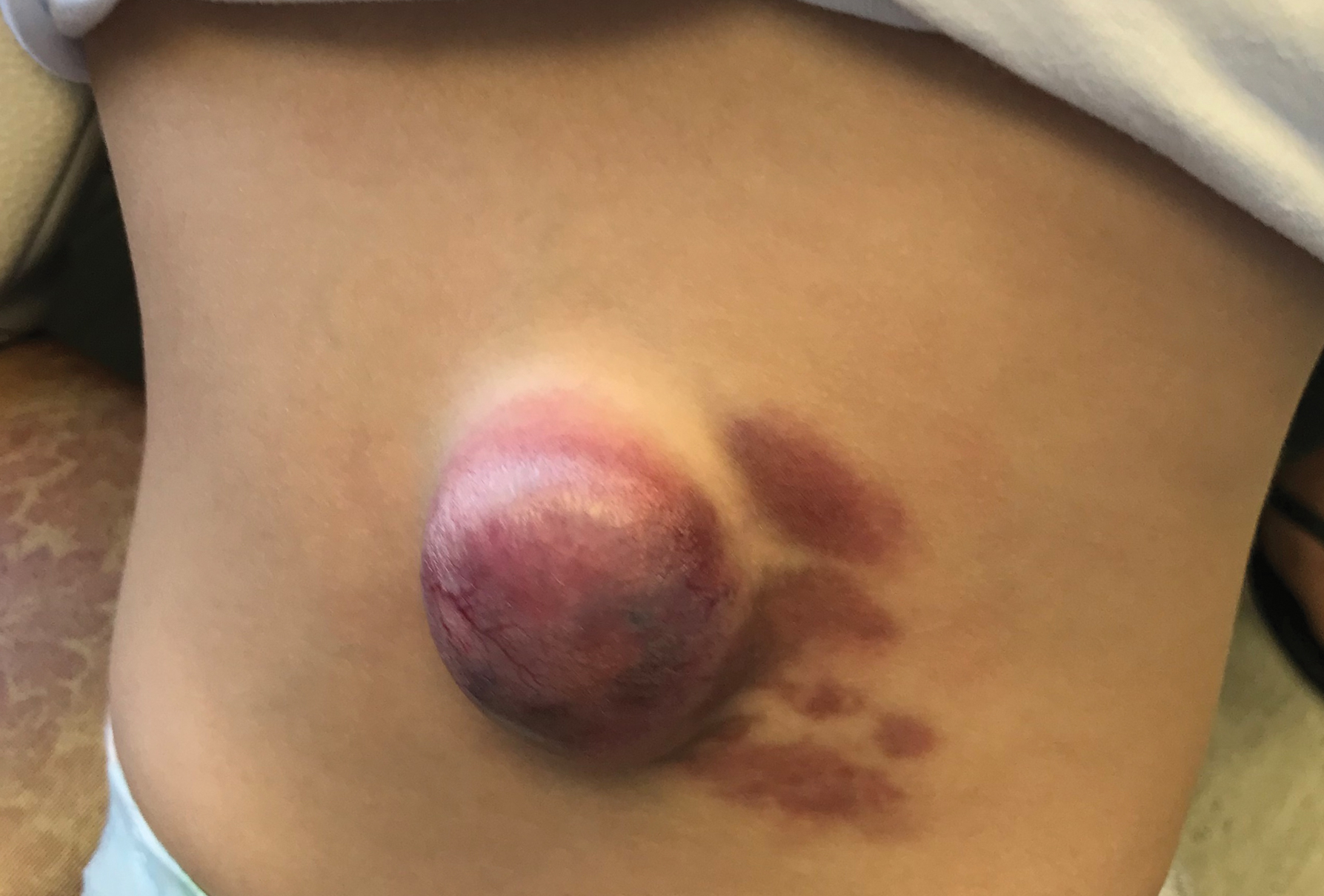
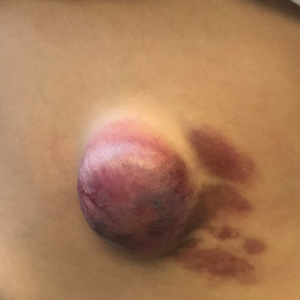
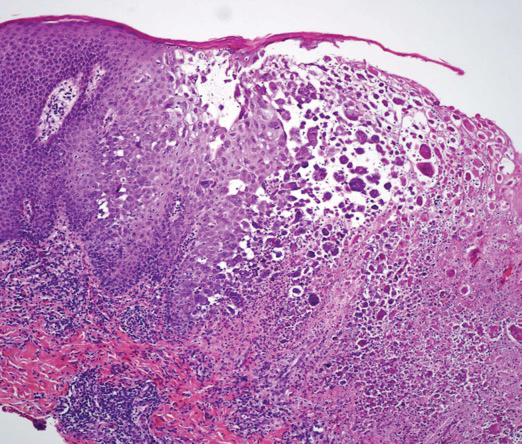
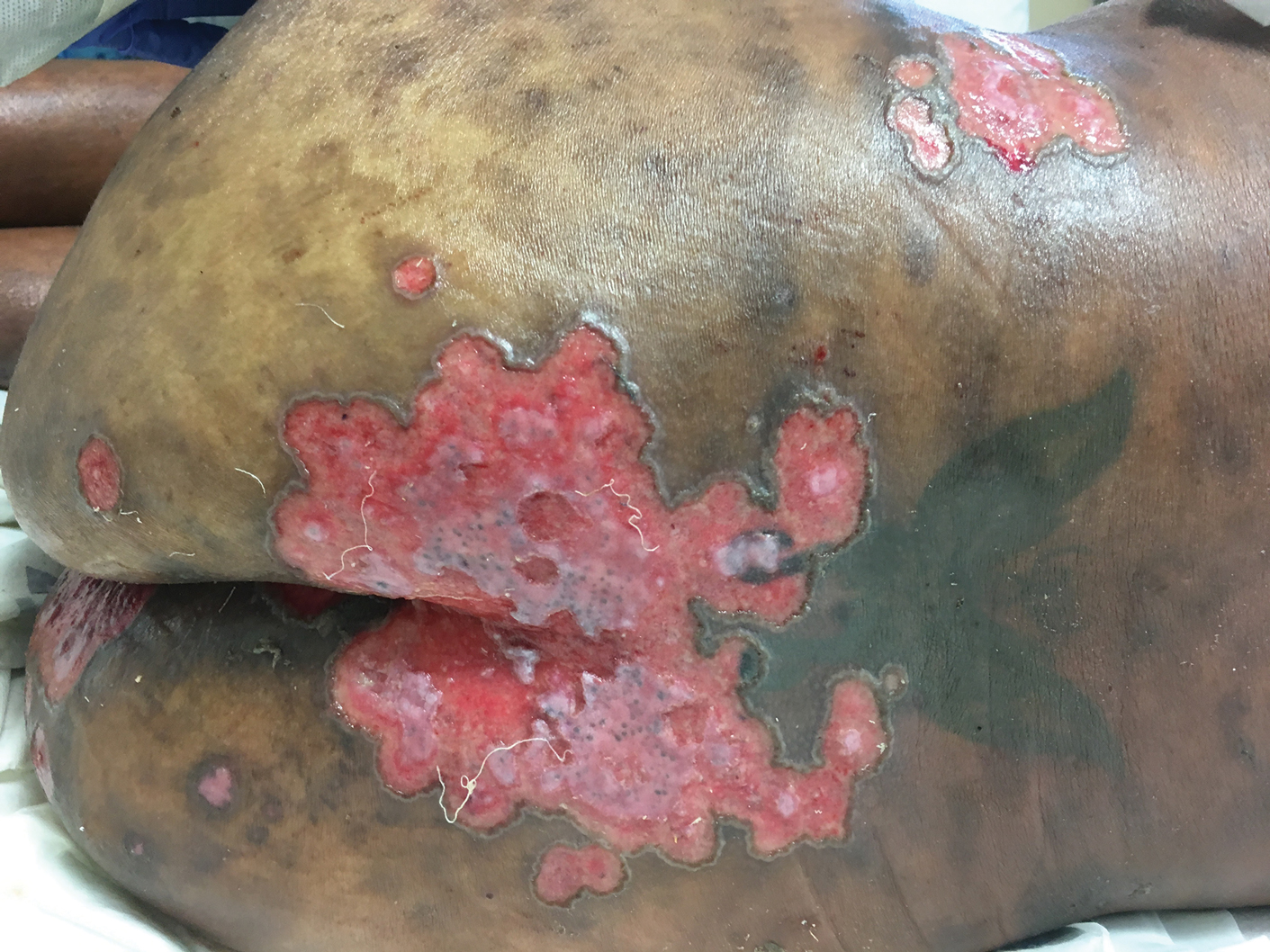
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Patch Testing in Children: Not Just Little Adults
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
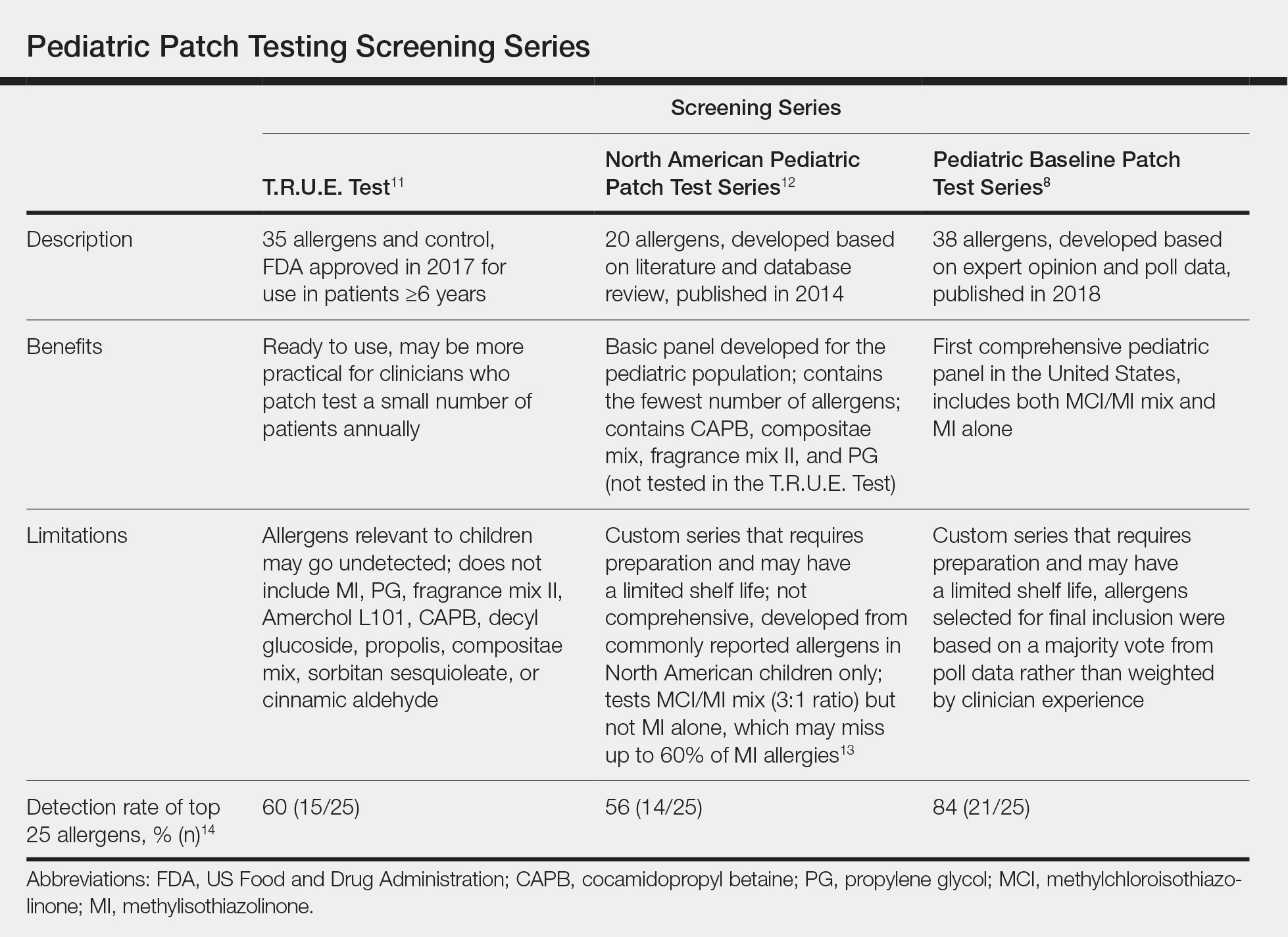
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
The pediatric population has a unique product exposure profile due to the many care products specifically marketed for use in children. In fact, the prevalence of allergic contact dermatitis (ACD) in children may be as high as 24.5% in the United States.1 In patch tested children, relevant positive reaction rates of 56.7% and 48% have been reported by the North American Contact Dermatitis Group and the Pediatric Contact Dermatitis Registry, respectively.2,3 In this article, we provide an overview of current trends in pediatric patch testing as well as specific considerations in this patient population.
