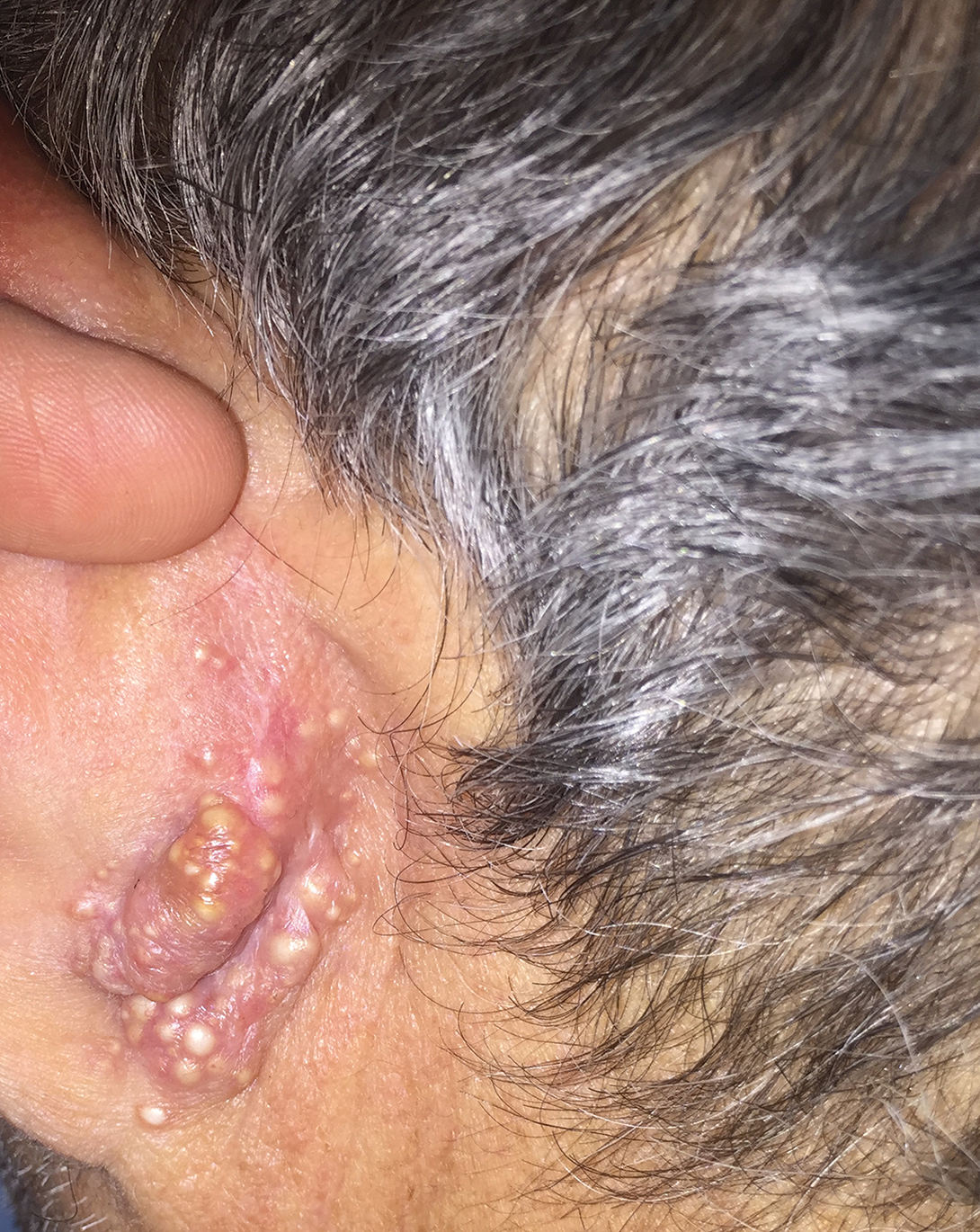
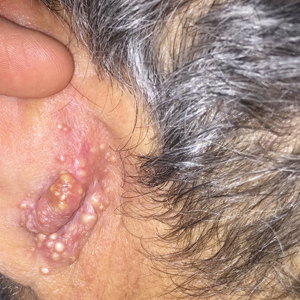
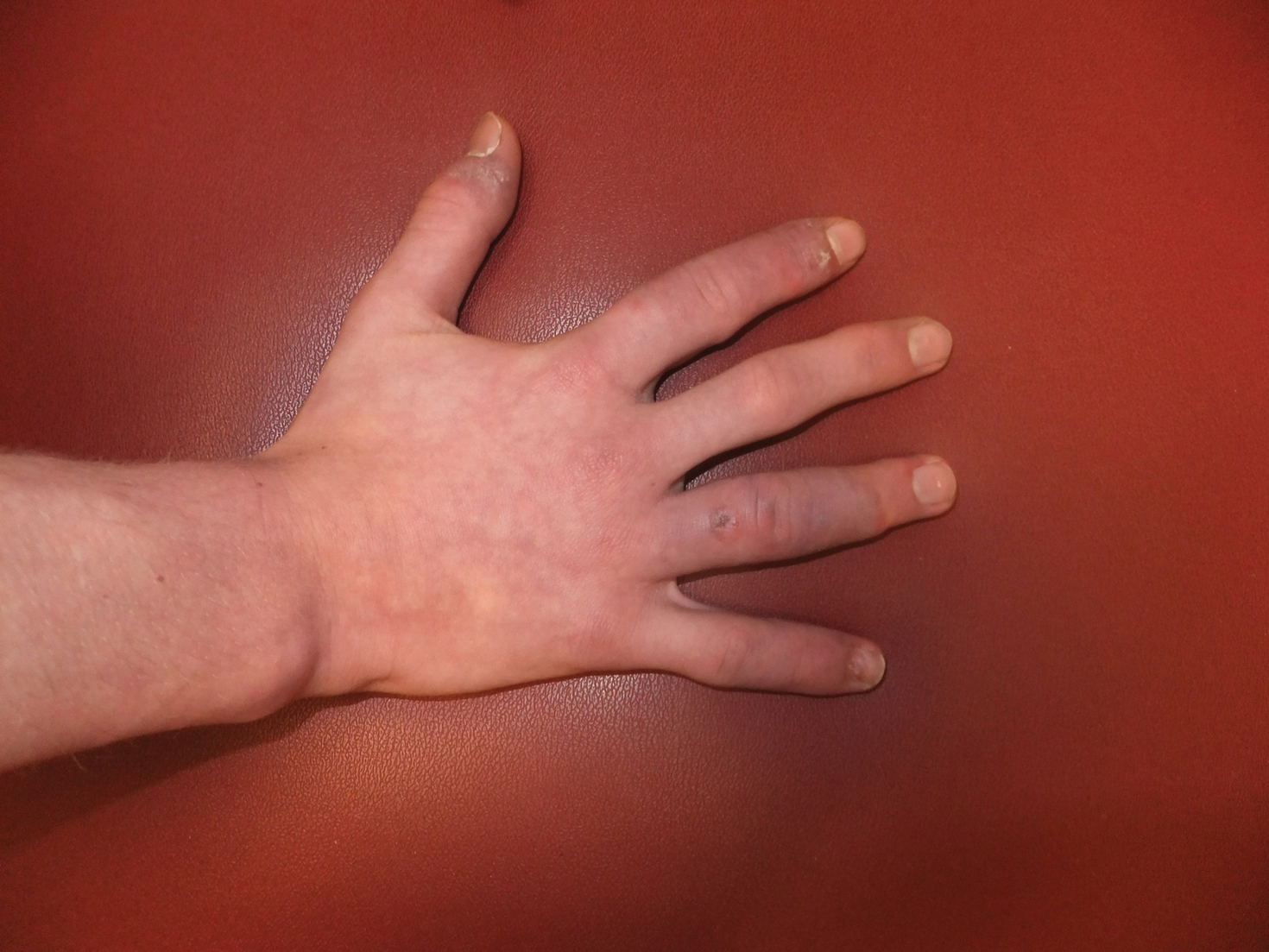
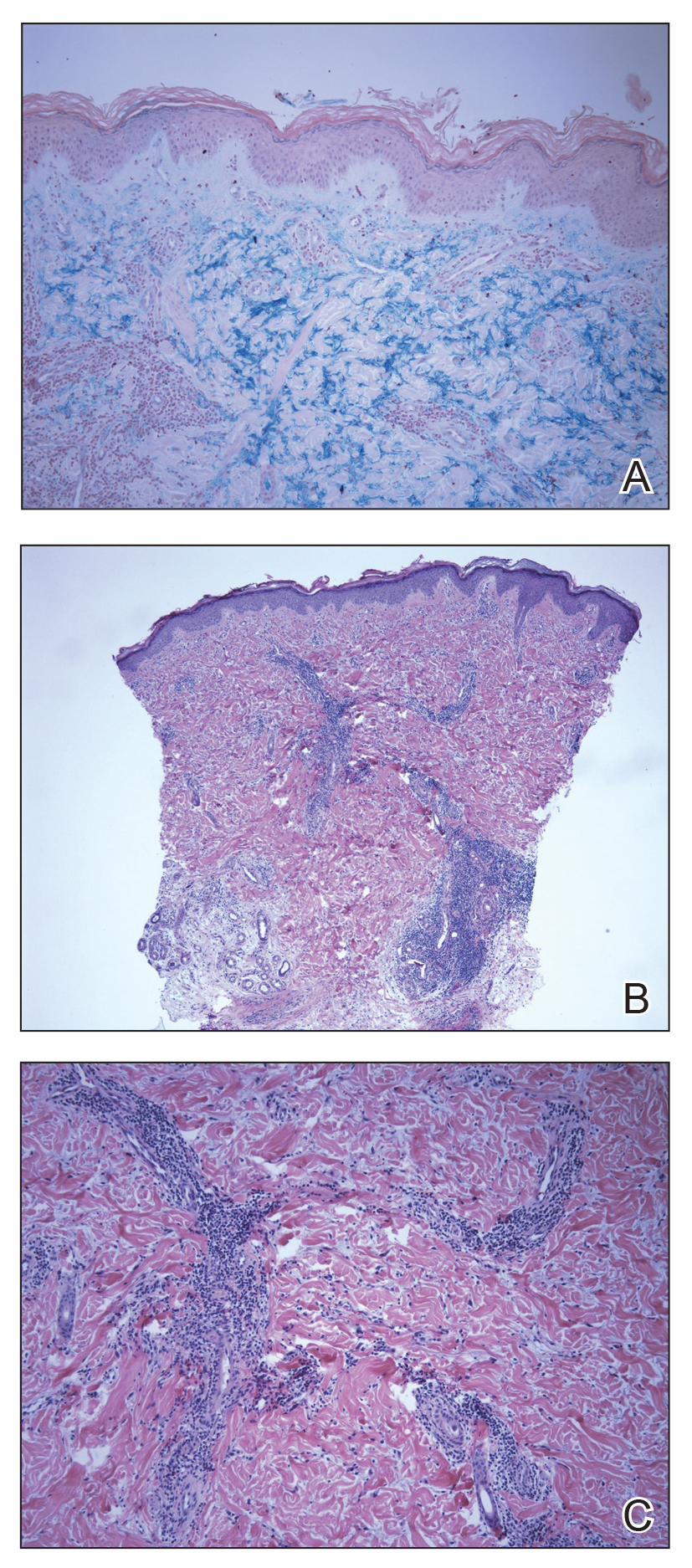
User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Noninvasive Imaging Tools in Dermatology
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
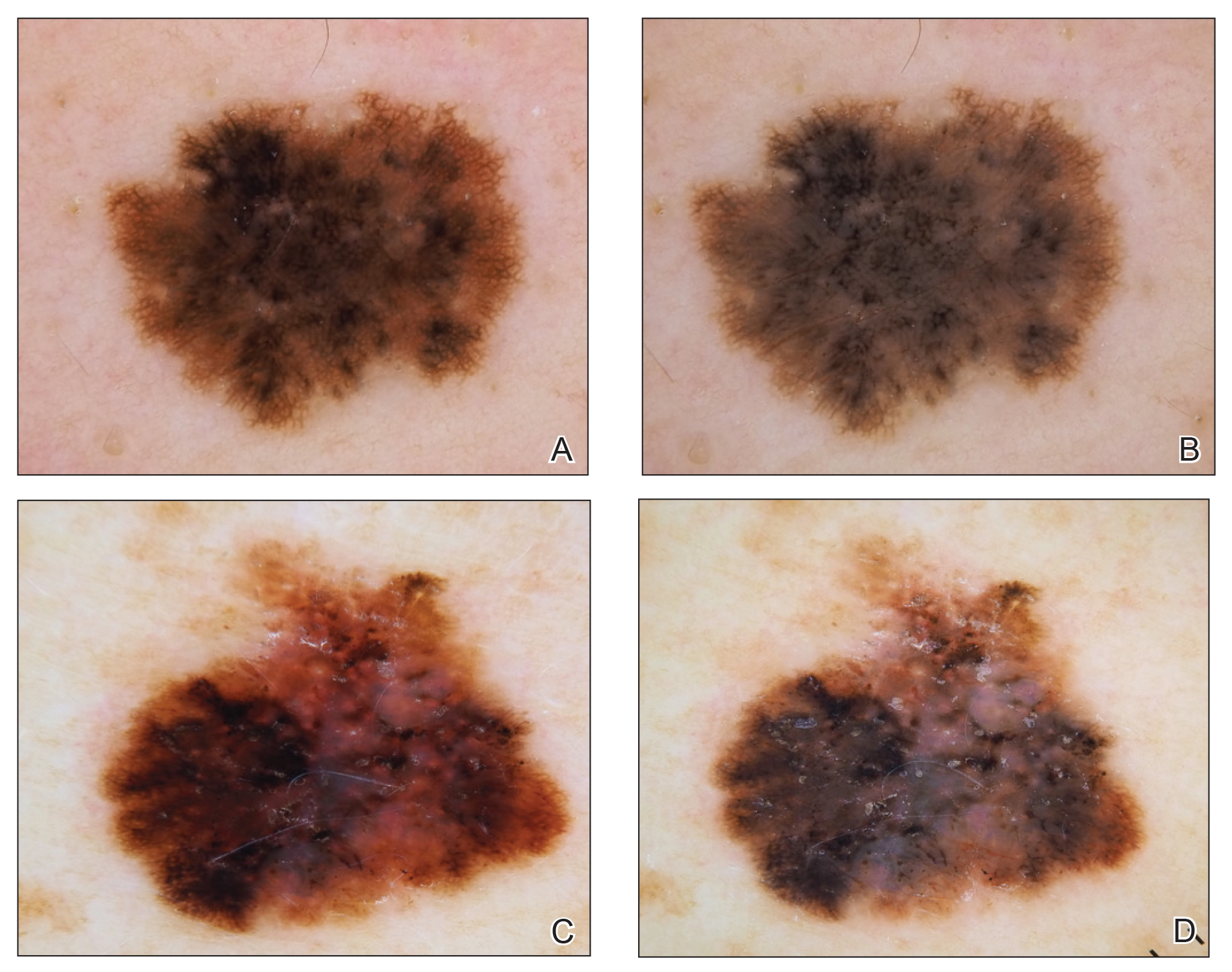
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
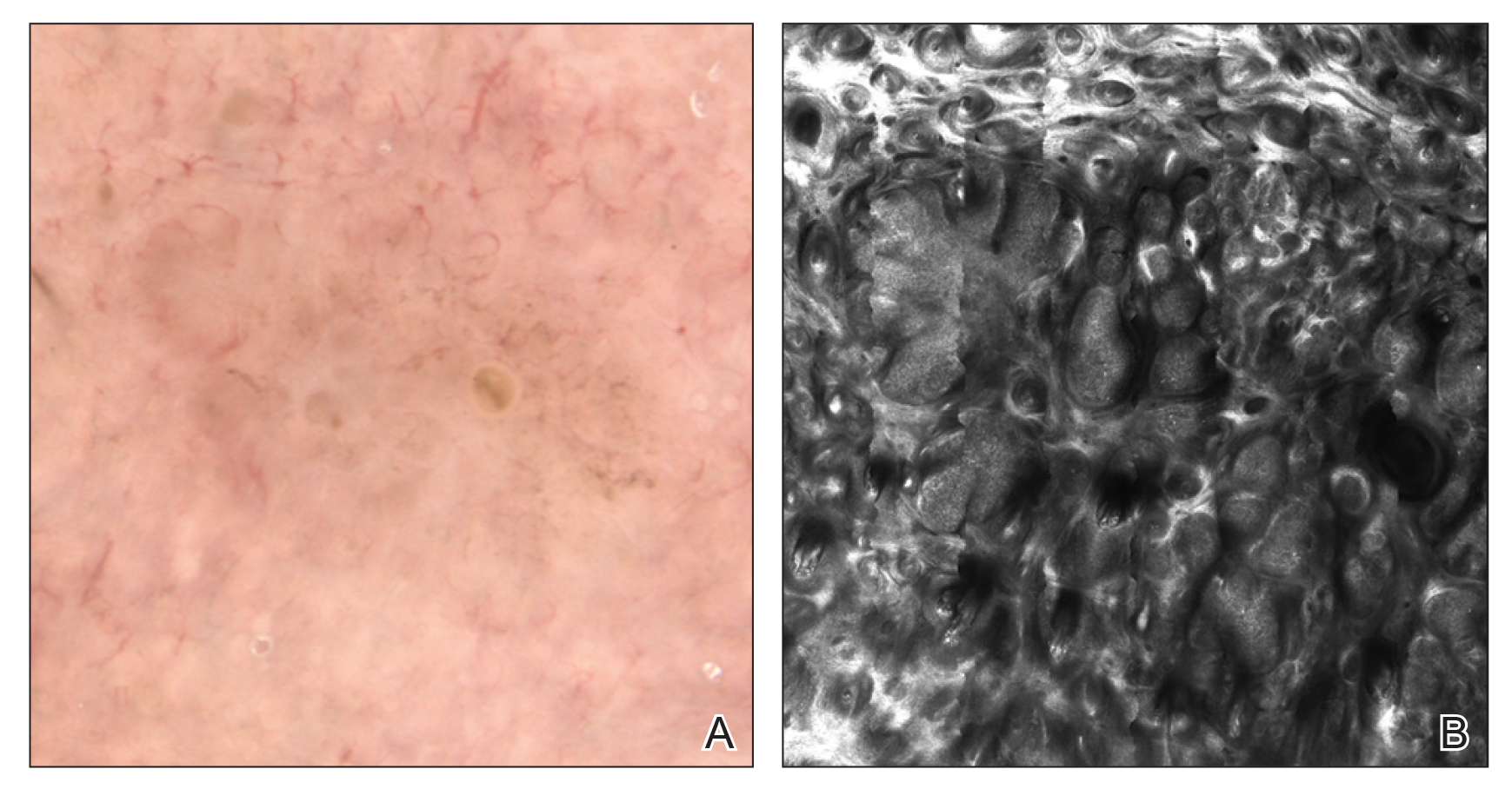
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
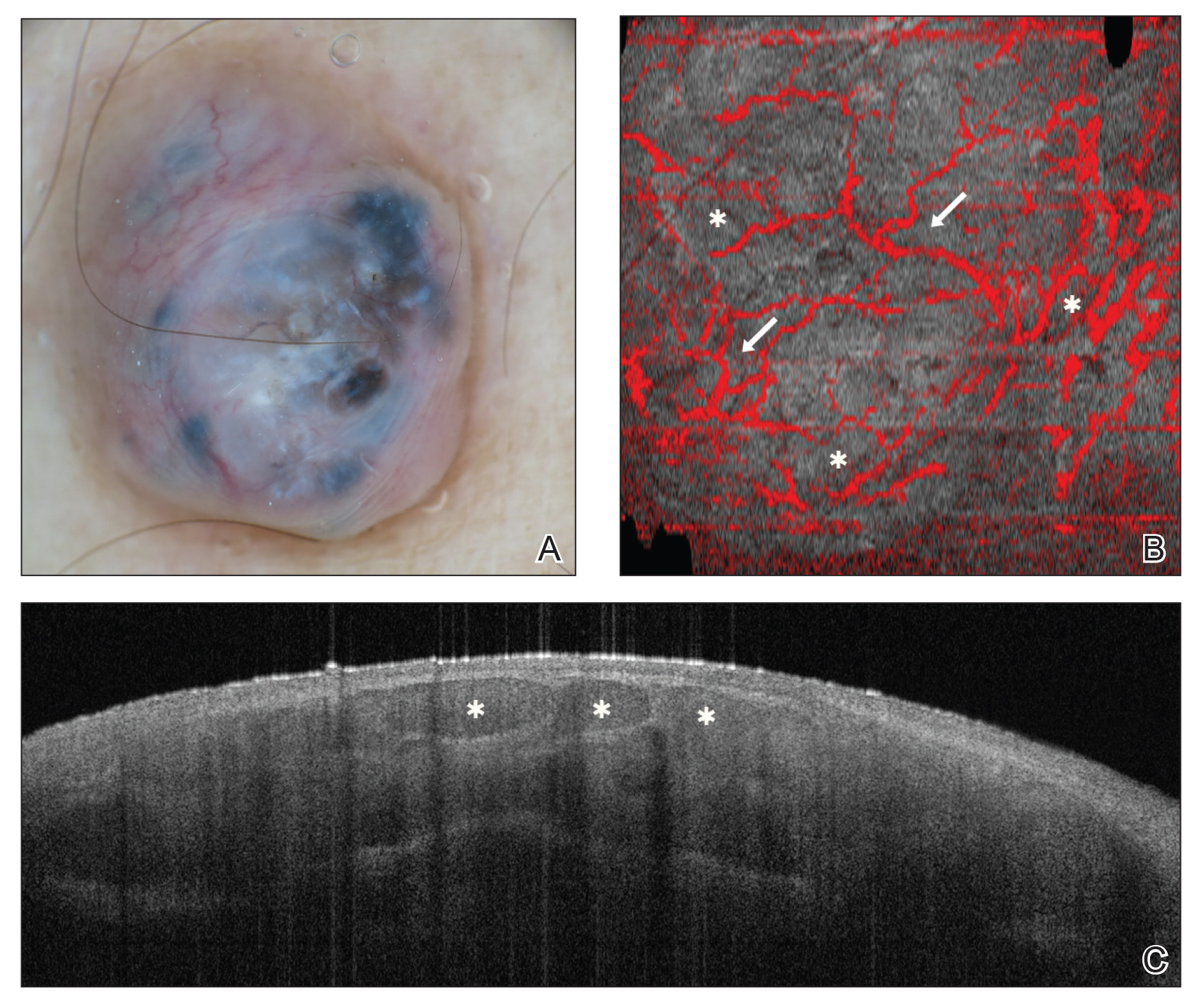
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
Traditionally, diagnosis of skin disease relies on clinical inspection, often followed by biopsy and histopathologic examination. In recent years, new noninvasive tools have emerged that can aid in clinical diagnosis and reduce the number of unnecessary benign biopsies. Although there has been a surge in noninvasive diagnostic technologies, many tools are still in research and development phases, with few tools widely adopted and used in regular clinical practice. In this article, we discuss the use of dermoscopy, reflectance confocal microscopy (RCM), and optical coherence tomography (OCT) in the diagnosis and management of skin disease.
Dermoscopy
Dermoscopy, also known as epiluminescence light microscopy and previously known as dermatoscopy, utilizes a ×10 to ×100 microscope objective with a light source to magnify and visualize structures present below the skin’s surface, such as melanin and blood vessels. There are 3 types of dermoscopy: conventional nonpolarized dermoscopy, polarized contact dermoscopy, and nonpolarized contact dermoscopy (Figure 1). Traditional nonpolarized dermoscopy requires a liquid medium and direct contact with the skin, and it relies on light reflection and refraction properties.1 Cross-polarized light sources allow visualization of deeper structures, either with or without a liquid medium and contact with the skin surface. Although there is overall concurrence among the different types of dermoscopy, subtle differences in the appearance of color, features, and structure are present.1
Dermoscopy offers many benefits for dermatologists and other providers. It can be used to aid in the diagnosis of cutaneous neoplasms and other skin diseases. Numerous low-cost dermatoscopes currently are commercially available. The handheld, easily transportable nature of dermatoscopes have resulted in widespread practice integration. Approximately 84% of attending dermatologists in US academic settings reported using dermoscopy, and many refer to the dermatoscope as “the dermatologist’s stethoscope.”2 In addition, 6% to 15% of other US providers, including family physicians, internal medicine physicians, and plastic surgeons, have reported using dermoscopy in their clinical practices. Limitations of dermoscopy include visualization of the skin surface only and not deeper structures within the tissue, the need for training for adequate interpretation of dermoscopic images, and lack of reimbursement for dermoscopic examination.3
Many dermoscopic structures that correspond well with histopathology have been described. Dermoscopy has a sensitivity of 79% to 96% and specificity of 69% to 99% in the diagnosis of melanoma.4 There is variable data on the specificity of dermoscopy in the diagnosis of melanoma, with one meta-analysis finding no statistically significant difference in specificity compared to naked eye examination,5 while other studies report increased specificity and subsequent reduction in biopsy of benign lesions.6,7 Dermoscopy also can aid in the diagnosis of keratinocytic neoplasms, and dermoscopy also results in a sensitivity of 78.6% to 100% and a specificity of 53.8% to 100% in the diagnosis of basal cell carcinoma (BCC).8 Limitations of dermoscopy include false-positive diagnoses, commonly seborrheic keratoses and nevi, resulting in unnecessary biopsies, as well as false-negative diagnoses, commonly amelanotic and nevoid melanoma, resulting in delays in skin cancer diagnosis and resultant poor outcomes.9 Dermoscopy also is used to aid in the diagnosis of inflammatory and infectious skin diseases, as well as scalp, hair, and nail disorders.10
Reflectance Confocal Microscopy
