User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
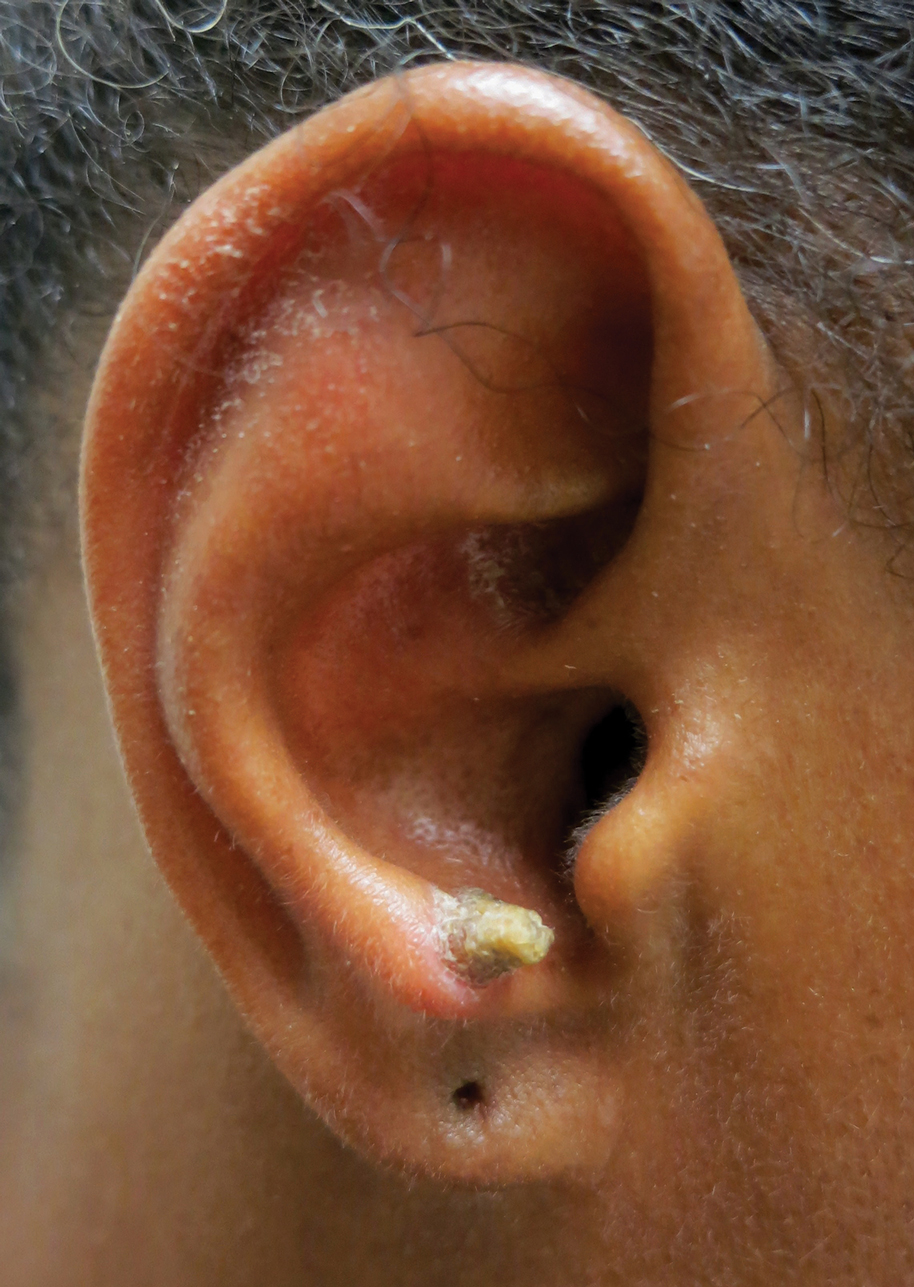
Solitary Papule on the Leg
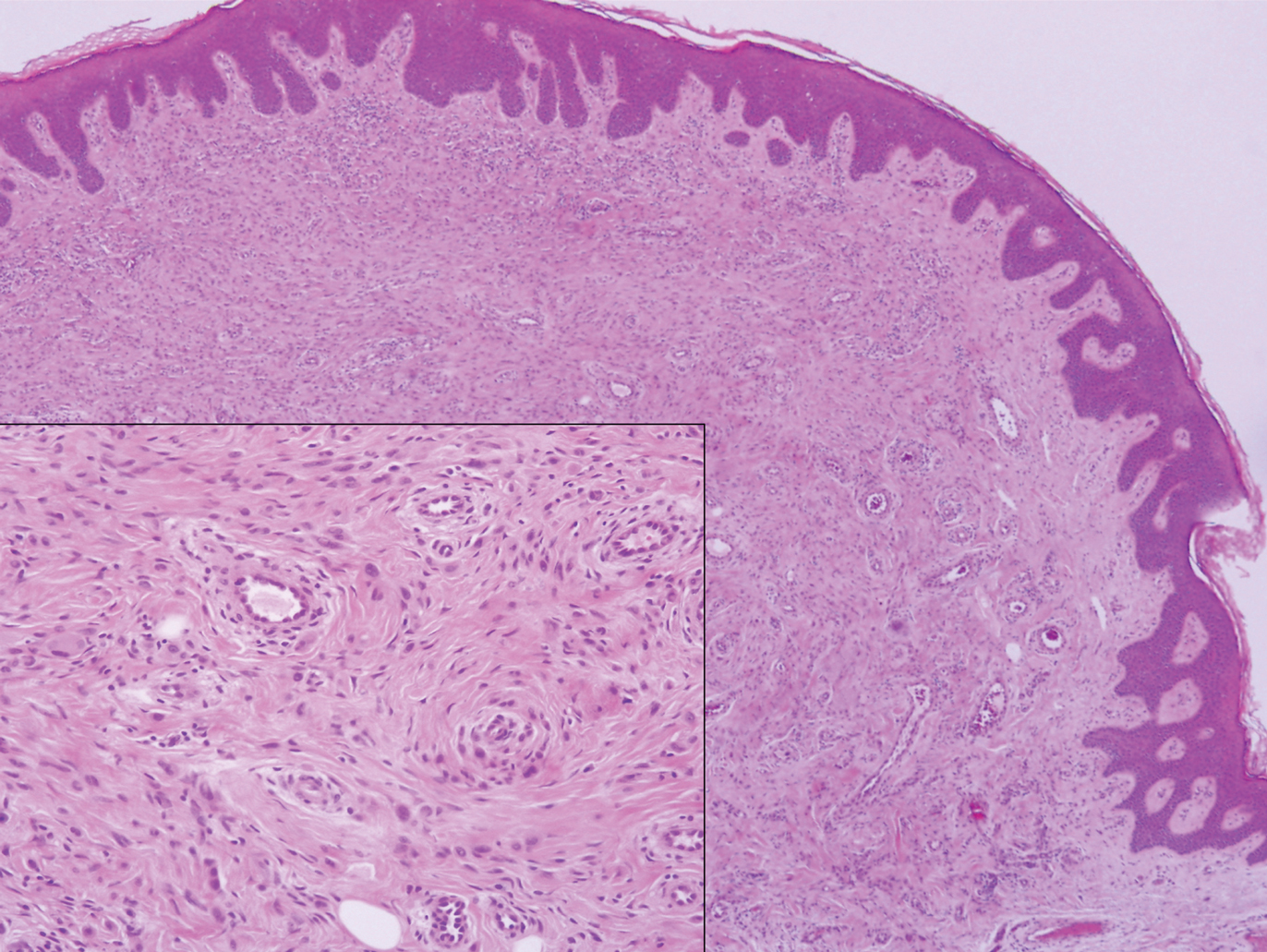
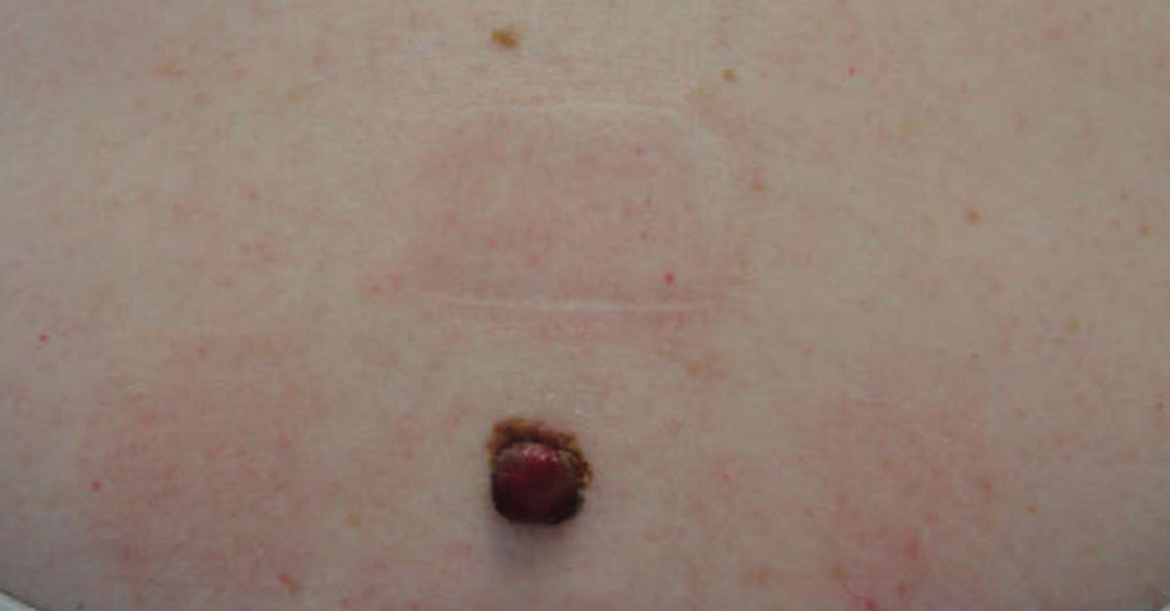
The Diagnosis: Epithelioid Histiocytoma
Epithelioid histiocytoma (EH), also known as epithelioid cell histiocytoma or epithelioid fibrous histiocytoma, is a rare benign fibrohistiocytic tumor first described in 1989.1 Epithelioid histiocytoma commonly presents in middle-aged adults with a slight predilection for males.2 The most frequently affected site is the lower extremity. The arms, trunk, head and neck, groin, and tongue also can be involved.3,4 It usually presents as a solitary asymptomatic papule or nodule, though cases with multiple lesions have been reported.5 Anaplastic lymphoma kinase rearrangement and overexpression have been confirmed and suggest that EH is distinct from conventional cutaneous fibrous histiocytoma.5
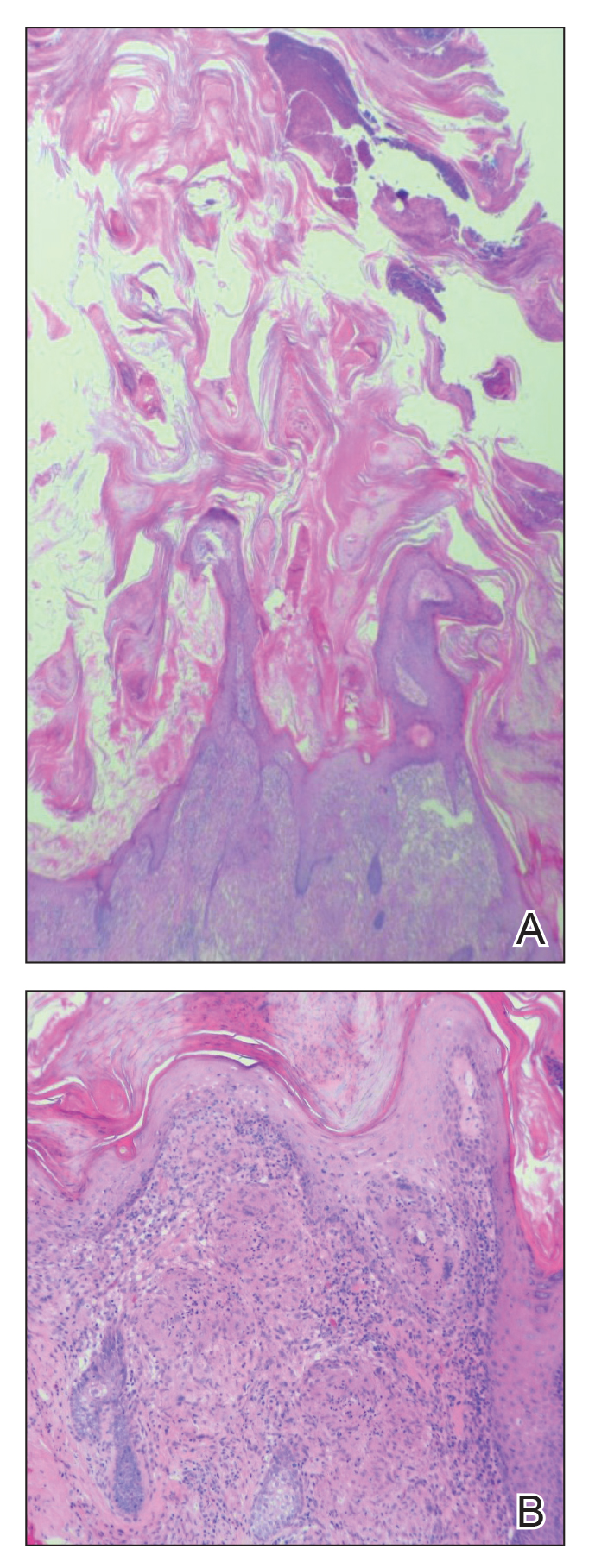
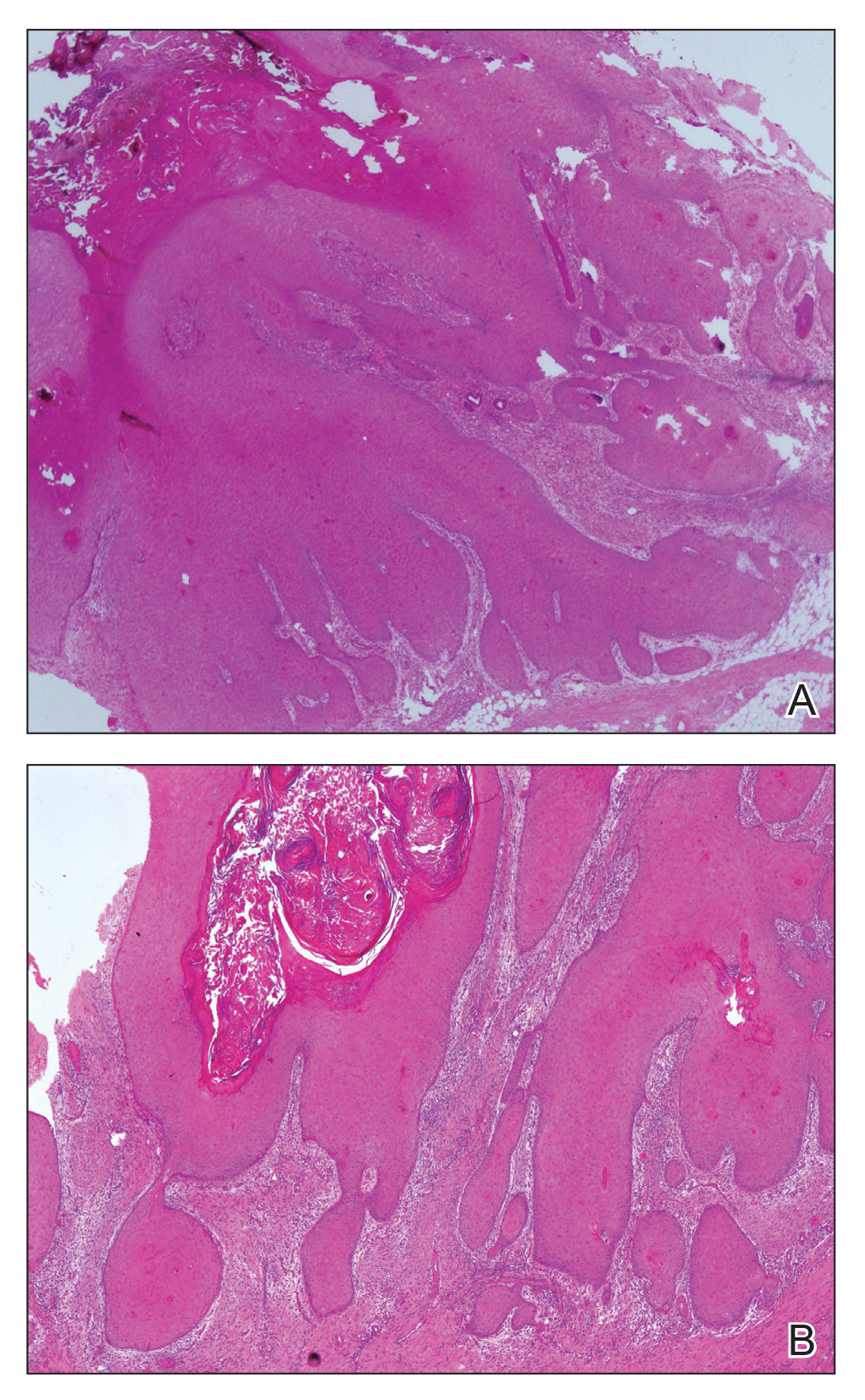
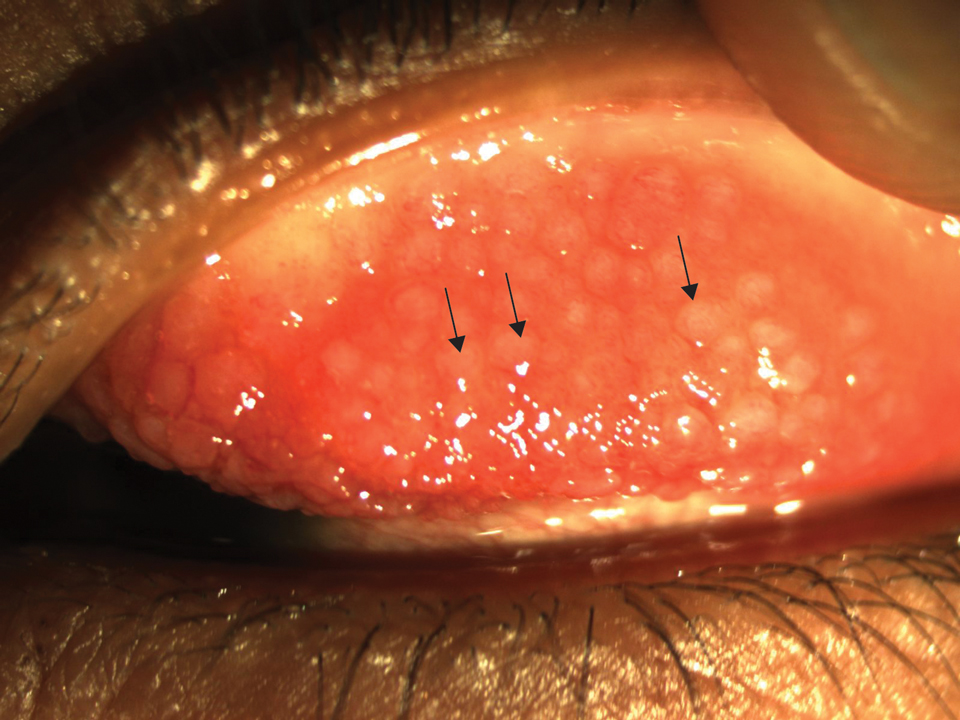
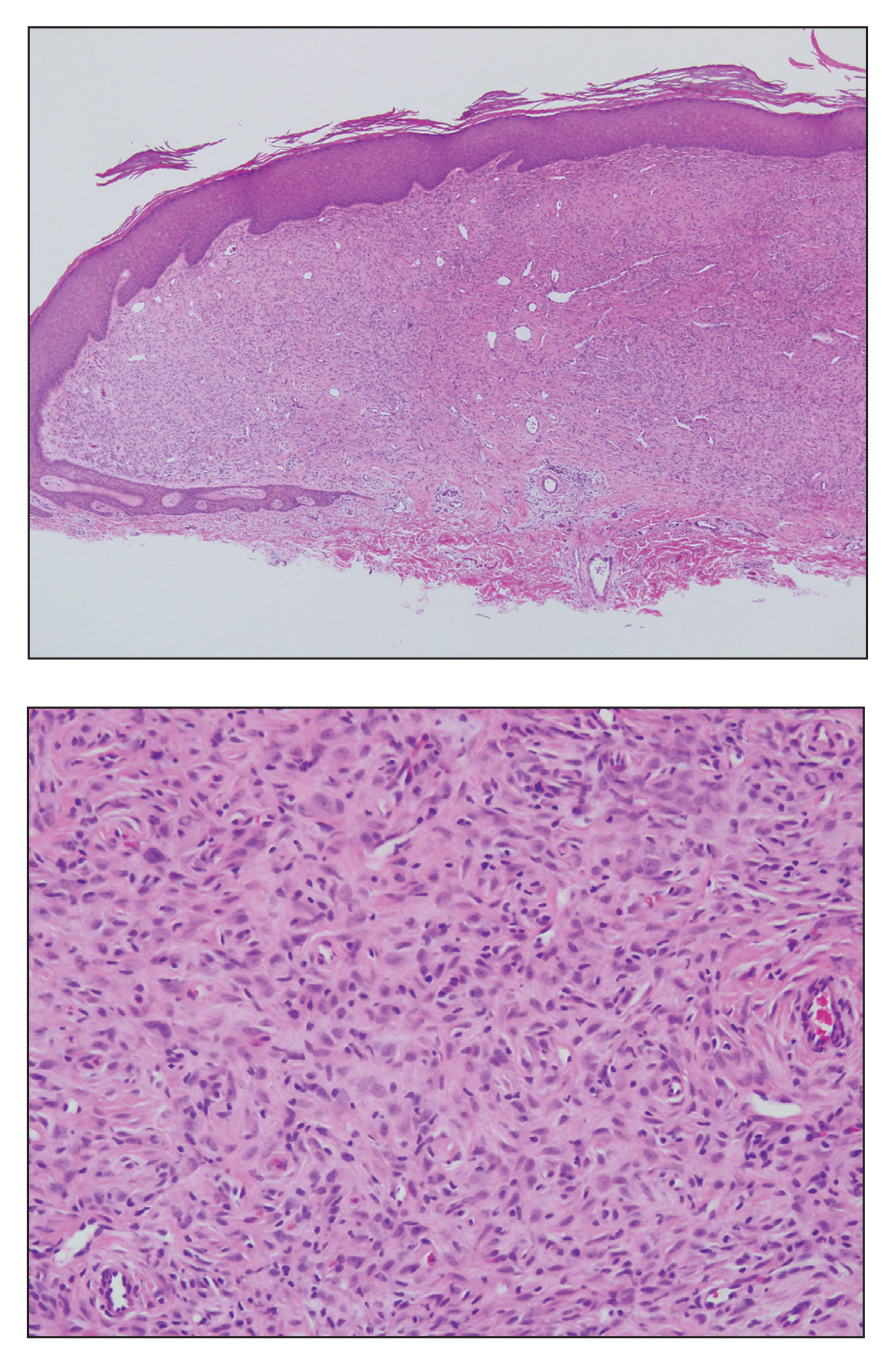
Histologically, EH appears as an exophytic, symmetric, and well-demarcated dermal nodule with a classic epidermal collarette. Prominent vascularity with perivascular accentuation of the epithelioid tumor cells is common. Older lesions may be hyalinized and sclerotic. Epithelioid cells commonly account for more than 50% of the tumor and are characterized by eosinophilic cytoplasm, vesicular nuclei, and small eosinophilic nucleoli. A small population of lymphocytes and mast cells are variably present (quiz image, bottom).1-3,7 A predominantly spindle cell variant has been reported.8 Other histopathologic variants include granular cell,9 cellular,10 and EH with perineuriomalike growth.11 Immunohistochemical staining shows anaplastic lymphoma kinase positivity in most cases, and more than half of cases stain positive for factor XIIIa and epithelial membrane antigen. Tumor cells consistently are negative for desmin and cytokeratins.6,10,12 Excision is curative.8
Polypoid Spitz nevus (PSN) is a benign nevus with a conspicuous polypoid or papillary exophytic architecture. The term was coined in 2000 by Fabrizi and Massi.13 Spitz nevus is a benign acquired melanocytic tumor that typically presents in children and adolescents and has a wide histologic spectrum.14 There is some debate on this entity, as some authors do not regard PSN as a distinct histologic variant; thus, it seems underreported in the literature.15 In a review of 349 cases of Spitz nevi, the authors found 7 cases of PSN.16 In another review of 74 cases of intradermal Spitz nevi, 14 cases of PSN were identified.14 This polypoid variant is easily mistaken for a polypoid melanoma because it can show cytologic atypia with large nuclei. Polypoid Spitz nevus usually lacks mitoses, notable pleomorphism, and sheetlike growth, unlike melanoma (Figure 1).13,14
Myopericytoma is an uncommon benign mesenchymal neoplasm that typically presents as a solitary, slowly enlarging and painless nodule with a predilection for the lower extremities, usually in adult males.17-20 Histologically, it consists of a well-circumscribed nodule with numerous thin-walled vessels and a proliferation of ovoid to spindled myopericytes exhibiting a concentric perivascular growth pattern (Figure 2). Myopericytoma usually is positive for smooth muscle actin and h-caldesmon but is negative or only focally positive for desmin. The prognosis is good with rare recurrence, despite incomplete excision.17,18
Solitary reticulohistiocytoma is a rare benign form of non-Langerhans cell histiocytosis.21,22 Unlike its multicentric counterpart, solitary reticulohistiocytoma rarely is associated with systemic disease. It presents as a small, dome-shaped, painless papule or nodule that can affect any part of the body.22,23 Solitary reticulohistiocytoma characteristically demonstrates cells with a ground glass-like appearance and 2-toned cytoplasm. A mixed inflammatory infiltrate including neutrophils, eosinophils, and lymphocytes commonly is present (Figure 3). The epithelioid histiocytes are positive for vimentin and histiocytic markers including CD68 and CD163.22
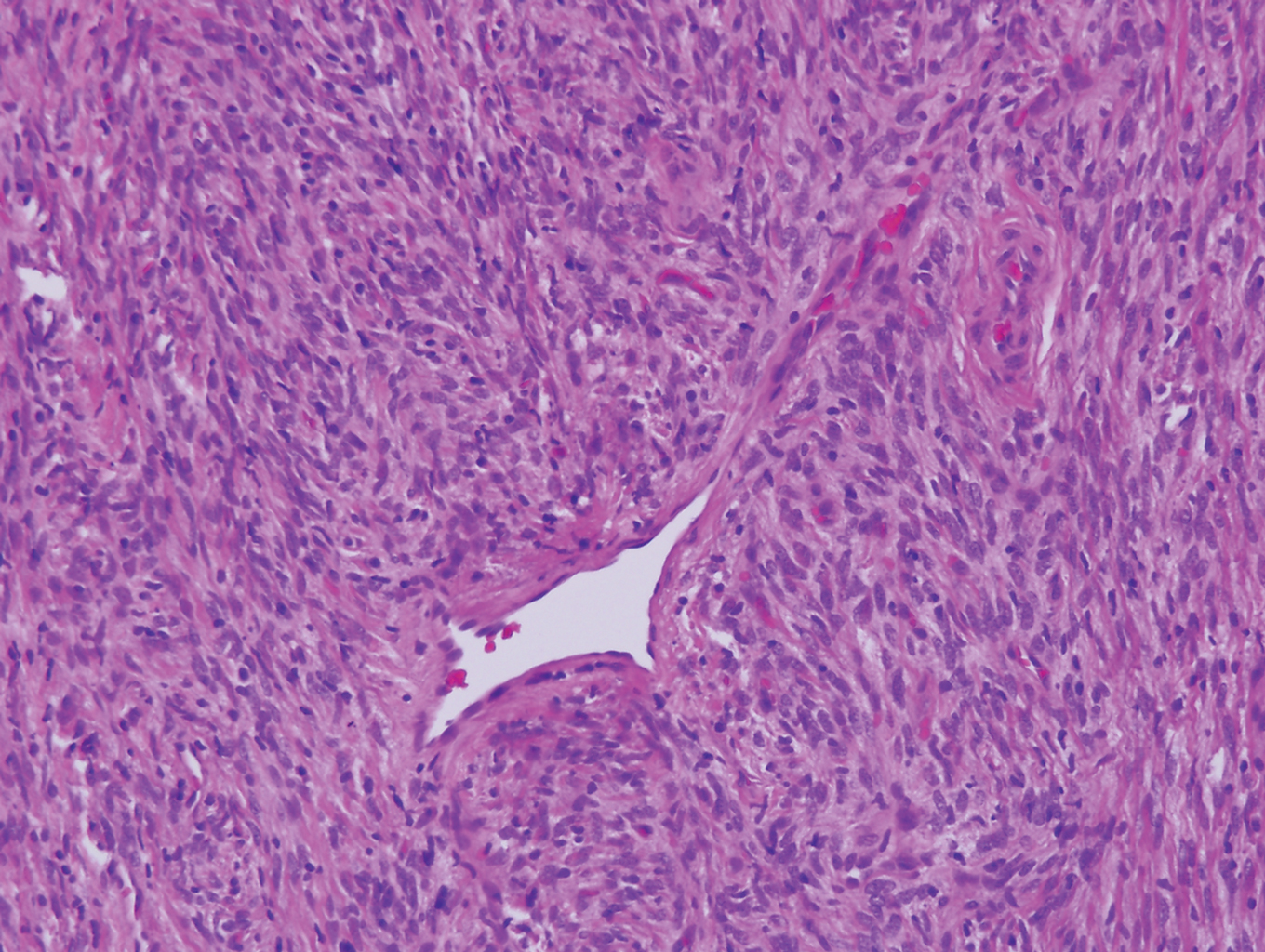
Solitary fibrous tumor (SFT) is an uncommon mesenchymal fibroblastic neoplasm that can arise at almost any anatomic site.24 Cutaneous SFTs are more common in women, most often involve the head, and appear to behave in an indolent manner.25 Solitary fibrous tumors are translocation-associated neoplasms with a NAB2-STAT6 gene fusion.26 The classic histology of SFT is a spindled fibroblastic proliferation arranged in a "patternless pattern" with interspersed stag horn-like, thin-walled blood vessels (Figure 4). Tumor cells usually are positive for CD34, CD99, and Bcl-2.27 In addition, STAT6 immunoreactivity is useful in diagnosis of SFT.25
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published online October 4, 2018]. Am J Dermatopathol. doi:10.1097/DAD.0000000000001272.
- Rawal YB, Kalmar JR, Shumway B, et al. Presentation of an epithelioid cell histiocytoma on the ventral tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:75-83.
- Cangelosi JJ, Prieto VG, Baker GF, et al. Unusual presentation of multiple epithelioid cell histiocytomas. Am J Dermatopathol. 2008;30:373-376.
- Doyle LA, Marino-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Silverman JS, Glusac EJ. Epithelioid cell histiocytoma--histogenetic and kinetics analysis of dermal microvascular unit dendritic cell subpopulations. J Cutan Pathol. 2003;30:415-422.
- Murigu T, Bhatt N, Miller K, et al. Spindle cell-predominant epithelioid fibrous histiocytoma. Histopathology. 2018;72:1233-1236.
- Rabkin MS, Vukmer T. Granular cell variant of epithelioid cell histiocytoma. Am J Dermatopathol. 2012;34:766-769.
- Glusac EJ, Barr RJ, Everett MA, et al. Epithelioid cell histiocytoma. a report of 10 cases including a new cellular variant. Am J Surg Pathol. 1994;18:583-590.
- Creytens D, Ferdinande L, Van Dorpe J. ALK Rearrangement and overexpression in an unusual cutaneous epithelioid tumor with a peculiar whorled "perineurioma-like" growth pattern: epithelioid fibrous histiocytoma. Appl Immunohistochem Mol Morphol. 2017;25:E46-E48.
- Doyle LA, Fletcher CD. EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol. 2011;38:697-703.
- Fabrizi G, Massi G. Polypoid Spitz naevus: the benign counterpart of polypoid malignant melanoma. Br J Dermatol. 2000;142:128-132.
- Plaza JA, De Stefano D, Suster S, et al. Intradermal Spitz nevi: a rare subtype of Spitz nevi analyzed in a clinicopathologic study of 74 cases. Am J Dermatopathol. 2014;36:283-294; quiz 295-287.
- Menezes FD, Mooi WJ. Spitz tumors of the skin. Surg Pathol Clin. 2017;10:281-298.
- Requena C, Requena L, Kutzner H, et al. Spitz nevus: a clinicopathological study of 349 cases. Am J Dermatopathol. 2009;31:107-116.
- Mentzel T, Dei Tos AP, Sapi Z, et al. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30:104-113.
- Aung PP, Goldberg LJ, Mahalingam M, et al. Cutaneous myopericytoma: a report of 3 cases and review of the literature. Dermatopathology (Basel). 2015;2:9-14.
- Morzycki A, Joukhadar N, Murphy A, et al. Digital myopericytoma: a case report and systematic literature review. J Hand Microsurg. 2017;9:32-36.
- LeBlanc RE, Taube J. Myofibroma, myopericytoma, myoepithelioma, and myofibroblastoma of skin and soft tissue. Surg Pathol Clin. 2011;4:745-759.
- Chisolm SS, Schulman JM, Fox LP. Adult xanthogranuloma, reticulohistiocytosis, and Rosai-Dorfman disease. Dermatol Clin. 2015;33:465-472; discussion 473.
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521-528.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatol Online J. 2014. pii:doj_21725.
- Soldano AC, Meehan SA. Cutaneous solitary fibrous tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008;30:54-58.
- Feasel P, Al-Ibraheemi A, Fritchie K, et al. Superficial solitary fibrous tumor: a series of 26 cases. Am J Surg Pathol. 2018;42:778-785.
- Thway K, Ng W, Noujaim J, et al. The current status of solitary fibrous tumor: diagnostic features, variants, and genetics. Int J Surg Pathol. 2016;24:281-292.
- Erdag G, Qureshi HS, Patterson JW, et al. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007;34:844-850.
The Diagnosis: Epithelioid Histiocytoma
Epithelioid histiocytoma (EH), also known as epithelioid cell histiocytoma or epithelioid fibrous histiocytoma, is a rare benign fibrohistiocytic tumor first described in 1989.1 Epithelioid histiocytoma commonly presents in middle-aged adults with a slight predilection for males.2 The most frequently affected site is the lower extremity. The arms, trunk, head and neck, groin, and tongue also can be involved.3,4 It usually presents as a solitary asymptomatic papule or nodule, though cases with multiple lesions have been reported.5 Anaplastic lymphoma kinase rearrangement and overexpression have been confirmed and suggest that EH is distinct from conventional cutaneous fibrous histiocytoma.5
