User login
Cardiology News is an independent news source that provides cardiologists with timely and relevant news and commentary about clinical developments and the impact of health care policy on cardiology and the cardiologist's practice. Cardiology News Digital Network is the online destination and multimedia properties of Cardiology News, the independent news publication for cardiologists. Cardiology news is the leading source of news and commentary about clinical developments in cardiology as well as health care policy and regulations that affect the cardiologist's practice. Cardiology News Digital Network is owned by Frontline Medical Communications.
Wegovy scores HFpEF benefits in people with obesity
AMSTERDAM – Adults with heart failure with preserved ejection fraction (HFpEF) but without diabetes showed significant improvements in their heart failure-related symptoms and physical limitations, exercise function, and weight loss when treated with a weight-reducing dose of semaglutide for 52 weeks, compared with placebo, in the randomized STEP-HFpEF trial.
The results, which also showed the treatment’s safety in these patients, “indicate that treatment with semaglutide is a valuable therapeutic approach in the management of patients with HFpEF and obesity,” Mikhail Kosiborod, MD, said at the annual congress of the European Society of Cardiology.
The findings establish semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist, as a second class of medication with proven efficacy and safety for people with HFpEF, joining two agents also proven beneficial for people with HFpEF, dapagliflozin (Farxiga) and empagliflozin (Jardiance), both from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors.
When administered at the approved dose for weight loss of 2.4 mg, injected subcutaneously weekly for 52 weeks, semaglutide (Wegovy) produced an average 7.8-point incremental improvement in patients’ scores on the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated measure of symptoms and functional limitations, compared with controls who received placebo injections, as well as an average incremental weight loss from baseline, compared with placebo, of 10.7%. Both were significant effects, compared with placebo, and clinically meaningful benefits for the study’s two primary endpoints.
Simultaneously with Kosiborod’s report the results also appeared in a report posted online in the New England Journal of Medicine.
A ‘paradigm shift’ for medical weight loss in cardiology
The findings from this study with 529 randomized patients immediately propelled the weight loss formulation of semaglutide into the ranks of agents used to treat and prevent cardiovascular disease events. This evolution in the indications for semaglutide will be driven not only by the STEP-HFpEF results but also by findings from the SELECT trial, which tested the same semaglutide weight-loss dose in people with obesity, established cardiovascular disease, and had positive top-line results for prevention of major cardiovascular adverse events, according to a press release from Novo Nordisk on Aug. 8.
The STEP-HFpEF and SELECT results will trigger “a paradigm shift” for cardiologists, who will now need to consider prescribing a weight-loss medication to many of their patients, agents that until now were not part of the usual pharmacologic toolbox for cardiologists, said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. This shift will require education to bring the clinical cardiology community on board, he added in an interview.
Given that semaglutide administered at this dose already has a Food and Drug Administration–approved indication for weight loss in people with obesity or overweight plus at least one comorbidity, clinicians could immediately start using the treatment in people with obesity and HFpEF, said Dr. Kosiborod and other cardiologists.
Weekly semaglutide injections “could be considered a treatment option right now” for people with obesity and HFpEF, Dr. Kosiborod said during a press briefing.
Other experts agreed, especially because the STEP-HFpEF results confirmed that weight loss treatment with semaglutide was safe in this population.
‘A terrific win for patients’
The new findings are “a terrific win and game changer for patients with HFpEF,” commented Gregg C. Fonarow, MD, professor and cochief of cardiology at the University of California, Los Angeles, who was not involved with the study.
“The magnitude of improvement in the patient-reported health status scores are large and impressive. These data support clinical use of this agent for individuals with HFpEF with a body mass index of 30 kg/m2, patients who already fall within existing indications,” Dr. Fonarow said in an interview.
“Given the improvements in clinical outcomes in the STEP-HFpEF and SELECT trials, cardiologists should be prescribing these medications to eligible patients without conditions,” he added. “The perception of [semaglutide] needs to shift and be viewed as a component of the comprehensive medical therapies provided to individuals with established cardiovascular disease or HFpEF who also have elevated body mass index to improve their clinical outcomes.”
Historically, cardiologists have had a concern that weight loss was potentially harmful in people with heart failure and that obesity was protective, a phenomenon known as the “obesity paradox,” but the STEP-HFpEF data help disprove that notion, commented Nancy K. Sweitzer, MD, PhD, a heart failure specialist and vice chair of clinical research in the department of medicine at Washington University in St. Louis, who also was not involved in the study.
No signal of an obesity paradox
“There’s been a concern in the heart failure community to use weight-loss strategies in people with heart failure because of this, but this evidence provides a lot of confidence that it’s safe to use this weight loss treatment. The results show that patients feel better and lose weight with no signal of harm,” Dr. Sweitzer said in an interview.
The “encouraging findings” for semaglutide in patients with HFpEF “potentially add a much needed extra option for these patients and provide another upstream treatment for patients with signs of this condition plus a high body mass index,” commented Yigal M. Pinto, MD, PhD, in an editorial that accompanied the published report.
“How these findings translate to hard end points remains to be established and will be important in determining the role of GLP-1 agonism,” wrote Dr. Pinto, a professor and heart failure specialist at Amsterdam University Medical Center.
But Dr. Kosiborod said that the improvement seen in the KCCQ score was itself an important benefit for patients. “Heart failure is defined clinically based on symptoms,” he noted, and results in prior studies documented that patients value improvements in symptoms and physical limitations even more than they value “hard endpoints” such as survival.
The new findings, which indicate that two different and expensive classes of medications are now standard of care for many people with HFpEF and obesity – the SGLT2 inhibitors and the GLP-1 receptor agonist semaglutide – also raise concerns over patient access and affordability, as many U.S. insurers have a history of requiring prior authorization, high copays, or coverage denials for these two medical classes.
But Dr. Sweitzer and Dr. Kosiborod both said that the insurance-coverage climate seems, in just the past couple of years or so, to have dramatically improved, although it’s still not ideal.
Prior authorization hoops have decreased
“We still have prior-authorization hoops to jump through, but I expect these will continue to decrease over time as evidence for clinical benefits [from weight loss] continues to accumulate,” said Dr. Sweitzer.
And “the SELECT data mean that cardiologists will need to become comfortable prescribing GLP-1 receptor agonists,” she added.
“It’s not okay for insurers to say we are not going to cover weight loss medications because it’s a cosmetic indication,” said Dr. Kosiborod. “Obesity appears to be very important in the pathogenesis and progression of heart failure, and if patients derive substantial benefit, they should have access to this treatment.”
The improvements in KCCQ score, as well as in several secondary and exploratory endpoints including a significant reduction in C-reactive protein (an indication of a potent anti-inflammatory effect), an average 20 m increase in 6-minute walk distance, a significant average drop in N-terminal pro-brain natriuretic peptide, and a drop in heart failure hospitalizations or urgent heart failure visits (although the trial was not powered to show differences in clinical events), “were the largest benefits in these outcomes we’ve seen,” compared with any other medical intervention in people with HFpEF, he noted.
“About 80% of U.S. patients with HFpEF have obesity or overweight,” Dr. Kosiborod noted. Using semaglutide on these patients “is an issue of access and insurance coverage. My hope is that these and other data will favorably change this.”
A related trial with a similar design, STEP-HFpEF DM, is still in progress and testing the same semaglutide treatment in adults with HFpEF, obesity, and type 2 diabetes, noted Dr. Kosiborod, who is also lead investigator for that study. He said those results will likely become available before the end of 2023.
The study was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to and has received honoraria from Novo Nordisk. He has also been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Fonarow has been a consultant to Abbott, Amgen, AstraZeneca, CHF Solutions, Cytokinetics, Edwards, Janssen, Medtronic, Merck, Novartis, and Regeneron. Dr. Sweitzer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Adults with heart failure with preserved ejection fraction (HFpEF) but without diabetes showed significant improvements in their heart failure-related symptoms and physical limitations, exercise function, and weight loss when treated with a weight-reducing dose of semaglutide for 52 weeks, compared with placebo, in the randomized STEP-HFpEF trial.
The results, which also showed the treatment’s safety in these patients, “indicate that treatment with semaglutide is a valuable therapeutic approach in the management of patients with HFpEF and obesity,” Mikhail Kosiborod, MD, said at the annual congress of the European Society of Cardiology.
The findings establish semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist, as a second class of medication with proven efficacy and safety for people with HFpEF, joining two agents also proven beneficial for people with HFpEF, dapagliflozin (Farxiga) and empagliflozin (Jardiance), both from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors.
When administered at the approved dose for weight loss of 2.4 mg, injected subcutaneously weekly for 52 weeks, semaglutide (Wegovy) produced an average 7.8-point incremental improvement in patients’ scores on the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated measure of symptoms and functional limitations, compared with controls who received placebo injections, as well as an average incremental weight loss from baseline, compared with placebo, of 10.7%. Both were significant effects, compared with placebo, and clinically meaningful benefits for the study’s two primary endpoints.
Simultaneously with Kosiborod’s report the results also appeared in a report posted online in the New England Journal of Medicine.
A ‘paradigm shift’ for medical weight loss in cardiology
The findings from this study with 529 randomized patients immediately propelled the weight loss formulation of semaglutide into the ranks of agents used to treat and prevent cardiovascular disease events. This evolution in the indications for semaglutide will be driven not only by the STEP-HFpEF results but also by findings from the SELECT trial, which tested the same semaglutide weight-loss dose in people with obesity, established cardiovascular disease, and had positive top-line results for prevention of major cardiovascular adverse events, according to a press release from Novo Nordisk on Aug. 8.
The STEP-HFpEF and SELECT results will trigger “a paradigm shift” for cardiologists, who will now need to consider prescribing a weight-loss medication to many of their patients, agents that until now were not part of the usual pharmacologic toolbox for cardiologists, said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. This shift will require education to bring the clinical cardiology community on board, he added in an interview.
Given that semaglutide administered at this dose already has a Food and Drug Administration–approved indication for weight loss in people with obesity or overweight plus at least one comorbidity, clinicians could immediately start using the treatment in people with obesity and HFpEF, said Dr. Kosiborod and other cardiologists.
Weekly semaglutide injections “could be considered a treatment option right now” for people with obesity and HFpEF, Dr. Kosiborod said during a press briefing.
Other experts agreed, especially because the STEP-HFpEF results confirmed that weight loss treatment with semaglutide was safe in this population.
‘A terrific win for patients’
The new findings are “a terrific win and game changer for patients with HFpEF,” commented Gregg C. Fonarow, MD, professor and cochief of cardiology at the University of California, Los Angeles, who was not involved with the study.
“The magnitude of improvement in the patient-reported health status scores are large and impressive. These data support clinical use of this agent for individuals with HFpEF with a body mass index of 30 kg/m2, patients who already fall within existing indications,” Dr. Fonarow said in an interview.
“Given the improvements in clinical outcomes in the STEP-HFpEF and SELECT trials, cardiologists should be prescribing these medications to eligible patients without conditions,” he added. “The perception of [semaglutide] needs to shift and be viewed as a component of the comprehensive medical therapies provided to individuals with established cardiovascular disease or HFpEF who also have elevated body mass index to improve their clinical outcomes.”
Historically, cardiologists have had a concern that weight loss was potentially harmful in people with heart failure and that obesity was protective, a phenomenon known as the “obesity paradox,” but the STEP-HFpEF data help disprove that notion, commented Nancy K. Sweitzer, MD, PhD, a heart failure specialist and vice chair of clinical research in the department of medicine at Washington University in St. Louis, who also was not involved in the study.
