User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Inflammatory Linear Verrucous Epidermal Nevus Responsive to 308-nm Excimer Laser Treatment
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
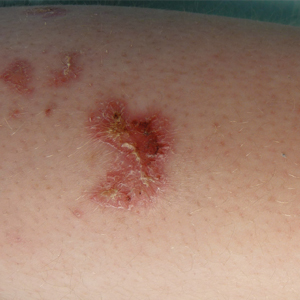
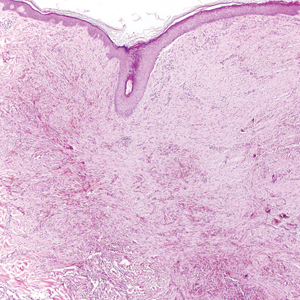
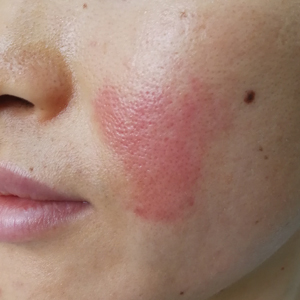
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
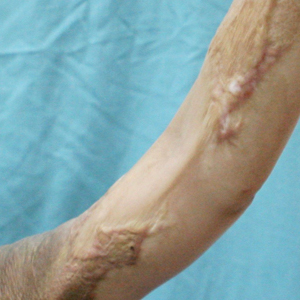
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
Inflammatory linear verrucous epidermal nevus (ILVEN) is a rare entity that presents with linear and pruritic psoriasiform plaques and most commonly occurs during childhood. It represents a dysregulation of keratinocytes exhibiting genetic mosaicism.1,2 Epidermal nevi may derive from keratinocytic, follicular, sebaceous, apocrine, or eccrine origin. Inflammatory linear verrucous epidermal nevus is classified under the keratinocytic type of epidermal nevus and represents approximately 6% of all epidermal nevi.3 The condition presents as erythematous and verrucous plaques along the lines of Blaschko.2,4 There is a predilection for the legs, and girls are 4 times more commonly affected than boys.1 Cases of ILVEN are predominantly sporadic, though rare familial cases have been reported.4
Inflammatory linear verrucous epidermal nevus is notoriously refractory to treatment. First-line therapies include topical agents such as corticosteroids, calcipotriol, retinoids, and 5-fluorouracil.3,4 Other treatments include intralesional corticosteroids, cryotherapy, electrodesiccation and curettage, and surgical excision.3 Several case reports have shown promising results using the pulsed dye and ablative CO2 lasers.5-8
Case Report
An otherwise healthy 20-year-old woman presented with dry, pruritic, red lesions on the right leg that had been present and stable since she was an infant (2 weeks of age). Her medical history included acne vulgaris, but she denied any personal or family history of psoriasis as well as any arthralgia or arthritis. Physical examination revealed discrete, oval, hyperkeratotic, scaly, red plaques on the lateral right leg with a larger hyperkeratotic, linear, red plaque extending from the right popliteal fossa to the posterior thigh (Figure 1A). The nails, scalp, buttocks, and upper extremities were unaffected. Bacterial culture of the right leg demonstrated Staphylococcus aureus colonization. Biopsy of the right popliteal fossa showed psoriasiform dermatitis with psoriasiform hyperplasia, a slightly verruciform surface, broad zones of superficial pallor, and parakeratosis with conspicuous colonies of bacteria (Figure 2).


Following the positive bacterial culture, the patient was treated with a short course of oral doxycycline, which did not alter the clinical appearance of the lesions or improve symptoms of pruritus. Pruritus improved moderately with topical corticosteroid treatment, but clinically the lesions appeared unchanged. The plaque on the superior right leg was treated with a superpulsed CO2 laser and the plaque on the inferior right leg was treated with a fractional CO2 laser, both with minimal improvement.
Because of the clinical and histopathologic similarities of the patient's lesions to psoriasis, a trial of the UV 308-nm excimer laser was initiated. Following initial test spots, she completed a total of 18 treatments to all lesions with noticeable clinical improvement (Figure 1B). Initially, the patient returned for treatment biweekly for approximately 5 weeks with 2 small spots being targeted at each session, with an average surface area of approximately 16 cm2. She was started at 225 mJ/cm2 with 25% increases at each session and ultimately reached up to 1676 mJ/cm2 at the end of the 10 sessions. She tolerated the procedure well with some minor blistering. Treatment was deferred for 3 months due to the patient's schedule, then biweekly treatments resumed for 4 weeks, totaling 8 more sessions. At that time, all lesions on the right leg were targeted, with an average surface area of approximately 100 cm2. The laser settings were initiated at 225 mJ/cm2 with 20% increases at each session and ultimately reached 560 mJ/cm2. The treatment was well tolerated throughout; however, the patient initially reported residual pruritus. The plaques continued to improve, and most notably, there was thinning of the hyperkeratotic scale of the plaques in addition to decreased erythema and complete resolution of pruritus. Ultimately, treatment was discontinued because of lack of insurance coverage and financial burden. The patient was lost to follow-up.
Comment
Presentation
Inflammatory linear verrucous epidermal nevus is a rare type of keratinocytic epidermal nevus4 that clinically presents as small, discrete, pruritic, scaly plaques coalescing into a linear plaque along the lines of Blaschko.9 Considerable pruritus and resistance to treatment are hallmarks of the disease.10 Histopathologically, ILVEN is characterized by alternating orthokeratosis and parakeratosis with a lack of neutrophils in an acanthotic epidermis.11-13 Inflammatory linear verrucous epidermal nevus presents at birth or in early childhood. Adult onset is rare.9,14 Approximately 75% of lesions present by 5 years of age, with a majority occurring within the first 6 months of life.15 The differential diagnosis includes linear psoriasis, epidermal nevi, linear lichen planus, linear verrucae, linear lichen simplex chronicus, and mycosis fungoides.4,11
Differentiation From Psoriasis
Despite the histopathologic overlap with psoriasis, ILVEN exhibits fewer Ki-67-positive keratinocyte nuclei (proliferative marker) and more cytokeratin 10-positive cells (epidermal differentiation marker) than psoriasis.16 Furthermore, ILVEN has demonstrated fewer CD4−, CD8−, CD45RO−, CD2−, CD25−, CD94−, and CD161+ cells within the dermis and epidermis than psoriasis.16
The clinical presentations of ILVEN and psoriasis may be similar, as some patients with linear psoriasis also present with psoriatic plaques along the lines of Blaschko.17 Additionally, ILVEN may be a precursor to psoriasis. Altman and Mehregan1 found that ILVEN patients who developed psoriasis did so in areas previously affected by ILVEN; however, they continued to distinguish the 2 pathologies as distinct entities. Another early report also hypothesized that the dermoepidermal defect caused by epidermal nevi provided a site for the development of psoriatic lesions because of the Koebner phenomenon.18
Patients with ILVEN also have been found to have extracutaneous manifestations and symptoms commonly seen in psoriasis patients. A 2012 retrospective review revealed that 37% (7/19) of patients with ILVEN also had psoriatic arthritis, cutaneous psoriatic lesions, and/or nail pitting. The authors concluded that ILVEN may lead to the onset of psoriasis later in life and may indicate an underlying psoriatic predisposition.19 Genetic theories also have been proposed, stating that ILVEN may be a mosaic of psoriasis2 or that a postzygotic mutation leads to the predisposition for developing psoriasis.20
Treatment
Inflammatory linear verrucous epidermal nevus frequently is refractory to treatment; however, the associated pruritus and distressing cosmesis make treatment attempts worthwhile.11 No single therapy has been found to be successful in all patients. A widely used first-line treatment is topical or intralesional corticosteroids, with the former typically used with occlusion.13 Other treatments include adalimumab, calcipotriol,22,23 tretinoin,24 and 5-fluorouracil.24 Physical modalities such as cryotherapy, electrodesiccation, and dermabrasion have been reported with varying success.15,24 Surgical treatments include tangential25 and full-thickness excisions.26
The CO2 laser also has demonstrated success. One study showed considerable improvement of pruritus and partial resolution of lesions only 5 weeks following a single CO2 laser treatment.5 Another study showed promising results when combining CO2 pulsed laser therapy with fractional CO2 laser treatment.6 Other laser therapies including the argon27 and flashlamp-pumped pulsed dye lasers8 have been used with limited success. The use of light therapy and lasers in psoriasis have now increased the treatment options for ILVEN based on the rationale of their shared histopathologic characteristics. Photodynamic therapy also has been attempted because of its successful use in psoriasis patients. It has been found to be successful in diminishing ILVEN lesions and associated pruritus after a few weeks of therapy; however, treatment is limited by the associated pain and requirement for local anesthesia.28
The excimer laser is a form of targeted phototherapy that emits monochromatic light at 308 nm.29 It is ideal for inflammatory skin lesions because the UVB light induces apoptosis.30 Psoriasis lesions treated with the excimer laser show a decrease in keratinocyte proliferation, which in turn reverses epidermal acanthosis and causes T-cell depletion due to upregulation of p53.29,31 This mechanism of action addresses the overproliferation of keratinocytes mediated by T cells in psoriasis and contributes to the success of excimer laser treatment.31 A considerable advantage is its localized treatment, resulting in lower cumulative doses of UVB and reducing the possible carcinogenic and phototoxic risks of whole-body phototherapy.32
One study examined the antipruritic effects of the excimer laser following the treatment of epidermal hyperinnervation leading to intractable pruritus in patients with atopic dermatitis. The researchers suggested that a potential explanation for the antipruritic effect of the excimer laser may be secondary to nerve degeneration.33 Additionally, low doses of UVB light also may inhibit mast cell degranulation and prevent histamine release, further supporting the antipruritic properties of excimer laser.34
In our patient, failed treatment with other modalities led to trial of excimer laser therapy because of the overlapping clinical and histopathologic findings with psoriasis. Excimer laser improved the clinical appearance and overall texture of the ILVEN lesions and decreased pruritus. The reasons for treatment success may be two-fold. By decreasing the number of keratinocytes and mast cells, the excimer laser may have improved the epidermal hyperplasia and pruritus in the ILVEN lesions. Alternatively, because the patient had ILVEN lesions since infancy, psoriasis may have developed in the location of the ILVEN lesions due to koebnerization, resulting in the clinical response to excimer therapy; however, she had no other clinical evidence of psoriasis.
Because of the recalcitrance of ILVEN lesions to conventional therapies, it is important to investigate therapies that may be of possible benefit. Our novel case documents successful use of the excimer laser in the treatment of ILVEN.
Conclusion
Our case of ILVEN in a woman that had been present since infancy highlights the disease pathology as well as a potential new treatment modality. The patient was refractory to first-line treatments and was concerned about the cosmetic appearance of the lesions. The patient was subsequently treated with a trial of a 308-nm excimer laser with clinical improvement of the lesions. It is possible that the similarity of ILVEN and psoriasis may have contributed to the clinical improvement in our patient, but the mechanism of action remains unknown. Due to the paucity of evidence regarding optimal treatment of ILVEN, the current case offers dermatologists an option for patients who are refractory to other treatments.
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
- Altman J, Mehregan AH. Inflammatory linear verrucose epidermal nevus. Arch Dermatol. 1971;104:385-389.
- Hofer T. Does inflammatory linear verrucous epidermal nevus represent a segmental type 1/type 2 mosaic of psoriasis? Dermatology. 2006;212:103-107.
- Rogers M, McCrossin I, Commens C. Epidermal nevi and the epidermal nevus syndrome: a review of 131 cases. J Am Acad Dermatol. 1989;20:476-488.
- Khachemoune A, Janjua S, Guldbakke K. Inflammatory linear verrucous epidermal nevus: a case report and short review of the literature. Cutis. 2006;78:261-267.
- Ulkur E, Celikoz B, Yuksel F, et al. Carbon dioxide laser therapy for an inflammatory linear verrucous epidermal nevus: a case report. Aesthetic Plast Surg. 2004;28:428-430.
- Conti R, Bruscino N, Campolmi P, et al. Inflammatory linear verrucous epidermal nevus: why a combined laser therapy. J Cosmet Laser Ther. 2013;15:242-245.
- Alonso-Castro L, Boixeda P, Reig I, et al. Carbon dioxide laser treatment of epidermal nevi: response and long-term follow-up. Actas Dermosifiliogr. 2012;103:910-918.
- Alster TS. Inflammatory linear verrucous epidermal nevus: successful treatment with the 585 nm flashlamp-pumped dye laser. J Am Acad Dermatol. 1994;31:513-514.
- Kruse LL. Differential diagnosis of linear eruptions in children. Pediatr Ann. 2015;44:194-198.
- Renner R, Colsman A, Sticherling M. ILVEN: is it psoriasis? debate based on successful treatment with etanercept. Acta Derm Venereol. 2008;88:631-632.
- Lee SH, Rogers M. Inflammatory linear verrucous epidermal naevi: a review of 23 cases. Australas J Dermatol. 2001;42:252-256.
- Ito M, Shimizu N, Fujiwara H, et al. Histopathogenesis of inflammatory linear verrucose epidermal nevus: histochemistry, immunohistochemistry and ultrastructure. Arch Dermatol Res. 1991;283:491-499.
- Cerio R, Jones EW, Eady RA. ILVEN responding to occlusive potent topical steroid therapy. Clin Exp Dermatol. 1992;17:279-281.
- Kawaguchi H, Takeuchi M, Ono H, et al. Adult onset of inflammatory linear verrucous epidermal nevus. J Dermatol. 1999;26:599-602.
- Behera B, Devi B, Nayak BB, et al. Giant inflammatory linear verrucous epidermal nevus: successfully treated with full thickness excision and skin grafting. Indian J Dermatol. 2013;58:461-463.
- Vissers WH, Muys L, Erp PE, et al. Immunohistochemical differentiation between ILVEN and psoriasis. Eur J Dermatol. 2004;14:216-220.
- Agarwal US, Besarwal RK, Gupta R, et a. Inflammatory linear verrucous epidermal nevus with psoriasiform histology. Indian J Dermatol. 2014;59:211.
- Bennett RG, Burns L, Wood MG. Systematized epidermal nevus: a determinant for the localization of psoriasis. Arch Dermatol. 1973;108:705-757.
- Tran K, Jao-Tan C, Ho N. ILVEN and psoriasis: a retrospective study among pediatric patients. J Am Acad Dermatol. 2012;66(suppl 1):AB163.
- Happle R. Superimposed linear psoriasis: a historical case revisited. J Dtsch Dermatol Ges. 2011;9:1027-1028; discussion 1029.
- Özdemir M, Balevi A, Esen H. An inflammatory verrucous epidermal nevus concomitant with psoriasis: treatment with adalimumab. Dermatol Online J. 2012;18:11.
- Zvulunov A, Grunwald MH, Halvy S. Topical calcipotriol for treatment of inflammatory linear verrucous epidermal nevus. Arch Dermatol. 1997;133:567-568.
- Gatti S, Carrozzo AM, Orlandi A, et al. Treatment of inflammatory linear verrucous epidermal naevus with calcipotriol. Br J Dermatol. 1995;132:837-839.
- Fox BJ, Lapins NA. Comparison of treatment modalities for epidermal nevus: a case report and review. J Dermatol Surg Oncol. 1983;9:879-885.
- Pilanci O, Tas B, Ceran F, et al. A novel technique used in the treatment of inflammatory linear verrucous epidermal nevus: tangential excision. Aesthetic Plast Surg. 2014;38:1066-1067.
- Lee BJ, Mancini AJ, Renucci J, et al. Full-thickness surgical excision for the treatment of inflammatory linear verrucous epidermal nevus. Ann Plast Surg. 2001;47:285-292.
- Hohenleutner U, Landthaler M. Laser therapy of verrucous epidermal naevi. Clin Exp Dermatol. 1993;18:124-127.
- Parera E, Gallardo F, Toll A, et al. Inflammatory linear verrucous epidermal nevus successfully treated with methyl-aminolevulinate photodynamic therapy. Dermatol Surg. 2010;36:253-256.
- Situm M, Bulat V, Majcen K, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253.
- Beggs S, Short J, Rengifo-Pardo M, et al. Applications of the excimer laser: a review. Dermatol Surg. 2015;41:1201-1211.
- Bianchi B, Campolmi P, Mavilia L, et al. Monochromatic excimer light (308 nm): an immunohistochemical study of cutaneous T cells and apoptosis-related molecules in psoriasis. J Eur Acad Dermatol Venereol. 2003;17:408-413.
- Mudigonda T, Dabade TS, Feldman SR. A review of targeted ultraviolet B phototherapy for psoriasis. J Am Acad Dermatol. 2012;66:664-672.
- Kamo A, Tominaga M, Kamata Y, et al. The excimer lamp induces cutaneous nerve degeneration and reduces scratching in a dry-skin mouse model. J Invest Dermatol. 2014;134:2977-2984.
- Bulat V, Majcen K, Dzapo A, et al. Benefits of controlled ultraviolet radiation in the treatment of dermatological diseases. Coll Antropol. 2014;38:1249-1253
Melasma Treatment With Oral Tranexamic Acid and a Novel Adjuvant Topical Therapy
To the Editor:
I read with interest the informative article by Sheu1 published online in Cutis in February 2018, which succinctly described the pharmacologic characteristics of tranexamic acid, a synthetic lysine derivative, and its mechanism of action in the management of melasma by mitigating UV radiation-induced melanogenesis and neovascularization by inhibiting plasminogen activation. Additionally, the author summarized a study in which oral tranexamic acid was used to successfully treat melasma patients. After 4 months of treatment, 90% of 561 patients treated at a single center in Singapore demonstrated improvement in melasma severity.2 Sheu1 also discussed daily oral doses of tranexamic acid (500-1500 mg) that demonstrated improvement in melasma patients and reviewed potential adverse events (eg, abdominal pain and bloating, deep venous thrombosis, pulmonary embolism) for which patients should be screened and counseled prior to initiating treatment.
