User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Pulmonary Hemorrhage as the Initial Presentation of AIDS-Related Kaposi Sarcoma
To the Editor:
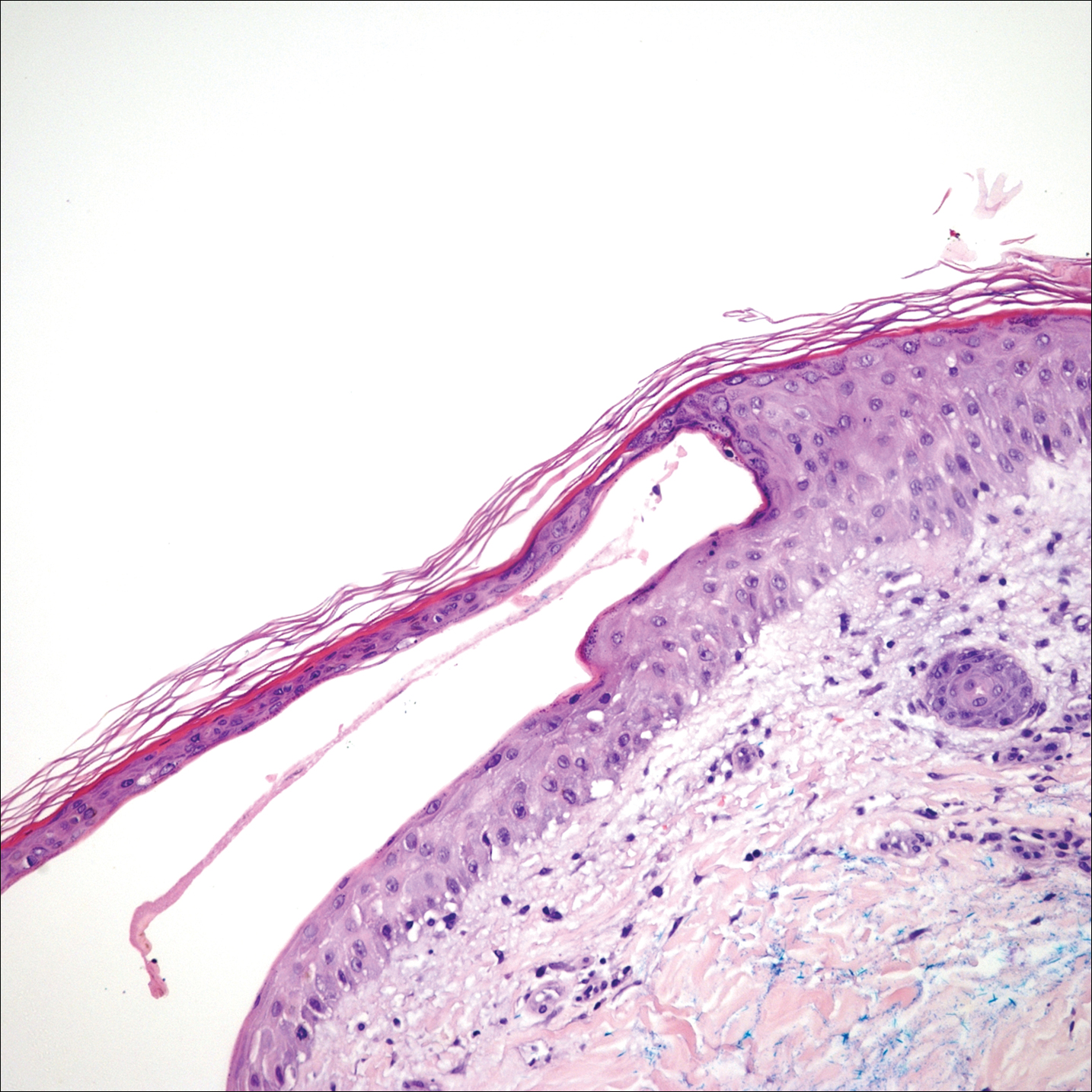
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
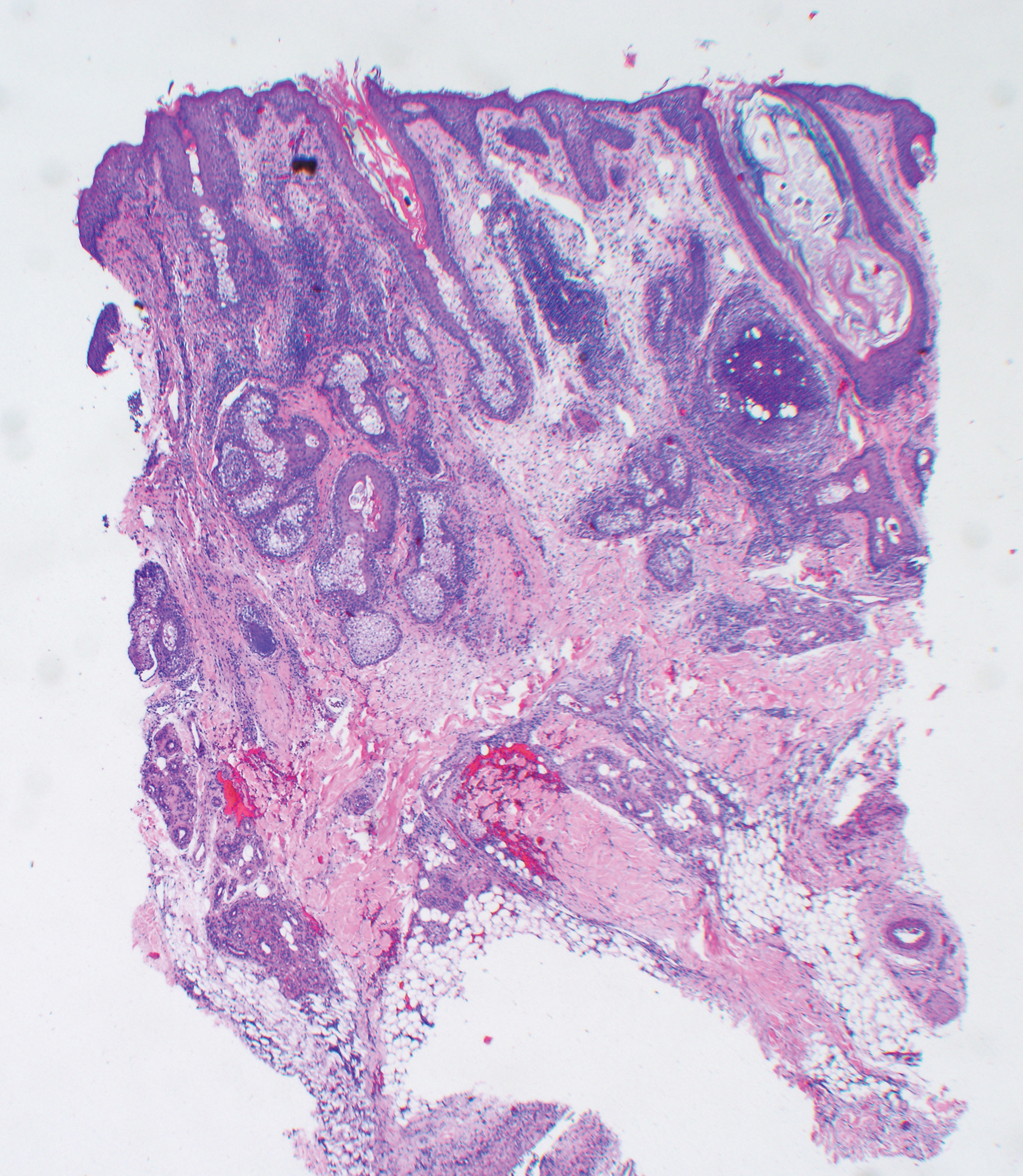
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
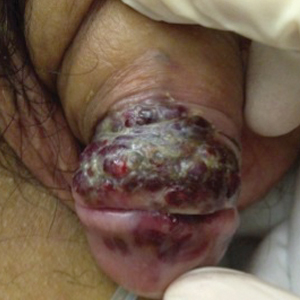
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.
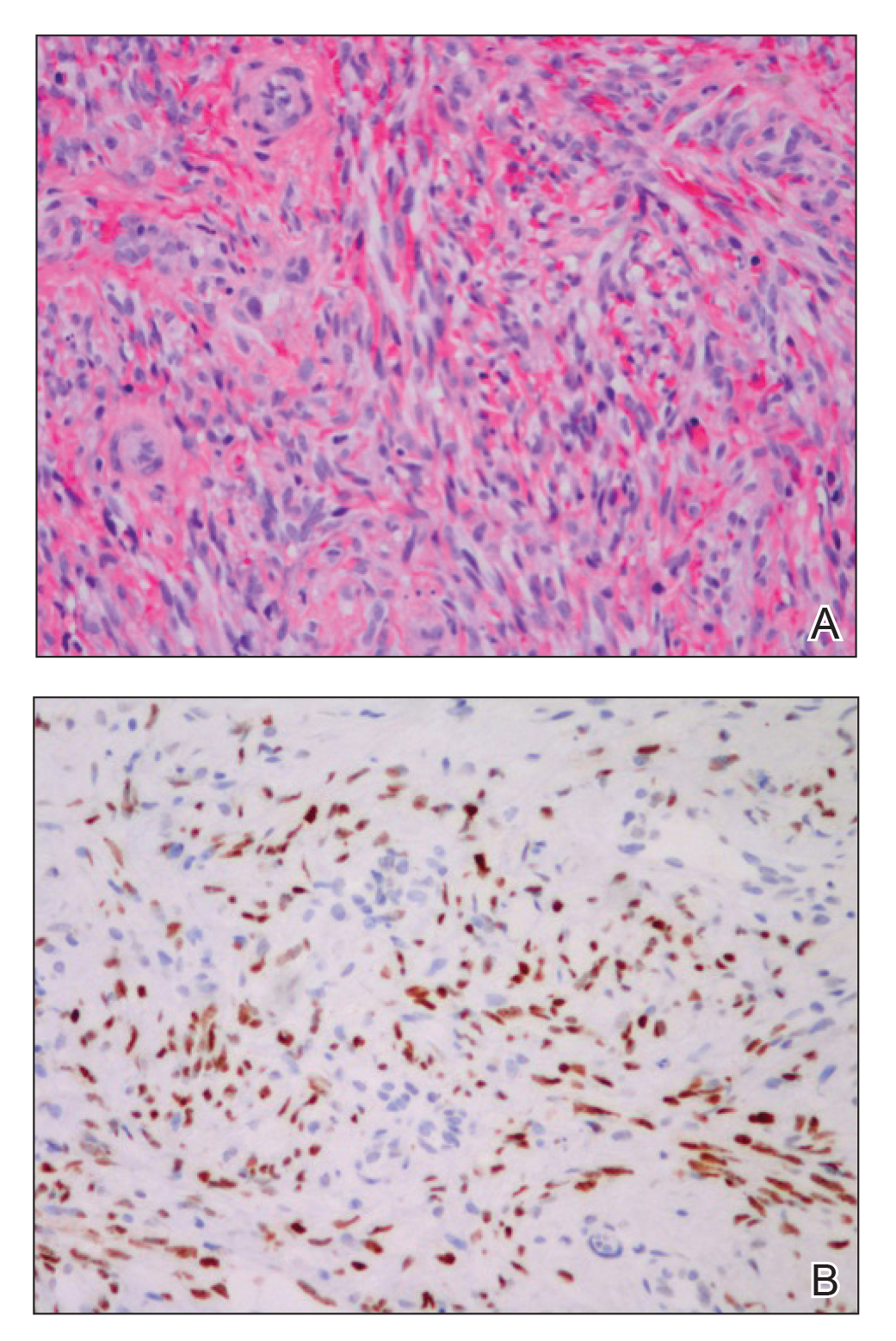
Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.
This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.
Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.
This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
To the Editor:
Kaposi sarcoma (KS) is an angioproliferative tumor of endothelial origin associated with human herpesvirus 8 infection. It is one of the most prevalent opportunistic infections associated with AIDS and is considered an AIDS-defining illness. In the general population, the incidence of KS is 1 in 100,000 worldwide.1 At the onset of the human immunodeficiency virus (HIV) epidemic in the early 1980s, 25% of individuals with AIDS were found to have KS at the time of AIDS diagnosis. Beginning in the mid-1980s and early 1990s with the introduction of highly active antiretroviral therapy (HAART), the incidence of KS declined to 2% to 4%,2 likely secondary to restoration of immune response.3
The clinical course of KS ranges from benign to severe, involving both cutaneous and visceral forms of disease. Cutaneous KS is the most common form of disease and typically characterizes the initial presentation. It is classically described as violaceous patches, papules, or plaques that can become confluent, forming larger tumors over time. Biopsy of cutaneous lesions may vary based on the clinical morphology. The patch stage typically is characterized by abnormal proliferating vessels surrounding larger ectatic vessels.4 Vascular spaces are more jagged and lined by thin endothelial cells extending into the dermis, forming the classic promontory sign.5 In the plaque stage, the vascular infiltrate becomes more diffuse, involving the dermis and subcutis, and there is proliferation of spindle cells.4 In the nodular stage, spindle-shaped tumor cells form fascicles and vascular spaces become more dilated.4,5 Advanced lesions are further associated with hyaline globules staining positive with periodic acid–Schiff.4 Lymphocytes, plasma cells, and hemosiderin-laden macrophages are admixed within this pathologic architecture.4,5
Visceral KS most commonly occurs in the oropharynx, respiratory tract, and gastrointestinal tract, and rarely is the initial presentation of disease. Classically, visceral KS is an aggressive, potentially life-threatening form of disease and has been found to have a much worse prognosis than cutaneous KS alone. Pulmonary involvement is the second most common site of extracutaneous KS and is known as the most severely life-threatening form of disease.1 Interestingly, since the advent of HAART, the incidence of KS with involvement of the visceral organs has declined at a more dramatic rate than cutaneous KS alone.3 Therefore, although more aggressive in nature, KS with visceral features has become increasingly rare and should be largely preventable given advances in AIDS therapy. We present a case of advanced AIDS-related KS with pulmonary involvement that is rarely seen after the advent of HAART.