Reflectance confocal microscopy utilizes an 830-nm laser to capture horizontal en face images of the skin with high resolution. Different structures of the skin have varying indices of refraction: keratin, melanin, and collagen appear bright white, while other components appear dark, generating black-and-white RCM images.11 Currently, there are 2 reflectance confocal microscopes that are commercially available in the United States. The Vivascope 1500 (Caliber ID) is the traditional model that captures 8×8-mm images, and the Vivascope 3000 (Caliber ID) is a smaller handheld model that captures 0.5×0.5-mm images. The traditional model provides the advantages of higher-resolution images and the ability to capture larger surface areas but is best suited to image flat areas of skin to which a square window can be adhered. The handheld model allows improved contact with the varying topography of skin; does not require an adhesive window; and can be used to image cartilaginous, mucosal, and sensitive surfaces. However, it can be difficult to correlate individual images captured by the handheld RCM with the location relative to the lesion, as it is exquisitely sensitive to motion and also is operator dependent. Although complex algorithms are under development to stitch individual images to provide better correlation with the geography of the lesion, such programs are not yet widely available.12
Reflectance confocal microscopy affords many benefits for patients and providers. It is noninvasive and painless and is capable of imaging in vivo live skin as compared to clinical examination and dermoscopy, which only allow for visualization of the skin’s surface. Reflectance confocal microscopy also is time efficient, as imaging of a single lesion can be completed in 10 to 15 minutes. This technology generates high-resolution images, and RCM diagnosis has consistently demonstrated high sensitivity and specificity when compared to histopathology.13 Additionally, RCM imaging can spare biopsy and resultant scarring on cosmetically sensitive areas. Recently, RCM imaging of the skin has been granted Category I Current Procedural Terminology reimbursement codes that allow provider reimbursement and integration of RCM into daily practice14; however, private insurance coverage in the United States is variable. Limitations of RCM include a maximum depth of 200 to 300 µm, high cost to procure a reflectance confocal microscope, and the need for considerable training and practice to accurately interpret grayscale en face images.15
There has been extensive research regarding the use of RCM in the evaluation of cutaneous neoplasms and other skin diseases. Numerous features and patterns have been identified and described that correspond with different skin diseases and correspond well with histopathology (Figure 2).13,16,17 Reflectance confocal microscopy has demonstrated consistently high accuracy in the diagnosis of melanocytic lesions, with a sensitivity of 93% to 100% and a specificity of 75% to 99%.18-21 Reflectance confocal microscopy is especially useful in the evaluation of clinically or dermoscopically equivocal pigmented lesions due to greater specificity, resulting in a reduction of unnecessary biopsies.22,23 It also has high accuracy in the diagnosis of keratinocytic neoplasms, with a sensitivity of 82% to 100% and a specificity of 78% to 97% in the diagnosis of BCC,24 and a sensitivity of 74% to 100% and specificity of 78% to 100% in the diagnosis of squamous cell carcinoma (SCC).25,26 Evaluation of SCC and actinic keratosis (AK) using RCM may be limited by considerable hyperkeratosis and ulceration. In addition, it can be challenging to differentiate AK and SCC on RCM, and considerable expertise is required to accurately grade cytologic and architectural atypia.27 However, RCM has been used to discriminate between in situ and invasive proliferations.28 Reflectance confocal microscopy has wide applications in the diagnosis and management of cutaneous infections29,30 and inflammatory skin diseases.29,31-33 Recent RCM research explored the use of RCM to identify biopsy sites,34 delineate presurgical tumor margins,35,36 and monitor response to noninvasive treatments.37,38
Optical Coherence Tomography
Optical coherence tomography is an imaging modality that utilizes light backscatter from infrared light to produce grayscale cross-sectional or vertical images and horizontal en face images.39 Optical coherence tomography can visualize structures in the epidermis, dermoepidermal junction, and upper dermis.40 It can image boundaries of structures but cannot visualize individual cells.
