User login
Study explores why RA patients discontinue methotrexate
SAN DIEGO – Despite the well-recognized role of methotrexate in the management of rheumatoid arthritis, about 30% of patients with RA discontinue treatment with methotrexate 1-2 years after starting it, according to results from a registry study.
“We know that methotrexate is an acceptable and certainly well-characterized treatment for RA,” study author Jeffrey R. Curtis, MD, said at the annual meeting of the American College of Rheumatology. “Despite, this, though, patterns of persistence, intolerance, and inadequate response with methotrexate [MTX] are not well characterized in real-world settings. We all get the sense that there are patients who greatly dislike it.”
The researchers analyzed patients not previously treated with a biologic DMARD (bDMARD) or a targeted synthetic DMARD (tsDMARD). They compared 1,488 patients who initiated MTX monotherapy with 656 patients who initiated MTX in combination with csDMARDs during October 2001 through February 2017 and had at least one follow-up visit after treatment initiation.
The mean age of patients was 57 years, 75% were female, and 84% were white. Patients in the MTX combination therapy group were more likely to be white, better educated, and employed, but were slightly less likely to have active disease. Dr. Curtis reported that the proportion of MTX monotherapy patients who discontinued MTX was 24% at 1 year, 37% at 2 years, and 46% at 3 years, and was not significantly different from the figures for the combination MTX therapy group (P = .99). “The survival curve was overlapping and nonsignificant,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham. “For example, at 12 months, 24.4% of people stopped methotrexate if they had started it alone, but it was 26% if they were on combination therapy. Flipping that around, 75% stayed on MTX at 1 year’s time.”
In contrast, the researchers also found that patients in the MTX monotherapy group were significantly more likely to start a bDMARD or a tsDMARD earlier than patients in the MTX combination therapy group (P less than .001). In an adjusted Cox proportional hazards model, higher risk for MTX discontinuation was associated with being disabled (hazard ratio, 1.33), being retired (HR, 1.37), or using alcohol regularly (the HR ranged from 1.22 to 2.03 depending on the mean units consumed per day). MTX discontinuation was less likely in patients with older age (HR, 0.94), longer duration of RA (HR, 0.92), or higher baseline Clinical Disease Activity Index score (HR, 0.96).
About 20% of physicians in the registry provided reasons for discontinuation of MTX. The top reasons they gave were the presence of infection and cancer (about 85% and 15%, respectively). The reasons patients gave for discontinuation of MTX were wide ranging and included unusual fatigue (about 67%), followed by stomach problems (48%), hair loss (about 36%), mental fog (about 29%), sores in the mouth (about 25%), nausea (about 19%), and diarrhea (about 19%). “Patients are telling us very different things about why this drug is being stopped,” Dr. Curtis said. “Doctors in this registry could specify a reason for stopping that would map to these reasons, and yet they didn’t.”
He acknowledged certain limitations of the study, including the potential for missing data and the possibility that some of the patients may have reinitiated MTX. “We know from other data that people may stop MTX for a period of time but then may resume it,” Dr. Curtis said. Going forward, he called for strategies “to better identify patients with suboptimal persistence to MTX and predict those most likely to not tolerate MTX, to optimize overall RA treatment.”
The study was supported by Corrona, and the analysis was funded by Pfizer. Dr. Curtis reported that he has received research grants from Amgen, Corrona, Crescendo Bioscience, and Pfizer, and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Myriad, Pfizer, Roche/Genentech, and UCB.
SAN DIEGO – Despite the well-recognized role of methotrexate in the management of rheumatoid arthritis, about 30% of patients with RA discontinue treatment with methotrexate 1-2 years after starting it, according to results from a registry study.
“We know that methotrexate is an acceptable and certainly well-characterized treatment for RA,” study author Jeffrey R. Curtis, MD, said at the annual meeting of the American College of Rheumatology. “Despite, this, though, patterns of persistence, intolerance, and inadequate response with methotrexate [MTX] are not well characterized in real-world settings. We all get the sense that there are patients who greatly dislike it.”
The researchers analyzed patients not previously treated with a biologic DMARD (bDMARD) or a targeted synthetic DMARD (tsDMARD). They compared 1,488 patients who initiated MTX monotherapy with 656 patients who initiated MTX in combination with csDMARDs during October 2001 through February 2017 and had at least one follow-up visit after treatment initiation.
The mean age of patients was 57 years, 75% were female, and 84% were white. Patients in the MTX combination therapy group were more likely to be white, better educated, and employed, but were slightly less likely to have active disease. Dr. Curtis reported that the proportion of MTX monotherapy patients who discontinued MTX was 24% at 1 year, 37% at 2 years, and 46% at 3 years, and was not significantly different from the figures for the combination MTX therapy group (P = .99). “The survival curve was overlapping and nonsignificant,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham. “For example, at 12 months, 24.4% of people stopped methotrexate if they had started it alone, but it was 26% if they were on combination therapy. Flipping that around, 75% stayed on MTX at 1 year’s time.”
In contrast, the researchers also found that patients in the MTX monotherapy group were significantly more likely to start a bDMARD or a tsDMARD earlier than patients in the MTX combination therapy group (P less than .001). In an adjusted Cox proportional hazards model, higher risk for MTX discontinuation was associated with being disabled (hazard ratio, 1.33), being retired (HR, 1.37), or using alcohol regularly (the HR ranged from 1.22 to 2.03 depending on the mean units consumed per day). MTX discontinuation was less likely in patients with older age (HR, 0.94), longer duration of RA (HR, 0.92), or higher baseline Clinical Disease Activity Index score (HR, 0.96).
About 20% of physicians in the registry provided reasons for discontinuation of MTX. The top reasons they gave were the presence of infection and cancer (about 85% and 15%, respectively). The reasons patients gave for discontinuation of MTX were wide ranging and included unusual fatigue (about 67%), followed by stomach problems (48%), hair loss (about 36%), mental fog (about 29%), sores in the mouth (about 25%), nausea (about 19%), and diarrhea (about 19%). “Patients are telling us very different things about why this drug is being stopped,” Dr. Curtis said. “Doctors in this registry could specify a reason for stopping that would map to these reasons, and yet they didn’t.”
He acknowledged certain limitations of the study, including the potential for missing data and the possibility that some of the patients may have reinitiated MTX. “We know from other data that people may stop MTX for a period of time but then may resume it,” Dr. Curtis said. Going forward, he called for strategies “to better identify patients with suboptimal persistence to MTX and predict those most likely to not tolerate MTX, to optimize overall RA treatment.”
The study was supported by Corrona, and the analysis was funded by Pfizer. Dr. Curtis reported that he has received research grants from Amgen, Corrona, Crescendo Bioscience, and Pfizer, and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Myriad, Pfizer, Roche/Genentech, and UCB.
SAN DIEGO – Despite the well-recognized role of methotrexate in the management of rheumatoid arthritis, about 30% of patients with RA discontinue treatment with methotrexate 1-2 years after starting it, according to results from a registry study.
“We know that methotrexate is an acceptable and certainly well-characterized treatment for RA,” study author Jeffrey R. Curtis, MD, said at the annual meeting of the American College of Rheumatology. “Despite, this, though, patterns of persistence, intolerance, and inadequate response with methotrexate [MTX] are not well characterized in real-world settings. We all get the sense that there are patients who greatly dislike it.”
The researchers analyzed patients not previously treated with a biologic DMARD (bDMARD) or a targeted synthetic DMARD (tsDMARD). They compared 1,488 patients who initiated MTX monotherapy with 656 patients who initiated MTX in combination with csDMARDs during October 2001 through February 2017 and had at least one follow-up visit after treatment initiation.
The mean age of patients was 57 years, 75% were female, and 84% were white. Patients in the MTX combination therapy group were more likely to be white, better educated, and employed, but were slightly less likely to have active disease. Dr. Curtis reported that the proportion of MTX monotherapy patients who discontinued MTX was 24% at 1 year, 37% at 2 years, and 46% at 3 years, and was not significantly different from the figures for the combination MTX therapy group (P = .99). “The survival curve was overlapping and nonsignificant,” said Dr. Curtis, professor of medicine in the division of clinical immunology and rheumatology at the University of Alabama at Birmingham. “For example, at 12 months, 24.4% of people stopped methotrexate if they had started it alone, but it was 26% if they were on combination therapy. Flipping that around, 75% stayed on MTX at 1 year’s time.”
In contrast, the researchers also found that patients in the MTX monotherapy group were significantly more likely to start a bDMARD or a tsDMARD earlier than patients in the MTX combination therapy group (P less than .001). In an adjusted Cox proportional hazards model, higher risk for MTX discontinuation was associated with being disabled (hazard ratio, 1.33), being retired (HR, 1.37), or using alcohol regularly (the HR ranged from 1.22 to 2.03 depending on the mean units consumed per day). MTX discontinuation was less likely in patients with older age (HR, 0.94), longer duration of RA (HR, 0.92), or higher baseline Clinical Disease Activity Index score (HR, 0.96).
