User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Reflectance Confocal Microscopy as a Diagnostic Aid in Allergic Contact Dermatitis to Mango Sap
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
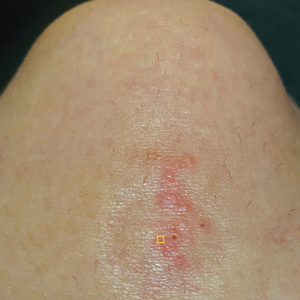
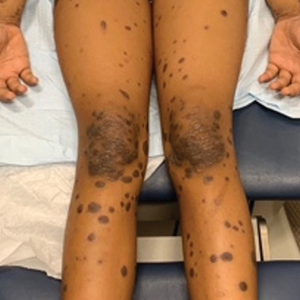
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.
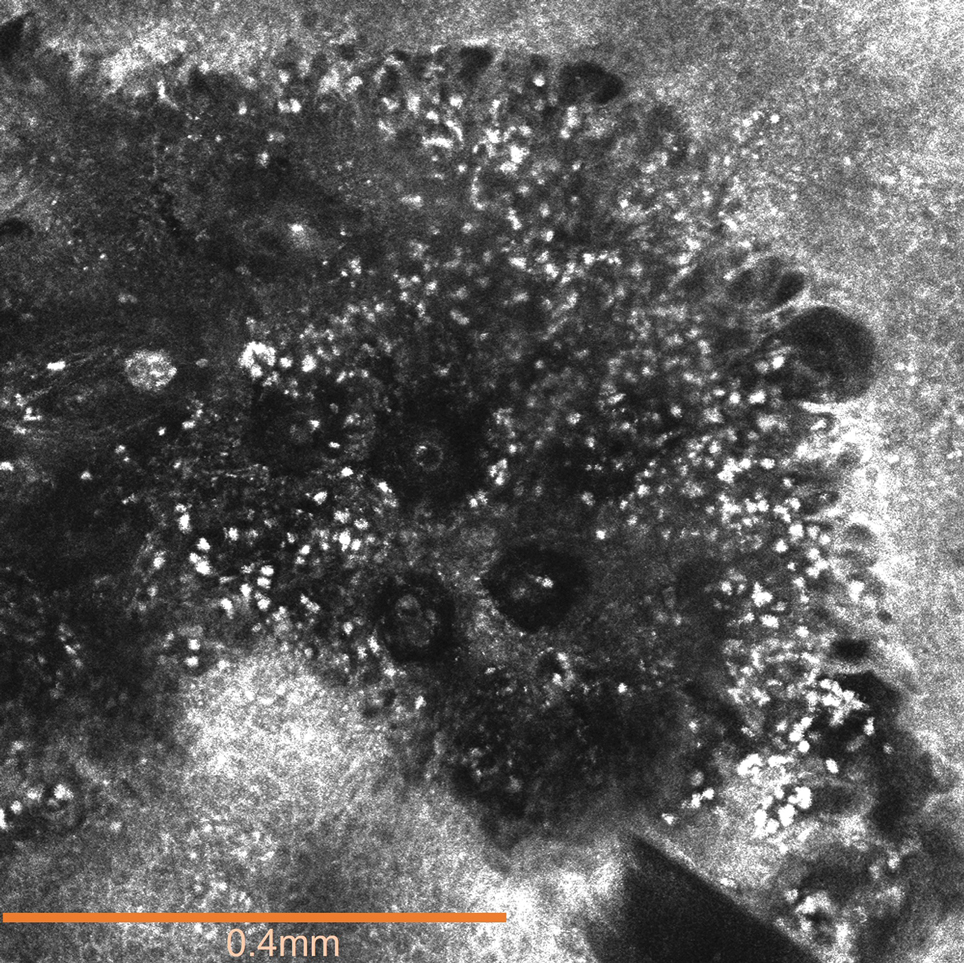
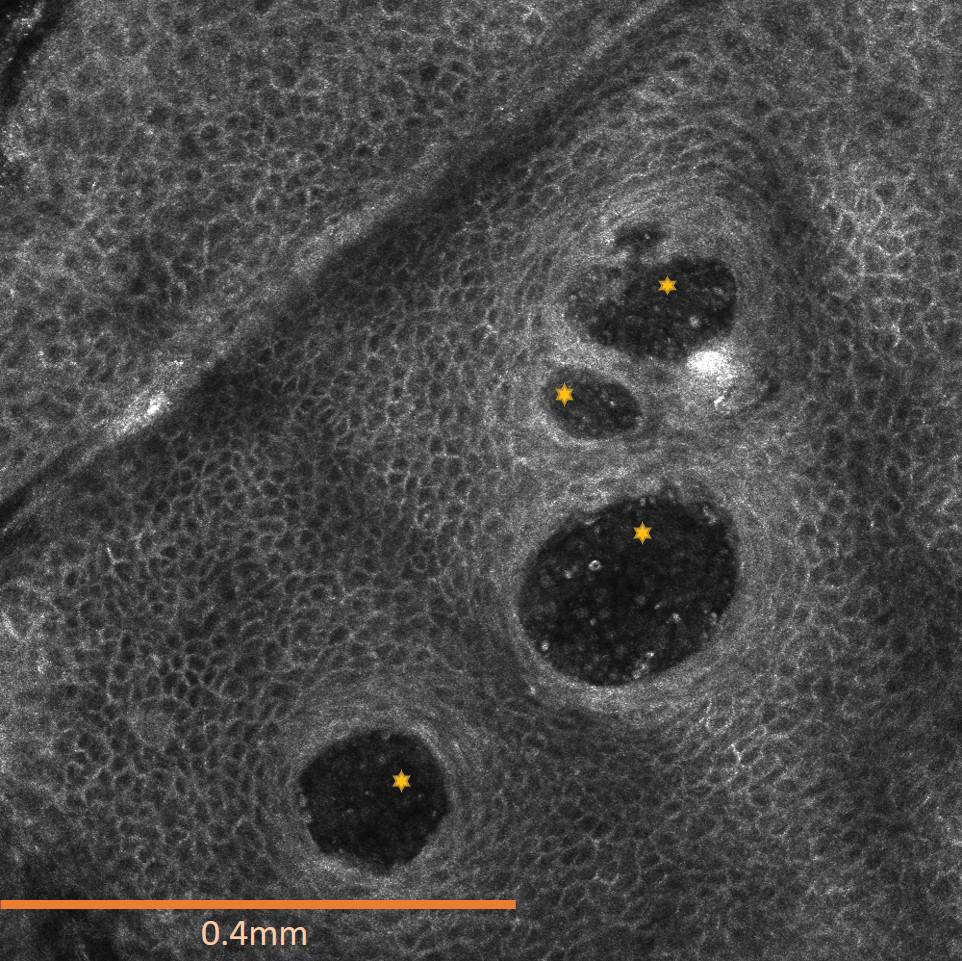
The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.
Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
The mango tree (Mangifera indica) produces nutrient-dense fruit—known colloquially as the “king of fruits”—that is widely consumed across the world. Native to southern Asia, the mango tree is a member of the Anacardiaceae family, a large family of flowering, fruit-bearing plants.1 Many members of the Anacardiaceae family, which includes poison ivy and poison oak, are known to produce urushiol, a skin irritant associated with allergic contact dermatitis (ACD).2 Interestingly, despite its widespread consumption and categorization in the Anacardiaceae family, allergic reactions to mango are comparatively rare; they occur as either immediate type I hypersensitivity reactions manifesting with rapid-onset symptoms such as urticaria, wheezing, and angioedema, or delayed type IV hypersensitivity reactions manifesting as ACD.3 Although exposure to components of the mango tree has been most characteristically linked to type IV hypersensitivity reactions, there remain fewer than 40 reported cases of mango-induced ACD since it was first described in 1939.4
Evaluation of ACD most commonly includes a thorough clinical assessment with diagnostic support from patch testing and histopathologic review following skin biopsy. In recent years, reflectance confocal microscopy (RCM) has shown promising potential to join the repertoire of diagnostic tools for ACD by enabling dynamic and high-resolution imaging of contact dermatitis in vivo.5-10 Reflectance confocal microscopy is a noninvasive optical imaging technique that uses a low-energy diode laser to penetrate the layers of the skin. The resulting reflected light generates images that facilitate visualization of cutaneous structures to the depth of the papillary dermis.11 While it is most commonly used in skin cancer diagnostics, preliminary studies also have shown an emerging role for RCM in the evaluation of eczematous and inflammatory skin disease, including contact dermatitis.5-10 Herein, we present a unique case of mango sap–induced ACD imaged and diagnosed in real time via RCM.
Case Report
A 39-year-old woman presented to our clinic with a pruritic vesicular eruption on the right leg of 2 weeks’ duration that initially had developed within 7 days of exposure to mango tree sap (Figure 1). The patient reported having experienced similar pruritic eruptions in the past following contact with mango sap while eating mangos but denied any history of reactions from ingestion of the fruit. She also reported a history of robust reactions to poison ivy; however, a timeline specifying the order of first exposure to these irritants was unknown. She denied any personal or family history of atopic conditions.

The affected skin was imaged in real time during clinic using RCM, which showed an inflammatory infiltrate represented by dark spongiotic vesicles containing bright cells (Figure 2). Additional RCM imaging at the level of the stratum spinosum showed dark spongiotic areas with bright inflammatory cells infiltrating the vesicles, which were surrounded by normal skin showing a typical epidermal honeycomb pattern (Figure 3). These findings were diagnostic of ACD secondary to exposure to mango sap. The patient was advised to apply clobetasol cream 0.05% to the affected area. Notable improvement of the rash was noted within 10 days of treatment.


Comment
Exposure to the mango tree and its fruit is a rare cause of ACD, with few reported cases in the literature. The majority of known instances have occurred in non–mango-cultivating countries, largely the United States, although cases also have been reported in Canada, Australia, France, Japan, and Thailand.3,12 Mango-induced contact allergy follows a roughly equal distribution between males and females and most often occurs in young adults during the third and fourth decades of life.4,12-21 Importantly, delayed-type hypersensitivity reactions to mango can manifest as either localized or systemic ACD. Localized ACD can be induced via direct contact with the mango tree and its components or ingestion of the fruit.3,12,22 Conversely, systemic ACD is primarily stimulated by ingestion of the fruit. In our case, the patient had no history of allergy following mango ingestion, and her ACD was prompted by isolated contact with mango sap. The time from exposure to symptom onset of known instances of mango ACD varies widely, ranging from less than 24 hours to as long as 9 days.3,12 Diagnosis of mango-induced ACD largely is guided by clinical findings. Presenting symptoms often include an eczematous, vesicular, pruritic rash on affected areas of the skin, frequently the head, neck, and extremities. Patients also commonly present with linear papulovesicular lesions and periorbital or perioral edema.
The suspected allergens responsible for mango-induced ACD are derived from resorcinol—specifically heptadecadienyl resorcinol, heptadecenyl resorcinol, and pentadecyl resorcinol, which are collectively known as mango allergens.23 These allergens can be found within the pulp and skin of the mango fruit as well as in the bark and leaves of the mango tree, which may explain observed allergic reactions to components of both the mango fruit and tree.12 Similar to these resorcinol derivatives, the urushiol resin found in poison ivy and poison oak is a catechol derivative.2 Importantly, both resorcinols and catechols are isomers of the same aromatic phenol—dihydroxybenzene. Because of these similarities, it is thought that the allergens in mangos may cross-react with urushiol in poison ivy or poison oak.23 Alongside their shared categorization in the Anacardiaceae family, it is hypothesized that this cross-reactivity underlies the sensitization that has been noted between mango and poison ivy or poison oak exposure.12,23,24 Thus, ACD often can occur on initial contact with the mango tree or its components, as a prior exposure to poison ivy or poison oak may serve as the inciting factor for hypersensitization. The majority of reported cases in the literature also occurred in countries where exposure to poison ivy and poison oak are common, further supporting the notion that these compounds may provide a sensitizing trigger for a future mango contact allergy.12
A detailed clinical history combined with adjunctive diagnostic support from patch testing and histopathology of biopsied skin lesions classically are used in the diagnosis of mango-induced ACD. Due to its ability to provide quick and noninvasive in vivo imaging of cutaneous lesions, RCM's applications have expanded to include evaluation of inflammatory skin diseases such as contact dermatitis. Many features of contact dermatitis identified via RCM are common between ACD and irritant contact dermatitis (ICD) and include disruption of the stratum corneum, parakeratosis, vesiculation, spongiosis, and exocytosis.6,10,25 Studies also have described features shown via RCM that are unique to ACD, including vasodilation and intercellular edema, compared to more distinct targetoid keratinocytes and detached corneocytes seen in ICD.6,10,25 Studies by Astner et al5,6 demonstrated a wide range of sensitivity from 52% to 96% and a high specificity of RCM greater than 95% for many of the aforementioned features of contact dermatitis, including disruption of the stratum corneum, parakeratosis, spongiosis, and exocytosis. Additional studies have further strengthened these findings, demonstrating sensitivity and specificity values of 83% and 92% for contact dermatitis under RCM, respectively.26 Importantly, given the similarities and potentially large overlap of features between ACD and ICD identified via RCM as well as findings seen on physical examination and histopathology, an emphasis on clinical correlation is essential when differentiating between these 2 variants of contact dermatitis. Thus, taken in consideration with clinical contexts, RCM has shown potent diagnostic accuracy and great potential to support the evaluation of ACD alongside patch testing and histopathology.
Final Thoughts
Contact allergy to the mango tree and its components is uncommon. We report a unique case of mango sap–induced ACD evaluated and diagnosed via dynamic visualization under RCM. As a noninvasive and reproducible imaging technique with resolutions comparable to histopathologic analysis, RCM is a promising tool that can be used to support the diagnostic evaluation of ACD.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
- Shah KA, Patel MB, Patel RJ, et al. Mangifera indica (mango). Pharmacogn Rev. 2010;4:42-48.
- Lofgran T, Mahabal GD. Toxicodendron toxicity. StatPearls [Internet]. Updated May 16, 2023. Accessed September 19, 2024. https://www.ncbi.nlm.nih.gov/books/NBK557866
- Sareen R, Shah A. Hypersensitivity manifestations to the fruit mango. Asia Pac Allergy. 2011;1:43-49.
- Zakon SJ. Contact dermatitis due to mango. JAMA. 1939;113:1808.
- Astner S, Gonzalez E, Cheung A, et al. Pilot study on the sensitivity and specificity of in vivo reflectance confocal microscopy in the diagnosis of allergic contact dermatitis. J Am Acad Dermatol. 2005;53:986-992.
- Astner S, Gonzalez S, Gonzalez E. Noninvasive evaluation of allergic and irritant contact dermatitis by in vivo reflectance confocal microscopy. Dermatitis. 2006;17:182-191.
- Csuka EA, Ward SC, Ekelem C, et al. Reflectance confocal microscopy, optical coherence tomography, and multiphoton microscopy in inflammatory skin disease diagnosis. Lasers Surg Med. 2021;53:776-797.
- Guichard A, Fanian F, Girardin P, et al. Allergic patch test and contact dermatitis by in vivo reflectance confocal microscopy [in French]. Ann Dermatol Venereol. 2014;141:805-807.
- Sakanashi EN, Matsumura M, Kikuchi K, et al. A comparative study of allergic contact dermatitis by patch test versus reflectance confocal laser microscopy, with nickel and cobalt. Eur J Dermatol. 2010;20:705-711.
- Swindells K, Burnett N, Rius-Diaz F, et al. Reflectance confocal microscopy may differentiate acute allergic and irritant contact dermatitis in vivo. J Am Acad Dermatol. 2004;50:220-228.
- Shahriari N, Grant-Kels JM, Rabinovitz H, et al. Reflectance confocal microscopy: principles, basic terminology, clinical indications, limitations, and practical considerations. J Am Acad Dermatol. 2021;84:1-14.
- Berghea EC, Craiu M, Ali S, et al. Contact allergy induced by mango (Mangifera indica): a relevant topic? Medicina (Kaunas). 2021;57:1240.
- O’Hern K, Zhang F, Zug KA, et al. “Mango slice” dermatitis: pediatric allergic contact dermatitis to mango pulp and skin. Dermatitis. 2022;33:E46-E47.
- Raison-Peyron N, Aljaber F, Al Ali OA, et al. Mango dermatitis: an unusual cause of eyelid dermatitis in France. Contact Dermatitis. 2021;85:599-600.
- Alipour Tehrany Y, Coulombe J. Mango allergic contact dermatitis. Contact Dermatitis. 2021;85:241-242.
- Yoo MJ, Carius BM. Mango dermatitis after urushiol sensitization. Clin Pract Cases Emerg Med. 2019;3:361-363.
- Miyazawa H, Nishie W, Hata H, et al. A severe case of mango dermatitis. J Eur Acad Dermatol Venereol. 2018;32:E160-E161.
- Trehan I, Meuli GJ. Mango contact allergy. J Travel Med. 2010;17:284.
- Wiwanitkit V. Mango dermatitis. Indian J Dermatol. 2008;53:158.
- Weinstein S, Bassiri-Tehrani S, Cohen DE. Allergic contact dermatitis to mango flesh. Int J Dermatol. 2004;43:195-196.
- Calvert ML, Robertson I, Samaratunga H. Mango dermatitis: allergic contact dermatitis to Mangifera indica. Australas J Dermatol. 1996;37:59-60.
- Thoo CH, Freeman S. Hypersensitivity reaction to the ingestion of mango flesh. Australas J Dermatol. 2008;49:116-119.
- Oka K, Saito F, Yasuhara T, et al. A study of cross-reactions between mango contact allergens and urushiol. Contact Dermatitis. 2004;51:292-296.
- Keil H, Wasserman D, Dawson CR. Mango dermatitis and its relationship to poison ivy hypersensitivity. Ann Allergy. 1946;4: 268-281.
- Maarouf M, Costello CM, Gonzalez S, et al. In vivo reflectance confocal microscopy: emerging role in noninvasive diagnosis and monitoring of eczematous dermatoses. Actas Dermosifiliogr (Engl Ed). 2019;110:626-636.
- Koller S, Gerger A, Ahlgrimm-Siess V, et al. In vivo reflectance confocal microscopy of erythematosquamous skin diseases. Exp Dermatol. 2009;18:536-540.
Practice Points
- Contact with mango tree sap can induce allergic contact dermatitis.
- Reflectance confocal microscopy (RCM) is a noninvasive imaging technique that can provide real-time in vivo visualization of affected skin in contact dermatitis.
- Predominant findings of contact dermatitis under RCM include disruption of the stratum corneum; parakeratosis; vesiculation; spongiosis; and exocytosis, vasodilation, and intercellular edema more specific to the allergic subtype.
Transient Eruption of Verrucous Keratoses During Encorafenib Therapy: Adverse Event or Paraneoplastic Phenomenon?
