User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Group Clinic for Chemoprevention of Squamous Cell Carcinoma: A Pilot Study
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
Squamous cell carcinoma (SCC) has an estimated incidence of more than 2.5 million cases per year in the United States.1 Its precursor lesion, actinic keratosis (AK), had an estimated prevalence of 39.5 million cases in the United States in 2004.2 The dermatology clinic at the Providence VA Medical Center in Rhode Island exerts consistent efforts to treat both SCC and AK by prescribing topical 5-fluorouracil (5-FU) and lifestyle changes that include avoiding sun exposure, wearing protective clothing, and using effective sunscreen.3 A single course of topical 5-FU in veterans has been shown to decrease the risk for SCC by 74% during the year after treatment and also improve AK clearance rates.4,5
Effectiveness of 5-FU for secondary prevention can be decreased by patient misunderstandings, such as applying 5-FU for too short a time or using the corticosteroid cream prematurely, as well as patient nonadherence due to expected adverse skin reactions to 5-FU.6 Education and reassurance before and during therapy maximize patient compliance but can be difficult to accomplish in clinics when time is in short supply. During standard 5-FU treatment at the Providence VA Medical Center, the provider prescribes 5-FU and posttherapy corticosteroid cream at a clinic visit after an informed consent process that includes reviewing with the patient a color handout depicting the expected adverse skin reaction. Patients who later experience severe inflammation and anxiety call the clinic and are overbooked as needed.
To address the practical obstacles to the patient experience with topical 5-FU therapy, we developed a group chemoprevention clinic based on the shared medical appointment (SMA) model. Shared medical appointments, during which multiple patients are scheduled at the same visit with 1 or more health care providers, promote patient risk reduction and guideline adherence in complex diseases, such as chronic heart failure and diabetes mellitus, through efficient resource use, improvement of access to care, and promotion of behavioral changes through group support.7-13 To increase efficiency in the group chemoprevention clinic, we integrated dermatology nurses and nurse practitioners from the chronic care model into the group medical visits, which ran from September 2016 through March 2017. Because veterans could interact with peers undergoing the same treatment, we hypothesized that use of the cream in a group setting would provide positive reinforcement during the course of therapy, resulting in a positive treatment experience. We conducted a retrospective review of medical records of the patients involved in this pilot study to evaluate this model.
Methods
Institutional review board approval was obtained from the Providence VA Medical Center. Informed consent was waived because this study was a retrospective review of medical records.
Study Population
We offered participation in a group chemoprevention clinic based on the SMA model for patients of the dermatology clinic at the Providence VA Medical Center who were planning to start 5-FU in the fall of 2016. Patients were asked if they were interested in participating in a group clinic to receive their 5-FU treatment. Patients who were established dermatology patients within the Veterans Affairs system and had scheduled annual full-body skin examinations were included; patients were not excluded if they had a prior diagnosis of AK but had not been previously treated with 5-FU.
Design
Each SMA group consisted of 3 to 4 patients who met initially to receive the 5-FU medication and attend a 10-minute live presentation that included information on the dangers and causes of SCC and AK, treatment options, directions for using 5-FU, expected spectrum of side effects, and how to minimize the discomfort of treatment side effects. Patients had field treatment limited to areas with clinically apparent AKs on the face and ears. They were prescribed 5-FU cream 5% twice daily.
One physician, one nurse practitioner, and one registered nurse were present at each 1-hour clinic. Patients arrived and were checked in individually by the providers. At check-in, the provider handed the patient a printout of his/her current medication list and a pen to make any necessary corrections. This list was reviewed privately with the patient so the provider could reconcile the medication list and review the patient’s medical history and so the patient could provide informed consent. After, the patient had the opportunity to select a seat from chairs arranged in a circle. There was a live PowerPoint presentation given at the beginning of the clinic with a question-and-answer session immediately following that contained information about the disease and medication process. Clinicians assisted the patients with the initial application of 5-FU in the large group room, and each patient received a handout with information about AKs and a 40-g tube of the 5-FU cream.
This same group then met again 2 weeks later, at which time most patients were experiencing expected adverse skin reactions. At that time, there was a 10-minute live presentation that congratulated the patients on their success in the treatment process, reviewed what to expect in the following weeks, and reinforced the importance of future sun-protective practices. At each visit, photographs and feedback about the group setting were obtained in the large group room. After photographing and rating each patient’s skin reaction severity, the clinicians advised each patient either to continue the 5-FU medication for another week or to discontinue it and apply the triamcinolone cream 0.1% up to 4 times daily as needed for up to 7 days. Each patient received the prescription corticosteroid cream and a gift, courtesy of the VA Voluntary Service Program, of a 360-degree brimmed hat and sunscreen. Time for questions or concerns was available at both sessions.
Data Collection
We reviewed medical records via the Computerized Patient Record System, a nationally accessible electronic health record system, for all patients who participated in the SMA visits from September 2016 through March 2017. Any patient who attended the initial visit but declined therapy at that time was excluded.
Outcomes included attendance at both appointments, stated completion of 14 days of 5-FU treatment, and evidence of 5-FU use according to a validated numeric scale of skin reaction severity.14 We recorded telephone calls and other dermatology clinic and teledermatology appointments during the 3 weeks after the first appointment and the number of dermatology clinic appointments 6 months before and after the SMA for side effects related to 5-FU treatment. Feedback about treatment in the group setting was obtained at both visits.
Results
A total of 16 male patients attended the SMAs, and 14 attended both sessions. Of the 2 patients who were excluded from the study, 1 declined to be scheduled for the second group appointment, and the other was scheduled and confirmed but did not come for his appointment. The mean age was 72 years.
Of the 14 study patients who attended both sessions of the group clinic, 10 stated that they completed 2 weeks of 5-FU therapy, and the other 4 stated that they completed at least 11 days. Results of the validated scale used by clinicians during the second visit to grade the patients’ 5-FU reactions showed that all 14 patients demonstrated at least some expected adverse reactions (eTable). Eleven of 14 patients showed crusting and erosion; 13 showed grade 2 or higher erythema severity. One patient who stopped treatment after 11 days telephoned the dermatology clinic within 1 week of his second SMA. Another patient who stopped treatment after 11 days had a separate dermatology surgery clinic appointment within the 3-week period after starting 5-FU for a recent basal cell carcinoma excision. None of the 14 patients had a dermatology appointment scheduled within 6 months before or after for a 5-FU adverse reaction. One patient who completed the 14-day course was referred to teledermatology for insect bites within that period.
None of the patients were prophylaxed for herpes simplex virus during the treatment period, and none developed a herpes simplex virus eruption during this study. None of the patients required antibiotics for secondary impetiginization of the treatment site.
The verbal feedback about the group setting from patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers. At the conclusion of the second appointment, all of the patients reported an increased understanding of their condition and the importance of future sun-protective behaviors.
Comment
Shared medical appointments promote treatment adherence in patients with chronic heart failure and diabetes mellitus through efficient resource use, improvement of access to care, and promotion of behavioral change through group support.7-13 Within the dermatology literature, SMAs are more profitable than regular clinic appointments.15 In SMAs designed to improve patient education for preoperative consultations for Mohs micrographic surgery, patient satisfaction reported in postvisit surveys was high, with 84.7% of 149 patients reporting they found the session useful, highlighting how SMAs have potential as practical alternatives to regular medical appointments.16 Similarly, the feedback about the group setting from our patients who completed both appointments was uniformly positive, with specific appreciation for the normalization of the treatment process and opportunity to ask questions with their peers.
The group setting—where patients were interacting with peers undergoing the same treatment—provided an encouraging environment during the course of 5-FU therapy, resulting in a positive treatment experience. Additionally, at the conclusion of the second visit, patients reported an increased understanding of their condition and the importance of future sun-protective behaviors, further demonstrating the impact of this pilot initiative.
The Veterans Affairs’ Current Procedural Terminology code for a group clinic is 99078. Veterans Affairs medical centers and private practices have different approaches to billing and compensation. As more accountable care organizations are formed, there may be a different mixture of ways for handling these SMAs.
Limitations
Our study is limited by the small sample size, selection bias, and self-reported measure of adherence. Adherence to 5-FU is excellent without group support, and without a control group, it is unclear how beneficial the group setting was for adherence.17 The presence of the expected skin reactions at the 2-week return visit cannot account for adherence during the interval between the visits, and this close follow-up may be responsible for the high adherence in this group setting. The major side effects with 5-FU are short-term. Nonetheless, longer-term follow-up would be helpful and a worthy future endeavor.
Veterans share a common bond of military service that may not be shared in a typical private practice setting, which may have facilitated success of this pilot study. We recommend group clinics be evaluated independently in private practices and other systems. However, despite these limitations, the patients in the SMAs demonstrated positive reactions to 5-FU therapy, suggesting the potential for utilizing group clinics as a practical alternative to regular medical appointments.
Conclusion
Our pilot group clinics for AK treatment and chemoprevention of SCC with 5-FU suggest that this model is well received. The group format, which demonstrated uniformly positive reactions to 5-FU therapy, shows promise in battling an epidemic of skin cancer that demands cost-effective interventions.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Bickers DR, Lim HW, Margolis D, et al. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J Am Acad Dermatol. 2006;55:490-500.
