User login
American College of Rheumatology (ACR): Annual Scientific Meeting
Mycophenolate puts lupus patients in remission faster than azathioprine
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Mycophenolate sodium conferred complete remission sooner and more often than did azathioprine in a 24-month, open-label, randomized trial of patients with nonrenal systemic lupus erythematosus.
The enteric-coated version of mycophenolate used in the study was well tolerated, too, with just 7.5% of patients reporting gastrointestinal symptoms – a distinct advantage, Josefina Cortés-Hernández, MD, reported at the annual meeting of the American College of Rheumatology.
But azathioprine still holds one critical advantage over mycophenolate, said Dr. Cortés-Hernández of Vall d´Hebron Hospital, Barcelona: It is safe for use in pregnancy. “This is a very important advantage that we should not lose sight of,” she said.
Dr. Cortés-Hernández and her associates at 13 centers across Spain randomized 240 patients with nonrenal, active systemic lupus erythematosus (SLE) to either azathioprine (target dose, 2 mg/kg per day) or the enteric-coated mycophenolate sodium (target dose, 1,440 mg/day). Patients could add both a corticosteroid and an antimalarial as needed. No patient was allowed to take immunosuppressive therapy for at least 3 months before randomization.
The primary endpoint of the trial was complete remission at 3 months and 24 months, defined as an SLE Disease Activity Index (SLEDAI) less than 4 and/or absence of any BILAG (British Isles Lupus Assessment Group) A or B flares. Secondary endpoints were time to remission, time to first flare, and changes in prednisone use.
The patients were primarily women with a mean age of 41 years and mean disease duration of 5 years. The mean SLEDAI was 9.5; 45% had a mean SLEDAI of 10 or higher. The mean BILAG score was about 19. All the patients had autoantibodies, and about half had anti–double-stranded DNA antibodies. Half of the patients also had low complement C3 and/or C4 levels. About 80% were taking an antimalarial and 95%, a corticosteroid; the mean daily prednisone dose equivalent was about 19 mg.
In an intent-to-treat analysis, patients treated with mycophenolate had a higher remission rate at both 3 and 24 months. At 3 months, the complete remission rate was 33% for mycophenolate, compared with 19% for azathioprine. Clinical response improved over time in both groups, but mycophenolate remained significantly more effective over the entire study period. By 24 months, complete remission was present in 71% of those taking mycophenolate versus 48% of those taking azathioprine.
Both groups had significant improvements over baseline in both the SLEDAI and BILAG scores. By 24 months, the SLEDAI had dropped to a mean of 3 in the mycophenolate group and 4 in the azathioprine group. The BILAG dropped from a mean of 19 at baseline to 2 in the mycophenolate group and 5 in the azathioprine group.
BILAG A/B flares occurred in 50% of the mycophenolate group and 72% of the azathioprine group. The time to first flare was longer with mycophenolate, as was the time to a severe flare. New BILAG A flares occurred in 8% of the mycophenolate group and 22% of the azathioprine group. Azathioprine was associated with more renal flares (7% vs. 2%) and more hematologic flares (7.5% vs. 2.5%)
Steroid use declined to a prednisone equivalent of less than 7.5 mg/day in significantly more patients taking mycophenolate than azathioprine (95% vs. 85%).
Adverse events occurred in about 70% of each group; these were serious in about 10% of each group. Three patients taking mycophenolate and 10 taking azathioprine discontinued because of an adverse event, but this wasn’t statistically significant. Infections were the most common problem, occurring in about a quarter of each group; these were serious in five mycophenolate patients and seven azathioprine patients. There were two deaths, one in each group. Cancers occurred in three taking azathioprine and one taking mycophenolate. Leukopenia occurred in five patients taking azathioprine but in no one taking mycophenolate.
The study was funded by the Spanish Ministry of Health. Dr. Cortés-Hernández had no relevant financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
Key clinical point:
Major finding: Complete remission rates at 3 months were 32% for mycophenolate and 19% for azathioprine.
Data source: A 24-month, open-label, randomized trial of 240 patients.
Disclosures: The Spanish Ministry of Health funded the study. Dr. Cortés-Hernández had no relevant financial disclosures.
Early-onset preeclampsia more likely to occur in women with lupus
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Women with systemic lupus erythematosus are nine times more likely to develop early-onset preeclampsia during a first pregnancy than are women without the disease, according a study of two Swedish national population-based registries.
The risk of preeclampsia occurring before 34 weeks’ gestation declined with subsequent pregnancies, but it remained significantly elevated above the background risk, Julia F. Simard, ScD, said at the annual meeting of the American College of Rheumatology.
During the study period, 742 births to women with SLE were matched with 10,484 births to women without the disease. The mean age of the patients was 31 years.
Of the women with SLE, 5% had pregestational hypertension and 3% had pregestational diabetes. Antiphospholipid antibodies were present in 2%. Among the controls, less than 1% had pregestational hypertension, and 1.3% had pregestational diabetes. There were no healthy controls with antiphospholipid antibodies.
In the entire cohort, there were 438 cases of preeclampsia: 82 in the SLE group and 356 in the control group. In the fully adjusted model, this translated to nearly a tripling of relative risk (RR, 2.7).
Preeclampsia more commonly occurred in first births, based on 56 cases in the SLE group and 225 in the control group. SLE patients in their first pregnancy also had a tripling of risk (RR, 3.2). Among subsequent births, there were 157 cases: 26 in the SLE group and 131 in the control group. The relative risk for preeclampsia was lower, but still significantly elevated (RR, 2).
There were 87 cases of early-onset preeclampsia: 32 in the SLE group and 55 in the control group. Women with SLE were more than six times more likely to develop the disorder (RR, 6.3). Early-onset preeclampsia was more common in first births for both groups: 24 in the SLE group and 34 in the control group, for a ninefold increased risk (RR, 9.3).
Again, the incidence decreased with subsequent births in both groups: 8 cases in the SLE group and 21 in the control group. But SLE patients still faced a significant threefold increase in risk (RR, 2.8).
“Antiphospholipid antibodies appear to be an important risk factor that needs to more fully understood,” Dr. Simard said. “But the risk seems to be independent of other traditional risk factors, like pregestational hypertension, body mass index, and smoking.”