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
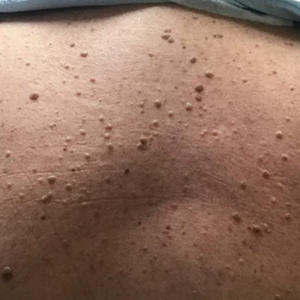
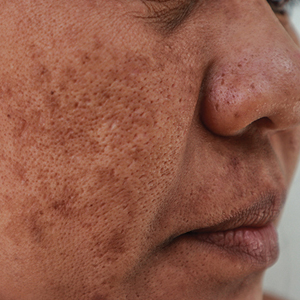
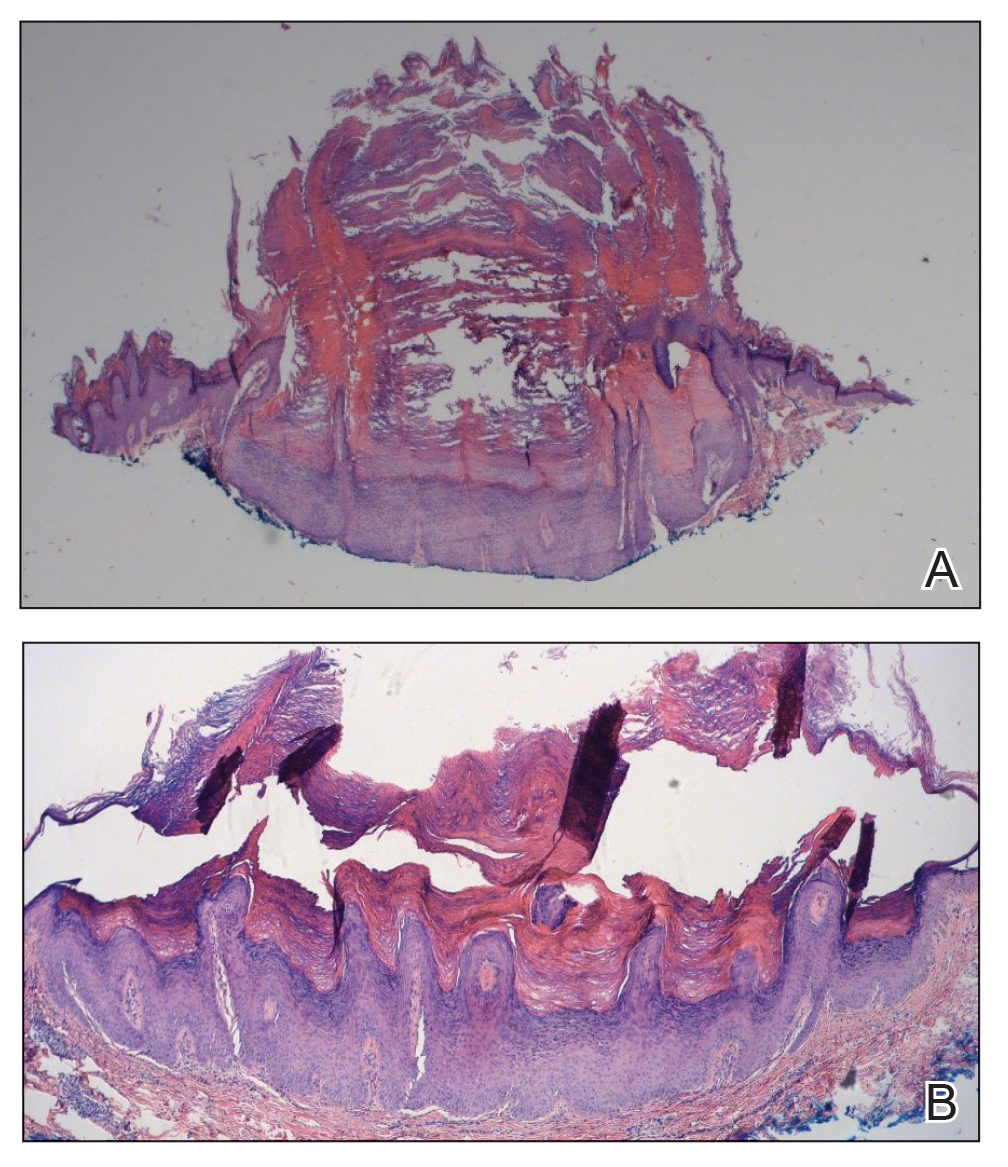
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.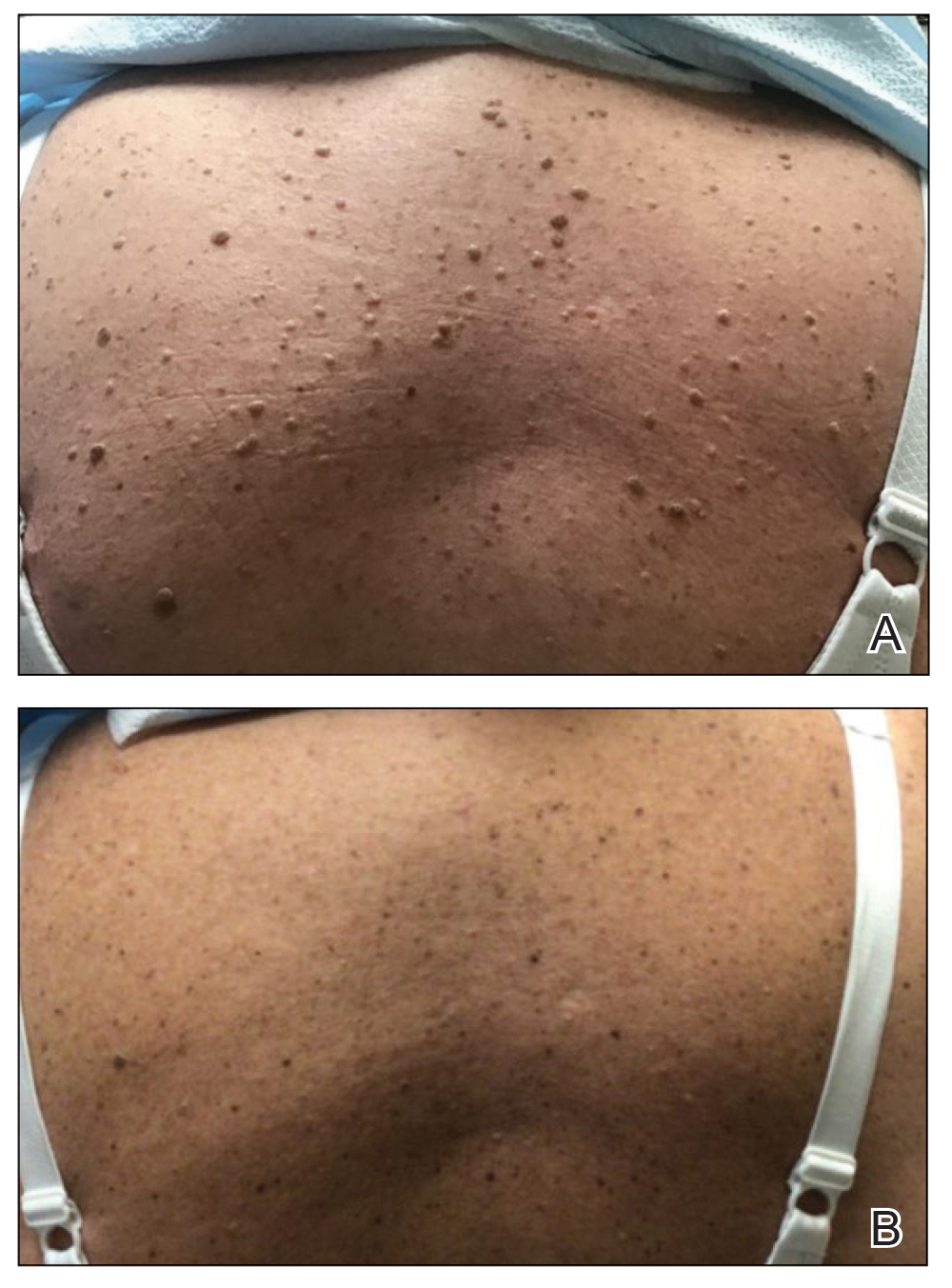
Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.

Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
To the Editor:
Mutations of the BRAF protein kinase gene are implicated in a variety of malignancies.1BRAF mutations in malignancies cause the mitogen-activated protein kinase (MAPK) pathway to become constitutively active, which results in unchecked cellular proliferation,2,3 making the BRAF mutation an attractive target for inhibition with pharmacologic agents to potentially halt cancer growth.4 Vemurafenib—the first selective BRAF inhibitor used in clinical practice—initially was approved by the US Food and Drug Administration in 2011. The approval of dabrafenib followed in 2013 and most recently encorafenib in 2018.5
Although targeted treatment of BRAF-mutated malignancies with BRAF inhibitors has become common, it often is associated with cutaneous adverse events (AEs), such as rash, pruritus, photosensitivity, actinic keratosis, and verrucous keratosis. Some reports demonstrate these events in up to 95% of patients undergoing BRAF inhibitor treatment.6 In several cases the eruption of verrucous keratoses is among the most common cutaneous AEs seen among patients receiving BRAF inhibitor treatment.5-7
In general, lesions can appear days to months after therapy is initiated and may resolve after switching to dual therapy with a MEK inhibitor or with complete cessation of BRAF inhibitor therapy.5,7,8 One case of spontaneous resolution of vemurafenib-associated panniculitis during ongoing BRAF inhibitor therapy has been reported9; however, spontaneous resolution of cutaneous AEs is uncommon. Herein, we describe verrucous keratoses in a patient undergoing treatment with encorafenib that resolved spontaneously despite ongoing BRAF inhibitor therapy.
A 61-year-old woman presented to the emergency department with pain in the right lower quadrant. Computed tomography (CT) of the abdomen and pelvis revealed a large ovarian mass. Subsequent bloodwork revealed elevated carcinoembryonic antigen levels. The patient underwent a hysterectomy, bilateral salpingo-oophorectomy, omentectomy, right hemicolectomy with ileotransverse side-to-side anastomosis, right pelvic lymph node reduction, and complete cytoreduction. Histopathology revealed an adenocarcinoma of the cecum with tumor invasion into the visceral peritoneum and metastases to the left ovary, fallopian tube, and omentum. A BRAF V600E mutation was detected.
Two months after the initial presentation, the patient started her first cycle of chemotherapy with a combination of folinic acid, fluorouracil, and oxaliplatin. She completed 11 cycles of this regimen, then was switched to capecitabine and oxaliplatin for an additional 2 cycles due to insurance concerns. At the end of treatment, there was no evidence of disease on CT, thus the patient was followed with observation. However, she presented 10 months later to the emergency department with abdominal pain, and CT revealed new lesions in the liver that were concerning for potential metastases. She started oral encorafenib 300 mg/d and intravenous cetuximab 500 mg weekly; after 1 week, encorafenib was reduced to 150 mg/d due to nausea and loss of appetite. Within 2 weeks of starting treatment, the patient reported the relatively abrupt appearance of more than 50 small papules across the shoulders and back (Figure 1A). She was referred to dermatology, and shave biopsies of 2 lesions—one from the left anterior thigh, the other from the right posterior shoulder—revealed verrucous keratosis pathology (Figure 2). At this time, encorafenib was increased again to 300 mg/d as the patient had been tolerating the reduced dose. She continued to report the appearance of new lesions for the next 3 months, after which the lesions were stable for approximately 2 months. By 2.5 months after initiation of therapy, the patient had undergone CT demonstrating resolution of the liver lesions. At 5 months of therapy, the patient reported a stable to slightly reduced number of skin lesions but had begun to experience worsening joint pain, and the dosage of encorafenib was reduced to 225 mg/d. At 7 months of therapy, the dosage was further reduced to 150 mg/d due to persistent arthralgia. A follow-up examination at 10 months of therapy showed improvement in the number and size of the verrucous keratoses, and near resolution was seen by 14 months after the initial onset of the lesions (Figure 1B). At 20 months after initial onset, only 1 remaining verrucous keratosis was identified on physical examination and biopsy. The patient had continued a regimen of encorafenib 150 mg/d and weekly intravenous 500 mg cetuximab up to this point. Over the entire time period that the patient was seen, up to 12 lesions located in high-friction areas had become irritated and were treated with cryotherapy, but this contributed only minorly to the patient’s overall presentation.

Verrucous keratosis is a known cutaneous AE of BRAF inhibitor treatment with vemurafenib and dabrafenib, with fewer cases attributed to encorafenib.5,6 Within the oncologic setting, the eruption of verrucous papules as a paraneoplastic phenomenon is heavily debated in the literature and is known as the Leser-Trélat sign. This phenomenon is commonly associated with adenocarcinomas of the gastrointestinal tract, as seen in our patient.10 Based on Curth’s postulates—the criteria used to evaluate the relationship between an internal malignancy and a cutaneous disorder—this was unlikely in our patient. The criteria, which do not all need to be met to suggest a paraneoplastic phenomenon, include concurrent onset of the malignancy and the dermatosis, parallel course, association of a specific dermatosis with a specific malignancy, statistical significance of the association, and the presence of a genetic basis for the association.11 Several features favored a drug-related cutaneous eruption vs a paraneoplastic phenomenon: (1) the malignancy was identified months before the cutaneous eruptions manifested; (2) the cutaneous lesions appeared once treatment had already been initiated; and (3) the cutaneous lesions persisted long after the malignancy was no longer identifiable on CT. Indeed, eruption of the papules temporally coincided closely with the initiation of BRAF inhibitor therapy, arguing for correlation.
As a suspected BRAF inhibitor–associated cutaneous AE, the eruption of verrucous keratoses in our patient is remarkable for its spontaneous resolution despite ongoing therapy. It is speculated that keratinocytic proliferation while on BRAF inhibitor therapy may be caused by a paradoxical increase in signaling through CRAF, another Raf isoform that plays a role in the induction of terminal differentiation of keratinocytes, with a subsequent increase in MAPK signaling.12-14 Self-resolution of this cycle despite continuing BRAF inhibitor therapy suggests the possible involvement of balancing and/or alternative mechanistic pathways that may be related to the immune system. Although verrucous keratoses are considered benign proliferations and do not necessarily require any specific treatment or reduction in BRAF inhibitor dosage, they may be treated with cryotherapy, electrocautery, shave removal, or excision,15 which often is done if the lesions become inflamed and cause pain. Additionally, some patients may feel distress from the appearance of the lesions and desire treatment for this reason. Understanding that verrucous keratoses can be a transient cutaneous AE rather than a persistent one may be useful to clinicians as they manage AEs during BRAF inhibitor therapy.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
- Pakneshan S, Salajegheh A, Smith RA, Lam AK. Clinicopathological relevance of BRAF mutations in human cancer. Pathology. 2013;45:346-356. doi:10.1097/PAT.0b013e328360b61d
- Dhomen N, Marais R. BRAF signaling and targeted therapies in melanoma. Hematol Oncol Clin North Am. 2009;23:529-545. doi:10.1016/j.hoc.2009.04.001
- Long GV, Menzies AM, Nagrial AM, et al. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J Clin Oncol. 2011;29:1239-1246. doi:10.1200/JCO.2010.32.4327
- Ji Z, Flaherty KT, Tsao H. Targeting the RAS pathway in melanoma. Trends Mol Med. 2012;18:27-35. doi:10.1016/j.molmed.2011.08.001
- Gouda MA, Subbiah V. Precision oncology for BRAF-mutant cancers with BRAF and MEK inhibitors: from melanoma to tissue-agnostic therapy. ESMO Open. 2023;8:100788. doi:10.1016/j.esmoop.2023.100788
- Gençler B, Gönül M. Cutaneous side effects of BRAF inhibitors in advanced melanoma: review of the literature. Dermatol Res Pract. 2016;2016:5361569. doi:10.1155/2016/5361569.
- Chu EY, Wanat KA, Miller CJ, et al. Diverse cutaneous side effects associated with BRAF inhibitor therapy: a clinicopathologic study. J Am Acad Dermatol. 2012;67:1265-1272. doi:10.1016/j.jaad.2012.04.008
- Naqash AR, File DM, Ziemer CM, et al. Cutaneous adverse reactions in B-RAF positive metastatic melanoma following sequential treatment with B-RAF/MEK inhibitors and immune checkpoint blockade or vice versa. a single-institutional case-series. J Immunother Cancer. 2019;7:4. doi:10.1186/s40425-018-0475-y
- Maldonado-Seral C, Berros-Fombella JP, Vivanco-Allende B, et al. Vemurafenib-associated neutrophilic panniculitis: an emergent adverse effect of variable severity. Dermatol Online J. 2013;19:16. doi:10.5070/d370x41670
- Mirali S, Mufti A, Lansang RP, et al. Eruptive seborrheic keratoses are associated with a co-occurring malignancy in the majority of reported cases: a systematic review. J Cutan Med Surg. 2022;26:57-62. doi:10.1177/12034754211035124
- Thiers BH, Sahn RE, Callen JP. Cutaneous manifestations of internal malignancy. CA Cancer J Clin. 2009;59:73-98. doi:10.3322/caac.20005
- Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435. doi:10.1038/nature08833
- Heidorn SJ, Milagre C, Whittaker S, et al. Kinase-dead BRAF and oncogenic RAS cooperate to drive tumor progression through CRAF. Cell. 2010;140:209-221. doi:10.1016/j.cell.2009.12.040
- Poulikakos PI, Zhang C, Bollag G, et al. RAF inhibitors transactivate RAF dimers and ERK signaling in cells with wild-type BRAF. Nature. 2010;464:427-430. doi:10.1038/nature08902
- Hayat MA. Brain Metastases from Primary Tumors, Volume 3: Epidemiology, Biology, and Therapy of Melanoma and Other Cancers. Academic Press; 2016.
Practice Points
- Verrucous keratoses are common cutaneous adverse events (AEs) associated with BRAF inhibitor therapy.
- Verrucous papules may be a paraneoplastic phenomenon and can be differentiated from a treatment-related AE based on the timing and progression in relation to tumor burden.
- Although treatment of particularly bothersome lesions with cryotherapy may be warranted, verrucous papules secondary to BRAF inhibitor therapy may resolve spontaneously.
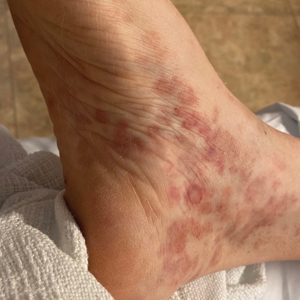
Nonscaly Red-Brown Macules on the Feet and Ankles
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.
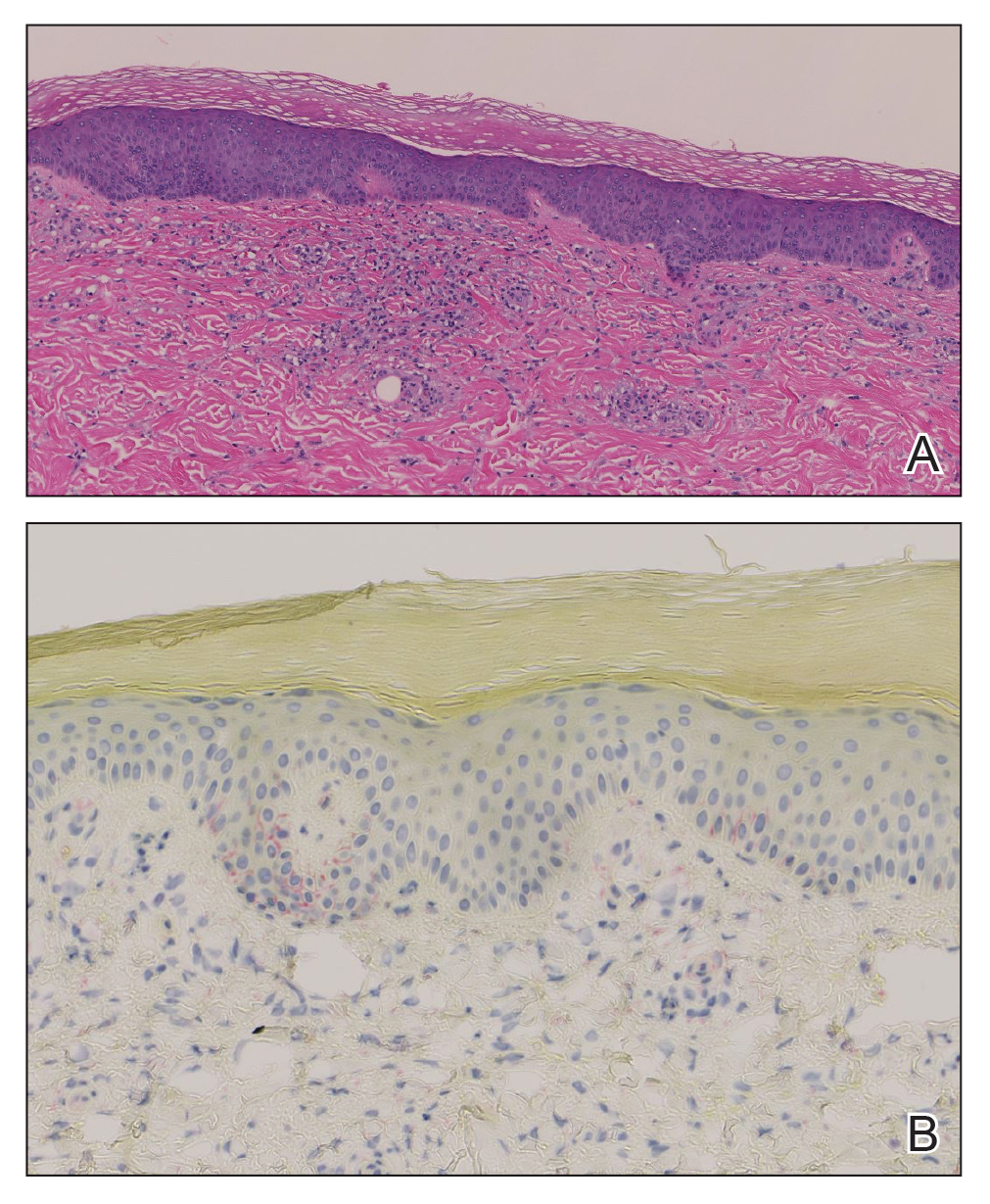
Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
THE DIAGNOSIS: Secondary Syphilis
Histopathology demonstrated a mild superficial perivascular and interstitial infiltrate composed of lymphocytes, histiocytes, and rare plasma cells with a background of extravasated erythrocytes (Figure, A). Treponema pallidum staining highlighted multiple spirochetes along the dermoepidermal junction and in the superficial dermis (Figure, B). Direct immunofluorescence was negative. Laboratory workup revealed a reactive rapid plasma reagin screen with a titer of 1:16 and positive IgG and IgM treponemal antibodies. The patient was diagnosed with secondary syphilis and was treated with a single dose of 2.4 million U of intramuscular benzathine penicillin G, with notable improvement of the rash and arthritis symptoms at 2-week follow-up.

Syphilis is a sexually transmitted infection caused by the spirochete T pallidum that progresses through active and latent stages. The incidence of both the primary and secondary stages of syphilis was at a historic low in the year 2000 and has increased annually since then.1 Syphilis is more common in men, and men who have sex with men (MSM) are disproportionately affected. Although the incidence of syphilis in MSM has increased since 2000, rates have slowed, with slight decreases in this population between 2019 and 2020.1 Conversely, rates among women have increased substantially in recent years, suggesting a more recent epidemic affecting heterosexual men and women.2
Classically, the primary stage of syphilis manifests as an asymptomatic papule followed by a painless ulcer (chancre) that heals spontaneously. The secondary stage of syphilis results from dissemination of T pallidum and is characterized by a wide range of mucocutaneous manifestations and prodromal symptoms. The most common cutaneous manifestation is a diffuse, nonpruritic, papulosquamous rash with red-brown scaly macules or papules on the trunk and extremities.3 The palms and soles commonly are involved. Mucosal patches, “snail-track” ulcers in the mouth, and condylomata lata are the characteristic mucosal lesions of secondary syphilis. Mucocutaneous findings typically are preceded by systemic signs including fever, malaise, myalgia, and generalized lymphadenopathy. However, syphilis is considered “the great mimicker,” with new reports of unusual presentations of the disease. In addition to papulosquamous morphologies, pustular, targetoid, psoriasiform, and noduloulcerative (also known as lues maligna) forms of syphilis have been reported.3-5
The histopathologic features of secondary syphilis also are variable. Classically, secondary syphilis demonstrates vacuolar interface dermatitis and acanthosis with slender elongated rete ridges. Other well-known features include endothelial swelling and the presence of plasma cells in most cases.6 However, the histopathologic features of secondary syphilis may vary depending on the morphology of the skin eruption and when the biopsy is taken. Our patient lacked the classic histopathologic features of secondary syphilis. However, because syphilis was in the clinical differential diagnosis, a treponemal stain was ordered and confirmed the diagnosis. Immunohistochemical stains using antibodies to treponemal antigens have a reported sensitivity of 71% to 100% and are highly specific.7 Although the combination of endothelial swelling, interstitial inflammation, irregular acanthosis, and elongated rete ridges should raise the possibility of syphilis, a treponemal stain may be useful to identify spirochetes if clinical suspicion exists.8
Given our patient’s known history of GPA, leukocytoclastic vasculitis was high on the list of differential diagnoses. However, leukocytoclastic vasculitis most classically manifests as petechiae and palpable purpura, and unlike in secondary syphilis, the palms and soles are less commonly involved. Because our patient’s rash was mainly localized to the lower limbs, the differential also included 2 pigmented purpuric dermatoses (PPDs): progressive pigmentary purpura (Schamberg disease) and purpura annularis telangiectodes (Majocchi disease). Progressive pigmentary purpura is the most common manifestation of PPD and appears as cayenne pepper–colored macules that coalesce into golden brown–pigmented patches on the legs.9 Purpura annularis telangiectodes is another variant of PPD that manifests as pinpoint telangiectatic macules that progress to annular hyperpigmented patches with central clearing. Although PPDs frequently occur on the lower extremities, reports of plantar involvement are rare.10 Annular lichen planus manifests as violaceous papules with a clear center; however, it would be atypical for these lesions to be restricted to the feet and ankles. Palmoplantar lichen planus can mimic secondary syphilis clinically, but these cases manifest as hyperkeratotic pruritic papules on the palms and soles in contrast to the faint brown asymptomatic macules noted in our case.11
Our case highlights an unusual presentation of secondary syphilis and demonstrates the challenge of diagnosing this entity on clinical presentation alone. Because this patient lacked the classic clinical and histopathologic features of secondary syphilis, a skin biopsy with positive immunohistochemical staining for treponemal antigens was necessary to make the diagnosis. Given the variability in presentation of secondary syphilis, a biopsy or serologic testing may be necessary to make a proper diagnosis.