Histologically, EH appears as an exophytic, symmetric, and well-demarcated dermal nodule with a classic epidermal collarette. Prominent vascularity with perivascular accentuation of the epithelioid tumor cells is common. Older lesions may be hyalinized and sclerotic. Epithelioid cells commonly account for more than 50% of the tumor and are characterized by eosinophilic cytoplasm, vesicular nuclei, and small eosinophilic nucleoli. A small population of lymphocytes and mast cells are variably present (quiz image, bottom).1-3,7 A predominantly spindle cell variant has been reported.8 Other histopathologic variants include granular cell,9 cellular,10 and EH with perineuriomalike growth.11 Immunohistochemical staining shows anaplastic lymphoma kinase positivity in most cases, and more than half of cases stain positive for factor XIIIa and epithelial membrane antigen. Tumor cells consistently are negative for desmin and cytokeratins.6,10,12 Excision is curative.8
Polypoid Spitz nevus (PSN) is a benign nevus with a conspicuous polypoid or papillary exophytic architecture. The term was coined in 2000 by Fabrizi and Massi.13 Spitz nevus is a benign acquired melanocytic tumor that typically presents in children and adolescents and has a wide histologic spectrum.14 There is some debate on this entity, as some authors do not regard PSN as a distinct histologic variant; thus, it seems underreported in the literature.15 In a review of 349 cases of Spitz nevi, the authors found 7 cases of PSN.16 In another review of 74 cases of intradermal Spitz nevi, 14 cases of PSN were identified.14 This polypoid variant is easily mistaken for a polypoid melanoma because it can show cytologic atypia with large nuclei. Polypoid Spitz nevus usually lacks mitoses, notable pleomorphism, and sheetlike growth, unlike melanoma (Figure 1).13,14
Myopericytoma is an uncommon benign mesenchymal neoplasm that typically presents as a solitary, slowly enlarging and painless nodule with a predilection for the lower extremities, usually in adult males.17-20 Histologically, it consists of a well-circumscribed nodule with numerous thin-walled vessels and a proliferation of ovoid to spindled myopericytes exhibiting a concentric perivascular growth pattern (Figure 2). Myopericytoma usually is positive for smooth muscle actin and h-caldesmon but is negative or only focally positive for desmin. The prognosis is good with rare recurrence, despite incomplete excision.17,18
Solitary reticulohistiocytoma is a rare benign form of non-Langerhans cell histiocytosis.21,22 Unlike its multicentric counterpart, solitary reticulohistiocytoma rarely is associated with systemic disease. It presents as a small, dome-shaped, painless papule or nodule that can affect any part of the body.22,23 Solitary reticulohistiocytoma characteristically demonstrates cells with a ground glass-like appearance and 2-toned cytoplasm. A mixed inflammatory infiltrate including neutrophils, eosinophils, and lymphocytes commonly is present (Figure 3). The epithelioid histiocytes are positive for vimentin and histiocytic markers including CD68 and CD163.22
Solitary fibrous tumor (SFT) is an uncommon mesenchymal fibroblastic neoplasm that can arise at almost any anatomic site.24 Cutaneous SFTs are more common in women, most often involve the head, and appear to behave in an indolent manner.25 Solitary fibrous tumors are translocation-associated neoplasms with a NAB2-STAT6 gene fusion.26 The classic histology of SFT is a spindled fibroblastic proliferation arranged in a "patternless pattern" with interspersed stag horn-like, thin-walled blood vessels (Figure 4). Tumor cells usually are positive for CD34, CD99, and Bcl-2.27 In addition, STAT6 immunoreactivity is useful in diagnosis of SFT.25
The Diagnosis: Epithelioid Histiocytoma
Epithelioid histiocytoma (EH), also known as epithelioid cell histiocytoma or epithelioid fibrous histiocytoma, is a rare benign fibrohistiocytic tumor first described in 1989.1 Epithelioid histiocytoma commonly presents in middle-aged adults with a slight predilection for males.2 The most frequently affected site is the lower extremity. The arms, trunk, head and neck, groin, and tongue also can be involved.3,4 It usually presents as a solitary asymptomatic papule or nodule, though cases with multiple lesions have been reported.5 Anaplastic lymphoma kinase rearrangement and overexpression have been confirmed and suggest that EH is distinct from conventional cutaneous fibrous histiocytoma.5
Histologically, EH appears as an exophytic, symmetric, and well-demarcated dermal nodule with a classic epidermal collarette. Prominent vascularity with perivascular accentuation of the epithelioid tumor cells is common. Older lesions may be hyalinized and sclerotic. Epithelioid cells commonly account for more than 50% of the tumor and are characterized by eosinophilic cytoplasm, vesicular nuclei, and small eosinophilic nucleoli. A small population of lymphocytes and mast cells are variably present (quiz image, bottom).1-3,7 A predominantly spindle cell variant has been reported.8 Other histopathologic variants include granular cell,9 cellular,10 and EH with perineuriomalike growth.11 Immunohistochemical staining shows anaplastic lymphoma kinase positivity in most cases, and more than half of cases stain positive for factor XIIIa and epithelial membrane antigen. Tumor cells consistently are negative for desmin and cytokeratins.6,10,12 Excision is curative.8
Polypoid Spitz nevus (PSN) is a benign nevus with a conspicuous polypoid or papillary exophytic architecture. The term was coined in 2000 by Fabrizi and Massi.13 Spitz nevus is a benign acquired melanocytic tumor that typically presents in children and adolescents and has a wide histologic spectrum.14 There is some debate on this entity, as some authors do not regard PSN as a distinct histologic variant; thus, it seems underreported in the literature.15 In a review of 349 cases of Spitz nevi, the authors found 7 cases of PSN.16 In another review of 74 cases of intradermal Spitz nevi, 14 cases of PSN were identified.14 This polypoid variant is easily mistaken for a polypoid melanoma because it can show cytologic atypia with large nuclei. Polypoid Spitz nevus usually lacks mitoses, notable pleomorphism, and sheetlike growth, unlike melanoma (Figure 1).13,14
Myopericytoma is an uncommon benign mesenchymal neoplasm that typically presents as a solitary, slowly enlarging and painless nodule with a predilection for the lower extremities, usually in adult males.17-20 Histologically, it consists of a well-circumscribed nodule with numerous thin-walled vessels and a proliferation of ovoid to spindled myopericytes exhibiting a concentric perivascular growth pattern (Figure 2). Myopericytoma usually is positive for smooth muscle actin and h-caldesmon but is negative or only focally positive for desmin. The prognosis is good with rare recurrence, despite incomplete excision.17,18
Solitary reticulohistiocytoma is a rare benign form of non-Langerhans cell histiocytosis.21,22 Unlike its multicentric counterpart, solitary reticulohistiocytoma rarely is associated with systemic disease. It presents as a small, dome-shaped, painless papule or nodule that can affect any part of the body.22,23 Solitary reticulohistiocytoma characteristically demonstrates cells with a ground glass-like appearance and 2-toned cytoplasm. A mixed inflammatory infiltrate including neutrophils, eosinophils, and lymphocytes commonly is present (Figure 3). The epithelioid histiocytes are positive for vimentin and histiocytic markers including CD68 and CD163.22
Solitary fibrous tumor (SFT) is an uncommon mesenchymal fibroblastic neoplasm that can arise at almost any anatomic site.24 Cutaneous SFTs are more common in women, most often involve the head, and appear to behave in an indolent manner.25 Solitary fibrous tumors are translocation-associated neoplasms with a NAB2-STAT6 gene fusion.26 The classic histology of SFT is a spindled fibroblastic proliferation arranged in a "patternless pattern" with interspersed stag horn-like, thin-walled blood vessels (Figure 4). Tumor cells usually are positive for CD34, CD99, and Bcl-2.27 In addition, STAT6 immunoreactivity is useful in diagnosis of SFT.25
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published online October 4, 2018]. Am J Dermatopathol. doi:10.1097/DAD.0000000000001272.
