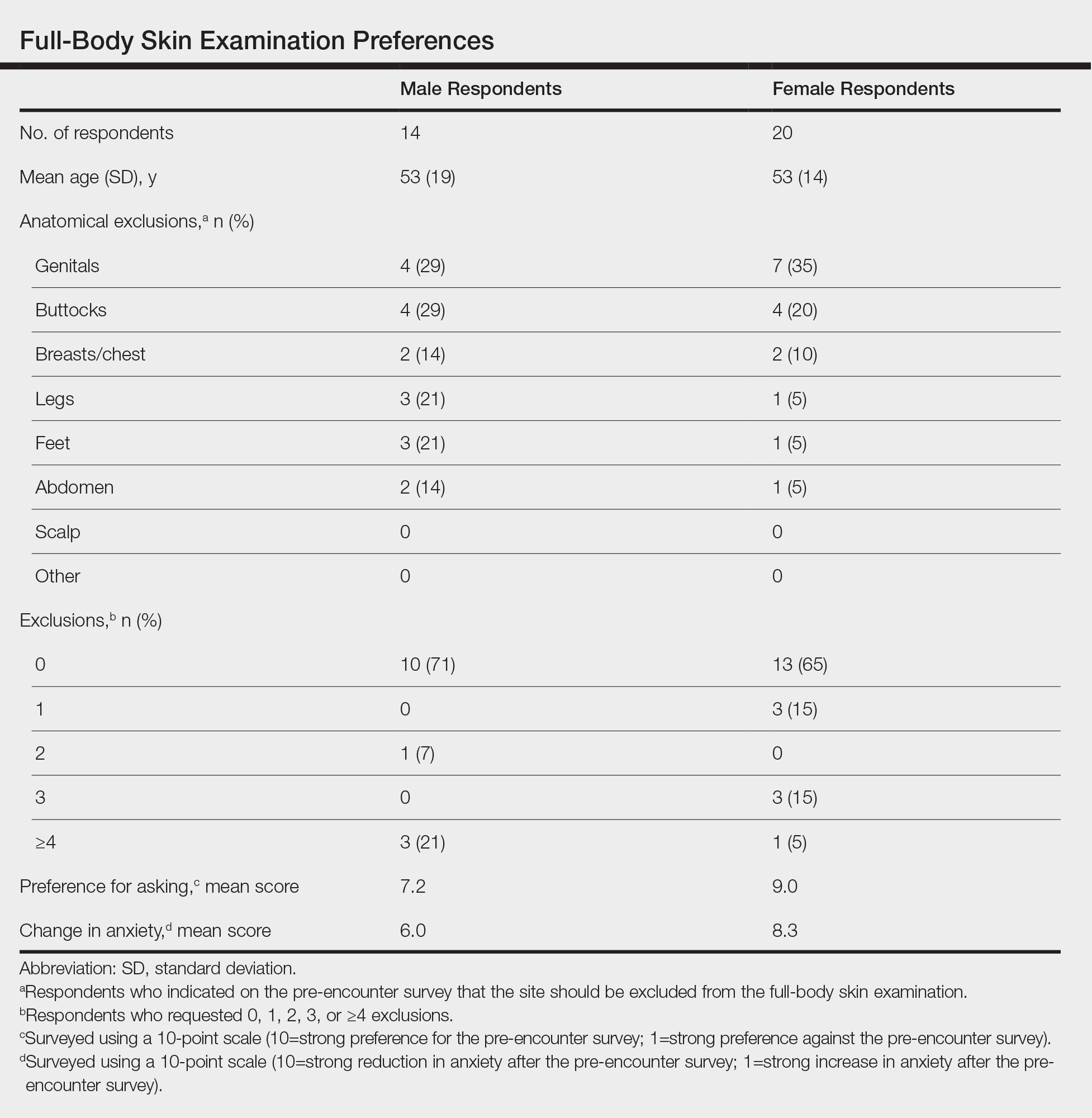
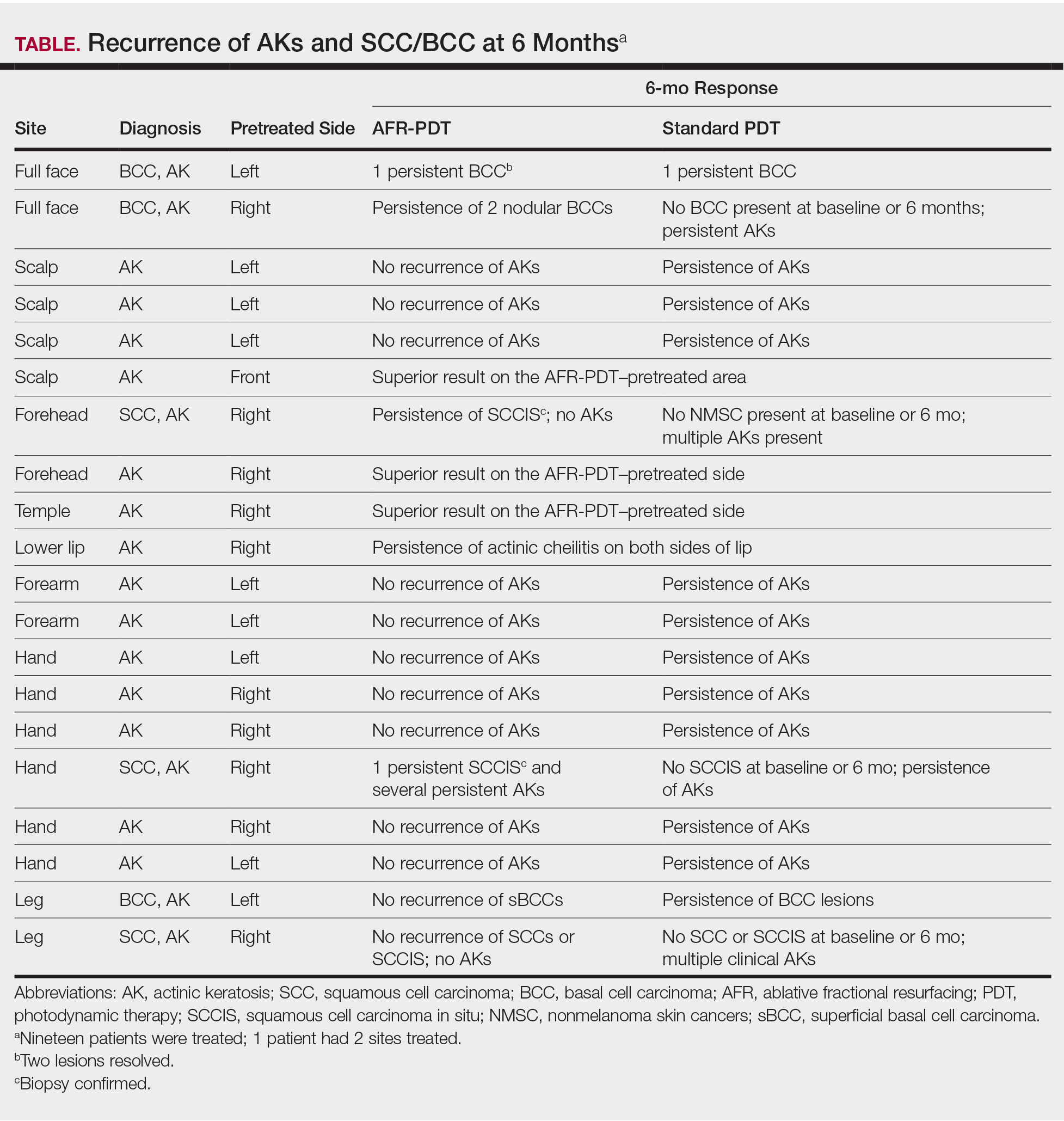
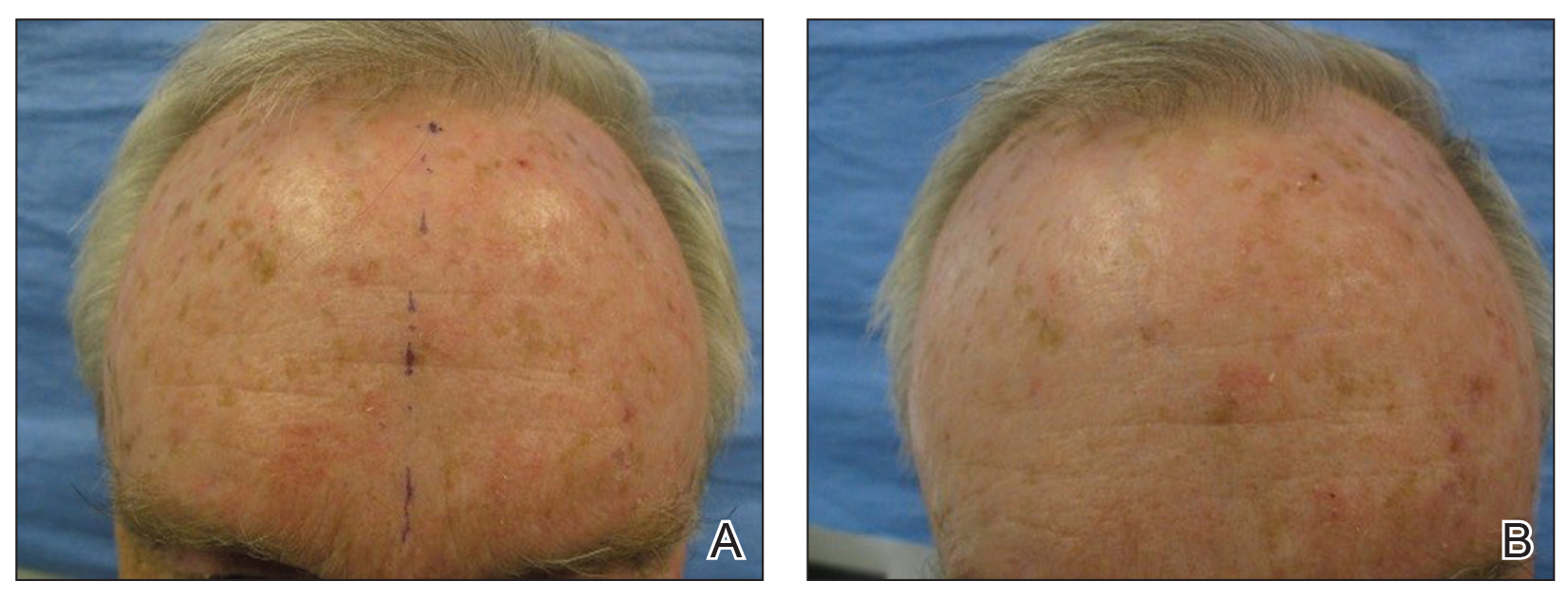
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Over-the-counter Topical Products in Dermatology
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
Over-the-counter (OTC) topical products commonly are discussed during dermatology encounters. Unsurprisingly, dermatologists recommend OTC topical formulations at the highest rate of all medical specialists.1,2 These products may aid in the treatment of skin disease and include shampoo for seborrheic dermatitis, moisturizer for atopic dermatitis, and an armamentarium of products for acne. Conversely, an incorrect selection of OTC topicals can cause or exacerbate skin conditions or result in systemic toxicity. This article addresses how dermatology residents may become familiar with the safety, utility, and tolerability of these products.
Safety and Regulation
Over-the-counter products fall into one or more US Food and Drug Administration (FDA) categories, each of which is subject to a unique set of regulations. The FDA website (www.fda.gov/cosmetics and www.fda.gov/drugs) is an excellent resource for comprehensive and up-to-date information about categorization, safety, and regulation of these products.
Many OTC products are categorized as drugs, including topical steroids, antimicrobials, and sunscreens.3 Most of these products previously were available by prescription and became available OTC after sufficient postmarketing safety information.4 Once a drug becomes available OTC, monitoring relies on reporting from health care professionals.5 Notably, the safety of chemical sunscreens is being re-evaluated in light of recent data demonstrating serum levels in humans above the FDA limit for drugs exempt from further testing for carcinogenicity and reproductive and developmental effects.6-8
The FDA has the authority to regulate imported cosmetic products.
Another category relevant to dermatologists includes dietary supplements. The FDA is responsible for evaluating safety and labeling of products before marketing and taking action against any adulterated or misbranded dietary supplement.14 The FDA does not directly test products, though third-party agencies including NSF International and United States Pharmacopeia impart certification after verification that labeled ingredients are present in the product and test for contaminants.15,16
Utility and Pharmacology
Dermatology residents may have less experience and comfort with the safety profiles and indications of nondrug ingredients in topical products. The textbook Comprehensive Dermatologic Drug Therapy17 is an excellent initial resource for learning about the mechanism of action, efficacy, pharmacology, and side effects of such ingredients, including hydroxy acids, shampoos, cleansers, sunscreens, insect repellents, and topical antioxidants. Dermatology residents also need to be familiar with ingredients causing allergic contact dermatitis, and Fisher’s Contact Dermatitis18 is an excellent resource.
When patients indicate use of a particular product, clinicians may not be certain about specific ingredients. In this case, they may refer to the Walgreens website (www.walgreens.com), which provides an ingredient list for all products that they sell. Additionally, the Environmental Working Group’s Skin Deep program (www.ewg.org/skindeep) maintains a database of more than 85,000 personal care products, which may be accessed online or using their mobile application (Healthy Living), which allows one to scan a product’s barcode.
Trying Them Out
Lastly, it is helpful for dermatologists to be personally familiar with a variety of products to address patients’ concerns regarding tolerability of products (eg, greasiness, inability to “rub in,” sunscreens leaving a white cast, drying effect of cleansers). Samples at conferences including the annual meeting of the American Academy of Dermatology provide a cost-effective way for residents to try out a variety of products. Additionally, residents may purchase different products each time they restock their own supply of personal care products to sample a variety.
Final Thoughts
The FDA website contains up-to-date information on the safety of OTC products, which is constantly in flux. This article provides additional references for dermatology residents to begin to learn about the safety, utility, and pharmacology of topical OTC products. Firsthand experience by sampling products helps dermatologists
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
- Vogel CA, Balkrishnan R, Fleischer AB, et al. Over-the-counter topical skin products—a common component of skin disease management. Cutis. 2004;74:55-67.
- Nolan BV, Levender MM, Davis SA, et al. Trends in the use of topical over the counter products in the management of dermatologic disease in the United States. Dermatol Online J. 2012;18:1.
