User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Challenges of Treating Primary Psychiatric Disease in Dermatology
Dermatology patients experience a high burden of mental health disturbance. Psychiatric disease is seen in an estimated 30% to 60% of our patients.1 It can be secondary to or comorbid with dermatologic disorders, or it can be the primary condition that is driving cutaneous disease. Patients with secondary or comorbid psychiatric disorders often are amenable to referral to a mental health provider or are already participating in some form of mental health treatment; however, patients with primary psychiatric disease who present to dermatology generally do not accept these referrals.2 Therefore, if these patients are to receive care anywhere in the health care system, it often must be in the department of dermatology.
What primary psychiatric conditions do we see in dermatology?
Common primary psychiatric conditions seen in dermatology include delusional infestation, obsessive-compulsive disorder and related disorders, and dermatitis artefacta.
Delusional Infestation
Also known as delusions of parasitosis or delusional parasitosis, delusional infestation presents as a fixed false belief that there is an organism or other foreign entity that is present in the skin and is the cause of cutaneous disruption. It often is an isolated delusion but can have a notable psychosocial impact. The term delusional infestation is sometimes preferred, as it is inclusive of delusions focused on any type of organism, not just parasites. It also encompasses delusions of infestation with nonliving matter such as fibers, also known as Morgellons disease.3
Obsessive-Compulsive Disorder and Related Disorders
This broad category includes several conditions encountered in dermatology. Body dysmorphic disorder (BDD), olfactory reference syndrome (ORS), excoriation disorder, and trichotillomania are some of the most common variants. In patients with BDD, skin and hair are the 2 most common preoccupations. It has been estimated that 12% of dermatology patients experience BDD. Unsurprisingly, it is more common in patients presenting to cosmetic dermatology, but general dermatology patients also are affected at a rate of 7%.2 Patients with ORS falsely believe they have body odor and/or halitosis. Excoriation disorder manifests as repetitive skin picking, either of normal skin or of lesions such as pimples and scabs. Trichotillomania presents as repeated hair pulling, and trichophagia (eating the pulled hair) also may be present.
Dermatitis Artefacta
Almost 1 in 4 patients who seek dermatologic evaluation for primarily psychiatric disorders have dermatitis artefacta, the presence of deliberately self-inflicted skin lesions.2 Patients with dermatitis artefacta have unconscious motives for their behavior and should be distinguished from malingering patients who have a conscious goal of secondary gain.
What treatments are available?
Antidepressants
Selective serotonin reuptake inhibitors are one of the first-line treatments for BDD and may be useful in ORS. In excoriation disorder and trichotillomania, selective serotonin reuptake inhibitors are the most commonly prescribed pharmacotherapy, but they have limited efficacy.2
Antipsychotics
The recommended treatment of delusional infestation is antipsychotic pharmacotherapy. Treatment with risperidone and olanzapine has been reported to achieve full or partial remission in more than two-thirds of cases.4 Aripiprazole, a newer antipsychotic, has fewer side effects and has been successful in several case reports.5-7
Cognitive Behavioral Therapy
Psychotherapy, most often in the form of cognitive behavioral therapy, has been reported as effective treatment of several psychocutaneous diseases. Cognitive behavioral therapy is considered first-line treatment of body-focused repetitive behavior disorders such as excoriation disorder and trichotillomania.2 It addresses maladaptive thought patterns to modify behavior.
Who treats patients with neurodermatoses?
If a patient presents to dermatology with a rash found to be related to an underlying thyroid disorder, the treatment plan likely would include referral to an endocrinologist. Similarly, patients with primary psychiatric conditions presenting to dermatology should ideally be referred to psychiatrists or psychotherapists, the providers most thoroughly trained and best equipped to treat them. The challenge in psychodermatology is that patients often are resistant to the assessment that the primary pathology is psychiatric. Patients may deny that they are “crazy” and see numerous providers in search of a dermatologist who “believes” them.8
Referral to mental health professionals almost always is refused by patients with primarily psychiatric neurodermatoses, which presents dermatologists with a dilemma. As the authors of the “Psychotropic Agents” chapter of Comprehensive Dermatologic Drug Therapy put it: “A dermatologist has two choices. The first is to try to ‘look the other way’ and pacify the patient by providing relatively benign, but minimally effective treatments. The other option is to try to directly address the psychological/psychiatric problems.” The chapter then provides a thorough guide for the use of psychotropic medications in the dermatology population, advocating for option 2: treatment by dermatologists.9
Should a dermatologist prescribe psychotropic drugs?
In Dermatology, the principle reference textbook in many dermatology training programs, it is stated that “[a]lthough less comprehensive than treatment delivered in collaboration with a psychiatrist, in the authors’ opinion, management of these issues by a dermatologist is better than no treatment at all.”10 Recent reviews in the dermatologic literature of psychiatric diseases and drugs in dermatology agree that dermatologists should feel comfortable with prescribing pharmacologic treatment.2,8,11 Performance of psychotherapy by dermatologists, on the other hand, is not recommended based on time constraints and lack of training.
Despite the apparent agreement in the texts and literature that pharmacotherapy of psychiatric neurodermatoses is within our scope of practice in dermatology, most dermatologists do not prescribe psychotropic agents. Dermatology residencies generally do not provide thorough training in psychopharmacotherapy.9 Unsurprisingly, a survey of 40 dermatologists at one academic institution found that only 11% felt comfortable prescribing an antidepressant and a mere 3% were comfortable prescribing an antipsychotic.12
Final Thoughts
The challenges involved in managing patients with primary psychiatric disease in dermatology are great and many patients are undertreated despite the availability of effective, evidence-based treatment options. We need to continue to work toward providing better access to these treatments in a way that maximizes the chance that our patients will accept our care.
- Korabel H, Dudek D, Jaworek A, et al. Psychodermatology: psychological and psychiatrical aspects of dermatology [in Polish]. Przegl Lek. 2008;65:244-248.
- Krooks JA, Weatherall AG, Holland PJ. Review of epidemiology, clinical presentation, diagnosis, and treatment of common primary psychiatric causes of cutaneous disease. J Dermatolog Treat. 2018;29:418-427.
- Bewley AP, Lepping P, Freundenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol. 2018;163:1-2.
- Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28:500-508.
- Miyamoto S, Miyake N, Ogino S, et al. Successful treatment of delusional disorder with low-dose aripiprazole. Psychiatry Clin Neurosci. 2008;62:369.
- Ladizinski B, Busse KL, Bhutani T, et al. Aripiprazole as a viable alternative for treating delusions of parasitosis. J Drugs Dermatol. 2010;9:1531-1532.
- Huang WL, Chang LR. Aripiprazole in the treatment of delusional parasitosis with ocular and dermatologic presentations. J Clin Psychopharmacol. 2013;33:272-273.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80:1428-1434.
- Bhutani T, Lee CS, Koo JYM. Psychotropic agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Elsevier Saunders; 2013:375-388.
- Duncan KO, Koo JYM. Psychocutaneous diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:128-137.
- Shah B, Levenson JL. Use of psychotropic drugs in the dermatology patient: when to start and stop? Clin Dermatol. 2018;36:748-755.
- Gee SN, Zakhary L, Keuthen N, et al. A survey assessment of the recognition and treatment of psychocutaneous disorders in the outpatient dermatology setting: how prepared are we? J Am Acad Dermatol. 2013;68:47-52.
Dermatology patients experience a high burden of mental health disturbance. Psychiatric disease is seen in an estimated 30% to 60% of our patients.1 It can be secondary to or comorbid with dermatologic disorders, or it can be the primary condition that is driving cutaneous disease. Patients with secondary or comorbid psychiatric disorders often are amenable to referral to a mental health provider or are already participating in some form of mental health treatment; however, patients with primary psychiatric disease who present to dermatology generally do not accept these referrals.2 Therefore, if these patients are to receive care anywhere in the health care system, it often must be in the department of dermatology.
What primary psychiatric conditions do we see in dermatology?
Common primary psychiatric conditions seen in dermatology include delusional infestation, obsessive-compulsive disorder and related disorders, and dermatitis artefacta.
Delusional Infestation
Also known as delusions of parasitosis or delusional parasitosis, delusional infestation presents as a fixed false belief that there is an organism or other foreign entity that is present in the skin and is the cause of cutaneous disruption. It often is an isolated delusion but can have a notable psychosocial impact. The term delusional infestation is sometimes preferred, as it is inclusive of delusions focused on any type of organism, not just parasites. It also encompasses delusions of infestation with nonliving matter such as fibers, also known as Morgellons disease.3
Obsessive-Compulsive Disorder and Related Disorders
This broad category includes several conditions encountered in dermatology. Body dysmorphic disorder (BDD), olfactory reference syndrome (ORS), excoriation disorder, and trichotillomania are some of the most common variants. In patients with BDD, skin and hair are the 2 most common preoccupations. It has been estimated that 12% of dermatology patients experience BDD. Unsurprisingly, it is more common in patients presenting to cosmetic dermatology, but general dermatology patients also are affected at a rate of 7%.2 Patients with ORS falsely believe they have body odor and/or halitosis. Excoriation disorder manifests as repetitive skin picking, either of normal skin or of lesions such as pimples and scabs. Trichotillomania presents as repeated hair pulling, and trichophagia (eating the pulled hair) also may be present.
Dermatitis Artefacta
Almost 1 in 4 patients who seek dermatologic evaluation for primarily psychiatric disorders have dermatitis artefacta, the presence of deliberately self-inflicted skin lesions.2 Patients with dermatitis artefacta have unconscious motives for their behavior and should be distinguished from malingering patients who have a conscious goal of secondary gain.
What treatments are available?
Antidepressants
Selective serotonin reuptake inhibitors are one of the first-line treatments for BDD and may be useful in ORS. In excoriation disorder and trichotillomania, selective serotonin reuptake inhibitors are the most commonly prescribed pharmacotherapy, but they have limited efficacy.2
Antipsychotics
The recommended treatment of delusional infestation is antipsychotic pharmacotherapy. Treatment with risperidone and olanzapine has been reported to achieve full or partial remission in more than two-thirds of cases.4 Aripiprazole, a newer antipsychotic, has fewer side effects and has been successful in several case reports.5-7
Cognitive Behavioral Therapy
Psychotherapy, most often in the form of cognitive behavioral therapy, has been reported as effective treatment of several psychocutaneous diseases. Cognitive behavioral therapy is considered first-line treatment of body-focused repetitive behavior disorders such as excoriation disorder and trichotillomania.2 It addresses maladaptive thought patterns to modify behavior.
Who treats patients with neurodermatoses?
If a patient presents to dermatology with a rash found to be related to an underlying thyroid disorder, the treatment plan likely would include referral to an endocrinologist. Similarly, patients with primary psychiatric conditions presenting to dermatology should ideally be referred to psychiatrists or psychotherapists, the providers most thoroughly trained and best equipped to treat them. The challenge in psychodermatology is that patients often are resistant to the assessment that the primary pathology is psychiatric. Patients may deny that they are “crazy” and see numerous providers in search of a dermatologist who “believes” them.8
Referral to mental health professionals almost always is refused by patients with primarily psychiatric neurodermatoses, which presents dermatologists with a dilemma. As the authors of the “Psychotropic Agents” chapter of Comprehensive Dermatologic Drug Therapy put it: “A dermatologist has two choices. The first is to try to ‘look the other way’ and pacify the patient by providing relatively benign, but minimally effective treatments. The other option is to try to directly address the psychological/psychiatric problems.” The chapter then provides a thorough guide for the use of psychotropic medications in the dermatology population, advocating for option 2: treatment by dermatologists.9
Should a dermatologist prescribe psychotropic drugs?
In Dermatology, the principle reference textbook in many dermatology training programs, it is stated that “[a]lthough less comprehensive than treatment delivered in collaboration with a psychiatrist, in the authors’ opinion, management of these issues by a dermatologist is better than no treatment at all.”10 Recent reviews in the dermatologic literature of psychiatric diseases and drugs in dermatology agree that dermatologists should feel comfortable with prescribing pharmacologic treatment.2,8,11 Performance of psychotherapy by dermatologists, on the other hand, is not recommended based on time constraints and lack of training.
Despite the apparent agreement in the texts and literature that pharmacotherapy of psychiatric neurodermatoses is within our scope of practice in dermatology, most dermatologists do not prescribe psychotropic agents. Dermatology residencies generally do not provide thorough training in psychopharmacotherapy.9 Unsurprisingly, a survey of 40 dermatologists at one academic institution found that only 11% felt comfortable prescribing an antidepressant and a mere 3% were comfortable prescribing an antipsychotic.12
Final Thoughts
The challenges involved in managing patients with primary psychiatric disease in dermatology are great and many patients are undertreated despite the availability of effective, evidence-based treatment options. We need to continue to work toward providing better access to these treatments in a way that maximizes the chance that our patients will accept our care.
Dermatology patients experience a high burden of mental health disturbance. Psychiatric disease is seen in an estimated 30% to 60% of our patients.1 It can be secondary to or comorbid with dermatologic disorders, or it can be the primary condition that is driving cutaneous disease. Patients with secondary or comorbid psychiatric disorders often are amenable to referral to a mental health provider or are already participating in some form of mental health treatment; however, patients with primary psychiatric disease who present to dermatology generally do not accept these referrals.2 Therefore, if these patients are to receive care anywhere in the health care system, it often must be in the department of dermatology.
What primary psychiatric conditions do we see in dermatology?
Common primary psychiatric conditions seen in dermatology include delusional infestation, obsessive-compulsive disorder and related disorders, and dermatitis artefacta.
