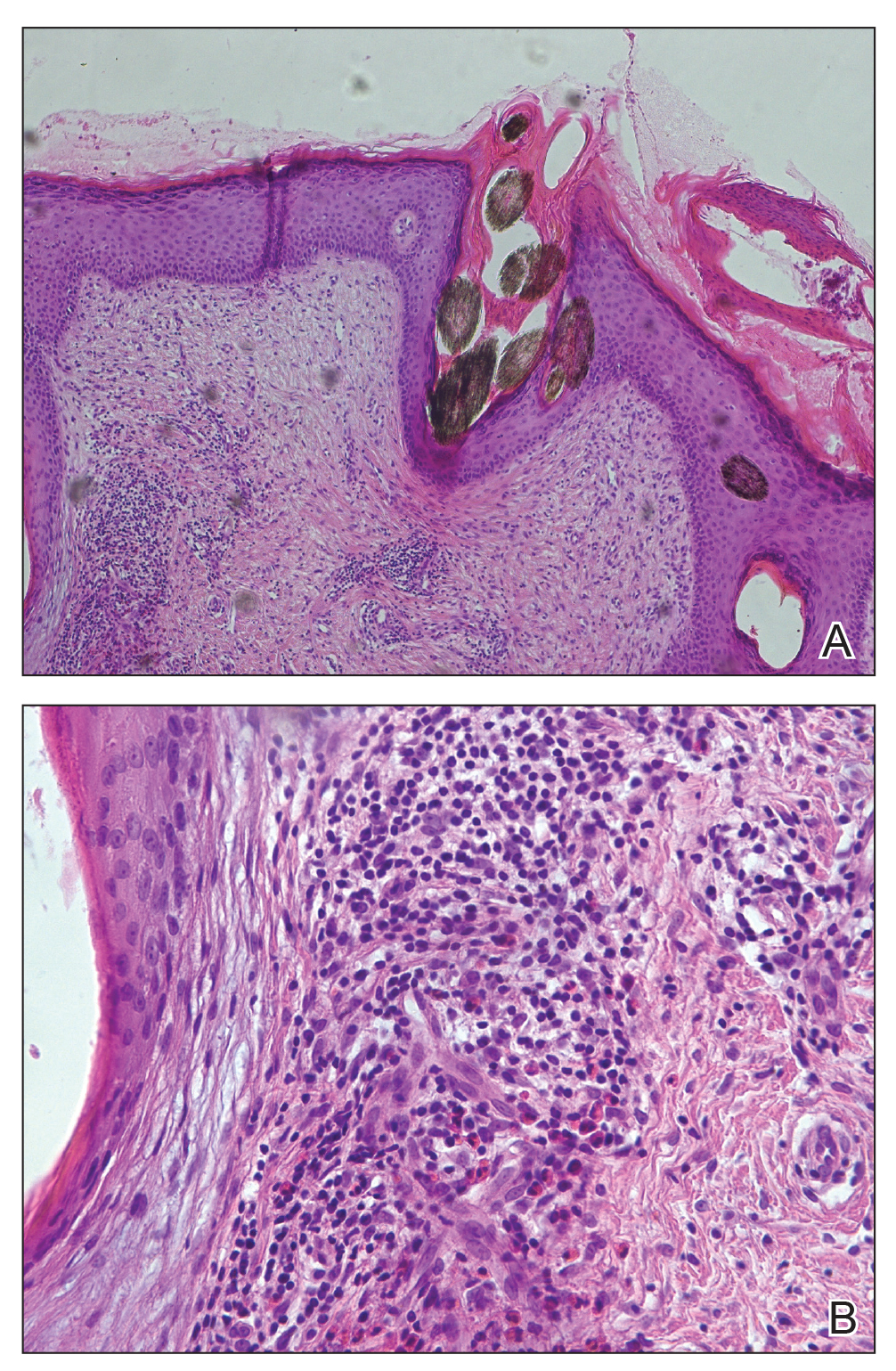
Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.


Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
The Diagnosis: Tufted Hair Folliculitis
Dermoscopic examination revealed multiple hair tufts of 5 to 20 normal hairs emerging from single dilated follicular openings (Figure 1). The density of hair follicles was reduced with adherent yellow-white scales that encircled the dilated follicular orifices. Histopathology revealed hyperkeratosis and parakeratosis in the stratum corneum. Infiltration of lymphocytes, neutrophils, plasma cells, and eosinophils around the upper portions of the follicles also was found. Multiple hairs emerging from a single dilated follicular ostia with prominent fibrosis of the dermis were seen (Figure 2). Based on the clinical and histopathological findings, the patient was diagnosed with tufted hair folliculitis (THF). She was treated with minocycline 100 mg once daily and an intralesional betamethasone injection 5 mg once daily. After 2 weeks of treatment, the lesion improved and decreased in size to 1×1 cm in diameter; however, the hair tufts and scarring alopecia remained.