There are different types of OCT devices available, including frequency-domain OCT (FD-OCT), or conventional OCT, and high-definition OCT (HD-OCT). With FD-OCT, images are captured at a maximum depth of 1 to 2 mm but with limited resolution. High-definition OCT has superior resolution compared to FD-OCT but is restricted to a shallower depth of 750 μm.39 The main advantage of OCT is the ability to noninvasively image live tissue and visualize 2- to 5-times greater depth as compared to RCM. Several OCT devices have obtained US Food and Drug Administration approval; however, OCT has not been widely adopted into clinical practice and is available only in tertiary academic centers. Additionally, OCT imaging in dermatology is rarely reimbursed. Other limitations of OCT include poor resolution of images, high cost to procure an OCT device, and the need for advanced training and experience to accurately interpret images.40,41
Optical coherence tomography primarily is used to diagnose cutaneous neoplasms. The best evidence of the diagnostic accuracy of OCT is in the setting of BCC, with a recent systematic review reporting a sensitivity of 66% to 96% and a specificity of 75% to 86% for conventional FD-OCT.42 The use of FD-OCT results in an increase in specificity without a significant change in sensitivity when compared to dermoscopy in the diagnosis of BCC.43 Melanoma is difficult to diagnose via FD-OCT, as the visualization of architectural features often is limited by poor resolution.44 A study of HD-OCT in the diagnosis of melanoma with a limited sample size reported a sensitivity of 74% to 80% and a specificity of 92% to 93%.45 Similarly, a study of HD-OCT used in the diagnosis of AK and SCC revealed a sensitivity and specificity of 81.6% and 92.6%, respectively, for AK and 93.8% and 98.9%, respectively, for SCC.46
Numerous algorithms and scoring systems have been developed to further explore the utility of OCT in the diagnosis of cutaneous neoplasms.47,48 Recent research investigated the utility of dynamic OCT, which can evaluate microvasculature in the diagnosis of cutaneous neoplasms (Figure 3)49; the combination of OCT with other imaging modalities50,51; the use of OCT to delineate presurgical margins52,53; and the role of OCT in the diagnosis and monitoring of inflammatory and infectious skin diseases.54,55
Final Thoughts
In recent years, there has been a surge of interest in noninvasive techniques for diagnosis and management of skin diseases; however, noninvasive tools exist on a spectrum in dermatology. Dermoscopy provides low-cost imaging of the skin’s surface and has been widely adopted by dermatologists and other providers to aid in clinical diagnosis. Reflectance confocal microscopy provides reimbursable in vivo imaging of live tissue with cellular-level resolution but is limited by depth, cost, and need for advanced training; thus, RCM has only been adopted in some clinical practices. Optical coherence tomography offers in vivo imaging of live tissue with substantial depth but poor resolution, high cost, need for advanced training, and rare reimbursement for providers. Future directions include combination of complementary imaging modalities, increased clinical practice integration, and education and reimbursement for providers.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
- Benvenuto-Andrade C, Dusza SW, Agero AL, et al. Differences between polarized light dermoscopy and immersion contact dermoscopy for the evaluation of skin lesions. Arch Dermatol. 2007;143:329-338.
- Terushkin V, Oliveria SA, Marghoob AA, et al. Use of and beliefs about total body photography and dermatoscopy among US dermatology training programs: an update. J Am Acad Dermatol. 2010;62:794-803.
- Morris JB, Alfonso SV, Hernandez N, et al. Use of and intentions to use dermoscopy among physicians in the United States. Dermatol Pract Concept. 2017;7:7-16.
- Yélamos O, Braun RP, Liopyris K, et al. Dermoscopy and dermatopathology correlates of cutaneous neoplasms. J Am Acad Dermatol. 2019;80:341-363.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Carli P, de Giorgi V, Chiarugi A, et al. Addition of dermoscopy to conventional naked-eye examination in melanoma screening: a randomized study. J Am Acad Dermatol. 2004;50:683-668.
- Lallas A, Zalaudek I, Argenziano G, et al. Dermoscopy in general dermatology. Dermatol Clin. 2013;31:679-694.
- Reiter O, Mimouni I, Gdalvevich M, et al. The diagnostic accuracy of dermoscopy for basal cell carcinoma: a systematic review and meta-analysis. J Am Acad Dermatol. 2019;80:1380-1388.
- Papageorgiou V, Apalla Z, Sotiriou E, et al. The limitations of dermoscopy: false-positive and false-negative tumours. J Eur Acad Dermatol Venereol. 2018;32:879-888.
- Micali G, Verzì AE, Lacarrubba F. Alternative uses of dermoscopy in daily clinical practice: an update. J Am Acad Dermatol. 2018;79:1117-1132.e1.
- Rajadhyaksha M, Grossman M, Esterowitz D, et al. In vivo confocal scanning laser microscopy of human skin: melanin provides strong contrast. J Invest Dermatol. 1995;104:946-952.
- Kose K, Gou M, Yélamos O, et al. Automated video-mosaicking approach for confocal microscopic imaging in vivo: an approach to address challenges in imaging living tissue and extend field of view. Sci Rep. 2017;7:10759.
- Rao BK, John AM, Francisco G, et al. Diagnostic accuracy of reflectance confocal microscopy for diagnosis of skin lesions [published online October 8, 2018]. Arch Pathol Lab Med. 2019;143:326-329.
- Current Procedural Terminology, Professional Edition. Chicago IL: American Medical Association; 2016. The preliminary physician fee schedule for 2017 is available at https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Federal-Regulation-Notices-Items/CMS-1654-P.html.
- Jain M, Pulijal SV, Rajadhyaksha M, et al. Evaluation of bedside diagnostic accuracy, learning curve, and challenges for a novice reflectance confocal microscopy reader for skin cancer detection in vivo. JAMA Dermatol. 2018;154:962-965.
- Rao BK, Pellacani G. Atlas of Confocal Microscopy in Dermatology: Clinical, Confocal, and Histological Images. New York, NY: NIDIskin LLC; 2013.
- Scope A, Benvenuto-Andrande C, Agero AL, et al. In vivo reflectance confocal microscopy imaging of melanocytic skin lesions: consensus terminology glossary and illustrative images. J Am Acad Dermatol. 2007;57:644-658.
- Gerger A, Hofmann-Wellenhof R, Langsenlehner U, et al. In vivo confocal laser scanning microscopy of melanocytic skin tumours: diagnostic applicability using unselected tumour images. Br J Dermatol. 2008;158:329-333.
- Stevenson AD, Mickan S, Mallett S, et al. Systematic review of diagnostic accuracy of reflectance confocal microscopy for melanoma diagnosis in patients with clinically equivocal skin lesions. Dermatol Pract Concept. 2013;3:19-27.
- Alarcon I, Carrera C, Palou J, et al. Impact of in vivo reflectance confocal microscopy on the number needed to treat melanoma in doubtful lesions. Br J Dermatol. 2014;170:802-808.
- Lovatto L, Carrera C, Salerni G, et al. In vivo reflectance confocal microscopy of equivocal melanocytic lesions detected by digital dermoscopy follow-up. J Eur Acad Dermatol Venereol. 2015;29:1918-1925.
- Guitera P, Pellacani G, Longo C, et al. In vivo reflectance confocal microscopy enhances secondary evaluation of melanocytic lesions. J Invest Dermatol. 2009;129:131-138.
- Xiong YQ, Ma SJ, Mo Y, et al. Comparison of dermoscopy and reflectance confocal microscopy for the diagnosis of malignant skin tumours: a meta-analysis. J Cancer Res Clin Oncol. 2017;143:1627-1635.
- Kadouch DJ, Schram ME, Leeflang MM, et al. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol. 2015;29:1890-1897.
- Dinnes J, Deeks JJ, Chuchu N, et al; Cochrane Skin Cancer Diagnostic Test Accuracy Group. Reflectance confocal microscopy for diagnosing keratinocyte skin cancers in adults. Cochrane Database Syst Rev. 2018;12:CD013191.
- Nguyen KP, Peppelman M, Hoogedoorn L, et al. The current role of in vivo reflectance confocal microscopy within the continuum of actinic keratosis and squamous cell carcinoma: a systematic review. Eur J Dermatol. 2016;26:549-565.
- Pellacani G, Ulrich M, Casari A, et al. Grading keratinocyte atypia in actinic keratosis: a correlation of reflectance confocal microscopy and histopathology. J Eur Acad Dermatol Venereol. 2015;29:2216-2221.
- Manfredini M, Longo C, Ferrari B, et al. Dermoscopic and reflectance confocal microscopy features of cutaneous squamous cell carcinoma. J Eur Acad Dermatol Venereol. 2017;31:1828-1833.
- Hoogedoorn L, Peppelman M, van de Kerkhof PC, et al. The value of in vivo reflectance confocal microscopy in the diagnosis and monitoring of inflammatory and infectious skin diseases: a systematic review. Br J Dermatol. 2015;172:1222-1248.
- Cinotti E, Perrot JL, Labeille B, et al. Reflectance confocal microscopy for cutaneous infections and infestations. J Eur Acad Dermatol Venereol. 2016;30:754-763.
- Ardigo M, Longo C, Gonzalez S; International Confocal Working Group Inflammatory Skin Diseases Project. Multicentre study on inflammatory skin diseases from The International Confocal Working Group: specific confocal microscopy features and an algorithmic method of diagnosis. Br J Dermatol. 2016;175:364-374.
- Ardigo M, Agozzino M, Franceschini C, et al. Reflectance confocal microscopy algorithms for inflammatory and hair diseases. Dermatol Clin. 2016;34:487-496.
- Manfredini M, Bettoli V, Sacripanti G, et al. The evolution of healthy skin to acne lesions: a longitudinal, in vivo evaluation with reflectance confocal microscopy and optical coherence tomography [published online April 26, 2019]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.15641.
- Navarrete-Dechent C, Mori S, Cordova M, et al. Reflectance confocal microscopy as a novel tool for presurgical identification of basal cell carcinoma biopsy site. J Am Acad Dermatol. 2019;80:e7-e8.
- Pan ZY, Lin JR, Cheng TT, et al. In vivo reflectance confocal microscopy of basal cell carcinoma: feasibility of preoperative mapping of cancer margins. Dermatol Surg. 2012;38:1945-1950.
- Venturini M, Gualdi G, Zanca A, et al. A new approach for presurgical margin assessment by reflectance confocal microscopy of basal cell carcinoma. Br J Dermatol. 2016;174:380-385.
- Sierra H, Yélamos O, Cordova M, et al. Reflectance confocal microscopy‐guided laser ablation of basal cell carcinomas: initial clinical experience. J Biomed Opt. 2017;22:1-13.
- Maier T, Kulichova D, Ruzicka T, et al. Noninvasive monitoring of basal cell carcinomas treated with systemic hedgehog inhibitors: pseudocysts as a sign of tumor regression. J Am Acad Dermatol. 2014;71:725-730.
- Levine A, Wang K, Markowitz O. Optical coherence tomography in the diagnosis of skin cancer. Dermatol Clin. 2017;35:465-488.
- Schneider SL, Kohli I, Hamzavi IH, et al. Emerging imaging technologies in dermatology: part I: basic principles. J Am Acad Dermatol. 2019;80:1114-1120.
- Mogensen M, Joergensen TM, Nümberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non‐melanoma skin cancer and benign lesions versus normal skin: observer‐blinded evaluation by dermatologists and pathologists. Dermatol Surg. 2009;35:965-972.
- Ferrante di Ruffano L, Dinnes J, Deeks JJ, et al. Optical coherence tomography for diagnosing skin cancer in adults. Cochrane Database Syst Rev. 2018;12:CD013189.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Wessels R, de Bruin DM, Relyveld GM, et al. Functional optical coherence tomography of pigmented lesions. J Eur Acad Dermatol Venereol. 2015;29:738‐744.
- Gambichler T, Schmid-Wendtner MH, Plura I, et al. A multicentre pilot study investigating high‐definition optical coherence tomography in the differentiation of cutaneous melanoma and melanocytic naevi. J Eur Acad Dermatol Venereol. 2015;29:537‐541.
- Marneffe A, Suppa M, Miyamoto M, et al. Validation of a diagnostic algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma by means of high-definition optical coherence tomography. Exp Dermatol. 2016;25:684-687.
- Boone MA, Suppa M, Dhaenens F, et al. In vivo assessment of optical properties of melanocytic skin lesions and differentiation of melanoma from non-malignant lesions by high-definition optical coherence tomography. Arch Dermatol Res. 2016;308:7-20.
- Boone MA, Suppa M, Marneffe A, et al. A new algorithm for the discrimination of actinic keratosis from normal skin and squamous cell carcinoma based on in vivo analysis of optical properties by high-definition optical coherence tomography. J Eur Acad Dermatol Venereol. 2016;30:1714-1725.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1655-1662.
- Alex A, Weingast J, Weinigel M, et al. Three-dimensional multiphoton/optical coherence tomography for diagnostic applications in dermatology. J Biophotonics. 2013;6:352-362.
- Iftimia N, Yélamos O, Chen CJ, et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt. 2017;22:76006.
- Wang KX, Meekings A, Fluhr JW, et al. Optical coherence tomography-based optimization of Mohs micrographic surgery of basal cell carcinoma: a pilot study. Dermatol Surg. 2013;39:627-633.
- Chan CS, Rohrer TE. Optical coherence tomography and its role in Mohs micrographic surgery: a case report. Case Rep Dermatol. 2012;4:269-274.
- Gambichler T, Jaedicke V, Terras S. Optical coherence tomography in dermatology: technical and clinical aspects. Arch Dermatol Res. 2011;303:457-473.
- Manfredini M, Greco M, Farnetani F, et al. Acne: morphologic and vascular study of lesions and surrounding skin by means of optical coherence tomography. J Eur Acad Dermatol Venereol. 2017;31:1541-1546.
Practice Points
- There are several new noninvasive imaging tools in dermatology that can be utilized to aid in the diagnosis and management of skin disease, including dermoscopy, reflectance confocal microscopy, and optical coherence tomography.
