User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Solar Urticaria Treated With Omalizumab
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
To the Editor:
First documented in 1904,1 solar urticaria is an IgE-induced condition that predominantly occurs in women aged 20 to 50 years. Worldwide prevalence and incidence information is lacking, but it is known to occur in up to 0.4% of urticaria cases.2 Solar urticaria is characterized by pruritus of the skin with erythematous wheals and flares in reaction to sunlight exposure, even despite partial protection by barriers such as glass or clothing.2,3 It can have an acute or chronic presentation caused by visible or UV light wavelengths. Solar urticaria can lead to debilitating symptoms and psychological stressors that can severely impact a patient’s well-being and also may be accompanied by conditions such as polymorphous light eruption, angioedema, or vasculitis.4 Standard treatments include first- and second-generation antihistamines, which are efficacious approximately 50% of the time, as well as phototherapy, which can be time consuming and a burden on patients who work or go to school full time.2 Other possible treatment modalities include plasmapheresis, intravenous immunoglobulins, steroids, cyclosporine, and anti-IgE recombinant monoclonal antibody injections.5,6 We present the case of a patient who was successfully treated with subcutaneous injections of omalizumab every 3 weeks to add to the growing number of case reports of treatment of solar urticaria.
A 30-year-old woman with Fitzpatrick skin type III and a 9-year history of solar urticaria was referred to the Department of Allergy and Immunology by her primary care physician. The patient reported that redness, swelling, and itching would occur on sun-exposed areas of the skin after approximately 10 minutes of exposure despite daily sunscreen application. She had been successfully treated with hydroxychloroquine 400 mg once daily after her first formal evaluation by dermatology 4 years prior to the current presentation. She subsequently self-discontinued treatment after 8 months of treatment due to resolution of symptoms. She noted the symptoms had returned upon relocating to Hawaii after living in the continental United States and Italy. Initially she was restarted on hydroxychloroquine 200 mg once daily and 4-times the recommended daily dose of second-generation antihistamines without relief. The hydroxychloroquine dosage subsequently was increased to 400 mg once daily, but her symptoms did not resolve.
On physical examination, sun-exposed areas of the skin showed marked macular erythema with discrete erythematous lines of demarcation observed between exposed and unexposed skin. The patient also reported concomitant pruritus, which antihistamines did not alleviate. A maximum 1-year course of cyclosporine 300 mg once daily initially was planned but was discontinued due to immediate onset of severe nausea and emesis after the first dose as well as continued outbreaks of urticaria for 1 month after incrementally increasing by 100 mg from a starting dose of 100 mg.
After discussion with the dermatology department, a trial of omalizumab was started because the daily impact of a UV light sensitization course was not feasible with her work schedule, and serum IgE blood level was 560.4 µg/L (reference range, 0–1500 µg/L). The patient was started on a regimen of omalizumab 300 mg (subcutaneous injections) every 2 weeks with noted improvement after the third dose, with no urticarial symptoms after sun exposure. After 2 months, the dosage interval was increased to every 4 weeks given her level of improvement, but her symptoms recurred. The treatment regimen was then changed to every 3 weeks. The patient was symptom free for a period of 10 months on this regimen, followed by only 1 outbreak of erythema and urticaria, which occurred 1 day prior to a scheduled omalizumab injection. Symptoms have otherwise been well controlled to date on omalizumab.
Solar urticaria is a poorly understood phenomenon that has no clear prognostic indicators; therefore, diagnosis often is made based on the patient’s history and physical examination. Further testing to confirm the diagnosis can be performed using specific wavelengths of UV light to determine which band of light affects patients most; however, the wavelength can change over time, leading to less clinical significance, and may decrease efficacy of phototherapy.2 Solar urticaria has no clear predisposing factors, and treatments to date have been moderately successful. Exposure to sunlight is thought to initiate an alteration in a skin or serum chromophore or photoallergen, which then causes subsequent cross-linking and IgE-dependent release of histamine as well as other mediators such as cytokines, eicosanoids, and proteases with mast cell degranulation.7
Omalizumab is a recombinant humanized monoclonal IgG1 antibody targeting the methylated IgE Cε3 domain that initially was marketed toward controlling IgE-mediated moderate to severe asthma recalcitrant to standard treatments. It has since received approval from the US Food and Drug Administration for treatment of chronic idiopathic urticaria after first being noticed to serendipitously treat a patient with cold urticaria and asthma in 2006.4,7,8 It was then first documented to successfully treat solar urticaria in 2008.6 The safety profile of omalizumab makes it a more favorable choice when compared to other immunomodulating treatments, with the most serious adverse reaction being anaphylaxis, occurring in 0.2% of patients in a postmarketing study.9 It functions through binding to free IgE at a region necessary for IgE to bind at low- and high-affinity receptors but not to immunoglobulins already bound to cells, thus theoretically preventing activation of mast cells or basophils.10 It also has been suggested that low steady-state values are needed to see continued benefit from the drug,10 which may have been seen in our patient after having an outbreak just prior to receiving an injection; however, prior reports have shown benefit unrelated to total IgE levels, with improvement after days to 4 months.4,10,11 One case report showed no response after 4 doses; it is unknown if this patient was tested for clinical improvement to omalizumab through further immunoglobulin analysis, but treatment response is important to consider when deciding on whether to use this drug in future patients.12 It is unknown why some patients will respond to omalizumab, others will partially respond, and others will not respond, which can be ascertained either through quality-of-life improvement or lack thereof.
In our experience, omalizumab is a viable option to consider in patients with solar urticaria that is recalcitrant to standard treatments and elevated IgE levels for whom other treatments are either too time consuming or have side-effect profiles that are not tolerable to the patient. If the patient has concomitant asthma, there may be additional therapeutic benefit. Further research is needed with regard to a cost-benefit analysis of omalizumab and whether using such a costly drug outweighs the cost associated with time and resources utilized with repeat clinic visits if other standard treatments are not effective.13
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
- Merkin P. Pratique Dermatologique. Paris, France: Masso; 1904.
- Beattie PE, Dawe RS, Ibbotson SH, et al. Characteristics and prognosis of idiopathic solar urticaria: a cohort of 87 cases. Arch Dermatol. 2003;139:1149-1154.
- Kaplan AP. Therapy of chronic urticaria: a simple, modern approach. Ann Allergy Asthma Immunol. 2014;112:419-425.
- Metz M, Maurer M. Omalizumab in chronic urticaria. Curr Opin Allergy Clin Immunol. 2012;12:406-410.
- Aubin F, Porcher R, Jeanmougin M, et al. Severe and refractory solar urticaria treated with intravenous immunoglobulins: a phase II multicenter study. J Am Acad Dermatol. 2014;71:948-953.e1.
- Güzelbey O, Ardelean E, Magerl M, et al. Successful treatment of solar urticaria with anti-immunoglobulin E therapy. Allergy. 2008;63:1563-1565.
- Wu K, Jabbar-Lopez Z. Omalizumab, an anti-IgE mAb, receives approval for the treatment of chronic idiopathic/spontaneous urticaria. J Invest Dermatol. 2015;135:13-15.
- Boyce JA. Successful treatment of cold-induced urticaria/anaphylaxis with anti-IgE. J Allergy Clin Immunol. 2006;117:1415-1418.
- Corren J, Casale TB, Lanier B, et al. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39:788-797.
- Wu K, Long H. Omalizumab for chronic urticaria. N Engl J Med. 2013;368:2527-2528.
- Morgado-Carrasco D, Giacaman-Von der Weth M, Fusta-Novell X, et al. Clinical response and long-term follow-up of 20 patients with refractory solar urticarial under treatment with omalizumab [published online May 28, 2019]. J Am Acad Dermatol. doi:10.1016/j.jaad.2019.05.070.
- Duchini G, Bäumler W, Bircher AJ, et al. Failure of omalizumab (Xolair®) in the treatment of a case of solar urticaria caused by ultraviolet A and visible light. Photodermatol Photoimmunol Photomed. 2011;27:336-337.
- Bernstein JA, Lang DM, Khan DA, et al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014;133:1270-1277.
Practice Points
- Recurrent solar urticaria can be recalcitrant to treatment.
- Omalizumab may be an effective treatment option for solar urticaria, especially in patients with a concomitant asthma diagnosis.