About 20% of physicians in the registry provided reasons for discontinuation of MTX. The top reasons they gave were the presence of infection and cancer (about 85% and 15%, respectively). The reasons patients gave for discontinuation of MTX were wide ranging and included unusual fatigue (about 67%), followed by stomach problems (48%), hair loss (about 36%), mental fog (about 29%), sores in the mouth (about 25%), nausea (about 19%), and diarrhea (about 19%). “Patients are telling us very different things about why this drug is being stopped,” Dr. Curtis said. “Doctors in this registry could specify a reason for stopping that would map to these reasons, and yet they didn’t.”
He acknowledged certain limitations of the study, including the potential for missing data and the possibility that some of the patients may have reinitiated MTX. “We know from other data that people may stop MTX for a period of time but then may resume it,” Dr. Curtis said. Going forward, he called for strategies “to better identify patients with suboptimal persistence to MTX and predict those most likely to not tolerate MTX, to optimize overall RA treatment.”
The study was supported by Corrona, and the analysis was funded by Pfizer. Dr. Curtis reported that he has received research grants from Amgen, Corrona, Crescendo Bioscience, and Pfizer, and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Myriad, Pfizer, Roche/Genentech, and UCB.
AT ACR 2017
Key clinical point: Nearly one-third of patients with RA discontinue methotrexate 1–2 years after initiation.
Major finding: The proportion of MTX monotherapy patients who discontinued MTX was 24% at 1 year, 37% at 2 years, and 46% at 3 years, and was not significantly different from the combination MTX therapy group (P = .99).
Study details: A registry study that compared 1,488 patients who initiated MTX monotherapy with 656 patients who initiated MTX in combination with conventional synthetic DMARDs.
Disclosures: The study was supported by Corrona, and the analysis was funded by Pfizer. Dr. Curtis reported that he has received research grants from Amgen, Corrona, Crescendo Bioscience, and Pfizer, and consulting fees from AbbVie, Amgen, Bristol-Myers Squibb, Corrona, Myriad, Pfizer, Roche/Genentech, and UCB.
Anabasum shows promise in treating skin-predominant dermatomyositis, systemic sclerosis
SAN DIEGO – Results from two phase 2 studies of the investigational agent anabasum provide evidence supporting its safety and efficacy in patients with refractory skin-predominant dermatomyositis or diffuse cutaneous systemic sclerosis.
Anabasum (JBT-101) is a nonimmunosuppressive, synthetic, oral cannabinoid receptor type 2 agonist being developed by Norwood, Mass.–based Corbus Pharmaceuticals. It works by triggering resolution of innate immune responses, said Barbara White, MD, a rheumatologist who is chief medical officer for Corbus. “It restores homeostasis, gets rid of inflammation, turns off active fibrotic processes, and helps clear bacteria if it is present – all without immunosuppression,” she explained in an interview at the annual meeting of the American College of Rheumatology. “A drug that would restore homeostasis in the setting of an ongoing immune response has the potential to be helpful in multiple autoimmune diseases.”
The mean age of the study participants was 53 years and most were white. Even though 19 of the patients were on immunosuppressants at baseline, both cohorts had mean CDASI scores in the 33-35 range, which can be considered severe.
The investigators, led by Victoria P. Werth, MD, of the University of Pennsylvania, Philadelphia, reported that anabasum-treated subjects experienced a medically meaningful improvement in mean CDASI scores, with a reduction of at least 5 points at all visits after week 4 and reaching –9.3 at the end of the study, compared with –3.7 points in the placebo group (P = .02). They also found that 56% of subjects in the anabasum group had a 10-point reduction or more in CDASI score, compared with only 18% in the placebo group (P = .09). In addition, no subjects in the anabasum group developed skin erosions during the active dosing period, compared with 36% of subjects in the placebo group (P = .05).
The researchers also observed that, compared with subjects in the placebo group, those in the anabasum group had greater improvement in patient-reported global skin disease and overall disease assessments, skin symptoms (including photosensitivity and itch), fatigue, sleep, interference with activities, pain, and physical function. No serious, severe, or unexpected adverse events occurred in the anabasum group. Adverse events included dizziness, dyspepsia, headache, and increased appetite.
There is not a Food and Drug Administration or European Medicines Agency–approved drug for refractory skin-predominant dermatomyositis, “so this is encouraging,” Dr. White said.
Dr. Spiera reported that patients in the anabasum group had greater improvement in ACR CRISS, compared with placebo-treated subjects over 16 weeks (P = .044). They also had greater improvement and less worsening in individual CRISS core measures, including modified Rodnan Skin Score, Patient Global Assessment, Physician Global Assessment, and the Health Assessment Questionnaire Disability Index. Patient-reported outcomes of systemic sclerosis skin symptoms, itch, and the Patient-Reported Outcomes Measurement Information System–29 (PROMIS-29) domains of physical function, pain interference, and sleep also improved for the anabasum group, compared with the placebo group (P less than .05 for all).
An analysis of paired skin biopsies before and after treatment with the assessor blinded to treatment assignment demonstrated that patients treated with anabasum were more likely to show improvement in fibrosis and inflammation and less likely to show worsening than were those treated with placebo, consistent with what was observed clinically. In a related poster presented at the meeting, gene expression analysis from specimens before and after treatment also revealed that anabasum treatment (as opposed to treatment with placebo) was associated with changes relevant to pathways involved in fibrosis and inflammation.
“This is the first double-blind, randomized, placebo controlled trial in diffuse cutaneous systemic sclerosis to demonstrate a clinical benefit using the CRISS as an endpoint, with a drug that was safe and well tolerated in the trial,” Dr. Spiera said. “These results bring hope to patients and their physicians that anabasum may be an effective drug for systemic sclerosis where currently there are no proven treatments.”
Both studies were sponsored by Corbus, and the dermatomyositis study was also sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some of the investigators in both studies were employees of Corbus. Dr. Spiera reported receiving research support from Corbus and many other pharmaceutical companies. He also is a consultant to Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, and CSL Behring. He is a member of the Rheumatology News editorial advisory board.
SAN DIEGO – Results from two phase 2 studies of the investigational agent anabasum provide evidence supporting its safety and efficacy in patients with refractory skin-predominant dermatomyositis or diffuse cutaneous systemic sclerosis.
Anabasum (JBT-101) is a nonimmunosuppressive, synthetic, oral cannabinoid receptor type 2 agonist being developed by Norwood, Mass.–based Corbus Pharmaceuticals. It works by triggering resolution of innate immune responses, said Barbara White, MD, a rheumatologist who is chief medical officer for Corbus. “It restores homeostasis, gets rid of inflammation, turns off active fibrotic processes, and helps clear bacteria if it is present – all without immunosuppression,” she explained in an interview at the annual meeting of the American College of Rheumatology. “A drug that would restore homeostasis in the setting of an ongoing immune response has the potential to be helpful in multiple autoimmune diseases.”
The mean age of the study participants was 53 years and most were white. Even though 19 of the patients were on immunosuppressants at baseline, both cohorts had mean CDASI scores in the 33-35 range, which can be considered severe.
The investigators, led by Victoria P. Werth, MD, of the University of Pennsylvania, Philadelphia, reported that anabasum-treated subjects experienced a medically meaningful improvement in mean CDASI scores, with a reduction of at least 5 points at all visits after week 4 and reaching –9.3 at the end of the study, compared with –3.7 points in the placebo group (P = .02). They also found that 56% of subjects in the anabasum group had a 10-point reduction or more in CDASI score, compared with only 18% in the placebo group (P = .09). In addition, no subjects in the anabasum group developed skin erosions during the active dosing period, compared with 36% of subjects in the placebo group (P = .05).
The researchers also observed that, compared with subjects in the placebo group, those in the anabasum group had greater improvement in patient-reported global skin disease and overall disease assessments, skin symptoms (including photosensitivity and itch), fatigue, sleep, interference with activities, pain, and physical function. No serious, severe, or unexpected adverse events occurred in the anabasum group. Adverse events included dizziness, dyspepsia, headache, and increased appetite.
There is not a Food and Drug Administration or European Medicines Agency–approved drug for refractory skin-predominant dermatomyositis, “so this is encouraging,” Dr. White said.
Dr. Spiera reported that patients in the anabasum group had greater improvement in ACR CRISS, compared with placebo-treated subjects over 16 weeks (P = .044). They also had greater improvement and less worsening in individual CRISS core measures, including modified Rodnan Skin Score, Patient Global Assessment, Physician Global Assessment, and the Health Assessment Questionnaire Disability Index. Patient-reported outcomes of systemic sclerosis skin symptoms, itch, and the Patient-Reported Outcomes Measurement Information System–29 (PROMIS-29) domains of physical function, pain interference, and sleep also improved for the anabasum group, compared with the placebo group (P less than .05 for all).
An analysis of paired skin biopsies before and after treatment with the assessor blinded to treatment assignment demonstrated that patients treated with anabasum were more likely to show improvement in fibrosis and inflammation and less likely to show worsening than were those treated with placebo, consistent with what was observed clinically. In a related poster presented at the meeting, gene expression analysis from specimens before and after treatment also revealed that anabasum treatment (as opposed to treatment with placebo) was associated with changes relevant to pathways involved in fibrosis and inflammation.