She and her associates had no financial disclosures.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
ACR ANNUAL MEETING
Key clinical point:
Major finding: Women with SLE in a first pregnancy had a ninefold greater risk of early-onset preeclampsia than did healthy control patients in their first pregnancy.
Data source: The data were extracted from two Swedish national population-based registries.
Disclosures: Dr, Simard and her associates had no financial disclosures.
Low-dose IL-2 shows promise for refractory lupus
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
WASHINGTON – A novel biologic treatment strategy involving subcutaneous low-dose interleukin-2 therapy for refractory systemic lupus erythematosus (SLE) showed promise in a single-center, combined phase I/IIa trial.
In 12 patients with active and refractory SLE – that is, patients with SLE disease activity index (SLEDAI) score of at least 6 who were on at least two different immunosuppressive therapies – low-dose IL-2 treatment led to an effective and cycle-dependent increase in the percentage of CD25hi cells among regulatory T cells (Treg). The increase was statistically significant (P less than .001), Jens Humrich, MD, and his colleagues reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, a reduction in levels of anti-dsDNA antibodies was not observed, said Dr. Humrich of University Hospital Schleswig-Holstein in Lubeck, Germany.
Treatment was safe; treatment-related adverse events were generally mild and transient, Dr. Humrich noted.
Study subjects received four treatment cycles each, with daily subcutaneous injections of recombinant human IL-2 (aldesleukin) at single doses of 0.75, 1.5, or 3.0 million IU on 5 consecutive days. Cycles were separated by a washout period of 9-16 days. Subjects were then followed for 9 weeks.
IL-2 is crucial for the growth and survival of Treg (and thus for the control of autoimmunity). Prior studies demonstrated the significance of acquired IL-2 deficiency and related Treg defects in the pathogenesis of SLE – and that compensation for IL-2 deficiency with low-dose IL-2 can correct these defects, he explained.
In the current study, the primary aim was to show at least a twofold increase in the percentage of CD25hi cells among CD3+CD4+Foxp3+CD127lo Treg cells after the fourth treatment cycle vs. baseline, and secondary aims included clinical responses assessed by SLEDAI and changes in serologic and other immunologic parameters, he said.
The findings suggest that low-dose IL-2 therapy can safely and selectively expand the Treg population and decrease disease activity in patients with active and refractory SLE.
“This study provides the basis for larger and placebo-controlled clinical studies aiming to prove the efficacy of this novel biologic treatment strategy,” the investigators concluded.
Dr. Humrich reported having no disclosures.
Key clinical point:
Major finding: A reduction in SLEDAI was seen in 10 patients (83.3%), and a clinical response occurred in 8 (66.7%).
Data source: A combined phase I/IIa trial involving 12 patients.
Disclosures: Dr. Humrich reported having no disclosures.
VIDEO: Denosumab trumps risedronate in bone building for glucocorticoid-induced osteoporosis
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
WASHINGTON – Denosumab built significantly more bone at the hip and lumbar spine than did risedronate when given for 1 year to patients with glucocorticoid-induced osteoporosis in an ongoing 2-year, head-to-head, randomized trial.
Denosumab (Prolia) is currently approved for the treatment of postmenopausal osteoporosis, and it performed so well in the trial that it could be put forward for the indication of glucocorticoid-induced osteoporosis as well, Kenneth Saag, MD, said at the annual meeting of the American College of Rheumatology.
“I would say there is definitely potential for this as a new therapeutic option for these patients,” he said in a video interview about the trial’s primary outcome of denosumab’s noninferiority to risedronate in percentage change in bone mineral density (BMD) at the lumbar spine after 1 year and secondary outcomes of the superiority of denosumab over risedronate in total hip and lumbar spine BMD at 1 year.
Denosumab is a particularly intriguing treatment option for patients with glucocorticoid-induced osteoporosis. They experience a double hit on bone health: increased RANKL, a protein that stimulates osteoclast development, and decreased osteoprotegerin, a protein that inhibits osteoclasts. Denosumab is a RANKL-inhibitor and, as such, tamps down on osteoclastic bone remodeling, said Dr. Saag, vice chair of the department of medicine and director of the Center for Education and Research on Therapeutics at the University of Alabama at Birmingham.
The phase III trial comprised 795 patients who were taking corticosteroids for a variety of rheumatic diseases, including rheumatoid arthritis, polymyalgia rheumatica, and systemic lupus erythematosus, and randomized them to denosumab or risedronate, which is already FDA approved for glucocorticoid-induced bone loss. Patients were randomized to 24 months of subcutaneous denosumab 60 mg given every 6 months or oral risedronate 5-mg daily. The study is still ongoing to test secondary outcomes at 24 months.
The patients were split into those who were continuing glucocorticoid therapy (505) and those who were just initiating it (290). Patients’ mean age ranged from 61 to 67 years, with the glucocorticoid-initiating group (GC-I) being somewhat older. The mean daily prednisone-equivalent dose was 16 mg in that group and 12 mg in the glucocorticoid-continuing group (GC-C). The mean BMD T-scores in the GC-C group were –1.96 at the lumbar spine and –1.56 at the total hip. In the GC-I group, BMD T-scores were –1.06 at the lumbar spine and –0.98 at the total hip.
In the GC-C group, denosumab increased BMD significantly more than risedronate at both spine and hip. At the lumbar spine, denosumab was associated with a mean increase of 4.4% over baseline, compared with a 2.3% increase with risedronate. Total hip BMD increased 2.1% with denosumab and 0.6% with risedronate.
The results were similar in the GC-I group. Denosumab increased lumbar spine BMD by 3.8% over baseline, compared with an increase of 0.8% with risedronate. Total hip BMD increased 1.7% with denosumab and 0.2% with risedronate.
Denosumab was also associated with significantly greater increases in femoral neck BMD in both groups, Dr. Saag noted. There were no significant differences in markers of bone turnover between the treatment groups. Adverse events, including pneumonia, diverticulitis, and bronchitis, were similar.
Amgen, manufacturer of denosumab, is sponsoring the 24-month study. Dr. Saag has been a consultant for Amgen. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
msullivan@frontlinemedcom.com
On Twitter @alz_gal
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: In patients on continuous glucocorticoid therapy, denosumab increased BMD by 4.4% at the lumbar spine and 2.1% at the total hip, compared with increases of 2.3% and 0.6% with risedronate.