- Rawal YB, Kalmar JR, Shumway B, et al. Presentation of an epithelioid cell histiocytoma on the ventral tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:75-83.
- Cangelosi JJ, Prieto VG, Baker GF, et al. Unusual presentation of multiple epithelioid cell histiocytomas. Am J Dermatopathol. 2008;30:373-376.
- Doyle LA, Marino-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Silverman JS, Glusac EJ. Epithelioid cell histiocytoma--histogenetic and kinetics analysis of dermal microvascular unit dendritic cell subpopulations. J Cutan Pathol. 2003;30:415-422.
- Murigu T, Bhatt N, Miller K, et al. Spindle cell-predominant epithelioid fibrous histiocytoma. Histopathology. 2018;72:1233-1236.
- Rabkin MS, Vukmer T. Granular cell variant of epithelioid cell histiocytoma. Am J Dermatopathol. 2012;34:766-769.
- Glusac EJ, Barr RJ, Everett MA, et al. Epithelioid cell histiocytoma. a report of 10 cases including a new cellular variant. Am J Surg Pathol. 1994;18:583-590.
- Creytens D, Ferdinande L, Van Dorpe J. ALK Rearrangement and overexpression in an unusual cutaneous epithelioid tumor with a peculiar whorled "perineurioma-like" growth pattern: epithelioid fibrous histiocytoma. Appl Immunohistochem Mol Morphol. 2017;25:E46-E48.
- Doyle LA, Fletcher CD. EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol. 2011;38:697-703.
- Fabrizi G, Massi G. Polypoid Spitz naevus: the benign counterpart of polypoid malignant melanoma. Br J Dermatol. 2000;142:128-132.
- Plaza JA, De Stefano D, Suster S, et al. Intradermal Spitz nevi: a rare subtype of Spitz nevi analyzed in a clinicopathologic study of 74 cases. Am J Dermatopathol. 2014;36:283-294; quiz 295-287.
- Menezes FD, Mooi WJ. Spitz tumors of the skin. Surg Pathol Clin. 2017;10:281-298.
- Requena C, Requena L, Kutzner H, et al. Spitz nevus: a clinicopathological study of 349 cases. Am J Dermatopathol. 2009;31:107-116.
- Mentzel T, Dei Tos AP, Sapi Z, et al. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30:104-113.
- Aung PP, Goldberg LJ, Mahalingam M, et al. Cutaneous myopericytoma: a report of 3 cases and review of the literature. Dermatopathology (Basel). 2015;2:9-14.
- Morzycki A, Joukhadar N, Murphy A, et al. Digital myopericytoma: a case report and systematic literature review. J Hand Microsurg. 2017;9:32-36.
- LeBlanc RE, Taube J. Myofibroma, myopericytoma, myoepithelioma, and myofibroblastoma of skin and soft tissue. Surg Pathol Clin. 2011;4:745-759.
- Chisolm SS, Schulman JM, Fox LP. Adult xanthogranuloma, reticulohistiocytosis, and Rosai-Dorfman disease. Dermatol Clin. 2015;33:465-472; discussion 473.
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521-528.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatol Online J. 2014. pii:doj_21725.
- Soldano AC, Meehan SA. Cutaneous solitary fibrous tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008;30:54-58.
- Feasel P, Al-Ibraheemi A, Fritchie K, et al. Superficial solitary fibrous tumor: a series of 26 cases. Am J Surg Pathol. 2018;42:778-785.
- Thway K, Ng W, Noujaim J, et al. The current status of solitary fibrous tumor: diagnostic features, variants, and genetics. Int J Surg Pathol. 2016;24:281-292.
- Erdag G, Qureshi HS, Patterson JW, et al. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007;34:844-850.
- Jones EW, Cerio R, Smith NP. Epithelioid cell histiocytoma: a new entity. Br J Dermatol. 1989;120:185-195.
- Singh Gomez C, Calonje E, Fletcher CD. Epithelioid benign fibrous histiocytoma of skin: clinico-pathological analysis of 20 cases of a poorly known variant. Histopathology. 1994;24:123-129.
- Felty CC, Linos K. Epithelioid fibrous histiocytoma: a concise review [published online October 4, 2018]. Am J Dermatopathol. doi:10.1097/DAD.0000000000001272.
- Rawal YB, Kalmar JR, Shumway B, et al. Presentation of an epithelioid cell histiocytoma on the ventral tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:75-83.
- Cangelosi JJ, Prieto VG, Baker GF, et al. Unusual presentation of multiple epithelioid cell histiocytomas. Am J Dermatopathol. 2008;30:373-376.
- Doyle LA, Marino-Enriquez A, Fletcher CD, et al. ALK rearrangement and overexpression in epithelioid fibrous histiocytoma. Mod Pathol. 2015;28:904-912.
- Silverman JS, Glusac EJ. Epithelioid cell histiocytoma--histogenetic and kinetics analysis of dermal microvascular unit dendritic cell subpopulations. J Cutan Pathol. 2003;30:415-422.
- Murigu T, Bhatt N, Miller K, et al. Spindle cell-predominant epithelioid fibrous histiocytoma. Histopathology. 2018;72:1233-1236.
- Rabkin MS, Vukmer T. Granular cell variant of epithelioid cell histiocytoma. Am J Dermatopathol. 2012;34:766-769.
- Glusac EJ, Barr RJ, Everett MA, et al. Epithelioid cell histiocytoma. a report of 10 cases including a new cellular variant. Am J Surg Pathol. 1994;18:583-590.
- Creytens D, Ferdinande L, Van Dorpe J. ALK Rearrangement and overexpression in an unusual cutaneous epithelioid tumor with a peculiar whorled "perineurioma-like" growth pattern: epithelioid fibrous histiocytoma. Appl Immunohistochem Mol Morphol. 2017;25:E46-E48.
- Doyle LA, Fletcher CD. EMA positivity in epithelioid fibrous histiocytoma: a potential diagnostic pitfall. J Cutan Pathol. 2011;38:697-703.
- Fabrizi G, Massi G. Polypoid Spitz naevus: the benign counterpart of polypoid malignant melanoma. Br J Dermatol. 2000;142:128-132.
- Plaza JA, De Stefano D, Suster S, et al. Intradermal Spitz nevi: a rare subtype of Spitz nevi analyzed in a clinicopathologic study of 74 cases. Am J Dermatopathol. 2014;36:283-294; quiz 295-287.
- Menezes FD, Mooi WJ. Spitz tumors of the skin. Surg Pathol Clin. 2017;10:281-298.
- Requena C, Requena L, Kutzner H, et al. Spitz nevus: a clinicopathological study of 349 cases. Am J Dermatopathol. 2009;31:107-116.
- Mentzel T, Dei Tos AP, Sapi Z, et al. Myopericytoma of skin and soft tissues: clinicopathologic and immunohistochemical study of 54 cases. Am J Surg Pathol. 2006;30:104-113.
- Aung PP, Goldberg LJ, Mahalingam M, et al. Cutaneous myopericytoma: a report of 3 cases and review of the literature. Dermatopathology (Basel). 2015;2:9-14.
- Morzycki A, Joukhadar N, Murphy A, et al. Digital myopericytoma: a case report and systematic literature review. J Hand Microsurg. 2017;9:32-36.
- LeBlanc RE, Taube J. Myofibroma, myopericytoma, myoepithelioma, and myofibroblastoma of skin and soft tissue. Surg Pathol Clin. 2011;4:745-759.
- Chisolm SS, Schulman JM, Fox LP. Adult xanthogranuloma, reticulohistiocytosis, and Rosai-Dorfman disease. Dermatol Clin. 2015;33:465-472; discussion 473.
- Miettinen M, Fetsch JF. Reticulohistiocytoma (solitary epithelioid histiocytoma): a clinicopathologic and immunohistochemical study of 44 cases. Am J Surg Pathol. 2006;30:521-528.
- Cohen PR, Lee RA. Adult-onset reticulohistiocytoma presenting as a solitary asymptomatic red knee nodule: report and review of clinical presentations and immunohistochemistry staining features of reticulohistiocytosis. Dermatol Online J. 2014. pii:doj_21725.
- Soldano AC, Meehan SA. Cutaneous solitary fibrous tumor: a report of 2 cases and review of the literature. Am J Dermatopathol. 2008;30:54-58.
- Feasel P, Al-Ibraheemi A, Fritchie K, et al. Superficial solitary fibrous tumor: a series of 26 cases. Am J Surg Pathol. 2018;42:778-785.
- Thway K, Ng W, Noujaim J, et al. The current status of solitary fibrous tumor: diagnostic features, variants, and genetics. Int J Surg Pathol. 2016;24:281-292.
- Erdag G, Qureshi HS, Patterson JW, et al. Solitary fibrous tumors of the skin: a clinicopathologic study of 10 cases and review of the literature. J Cutan Pathol. 2007;34:844-850.
A 28-year-old man presented with a growing asymptomatic papule on the right leg.
Severe Pretibial Myxedema Refractory to Systemic Immunosuppressants
To the Editor:
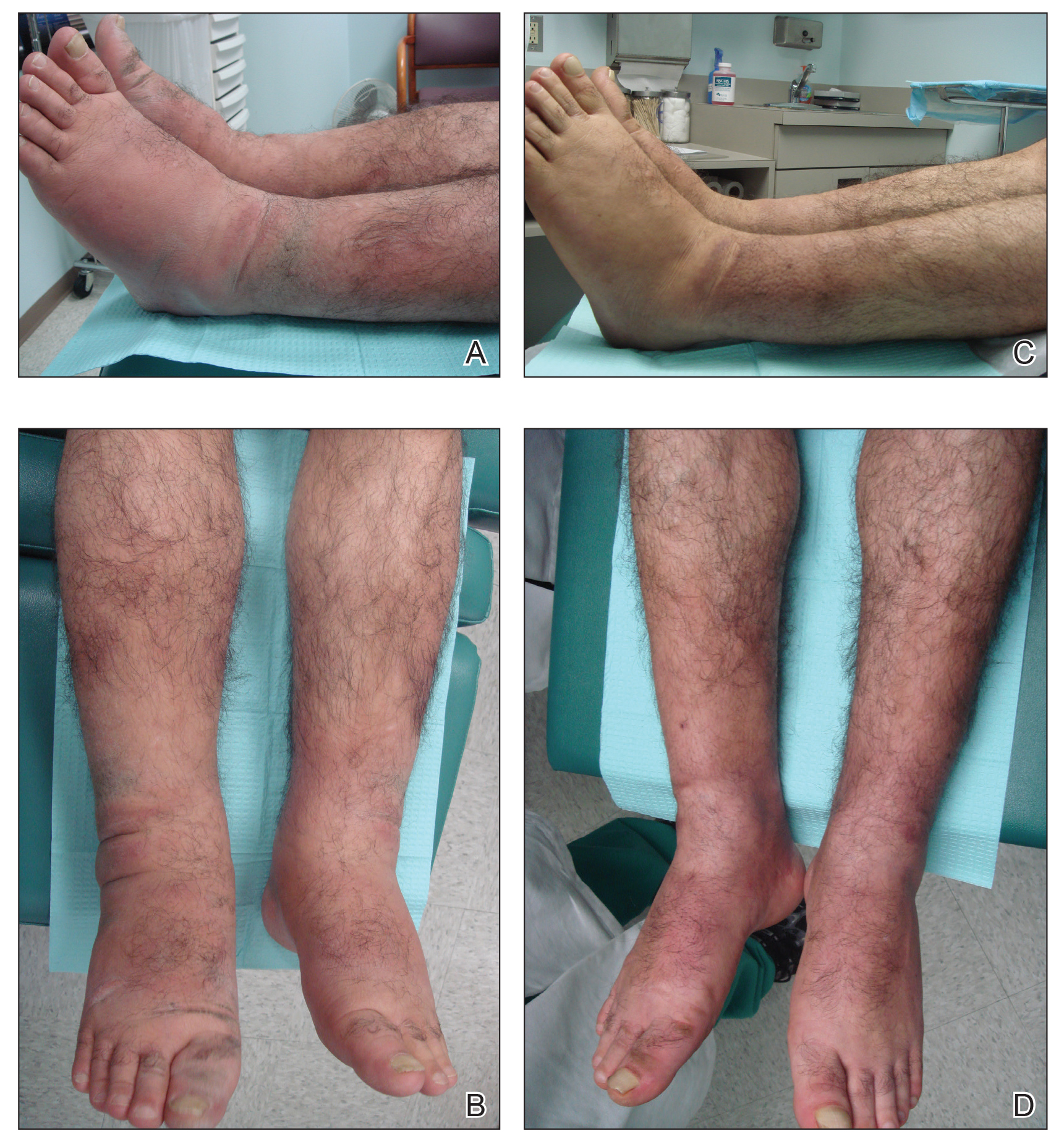
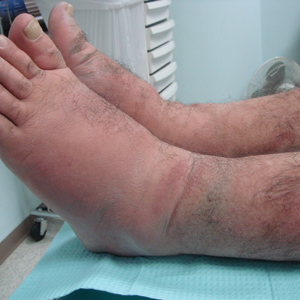
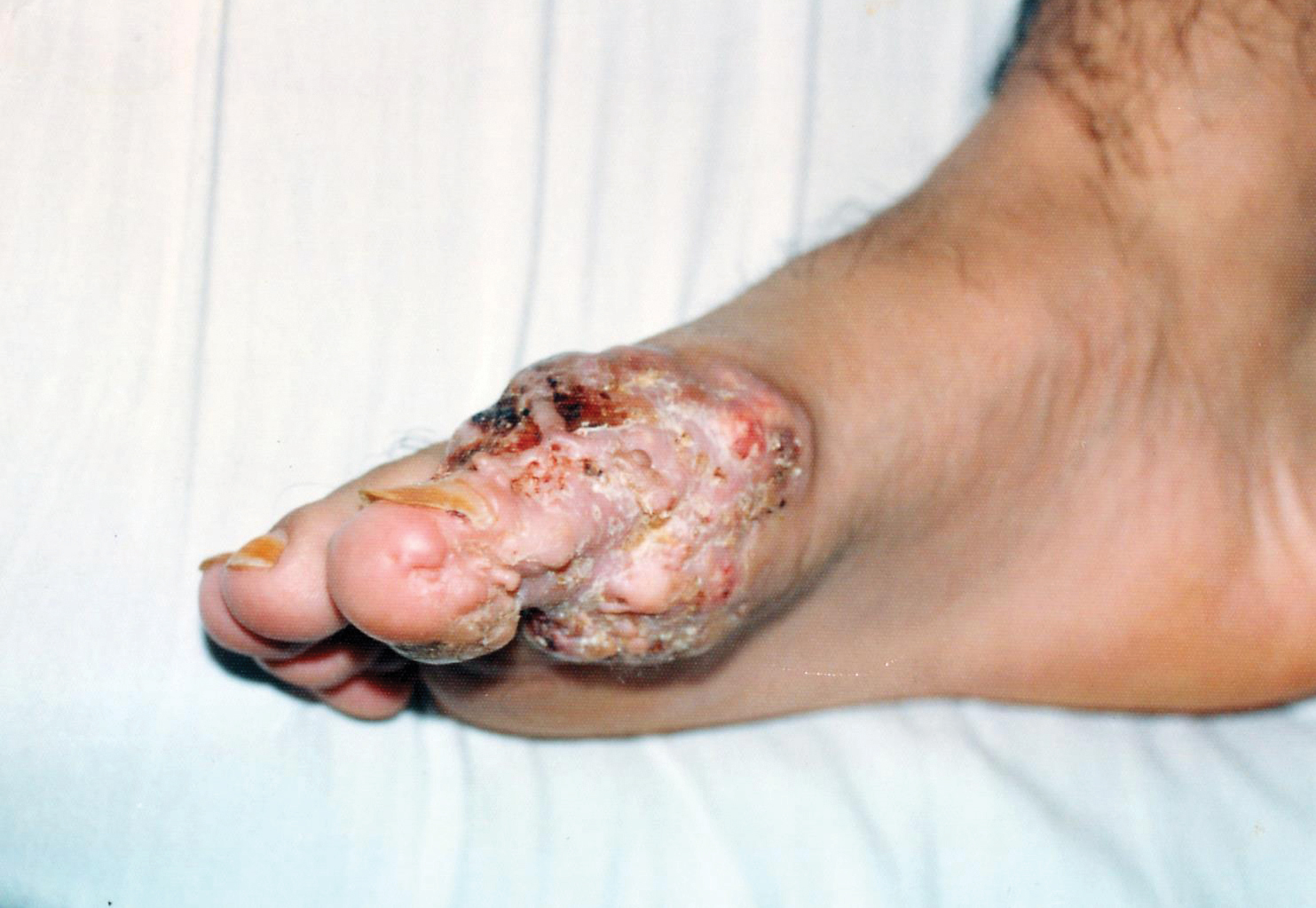
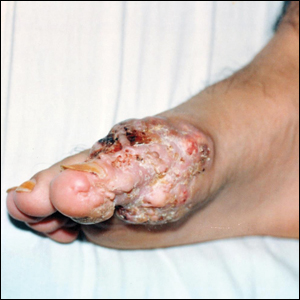
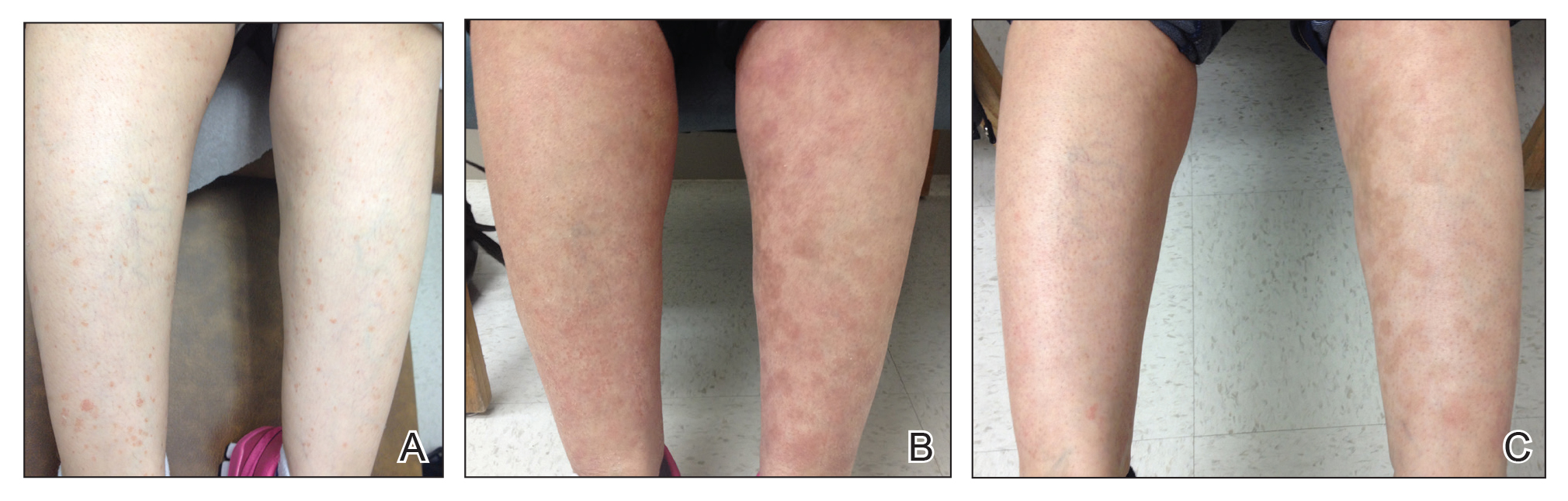
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
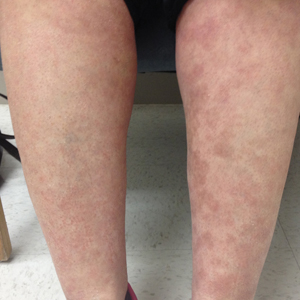
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
To the Editor:
A 55-year-old man with a history of Graves disease treated with radioactive iodine and Graves ophthalmopathy was referred to our dermatology clinic by his endocrinologist with a 2-year history of severe pretibial myxedema (PM) that had failed treatment with systemic immunosuppressants after diagnosis by an outside dermatologist in the United Kingdom approximately 2 years prior. In addition to burning pain and difficulty walking associated with progressive “enlarging” of the lower legs and feet (Figure, A and B), the patient reported that he consistently had to find larger shoes (size 13 at the current presentation). His medications included gabapentin for foot pain and levothyroxine for hypothyroidism.
