User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Follicular Traction Urticaria Induced by Electric Epilation
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
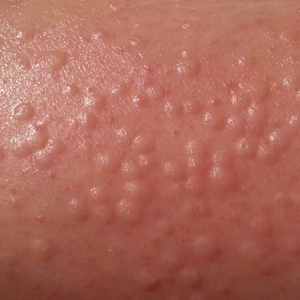
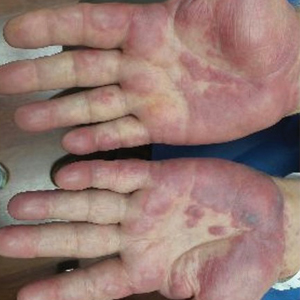
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.
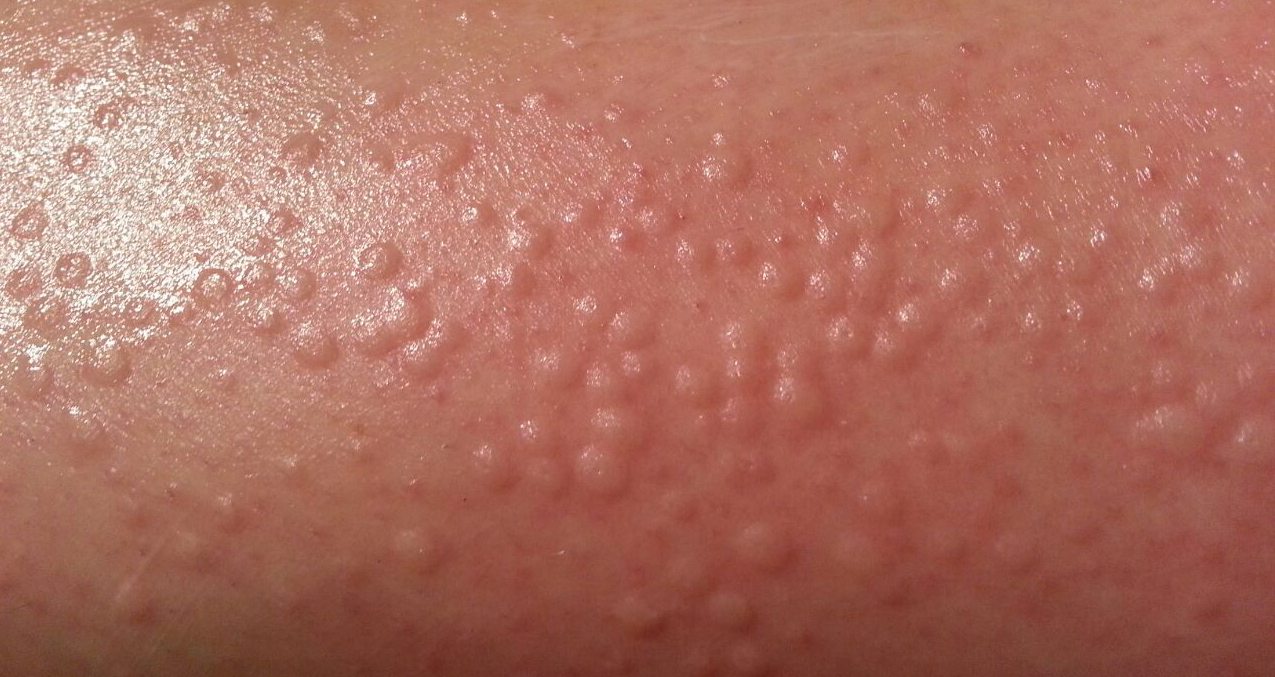
Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
To the Editor:
A 33-year-old woman who was otherwise healthy presented with itchy wheals that developed within 15 to 20 minutes of removing leg hair with an electric epilator. Furthermore, she reported that small hives often developed after waxing the legs with warm wax. All lesions spontaneously disappeared within 3 hours; depilatory creams and shaving did not trigger urticarial lesions. She had no history of atopy or prior episodes of spontaneous urticaria. Symptomatic dermographism also was not reported. Classic physical stimuli that could be associated with the use of an electric epilator, such as heat, vibration, and pressure, did not elicit lesions.
Physical examination showed no active lesions. Dermographism was not inducible by stroking the patient’s skin with a blunt object. She brought personal photographs that showed erythematous follicular hives measuring 1 to 3 mm in diameter located on the distal legs (Figure). In accordance with these findings, she was diagnosed with an unusual form of physical urticaria likely resulting from hair traction and was prescribed oral H1 antihistamines to be taken a few days before and after hair removal.

Physical urticaria are characterized by the presence of reddish, edematous, and pruritic wheals developing in response to a variety of exogenous physical stimuli such as heat, cold, vibration, dermographism, and pressure. These variants are widely described; nonetheless, follicular traction urticaria has been proposed as a new form of physical urticaria elicited by traction of hair, which would cause tension on and around hair follicles on a secondary basis.1 A PubMed search of articles indexed for MEDLINE using the term traction urticaria revealed 6 other cases. In 3 cases, hives were triggered by waxing or using an electric epilator.1-3 In 1 case, urticaria was elicited by shaving with a wet straight razor,whereas the other 2 cases were induced by the removal of patch tests.4-6 Sheraz et al7 investigated the role of dermographism in erythematous reactions during patch testing and concluded that some of these reactions might be caused by traction urticaria instead of being a form of dermographism.
Özkaya and Yazganog˘lu1 proposed that follicular dermographism should be differentiated from physical urticaria. This variant of dermographism is characterized by discrete urticarial papules appearing at the location of hair follicles after having stroked the skin with a blunt object.1,8 These lesions usually disappear within 30 minutes.8 Given that none of the reported cases presented dermographism on examination tests, we agree with Özkaya and Yazganog˘lu1 that this phenomenon of traction urticaria likely is a different condition than follicular dermographism, even though intraindividual variability sometimes can be seen in dermographism skin tests.7
We present a unique form of urticaria that easily can be misdiagnosed as pseudofolliculitis, which tends to be more commonly associated with the use of electric epilators.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
- Özkaya E, Yazganog˘lu KD. Follicular traction urticaria. J Am Acad Dermatol. 2012;67:E234-E236.
- Duman H, Topal IO, Kocaturk E. Follicular traction urticaria. An Bras Dermatol. 2016;91:64-65.
- Raison-Peyron N, Reymann V, Bessis D. Follicular traction urticaria: a new form of chronic inducible urticaria? Acta Derm Venereol. 2017;97:522-523.
- Patel SS, Lockey RF. Follicular traction urticaria. J Allergy Clin Immunol Pract. 2018;6:1383.
- Gallo R, Fausti V, Parodi A. Traction urticaria. Contact Dermatitis. 2009;61:301-302.
- Özkaya E. Follicular traction urticaria: an occult case diagnosed by patch testing. Dermatitis. 2019;30:171-173.
- Sheraz A, Simms MJ, White IR, et al. Erythematous reactions on removal of Scanpor® tape in patch testing are not necessarily caused by dermographism. Contact Dermatitis. 2014;71:62-64.
- Bhute D, Doshi B, Pande S, et al. Dermatographism. Indian J Dermatol Venereol Leprol. 2008;74:177-179.
Practice Points
- Follicular traction urticaria is an unusual form of chronic inducible urticaria.
- Follicular traction urticaria consists of follicular hives that develop after being triggered by hair traction.
Making the World's Skin Crawl: Dermatologic Implications of COVID-19
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
Coronaviruses (CoVs) are among the most common causes of the common cold but also can lead to severe respiratory disease.1 In recent years, CoVs have been responsible for outbreaks of severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), caused by SARS-CoV and MERS-CoV, respectively. Severe acute respiratory syndrome emerged from China in 2002, and MERS started in Saudi Arabia in 2012. In December 2019, several cases of unexplained pneumonia were reported in Wuhan, China.1 A novel CoV--SARS-CoV-2--was isolated in these patients and is now known to cause coronavirus disease 19 (COVID-19).1 Coronavirus disease 19 can cause acute respiratory distress and multiorgan failure.1,2 It spread quickly throughout the world and was declared a pandemic by the World Health Organization on March 11, 2020. According to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html), there were approximately 14,500 COVID-19 cases diagnosed worldwide on February 1, 2020; by May 22, 2020, there were more than 5,159,600 cases. Thus, heightened measures for infection prevention and control were put in place around the globe in an attempt to slow the spread of disease.1
In this article, we describe the dermatologic implications of COVID-19, including the clinical manifestations of the disease, risk reduction techniques for patients and providers, personal protective equipment-associated adverse reactions, and the financial impact on dermatologists.
Clinical Manifestations
At the start of the COVID-19 outbreak, little was known about the skin manifestations of the disease. Providers speculated that COVID-19 could have nonspecific skin findings similar to many other viral illnesses.3,4 Research throughout the pandemic has found many cutaneous manifestations of the disease.3-6 A case report from Thailand described a patient who presented with petechiae in addition to fever and thrombocytopenia, which led to an initial misdiagnosis of Dengue fever; however, when the patient began having respiratory symptoms, the diagnosis of COVID-19 was discovered.5 Furthermore, a study from Italy (N=88) showed dermatologic findings in 20.4% (18/88) of patients, including erythematous rash (77.8% [14/18]), widespread urticaria (16.7% [3/18]), and chickenpoxlike vesicles (5.6% [1/18]). A recent study from Spain (N=375) found 5 cutaneous patterns associated with COVID-19: pseudochilblain--acral areas of erythema with vesicles and/or pustules--lesions (19%), vesicular eruptions (9%), urticarial lesions (19%), maculopapular eruptions (47%), and livedoid/necrotic lesions (6%).6 Pseudochilblain lesions appeared in younger patients, occurred later in the disease course, and were associated with less severe disease. Vesicular lesions often were found in middle-aged patients prior to the onset of other COVID-19 symptoms, and they were associated with intermediate disease severity. Urticarial and maculopapular lesions typically paralleled other COVID-19 symptoms in timing and were associated with more severe disease. Likewise, livedoid and necrotic lesions were associated with more severe disease; they occurred more frequently in older patients.6 Clinicians at Cleveland Clinic found similar cutaneous lesions in COVID-19 patients, including morbilliform rashes, acral purpura resembling perniosis, and livedoid lesions.3 Initial biopsies of these lesions pointed to viral exanthema and thrombotic vasculopathy as potential etiologies of morbilliform and livedoid lesions, respectively. Interestingly, patients may present with multiple cutaneous morphologies of the disease at the same time.3 The acral lesions ("COVID toes") have been popularized throughout the media and thus may be the best-known cutaneous manifestation of the disease at this time. New findings continuously arise, and further research is warranted as lesions that develop in hospitalized COVID-19 patients could be virus related or secondary to hospital-induced skin irritation, stressors, or medications.3 Importantly, clinicians should be aware of these cutaneous signs of COVID-19, especially when triaging patients.
Risk Reduction
The current health crisis could have a drastic impact on dermatology patients and providers. One factor that may increase COVID-19 risk in dermatology patients is immunosuppression. Many patients are on immunomodulators and biologics for skin conditions, which can cause immunosuppression directly and indirectly. Immunosuppression is a risk factor for severe disease in patients with COVID-19, so this population is at higher risk for serious infection.7 Telemedicine for nonemergent cases and follow-ups should be considered to decrease traffic in high-risk hospitals; to limit the number of people in waiting rooms; and to protect staff, providers, and patients alike.1 Recommendations for teledermatology consultation during this time include the following: First, have patients take photographs of their skin lesions and send them remotely to the consulting physician. If the lesion is easily recognizable, treatment recommendations can be made remotely; if the diagnosis is ambiguous, the dermatologist can set up an in-person appointment.1
Personal Protective Equipment
Moreover, the current need to wear personal protective equipment (PPE) and wash hands frequently may lead to skin disease among health care providers. Facial rashes may arise from wearing masks and goggles, and repeated handwashing and wearing gloves may lead to hand dermatitis.8 One study examined adverse skin reactions among health care workers (N=322) during the SARS outbreak in 2003. More than one-third (35.5%) of staff members who wore masks regularly during the outbreak reported adverse skin reactions, including acne (59.6%), facial itching (51.4%), and rash (35.8%).8 The acne etiology likely is multifactorial. Masks increase heat and humidity in the covered facial region, which can cause acne flare-ups due to increased sebum production and Cutibacterium acnes growth.8 Additionally, tight N95 masks may occlude the pilosebaceous glands, causing acne to flare. In the SARS study, facial itchiness and rashes likely were due to irritant contact dermatitis to the N95 masks. All of the respondents with adverse skin reactions from masks developed them after using N95 masks; those who wore surgical masks did not report reactions.8 Because N95 masks are recommended for health care workers caring for patients with highly transmissible respiratory infections such as SARS and COVID-19, it will be difficult to avoid wearing them during the current crisis. For this reason, topical retinoids and topical benzoyl peroxide should be the first-line treatment of mask-induced acne, and moisturization and topical corticosteroids should be used for facial erythema. Additionally, 21.4% of respondents reported adverse skin reactions from latex gloves during the SARS outbreak, including dry skin, itchiness, rash, and wheals.8 These skin reactions may have been type I IgE-mediated hypersensitivity reactions or irritant contact dermatitis due to latex sensitization and frequent handwashing. No respondents reported skin reactions to plastic gloves.8 For this reason, health care providers should consider wearing plastic gloves in lieu of or under latex gloves to prevent hand dermatitis during this time. Moisturization, barrier creams, and topical corticosteroids also can help treat hand dermatitis. Frequently changing PPE may help prevent skin disease among the frontline health care workers,8 which posed a problem at the beginning of the COVID-19 outbreak as there was a PPE shortage. With industry and individuals coming together to make and donate PPE, it is now more widely available for our frontline providers.