Patch Test Reactions in Children
Several publications have documented pediatric patch test reactions. The North American Contact Dermatitis Group reported patch test results in 883 children from the United States and Canada (2005-2012).2 The most common reactions were nickel (28.1%), cobalt (12.3%), neomycin (7.1%), balsam of Peru (5.7%), lanolin (5.5%), and fragrance mix I (5.2%). When compared to adults, children were more likely to have relevant positive patch tests to nickel, cobalt, and compositae mix.2 In comparison, data from the Pediatric Contact Dermatitis Registry showed that the most common reactions in 1142 children in the United States (2015-2016) were nickel (22%), fragrance mix I (11%), cobalt (9.1%), balsam of Peru (8.4%), neomycin (7.2%), and propylene glycol (6.8%).3
Allergen sensitivities may vary based on geographic region. In Spain, children showed the highest sensitivities to thiomersal (10.2%), cobalt (9.1%), colophony (9.1%), paraphenylenediamine (8.3%), mercury (7.9%), potassium dichromate (7.9%), and nickel (6.4%).4
Pediatric Patch Testing Pearls
History of Product Use
From diapers to drama club, pediatric exposures and sources of ACD are not the same as those seen in adults. Because obtaining a medical history from a toddler can be exasperating, the patient’s caregivers should be asked about potential exposures, ranging from personal care products and diapers to school activities, hobbies, and sports.5,6 It is important to keep in mind that the patient’s primary caregiver may not be the only individual who applies products to the child.7
Application of Allergens
Children are not merely small adults, but they usually do have smaller backs than adult patients. This reduced surface area means that the patch tester must carefully select the allergens to be patch tested. For reference, the back of a typical 6-year-old child can fit 40 to 60 allergens during patch testing.8
Patch Test Chambers
In children, the use of plastic patch test chambers may be preferred over aluminum chambers. Children with persistent pruritic subcutaneous nodules induced by aluminum-based vaccines also may have delayed-type sensitivity reactions to aluminum.9 These patients could react to the aluminum present in some patch test chambers, making interpretation of the results difficult. The authors (A.R.A. and M.R.) typically use plastic chambers in the pediatric population.
Managing Expectations
As with other procedures in the pediatric population, patch testing can elicit emotions of fear, anxiety, and distrust. Video distraction and/or role-playing games may help capture the attention of children and can be particularly helpful during patch application. Children may be apprehensive about the term allergy testing if they are familiar with the term needle testing from previous allergies.5
Securing Patches
Young children can be quite active, posing another challenge for keeping patches in place. We recommend using extra tape to secure the patches in place on a child’s back. In addition, a large transparent film dressing (ie, 12×8 in) can be used if quick application is needed. For extra precaution, the use of a tight T-shirt or favorite onesie during the patch test process may be helpful, making it more difficult for little fingers to remove tape edges.
Duration of Patch Testing
Some authors have proposed application of patch tests for 24 hours in pediatric patients, as compared to 48 hours in adults.10 This recommendation is based on a theory that the reduced application period will decrease the risk for irritant reactions in pediatric patients.
Pediatric Patch Test Screening Series
A summary of the published screening series for patch testing in the pediatric population is provided (Table).
The T.R.U.E. Test (SmartPractice) is approved by the US Food and Drug Administration for use in patients 6 years and older11; however, it may not adequately represent allergen exposures in the pediatric population. Brankov and Jacob14 found that 10 (40%) of their proposed top 25 pediatric allergens were not detected using the T.R.U.E. Test.
In 2014, the North American Pediatric Patch Test Series was proposed as a basic screening panel for children aged 6 to 12 years.12 This series of 20 allergens was developed based on a literature review of pediatric patch test results and case reports as well as a database review. The authors proposed additional allergens to be considered based on patient history.12
More recently, a 2017 American Contact Dermatitis Society physician work group proposed the Pediatric Baseline Patch Test Series. This series of 38 allergens for children aged 6 to 18 years was developed based on expert consensus.8 Studies to determine the efficacy of this series have yet to be conducted, but it may have high sensitivity in detecting relevant allergens in children as demonstrated by a theoretical detection rate of 84%.14
There are 2 recommended patch test series for allergic diaper dermatitis.15 The first series focuses on 23 potential allergens found in wet wipes and topical diaper preparations. The second series contains 10 potential allergens found in diapers. These series contain common topical medications for children including corticosteroids, antimicrobials, and sensitizers specific to diapers such as rubbers and adhesives.15
Similar to adults, it may be difficult to designate one screening panel that can identify all relevant allergens in children; thus, it is always important to obtain a thorough exposure history and customize testing to suspected allergens and/or patient products based on history and clinical relevance.
Unique Pediatric Allergens
Hobbies
Sports gear such as shin guards and splints often contain allergens such as formaldehyde resin, thiuram mix, and dialkyl thioureas.16 Perioral dermatitis may be caused by musical instrument mouthpieces containing nickel.6
Preservatives
Commonly reported causes of ACD in children include methylisothiazolinone (MI) and methylchloroisothiazolinone (MCI) found in wet wipes. A 2016 analysis of diaper wipes showed a low prevalence of MI (6.3%) and MCI (1.6%) in these products, which may reflect the industry’s awareness of these potential allergens and a subsequent change in the preservatives they utilize.17 However, the prevalence of MCI/MI contact allergy may be on the rise due to the popularity of homemade slime, which is made from common household products such as laundry detergent, dishwashing soap, and liquid glue. The Pediatric Baseline Patch Test Series captures most of the potential allergens in these homemade slime recipes and is recommended for use in pediatric patients suspected of having dermatitis secondary to playing with slime.8,18
Toilet Seat Dermatitis
Toilet seat dermatitis presents as a pruritic dermatitis on the posterior upper thighs and buttocks. Although most cases of toilet seat dermatitis are irritant rather than allergic, potential allergens include plastics, fragrances, and components of cleaning products. Thus, physicians should maintain a high index of suspicion for ACD to toilet seats.19
Fragrance and Natural Ingredients
A 2018 study evaluating personal care products marketed specifically for infants and children found that 55% of products (294/533) contained at least 1 common allergen, with fragrance being the most common (48% [255/533]). Other common allergens include betaines (18%), propylene glycol (9%), lanolin (6%), and MCI/MI (3%).20 Caregivers should be advised against the myth that natural products are safer and less allergenic and should be provided with resources such as the Contact Allergen Management Program (CAMP) database (https://www.contactderm.org/resources/acds-camp) for safe alternative personal care products.
Metal Allergens
Nickel, the American Contact Dermatitis Society 2008 Allergen of the Year, is another common allergen that affects children. Nickel allergy, commonly thought to affect the ears due to jewelry and ear piercing, may actually be found in a wide range of daily items such as braces, eyeglasses, keys, zippers, school chairs, electronics, toys, and even food.3,6,21,22 With increased use of electronics in children of all ages, nickel found in mobile phones and other devices may be of particular concern. Caregivers can use a case or cover for metallic-appearing electronics.
Final Interpretation
Pediatric ACD is common. With limited surface area for patch testing in children, we recommend customized panels based on patient history and exposure. It is important for clinicians to recognize the unique causes of ACD in children and develop age-appropriate management plans.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
- Bruckner AL, Weston WL, Morelli JG. Does sensitization to contact allergens begin in infancy? Pediatrics. 2000;105:e3.
- Zug KA, Pham AK, Belsito DV, et al. Patch testing in children from 2005 to 2012: results from the North American contact dermatitis group. Dermatitis. 2014;25:345-355.
- Goldenberg A, Mousdicas N, Silverberg N, et al. Pediatric Contact Dermatitis Registry inaugural case data. Dermatitis. 2016;27:293-302.
- Ortiz Salvador JM, Esteve Martinez A, Subiabre Ferrer D, et al. Pediatric allergic contact dermatitis: clinical and epidemiological study in a tertiary hospital. Actas Dermosifiliogr. 2017;108:571-578.
- Jacob SE, Steele T, Brod B, et al. Dispelling the myths behind pediatric patch testing—experience from our tertiary care patch testing centers. Pediatr Dermatol. 2008;25:296-300.
- Brod BA, Treat JR, Rothe MJ, et al. Allergic contact dermatitis: kids are not just little people. Clin Dermatol. 2015;33:605-612.
- Elliott JF, Ramzy A, Nilsson U, et al. Severe intractable eyelid dermatitis probably caused by exposure to hydroperoxides of linalool in a heavily fragranced shampoo. Contact Dermatitis. 2017;76:114-115.
- Yu J, Atwater AR, Brod B, et al. Pediatric Baseline Patch Test Series: Pediatric Contact Dermatitis Workgroup. Dermatitis. 2018;29:206-212.
- Bergfors E, Inerot A, Falk L, et al. Patch testing children with aluminium chloride hexahydrate in petrolatum: a review and a recommendation. Contact Dermatitis. 2019;81:81-88.