- Is it a cosmetic, a drug, or both? (or is it soap?). US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/it-cosmetic-drug-or-both-or-it-soap. Updated August 2, 2018. Accessed April 30, 2020.
- Clarke P. How FDA strives to ensure safety of OTC products. US Food and Drug Administration website. https://www.fda.gov/drugs/special-features/how-fda-strives-ensure-safety-otc-products. Updated March 10, 2016. Accessed April 30, 2020.
- Bond C, Hannaford P. Issues related to monitoring the safety of over-the-counter (OTC) medicines. Drug Saf. 2003;26:1065-1074.
- Matta MK, Zusterzeel R, Pilli NR, et al. Effect of sunscreen application under maximal use conditions on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2019;321:2082-2091.
- Matta MK, Florian J, Zusterzeel R, et al. Effect of sunscreen application on plasma concentration of sunscreen active ingredients: a randomized clinical trial. JAMA. 2020;323:256-267.
- FDA advances new proposed regulation to make sure that sunscreens are safe and effective. US Food and Drug Administration website. https://www.fda.gov/news-events/press-announcements/fda-advances-new-proposed-regulation-make-sure-sunscreens-are-safe-and-effective. Published February 21, 2019. Accessed May 1, 2020.
- FDA authority over cosmetics: how cosmetics are not FDA-approved, but are FDA-regulated. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-laws-regulations/fda-authority-over-cosmetics-how-cosmetics-are-not-fda-approved-are-fda-regulated. Updated July 24, 2018. Accessed May 1, 2020.
- Inspection of cosmetics. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-compliance-enforcement/inspection-cosmetics. Updated November 3, 2017. Accessed May 1, 2020.
- Cosmetics imports. US Food and Drug Administration website. https://www.fda.gov/cosmetics/cosmetics-international-activities/cosmetics-importers. Updated September 14, 2018. Accessed May 1, 2020.
- Mercury poisoning linked to use of skin-lightening creams from Mexico. California Department of Health website. https://www.cdph.ca.gov/Programs/CCDPHP/DEODC/EHIB/CPE/CDPH%20Document%20Library/Mercury%20in%20Skin%20Creams_HealthAlert%202019.pdf. Accessed May 1, 2020.
- Otley CC, Sober A. Over-the-counter clobetasol propionate. Arch Dermatol. 1994;130:121.
- Dietary supplements. US Food and Drug Administration website. https://www.fda.gov/food/dietary-supplements. Updated August 16, 2019. Accessed May 1, 2020.
- Supplement and vitamin certification. NSF website. https://www.nsf.org/consumer-resources/health-beauty/supplements-vitamins/supplement-vitamin-certification. Accessed May 1, 2020.
- USP Verified Mark. The United States Pharmacopeial Convention website. https://www.usp.org/verification-services/verified-mark. Accessed May 1, 2020.
- Wolverton SE. Comprehensive Dermatologic Drug Therapy. 3rd ed. New York, NY: Elsevier Saunders; 2013.
- Fowler JF, Zirwas MJ, eds. Fisher’s Contact Dermatitis. 7th ed. Phoenix, AZ: Contact Dermatitis Institute; 2019.
Resident Pearls
- Several branches of the US Food and Drug Administration are responsible for regulation of overthe-counter (OTC) topical products with both direct and indirect oversight.
- There are several excellent resources available to dermatologists in training who are interested in learning about pharmacology and tolerability of OTC products.
- Firsthand experience in personally sampling a variety of products also helps clinicians provide practical recommendations to patients.
Solitary Papule on the Shoulder
The Diagnosis: Dermatofibroma With Sebaceous Induction
The biopsy of the lesion revealed a fibrohistiocytic dermal pattern with overlying benign epidermal and sebaceous hyperplasia with a proliferation of fibroblasts in the dermis. Other sections revealed hyperplastic sebaceous glands of the superficial and mid dermis. These findings were suggestive of a dermatofibroma (DF) that had induced epidermal and sebaceous hyperplasia.
Dermatofibromas are common benign fibrous soft tissue growths that account for approximately 3% of dermatopathology specimens.1 The etiology of DFs is unknown; however, they are thought to arise from sites of prior trauma or arthropod bites. Multiple or eruptive DFs have been reported in patients with lupus and atopic dermatitis.2 They commonly appear as round firm nodules measuring less than 1 cm in diameter on the extremities of young adults. Eruptive dermatofibromas also have been reported in human immunodeficiency virus-positive and immunosuppressed patients.3,4 On physical examination, gently pinching the lesion causes a downward movement known as the "dimple sign." If left undisturbed, DFs persist but may undergo partial regression, especially in the center; they also may be excised if symptomatic.
The clinical differential for this papule included a scar and sebaceous hyperplasia. The lack of history of skin cancer or prior procedure made a scar less likely. Sebaceous glands are less prominent on the shoulders, making sebaceous hyperplasia less likely, though dermoscopy showed pale yellow lobules. Sebaceous adenomas most commonly are seen on the head or neck and present as a flesh-colored papule. Sebaceous induction by DFs is rare but has been reported in the literature.5,6
The histology of DFs is described as a nodular proliferation of spindle-shaped fibroblasts and myofibroblasts with short intersecting fascicles. A predilection for sebaceous induction from an underlying DF on the shoulder has been reported.5 Sebaceous differentiation has been reported in 16% to 31.6% of DFs.5,6 Seborrheic keratosis-like epidermal hyperplasia frequently has been seen in DFs with sebaceous induction in comparison to DFs without sebaceous induction.5 Immunohistochemical stains are important to help differentiate DF from dermatofibrosarcoma protuberans, especially when approaching the subcutis. Dermatofibromas stain positive for factor XIIIa and negative for CD34, whereas dermatofibrosarcoma protuberans stain negative for factor XIIIa and positive for CD34.7 Dermatofibromas also demonstrate positive immunostaining for vimentin, stromelysin 3,8 muscle-specific actin, and CD68.
- Rahbari H, Mehregan AH. Adnexal displacement and regression in association with histiocytoma (dermatofibroma). J Cutan Pathol. 1985;12:94-102.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Kanitakis J, Carbonnel E, Delmonte S, et al. Multiple eruptive dermatofibromas in a patient with HIV infection: case report and literature review. J Cutan Pathol. 2000;27:54-56.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Shuweiter M, Böer A. Spectrum of follicular and sebaceous differentiation induced by dermatofibroma. Am J Dermatopathol. 2009;31:778.
- Abenoza P, Lillemoe T. CD34 and factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Am J Dermatopathol. 1993;15:429-434.
- Kim HJ, Lee JY, Kim SH, et al. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: comparison with factor XIIIa and CD34. Br J Dermatol. 2007;157:319-324.
The Diagnosis: Dermatofibroma With Sebaceous Induction
The biopsy of the lesion revealed a fibrohistiocytic dermal pattern with overlying benign epidermal and sebaceous hyperplasia with a proliferation of fibroblasts in the dermis. Other sections revealed hyperplastic sebaceous glands of the superficial and mid dermis. These findings were suggestive of a dermatofibroma (DF) that had induced epidermal and sebaceous hyperplasia.
Dermatofibromas are common benign fibrous soft tissue growths that account for approximately 3% of dermatopathology specimens.1 The etiology of DFs is unknown; however, they are thought to arise from sites of prior trauma or arthropod bites. Multiple or eruptive DFs have been reported in patients with lupus and atopic dermatitis.2 They commonly appear as round firm nodules measuring less than 1 cm in diameter on the extremities of young adults. Eruptive dermatofibromas also have been reported in human immunodeficiency virus-positive and immunosuppressed patients.3,4 On physical examination, gently pinching the lesion causes a downward movement known as the "dimple sign." If left undisturbed, DFs persist but may undergo partial regression, especially in the center; they also may be excised if symptomatic.
The clinical differential for this papule included a scar and sebaceous hyperplasia. The lack of history of skin cancer or prior procedure made a scar less likely. Sebaceous glands are less prominent on the shoulders, making sebaceous hyperplasia less likely, though dermoscopy showed pale yellow lobules. Sebaceous adenomas most commonly are seen on the head or neck and present as a flesh-colored papule. Sebaceous induction by DFs is rare but has been reported in the literature.5,6
The histology of DFs is described as a nodular proliferation of spindle-shaped fibroblasts and myofibroblasts with short intersecting fascicles. A predilection for sebaceous induction from an underlying DF on the shoulder has been reported.5 Sebaceous differentiation has been reported in 16% to 31.6% of DFs.5,6 Seborrheic keratosis-like epidermal hyperplasia frequently has been seen in DFs with sebaceous induction in comparison to DFs without sebaceous induction.5 Immunohistochemical stains are important to help differentiate DF from dermatofibrosarcoma protuberans, especially when approaching the subcutis. Dermatofibromas stain positive for factor XIIIa and negative for CD34, whereas dermatofibrosarcoma protuberans stain negative for factor XIIIa and positive for CD34.7 Dermatofibromas also demonstrate positive immunostaining for vimentin, stromelysin 3,8 muscle-specific actin, and CD68.
The Diagnosis: Dermatofibroma With Sebaceous Induction
The biopsy of the lesion revealed a fibrohistiocytic dermal pattern with overlying benign epidermal and sebaceous hyperplasia with a proliferation of fibroblasts in the dermis. Other sections revealed hyperplastic sebaceous glands of the superficial and mid dermis. These findings were suggestive of a dermatofibroma (DF) that had induced epidermal and sebaceous hyperplasia.
Dermatofibromas are common benign fibrous soft tissue growths that account for approximately 3% of dermatopathology specimens.1 The etiology of DFs is unknown; however, they are thought to arise from sites of prior trauma or arthropod bites. Multiple or eruptive DFs have been reported in patients with lupus and atopic dermatitis.2 They commonly appear as round firm nodules measuring less than 1 cm in diameter on the extremities of young adults. Eruptive dermatofibromas also have been reported in human immunodeficiency virus-positive and immunosuppressed patients.3,4 On physical examination, gently pinching the lesion causes a downward movement known as the "dimple sign." If left undisturbed, DFs persist but may undergo partial regression, especially in the center; they also may be excised if symptomatic.
The clinical differential for this papule included a scar and sebaceous hyperplasia. The lack of history of skin cancer or prior procedure made a scar less likely. Sebaceous glands are less prominent on the shoulders, making sebaceous hyperplasia less likely, though dermoscopy showed pale yellow lobules. Sebaceous adenomas most commonly are seen on the head or neck and present as a flesh-colored papule. Sebaceous induction by DFs is rare but has been reported in the literature.5,6
The histology of DFs is described as a nodular proliferation of spindle-shaped fibroblasts and myofibroblasts with short intersecting fascicles. A predilection for sebaceous induction from an underlying DF on the shoulder has been reported.5 Sebaceous differentiation has been reported in 16% to 31.6% of DFs.5,6 Seborrheic keratosis-like epidermal hyperplasia frequently has been seen in DFs with sebaceous induction in comparison to DFs without sebaceous induction.5 Immunohistochemical stains are important to help differentiate DF from dermatofibrosarcoma protuberans, especially when approaching the subcutis. Dermatofibromas stain positive for factor XIIIa and negative for CD34, whereas dermatofibrosarcoma protuberans stain negative for factor XIIIa and positive for CD34.7 Dermatofibromas also demonstrate positive immunostaining for vimentin, stromelysin 3,8 muscle-specific actin, and CD68.
- Rahbari H, Mehregan AH. Adnexal displacement and regression in association with histiocytoma (dermatofibroma). J Cutan Pathol. 1985;12:94-102.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Kanitakis J, Carbonnel E, Delmonte S, et al. Multiple eruptive dermatofibromas in a patient with HIV infection: case report and literature review. J Cutan Pathol. 2000;27:54-56.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Shuweiter M, Böer A. Spectrum of follicular and sebaceous differentiation induced by dermatofibroma. Am J Dermatopathol. 2009;31:778.
- Abenoza P, Lillemoe T. CD34 and factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Am J Dermatopathol. 1993;15:429-434.
- Kim HJ, Lee JY, Kim SH, et al. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: comparison with factor XIIIa and CD34. Br J Dermatol. 2007;157:319-324.
- Rahbari H, Mehregan AH. Adnexal displacement and regression in association with histiocytoma (dermatofibroma). J Cutan Pathol. 1985;12:94-102.
- Yazici AC, Baz K, Ikizoglu G, et al. Familial eruptive dermatofibromas in atopic dermatitis. J Eur Acad Dermatol Venereol. 2006;20:90-92.
- Kanitakis J, Carbonnel E, Delmonte S, et al. Multiple eruptive dermatofibromas in a patient with HIV infection: case report and literature review. J Cutan Pathol. 2000;27:54-56.
- Zaccaria E, Rebora A, Rongioletti F. Multiple eruptive dermatofibromas and immunosuppression: report of two cases and review of the literature. Int J Dermatol. 2008;47:723-727.
- Zeidi M, North JP. Sebaceous induction in dermatofibroma: a common feature of dermatofibromas on the shoulder. J Cutan Pathol. 2015;42:400-405.
- Shuweiter M, Böer A. Spectrum of follicular and sebaceous differentiation induced by dermatofibroma. Am J Dermatopathol. 2009;31:778.
- Abenoza P, Lillemoe T. CD34 and factor XIIIa in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans. Am J Dermatopathol. 1993;15:429-434.
- Kim HJ, Lee JY, Kim SH, et al. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: comparison with factor XIIIa and CD34. Br J Dermatol. 2007;157:319-324.