Financial Impact
Finally, the pandemic is having an immense financial impact on dermatology.9 At the onset of the outbreak, our role as health care providers was to help slow the spread of COVID-19; for this reason, most elective procedures were cancelled, and many outpatient clinics closed. Both elective procedures and outpatient visits are central to dermatology, so many dermatologists worked less or not at all during this time, leading to a loss of revenue. The goals of these measures were to reduce transmissibility of the disease, to prevent the health care system from being overwhelmed with critical COVID-19 cases, and to allocate resources to the frontline providers.9 Although these measures were beneficial for slowing the spread of disease, they were detrimental to some providers' and practices' financial stability. Many dermatology practices have begun to reopen with COVID-19 precautions in place. For example, practices are limiting the number of patients that can be in the office at one time, mandating temperature readings upon check-in, and requiring masks be worn throughout the entire visit. With continued recommendations for individuals to stay at home as much as possible, the number of patients being seen in dermatology clinics on a daily basis remains less than normal. One potential solution is telemedicine, which would allow patients' concerns to be addressed while keeping providers practicing with a normal patient volume during this time.9 Keeping providers financially afloat is vital for private practices to continue operating after the pandemic. Dermatology appointments are in high demand with long waiting lists during nonpandemic times; without dermatologists practicing at full capacity, there will be an accumulation of patients with dermatologic conditions with even longer waiting times after the pandemic. Telemedicine may help reduce this potential accumulation of patients and allow patients to be treated in a more timely manner while alleviating financial pressures for providers.
Final Thoughts
The COVID-19 pandemic has spread across the world, infecting millions of people. Although the trends have slowed, more than 106,100 cases are still being diagnosed daily according to the Johns Hopkins University Coronavirus Resource Center (https://coronavirus.jhu.edu/map.html). Patients with COVID-19 may present with a variety of cutaneous lesions. Wearing PPE to take care of COVID-19 patients may lead to skin irritation, so care should be taken to address these adverse skin reactions to maintain the safety of providers. Finally, dermatologists should consider telemedicine during this time to protect high-risk patients, prevent a postpandemic surge of patients, and alleviate financial stressors caused by COVID-19.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
- Tao J, Song Z, Yang L, et al. Emergency management for preventing and controlling nosocomial infection of 2019 novel coronavirus: implications for the dermatology department [published online March 5, 2020]. Br J Dermatol. doi:10.1111/bjd.19011.
- Lippi G, Plebani M, Michael HB. Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID-19) infections: a meta-analysis [published online March 13, 2020]. Clin Chim Acta. doi:10.1016/j.cca.2020.03.022.
- Young S, Fernandez AP. Skin manifestations of COVID-19 [published online May 14, 2020]. Cleve Clin J Med. doi:10.3949/ccjm.87a.ccc031.
- Recalcati S. Cutaneous manifestations in COVID-19: a first perspective [published online March 26, 2020]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.16387.
- Joob B, Wiwanitkit V. COVID-19 can present with a rash and be mistaken for Dengue [published online March 22, 2020]. J Am Acad Dermatol. doi:10.1016/j.jaad.2020.03.036.
- Casas CG, Catalá A, Hernández GC, et al. Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases [published online April 29, 2020]. Br J Dermatol. doi:10.1111/bjd.19163.
- Conforti C, Giuffrida R, Dianzani C, et al. COVID-19 and psoriasis: is it time to limited treatment with immunosuppressants? a call for action [published online March 11, 2020]. Dermatol Ther. doi:10.1111/dth.13298.
- Foo CC, Goon AT, Leow YH, et al. Adverse skin reactions to personal protective equipment against severe respiratory syndrome--a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294.
- Heymann WR. The profound dermatological manifestations of COVID-19 [published online March 18, 2020]. Dermatology World Insights and Inquiries. https://www.aad.org/dw/dw-insights-and-inquiries/2020-archive/march/dermatological-manifestations-covid-19. Accessed May 21, 2020.
Practice Points
- Clinicians should be aware of the skin manifesta-tions of coronavirus disease 19, especially when triaging patients.
- Health care providers may develop skin diseases from wearing the extensive personal protective equipment required during the current health crisis.
- Coronavirus disease 19 has had a substantial finan-cial impact on dermatologists, and telemedicine may be a potential solution.
Asymptomatic Transient Lingual Hyperpigmentation
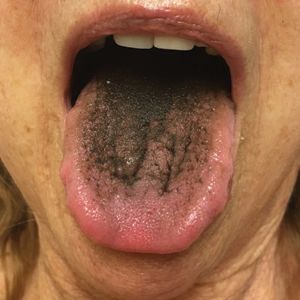
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
The Diagnosis: Pseudo-Black Hairy Tongue
Pseudo-black hairy tongue is a benign and painless disorder characterized by transient hyperpigmentation of the tongue with a substance that can be easily scraped off. In this case, the patient's lingual discoloration was secondary to the ingestion of bismuth salicylate. The phenomenon is thought to occur due to a reaction between bismuth and sulfur-containing compounds in the saliva, resulting in the characteristic black substance on the surface of the tongue that nestles between the lingual papillae.1 An associated feature may include black stools. Other etiologic factors involved in pseudo-black hairy tongue include food coloring, tobacco, and other drugs such antibiotics and antidepressants.2
The differential diagnosis of lingual hyperpigmentation includes lingua villosa nigra (also known as black hairy tongue), pigmented fungiform papillae of the tongue, acanthosis nigricans, and oral hairy leukoplakia. Lingua villosa nigra is a similar condition in which individuals present with a black tongue; however, the tongue also appears hairy. The tongue may appear as other colors such as brown, yellow, or green. Patients additionally may have symptoms of burning, dysgeusia, halitosis, or gagging. Poor oral hygiene, xerostomia, use of tobacco or alcohol, and different medications including antibiotics and antipsychotic medications increase the risk for developing lingua villosa nigra.2,3 This condition is distinguished from pseudo-black hairy tongue by proliferation and elongation of the filiform papillae.3 Pigmented fungiform papillae of the tongue is a normal variant of tongue morphology, is more common in individuals with darker skin types, and primarily affects the lateral aspect and apex of the tongue.4 Acanthosis nigricans can appear in the oral cavity as multiple pigmented papillary lesions on the dorsal and lateral regions of the tongue and frequently involves the lips; this condition may be associated with metabolic disorders or underlying malignancy.2,3 Oral hairy leukoplakia is caused by Epstein-Barr virus infection and typically presents as white plaques on the dorsal and ventral surfaces of the tongue; this condition largely is found in immunocompromised patients.5
In our patient there was an acute onset of tongue discoloration associated with ingestion of bismuth salicylate, no hypertrophy or lengthening of the lingual papillae, and no involvement of the patient's lips, which was consistent with the diagnosis of pseudo-black hairy tongue. Pseudo-black hairy tongue is transient and treated by discontinuation of offending agents and proper hygiene practices.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
- Bradley B, Singleton M, Lin Wan Po A. Bismuth toxicity--a reassessment. J Clin Pharm Ther. 1989;14:423-441.
- Gurvits GE, Tan A. Black hairy tongue syndrome. World J Gastroenterol. 2014;20:10845-10850.
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569.
- Mangold AR, Torgerson RR, Rogers RS. Diseases of the tongue. Clin Dermatol. 2016;34:458-469.
- Husak R, Garbe C, Orfanos CE. Oral hairy leukoplakia in 71 HIV-seropositive patients: clinical symptoms, relation to immunologic status, and prognostic significance. J Am Acad Dermatol. 1996;35:928-934.
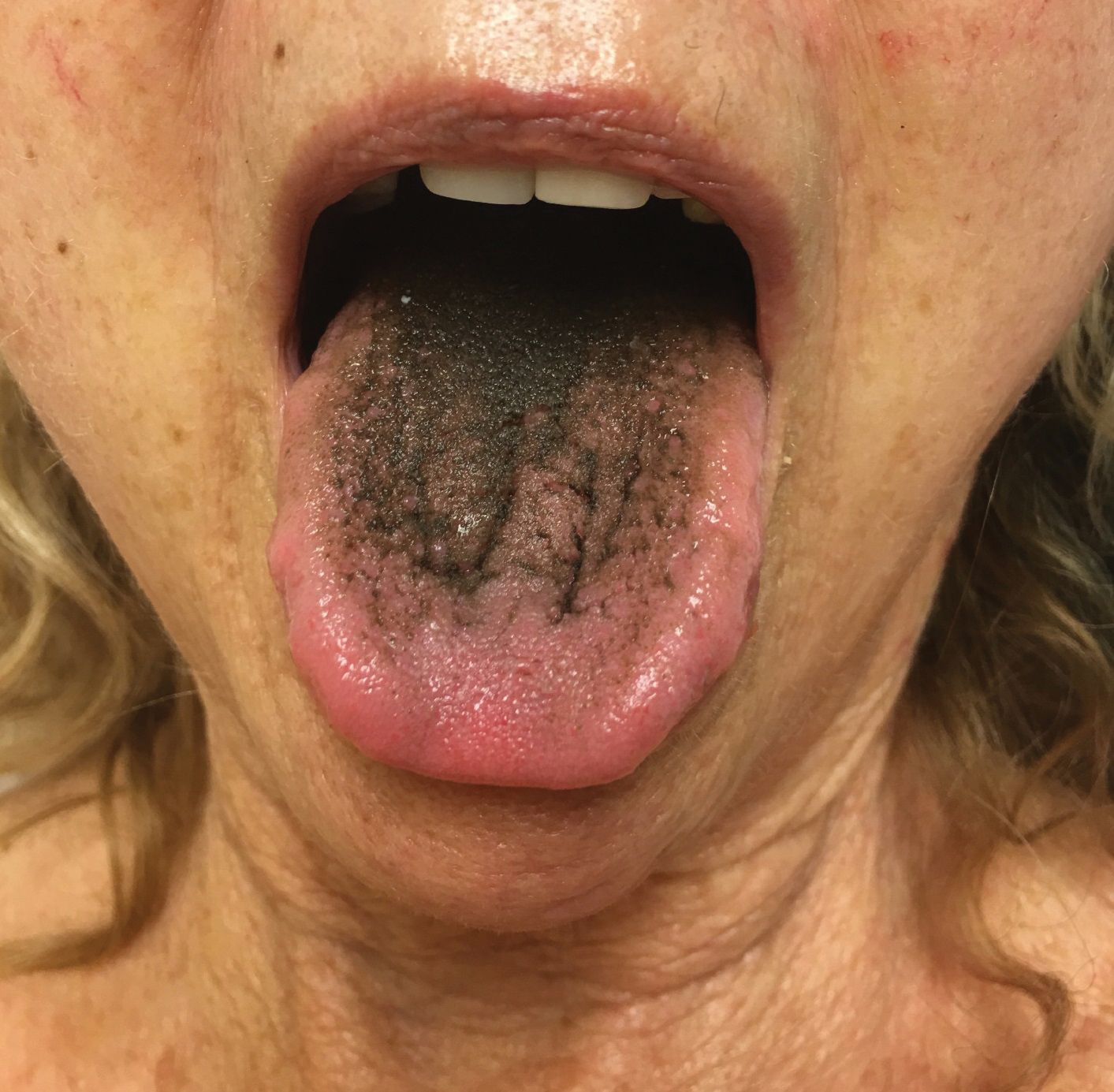
A 77-year-old woman incidentally was noted to have black discoloration of the tongue during a routine dermatologic examination. The patient was unaware of the tongue discoloration and reported that her tongue appeared normal the day prior. The tongue was asymptomatic. Clinical examination revealed black hyperpigmentation on the dorsal aspect of the tongue without appreciable hypertrophy or hyperkeratosis of the filiform papillae. The patient had a half-pack daily smoking habit for many years but had abstained from any smoking or tobacco use for the last 15 years. The patient endorsed good oral hygiene. Upon further questioning, the patient revealed that she had ingested 1 tablet of bismuth salicylate the prior night to relieve postprandial dyspepsia. A cotton-tipped applicator was rubbed gently against the affected area and removed some of the black pigment.
Cutaneous Metastatic Breast Adenocarcinoma
To the Editor:
Cutaneous metastases occur more often in the setting of breast carcinoma than other malignancies in women.1 Although interventions are aimed at halting disease progression, cutaneous metastases indicate widespread disease and are associated with poor prognosis. We present the case of a patient with metastatic breast adenocarcinoma who developed cutaneous metastasis on the trunk after a double mastectomy. The widespread distribution and wide range of clinical manifestations are unique.
An 81-year-old woman presented to the dermatology office for evaluation of a skin eruption that started along a mastectomy scar on the left breast a few months postoperatively. She had a history of stage IV breast adenocarcinoma metastatic to the chest wall that was treated with a double mastectomy 2 years prior. The patient denied associated pain or pruritus and mainly was concerned with the cosmetic appearance. At the time of the initial diagnosis of breast adenocarcinoma, the patient was offered chemotherapy, which she did not tolerate. The patient opted against radiation therapy, as she preferred a more natural approach, such as anticancer shakes, which she was taking from a homeopathic source. She was unaware of the ingredients used in the shakes.
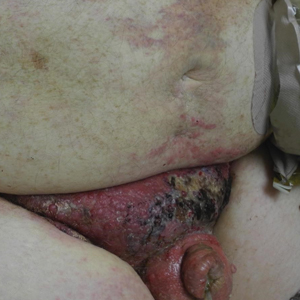
Physical examination revealed multiple grouped, firm, purpuric papules, nodules, and pseudovesicles on a background of violaceous erythema on the chest, abdomen, and flank (Figure 1). The background erythema had a mosaic pattern that extended toward the central back (Figure 2). A scoop shave biopsy of one of the purpuric nodules revealed highly atypical cells with abundant cytoplasm, large nuclei, and prominent nucleoli (Figure 3). Focally, the cells appeared to form glandular structures. Numerous atypical mitotic figures were present. Lymphatic invasion and microcalcifications were identified (Figure 3 [inset]). Immunohistochemical staining for cytokeratin 7 and gross cystic disease fluid protein 15 were strongly positive (Figure 4). Based on the histopathologic and immunohistochemical findings, a diagnosis of cutaneous metastatic breast adenocarcinoma was made. The patient opted to continue the homeopathic anticancer shakes and was subsequently lost to follow-up.
