User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
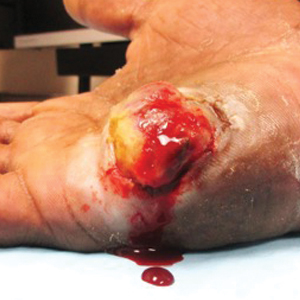
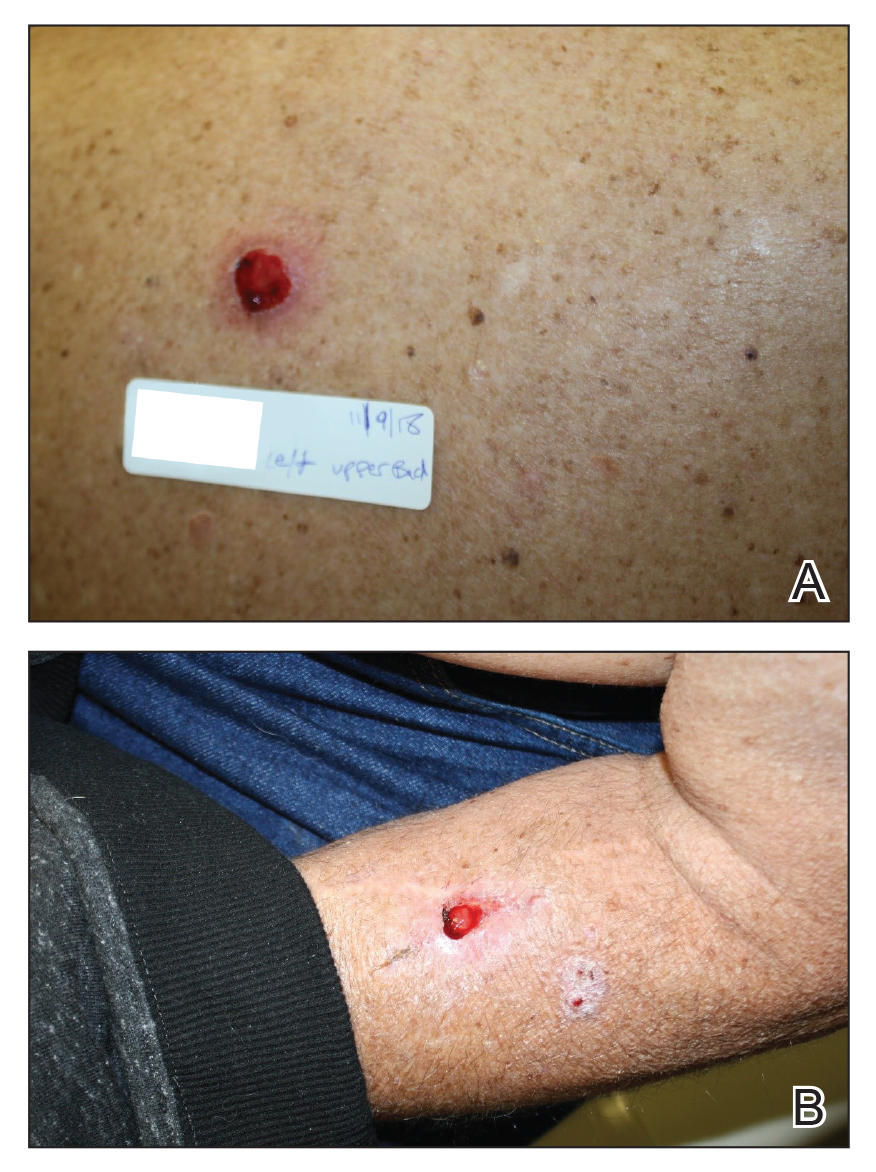
Well-Circumscribed Tumor on the Hand
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
The Diagnosis: Nodular Kaposi Sarcoma
Epidemic Kaposi sarcoma (KS) primarily affects patients with human immunodeficiency virus (HIV) infection. Kaposi sarcoma can appear as brown, red, or blue-black macules, plaques, patches, nodules, or tumors, and it often is observed as multifocal cutaneous lesions located on the head, neck, and upper aspects of the trunk in a fulminant manner. Kaposi sarcoma portends a poor prognosis and is an AIDS-defining malignancy.1-3 Importantly, antiretroviral therapy does not preclude its consideration in those without AIDS-defining CD4 cell counts and undetectable HIV viremia presenting with cutaneous manifestations.2,3 A retrospective review by Daly et al4 reported KS lesions in patients with CD4 lymphocyte counts greater than 300 cells/µL, most of whom were antiretroviral therapy-naïve patients. Also, those with higher CD4 counts tended to have a solitary KS lesion at presentation, while those with CD4 counts less than 300 cells/µL tended to present with multiple foci.4 Epidemic KS lesions are clinically indistinguishable from other common cutaneous conditions in the differential diagnosis of KS, necessitating biopsy for histopathologic examination. Light microscopy findings help to delineate the diagnosis of KS. Immunohistochemical staining to the latent nuclear antigen 1 of human herpesvirus 8 (HHV-8) confirms the KS diagnosis.5,6 Our patient's presentation as a solitary acral lesion was atypical for KS.
Light microscopy of our patient's biopsy demonstrated a large tumor on the acral surface of the right hand. Dermal collections of basophilic spindled cells clustered with small slitlike vascular spaces with abundant erythrocyte extravasation and numerous large ectatic vessels at the periphery were seen (Figure, A). At higher magnification, interlaced bundles of spindle cells with slitlike vessels with scattered lymphocytes and plasma cells were seen (Figure, B). An immunohistochemical stain for HHV-8 was positive and largely confined to spindle cells (Figure, C). These findings confirmed KS and met AIDS-defining criteria. Awareness of these histopathologic features is key in differentiating KS from other conditions in the differential diagnosis.
The patient's history of late latent syphilis coinfected with HIV and persistently elevated rapid plasma reagin that was recalcitrant to therapy placed an atypical nodular presentation within reason for the differential diagnosis. Deviations from the typical papulosquamous presentation with acral involvement in an immunocompromised patient mandates a consideration for syphilis with an atypical presentation. Atypical presentations include nodular, annular, pustular, lues maligna, frambesiform, corymbose, and photosensitive distributions.7,8 Notably, coinfection with HIV modifies the clinical presentation, serology, and efficacy of treatment.7-10 Atypical presentations are more common in coinfected HIV-positive patients, mandating a high degree of suspicion. Nodular secondary syphilis and the noduloulcerative form (lues maligna) often spare the palmar and plantar surfaces, and patients often have constitutional symptoms accompanying the cutaneous eruptions. In questionable cases, a biopsy lends clarification. Light microscopy on hematoxylin and eosin (H&E) staining may display acanthosis, superficial and deep perivascular swelling, plasma, histiocyte infiltrates, dermoepidermal junction changes, mixed patterns, epidermal hyperplasia, and dermal vascular thickening.7-9,11 Spirochetes may be observed on Warthin-Starry stain; however, artifact obscuration from melanin granules and reticular fibers or paucity of organisms can make identification difficult. Immunohistochemical staining may prove useful when H&E stains are atypical or have a paucity of organisms or plasma cells or when silver stains have artifactual obscuration.9 Our patient's solitary palmar lesion without constitutional symptoms made an atypical nodular secondary syphilis presentation less likely. Ultimately, the histopathologic findings were consistent with KS.
Bacillary angiomatosis (BA) is caused by Bartonella species and results in vascular proliferation with cutaneous manifestation. It frequently is observed in patients with HIV or other immunosuppressive conditions as well as patients with exposure to mammals or their vectors. Protean cutaneous manifestations and distributions of BA exist. The number of lesions can be singular to thousands. Solitary superficial pyogenic granuloma-like lesions can be clinically indistinguishable from both KS and pyogenic granuloma (PG). Superficial lesions often begin as red, violaceous, or flesh-colored papules that hemorrhage easily with trauma. The morphology of the papule can progress to be exophytic with dome-shaped or ulcerative surface features and is rubbery on palpation.12 Biopsy is required to differentiate BA from KS. Bacillary angiomatosis on light microscopy with H&E shows protuberant, lobulated, round vessels with plump endothelial cells with or without necrosis. A neutrophil infiltrate in close proximity to bacilli may be noted. Warthin-Starry stain demonstrates numerous bacilli juxtaposed to these endothelial cells. The lack of immunohistochemical staining for HHV-8 also differentiates BA from KS.12,13
Pyogenic granuloma is resultant from proliferation of endothelial cells with a lobular architecture. Pyogenic granulomas are benign, rapidly progressive, acquired lesions presenting in the skin and mucous membranes. Pyogenic granuloma often presents as a single painless papule or nodule with a glistening red-violaceous color that occasionally appears with a perilesional collarette. The lesions are friable and easily hemorrhage. Pyogenic granuloma has been associated with local skin trauma and estrogen hormones. Histopathologic examination of PG assists with differentiation from other nodular lesions. Light microscopy with standard H&E staining demonstrates a network of capillaries arranged into a lobule surrounded by a fibrous matrix. Endothelial cells appear round and protrude into the vascular lamina. Mitotic activity is increased. Lack of findings on Warthin-Starry stain assists with differentiating PG from BA, while the microscopy architecture and immunohistochemical staining differentiates PG from KS.6,13,14
Squamous cell carcinoma (SCC) is the primary malignant cancer of the hand. The dorsal aspect of the hand is the most common location; SCC less commonly is located on the palmar surface, fingers, nail bed, or intertriginous areas.15-17 Chakrabarti et al16 found that these lesions were invasive SCC when located on the palmar surface. Morphologically, SCC takes an exophytic papular, nodular, or scaly appearance with a red to flesh-colored appearance and poor demarcation of the borders. Progression to large ulcerated or secondarily infected lesions also can occur. The inflammatory reaction may cause tenderness to palpation and hemorrhage with trauma. Histopathologic examination of invasive SCC reveals atypical keratinocytes violating the basement membrane and abundant cytoplasm. Our patient's clinical presentation placed invasive SCC low on the differential diagnosis, and the histopathologic and immunohistochemical results eliminated SCC as the diagnosis.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
- Antman K, Chang Y. Kaposi's sarcoma. N Engl J Med. 2000;342:1027-1038.
- Pipette WW. The incidence of second malignancies in subsets of Kaposi's sarcoma. J Am Acad Dermatol. 1987;16:855-861.
- Shiels MS, Engels EA. Evolving epidemiology of HIV-associated malignancies. Curr Opin HIV AIDS. 2017;12:6-11.
- Daly ML, Fogo A, McDonald C, et al. Kaposi sarcoma: no longer an AIDS-defining illness? a retrospective study of Kaposi sarcoma cases with CD4 counts above 300/mm³ at presentation. Clin Exp Dermatol. 2014;39:7-12.
- Broccolo F, Tassan Din C, Viganò MG, et al. HHV-8 DNA replication correlates with the clinical status in AIDS-related Kaposi's sarcoma. J Clin Virol. 2016;78:47-52.
- Pereira PF, Cuzzi T, Galhardo MC. Immunohistochemical detection of the latent nuclear antigen-1 of the human herpesvirus type 8 to differentiate cutaneous epidemic Kaposi sarcoma and its histological simulators. An Bras Dermatol. 2013;88:243-246.
- Gevorgyan O, Owen BD, Balavenkataraman A, et al. A nodular-ulcerative form of secondary syphilis in AIDS. Proc (Bayl Univ Med Cent). 2017;30:80-82.
- Balagula Y, Mattei PL, Wisco OJ, et al. The great imitator revisited: the spectrum of atypical cutaneous manifestations of secondary syphilis. Int J Dermatol. 2014;53:1434-1441.
- Hoang MP, High WA, Molberg KH. Secondary syphilis: a histologic and immunohistochemical evaluation. J Cutan Pathol. 2004;31:595-599.
- Yayli S, della Torre R, Hegyi I, et al. Late secondary syphilis with nodular lesions mimicking Kaposi sarcoma in a patient with human immunodeficiency virus. Int J Dermatol. 2014;53:E71-E73.
- Jeerapaet P, Ackerman AB. Histologic patterns of secondary syphilis. Arch Dermatol. 1973;107:373-377.
- Cockerell CJ, LeBoit PE. Bacillary angiomatosis: a newly characterized, pseudoneoplastic, infectious, cutaneous vascular disorder. J Am Acad Dermatol. 1990;22:501-512.
- Forrestel AK, Naujokas A, Martin JN, et al. Bacillary angiomatosis masquerading as Kaposi's sarcoma in East Africa. J Int Assoc Provid AIDS Care. 2015;14:21-25.
- Fortna RR, Junkins-Hopkins JM. A case of lobular capillary hemangioma (pyogenic granuloma), localized to the subcutaneous tissue, and a review of the literature. Am J Dermatopathol. 2007;29:408-411.
- Marks R. Squamous cell carcinoma. Lancet. 1996;347:735-738.
- Chakrabarti I, Watson JD, Dorrance H. Skin tumours of the hand. a 10-year review. J Hand Surg Br. 1993;18:484-486.
- Sobanko JF, Dagum AB, Davis IC, et al. Soft tissue tumors of the hand. 2. malignant. Dermatol Surg. 2007;33:771-785.
A 52-year-old man presented to the dermatology clinic with a 2×3-cm, fungating, dome-shaped, ulcerative, moist, well-circumscribed tumor with peripheral maceration on the volar aspect of the right hand of 3 months’ duration. The tumor was malodorous, painful, and hemorrhaged easily with minimal trauma. The patient’s medical history was notable for human immunodeficiency virus and latent syphilis, with elevated rapid plasma reagin titers and a positive Treponema palladium antibody on chemiluminescent immunoassay, that was refractory to 3 treatments with penicillin. The patient was not on antiretroviral therapy. He had a CD4+ lymphocyte count of 980 cells/µL (reference range, 359–1519 cells/µL) and a viral load of 8560 copies/mL (reference range, <200 copies/mL). No other skin or systemic concerns were noted, and the patient denied any recent travel, exposure to animals, or constitutional symptoms. A deep shave biopsy of the lesion was performed.
The Impact of Diet on Psoriasis
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
Psoriasis is a chronic cutaneous disease associated with immune-mediated inflammation. The disease has a complex etiology, with factors such as genetics, smoking, alcohol use, diet, and stress all believed to be implicated in its appearance and severity. Specific factors, including increased body mass index and weight gain, have been associated with a higher prevalence of psoriasis and are risk factors for the disease. Because psoriasis varies in severity and incidence, patients often can experience a substantial negative impact on their quality of life, with increased incidences of anxiety and depression.1 Because diet is an accessible and controllable variable, many patients choose to alter their diets to help relieve symptoms of the disease. This article aims to review and summarize the existing literature for possible relationships and correlations between diet and psoriasis.
Because diet is a factor contributing to psoriasis, it is a lifestyle change that patients often make. In a 2017 survey of 1206 patients with psoriasis, 86% reported modifying their diets.2 Furthermore, when patients were compared with control individuals of the same sex and of similar age, it was shown that those with psoriasis consumed statistically significant lower amounts of sugar, whole-grain fiber, dairy products, and calcium (P<.001). The survey also found that patient diets included significantly more fruits, vegetables, and legumes (P<.01). Although no single diet was adhered to by patients, 40% did report attempting a specialized diet to improve their psoriasis. The most common diets were gluten free (35.6%), low carbohydrate/high protein (16.6%), and Paleolithic (11.6%). In addition to these diets, the Mediterranean diet and a vegetarian diet were both among those reported to improve psoriatic symptoms. Finally, certain foods stood out as more frequently reported to affect symptoms, particularly fish oil, fruits, vegetables, and water, which were all reported by at least 10% of respondents to positively affect their psoriasis. Reductions in consumption of alcohol, gluten, nightshades, and junk foods were associated with skin improvements in at least 50% of patients.2 These baseline differences in diet informed our search of the literature and showed that dietary changes can serve as an important adjunct to treatment for many patients.