Delusional Infestation
Also known as delusions of parasitosis or delusional parasitosis, delusional infestation presents as a fixed false belief that there is an organism or other foreign entity that is present in the skin and is the cause of cutaneous disruption. It often is an isolated delusion but can have a notable psychosocial impact. The term delusional infestation is sometimes preferred, as it is inclusive of delusions focused on any type of organism, not just parasites. It also encompasses delusions of infestation with nonliving matter such as fibers, also known as Morgellons disease.3
Obsessive-Compulsive Disorder and Related Disorders
This broad category includes several conditions encountered in dermatology. Body dysmorphic disorder (BDD), olfactory reference syndrome (ORS), excoriation disorder, and trichotillomania are some of the most common variants. In patients with BDD, skin and hair are the 2 most common preoccupations. It has been estimated that 12% of dermatology patients experience BDD. Unsurprisingly, it is more common in patients presenting to cosmetic dermatology, but general dermatology patients also are affected at a rate of 7%.2 Patients with ORS falsely believe they have body odor and/or halitosis. Excoriation disorder manifests as repetitive skin picking, either of normal skin or of lesions such as pimples and scabs. Trichotillomania presents as repeated hair pulling, and trichophagia (eating the pulled hair) also may be present.
Dermatitis Artefacta
Almost 1 in 4 patients who seek dermatologic evaluation for primarily psychiatric disorders have dermatitis artefacta, the presence of deliberately self-inflicted skin lesions.2 Patients with dermatitis artefacta have unconscious motives for their behavior and should be distinguished from malingering patients who have a conscious goal of secondary gain.
What treatments are available?
Antidepressants
Selective serotonin reuptake inhibitors are one of the first-line treatments for BDD and may be useful in ORS. In excoriation disorder and trichotillomania, selective serotonin reuptake inhibitors are the most commonly prescribed pharmacotherapy, but they have limited efficacy.2
Antipsychotics
The recommended treatment of delusional infestation is antipsychotic pharmacotherapy. Treatment with risperidone and olanzapine has been reported to achieve full or partial remission in more than two-thirds of cases.4 Aripiprazole, a newer antipsychotic, has fewer side effects and has been successful in several case reports.5-7
Cognitive Behavioral Therapy
Psychotherapy, most often in the form of cognitive behavioral therapy, has been reported as effective treatment of several psychocutaneous diseases. Cognitive behavioral therapy is considered first-line treatment of body-focused repetitive behavior disorders such as excoriation disorder and trichotillomania.2 It addresses maladaptive thought patterns to modify behavior.
Who treats patients with neurodermatoses?
If a patient presents to dermatology with a rash found to be related to an underlying thyroid disorder, the treatment plan likely would include referral to an endocrinologist. Similarly, patients with primary psychiatric conditions presenting to dermatology should ideally be referred to psychiatrists or psychotherapists, the providers most thoroughly trained and best equipped to treat them. The challenge in psychodermatology is that patients often are resistant to the assessment that the primary pathology is psychiatric. Patients may deny that they are “crazy” and see numerous providers in search of a dermatologist who “believes” them.8
Referral to mental health professionals almost always is refused by patients with primarily psychiatric neurodermatoses, which presents dermatologists with a dilemma. As the authors of the “Psychotropic Agents” chapter of Comprehensive Dermatologic Drug Therapy put it: “A dermatologist has two choices. The first is to try to ‘look the other way’ and pacify the patient by providing relatively benign, but minimally effective treatments. The other option is to try to directly address the psychological/psychiatric problems.” The chapter then provides a thorough guide for the use of psychotropic medications in the dermatology population, advocating for option 2: treatment by dermatologists.9
Should a dermatologist prescribe psychotropic drugs?
In Dermatology, the principle reference textbook in many dermatology training programs, it is stated that “[a]lthough less comprehensive than treatment delivered in collaboration with a psychiatrist, in the authors’ opinion, management of these issues by a dermatologist is better than no treatment at all.”10 Recent reviews in the dermatologic literature of psychiatric diseases and drugs in dermatology agree that dermatologists should feel comfortable with prescribing pharmacologic treatment.2,8,11 Performance of psychotherapy by dermatologists, on the other hand, is not recommended based on time constraints and lack of training.
Despite the apparent agreement in the texts and literature that pharmacotherapy of psychiatric neurodermatoses is within our scope of practice in dermatology, most dermatologists do not prescribe psychotropic agents. Dermatology residencies generally do not provide thorough training in psychopharmacotherapy.9 Unsurprisingly, a survey of 40 dermatologists at one academic institution found that only 11% felt comfortable prescribing an antidepressant and a mere 3% were comfortable prescribing an antipsychotic.12
Final Thoughts
The challenges involved in managing patients with primary psychiatric disease in dermatology are great and many patients are undertreated despite the availability of effective, evidence-based treatment options. We need to continue to work toward providing better access to these treatments in a way that maximizes the chance that our patients will accept our care.
- Korabel H, Dudek D, Jaworek A, et al. Psychodermatology: psychological and psychiatrical aspects of dermatology [in Polish]. Przegl Lek. 2008;65:244-248.
- Krooks JA, Weatherall AG, Holland PJ. Review of epidemiology, clinical presentation, diagnosis, and treatment of common primary psychiatric causes of cutaneous disease. J Dermatolog Treat. 2018;29:418-427.
- Bewley AP, Lepping P, Freundenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol. 2018;163:1-2.
- Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28:500-508.
- Miyamoto S, Miyake N, Ogino S, et al. Successful treatment of delusional disorder with low-dose aripiprazole. Psychiatry Clin Neurosci. 2008;62:369.
- Ladizinski B, Busse KL, Bhutani T, et al. Aripiprazole as a viable alternative for treating delusions of parasitosis. J Drugs Dermatol. 2010;9:1531-1532.
- Huang WL, Chang LR. Aripiprazole in the treatment of delusional parasitosis with ocular and dermatologic presentations. J Clin Psychopharmacol. 2013;33:272-273.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80:1428-1434.
- Bhutani T, Lee CS, Koo JYM. Psychotropic agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Elsevier Saunders; 2013:375-388.
- Duncan KO, Koo JYM. Psychocutaneous diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:128-137.
- Shah B, Levenson JL. Use of psychotropic drugs in the dermatology patient: when to start and stop? Clin Dermatol. 2018;36:748-755.
- Gee SN, Zakhary L, Keuthen N, et al. A survey assessment of the recognition and treatment of psychocutaneous disorders in the outpatient dermatology setting: how prepared are we? J Am Acad Dermatol. 2013;68:47-52.
- Korabel H, Dudek D, Jaworek A, et al. Psychodermatology: psychological and psychiatrical aspects of dermatology [in Polish]. Przegl Lek. 2008;65:244-248.
- Krooks JA, Weatherall AG, Holland PJ. Review of epidemiology, clinical presentation, diagnosis, and treatment of common primary psychiatric causes of cutaneous disease. J Dermatolog Treat. 2018;29:418-427.
- Bewley AP, Lepping P, Freundenmann RW, et al. Delusional parasitosis: time to call it delusional infestation. Br J Dermatol. 2018;163:1-2.
- Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28:500-508.
- Miyamoto S, Miyake N, Ogino S, et al. Successful treatment of delusional disorder with low-dose aripiprazole. Psychiatry Clin Neurosci. 2008;62:369.
- Ladizinski B, Busse KL, Bhutani T, et al. Aripiprazole as a viable alternative for treating delusions of parasitosis. J Drugs Dermatol. 2010;9:1531-1532.
- Huang WL, Chang LR. Aripiprazole in the treatment of delusional parasitosis with ocular and dermatologic presentations. J Clin Psychopharmacol. 2013;33:272-273.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80:1428-1434.
- Bhutani T, Lee CS, Koo JYM. Psychotropic agents. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. China: Elsevier Saunders; 2013:375-388.
- Duncan KO, Koo JYM. Psychocutaneous diseases. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. China: Elsevier; 2018:128-137.
- Shah B, Levenson JL. Use of psychotropic drugs in the dermatology patient: when to start and stop? Clin Dermatol. 2018;36:748-755.
- Gee SN, Zakhary L, Keuthen N, et al. A survey assessment of the recognition and treatment of psychocutaneous disorders in the outpatient dermatology setting: how prepared are we? J Am Acad Dermatol. 2013;68:47-52.
Resident Pearl