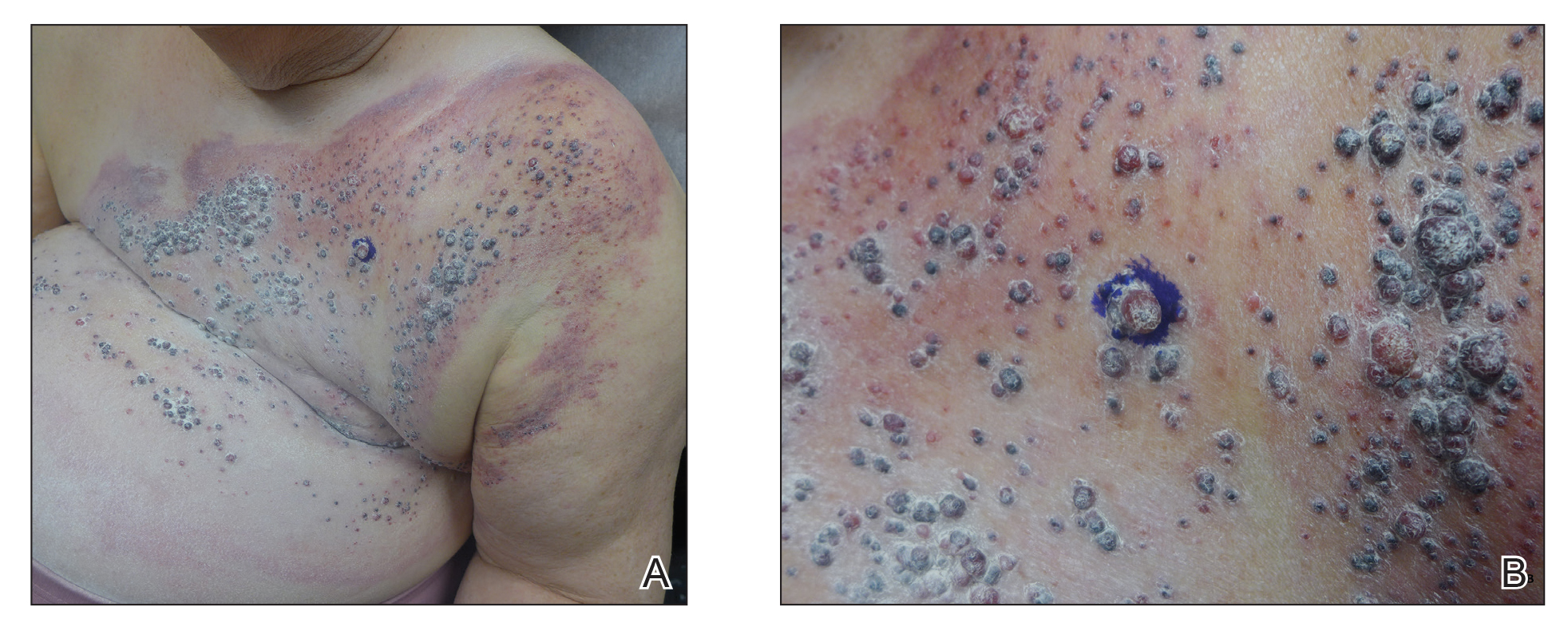

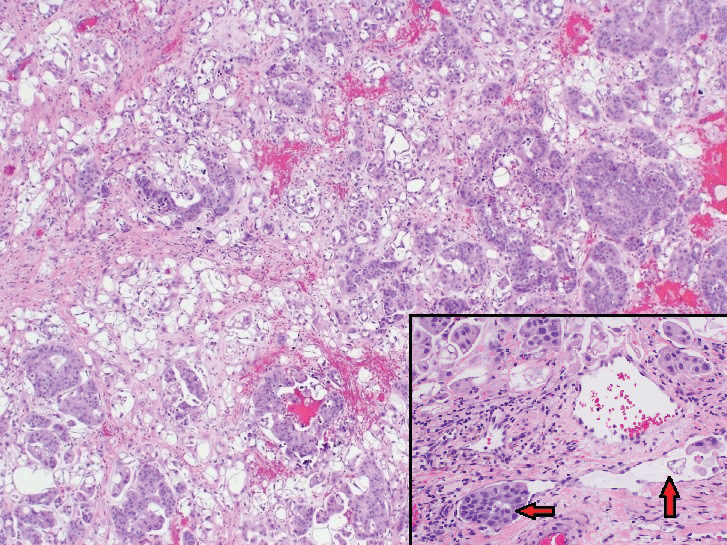
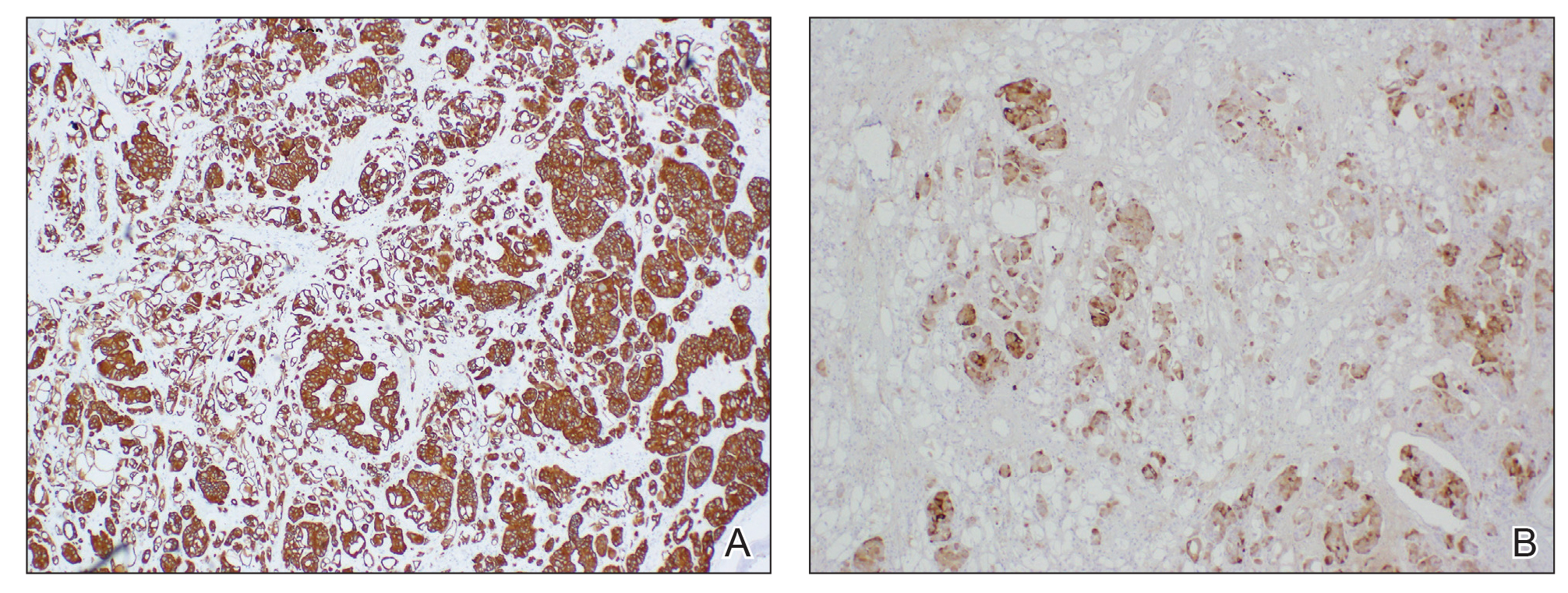
Cutaneous metastases of internal malignancies make up only 2% of all skin tumors,1 making them relatively uncommon in the dermatologic setting. However, cutaneous metastasis occurs in 23.9% of patients with breast carcinoma, making it the most common tumor after malignant melanoma to metastasize to the skin.2 The most common sites for breast carcinoma cutaneous metastasis (BCCM) are the chest wall and abdomen; other sites include the head/neck region and the extremities. The clinical presentation of BCCM varies depending on the mode of dissemination—lymphatic, hematogenous, contiguous growth, or iatrogenic implantation. The most common presentation is nodular carcinoma (47%–80%).2,3 Other presentations include carcinoma telangiectoides (8%–11%),2,3 alopecia neoplastica (2%–12%),2,3 and carcinoma erysipeloides (3%–6%).2,3 Carcinoma en cuirasse is rare.3
Nodular BCCM may present as firm solitary or grouped papules and nodules that are painless and range in color from flesh colored or pink to red-brown. Histologically, they are composed of atypical neoplastic cells arranged in small nests and cords, usually in a single-file line within the collagen bundles of the dermis.4 Carcinoma telangiectoides is characterized by its violaceous hue due to the dilated vascular channels. The lesions are purpuric papules and pseudovesicles appearing on an erythematous base, most commonly contiguous with the surgical scar. Histologically, collections of atypical tumor cells and erythrocytes are present along with dilated blood vessels in the papillary dermis.2 Alopecia neoplastica presents as singular or grouped cicatricial patches of hair loss. Lesions of carcinoma erysipeloides present as warm, erythematous, tender, well-defined patches or plaques. Carcinoma en cuirasse is characterized by an erythematous sclerodermoid plaque on the chest wall.2
Our patient’s presentation was unique due to the widespread distribution, unusual pattern, and variable clinical morphologies of the cutaneous metastases. Our patient had findings of both carcinoma telangiectoides and nodular carcinoma. The mosaic violaceous erythema extending toward the mid-back rarely is reported in the literature and indicates extensive intravascular spread of tumor cells in the dermis.
Metastatic breast cancer is associated with a poor prognosis because it typically occurs in advanced stages and often does not respond to treatment.5 Although chemotherapy, hormonal therapy, and/or radiation therapy may improve survival, the choice in regimen is guided by cancer histology as well as prior treatments. In our case, the patient chose to continue her homeopathic therapy.
- Nashan D, Meiss F, Braun-Falco M, et al. Cutaneous metastasis from internal malignancies. Dermatol Ther. 2010;23:567-580.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Mordenti C, Peris K, Concetta Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerologica. 2000;9:143-148.
- Nava G, Greer K, Patterson J, et al. Metastatic cutaneous breast carcinoma: a case report and review of the literature. Can J Plast Surg. 2009;17:25-27.
- Kalmykow B, Walker S. Cutaneous metastasis in breast cancer. Clin J Oncol Nurs. 2001;15:99-101.
To the Editor:
Cutaneous metastases occur more often in the setting of breast carcinoma than other malignancies in women.1 Although interventions are aimed at halting disease progression, cutaneous metastases indicate widespread disease and are associated with poor prognosis. We present the case of a patient with metastatic breast adenocarcinoma who developed cutaneous metastasis on the trunk after a double mastectomy. The widespread distribution and wide range of clinical manifestations are unique.
An 81-year-old woman presented to the dermatology office for evaluation of a skin eruption that started along a mastectomy scar on the left breast a few months postoperatively. She had a history of stage IV breast adenocarcinoma metastatic to the chest wall that was treated with a double mastectomy 2 years prior. The patient denied associated pain or pruritus and mainly was concerned with the cosmetic appearance. At the time of the initial diagnosis of breast adenocarcinoma, the patient was offered chemotherapy, which she did not tolerate. The patient opted against radiation therapy, as she preferred a more natural approach, such as anticancer shakes, which she was taking from a homeopathic source. She was unaware of the ingredients used in the shakes.
Physical examination revealed multiple grouped, firm, purpuric papules, nodules, and pseudovesicles on a background of violaceous erythema on the chest, abdomen, and flank (Figure 1). The background erythema had a mosaic pattern that extended toward the central back (Figure 2). A scoop shave biopsy of one of the purpuric nodules revealed highly atypical cells with abundant cytoplasm, large nuclei, and prominent nucleoli (Figure 3). Focally, the cells appeared to form glandular structures. Numerous atypical mitotic figures were present. Lymphatic invasion and microcalcifications were identified (Figure 3 [inset]). Immunohistochemical staining for cytokeratin 7 and gross cystic disease fluid protein 15 were strongly positive (Figure 4). Based on the histopathologic and immunohistochemical findings, a diagnosis of cutaneous metastatic breast adenocarcinoma was made. The patient opted to continue the homeopathic anticancer shakes and was subsequently lost to follow-up.




Cutaneous metastases of internal malignancies make up only 2% of all skin tumors,1 making them relatively uncommon in the dermatologic setting. However, cutaneous metastasis occurs in 23.9% of patients with breast carcinoma, making it the most common tumor after malignant melanoma to metastasize to the skin.2 The most common sites for breast carcinoma cutaneous metastasis (BCCM) are the chest wall and abdomen; other sites include the head/neck region and the extremities. The clinical presentation of BCCM varies depending on the mode of dissemination—lymphatic, hematogenous, contiguous growth, or iatrogenic implantation. The most common presentation is nodular carcinoma (47%–80%).2,3 Other presentations include carcinoma telangiectoides (8%–11%),2,3 alopecia neoplastica (2%–12%),2,3 and carcinoma erysipeloides (3%–6%).2,3 Carcinoma en cuirasse is rare.3
Nodular BCCM may present as firm solitary or grouped papules and nodules that are painless and range in color from flesh colored or pink to red-brown. Histologically, they are composed of atypical neoplastic cells arranged in small nests and cords, usually in a single-file line within the collagen bundles of the dermis.4 Carcinoma telangiectoides is characterized by its violaceous hue due to the dilated vascular channels. The lesions are purpuric papules and pseudovesicles appearing on an erythematous base, most commonly contiguous with the surgical scar. Histologically, collections of atypical tumor cells and erythrocytes are present along with dilated blood vessels in the papillary dermis.2 Alopecia neoplastica presents as singular or grouped cicatricial patches of hair loss. Lesions of carcinoma erysipeloides present as warm, erythematous, tender, well-defined patches or plaques. Carcinoma en cuirasse is characterized by an erythematous sclerodermoid plaque on the chest wall.2
Our patient’s presentation was unique due to the widespread distribution, unusual pattern, and variable clinical morphologies of the cutaneous metastases. Our patient had findings of both carcinoma telangiectoides and nodular carcinoma. The mosaic violaceous erythema extending toward the mid-back rarely is reported in the literature and indicates extensive intravascular spread of tumor cells in the dermis.
Metastatic breast cancer is associated with a poor prognosis because it typically occurs in advanced stages and often does not respond to treatment.5 Although chemotherapy, hormonal therapy, and/or radiation therapy may improve survival, the choice in regimen is guided by cancer histology as well as prior treatments. In our case, the patient chose to continue her homeopathic therapy.
To the Editor:
Cutaneous metastases occur more often in the setting of breast carcinoma than other malignancies in women.1 Although interventions are aimed at halting disease progression, cutaneous metastases indicate widespread disease and are associated with poor prognosis. We present the case of a patient with metastatic breast adenocarcinoma who developed cutaneous metastasis on the trunk after a double mastectomy. The widespread distribution and wide range of clinical manifestations are unique.