- Worm M, Aberer W, Agathos M, et al. Patch testing in children—recommendations of the German Contact Dermatitis Research Group (DKG). J Dtsch Dermatol Ges. 2007;5:107-109.
- T.R.U.E. Test (Thin-Layer Rapid Use Epicutaneous Patch Test) [package insert]. Hillerød, Denmark: SmartPractice Denmark ApS; 2017.
- Jacob SE, Admani S, Herro EM. Invited commentary: recommendation for a North American pediatric patch test series. Curr Allergy Asthma Rep. 2014;14:444.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6.
- Brankov N, Jacob SE. Pre-emptive avoidance strategy 2016: update on pediatric contact dermatitis allergens. Expert Rev Clin Immunol. 2017;13:93-95.
- Yu J, Treat J, Brod B. Patch test series for allergic perineal dermatitis in the diapered infant. Dermatitis. 2017;28:70-75.
- Sung CT, McGowan MA, Jacob SE. Allergic contact dermatitis evaluation: strategies for the preschooler. Curr Allergy Asthma Rep. 2018;18:49.
- Yu J, Treat J, Chaney K, et al. Potential allergens in disposable diaper wipes, topical diaper preparations, and disposable diapers: under-recognized etiology of pediatric perineal dermatitis. Dermatitis. 2016;27:110-118.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Dorfman CO, Barros MA, Zaenglein AL. Contact dermatitis to training toilet seat (potty seat dermatitis). Pediatr Dermatol. 2018;35:e251-e252.
- Bonchak JG, Prouty ME, de la Feld SF. Prevalence of contact allergens in personal care products for babies and children. Dermatitis. 2018;29:81-84.
- Chen JK, Jacob SE, Nedorost ST, et al. A pragmatic approach to patch testing atopic dermatitis patients: clinical recommendations based on expert consensus opinion. Dermatitis. 2016;27:186-192.
- Goldenberg A, Silverberg N, Silverberg JI, et al. Pediatric allergic contact dermatitis: lessons for better care. J Allergy Clin Immunol Pract. 2015;3:661-667; quiz 668.
Practice Points
- Pediatric allergic contact dermatitis (ACD) is common with children having unique product exposures.
- Children suspected to have ACD should be patch tested with customized panels based on history and exposure.
- Common pediatric allergens have been identified in personal care products, household products, and recreational gear and toys.
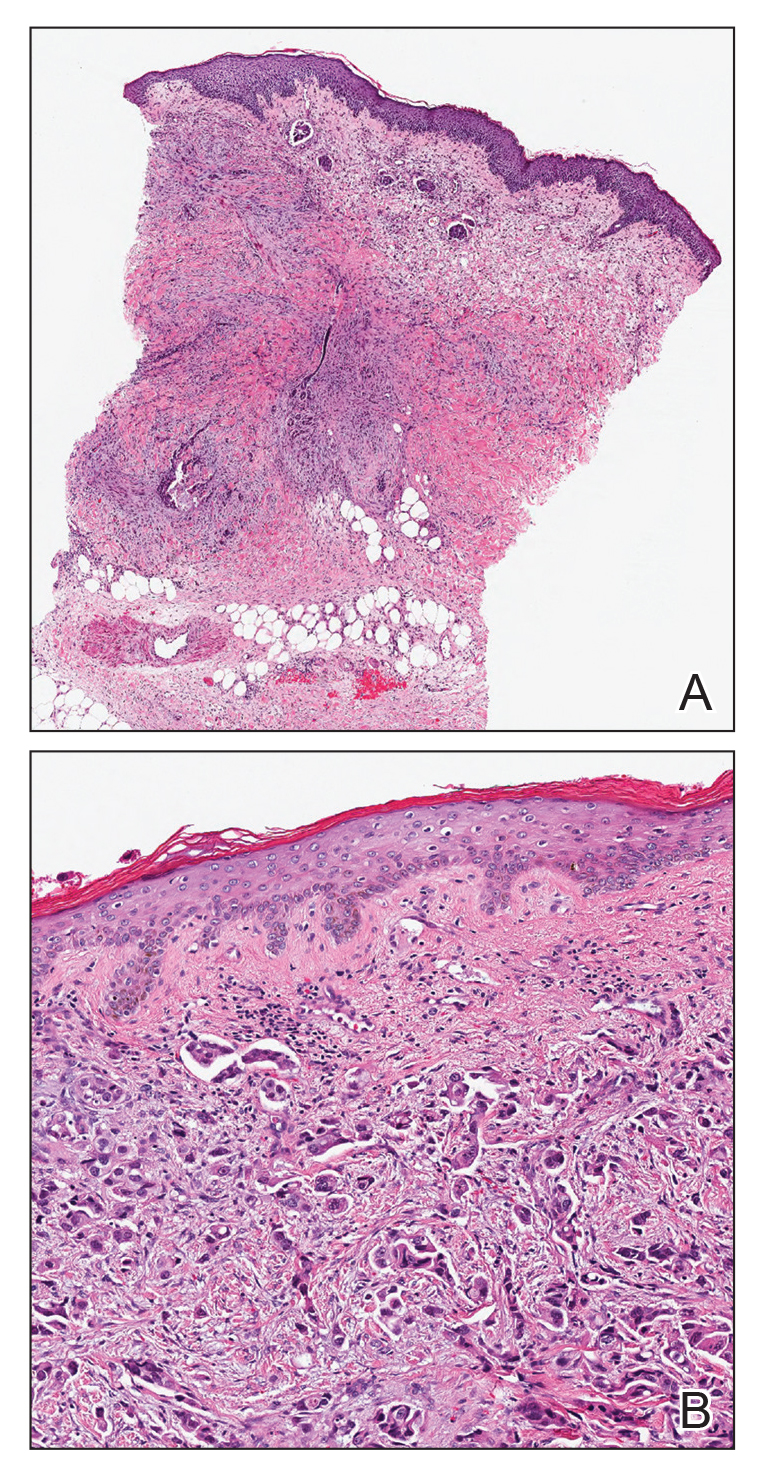
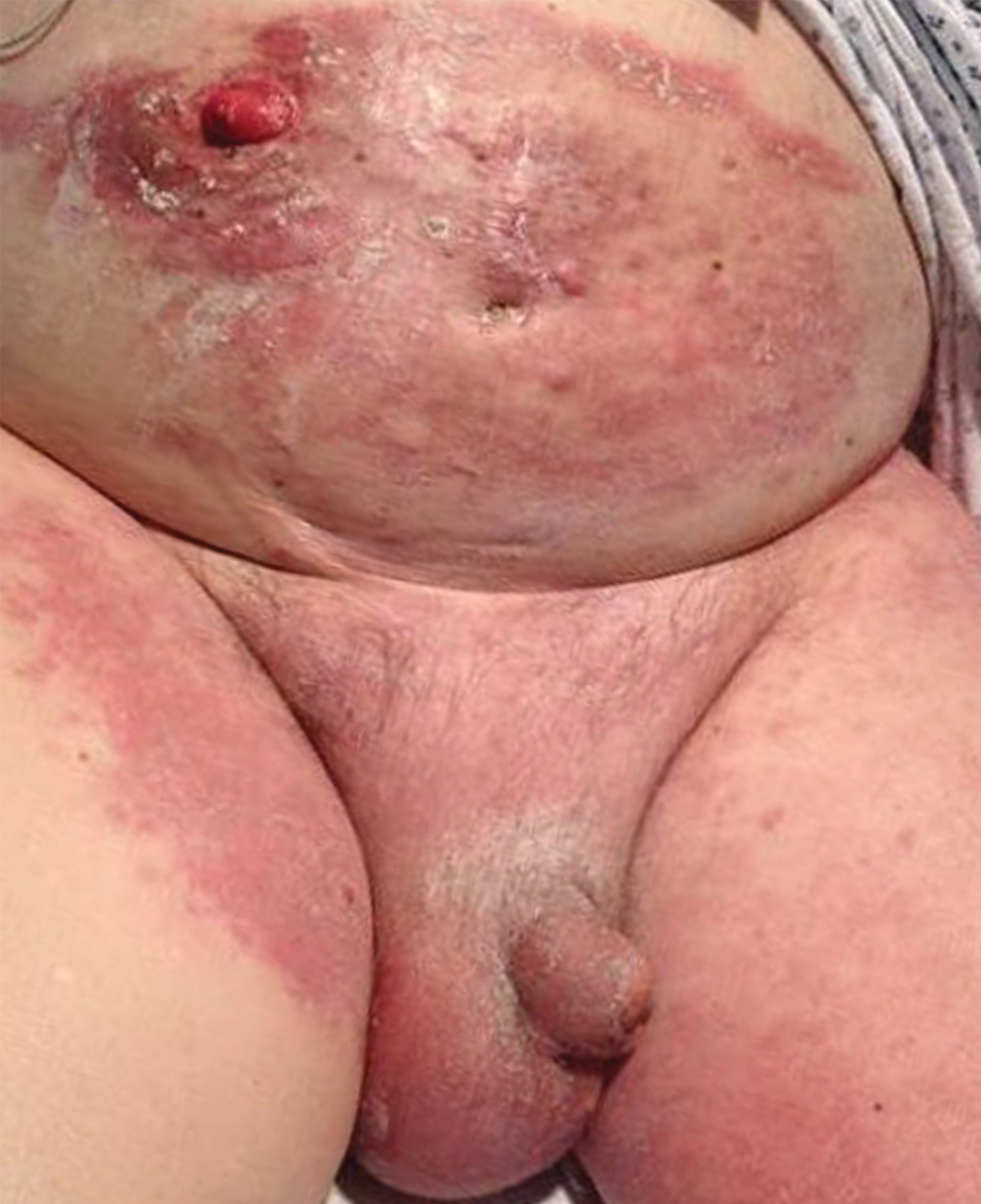
Current Controversies in Mohs Micrographic Surgery
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
Mohs micrographic surgery (MMS) has been met with controversy since its inception in the 1930s. Current debate centers on the types of tumors treated with MMS, increasing utilization, third-party payer reimbursement, the Appropriate Use Criteria (AUC), and subspecialty certification.