Recently, another study showed oral tranexamic acid to be an effective treatment in women with moderate to severe melasma. An important observation by the investigators was that once the initial phase of their study--250 mg of oral tranexamic acid twice daily and sunscreen applied to the face each morning and every 2 hours during daylight hours for 3 months--concluded and a second phase during which all participants only applied sunscreen for an additional 3 months, those with severe melasma lost most of their improvement.3 An adjuvant topical treatment, such as tranexamic acid or an inhibitor of tyrosinase (hydroquinone), might improve the results; however, initiating therapy with a topical agent whose mode of action is directed toward other melasma etiologic factors, such as the increased expression of estrogen receptors and vascular endothelial growth factor in affected skin, might be more beneficial.4,5
I recently proposed a novel approach for melasma management that would be appropriate as an adjuvant topical therapy for patients concurrently being treated with oral tranexamic acid.6 The therapeutic intervention utilizes active agents that specifically affect etiologic factors in the pathogenesis of melasma--estrogen and angiogenesis--that previously have not been targeted topically. Indeed, the topical agent contains an antiestrogen--either a selective estrogen receptor modulator (eg, tamoxifen, raloxifene), aromatase inhibitor (eg, anastrozole, letrozole, exemestane), or a selective estrogen receptor degrader (eg, fulvestrant)--and a vascular endothelial growth factor inhibitor (eg, bevacizumab).6
In conclusion, the therapeutic armamentarium for managing patients with melasma includes topical agents, oral therapies, and physical modalities. Optimizing the approach to treating melasma patients should incorporate therapies that are specifically directed toward various etiologic factors of the condition. The concurrent use of a topical agent that contains an antiestrogen and an inhibitor of vascular endothelial growth factor in women with melasma who are being treated with oral tranexamic acid warrants further investigation to assess not only for enhanced but also sustained reduction in facial skin pigmentation.
- Sheu SL. Treatment of melasma using tranexamic acid: what's known and what's next. Cutis. 2018;101:E7-E8.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective study. J Am Acad Dermatol. 2016;75:385-392.
- Del Rosaria E, Forez-Pollack S, Zapata L Jr, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369.
- Jang YH, Lee JY, Kang HY, et al. Oestrogen and progesterone receptor expression in melasma: an immunohistochemical analysis. J Eur Acad Dermatol Venereol. 2010;24:1312-1316.
- Kim EH, Kim YC, Lee ES, et al. The vascular characteristics of melasma. J Dermatol Sci. 2007;46:111-116.
- Cohen PR. Melasma treatment: a novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor [published online February 3, 2017]. Med Hypotheses. 2017;101:1-5.
To the Editor:
I read with interest the informative article by Sheu1 published online in Cutis in February 2018, which succinctly described the pharmacologic characteristics of tranexamic acid, a synthetic lysine derivative, and its mechanism of action in the management of melasma by mitigating UV radiation-induced melanogenesis and neovascularization by inhibiting plasminogen activation. Additionally, the author summarized a study in which oral tranexamic acid was used to successfully treat melasma patients. After 4 months of treatment, 90% of 561 patients treated at a single center in Singapore demonstrated improvement in melasma severity.2 Sheu1 also discussed daily oral doses of tranexamic acid (500-1500 mg) that demonstrated improvement in melasma patients and reviewed potential adverse events (eg, abdominal pain and bloating, deep venous thrombosis, pulmonary embolism) for which patients should be screened and counseled prior to initiating treatment.
Recently, another study showed oral tranexamic acid to be an effective treatment in women with moderate to severe melasma. An important observation by the investigators was that once the initial phase of their study--250 mg of oral tranexamic acid twice daily and sunscreen applied to the face each morning and every 2 hours during daylight hours for 3 months--concluded and a second phase during which all participants only applied sunscreen for an additional 3 months, those with severe melasma lost most of their improvement.3 An adjuvant topical treatment, such as tranexamic acid or an inhibitor of tyrosinase (hydroquinone), might improve the results; however, initiating therapy with a topical agent whose mode of action is directed toward other melasma etiologic factors, such as the increased expression of estrogen receptors and vascular endothelial growth factor in affected skin, might be more beneficial.4,5
I recently proposed a novel approach for melasma management that would be appropriate as an adjuvant topical therapy for patients concurrently being treated with oral tranexamic acid.6 The therapeutic intervention utilizes active agents that specifically affect etiologic factors in the pathogenesis of melasma--estrogen and angiogenesis--that previously have not been targeted topically. Indeed, the topical agent contains an antiestrogen--either a selective estrogen receptor modulator (eg, tamoxifen, raloxifene), aromatase inhibitor (eg, anastrozole, letrozole, exemestane), or a selective estrogen receptor degrader (eg, fulvestrant)--and a vascular endothelial growth factor inhibitor (eg, bevacizumab).6
In conclusion, the therapeutic armamentarium for managing patients with melasma includes topical agents, oral therapies, and physical modalities. Optimizing the approach to treating melasma patients should incorporate therapies that are specifically directed toward various etiologic factors of the condition. The concurrent use of a topical agent that contains an antiestrogen and an inhibitor of vascular endothelial growth factor in women with melasma who are being treated with oral tranexamic acid warrants further investigation to assess not only for enhanced but also sustained reduction in facial skin pigmentation.
To the Editor:
I read with interest the informative article by Sheu1 published online in Cutis in February 2018, which succinctly described the pharmacologic characteristics of tranexamic acid, a synthetic lysine derivative, and its mechanism of action in the management of melasma by mitigating UV radiation-induced melanogenesis and neovascularization by inhibiting plasminogen activation. Additionally, the author summarized a study in which oral tranexamic acid was used to successfully treat melasma patients. After 4 months of treatment, 90% of 561 patients treated at a single center in Singapore demonstrated improvement in melasma severity.2 Sheu1 also discussed daily oral doses of tranexamic acid (500-1500 mg) that demonstrated improvement in melasma patients and reviewed potential adverse events (eg, abdominal pain and bloating, deep venous thrombosis, pulmonary embolism) for which patients should be screened and counseled prior to initiating treatment.
Recently, another study showed oral tranexamic acid to be an effective treatment in women with moderate to severe melasma. An important observation by the investigators was that once the initial phase of their study--250 mg of oral tranexamic acid twice daily and sunscreen applied to the face each morning and every 2 hours during daylight hours for 3 months--concluded and a second phase during which all participants only applied sunscreen for an additional 3 months, those with severe melasma lost most of their improvement.3 An adjuvant topical treatment, such as tranexamic acid or an inhibitor of tyrosinase (hydroquinone), might improve the results; however, initiating therapy with a topical agent whose mode of action is directed toward other melasma etiologic factors, such as the increased expression of estrogen receptors and vascular endothelial growth factor in affected skin, might be more beneficial.4,5
I recently proposed a novel approach for melasma management that would be appropriate as an adjuvant topical therapy for patients concurrently being treated with oral tranexamic acid.6 The therapeutic intervention utilizes active agents that specifically affect etiologic factors in the pathogenesis of melasma--estrogen and angiogenesis--that previously have not been targeted topically. Indeed, the topical agent contains an antiestrogen--either a selective estrogen receptor modulator (eg, tamoxifen, raloxifene), aromatase inhibitor (eg, anastrozole, letrozole, exemestane), or a selective estrogen receptor degrader (eg, fulvestrant)--and a vascular endothelial growth factor inhibitor (eg, bevacizumab).6
In conclusion, the therapeutic armamentarium for managing patients with melasma includes topical agents, oral therapies, and physical modalities. Optimizing the approach to treating melasma patients should incorporate therapies that are specifically directed toward various etiologic factors of the condition. The concurrent use of a topical agent that contains an antiestrogen and an inhibitor of vascular endothelial growth factor in women with melasma who are being treated with oral tranexamic acid warrants further investigation to assess not only for enhanced but also sustained reduction in facial skin pigmentation.
- Sheu SL. Treatment of melasma using tranexamic acid: what's known and what's next. Cutis. 2018;101:E7-E8.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective study. J Am Acad Dermatol. 2016;75:385-392.
- Del Rosaria E, Forez-Pollack S, Zapata L Jr, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369.
- Jang YH, Lee JY, Kang HY, et al. Oestrogen and progesterone receptor expression in melasma: an immunohistochemical analysis. J Eur Acad Dermatol Venereol. 2010;24:1312-1316.
- Kim EH, Kim YC, Lee ES, et al. The vascular characteristics of melasma. J Dermatol Sci. 2007;46:111-116.
- Cohen PR. Melasma treatment: a novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor [published online February 3, 2017]. Med Hypotheses. 2017;101:1-5.
- Sheu SL. Treatment of melasma using tranexamic acid: what's known and what's next. Cutis. 2018;101:E7-E8.
- Lee HC, Thng TG, Goh CL. Oral tranexamic acid (TA) in the treatment of melasma: a retrospective study. J Am Acad Dermatol. 2016;75:385-392.
- Del Rosaria E, Forez-Pollack S, Zapata L Jr, et al. Randomized, placebo-controlled, double-blind study of oral tranexamic acid in the treatment of moderate-to-severe melasma. J Am Acad Dermatol. 2018;78:363-369.
- Jang YH, Lee JY, Kang HY, et al. Oestrogen and progesterone receptor expression in melasma: an immunohistochemical analysis. J Eur Acad Dermatol Venereol. 2010;24:1312-1316.
- Kim EH, Kim YC, Lee ES, et al. The vascular characteristics of melasma. J Dermatol Sci. 2007;46:111-116.
- Cohen PR. Melasma treatment: a novel approach using a topical agent that contains an anti-estrogen and a vascular endothelial growth factor inhibitor [published online February 3, 2017]. Med Hypotheses. 2017;101:1-5.
Going Digital With Dermoscopy
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
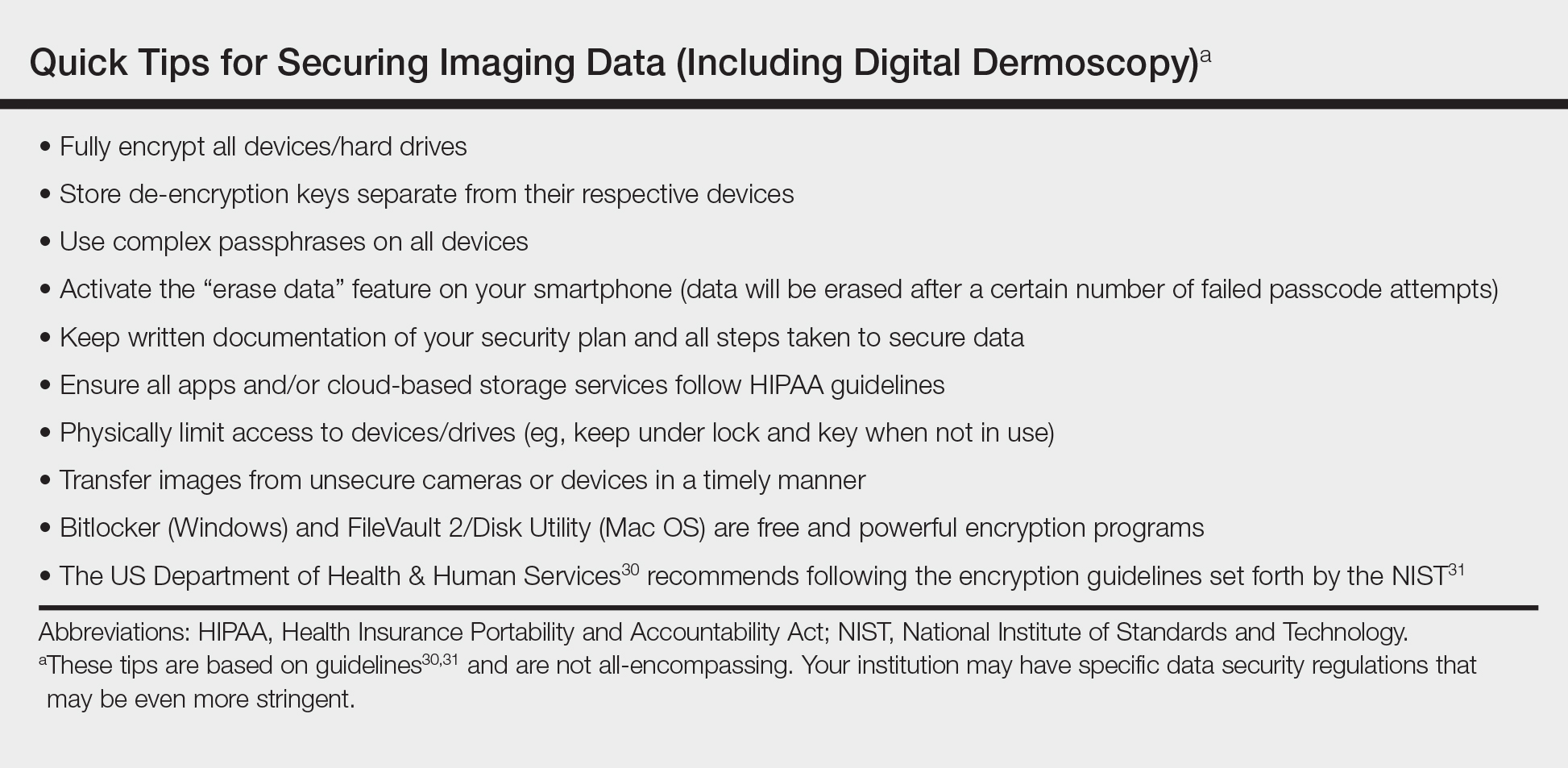
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
Dermoscopic examination has been proven to increase diagnostic accuracy and decrease unnecessary biopsies of both melanoma and nonmelanoma skin cancers.1,2 Digital dermoscopy refers to acquiring and storing digital dermoscopic photographs via digital camera, smart image capture devices such as smartphones and tablets, or any other devices used for image acquisition. The stored images may then be used in a variety of ways, including sequential digital monitoring, teledermoscopy, and machine learning.
Sequential Digital Monitoring
Sequential digital dermoscopy imaging (SDDI) is the capture and storage of dermoscopic images of suspicious lesions that are then monitored over time for changes. Studies have shown that SDDI allows for early detection of melanomas and leads to a decrease in the number of unnecessary excisions.3,4 A meta-analysis of SDDI found that the chance of detecting melanoma increased with the length of monitoring, which suggests that continued follow-up, especially in high-risk groups, is crucial.4
Teledermoscopy
Teledermatology (telederm) is on the rise in the United States, with the number of programs and consultations increasing yearly. One study showed a 48% increase in telederm programs in the last 5 years.5 Studies have shown the addition of digital dermoscopic images improved the diagnostic accuracy in telederm skin cancer screenings versus clinical images alone.6,7
Telederm currently is practiced in 2 main models: live-interactive video consultation and storage of images for future consultation (store and forward). Medicare currently only reimburses live-interactive telederm for patients in nonmetropolitan areas and store-and-forward telederm pilot programs in Alaska and Hawaii; however, Medicaid does reimburse for store and forward in a handful of states.8 Similar to dermatoscope use during clinical examination, there currently is no additional reimbursement for teledermoscopy. Of note, a willingness-to-pay survey of 214 students from a southwestern university health center showed that participants were willing to pay an average (SD) of $55.27 ($39.11) out of pocket for a teledermoscopy/telederm evaluation, citing factors such as convenience.9
Direct-to-consumer telederm offers a new way for patients to receive care.10 Some dermatoscopes (eg, DermLite HÜD [3Gen], Molescope/Molescope II [Metaoptima Technology Inc]) currently are marketed directly to consumers along with telederm services to facilitate direct-to-patient teledermoscopy.11,12
Machine Learning
Big data and machine learning has been hailed as the future of medicine and dermatology alike.13 Machine learning is a type of artificial intelligence that uses computational algorithms (eg, neural networks) that allow computer programs to automatically improve their accuracy (learn) by analyzing large data sets. In dermatology, machine learning has been most notably used to train computers to identify images of skin cancer by way of large image databases.14-17 One algorithm, a convolutional neural network (CNN), made headlines in 2017 when it was able to identify dermoscopic and clinical images of skin cancer with comparable accuracy to a group of 21 dermatologists.14 In 2018, the International Skin Imaging Collaboration (ISIC) published results of a study of the diagnostic accuracy of 25 computer algorithms compared to 8 dermatologists using a set of 100 dermoscopic images of melanoma and benign nevi.15 Using the average sensitivity of the dermatologists (82%), the top fusion algorithm in the study had a sensitivity of 76% versus 59% for the dermatologists (P=.02). These results compared the mean sensitivity of the dermatologists, as some individual dermatologists outperformed the algorithm.15 More recently, another CNN was compared to 58 international dermatologists in the classification of a set of 100 dermoscopic images (20 melanoma and 80 melanocytic nevi).16 Using the mean sensitivity of the dermatologists (86.6%), the CNN had a specificity of 92.5% versus 71.3% for dermatologists (P<.01). In the second part of the study, the dermatologists were given some clinical information and close-up photographs of the lesions, which improved their average (SD) sensitivity and specificity to 88.9% (9.6%)(P=.19) and 75.7% (11.7%)(P<.05), respectively. When compared to the CNN at this higher sensitivity, the CNN still had a higher specificity than the dermatologists (82.5% vs 75.7% [P<.01]).16 However, in real-life clinical practice dermatologists perform better, not only because they can collect more in-person clinical information but also because humans gather more information during live examination than when they are interpreting close-up clinical and/or dermoscopic images. In a sense, we currently are limited to comparing data that is incommensurable.