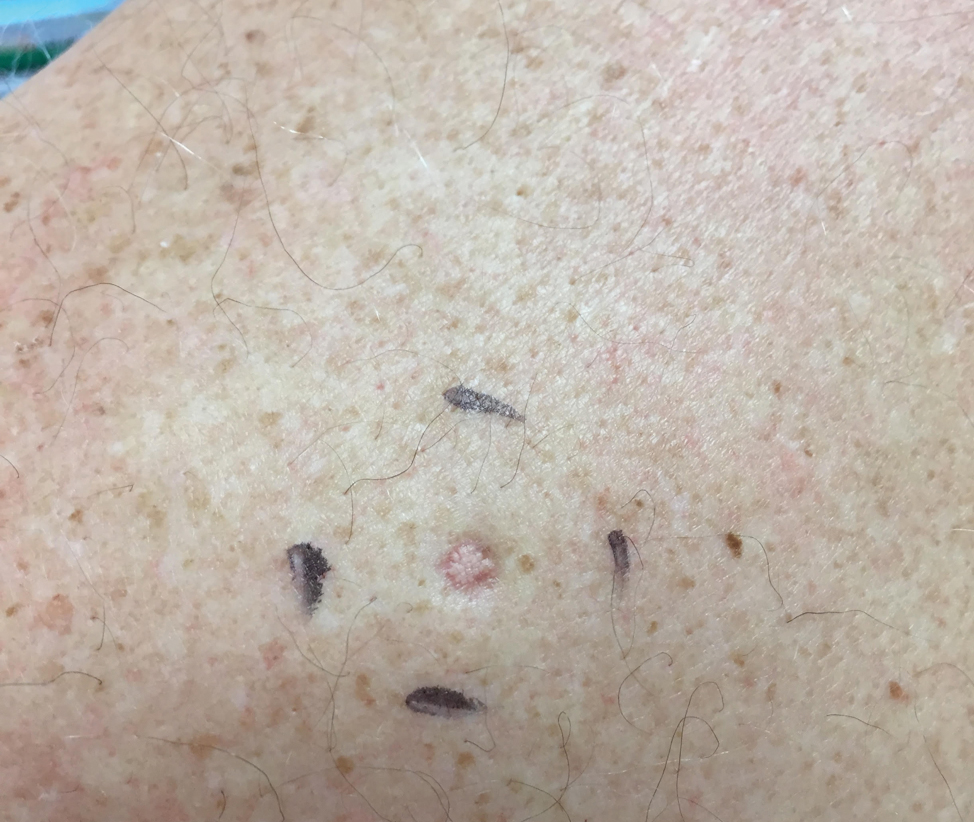

A 64-year-old man presented to dermatology for a full-body skin examination. He had no history of skin cancer. Physical examination revealed an asymptomatic, 4-mm, yellowish pink papule on the left posterior shoulder (top). Dermoscopy revealed yellow globules (bottom). The patient was unsure of the duration of the lesion and denied any prior trauma or medical procedure to the area. Subsequently, a shave biopsy was performed.
Keratotic Papule on the Abdomen
The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
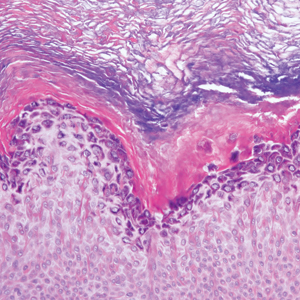
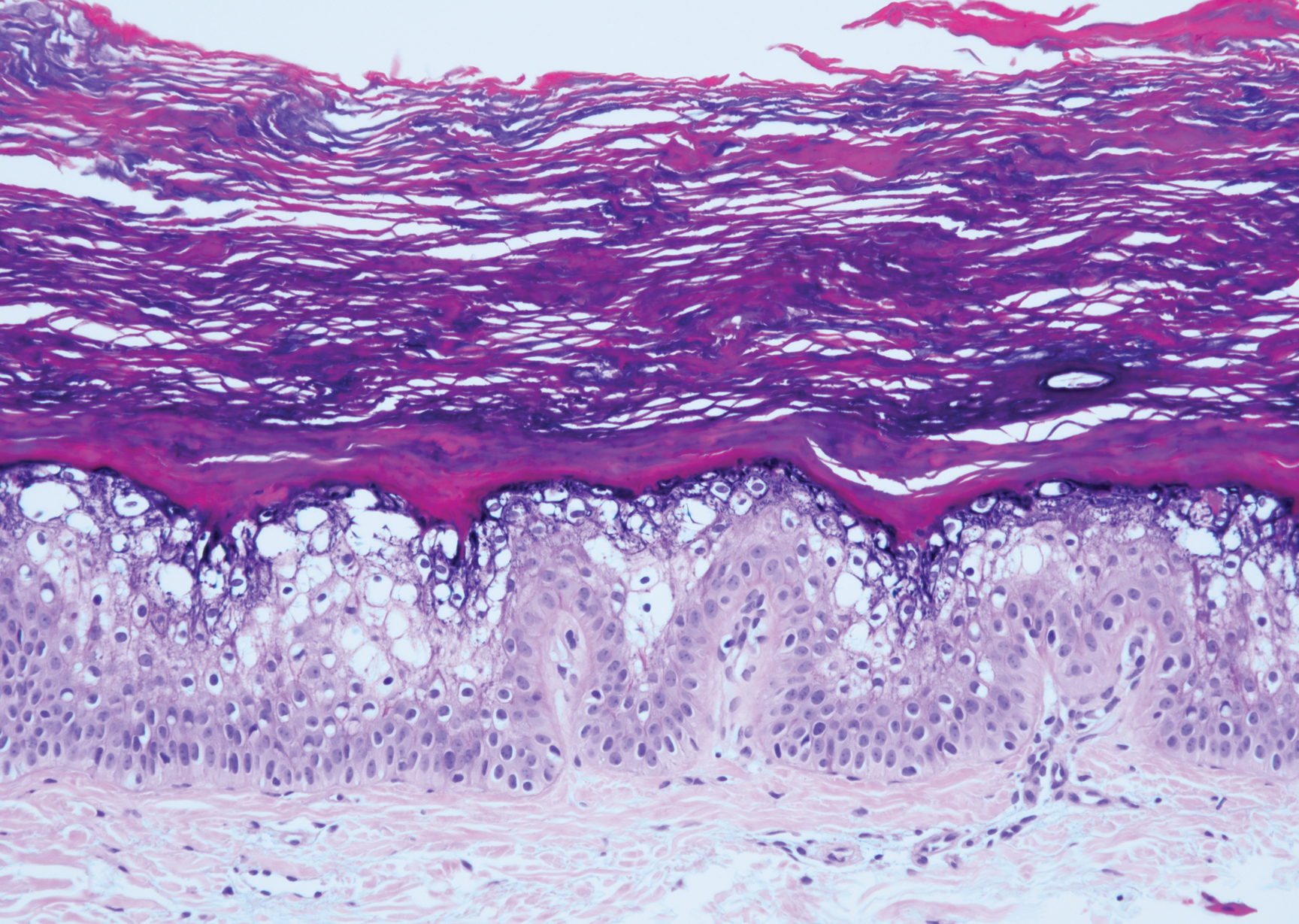
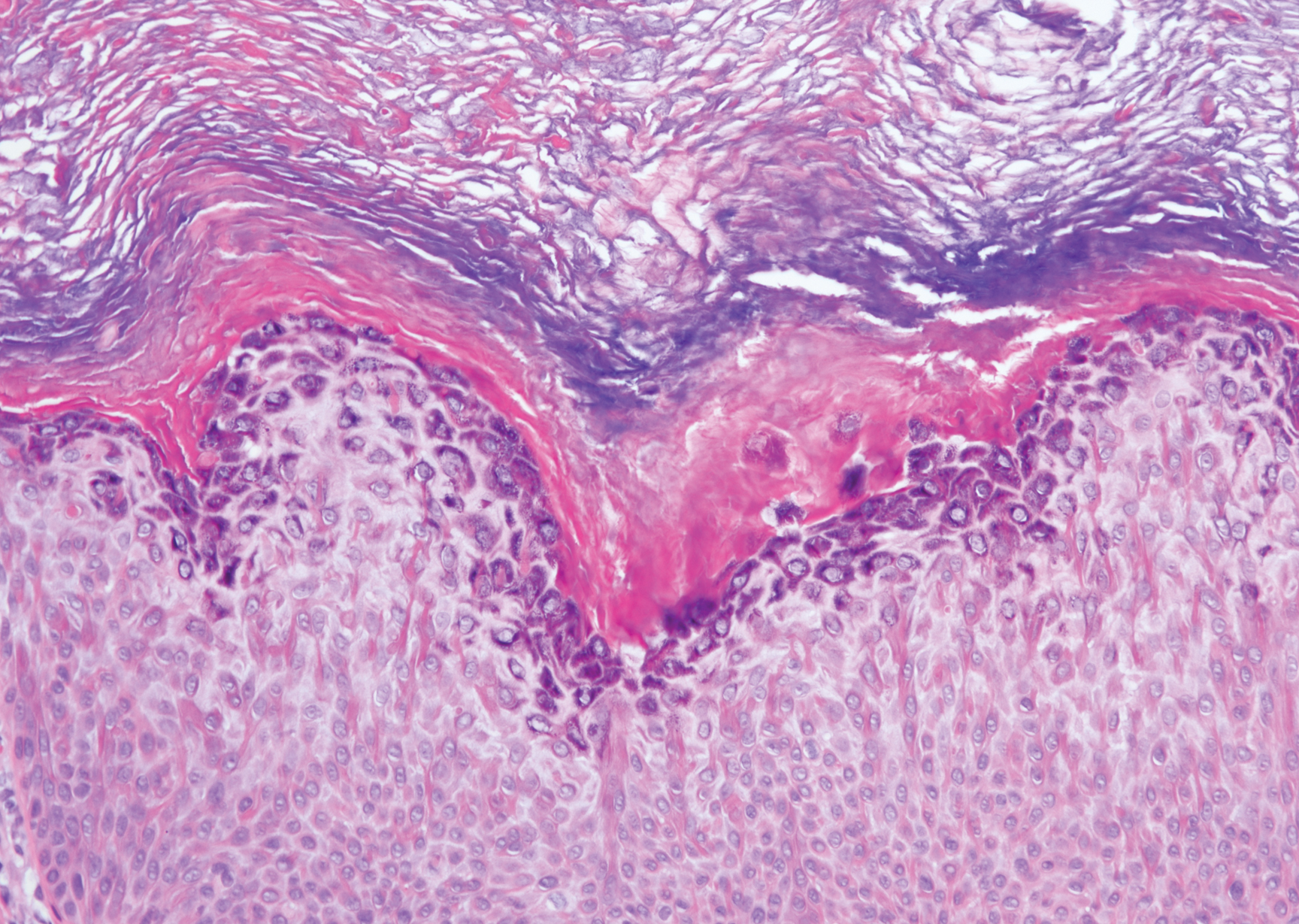
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5
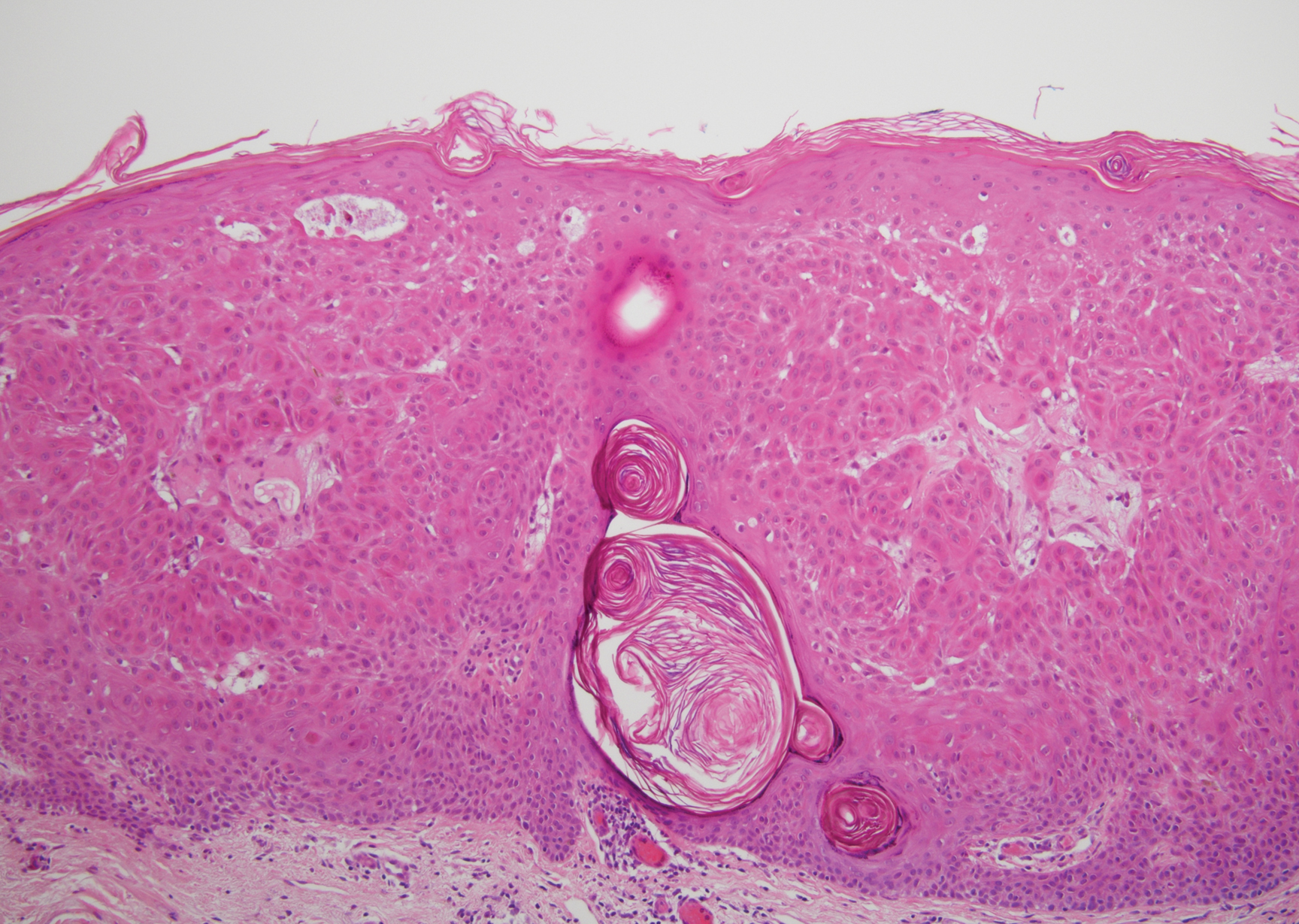
Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8
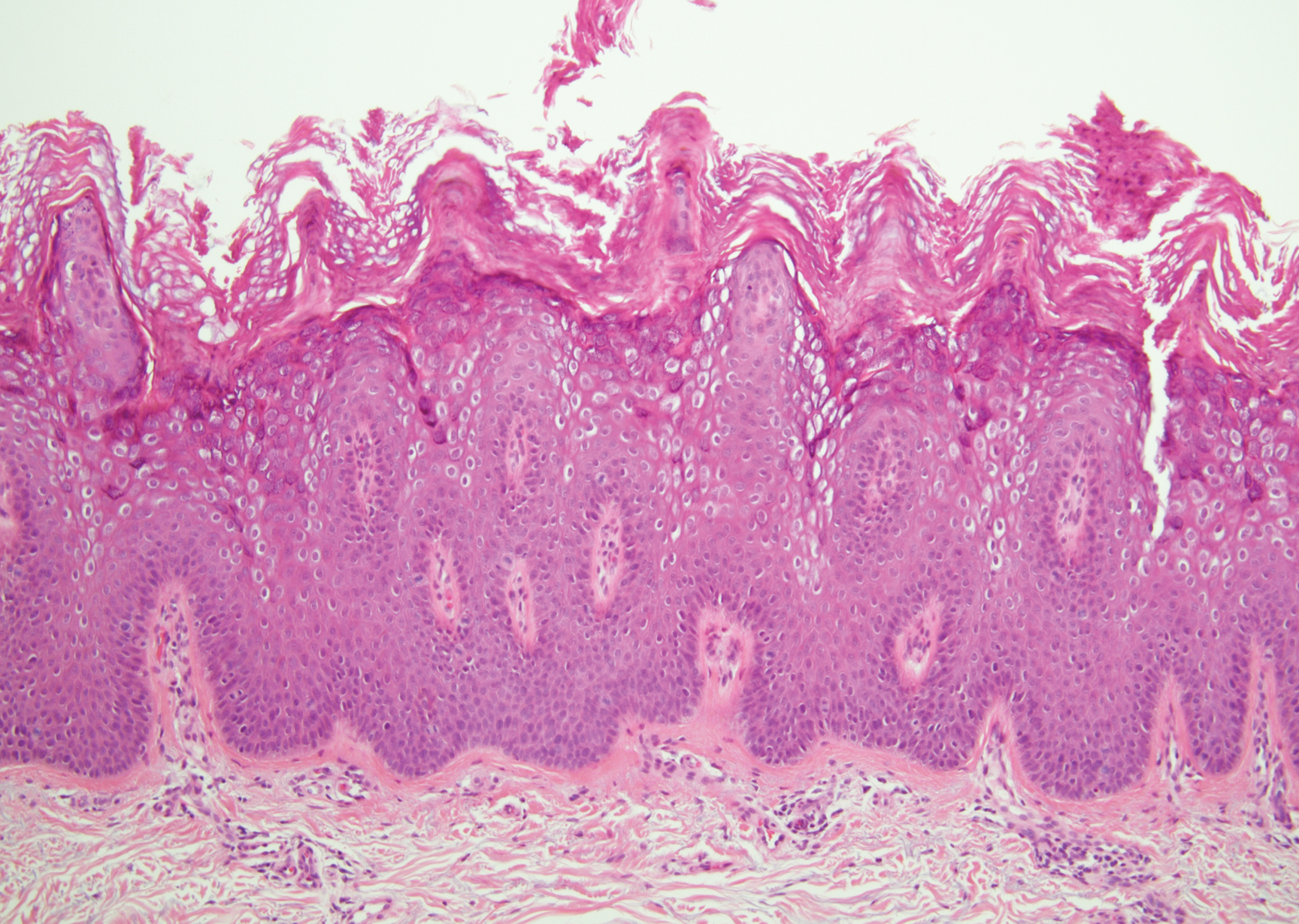
Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10
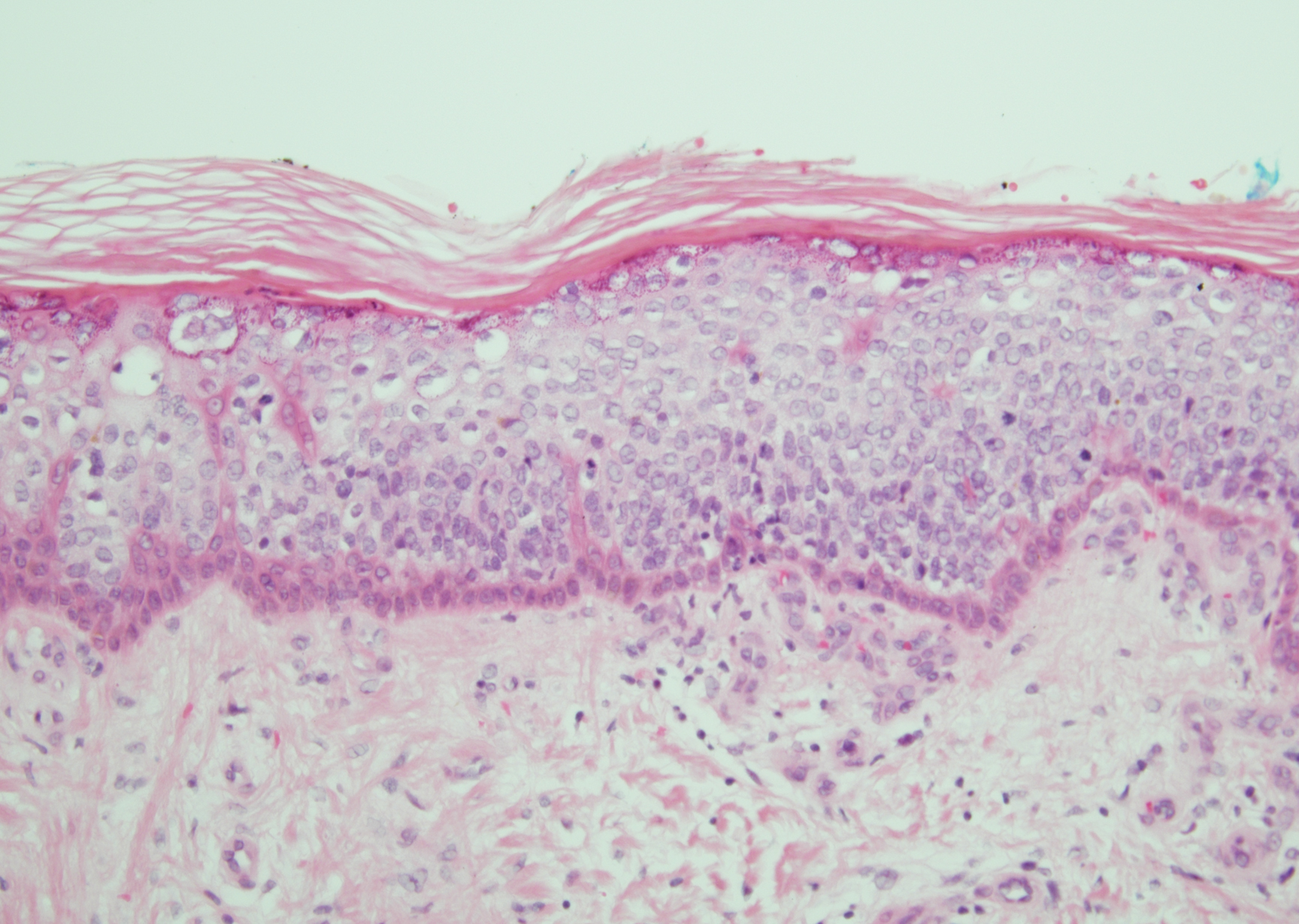
Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.
- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5

Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8

Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10

Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.

The Diagnosis: Hypergranulotic Dyscornification
Hypergranulotic dyscornification (HD) is a rarely reported reaction pattern present in benign solitary keratoses with only few reports to date. It may be an underrecognized reaction pattern based on the paucity of reported cases as well as the histologic similarities to other entities. It has been hypothesized that this pattern reflects an underlying keratin mutation or disorder of keratinization.1
Clinically, HD most commonly presents as a waxy, tan-colored, solitary keratosis generally found on the lower limbs, trunk, or back in individuals aged 20 to 60 years.1,2 Histopathology shows marked hyperkeratosis, papillomatosis, and clumped basophilic keratohyalin granules within the corneocytes with digitated epidermal hyperplasia. There is abnormal cornification across the entire lesion with papillomatosis and marked hypergranulosis.3 There often are homogeneous orthokeratotic mounds of large, dull, eosinophilic-staining anucleate keratinocytes that are sharply demarcated from the thickened granular layer.1,2 Within the spinous, granular, and corneal layers, there is a pale, gray-staining, basophilic, cytoplasmic substance intercellularly.1
Histopathologically, HD may be mistaken for several other entities both benign and malignant.1 Epidermolytic hyperkeratosis can be a genetic disorder, an incidental finding in a variety of skin conditions, or an isolated lesion.4 The genetic syndrome, caused by mutation in keratins 1 or 10, clinically presents with hyperkeratosis, erosions, blisters, and thickening of the epidermis, often with a corrugated appearance. Epidermal nevi findings often are seen in conjunction with histologic changes of epidermolytic hyperkeratosis caused by mutation. Solitary lesions also can resemble seborrheic keratosis or verruca. In all examples of epidermolytic hyperkeratosis, the histopathologic findings are identical.4 The granular layer is thickened, and coarse keratohyalin granules aggregate in the suprabasal cells.5 There is acantholysis with perinuclear vacuolization in the spinous and granular layers with characteristic pale cytoplasmic areas devoid of keratin filaments (Figure 1). The basal layer may be hyperproliferative.5