Data source: 12-month results of the 24-month, phase III study of 795 patients.
Disclosures: Amgen sponsored the study. Dr. Saag has been a consultant for the company. One coauthor is an employee of Amgen, and others disclosed financial relationships with Amgen and other pharmaceutical companies.
VIDEO: Statins cut mortality in ankylosing spondylitis, psoriatic arthritis
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
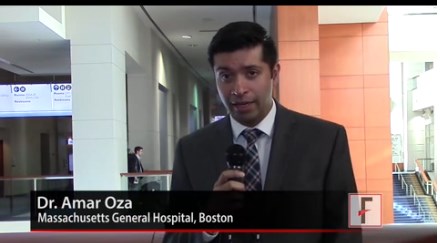
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Statins lowered all-cause mortality by 32% in patients with ankylosing spondylitis (AS) and psoriatic arthritis (PsA) in a retrospective cohort study.
The magnitude of benefit from statins in these two disease states is greater than that found in the general population (estimated 9%-14% reduction in all-cause mortality) and than that reported in patients with rheumatoid arthritis (RA, 21% reduction), said Amar Oza, MD, a second-year rheumatology fellow at Massachusetts General Hospital and Harvard Medical School, both in Boston.
“This is a unique study. The benefit of statins has not been looked at in AS and PsA, specifically,” Dr. Oza explained. “More data are needed” to establish this benefit with certainty, he added.
The data were presented at the annual meeting of the American College of Rheumatology, and Dr. Oza discussed the findings in a video interview.
The study compared 2,904 patients with AS or PsA who initiated statins between 2000 and 2014 with 2,904 propensity-matched AS or PsA patients who did not initiate statins during that period. Patients were drawn from a United Kingdom general population database.
The investigators used a propensity score that accounted for 50 confounding variables to match the two cohorts. These variables included, but were not limited to, disease duration, socioeconomic status, body mass index, lifestyle factors, and medication use.
“This study is the first step in elucidating the benefit of statins in AS and PsA. It is a good step forward. If additional data substantiate that AS and PsA patients have a low threshold for statins, I can envision statins for both primary and secondary prevention in this patient population,” Dr. Oza stated.
The authors had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Myeloablative HSCT bests IV CYC for systemic scleroderma
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
WASHINGTON – Myeloablative autologous hematopoietic stem cell transplantation was superior to monthly intravenous cyclophosphamide in patients with severe scleroderma with internal organ involvement in the randomized, multicenter Scleroderma: Cyclophosphamide or Transplantation (SCOT) trial.
At both 54 and 48 months, a comparison of global rank composite scores favored myeloablation followed by CD34+-selected autologous hematopoietic stem cell transplantation (HSCT) over 12 monthly pulses of 750 mg/m2 cyclophosphamide in the intention-to-treat population of 36 and 39 subjects with diffuse cutaneous systemic sclerosis and high-risk lung and/or renal involvement who were randomized to the groups, respectively (P = .013 at 54 months and P = .008 at 48 months). In subjects who actually underwent HSCT or received at least nine cyclophosphamide doses, the effect was even stronger (P = .004 at 54 months and P = .003 at 48 months), Keith M. Sullivan, MD, reported at the annual meeting of the American College of Rheumatology.
The global rank composite scores were derived based on a hierarchy of outcomes including – in rank order – death, event-free survival, forced vital capacity, scleroderma health assessment questionnaire score, and modified Rodnan skin score (mRSS); each patient was compared, based on the hierarchy, with every other patient in the study.
HSCT was superior overall and within each of the components of the score, Dr. Sullivan said.
The findings were supported by secondary analyses showing that at 54 months, event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively; the differences between the groups were statistically significant, said Dr. Sullivan of Duke University, Durham, N.C.
“In advanced scleroderma, that is a remarkable finding,” he said of the nearly 30-point difference in event-free survival rates between the groups.
Further, disease-modifying antirheumatic drugs were initiated by 54 months in 9% of the HSCT recipients vs. 44% of those in the cyclophosphamide group, he noted.
The rate of serious adverse events was similar in the two groups, although grade 3 or greater adverse events, including treatment-related cytopenias, were more common in the HSCT group, as was herpes zoster. Remarkably, most serious adverse events and adverse events occurred in the first 14 months, Dr. Sullivan noted.
Treatment-related mortality at 54 months was 3% in the HSCT group, and 0% in the cyclophosphamide group, he said.
Study subjects were adults aged 18-69 years with mean baseline modified Rodnan skin score of 30, mean baseline forced vital capacity of 74%, and mean diffusing capacity of the lung for carbon monoxide (DLCO) of 53% of predicted. All but two had lung involvement.
“So [there was] significant skin and pulmonary impairment,” he said.
There are, unquestionably, risks with transplant, but the SCOT findings support myeloablative autologous HSCT as a significant advance in the care of diffuse cutaneous systemic scleroderma with internal organ involvement, as this approach provided superior long-term outcomes, compared with 12 months of IV cyclophosphamide, he said, adding that the global rank composite score developed for this study was a useful measure of scleroderma outcomes.
“Therefore it would be prudent to consider early referral to the transplant center for consultation, which would allow patients, earlier in their disease – before the mRSS hit 30 and the DLCOs hit 50 – to make informed decisions,” he concluded.
The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: Event-free survival was 79% in the HSCT group vs. 50% in the cyclophosphamide group, and overall survival was 91% vs. 77% in the groups, respectively.
Data source: The randomized, multicenter SCOT trial of 75 systemic scleroderma patients.
Disclosures: The SCOT trial was funded by the National Institute of Allergy and Infectious Diseases. The authors reported having no disclosures.
ADDRESS II study: Atacicept shows promise for SLE
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
WASHINGTON – The recombinant fusion protein atacicept, which targets B-cell stimulating factors BLyS and APRIL, had a favorable safety profile and showed some evidence of efficacy in systemic lupus erythematosus patients – particularly those with high disease activity and serologically active disease – in the randomized, phase IIb ADDRESS II study.