Mediterranean Diet
The Mediterranean diet consists of a high amount of fruits, vegetables, nuts and legumes, cereals, and olive oil, while restricting consumption of red meats, dairy products, and alcohol (besides red wine) at meals.3 Adherence to the diet has been associated with a reduced risk for cardiovascular diseases,4 rheumatoid arthritis, and Crohn disease,3 among others, possibly because the diet contains a high proportion and variety of foods that contain antioxidants and anti-inflammatory compounds, including the monounsaturated fatty acids (MUFAs) in olive oil and the polyphenols in fruits and vegetables. Consumption of both MUFAs and highly anti-inflammatory nutrients has been associated with reduced prevalence of risk factors for chronic inflammatory diseases, and consumption levels of MUFAs in particular have been reported to be a predictive factor in psoriasis severity.3
Recent studies have tried to quantify an association between consumption of the Mediterranean diet and psoriasis. One cross-sectional study in 2015 evaluated 62 patients with psoriasis for their adherence to the Mediterranean diet and psoriasis severity.4 Utilizing a 14-question evaluation, the study found that patients with a higher severity of psoriasis, as evaluated by a psoriasis area and severity index (PASI) score and C-reactive protein levels, had a lower adherence to the diet. Notably, consumption of extra-virgin olive oil was found to be an independent predictor of PASI score, and consumption of fish was an independent predictor of C-reactive protein levels.4
A second cross-sectional questionnaire study found similar results in a larger population of 3557 patients. The same association between patients with severe psoriasis and low levels of adherence to the Mediterranean diet was reported.3 Although neither study showed a causal relationship between the diet and psoriasis severity, both did report the potential impacts of proinflammatory and anti-inflammatory foods. General foods and nutrients listed by the studies as having anti-inflammatory properties include MUFAs; fish; vitamins A, C, D, and E; and omega-3 fatty acids.3 Because of the large number of confounding factors in dietary studies that rely on questionnaires, it is hard to definitively label the Mediterranean diet as beneficial topsoriasis. However, individual components of the diet may be used as predictors of psoriasis severity, and the diet itself may be used in tandem with other treatments for psoriasis.
Gluten-Free Diet
Celiac disease is an inflammatory enteropathy caused by an immune reaction to the protein gliadin, which is found in foods containing gluten, such as wheat.5 Immune system assault on the intestinal enterocytes leads to the stripping away of villi, negatively affecting nutrient absorption. Multiple studies have reported an association between having psoriasis and having celiac disease as well as the reverse, including a 3-fold increased risk of celiac disease for patients with psoriasis in a 2017 meta-analysis.6 Even if patients with psoriasis did not have celiac disease, studies have found that a notable percentage of patients with psoriasis have elevated antigliadin IgA antibody levels.7 Many hypotheses have been proposed to explain this association. One article suggested that the malabsorption associated with celiac disease predisposes patients to vitamin D deficiency, which is a contributing risk factor for psoriasis.8 Other explanations involve common immune cells involved in the response to both diseases and a shared genetic background between the 2 diseases.8 As a gluten-free diet is standard for patients with celiac disease, it stands to reason that IgA could be used as a serum biomarker for patients who also could see improvements by adopting the diet.
This result could help explain the proportion of respondents to the 2017 survey who experienced improvements to their psoriasis if the gluten-free diet was in fact not triggering the inflammatory effects that a regular diet would, which also may help to explain the mixed results that the gluten-free diet has had as a treatment for psoriasis. One 3-month study of patients who were positive for antigliadin antibodies found that the majority (82%) experienced a decrease in antibody levels and affected skin area after following a gluten-free diet. Only half the patients had been diagnosed with celiac disease prior to the study, lending credibility to the idea that antigliadin antibody could be used as a marker for patients with psoriasis who would benefit from a gluten-free diet.9 Other case studies have reported no improvement of psoriasis following implementation of a gluten-free diet,10 despite the patients having elevated gliadin antibodies or celiac disease. More studies are required to discern the exact nature of the benefits of a gluten-free diet on psoriasis; however, it does serve as a promising option for patients with both psoriasis and celiac disease.
Ketogenic Diet
As obesity and weight gain are factors associated with psoriasis, some patients turn to diets that restrict calories with the goal of losing weight to improve their symptoms. One 2015 case report studied a patient who restored her response to systemic treatment of psoriasis following an intensive 4-week, calorie-restricted ketogenic diet.11 The ketogenic diet is a high-fat, adequate-protein, low-carbohydrate diet. Animal studies have shown the diet to have anti-inflammatory effects, including lowering levels of proinflammatory cytokines and reduced fever.12 In the 2015 case report, the rapid and consistent weight loss experienced by the patient because of the ketogenic diet was thought to be the cause of the restoration of treatment effectiveness,11 which is interesting, since the role of the ketogenic diet was not to supplement any deficiencies but to move the patient to a physiologic state that was once again receptive to treatment. This finding suggests that a variety of diets could improve psoriasis symptoms, so long as they do not cause inflammation or reduce overall body mass. One study of patients on a calorie-restricted diet over 8 weeks did see a trend of patients on the diet showing improvement in both their PASI scores and Dermatology Life Quality Index, though the improvement was not statistically significant.13 To determine if the ketogenic diet has a significant association with psoriasis improvement, controlled, large-population studies should be performed in the future with age, sex, and weight-matched controls, which may be difficult to do. Further studies looking at the association between weight loss and psoriasis also could be another direction.
Vegetarian Diet
Both vegetarian and vegan diets have been evaluated for their efficacy in relieving symptoms of chronic inflammatory disorders. Although the 2 diets are similar in avoiding consumption of meat, fish, and poultry, vegan diets often have additional food restrictions, including avoiding eggs, honey, and dairy products. One study noted the impact of these diets on patients with a variety of skin conditions following a period of fasting. It was observed that some patients with psoriasis saw an improvement in their symptoms during the period when they were eating a vegetarian or vegan diet, which was attributed to a return to normal levels of activity of neutrophils, extrapolated from serum levels of lactoferrin.14 Vegetarian diets have been shown to be associated with higher ratios of anti-inflammatory to proinflammatory adipokines compared to omnivorous diets,15 as well as lower expression levels of proinflammatory genes in the gut microbiota and lower expression levels of IgE.16 Perhaps the anti-inflammatory impacts of the diet affected the symptoms of psoriasis. The benefits of a vegetarian diet also have been attributed to the high amount of potassium consumed,17 which is used in the body to synthesize cortisol, a common treatment for psoriasis. Potassium supplementation has been shown to raise serum cortisol levels in patients.6 Although additional studies are needed to discern the significance of potassium in the vegetarian diet, both hypotheses are reasonable explanations for the observations seen in these studies.
Vitamin D and Other Nutritional Supplements
Because it is not always feasible for patients to alter their diets, many have turned to dietary supplements as an alternative method of treatment and lifestyle change. Two of the more prominently represented nutritional additives in the literature are fish oils and vitamin D.18 Supplemental vitamin D is a prohormone that can be endogenously converted to its active 1,25-dihydroxyvitamin D.19 Vitamin D plays important roles in the regulation of calcium and magnesium in the bones as well as the maturation and differentiation of keratinocytes in the skin.16 Topical vitamin D analogues are standard treatments for psoriasis, as they are used to modulate the immune system to great effect.20 Some patients with psoriasis present with vitamin D insufficiency,21 and it stands to reason that oral supplementation may be a treatment option. There have been multiple studies assessing the efficacy of oral vitamin D for the treatment of psoriasis; however, in the only randomized and placebo-controlled trial, there was only a slight nonsignificant improvement in the group supplemented with vitamin D.20 Another small, open-label study reported remarkably improved PASI scores in 9 vitamin D–supplemented, dietary calcium–restricted patients.22 The lack of recent, large-sample studies makes it hard to draw notable conclusions from these studies.
The polyunsaturated fatty acids found in fish oils also have been considered as a treatment option for psoriasis.23 Millsop et al20 conducted an analysis of the literature reviewing the efficacy of fish oil in the treatment of psoriasis. Twelve of 15 compiled trials showed an improvement in psoriasis, ranging from slight improvements from baseline levels of the disease to statistically significant decreases in PASI scores (P<.05). It is notable that the amount of fish oil given in these studies varied widely, but the amount given did not necessarily correlate with strength of impact.20 For example, Mayser et al,24 Bittiner et al,25 and Grimminger et al26 each performed prospective, double-blind studies with
Studies also have shown little to no improvement in the use of fish oil to treat psoriasis. One such study was conducted by Soyland et al27 in 1993 in Norway. Utilizing a prospective, double-blind, placebo-controlled design over 4 months on 145 patients with moderate to severe psoriasis, researchers evaluated the treatment effectiveness via PASI scores; subjective reports from the patients; clinical manifestations; and factors such as cellular infiltration, desquamation, and redness. The results were mixed, with the placebo (corn oil) group having less redness and cellular desquamation and the fish oil group showing less cellular infiltration. In the other categories, there was no significant difference between the 2 groups, and researchers concluded there was no significant benefit to treating psoriasis using fish oil vs corn oil.27 As with many of the other diets, there have been no recent, large-scale studies performed on the effect of fish oil supplementation on psoriasis; however, of the studies we reviewed, none showed fish oil supplementation to have a significant negative impact on psoriasis.
Conclusion
Dietary modifications have a complex multifactorial effect on psoriasis, often dependent on the variations of psoriasis and the lifestyle of the patient, including level of exercise, activities such as smoking and drinking, and genetic susceptibilities to conditions such as obesity. Thus, it is difficult for one diet to have a significant impact on psoriasis symptoms that applies to the majority of individuals. However, it appears that certain foods or nutritional supplements can be modified from all diets for general improvement. Foods with systemic anti-inflammatory effects, such as olive oil and fish oil, seem to be beneficial in treating psoriasis. As an extension, a gluten-free diet may help psoriasis patients with celiac disease by reducing the inflammatory environment of the body. On the opposite side of the spectrum, proinflammatory foods such as dietary fat and alcohol should be avoided.28
In general, larger and more recent population-based studies are needed to add to the literature on this subject. Nationwide voluntary web-based surveys such as the NutriNet-Santé study in France may be one way to quickly amass large quantities of data (ClinicalTrials.gov Identifier NCT03335644). Participants are recruited through multimedia campaigns and return online questionnaires annually for 1 decade. A subset of participants also contributes biologic samples and participates in clinical examinations. This type of data gathering would capture many variables, provide a large sample size, and perhaps shed light on regional differences in diet and lifestyle that could then be targeted with treatments.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
- Madrid Álvarez MB, Carretero Hernández G, González Quesada A, et al. Measurement of the psychological impact of psoriasis on patients receiving systemic treatment. Actas Dermosifiliogr (English edition). 2018;109:733-740.
- Afifi L, Danesh MJ, Lee KM, et al. Dietary behaviors in psoriasis: patient-reported outcomes from a U.S. national survey. Dermatol Ther (Heidelb). 2017;7:227-242.
- Phan C, Touvier M, Kesse-Guyot E, et al. Association between Mediterranean anti-inflammatory dietary profile and severity of psoriasis: results from the NutriNet-Santé cohort. JAMA Dermatol. 2018;154:1017-1024.
- Barrea L, Balato N, Di Somma C, et al. Nutrition and psoriasis: is there any association between the severity of the disease and adherence to the Mediterranean diet? J Transl Med. 2015;13:18.
- Bhatia BK, Millsop JW, Debbaneh M, et al. Diet and psoriasis, part II: celiac disease and role of a gluten-free diet. J Am Acad Dermatol. 2014;71:350-358.
- Ungprasert P, Wijarnpreecha K, Kittanamongkolchai W. Psoriasis and risk of celiac disease: a systematic review and meta-analysis. Indian J Dermatol. 2017;62:41-46.
- Kolchak NA, Tetarnikova MK, Theodoropoulou MS, et al. Prevalence of antigliadin IgA antibodies in psoriasis vulgaris and response of seropositive patients to a gluten-free diet. J Multidiscip Healthc. 2017;11:13-19.
- Ludvigsson JF, Lindelöf B, Zingone F, et al. Psoriasis in a nationwide cohort study of patients with celiac disease. J Invest Dermatol. 2011;131:2010-2016.
- De Bastiani R, Gabrielli M, Lora L, et al. Association between coeliac disease and psoriasis: Italian primary care multicentre study. Dermatology. 2015;230:156-160.
- Pietrzak D, Pietrzak A, Krasowska D, et al. Digestive system in psoriasis: an update. Arch Dermatol Res. 2017;309:679-693.
- Castaldo G, Galdo G, Rotondi Aufiero F, et al. Very low-calorie ketogenic diet may allow restoring response to systemic therapy in relapsing plaque psoriasis [published online November 11, 2015]. Obes Res Clin Pract. 2016;10:348-352.
- Dupuis N, Curatolo N, Benoist J-F, et al. Ketogenic diet exhibits anti-inflammatory properties. Epilepsia. 2015;56:e95-e98.
- Jensen P, Zachariae C, Christensen R, et al. Effect of weight loss on the severity of psoriasis: a randomized clinical study. JAMA Dermatol. 2013;149:795-801.
- Lithell H, Bruce A, Gustafsson IB, et al. A fasting and vegetarian diet treatment trial on chronic inflammatory disorders. Acta Derm Venereol. 1983;63:397-403.
- Ambroszkiewicz J, Chełchowska M, Rowicka G, et al. Anti-inflammatory and pro-inflammatory adipokine profiles in children on vegetarian and omnivorous diets. Nutrients. 2018;10;pii E1241.
- Rastmanesh R. Psoriasis and vegetarian diets: a role for cortisol and potassium? Med Hypotheses. 2009;72:368.
- Zhang C, Björkman A, Cai K, et al. Impact of a 3-months vegetarian diet on the gut microbiota and immune repertoire. Front Immunol. 2018;9:908.
- Wolters M. Diet and psoriasis: experimental data and clinical evidence. Br J Dermatol. 2005;153:706-714.
- Zuccotti E, Oliveri M, Girometta C, et al. Nutritional strategies for psoriasis: current scientific evidence in clinical trials. Eur Rev Med Pharmacol Sci. 2018;22:8537-8551.
- Millsop JW, Bhatia BK, Debbaneh M, et al. Diet and psoriasis: part 3. role of nutritional supplements. J Am Acad Dermatol. 2014;71:561-569.
- El-Moaty Zaher HA, El-Komy MHM, Hegazy RA, et al. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69:840-842.
- Finamor DC, Sinigaglia-Coimbra R, Neves LCM, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol. 2013;5:222-234.
- Pona A, Haidari W, Kolli SS, et al. Diet and psoriasis. Dermatol Online J. 2019;25. https://escholarship.org/uc/item/1p37435s. Accessed April 14, 2019.
- Mayser P, Mrowietz U, Arenberger P, et al. ω-3 fatty acid–based lipid infusion in patients with chronic plaque psoriasis: results of a double-blind, randomized, placebo-controlled, multicenter trial. J Am Acad Dermatol. 1998;38:539-547.
- Bittiner SB, Tucker WF, Cartwright I, et al. A double-blind, randomised, placebo-controlled trial of fish oil in psoriasis. Lancet. 1988;1:378-380.
