User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
Decline in non-Hodgkin lymphoma deaths to continue in 2018
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.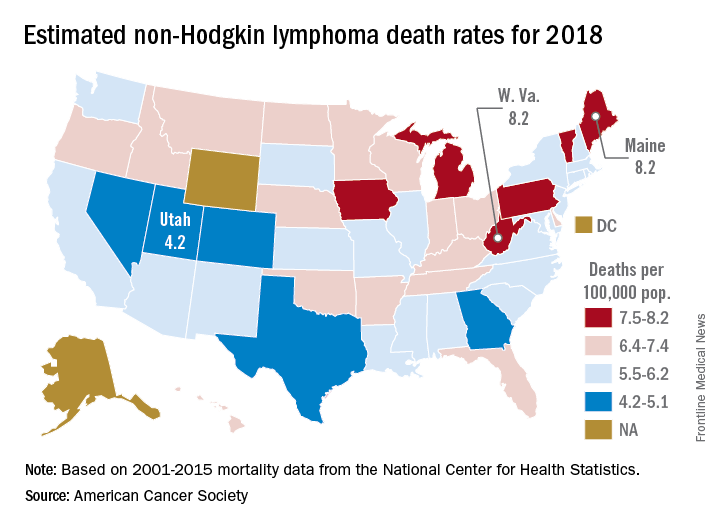
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
Mortality from non-Hodgkin lymphoma is expected to be about 6.1 per 100,000 population in 2018, with the highest rate in Maine and West Virginia and the lowest in Utah.
in its Cancer Facts & Figures 2018, based on analysis of 2001-2015 data from the National Center for Health Statistics. That figure is down from the 20,140 predicted for 2017, as the trend in the death rate since 2006 has been a decline of about 2% per year.
Nationally, death rates for NHL were 7.4 per 100,000 for males and 4.5 for females for 2011-2015, and incidence rates were 22.9 per 100,000 for males and 15.8 for females for 2010-2014, the ACS reported.
Over time, the relative survival rate for NHL has gone from 47% in 1975-1977 to 51% in 1987-1989 to 73% in 2007-2013, although there is some disparity between whites, whose respective rates are 47%, 51%, and 74%, and blacks, who have rates of 49%, 46%, and 67%, respectively, the ACS said.
TRANSCEND NHL trial identifies window for CAR T expansion
for CAR T expansion
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
sworcester@frontlinemedcom.com
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
sworcester@frontlinemedcom.com
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
SAN FRANCISCO – The CD19-directed 4-1BB chimeric antigen receptor (CAR) T-cell product JCAR017 demonstrated increased CAR T-cell expansion and persistence, and higher durability of response at higher dose levels – with manageable toxicities – in a pivotal phase 1 trial of relapsed/refractory B-cell non-Hodgkin lymphoma.
However, preliminary modeling data suggest that a therapeutic window exists for the CAR T-cell expansion, which means that development of strategies for pushing patients into that window could enhance efficacy and limit toxicity associated with JCAR017, Tanya Siddiqi, MD, of City of Hope Comprehensive Cancer Center, Duarte, Calif., reported at the ASCO-SITC Clinical Immuno-Oncology Symposium.
TRANSCEND NHL 001 is a multicenter, seamless design, pivotal trial, which started as a phase 1 first-in-human study of JCAR017, a defined composition CAR T-cell product also known as lisocabtagene maraleucel and administered at precise doses of CD4+ and CD8+ CAR T cells. Dose-finding and dose-expansion cohorts have been investigated, and currently the pivotal diffuse large B-cell lymphoma (DLBCL) cohort is enrolling, Dr. Saddiqi said, noting that dose level 2 (1 x 108 cells given as a single dose) was selected for that cohort.
The current findings are based on the TRANSCEND core population – a set of patients selected from the dose-finding and dose-expansion cohorts. This population includes patients with DLBCL not otherwise specified, transformed follicular lymphoma, or high-grade double- or triple-hit lymphomas, she said, explaining that she and her colleagues looked at prelymphodepletion baseline patient characteristics and biomarkers to assess how they related to outcomes and toxicities. This precise dosing of JCAR017 reduces variability, enabling the identification of potential patient factors associated with clinical outcomes, she said.