“This is the first double-blind, randomized, placebo controlled trial in diffuse cutaneous systemic sclerosis to demonstrate a clinical benefit using the CRISS as an endpoint, with a drug that was safe and well tolerated in the trial,” Dr. Spiera said. “These results bring hope to patients and their physicians that anabasum may be an effective drug for systemic sclerosis where currently there are no proven treatments.”
Both studies were sponsored by Corbus, and the dermatomyositis study was also sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some of the investigators in both studies were employees of Corbus. Dr. Spiera reported receiving research support from Corbus and many other pharmaceutical companies. He also is a consultant to Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, and CSL Behring. He is a member of the Rheumatology News editorial advisory board.
SAN DIEGO – Results from two phase 2 studies of the investigational agent anabasum provide evidence supporting its safety and efficacy in patients with refractory skin-predominant dermatomyositis or diffuse cutaneous systemic sclerosis.
Anabasum (JBT-101) is a nonimmunosuppressive, synthetic, oral cannabinoid receptor type 2 agonist being developed by Norwood, Mass.–based Corbus Pharmaceuticals. It works by triggering resolution of innate immune responses, said Barbara White, MD, a rheumatologist who is chief medical officer for Corbus. “It restores homeostasis, gets rid of inflammation, turns off active fibrotic processes, and helps clear bacteria if it is present – all without immunosuppression,” she explained in an interview at the annual meeting of the American College of Rheumatology. “A drug that would restore homeostasis in the setting of an ongoing immune response has the potential to be helpful in multiple autoimmune diseases.”
The mean age of the study participants was 53 years and most were white. Even though 19 of the patients were on immunosuppressants at baseline, both cohorts had mean CDASI scores in the 33-35 range, which can be considered severe.
The investigators, led by Victoria P. Werth, MD, of the University of Pennsylvania, Philadelphia, reported that anabasum-treated subjects experienced a medically meaningful improvement in mean CDASI scores, with a reduction of at least 5 points at all visits after week 4 and reaching –9.3 at the end of the study, compared with –3.7 points in the placebo group (P = .02). They also found that 56% of subjects in the anabasum group had a 10-point reduction or more in CDASI score, compared with only 18% in the placebo group (P = .09). In addition, no subjects in the anabasum group developed skin erosions during the active dosing period, compared with 36% of subjects in the placebo group (P = .05).
The researchers also observed that, compared with subjects in the placebo group, those in the anabasum group had greater improvement in patient-reported global skin disease and overall disease assessments, skin symptoms (including photosensitivity and itch), fatigue, sleep, interference with activities, pain, and physical function. No serious, severe, or unexpected adverse events occurred in the anabasum group. Adverse events included dizziness, dyspepsia, headache, and increased appetite.
There is not a Food and Drug Administration or European Medicines Agency–approved drug for refractory skin-predominant dermatomyositis, “so this is encouraging,” Dr. White said.
Dr. Spiera reported that patients in the anabasum group had greater improvement in ACR CRISS, compared with placebo-treated subjects over 16 weeks (P = .044). They also had greater improvement and less worsening in individual CRISS core measures, including modified Rodnan Skin Score, Patient Global Assessment, Physician Global Assessment, and the Health Assessment Questionnaire Disability Index. Patient-reported outcomes of systemic sclerosis skin symptoms, itch, and the Patient-Reported Outcomes Measurement Information System–29 (PROMIS-29) domains of physical function, pain interference, and sleep also improved for the anabasum group, compared with the placebo group (P less than .05 for all).
An analysis of paired skin biopsies before and after treatment with the assessor blinded to treatment assignment demonstrated that patients treated with anabasum were more likely to show improvement in fibrosis and inflammation and less likely to show worsening than were those treated with placebo, consistent with what was observed clinically. In a related poster presented at the meeting, gene expression analysis from specimens before and after treatment also revealed that anabasum treatment (as opposed to treatment with placebo) was associated with changes relevant to pathways involved in fibrosis and inflammation.
“This is the first double-blind, randomized, placebo controlled trial in diffuse cutaneous systemic sclerosis to demonstrate a clinical benefit using the CRISS as an endpoint, with a drug that was safe and well tolerated in the trial,” Dr. Spiera said. “These results bring hope to patients and their physicians that anabasum may be an effective drug for systemic sclerosis where currently there are no proven treatments.”
Both studies were sponsored by Corbus, and the dermatomyositis study was also sponsored by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Some of the investigators in both studies were employees of Corbus. Dr. Spiera reported receiving research support from Corbus and many other pharmaceutical companies. He also is a consultant to Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, and CSL Behring. He is a member of the Rheumatology News editorial advisory board.
AT ACR 2017
Key clinical point: Anabasum had a favorable safety profile and was well tolerated in patients with either refractory skin-predominant dermatomyositis or diffuse cutaneous systemic sclerosis.
Major finding: Treatment with anabasum resulted in greater improvement in the Cutaneous Dermatomyositis Disease Area and Severity Index (CDASI) score at 12 weeks, compared with placebo (–9.3 vs. –3.7 points, respectively; P = .02) and also in the ACR Combined Response Index in Systemic Sclerosis (CRISS) at 16 weeks, compared with placebo (P = .044).
Study details: Two 12-week, phase 2 studies of anabasum in patients with dermatomyositis or systemic sclerosis.
Disclosures: The studies were funded by Corbus, and the dermatomyositis study was additionally supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Spiera reported receiving research support from Corbus and many other pharmaceutical companies. He is a consultant to Roche-Genentech, GlaxoSmithKline, Boehringer Ingelheim, and CSL Behring. Some of the investigators in both studies were employees of Corbus.
Novel agent found to benefit Sjögren’s patients
SAN DIEGO – according to results of a proof-of-concept study.
CFZ533 is a novel monoclonal antibody being developed by Novartis that potently and selectively blocks CD40, a costimulatory pathway receptor essential for germinal center reactions and other immune-mediated functions implicated in primary Sjögren’s syndrome pathogenesis.
“Sjögren’s syndrome is characterized by focal infiltration of lymphocytes in a periductal distribution in exocrine glands,” lead study author Benjamin Fisher, MD, said at the annual meeting of the American College of Rheumatology.
“These lymphocytic foci often show organization and a proportion will show a germinal center formation. The lymphocytic infiltrates are associated with profound dryness, conjunctival erosions, fatigue that’s disabling, and in at least one-third of patients, systemic manifestations. There are no proven immunomodulatory treatments for this Sjögren’s syndrome. The lymphocytic infiltrates are also characterized by the expression of the costimulatory molecule CD40 and its ligand, also known as CD154,” he said.
For the study, subjects with an ESSDAI (EULAR Sjögren’s Syndrome Disease Activity Index) score of 6 or more were randomized 2:1 to receive 10 mg/kg of CFZ533 or placebo over a 12-week period. Four additional doses were administered in an open-label extension for 12 weeks. The primary goals were to evaluate the drug’s safety and tolerability as assessed by adverse events, and also to assess disease activity, with the primary outcome being the ESSDAI score. “The proof-of-concept criteria were stringent,” Dr. Fisher said. They “required a reduction in the ESSDAI score after 12 weeks to be 5 or more points greater in the CFZ533 group than the placebo group. The minimal clinically important difference is 3 points.”
He reported results from 21 patients in the CFZ533 and 11 in the placebo group. The baseline ESSDAI scores were 10.6 and 11 in the CFZ533 group and placebo groups, respectively. Patients also showed high symptomatic burden as assessed by the ESSPRI (EULAR Sjögren’s Syndrome Patient Reported Index), which measures dryness, fatigue, and pain on a 0-10 scale (mean scores of 6.7 and 7.2, respectively).
At 24 weeks, CFZ533 was found to be well tolerated, with no unexpected safety findings. “There were no serious infectious complications and there were no thromboembolic events,” Dr. Fisher said. “We did have one serious adverse event at the end of the open-label period. It occurred on the last day of that subject’s follow-up period. This was a case of atrial fibrillation. However, the patient had a predisposing medical condition and it was thought to be unrelated to the study drug.” Evaluation of pharmacokinetic data revealed that plasma levels throughout the study were above the level which previous data suggest is associated with inhibition of the CD40 pathway in tissue. “These plasma levels are also associated with inhibition of germinal center formation and with inhibition of T-dependent antigen response,” he said.
At the end of 12 weeks, CFZ533 was associated with a statistically significant reduction in ESSDAI scores, compared with the placebo group. The difference between groups was 5.6 points, which exceeded the proof-of-concept criteria. “This response was maintained through the open-label period,” Dr. Fisher said. “We also saw a reduction in the placebo group once they’d been switched to CFZ533 in the open-label period.”
The researchers also observed clinically meaningful improvements in other measures that favored CFZ533 over placebo, including the Physician Global Assessment, the physician and patient ratings on the Visual Analog Scale, and the Short Form–36 physical and mental health measures. “These data support continuation of the CFZ533 clinical program in primary Sjögren’s syndrome,” Dr. Fisher concluded.
Novartis sponsored the study. Dr. Fisher disclosed having received research support from Novartis, Roche, Bristol-Myers Squibb, and Virtualscopics.