Irritated seborrheic keratosis presents as an exophytic, waxy, dark, sharply demarcated plaque with a stuck-on appearance.6 There is visible keratinization with comedolike openings, fissures and ridges, and scale; it also can contain milialike cysts. Histopathologically there is papillomatosis with prominent rete ridges, often including keratin pseudohorn cysts and squamous eddies. Enlarged capillaries can be seen in the dermal papillae. There is normal cytology with benign sheets of basaloid cells (Figure 2).7 Activating mutation in fibroblast growth factor receptor 3 leads to the growth and thickness of the epidermis that has been identified in these benign lesions.8

Verruca plana appears as a flesh-colored or reddish, warty, flat-topped papule that often forms clusters. Histopathologically it shows prominent hypergranulosis, thickened stratum spinosum, and vacuolized keratinocytes.9 The nuclei demonstrate a characteristic cytopathic effect of the virion, blurring the nuclear chromatin due to viral particle accumulation, known as koilocytes (Figure 3). The cause is the double-stranded DNA human papillomavirus types 2, 3, and 10.10

Bowen disease is a form of squamous cell carcinoma in situ characterized by an enlarging, well-demarcated, erythematous plaque with an irregular border and crusting or scaling. Histopathology reveals pleomorphic epidermal keratinization that becomes incorporated in the stratum corneum as parakeratotic nuclei. There is acanthosis, elongation of the rete ridges, and disorganized keratinocytes with atypia.11 The granular and spinous layers show an atypical honeycomb pattern with atypical cellular morphology (Figure 4).12 Bowen disease is a malignant lesion commonly found in older adults on sun-exposed skin that can evolve into invasive squamous cell carcinoma.

- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
- Roy SF, Ko CJ, Moeckel GW, et al. Hypergranulotic dyscornification: 30 cases of a striking epithelial reaction pattern. J Cutan Pathol. 2019;46:742-747.
- Dohse L, Elston D, Lountzis N, et al. Benign hypergranulotic keratosis with dyscornification. J Am Acad Dermatol. 2010;62:AB52.
- Reichel M. Hypergranulotic dyscornification. Am J Dermatopathol. 1999;21:21-24.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Peter Rout D, Nair A, Gupta A, et al. Epidermolytic hyperkeratosis: clinical update. Clin Cosmet Investig Dermatol. 2019;12:333-344.
- Ingraffea A. Benign skin neoplasms. Facial Plast Surg Clin North Am. 2013;21:21-32.
- Braun R. Dermoscopy of pigmented seborrheic keratosis. Arch Dermatol. 2002;138:1556.
- Duperret EK, Oh SJ, McNeal A, et al. Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle. 2014;13:1551-1559.
- Liu H, Chen S, Zhang F, et al. Seborrheic keratosis or verruca plana? a pilot study with confocal laser scanning microscopy. Skin Res Technol. 2010;16:408-412.
- Prieto-Granada CN, Lobo AZC, Mihm MC. Skin infections. In: Kradin RL, ed. Diagnostic Pathology of Infectious Disease. Philadelphia, PA: Saunders Elsevier; 2010:519-616.
- DeCoste R, Moss P, Boutilier R, et al. Bowen disease with invasive mucin-secreting sweat gland differentiation: report of a case and review of the literature. J Cutan Pathol. 2019;46:425-430.
- Ulrich M, Kanitakis J, González S, et al. Evaluation of Bowen disease by in vivo reflectance confocal microscopy. Br J Dermatol. 2011;166:451-453.
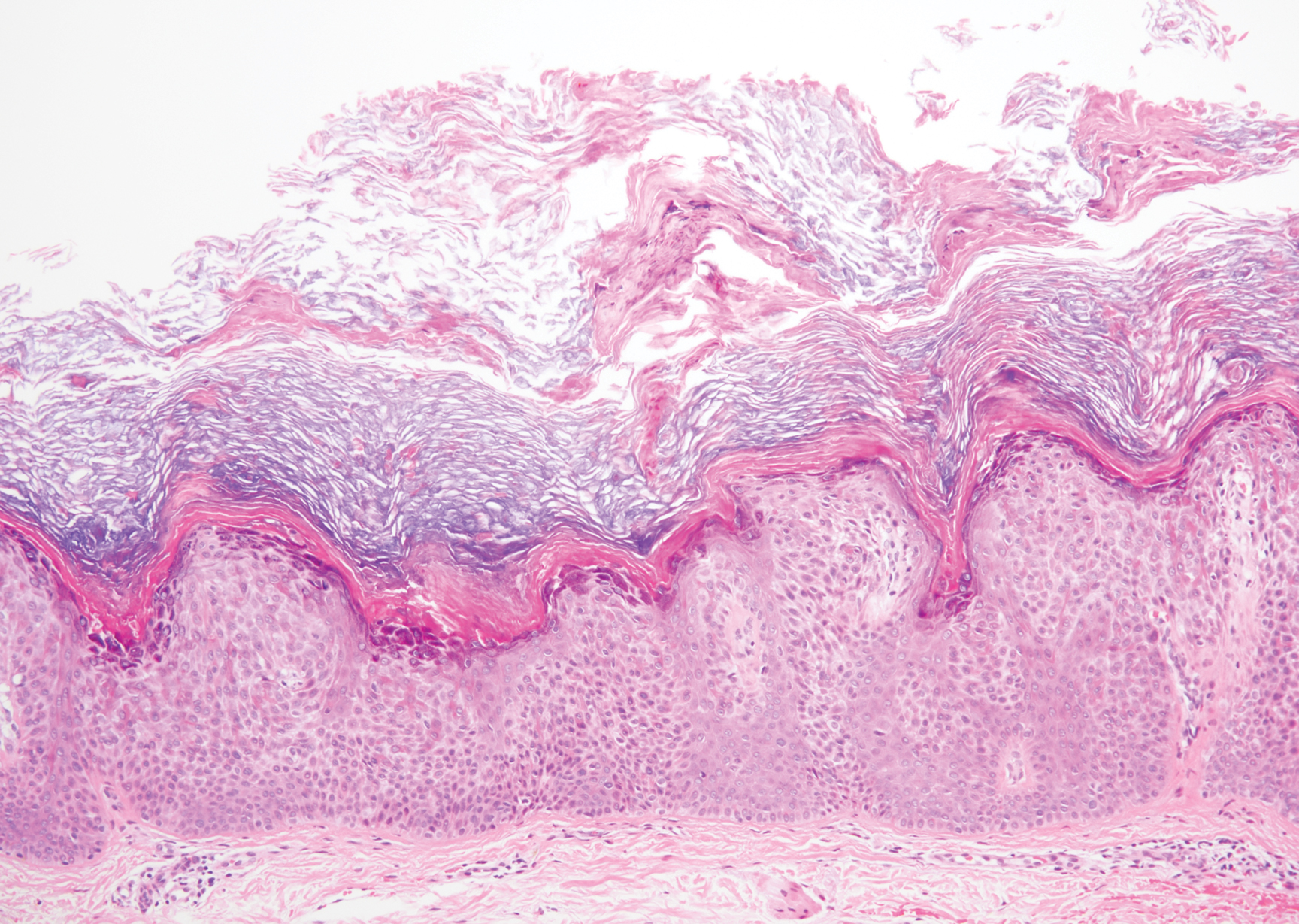
A 59-year-old woman with a history of basal cell carcinoma, uterine and ovarian cancer, and verrucae presented with an asymptomatic 3-mm lesion on the left side of the lower abdomen. Physical examination revealed a waxy, tan-colored, solitary keratosis. A shave biopsy was performed. Histopathology showed hyperkeratosis, focal parakeratosis, papillomatosis, and marked hypergranulosis with pale gray cytoplasm of the spinous-layer keratinocytes.