Response rates among 27 patients who received dose level 2 were high, with an 81% overall response rate and a 63% complete response rate.
“Patients with complete remission seemed to have more of a durable response,” she said. “In the core population at dose level 2, 50% with 6 months of follow-up seemed to remain in complete response, so there’s a dose-response effect in these patients.”
It doesn’t appear that the dose affects development of cytokine release syndrome (CRS) or neurotoxicity (NT), as the rates of these (30% and 20%, respectively) did not differ by dose level or schedule, but certain baseline features, such as a lactate dehydrogenase (LDH) level above 500 U/L and tumor burden measured as the sum of the product of diameters of 50 cm or greater, do appear to affect the development of these toxicities.
For example, 10 of 13 (77%) of patients with both of those baseline characteristics developed CRS, and 7 of 13 (54%) developed NT; for those without these characteristics, the odds of developing CRS and NT were significantly lower, she said.
Of note, higher Cmax, or peak expansion of CAR T cells in vivo, was seen at dose level 2, and exposure (area under the curve) was also higher, as was expected at that dose level, Dr. Siddiqi said.
“It also appears that there is a trend of patients with higher tumor burden to have higher expansion of their CAR T cells in vivo,” she said, noting that some of those patients were “superexpanders” with very high expansion of CAR T cells. “Similarly there were certain cytokines at the prelymphodepletion time point that seem to be higher … in patients who also go on to have higher expansion of their CAR T cells.”
These included interleukin-7, IL-15, MIP (macrophage inflammatory protein)–1alpha, and tumor necrosis factor (TNF)–alpha.
“Patients with certain higher inflammatory cytokines and biomarkers of inflammation also were noted to have higher events of CRS and neurotoxicity, so not just higher tumor burden, but also higher inflammatory state at baseline seems to affect patients in terms of getting any grade CRS or any grade neurotoxicity,” she said.
Those associated with CRS included ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNF-alpha, and MIP-1beta levels, and those associated with NT included ferritin, CRP, d-dimer, IL-6, IL-15, TNF-alpha, and MIP-1alpha levels.
“Interestingly, there’s an inverse relationship between the expression of some of these biomarkers and inflammatory markers and the durability of response at 3 months,” she said.
Patients with higher tumor burden, LDH, and other markers either had no response at 3 months, or they had a very rapid response at the 1-month mark and then lost their response by 3 months, she noted.
“One of the theories could be that these patients with higher tumor burden, higher inflammatory state – they have such a high peak expansion rapidly after receiving their CAR T cells that potentially those CAR T cells may be getting exhausted or dying off very quickly, and therefore patients can lose their response,” she said.
When these data are considered together and modeled, there seems to be a therapeutic window where patients who have optimal expansion of CAR T cells in vivo may have lesser toxicity, higher overall response rates, and better durability of response, she said.
That is, on one end of the spectrum, there are patients with lower CAR T-cell expansion who have lower toxicities, but who also have lower overall response rates and lower durability of response, and on the other end, there are patients with superhigh expansion of CAR T cells, who have higher response rates, but also higher rates of toxicity, and who lose their response quickly.
“So if we can identify patients who would be in this window of optimal target expansion of CAR T cells, and if we could find strategies or mechanisms to move patients who are on either end of that spectrum into this window, we may be able to get patients with better efficacy and lower toxicities, and there could be combination strategies that could help get us there,” she said.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
sworcester@frontlinemedcom.com
SOURCE: Siddiqi T et al. ASCO-SITC, abstract 122.
for CAR T expansion
for CAR T expansion
REPORTING FROM THE CLINICAL IMMUNO-ONCOLOGY SYNDROME
Key clinical point:
Major finding: In all, 77% of patients with a lactate dehydrogenase level greater than 500 U/L and a sum of the product of diameters of 50 cm or greater developed cytokine release syndrome, and 54% of patients with those characteristics developed neurotoxicity.