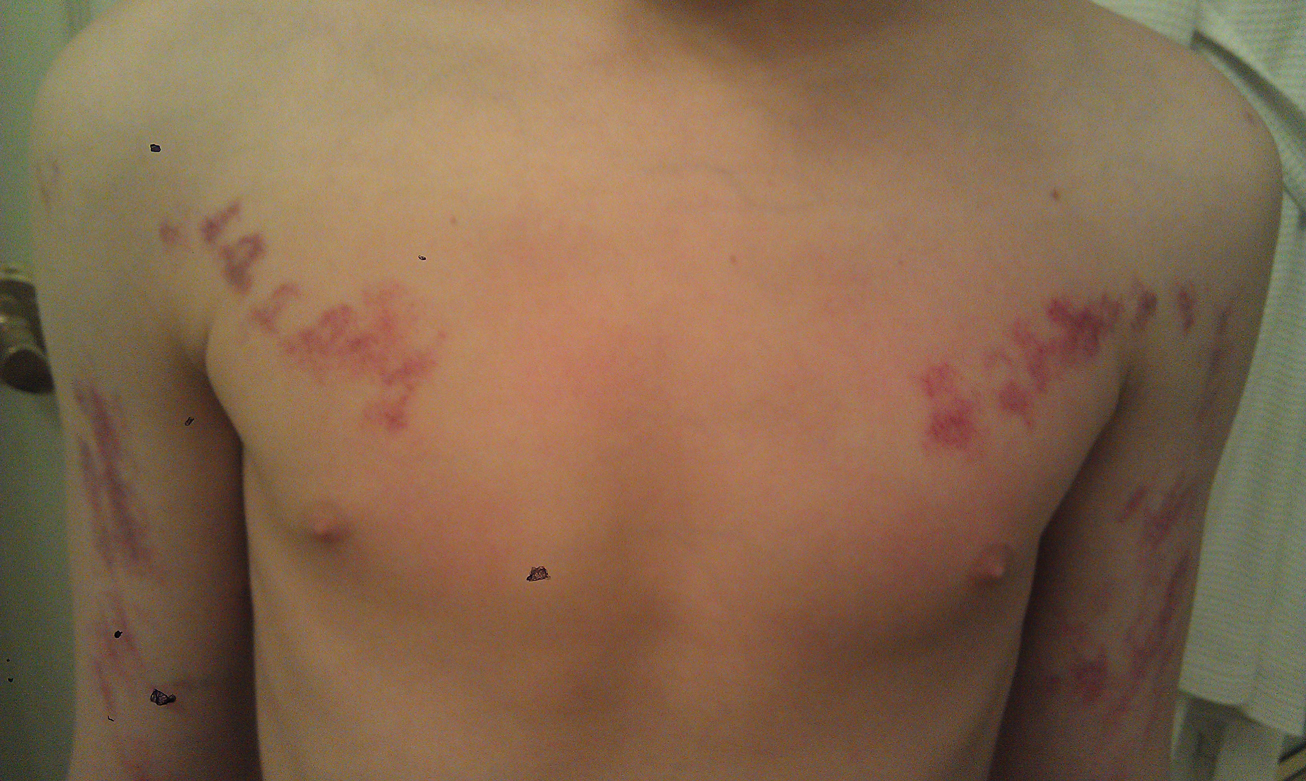
Painless Purple Streaks on the Arms and Chest
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
The Diagnosis: Factitial Purpura
Factitial dermatologic disorders are characterized by skin findings triggered by deliberate manipulation of the skin with objects to create lesions and feign signs of a dermatologic condition to seek emotional and psychological benefit.1 The etiology of the lesions is unclear, and the patient's history of the injury is hollow.2 Most often, there is sudden onset of the lesions without any warning or symptoms. When giving the history, the patient may appear unemotional, does not report pain, and denies self-infliction.1
In factitial purpura, the purple patches are clearly demarcated from uninvolved skin and have an unusual angular or geometric shape. The pattern typically takes the shape of the object used to create the purpura and lacks the features of recognizable dermatoses.2 In our patient and those with similar linear purpuric streaks, we use the term penny purpura to indicate that the lesions resulted from rubbing with a penny or other blunt object, similar to coining. The lesions occur in areas that are easily accessible and visible such as the arms, chest, or chin. It is suggested that the child unconsciously wants the lesions to be seen. Histologic findings in factitial purpura include disruption of collagen fiber bundles and extravasated red blood cells in the dermis.3 Unfortunately, evolving lesions may give nonspecific histologic findings; when the clinical lesions are typical, skin biopsy usually is unnecessary and may be misleading. Laboratory test results such as complete blood cell count, prothrombin time, and partial thromboplastin time usually are within reference range, as in our patient.
When evaluating these patients, confrontation is not recommended. More than two-thirds of affected patients have a history of trauma such as sexual/physical abuse or neglect, and the lesions typically arise during times of stress.1,3 Thus, treatment includes nonaccusatory measures and referral for psychologic evaluation. The purpura will rapidly heal when covered with an occlusive dressing.2
The differential diagnosis for penny purpura includes lesions that evolve from cupping and coining. Cupping is a type of complementary and alternative medicine that acts by correcting imbalances in the internal biofield and restoring the flow of qi, which determines the state of one's health and life span.4 Cupping is performed by placing a glass cup over a painful body part. A partial vacuum is created by flaming, mechanical withdrawal, or thermal cooling of the entrapped air under the cup. When the flame exhausts the supply of oxygen, the skin is sucked into the mouth of the glass, and the skin is bruised painlessly.4
The differential also includes child maltreatment syndrome and other disorders that would potentiate bruising. Intravascular etiologies include idiopathic thrombocytopenic purpura, leukemia, coagulation disorders, and other causes of thrombocytopenia or platelet dysfunction.3 Extravascular etiologies include hereditary collagen vascular disease (eg, Ehlers-Danlos syndrome), malnutrition, and other disorders associated with a decrease in collagen and other tissues that support cutaneous vessels. Vascular etiologies include infectious (eg, Rocky Mountain spotted fever, meningococcemia) and noninfectious vasculitis (eg, Henoch-Schönlein purpura), leaky capillary syndrome, drug reactions, and other disorders associated with a loss of vascular integrity.3
It is important to be able to differentiate self-inflicted lesions in a person who repeatedly acts as if he/she has a physical disorder from those that are created during the practices of cupping or any other cultural healing practice. Vascular disorders, malnutrition, and child abuse also should be excluded.3
For our patient with factitial purpura, we gently encouraged the family to work with the child's pediatrician and a pediatric psychologist to deal with stress related to the recurrent rash and asked them to think of the rash as a result of an external cause; however, we were careful not to blame anyone for the rash.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372; quiz 373.
- Al Hawsawi K, Pope E. Pediatric psychocutaneous disorders: a review of primary psychiatric disorders with dermatologic manifestations. Am J Clin Dermatol. 2011;12:247-257.
- Ring HC, Miller IM, Benfeldt E, et al. Artefactual skin lesions in children and adolescents: review of the literature and two cases of factitious purpura. Int J Dermatol. 2015;54:E27-E32.
- Mehta P, Dhapte V. Cupping therapy: a prudent remedy for a plethora of medical ailments. J Tradit Complement Med. 2015;5:127-134.
A 10-year-old boy presented with painless purple streaks on the arms and chest of 2 months' duration. The rash recurred several times per month and cleared without treatment in 3 to 5 days. There was no history of trauma or medication exposure, and he was growing and developing normally.
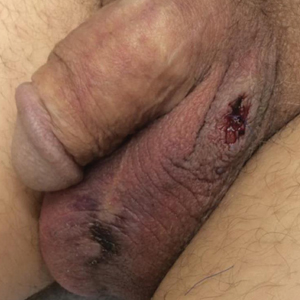
Scrotal Ulceration: A Complication of Hyperthermic Intraperitoneal Chemotherapy and Subsequent Treatment With Dimethyl Sulfoxide
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
To the Editor:
A 54-year-old man with a history of stage IV appendiceal carcinoid adenocarcinoma treated approximately 3 months prior with intraoperative hyperthermic intraperitoneal chemotherapy (HIPEC) presented to our clinic with scrotal pain of 5 days’ duration. He had no history of genital herpes, topical contactants, other cutaneous lesions on the body, fever, or chills. On physical examination the patient had an erythematous, purpuric, indurated, tender plaque on the left anterolateral and anterior midline of the scrotum (Figure 1). No other areas of acral purpura or livedoid cutaneous changes were identified. There was no inguinal lymphadenopathy. Biopsy was performed for histologic examination as well as tissue culture. Histology demonstrated epidermal necrosis without evidence of vasculitis. Tissue culture was unremarkable.
Two days after clinic evaluation, the patient presented to the emergency department with progression of the lesions, and he was admitted to the hospital for pain control. Computed tomography of the pelvis showed bilateral hydroceles without evidence of abscess. Ultrasonography showed scrotal thickening without abscess or fluid collection. On day 5 in the hospital, a regimen of topical 60% dimethyl sulfoxide (DMSO) was applied every 8 hours to the affected area. The patient experienced notable pain relief and a decrease in erythema within 7 hours of application (Figure 2). This regimen was continued for 7 days with improvement in surrounding erythema and pain; however, the patient’s pain persisted in the areas of necrosis. Fourteen days following completion of therapy (27 days following presentation), the patient underwent debridement and partial scrotal resection for eschar removal. Histologic examination of the debrided scrotal tissue showed necrosis extending into the dermis and no evidence of vasculitis.
Our case demonstrates a unique presentation of scrotal necrosis secondary to mitomycin C (MitC) extravasation subsequently managed with DMSO. Imaging and biopsy findings effectively ruled out infection or vasculitis and led us to consider extravasation reactions that typically occur at peripheral intravenous (IV) infusion sites. Suspected cases of scrotal necrosis following HIPEC with MitC have been reported in the literature, along with hypothesized pathophysiology.1-3
In consideration of the proposed pathophysiology, individuals with hydroceles may be more likely to experience this complication due to an abnormal but not uncommon communication between the intraperitoneal cavity and the scrotum via a patent processus vaginalis. The location of necrosis on the anterior scrotum remains unexplained. It may be a consequence of the anatomic location of the hydrocele, a collection of fluid within the tunica vaginalis. The tunica vaginalis is composed of an inner visceral and outer parietal layer, enveloping the testis at the anterior border but not the superior or posterior border. Thus, sequestration of MitC in a hydrocele would correlate anatomically to necrosis of the anterior wall of the scrotum.
Akhavan et al1 proposed the testes are unaffected because of the presence of the tough fibrous coat of the tunica albuginea that directly adheres to the testes, in addition to the adjacent visceral layer of the tunica vaginalis. These 2 layers separating the testes and the hydrocele may provide a double barrier of protection for the testes.1
According to a PubMed search of articles indexed for MEDLINE using the terms scrotal or cutaneous, pain or ulceration, and HIPEC or hyperthermic in
Hyperthermic intraperitoneal chemotherapy involves installation of high-concentration chemotherapeutics into the peritoneal cavity at the conclusion of surgical cytoreductive therapy. Cell cycle–nonspecific agents such as MitC commonly are used for this procedure.4 It is classified as a vesicant, which is the designation given to drugs known to produce the most severe extravasation reactions of skin ulceration and necrosis.5,6 Symptoms typically include an early area of localized edema, erythema, and severe pain that progresses to superficial soft tissue and skin necrosis.7 Unfortunately, no well-studied antidote exists for MitC, though empirical guidelines suggest therapeutic management with DMSO and ice packs.6,8
Dimethyl sulfoxide is thought to work as a free radical scavenger as well as a solvent that facilitates diffusion of chemotherapeutics through tissues and thus down a concentration gradient, ideal in the circumstance of an extravasation reaction.8 Topical DMSO has been studied as a nonsurgical treatment in a small number of patients to prevent progression to necrosis following MitC extravasation.5,7 However, these cases only report extravasation reactions from IV infiltration.5,7,9 Dimethyl sulfoxide is rapidly absorbed and acts as a theoretical carrier for MitC as well as other topical substances.5,10,11 Caution is advised when using topical lidocaine or steroids in combination with DMSO, as they will be rapidly absorbed systemically. Patients also should be informed about a mild local burning sensation after DMSO application and a garliclike odor of the breath, which have occurred in 5.5% and 27.5% of patients, respectively (N=144).5 Dimethyl sulfoxide has no known toxic side effects but can cause erythema, pruritus, and very rarely allergic contact dermatitis.5,12 Abdul Aziz et al2 postulated that DMSO might be used as a method to prevent the progression of necrosis in symptomatic patients following HIPEC with MitC. Reports of its use on the scrotum are absent in the current available literature.