Physical examination revealed diffuse, waxy, indurated, flesh-colored and erythematous plaques and nodules with a peau d’orange appearance on the dorsal feet, ankles, and lower legs. Laboratory evaluation revealed a thyroid stimulating immunoglobulin level of 617% (reference range, <140%) and mild anemia. His thyroid stimulating hormone and free T4 levels, a comprehensive metabolic panel, and lipid panel were all within reference range.
Treatment with oral, intravenous, and intralesional steroids; cyclosporine; and azathioprine were tried prior to presentation to our clinic with no improvement. The patient was started on pentoxifylline (400 mg 3 times daily), intralesional triamcinolone acetonide (5 mg/mL every 3–4 weeks), clobetasol propionate ointment 0.05% under occlusion twice daily, short-stretch bandages, and compression stockings (20–30 mm Hg). The baseline circumference of the extremities also were measured (right ankle, 12 in; left ankle, 11.5 in; right and left mid-plantar feet, 12 in).
At 3-week follow-up, the lesions had flattened with softening of the skin. The patient reported his legs were smaller and he had bought a new pair of shoes at size 8.5 (Figure, C). He noted less pain and difficulty with walking. The circumference of the extremities was measured again (right ankle, 10.2 in; left ankle, 10 in; right and left mid-plantar feet, 10.5 in). The patient continued treatment and was followed for 3 months. At each visit, clinical improvement was noted as well as report of decreased pain while walking (Figure, D).
Pretibial myxedema is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy. Pretibial myxedema occurs in 0.5% to 4.3% of patients with Graves disease and variably manifests as diffuse nonpitting edema or localized, waxy, indurated plaques or nodules.1,2
The proposed pathogenesis of PM is that autoantibodies directed against the thyroid receptors cross-react with the fibroblasts of the skin,2,3 which stimulates the fibroblasts to produce high amounts of glycosaminoglycans, especially hyaluronic acid, in the dermis and subcutis of the pretibial area. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position (ie, leg position is lower than the level of the heart) may be involved.4
The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required. Systemic immunosuppressants such as cyclosporine, azathioprine, and corticosteroids have proven useful in some but not all cases.5,6
Our patient did not respond to treatment with systemic and intralesional corticosteroids, cyclosporine, or azathioprine before he presented to our clinic; however, the lesions were dramatically improved after 3 weeks of treatment with pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression stockings.
Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves ophthalmology and PM.7 It has been shown to reduce thickness of skin lesions when used in combination with topical or intralesional steroids.3,8 Corticosteroids are thought to block fibroblast-mediated glycosaminoglycan production.3,9 The deposition of mucin, which is comprised of glycosaminoglycans, expands the dermal tissue and causes fluid to accumulate; it also causes compression of dermal lymphatics, worsening the dermal edema. Because fluid accumulates, the use of short-stretch bandages and compression stockings may provide additional benefit, as was seen in our patient, whose shoe size decreased from a 13 to an 8.5 within 3 weeks of treatment.
In conclusion, the combination of pentoxifylline, intralesional and topical corticosteroids under occlusion, short-stretch bandages, and compression garments can cause substantial improvement in severe PM refractory to systemic immunosuppressants.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
- Susser WS, Heermans AG, Chapman MS, et al. Elephantiasic pretibial myxedema: a novel treatment for an uncommon disorder. J Am Acad Dermatol. 2002;46:723-726.
- Kriss J. Pathogenesis and treatment of pretibial myxedema. Endocrinol Metab Clin North Am. 1987;16:409-415.
- Pineda AM, Tianco EA, Tan JB, et al. Oral pentoxifylline and topical clobetasol propionate ointment in the treatment of pretibial myxoedema, with concomitant improvement of Graves’ ophthalmopathy. J Eur Acad Dermatol Venereol. 2007; 21:1441-1443.
- Fatourechi V. Pretibial myxedema. Am J Clin Dermatol. 2005;6:295-309.
- Benoit FL, Greenspan FS. Corticoid therapy for pretibial myxedema: observations on the long-acting thyroid stimulator. Ann Intern Med. 1967;66:711-720.
- Hanke CW, Bergfeld WF, Guirguis MN, et al. Pretibial myxedema (elephantiasis form): treatment with cytotoxic therapy. Cleve Clin Q. 1983;50:183-188.
- Chang CC, Chang TC, Kao SC, et al. Pentoxifylline inhibits the proliferation and glycosaminoglycan synthesis of cultured fibroblasts derived from patients with Graves’ ophthalmopathy and pretibial myxoedema. Acta Endocrinol (Copenh). 1993;129:322-327.
- Engin B, Gümüs¸el M, Ozdemir M, et al. Successful combined pentoxifylline and intralesional triamcinolone acetonide treatment of severe pretibial myxedema. Dermatol Online J. 2007;13:16.
- Lang PG, Sisson JC, Lynch PJ. Intralesional triamcinolone therapy for pretibial myxedema. Arch Dermatol. 1975;111:197-202.
Practice Points
- Pretibial myxedema (PM) is a known manifestation of Graves disease that almost always occurs in the presence of Graves ophthalmopathy.
- The proposed pathogenesis of PM is cross-reaction of autoantibodies directed against the thyroid receptors with the fibroblasts of the skin. It is not known why there is a predilection for the anterior shins, but mechanical factors and dependent position may be involved.
- The mainstay of treatment for PM is topical and intralesional corticosteroids, which may have a benefit in mild to moderate disease; however, in cases of severe disease that is refractory to intralesional and topical corticosteroids under occlusion, more aggressive treatment is required.
Painful and Pruritic Erosions on the Back
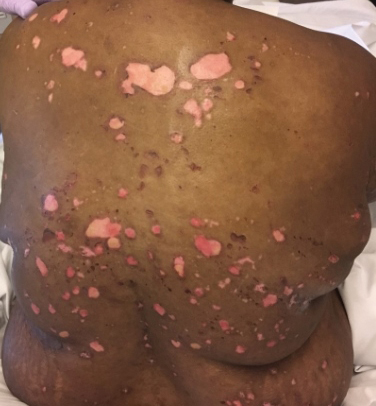
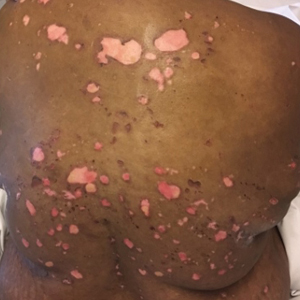
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
The Diagnosis: Bullous Systemic Lupus Erythematosus
Bullous systemic lupus erythematosus (BSLE) is a rare blistering disease that affects patients with systemic lupus erythematosus (SLE). Our patient had a several-year history of SLE and was being managed by a rheumatologist. She was taking hydroxychloroquine at the time of the flare. Although BSLE tends to present in those with SLE that has already been diagnosed, BSLE has been reported as a possible initial manifestation of SLE.1
Bullous systemic lupus erythematosus is estimated to occur in less than 5% of patients with SLE and is more common in black women between the second and third decades of life,2 though it also can be seen in the pediatric population.3 The lesions of BSLE usually present as subepidermal blisters often located on the face, neck, and arms on an erythematous or possibly urticarial base. Although non-BSLE vesiculobullous eruptions may be seen in patients with SLE, BSLE is differentiated from these other eruptions by its appearance on sun-exposed and non-sun-exposed areas of the body, while other vesiculobullous eruptions associated with SLE typically are limited to sun-exposed sites.4
Due to its clinical presentation overlapping with several vesiculobullous conditions, a set of diagnostic criteria have been suggested for BSLE, including the following: (1) fulfillment of the American Rheumatism Association's criteria for SLE5; (2) a new-onset vesiculobullous eruption, primarily on sun-exposed skin; (3) histology showing a subepidermal blister with a predominantly neutrophilic infiltrate; (4) presence of IgG, IgA, IgM, and C3 at the basement membrane zone; (5) evidence of antibodies to type VII collagen; and (6) immunoelectron microscopy showing codistribution of immunoglobulin deposits with anchoring fibrils/type VII collagen. To meet the diagnosis of type I BSLE, all 6 criteria must be satisfied. To meet the diagnosis of type II BSLE, only criteria 1 to 4 need to be satisfied.6
Patients with BSLE may be presumed to have a different but clinically similar vesiculobullous condition (eg, bullous pemphigoid, cutaneous manifestations of SLE) and may be started on systemic corticosteroids. However, BSLE patients often do not show great improvement while on corticosteroids and may even flare shortly after beginning systemic corticosteroid treatment. The current treatment of choice for BSLE is dapsone, a sulfa drug that is thought to exhibit its anti-inflammatory properties via the inhibition of the alternative pathway of the complement system and through the inhibition of polymorphonuclear leukocyte functions.7 A response to dapsone helps differentiate BSLE from histopathologically and immunopathologically identical conditions such as epidermolysis bullosa acquisita.4 Bullous systemic lupus erythematosus can be differentiated from dermatitis herpetiformis with the presence of antigliadin and antitissue transglutaminase antibodies, which are found in the latter. Additionally, BSLE may show the presence of IgG and IgM deposition in addition to IgA deposition, as opposed to dermatitis herpetiformis where only IgA is found.8 The presence of these additional antibody depositions also help differentiate BSLE from linear IgA bullous dermatosis (LABD), as LABD will only have IgA depositions and often presents with an annular, crown of jewels-like appearance. Finally, there is a well-described phenomenon of LABD being drug induced, particularly after a course of vancomycin,9 and such an association with vancomycin has not been documented for BSLE.
Our patient was diagnosed with BSLE following the flare approximately 1.5 years prior to the current presentation. She had been started on dapsone 75 mg daily at that time and was taking 75 mg at the time of presentation. She was admitted and treated as an inpatient with high-dose (1 mg/kg) intravenous prednisone due to the extensive current flare.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
- Fujimoto W, Hamada T, Yamada J, et al. Bullous systemic lupus erythematosus as an initial manifestation of SLE. J Dermatol. 2005;32:1021-1027.
- Miziara ID, Mahmoud A, Chagury AA, et al. Bullous systemic lupus erythematosus: case report. Int Arch Otorhinolaryngol. 2013;17:344-346.
- Tincopa M, Puttgen KB, Sule S, et al. Bullous lupus: an unusual initial presentation of systemic lupus erythematosus in an adolescent girl. Pediatr Dermatol. 2010;27:373-376.
- Grover C, Khurana A, Sharma S, et al. Bullous systemic lupus erythematosus. Indian J Dermatol. 2013;58:492.
- Aringer M, Costenbader K, Daikh D, et al. 2019 European League Against Rheumatism/American College of RheumatologyClassification Criteria for Systemic Lupus Erythematosus [published online August 6, 2019]. Arthritis Rheumatol. 2019;71:1400-1412.
- Gammon WR, Briggaman RA. Bullous SLE: a phenotypically distinctive but immunologically heterogeneous bullous disorder. J Invest Dermatol. 1993;100:28S-34S.
- Duan L, Chen L, Zhong S, et al. Treatment of bullous systemic lupus erythematosus. J Immunol Res. 2015;2015:6.
- Barbosa WS, Rodarte CM, Guerra JG, et al. Bullous systemic lupus erythematosus: differential diagnosis with dermatitis herpetiformis. An Bras Dermatol. 2011;86(4 suppl 1):S92-S95.
- Yordanova I, Valtchev V, Gospodinov D, et al. IgA linear bullous dermatosis in childhood. J IMAB. 2015;21:1012-1014.
A 51-year-old black woman presented to the dermatology clinic with painful and pruritic erosions on the back, abdomen, neck, and arms of approximately 2 months' duration. The lesions started on the back and spread in a cephalocaudal manner. The patient denied any new changes in medication. Physical examination revealed large erosions with mild weeping of serosanguineous fluid on the back, abdomen, neck, and upper extremities. A few tense bullae were present on the dorsal aspect of the right hand. She had experienced a similar flare approximately 1.5 years prior to the current presentation. At that time, 2 shave biopsies from vesiculobullous lesions on the right side of the neck were sent for hematoxylin and eosin staining and direct immunofluorescence. Biopsy results showed a subepidermal blister that extended along the course of the hair follicle and was associated with an infiltrate of neutrophilic granulocytes that also extended along the course of the hair follicle. Direct immunofluorescence showed IgG and C3 deposition in the basement membrane zone extending along the floor of the blister where the epidermis was separated from the dermis.