- Patients often present to dermatology with primary psychologic disorders such as delusional infestation or trichotillomania. Treatment of such conditions with antidepressants and antipsychotics can be highly effective and is within our scope of practice. Increased emphasis on psychopharmacotherapy in dermatology training would increase access to appropriate care for this patient population.
Bimatoprost-Induced Iris Hyperpigmentation: Beauty in the Darkened Eye of the Beholder
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
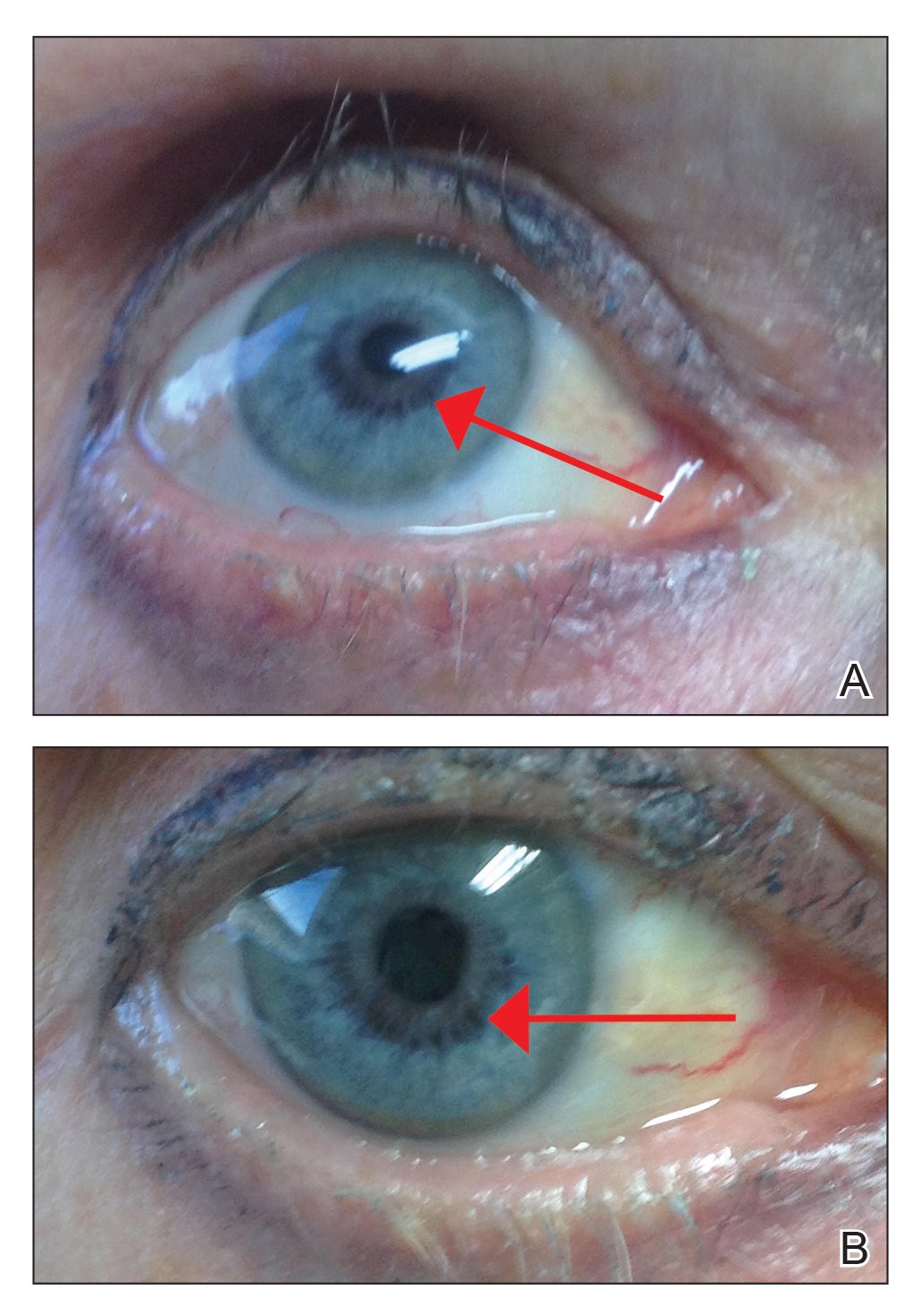
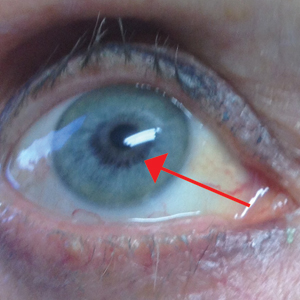
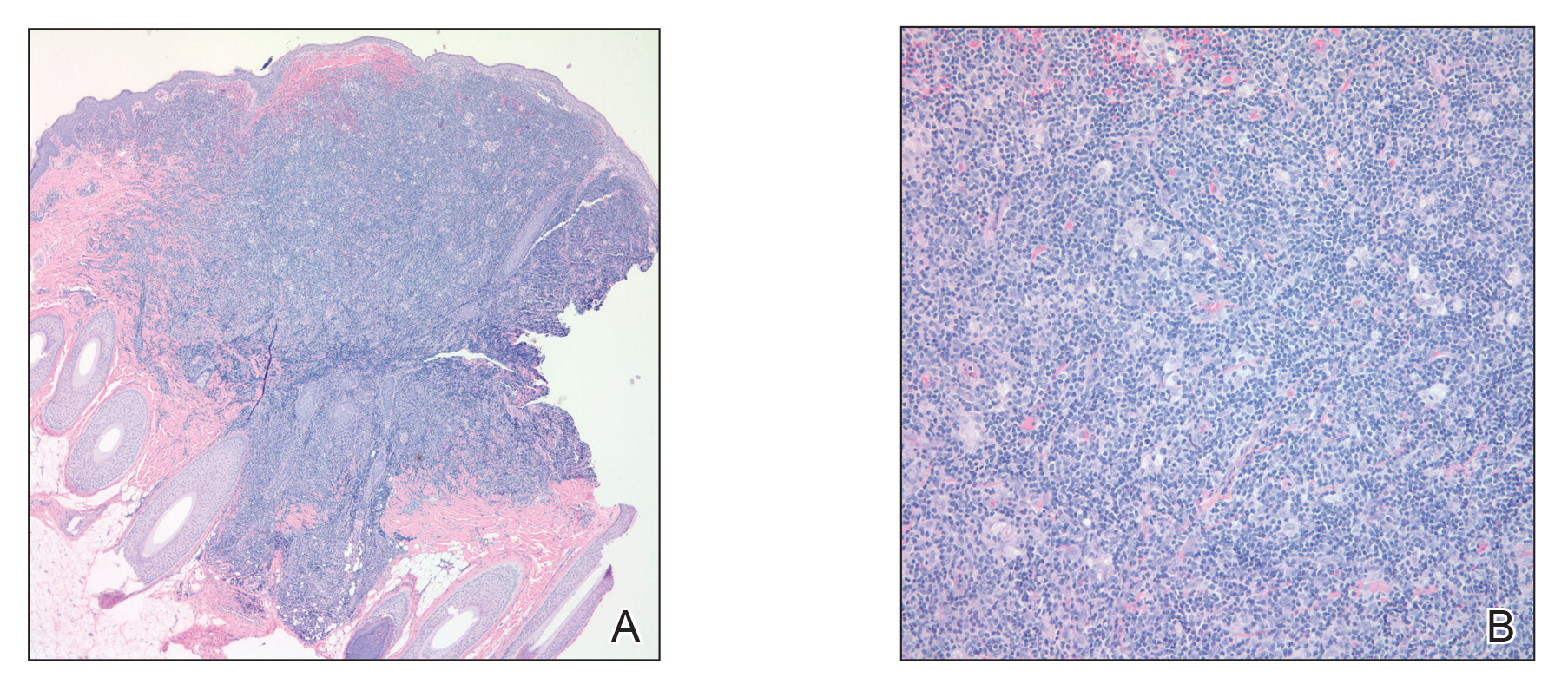
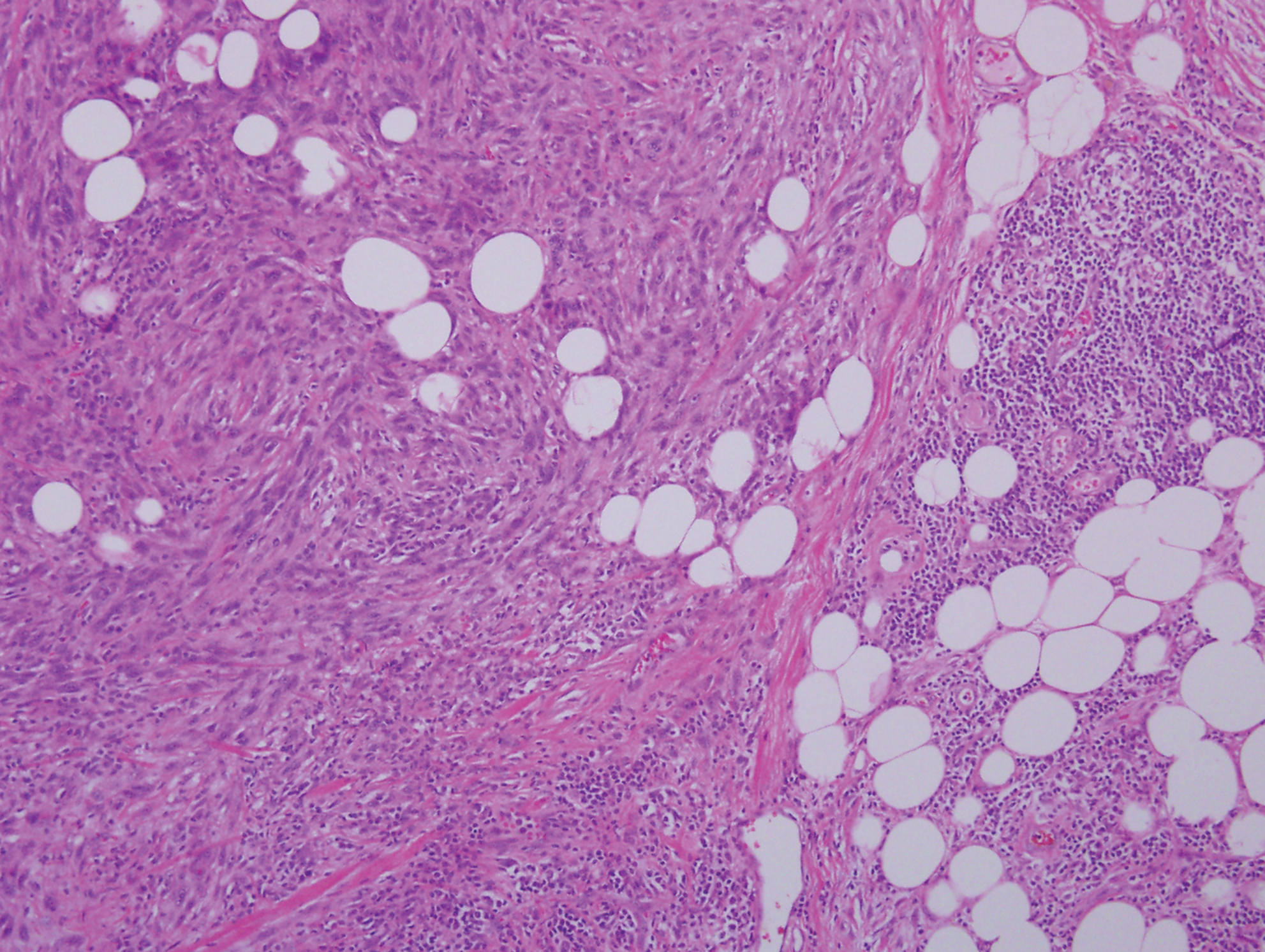
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).
The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).
The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
To the Editor:
Long, dark, and thick eyelashes have been a focal point of society’s perception of beauty for thousands of years,1 and the use of makeup products such as mascaras, eyeliners, and eye shadows has further increased the perception of attractiveness of the eyes.2 Many eyelash enhancement methods have been developed or in some instances have been serendipitously discovered. Bimatoprost ophthalmic solution 0.03% originally was developed as an eye drop that was approved by the US Food and Drug Association (FDA) in 2001 for the reduction of elevated intraocular pressure in patients with open-angle glaucoma or ocular hypertension. An unexpected side effect of this product was eyelash hypertrichosis.3,4 As a result, the FDA approved
Because all follicular development occurs during embryogenesis, the number of eyelash follicles does not increase over time.6 Bitmatoprost eyelash solution works by prolonging the anagen (growth) phase of the eyelashes and stimulating the transition from the telogen (dormant) phase to the anagen phase. It also has been shown to increase the hair bulb diameter of follicles undergoing the anagen phase, resulting in thicker eyelashes.7 Although many patients have enjoyed this unexpected indication, prostaglandin (PG) analogues such as bimatoprost and latanoprost have a well-documented history of ocular side effects when applied directly to the eye. The most common adverse reactions include eye pruritus, conjunctival hyperemia, and eyelid pigmentation.3 The product safety information indicates that eyelid pigmentation typically is reversible.3,5 Iris pigmentation is perhaps the least desirable side effect of PG analogues and was first noted in latanoprost studies on primates.8 The underlying mechanism appears to be due to an increase in melanogenesis that results in an increase in melanin granules without concomitant proliferation of melanocytes, cellular atypia, or evidence of inflammatory reaction. Unfortunately, this pigmentation typically is permanent.3,5,9
Studies have shown that
An otherwise healthy 63-year-old woman presented to our clinic for an annual skin examination. She noted that she had worsening dark pigmentation of the bilateral irises. The patient did not have any personal or family history of melanoma or ocular nevi, and there were no associated symptoms of eye tearing, pruritus, burning, or discharge. No prior surgical procedures had been performed on or around the eyes, and the patient never used contact lenses. She had been intermittently using bimatoprost eyelash solution prescribed by an outside physician for approximately 3 years to enhance her eyelashes. Although she never applied the product directly into her eyes, she noted that she often was unmethodical in application of the product and that runoff from the product may have occasionally leaked into the eyes. Physical examination revealed bilateral blue irises with ink spot–like, grayish black patches encircling the bilateral pupils (Figure).
The patient was advised to stop using the product, but no improvement of the iris hyperpigmentation was appreciated at 6-month follow-up. The patient declined referral to ophthalmology for evaluation to confirm a diagnosis and discuss treatment because the hyperpigmentation did not bother her.
There have been several studies of iris hyperpigmentation with use of PG analogues in the treatment of glaucoma. In a phase 3 clinical trial of the safety and efficacy of latanoprost for treatment of ocular hypertension, it was noted that 24 (12%) of 198 patients experienced iris hyperpigmentation and that patients with heterogeneous pigmentation (ie, hazel irises and mixed coloring) were at an increased risk.11 Other studies also have shown an increased risk of iris hyperpigmentation due to heterogeneous phenotype12 as well as older age.13
Reports of bimatoprost eye drops used for treatment of glaucoma have shown a high incidence of iris hyperpigmentation with long-term use. A prospective study conducted in 2012 investigated the adverse events of bimatoprost eye drops in 52 Japanese patients with glaucoma or ocular hypertension. Clinical photographs of the irises, eyelids, and eyelashes were taken at baseline and after 6 months of treatment. It was noted that 50% (26/52) of participants experienced iris hyperpigmentation upon completion of treatment.10
In our patient, bimatoprost eyelash solution was applied to the top eyelid margins using an applicator; our patient did not use the eye drop formulation, which is directed for use in ocular hypertension or glaucoma. A PubMed search of articles indexed for MEDLINE using the terms bimatoprost and iris hyperpigmentation yielded no published peer-reviewed studies or case reports of iris hyperpigmentation caused by bimatoprost eyelash solution for treatment of eyelid hypotrichosis, which makes this case report novel. With that said, the package insert states iris hyperpigmentation as a side effect in the prescribing information for both a bimatoprost eye drop formulation used to treat ocular hypertension3 as well as a formulation for topical application on the eyelids/eyelashes.5 A 2014 retrospective review of long-term safety with bimatoprost eyelash solution for eyelash hypotrichosis reported 4 instances (0.7%) of documented adverse events after 12 months of use in 585 patients, including dry eye, eyelid erythema, ocular pruritus, and low ocular pressure. Iris hyperpigmentation was not reported.14
The method of bimatoprost application likely is a determining factor in the number of reported adverse events. Studies with similar treatment periods have demonstrated more adverse events associated with bimatoprost eye drops vs eyelash solution.15,16 When bimatoprost is used in the eye drop formulation for treatment of glaucoma, iris hyperpigmentation has been estimated to occur in 1.5%4 to 50%9 of cases. To our knowledge, there are no documented cases when bimatoprost eyelash solution is applied with a dermal applicator for treatment of eyelash hypotrichosis.15,17 These results may be explained using an ocular splash test. In one study using lissamine green dye, decreased delivery of bimatoprost eyelash solution with the dermal applicator was noted vs eye drop application. Additionally, it has been demonstrated that approximately 5% (based on weight) of a one-drop dose of bimatoprost eyelash solution applied to the dermal applicator is actually delivered to the patient.18 The rest of the solution remains on the applicator.
It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information (eg, clean the face, remove makeup and contact lenses prior to applying the product). The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye. One drop of bimatoprost eyelash solution should be applied to the applicator supplied by the manufacturer and distributed evenly along the skin of the upper eyelid margin at the base of the eyelashes. It is important to blot any excess solution runoff outside the upper eyelid margin.5 Of note, our patient admitted to not always doing this step, which may have contributed to her susceptibility to this rare side effect.
Prostaglandin analogues have been observed to cause iris hyperpigmentation when applied directly to the eye for use in the treatment of glaucoma.19 Theoretically, the same side-effect profile should apply in their use as a dermal application on the eyelids. For this reason, one manufacturer includes iris hyperpigmentation as an adverse side effect in the prescribing information.5 It is important for physicians who prescribe bimatoprost eyelash solution to inform patients of this rare yet possible side effect and to instruct patients on proper application to minimize hyperpigmentation.
Our literature review did not demonstrate previous cases of iris hyperpigmentation associated with bimatoprost eyelash solution. One study suggested that 2 patients experienced hypopigmentation; however, this was not clinically significant and was not consistent with the proposed iris pigmentation thought to be caused by bimatoprost eyelash solution.20
Potential future applications and off-label uses of bimatoprost include treatment of eyelash hypotrichosis on the lower eyelid margin and eyebrow hypertrichosis, as well as androgenic alopecia, alopecia areata, chemotherapy-induced alopecia, vitiligo, and hypopigmented scarring.21 Currently, investigational studies are looking at bimatoprost ophthalmic solution 0.03% for chemotherapy-induced eyelash hypotrichosis with positive results.22 In the future, bimatoprost may be used for other off-label and possibly FDA-approved uses.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
- Draelos ZD. Special considerations in eye cosmetics. Clin Dermatol. 2001;19:424-430.