Controversies in Applications
Controversy surrounding treatment with MMS for certain tumor types is abundant, in large part due to a lack of well-designed studies. Perhaps most notably, the surgical management of melanoma has been hotly contested for decades.1 An increasing number of Mohs surgeons advocate the use of MMS for treatment of melanoma. Advocates reason that tumor margins may be ill-defined, necessitating histologic examination of the margin for tumor clearance. In a study by Zitelli et al,2 5-year survival and metastatic rates for 535 patients with melanomas treated by MMS with frozen sections were the same or better when compared to historical controls treated with conventional wide local excision. Melanoma-associated antigen recognized by T cells (MART-1) immunostaining may offer improved diagnostic accuracy.3 Others believe that staged excision with permanent sections processed vertically, en face, or horizontally (“slow Mohs”) is more accurate and efficacious for the treatment of melanoma.1 Advocates of this approach maintain that when compared to MMS with frozen sections, staged excision with permanent sections enables more accurate interpretation of residual melanoma and atypical junctional melanocytic hyperplasia as well as circumvents difficulty in interpreting freeze artifact.4
Although Merkel cell carcinoma has traditionally been treated with wide local excision, MMS with or without adjuvant radiotherapy has gained traction as a treatment option. Advocates for treatment by MMS hold that Merkel cell carcinoma is a contiguous tumor with a high rate of residual tumor persistence, making histologic margin control an ideal characteristic of treatment. However, in the absence of large randomized controlled studies comparing MMS to wide local excision, controversy surrounds the most appropriate surgical approach.1 In a retrospective study of 86 patients by O’Connor et al,5 MMS was demonstrated to compare favorably to standard surgical excision. Standard surgical excision was associated with a 31.7% (13/41) local persistence rate and 48.8% (20/41) regional metastasis rate compared to 8.3% (1/12) and 33.3% (4/12) for MMS, respectively.5
Controversies in Increasing Utilization
The incidence of skin cancers has increased in recent years. As a result, it is reasonable to expect the rates of MMS to increase. Nonetheless, there is escalating concern among groups of third-party payers, the public, and physicians that MMS is being overused.6 Growth of the body of evidence supporting the appropriateness of MMS remains essential. Such studies continue to support reasons for increased MMS usage, demonstrating the stability of the percentage of skin cancers treated with MMS in the setting of increasing skin cancer incidence, the procedure’s superior efficacy for appropriately chosen cases, its expanding application to melanoma and other tumors, and an emphasis of MMS in residency training programs.6-9
A current hot topic of controversy focuses on the wide variation among Mohs surgeons in the mean number of stages used to resect a tumor. Overuse among outliers has been proposed to stem from lack of technical expertise or from abuse of the current fee-for-service payment model, which bases compensation on the number of stages performed. A study by Krishnan et al10 determined that the mean number of stages per tumor in the studied population (all physicians [N=2305] receiving Medicare payments for MMS from January 2012 to December 2014) was 1.74, with a range of 1.09 to 4.11. Persistently high outliers were more likely to perform MMS in a solo practice, with an odds ratio of 2.35.10 In response to the wide variation in mean stages used to resect a skin cancer and its implications on increased financial burden and surgery to individual patients, intervention has been proposed. Notably, it has been demonstrated that mailing out individual reports of practice patterns to high-outlier physicians resulted in a reduction in mean stages per tumor as well as an associated cost savings when compared to outlier physicians who did not receive these reports.11
Controversies in Reimbursement
Mohs micrographic surgery also has been in the spotlight for debate regarding reimbursement. The procedure has been targeted partly in response to its substantial contribution to total Medicare reimbursements paid out. In 2013, primary MMS billing codes constituted nearly 19% of total reimbursements to dermatologists and approximately 0.5% of total reimbursements to all physicians participating in Medicare.12 Mohs micrographic surgery codes have correspondingly received frequent review by the Relative Value Scale Update Committee and remained on a list of potentially misvalued services according to the Centers for Medicare & Medicaid Services for years.13 Due to continued scrutiny and review, especially by the Relative Value Scale Update Committee and Centers for Medicare & Medicaid Services, reimbursement to perform MMS and reconstructive surgery has gone down by more than 20% in the last 15 years.14 Public perception mirrors third-party payer concerns for overcompensation. An article title in the New York Times theatrically postures “Patients’ Costs Skyrocket, Specialists’ Incomes Soar.” The article recounts an MMS patient’s “outrage at charges” associated with treatment of her “minor medical problem” and the simultaneous “sharp climb” in dermatologist income over the last 2 decades.15
However, studies continue to demonstrate the cost-effectiveness of MMS. A study by Ravitskiy et al16 demonstrates the cost-effectiveness of MMS, regardless of place of service or type of tumor. Of 406 tumors studied, MMS was the least expensive surgical procedure evaluated ($805 per tumor) when compared to standard surgical excision with permanent margins ($1026 per tumor), standard surgical excision with frozen margins ($1200 per tumor), and ambulatory surgery center standard surgical excision ($2507 per tumor). Furthermore, adjusted for inflation, the cost of MMS was lower in 2009 vs 1998.16 Similar results have been consistently demonstrated.17
Controversies in the AUC
To provide clinicians, policy makers, and insurers guidance for utilization of MMS in the setting of concerns for overutilization, overcompensation, and inappropriate application, the MMS AUC were established in 2012. The guidelines were developed by a process integrating evidence-based medicine, clinical experience, and expert opinion and is applicable to 270 clinical scenarios.18
A unique set of debate accompanies the guidelines. Namely, controversy has surrounded the classification of most primary superficial basal cell carcinomas as appropriate for treatment by MMS. These tumors have comparable cure rates when treated by MMS or curettage and cryosurgery, are often multifocal and require more Mohs stages than other basal cell carcinoma subtypes, and largely lack data on recurrence and invasion.19 The guidelines also have been scrutinized for including only studies from the United States.20 Furthermore, the report is largely based on expert opinion rather than evidence.
Some Mohs surgeons have concerns that the guidelines will minimize clinical judgment. Nonetheless, deviations from the AUC practiced by Mohs surgeons have been reported where clinical judgment supplants guideline criteria. The most commonly cited reasons for performing MMS on tumors classified as uncertain or inappropriate, according to one study by Ruiz et al,21 included performing multiple MMSs on the same day, tumor location on the lower legs, and incorporation into an adjacent wound. Reported discrepancies in the AUC further emphasize the importance of clinical judgment and call into question the need for future revision of the criteria.22 For example, a primary squamous cell carcinoma in situ greater than or equal to 2 cm located on the trunk and extremities (excluding pretibial surfaces, hands, feet, nail units, and ankles) in a healthy patient is categorized as appropriate, while a recurrent but otherwise identical squamous cell carcinoma in situ is categorized as uncertain. These counterintuitive criteria are unsupported by existing studies.
Controversies in Subspecialty Certification
Recently, debate also has surfaced regarding subspecialty certification. Over the last decade, proponents of subspecialty certification have argued that board certification would bring consistency and decrease divisiveness among dermatologists; help to prevent exclusion of Mohs surgeons from insurance networks and teaching opportunities at the Veterans Administration; and demonstrate competence to patients, the media, and payers. Those in opposition contest that practices may be restricted by insurers using lack of certification to eliminate dermatologists from their networks, economic credentialing may be applied to dermatologists such that those without the subspecialty certification may not be deemed qualified to manage skin cancer, major limitations may be set determining which dermatologists can sit for the certification examination, and subspecialty certification could create disenfranchisement of many dermatologists. A 2017 American Academy of Dermatology member survey demonstrated ambivalence regarding subcertification, with 51% of respondents pro-subcertification and 48% anti-subcertification.23
Nonetheless, after years of debate the American Board of Dermatology proposed subspecialty certification in Micrographic Dermatologic Surgery, which was approved by the American Board of Medical Specialties on October 26, 2018. The first certification examination will likely take place in 2 years, and a maintenance of certification examination will be required every 10 years.24
Final Thoughts
Further investigation is needed to elucidate and optimize solutions to many of the current controversies associated with MMS.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
- Levy RM, Hanke CW. Mohs micrographic surgery: facts and controversies. Clin Dermatol. 2010;28:269-274.
- Zitelli JA, Brown C, Hanusa BH. Surgical margins for excision of primary cutaneous melanoma. J Am Acad Dermatol. 1997;37:422-429.
- Albertini JG, Elston DM, Libow LF, et al. Mohs micrographic surgery for melanoma: a case series, a comparative study of immunostains, an informative case report, and a unique mapping technique. Dermatol Surg. 2002;28:656-665.
- Walling HW, Scupham RK, Bean AK, et al. Staged excision versus Mohs micrographic surgery for lentigo maligna and lentigo maligna melanoma. J Am Acad Dermatol. 2007;57:659-664.
- O’Connor WJ, Roenigk RK, Brodland DG. Merkel cell carcinoma. comparison of Mohs micrographic surgery and wide excision in eighty-six patients. Dermatol Surg. 1997;23:929-933.
- Reeder VJ, Gustafson CJ, Mireku K, et al. Trends in Mohs surgery from 1995 to 2010: an analysis of nationally representative data. Dermatol Surg. 2015;41:397-403.
- Mosterd K, Krekels GA, Nieman FH, et al. Surgical excision versus Mohs’ micrographic surgery for primary and recurrent basal-cell carcinoma of the face: a prospective randomised controlled trial with 5-years’ follow-up. Lancet Oncol. 2008;9:1149-1156.
- Viola KV, Rezzadeh KS, Gonsalves L, et al. National utilization patterns of Mohs micrographic surgery for invasive melanoma and melanoma in situ. J Am Acad Dermatol. 2015;72:1060-1065.
- Todd MM, Miller JJ, Ammirati CT. Dermatologic surgery training in residency. Dermatol Surg. 2002;28:547-549.
- Krishnan A, Xu T, Hutfless S, et al; American College of Mohs Surgery Improving Wisely Study Group. Outlier practice patterns in Mohs micrographic surgery: defining the problem and a proposed solution. JAMA Dermatol. 2017;153:565-570.
- Albertini JG, Wang P, Fahim C, et al. Evaluation of a peer-to-peer data transparency intervention for Mohs micrographic surgery overuse [published online May 5, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1259.
- Johnstone C, Joiner KA, Pierce J, et al. Mohs micrographic surgery volume and payment patterns among dermatologists in the Medicare population, 2013. Am J Clin Oncol. 2018;41:1199-1203.
- Donaldson MR, Coldiron BM. Mohs micrographic surgery utilization in the Medicare population, 2009. Dermatol Surg. 2012;38:1427-1434.
- Bath C. Dermatologists defend Mohs surgery as effective and cost-efficient with low rate of recurrence. ASCO Post. March 15, 2014. https://www.ascopost.com/issues/march-15-2014/dermatologists-defend-mohs-surgery-as-effective-and-cost-efficient-with-low-rate-of-recurrence. Accessed October 23, 2019.
- Rosenthal E. Patients’ costs skyrocket; specialists’ incomes soar. New York Times. January 18, 2004. https://www.nytimes.com/2014/01/19/health/patients-costs-skyrocket-specialists-incomes-soar.html. Accessed October 23, 2019.
- Ravitskiy L, Brodland DG, Zitelli JA. Cost analysis: Mohs micrographic surgery. Dermatol Surg. 2012;38:585-594.
- Tierney EP, Hanke CW. Cost effectiveness of Mohs micrographic surgery: review of the literature. J Drugs Dermatol. 2009;8:914-922.
- Connolly SM, Baker DR, Coldiron BM, et al. AAD/ACMS/ASDSA/ASMS 2012 appropriate use criteria for Mohs micrographic surgery: a report of the American Academy of Dermatology, American College of Mohs Surgery, American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery. J Am Acad Dermatol. 2012;67:531-550.