- Among these tools, there are several differences in cost, clinical integration, reimbursement, and accuracy.
Barriers and Job Satisfaction Among Dermatology Hospitalists
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
Consultative dermatologists, or dermatology hospitalists (DHs), perform a critical role in the care of inpatients with skin disease, providing efficient diagnosis and management of patients with complex skin conditions as well as education of patients and trainees in the hospital setting.1 In 2013, 27% of the US population was seen by a physician for a skin disease.2 In 2014, there were nearly 650,000 hospital admissions principally for skin disease.3 Input by dermatologists facilitates accurate diagnosis and management of inpatients with skin disease,4 including a substantial number of cutaneous malignancies diagnosed in the inpatient setting.5 Several studies have highlighted the generally low level of diagnostic concordance between referring services and dermatology consultants,4,6 with dermatology consultants frequently noting diagnoses not considered by referring services,7 reinforcing the importance of having access to dermatologists in the hospital setting.
The care of skin disease in the inpatient setting has become increasingly complex. The Society for Dermatology Hospitalists (SDH) was created in 2009 to address this complexity, with the goal to “strive to develop the highest standards of clinical care of hospitalized patients with skin disease.”8 A recent survey found that 50% of DHs spend between 41 to 52 weeks per year on service.9 Despite this degree of commitment, there are considerable barriers that prevent the majority of dermatologists from efficiently providing inpatient consultative care. The inpatient and outpatient provision of dermatology care varies greatly, including the variety of ethical situations encountered and the diversity of skin conditions treated.10-12 Additionally, the transition between inpatient and outpatient care can be challenging for providers.13
The goal of this study was to evaluate the overall job satisfaction of DHs and further describe potential barriers to inpatient dermatology consultations.
Methods
An anonymous 31-question electronic survey was sent via email to all current members of the SDH from November 20 to December 10, 2018. The study was reviewed and determined to be exempt from federal human subjects regulations by the University of Washington Human Subjects Division (Seattle, Washington)(STUDY00005832).
Results
At the time of survey distribution, the SDH had 145 members, including attending-level dermatologists and resident members. Thirty-seven self-identified DHs (46% [17/37] women; 54% [20/37] men) completed the survey. The majority of respondents were junior faculty, with 46% (17/37) assistant professors, 5% (2/37) acting instructors, 32% (12/37) associate professors, and 16% (6/37) professors. All regions of the United States were represented.
Time Dedicated to Providing Inpatient Dermatology Consultations
The majority of those surveyed were satisfied or very satisfied (68% [25/37]) with the amount of time allotted for inpatient dermatology consultations, while 14% (5/37) were unsatisfied or very unsatisfied. Of those surveyed, 46% (17/37) reported that 21% to 50% of their time is dedicated to inpatient dermatology consultations. The majority (57% [21/37]) reported that their outpatient clinic efforts are reduced when providing dermatology inpatient consultations.
Regarding travel to the inpatient practice site, 60% (22/37) rated their travel time/effort as very easy, with 38% (14/37) reporting that the sites at which they provide inpatient dermatology consultations and their main outpatient clinics are the same physical location; 38% (14/37) reported travel times of less than 15 minutes between clinical practice sites.
Eighty-nine percent (33/37) of respondents said they are able to spend more time teaching trainees when providing inpatient dermatology consultations compared to their time spent in clinic. Similarly, 70% (26/37) said they are able to spend more time learning about patients and their conditions when providing inpatient dermatology consultations. Respondents also reported additional time expenditures because of inpatient dermatology consultations, primarily additional teaching requirements (49% [18/37]), additional electronic medical record training (35% [13/37]), and credentialing requirements (24% [9/37]).
Infrastructure for Providing Inpatient Dermatology Consultations
For many respondents (30% [11/37]), only 2 faculty dermatologists regularly provide inpatient dermatology consultations at their institutions. Four respondents reported having at least 5 faculty dermatologists who regularly provide inpatient dermatology consultations; excluding these, the average number of DHs was 2.42 faculty per institution.
Most respondents (57% [21/37]) reported their institutions support inpatient dermatology services by providing salary support for residents to cover services. Other methods of support included dedicated office spaces (30% [11/37]), free hospital parking while providing inpatient consultations (24% [9/37]), and administrative support (11% [4/37]).
Consultation Composition
Respondents indicated that requests for DH consultations most often come from medical services, including medical intensive care, internal medicine, and family medicine (95% [35/37]); the emergency department (95% [35/37]); surgical services (92% [34/37]); and hematology/oncology (89% [33/37]). Fewer DHs reported receiving consultation requests from pediatrics (70% [26/37]).
Many respondents (49% [18/37]) reported consulting for patients with skin disorders that they considered to be life-threatening or potentially life-threatening either very frequently (daily) or frequently (several times weekly), with only 16% (6/37) responding that they see such patients about once per month.
Compensation for Inpatient Dermatology Consultation
The most commonly reported compensation models for DHs were fixed salary plus productivity or performance incentives and fixed salary only models (49% [18/37] and 32% [12/37], respectively), with relative value unit (RVU) models and other models less frequently reported (16% [6/37] and 3% [1/37], respectively). Only 46% (17/37) of respondents were satisfied or very satisfied with their institutions’ compensation models; the remainder (54% [20/37]) were either neutral, unsatisfied, or very unsatisfied regarding their institutions’ compensation models. Overall compensation satisfaction was higher, with 60% (22/37) of DHs reporting they were satisfied or very satisfied with their salaries and 41% (15/37) reporting they were either neutral or not satisfied. The majority (60% [2/37]) of respondents felt that fixed salary plus productivity or performance incentives models would be the ideal compensation model for DHs.
Of the DHs whose compensations models were RVU based (6/37 [16%]), 67% (4/6) said they receive incentive pay upon meeting their RVU targets. No respondents reported that they were expected to generate an equivalent number of RVUs when performing inpatient consultations as compared to an outpatient session. Only 32% (12/37) of respondents reported keeping the revenue/RVUs generated by inpatient dermatology consultations; most (57% [21/37]) noted that their dermatology divisions/departments keep the revenue/RVUs, followed by university hospitals (27% [10/37]), schools of medicine (11% [4/37]), and departments of medicine (3% [1/37]). The remainder of respondents (22% [8/37]) were unsure who keeps the revenue/RVUs generated by inpatient dermatology consultations.
Most respondents (70% [26/37]) reported that the revenue (or RVU equivalent) generated by inpatient dermatology consultations does not fully support their salary for the time spent as consultants. Rather, these DHs noted sources of additional financial support, primarily the DHs themselves (69% [18/26]), followed by dermatology divisions/departments (50% [13/26]), departments of medicine (23% [6/26]), university hospitals (23% [6/26]), and schools of medicine (12% [3/26]).
Job Fulfillment Among DHs
Most respondents said they choose to provide inpatient dermatology consultations due to their interest in complex medical dermatology and their desire to work with other medical teams and specialties (92% [34/37] and 76% [28/37], respectively). Seventy percent (26/37) said they choose to provide inpatient consultations to be able to teach medical students and residents as well as to take advantage of the added opportunities to practice in a variety of settings beyond their outpatient clinics (57% [21/37]). Only 3% (1/37) of respondents reported that they provide inpatient dermatology consultations because they are “required to do so.”
Most DHs (84% [31/37]) said they feel their institutions as well as their dermatology divisions/departments value having access to inpatient dermatology services, though some did not feel this way (16% [6/37] neutral or strongly disagree). Nearly all respondents (97% [36/37]) felt they provide a critical service when performing inpatient dermatology consultations. All respondents (100%) said they found providing inpatient dermatology consultations fulfilling, and 65% (24/37) said they prefer providing inpatient dermatology consultations to spending time in clinic. Of the DHs who were surveyed, 68% (25/37) said they were satisfied with the balance of outpatient and inpatient services in their clinical practice and 30% (11/37) said they were not.
Comment
Factors such as patient care, hospital infrastructure, and procedural support have all been cited by DHs as crucial aspects of their contributions to the care of hospitalized pa
Dermatology is primarily an outpatient specialty, and our study highlighted several important challenges for providers performing inpatient dermatology consultations. A major issue is time expenditures, including additional teaching requirements, additional electronic medical record training, and credentialing requirements. Travel time to inpatient hospital sites does not appear to be one of these hindering factors; nearly 60% of respondents rated their travel time/effort as very easy, with approximately 75% of respondents’ consultation locations being either at the same physical location as their main outpatient clinic or less than 15 minutes away. Maintaining easy travel between outpatient and inpatient settings is important to the success of the DH.
Our data suggest that compensation of DHs is a potential limitation to providing inpatient dermatology care. Our survey reinforced that providers who do inpatient dermatology consultations generally do not generate the revenue necessary to cover these efforts. More than 40% of DH respondents said they either feel neutral about or unsatisfied with their overall salary, and more than half said they feel similarly regarding their institutions’ compensation models. Most respondents said that a fixed salary model plus productivity or performance incentives is the ideal compensation model for those providing inpatient dermatology consultations, though only half said they actually are compensated according to this model. This discrepancy highlights the disconnect between the current accepted compensation models and the DH’s ideal model and provides direction for dermatology chairs and division heads as to what compensation model is preferable to support the success of DHs at their institutions.
Despite the barriers and compensation constraints we identified, DHs report high job satisfaction, which we hypothesize could combat burnout. In our study, all DHs surveyed say they find providing inpatient dermatology consultations fulfilling, and most were satisfied with the amount of time allotted for consultations. Some of the possible reasons why DHs may find their work fulfilling include increased time for teaching trainees and learning about patients and their conditions while consulting, as well as a preference for providing inpatient dermatology consultations to spending time in clinic. Most DHs said they choose to provide inpatient dermatology consultation rather than do so as a requirement, primarily due to their interest in complex medical dermatology and their desire to work with other medical teams/specialties; thankfully, only a small percentage said they provide these consultations because they are required to do so.
This study was conducted to analyze job satisfaction among DHs who provided inpatient dermatology consultations and determine common barriers and obstacles to their job satisfaction. Limitations to our study included the small sample size and the possibly limited representation of the intended population, as only the members of the SDH were surveyed, potentially excluding providers who regularly perform inpatient dermatology consultations but are not members of the SDH. Further limitations included recall bias and the qualitative nature of the survey instrument.
Final Thoughts
There was near-unanimous agreement among the DHs we surveyed regarding the importance of the role they play in their divisions/departments, but there are clear barriers to provision of inpatient dermatology consultation, specifically relating to extraneous time expenditures and compensation. Despite these barriers, the majority of respondents said they are very satisfied with the role they play in the inpatient setting and feel that their contributions are valued by the institutions where they work. Protecting these benefits of providing dermatology hospital consultations will be critical for maintaining this high job satisfaction and balancing out the barriers to providing these consultations. Protecting the time required to provide consultations is paramount so DHs continue to gain fulfillment from teaching trainees, caring for complex patients, and maintaining their place as valuable colleagues in the hospital setting.
Acknowledgment
The authors thank the members of the SDH for their participation in this survey.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
- Biesbroeck LK, Shinohara MM. Inpatient consultative dermatology. Med Clin North Am. 2015;99:1349-1364.
- Lim HW, Collins SAB, Resneck JS Jr, et al. The burden of skin disease in the United States. J Am Acad Dermatol. 2017;76:958-972.e2.
- Arnold JD, Yoon SJ, Kirkorian AY. The national burden of inpatient dermatology in adults. J Am Acad Dermatol. 2018;80:425-432.
- Mancusi S, Festa Neto C. Inpatient dermatological consultations in a university hospital. Clinics (Sao Paulo). 2010;65:851-855.
- Tsai S, Scott JF, Keller JJ, et al. Cutaneous malignancies identified in an inpatient dermatology consultation service. Br J Dermatol. 2017;177:e116-e118.
- Pereira AR, Porro AM, Seque CA, et al. Inpatient dermatology consultations in renal transplant recipients. Actas Dermosifiliogr. 2018;109:900-907.
- Tracey EH, Forrestel A, Rosenbach M, et al. Inpatient dermatology consultation in patients with hematologic malignancies. J Am Acad Dermatol. 2016;75:835-836.
- Fox LP, Cotliar J, Hughey L, et al. Hospitalist dermatology. J Am Acad Dermatol. 2009;61:153-154.
- Ko LN, Kroshinsky D. Dermatology hospitalists: a multicenter survey study characterizing the infrastructure of consultative dermatology in select American hospitals. Int J Dermatol. 2018;57:553-558.
- Hansra NK, Shinkai K, Fox LP. Ethical issues in inpatient consultative dermatology. Clin Dermatol. 2012;30:496-500.
- El-Azhary R, Weenig RH, Gibson LE. The dermatology hospitalist: creating value by rapid clinical pathologic correlation in a patient-centered care model. Int J Dermatol. 2012;51:1461-1466.
- Ahronowitz I, Fox LP. Herpes zoster in hospitalized adults: practice gaps, new evidence, and remaining questions. J Am Acad Dermatol. 2018;78:223-230.e3.
- Rosenbach M. The logistics of an inpatient dermatology service. Semin Cutan Med Surg. 2017;36:3-8.
- Ackerman L, Kessler M. The efficient, effective community hospital inpatient dermatology consult. Semin Cutan Med Surg. 2017;36:9-11.
Practice Points
• Dermatology hospitalists play a critical role in the specialized care of hospitalized patients with
skin conditions.
• Dermatology hospitalists have high job satisfaction, with opportunities to teach trainees and practice complex medical dermatology.
• Most dermatology hospitalists do not generate sufficient revenue providing inpatient dermatology consultations to fully support their salary for the time spent as consultants; alternate payment models are needed to maintain dermatology’s presence in the hospital.