Machine learning studies have other notable limitations, such as data sets that do not contain a full spectrum of skin lesions or less common lesions (eg, pigmented seborrheic keratoses, amelanotic melanomas) and variation in image databases used.15,16 For machine algorithms to improve, they require access to high-quality and ideally standardized digital dermoscopic image databases. The ISIC and other organizations currently have databases specifically for this purpose, but more images are needed.18 As additional practitioners incorporate digital dermoscopy in their clinical practice, the potential for larger databases and more accurate algorithms becomes a possibility.
Image Acquisition
Many devices are available for digital dermoscopic image acquisition, including dermatoscopes that attach to smartphones and/or digital cameras and all-in-one systems (eTable). The exact system employed will depend on the practitioner's requirements for price, portability, speed, image quality, and software. Digital single-lens reflex (DSLR) cameras boast the highest image quality, while video dermoscopy traditionally yields stored images with poor resolution.19 Macroscopic images obtained by other imaging devices, including spectral imaging devices and reflectance confocal microscopy, usually are yielded via video dermoscopy or a video camera to capture images; thus, stored images generally are not as high quality.
Smartphones are increasingly used for clinical imaging in dermatology.20 Although DSLR cameras still take the highest-quality images, current smartphone image quality is comparable to digital cameras.21,22 Computational photography uses computer processing power to enhance image quality and may bring smartphone image quality closer to DSLR cameras.22,23 Smartphones with newer dual-lens cameras have been reported to further improve image quality.21 Current smartphones have the option of enabling high-dynamic-range imaging, which combines multiple images taken with different exposures to create a single image with improved dynamic range of luminosity. It has been reported that high-dynamic-range imaging may even enhance dermoscopic features of more challenging hypopigmented skin cancers.24
Standardizing Imaging
There has been a concerted effort to standardize digital dermatologic image acquisition.25,26 Standardization promises to facilitate data analysis, improve collaboration, protect patient privacy, and improve patient care.13,26,27 At the forefront of image standardization is the ISIC organization, which recently published its Delphi consensus guidelines on standards for lesion imaging, including dermoscopy.26
The true holy grail of image standardization is the Digital Imaging and Communications in Medicine (DICOM) standard.26-28 The DICOM is a comprehensive imaging standard for storage, annotation, transfer, and display of images, and it is most notable for its use in radiology. The DICOM also could be applied to new imaging modalities in dermatology (eg, optical coherence tomography, reflectance confocal microscopy). Past efforts to develop a DICOM standard for dermatology were undertaken by a working group that has since disbanded.27 Work by the ISIC and many others will hopefully lead to adoption of the DICOM standard by dermatology at some point in the future.
Protected Health Information
The Health Insurance Portability and Accountability Act (HIPAA) requires protected health information (PHI) to be stored in a secure manner with limited access that sufficiently protects identifiable patient information. Although dermoscopic images generally are deidentified, they often are stored alongside clinical photographs and data that contains PHI in clinical practice.
Image storage can take 2 forms: (1) physical local storage on internal and external hard drives or (2) remote storage (eg, cloud-based storage). Encryption is essential regardless of the method of storage. It is required by law that loss of nonencrypted PHI be reported to all potentially affected patients, the US Department of Health & Human Services, and local/state media depending on the number of patients affected. Loss of PHI can result in fines of up to $1.5 million.29 On the contrary, loss of properly encrypted data would not be required to be reported.30
As smart image acquisition devices begin to dominate the clinical setting, practitioners need to be vigilant in securing patient PHI. There are multiple applications (apps) that allow for secure encrypted digital dermoscopic image acquisition and storage on smartphones. Additionally, it is important to secure smartphones with complex passcodes (eg, a mix of special characters, numbers, uppercase and lowercase letters). Most dermatoscope manufacturers have apps for image acquisition and storage that can be tied into other platforms or storage systems (eg, DermLite app [3Gen], Handyscope [FotoFinder Systems GmbH], VEOS app [Canfield Scientific, Inc]).28 Other options include syncing images with current electronic medical record technologies, transferring photographs to HIPAA-compliant cloud storage, or transferring photographs to an encrypted computer and/or external hard drive. Some tips for securing data based on HIPAA and other guidelines are listed in the Table.30,31
Conclusion
The expansion of teledermoscopy alongside direct-to-patient services may create additional incentives for clinicians to incorporate digital dermoscopy into their practice. As more practitioners adopt digital dermoscopy, machine learning driven by technological advancements and larger image data sets could influence the future practice of dermatology. With the rise in digital dermoscopy by way of smartphones, additional steps must be taken to ensure patients' PHI is safeguarded. Digital dermoscopy is a dynamic field that will likely see continued growth in the coming years.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
- Vestergaard ME, Macaskill P, Holt PE, et al. Dermoscopy compared with naked eye examination for the diagnosis of primary melanoma: a meta-analysis of studies performed in a clinical setting. Br J Dermatol. 2008;159:669-676.
- Rosendahl C, Tschandl P, Cameron A, et al. Diagnostic accuracy of dermatoscopy for melanocytic and nonmelanocytic pigmented lesions. J Am Acad Dermatol. 2011;64:1068-1073.
- Salerni G, Lovatto L, Carrera C, et al. Melanomas detected in a follow-up program compared with melanomas referred to a melanoma unit. Arch Dermatol. 2011;147:549-555.
- Salerni G, Terán T, Puig S, et al. Meta-analysis of digital dermoscopy follow-up of melanocytic skin lesions: a study on behalf of the International Dermoscopy Society. J Eur Acad Dermatol Venereol. 2013;27:805-814.
- Yim KM, Armstrong AW, Oh DH, et al. Teledermatology in the United States: an update in a dynamic era [published online January 22, 2018]. Telemed J E Health. doi:10.1089/tmj.2017.0253.
- Ferrándiz L, Ojeda-Vila T, Corrales A, et al. Internet-based skin cancer screening using clinical images alone or in conjunction with dermoscopic images: a randomized teledermoscopy trial. J Am Acad Dermatol. 2017;76:676-682.
- Şenel E, Baba M, Durdu M. The contribution of teledermatoscopy to the diagnosis and management of non-melanocytic skin tumours. J Telemed Telecare. 2013;19:60-63.
- State telehealth laws and Medicaid program policies: a comprehensive scan of the 50 states and District of Columbia. Public Health Institute Center for Connected Health Policy website. http://www.cchpca.org/sites/default/files/resources/
50%20State%20FINAL%20April%202016.pdf. Published March 2016. Accessed July 2, 2018. - Raghu TS, Yiannias J, Sharma N, et al. Willingness to pay for teledermoscopy services at a university health center. J Patient Exp. 2018. doi:10.11772374373517748657.
- Fogel AL, Sarin KY. A survey of direct-to-consumer teledermatology services available to US patients: explosive growth, opportunities and controversy. J Telemed Telecare. 2017;23:19-25.
- MoleScope. MetaOptima Technology Inc website. https://molescope.com/product/. Accessed July 2, 2018.
- DermLite HÜD. 3Gen website. https://dermlite.com/products/dermlite-hud. Accessed July 2, 2018.
- Park AJ, Ko JM, Swerlick RA. Crowdsourcing dermatology: DataDerm, big data analytics, and machine learning technology. J Am Acad Dermatol. 2018;78:643-644.
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115-118.
- Marchetti MA, Codella NCF, Dusza SW, et al; International Skin Imaging Collaboration. results of the 2016 International Skin Imaging Collaboration International Symposium on Biomedical Imaging challenge: comparison of the accuracy of computer algorithms to dermatologists for the diagnosis of melanoma from dermoscopic images. J Am Acad Dermatol. 2018;78:270-277.
- Haenssle HA, Fink C, Schneiderbauer R, et al. Man against machine: diagnostic performance of a deep learning convolutional neural network for dermoscopic melanoma recognition in comparison to 58 dermatologists [published online May 28, 2018]. doi:10.1093/annonc/mdy166.
- Prado G, Kovarik C. Cutting edge technology in dermatology: virtual reality and artificial intelligence. Cutis. 2018;101:236-237.
- Sultana NN, Puhan NB. Recent deep learning methods for melanoma detection: a review. In: Ghosh D, Giri D, Mohapatra R, et al, eds. Mathematics and Computing. Singapore: Springer Nature; 2018:118-132.
- Lake A, Jones B. Dermoscopy: to cross-polarize, or not to cross-polarize, that is the question. J Vis Commun Med. 2015;38:36-50.
- Abbott LM, Magnusson RS, Gibbs E, et al. Smartphone use in dermatology for clinical photography and consultation: current practice and the law [published online February 28, 2017]. Australas J Dermatol. 2018;59:101-107.
- Hauser W, Neveu B, Jourdain JB, et al. Image quality benchmark of computational bokeh. Electron Imaging. 2018;2018:1-10.
- Ignatov A, Kobyshev N, Timofte R, et al. DSLR-quality photos on mobile devices with deep convolutional networks. 2017 IEEE International Conference on Computer Vision (ICCV). Venice, Italy: IEEE; 2017:3297-3305.
- Greengard S. Computational photography comes into focus. Commun ACM. 2014;57:19-21.
- Braun RP, Marghoob A. High-dynamic-range dermoscopy imaging and diagnosis of hypopigmented skin cancers. JAMA Dermatol. 2015;151:456-457.
- Quigley EA, Tokay BA, Jewell ST, et al. Technology and technique standards for camera-acquired digital dermatologic images: a systematic review. JAMA Dermatol. 2015;151:883-890.
- Katragadda C, Finnane A, Soyer HP, et al. Technique standards for skin lesion imaging a delphi consensus statement. JAMA Dermatol. 2017;153:207-213.
- Caffery LJ, Clunie D, Curiel-Lewandrowski C, et al. Transforming dermatologic imaging for the digital era: metadata and standards [published online January 17, 2018]. J Digit Imaging. doi:10.1007/s10278-017-0045-8.
- Pagliarello C, Stanganelli I, Fabrizi G, et al. Digital dermoscopy monitoring: is it time to define a quality standard? Acta Derm Venereol. 2017;97:864-865.
- HITECH Act Enforcement Interim Final Rule. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/special-topics/hitech-act-enforcement-interim-final-rule/index.html. Updated June 16, 2017. Accessed July 2, 2018.
- Guidance to render unsecured protected health information unusable, unreadable, or indecipherable to unauthorized individuals. US Department of Health & Human Services website. https://www.hhs.gov/hipaa/for-professionals/breach-notification/guidance/index.html. Updated July 26, 2013. Accessed July 2, 2018.
- Scarfone K, Souppaya M, Sexton M. Guide to Storage Encryption Technologies for End User Devices. Gaithersburg, MD: US Department of Commerce; 2007. NIST Special Publication 800-111.
Laser Scar Management: Focused and High-Intensity Medical Exchange in Vietnam
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.
Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
Over the last decade the treatment of traumatic scars with lasers has emerged as a core component of multidisciplinary management. Military dermatologists have played a fundamental role in this shift by helping to develop new applications for existing technology and promulgate the techniques to reach additional providers and patients. Beyond scar management, the repurposing of adjunctive procedural techniques, such as sweat and hair reduction in amputees, also promises to enhance rehabilitation for many patients.
International engagement is a prominent and highly attractive feature of military practice, and military dermatologists routinely participate in disaster response missions, such as the 2010 Haiti earthquake,1 and ongoing planned operations, such as Pacific Partnership in the Indo-Asia-Pacific region led by the US Navy.2 In this article, I present a military perspective on the emerging niche of trauma dermatology and outline my more than 5 years of experience leveraging these skills to lead a multidisciplinary exchange in restorative medicine and burn scar management in Vietnam.
Trauma Dermatology
Over the course of the last decade, traumatic scar management has emerged as a staple of dermatologic surgery practice in some centers. Dermatologists hold the key to increasing patient access to effective outpatient care for symptomatic traumatic scars and other related issues using devices and techniques initially conceived for cosmetic applications.3 A major impetus for the considerable remodeling in our collective thoughts about traumatic scar management was the emergence of fractional laser technology in the mid-2000s. The remarkable, safe, reproducible, and durable benefits of fractional laser treatment of various scar types have created substantial momentum in recent years. The Naval Medical Center San Diego in California houses 1 of 3 centers of excellence in rehabilitation in the US military. Mastery of minimally invasive procedures to manage scars and other issues associated with trauma for the first time has established dermatologists as important partners in the overall rehabilitative effort.
My perspective on laser scar management has been previously described.4,5 Ablative fractional laser resurfacing is the backbone of rehabilitative scar management.6 Although the literature in this field is still relatively immature, higher-quality studies are accumulating rapidly as the burn and surgical communities adopt the procedure more widely.7-10 A considerable step forward in the dissemination of the procedure occurred recently with the development of category III Current Procedural Terminology (CPT) codes for ablative laser treatment of traumatic scars.11 Category III CPT codes are temporary codes used for emerging procedures that have not yet been deemed medically necessary. Although individual insurance carriers can determine whether to cover these procedures and the corresponding level of reimbursement, regular use is important for ultimate elevation to category I codes by the American Medical Association over a 5-year observation period. The CPT codes 0479T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; first 100 cm2 or part thereof, or 1% of body surface area of infants and children) and 0480T (fractional ablative laser fenestration of burn and traumatic scars for functional improvement; each additional 100 cm2, or each additional 1% of body surface area of infants and children, or part thereof [list separately in addition to code for primary procedure]) are examples of these category III codes.11
Nonablative fractional lasers; vascular-specific devices for erythematous scars; and long- and short-pulsed pigment-specific devices for hair and traumatic tattoo treatment, respectively, round out the commonly used laser platforms. For example, laser hair reduction can help improve the fit and comfort of prosthetic devices and has been shown to improve the overall quality of life for amputees.12 Botulinum toxin can be an important component of treatment of excessive sweating induced by occlusive liners in prosthetics, and microwave eccrine ablation is an emerging potential option for longer-lasting sweat reduction in this population.13-15 In addition to providing direct dermatology care and education, having members of the specialty in uniform has been a key to adopting new practical solutions to unsolved problems.
Pacific Partnership
Pacific Partnership is the largest annual multinational humanitarian assistance and disaster preparedness mission in the Indo-Asia-Pacific region.16 It was started in 2006 following the tsunami that devastated parts of South and Southeast Asia in 2004. The recently concluded Pacific Partnership 2018 marked the 13th iteration of the annual mission led by the US Navy in collaboration with other partner nations, which in 2018 included Japan, Australia, Canada, the United Kingdom, France, Singapore, Korea, and Peru, as well as nongovernmental organizations and international governmental agencies. Host nation mission locations vary somewhat from year to year, but 2018 included visits of the hospital ship USNS Mercy and more than 800 personnel to Indonesia, Malaysia, Sri Lanka, and Vietnam. Medical/dental, engineering, and veterinary teams join with their counterparts in each host nation to conduct civic action projects, community health exchanges, medical care, and disaster response training activities.16
Rehabilitation As a Vehicle for Medical Exchange
Since approximately 2012 there has been an evolving paradigm in Pacific Partnership from an emphasis on maximizing direct patient care in changing locations to one focused on building lasting partnerships through subject matter expert exchange. Multidisciplinary scar management, including surgical and laser scar revision and physical and occupational therapy, is a very promising model for engagement. Potential advantages of this type of exchange include the following: developing nations have relatively high rates of burns and other forms of trauma as well as uneven access to acute and ongoing rehabilitative care; patients often are otherwise healthy and young; results are frequently profound and readily demonstrable; and it is a skill set that has become highly developed in the military system. Just as dermatologists are illustrating their utility in trauma rehabilitation at home, these procedural skills provide fertile ground for exchange overseas.
The Overseas Humanitarian Assistance Shared Information System is an online platform that allows users to apply for grants under the Asia-Pacific Regional Initiative. In 2013, I started the Burn Scar Treatment/Restorative Medicine exchange with a grant under this program. A multidisciplinary team representing the specialties of dermatology, hand surgery, plastic surgery, physical medicine and rehabilitation, and pulmonary critical care participated in the 2013 Asia Pacific Burn Congress hosted by the National Institute of Burns (NIB) in Hanoi, Vietnam, and then followed up with didactics and patient care alongside Vietnamese physicians in the management of disfiguring and debilitating scars from burns and other trauma. This pilot project consisted of three 2- to 3-week phases: 2 at the NIB in Hanoi and 1 with a delegation from the NIB visiting the Naval Medical Center San Diego. When initial project funds expired in 2014, the exchange was absorbed into Pacific Partnership 2014, which began a string of 4 consecutive annual Pacific Partnership engagements at Da Nang General Hospital in Vietnam. The 2 most recent exchanges, including the exchange associated with Pacific Partnership 2018, have taken place at Khanh Hoa General Hospital in Nha Trang, Vietnam. During this time the team has grown to include physical and occupational therapists as well as a wound care nurse.
The Burn Scar Treatment/Restorative Medicine exchange consists of side-by-side laser and surgical scar revision performed with our Vietnamese hosts in their own hospital. Our Vietnamese partners perform a large volume of reconstructive surgeries in their usual practice, so it truly has been a bilateral exchange incorporating some advanced technology and techniques with an emphasis on longitudinal multidisciplinary care. Importantly, the procedures are supplemented with preoperative and postoperative care as well as instruction provided by physical and occupational therapy and wound care professionals working alongside host nation support staff. Because the areas of involvement often are extensive and a patient may only be seen once in this setting, laser and surgical procedures often are performed concurrently in the host nation operating room. Anesthesia support is provided by the host nation. Basic consumable surgical supplies (eg, sutures, gloves, marking pens, staplers) are supplemented with mission funds. Special adjuncts for the most severe contractures have included negative pressure wound therapy and a collagen-based bilayer matrix wound dressing. Laser treatments have been performed on the vast majority of patients with an ablative fractional CO2 laser and laser-assisted delivery of corticosteroid in hypertrophic areas. Of note, use of the laser has been provided to our hosts by the manufacturer for each of the 7 iterations of the exchange, and the wound dressing manufacturer also has donated some of their product to the exchange through the nongovernmental organization Project Hope for 2 missions. To date, more than 300 patients have safely received life-changing treatment during the exchange, with some receiving multiple treatments (Figure). Although multiple treatments over time are ideal, even a single treatment session can result in considerable and lasting improvements in function and symptoms.17 The hospital ship USNS Mercy has the same laser technology and has brought advanced scar treatment techniques to the far corners of the Pacific.