- Siegel JA, Korgavkar K, Weinstock MA. Current perspective on actinic keratosis: a review. Br J Dermatol. 2017;177:350-358.
- Weinstock MA, Thwin SS, Siegel JA, et al. Chemoprevention of basal and squamous cell carcinoma with a single course of fluorouracil, 5%, cream: a randomized clinical trial. JAMA Dermatol. 2018;154:167-174.
- Pomerantz H, Hogan D, Eilers D, et al. Long-term efficacy of topical fluorouracil cream, 5%, for treating actinic keratosis: a randomized clinical trial. JAMA Dermatol. 2015;151:952-960.
- Foley P, Stockfleth E, Peris K, et al. Adherence to topical therapies in actinic keratosis: a literature review. J Dermatolog Treat. 2016;27:538-545.
- Desouza CV, Rentschler L, Haynatzki G. The effect of group clinics in the control of diabetes. Prim Care Diabetes. 2010;4:251-254.
- Edelman D, McDuffie JR, Oddone E, et al. Shared Medical Appointments for Chronic Medical Conditions: A Systematic Review. Washington, DC: Department of Veterans Affairs; 2012.
- Edelman D, Gierisch JM, McDuffie JR, et al. Shared medical appointments for patients with diabetes mellitus: a systematic review. J Gen Intern Med. 2015;30:99-106.
- Trento M, Passera P, Tomalino M, et al. Group visits improve metabolic control in type 2 diabetes: a 2-year follow-up. Diabetes Care. 2001;24:995-1000.
- Wagner EH, Grothaus LC, Sandhu N, et al. Chronic care clinics for diabetes in primary care: a system-wide randomized trial. Diabetes Care. 2001;24:695-700.
- Harris MD, Kirsh S, Higgins PA. Shared medical appointments: impact on clinical and quality outcomes in veterans with diabetes. Qual Manag Health Care. 2016;25:176-180.
- Kirsh S, Watts S, Pascuzzi K, et al. Shared medical appointments based on the chronic care model: a quality improvement project to address the challenges of patients with diabetes with high cardiovascular risk. Qual Saf Health Care. 2007;16:349-353.
- Pomerantz H, Korgavkar K, Lee KC, et al. Validation of photograph-based toxicity score for topical 5-fluorouracil cream application. J Cutan Med Surg. 2016;20:458-466.
- Sidorsky T, Huang Z, Dinulos JG. A business case for shared medical appointments in dermatology: improving access and the bottom line. Arch Dermatol. 2010;146:374-381.
- Knackstedt TJ, Samie FH. Shared medical appointments for the preoperative consultation visit of Mohs micrographic surgery. J Am Acad Dermatol. 2015;72:340-344.
- Yentzer B, Hick J, Williams L, et al. Adherence to a topical regimen of 5-fluorouracil, 0.5%, cream for the treatment of actinic keratoses. JAMA Dermatol. 2009;145:203-205.
Practice Points
- Shared medical appointments (SMAs) enhance patient experience with topical 5-fluorouracil (5-FU) treatment of actinic keratosis (AK).
- Dermatologists should consider utilizing the SMA model for their patients being treated with 5-FU, as patients demonstrated a positive emotional response to 5-FU therapy in the group clinic setting.
What’s Eating You? Bark Scorpions (Centruroides exilicauda and Centruroides sculpturatus)
Epidemiology and Identification
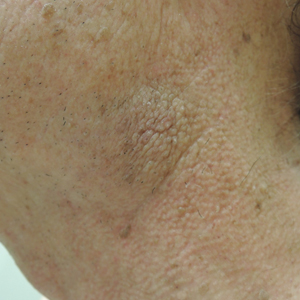
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
Epidemiology and Identification
Centruroides is a common genus of bark scorpions in the United States with at least 21 species considered to be medically important, including the closely related Centruroides exilicauda and Centruroides sculpturatus.1 Scorpions can be recognized by a bulbous sac and pointed stinger at the end of a tail-like abdomen. They also have long lobsterlike pedipalps (pincers) for grasping their prey. Identifying characteristics for C exilicauda and C sculpturatus include a small, slender, yellow to light brown or tan body typically measuring 1.3 to 7.6 cm in length with a subaculear tooth or tubercle at the base of the stinger, a characteristic that is common to all Centruroides species (Figure).2 Some variability in size has been shown, with smaller scorpions found in increased elevations and cooler temperatures.1,3 Both C exilicauda and C sculpturatus are found in northern Mexico as well as the southwestern United States (eg, Arizona, New Mexico, Texas, California, Nevada).1 They have a preference for residing in or around trees and often are found on the underside of bark, stones, or tables as well as inside shoes or small cracks and crevices. Scorpions typically sting in self-defense, and stings commonly occur when humans attempt to move tables, put on shoes, or walk barefoot in scorpion-infested areas. Most stings occur from the end of spring through the end summer, but many may go unreported.1,4

The venom of the Centruroides genus includes peptides and proteins that play a fundamental role in toxic activity by impairing potassium, sodium, and calcium ion channels.1,3 Toxins have been shown to be species specific, functioning either in capturing prey or deterring predators. Intraspecies variability in toxins has been demonstrated, which may complicate the production of adequate antivenin.3 Many have thought that C exilicauda Wood and C sculpturatus Ewing are the same species, and the names have been used synonymously in the past; however, genetic and biochemical studies of their venom components have shown that they are distinct species and that C sculpturatus is the more dangerous of the two.5 The median lethal dose 50% of C sculpturatus was found to be 22.7 μg in CD1 mice.6
Envenomation and Clinical Manifestations
Stings from C exilicauda and C sculpturatus have been shown to cause fatality in children more often than in adults.7 In the United States, Arizona has the highest frequency of serious symptoms of envenomation as well as the highest hospital and intensive care unit admission rates.6 Envenomation results in an immediate sharp burning pain followed by numbness.4 Wounds can produce some regional lymph node swelling, ecchymosis, paresthesia, and lymphangitis. More often than not, however, wounds have little to no inflammation and are characterized only by pain.4 The puncture wound is too small to be seen, and C exilicauda and C sculpturatus venom do not cause local tissue destruction, an important factor in distinguishing it from other scorpion envenomations.
More severe complications that may follow are caused by the neurotoxin released by Centruroides stings. The toxin components can increase the duration and amplitude of the neuronal action potential and enhance the release of neurotransmitters such as acetylcholine and norepinephrine.8 Stings can lead to cranial nerve dysfunction and somatic skeletal neuromuscular dysfunction as well as autonomic dysfunction, specifically salivation, fever, tongue and muscle fasciculations, opsoclonus, vomiting, bronchoconstriction, diaphoresis, nystagmus, blurred vision, slurred speech, hypertension, rhabdomyolysis, stridor, wheezing, aspiration, anaphylaxis, and tachycardia, leading to cardiac and respiratory compromise.4,8 Some patients have experienced a decreased sense of smell or hearing and decreased fine motor movements.7 Although pancreatitis may occur with scorpion stings, it is not common for C exilicauda.9 Comorbidities such as cardiac disease and substance use disorders contribute to prolonged length of hospital stay and poor outcome.8
Treatment
Most Centruroides stings can be managed at home, but patients with more serious symptoms and children younger than 2 years should be taken to a hospital for treatment.7 If a patient reports only pain but shows no other signs of neurotoxicity, observation and pain relief with rest, ice, and elevation is appropriate management. Patients with severe manifestations have been treated with various combinations of lorazepam, glycopyrrolate, ipratropium bromide, and ondansetron, but the only treatment definitively shown to decrease time to symptom abatement is antivenin.7 It has been demonstrated that C exilicauda and C sculpturatus antivenin is relatively safe.7 Most patients, especially adults, do not die from C exilicauda and C sculpturatus stings; therefore, antivenin more commonly is symptom abating than it is lifesaving.10 In children, time to symptom resolution was decreased to fewer than 4 hours with antivenin, and there is a lower rate of inpatient admission when antivenin is administered.4,10,11 There is a low incidence of anaphylactic reaction after antivenin, but there have been reported cases of self-limited serum sickness after antivenin use that generally can be managed with antihistamines and corticosteroids.4,7
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
- Gonzalez-Santillan E, Possani LD. North American scorpion species of public health importance with reappraisal of historical epidemiology. Acta Tropica. 2018;187:264-274.
- Goldsmith LA, Katz SI, Gilchrest BA, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012.
- Carcamo-Noriega EN, Olamendi-Portugal T, Restano-Cassulini R, et al. Intraspecific variation of Centruroides sculpturatus scorpion venom from two regions of Arizona. Arch Biochem Biophys. 2018;638:52-57.
- Kang AM, Brooks DE. Nationwide scorpion exposures reported to US Poison Control centers from 2005 to 2015. J Med Toxicol. 2017;13:158-165.
- Valdez-Cruz N, Dávila S, Licea A, et al. Biochemical, genetic and physiological characterization of venom components from two species of scorpions: Centruroides exilicauda Wood and Centruroides sculpturatus Ewing. Biochimie. 2004;86:387-396.