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
- Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2020. Accessed September 4, 2024. https://www.cdc.gov/std/statistics/2020/2020-SR-4-10-2023.pdf
- Ghanem KG, Ram S, Rice PA. The modern epidemic of syphilis. N Engl J Med. 2020;382:845-854. doi:10.1056/NEJMra1901593
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: historical aspects, microbiology, epidemiology, and clinical manifestations. J Am Acad Dermatol. 2020;82:1-14. doi:10.1016/j.jaad.2019.02.073
- Wu MC, Hsu CK, Lee JY, et al. Erythema multiforme-like secondary syphilis in a HIV-positive bisexual man. Acta Derm Venereol. 2010;90:647-648. doi:10.2340/00015555-0920
- Kopelman H, Lin A, Jorizzo JL. A pemphigus-like presentation of secondary syphilis. JAAD Case Rep. 2019;5:861-864. doi:10.1016/j.jdcr.2019.07.021
- Liu XK, Li J. Histologic features of secondary syphilis. Dermatology. 2020;236:145-150. doi:10.1159/000502641
- Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: laboratory diagnosis, management, and prevention. J Am Acad Dermatol. 2020;82:17-28. doi:10.1016/j.jaad.2019.02.074
- Flamm A, Parikh K, Xie Q, et al. Histologic features of secondary syphilis: a multicenter retrospective review. J Am Acad Dermatol. 2015;73:1025-1030. doi:10.1016/j.jaad.2015.08.062
- Kim DH, Seo SH, Ahn HH, et al. Characteristics and clinical manifestations of pigmented purpuric dermatosis. Ann Dermatol. 2015;27:404-410. doi:10.5021/ad.2015.27.4.404
- Sivendran M, Mowad C. Hyperpigmented patches on shins, palms, and soles. JAMA Dermatol. 2013;149:223. doi:10.1001/2013.jamadermatol.652a
- Kim YS, Kim MH, Kim CW, et al. A case of palmoplantar lichen planus mimicking secondary syphilis. Ann Dermatol. 2009;21:429-431.doi:10.5021/ad.2009.21.4.429
A 59-year-old man presented with a nontender nonpruritic rash on the feet of 2 days’ duration. The patient had a several-year history of granulomatosis with polyangiitis (GPA) and was taking methotrexate and prednisone. The rash appeared suddenly—first on the right foot and then on the left foot—and was preceded by 1 week of worsening polyarthralgia, most notably in the ankles. He denied any fever, chills, sore throat, or weight loss. His typical GPA symptoms included inflammatory arthritis, scleritis, leukocytoclastic vasculitis, and sinonasal and renal involvement. He recently experienced exacerbation of inflammatory arthritis that required an increase in the prednisone dosage (from 40 mg to 60 mg daily), but there were no other GPA symptoms. He had a history of multiple female sexual partners but no known history of HIV and no recent testing for sexually transmitted infections. Hepatitis C antibody testing performed 5 years earlier was nonreactive. He denied any illicit drug use, recent travel, sick contacts, or new medications.
Dermatologic examination revealed nonscaly, clustered, red-brown macules, some with central clearing, on the medial and lateral aspects of the feet and ankles with a few faint copper-colored macules on the palms and soles. The ankles had full range of motion with no edema or effusion. There were no oral or genital lesions. The remainder of the skin examination was normal. Punch biopsies of skin on the left foot were obtained for histopathology and direct immunofluorescence.
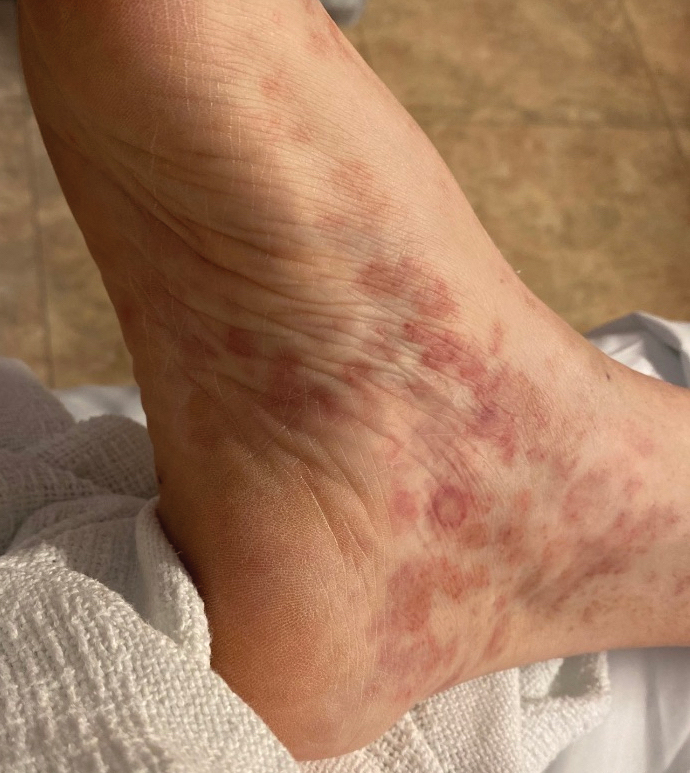
Rare Case of Photodistributed Hyperpigmentation Linked to Kratom Consumption
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.
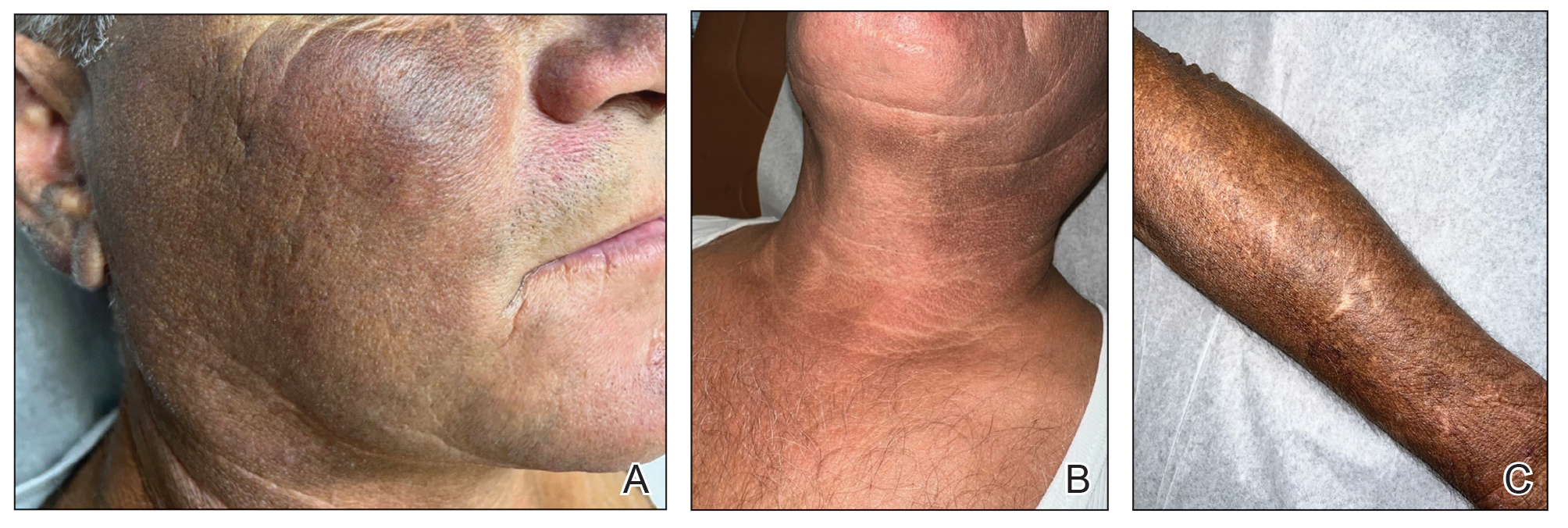
When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).
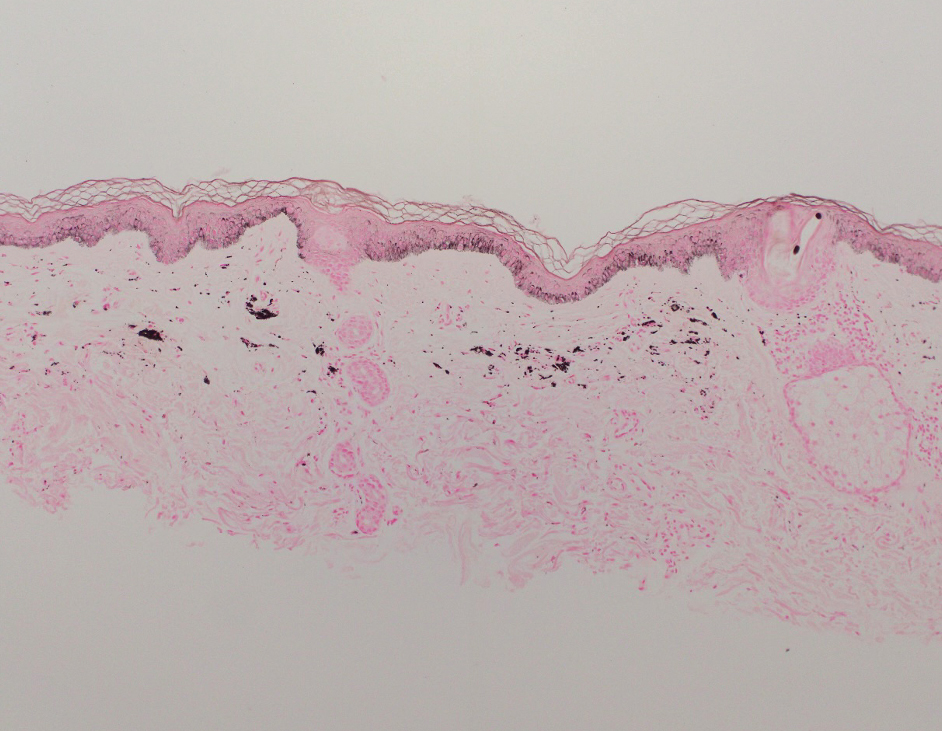
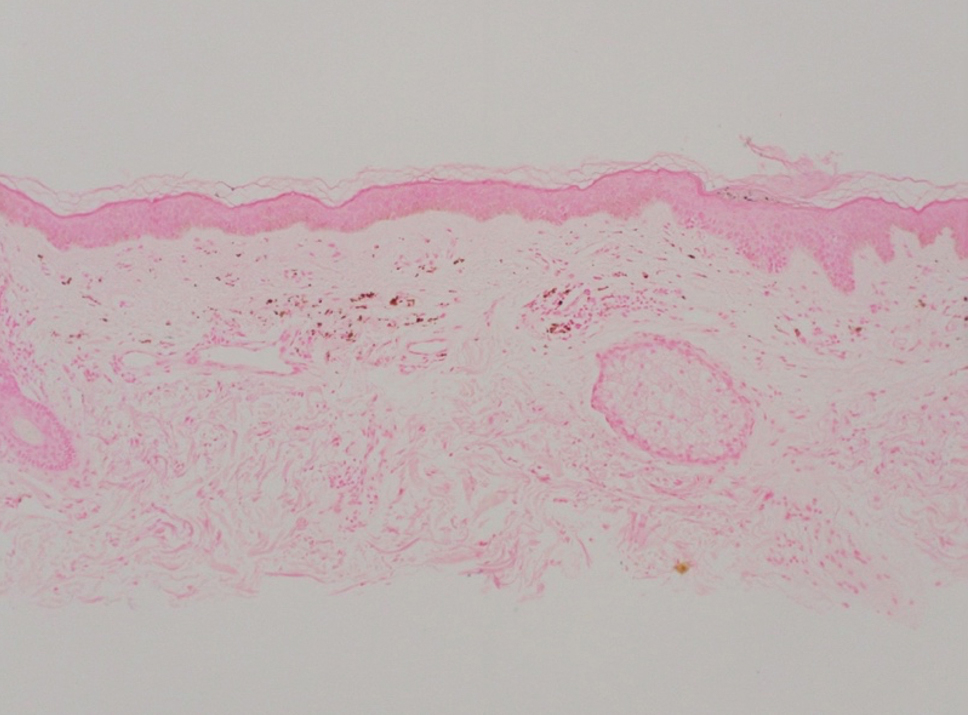
Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
To the Editor:
Kratom (Mitragyna speciosa) is an evergreen tree native to Southeast Asia.1 Its leaves contain psychoactive compounds including mitragynine and 7-hydroxymitragynine, which exert dose-dependent effects on the central nervous system through opioid and monoaminergic receptors.2,3 At low doses (1–5 g), kratom elicits mild stimulant effects such as increased sociability, alertness, and talkativeness. At high doses (5–15 g), kratom has depressant effects that can provide relief from pain and opioid-withdrawal symptoms.3
Traditionally, kratom has been used in Southeast Asia for recreational and ceremonial purposes, to ease opioid-withdrawal symptoms, and to reduce fatigue from manual labor.4 In the 21st century, availability of kratom expanded to Europe, Australia, and the United States, largely facilitated by widespread dissemination of deceitful marketing and unregulated sales on the internet.1 Although large-scale epidemiologic studies evaluating kratom’s prevalence are scarce, available evidence indicates rising worldwide usage, with a notable increase in kratom-related poison center calls between 2011 and 2017 in the United States.5 In July 2023, kratom made headlines due to the death of a woman in Florida following use of the substance.6
A cross-sectional study revealed that in the United States, kratom typically is used by White individuals for self-treatment of anxiety, depression, pain, and opioid withdrawal.7 However, the potential for severe adverse effects and dependence on kratom can outweigh the benefits.6,8 Reported adverse effects of kratom include tachycardia, hypercholesteremia, liver injury, hallucinations, respiratory depression, seizure, coma, and death.9,10 We present a case of kratom-induced photodistributed hyperpigmentation.
A 63-year-old man presented to the dermatology clinic with diffuse tender, pruritic, hyperpigmented skin lesions that developed over the course of 1 year. The lesions were distributed on sun-exposed areas, including the face, neck, and forearms (Figure 1). The patient reported no other major symptoms, and his health was otherwise unremarkable. He had a medical history of psoriasiform and spongiotic dermatitis consistent with eczema, psoriasis, hypercholesteremia, and hyperlipidemia. The patient was not taking any medications at the time of presentation. He had a family history of plaque psoriasis in his father. Five years prior to the current presentation, the patient was treated with adalimumab for steroid-resistant psoriasis; however, despite initial improvement, he experienced recurrence of scaly erythematous plaques and had discontinued adalimumab the year prior to presentation.

When adalimumab was discontinued, the patient sought alternative treatment for the skin symptoms and began self-administering kratom in an attempt to alleviate associated physical discomfort. He ingested approximately 3 bottles of liquid kratom per day, with each bottle containing 180 mg of mitragynine and less than 8 mg of 7-hydroxymitragynine. Although not scientifically proven, kratom has been colloquially advertised to improve psoriasis.11 The patient reported no other medication use or allergies.
Shave biopsies of hyperpigmented lesions on the right side of the neck, ear, and forearm were performed. Histopathology revealed a sparse superficial, perivascular, lymphocytic infiltrate accompanied by a prominent number of melanophages in the superficial dermis (Figure 2). Special stains further confirmed that the pigment was melanin; the specimens stained positive with Fontana-Masson stain (Figure 3) and negative with an iron stain (Figure 4).



Adalimumab-induced hyperpigmentation was considered. A prior case of adalimumab-induced hyperpigmentation manifested on the face. Histopathology was consistent with a superficial, perivascular, lymphocytic infiltrate with melanophages in the dermis; however, hyperpigmentation was absent in the periorbital area, and affected areas faded 4 months after discontinuation of adalimumab.12 Our patient presented with hyperpigmentation 1 year after adalimumab cessation, and the hyperpigmented areas included the periorbital region. Because of the distinct temporal and clinical features, adalimumab-induced hyperpigmentation was eliminated from the differential diagnosis.
Based on the photodistributed pattern of hyperpigmentation, histopathology, and the temporal relationship between hyperpigmentation onset and kratom usage, a diagnosis of kratom-induced photodistributed hyperpigmentation was made. The patient was advised to discontinue kratom use and use sun protection to prevent further photodamage. The patient subsequently was lost to follow-up.
Kratom alkaloids bind all 3 opioid receptors—μOP, δOP, and κOPs—in a G-protein–biased manner with 7-hydroxymitragynine, the most pharmacologically active alkaloid, exhibiting a higher affinity for μ-opioid receptors.13,14 In human epidermal melanocytes, binding between μ-opioid receptors and β-endorphin, an endogenous opioid, is associated with increased melanin production. This melanogenesis has been linked to hyperpigmentation.15 Given the similarity between kratom alkaloids and β-endorphin in opioid-receptor binding, it is possible that kratom-induced hyperpigmentation may occur through a similar mechanism involving μ-opioid receptors and melanogenesis in epidermal melanocytes. Moreover, some researchers have theorized that sun exposure may result in free radical formation of certain drugs or their metabolites. These free radicals then can interact with cellular DNA, triggering the release of pigmentary mediators and resulting in hyperpigmentation.16 This theory may explain the photodistributed pattern of kratom-induced hyperpigmentation. Further studies are needed to understand the mechanism behind this adverse reaction and its implications for patient treatment.
Literature on kratom-induced hyperpigmentation is limited. Powell et al17 reported a similar case of kratom-induced photodistributed hyperpigmentation—a White man had taken kratom to reduce opioid use and subsequently developed hyperpigmented patches on the arms and face. Moreover, anonymous Reddit users have shared anecdotal reports of hyperpigmentation following kratom use.18
Physicians should be aware of hyperpigmentation as a potential adverse reaction of kratom use as its prevalence increases globally. Further research is warranted to elucidate the mechanism behind this adverse reaction and identify risk factors.
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
- Prozialeck WC, Avery BA, Boyer EW, et al. Kratom policy: the challenge of balancing therapeutic potential with public safety. Int J Drug Policy. 2019;70:70-77. doi:10.1016/j.drugpo.2019.05.003
- Bergen-Cico D, MacClurg K. Kratom (Mitragyna speciosa) use, addiction potential, and legal status. In: Preedy VR, ed. Neuropathology of Drug Addictions and Substance Misuse. 2016:903-911. doi:10.1016/B978-0-12-800634-4.00089-5
- Warner ML, Kaufman NC, Grundmann O. The pharmacology and toxicology of kratom: from traditional herb to drug of abuse. Int J Legal Med. 2016;130:127-138. doi:10.1007/s00414-015-1279-y
- Transnational Institute. Kratom in Thailand: decriminalisation and community control? May 3, 2011. Accessed August 23, 2024. https://www.tni.org/en/publication/kratom-in-thailand-decriminalisation-and-community-control
- Eastlack SC, Cornett EM, Kaye AD. Kratom—pharmacology, clinical implications, and outlook: a comprehensive review. Pain Ther. 2020;9:55-69. doi:10.1007/s40122-020-00151-x
- Reyes R. Family of Florida mom who died from herbal substance kratom wins $11M suit. New York Post. July 30, 2023. Updated July 31, 2023. Accessed August 23, 2024. https://nypost.com/2023/07/30/family-of-florida-mom-who-died-from-herbal-substance-kratom-wins-11m-suit/
- Garcia-Romeu A, Cox DJ, Smith KE, et al. Kratom (Mitragyna speciosa): user demographics, use patterns, and implications for the opioid epidemic. Drug Alcohol Depend. 2020;208:107849. doi:10.1016/j.drugalcdep.2020.107849
- Mayo Clinic. Kratom: unsafe and ineffective. Accessed August 23, 2024. https://www.mayoclinic.org/healthy-lifestyle/consumer-health/in-depth/kratom/art-20402171
- Sethi R, Hoang N, Ravishankar DA, et al. Kratom (Mitragyna speciosa): friend or foe? Prim Care Companion CNS Disord. 2020;22:19nr02507.
- Eggleston W, Stoppacher R, Suen K, et al. Kratom use and toxicities in the United States. Pharmacother J Hum Pharmacol Drug Ther. 2019;39:775-777. doi:10.1002/phar.2280
- Qrius. 6 benefits of kratom you should know for healthy skin. March 21, 2023. Accessed August 23, 2024. https://qrius.com/6-benefits-of-kratom-you-should-know-for-healthy-skin/
- Blomberg M, Zachariae COC, Grønhøj F. Hyperpigmentation of the face following adalimumab treatment. Acta Derm Venereol. 2009;89:546-547. doi:10.2340/00015555-0697
- Matsumoto K, Hatori Y, Murayama T, et al. Involvement of μ-opioid receptors in antinociception and inhibition of gastrointestinal transit induced by 7-hydroxymitragynine, isolated from Thai herbal medicine Mitragyna speciosa. Eur J Pharmacol. 2006;549:63-70. doi:10.1016/j.ejphar.2006.08.013
- Jentsch MJ, Pippin MM. Kratom. In: StatPearls. StatPearls Publishing; 2023.