Rubbery Nodule on the Face of an Infant
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 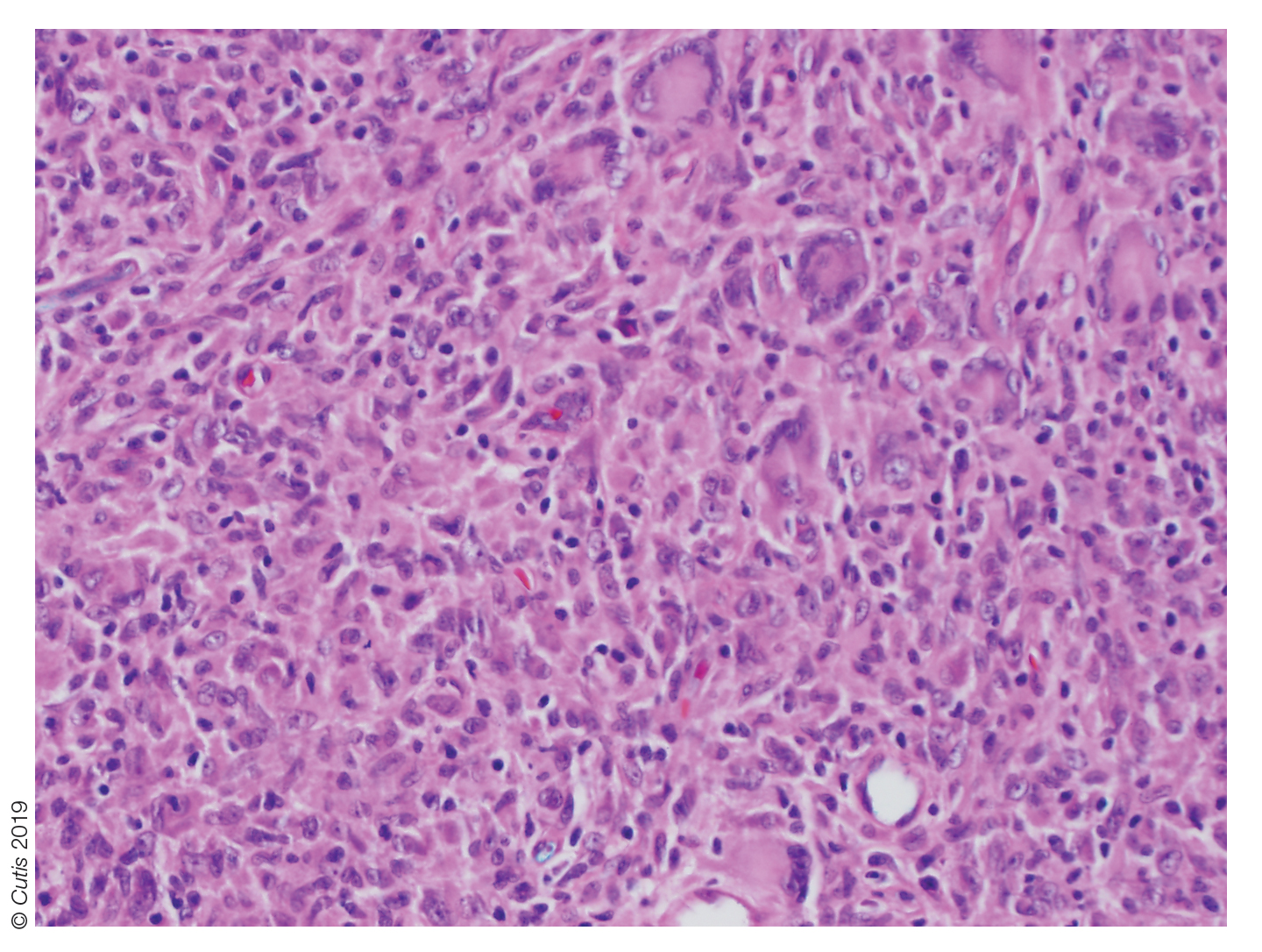
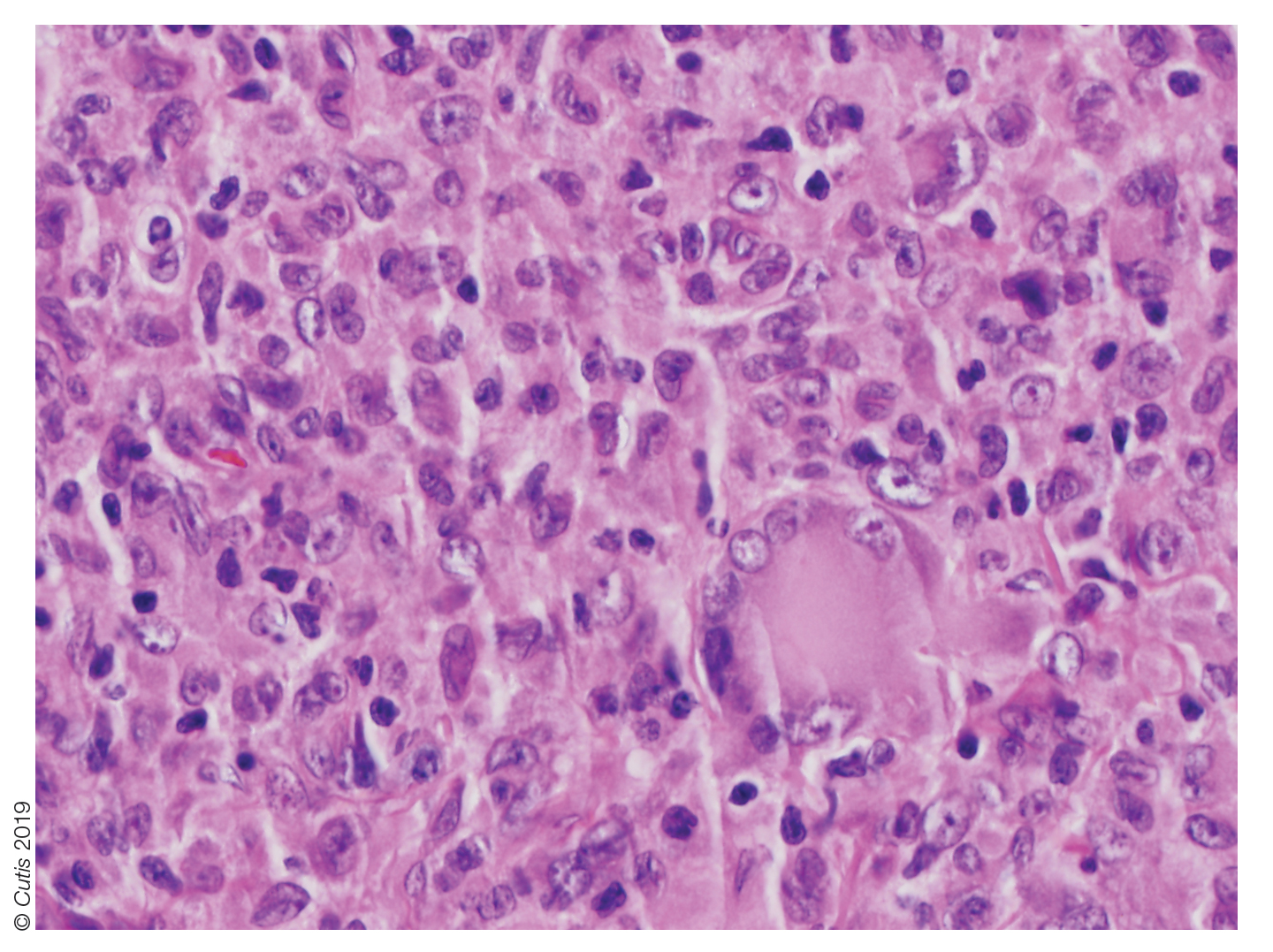
Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13
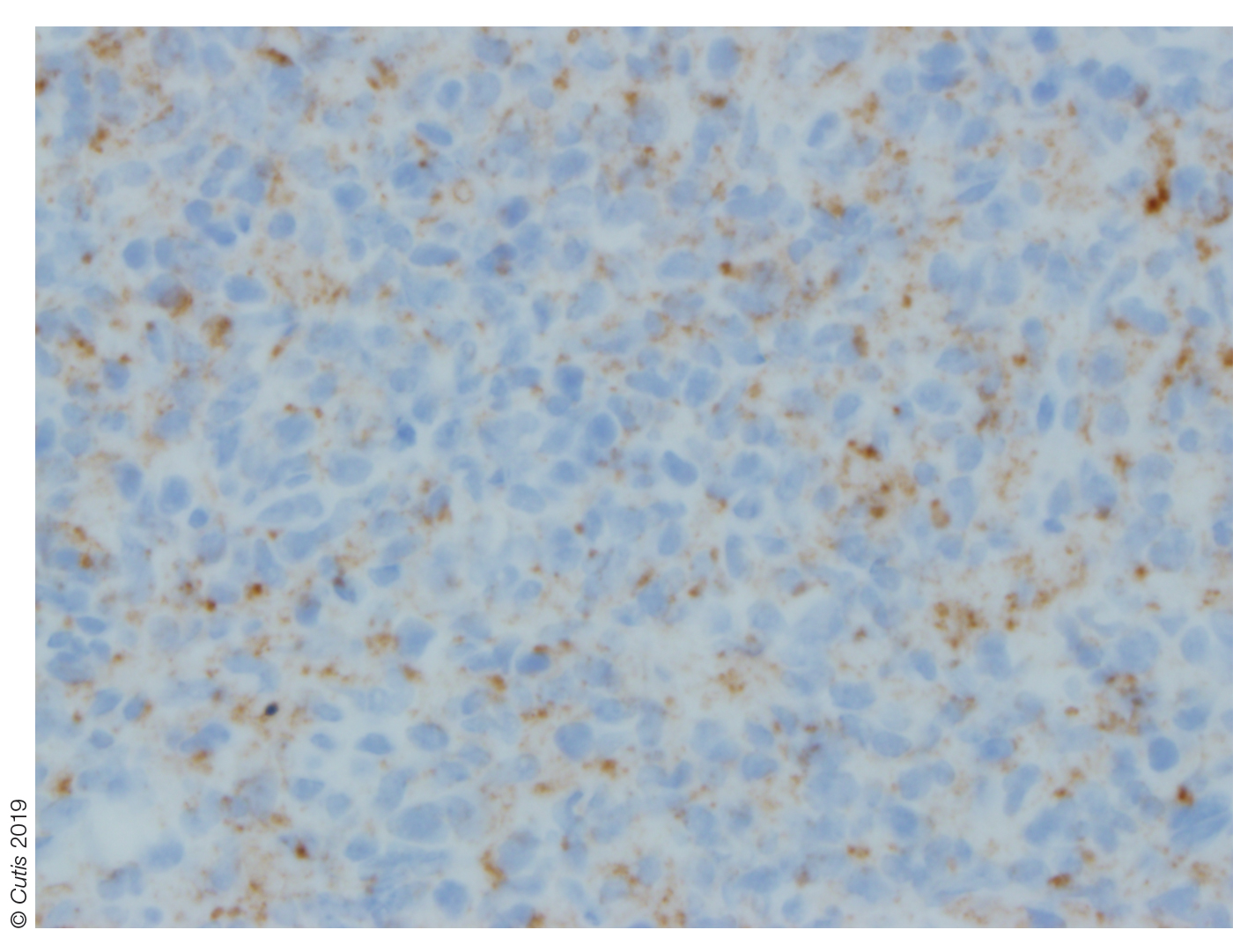
Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
The Diagnosis: Juvenile Xanthogranuloma
Juvenile xanthogranuloma (JXG) was first described in 1905 by Adamson1 as solitary or multiple plaquiform or nodular lesions that are yellow to yellowish brown. In 1954, Helwig and Hackney2 coined the term juvenile xanthogranuloma to define this histiocytic cutaneous granulomatous tumor.
Juvenile xanthogranuloma is a rare dermatologic disorder that may be present at birth and primarily affects infants and young children. The benign lesions generally occur in the first 4 years of life, with a median age of onset of 2 years.3 Lesions range in size from millimeters to several centimeters in diameter.4 The skin of the head and neck is the most commonly involved site in JXG. The most frequent noncutaneous site of JXG involvement is the eye, particularly the iris, accounting for 0.4% of cases.5,6 Extracutaneous sites such as the heart, liver, lungs, spleen, oral cavity, and brain also may be involved.4
Most children with JXG are asymptomatic. Skin lesions present as well-demarcated, rubbery, tan-orange papules or nodules. They usually are solitary, and multiple nodules can increase the risk for extracutaneous involvement.4 A case series of patients with neurofibromatosis type 1 showed 14 of 77 (18%) patients examined in the first year of life presented with JXG or other non–Langerhans cell histiocytosis.7 The adult form of cutaneous xanthogranuloma often presents with severe bronchial asthma.8
Histopathologic examination of a biopsy of the lesion typically demonstrates well-circumscribed nodules with dense infiltrates of polyhedral histiocytes with vaculoles.3-7 In 85% of cases, Touton giant cells are present.4,9 A prominent vascular network often is present, which was observed in our patient’s biopsy (Figure 1). Immunohistochemistry typically is positive for CD14, CD68, CD163, fascin, and factor XIIIa.4,10 Classically, the cells are negative for S-100 and CD1a, which differentiates these lesions from Langerhans cell histiocytosis.4-7,10 Our patient demonstrated scattered S-100–positive cells representing background dendritic cells and macrophages (Figure 2). The remainder of the clinical, morphologic, and immunophenotypic findings were consistent with non–Langerhans cell histiocytosis, specifically JXG. 

Biopsy should be performed in all cases, and basic laboratory test results such as a complete blood cell count and basic metabolic panel also are appropriate. Routine referral of all patients with cutaneous JXG for ophthalmologic evaluation is not recommended.11 Most patients with ocular involvement present with acute ocular concerns; asymptomatic eye involvement is rare. It is reasonable to consider referral to ophthalmology for patients younger than 2 years who present with multiple lesions, as they may have a higher risk for ocular involvement.11
Juvenile xanthogranuloma usually is a benign disorder with management involving observation, as the lesions typically involute spontaneously.3-7,9,10,12 Systemic or intralesional corticosteroids may be used for treatment in lesions that do not resolve. Ocular JXG refractory to steroid treatment has been managed in several cases with intravitreal bevacizumab.13 Additionally, surgical excision can be considered if malignancy is suspected, the lesion does not resolve with observation or steroid treatment, or the lesion is located near vital structures.4-7,9-13

Spitz nevus presents as a single dome-shaped papule, but histology shows a symmetrical proliferation of spindle and epithelioid cells. Trachoma can present in and around the eye as a follicular hypertrophy but most commonly is seen in the conjunctiva. Dermoid cysts present as solitary subcutaneous cystic lesions; histology demonstrates a lining of keratinizing squamous epithelium with the presence of pilosebaceous structures. Dermatofibroma appears as a tan to reddish-brown papule in an area of prior minor trauma; pathology demonstrates an acanthotic epidermis with an underlying zone of normal papillary dermis and unencapsulated lesions with spindle cells overlapping in fascicles and whorls at the periphery.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
1. Adamson HG. Society intelligence: the Dermatological Society of
London. Br J Dermatol. 1905;17:222-223.
2. Helwig E, Hackney VC. Juvenile xanthogranuloma (nevoxanthoendothelioma).
Am J Pathol. 1954;30:625-626.