- Jiménez-Vargas JM, Quintero-Hernández V, Gonzáles-Morales L, et al. Design and expression of recombinant toxins from Mexican scorpions of the genus Centruroides for production of antivenoms. Toxicon. 2017;128:5-14.
- Hurst NB, Lipe DN, Karpen SR, et al. Centruroides sculpturatus envenomation in three adult patients requiring treatment with antivenom. Clin Toxicol (Phila). 2018;56:294-296.
- O’Connor A, Padilla-Jones A, Ruha A. Severe bark scorpion envenomation in adults. Clin Toxicol. 2018;56:170-174.
- Berg R, Tarantino M. Envenomation by the scorpion Centruroides exilicauda (C sculpturatus): severe and unusual manifestations. Pediatrics. 1991;87:930-933.
- LoVecchio F, McBride C. Scorpion envenomations in young children in central Arizona. J Toxicol Clin Toxicol. 2003;41:937-940.
- Rodrigo C, Gnanathasan A. Management of scorpion envenoming: a systematic review and meta-analysis of controlled clinical trials. Syst Rev. 2017;6:74.
Practice Points
- Centruroides scorpions can inflict painful stings.
- Children are at greatest risk for systemic toxicity.
Acne Keloidalis Nuchae in the Armed Forces
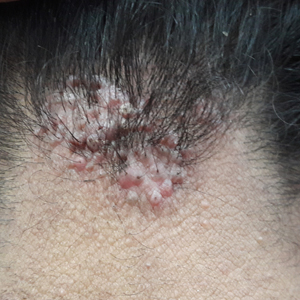
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
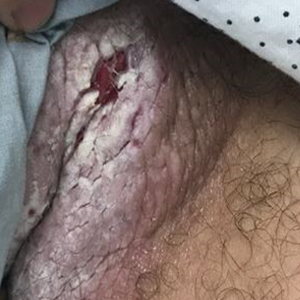
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder most commonly involving the occipital scalp and posterior neck characterized by the development of keloidlike papules, pustules, and plaques. If left untreated, this condition may progress to scarring alopecia. It primarily affects males of African descent, but it also may occur in females and in other ethnic groups. Although the exact underlying pathogenesis is unclear, close haircuts and chronic mechanical irritation to the posterior neck and scalp are known inciting factors. For this reason, AKN disproportionately affects active-duty military servicemembers who are held to strict grooming standards. The US Military maintains these grooming standards to ensure uniformity, self-discipline, and serviceability in operational settings.1 Regulations dictate short tapered hair, particularly on the back of the neck, which can require weekly to biweekly haircuts to maintain.1-5
First-line treatment of AKN is prevention by avoiding short haircuts and other forms of mechanical irritation.1,6,7 However, there are considerable barriers to this strategy within the military due to uniform regulations as well as personal appearance and grooming standards. Early identification and treatment are of utmost importance in managing AKN in the military population to ensure reduction of morbidity, prevention of late-stage disease, and continued fitness for duty. This article reviews the clinical features, epidemiology, and treatments available for management of AKN, with a special focus on the active-duty military population.
Clinical Features and Epidemiology
Acne keloidalis nuchae is a chronic inflammatory disorder characterized by the development of keloidlike papules, pustules, and plaques on the posterior neck and occipital scalp.6 Also known as folliculitis keloidalis nuchae, AKN is seen primarily in men of African descent, though cases also have been reported in females and in a few other ethnic groups.6,7 In black males, the AKN prevalence worldwide ranges from 0.5% to 13.6%. The male to female ratio is 20 to 1.7 Although the exact cause is unknown, AKN appears to develop from chronic irritation and inflammation following localized skin injury and/or trauma. Chronic irritation from close-shaved haircuts, tight-fitting shirt collars, caps, and helmets have all been implicated as considerable risk factors.6-8
Symptoms generally develop hours to days following a close haircut and begin with the early formation of inflamed irritated papules and notable erythema.6,7 These papules may become secondarily infected and develop into pustules and/or abscesses, especially in cases in which the affected individual continues to have the hair shaved. Continued use of shared razors increases the risk for secondary infection and also raises the concern for transmission of blood-borne pathogens, as AKN lesions are quick to bleed with minor trauma.7
Over time, chronic inflammation and continued trauma of the AKN papules leads to widespread fibrosis and scar formation, as the papules coalesce into larger plaques and nodules. If left untreated, these later stages of disease can progress to chronic scarring alopecia.6
Prevention
In the general population, first-line therapy of AKN is preventative. The goal is to break the cycle of chronic inflammation, thereby preventing the development of additional lesions and subsequent scarring.7 Patients should be encouraged to avoid frequent haircuts, close shaves, hats, helmets, and tight shirt collars.6-8
A 2017 cross-sectional study by Adotama et al9 investigated recognition and management of AKN in predominantly black barbershops in an urban setting. Fifty barbers from barbershops in Oklahoma City, Oklahoma, were enrolled and interviewed for the study. Of these barbers, only 44% (22/50) were able to properly identify AKN from a photograph. Although the vast majority (94% [47/50]) were aware that razor use would aggravate the condition, only 46% (23/50) reported avoidance of cutting hair for clients with active AKN.9 This study, while limited by its small sample size, showed that many barbers may be unaware of AKN and therefore unknowingly contribute to the disease process by performing haircuts on actively inflamed scalps. For this reason, it is important to educate patients about their condition and strongly recommend lifestyle and hairstyle modifications in the management of their disease.
Acne keloidalis nuchae that is severe enough to interfere with the proper use and wear of military equipment (eg, Kevlar helmets) or maintenance of regulation grooming standards does not meet military admission standards.10,11 However, mild undiagnosed cases may be overlooked during entrance physical examinations, while many servicemembers develop AKN after entering the military.10 For these individuals, long-term avoidance of haircuts is not a realistic or obtainable therapeutic option.
Treatment
Topical Therapy
Early mild to moderate cases of AKN—papules less than 3 mm, no nodules present—may be treated with potent topical steroids. Studies have shown 2-week alternating cycles of high-potency topical steroids (2 weeks of twice-daily application followed by 2 weeks without application) for 8 to 12 weeks to be effective in reducing AKN lesions.8,12 Topical clindamycin also may be added and has demonstrated efficacy particularly when pustules are present.7,8
Intralesional Steroids
For moderate cases of AKN—papules more than 3 mm, plaques, and nodules—intralesional steroid injections may be considered. Triamcinolone may be used at a dose of 5 to 40 mg/mL administered at 4-week intervals.7 More concentrated doses will produce faster responses but also carry the known risk of side effects such as hypopigmentation in darker-skinned individuals and skin atrophy.
Systemic Therapy
Systemic therapy with oral antibiotics may be warranted as an adjunct to mild to moderate cases of AKN or in cases with clear evidence of secondary infection. Long-term tetracycline antibiotics, such as minocycline and doxycycline, may be used concurrently with topical and/or intralesional steroids.6,7 Their antibacterial and anti-inflammatory effects are useful in controlling secondary infections and reducing overall chronic inflammation.
When selecting an appropriate antibiotic for long-term use in active-duty military patients, it is important to consider their effects on duty status. Doxycycline is preferred for active-duty servicemembers because it is not duty limiting or medically disqualifying.10,13-15 However, minocycline, is restricted for use in aviators and aircrew members due to the risk for central nervous system side effects, which may include light-headedness, dizziness, and vertigo.
UV Light Therapy
UV radiation has known anti-inflammatory, immunosuppressive, and antifibrotic effects and commonly is used in the treatment of many dermatologic conditions.16 Within the last decade, targeted UVB (tUVB) radiation has shown promise as an effective alternative therapy for AKN. In 2014, Okoye et al16 conducted a prospective, randomized, split-scalp study in 11 patients with AKN. Each patient underwent treatment with a tUVB device (with peaks at 303 and 313 nm) to a randomly selected side of the scalp 3 times weekly for 16 weeks. Significant reductions in lesion count were seen on the treated side after 8 (P=.03) and 16 weeks (P=.04), with no change noted on the control side. Aside from objective lesion counts, patients completed questionnaires (n=6) regarding their treatment outcomes. Notably, 83.3% (5/6) reported marked improvement in their condition. Aside from mild transient burning and erythema of the treated area, no serious side effects were reported.16
Targeted UVB phototherapy has limited utility in an operational setting due to accessibility and operational tempo. Phototherapy units typically are available only at commands in close proximity to large medical treatment facilities. Further, the vast majority of servicemembers have duty hours that are not amenable to multiple treatment sessions per week for several months. For servicemembers in administrative roles or serving in garrison or shore billets, tUVB or narrowband UV phototherapy may be viable treatment options.