An 81-year-old woman presented to the dermatology office for evaluation of a skin eruption that started along a mastectomy scar on the left breast a few months postoperatively. She had a history of stage IV breast adenocarcinoma metastatic to the chest wall that was treated with a double mastectomy 2 years prior. The patient denied associated pain or pruritus and mainly was concerned with the cosmetic appearance. At the time of the initial diagnosis of breast adenocarcinoma, the patient was offered chemotherapy, which she did not tolerate. The patient opted against radiation therapy, as she preferred a more natural approach, such as anticancer shakes, which she was taking from a homeopathic source. She was unaware of the ingredients used in the shakes.
Physical examination revealed multiple grouped, firm, purpuric papules, nodules, and pseudovesicles on a background of violaceous erythema on the chest, abdomen, and flank (Figure 1). The background erythema had a mosaic pattern that extended toward the central back (Figure 2). A scoop shave biopsy of one of the purpuric nodules revealed highly atypical cells with abundant cytoplasm, large nuclei, and prominent nucleoli (Figure 3). Focally, the cells appeared to form glandular structures. Numerous atypical mitotic figures were present. Lymphatic invasion and microcalcifications were identified (Figure 3 [inset]). Immunohistochemical staining for cytokeratin 7 and gross cystic disease fluid protein 15 were strongly positive (Figure 4). Based on the histopathologic and immunohistochemical findings, a diagnosis of cutaneous metastatic breast adenocarcinoma was made. The patient opted to continue the homeopathic anticancer shakes and was subsequently lost to follow-up.




Cutaneous metastases of internal malignancies make up only 2% of all skin tumors,1 making them relatively uncommon in the dermatologic setting. However, cutaneous metastasis occurs in 23.9% of patients with breast carcinoma, making it the most common tumor after malignant melanoma to metastasize to the skin.2 The most common sites for breast carcinoma cutaneous metastasis (BCCM) are the chest wall and abdomen; other sites include the head/neck region and the extremities. The clinical presentation of BCCM varies depending on the mode of dissemination—lymphatic, hematogenous, contiguous growth, or iatrogenic implantation. The most common presentation is nodular carcinoma (47%–80%).2,3 Other presentations include carcinoma telangiectoides (8%–11%),2,3 alopecia neoplastica (2%–12%),2,3 and carcinoma erysipeloides (3%–6%).2,3 Carcinoma en cuirasse is rare.3
Nodular BCCM may present as firm solitary or grouped papules and nodules that are painless and range in color from flesh colored or pink to red-brown. Histologically, they are composed of atypical neoplastic cells arranged in small nests and cords, usually in a single-file line within the collagen bundles of the dermis.4 Carcinoma telangiectoides is characterized by its violaceous hue due to the dilated vascular channels. The lesions are purpuric papules and pseudovesicles appearing on an erythematous base, most commonly contiguous with the surgical scar. Histologically, collections of atypical tumor cells and erythrocytes are present along with dilated blood vessels in the papillary dermis.2 Alopecia neoplastica presents as singular or grouped cicatricial patches of hair loss. Lesions of carcinoma erysipeloides present as warm, erythematous, tender, well-defined patches or plaques. Carcinoma en cuirasse is characterized by an erythematous sclerodermoid plaque on the chest wall.2
Our patient’s presentation was unique due to the widespread distribution, unusual pattern, and variable clinical morphologies of the cutaneous metastases. Our patient had findings of both carcinoma telangiectoides and nodular carcinoma. The mosaic violaceous erythema extending toward the mid-back rarely is reported in the literature and indicates extensive intravascular spread of tumor cells in the dermis.
Metastatic breast cancer is associated with a poor prognosis because it typically occurs in advanced stages and often does not respond to treatment.5 Although chemotherapy, hormonal therapy, and/or radiation therapy may improve survival, the choice in regimen is guided by cancer histology as well as prior treatments. In our case, the patient chose to continue her homeopathic therapy.
- Nashan D, Meiss F, Braun-Falco M, et al. Cutaneous metastasis from internal malignancies. Dermatol Ther. 2010;23:567-580.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Mordenti C, Peris K, Concetta Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerologica. 2000;9:143-148.
- Nava G, Greer K, Patterson J, et al. Metastatic cutaneous breast carcinoma: a case report and review of the literature. Can J Plast Surg. 2009;17:25-27.
- Kalmykow B, Walker S. Cutaneous metastasis in breast cancer. Clin J Oncol Nurs. 2001;15:99-101.
- Nashan D, Meiss F, Braun-Falco M, et al. Cutaneous metastasis from internal malignancies. Dermatol Ther. 2010;23:567-580.
- De Giorgi V, Grazzini M, Alfaioli B, et al. Cutaneous manifestations of breast carcinoma. Dermatol Ther. 2010;23:581-589.
- Mordenti C, Peris K, Concetta Fargnoli M, et al. Cutaneous metastatic breast carcinoma. Acta Dermatovenerologica. 2000;9:143-148.
- Nava G, Greer K, Patterson J, et al. Metastatic cutaneous breast carcinoma: a case report and review of the literature. Can J Plast Surg. 2009;17:25-27.
- Kalmykow B, Walker S. Cutaneous metastasis in breast cancer. Clin J Oncol Nurs. 2001;15:99-101.
Practice Points
- Breast carcinoma is one of the most common malignancies to metastasize to the skin in women.
- Although interventions are aimed at halting disease progression, cutaneous metastases indicate widespread disease and are associated with a poor prognosis.
Penile Paraffinoma: Dramatic Recurrence After Surgical Resection
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.
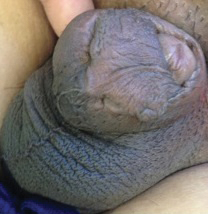
The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
To the Editor:
The term paraffinoma refers to a chronic granulomatous response to injection of paraffin, silicone, or other mineral oils into skin and soft tissue. Paraffinomas develop when the material is injected into the skin for cosmetic purposes to augment or enhance one’s appearance. Although they may occur in any location, the most common sites include the breasts and buttocks. The penis is a rare but emerging site for paraffinomas.1-3 We present a rare case of recurrence of a penile paraffinoma following surgical resection.
A 26-year-old uncircumcised Trinidadian man presented with a 5-cm, exquisitely tender tumor involving the penile shaft and median raphe that rapidly evolved over the course of 3 weeks (Figure 1). He presented with inability to urinate, attain an erection, or ambulate without notable tenderness. Additionally, he developed swelling of the penis and surrounding tissue. He had no other medical comorbidities; however, 1 year prior he presented to a urologist with a 1-cm nodule involving the median raphe that was surgically resected and required circumcision. Biopsy at the time of his surgical procedure revealed an exuberant foreign body giant cell reaction with surrounding empty spaces in the dermis resembling Swiss cheese, consistent with a paraffinoma (Figure 2). The recurrent tumor, which was 5 times the size of the initial nodule, was biopsied. Again, histopathologic findings were consistent with a paraffinoma with extensive dermal fibrosis and absence of polarizable material.


The patient underwent extensive reconstructive surgery requiring skin grafting to the penile shaft. Given the size and location of this recurrent tumor with the ability to destroy vital urologic and reproductive function, consideration for prevention of recurrent episodes included novel therapeutic treatment options to suppress inflammation and fibrosis with doxycycline and nicotinamide.
Paraffin injections are used for cosmetic enhancement and most often occur in a nonclinical setting without medical supervision, as they are not US Food and Drug Administration–approved medical injectable materials. Examples of oils injected include paraffin, camphorated oil, cottonseed or sesame oil, mineral oil, petroleum jelly, and beeswax. These oils are not hydrolyzed by tissue lipases but are instead treated as a foreign body substance with subsequent granuloma formation (also known as sclerosing lipogranuloma), which can occur many years after injection.4 The granulomatous response may be observed months to years after injection. The paraffinoma normally affects the injection site; however, regional lymphadenopathy and systemic disease has been reported.2 Histopathologic findings are characteristic and consist of a foreign body giant cell reaction, variably sized round to oval cavities within the dermis, and varying degrees of dermal fibrosis.5
In 1899, mineral oil was first injected into male genitalia to restore architecture in a patient’s testicles following bilateral orchiectomy. After the success of this endeavor, mineral oil injections were used as filler for other defects.3 However, by 1906 the complications of these injections became public knowledge when 2 patients developed subcutaneous nodules after receiving injections for facial wrinkles.2 Despite public knowledge of these complications, penile paraffin injections continued to occur both in medical and eventually nonmedical settings.
In 1947, Quérnu and Pérol6 described 6 penile paraffinoma cases outside the United States. Patients had petroleum jelly injections that eventuated in penile paraffinomas, and all of them lost the ability to attain an erection.6 Four years later, Bradley and Ehrgott7 described a case of penile paraffinoma likely caused by application of paraffin in association with occupational exposure. In 1956, May and Pickering8 cited a case of penile paraffinoma affecting the entire penile shaft in which the patient had undergone paraffin injection 7 years prior to treat premature ejaculation. Unfortunately, the injection resulted in a painful and unsatisfactory erection without resolution of premature ejaculation.8 Lee et al9 analyzed 26 cases of penile paraffinomas that occurred from 1981 to 1993. They found that all patients underwent injections of paraffin or petroleum jelly performed by nonmedical personnel with the predominant goal of enhancing penis size. Within 18.5 months of injection, 19 patients already experienced tenderness at the injection site. The remaining 7 patients experienced penile skin discoloration and abnormal contouring of the penis. Biopsy specimens revealed hyaline necrosis of subcutaneous adipose septa, cystlike spaces throughout involved tissue, and macrophages engulfing adipose tissue were found near blood vessels.9 In 2007, Eandi et al4 reported a case of penile paraffinoma with a 40-year delay of onset. Four years later, Manny et al10 reported penile paraffinomas in 3 Laotian men who injected a mineral oil.
Currently, paraffin injections are uncommon but still are being performed in some countries in Eastern Europe and the Far East11; they rarely are reported in the United States. Injections can occur in unusual sites such as the knee, and paraffinomas can develop many years after the procedure.12 Additionally, paraffinomas can obscure proper diagnosis of carcinomas, as described by Lee et al13 in a case in which a cervical paraffin injection confounded the diagnosis of a thyroid tumor. Furthermore, these injections usually are performed by nonmedical personnel and typically are repeated multiple times to reach cosmetic goals, rendering the patient vulnerable to early complications including allergic reactions, paraphimosis, infection, and inflammation.3
The clinical presentation of a penile paraffinoma may be a mimicker of several different entities, which are important to consider in the evaluation of a presenting patient. Infectious etiologies must be considered including lymphogranuloma venereum, granuloma inguinale, atypical mycobacteria, lupus vulgaris, and sexually transmitted infections. Importantly, neoplasms must be ruled out including squamous cell carcinoma, soft tissue sarcomas, melanoma, adenocarcinoma, or metastasis. Lymphedema, prior surgical procedures, trauma, and inflammatory etiologies also are in the differential diagnosis.14 Nonetheless, physicians must have a high clinical suspicion in the evaluation of a possible paraffinoma, as patients may not be forthcoming with relevant clinical history regarding a prior injection to the affected site, particularly if the injection occurred many years ago. As such, the patient may not consider this history relevant or may not even remember the event occurred, as was observed in our case. Furthermore, embarrassment, social taboo, and stigma may be associated with the behavior of undergoing injections in nonclinical settings without medical supervision.15
Patients may be motivated to undergo dangerous procedures to potentially alter their appearance due to perceived enhanced sexual ability, influence by loved ones, cultural rituals, and societal pressure.15,16 Furthermore, patients may not be aware of the material being injected or the volume. Given that these injections often are used with the goal of cosmetic enhancement, biopsies in cosmetically sensitive areas must be given careful consideration, and a thorough clinical history must support the decision to pursue a biopsy to obtain a definitive diagnosis.
The definitive diagnosis of a paraffinoma is determined by histopathology. However, the use of imaging modalities such as magnetic resonance imaging and computed tomography have been employed to delineate the extent of involvement. Imaging studies allow for surgical planning and may assist in narrowing a differential diagnosis.17 Currently, wide and complete surgical resection is the only definitive treatment of paraffinomas, including penile paraffinomas, as there is no evidence of spontaneous regression.3 A report of a reconstructive surgery involving penile resurfacing without T-style anastomosis has been found effective at preventing necrosis of the ventral penile skin. Not all paraffinomas behave similarly, and there is no reliable method to determine which paraffinoma may possess a more aggressive clinical course compared to those which have a more indolent course.18 As such, early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance. In the case of a large penile paraffinoma with the ability to destroy vital urologic and reproductive function, physicians must consider prevention of recurrent episodes through suppression of inflammation and fibrosis with doxycycline and nicotinamide.19 Other medical treatments reported with varying success include corticosteroids, imiquimod, and isotretinoin.19-24 Employing adjunctive medical treatment may decrease the size of the mass, reducing the surgical defect size and preserving tissue vitality. Ultimately, the most crucial aspect in treatment is prevention, as injection of foreign materials elicits a foreign body response and can lead to notable morbidity.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
- De Siati M, Selvaggio O, Di Fino G, et al. An unusual delayed complication of paraffin self-injection for penile girth augmentation. BMC Urol. 2013;13:66.
- Sejben I, Rácz A, Svébis M, et al. Petroleum jelly-induced penile paraffinoma with inguinal lymphadenitis mimicking incarcerated inguinal hernia. Can Urol Assoc J. 2012;6:E137-E139.
- Bayraktar N, Basar I. Penile paraffinoma [published online September 17, 2012]. Case Rep Urol. 2012;2012:202840.
- Eandi JA, Yao AP, Javidan J. Penile paraffinoma: the delayed presentation. Int Urol Nephrol. 2007;29:553-555.
- HirshBCJohnsonWC. Pathology of granulomatous diseases. foreign body granulomas. Int J Dermatol. 1984;23:531-538.
- Quérnu J, Pérol E. Paraffinomas of the penis. J Chir Par. 1947;63:345.
- Bradley, RH, Ehrgott WA. Paraffinoma of the penis: case report. J Urol. 1951;65:453.
- May JA, Pickering PP. Paraffinoma of the penis. Calif Med. 1956;85:42-44.
Yonsei Med J. 1994;35:344-348. - Lee T, Choi HR, Lee YT, et al. Paraffinoma of the penis.
- Manny T, Pettus J, Hemal A, et al. Penile sclerosing lipogranulomas and disfigurement from use of “1Super Extenze” among Laotian immigrants. J Sex Med. 2011;8:3505-3510.
- Akkus E Paraffinoma and ulcer of the external genitalia after self-injection of vaseline. J Sex Med. 2006;3:170-172.