- Bigliardi PL, Tobin DJ, Gaveriaux-Ruff C, et al. Opioids and the skin—where do we stand? Exp Dermatol. 2009;18:424-430.
- Boyer M, Katta R, Markus R. Diltiazem-induced photodistributed hyperpigmentation. Dermatol Online J. 2003;9:10. doi:10.5070/D33c97j4z5
- Powell LR, Ryser TJ, Morey GE, et al. Kratom as a novel cause of photodistributed hyperpigmentation. JAAD Case Rep. 2022;28:145-148. doi:10.1016/j.jdcr.2022.07.033
- Haccoon. Skin discoloring? Reddit. June 30, 2019. Accessed August 23, 2024. https://www.reddit.com/r/quittingkratom/comments/c7b1cm/skin_discoloring/
Practice Points
- Clinicians should be aware of photodistributed hyperpigmentation as a potential adverse effect of kratom usage.
- Kratom-induced photodistributed hyperpigmentation should be suspected in patients with hyperpigmented lesions in sun-exposed areas of the skin following kratom use. A biopsy of lesions should be obtained to confirm the diagnosis.
- Cessation of kratom should be recommended.
Unlocking the Potential of Baricitinib for Vitiligo
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
Vitiligo, the most common skin pigmentation disorder, has affected patients for thousands of years.1 The psychological and social impacts on patients include sleep and sexual disorders, low self-esteem, low quality of life, anxiety, and depression when compared to those without vitiligo.2,3 There have been substantial therapeutic advancements in the treatment of vitiligo, with the recent approval of ruxolitinib cream 1.5% by the US Food and Drug Administration (FDA) in 2022 and by the European Medicines Agency in 2023.4 Ruxolitinib is the first topical Janus kinase (JAK) inhibitor approved by the FDA for the treatment of nonsegmental vitiligo in patients 12 years and older, ushering in the era of JAK inhibitors for patients affected by vitiligo. The efficacy and safety of ruxolitinib was supported by 2 randomized clinical trials.4 It also is FDA approved for the intermittent and short-term treatment of mild to moderate atopic dermatitis in nonimmunocompromised patients 12 years and older whose disease is not adequately controlled with other topical medications.5
Vitiligo is characterized by an important inflammatory component, with the JAK/STAT (signal transducer and activator of transcription) pathway playing a crucial role in transmitting signals of inflammatory cytokines. In particular, IFN-γ and chemokines CXCL9 and CXCL10 are major contributors to the development of vitiligo, acting through the JAK/STAT pathway in local keratinocytes. Inhibiting JAK activity helps mitigate the effects of IFN-γ and downstream chemokines.6
Currently, baricitinib is not FDA approved for the treatment of vitiligo; it is FDA approved for moderate to severe active rheumatoid arthritis, severe alopecia areata, and in specific cases for COVID-19.7 Mumford et al8 first reported the use of oral baricitinib for the treatment of nonsegmental vitiligo. This patient experienced poor improvement using the oral JAK inhibitor tofacitinib for 5 months but achieved near-complete repigmentation after switching to baricitinib for 8 months (4 mg daily).8 Furthermore, a recent study found that in vitro baricitinib could increase tyrosinase activity and melanin content as well as stimulate the expression of genes related to tyrosinase in damaged melanocytes.9
A recent study by Li et al10 has shown satisfactory repigmentation and good tolerance in 2 cases of vitiligo treated with oral baricitinib in combination with narrowband UVB (NB-UVB) phototherapy. These findings are supported by a prior study of oral tofacitinib and NB-UVB phototherapy in 10 cases; the JAK inhibitor treatment demonstrated enhanced effectiveness when combined with light exposure.11
Large-scale randomized clinical trials are needed to evaluate the efficacy and safety of oral baricitinib for vitiligo treatment. Currently, a clinical trial is underway (recruiting phase) to compare the efficacy and safety of combining baricitinib and excimer lamp phototherapy vs phototherapy alone.12 The results of this trial can provide valuable information about whether baricitinib is promising as part of the therapeutic arsenal for vitiligo treatment in the future. A recently completed multicenter, randomized, double-blind clinical trial assessed the efficacy and tolerability of oral baricitinib in combination with NB-UVB phototherapy for the treatment of vitiligo. The trial included 49 patients and may provide valuable insights for the potential future application of baricitinib in the treatment of vitiligo.13 If the results of these clinical trials are favorable, approval of the first orally administered JAK inhibitor for repigmentation treatment in patients with vitiligo could follow, which would be a major breakthrough.
The off-label use of baricitinib—alone or in combination with phototherapy—appears to be promising in studies with a small sample size (an important limitation). The results of clinical trials will help us elucidate the efficacy and safety of baricitinib for vitiligo treatment, which could be a subject of debate. Recently, the FDA issued a warning due to findings showing that the use of tofacitinib has been associated with an increased risk of serious heart-related events, such heart attack, stroke, cancer, blood clots, and death.14 In response, the FDA issued warnings for 2 other JAK inhibitors—baricitinib and upadacitinib. Unlike tofacitinib, baricitinib and upadacitinib have not been studied in large safety clinical trials, and as a result, their risks have not been adequately evaluated. However, due to the shared mechanisms of action of these drugs, the FDA believes that these medications may pose similar risks as those observed in the tofacitinib safety trial.14
Disadvantages of JAK inhibitors include the high cost, immune-related side effects, potential cardiovascular adverse effects, and limited availability worldwide. If current and future clinical trials obtain objective evidence with a large sample size that yields positive outcomes with tolerable or acceptable side effects, and if the drug is affordable for hospitals and patients, the use of oral or topical baricitinib will be embraced and may be approved for vitiligo.
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Berger BJ, Rudolph RI, Leyden JJ. Letter: transient acantholytic dermatosis. Arch Dermatol. 1974;109:913. doi:10.1001/archderm.1974.01630060081033
- Hu Z, Wang T. Beyond skin white spots: vitiligo and associated comorbidities. Front Med (Lausanne). 2023;10:1072837. doi:10.3389/fmed.2023.1072837
- Rzepecki AK, McLellan BN, Elbuluk N. Beyond traditional treatment: the importance of psychosocial therapy in vitiligo. J Drugs Dermatol. 2018;17:688-691.
- Topical ruxolitinib evaluation in vitiligo study 1 (TRuE-V1). ClinicalTrials.gov identifier: NCT04052425. Updated September 21, 2022. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04052425
- US Food and Drug Administration. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. July 19, 2022. Accessed August 16, 2024. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Harris JE, Harris TH, Weninger W, et al. A mouse model of vitiligo with focused epidermal depigmentation requires IFN-γ for autoreactive CD8+ T-cell accumulation in the skin. J Invest Dermatol. 2012;132:1869-1876. doi:10.1038/jid.2011.463
- Garcia-Melendo C, Cubiró X, Puig L. Janus kinase inhibitors in dermatology: part 1—general considerations and applications in vitiligo and alopecia areata. Actas Dermosifiliogr. 2021;112:503-515. doi:10.1016/j.ad.2020.12.003
- Mumford BP, Gibson A, Chong AH. Repigmentation of vitiligo with oral baricitinib. Australas J Dermatol. 2020;61:374-376. doi:10.1111/ajd.13348
- Dong J, Huang X, Ma LP, et al. Baricitinib is effective in treating progressing vitiligo in vivo and in vitro. Dose Response. 2022;20:15593258221105370. doi:10.1177/15593258221105370
- Li X, Sun Y, Du J, et al. Excellent repigmentation of generalized vitiligo with oral baricitinib combined with NB-UVB phototherapy. Clin Cosmet Investig Dermatol. 2023;16:635-638. doi:10.2147/CCID.S396430
- Liu LY, Strassner JP, Refat MA, et al. Repigmentation in vitiligo using the Janus kinase inhibitor tofacitinib may require concomitant light exposure. J Am Acad Dermatol. 2017;77:675-682.e1. doi:10.1016/j.jaad.2017.05.043
- Evaluation safety, efficacy baricitinib plus excimer light versus excimer light alone in non segmental vitiligo. ClinicalTrials.gov identifier: NCT05950542. Updated July 18, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT05950542
- Evaluation of effect and tolerance of the association of baricitinib and phototherapy versus phototherapy in adults with progressive vitiligo (BARVIT). ClinicalTrials.gov identifier: NCT04822584. Updated June 13, 2023. Accessed August 16, 2024. https://clinicaltrials.gov/study/NCT04822584
- US Food and Drug Administration. FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. December 7, 2021. Accessed August 16, 2024. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
Enhanced Care for Pediatric Patients With Generalized Lichen Planus: Diagnosis and Treatment Tips
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.
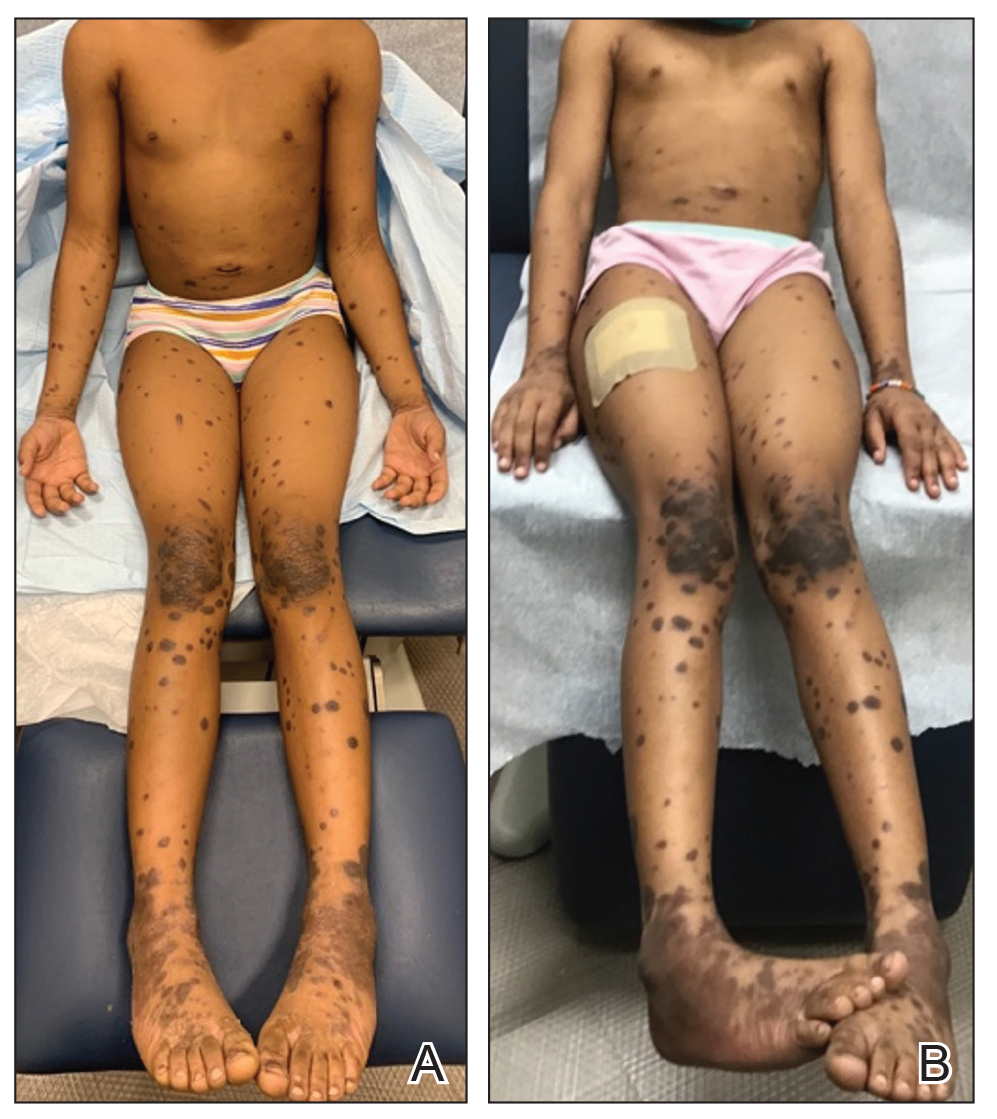
The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
Practice Gap
Lichen planus (LP) is an inflammatory cutaneous disorder. Although it often is characterized by the 6 Ps—pruritic, polygonal, planar, purple, papules, and plaques with a predilection for the wrists and ankles—the presentation can vary in morphology and distribution.1-5 With an incidence of approximately 1% in the general population, LP is undoubtedly uncommon.1 Its prevalence in the pediatric population is especially low, with only 2% to 3% of cases manifesting in individuals younger than 20 years.2
Generalized LP (also referred to as eruptive or exanthematous LP) is a rarely reported clinical subtype in which lesions are disseminated or spread rapidly.5 The rarity of generalized LP in children often leads to misdiagnosis or delayed treatment, impacting the patient’s quality of life. Thus, there is a need for heightened awareness among clinicians on the variable presentation of LP in the pediatric population. Incorporating a punch biopsy for the diagnosis of LP when lesions manifest as widespread, erythematous to violaceous, flat-topped papules or plaques, along with the addition of an intramuscular (IM) injection in the treatment plan, improves overall patient outcomes.
Tools and Techniques
A detailed physical examination followed by a punch biopsy was critical for the diagnosis of generalized LP in a 7-year-old Black girl. The examination revealed a widespread distribution of dark, violaceous, polygonal, shiny, flat-topped, firm papules coalescing into plaques across the entire body, with a greater predilection for the legs and overlying joints (Figure, A). Some lesions exhibited fine, silver-white, reticular patterns consistent with Wickham striae. Notably, there was no involvement of the scalp, nails, or mucosal surfaces.

The patient had no relevant medical or family history of skin disease and no recent history of illness. She previously was treated by a pediatrician with triamcinolone cream 0.1%, a course of oral cephalexin, and oral cetirizine 10 mg once daily without relief of symptoms.
Although the clinical presentation was consistent with LP, the differential diagnosis included lichen simplex chronicus, atopic dermatitis, psoriasis, and generalized granuloma annulare. To address the need for early recognition of LP in pediatric patients, a punch biopsy of a lesion on the left anterior thigh was performed and showed lichenoid interface dermatitis—a pivotal finding in distinguishing LP from other conditions in the differential.
Given the patient’s age and severity of the LP, a combination of topical and systemic therapies was prescribed—clobetasol cream 0.025% twice daily and 1 injection of 0.5 cc of IM triamcinolone acetonide 40 mg/mL. This regimen was guided by the efficacy of IM injections in providing prompt symptomatic relief, particularly for patients with extensive disease or for those whose condition is refractory to topical treatments.6 Our patient achieved remarkable improvement at 2-week follow-up (Figure, B), without any observed adverse effects. At that time, the patient’s mother refused further systemic treatment and opted for only the topical therapy as well as natural light therapy.
Practice Implications
Timely and accurate diagnosis of LP in pediatric patients, especially those with skin of color, is crucial. Early intervention is especially important in mitigating the risk for chronic symptoms and preventing potential scarring, which tends to be more pronounced and challenging to treat in individuals with darker skin tones.7 Although not present in our patient, it is important to note that LP can affect the face (including the eyelids) as well as the palms and soles in pediatric patients with skin of color.
The most common approach to management of pediatric LP involves the use of a topical corticosteroid and an oral antihistamine, but the recalcitrant and generalized distribution of lesions warrants the administration of a systemic corticosteroid regardless of the patient’s age.6 In our patient, prompt administration of low-dose IM triamcinolone was both crucial and beneficial. Although an underutilized approach, IM triamcinolone helps to prevent the progression of lesions to the scalp, nails, and mucosa while also reducing inflammation and pruritus in glabrous skin.8
Triamcinolone acetonide injections—administered at concentrations of 5 to 40 mg/mL—directly into the lesion (0.5–1 cc per 2 cm2) are highly effective in managing recalcitrant thickened lesions such as those seen in hypertrophic LP and palmoplantar LP.6 This treatment is particularly beneficial when lesions are unresponsive to topical therapies. Administered every 3 to 6 weeks, these injections provide rapid symptom relief, typically within 72 hours,6 while also contributing to the reduction of lesion size and thickness over time. The concentration of triamcinolone acetonide should be selected based on the lesion’s severity, with higher concentrations reserved for thicker, more resistant lesions. More frequent injections may be warranted in cases in which rapid lesion reduction is necessary, while less frequent sessions may suffice for maintenance therapy. It is important to follow patients closely for adverse effects, such as signs of local skin atrophy or hypopigmentation, and to adjust the dose or frequency accordingly. To mitigate these risks, consider using the lowest effective concentration and rotating injection sites if treating multiple lesions. Additionally, combining intralesional corticosteroids with topical therapies can enhance outcomes, particularly in cases in which monotherapy is insufficient.
Patients should be monitored vigilantly for complications of LP. The risk for postinflammatory hyperpigmentation is a particular concern for patients with skin of color. Other complications of untreated LP include nail deformities and scarring alopecia.9 Regular and thorough follow-ups every few months to monitor scalp, mucosal, and genital involvement are essential to manage this risk effectively.
Furthermore, patient education is key. Informing patients and their caregivers about the nature of LP, the available treatment options, and the importance of ongoing follow-up can help to enhance treatment adherence and improve overall outcomes.
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
- Le Cleach L, Chosidow O. Clinical practice. Lichen planus. N Engl J Med. 2012;366:723-732. doi:10.1056/NEJMcp1103641
- Handa S, Sahoo B. Childhood lichen planus: a study of 87 cases. Int J Dermatol. 2002;41:423-427. doi:10.1046/j.1365-4362.2002.01522.x
- George J, Murray T, Bain M. Generalized, eruptive lichen planus in a pediatric patient. Contemp Pediatr. 2022;39:32-34.
- Arnold DL, Krishnamurthy K. Lichen planus. StatPearls [Internet]. Updated June 1, 2023. Accessed August 12, 2024. https://www.ncbi.nlm.nih.gov/books/NBK526126/
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149. doi:10.1016/j.ijwd.2015.04.001
- Mutalik SD, Belgaumkar VA, Rasal YD. Current perspectives in the treatment of childhood lichen planus. Indian J Paediatr Dermatol. 2021;22:316-325. doi:10.4103/ijpd.ijpd_165_20
- Usatine RP, Tinitigan M. Diagnosis and treatment of lichen planus. Am Fam Physician. 2011;84:53-60.
- Thomas LW, Elsensohn A, Bergheim T, et al. Intramuscular steroids in the treatment of dermatologic disease: a systematic review. J Drugs Dermatol. 2018;17:323-329.
- Gorouhi F, Davari P, Fazel N. Cutaneous and mucosal lichen planus: a comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. ScientificWorldJournal. 2014;2014:742826. doi:10.1155/2014/742826
Top DEI Topics to Incorporate Into Dermatology Residency Training: An Electronic Delphi Consensus Study
Diversity, equity, and inclusion (DEI) programs seek to improve dermatologic education and clinical care for an increasingly diverse patient population as well as to recruit and sustain a physician workforce that reflects the diversity of the patients they serve.1,2 In dermatology, only 4.2% and 3.0% of practicing dermatologists self-identify as being of Hispanic and African American ethnicity, respectively, compared with 18.5% and 13.4% of the general population, respectively.3 Creating an educational system that works to meet the goals of DEI is essential to improve health outcomes and address disparities. The lack of robust DEI-related curricula during residency training may limit the ability of practicing dermatologists to provide comprehensive and culturally sensitive care. It has been shown that racial concordance between patients and physicians has a positive impact on patient satisfaction by fostering a trusting patient-physician relationship.4
It is the responsibility of all dermatologists to create an environment where patients from any background can feel comfortable, which can be cultivated by establishing patient-centered communication and cultural humility.5 These skills can be strengthened via the implementation of DEI-related curricula during residency training. Augmenting exposure of these topics during training can optimize the delivery of dermatologic care by providing residents with the tools and confidence needed to care for patients of culturally diverse backgrounds. Enhancing DEI education is crucial to not only improve the recognition and treatment of dermatologic conditions in all skin and hair types but also to minimize misconceptions, stigma, health disparities, and discrimination faced by historically marginalized communities. Creating a culture of inclusion is of paramount importance to build successful relationships with patients and colleagues of culturally diverse backgrounds.6
There are multiple efforts underway to increase DEI education across the field of dermatology, including the development of DEI task forces in professional organizations and societies that serve to expand DEI-related research, mentorship, and education. The American Academy of Dermatology has been leading efforts to create a curriculum focused on skin of color, particularly addressing inadequate educational training on how dermatologic conditions manifest in this population.7 The Skin of Color Society has similar efforts underway and is developing a speakers bureau to give leading experts a platform to lecture dermatology trainees as well as patient and community audiences on various topics in skin of color.8 These are just 2 of many professional dermatology organizations that are advocating for expanded education on DEI; however, consistently integrating DEI-related topics into dermatology residency training curricula remains a gap in pedagogy. To identify the DEI-related topics of greatest relevance to the dermatology resident curricula, we implemented a modified electronic Delphi (e-Delphi) consensus process to provide standardized recommendations.
Methods
A 2-round modified e-Delphi method was utilized (Figure). An initial list of potential curricular topics was formulated by an expert panel consisting of 5 dermatologists from the Association of Professors of Dermatology DEI subcommittee and the American Academy of Dermatology Diversity Task Force (A.M.A., S.B., R.V., S.D.W., J.I.S.). Initial topics were selected via several meetings among the panel members to discuss existing DEI concerns and issues that were deemed relevant due to education gaps in residency training. The list of topics was further expanded with recommendations obtained via an email sent to dermatology program directors on the Association of Professors of Dermatology listserve, which solicited voluntary participation of academic dermatologists, including program directors and dermatology residents.