Laser Therapy
Various lasers have been used to treat AKN, including the CO2 laser, pulsed dye laser, 810-nm diode laser, and 1064-nm Nd:YAG laser.6 Kantor et al17 utilized a CO2 laser with a focused beam for surgical excision of a late-stage AKN case as early as 1986. In these patients, it was demonstrated that focused CO2 laser could be used to remove fibrotic lesions in an outpatient setting with only local anesthesia. Although only 8 patients were treated in this report, no relapses occurred.17
CO2 laser evaporation using the unfocused beam setting with 130 to 150 J/cm2 has been less successful, with relapses reported in multiple cases.6 Dragoni et al18 attempted treatment with a 595-nm pulsed dye laser with 6.5-J/cm2 fluence and 0.5-millisecond pulse but faced similar results, with lesions returning within 1 month.
There have been numerous reports of clinical improvement of AKN with the use of the 1064-nm Nd:YAG laser.6,19 Esmat et al19 treated 16 patients with a fluence of 35 to 45 J/cm2 and pulse duration of 10 to 30 milliseconds adjusted to skin type and hair thickness. An overall 82% reduction in lesion count was observed after 5 treatment sessions. Biopsies following the treatment course demonstrated a significant reduction in papule and plaque count (P=.001 and P=.011, respectively), and no clinical recurrences were noted at 12 months posttreatment.19 Similarly, Woo et al20 conducted a single-blinded, randomized, controlled trial to assess the efficacy of the Nd:YAG laser in combination with topical corticosteroid therapy vs topical corticosteroid monotherapy. Of the 20 patients treated, there was a statistically significant improvement in patients with papule-only AKN who received the laser and topical combination treatment (P=.031).20
Laser therapy may be an available treatment option for military servicemembers stationed within close proximity to military treatment facilities, with the Nd:YAG laser typically having the widest availability. Although laser therapy may be effective in early stages of disease, servicemembers would have to be amenable to limitation of future hair growth in the treated areas.
Surgical Excision
Surgical excision may be considered for large, extensive, disfiguring, and/or refractory lesions. Excision is a safe and effective method to remove tender, inflamed, keloidlike masses. Techniques for excision include electrosurgical excision with secondary intention healing, excision of a horizontal ellipse involving the posterior hairline with either primary closure or secondary intention healing, and use of a semilunar tissue expander prior to excision and closure.6 Regardless of the technique, it is important to ensure that affected tissue is excised at a depth that includes the base of the hair follicles to prevent recurrence.21
Final Thoughts
Acne keloidalis nuchae is a chronic inflammatory disease that causes considerable morbidity and can lead to chronic infection, alopecia, and disfigurement of the occipital scalp and posterior neck. Although easily preventable through the avoidance of mechanical trauma, irritation, and frequent short haircuts, the active-duty military population is restricted in their preventive measures due to current grooming and uniform standards. In this population, early identification and treatment are necessary to manage the disease to reduce patient morbidity and ensure continued operational and medical readiness. Topical and intralesional steroids may be used in mild to moderate cases. Topical and/or systemic antibiotics may be added to the treatment regimen in cases of secondary bacterial infection. For more severe refractory cases, laser therapy or complete surgical excision may be warranted.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Weiss AN, Arballo OM, Miletta NR, et al. Military grooming standards and their impact on skin diseases of the head and neck. Cutis. 2018;102:328, 331-333.
- US Department of the Army. Wear and Appearance of Army Uniforms and Insignia: Army Regulation 670-1. Washington, DC: Department of the Army; 2017. https://history.army.mil/html/forcestruc/docs/AR670-1.pdf. Accessed April 14, 2020.
- U.S. Headquarters Marine Corps. Marine Corps Uniform Regulations: Marine Corps Order 1020.34H. Quantico, VA: United States Marine Corps, 2018. https://www.marines.mil/portals/1/Publications/MCO%201020.34H%20v2.pdf?ver=2018-06-26-094038-137. Accessed April 14, 2020.
- Grooming standards. In: US Department of the Navy. United States Navy Uniform Regulations: NAVPERS 15665I. https://www.public.navy.mil/bupers-npc/support/uniforms/uniformregulations/chapter2/Pages/2201PersonalAppearance.aspx. Updated May 2019. Accessed April 14, 2020.
- Department of the Air Force. AFT 36-2903, Dress and Personal Appearance of Air Force Personnel. Washington, DC: Department of the Air Force, 2019. https://static.e-publishing.af.mil/production/1/af_a1/publication/afi36-2903/afi36-2903.pdf. Accessed April 14, 2020.
- Maranda EL, Simmons BJ, Nguyen AH, et al. Treatment of acne keloidalis nuchae: a systemic review of the literature. Dermatol Ther (Heidelb). 2016;6:362-378.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191.
- Adotama P, Tinker D, Mitchell K, et al. Barber knowledge and recommendations regarding pseudofolliculitis barbae and acne keloidalis nuchae in an urban setting. JAMA Dermatol. 2017;12:1325.
- Burke KR, Larrymore DC, Cho S. Treatment considerations for US military members with sin disease. Cutis. 2019;6:329-332.
- Medical standards for Appointment, Enlistment, or Induction Into the Military Services (DoD Instruction 6130.03). Washington, DC: Department of Defense; May 6, 2018. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003p.pdf. Accessed April 27, 2020.
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- US Department of the Army. Standards of medical fitness. https://www.qmo.amedd.army.mil/diabetes/AR40_5012011.pdf. Published December 14, 2007. Accessed April 27, 2020.
- US Department of the Air Force. Medical examinations and standards. https://static.e-publishing.af.mil/production/1/af_sg/publication/afi48-123/afi48-123.pdf. Published November 5, 2013. Accessed April 27, 2020.
- US Navy Aeromedical Reference and Waiver Guide. https://www.med.navy.mil/sites/nmotc/nami/arwg/Documents/WaiverGuide/Complete_Waiver_Guide.pdf. Published September 4, 2019. Accessed April 14, 2020.
- Okoye GA, Rainer BM, Leung SG, et al. Improving acne keloidalis nuchae with targeted ultraviolet B treatment: a prospective, randomized split-scalp study. Br J Dermatol. 2014;17:1156-1163.
- Kantor GR, Ratz JL, Wheeland RG. Treatment of acne keloidalis nuchae with carbon dioxide laser. J Am Acad Dermatol. 1986;14(2, pt 1):263-267.
- 18. Dragoni F, Bassi A, Cannarozzo G, et al. Successful treatment of acne keloidalis nuchae resistant to conventional therapy with 1064-nm Nd:YAG laser. G Ital Dermatol Venereol. 2013;148:231-232.
- Esmat SM, Hay RMA, Zeid OMA, et al. The efficacy of laser assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650.
- Woo DK, Treyger G, Henderson M, et al. Prospective controlled trial for the treatment of acne keloidalis nuchae with a long-pulsed neodymium-doped yttrium-aluminum-garnet laser. J Cutan Med Surg. 2018;22:236-238.
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
Practice Points
- Acne keloidalis nuchae (AKN) is a chronic inflammatory disorder of the occipital scalp and posterior neck characterized by keloidlike papules, pustules, and plaques that develop following mechanical irritation.
- Military members are required to maintain short haircuts and may be disproportionately affected by AKN.
- In the military population, early identification and treatment, which includes topical steroids, oral antibiotics, UV light therapy, lasers, and surgical excision, can prevent further scarring, permanent hair loss, and disfigurement from AKN.
Tip Sheet: Teledermatology 101
Teledermatology Fast Facts
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
Due to the impact of the coronavirus disease 2019 (COVID-19) pandemic, many patients are working from home, which has led to a unique opportunity for dermatologists to step in and continue to care for their patients at home via telemedicine. With recent waivers and guidance from the Centers for Medicare & Medicaid Services (CMS), insurance coverage has been expanded for telehealth services, usually at the same level as an in-person visit. This editorial provides guidance for implementing telehealth services in your practice, and a tip sheet is available online for you to save and print. Please note that this information is changing on a day-to-day basis, so refer to the resources in the Table to get the latest updates.
Billing and Coding
The best reimbursements are for live telemedicine that emulates an outpatient visit and is billed using the same Current Procedural Terminology (CPT) codes (99201–99215). Previously, Medicare did not allow direct-to-patient visits to be billed, instead requiring a waiver for these services to be provided in underserved areas. During the COVID-19 pandemic, this requirement has been lifted, allowing all patients to be seen from any originating site (eg, the patient’s home).
Previously, the CMS had issued guidelines for telehealth visits that required that a physician-patient relationship be established in person prior to conducting telemedicine visits. These guidelines also have been waived for the duration of this public health emergency, allowing physicians to conduct new patient visits via telehealth and bill Medicare. Many commercial payors also are covering new patient visits via telehealth; however, it is best to check the patient’s plan first, as some plans may have different requirements or restrictions on allowable CPT codes and/or place of service. Prior requirements that physicians at a distant site (ie, the physician providing telemedicine services) be located at a site of clinical care also have been relaxed, thus allowing physicians to be located anywhere while providing services, even for those who are confined to their homes.
In general, commercial payors are covering telehealth visits at 100% of an in-person visit. Although COVID-19–related visits are covered by law, many payors including Aetna, Anthem, Blue Cross Blue Shield, Cigna, Emblem Health, Humana, and United Healthcare have indicated that they will waive all telehealth co-pays for a limited time, including visits not related to COVID-19. At the time of publication, only Aetna has issued a formal policy to this effect, so it is best to check with the insurer.1,2 However, it is important to note that regional and employer-specific plans may have different policies, so it is best to check with the insurance plans directly to confirm coverage and co-pay status.