- Grassetti L, Lazzeri D, Torresetti M, et al. Paraffinoma of the knee 60 years after primary infection. Arch Plast Surg. 2013;40:789-790.
- Lee YS, Son EJ, Kim BW, et al. Difficult evaluation of thyroid cancer due to cervical paraffin injection. J Korean Surg Soc. 2011;81(suppl 1):S17-S20.
- Gómez-Armayones S, Penín R, Marcoval J. Penile paraffinoma [in Spanish]. Actas Dermosifiliogr. 2014;105:957-959.
- Moon DG, Yoo JW, Bae JH, et al. Sexual function and psychological characteristics of penile paraffinoma. Asian J Androl. 2003;5:191-194.
- Pehlivanov G, Kavaklieva S, Kazandjieva J, et al. Foreign-body granuloma of the penis in sexually active individuals (penile paraffinoma). J Eur Acad Dermatol Venereol. 2008;22:845-851.
- Cormio L, Di Fino G, Scavone C, et al. Magnetic resonance imaging of penile paraffinoma: case report. BMC Med Imaging. 2014;14:39.
- Shin YS, Zhao C, Park JK. New reconstructive surgery for penile paraffinoma to prevent necrosis of ventral penile skin. Urology. 2013;81:437-441.
- Feldmann R, Harms M, Chavaz P, et al. Orbital and palpebral paraffinoma. J Am Acad Dermatol. 1992;26:833-835.
- MastruserioDNPesqueiraMJCobbMW. Severe granulomatous reaction and facial ulceration occurring after subcutaneous silicone injection. J Am Acad Dermatol. 1996;34:849-852.
- HoWS Management of paraffinoma of the breast. Br J Plast Surg. 2001;54:232-234.
- LloretPSuccessful treatment of granulomatous reactions secondary to injection of esthetic implants. Dermatol Surg. 2005;31:486-490.
- RosenbergEThree cases of penile paraffinoma. Urology. 2007;70:372.
- Baumann LS, Halem ML. Lip silicone granulomatous foreign body reaction treated with Aldara (imiquimod 5%). Dermatol Surg. 2003;29:429-432.
Practice Points
- Taking a thorough history in patients with possible paraffinomas is vital, including a history of injectables even in the genital region.
- Biopsies in cosmetically sensitive areas must be given careful consideration. Clinical history must support the decision to pursue a definitive diagnosis.
- Early detection is critical in the management of paraffinomas, especially in anatomic locations where tissue preservation is of utmost importance.
Complex Regional Pain Syndrome Type II After a Brachial Plexus and C6 Nerve Root Injury
To the Editor:
A 62-year-old man presented with an atrophied painful left arm of 17 years’ duration that began when he was hit by a car as a pedestrian. He sustained severe multisystem injuries from the accident, including left brachial plexus and C6 nerve root avulsion injury. When he regained consciousness after 6 weeks in the intensive care unit, he immediately noted diffuse pain throughout the body, especially in the left arm. Since the accident, the patient continued to have diminished sensation to touch and temperature in the left arm. He also had burning, throbbing, and electrical pain in the left arm with light touch as well as spontaneously. He was thoroughly evaluated by a neurologist and was diagnosed with complex regional pain syndrome (CRPS) type II. For the treatment of pain, dorsal column stimulation and hemilaminectomy with exploration of the avulsed nerve root were attempted, both of which had minimal effect. He was maintained on hydromorphone, methadone, and oxazepam. He reported that for many years he was unable move out of bed due to the unbearable pain. With pain medications, he was able to regain most of his independence in his daily life, though the pain and other clinical aspects of CRPS still completely limited his use of the left arm.
Physical examination revealed glossy, cold, hairless skin with hypohidrosis of the left arm, forearm, and hand (Figures 1 and 2A). The left arm was conspicuously atrophied, with the forearm and hand erythematous. The fingers were taut, contracted, and edematous (Figure 2B), and the skin was unable to be pinched. The fingernails on the left hand had dystrophic changes including yellow color and brittleness with longitudinal ridges (Figure 3). The patient could activate the left bicep and tricep muscles against gravity but had minimal function of the deltoid muscle. He also had minimal movement of the left index finger and was unable to move any other digits of the left hand. The patient was continued on pain management treatments and physical therapy for his condition.

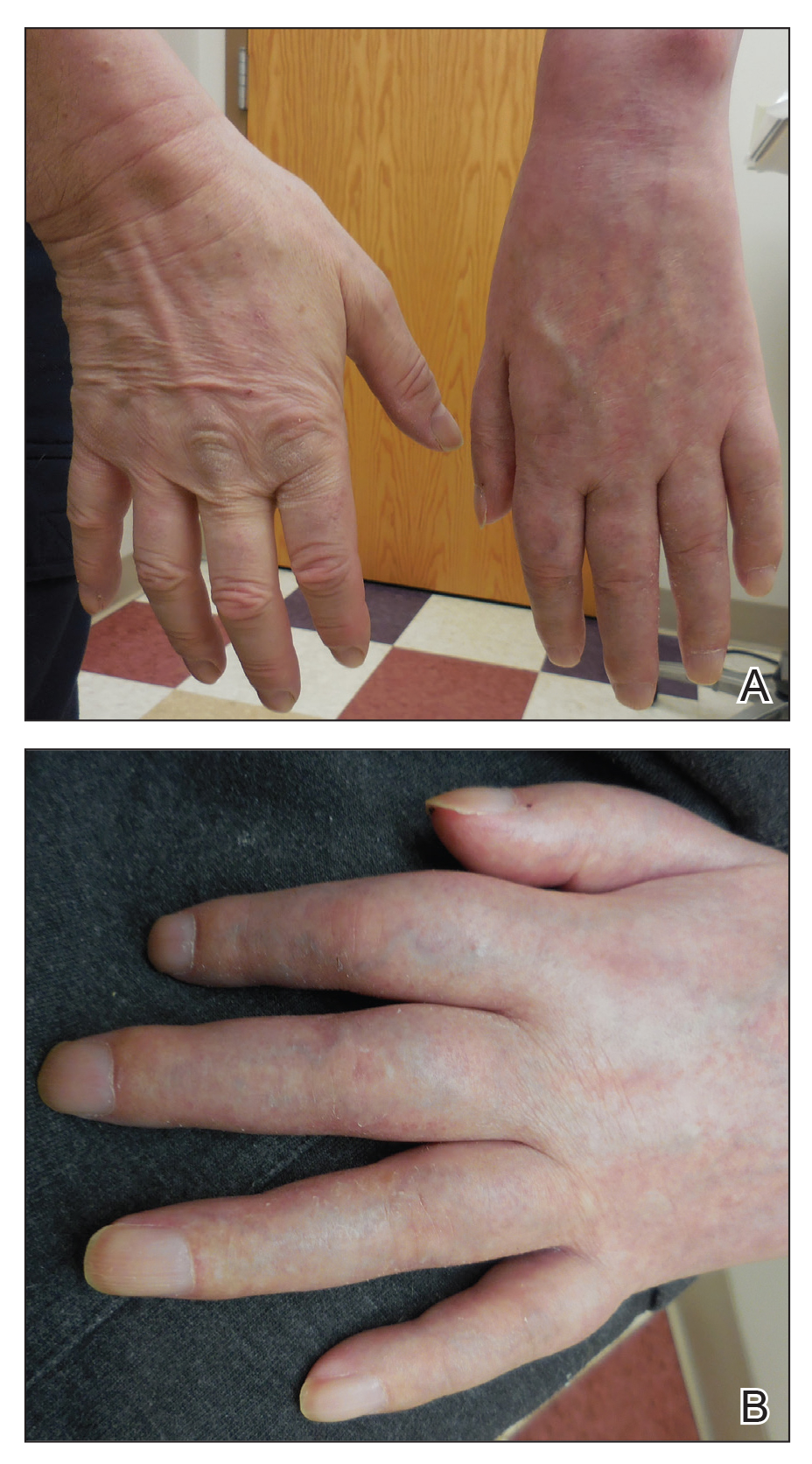
Complex regional pain syndrome is a neuropathic disorder of the extremities characterized by pain and a variety of autonomic and motor disturbances such as local edema, limited active range of motion, and vasomotor and trophic skin changes. There are 2 types of CRPS: type II is marked by explicit nerve injury and type I is not. The pathophysiology of CRPS is unknown.1-3
There is no definite set of diagnostic criteria for CRPS. The lack of any gold-standard diagnostic test for CRPS has made arriving at one valid, widely accepted set of diagnostic criteria impossible.1 There are 4 widely used sets of diagnostic criteria. One is the International Association for the Study of Pain diagnostic criteria defined in 1994.4 However, the criteria rely entirely on subjective symptoms and have been under great scrutiny due to their questionable validity.2 Veldman et al5 presented other widely used CRPS diagnostic criteria in their prospective study of 829 reflex sympathetic dystrophy patients, which paid particular attention to the early clinical manifestations of CRPS. In 1999, Bruehl et al2 proposed their own modified diagnostic criteria, which required physician-assessed signs in 2 of 4 categories to avoid the practice of exclusively relying on subjective symptoms. In addition, during a consensus meeting in Budapest, Hungary, a modified version of the Bruehl criteria was proposed.6 All 4 criteria rely solely on detailed history and physical examination, and the choice of diagnostic criteria remains subjective.
The pathophysiology of CRPS also remains unclear. There are several proposed mechanisms such as sympathetic nervous system dysfunction, abnormal inflammatory response, and central nervous system involvement.1 Psychologic factors, sequelae of nerve injury, and genetic predisposition also have been implicated in the pathophysiology of CRPS.1 It is likely that several mechanisms variably contribute to each presentation of CRPS.
Many dermatologic findings, in addition to neuromuscular symptoms, accompany CRPS and serve as important clues to making the clinical diagnosis. Complex regional pain syndrome has been thought to have 3 distinct sequential stages of CRPS.1,3,7 Stage 1—the acute stage—is marked by hyperalgesia, allodynia, sudomotor disturbances, and prominent edema. Stage 2—the dystrophic stage—is characterized by more marked pain and sensory dysfunction, vasomotor dysfunction, development of motor dysfunction, soft tissue edema, skin and articular soft tissue thickening, and development of dystrophic nail changes. Stage 3—the atrophic stage—is marked by decreased pain and sensory disturbances, markedly increased motor dysfunction, waxy atrophic skin changes, progression of dystrophic nail changes, and skeletal cystic and subchondral erosions with diffuse osteoporosis.1,3,7
The staging model, however, has been called into question.3 In a cluster analysis, Bruehl et al3 arrived at 3 relatively consistent CRPS patient subgroups that did not have notably different pain duration, suggesting the existence of 3 CRPS subtypes, not stages. Their study found that one of the subgroups best represented the clinical presentation of CRPS type II. This subgroup had the greatest pain and sensory abnormalities and the least vasomotor dysfunction of all 3 subgroups. Nonetheless, this study has not settled the discussion, as it only included 113 patients.3 Thus, with future studies, our understanding of CRPS in stages may change, which likely will impact how the clinical diagnosis is made.
There is a lack of high-quality evidence for most treatment interventions for CRPS8; however, the current practice is to use an interdisciplinary approach.1,9,10 The main therapeutic arm of this approach is rehabilitation; physical and occupational therapy can help improve range of motion, contracture, and atrophy. The other 2 arms of the approach are psychologic therapy to improve quality of life and pain management with pharmacologic therapy and/or invasive interventions. The choice of therapy remains empirical; trial and error should be expected in developing an adequate treatment plan for each individual patient.
Many aspects of CRPS remain unclear, and even our current understanding of the disease will inevitably change over time. The syndrome can cause life-changing morbidities in patients, and late diagnosis and treatment are associated with poor prognosis. Because there are many dermatologic findings associated with the disorder, it is crucial for dermatologists to clinically recognize the disorder and to refer patients to appropriate channels so that treatment can be started as soon as possible.
- Borchers A, Gershwin M. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242-265.
- Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147-154.
- Bruehl S, Harden RN, Gaker BS, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119-124.
- Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
- Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012-1016.
- Harden RN, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150:268-274.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307.
- O’Connell NE, Wand BM, McAuley J, et al. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev. 2013;4:CD009416.
- Hsu ES. Practical management of complex regional pain syndrome. Am J Ther. 2009;16:147-154.
- Stanton-Hicks MD, Burton AW, Bruehl SP, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002;2:1-16.
To the Editor:
A 62-year-old man presented with an atrophied painful left arm of 17 years’ duration that began when he was hit by a car as a pedestrian. He sustained severe multisystem injuries from the accident, including left brachial plexus and C6 nerve root avulsion injury. When he regained consciousness after 6 weeks in the intensive care unit, he immediately noted diffuse pain throughout the body, especially in the left arm. Since the accident, the patient continued to have diminished sensation to touch and temperature in the left arm. He also had burning, throbbing, and electrical pain in the left arm with light touch as well as spontaneously. He was thoroughly evaluated by a neurologist and was diagnosed with complex regional pain syndrome (CRPS) type II. For the treatment of pain, dorsal column stimulation and hemilaminectomy with exploration of the avulsed nerve root were attempted, both of which had minimal effect. He was maintained on hydromorphone, methadone, and oxazepam. He reported that for many years he was unable move out of bed due to the unbearable pain. With pain medications, he was able to regain most of his independence in his daily life, though the pain and other clinical aspects of CRPS still completely limited his use of the left arm.
Physical examination revealed glossy, cold, hairless skin with hypohidrosis of the left arm, forearm, and hand (Figures 1 and 2A). The left arm was conspicuously atrophied, with the forearm and hand erythematous. The fingers were taut, contracted, and edematous (Figure 2B), and the skin was unable to be pinched. The fingernails on the left hand had dystrophic changes including yellow color and brittleness with longitudinal ridges (Figure 3). The patient could activate the left bicep and tricep muscles against gravity but had minimal function of the deltoid muscle. He also had minimal movement of the left index finger and was unable to move any other digits of the left hand. The patient was continued on pain management treatments and physical therapy for his condition.