A 39-year-old man with HIV diagnosed 8 years prior presented with fever, chest pain, progressive dyspnea, and hemoptysis of 5 months’ duration. At the time, he was nonadherent to medications and had poor follow-up with primary care physicians. At presentation he was tachycardic (149 beats per minute), tachypneic (26 breaths per minute), and his oxygen saturation was 80% on room air. Physical examination of the skin revealed asymptomatic violaceous penile lesions that the patient reported had been present for the last 8 months (Figure 1). Pertinent laboratory values included an HIV-1 viral load of 480,135 copies/mL (reference range, <20 copies/mL) and CD4 count of 14 cells/mm3 (reference range, 480–1700 cells/mm3). A chest radiograph was obtained and revealed bibasilar opacities compatible with a pleural and/or parenchymal process. Bronchoscopy was then performed and revealed bloody secretions throughout the tracheobronchial tree.
Histologic examination of biopsies of the penile lesions revealed spindle cell proliferation with hemorrhage (Figure 2A) that stained positively for HHV-8 (Figure 2B), consistent with KS. Biopsies taken during bronchoscopy similarly revealed spindle cells with hemorrhage (Figure 3). The patient was diagnosed with AIDS-related KS with visceral involvement of the lung parenchyma and tracheobronchial tree. The patient was then admitted to the medical intensive care unit and intubated. Therapy with HAART and paclitaxel was initiated. After 7 days of poor response to therapy, the family opted for terminal extubation and comfort care measures. The patient died hours later.
This case report describes the classic phenomenon of AIDS-related KS in a patient with a long-standing history of immunocompromise. Even in the era of HAART, this patient developed a severe form of visceral KS with involvement of the respiratory tract and lung parenchyma.
Since the advent of HAART for the treatment of HIV/AIDS, the incidence of KS, both visceral and cutaneous forms, has dramatically declined; the risk for visceral KS declined by more than 50% but less than 30% for cutaneous KS, supporting the observation that although visceral involvement has classically been noted as the more aggressive and life-threatening form of disease, HAART appears to have a stronger effect on visceral disease than cutaneous disease.3 Although the overall impact of AIDS-defining illnesses has substantially improved over the years, those with AIDS infection remain at risk for opportunistic illness.2
It has been shown that HAART therapy leads to response in more than 50% of cases of KS.5 The administration of HAART in KS patients is associated with improved survival and an 80% reduced risk of death, even when started after KS is diagnosed.6 In a comparison of the differences in clinical manifestations of KS between patients who were already receiving HAART at the time of KS diagnosis to those who were not on HAART, it was shown that patients already on therapy presented with less aggressive clinical features. A smaller percentage of patients who were already on HAART at KS diagnosis presented with visceral disease compared to those who were not on therapy.7
It is evident that treatment of AIDS patients with HAART is not only first-line therapy for the disease but also the best preventative measure against development of KS. Management of KS also centers around the initiation of HAART if the patient is not already maintained on the proper therapy.8 In addition to HAART, treatment options for visceral KS include a variety of chemotherapeutic agents, including but not limited to the use of single-agent adriamycin, vinblastine, paclitaxel, and thalidomide, or combination therapies.
Although notable advances have been made in the management of AIDS patients, this case highlights the need for clinicians to be aware of the risk for KS in the context of immunocompromise. Specifically, patients with advanced AIDS who are not adherent to HAART or who have a poor response to therapy have an amplified risk for developing KS in general as well as an increased risk for developing more severe visceral KS. Maintenance of patients with HAART is shown to greatly reduce the risk for both cutaneous and visceral KS; therefore, patient adherence with therapy is of utmost importance in preventing the occurrence of this deadly disease and its complications. Appropriate follow-up should be made, ensuring that these patients at high risk are adherent to therapy and have proper access to medical care to allow for prevention and early identification of potential complications.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
- La Ferla L, Pinzone MR, Nunnari G, et al. Kaposi’s sarcoma in HIV-positive patients: the state of art in the HARRT-era. Eur Rev Med Pharmacol Sci. 2013;17:2354-2365.
- Engels EA, Pfeiffer RM, Goedert JJ, et al; HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980-2002. AIDS. 2006;20:1645-1654.
- Grabar S, Abraham B, Mahamat A, et al. Differential impact of combination antiretroviral therapy in preventing Kaposi’s sarcoma with and without visceral involvement. JCO. 2006;24:3408-3414.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma [published online July 25, 2008]. Diagn Pathol. 2008;3:31.
- Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
- Tam HK, Zhang ZF, Jacobson LP, et al. Effect of highly active antiretroviral therapy on survival among HIV-infected men with Kaposi sarcoma or non-Hodgkin lymphoma. Int J Cancer. 2002;98:916-922.
- Nasti G, Martellotta F, Berretta M, et al. Impact of highly active antiretroviral therapy on the presenting features and outcome of patients with acquired immunodeficiency syndrome-related Kaposi sarcoma. Cancer. 2003;98:2440-2446.
- Dupont C, Vasseur E, Beauchet A, et al. Long-term efficacy on Kaposi’s sarcoma of highly active antriretroviral therapy in a cohort of HIV-positive patients. AIDS. 2000;14:987-993.
Practice Points
- Visceral Kaposi sarcoma (KS) should be considered in patients with unexplained systemic symptoms in the setting of poorly controlled human immunodeficiency virus (HIV).
- If cutaneous KS is diagnosed in an HIV patient, a detailed history and physical examination should be undertaken to evaluate for signs of systemic disease.
Crusted Demodicosis in an Immunocompetent Patient
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
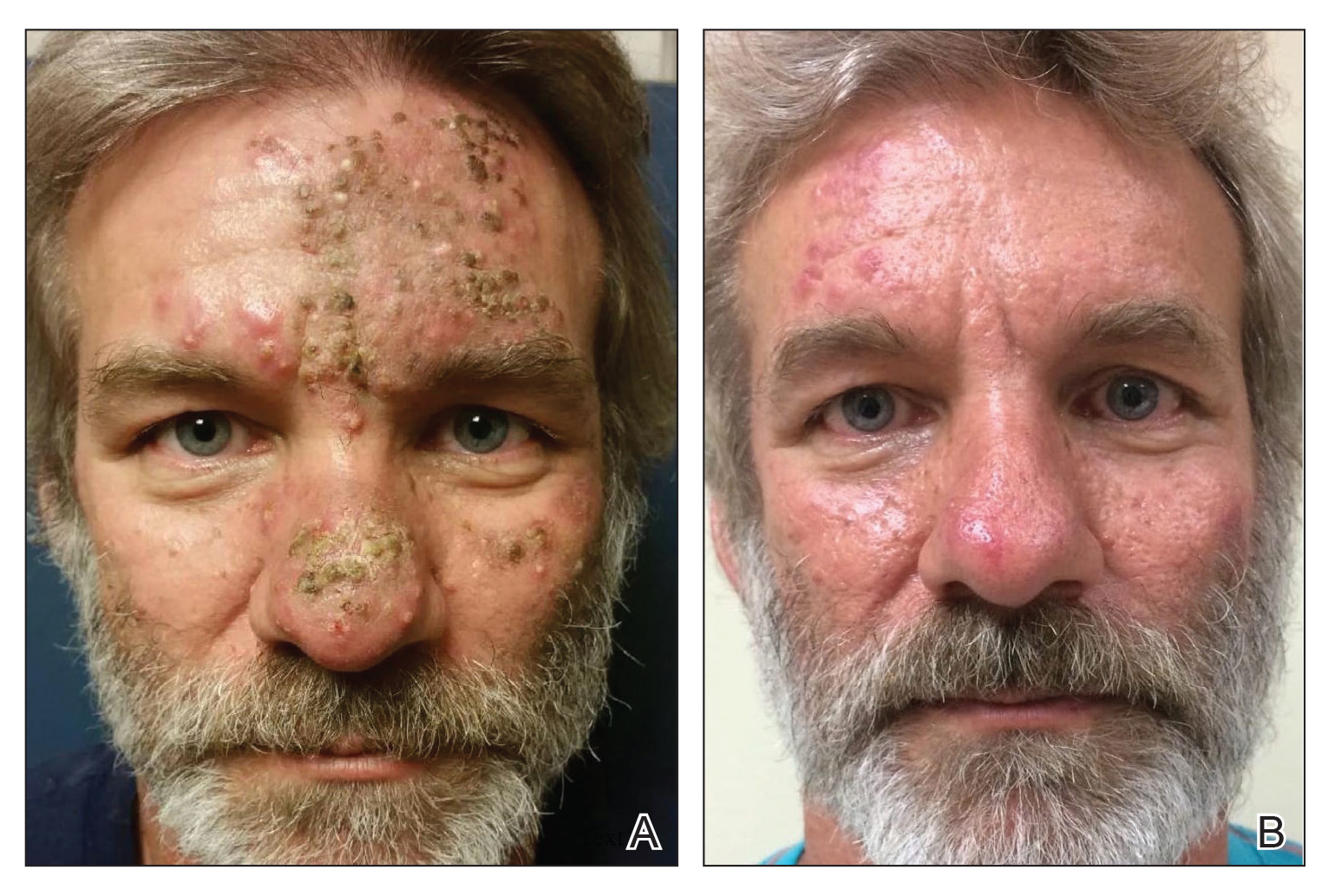
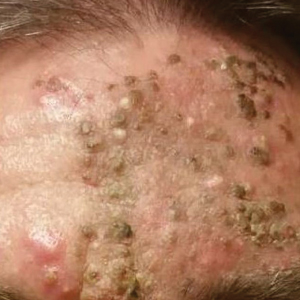
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
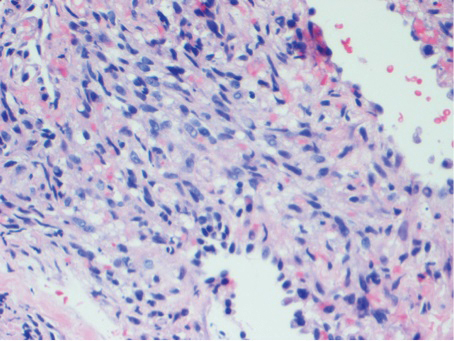
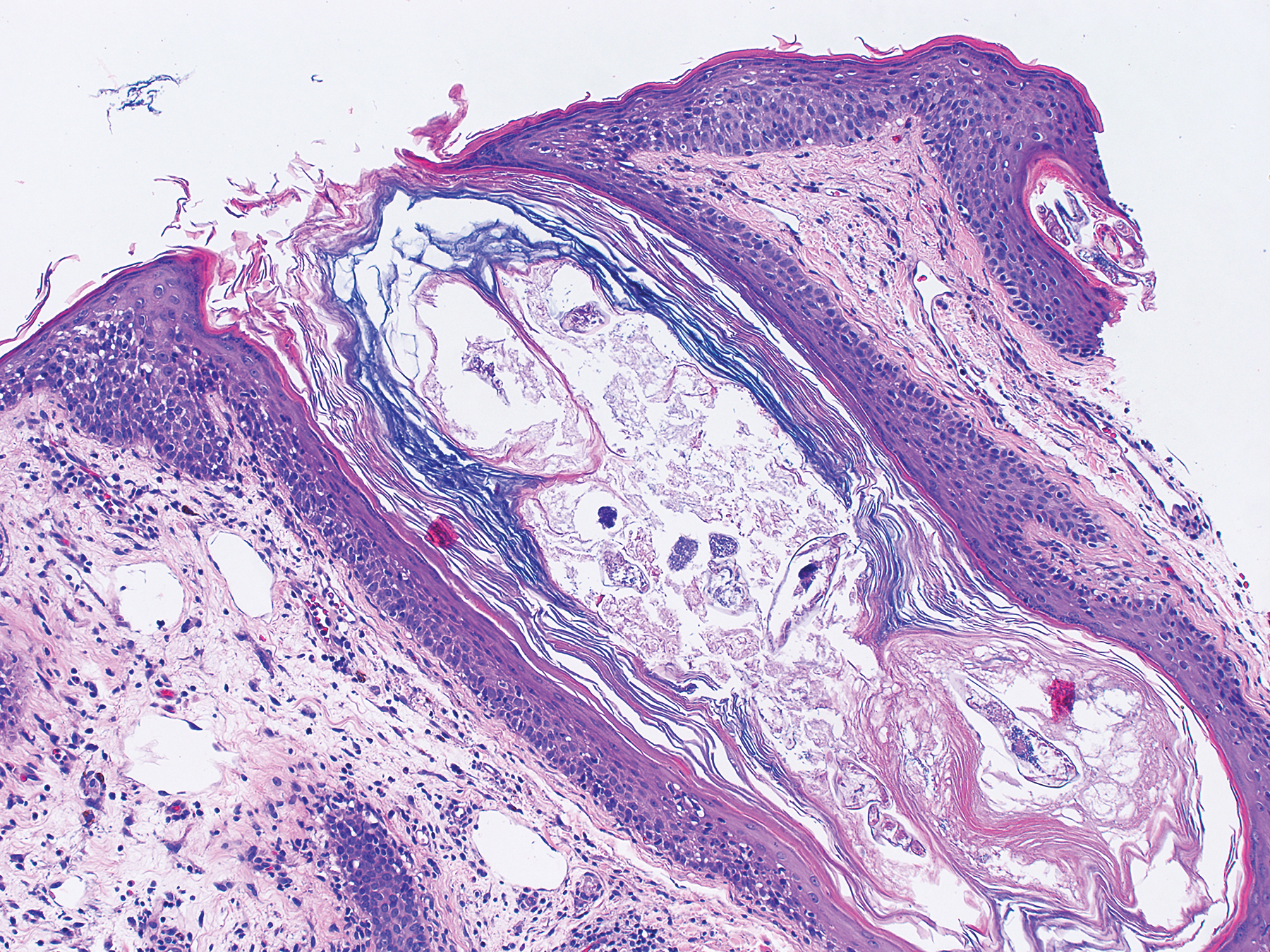
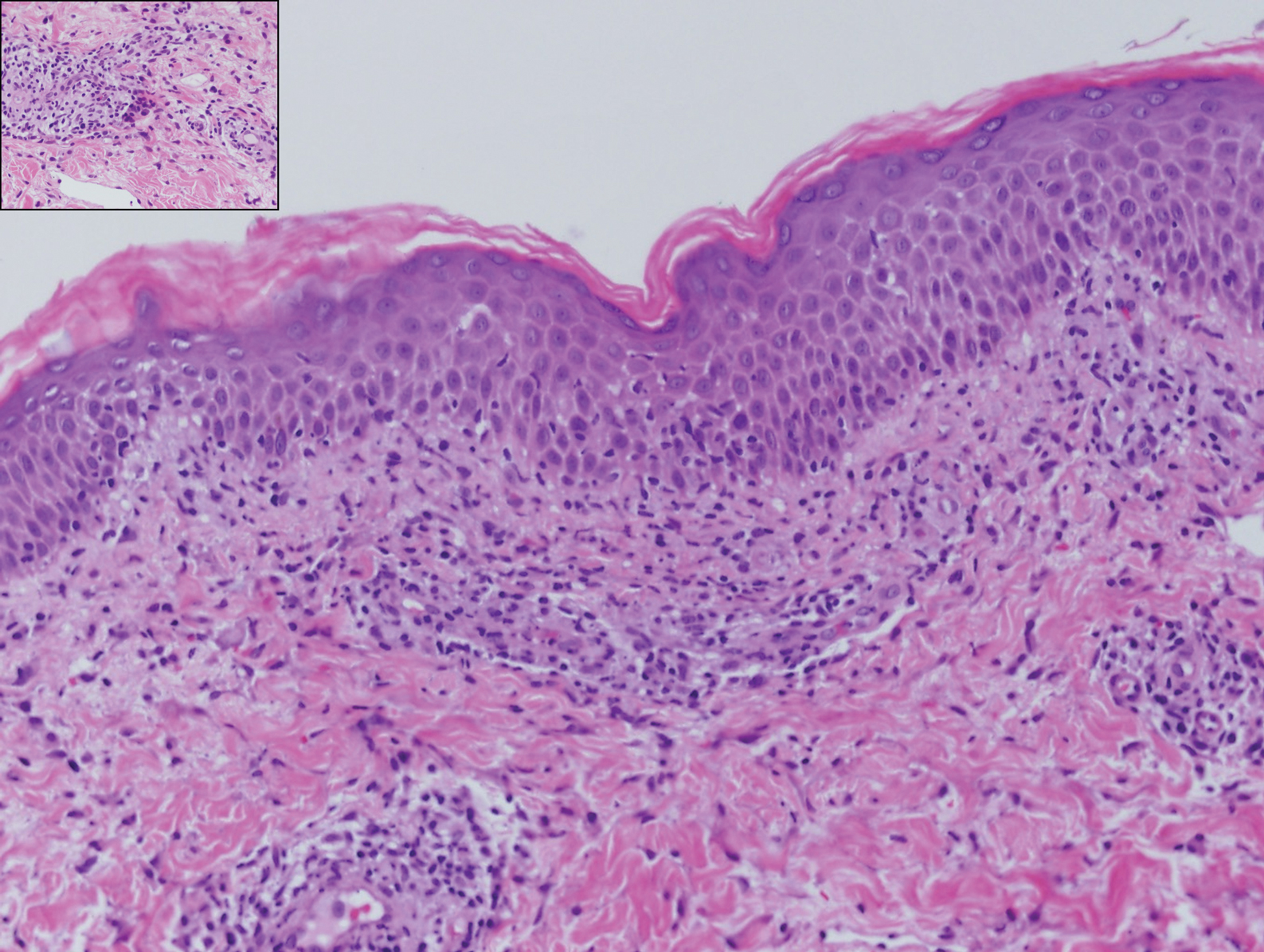
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
To the Editor:
Demodicosis is an infection of humans caused by species of the genus of saprophytic mites Demodex (most commonly Demodex brevis and Demodex folliculorum) that feed on the pilosebaceous unit.1Demodex mites are believed to be a commensal species in humans; an increase in mite concentration or mite penetration of the dermis, however, can cause a shift from a commensal to a pathologic form.2 Demodicosis manifests in a variety of forms, including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis. The likelihood of colonization increases with age; the mite rarely is observed in children but is found at a rate approaching 100% in the elderly population.3 It is hypothesized that manifestation of disease might be due to a decrease in immune function or an inherited HLA antigen that causes local immunosuppression.4
A 51-year-old man who was otherwise healthy presented to our clinic with a crusting rash on the face of 9 weeks’ duration. The rash began a few days after he demolished a rotting wooden shed in his backyard. Lesions began as pustules on the left cheek, which then developed notable crusting over the next 5 to 7 days and spread to involve the forehead, nose, and right cheek (Figure 1A).