Sniffing Out Malignant Melanoma: A Case of Canine Olfactory Detection
To the Editor:
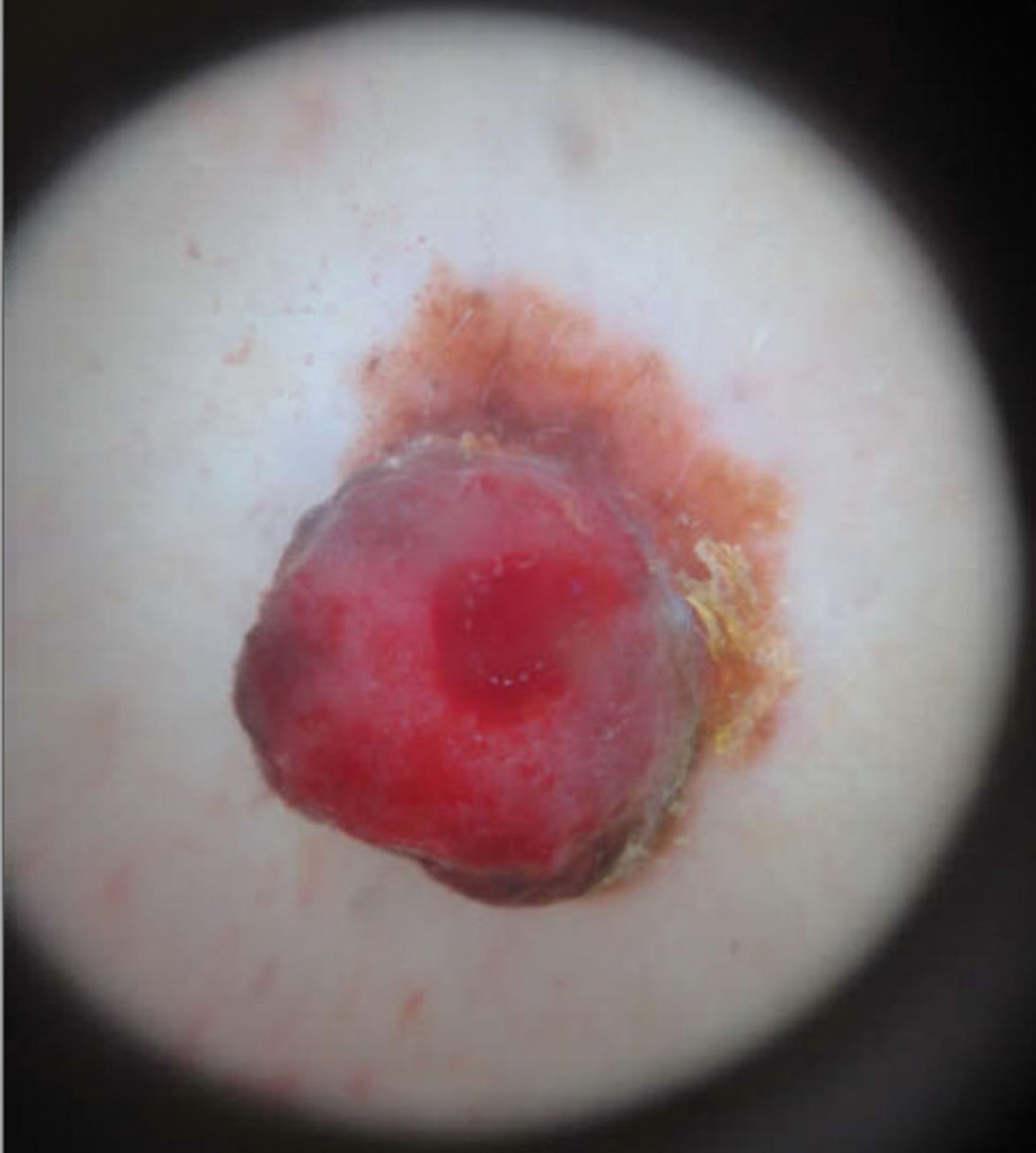
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
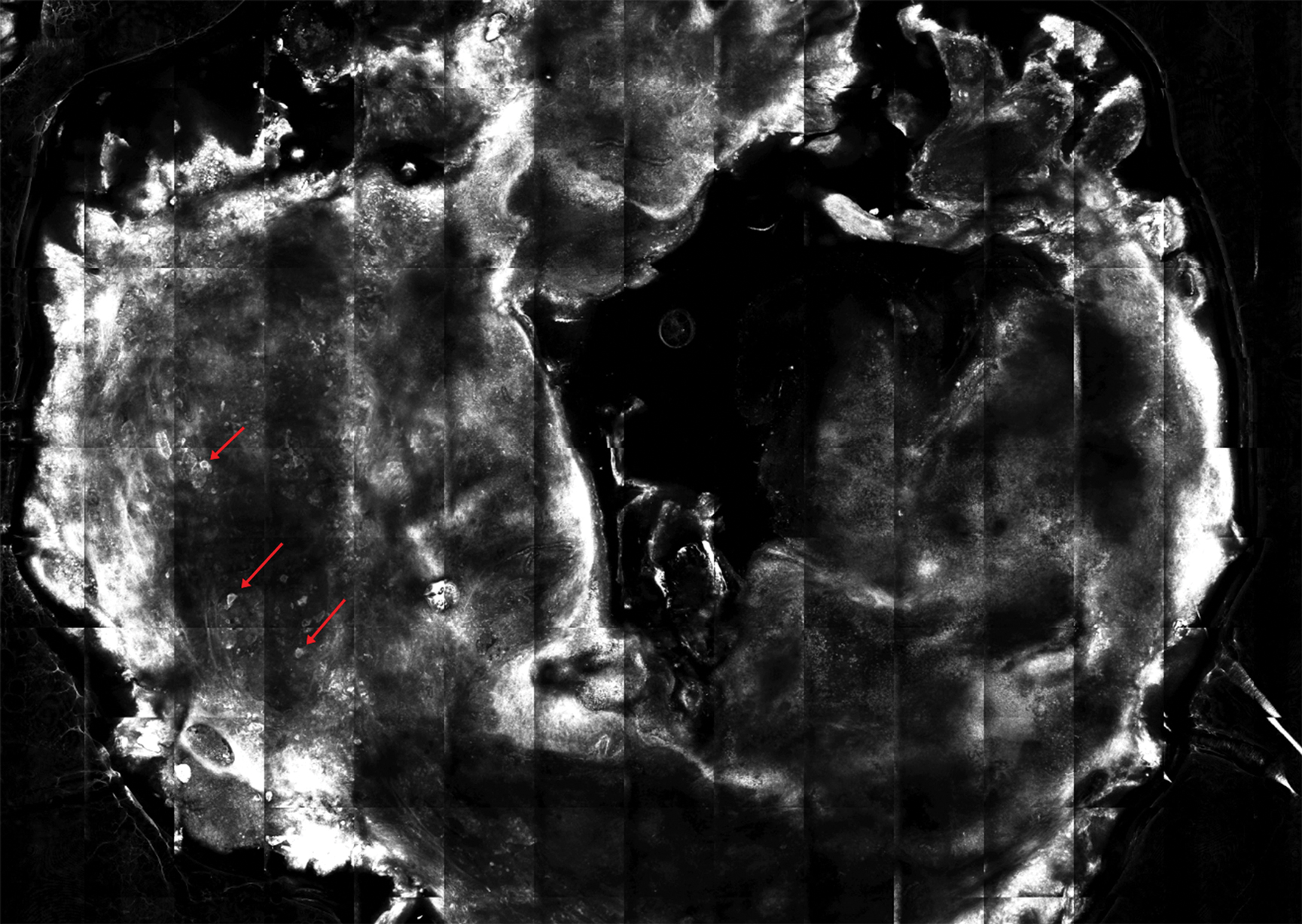
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
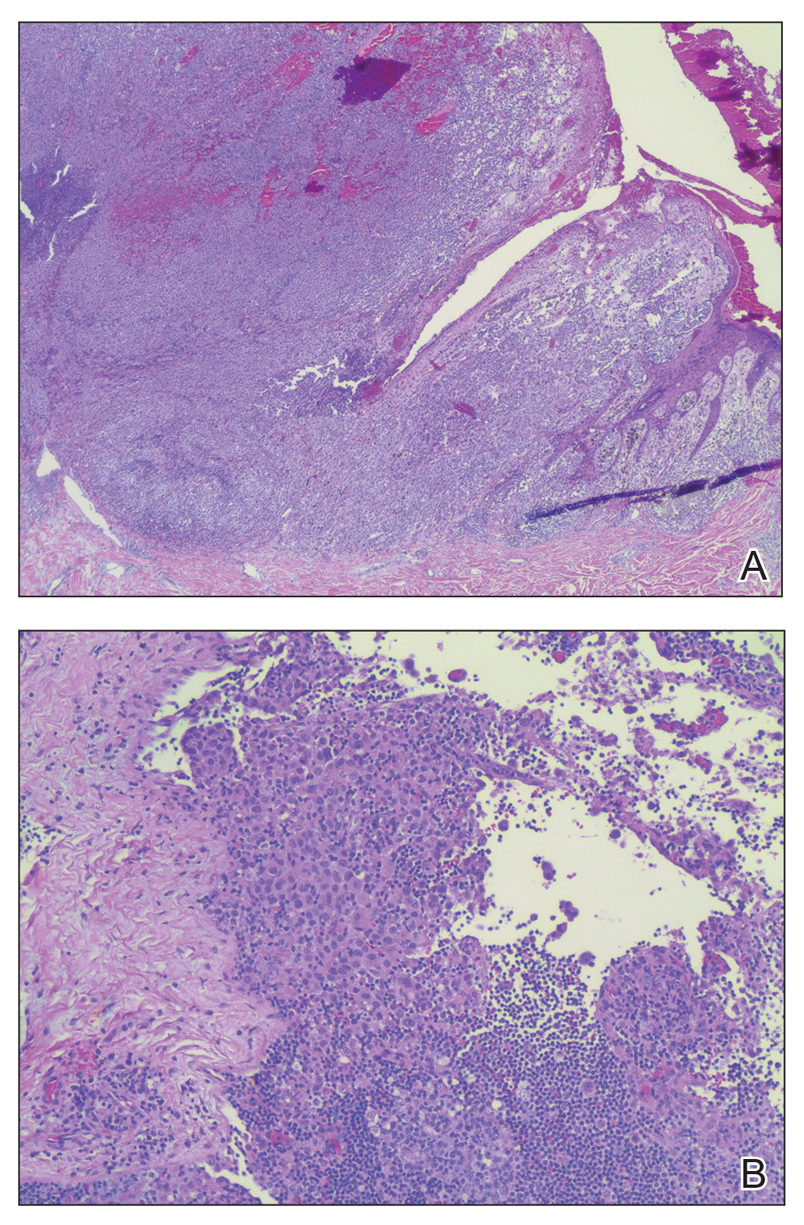
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
To the Editor:
A 43-year-old woman presented with a mole on the central back that had been present since childhood and had changed and grown over the last few years. The patient reported that her 2-year-old rescue dog frequently sniffed the mole and would subsequently get agitated and try to scratch and bite the lesion. This behavior prompted the patient to visit a dermatologist.
She reported no personal history of melanoma or nonmelanoma skin cancer, tanning booth exposure, blistering sunburns, or use of immunosuppressant medications. Her family history was remarkable for basal cell carcinoma in her father but no family history of melanoma. Physical examination revealed a 1.2×1.5-cm brown patch along with a 1×1-cm ulcerated nodule on the lower aspect of the lesion (Figure 1). Dermoscopy showed a blue-white veil and an irregular vascular pattern (Figure 2). No cervical, axillary, or inguinal lymphadenopathy was appreciated on physical examination. Reflectance confocal microscopy showed pagetoid spread of atypical round melanocytes as well as melanocytes in the stratum corneum (Figure 3).
The patient was referred to a surgical oncologist for wide local excision and sentinel lymph node biopsy. Pathology showed a 4-mm-thick melanoma with numerous positive lymph nodes (Figure 4). The patient subsequently underwent a right axillary lymphadenectomy and was diagnosed with stage IIIB malignant melanoma. After surgery, the patient reported that her dog would now sniff her back and calmly rest his head in her lap.
She was treated with ipilimumab but subsequently developed panhypopituitarism, so she was taken off the ipilimumab. Currently, the patient is doing well. She follows up annually for full-body skin examinations and has not had any recurrence in the last 7 years. The patient credits her dog for prompting her to see a dermatologist and saving her life.
Both anecdotal and systematic evidence have emerged on the role of canine olfaction in the detection of lung, breast, colorectal, ovarian, prostate, and skin cancers, including malignant melanoma.1-6 A 1989 case report described a woman who was prompted to seek dermatologic evaluation of a pigmented lesion because her dog consistently targeted the lesion. Excision and subsequent histopathologic examination of the lesion revealed that it was malignant melanoma.5 Another case report described a patient whose dog, which was not trained to detect cancers in humans, persistently licked a lesion behind the patient’s ear that eventually was found to be malignant melanoma.6 These reports have inspired considerable research interest regarding canine olfaction as a potential method to noninvasively screen for and even diagnose malignant melanomas in humans.
Both physiologic and pathologic metabolic processes result in the production of volatile organic compounds (VOCs), or small odorant molecules that evaporate at normal temperatures and pressures.1 Individual cells release VOCs in extremely low concentrations into the blood, urine, feces, and breath, as well as onto the skin’s surface, but there are methods for detecting these VOCs, including gas chromatography–mass spectrometry and canine olfaction.7,8 Pathologic processes, such as infection and malignancy, result in irregular protein synthesis and metabolism, producing new VOCs or differing concentrations of VOCs as compared to normal processes.1
Dimethyl disulfide and dimethyl trisulfide compounds have been identified in malignant melanoma, and these compounds are not produced by normal melanocytes.7 Furthermore, malignant melanoma produces differing quantities of these compounds as compared to normal melanocytes, including isovaleric acid, 2-methylbutyric acid, isoamyl alcohol (3-methyl-1-butanol), and 2-methyl-1-butanol, resulting in a distinct odorant profile that previously has been detected via canine olfaction.7 Canine olfaction can identify odorant molecules at up to 1 part per trillion (a magnitude more sensitive than the currently available gas chromatography–mass spectrometry technologies) and can detect the production of new VOCs or altered VOC ratios due to pathologic processes.1 Systematic studies with dogs that are trained to detect cancers in humans have shown that canine olfaction correctly identified malignant melanomas against healthy skin, benign nevi, and even basal cell carcinomas at higher rates than what would have been expected by chance alone.2,3
Canine olfaction can identify new or altered ratios of odorant VOCs associated with pathologic metabolic processes, and canines can be trained to target odor profiles associated with specific diseases.1 Canine olfaction for melanoma screening and diagnosis may seem appealing, as it provides an easily transportable, real-time, low-cost method compared to other techniques such as gas chromatography–mass spectrometry.1 Although preliminary results have shown that canine olfaction detects melanoma at higher rates than would be expected by chance alone, these findings have not approached clinical utility for the widespread use of canine olfaction as a screening method for melanoma.2,3,9 Further studies are needed to understand the role of canine olfaction in melanoma screening and diagnosis as well as to explore methods to optimize sensitivity and specificity. Until then, patients and dermatologists should not ignore the behavior of dogs toward skin lesions. Dogs may be beneficial in the detection of melanoma and help save lives, as was seen in our case.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
- Angle C, Waggoner LP, Ferrando A, et al. Canine detection of the volatilome: a review of implications for pathogen and disease detection. Front Vet Sci. 2016;3:47.
- Pickel D, Mauncy GP, Walker DB, et al. Evidence for canine olfactory detection of melanoma. Applied Animal Behaviour Science. 2004;89:107-116.
- Willis CM, Britton LE, Swindells MA, et al. Invasive melanoma in vivo can be distinguished from basal cell carcinoma, benign naevi and healthy skin by canine olfaction: a proof‐of‐principle study of differential volatile organic compound emission. Br J Dermatol. 2016;175:1020-1029.
- Jezierski T, Walczak M, Ligor T, et al. Study of the art: canine olfaction used for cancer detection on the basis of breath odour. perspectives and limitations. J Breath Res. 2015;9:027001.
- Williams H, Pembroke A. Sniffer dogs in the melanoma clinic? Lancet. 1989;1:734.
- Campbell LF, Farmery L, George SM, et al. Canine olfactory detection of malignant melanoma. BMJ Case Rep. 2013. doi:10.1136/bcr-2013-008566.
- Kwak J, Gallagher M, Ozdener MH, et al. Volatile biomarkers from human melanoma cells. J Chromotogr B Analyt Technol Biomed Life Sci. 2013;931:90-96.
- D’Amico A, Bono R, Pennazza G, et al. Identification of melanoma with a gas sensor array. Skin Res Technol. 2008;14:226-236.
- Elliker KR, Williams HC. Detection of skin cancer odours using dogs: a step forward in melanoma detection training and research methodologies. Br J Dermatol. 2016;175:851-852.
Practice Points
- Physiologic and pathologic processes produce volatile organic compounds in the skin and other tissues.
- Malignant melanocytes release unique volatile organic compounds (VOCs) as well as differing combinations and quantities of VOCs as compared to normal melanocytes.
- Volatile organic compounds released at the skin’s surface can be detected by various methods, including canine olfaction; therefore, unusual canine behavior toward skin lesions should not be ignored.
Cutaneous Sarcoidosis Presenting as a Cutaneous Horn
To the Editor:
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
To the Editor:
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
To the Editor:
A 53-year-old woman presented to our dermatology clinic with a painful growth on the right ear of 2 months’ duration. A complete review of systems was negative except for an isolated episode of shortness of breath prior to presentation that resolved without intervention. During this episode, her primary care physician made a diagnosis of chronic obstructive pulmonary disease based on a chest radiograph. The patient reported minimal tobacco use, specifically that she had smoked a few cigarettes daily for several years but had quit 6 months prior to the current presentation.
Cutaneous horn is a clinical term used to describe hyperkeratotic horn-shaped growths of highly variable shapes and sizes. Although the pathogenesis and incidence of cutaneous horns remain unknown, these lesions most often are the result of a neoplastic rather than an inflammatory process. The differential diagnosis typically includes entities characterized by marked hyperkeratosis, including hypertrophic actinic keratosis, squamous cell carcinoma (SCC), seborrheic keratosis, and verruca vulgaris. The base of the horn must be biopsied to determine the underlying etiology, paying careful attention to avoid a superficial biopsy, as it may be nondiagnostic.
Studies analyzing the underlying diagnoses and clinical features of cutaneous horns are limited. In a large retrospective study of 643 cutaneous horns, 61% were benign, 23% were premalignant, and 16% were malignant. In this study, 4 features were associated with premalignant or malignant pathology: (1) older age (mid- 60s to 70s); (2) male sex; (3) location on the nose, pinnae, dorsal hands, scalp, forearms, or face; and (4) a wide base (4.4 mm or larger) and a lower height-to-base ratio than benign lesions.1 Two additional studies of more than 200 horns each showed higher rates of premalignant horns (42% and 38%, respectively) with malignancy found in 7% and 20% of horns, respectively.2,3 One prospective study sought to identify clinical and dermatoscopic features of SCCs underlying cutaneous horns, concluding that SCC diagnosis was more likely if a horn had (1) a height less than the diameter of its base, (2) a lack of terrace morphology (a dermatoscopic feature defined as horizontal parallel layers of keratin), (3) erythema at the base, and (4) the presence of pain.4
Our patient had a cutaneous horn on the pinna that was painful, wider than it was tall, and erythematous at the base, suggesting a malignant process; however, a complete cutaneous physical examination revealed other skin lesions that were concerning for sarcoidosis and raised suspicion that the horn also was a manifestation of the same inflammatory process.
Although unusual, cutaneous sarcoidosis presenting as a cutaneous horn is not unexpected. In a histopathologic study of 62 cases of cutaneous sarcoidosis, 79% (49/62) showed epidermal changes and 13% (8/62) demonstrated hyperkeratosis. Other epidermal changes included parakeratosis (16% [10/62]), acanthosis (10% [6/62]), and epidermal atrophy (57% [35/62]).5 The spectrum of epidermal pathology in cutaneous sarcoidosis is evident in its well-documented verrucous, psoriasiform, and ichthyosiform presentations. For completeness, cutaneous horn is added to the list of clinical morphologies for this “great imitator” of cutaneous diseases.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
- Yu RC, Pryce DW, Macfarlane AW, et al. A histopathological study of 643 cutaneous horns. Br J Dermatol. 1991;124:449-452.
- Schosser RH, Hodge SJ, Gaba CR, et al. Cutaneous horns: a histopathologic study. South Med J. 1979;72:1129-1131.
- Mantese SA, Diogo PM, Rocha A, et al. Cutaneous horn: a retrospective histopathological study of 222 cases. An Bras Dermatol. 2010;85:157-163.
- Pyne J, Sapkota D, Wong JC. Cutaneous horns: clues to invasive squamous cell carcinoma being present in the horn base. Dermatol Pract Concept. 2013;3:3-7.
- Hiroyuki O. Epidermal changes in cutaneous lesions of sarcoidosis. Am J Dermatopathol. 1999;21:229-233.
Practice Points
- Biopsy of a cutaneous horn should be deep enough to capture the neoplastic or inflammatory process at the base of the lesion.
- Cutaneous sarcoidosis can present with variable morphologies including the epidermal changes of a cutaneous horn.
Unusually Early-Onset Plantar Verrucous Carcinoma
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
To the Editor:
Verrucous carcinoma (VC) is a rare type of squamous cell carcinoma characterized by a well-differentiated low-grade tumor with a high degree of keratinization. First described by Ackerman1 in 1948, VC presents on the skin or oral and genital mucosae with minimal atypical cytologic findings.1-3 It most commonly is seen in late middle-aged men (85% of cases) and presents as a slow-growing mass, often of more than 10 years’ duration.2,3 Verrucous carcinoma frequently is observed at 3 particular anatomic sites: the oral cavity, known as oral florid papillomatosis; the anogenital area, known as Buschke-Löwenstein tumor; and on the plantar surface, known as epithelioma cuniculatum.2-13
A 19-year-old man presented with an ulcerous lesion on the right big toe of 2 years’ duration. He reported that the lesion had gradually increased in size and was painful when walking. Physical examination revealed an ulcerated lesion on the right big toe with purulent inflammation and necrosis, unclear edges, and border nodules containing a fatty, yellowish, foul-smelling material (Figure 1). Histologic examination of purulent material from deep within the primary lesion revealed gram-negative rods and gram-positive diplococci. Erlich-Ziehl-Neelsen staining and culture in Lowenstein-Jensen medium were negative for mycobacteria. Histologic examination and fungal culture were not diagnostic for fungal infection.