Complex regional pain syndrome is a neuropathic disorder of the extremities characterized by pain and a variety of autonomic and motor disturbances such as local edema, limited active range of motion, and vasomotor and trophic skin changes. There are 2 types of CRPS: type II is marked by explicit nerve injury and type I is not. The pathophysiology of CRPS is unknown.1-3
There is no definite set of diagnostic criteria for CRPS. The lack of any gold-standard diagnostic test for CRPS has made arriving at one valid, widely accepted set of diagnostic criteria impossible.1 There are 4 widely used sets of diagnostic criteria. One is the International Association for the Study of Pain diagnostic criteria defined in 1994.4 However, the criteria rely entirely on subjective symptoms and have been under great scrutiny due to their questionable validity.2 Veldman et al5 presented other widely used CRPS diagnostic criteria in their prospective study of 829 reflex sympathetic dystrophy patients, which paid particular attention to the early clinical manifestations of CRPS. In 1999, Bruehl et al2 proposed their own modified diagnostic criteria, which required physician-assessed signs in 2 of 4 categories to avoid the practice of exclusively relying on subjective symptoms. In addition, during a consensus meeting in Budapest, Hungary, a modified version of the Bruehl criteria was proposed.6 All 4 criteria rely solely on detailed history and physical examination, and the choice of diagnostic criteria remains subjective.
The pathophysiology of CRPS also remains unclear. There are several proposed mechanisms such as sympathetic nervous system dysfunction, abnormal inflammatory response, and central nervous system involvement.1 Psychologic factors, sequelae of nerve injury, and genetic predisposition also have been implicated in the pathophysiology of CRPS.1 It is likely that several mechanisms variably contribute to each presentation of CRPS.
Many dermatologic findings, in addition to neuromuscular symptoms, accompany CRPS and serve as important clues to making the clinical diagnosis. Complex regional pain syndrome has been thought to have 3 distinct sequential stages of CRPS.1,3,7 Stage 1—the acute stage—is marked by hyperalgesia, allodynia, sudomotor disturbances, and prominent edema. Stage 2—the dystrophic stage—is characterized by more marked pain and sensory dysfunction, vasomotor dysfunction, development of motor dysfunction, soft tissue edema, skin and articular soft tissue thickening, and development of dystrophic nail changes. Stage 3—the atrophic stage—is marked by decreased pain and sensory disturbances, markedly increased motor dysfunction, waxy atrophic skin changes, progression of dystrophic nail changes, and skeletal cystic and subchondral erosions with diffuse osteoporosis.1,3,7
The staging model, however, has been called into question.3 In a cluster analysis, Bruehl et al3 arrived at 3 relatively consistent CRPS patient subgroups that did not have notably different pain duration, suggesting the existence of 3 CRPS subtypes, not stages. Their study found that one of the subgroups best represented the clinical presentation of CRPS type II. This subgroup had the greatest pain and sensory abnormalities and the least vasomotor dysfunction of all 3 subgroups. Nonetheless, this study has not settled the discussion, as it only included 113 patients.3 Thus, with future studies, our understanding of CRPS in stages may change, which likely will impact how the clinical diagnosis is made.
There is a lack of high-quality evidence for most treatment interventions for CRPS8; however, the current practice is to use an interdisciplinary approach.1,9,10 The main therapeutic arm of this approach is rehabilitation; physical and occupational therapy can help improve range of motion, contracture, and atrophy. The other 2 arms of the approach are psychologic therapy to improve quality of life and pain management with pharmacologic therapy and/or invasive interventions. The choice of therapy remains empirical; trial and error should be expected in developing an adequate treatment plan for each individual patient.
Many aspects of CRPS remain unclear, and even our current understanding of the disease will inevitably change over time. The syndrome can cause life-changing morbidities in patients, and late diagnosis and treatment are associated with poor prognosis. Because there are many dermatologic findings associated with the disorder, it is crucial for dermatologists to clinically recognize the disorder and to refer patients to appropriate channels so that treatment can be started as soon as possible.
To the Editor:
A 62-year-old man presented with an atrophied painful left arm of 17 years’ duration that began when he was hit by a car as a pedestrian. He sustained severe multisystem injuries from the accident, including left brachial plexus and C6 nerve root avulsion injury. When he regained consciousness after 6 weeks in the intensive care unit, he immediately noted diffuse pain throughout the body, especially in the left arm. Since the accident, the patient continued to have diminished sensation to touch and temperature in the left arm. He also had burning, throbbing, and electrical pain in the left arm with light touch as well as spontaneously. He was thoroughly evaluated by a neurologist and was diagnosed with complex regional pain syndrome (CRPS) type II. For the treatment of pain, dorsal column stimulation and hemilaminectomy with exploration of the avulsed nerve root were attempted, both of which had minimal effect. He was maintained on hydromorphone, methadone, and oxazepam. He reported that for many years he was unable move out of bed due to the unbearable pain. With pain medications, he was able to regain most of his independence in his daily life, though the pain and other clinical aspects of CRPS still completely limited his use of the left arm.
Physical examination revealed glossy, cold, hairless skin with hypohidrosis of the left arm, forearm, and hand (Figures 1 and 2A). The left arm was conspicuously atrophied, with the forearm and hand erythematous. The fingers were taut, contracted, and edematous (Figure 2B), and the skin was unable to be pinched. The fingernails on the left hand had dystrophic changes including yellow color and brittleness with longitudinal ridges (Figure 3). The patient could activate the left bicep and tricep muscles against gravity but had minimal function of the deltoid muscle. He also had minimal movement of the left index finger and was unable to move any other digits of the left hand. The patient was continued on pain management treatments and physical therapy for his condition.



Complex regional pain syndrome is a neuropathic disorder of the extremities characterized by pain and a variety of autonomic and motor disturbances such as local edema, limited active range of motion, and vasomotor and trophic skin changes. There are 2 types of CRPS: type II is marked by explicit nerve injury and type I is not. The pathophysiology of CRPS is unknown.1-3
There is no definite set of diagnostic criteria for CRPS. The lack of any gold-standard diagnostic test for CRPS has made arriving at one valid, widely accepted set of diagnostic criteria impossible.1 There are 4 widely used sets of diagnostic criteria. One is the International Association for the Study of Pain diagnostic criteria defined in 1994.4 However, the criteria rely entirely on subjective symptoms and have been under great scrutiny due to their questionable validity.2 Veldman et al5 presented other widely used CRPS diagnostic criteria in their prospective study of 829 reflex sympathetic dystrophy patients, which paid particular attention to the early clinical manifestations of CRPS. In 1999, Bruehl et al2 proposed their own modified diagnostic criteria, which required physician-assessed signs in 2 of 4 categories to avoid the practice of exclusively relying on subjective symptoms. In addition, during a consensus meeting in Budapest, Hungary, a modified version of the Bruehl criteria was proposed.6 All 4 criteria rely solely on detailed history and physical examination, and the choice of diagnostic criteria remains subjective.
The pathophysiology of CRPS also remains unclear. There are several proposed mechanisms such as sympathetic nervous system dysfunction, abnormal inflammatory response, and central nervous system involvement.1 Psychologic factors, sequelae of nerve injury, and genetic predisposition also have been implicated in the pathophysiology of CRPS.1 It is likely that several mechanisms variably contribute to each presentation of CRPS.
Many dermatologic findings, in addition to neuromuscular symptoms, accompany CRPS and serve as important clues to making the clinical diagnosis. Complex regional pain syndrome has been thought to have 3 distinct sequential stages of CRPS.1,3,7 Stage 1—the acute stage—is marked by hyperalgesia, allodynia, sudomotor disturbances, and prominent edema. Stage 2—the dystrophic stage—is characterized by more marked pain and sensory dysfunction, vasomotor dysfunction, development of motor dysfunction, soft tissue edema, skin and articular soft tissue thickening, and development of dystrophic nail changes. Stage 3—the atrophic stage—is marked by decreased pain and sensory disturbances, markedly increased motor dysfunction, waxy atrophic skin changes, progression of dystrophic nail changes, and skeletal cystic and subchondral erosions with diffuse osteoporosis.1,3,7
The staging model, however, has been called into question.3 In a cluster analysis, Bruehl et al3 arrived at 3 relatively consistent CRPS patient subgroups that did not have notably different pain duration, suggesting the existence of 3 CRPS subtypes, not stages. Their study found that one of the subgroups best represented the clinical presentation of CRPS type II. This subgroup had the greatest pain and sensory abnormalities and the least vasomotor dysfunction of all 3 subgroups. Nonetheless, this study has not settled the discussion, as it only included 113 patients.3 Thus, with future studies, our understanding of CRPS in stages may change, which likely will impact how the clinical diagnosis is made.
There is a lack of high-quality evidence for most treatment interventions for CRPS8; however, the current practice is to use an interdisciplinary approach.1,9,10 The main therapeutic arm of this approach is rehabilitation; physical and occupational therapy can help improve range of motion, contracture, and atrophy. The other 2 arms of the approach are psychologic therapy to improve quality of life and pain management with pharmacologic therapy and/or invasive interventions. The choice of therapy remains empirical; trial and error should be expected in developing an adequate treatment plan for each individual patient.
Many aspects of CRPS remain unclear, and even our current understanding of the disease will inevitably change over time. The syndrome can cause life-changing morbidities in patients, and late diagnosis and treatment are associated with poor prognosis. Because there are many dermatologic findings associated with the disorder, it is crucial for dermatologists to clinically recognize the disorder and to refer patients to appropriate channels so that treatment can be started as soon as possible.
- Borchers A, Gershwin M. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242-265.
- Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147-154.
- Bruehl S, Harden RN, Gaker BS, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119-124.
- Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
- Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012-1016.
- Harden RN, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150:268-274.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307.
- O’Connell NE, Wand BM, McAuley J, et al. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev. 2013;4:CD009416.
- Hsu ES. Practical management of complex regional pain syndrome. Am J Ther. 2009;16:147-154.
- Stanton-Hicks MD, Burton AW, Bruehl SP, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002;2:1-16.
- Borchers A, Gershwin M. Complex regional pain syndrome: a comprehensive and critical review. Autoimmun Rev. 2014;13:242-265.
- Bruehl S, Harden RN, Galer BS, et al. External validation of IASP diagnostic criteria for complex regional pain syndrome and proposed research diagnostic criteria. International Association for the Study of Pain. Pain. 1999;81:147-154.
- Bruehl S, Harden RN, Gaker BS, et al. Complex regional pain syndrome: are there distinct subtypes and sequential stages of the syndrome? Pain. 2002;95:119-124.
- Merskey H, Bogduk N. Classification of Chronic Pain: Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. 2nd ed. Seattle, WA: IASP Press; 1994.
- Veldman PH, Reynen HM, Arntz IE, et al. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012-1016.
- Harden RN, Bruehl S, Perez RS, et al. Validation of proposed diagnostic criteria (the “Budapest Criteria”) for complex regional pain syndrome. Pain. 2010;150:268-274.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307.
- O’Connell NE, Wand BM, McAuley J, et al. Interventions for treating pain and disability in adults with complex regional pain syndrome. Cochrane Database Syst Rev. 2013;4:CD009416.
- Hsu ES. Practical management of complex regional pain syndrome. Am J Ther. 2009;16:147-154.
- Stanton-Hicks MD, Burton AW, Bruehl SP, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Pract. 2002;2:1-16.
Practice Points
- Complex regional pain syndrome (CRPS) is a neuropathic disorder of the extremities characterized by pain, a variety of autonomic and motor disturbances, and dermatologic findings.
- Early recognition of CRPS is critical, as it presents life-changing morbidities to patients.
- A multidisciplinary treatment approach with physical therapy, occupational therapy, psychological support, and pain control is needed for the management of CRPS.
Painful Indurated Plaque on the Groin
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.
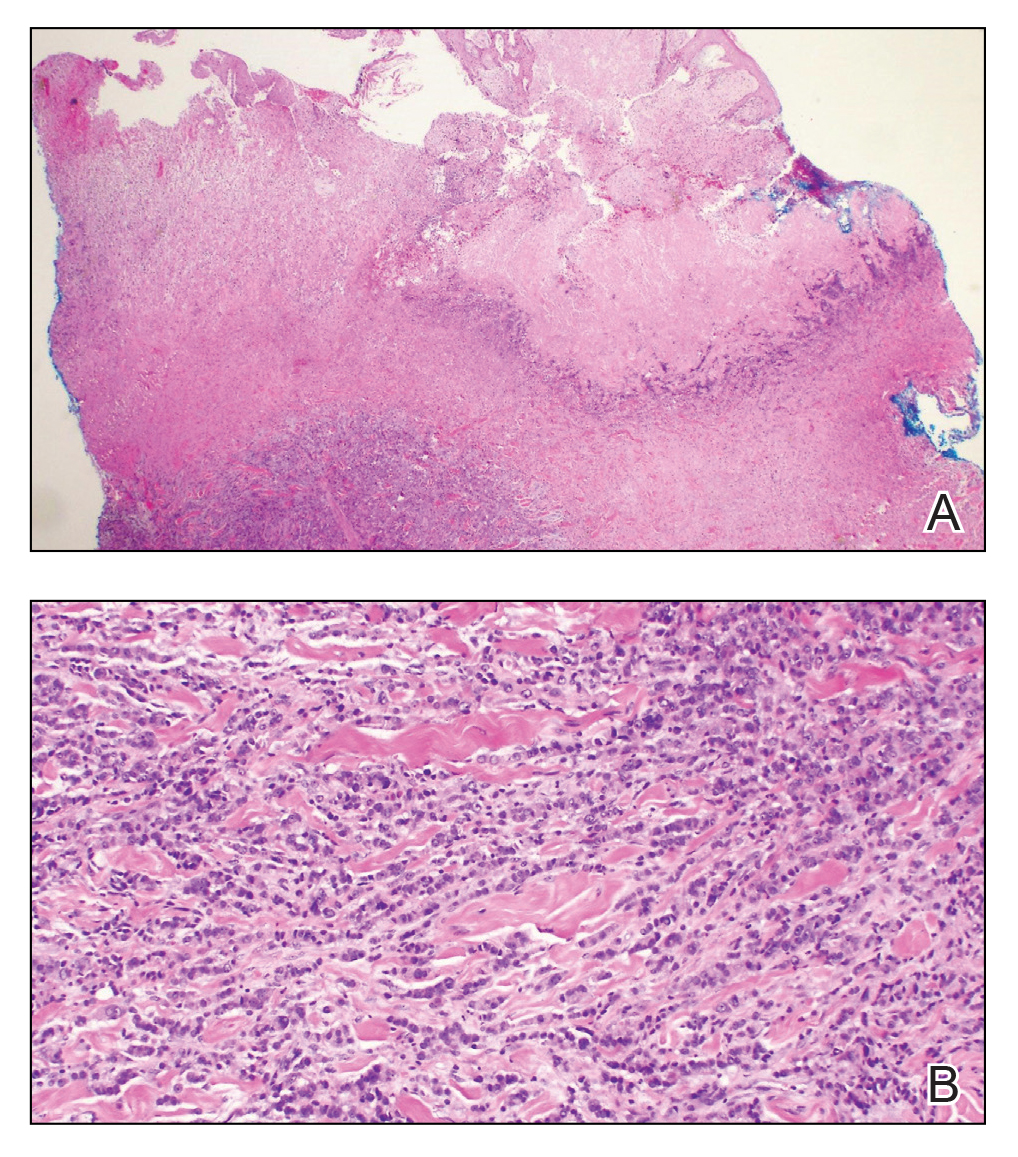
Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
The Diagnosis: Cutaneous Metastasis
Histopathology demonstrated ulceration of the epidermis with necrosis of the papillary dermis. There was a diffuse infiltration of pleomorphic and atypical epithelioid cells in the reticular dermis (Figure). Focally there was ductal and glandular differentiation. The stroma was sclerotic. At the deep aspect of the biopsy specimen, tumor cells intercalated between collagen bundles in linear strands. Atypical mitoses were common, and necrosis en masse was seen. An immunohistochemical panel also was performed. Tissue from the biopsy was strongly positive for CDX-2 and cytokeratin 20 and diffusely negative for cytokeratin 7, gross cystic disease fluid protein 15, and prostate-specific antigen. The other biopsy was sent for cultures and grew no organisms, which confirmed the diagnosis of cutaneous metastasis from the patient's primary colonic adenocarcinoma. Due to the poor prognosis and his overall poor health, our patient opted for palliative care.