Tufted hair folliculitis is a rare inflammatory condition of the scalp characterized by a peculiar tufting of hair that was first described by Smith and Sanderson1 in 1978. Most patients present with a patch or plaque on the parietal or occipital region of the scalp. The condition may lead to the destruction of follicular units, resulting in permanent scarring alopecia.2 Histopathology in our patient revealed perifollicular inflammation, and several follicles could be seen converging toward a common follicular duct with a widely dilated opening, consistent with the diagnosis of THF.
The pathogenic mechanisms of THF are unclear. Primary hair tufting, local trauma, tinea capitis, nevoid malformation, and Staphylococcus aureus infection have been proposed as causative pathomechanisms.3 Typically there is no history of underlying disease or trauma on the scalp; however, secondary changes may have occurred following unrecognized trauma or repeated stimuli. Staphylococcal infections may play a notable role in inducing THF. Ekmekci and Koslu4 reported that a local inflammatory process led to the destruction of adjacent follicles, which subsequently amalgamated to form a common follicular duct due to local fibrosis and scarring. However, Powell et al5 found no evidence of local immune suppression or immune failure that could explain the abnormal host response to a certain presumptive superantigen. In our patient, the inflammatory injury was mild, and no purulent exudation was found from the dilated follicular openings. Because the patient had applied an antibiotic ointment prior to presentation, bacterial cultures from biopsy specimens were not appropriate.
The differential diagnosis of THF includes folliculitis decalvans, folliculitis keloidalis nuchae, dissecting cellulitis of the scalp, and follicular lichen planus.6 In our patient, folliculitis keloidalis nuchae and dissecting cellulitis of the scalp were excluded because no keloid or purulent inflammation was found. The diagnosis of follicular lichen planus was not taken into consideration because characteristic pathology such as liquefaction degeneration of basal cells was not observed. Folliculitis decalvans was considered to be a possible cause of the alopecia in our patient. It also was suggested that hair tufting could be a secondary phenomenon, occurring in several inflammatory disorders of the scalp. Powell et al5 concluded that THF should be considered as a distinctive clinicohistologic variant of folliculitis decalvans characterized by multiple hair tufts with patches of scarring alopecia. This hypothesis corresponded with our patient's clinical manifestation and histopathology.
Conventional treatment of THF includes topical antiseptics and oral antibiotics (eg, flucloxacillin, erythromycin, tetracycline, doxycycline), but reduction in hair bundling rarely has been observed after antibiotic treatment. Although good prognosis has been reported after surgical excision of the involved areas, it can only be performed in small lesions.6 Pranteda et al7 reported that combination therapy with oral rifampin and oral clindamycin can prevent relapse long-term. Combination therapy for 10 weeks also was effective in 10 of 18 patients with THF.5 Rifampin is an effective therapeutic modality to control the progression of THF as well as prevent relapse; however, long-term use should be avoided to prevent hepatic or renal side effects.7 Our patient was successfully treated with intralesional betamethasone and oral minocycline to reduce the inflammation and prevent the expansion of scarring alopecia.
Acknowledgment
The authors thank Xue Chen, MD (Beijing, China), for writing support.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.
- Smith NP, Sanderson KV. Tufted folliculitis of the scalp. J R Soc Med. 1978;71:606-608.
- Broshtilova V, Bardarov E, Kazandjieva J, et al. Tufted hair folliculitis: a case report and literature review. Acta Dermatovenerol Alp Pannonica Adriat. 2011;20:27-29.
- Gungor S, Yuksel T, Topal I. Tufted hair folliculitis associated with Melkersson-Rosenthal syndrome and hidradenitis suppurativa. Indian J Dermatol Venereol Leprol. 2014;80:484-487.
- Ekmekci TR, Koslu A. Tufted hair folliculitis causing skullcap-pattern cicatricial alopecia. J Eur Acad Dermatol Venereol. 2006;20:227-229.
- Powell JJ, Dawber RP, Gatter K. Folliculitis decalvans including tufted folliculitis: clinical, histological and therapeutic findings. Br J Dermatol. 1999;140:328-333.
- Baroni A, Romano F. Tufted hair folliculitis in a patient affected by pachydermoperiostosis: case report and videodermoscopic features. Skinmed. 2011;9:186-188.
- Pranteda G, Grimaldi M, Palese E, et al. Tufted hair folliculitis: complete enduring response after treatment with rifampicin. J Dermatolog Treat. 2004;15:396-398.

A 37-year-old woman presented with a 2×6-cm, firm, erythematous plaque on the parietal region of the scalp of 1 year’s duration. No history of injury to the scalp was noted. The patient noticed hair loss in the affected area in the month prior to presentation. She was afebrile and otherwise asymptomatic. She denied a family history of similar scalp disorders.
Applications for the CUTIS 2021 Resident Corner Column
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!
The Cutis Editorial Board is now accepting applications for the 2021 Resident Corner column. The Editorial Board will select 2 to 3 residents to serve as the Resident Corner columnists for 1 year. Articles are posted online only at www.mdedge.com/dermatology but will be referenced in Index Medicus. All applicants must be current residents and will be in residency throughout 2021.
Columnists also will participate in a monthly resident takeover of our Dermatology Weekly podcast.
For consideration, send your curriculum vitae along with a brief (not to exceed 500 words) statement of why you enjoy Cutis and what you can offer your fellow residents in contributing a monthly column.
A signed letter of recommendation from the Director of the dermatology residency program also should be supplied.
All materials should be submitted via email to Melissa Sears (msears@mdedge.com) by October 15. The residents who are selected to write the column for the upcoming year will be notified by November 2.
We look forward to continuing to educate dermatology residents on topics that are most important to them!