Petechiae and Ecchymoses on the Arm
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
The Diagnosis: Rumpel-Leede Phenomenon
Rumpel-Leede (R-L) phenomenon describes the rare benign occurrence of dermal capillaries acutely rupturing following the administration of a tourniquetlike force on an extremity,1 which manifests as asymptomatic petechiae and ecchymoses on a distal extremity, usually following noninvasive measurement of blood pressure.2 Rumpel-Leede phenomenon represents an underrecognized entity that either is excluded or minimally referenced in most dermatology textbooks. The R-L sign initially was described in the early 1900s after it was observed that tourniquets applied to the arms of patients with scarlet fever would lead to the development of petechiae in that extremity.3 It later was elucidated that underlying vascular disease predisposes to dermal capillary fragility, a risk factor for R-L phenomenon to occur upon application of a tourniquet. Rumpel-Leede phenomenon has been noted in patients with diabetes mellitus, acute or chronic hypertension, and thrombocytopenia.4 In addition to being hypertensive and diabetic, our patient had been taking amlodipine and diltiazem. Calcium channel blockers have been linked to R-L phenomenon in case reports as well as in a study of calcium channel blockers inducing capillary fragility in vivo.5 Rumpel-Leede phenomenon also has been noted in patients with tightly fitting garments and infants carried in baby carriers.1
The differential diagnosis for R-L phenomenon includes actinic purpura, small vessel vasculitis, disseminated intravascular coagulation (DIC), and deep vein thrombosis (DVT). Actinic purpura, also called solar purpura or senile purpura, represents the petechiae and ecchymoses that are associated with aging skin. It is thought to occur when DNA damage, UV-induced solar elastosis, and decreased lipids in the stratum corneum cause a weakened ability to contain red blood cell extravasation from capillaries.6 Due to the lack of history of trauma and clear association with the blood pressure cuff placement in our patient, a diagnosis of actinic purpura was unlikely. In small vessel vasculitis, patients classically present with nonblanching palpable purpura frequently distributed over the lower extremities. The isolation of the lesions to only the left arm lowered the suspicion for vasculitis. Cutaneous manifestations of DIC and other hypercoagulable states may include purpura, livedo reticularis, atrophie blanche, and in extreme cases purpura fulminans. Routine laboratory examination reveals thrombocytopenia, prolonged prothrombin time/partial thromboplastin time, and hemolytic anemia.7 Although our patient had the risk factors of recent infection and surgery, a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL) and platelet count of 279,000/µL (reference range, 150,000-350,000/µL) excluded DIC as the probable diagnosis. Our patient was at an overall increased risk for DVT due to his prolonged hospital stay, increased age, and other factors. Despite these risk factors, the lack of pain or swelling made this diagnosis unlikely. Furthermore, our patient was heparinized throughout his hospital stay, and upper extremity DVT accounts for only 4% to 10% of the total DVT incidence.5
Although R-L phenomenon is a benign, self-limited condition, it may be necessary in some cases to rule out more serious underlying etiologies with investigative workup comprised of a complete blood cell count, coagulation profile, and basic metabolic panel. However, recognition of the R-L phenomenon in the right clinical context of localized petechiae or ecchymoses with a history of a tourniquetlike force may prevent an unnecessary and costly workup. Patients should be encouraged that R-L phenomenon will resolve over time with identification and correction of the tourniquetlike force. In this case, we recommended loosening of the sphygmomanometer cuff and alternating extremities to which the cuff was to be placed, which resulted in complete clearance of the petechiae and ecchymoses within 5 days.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
- Nguyen T, Garcia D, Wang A, et al. Rumpel-Leede phenomenon associated with tourniquet-like forces of baby carriers in otherwise healthy infants. JAMA Dermatol. 2016;152:728-730.
- Chester M, Barwise J, Holzman M, et al. Acute dermal capillary rupture associated with noninvasive blood pressure monitoring. J Clin Anesth. 2007;19:473-475.
- Hartley A, Lim PB, Hayat SA. Rumpel-Leede phenomenon in a hypertensive patient due to mechanical trauma: a case report. J Med Case Rep. 2016;10:150.
- Varela D, Tran D, Ngamdu K, et al. Rumpel-Leede phenomenon presenting as a hypertensive urgency. Proc (Bayl Univ Med Cent). 2016;29:200-201.
- Cox NH, Walsh ML, Robson RH. Purpura and bleeding due to calcium-channel blockers: an underestimated problem? case reports and a pilot study. Clin Exp Dermatol. 2009;34:487-491.
- Ceilley RI. Treatment of actinic purpura. J Clin Aesthet Dermatol. 2017;10:44-50.
- Rajagopal R, Thachil J, Monagle P. Disseminated intravascular coagulation in paediatrics. Arch Dis Child. 2017;102:187-193.
- Kraaijpoel N, van Es N, Porreca E, et al. The diagnostic management of upper extremity deep venous thrombosis: a review of the literature. Thromb Res. 2017;156:54-59.
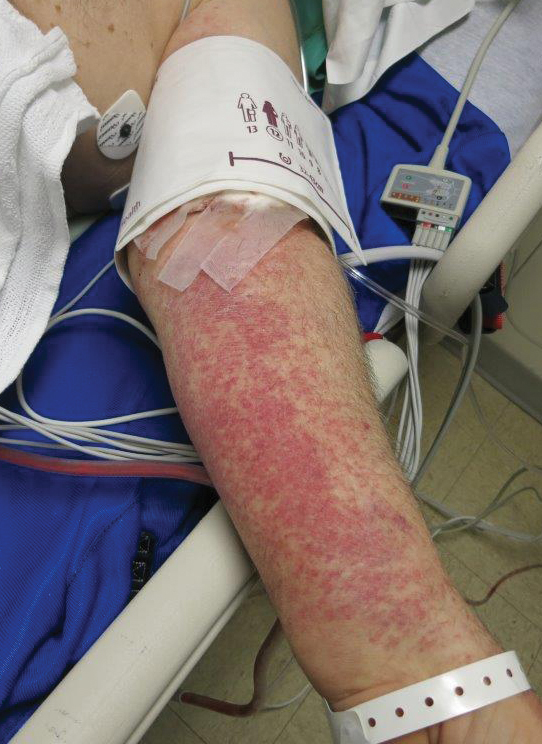
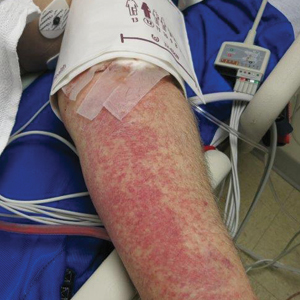
A 70-year-old man who had been admitted to the hospital a week prior for a right groin abscess overlying the site of a femoral graft developed a purpuric rash isolated to the left arm of 1 day's duration. The dermatology service was consulted by vascular surgery. The patient denied prior episodes of a similar rash, and there was no associated pruritus or pain. There was no history of trauma to the area. His medical history was remarkable for type 2 diabetes mellitus, hypertension, and peripheral vascular disease. His surgical history was notable for bilateral popliteal aneurysm repair and right femoral aneurysm repair. No pertinent family history was elicited. Cefepime, metronidazole, and vancomycin were administered for the groin abscess. Additional medications included amlodipine, atorvastatin, diltiazem, gabapentin, heparin, insulin, oxycodone, and acetaminophen.
Physical examination revealed broad ecchymoses on the left forearm with scattered petechiae as well as linear ecchymoses on the left upper arm distributed in the area where a sphygmomanometer cuff was applied. Full-body skin examination confirmed that the distribution of the petechiae and ecchymoses was limited to the left arm. The patient was normotensive at the time of examination. Laboratory evaluation revealed a hemoglobin level of 10.9 g/dL (reference range, 14.0-17.5 g/dL), a platelet count of 279,000/µL (reference range, 150,000-350,000/µL), and a glucose level of 121 mg/dL (reference range, 70-110 mg/dL).
Hand Hygiene in Preventing COVID-19 Transmission
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8
Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
Handwashing with antimicrobial soaps or alcohol-based sanitizers is an effective measure in preventing microbial disease transmission. In the context of coronavirus disease 2019 (COVID-19) prevention, the World Health Organization and Centers for Disease Control and Prevention have recommended handwashing with soap and water after coughing/sneezing, visiting a public place, touching surfaces outside the home, and taking care of a sick person(s), as well as before and after eating. When soap and water are not available, alcohol-based sanitizers may be used.1,2
Irritant contact dermatitis (ICD) is most commonly associated with wet work and is frequently seen in health care workers in relation to hand hygiene, with survey-based studies reporting 25% to 55% of nurses affected.3-5 In a prospective study (N=102), health care workers who washed their hands more than 10 times per day were55% more likely to develop hand dermatitis.6 Frequent ICD of the hands has been reported in Chinese health care workers in association with COVID-19.7 Handwashing and/or glove wearing may be newly prioritized by workers who handle frequently touched goods and surfaces, such as flight attendants (Figure). Patients with obsessive-compulsive disorder may be another vulnerable population.8

Alcohol-based sanitizers and detergents or antimicrobials in soaps may cause ICD of the hands by denaturation of stratum corneum proteins, depletion of intercellular lipids, and decreased corneocyte cohesion. These agents alter the skin flora, with increased colonization by staphylococci and gram-negative bacilli.9 Clinical findings include xerosis, scaling, fissuring, and bleeding. Physicians may evaluate severity of ICD of the hands using the
Cleansing the hands with alcohol-based sanitizers has consistently shown equivalent or greater efficacy than antimicrobial soaps for eradication of most microbes, with exception of bacterial spores and protozoan oocysts.11 In an in vivo experiment, 70% ethanol solution was more effective in eradicating rotavirus from the fingerpads of adults than 10% povidone-iodine solution, nonmedicated soaps, and soaps containing chloroxylenol 4.8% or chlorhexidine gluconate 4%.12 Coronavirus disease 2019 is a lipophilic enveloped virus. The lipid-dissolving effects of alcohol-based sanitizers is especially effective against these kinds of viruses. An in vitro experiment showed that alcohol solutions are effective against enveloped viruses including severe acute respiratory syndrome coronavirus, Ebola virus, and Zika virus.13 There are limited data for the virucidal efficacy of non–alcohol-based sanitizers containing quaternary ammonium compounds (most commonly benzalkonium chloride) and therefore they are not recommended for protection against COVID-19. Handwashing is preferred over alcohol-based solutions when hands are visibly dirty.
Alcohol-based sanitizers typically are less likely to cause ICD than handwashing with detergent-based or antimicrobial soaps. Antimicrobial ingredients in soaps such as chlorhexidine, chloroxylenol, and triclosan are frequent culprits.11 Detergents in soap such as sodium laureth sulfate cause more skin irritation and transepidermal water loss than alcohol14; however, among health care workers, alcohol-based sanitizers often are perceived as more damaging to the skin.15 During the 2014 Ebola outbreak, use of alcohol-based sanitizers vs handwashing resulted in lower hand eczema severity index scores (n=108).16
Propensity for ICD is a limiting factor in hand hygiene adherence.17 In a double-blind randomized trial (N=54), scheduled use of an oil-containing lotion was shown to increase compliance with hand hygiene protocols in health care workers by preventing cracks, scaling, and pain.18 Using sanitizers containing humectants (eg, aloe vera gel) or moisturizers with petrolatum, liquid paraffin, glycerin, or mineral oil have all been shown to decrease the incidence of ICD in frequent handwashers.19,20 Thorough hand drying also is important in preventing dermatitis. Drying with disposable paper towels is preferred over automated air dryers to prevent aerosolization of microbes.21 Because latex has been implicated in development of ICD, use of latex-free gloves is recommended.22
Alcohol-based sanitizer is not only an effective virucidal agent but also is less likely to cause ICD, therefore promoting hand hygiene adherence. Handwashing with soap still is necessary when hands are visibly dirty but should be performed less frequently if feasible. Hand hygiene and emollient usage education is important for physicians and patients alike, particularly during the COVID-19 crisis.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
- Centers for Disease Control and Prevention. Coronavirus disease 2019. how to protect yourself & others. https://www.cdc.gov/coronavirus/2019-ncov/prepare/prevention.html. Updated April 13, 2020. Accessed April 21, 2020.
- World Health Organization. Coronavirus disease (COVID-19) advice for the public. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/advice-for-public. Updated March 31, 2020. Accessed April 21, 2020.
- Carøe TK, Ebbehøj NE, Bonde JPE, et al. Hand eczema and wet work: dose-response relationship and effect of leaving the profession. Contact Dermatitis. 2018;78:341-347.