Although the primary endpoint of the study – percentage of patients achieving SLE responder index-4 (SRI-4) response at 24 weeks – was not met, a reduction in both disease activity and severe flares was observed in patients in the multicenter study who received atacicept vs. placebo, Joan T. Merrill, MD, of the Oklahoma Medical Research Foundation, Oklahoma City, reported in a late-breaking poster at the annual meeting of the American College of Rheumatology.
However, in a prespecified sensitivity analysis using study day 1 rather than screening visit as baseline, significantly more patients in the atacicept groups achieved SRI-4 response at week 24. For example, compared with a 41% SRI-4 response rate in the placebo group, the rate was 55.9% for the 75-mg atacicept group (odds ratio, 1.88) and 55.8% for the 150-mg atacicept group (odds ratio, 1.96), Dr. Merrill said.
Further, enhanced improvements in both SRI-4 and SRI-6 (a composite measure including reduction of at least 6 points on the SLEDAI-2K) were seen in the atacicept groups vs. the placebo group in 158 patients with high disease activity (defined as SLEDAI-2K of 10 or greater), in 84 patients with serologically active disease (those with dsDNA antibody-positive disease and low complement), and in 69 patients with both.
For example, SRI-4 response rates in patients with high disease activity were significantly greater with atacicept 150 mg vs. placebo at week 24 (62.7% vs. 42.3%; odds ratio, 2.43), and the corresponding SRI-6 rates were 54.9% vs. 28.8% with an odds ratio of 3.30.
The SRI-4 rates at week 24 in those with both high disease activity and serologically active disease who received 150 mg atacicept vs. placebo were 65% vs. 25% (odds ratio, 7.48), and the SRI-6 rates in those patients were 55% vs. 16.7% (odds ratio, 7.13).
In patients with high disease activity, severe flare was reduced with both the 75-mg and 150-mg doses of atacicept, Dr. Merrill said, noting that in the intent-to-treat population, BILAG A flare was significantly reduced with atacicept 75 mg vs. placebo, and severe flares (as measured with the SLEDAI flare index) was reduced with atacicept 150 mg.
Atacicept was associated with increased serum complement C3/C4, and with decreased IgG, IgM, IgA, and anti-dsDNA antibodies over time, she said.
Treatment-emergent adverse events occurred in similar percentages of patients in the groups (71% with placebo, 81.4% with 75 mg atacicept, and 80.8% with 150 mg atacicept). Serious treatment-emergent adverse events occurred in 11%, 8.8%, and 5.8%, respectively.
In a press statement, Dr. Merrill said the results are impressive, particularly for a small, 24-week study.
“If confirmed in future studies, this could hold exciting possibilities for our patients,” she said.
ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: In a prespecified sensitivity analysis, SLI-4 response was 41% with placebo, 55.9% with atacicept 75 mg (odds ratio, 1.88), and 55.8% with atacicept 150 mg (odds ratio 1.96).
Data source: The multicenter, randomized, phase IIb ADDRESS II study of 306 patients.
Disclosures: ADDRESS II was sponsored by EMD Serono. Dr. Merrill reported financial relationships with Anthera Pharmaceuticals and Lilly. Other authors reported financial relationships with EMD Serono/Merck Serono.
Canakinumab controls periodic fever syndromes in post-flare and maintenance dosing
WASHINGTON – Prolonged dosing with canakinumab, an anti-interleukin-1beta monoclonal antibody, confirmed its efficacy in controlling flares in three rare autoinflammatory diseases grouped as periodic fever syndromes: familial Mediterranean fever, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency, and TNF receptor–associated periodic syndrome.
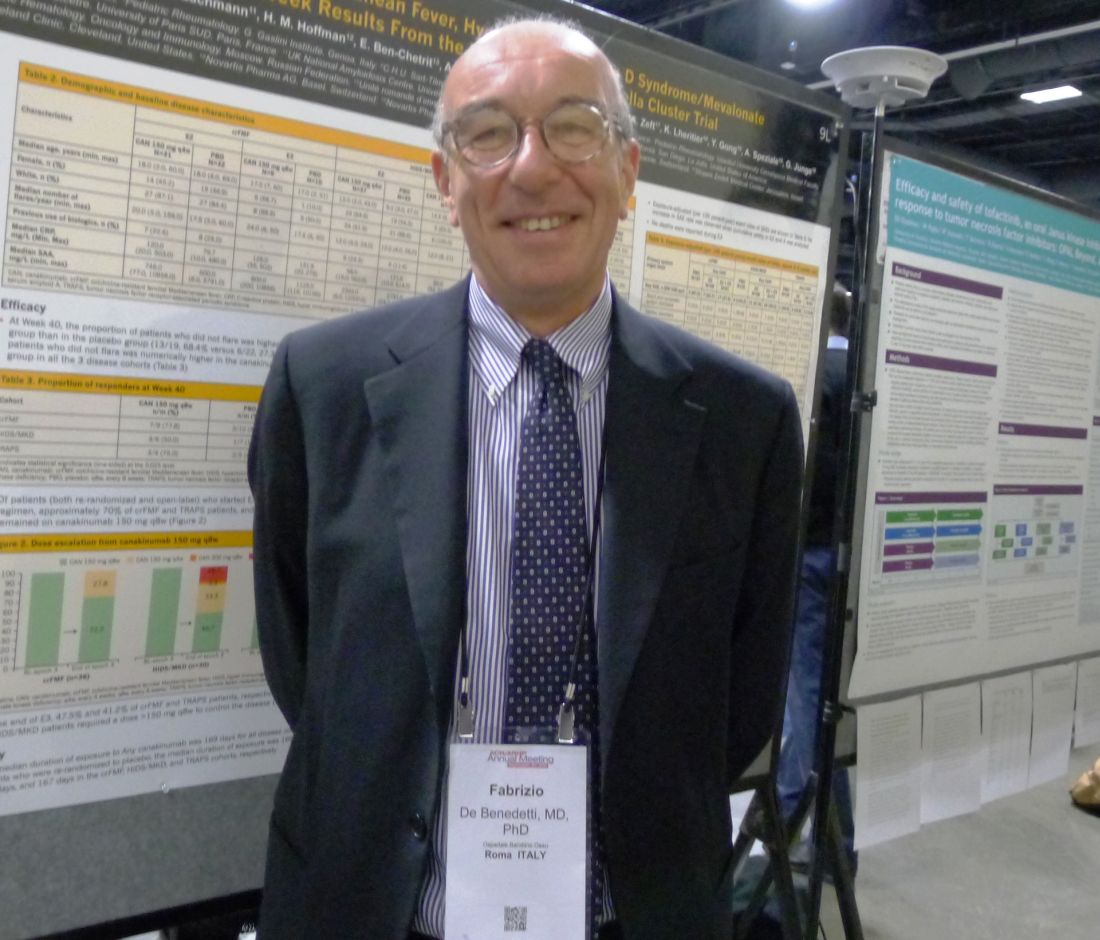
“Colchicine is effective in the majority of patients with FMF [familial Mediterranean fever]. However, patients with resistance to colchicine and patients with HIDS/MKD [hyperimmunoglobulin D syndrome/mevalonate kinase deficiency] or TRAPS [TNF receptor–associated periodic syndrome] have no available therapy until now. The diseases have different mechanisms and different genetic causes, but overlapping and different clinical features and a common mediator. They are all characterized by recurrent fever, joint pain, and involvement of various organs. In addition to greatly affecting the quality of life, the major complication is amyloidosis. Clinical studies and animal studies suggested that IL-1beta was involved in the pathogenesis of these diseases, and canakinumab [Ilaris] targets IL-1beta,” explained Fabrizio De Benedetti, MD, a pediatric rheumatologist at Bambino Gesu Children’s Hospital in Rome.