Treatment with DMSO was attempted in our patient with limited success secondary to delayed recognition and lack of supporting literature for DMSO treatment of scrotal necrosis. Treatment was delayed by 11 days after the onset of symptoms, which is far beyond the recommendation of starting within 10 minutes.8 Irreversible tissue necrosis had already occurred as evidenced by the presence of eschar. However, it seems apparent that DMSO provided some benefit given the clear improvement in erythema and pain 7 hours after application (Figure 2). It is unknown to what extent the necrosis would have progressed if not treated with DMSO.
Scrotal necrosis following HIPEC with MitC is a rare and incompletely understood but important chemotherapy reaction. The presentation is fairly specific with the presence of intractable and constant scrotal pain along with erythema and induration progressing to eschar. Although DMSO has been found to be effective for certain vesicant extravasation reactions at IV sites, it is not well studied for MitC, and no reports exist regarding its use on the scrotum. The presented characterization and explanation of the pathophysiology of this entity will aid in early recognition and timely institution of topical mitigating agents such as DMSO, which may prevent progression to scrotal necrosis and need for surgical debridement. More effective strategies may be geared toward prevention with thorough washout following HIPEC, preprocedural radiologic imaging or intraoperative visualization of the patent processus vaginalis, internal inguinal canal plugs, and patient education with anticipatory guidance should a reaction occur.2
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
- Akhavan A, Yin M, Benoit R. Scrotal ulcer after intraperitoneal hyperthermic chemotherapy. Urology. 2007;69:778.E9-E10.
- Abdul Aziz NH, Wang W, Teo MC. Scrotal pain and ulceration post HIPEC: a case report. J Gastrointest Cancer. 2015;46:60-63.
- Silva F, Avancini J, Criado P, et al. Scrotum ulcer developed after intraperitoneal hyperthermic chemotherapy with mitomycin-C [published October 21, 2012]. Bjui International. doi:10.1002/BJUIw-2012-019-web.
- González-Moreno S, González-Bayón LA, Ortega-Pérez G.Hyperthermic intraperitoneal chemotherapy: rationale and technique. World J Gastrointest Oncol. 2010;15:68-75.
- Bertelli G, Gozza A, Forno GB, et al. Topical dimethyl sulfoxide for the prevention of soft tissue injury after extravasation of vesicant cytotoxic drugs: a prospective clinical study. J Clin Oncol. 1995;13:2851-2855.
- Bertelli G. Prevention and management of extravasation of cytotoxic drugs. Drug Saf. 1995;12:245-255.
- Alberts DS, Dorr RT. Case report: topical DMSO for mitomycin-C-induced skin ulceration. Oncol Nurs Forum. 1991;18:693-695.
- Pérez Fidalgo JA, García Fabregat L, Cervantes A, et al; ESMO Guidelines Working Group. Management of chemotherapy extravasation: ESMO-EONS Clinical Practice Guidelines. Ann Oncol. 2012;23(suppl 5):167-173.
- Ludwig CU, Stoll HR, Obrist R, et al. Prevention of cytotoxic drug induced skin ulcers with dimethyl sulfoxide (DMSO) and alpha-tocopherole. Eur J Cancer Clin Oncol. 1987;23:327-329.
- Groel JT. Dimethyl sulfoxide as a vehicle for corticosteroids. a comparison with the occlusive dressing technique. Arch Dermatol. 1968;97:110-114.
- Simon LS, Grierson LM, Naseer Z. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis [published online April 19, 2009]. Pain. 2009;143:238-245.
- Nishimura M, Takano Y, Toshitani S. Systemic contact dermatitis medicamentosa occurring after intravesical dimethyl sulfoxide treatment for interstitial cystitis. Arch Dermatol. 1988;124:182-183.
Practice Points
- Scrotal ulceration following hyperthermic intraperitoneal chemotherapy has been reported only a few times in the literature and is likely underreported. The presentation in all reported cases was similar, with a delay in symptom onset of weeks to months, involvement of the anterior scrotum, and pain.
- Dimethyl sulfoxide, used in other vesicant reactions, may have a role in mitigating tissue damage. Alternatively, methods to prevent sequestration of vesicants in the potential space of the tunica vaginalis layers can be employed.
Ill-Defined Macule on the Abdomen