- Steinman HK, Dixon A, Zachary CB. Reevaluating Mohs surgery appropriate use criteria for primary superficial basal cell carcinoma. JAMA Dermatol. 2018;154:755-756.
- Kelleners-Smeets NW, Mosterd K. Comment on 2012 appropriate use criteria for Mohs micrographic surgery. J Am Acad Dermatol. 2013;69:317-318.
- Ruiz ES, Karia PS, Morgan FC, et al. Multiple Mohs micrographic surgery is the most common reason for divergence from the appropriate use criteria: a single institution retrospective cohort study. J Am Acad Dermatol. 2016;75:830-831.
- Croley JA, Joseph AK, Wagner RF Jr. Discrepancies in the Mohs Micrographic Surgery appropriate use criteria [published online December 23, 2018]. J Am Acad Dermatol. doi:10.1016/j.jaad.2018.11.064.
- Weinstein A. The ABD’s push for subspecialty certification in Mohs surgery will fracture dermatology. Pract Dermatol. April 2018:37-39. https://practicaldermatology.com/articles/2018-apr/perspective-the-abds-push-for-subspecialty-certification-in-mohs-surgery-will-fracture-dermatology. Accessed Oc
tober 30, 2019. - ABD Micrographic Dermatologic Surgery (MDS) Subspecialty Certification Questions & Answers. American Board of Dermatology website. https://www.abderm.org/residents-and-fellows/fellowship-training/micrographic-dermatologic-surgery-mds-questions-and-answers-1.aspx. Accessed October 23, 2019.
Resident Pearl:
• Further investigation is needed to elucidate and optimize solutions to current controversies in Mohs micrographic surgery.
Neonatal Consultations: Vascular Lumps, Bumps, and Tumors in the Neonate
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).
Congenital Hemangioma
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4
Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.
Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).
Congenital Hemangioma
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4
Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.
Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
Although most neonatal vascular lumps, bumps, and tumors are benign, proper diagnosis is important for prognosis and management. Therefore, knowledge of both common and rare conditions is important when evaluating a neonatal nodule. Differential diagnosis of neonatal vascular nodules must focus on important diagnostic clues that should prompt consideration and evaluation for less common and/or potentially threatening conditions. Infantile hemangioma (IH), congenital hemangioma (CH), venous malformation (VM), lymphatic malformation (LM), kaposiform hemangioendothelioma (KHE) and tufted angioma, and malignant tumors are reviewed here.
Infantile Hemangioma
Infantile hemangioma, a benign proliferation of capillaries, is the most common tumor of infancy with reported incidence of up to 5% in neonates.1 As such, suspicion for less common lesions is often predicated on identifying features that would be atypical for an IH. A superficial IH presents as a bright red papule, nodule, or plaque, while a deep IH presents as a flesh-colored to bluish nodule. Mixed IHs combine features of both superficial and deep lesions. The distribution may be focal or segmental, with segmental lesions encompassing a larger territory–like distribution and frequently displaying a thin, coarsely telangiectatic appearance.
Knowledge of the natural history of IH generally is crucial in differentiating it from other neonatal lesions. Infantile hemangiomas display a natural history that is distinct and predictable. They typically manifest within the first few weeks of life, though up to 30% present at birth with a premonitory mark, which may be a light red, pink, bluish, or vasoconstricted patch. Thus, mere presence of a lesion at birth is not the feature that distinguishes other congenital lesions from an IH. After initial appearance, IHs undergo a period of proliferation that occurs over 4 to 6 months in most patients. In some cases, areas of proliferation may be subtle, but nonetheless the presence of some areas of increased redness and/or volumetric growth generally is required to firmly establish the diagnosis of IH. Thereafter, IH will involute, a process that begins before 1 year of age in most cases and continues over years. Although IHs undergo involution, complete clearance may not occur, as nearly 70% will leave permanent residua such as fibrofatty masses or anetodermic skin.2 Nevertheless, the presence of a proliferative phase followed by a slower period of involution is a hallmark feature of the IH.
Biopsy and imaging rarely are required for establishing diagnosis of an IH. Histopathology showing a proliferation of capillaries with positive glucose transporter 1 (GLUT-1) staining is characteristic. Imaging with ultrasound reveals a fast-flow lesion. Apart from exceptionally rare cases, a cutaneous IH typically does not cross muscle fascia, and thus alternative diagnoses should be considered for a cutaneous lesion that demonstrates infiltration into nerve, bone, joint, or other deeper tissues. Most IHs do not require treatment; however, a small subset may be associated with complications and thus require intervention. Complications of IH may include impairment of function (eg, vision, feeding, respiratory), ulceration, and risk for permanent disfigurement. When treatment is indicated, the most commonly employed options during the proliferative phase are the topical beta-blocker timolol and the oral beta-blocker propranolol. In addition, certain IHs may be associated with either syndromic presentations and/or visceral involvement, thus requiring further workup (Table).
Congenital Hemangioma
A CH is an uncommon benign neonatal tumor that is distinct from an IH in behavior, biology, and treatment. Congenital hemangiomas may have a rapidly involuting course, referred to as RICH (rapidly involuting congenital hemangioma), or a noninvoluting course, referred to as NICH (noninvoluting congenital hemangioma). Partially involuting types also have been described.3 A RICH typically presents as a highly vascular, red-violaceous or bluish plaque, nodule, or large mass at birth. An NICH presents as a red-violaceous or bluish, coarsely telangiectatic patch, plaque, or nodule. A characteristic feature of the CH is the rim of vasoconstriction around the lesion, which is an important diagnostic clue (Figure 1). In contrast to IH, multifocal lesions are highly unlikely in CH, though it rarely has been reported.4
Regardless of subtype, CHs are fully developed at birth. Infantile hemangiomas, on the other hand, are either minimally present or not present at birth and thereafter proliferate. After birth, a RICH rapidly involutes over the first 9 to 12 months of life. This process generally is evident even in the first few weeks of life, which would not be expected of an IH and is therefore a major distinguishing factor. A NICH, on the other hand, is expected to be persistent, for the most part neither showing signs of proliferation nor involution.
Complications of CHs may include ulceration, functional impairment, or risk for permanent disfigurement depending on location. In addition, due to their fast-flow state and potential large size, some CHs may be complicated by high-output heart failure in the neonate. Distinguishing an IH from a CH is important not only for prognosis but also treatment. Beta-blocker therapy generally is not useful for CHs, and management usually is supportive in the neonatal period.
In the majority of cases, diagnosis can be achieved solely on clinical features. Biopsy with immunohistochemistry shows negative GLUT-1 staining, which will distinguish this lesion from an IH. At times, the highly vascular nature and/or striking size of a CH may lead some to consider the potential diagnosis of an arteriovenous malformation. However, soft-tissue arteriovenous malformations involving the skin are almost never fully developed in the neonatal period and generally take years to evolve from a quiescent state to a destructive lesion.
Venous Malformation
Venous malformations are congenital malformations of veins that may be apparent at birth or later. They appear as bluish to flesh-colored, compressible nodules or plaques. They tend to increase in size when the affected body part is in a dependent position, and this maneuver can be a helpful distinguishing clue. Although the majority of patients have a single lesion, multifocal involvement may occur uncommonly (Table). The diagnosis of VM usually is clinical, though at times, a VM may be difficult to distinguish from a purely deep IH. However, a VM will persist over time, growing in proportion to the patient. In addition, a VM displays low flow on ultrasound, a distinguishing feature from the fast-flow IH. Magnetic resonance imaging with and without contrast is the imaging study of choice. At times, cutaneous VMs will demonstrate infiltration into other tissue planes such as muscle and joint. Pain may occur secondary to thrombus formation within the malformation. In extensive lesions, intravascular coagulation may be notable, as reflected in elevated D-dimer and decreased fibrinogen levels. Treatment with sclerotherapy or surgery may be considered in select cases during infancy; however, in general, an asymptomatic VM may be observed early on in life.
Lymphatic Malformation
A lymphatic malformation (LM) is a congenital malformation of lymphatic vessels and may be further differentiated into microcystic, macrocystic, or mixed types depending on the size of the channels. An LM may present at birth or later and persists over time. Superficial microcystic LMs, synonymous with the term lymphangioma circumscriptum, characteristically appear as a group of clear and violaceous hemorrhagic vesicles on the skin. Deeper LMs appear as a tense or spongy, flesh-colored nodule or mass. Involvement of the head and neck is common. Complications frequently occur in LMs. Cutaneous LMs may ooze or bleed. Infection and hemorrhage into cysts may occur, which will cause acute pain, redness, swelling, and induration. Cervicofacial lesions may result in respiratory distress. Thus, the majority of LMs require treatment, though asymptomatic lesions may be observed in the neonate. An ultrasound will demonstrate a low-flow lesion, and magnetic resonance imaging is the diagnostic modality of choice for diagnosis and definition of extent.
KHE and Tufted Angioma
Kaposiform hemangioendothelioma is a rare, locally aggressive, vascular tumor that is frequently associated with a potentially life-threatening coagulopathy, Kasabach-Merritt phenomenon. Tufted angiomas are now understood to belong on a spectrum with KHEs, which usually present in the neonatal period or infancy as firm, red-violaceous plaques, nodules, or large tumors. Infiltration into nerve, muscle, and bone may occur. The firm/hard nature and deep violaceous appearance generally are initial clues that it is not an IH. Kasabach-Merritt phenomenon manifests as thrombocytopenia as well as low fibrinogen and elevated D-dimer levels. Thrombocytopenia is generally profound in Kasabach-Merritt phenomenon and results from platelet trapping within the vascular tumor. Given these potential complications, KHEs generally require immediate medical attention, and various treatment protocols including prednisone, vincristine, and sirolimus are utilized for complicated cases.5 The diagnosis may require biopsy to distinguish it from malignant tumors, particularly sarcomas.
Malignant Tumors
Various malignancies, including congenital leukemia, neuroblastoma, Langerhans cell histiocytosis, infantile fibrosarcoma, and rhabdomyosarcoma, rarely may present as cutaneous nodules or masses in a neonate mimicking hemangiomas or other vascular lesions (Figure 2). Neonates may present with multiple bluish papules and nodules resembling a blueberry muffin baby; multiple violaceous-red nodules; or a single red-violaceous, highly vascular–appearing mass mimicking hemangiomas. Malignant tumors may display vascularity on imaging, and thus the presence of vascular flow on ultrasound should not dissuade one from the possibility of a malignancy if other clinical features are atypical or unusual for a hemangioma. When a neonatal malignancy is suspected, a large punch biopsy or incisional biopsy is required for workup.
Final Thoughts
Although IHs are the most common vascular nodules in neonates and young infants, other conditions such as VMs, LMs, CHs, KHEs, and malignancy may occur less commonly. Identifying features that would be considered atypical for IH is crucial to recognize these less common possibilities.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
- Kanada KN, Merin MR, Munden A, et al. A prospective study of cutaneous findings in newborns in the United States: correlation with race, ethnicity, and gestational status using updated classification and nomenclature. J Pediatr. 2012;161:240-245.