3. Farrugia EJ, Stephen AP, Raza SA. Juvenile xanthogranuloma of
temporal bone—a case report. J Laryngol Otol. 1997;111:63-65.
4. Cypel TK, Zuker RM. Juvenile xanthogranuloma: case report and review
of the literature. Can J Plast Surg. 2008;16:175-177.
5. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445-449.
6. Zimmerman LE. Ocular lesions of juvenile xanthogranuloma.
nevoxanthoendothelioma. Am J Ophthalmol. 1965;60:1011-1035.
7. Cambiaghi S, Restano L, Caputo R. Juvenile xanthogranuloma associated
with neurofibromatosis 1: 14 patients without evidence of hematologic
malignancies. Pediatr Dermatol. 2004;21:97-101.
8. Stover DG, Alapati S, Regueira O, et al. Treatment of juvenile xanthogranuloma.
Pediatr Blood Cancer. 2008;51:130-133.
9. Dehner LP. Juvenile xanthogranulomas in the first two decades of life. a
clinicopathologic study of 174 cases with cutaneous and extracutaneous
manifestations. Am J Surg Pathol. 2003;27:579-593.
10. Weitzman S, Jaffe R. Uncommon histiocytic disorders: the non-
Langerhans cell histiocytoses. Pediatr Blood Cancer. 2005;45:256-264.
11. Chang MW, Frieden IJ, Good W. The risk of intraocular juvenile
xanthogranuloma: survey of current practices and assessment of risk.
J Am Acad Dermatol. 1996;34:445.
12. Eggli KD, Caro P, Quioque T, et al. Juvenile xanthogranuloma: non-X
histiocytosis with systemic involvement. Pediatr Radiol. 1992;22:374-376.
13. Ashkenazy N, Henry CR, Abbey AM, et al. Successful treatment of juvenile
xanthogranuloma using bevacizumab. J AAPOS. 2014;18:295-297.
A 10-month-old girl presented with a facial nodule of 7 months’ duration that started as a small lesion. On physical examination, a single 10×10-mm, nontender, well-circumscribed, firm, freely mobile nodule was observed in the left infraorbital area. The patient was born full term at 37 weeks’ gestation via spontaneous vaginal delivery and had no other notable findings on physical examination. Excision was performed by an oculoplastic surgeon. Pathology revealed a relatively well-circumscribed, diffuse, dermal infiltrate of cells arranged in short fascicles and a storiform pattern. The cells had abundant clear to amphophilic cytoplasm, ovoid to reniform nuclei with vesicular chromatin and focal grooves, and diffuse CD68+ immunoreactivity, as well as scattered S-100–positive cells. The patient did well with the excision and no new lesions have developed.
Darkening and Eruptive Nevi During Treatment With Erlotinib
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
To the Editor:
Erlotinib is a small-molecule selective tyrosine kinase inhibitor that functions by blocking the intracellular portion of the epidermal growth factor receptor (EGFR)1,2; EGFR normally is expressed in the basal layer of the epidermis, sweat glands, and hair follicles, and is overexpressed in some cancers.1,3 Normal activation of EGFR leads to signal transduction through the mitogen-activated protein kinase (MAPK) signaling pathway, which stimulates cell survival and proliferation.4,5 Erlotinib-induced inhibition of EGFR prevents tyrosine kinase phosphorylation and aims to decrease cell proliferation in these tumors.
Erlotinib is indicated as once-daily oral monotherapy for the treatment of advanced-stage non–small cell lung cancer (NSCLCA) and in combination with gemcitabine for treatment of advanced-stage pancreatic cancer.1 A number of cutaneous side effects have been reported, including acneform eruption, xerosis, paronychia, and pruritus.6 Other tyrosine kinase inhibitors, which also decrease signal transduction through the MAPK pathway, have some overlapping side effects; among these are vemurafenib, a selective BRAF inhibitor, and sorafenib, a multikinase inhibitor.7,8
A 70-year-old man with NSCLCA presented with eruptive nevi and darkening of existing nevi 3 months after starting monotherapy with erlotinib. Physical examination demonstrated the simultaneous appearance of scattered acneform papules and pustules; diffuse xerosis; and numerous dark brown to black nevi on the trunk, arms, and legs. Compared to prior clinical photographs taken in our office, darkening of existing medium brown nevi was noted, and new nevi developed in areas where no prior nevi had been visible (Figure 1).
The patient’s medical history included 3 invasive melanomas, all of which were diagnosed at least 7 years prior to the initiation of erlotinib and were treated by surgical excision alone. Prior treatment of NSCLCA consisted of a left lower lobectomy followed by docetaxel, carboplatin, pegfilgrastim, dexamethasone, and pemetrexed. A thorough review of all of the patient’s medications revealed no associations with changes in nevi.
A review of the patient’s treatment timeline revealed that all other chemotherapeutic medications had been discontinued a minimum of 5 weeks before starting erlotinib. A complete cutaneous examination performed in our office after completion of these chemotherapeutic agents and prior to initiation of erlotinib was unremarkable for abnormally dark or eruptive nevi.
Since starting erlotinib treatment, the patient underwent 10 biopsies of clinically suspicious dark nevi performed by a dermatologist in our office. Two of these were diagnosed as melanoma in situ and one as an atypical nevus. A temporal association of the darkening and eruptive nevi with erlotinib treatment was established; however, because erlotinib was essential to his NSCLCA treatment, he continued erlotinib with frequent complete cutaneous examinations.
A number of cutaneous side effects have been described during treatment with erlotinib, the most common being acneform eruption.6 The incidence and severity of acneform eruptions have been positively correlated to survival in patients with NSCLCA.3,5,6 Other common side effects include xerosis, paronychia, and pruritus.1,5,6 Less common side effects include periungual pyogenic granulomas and hair growth abnormalities.1
Eruptive nevi previously were reported in a patient who was treated with erlotinib.1 Other tyrosine kinase inhibitors that also decrease signal transduction through the MAPK pathway, including sorafenib and vemurafenib, have been reported to cause eruptive nevi. There are 7 reports of eruptive nevi with sorafenib and 5 reports with vemurafenib.7-9 Development of nevi were noted within a few months of initiating treatment with these medications.7
A PubMed search of articles indexed for MEDLINE using the terms erlotinib and melanoma and erlotinib and nevi yielded no prior reports of darkening of existing nevi or the development of melanoma during treatment with erlotinib. However, vemurafenib has been reported to cause dysplastic nevi, melanomas, and darkening of existing nevi, in addition to eruptive nevi.8-10 The side effects of vemurafenib have been ascribed to a paradoxical upregulation of MAPK in BRAF wild-type cells. This effect has been well documented and demonstrated in vivo.8,10 Perhaps erlotinib has a similar potential to paradoxically upregulate the MAPK pathway, thus stimulating cellular proliferation and survival.
Another tyrosine kinase receptor, c-KIT, is found on the cell membrane of melanocytes along with EGFR.11,12 The c-KIT receptor also activates the MAPK pathway and is critical to the development, migration, and survival of melanocytes.11,13 Stimulation of the c-KIT tyrosine kinase receptor also can induce melanocyte proliferation and melanogenesis.11 The c-KIT receptor is encoded by the KIT gene (KIT proto-oncogene receptor tyrosine kinase). Mutations in this gene are associated with melanocytic disorders. Inherited KIT mutation leading to c-KIT receptor deficiency is associated with piebaldism. Acquired activating KIT mutations increasing c-KIT expression are associated with acral and mucosal melanomas as well as melanomas in chronically sun-damaged skin.13
We hypothesized that erlotinib-induced inhibition of the MAPK pathway could lead to a reactive increase in expression of c-KIT and thus stimulate melanocyte proliferation and pigment production. Similar feedback upregulation of an MAPK pathway stimulating receptor during downstream MAPK inhibition has been demonstrated in colon adenocarcinoma; in this setting, BRAF inhibitors blocking the MAPK pathway leads to upregulation of EGFR.14 In our patient, c-KIT immunostaining revealed a mild to moderate increase in intensity (ie, the darkness of the staining) in nevi and melanomas during treatment with erlotinib compared to nevi biopsied before erlotinib treatment (Figure 2). The increased intensity of c-KIT immunostaining was further confirmed via semiquantitative digital image analysis. Using this method, a darkened nevus biopsied during treatment with erlotinib demonstrated 43.16% of cells (N=31,451) had very strong c-KIT staining, while a nevus biopsied before treatment with erlotinib demonstrated only 3.32% of cells (N=7507) with very strong c-KIT staining. Increased expression of c-KIT, possibly reactive to downstream inhibition the MAPK pathway from erlotinib, could be implicated in our case of eruptive nevi.
In summary, we report a rare case of darkening of existing nevi and development of melanoma in situ during treatment with erlotinib. The patient’s therapeutic timeline and concurrence of other well-documented side effects provided support for erlotinib as the causative agent in our patient. Additional support is provided through reports of other medications affecting the same pathway as erlotinib causing eruptive nevi, darkening of existing nevi, and melanoma in situ.7-10 Through c-KIT immunostaining, we demonstrated that increased expression of c-KIT might be responsible for the changes in nevi in our patient. We, therefore, suggest frequent full-body skin examinations in patients treated with erlotinib to monitor for the possible development of malignant melanomas.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
- Santiago F, Goncalo M, Reis J, et al. Adverse cutaneous reactions to epidermal growth factor receptor inhibitors: a study of 14 patients. An Bras Dermatol 2011;86:483-490.
- Lubbe J, Masouye I, Dietrich P. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva). Dermatology. 2008;216:247-249.
- Dessinioti C, Antoniou C, Katsambas A. Acneiform eruptions. Clin Dermatol. 2014;32:24-34.
- Herbst R, Fukuoka M, Baselga J. Gefitinib—a novel targeted approach to treating cancer. Nat Rev Cancer. 2004;4:979-987.
- Brodell L, Hepper D, Lind A, et al. Histopathology of acneiform eruptions in patients treated with epidermal growth factor receptor inhibitors. J Cutan Pathol. 2013;40:865-870.
- Kiyohara Y, Yamazaki N, Kishi A. Erlotinib-related skin toxicities: treatment strategies in patients with metastatic non-small cell lung cancer. J Am Acad Dermatol 2013;69:463-472.
- Uhlenhake E, Watson A, Aronson P. Sorafenib induced eruptive melanocytic lesions. Dermatol Online J. 2013;19:181-84.
- Chu E, Wanat K, Miller C, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol 2012;67:1265-1272.
- Boussemart L, Routier E, Mateus C, et al. Prospective study of cutaneous side-effects associated with the BRAF inhibitor vemurafenib: a study of 42 patients. Ann Oncol. 2013;24:1691-1697.
- Cohen P, Bedikian A, Kim K. Appearance of new vemurafenib-associated melanocytic nevi on normal-appearing skin: case series and a review of changing or new pigmented lesions in patients with metastatic malignant melanoma after initiating treatment with vemurafenib. J Clin Aesthet Dermatol. 2013;6:27-37.
- Longley B, Tyrrell L, Lu S, et al. Somatic c-KIT activating mutation in urticaria pigmentosa and aggressive mastocytosis: establishment of clonality in a human mast cell neoplasm. Nat Genet. 1996;12:312-314.
- Yun W, Bang S, Min K, et al. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol Surg. 2013;39:1903-1911.
- Swick J, Maize J. Molecular biology of melanoma. J Am Acad Dermatol. 2012;67:1049-1054.
- Sun C, Wang L, Huang S, et al. Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature. 2014;508:118-122.
Practice Points
- Cutaneous side effects of erlotinib include acneform eruption, xerosis, paronychia, and pruritus.
- Clinicians should monitor patients for darkening and/or eruptive nevi as well as melanoma during treatment with erlotinib.
Metastatic Adamantinoma Presenting as a Cutaneous Papule
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
To the Editor:
A 34-year-old woman with a history of adamantinoma of the right tibia that had been surgically resected with tibial reconstruction 5 years prior presented with a mildly tender, enlarging lesion on the right distal shin of 6 months’ duration that had started to change color. Review of systems was otherwise negative. Physical examination revealed an 8-mm, slightly tender, rubbery, pink papule adjacent to the surgical scar over the right tibia (Figure 1). Given the rapid growth of the lesion and its proximity to the surgical site, a punch biopsy was performed.
Histopathologic examination demonstrated a densely cellular dermal tumor composed of spindle cells with large hyperchromatic nuclei, numerous mitotic figures, and minimal eosinophilic cytoplasm (Figure 2A). Immunohistochemical studies revealed that approximately 40% of the tumor nuclei were immunoreactive to Ki-67 (Figure 2B), and total cytokeratin was focally positive (Figure 2C). A diagnosis of metastatic adamantinoma was made. Positron emission tomography and magnetic resonance imaging revealed new lytic lesions involving the T10 and L2 vertebrae (without frank spinal cord compression) and the right superior sacrum. Additionally, a small pulmonary nodule on the left upper lobe was noted on positron emission tomography, but it was below the size threshold for reliable detection. A computed tomography–guided biopsy of the T10 lesion demonstrated metastatic adamantinoma. The patient underwent a spinal stabilization procedure and discussed options regarding further oncologic and palliative management.
Adamantinoma is an extremely rare primary malignant bone tumor that typically involves the anterior portion of the tibial metaphysis or diaphysis in approximately 90% of cases. Young adults most commonly are affected in the third or fourth decades of life.1 Although the histogenesis is not clearly understood, experts have theorized that fetal implantation during embryogenesis or traumatic implantation of epithelial cells may be causes of this tumor and may explain the close pathologic similarity to basal cell carcinoma.2
Adamantinomas are slow growing, and as a result, patients often present with gradual onset of pain and swelling that persists for years.3,4 Metastasis occurs in 10% to 30% of patients, typically located in regional lymph nodes, the lungs, and distant bone.1,4 Our case represents a rare instance of adamantinoma metastasis to the skin. Although primary adamantinomas consist of both epithelial and stromal components, the typical metastatic lesions of adamantinomas are solely epithelial (often in a spindle-cell pattern),1 as was seen in our patient.
Operative removal via amputation or en bloc resection with limb salvage is the current treatment of choice. Adamantinomas are highly radioresistant, and chemotherapy has shown minimal efficacy.3,5
In conclusion, the presence of cutaneous metastasis from an adamantinoma is rare. Our case emphasizes this tumor’s potential for late metastasis as well as late recurrence.3,6 Most importantly, dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
- Schowinsky JT, Ormond DR, Kleinschmidt-DeMasters BK. Tibial adamantinoma: late metastasis to the brain. J Neuropathol Exp Neurol. 2015;74:95-97.
- Jain D, Jain VK, Vasishta RK, et al. Adamantinoma: a clinicopathological review and update. Diagn Pathol. 2008;3:8.