The Diagnosis: Microvenular Hemangioma
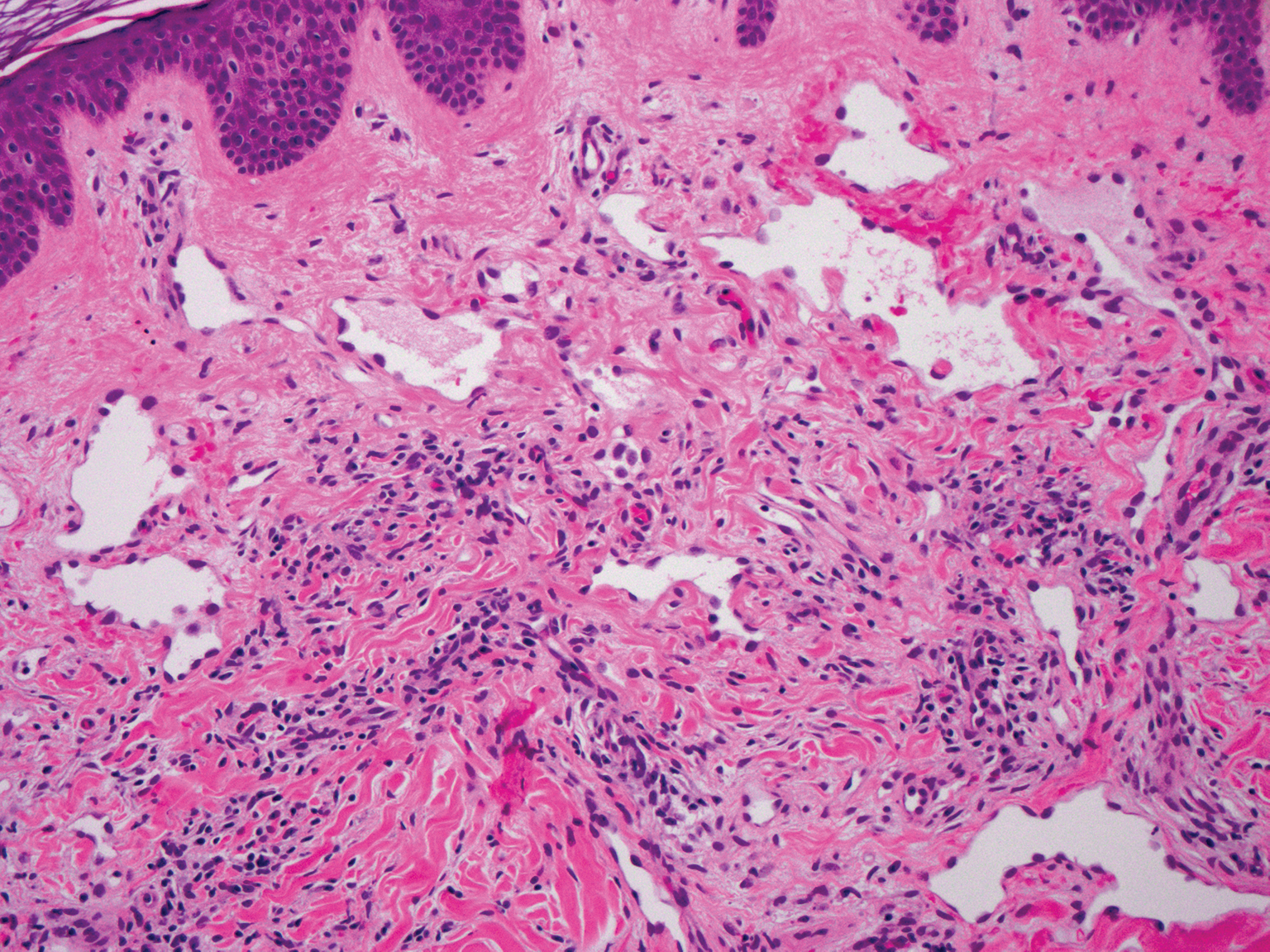
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
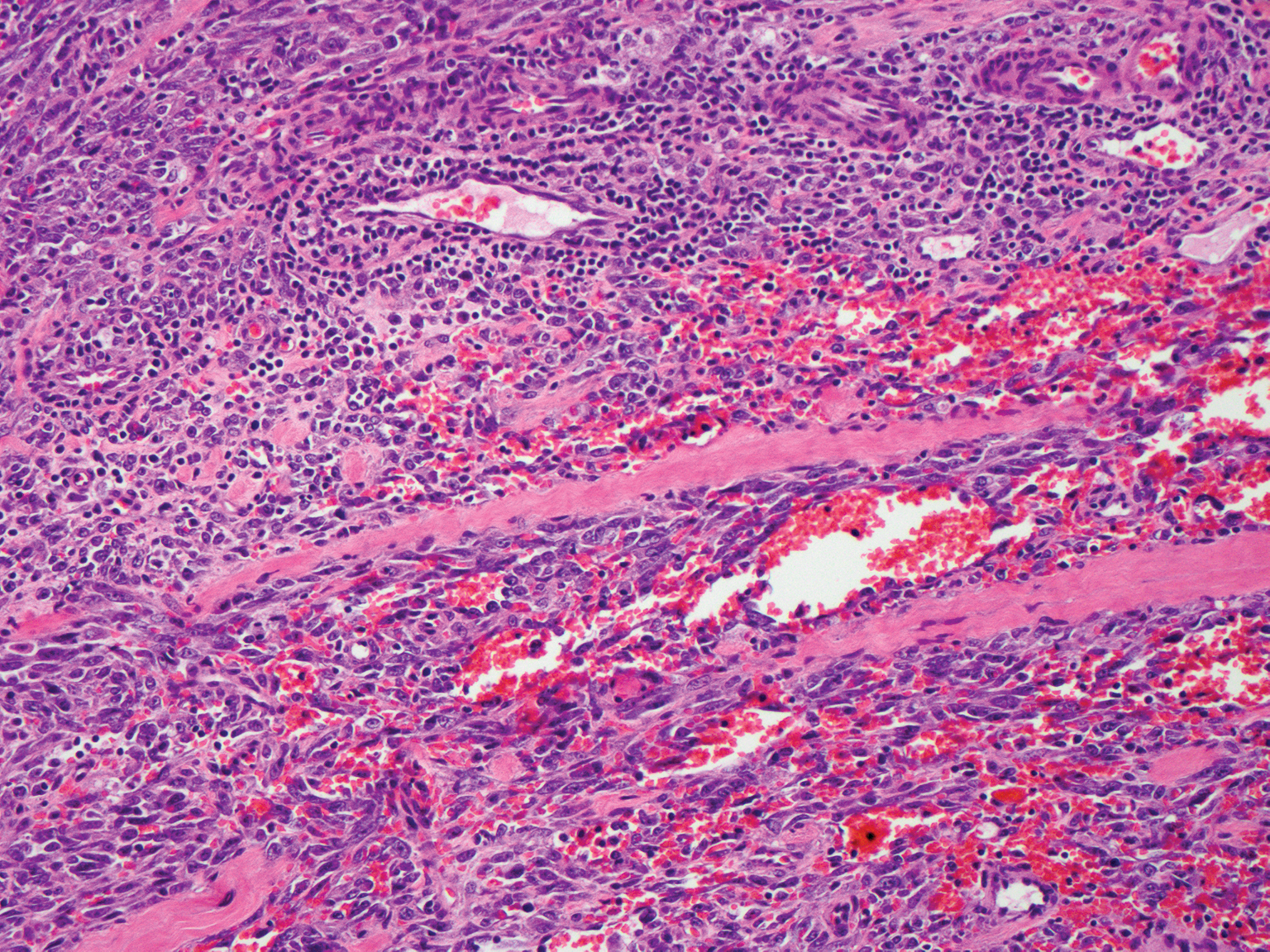
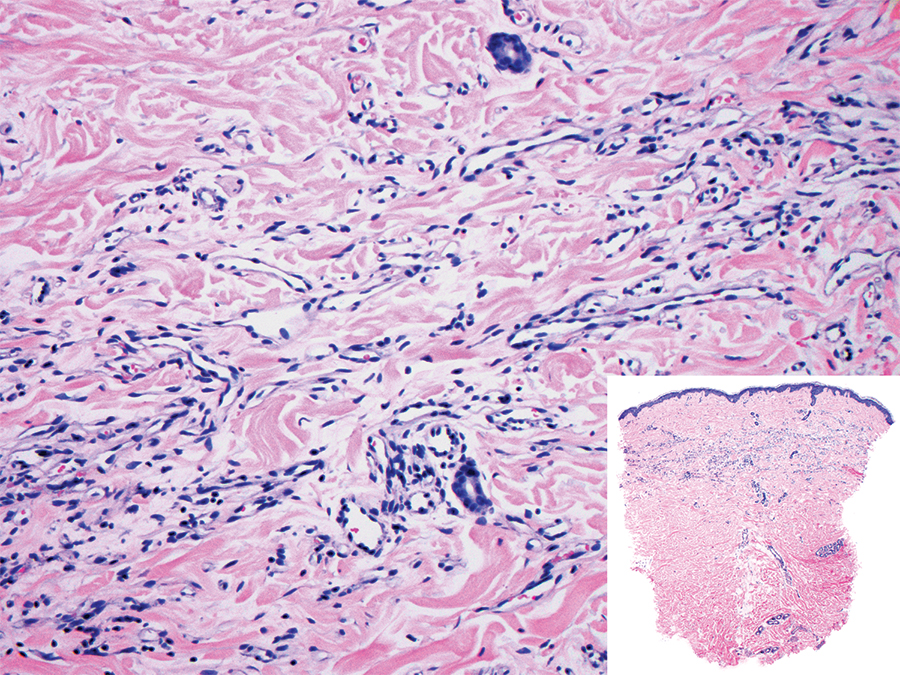
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
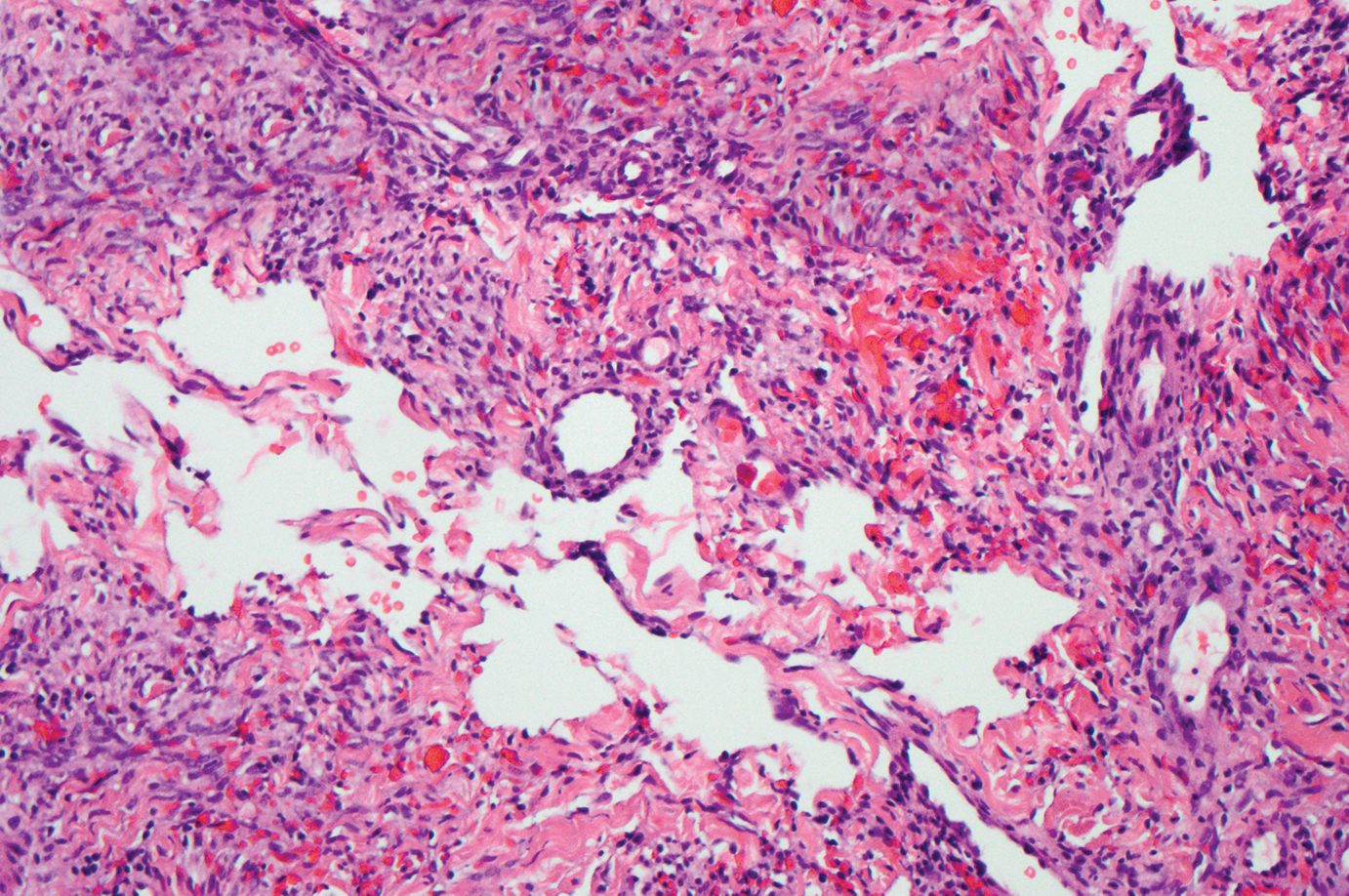
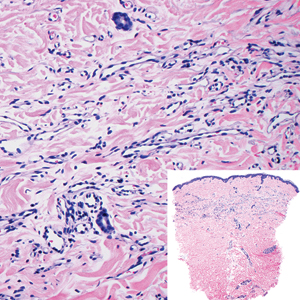
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
The Diagnosis: Microvenular Hemangioma
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
The Diagnosis: Microvenular Hemangioma
Microvenular hemangioma is an acquired benign vascular neoplasm that was described by Hunt et al1 in 1991, though Bantel et al2 reported a similar entity termed micropapillary angioma in 1989. Microvenular hemangioma typically presents as a solitary, slowly enlarging, red to violaceous, asymptomatic papule, plaque, or nodule measuring 5 to 20 mm in diameter. It usually is located on the trunk, arms, or legs of young adults without any gender predilection. Microvenular hemangioma is rare.3 The etiology has not been elucidated, though a relationship with hormonal factors such as pregnancy or hormonal contraceptives has been described.2
Histopathologically, microvenular hemangioma has a characteristic morphology. It is comprised of a well-circumscribed collection of thin-walled blood vessels with narrow lumens (quiz image).4 The blood vessels tend to infiltrate the superficial and deep dermis and are surrounded by a collagenous or desmoplastic stroma. The endothelial cells are normal in size without atypia, mitotic figures, or pleomorphism. A mild lymphoplasmacytic inflammatory infiltrate sometimes is present. Microvenular hemangioma expresses many vascular markers confirming its endothelial origin, including CD34, CD31, WT1, factor VIII-related antigen, and von Willebrand factor.3 Moreover, WT1 staining suggests the lesion is a vascular proliferative growth, as it usually is negative in vascular malformations due to errors of endothelial development.5 In addition, it lacks expression of podoplanin (D2-40), which also supports a vascular as opposed to a lymphatic origin.4
Cutaneous angiosarcoma is a rare and highly aggressive malignant neoplasm of the vascular endothelium with a predilection for the skin and superficial soft tissue. Clinical presentation is variable, as it can arise sporadically, commonly on the scalp and face of elderly patients, in areas of chronic radiation therapy, or in association with chronic lymphedema (Stewart-Treves syndrome).6 Sporadic neoplasms appear clinically as purpuric macules, plaques, or nodules and are more common in elderly men than women. They are aggressive tumors that tend to recur and metastasize despite aggressive therapy and therefore carry a poor prognosis.7 Histopathologically, well-differentiated tumors are characterized by irregular dissecting vessels lined with crowded inconspicuous endothelial cells (Figure 1). Cutaneous angiosarcoma is poorly circumscribed with marked cytologic atypia, and the vessels can take on a sinusoidal growth pattern.8
Kaposi sarcoma (KS) is a virally induced lymphoangioproliferative disease, with human herpesvirus 8 as the implicated agent. There are 4 principal clinical variants of KS: epidemic or AIDS-associated KS, endemic or African KS, KS due to iatrogenic immunosuppression, and Mediterranean or classic KS.9 Cutaneous lesions vary from pink patches to dark purple plaques or nodules that commonly occur on the lower legs10; however, the clinical appearance of KS varies depending on the clinical variant and stage. Histopathologically, early lesions of KS exhibit a superficial dermal proliferation of small angulated and jagged vessels that tend to separate into collagen bundles and are surrounded by a lymphoplasmacytic perivascular infiltrate. These native vascular structures often are surrounded by more ectatic neoplastic channels with plump endothelial cells, known as the promontory sign (Figure 2).11 With more advanced lesions, the proliferation of slitlike vessels becomes more cellular and extends deeper into the dermis and subcutis. Although the histopathologic features vary with the stage of the lesion, they do not notably vary between clinical subtypes.