Following the 16-week, double-blind, randomized treatment phase of the CLUSTER trial, responders at the standard dose of 150 mg (or 2 mg/kg for patients weighing 40 kg or less) every 4 weeks were rerandomized in a 24-week randomized withdrawal phase, followed by a 72-week open-label phase.
At the annual meeting of the American College of Rheumatology, Dr. De Benedetti presented results of the 24-week randomized withdrawal phase in which 42 patients who showed complete response (defined as resolution of index flare and no new flare in the 16 week duration of the randomized controlled phase of the study) after being initially randomized to canakinumab 150 mg every 4 weeks were then rerandomized 1:1 to canakinumab 150 mg every 8 weeks versus placebo. He also presented results for patients who failed placebo during the initial 16-week randomized treatment period; went to open-label rescue treatment with canakinumab; and then, in the 24-week maintenance period, began taking open-label canakinumab 150 mg every 8 weeks. Overall, 160 patients had evaluable data for the maintenance dosing period.
The end point of this phase on a maintenance dose of canakinumab 150 mg every 8 weeks was the proportion of patients who maintained disease control and had no flare, meaning Physician Global Assessment was less than 2 and C-reactive protein was less than 30 mg/L between week 16 and week 40 after rerandomization. Patients who experienced a flare during this time could be escalated up to canakinumab 300 mg every 4 weeks.
At week 40, the proportion of responders was higher in the canakinumab-treated group, compared with placebo, at 68% versus 27%, respectively, but the difference was not statistically significant, Dr. De Benedetti reported.
The percentage of patients on canakinumab who maintained their response by week 40 was higher in each disease group, compared with placebo, but not to a statistically significant extent. The response rates were 78% vs. 30% for FMF; 50% vs. 14% for HIDS/MKD; and 75% vs. 40% for TRAPS. Only 10% of FMF and 8% of TRAPS patients required titration of canakinumab up to 300 mg every 4 weeks, compared with 29% of HIDS/MKD patients, he said.
No new safety findings were reported and there was no accumulation of toxicity. A total of 139 adverse events was reported among all patients in the placebo arm (including the double-blind and prolonged dosing phases), and 8 of these were deemed serious adverse events. The rate of serious adverse events was 85 per 100 patient-years. Among canakinumab-treated patients, the rates of serious adverse events were 34 per 100 patient-years for FMF, 34 per 100 for HIDS/MKD, and 19 per 100 for TRAPS.
“The results of this trial confirm the long-term efficacy of canakinumab in these rare diseases and provide information on the long-term dose needed to control disease, with about half of the patients with FMF or TRAPS and about one-third of those with HIDS/MKD showing no flare at a prolonged dose of 150 mg every 8 weeks,” Dr. De Benedetti said.
The study was supported by Novartis, which markets canakinumab. Dr. De Benedetti disclosed financial ties with Novartis, Pfizer, AbbVie, Roche, Novimmune, and Bristol-Myers Squibb.
WASHINGTON – Prolonged dosing with canakinumab, an anti-interleukin-1beta monoclonal antibody, confirmed its efficacy in controlling flares in three rare autoinflammatory diseases grouped as periodic fever syndromes: familial Mediterranean fever, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency, and TNF receptor–associated periodic syndrome.
“Colchicine is effective in the majority of patients with FMF [familial Mediterranean fever]. However, patients with resistance to colchicine and patients with HIDS/MKD [hyperimmunoglobulin D syndrome/mevalonate kinase deficiency] or TRAPS [TNF receptor–associated periodic syndrome] have no available therapy until now. The diseases have different mechanisms and different genetic causes, but overlapping and different clinical features and a common mediator. They are all characterized by recurrent fever, joint pain, and involvement of various organs. In addition to greatly affecting the quality of life, the major complication is amyloidosis. Clinical studies and animal studies suggested that IL-1beta was involved in the pathogenesis of these diseases, and canakinumab [Ilaris] targets IL-1beta,” explained Fabrizio De Benedetti, MD, a pediatric rheumatologist at Bambino Gesu Children’s Hospital in Rome.
Following the 16-week, double-blind, randomized treatment phase of the CLUSTER trial, responders at the standard dose of 150 mg (or 2 mg/kg for patients weighing 40 kg or less) every 4 weeks were rerandomized in a 24-week randomized withdrawal phase, followed by a 72-week open-label phase.
At the annual meeting of the American College of Rheumatology, Dr. De Benedetti presented results of the 24-week randomized withdrawal phase in which 42 patients who showed complete response (defined as resolution of index flare and no new flare in the 16 week duration of the randomized controlled phase of the study) after being initially randomized to canakinumab 150 mg every 4 weeks were then rerandomized 1:1 to canakinumab 150 mg every 8 weeks versus placebo. He also presented results for patients who failed placebo during the initial 16-week randomized treatment period; went to open-label rescue treatment with canakinumab; and then, in the 24-week maintenance period, began taking open-label canakinumab 150 mg every 8 weeks. Overall, 160 patients had evaluable data for the maintenance dosing period.