Coding should be performed using the usual new/established patient visit codes for outpatients (99201–99215). A place of service (POS) code of 02 previously was used for all telehealth visits; however, the CMS is allowing offices to bill with their usual POS (generally POS 11) and modifier -95 in an updated rule that is active during this public health crisis. This change allows access to higher reimbursements, as POS 02 visits are paid at lower facility fee rates. Commercial insurers have varying policies on POS that are changing, so it is best to check with them individually.
In certain states, store-and-forward services may be billed using a GQ modifier for Medicaid; however, the remote check-in and telephone codes for Medicare do not reimburse well and generally are best avoided if a live telemedicine encounter is possible, as it provides better patient care and direct counseling capabilities, similar to an in-person visit. The CMS has indicated that it is now covering telephone visits (99441-99443) so that providers can contact patients through an audio-only device and bill for the encounter. Generally speaking, telephone visits reimburse the same or more than the virtual check-in codes (G2010/G2012) as long as the telephone encounter is more than 5-minutes long. Digital visits also are available (99421-99423), which include both store-and-forward photographs and a telephone call, but the reimbursements are similar to the telephone-only visit codes.3
Although the CMS has relaxed regulations for physicians to provide care across state lines, not all state licensing authorities have adopted similar measures, and the CMS waiver only applies to federally funded programs. It is important to check with state medical licensing authorities to see whether you are authorized to provide care if your patient is not located within the state where you hold your license at the time of the visit. Many states, but not all, have waived this requirement or have set up very expedient ways to apply for telemedicine licenses.
The CMS also released guidance that rules for documentation requirements have been temporarily relaxed,3 such that visits should be billed at a level of service consistent with either medical decision-making or total time spent by the provider, including face-to-face and non–face-to-face time spent on the patient. (Note: If billing by time, which usually is not advised, use the CMS definitions of time-based coding.) History and physical examination criteria do not have to be met.
Workflow
In general, it is best to maintain your current workflow as much as possible, with a live video encounter replacing only the patient interaction portion of the visit. You will need to maintain an infrastructure for scheduling visits, collecting co-pays (eg, over the telephone prior to the video visit), and documentation/billing.
It is best to have one device for conducting the actual video visit (eg, a laptop, tablet, or smartphone) and a separate device to use for documentation (eg, another device to access the electronic medical record). The CMS has advised that it will not enforce Health Insurance Portability and Accountability Act (HIPAA) rules,4 allowing physicians to use video conferencing and chat applications such as FaceTime, Skype, or Google Hangouts; however, patient safety is still an issue, and it is imperative to make sure you identify the patient correctly upon starting the visit. During the COVID-19 pandemic, numerous telehealth companies are offering temporary free video conferencing software that is HIPAA compliant, such as Doximity, VSee, Doxy.me, and Medweb. If you are able to go through one of these vendors, you will be able to continue conducting some telemedicine visits after the public health emergency, which may be helpful to your practice.
For some visits, such as acne patients on isotretinoin, you can write for a standing laboratory order that can be drawn at a laboratory center near your patient, and you can perform the counseling via telemedicine. For patients on isotretinoin, iPledge has issued a program update allowing the use of at-home pregnancy tests during the pandemic. The results must be communicated to the provider and documented with a time/date.5
Video Visit Tips and Pearls
Make sure to have well-defined parameters about what can be triaged via a single video visit. Suggestions include no total-body skin examinations and a limit of 1 rash or 2 lesions. Provide a disclaimer that it is not always possible to tell whether or not a lesion is concerning via a video visit, and the patient may have to come in for a biopsy at some point.
It is better to overcall via telemedicine than to undercall. Unless something is a very obvious seborrheic keratosis, skin tag, cherry angioma, or other benign lesion, it might be reasonable to tell a patient to come in for further evaluation of a worrisome lesion after things get back to normal. A static photograph from the patient can be helpful so it is clear what lesion is being examined during the current visit. If the patient has a skin cancer at a distant site in the future, there will be no doubt as to what lesion you examined. Having the capability to receive static images from the patient to serve as representative photographs of their chief concern is very helpful before the visit. Often, these images turn out to be better diagnostically than the live video itself, which can be compressed and show inaccurate colors. Some of the telemedicine vendors have this feature built-in, which is preferable. If you are asking patients to send you emails, it is better to have access to a HIPAA-compliant email inbox to avoid any potential issues down the line.
When scheduling a video visit, have your schedulers specifically tell patients that they should be on a high-speed Wi-Fi connection with good lighting in the room. You would be surprised that this is not intuitive for everyone!
Finally, most telemedicine visits are relatively short and to the point. In the beginning, start by scheduling patients every 15 to 20 minutes to allow for technical difficulties, but ultimately plan to be seeing patients at least every 10 minutes—it can be quite efficient!
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
- America’s Health Insurance Providers. Health insurance providers respond to coronavirus (COVID-19). https://www.ahip.org/health-insurance-providers-respond-to-coronavirus-covid-19/. Published April 22, 2020. Accessed April 23, 2020.
- Private payer coverage during COVID-19. American College of Physicians website. https://www.acponline.org/system/files/documents/clinical_information/resources/covid19/payer_chart_covid-19.pdf. Updated April 22, 2020. Accessed April 23, 2020.
- Centers for Medicare & Medicaid Services. Medicare and Medicaid programs; policy and regulatory revisions in response to the COVID-19 public health emergency. https://www.cms.gov/files/document/covid-final-ifc.pdf. Published March 26, 2020. Accessed April 23, 2020.
- Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. US Department of Health and Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed April 23, 2020.
- Program update. iPledge website. https://www.ipledgeprogram.com/iPledgeUI/home.u. Accessed April 23, 2020.
Minimally Hyperpigmented Plaque With Skin Thickening on the Neck
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
The Diagnosis: Fiddler's Neck
A thorough patient history revealed that the patient was retired and played violin regularly in the local orchestra. Fiddler's neck, or violin hickey, is an uncommon physical examination finding and often is considered a badge of honor by musicians who develop it. Fiddler's neck is a hobby-related callus seen in highly dedicated violin and viola players, and in some circles, it is known as a mark of greatness. In one instance, members of the public were asked to display a violin hickey before they were allowed to play a $3.5 million violin on public display in London, England.1 Fiddler's neck is a benign submandibular lesion caused by pressure and friction on the skin from extensive time spent playing the instrument. The primary cause is thought to be mechanical, but it is not fully understood why the lesion occurs in some musicians and not others, regardless of playing time.1 This submandibular fiddler's neck is distinct from a similarly named supraclavicular lesion, which represents an allergic contact dermatitis to the nickel bracket of the instrument's chin rest and presents with eczematous scale and/or vesicles.2,3 Submandibular fiddler's neck presents with some combination of erythema, edema, lichenification, and scarring just below the angle of the jaw. Occasionally, papules, pustules, and even cyst formation may be noted. Lesions are sometimes mistaken for malignancy or lymphedema. Therefore, a thorough history and clinical expertise are important, as surgical excision should be avoided.2
Depending on presentation, the differential diagnosis also may include malignant melanoma due to irregular pigmentation, branchial cleft cyst or sialolithiasis due to location and texture, or a tumor of the salivary gland.
Management of fiddler's neck may include topical steroids, neck or instrument padding, or decreased playing time. However, the lesion often is worn with pride, seen as a testament to the musician's dedication, and reassurance generally is most appropriate.1
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
- Roberts C. How to prevent or even cure a violin hickey. Strings. February 1, 2011. https://stringsmagazine.com/how-to-prevent-or-even-cure-a-violin-hickey/. Accessed January 31, 2020.
- Myint CW, Rutt AL, Sataloff RT. Fiddler's neck: a review. Ear Nose Throat J. 2017;96:76-79.
- Jue MS, Kim YS, Ro YS. Fiddler's neck accompanied by allergic contact dermatitis to nickel in a viola player. Ann Dermatol. 2010;22:88-90.