- Mulhern R, Fieldman G, Hussey T, et al. Do cosmetics enhance female Caucasian facial attractiveness? Int J Cosmet Sci. 2003;25:199-205.
- Lumigan [package insert]. Irvine, CA: Allergan, Inc; 2012.
- Higginbotham EJ, Schuman JS, Goldberg I, et al; Bimatoprost Study Groups 1 and 2. one-year, randomized study comparing bimatoprost and timolol in glaucoma and ocular hypertension. Arch Ophthalmol. 2002;120:1286-1293.
- Latisse [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Hair diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Treatment. 4th ed. St. Louis, MO: C.V. Mosby Company; 2003. 7. Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Selen G, Stjernschantz J, Resul B. Prostaglandin-induced iridial pigmentation in primates. Surv Opthalmol. 1997;41(suppl 2):S125-128.
- Stjernschantz JW, Albert DM, Hu D-N, et al. Mechanism and clinical significance of prostaglandin-induced iris pigmentation. Surv Ophthalmol. 2002;47(suppl 1):162S-S175S.
- Inoue K, Shiokawa M, Sugahara M, et al. Iris and periocular adverse reactions to bimatoprost in Japanese patients with glaucoma or ocular hypertension. Clin Ophthalmol. 2012;6:111-116.
- Alm A, Camras C, Watson P. Phase III latanoprost studies in Scandinavia, the United Kingdom and the United States. Surv Ophthalmol. 1997;41(suppl 2):S105-S110.
- Wistrand PJ, Stjernschantz J, Olsson K. The incidence and time-course of latanoprost-induced iridial pigmentation as a function of eye color. Surv Ophthalmol. 1997;41(suppl 2):S129-S138.
- Arranz-Marquez E, Teus MA. Effect of age on the development of a latanoprost-induced increase in iris pigmentation. Ophthalmology. 2007;114:1255-1258.
- Yoelin S, Fagien S, Cox S, et al. A retrospective review and observational study of outcomes and safety of bimatoprost ophthalmic solution 0.03% for treating eyelash hypotrichosis. Dermatol Surg. 2014;40:1118-1124.
- Brandt JD, VanDenburgh AM, Chen K, et al; Bimatoprost Study Group. Comparison of once- or twice-daily bimatoprost with twice-daily timolol in patients with elevated IOP: a 3-month clinical trial. Ophthalmology. 2001;108:1023-1031; discussion 1032.
- Fagien S, Walt JG, Carruthers J, et al. Patient-reported outcomes of bimatoprost for eyelash growth: results from a randomized, double-masked, vehicle-controlled, parallel-group study. Aesthet Surg J. 2013;33:789-798.
- Yoelin S, Walt JG, Earl M. Safety, effectiveness, and subjective experience with topical bimatoprost 0.03% for eyelash growth. Dermatol Surg. 2010;36:638-649.
- Fagien S. Management of hypotrichosis of the eyelashes: focus on bimatoprost. Clin Cosmet Investig Dermatol. 2010;2:29-48.
- Rodríguez-Agramonte F, Jiménez JC, Montes JR. Periorbital changes associated with topical prostaglandins analogues in a Hispanic population. P R Health Sci J. 2017;36:218-222.
- Wirta D, Baumann L, Bruce S, et al. Safety and efficacy of bimatoprost for eyelash growth in postchemotherapy subjects. J Clin Aesthet Dermatol. 2015;8:11-20.
- Choi YM, Diehl J, Levins PC. Promising alternative clinical uses of prostaglandin F2α analogs: beyond the eyelashes [published online January 16, 2015]. J Am Acad Dermatol. 2015;72:712-716.
- Ahluwalia GS. Safety and efficacy of bimatoprost solution 0.03% topical application in patients with chemotherapy-induced eyelash loss. J Investig Dermatol Symp Proc. 2013;16:S73-S76.
Practice Points
- Bimatoprost ophthalmic solution 0.03% was approved by the US Food and Drug Administration in 2008 as an eyelash solution with an eyelid applicator for treatment of eyelash hypotrichosis.
- Iris hyperpigmentation can occur when bimatoprost eye drops are applied to the eyes for treatment of ocular hypertension and glaucoma, but reports associated with bimatoprost eyelash solution are rare.
- It is important that patients use bimatoprost eyelash solution as instructed in the prescribing information to avoid potential adverse events. The eyelid should not be rinsed after application, which limits the possibility of the bimatoprost solution from contacting or pooling in the eye.
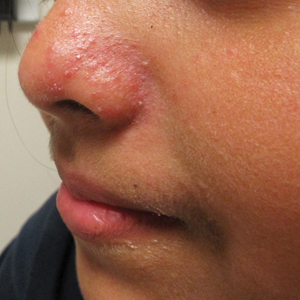
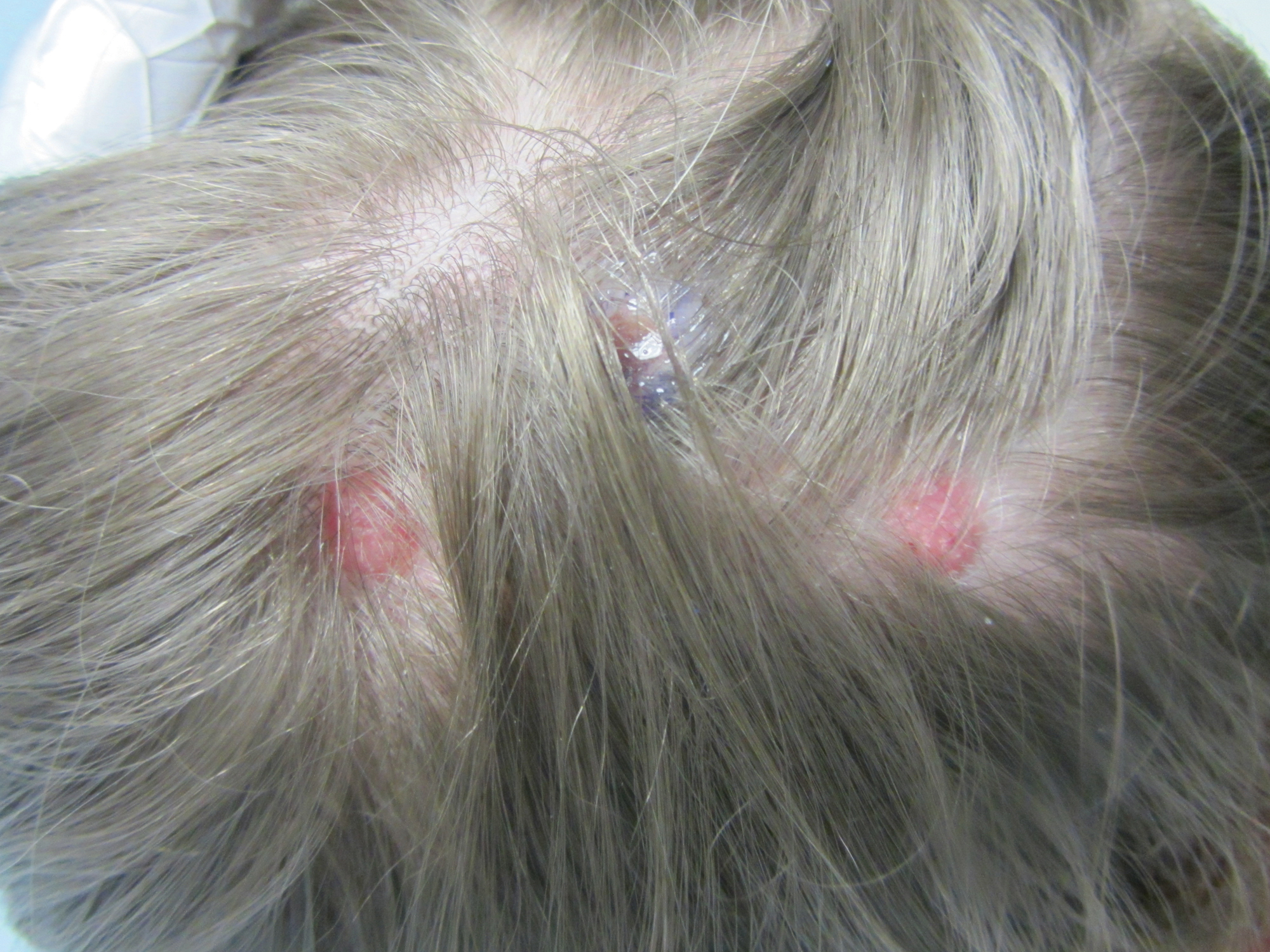
Erythematous Papules and Pustules on the Nose
The Diagnosis: Granulosis Rubra Nasi
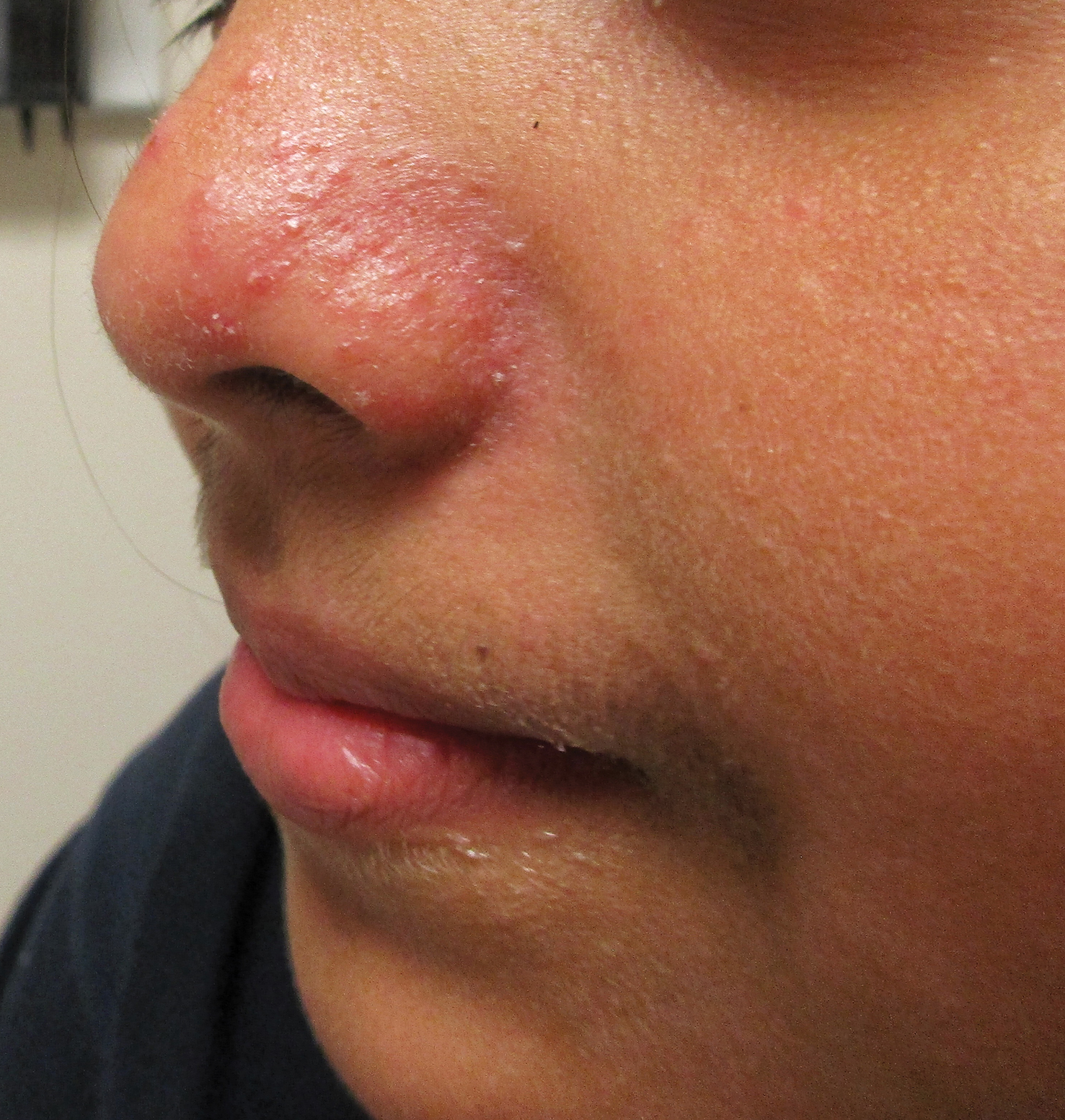
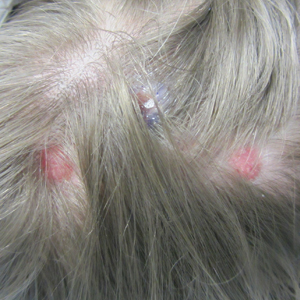
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
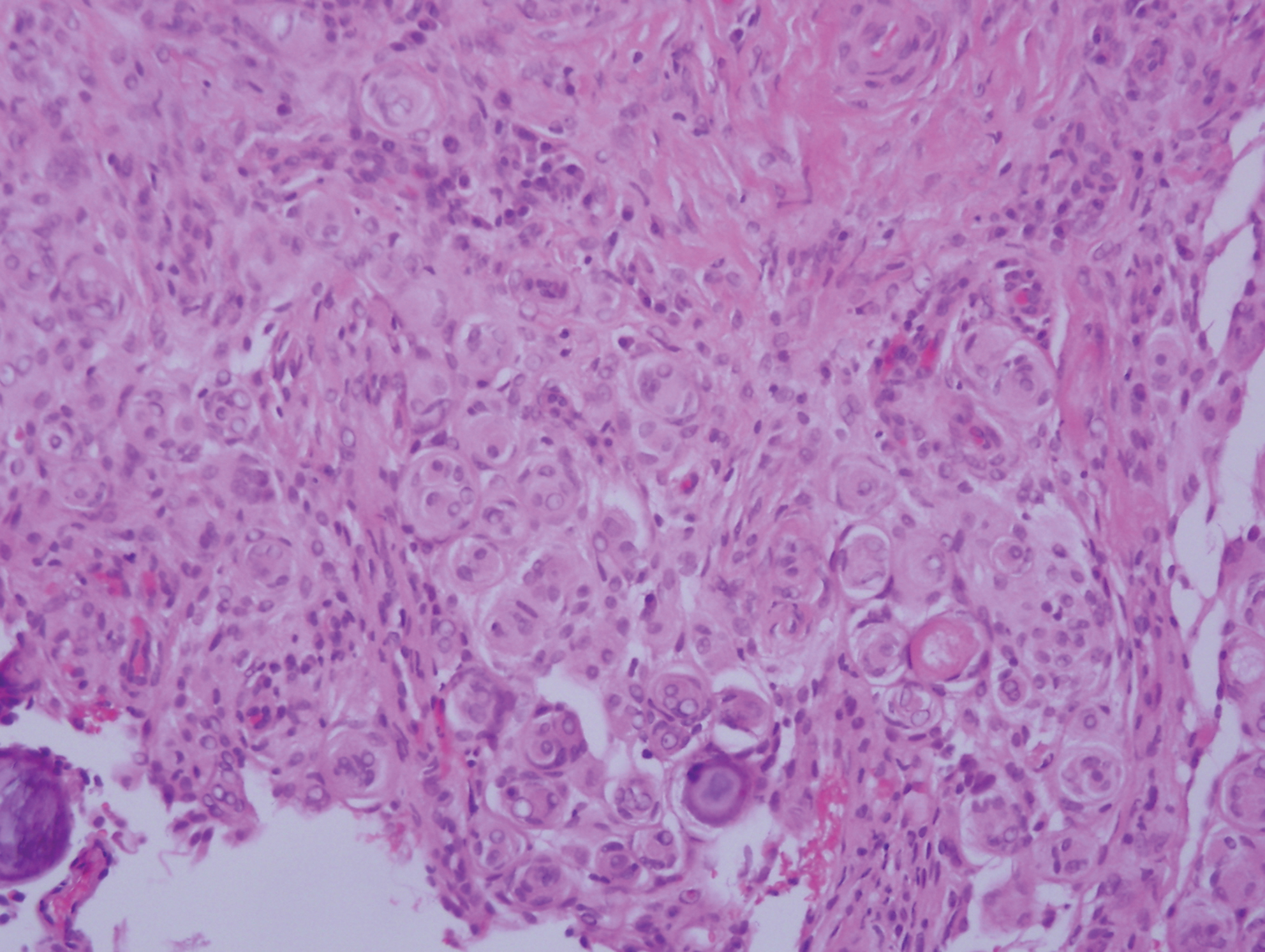
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
The Diagnosis: Granulosis Rubra Nasi
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
The Diagnosis: Granulosis Rubra Nasi
A history of prominent nasal sweating was later elicited and the patient was subsequently diagnosed with granulosis rubra nasi. She was instructed to continue daily use of topical pimecrolimus with the addition of topical atropine, resulting in complete resolution of the eruption at 6-week follow-up (Figure, A). She was then maintained on topical atropine monotherapy, only noting recurrence with cessation of the atropine (Figure, B).