Targetoid hemosiderotic hemangioma, also known as hobnail hemangioma, is a small, benign, vascular tumor that usually affects the trunk, arms, and legs in young to middle-aged adults without a gender predilection. Clinically, it appears as a small, solitary, red to purple papule or macule that typically is surrounded by a pale thin area and a peripheral ecchymotic ring, creating a targetoid appearance, thus the term targetoid hemosiderotic hemangioma.12 Histopathologically, there is a prominent dermal vascular proliferation. In the papillary dermis, there are dilated superficial vessels lined with a single layer of endothelial cells characterized by a plump, hobnail-like appearance that protrude into the lumen (Figure 3). In the deeper dermis, the vascular spaces are angulated and slitlike and appear to dissect through collagen bundles. Hemosiderin, thrombi, extravasated erythrocytes, and a lymphocytic infiltrate also are often seen.13
Tufted angioma is a rare benign vascular lesion that usually presents as an acquired lesion in children and young adults, though it may be congenital. It is commonly localized to the skin and subcutaneous tissues. Clinically, the lesions appear as red to purple patches and plaques that typically are located on the neck or trunk. More than 50% of cases present during the first year of life and slowly spread to involve large areas before stabilizing in size.14 Partial spontaneous regression may occur, but complete regression is rare.15 Lesions usually are asymptomatic but may be painful during periods of platelet trapping (Kasabach-Merritt phenomenon), which may develop in congenital cases. Tufted angioma is named for its characteristic histopathologic appearance, which consists of multiple discrete lobules or tufts of tightly packed capillaries in a cannonball-like appearance throughout the dermis and subcutis (Figure 4).14,15
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
- Hunt SJ, Santa Cruz DJ, Barr RJ. Microvenular hemangioma. J Cutan Pathol. 1991;18:235-240.
- Bantel E, Grosshans E, Ortonne JP. Understanding microcapillary angioma, observations in pregnant patients and in females treated with hormonal contraceptives [in German]. Z Hautkr. 1989;64:1071-1074.
- Mansur AT, Demirci GT, Ozbal Koc E, et al. An unusual lesion on the nose: microvenular hemangioma. Dermatol Pract Concept. 2018;8:7-11.
- Napekoski KM, Fernandez AP, Billings SD. Microvenular hemangioma: a clinicopathologic review of 13 cases. J Cutan Pathol. 2014;41:816-822.
- Trinidade F, Tellechea O, Torrelo A, et al. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33:569-572.
- Shustef E, Kazlouskaya V, Prieto VG, et al. Cutaneous angiosarcoma: a current update. J Clin Pathol. 2017;70:917-925.
- Morgan M, Swann M, Somach S, et al. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50:867-874.
- Shon W, Billings SD. Cutaneous malignant vascular neoplasms. Clin Lab Med. 2017;37:633-646.
- Régnier-Rosencher E, Guillot B, Dupin N. Treatments for classic Kaposi sarcoma: a systematic review of the literature. J Am Acad Dermatol. 2013;68:313-331.
- Tappero JW, Conant MA, Wolfe SF, et al. Kaposi's sarcoma: epidemiology, pathogenesis, histology, clinical spectrum, staging criteria and therapy. J Am Acad Dermatol. 1993;28:371-395.
- Grayson W, Pantanowitz L. Histological variants of cutaneous Kaposi sarcoma. Diagn Pathol. 2008;3:31.
- Mentzel T, Partanen TA, Kutzner H. Hobnail hemangioma ("targetoid hemosiderotic hemangioma"): clinicopathologic and immunohistochemical analysis of 62 cases. J Cutan Pathol. 1999;26:279-286.
- Morales-Callaghan AM, Martinez-Garcia G, Aragoneses-Fraile H, et al. Targetoid hemosiderotic hemangioma: clinical and dermoscopical findings. J Eur Acad Dermatol Venereol. 2007;21:267-269.
- Kamath GH, Bhat RM, Kumar S. Tufted angioma. Int J Dermatol. 2005;44:1045-1047.
- Prasuna A, Rao P. A tufted angioma. Indian Dermatol Online J. 2015;6:266-268.
A 38-year-old woman presented with an asymptomatic lesion on the abdomen. On physical examination, there was a 5×2-mm, solitary, ill-defined pink macule on the right side of the abdomen. The patient denied recent change in size or color of the lesion, prior trauma, or a personal or family history of similar lesions. Due to the uncertain diagnostic appearance, a punch biopsy was performed.
Product News July 2019
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
AbbVie to Acquire Allergan
AbbVie Inc and Allergan announce that the companies have entered into a definitive transaction agreement under which AbbVie will acquire Allergan in a cash and stock transaction. The combined company will consist of several franchises with leadership positions across immunology, hematologic oncology, medical aesthetics, neuroscience, women’s health, eye care, and virology. Furthermore, Allergan’s product portfolio will be enhanced by AbbVie’s commercial strength, expertise, and international infrastructure. For more information, visit www.abbvie.com and www.allergan.com.
Avène Launches Mineral Sunscreen Fluids
Pierre Fabre Dermo-Cosmetique introduces Avène Mineral Sunscreen Fluids SPF 50+ in both tinted and nontinted varieties. With active ingredients titanium dioxide (11.4%) and zinc oxide (14.6%), both products provide broad-spectrum UVA and UVB protection and are free from octinoxate and oxybenzone (reef friendly). These lightweight lotions are ideal for use on the face; they absorb quickly and can be layered invisibly under makeup. Additionally, the tinted fluid offers protection against blue light. For more information, visit www.aveneusa.com.
CoolTone Device Clearance Expanded
Allergan announces US Food and Drug Administration clearance of the nonsurgical CoolTone device for the improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone also is indicated for the strengthening, toning, and firming of buttocks and thighs. Using magnetic muscle stimulation, CoolTone technology penetrates into the muscle layers and induces involuntary muscle contractions. The body responds to these contractions by strengthening its muscle fibers, resulting in a more defined and toned appearance. For more information, visit www.cooltonebycoolsculpting.com.
Duobrii Lotion for Plaque Psoriasis Now Available
Ortho Dermatologics announces the US launch of Duobrii (halobetasol propionate and tazarotene) Lotion 0.01%/0.045%. Duobrii was approved by the US Food and Drug Administration in April 2019 for the treatment of plaque psoriasis in adult patients, offering psoriasis patients a treatment with strong efficacy and an extended duration of use in a once-daily lotion that can be dosed to clearance. When used separately to treat plaque psoriasis, the duration of use of halobetasol propionate is limited to 2 to 4 weeks, and the use of tazarotene can be limited due to tolerability concerns. By combining halobetasol propionate and tazarotene in a patented once-daily moisturizing lotion, the Duobrii formulation ensures uniform distribution, allowing for simultaneous contact with the skin surface. Unlike other topical products that either contain steroids or are steroids on their own, Duobrii is not restricted to 8 weeks or less of use. The approved labeling for Duobrii does not include a duration limitation; it can be dosed to clearance as long as local skin reactions do not occur, and treatment should be discontinued once clearance is achieved. For more information, visit www.duobrii.com.
Zika Virus Diagnostic Test Receives Marketing Authorization
The US Food and Drug Administration authorizes marketing of the ZIKV Detect 2.0 IgM Capture ELISA (enzymelinked immunosorbent assay)(InBios International, Inc) for the qualitative detection of Zika virus IgM antibodies in human blood. The ZIKV Detect 2.0 IgM Capture ELISA is designed to identify proteins (antibodies) produced by the body’s immune system when it tests for Zika virus infection in the blood; IgM antibodies indicate an early immune response. The test is for use only in patients with clinical signs and symptoms consistent with Zika virus infection and/or those who meet the Centers for Disease Control and Prevention’s Zika virus epidemiologic criteria, such as history of residence in or travel to a geographic region with active Zika transmission at the time of travel. For more information, visit www.inbios.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at cutis@mdedge.com.
Establishing the Diagnosis of Rosacea in Skin of Color Patients
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
Rosacea is a chronic inflammatory cutaneous disorder that affects the vasculature and pilosebaceous units of the face. Delayed and misdiagnosed rosacea in the SOC population has led to increased morbidity in this patient population. 1-3 It is characterized by facial flushing and warmth, erythema, telangiectasia, papules, and pustules. The 4 major subtypes include erythematotelangiectatic, papulopustular, phymatous, and ocular rosacea. 4 Granulomatous rosacea is considered to be a unique variant of rosacea. Until recently, rosacea was thought to predominately affect lighter-skinned individuals of Celtic and northern European origin. 5,6 A paucity of studies and case reports in the literature have contributed to the commonly held belief that rosacea occurs infrequently in patients with skin of color (SOC). 1 A PubMed search of articles indexed for MEDLINE revealed 32 results using the terms skin of color and rosacea vs 3786 using the term rosacea alone. It is possible that the nuance involved in appreciating erythema or other clinical manifestations of rosacea in SOC patients has led to underdiagnosis. Alternatively, these patients may be unaware that their symptoms represent a disease process and do not seek treatment. Many patients with darker skin will have endured rosacea for months or even years because the disease has been unrecognized or misdiagnosed. 6-8 Another factor possibly accounting for the perception that rosacea occurs infrequently in patients with SOC is misdiagnosis of rosacea as other diseases that are known to occur more commonly in the SOC population. Dermatologists should be aware that rosacea can affect SOC patients and that there are several rosacea mimickers to be considered and excluded when making the rosacea diagnosis in this patient population. To promote accurate and timely diagnosis of rosacea, we review several possible rosacea mimickers in SOC patients and highlight the distinguishing features.