Based on large retrospective studies, the frequency of cutaneous metastasis for patients diagnosed with any malignancy is 0.7% to 9.0%.1-4 The third most common malignancy in both sexes is colorectal cancer, affecting approximately 5% of the US population.3 The frequency of cutaneous metastases from colorectal cancer is 0.81% to 3.9%.1,2,4,5 Generally, cutaneous metastases present within 2 to 3 years from diagnosis of primary malignancy.6,7 The most common sites for cutaneous metastases in a patient with colorectal cancer are the abdomen and pelvic region, often at surgical sites.1-4,6-9
The clinical presentation of cutaneous metastases varies greatly, and as a result, they commonly are misdiagnosed.6,7 Although treatment with many antibiotics and antifungals had failed in our patient, the examination still was concerning for a possible granulomatous infection vs malignancy. With the history of colon cancer, radiation treatment, and chemotherapy, the possible malignancy diagnoses included primary skin cancers, viral tumors, and cutaneous metastasis. The initial evaluations had focused on infectious causes and resulted in 6 weeks of misdiagnosis and inappropriate therapy. Despite cutaneous metastases being uncommon, there should be a high index of suspicion for lesions in patients who have a history of cancer, especially if the lesion does not respond to treatment.2,6,7
Physical examination in our patient showed a high tumor burden as well as evidence of carcinoma erysipeloides on the lower abdomen and thighs, in addition to carcinoma en cuirasse throughout the pubic region. Carcinoma erysipeloides was first described in 1893 in a patient with breast cancer: "The erythematous infiltration of the skin was very superficial, and was attended simply by redness with a slight degree of induration. Until touched by the finger the condition might easily have been taken for a slightly-marked form of erysipelas."10 The clinical findings are a result of lymphatic and vascular obstruction.3,9 The breast is the most common location to find carcinoma erysipeloides.3 It is an unusual occurrence to find it on the abdomen from colonic adenocarcinoma. The term cancer en cuirasse was coined in 1838 to describe the cutaneous manifestation of breast cancer that caused the skin to resemble the metal breastplate of a cuirasser.4 Similar to carcinoma erysipeloides, carcinoma en cuirasse most commonly is found as cutaneous metastasis from breast cancer, not from colonic adenocarcinoma.3
The histologic characteristics of cutaneous metastases in general are similar to the primary malignancy but can be more poorly differentiated.7 Generally, neoplastic cells are seen in the lymphatic and blood vessels, and a large portion of the tumor is confined to the deep dermis and in the subcutaneous fat.3,6 Histologic features of colonic adenocarcinoma metastases can demonstrate a well-differentiated, glandular architecture with mucin-secreting cells.3,8,9 There also is a histologic pattern of neoplastic cells arranging themselves between collagen bundles in linear strands; this finding more commonly is seen in adenocarcinoma of the breast but also was seen in our patient.3,9 With immunohistochemical staining, a truncated panel of cytokeratin 7, cytokeratin 20, and S-100 had a diagnostic accuracy of 100% for cutaneous metastases from colonic adenocarcinoma in one study. The pattern of all colonic adenocarcinomas was cytokeratin 20 positive and cytokeratin 7 and S-100 negative.6
Cutaneous metastases typically demonstrate widespread and rapidly progressive disease.3,9 Survival studies of cutaneous metastases showed that 48% to 66% of patients died within the first 6 months.3,6 Specifically, cutaneous metastases from colorectal cancers showed a median survival of 3 to 5 months.6,7 Currently there are no treatment guidelines for cutaneous metastases.
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
- Lookingbill DP, Spangler N, Helm K. Cutaneous metastases in patients with metastatic carcinoma: a retrospective study of 4020 patients. J Am Acad Dermatol. 1993;29(2 pt 1):228-236.
- Gul U, Kilic A, Gonul M, et al. Spectrum of cutaneous metastases in 1287 cases of internal malignancies: a study from Turkey. Acta Derm Venereol. 2007;87:160-162.
- Hussein MR. Skin metastasis: a pathologist's perspective. J Cutan Pathol. 2010;37:E1-E20.
- Schwartz RA. Cutaneous metastatic disease. J Am Acad Dermatol. 1995;33(2 pt 1):161-182; quiz 183-186.
- Hu S, Chen G, Wu C, et al. Rates of cutaneous metastases from different internal malignancies: experience from a Taiwanese medical center. J Am Acad Dermatol. 2009;60:379-387.
- Saeed S, Keehn C, Morgan M. Cutaneous metastasis: a clinical, pathological, and immunohistochemical appraisal. J Cutan Pathol. 2004;31:419-430.
- Sariya D, Ruth K, Adams-McDonnell R. Clinicopathologic correlation of cutaneous metastases: experience of a cancer center. Arch Dermatol. 2007;143:613-620.
- Brownstein M, Helwig E. Metastatic tumors of the skin. Cancer. 1972;29:1298-1307.
- McKee PH. Cutaneous metastases. J Cutan Pathol. 1985;12:239-250.
- Hutchinson J. Notes from congresses and continental hospitals: erythema-scirrhus of the skin in association with cancer of the breast. Arch Surg (London). 1893;4:220-222
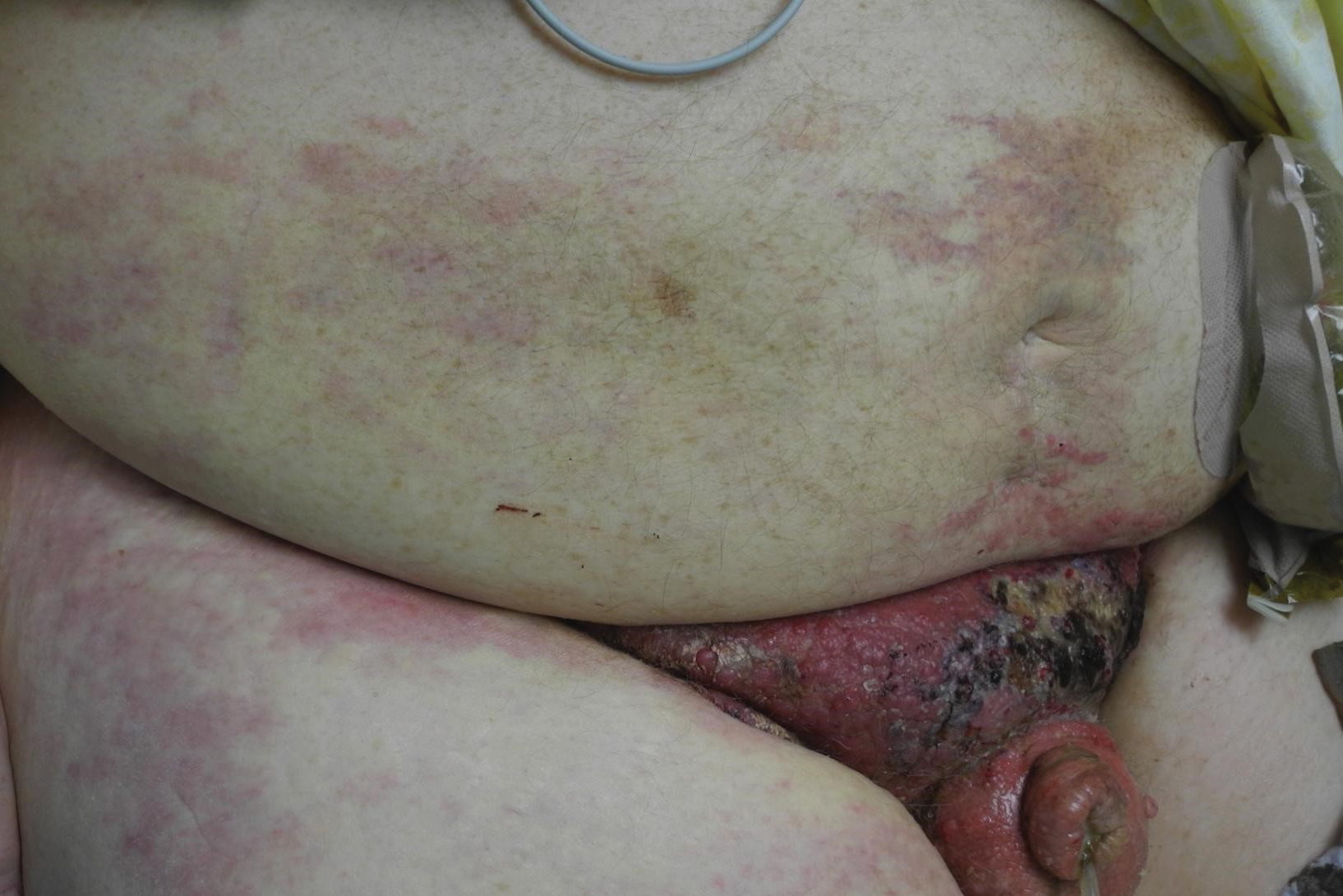
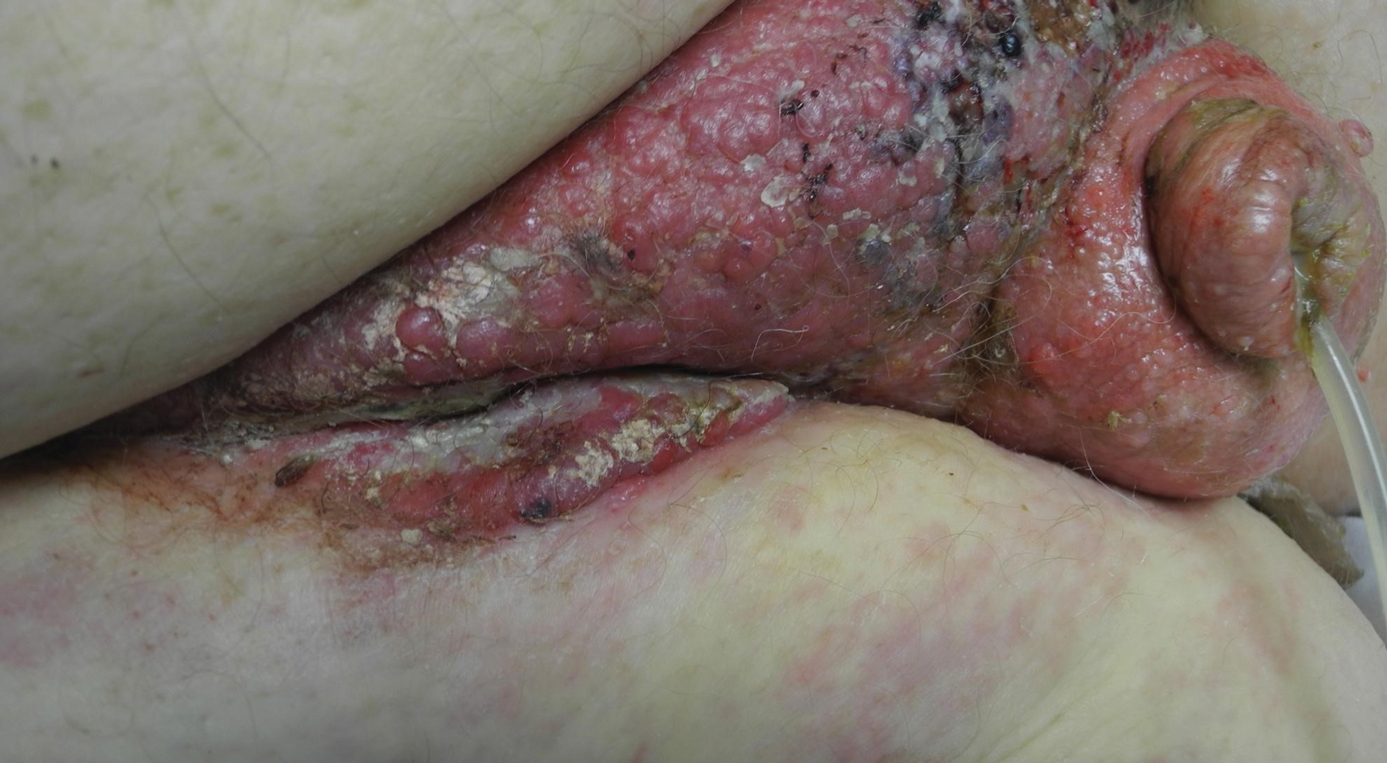
A 67-year-old man presented with a chronic lesion on the groin of 6 weeks' duration. The patient had a history of type 2 diabetes mellitus and colonic adenocarcinoma diagnosed 4 years prior that was treated with a colectomy, radiation therapy, and chemotherapy. Six weeks prior to the current presentation, the patient first sought treatment of swelling, redness, pain, and a bumpy texture on the groin. He was unsuccessfully managed by several physicians including at a long-term care facility where he was admitted and treated for presumed cellulitis. Attempted treatments included a topical antifungal, fluconazole, ciprofloxacin, metronidazole, cefepime, clindamycin, daptomycin, and vancomycin. The affected area continued to worsen along with the patient's overall health. He was transferred to the hospital for more advanced care and was evaluated by inpatient dermatology. Physical examination revealed firm, pink to red-brown, ulcerating papulonodules that coalesced into a large indurated plaque over the pubis, scrotum, penis, and inguinal folds (top). There also were red-violet, indurated plaques on the lower abdomen and bilateral proximal thighs (bottom). Punch biopsies were taken from the indurated area on the left side of the pubis--one for histopathologic evaluation and the other for bacterial, fungal, atypical mycobacterial, and Nocardia tissue cultures.