- Grimminger F, Mayser P, Papavassilis C, et al. A double-blind, randomized, placebo-controlled trial of n-3 fatty acid based lipid infusion in acute, extended guttate psoriasis: rapid improvement of clinical manifestations and changes in neutrophil leukotriene profile. Clin Investig. 1993;71:634-643.
- Soyland E, Funk J, Rajka G, et al. Effect of dietary supplementation with very-long-chain n-3 fatty acids in patients with psoriasis. N Engl J Med. 1993;328:1812-1816.
- Cunningham E. Is there research to support a specific diet for psoriasis? J Acad Nutr Diet. 2014;114:508.
Practice Points
- No single food, supplement, or diet has been shown to have a notable positive impact on all variations of psoriasis. However, foods with systemic anti-inflammatory effects may be worth testing and adding to the patient’s diet.
- A considerable proportion of patients with psoriasis also have elevated levels of antigliadin antibody. If patients have celiac disease or high levels of antigliadin antibody, switching them to a gluten-free diet could have a positive impact on their psoriasis.
- Elevated body mass index, weight gain, smoking, and obesity are all associated with a higher risk for psoriasis appearance and severity.
Translating the 2019 AAD-NPF Guidelines of Care for the Management of Psoriasis With Biologics to Clinical Practice
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.
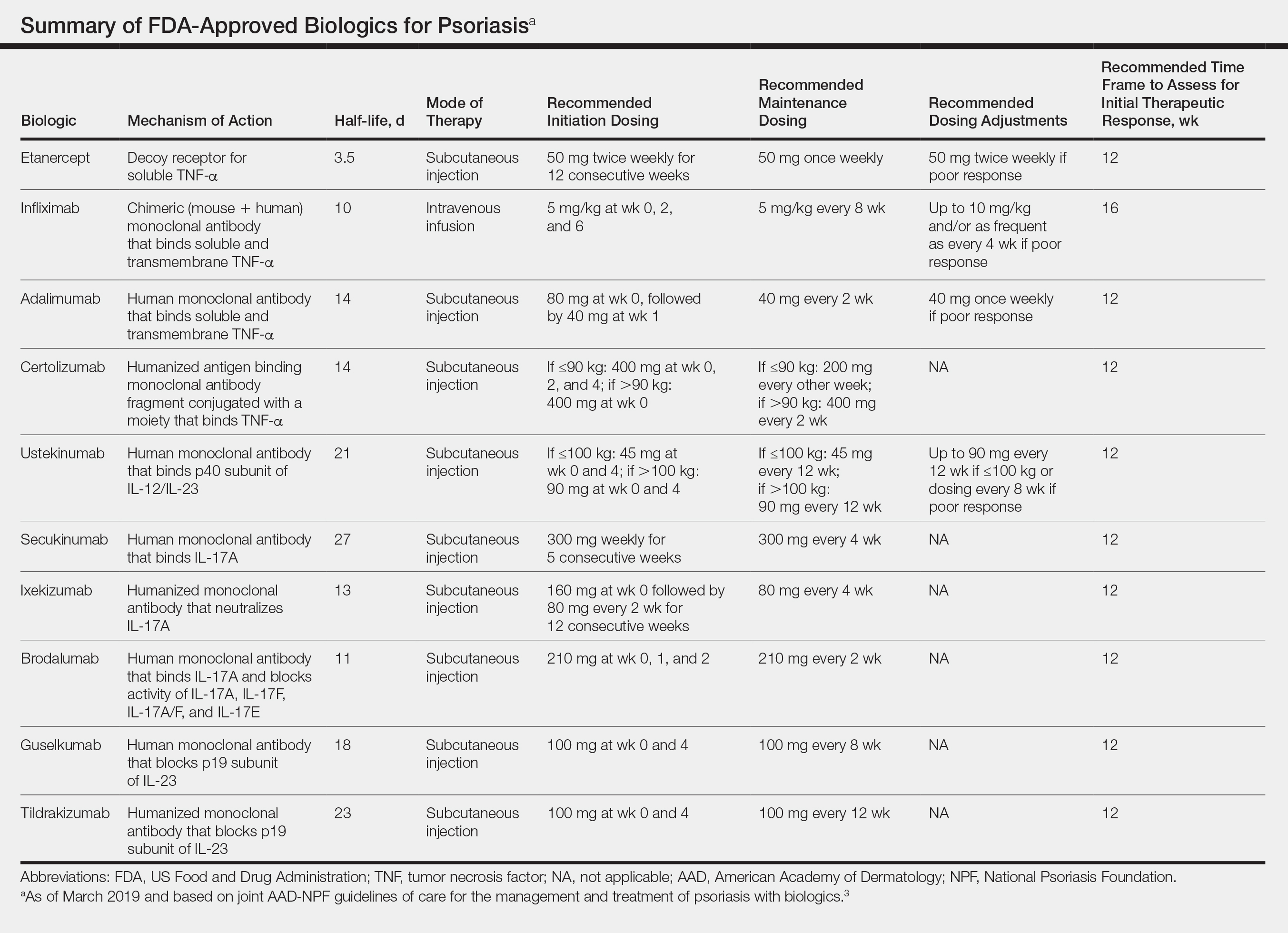
In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
Psoriasis is a systemic immune-mediated disorder characterized by erythematous, scaly, well-demarcated plaques on the skin that affects approximately 3% of the world’s population.1 The disease is moderate to severe for approximately 1 in 6 individuals with psoriasis.2 These patients, particularly those with symptoms that are refractory to topical therapy and/or phototherapy, can benefit from the use of biologic agents, which are monoclonal antibodies and fusion proteins engineered to inhibit the action of cytokines that drive psoriatic inflammation.
In February 2019, the American Academy of Dermatology (AAD) and National Psoriasis Foundation (NPF) released an updated set of guidelines for the use of biologics in treating adult patients with psoriasis.3 The prior guidelines were released in 2008 when just 3 biologics—etanercept, infliximab, and adalimumab—were approved by the US Food and Drug Administration (FDA) for the management of psoriasis. These older recommendations were mostly based on studies of the efficacy and safety of biologics for patients with psoriatic arthritis.4 Over the last 11 years, 8 novel biologics have gained FDA approval, and numerous large phase 2 and phase 3 trials evaluating the risks and benefits of biologics have been conducted. The new guidelines contain considerably more detail and are based on evidence more specific to psoriasis rather than to psoriatic arthritis. Given the large repertoire of biologics available today and the increased amount of published research regarding each one, these guidelines may aid dermatologists in choosing the optimal biologic and managing therapy.
The AAD-NPF recommendations discuss the mechanism of action, efficacy, safety, and adverse events of the 10 biologics that have been FDA approved for the treatment of psoriasis as of March 2019, plus risankizumab, which was pending FDA approval at the time of publication and was later approved in April 2019. They also address dosing regimens, potential to combine biologics with other therapies, and different forms of psoriasis for which each may be effective.3 The purpose of this discussion is to present these guidelines in a condensed form to prescribers of biologic therapies and review the most clinically significant considerations during each step of treatment. Of note, we highlight only treatment of adult patients and do not discuss information relevant to risankizumab, as it was not FDA approved when the AAD-NPF guidelines were released.
Choosing a Biologic
Biologic therapy may be considered for patients with psoriasis that affects more than 3% of the body’s surface and is recalcitrant to localized therapies. There is no particular first-line biologic recommended for all patients with psoriasis; rather, choice of therapy should be individualized to the patient, considering factors such as body parts affected, comorbidities, lifestyle, and drug cost.
All 10 FDA-approved biologics (Table) have been ranked by the AAD and NPF as having grade A evidence for efficacy as monotherapy in the treatment of moderate to severe plaque-type psoriasis. Involvement of difficult-to-treat areas may be considered when choosing a specific therapy. The tumor necrosis factor α (TNF-α) inhibitors etanercept and adalimumab, the IL-17 inhibitor secukinumab, and the IL-23 inhibitor guselkumab have the greatest evidence for efficacy in treatment of nail disease. For scalp involvement, etanercept and guselkumab have the highest-quality evidence, and for palmoplantar disease, adalimumab, secukinumab, and guselkumab are considered the most effective. The TNF-α inhibitors are considered the optimal treatment option for concurrent psoriatic arthritis, though the IL-12/IL-23 inhibitor ustekinumab and the IL-17 inhibitors secukinumab and ixekizumab also have shown grade A evidence of efficacy. Of note, because TNF-α inhibitors received the earliest FDA approval, there is most evidence available for this class. Therapies with lower evidence quality for certain forms of psoriasis may show real-world effectiveness in individual patients, though more trials will be necessary to generate a body of evidence to change these clinical recommendations.

In pregnant women or those are anticipating pregnancy, certolizumab may be considered, as it is the only biologic shown to have minimal to no placental transfer. Other TNF-α inhibitors may undergo active placental transfer, particularly during the latter half of pregnancy,5 and the greatest theoretical risk of transfer occurs in the third trimester. Although these drugs may not directly harm the fetus, they do cause fetal immunosuppression for up to the first 3 months of life. All TNF-α inhibitors are considered safe during lactation. There are inadequate data regarding the safety of other classes of biologics during pregnancy and lactation.
Overweight and obese patients also require unique considerations when choosing a biologic. Infliximab is the only approved psoriasis biologic that utilizes proportional-to-weight dosing and hence may be particularly efficacious in patients with higher body mass. Ustekinumab dosing also takes patient weight into consideration; patients heavier than 100 kg should receive 90-mg doses at initiation and during maintenance compared to 45 mg for patients who weigh 100 kg or less. Other approved biologics also may be utilized in these patients but may require closer monitoring of treatment efficacy.
There are few serious contraindications for specific biologic therapies. Any history of allergic reaction to a particular therapy is an absolute contraindication to its use. In patients for whom IL-17 inhibitor treatment is being considered, inflammatory bowel disease (IBD) should be ruled out given the likelihood that IL-17 could reactivate or worsen IBD. Of note, TNF-α inhibitors and ustekinumab are approved therapies for patients with IBD and may be recommended in patients with comorbid psoriasis. Phase 2 and phase 3 trials have found no reactivation or worsening of IBD in patients with psoriasis who were treated with the IL-23 inhibitor tildrakizumab,6 and phase 2 trials of treatment of IBD with guselkumab are currently underway (ClinicalTrials.gov Identifier NCT03466411). In patients with New York Heart Association class III and class IV congestive heart failure or multiple sclerosis, initiation of TNF-α inhibitors should be avoided. Among 3 phase 3 trials encompassing nearly 3000 patients treated with the IL-17 inhibitor brodalumab, a total of 3 patients died by suicide7,8; hence, the FDA has issued a black box warning cautioning against use of this drug in patients with history of suicidal ideation or recent suicidal behavior. Although a causal relationship between brodalumab and suicide has not been well established,9 a thorough psychiatric history should be obtained in those initiating treatment with brodalumab.
Initiation of Therapy
Prior to initiating biologic therapy, it is important to obtain a complete blood cell count, complete metabolic panel, tuberculosis testing, and hepatitis B virus (HBV) and hepatitis C virus serologies. Testing for human immunodeficiency virus may be pursued at the clinician’s discretion. It is important to address any positive or concerning results prior to starting biologics. In patients with active infections, therapy may be initiated alongside guidance from an infectious disease specialist. Those with a positive purified protein derivative test, T-SPOT test, or QuantiFERON-TB Gold test must be referred for chest radiographs to rule out active tuberculosis. Patients with active HBV infection should receive appropriate referral to initiate antiviral therapy as well as core antibody testing, and those with active hepatitis C virus infection may only receive biologics under the combined discretion of a dermatologist and an appropriate specialist. Patients with human immunodeficiency virus must concurrently receive highly active antiretroviral therapy, show normal CD4+ T-cell count and undetectable viral load, and have no recent history of opportunistic infection.
Therapy should be commenced using specific dosing regimens, which are unique for each biologic (Table). Patients also must be educated on routine follow-up to assess treatment response and tolerability.
Assessment and Optimization of Treatment Response
Patients taking biologics may experience primary treatment failure, defined as lack of response to therapy from initiation. One predisposing factor may be increased body mass; patients who are overweight and obese are less likely to respond to standard regimens of TNF-α inhibitors and 45-mg dosing of ustekinumab. In most cases, however, the cause of primary nonresponse is unpredictable. For patients in whom therapy has failed within the recommended initial time frame (Table), dose escalation or shortening of dosing intervals may be pursued. Recommended dosing adjustments are outlined in the Table. Alternatively, patients may be switched to a different biologic.
If desired effectiveness is not reached with biologic monotherapy, topical corticosteroids, topical vitamin D analogues, or narrowband UVB light therapy may be concurrently used for difficult-to-treat areas. Evidence for safety and effectiveness of systemic adjuncts to biologics is moderate to low, warranting caution with their use. Methotrexate, cyclosporine, and apremilast have synergistic effects with biologics, though they may increase the risk for immunosuppression-related complications. Acitretin, an oral retinoid, likely is the most reasonable systemic adjunct to biologics because of its lack of immunosuppressive properties.
In patients with a suboptimal response to biologics, particularly those taking therapies that require frequent dosing, poor compliance should be considered.10 These patients may be switched to a biologic with less-frequent maintenance dosing (Table). Ustekinumab and tildrakizumab may be the best options for optimizing compliance, as they require dosing only once every 12 weeks after administration of loading doses.
Secondary treatment failure is diminished efficacy of treatment following successful initial response despite no changes in regimen. The best-known factor contributing to secondary nonresponse to biologics is the development of antidrug antibodies (ADAs), a phenomenon known as immunogenicity. The development of efficacy-limiting ADAs has been observed in response to most biologics, though ADAs against etanercept and guselkumab do not limit therapeutic response. Patients taking adalimumab and infliximab have particularly well-documented efficacy-limiting immunogenicity, and those who develop ADAs to infliximab are considered more prone to developing infusion reactions. Methotrexate, which limits antibody formation, may concomitantly be prescribed in patients who experience secondary treatment failure. It should be considered in all patients taking infliximab to increase efficacy and tolerability of therapy.
Considerations During Active Therapy
In addition to monitoring adherence and response to regimens, dermatologists must be heavily involved in counseling patients regarding the risks and adverse effects associated with these therapies. During maintenance therapy with biologics, patients must follow up with the prescriber at minimum every 3 to 6 months to evaluate for continued efficacy of treatment, extent of side effects, and effects of treatment on overall health and quality of life. Given the immunosuppressive effects of biologics, annual testing for tuberculosis should be considered in high-risk individuals. In those who are considered at low risk, tuberculosis testing may be done at the discretion of the dermatologist. In those with a history of HBV infection, HBV serologies should be pursued routinely given the risk for reactivation.
Annual screening for nonmelanoma skin cancer should be performed in all patients taking biologics. Tumor necrosis factor α inhibitor therapy in particular confers an elevated risk for cutaneous squamous cell carcinoma, especially in patients who are immunosuppressed at baseline and those with history of UV phototherapy. Use of acitretin alongside TNF-α inhibitors or ustekinumab may prevent squamous cell carcinoma formation in high-risk patients.