No signal of an obesity paradox
“There’s been a concern in the heart failure community to use weight-loss strategies in people with heart failure because of this, but this evidence provides a lot of confidence that it’s safe to use this weight loss treatment. The results show that patients feel better and lose weight with no signal of harm,” Dr. Sweitzer said in an interview.
The “encouraging findings” for semaglutide in patients with HFpEF “potentially add a much needed extra option for these patients and provide another upstream treatment for patients with signs of this condition plus a high body mass index,” commented Yigal M. Pinto, MD, PhD, in an editorial that accompanied the published report.
“How these findings translate to hard end points remains to be established and will be important in determining the role of GLP-1 agonism,” wrote Dr. Pinto, a professor and heart failure specialist at Amsterdam University Medical Center.
But Dr. Kosiborod said that the improvement seen in the KCCQ score was itself an important benefit for patients. “Heart failure is defined clinically based on symptoms,” he noted, and results in prior studies documented that patients value improvements in symptoms and physical limitations even more than they value “hard endpoints” such as survival.
The new findings, which indicate that two different and expensive classes of medications are now standard of care for many people with HFpEF and obesity – the SGLT2 inhibitors and the GLP-1 receptor agonist semaglutide – also raise concerns over patient access and affordability, as many U.S. insurers have a history of requiring prior authorization, high copays, or coverage denials for these two medical classes.
But Dr. Sweitzer and Dr. Kosiborod both said that the insurance-coverage climate seems, in just the past couple of years or so, to have dramatically improved, although it’s still not ideal.
Prior authorization hoops have decreased
“We still have prior-authorization hoops to jump through, but I expect these will continue to decrease over time as evidence for clinical benefits [from weight loss] continues to accumulate,” said Dr. Sweitzer.
And “the SELECT data mean that cardiologists will need to become comfortable prescribing GLP-1 receptor agonists,” she added.
“It’s not okay for insurers to say we are not going to cover weight loss medications because it’s a cosmetic indication,” said Dr. Kosiborod. “Obesity appears to be very important in the pathogenesis and progression of heart failure, and if patients derive substantial benefit, they should have access to this treatment.”
The improvements in KCCQ score, as well as in several secondary and exploratory endpoints including a significant reduction in C-reactive protein (an indication of a potent anti-inflammatory effect), an average 20 m increase in 6-minute walk distance, a significant average drop in N-terminal pro-brain natriuretic peptide, and a drop in heart failure hospitalizations or urgent heart failure visits (although the trial was not powered to show differences in clinical events), “were the largest benefits in these outcomes we’ve seen,” compared with any other medical intervention in people with HFpEF, he noted.
“About 80% of U.S. patients with HFpEF have obesity or overweight,” Dr. Kosiborod noted. Using semaglutide on these patients “is an issue of access and insurance coverage. My hope is that these and other data will favorably change this.”
A related trial with a similar design, STEP-HFpEF DM, is still in progress and testing the same semaglutide treatment in adults with HFpEF, obesity, and type 2 diabetes, noted Dr. Kosiborod, who is also lead investigator for that study. He said those results will likely become available before the end of 2023.
The study was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to and has received honoraria from Novo Nordisk. He has also been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Fonarow has been a consultant to Abbott, Amgen, AstraZeneca, CHF Solutions, Cytokinetics, Edwards, Janssen, Medtronic, Merck, Novartis, and Regeneron. Dr. Sweitzer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AMSTERDAM – Adults with heart failure with preserved ejection fraction (HFpEF) but without diabetes showed significant improvements in their heart failure-related symptoms and physical limitations, exercise function, and weight loss when treated with a weight-reducing dose of semaglutide for 52 weeks, compared with placebo, in the randomized STEP-HFpEF trial.
The results, which also showed the treatment’s safety in these patients, “indicate that treatment with semaglutide is a valuable therapeutic approach in the management of patients with HFpEF and obesity,” Mikhail Kosiborod, MD, said at the annual congress of the European Society of Cardiology.
The findings establish semaglutide, a glucagonlike peptide–1 (GLP-1) receptor agonist, as a second class of medication with proven efficacy and safety for people with HFpEF, joining two agents also proven beneficial for people with HFpEF, dapagliflozin (Farxiga) and empagliflozin (Jardiance), both from the class of sodium-glucose cotransporter 2 (SGLT2) inhibitors.
When administered at the approved dose for weight loss of 2.4 mg, injected subcutaneously weekly for 52 weeks, semaglutide (Wegovy) produced an average 7.8-point incremental improvement in patients’ scores on the Kansas City Cardiomyopathy Questionnaire (KCCQ), a validated measure of symptoms and functional limitations, compared with controls who received placebo injections, as well as an average incremental weight loss from baseline, compared with placebo, of 10.7%. Both were significant effects, compared with placebo, and clinically meaningful benefits for the study’s two primary endpoints.
Simultaneously with Kosiborod’s report the results also appeared in a report posted online in the New England Journal of Medicine.
A ‘paradigm shift’ for medical weight loss in cardiology
The findings from this study with 529 randomized patients immediately propelled the weight loss formulation of semaglutide into the ranks of agents used to treat and prevent cardiovascular disease events. This evolution in the indications for semaglutide will be driven not only by the STEP-HFpEF results but also by findings from the SELECT trial, which tested the same semaglutide weight-loss dose in people with obesity, established cardiovascular disease, and had positive top-line results for prevention of major cardiovascular adverse events, according to a press release from Novo Nordisk on Aug. 8.
The STEP-HFpEF and SELECT results will trigger “a paradigm shift” for cardiologists, who will now need to consider prescribing a weight-loss medication to many of their patients, agents that until now were not part of the usual pharmacologic toolbox for cardiologists, said Dr. Kosiborod, a cardiologist and codirector of the Haverty Cardiometabolic Center of Excellence at Saint Luke’s Mid America Heart Institute in Kansas City, Mo. This shift will require education to bring the clinical cardiology community on board, he added in an interview.
Given that semaglutide administered at this dose already has a Food and Drug Administration–approved indication for weight loss in people with obesity or overweight plus at least one comorbidity, clinicians could immediately start using the treatment in people with obesity and HFpEF, said Dr. Kosiborod and other cardiologists.
Weekly semaglutide injections “could be considered a treatment option right now” for people with obesity and HFpEF, Dr. Kosiborod said during a press briefing.
Other experts agreed, especially because the STEP-HFpEF results confirmed that weight loss treatment with semaglutide was safe in this population.
‘A terrific win for patients’
The new findings are “a terrific win and game changer for patients with HFpEF,” commented Gregg C. Fonarow, MD, professor and cochief of cardiology at the University of California, Los Angeles, who was not involved with the study.
“The magnitude of improvement in the patient-reported health status scores are large and impressive. These data support clinical use of this agent for individuals with HFpEF with a body mass index of 30 kg/m2, patients who already fall within existing indications,” Dr. Fonarow said in an interview.
“Given the improvements in clinical outcomes in the STEP-HFpEF and SELECT trials, cardiologists should be prescribing these medications to eligible patients without conditions,” he added. “The perception of [semaglutide] needs to shift and be viewed as a component of the comprehensive medical therapies provided to individuals with established cardiovascular disease or HFpEF who also have elevated body mass index to improve their clinical outcomes.”
Historically, cardiologists have had a concern that weight loss was potentially harmful in people with heart failure and that obesity was protective, a phenomenon known as the “obesity paradox,” but the STEP-HFpEF data help disprove that notion, commented Nancy K. Sweitzer, MD, PhD, a heart failure specialist and vice chair of clinical research in the department of medicine at Washington University in St. Louis, who also was not involved in the study.
No signal of an obesity paradox
“There’s been a concern in the heart failure community to use weight-loss strategies in people with heart failure because of this, but this evidence provides a lot of confidence that it’s safe to use this weight loss treatment. The results show that patients feel better and lose weight with no signal of harm,” Dr. Sweitzer said in an interview.
The “encouraging findings” for semaglutide in patients with HFpEF “potentially add a much needed extra option for these patients and provide another upstream treatment for patients with signs of this condition plus a high body mass index,” commented Yigal M. Pinto, MD, PhD, in an editorial that accompanied the published report.
“How these findings translate to hard end points remains to be established and will be important in determining the role of GLP-1 agonism,” wrote Dr. Pinto, a professor and heart failure specialist at Amsterdam University Medical Center.
But Dr. Kosiborod said that the improvement seen in the KCCQ score was itself an important benefit for patients. “Heart failure is defined clinically based on symptoms,” he noted, and results in prior studies documented that patients value improvements in symptoms and physical limitations even more than they value “hard endpoints” such as survival.
The new findings, which indicate that two different and expensive classes of medications are now standard of care for many people with HFpEF and obesity – the SGLT2 inhibitors and the GLP-1 receptor agonist semaglutide – also raise concerns over patient access and affordability, as many U.S. insurers have a history of requiring prior authorization, high copays, or coverage denials for these two medical classes.
But Dr. Sweitzer and Dr. Kosiborod both said that the insurance-coverage climate seems, in just the past couple of years or so, to have dramatically improved, although it’s still not ideal.
Prior authorization hoops have decreased
“We still have prior-authorization hoops to jump through, but I expect these will continue to decrease over time as evidence for clinical benefits [from weight loss] continues to accumulate,” said Dr. Sweitzer.
And “the SELECT data mean that cardiologists will need to become comfortable prescribing GLP-1 receptor agonists,” she added.
“It’s not okay for insurers to say we are not going to cover weight loss medications because it’s a cosmetic indication,” said Dr. Kosiborod. “Obesity appears to be very important in the pathogenesis and progression of heart failure, and if patients derive substantial benefit, they should have access to this treatment.”
The improvements in KCCQ score, as well as in several secondary and exploratory endpoints including a significant reduction in C-reactive protein (an indication of a potent anti-inflammatory effect), an average 20 m increase in 6-minute walk distance, a significant average drop in N-terminal pro-brain natriuretic peptide, and a drop in heart failure hospitalizations or urgent heart failure visits (although the trial was not powered to show differences in clinical events), “were the largest benefits in these outcomes we’ve seen,” compared with any other medical intervention in people with HFpEF, he noted.
“About 80% of U.S. patients with HFpEF have obesity or overweight,” Dr. Kosiborod noted. Using semaglutide on these patients “is an issue of access and insurance coverage. My hope is that these and other data will favorably change this.”
A related trial with a similar design, STEP-HFpEF DM, is still in progress and testing the same semaglutide treatment in adults with HFpEF, obesity, and type 2 diabetes, noted Dr. Kosiborod, who is also lead investigator for that study. He said those results will likely become available before the end of 2023.
The study was funded by Novo Nordisk, the company that markets semaglutide (Wegovy). Dr. Kosiborod has been a consultant and adviser to and has received honoraria from Novo Nordisk. He has also been a consultant to numerous other companies, received research grants from AstraZeneca, Boehringer Ingelheim, and Pfizer, honoraria from AstraZeneca, and is a stockholder in Artera Health and Saghmos Therapeutics. Dr. Fonarow has been a consultant to Abbott, Amgen, AstraZeneca, CHF Solutions, Cytokinetics, Edwards, Janssen, Medtronic, Merck, Novartis, and Regeneron. Dr. Sweitzer reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT THE ESC CONGRESS 2023
FDA panel split on efficacy of Spyral renal denervation system
The Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed that the Symplicity Spyral Renal Denervation System (Medtronic) is safe, but the panel was split on its efficacy and whether the benefits outweighed the risks associated with its use.