Study details: A cohort of 27 patients from the pivotal phase 1 TRANSCEND NHL 001 trial.
Disclosures: TRANSCEND NHL 001 is sponsored by Juno Therapeutics. Dr. Siddiqi reported serving as a consultant or adviser for Juno Therapeutics, and as a member of the speakers bureau for Pharmacyclics/Janssen and Seattle Genetics. Research funding was provided to her institution by Juno Therapeutics and several other companies.
Source: Siddiqi T et al. ASCO-SITC, abstract 122.
Triple therapy ups response in refractory mantle cell lymphoma
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
A combination of ibrutinib, lenalidomide, and rituximab produced an overall response rate of 76% at 17.8 months median follow-up among 50 adults with relapsed or refractory mantle cell lymphoma, according to an open-label, single-arm, phase 2 trial.
There were complete responses in 28 patients (56%) and partial responses in 10 (20%). Median progression-free survival was 16 months and median overall survival was 22 months. Similar proportions of patients, with and without TP53 mutations, had overall and complete responses, suggesting that triple therapy might be particularly useful in patients with high-risk genetic features.
“Our results provide preliminary evidence that the triplet combination of ibrutinib, lenalidomide, and rituximab is an active regimen in patients with relapsed or refractory mantle cell lymphoma, and should be evaluated in a prospective randomized controlled trial,” wrote Mats Jerkeman, MD, of Lund University, Sweden, and colleagues. The report was published in The Lancet Haematology.
“Addition of lenalidomide to ibrutinib and rituximab might increase the proportion of patients who have complete remission ... Previous studies reported complete responses in 44% of patients on ibrutinib and rituximab, in 36% of patients on rituximab and lenalidomide, and in 19% of patients on ibrutinib alone,” they wrote.
Treatment was divided into an induction phase of 12 cycles of 28 days with all three drugs and a maintenance phase with ibrutinib and rituximab only, given until disease progression or unacceptable toxicity. All the patients had previously been treated with at least one rituximab-containing regimen.
Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
SOURCE: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
FROM THE LANCET HAEMATOLOGY
Key clinical point:
Major finding: The overall response from for the combination of the three drugs was 76% at 17.8 months median follow-up.
Study details: An open-label, single-arm, phase 2 trial of 50 adults with relapsed/refractory MCL.
Disclosures: Janssen and Celgene funded the work. Dr. Jerkeman reported ties to Janssen and Celgene, as well as AbbVie and Gilead.
Source: Jerkeman M et al. Lancet Haematol. 2018 Jan 29. doi: 10.1016/S2352-3026(18)30018-8.
Background color a dermoscopic clue to cutaneous B-cell lymphoma
A salmon-colored background and prominent serpentine blood vessels are two characteristic features of primary cutaneous B-cell lymphoma (PCBCL) that can be identified dermoscopically and may aid diagnosis, researchers say.
In the January issue of the Journal of the European Academy of Dermatology and Venereology, researchers reported the results of a retrospective observational study using the dermoscopic images of 58 biopsy-confirmed primary cutaneous B-cell lymphoma lesions in 51 patients.
While all the lesions were nonpigmented, 46 (79.3%) of them showed salmon- or yellow- to orange- colored background areas. More than three-quarters of the lesions also featured prominent blood vessels (77.6%), the majority of which were serpentine in nature.
, while only 8.6% of the lesions showed neither feature.
Of the 58 lesions, the authors selected 17 to be evaluated by two dermoscopy experts who were blinded to the diagnosis. In 70.6% of these cases they included cutaneous B-cell lymphoma in the differential diagnosis, while other diagnoses included spider bite (58.8%), basal cell carcinoma (52.9%), amelanotic melanoma (47.1%), and scar/keloid (47.1%). Overall, the two experts did not agree on almost 30% of the suggested differential diagnoses.