Because infliximab treatment poses an elevated risk of liver injury,11 liver function tests should be repeated 3 months following initiation of treatment and then every 6 to 12 months subsequently if results are normal. Periodic assessment of suicidal ideation is recommended in patients on brodalumab therapy, which may necessitate more frequent follow-up visits and potentially psychiatry referrals in certain patients. Patients taking IL-17 inhibitors, particularly those who are concurrently taking methotrexate, are at increased risk for developing mucocutaneous Candida infections; these patients should be monitored for such infections and treated appropriately.12
It is additionally important for prescribing dermatologists to ensure that patients on biologics are following up with their general providers to receive timely age-appropriate preventative screenings and vaccines. Inactivated vaccinations may be administered during therapy with any biologic; however, live vaccinations may induce systemic infection in those who are immunocompromised, which theoretically includes individuals taking biologic agents, though incidence data in this patient population are scarce.13 Some experts believe that administration of live vaccines warrants temporary discontinuation of biologic therapy for 2 to 3 half-lives before and after vaccination (Table). Others recommend stopping treatment at least 4 weeks before and until 2 weeks after vaccination. For patients taking biologics with half-lives greater than 20 days, which would theoretically require stopping the drug 2 months prior to vaccination, the benefit of vaccination should be weighed against the risk of prolonged discontinuation of therapy. Until recently, this recommendation was particularly important, as a live herpes zoster vaccination was recommended by the Centers for Disease Control and Prevention for adults older than 60 years. In 2017, a new inactivated herpes zoster vaccine was introduced and is now the preferred vaccine for all patients older than 50 years.14 It is especially important that patients on biologics receive this vaccine to avoid temporary drug discontinuation.
Evidence that any particular class of biologics increases risk for solid tumors or lymphoreticular malignancy is limited. One case-control analysis reported that more than 12 months of treatment with TNF-α inhibitors may increase risk for malignancy; however, the confidence interval reported hardly allows for statistical significance.15 Another retrospective cohort study found no elevated incidence of cancer in patients on TNF-α inhibitors compared to nonbiologic comparators.16 Ustekinumab was shown to confer no increased risk for malignancy in 1 large study,15 but no large studies have been conducted for other classes of drugs. Given the limited and inconclusive evidence available, the guidelines recommend that age-appropriate cancer screenings recommended for the general population should be pursued in patients taking biologics.
Surgery while taking biologics may lead to stress-induced augmentation of immunosuppression, resulting in elevated risk of infection.17 Low-risk surgeries that do not warrant discontinuation of treatment include endoscopic, ophthalmologic, dermatologic, orthopedic, and breast procedures. In patients preparing for elective surgery in which respiratory, gastrointestinal, or genitourinary tracts will be entered, biologics may be discontinued at least 3 half-lives (Table) prior to surgery if the dermatologist and surgeon collaboratively deem that risk of infection outweighs benefit of continued therapy.18 Therapy may be resumed within 1 to 2 weeks postoperatively if there are no surgical complications.
Switching Biologics
Changing therapy to another biologic should be considered if there is no response to treatment or the patient experiences adverse effects while taking a particular biologic. Because evidence is limited regarding the ideal time frame between discontinuation of a prior medication and initiation of a new biologic, this interval should be determined at the discretion of the provider based on the patient’s disease severity and response to prior treatment. For individuals who experience primary or secondary treatment failure while maintaining appropriate dosing and treatment compliance, switching to a different biologic is recommended to maximize treatment response.19 Changing therapy to a biologic within the same class is generally effective,20 and switching to a biologic with another mechanism of action should be considered if a class-specific adverse effect is the major reason for altering the regimen. Nonetheless, some patients may be unresponsive to biologic changes. Further research is necessary to determine which biologics may be most effective when previously used biologics have failed and particular factors that may predispose patients to biologic unresponsiveness.
Resuming Biologic Treatment Following Cessation
In cases where therapy is discontinued for any reason, it may be necessary to repeat initiation dosing when resuming treatment. In patients with severe or flaring disease or if more than 3 to 4 half-lives have passed since the most recent dose, it may be necessary to restart therapy with the loading dose (Table). Unfortunately, restarting therapy may preclude some patients from experiencing the maximal response that they attained prior to cessation. In such cases, switching biologic therapy to a different class may prove beneficial.
Final Thoughts
These recommendations contain valuable information that will assist dermatologists when initiating biologics and managing outcomes of their psoriasis patients. It is, however, crucial to bear in mind that these guidelines serve as merely a tool. Given the paucity of comprehensive research, particularly regarding some of the more recently approved therapies, there are many questions that are unanswered within the guidelines. Their utility for each individual patient situation is therefore limited, and clinical judgement may outweigh the information presented. The recommendations nevertheless provide a pivotal and unprecedented framework that promotes discourse among patients, dermatologists, and other providers to optimize the efficacy of biologic therapy for psoriasis.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
- Michalek IM, Loring B, John SM. A systematic review of worldwide epidemiology of psoriasis. J Eur Acad Dermatol Venereol. 2017;31:205-212.
- Kurd SK, Gelfand JM. The prevalence of previously diagnosed and undiagnosed psoriasis in US adults: results from NHANES 2003-2004. J Am Acad Dermatol. 2009;60:218-224.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics [published online February 13, 2019]. J Am Acad Dermatol. 2019;80:1029-1072.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Förger F, Villiger PM. Treatment of rheumatoid arthritis during pregnancy: present and future. Expert Rev Clin Immunol. 2016;12:937-944.
- Gooderham M, Elewski B, Pariser D, et al. Incidence of serious gastrointestinal events and inflammatory bowel disease among tildrakizumab-treated patients with moderate-to-severe plaque psoriasis: data from 3 large randomized clinical trials [abstract]. J Am Acad Dermatol. 2018;79(suppl 1):AB166.
- Lebwohl M, Strober B, Menter A, et al. Phase 3 studies comparing brodalumab with ustekinumab in psoriasis. N Engl J Med. 2015;373:1318-328.
- Papp KA, Reich K, Paul C, et al. A prospective phase III, randomized, double-blind, placebo-controlled study of brodalumab in patients with moderate-to-severe plaque psoriasis. Br J Dermatol. 2016;175:273-286
- Beck KM, Koo J. Brodalumab for the treatment of plaque psoriasis: up-to-date. Expert Opin Biol Ther. 2019;19:287-292.
- Fouéré S, Adjadj L, Pawin H. How patients experience psoriasis: results from a European survey. J Eur Acad Dermatol Venereol. 2005;19(suppl 3):2-6.
- Björnsson ES, Bergmann OM, Björnsson HK, et al. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1-3; quiz e19-20.
- Saunte DM, Mrowietz U, Puig L, et al. Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Br J Dermatol. 2017;177:47-62.
- Huber F, Ehrensperger B, Hatz C, et al. Safety of live vaccines on immunosuppressive or immunomodulatory therapy—a retrospective study in three Swiss Travel Clinics [published online January 1, 2018]. J Travel Med. doi:10.1093/jtm/tax082.
- Dooling KL, Guo A, Patel M, et al. Recommendations of the Advisory Committee on Immunization Practices for Use of Herpes Zoster Vaccines. MMWR Morb Mortal Wkly Rep. 2018;67:103-108.
- Fiorentino D, Ho V, Lebwohl MG, et al. Risk of malignancy with systemic psoriasis treatment in the Psoriasis Longitudinal Assessment Registry. J Am Acad Dermatol. 2017;77:845-854.e5.
- Haynes K, Beukelman T, Curtis JR, et al. Tumor necrosis factor α inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum. 2013;65:48-58.
- Fabiano A, De Simone C, Gisondi P, et al. Management of patients with psoriasis treated with biologic drugs needing a surgical treatment. Drug Dev Res. 2014;75(suppl 1):S24-S26.
- Choi YM, Debbaneh M, Weinberg JM, et al. From the Medical Board of the National Psoriasis Foundation: perioperative management of systemic immunomodulatory agents in patients with psoriasis and psoriatic arthritis. J Am Acad Dermatol. 2016;75:798-805.e7.
- Honda H, Umezawa Y, Kikuchi S, et al. Switching of biologics in psoriasis: reasons and results. J Dermatol. 2017;44:1015-1019.
- Bracke S, Lambert J. Viewpoint on handling anti-TNF failure in psoriasis. Arch Dermatol Res. 2013;305:945-950.
Practice Points
- There are currently 11 biologics approved for psoriasis, but there is no first-line or optimalbiologic. The choice must be made using clinical judgment based on a variety of medical and social factors.
- Frequent assessment for efficacy of and adverse events due to biologic therapy is warranted, as lack of response, loss of response, or severe side effects may warrant addition of concurrent therapies or switching to a different biologic.
- There are important considerations to make when immunizing and planning for surgery in patients on biologics.
Systemic Therapies in Psoriasis: An Update on Newly Approved and Pipeline Biologics and Oral Treatments
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
Recent advances in our understanding of psoriatic immune pathways have led to new generations of targeted therapies developed over the last 5 years. Although the pathogenesis of psoriasis remains to be fully elucidated, the success of these targeted therapies has confirmed a critical role of the IL-23/helper T cell (TH17) axis in maintaining the psoriatic immune cascade, a positive feedback loop in which IL-17, IL-12, and IL-23 released from myeloid dendritic cells lead to activation of helperT cells. Activated helper T cells—namely TH1, TH17, and TH22—release IL-17, IL-22, and other proinflammatory cytokines, amplifying the immune response and leading to keratinocyte proliferation and immune cell migration to psoriatic lesions. Inhibition of IL-17 and IL-23 by several biologics disrupts this aberrant inflammatory cascade and has led to dramatic improvements in outcomes, particularly among patients with moderate to severe disease.
Numerous biologics targeting these pathways and several oral treatments have been approved by the US Food and Drug Administration (FDA) for the treatment of psoriasis; in addition, a number of promising therapies are on the horizon, and knowledge of these medications might help guide our treatment approach to the patient with psoriasis. This article provides an update on the most recent (as of 2019) approved therapies and medications in the pipeline for moderate to severe plaque psoriasis, with a focus on systemic agents in phase 3 clinical trials. (Medications targeting psoriatic arthritis, biosimilars, and existing medications approved by the FDA prior to 2019 will not be discussed.)
Risankizumab
Risankizumab-rzaa (formerly BI 655066) is a humanized IgG1 monoclonal antibody that targets the p19 subunit of IL-23, selectively inhibiting the role of this critical cytokine in psoriatic inflammation.
Phase 1 Trial
In a phase 1 proof-of-concept study, 39 patients with moderate to severe plaque psoriasis received varying dosages of intravenous or subcutaneous risankizumab or placebo.1 At week 12, the percentage of risankizumab-treated patients achieving reduction in the psoriasis area and severity index (PASI) score by 75% (PASI 75), 90% (PASI 90), and 100% (PASI 100) was 87% (27/31; P<.001 vs placebo), 58% (18/31; P=.007 vs placebo), and 16% (5/31; P=.590 vs placebo), respectively. Improvements in PASI scores were observed as early as week 2. Adverse events (AEs) were reported by 65% of the risankizumab group and 88% of the placebo group. Serious AEs were reported in 4 patients receiving risankizumab, none of which were considered related to the study medication.1
Phase 2 Trial
A phase 2 comparator trial demonstrated noninferiority at higher dosages of risankizumab in comparison to the IL-12/IL-23 inhibitor ustekinumab.2 Among 166 participants with moderate to severe plaque psoriasis, PASI 90 at week 12 was met by 77% of participants receiving 90 or 180 mg of risankizumab compared to 40% receiving ustekinumab (P<.001). Onset of activity with risankizumab was faster and the duration of effect longer vs ustekinumab; by week 8, at least PASI 75 was achieved by approximately 80% of participants in the 90-mg and 180-mg risankizumab groups compared to 60% in the ustekinumab group; PASI score reductions generally were maintained for as long as 20 weeks after the final dose of risankizumab was administered.2
Phase 3 Trials
The 52-week UltIMMa-1 and UltIMMa-2 phase 3 trials compared subcutaneous risankizumab (150 mg) to ustekinumab (45 or 90 mg [weight-based dosing]) or placebo administered at weeks 0, 4, 16, 28, and 40 in approximately 1000 patients with moderate to severe plaque psoriasis.3 Patients initially assigned to placebo switched to risankizumab 150 mg at week 16. At week 16, PASI 90 was achieved by 75.3% of risankizumab-treated patients, 42.0% of ustekinumab-treated patients, and 4.9% of placebo-treated patients in UltIMMa-1, and by 74.8% of risankizumab-treated patients, 47.5% of ustekinumab-treated patients, and 2.0% of placebo-treated patients in UltIMMa-2 (P<.0001 vs placebo and ustekinumab for both studies). Achievement of a static physician’s global assessment (sPGA) score of 0 or 1 at week 16 similarly favored risankizumab, with 87.8%, 63.0%, and 7.8% of patients in UltIMMa-1 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively, and 83.7%, 61.6%, and 5.1% in UltIMMa-2 meeting an sPGA score of 0 or 1 in the risankizumab, ustekinumab, and placebo groups, respectively (P<.0001 vs placebo and ustekinumab for both studies). Among patients initially assigned to risankizumab, improvements in PASI and sPGA continued to increase until week 52, with 81.9% achieving PASI 90 at week 52 compared to 44.0% on ustekinumab in UltIMMa-1, and 80.6% achieving PASI 90 at week 52 compared to 50.5% on ustekinumab in UltIMMa-2 (P<.0001 vs ustekinumab for both studies). Treatment-emergent AE profiles were similar for risankizumab and ustekinumab in both studies, and there were no unexpected safety findings.3
Risankizumab received FDA approval for the treatment of moderate to severe plaque psoriasis in April 2019.
Bimekizumab
Bimekizumab (UCB4940), a humanized IgG1 monoclonal antibody, selectively neutralizes the biologic functions of IL-17A and IL-17F, the latter of which has only recently been implicated in contributing to the psoriatic immune cascade.4
First-in-Human Study
Thirty-nine participants with mild psoriasis demonstrated efficacy after single-dose intravenous bimekizumab, with maximal improvements in all measures of disease activity observed between weeks 8 and 12 in participants receiving 160 to 640 mg.5
Proof-of-Concept Phase 1b Study
A subsequent trial of 53 participants with psoriatic arthritis demonstrated sustained efficacy to week 20 with varying dosages of intravenous bimekizumab.6 At week 8, PASI 100 was met by 86.7% of participants receiving the top 3 dosages of bimekizumab compared to none of the placebo-treated participants. Treatment-emergent AEs, including neutropenia and elevation of liver transaminases, were mostly mild to moderate and resolved spontaneously. There were 3 severe AEs and 3 serious AEs, none of which were related to treatment.6
Importantly, bimekizumab was shown in this small study to have the potential to be highly effective at treating psoriatic arthritis. American College of Rheumatology ACR20, ACR50, and ACR70 response criteria were very high, with an ACR20 of 80% and an ACR50 of 40%.6 Further trials are necessary to gather more data and confirm these findings; however, these levels of response are higher than those of any other biologic on the market.
Phase 2b Dose-Ranging Study
In this trial, 250 participants with moderate to severe plaque psoriasis received either 64 mg, 160 mg with a 320-mg loading dose, 320 mg, or 480 mg of subcutaneous bimekizumab or placebo at weeks 0, 4, and 8.7 At week 12, PASI 90 was achieved by significantly more patients in all bimekizumab-treated groups compared to the placebo group (46.2%–79.1% vs 0%; P<.0001 for all dosages); PASI 100 also was achieved by significantly more bimekizumab-treated patients (27.9%–60.0% vs 0%; P<.0002). Improvement began as early as week 4, with clinically meaningful responses observed in all bimekizumab groups across all measures of disease activity. Treatment-emergent AEs occurred more frequently in bimekizumab-treated participants (61%) than in placebo-treated participants (36%); the most common AEs were nasopharyngitis and upper respiratory tract infection. Of note, fungal infections were reported by 4.3% of participants receiving bimekizumab; all cases were localized superficial infection, and none led to discontinuation. Three serious AEs were reported, none of which were considered related to the study treatment.7
Mirikizumab
Mirikizumab (LY3074828) is a humanized IgG4 monoclonal antibody that selectively binds and inhibits the p19 subunit of IL-23, with no action on IL-12.