There were 2 voting rounds, with each round consisting of questions scored on a Likert scale ranging from 1 to 5 (1=not essential, 2=probably not essential, 3=neutral, 4=probably essential, 5=definitely essential). The inclusion criteria to classify a topic as necessary for integration into the dermatology residency curriculum included 95% (18/19) or more of respondents rating the topic as probably essential or definitely essential; if more than 90% (17/19) of respondents rated the topic as probably essential or definitely essential and less than 10% (2/19) rated it as not essential or probably not essential, the topic was still included as part of the suggested curriculum. Topics that received ratings of probably essential or definitely essential by less than 80% (15/19) of respondents were removed from consideration. The topics that did not meet inclusion or exclusion criteria during the first round of voting were refined by the e-Delphi steering committee (V.S.E-C. and F-A.R.) based on open-ended feedback from the voting group provided at the end of the survey and subsequently passed to the second round of voting.
Results
Participants—A total of 19 respondents participated in both voting rounds, the majority (80% [15/19]) of whom were program directors or dermatologists affiliated with academia or development of DEI education; the remaining 20% [4/19]) were dermatology residents.
Open-Ended Feedback—Voting group members were able to provide open-ended feedback for each of the sets of topics after the survey, which the steering committee utilized to modify the topics as needed for the final voting round. For example, “structural racism/discrimination” was originally mentioned as a topic, but several participants suggested including specific types of racism; therefore, the wording was changed to “racism: types, definitions” to encompass broader definitions and types of racism.
Survey Results—Two genres of topics were surveyed in each voting round: clinical and nonclinical. Participants voted on a total of 61 topics, with 23 ultimately selected in the final list of consensus curricular topics. Of those, 9 were clinical and 14 nonclinical. All topics deemed necessary for inclusion in residency curricula are presented in eTables 1 and 2.
During the first round of voting, the e-Delphi panel reached a consensus to include the following 17 topics as essential to dermatology residency training (along with the percentage of voters who classified them as probably essential or definitely essential): how to mitigate bias in clinical and workplace settings (100% [40/40]); social determinants of health-related disparities in dermatology (100% [40/40]); hairstyling practices across different hair textures (100% [40/40]); definitions and examples of microaggressions (97.50% [39/40]); definition, background, and types of bias (97.50% [39/40]); manifestations of bias in the clinical setting (97.44% [38/39]); racial and ethnic disparities in dermatology (97.44% [38/39]); keloids (97.37% [37/38]); differences in dermoscopic presentations in skin of color (97.30% [36/37]); skin cancer in patients with skin of color (97.30% [36/37]); disparities due to bias (95.00% [38/40]); how to apply cultural humility and safety to patients of different cultural backgrounds (94.87% [37/40]); best practices in providing care to patients with limited English proficiency (94.87% [37/40]); hair loss in patients with textured hair (94.74% [36/38]); pseudofolliculitis barbae and acne keloidalis nuchae (94.60% [35/37]); disparities regarding people experiencing homelessness (92.31% [36/39]); and definitions and types of racism and other forms of discrimination (92.31% [36/39]). eTable 1 provides a list of suggested resources to incorporate these topics into the educational components of residency curricula. The resources provided were not part of the voting process, and they were not considered in the consensus analysis; they are included here as suggested educational catalysts.
During the second round of voting, 25 topics were evaluated. Of those, the following 6 topics were proposed to be included as essential in residency training: differences in prevalence and presentation of common inflammatory disorders (100% [29/29]); manifestations of bias in the learning environment (96.55%); antiracist action and how to decrease the effects of structural racism in clinical and educational settings (96.55% [28/29]); diversity of images in dermatology education (96.55% [28/29]); pigmentary disorders and their psychological effects (96.55% [28/29]); and LGBTQ (lesbian, gay, bisexual, transgender, and queer) dermatologic health care (96.55% [28/29]). eTable 2 includes these topics as well as suggested resources to help incorporate them into training.
Comment
This study utilized a modified e-Delphi technique to identify relevant clinical and nonclinical DEI topics that should be incorporated into dermatology residency curricula. The panel members reached a consensus for 9 clinical DEI-related topics. The respondents agreed that the topics related to skin and hair conditions in patients with skin of color as well as textured hair were crucial to residency education. Skin cancer, hair loss, pseudofolliculitis barbae, acne keloidalis nuchae, keloids, pigmentary disorders, and their varying presentations in patients with skin of color were among the recommended topics. The panel also recommended educating residents on the variable visual presentations of inflammatory conditions in skin of color. Addressing the needs of diverse patients—for example, those belonging to the LGBTQ community—also was deemed important for inclusion.
The remaining 14 chosen topics were nonclinical items addressing concepts such as bias and health care disparities as well as cultural humility and safety.9 Cultural humility and safety focus on developing cultural awareness by creating a safe setting for patients rather than encouraging power relationships between them and their physicians. Various topics related to racism also were recommended to be included in residency curricula, including education on implementation of antiracist action in the workplace.
Many of the nonclinical topics are intertwined; for instance, learning about health care disparities in patients with limited English proficiency allows for improved best practices in delivering care to patients from this population. The first step in overcoming bias and subsequent disparities is acknowledging how the perpetuation of bias leads to disparities after being taught tools to recognize it.
Our group’s guidance on DEI topics should help dermatology residency program leaders as they design and refine program curricula. There are multiple avenues for incorporating education on these topics, including lectures, interactive workshops, role-playing sessions, book or journal clubs, and discussion circles. Many of these topics/programs may already be included in programs’ didactic curricula, which would minimize the burden of finding space to educate on these topics. Institutional cultural change is key to ensuring truly diverse, equitable, and inclusive workplaces. Educating tomorrow’s dermatologists on these topics is a first step toward achieving that cultural change.
Limitations—A limitation of this e-Delphi survey is that only a selection of experts in this field was included. Additionally, we were concerned that the Likert scale format and the bar we set for inclusion and exclusion may have failed to adequately capture participants’ nuanced opinions. As such, participants were able to provide open-ended feedback, and suggestions for alternate wording or other changes were considered by the steering committee. Finally, inclusion recommendations identified in this survey were developed specifically for US dermatology residents.
Conclusion
In this e-Delphi consensus assessment of DEI-related topics, we recommend the inclusion of 23 topics into dermatology residency program curricula to improve medical training and the patient-physician relationship as well as to create better health outcomes. We also provide specific sample resource recommendations in eTables 1 and 2 to facilitate inclusion of these topics into residency curricula across the country.
- US Census Bureau projections show a slower growing, older, more diverse nation a half century from now. News release. US Census Bureau. December 12, 2012. Accessed August 14, 2024. https://www.census.gov/newsroom/releases/archives/population/cb12243.html#:~:text=12%2C%202012,U.S.%20Census%20Bureau%20Projections%20Show%20a%20Slower%20Growing%2C%20Older%2C%20More,by%20the%20U.S.%20Census%20Bureau
- Lopez S, Lourido JO, Lim HW, et al. The call to action to increase racial and ethnic diversity in dermatology: a retrospective, cross-sectional study to monitor progress. J Am Acad Dermatol. 2020;86:E121-E123. doi:10.1016/j.jaad.2021.10.011
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Street RL Jr, O’Malley KJ, Cooper LA, et al. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6:198-205. doi:10.1370/afm.821
- Dadrass F, Bowers S, Shinkai K, et al. Diversity, equity, and inclusion in dermatology residency. Dermatol Clin. 2023;41:257-263. doi:10.1016/j.det.2022.10.006
- Diversity and the Academy. American Academy of Dermatology website. Accessed August 22, 2024. https://www.aad.org/member/career/diversity
- SOCS speaks. Skin of Color Society website. Accessed August 22, 2024. https://skinofcolorsociety.org/news-media/socs-speaks
- Solchanyk D, Ekeh O, Saffran L, et al. Integrating cultural humility into the medical education curriculum: strategies for educators. Teach Learn Med. 2021;33:554-560. doi:10.1080/10401334.2021.1877711
Diversity, equity, and inclusion (DEI) programs seek to improve dermatologic education and clinical care for an increasingly diverse patient population as well as to recruit and sustain a physician workforce that reflects the diversity of the patients they serve.1,2 In dermatology, only 4.2% and 3.0% of practicing dermatologists self-identify as being of Hispanic and African American ethnicity, respectively, compared with 18.5% and 13.4% of the general population, respectively.3 Creating an educational system that works to meet the goals of DEI is essential to improve health outcomes and address disparities. The lack of robust DEI-related curricula during residency training may limit the ability of practicing dermatologists to provide comprehensive and culturally sensitive care. It has been shown that racial concordance between patients and physicians has a positive impact on patient satisfaction by fostering a trusting patient-physician relationship.4
It is the responsibility of all dermatologists to create an environment where patients from any background can feel comfortable, which can be cultivated by establishing patient-centered communication and cultural humility.5 These skills can be strengthened via the implementation of DEI-related curricula during residency training. Augmenting exposure of these topics during training can optimize the delivery of dermatologic care by providing residents with the tools and confidence needed to care for patients of culturally diverse backgrounds. Enhancing DEI education is crucial to not only improve the recognition and treatment of dermatologic conditions in all skin and hair types but also to minimize misconceptions, stigma, health disparities, and discrimination faced by historically marginalized communities. Creating a culture of inclusion is of paramount importance to build successful relationships with patients and colleagues of culturally diverse backgrounds.6
There are multiple efforts underway to increase DEI education across the field of dermatology, including the development of DEI task forces in professional organizations and societies that serve to expand DEI-related research, mentorship, and education. The American Academy of Dermatology has been leading efforts to create a curriculum focused on skin of color, particularly addressing inadequate educational training on how dermatologic conditions manifest in this population.7 The Skin of Color Society has similar efforts underway and is developing a speakers bureau to give leading experts a platform to lecture dermatology trainees as well as patient and community audiences on various topics in skin of color.8 These are just 2 of many professional dermatology organizations that are advocating for expanded education on DEI; however, consistently integrating DEI-related topics into dermatology residency training curricula remains a gap in pedagogy. To identify the DEI-related topics of greatest relevance to the dermatology resident curricula, we implemented a modified electronic Delphi (e-Delphi) consensus process to provide standardized recommendations.
Methods
A 2-round modified e-Delphi method was utilized (Figure). An initial list of potential curricular topics was formulated by an expert panel consisting of 5 dermatologists from the Association of Professors of Dermatology DEI subcommittee and the American Academy of Dermatology Diversity Task Force (A.M.A., S.B., R.V., S.D.W., J.I.S.). Initial topics were selected via several meetings among the panel members to discuss existing DEI concerns and issues that were deemed relevant due to education gaps in residency training. The list of topics was further expanded with recommendations obtained via an email sent to dermatology program directors on the Association of Professors of Dermatology listserve, which solicited voluntary participation of academic dermatologists, including program directors and dermatology residents.

There were 2 voting rounds, with each round consisting of questions scored on a Likert scale ranging from 1 to 5 (1=not essential, 2=probably not essential, 3=neutral, 4=probably essential, 5=definitely essential). The inclusion criteria to classify a topic as necessary for integration into the dermatology residency curriculum included 95% (18/19) or more of respondents rating the topic as probably essential or definitely essential; if more than 90% (17/19) of respondents rated the topic as probably essential or definitely essential and less than 10% (2/19) rated it as not essential or probably not essential, the topic was still included as part of the suggested curriculum. Topics that received ratings of probably essential or definitely essential by less than 80% (15/19) of respondents were removed from consideration. The topics that did not meet inclusion or exclusion criteria during the first round of voting were refined by the e-Delphi steering committee (V.S.E-C. and F-A.R.) based on open-ended feedback from the voting group provided at the end of the survey and subsequently passed to the second round of voting.
Results
Participants—A total of 19 respondents participated in both voting rounds, the majority (80% [15/19]) of whom were program directors or dermatologists affiliated with academia or development of DEI education; the remaining 20% [4/19]) were dermatology residents.
Open-Ended Feedback—Voting group members were able to provide open-ended feedback for each of the sets of topics after the survey, which the steering committee utilized to modify the topics as needed for the final voting round. For example, “structural racism/discrimination” was originally mentioned as a topic, but several participants suggested including specific types of racism; therefore, the wording was changed to “racism: types, definitions” to encompass broader definitions and types of racism.
Survey Results—Two genres of topics were surveyed in each voting round: clinical and nonclinical. Participants voted on a total of 61 topics, with 23 ultimately selected in the final list of consensus curricular topics. Of those, 9 were clinical and 14 nonclinical. All topics deemed necessary for inclusion in residency curricula are presented in eTables 1 and 2.
During the first round of voting, the e-Delphi panel reached a consensus to include the following 17 topics as essential to dermatology residency training (along with the percentage of voters who classified them as probably essential or definitely essential): how to mitigate bias in clinical and workplace settings (100% [40/40]); social determinants of health-related disparities in dermatology (100% [40/40]); hairstyling practices across different hair textures (100% [40/40]); definitions and examples of microaggressions (97.50% [39/40]); definition, background, and types of bias (97.50% [39/40]); manifestations of bias in the clinical setting (97.44% [38/39]); racial and ethnic disparities in dermatology (97.44% [38/39]); keloids (97.37% [37/38]); differences in dermoscopic presentations in skin of color (97.30% [36/37]); skin cancer in patients with skin of color (97.30% [36/37]); disparities due to bias (95.00% [38/40]); how to apply cultural humility and safety to patients of different cultural backgrounds (94.87% [37/40]); best practices in providing care to patients with limited English proficiency (94.87% [37/40]); hair loss in patients with textured hair (94.74% [36/38]); pseudofolliculitis barbae and acne keloidalis nuchae (94.60% [35/37]); disparities regarding people experiencing homelessness (92.31% [36/39]); and definitions and types of racism and other forms of discrimination (92.31% [36/39]). eTable 1 provides a list of suggested resources to incorporate these topics into the educational components of residency curricula. The resources provided were not part of the voting process, and they were not considered in the consensus analysis; they are included here as suggested educational catalysts.
During the second round of voting, 25 topics were evaluated. Of those, the following 6 topics were proposed to be included as essential in residency training: differences in prevalence and presentation of common inflammatory disorders (100% [29/29]); manifestations of bias in the learning environment (96.55%); antiracist action and how to decrease the effects of structural racism in clinical and educational settings (96.55% [28/29]); diversity of images in dermatology education (96.55% [28/29]); pigmentary disorders and their psychological effects (96.55% [28/29]); and LGBTQ (lesbian, gay, bisexual, transgender, and queer) dermatologic health care (96.55% [28/29]). eTable 2 includes these topics as well as suggested resources to help incorporate them into training.
Comment
This study utilized a modified e-Delphi technique to identify relevant clinical and nonclinical DEI topics that should be incorporated into dermatology residency curricula. The panel members reached a consensus for 9 clinical DEI-related topics. The respondents agreed that the topics related to skin and hair conditions in patients with skin of color as well as textured hair were crucial to residency education. Skin cancer, hair loss, pseudofolliculitis barbae, acne keloidalis nuchae, keloids, pigmentary disorders, and their varying presentations in patients with skin of color were among the recommended topics. The panel also recommended educating residents on the variable visual presentations of inflammatory conditions in skin of color. Addressing the needs of diverse patients—for example, those belonging to the LGBTQ community—also was deemed important for inclusion.
The remaining 14 chosen topics were nonclinical items addressing concepts such as bias and health care disparities as well as cultural humility and safety.9 Cultural humility and safety focus on developing cultural awareness by creating a safe setting for patients rather than encouraging power relationships between them and their physicians. Various topics related to racism also were recommended to be included in residency curricula, including education on implementation of antiracist action in the workplace.
Many of the nonclinical topics are intertwined; for instance, learning about health care disparities in patients with limited English proficiency allows for improved best practices in delivering care to patients from this population. The first step in overcoming bias and subsequent disparities is acknowledging how the perpetuation of bias leads to disparities after being taught tools to recognize it.
Our group’s guidance on DEI topics should help dermatology residency program leaders as they design and refine program curricula. There are multiple avenues for incorporating education on these topics, including lectures, interactive workshops, role-playing sessions, book or journal clubs, and discussion circles. Many of these topics/programs may already be included in programs’ didactic curricula, which would minimize the burden of finding space to educate on these topics. Institutional cultural change is key to ensuring truly diverse, equitable, and inclusive workplaces. Educating tomorrow’s dermatologists on these topics is a first step toward achieving that cultural change.
Limitations—A limitation of this e-Delphi survey is that only a selection of experts in this field was included. Additionally, we were concerned that the Likert scale format and the bar we set for inclusion and exclusion may have failed to adequately capture participants’ nuanced opinions. As such, participants were able to provide open-ended feedback, and suggestions for alternate wording or other changes were considered by the steering committee. Finally, inclusion recommendations identified in this survey were developed specifically for US dermatology residents.
Conclusion
In this e-Delphi consensus assessment of DEI-related topics, we recommend the inclusion of 23 topics into dermatology residency program curricula to improve medical training and the patient-physician relationship as well as to create better health outcomes. We also provide specific sample resource recommendations in eTables 1 and 2 to facilitate inclusion of these topics into residency curricula across the country.
Diversity, equity, and inclusion (DEI) programs seek to improve dermatologic education and clinical care for an increasingly diverse patient population as well as to recruit and sustain a physician workforce that reflects the diversity of the patients they serve.1,2 In dermatology, only 4.2% and 3.0% of practicing dermatologists self-identify as being of Hispanic and African American ethnicity, respectively, compared with 18.5% and 13.4% of the general population, respectively.3 Creating an educational system that works to meet the goals of DEI is essential to improve health outcomes and address disparities. The lack of robust DEI-related curricula during residency training may limit the ability of practicing dermatologists to provide comprehensive and culturally sensitive care. It has been shown that racial concordance between patients and physicians has a positive impact on patient satisfaction by fostering a trusting patient-physician relationship.4
It is the responsibility of all dermatologists to create an environment where patients from any background can feel comfortable, which can be cultivated by establishing patient-centered communication and cultural humility.5 These skills can be strengthened via the implementation of DEI-related curricula during residency training. Augmenting exposure of these topics during training can optimize the delivery of dermatologic care by providing residents with the tools and confidence needed to care for patients of culturally diverse backgrounds. Enhancing DEI education is crucial to not only improve the recognition and treatment of dermatologic conditions in all skin and hair types but also to minimize misconceptions, stigma, health disparities, and discrimination faced by historically marginalized communities. Creating a culture of inclusion is of paramount importance to build successful relationships with patients and colleagues of culturally diverse backgrounds.6
There are multiple efforts underway to increase DEI education across the field of dermatology, including the development of DEI task forces in professional organizations and societies that serve to expand DEI-related research, mentorship, and education. The American Academy of Dermatology has been leading efforts to create a curriculum focused on skin of color, particularly addressing inadequate educational training on how dermatologic conditions manifest in this population.7 The Skin of Color Society has similar efforts underway and is developing a speakers bureau to give leading experts a platform to lecture dermatology trainees as well as patient and community audiences on various topics in skin of color.8 These are just 2 of many professional dermatology organizations that are advocating for expanded education on DEI; however, consistently integrating DEI-related topics into dermatology residency training curricula remains a gap in pedagogy. To identify the DEI-related topics of greatest relevance to the dermatology resident curricula, we implemented a modified electronic Delphi (e-Delphi) consensus process to provide standardized recommendations.
Methods
A 2-round modified e-Delphi method was utilized (Figure). An initial list of potential curricular topics was formulated by an expert panel consisting of 5 dermatologists from the Association of Professors of Dermatology DEI subcommittee and the American Academy of Dermatology Diversity Task Force (A.M.A., S.B., R.V., S.D.W., J.I.S.). Initial topics were selected via several meetings among the panel members to discuss existing DEI concerns and issues that were deemed relevant due to education gaps in residency training. The list of topics was further expanded with recommendations obtained via an email sent to dermatology program directors on the Association of Professors of Dermatology listserve, which solicited voluntary participation of academic dermatologists, including program directors and dermatology residents.

There were 2 voting rounds, with each round consisting of questions scored on a Likert scale ranging from 1 to 5 (1=not essential, 2=probably not essential, 3=neutral, 4=probably essential, 5=definitely essential). The inclusion criteria to classify a topic as necessary for integration into the dermatology residency curriculum included 95% (18/19) or more of respondents rating the topic as probably essential or definitely essential; if more than 90% (17/19) of respondents rated the topic as probably essential or definitely essential and less than 10% (2/19) rated it as not essential or probably not essential, the topic was still included as part of the suggested curriculum. Topics that received ratings of probably essential or definitely essential by less than 80% (15/19) of respondents were removed from consideration. The topics that did not meet inclusion or exclusion criteria during the first round of voting were refined by the e-Delphi steering committee (V.S.E-C. and F-A.R.) based on open-ended feedback from the voting group provided at the end of the survey and subsequently passed to the second round of voting.
Results
Participants—A total of 19 respondents participated in both voting rounds, the majority (80% [15/19]) of whom were program directors or dermatologists affiliated with academia or development of DEI education; the remaining 20% [4/19]) were dermatology residents.
Open-Ended Feedback—Voting group members were able to provide open-ended feedback for each of the sets of topics after the survey, which the steering committee utilized to modify the topics as needed for the final voting round. For example, “structural racism/discrimination” was originally mentioned as a topic, but several participants suggested including specific types of racism; therefore, the wording was changed to “racism: types, definitions” to encompass broader definitions and types of racism.
Survey Results—Two genres of topics were surveyed in each voting round: clinical and nonclinical. Participants voted on a total of 61 topics, with 23 ultimately selected in the final list of consensus curricular topics. Of those, 9 were clinical and 14 nonclinical. All topics deemed necessary for inclusion in residency curricula are presented in eTables 1 and 2.