The moderator’s tiebreaker vote went against the benefit-risk profile, for a final vote of 6 yes, 7 no, and 1 abstention.
The Symplicity Spyral system provides a catheter-based approach to denervate the renal arteries using radiofrequency energy. The proposed indication is for reduction of blood pressure in patients with uncontrolled hypertension despite their use of antihypertensive medications, or in patients who cannot tolerate antihypertensive medications.
The Spyral device received breakthrough device designation in March 2020. The FDA determined that the device met the criteria for inclusion in the program because it was a novel technology and had the potential to provide more effective treatment for patients with resistant or uncontrolled hypertension.
Medtronic presented data from two studies, the SPYRAL HTN-OFF and SPYRAL HTN-ON randomized trials.
HTN-OFF enrolled patients with hypertension whose medications could be discontinued at the start of the trial. The primary effectiveness endpoint was the mean difference in the baseline adjusted 24-hour ambulatory systolic blood pressure (ASBP) from baseline to 3 months post renal denervation or sham procedure. The study showed a statistically significant reduction of 3.9 mm Hg ASBP in patients who received the device compared with sham control patients.
HTN-ON evaluated patients with uncontrolled hypertension who continued taking their blood pressure medications during treatment with either the Spyral renal denervation device or a sham device. The primary endpoint was the mean difference in the baseline adjusted 24 hour ambulatory systolic blood pressure at 6 months. The study showed a nonsignificant 24-hour 0.03–mm Hg reduction in ambulatory systolic blood pressure in active-treatment patients compared with sham control patients.
Many on the panel agreed that the device was safe and effective. Randall Starling, MD, professor of medicine in the Heart, Vascular, and Thoracic Institute at Cleveland Clinic, said that he was comfortable with the data presented by Medtronic and that his affirmative vote reflected his recognition that hypertension is not effectively treated with current medications and that another tool in the armamentarium to treat patients is needed.
Matthew Corriere, MD, Frankel Professor of Cardiovascular Surgery at the University of Michigan, Ann Arbor, abstained from voting on whether the benefits of the system outweighed its risks. “I think there is potential benefit, but we don’t know which patients are most likely to have a benefit that outweighs any risks. More selective indications for this product could potentially tip the balance of the benefit outweighing the risks,” he said.
Robert Yeh, MD, director, Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, said he believed that the device was safe and effective and that its use resulted in a favorable risk-benefit ratio for patients. He pointed to the wide variability in effectiveness across the patient population and suggested that as the device becomes more widely used, experience will show which patients will benefit the most from its use.
Keith Allen, MD, director of surgical research at St. Luke’s Hospital of Kansas City, Kansas City, Mo., said the data presented by Medtronic reassured him that the device was safe, but he said he remained unconvinced that the device was effective. “I think that, while this is a safe procedure, the efficacy was mild at best, and that was only at 3 months,” he said.
Other panel members agreed.
“Yes as to safety, but no as to effectiveness,” said Mark Lockhart, MD, professor, department of radiology, University of Alabama, Birmingham. “There is too much uncertainty about there actually being a real benefit to outweigh even a small risk of an invasive procedure,” he said.
One of the statisticians on the panel, Benjamin Saville, PhD, director and senior statistical scientist, Berry Consultants, Austin, Texas, said he did not feel that effectiveness was adequately demonstrated in the trial data presented by Medtronic.
He agreed there is a small but potentially clinically meaningful benefit but voted no because he did not think benefit was demonstrated for those patients in the proposed indication. “For me, I think I would need additional randomized data to convince me that the benefits outweigh the risks.”
Julia Lewis, MD, professor of medicine at Vanderbilt University, Nashville, Tenn., voted against endorsing the device for efficacy. “We have one study that is negative and one that is minimally positive,” and there is no reason to think one of those results is more valid than the other, she said.
“So as far as I’m concerned, we still don’t know the efficacy of this, and if it gets on the market, the anecdotal, small sample size of each individual physician using this intervention will not allow them to select out the patients that will benefit from those who won’t benefit, and to not have a definitive study that better defines that it is efficacious and in whom is actually a disservice to the public,” she concluded.
After the panel meeting, Medtronic issued a statement on the result.
“We appreciate the robust conversation that occurred prior to the vote,” Jason Weidman, senior vice-president of the coronary and renal denervation business at Medtronic, said in the statement. “We will continue to collaborate with the FDA on bringing a new option to the millions of people living with high blood pressure.”
The lead investigator of the SPYRAL HTN-ON MED trial, David Kandzari, MD, chief at Piedmont Heart Institute and Cardiovascular Services, added, “The totality of the evidence demonstrated that there is a benefit with the SPYRAL RDN catheter, and there is no question about the safety of the procedure.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed that the Symplicity Spyral Renal Denervation System (Medtronic) is safe, but the panel was split on its efficacy and whether the benefits outweighed the risks associated with its use.
The moderator’s tiebreaker vote went against the benefit-risk profile, for a final vote of 6 yes, 7 no, and 1 abstention.
The Symplicity Spyral system provides a catheter-based approach to denervate the renal arteries using radiofrequency energy. The proposed indication is for reduction of blood pressure in patients with uncontrolled hypertension despite their use of antihypertensive medications, or in patients who cannot tolerate antihypertensive medications.
The Spyral device received breakthrough device designation in March 2020. The FDA determined that the device met the criteria for inclusion in the program because it was a novel technology and had the potential to provide more effective treatment for patients with resistant or uncontrolled hypertension.
Medtronic presented data from two studies, the SPYRAL HTN-OFF and SPYRAL HTN-ON randomized trials.
HTN-OFF enrolled patients with hypertension whose medications could be discontinued at the start of the trial. The primary effectiveness endpoint was the mean difference in the baseline adjusted 24-hour ambulatory systolic blood pressure (ASBP) from baseline to 3 months post renal denervation or sham procedure. The study showed a statistically significant reduction of 3.9 mm Hg ASBP in patients who received the device compared with sham control patients.
HTN-ON evaluated patients with uncontrolled hypertension who continued taking their blood pressure medications during treatment with either the Spyral renal denervation device or a sham device. The primary endpoint was the mean difference in the baseline adjusted 24 hour ambulatory systolic blood pressure at 6 months. The study showed a nonsignificant 24-hour 0.03–mm Hg reduction in ambulatory systolic blood pressure in active-treatment patients compared with sham control patients.
Many on the panel agreed that the device was safe and effective. Randall Starling, MD, professor of medicine in the Heart, Vascular, and Thoracic Institute at Cleveland Clinic, said that he was comfortable with the data presented by Medtronic and that his affirmative vote reflected his recognition that hypertension is not effectively treated with current medications and that another tool in the armamentarium to treat patients is needed.
Matthew Corriere, MD, Frankel Professor of Cardiovascular Surgery at the University of Michigan, Ann Arbor, abstained from voting on whether the benefits of the system outweighed its risks. “I think there is potential benefit, but we don’t know which patients are most likely to have a benefit that outweighs any risks. More selective indications for this product could potentially tip the balance of the benefit outweighing the risks,” he said.
Robert Yeh, MD, director, Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, said he believed that the device was safe and effective and that its use resulted in a favorable risk-benefit ratio for patients. He pointed to the wide variability in effectiveness across the patient population and suggested that as the device becomes more widely used, experience will show which patients will benefit the most from its use.
Keith Allen, MD, director of surgical research at St. Luke’s Hospital of Kansas City, Kansas City, Mo., said the data presented by Medtronic reassured him that the device was safe, but he said he remained unconvinced that the device was effective. “I think that, while this is a safe procedure, the efficacy was mild at best, and that was only at 3 months,” he said.
Other panel members agreed.
“Yes as to safety, but no as to effectiveness,” said Mark Lockhart, MD, professor, department of radiology, University of Alabama, Birmingham. “There is too much uncertainty about there actually being a real benefit to outweigh even a small risk of an invasive procedure,” he said.
One of the statisticians on the panel, Benjamin Saville, PhD, director and senior statistical scientist, Berry Consultants, Austin, Texas, said he did not feel that effectiveness was adequately demonstrated in the trial data presented by Medtronic.
He agreed there is a small but potentially clinically meaningful benefit but voted no because he did not think benefit was demonstrated for those patients in the proposed indication. “For me, I think I would need additional randomized data to convince me that the benefits outweigh the risks.”
Julia Lewis, MD, professor of medicine at Vanderbilt University, Nashville, Tenn., voted against endorsing the device for efficacy. “We have one study that is negative and one that is minimally positive,” and there is no reason to think one of those results is more valid than the other, she said.
“So as far as I’m concerned, we still don’t know the efficacy of this, and if it gets on the market, the anecdotal, small sample size of each individual physician using this intervention will not allow them to select out the patients that will benefit from those who won’t benefit, and to not have a definitive study that better defines that it is efficacious and in whom is actually a disservice to the public,” she concluded.
After the panel meeting, Medtronic issued a statement on the result.
“We appreciate the robust conversation that occurred prior to the vote,” Jason Weidman, senior vice-president of the coronary and renal denervation business at Medtronic, said in the statement. “We will continue to collaborate with the FDA on bringing a new option to the millions of people living with high blood pressure.”
The lead investigator of the SPYRAL HTN-ON MED trial, David Kandzari, MD, chief at Piedmont Heart Institute and Cardiovascular Services, added, “The totality of the evidence demonstrated that there is a benefit with the SPYRAL RDN catheter, and there is no question about the safety of the procedure.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration’s Circulatory System Devices Panel unanimously agreed that the Symplicity Spyral Renal Denervation System (Medtronic) is safe, but the panel was split on its efficacy and whether the benefits outweighed the risks associated with its use.
The moderator’s tiebreaker vote went against the benefit-risk profile, for a final vote of 6 yes, 7 no, and 1 abstention.
The Symplicity Spyral system provides a catheter-based approach to denervate the renal arteries using radiofrequency energy. The proposed indication is for reduction of blood pressure in patients with uncontrolled hypertension despite their use of antihypertensive medications, or in patients who cannot tolerate antihypertensive medications.
The Spyral device received breakthrough device designation in March 2020. The FDA determined that the device met the criteria for inclusion in the program because it was a novel technology and had the potential to provide more effective treatment for patients with resistant or uncontrolled hypertension.
Medtronic presented data from two studies, the SPYRAL HTN-OFF and SPYRAL HTN-ON randomized trials.
HTN-OFF enrolled patients with hypertension whose medications could be discontinued at the start of the trial. The primary effectiveness endpoint was the mean difference in the baseline adjusted 24-hour ambulatory systolic blood pressure (ASBP) from baseline to 3 months post renal denervation or sham procedure. The study showed a statistically significant reduction of 3.9 mm Hg ASBP in patients who received the device compared with sham control patients.
HTN-ON evaluated patients with uncontrolled hypertension who continued taking their blood pressure medications during treatment with either the Spyral renal denervation device or a sham device. The primary endpoint was the mean difference in the baseline adjusted 24 hour ambulatory systolic blood pressure at 6 months. The study showed a nonsignificant 24-hour 0.03–mm Hg reduction in ambulatory systolic blood pressure in active-treatment patients compared with sham control patients.
Many on the panel agreed that the device was safe and effective. Randall Starling, MD, professor of medicine in the Heart, Vascular, and Thoracic Institute at Cleveland Clinic, said that he was comfortable with the data presented by Medtronic and that his affirmative vote reflected his recognition that hypertension is not effectively treated with current medications and that another tool in the armamentarium to treat patients is needed.