Phase 1 Trial
Mirikizumab was shown to improve PASI scores in patients with plaque psoriasis.8
Phase 2 Trial
Subsequently, a trial of 205 participants with moderate to severe plaque psoriasis compared 3 dosing regimens of subcutaneous mirikizumab—30, 100, or 300 mg—at weeks 0 and 8 compared to placebo.9 Primary end point results at week 16 demonstrated PASI 90 response rates of 0%, 29% (P=.009), 59% (P<.001), and 67% (P<.001) in the placebo, 30-mg, 100-mg, and 300-mg mirikizumab groups, respectively. Complete clearance of psoriasis, measured by PASI 100 and sPGA 0, was achieved by 0%, 16%, 31%, and 31%, respectively (P=.039 for 30 mg vs placebo; P=.007 for the higher dosage groups vs placebo). Response rates for all efficacy outcomes were statistically significantly higher for all mirikizumab treatment groups compared to placebo and were highest in the 100-mg and 300-mg treatment groups. Frequencies of participants reporting AEs were similar across treatment and placebo groups.9
Oral Medications
Only a few small-molecule, orally bioavailable therapies are on the market for the treatment of psoriasis, some of which are associated with unfavorable side-effect profiles that preclude long-term therapy.
BMS-986165
The intracellular signaling enzyme tyrosine kinase 2 is involved in functional responses of IL-12 and IL-23. BMS-986165, a potent oral inhibitor of tyrosine kinase 2 with greater selectivity than other tyrosine kinase inhibitors, demonstrated efficacy in a phase 2 trial of 267 participants with moderate to severe plaque psoriasis receiving any of 5 dosing regimens—3 mg every other day, 3 mg daily, 3 mg twice daily, 6 mg twice daily, and 12 mg daily—compared to placebo.10 At week 12, the percentage of patients with a 75% or greater reduction in PASI was 7% with placebo, 9% with 3 mg every other day (P=.49 vs placebo), 39% with 3 mg daily (P<.001 vs placebo), 69% with 3 mg twice daily (P<.001 vs placebo), 67% with 6 mg twice daily (P<.001 vs placebo), and 75% with 12 mg once daily (P<.001 vs placebo). Adverse events occurred in 51% of patients in the placebo group and in 55% to 80% of BMS-986165–treated patients; the most common AEs were nasopharyngitis, headache, diarrhea, nausea, and upper respiratory tract infection.10
A phase 3 trial comparing BMS-986165 with placebo and apremilast is underway (ClinicalTrials.gov Identifier NCT03611751).
Piclidenoson (CF101)
A novel small molecule that binds the Gi protein–associated A3 adenosine receptor piclidenoson induces an anti-inflammatory response via deregulation of the Wnt and nuclear factor κB signal transduction pathways, leading to downregulation of proinflammatory cytokines, including IL-17 and IL-23.11
In a phase 2 dose-ranging study, 75 patients with moderate to severe plaque psoriasis received varying dosages—1, 2, or 4 mg—of oral piclidenoson or placebo twice daily for 12 weeks.12 Progressive improvement in the mean change from baseline PASI score was observed in the 2-mg group, with statistically significant differences at weeks 8 and 12 compared to placebo (P=.047 and P=.031, respectively). At week 12, 35.3% of the 2-mg group achieved at least PASI 50. Improvements in PASI were less pronounced in the 4-mg group, and no therapeutic benefit was observed in the 1-mg group. Of the 20 AEs reported, 15 possibly were related to the study drug; 1 AE was severe.12
In a subsequent phase 2/3 trial, patients with moderate to severe plaque psoriasis received piclidenoson—1 or 2 mg—or placebo twice daily.13 At week 12, PASI 75 was achieved by 8.5% of patients in the 2-mg group and by 6.9% of patients receiving placebo (P=.621), thereby not meeting the primary study end point. Results at week 32 were more encouraging. In the 2-mg group, PASI mean percentage improvement was 57% (P<.002) compared to baseline, with linear improvements observed in PASI 50 (63.5%), PASI 75 (35.5%), PASI 90 (24.7%), and PASI 100 (10.6%).13
A phase 3 trial comparing piclidenoson 2 and 3 mg to apremilast and placebo is in progress (ClinicalTrials.gov Identifier NCT03168256).
Future Directions
Despite abundant options for treating moderate to severe plaque psoriasis and psoriatic arthritis, the pipeline remains rich. Novel treatments might have improved efficacy, favorable safety profiles, and different modes of administration compared to current medications. In addition to the novel therapeutics covered here, several treatments are in development further down the pipeline, with only phase 1 or 2 data available. Remtolumab (ABT-122), a tumor necrosis factor α– and IL-17A–targeted immunoglobulin, is unique among biologics, given its dual inhibition of tumor necrosis factor α and IL-17A.14 M1095 (ALX-0761), a novel trivalent bispecific nanobody, is another intriguing candidate. This dual inhibitor of IL-17A/F might exhibit a number of advantages over conventional antibodies, including better tissue penetration, reduced immunogenicity, and a longer half-life (ClinicalTrials.gov Identifier NCT03384745).15,16
As always with drug development, numerous medications that were under development failed to meet primary end points in phase 2 trials and have therefore been discontinued, including namilumab and prurisol. It is reassuring that the pace of drug discovery and development in psoriasis does not seem to be slowing; to our patients’ benefit, we will have an array of treatments available to tailor therapy to the individual.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
- Krueger JG, Ferris LK, Menter A, et al. Anti-IL-23A mAb BI 655066 for treatment of moderate-to-severe psoriasis: safety, efficacy, pharmacokinetics, and biomarker results of a single-rising-dose, randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2015;136:116-124.e7.
- Papp KA, Blauvelt A, Bukhalo M, et al. Risankizumab versus ustekinumab for moderate-to-severe plaque psoriasis. N Engl J Med. 2017;376:1551-1560.
- Gordon KB, Strober B, Lebwohl M, et al. Efficacy and safety of risankizumab in moderate-to-severe plaque psoriasis (UltIMMa-1 and UltIMMa-2): results from two double-blind, randomised, placebo-controlled and ustekinumab-controlled phase 3 trials. Lancet. 2018;392:650-661.
- Maroof A, Baeten D, Archer S, et al. 02.13 Il-17f contributes to human chronic inflammation in synovial tissue: preclinical evidence with dual IL-17a and IL-17f inhibition with bimekizumab in psoriatic arthritis. Ann Rheum Dis. 2017;76(Suppl 1):A13.
- Glatt S, Helmer E, Haier B, et al. First-in-human randomized study of bimekizumab, a humanized monoclonal antibody and selective dual inhibitor of IL-17A and IL-17F, in mild psoriasis. Br J Clin Pharmacol. 2017;83:991-1001.
- Glatt S, Baeten D, Baker T, et al. Dual IL-17A and IL-17F neutralisation by bimekizumab in psoriatic arthritis: evidence from preclinical experiments and a randomised placebo-controlled clinical trial that IL-17F contributes to human chronic tissue inflammation. Ann Rheum Dis. 2018;77:523-532.
- Papp KA, Merola JF, Gottlieb AB, et al. Dual neutralization of bothinterleukin 17A and interleukin 17F with bimekizumab in patients with psoriasis: results from BE ABLE 1, a 12-week randomized, double-blinded, placebo-controlled phase 2b trial. J Am Acad Dermatol. 2018;79:277-286.e10.
- Maari C. Safety, efficacy, and pharmacokinetics of a p19-directed IL-23 antibody in patients with plaque psoriasis and healthy subjects. Presented at: 25th European Academy of Dermatology and Venereology Congress; Vienna, Austria; September 28-October 2, 2016.
- Reich K, Rich P, Maari C, et al. Efficacy and safety of mirikizumab (LY3074828) in the treatment of moderate-to-severe plaque psoriasis: results from a randomized phase II study. Br J Dermatol. 2019;181:88-95.
- Papp K, Gordon K, Thaçi D, et al. Phase 2 trial of selective tyrosine kinase 2 inhibition in psoriasis. N Engl J Med. 2018;379:1313-1321.
- Cohen S, Barer F, Itzhak I, et al. Inhibition of IL-17 and IL-23 in human keratinocytes by the A3 adenosine receptor agonist piclidenoson. J Immunol Res. 2018;2018:2310970.
- David M, Akerman L, Ziv M, et al. Treatment of plaque-type psoriasis with oral CF101: data from an exploratory randomized phase 2 clinical trial. J Eur Acad Dermatol Venereol. 2012;26:361-367.
- 13. David M, Gospodinov DK, Gheorghe N, et al. Treatment of plaque-type psoriasis with oral CF101: data from a phase II/III multicenter, randomized, controlled trial. J Drugs Dermatol. 2016;15:931-938.
- Mease PJ, Genovese MC, Weinblatt ME, et al. Phase II study of ABT-122, a tumor necrosis factor- and interleukin-17A-targeted dual variable domain immunoglobulin, in patients with psoriatic arthritis with an inadequate response to methotrexate. Arthritis Rheumatol. 2018;70:1778-1789.
- Nanobodies’ competitive features. Ablynx website. http://www.ablynx.com/technology-innovation/nanobodies-competitive-features. Accessed July 4, 2019.
- Svecova D, Lubell MW, Casset-Semanaz F, et al. A randomized, double-blind, placebo-controlled phase 1 study of multiple ascending doses of subcutaneous M1095, an anti-interleukin-17A/F nanobody, in moderate-to-severe psoriasis. J Am Acad Dermatol. 2019;81:196-203.
Practice Points
- New systemic options for the treatment of psoriasis continue to emerge.
- With more choices, we can now tailor therapeutic approaches to the patient rather than base treatment choices purely on efficacy.
- New and upcoming biologics may offer improved skin clearance in line with the National Psoriasis Foundation’s treat-to-target approach, while others may offer increased efficacy in treating psoriatic arthritis.
- Novel small-molecule oral medications are in development and may have improved efficacy over current options.
Comment on “Analysis of Nail-Related Content of the Basic Dermatology Curriculum”
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
To the Editor:
In the April 2019 Cutis article by John and Lipner,1 the authors critiqued the American Academy of Dermatology Basic Dermatology Curriculum (BDC) for not providing an adequate scaffolding of nail findings on which dermatology residents can build their knowledge base; however, that criticism belies a misunderstanding of the BDC’s purpose. It was carefully designed to address the needs of undifferentiated medical students and primary care learners based on needs assessments from practicing primary care physicians and experienced dermatology educators.2,3 Given the limited amount of time to teach, a basic curriculum must focus on the most high-yield items. The BDC work group developed goals and objectives based on needs assessments for primary care practice with 38 core dermatology diagnoses, including 3 diagnoses with important nail findings: onychomycosis, melanoma, and psoriasis. Much repetition is built into the BDC, and the same diagnosis is used in multiple cases in different modules to encourage retention of information. Therefore, “analysis of nail-related content” should focus on diagnoses rather than cases, and for each diagnosis, note whether the nail findings are a pertinent negative or pertinent positive. In cases of the other 35 diagnoses covered in the BDC, nail findings are omitted for space because they are not relevant (eg, in cases of seborrheic dermatitis or rosacea). Normal nail findings are not pertinent negatives for most diagnoses in the BDC, except in cases with diagnoses for which psoriasis is in the differential, such as nummular dermatitis or pityriasis rosea.
Furthermore, a true analysis of the needs of medical students and primary care learners with regard to nail findings would begin with a needs assessment of the most common nail conditions evaluated in the primary care and urgent care settings. Ingrown nails, paronychia, onychomycosis, and subungual hematomas and other nail traumas are the most common nail conditions encountered in primary care and urgent care,4-10 but John and Lipner1 failed to perform analysis or needs assessment based on the incidence of nail diagnoses in these settings.
Other sources for medical students and primary care residents include excellent introductions to nail findings. The newly revised skin chapter of Bates’ Guide to Physical Examination and History Taking11 includes updated photographs of common nail findings and discusses the importance of examining nails in the full-body skin examination. Additionally, Clinical Dermatology: A Color Guide to Diagnosis and Therapy,12Lookingbill and Marks’ Principles of Dermatology,13 and The Color Atlas and Synopsis of Family Medicine14 cover nail disease beautifully for medical students and primary care learners. The BDC was never meant to supplant these bountiful resources.
The authors referred to lack of confidence in nail diagnoses among dermatology residents,1 which is a very real problem that must be addressed by dermatology residency programs. The BDC is not the proper vehicle for training dermatology residents about these conditions; that is the responsibility and challenge of our dermatology residency programs. The authors also suggested teaching how to perform nail biopsies in the BDC.1 It not reasonable to expect that our primary care colleagues will be performing nail biopsies. A more appropriate level of expectation is that they would know when to refer patients to dermatology; for example, they should know that a pigmented streak on a single nail that is expanding is an indication for referral to a dermatologist.
If the authors or others were to propose an additional nail module to the BDC work group, they would need to include an analysis of the literature regarding the incidence of nail disease seen in primary care and urgent care settings rather than the nail conditions seen by referral bias experienced by consulting dermatologists. The analysis would be worth considering and worthy of the goodwill engendered by the creation of the BDC in the first place.
Sincerely,
Patrick E. McCleskey, MD
From the Department of Dermatology, Kaiser Permanente Oakland Medical Center, California.
Dr. McCleskey previously served as Chair of the American Academy of Dermatology Basic Dermatology Curriculum Work Group (2013-2017) .
Correspondence: Patrick E. McCleskey, MD, 3701 Broadway, 4th Floor, Oakland, CA 94611 (Patrick.e.mccleskey@kp.org).
References
1. John JJ, Lipner SR. Analysis of nail-related content in the basic dermatology curriculum. Cutis. 2019;103:214-216.
2. Hansra NK, O’Sullivan P, Chen CL, et al. Medical school dermatology curriculum: are we adequately preparing primary care physicians? J Am Acad Dermatol. 2009;61:23-29.
3. McCleskey PE, Gilson RT, Devillez R. Medical student core curriculum in dermatology survey. J Am Acad Dermatol. 2009;61:30-35.
4. Vierhoeven EWM, Kraaimaat FW, van Wheel C, et al. Skin diseases in family medicine: prevalence and health care use. Ann Fam Med. 2008;6:349-354.
5. Fleisher AB, Herbert CR, Feldman SR, et al. Diagnosis of skin disease by non-dermatologists. Am J Manag Care. 2000;6;1149-1156.
6. Akbas A, Kilinc F, Yakut HI, et al. Nail disorders in children, a clinical study. Our Dermatol Online. 2016;7:149-154.
7. Nadkarni A, Domeisen N, Hill D, et al. The most common dermatology diagnoses in the emergency department. J Am Acad Dermatol. 2016;75:1261-1262.
8. Baibergenova A, Shear NH. Skin conditions that bring patients to emergency departments. Arch Dermatol. 2011;147:118-120.
9. Wang E, Lim BL, Than KY. Dermatological conditions presenting at an emergency department in Singapore. Singapore Med J. 2009;50:881-884.
10. Lai-Kwon J, Weiland TJ, Chong AH, et al. Which dermatological conditions present to an emergency department in Australia? Emerg Med Int. 2014;2014:463026.
11. McCleskey PE. The skin, hair, and nails. In: Bickley L, ed. Bates’ Guide to Physical Examination and History Taking. 12th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2017:173-214.