The differential diagnosis included tuberculosis cutis verrucosa, subcutaneous mycoses, swimming pool granuloma, leishmania cutis, chronic pyoderma vegetans, and VC. A punch biopsy of the lesion showed chronic nonspecific inflammation, hyperkeratosis, parakeratosis, and pseudoepitheliomatous hyperplasia. A repeat biopsy performed 15 days later also showed a nonspecific inflammation. At the initial presentation, an anti–human immunodeficiency virus test was negative. A purified protein derivative (PPD) skin test was positive and showed a 17-mm induration, and a sputum test was negative for Mycobacterium tuberculosis. A chest radiograph was normal. We considered the positive PPD skin test to be clinically insignificant; we did not find an accompanying tuberculosis infection, and the high exposure to atypical tuberculosis in developing countries such as Turkey, which is where the patient resided, often explains a positive PPD test.
At the initial presentation, radiography of the right big toe revealed porotic signs and cortical irregularity of the distal phalanx. A deep incisional biopsy of the lesion was performed for pathologic and microbiologic analysis. Erlich-Ziehl-Neelsen staining was negative, fungal elements could not be observed, and there was no growth in Lowenstein-Jensen medium or Sabouraud dextrose agar. Polymerase chain reaction for human papillomavirus, M tuberculosis, and atypical mycobacterium was negative. Periodic acid–Schiff staining was negative for fungal elements. Histopathologic examination revealed an exophytic as well as endophytic squamous cell proliferation infiltrating deeper layers of the dermis with a desmoplastic stroma (Figure 2). Slight cytologic atypia was noted. A diagnosis of VC was made based on the clinical and histopathologic findings. The patient’s right big toe was amputated by plastic surgery 6 months after the initial presentation.
The term epithelioma cuniculatum was first used in 1954 to describe plantar VC. The term cuniculus is Latin for rabbit nest.3 At the distal part of the plantar surface of the foot, VC presents as an exophytic funguslike mass with abundant keratin-filled sinuses.14 When pressure is applied to the lesion, a greasy, yellowish, foul-smelling material with the consistency of toothpaste emerges from the sinuses. The lesion resembles pyoderma vegetans and may present with secondary infections (eg, Staphylococcus aureus, gram-negative bacteria, fungal infection) and/or ulcerations. Its appearance resembles an inflammatory lesion more than a neoplasm.6 Sometimes the skin surrounding the lesion may be a yellowish color, giving the impression of a plantar wart.3,4 In most cases, in situ hybridization demonstrates a human papillomavirus genome.2-5,10 Other factors implicated in the etiopathogenesis of VC include chronic inflammation; a cicatrice associated with a condition such as chronic cutaneous tuberculosis, ulcerative leprosy, dystrophic epidermolysis bullosa, or chronic osteomyelitis4; recurrent trauma3; and/or lichen planus.2,4 In spite of its slow development and benign appearance, VC may cause severe destruction affecting surrounding bony structures and may ultimately require amputation.2,4 In its early stages, VC can be mistaken for a benign tumor or other benign lesion, such as giant seborrheic keratosis, giant keratoacanthoma, eccrine poroma, or verruciform xanthoma, potentially leading to an incorrect diagnosis.5
Histopathologic examination, especially of superficial biopsies, generally reveals squamous cell proliferation demonstrating minimal pleomorphism and cytologic atypia with sparse mitotic figures.4-6 Diagnosis of VC can be challenging if the endophytic proliferation, which characteristically pushes into the dermis and even deeper tissues at the base of the lesion, is not seen. This feature is uncommon in squamous cell carcinomas.3,4,6 Histopathologic detection of koilocytes can lead to difficulty in distinguishing VC from warts.5 The growth of lesions is exophytic in plantar verrucae, whereas in VC it may be either exophytic or endophytic.4 At early stages, it is too difficult to distinguish VC from pseudoepitheliomatous hyperplasia caused by chronic inflammation, as well as from tuberculosis and subcutaneous mycoses.3,6 In these situations, possible responsible microorganisms must be sought out. Amelanotic malignant melanoma and eccrine poroma also should be considered in the differential diagnosis.3,5 If the biopsy specimen is obtained superficially and is fragmented, the diagnosis is more difficult, making deep biopsies essential in suspicious cases.4 Excision is the best treatment, and Mohs micrographic surgery may be required in some cases.2,3,11 It is important to consider that radiotherapy may lead to anaplastic transformation and metastasis.2 Metastasis to lymph nodes is very rare, and the prognosis is excellent when complete excision is performed.2 Recurrence may be observed.4
Our case of plantar VC is notable because of the patient’s young age, which is uncommon, as the typical age for developing VC is late middle age (ie, fifth and sixth decades of life). A long-standing lesion that is therapy resistant and without a detectable microorganism should be investigated for malignancy by repetitive deep biopsy regardless of the patient’s age, as demonstrated in our case.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
- Ackerman LV. Verrucous carcinoma of the oral cavity. Surgery. 1948;23:670-678.
- Schwartz RA. Verrucous carcinoma of the skin and mucosal. J Am Acad Dermatol. 1995;32:1-21.
- Kao GF, Graham JH, Helwig EB. Carcinoma cuniculatum (verrucous carcinoma of the skin): a clinicopathologic study of 46 cases with ultrastructural observations. Cancer. 1982;49:2395-2403.
- Mc Kee PH, ed. Pathology of the Skin. 2nd ed. London, England: Mosby-Wolfe; 1996.
- Schwartz RA, Stoll HL. Squamous cell carcinoma. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Fitzpatrick’s Dermatology in General Medicine. 5th ed. New York, NY: Mc-Graw Hill; 1999:840-856.
- MacKie RM. Epidermal skin tumours. In: Rook A, Wilkinson DS, Ebling FJG, et al, eds. Textbook of Dermatology. 5th ed. Oxford, United Kingdom: Blackwell Scientific; 1992:1500-1556.
- Yoshtatsu S, Takagi T, Ohata C, et al. Plantar verrucous carcinoma: report of a case treated with Boyd amputation followed by reconstruction with a free forearm flap. J Dermatol. 2001;28:226-230.
- Van Geertruyden JP, Olemans C, Laporte M, et al. Verrucous carcinoma of the nail bed. Foot Ankle Int. 1998;19:327-328.
- Sanchez-Yus E, Velasco E, Robledo A. Verrucous carcinoma of the back. J Am Acad Dermatol. 1986;14(5 pt 2):947-950.
- Noel JC, Peny MO, Detremmerie O, et al. Demonstration of human papillomavirus type 2 in a verrucous carcinoma of the foot. Dermatology. 1993;187:58-61.
- Mora RG. Microscopically controlled surgery (Mohs’ chemosurgery) for treatment of verrucous squamous cell carcinoma of the foot (epithelioma cuniculatum). J Am Acad Dermatol. 1983;8:354-362.
- Kathuria S, Rieker J, Jablokow VR, et al. Plantar verrucous carcinoma (epithelioma cuniculatum): case report with review of the literature. J Surg Oncol. 1986;31:71-75.
- Brownstein MH, Shapiro L. Verrucous carcinoma of skin: epithelioma cuniculatum plantare. Cancer. 1976;38:1710-1716.
- Ho J, Diven DG, Butler PJ, et al. An ulcerating verrucous plaque on the foot. verrucous carcinoma (epithelioma cuniculatum). Arch Dermatol. 2000;136:547-548, 550-551.
Practice Points
- Verrucous carcinoma (VC) frequently is observed at 3 particular anatomic sites: the oral cavity, the anogenital area, and on the plantar surface.
- Plantar VC is rare, with a male predominance and most patients presenting in the fifth to sixth decades of life.
- Differentiating VS from benign tumors may be difficult, especially if only superficial biopsies are taken. Multiple biopsies and a close clinical correlation are required before a definite diagnosis is possible.
Postinflammatory Hyperpigmentation Following Treatment of Hyperkeratosis Lenticularis Perstans With Tazarotene Cream 0.1%
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.
Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.
Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
To the Editor:
Hyperkeratosis lenticularis perstans (HLP), or Flegel disease, is a rare keratinization disorder characterized by asymptomatic, red-brown, 1- to 5-mm papules with irregular horny scales commonly seen on the dorsal feet and lower legs.1 Hyperkeratosis lenticularis perstans is notorious for being difficult to treat. Various treatment options, including 5-fluorouracil, topical and oral retinoids, vitamin D3 derivatives, psoralen plus UVA therapy, and dermabrasion, have been explored but none have proven to be consistently effective.
A woman in her 50s presented with an asymptomatic eruption on the legs and thighs that had been present for the last 20 years. She had been misdiagnosed by multiple outside providers with atopic dermatitis and was treated with topical steroids without considerable improvement. Upon initial presentation to our clinic , physical examination revealed a woman with Fitzpatrick skin type II with multiple hyperpigmented, red-brown, 2- to 6-mm papules on the extensor surfaces of the lower legs and upper thighs (Figure, A). A 3-mm punch biopsy of a lesion on the right upper thigh revealed hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate. The clinical and histopathologic findings were consistent with HLP.
The patient was started on treatment with 5-fluorouracil cream on the right leg and tazarotene cream 0.1% on the left leg to determine which agent would work best. After 9 weeks of treatment, slight improvement was observed on both legs, but the lesions were still erythematous (Figure, B). Treatment was continued, and after 14 weeks complete resolution of the lesions was noted on both legs; however, postinflammatory hyperpigmentation (PIH) was observed on the left leg, which had been treated with tazarotene (Figure, C). The patient was lost to follow-up prior to treatment of the PIH.
Postinflammatory hyperpigmentation is an acquired excess of pigment due to a prior disease process such as an infection, allergic reaction, trauma, inflammatory disease, or drug reaction. In our patient, this finding was unusual because tazarotene has been shown to be an effective treatment of PIH.2,3
In PIH, there is either abnormal production or distribution of melanin pigment in the epidermis and/or dermis. Several mechanisms for PIH have been suggested. One potential mechanism is disruption of the basal cell layer due to dermal lymphocytic inflammation, causing melanin to be released and trapped by macrophages present in the dermal papillae. Another possible mechanism is epidermal hypermelanosis, in which the release and oxidation of arachidonic acid to prostaglandins and leukotrienes alters immune cells and melanocytes, causing an increase in melanin and increased transfer of melanin to keratinocytes in the surrounding epidermis.4
Treatment of PIH can be a difficult and prolonged process, especially when a dermal rather than epidermal melanosis is observed. Topical retinoids, topical hydroquinone, azelaic acid, corticosteroids, tretinoin cream, glycolic acid, and trichloroacetic acid have been shown to be effective in treating epidermal PIH. Tazarotene is a synthetic retinoid that has been proven to be an effective treatment of PIH3; however, in our patient the PIH progressed with treatment. One plausible explanation is that irritation caused by the medication led to further PIH.2,5
It is uncommon for tazarotene to cause PIH. Hyperpigmentation is listed as an adverse effect observed during the postmarketing experience according to one manufacturer6 and the US Food and Drug Administration; however, details about prior incidents of hyperpigmentation have not been reported in the literature. Our case is unique because both treatments showed considerable improvement in HLP, but more PIH was observed on the tazarotene-treated leg.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
- Bean SF. Hyperkeratosis lenticularis perstans. a clinical, histopathologic, and genetic study. Arch Dermatol. 1969;99:705-709.
- Callender V, St. Surin-Lord S, Davis E, et al. Postinflammatory hyperpigmentation: etiologic and therapeutic considerations. Am J Clin Dermatol. 2011;12:87-99.
- McEvoy G. Tazarotene (topical). In: AHFS Drug Information. Bethesda, MD: American Society of Health-System Pharmacists, Inc; 2014:84-92.
- Lacz N, Vafaie J, Kihiczak N, et al. Postinflammatory hyperpigmentation: a common but troubling condition. Int J Dermatol. 2004;43:362-365.
- Tazorac (tazarotene) cream [package insert]. Irvine, CA: Allergan, Inc; 2013.
- Tazorac (tazarotene) gel [package insert]. Irvine, CA: Allergan, Inc; 2014.
Practice Points
- Hyperkeratosis lenticularis perstans is a rare keratinization disorder that presents with asymptomatic red-brown papules with irregular horny scales on the lower extremities.
- Hyperkeratosis lenticularis perstans can be difficult to diagnose and treat. Hematoxylin and eosin staining generally will show hyperkeratosis and parakeratosis with basal layer degeneration and a perivascular lymphocytic infiltrate.
- Tazarotene cream 0.1% is a synthetic retinoid sometimes used for treatment of hyperpigmentation, but it also can cause postinflammatory hyperpigmentation.
#Dermlife and the Burned-out Resident
Dermatologist Dr. Jeffrey Benabio quipped, “The phrase ‘dermatologist burnout’ may seem as oxymoronic as jumbo shrimp, yet both are real.”1 Indeed, dermatologists often self-report as among the happiest specialists both at work and home, according to the annual Medscape Physician Lifestyle and Happiness Report.2 Similarly, others in the medical field may perceive dermatologists as low-stress providers—well-groomed, well-rested rays of sunshine, getting out of work every day at 5:00
Burnout in Dermatology Residents
Burnout is a syndrome of emotional exhaustion, depersonalization, and a sense of reduced personal accomplishment that affects residents of all specialties3; however, there is a paucity of literature on burnout as it relates to dermatology. Although long work hours and schedule volatility have captured the focus of resident burnout conversations, a less discussed set of factors may contribute to dermatology resident burnout, such as increasing patient load, intensifying regulations, and an unrelenting pace of clinic. A recent survey study by Shoimer et al3 found that 61% of 116 participating Canadian dermatology residents cited examinations (including the board certifying examination) as their top stressor, followed by work (27%). Other stressors included family, relationships, finances, pressure from staff, research, and moving. More than 50% of dermatology residents surveyed experienced high levels of emotional exhaustion and depersonalization, while 40% demonstrated a low sense of personal accomplishment, all of which are determinants of the burnout syndrome.3
Comparison to Residents in Other Specialties
Although dermatology residents experience lower burnout rates than colleagues in other specialties, the absolute prevalence warrants attention. A recent study published in the Journal of the American Medical Association of 3588 second-year medical residents in the United States found that rates of burnout symptoms across clinical specialties ranged from 29.6% to 63.8%. The highest rates of burnout were found in urology (63.8%), neurology (61.6%), and ophthalmology (55.8%), but the lowest reported rate of burnout was demonstrated in dermatology (29.6%).4 Although dermatology ranked the lowest, that is still nearly a whopping 1 in 3 dermatology residents with burnout symptoms. The absolute prevalence should not be obscured by the ranking among other specialties.
Preventing Burnout
Several burnout prevention and coping strategies across specialties have been suggested.
Mindfulness and Self-awareness
A study by Chaukos et al5 found that mindfulness and self-awareness are resilience factors associated with resident burnout. Counseling is one strategy demonstrated to increase self-awareness. Mindfulness may be practiced through meditation or yoga. Regular meditation has been shown to improve mood and emotional stress.6 Similarly, yoga has been shown to yield physical, emotional, and psychological benefits to resdients.7
Work Factors
A supportive clinical faculty and receiving constructive monthly performance feedback have been negatively correlated with dermatology resident burnout.3 Other workplace interventions demonstrating utility in decreasing resident burnout include increasing staff awareness about burnout, increasing support for health professionals treating challenging populations, and ensuring a reasonable workload.6
Sleep
It has been demonstrated that sleeping less than 7 hours per night also is associated with resident burnout,7 yet it has been reported that 72% of dermatology residents fall into this category.3 Poor sleep quality has been shown to be a predictor of lower academic performance. It has been proposed that to minimize sleep deprivation and poor sleep quality, institutions should focus on programs that promote regular exercise, sleep hygiene, mindfulness, and time-out activities such as meditation.7
Social Support
Focusing on peers may foster the inner strength to endure suffering.1 Venting, laughing, and discussing care with colleagues has been demonstrated to decrease anxiety.6 Work-related social networks may be strengthened through attendance at conferences, lectures, and professional organizations.7 Additionally, social supports and spending quality time with family have been demonstrated as negative predictors of dermatology resident burnout.3
Physical Exercise
Exercise has been demonstrated to improve mood, anxiety, and depression, thereby decreasing resident burnout.6
Final Thoughts
Burnout among dermatology residents warrants awareness, as it does in other medical specialties. Awareness may facilitate identification and prevention, the latter of which is perhaps best summarized by the words of psychologist Dr. Christina Maslach: “If all of the knowledge and advice about how to beat burnout could be summed up in 1 word, that word would be balance—balance between giving and getting, balance between stress and calm, balance between work and home.”8
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed August 14, 2019.
- Martin KL. Medscape Physician Lifestyle & Happiness Report 2019. Medscape website. https://www.medscape.com/slideshow/2019-lifestyle-happiness-6011057. Published January 9, 2019. Accessed August 14, 2019.
- Shoimer I, Patten S, Mydlarski P. Burnout in dermatology residents: a Canadian perspective. Br J Dermatol. 2018;178:270-271.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among us resident physicians. JAMA. 2018;320:1114-1130.
- Chaukos D, Chaed-Friedman E, Mehta D, et al. Risk and resilience factors associated with resident burnout. Acad Psychiatry. 2017;41:189-194.
- Ishak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;2:236-242.
- Tolentino J, Guo W, Ricca R, et al. What’s new in academic medicine: can we effectively address the burnout epidemic in healthcare? Int J Acad Med. 2017;3.
- Maslach C. Burnout: a multidimensional perspective. In: Schaufeli W, Maslach C, Marek T, eds. Professional Burnout: Recent Developments in Theory and Research. Washington, DC: Taylor and Francis; 1993:19-32.
Dermatologist Dr. Jeffrey Benabio quipped, “The phrase ‘dermatologist burnout’ may seem as oxymoronic as jumbo shrimp, yet both are real.”1 Indeed, dermatologists often self-report as among the happiest specialists both at work and home, according to the annual Medscape Physician Lifestyle and Happiness Report.2 Similarly, others in the medical field may perceive dermatologists as low-stress providers—well-groomed, well-rested rays of sunshine, getting out of work every day at 5:00
Burnout in Dermatology Residents
Burnout is a syndrome of emotional exhaustion, depersonalization, and a sense of reduced personal accomplishment that affects residents of all specialties3; however, there is a paucity of literature on burnout as it relates to dermatology. Although long work hours and schedule volatility have captured the focus of resident burnout conversations, a less discussed set of factors may contribute to dermatology resident burnout, such as increasing patient load, intensifying regulations, and an unrelenting pace of clinic. A recent survey study by Shoimer et al3 found that 61% of 116 participating Canadian dermatology residents cited examinations (including the board certifying examination) as their top stressor, followed by work (27%). Other stressors included family, relationships, finances, pressure from staff, research, and moving. More than 50% of dermatology residents surveyed experienced high levels of emotional exhaustion and depersonalization, while 40% demonstrated a low sense of personal accomplishment, all of which are determinants of the burnout syndrome.3
Comparison to Residents in Other Specialties
Although dermatology residents experience lower burnout rates than colleagues in other specialties, the absolute prevalence warrants attention. A recent study published in the Journal of the American Medical Association of 3588 second-year medical residents in the United States found that rates of burnout symptoms across clinical specialties ranged from 29.6% to 63.8%. The highest rates of burnout were found in urology (63.8%), neurology (61.6%), and ophthalmology (55.8%), but the lowest reported rate of burnout was demonstrated in dermatology (29.6%).4 Although dermatology ranked the lowest, that is still nearly a whopping 1 in 3 dermatology residents with burnout symptoms. The absolute prevalence should not be obscured by the ranking among other specialties.