The patient had no underlying immunosuppressive disease; a human immunodeficiency virus screen, complete blood cell count, and tests of hepatic function were all unremarkable. He denied a history of frequent or recurrent sinopulmonary infections, skin infections, or infectious diarrheal illnesses. He had been seen by his primary care physician who had treated him for herpes zoster without improvement.
At our initial evaluation, biopsy was performed; specimens were sent for histopathologic analysis and culture. Findings included a dermal neutrophilic inflammation, a dense perivascular and perifollicular lymphoplasmacytic infiltrate with foci of neutrophilic pustules within the follicles (Figure 2), numerous intrafollicular Demodex mites (Figure 3), perifollicular vague noncaseating granuloma, and mild sebaceous hyperplasia. Grocott methenamine-silver stain and acid-fast bacilli stain were negative.
Review of clinical and pathological data yielded a final diagnosis of crusted demodicosis with a background of rosacea. The patient was ultimately treated with a single dose of oral ivermectin 15 mg with a second dose 7 days later in addition to daily application of ivermectin cream 1% to affected areas of his rash. He had notable improvement with this regimen, with complete resolution within 6 weeks (Figure 1B). The patient noted mild recurrence 14 to 21 days after discontinuing topical ivermectin.
The 2 species of Demodex that cause disease in humans each behave distinctively: D folliculorum, with a cigar-shaped body, favors superficial hair follicles; D brevis, a smaller form, burrows deeper into skin where it feeds on the pilosebaceous unit.1 Colonization occurs through direct skin-skin contact that begins as early as infancy and becomes more common with age due to development of sebaceous glands, the main source of nourishment for the mites.2
Demodicosis is classified as primary and secondary. In a prospective study of patients with clinical findings of demodicosis, Akilov et al1 discovered that the 2 forms can be differentiated by skin distribution, seasonality, mite species, and preexisting dermatoses. Primary demodicosis is categorized by sudden onset of symptoms on healthy skin, usually the face. Secondary demodicosis develops progressively in patients with preexisting skin disease, such as rosacea, and can have a broader distribution, involving the face and trunk.2 Clinical manifestations of demodicosis are broad and include pruritic papulopustular, nodulocystic, crusted, and abscesslike lesions.5
Most cases of demodicosis reported in the literature are associated with either local or systemic immunosuppression.6-8 In a case report, an otherwise immunocompetent child developed facial demodicosis after local immunosuppression from chronic use of 2 topical steroid agents.9
Demodex infestation can be diagnosed using a variety of methods, including standardized skin surface biopsy, punch biopsy, and potassium hydroxide analysis. Standardized skin surface biopsy is the preferred method to diagnose demodicosis because it is noninvasive and samples the superficial follicle where Demodex mites typically reside. Diagnosis is made by identifying 5 or more Demodex mites in a low-power field or more than 5 mites per square centimeter in standardized skin surface biopsy.2 Other potential diagnostic tools reported in the literature include dermoscopy and confocal laser scanning microscopy.10,11
There is no standard therapeutic regimen for demodicosis because evidence-based trials regarding the efficacy of treatments are lacking. Oral ivermectin 200 µg/kg in a single dose is considered the preferred treatment; it can be combined with oral erythromycin, topical permethrin, or topical metronidazole.5-7,9
Our case is unique, as crusted demodicosis developed in an immunocompetent adult. Demodicosis usually causes severe eruptions in immunocompromised persons, with only 1 case report detailing a papulopustular rash in an immunocompetent adult.12,13
The pathogenesis of demodicosis remains unclear. Many mechanisms have been hypothesized to play a role in its pathogenesis, including mechanical obstruction of hair follicles, hypersensitivity reaction to Demodex mites, immune dysregulation, and a foreign-body granulomatous reaction to the skeleton of the mite.2,3 Our patient’s particular infestation could have been caused by an exuberant reaction to Demodex; however, it is likely that many factors played a role in his disease process to cause an increase in mite density and subsequent manifestations of disease.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
- Akilov OE, Butov YS, Mumcuoglu KY. A clinico-pathological approach to the classification of human demodicosis. J Dtsch Dermatol Ges. 2005;3:607-614.
- Karincaoglu Y, Bayram N, Aycan O, et al. The clinical importance of Demodex folliculorum presenting with nonspecific facial signs and symptoms. J Dermatol. 2004;31:618-626.
- Baima B, Sticherling M. Demodicidosis revisited. Acta Derm Venereol. 2002;82:3-6.
- Noy ML, Hughes S, Bunker CB. Another face of demodicosis. Clin Exp Dermatol. 2016;41:958-959.
- Chen W, Plewig G. Human demodicosis: revisit and a proposed classification. Br J Dermatol. 2014;170:1219-1225.
- Morrás PG, Santos SP, Imedio IL, et al. Rosacea-like demodicidosis in an immunocompromised child. Pediatr Dermatol. 2003;20:28-30.
- Damian D, Rogers M. Demodex infestation in a child with leukaemia: treatment with ivermectin and permethrin. Int J Dermatol. 2003;42:724-726.
- Clyti E, Nacher M, Sainte-Marie D, et al. Ivermectin treatment of three cases of demodecidosis during human immunodeficiency virus infection. Int J Dermatol. 2006;45:1066-1068.
- Guerrero-González GA, Herz-Ruelas ME, Gómez-Flores M, et al. Crusted demodicosis in an immunocompetent pediatric patient. Case Rep Dermatol Med. 2014;2014:458046.
- Friedman P, Sabban EC, Cabo H. Usefulness of dermoscopy in the diagnosis and monitoring treatment of demodicidosis. Dermatol Pract Concept. 2017;7:35-38.
- Harmelin Y, Delaunay P, Erfan N, et al. Interest of confocal laser scanning microscopy for the diagnosis and treatment monitoring of demodicosis. J Eur Acad Dermatol Venereol. 2014;28:255-257.
- Elston CA, Elston DM. Demodex mites. Clin Dermatol. 2014;32:739-743.
- Kaur T, Jindal N, Bansal R, et al. Facial demodicidosis: a diagnostic challenge. Indian J Dermatol. 2012;57:72-73.
Practice Points
- The Demodex mite, believed to be a commensal species in humans, has the ability to shift to a pathologic form in immunocompromised patients.
- Demodicosis can manifest in a variety of forms including pityriasis folliculorum, rosacealike demodicosis, and demodicosis gravis.
Discoloration and Bullous Lesions on the Hands
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
The Diagnosis: Irritant Contact Dermatitis and Hyperpigmentation Due to Juglone
Clinical suspicion, resemblance to similar cases, and questioning the patient about his behavior prior to the onset of symptoms led to the diagnosis of irritant contact dermatitis and hyperpigmentation due to juglone in this case. Walnuts belong to the botanical family of Juglandaceae and are the seed of the trees of the genus Juglans, which encompass 24 different species. The nuts from all species included in this genus are edible.1 The most well-known species of walnut is the common walnut (Juglans regia), which is native to the Balkans region in southeast Europe, southwest and central Asia extending to the Himalayas, and southwest China.1
Walnut fruits are rich in phenolic compounds. Thirteen phenolic compounds have been identified in walnut husks including chlorogenic acid, caffeic acid, ferulic acid, sinapic acid, gallic acid, ellagic acid, protocatechuic acid, syringic acid, vanillic acid, catechin, epicatechin, myricetin, and juglone.2 Juglone, also called 5-hydroxy-1,4-napthoquinone, is a yellow naphthoquinone pigment that occurs naturally in the leaves, roots, husks, and bark of plants in the Juglandaceae family, particularly the black walnut (Juglans nigra).3,4
Juglans regia, also known as English or Persian walnut, contains potent chemical constituents and has been used to treat diverse ailments such as diarrhea, hyperglycemia, cancer, infectious diseases, anorexia, asthma, helminthiasis, arthritis, sinusitis, stomachache, and skin disorders (eg, eczema; acne; alopecia; scalp itching, peeling, and dandruff), and as an adjunctive emollient and itch-relieving treatment.5,6
The juice of walnut shells from the J regia tree have been used for centuries to color the skin and hair.7 Irritation and skin hyperpigmentation have been associated with topical walnut use.5 As a naphthoquinone, juglone also is reported to exert some toxic effects on normal tissues including acute irritant contact dermatitis.4 As the active ingredient from the green husk of walnuts, it has been considered a strong sensitizer in guinea pigs,1 but contact sensitivity in humans rarely has been reported.7
Juglone is known to react with the keratin proteins present in the skin to form sclerojuglonic compounds, which have UV protection properties and a red-brown color.8 The resulting reaction gives rise to chromophore groups with a strong pigmenting action that absorbs visible colors (especially violet) and reflects yellow and red, resulting in the coloration ranging from red to deep brown.7 The mechanism of skin pigmentation does not involve the melanocytes. Hyperchromia involving the hands--particularly the palms, fingers, and nails--lasts 1 to 4 weeks depending on the intensity of the pigmentation. Housewives and agricultural workers are the at-risk population.7 Acute irritant contact dermatitis and hyperpigmentation due to juglone mainly has been observed during the early autumn in agricultural workers and housewives who remove the green husk of walnuts.9
Addison disease can present with pigmentary changes in the skin and mucous membranes; it also is accompanied by fatigue, anorexia, weakness, and weight loss, none of which were noted in our patient. A fixed drug eruption tends to have an annular or oval form and is related to the intake of medication (mostly antibiotics) up to 2 weeks prior to the onset of the dermatosis. Our patient did not have any chronic disease or take any medication prior to the dermatosis and lacked the classic clinical morphology of this entity. Hemochromatosis affects not only the skin but also the liver, myocardial fibers, and other internal organs. Our patient did not have any clinical manifestations of liver or heart failure or diabetes mellitus.
Our patient was treated with drainage of the blisters. Due to the extent of the dermatosis, prednisone 25 mg/d also was initiated. The patient was instructed to avoid direct contact with the husk of walnuts. At 1-month follow-up, the hyperpigmentation had resolved with no relapse (Figure).
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
- Costa J, Carrapatoso I, Oliveira MB, et al. Walnut allergens: molecular characterization, detection and clinical relevance. Clin Exp Allergy. 2013;44:319-341.
- Cosmulescu S, Trandafir I, Achim G, et al. Phenolics of green husk in mature walnut fruits. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2010;38:53-56.
- Cosmulescu S, Trandafir I, Achim G, et al. Juglone content in leaf and green husk of five walnut (Juglans regia L.) cultivars. Notulae Botanicae Horti Agrobotanici Cluj-Napoca. 2011;39:237-240.