- Darrow DH, Greene AK, Mancini AJ, et al. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136:E1060-E1104.
- Nasseri E, Piram M, McCuaig CC, et al. Partially involuting congenital hemangiomas: a report of 8 cases and review of the literature. J Am Acad Dermatol. 2014;70:75-79.
- Blumenthal S, Stefanko N, Cossio M, et al. Multifocal congenital hemangioma: expanding the pathogenesis of “neonatal hemangiomatosis.” Pediatr Dermatol. 2019;36:720-722.
- Croteau SE, Gupta D. The clinical spectrum of kaposiform hemangioendothelioma and tufted angioma. Semin Cutan Med Surg. 2016;35:147-152.
Pink Polycyclic Ulcerations on the Lower Back and Buttocks
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
The Diagnosis: Herpes Simplex Virus
A skin biopsy was sent for tissue culture and was negative for mycobacterial, bacterial, and fungal growth. Histopathologic examination showed ballooning degeneration of keratinocytes with herpetic cytopathic effect consistent with herpetic ulceration (Figure). A swab of the lesion on the buttock was sent for human herpesvirus (HHV) and varicella-zoster virus nucleic acid testing, which was positive for HHV-2. She was started on oral valacyclovir 1000 mg twice daily for 10 days and then was continued on chronic suppression with 500 mg once daily. The patient's ulcerations healed slowly over the following few weeks.
Human herpesvirus 2 is the most common cause of genital ulcer disease and may present as chronic and recurrent ulcers in immunocompromised patients.1 It usually is spread by sexual contact. Primary infection typically occurs in the cells of the dermis and epidermis. Two weeks after the primary infection, extragenital lesions can occur in the lumbosacral area on the buttocks, fingers, groin, or thighs, as seen in our patient,2 which is a direct result of viral shedding and spread. Reactivation of HHV from the ganglia can occur with or without symptoms. Common locations for viral shedding in women are the cervix, vulva, and perianal areas.3 Patients should be counseled to avoid sexual contact during recurrences.
Cancer patients have a particularly increased risk for developing HHV-2 due to their limited cell-mediated immunity and exposure to immunosuppressive drugs.4 Moreover, approximately 5% of immunocompromised patients develop resistance to antiviral therapy.5 Although this phenomenon was not observed in our patient, identification of novel strategies to treat these new groups of patients will be essential.
The differential diagnosis includes perianal candidiasis, which is classified by erythematous plaques with satellite vesicles and pustules. Contact dermatitis is common in the buttock area and usually secondary to ingredients in cleansing wipes and topical treatments. It is defined by a well-demarcated, symmetric rash, which is more eczematous in nature. Cutaneous T-cell lymphoma was high in our differential given the patient's history of the disease. There are many variants, and tumor-stage disease may result in ulceration of the skin. Cutaneous T-cell lymphoma is differentiated by histology with immunophenotyping in conjunction with the clinical picture. Epstein-Barr virus (EBV) may cause genital ulcerations, which can be diagnosed with a positive EBV serology and detection of EBV by a polymerase chain reaction swab of the ulceration.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
- Schiffer JT, Corey L. New concepts in understanding genital herpes. Curr Infect Dis Rep. 2009;11:457-464.
- Vassantachart JM, Menter A. Recurrent lumbosacral herpes simplex. Proc (Bayl Univ Med Cent). 2016;29:48-49.
- Tata S, Johnston C, Huang ML, et al. Overlapping reactivations of HSV-2 in the genital and perianal mucosa. J Infect Dis. 2010;201:499-504.
- Tang IT, Shepp DH. Herpes simplex virus infection in cancer patients: prevention and treatment. Oncology (Williston Park). 1992;6:101-106.
- Jiang YC, Feng H, Lin YC, et al. New strategies against drug resistance to herpes simplex virus. Int J Oral Sci. 2016;8:1-6.
A 32-year-old woman with stage IV cutaneous T-cell lymphoma was admitted with pancytopenia and septic shock secondary to methicillin-susceptible Staphylococcus aureus bacteremia. Dermatology was consulted regarding sacral ulcerations. The lesions were asymptomatic and had been slowly enlarging over the course of 1 month. Physical examination revealed well-demarcated, pink, polycyclic ulcerations on the lower back and buttocks extending onto the perineum. There was no pain or tingling associated with the ulcerations. She denied a history of cold sore lesions on the lips or genitals. A skin biopsy was sent for tissue culture and histopathologic examination.
Infographic: Inpatient Dermatology Consultations
Secondary Syphilis Mimicking Molluscum Contagiosum in the Beard Area of an AIDS Patient
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
To the Editor:
A 46-year-old man with a history of AIDS (viral load, 28,186 copies/mL; CD4 count, 22 cells/μL) presented with a 40-lb weight loss over the last 6 months as well as dysphagia and a new-onset pruritic facial eruption of 1 week’s duration. The facial lesions quickly spread to involve the beard area and the upper neck. His medical history was notable for nicotine dependence, seborrheic dermatitis, molluscum contagiosum (MC), treated neurosyphilis and latent tuberculosis, hypertension, a liver mass suspected to be a hemangioma, and erythrocytosis. He was diagnosed with human immunodeficiency virus infection 19 years prior to presentation and was not compliant with the prescribed highly active antiretroviral therapy.
Skin examination revealed multiple discrete and coalescing, 2- to 12-mm, nonumbilicated, hyperkeratotic papules and nodules localized to the left and right beard areas (Figure 1A). A few discrete, 2- to 5-mm, umbilicated papules were noted in the right beard area (Figure 1B), as well as on the right side of the neck (Figure 1C), buttocks, and legs. Mild erythema with yellow-white scale was present in the alar creases. Examination of the oropharyngeal mucosa revealed multiple thick white plaques that were easily scraped off with a tongue depressor. Examination of the palms, soles, and anogenital areas was normal.
A punch biopsy of a nonumbilicated hyperkeratotic papule from the left beard area demonstrated spongiosis; neutrophilic microabscess formation; plasma cells; and a superficial and deep perivascular, predominantly lymphohistiocytic infiltrate (Figure 2A). Spirochete immunohistochemical staining of tissue highlighted abundant organisms in the dermoepidermal junction and vascular endothelial cells (Figure 2B). Other tissue stains for bacteria, including acid-fast bacilli, and fungi were negative. Bacterial culture of tissue from the lesion in the left beard area grew Staphylococcus aureus. Results of acid-fast and fungal cultures of tissue were negative. Shave biopsy of the umbilicated papule on the right side of the neck demonstrated classic invagination of the epidermis into the dermis and intracytoplasmic viral inclusions consistent with MC (Figure 2C). Spirochete immunohistochemical staining of the same tissue sample was negative (Figure 2D).
Serum rapid plasma reagin was reactive with a titer of 1:128 compared to the last known reactive rapid plasma reagin titer of 1:1 five years prior to presentation. A fluorescent treponemal antibody absorption test and VDRL test of cerebrospinal fluid was nonreactive. Fungal, bacterial, and acid-fast cultures of cerebral spinal fluid and a cryptococcal antigen test were negative. Serum cryptococcal antigen and coccidioides complement fixation tests were negative. Cytomegalovirus plasma polymerase chain reaction and urine histoplasma antigen testing were negative. Computed tomography of the chest revealed a new 1.9×1.6×2.1-cm3 cavitary lesion with distal tree-in-bud opacities in the lingula of the left lung. Acid-fast blood culture was negative, and acid-fast sputum culture was positive for Mycobacterium kansasii.
The cutaneous pathology findings and serologic findings confirmed the diagnoses of cutaneous secondary syphilis (SS) in the beard area and MC on the right side of the neck. Clinical diagnoses of seborrheic dermatitis of the alar creases and esophageal candidiasis also were made. The patient was treated with intramuscular penicillin G 2.4 million U once weekly for 3 weeks. The lesions confined to the beard area rapidly resolved within 7 days after the first dose of antibiotics, which further supported the diagnosis of localized cutaneous SS. Fluconazole 100 mg once daily was prescribed for the esophageal candidiasis, and he also was started on a regimen of rifampin 600 mg once daily, isoniazid 300 mg once daily, ethambutol 1200 mg once daily, and pyrazinamide 1500 mg once daily.
Syphilis is well known as the great masquerader due to its many possible manifestations. Many patients present with typical palmar and plantar dermatoses.1 Other documented SS presentations include eruptions ranging from a few to diffusely disseminated maculopapular lesions with or without scale on the trunk and upper extremities; pustular and nodular lesions of the face; alopecia; grayish white patches on the oral mucosa; and ulcerative, psoriasiform, follicular, and lichenoid lesions.2 Cutaneous SS has not been commonly reported in a localized distribution to the beard area with a clinical appearance mimicking hyperkeratotic MC lesions.3 Secondary syphilis is not known to spread through autoinoculation, presumably from shaving (as in our case), as might occur with other cutaneous infectious processes such as MC, verruca vulgaris, S aureus, and dermatophytosis in the beard area.
The differential diagnosis for hyperkeratotic papules and nodules localized to the beard area in human immunodeficiency virus–infected males includes MC, verruca vulgaris, chronic verrucous varicella-zoster virus, crusted scabies, tuberculosis verrucosa cutis, hypertrophic lichen planus, and disseminated deep fungal infections including cryptococcosis and coccidioidomycosis. In the setting of immunosuppression, the diagnosis of hyperkeratotic MC was favored in our patient given the co-location of classic umbilicated MC lesions with the hyperkeratotic papules and nodules. It is common to see MC autoinoculated in the beard area in men from shaving, as well as for MC to present in an atypical manner, particularly as hyperkeratotic lesions, in patients with AIDS.4 The predominant localized beard distribution and lack of other mucocutaneous manifestations of SS at presentation supported a clinical diagnosis of hyperkeratotic MC in our patient.
Unique presentations of SS have been documented, including nodular lesions of the face, but they typically have been accompanied by other stigmata of SS such as the classic palmoplantar or truncal maculopapular rash.3 One notable difference in our case was the localized beard distribution of the syphilitic cutaneous lesions in a man with AIDS. Our case reinforces the importance of cutaneous biopsies in immunocompromised patients. It is known that SS spreads hematogenously; however, in our case it was suspected that the new lesions may have spread locally through autoinoculation via beard hair removal, as the hyperkeratotic lesions were limited to the beard area. Koebnerization secondary to trauma induced by beard hair removal was considered in this case; however, koebnerization is known to occur in noninfectious dermatologic conditions, such as psoriasis, lichen planus, lichen nitidus, and vitiligo, but not in infections such as syphilis. Our case is pivotal in raising the question of whether SS can be autoinoculated in the beard area.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
- Baughn RE, Musher DM. Secondary syphilitic lesions. Clin Microbiol Rev. 2005;18:205-216.
- Dourmishev LA, Dourmishev AL. Syphilis: uncommon presentations in adults. Clin Dermatol. 2005;23:555-564.