Other successful treatment regimens of granulosis rubra nasi include injection of botulinum toxin into the nose,1 monotherapy with topical tacrolimus,2 topical indomethacin, steroids, and cryotherapy, among other modalities.1 Topical atropine and pimecrolimus were selected as first-line agents for treating our pediatric patient due to tolerability and their anti-inflammatory and anticholinergic properties.
Granulosis rubra nasi is a form of focal hyperhidrosis that presents as erythematous papules, pustules, and vesicles of the midface, especially the nose.3 It is a fairly rare condition that can mimic many other common clinical entities, including comedonal acne, nevus comedonicus, periorificial dermatitis, and tinea faciei, but is resistant to treatments aimed at these disorders. It was first described as a "peculiar disease of the skin of the nose in children" in a case report by Jadassohn4 in 1901. It is most common in children aged 7 to 12 years and typically resolves at puberty; adults rarely are affected. Although the etiology has not yet been elucidated, autosomal-dominant transmission has been described, and the cutaneous changes are hypothesized to be secondary to hyperhidrosis.5 This postulation is further corroborated by a case report of a pheochromocytoma-associated granulosis rubra nasi that resolved with surgical excision of the pheochromocytoma.6 It is not uncommon for patients to have concomitant palmoplantar hyperhidrosis and acrocyanosis.5 Histopathologic examination is not necessary for diagnosis, but when performed, it discloses a mononuclear cellular infiltrate surrounding eccrine sweat ducts, blood vessels, and lymphatics without other abnormalities of the epidermis or pilosebaceous unit.1-3,7
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
- Grazziotin TC, Buffon RB, Da Silva Manzoni AP, et al. Treatment of granulosis rubra nasi with botulinum toxin. Dermatol Surg. 2009;35:1298-1299.
- Kumar P, Gosai A, Mondal AK, et al. Granulosis rubra nasi: a rare condition treated successfully with topical tacrolimus. Dermatol Reports. 2012;4:E5.
- Sargunam C, Thomas J, Ahmed NA. Granulosis rubra nasi. Indian Dermatol Online J. 2013;4:208-209.
- Jadassohn J. Ueber eine eigenartige erkrankung der nasenhaut bei kindern. Arch Derm Syph. 1901;58:145-158.
- Hellier FF. Granulosis rubra nasi in a mother and daughter. Br Med J. 1937;2:1068.
- Heid E, Samain F, Jelen G, et al. Granulosis rubra nasi and pheochromocytoma. Ann Dermatol Venereol. 1996;123:106-108.
- Akhdari N. Granulosis rubra nasi. Int J Dermatol. 2007;46:396.
A healthy 9-year-old girl presented with a 2-year history of erythematous papules and pustules on the nose. There was no involvement of the rest of the face or body. At the time of presentation, she had been treated with several topical therapies including steroids, calcineurin inhibitors, antibiotics, and retinoids without improvement. A potassium hydroxide preparation from a pustule was performed and revealed only normal keratinocytes.
Monitoring Acne Patients on Oral Therapy: Survey of the Editorial Board
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
To improve patient care and outcomes, leading dermatologists from the Cutis and Dermatology News Editorial Boards answered 5 questions on monitoring acne patients on oral therapy. Here’s what we found.
Do you check potassium levels for patients taking spironolactone for acne?
Half of dermatologists surveyed never check potassium levels for patients taking spironolactone for acne. For those who do check levels, 8% do it at baseline only, 8% at baseline and every 6 months, 23% at baseline and yearly, and 13% at baseline and for dosing changes.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Although some dermatologists are still checking for potassium levels in patients taking spironolactone for acne, there is a clear trend toward foregoing laboratory monitoring. This change was likely spurred by a retrospective study of healthy young women taking spironolactone for acne that found a hyperkalemia rate of 0.72%, which is practically equivalent to the 0.76% baseline rate of hyperkalemia in this age group. Furthermore, since repeat testing in 6 of 13 patients showed normal values, the original potassium measurements may have been erroneous. Based on this study, routine potassium monitoring is likely unnecessary for healthy young women taking spironolactone for acne (Plovanich et al). In another retrospective study of women aged 18 to 65 years taking spironolactone for acne, women aged 46 to 65 years had a significantly higher rate of hyperkalemia with spironolactone compared with women aged 18 to 45 years (2/12 women [16.7%] vs 1/112 women [<1%]; P=.0245). Based on this study, potassium monitoring may be indicated for women older than 45 years taking spironolactone for acne (Thiede et al).
Next page: Cholesterol levels
Do you monitor cholesterol levels in patients taking isotretinoin?
Almost two-thirds of dermatologists indicated that they monitor all cholesterol levels in patients taking isotretinoin, including low-density lipoprotein, high-density lipoprotein, very low-density lipoprotein, and triglycerides, but almost one-third monitor triglycerides only. Five percent do not monitor cholesterol levels.
Do you routinely monitor cholesterol levels in patients taking isotretinoin?
More than 80% of dermatologists surveyed routinely monitor cholesterol levels in patients taking isotretinoin, with almost half (45%) at baseline and every 2 to 3 months. Eight percent check levels at baseline only, 28% at baseline and monthly, and 3% at baseline and end of therapy. Eighteen percent indicated they do not routinely monitor cholesterol levels.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
In this survey, dermatologists most often check cholesterol levels at baseline and then every 2 to 3 months, with most monitoring all cholesterol types. Elevations in cholesterol are by far the most common laboratory abnormality seen with isotretinoin therapy. In a retrospective study of 515 patients undergoing isotretinoin treatment of acne, mild to moderate triglyceride elevations were seen in 23.5% of patients (Hansen et al). At least in part, these elevations are likely due to the fact the levels were not drawn during fasting. Keep in mind that triglyceride-induced pancreatitis due to isotretinoin is remarkably rare, so monthly screening for triglycerides is likely not warranted. It is reasonable to monitor triglyceride levels during isotretinoin dose adjustments and for patients whose values are trending upward.
Next page: Monitoring CBC
Do you routinely monitor complete blood cell count (CBC) in patients taking isotretinoin?
More than half (55%) of dermatologists surveyed routinely monitor complete blood cell (CBC) counts in patients taking isotretinoin, while 45% do not. Of those who do monitor CBC, 13% do so at baseline only, 26% at baseline and monthly, 13% at baseline only and every 2 to 3 months, and only 3% at baseline and end of therapy.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
Slightly more than half of dermatologists in this survey are obtaining CBC for their patients taking isotretinoin for acne and many of those are performing the test at baseline and monthly. Multiple studies as well as American Academy of Dermatology guidelines have substantiated that routine CBC monitoring is unwarranted in healthy patients, as abnormal values usually resolve or remain stable with therapy (American Academy of Dermatology, Isotretinoin: Recommendations). Therefore, it is worthwhile to consider foregoing CBC testing or obtaining just a baseline CBC in healthy patients being treated with isotretinoin.
Next page: Pregnancy testing
Which pregnancy test do you perform on female patients taking isotretinoin?
More than 40% of dermatologists surveyed use the urine β-human chorionic gonadotropin (hCG) pregnancy test for female patients taking isotretinoin, while 30% use the serum B-hCG test; 28% indicated that they use both tests.
Expert Commentary
Provided by Shari R. Lipner, MD, PhD (New York, New York)
The iPLEDGE program was implemented in 2006 to avoid fetal exposure to isotretinoin and requires pregnancy testing (urine or serum) for females of childbearing potential taking isotretinoin. In a study of pregnancy-related adverse events associated with isotretinoin reported to the US Food and Drug Administration, 6740 total pregnancies were reported from 1997 to 2017. The rate peaked with 768 pregnancies in 2006 and then decreased. Because several hundred pregnancies in women taking isotretinoin have been reported yearly in the last 10 years, there is a clear need to have better systems in place and patient education to prevent fetal exposure to isotretinoin.
Next page: More tips from derms
More Tips From Dermatologists
The dermatologists we polled had the following advice for their peers:
I see lab monitoring as an opportunity to engage patients and families in co-directing their care (ie, practice patient- and family-centered care). Some families and patients like frequent monitoring and some want as few blood draws as possible. I do my best to make sure the decision includes components of the patients’ preferences, medical evidence and my best clinical judgement.—Craig Burkhart, MD, MS, MPH (Chapel Hill, North Caroline)
Being familiar with and following the standard of care guidelines for the individual oral therapies used in the treatment of acne is very important. However, it is equally as important to assure the individual patient (medical history, physical examination, social history, etc) is taken into consideration to determine if additional monitoring is required.—Fran E. Cook-Bolden, MD (New York, New York)
The trend seems to be towards less routine monitoring other than pregnancy. Baseline tests may pick out the occasional patient with comorbidities that would preclude or delay treatment, but the majority of patients may not need the repetitive and costly testing that we have done in the past.—Richard Glogau, MD (San Francisco, California)
I have loosened my lab monitoring with isotretinoin over the past few years. If a patient has normal lipid values, comprehensive panel and complete blood cell count for the first 3 months of tests, I skip labs until the end of therapy.—Lawrence J. Green, MD (Washington, DC)
Interestingly, we focus quite a bit of attention on the risk of pregnancy with isotretinoin, and often don't focus enough on the risk with spironolactone. In our practice, we are careful to warn the patients on spironolactone about pregnancy prevention.—Stephen Stone, MD (Springfield, Illinois)
About This Survey
The survey was fielded electronically to Cutis and Dermatology News Editorial Board Members within the United States from May 5, 2019, to June 23, 2019. A total of 40 usable responses were received.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
American Academy of Dermatology. Isotretinoin: recommendations. https://www.aad.org/practicecenter/quality/clinical-guidelines/acne/isotretinoin. Accessed August 20, 2019.