SAN DIEGO – according to results of a proof-of-concept study.
CFZ533 is a novel monoclonal antibody being developed by Novartis that potently and selectively blocks CD40, a costimulatory pathway receptor essential for germinal center reactions and other immune-mediated functions implicated in primary Sjögren’s syndrome pathogenesis.
“Sjögren’s syndrome is characterized by focal infiltration of lymphocytes in a periductal distribution in exocrine glands,” lead study author Benjamin Fisher, MD, said at the annual meeting of the American College of Rheumatology.
“These lymphocytic foci often show organization and a proportion will show a germinal center formation. The lymphocytic infiltrates are associated with profound dryness, conjunctival erosions, fatigue that’s disabling, and in at least one-third of patients, systemic manifestations. There are no proven immunomodulatory treatments for this Sjögren’s syndrome. The lymphocytic infiltrates are also characterized by the expression of the costimulatory molecule CD40 and its ligand, also known as CD154,” he said.
For the study, subjects with an ESSDAI (EULAR Sjögren’s Syndrome Disease Activity Index) score of 6 or more were randomized 2:1 to receive 10 mg/kg of CFZ533 or placebo over a 12-week period. Four additional doses were administered in an open-label extension for 12 weeks. The primary goals were to evaluate the drug’s safety and tolerability as assessed by adverse events, and also to assess disease activity, with the primary outcome being the ESSDAI score. “The proof-of-concept criteria were stringent,” Dr. Fisher said. They “required a reduction in the ESSDAI score after 12 weeks to be 5 or more points greater in the CFZ533 group than the placebo group. The minimal clinically important difference is 3 points.”
He reported results from 21 patients in the CFZ533 and 11 in the placebo group. The baseline ESSDAI scores were 10.6 and 11 in the CFZ533 group and placebo groups, respectively. Patients also showed high symptomatic burden as assessed by the ESSPRI (EULAR Sjögren’s Syndrome Patient Reported Index), which measures dryness, fatigue, and pain on a 0-10 scale (mean scores of 6.7 and 7.2, respectively).
At 24 weeks, CFZ533 was found to be well tolerated, with no unexpected safety findings. “There were no serious infectious complications and there were no thromboembolic events,” Dr. Fisher said. “We did have one serious adverse event at the end of the open-label period. It occurred on the last day of that subject’s follow-up period. This was a case of atrial fibrillation. However, the patient had a predisposing medical condition and it was thought to be unrelated to the study drug.” Evaluation of pharmacokinetic data revealed that plasma levels throughout the study were above the level which previous data suggest is associated with inhibition of the CD40 pathway in tissue. “These plasma levels are also associated with inhibition of germinal center formation and with inhibition of T-dependent antigen response,” he said.
At the end of 12 weeks, CFZ533 was associated with a statistically significant reduction in ESSDAI scores, compared with the placebo group. The difference between groups was 5.6 points, which exceeded the proof-of-concept criteria. “This response was maintained through the open-label period,” Dr. Fisher said. “We also saw a reduction in the placebo group once they’d been switched to CFZ533 in the open-label period.”
The researchers also observed clinically meaningful improvements in other measures that favored CFZ533 over placebo, including the Physician Global Assessment, the physician and patient ratings on the Visual Analog Scale, and the Short Form–36 physical and mental health measures. “These data support continuation of the CFZ533 clinical program in primary Sjögren’s syndrome,” Dr. Fisher concluded.
Novartis sponsored the study. Dr. Fisher disclosed having received research support from Novartis, Roche, Bristol-Myers Squibb, and Virtualscopics.
SAN DIEGO – according to results of a proof-of-concept study.
CFZ533 is a novel monoclonal antibody being developed by Novartis that potently and selectively blocks CD40, a costimulatory pathway receptor essential for germinal center reactions and other immune-mediated functions implicated in primary Sjögren’s syndrome pathogenesis.
“Sjögren’s syndrome is characterized by focal infiltration of lymphocytes in a periductal distribution in exocrine glands,” lead study author Benjamin Fisher, MD, said at the annual meeting of the American College of Rheumatology.
“These lymphocytic foci often show organization and a proportion will show a germinal center formation. The lymphocytic infiltrates are associated with profound dryness, conjunctival erosions, fatigue that’s disabling, and in at least one-third of patients, systemic manifestations. There are no proven immunomodulatory treatments for this Sjögren’s syndrome. The lymphocytic infiltrates are also characterized by the expression of the costimulatory molecule CD40 and its ligand, also known as CD154,” he said.
For the study, subjects with an ESSDAI (EULAR Sjögren’s Syndrome Disease Activity Index) score of 6 or more were randomized 2:1 to receive 10 mg/kg of CFZ533 or placebo over a 12-week period. Four additional doses were administered in an open-label extension for 12 weeks. The primary goals were to evaluate the drug’s safety and tolerability as assessed by adverse events, and also to assess disease activity, with the primary outcome being the ESSDAI score. “The proof-of-concept criteria were stringent,” Dr. Fisher said. They “required a reduction in the ESSDAI score after 12 weeks to be 5 or more points greater in the CFZ533 group than the placebo group. The minimal clinically important difference is 3 points.”
He reported results from 21 patients in the CFZ533 and 11 in the placebo group. The baseline ESSDAI scores were 10.6 and 11 in the CFZ533 group and placebo groups, respectively. Patients also showed high symptomatic burden as assessed by the ESSPRI (EULAR Sjögren’s Syndrome Patient Reported Index), which measures dryness, fatigue, and pain on a 0-10 scale (mean scores of 6.7 and 7.2, respectively).
At 24 weeks, CFZ533 was found to be well tolerated, with no unexpected safety findings. “There were no serious infectious complications and there were no thromboembolic events,” Dr. Fisher said. “We did have one serious adverse event at the end of the open-label period. It occurred on the last day of that subject’s follow-up period. This was a case of atrial fibrillation. However, the patient had a predisposing medical condition and it was thought to be unrelated to the study drug.” Evaluation of pharmacokinetic data revealed that plasma levels throughout the study were above the level which previous data suggest is associated with inhibition of the CD40 pathway in tissue. “These plasma levels are also associated with inhibition of germinal center formation and with inhibition of T-dependent antigen response,” he said.
At the end of 12 weeks, CFZ533 was associated with a statistically significant reduction in ESSDAI scores, compared with the placebo group. The difference between groups was 5.6 points, which exceeded the proof-of-concept criteria. “This response was maintained through the open-label period,” Dr. Fisher said. “We also saw a reduction in the placebo group once they’d been switched to CFZ533 in the open-label period.”
The researchers also observed clinically meaningful improvements in other measures that favored CFZ533 over placebo, including the Physician Global Assessment, the physician and patient ratings on the Visual Analog Scale, and the Short Form–36 physical and mental health measures. “These data support continuation of the CFZ533 clinical program in primary Sjögren’s syndrome,” Dr. Fisher concluded.
Novartis sponsored the study. Dr. Fisher disclosed having received research support from Novartis, Roche, Bristol-Myers Squibb, and Virtualscopics.
AT ACR 2017
Key clinical point: Data support continuation of CFZ533 in clinical trials.
Major finding: At the end of 12 weeks, patients in the treatment group had a mean improvement of 5.6 points on the EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) score.
Study details: A proof of concept study of 42 Sjögren’s patients.
Disclosures: Novartis sponsored the study. Dr. Fisher disclosed having received research support from Novartis, Roche, Bristol-Myers Squibb, and Virtualscopics.
Higher water intake linked to less hyperuricemia in gout
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
SAN DIEGO – While more hydration seems to improve gout, there’s little research into the connection between the two. Now, a new study suggests a strong link between low water consumption and hyperuricemia, a possible sign that boosting water intake will help some gout patients.
While it’s too early to confirm a clinically relevant connection, “there is a statistically significant inverse association between water consumption and high uric acid levels,” said Patricia Kachur, MD, a third-year internal medicine resident at the University of Central Florida, Ocala (Fla.) Regional Medical Center. Dr. Kachur, who spoke about the findings in an interview, is lead author of a study presented at the annual meeting of the American College of Rheumatology.
An abstract presented at the 2009 ACR annual meeting reported fewer gout attacks (adjusted odds ratio, 0.54; 95% confidence interval, 0.32-0.90) in 535 gout patients who reported drinking more than eight glasses of water over a 24-hour period, compared with those who drank one or fewer.
For the new study, Dr. Kachur and her colleagues examined findings from 539 participants with gout (but not chronic kidney disease) out of 17,321 individuals who took part in the National Health and Nutrition Examination Survey from 2009 to 2014.
Of the 539 participants, 39% were defined as having hyperuricemia (6.0 mg/dL or greater), with the rest having a low or normal level. Those with hyperuricemia were significantly more likely to be male and have obesity and hypertension.
The investigators defined high water intake as three or more liters of water per day for men and 2.2 or more liters for women. Of the 539 participants, 116 (22%) had high water intake.
The researchers found a lower risk of developing hyperuricemia in those with higher water intake, compared with those with lower intake (adjusted OR, 0.421; 95% CI, 0.262-0.679; P = .0007).