Measuring overall success—treatment and international relations—in this setting can be challenging. On an individual patient level, the benefits of restoring the ability to walk and work as well as reducing pain and itching are manifest and transformative for both the patient and family; however, aggregating this information into high-quality outcome data is difficult given the heterogeneous nature of traumatic injuries, which is compounded in the setting of international engagement where the intersection between patient and visiting provider may be singular or difficult to predict, funding is limited, language frequently is a barrier, and documentation, privacy, and medical research guidelines may be unfamiliar or contradictory. The cumulative impact of these types of exchanges on the relationship between nations also is critical but difficult to measure. It is common sense that deepening personal and professional relationships in the medical setting over time can increase trust and mutual understanding, perhaps setting the stage for broader engagement in other more sensitive areas. Trust and understanding are rather nebulous concepts, but earlier this year marked the first visit of an American aircraft carrier to Da Nang since 1975, following 4 consecutive annual Pacific Partnership missions in the same city, which does carry the patina of successful engagement on a systemic level.
Final Thoughts
Based on my personal experience, I provide the following tips for building a successful, focused, long-term medical exchange.
- Leverage your strengths and respect the strengths and style of practice of your hosts. A mind-set of exchange and not simply humanitarian care will be more successful. Your hosts are experts in a style of practice adapted to their surroundings and introducing new techniques that are grounded in the local practice patterns are more likely to be perpetuated.
- Collaboration with nongovernmental organizations and industry can be extremely helpful. Military and governmental organizations often are limited in funding, in the ways they can spend available funding, and in the receipt of donations. Appropriate coordination with civilian entities can elevate the exchange considerably by adding expertise and available assets as well as broadening the overall impact.
- Engage the support staff as well as the physicians. You will leverage contact with families and enhance care over the long-term.
- The benefits of multiple interactions over time are manifest, for both the patients and the participants. Personal and professional relationships are intertwined and naturally mature over time. Go for singles and doubles first before swinging for the fences.
- Multidisciplinary work overseas informs and enhances collaboration at home.
- Adding regional experts in international research and assessment to these specialized medical teams may better capture the impact of future exchanges of any flavor.
- The model of creating a focused exchange with independent funding followed by incorporation of successful concepts into larger missions seems to be a worthy and reproducible approach for future projects of any variety.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
- Galeckas K. Dermatology aboard the USNS Comfort: disaster relief operations in Haiti after the 2010 earthquake. Dermatol Clin. 2011;29:15-19.
- Satter EK. The role of the dermatologist on military humanitarian missions. Cutis. 2010;85:85-89.
- Miletta NR, Donelan MB, Hivnor CM. Management of trauma and burn scars; the dermatologist's role in expanding patient access to care. Cutis. 2017;100:18-20.
- Shumaker PR. Laser treatment of traumatic scars: a military perspective. Semin Cutan Med Surg. 2015;34:17-23.
- Shumaker PR, Beachkofsky T, Basnett A, et al. A military perspective. In: Krakowski AC, Shumaker PR, eds. The Scar Book: Formation, Mitigation, Rehabilitation and Prevention. Philadelphia, PA: Wolters Kluwer; 2017:327-338.
- Anderson RR, Donelan MB, Greeson E, et al. Consensus report: laser treatment of traumatic scars with an emphasis on ablative fractional resurfacing. JAMA Dermatol. 2014;150:187-193.
- Hultman CS, Friedstat JS, Edkins RE, et al. Laser resurfacing and remodeling of hypertrophic burn scars: the results of a large, prospective, before and after cohort study, with long-term follow-up. Ann Surg. 2014;260:519-532.
- Blome-Eberwein S, Gogal C, Weiss MJ, et al. Prospective evaluation of fractional CO2 laser treatment of mature burn scars. J Burn Care Res. 2016;37:379-387.
- Issler-Fisher AC, Fisher OM, Smialkowski AO, et al. Ablative fractional CO2 laser for burn scar reconstruction: an extensive subjective and objective short-term outcome analysis of a prospective treatment cohort. Burns. 2017;43:573-582.
- Zuccaro J, Zlolkowski N, Fish J. A systematic review of the effectiveness of laser therapy for hypertrophic burn scars. Clin Plast Surg. 2017;44:767-779.
- Miller A. CPT 2018: What's new, part 2. American Academy of Dermatology website. https://www.aad.org/dw/monthly/2018/january/cpt-2018-whats-new-part-2. Accessed July 24, 2018.
- Miletta NR, Kim S, Lezanski-Gujda A, et al. Improving health-related quality of life in wounded warriors: the promising benefits of laser hair removal to the residual limb-prosthetic interface. Dermatol Surg. 2016;42:1182-1187.
- Gratrix M, Hivnor C. Botulinum toxin for hyperhidrosis in patients with prosthetic limbs. Arch Dermatol. 2010;146:1314-1315.
- Pace S, Kentosh J. Managing residual limb hyperhidrosis in wounded warriors. Cutis. 2016;97:401-403.
- Mula KN, Winston J, Pace S, et al. Use of a microwave device for treatment of amputation residual limb hyperhidrosis. Dermatol Surg. 2017;43:149-152.
- USNS Mercy deploys in support of Pacific Partnership 2018 [news release]. Washington, DC: US Department of Defense; February 26, 2018. https://www.defense.gov/News/Article/Article/1450292/usns-mercy-deploys-in-support-of-pacific-partnership-2018/. Accessed July 11, 2018.
- Burns C, Basnett A, Valentine J, et al. Ablative fractional resurfacing: a powerful tool to help restore form and function during international medical exchange. Lasers Surg Med. 2017;49:471-474.
Pigmented Lesion on the Forearm
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3
Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4
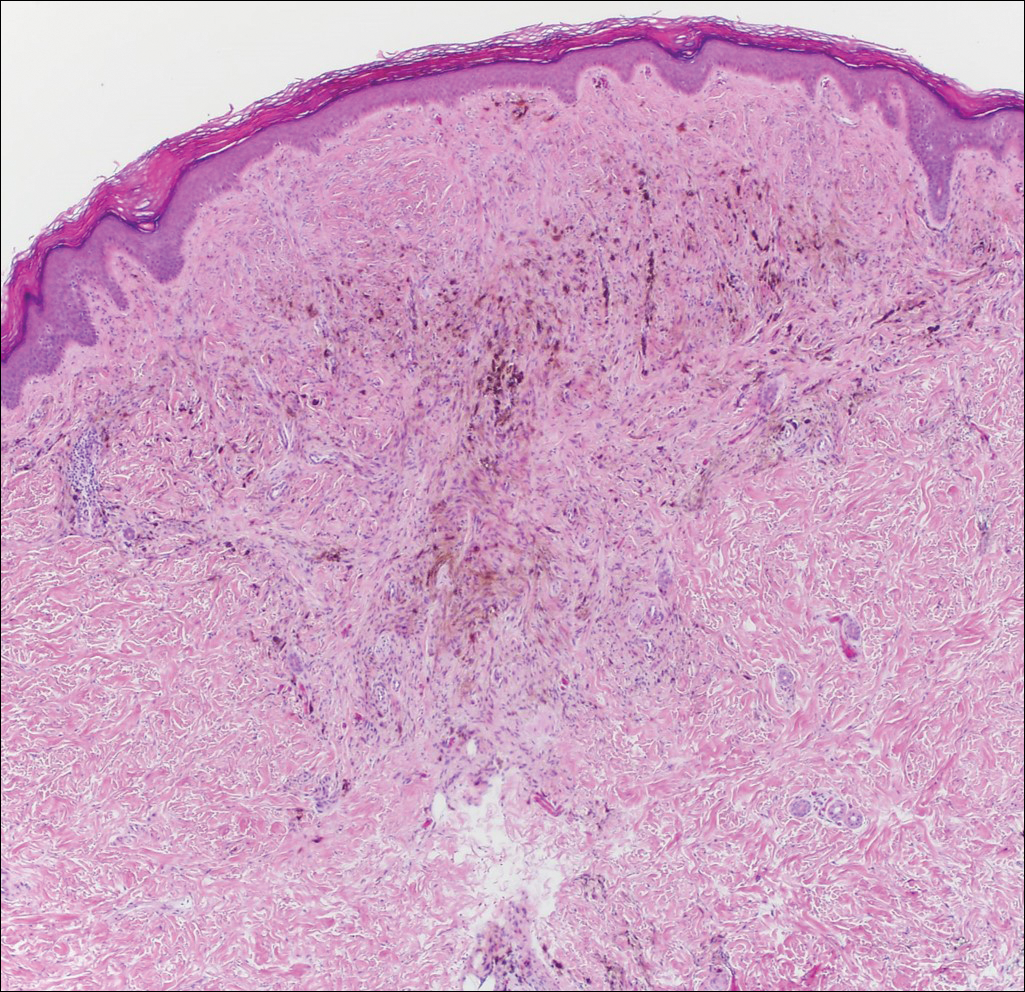
Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8
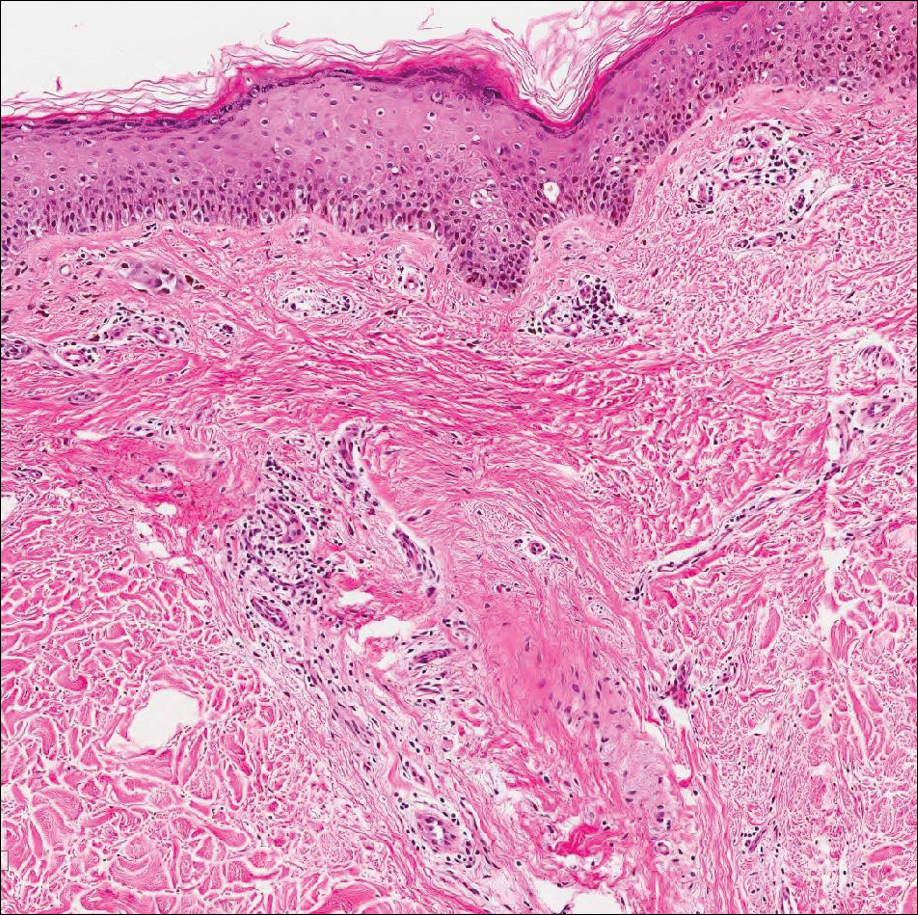
Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
The Diagnosis: Monsel Solution Reaction
Exogenous substances can cause interesting incongruities in cutaneous biopsies of which pathologists and dermatologists should be cognizant. Exogenous lesions are caused by externally introduced foreign bodies, substances, or materials, such as sterile compressed sponges, aluminum chloride hexahydrate and anhydrous ethyl alcohol, silica, paraffin, and Monsel solution. Monsel solution reaction is a florid fibrohistiocytic proliferation stimulated by the application of Monsel solution. Monsel solution is a ferric subsulfate that often is used to achieve hemostasis after shave biopsies. Hemostasis is thought to result from the ability of ferric ions to denature and agglutinate proteins such as fibrinogen.1,2 Application of Monsel solution likely causes ferrugination of fibrin, dermal collagen, and striated muscle fibers. Some ferruginated collagen fibers are eliminated through the epidermis as the epidermis regenerates, while some fibers become calcified. Siderophages (iron-containing macrophages) are present in these areas. The ferrugination of collagen fibers becomes less pronounced as the biopsy sites heal and the iron pigment subsequently is absorbed by macrophages. Ferruginated skeletal muscles can act as foreign bodies and may elicit granulomatous reactions.2
It is currently unclear why fibrohistiocytic responses occur in some instances but not others. Iron stains (eg, Perls Prussian blue stain) make interpretation clear, provided the pathologist is familiar with Monsel solution. The primary differential diagnosis of these lesions centers on heavily pigmented melanocytic proliferations. It is critical to review prior biopsy sections or to have definite knowledge of the prior biopsy diagnosis. Histologically, the epidermis may demonstrate nonspecific reactive changes such as hyperkeratosis with foci of irregular acanthosis. The prominent features are present in the dermis where there is a proliferation of spindle- and polyhedral-shaped cells that may show cytologic atypia and occasional mitotic figures. The cells contain refractile brown pigment scattered in the dermis and deposited on collagen fibers (quiz images). Occasional large black or brown encrustations may be identified. Monsel-containing cells may indiscernibly blend with foci of more blatantly fibrohistiocytic differentiation, in which case iron stains are strongly positive (Figure 1). If the clinician uses Monsel solution for hemostasis during the removal of a nevomelanocytic neoplasm, it might be necessary to use melanin stains or immunohistochemistry on the reexcision specimen to distinguish between residual nevomelanocytic and fibrohistiocytic cells.3

Common blue nevus is a benign, typically intradermal melanocytic lesion. It most frequently occurs in young adults and has a predilection for females. Clinically, it can be found anywhere on the body as a single, asymptomatic, well-circumscribed, blue-black, dome-shaped papule measuring less than 1 cm in diameter. Histologically, it is characterized by pigmented, dendritic, spindle-shaped melanocytes that typically are separated by thick collagen bundles (Figure 2). The melanocytes typically have small nuclei with occasional basophilic nucleolus. Melanocytes typically are diffusely positive for melanocytic markers including human melanoma black (HMB) 45, S-100, Melan-A, and microphthalmia transcription factor 1. In contrast to most other benign melanocytic nevi, HMB-45 strongly stains the entire lesion in blue nevi.4

Desmoplastic melanoma accounts for 1% to 4% of all melanomas. The median age at diagnosis is 62 years and, as in other types of melanoma, men are more commonly affected.5 Clinically, desmoplastic melanoma typically presents on the head and neck as a painless indurated plaque, though it can present as a small papule or nodule. Nearly half of desmoplastic melanomas lack obvious pigmentation, which may lead to the misdiagnosis of basal cell carcinoma or a scar. Histologically, desmoplastic melanomas are composed of spindled melanocytes separated by collagen fibers or fibrous stroma (Figure 3). Histology displays variable cytologic atypia and stromal fibrosis. Characteristically there are small islands of lymphocytes and plasma cells within or at the edge of the tumor. The spindle cells stain positive with S-100 and Sry-related HMg-box gene 10, SOX10. Type IV collagen and laminin often are expressed in desmoplastic melanoma. In contrast to many other subtypes of melanoma, HMB-45 and Melan-A usually are negative.6

Animal-type melanoma is a rare neoplasm that differs from other subtypes of melanoma both clinically and histologically. Most frequently, animal-type melanoma affects younger adults (median age, 27 years) and arises on the arms and legs, head and neck, or trunk; men and women are affected equally.7 It most commonly presents with a blue or blue-black nodule with a blue-white veil or irregular white areas. Histologically, animal-type melanoma is a predominantly dermal-based melanocytic proliferation with heavily pigmented epithelioid and spindled melanocytes (Figure 4). The pigmentation pattern ranges widely from fine, granular, light brown deposits to coarse dark brown deposits with malignant cells often arranged in fascicles or sheets. Frequently, there is periadnexal and perieccrine spread. Often, there is epidermal hyperplasia above the dermis. As with conventional melanoma, the immunohistochemistry of animal-type melanoma is positive for S-100 protein, HMB-45, SOX10, and Melan-A.7

Recurrent nevi typically arise within 6 months of a previously biopsied melanocytic nevus. Most recurrent nevi originate from common banal nevi (most often a compound nevus). Recurrent nevi also may arise from congenital, atypical/dysplastic, and Spitz nevi. Most often they are found on the back of women aged 20 to 30 years.8 Clinically, they manifest as a macular area of scar with variegated hyperpigmentation and hypopigmentation as well as linear streaking. They may demonstrate variable diffuse, stippled, and halo pigmentation patterns. Classically, recurrent nevi present with a trizonal histologic pattern. Within the epidermis there is a proliferation of melanocytes along the dermoepidermal junction, which may show varying degrees of atypia and pagetoid migration. The melanocytes often are described as epithelioid with round nuclei and even chromatin (Figure 5). The atypical features should be confined to the epidermis overlying the prior biopsy site. Within the dermis there is dense dermal collagen and fibrosis with vertically oriented blood vessels. Finally, features of the original nevus may be seen at the base of the lesion. Although immunohistochemistry may be helpful in some cases, an appropriate clinical history and comparison to the prior biopsy can be invaluable.8

Host tissue reactions resulting in artefactual changes caused by foreign bodies or substances may confound the untrained eye. Monsel solution reaction may be confused for a blue nevus, desmoplastic melanoma, animal-type melanoma, and a residual/recurrent nevus. This confusion could lead to serious diagnostic errors that could cause an unfavorable outcome for the patient. It is critical to know the salient points in the patient's clinical history. Knowledge of the Monsel solution reaction and other exogenous lesions as well as the subsequent unique tissue reaction patterns can aid in facilitating an accurate and prompt pathologic diagnosis.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
- Olmstead PM, Lund HZ, Leonard DD. Monsel's solution: a histologic nuisance. J Am Acad Dermatol. 1980;3:492-498.