Hansen TJ, Lucking S, Miller JJ, et al. Standardized laboratory monitoring with use of isotretinoin in acne. J Am Acad Dermatol. 2016;75:323-328.
Plovanich M, Weng QY, Mostaghimi A. Low usefulness of potassium monitoring among healthy young women taking spironolactone for acne. JAMA Dermatol. 2015;151:941-944.
Thiede RM, Rastogi S, Nardone B, et al. Hyperkalemia in women with acne exposed to oral spironolactone: a retrospective study from the RADAR (Research on Adverse Drug Events and Reports) program. Int J Womens Dermatol. 2019;5:155-157.
Tkachenko E, Singer S, Sharma P, et al. US Food and Drug Administration reports of pregnancy and pregnancy-related adverse events associated with isotretinoin [published online July 17, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2019.1388.
Considerations for Psoriasis in Pregnancy
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
1. Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
2. Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
3. Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
4. Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
5. Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
6. Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
7. Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
8. Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
9. Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
10. Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
11. Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
12. Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
13. Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrow¬band UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
Psoriasis in Pregnancy
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
Pustular psoriasis of pregnancy (PPP) is a rare but potentially serious dermatosis of pregnancy. Left untreated, PPP can be fatal for both the mother and the fetus.1,2 Contrary to many other pregnancy dermatoses, which are typically limited to the skin, systemic signs and symptoms often accompany PPP, including fatigue, fever, diarrhea, delirium, elevated markers of inflammation such as an increased erythrocyte sedimentation rate, and increased white blood cell counts.1,3,4 Progression of the rash to erythroderma with subsequent dangerous fluid and electrolyte imbalances, loss of thermoregulation in the skin, and the risk for secondary infection and sepsis can occur in severe cases.1,5
Increasing evidence suggests that PPP is likely a variant of generalized pustular psoriasis (GPP), which can be vulnerable to a variety of triggers, including metabolic disturbances, systemic steroid withdrawals, and pregnancy; however, classification of the disease as either a variant of disease or a distinct disease state remains controversial.1,6 Early recognition and prompt treatment are critically important given the potential for fetal and maternal morbidity and mortality that is associated with PPP.1,6,7
Clinical Presentation
Most cases of PPP involve presentation in the early part of the third trimester of pregnancy; postpartum PPP has been reported but is exceedingly rare.1 Typically, lesions develop in the skin folds and spread centrifugally.2,6 The lesions usually begin as erythematous plaques with a pustular ring with a central erosion. The face, palms, and soles of the feet usually are spared; occasionally, involvement of oral and esophageal mucosae is seen. Biopsy findings usually comprise spongiform pustules with neutrophil invasion into the epidermis. Characteristic laboratory findings include electrolyte derangements with elevated erythrocyte sedimentation rate and leukocytosis.2,8
As was seen in the case presentation, PPP largely resolves following childbirth; however, there is a significant risk for recurrence in subsequent pregnancies, which may be more severe and present earlier.1,6 Menstrual cycle changes and the use of oral contraceptives, particularly those containing progesterone, also have been associated with PPP flares.1,6 Although its pathophysiology is not entirely understood, the development of PPP is believed to be associated with the hormonal changes that occur in the third trimester, in particular elevated progesterone levels.6
Treatment
Oral corticosteroids remain the mainstay of treatment for PPP.6 Lower dosages ranging from 15 to 30 mg daily can be used for mild cases.1 More severe cases usually are treated with an initial trial of prednisone or prednisolone with dosages ranging from 30 mg daily to as high as 60 to 80 mg daily. Higher doses should be used with caution, however, as they may result in reduced fetal reactivity on fetal monitoring.1,9 Treatment at least throughout the remainder of a patient’s pregnancy usually is required, with subsequent gradual tapering of the medication.1
Although it was once reserved for severe or refractory PPP, in 2012, a task force from the National Psoriasis Foundation categorized cyclosporine as an appropriate first-line therapy for PPP.10 Published case reports have documented the successful treatment of PPP with cyclosporine in cases that did not respond to systemic steroids.6,11
Several case reports have documented the safe use of anti–tumor necrosis factors (TNFs), primarily infliximab, for PPP.12 Infliximab and other TNF-α antibodies are pregnancy category B, but limited controlled human data exist regarding their safety in pregnancy.1
Finally, the addition of narrowband UVB light therapy to oral corticosteroids also has been proposed as a safe treatment of refractory disease.6,13 Unlike psoralen plus UVA, which is usually reserved for postpartum use, narrowband UVB light therapy has been shown to be safe for use during pregnancy.1
Future Directions
Genetic and pathogenesis studies have described involvement of IL-1 and IL-36 cytokines in GPP.1 These interleukins are important in neutrophil chemotaxis leading to pustule formation. In addition, as in other psoriasis variants, TNF-α and IL-17α are important. Such findings may help to pave the way for the development of future therapies for both GPP and PPP.
Bottom Line
Clinicians should have a high index of suspicion for PPP in pregnant women who present with widespread cutaneous eruptions. Presently, oral corticosteroids paired with close involvement of obstetric care remains the cornerstone of treatment for PPP. As the case report illustrates, effective diagnosis, treatment, and monitoring are essential for safe outcomes for both mother and baby.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
- Trivedi MK, Vaughn AR, Murase JE. Pustular psoriasis of pregnancy: current perspectives. Int J Womens Health. 2018;10:109-115.
- Kondo RN, Araújo FM, Pereira AM, et al. Pustular psoriasis of pregnancy (impetigo herpetiformis)—case report. An Bras Dermatol. 2013;88(6 suppl 1):186-189.
- Oumeish OY, Farraj SE, Bataineh AS. Some aspects of impetigo herpetiformis. Arch Dermatol. 1982;118:103-105.
- Flynn A, Burke N, Byrne B, et al. Two case reports of generalized pustular psoriasis of pregnancy: different outcomes. Obstet Med. 2016;9:55-59.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine. BMJ Case Rep. 2011;2011:bcr0220113915.
- Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
- Namazi N, Dadkhahfar S. Impetigo herpetiformis: review of pathogenesis, complication, and treatment [published April 4, 2018]. Dermatol Res Pract. 2018;2018:5801280. doi:10.1155/2018/5801280. eCollection 2018.
- Lehrhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Ulubay M, Keskin U, Fidan U, et al. Case report of a rare dermatosis in pregnancy: impetigo herpetiformis. J Obstet Gynaecol Res. 2015;41:301-303.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB [published online January 20, 2012]. Cutan Ocul Toxicol. 2012;31:67-69.
The Case
An otherwise healthy 29-year-old woman at 32 weeks’ gestation presented to the emergency department with a 1-week history of a pruritic burning rash that began on the thighs and then spread diffusely. She denied any similar rash in her prior pregnancy. The patient was not taking any medications except for prenatal vitamins, and she denied any systemic symptoms. Three days prior, treatment with methylprednisolone 50 mg once daily was initiated by the patient’s obstetrician for the rash, but the patient reported no improvement in symptoms. Physical examination revealed edematous pink plaques studded with 1- to 2-mm collarettes of scaling and sparse 1-mm pustules involving the arms, chest, abdomen, back, groin, buttocks, and legs (Figure 1). A peripheral rim of desquamative scaling was noted on the plaques on the back and inner thighs. There were pink macules on the palms, and superficial desquamation was noted on the lips; no other involvement of the oral mucosa was noted.
Biopsy specimens from the left arm revealed discrete subcorneal pustules with mild acanthosis of the epidermis with spongiosis (Figure 2). The papillary dermis showed a sparse infiltrate of neutrophils with numerous marginated neutrophils within vessels. Direct immunofluorescence was negative for human IgG, IgA, IgM, complement component 3, and fibrinogen. Laboratory workup revealed leukocytosis of 21.5×109/L (reference range, 4.5–11.0×109/L) with neutrophilic predominance of 73.6% (reference range, 56%), an elevated erythrocyte sedimentation rate of 40 mm/h (reference range, 0–20 mm/h), and normal calcium of 8.6 mg/dL (reference range, 8.2–10.2 mg/dL).
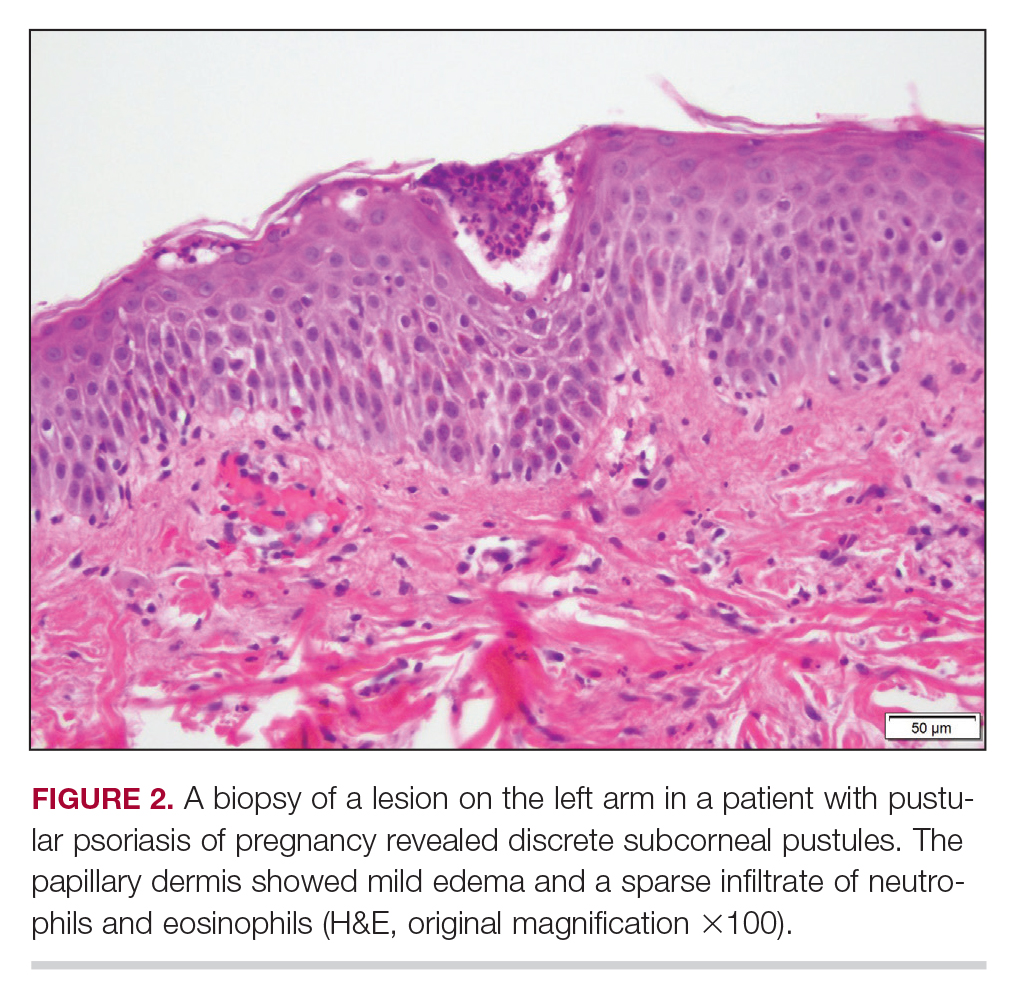
Treatment
The patient was started on methylprednisone 40 mg once daily with a plan to taper the dose by 8 mg every 5 days.
Patient Outcomes
Three weeks following the initial presentation (35 weeks’ gestation), the patient continued to report pruritus and burning in the areas where the rash had developed. The morphology of the rash had changed considerably, as she now had prominent, annular, pink plaques with central clearing, trailing scaling, and a border of subtle pustules on the legs. Rings of desquamative scaling also were noted on the palms. During follow-up at 37 weeks’ gestation, the back, chest, and abdomen were improved from the initial presentation, and annular pink plaques with central clearing were noted on the legs (Figure 3). Given the clinical features and histopathologic findings, a diagnosis of pustular psoriasis of pregnancy (PPP) was made. Increased fetal surveillance with close obstetric follow-up was recommended. Weekly office visits with obstetrics and twice-weekly Doppler ultrasounds and fetal nonstress tests were deemed appropriate management. Given the risk for potential harm to the fetus PPP conveys, the patient was scheduled for induction at 39 weeks’ gestation. She was maintained on low-dose methylprednisolone 4 mg once daily for the duration of the pregnancy, and gradual improvement of the rash continued to be noted at the low treatment dose.