- Aithal BK, Sunil Kumar MR, Rao BN, et al. Evaluation of pharmacokinetic, biodistribution, pharmacodynamic, and toxicity profile of free juglone and its sterically stabilized liposomes. J Pharm Sci. 2011;100:3517-3528.
- Panth N, Paudel KR, Karki R. Phytochemical profile and biological activity of Juglans regia. J Integr Med. 2016;14:359-373.
- Aburjai T, Natsheh FM. Plants used in cosmetics. Phytother Res. 2003;17:987-1000.
- Bonamonte D, Foti C, Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermatitis. 2001;44:101-102.
- Dweck AC. Natural ingredients for colouring and styling. Int J Cosmet Sci. 2002;24:287-302.
- Neri I, Bianchi F, Giacomini F, et al. Acute irritant contact dermatitis due to Juglans regia. Contact Dermatitis. 2006;55:62-63.
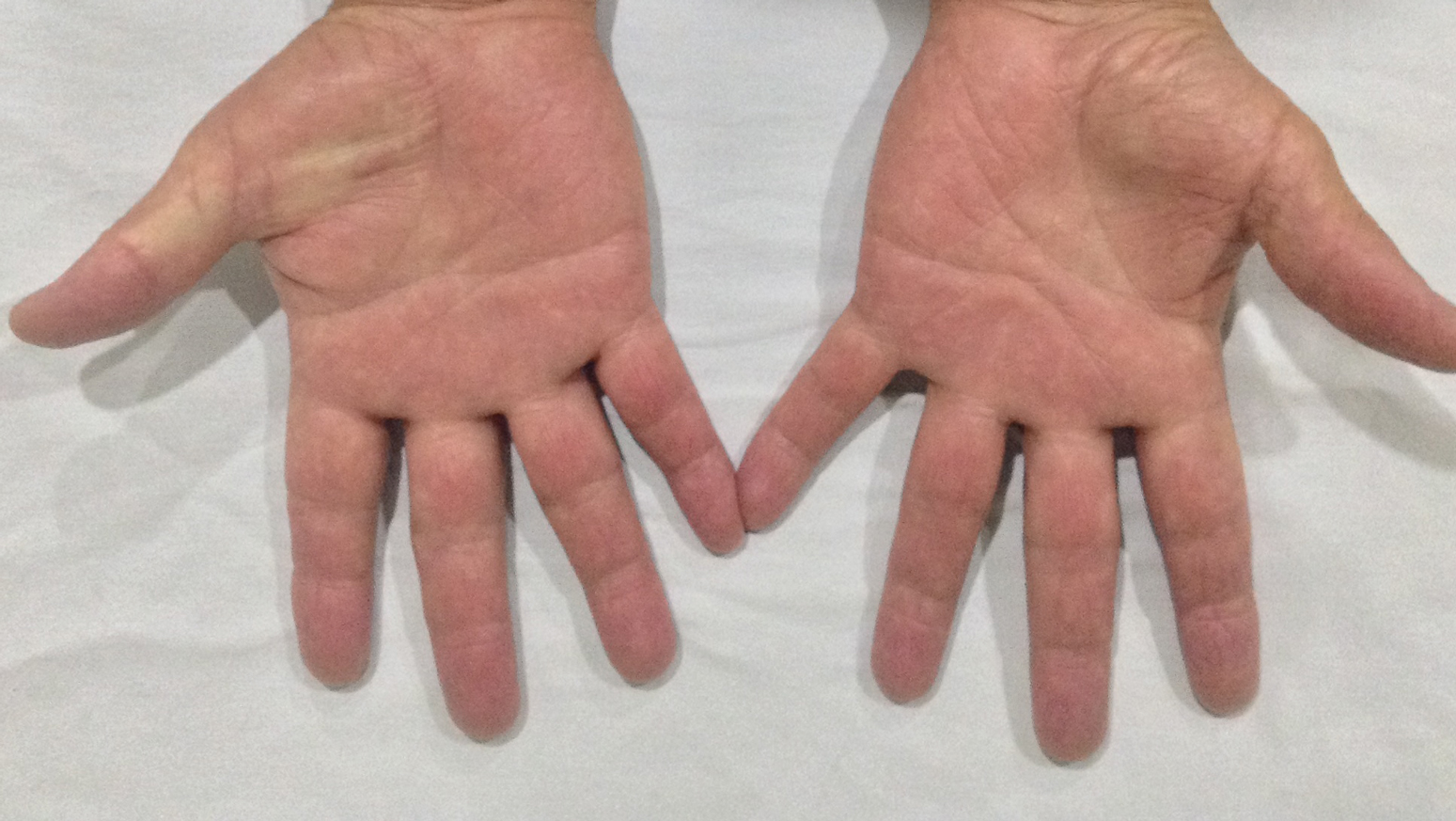
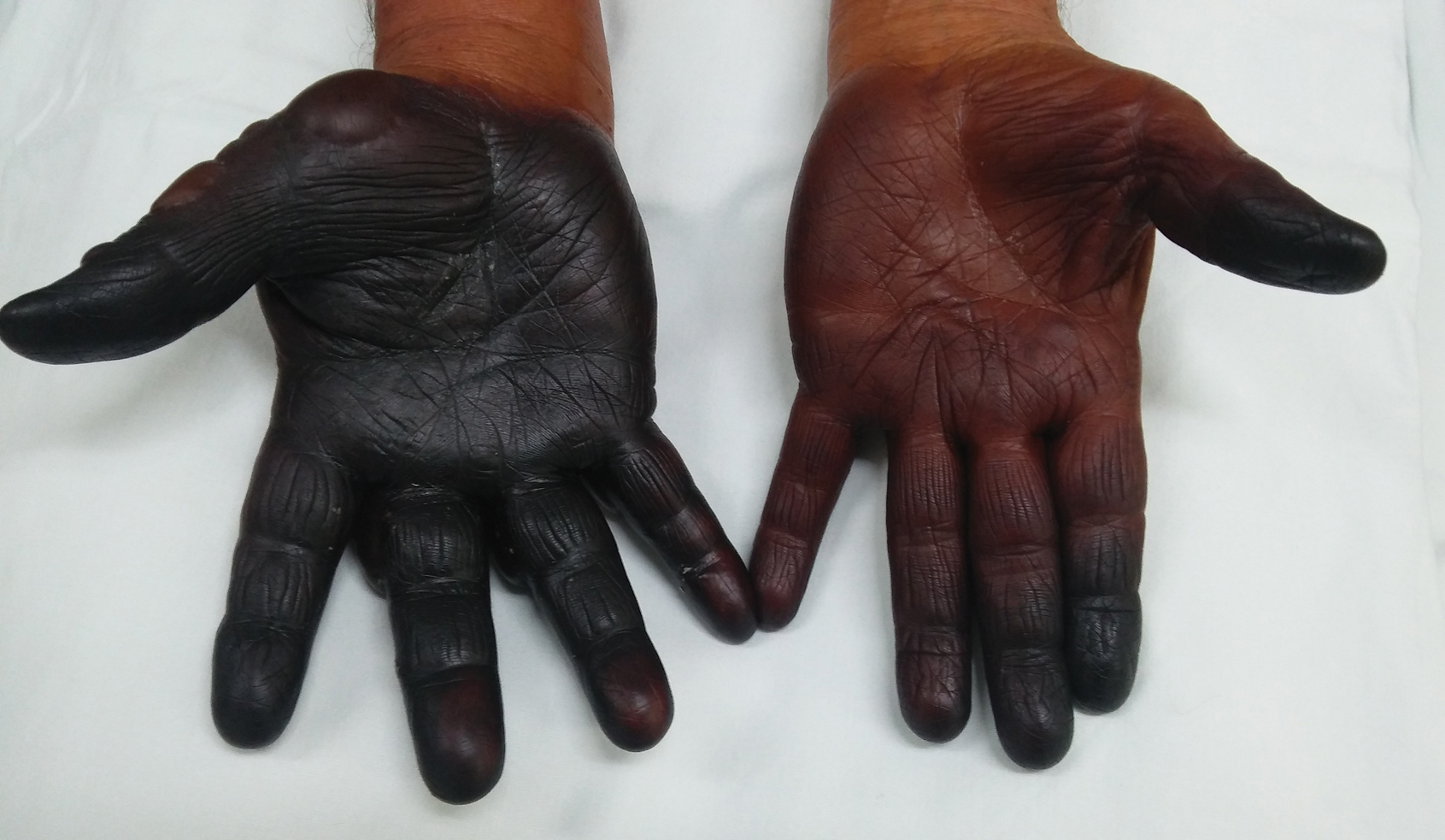
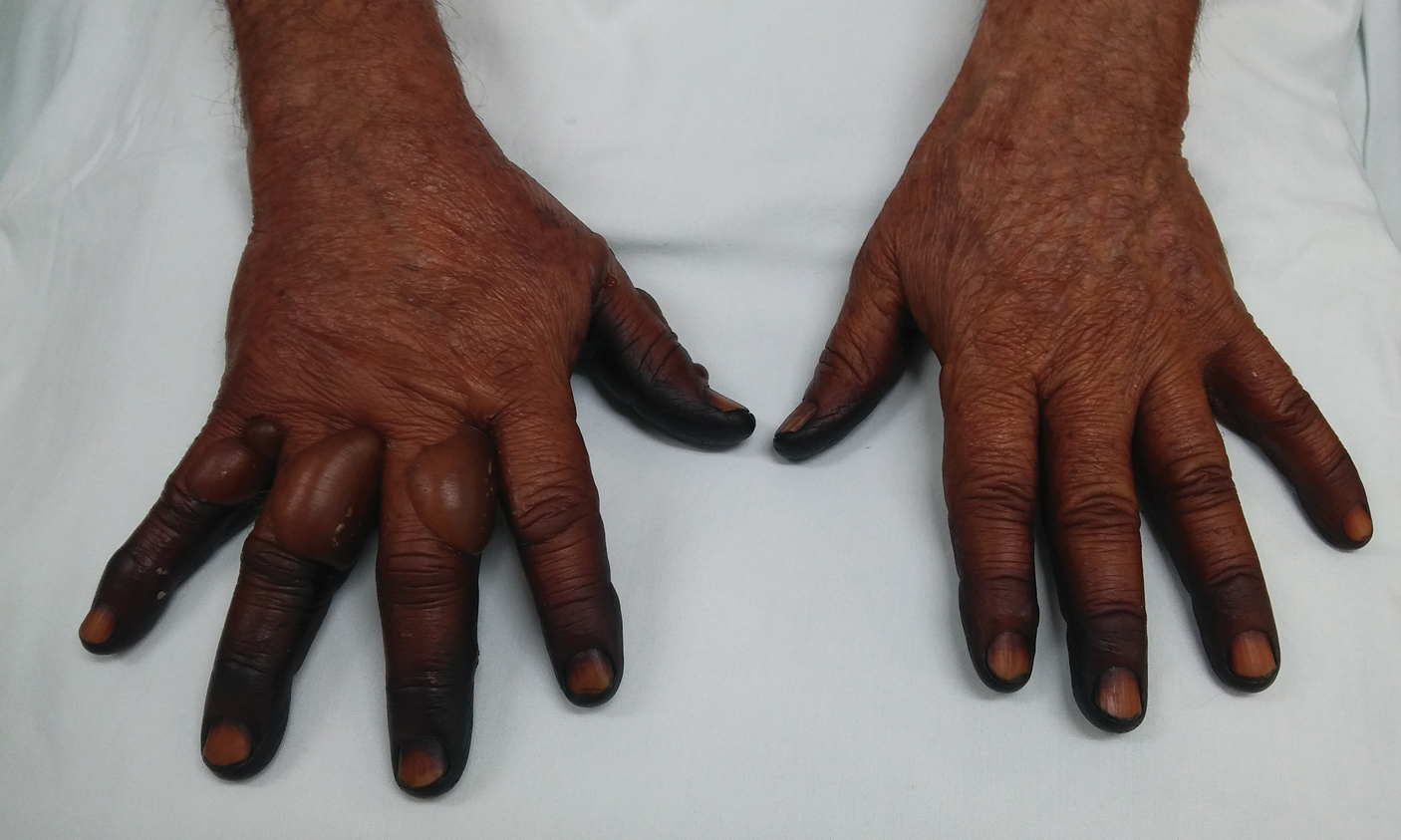
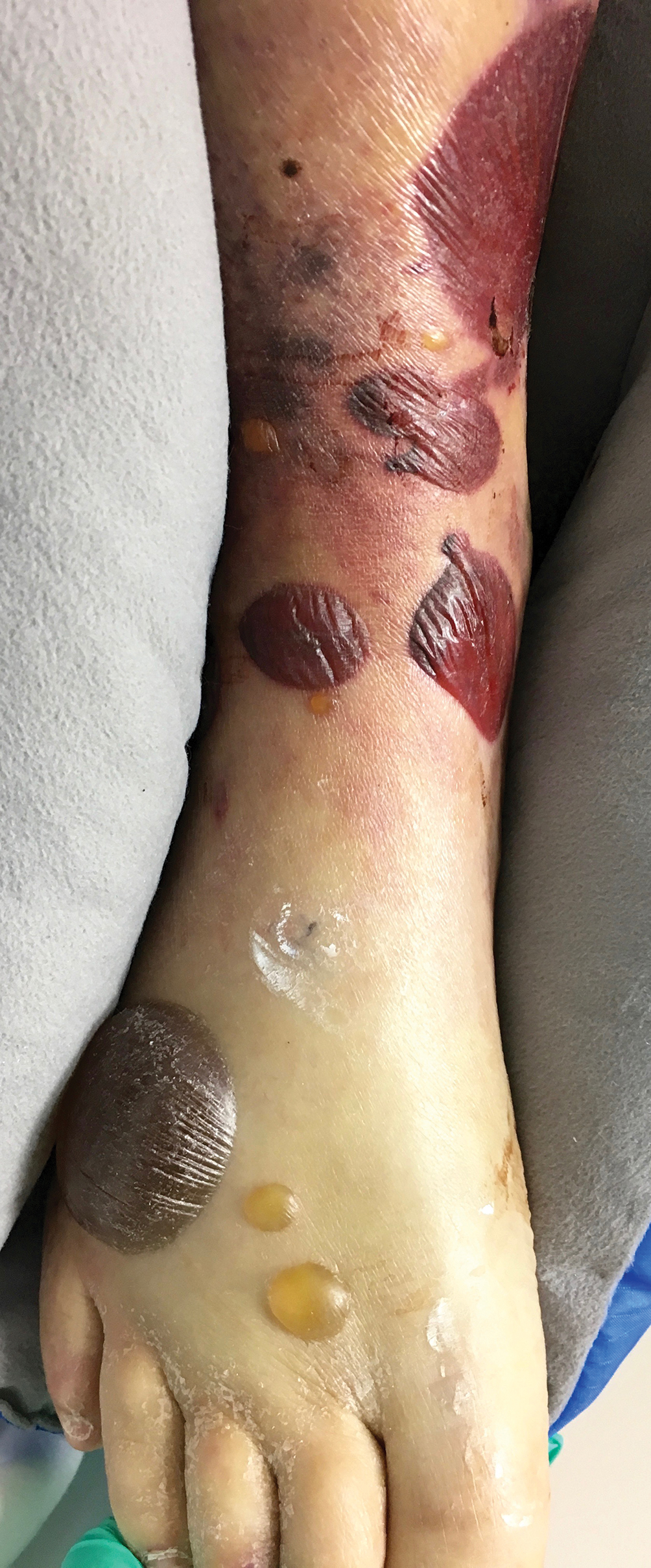
A 71-year-old man presented for evaluation of discoloration and blisters of 1 day's duration on both hands that were more severe on the right hand. The lesions were preceded by a sensation of stinging pain. One hour prior to the onset of symptoms, he had peeled approximately 100 walnuts. He had no relevant medical history. Physical examination revealed dark brown to black discoloration involving both hands (top) extending to the fingernails. Blisters filled with clear fluid also were present on the fingers (bottom).