Epidemiology
In 2018, a meta-analysis of published studies on rosacea estimated the global prevalence in all adults to be 5.46%.9 A multicenter study across 6 cities in Colombia identified 291 outpatients with rosacea; of them, 12.4% had either Fitzpatrick skin types IV or V.10 A study of 2743 Angolan adults with Fitzpatrick skin types V and VI reported that only 0.4% of patients had a diagnosis of rosacea.11 A Saudi study of 50 dark-skinned female patients with rosacea revealed 40% (20/50), 18% (9/50), and 42% (21/50) were Fitzpatrick skin types IV, V, and VI, respectively.12 The prevalence of rosacea in SOC patients in the United States is less defined. Data from the US National Ambulatory Medical Care Survey (1993-2010) of 31.5 million rosacea visits showed that 2% of rosacea patients were black, 2.3% were Asian or Pacific Islander, and 3.9% were Hispanic or Latino.8
Clinical Features
Each of the 4 major rosacea subtypes can present in the SOC population. The granulomatous variant has been predominantly reported in black patients.13 This predilection has been attributed to either an increased susceptibility in black patients to develop this variant or a delay in diagnosis of earlier phases of inflammatory rosacea.7
In a Saudi study (N=50), severe erythematotelangiectatic rosacea was diagnosed in 42% (21/50) of patients, with the majority having Fitzpatrick skin type IV. The severe papulopustular subtype was seen in 14% (7/50) of patients, with 20% (10/50) and 14% (7/50) having Fitzpatrick skin types IV and VI, respectively.12 In a Tunisian study (N=244), erythematotelangiectatic rosacea was seen in 12% of patients, papulopustular rosacea in 69%, phymatous rosacea in 4%, and ocular rosacea in 16%. Less frequently, the granulomatous variant was seen in 3% of patients, and steroid rosacea was noted in 12% patients.14
Recognizing the signs of rosacea may be a challenge, particularly erythema and telangiectasia. Tips for making an accurate diagnosis include use of adequate lighting, blanching of the skin (Figure 1), photography of the affected area against a dark blue background, and dermatoscopic examination.3 Furthermore, a thorough medical history, especially when evaluating the presence of facial erythema and identifying triggers, may help reach the correct diagnosis. Careful examination of the distribution of papules and pustules as well as the morphology and color of the papules in SOC patients also may provide diagnostic clues.
Differential Diagnosis and Distinguishing Features
Several disorders are included in the differential diagnosis of rosacea and may confound a correct rosacea diagnosis, including systemic lupus erythematosus (SLE), seborrheic dermatitis, dermatomyositis, acne vulgaris, sarcoidosis, and steroid dermatitis. Many of these disorders also occur more commonly in patients with SOC; therefore, it is important to clearly distinguish these entities from rosacea in this population.
Systemic Lupus Erythematosus
Systemic lupus erythematosus is an autoimmune disease that commonly presents with erythema as well as erythematous inflammatory facial lesions similar to rosacea. The classic clinical appearance of SLE is the butterfly or malar rash, an erythematous macular eruption on the malar region of the face that also may involve the nose. This rash can appear similar to rosacea; however, the malar rash classically spares the nasolabial folds, while erythema of rosacea often involves this anatomic boundary. Although the facial erythema in both SLE and early stages of rosacea may be patchy and similar in presentation, the presence of papules and pustules rarely occurs in SLE and may help to differentiate SLE from certain variants of rosacea.15
Both SLE and rosacea may be exacerbated by sun exposure, and patients may report burning and stinging.16-18 Performing a complete physical examination, performing a skin biopsy with hematoxylin and eosin and direct immunofluorescence, and checking serologies including antinuclear antibody (ANA) can assist in making the diagnosis. It is important to note that elevated ANA, albeit lower than what is typically seen in SLE, has been reported in rosacea patients.19 If ANA is elevated, more specific SLE antibodies should be tested (eg, double-stranded DNA). Additionally, SLE can be differentiated on histology by a considerably lower CD4:CD8 ratio, fewer CD4+CD25+ regulatory T cells, and more CD123+ plasmacytoid dendritic cells compared to rosacea.20
Seborrheic Dermatitis
Seborrheic dermatitis is a frequent cause of facial erythema linked to the Malassezia yeast species in susceptible individuals. Seborrheic dermatitis has a notable prevalence in women of African descent and often is considered normal by these patients.21 Rosacea and seborrheic dermatitis are relatively common dermatoses and therefore can present concurrently. In both diseases, facial erythema may be difficult to discern upon cursory inspection. Seborrheic dermatitis may be distinguished from rosacea by the clinical appearance of erythematous patches and plaques involving the scalp, anterior and posterior hairlines, preauricular and postauricular areas, and medial eyebrows. Both seborrheic dermatitis and rosacea may involve the nasolabial folds, but the presence of scale in seborrheic dermatitis is a distinguishing feature. Scale may vary in appearance from thick, greasy, and yellowish to fine, thin, and whitish.22 In contrast to rosacea, the erythematous lesions of seborrheic dermatitis often are annular in configuration. Furthermore, postinflammatory hypopigmentation and, to a lesser extent, postinflammatory hyperpigmentation are key clinical components of seborrheic dermatitis in SOC patients but are not as commonly observed in rosacea.
Dermatomyositis
Dermatomyositis is a systemic autoimmune disease characterized by progressive and symmetric proximal musculoskeletal weakness and cutaneous findings. Facial erythema in the malar and nasolabial folds can be seen in patients with dermatomyositis18; however, the facial erythema seen in dermatomyositis, known as heliotrope rash, has a violaceous dusky quality and also involves the periorbital region. The violaceous hue and periorbital involvement are distinguishing features from rosacea. Okiyama et al23 described facial macular violaceous erythema with scale and edema in Japanese patients with dermatomyositis on the nasolabial folds, eyebrows, chin, cheeks, and ears; they also described mild atrophy with telangiectasia. Other clinical signs to help distinguish rosacea from dermatomyositis are the presence of edema of the face and extremities, Gottron papules, and poikiloderma. Dermatomyositis is a disease that affects all races; however, it is 4 times more common in black vs white patients,24 making it even more important to be able to distinguish between these conditions.
Acne Vulgaris
Acne vulgaris, the most commonly diagnosed dermatosis in patients with SOC, is characterized by papules, pustules, cysts, nodules, open and closed comedones, and hyperpigmented macules on the face, chest, and back.25,26 The absence of comedonal lesions and the presence of hyperpigmented macules distinguishes acne vulgaris from rosacea in this population.1 In addition, the absence of telangiectasia and flushing are important distinguishing factors when making the diagnosis of acne vulgaris.
Sarcoidosis
Sarcoidosis is a multisystem inflammatory disease characterized histologically by the presence of noncaseating granulomas in sites such as the lungs, lymph nodes, eyes, nervous system, liver, spleen, heart, and skin.27 Cutaneous sarcoidosis is known as a great mimicker of many other dermatoses, as it may present with multiple morphologic features. Cutaneous sarcoidosis most typically presents as papules, but nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars also have been identified.28 Sarcoid papules typically are 1 to 5 mm in size on the face, neck, and periorbital skin29; they are initially orange or yellow-brown in color, turn brownish red or violaceous, then involute to form faint macules.30 Papular lesions may either resolve or evolve into plaques, particularly on the extremities, face, scalp, back, and buttocks. Additionally, there are a few case reports of patients with cutaneous sarcoidosis presenting with large bulbous nasal masses initially thought to be rhinophyma.31-33 Finally, it may be difficult to distinguish sarcoidosis from granulomatous rosacea, which is characterized by firm yellow, brown, violaceous, red, or flesh-colored monomorphic papules or nodules affecting the perioral, periocular, medial, and/or lateral areas of the face (Figure 2).4,34 Patients also can have unilateral disease.35 Patients with granulomatous rosacea lack flushing and erythema as seen in more characteristic presentations of rosacea. They may report pain, pruritus, or burning, or they may be asymptomatic.36 Features that distinguish granulomatous rosacea from sarcoidosis include the absence of nodules, plaques, lupus pernio, subcutaneous infiltrates, and infiltration of scars. Clinical, histological, and radiographic evaluation are necessary to make the diagnosis of sarcoidosis over rosacea.
Steroid Dermatitis
Steroid dermatitis involving the face may mimic rosacea. It is caused by the application of a potent corticosteroid to the facial skin for a prolonged period of time. In a report from a teaching hospital in Baghdad, the duration of application was 0.25 to 10 years on average.37 Reported characteristics of steroid dermatitis included facial erythema, telangiectasia, papules, pustules, and warmth to the touch. Distinguishing features from rosacea may be the presence of steroid dermatitis on the entire face, whereas rosacea tends to occur on the center of the face. Diagnosis of steroid dermatitis is made based on a history of chronic topical steroid use with rebound flares upon discontinuation of steroid.
Final Thoughts
Rosacea has features common to many other facial dermatoses, making the diagnosis challenging, particularly in patients with SOC. This difficulty in diagnosis may contribute to an underestimation of the prevalence of this disease in SOC patients. An understanding of rosacea, its nuances in clinical appearance, and its mimickers in SOC patients is important in making an accurate diagnosis.
References
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
- Alexis AF. Rosacea in patients with skin of color: uncommon but not rare. Cutis. 2010;86:60-62.
- Kim NH, Yun SJ, Lee JB. Clinical features of Korean patients with rhinophyma. J Dermatol. 2017;44:710-712.
- Hua TC, Chung PI, Chen YJ, et al. Cardiovascular comorbidities in patients with rosacea: a nationwide case-control study from Taiwan. J Am Acad Dermatol. 2015;73:249-254.
- Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the National Rosacea Society Expert Committee on the Classification and Staging of Rosacea. J Am Acad Dermatol. 2002;46:584-587.
- Elewski BE, Draelos Z, Dreno B, et al. Global diversity and optimized outcome: proposed international consensus from the Rosacea International Expert Group. J Eur Acad Dermatol Venereol. 2011;25:188-200.
- Alexis AF, Callender VD, Baldwin HE, et al. Global epidemiology and clinical spectrum of rosacea, highlighting skin of color: review and clinical practice experience [published online September 19, 2018]. J Am Acad Dermatol. 2019;80:1722-1729.e7.