“The presentation of cutaneous lymphomas in general and of PCBCLs in particular can be nonspecific, and a biopsy is essential for a definitive diagnosis,” wrote Shamir Geller, MD, of the dermatology service at Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The 58 PCBCLs analyzed were among 172 biopsy-proven PCBCL lesions in the study, which were newly diagnosed and whose pathology reports included the clinical differential diagnosis in the pathology requisition slip, in patients referred to the cancer center between 1992 and 2016. In only 16.3% of these cases, the clinician suspected cutaneous lymphoma. Skin malignancies were suspected in 54.7% of cases, with the leading diagnosis being basal cell carcinoma in 17.4% of cases. Basal cell carcinoma was considered in nearly one-third of lesions, particularly those on the head and neck.
Nonneoplastic conditions suspected by clinicians included cyst in 21.5% of cases, granulomatous processes in 15.7%, and infectious disease in 4.7%.
The authors commented that a low index of suspicion for skin lymphoma was seen regardless of the subtype or site.
“While dermoscopy offers a bridge between the naked eye examination and the histopathological appearance, cutaneous lymphoma is diagnosed on a cellular level using histopathology, immunohistochemistry and molecular studies,” they wrote. “Therefore, dermoscopy may serve as an ancillary tool in PCBCL; however, it cannot be diagnostic.”
The study was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center. Dr. Geller is a recipient of a grant from the American Physicians and Friends For Medicine in Israel. No conflicts of interest were declared.
SOURCE: Geller S et al. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):53-6.
A salmon-colored background and prominent serpentine blood vessels are two characteristic features of primary cutaneous B-cell lymphoma (PCBCL) that can be identified dermoscopically and may aid diagnosis, researchers say.
In the January issue of the Journal of the European Academy of Dermatology and Venereology, researchers reported the results of a retrospective observational study using the dermoscopic images of 58 biopsy-confirmed primary cutaneous B-cell lymphoma lesions in 51 patients.
While all the lesions were nonpigmented, 46 (79.3%) of them showed salmon- or yellow- to orange- colored background areas. More than three-quarters of the lesions also featured prominent blood vessels (77.6%), the majority of which were serpentine in nature.
, while only 8.6% of the lesions showed neither feature.
Of the 58 lesions, the authors selected 17 to be evaluated by two dermoscopy experts who were blinded to the diagnosis. In 70.6% of these cases they included cutaneous B-cell lymphoma in the differential diagnosis, while other diagnoses included spider bite (58.8%), basal cell carcinoma (52.9%), amelanotic melanoma (47.1%), and scar/keloid (47.1%). Overall, the two experts did not agree on almost 30% of the suggested differential diagnoses.
“The presentation of cutaneous lymphomas in general and of PCBCLs in particular can be nonspecific, and a biopsy is essential for a definitive diagnosis,” wrote Shamir Geller, MD, of the dermatology service at Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The 58 PCBCLs analyzed were among 172 biopsy-proven PCBCL lesions in the study, which were newly diagnosed and whose pathology reports included the clinical differential diagnosis in the pathology requisition slip, in patients referred to the cancer center between 1992 and 2016. In only 16.3% of these cases, the clinician suspected cutaneous lymphoma. Skin malignancies were suspected in 54.7% of cases, with the leading diagnosis being basal cell carcinoma in 17.4% of cases. Basal cell carcinoma was considered in nearly one-third of lesions, particularly those on the head and neck.
Nonneoplastic conditions suspected by clinicians included cyst in 21.5% of cases, granulomatous processes in 15.7%, and infectious disease in 4.7%.
The authors commented that a low index of suspicion for skin lymphoma was seen regardless of the subtype or site.
“While dermoscopy offers a bridge between the naked eye examination and the histopathological appearance, cutaneous lymphoma is diagnosed on a cellular level using histopathology, immunohistochemistry and molecular studies,” they wrote. “Therefore, dermoscopy may serve as an ancillary tool in PCBCL; however, it cannot be diagnostic.”