Matthew Corriere, MD, Frankel Professor of Cardiovascular Surgery at the University of Michigan, Ann Arbor, abstained from voting on whether the benefits of the system outweighed its risks. “I think there is potential benefit, but we don’t know which patients are most likely to have a benefit that outweighs any risks. More selective indications for this product could potentially tip the balance of the benefit outweighing the risks,” he said.
Robert Yeh, MD, director, Center for Outcomes Research in Cardiology, Beth Israel Deaconess Medical Center, Boston, said he believed that the device was safe and effective and that its use resulted in a favorable risk-benefit ratio for patients. He pointed to the wide variability in effectiveness across the patient population and suggested that as the device becomes more widely used, experience will show which patients will benefit the most from its use.
Keith Allen, MD, director of surgical research at St. Luke’s Hospital of Kansas City, Kansas City, Mo., said the data presented by Medtronic reassured him that the device was safe, but he said he remained unconvinced that the device was effective. “I think that, while this is a safe procedure, the efficacy was mild at best, and that was only at 3 months,” he said.
Other panel members agreed.
“Yes as to safety, but no as to effectiveness,” said Mark Lockhart, MD, professor, department of radiology, University of Alabama, Birmingham. “There is too much uncertainty about there actually being a real benefit to outweigh even a small risk of an invasive procedure,” he said.
One of the statisticians on the panel, Benjamin Saville, PhD, director and senior statistical scientist, Berry Consultants, Austin, Texas, said he did not feel that effectiveness was adequately demonstrated in the trial data presented by Medtronic.
He agreed there is a small but potentially clinically meaningful benefit but voted no because he did not think benefit was demonstrated for those patients in the proposed indication. “For me, I think I would need additional randomized data to convince me that the benefits outweigh the risks.”
Julia Lewis, MD, professor of medicine at Vanderbilt University, Nashville, Tenn., voted against endorsing the device for efficacy. “We have one study that is negative and one that is minimally positive,” and there is no reason to think one of those results is more valid than the other, she said.
“So as far as I’m concerned, we still don’t know the efficacy of this, and if it gets on the market, the anecdotal, small sample size of each individual physician using this intervention will not allow them to select out the patients that will benefit from those who won’t benefit, and to not have a definitive study that better defines that it is efficacious and in whom is actually a disservice to the public,” she concluded.
After the panel meeting, Medtronic issued a statement on the result.
“We appreciate the robust conversation that occurred prior to the vote,” Jason Weidman, senior vice-president of the coronary and renal denervation business at Medtronic, said in the statement. “We will continue to collaborate with the FDA on bringing a new option to the millions of people living with high blood pressure.”
The lead investigator of the SPYRAL HTN-ON MED trial, David Kandzari, MD, chief at Piedmont Heart Institute and Cardiovascular Services, added, “The totality of the evidence demonstrated that there is a benefit with the SPYRAL RDN catheter, and there is no question about the safety of the procedure.”
A version of this article first appeared on Medscape.com.
Long COVID leads to greater health risks, research finds
That is the finding of a new study from Washington University in St. Louis. The school distributed a press release about the study, which was published in the journal Nature Medicine.
“Some estimates show more than 90% of the U.S. population has been infected with COVID-19,” Ziyad Al-Aly, chief of research and development at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University, told the St. Louis Post–Dispatch. “Doctors need to realize that their patients could be at risk of these conditions, be it heart disease or lung problems or brain problems – they’re at risk.”
The researchers compared the health records for 138,000 patients who had been infected with those of 6 million who had not. They followed 80 health conditions associated with long COVID for 2 years. They used unnamed records from the VA.
“There was really nothing at all looking at what happens to people at two years after the infection,” Dr. Al-Aly said. “So we decided to take a look.”
Patients who hadn’t been hospitalized within 30 days of infection had a higher risk of death 6 months after recovery, and a higher risk of hospitalization within 18 months. They had higher risk of diabetes, fatigue, joint pain, and other problems compared with people who had not been infected.
“In the nonhospitalized group, risks remained elevated for several problems, for several organ systems,” Dr. Al-Aly said. “For the people who were hospitalized, the risk was ubiquitous across all organ systems. It really spans the gamut with respect to the organ systems that are affected.”
People who had been hospitalized had a 65% greater risk of illnesses after 2 years. Nonhospitalized patients had just a 35% greater risk.
A version of this article first appeared on WebMD.com.
That is the finding of a new study from Washington University in St. Louis. The school distributed a press release about the study, which was published in the journal Nature Medicine.
“Some estimates show more than 90% of the U.S. population has been infected with COVID-19,” Ziyad Al-Aly, chief of research and development at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University, told the St. Louis Post–Dispatch. “Doctors need to realize that their patients could be at risk of these conditions, be it heart disease or lung problems or brain problems – they’re at risk.”
The researchers compared the health records for 138,000 patients who had been infected with those of 6 million who had not. They followed 80 health conditions associated with long COVID for 2 years. They used unnamed records from the VA.
“There was really nothing at all looking at what happens to people at two years after the infection,” Dr. Al-Aly said. “So we decided to take a look.”
Patients who hadn’t been hospitalized within 30 days of infection had a higher risk of death 6 months after recovery, and a higher risk of hospitalization within 18 months. They had higher risk of diabetes, fatigue, joint pain, and other problems compared with people who had not been infected.
“In the nonhospitalized group, risks remained elevated for several problems, for several organ systems,” Dr. Al-Aly said. “For the people who were hospitalized, the risk was ubiquitous across all organ systems. It really spans the gamut with respect to the organ systems that are affected.”
People who had been hospitalized had a 65% greater risk of illnesses after 2 years. Nonhospitalized patients had just a 35% greater risk.
A version of this article first appeared on WebMD.com.
That is the finding of a new study from Washington University in St. Louis. The school distributed a press release about the study, which was published in the journal Nature Medicine.
“Some estimates show more than 90% of the U.S. population has been infected with COVID-19,” Ziyad Al-Aly, chief of research and development at Veterans Affairs St. Louis Health Care System and clinical epidemiologist at Washington University, told the St. Louis Post–Dispatch. “Doctors need to realize that their patients could be at risk of these conditions, be it heart disease or lung problems or brain problems – they’re at risk.”
The researchers compared the health records for 138,000 patients who had been infected with those of 6 million who had not. They followed 80 health conditions associated with long COVID for 2 years. They used unnamed records from the VA.
“There was really nothing at all looking at what happens to people at two years after the infection,” Dr. Al-Aly said. “So we decided to take a look.”
Patients who hadn’t been hospitalized within 30 days of infection had a higher risk of death 6 months after recovery, and a higher risk of hospitalization within 18 months. They had higher risk of diabetes, fatigue, joint pain, and other problems compared with people who had not been infected.
“In the nonhospitalized group, risks remained elevated for several problems, for several organ systems,” Dr. Al-Aly said. “For the people who were hospitalized, the risk was ubiquitous across all organ systems. It really spans the gamut with respect to the organ systems that are affected.”
People who had been hospitalized had a 65% greater risk of illnesses after 2 years. Nonhospitalized patients had just a 35% greater risk.
A version of this article first appeared on WebMD.com.
FROM NATURE MEDICINE
Triglyceride puzzle: Do TG metabolites better predict risk?
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.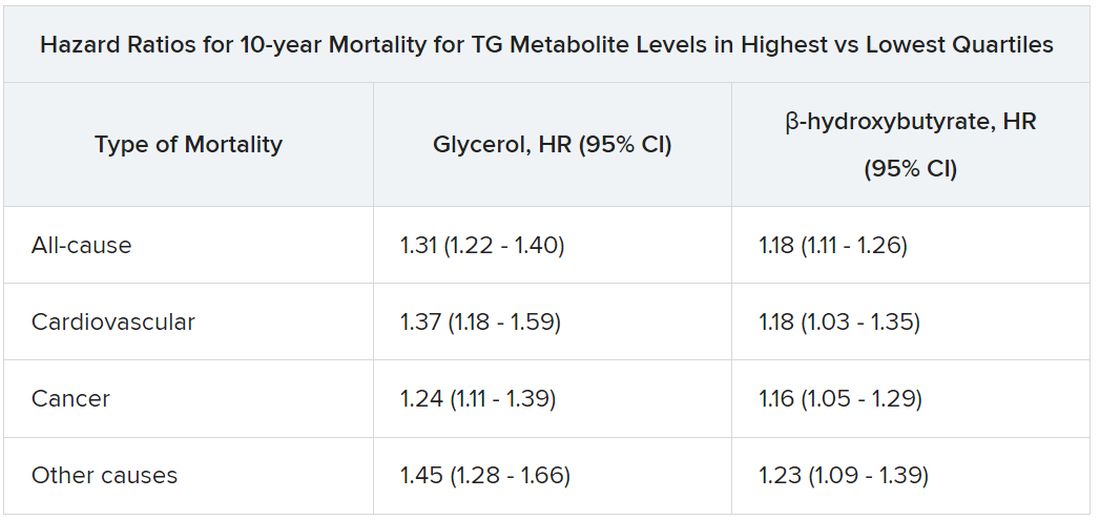
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
Triglyceride levels are a measure of cardiovascular risk and a target for therapy, but a focus on TG levels as a bad guy in CV risk assessments may be missing the mark, a population-based cohort study suggests.
The analysis, based on 30,000 participants in the Copenhagen General Population Study, saw sharply increased risks for all-cause mortality, CV mortality, and cancer mortality over 10 years among those with robust TG metabolism.
Those significant risks, gauged by concentrations of two molecules considered markers of TG metabolic rate, were independent of body mass index (BMI) and a range of other TG-linked risk factors, including plasma TG levels themselves.
All-cause mortality jumped 31% for plasma levels of glycerol in the highest versus lowest quartiles and rose 18% for highest-quartile levels of beta-hydroxybutyrate. In parallel, CV mortality climbed 37% for glycerol and 18% for beta-hydroxybutyrate in the study, published in the European Heart Journal.
The findings “implicate triglyceride metabolic rate as a risk factor for mortality not explained by high plasma triglycerides or high BMI,” the report states. The study, it continues, may be the first to link increased mortality to more active TG metabolism – according to levels of the two biomarkers – in the general population.
The results were “really, really surprising,” senior author Børge G. Nordestgaard, MD, DMSc, said in an interview. They are “completely novel” and “may make people think differently” about TG and mortality risk.
Given their unexpected findings, the group conducted further analyses for evidence that the metabolite-mortality associations weren’t independent. “We tried to stratify them away, but they stayed,” said Dr. Nordestgaard, of the University of Copenhagen.
In a weight-stratified analysis, for example, findings were similar in people with normal weight and with overweight and who were obese, Dr. Nordestgaard observed. “Even in the ones with normal weight by World Health Organization criteria, we saw the same and maybe even stronger relationships” between TG metabolism and mortality.
The study authors were is careful to note the retrospective cohort study’s limitations, but its findings “at most support an association, not causation,” Michael Miller, MD, Hospital of the University of Pennsylvania, Philadelphia, observed in an interview. Therefore, it can’t answer “whether and to what extent glycerol and/or beta-hydroxybutyrate independently contribute to mortality beyond triglyceride levels per se.”
Assessing levels of the two biomarkers “was an interesting way to indirectly assess whole-body TG metabolism,” but they were not fasting levels, said Dr. Miller, who wasn’t part of the study.