Role of Psoriasis in the Development of Merkel Cell Carcinoma
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
1. O’Brien T, Power DG. Metastatic Merkel-cell carcinoma: the dawn of a new era. BMJ Case Rep. 2018;11:2018. doi:10.1136/bcr-2018-224924.
2. Del Marmol V, Lebbé C. New perspectives in Merkel cell carcinoma. Curr Opin Oncol. 2019;31:72-83.
3. Garcia-Carbonero R, Marquez-Rodas I, de la Cruz-Merino L, et al. Recent therapeutic advances and change in treatment paradigm of patients with Merkel cell carcinoma [published online April 8, 2019]. Oncologist. doi:10.1634/theoncologist.2018-0718.
4. Samimi M, Gardair C, Nicol JT, et al. Merkel cell polyomavirus in Merkel cell carcinoma: clinical and therapeutic perspectives. Semin Oncol. 2015;42:347-358.
5. Kitamura N, Tomita R, Yamamoto M, et al. Complete remission of Merkel cell carcinoma on the upper lip treated with radiation monotherapy and a literature review of Japanese cases. World J Surg Oncol. 2015;13:152.
6. Timmer FC, Klop WM, Relyveld GN, et al. Merkel cell carcinoma of the head and neck: emphasizing the risk of undertreatment. Eur Arch Otorhinolaryngol. 2016;273:1243-1252.
7. Açıkalın A, Paydas¸ S, Güleç ÜK, et al. A unique case of Merkel cell carcinoma with ovarian metastasis. Balkan Med J. 2014;31:356-359.
8. Yousif J, Yousif B, Kuriata MA. Complete remission of metastatic Merkel cell carcinoma in a patient with severe psoriasis. Cutis. 2018;101:E24-E27.
9. Grandhaye M, Teixeira PG, Henrot P, et al. Focus on Merkel cell carcinoma: diagnosis and staging. Skeletal Radiol. 2015;44:777-786.
10. Chatzinasiou F, Papadavid E, Korkolopoulou P, et al. An unusual case of diffuse Merkel cell carcinoma successfully treated with low dose radiotherapy. Dermatol Ther. 2015;28:282-286.
11. Pang C, Sharma D, Sankar T. Spontaneous regression of Merkel cell carcinoma: a case report and review of the literature. Int J Surg Case Rep. 2015;7C:104-108.
12. National Comprehensive Cancer Network. Merkel cell carcinoma. Published October 3, 2016. http://merkelcell.org/wp-content/uploads/2015/10/MccNccn.pdf. Accessed September 10, 2019.
13. Coggshall K, Tello TL, North JP, Yu SS. Merkel cell carcinoma: an update and review: pathogenesis, diagnosis, and staging. J Am Acad Dermatol. 2018;78:433-442.
14. Lanoy E, Engels EA. Skin cancers associated with autoimmune conditions among elderly adults. Br J Cancer. 2010;103:112-114.
15. Mertz KD, Junt T, Schmid M, et al. Inflammatory monocytes are a reservoir for Merkel cell polyomavirus. J Invest Dermatol. 2009;130:1146-1151.
Atopic Dermatitis Affects Sleep and Work Productivity
Read the full Cutis article, “Quality of Life in Patients With Atopic Dermatitis.”
Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
Read the full Cutis article, “Quality of Life in Patients With Atopic Dermatitis.”
Read the full Cutis article, “Quality of Life in Patients With Atopic Dermatitis.”
Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
Koszorú K, Borza J, Gulácsi L, et al. Quality of life in patients with atopic dermatitis. Cutis. 2019;104:174-177.
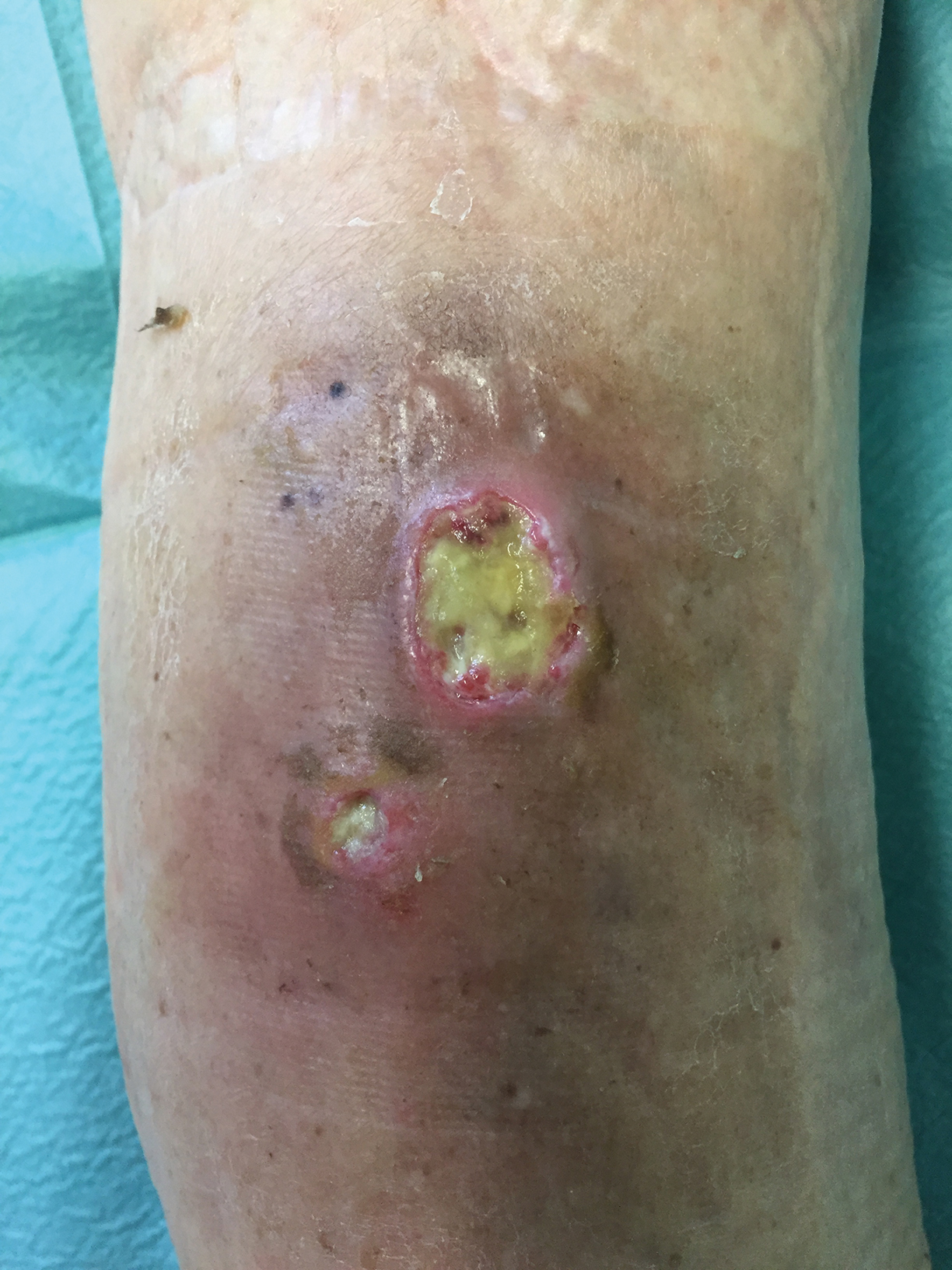
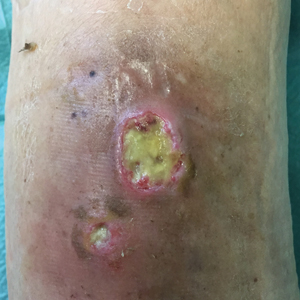
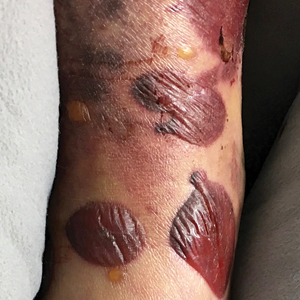
Painless Round Ulcers on the Leg
The Diagnosis: Cutaneous Tuberculosis
The patient's medical history was notable for bone tuberculosis (TB) treated in childhood. Skin biopsy revealed neutrophilic infiltrates with necrosis without granulomas. A real-time polymerase chain reaction test detected Mycobacterium tuberculosis complex in the skin fragment, which was confirmed by culture of the biopsy specimen using a liquid growth medium that grew M tuberculosis. Tuberculotic foci were not present on the lungs, gastrointestinal tract, kidneys, and bones by radiologic, microbiologic, and ultrasonographic investigations. The patient was started on 4 antituberculotic drugs--isoniazid 300 mg, rifampicin 600 mg, ethambutol 1200 mg, pyrazinamide 1500 mg--once daily for 2 months followed by isoniazid 300 mg and rifampicin 600 mg once daily for another 4 months with resolution of the skin lesions.
Cutaneous TB is an infectious disease caused by M tuberculosis and accounts for only 1.5% of extrapulmonary TB cases.1,2 Similar to other forms of TB, a resurgence of cutaneous TB has been noted in parts of the world where human immunodeficiency virus infection is prevalent and remains to be one of the most elusive and more difficult diseases to diagnose.3 Thought to be a predominantly occupational disease, it is being encountered more frequently in healthy individuals where the source of infection remains unidentified in most cases.4 The clinical types depend on the method of infection, virulence of the bacillus, immune status of the host, and presence or absence of host sensitization to M tuberculosis.2 The route of infection is used to classify cutaneous mycobacteriosis.5 Inoculation from an exogenous source can produce TB verrucosa cutis in individuals who have previously been sensitized to M tuberculosis or tuberculous chancre in individuals without prior exposure to the bacterium.4 Cutaneous TB resulting from direct spread to the skin from an underlying contiguous structure in most cases spreads from lymph nodes and bone (scrofuloderma). Immunosuppressed patients with advanced TB of the lung, gastrointestinal tract, or the genitourinary tract may present with periorificial TB.4 Dissemination to the skin caused by hematogenous spread can occur in the form of lupus vulgaris, miliary TB, or metastatic tuberculous abscesses (gummas).4,5 A fourth category--cutaneous TB from paradoxical expansion--also was proposed. Paradoxical expansion is defined as the transient expansion of a preexisting lesion or the appearance of new lesions during appropriate anti-TB therapy.5
Although histopathology and protein chain reaction tests are useful, the gold standard for diagnosis is still the isolation of M tuberculosis on culture.3,6 Treatment regimens of cutaneous TB are similar to those of pulmonary TB, with a 4-agent regimen given for 2 months followed by a 2-drug regimen for the next 4 months.1,7 The differential diagnosis of leg ulcers includes stasis ulcer, necrobiotic xanthogranuloma, pyoderma gangrenosum, and squamous cell carcinoma, among others. Cutaneous biopsy, microbiological culture, and a high degree of suspicion are fundamental for the final diagnosis. Cutaneous TB should be suspected in immunocompetent as well as in immunosuppressed patients who present with ulcerated lesions that do not respond to antibacterial treatment.
- Karoney MJ, Kaumbuki EK, Koech MK, et al. Primary cutaneous tuberculosis in a 27-year-old medical intern from needle-stick injury: a case report. Clin Case Rep. 2015;3:39-42.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area of tuberculosis, Vitória, Espírito Santo, Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Sahin N, Aydin NE, Senol M, et al. Longstanding skin ulcers due to Mycobacterium tuberculosis in a healthy man. Trop Biomed. 2010;27:120-124.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Ram R, Uppin S, Swarnalatha G, et al. Isolated skin ulcers due to Mycobacterium tuberculosis in a renal allograft recipient. Nat Clin Pract Nephrol. 2007;3:688-693.
- Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
- Handog EB, Gabriel TG, Pineda RT. Management of cutaneous tuberculosis. Dermatol Ther. 2008;21:154-161.
The Diagnosis: Cutaneous Tuberculosis
The patient's medical history was notable for bone tuberculosis (TB) treated in childhood. Skin biopsy revealed neutrophilic infiltrates with necrosis without granulomas. A real-time polymerase chain reaction test detected Mycobacterium tuberculosis complex in the skin fragment, which was confirmed by culture of the biopsy specimen using a liquid growth medium that grew M tuberculosis. Tuberculotic foci were not present on the lungs, gastrointestinal tract, kidneys, and bones by radiologic, microbiologic, and ultrasonographic investigations. The patient was started on 4 antituberculotic drugs--isoniazid 300 mg, rifampicin 600 mg, ethambutol 1200 mg, pyrazinamide 1500 mg--once daily for 2 months followed by isoniazid 300 mg and rifampicin 600 mg once daily for another 4 months with resolution of the skin lesions.