During the first round of voting, the e-Delphi panel reached a consensus to include the following 17 topics as essential to dermatology residency training (along with the percentage of voters who classified them as probably essential or definitely essential): how to mitigate bias in clinical and workplace settings (100% [40/40]); social determinants of health-related disparities in dermatology (100% [40/40]); hairstyling practices across different hair textures (100% [40/40]); definitions and examples of microaggressions (97.50% [39/40]); definition, background, and types of bias (97.50% [39/40]); manifestations of bias in the clinical setting (97.44% [38/39]); racial and ethnic disparities in dermatology (97.44% [38/39]); keloids (97.37% [37/38]); differences in dermoscopic presentations in skin of color (97.30% [36/37]); skin cancer in patients with skin of color (97.30% [36/37]); disparities due to bias (95.00% [38/40]); how to apply cultural humility and safety to patients of different cultural backgrounds (94.87% [37/40]); best practices in providing care to patients with limited English proficiency (94.87% [37/40]); hair loss in patients with textured hair (94.74% [36/38]); pseudofolliculitis barbae and acne keloidalis nuchae (94.60% [35/37]); disparities regarding people experiencing homelessness (92.31% [36/39]); and definitions and types of racism and other forms of discrimination (92.31% [36/39]). eTable 1 provides a list of suggested resources to incorporate these topics into the educational components of residency curricula. The resources provided were not part of the voting process, and they were not considered in the consensus analysis; they are included here as suggested educational catalysts.
During the second round of voting, 25 topics were evaluated. Of those, the following 6 topics were proposed to be included as essential in residency training: differences in prevalence and presentation of common inflammatory disorders (100% [29/29]); manifestations of bias in the learning environment (96.55%); antiracist action and how to decrease the effects of structural racism in clinical and educational settings (96.55% [28/29]); diversity of images in dermatology education (96.55% [28/29]); pigmentary disorders and their psychological effects (96.55% [28/29]); and LGBTQ (lesbian, gay, bisexual, transgender, and queer) dermatologic health care (96.55% [28/29]). eTable 2 includes these topics as well as suggested resources to help incorporate them into training.
Comment
This study utilized a modified e-Delphi technique to identify relevant clinical and nonclinical DEI topics that should be incorporated into dermatology residency curricula. The panel members reached a consensus for 9 clinical DEI-related topics. The respondents agreed that the topics related to skin and hair conditions in patients with skin of color as well as textured hair were crucial to residency education. Skin cancer, hair loss, pseudofolliculitis barbae, acne keloidalis nuchae, keloids, pigmentary disorders, and their varying presentations in patients with skin of color were among the recommended topics. The panel also recommended educating residents on the variable visual presentations of inflammatory conditions in skin of color. Addressing the needs of diverse patients—for example, those belonging to the LGBTQ community—also was deemed important for inclusion.
The remaining 14 chosen topics were nonclinical items addressing concepts such as bias and health care disparities as well as cultural humility and safety.9 Cultural humility and safety focus on developing cultural awareness by creating a safe setting for patients rather than encouraging power relationships between them and their physicians. Various topics related to racism also were recommended to be included in residency curricula, including education on implementation of antiracist action in the workplace.
Many of the nonclinical topics are intertwined; for instance, learning about health care disparities in patients with limited English proficiency allows for improved best practices in delivering care to patients from this population. The first step in overcoming bias and subsequent disparities is acknowledging how the perpetuation of bias leads to disparities after being taught tools to recognize it.
Our group’s guidance on DEI topics should help dermatology residency program leaders as they design and refine program curricula. There are multiple avenues for incorporating education on these topics, including lectures, interactive workshops, role-playing sessions, book or journal clubs, and discussion circles. Many of these topics/programs may already be included in programs’ didactic curricula, which would minimize the burden of finding space to educate on these topics. Institutional cultural change is key to ensuring truly diverse, equitable, and inclusive workplaces. Educating tomorrow’s dermatologists on these topics is a first step toward achieving that cultural change.
Limitations—A limitation of this e-Delphi survey is that only a selection of experts in this field was included. Additionally, we were concerned that the Likert scale format and the bar we set for inclusion and exclusion may have failed to adequately capture participants’ nuanced opinions. As such, participants were able to provide open-ended feedback, and suggestions for alternate wording or other changes were considered by the steering committee. Finally, inclusion recommendations identified in this survey were developed specifically for US dermatology residents.
Conclusion
In this e-Delphi consensus assessment of DEI-related topics, we recommend the inclusion of 23 topics into dermatology residency program curricula to improve medical training and the patient-physician relationship as well as to create better health outcomes. We also provide specific sample resource recommendations in eTables 1 and 2 to facilitate inclusion of these topics into residency curricula across the country.
- US Census Bureau projections show a slower growing, older, more diverse nation a half century from now. News release. US Census Bureau. December 12, 2012. Accessed August 14, 2024. https://www.census.gov/newsroom/releases/archives/population/cb12243.html#:~:text=12%2C%202012,U.S.%20Census%20Bureau%20Projections%20Show%20a%20Slower%20Growing%2C%20Older%2C%20More,by%20the%20U.S.%20Census%20Bureau
- Lopez S, Lourido JO, Lim HW, et al. The call to action to increase racial and ethnic diversity in dermatology: a retrospective, cross-sectional study to monitor progress. J Am Acad Dermatol. 2020;86:E121-E123. doi:10.1016/j.jaad.2021.10.011
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Street RL Jr, O’Malley KJ, Cooper LA, et al. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6:198-205. doi:10.1370/afm.821
- Dadrass F, Bowers S, Shinkai K, et al. Diversity, equity, and inclusion in dermatology residency. Dermatol Clin. 2023;41:257-263. doi:10.1016/j.det.2022.10.006
- Diversity and the Academy. American Academy of Dermatology website. Accessed August 22, 2024. https://www.aad.org/member/career/diversity
- SOCS speaks. Skin of Color Society website. Accessed August 22, 2024. https://skinofcolorsociety.org/news-media/socs-speaks
- Solchanyk D, Ekeh O, Saffran L, et al. Integrating cultural humility into the medical education curriculum: strategies for educators. Teach Learn Med. 2021;33:554-560. doi:10.1080/10401334.2021.1877711
- US Census Bureau projections show a slower growing, older, more diverse nation a half century from now. News release. US Census Bureau. December 12, 2012. Accessed August 14, 2024. https://www.census.gov/newsroom/releases/archives/population/cb12243.html#:~:text=12%2C%202012,U.S.%20Census%20Bureau%20Projections%20Show%20a%20Slower%20Growing%2C%20Older%2C%20More,by%20the%20U.S.%20Census%20Bureau
- Lopez S, Lourido JO, Lim HW, et al. The call to action to increase racial and ethnic diversity in dermatology: a retrospective, cross-sectional study to monitor progress. J Am Acad Dermatol. 2020;86:E121-E123. doi:10.1016/j.jaad.2021.10.011
- El-Kashlan N, Alexis A. Disparities in dermatology: a reflection. J Clin Aesthet Dermatol. 2022;15:27-29.
- Laveist TA, Nuru-Jeter A. Is doctor-patient race concordance associated with greater satisfaction with care? J Health Soc Behav. 2002;43:296-306.
- Street RL Jr, O’Malley KJ, Cooper LA, et al. Understanding concordance in patient-physician relationships: personal and ethnic dimensions of shared identity. Ann Fam Med. 2008;6:198-205. doi:10.1370/afm.821
- Dadrass F, Bowers S, Shinkai K, et al. Diversity, equity, and inclusion in dermatology residency. Dermatol Clin. 2023;41:257-263. doi:10.1016/j.det.2022.10.006
- Diversity and the Academy. American Academy of Dermatology website. Accessed August 22, 2024. https://www.aad.org/member/career/diversity
- SOCS speaks. Skin of Color Society website. Accessed August 22, 2024. https://skinofcolorsociety.org/news-media/socs-speaks
- Solchanyk D, Ekeh O, Saffran L, et al. Integrating cultural humility into the medical education curriculum: strategies for educators. Teach Learn Med. 2021;33:554-560. doi:10.1080/10401334.2021.1877711
PRACTICE POINTS
- Advancing curricula related to diversity, equity, and inclusion in dermatology training can improve health outcomes, address health care workforce disparities, and enhance clinical care for diverse patient populations.
- Education on patient-centered communication, cultural humility, and the impact of social determinants of health results in dermatology residents who are better equipped with the necessary tools to effectively care for patients from diverse backgrounds.
Melasma Risk Factors: A Matched Cohort Study Using Data From the All of Us Research Program
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.
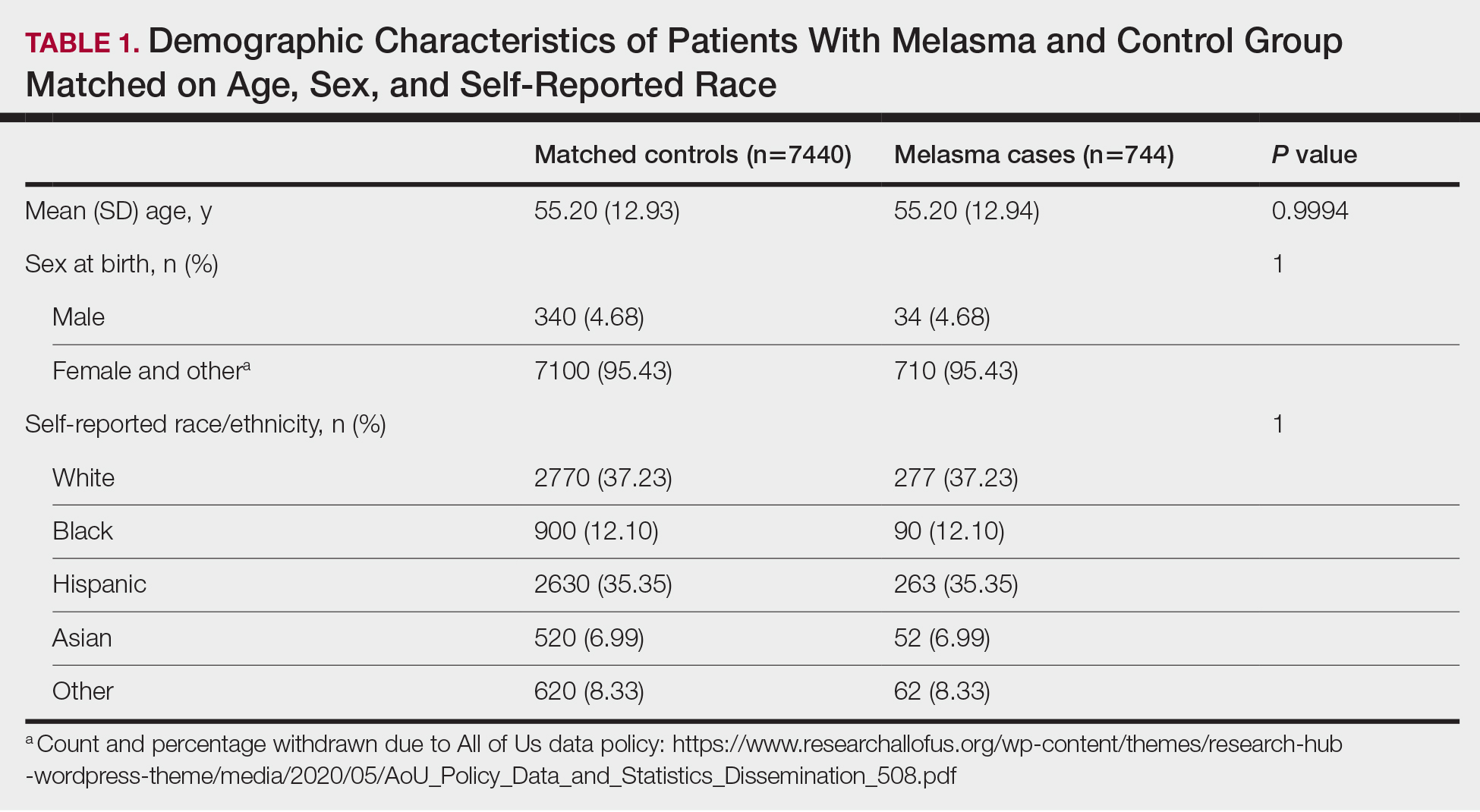
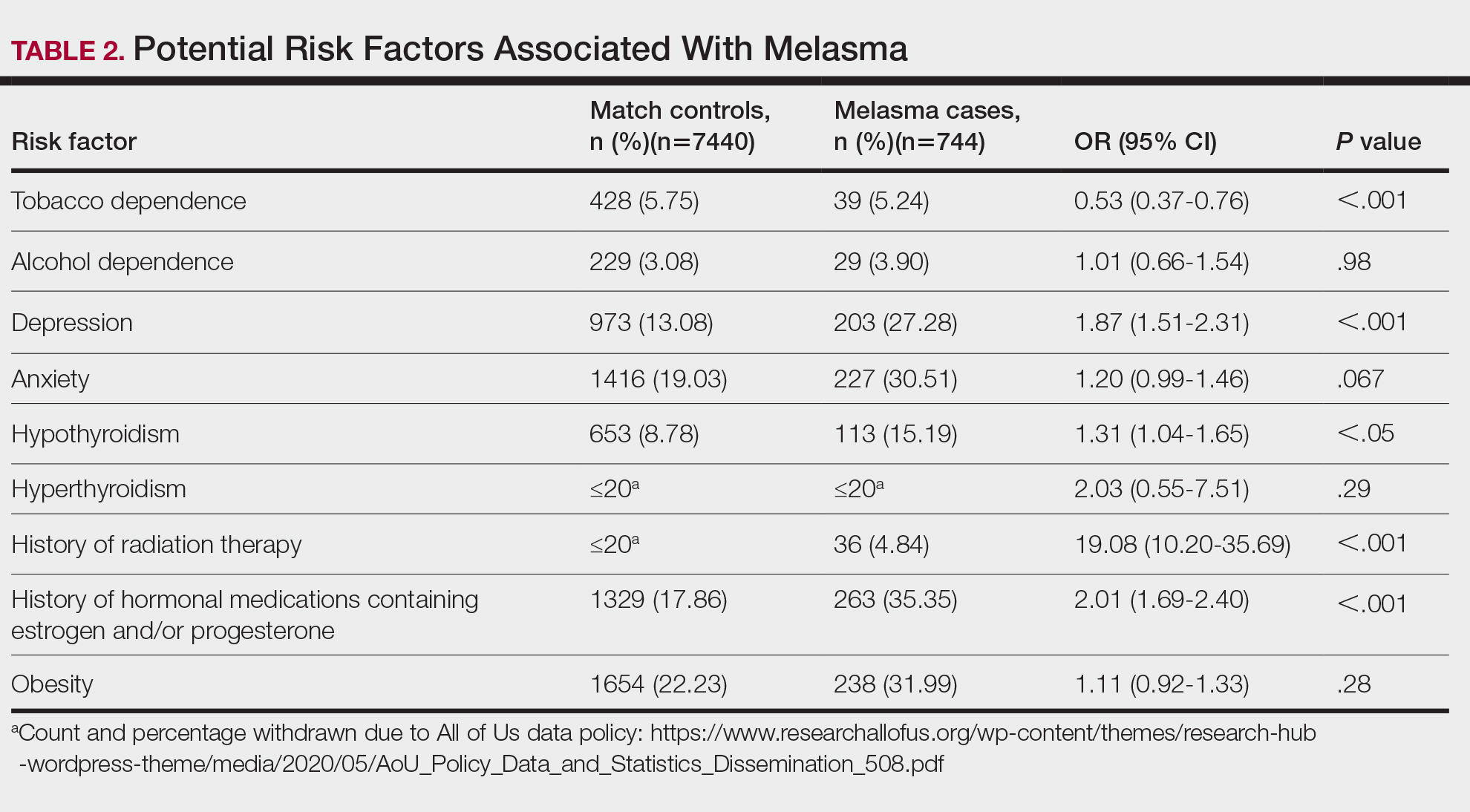
Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
To the Editor:
Melasma (also known as chloasma) is characterized by symmetric hyperpigmented patches affecting sun-exposed areas. Women commonly develop this condition during pregnancy, suggesting a connection between melasma and increased female sex hormone levels.1 Other hypothesized risk factors include sun exposure, genetic susceptibility, estrogen and/or progesterone therapy, and thyroid abnormalities but have not been corroborated.2 Treatment options are limited because the pathogenesis is poorly understood; thus, we aimed to analyze melasma risk factors using a national database with a nested case-control approach.
We conducted a matched case-control study using the Registered Tier dataset (version 7) from the National Institute of Health’s All of Us Research Program (https://allofus.nih.gov/), which is available to authorized users through the program’s Researcher Workbench and includes more than 413,000 total participants enrolled from May 1, 2018, through July 1, 2022. Cases included patients 18 years and older with a diagnosis of melasma (International Classification of Diseases, Tenth Revision, Clinical Modification code L81.1 [Chloasma]; concept ID 4264234 [Chloasma]; and Systematized Nomenclature of Medicine [SNOMED] code 36209000 [Chloasma]), and controls without a diagnosis of melasma were matched in a 1:10 ratio based on age, sex, and self-reported race. Concept IDs and SNOMED codes were used to identify individuals in each cohort with a diagnosis of alcohol dependence (concept IDs 433753, 435243, 4218106; SNOMED codes 15167005, 66590003, 7200002), depression (concept ID 440383; SNOMED code 35489007), hypothyroidism (concept ID 140673; SNOMED code 40930008), hyperthyroidism (concept ID 4142479; SNOMED code 34486009), anxiety (concept IDs 441542, 442077, 434613; SNOMED codes 48694002, 197480006, 21897009), tobacco dependence (concept IDs 37109023, 437264, 4099811; SNOMED codes 16077091000119107, 89765005, 191887008), or obesity (concept IDs 433736 and 434005; SNOMED codes 414916001 and 238136002), or with a history of radiation therapy (concept IDs 4085340, 4311117, 4061844, 4029715; SNOMED codes 24803000, 85983004, 200861004, 108290001) or hormonal medications containing estrogen and/or progesterone, including oral medications and implants (concept IDs 21602445, 40254009, 21602514, 21603814, 19049228, 21602529, 1549080, 1551673, 1549254, 21602472, 21602446, 21602450, 21602515, 21602566, 21602473, 21602567, 21602488, 21602585, 1596779, 1586808, 21602524). In our case cohort, diagnoses and exposures to treatments were only considered for analysis if they occurred prior to melasma diagnosis.
Multivariate logistic regression was performed to calculate odds ratios and P values between melasma and each comorbidity or exposure to the treatments specified. Statistical significance was set at P<.05.
We identified 744 melasma cases (mean age, 55.20 years; 95.43% female; 12.10% Black) and 7440 controls with similar demographics (ie, age, sex, race/ethnicity) between groups (all P>.05 [Table 1]). Patients with a melasma diagnosis were more likely to have a pre-existing diagnosis of depression (OR, 1.87; 95% CI, 1.51-2.31 [P<.001]) or hypothyroidism (OR, 1.31; 95% CI, 1.04-1.65 [P<.05]), or a history of radiation therapy (OR, 19.08; 95% CI, 10.20-35.69 [P<.001]) and/or estrogen and/or progesterone therapy (OR, 2.01; 95% CI, 1.69-2.40 [P<.001]) prior to melasma diagnosis. A diagnosis of anxiety prior to melasma diagnosis trended toward an association with melasma (P=.067). Pre-existing alcohol dependence, obesity, and hyperthyroidism were not associated with melasma (P=.98, P=.28, and P=.29, respectively). A diagnosis of tobacco dependence was associated with a decreased melasma risk (OR, 0.53, 95% CI, 0.37-0.76)[P<.001])(Table 2).
Our study results suggest that pre-existing depression was a risk factor for subsequent melasma diagnosis. Depression may exacerbate stress, leading to increased activation of the hypothalamic-pituitary-adrenal axis as well as increased levels of cortisol and adrenocorticotropic hormone, which subsequently act on melanocytes to increase melanogenesis.3 A retrospective study of 254 participants, including 127 with melasma, showed that increased melasma severity was associated with higher rates of depression (P=.002)2; however, the risk for melasma following a depression diagnosis has not been reported.
Our results also showed that hypothyroidism was associated with an increased risk for melasma. On a cellular level, hypothyroidism can cause systemic inflammation, potentailly leading to increased stress and melanogenesis via activation of the hypothalamic-pituitary-adrenal axis.4 These findings are similar to a systematic review and meta-analysis reporting increased thyroid-stimulating hormone, anti–thyroid peroxidase, and antithyroglobulin antibody levels associated with increased melasma risk (mean difference between cases and controls, 0.33 [95% CI, 0.18-0.47]; pooled association, P=.020; mean difference between cases and controls, 0.28 [95% CI, 0.01-0.55], respectively).5
Patients in our cohort who had a history of radiation therapy were 19 times more likely to develop melasma, similar to findings of a survey-based study of 421 breast cancer survivors in which 336 (79.81%) reported hyperpigmentation in irradiated areas.6 Patients in our cohort who had a history of estrogen and/or progesterone therapy were 2 times more likely to develop melasma, similar to a case-control study of 207 patients with melasma and 207 controls that showed combined oral contraceptives increased risk for melasma (OR, 1.23 [95% CI, 1.08-1.41; P<.01).3
Tobacco use is not a well-known protective factor against melasma. Prior studies have indicated that tobacco smoking activates melanocytes via the Wnt/β-Catenin pathway, leading to hyperpigmentation.7 Although exposure to cigarette smoke decreases angiogenesis and would more likely lead to hyperpigmentation, nicotine exposure has been shown to increase angiogenesis, which could lead to increased blood flow and partially explain the protection against melasma demonstrated in our cohort.8 Future studies are needed to explore this relationship.


Limitations of our study include lack of information about melasma severity and information about prior melasma treatment in our cohort as well as possible misdiagnosis reported in the dataset.
Our results demonstrated that pre-existing depression and hypothyroidism as well as a history of radiation or estrogen and/or progesterone therapies are potential risk factors for melasma. Therefore, we recommend that patients with melasma be screened for depression and thyroid dysfunction, and patients undergoing radiation therapy or starting estrogen and/or progesterone therapy should be counseled on their increased risk for melasma. Future studies are needed to determine whether treatment of comorbidities such as hypothyroidism and depression improve melasma severity. The decreased risk for melasma associated with tobacco use also requires further investigation.