- Amazon K, Robinson MJ, Rywlin AM. Ferrugination caused by Monsel's solution. clinical observations and experimentations. Am J Dermatopathol. 1980;2:197-205.
- Del Rosario RN, Barr RJ, Graham BS, et al. Exogenous and endogenous cutaneous anomalies and curiosities. Am J Dermatopathol. 2005;27:259-267.
- Calonje E, Blessing K, Glusac E, et al. Blue naevi. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:95-99.
- Jain S, Allen PW. Desmoplastic malignant melanoma and its variants. a study of 45 cases. Am J Surg Pathol. 1989;13:358-373.
- McCarthy SW, Crotty KA, Scolyer RA. Desmoplastic melanoma and desmoplastic neurotropic melanoma. In: LeBoit PE, Burg G, Weedon D, et al, eds. World Health Organization Classification of Tumours, Pathology and Genetics of Skin Tumours. Lyon, France: IARC Press; 2006:76-78.
- Vyas R, Keller JJ, Honda K, et al. A systematic review and meta-analysis of animal-type melanoma. J Am Acad Dermatol. 2015;73:1031-1039.
- Fox JC, Reed JA, Shea CR. The recurrent nevus phenomenon: a history of challenge, controversy, and discovery. Arch Pathol Lab Med. 2011;135:842-846.
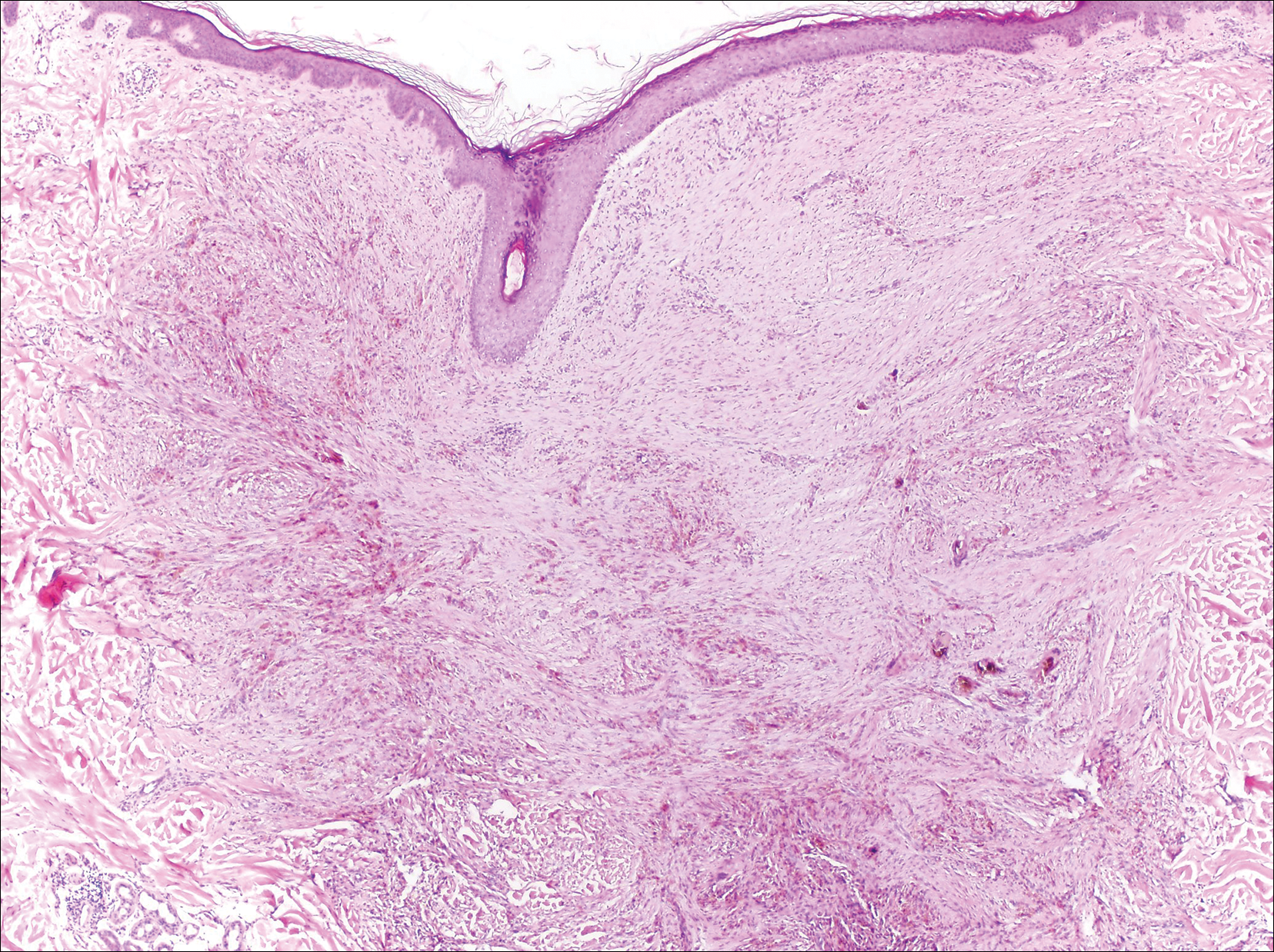
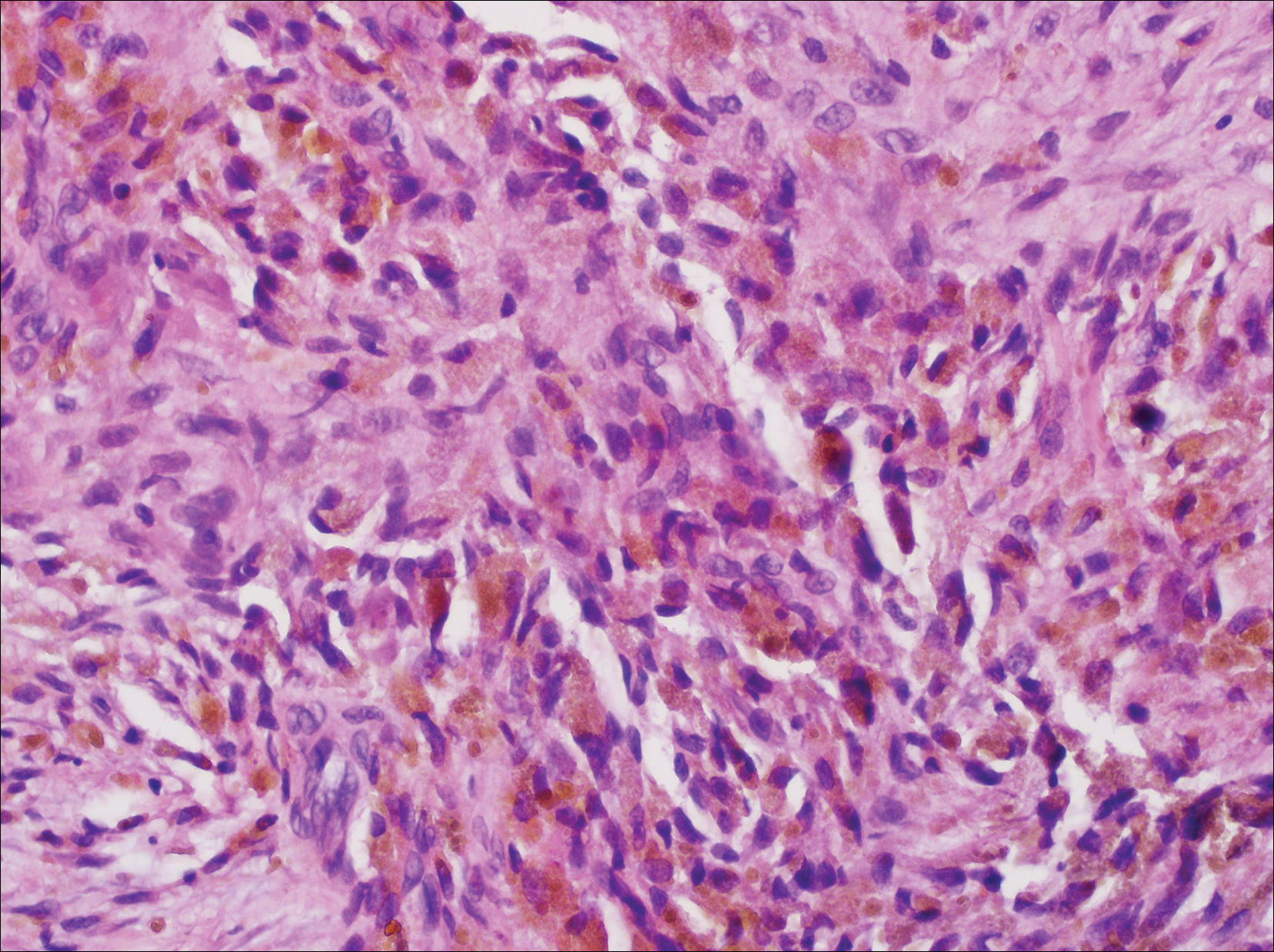
A 67-year-old man presented to the dermatology clinic with a 2-cm pigmented lesion on the forearm. An excisional biopsy was obtained.
Wound Closure Tips
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
What does your patient need to know preoperatively?
Patients should be educated on all aspects of the procedure as well as the expected postoperative course of healing. Manage patient expectations in advance to minimize any surprises for everyone involved. Swelling and bruising are not uncommon in the immediate postoperative phase, and for surgery near the eyes, both may be worse, making it prudent for patients to schedule any procedures after big events or vacations.
The sutured wound initially can appear lumpy, bumpy, and pink, and it may take potentially 3 to 6 months, or even longer, for the scar to fully mature depending on the type of repair performed. Sutured wounds require activity restrictions, which is especially important for young active patients as well as patients who may have labor-intensive occupations. I often recommend 1 to 2 weeks before resuming most forms of strenuous exercise and/or physical labor. Skin grafts may require even longer limitations. Although the overall risk for infection is low (approximately 1%), patients should be instructed to monitor for purulent drainage, fever, and worsening pain and redness, and to inform the dermatologist immediately of any concerning symptoms.
What is your go-to approach for wound closure?
My motto is: Simplest is often best. For the patient who prioritizes returning to full activity as soon as possible, the wound may be able to heal by secondary intention in select anatomic locations, and this approach can often yield excellent cosmetic results. If wound closure with sutures is indicated, then I use the following treatment algorithm:
- Primary closure is used if I can close a wound in a linear fashion without distorting free margins, especially if I can hide the lines within cosmetic subunit junctions and/or relaxed skin tension lines.
- Local flap is used for defects when repair in a linear fashion is not always ideal for various reasons. Recruit local skin with various flap options for the best color and texture match. This approach may be more involved but often provides the best long-term cosmetic outcome; however, it usually results in a longer recovery time and may even require staged procedures.
- Graft usually is our last preferred option because it may appear as a sewn-in patch; however, in certain anatomic locations and in the right patient, skin grafts also can yield acceptable cosmetic results.
I give trainees the following surgical technique pearls:
- Use buried vertical mattress sutures to achieve eversion of wound edges with deep sutures
- Dermal pulley as well as epidermal pulley sutures can offset tension wonderfully, especially in high-tension areas such as the back and scalp
- Placement of a running subcuticular suture in place of epidermal stitches on the trunk and extremities can prevent track marks
How do you keep patients compliant with wound care instructions?
Two keys to high patient compliance with wound care are making instructions as simple as possible and providing detailed written instructions. We instruct patients to keep the pressure dressing in place for 48 hours. Once removed, we recommend patients clean the wound with regular soap and water daily, followed by application of petrolatum ointment. For hard-to-reach areas or on non-hair-bearing skin, my surgical assistants apply adhesive strips over the sutures, eliminating the need for daily wound care. For full-thickness skin grafts, we commonly place a bolster pressure dressing that stays in place until the patient returns to our clinic for a postoperative visit. We provide every patient with detailed written instructions as a patient handout that is specific to the type of wound closure performed.
What do you do if the patient refuses your recommendation for wound closure?
It is important to explain all wound closure options to the patient and the risks and benefits of each. I always show patients the proposed plan using a mirror and/or textbook images so that they can better understand the process. In rare cases when the patient refuses the preferred method of closure, we ensure that he/she understands the advantages and disadvantages of the proposed procedure and why the recommendation was made. If the patient still refuses, we document our lengthy discussion in the medical record. For patients who refuse our recommended plan of sutures and opt to heal by secondary intention, we will see these patients almost weekly to ensure appropriate healing as well as provide further recommendations such as a delayed repair if there is any evidence of functional impairment and/or notable cosmetic implications. A patient completely refusing a planned repair is rare.
More commonly, patients request a "simpler" repair, even if the cosmetic outcome may be suboptimal. For example, some elderly patients with large nasal defects do not want to undergo a staged flap, even though it would give a superior cosmetic result. Instead, we do the best we can with a skin graft or single-stage flap.
What resources do you provide to patients for wound care instructions?
We recommend that physicians prepare comprehensive handouts on wound care instructions that address both short-term and long-term expectations, provide instructions regarding follow-up, and encourage good sun protection behaviors. Some physicians post videos demonstrating proper wound care on their websites, which may be another useful tool.
Acknowledgment
The author thanks Daniel Condie, MD (Dallas, Texas), for his contributions.
Suggested Readings
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part I. cutting tissue: incising, excising, and undermining. J Am Acad Dermatol. 2015;72:377-387.
Miller CJ, Antunes MB, Sobanko JF. Surgical technique for optimal outcomes: part II. repairing tissue: suturing. J Am Acad Dermatol. 2015;72:389-402.
Acute Painful Rash on the Cheek
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.
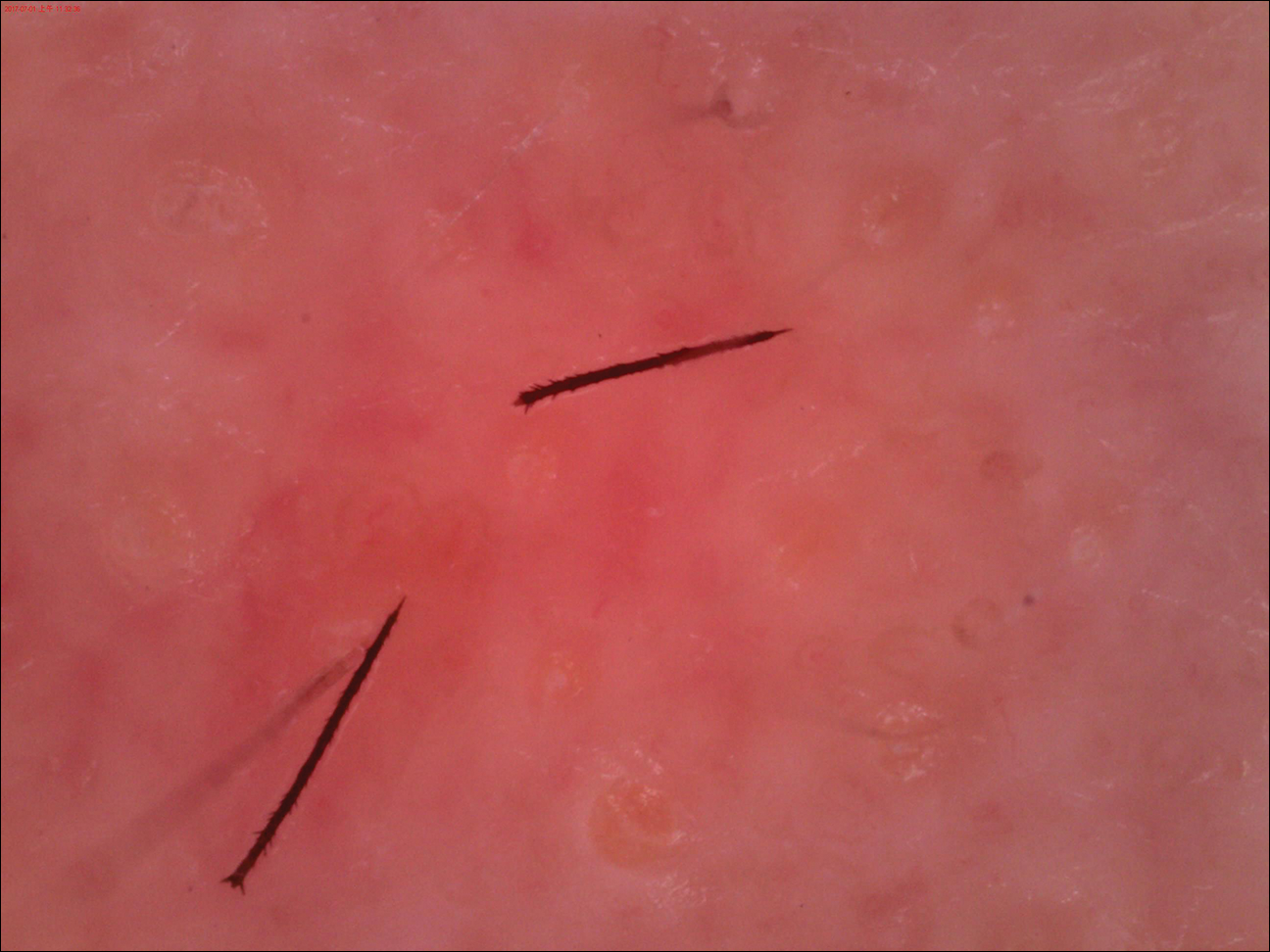
Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
The Diagnosis: Acute Contact Dermatitis
Dermoscopy demonstrated caterpillar hairs (Figure) and established the diagnosis of acute contact dermatitis due to a caterpillar. Upon further questioning, the patient recalled that something dustlike fell on the left cheek as she walked under some trees. The clinical and dermoscopic findings suggested a diagnosis of caterpillar dermatitis (family Limacodidae or Lymantriinae, order Lepidoptera). During the life cycle from young larvae to mature larvae, the quantities of the toxic thorns and hairs increase from 60,000 to 80,000 to 2,000,000 to 3,000,000. The toxic hairs measure 0.5 to 2.0 mm in length. They drop from the mature larvae's skin during desquamation as well as from the cocoon curing maturation to a moth. The hairs appear tubular and arrowlike with terminal spines.1 The larvae are called "the fiery hot" in Chinese, which vividly describes the swelling and sensation of burning with immediate contact.