“These findings do not say anything about water and gout – not yet anyway,” Dr. Kachur said. “Rather there is a possibility that outpatient water intake has an association with lower uric acid levels in people afflicted by gout even after considering multiple other factors such as gender, race, BMI, age, hypertension, and diabetes mellitus.”
Indeed, she and her colleagues decided against publishing the results of their 2009 study “because there is a major challenge in interpreting these data.”
“Given that people only consume a finite amount of liquids each day, is it that consuming more water is beneficial or that drinking less of ‘bad’ fluids (for example, sodas, sugar-sweetened juices) is beneficial? We were not able to disentangle this issue,” she explained.
Still, she said, there are explanations about why water intake could be beneficial for gout. “Intravascular volume depletion increases the concentration of serum urate, and increased serum urate beyond the saturation threshold can result in crystallization,” she said. “With heat-related dehydration, there may also be metabolic acidosis and/or electrolyte abnormalities that can lead to decreased urate secretion in renal tubules, and an acidic pH can decrease solubility of serum urate.”
Dr. Neogi does encourage appropriate gout patients to make sure they drink enough water, especially if it is hot. She cowrote a 2014 study that linked gout flares to high temperatures and extremes of humidity, which can lead to dehydration (Am J Epidemiol. 2014 Aug 15;180[4]:372-7).
“The amount of water intake that is beneficial for gout is not known, so patients should follow general recommendations for water intake. In addition, I strongly encourage patients with gout to avoid or limit the amount of liquid consumed in the form of regular sodas and sweetened drinks or juices, particularly those with high-fructose corn syrup, and alcohol,” she said. “With regards to tea or coffee, if patients drink either tea or coffee, they can continue to do so and to use only low-fat or nonfat milk and little or no sugar.”
Meanwhile, she said, “there are some data to suggest that cherry juice – true natural cherry juice from fruit, not ‘cherry drinks’ – can be beneficial for gout. We are formally testing cherry juice in a trial.”
What’s next for research into water intake and gout? “The clinical correlation is missing in the study,” said Dr. Kachur, lead author of the new study. “Targeted surveys of gout patients, hopefully followed by a randomized controlled trial regulating water intake, can help make those connections.”
Dr. Kachur and other study authors reported having no relevant disclosures. Dr. Neogi reported having no relevant disclosures. No specific study funding was reported.
AT ACR 2017
Key clinical point:
Major finding: Gout patients with the highest water intake were less likely than others to have hyperuricemia (aOR, 0.421).
Data source: 539 participants with gout (but not chronic kidney disease) out of 17,321 in the National Health and Nutrition Examination Survey, 2009-2014.
Disclosures: The study authors reported having no relevant disclosures. No specific study funding was reported.
Race may transcend social, geographical parameters in lupus mortality
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
dbrunk@frontlinemedcom.com
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
dbrunk@frontlinemedcom.com
SAN DIEGO – Blacks with systemic lupus erythematosus (SLE) who share the same social and geographic contexts as whites with the disease were disproportionately more likely to die young and to show severe patterns of mortality, according to a study of death certificate data.
“One of the most salient aspects of the epidemiology of lupus is the predilection of the disease for women and racial minorities,” lead study author Titilola Falasinnu, PhD, said at the annual meeting of the American College of Rheumatology.
Dr. Falasinnu, a postdoctoral fellow in Stanford (Calif.) University’s department of health research and policy, and her associates examined SLE mortality across eight groups of race-county combinations published in 2006 and known as the “Eight Americas” (PLoS Med. 2006 Sep 12;3[9]:e260). This seminal work, which has been validated across multiple disease states, jointly characterized race, socioeconomic status, and geographical location in relation to health disparities in the United States to demonstrate the most important factors accounting for these disparities within the Eight Americas.
For the current analysis, Dr. Falasinnu and her associates were most interested in America 6 (black middle America), America 7 (Southern low-income rural blacks), and America 8 (high-risk urban blacks). “The question we wanted to ask is whether, on average, poorer individuals have more severe SLE mortality experiences, compared with richer individuals in the black community,” she said. “What happens when you hold race constant and you vary socioeconomic indices?” Using death certificate data from the National Center for Health Statistics Multiple Causes of Death database, they identified SLE-related deaths between 2003 and 2014. Next, they compared these data with mortality statistics from each of the Eight Americas.
In all, there were nearly 25,000 SLE-related deaths, of which 85% were female. More than one-third of deaths occurred among those aged 45-64 years, and the mean age at death was 57 years. More than half of deaths (64%) occurred among whites, and 31% among blacks. Among SLE decedents, northern rural whites in America 2 had the lowest mortality rates. Blacks in America 6, 7, and 8 had the highest mortality, yet there were no significant differences in the death rates among those three groups. “We found that in general, blacks were three times more likely to die with SLE, compared with those in America 3 [middle America],” Dr. Falasinnu said.
Asians, Native Americans, and blacks with SLE died at an average age of 48-49 years, regardless of their social context, while Northern whites had the highest life expectancy: an average age of 69 years. They also found that 17% of SLE deaths in America 2 occurred before the age of 50 years, compared with more than 50% in Americas 6, 7, and 8. The most frequently reported associated causes of death were cardiovascular disease (about 50% of all SLE-related deaths) and kidney manifestations (about 20% of all SLE-related deaths). Compared with those in America 3, racial minorities had a 23%-53% higher risk of infections, a 5%-64% higher risk of kidney disease, a 7%-23% lower risk of cardiovascular disease, and a 20%-52% lower risk of cancers.
“Although blacks inhabited three vastly different geographical and social contexts, SLE mortality parameters did not vary among socially advantaged and disadvantaged black Americas,” Dr. Falasinnu concluded. “Blacks sharing the same social and geographical contexts as whites were disproportionately more likely to die young and exhibit patterns of mortality associated with active SLE disease.”
She acknowledged certain limitations of the study, including that differences in the degree of underreporting on death certificates across racial groups could bias the results. “The Eight Americas framework does not allow for evaluation of ethnicity,” she added. “We were also unable to examine causes for the disparities in SLE mortality. One could argue that there are a lot of other social factors that are likely race related that are not necessarily captured by the Eight Americas. Also, as with most epidemiological studies, we cannot rule out the role that ecological fallacy may play where the population average may not be appropriate in estimating an individual’s risk of mortality.”
One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
dbrunk@frontlinemedcom.com
AT ACR 2017
Key clinical point: Blacks sharing the same social and geographical contexts as whites were more likely to die young and exhibit patterns of mortality associated with active SLE disease.
Major finding: Blacks in three race-geographic contexts were about three times more likely than whites in middle America to die with SLE.
Study details: An analysis of nearly 25,000 SLE-related deaths from the National Center for Health Statistics Multiple Causes of Death database.
Disclosures: One of the study coauthors reported receiving partial salary support through the Dr. Elaine Lambert Lupus Foundation via the John and Marcia Goldman Foundation and previously receiving salary support through a lupus-related grant from the Genomics Institute of the Novartis Research Foundation. The other coauthors reported having no relevant disclosures.
Proposed SLE classification criteria prove highly sensitive, specific
SAN DIEGO – yielded a sensitivity of 98% and a specificity of 97%.
The development is part of a four-phase joint effort by the American College of Rheumatology and the European League Against Rheumatism, first launched in 2014, to improve classification of patients with SLE for the purposes of clinical trials of new therapies and clinical research into the causes and outcomes of the disease. “The ACR and EULAR have long recognized the importance of classification criteria, so that we have more homogeneous groups in these research studies, and then we can compare results from studies,” Sindhu Johnson, MD, PhD, said during a press briefing at the annual meeting of the American College of Rheumatology.
A synopsis of the first three phases from the ongoing effort was presented at the June 2017 EULAR meeting. It included results from a systematic review of the literature and a meta-regression analysis to determine whether antinuclear autoantibodies should be required in the classification of SLE. The analysis showed that an antinuclear antibody titer of greater than or equal to 1:80 by immunofluorescence on human epithelial type 2 cells had a sensitivity of 98.4% for correctly capturing SLE. This prompted the EULAR/ACR steering committee to propose this titer as an “entry criterion” for SLE classification.
At the ACR meeting, Dr. Johnson, a rheumatologist at the University of Toronto who also cochairs the ACR Classification and Response Criteria subcommittee, discussed the fourth phase of the collaboration, which involves fine-tuning and validating the proposed SLE classification criteria.
To date, 189 investigators and more than 3,500 patients have contributed to the effort. “In the most recent phase, 36 international lupus centers were approached to contribute 100 cases and 100 controls,” she explained. “We then had each case independently adjudicated by three lupus experts at three different lupus centers to make sure there was consensus on the diagnosis. Next, we randomly selected 500 cases and 500 controls, resulting in a derivation cohort of 1,000 subjects to test our draft criteria system.”
Dr. Johnson and her associates found that the proposed SLE criteria had a sensitivity of 98% and a specificity of 97%, which exceeds that of the old ACR criteria. “We have defined a system of criteria which produces a measure of the relative probability that a particular case with a combination of clinical symptoms or features has SLE,” she said.