- Qureshi AA, Shott S, Mallin BA, et al. Current trends in the management of adamantinoma of long bones. an international study. J Bone Joint Surg Am. 2000;82-A:1122-1131.
- Desai SS, Jambhekar N, Agarwal M, et al. Adamantinoma of tibia: a study of 12 cases. J Surg Oncol. 2006;93:429-433.
- Weiss SW, Dorfman HD. Adamantinoma of long bone. an analysis of nine new cases with emphasis on metastasizing lesions and fibrous dysplasia-like changes. Hum Pathol. 1977;8:141-153.
- Szendroi M, Antal I, Arató G. Adamantinoma of long bones: a long-term follow-up study of 11 cases. Pathol Oncol Res. 2009;15:209-216.
Practice Points
- Metastatic adamantinoma of the skin is a rare clinical scenario.
- Dermatologists should be made aware of this rare bone tumor and its unusual presentation, as early detection can aid in prognosis.
Rapidly Growing Retroauricular Tumor
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
The Diagnosis: Milia En Plaque
Biopsy results revealed a normal epidermis; the dermis showed multiple small cystic structures lined by a stratified squamous epithelium containing eosinophilic keratin surrounded by a mononuclear cell infiltrate and some melanophages (Figure).
Milia en plaque was first described in 1903 by Balzer and Fouquet.1 In 1978, Hubler et al2 presented 2 cases with an asymptomatic, erythematous, and edematous plaque and white milialike lesions. On histopathology, they showed multiple cystic structures characterized by central laminated keratin and an intense polymorphic inflammatory reaction surrounding the cyst and epidermal appendages. Both patients were treated with topical tretinoin with complete response at 3 months. The authors suggested the term milia en plaque to describe this clinical entity.2
Milia en plaque is described as an infrequent condition that more often presents on the head, neck, and trunk, as well as the periocular, periauricular, and perinasal areas. It has been reported to occur at any age3 but appears more frequently in middle-aged adults and females. A congenital case also has been reported.4 It has been associated with pseudoxanthoma elasticum, lichen planus, trauma, kidney transplant, and cyclosporine use, but it also can present in healthy individuals,3 as in our patient. No clear cause has been identified.
Pathology is characteristic, with multiple cysts filled with keratin and surrounded by 2 or 3 layers of epithelial cells, associated with a mononuclear, nonlichenoid, mononuclear infiltrate.5 Structures similar to follicular infundibular tumors have been described, suggesting a common origin of follicular lesions as milia en plaque.6
Treatment includes surgical excision, cryosurgery, dermabrasion, electrodesiccation, trichloroacetic acid, photodynamic therapy, CO2 and erbium lasers, topical retinoids, minocycline, and etretinate.7 We performed a complete surgical excision in our patient.
In acneform reactions, erythematous papules and pustules can be found on the cheeks and forehead. Nevus comedonicus appears during childhood and presents with multiple open comedones. Postinflammatory milia is present in chronic inflammatory pathologies such as porphyria cutanea tarda. Histopathologic findings in adnexal tumors show a benign proliferation of any cellular type of a cutaneous annex.
Milia en plaque is an unusual but benign condition that is distinguished clinically by its characteristic presentation.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
- Balzer F, Fouquet C. Milium confluent retroauricularies bilateral. Bull Soc Fr Dermatol Syphiligr. 1903;14:361.
- Hubler WR, Rudolph AH, Kelleher RM. Milia en plaque. Cutis. 1978;22:67-70.
- Berk DR, Bayliss SJ. Milia: a review and classification. J Am Acad Dermatol. 2008;59:1050-1063.
- Wang AR, Bercovitch L. Congenital milia en plaque. Pediatr Dermatol. 2016;33:258-259.
- Muñoz-Martínez R, Santamarina-Albertos A, Sanz-Muñoz C, et al. Milia en plaque. Actas Dermosifiliogr. 2013;104:638-640.
- Terui H, Hashimoto A, Yamasaki K, et al. Milia en plaque as a distinct follicular hamartoma with cystic trichoepitheliomatous features. Am J Dermatopathol. 2016;38:212-217.
- Tenna S, Filoni A, Pagliarello C, et al. Eyelid milia en plaque: a treatment challenge with a new CO2 fractional laser. Dermatol Ther. 2014;27:65-67.
A 72-year-old man with a history of hypertension presented with a rapidly growing left retroauricular tumor of 3 months' duration. When manipulated, whitish material with a foul-smelling odor was expressed from the lesion. Physical examination showed an erythematous 3.2 ×1-cm tumor on the left posterior ear with multiple 1- to 2-mm white-yellow papules on its surface. A biopsy of the lesion was performed.
Impact of Psoriasis Treatment on Comorbidities
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
1. Elmets CA, Leonardi CL, Davis DMR, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with awareness and attention to comorbidities. J Am Acad Dermatol. 2019;80:1073-1113.
2. Davidovici BB, Sattar N, Prinz J, et al. Psoriasis and systemic inflammatory diseases: potential mechanistic links between skin disease and co-morbid conditions. J Invest Dermatol. 2010;130:1785-1796.
3. Oliveira Mde F, Rocha Bde O, Duarte GV. Psoriasis: classical and emerging comorbidities. An Bras Dermatol. 2015;90:9-20.
4. Shah K, Mellars L, Changolkar A, Feldman SR. Real-world burden of comorbidities in US patients with psoriasis. J Am Acad Dermatol. 2017;77:287-292.
5. Hu SC, Lan CE. Psoriasis and cardiovascular comorbidities: focusing on severe vascular events, cardiovascular risk factors and implications for treatment [published online October 21, 2017]. Int J Mol Sci. doi:10.3390/ijms18102211.
6. Hugh J, Van Voorhees AS, Nijhawan RI, et al. From the Medical Board of The National Psoriasis Foundation: the risk of cardiovascular disease in individuals with psoriasis and the potential impact of current therapies. J Am Acad Dermatol. 2014;70:168-177.
7. Churton S, Brown L, Shin TM, et al. Does treatment of psoriasis reduce the risk of cardiovascular disease? Drugs. 2014;74:169-182.
8. Prodanovich S, Ma F, Taylor J, et al. Methotrexate reduces incidence of vascular diseases in veterans with psoriasis or rheumatoid arthritis. J Am Acad Dermatol. 2005;52:262-226.
9. Gulliver WP, Young HM, Bachelez H, et al. Psoriasis patients treated with biologics and methotrexate have a reduced rate of myocardial infarction: a collaborative analysis using international cohorts. J Cutan Med Surg. 2016;20:550-554.
10. Ahlehoff O, Skov L, Gislason G, et al. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197-204.
11. Wu JJ, Poon KY, Channual JC, et al. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148:1244-1250.
12. Wu JJ, Poon KY. Association of ethnicity, tumor necrosis factor inhibitor therapy, and myocardial infarction risk in patients with psoriasis. J Am Acad Dermatol. 2013;69:167-168.
13. Wu JJ, Poon KY, Bebchuk JD. Association between the type and length of tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. J Drugs Dermatol. 2013;12:899-903.
14. Wu JJ, Poon KY, Bebchuk JD. Tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis, psoriatic arthritis, or both. J Drugs Dermatol. 2014;13:932-934.
15. Famenini S, Sako EY, Wu JJ. Effect of treating psoriasis on cardiovascular co-morbidities: focus on TNF inhibitors. Am J Clin Dermatol. 2014;15:45-50.
16. Nguyen T, Wu JJ. Relationship between tumor necrosis factor-alpha inhibitors and cardiovascular disease in psoriasis: a review. Perm J. 2014;18:49-54.
17. Shaaban D, Al-Mutairi N. The effect of tumour necrosis factor inhibitor therapy on the incidence of myocardial infarction in patients with psoriasis: a retrospective study [published online November 17, 2017]. J Dermatol Treat. doi:10.1080/09546634.2016.1254145.
18. Wu D, Hou SY, Zhao S, et al. Efficacy and safety of interleukin-17 antagonists in patients with plaque psoriasis: A meta-analysis from phase 3 randomized controlled trials. J Eur Acad Dermatol Venereol. 2017;31:992-100.
19. Yang ZS, Lin NN, Li L, et al. The effect of TNF inhibitors on cardiovascular events in psoriasis and psoriatic arthritis: an updated meta-analysis. Clin Rev Allergy Immunol. 2016;51:240-247.
20. Heredi E, Vegh J, Pogacsas L, et al. Subclinical cardiovascular disease and it’s improvement after long-term TNF-alpha inhibitor therapy in severe psoriatic patients. J Eur Acad Dermatol Venereol. 2016;30:1531-1536.
21. Pina T, Corrales A, Lopez-Mejias R, et al. Anti-tumor necrosis factor-alpha therapy improves endothelial function and arterial stiffness in patients with moderate to severe psoriasis: a 6-month prospective study. J Dermatol. 2016;43:1267-1272.
22. Piaserico S, Osto E, Famoso G, et al. Treatment with tumor necrosis factor inhibitors restores coronary microvascular function in young patients with severe psoriasis. Atherosclerosis. 2016;251:25-30.
23. Van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: a pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol. 2016;75:83-98.
24. Wu JJ, Guerin A, Sundaram M, et al. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor-alpha inhibitors versus methotrexate. J Am Acad Dermatol. 2017;76:81-90.
25. Torres T, Raposo I, Selores M. IL-17 blockade in psoriasis: friend or foe in cardiovascular risk? Am J Clin Dermatol. 2016;17:107-112.
26. Deeks ED. Apremilast: a review in psoriasis and psoriatic arthritis. Drugs. 2015;75:1393-1403.
27. Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >/= 156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.
28. Kavanaugh A, Mease PJ, Gomez-Reino JJ, et al. Treatment of psoriatic arthritis in a phase 3 randomised, placebo-controlled trial with apremilast, an oral phosphodiesterase 4 inhibitor. Ann Rheum Dis. 2014;73:1020-1026.
29. Daudén E, Griffiths CE, Ortonne JP, et al. Improvements in patient-reported outcomes in moderate-to-severe psoriasis patients receiving continuous or paused etanercept treatment over 54 weeks: the CRYSTEL study. J Eur Acad Dermatol Venereol. 2009;23:1374-1382.
30. Menter A, Augustin M, Signorovitch J, et al. The effect of adalimumab on reducing depression symptoms in patients with moderate to severe psoriasis: a randomized clinical trial. J Am Acad Dermatol. 2010;62:812-818.
31. Tyring S, Gottlieb A, Papp K, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29-35.
32. Strober B, Gooderham M, de Jong EMGJ, et al. Depressive symptoms, depression, and the effect of biologic therapy among patients in Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Am Acad Dermatol. 2018;78:70-80.
33. Egeberg A, Khalid U, Gislason GH, et al. Association of psoriatic disease with uveitis: a Danish nationwide cohort study. JAMA Dermatol. 2015;151:1200-1205.
34. Huynh N, Cervantes-Castaneda RA, Bhat P, et al. Biologic response modifier therapy for psoriatic ocular inflammatory disease. Ocul Immunol Inflamm. 2008;16:89-93.
35. Pulusani S, McMurray SL, Jensen K, et al. Psoriasis treatment in patients with sickle cell disease Cutis. 2019;103:93-94.
36. Nnodim J, Meludu SC, Dioka CE, et al. Cytokine expression in homozygous sickle cell anaemia. JKIMSU. 2015;4:34-37.
The ABCs of COCs: A Guide for Dermatology Residents on Combined Oral Contraceptives
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4

Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4

Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
The American Academy of Dermatology confers combined oral contraceptives (COCs) a strength A recommendation for the treatment of acne based on level I evidence, and 4 COCs are approved for the treatment of acne by the US Food and Drug Administration (FDA).1 Furthermore, when dermatologists prescribe isotretinoin and thalidomide to women of reproductive potential, the iPLEDGE and THALOMID Risk Evaluation and Mitigation Strategy (REMS) programs require 2 concurrent methods of contraception, one of which may be a COC. In addition, COCs have several potential off-label indications in dermatology including idiopathic hirsutism, female pattern hair loss, hidradenitis suppurativa, and autoimmune progesterone dermatitis.
Despite this evidence and opportunity, research suggests that dermatologists underprescribe COCs. The National Ambulatory Medical Care Survey found that between 1993 and 2008, dermatologists in the United States prescribed COCs to only 2.03% of women presenting for acne treatment, which was less often than obstetricians/gynecologists (36.03%) and internists (10.76%).2 More recently, in a survey of 130 US dermatologists conducted from 2014 to 2015, only 55.4% reported prescribing COCs. This survey also found that only 45.8% of dermatologists who prescribed COCs felt very comfortable counseling on how to begin taking them, only 48.6% felt very comfortable counseling patients on side effects, and only 22.2% felt very comfortable managing side effects.3
In light of these data, this article reviews the basics of COCs for dermatology residents, from assessing patient eligibility and selecting a COC to counseling on use and managing risks and side effects. Because there are different approaches to prescribing COCs, readers are encouraged to integrate the information in this article with what they have learned from other sources.
Assess Patient Eligibility
In general, patients should be at least 14 years of age and have waited 2 years after menarche to start COCs. They can be taken until menopause.1,4 Contraindications can be screened for by taking a medical history and measuring a baseline blood pressure (Tables 1 and 2).5 In addition, pregnancy should be excluded with a urine or serum pregnancy test or criteria provided in Box 2 of the 2016 US Selected Practice Recommendations for Contraceptive Use from the Centers for Disease Control and Prevention (CDC).4 Although important for women’s overall health, a pelvic examination is not required to start COCs according to the CDC and the American Academy of Dermatology.1,4

Select the COC
Combined oral contraceptives combine estrogen, usually in the form of ethinyl estradiol, with a progestin. Data suggest that all COCs effectively treat acne, but 4 are specifically FDA approved for acne: ethinyl estradiol–norethindrone acetate–ferrous fumarate, ethinyl estradiol–norgestimate, ethinyl estradiol–drospirenone, and ethinyl estradiol–drospirenone–levomefolate.1 Ethinyl estradiol–desogestrel and ethinyl estradiol–drospirenone are 2 go-to COCs for some of the attending physicians at my residency program. All COCs are FDA approved for contraception. When selecting a COC, one approach is to start with the patient’s drug formulary, then consider the following characteristics.
Monophasic vs Multiphasic
All the hormonally active pills in a monophasic formulation contain the same dose of estrogen and progestin; however, these doses change per pill in a multiphasic formulation, which requires that patients take the pills in a specific order. Given this greater complexity and the fact that multiphasic formulations often are more expensive and lack evidence of superiority, a 2011 Cochrane review recommended monophasic formulations as first line.6 In addition, monophasic formulations are preferred for autoimmune progesterone dermatitis because of the stable progestin dose.