Also, the analysis doesn’t account for heparinization and other factors “that artificially raise glycerol levels” and suffers in other ways “from the inherent limitations of residual confounding,” said Dr. Miller, who is also chief of medicine at Corporal Michael J Crescenz VA Medical Center, Philadelphia.
The analysis tracked 30,000 men and women, participants in the much larger Copenhagen General Population Study cohort, for a median of 10.7 years. During that time, 9,897 of them died.
Plasma levels of glycerol and beta-hydroxybutyrate, the study authors noted, were measured using high-throughput nuclear magnetic resonance spectroscopy.
Glycerol levels greater than 80 mcmol/L represented the highest quartile and those less than 52 mcmol/L the lowest quartile. The corresponding beta-hydroxybutyrate quartiles were greater than 154 mcmol/L and less than 91 mcmol/L, respectively.
Mortality risks were independent not only of BMI and TG levels but also of age, greater waist circumference, many other standard CV risk factors, chronic obstructive pulmonary disease, diabetes, insulin use, and CV comorbidities and medications.
Dr. Nordestgaard, who also stressed that the findings are only hypothesis generating, speculated that glycerol and beta-hydroxybutyrate could potentially serve as biomarkers for predicting risk or guiding therapy and, indeed, might be amenable to risk-factor modification. “But I have absolutely no data to support that.”
The study was funded by the Independent Research Fund, and by Johan Boserup and Lise Boserups Grant. Dr. Nordestgaard reported consulting for or giving talks sponsored by AstraZeneca, Sanofi, Regeneron, Akcea, Amgen, Kowa, Denka, Amarin, Novartis, Novo Nordisk, Esperion, and Silence Therapeutics. The other authors reported no conflicts. Dr. Miller disclosed serving as a scientific adviser for Amarin and 89bio.
A version of this article first appeared on Medscape.com.
FROM THE EUROPEAN HEART JOURNAL
How should muscle mass be measured in heart failure?
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
, research suggests.
In a single-center, retrospective study that included more than 800 patients, high MUAC (hazard ratio for combined events, 0.590) and high AMC (HR for combined events, 0.529) were associated with significantly better prognoses than low MUAC and low AMC.
The findings were “surprising,” Kentaro Kamiya, PT, PhD, and Shota Uchida, PT, PhD, of Kitasato University School of Allied Health Sciences in Kanagawa, Japan, said in an interview.
“These findings challenge the current recommendations found in sarcopenia guidelines,” they noted. The European Working Group on Sarcopenia in Older People and the Asian Working Group for Sarcopenia recommend SMI, as measured by bioelectrical impedance analysis (BIA), and CC as methods for screening skeletal muscle mass.
The study was published online in the Canadian Journal of Cardiology.
Arm measures prognostic
Sarcopenia, which is marked by a loss of skeletal muscle mass and strength, is associated with risks of adverse outcomes. Patients with heart failure have a high rate of sarcopenia, but assessing skeletal muscle mass in these patients is difficult because of the fluid retention they often have.
The investigators examined the association between skeletal muscle mass metrics, measured using bioelectrical impedance analysis, and anthropometric measures and prognosis in patients with heart failure.
SMI was calculated using the BIA by dividing appendicular skeletal muscle mass by height squared. MUAC and CC were measured to the nearest 1 mm using a plastic tape measure. AMC was calculated as follows: MUAC (cm) − (0.314 x triceps skinfold [TSF]). The TSF was measured to the nearest 2 mm with a skinfold caliper. The measuring spot for TSF was the same measuring spot for MUAC. MUAC, CC, and TSF were measured by trained physiotherapists or nurses.
The investigators identified 1,930 consecutive patients with heart failure who underwent cardiac rehabilitation during their hospitalization. They excluded from their analysis 1,013 patients who did not undergo a skeletal mass metrics evaluation and 48 who could not be followed up.
The analysis included 869 patients (median age, 73 years; 62% men). Patients were separated into three groups on the basis of the sex-specific tertiles of skeletal muscle mass. The study endpoint was all-cause death or readmission due to heart failure, and the median follow-up period was 1.24 years.
After the investigators adjusted the data for age, sex, New York Heart Association functional class III or IV, left ventricular ejection fraction (LVEF), ischemic etiology, prior heart failure, diabetes, chronic obstructive pulmonary disease, log-transformed B-type natriuretic peptide (BNP), and estimated glomerular filtration rate (eGFR), the high MUAC and high AMC groups were associated with significantly better prognoses than their respective low groups. By contrast, high SMI and high CC were not associated with better prognoses.
Subgroup analyses showed no interactions between MUAC and age, sex, LVEF, BNP, eGFR, prior heart failure, beta-blocker use, and angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker use. However, diuretic agents significantly interacted with AMC (P = .03).
“These results support the use of MUAC and AMC to determine the risk stratification of sarcopenia and a poor prognosis in patients with heart failure and suggest that they may be useful in developing treatment strategies in patients with heart failure,” wrote the authors.
“When caring for patients with heart failure, it seems that the often overlooked and simple measure of arm circumference might carry significant prognostic value,” said Dr. Kamiya and Dr. Uchida. “So, as you cuff the arm for routine blood pressure measurement, it might be worthwhile to also pay attention to arm girth.”
Although the findings provide valuable insights, they should be approached with caution, Dr. Kamiya and Dr. Uchida added. “Before considering them practice-changing, further research is needed to validate these results in diverse patient cohorts.”
Prospective study needed
Commenting on the study for this news organization, Jonathan H. Whiteson, MD, vice chair of clinical operations and medical director of cardiac and pulmonary rehabilitation at NYU Langone Health’s Rusk Rehabilitation in New York, expressed concerns about the study methodology.
Methodologic weaknesses include the retrospective, observational nature of the study and the fact that incomplete data collection led to the exclusion of more than half of the potential participants, he said. In addition, “anthropometric measurements are prone to inter-rater error. It is not clear if the same or different researchers conducted these measurements.
“Furthermore, hospitalized patients may not be clinically stable when discharged,” said Dr. Whiteson. “It is recommended that biometrics for muscle mass and fluid retention be done when patients are at an optimized clinical state: that is, stabilized outpatients.”
For now, Dr. Whiteson concluded, the findings should be considered “interesting and suggestive of further study.” What’s needed is “a prospective study including all patients admitted with heart failure, but measurements done when the patient is stabilized as an outpatient.”
The study was partially supported by the Japan Society for the Promotion of Science KAKENHI. Dr. Kamiya, Dr. Uchida, and Dr. Whiteson reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN JOURNAL OF CARDIOLOGY
Aspirin for secondary CVD prevention underused worldwide
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
Nationally representative survey data from 51 countries showed that fewer than half of eligible people overall, including less than one-quarter in low-income and lower-middle–income countries, were taking aspirin for secondary CVD prevention.
“Our findings were not surprising but rather disappointing,” first author Sang Gune Yoo, MD, fellow in cardiovascular disease at Washington University in St Louis, said in an interview.
“We had hoped that the rates of aspirin use for secondary prevention would have increased after decades of effort to promote cardiovascular health worldwide,” Dr. Yoo said.
In high-income countries, such as the United States, rates of aspirin use for secondary CVD prevention were higher – at around 65% – but that’s also “really low and not particularly good or anything to be proud of,” Deepak Bhatt, MD, MPH, director of Mount Sinai Heart, New York, who wasn’t involved in the study, said in an interview.
The study was published online in JAMA. It provides the most extensive and up-to-date estimates of the worldwide use of aspirin for secondary prevention of CVD.
The researchers did a cross-sectional analysis using pooled, individual participant data from nationally representative health surveys conducted from 2013 to 2020 in 51 low-, middle-, and high-income countries.
The overall pooled sample included 124,505 nonpregnant adults (mean age, 52 years; 51% women). A total of 10,589 (8.1%) had a self-reported history of CVD and about 40% of these individuals were taking aspirin.
However, rates differed markedly by country, with use rates lowest in low-income and lower-middle–income countries and highest in upper-middle–income and high-income countries.
Primary vs. secondary prevention
The study did not explore the factors or reasons behind suboptimal aspirin use for secondary CVD prevention.
For example, it did not investigate whether data demonstrating that aspirin is not helpful in primary prevention is having a negative effect on use rates for secondary prevention. However, “rates of aspirin use for secondary prevention were low previously and remain suboptimal,” Dr. Yoo said.
Dr. Bhatt said that the “suboptimal” use of aspirin for secondary prevention is “a bit perplexing because this is a medicine that’s familiar, the data in secondary prevention are broadly known to physicians and it’s a cheap medicine so we can’t, in this case, blame high cost.”
Dr. Bhatt said it’s possible that coverage in the lay media of “negative” aspirin trials that may not distinguish between a primary and secondary prevention trial may contribute to confusion about aspirin. “In some cases, the doctor may think the patient is taking aspirin, but self discontinues it based on something they read or saw on the Internet.”
Dr. Yoo and colleagues said that, to meet the goal of reducing premature mortality from noncommunicable diseases, including CVD, “national health policies and health systems must develop, implement and evaluate strategies to promote evidence-based use of aspirin.”
“Strategies to boost appropriate aspirin use must be contextualized to the country and its health system,” Dr. Yoo added.
The study had no commercial funding. Dr. Yoo has disclosed no relevant financial relationships. Dr. Bhatt disclosed receiving grants and/or personal fees from many companies, publications, and organizations.
A version of this article appeared on Medscape.com.
FROM JAMA
ReCor renal denervation system safe, effective: FDA panel
in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
The device is intended to be used in renal arteries with diameters of 3.0 to 8.0 mm.
After hearing data from three trials, RADIANCE-HTN SOLO, RADIANCE II, and RADIANCE-HTN TRIO, the 12-member panel unanimously agreed that there was “reasonable assurance” that the ReCor Paradise Ultrasound Renal Denervation System (ReCor Medical) was safe.
However, while most of the panel felt the device was effective, a few disagreed.
Keith Allen, MD, director of surgical research for the Mid-America Heart and Lung Surgeons, Kansas City, Mo., who was one of the three panel members who voted no regarding efficacy, stated that he had concerns about the duration and the degree of efficacy shown in the trials.
Mark Lockhart, MD, University of Alabama, Birmingham, also voted no. “I do think there was an effect for 2 months, but the duration of that positive effect appears to decline after that period of time.”
Benjamin Saville, PhD, echoed Dr. Lockhart’s concern: “The benefit is more short term, it is unclear what the long term benefit would be.”
Data from all three trials showed a significant drop in blood pressure with the device compared with pharmacologic therapy, but after 2 months of follow-up, that advantage disappeared.
The FDA highlighted data from the trials that showed that at 2 months, uRDN patients experienced significant reductions in systolic blood pressure compared with those treated with a sham device; however, by 6 months, there was a difference of only 1 mm Hg between the two groups.
“It seems when I look at 6 months and 12 months, the benefit is very tiny. We know the safety is fine, but a benefit of less than 1 mm Hg difference would not make me want to have an intervention,” said statistician Janet Wittes, PhD.
“I think the device is efficacious, even though there is not much difference between sham and treatment, but a big issue is the fact that half of our patients are not compliant. That will make the benefits over sham more clear,” noted Jim Blankenship, MD, professor of medicine and director of the division of cardiology at the University of New Mexico in Albuquerque.
John Hirshfeld Jr., MD, professor emeritus of medicine at the University of Pennsylvania, Philadelphia, said he voted yes on safety and efficacy but admitted he had some misgivings. “The sample size was small, but it is a novel tool to add to our tool box, and hopefully it will be used responsibly,” he said.