Dr. Johnson emphasized that the proposed criteria are intended for the classification of SLE, not for diagnosing the condition. “The diagnosis of lupus still remains in the hands of the physician, who will take into account all of the symptoms, signs, and other investigations,” she said. “We have identified the highest yield of those, but there will be some people who do not fulfill classification criteria yet do have a diagnosis of SLE.”
The next few weeks is a period to solicit feedback from stakeholders, after which members of the EULAR/ACR steering committee will be weighing feedback on the proposed criteria. “After that, we will see if there are any final revisions that need to be made,” Dr. Johnson said. “If we’re happy with the product, then the final validation will occur. We still have more than 1,000 patients from the validation core that has been reserved for that final validation.”
Data from the final validation are expected to be presented at the June 2018 EULAR meeting and ultimately published in Arthritis and Rheumatism and the Annals of Rheumatic Diseases. “Before we can get there, though, it needs to be formally reviewed by the ACR and EULAR for their formal endorsement. We expect that will take another 6 months.” She reported having no disclosures.
SAN DIEGO – yielded a sensitivity of 98% and a specificity of 97%.
The development is part of a four-phase joint effort by the American College of Rheumatology and the European League Against Rheumatism, first launched in 2014, to improve classification of patients with SLE for the purposes of clinical trials of new therapies and clinical research into the causes and outcomes of the disease. “The ACR and EULAR have long recognized the importance of classification criteria, so that we have more homogeneous groups in these research studies, and then we can compare results from studies,” Sindhu Johnson, MD, PhD, said during a press briefing at the annual meeting of the American College of Rheumatology.
A synopsis of the first three phases from the ongoing effort was presented at the June 2017 EULAR meeting. It included results from a systematic review of the literature and a meta-regression analysis to determine whether antinuclear autoantibodies should be required in the classification of SLE. The analysis showed that an antinuclear antibody titer of greater than or equal to 1:80 by immunofluorescence on human epithelial type 2 cells had a sensitivity of 98.4% for correctly capturing SLE. This prompted the EULAR/ACR steering committee to propose this titer as an “entry criterion” for SLE classification.
At the ACR meeting, Dr. Johnson, a rheumatologist at the University of Toronto who also cochairs the ACR Classification and Response Criteria subcommittee, discussed the fourth phase of the collaboration, which involves fine-tuning and validating the proposed SLE classification criteria.
To date, 189 investigators and more than 3,500 patients have contributed to the effort. “In the most recent phase, 36 international lupus centers were approached to contribute 100 cases and 100 controls,” she explained. “We then had each case independently adjudicated by three lupus experts at three different lupus centers to make sure there was consensus on the diagnosis. Next, we randomly selected 500 cases and 500 controls, resulting in a derivation cohort of 1,000 subjects to test our draft criteria system.”
Dr. Johnson and her associates found that the proposed SLE criteria had a sensitivity of 98% and a specificity of 97%, which exceeds that of the old ACR criteria. “We have defined a system of criteria which produces a measure of the relative probability that a particular case with a combination of clinical symptoms or features has SLE,” she said.
Dr. Johnson emphasized that the proposed criteria are intended for the classification of SLE, not for diagnosing the condition. “The diagnosis of lupus still remains in the hands of the physician, who will take into account all of the symptoms, signs, and other investigations,” she said. “We have identified the highest yield of those, but there will be some people who do not fulfill classification criteria yet do have a diagnosis of SLE.”
The next few weeks is a period to solicit feedback from stakeholders, after which members of the EULAR/ACR steering committee will be weighing feedback on the proposed criteria. “After that, we will see if there are any final revisions that need to be made,” Dr. Johnson said. “If we’re happy with the product, then the final validation will occur. We still have more than 1,000 patients from the validation core that has been reserved for that final validation.”
Data from the final validation are expected to be presented at the June 2018 EULAR meeting and ultimately published in Arthritis and Rheumatism and the Annals of Rheumatic Diseases. “Before we can get there, though, it needs to be formally reviewed by the ACR and EULAR for their formal endorsement. We expect that will take another 6 months.” She reported having no disclosures.
SAN DIEGO – yielded a sensitivity of 98% and a specificity of 97%.
The development is part of a four-phase joint effort by the American College of Rheumatology and the European League Against Rheumatism, first launched in 2014, to improve classification of patients with SLE for the purposes of clinical trials of new therapies and clinical research into the causes and outcomes of the disease. “The ACR and EULAR have long recognized the importance of classification criteria, so that we have more homogeneous groups in these research studies, and then we can compare results from studies,” Sindhu Johnson, MD, PhD, said during a press briefing at the annual meeting of the American College of Rheumatology.
A synopsis of the first three phases from the ongoing effort was presented at the June 2017 EULAR meeting. It included results from a systematic review of the literature and a meta-regression analysis to determine whether antinuclear autoantibodies should be required in the classification of SLE. The analysis showed that an antinuclear antibody titer of greater than or equal to 1:80 by immunofluorescence on human epithelial type 2 cells had a sensitivity of 98.4% for correctly capturing SLE. This prompted the EULAR/ACR steering committee to propose this titer as an “entry criterion” for SLE classification.
At the ACR meeting, Dr. Johnson, a rheumatologist at the University of Toronto who also cochairs the ACR Classification and Response Criteria subcommittee, discussed the fourth phase of the collaboration, which involves fine-tuning and validating the proposed SLE classification criteria.
To date, 189 investigators and more than 3,500 patients have contributed to the effort. “In the most recent phase, 36 international lupus centers were approached to contribute 100 cases and 100 controls,” she explained. “We then had each case independently adjudicated by three lupus experts at three different lupus centers to make sure there was consensus on the diagnosis. Next, we randomly selected 500 cases and 500 controls, resulting in a derivation cohort of 1,000 subjects to test our draft criteria system.”
Dr. Johnson and her associates found that the proposed SLE criteria had a sensitivity of 98% and a specificity of 97%, which exceeds that of the old ACR criteria. “We have defined a system of criteria which produces a measure of the relative probability that a particular case with a combination of clinical symptoms or features has SLE,” she said.
Dr. Johnson emphasized that the proposed criteria are intended for the classification of SLE, not for diagnosing the condition. “The diagnosis of lupus still remains in the hands of the physician, who will take into account all of the symptoms, signs, and other investigations,” she said. “We have identified the highest yield of those, but there will be some people who do not fulfill classification criteria yet do have a diagnosis of SLE.”
The next few weeks is a period to solicit feedback from stakeholders, after which members of the EULAR/ACR steering committee will be weighing feedback on the proposed criteria. “After that, we will see if there are any final revisions that need to be made,” Dr. Johnson said. “If we’re happy with the product, then the final validation will occur. We still have more than 1,000 patients from the validation core that has been reserved for that final validation.”
Data from the final validation are expected to be presented at the June 2018 EULAR meeting and ultimately published in Arthritis and Rheumatism and the Annals of Rheumatic Diseases. “Before we can get there, though, it needs to be formally reviewed by the ACR and EULAR for their formal endorsement. We expect that will take another 6 months.” She reported having no disclosures.
AT ACR 2017
For women with RA, small-joint surgery rate nearly twice that of men
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
SAN DIEGO – but the rate of small-joint procedures is declining for both sexes. However, no differences in rates of large-joint procedures between sexes were observed during the same time period.
Those are key findings from a retrospective study which set out to determine if there are sex differences in the incidence and trends of large- versus small-joint surgery rates in rheumatoid arthritis over time. “Why is orthopedic surgery important to rheumatology? The main reason is because it’s a surrogate for failed medical management,” lead study author Michael D. Richter, MD, said at the annual meeting of the American College of Rheumatology.
Dr. Richter, an internal medicine resident at Mayo Clinic, Rochester, Minn., said that women with RA generally present with more severe symptoms and higher rates of disability, while men have a better treatment response and a higher remission rate. For example, results from the multinational Quantitative Standard Monitoring of Patients with RA study found that remission rates were around 30% in men and 17% in women (Arthritis Res Ther. 2009;11[1]:R7). “However, a lot of these studies are criticized because it’s thought that gender can play a role in the disease measures,” he said. “By looking at joint surgery we have an objective outcome, and we can look at differences in treatment efficacy.”
Dr. Richter and his associates drew from the Rochester Epidemiology Project to identify 1,077 patients from Olmstead County, Minn., who fulfilled ACR criteria for RA between 1980 and 2013, and who were followed up until death, migration, or July 1, 2016. They classified surgeries as small joint (wrist, hand, or foot) or large joint (shoulder, elbow, hip, knee, or ankle). A majority of the patients (70%) were women. Compared with women, men were slightly older at diagnosis (a mean of 58 years vs. 55 years, respectively), were more likely to have a history of smoking (67% vs. 46%), and were more likely to have large-joint swelling upon initial presentation (49% vs. 42%). The mean follow-up was 12 years. No differences between men and women were noted in obesity, inflammatory biomarkers, or seropositivity.
During the study period, 112 patients underwent at least one small-joint surgery, 90 of whom were women (80%). The cumulative incidence of small-joint surgery at 15 years was nearly double that of men: 14.4% vs. 7.6%, respectively (P = .008). “Prior to the year 2000 there were no significant trends in the rate of small-joint surgery but it was more common in women,” he said. “After 2000 there was a significant decline for men and women (P = .002), but no significant difference between sexes.”