Eruption severity and distribution depend on exposure modality and intensity. Exposed body parts, including the face, neck, forearms, interdigital spaces, and dorsal aspects of the hands, most commonly are involved. The eruption can be immediate or delayed, occurring hours or even days after the first contact.2 Itching is intense and continuous, with intermittent worsening. Clinically, the eruption manifests with rose to bright red, round macules and papules. Although rare, skin manifestations can be accompanied by systemic symptoms, such as malaise, fever, and anaphylaxis syndrome.3 The cutaneous lesions may last for 3 to 4 days and subside, leaving a brownish macule.1,4
The differential diagnosis includes acute herpes simplex, which presents as grouped vesicles on an erythematous base with itching or burning, and recurrences in the same location are common. Acute Sweet syndrome may appear as erythematous or edematous painful plaques with fever and neutrophilia. Acute urticaria appears as wheals with severe pruritus, and individual lesions can resolve within several hours. Insect bites often appear as itching or painful erythema or papules.
We sterilized the lesion with alcohol, removed the thorns as much as possible with ophthalmic forceps under the guidance of dermoscopy, and prescribed chloramphenicol ointment 1% twice daily. Our patient was completely cured within 24 hours with no systemic symptoms or pigmentation.
This case directly showed a novel usage of dermoscopy in diagnosis and therapy, especially in acute contact dermatitis. Small irritants such as caterpillar thorns and hairs easily can be observed and removed by dermoscopy devices with higher magnification.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
- Fangan H, Yun H, Yuhua G, et al. Observations on the pathogenicity of Lepidoptera, Euileidae caterpillar and the clinical pathological pictures of patients with dermatitis. Chinese J Zoonoses. 2005,21:414-416.
- Bonamonte D, Foti C, Vestita M, et al. Skin reactions to pine processionary caterpillar Thaumetopoea pityocampa Schiff. ScientificWorldJournal. 2013;2013:867431.
- Burns T, Breathnach S, Cox N, et al, eds. Rook's Textbook of Dermatology. 8th ed. Vol 2. Oxford, United Kingdom: Blackwell; 2010.
- Henwood BP, MacDonald DM. Caterpillar dermatitis. Clin Exp Dermatol. 1983;8:77-93.
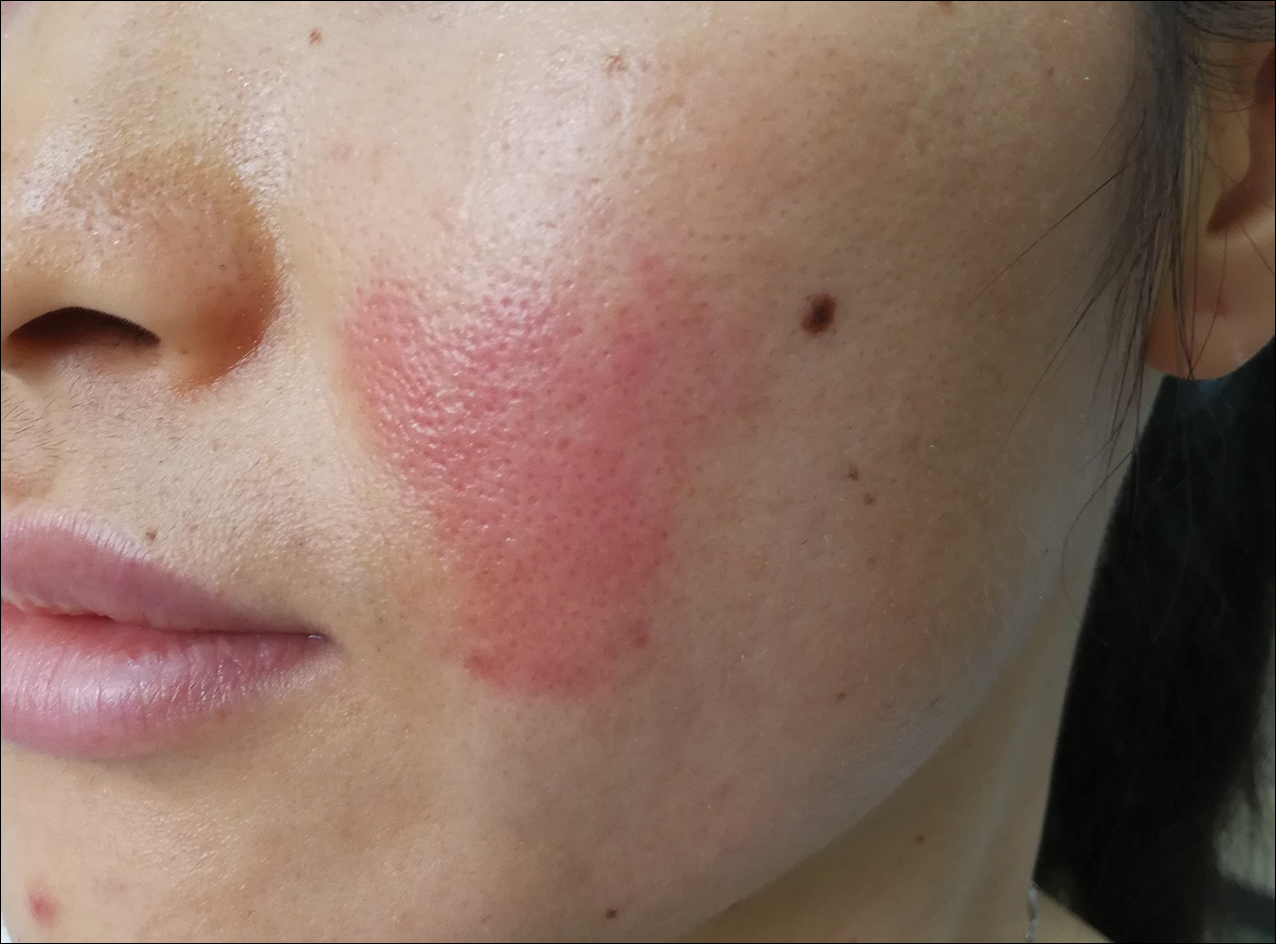
A 31-year-old woman presented to an outpatient dermatology department with acute pruritus, burning, and moderate swelling of the left cheek of 10 minutes' duration that occurred while waiting to see a hematologist in the same building. The patient was diagnosed with aplastic anemia 11 years prior and was awaiting bone marrow transplantation. Physical examination showed an edematous erythematous wheal with a relatively distinct border measuring 3 cm in diameter. No foreign material could be identified on the surface with the naked eye. Dermoscopy was performed.
Electronic Medical Records in Dermatology: The Good, the Bad, and the Ugly
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
The American Recovery and Reinvestment Act of 2009 introduced the Health Information Technology for Economic and Clinical Health (HITECH) Act and allocated $19.2 billion to promote the implementation of an electronic medical record (EMR) by hospitals as well as physicians in private practices.1 The EMR stores longitudinal health information and constructs a comprehensive picture of a patient's medical history.2,3 Following its debut, the US Department of Health & Human Services set forth meaningful use (MU) criteria,1 which aimed to increase quality of care, safety, and efficiency. Meaningful use criteria also sought to decrease health disparities; improve coordination of care; engage patients in their care; refine population and public health measures; and finally ensure accessibility, privacy, and security of patient data.1,2
The EMR offers potential gains at multiple levels of the patient-physician-public health hierarchy: a decrease in medical errors and duplicate services, timely access to test results and records, timely notification of patients in need for preventive services, and preservation of medical records in the event of an environmental disaster.1-3 Furthermore, physicians can take advantage of informational reciprocity with other providers, gain remote access to medical records, be reminded of need for service, e-prescribe, monitor for drug interactions, and utilize clinical information for research purposes.1,3 Lastly, public health organizations can use EMRs to improve outcomes by employing surveillance measures and creating patient registries that serve to protect the society at large.1
Although it seems that the broad-scale implementation of EMRs will undoubtedly enhance the quality of patient care in the years to come, many obstacles must be overcome to reach this potential. Certification of EMR systems and implementation of confidentiality measures that are compatible with the Health Insurance Portability and Accountability Act are the forerunning concerns, and the interconnectivity of EMR systems becomes more important as MU enters its later stages (eg, increased electronic transmission of patient data during phases of care, reliance on e-prescribing, population-level analysis of patient data to improve health outcomes). Additionally, the cost to implement and maintain an EMR deters many physicians in smaller practices from shouldering the charges, as they do not necessarily see increased productivity with this technology.2 Last but not least, unintended consequences of EMR implementation can involve the dangers of upcoding and overdocumentation.1
Dermatology is a visually dominant field serving a high volume of patients that require both medical and surgical care. These factors do not preclude the implementation of EMRs in our specialty but rather necessitate the utilization of a system specifically designed to cater to the needs of specialists in dermatology. This editorial will address some fundamental considerations in implementing an EMR in dermatology; discuss what platforms and patient interfaces are most practical; reiterate the importance of interoperability; and highlight the implications of this powerful tool in education, research, training, and monitoring quality of care.
Implementation and Specific Considerations in Dermatology
One of the biggest areas of concern in adopting an EMR is the associated financial burden.2-5 Although government incentives of MU cover a part of the initial cost of purchasing an EMR, expert maintenance costs, changes in workflow dynamics, and a steep learning curve can translate into lost productivity and revenue, discouraging many dermatologists from implementing an EMR.3,5 Furthermore, physicians who are nearing the end of their careers may not realize the longer-term benefits of implementing an EMR and therefore may decline to do so despite the disincentives of lower Medicare reimbursements.2 In fact, when juxtaposing the upfront cost of implementing an EMR against the expected increase in revenue after its implementation, there are general concerns that there will be a net loss on the part of the provider, which is a barrier to adoption of EMRs.3
Beyond the cost-benefit analysis, dermatologists often report multiple lesions in different anatomic sites or identify multiple biopsy sites that have been conveniently recorded on body templates included in their paper-based examination forms. Converting that information into words to be entered into an EMR can be excruciatingly time consuming and not easily comprehensible upon follow-up visits with the patient, which again results in decreased efficiency and productivity.4 Therefore, selecting a dermatology-compatible EMR that aims to make this transition easier is of utmost importance. One developer introduced an EMR with a touch interface containing a human body in all its facets that can be rotated, zoomed, and marked multiple times for accurate and convenient recording of lesions and intended procedure sites. This system automatically produces codes for examinations and procedures; facilitates e-prescription and ordering of laboratory results; prints pathology requests and consent forms; and includes information for patient education regarding their care. For example, the physical examination section of an H&P (history and physical examination) can be generated with a few taps on the screen, translating into increased efficiency and productivity.
The visual nature of dermatology demands the use of images, and as such, photographs have become integral in the diagnosis and follow-up of dermatology patients. Digital and dermatoscopic images not only help to eliminate unnecessary biopsies but also can promote early detection and management of malignancies.6 Thus, the capability to link photographs to a patient's medical record using an EMR is an invaluable gain. However, employing this feature in clinical practice has ethical implications that must be addressed, given that taking photographs can evoke an avoidable fear in patients regarding unlawful dissemination or unnecessary exposure of these images to physicians who do not need to access them.6 Thus, guidelines must be set for uploading, de-identifying, and annotating patient photographs, and only physicians involved in the care of the patient should be allocated access.4,6
EMR Platforms
When computers were first introduced into examination rooms, many physicians were reluctant, as computers were thought to disconnect physicians from patients, arousing a sense of remoteness and further depersonalizing the encounter as physicians spent more time typing and less time making meaningful eye contact with patients.5 The practice of dermatology requires patient-centered communication that serves to enhance the quality of care while at the same time allowing physicians to fulfill professional competencies and reduce medical errors, which may ultimately translate into patient satisfaction.7 In fact, when the patient-physician relationship is interrupted, patients are more likely to pursue legal action in the wake of a bad treatment outcome.5
Employing a tablet-friendly EMR can help circumvent (or at least minimize) this problem by providing the physician with a light, user-friendly device that eliminates the need for laptops or desktop computers.4 Going one step further, the utilization of tablets eliminates the need for accessory digital cameras, as most tablets come with built-in high-resolution cameras for capturing clinical photographs and immediately linking them to the patient's medical record. Lastly, tablet technology allows physicians to access consent forms while in the examination room with the patient to more readily obtain a signature for procedural or research consent.4,8
Interoperability, MU, and Quality of Care
The real goal in nationwide implementation of EMR technology is to accomplish MU criteria. Different EMRs should not only allow data to be imported and exported but ideally should be compatible and interoperable with one another.2 The myriad of different EMR platforms available impedes maximal functionality as MU moves into its final stage. For example, if an academic dermatology program in the setting of a larger hospital is required to use the generic hospital EMR, the dermatologist's specific needs may not be effectively met; on the other hand, if a dermatology-specific EMR is implemented, access to the hospital's larger database of patient information may be sacrificed. Optimal EMR systems should be designed to allow specificity for a given specialty while being able to receive and integrate laboratory values, dermatopathology and radiology results, and notes from consultations by other physicians. Such integration may reduce duplicate services, increase patient satisfaction, and fulfill MU criteria. In fact, the fear of many physicians, especially those in a field such as dermatology, are the unwanted costs that come with implementation of an EMR system that will soon become impractical due to compatibility issues.2 As a result, until a system that can meet the needs of multiple specialties is developed, dermatologists and other physicians upgrading to an EMR should consider implementing a system that is compatible with nearby hospitals, other specialists' offices, and diagnostic centers to maximize interoperability at the local level.2,3
Electronic medical record systems that interoperate also provide the ability to set forth performance measures for physicians aimed at improving quality of care. As our health care system moves toward a pay-for-performance model, EMRs will become a tool to determine if standards of care have been met, unnecessary diagnostic tests have been avoided, and unwanted outcomes have been minimized. These measures will usher in a new era of medicine in which physicians strive to improve the care provided to their patients and receive increases in their reimbursements, while patient outcomes and satisfaction are improved.9
Academics, Education, Research, and Residency
The practicality of EMRs in dermatology may best be appreciated in academic settings. Electronic medical records serve as a repository of coded information that is neatly organized and can be rapidly searched, allowing for use as a powerful research tool. As an example, physicians can use EMR systems to identify patients with specific qualifications and study outcome variables over time.2,3 Additionally, with the rise of interoperable systems, we can expect a new dawn in medical research as more information becomes available to clinical investigators, opening doors to endless possibilities for evidence-based care.2,3,8
Another advantage of EMRs is their utility in residency programs that are charged with the task of ensuring resident competency via exposure to a comprehensive host of clinical encounters. An EMR system uniquely allows residents and attending dermatologists to monitor adequate exposure to general, pediatric, complex medical, procedural, and dermatopathologic cases, and to track the number of procedures performed by the residents by directly linking the information into the Accreditation Council for Graduate Medical Education procedure log.
Furthermore, due to the dominance of digital and dermatoscopic images in the field, interoperable EMRs could be used to construct a database of clinical and dermatopathologic specimens that not only can be used in educating residents but also may serve as a powerful reference tool in the diagnosis of complex and rare cases.8 Another often unrecognized advantage of EMRs is their utility in teledermatology. With the interoperable EMRs within academic institutions, teledermatology can be used locally and nationally for rapid consultation with high diagnostic validity,10 which has the burgeoning potential of providing patients with quicker time to diagnosis considering the dermatologist shortage in various parts of the country.
Lastly, the implementation of EMRs in residency programs has the additional benefit of exposing residents and medical students to emerging technology early on in their careers and fosters a degree of familiarity and comfort that may lead to implementation of EMRs in their future practices.2 For dermatologists in-training, early exposure to these technologies also may serve as a way to develop an interactive interview style and adapt to the presence of EMRs in examination rooms without sacrificing quality of care and meaningful patient interaction.7
Conclusion
Electronic medical records are already becoming an integral part of many hospitals and private dermatology practices. Although EMRs provide potential benefits that can be expected to ultimately outweigh the associated costs in larger settings such as hospitals, residency programs, and multidisciplinary practices, EMRs may not be immediately beneficial to physicians in private practices or those approaching the end of their careers. Although a perfect system may be unattainable, development of interoperable systems designed to meet the needs of specialties such as dermatology are essential in attaining a comprehensive patient medical profile, improving quality of care, minimizing costs, reducing medical errors, and maximizing patient satisfaction.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
- Health IT and health information exchange basics. Office of the National Coordinator for Health Information Technology website. http://www.healthit.gov/providers-professionals/learn-ehr-basics. Reviewed January 8, 2018. Accessed July 10, 2018.