12. Habif TP. Nail diseases. In: Habif TP, ed. Clinical Dermatology: A Color Guide to Diagnosis and Therapy. 6th ed. China: Elsevier; 2016:960-985.
13. Marks JG, Miller JJ. Nail disorders. In: Marks JG, Miller JJ, eds. Lookingbill and Marks’ Principles of Dermatology. 6th ed. China: Elsevier; 2019:277-282.
14. Mayeaux EJ Jr, Williams J. Hair and nail conditions. In: Usatine RP, Smith MA, Mayeaux EJ Jr, et al. The Color Atlas and Synopsis of Family Medicine. 3rd ed. New York, NY: McGraw-Hill Education; 2019.
Author Response
I thank Dr. McCleskey for his interest in our article. Although I acknowledge that the Basic Dermatology Curriculum (BDC) serves as an introduction to dermatology for medical students and primary care physicians, I disagree that the current curriculum should be limited to only 3 diagnoses with important nail findings—onychomycosis, melanoma, and psoriasis—and exclude other common and potentially fatal nail diseases.
To characterize the overall nail burden of ambulatory care visits in the United States, data from the National Ambulatory Medical Care Survey from 2007 to 2016 were analyzed and there were more than 20 million outpatient visits for nail concerns during this period; furthermore, although many patients were seen by dermatologists, a considerable number were seen by pediatricians and general practitioners (Lipner SR, Hancock J, Fleischer AB Jr; unpublished data; July 2019). These findings underscore the importance of educating medical students and primary care physicians on the diagnosis and appropriate referral of patients with nail diseases.
Some limited information on nail unit melanomas is included in the BDC, but it is essential that medical students and general practitioners be educated about early diagnosis of squamous cell carcinomas and melanomas of the nail unit, which may help avoid unnecessary amputations and decrease mortality.1 Unfortunately, the vast majority of nail unit melanomas are diagnosed at stage II or later, which has been partially attributed to clinical knowledge gaps in the understanding of nail disease.2
Several studies have shown that many physicians fail to examine their patients’ nails during physical examinations, either due to concealment with nail polish or lack of clinical awareness. In a survey-based study analyzing patients’ awareness of longitudinal melanonychia and worrisome signs of nail unit melanoma, only 12% of patients (43/363) stated that their dermatologist or internist specifically asked them about nail changes.3 Furthermore, in another survey-based study of nail examinations at a free cancer screening by the American Academy of Dermatology, more than half of female participants (47/87 [54%]) stated that they were wearing nail polish at the time of screening.4,5 Therefore, examinations of the nails were not performed as part of the total-body skin examination.
In summary, nail diseases are an important concern in clinical practice with aesthetic and functional consequences. There is a strong need to emphasize the importance of nail examinations for diagnostic purposes and to incorporate more expansive nail-related content into the BDC, which can result in longer and more functional lives for our patients.
Sincerely,
Shari R. Lipner, MD, PhD
From the Department of Dermatology, Weill Cornell Medicine, New York, New York.
The author reports no conflict of interest.
References
1. Lipner SR. Ulcerated nodule of the fingernail. JAMA. 2018;319:713.
2. Tan KB, Moncrieff M, Thompson JF, et al. Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol. 2007;31:1902-1912.
3. Halteh P, Scher R, Artis A, et al. Assessment of patient knowledge of longitudinal melanonychia: a survey study of patients in outpatient clinics. Skin Appendage Disord. 2017;2:156-161.
4. Ko D, Lipner SR. A survey-based study on nail examinations at an American Academy of Dermatology free skin cancer screening. J Am Acad Dermatol. 2018;79:975-978.
5. Ko D, Lipner SR. Comment on: “The first 30 years of the American Academy of Dermatology skin cancer screening program: 1985-2014.” J Am Acad Dermatol. 2019;80:e23.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
In response to a recent analysis of nail-related content in the Basic Dermatology Curriculum (BDC), the author suggests the BDC is not the proper vehicle for training dermatology residents about nail conditions and proposes alternative sources for mastering this material.
How Do Drug Shortages Affect Dermatologists?
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
The frequency of drug shortages in the United States has considerably increased over the last decade, affecting different areas of health care practice.1,2 Basic products needed to care for patients in hospitals and clinics are many of the same drugs that are in short supply.3 This issue has become an ongoing public health concern that directly affects health care providers and their patients.4 In dermatology, similar to other specialties, success often is influenced by the efficacy of medications used to treat patients, and lack of appropriate medications has the potential to diminish health outcomes. Therefore, it is imperative for dermatology providers to recognize the factors that contribute to this issue, understand the effects of drug shortages on patients, and learn how they can improve stewardship of scarce resources and contribute to the solution.
Causes of Drug Shortages
Drug shortages can occur due to discontinuations, delays, or manufacturing and quality problems.5 Shortages of the most basic hospital products represent market failure.1 In such cases, a small number of manufacturers supply these products, and if a manufacturer discontinues a particular product—as in the case of lidocaine with epinephrine—a shortage results, as the current system does not have the capacity to deal with such as issue.1,6
An important playmaker affecting the market for medical supplies and drugs are group purchasing organizations (GPOs). The 4 largest GPOs in the United States account for 90% of the medical supply market.7 Although they have simplified the process for hospitals to purchase supplies by taking on the work and expense of dealing with hundreds of manufacturers, GPOs have considerable power to affect the supply chain. By allowing certain manufacturers to become the sole suppliers of products in return for premium fees, GPOs have narrowed the supply chain of key products to sometimes only 1 or 2 manufacturers.7 This practice may lead to decreased capacity of regional and national supply chains, setting up the system to eventual product shortage in scenarios of production problems or a decrease in the already limited number of manufacturers.
The US Food and Drug Administration (FDA) works closely with manufacturers to prevent or reduce the impact of drug shortages. Although the FDA recently has taken more action to address the issue, solutions such as allowing imported products and underlying or approving new suppliers are only temporary fixes.1 The root of the problem needs to be dealt with by ensuring there is a broad competitive supply chain.
Impact on Dermatologists
The nationwide shortage of lidocaine with epinephrine that occurred in 2017 is a specific example of how drug shortages affect dermatologists.6 This product is used in the typical dermatology clinic on a daily basis for biopsies. Possible solutions to decrease usage include drawing up 1.5 mL lidocaine with epinephrine instead of 3 mL and mixing readily available normal saline with lidocaine to produce a 1:200,000 mixture to yield a 0.5% concentration that still maintains good vasoconstrictor effects. Options for dermatologists who run out of lidocaine with epinephrine are to either use lidocaine without epinephrine, which disrupts optimal patient care, or to purchase 1% lidocaine with epinephrine at a much higher cost.6 A study that analyzed changes in drug pricing following shortages in the United States indicated that prices of drugs facing a shortage increased more than twice as quickly as expected between 2015 and 2016 vs those that were not in shortage, which may reflect opportunistic behaviors of drug manufacturers during shortages.8
The American Academy of Dermatology Association has created a letter and encouraged patients to notify their lawmakers about the severity of the drug shortage issue. Given the shortage of local anesthetics and their importance to the practice of dermatology, the American Academy of Dermatology Association also has created guidelines discussing local anesthetics that could be an alternative to lidocaine for office-based dermatologic surgery.9
Final Thoughts
Dermatology practitioners should be aware of current shortages impacting their practice and address the potential shortage proactively. We propose that dermatology clinics should keep an emergency reservoir of products routinely used in practice that currently are on the FDA drug shortage list, particularly lidocaine hydrochloride (with and without epinephrine) and sodium bicarbonate,10 which may diminish the negative impact a shortage may have on the high quality of health care we strive to provide. On a bigger scale, providers should be more proactive to have their voices heard and get involved with policymaking given the potential for patient harm and suboptimal care associated with drug shortages.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
- Mazer-Amirshahi M, Fox ER, Zocchi MS, et al. Longitudinal trends in US shortages of sterile solutions, 2001-17. Am J Health Syst Pharm. 2018;75:1903-1908.
- Fox ER, Sweet BV, Jensen V. Drug shortages: a complex health care crisis. Mayo Clin Proc. 2014;89:361-373.
- Drug shortages roundtable: minimizing impact on patient care [published online March 15, 2018]. Am J Health Syst Pharm. 2018;75:816-820.
- Fox ER, McLaughlin MM. ASHP guidelines on managing drug product shortages. Am J Health Syst Pharm. 2018;75:1742-1750.
- Bowles SK. Drug shortages: more than just a background noise [published online February 28, 2018]. Can J Hosp Pharm. 2019;72:3-4.
- Bodie B, Brodell RT, Helms SE. Shortage of lidocaine with epinephrine: causes and solutions. J Am Acad Dermatol. 2018;79:392-393.
- Bruhn WE, Fracica EA, Makary MA. Group purchasing organizations, health care costs, and drug shortages. JAMA. 2018;320:1859-1860.
- Hernandez I, Sampathkumar S, Good CB, et al. Changes in drug pricing after drug shortages in the United States. Ann Intern Med. 2018;170:74-76.
- AADA, other specialties continue pressing FDA on drug shortages American Academy of Dermatology Association website.
https://www.aad.org/advocacy/news/news/2018/02/aada-other-specialties-continue-pressing-fda-on-drug-shortages. Published February 23, 2018. Accessed July 24, 2019. - FDA drug shortages. US Food & Drug Administration website. https://www.aad.org/advocacy/drug-pricing-and-availability/dermatologic-drug-shortages. Accessed July 24, 2019.
Intraoperative Electrosurgical Smoke During Outpatient Surgery: A Survey of Dermatologic Surgeon and Staff Preferences
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
A growing body of evidence shows that electrosurgical smoke contains both harmful chemicals as well as live material, including blood particles, bacteria, and viruses.1 Both human immunodeficiency virus and human papillomavirus have been identified in surgical smoke plumes, and bacterial colony growth has been demonstrated from electrosurgical smoke specimens, specifically Staphylococcus, Corynebacterium, and Neisseria species.2-8 Treating 1 g of tissue with electrocoagulation produces chemical by-products equivalent to burning 6 unfiltered cigarettes,9 which is twice the amount of chemical by-products produced by CO2 laser vaporization of the same quantity of tissue. It is a common misconception that electrosurgical smoke is less hazardous than smoke produced by ablative CO2 procedures.9 Many chemicals are present in electrosurgical smoke plumes, including nitriles, benzenes, carbon monoxide, hydrogen cyanide, indoles, phenols, pyridine, pyrrole, styrene, toluene, and xylene.10-12 In animal model studies of rat lungs exposed to surgical smoke, pathologic changes, including interstitial pneumonia, bronchiolitis, and emphysema, have been shown in a dose-dependent manner.1,13-16 Diseases and symptoms linked to inhalation of electrosurgical smoke in humans include anemia, eye irritation, hypoxia, dizziness, nasopharyngeal lesions, vomiting, sneezing, throat irritation, and weakness.1,8,17-19 A study of 153 dermatology residents found that more than 70% reported receiving no formal education on the hazards of electrosurgical smoke.20 Approximately 45% were unaware if they had access to smoke evacuation in rooms where electrosurgery was performed. More than 76% were concerned with the infectious risk of electrosurgical smoke, and more than 71% were concerned with its potential carcinogenic risk.20
We surveyed dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences.
Materials and Methods
Survey Instrument
We developed a REDCap survey consisting of 17 questions that was approved by the executive committees of the American College of Mohs Surgery and the American Society for Dermatologic Surgery for distribution to their dermatologist memberships. It was emailed to eligible participants using their mailing lists. Although the survey was sent directly to member physicians, it was recommended that they forward the survey to their clinical staff to complete.
After responding to an initial set of survey questions, respondents were informed that there is growing evidence of potential harms of inhalation of surgical smoke. They then were asked the same series of survey questions in light of this information.
Statistical Analysis
Statistical analysis of the survey responses was then completed, and free-text responses as a final question of the survey were assessed for themes. Preintervention responses of staff and clinicians noticing smoke and being bothered by smoke were assessed using proportions and 95% confidence interval (CI) estimates of the proportions. On most questions, respondents could answer on a scale of 1 to 10. Responses of 5 to 10 on noticing smoke and 5 to 10 on being bothered or troubled by the smoke smell were grouped for analyses. A cross-tabulation using the Bhapkar test for marginal homogeneity was used to assess if information presented on potential smoke hazards changed responses. A Cochran-Mantel-Haenszel test for ordinal responses was used to determine differences between surgeons and staff. A McNemar test was used to determine statistical significance of change in responses to cost. Statistical analysis was performed using SAS version 9.
Results
There was a total of 443 responses to our questionnaire. Two respondents answered that they did not work in an office where skin surgery was performed, and 4 respondents did not answer any questions and were therefore excluded, leaving a total of 437 responses (402 physicians and 35 staff members). A summary of the characteristics of the respondents is shown in the Table. Some respondents did not answer each question, leading to fewer than 437 answers for some questions.
Two hundred eighty-two respondents (64.5%) never or very rarely used smoke evacuation during skin surgical procedures, and only 85 (19.5%) used smoke evacuation with nearly every case. The remaining respondents sometimes used smoke evacuation (Figure 1).
Prior to being presented with the potential dangers of electrosurgical smoke and using a value of 5 to 10 to determine if respondents noticed smoke, 54.4% (95% CI, 49.5%-59.1%) did notice intraoperative smoke during procedures. Using a value of 5 to 10 to indicate if respondents were bothered or troubled by the smoke smell, 35.5% (95% CI, 31.0%-40.2%) were bothered or troubled by intraoperative smoke prior to potential hazards being presented.
Regarding acceptable increase in cost per procedure for smoke evacuation at baseline, 68.9% of respondents favored additional cost; 57.8% of respondents chose the lowest cost grouping of $1 to $30. After being presented with information about the potential harm of intraoperative smoke, the respondents in favor of additional cost increased to 71.5%, which was a small but statistically significant change (P=.0075)(Figure 2).
Respondents were sorted into groups consisting of those who never used smoke evacuation, those who used it occasionally, and those who used it with all smoke-producing procedures. The degree to which respondents noticed intraoperative smoke was strongly correlated with their use of smoke evacuation; those who never used smoke evacuation noticed the presence of smoke more, and those who always used smoke evacuation noticed it less (P=.0002). Similar trends were noted regarding if the smoke smell bothered or troubled respondents (P=.0014).
After being presented with the potential risks of electrosurgical smoke, 29 more respondents answered that they were severely bothered by electrosurgical smoke, whereas 45 fewer respondents selected that they were not bothered or troubled at all by electrosurgical smoke (Figure 3). This difference was statistically significant (P<.0001). Fifteen more respondents answered that they would be much more likely to choose to work at a practice with smoke evacuation once the potential harm of electrosurgical smoke was introduced, and 11 were somewhat more likely to choose a practice with smoke evacuation (P<.0001).
Information about the potential harm of electrosurgical smoke did not statistically significantly affect satisfaction with work environment (P=.3139)(Figure 4).
There were no statistically significant differences between surgeon and staff responses on any questions.
Comment
Developing evidence of health risks associated with electrosurgical smoke plumes has led to an increasing interest in the use of smoke protection or remediation tools during surgical procedures. High-filtration face masks and smoke-evacuation devices protect physicians, staff members, and patients, as well as improve the patient’s clinical experience.