John Somberg, MD, professor emeritus of medicine, cardiology, and pharmacology, Rush University, Chicago, said the data on this procedure show “that antihypertensive medication works. Denervation is not superior to medications. It lowers blood pressure and is persistent, but when you can take the sham group to almost as good control as you get in the denervation group, that shows it can also be done with medicines.”
The panel wants to see results from additional studies in important subpopulations who are affected by hypertension, including Black people, women, the elderly, and people who already have cardiovascular risk factors, such as diabetes and heart failure.
Deneen Hesser, RN, the panel’s patient representative, called for any postmarketing studies that may be conducted by ReCor to include a good patient education program and also a way of documenting patient-reported outcomes.
“This would help us ascertain how happy people were if they were able to reduce their medication burden by, for example, one drug, or if they were willing to undergo a procedure to be able to stop taking so many antihypertensive medications,” she said.
The panel will meet again to review data on Medtronic’s Simplicity Spyral Renal Denervation System, which is also for patients with uncontrolled hypertension.
A version of this article appeared on Medscape.com.
in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
The device is intended to be used in renal arteries with diameters of 3.0 to 8.0 mm.
After hearing data from three trials, RADIANCE-HTN SOLO, RADIANCE II, and RADIANCE-HTN TRIO, the 12-member panel unanimously agreed that there was “reasonable assurance” that the ReCor Paradise Ultrasound Renal Denervation System (ReCor Medical) was safe.
However, while most of the panel felt the device was effective, a few disagreed.
Keith Allen, MD, director of surgical research for the Mid-America Heart and Lung Surgeons, Kansas City, Mo., who was one of the three panel members who voted no regarding efficacy, stated that he had concerns about the duration and the degree of efficacy shown in the trials.
Mark Lockhart, MD, University of Alabama, Birmingham, also voted no. “I do think there was an effect for 2 months, but the duration of that positive effect appears to decline after that period of time.”
Benjamin Saville, PhD, echoed Dr. Lockhart’s concern: “The benefit is more short term, it is unclear what the long term benefit would be.”
Data from all three trials showed a significant drop in blood pressure with the device compared with pharmacologic therapy, but after 2 months of follow-up, that advantage disappeared.
The FDA highlighted data from the trials that showed that at 2 months, uRDN patients experienced significant reductions in systolic blood pressure compared with those treated with a sham device; however, by 6 months, there was a difference of only 1 mm Hg between the two groups.
“It seems when I look at 6 months and 12 months, the benefit is very tiny. We know the safety is fine, but a benefit of less than 1 mm Hg difference would not make me want to have an intervention,” said statistician Janet Wittes, PhD.
“I think the device is efficacious, even though there is not much difference between sham and treatment, but a big issue is the fact that half of our patients are not compliant. That will make the benefits over sham more clear,” noted Jim Blankenship, MD, professor of medicine and director of the division of cardiology at the University of New Mexico in Albuquerque.
John Hirshfeld Jr., MD, professor emeritus of medicine at the University of Pennsylvania, Philadelphia, said he voted yes on safety and efficacy but admitted he had some misgivings. “The sample size was small, but it is a novel tool to add to our tool box, and hopefully it will be used responsibly,” he said.
John Somberg, MD, professor emeritus of medicine, cardiology, and pharmacology, Rush University, Chicago, said the data on this procedure show “that antihypertensive medication works. Denervation is not superior to medications. It lowers blood pressure and is persistent, but when you can take the sham group to almost as good control as you get in the denervation group, that shows it can also be done with medicines.”
The panel wants to see results from additional studies in important subpopulations who are affected by hypertension, including Black people, women, the elderly, and people who already have cardiovascular risk factors, such as diabetes and heart failure.
Deneen Hesser, RN, the panel’s patient representative, called for any postmarketing studies that may be conducted by ReCor to include a good patient education program and also a way of documenting patient-reported outcomes.
“This would help us ascertain how happy people were if they were able to reduce their medication burden by, for example, one drug, or if they were willing to undergo a procedure to be able to stop taking so many antihypertensive medications,” she said.
The panel will meet again to review data on Medtronic’s Simplicity Spyral Renal Denervation System, which is also for patients with uncontrolled hypertension.
A version of this article appeared on Medscape.com.
in lowering blood pressure for adults with uncontrolled hypertension who may be inadequately responsive to, or who are intolerant of, antihypertensive medications.
The device is intended to be used in renal arteries with diameters of 3.0 to 8.0 mm.
After hearing data from three trials, RADIANCE-HTN SOLO, RADIANCE II, and RADIANCE-HTN TRIO, the 12-member panel unanimously agreed that there was “reasonable assurance” that the ReCor Paradise Ultrasound Renal Denervation System (ReCor Medical) was safe.
However, while most of the panel felt the device was effective, a few disagreed.
Keith Allen, MD, director of surgical research for the Mid-America Heart and Lung Surgeons, Kansas City, Mo., who was one of the three panel members who voted no regarding efficacy, stated that he had concerns about the duration and the degree of efficacy shown in the trials.
Mark Lockhart, MD, University of Alabama, Birmingham, also voted no. “I do think there was an effect for 2 months, but the duration of that positive effect appears to decline after that period of time.”
Benjamin Saville, PhD, echoed Dr. Lockhart’s concern: “The benefit is more short term, it is unclear what the long term benefit would be.”
Data from all three trials showed a significant drop in blood pressure with the device compared with pharmacologic therapy, but after 2 months of follow-up, that advantage disappeared.
The FDA highlighted data from the trials that showed that at 2 months, uRDN patients experienced significant reductions in systolic blood pressure compared with those treated with a sham device; however, by 6 months, there was a difference of only 1 mm Hg between the two groups.
“It seems when I look at 6 months and 12 months, the benefit is very tiny. We know the safety is fine, but a benefit of less than 1 mm Hg difference would not make me want to have an intervention,” said statistician Janet Wittes, PhD.
“I think the device is efficacious, even though there is not much difference between sham and treatment, but a big issue is the fact that half of our patients are not compliant. That will make the benefits over sham more clear,” noted Jim Blankenship, MD, professor of medicine and director of the division of cardiology at the University of New Mexico in Albuquerque.
John Hirshfeld Jr., MD, professor emeritus of medicine at the University of Pennsylvania, Philadelphia, said he voted yes on safety and efficacy but admitted he had some misgivings. “The sample size was small, but it is a novel tool to add to our tool box, and hopefully it will be used responsibly,” he said.
John Somberg, MD, professor emeritus of medicine, cardiology, and pharmacology, Rush University, Chicago, said the data on this procedure show “that antihypertensive medication works. Denervation is not superior to medications. It lowers blood pressure and is persistent, but when you can take the sham group to almost as good control as you get in the denervation group, that shows it can also be done with medicines.”
The panel wants to see results from additional studies in important subpopulations who are affected by hypertension, including Black people, women, the elderly, and people who already have cardiovascular risk factors, such as diabetes and heart failure.
Deneen Hesser, RN, the panel’s patient representative, called for any postmarketing studies that may be conducted by ReCor to include a good patient education program and also a way of documenting patient-reported outcomes.
“This would help us ascertain how happy people were if they were able to reduce their medication burden by, for example, one drug, or if they were willing to undergo a procedure to be able to stop taking so many antihypertensive medications,” she said.
The panel will meet again to review data on Medtronic’s Simplicity Spyral Renal Denervation System, which is also for patients with uncontrolled hypertension.
A version of this article appeared on Medscape.com.
As MOC debate heats up, cardiology societies weigh in
It’s no secret that many physicians question the value of Maintenance of Certification requirements and are concerned about the amount of time, effort, and money the process takes. Now, they and at least two cardiology societies are starting to speak up.
MOC is an initiative from the American Board of Internal Medicine that requires an initial certification that costs thousands of dollars and must be repeated every 10 years. Annual MOC requirements involve tests that cost $220 for the first certificate a physician holds and about $120 for each subsequent one.
Interventional cardiologists (ICs) and other subspecialists have additional fees and requirements.
MOC ‘burdensome,’ ‘costly,’ ‘complex’
On July 21, hematologist-oncologist Aaron Goodman, MD, an associate professor at the University of California, San Diego, posted a petition on behalf of ABIM diplomates.
As of August 22, the petition had garnered more than 18,000 signatures.
Dr. Goodman recently debated ABIM President and CEO Richard J. Baron, MD, in a Healthcare Unfiltered podcast. Before the debate, host Chadi Nabhan, MD, MBA, tweeted that he could not find a single physician who would defend the MOC and recertification.
The debate touched on topics such as fees, evidence of value, the certification test format, and the cost and requirements to maintain more than one board certification. Overall, Dr. Goodman made the analogy to giving a patient chemotherapy: Because there are harms, he better know that there are also benefits. He cited that the harms associated with MOC include “financial toxicity, time toxicity, and stress toxicity,” with the latter being particularly toxic to him personally.
Though the podcast gave both participants ample opportunities to express their views, it’s not clear that either participant persuaded the other.
Cardiologists who are unhappy with MOC are speaking up on X, formerly known as Twitter. IC Matthew Sample, MD, listed five things he’s done to improve his practice since IC graduation, for which he received no MOC points.
In response, internist Artem Minalyan, MD, asked: “Hypothetically, if Dr. Baron required an IC procedure, I wonder if he would request you to get all your MOC points prior to consenting.”
SCAI and HRS weigh in
Some professional societies have responded to the ABIM’s threat to revoke the certifications of cardiologists who don’t participate in periodic MOC activities.
The Society for Cardiovascular Angiography & Interventions published its “Position on ABIM Revocation of Certification for Not Participating in MOC.” In it, SCAI states that ABIM diplomates who pass their exams and report procedural volumes as required should be “indisputably” recognized as “certified” for the relevant time frame (for example, 10 years), regardless of whether they participate in any other MOC activities.
SCAI President George D. Dangas, MD, PhD, said in an interview that “many of our members have expressed their frustration surrounding the confusion regarding their MOC requirements, including myself. We felt that this confusion could endanger the certified status of members, which would inevitably impact patient care, which is our greatest concern.”
The society has received an “overwhelmingly positive response” to its statement, he said. “Our hope is that ABIM will consider simpler, transparent regulations that are reflective of the feedback received from their constituents.”
In response to the COVID-19 pandemic, ABIM extended the deadline for diplomates whose certificate expired in 2020 or 2021 until the end of 2022; Dr. Dangas suggested ABIM further extend the deadline to enroll in or renew the MOC to the end of 2024 and that ABIM should “develop a recertification program that can be explained in a single slide/page.”
Other subspecialty groups are following SCAI’s lead including the EP Advocacy Foundation, and the Heart Rhythm Society.
MOC alternatives
The ABIM touts the value of MOC on its website, stating: “There is compelling evidence showing that MOC improves value of care without sacrificing quality and that board certified physicians command higher salaries.”
Alternative options that are arguably less arduous are available.
In collaboration with ABIM, the American College of Cardiology launched the ABIM/ACC Collaborative Maintenance Pathway in 2019 as an alternative MOC assessment option.
The CMP “focuses on one or a small group of topics within cardiology each year, incorporating learning activities as well as a pre-/postformative knowledge assessment,” Janice Sibley, ACC’s executive vice president of education and publishing said in an interview, adding that the program continues to evolve.
In 2022, she noted that the ACC increased the flexibility of the CMP by removing the 7-hour learning engagement requirement, allowing users to choose how much time to spend learning in the CMP program. They also extended the performance assessment windows from 7 to 9 days each, covering 2 weekends for each.