At the same time, 204 patients underwent at least one large-joint surgery during the time period, 141 of whom were women (69%). The cumulative incidence of large-joint surgery at 15 years was 20.2% for women and 18.8% for men, which was statistically similar (P = .55). “We saw no significant change over time in the rate of large-joint surgery from 1980 to 2016,” Dr. Richter said. “This is in contrast to what we see in the general population, where orthopedic procedures for osteoarthritis are more common.”
He acknowledged certain limitations of the study, including its retrospective design and the fact that the researchers were unable to include specific surgical indications in the analysis. “This becomes particularly important for the large-joint procedures,” he said. “We don’t know if osteoarthritis or chronic inflammatory arthritis is leading to the large-joint procedure.”
The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
AT ACR 2017
Key clinical point: Women with RA had a higher rate of small-joint surgery, compared with men.
Major finding: The cumulative incidence of small-joint surgery was significantly higher among women, compared with men (14.4% vs. 7.6%, respectively), but there were no differences between sexes in the rates of large-joint surgery.
Study details: A retrospective, population-based study of 1,077 patients with RA.
Disclosures: The National Institute of Arthritis and Musculoskeletal and Skin Diseases and the National Institute on Aging funded the study. Dr. Richter reported having no financial disclosures.
Mixed results for rheumatologists on Medicare quality measures
SAN DIEGO – As quality measures are poised to become crucial to U.S. physician incomes in 2019, an analysis of a nationwide registry sample finds that rheumatologists overall have decent scores on several fronts. But they still need to boost their record on preventive measures that are not geared specifically to their specialty.
“Rheumatologists are performing well, so far, on aspects of care coordination, such as communication with the health care team post osteoporotic fracture and on several patient safety measures, such as avoiding high-risk drugs in the elderly,” said Jinoos Yazdany, MD, of the University of California, San Francisco. However, “in some preventive care measures, such as tobacco screening, there is room for improvement compared to benchmarks that the Centers for Medicare & Medicaid Services has set across providers.”
The ACR created the registry “to help rheumatology harness the power of electronic health record data to advance our understanding of the natural history, treatment, and outcomes of rheumatic disease,” Dr. Yazdany said. “Another important goal was to harness the power of a national registry to measure and improve quality of care and outcomes. Practices can use RISE to see where they are performing well and where there is room for improvement.”
Since 2016, the registry has passively collected data on 2.5 million patients and 13.7 million encounters.
“The quality measures in RISE serve several purposes,” Dr. Yazdany said. “First, they fulfill reporting requirements to CMS through the Merit-Based Incentive Payment System [MIPS]. Second, the measures provide information to practices that can be used to track quality improvement and population health management. Finally, the measures create unprecedented opportunities to learn from practices that are excelling and to adapt successful work flows to improve care for our patients.”
These measures matter. In 2019, payments for many physicians under Medicare Part B will be adjusted based on their performance in these areas in previous years. Most rheumatologists will take part, Dr. Yazdany said.
“Rather than focusing on a single measure, the key number in 2017 for the MIPS program is 70 points across the three domains of Quality, Advancing Care Information, and Improvement Activities,” she said. “Above that threshold, rheumatologists will qualify for an ‘exceptional performance bonus.’ That means they will get a minimum of an additional 0.5% on their Medicare billing.”
She added that “there is no reason that most rheumatologists should not cross the 70-point threshold. Proactively monitoring their progress in RISE will help them succeed.”
The ACR session focused on a registry sample of 225 practices and 750 rheumatologists. The analysis measured their performance from January to September 2017 in the Quality, Advancing Care Information, and Improvement Activities areas.
In terms of meeting benchmarks, the rheumatologists in the sample performed especially well in several areas.
On the drug safety front, across elderly patients, an average of just 3.6% were prescribed one or more high-risk medications, and 0.2% were prescribed two or more.
On rheumatoid arthritis measures, 52% of patients had documentation of tuberculosis screening before biologics, and 46.3% underwent functional status assessments. And in the care coordination and documentation measure, 92.9% documented current medications in the EHR.
Rheumatologists lagged in terms of preventive care, compared with other physicians nationally: The average performance across patients was 77.2% for tobacco screening and counseling, 42.7% for body mass index screening and counseling, and 60.2% for blood pressure management.
Why are these preventive care measures being tracked in rheumatology instead of more rheumatology-specific measures? “CMS requires that physicians submit an outcome measure. Unfortunately, we don’t have validated outcome measures in rheumatology, so we had to adopt outcome measures like controlling blood pressure,” Dr. Yazdany said. “Also, many preventive care measures are designated ‘high priority,’ which enables physicians to get bonus points. We wanted rheumatologists to have access to these extra points and therefore included these measures in RISE.”
The ACR is working on developing outcome measures, she said, “and hopefully we’ll have outcomes to put in the registry in coming years.”
What are the chances that rheumatologists will do well? “Our analyses show that most rheumatologists participating in RISE are well positioned to succeed. If they complete their improvement activities (15% of MIPS), and advancing care information (25% of MIPS) modules, that gets them to 40 points. That means they only need 30 additional points in the quality domain to get to the exceptional performance threshold and qualify for a bonus,” she said. “All 15 of the rheumatologists who have completed all three MIPS categories have reached 70 points, and we anticipate that many others will by the end of the year.”
Even just participating in RISE will boost points, she said. “It is clear that CMS is encouraging the large-scale development of quality improvement registries like RISE.”
In the big picture, she said, “the key point is that rheumatologists should be proactive. They need to understand their performance on measures, pick areas to focus on, including areas where they can easily improve their scores.”
Dr. Yazdany reported no relevant disclosures. The study was funded by ACR.
SAN DIEGO – As quality measures are poised to become crucial to U.S. physician incomes in 2019, an analysis of a nationwide registry sample finds that rheumatologists overall have decent scores on several fronts. But they still need to boost their record on preventive measures that are not geared specifically to their specialty.
“Rheumatologists are performing well, so far, on aspects of care coordination, such as communication with the health care team post osteoporotic fracture and on several patient safety measures, such as avoiding high-risk drugs in the elderly,” said Jinoos Yazdany, MD, of the University of California, San Francisco. However, “in some preventive care measures, such as tobacco screening, there is room for improvement compared to benchmarks that the Centers for Medicare & Medicaid Services has set across providers.”
The ACR created the registry “to help rheumatology harness the power of electronic health record data to advance our understanding of the natural history, treatment, and outcomes of rheumatic disease,” Dr. Yazdany said. “Another important goal was to harness the power of a national registry to measure and improve quality of care and outcomes. Practices can use RISE to see where they are performing well and where there is room for improvement.”
Since 2016, the registry has passively collected data on 2.5 million patients and 13.7 million encounters.
“The quality measures in RISE serve several purposes,” Dr. Yazdany said. “First, they fulfill reporting requirements to CMS through the Merit-Based Incentive Payment System [MIPS]. Second, the measures provide information to practices that can be used to track quality improvement and population health management. Finally, the measures create unprecedented opportunities to learn from practices that are excelling and to adapt successful work flows to improve care for our patients.”
These measures matter. In 2019, payments for many physicians under Medicare Part B will be adjusted based on their performance in these areas in previous years. Most rheumatologists will take part, Dr. Yazdany said.
“Rather than focusing on a single measure, the key number in 2017 for the MIPS program is 70 points across the three domains of Quality, Advancing Care Information, and Improvement Activities,” she said. “Above that threshold, rheumatologists will qualify for an ‘exceptional performance bonus.’ That means they will get a minimum of an additional 0.5% on their Medicare billing.”
She added that “there is no reason that most rheumatologists should not cross the 70-point threshold. Proactively monitoring their progress in RISE will help them succeed.”
The ACR session focused on a registry sample of 225 practices and 750 rheumatologists. The analysis measured their performance from January to September 2017 in the Quality, Advancing Care Information, and Improvement Activities areas.
In terms of meeting benchmarks, the rheumatologists in the sample performed especially well in several areas.
On the drug safety front, across elderly patients, an average of just 3.6% were prescribed one or more high-risk medications, and 0.2% were prescribed two or more.
On rheumatoid arthritis measures, 52% of patients had documentation of tuberculosis screening before biologics, and 46.3% underwent functional status assessments. And in the care coordination and documentation measure, 92.9% documented current medications in the EHR.
Rheumatologists lagged in terms of preventive care, compared with other physicians nationally: The average performance across patients was 77.2% for tobacco screening and counseling, 42.7% for body mass index screening and counseling, and 60.2% for blood pressure management.
Why are these preventive care measures being tracked in rheumatology instead of more rheumatology-specific measures? “CMS requires that physicians submit an outcome measure. Unfortunately, we don’t have validated outcome measures in rheumatology, so we had to adopt outcome measures like controlling blood pressure,” Dr. Yazdany said. “Also, many preventive care measures are designated ‘high priority,’ which enables physicians to get bonus points. We wanted rheumatologists to have access to these extra points and therefore included these measures in RISE.”