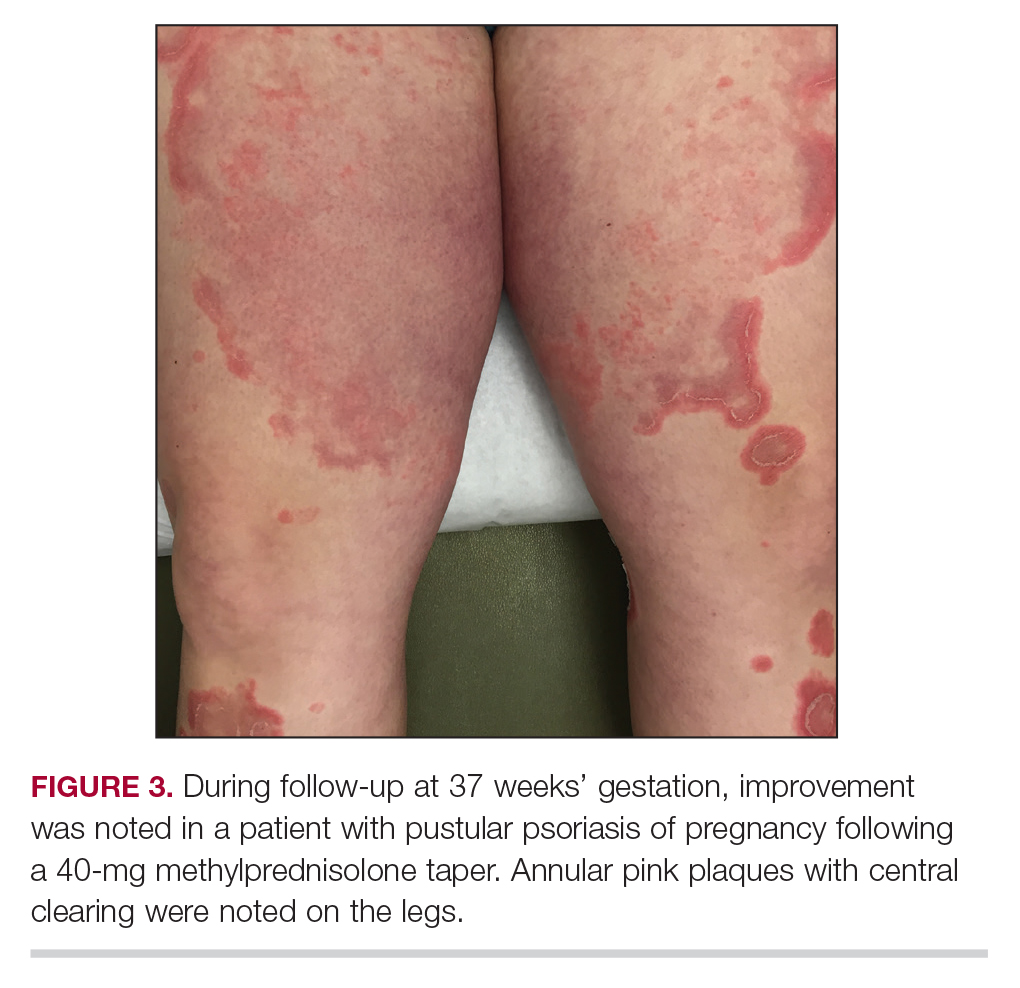
Following induction at 39 weeks’ gestation, the patient vaginally delivered a healthy, 6-lb male child at an outside hospital. She reported that the burning sensation improved within hours of delivery, and systemic steroids were stopped after delivery. At a follow-up visit 3 weeks postpartum, considerable improvement of the rash was noted with no evidence of pustules. Fading pink patches with a superficial scaling were noted on the back, chest, abdomen, arms, legs (Figure 4), and fingertips. The patient was counseled that PPP could recur in subsequent pregnancies and that she should be aware of the potential risks to the fetus.
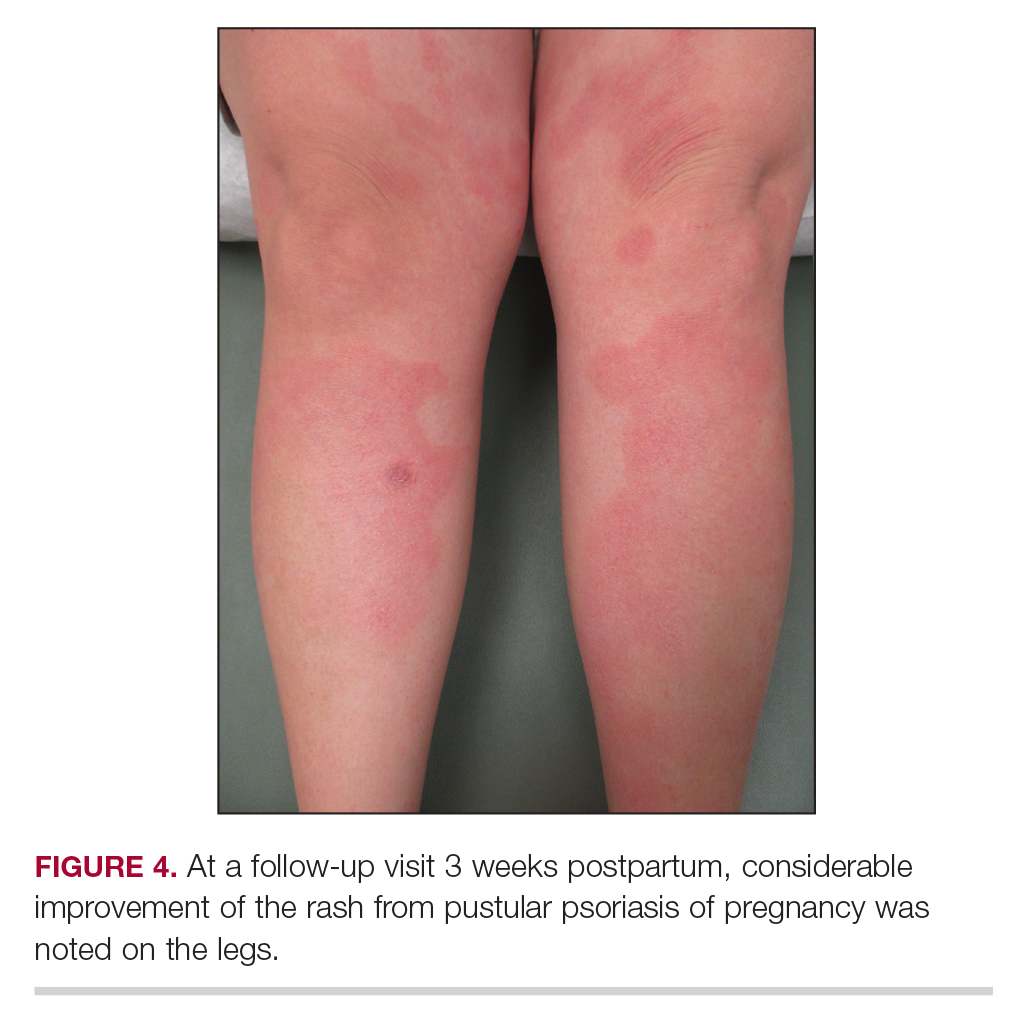
This case was adapted from Pitch M, Somers K, Scott G, et al. A case of pustular psoriasis of pregnancy with positive maternal-fetal outcomes. Cutis. 2018;101:278-280.
Lamotrigine-Induced Cutaneous Pseudolymphoma
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.
Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).
Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.
Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).
Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
To the Editor:
An 8-year-old girl presented with new lesions on the scalp that were mildly painful to palpation and had been increasing in size and number over the last 2 months. Her medical history was remarkable for seizures, keratosis pilaris, and seborrheic dermatitis. The seizures had been well controlled on oxcarbazepine; however, she was switched to lamotrigine 6 months prior to presentation under the care of her neurologist. The patient was not taking other oral medications, and she denied any trauma/insect bites to the affected area or systemic symptoms such as fever, fatigue, weight loss, nausea, swollen lymph nodes, or night sweats. Physical examination revealed 3 well-circumscribed, pink, slightly scaly, indurated nodules on the frontal and vertex scalp (Figure 1). She reported pain on palpation of the lesions. Treatment with ketoconazole shampoo and high-potency topical corticosteroids was ineffective.
Over a period of 2 months after the initial presentation, the patient developed a total of 9 scalp lesions. Testing was performed 4 months after presentation of lesions. Bacterial and fungal cultures of the lesional skin of the scalp were negative. Two biopsies of lesions on the scalp were performed, the first of which showed a nonspecific lymphohistiocytic infiltrate. The second biopsy revealed a dense, nodular, atypical dermal lymphoid infiltrate composed primarily of round regular lymphocytes intermixed with some larger, more irregular lymphocytes and few scattered mitoses (Figure 2).
Immunohistochemical studies revealed small B-cell lymphoma 2–positive lymphocytes with a 2:1 mixture of CD3+ T cells and CD20+CD79a+ B cells. The T cells expressed CD2, CD5, and CD43, and a subset showed a loss of CD7. The CD4:CD8 ratio was 10 to 1. No follicular dendritic networks were noted with CD21 and CD23. Rare, scattered, medium-sized CD30 cells were noted. Staining for CD10, B-cell lymphoma 6, anaplastic lymphoma kinase, Epstein-Barr virus–encoded RNA 1, IgD, and IgM were negative. The plasma cells had a κ/λ free light chain ratio of 2 to 1. Ki-67 was positive in 15% of lymphoid cells. Polymerase chain reaction analysis of T-cell receptor gene rearrangement revealed a peak at 228 bp in a predominantly polyclonal background. A thorough systemic workup including complete blood cell count, immunoglobulin assay, bone marrow transplant panel, comprehensive metabolic panel, lactate dehydrogenase test, inflammatory markers, and viral testing failed to reveal any evidence of underlying malignancy.
After conferring with the patient’s neurologist, lamotrigine was discontinued. Within a few weeks of cessation, the scalp lesions resolved without recurrence at 9-month follow-up. In addition to the lack of clinical, histological, or immunohistochemical evidence of underlying malignancy, the temporal association of the development of lesions after starting lamotrigine and rapid resolution upon its discontinuation suggested a diagnosis of lamotrigine-induced cutaneous pseudolymphoma.
Cutaneous pseudolymphoma is a term used to describe a heterogenous group of benign reactive T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.1 Historically, these types of proliferations have been classified under many alternative names that originally served to describe only B-cell–type proliferations. With advances in immunohistochemistry allowing for more specific cell marker identification, cutaneous pseudolymphomas often are found to contain a mixture of T-cell and B-cell populations, which also led to identifying and describing T-cell–type pseudolymphomas.2
The clinical appearance of cutaneous pseudolymphoma is variable, ranging from discrete nodules or papules to even confluent erythroderma in certain cases.2 The high clinical variability further complicates diagnosis. Although our patient presented with 9 individual nodular lesions, this finding alone is not sufficient to have high suspicion for cutaneous pseudolymphoma without including a much broader differential diagnosis. In our case, the differential diagnosis also included cutaneous lymphoma, arthropod bite reaction, lymphomatoid papulosis, tumid lupus, follicular mucinosis, lymphocytic infiltrate of Jessner, and leukemia cutis.
The primary concern regarding diagnosis of cutaneous pseudolymphoma is the clinician’s ability to effectively differentiate this entity from a true malignant lymphoma. Immunostaining has some value by identification of heterogeneous cell–type populations with a mixed T-cell and B-cell infiltrate that is more characteristic of a benign reactive process. Subsequent polymerase chain reaction analysis can detect the presence or absence of monoclonal T-cell receptor gene rearrangement or immunoglobulin heavy chain rearrangement.3 If these monoclonal rearrangements are absent, a benign diagnosis is favored; however, these rearrangements also have been shown to exist in a case of cutaneous pseudolymphoma that earned the final diagnosis when removal of the offending agent led to spontaneous lesion regression, similar to our case.4
Many different entities have been described as causative factors for the development of cutaneous pseudolymphoma. Of those that have been considered causative, simple categories have emerged, including endogenous, exogenous, and iatrogenic causes. One potential endogenous etiology of cutaneous pseudolymphoma is IgG4-related disease.5 A multitude of exogenous causes have been reported, including several cases of cutaneous pseudolymphoma developing in a prior tattoo site.6 Viruses, specifically molluscum contagiosum, also have been implicated as exogenous causes, and a report of cutaneous pseudolymphoma development at a prior site of herpes zoster lesions has been described.7 Development of cutaneous pseudolymphoma in vaccination sites also has been reported,8 as well as more obscure inciting events such as Leishmania donovani infection and medicinal leech therapy.9
A considerable number of reported cases of cutaneous pseudolymphoma have been attributed to drugs, including monoclonal antibodies,10 herbal supplements,11 and a multitude of other medications.1 As a class, anticonvulsants are considered more likely to cause lymph node pseudolymphomas than strictly cutaneous pseudolymphomas12; however, many drugs in this class of medications have been described in the development of cutaneous pseudolymphoma.3 A review of the literature by Ploysangam et al1 revealed reports of the development of cutaneous pseudolymphomas after administration of phenytoin, carbamazepine, mephenytoin, trimethadione, phenobarbital, primidone, butabarbital, methsuximide, phensuximide, and valproic acid.
Our patient represents a rare case of strictly cutaneous pseudolymphoma caused by administration of lamotrigine. Our case demonstrated a clear temporal relation between the cessation of lamotrigine and rapid and spontaneous disappearance of cutaneous lesions. We found another case of pseudolymphoma in which lamotrigine was deemed causative, but only lymph node involvement was observed.12
Proper diagnosis of cutaneous pseudolymphoma is important not only with regard to the initial differentiation from true malignant lymphoma but in allowing for appropriate follow-up and vigilant surveillance. Cases of progression from cutaneous pseudolymphoma to true lymphoma have been reported.1,2 It is recommended that watchful follow-up for these patients be carried out until at least 5 years after the diagnosis of cutaneous pseudolymphoma is made to rule out the possibility of malignant transformation, particularly in idiopathic cases.13
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
- Ploysangam T, Breneman D, Mutasim D. Cutaneous pseudolymphomas. J Am Acad Dermatol. 1998;38:877-898.
- Bergman R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28:568-574.
- Braddock S, Harrington D, Vose J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. J Am Acad Dermatol. 1992;27:337-340.
- Cogrel O, Beylot-Barry M, Vergier B, et al. Sodium valproate-induced cutaneous pseudolymphoma followed by recurrence with carbamazepine. Br J Dermatol. 2001;144:1235-1238.