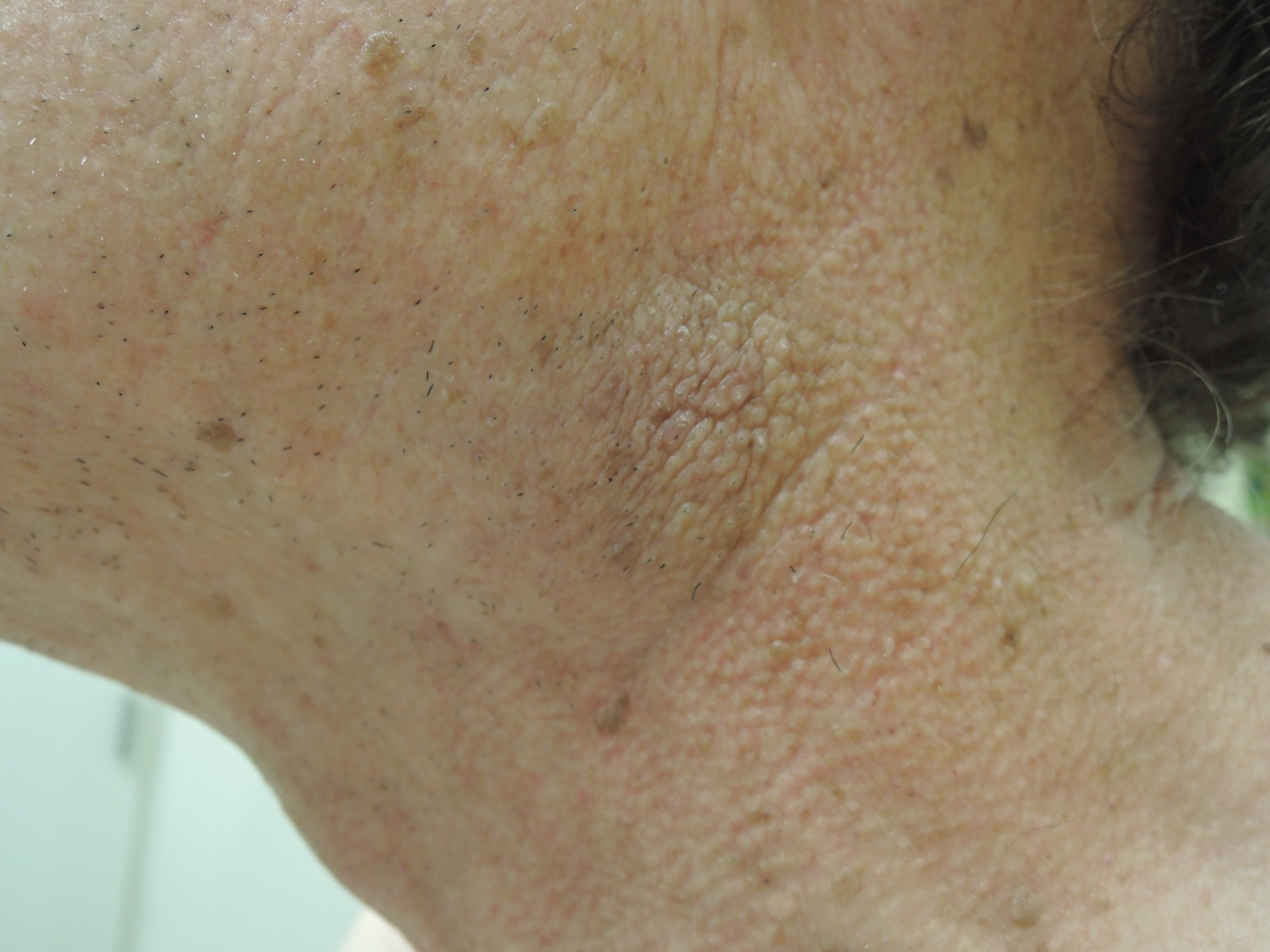
A 74-year-old man with a history of melanoma and basal cell carcinoma presented for an annual skin examination and displayed asymptomatic stable thickening of skin on the left side of the neck below the jawline of several years' duration. Physical examination revealed a 4×2-cm minimally hyperpigmented plaque with skin thickening and a pebbly appearing surface on the left lateral neck just inferior to the angle of the mandible.
Phytophotodermatitis in a Butterfly Enthusiast Induced by Common Rue
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.
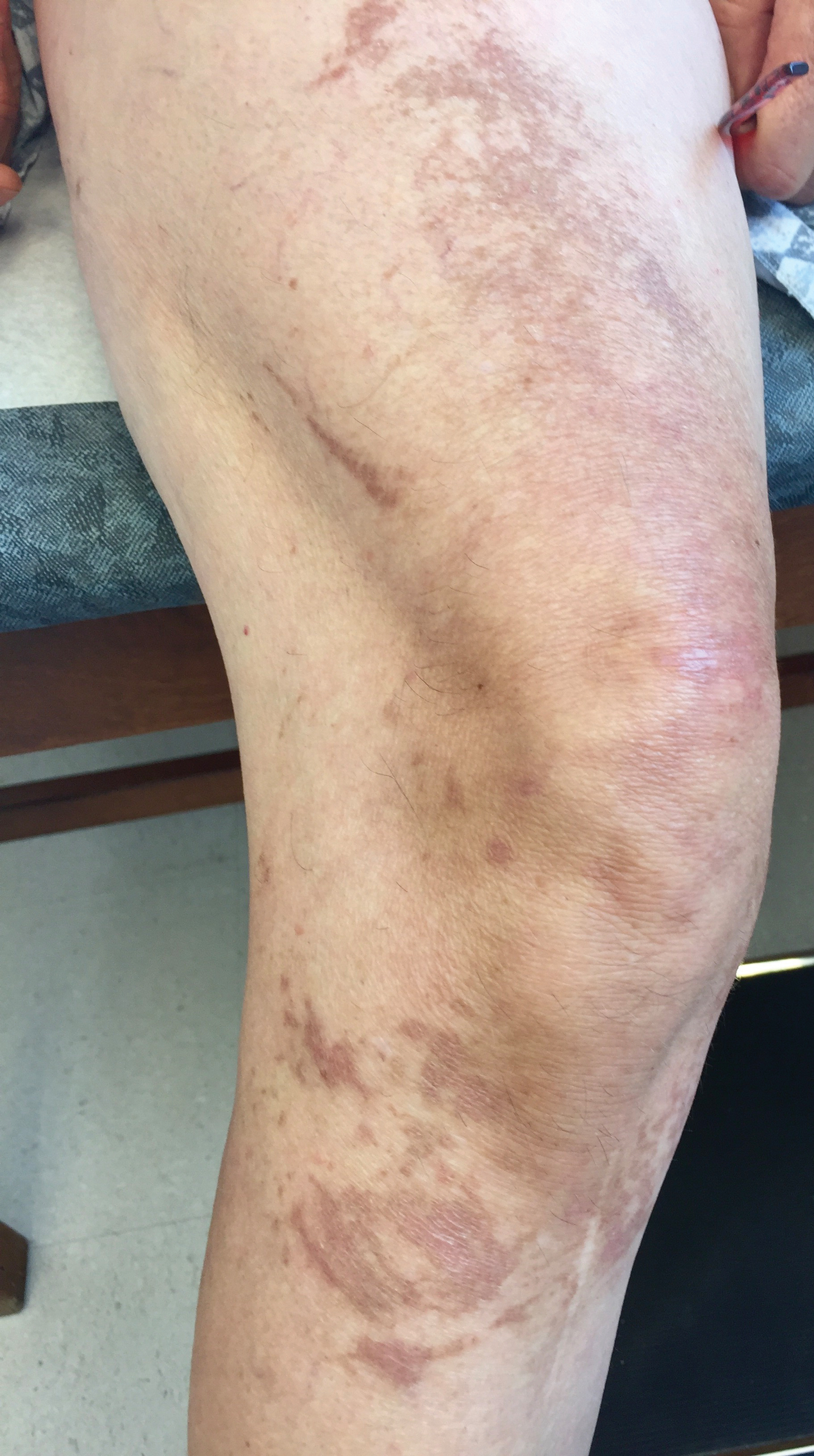
In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
To the Editor:
Phytophotodermatitis is common in dermatology during the summer months, especially in individuals who spend time outdoors; however, identification of the offending plant can be challenging. We report a case of phytophotodermatitis in which the causative plant, common rue, was not identified until it was revealed that the patient was a butterfly enthusiast.
A 60-year-old woman presented to the outpatient dermatology clinic in late summer for a routine skin examination. An eruption was noted over the right thigh and knee that had first appeared approximately 2 weeks prior. The rash started as pruritic blisters but gradually progressed to erythema and then eventually to brown markings, which were observed at the current presentation. Physical examination revealed hyperpigmented, brown, streaky, linear patches and plaques over the right thigh, knee, and lower leg (Figure). When asked about her hobbies, the patient reported an affinity for butterflies and noted that she attracts them with specific species of plants in her garden. She recalled recently planting the herb of grace, or common rue, to attract the giant swallowtail butterfly (Papilio cresphontes). Upon further inquiry, she remembered working in the garden on her knees and digging up roots near the common rue plant while wearing shorts approximately 2 weeks prior to the current presentation. Given the streaky linear pattern of the eruption along with recent sun exposure and exposure to the common rue plant, a diagnosis of phytophotodermatitis was made. No further treatment was sought, as the eruption was not bothersome to her. She was intrigued that the common rue plant had caused the dermatitis and planned on taking proper precautions when working near the plant in the future.