Our study was designed to query dermatologists who perform skin surgery as well as staff members with respect to their experiences with electrosurgical smoke and to observe any difference that information on the potential hazards of electrosurgical smoke may have on their attitudes and preferences. We received 437 responses to our survey (Table). At baseline, 54.4% of respondents noticed and 35.5% were bothered or troubled by the smoke smell produced during skin electrosurgery. These data were intuitively associated in a statistically significant manner with the use of smoke evacuation for respondents; those respondents who more commonly used smoke evacuation were bothered less by electrosurgical smoke, and those respondents who used smoke evacuation less often were more likely to notice and be bothered by surgical smoke.
Once our respondents were presented with the potentially harmful effects of electrosurgical smoke, they became significantly more likely to be bothered by electrosurgical smoke and to want to work in a practice where smoke evacuation was available. This information, however, did not change respondents’ satisfaction with their work environment, and no statistically significant differences were noted between physicians and staff.
At baseline, 68.9% of respondents favored additional cost for smoke evacuation, with approximately 58% favoring the lowest cost category we presented ($1–$30). After being presented with information about the potential dangers of electrosurgical smoke, 71.5% were in favor of increased cost for smoke evacuation, which was a small but statistically significant increase.
The open-comment section of the survey provided interesting insight into the opinions of our respondents on smoke remediation. It is important to note that statistical analysis cannot be performed with these data, and firm generalizable conclusions cannot be drawn from them; however, they reveal topics that may guide further research and policy and certainly merit mention. Of 437 respondents, 108 left free-text comments. Twenty-six percent were categorized as unqualified proponents (in favor of smoke remediation) and 45% as qualified proponents (defined as an individual who verbalized a desire for smoke remediation but also cited a factor limiting their ability to use it, such as cost or staff availability). Only 12% were firmly against smoke remediation, while the remaining 17% did not comment discernibly for or against smoke remediation, indicating that a majority (71% of our comment section respondents) were in favor of some type of smoke remediation, especially if obstacles such as cost could be addressed. Only a small minority was firmly against smoke remediation.
The comments section of our survey highlighted some of the concerns that dermatologic surgeons and their staff have with electrosurgical smoke evacuation. Thirty percent cited cost as an obstacle to use of these devices, and several comments raised concern about increasing overhead and regulatory demands placed on practices. Many indicated that, without sufficient evidence of the harm caused by electrosurgical smoke, regulation that forces use of smoke remediation devices would represent a costly unfunded mandate. Others referenced the logistical challenges of smoke evacuation and the need for staff assistance. Newer smoke-evacuation wands built into cautery pens address much of this concern regarding logistical and staff challenges and further allow the evacuator tip to be located where it is most effective: 1 cm to 2 in from the point of cautery.21,22
Additionally, 12% of commenters noted that their patients were bothered by the smell of electrosurgical smoke, which is a point that requires further research and is the focus of a current randomized trial at our institution (ClinicalTrials.gov Identifier NCT02958826).
Our current study is limited in that it is a survey and therefore is subject to response bias. Further, some may assert that the hazards of electrosurgical smoke are not settled science, and although we agree with this point on some level, the study aim was not to prove risk but rather to assess current attitudes and see if awareness of a potential risk influenced those attitudes. Additionally, most responses were from physicians—only 35 responses were from nonphysician staff—so it may be difficult to generalize the findings of this study to staff. The large number of physician respondents, however, can be seen as a strength, and the findings are likely much more generalizable to providers who routinely perform clinic-based surgical procedures involving electrosurgery.
Conclusion
Our study shows that most dermatologists who perform skin surgery notice and are bothered by the smoke produced by electrosurgery to at least some extent. When presented with the possibility that inhaling electrosurgical smoke may be harmful, dermatologists were more likely to be bothered by electrosurgical smoke, more likely to prefer a practice environment where smoke evacuation was available, and more likely to be willing to bear additional cost for smoke evacuation. The free-text comments on our survey highlighted that many dermatologic surgeons are proponents of smoke evacuation but have concerns about cost and potential regulatory challenges associated with smoke evacuation, especially if the potential risks are not settled science. Many logistical concerns for smoke evacuation are addressed with the use of integrated devices. More research is needed to determine the health effects of the surgical smoke we are exposed to daily and the optimal way to limit any risk.
Acknowledgment
The authors would like to thank Richard W. Madsen, PhD (Columbia, Missouri), biostatistician, for his valuable guidance in the statistical analysis of data, interpretation of results, and editorial support in finalizing the manuscript.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
- Lewin J, Brauer J, Ostad A. Surgical smoke and the dermatologist. J Am Acad Dermatol. 2011;65:636-641.
- Garden JM, O’Banion MK, Shelnitz LS, et al. Papillomavirus in the vapor of carbon dioxide laser-treated verrucae. JAMA. 1988;259:1199-1202.
- Sawchuk WS, Weber PJ, Lowy DR, et al. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41-49.
- Baggish MS, Poiesz BJ, Joret D, et al. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197-203.
- Capizzi PJ, Clay RP, Battey MJ. Microbiologic activity in laser resurfacing plume and debris. Lasers Surg Med. 1998;23:172-174.
- Sebben JE. The hazards of electrosurgery. J Am Acad Dermatol. 1987;16:869-872.
- Bigony L. Risks associated with exposure to surgical smoke plume: a review of the literature. AORN J. 2007;86:1013-1020.
- Barrett WL, Garber SM. Surgical smoke: a review of the literature. Surg Endosc. 2003;17:979-987.
- Tomita Y, Mihashi S, Nagata K, et al. Mutagenicity of smoke condensates induced by CO2-laser irradiation and electrocauterization. Mutat Res. 1981;89:145-149.
- Hollmann R, Hort CE, Kammer E, et al. Smoke in the operating theater: an unregarded source of danger. Plast Reconstr Surg. 2004;114:458-463.
- Hensman C, Baty D, Willis RG, et al. Chemical composition of smoke produced by high-frequency electrosurgery in a closed gaseous environment. An in vitro study. Surg Endosc. 1998;12:1017-1019.
- Ulmer B. The hazards of surgical smoke. AORN J. 2008;87:721-734; quiz 735-738.
- Baggish MS, Baltoyannis P, Sze E. Protection of the rat lung from the harmful effects of laser smoke. Lasers Surg Med. 1988;8:248-253.
- Baggish MS, Elbakry M. The effects of laser smoke on the lungs of rats. Am J Obstet Gynecol. 1987;156:1260-1265.
- Freitag L, Chapman GA, Sielczak M, et al. Laser smoke effect on the bronchial system. Lasers Surg Med. 1987;7:283-288.
- Gracie KW. Hazards of vaporized tissue plume. Surgical Technologist. 2001;33:20-26.
- Giordano BP. Don’t be a victim of surgical smoke. AORN J. 1996;63:520, 522.
- Dikes CN. Is it safe to allow smoke in our operating room? Todays Surg Nurse. 1999;21:15-21; quiz 38-39.
- Wu MP, Ou CS, Chen SL, et al. Complications and recommended practices for electrosurgery in laparoscopy. Am J Surg. 2000;179:67-73.
- Chapman LW, Korta DZ, Lee PK, et al. Awareness of surgical smoke risks and assessment of safety practices during electrosurgery among US dermatology residents. JAMA Dermatol. 2017;153:467-468.
- Trevor M. Presence of virus in CO2 laser plumes raises infection concern. Hosp Infect Control. 1987;14:166-167.
- Smith JP, Moss CE, Bryant CJ, et al. Evaluation of a smoke evacuator used for laser surgery. Lasers Surg Med. 1989;9:276-281.
Practice Points
- Growing evidence suggests that the surgical smoke plume generated during electrosurgery may be harmful if inhaled.
- Our survey indicates that this information may affect clinician and staff perceptions about exposure to electrosurgical smoke and its remediation.
Diversity and Inclusivity Are Essential to the Future of Dermatology
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
Over the last 5 years, there has been an important dialogue among dermatologists about diversity in our specialty that has shifted the mind-set of the dermatology community and highlighted an intent to build a diverse workforce. It is important to reflect on this effort and acknowledge the progress that has been made. Additionally, it also is important to envision what our ideal specialty will look like 10 years from now and to discuss specific ways that we can achieve that vision for the future of dermatology.
At the 2015 Annual Meeting of the American Academy of Dermatology (AAD), Bruce E. Wintroub, MD, highlighted the importance of diversity in dermatology when he presented the Clarence S. Livingood lecture.1 His discussion was followed by a call to action from Pandya et al2 in 2016, which described the lack of diversity in our specialty (the second least diverse specialty in medicine) and proposed specific steps that can be taken by individuals and organizations to address the issue. In line with this effort, the AAD’s Diversity Task Force, Diversity Mentorship Program,3 and Diversity Champion Initiative were created. The latter program enlisted dermatology residency programs across the country to select a diversity champion who would lead efforts to increase diversity in each participating department, including mentorship of underrepresented-in-medicine college and medical students. The AAD’s 2019 Diversity Champion Workshop4 (September 12–13, 2019) will be held for the first time prior to the Association of Professors of Dermatology Annual Meeting (September 13–14, 2019) in an attempt to scale up the Diversity Champion Initiative. This workshop has galvanized widespread support and will be collaboratively hosted by the AAD, Association of Professors of Dermatology, Skin of Color Society, Society for Investigative Dermatology, and Women’s Dermatologic Society.
Current diversity efforts have largely focused on increasing representation in the dermatology workforce. A publication in 2017 challenged the tenets of dermatology resident selection and advocated for holistic review of residency program applicants as one way to address the lack of diversity in dermatology.5 This viewpoint highlighted that dermatology’s traditional focus on US Medical Licensing Examination scores and Alpha Omega Alpha Honor Medical Society membership leads to bias6-8; the viewpoint proposed several ways to change the resident selection process to enhance diversity.5 A recent proposal to eliminate numerical scores on the US Medical Licensing Examination Step 1 and move to a pass/fail grading system aligns well with this viewpoint.9 Defining best practices to perform holistic reviews is an ongoing effort and challenge for many programs, one that will be discussed at the AAD’s 2019 Diversity Champion Workshop. Implementing best practices will require individual residency programs to develop review processes tailored to departmental resources and strengths. Achieving increased representation must be an active process starting with an explicit commitment to improving diversity.
Through these efforts, we are poised to improve our specialty; however, it is critical to recognize that simply increasing the number of underrepresented dermatologists is not enough to improve diversity in dermatology. What does meaningful change look like? In 10 years, we hope that, in addition to a more inclusive workforce, we will see expanded diversity efforts beyond race and ethnicity; improved cultural competence within dermatology departments and organizations that creates more inclusive places to work, learn, and practice medicine; intentional broader representation in dermatology leadership; high-quality, evidence-based, inclusive, and culturally competent education, patient care, and research; and equal and improved outcomes for all of our patients, particularly those who traditionally experience health care disparities. To this end, ensuring diversity in research and publications is paramount. Academic journals should be actively working to include articles in the literature that help us better understand health care differences, including research that examines the presentations of skin disease in a broad spectrum of study populations, as well as to spotlight and solicit content from diverse voices. Inclusion of a diverse range of participants in research based on human subjects should be a requirement for publication, which would ensure more generalizable data. Diversity in clinical trials is improving,10 but more effort should be devoted to further increasing diversity in medical research. In particular, we need to broaden the inclusivity of dermatology research efforts and outcomes data to include more patients with skin of color as well as other underrepresented groups, thus helping to improve our understanding of the differential effects of certain interventions.
We also must educate trainees and practicing dermatologists to better understand the diagnosis and management of skin diseases in all populations; to this end, it is essential to develop a culturally competent curriculum and continuing medical education on diseases of the skin and hair that affect patients with skin of color as well as cutaneous conditions that present in groups such as sexual and gender minorities.11,12 All dermatologists—not just the experts in academic skin of color and other specialty clinics—should have expertise in the dermatologic care of diverse patients.
We have made notable and important strides with regard to diversity in dermatology by beginning this conversation, identifying problems, coming up with solutions, and implementing them.13 This progress has been made relatively quickly and is commendable; however, we have more work to do before our specialty is inclusive of underrepresented-in-medicine physicians and provides excellent care to all patients.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
- Wintroub BE. Dermatology: insuring the future for the patients we serve. Presented at: 73rd Annual Meeting of the American Academy of Dermatology; March 20-24, 2015; San Francisco, California.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Diversity Mentorship Program: current mentors. American Academy of Dermatology website. https://www.aad.org/members/leadership-institute/mentoring/diversity-mentorship-program-current-mentors. Accessed July 17, 2019.
- Diversity Champion Workshop. American Academy of Dermatology website. https://www.aad.org/meetings/diversity-champion-workshop. Accessed July 17, 2019.
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260.
- McGaghie WC, Cohen ER, Wayne DB. Are United States Medical Licensing Exam Step 1 and 2 scores valid measures for postgraduate medical residency selection decisions? Acad Med. 2011;86:48-52.
- Edmond MB, Deschenes JL, Eckler M, et al. Racial bias in using USMLE step 1 scores to grant internal medicine residency interviews. Acad Med. 2001;76:1253-1256.
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha Honor Society. JAMA Intern Med. 2017;177:659-665.
- The conversation continues: exploring possible changes to USMLE score reporting. US Medical Licensing Examination website. https://www.usmle.org/usmlescoring/. Accessed July 17, 2019.
- Charrow A, Xia FD, Joyce C, et al. Diversity in dermatology clinical trials: a systematic review. JAMA Dermatol. 2017;153:193-198.
- Vashi NA, Patzelt N, Wirya S, et al. Dermatoses caused by cultural practices: therapeutic cultural practices. J Am Acad Dermatol. 2018;79:1-16.
- Yeung H, Luk KM, Chen SC, et al. Dermatologic care for lesbian, gay, bisexual, and transgender persons: epidemiology, screening, and disease prevention. J Am Acad Dermatol. 2019;80:591-602.
- Pritchett EN, Pandya AG, Ferguson NN, et al. Diversity in dermatology: roadmap for improvement. J Am Acad Dermatol. 2018;79:337-341.
Methylisothiazolinone and Isothiazolinone Allergy
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
Unless you have been living under a rock, you probably already know that the preservative methylisothiazolinone (MI) has caused an epidemic of allergic contact dermatitis (ACD) and was named the 2013 American Contact Dermatitis Society Allergen of the Year.1 Methylisothiazolinone is not new on the market, but its solo use as a preservative is relatively new. In this article, we review the emergence of MI as a common allergen, discuss North American MI patch test results, and describe common and uncommon sources of MI exposure. We also explore the related isothiazolinones, benzisothiazolinone (BIT) and octylisothiazolinone (OIT).
Background
Methylchloroisothiazolinone (MCI) and MI have been utilized as a preservative in a 3:1 ratio since the 1980s. In 2005, MI was first used alone as a preservative in personal care products in concentrations of up to 100 ppm, which represented a 25-fold increase in exposure to MI in personal care products and thus unleashed an epidemic of ACD.1 In the 2015 to 2016 cycle of the North American Contact Dermatitis Group (NACDG) patch testing results, MI was found to be positive in 13.4% of patch tested patients (N=5597) and also had the highest significance-prevalence index number, a calculation that represents the relevance of positive reactions in relationship to prevalence.2 In Europe, MI is banned in leave-on products and is allowed in rinse-off products in concentrations of up to 15 ppm. In the United States, the Cosmetic Ingredient Review panel concluded that MI is safe at a maximum concentration up to 100 ppm in rinse-off products and safe in leave-on products when formulated to be nonsensitizing, which may be determined based on a quantitative risk assessment.3
It is recommended that MI be patch tested at a concentration of 2000 ppm (0.2% aqueous).4 Testing at lower concentrations may result in missed positives. In addition, it should be noted that MCI/MI is present in the T.R.U.E. Test (SmartPractice), but MI alone is not.