Vulvar Syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.

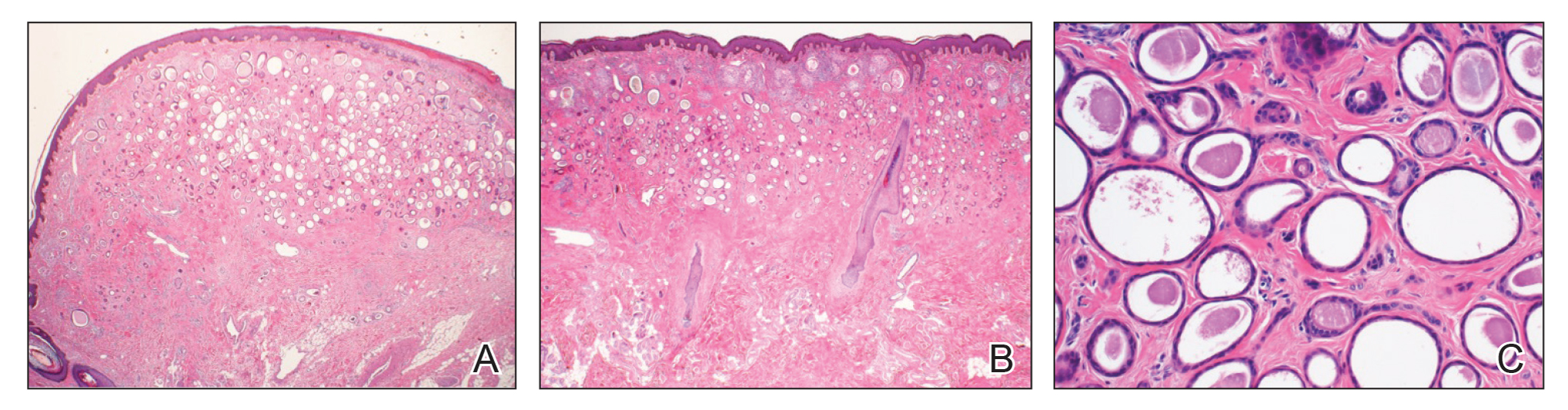
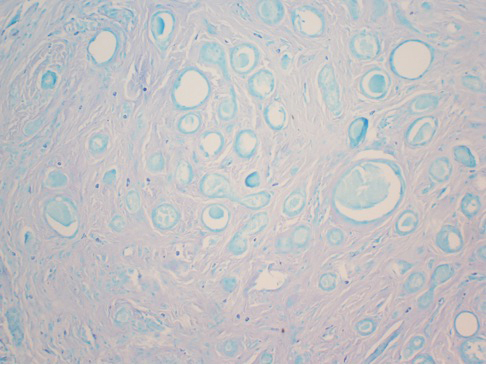
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (Figure 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (Figures 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (Figure 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (Figure 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulgar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (Figures 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (Figure 3). Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas Dermo-Sifiliográficas. 2008;99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995;22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinoma - aggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369-372.
Practice Points
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype.
Sweet Syndrome With Marked Eosinophilic Infiltrate
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.
Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.
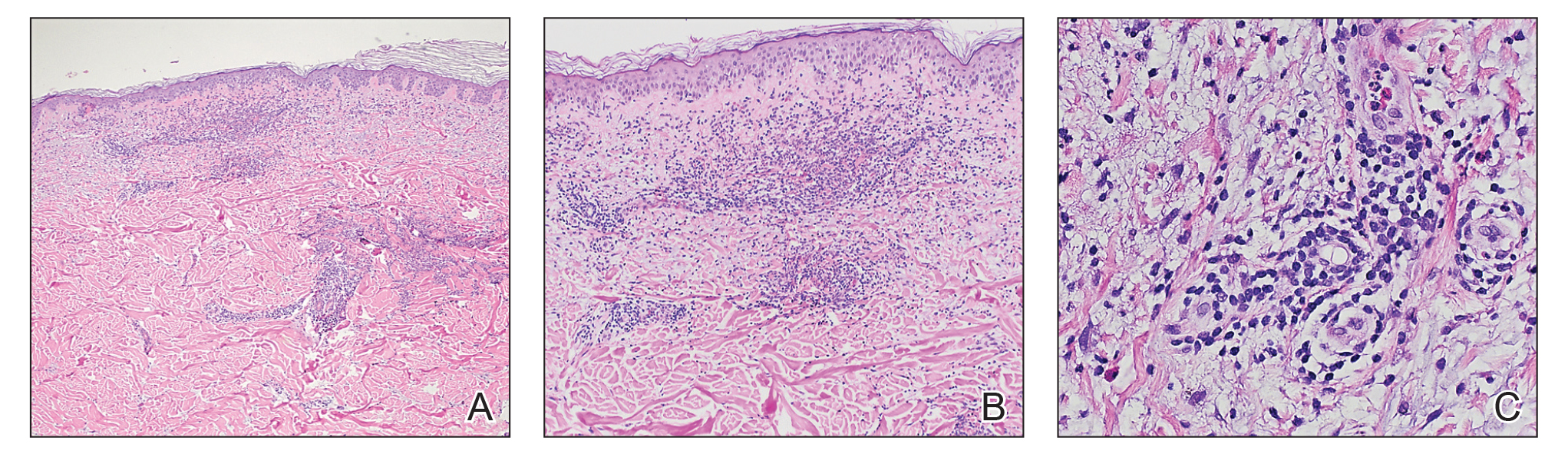
Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.

Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.

Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
To the Editor:
Sweet syndrome (SS), also known as acute febrile neutrophilic dermatosis, is an uncommon inflammatory skin disorder characterized by sudden onset of fever, leukocytosis, neutrophilia, and tender erythematous papules or plaques or both. Skin biopsy usually reveals extensive infiltration of neutrophils into the epidermis and dermis.1-3 Although rare, cases of eosinophil-rich SS have been reported in patients with drug-induced and malignancy-associated SS.4,5 We report a case of a patient with classical SS with dermal eosinophilic infiltration.
An 80-year-old Hispanic man presented with abrupt onset of a rash on the posterior scalp, left ear, back, and hands of 5 days’ duration. The lesions were painful and had progressed to the point of impairing hand grip. The patient’s medical history included a reported common cold the week prior, hyperlipidemia, and hypertension, for which he took metoprolol, simvastatin, aspirin, and clopidogrel. He denied oral lesions and medication changes. He was afebrile and did not experience dietary changes, weight loss, or fatigue. He recently returned from travel to the Dominican Republic.
Physical examination revealed tender, well demarcated, pink to violaceous, pseudovesicular papules and plaques on the palms and dorsal hands (Figure 1), the posterior scalp, left ear, proximal left arm, and back. Pink, juicy, targetoid papules were also found on the scalp, back, and left arm. There was no evidence of lymphadenopathy. Laboratory test results revealed an elevated white blood cell count (11,500/µL [reference range, 3800-10,800/µL]), absolute neutrophil count (8073/µL [reference range, 1500–7800/µL]), and eosinophil count (610/µL [reference range, 15–500/µL]). These results indicated leukocytosis with neutrophilia and mild eosinophilia. The patient also was anemic (hemoglobin, 11.5 g/dL [reference range, 13.2–17.1 g/dL]; hematocrit, 35.1% [reference range, 38.5%–50%]). Urine testing revealed altered renal function (serum creatinine, 2.42 mg/dL [reference range, 0.7–1.1 mg/dL]; blood urea nitrogen, 34 mg/dL [reference range, 7–25 mg/dL]; glomerular filtration rate, 4 mL/min/1.73 m2 (reference range, ≥60 mL/min/1.73 m2]), suggesting stage 4 chronic kidney disease. Urinalysis showed mild hematuria and proteinuria.

Histopathology of biopsies taken from plaques on the left arm and lower back revealed a dense neutrophilic infiltrate with numerous scattered eosinophils in the dermis. Some neutrophils were intact; others were fragmented without evidence of vasculitis. A subtle subepidermal edema also was noted (Figure 2). A diagnosis of SS was made.

Initial treatment included prednisone (40 mg daily, tapered by 5 mg every 3 days) and erythromycin (500 mg 4 times daily) for 7 days because of suspected Mycoplasma infection. The rash resolved in 1 week. No recurrence was noted during 4 months of follow-up. The white blood cell count returned to within reference range (8400/µL), ruling out the possibility of a smoldering myeloid process.
Acute febrile neutrophilic dermatosis was first described in a case series of 8 women by Sweet6 in 1964. Patients typically present first with fever, which can precede cutaneous symptoms for days or weeks. Skin lesions generally are asymmetric and located on the face, neck, and upper extremities. Lesions can be described as painful, purple to red papules, plaques, or nodules. Sweet syndrome can present as 3 subtypes based on cause7: (1) classical SS, also known as idiopathic SS, can be preceded by an upper respiratory tract or gastrointestinal tract infection or vaccination, or can be pregnancy associated2; (2) drug-induced SS usually follows use of granulocyte colony-stimulating factor, or other causative drugs including trimethoprim-sulfamethoxazole, nitrofurantoin, quinolones, oral contraceptives, furosemide, hydralazine, diazepam, clozapine, abacavir, imatinib, bortezomib, azathioprine, and celecoxib2,3,8; and (3) malignancy-associated SS can occur as a paraneoplastic syndrome and generally is associated with hematologic malignancy or a solid tumor.1,9
In our patient, the observed clinical and histological findings were consistent with a diagnosis of SS,2,10 specifically tender erythematous plaques of sudden onset, fast response to systemic corticosteroid therapy, a dermal neutrophilic infiltrate without evidence of leukocytoclastic vasculitis, and leukocytosis greater than 8000/µL with more than 70% neutrophils. He also exhibited targetoid lesions, which have been reported in 7% to 12% of SS patients.10,11
The predominant cells involved in the dermis of SS lesions are mature neutrophils; however, eosinophils have been observed in small numbers within dermal infiltrates in skin lesions of patients with either classical SS or drug-induced dermatosis.2 In 2 studies of cases of SS (N=73 and N=31), eosinophils were reported in 35% and 41% of skin biopsies, respectively.4,5 Nevertheless, cases with dense eosinophilic infiltrates are rare. Furthermore, Masuda et al12 reported a case of eosinophil-rich SS in a 29-year-old woman after treatment of an upper respiratory tract infection with an antibiotic, and Soon et al13 described an eosinophil-rich case of SS in the setting of new-onset enteropathy-associated T-cell lymphoma.
Our patient was considered to have classical SS because he had an episode of an upper respiratory tract infection 1 week prior to onset of clinical manifestations. The histologic finding of numerous eosinophils in our case was unusual for idiopathic SS. This finding might suggest a drug hypersensitivity reaction, but the lack of any change in the patient’s long-term medication list and the lack of any other episodes made a diagnosis of drug-induced SS less likely in our patient.
Eosinophilic dermatosis of hematologic malignancy is a rare cutaneous condition in which nodules, pruritic papules, and vesicles arise in patients with a hematologic malignancy, such as chronic lymphocytic leukemia and mantle cell lymphoma,13 in which a deep perivascular lymphocytic infiltrate and numerous eosinophils are observed. Malignancy was ruled out in our patient because of the lack of characteristic abnormalities in blood testing, the fast response to corticosteroid therapy, and the lack of recurrence posttreatment or additional systemic concerns.
The typical pathology findings of SS consist of mature neutrophils found in the dermis without evidence of leukocytoclastic vasculitis. Eosinophil-rich infiltration, however rare, has been reported in SS. This report highlights a case of classical SS with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses. Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of this disorder.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
- Herbert-Cohen D, Jour G, Saul T. Sweet’s syndrome. J Emerg Med. 2015;49:e95-e97.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34.
- Villarreal-Villarreal CD, Ocampo-Candiani J, Villarreal-Martínez A. Sweet syndrome: a review and update. Actas Dermosifiliogr. 2016;107:369-378.
- Rochael MC, Pantaleão L, Vilar EA, et al. Sweet’s syndrome: study of 73 cases, emphasizing histopathological findings. An Bras Dermatol. 2011;86:702-707.
- Ratzinger G, Burgdorf W, Zelger BG, et al. Acute febrile neutrophilic dermatosis: a histopathologic study of 31 cases with review of literature. Am J Dermatopathol. 2007;29:125-133.
- Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol. 1964;76:349-356.
- Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol. 2003;42:761-778.
- Polimeni G, Cardillo R, Garaffo E, et al. Allopurinol-induced Sweet’s syndrome. Int J Immunopathol Pharmacol. 2016;29:329-332.
- Paydas S. Sweet’s syndrome: a revisit for hematologists and oncologists. Crit Rev Oncol Hematol. 2013;86:85-95.
- Amouri M, Masmoudi A, Ammar M, et al. Sweet’s syndrome: a retrospective study of 90 cases from a tertiary care center. Int J Dermatol. 2016;55:1033-1039.
- Marcoval J, Martín-Callizo C, Valentí-Medina F, et al. Sweet syndrome: long-term follow-up of 138 patients. Clin Exp Dermatol. 2016;41:741-746.
- Masuda T, Abe Y, Arata J, et al. Acute febrile neutrophilic dermatosis (Sweet’s syndrome) associated with extreme infiltration of eosinophils. J Dermatol. 1994;21:341-346.
- Soon CW, Kirsch IR, Connolly AJ, et al. Eosinophil-rich acute febrile neutrophilic dermatosis in a patient with enteropathy-associated T-cell lymphoma, type 1. Am J Dermatopathol. 2016;38:704-708.
Practice Points
- This report highlights a case of classical Sweet syndrome (SS) with a particularly dense eosinophilic infiltrate, which could be mistaken for other eosinophilic dermatoses.
- Dermatologists should be aware of the possibility of marked eosinophilic infiltration in all subtypes of SS.