In this case, the observed phototoxic skin findings resulted from exposure to common rue (Ruta graveolens),a pungently scented evergreen shrub native to the Mediterranean region and a member of the Rutaceae family. Extracts have been used in homeopathic practices for bruises, sprains, headache, neck stiffness, rheumatologic pain, neuralgia, stomach problems, and phlebitis, as well as in seasonings, soaps, creams, and perfumes.1 The most commonly encountered plants known to cause phytophotodermatitis belong to the Apiaceae and Rutaceae families.2 Members of Apiaceae include angelica, celery, dill, fennel, hogweed, parsley, and parsnip. Aside from the common rue plant, the Rutaceae family also includes bergamot orange, bitter orange, burning bush (or gas plant), grapefruit, lemon, and lime. Other potential offending agents are fig, mustard, buttercup, St. John’s wort, and scurfpea. The phototoxic properties are due to furocoumarins, which include psoralens and angelicins. They are inert until activated by UVA radiation, which inflicts direct cellular damage, causing vacuolization and apoptosis of keratinocytes, similar to a sunburn.3 Clinical findings typically present 24 hours after sun exposure with erythema, edema, pain, and occasionally vesicles or bullae in severe cases. Unlike sunburn, lesions often present in linear, streaky, or bizarre patterns, reflective of the direct contact with the plant. The lesions eventually transition to hyperpigmentation, which may take months to years to resolve.
Other considerations in cases of suspected phytophotodermatitis include polymorphic light eruption, actinic prurigo, hydroa vacciniforme, chronic actinic dermatitis, solar urticaria, drug reactions, porphyria, Smith-Lemli-Opitz syndrome, lupus erythematosus, and dermatomyositis.4 Clinicians should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery. As in our case, phytophotodermatitis also should be considered in butterfly enthusiasts trying to attract the giant swallowtail butterfly. The caterpillars feed on the leaves of the common rue plant, one of a select few plants that giant swallowtail butterflies use as a host due to its bitter leaves that aid in avoiding predators.5
This case illustrates a unique perspective of phytophotodermatitis, as butterfly enthusiasm is not commonly reported in association with the common rue plant with respect to phytophotodermatitis. This case underscores the importance of inquiring about patients’ professions and hobbies, both in dermatology and other specialties.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
- Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117-124.
- McGovern TW. Dermatoses due to plants. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. 2nd ed. Edinburgh, Scotland: Mosby; 2007:265-283.
- Hawk JLM, Calonje E. The photosensitivity disorders. In: Elder DE, ed. Lever’s Histopathology of the Skin. 9th ed. Philadelphia, Pennsylvania: Lippincott Williams and Wilkins; 2005:345-353.
- Lim HW. Abnormal responses to ultraviolet radiation: photosensitivity induced by exogenous agents. In: Wolff K, Goldsmith LA, Katz SI, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 8th ed. New York, NY: McGraw-Hill; 2012:1066-1074.
- McAuslane H. Giant swallowtail. University of Florida Department of Entomology and Nematology Featured Creatures website. http://entnemdept.ufl.edu/creatures/citrus/giantswallowtail.htm. Revised January 2018. Accessed April 10, 2020.
Practice Points
- It is important to inquire about patients’ professions and hobbies, which may lead to the diagnosis, as in this case of a butterfly enthusiast trying to attract the giant swallowtail butterfly with the common rue plant.
- One should suspect phytophotodermatitis with phototoxic findings in bartenders, citrus farm workers, gardeners, chefs, and kitchen workers, especially those handling limes and celery
Tender White Lesions on the Groin
The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).
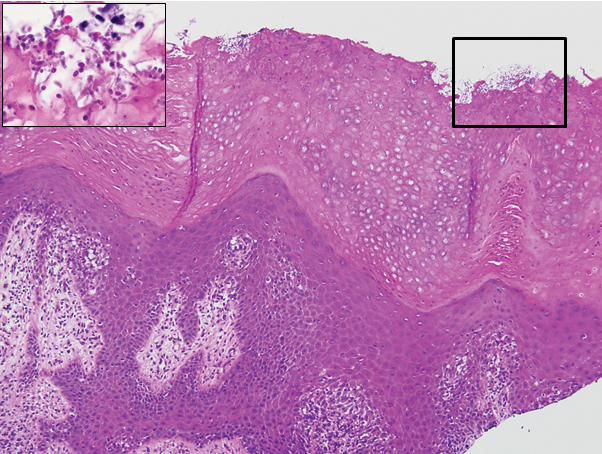
Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1
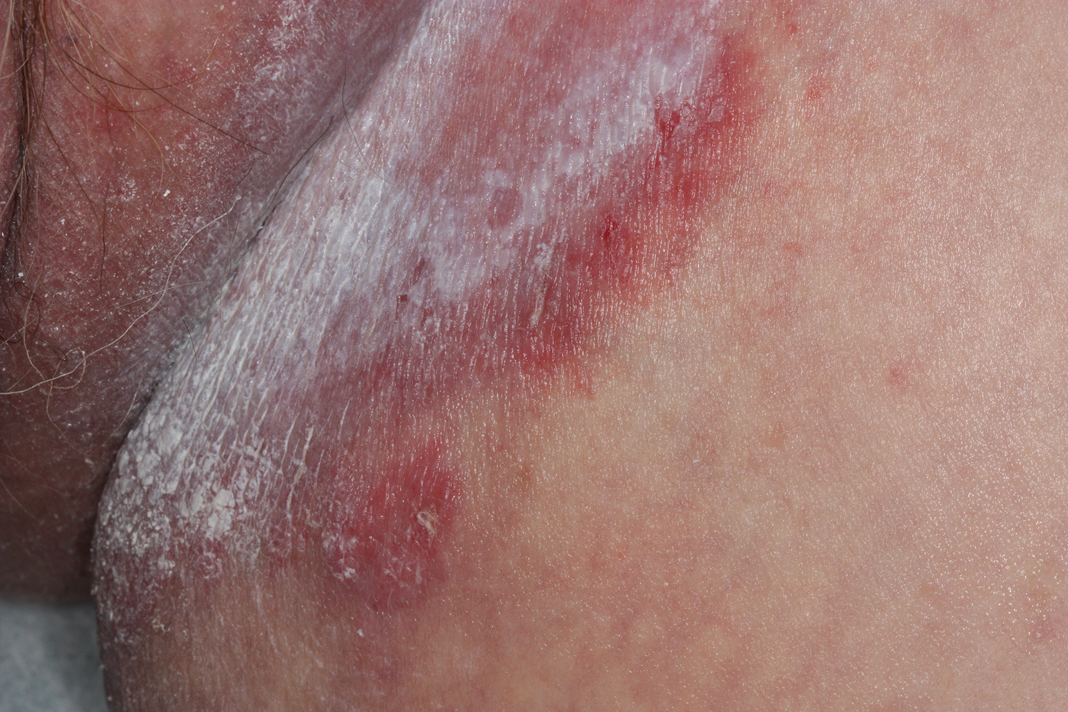
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

The Diagnosis: Candidal Intertrigo
The biopsy confirmed a diagnosis of severe hyperkeratotic candidal intertrigo with no evidence of Hailey-Hailey disease. Hematoxylin and eosin- stained sections demonstrated irregular acanthosis and variable spongiosis. The stratum corneum was predominantly orthokeratotic with overlying psuedohyphae and yeast fungal elements (Figure 1).

Hyperimmunoglobulinemia E syndrome (HIES), also known as hyper-IgE syndrome or Job syndrome, is a rare immunodeficiency disorder characterized by an eczematous dermatitis-like rash, recurrent skin and lung abscesses, eosinophilia, and elevated serum IgE. Facial asymmetry, prominent forehead, deep-set eyes, broad nose, and roughened facial skin with large pores are characteristic of the sporadic and autosomal-recessive forms. Other common findings include retained primary teeth, hyperextensible joints, and recurrent mucocutaneous candidiasis.1
Although autosomal-dominant and autosomal-recessive inheritance patterns exist, sporadic mutations are the most common cause of HIES.2 Several genes have been implicated depending on the inheritance pattern. The majority of autosomal-dominant cases are associated with inactivating STAT3 (signal transducer and activator of transcription 3) mutations, whereas the majority of autosomal-recessive cases are associated with inactivating DOCK8 (dedicator of cytokinesis 8) mutations.1 Ultimately, all of these mutations lead to an impaired helper T cell (TH17) response, which is crucial for clearing fungal and extracellular bacterial infections.3
Skin eruptions typically are the first manifestation of HIES; they appear within the first week to month of life as papulopustular eruptions on the face and scalp and rapidly generalize to the rest of the body, favoring the shoulders, arms, chest, and buttocks. The pustules then coalesce into crusted plaques that resemble atopic dermatitis, frequently with superimposed Staphylococcus aureus infection. On microscopy, the pustules are folliculocentric and often contain eosinophils, whereas the plaques may contain intraepidermal collections of eosinophils.1
Mucocutaneous candidiasis is seen in approximately 60% of HIES cases and is closely linked to STAT3 inactivating mutations.3 Histologically, there is marked acanthosis with neutrophil exocytosis and abundant yeast and pseudohyphal forms within the stratum corneum (Figure 2).4 Cutaneous candidal infections typically require both oral and topical antifungal agents to clear the infection.3 Most cases of mucocutaneous candidiasis are caused by Candida albicans; however, other known culprits include Candida glabrata, Candida tropicalis, Candida parapsilosis, and Candida krusei.5,6 Of note, species identification and antifungal susceptibility studies may be useful in refractory cases, especially with C glabrata, which is known to acquire resistance to azoles, such as fluconazole, with emerging resistance to echinocandins.6
The differential diagnosis of this groin eruption included Hailey-Hailey disease; pemphigus vegetans, Hallopeau type; tinea cruris; and inverse psoriasis. Hailey-Hailey disease can be complicated by a superimposed candidal infection with similar clinical features, and biopsy may be required for definitive diagnosis. Hailey-Hailey disease typically presents with macerated fissured plaques that resemble macerated tissue paper with red fissures (Figure 3). Biopsy confirms full-thickness acantholysis resembling a dilapidated brick wall with minimal dyskeratosis.1 Pemphigus vegetans is a localized variant of pemphigus vulgaris with a predilection for flexural surfaces. The lesions progress to vegetating erosive plaques.4 The Hallopeau type often is studded with pustules and typically remains more localized than the Neumann type. Direct immunofluorescence demonstrates intercellular deposition of IgG and C3, and routine sections characteristically show pseudoepitheliomatous hyperplasia with intraepidermal eosinophilic microabscesses.1,4 Tinea cruris is characterized by erythematous annular lesions with raised scaly borders spreading down the inner thighs.7 The epidermis is variably spongiotic with parakeratosis, and neutrophils often present in a layered stratum corneum with basketweave keratin above a layer of more compact and eosinophilic keratin. Fungal stains, such as periodic acid-Schiff, will highlight the fungal hyphae within the stratum corneum. The inguinal folds are a typical location for inverse psoriasis, which generally appears as thin, sharply demarcated, shiny red plaques with less scale than plaque psoriasis.1 Psoriasiform hyperplasia with a diminished granular layer and tortuous papillary dermal vessels would be expected histologically.1

- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
- James WD, Berger TG, Elston DM. Andrews' Diseases of the Skin. 12th ed. Philadelphia, PA: Elsevier; 2016.