Hormone-Free Interval
Some COCs include placebo pills during which hormone withdrawal symptoms such as bleeding, pelvic pain, mood changes, and headache may occur. If a patient is concerned about these symptoms, choose a COC with no or fewer placebo pills, or have the patient skip the hormone-free interval altogether and start the next pack early7; in this case, the prescription should be written with instructions to allow the patient to get earlier refills from the pharmacy.
Estrogen Dose
To minimize estrogen-related side effects, the lowest possible dose of ethinyl estradiol that is effective and tolerable should be prescribed7,8; 20 μg of ethinyl estradiol generally is the lowest dose available, but it may be associated with more frequent breakthrough bleeding.9 The International Planned Parenthood Federation recommends starting with COCs that contain 30 to 35 μg of estrogen.10 Synthesizing this information, one option is to start with 20 μg of ethinyl estradiol and increase the dose if breakthrough bleeding persists after 3 cycles.
Progestin Type
First-generation progestins (eg, norethindrone), second-generation progestins (eg, norgestrel, levonorgestrel), and third-generation progestins (eg, norgestimate, desogestrel) are derived from testosterone and therefore are variably androgenic; second-generation progestins are the most androgenic, and third-generation progestins are the least. On the other hand, drospirenone, the fourth-generation progestin available in the United States, is derived from 17α-spironolactone and thus is mildly antiandrogenic (3 mg of drospirenone is considered equivalent to 25 mg of spironolactone).
Although COCs with less androgenic progestins should theoretically treat acne better, a 2012 Cochrane review of COCs and acne concluded that “differences in the comparative effectiveness of COCs containing varying progestin types and dosages were less clear, and data were limited for any particular comparison.”11 As a result, regardless of the progestin, all COCs are believed to have a net antiandrogenic effect due to their estrogen component.1
Counsel on Use
Combined oral contraceptives can be started on any day of the menstrual cycle, including the day the prescription is given. If a patient begins a COC within 5 days of the first day of her most recent period, backup contraception is not needed.4 If she begins the COC more than 5 days after the first day of her most recent period, she needs to use backup contraception or abstain from sexual intercourse for the next 7 days.4 In general, at least 3 months of therapy are required to evaluate the effectiveness of COCs for acne.1
Manage Risks and Side Effects
Breakthrough Bleeding
The most common side effect of breakthrough bleeding can be minimized by taking COCs at approximately the same time every day and avoiding missed pills. If breakthrough bleeding does not stop after 3 cycles, consider increasing the estrogen dose to 30 to 35 μg and/or referring to an obstetrician/gynecologist to rule out other etiologies of bleeding.7,8
Nausea, Headache, Bloating, and Breast Tenderness
These symptoms typically resolve after the first 3 months. To minimize nausea, patients should take COCs in the early evening and eat breakfast the next morning.7,8 For headaches that occur during the hormone-free interval, consider skipping the placebo pills and starting the next pack early. Switching the progestin to drospirenone, which has a mild diuretic effect, can help with bloating as well as breast tenderness.7 For persistent symptoms, consider a lower estrogen dose.7,8
Changes in Libido
In a systemic review including 8422 COC users, 64% reported no change in libido, 22% reported an increase, and 15% reported a decrease.12
Weight Gain
Although patients may be concerned that COCs cause weight gain, a 2014 Cochrane review concluded that “available evidence is insufficient to determine the effect of combination contraceptives on weight, but no large effect is evident.”13 If weight gain does occur, anecdotal evidence suggests it tends to be not more than 5 pounds. If weight gain is an issue, consider a less androgenic progestin.8
Venous Thromboembolism
Use the 3-6-9-12 model to contextualize venous thromboembolism (VTE) risk: a woman’s annual VTE risk is 3 per 10,000 women at baseline, 6 per 10,000 women with nondrospirenone COCs, 9 per 10,000 women with drospirenone-containing COCs, and 12 per 10,000 women when pregnant.14 Patients should be counseled on the signs and symptoms of VTE such as unilateral or bilateral leg or arm swelling, pain, warmth, redness, and/or shortness of breath. The British Society for Haematology recommends maintaining mobility as a reasonable precaution when traveling for more than 3 hours.15
Cardiovascular Disease
A 2015 Cochrane review found that the risk for myocardial infarction or ischemic stroke is increased 1.6‐fold in COC users.16 Despite this increased relative risk, the increased absolute annual risk of myocardial infarction in nonsmoking women remains low: increased from 0.83 to 3.53 per 10,000,000 women younger than 35 years and from 9.45 to 40.4 per 10,000,000 women 35 years and older.17
Breast Cancer and Cervical Cancer
Data are mixed on the effect of COCs on the risk for breast cancer and cervical cancer.1 According to the CDC, COC use for 5 or more years might increase the risk of cervical carcinoma in situ and invasive cervical carcinoma in women with persistent human papillomavirus infection.5 Regardless of COC use, women should undergo age-appropriate screening for breast cancer and cervical cancer.
Melasma
Melasma is an estrogen-mediated side effect of COCs.8 A study from 1967 found that 29% of COC users (N=212) developed melasma; however, they were taking COCs with much higher ethinyl estradiol doses (50–100 μg) than typically used today.18 Nevertheless, as part of an overall skin care regimen, photoprotection should be encouraged with a broad-spectrum, water-resistant sunscreen that has a sun protection factor of at least 30. In addition, sunscreens with iron oxides have been shown to better prevent melasma relapse by protecting against the shorter wavelengths of visible light.19
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945-973.e933.
- Landis ET, Levender MM, Davis SA, et al. Isotretinoin and oral contraceptive use in female acne patients varies by physician specialty: analysis of data from the National Ambulatory Medical Care Survey. J Dermatolog Treat. 2012;23:272-277.
- Fitzpatrick L, Mauer E, Chen CL. Oral contraceptives for acne treatment: US dermatologists’ knowledge, comfort, and prescribing practices. Cutis. 2017;99:195-201.
- Curtis KM, Jatlaoui TC, Tepper NK, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-66.
- Curtis KM, Tepper NK, Jatlaoui TC, et al. U.S. Medical Eligibility Criteria for Contraceptive Use, 2016. MMWR Recomm Rep. 2016;65:1-103.
- Van Vliet HA, Grimes DA, Lopez LM, et al. Triphasic versus monophasic oral contraceptives for contraception. Cochrane Database Syst Rev. 2011:CD003553.
- Stewart M, Black K. Choosing a combined oral contraceptive pill. Aust Prescr. 2015;38:6-11.
- McKinney K. Understanding the options: a guide to oral contraceptives. https://www.cecentral.com/assets/2097/022%20Oral%20Contraceptives%2010-26-09.pdf. Published November 5, 2009. Accessed June 20, 2019.
- Gallo MF, Nanda K, Grimes DA, et al. 20 microg versus >20 microg estrogen combined oral contraceptives for contraception. Cochrane Database Syst Rev. 2013:CD003989.
- Terki F, Malhotra U. Medical and Service Delivery Guidelines for Sexual and Reproductive Health Services. London, United Kingdom: International Planned Parenthood Federation; 2004.
- Arowojolu AO, Gallo MF, Lopez LM, et al. Combined oral contraceptive pills for treatment of acne. Cochrane Database Syst Rev. 2012:CD004425.
- Pastor Z, Holla K, Chmel R. The influence of combined oral contraceptives on female sexual desire: a systematic review. Eur J Contracept Reprod Health Care. 2013;18:27-43.
- Gallo MF, Lopez LM, Grimes DA, et al. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 2014:CD003987.
- Birth control pills for acne: tips from Julie Harper at the Summer AAD. Cutis. https://www.mdedge.com/dermatology/article/144550/acne/birth-control-pills-acne-tips-julie-harper-summer-aad. Published August 14, 2017. Accessed June 24, 2019.
- Watson HG, Baglin TP. Guidelines on travel-related venous thrombosis. Br J Haematol. 2011;152:31-34.
- Roach RE, Helmerhorst FM, Lijfering WM, et al. Combined oral contraceptives: the risk of myocardial infarction and ischemic stroke. Cochrane Database Syst Rev. 2015:CD011054.
- Acute myocardial infarction and combined oral contraceptives: results of an international multicentre case-control study. WHO Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Lancet. 1997;349:1202-1209.
- Resnik S. Melasma induced by oral contraceptive drugs. JAMA. 1967;199:601-605.
- Boukari F, Jourdan E, Fontas E, et al. Prevention of melasma relapses with sunscreen combining protection against UV and short wavelengths of visible light: a prospective randomized comparative trial. J Am Acad Dermatol. 2015;72:189-190.e181.
Resident Pearls
- Screen for contraindications to combined oral contraceptives (COCs) by taking a medical history, measuring a baseline blood pressure, and excluding pregnancy. A baseline pelvic examination is unnecessary.
- Characteristics to consider when selecting a COC include the formulation, hormone-free interval, estrogen dose, and progestin type.
- Combined oral contraceptives can be initiated on any day of the menstrual cycle, with the need for backup contraception based on the number of days since the first day of the patient’s most recent period.
- Management of risks and side effects includes simple lifestyle changes, skipping the hormone-free interval, switching the COC, and referring to an obstetrician/gynecologist.
A Unique Presentation of Lupus Erythematosus Tumidus in an Adolescent Boy
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
To the Editor:
Lupus erythematosus tumidus (LET) is a rarely diagnosed condition that was first described in 1909 by Hoffmann.1 Limited cases have been reported in the literature, with few documenting the disease in children.2 We report a unique clinical case of LET in a 14-year-old adolescent boy that was distributed solely on the hands. With slight heterogeneity in regards to clinical presentation and histopathology, there is a need for further exploration with regard to LET.
A 14-year-old adolescent boy presented to the dermatology clinic with progressive bilateral edema of 1 year’s duration with plaques and some scaling on the dorsal aspects of the digits and the nail bases predominantly on the right hand (Figure 1) and to a lesser extent on the left hand. The edema, erythema, and tenderness started in the right fifth digit; soon after the edema appeared, plaques began to form at the base of each nail bed, and the edema and erythema progressively spread to the other digits. He denied worsening of symptoms when exposed to cold temperatures. A complete review of systems was negative. The differential diagnoses included chilblain lupus erythematosus, perniosis, dermatomyositis, and polymorphous light eruption. A punch biopsy from the right fourth digit was performed.
The biopsy showed superficial and deep perivascular and periadnexal mononuclear inflammation with large amounts of interstitial mucin deposition (Figure 2). The epidermis exhibited a loose orthokeratotic scale with no signs of interface damage. A diagnosis of perniosis was entertained but was ruled out due to the lack of papillary dermal edema and large amounts of mucin. With the lack of interface change and large amounts of mucin, a diagnosis of LET was favored over chilblain lupus erythematosus, as the latter diagnosis typically demonstrates interface change. The patient was started on hydroxychloroquine 200 mg twice daily and a short course of prednisone, and improvement of the lesions/plaques was noted at follow-up 6 weeks later. Continued improvement was noted 2 years after the initial presentation. His condition recurred when the hydroxychloroquine dosage was reduced to 200 mg once daily after 1 year. The patient did not report any adverse sequelae to treatment.
Histopathologic findings of superficial and deep perivascular and periadnexal lymphocytic infiltrates and interstitial dermal deposition of mucin in LET have remained consistent in the literature. Direct immunofluorescence has not revealed any complement or immunoglobulin deposition on the basement membrane.3,4 The epidermal characteristics are not as uniform, with the majority of cases in one review showing no epidermal changes and a minority showing minimal epidermal changes (eg, epidermal atrophy, hyperkeratosis, parakeratosis, acanthosis, spongiosis).5 When working up patients for LET, blood work usually is unremarkable, as LET rarely is associated with antinuclear antibodies or anti-Ro, anti-La, and anti-DNA antibodies.3,4 Lupus erythematosus tumidus generally is an independent process, but it has been reported to coexist with discoid lupus erythematosus and systemic lupus erythematosus in rare cases.6
The lesions of LET have been consistently described in the literature as photosensitive, erythematous, non-scarring, annular plaques and papules commonly occurring on the head/neck and other sun-exposed areas that do not cause hypopigmentation.3 Treatment of LET consists of systemic treatment with antimalarial drugs, sunscreens, and topical steroids for flares.
Lupus erythematosus tumidus is rare in children, with few case reports noted in the literature. Sonntag et al2 documented the disease in 3 children ranging from 3 to 8 years of age. Furthermore, Ruiz and Sanchez7 reported a case of LET in a 16-year-old adolescent girl. Our case is unique in that the lesions only occurred on the hands, whereas most case reports document distribution of the lesions on the head, neck, face, arms, back, and chest. Our patient’s age and the location of the lesions make it a unique clinical presentation of LET.
Reports in the literature show evidence of heterogeneity in the presentation, classification, and some of the histopathologic features of LET; however, there are minimal data on childhood LET. Further research and investigations are needed to more precisely define this condition.
Acknowledgment
The authors acknowledge Richard Schwartz, MD (Akron, Ohio), for reading the biopsy reports and assisting with photomicrographs.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
- Hoffmann E. Demonstrationen: lupus erythematosus tumidus. Derm Zeitschr. 1909;16:159-160.
- Sonntag M, Lehmann P, Megahed M, et al. Lupus erythematosus tumidus in childhood. Dermatology. 2003;207:188-192.
- Schmitt V, Meuth AM, Amler S, et al. Lupus erythematosus tumidus is a separate subtype of cutaneous lupus erythematosus. Br J Dermatol. 2010;162:64-73.
- Vieira V, Del Pozo J, Yebra-Pimentel MT, et al. Lupus erythematosus tumidus: a series of 26 cases. Int J Dermatol. 2006;45:512-517.
- Kuhn A, Richter-Hintz D, Oslislo C, et al. Lupus erythematosus tumidus—a neglected subset of cutaneous lupus erythematosus: report of 40 cases. Arch Dermatol. 2000;136:1033-1041.
- Chen X, Wang S, Li L. A case report of lupus erythematosus tumidus converted from discoid lupus erythematosus. Medicine (Baltimore). 2018;97:e0375.
- Ruiz H, Sanchez J. Tumid lupus erythematosus. Am J Dermatopathol. 1999;21:356-360.
Practice Points
- Lupus erythematosus tumidus rarely occurs in the pediatric population.
- Lupus erythematosus tumidus is a unique subset of lupus associated with lack of interface change on histology and large amounts of mucin.
- Lesions typically present on the face and trunk but can very rarely present on the extremities and hands.