She said that, to date, more than “6,400 learners” are enrolled in the CMP program.
Though the collaboration seems to make MOC less onerous, some cardiologists think it makes the ACC “complicit.”
A certification program that is independent of the ABIM launched in 2015. The National Board of Physicians and Surgeons is a nonprofit organization led by an advisory board of unpaid physicians. NBPAS seems to be gaining momentum and acceptance.
Cardiologist Melissa Walton-Shirley, MD, recounted her recertification experience with the NBPAS late in 2022. She now maintains a “hybrid” certification with both ABIM and NBPAS. Though she wants to support the latter, she found that the alternative certification option still requires an initial ABIM certification and is not recognized in all states or by many insurers and hospitals.
Will MOC ever disappear? Ms. Sibley said that the ACC is always looking to improve and enhance their offerings. “It is time to lead a change in the conversation from certification to continuous competency, from punitive to supportive options, from random knowledge testing to focused assessing knowledge gaps and lifelong learning. This will require innovation, technology, and new ways of thinking that offer cardiologists flexibility, relevance, and value and ultimately benefit the patients they serve.”
Many physicians, including cardiologists, are hoping that Dr. Goodman’s petition and further pressure from professional societies may finally translate into action.
A version of this article first appeared on Medscape.com.
It’s no secret that many physicians question the value of Maintenance of Certification requirements and are concerned about the amount of time, effort, and money the process takes. Now, they and at least two cardiology societies are starting to speak up.
MOC is an initiative from the American Board of Internal Medicine that requires an initial certification that costs thousands of dollars and must be repeated every 10 years. Annual MOC requirements involve tests that cost $220 for the first certificate a physician holds and about $120 for each subsequent one.
Interventional cardiologists (ICs) and other subspecialists have additional fees and requirements.
MOC ‘burdensome,’ ‘costly,’ ‘complex’
On July 21, hematologist-oncologist Aaron Goodman, MD, an associate professor at the University of California, San Diego, posted a petition on behalf of ABIM diplomates.
As of August 22, the petition had garnered more than 18,000 signatures.
Dr. Goodman recently debated ABIM President and CEO Richard J. Baron, MD, in a Healthcare Unfiltered podcast. Before the debate, host Chadi Nabhan, MD, MBA, tweeted that he could not find a single physician who would defend the MOC and recertification.
The debate touched on topics such as fees, evidence of value, the certification test format, and the cost and requirements to maintain more than one board certification. Overall, Dr. Goodman made the analogy to giving a patient chemotherapy: Because there are harms, he better know that there are also benefits. He cited that the harms associated with MOC include “financial toxicity, time toxicity, and stress toxicity,” with the latter being particularly toxic to him personally.
Though the podcast gave both participants ample opportunities to express their views, it’s not clear that either participant persuaded the other.
Cardiologists who are unhappy with MOC are speaking up on X, formerly known as Twitter. IC Matthew Sample, MD, listed five things he’s done to improve his practice since IC graduation, for which he received no MOC points.
In response, internist Artem Minalyan, MD, asked: “Hypothetically, if Dr. Baron required an IC procedure, I wonder if he would request you to get all your MOC points prior to consenting.”
SCAI and HRS weigh in
Some professional societies have responded to the ABIM’s threat to revoke the certifications of cardiologists who don’t participate in periodic MOC activities.
The Society for Cardiovascular Angiography & Interventions published its “Position on ABIM Revocation of Certification for Not Participating in MOC.” In it, SCAI states that ABIM diplomates who pass their exams and report procedural volumes as required should be “indisputably” recognized as “certified” for the relevant time frame (for example, 10 years), regardless of whether they participate in any other MOC activities.
SCAI President George D. Dangas, MD, PhD, said in an interview that “many of our members have expressed their frustration surrounding the confusion regarding their MOC requirements, including myself. We felt that this confusion could endanger the certified status of members, which would inevitably impact patient care, which is our greatest concern.”
The society has received an “overwhelmingly positive response” to its statement, he said. “Our hope is that ABIM will consider simpler, transparent regulations that are reflective of the feedback received from their constituents.”
In response to the COVID-19 pandemic, ABIM extended the deadline for diplomates whose certificate expired in 2020 or 2021 until the end of 2022; Dr. Dangas suggested ABIM further extend the deadline to enroll in or renew the MOC to the end of 2024 and that ABIM should “develop a recertification program that can be explained in a single slide/page.”
Other subspecialty groups are following SCAI’s lead including the EP Advocacy Foundation, and the Heart Rhythm Society.
MOC alternatives
The ABIM touts the value of MOC on its website, stating: “There is compelling evidence showing that MOC improves value of care without sacrificing quality and that board certified physicians command higher salaries.”
Alternative options that are arguably less arduous are available.
In collaboration with ABIM, the American College of Cardiology launched the ABIM/ACC Collaborative Maintenance Pathway in 2019 as an alternative MOC assessment option.
The CMP “focuses on one or a small group of topics within cardiology each year, incorporating learning activities as well as a pre-/postformative knowledge assessment,” Janice Sibley, ACC’s executive vice president of education and publishing said in an interview, adding that the program continues to evolve.
In 2022, she noted that the ACC increased the flexibility of the CMP by removing the 7-hour learning engagement requirement, allowing users to choose how much time to spend learning in the CMP program. They also extended the performance assessment windows from 7 to 9 days each, covering 2 weekends for each.
She said that, to date, more than “6,400 learners” are enrolled in the CMP program.
Though the collaboration seems to make MOC less onerous, some cardiologists think it makes the ACC “complicit.”
A certification program that is independent of the ABIM launched in 2015. The National Board of Physicians and Surgeons is a nonprofit organization led by an advisory board of unpaid physicians. NBPAS seems to be gaining momentum and acceptance.
Cardiologist Melissa Walton-Shirley, MD, recounted her recertification experience with the NBPAS late in 2022. She now maintains a “hybrid” certification with both ABIM and NBPAS. Though she wants to support the latter, she found that the alternative certification option still requires an initial ABIM certification and is not recognized in all states or by many insurers and hospitals.
Will MOC ever disappear? Ms. Sibley said that the ACC is always looking to improve and enhance their offerings. “It is time to lead a change in the conversation from certification to continuous competency, from punitive to supportive options, from random knowledge testing to focused assessing knowledge gaps and lifelong learning. This will require innovation, technology, and new ways of thinking that offer cardiologists flexibility, relevance, and value and ultimately benefit the patients they serve.”
Many physicians, including cardiologists, are hoping that Dr. Goodman’s petition and further pressure from professional societies may finally translate into action.
A version of this article first appeared on Medscape.com.
It’s no secret that many physicians question the value of Maintenance of Certification requirements and are concerned about the amount of time, effort, and money the process takes. Now, they and at least two cardiology societies are starting to speak up.
MOC is an initiative from the American Board of Internal Medicine that requires an initial certification that costs thousands of dollars and must be repeated every 10 years. Annual MOC requirements involve tests that cost $220 for the first certificate a physician holds and about $120 for each subsequent one.
Interventional cardiologists (ICs) and other subspecialists have additional fees and requirements.
MOC ‘burdensome,’ ‘costly,’ ‘complex’
On July 21, hematologist-oncologist Aaron Goodman, MD, an associate professor at the University of California, San Diego, posted a petition on behalf of ABIM diplomates.
As of August 22, the petition had garnered more than 18,000 signatures.
Dr. Goodman recently debated ABIM President and CEO Richard J. Baron, MD, in a Healthcare Unfiltered podcast. Before the debate, host Chadi Nabhan, MD, MBA, tweeted that he could not find a single physician who would defend the MOC and recertification.
The debate touched on topics such as fees, evidence of value, the certification test format, and the cost and requirements to maintain more than one board certification. Overall, Dr. Goodman made the analogy to giving a patient chemotherapy: Because there are harms, he better know that there are also benefits. He cited that the harms associated with MOC include “financial toxicity, time toxicity, and stress toxicity,” with the latter being particularly toxic to him personally.
Though the podcast gave both participants ample opportunities to express their views, it’s not clear that either participant persuaded the other.
Cardiologists who are unhappy with MOC are speaking up on X, formerly known as Twitter. IC Matthew Sample, MD, listed five things he’s done to improve his practice since IC graduation, for which he received no MOC points.
In response, internist Artem Minalyan, MD, asked: “Hypothetically, if Dr. Baron required an IC procedure, I wonder if he would request you to get all your MOC points prior to consenting.”
SCAI and HRS weigh in
Some professional societies have responded to the ABIM’s threat to revoke the certifications of cardiologists who don’t participate in periodic MOC activities.
The Society for Cardiovascular Angiography & Interventions published its “Position on ABIM Revocation of Certification for Not Participating in MOC.” In it, SCAI states that ABIM diplomates who pass their exams and report procedural volumes as required should be “indisputably” recognized as “certified” for the relevant time frame (for example, 10 years), regardless of whether they participate in any other MOC activities.
SCAI President George D. Dangas, MD, PhD, said in an interview that “many of our members have expressed their frustration surrounding the confusion regarding their MOC requirements, including myself. We felt that this confusion could endanger the certified status of members, which would inevitably impact patient care, which is our greatest concern.”
The society has received an “overwhelmingly positive response” to its statement, he said. “Our hope is that ABIM will consider simpler, transparent regulations that are reflective of the feedback received from their constituents.”
In response to the COVID-19 pandemic, ABIM extended the deadline for diplomates whose certificate expired in 2020 or 2021 until the end of 2022; Dr. Dangas suggested ABIM further extend the deadline to enroll in or renew the MOC to the end of 2024 and that ABIM should “develop a recertification program that can be explained in a single slide/page.”
Other subspecialty groups are following SCAI’s lead including the EP Advocacy Foundation, and the Heart Rhythm Society.
MOC alternatives
The ABIM touts the value of MOC on its website, stating: “There is compelling evidence showing that MOC improves value of care without sacrificing quality and that board certified physicians command higher salaries.”
Alternative options that are arguably less arduous are available.
In collaboration with ABIM, the American College of Cardiology launched the ABIM/ACC Collaborative Maintenance Pathway in 2019 as an alternative MOC assessment option.
The CMP “focuses on one or a small group of topics within cardiology each year, incorporating learning activities as well as a pre-/postformative knowledge assessment,” Janice Sibley, ACC’s executive vice president of education and publishing said in an interview, adding that the program continues to evolve.
In 2022, she noted that the ACC increased the flexibility of the CMP by removing the 7-hour learning engagement requirement, allowing users to choose how much time to spend learning in the CMP program. They also extended the performance assessment windows from 7 to 9 days each, covering 2 weekends for each.
She said that, to date, more than “6,400 learners” are enrolled in the CMP program.
Though the collaboration seems to make MOC less onerous, some cardiologists think it makes the ACC “complicit.”
A certification program that is independent of the ABIM launched in 2015. The National Board of Physicians and Surgeons is a nonprofit organization led by an advisory board of unpaid physicians. NBPAS seems to be gaining momentum and acceptance.
Cardiologist Melissa Walton-Shirley, MD, recounted her recertification experience with the NBPAS late in 2022. She now maintains a “hybrid” certification with both ABIM and NBPAS. Though she wants to support the latter, she found that the alternative certification option still requires an initial ABIM certification and is not recognized in all states or by many insurers and hospitals.
Will MOC ever disappear? Ms. Sibley said that the ACC is always looking to improve and enhance their offerings. “It is time to lead a change in the conversation from certification to continuous competency, from punitive to supportive options, from random knowledge testing to focused assessing knowledge gaps and lifelong learning. This will require innovation, technology, and new ways of thinking that offer cardiologists flexibility, relevance, and value and ultimately benefit the patients they serve.”