- Cohen SE, Klausner JD, Engelman J, et al. Syphilis in the modern era: an update for physicians. Infect Dis Clin North Am. 2013;27:705-722.
- Filo-Rogulska M, Pindycka-Plaszcznska M, Januszewski K, et al. Disseminated atypical molluscum contagiosum as a presenting symptom of HIV infection. Postepy Dermatol Alergol. 2013;30:56-58.
Practice Points
- Recognize typical and atypical presentations of secondary syphilis (SS).
- This case reinforces the importance of cutaneous biopsies in immunocompromised patients.
- Consider the possibility of autoinoculation in SS.
Erythematous Plaques and Nodules on the Abdomen and Groin
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
The Diagnosis: Inflammatory Urothelial Carcinoma
Microscopic examination revealed metastatic carcinoma with extensive dermal lymphatic invasion (Figure). Immunohistochemical stains were positive for p63 and GATA3, markers for urothelial carcinomas, and negative for S-100 and Melan-A, markers for melanoma. Thus, the biopsy was compatible with a diagnosis of urothelial carcinoma. Gram and Grocott-Gomori methenamine-silver stains were negative for bacterial or fungal organisms. An additional 4-mm punch biopsy was performed of the left thigh at the distal-most aspect of the eruption to determine the extent of cutaneous metastasis. Pathology again showed metastatic urothelial carcinoma with extensive dermal lymphatic involvement and overlying epidermal spongiosis.
The patient had a history of bladder cancer diagnosed 1.5 years prior to presentation. It was a high-grade (World Health Organization) urothelial carcinoma that penetrated the bladder muscular wall, focally infiltrating into pericystic fat with multifocal seeding of pericystic lymphatics. It was unresponsive to bacillus Calmette-Guérin therapy. He underwent a cystoprostatectomy and bilateral staging lymph node dissection with clear surgical margins without adjuvant chemotherapy or radiation. He also reported a history of 2 prior cutaneous melanomas that were excised without sentinel lymph node biopsy.
Four months prior to presentation, he developed a mildly pruritic cutaneous eruption on the abdomen that was treated with topical miconazole for presumed tinea cruris without improvement. He also was previously diagnosed with candidiasis of his urostomy and was taking oral fluconazole. The patient was admitted for the abdominal pain and distension, and computed tomography of the abdomen and pelvis revealed peritoneal carcinomatosis resulting in mechanical small bowel obstruction as well as enlarged pelvic and retroperitoneal lymph nodes. Confirmation of metastatic disease via skin biopsy avoided an invasive peritoneal biopsy. He was treated with triamcinolone acetonide ointment 0.1% with moderate relief of pruritus, and a palliative percutaneous endoscopic gastrostomy tube was placed for bowel decompression. The patient's hospital course was complicated by Proteus mirabilis bacteremia requiring cefepime. He was transitioned to home hospice and died 1 month after presentation.
Inflammatory carcinoma, also called carcinoma erysipeloides, is a type of cutaneous metastasis most commonly seen in breast adenocarcinoma. Reported cases secondary to urothelial carcinoma are rare and most often involve the abdomen, groin, and lower extremities.1-5 Clinically, inflammatory carcinoma presents as erythematous indurated patches or plaques with well-defined borders, often with edema, warmth, and tenderness. Its morphologic appearance is due to the obstruction of lymphatic vessels by tumor cells and the release of inflammatory cytokines. Its presentation can mimic other dermatoses such as cellulitis, erysipelas, fungal infection, radiation dermatitis, Majocchi granuloma, or contact dermatitis.6 Cutaneous metastases may be the first clinical manifestations of metastatic disease, and they may occur due to hematogenous and lymphatic spread, direct contiguous tissue invasion, or iatrogenic implantation following surgical excision of the primary tumor. Histologically, nuclear markers GATA3 and p63 stain positively in urothelial carcinomas and are negative in prostatic adenocarcinomas.7,8 Other markers may be used such as cytokeratins 7 and 20, which are cytoplasmic epithelial markers that both stain positive in urothelial neoplasms.9
Inflammatory carcinoma may be treated with radiation or systemic chemotherapy depending on the extent of systemic involvement in the patient; however, its presence portends a poor prognosis. Less than 1% of genitourinary malignancies have cutaneous involvement, and median disease-specific survival is less than 6 months from presentation of the cutaneous metastasis.10 Clinicians faced with a recalcitrant inflammatory cutaneous eruption should maintain a high index of suspicion for cutaneous metastases, particularly in patients with a history of cancer. Early dermatology referral may help establish the diagnosis and guide disease-targeted therapy or goals of care discussions.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
- Grace SA, Livingood MR, Boyd AS. Metastatic urothelial carcinoma presenting as carcinoma erysipeloides. J Cutan Pathol. 2017;44:513-515.
- Zangrilli A, Saraceno R, Sarmati L, et al. Erysipeloid cutaneous metastasis from bladder carcinoma. Eur J Dermatol. 2007;17:534-536.
- Chang CP, Lee Y, Shih HJ. Unusual presentation of cutaneous metastasis from bladder urothelial carcinoma. Chin J Cancer Res. 2013;25:362-365.
- Aloi F, Solaroli C, Paradiso M, et al. Inflammatory type cutaneous metastasis of bladder neoplasm: erysipeloid carcinoma [in Italian]. Minerva Urol Nefrol. 1998;50:205-208.
- Alcaraz I, Cerroni L, Rutten A, et al. Cutaneous metastases from internal malignancies: a clinicopathologic and immunohistochemical review. Am J Dermatopathol. 2012;34:347-393.
- Al Ameer A, Imran M, Kaliyadan F, et al. Carcinoma erysipeloides as a presenting feature of breast carcinoma: a case report and brief review of literature. Indian Dermatol Online J. 2015;6:396-398.
- Chang A, Amin A, Gabrielson E, et al. Utility of GATA3 immunohistochemistry in differentiating urothelial carcinoma from prostate adenocarcinoma and squamous cell carcinomas of the uterine cervix, anus, and lung. Am J Surg Pathol. 2012;36:1472-1476.
- Ud Din N, Qureshi A, Mansoor S. Utility of p63 immunohistochemical stain in differentiating urothelial carcinomas from adenocarcinomas of prostate. Indian J Pathol Microbiol. 2011;54:59-62.
- Bassily NH, Vallorosi CJ, Akdas G, et al. Coordinate expression of cytokeratins 7 and 20 in prostate adenocarcinoma and bladder urothelial carcinoma. Am J Clin Pathol. 2000;113:383-388.
- Mueller TJ, Wu H, Greenberg RE, et al. Cutaneous metastases from genitourinary malignancies. Urology. 2004;63:1021-1026.
An 82-year-old man presented with acute abdominal pain and distension as well as an abdominal rash of 4 months' duration that was expanding despite treatment with topical miconazole. He had a history of melanoma and bladder cancer treated with cystoprostatectomy. He previously was diagnosed with candidiasis of his urostomy and was taking oral fluconazole. Physical examination revealed a large, well-demarcated, erythematous, smooth plaque covering the entire abdomen, scrotum, penis, inguinal folds, and bilateral upper thighs, with several satellite plaques and firm nodules clustered around the umbilicus. An 8-mm punch biopsy of a periumbilical nodule was performed.
Basal Cell Carcinoma Arising in Nevus Sebaceous During Pregnancy
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
To the Editor:
Nevus sebaceous of Jadassohn (or nevus sebaceous [NS]) is a congenital hamartomatous disorder initially described by Jadassohn1 in 1895. Nevus sebaceous occurs in 0.3% of newborns2 and is most commonly identified on the face and scalp.3,4 Mehregan and Pinkus5 characterized NS as an organoid tumor containing multiple skin components with 3 life stages. The first stage—occurring during infancy—consists of immature hair follicles and sebaceous glands. The second stage—beginning at puberty—shows development of sebaceous glands, epidermal hyperplasia, and maturation of apocrine glands. The final stage involves formation of secondary benign and malignant neoplasms.
Historically, basal cell carcinoma (BCC) was thought to be the most common neoplasm arising in NS.5-8 In 1993, Ackerman et al9 introduced a new definition of trichoblastoma (TB), expanding the definition to encompass previously excluded benign follicular neoplasms. Large studies conducted after this new definition was proposed suggested that syringocystadenoma papilliferum and TB develop more frequently than does BCC.3,4,10-15 Furthermore, Cribier et al4 and Merrot et al15 reviewed prior cases of NS using the new definition and asserted that the majority of previously diagnosed cases of BCC were considered to be TB under the new criteria. With the advent of modern diagnostic testing, the rate of secondary benign neoplasm growth is now thought to be between 7% and 19%, with syringocystadenoma papilliferum arising in 2% to 13% of cases and TB in 1.5% to 7%.3,4,10-14 Malignant neoplasms are observed much less frequently, with BCC arising in 0% to 1% of NS cases.
Nevus sebaceous lesions typically enlarge during puberty, while malignant neoplasms occur almost exclusively in adulthood,4,10-12 suggesting that hormones contribute to NS stage progression. We present the case of a woman who developed BCC in a previously asymptomatic NS during pregnancy.
A 32-year-old woman who was otherwise healthy presented to our dermatology clinic with a pink-yellow verrucous plaque on the right temporal hairline extending to the preauricular area of the face. The patient had no personal or family history of skin cancer and no history of tanning bed use. She reported that the lesion had been present since birth. A diagnosis of NS was made.
Two years later, she presented with a new bleeding growth atop the previously diagnosed NS that had been present for approximately 4 months (Figure). At this visit she was pregnant (30 weeks’ gestation). Physical examination revealed a 4-mm, brown, pearly papule at the inferior margin of the previously noted pink verrucous plaque on the right temporal hairline. A biopsy was performed and histopathology displayed aggregates of basaloid cells with a high nuclear to cytoplasmic ratio, peripheral palisading, and abundant melanin, consistent with pigmented BCC. The patient was referred for Mohs micrographic surgery; the lesion was removed with clear margins. The patient had no recurrence of BCC at 36-month follow-up.
Few studies have looked at the signal transduction pathways leading to malignant neoplasm formation in NS. Nevus sebaceous lesions are theorized to result from postzygotic genetic mutations in HRAS and KRAS oncogenes,16,17 which also are altered in squamous cell carcinoma and BCC.18 Similarly, Xin et al19 detected loss of heterozygosity of the human patched gene, PTCH, a tumor suppressor in the hedgehog pathway that has been implicated in sporadic BCC formation, suggesting that this loss of heterozygosity may predispose to secondary BCC formation.20,21 However, loss of PTCH heterozygosity could not be replicated by Takata et al22 and Levinsohn et al.16
Increased numbers of androgen receptors have been demonstrated in NS basal keratinocytes and sebaceous glands.23 Nevus sebaceous lesions enlarge during puberty,5 and malignant neoplasms arise almost exclusively in adulthood.3,4,10-13 The androgen surge during puberty and increased androgen levels in adulthood may promote sebaceous gland development and epidermal hyperplasia that result in progression of NS lesions from the first stage to the second stage. Basal cell carcinomas also express androgen receptors and have abnormal androgen hormone metabolism,24,25 though they do not display a notable number of estrogen or progesterone receptors.26 Therefore, increased androgen levels in adulthood also may contribute to progression to secondary neoplasm formation in the third stage.