The end point of this phase on a maintenance dose of canakinumab 150 mg every 8 weeks was the proportion of patients who maintained disease control and had no flare, meaning Physician Global Assessment was less than 2 and C-reactive protein was less than 30 mg/L between week 16 and week 40 after rerandomization. Patients who experienced a flare during this time could be escalated up to canakinumab 300 mg every 4 weeks.
At week 40, the proportion of responders was higher in the canakinumab-treated group, compared with placebo, at 68% versus 27%, respectively, but the difference was not statistically significant, Dr. De Benedetti reported.
The percentage of patients on canakinumab who maintained their response by week 40 was higher in each disease group, compared with placebo, but not to a statistically significant extent. The response rates were 78% vs. 30% for FMF; 50% vs. 14% for HIDS/MKD; and 75% vs. 40% for TRAPS. Only 10% of FMF and 8% of TRAPS patients required titration of canakinumab up to 300 mg every 4 weeks, compared with 29% of HIDS/MKD patients, he said.
No new safety findings were reported and there was no accumulation of toxicity. A total of 139 adverse events was reported among all patients in the placebo arm (including the double-blind and prolonged dosing phases), and 8 of these were deemed serious adverse events. The rate of serious adverse events was 85 per 100 patient-years. Among canakinumab-treated patients, the rates of serious adverse events were 34 per 100 patient-years for FMF, 34 per 100 for HIDS/MKD, and 19 per 100 for TRAPS.
“The results of this trial confirm the long-term efficacy of canakinumab in these rare diseases and provide information on the long-term dose needed to control disease, with about half of the patients with FMF or TRAPS and about one-third of those with HIDS/MKD showing no flare at a prolonged dose of 150 mg every 8 weeks,” Dr. De Benedetti said.
The study was supported by Novartis, which markets canakinumab. Dr. De Benedetti disclosed financial ties with Novartis, Pfizer, AbbVie, Roche, Novimmune, and Bristol-Myers Squibb.
WASHINGTON – Prolonged dosing with canakinumab, an anti-interleukin-1beta monoclonal antibody, confirmed its efficacy in controlling flares in three rare autoinflammatory diseases grouped as periodic fever syndromes: familial Mediterranean fever, hyperimmunoglobulin D syndrome/mevalonate kinase deficiency, and TNF receptor–associated periodic syndrome.
“Colchicine is effective in the majority of patients with FMF [familial Mediterranean fever]. However, patients with resistance to colchicine and patients with HIDS/MKD [hyperimmunoglobulin D syndrome/mevalonate kinase deficiency] or TRAPS [TNF receptor–associated periodic syndrome] have no available therapy until now. The diseases have different mechanisms and different genetic causes, but overlapping and different clinical features and a common mediator. They are all characterized by recurrent fever, joint pain, and involvement of various organs. In addition to greatly affecting the quality of life, the major complication is amyloidosis. Clinical studies and animal studies suggested that IL-1beta was involved in the pathogenesis of these diseases, and canakinumab [Ilaris] targets IL-1beta,” explained Fabrizio De Benedetti, MD, a pediatric rheumatologist at Bambino Gesu Children’s Hospital in Rome.
Following the 16-week, double-blind, randomized treatment phase of the CLUSTER trial, responders at the standard dose of 150 mg (or 2 mg/kg for patients weighing 40 kg or less) every 4 weeks were rerandomized in a 24-week randomized withdrawal phase, followed by a 72-week open-label phase.
At the annual meeting of the American College of Rheumatology, Dr. De Benedetti presented results of the 24-week randomized withdrawal phase in which 42 patients who showed complete response (defined as resolution of index flare and no new flare in the 16 week duration of the randomized controlled phase of the study) after being initially randomized to canakinumab 150 mg every 4 weeks were then rerandomized 1:1 to canakinumab 150 mg every 8 weeks versus placebo. He also presented results for patients who failed placebo during the initial 16-week randomized treatment period; went to open-label rescue treatment with canakinumab; and then, in the 24-week maintenance period, began taking open-label canakinumab 150 mg every 8 weeks. Overall, 160 patients had evaluable data for the maintenance dosing period.
The end point of this phase on a maintenance dose of canakinumab 150 mg every 8 weeks was the proportion of patients who maintained disease control and had no flare, meaning Physician Global Assessment was less than 2 and C-reactive protein was less than 30 mg/L between week 16 and week 40 after rerandomization. Patients who experienced a flare during this time could be escalated up to canakinumab 300 mg every 4 weeks.
At week 40, the proportion of responders was higher in the canakinumab-treated group, compared with placebo, at 68% versus 27%, respectively, but the difference was not statistically significant, Dr. De Benedetti reported.
The percentage of patients on canakinumab who maintained their response by week 40 was higher in each disease group, compared with placebo, but not to a statistically significant extent. The response rates were 78% vs. 30% for FMF; 50% vs. 14% for HIDS/MKD; and 75% vs. 40% for TRAPS. Only 10% of FMF and 8% of TRAPS patients required titration of canakinumab up to 300 mg every 4 weeks, compared with 29% of HIDS/MKD patients, he said.
No new safety findings were reported and there was no accumulation of toxicity. A total of 139 adverse events was reported among all patients in the placebo arm (including the double-blind and prolonged dosing phases), and 8 of these were deemed serious adverse events. The rate of serious adverse events was 85 per 100 patient-years. Among canakinumab-treated patients, the rates of serious adverse events were 34 per 100 patient-years for FMF, 34 per 100 for HIDS/MKD, and 19 per 100 for TRAPS.
“The results of this trial confirm the long-term efficacy of canakinumab in these rare diseases and provide information on the long-term dose needed to control disease, with about half of the patients with FMF or TRAPS and about one-third of those with HIDS/MKD showing no flare at a prolonged dose of 150 mg every 8 weeks,” Dr. De Benedetti said.
The study was supported by Novartis, which markets canakinumab. Dr. De Benedetti disclosed financial ties with Novartis, Pfizer, AbbVie, Roche, Novimmune, and Bristol-Myers Squibb.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: At week 40, the proportion of responders was higher in the canakinumab-treated group (68%), compared with placebo (27%), but the difference was not statistically significant.