- Larson E, Friedman C, Cohran J, et al. Prevalence and correlates of skin damage on the hands of nurses. Heart Lung. 1997;26:404-412.
- Lampel HP, Patel N, Boyse K, et al. Prevalence of hand dermatitis in inpatient nurses at a United States hospital. Dermatitis. 2007;18:140-142.
- Callahan A, Baron E, Fekedulegn D, et al. Winter season, frequent hand washing, and irritant patch test reactions to detergents are associated with hand dermatitis in health care workers. Dermatitis. 2013;24:170-175.
- Lan J, Song Z, Miao X, et al. Skin damage among healthcare workers managing coronavirus disease-2019 [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1215-1216.
- Katz RJ, Landau P, DeVeaugh-Geiss J, et al. Pharmacological responsiveness of dermatitis secondary to compulsive washing. Psychiatry Res. 1990;34:223-226.
- Larson EL, Hughes CA, Pyrek JD, et al. Changes in bacterial flora associated with skin damage on hands of health care personnel. Am J Infect Control. 1998;26:513-521.
- Held E, Skoet R, Johansen JD, et al. The hand eczema severity index (HECSI): a scoring system for clinical assessment of hand eczema. a study of inter- and intraobserver reliability. Br J Dermatol. 2005;152:302-307.
- Boyce JM, Pittet D, Healthcare Infection Control Practices Advisory Committee, et al. Guideline for Hand Hygiene in Health-Care Settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HIPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Am J Infect Control. 2002;30:S1-S46.
- Ansari SA, Sattar SA, Springthorpe VS, et al. Invivo protocol for testing efficacy of hand-washing agents against viruses and bacteria—experiments with rotavirus and Escherichi coli. Appl Environ Microbiol. 1989;55:3113-3118.
- Siddharta A, Pfaender S, Vielle NJ, et al. virucidal activity of world health organization-recommended formulations against enveloped viruses, including Zika, Ebola, and emerging coronaviruses. J Infect Dis. 2017;215:902-906.
- Pedersen LK, Held E, Johansen JD, et al. Less skin irritation from alcohol-based disinfectant than from detergent used for hand disinfection. Br J Dermatol. 2005;153:1142-1146.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Wolfe MK, Wells E, Mitro B, et al. Seeking clearer recommendations for hand hygiene in communities facing Ebola: a randomized trial investigating the impact of six handwashing methods on skin irritation and dermatitis. PLoS One. 2016;11:e0167378.
- Pittet D, Allegranzi B, Storr J. The WHO Clean Care is Safer Care programme: field-testing to enhance sustainability and spread of hand hygiene improvements. J Infect Public Health. 2008;1:4-10.
- McCormick RD, Buchman TL, Maki DG. Double-blind, randomized trial of scheduled use of a novel barrier cream and an oil-containing lotion for protecting the hands of health care workers. Am J Infect Control. 2000;28:302-310.
- Berndt U, Wigger-Alberti W, Gabard B, et al. Efficacy of a barrier cream and its vehicle as protective measures against occupational irritant contact dermatitis. Contact Dermatitis. 2000;42:77-80.
- Kampf G, Ennen J. Regular use of a hand cream can attenuate skin dryness and roughness caused by frequent hand washing. BMC Dermatol. 2006;6:1.
- Gammon J, Hunt J. The neglected element of hand hygiene - significance of hand drying, efficiency of different methods, and clinical implication: a review. J Infect Prev. 2019;20:66-74.
- Elston DM. Letter from the editor: occupational skin disease among healthcare workers during the coronavirus (COVID-19) epidemic [published online March 18, 2020]. J Am Acad Dermatol. 2020;82:1085-1086.
Practice Points
- Alcohol-based sanitizers are as or even more effective as handwashing with soap and water for preventing disease transmission of enveloped viruses such as severe acute respiratory syndrome coronavirus.
- Although perceived as more irritating, alcohol-based sanitizers are less likely to cause irritant contact dermatitis of the hands than handwashing with soap and water.
- Use of humectants, moisturizers, and/or emollients in combination with alcohol-based sanitizers allows for effective hand hygiene without irritating the skin.
Analysis of Education on Nail Conditions at the American Academy of Dermatology Annual Meetings
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.

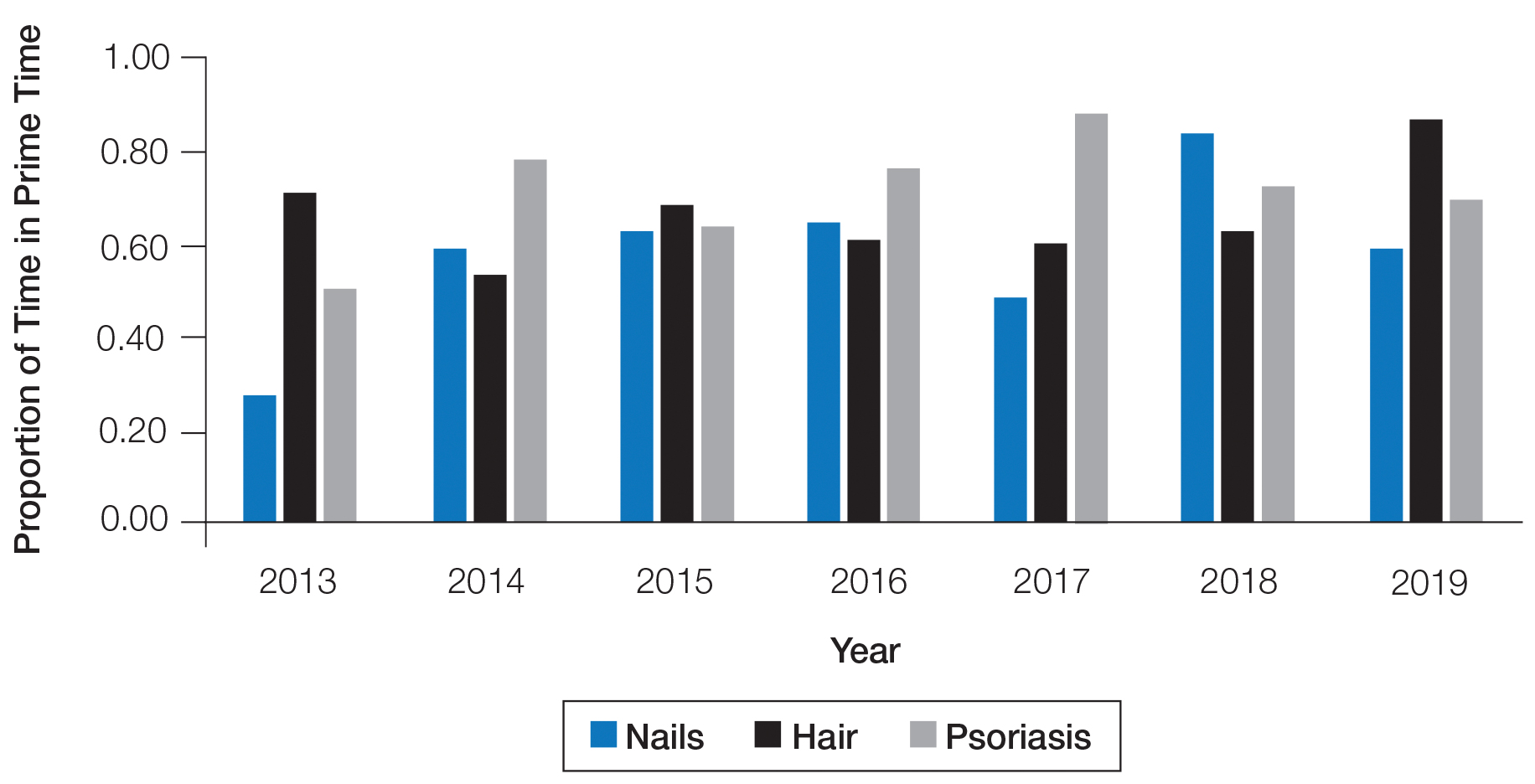
Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
To the Editor:
The diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.1 The American Academy of Dermatology (AAD) annual meeting is one of the largest and most highly attended dermatology educational conferences worldwide. We sought to determine the number of hours dedicated to nail-related topics at the AAD annual meetings from 2013 to 2019.
We accessed programs from the AAD annual meetings archive online (https://www.aad.org/meetings/previous-meetings-archive), and we used hair and psoriasis content for comparison. Event titles and descriptions were searched for nail-related content (using search terms nail, onychia, and onycho), hair-related content (hair, alopecia, trichosis, hirsutism), and psoriasis content (psoriasis). Data acquired for each event included the date, hours, title, and event type (eg, forum, course, focus session, symposium, discussion group, workshop, plenary session).
The number of hours dedicated to nail education consistently lagged behind those related to hair and psoriasis content during the study period (Figure 1). According to the AAD, the conference runs Friday to Tuesday with higher attendance Friday to Sunday (Tim Moses, personal communication, July 9, 2019). Lectures during the weekend are likely to have a broader reach than lectures on Monday and Tuesday. The proportion of nail content during weekend prime time slots was similar to that of hair and psoriasis (Figure 2). Plenary sessions often are presented by renowned experts on hot topics in dermatology. Notably, hair (2014-2015) and psoriasis (2015-2017) content were represented in the plenary sessions during the study period, while nail content was not featured.


Our study shows that nail-related education was underrepresented at the AAD annual meetings from 2013 to 2019 compared to hair- and psoriasis-related content. Educational gaps in the diagnosis of fignail conditions previously have been delineated, and prioritization of instruction on nail disease pathology and diagnostic procedures has been recommended to improve patient care.1 The majority of nail unit melanomas are diagnosed at late stages, which has been attributed to deficiencies in clinical knowledge and failure to perform or inadequate biopsy techniques.2 Notably, a survey of third-year dermatology residents (N=240) assessing experience in procedural dermatology showed that 58% performed 10 or fewer nail procedures and 30% did not feel competent in performing nail surgery.3 Furthermore, a survey examining the management of longitudinal melanonychia among attending and resident dermatologists (N=402) found that 62% of residents and 28% of total respondents were not confident in managing melanonychia.4
A limitation of this study was the lack of online data available for AAD annual meetings before 2013, so we were unable to characterize any long-term trends. Furthermore, we were unable to assess the educational reach of these sessions, as data on attendance are lacking.
This study demonstrates a paucity of nail-related content at the AAD annual meetings. The introduction of the “Hands-on: Nail Surgery” in 2015 is an important step forward to diminish the knowledge gap in the diagnosis of various nail diseases and malignancies. We recommend increasing the number of hours and overall content of didactic nail sessions at the AAD annual meeting to further the knowledge and procedural skills of dermatologists in caring for patients with nail disorders.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
- Hare AQ, R ich P. Clinical and educational gaps in diagnosis of nail disorders. Dermatol Clin. 2016;34:269-273.
- Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
- Lee EH, Nehal KS, Dusza SW, et al. Procedural dermatology training during dermatology residency: a survey of third-year dermatology residents. J Am Acad Dermatol. 2011;64:475-483.
- Halteh P, Scher R, Artis A, et al. A survey-based study of management of longitudinal melanonychia amongst attending and resident dermatologists. J Am Acad Dermatol. 2017;76:994-996.
Practice Points
- Diagnosis and treatment of nail conditions are necessary competencies for board-certified dermatologists, but appropriate education often is lacking.
- We recommend increasing the number of hours and overall content of didactic nail sessions at the American Academy of Dermatology annual meeting to further the knowledge and procedural skills of dermatologists caring for patients with nail disorders.
Facial Malignancies in Patients Referred for Mohs Micrographic Surgery: A Retrospective Review of the Impact of Hair Growth on Tumor and Defect Size
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
Male facial hair trends are continuously changing and are influenced by culture, geography, religion, and ethnicity.1 Although the natural pattern of these hairs is largely androgen dependent, the phenotypic presentation often is a result of contemporary grooming practices that reflect prevailing trends.2 Beards are common throughout adulthood, and thus, preserving this facial hair pattern is considered with reconstructive techniques.3,4 Male facial skin physiology and beard hair biology are a dynamic interplay between both internal (eg, hormonal) and external (eg, shaving) variables. The density of beard hair follicles varies within different subunits, ranging between 20 and 80 follicles/cm2. Macroscopically, hairs vary in length, diameter, color, and growth rate across individuals and ethnicities.1,5
There is a paucity of literature assessing if male facial hair offers a protective role for external insults. One study utilized dosimetry to examine the effectiveness of facial hair on mannequins with varying lengths of hair in protecting against erythemal UV radiation (UVR). The authors concluded that, although facial hair provides protection from UVR, it is not significant.6 In a study of 200 male patients with
We sought to determine if facial hair growth is implicated in the diagnosis and treatment of cutaneous malignancies. Specifically, we hypothesized that the presence of facial hair leads to a delay in diagnosis with increased subclinical growth given that tumors may be camouflaged and go undetected. Although there is a lack of literature, our anecdotal evidence suggests that male patients with facial hair have larger tumors compared to patients who do not regularly maintain any facial hair.
Methods
We performed a retrospective chart review following approval from the institutional review board at The University of North Carolina at Chapel Hill. We identified all male patients with a cutaneous malignancy located on the face who were treated from January 2015 to December 2018. Photographs were reviewed and patients with tumors located within the following facial hair-bearing anatomic subunits were included: lip, melolabial fold, chin, mandible, preauricular cheek, buccal cheek, and parotid-masseteric cheek. Tumors located within the medial cheek were excluded.
Facial hair growth was determined via image review. Because biopsy photographs were not uploaded into the health record for patients who were referred externally, we reviewed all historical photographs for patients who had undergone prior Mohs micrographic surgery at The University of North Carolina at Chapel Hill, preoperative photographs, and follow-up photographs as a proxy to determine facial hair status. Postoperative photographs taken within 2 weeks following surgery were not reviewed, as any facial hair growth was likely due to disinclination on behalf of the patient to shave near or over the incision. Age, number of days from biopsy to surgery, pathology, preoperative tumor size, number of Mohs layers, and defect size also were extrapolated from our chart review.
Statistical Analysis
Summary statistics were applied to describe demographic and clinical characteristics. An unpaired 2-tailed t test was utilized to test the null hypothesis that the mean difference was zero. The χ2 test was used for categorical variables. Results achieving P<.05 were considered statistically significant.