- Dlova NC, Mosam A. Rosacea in black South Africans with skin phototypes V and VI. Clin Exp Dermatol. 2017;42:670-673.
- Al-Dabagh A, Davis SA, McMichael AJ, et al. Rosacea in skin of color: not a rare diagnosis [published online October 15, 2014]. Dermatol Online J. 2014;20. pii:13030/qt1mv9r0ss.
- Gether L, Overgaard LK, Egeberg A, et al. Incidence and prevalence of rosacea: a systematic review and meta-analysis. Br J Dermatol. 2018;179:282-289.
- Rueda LJ, Motta A, Pabon JG, et al. Epidemiology of rosacea in Colombia. Int J Dermatol. 2017;56:510-513.
- De Luca DA, Maianski Z, Averbukh M. A study of skin disease spectrum occurring in Angola phototype V-VI population in Luanda. Int J Dermatol. 2018;57:849-855.
- Al Balbeesi AO, Halawani MR. Unusual features of rosacea in Saudi females with dark skin. Ochsner J. 2014;14:321-327.
- Rosen T, Stone MS. Acne rosacea in blacks. J Am Acad Dermatol. 1987;17:70-73.
- Khaled A, Hammami H, Zeglaoui F, et al. Rosacea: 244 Tunisian cases. Tunis Med. 2010;88:597-601.
- Usatine RP, Smith MA, Chumley HS, et al. The Color Atlas of Family Medicine. 2nd ed. New York, NY: The McGraw-Hill Companies; 2013.
- O'Gorman SM, Murphy GM. Photoaggravated disorders. Dermatol Clin. 2014;32:385-398, ix.
- Foering K, Chang AY, Piette EW, et al. Characterization of clinical photosensitivity in cutaneous lupus erythematosus. J Am Acad Dermatol. 2013;69:205-213.
- Saleem MD, Wilkin JK. Evaluating and optimizing the diagnosis of erythematotelangiectatic rosacea. Dermatol Clin. 2018;36:127-134.
- Black AA, McCauliffe DP, Sontheimer RD. Prevalence of acne rosacea in a rheumatic skin disease subspecialty clinic. Lupus. 1992;1:229-237.
- Brown TT, Choi EY, Thomas DG, et al. Comparative analysis of rosacea and cutaneous lupus erythematosus: histopathologic features, T-cell subsets, and plasmacytoid dendritic cells. J Am Acad Dermatol. 2014;71:100-107.
- Taylor SC, Barbosa V, Burgess C, et al. Hair and scalp disorders in adult and pediatric patients with skin of color. Cutis. 2017;100:31-35.
- Gary G. Optimizing treatment approaches in seborrheic dermatitis. J Clin Aesthet Dermatol. 2013;6:44-49.
- Okiyama N, Kohsaka H, Ueda N, et al. Seborrheic area erythema as a common skin manifestation in Japanese patients with dermatomyositis. Dermatology. 2008;217:374-377.
- Taylor SC, Kyei A. Defining skin of color. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Taylor and Kelly's Dermatology for Skin of Color. 2nd ed. New York, NY: McGraw-Hill; 2016:9-15.
- Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
- Taylor SC, Cook-Bolden F, Rahman Z, et al. Acne vulgaris in skin of color. J Am Acad Dermatol. 2002;46(2 suppl understanding):S98-S106.
- Wick MR. Granulomatous & histiocytic dermatitides. Semin Diagn Pathol. 2017;34:301-311.
- Ball NJ, Kho GT, Martinka M. The histologic spectrum of cutaneous sarcoidosis: a study of twenty-eight cases. J Cutan Pathol. 2004;31:160-168.
- Marchell RM, Judson MA. Chronic cutaneous lesions of sarcoidosis. Clin Dermatol. 2007;25:295-302.
- Mahajan VK, Sharma NL, Sharma RC, et al. Cutaneous sarcoidosis: clinical profile of 23 Indian patients. Indian J Dermatol Venereol Leprol. 2007;73:16-21.
- Goldenberg JD, Kotler HS, Shamsai R, et al. Sarcoidosis of the external nose mimicking rhinophyma. case report and review of the literature. Ann Otol Rhinol Laryngol. 1998;107:514-518.
- Gupta-Elera G, Lam C, Chung C, et al. Violaceous plaque on the nose referred for rhinophyma surgery. Int J Dermatol. 2015;54:1011-1013.
- Leonard AL. A case of sarcoidosis mimicking rhinophyma. J Drugs Dermatol. 2003;2:333-334.
- Kelati A, Mernissi FZ. Granulomatous rosacea: a case report. J Med Case Rep. 2017;11:230.
- Crawford GH, Pelle MT, James WD. Rosacea: I. etiology, pathogenesis, and subtype classification. J Am Acad Dermatol. 2004;51:327-341; quiz 342-324.
- Reinholz M, Ruzicka T, Steinhoff M, et al. Pathogenesis and clinical presentation of rosacea as a key for a symptom-oriented therapy. J Dtsch Dermatol Ges. 2016;14(suppl 6):4-15.
- Hameed AF. Steroid dermatitis resembling rosacea: a clinical evaluation of 75 patients. ISRN Dermatol. 2013;2013:491376.
Practice Points
- The clinical signs of rosacea may be similar in all skin types; however, dermatologists must have a high clinical index of suspicion for rosacea in patients with skin of color (SOC).
- Dermatologists should consider a wide differential diagnosis when presented with an SOC patient with facial erythema and/or papules and pustules.
Psoriasis Treatment in Patients With HIV
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
- Nakamura M, Abrouk M, Farahnik B, et al. Psoriasis treatment in HIV-positive patients: a systematic review of systemic immunosuppressive therapies. Cutis. 2018;101:38, 42, 56.
- Patel RV, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 2: review of treatment. Cutis. 2008;82:202-210.
- Ceccarelli M, Venanzi Rullo E, Vaccaro M, et al. HIV‐associated psoriasis: epidemiology, pathogenesis, and management [published online January 6, 2019]. Dermatol Ther. 2019;32:e12806. doi:10.1111/dth.12806.
- Zarbafian M, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193.
- Menon K, Van Vorhees AS, Bebo, BF, et al. Psoriasis in patients with HIV infection: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62:291-299.
- Mallon E, Bunker CB. HIV-associated psoriasis. AIDS Patient Care STDS. 2000;14:239-246.
- Patel VA, Weinberg JM. Psoriasis in the patient with human immunodeficiency virus, part 1: review of pathogenesis. Cutis. 2008;82:117-122.
- Castillo RL, Racaza GZ, Dela Cruz Roa F. Ostraceous and inverse psoriasis with psoriatic arthritis as the presenting features of advanced HIV infection. Singapore Med J. 2014;55:e60-e63.
- Duvic M, Crane MM, Conant M, et al. Zidovudine improves psoriasis in human immunodeficiency virus- positive males. Arch Dermatol. 1994;130:447.
- Jaffee D, May LP, Sanchez M, et al. Staphylococcal sepsis in HIV antibody seropositive psoriasis patients. J Am Acad Dermatol. 1991;24:970-972.
- King LE, Dufresne RG, Lovette GL, et al. Erythroderma: review of 82 cases. South Med J. 1986;79:1210-1215.
- Kaminetsky J, Aziz M, Kaushik S. A review of biologics and other treatment modalities in HIV-associated psoriasis. Skin. 2018;2:389-401.
- Wolff K. Side effects of psoralen photochemotherapy (PUVA). Br J Dermatol. 1990;122:117-125.
- Stern RS, Mills DK, Krell K, et al. HIV-positive patients differ from HIV-negative patients in indications for and type of UV therapy used. J Am Acad Dermatol. 1998;39:48-55.
- Oracion RM, Skiest DJ, Keiser PH, et al. HIV-related skin diseases. Prog Dermatol. 1999;33:1-6.
- Finkelstein M, Berman B. HIV and AIDS in inpatient dermatology: approach to the consultation. Dermatol Clin. 2000;18:509-520.
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53.
- Sellam J, Bouvard B, Masson C, et al. Use of infliximab to treat psoriatic arthritis in HIV-positive patients. Joint Bone Spine. 2007;74:197-200.
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E1-E7.
- Otezla (apremilast). Summit, NJ: Celgene Corporation; 2017.
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590.
Infographic: Step-by-Step Guide to Managing Ocular Chemical Burns
Update on Diet and Acne
Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.
Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.
Acne is a common condition that most often affects adolescents but is not uncommon in adults. It can result in considerable anxiety, depression, and medical and pharmaceutical costs. Additionally, oral antibiotics, the standard treatment for acne, are increasingly under suspicion for causing bacterial resistance as well as disruption of the cutaneous and gut microbiomes.1,2 These factors are among those that often drive patients and physicians to search for alternative and complementary treatments, including dietary modification.
Over the last few decades, the interaction between diet and acne has been one of the most fluid areas of research in dermatology. The role of diet in acne incidence and presentation has evolved from the general view in the 1970s that there was no connection to today’s more data-driven understanding that the acne disease course likely is modified by specific dietary components. Better designed and more rigorous studies have supported a link between acne severity and glycemic index (GI)/glycemic load (GL) and possibly dairy consumption. The ability to use data-driven evidence to counsel patients regarding dietary treatment of acne is increasingly important to counteract the pseudoadvice that patients can easily find on the Internet.
This article summarizes the history of beliefs about diet and acne, reviews more recent published data regarding dietary components that can modify acne severity, and outlines the current American Academy of Dermatology (AAD) guidelines and recommendations for diet and acne.