Preventing Burnout
Several burnout prevention and coping strategies across specialties have been suggested.
Mindfulness and Self-awareness
A study by Chaukos et al5 found that mindfulness and self-awareness are resilience factors associated with resident burnout. Counseling is one strategy demonstrated to increase self-awareness. Mindfulness may be practiced through meditation or yoga. Regular meditation has been shown to improve mood and emotional stress.6 Similarly, yoga has been shown to yield physical, emotional, and psychological benefits to resdients.7
Work Factors
A supportive clinical faculty and receiving constructive monthly performance feedback have been negatively correlated with dermatology resident burnout.3 Other workplace interventions demonstrating utility in decreasing resident burnout include increasing staff awareness about burnout, increasing support for health professionals treating challenging populations, and ensuring a reasonable workload.6
Sleep
It has been demonstrated that sleeping less than 7 hours per night also is associated with resident burnout,7 yet it has been reported that 72% of dermatology residents fall into this category.3 Poor sleep quality has been shown to be a predictor of lower academic performance. It has been proposed that to minimize sleep deprivation and poor sleep quality, institutions should focus on programs that promote regular exercise, sleep hygiene, mindfulness, and time-out activities such as meditation.7
Social Support
Focusing on peers may foster the inner strength to endure suffering.1 Venting, laughing, and discussing care with colleagues has been demonstrated to decrease anxiety.6 Work-related social networks may be strengthened through attendance at conferences, lectures, and professional organizations.7 Additionally, social supports and spending quality time with family have been demonstrated as negative predictors of dermatology resident burnout.3
Physical Exercise
Exercise has been demonstrated to improve mood, anxiety, and depression, thereby decreasing resident burnout.6
Final Thoughts
Burnout among dermatology residents warrants awareness, as it does in other medical specialties. Awareness may facilitate identification and prevention, the latter of which is perhaps best summarized by the words of psychologist Dr. Christina Maslach: “If all of the knowledge and advice about how to beat burnout could be summed up in 1 word, that word would be balance—balance between giving and getting, balance between stress and calm, balance between work and home.”8
Dermatologist Dr. Jeffrey Benabio quipped, “The phrase ‘dermatologist burnout’ may seem as oxymoronic as jumbo shrimp, yet both are real.”1 Indeed, dermatologists often self-report as among the happiest specialists both at work and home, according to the annual Medscape Physician Lifestyle and Happiness Report.2 Similarly, others in the medical field may perceive dermatologists as low-stress providers—well-groomed, well-rested rays of sunshine, getting out of work every day at 5:00
Burnout in Dermatology Residents
Burnout is a syndrome of emotional exhaustion, depersonalization, and a sense of reduced personal accomplishment that affects residents of all specialties3; however, there is a paucity of literature on burnout as it relates to dermatology. Although long work hours and schedule volatility have captured the focus of resident burnout conversations, a less discussed set of factors may contribute to dermatology resident burnout, such as increasing patient load, intensifying regulations, and an unrelenting pace of clinic. A recent survey study by Shoimer et al3 found that 61% of 116 participating Canadian dermatology residents cited examinations (including the board certifying examination) as their top stressor, followed by work (27%). Other stressors included family, relationships, finances, pressure from staff, research, and moving. More than 50% of dermatology residents surveyed experienced high levels of emotional exhaustion and depersonalization, while 40% demonstrated a low sense of personal accomplishment, all of which are determinants of the burnout syndrome.3
Comparison to Residents in Other Specialties
Although dermatology residents experience lower burnout rates than colleagues in other specialties, the absolute prevalence warrants attention. A recent study published in the Journal of the American Medical Association of 3588 second-year medical residents in the United States found that rates of burnout symptoms across clinical specialties ranged from 29.6% to 63.8%. The highest rates of burnout were found in urology (63.8%), neurology (61.6%), and ophthalmology (55.8%), but the lowest reported rate of burnout was demonstrated in dermatology (29.6%).4 Although dermatology ranked the lowest, that is still nearly a whopping 1 in 3 dermatology residents with burnout symptoms. The absolute prevalence should not be obscured by the ranking among other specialties.
Preventing Burnout
Several burnout prevention and coping strategies across specialties have been suggested.
Mindfulness and Self-awareness
A study by Chaukos et al5 found that mindfulness and self-awareness are resilience factors associated with resident burnout. Counseling is one strategy demonstrated to increase self-awareness. Mindfulness may be practiced through meditation or yoga. Regular meditation has been shown to improve mood and emotional stress.6 Similarly, yoga has been shown to yield physical, emotional, and psychological benefits to resdients.7
Work Factors
A supportive clinical faculty and receiving constructive monthly performance feedback have been negatively correlated with dermatology resident burnout.3 Other workplace interventions demonstrating utility in decreasing resident burnout include increasing staff awareness about burnout, increasing support for health professionals treating challenging populations, and ensuring a reasonable workload.6
Sleep
It has been demonstrated that sleeping less than 7 hours per night also is associated with resident burnout,7 yet it has been reported that 72% of dermatology residents fall into this category.3 Poor sleep quality has been shown to be a predictor of lower academic performance. It has been proposed that to minimize sleep deprivation and poor sleep quality, institutions should focus on programs that promote regular exercise, sleep hygiene, mindfulness, and time-out activities such as meditation.7
Social Support
Focusing on peers may foster the inner strength to endure suffering.1 Venting, laughing, and discussing care with colleagues has been demonstrated to decrease anxiety.6 Work-related social networks may be strengthened through attendance at conferences, lectures, and professional organizations.7 Additionally, social supports and spending quality time with family have been demonstrated as negative predictors of dermatology resident burnout.3
Physical Exercise
Exercise has been demonstrated to improve mood, anxiety, and depression, thereby decreasing resident burnout.6
Final Thoughts
Burnout among dermatology residents warrants awareness, as it does in other medical specialties. Awareness may facilitate identification and prevention, the latter of which is perhaps best summarized by the words of psychologist Dr. Christina Maslach: “If all of the knowledge and advice about how to beat burnout could be summed up in 1 word, that word would be balance—balance between giving and getting, balance between stress and calm, balance between work and home.”8
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed August 14, 2019.
- Martin KL. Medscape Physician Lifestyle & Happiness Report 2019. Medscape website. https://www.medscape.com/slideshow/2019-lifestyle-happiness-6011057. Published January 9, 2019. Accessed August 14, 2019.
- Shoimer I, Patten S, Mydlarski P. Burnout in dermatology residents: a Canadian perspective. Br J Dermatol. 2018;178:270-271.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among us resident physicians. JAMA. 2018;320:1114-1130.
- Chaukos D, Chaed-Friedman E, Mehta D, et al. Risk and resilience factors associated with resident burnout. Acad Psychiatry. 2017;41:189-194.
- Ishak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;2:236-242.
- Tolentino J, Guo W, Ricca R, et al. What’s new in academic medicine: can we effectively address the burnout epidemic in healthcare? Int J Acad Med. 2017;3.
- Maslach C. Burnout: a multidimensional perspective. In: Schaufeli W, Maslach C, Marek T, eds. Professional Burnout: Recent Developments in Theory and Research. Washington, DC: Taylor and Francis; 1993:19-32.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed August 14, 2019.
- Martin KL. Medscape Physician Lifestyle & Happiness Report 2019. Medscape website. https://www.medscape.com/slideshow/2019-lifestyle-happiness-6011057. Published January 9, 2019. Accessed August 14, 2019.
- Shoimer I, Patten S, Mydlarski P. Burnout in dermatology residents: a Canadian perspective. Br J Dermatol. 2018;178:270-271.
- Dyrbye LN, Burke SE, Hardeman RR, et al. Association of clinical specialty with symptoms of burnout and career choice regret among us resident physicians. JAMA. 2018;320:1114-1130.
- Chaukos D, Chaed-Friedman E, Mehta D, et al. Risk and resilience factors associated with resident burnout. Acad Psychiatry. 2017;41:189-194.
- Ishak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;2:236-242.
- Tolentino J, Guo W, Ricca R, et al. What’s new in academic medicine: can we effectively address the burnout epidemic in healthcare? Int J Acad Med. 2017;3.
- Maslach C. Burnout: a multidimensional perspective. In: Schaufeli W, Maslach C, Marek T, eds. Professional Burnout: Recent Developments in Theory and Research. Washington, DC: Taylor and Francis; 1993:19-32.
Resident Pearl
- Reported techniques for preventing and coping with resident burnout include mindfulness and self-awareness, optimization of workplace factors, adequate sleep, social support, and physical exercise.
Ocular Complications of Atopic Dermatitis
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
Atopic dermatitis (AD) is a chronic inflammatory skin condition with a lifetime prevalence of 15% to 20% in industrialized countries.1 It affects both children and adults and is predominantly characterized by a waxing and waning course of eczematous skin lesions and pruritus. In recent years, there is increasing recognition that AD can present with extracutaneous findings. Large-scale epidemiologic studies have reported a notably higher prevalence of ophthalmic complications in the AD population compared to the general population, in a severity-dependent manner.2,3 Potential complications include blepharitis, keratoconjunctivitis, keratoconus, glaucoma, cataracts, retinal detachment, ophthalmic herpes simplex virus infections, and dupilumab-associated ocular complications.
The etiology of each ocular complication in the context of AD is complex and likely multifactorial. Intrinsic immune dysregulation, physical trauma from eye rubbing, AD medication side effects, and genetics all have been speculated to play a role.2 Some of these ocular complications have a chronic course, while others present with sudden onset of symptoms; many of them can result in visual impairment if undiagnosed or left untreated. This article reviews several of the most common ocular comorbidities associated with AD. We discuss the clinical presentation, pathophysiology, and management strategies for each condition.
Blepharitis
Blepharitis, an inflammatory condition of the eyelids, is estimated to affect more than 6% of patients with AD compared to less than 1% of the general population.2 Blepharitis can be classified as anterior or posterior, based on the anatomic location of the affected region relative to the lash margin. Affected individuals may experience pruritus and irritation of the eyelids, tearing, a foreign body or burning sensation, crusting of the eyelids, and photophobia.4 Anterior blepharitis commonly is due to staphylococcal disease, and posterior blepharitis is secondary to structural changes and obstruction of meibomian gland orifices.
Although the pathophysiology is not well defined, xerosis in atopic patients is accompanied by barrier disruption and transepidermal water loss, which promote eyelid skin inflammation.
The mainstay of therapy for atopic blepharitis consists of conventional lid hygiene regimens, such as warm compresses and gentle scrubbing of the lid margins to remove crust and debris, which can be done with nonprescription cleansers, pads, and baby shampoos. Acute exacerbations may require topical antibiotics (ie, erythromycin or bacitracin applied to the lid margins once daily), topical calcineurin inhibitors (ie, cyclosporine ophthalmic emulsion 0.05%), or low-potency topical corticosteroids (ie, fluorometholone 0.1% or loteprednol etabonate 0.5% ophthalmic suspensions).5 Due to potential side effects of medications, especially topical corticosteroids, patients should be referred to ophthalmologists for definitive diagnosis and treatment.
Keratoconjunctivitis
Atopic keratoconjunctivitis (AKC) is a noninfectious inflammatory condition of the cornea and conjunctiva that occurs in an estimated 25% to 42% of patients with AD.6,7 It frequently presents in late adolescence and has a peak incidence between 30 and 50 years of age.8 The symptoms of AKC include ocular pruritus, redness, ropy mucoid discharge, burning discomfort, photophobia, and blurring of vision. Corneal involvement can progress to corneal neovascularization and punctate or macroepithelial erosions and ulcerations, which increase the risk for corneal scarring and visual impairment.7
Keratoconjunctivitis is a complex inflammatory disease characterized by infiltration of the conjunctival epithelium by eosinophils, mast cells, and lymphocytes. On examination, patients frequently are found to have concurrent AD of the periorbital skin as well as papillary hypertrophy of the tarsal conjunctiva with accompanying fibrosis, which can lead to entropion (turning inward of the lid margins and lashes) in severe cases.7 Ophthalmic evaluation is strongly recommended for patients with AKC to control symptoms, to limit exacerbations, and to prevent sight-threatening inflammation leading to vision loss. Treatment can be challenging given the chronicity of the condition and may require multiple treatment arms. Conservative measures include cool compresses and treatment with ophthalmic eye drops containing antihistamines (ie, ketotifen 0.025% [available over-the-counter]) and mast cell stabilizers (ie, olopatadine ophthalmic solution 0.1%).8 Atopic keratoconjunctivitis exacerbations may require short-term use of topical steroids or calcineurin inhibitors, or systemic equivalents for refractory cases.6 Long-term maintenance therapy typically consists of proper eye hygiene and steroid-sparing agents that reduce ocular inflammation, such as topical cyclosporine and tacrolimus, neither of which are associated with increased intraocular pressure (IOP)(Figure 1).8 Cornea disease resulting from chronic conjunctival/lid microtrauma can be managed with soft or scleral contact lenses.
Keratoconus
Keratoconus is a noninflammatory ocular disorder characterized by progressive thinning and conelike protrusion of the cornea. The corneal topographic changes result in high irregular astigmatism and reduced visual acuity, which can manifest as image blurring or distortion (Figure 2).2,9 Multiple case series and controlled studies have reported a positive association between keratoconus and a history of atopic disease.10,11
The precise etiology of keratoconus in the context of AD is unclear and likely is multifactorial. Habitual eye rubbing from periocular pruritus and discomfort has been reported to be a notable contributor to keratoconus.12 In addition, intrinsic inflammation and imbalance of cytokines and proteases also may contribute to development of keratoconus.13
Keratoconus is a progressive condition that can severely impact vision, making it critical to diagnose patients before irreversible vision loss occurs. Individuals with risk factors, such as AD of the eyelids, history of eye rubbing, or family history of keratoconus, should be advised to receive routine vision screening for worsening astigmatism, especially during the first few decades of life when keratoconus progresses rapidly.
The conservative management for early keratoconus includes glasses and gas-permeable contact lenses for correction of visual acuity and astigmatism. For advanced keratoconus, scleral lenses often are prescribed. These large-diameter, gas-permeable lenses are designed to rest on the sclera and arch over the entire cornea.9 Alternatively, corneal collagen cross-linking is a newer technique that utilizes riboflavin and UVA irradiation to strengthen the corneal tissue. It has proven to be safe and effective in slowing or stopping the progression of keratoconus, particularly when treated at the early stage, and received US Food and Drug Administration approval in 2016.9
Glaucoma
Glaucoma is a well-known complication of AD and can lead to irreversible ocular hypertension and optic nerve damage. Corticosteroid use is a major risk factor for glaucoma, and the rise in IOP is thought to be due to increased aqueous outflow resistance.14
Multiple case reports have linked glaucoma to long-term use of potent topical corticosteroids in the facial and palpebral regions, which has been attributed to direct steroid contact and absorption by ocular tissues, as glaucoma rarely occurs with topical steroid application elsewhere on the body.15-17 Systemic steroids (ie, prednisolone) taken for more than 8 weeks also have been associated with a marked rise in IOP.18
Certain risk factors may predispose a steroid user to increased IOP, including existing open-angle glaucoma, diabetes mellitus, collagen disease, and high myopia.15,19 Steroid responders and younger individuals also demonstrate increased sensitivity to steroids.20
Given that glaucoma often is asymptomatic until advanced stages, early detection is the key for proper intervention. Periodic glaucoma screening by an ophthalmologist would be appropriate for known steroid responders, as well as patients with a prolonged history of topical steroid application in the palpebral region and systemic steroid use, family history of glaucoma, or known ocular pathology.21 Furthermore, patients with concurrent glaucoma and AD should be jointly managed by dermatology and ophthalmology, and systemic and topical corticosteroid use should be minimized in favor of alterative agents such as calcineurin inhibitors.22
In addition to steroid-induced glaucoma, intrinsic atopic glaucoma recently has been proposed as a clinical entity and is characterized by increased inflammatory cytokines—IL-8 and CCL2—in the aqueous humor and abnormal accumulation of fibers in corneoscleral meshwork.23
Cataracts
Cataracts are estimated to affect 8% to 25% of patients with AD.21,24 Unlike age-related cataracts, cataracts associated with AD are observed in adolescents and young adults in addition to the older population. The progression of lenticular opacity can rapidly occur and has been reported to coincide with AD flares.25,26
Patients with AD typically present with anterior or posterior subcapsular cataracts instead of nuclear and cortical cataracts, which are more common in the general population.27,28 Anterior subcapsular cataracts are more specific to AD, whereas posterior subcapsular cataracts are associated with both prolonged corticosteroid use and AD.26 Children generally are more sensitive to steroids than adults and may develop cataracts more rapidly and at lower concentrations.29
The pathophysiology of cataract formation and progression in the context of AD is multifactorial. Cataract patients with AD have compromised blood-retinal barrier integrity as well as increased oxidative damage in the lens.30,31 Genetics and blunt trauma from eye rubbing are thought to play a role, and the latter has been associated with faster progression of cataracts.28 In contrast, corticosteroid-induced cataracts likely are caused by transcriptional changes and disrupted osmotic balance in the lens fibers, which can lead to fiber rupture and lens opacification.26,32 Systemic corticosteroids show the strongest association with cataract development, but inhaled and topical steroids also have been implicated.26
Although cataracts can be surgically corrected, prevention is critical. Patients with early-onset periorbital AD, prolonged use of topical or systemic corticosteroids, and family history of cataracts should be routinely screened. Anterior and posterior subcapsular cataracts are diagnosed with red reflex examinations that can be readily performed by the primary care physician or ophthalmologist.33 Atopic dermatitis patients with cataracts should be advised to use calcineurin inhibitors and alternative treatments in place of corticosteroids.
Retinal Detachment
Retinal detachment (RD) is a serious complication of AD that can present in individuals younger than 35 years. The incidence of RD in patients with AD has been estimated to be 4% to 8%.34 Retinal detachment manifests with visual disturbances such as flashing lights, shadows, visual field defect, and blurring of vision, but also may occur in the absence of vision changes.35,36
Across multiple case series, patients who developed RD were consistently found to have AD in the facial or periorbital region and a history of chronic eye rubbing. Multiple patients also presented with concurrent proliferative vitreoretinopathy, lens subluxation, and/or cataracts.35,37 The mechanism for RD has been attributed to ocular contusion from vigorous eye rubbing, as fundus findings between traumatic and AD-associated RD are similarly characterized by tractional breaks in the retina at vitreous base borders.37
Avoidance of eye rubbing and optimized treatment of facial AD may help prevent RD in patients with AD. Furthermore, all patients with symptoms of RD should be immediately referred to ophthalmology for surgical repair.
Herpetic Ocular Disease
Ocular herpes simplex virus infections cause ocular pain and are associated with notable visual morbidity, as recurrences can result in irreversible corneal scarring and neovascularization. Two retrospective case-control studies independently reported that individuals with a history of AD are at greater risk for herpetic ocular disease compared to age-matched controls.38,39 Furthermore, atopic disease is associated with higher recurrence rates and slower regeneration of the corneal epithelium.40
These findings suggest that AD patients with a history of recurrent herpetic ocular diseases should be closely monitored and treated with antiviral prophylaxis and/or topical corticosteroids, depending on the type of keratitis (epithelial or stromal).40 Furthermore, active ocular herpetic infections warrant urgent referral to an ophthalmologist.