Sources of MI Exposure
The first few case reports of MI contact allergy were associated with occupational exposures. In 2004, Isaksson et al5 reported 2 cases of MI allergy following exposure to wallpaper glue and a chemical burn from a biocide, respectively. Soon after, Thyssen et al6 reported 4 occupational cases of MI allergy at a paint manufacturing plant.
An early case series of MI contact allergy associated with personal care products was published in 2010 in which the authors described adults with ACD from wet wipes and a makeup remover that contained MI.7 A more recent report indicated that MI is now an infrequent ingredient in wet wipes but is still found in a wide variety of household and personal care products.8 A 2017 query of the American Contact Dermatitis Society’s Contact Allergy Management Program (CAMP) database revealed that 12.9% of all products contained MI. Furthermore, CAMP data revealed that MI was the most commonly found preservative in both hair care and household products.9 An additional CAMP database study revealed that 53% of shampoos and 45% of conditioners contained MI, and it also was commonly found in hair dyes, soaps and cleansers, hand cleaners and sanitizers, vaginal hygiene products, sunscreens, and moisturizers.10
Household products represent an important source of MI exposure. A chemical analysis of water-based paints identified the presence of isothiazolinones. Contact allergy from isothiazolinones in paint can present as either direct or airborne-pattern contact dermatitis.11 Sodium bisulfite has been used to inactivate MCI/MI in wall paint and could be utilized in severe cases of airborne contact dermatitis.12 Off-gassing may take up to 5.5 weeks before the paint cures and the isothiazolinone level decreases.13 A 2016 analysis of household products in the CAMP database revealed that MI commonly was found in dishwashing soap (64%), followed by household cleaners (47%), laundry softeners/additives (30%), surface disinfectants (27%), and laundry detergents (13%).10 Because certain chemical ingredients are not always listed on household product labels, patients with MI contact allergy may be at higher risk for unanticipated exposure to this allergen.
Dear reader, we know that you know all of this. We know that you have been watching the MI epidemic and have followed its every turn. But something that may be new to you are the unique MI exposures identified over the last several years.
In 2017, MI was identified in the glue used to affix 3 layers of the upper portion of a shoe.14 In addition, a recent chemical analysis of US consumer adhesives confirmed the presence of isothiazolinones in 50% (19/38) of products; 44.7% (17/38) specifically contained MI.15 Slime, the sticky play substance that children concoct out of household materials, has caused ACD, and not surprisingly, MI has been identified as a culprit allergen. In one case report, contact allergy was caused by MI present in a slime mixture made up of laundry detergent, dish soap, shampoo, and hand cream.16 In another case series, 3 children with MI contact allergy had played with slime that included dishwashing liquid, which contained MI, along with polyvinyl acetate glue and liquid soap components.17 Another case report documented slime made from MI-containing school glue as the source of ACD.18 Isothiazolinones also have been identified as causative allergens in “noise putty,” another homemade play item.19
Additionally, there has been a report of contact allergy to MI in a designer eyeglass frame.20 There also have been several documented cases of ACD to MCI/MI aerosolized from water used during ironing.21,22
There also have been several reports of photoaggravated ACD and possible photoallergic contact dermatitis from MI.23,24 In such cases, patients also may have transient photosensitivity even when MI exposure is discontinued; therefore, MI should be considered for inclusion in photopatch test panels when relevant.
Methylisothiazolinone contact allergy also should be considered for products that do not list MI on the label, which presents another potential exposure. In products that do not list MI as an ingredient on the label, its presence may be due to inclusion of the preservative in raw materials used in production. For example, a patient who reacted to a facial mask gel had a positive patch test reaction to MI, the facial mask gel, and sodium hyaluronate, the raw ingredient in the gel. Further analysis revealed that MI was unexpectedly present in the sodium hyaluronate.25 Similar scenarios have been reported in association with facial wet wipes,26 an exfoliating facial sponge,27 and a polyurethane sponge from a wound vacuum pump,28 among others.
Other Isothiazolinones
Other isothiazolinones also are known to cause ACD, albeit less commonly than MI. Benzisothiazolinone has been identified in glues, cleaning agents, paints, and industrial chemicals; unlike MI, the presence of BIT is infrequent in personal care products.15,29 This chemical is not commonly included in patch test screening series in the United States but is currently present in the NACDG screening series as BIT 0.1% in petrolatum.
Octylisothiazolinone (OIT) has been reported in leather furniture, belts, shoes, and watchbands, as well as industrial chemicals.30,31 Similar to BIT, OIT is not commonly tested in screening series in the United States; the NACDG tests this chemical as OIT 0.025% in petrolatum.
The cross-reaction patterns between the isothiazolinones remain uncertain. A study in mice supported cross-reactivity between MI, OIT, and BIT32; however, several clinical epidemiologic studies suggested that although there is evidence that there may be cross-reactivity between OIT and MI, concomitant positive BIT and MI reactions more likely represent cosensitization.33-35
Final Interpretation
Methylisothiazolinone continues to have high positive patch test rates in North American patch test populations and should be tested at a concentration of 2000 ppm (0.2% aqueous). Methylisothiazolinone may now be rare in wet wipes, but it is still present in numerous personal care products including hair care products, liquid soaps, and cleaning products. Novel exposures to MI include paint, slime, and glues. It also is important to remember that MI can cause photoaggravated or photoallergic contact dermatitis and might be a worthy addition to photopatch test trays. Finally, keep a look out for BIT and OIT, which may be present in industrial chemicals, glues, paints, cleaning products, and leather items
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
- Castanedo-Tardana MP, Zug KA. Methylisothiazolinone. Dermatitis. 2013;24:2-6. 2. DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- DeKoven JG, Warshaw EM, Zug KA, et al. North American Contact Dermatitis Group patch test results: 2015-2016. Dermatitis. 2018;29:297-309.
- Cosmetic Ingredient Review. Amended safety assessment of methylisothiazolinone as used in cosmetics. https://www.cir-safety.org/sites/default/files/mthiaz092014FR_final.pdf. Released October 8, 2014. Accessed July 9, 2019.
- Isaksson M, Ale I, Andersen KE, et al. Multicenter patch testing with methylisothiazolinone and methylchloroisothiazolinone/methylisothiazolinone within the International Contact Dermatitis Research Group. Dermatitis. 2017;28:210-214.
- Isaksson M, Gruvberger B, Bruze M. Occupational contact allergy and dermatitis from methylisothiazolinone after contact with wallcovering glue and after a chemical burn from a biocide. Dermatitis. 2004;15:201-205.
- Thyssen JP, Sederberg-Olsen N, Thomsen JF, et al. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis. 2006;54:322-324.
- García-Gavín J, Vansina S, Kerre S, et al. Methylisothiazolinone, an emerging allergen in cosmetics? Contact Dermatitis. 2010;63:96-101.
- Hamann CR, Sahni S, Zug KA. Methylisothiazolinone: still on leave-on products, but no longer on baby wipes. Dermatitis. 2019;30:173-174.
- Beene KM, Scheman A, Severson D, et al. Prevalence of preservatives across all product types in the Contact Allergen Management Program. Dermatitis. 2017;28:81-87.
- Scheman A, Severson D. American Contact Dermatitis Society Contact Allergy Management Program: an epidemiologic tool to quantify ingredient usage. Dermatitis. 2016;27:11-13.
- Goodier MC, Siegel PD, Zang LY, et al. Isothiazolinone in residential interior wall paint: a high-performance liquid chromatographic-mass spectrometry analysis. Dermatitis. 2018;29:332-338.
- Bohn S, Niederer M, Brehm K, et al. Airborne contact dermatitis from methylchloroisothiazolinone in wall paint. abolition of symptoms by chemical allergen inactivation. Contact Dermatitis. 2000;42:196-201.
- Amsler E, Aerts O, Raison-Peyron N, et al; Dermatology Allergy Group (DAG) of the French Society of Dermatology. Airborne allergic contact dermatitis caused by isothiazolinones in water-based paints: a retrospective study of 44 cases. Contact Dermatitis. 2017;77:163-170.
- Silva CA, El-Houri RB, Christensen LP, et al. Contact allergy caused by methylisothiazolinone in shoe glue. Contact Dermatitis. 2017;77:175-176.
- Goodier MC, Zang LY, Siegel PD, et al. Isothiazolinone content of US consumer adhesives: ultrahigh-performance liquid chromatographic mass spectrometry analysis. Dermatitis. 2019;30:129-134.
- Anderson LE, Treat JR, Brod BA, et al. “Slime” contact dermatitis: case report and review of relevant allergens. Pediatr Dermatol. 2019;36:335-337.
- Salman A, Demir G, Apti O. “Slime”: a trending cause of isothiazolinone contact allergy in children. Contact Dermatitis. 2019;80:409-411.
- Zhang AJ, Boyd AH, Asch S, et al. Allergic contact dermatitis to slime: the epidemic of isothiazolinone allergy encompasses school glue. Pediatr Dermatol. 2019;36:e37-e38.
- Ducharme O, Labadie M, Briand SM, et al. Allergic contact dermatitis in a child caused by isothiazolinones in a “noise putty.” Contact Dermatitis. 2018;79:393-394.
- El-Houri RB, Christensen LP, Persson C, et al. Methylisothiazolinone in a designer spectacle frame—a surprising finding. Contact Dermatitis. 2016;75:310-312.
- Atkar R, Todd P. Four cases of allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2016;75:316-317.
- Hunter KJ, Shelley JC, Haworth AE. Airborne allergic contact dermatitis to methylchloroisothiazolinone/methylisothiazolinone in ironing water. Contact Dermatitis. 2008;58:183-184.
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Trokoudes D, Banerjee P, Fityan A, et al. Photoaggravated contact dermatitis caused by methylisothiazolinone. Contact Dermatitis. 2017;76:303-304.
- Kerre S, Naessens T, Theunis M, et al. Facial dermatitis caused by undeclared methylisothiazolinone in a gel mask: is the preservation of raw materials in cosmetics a cause of concern? Contact Dermatitis. 2018;78:421-424.
- Isaksson M, Persson L. ‘Mislabelled’ make-up remover wet wipes as a cause of severe, recalcitrant facial eczema [published online March 27, 2015]. Contact Dermatitis. 2015;73:56-59.
- Madsen JT, Andersen KE, Nielsen DT, et al. Undisclosed presence of methylisothiazolinone in ‘100% natural’ Konjac® sponge. Contact Dermatitis. 2016;75:308-309.
- Schliemann S, Isaksson M, Persson C, et al. Allergic contact dermatitis caused by methylchloroisothiazolinone/methylisothiazolinone in a medical device. Contact Dermatitis. 2016;75:312-314.
- Kaur-Knudsen D, Menné T, Christina Carlsen B. Systemic allergic dermatitis following airborne exposure to 1,2-benzisothiazolin-3-one. Contact Dermatitis. 2012;67:310-312.
- Aerts O, Meert H, Romaen E, et al. Octylisothiazolinone, an additional cause of allergic contact dermatitis caused by leather: case series and potential implications for the study of cross-reactivity with methylisothiazolinone. Contact Dermatitis. 2016;75:276-284.
- Alipour Tehrany Y, Quenan S, Bugey A, et al. Allergic contact dermatitis caused by octylisothiazolinone in a leather sofa. Contact Dermatitis. 2018;79:188-189.
- Schwensen JF, Menné Bonefeld C, Zachariae C, et al. Cross-reactivity between methylisothiazolinone, octylisothiazolinone and benzisothiazolinone using a modified local lymph node assay. Br J Dermatol. 2017;176:176-183.
- Aalto-Korte K, Suuronen K. Patterns of concomitant allergic reactions in patients suggest cross-sensitization between octylisothiazolinone and methylisothiazolinone. Contact Dermatitis. 2017;77:385-389.
- Craig S, Urwin R, Latheef F, et al. Patch test clinic experience of potential cross-reactivity of isothiazolinones. Contact Dermatitis. 2017;76:299-300.
- Geier J, Lessmann H, Schnuch A, et al. Concomitant reactivity to methylisothiazolinone, benzisothiazolinone, and octylisothiazolinone. International Network of Departments of Dermatology data, 2009-2013. Contact Dermatitis. 2015;72:337-339.
Practice Points
- Methylisothiazolinone (MI) is a preservative found in water-based personal care products and is a common allergen in patch-tested populations.
- Methylisothiazolinone also has been identified in household products, industrial chemicals, paint, adhesives, and other unique sources.
- Benzisothiazolinone and octylisothiazolinone are structurally similar to MI, and a subset of MI-allergic patients may need to avoid them.
Clinical Pearl: Topical Timolol for Refractory Hypergranulation
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
Practice Gap
Hypergranulation is a frequent complication of dermatologic surgery, especially when surgical defects are left to heal by secondary intention (eg, after electrodesiccation and curettage). Although management of postoperative hypergranulation with routine wound care, superpotent topical corticosteroids, and/or topical silver nitrate often is effective, refractory cases pose a difficult challenge given the paucity of treatment options. Effective management of these cases is important because hypergranulation can delay wound healing, cause patient discomfort, and lead to poor wound cosmesis.
The Technique
If refractory hypergranulation fails to respond to treatment with routine wound care and topical silver nitrate, we prescribe twice-daily application of timolol maleate ophthalmic gel forming solution 0.5% for up to 14 days or until complete resolution of the hypergranulation is achieved. We counsel patients to continue routine wound care with daily dressing changes in conjunction with topical timolol application.
We initiated treatment with topical timolol in a patient who developed hypergranulation at 2 separate electrodesiccation and curettage sites that was refractory to 6 weeks of routine wound care with white petrolatum under nonadherent sterile gauze dressings and 2 subsequent topical silver nitrate applications (Figure 1). After 2 weeks of treatment with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 2). Another patient presented with hypergranulation that developed following a traumatic injury on the left upper arm and had been treated unsuccessfully for several months at a wound care clinic with daily nonadherent sterile gauze dressings and both topical and oral antibiotics (Figure 3A). After treatment for 9 days with topical timolol, resolution of the hypergranulation and re-epithelialization of the surgical sites was observed (Figure 3B).
Practice Implications
Beta-blockers are increasingly being used for management of chronic nonhealing wounds since the 1990s when oral administration of propranolol initially was reported to be an effective adjuvant therapy for managing severe burns.1 Since then, topical beta-blockers have been reported to be effective for management of ulcerated hemangiomas, venous stasis ulcers, chronic diabetic ulcers, and chronic nonhealing surgical wounds; however, there are no known reports of using topical beta-blockers for management of hypergranulation.2-5 We found timolol ophthalmic gel to be an excellent second-line therapy for management of postoperative hypergranulation if prior treatment with routine wound care and superpotent topical corticosteroids has failed. To date, we have found no reported adverse effects from the use of topical timolol for this indication that have required discontinuation of the medication. Use of this simple and safe intervention can be effective as a solution to a common postoperative condition.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.
- Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223-1229.
- Pope E, Chakkittakandiyil A. Topical timolol gel for infantile hemangiomas: a pilot study. Arch Dermatol. 2010;146:564-565.
- Braun L, Lamel S, Richmond N, et al. Topical timolol for recalcitrant wounds. JAMA Dermatol. 2013;149:1400-1402.
- Thomas B, Kurien J, Jose T, et al. Topical timolol promotes healing of chronic leg ulcer. J Vasc Surg. 2017;5:844-850.
- Tang J, Dosal J, Kirsner RS. Topical timolol for a refractory wound. Dermatol Surg. 2012;38:135-138.