Cutaneous TB is an infectious disease caused by M tuberculosis and accounts for only 1.5% of extrapulmonary TB cases.1,2 Similar to other forms of TB, a resurgence of cutaneous TB has been noted in parts of the world where human immunodeficiency virus infection is prevalent and remains to be one of the most elusive and more difficult diseases to diagnose.3 Thought to be a predominantly occupational disease, it is being encountered more frequently in healthy individuals where the source of infection remains unidentified in most cases.4 The clinical types depend on the method of infection, virulence of the bacillus, immune status of the host, and presence or absence of host sensitization to M tuberculosis.2 The route of infection is used to classify cutaneous mycobacteriosis.5 Inoculation from an exogenous source can produce TB verrucosa cutis in individuals who have previously been sensitized to M tuberculosis or tuberculous chancre in individuals without prior exposure to the bacterium.4 Cutaneous TB resulting from direct spread to the skin from an underlying contiguous structure in most cases spreads from lymph nodes and bone (scrofuloderma). Immunosuppressed patients with advanced TB of the lung, gastrointestinal tract, or the genitourinary tract may present with periorificial TB.4 Dissemination to the skin caused by hematogenous spread can occur in the form of lupus vulgaris, miliary TB, or metastatic tuberculous abscesses (gummas).4,5 A fourth category--cutaneous TB from paradoxical expansion--also was proposed. Paradoxical expansion is defined as the transient expansion of a preexisting lesion or the appearance of new lesions during appropriate anti-TB therapy.5
Although histopathology and protein chain reaction tests are useful, the gold standard for diagnosis is still the isolation of M tuberculosis on culture.3,6 Treatment regimens of cutaneous TB are similar to those of pulmonary TB, with a 4-agent regimen given for 2 months followed by a 2-drug regimen for the next 4 months.1,7 The differential diagnosis of leg ulcers includes stasis ulcer, necrobiotic xanthogranuloma, pyoderma gangrenosum, and squamous cell carcinoma, among others. Cutaneous biopsy, microbiological culture, and a high degree of suspicion are fundamental for the final diagnosis. Cutaneous TB should be suspected in immunocompetent as well as in immunosuppressed patients who present with ulcerated lesions that do not respond to antibacterial treatment.
The Diagnosis: Cutaneous Tuberculosis
The patient's medical history was notable for bone tuberculosis (TB) treated in childhood. Skin biopsy revealed neutrophilic infiltrates with necrosis without granulomas. A real-time polymerase chain reaction test detected Mycobacterium tuberculosis complex in the skin fragment, which was confirmed by culture of the biopsy specimen using a liquid growth medium that grew M tuberculosis. Tuberculotic foci were not present on the lungs, gastrointestinal tract, kidneys, and bones by radiologic, microbiologic, and ultrasonographic investigations. The patient was started on 4 antituberculotic drugs--isoniazid 300 mg, rifampicin 600 mg, ethambutol 1200 mg, pyrazinamide 1500 mg--once daily for 2 months followed by isoniazid 300 mg and rifampicin 600 mg once daily for another 4 months with resolution of the skin lesions.
Cutaneous TB is an infectious disease caused by M tuberculosis and accounts for only 1.5% of extrapulmonary TB cases.1,2 Similar to other forms of TB, a resurgence of cutaneous TB has been noted in parts of the world where human immunodeficiency virus infection is prevalent and remains to be one of the most elusive and more difficult diseases to diagnose.3 Thought to be a predominantly occupational disease, it is being encountered more frequently in healthy individuals where the source of infection remains unidentified in most cases.4 The clinical types depend on the method of infection, virulence of the bacillus, immune status of the host, and presence or absence of host sensitization to M tuberculosis.2 The route of infection is used to classify cutaneous mycobacteriosis.5 Inoculation from an exogenous source can produce TB verrucosa cutis in individuals who have previously been sensitized to M tuberculosis or tuberculous chancre in individuals without prior exposure to the bacterium.4 Cutaneous TB resulting from direct spread to the skin from an underlying contiguous structure in most cases spreads from lymph nodes and bone (scrofuloderma). Immunosuppressed patients with advanced TB of the lung, gastrointestinal tract, or the genitourinary tract may present with periorificial TB.4 Dissemination to the skin caused by hematogenous spread can occur in the form of lupus vulgaris, miliary TB, or metastatic tuberculous abscesses (gummas).4,5 A fourth category--cutaneous TB from paradoxical expansion--also was proposed. Paradoxical expansion is defined as the transient expansion of a preexisting lesion or the appearance of new lesions during appropriate anti-TB therapy.5
Although histopathology and protein chain reaction tests are useful, the gold standard for diagnosis is still the isolation of M tuberculosis on culture.3,6 Treatment regimens of cutaneous TB are similar to those of pulmonary TB, with a 4-agent regimen given for 2 months followed by a 2-drug regimen for the next 4 months.1,7 The differential diagnosis of leg ulcers includes stasis ulcer, necrobiotic xanthogranuloma, pyoderma gangrenosum, and squamous cell carcinoma, among others. Cutaneous biopsy, microbiological culture, and a high degree of suspicion are fundamental for the final diagnosis. Cutaneous TB should be suspected in immunocompetent as well as in immunosuppressed patients who present with ulcerated lesions that do not respond to antibacterial treatment.
- Karoney MJ, Kaumbuki EK, Koech MK, et al. Primary cutaneous tuberculosis in a 27-year-old medical intern from needle-stick injury: a case report. Clin Case Rep. 2015;3:39-42.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area of tuberculosis, Vitória, Espírito Santo, Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Sahin N, Aydin NE, Senol M, et al. Longstanding skin ulcers due to Mycobacterium tuberculosis in a healthy man. Trop Biomed. 2010;27:120-124.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Ram R, Uppin S, Swarnalatha G, et al. Isolated skin ulcers due to Mycobacterium tuberculosis in a renal allograft recipient. Nat Clin Pract Nephrol. 2007;3:688-693.
- Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
- Handog EB, Gabriel TG, Pineda RT. Management of cutaneous tuberculosis. Dermatol Ther. 2008;21:154-161.
- Karoney MJ, Kaumbuki EK, Koech MK, et al. Primary cutaneous tuberculosis in a 27-year-old medical intern from needle-stick injury: a case report. Clin Case Rep. 2015;3:39-42.
- Spelta K, Diniz LM. Cutaneous tuberculosis: a 26-year retrospective study in an endemic area of tuberculosis, Vitória, Espírito Santo, Brazil. Rev Inst Med Trop Sao Paulo. 2016;58:49.
- Sahin N, Aydin NE, Senol M, et al. Longstanding skin ulcers due to Mycobacterium tuberculosis in a healthy man. Trop Biomed. 2010;27:120-124.
- Semaan R, Traboulsi R, Kanj S. Primary Mycobacterium tuberculosis complex cutaneous infection: report of two cases and literature review. Int J Infect Dis. 2008;12:472-477.
- Ram R, Uppin S, Swarnalatha G, et al. Isolated skin ulcers due to Mycobacterium tuberculosis in a renal allograft recipient. Nat Clin Pract Nephrol. 2007;3:688-693.
- Bravo FG, Gotuzzo E. Cutaneous tuberculosis. Clin Dermatol. 2007;25:173-180.
- Handog EB, Gabriel TG, Pineda RT. Management of cutaneous tuberculosis. Dermatol Ther. 2008;21:154-161.
A 78-year-old man was referred to our clinic for evaluation of 2 painless round ulcers with an undermined edge and purulent discharge on the left posterior leg of 2 months' duration. The ulcers had appeared following a presumed trauma. He had received repeated courses of oral antibiotics and antifungals without improvement. No regional lymphadenopathy could be detected. Biochemical analyses were within reference range. Human immunodeficiency virus 1 and 2, hepatitis B and C antibodies, and a VDRL test were all negative.
Larval Tick Infestation Causing an Eruption of Pruritic Papules and Pustules
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.
Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.
Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.
Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.
Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
Case Reports
Patient 1
A 65-year-old woman presented to the dermatology clinic in July with a pruritic rash of 2 days’ duration that started on the back and spread diffusely. The patient gardened regularly. Physical examination showed inflammatory papules and pustules on the back (Figure 1), as well as the groin, breasts, and ears. There was a punctate black dot in the center of some papules, and dermoscopy revealed ticks (Figure 2). Removal and microscopic examination confirmed larval-stage lone star ticks (Figure 3). The patient was prescribed topical steroids for pruritus as well as oral doxycycline for prophylaxis against tick-borne illnesses.
Patient 2
A 54-year-old man presented to the same clinic in July with pruritic lesions on the back, legs, ankles, and scrotum of 3 days’ duration that first appeared 24 hours after performing yardwork. Physical examination revealed diffusely distributed papules, pustules, and vesicles on the back (Figure 4). Some papules featured a punctate black dot in the center (similar to patient 1), and dermoscopy again revealed ticks. Removal and microscopic examination confirmed larval-stage ticks. The patient was treated with topical steroids and oral antihistamines for pruritus as well as prophylactic oral doxycycline.
Comment
Ticks are well-known human parasites, representing the second most common vector of human infectious disease.1 Ticks have 3 motile stages: larva (or “seed”), nymph, and adult. They can bite humans during all stages. Larval ticks, distinguished by having 6 legs rather than 8 legs in nymphs and adults, can attack in droves and cause an infestation that presents as diffuse, pruritic, erythematous papules and pustules.2-4 The first report of larval tick infestation in humans may have been in 1728 by William Byrd who described finding ticks on the skin that were too small to see without a microscope.5
Identification
The ticks in both of our cases were lone star ticks (Amblyomma americanum). The larval stage of A americanum is a proven cause of cutaneous reaction.6,7 A PubMed search of articles indexed for MEDLINE as well as a Google Scholar search using the terms tick, seed tick, or tick bite in combination with rash, eruption, infestation, papule, pustule, or pruritic revealed 6 reported cases of larval tick infestation in the literature (including our case); 5 were caused by A americanum and 1 by Ixodes dammini (now known as Ixodes scapularis); all occurred in July or August.3,7-10 This time frame is consistent with the general tick life cycle across species: Adults feed from April to June, then lay eggs that hatch into larval ticks within 4 to 6 weeks. After hatching, larval ticks climb grass and weeds awaiting a passing host.4
Diagnosis
Larval tick infestation remains a frequently misdiagnosed etiology of diffuse pruritic papules and pustules, especially in urban settings where physicians are less likely to be familiar with this type of manifestation.3,9-11 Larval ticks are submillimeter in size and difficult to appreciate with the naked eye, contributing to misdiagnosis. A punctate black dot may sometimes be seen in papules; however, dermoscopy is critical for accurate diagnosis, as hemorrhagic crust is a frequent misdiagnosis.
Management
In addition to symptomatic therapy, both of our patients received doxycycline as antibiotic prophylaxis for tick-borne illnesses given that a high number of ticks had been attached for more than 2 days.12,13 Antibiotic prophylaxis for tick-borne illness is controversial. The exception is Lyme disease transmitted by nymphal or adult I scapularis when specific conditions are met: the bite must have occurred in an endemic area, doxycycline cannot be contraindicated, estimated duration of attachment is at least 36 hours, and prophylaxis must be started within 72 hours of tick removal.13 There are no official recommendations for the A americanum species or for larval-stage ticks of any species. Larval-stage ticks acting as vectors for disease transmission is not well documented in recent literature, and there currently is limited evidence supporting prophylactic antibiotics for larval tick bites. The presence of spotted fever rickettsioses has been reported (with the exception of Rickettsia rickettsii and Ehrlichia chaffeensis) in larval A americanum ticks, suggesting a theoretical possibility that they could act as disease vectors.3,8,11,14-17 At a minimum, both prompt tick removal and close patient follow-up is warranted.
Conclusion
Human infestation with larval ticks is a common occurrence but can present a diagnostic challenge to an unfamiliar physician. We encourage consideration of larval tick infestation as the etiology of multiple or diffuse pruritic papules with a history of outdoor exposure.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
- Sonenshine DE. Biology of Ticks. New York, NY: Oxford University; 1991.
- Alexander JOD. The effects of tick bites. In: Alexander JOD. Arthropods and Human Skin. London, England: Springer London; 1984:363-382.
- Duckworth PF Jr, Hayden GF, Reed CN. Human infestation by Amblyomma americanum larvae (“seed ticks”). South Med J. 1985;78:751-753.
- Parola P, Raoult D. Ticks and tickborne bacterial diseases in humans: an emerging infectious threat. Clin Infect Dis. 2001;32:897-928.
- Cropley TG. William Byrd on ticks, 1728. Arch Dermatol. 2009;145:187.
- Goddard J. A ten-year study of tick biting in Mississippi: implications for human disease transmission. J Agromedicine. 2002;8:25-32.
- Goddard J, Portugal JS. Cutaneous lesions due to bites by larval Amblyomma americanum ticks. JAMA Dermatol. 2015;151:1373-1375.
- Fibeger EA, Erickson QL, Weintraub BD, et al. Larval tick infestation: a case report and review of tick-borne disease. Cutis. 2008;82:38-46.
- Jones BE. Human ‘seed tick’ infestation: Amblyomma americanum larvae. Arch Dermatol. 1981;117:812-814.
- Fisher EJ, Mo J, Lucky AW. Multiple pruritic papules from lone star tick larvae bites. Arch Dermatol. 2006;142:491-494.
- Culp JS. Seed ticks. Am Fam Physician. 1987;36:121-123.
- Perea AE, Hinckley AF, Mead PS. Tick bite prophylaxis: results from a 2012 survey of healthcare providers. Zoonoses Public Health. 2015;62:388-392.
- Tick bites/prevention. Centers for Disease Control and Prevention website. https://www.cdc.gov/ticks/tickbornediseases/tick-bites-prevention.html. Revised January 10, 2019. Accessed September 17, 2019.
- Moncayo AC, Cohen SB, Fritzen CM, et al. Absence of Rickettsia rickettsii and occurrence of other spotted fever group rickettsiae in ticks from Tennessee. Am J Trop Med Hyg. 2010;83:653-657.
- Castellaw AH, Showers J, Goddard J, et al. Detection of vector-borne agents in lone star ticks, Amblyomma americanum (Acari: Ixodidae), from Mississippi. J Med Entomol. 2010;47:473-476.
- Stromdahl EY, Vince MA, Billingsley PM, et al. Rickettsia amblyommii infecting Amblyomma americanum larvae. Vector Borne Zoonotic Dis. 2008;8:15-24.
- Long SW, Zhang X, Zhang J, et al. Evaluation of transovarial transmission and transmissibility of Ehrlichia chaffeensis (Rickettsiales: Anaplasmataceae) in Amblyomma americanum (Acari: Ixodidae). J Med Entomol. 2003;40:1000-1004.
Practice Points
- Larval (“seed”) ticks can attack in droves, causing a widespread rash consisting of pruritic erythematous papules and pustules.