Dupilumab-Associated Ocular Complications
Dupilumab, a monoclonal antibody that blocks IL-4 and IL-13 signaling, is the first biologic therapy to be approved for treatment of moderate to severe AD. Prior clinical trials have described a higher incidence of anterior conjunctivitis in dupilumab-treated AD patients (5%–28%) compared to placebo (2%–11%).41 Of note, the incidence may be as high as 70%, as reported in a recent case series.42 Interestingly, independent trials assessing dupilumab treatment in asthma, nasal polyposis, and eosinophilic esophagitis patients did not observe a higher incidence of conjunctivitis in dupilumab-treated patients compared to placebo, suggesting an AD-specific mechanism.43
Prominent features of dupilumab-associated conjunctivitis include hyperemia of the conjunctiva and limbus, in addition to ocular symptoms such as tearing, burning, and bilateral decrease in visual acuity. Marked reduction of conjunctival goblet cells has been reported.44 In addition to conjunctivitis, blepharitis also has been reported during dupilumab treatment.45
Standardized treatment guidelines for dupilumab-associated ocular complications have not yet been established. Surprisingly, antihistamine eye drops appear to be inefficacious in the treatment of dupilumab-associated conjunctivitis.41 However, the condition has been successfully managed with topical steroids (fluorometholone ophthalmic suspension 0.1%) and tacrolimus ointment 0.03%.41 Lifitegrast, an anti-inflammatory agent approved for chronic dry eye, also has been suggested as a treatment option for patients refractory to topical steroids.45 Alternatively, cessation of dupilumab could be considered in AD patients who experience severe ocular complications. Atopic dermatitis patients taking dupilumab who have any concerning signs for ocular complications should be referred to an ophthalmologist for further diagnosis and management.
Conclusion
Practicing dermatologists likely will encounter patients with concurrent AD and ocular complications. Although eye examinations are not routinely performed in the care of AD patients, dermatologists can proactively inquire about ocular symptoms and monitor patients longitudinally. Early diagnosis and treatment of these ocular conditions can prevent vision loss in these patients. Furthermore, symptomatic control of AD and careful consideration of the side-effect profiles of medications can potentially reduce the incidence of ocular complications in individuals with AD.
Patients with visual concerns or risk factors, such as a history of vigorous eye rubbing or chronic corticosteroid use, should be jointly managed with an ophthalmologist for optimized care. Moreover, acute exacerbations of ocular symptoms and visual deterioration warrant urgent referral to ophthalmology.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
- Weidinger S, Beck LA, Bieber T, et al. Atopic dermatitis. Nat Rev Dis Primers. 2018;4:1.
- Govind K, Whang K, Khanna R, et al. Atopic dermatitis is associated with increased prevalence of multiple ocular comorbidities. J Allergy Clin Immunol Pract. 2019;7:298-299.
- Thyssen JP, Toft PB, Halling-Overgaard AS, et al. Incidence, prevalence, and risk of selected ocular disease in adults with atopic dermatitis. J Am Acad Dermatol. 2017;77:280-286.e281.
- Putnam CM. Diagnosis and management of blepharitis: an optometrist’s perspective. Clin Optom (Auckl). 2016;8:71-78.
- Amescua G, Akpek EK, Farid M, et al. Blepharitis Preferred Practice Pattern®. Ophthalmology. 2019;126:P56-P93.
- Bielory B, Bielory L. Atopic dermatitis and keratoconjunctivitis. Immunol Allergy Clin North Am. 2010;30:323-336.
- Guglielmetti S, Dart JK, Calder V. Atopic keratoconjunctivitis and atopic dermatitis. Curr Opin Allergy Clin Immunol. 2010;10:478-485.
- Chen JJ, Applebaum DS, Sun GS, et al. Atopic keratoconjunctivitis: a review. J Am Acad Dermatol. 2014;70:569-575.
- Andreanos KD, Hashemi K, Petrelli M, et al. Keratoconus treatment algorithm. Ophthalmol Ther. 2017;6:245-262.
- Rahi A, Davies P, Ruben M, et al. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761-764.
- Gasset AR, Hinson WA, Frias JL. Keratoconus and atopic diseases. Ann Ophthalmol. 1978;10:991-994.
- Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834-836.
- Galvis V, Sherwin T, Tello A, et al. Keratoconus: an inflammatory disorder? Eye (Lond). 2015;29:843-859.
- Clark AF, Wordinger RJ. The role of steroids in outflow resistance. Exp Eye Res. 2009;88:752-759.
- Daniel BS, Orchard D. Ocular side-effects of topical corticosteroids: what a dermatologist needs to know. Australas J Dermatol. 2015;56:164-169.
- Garrott HM, Walland MJ. Glaucoma from topical corticosteroids to the eyelids. Clin Exp Ophthalmol. 2004;32:224-226.
- Aggarwal RK, Potamitis T, Chong NH, et al. Extensive visual loss with topical facial steroids. Eye (Lond). 1993;7(pt 5):664-666.
- Mandapati JS, Metta AK. Intraocular pressure variation in patients on long-term corticosteroids. Indian Dermatol Online J. 2011;2:67-69.
- Jones R 3rd, Rhee DJ. Corticosteroid-induced ocular hypertension and glaucoma: a brief review and update of the literature. Curr Opin Ophthalmol. 2006;17:163-167.
- Tamagawa-Mineoka R, Yasuoka N, Ueta M, et al. Influence of topical steroids on intraocular pressure in patients with atopic dermatitis. Allergol Int. 2018;67:388-391.
- Bercovitch L. Screening for ocular complications in atopic dermatitis. Arch Dermatol. 2011;147:588-589.
- Abramovits W, Hung P, Tong KB. Efficacy and economics of topical calcineurin inhibitors for the treatment of atopic dermatitis. Am J Clin Dermatol. 2006;7:213-222.
- Takakuwa K, Hamanaka T, Mori K, et al. Atopic glaucoma: clinical and pathophysiological analysis. J Glaucoma. 2015;24:662-668.
- Haeck IM, Rouwen TJ, Timmer-de Mik L, et al. Topical corticosteroids in atopic dermatitis and the risk of glaucoma and cataracts. J Am Acad Dermatol. 2011;64:275-281.
- Amemiya T, Matsuda H, Uehara M. Ocular findings in atopic dermatitis with special reference to the clinical features of atopic cataract. Ophthalmologica. 1980;180:129-132.
- Tatham A. Atopic dermatitis, cutaneous steroids and cataracts in children: two case reports. J Med Case Rep. 2008;2:124.
- Chew M, Chiang PP, Zheng Y, et al. The impact of cataract, cataract types, and cataract grades on vision-specific functioning using Rasch analysis. Am J Ophthalmol. 2012;154:29-38.
- Nagaki Y, Hayasaka S, Kadoi C. Cataract progression in patients with atopic dermatitis. J Cataract Refract Surg. 1999;25:96-99.
- Kaye LD, Kalenak JW, Price RL, et al. Ocular implications of long-term prednisone therapy in children. J Pediatr Ophthalmol Strabismus. 1993;30:142-144.
- Matsuo T, Saito H, Matsuo N. Cataract and aqueous flare levels in patients with atopic dermatitis. Am J Ophthalmol. 1997;124:36-39.
- Namazi MR, Handjani F, Amirahmadi M. Increased oxidative activity from hydrogen peroxide may be the cause of the predisposition to cataracts among patients with atopic dermatitis. Med Hypotheses. 2006;66:863-864.
- James ER. The etiology of steroid cataract. J Ocul Pharmacol Ther. 2007;23:403-420.
- Lambert SR, Teng JMC. Assessing whether the cataracts associated with atopic dermatitis are associated with steroids or inflammatory factors. JAMA Ophthalmol. 2018;136:918-919.
- Sasoh M, Mizutani H, Matsubara H, et al. Incidence of retinal detachment associated with atopic dermatitis in Japan: review of cases from 1992 to 2011. Clin Ophthalmol. 2015;9:1129-1134.
- Yoneda K, Okamoto H, Wada Y, et al. Atopic retinal detachment. report of four cases and a review of the literature. Br J Dermatol. 1995;133:586-591.
- Gnana Jothi V, McGimpsey S, Sharkey JA, et al. Retinal detachment repair and cataract surgery in patients with atopic dermatitis. Eye (Lond). 2017;31:1296-1301.
- Oka C, Ideta H, Nagasaki H, et al. Retinal detachment with atopic dermatitis similar to traumatic retinal detachment. Ophthalmology. 1994;101:1050-1054.
- Prabriputaloong T, Margolis TP, Lietman TM, et al. Atopic disease and herpes simplex eye disease: a population-based case-control study. Am J Ophthalmol. 2006;142:745-749.
- Borkar DS, Gonzales JA, Tham VM, et al. Association between atopy and herpetic eye disease: results from the pacific ocular inflammation study. JAMA Ophthalmol. 2014;132:326-331.
- Rezende RA, Hammersmith K, Bisol T, et al. Comparative study of ocular herpes simplex virus in patients with and without self-reported atopy. Am J Ophthalmol. 2006;141:1120-1125.
- Wollenberg A, Ariens L, Thurau S, et al. Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment. J Allergy Clin Immunol Pract. 2018;6:1778-1780.e1.
- Ivert LU, Wahlgren CF, Ivert L, et al. Eye complications during dupilumab treatment for severe atopic dermatitis. Acta Derm Venereol. 2019;99:375-378.
- Akinlade B, Guttman-Yassky E, de Bruin-Weller M, et al. Conjunctivitis in dupilumab clinical trials [published online March 9, 2019]. Br J Dermatol. doi:10.1111/bjd.17869.
- Bakker DS, Ariens LFM, van Luijk C, et al. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019;180:1248-1249.
- Zirwas MJ, Wulff K, Beckman K. Lifitegrast add-on treatment for dupilumab-induced ocular surface disease (DIOSD): a novel case report. JAAD Case Rep. 2019;5:34-36.
Practice Points
- Atopic dermatitis (AD) is associated with various ocular comorbidities that can result in permanent vision loss if untreated.
- Timely recognition of ocular complications in AD patients is critical, and dermatologists should proactively inquire about ocular symptoms in the review of systems.
- Patients with ocular symptoms should be jointly managed with ophthalmology.
Allergic Contact Dermatitis From Sorbitans in Beer and Bread
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
Sorbitan sesquioleate (SSO), sorbitan monooleate (SMO), and related compounds are increasingly recognized contact allergens. Sorbitan sesquioleate and SMO are nonionic emulsifying agents derived from sorbitol.1
Sorbitan sesquioleate, SMO, and other sorbitol derivatives are used as emulsifiers and dispersing agents in cosmetics, topical medications, topical emollients, produce, and other commercial products. Related compounds also are found in foods such as apples, berries, cherries, and sucrose-free cakes and cookies.1 We present a case of allergic contact dermatitis (ACD) with positive patch testing to sorbitans and clinical correlation with beer and bread exposure.
Case Report
A 62-year-old man presented with a persistent pruritic rash of 6 months’ duration. Erythematous eczematous papules and plaques were observed on the face, neck, chest, abdomen, back, and upper and lower extremities, affecting approximately 60% of the body surface area. His current list of medications was reviewed and included a multivitamin, fish oil, and vitamin C. A punch biopsy revealed spongiotic dermatitis with eosinophils. Patch testing using the North American Contact Dermatitis Group Standard Series with supplemental allergens found in toiletries revealed a positive reaction to SSO and SMO that was persistent at 48 and 96 hours. Notably, patch testing for sodium benzoate, nickel, potassium dichromate, and balsam of Peru were negative. Investigation into the personal care products the patient used identified the presence of sorbitol solution in Vanicream bar soap and Vanicream moisturizing cream (Pharmaceutical Specialties Inc). These products were started after the development of the rash and were discontinued after positive patch testing, but the patient continued to experience the eruption with no improvement.
Retrospectively, the patient was able to correlate exacerbations with drinking beer and eating sandwiches. He habitually ate a sandwich on the same type of bread every single day and enjoyed the same brand of beer 2 to 4 times per week without much variation. To limit allergens, the patient gave up the daily sandwich and avoided bread altogether, noting remarkable clinical improvement over a few weeks. Later, he described even more improvement while on a trip where he did not have access to his usual beer. The eruption recurred when he returned home and excessively indulged in his favorite beer. He also noted recurrence with exposure to certain breads. No new lesions developed with avoidance of beer and bread, and he had less than 1% body surface area involvement at 2-month follow-up and 0% involvement at 1 year. For educational purposes, follow-up patch testing was performed using Vanicream sorbitol solution and the specific beer and bread the patient consumed. The Vanicream solution was obtained from the manufacturer. The beer was placed directly onto a test disc. The bread was moistened with a drop of saline and then placed directly onto a test disc. All were negative at 48 and 96 hours.
Comment
Sorbitol Ingredients
We report a case of systemic ACD with a positive patch test to sorbitans that was exacerbated with consumption of beer and bread and resolved with avoidance of these products. Although it was determined that the patient used personal care products containing a sorbitol solution, discontinuation did not result in clinical improvement. Sorbitol, sorbitans, and sorbitol derivatives are not commonly reported in the ingredient lists of foods such as beer and bread. Both beer and bread are created with the addition of yeast cultures, for fermentation in beer and for leavening in bread. Sorbitol is used as an osmotic stabilizer in the preparation of yeast strains2 and also is a by-product of fermentation by certain bacteria3 found in beer. Additionally, review of commercially available preparations of baker’s and brewer’s yeasts, such as Fleischmann’s and Red Star, list sorbitan monostearate in the ingredients.4-7 We propose that trace amounts are present in the yeast preparations for brewing and baking.
In this case, the offending beer and bread were locally made products (Abita Beer, Covington, Louisiana; Leidenheimer Bread, New Orleans, Louisiana). Both companies were unable to share their yeast sources, limiting our ability to confirm the use of sorbitol in their preparation. We hypothesize that if sorbitol is commonly used in yeast culture preparation and can be a by-product of fermentation, then it is present in trace amounts in many beers and breads and is not specific to these two products.
Contact Allergy
There are few prior reports of ACD due to beer. A case series in 1969 described 4 patients with positive patch testing to ethanol and alcohol by-products and clinical resolution with avoidance of alcohol.8 Another case from 1985 described ACD to beer where patch testing was positive to the beer itself.9 Other published cases of cutaneous reactions to beer demonstrated immediate-type hypersensitivity resulting from both ingestion and skin contact, which is thought to be caused by IgE antibodies to malt and barley proteins.10,11
It is important to distinguish between systemic ACD and oral allergy syndrome (OAS). Although the defining features and criteria for diagnosing OAS have not been officially established, OAS is an IgE-mediated immune reaction commonly described as itching, tingling, or swelling, usually confined to the oral cavity after recent consumption of foods such as raw fruits, vegetables, and nuts.12 Oral allergy syndrome is treated with antihistamines and avoidance of known food allergens. In comparison, ACD is a type IV hypersensitivity, delayed cell-mediated reaction, commonly presenting with widespread rash.
Occupational contact dermatitis is common in bakers and food handlers and is more often irritant than allergic. Several relevant allergens have been identified in these groups13,14 and do not include sorbitans; our patient tested positive to both SSO and SMO. Sorbitan sesquioleate and SMO have been increasingly recognized as contact allergens over the last several years, both as standalone allergens and as potential cross-reactors.1 Sorbitan sesquioleate, SMO, and other sorbitol derivatives are found in cosmetics, topical and oral medications, topical emollients, produce, and other commercial products, including but not limited to topical clindamycin, topical metronidazole, topical ketoconazole, tazarotene cream 0.05% and 0.1%, toothpastes, acetaminophen maximum strength liquid, apples, berries, and sucrose-free cakes and cookies.1,15,16
In 2014, a study evaluated 12 oral antihistamines as potential sources for systemic contact allergens; 55% of these 12 oral antihistamine preparations included at least 1 of 10 allergen groups specifically identified. The sorbitans and sorbitol derivatives group ranked highest among the group of allergens found listed in these oral medications.17
Most patients found to have a contact allergy to the products containing SSO, SMO, or sorbitol derivatives reported notable improvement with discontinuation and change to sorbitol-free product use.1,18 It should be noted that SSO is added as an emulsifier to many of the fragrances used for patch testing. A positive patch test to fragrance mix without concomitant sorbitan testing may incorrectly diagnose the allergen.19
Patients with atopic dermatitis, particularly those with a filaggrin mutation, are at increased risk for ACD to sorbitans due to a compromised skin barrier and frequent use of topical steroids. In one study, 75% of patients (n=12) with a positive patch test to SSO were using a topical steroid emulsified with sorbitol or sorbitan derivatives.19
Conclusion
Sorbitan sesquioleate and SMO are increasingly relevant contact allergens. Sorbitol and related substances have been identified in numerous products and may be present in yeast-fermented and leavened goods. When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread, in addition to inventorying other dietary preferences, prescription and over-the-counter medications, and personal care products. We suggest dietary considerations only if topical exposures have been eliminated and the rash has not improved.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
- Asarch A, Scheinman PL. Sorbitan sesquioleate: an emerging contact allergen. Dermatitis. 2008;19:339-341.
- Lundblad V, Struhl K. Yeast. In: Adelman K, Ausubel F, Brent R, et al. Current Protocols in Molecular Biology, Supplement 64. New York, NY: John Wiley & Sons, Inc; 2008:13.0.1-13.0.4. https://onlinelibrary.wiley.com. Accessed August 19, 2019.
- Spitaels F, Wieme A, Balzarini T, et al. Gluconobacter cerevisiae sp. nov., isolated from the brewery environment. Int J Sys Evol Microbiol. 2014;64(pt 4):1134-1141.
- Fleischmann’s, n.d. Product Label for Rapid Rise Instant Yeast. Memphis, TN. 2017.
- Fleischmann’s, n.d. Product Label for Active Dry Yeast. Memphis, TN. 2017.
- Red Star, n.d. Product Label for Quick-Rise. Milwaukee, WI. 2017.
- Red Star, n.d. Product Label for Platinum Superior Baking Yeast. Milwaukee, WI. 2017.
- Fregert S, Groth O, Hjorth N, et al. Alcohol dermatitis. Acta Derm Venereol. 1969;49:493-497.
- Clarke P. Contact dermatitis due to beer. Med J Aust. 1985;143:92.
- Koelemij I, Van Zuuren EJ. Contact urticaria from beer. Clin Exp Dermatol. 2014;39:395-407.
- Santucci B, Cristaudo A, Cannistraci C, et al. Urticaria from beer in 3 patients. Contact Dermatitis. 1996;34:368.
- Kohn JB. What is oral allergy syndrome? J Acad Nutr Diet. 2017;117:988.
- Vincenzi C, Stinchi C, Ricci C, et al. Contact dermatitis due to an emulsifying agent in a baker. Contact Dermatitis. 1995;32:57.
- Nethercott JR, Holness DL. Occupational dermatitis in food handlers and bakers. J Am Acad Dermatol. 1989;21:485-490.
- Pereira F, Cunha H, Dias M. Contact dermatitis due to emulsifiers. Contact Dermatitis. 1997;36:114.
- Gao Z, Maurousset L, Lemoine R, et al. Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol. 2003;131:1566-1575.
- McEnery-Stonelake M, Silvestri DL. Contact allergens in oral antihistamines. Dermatitis. 2014;25:83-88.
- Asarch A, Scheinman PL. Sorbitan sesquioleate, a common emulsifier in topical steroids, is an important contact allergen. Dermatitis. 2008;19:323-327.
- Hald M, Menné T, Johansen JD, et al. Allergic contact dermatitis caused by sorbitan sesquioleate imitating severe glove dermatitis in a patient with filaggrin mutation. Contact Dermatitis. 2013;69:311-322.
Practice Points
- Sorbitan sesquioleate (SSO) and sorbitan monooleate (SMO) are increasingly relevant contact allergens that may be present in yeast-fermented and leavened products.
- When patch testing is positive to SSO and SMO, the dermatologist should inquire about dietary habits with specific attention to beer and bread.
- Consider elimination of beer, bread, and other leavened products when rash persists after avoidance of topical exposures.