History of Diet and Acne
In most of the current literature, acne frequently is referred to as a disease of modern civilization or a consequence of the typical Western diet.3 For clarity, the Western diet is most commonly described as “a dietary regimen characterized by high amounts of sugary desserts, refined grains, high protein, high-fat dairy products, and high-sugar drinks.”4 The role of dairy in the etiology of acne typically is discussed separately from the Western diet. It has been reported that acne is not found in nonwesternized populations where a Paleolithic diet, which does not include consumption of high-GI carbohydrates, milk, or other dairy products, is common.5
Extending this line of argument, acne vulgaris has been called a metabolic syndrome of the sebaceous follicle and one of the mammalian target of rapamycin complex 1–driven diseases of civilization, along with cancer, obesity, and diabetes mellitus.3 This view seems somewhat extreme and discounts other drivers of acne incidence and severity. Twin studies have shown that acne is highly heritable, with 81% of the population variance attributed to genetic factors.6 Similar incidence numbers for acne vulgaris have been reported worldwide, and global incidence in late adolescence is rising; however, it is unknown whether this increase is a result of the adoption of the Western diet, which is thought to encourage early onset of puberty; genetic drift; changes in regional and cultural understanding and reporting of acne; or a byproduct of unknown environmental factors.4 More nuanced views acknowledge that acne is a multifactorial disease,7 and therefore genetic and possibly epigenetic factors as well as the cutaneous and gut microbiomes also must be taken into account. An interesting historical perspective on acne by Mahmood and Shipman8 outlined acne descriptions, diagnoses, topical treatments, and dietary advice going back to ancient Greek and Egyptian civilizations. They also cited recommendations from the 1930s that suggested avoiding “starchy foods, bread rolls, noodles, spaghetti, potatoes, oily nuts, chop suey, chow mein, and waffles” and listed the following foods as suitable to cure acne: “cooked and raw fruit, farina, rice, wheat, oatmeal, green vegetables, boiled or broiled meat and poultry, clear soup, vegetable soup, and an abundance of water.”8
More Recent Evidence of Dietary Influence on Acne
Importantly, the available research does not demonstrate that diet causes acne but rather that it may influence or aggravate existing acne. Data collection for acne studies also can be confounded by the interplay of many factors, such as increased access to health care, socioeconomic status, and shifting cultural perceptions of skin care and beauty.4 An important facet of any therapeutic recommendation is that it should be supported by confirmable mechanistic pathways.
GI and GL
Over the last few decades, a number of observational and intervention studies have focused on the possible influence of the GI/GL of foods on acne incidence and/or severity. A high GI diet is characterized by a relatively high intake of carbohydrate-containing foods that are quickly digested and absorbed, increasing blood glucose and insulin concentrations. Glycemic load takes the portion size of dietary carbohydrates into consideration and therefore is a measure of both the quality and quantity of carbohydrate-containing foods.9 TheGI/GL values of more than 2480 food items are available in the literature.10
Evidence from several studies supports the role of high GI/GL diets in exacerbating acne and suggests that transitioning to low GI/GL diets may lead to decreased lesion counts after 12 weeks.11-13 In one randomized controlled trial, male participants aged 15 to 25 years with mild to moderate facial acne were instructed either to eat a high protein/low GI diet or a conventional high GL control diet.13 After 12 weeks, total lesion counts had decreased more in the low GI diet group than the control. As partial confirmation of a mechanistic pathway for a high GI diet and acne, the low GI group demonstrated lower free androgen index and insulin levels than the control group.13 In a Korean study, a 10-week low GL regimen led to a reduction in acne lesion count, a decrease in sebaceous gland size, decreased inflammation, and reduced expression of sterol regulatory element-binding protein 1 and IL-8.14
More recent studies have further solidified the role of high GI/GL diets in acne severity.9,15,16 High GI/GL diets are believed to stimulate acne pathways by stimulating insulinlike growth factor 1 (IGF-1), which induces proliferation of both keratinocytes and sebocytes and simulates androgen production.17 An excellent diagram showing the connection between high GI diets (and dairy) and IGF-1, insulin and its receptors, androgen and its receptors, mammalian target of rapamycin, and the pilosebaceous unit was published in the literature in 2016.4 Interestingly, metformin has been shown to be an effective adjunctive therapy in the treatment of moderate to severe acne vulgaris.18,19
Milk and Dairy Consumption
Milk consumption also has been examined for its potential role in the pathogenesis of acne, including its ability to increase insulin and IGF-1 levels and bind to the human IGF-1 receptor as well as the fact that it contains bovine IGF-1 and dihydrotestosterone precursors.20 Although not studied quite as extensively or rigorously as GI/GL, consumption of milk and dairy products does appear to have the potential to exacerbate acne lesions. Beginning with a series of retrospective and prospective epidemiologic studies published from 2005 to 2008,21-23 a link between clinical acne and milk or dairy consumption in adolescent subjects was reported. A recent meta-analysis found a positive relationship between dairy, total milk, whole milk, low-fat milk, and skim milk consumption and acne occurrence but no significant association between yogurt/cheese consumption and acne development.24
AAD Guidelines
In their public forum, the AAD has advised that a low-glycemic diet may reduce the number of lesions in acne patients and highlighted data from around the world that support the concept that a high-glycemic diet and dairy are correlated with acne severity. They stated that consumption of milk—whole, low fat, and skim—may be linked to an increase in acne breakouts but that no studies have found that products made from milk, such as yogurt or cheese, lead to more breakouts.25
Other Considerations
Acne can be a serious quality-of-life issue with considerable psychological distress, physical morbidity, and social prejudice.9 Consequently, acne patients may be more willing to accept nonprofessional treatment advice, and there is no shortage of non–health care “experts” willing to provide an array of unfounded and fantastical advice. Dietary recommendations found online range from specific “miracle” foods to the more data-driven suggestions to “avoid dairy” or “eat low GI foods.” An important study recently published in Cutis concluded that most of the information found online regarding diet and acne is unfounded and/or misleading.26
Two additional reasons for recommending that acne patients consider dietary modification are not directly related to the disease: (1) the general health benefits of a lower GI/GL diet, and (2) the potential for decreasing the use of antibiotics. Antibiotic resistance is a growing problem across medicine, and dermatologists prescribe more antibiotics per provider than any other specialty.17 Dietary modification, where appropriate, could provide an approach to limiting the use of antibiotics in acne.
Final Thoughts
When advising acne patients, dermatologists can refer to the Table for general guidelines that incorporate the most current data-driven information on the relationship between diet and acne. Dietary modification, of course, will not work for all but can be safely recommended in cases of mild to moderate acne.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. doi:10.1001/jamadermatol.2018.4944.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limit systemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments. J Am Acad Dermatol. 2019;80:538-549.
- Melnik BC. Acne vulgaris: the metabolic syndrome of the pilosebaceous follicle [published online September 8, 2017]. Clin Dermatol. 2018;36:29-40.
- Lynn DD, Umari T, Dunnick CA, et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13-25.
- Cordain L, Lindeberg S, Hurtado M, et al. Acne vulgaris: a disease of Western civilization. Arch Dermatol. 2002;138:1584-1590.
- Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris [published online February 17, 2016]. J Am Acad Dermatol. 2016;74:945.e33-973.e33.
- Rezakovic´ S, Bukvic´ Mokos Z, Basta-Juzbašic´ A. Acne and diet: facts and controversies. Acta Dermatovenerol Croat. 2012;20:170-174.
- Mahmood NF, Shipman AR. The age-old problem of acne. Int J Womens Dermatol. 2017;3:71-76.
- Burris J, Shikany JM, Rietkerk W, et al. A low glycemic index and glycemic load diet decreases insulin-like growth factor-1 among adults with moderate and severe acne: a short-duration, 2-week randomized controlled trial. J Acad Nutr Diet. 2018;118:1874-1885.
- Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008 [published online October 3, 2008]. Diabetes Care. 2008;31:2281-2283.
- Smith RN, Braue A, Varigos GA, et al. The effect of a low glycemic load diet on acne vulgaris and the fatty acid composition of skin surface triglycerides. J Dermatol Sci. 2008;50:41-52
- Smith RN, Braue A, Varigos GA, et al. A low-glycemic-load diet improves symptoms in acne vulgaris patients: a randomized controlled trial. Am J Clin Nutr. 2007;86:107-115.
- Smith RN, Mann NJ, Braue A, et al. The effect of a high-protein, low glycemic-load diet versus a conventional, high glycemic-load diet on biochemical parameters associated with acne vulgaris: a randomized, investigator-masked, controlled trial. J Am Acad Dermatol. 2007;57:247-256.
- Kwon HH, Yoon JY, Hong JS, et al. Clinical and histological effect of a low glycaemic load diet in treatment of acne vulgaris in Korean patients: a randomized, controlled trial. Acta Derm Venereol. 2012;92:241-246.
- Burris J, Rietkerk W, Woolf K. Differences in dietary glycemic load and hormones in New York City adults with no and moderate/severe acne. J Acad Nutr Diet. 2017;117:1375-1383.
- Burris J, Rietkerk W, Woolf K. Relationships of self-reported dietary factors and perceived acne severity in a cohort of New York young adults [published online January 9, 2014]. J Acad Nutr Diet. 2014;114:384-392.
- Barbieri JS, Bhate K, Hartnett KP, et al. Trends in oral antibiotic prescription in dermatology, 2008 to 2016 [published online January 16, 2019]. JAMA Dermatol. 2019. doi:10.1001/jamadermatol.2018.4944.
- Lee JK, Smith AD. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris [published online November 15, 2017]. Dermatol Online J. 2017;23. pii:13030/qt53m2q13s.
- Robinson S, Kwan Z, Tang MM. Metformin as an adjunct therapy for the treatment of moderate to severe acne vulgaris: a randomized open-labeled study [published online May 1, 2019]. Dermatol Ther. 2019. doi:10.1111/dth.12953.
- Barbieri JS, Spaccarelli N, Margolis DJ, et al. Approaches to limitsystemic antibiotic use in acne: systemic alternatives, emerging topical therapies, dietary modification, and laser and light-based treatments [published online October 5, 2018]. J Am Acad Dermatol. 2019;80:538-549.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in adolescent girls. Dermatol Online J. 2006;12:1.
- Adebamowo CA, Spiegelman D, Berkey CS, et al. Milk consumption and acne in teenaged boys. J Am Acad Dermatol. 2008;58:787-793.
- Adebamowo CA, Spiegelman D, Danby FW, et al. High school dietary dairy intake and teenage acne. J Am Acad Dermatol. 2005;52:207-214.
- Aghasi M, Golzarand M, Shab-Bidar S, et al. Dairy intake and acne development: a meta-analysis of observational studies. Clin Nutr. 2019;38:1067-1075.
- Can the right diet get rid of acne? American Academy of Dermatology website. https://www.aad.org/public/diseases/acne-and-rosacea/can-the-right-diet-get-rid-of-acne. Accessed June 13, 2019.
- Khanna R, Shifrin N, Nektalova T, et al. Diet and dermatology: Google search results for acne, psoriasis, and eczema. Cutis. 2018;102:44-46, 48.