Acknowledgments—The All of Us Research Program is supported by the National Institutes of Health, Office of the Director: Regional Medical Centers: 1 OT2 OD026549; 1 OT2 OD026554; 1 OT2 OD026557; 1 OT2 OD026556; 1 OT2 OD026550; 1 OT2 OD 026552; 1 OT2 OD026553; 1 OT2 OD026548; 1 OT2 OD026551; 1 OT2 OD026555; IAA #: AOD 16037; Federally Qualified Health Centers: HHSN 263201600085U; Data and Research Center: 5 U2C OD023196; Biobank: 1 U24 OD023121; The Participant Center: U24 OD023176; Participant Technology Systems Center: 1 U24 OD023163; Communications and Engagement: 3 OT2 OD023205; 3 OT2 OD023206; and Community Partners: 1 OT2 OD025277; 3 OT2 OD025315; 1 OT2 OD025337; 1 OT2 OD025276.
In addition, the All of Us Research Program would not be possible without the partnership of its participants, who we gratefully acknowledge for their contributions and without whom this research would not have been possible. We also thank the All of Us Research Program for making the participant data examined in this study available to us.
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
- Filoni A, Mariano M, Cameli N. Melasma: how hormones can modulate skin pigmentation. J Cosmet Dermatol. 2019;18:458-463. doi:10.1111/jocd.12877
- Platsidaki E, Efstathiou V, Markantoni V, et al. Self-esteem, depression, anxiety and quality of life in patients with melasma living in a sunny mediterranean area: results from a prospective cross-sectional study. Dermatol Ther (Heidelb). 2023;13:1127-1136. doi:10.1007/s13555-023-00915-1
- Handel AC, Lima PB, Tonolli VM, et al. Risk factors for facial melasma in women: a case-control study. Br J Dermatol. 2014;171:588-594. doi:10.1111/bjd.13059
- Erge E, Kiziltunc C, Balci SB, et al. A novel inflammatory marker for the diagnosis of Hashimoto’s thyroiditis: platelet-count-to-lymphocyte-count ratio (published January 22, 2023). Diseases. 2023;11:15. doi:10.3390/diseases11010015
- Kheradmand M, Afshari M, Damiani G, et al. Melasma and thyroid disorders: a systematic review and meta-analysis. Int J Dermatol. 2019;58:1231-1238. doi:10.1111/ijd.14497
- Chu CN, Hu KC, Wu RS, et al. Radiation-irritated skin and hyperpigmentation may impact the quality of life of breast cancer patients after whole breast radiotherapy (published March 31, 2021). BMC Cancer. 2021;21:330. doi:10.1186/s12885-021-08047-5
- Nakamura M, Ueda Y, Hayashi M, et al. Tobacco smoke-induced skin pigmentation is mediated by the aryl hydrocarbon receptor. Exp Dermatol. 2013;22:556-558. doi:10.1111/exd.12170
- Ejaz S, Lim CW. Toxicological overview of cigarette smoking on angiogenesis. Environ Toxicol Pharmacol. 2005;20:335-344. doi:10.1016/j.etap.2005.03.011
Practice Points
- Treatment options for melasma are limited due to its poorly understood pathogenesis.
- Depression and hypothyroidism and/or history of exposure to radiation and hormonal therapies may increase melasma risk.
- We recommend that patients with melasma be screened for depression and thyroid dysfunction. Patients undergoing radiation therapy or starting estrogen and/ or progesterone therapy should be counseled on the increased risk for melasma.
Moving Beyond Traditional Methods for Treatment of Acne Keloidalis Nuchae
The Comparison
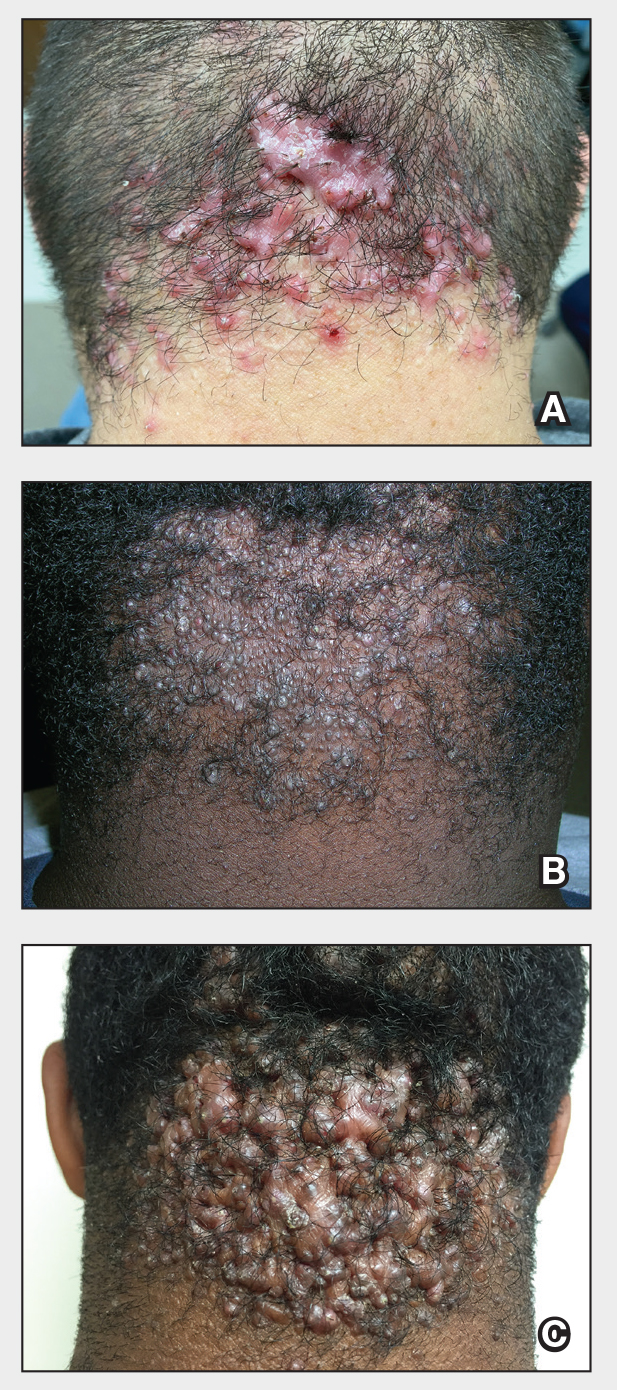
Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
The Comparison

Acne keloidalis nuchae (AKN) is a chronic inflammatory condition commonly affecting the occipital scalp and posterior neck. It causes discrete or extensive fibrosing papules that may coalesce to form pronounced tumorlike masses1,2 with scarring alopecia (Figure, A–C).3 Pustules, hair tufts, secondary bacterial infections, abscesses, and sinus tracts also may occur.1 The pathogenesis of AKN has been characterized as varying stages of follicular inflammation at the infundibular and isthmus levels followed by fibrotic occlusion of the follicular lumen.4 Pruritus, pain, bleeding, oozing, and a feeling of scalp tightness may occur.1,5
Umar et al6 performed a retrospective review of 108 men with AKN—58% of African descent, 37% Hispanic, 3% Asian, and 2% Middle Eastern—and proposed a 3-tier classification system for AKN. Tier 1 focused on the distribution and sagittal spread of AKN lesions between the clinical demarcation lines of the occipital notch and posterior hairline. Tier 2 focused on the type of lesions present—discrete papules or nodules, coalescing/abutting lesions, plaques (raised, atrophic, or indurated), or dome-shaped tumoral masses. Tier 3 focused on the presence or absence of co-existing dissecting cellulitis or folliculitis decalvans.6
Epidemiology
Acne keloidalis nuchae primarily manifests in adolescent and adult men of African or Afro-Caribbean descent.7 Among African American men, the prevalence of AKN ranges from 0.5% to 13.6%.8 Similar ranges have been reported among Nigerian, South African, and West African men.1 Acne keloidalis nuchae also affects Asian and Hispanic men but rarely is seen in non-Hispanic White men or in women of any ethnicity.9,10 The male to female ratio is 20:1.1,11 Hair texture, hairstyling practices such as closely shaved or faded haircuts, and genetics likely contribute to development of AKN. Sports and occupations that require the use of headgear or a tight collar may increase the risk for AKN.12
Key clinical features in people with darker skin tones
- The lesions of AKN range in color from pink to dark brown or black. Postinflammatory hyperpigmentation or hyperchromia may be present around AKN lesions.
- Chronicity of AKN may lead to extended use of high-potency topical or intralesional corticosteroids, which causes transient or long-lasting hypopigmentation, especially in those with darker skin tones.
Worth noting
- Acne keloidalis nuchae can be disfiguring, which negatively impacts quality of life and self-esteem.12
- Some occupations (eg, military, police) have hair policies that may not be favorable to those with or at risk for AKN.
- Patients with AKN are 2 to 3 times more likely to present with metabolic syndrome, hypertension, type 2 diabetes mellitus, or obesity.13
Treatment
There are no treatments approved by the US Food and Drug Administration specifically for AKN. Treatment approaches are based on the pathophysiology, secondary impacts on the skin, and disease severity. Growing out the hair may prevent worsening and/or decrease the risk for new lesions.6
- Options include but are not limited to topical and systemic therapies (eg, topical corticosteroids, oral or topical antibiotics, isotretinoin, topical retinoids, imiquimod, pimecrolimus), light devices (eg, phototherapy, laser), ablative therapies (eg, laser, cryotherapy, radiotherapy), and surgery (eg, excision, follicular unit excision), often in combination.6,14,15
- Intralesional triamcinolone injections are considered standard of care. Adotama et al16 found that injecting triamcinolone into the deep dermis in the area of flat or papular AKN yielded better control of inflammation and decreased appearance of lesions compared with injecting individual lesions.
- For extensive AKN lesions that do not respond to less-invasive therapies, consider surgical techniques,6,17 such as follicular unit excision18 and more extensive surgical excisions building on approaches from pioneers Drs. John Kenney and Harold Pierce.19 An innovative surgical approach for removal of large AKNs is the bat excision technique—wound shape resembles a bat in a spread-eagled position—with secondary intention healing with or without debridement and/or tension sutures. The resulting linear scar acts as a new posterior hair line.20
Health disparity highlights
Access to a dermatologic or plastic surgeon with expertise in the surgical treatment of large AKNs may be challenging but is needed to reduce risk for recurrence and adverse events.
Close-cropped haircuts on the occipital scalp, which are particularly popular among men of African descent, increase the risk for AKN.5 Although this grooming style may be a personal preference, other hairstyles commonly worn by those with tightly coiled hair may be deemed “unprofessional” in society or the workplace,21 which leads to hairstyling practices that may increase the risk for AKN.
Acne keloidalis nuchae remains an understudied entity that adversely affects patients with skin of color.
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
- Ogunbiyi A. Acne keloidalis nuchae: prevalence, impact, and management challenges. Clin Cosmet Investig Dermatol. 2016;9:483-489. doi:10.2147/CCID.S99225
- Al Aboud DM, Badri T. Acne keloidalis nuchae. In: StatPearls [Internet]. Updated July 31, 2023. Accessed August 2, 2024. https://www.ncbi.nlm.nih.gov/books/NBK459135/ 3.
- Sperling LC, Homoky C, Pratt L, et al. Acne keloidalis is a form of primary scarring alopecia. Arch Dermatol. 2000;136:479-484.
- Herzberg AJ, Dinehart SM, Kerns BJ, et al. Acne keloidalis: transverse microscopy, immunohistochemistry, and electron microscopy. Am J Dermatopathol. 1990;12:109-121. doi:10.1097/00000372-199004000-00001
- Saka B, Akakpo A-S, Téclessou JN, et al. Risk factors associated with acne keloidalis nuchae in black subjects: a case-control study. Ann Dermatol Venereol. 2020;147:350-354. doi:10.1016/j.annder.2020.01.007
- Umar S, Lee DJ, Lullo JJ. A retrospective cohort study and clinical classification system of acne keloidalis nuchae. J Clin Aesthet Dermatol. 2021;14:E61-E67.
- Reja M, Silverberg NB. Acne keloidalis nuchae. In: Silverberg NB, Durán-McKinster C, Tay YK, eds. Pediatric Skin of Color. Springer; 2015:141-145. doi:10.1007/978-1-4614-6654-3_16 8.
- Knable AL Jr, Hanke CW, Gonin R. Prevalence of acne keloidalis nuchae in football players. J Am Acad Dermatol. 1997;37:570-574. doi:10.1016/s0190-9622(97)70173-7
- Umar S, Ton D, Carter MJ, et al. Unveiling a shared precursor condition for acne keloidalis nuchae and primary cicatricial alopecias. Clin Cosmet Investig Dermatol. 2023;16:2315-2327. doi:10.2147/CCID.S422310
- Na K, Oh SH, Kim SK. Acne keloidalis nuchae in Asian: a single institutional experience. PLoS One. 2017;12:e0189790. doi:10.1371/journal.pone.0189790
- Ogunbiyi A, George A. Acne keloidalis in females: case report and review of literature. J Natl Med Assoc. 2005;97:736-738.
- Alexis A, Heath CR, Halder RM. Folliculitis keloidalis nuchae and pseudofolliculitis barbae: are prevention and effective treatment within reach? Dermatol Clin. 2014;32:183-191. doi:10.1016/j.det.2013.12.001
- Kridin K, Solomon A, Tzur-Bitan D, et al. Acne keloidalis nuchae and the metabolic syndrome: a population-based study. Am J Clin Dermatol. 2020;21:733-739. doi:10.1007/s40257-020-00541-z
- Smart K, Rodriguez I, Worswick S. Comorbidities and treatment options for acne keloidalis nuchae. Dermatol Ther. Published online May 25, 2024. doi:10.1155/2024/8336926
- Callender VD, Young CM, Haverstock CL, et al. An open label study of clobetasol propionate 0.05% and betamethasone valerate 0.12% foams in the treatment of mild to moderate acne keloidalis. Cutis. 2005;75:317-321.
- Adotama P, Grullon K, Ali S, et al. How we do it: our method for triamcinolone injections of acne keloidalis nuchae. Dermatol Surg. 2023;49:713-714. doi:10.1097/DSS.0000000000003803
- Beckett N, Lawson C, Cohen G. Electrosurgical excision of acne keloidalis nuchae with secondary intention healing. J Clin Aesthet Dermatol. 2011;4:36-39.
- Esmat SM, Abdel Hay RM, Abu Zeid OM, et al. The efficacy of laser-assisted hair removal in the treatment of acne keloidalis nuchae; a pilot study. Eur J Dermatol. 2012;22:645-650. doi:10.1684/ejd.2012.1830
- Dillard AD, Quarles FN. African-American pioneers in dermatology. In: Taylor SC, Kelly AP, Lim HW, et al, eds. Dermatology for Skin of Color. 2nd ed. McGraw-Hill Education; 2016:717-730.
- Umar S, David CV, Castillo JR, et al. Innovative surgical approaches and selection criteria of large acne keloidalis nuchae lesions. Plast Reconstr Surg Glob Open. 2019;7:E2215. doi:10.1097/GOX.0000000000002215
- Lee MS, Nambudiri VE. The CROWN act and dermatology: taking a stand against race-based hair discrimination. J Am Acad Dermatol. 2021;84:1181-1182. doi:10.1016/j.jaad.2020.11.065
Benefits of High-Dose Vitamin D in Managing Cutaneous Adverse Events Induced by Chemotherapy and Radiation Therapy
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22=5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22=13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22=5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22=13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
Vitamin D (VD) regulates keratinocyte proliferation and differentiation, modulates inflammatory pathways, and protects against cellular damage in the skin. 1 In the setting of tissue injury and acute skin inflammation, active vitamin D—1,25(OH) 2 D—suppresses signaling from pro-inflammatory chemokines and cytokines such as IFN- γ and IL-17. 2,3 This suppression reduces proliferation of helper T cells (T H 1, T H 17) and B cells, decreasing tissue damage from reactive oxygen species release while enhancing secretion of the anti-inflammatory cytokine IL-10 by antigen-presenting cells. 2-4
Suboptimal VD levels have been associated with numerous health consequences including malignancy, prompting interest in VD supplementation for improving cancer-related outcomes.5 Beyond disease prognosis, high-dose VD supplementation has been suggested as a potential therapy for adverse events (AEs) related to cancer treatments. In one study, mice that received oral vitamin D3 supplementation of 11,500 IU/kg daily had fewer doxorubicin-induced cardiotoxic effects on ejection fraction (P<.0001) and stroke volume (P<.01) than mice that received VD supplementation of 1500 IU/kg daily.6
In this review, we examine the impact of chemoradiation on 25(OH)D levels—which more accurately reflects VD stores than 1,25(OH)2D levels—and the impact of suboptimal VD on cutaneous toxicities related to chemoradiation. To define the suboptimal VD threshold, we used the Endocrine Society’s clinical practice guidelines, which characterize suboptimal 25(OH)D levels as insufficiency (21–29 ng/mL [52.5–72.5 nmol/L]) or deficiency (<20 ng/mL [50 nmol/L])7; deficiency can be further categorized as severe deficiency (<12 ng/mL [30 nmol/L]).8 This review also evaluates the evidence for vitamin D3 supplementation to alleviate the cutaneous AEs of chemotherapy and radiation treatments.
Effects of Chemotherapy on Vitamin D Levels
A high prevalence of VD deficiency is seen in various cancers. In a retrospective review of 25(OH)D levels in 2098 adults with solid tumors of any stage (6% had metastatic disease [n=124]), suboptimal levels were found in 69% of patients with breast cancer (n=617), 75% with colorectal cancer (n=84), 72% with gynecologic cancer (n=65), 79% with kidney and bladder cancer (n=145), 83% with pancreatic and upper gastrointestinal tract cancer (n=178), 73% with lung cancer (n=73), 69% with prostate cancer (n=225), 61% with skin cancer (n=399), and 63% with thyroid cancer (n=172).5 Suboptimal VD also has been found in hematologic malignancies. In a prospective cohort study, mean serum 25(OH)D levels in 23 patients with recently diagnosed acute myeloid leukemia demonstrated VD deficiency (mean [SD], 18.6 [6.6] nmol/L).9 Given that many patients already exhibit a baseline VD deficiency at cancer diagnosis, it is important to understand the relationship between VD and cancer treatment modalities.5
In the United States, breast and colorectal cancers were estimated to be the first and fourth most common cancers, with 313,510 and 152,810 predicted new cases in 2024, respectively.10 This review will focus on breast and colorectal cancer when describing VD variation associated with chemotherapy exposure due to their high prevalence.
Effects of Chemotherapy on Vitamin D Levels in Breast Cancer—Breast cancer studies have shown suboptimal VD levels in 76% of females 75 years or younger with any T1, T2, or T3; N0 or N1; and M0 breast cancer, in which 38.5% (n=197) had insufficient and 37.5% (n=192) had deficient 25(OH)D levels.11 In a study of female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), VD deficiency was seen in 60% of patients not receiving VD supplementation.12,13 A systematic review that included 7 studies of different types of breast cancer suggested that circulating 25(OH)D may be associated with improved prognosis.14 Thus, studies have investigated risk factors associated with poor or worsening VD status in individuals with breast cancer, including exposure to chemotherapy and/or radiation treatment.12,15-18
A prospective cohort study assessed 25(OH)D levels in 95 patients with any breast cancer (stages I, II, IIIA, IIIB) before and after initiating chemotherapy with docetaxel, doxorubicin, epirubicin, 5-fluorouracil, or cyclophosphamide, compared with a group of 52 females without cancer.17 In the breast cancer group, approximately 80% (76/95) had suboptimal and 50% (47/95) had deficient VD levels before chemotherapy initiation (mean [SD], 54.1 [22.8] nmol/L). In the comparison group, 60% (31/52) had suboptimal and 30% (15/52) had deficient VD at baseline (mean [SD], 66.1 [23.5] nmol/L), which was higher than the breast cancer group (P=.03). A subgroup analysis excluded participants who started, stopped, or lacked data on dietary supplements containing VD (n=39); in the remaining 56 participants, a significant decrease in 25(OH)D levels was observed shortly after finishing chemotherapy compared with the prechemotherapy baseline value (mean, −7.9 nmol/L; P=.004). Notably, 6 months after chemotherapy completion, 25(OH)D levels increased (mean, +12.8 nmol/L; P<.001). Vitamin D levels remained stable in the comparison group (P=.987).17
Consistent with these findings, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of chemotherapy was associated with increased odds of 25(OH)D levels less than 20 ng/mL compared with breast cancer patients with no prior chemotherapy (odds ratio, 1.86; 95% CI, 1.03-3.38).12 Although the study data included chemotherapy history, no information was provided on specific chemotherapy agents or regimens used in this cohort, limiting the ability to detect the drugs most often implicated.
Both studies indicated a complex interplay between chemotherapy and VD levels in breast cancer patients. Although Kok et al17 suggested a transient decrease in VD levels during chemotherapy with a subsequent recovery after cessation, Fassio et al12 highlighted the increased odds of VD deficiency associated with chemotherapy. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status in breast cancer patients.