Results
We reviewed medical records for 171 patients with facial hair and 336 patients without facial hair. The primary outcomes for this study assessed tumor and defect size in patients with facial hair compared to patients with no facial hair (Table 1). On average, patients who had facial hair were younger (67.5 years vs 74.0 years, P<.001). The median number of days from biopsy to surgery (43.0 vs 44.0 days) was comparable across both groups. The majority of patients (47%) exhibited a beard, while 30% had a mustache and 23% had a goatee. The most common tumor location was the preauricular cheek for both groups (29% and 28%, respectively). The mean preoperative tumor size in the facial hair cohort was 1.40 cm compared to 1.22 cm in the group with no facial hair (P=.03). The mean number of Mohs layers in the facial hair cohort was 1.53 compared to 1.33 in the group with no facial hair (P=.03). The facial hair cohort also had a larger mean postoperative defect size (2.18 cm) compared to the group with no facial hair (1.98 cm); however, this finding was not significant (P=.05).
We then stratified our data to analyze only lip tumors in patients with and without a mustache (Table 2). The mean preoperative tumor size in the mustache cohort was 1.10 cm compared to 0.82 cm in the group with no mustaches (P=.046). The mean number of Mohs layers in the mustache cohort was 1.57 compared to 1.42 in the group with no mustaches (P=.43). The mustache cohort also had a larger mean postoperative defect size (1.63 cm) compared to the group with no facial hair (1.33 cm), though this finding also did not reach significance (P=.13).
Comment
Our findings support anecdotal observations that tumors in men with facial hair are larger, require more Mohs layers, and result in larger defects compared with patients who are clean shaven. Similarly, in lip tumors, men with a mustache had a larger preoperative tumor size. Although these patients also required more Mohs layers to clear and a larger defect size, these parameters did not reach significance. These outcomes may, in part, be explained by a delay in diagnosis, as patients with facial hair may not notice any new suspicious lesions within the underlying skin as easily as patients with glabrous skin.
Although facial hair may shield skin from UVR, we agree with Parisi et al6 that this protection is marginal at best and that early persistent exposure to UVR plays a much more notable role in cutaneous carcinogenesis. As more men continue to grow facial hairstyles that emulate historical or contemporary trends, dermatologists should emphasize the risk for cutaneous malignancies within these sun-exposed areas of the face. Although some facial hair practices may reflect cultural or ethnic settings, the majority reflect a desired appearance that is achieved with grooming or otherwise.
Skin cancer screening in men with facial hair, particularly those with a strong history of UVR exposure and/or family history, should be discussed and encouraged to diagnose cutaneous tumors earlier. We encourage men with facial hair to be cognizant that cutaneous malignancies can arise within hair-bearing skin and to incorporate self–skin checks into grooming routines, which is particularly important in men with dense facial hair who forego regular self-care grooming or trim intermittently. Furthermore, we urge dermatologists to continue to thoroughly examine the underlying skin, especially in patients with full beards, during skin examinations. Diagnosing and treating cutaneous malignancies early is imperative to maximize ideal functional and cosmetic outcomes, particularly within perioral and lip subunits, where marginal millimeters can impact reconstructive complexity.
Conclusion
Men with facial hair who had cutaneous tumors in our study exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men without any facial hair growth. Similar findings also were noted when we stratified and compared lip tumors in patients with and without mustaches. Given these observations, patients and dermatologists should continue to have a high index of suspicion for any concerning lesion located within skin underlying facial hair. Regular screening in men with facial hair should be discussed and encouraged to diagnose and treat potential cutaneous tumors earlier.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
- Wu Y, Konduru R, Deng D. Skin characteristics of Chinese men and their beard removal habits. Br J Dermatol. 2012;166:17-21.
- Janif ZJ, Brooks RC, Dixson BJ. Negative frequency-dependent preferences and variation in male facial hair. Biol Lett. 2014;10:20130958.
- Benjegerdes KE, Jamerson J, Housewright CD. Repair of a large submental defect. Dermatol Surg. 2019;45:141-143.
- Ninkovic M, Heidekruegger PI, Ehri D, et al. Beard reconstruction: a surgical algorithm. J Plast Reconstr Aesthet Surg. 2016;69:E111-E118.
- Maurer M, Rietzler M, Burghardt R, et al. The male beard hair and facial skin–challenges for shaving. Int J Cosmet Sci. 2016;38(suppl 1):3-9.
- Parisi AV, Turnbull DJ, Downs N, et al. Dosimetric investigation of the solar erythemal UV radiation protection provided by beards and moustaches. Radiat Prot Dosimetry. 2012;150:278-282.
- Liu DY, Gul MI, Wick J, et al. Long-term sheltering mustaches reduce incidence of lower lip actinic keratosis. J Am Acad Dermatol. 2019;80:1757-1758.e1.
Practice Points
- In our study, men with cutaneous tumors who had facial hair exhibited larger tumors, required more Mohs layers, and had a larger defect size compared to men who do not have any facial hair growth.
- Both patients and dermatologists should have a high index of suspicion for any concerning lesion contained within skin underlying facial hair to ensure prompt diagnosis and treatment of cutaneous tumors.
More on How to Decrease Dermatology Interview Costs
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
To the Editor:
Ongoing concern about the high costs of dermatology residency interviews has led to several cost-saving proposals, as presented by Hussain1 in the Cutis article, “Reducing the Cost of Dermatology Residency Applications: An Applicant’s Perspective.” Additional strategies to reduce applicant costs include eliminating travel costs through video or telephone interviews, interviewing students who are visiting during their away rotation, and developing and implementing a mechanism to exempt students from participating in the Electronic Residency Application Service (
First, because applicants would be limited to 1 application to participate in the early decision program, they must realistically consider the strength of their application and weigh their chances for acceptance to that program. Programs could facilitate the process by becoming more transparent about the type of applicants that have previously matched in their program.2 If an early-decision applicant successfully matches, that applicant would be prohibited from applying to additional dermatology residency programs through
Second, early-decision actions by programs—probably by August 1, a time when most third-year medical students have completed their academic year—would be determined before ERAS releases applications to residency programs. This timeline would remove successful applicants in the early decision program from going to additional interviews and incurring the associated travel costs.
Third, early decision could be potentially beneficial to applicants who are tied to a specific geographic region for training and to programs with specific program needs, such as expertise in specific areas of dermatology research or areas of clinical need (eg, adding a dermatopathologist, plastic surgeon, internist, or a pediatrician to the residency program who now wants dermatology training) or other program needs.
Fourth, application costs could potentially be lower for early-decision applicants than through the present application process if participating institutions waived application fees. Applicants would still be responsible for submitting requested academic transcripts, letters of recommendation, and travel expenses if an on-site interview is requested by the program.
Finally, highly desirable applicants who are offered a position through early decision would result in more opportunities for other applicants to interview for the remaining available residency positions through ERAS/NRMP.
Downsides to early decision for dermatology residency include the inability of applicants to compare programs to one another through their personal experiences, such as prior rotations or interviews, and for programs to compare applicants though the interview process and away rotations. In addition, US Medical Licensing Examination Step 2 scores and Alpha Omega Alpha honor medical society status and other academic honors may not be available to programs to consider at the time of early decision. Cooperation would be needed with ERAS and NRMP to create an early decision program for dermatology residency.
One other potential consequence of the early match could involve instances of strained relationships between research fellows and their sponsoring institution or dermatology program. Research fellows often match at their research institution, and failing to early match could potentially sour the relationship between the applicant and the program, thus leading to a less productive year. However, many programs participating in an early match will probably have additional residency positions remaining in the traditional match that would be still available to the fellows.
The concept of an early-binding residency match process has the potential to save both time and money for programs and applicants. Although an early-match process would have many positive effects, there also would be inherent downsides that accompany such a system. Nonetheless, an early-match process in dermatology has the prospect of efficiently pairing applicants and programs that feel strongly about each other while simplifying the match process and reducing costs for all parties involved.
References
1. Hussain AN.
2. Weisert E, Phan M. Thoughts on reducing the cost for dermatology residency applications. DIG@UTMB blog. http://digutmb.blogspot.com/2019/12/thoughts-on-reducing-cost-for.html. Published December 23, 2019. Accessed April 17, 2020.
3. Early decision program. Association of American Medical Colleges website. https://students-residents.aamc.org/applying-medical-school/article/early-decision-program/. Accessed April 8, 2020.
Author’s Response
The early decision option for dermatology residency applications would be a welcomed addition to the process but may be complicated by 2 recent events: the coronavirus disease 2019 (COVID-19) pandemic and the change of US Medical Licensing Examination (USMLE) Step 1 score reporting to a pass/fail system.
The COVID-19 pandemic has caused remarkable economic distress and likely affects medical students more acutely given their high levels of debt. As Ryan and Wagner observed, one advantage of the early-decision option would be financial relief for certain students. If applicants successfully match during the early-decision phase, they will not need to apply to any additional dermatology programs and also can target their preliminary-year applications to the geographic region where they have already matched.
In addition, the COVID-19 pandemic may further reduce early applicants’ ability to visit programs in person. Various medical schools have curtailed away rotations, and programs may opt for virtual interviews in accordance with social distancing guidelines.1 Thus, early applicants will have even fewer opportunities to compare programs before they must make a binding decision about their residency placement. Although away rotations and interview travel are some of the largest drivers of application cost,2 reducing costs in this way might shortchange both students and programs.
Arguably, the change in USMLE Step 1 score reporting beginning in 2022 may impact residency selection for a longer period of time than the COVID-19 pandemic. Program directors cited USMLE Step 1 scores as one of the main factors determining which applicants may be invited to interview.3 The lack of numerical USMLE Step 1 scores may encourage programs to place more weight on other metrics such as USMLE Step 2 CK scores or Alpha Omega Alpha membership.4 However, as Ryan and Wagner point out, such metrics may not be available in time for early-decision applicants.
As such, future program directors will have precious little information to screen early-decision applicants and may need to conduct holistic application review. This would require increased time and manpower compared to screening based on traditional metrics but may lead to a better “fit” for an applicant with a residency.
In general, implementation of any early decision program would benefit dermatology applicants as a group by removing elite candidates from the applicant pool. According to National Resident Matching Program data, just 3% of dermatology applicants account for more than 12% of overall interviews.5 In other words, a small group of the strongest applicants receives a lion’s share of interviews, crowding out many other candidates. Removing these top-tier applicants likely would provide remaining applicants with a higher return on investment per application, and students may choose to save money by applying to fewer programs.
Adopting early-decision options within the dermatology match may be complicated given the COVID-19 pandemic and USMLE score changes but may spur positive changes in the process while also reducing the financial burden on applicants.
Aamir N. Hussain, MD, MAPP
From Northwell Health, Manhasset, New York.
The author reports no conflict of interest.
Correspondence: Aamir N. Hussain, MD, MAPP (aamir.nav.hussain@gmail.com).
References
1. Coronavirus (COVID-19) and the VSLO program. Association of American Medical Colleges website. https://students-residents.aamc.org/attending-medical-school/article/coronavirus-covid-19-and-vslo-program/. Accessed April 17, 2020.
2. Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756.
3. National Resident Matching Program, Data Release and Research Committee. Results of the 2018 NRMP Program Director Survey. Washington, DC: National Resident Matching Program; 2018. https://www.nrmp.org/wp-content/uploads/2018/07/NRMP-2018-Program-Director-Survey-for-WWW.pdf. Published June 2018. Accessed April 17, 2020.
4. Crane MA, Chang HA, Azamfirei R. Medical education takes a step in the right direction: where does that leave students? [published online March 6, 2020]. JAMA. doi:10.1001/jama.2020.2950.
5. Lee AH, Young P, Liao R, et al. I dream of Gini: quantifying inequality in otolaryngology residency interviews. Laryngoscope. 2019;129:627-633.
Patient Questionnaire to Reduce Anxiety Prior to Full-Body Skin Examination
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
To the Editor:
A thorough full-body skin examination (FBSE) is an integral component of a dermatologic encounter and helps identify potentially malignant and high-risk lesions, particularly in areas that are difficult for the patient to visualize.1 Despite these benefits, many patients experience discomfort and anxiety about this examination because it involves sensitive anatomical areas. The true psychological impact of an FBSE is not clearly understood; however, research into improving patient comfort in these circumstances can have a broad positive impact.2 The purpose of this pilot study was to establish patients’ willingness to complete a pre-encounter questionnaire that defines their FBSE preferences as well as to identify the anatomical areas that are of most concern.
This study was approved by the University of Kansas institutional review board as nonhuman subjects research. A pre-encounter questionnaire that included information about the benefits of FBSEs was administered to 34 patients, allowing them to identify anatomic locations that they wanted to exclude from the FBSE.
Following the patient visit (in which the identified anatomical locations were excluded), patients were given a brief exit survey that asked about (1) their preference for a pre-encounter FBSE questionnaire and (2) the impact of the questionnaire on their anxiety level throughout the encounter. Preference for asking was surveyed using a 10-point scale (10=strong preference for the pre-encounter survey; 1=strong preference against the pre-encounter survey). Change in anxiety was surveyed using a 10-point scale (10=strong reduction in anxiety after the pre-encounter survey; 1=strong increase in anxiety after the pre-encounter survey). Statistical analysis was performed using 2-tailed unpaired t tests, with P<.05 considered statistically significant.
Twenty female and 14 male patients were enrolled (mean age, 53 years)(Table). The most commonly excluded anatomical location on the pre-encounter survey was the genitals, followed by the buttocks, breasts/chest, legs, feet, and abdomen (Table); 10 (71%) male and 13 (65%) female respondents did not exclude any component of the FBSE.
After the provider visit, females had a higher preference for the pre-encounter survey (mean score, 9.0) compared to males (mean score, 7.2; P=.021). Similarly, females had reduced anxiety about the office visit after survey administration compared to males (mean score, 8.3 vs 6.0; P=.001)(Table).
The results of our pilot study showed that a brief pre-encounter questionnaire may reduce the distress associated with an FBSE. Our survey took less than 1 minute to complete and served as a useful guide to direct the provider during the FBSE. Moreover, recognizing that patients do not want certain anatomic locations examined can serve as an opportunity for the dermatologist to provide helpful home skin check instructions and recommendations.
The small sample size was a limitation of this study. Future studies can assess with greater precision the clear benefits of a pre-encounter survey as well as the benefits or drawbacks of a survey compared to other modalities that are aimed at reducing patient anxiety about the FBSE, such as having the physician directly ask the patient about areas to avoid during the examination.