Many physicians, including cardiologists, are hoping that Dr. Goodman’s petition and further pressure from professional societies may finally translate into action.
A version of this article first appeared on Medscape.com.
AHA advocates normothermia for most comatose OHCA patients
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
a new American Heart Association (AHA) scientific advisory suggests.
On the basis of data from recent trials, the International Liaison Committee on Resuscitation and other organizations have altered their treatment recommendations for temperature management after cardiac arrest.
The AHA will present guidelines on this topic in a focused update to be published later in the year. Meanwhile, AHA’s Emergency Cardiovascular Care Committee convened a writing group to review the Hypothermia Versus Normothermia After Out-of-Hospital Cardiac Arrest (TTM2) trial in the context of other recent evidence and rendered an expert opinion on how the trial may influence clinical practice. These findings will be incorporated into the upcoming guidelines.
“Many centers have already moved toward controlled normothermia for post-arrest patients, so we think this guidance will be welcomed by many,” said Sarah Perman, MD, of the Yale University, and Kate Berg, MD, of Beth Israel Deaconess Medical Center, who are both members of the AHA Emergency Cardiovascular Care Committee that authored the advisory.
“For those who continue to favor temperatures in the 32° to 36° range for some or even all patients, the guidance that we have drafted leaves room for clinicians to make patient-centered decisions,” they told this news organization.
“Certainly, a finite guideline that recommends one temperature for all would be easier to apply,” the authors acknowledged. “However, cardiac arrest is a heterogeneous event and brain injury is variable, and definitive evidence that one temperature in the range of 32-37.5 is superior to another is lacking. We hope that clinicians find that this guidance supports and informs their practice.”
The advisory was published online in Circulation.
TTM2 key
The new guidance is based largely on findings from the TTM2 trial, a multicenter, randomized clinical trial of temperature management for neuroprotection after cardiac arrest that included 1,900 unresponsive adult patients successfully resuscitated from OHCA.
Patients were randomly assigned to receive hypothermia, defined as a target temperature of 33° C for 28 hours, followed by gradual rewarming to 37° C, or normothermia, defined as a target temperature < 37.8° C, with early treatment of fever.
No significant between-group difference was seen in the primary outcome of death at 6 months, nor were there any significant differences by subgroups of sex, age, time to return of spontaneous circulation, initial rhythm, or circulatory shock on admission.
Although it’s still not clear whether certain patients might benefit from lower target temperatures, the authors noted, major international organizations now suggest a target post–cardiac arrest temperature of less than 37.5° C.
By contrast, current AHA guidelines endorse targeting a temperature between 32° C and 36° C for 24 hours.
Between now and the forthcoming formal guidance in the “2023 American Heart Association Focused Update on Advanced Cardiovascular Life Support,” the scientific advisory writing group agreed: “For unresponsive post–cardiac arrest adult patients with characteristics similar to those of individuals included in the TTM2 trial (OHCA of cardiac or unknown cause, excluding those with unwitnessed asystole), controlling patient temperature to < 37.5° C is a reasonable and evidence-based approach.
“For the broader group of patients with in-hospital cardiac arrest or OHCA of noncardiac (other medical) cause, evidence for the ideal approach to temperature management after return of spontaneous circulation is less certain; whether some of these patients might benefit from temperature control at temperatures between 33° C and 37.5° C remains unclear.”
Unless a catastrophic brain injury results from OHCA, the group wrote, “strictly preventing fever with continuous temperature monitoring, providing comprehensive critical care support, and deploying multimodal evidence-based strategies for neuroprognostication at a minimum of 72 hours after normothermia remain essential ...”
Dr. Perman and Dr. Berg concluded, “We hope that this guidance continues to encourage aggressive post-arrest care that includes focus on temperature control as well as the other major contributors to post-arrest bundles of care including hemodynamic optimization and guideline concordant neuroprognostication.”
No funding was reported. Dr. Berg has received grant support from AHA/ILCOR, and Dr. Perman, from NIH/NHLBI.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Artificial sweeteners no help for weight loss: Review
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
It also shows evidence that these products are not beneficial for controlling excess weight.
Francisco Gómez-Delgado, MD, PhD, and Pablo Pérez-Martínez, MD, PhD, are members of the Spanish Society of Arteriosclerosis and of the Spanish Society of Internal Medicine. They have coordinated an updated review of the leading scientific evidence surrounding artificial sweeteners: evidence showing that far from positively affecting our health, they have “negative effects for the cardiometabolic system.”
The paper, published in Current Opinion in Cardiology, delves into the consumption of these sweeteners and their negative influence on the development of obesity and of several of the most important cardiometabolic risk factors (hypertension, dyslipidemia, and diabetes).
Globalization and the increase in consumption of ultraprocessed foods have led to a need for greater knowledge on the health impacts of certain nutrients such as artificial sweeteners (nutritive and nonnutritive). This review aims to analyze their role and their effect on cardiometabolic and cardiovascular disease risk.
Cardiovascular risk
The detrimental effects of a high-calorie, high-sugar diet have been well established. For this reason, health authorities recommend limiting sugar consumption. The recommendation has led the food industry to develop different artificial sweeteners with specific properties, such as flavor and stability (nutritive artificial sweeteners), and others aimed at limiting sugar in the diet (nonnutritive artificial sweeteners). Recent evidence explores the influence of these two types of artificial sweeteners on cardiovascular disease risk through risk factors such as obesity and type 2 diabetes, among others.
Initially, the consumption of artificial sweeteners was presented as an alternative for reducing calorie intake in the diet as an option for people with excess weight and obesity. However, as this paper explains, the consumption of these artificial sweeteners favors weight gain because of neuroendocrine mechanisms related to satiety that are abnormally activated when artificial sweeteners are consumed.
Weight gain
On the other hand, evidence shows that consuming artificial sweeteners does not encourage weight loss. “Quite the contrary,” Dr. Pérez-Martínez, scientific director at the Maimonides Biomedical Research Institute and internist at the University Hospital Reina Sofia, both in Córdoba, told this news organization. “There is evidence showing weight gain resulting from the effect that artificial sweetener consumption has at the neurohormonal level by altering the mechanisms involved in regulating the feeling of satiety.”
However, on the basis of current evidence, sugar cannot be claimed to be less harmful. “What we do know is that in both cases, we should reduce or remove them from our diets and replace them with other healthier alternatives for weight management, such as eating plant-based products or being physically active.”
Confronting ignorance
Nonetheless, these recommendations are conditional, “because the weight of the evidence is not extremely high, since there have not been a whole lot of studies. All nutritional studies must be viewed with caution,” Manuel Anguita, MD, PhD, said in an interview. Dr. Anguita is department head of clinical cardiology at the University Hospital Reina Sofia in Córdoba and past president of the Spanish Society of Cardiology.
“It’s something that should be included within the medical record when you’re assessing cardiovascular risk. In addition to identifying patients who use artificial sweeteners, it’s especially important to emphasize that it’s not an appropriate recommendation for weight management.” Healthier measures include moderate exercise and the Mediterranean diet.
Explaining why this research is valuable, he said, “It’s generally useful because there’s ignorance not only in the population but among physicians as well [about] these negative effects of sweeteners.”
Diabetes and metabolic syndrome
Artificial sweeteners cause significant disruptions in the endocrine system, leading our metabolism to function abnormally. The review revealed that consuming artificial sweeteners raises the risk for type 2 diabetes by between 18% and 24% and raises the risk for metabolic syndrome by up to 44%.
Dr. Gómez-Delgado, an internal medicine specialist at the University Hospital of Jaen in Spain and first author of the study, discussed the deleterious effects of sweeteners on metabolism. “On one hand, neurohormonal disorders impact appetite, and the feeling of satiety is abnormally delayed.” On the other hand, “they induce excessive insulin secretion in the pancreas,” which in the long run, encourages metabolic disorders that lead to diabetes. Ultimately, this process produces what we know as “dysbiosis, since our microbiota is unable to process these artificial sweeteners.” Dysbiosis triggers specific pathophysiologic processes that negatively affect cardiometabolic and cardiovascular systems.
No differences
Regarding the type of sweetener, Dr. Gómez-Delgado noted that currently available studies assess the consumption of special dietary products that, in most cases, include various types of artificial sweeteners. “So, it’s not possible to define specific differences between them as to how they impact our health.” Additional studies are needed to confirm this effect at the cardiometabolic level and to analyze the different types of artificial sweeteners individually.
“There’s enough evidence to confirm that consuming artificial sweeteners negatively interferes with our metabolism – especially glucose metabolism – and increases the risk of developing diabetes,” said Dr. Gómez-Delgado.
High-sodium drinks
When it comes to the influence of artificial sweeteners on hypertension, “there is no single explanation. The World Health Organization already discussed this issue 4-5 years ago, not only due to their carcinogenic risk, but also due to this cardiovascular risk in terms of a lack of control of obesity, diabetes, and hypertension,” said Dr. Anguita.
Another important point “is that this is not in reference to the sweeteners themselves, but to soft drinks containing those components, which is where we have more studies,” he added. There are two factors explaining this increase in hypertension, which poses a problem at the population level, with medium- to long-term follow-up. “The sugary beverages that we mentioned have a higher sodium content. That is, the sweeteners add this element, which is a factor that’s directly linked to the increase in blood pressure levels.” Another factor that can influence blood pressure is “the increase in insulin secretion that has been described as resulting from sweeteners. In the medium and long term, this is associated with increased blood pressure levels.”
Cardiovascular risk factor?
Are artificial sweeteners considered to be a new cardiovascular risk factor? “What they really do is increase the incidence of the other classic risk factors,” including obesity, said Dr. Anguita. It has been shown that artificial sweeteners don’t reduce obesity when used continuously. Nonetheless, “there is still not enough evidence to view it in the same light as the classic risk factors,” added Dr. Anguita. However, it is a factor that can clearly worsen the control of the other factors. Therefore, “it’s appropriate to sound an alarm and explain that it’s not the best way to lose weight; there are many other healthier choices.”
“We need more robust evidence to take a clear position on the use of this type of sweetener and its detrimental effect on health. Meanwhile, it would be ideal to limit their consumption or even avoid adding artificial sweeteners to coffee or teas,” added Dr. Pérez-Martínez.
Regulate consumption
Dr. Pérez-Martínez mentioned that the measures proposed to regulate the consumption of artificial sweeteners and to modify the current legislation must involve “minimizing the consumption of these special dietary products as much as possible and even avoiding adding these artificial sweeteners to the foods that we consume; for example, to coffee and tea.” On the other hand, “we must provide consumers with information that is as clear and simple as possible regarding the composition of the food they consume and how it impacts their health.”
However, “we need more evidence to be able to take a clear position on what type of sweeteners we can consume in our diet and also to what extent we should limit their presence in the foods we consume,” said Dr. Pérez-Martínez.
Last, “most of the evidence is from short-term observational studies that assess frequencies and patterns of consumption of foods containing these artificial sweeteners.” Of course, “we need studies that specifically analyze their effects at the metabolic level as well as longer-term studies where the nutritional follow-up of participants is more accurate and rigorous, especially when it comes to the consumption of this type of food,” concluded Dr. Gómez-Delgado.
This article was translated from the Medscape Spanish Edition. A version appeared on Medscape.com.
FROM CURRENT OPINION IN CARDIOLOGY