Data source: A phase III, randomized, double-blind, 16-week, placebo-controlled trial of 181 patients, followed by a 24-week withdrawal phase rerandomizing 42 responders, with all other patients continuing on canakinumab.
Disclosures: The study was supported by Novartis, which markets canakinumab. Dr. De Benedetti disclosed financial ties with Novartis, Pfizer, AbbVie, Roche, Novimmune, and Bristol-Myers Squibb.
VIDEO: Urate lowering therapy improved kidney function
WASHINGTON – Urate lowering therapy improved kidney function in patients with chronic kidney disease (CKD), according to a large retrospective study presented at the annual meeting of the American College of Rheumatology. Moreover, patients with CKD stage 3 derived the most benefit from urate lowering therapy, and those with CKD stage 2 also benefited to a lesser degree. Patients with CKD stage 4 had no benefit from urate lowering therapy.
“Two years ago we showed that urate lowering therapy did not worsen kidney function in patients with chronic kidney disease. This study shows that their kidney function improved [with urate lowering therapy],” said Gerald D. Levy, MD, MBA, a rheumatologist at Kaiser Permanente of Southern California, Downey, Calif.
The study was conducted from 2008 to 2014 and included 12,751 patients with serum urate levels of above 7 mg/dL and CKD Stages 2, 3, and 4 at the index date, defined as the first time this test result was reported. Patients were drawn from the Kaiser Permanente database and were treated by primary care physicians. Patients were followed for 1 year from the index date. The primary outcome measure was a 30% increase or a 30% decrease in glomerular filtration rate (GFR) from baseline to the last available result.
Of the 12,751 patients, 2,690 were on urate lowering therapy and 10,061 were not on urate lowering therapy. Goal serum urate (sUA) was achieved in 1,118 (42%) of patients on urate lowering therapy. Among patients who achieved goal sUA, a 30% improvement in GFR was observed in 17.1% versus 10.4% of patients who did not achieve sUA goal, for an absolute difference of 6.7% (P less than .001).
For patients at goal versus those not at goal, the ratio of improvement was 3.4 and 3.8, respectively.
“This study suggests that patients with CKD should be tested for uric acid independent of whether they have gout or not. Getting to goal is important. Stage 3 CKD is the sweet spot where patients got the most pronounced benefit from urate lowering therapy,” he stated. “Stage 4 CKD is too late.”
Dr. Levy discussed the findings in a video interview during the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Urate lowering therapy improved kidney function in patients with chronic kidney disease (CKD), according to a large retrospective study presented at the annual meeting of the American College of Rheumatology. Moreover, patients with CKD stage 3 derived the most benefit from urate lowering therapy, and those with CKD stage 2 also benefited to a lesser degree. Patients with CKD stage 4 had no benefit from urate lowering therapy.
“Two years ago we showed that urate lowering therapy did not worsen kidney function in patients with chronic kidney disease. This study shows that their kidney function improved [with urate lowering therapy],” said Gerald D. Levy, MD, MBA, a rheumatologist at Kaiser Permanente of Southern California, Downey, Calif.
The study was conducted from 2008 to 2014 and included 12,751 patients with serum urate levels of above 7 mg/dL and CKD Stages 2, 3, and 4 at the index date, defined as the first time this test result was reported. Patients were drawn from the Kaiser Permanente database and were treated by primary care physicians. Patients were followed for 1 year from the index date. The primary outcome measure was a 30% increase or a 30% decrease in glomerular filtration rate (GFR) from baseline to the last available result.
Of the 12,751 patients, 2,690 were on urate lowering therapy and 10,061 were not on urate lowering therapy. Goal serum urate (sUA) was achieved in 1,118 (42%) of patients on urate lowering therapy. Among patients who achieved goal sUA, a 30% improvement in GFR was observed in 17.1% versus 10.4% of patients who did not achieve sUA goal, for an absolute difference of 6.7% (P less than .001).
For patients at goal versus those not at goal, the ratio of improvement was 3.4 and 3.8, respectively.
“This study suggests that patients with CKD should be tested for uric acid independent of whether they have gout or not. Getting to goal is important. Stage 3 CKD is the sweet spot where patients got the most pronounced benefit from urate lowering therapy,” he stated. “Stage 4 CKD is too late.”
Dr. Levy discussed the findings in a video interview during the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – Urate lowering therapy improved kidney function in patients with chronic kidney disease (CKD), according to a large retrospective study presented at the annual meeting of the American College of Rheumatology. Moreover, patients with CKD stage 3 derived the most benefit from urate lowering therapy, and those with CKD stage 2 also benefited to a lesser degree. Patients with CKD stage 4 had no benefit from urate lowering therapy.
“Two years ago we showed that urate lowering therapy did not worsen kidney function in patients with chronic kidney disease. This study shows that their kidney function improved [with urate lowering therapy],” said Gerald D. Levy, MD, MBA, a rheumatologist at Kaiser Permanente of Southern California, Downey, Calif.
The study was conducted from 2008 to 2014 and included 12,751 patients with serum urate levels of above 7 mg/dL and CKD Stages 2, 3, and 4 at the index date, defined as the first time this test result was reported. Patients were drawn from the Kaiser Permanente database and were treated by primary care physicians. Patients were followed for 1 year from the index date. The primary outcome measure was a 30% increase or a 30% decrease in glomerular filtration rate (GFR) from baseline to the last available result.
Of the 12,751 patients, 2,690 were on urate lowering therapy and 10,061 were not on urate lowering therapy. Goal serum urate (sUA) was achieved in 1,118 (42%) of patients on urate lowering therapy. Among patients who achieved goal sUA, a 30% improvement in GFR was observed in 17.1% versus 10.4% of patients who did not achieve sUA goal, for an absolute difference of 6.7% (P less than .001).
For patients at goal versus those not at goal, the ratio of improvement was 3.4 and 3.8, respectively.
“This study suggests that patients with CKD should be tested for uric acid independent of whether they have gout or not. Getting to goal is important. Stage 3 CKD is the sweet spot where patients got the most pronounced benefit from urate lowering therapy,” he stated. “Stage 4 CKD is too late.”