- Grosshandler JA, Tulbert B, Kaufmann MD, et al. The electronic medical record in dermatology. Arch Dermatol. 2010;146:1031-1036.
- Menachemi N, Collum TH. Benefits and drawbacks of electronic health record systems. Risk Manag Healthc Policy. 2011;4:47-55.
- Kaufmann MD, Desai S. Special requirements for electronic health records in dermatology. Semin Cutan Med Surg. 2012;31:160-162.
- Wheeland RG. Separating EMR implementation hype from fact. Dermatology Times. http://www.dermatologytimes.com/modern-medicine-now/separating-emr-implementation-hype-fact. Published July 1, 2012. Accessed July 16, 2018.
- Lakdawala N, Bercovitch L, Grant-Kels JM. A picture is worth a thousand words: ethical dilemmas presented by storing digital photographs in electronic health records. J Am Acad Dermatol. 2013;69:473-475.
- Nguyen TV, Hong J, Prose NS. Compassionate care: enhancing physician-patient communication and education in dermatology: part I: patient-centered communication. J Am Acad Dermatol. 2013;68:353.e1-8.
- Ratner D, Thomas CO, Bickers D. The uses of digital photography in dermatology. J Am Acad Dermatol. 1999;41(5, pt 1):749-756.
- Wilson RL, Feldman SR. Physician performance measures in dermatology. J Am Acad Dermatol. 2010;63:E29-E35.
- Vañó-Galván S, Hidalgo A, Aguayo-Leiva I, et al. Store-and-forward teledermatology: assessment of validity in a series of 2000 observations [in Spanish]. Actas Dermosifiliogr. 2011;102:277-283.
Solitary Nodule on the Proximal Nail Fold
The Diagnosis: Superficial Acral Fibromyxoma
A shave biopsy revealed an uninvolved grenz zone and mildly cellular spindle cell dermal proliferation in a collagenous and myxoid background (Figure 1). Spindle cells were seen in a myxoid background among dense coarse collagen (Figure 2A). Spindle cells also were seen in a myxoid background with mast cells and capillary network (Figure 2B). Histopathologic examination of the biopsy specimen revealed spindle cells that were diffusely positive for CD34 (Figure 3); focally positive for epithelial membrane antigen; and negative for melanocytic markers, smooth muscle markers, and cytokeratin. A diagnosis of superficial acral fibromyxoma (SAFM) was made based on clinical, histopathologic, and immunohistochemical findings.
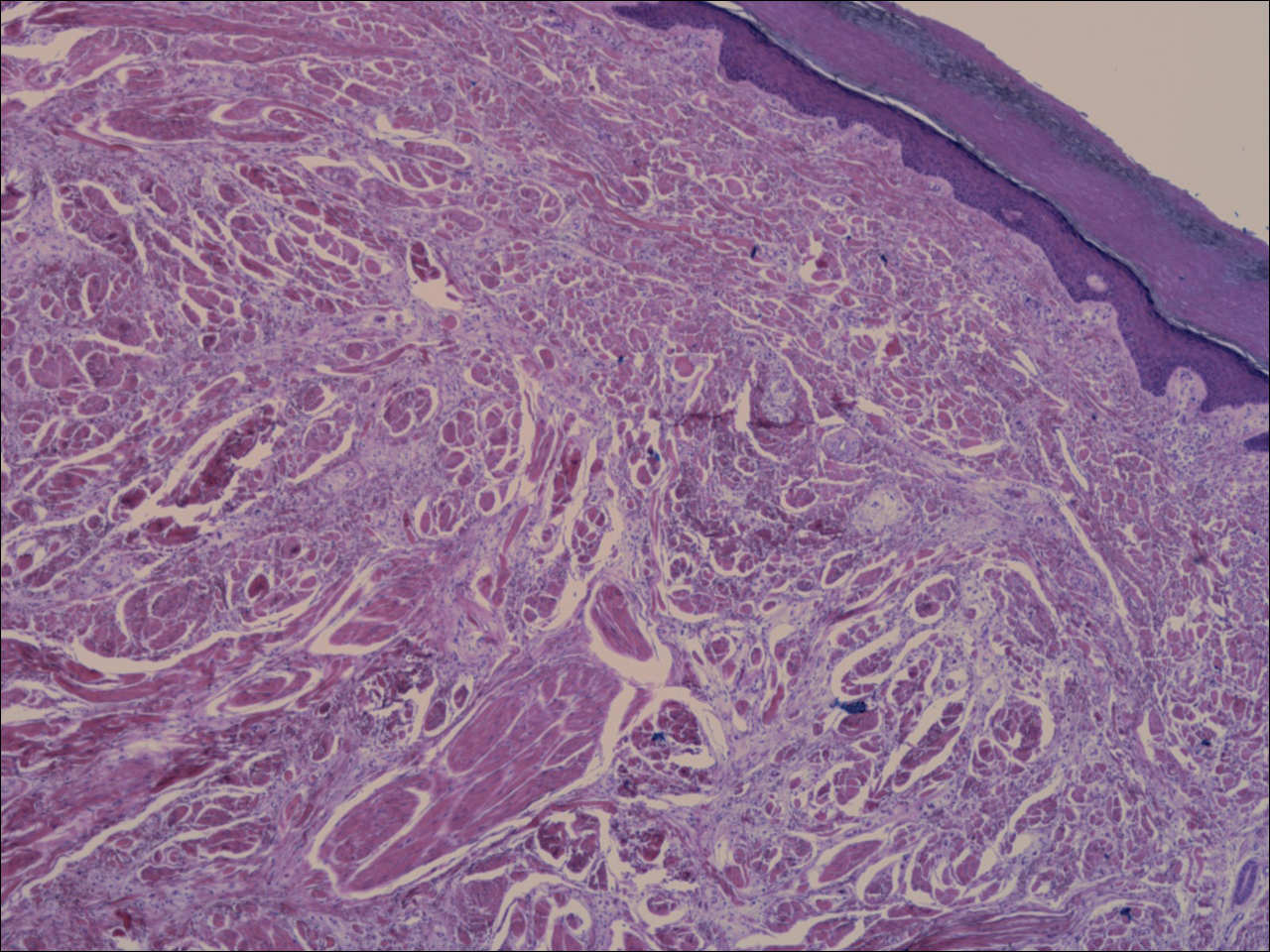
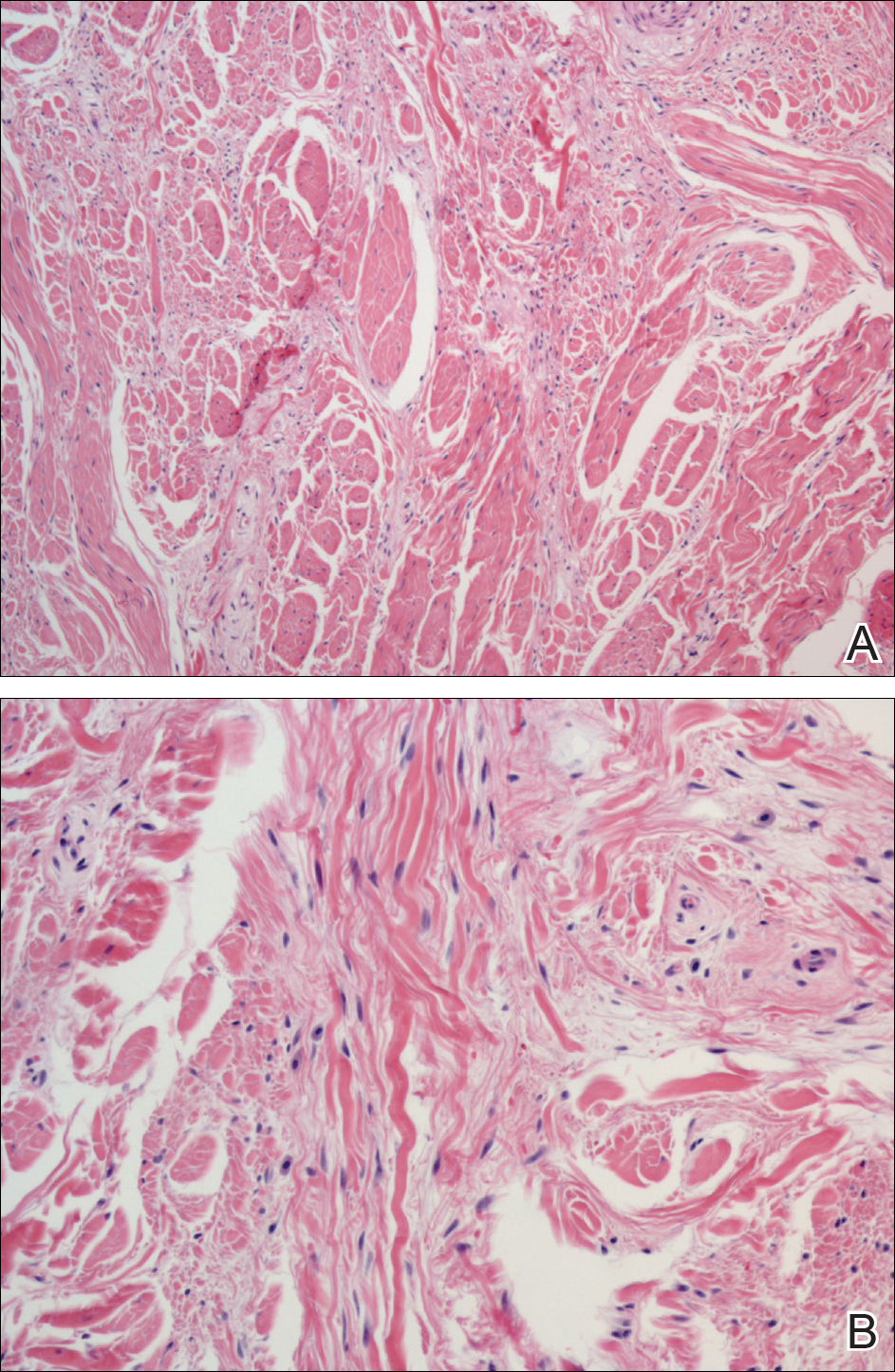
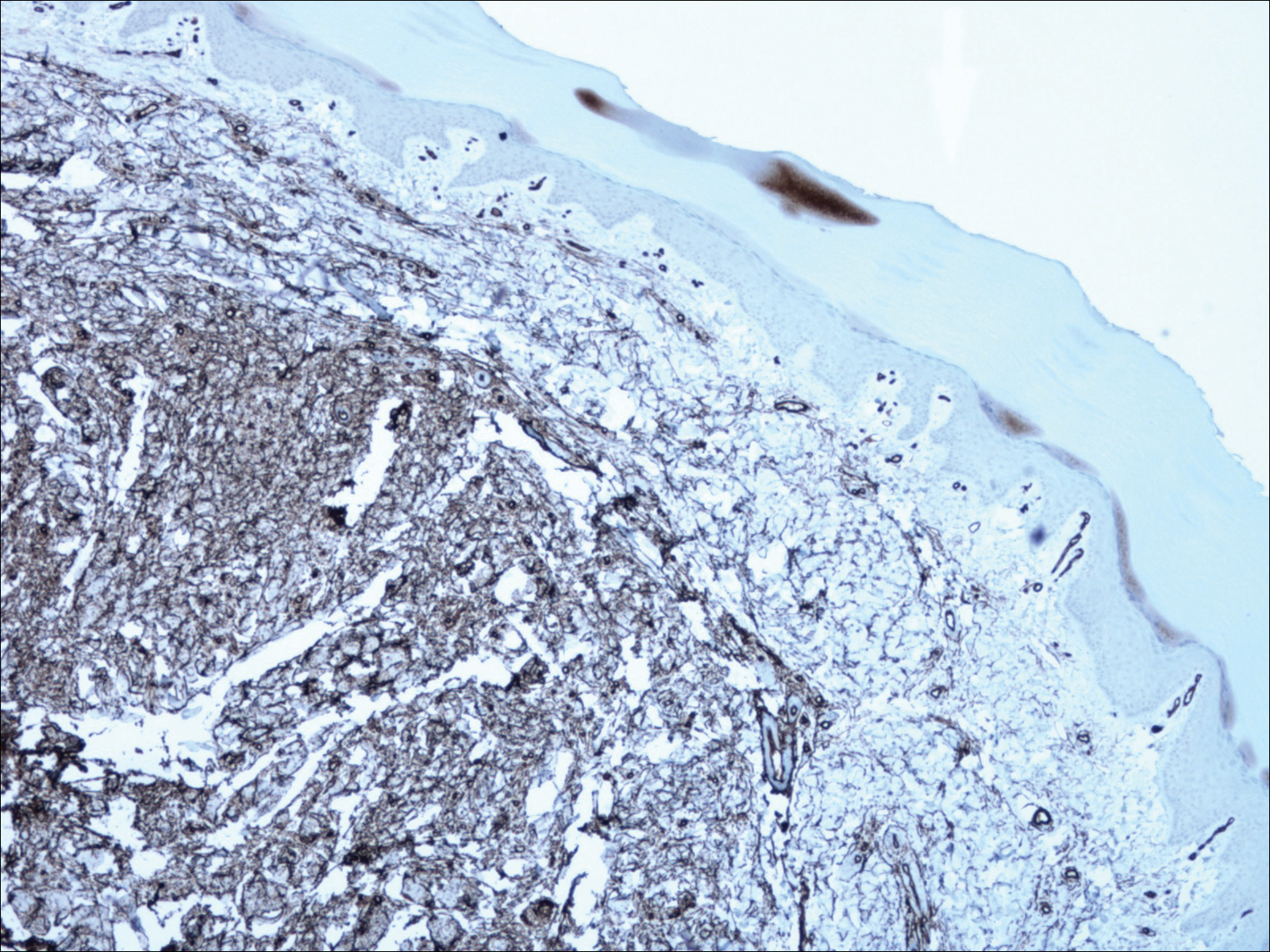
Superficial acral fibromyxomas, also known as digital fibromyxomas, are soft, slow-growing tumors that have a predilection for subungual or periungual regions of the hands and feet. Superficial acral fibromyxomas most frequently occur on the hallux and rarely occur on the ankle or leg. They can present as nodular, dome-shaped, polyploid, or verrucous masses. They can be soft to firm, gelatinous or solid, off-white to gray-white and can have fasciculate cut surfaces. Superficial acral fibromyxomas can be either painful or painless and present with a deformed nail in 9% of cases. Superficial acral fibromyxoma is a superficial lesion with frequent infiltration of the dermal collagen and subcutaneous tissue and may even erode or infiltrate into the underlying bone in rare cases.1-4 Although SAFMs are rare tumors, documented cases of SAFM have been reported at an increasing rate since the first published report by Fetsch et al2 in 2001.
Patients often delay seeking medical treatment and present with a solitary mass that has been slowly growing for months to years. In a study of 124 patients, Hollmann et al1 found that symptoms exist for a mean of 35 months and present with a small mass with a mean tumor size of 1.7 cm before biopsy or excision. Although the age range is broad, SAFM mostly affects middle-aged adults (median age, 49 years).1 Hollmann et al1 also reported a male predominance (1.3:1 ratio), and preexisting local trauma is reported in 25% of cases.2-4
The differential for SAFM should include dermatofibroma, keloid, dermatofibrosarcoma protuberans, acquired digital fibrokeratoma, infantile digital fibromatosis, neurolemmoma, sclerosing perineurioma, superficial angiomyxoma, low-grade fibromyxoid sarcoma, and acral myxoinflammatory fibroblastic sarcoma.1-4
Superficial acral fibromyxomas are composed of CD34+ spindle or stellate-shaped cells that are embedded in a myxoid and/or dense hyalinized collagenous stroma in a random or loosely fascicular growth pattern. The spindle or stellate-shaped cells in SAFMs also have been found to be focally positive for epithelial membrane antigen and CD99. Lesions have accentuated microvasculature and increased mast cells.5-8
Conservative management is reasonable, but patients presenting with persistent pain and/or local deformity should be definitively treated with complete excision and follow-up. Hollmann et al1 found that 24% of tumors recurred locally upon incomplete excision after a mean interval of 27 months. All recurrent tumors had positive margins at excision or initial biopsy.1 To date, no reports of tumors metastasizing have been documented.1-4
- Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
- Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
- Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
- Ashby-Richardson H, Rogers GS, Stadecker MJ. Superficial acral fibromyxoma: an overview. Arch Pathol Lab Med. 2011;135:1064-1066.
- Quaba O, Evans A, Al-Nafussi AA, et al. Superficial acral fibromyxoma. Br J Plast Surg. 2005;58:561-564.
- Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx: a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
- Meyerle J, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
- Kazakov DV, Mentzel T, Buro G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
The Diagnosis: Superficial Acral Fibromyxoma
A shave biopsy revealed an uninvolved grenz zone and mildly cellular spindle cell dermal proliferation in a collagenous and myxoid background (Figure 1). Spindle cells were seen in a myxoid background among dense coarse collagen (Figure 2A). Spindle cells also were seen in a myxoid background with mast cells and capillary network (Figure 2B). Histopathologic examination of the biopsy specimen revealed spindle cells that were diffusely positive for CD34 (Figure 3); focally positive for epithelial membrane antigen; and negative for melanocytic markers, smooth muscle markers, and cytokeratin. A diagnosis of superficial acral fibromyxoma (SAFM) was made based on clinical, histopathologic, and immunohistochemical findings.



Superficial acral fibromyxomas, also known as digital fibromyxomas, are soft, slow-growing tumors that have a predilection for subungual or periungual regions of the hands and feet. Superficial acral fibromyxomas most frequently occur on the hallux and rarely occur on the ankle or leg. They can present as nodular, dome-shaped, polyploid, or verrucous masses. They can be soft to firm, gelatinous or solid, off-white to gray-white and can have fasciculate cut surfaces. Superficial acral fibromyxomas can be either painful or painless and present with a deformed nail in 9% of cases. Superficial acral fibromyxoma is a superficial lesion with frequent infiltration of the dermal collagen and subcutaneous tissue and may even erode or infiltrate into the underlying bone in rare cases.1-4 Although SAFMs are rare tumors, documented cases of SAFM have been reported at an increasing rate since the first published report by Fetsch et al2 in 2001.