The study was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center. Dr. Geller is a recipient of a grant from the American Physicians and Friends For Medicine in Israel. No conflicts of interest were declared.
SOURCE: Geller S et al. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):53-6.
A salmon-colored background and prominent serpentine blood vessels are two characteristic features of primary cutaneous B-cell lymphoma (PCBCL) that can be identified dermoscopically and may aid diagnosis, researchers say.
In the January issue of the Journal of the European Academy of Dermatology and Venereology, researchers reported the results of a retrospective observational study using the dermoscopic images of 58 biopsy-confirmed primary cutaneous B-cell lymphoma lesions in 51 patients.
While all the lesions were nonpigmented, 46 (79.3%) of them showed salmon- or yellow- to orange- colored background areas. More than three-quarters of the lesions also featured prominent blood vessels (77.6%), the majority of which were serpentine in nature.
, while only 8.6% of the lesions showed neither feature.
Of the 58 lesions, the authors selected 17 to be evaluated by two dermoscopy experts who were blinded to the diagnosis. In 70.6% of these cases they included cutaneous B-cell lymphoma in the differential diagnosis, while other diagnoses included spider bite (58.8%), basal cell carcinoma (52.9%), amelanotic melanoma (47.1%), and scar/keloid (47.1%). Overall, the two experts did not agree on almost 30% of the suggested differential diagnoses.
“The presentation of cutaneous lymphomas in general and of PCBCLs in particular can be nonspecific, and a biopsy is essential for a definitive diagnosis,” wrote Shamir Geller, MD, of the dermatology service at Memorial Sloan Kettering Cancer Center, New York, and his coauthors.
The 58 PCBCLs analyzed were among 172 biopsy-proven PCBCL lesions in the study, which were newly diagnosed and whose pathology reports included the clinical differential diagnosis in the pathology requisition slip, in patients referred to the cancer center between 1992 and 2016. In only 16.3% of these cases, the clinician suspected cutaneous lymphoma. Skin malignancies were suspected in 54.7% of cases, with the leading diagnosis being basal cell carcinoma in 17.4% of cases. Basal cell carcinoma was considered in nearly one-third of lesions, particularly those on the head and neck.
Nonneoplastic conditions suspected by clinicians included cyst in 21.5% of cases, granulomatous processes in 15.7%, and infectious disease in 4.7%.
The authors commented that a low index of suspicion for skin lymphoma was seen regardless of the subtype or site.
“While dermoscopy offers a bridge between the naked eye examination and the histopathological appearance, cutaneous lymphoma is diagnosed on a cellular level using histopathology, immunohistochemistry and molecular studies,” they wrote. “Therefore, dermoscopy may serve as an ancillary tool in PCBCL; however, it cannot be diagnostic.”
The study was supported in part by the National Institutes of Health/National Cancer Institute Cancer Center. Dr. Geller is a recipient of a grant from the American Physicians and Friends For Medicine in Israel. No conflicts of interest were declared.
SOURCE: Geller S et al. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):53-6.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: A salmon-colored background and prominent serpentine blood vessels are two characteristic dermoscopic features of primary cutaneous B-cell lymphoma (PCBCL).
Major finding: Nearly 80% of PCBCLs had a salmon-colored background on dermoscopy.
Data source: A retrospective observational study that analyzed 172 biopsy-proven PCBCLs, including 58 PCBCL dermoscopic images.
Disclosures: The study was supported by the NIH/NCI Cancer Center. The lead author received a grant from the American Physicians and Friends for Medicine in Israel. No conflicts of interest were declared.
Source: Geller S et al. J Eur Acad Dermatol Venereol. 2018 Jan;32(1):53-6.
VIDEO: Practice changers out of ASH 2017
ATLANTA – There were a lot of new data presented during the annual meeting of the American Society of Hematology. But what findings could actually change the way you practice?
Robert A. Brodsky, MD, director of the division of hematology at Johns Hopkins University in Baltimore and the moderator for the late-breaking abstract session at ASH, highlighted results from two studies.