A pre-encounter survey about the FBSE can serve as an efficient means of determining patient preference and reducing self-reported anxiety about the visit.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Hoorens I, Vossaert K, Pil L, et al. Total-body examination vs lesion-directed skin cancer screening. JAMA Dermatol. 2016;152:27-34.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Practice Points
- Full-body skin examination (FBSE) is an assessment that requires examination of sensitive body areas, any of which can be seen as intrusive by certain patients.
- A pre-encounter survey on the FBSE can offer an efficient means by which to determine patient preference and reduce visit-associated anxiety.
CO2 Laser Ablative Fractional Resurfacing Photodynamic Therapy for Actinic Keratosis and Nonmelanoma Skin Cancer: A Randomized Split-Side Study
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
Actinic keratosis (AK) is the most common cutaneous lesion and is regarded as a precursor to nonmelanoma skin cancer (NMSC), particularly squamous cell carcinoma (SCC).1 Field cancerization refers to broad areas of chronically sun-exposed skin that show cumulative sun damage in the form of clinical and subclinical lesions. It is not feasible to treat large areas with multiple overt and subclinical lesions using surgical methods, and photodynamic therapy (PDT) has become a preferred method for treatment of field cancerization.2 Topical PDT uses the heme biosynthesis pathway precursors aminolevulinic acid (ALA) or methyl ALA (MAL), which localizes in the treatment area and is metabolized to protoporphyrin IX.3 After an incubation period, activation by a light source results in the formation of cytotoxic oxygen species,4 with reports of efficacy over large areas and excellent cosmetic outcomes.2
Laser ablative fractional resurfacing (AFR) also has been investigated as a treatment of AKs; CO2 laser AFR treatment resulted in a short-term reduction in the number of AK lesions and appeared to reduce the development of new lesions.5 However, case reports and small studies have indicated that pretreatment with laser AFR can increase the efficacy of PDT by creating microscopic vertical channels facilitating deeper penetration and uptake of the ALA.6 The use of erbium:YAG lasers in combination with PDT has demonstrated notable clinical and aesthetic improvements in treating basal cell carcinomas (BCCs)7 and AKs,8 with enhanced efficacy in moderate to thick AKs in particular. Hædersdal et al6 reported that CO2 laser AFR facilitated delivery of MAL into porcine skin, with AFR appearing to bypass the stratum corneum and deliver the treatment to the deep dermis.
The combination of CO2 laser AFR and PDT has shown statistically significant increases in efficacy for treatment of AKs compared to PDT alone (P<.001).9 In a small study, Alexiades10 reported a statistically significant improvement in AKs at 4 and 8 weeks posttreatment for 10 patients receiving CO2 laser AFR-PDT vs conventional PDT (P<.05). Studies of organ transplant recipients—who are at higher risk for AK and NMSC development—demonstrated favorable results for combined CO2 laser AFR and PDT vs either laser treatment11 or PDT9,12 alone, with significant reductions in the number of AKs (P=.002). Results were maintained for 3 to 4 months after treatment. Additional studies have shown that combining CO2 laser AFR and PDT may reduce the PDT incubation time or number of treatments required to achieve a response over conventional PDT.13,14
Our proof-of-concept study was designed to assess efficacy of CO2 laser AFR to enhance an approved drug delivery system in the treatment of AK and NMSC. The objective was to compare effect and durability of AFR-PDT vs standard ALA-PDT in the treatment of AK and NMSCs in a split-sided study of various body locations.
Methods
This randomized, split-sided study compared CO2 laser AFR-PDT to standard ALA-PDT for the treatment of AK and NMSC conducted at 1 site in Los Gatos, California. Patients who had a skin cancer screening and received a biopsy diagnosis of AK or NMSC were invited to attend an enrollment visit. Key inclusion criteria for enrollment were male or female patients aged 40 to 85 years with notable symmetrically comparable photodamage (at least 1 AK per square centimeter) in 1 or more skin areas—scalp, face, or distal extremities—with presence of clinically identifiable NMSCs proven by biopsy. Key exclusion criteria were patients who were pregnant; patients with epilepsy, seizures, or a photosensitive disorder; those taking photosensitizing medication (eg, doxycycline, hydrochlorothiazide); or immunocompromised patients. The study was approved by an institutional review board (Salus IRB [Austin, Texas]), and each participant underwent a complete and informed consent process.
Laterality for pretreatment with AFR followed by ALA-PDT vs ALA-PDT alone was determined at the time of treatment using a computer-based random number generator; even numbers resulted in pretreatment of the right side, and odd numbers resulted in pretreatment of the left side. Because of the difference in pretreatment methods for the 2 sides, it was not possible to perform the procedure under blinded conditions.
The treatment area was prepared by defatting the entire site with 70% isopropyl alcohol, followed by benzalkonium chloride antibacterial cleansing for the AFR pretreatment side. A 7% lidocaine/7% tetracaine ointment was applied under polyethylene wrap occlusion to the AFR pretreatment side for 20 minutes. Additionally, nerve blocks and field blocks with a mixture of 1.1% lidocaine with epinephrine/0.5% bupivacaine with epinephrine were performed wherever feasible. After 20 minutes, the lidocaine-tetracaine ointment was removed with isopropanol, and AFR treatment commenced immediately with the SmartXide DOT laser (DEKA)(1 pass of 25 W, 1200-microsecond duration at 500-µm spacing, 200-µm spot size, achieving 12% surface area ablation). Hyperkeratotic treated areas were debrided with saline and received a second pass with the laser. Aminolevulinic acid solution 20% (Levulan Kerastick; DUSA Pharmaceuticals, Inc)15 was applied to both sides of the treatment area and allowed to absorb for a 1-hour incubation period, which was followed by blue-light exposure at a power density of 10 mW/cm2 for 16 minutes and 40 seconds using the BLU-U Photodynamic Therapy Illuminator (DUSA Pharmaceuticals, Inc). Areas treated with AFR were then covered with a layer of Aquaphor ointment (Beiersdorf, Inc) and an absorptive hydrogel dressing for48 to 96 hours, with continued application of the ointment until resolution of all crusting. After treatment, patients were instructed to avoid direct sun exposure, wear a hat or visor for the first 2 weeks posttreatment when outdoors, and apply sunscreen with a sun protection factor greater than 30 once skin had healed.
Follow-up was conducted at 1 week, 1 month, 3 months, and 6 months after the PDT procedure. The primary end points were clinical clearance of NMSC lesions at 1, 3, and 6 months posttreatment and histological clearance at 6 months. Secondary end points assessed quality of life and functional improvements.
Results
Twenty-four potential participants experiencing AKs and/or NMSCs were screened for the study, with 19 meeting inclusion criteria. All participants were white, non-Hispanic, and had Fitzpatrick skin types I or II. Treated areas for all participants had field cancerization defined as at least 1 AK per square centimeter. All 19 participants enrolled in the study completed the posttreatment evaluations up to 6 months. All AFR-pretreated sites showed superior results in reduction in number, size, or hyperkeratosis of AKs at all follow-up visits, with a complete absence of new AK formation at the 6-month follow-up (Table). Conversely, sites treated with standard PDT only showed some recurrence of AKs at 6 months. Of the 3 participants who had biopsy-confirmed BCCs on the AFR-pretreated side, there were 3 persistent lesions after treatment at the 6-month visit. Two participants experienced persistence of a confirmed SCC in situ that was on the laser-pretreated side only (1 on the forehead and 1 on the hand), whereas 1 participant with an SCC on the leg at baseline had no recurrence at 6 months. A participant who received treatment on the lower lip had persistence of actinic cheilitis on both the AFR- and non–AFR-treated sides of the lip.
Scalp and facial sites healed fully in an average of 7 days, whereas upper extremities—forearm and hands—took approximately 14 days to heal completely. Lower extremity AFR-pretreated sites exhibited substantial weeping, resulting in prolonged healing of approximately 21 days for resolution of all scabbing.
Comment
In this split-sided study in patients with field cancerization, the use of CO2 laser AFR before treatment with PDT increased AK lesion clearance compared to ALA-PDT alone. Prior studies of fractional laser–assisted drug delivery on porcine skin using topical MAL showed that laser channels approximately 3-mm apart were able to distribute protoporphyrin through the entire skin.6 The ablative nature of AFR theoretically provides deeper and more effusive penetration of the ALA solution than using conventional PDT or erbium:YAG lasers with PDT.7,8 Helsing et al11 applied CO2 laser AFR MAL-PDT to AKs in organ transplant recipients and obtained complete responses in 73% of patients compared to a complete response of 31% for AFR alone. The results reported in our study are consistent with Helsing et al,11 showing a complete clinical response for 14 of 19 patients (74%), of whom 4 (21%) had no recurrence of NMSC and 10 (53%) had no recurrence of AK on the AFR-PDT–treated side.
The pretreatment process required for the laser AFR added time to the initial visit compared to conventional PDT, which is balanced by a reduced PDT incubation time (1 hour vs the approved indication of 14–18 hours for face/scalp or 3 hours for upper extremities under occlusion). The use of microneedling as an alternative pretreatment procedure before PDT also has been investigated, with the aim of decreasing the optimum ALA absorption time. The mean reduction in AKs (89.3%) was significantly greater than for PDT alone (69.5%; P<.05) in a small study by Spencer and Freeman.18 Although microneedling is less time-intensive and labor-intensive than laser AFR, the photocoagulative effect and subsequent microhemorrhages resulting from AFR should result in much deeper penetration of ALA solution than for microneedling.
The limitations of this proof-of-concept study arose from the small sample size of 19 participants and the short follow-up period of 6 months. Furthermore, the unblinded nature of the study could create selection, detection, or reporting bias. Further follow-up appointments would aid in determining the longevity of results, which may encourage future use of this technique, despite the time-consuming preparation. A larger study with follow-up greater than 1 year would be beneficial, particularly for monitoring remission from SCCs and BCCs.
Conclusion
Pretreatment with CO2 laser AFR before ALA-PDT provided superior clearance of AKs and thin NMSCs at 6 months compared to ALA-PDT alone (Figure). Additionally, the incubation period for ALA absorption can be reduced before PDT, leading to a shorter treatment time overall. The benefits of AFR pretreatment on AK clearance demonstrated in this study warrant further investigation in a larger trial with a longer follow-up period to monitor maintenance of response.
Acknowledgments
The authors thank the patients who participated in this study. Editorial assistance was provided by Louise Gildea, PhD, of JK Associates Inc, part of the Fishawack Group of Companies (Fishawack, United Kingdom), funded by Sun Pharmaceutical Industries, Inc.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
- Criscione VD, Weinstock MA, Naylor MF, et al. Actinic keratoses: natural history and risk of malignant transformation in the Veterans Affairs Topical Tretinoin Chemoprevention Trial. Cancer. 2009;115:2523-2530.
- Morton CA, McKenna KE, Rhodes LE. Guidelines for topical photodynamic therapy: update. Br J Dermatol. 2008;159:1245-1266.
- Casas A, Fukuda H, Di Venosa G, et al. Photosensitization and mechanism of cytotoxicity induced by the use of ALA derivatives in photodynamic therapy. Br J Cancer. 2001;85:279-284.
- Klotz LO, Fritsch C, Briviba K, et al. Activation of JNK and p38 but not ERK MAP kinases in human skin cells by 5-aminolevulinate-photodynamic therapy. Cancer Res. 1998;58:4297-4300.
- Gan SD, Hsu SH, Chuang G, et al. Ablative fractional laser therapy for the treatment of actinic keratosis: a split-face study. J Am Acad Dermatol. 2016;74:387-389.
- Hædersdal M, Sakamoto FH, Farinelli WA, et al. Fractional CO(2) laser-assisted drug delivery. Lasers Surg Med. 2010;42:113-122.
- Šmucler R, Vlk M. Combination of Er:YAG laser and photodynamic therapy in the treatment of nodular basal cell carcinoma. Lasers Surg Med. 2008;40:153-158.
- Ko DY, Jeon SY, Kim KH, et al. Fractional erbium:YAG laser-assisted photodynamic therapy for facial actinic keratoses: a randomized, comparative, prospective study. J Eur Acad Dermatol Venereol. 2014;28:1529-1539.
- Togsverd-Bo K, Lei U, Erlendsson AM, et al. Combination of ablative fractional laser and daylight-mediated photodynamic therapy for actinic keratosis in organ transplant recipients—a randomized controlled trial. Br J Dermatol. 2015;172:467-474.
- Alexiades M. Randomized, controlled trial of fractional carbon dioxide laser resurfacing followed by ultrashort incubation aminolevulinic acid blue light photodynamic therapy for actinic keratosis. Dermatol Surg. 2017;43:1053-1064.
- Helsing P, Togsverd-Bo K, Veierod MB, et al. Intensified fractional CO2 laser-assisted photodynamic therapy vs. laser alone for organ transplant recipients with multiple actinic keratoses and wart-like lesions: a randomized half-side comparative trial on dorsal hands. Br J Dermatol. 2013;169:1087-1092.
- Togsverd-Bo K, Haak CS, Thaysen-Petersen D, et al. Intensified photodynamic therapy of actinic keratoses with fractional CO2 laser: a randomized clinical trial. Br J Dermatol. 2012;166:1262-1269.
- Jang YH, Lee DJ, Shin J, et al. Photodynamic therapy with ablative carbon dioxide fractional laser in treatment of actinic keratosis. Ann Dermatol. 2013;25:417-422.
- Song HS, Jung SE, Jang YH, et al. Fractional carbon dioxide laser-assisted photodynamic therapy for patients with actinic keratosis. Photodermatol Photoimmunol Photomed. 2015;31:296-301.
- ALA Kerastick (aminolevulinic acid HCl) for topical solution, 20% [package insert]. Wilmington, MA: DUSA Pharmaceuticals; 2010.
- Data on file. Wilmington, MA: DUSA Pharmaceuticals; 2020.
- Campbell TM, Goldman MP. Adverse events of fractionated carbon dioxide laser: review of 373 treatments. Dermatol Surg. 2010;36:1645-1650.
- Spencer JM, Freeman SA. Microneedling prior to Levulan PDT for the treatment of actinic keratoses: a split-face, blinded trial. J Drugs Dermatol. 2016;15:1072-1074.
Practice Points
- Pretreatment with CO2 laser ablative fractional resurfacing (AFR) before photodynamic therapy (PDT) provided efficient clearance of actinic keratosis (AK).
- Superior clearance of lesions was seen at 6 months for AK and thin nonmelanoma skin cancers (NMSCs) on pretreated sites compared to PDT alone, with no novel adverse events reported.
- A reduced incubation period for aminolevulinic acid (ALA) absorption before PDT was used, leading to a shorter overall treatment time.