Similarly, cases of rapid growth of NS lesions during pregnancy, a state of increased testosterone production,27 have
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
- Jadassohn J. Bemerkugen zur Histologie der systematisirten Naevi und uber “Talgdru˝sen-Naevi”. Arch Dermatol Syph. 1895;33:355-372.
- Alper J, Holmes LB, Mihm MC Jr. Birthmarks with serious medical significance: nevocullular nevi, sebaceous nevi, and multiple café au lait spots. J Pediatr. 1979;95:696-700.
- Muñoz-Pérez MA, García-Hernandez MJ, Ríos JJ, et al. Sebaceus naevi: a clinicopathologic study. J Eur Acad Dermatol Venereol. 2002;16:319-324.
- Cribier B, Scrivener Y, Grosshans E. Tumors arising in nevus sebaceus: a study of 596 cases. J Am Acad Dermatol. 2000;42:263-268.
- Mehregan AH, Pinkus H. Life history of organoid nevi. Special reference to nevus sebaceus of Jadassohn. Arch Dermatol. 1965;91:574-588.
- Jones EW, Heyl T. Naevus sebaceus. a report of 140 cases with special regard to the development of secondary malignant tumours. Br J Dermatol. 1970;82:99-117.
- Serpas de López RM, Hernández-Pérez E. Jadassohn’s sebaceous nevus. J Dermatol Surg Oncol. 1985;11:68-72.
- Smolin T, Hundeiker M. Squamous epithelial and basal cell carcinomas in naevus sebaceus (Jadassohn). Z Hautkr. 1986;61:267-282.
- Ackerman B, Reddy VB, Soyer HP. Neoplasms with Follicular Differentiation. New York, NY: Ardor Scribendi; 1993.
- Kaddu S, Schäppi H, Kerl H, et al. Trichoblastoma and sebaceoma in nevus sebaceus. Am J Dermatopathol. 1999;21:552-556.
- Idriss MH, Elston DM. Secondary neoplasms associated with nevus sebaceus of Jadassohn: a study of 707 cases. J Am Acad Dermatol. 2014;70:332-337.
- Hsu MC, Liau JY, Hong JL, et al. Secondary neoplasms arising from nevus sebaceus: a retrospective study of 450 cases in Taiwan. J Dermatol. 2016;43:175-180.
- Santibanez-Gallerani A, Marshall D, Duarte AM, et al. Should nevus sebaceus of Jadassohn in children be excised? a study of 757 cases, and literature review. J Craniofac Surg. 2003;14:658-660.
- Jaqueti G, Requena L, Sánchez Yus E. Trichoblastoma is the most common neoplasm developed in nevus sebaceus of Jadassohn: a clinicopathologic study of a series of 155 cases. Am J Dermatopathol. 2000;22:108-118.
- Merrot O, Cotten H, Patenotre P, et al. Sebaceous hamartoma of Jadassohn: trichoblastoma mimicking basal cell carcinoma? Ann Chir Plast Esthet. 2002;47:210-213.
- Levinsohn JL, Tian LC, Boyden LM, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827-830.
- Groesser L, Herschberger E, Ruetten A, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783-787.
- Pierceall WE, Goldberg LH, Tainsky MA, et al. Ras gene mutation and amplification in human nonmelanoma skin cancers. Mol Carcinog. 1991;4:196-202.
- Xin H, Matt D, Qin JZ, et al. The sebaceous nevus: a nevus with deletions of the PTCH gene. Cancer Res. 1999;59:1834-1836.
- Johnson RL, Rothman AL, Xie J, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668-1671.
- Gailani MR, Ståhle-Bäckdahl M, Leffell DJ, et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nat Genet. 1996;14:78-81.
- Takata M, Tojo M, Hatta N, et al. No evidence of deregulated patched-hedgehog signaling pathway in trichoblastomas and other tumors arising within nevus sebaceous. J Invest Dermatol. 2001;117:1666-1670.
- Hamilton KS, Johnson S, Smoller BR. The role of androgen receptors in the clinical course of nevus sebaceus of Jadassohn. Mod Pathol. 2001;14:539-542.
- Moretti G, Cardo P, Rampini E, et al. Testosterone metabolism in basal cell epitheliomas. J Invest Dermatol. 1978;71:361-362.
- Bayer-Garner IB, Givens V, Smoller B. Immunohistochemical staining for androgen receptors: a sensitive marker of sebaceous differentiation. Am J Dermatopathol. 1999;21:426-431.
- Rogers GS, Flowers JL, Pollack SV, et al. Determination of sex steroid receptor in human basal cell carcinoma. J Am Acad Dermatol. 1988;18:1039-1043.
- Bammann BL, Coulam CB, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137:293-298.
- Terenzi V, Indrizzi E, Buonaccorsi S, et al. Nevus sebaceus of Jadassohn. J Craniofac Surg. 2006;17:1234-1239.
- Moody MN, Landau JM, Goldberg LH. Nevus sebaceous revisited. Pediatr Dermatol. 2012;29:15-23.
- Lillis PJ, Ceilley RI. Multiple tumors arising in nevus sebaceus. Cutis. 1979;23:310-314.
- Chun K, Vázquez M, Sánchez JL. Nevus sebaceus: clinical outcomeand considerations for prophylactic excision. Int J Dermatol. 1995;34:538-541.
Practice Points
- Benign neoplasms arise more frequently in nevus sebaceous (NS) lesions than do malignant neoplasms.
- The hormonal changes that occur during pregnancy and puberty appear to play a role in the development of neoplasms in NS lesions.
- Monitoring NS lesions more closely during periods of hormonal change may help diagnose malignant transformations in these patients.
Multiple Keratoacanthomas Arising Within Red Tattoo Pigment
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
To the Editor:
Keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) are rapidly evolving neoplasms of the epithelium that often spontaneously regress but rarely metastasize.1,2 Keratoacanthomas are thought to ascend from the hair follicle,1 and they clinically present as an enlarging solitary crateriform nodule with a keratin-filled center. Multiple KAs are rare2; histologically, KAs can be difficult to distinguish from conventional SCCs and are frequently treated by standard surgical excision.1 Reactive KAs are a subtype of KA that are induced by trauma including UV exposure, electromagnetic radiation, surgical procedures, chemical peels, laser treatments, and rarely tattoos.3-5
A 56-year-old man presented to the clinic with 3 asymptomatic enlarging papulonodules within a multicolored tattoo along the right forearm and elbow of 5 months’ duration (Figure 1). The lesions developed 1 month after the tattoo was placed and were localized to the areas of red pigment. The patient had several other tattoos. Histologic examination of the lesions revealed a well-differentiated squamous neoplasm with a crateriform invagination consistent with the superficial portion of a KA (Figures 2A–C). The specimen also revealed exogenous red pigment that was consistent with the background tattoo (Figure 2D). The patient underwent excisions of all 3 KAs, and free surgical margins were obtained.
Tattooing is a popular practice dating back to 3000
Cipollaro10 reported the first case of a KA in a tattoo in 1973. Although there have been reports of melanoma and basal cell carcinoma occurring within tattoos, KAs and conventional SCCs are the most common cutaneous neoplasms arising in tattoos.
The pathogenesis underlying the development of malignancies in tattoos is unclear. It has been hypothesized that trauma from tattooing may play a role given the temporal relationship between tattoo placement and malignancy development.11 Another theory is that tattoo pigment causes a chronic inflammatory foreign body reaction that triggers carcinogenesis.12 Lastly, it has been postulated that tattoo pigment may alter UV light absorption in the skin that could potentially impact mutagenesis.11
The most common treatment of KAs is standard surgical excision.4 Mohs micrographic surgery is an option if the KA is located in a cosmetically sensitive area. Although there are no reports of recurrence after excision of tattoo-related KAs, new KAs forming adjacent to a previously excised KA have been reported.13
Currently, tattoos are not regulated by the US Food and Drug Administration before going to market. Although many states regulate the practice of tattooing, few regulate the contents of tattoo ink, and ink is only investigated when safety issues arise.14 This case provides further evidence of an association between KAs, tattooing, and potentially carcinogenic pigments, especially in red dye, supporting the need for further research on the safety of pigment components and more regulation of tattoo ink.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
- Schwartz RA. Keratoacanthoma: a clinico-pathologic enigma. Dermatol Surg. 2004;30:326-333.
- Kwiek B, Schwartz RA. Keratoacanthoma (KA): an update and review. J Am Acad Dermatol. 2016;74:1220-1233.
- McGrouther DA, Downie PA, Thompson WD. Reactions to red tattoos. Br J Plas Surg. 1977;30:84-85.
- Sowden JM, Byrne JP, Smith AG, et al. Red tattoo reactions: x-ray microanalysis and patch-test studies. Br J Dermatol. 1991;124:576-580.
- Wiener DA, Scher RK. Basal cell carcinoma arising in a tattoo. Cutis. 1987;39:125-126.
- Pesapane F, Nazzaro G, Gianotti R, et al. A short history of tattoo. JAMA Dermatol. 2014;150:145.
- Junqueira AL, Wanat, KA, Farah RS. Squamous neoplasms arising within tattoos: clinical presentation, histopathology and management. Clin Exp Dermatol. 2017;42:601-606.
- Tammaro A, Toniolo C, Giulianelli V, et al. Chemical research on red pigments after adverse reactions to tattoo. Eur Ann Allergy Clin Immunol. 2016;48:46-48.
- Forbat E, Al-Niaimi F. Patterns of reactions to red pigment tattoo and treatment methods. Dermatol Therapy (Heidelb). 2016;6:13-23.
- Cipollaro VA. Keratoacanthoma developing in a tattoo. Cutis. 1973;11:809.
- Kluger N, Koljonen V. Tattoos, inks, and cancer. Lancet Oncol. 2012;13:E161-E168.
- Müller KM, Schmitz I, Hupe-Nörenberg L. Reaction patterns to cutaneous particulate and ornamental tattoos. Pathologe. 2002;23:46-53.
- Maxim E, Higgins H, D’Souza L. A case of multiple squamous cell carcinomas arising from red tattoo pigment. Int J Womens Dermatol. 2017;3:228-230.
- MacDonald J. Why doesn’t the FDA regulate tattoo ink? JSTOR Daily. September 21, 2017. https://daily.jstor.org/why-doesnt-the-fda-regulate-tattoo-ink/. Accessed October 15, 2019.
Practice Points
- Tattoo reactions range from infectious and inflammatory dermatoses to the development of malignant neoplasms.
- Red pigment is the most common cause of adverse tattoo reactions.
- The management of tattoo-associated keratoacanthoma (KA)–type squamous cell carcinomas (SCCs) has not been widely published, but they can be approached similarly to nontattoo-associated KA-SCCs.