Data from the MURANO trial showed robust results for a combination of venetoclax and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). At a median follow-up of 23.8 months, median progression-free survival -had not been reached in patients randomized to venetoclax/rituximab, while patients who received bendamustine plus rituximab had a median PFS of 17 months.
The based on the data presented, Dr. Brodsky said.
Another “enormously exciting and practice-changing” finding is that direct oral anticoagulants can be used safely in patients with cancer, Dr. Brodsky said in an interview.
In a randomized, open-label study, 12 months of daily treatment with edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in cancer patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – There were a lot of new data presented during the annual meeting of the American Society of Hematology. But what findings could actually change the way you practice?
Robert A. Brodsky, MD, director of the division of hematology at Johns Hopkins University in Baltimore and the moderator for the late-breaking abstract session at ASH, highlighted results from two studies.
Data from the MURANO trial showed robust results for a combination of venetoclax and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). At a median follow-up of 23.8 months, median progression-free survival -had not been reached in patients randomized to venetoclax/rituximab, while patients who received bendamustine plus rituximab had a median PFS of 17 months.
The based on the data presented, Dr. Brodsky said.
Another “enormously exciting and practice-changing” finding is that direct oral anticoagulants can be used safely in patients with cancer, Dr. Brodsky said in an interview.
In a randomized, open-label study, 12 months of daily treatment with edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in cancer patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – There were a lot of new data presented during the annual meeting of the American Society of Hematology. But what findings could actually change the way you practice?
Robert A. Brodsky, MD, director of the division of hematology at Johns Hopkins University in Baltimore and the moderator for the late-breaking abstract session at ASH, highlighted results from two studies.
Data from the MURANO trial showed robust results for a combination of venetoclax and rituximab in patients with relapsed/refractory chronic lymphocytic leukemia (CLL). At a median follow-up of 23.8 months, median progression-free survival -had not been reached in patients randomized to venetoclax/rituximab, while patients who received bendamustine plus rituximab had a median PFS of 17 months.
The based on the data presented, Dr. Brodsky said.
Another “enormously exciting and practice-changing” finding is that direct oral anticoagulants can be used safely in patients with cancer, Dr. Brodsky said in an interview.
In a randomized, open-label study, 12 months of daily treatment with edoxaban was noninferior to standard subcutaneous therapy with dalteparin for treatment of venous thromboembolism in cancer patients.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM ASH 2017
CLL drug in limited supply outside U.S.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Ofatumumab (Arzerra), a monoclonal antibody treatment for chronic lymphocytic leukemia, will soon be available outside the United States through compassionate use programs only. The drug will continue to be widely available in the United States.
Novartis announced in January that it would begin limiting the availability of the drug outside of the United States and would work with regulatory authorities to set up compassionate use programs for patients who are currently being treated with the drug. Patients who use these programs will receive the drug for free.
The decision was driven by the surge in CLL drugs that have become available over the last 5 years, according to Novartis.
The decision to pull the drug from international markets will not affect its use in ongoing clinical trials, particularly two phase 3 studies in relapsing multiple sclerosis and indolent non-Hodgkin lymphoma.
Gene therapy moves from promise to reality
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
Checkpoint inhibitors look safe in rheumatology patients
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
FROM ARTHRITIS AND RHEUMATOLOGY
Key clinical point:
Major finding: Six of 16 patients (38%) with rheumatologic disease and cancer had an IRAE or flare of their rheumatologic disease.
Study details: A single-center, retrospective records review to identify patients with rheumatologic diseases who had received checkpoint inhibitor therapy at Mayo Clinic between 2011 and 2016.
Disclosures: One of the authors reported advisory board membership with Bristol-Myers Squibb.
Source: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
FDA grants priority review to CAR T-cell therapy for DLBCL
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
AUDIO: Immunotherapy’s role in NHL
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
EXPERT ANALYSIS FROM ASH 2017