- Tiny black dots can be seen in some papules, which are the seed ticks themselves. Careful dermoscopic examination is critical to avoid easy misdiagnosis as hemorrhagic crust.
- We encourage providers to include larval tick infestation in the differential for eruptive pruritic papules and pustules with a history of outdoor exposure, especially during the summer months.
Infographic: Monitoring Acne Patients on Oral Therapy Survey Results and Practice Recommendations
Erythematous Papules on the Scrotum, Trunk, and Extremities
The Diagnosis: Lichenoid and Granulomatous Dermatitis in the Setting of Secondary Syphilis
Syphilis, an infectious disease that has risen in incidence and is most commonly reported in men who have sex with men, involves a vast array of clinical and histologic presentations.1 Clinically, secondary syphilis involves an erythematous maculopapular eruption on the face, trunk, palms, soles, or genital area.2 The characteristic histologic features for secondary syphilis include endothelial swelling, interstitial inflammatory array, irregular acanthosis, elongated rete ridges, and vacuolar interface dermatitis with lymphocytes and plasma cells.1 Syphilitic infection has been associated with lichenoid and granulomatous dermatitis, which is an inflammatory skin disease described by Magro and Crowson.3 Lichenoid and granulomatous dermatitis has been linked to various systemic disorders, including chronic hepatitis C, Crohn disease, rheumatoid arthritis, endocrinopathy, subacute cutaneous lupus erythematosus, secondary syphilis, prior herpes infection, tuberculoid leprosy, mycobacterial infection, and human immunodeficiency virus infection.3-7 For this patient, given histopathology findings, clinical presentation, and positive rapid plasma reagin serologies, a diagnosis of lichenoid and granulomatous dermatitis in the setting of a secondary syphilis infection was established. A comprehensive investigation should be conducted to consider secondary syphilis or other systemic diseases in patients with a histologic finding of lichenoid and granulomatous dermatitis.
Histologically, lichenoid and granulomatous dermatitis cases show a bandlike infiltrate of lymphocytes with neighboring histiocytes along the dermoepidermal junction, accompanied by epithelial changes of dyskeratosis, vasculopathy, and colloid body formation, in addition to a dermal histiocytic component.3 Our patient's biopsy showed a lichenoid reaction pattern with vacuolar interface changes, dyskeratosis, plump endothelial cells, and small collections of plasma cells. Additionally, there was a granulomatous component in the dermis with histiocytes admixed with lymphocytes and plasma cells. The presence of spirochetes was confirmed with antitreponemal immunohistochemical stain (Figure 1). Quantitative rapid plasma reagin was 1:64 (reference range, <1:1) and Treponema pallidum antibody was reactive.
Interstitial granulomatous dermatitis has a variable clinical presentation, often with red-purple annular plaques, hyperpigmented papules, and nodules frequently in a linear arrangement and predominantly on the trunk, thighs, groin, or buttocks.8,9 On histopathology, there are histiocytes in the reticular dermis and/or a macrophage infiltrate in the mid to deep dermis with collections of degenerated collagen (Figure 2).8,10 An interstitial infiltrate of eosinophils and neutrophils also may be appreciated, but mucin generally is absent.8,11 This condition often coexists with rheumatic and systemic autoimmune diseases.8-10
Interstitial granuloma annulare is a noninfectious granulomatous skin condition that often presents clinically as asymptomatic annular red-brown patches, usually on the extremities.11-13 On histopathology, an interstitial or palisaded inflammatory infiltrate with histiocytes and multinucleated giant cells may be seen along with collagen degeneration or collagen bundles without necrosis (Figure 3).9 Mucin often is associated with the histiocytes.11 Of note, our patient's skin biopsy shows interface dermatitis, differentiating it from both interstitial granuloma annulare and interstitial granulomatous dermatitis.
Postviral granulomatous reactions are the most frequently reported types of reactions to occur at the location of herpes zoster infection up to years after the initial disease. Wolf isotopic reaction encompasses skin reactions in the body region of formerly resolved skin disease, commonly herpesvirus infection.14,15 This manifestation may occur due to a hypersensitivity reaction from enduring viral proteins, resident memory T cells, or local neuroimmune imbalance from herpesvirus-induced injury to dermal sensory nerve fibers.14-17 Clinically, patients present with red-purple pruritic papules and plaques in a bandlike unilateral pattern, usually in the same region as the prior herpes infection and often accompanied by postherpetic neuralgia.16-19 Of note, our patient's clinical findings were more diffuse than the frequently localized and often linear distribution seen in postherpetic granulomatous reaction. On histopathology, granulomatous or lichenoid tissue reaction most commonly is appreciated.15 Specifically, interstitial granulomatous dermatitis with histiocytes, lymphocytes, and multinucleated giant cells showing elastophagocytosis and an inflammatory infiltrate with lymphocytes and plasma cells around vasculature, eccrine glands, and nerves can be noted (Figure 4).19
Lupus erythematosus is an autoimmune condition with a wide array of clinical features, including skin manifestations and systemic symptoms. Specifically, discoid lupus erythematosus presents with clearly outlined, red-pink macules or papules with scaling. Histologic features include keratotic follicular plugging, acanthosis, dermal mucin, thickening of the basement membrane zone, and dense lymphocytic infiltrate (Figure 5).20
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:325-330.
- Zeltser R, Kurban AK. Syphilis. Clin Dermatol. 2004;22:461-468.
- Magro CM, Crowson AN. Lichenoid and granulomatous dermatitis. Int J Dermatol. 2000;39:12-33.
- S Breza T Jr, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33:512-515.
- Granel B, Serratrice J, Rey J, et al. Chronic hepatitis C virus infection associated with a generalized granuloma annulare. J Am Acad Dermatol. 2000;43(5, pt 2):918-919.
- Jorizzo JL, Gonzalez EB, Apisarnthanarax P, et al. Pigmented purpuric eruption in a patient with rheumatoid arthritis. Arch Intern Med. 1982;142:2184-2185.
- Magro CM, Crowson AN, Regauer S. Granuloma annulare and necrobiosis lipoidica tissue reactions as a manifestation of systemic disease. Hum Pathol. 1996;27:50-56.
- Błażewicz I, Szczerkowska-Dobosz A, Pęksa R, et al. Interstitial granulomatous dermatitis: a characteristic histological pattern with variable clinical manifestations. Postepy Dermatol Alergol. 2015;32:475-477.
- Sezer E, Luzar B, Calonje E. Secondary syphilis with an interstitial granuloma annulare-like histopathologic pattern. J Cutan Pathol. 2011;38:439-442.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Sakiyama T, Hirai I, Konohana A, et al. Interstitial-type granuloma annulare associated with Sjögren syndrome. J Dtsch Dermatol Ges. 2014;12:415-416.
- Spring P, Vernez M, Maniu CM, et al. Localized interstitial granuloma annulare induced by subcutaneous injections for desensitization. Dermatol Online J. 2013;19:18572.
- Kluger N, Moguelet P, Chaslin-Ferbus D, et al. Generalized interstitial granuloma annulare induced by pegylated interferon-alpha. Dermatology. 2006;213:248-249.
- Ruocco E, Baroni A, Cutrì FT, et al. Granuloma annulare in a site of healed herpes zoster: Wolf's isotopic response. J Eur Acad Dermatol Venereol. 2003;17:686-688.
- Ise M, Tanese K, Adachi T, et al. Postherpetic Wolf's isotopic response: possible contribution of resident memory T cells to the pathogenesis of lichenoid reaction. Br J Dermatol. 2015;173:1331-1334.
- Lora V, Cota C, Kanitakis J. Zosteriform lichen planus after herpes zoster: report of a new case of Wolf's isotopic phenomenon and literature review. Dermatol Online J. 2014;20. pii:13030/qt5vf99178.
- Lin CH, Chen HC, Gao HW, et al. Wolf's post-herpetic isotopic response to tocilizumab for rheumatoid arthritis. Australas J Dermatol. 2018;59:E135-E137.
- Melgar E, Henry J, Valois A, et al. Extra-facial Lever granuloma on a herpes zoster scar: Wolf's isotopic response. Ann Dermatol Venereol. 2018;145:354-358.
- Ferenczi K, Rosenberg AS, McCalmont TH, et al. Herpes zoster granulomatous dermatitis: histopathologic findings in a case series. J Cutan Pathol. 2015;42:739-745.
- Li Q, Wu H, Liao W, et al. A comprehensive review of immune-mediated dermatopathology in systemic lupus erythematosus. J Autoimmun. 2018;93:1-15.
The Diagnosis: Lichenoid and Granulomatous Dermatitis in the Setting of Secondary Syphilis
Syphilis, an infectious disease that has risen in incidence and is most commonly reported in men who have sex with men, involves a vast array of clinical and histologic presentations.1 Clinically, secondary syphilis involves an erythematous maculopapular eruption on the face, trunk, palms, soles, or genital area.2 The characteristic histologic features for secondary syphilis include endothelial swelling, interstitial inflammatory array, irregular acanthosis, elongated rete ridges, and vacuolar interface dermatitis with lymphocytes and plasma cells.1 Syphilitic infection has been associated with lichenoid and granulomatous dermatitis, which is an inflammatory skin disease described by Magro and Crowson.3 Lichenoid and granulomatous dermatitis has been linked to various systemic disorders, including chronic hepatitis C, Crohn disease, rheumatoid arthritis, endocrinopathy, subacute cutaneous lupus erythematosus, secondary syphilis, prior herpes infection, tuberculoid leprosy, mycobacterial infection, and human immunodeficiency virus infection.3-7 For this patient, given histopathology findings, clinical presentation, and positive rapid plasma reagin serologies, a diagnosis of lichenoid and granulomatous dermatitis in the setting of a secondary syphilis infection was established. A comprehensive investigation should be conducted to consider secondary syphilis or other systemic diseases in patients with a histologic finding of lichenoid and granulomatous dermatitis.
Histologically, lichenoid and granulomatous dermatitis cases show a bandlike infiltrate of lymphocytes with neighboring histiocytes along the dermoepidermal junction, accompanied by epithelial changes of dyskeratosis, vasculopathy, and colloid body formation, in addition to a dermal histiocytic component.3 Our patient's biopsy showed a lichenoid reaction pattern with vacuolar interface changes, dyskeratosis, plump endothelial cells, and small collections of plasma cells. Additionally, there was a granulomatous component in the dermis with histiocytes admixed with lymphocytes and plasma cells. The presence of spirochetes was confirmed with antitreponemal immunohistochemical stain (Figure 1). Quantitative rapid plasma reagin was 1:64 (reference range, <1:1) and Treponema pallidum antibody was reactive.
Interstitial granulomatous dermatitis has a variable clinical presentation, often with red-purple annular plaques, hyperpigmented papules, and nodules frequently in a linear arrangement and predominantly on the trunk, thighs, groin, or buttocks.8,9 On histopathology, there are histiocytes in the reticular dermis and/or a macrophage infiltrate in the mid to deep dermis with collections of degenerated collagen (Figure 2).8,10 An interstitial infiltrate of eosinophils and neutrophils also may be appreciated, but mucin generally is absent.8,11 This condition often coexists with rheumatic and systemic autoimmune diseases.8-10
Interstitial granuloma annulare is a noninfectious granulomatous skin condition that often presents clinically as asymptomatic annular red-brown patches, usually on the extremities.11-13 On histopathology, an interstitial or palisaded inflammatory infiltrate with histiocytes and multinucleated giant cells may be seen along with collagen degeneration or collagen bundles without necrosis (Figure 3).9 Mucin often is associated with the histiocytes.11 Of note, our patient's skin biopsy shows interface dermatitis, differentiating it from both interstitial granuloma annulare and interstitial granulomatous dermatitis.
Postviral granulomatous reactions are the most frequently reported types of reactions to occur at the location of herpes zoster infection up to years after the initial disease. Wolf isotopic reaction encompasses skin reactions in the body region of formerly resolved skin disease, commonly herpesvirus infection.14,15 This manifestation may occur due to a hypersensitivity reaction from enduring viral proteins, resident memory T cells, or local neuroimmune imbalance from herpesvirus-induced injury to dermal sensory nerve fibers.14-17 Clinically, patients present with red-purple pruritic papules and plaques in a bandlike unilateral pattern, usually in the same region as the prior herpes infection and often accompanied by postherpetic neuralgia.16-19 Of note, our patient's clinical findings were more diffuse than the frequently localized and often linear distribution seen in postherpetic granulomatous reaction. On histopathology, granulomatous or lichenoid tissue reaction most commonly is appreciated.15 Specifically, interstitial granulomatous dermatitis with histiocytes, lymphocytes, and multinucleated giant cells showing elastophagocytosis and an inflammatory infiltrate with lymphocytes and plasma cells around vasculature, eccrine glands, and nerves can be noted (Figure 4).19
Lupus erythematosus is an autoimmune condition with a wide array of clinical features, including skin manifestations and systemic symptoms. Specifically, discoid lupus erythematosus presents with clearly outlined, red-pink macules or papules with scaling. Histologic features include keratotic follicular plugging, acanthosis, dermal mucin, thickening of the basement membrane zone, and dense lymphocytic infiltrate (Figure 5).20
The Diagnosis: Lichenoid and Granulomatous Dermatitis in the Setting of Secondary Syphilis
Syphilis, an infectious disease that has risen in incidence and is most commonly reported in men who have sex with men, involves a vast array of clinical and histologic presentations.1 Clinically, secondary syphilis involves an erythematous maculopapular eruption on the face, trunk, palms, soles, or genital area.2 The characteristic histologic features for secondary syphilis include endothelial swelling, interstitial inflammatory array, irregular acanthosis, elongated rete ridges, and vacuolar interface dermatitis with lymphocytes and plasma cells.1 Syphilitic infection has been associated with lichenoid and granulomatous dermatitis, which is an inflammatory skin disease described by Magro and Crowson.3 Lichenoid and granulomatous dermatitis has been linked to various systemic disorders, including chronic hepatitis C, Crohn disease, rheumatoid arthritis, endocrinopathy, subacute cutaneous lupus erythematosus, secondary syphilis, prior herpes infection, tuberculoid leprosy, mycobacterial infection, and human immunodeficiency virus infection.3-7 For this patient, given histopathology findings, clinical presentation, and positive rapid plasma reagin serologies, a diagnosis of lichenoid and granulomatous dermatitis in the setting of a secondary syphilis infection was established. A comprehensive investigation should be conducted to consider secondary syphilis or other systemic diseases in patients with a histologic finding of lichenoid and granulomatous dermatitis.