Dr. Levy discussed the findings in a video interview during the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE ACR ANNUAL MEETING
Long-term remission maintenance in ANCA-associated vasculitis leans toward rituximab over azathioprine
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
Over the long term, patients initially randomized to rituximab maintenance therapy in the initial phase of the MAINRITSAN trial continued to be more likely to remain in remission than were those who had been randomized to azathioprine. The maintenance therapy data for rituximab look better than for azathioprine.
In clinical practice, individualizing the timing of repeat rituximab may be favorable for remission maintenance rather than having the same fixed dose for all comers, as was used in the MAINRITSAN trial.
By tailoring therapy, it may be possible to use less medication. If patients’ B cells remain depleted and ANCA is stable, flare is unlikely in the next 3 months, but if B cells are reconstituting, particularly in concert with a rising ANCA, I would generally administer a “remission maintenance” dose of rituximab, particularly in an individual who has had relapsing disease in the past. Perhaps patients could use less cumulative rituximab if these parameters are used to make treatment decisions. The poster presentation by Pierre Charles, MD, suggested that this approach indeed is feasible.
Robert F. Spiera, MD, is director of the Scleroderma, Vasculitis, & Myositis Center at the Hospital for Special Surgery, New York. He is also professor of clinical medicine at Cornell University, New York. He made these comments in an interview. Dr. Spiera has received research funding and consulting fees from Roche/Genentech, which markets rituximab.
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
WASHINGTON – Rituximab was superior to azathioprine as maintenance therapy for antineutrophil cytoplasmic antibody–associated vasculitis over long-term follow-up of the MAINRITSAN trial. At 60 months, rituximab significantly improved overall survival and relapse-free survival, compared with azathioprine.
At 60 months, overall survival (OS) rates were 100% for rituximab (Rituxan) versus 93% for azathioprine (P = .045), and relapse-free survival (RFS) rates were 57.9% versus 37.3%, respectively (P = .012).
These long-term maintenance results build on the primary results of MAINRITSAN that were previously published in 2014 (N Engl J Med. 2014;Nov 6;371[19]:1771-80). The primary results showed the superiority of rituximab maintenance therapy versus the then-gold standard azathioprine in maintaining antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis (AAV) remission at 28 months following induction therapy with a cyclophosphamide/glucocorticoid regimen.
“Following publication of the primary results, some uncertainties remained as to the duration of remission on rituximab. There is a need for therapy that can prevent relapse over the longer term,” lead author Benjamin Terrier, MD, of the National Referral Center for Rare Systemic Autoimmune Diseases at Cochin Hospital and Paris Descartes University in France, said in his presentation of the long-term follow-up data at the annual meeting of the American College of Rheumatology.
The follow-up results indicate that “over the long term, despite late relapses after the 28-month initial follow-up period, maintenance therapy with rituximab remained significantly superior to azathioprine to maintain remission at 60 months and was associated with better survival,” Dr. Terrier said.
The study included 115 newly diagnosed or relapsing patients with AAV (granulomatosis with polyangiitis, microscopic polyangiitis, or eosinophilic granulomatosis with polyangiitis). Of these, 80% were newly diagnosed. After achieving remission on induction therapy, patients were randomized to rituximab infusion 500 mg on day 1, day 15, and 5.5 months later, then every 6 months for 18 months or to azathioprine for 22 months.
The investigators collected data prospectively on major and minor relapses and adverse events, using a Q-TWIST (Quality Adjusted Time Without Symptoms and Toxicity) analysis to show the trade-off between toxicity and disease activity.
For all relapses, major and minor, rituximab maintained superiority over azathioprine at 60 months. There were 24 events in the rituximab arm: 11 minor relapses and 13 major relapses. There were 36 events in the azathioprine arm: 10 minor relapses, 25 major relapses, and 1 death. Major RFS survival rates were 71.9% versus 49.4%, respectively (P = .003).
“There was an absolute difference of 12 months favoring rituximab for RFS,” Dr. Terrier said.
Serious infections were numerically increased in the rituximab-treated group: 30 compared with 20 in the azathioprine group. Cardiovascular event rates were similar for both groups.
Q-TWIST analysis found significantly longer quality-adjusted time without progression or toxicity in the rituximab arm (P less than .001). The cumulative dose of steroids was comparable between treatment groups.
Six cancers were found in the azathioprine arm (including four skin cancers), compared with two in the rituximab arm.
In the rituximab group, PR3 ANCA positivity or ANCA persistence 12 months after starting rituximab maintenance therapy were associated with higher major relapse rates.
“Combining these two factors allows discerning low relapse rate. Patients negative for ANCA and for PR3 ANCA were at low risk,” Dr. Terrier told the audience. “ANCA monitoring seems to be relevant to guide treatment duration.”
Best rituximab regimen?
A separate study presented at a poster session compared the systemic regimen used in MAINRITSAN (as a control group) versus an experimental regimen of fixed 500-mg rituximab infusions on day 0 post randomization and then every 3 months until month 18, based on ANCA status/titer and/or circulating CD19 B-cell reappearance. The open-label, randomized study included 163 patients with granulomatosis with polyangiitis or microscopic polyangiitis in complete remission after induction therapy with glucocorticoids and cyclophosphamide or rituximab or methotrexate.
At 28 months, the relapse rate was 8% in the control arm and 14% in the experimental arm, a difference that was not statistically significant.
“We found no difference in the primary endpoint of relapse between the two regimens, but the experimental arm received fewer infusions. From this study, we cannot make a strong recommendation for the experimental regimen, but we think it is better, because there is less cumulative exposure to rituximab,” stated lead author Pierre Charles, MD, also of Cochin Hospital.
Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles disclosed financial support from Hoffman-LaRoche.
AT THE ACR ANNUAL MEETING
Key clinical point:
Major finding: At 60 months, overall survival rates were 100% for rituximab versus 93% azathioprine (P = .045) and relapse-free survival rates were 57.9% versus 37.3%, respectively (P = .012).
Data source: 60-month follow-up of a randomized, controlled trial of 115 patients.
Disclosures: Both studies were funded by Hoffman-LaRoche. Dr. Terrier and Dr. Charles each disclosed financial support from Hoffman-LaRoche.