- Schwartz RA, Tarlow MM. Dermatologic manifestations of Job syndrome. Medscape website. https://emedicine.medscape.com/article/1050852-overview. Updated April 22, 2019. Accessed March 28, 2020.
- Minegishi Y, Saito M. Cutaneous manifestations of hyper IgE syndrome. Allergol Int. 2012;61:191-196.
- Patterson JW. Weedon's Skin Pathology. 4th ed. China: Churchill Livingstone Elsevier; 2016.
- Pappas PG, Kauffman CA, Andes DR, et al. Executive summary: clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409-417.
- Center for Disease Control and Prevention. Antifungal resistance. https://www.cdc.gov/fungal/antifungal-resistance.html. Updated March 17, 2020. Accessed April 20, 2020.
- Tinea cruris. DermNet NZ website. https://www.dermnetnz.org/topics/tinea-cruris/. Published 2003. Accessed March 28, 2020.
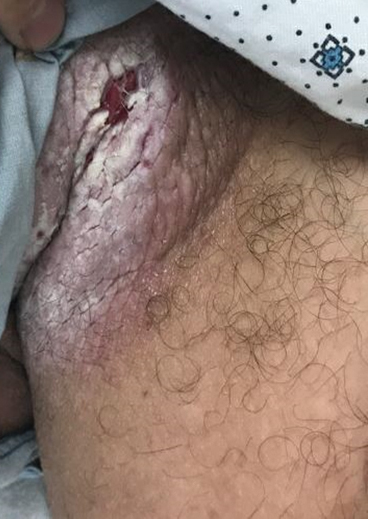
A 28-year-old man with a history of hyperimmunoglobulinemia E syndrome (previously known as Job syndrome), coarse facial features, and multiple skin and soft tissue infections presented to the university dermatology clinic with persistent white, macerated, fissured groin plaques that were present for months. The lesions were tender and pruritic with a burning sensation. Treatment with topical terbinafine and oral fluconazole was attempted without resolution of the eruption. A biopsy of the groin lesion was performed.
Sunless Tanner Caused Persistent Hyperpigmented Patches on the Hands
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
To the Editor:
The use of sunless tanners has become an alternative for individuals who wish to have tan skin without exposure to UV radiation.1 We present a case of a patient who experienced persistent hyperpigmented patches on the hands months after the use of a sunless tanner containing dihydroxyacetone (DHA), a carbohydrate that reacts with amino acids in the stratum corneum to produce pigments called melanoidins. The hyperpigmentation caused by DHA is due to the Maillard reaction, which is the nonenzymatic glycation of amino groups of proteins by the carbonyl groups of sugar.2 Many sunless tanners contain DHA at varying concentrations. Dermatologists should be aware of the benefits and potential side effects of these alternative products so that they can appropriately counsel patients.
A 20-year-old woman with no history of skin disease presented for evaluation of hyperpigmented patches on the dorsal hands of several months’ duration. Physical examination revealed ill-defined hyperpigmented patches on the dorsal fingers without associated scale or erythema (Figure 1). She had a remote history of Hodgkin lymphoma treated with chemotherapy and was in remission for 5 years prior to the current presentation. Her hematologists referred her to dermatology for evaluation, as they did not believe the patches could be related to her chemotherapy given that she had completed the treatment years before.
A punch biopsy of one of the patches was obtained to elucidate the origin of the hyperpigmentation, which had no obvious triggers according to the patient. Histopathologic examination revealed hyperpigmented parakeratosis and lentiginous hyperplasia along with pigmentation of the stratum corneum (Figures 2A and 2B) with black pigment, which stained positive with Fontana-Masson (Figure 2C).
Upon further questioning, it was revealed that our patient had used a sunless tanner 3 months prior to the development of the pigmented patches. She also used urea cream to hasten exfoliation, which resulted in lighter but still apparent hyperpigmentation at follow-up 6 months after the initial presentation.
There has been a rapid growth of the sunless tanning industry in the last several years due to effective public education against UV tanning. Generally, patients apply the sunless tanner and notice an increase in tan within the following 48 hours. Typically, the tan progressively fades with the normal skin exfoliation over the span of weeks. Although most of the DHA binds proteins in the stratum corneum, the US Food and Drug Administration released a report speculating that approximately 11% of the compound reaches the epidermis and dermis.3 There are limited data regarding the effects of the compound should it pass the stratum corneum into the living skin cells.
Products with DHA only confer a sun protection factor of approximately 34; although patients may appear tan, they have no actual decreased risk for sunburn after use. Reports have shown that the use of sunless tanners containing DHA can alter the appearance of melanocytic lesions clinically and has caused pseudochromhidrosis on the palms.3,5,6 A study performed on a human keratinocyte cell line, HaCaT, showed that DHA can induce DNA damage, cell-cycle block, and apoptosis.7 In addition, as described in our case, patients may experience prolonged hyperpigmentation after use.
This case demonstrates the potential for persistent hyperpigmentation months after the use of sunless tanners containing DHA. Asking patients specific questions regarding their history of tanning product use is essential in identifying the pathology. Although a skin biopsy may not be strictly indicated, it may aid diagnosis, especially when the history is unclear. As more dermatologists support the use of sunless tanner, we must be aware of this possible outcome, especially on more cosmetically sensitive areas such as the fingers in this patient. Clinicians should be aware that the US Food and Drug Administration recommends avoiding contact with mucous membranes when applying products containing DHA and also recommends use of a test spot prior to treating the entire body with the product.8 Patients must not only be educated on the benefits of using sunless tanners but on the potential side effects with use of these products as well.
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
- Garone M, Howard J, Fabrikant J. A review of common tanning methods. J Clin Aesthet Dermatol. 2015;8:43-47.
- Finot PA. Nonenzymatic browning products: physiologic effects and metabolic transit in relation to chemical structure. a review. Diabetes. 1982;31:22-28.
- Yourick JJ, Koenig ML, Yourick DL, et al. Fate of chemicals in skin after dermal application: does the in vitro skin reservoir affect the estimate of systemic absorption? Toxicol Appl Pharmacol. 2004;195:309-320.
- Nguyen B, Kochevar I. Influence of hydration on dihydroxyacetone-induced pigmentation of stratum corneum. J Invest Dermatol. 2003;120:655-661.
- Takita Y, Ichimiya M, Yamaguchi M, et al. A case of pseudochromhidrosis due to dihydroxyacetone. J Dermatol. 2006;33:230-231.
- Yoshida R, Kobayashi S, Amagai M, et al. Brown palm pseudochromhidrosis. Contact Dermatitis. 2002;46:237-238.
- Petersen AB, Wulf HC, Gniadecki R, et al. Dihydroxyacetone, the active browning ingredient in sunless tanning lotions, induces DNA damage, cell-cycle block and apoptosis in cultured HaCaT keratinocytes. Mutat Res. 2004;560:173-186.
- US Food and Drug Administration. Sunless tanners & bronzers. FDA website. http://www.fda.gov/Cosmetics/ProductsIngredients
/Products/ucm134064.htm. Updated March 6, 2018. Accessed April 23, 2020
Practice Points
- Patient education on the benefits and risks associated with sunless tanners is critical when using these products.
- Sunless tanners containing dihydroxyacetone potentially can lead to persistent hyperpigmented patches on areas of contact.
- Skin biopsy showing hyperpigmented parakeratosis along with pigmentation of the stratum corneum can aid in diagnosis.