Histologically, lichenoid and granulomatous dermatitis cases show a bandlike infiltrate of lymphocytes with neighboring histiocytes along the dermoepidermal junction, accompanied by epithelial changes of dyskeratosis, vasculopathy, and colloid body formation, in addition to a dermal histiocytic component.3 Our patient's biopsy showed a lichenoid reaction pattern with vacuolar interface changes, dyskeratosis, plump endothelial cells, and small collections of plasma cells. Additionally, there was a granulomatous component in the dermis with histiocytes admixed with lymphocytes and plasma cells. The presence of spirochetes was confirmed with antitreponemal immunohistochemical stain (Figure 1). Quantitative rapid plasma reagin was 1:64 (reference range, <1:1) and Treponema pallidum antibody was reactive.
Interstitial granulomatous dermatitis has a variable clinical presentation, often with red-purple annular plaques, hyperpigmented papules, and nodules frequently in a linear arrangement and predominantly on the trunk, thighs, groin, or buttocks.8,9 On histopathology, there are histiocytes in the reticular dermis and/or a macrophage infiltrate in the mid to deep dermis with collections of degenerated collagen (Figure 2).8,10 An interstitial infiltrate of eosinophils and neutrophils also may be appreciated, but mucin generally is absent.8,11 This condition often coexists with rheumatic and systemic autoimmune diseases.8-10
Interstitial granuloma annulare is a noninfectious granulomatous skin condition that often presents clinically as asymptomatic annular red-brown patches, usually on the extremities.11-13 On histopathology, an interstitial or palisaded inflammatory infiltrate with histiocytes and multinucleated giant cells may be seen along with collagen degeneration or collagen bundles without necrosis (Figure 3).9 Mucin often is associated with the histiocytes.11 Of note, our patient's skin biopsy shows interface dermatitis, differentiating it from both interstitial granuloma annulare and interstitial granulomatous dermatitis.
Postviral granulomatous reactions are the most frequently reported types of reactions to occur at the location of herpes zoster infection up to years after the initial disease. Wolf isotopic reaction encompasses skin reactions in the body region of formerly resolved skin disease, commonly herpesvirus infection.14,15 This manifestation may occur due to a hypersensitivity reaction from enduring viral proteins, resident memory T cells, or local neuroimmune imbalance from herpesvirus-induced injury to dermal sensory nerve fibers.14-17 Clinically, patients present with red-purple pruritic papules and plaques in a bandlike unilateral pattern, usually in the same region as the prior herpes infection and often accompanied by postherpetic neuralgia.16-19 Of note, our patient's clinical findings were more diffuse than the frequently localized and often linear distribution seen in postherpetic granulomatous reaction. On histopathology, granulomatous or lichenoid tissue reaction most commonly is appreciated.15 Specifically, interstitial granulomatous dermatitis with histiocytes, lymphocytes, and multinucleated giant cells showing elastophagocytosis and an inflammatory infiltrate with lymphocytes and plasma cells around vasculature, eccrine glands, and nerves can be noted (Figure 4).19
Lupus erythematosus is an autoimmune condition with a wide array of clinical features, including skin manifestations and systemic symptoms. Specifically, discoid lupus erythematosus presents with clearly outlined, red-pink macules or papules with scaling. Histologic features include keratotic follicular plugging, acanthosis, dermal mucin, thickening of the basement membrane zone, and dense lymphocytic infiltrate (Figure 5).20
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:325-330.
- Zeltser R, Kurban AK. Syphilis. Clin Dermatol. 2004;22:461-468.
- Magro CM, Crowson AN. Lichenoid and granulomatous dermatitis. Int J Dermatol. 2000;39:12-33.
- S Breza T Jr, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33:512-515.
- Granel B, Serratrice J, Rey J, et al. Chronic hepatitis C virus infection associated with a generalized granuloma annulare. J Am Acad Dermatol. 2000;43(5, pt 2):918-919.
- Jorizzo JL, Gonzalez EB, Apisarnthanarax P, et al. Pigmented purpuric eruption in a patient with rheumatoid arthritis. Arch Intern Med. 1982;142:2184-2185.
- Magro CM, Crowson AN, Regauer S. Granuloma annulare and necrobiosis lipoidica tissue reactions as a manifestation of systemic disease. Hum Pathol. 1996;27:50-56.
- Błażewicz I, Szczerkowska-Dobosz A, Pęksa R, et al. Interstitial granulomatous dermatitis: a characteristic histological pattern with variable clinical manifestations. Postepy Dermatol Alergol. 2015;32:475-477.
- Sezer E, Luzar B, Calonje E. Secondary syphilis with an interstitial granuloma annulare-like histopathologic pattern. J Cutan Pathol. 2011;38:439-442.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Sakiyama T, Hirai I, Konohana A, et al. Interstitial-type granuloma annulare associated with Sjögren syndrome. J Dtsch Dermatol Ges. 2014;12:415-416.
- Spring P, Vernez M, Maniu CM, et al. Localized interstitial granuloma annulare induced by subcutaneous injections for desensitization. Dermatol Online J. 2013;19:18572.
- Kluger N, Moguelet P, Chaslin-Ferbus D, et al. Generalized interstitial granuloma annulare induced by pegylated interferon-alpha. Dermatology. 2006;213:248-249.
- Ruocco E, Baroni A, Cutrì FT, et al. Granuloma annulare in a site of healed herpes zoster: Wolf's isotopic response. J Eur Acad Dermatol Venereol. 2003;17:686-688.
- Ise M, Tanese K, Adachi T, et al. Postherpetic Wolf's isotopic response: possible contribution of resident memory T cells to the pathogenesis of lichenoid reaction. Br J Dermatol. 2015;173:1331-1334.
- Lora V, Cota C, Kanitakis J. Zosteriform lichen planus after herpes zoster: report of a new case of Wolf's isotopic phenomenon and literature review. Dermatol Online J. 2014;20. pii:13030/qt5vf99178.
- Lin CH, Chen HC, Gao HW, et al. Wolf's post-herpetic isotopic response to tocilizumab for rheumatoid arthritis. Australas J Dermatol. 2018;59:E135-E137.
- Melgar E, Henry J, Valois A, et al. Extra-facial Lever granuloma on a herpes zoster scar: Wolf's isotopic response. Ann Dermatol Venereol. 2018;145:354-358.
- Ferenczi K, Rosenberg AS, McCalmont TH, et al. Herpes zoster granulomatous dermatitis: histopathologic findings in a case series. J Cutan Pathol. 2015;42:739-745.
- Li Q, Wu H, Liao W, et al. A comprehensive review of immune-mediated dermatopathology in systemic lupus erythematosus. J Autoimmun. 2018;93:1-15.
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:325-330.
- Zeltser R, Kurban AK. Syphilis. Clin Dermatol. 2004;22:461-468.
- Magro CM, Crowson AN. Lichenoid and granulomatous dermatitis. Int J Dermatol. 2000;39:12-33.
- S Breza T Jr, Magro CM. Lichenoid and granulomatous dermatitis associated with atypical mycobacterium infections. J Cutan Pathol. 2006;33:512-515.
- Granel B, Serratrice J, Rey J, et al. Chronic hepatitis C virus infection associated with a generalized granuloma annulare. J Am Acad Dermatol. 2000;43(5, pt 2):918-919.
- Jorizzo JL, Gonzalez EB, Apisarnthanarax P, et al. Pigmented purpuric eruption in a patient with rheumatoid arthritis. Arch Intern Med. 1982;142:2184-2185.
- Magro CM, Crowson AN, Regauer S. Granuloma annulare and necrobiosis lipoidica tissue reactions as a manifestation of systemic disease. Hum Pathol. 1996;27:50-56.
- Błażewicz I, Szczerkowska-Dobosz A, Pęksa R, et al. Interstitial granulomatous dermatitis: a characteristic histological pattern with variable clinical manifestations. Postepy Dermatol Alergol. 2015;32:475-477.
- Sezer E, Luzar B, Calonje E. Secondary syphilis with an interstitial granuloma annulare-like histopathologic pattern. J Cutan Pathol. 2011;38:439-442.
- Peroni A, Colato C, Schena D, et al. Interstitial granulomatous dermatitis: a distinct entity with characteristic histological and clinical pattern. Br J Dermatol. 2012;166:775-783.
- Sakiyama T, Hirai I, Konohana A, et al. Interstitial-type granuloma annulare associated with Sjögren syndrome. J Dtsch Dermatol Ges. 2014;12:415-416.
- Spring P, Vernez M, Maniu CM, et al. Localized interstitial granuloma annulare induced by subcutaneous injections for desensitization. Dermatol Online J. 2013;19:18572.
- Kluger N, Moguelet P, Chaslin-Ferbus D, et al. Generalized interstitial granuloma annulare induced by pegylated interferon-alpha. Dermatology. 2006;213:248-249.
- Ruocco E, Baroni A, Cutrì FT, et al. Granuloma annulare in a site of healed herpes zoster: Wolf's isotopic response. J Eur Acad Dermatol Venereol. 2003;17:686-688.
- Ise M, Tanese K, Adachi T, et al. Postherpetic Wolf's isotopic response: possible contribution of resident memory T cells to the pathogenesis of lichenoid reaction. Br J Dermatol. 2015;173:1331-1334.
- Lora V, Cota C, Kanitakis J. Zosteriform lichen planus after herpes zoster: report of a new case of Wolf's isotopic phenomenon and literature review. Dermatol Online J. 2014;20. pii:13030/qt5vf99178.
- Lin CH, Chen HC, Gao HW, et al. Wolf's post-herpetic isotopic response to tocilizumab for rheumatoid arthritis. Australas J Dermatol. 2018;59:E135-E137.
- Melgar E, Henry J, Valois A, et al. Extra-facial Lever granuloma on a herpes zoster scar: Wolf's isotopic response. Ann Dermatol Venereol. 2018;145:354-358.
- Ferenczi K, Rosenberg AS, McCalmont TH, et al. Herpes zoster granulomatous dermatitis: histopathologic findings in a case series. J Cutan Pathol. 2015;42:739-745.
- Li Q, Wu H, Liao W, et al. A comprehensive review of immune-mediated dermatopathology in systemic lupus erythematosus. J Autoimmun. 2018;93:1-15.
A 54-year-old man presented with painful, nonpruritic, erythematous papules that began on the scrotum. The eruption progressed to involve the trunk, arms, and legs.
Serous and Hemorrhagic Bullae on the Leg
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1
The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1
The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
The Diagnosis: Fracture Blisters
The shave biopsy pathology demonstrated a subepidermal bulla with re-epithelialization that was clinically consistent with fracture blisters (also known as fracture bullae)(Figure). Fracture blisters are a complication of bone fractures, usually occurring 24 to 48 hours after the trauma but possibly up to 3 weeks later. The skin usually is edematous with tense bullae overlying the fracture (in this case it was distal to the fracture); most blisters contain clear fluid, but older blisters tend to be more flaccid with hemorrhagic fluid.1 The cause is thought to be the result of skin strain during fracture formation.2 Edema and hypoxia from injured vessels and lymphatics contribute to the formation of bullae, which are seen as a dermoepidermal junction split on histology.1
The bullae are histologically indistinguishable from edema blisters. A clinical history can help to differentiate. Edema blisters occur in the setting of an acute exacerbation of chronic edema, usually on the lower extremities in the setting of fluid overload.3 Bullous cellulitis is associated with skin erythema, warmth, and systemic symptoms. Bullous pemphigoid can be localized to the lower legs at times; however, biopsy would show a subepidermal bulla with eosinophils along the dermoepidermal junction. Linear IgA bullous dermatosis can be drug induced from vancomycin; however, pathology would show a subepidermal blister with a neutrophil predominant infiltrate. Nonsteroidal anti-inflammatory medications such as naproxen are a common culprit for bullous drug eruptions, which can be localized or generalized and include diagnoses such as fixed drug eruption, toxic epidermal necrolysis, and drug-induced pseudoporphyria. Naproxen-induced pseudoporphyria more commonly presents with blisters, erosions, and scarring with a predilection for the dorsal hands. Histology also will demonstrate subepidermal bullae. Clues to differentiate pseudoporphyria from fracture blisters include festooning of the dermal papilla and caterpillar bodies consisting of basement membrane material and colloid bodies in the basal layer of the epidermis, though they are not always present.4
Fracture blisters can be localized to the injury site or extend beyond the fracture site. They usually are found where there is minimal subcutaneous tissue, such as the tibia, ankles, and elbows. Fractures treated within 24 hours are much less likely to have bullae formation.1 The bullae are sterile but may lead to wound healing complications, such as infections or delay in surgical management. However, there are no major adverse effects of postoperative fracture blisters.1 Fracture blisters are self-healing, though silver sulfadiazine has been shown to minimize soft-tissue complications by promoting re-epithelialization.5
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
- Varela CD, Vaughan TK, Carr JB, et al. Fracture blisters: clinical and pathological aspects. J Orthop Trauma. 1993;7:417-427.
- Giordano CP, Scott D, Kummer F, et al. Fracture blister formation: a laboratory study. J Trauma. 1995;38:907-909.
- Mascaro JM. Other vesicobullous diseases. In: Bolognia JL, Schafer JV, Cerroni L, eds. Dermatology. Vol 1. Philadelphia, PA: Elsevier; 2018:554-561.
- Patterson JW. The vesicobullous reaction pattern. In: Patterson JW. Weedon's Skin Pathology. 4th ed. Oxford, UK: Churchill Livingstone/Elsevier; 2016:135-187.
- Strauss EJ, Petrucelli G, Bong M, et al. Blisters associated with lower-extremity fracture: results of a prospective treatment protocol. J Orthop Trauma. 2006;20:618-622.
A 61-year-old wheelchair-bound man presented to the emergency department with increased swelling, bruising, and blister formation on the right lower leg over the last week. He had history of alcoholism and heavy smoking. Two weeks prior to presentation he had an open reduction and internal fixation of a right hip fracture. He recently started taking naproxen for pain and had taken a course of ciprofloxacin for a urinary tract infection. Physical examination showed a well-healed surgical wound along the right upper lateral thigh with no purulence or erythema. His right lower leg had extensive ecchymosis and pitting edema, and there was a cluster of well-defined, variably sized, serous and hemorrhagic bullae over the right lower ankle and dorsal aspect of the foot. He was hemodynamically stable and afebrile. Due to initial concern of cellulitis, he was given a dose of vancomycin in the emergency department. Computed tomography of the right leg showed diffuse edematous changes consistent with the recent surgery, and duplex ultrasonography showed no evidence of deep vein thrombosis. A shave biopsy was performed.