Patients often delay seeking medical treatment and present with a solitary mass that has been slowly growing for months to years. In a study of 124 patients, Hollmann et al1 found that symptoms exist for a mean of 35 months and present with a small mass with a mean tumor size of 1.7 cm before biopsy or excision. Although the age range is broad, SAFM mostly affects middle-aged adults (median age, 49 years).1 Hollmann et al1 also reported a male predominance (1.3:1 ratio), and preexisting local trauma is reported in 25% of cases.2-4
The differential for SAFM should include dermatofibroma, keloid, dermatofibrosarcoma protuberans, acquired digital fibrokeratoma, infantile digital fibromatosis, neurolemmoma, sclerosing perineurioma, superficial angiomyxoma, low-grade fibromyxoid sarcoma, and acral myxoinflammatory fibroblastic sarcoma.1-4
Superficial acral fibromyxomas are composed of CD34+ spindle or stellate-shaped cells that are embedded in a myxoid and/or dense hyalinized collagenous stroma in a random or loosely fascicular growth pattern. The spindle or stellate-shaped cells in SAFMs also have been found to be focally positive for epithelial membrane antigen and CD99. Lesions have accentuated microvasculature and increased mast cells.5-8
Conservative management is reasonable, but patients presenting with persistent pain and/or local deformity should be definitively treated with complete excision and follow-up. Hollmann et al1 found that 24% of tumors recurred locally upon incomplete excision after a mean interval of 27 months. All recurrent tumors had positive margins at excision or initial biopsy.1 To date, no reports of tumors metastasizing have been documented.1-4
The Diagnosis: Superficial Acral Fibromyxoma
A shave biopsy revealed an uninvolved grenz zone and mildly cellular spindle cell dermal proliferation in a collagenous and myxoid background (Figure 1). Spindle cells were seen in a myxoid background among dense coarse collagen (Figure 2A). Spindle cells also were seen in a myxoid background with mast cells and capillary network (Figure 2B). Histopathologic examination of the biopsy specimen revealed spindle cells that were diffusely positive for CD34 (Figure 3); focally positive for epithelial membrane antigen; and negative for melanocytic markers, smooth muscle markers, and cytokeratin. A diagnosis of superficial acral fibromyxoma (SAFM) was made based on clinical, histopathologic, and immunohistochemical findings.



Superficial acral fibromyxomas, also known as digital fibromyxomas, are soft, slow-growing tumors that have a predilection for subungual or periungual regions of the hands and feet. Superficial acral fibromyxomas most frequently occur on the hallux and rarely occur on the ankle or leg. They can present as nodular, dome-shaped, polyploid, or verrucous masses. They can be soft to firm, gelatinous or solid, off-white to gray-white and can have fasciculate cut surfaces. Superficial acral fibromyxomas can be either painful or painless and present with a deformed nail in 9% of cases. Superficial acral fibromyxoma is a superficial lesion with frequent infiltration of the dermal collagen and subcutaneous tissue and may even erode or infiltrate into the underlying bone in rare cases.1-4 Although SAFMs are rare tumors, documented cases of SAFM have been reported at an increasing rate since the first published report by Fetsch et al2 in 2001.
Patients often delay seeking medical treatment and present with a solitary mass that has been slowly growing for months to years. In a study of 124 patients, Hollmann et al1 found that symptoms exist for a mean of 35 months and present with a small mass with a mean tumor size of 1.7 cm before biopsy or excision. Although the age range is broad, SAFM mostly affects middle-aged adults (median age, 49 years).1 Hollmann et al1 also reported a male predominance (1.3:1 ratio), and preexisting local trauma is reported in 25% of cases.2-4
The differential for SAFM should include dermatofibroma, keloid, dermatofibrosarcoma protuberans, acquired digital fibrokeratoma, infantile digital fibromatosis, neurolemmoma, sclerosing perineurioma, superficial angiomyxoma, low-grade fibromyxoid sarcoma, and acral myxoinflammatory fibroblastic sarcoma.1-4
Superficial acral fibromyxomas are composed of CD34+ spindle or stellate-shaped cells that are embedded in a myxoid and/or dense hyalinized collagenous stroma in a random or loosely fascicular growth pattern. The spindle or stellate-shaped cells in SAFMs also have been found to be focally positive for epithelial membrane antigen and CD99. Lesions have accentuated microvasculature and increased mast cells.5-8
Conservative management is reasonable, but patients presenting with persistent pain and/or local deformity should be definitively treated with complete excision and follow-up. Hollmann et al1 found that 24% of tumors recurred locally upon incomplete excision after a mean interval of 27 months. All recurrent tumors had positive margins at excision or initial biopsy.1 To date, no reports of tumors metastasizing have been documented.1-4
- Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
- Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
- Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
- Ashby-Richardson H, Rogers GS, Stadecker MJ. Superficial acral fibromyxoma: an overview. Arch Pathol Lab Med. 2011;135:1064-1066.
- Quaba O, Evans A, Al-Nafussi AA, et al. Superficial acral fibromyxoma. Br J Plast Surg. 2005;58:561-564.
- Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx: a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
- Meyerle J, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
- Kazakov DV, Mentzel T, Buro G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
- Hollmann TJ, Bovée JV, Fletcher CD. Digital fibromyxoma (superficial acral fibromyxoma): a detailed characterization of 124 cases. Am J Surg Pathol. 2012;36:789-798.
- Fetsch JF, Laskin WB, Miettinen M. Superficial acral fibromyxoma: a clinicopathologic and immunohistochemical analysis of 37 cases of a distinctive soft tissue tumor with a predilection for the fingers and toes. Hum Pathol. 2001;32:704-714.
- Al-Daraji WI, Miettinen M. Superficial acral fibromyxoma: a clinicopathological analysis of 32 tumors including 4 in the heel. J Cutan Pathol. 2008;35:1020-1026.
- Ashby-Richardson H, Rogers GS, Stadecker MJ. Superficial acral fibromyxoma: an overview. Arch Pathol Lab Med. 2011;135:1064-1066.
- Quaba O, Evans A, Al-Nafussi AA, et al. Superficial acral fibromyxoma. Br J Plast Surg. 2005;58:561-564.
- Oteo-Alvaro A, Meizoso T, Scarpellini A, et al. Superficial acral fibromyxoma of the toe, with erosion of the distal phalanx: a clinical report. Arch Orthop Trauma Surg. 2008;128:271-274.
- Meyerle J, Keller RA, Krivda SJ. Superficial acral fibromyxoma of the index finger. J Am Acad Dermatol. 2004;50:134-136.
- Kazakov DV, Mentzel T, Buro G, et al. Superficial acral fibromyxoma: report of two cases. Dermatology. 2002;205:285-288.
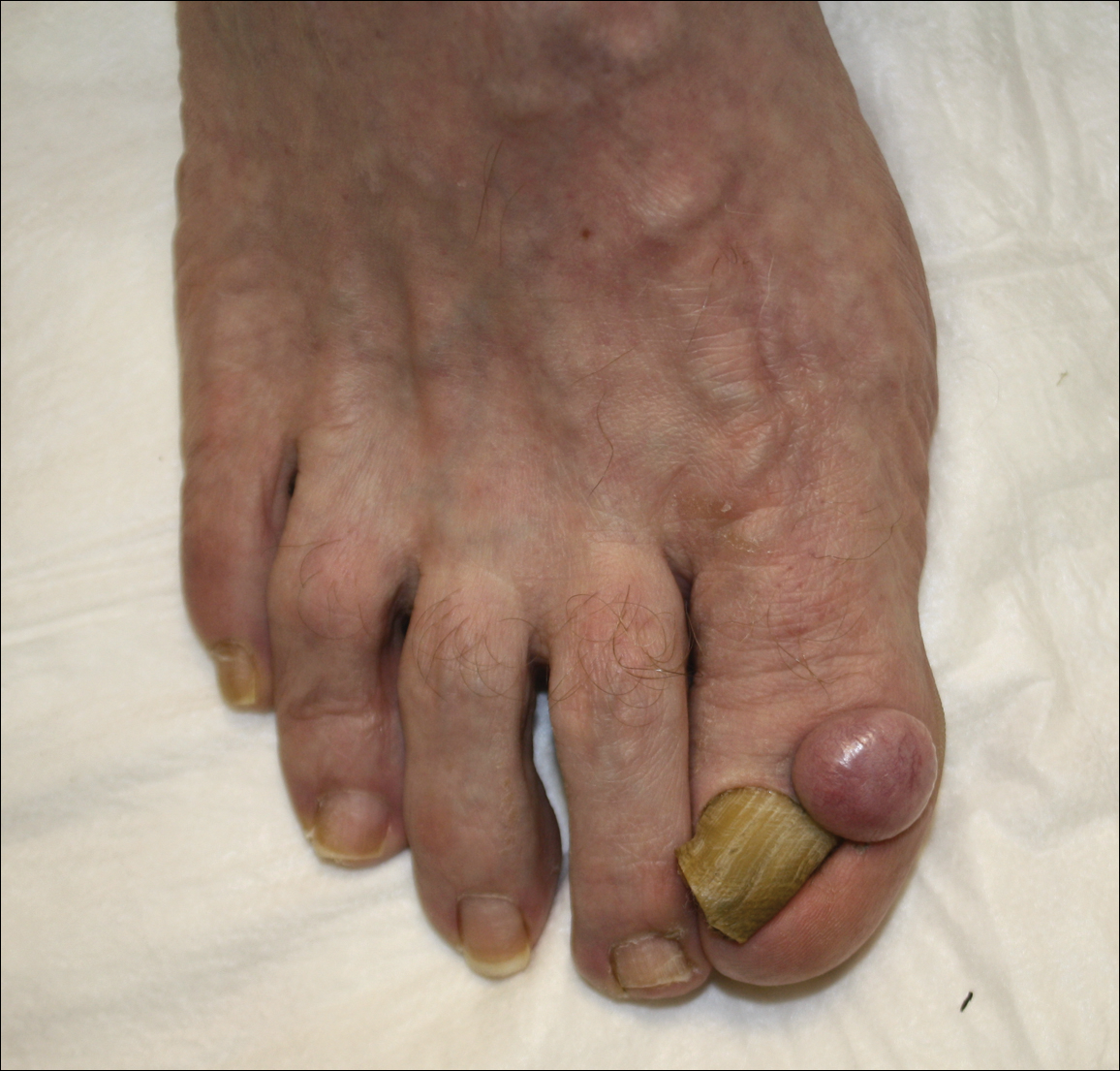
A 62-year-old man presented for evaluation of a slowly growing, nonpainful nodule on the first proximal toenail fold of the right foot of 6 years' duration. He reported that the nail plate of the affected toe was thickened and malaligned. He denied a history of trauma. Physical examination revealed a 2.0×1.6-cm, flesh-colored, nontender, well-defined, rubbery nodule with prominent overlying tortuous telangiectases on the medial aspect of the first proximal toenail fold of the right foot. The associated nail plate was yellow, thickened, and angled laterally into the second toe. Radiograph of the right hallux identified a soft tissue density contiguous with the dorsal aspect of the distal portion of the phalanx. There was no evidence of bony involvement. A shave saucerization biopsy specimen was obtained and sent for hematoxylin and eosin and immunohistochemical staining. The spindle cells were diffusely positive for CD34.
Physician Burnout in Dermatology
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
Many articles about physician burnout and more alarmingly depression and suicide include chilling statistics; however, the data are limited. The same study from Medscape about burnout broken down by medical specialty often is cited.1 Although dermatology fares better than many specialties in this research, the percentages are still abysmal.
I am writing as a physician, for physicians. I do not want to quote the data to you. If you are reading this article, you have probably felt some burnout, even transiently. Maybe you even feel it now, at this very moment. Physicians are competitive capable people. I do not want to present numbers and statistics that make you question the validity of your feelings, whether you fit with the average statistics, or make you try to calculate how many of your friends or colleagues match these statistics. The numbers are terrible, no matter how you look at them, and all trends show them worsening with time.
What is burnout?
To simply define burnout as fatigue or high workload would be to undervalue the term. Physicians are trained through college, medical school, and countless hours of residency to cope with both challenges. Maslach et al2 defined burnout as “a psychological syndrome in response to chronic interpersonal stressors on the job” leading to “overwhelming exhaustion, feelings of cynicism and detachment from the job, and a sense of ineffectiveness and lack of accomplishment.”
Who does burnout affect?
Physician burnout affects both the patient and the physician. It has been demonstrated that physician burnout leads to lower patient satisfaction and care as well as higher risk for medical errors. There are the more obvious and direct effects on the physician, with affected physicians having much higher employment turnover and risk for addiction and suicide.3 One could argue that there are even more downstream effects of burnout, including physicians’ families who may be directly affected and even societal effects when fully trained physicians leave the clinical arena to pursue other careers.
How do you recognize when you are burnt out?
The first time I recognized that I was burnt out was in medical school. I understood my burnout through the lens of my undergraduate training in anthropology as compassion fatigue, a term that has been used to describe the lack of empathy that can develop when any individual is presented with an overwhelming tragedy or horror. When you are in survival mode—waking up just to survive the next day or clinic shift or call—you are surviving but hardly thriving as a physician.3 I believe that humans have a tremendous capacity for survival, but when we are in survival mode we have little energy leftover for the pleasures of life, from family to hobbies. I would similarly argue that in survival mode we have limited ability to appreciate the pain and suffering our patients are experiencing. Survival mode limits our ability as physicians to connect with our patients and to engage in the full spectrum of emotion in our time outside of our job.
What are the causes of burnout in dermatology?
As dermatologists, we often have milder on-call schedules and fewer critically ill patients than many of our medical colleagues. For this reason, we may be afraid to address the real role of physician burnout in our field. Fellow dermatologist Jeffrey Benabio, MD (San Diego, California), notes that the phrase dermatologist burnout may even seem oxymoronic, but we face many of the same daily frustrations with electronic medical records, increasing patient volume, and insurance struggles.4 The electronic medical record looms large in many physicians’ complaints these days. A recent article in the New York Times described the physician as “the highest-paid clerical worker in the hospital,”5 which is not wrong. For every hour of patient time, we have nearly double that spent on paperwork.5
Dike Drummond, MD, a family practice physician who focuses on physician burnout, notes that physicians are taught very early to put the patient first, but it is never discussed when or how to turn this switch off.3 However, there is little written about dermatology-specific burnout. A problem that is not studied or even considered will be harder to fix.
Why does it matter?
I believe that addressing physician burnout is critical for 2 reasons: (1) we can improve patient care by improving patient satisfaction and decreasing medical error, and (2) we can find greater satisfaction and professional fulfillment while caring for our patients. Ultimately, patient care and physician care are intimately linked; as stated by Thomas et al,6 “[p]hysicians who are well can best serve their patients.”
As a resident in 2018, I recognize that my coresidents and I are training as physicians in the time of “duty hours” and an ongoing discussion of burnout. However, I sense a burnout fatigue setting in among residents, many who do not want to discuss wellness anymore. The newer data suggest that work hour restrictions do not improve patient safety, negating one of the driving reasons for the change.7 At the same time, residency programs are initiating wellness programs in response to the growing literature on physician burnout. These wellness programs vary in the types of activities included, from individual coping techniques such as mindfulness training to social gatherings for the residents. In general, these wellness initiatives focus on burnout at the individual level, but they do not take into account systemic or structural challenges that might contribute to this worsening epidemic.
Final Thoughts
As a profession, I believe that physicians have internalized the concept of burnout to equate with a personal individual failing. At various times in my training, I have felt that if I could just practice mediation, study more, or shift my perspective, I personally could overcome burnout. I have intermittently felt my burnout as proof that I should never have become a physician. As a woman and the first physician in my family, fighting the sense of burnout so early in my career seemed demoralizing and nearly drove me to change my career path. It exacerbated my sense of imposter syndrome: that I never truly belonged in medicine at all. After much soul-searching, I have concluded that burnout is a concept propagated by administrators and businesspeople to stigmatize the reaction by many physicians to the growing trends in medicine and cast it as a personal failure rather than as the symptom of a broken medical system.
If we continue to identify burnout as an individual failing and treat it as such, I believe that we will fail to stem the growing trend within dermatology and within medicine more broadly. We need to consider the driving factors behind dermatology burnout so that we can begin to address them at a structural level.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.
- Peckham C. Medscape national physician burnout & depression report 2018. Medscape website. https://www.medscape.com/slideshow/2018-lifestyle-burnout-depression-6009235. Published January 17, 2018. Accessed June 21, 2018.
- Maslach C, Schaufeli WB, Leiter MP. Job
burnout. Annu Rev Psychol. 2001;52:397-422. - Drummond D. Physician burnout: its origin, symptoms, and five main causes. Fam Pract Manag. 2015;22:42-47.
- Benabio J. Burnout. Dermatology News. November 14, 2017. https://www.mdedge.com/edermatologynews/article/152098/business-medicine/burnout. Accessed June 30, 2018.
- Verghese A. How tech can turn doctors into clerical workers. New York Times. May 16, 2018. https://www.nytimes.com/interactive/2018/05/16/magazine/health-issue-what-we-lose-with-data-driven-medicine.html. Accessed July 3, 2018.
- Thomas LR, Ripp JA, West CP. Charter on physician well-being. JAMA. 2018;319:1541-1542.
- Osborne R, Parshuram CS. Delinking resident duty hours from patient safety [published online December 11, 2014]. BMC Med Educ. 2014;14(suppl 1):S2.