- Cheuk W, Lee K, Chong L, et al. IgG4-related sclerosing disease: a potential new etiology of cutaneous pseudolymphoma. Am J Surg Pathol. 2009;33:1713-1719.
- Marchesi A, Parodi P, Brioschi M, et al. Tattoo ink-related cutaneous pseudolymphomas: a rare but significant complication. case report and review of the literature. Aesthetic Plast Surg. 2014;38:471-478.
- Gonzalez J, Sanz A, Martin T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47:502-504.
- Maubec E, Pinquier L, Viguier M, et al. Vaccination-induced cutaneous pseudolymphoma. J Am Acad Dermatol. 2005;52:623-629.
- Altamura D, Calonje E, Liau J, et al. Diffuse cutaneous pseudolymphoma due to therapy with medicinal leeches. JAMA Dermatol. 2014;150:783-784.
- Imafuku S, Ito K, Nakayama J. Cutaneous pseudolymphoma induced by adalimumab and reproduced by infliximab in a patient with arthopathic psoriasis. Br J Dermatol. 2011;166:675-678.
- Meyer S, Vogt T, Obermann EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214:94-96.
- Pathak P, McLachlan R. Drug-induced pseudolymphoma secondary to lamotrigine. Neurology. 1998;50:1509-1510.
- Prabu V, Shivani A, Pawar V. Idiopathic cutaneous pseudolymphoma: an enigma. Indian Dermatol Online J. 2014;5:224-226.
Practice Points
- Cutaneous pseudolymphomas are a heterogenous group of benign T-cell, B-cell, or mixed-cell lymphoproliferative processes that resemble cutaneous lymphomas clinically and/or histopathologically.
- Cutaneous pseudolymphomas have many causative factors, including medications, infections, tattoo ink, vaccinations, and insect bites.
- Lamotrigine is a potential inciting factor of cutaneous pseudolymphoma.
Pigmented Mass on the Shoulder
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
The Diagnosis: Pigmented Dermatofibrosarcoma Protuberans
Pigmented dermatofibrosarcoma protuberans (PDFSP), also known as Bednar tumor, is an uncommon variant of dermatofibrosarcoma protuberans (DFSP). Pigmented dermatofibrosarcoma protuberans constitutes 1% to 5% of all DFSP cases and most commonly is seen in nonwhite adults in the fourth decade of life, with occasional cases seen in pediatric patients, including some congenital cases. Typical sites of involvement include the shoulders, trunk, arms, legs, head, and neck.1,2 It also has been reported at sites of prior immunization, trauma, and insect bites.3
Histopathologic examination of our patient's shoulder nodule revealed an infiltrative neoplasm in the dermis and subcutaneous tissue composed of spindled cells with a storiform pattern and foci of scattered elongated dendritic pigmented cells. A narrow grenz zone separated the tumor from the epidermis, and characteristic honeycomb infiltration by tumor cells was noted in the subcutaneous fat. The nuclei were bland and monomorphous with areas of neuroid differentiation containing whorls and nerve cord-like structures (quiz image). The tumor cells were diffusely CD34 and vimentin positive, while S-100, SOX-10, neurofilament, smooth muscle actin, desmin, epithelial membrane antigen, and cytokeratins were negative. The immunophenotype excluded the possibility of neurogenic, pericytic, myofibroblastic, and myoid differentiation.
Wang and Yang4 previously reported a case of PDFSP with prominent meningothelial-like whorls focally resembling extracranial meningioma; however, the tumor cells were CD34 positive and epithelial membrane antigen negative, weighing against a diagnosis of meningioma. Most cases of PDFSP demonstrate the COL1A1-PDGFB (collagen type I α; 1/platelet-derived growth factor B-chain) fusion protein caused by the translocation t(17;22)(q22;q13), as in classic DFSP.5
Cellular blue nevus (CBN) is a benign melanocytic neoplasm that can present at any age and often occurs on the buttocks and in the sacrococcygeal region. Clinically, CBN presents as a firm, bluish black to bluish gray, dome-shaped nodule. The size varies from a few millimeters to several centimeters.6,7 Histologically, CBN is located completely in the dermis, extending along the adnexae into the subcutaneous tissue with a dumbbell-shaped outline (Figure 1).6-8 The tumor demonstrates oval epithelioid melanocytes with vesicular nuclei and prominent nucleoli. Immunohistochemically, tumor cells stain positively for melanocytic markers such as S-100, SOX-10, MART-1, and human melanoma black 45. CD34 expression rarely is reported in a subset of CBN.9
Pigmented neurofibroma is a rare variant of neurofibroma that produces melanin pigment and has a strong association with neurofibromatosis.10 It occurs most frequently in dark-skinned populations (Fitzpatrick skin types IV-VI). The most common location is the head and neck region.11,12 Histologically, pigmented neurofibroma resembles a diffuse neurofibroma admixed with melanin-producing cells (Figure 2).12 Immunostaining shows positivity for S-100 in both pigmented and Schwann cells; however, the pigmented cells stain positively for human melanoma black 45, Melan-A, and tyrosinase.10 CD34 can be fingerprint positive in neurofibroma, but a distinction from DFSP can be made by S-100 and SOX-10 immunostaining.13
Desmoplastic melanoma (DM) is an uncommon variant of malignant melanoma and has a higher tendency for persistent local growth and less frequent metastases than other variants of melanoma. It has a predilection for chronically sun-exposed areas such as the head and neck and occurs later in life. Clinically, DM appears as nonspecific, often amelanotic nodules or plaques or as scarlike lesions.14 Histologically, DM can be classified as mixed or pure based on the degree of desmoplasia and cellularity. A paucicellular proliferation of malignant spindled melanocytes within a densely fibrotic stroma with lymphoid nodules in the dermis is characteristic (Figure 3); perineural involvement is common.14,15 The most reliable confirmative stains are S-100 and SOX-10.16
Cutaneous meningioma is a rare tumor and could be subtyped into 3 groups. Type I is primary cutaneous meningioma and usually is present at birth on the scalp and paravertebral regions with a relatively good prognosis. Type II is ectopic soft-tissue meningioma that extends into the skin from around the sensory organs on the face. Type III is local invasion or true metastasis from a central nervous system meningioma. Types II and III develop later in life and the prognosis is poor.17,18 Clinically, lesions present as firm subcutaneous nodules or swellings. Cutaneous meningioma has several histopathologic variants. The classic presentation reveals concentric wrapping of tumor cells with round-oval nuclei containing delicate chromatin. Psammoma bodies are a common finding (Figure 4). Immunohistochemically, tumor cells are diffusely positive for epithelial membrane antigen and vimentin.18,19
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
- Amonkar GP, Rupani A, Shah A, et al. Bednar tumor: an uncommon entity. Dermatopathology (Basel). 2016;3:36-38.
- El Hachem M, Diociaiuti A, Latella E, et al. Congenital myxoid and pigmented dermatofibrosarcoma protuberans: a case report. Pediatr Dermatol. 2013;30:E74-E77.
- Anon-Requena MJ, Pico-Valimana M, Munoz-Arias G. Bednar tumor (pigmented dermatofibrosarcoma protuberans). Actas Dermosifiliogr. 2016;107:618-620.
- Wang J, Yang W. Pigmented dermatofibrosarcoma protuberans with prominent meningothelial-like whorls. J Cutan Pathol. 2008;35(suppl 1):65-69.
- Zardawi IM, Kattampallil J, Rode J. An unusual pigmented skin tumour. Bednar tumour, dorsum of left foot (pigmented dermatofibrosarcoma protuberans). Pathology. 2004;36:358-361.
- Sugianto JZ, Ralston JS, Metcalf JS, et al. Blue nevus and "malignant blue nevus": a concise review. Semin Diagn Pathol. 2016;33:219-224.
- Zembowicz A. Blue nevi and related tumors. Clin Lab Med. 2017;37:401-415.
- Zembowicz A, Granter SR, McKee PH, et al. Amelanotic cellular blue nevus: a hypopigmented variant of the cellular blue nevus: clinicopathologic analysis of 20 cases. Am J Surg Pathol. 2002;26:1493-1500.
- Smith K, Germain M, Williams J, et al. CD34-positive cellular blue nevi. J Cutan Pathol. 2001;28:145-150.
- Inaba M, Yamamoto T, Minami R, et al. Pigmented neurofibroma: report of two cases and literature review. Pathol Int. 2001;51:565-569.
- Fetsch JF, Michal M, Miettinen M. Pigmented (melanotic) neurofibroma: a clinicopathologic and immunohistochemical analysis of 19 lesions from 17 patients. Am J Surg Pathol. 2000;24:331-343.
- Motoi T, Ishida T, Kawato A, et al. Pigmented neurofibroma: review of Japanese patients with an analysis of melanogenesis demonstrating coexpression of c-met protooncogene and microphthalmia-associated transcription factor. Hum Pathol. 2005;36:871-877.
- Yeh I, McCalmont TH. Distinguishing neurofibroma from desmoplastic melanoma: the value of the CD34 fingerprint. J Cutan Pathol. 2011;38:625-630.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- Busam KJ. Desmoplastic melanoma. Clin Lab Med. 2011;31:321-330.
- Schleich C, Ferringer T. Desmoplastic melanoma. Cutis. 2015;96:306, 313-314, 335.
- Lopez DA, Silvers DN, Helwig EB. Cutaneous meningiomas--a clinicopathologic study. Cancer. 1974;34:728-744.
- Miedema JR, Zedek D. Cutaneous meningioma. Arch Pathol Lab Med. 2012;136:208-211.
- Bhanusali DG, Heath C, Gur D, et al. Metastatic meningioma of the scalp. Cutis. 2018;101:386-389.
A 37-year-old woman presented with an asymptomatic, indurated, pigmented, subcutaneous nodule on the right shoulder of more than 3 years' duration. The lesion had gradually increased in size with no associated symptoms. The patient had a history of endometrial adenocarcinoma and papillary thyroid carcinoma, which had been treated by hysterectomy-oophorectomy and right thyroidectomy, respectively. She had no other notable systemic abnormalities, and there was no family history of genetic disease or cancer. Physical examination demonstrated a 1.2×1.8-cm nontender, pigmented, subcutaneous nodule with a rough surface and indistinct borders. An excisional biopsy was performed.
Heart of the Matter
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
During the last year, the field of psoriasis has continued to expand, with new therapies, new guidelines, and further understanding of the impact of treatment on associated comorbidities.
One of the most exciting prospects of the treatment of psoriasis with biologics is the potential for the reduction in major adverse cardiovascular events (MACEs). It has been well established that psoriasis is associated with increased cardiovascular risk.1,2 Ahlehoff et al1 conducted a cohort study of the entire Danish population 18 years and older followed from 1997 to 2006 by individual-level linkage of nationwide registers. They concluded that psoriasis is associated with increased risk for adverse cardiovascular events and all-cause mortality. Young age, severe skin affection, and/or psoriatic arthritis (PsA) carry the most risk. They suggested that patients with psoriasis may be candidates for early cardiovascular risk factor modification.1
Ogdie et al2 endeavored to quantify the risk for MACE among patients with PsA, rheumatoid arthritis (RA), and psoriasis without known PsA compared to the general population after adjusting for traditional cardiovascular risk factors. Patients with PsA (N=8706), RA (N=41,752), psoriasis (N=138,424), and unexposed controls (N=81,573) were identified. After adjustment for traditional risk factors, the risk for MACE was higher in patients with PsA not prescribed a disease-modifying antirheumatic drug (DMARD)(hazard ratio [HR], 1.24; 95% confidence interval [CI], 1.03-1.49), patients with RA (no DMARD: HR, 1.39; 95% CI, 1.28-1.50; DMARD: HR, 1.58; 95% CI, 1.46-1.70), patients with psoriasis not prescribed a DMARD (HR, 1.08; 95% CI, 1.02-1.15), and patients with severe psoriasis (DMARD: HR, 1.42; 95% CI, 1.17-1.73).2 These data are one aspect of our increasing insight into the management of psoriasis.
To expand on the new guidelines and new therapies presented in 2019, this issue includes review articles looking at these facets. Wu and Weinberg3 review the impact of diet on psoriasis. Pithadia et al4 explain the American Academy of Dermatology and National Psoriasis Foundation guidelines of care for the management of psoriasis with biologics for the prescribing dermatologist, with an emphasis on the most clinically significant considerations during each step of treatment with biologic therapies (ie, choosing a biologic, initiating therapy, assessing treatment response, switching biologics). Havnaer et al5 provide an update on the newly approved and pipeline systemic agents for psoriasis.
We hope that you find this issue enjoyable and informative.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.
- Ahlehoff O, Gislason GH, Charlot M, et al. Psoriasis is associated with clinically significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. 2011;270:147-157.
- Ogdie A, Yu Y, Haynes K, et al. Risk of major cardiovascular events in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: a population-based cohort study. Ann Rheum Dis. 2015;74:326-332.
- Wu AG, Weinberg JM. The impact of diet on psoriasis. Cutis. 2019;104(suppl 2):7-10.
- Pithadia DJ, Reynolds KA, Lee EB, et al. Translating the 2019 AAD-NPF guidelines of care for the management of psoriasis with biologics to clinical practice. Cutis. 2019;104(suppl 2):12-16, 20.
- Havnaer A, Weinberg JM, Han G. Systemic therapies in psoriasis: an update on newly approved and pipeline biologics and oral treatments. Cutis. 2019;104(suppl 2):17-20.