Effects of Chemotherapy on Vitamin D Levels in Colorectal Cancer—Similar to patterns seen in breast cancer, a systematic review with 6 studies of different types of colorectal cancer suggested that circulating 25(OH)D levels may be associated with prognosis.14 Studies also have investigated the relationship between colorectal chemotherapy regimens and VD status.15,16,18,19
A retrospective study assessed 25(OH)D levels in 315 patients with any colorectal cancer (stage I–IV).15 Patients were included in the analysis if they received less than 400 IU daily of VD supplementation at baseline. For the whole study sample, the mean (SD) VD level was 23.7 (13.71) ng/mL. Patients who had not received chemotherapy within 3 months of the VD level assessment were categorized as the no chemotherapy group, and the others were designated as the chemotherapy group; the latter group was exposed to various chemotherapy regimens, including combinations of irinotecan, oxaliplatin, 5-fluorouracil, leucovorin, bevacizumab, or cetuximab. Multivariate analysis showed that the chemotherapy group was 3.7 times more likely to have very low VD levels (≤15 ng/mL) compared with those in the no chemotherapy group (P<.0001).15
A separate cross-sectional study examined serum 25(OH)D concentrations in 1201 patients with any newly diagnosed colorectal carcinoma (stage I–III); 91% of cases were adenocarcinoma.18 In a multivariate analysis, chemotherapy plus surgery was associated with lower VD levels than surgery alone 6 months after diagnosis (mean, −8.74 nmol/L; 95% CI, −11.30 to −6.18 nmol/L), specifically decreasing by a mean of 6.7 nmol/L (95% CI, −9.8 to −3.8 nmol/L) after adjusting for demographic and lifestyle factors.18 However, a prospective cohort study demonstrated different findings.19 Comparing 58 patients with newly diagnosed colorectal adenocarcinoma (stages I–IV) who underwent chemotherapy and 36 patients who did not receive chemotherapy, there was no significant change in 25(OH)D levels from the time of diagnosis to 6 months later. Median VD levels decreased by 0.7 ng/mL in those who received chemotherapy, while a minimal (and not significant) increase of 1.6 ng/mL was observed in those without chemotherapy intervention (P=.26). Notably, supplementation was not restricted in this cohort, which may have resulted in higher VD levels in those taking supplements.19
Since time of year and geographic location can influence VD levels, one prospective cohort study controlled for differential sun exposure due to these factors in their analysis.16 Assessment of 25(OH)D levels was completed in 81 chemotherapy-naïve cancer patients immediately before beginning chemotherapy as well as 6 and 12 weeks into treatment. More than 8 primary cancer types were represented in this study, with breast (34% [29/81]) and colorectal (14% [12/81]) cancer being the most common, but the cancer stages of the participants were not detailed. Vitamin D levels decreased after commencing chemotherapy, with the largest drop occurring 6 weeks into treatment. From the 6- to 12-week end points, VD increased but remained below the original baseline level (baseline: mean [SD], 49.2 [22.3] nmol/L; 6 weeks: mean [SD], 40.9 [19.0] nmol/L; 12 weeks: mean [SD], 45.9 [19.7] nmol/L; P=.05).16
Although focused on breast and colorectal cancers, these studies suggest that various chemotherapy regimens may confer a higher risk for VD deficiency compared with VD status at diagnosis and/or prior to chemotherapy treatment. However, most of these studies only discussed stage-based differences, excluding analysis of the variety of cancer subtypes that comprise breast and colorectal malignancies, which may limit our ability to extrapolate from these data. Ultimately, larger randomized controlled trials are needed to better understand the relationship between chemotherapy and VD status across various primary cancer types.
Effects of Radiation Therapy on Vitamin D Levels
Unlike chemotherapy, studies on the association between radiation therapy and VD levels are minimal, with most reports in the literature discussing the use of VD to potentiate the effects of radiation therapy. In one cross-sectional analysis of 1201 patients with newly diagnosed stage I, II, or III colorectal cancer of any type (94% were adenocarcinoma), radiation plus surgery was associated with slightly lower 25(OH)D levels than surgery alone for tumor treatment 6 months after diagnosis (mean, −3.17; 95% CI, −6.07 to −0.28 nmol/L). However, after adjustment for demographic and lifestyle factors, this decrease in VD levels attributable to radiotherapy was not statistically significant compared with the surgery-only cohort (mean, −1.78; 95% CI, −5.07 to 1.52 nmol/L).18
Similarly, a cross-sectional study assessing VD status in 394 female patients with primary breast cancer (stage I, II, or III and T1 with high Ki67 expression [≥30%], T2, or T3), found that a history of radiotherapy was not associated with a difference in serum 25(OH)D levels compared with those with breast cancer without prior radiotherapy (odds ratio, 0.90; 95% CI, 0.52-1.54).12 From the limited existing literature specifically addressing variations of VD levels with radiation, radiation therapy does not appear to significantly impact VD levels.
Vitamin D Levels and the Severity of Chemotherapy- or Radiation Therapy–Induced AEs
A prospective cohort of 241 patients did not find an increase in the incidence or severity of chemotherapy-induced cutaneous toxicities in those with suboptimal 1,25(OH)2D3 levels (≤75 nmol/L).20 Eight different primary cancer types were represented, including breast and colorectal cancer; the tumor stages of the participants were not detailed. Forty-one patients had normal 1,25(OH)2D3 levels, while the remaining 200 had suboptimal levels. There was no significant association between serum VD levels and the following dermatologic toxicities: desquamation (P=.26), xerosis (P=.15), mucositis (P=.30), or painful rash (P=.87). Surprisingly, nail changes and hand-foot reactions occurred with greater frequency in patients with normal VD levels (P=.01 and P=.03, respectively).20 Hand-foot reaction is part of the toxic erythema of chemotherapy (TEC) spectrum, which is comprised of a range of cytotoxic skin injuries that typically manifest within 2 to 3 weeks of exposure to the offending chemotherapeutic agents, often characterized by erythema, pain, swelling, and blistering, particularly in intertriginous and acral areas.21-23 Recovery from TEC generally takes at least 2 to 4 weeks and may necessitate cessation of the offending chemotherapeutic agent.21,24 Notably, this study measured 1,25(OH)2D3 levels instead of 25(OH)D levels, which may not reliably indicate body stores of VD.7,20 These results underscore the complex nature between chemotherapy and VD; however, VD levels alone do not appear to be a sufficient biomarker for predicting chemotherapy-associated cutaneous AEs.
Interestingly, radiation therapy–induced AEs may be associated with VD levels. A prospective cohort study of 98 patients with prostate, bladder, or gynecologic cancers (tumor stages were not detailed) undergoing pelvic radiotherapy found that females and males with 25(OH)D levels below a threshold of 35 and 40 nmol/L, respectively, were more likely to experience higher Radiation Therapy Oncology Group (RTOG) grade acute proctitis compared with those with VD above these thresholds.25 Specifically, VD below these thresholds was associated with increased odds of RTOG grade 2 or higher radiation-induced proctitis (OR, 3.07; 95% CI, 1.27-7.50 [P=.013]). Additionally, a weak correlation was noted between VD below these thresholds and the RTOG grade, with a Spearman correlation value of −0.189 (P=.031).25
One prospective cohort study included 28 patients with any cancer of the oral cavity, oropharynx, hypopharynx, or larynx stages II, III, or IVA; 93% (26/28) were stage III or IVA.26 The 20 (71%) patients with suboptimal 25(OH)D levels (≤75 nmol/L) experienced a higher prevalence of grade II radiation dermatitis compared with the 8 (29%) patients with optimal VD levels (χ22=5.973; P=.0505). This pattern persisted with the severity of mucositis; patients from the suboptimal VD group presented with higher rates of grades II and III mucositis compared with the VD optimal group (χ22=13.627; P=.0011).26 Recognizing the small cohort evaluated in the study, we highlight the importance of further studies to clarify these associations.
Chemotherapy-Induced Cutaneous Events Treated with High-Dose Vitamin D
Chemotherapeutic agents are known to induce cellular damage, resulting in a range of cutaneous AEs that can invoke discontinuation of otherwise effective chemotherapeutic interventions.27,28 Recent research has explored the potential of high-dose vitamin D3 as a therapeutic agent to mitigate cutaneous reactions.29,30
A randomized, double-blind, placebo-controlled trial investigated the use of a single high dose of oral 25(OH)D to treat topical nitrogen mustard (NM)–induced rash.29 To characterize baseline inflammatory responses from NM injury without intervention, clinical measures, serum studies, and tissue analyses from skin biopsies were performed on 28 healthy adults after exposure to topical NM—a chemotherapeutic agent classified as a DNA alkylator. Two weeks later, participants were exposed to topical NM a second time and were split into 2 groups: 14 patients received a single 200,000-IU dose of oral 25(OH)D while the other 14 participants were given a placebo. Using the inflammatory markers induced from baseline exposure to NM alone, posttreatment analysis revealed that the punch biopsies from
Although Ernst et al29 did not observe any clinically significant improvements with VD treatment, a case series of 6 patients with either glioblastoma multiforme, acute myeloid leukemia, or aplastic anemia did demonstrate clinical improvement of TEC after receiving high-dose vitamin D3.30 The mean time to onset of TEC was noted at 8.5 days following administration of the inciting chemotherapeutic agent, which included combinations of anthracycline, antimetabolite, kinase inhibitor, B-cell lymphoma 2 inhibitor, purine analogue, and alkylating agents. A combination of clinical and histologic findings was used to diagnose TEC. Baseline 25(OH)D levels were not established prior to treatment. The treatment regimen for 1 patient included 2 doses of 50,000 IU of VD spaced 1 week apart, totaling 100,000 IU, while the remaining 5 patients received a total of 200,000 IU, also split into 2 doses given 1 week apart. All patients received their first dose of VD within a week of the cutaneous eruption. Following the initial VD dose, there was a notable improvement in pain, pruritus, or swelling by the next day. Reduction in erythema also was observed within 1 to 4 days.30
No AEs associated with VD supplementation were reported, suggesting a potential beneficial role of high-dose VD in accelerating recovery from chemotherapy-induced rashes without evident safety concerns.
Radiation Therapy–Induced Cutaneous Events Treated with High-Dose Vitamin D
Radiation dermatitis is a common and often severe complication of radiation therapy that affects more than 90% of patients undergoing treatment, with half of these individuals experiencing grade 2 toxicity, according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events.31,32 Radiation damage to basal keratinocytes and hair follicle stem cells disrupts the renewal of the skin’s outer layer, while a surge of free radicals causes irreversible DNA damage.33 Symptoms of radiation dermatitis can vary from mild pink erythema to tissue ulceration and necrosis, typically within 1 to 4 weeks of radiation exposure.34 The resulting dermatitis can take 2 to 4 weeks to heal, notably impacting patient quality of life and often necessitating modifications or interruptions in cancer therapy.33
Prior studies have demonstrated the use of high-dose VD to improve the healing of UV-irradiated skin. A randomized controlled trial investigated high-dose vitamin D3 to treat experimentally induced sunburn in 20 healthy adults. Compared with those who received a placebo, participants receiving the oral dose of 200,000 IU of vitamin D3 demonstrated suppression of the pro-inflammatory mediators tumor necrosis factor α (P=.04) and inducible nitric oxide synthase (P=.02), while expression of tissue repair enhancer arginase 1 was increased (P<.005).35 The mechanism of this enhanced tissue repair was investigated using a mouse model, in which intraperitoneal 25(OH)D was administered following severe UV-induced skin injury. On immunofluorescence microscopy, mice treated with VD showed enhanced autophagy within the macrophages infiltrating UV-irradiated skin.36 The use of high-dose VD to treat UV-irradiated skin in these studies established a precedent for using VD to heal cutaneous injury caused by ionizing radiation therapy.
Some studies have focused on the role of VD for treating acute radiation dermatitis. A study of 23 patients with ductal carcinoma in situ or localized invasive ductal carcinoma breast cancer compared the effectiveness of topical calcipotriol to that of a standard hydrating ointment.37 Participants were randomized to 1 of 2 treatments before starting adjuvant radiotherapy to evaluate their potential in preventing radiation dermatitis. In 87% (20/23) of these patients, no difference in skin reaction was observed between the 2 treatments, suggesting that topical VD application may not offer any advantage over the standard hydrating ointment for the prevention of radiation dermatitis.37
Benefits of high-dose oral VD for treating radiation dermatitis also have been reported. Nguyen et al38 documented 3 cases in which patients with neuroendocrine carcinoma of the pancreas, tonsillar carcinoma, and breast cancer received 200,000 IU of oral ergocalciferol distributed over 2 doses given 7 days apart for radiation dermatitis. These patients experienced substantial improvements in pain, swelling, and redness within a week of the initial dose. Additionally, a case of radiation recall dermatitis, which occurred a week after vinorelbine chemotherapy, was treated with 2 doses totaling 100,000 IU of oral ergocalciferol. This patient also had improvement in pain and swelling but continued to have tumor-related induration and ulceration.39
Although topical VD did not show significant benefits over standard treatments for radiation dermatitis, high-dose oral VD appears promising in improving patient outcomes of pain and swelling more rapidly than current practices. Further research is needed to confirm these findings and establish standardized treatment protocols.
Final Thoughts
Suboptimal VD levels are prevalent in numerous cancer types. Chemotherapy often is associated with acute, potentially transient worsening of VD status in patients with breast and colorectal cancer. Although 25(OH)D levels have not corresponded with increased frequency of chemotherapy-related dermatologic AEs, suboptimal 25(OH)D levels appear to be associated with increased severity of radiation-induced mucositis and dermatitis.20,25,26 The use of high-dose VD as a therapeutic agent shows promise in mitigating chemotherapy-induced and radiation therapy–induced rashes in multiple cancer types with reduction of inflammatory markers and a durable anti-inflammatory impact. Although the mechanisms of cellular injury vary among chemotherapeutic agents, the anti-inflammatory and tissue repair properties of VD may make it an effective treatment for chemotherapy-induced cutaneous damage regardless of injury mechanism.2-4,35 However, reports of clinical improvement vary, and further objective studies to classify optimal dosing, administration, and outcome measures are needed. The absence of reported AEs associated with high-dose VD supplementation is encouraging, but selection of a safe and optimal dosing regimen can only occur with dedicated clinical trials.
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
- Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3-19. doi:10.1007/s11154-011-9194-0
- Penna G, Adorini L. 1α,25-Dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405-2411. doi:10.4049/jimmunol.164.5.2405
- Penna G, Amuchastegui S, Cossetti C, et al. Treatment of experimental autoimmune prostatitis in nonobese diabetic mice by the vitamin D receptor agonist elocalcitol. J Immunol. 2006;177:8504-8511. doi:10.4049/jimmunol.177.12.8504
- Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D3 promotes IL-10 production in human B cells. Eur J Immunol. 2008;38:2210-2218. doi:10.1002/eji.200838216
- Hauser K, Walsh D, Shrotriya S, et al. Low 25-hydroxyvitamin D levels in people with a solid tumor cancer diagnosis: the tip of the iceberg? Support Care Cancer. 2014;22:1931-1939. doi:10.1007/s00520-014-2154-y
- Lee KJ, Wright G, Bryant H, et al. Cytoprotective effect of vitamin D on doxorubicin-induced cardiac toxicity in triple negative breast cancer. Int J Mol Sci. 2021;22:7439. doi:10.3390/ijms22147439
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911-1930. doi:10.1210/jc.2011-0385
- Amrein K, Scherkl M, Hoffmann M, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. 2020;74:1498-1513. doi:10.1038/s41430-020-0558-y
- Thomas X, Chelghoum Y, Fanari N, et al. Serum 25-hydroxyvitamin D levels are associated with prognosis in hematological malignancies. Hematology. 2011;16:278-283. doi:10.1179/102453311X13085644679908
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12-49. doi:10.3322/caac.21820
- Goodwin PJ, Ennis M, Pritchard KI, et al. Prognostic effects of 25-hydroxyvitamin D levels in early breast cancer. J Clin Oncol. 2009;27:3757-3763. doi:10.1200/JCO.2008.20.0725
- Fassio A, Porciello G, Carioli G, et al. Post-diagnosis serum 25-hydroxyvitamin D concentrations in women treated for breast cancer participating in a lifestyle trial in Italy. Reumatismo. 2024;76:21-34.
- Augustin LSA, Libra M, Crispo A, et al. Low glycemic index diet, exercise and vitamin D to reduce breast cancer recurrence (DEDiCa): design of a clinical trial. BMC Cancer. 2017;17:69. doi:10.1186/s12885-017-3064-4
- Toriola AT, Nguyen N, Scheitler-Ring K, et al. Circulating 25-hydroxyvitamin D levels and prognosis among cancer patients: a systematic review. Cancer Epidemiol Biomarkers Prev. 2014;23:917-933. doi:10.1158/1055-9965.EPI-14-0053
- Fakih MG, Trump DL, Johnson CS, et al. Chemotherapy is linked to severe vitamin D deficiency in patients with colorectal cancer. Int J Colorectal Dis. 2009;24:219-224. doi:10.1007/s00384-008-0593-y
- Isenring EA, Teleni L, Woodman RJ, et al. Serum vitamin D decreases during chemotherapy: an Australian prospective cohort study. Asia Pac J Clin Nutr. 2018;27:962-967. doi:10.6133/apjcn.042018.01
- Kok DE, van den Berg MMGA, Posthuma L, et al. Changes in circulating levels of 25-hydroxyvitamin D3 in breast cancer patients receiving chemotherapy. Nutr Cancer. 2019;71:756-766. doi:10.1080/01635581.2018.1559938
- Wesselink E, Bours MJL, de Wilt JHW, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. 2020;199:105577. doi:10.1016/j.jsbmb.2020.105577
- Savoie MB, Paciorek A, Zhang L, et al. Vitamin D levels in patients with colorectal cancer before and after treatment initiation. J Gastrointest Cancer. 2019;50:769-779. doi:10.1007/s12029-018-0147-7
- Kitchen D, Hughes B, Gill I, et al. The relationship between vitamin D and chemotherapy-induced toxicity—a pilot study. Br J Cancer. 2012;107:158-160. doi:10.1038/bjc.2012.194
- Demircay Z, Gürbüz O, Alpdogan TB, et al. Chemotherapy-induced acral erythema in leukemic patients: a report of 15 cases. Int J Dermatol. 1997;36:593-598. doi:10.1046/j.1365-4362.1997.00040.x
- Valks R, Fraga J, Porras-Luque J, et al. Chemotherapy-induced eccrine squamous syringometaplasia. a distinctive eruption in patients receiving hematopoietic progenitor cells. Arch Dermatol. 1997;133;873-878. doi:10.1001/archderm.133.7.873
- Webber KA, Kos L, Holland KE, et al. Intertriginous eruption associated with chemotherapy in pediatric patients. Arch Dermatol. 2007;143:67-71. doi:10.1001/archderm.143.1.67
- Hunjan MK, Nowsheen S, Ramos-Rodriguez AJ, et al. Clinical and histopathological spectrum of toxic erythema of chemotherapy in patients who have undergone allogeneic hematopoietic cell transplantation. Hematol Oncol Stem Cell Ther. 2019;12:19-25. doi:10.1016/j.hemonc.2018.09.001
- Ghorbanzadeh-Moghaddam A, Gholamrezaei A, Hemati S. Vitamin D deficiency is associated with the severity of radiation-induced proctitis in cancer patients. Int J Radiat Oncol Biol Phys. 2015;92:613-618. doi:10.1016/j.ijrobp.2015.02.011
- Bhanu A, Waghmare CM, Jain VS, et al. Serum 25-hydroxy vitamin-D levels in head and neck cancer chemoradiation therapy: potential in cancer therapeutics. Indian J Cancer. Published online February 27, 2003. doi:10.4103/ijc.ijc_358_20
- Yang B, Xie X, Wu Z, et al. DNA damage-mediated cellular senescence promotes hand-foot syndrome that can be relieved by thymidine prodrug. Genes Dis. 2022;10:2557-2571. doi:10.1016/j.gendis.2022.10.004
- Lassere Y, Hoff P. Management of hand-foot syndrome in patients treated with capecitabine (Xeloda®). Eur J Oncol Nurs. 2004;8(suppl 1):S31-S40. doi:10.1016/j.ejon.2004.06.007
- Ernst MK, Evans ST, Techner JM, et al. Vitamin D3 and deconvoluting a rash. JCI Insight. 2023;8:E163789.
- Nguyen CV, Zheng L, Zhou XA, et al. High-dose vitamin d for the management of toxic erythema of chemotherapy in hospitalized patients. JAMA Dermatol. 2023;159:219-221. doi:10.1001/jamadermatol.2022.5397
- Fisher J, Scott C, Stevens R, et al. Randomized phase III study comparing best supportive care to biafine as a prophylactic agent for radiation-induced skin toxicity for women undergoing breast irradiation: Radiation Therapy Oncology Group (RTOG) 97-13. Int J Radiat Oncol Biol Phys. 2000;48:1307-1310. doi:10.1016/s0360-3016(00)00782-3
- Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol. 2008;26:2085-2092. doi:10.1200/JCO.2007.15.2488
- Hymes SR, Strom EA, Fife C. Radiation dermatitis: clinical presentation, pathophysiology, and treatment 2006. J Am Acad Dermatol. 2006;54:28-46. doi:10.1016/j.jaad.2005.08.054
- Ryan JL. Ionizing radiation: the good, the bad, and the ugly. J Invest Dermatol. 2012;132(3 pt 2):985-993. doi:10.1038/jid.2011.411
- Scott JF, Das LM, Ahsanuddin S, et al. Oral vitamin D rapidly attenuates inflammation from sunburn: an interventional study. J Invest Dermatol. 2017;137:2078-2086. doi:10.1016/j.jid.2017.04.040
- Das LM, Binko AM, Traylor ZP, et al. Vitamin D improves sunburns by increasing autophagy in M2 macrophages. Autophagy. 2019;15:813-826. doi:10.1080/15548627.2019.1569298
- Nasser NJ, Fenig S, Ravid A, et al. Vitamin D ointment for prevention of radiation dermatitis in breast cancer patients. NPJ Breast Cancer. 2017;3:10. doi:10.1038/s41523-017-0006-x
- Nguyen CV, Zheng L, Lu KQ. High-dose vitamin D for the management acute radiation dermatitis. JAAD Case Rep. 2023;39:47-50. doi:10.1016/j.jdcr.2023.07.001
- Nguyen CV, Lu KQ. Vitamin D3 and its potential to ameliorate chemical and radiation-induced skin injury during cancer therapy. Disaster Med Public Health Prep. 2024;18:E4. doi:10.1017/dmp.2023.211
Practice Points
- High-dose vitamin D supplementation may be considered in the management of cutaneous injury from chemotherapy or ionizing radiation.
- Optimal dosing has not been established, so patients given high-dose vitamin D supplementation should have close clinical follow-up; however, adverse events from high-dose vitamin D supplementation have not been reported.