The ACR is working on developing outcome measures, she said, “and hopefully we’ll have outcomes to put in the registry in coming years.”
What are the chances that rheumatologists will do well? “Our analyses show that most rheumatologists participating in RISE are well positioned to succeed. If they complete their improvement activities (15% of MIPS), and advancing care information (25% of MIPS) modules, that gets them to 40 points. That means they only need 30 additional points in the quality domain to get to the exceptional performance threshold and qualify for a bonus,” she said. “All 15 of the rheumatologists who have completed all three MIPS categories have reached 70 points, and we anticipate that many others will by the end of the year.”
Even just participating in RISE will boost points, she said. “It is clear that CMS is encouraging the large-scale development of quality improvement registries like RISE.”
In the big picture, she said, “the key point is that rheumatologists should be proactive. They need to understand their performance on measures, pick areas to focus on, including areas where they can easily improve their scores.”
Dr. Yazdany reported no relevant disclosures. The study was funded by ACR.
SAN DIEGO – As quality measures are poised to become crucial to U.S. physician incomes in 2019, an analysis of a nationwide registry sample finds that rheumatologists overall have decent scores on several fronts. But they still need to boost their record on preventive measures that are not geared specifically to their specialty.
“Rheumatologists are performing well, so far, on aspects of care coordination, such as communication with the health care team post osteoporotic fracture and on several patient safety measures, such as avoiding high-risk drugs in the elderly,” said Jinoos Yazdany, MD, of the University of California, San Francisco. However, “in some preventive care measures, such as tobacco screening, there is room for improvement compared to benchmarks that the Centers for Medicare & Medicaid Services has set across providers.”
The ACR created the registry “to help rheumatology harness the power of electronic health record data to advance our understanding of the natural history, treatment, and outcomes of rheumatic disease,” Dr. Yazdany said. “Another important goal was to harness the power of a national registry to measure and improve quality of care and outcomes. Practices can use RISE to see where they are performing well and where there is room for improvement.”
Since 2016, the registry has passively collected data on 2.5 million patients and 13.7 million encounters.
“The quality measures in RISE serve several purposes,” Dr. Yazdany said. “First, they fulfill reporting requirements to CMS through the Merit-Based Incentive Payment System [MIPS]. Second, the measures provide information to practices that can be used to track quality improvement and population health management. Finally, the measures create unprecedented opportunities to learn from practices that are excelling and to adapt successful work flows to improve care for our patients.”
These measures matter. In 2019, payments for many physicians under Medicare Part B will be adjusted based on their performance in these areas in previous years. Most rheumatologists will take part, Dr. Yazdany said.
“Rather than focusing on a single measure, the key number in 2017 for the MIPS program is 70 points across the three domains of Quality, Advancing Care Information, and Improvement Activities,” she said. “Above that threshold, rheumatologists will qualify for an ‘exceptional performance bonus.’ That means they will get a minimum of an additional 0.5% on their Medicare billing.”
She added that “there is no reason that most rheumatologists should not cross the 70-point threshold. Proactively monitoring their progress in RISE will help them succeed.”
The ACR session focused on a registry sample of 225 practices and 750 rheumatologists. The analysis measured their performance from January to September 2017 in the Quality, Advancing Care Information, and Improvement Activities areas.
In terms of meeting benchmarks, the rheumatologists in the sample performed especially well in several areas.
On the drug safety front, across elderly patients, an average of just 3.6% were prescribed one or more high-risk medications, and 0.2% were prescribed two or more.
On rheumatoid arthritis measures, 52% of patients had documentation of tuberculosis screening before biologics, and 46.3% underwent functional status assessments. And in the care coordination and documentation measure, 92.9% documented current medications in the EHR.
Rheumatologists lagged in terms of preventive care, compared with other physicians nationally: The average performance across patients was 77.2% for tobacco screening and counseling, 42.7% for body mass index screening and counseling, and 60.2% for blood pressure management.
Why are these preventive care measures being tracked in rheumatology instead of more rheumatology-specific measures? “CMS requires that physicians submit an outcome measure. Unfortunately, we don’t have validated outcome measures in rheumatology, so we had to adopt outcome measures like controlling blood pressure,” Dr. Yazdany said. “Also, many preventive care measures are designated ‘high priority,’ which enables physicians to get bonus points. We wanted rheumatologists to have access to these extra points and therefore included these measures in RISE.”
The ACR is working on developing outcome measures, she said, “and hopefully we’ll have outcomes to put in the registry in coming years.”
What are the chances that rheumatologists will do well? “Our analyses show that most rheumatologists participating in RISE are well positioned to succeed. If they complete their improvement activities (15% of MIPS), and advancing care information (25% of MIPS) modules, that gets them to 40 points. That means they only need 30 additional points in the quality domain to get to the exceptional performance threshold and qualify for a bonus,” she said. “All 15 of the rheumatologists who have completed all three MIPS categories have reached 70 points, and we anticipate that many others will by the end of the year.”
Even just participating in RISE will boost points, she said. “It is clear that CMS is encouraging the large-scale development of quality improvement registries like RISE.”
In the big picture, she said, “the key point is that rheumatologists should be proactive. They need to understand their performance on measures, pick areas to focus on, including areas where they can easily improve their scores.”
Dr. Yazdany reported no relevant disclosures. The study was funded by ACR.
AT ACR 2017
VIDEO: Advanced alternative payment model for RA set to undergo testing
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”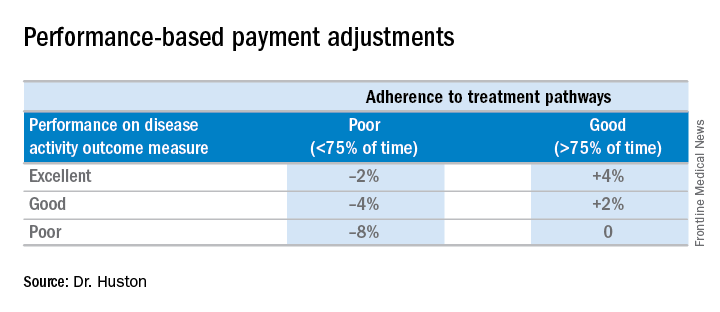
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
SAN DIEGO – for evaluation, in order to provide rheumatologists with another payment pathway under Medicare’s new Quality Payment Program.
The draft version of the rheumatoid arthritis advanced alternative payment model (APM), prepared by the American College of Rheumatology and unveiled at its annual meeting, aims to give rheumatologists a more focused opportunity to participate in value-based care and potentially earn greater incentive payments. The model is geared not only to private practice rheumatologists, but also to those in academia.
The Quality Payment Program, including its advanced APM track, was established by the Medicare and CHIP Reauthorization Act of 2015 (MACRA).
Other societies are working on or have submitted APMs for approval, Timothy Laing, MD, a member of the RA APM working group and also the ACR representative to the American Medical Association’s Relative Value Scale Update Committee and CPT Advisory Committee, said at the ACR meeting.
After presenting a draft of the RA APM at an AMA workshop in October, Dr. Laing came away encouraged by the attendees’ response to the usefulness and flexibility of the model to pay for services that rheumatologists are currently frustrated by in a fee-for-services system. “It’s a big if, but I think if we can make the money work, this will be a shift and it will have a lot of impact on how we practice, and I think it will be generalizable to more than one condition.”
The cochair of the RA APM working group, Kwas Huston, MD, presented the draft at the meeting. He noted that advanced APMs could be developed just for specific diseases, for all inflammatory arthritis, all types of vasculitis, or for all rheumatic diseases. RA was chosen first because the ACR is new to the development of APMs and “needed to start somewhere.”
“This model allows us to improve our ability to care for patients and is more sustainable over time for rheumatologists from a revenue standpoint,” Dr. Huston, a rheumatologist with Kansas City (Mo.) Physician Partners, said in an interview.
The RA APM helps to reduce barriers to good care by providing adequate reimbursement for cognitive services through monthly payments rather than relying on payment for separate office visits, Dr. Huston said. The model allows for more time spent in shared decision making, educational activities, and improving treatment adherence. It also builds in payment for non–face-to-face communication between rheumatologists, primary care physicians, and other specialists; interaction with patients via phone calls, email, and telemedicine; and using nurses or other staff to help with chronic disease management. It’s meant to be flexible for use in diverse settings by allowing comanagement of patients in rural areas and places where there is a shortage of rheumatologists or travel is difficult.
Why join an advanced APM?
Rheumatologists may want to join an advanced APM because of the potentially unsustainable, “zero-sum” nature of MACRA’s Merit-based Incentive Payment System (MIPS), in which the losers pay for the winners, said Angus Worthing, MD, chair of the ACR’s Government Affairs Committee and a member of the RA APM working group. In MIPS, there are expected over time to be fewer and fewer “losers” in the program, either because participants perform better or the losers drop out. In addition, advanced APM participants will receive a 5% bonus in 2019-2024 and Medicare payment updates will be higher for advanced APMs in 2026 and beyond than for MIPS (0.75% vs. 0.25%).
Beyond the financial practicalities of MACRA, it’s beneficial for rheumatologists to have their own advanced APM because it’s better than being stuck in one that’s “written by the government and doesn’t cater to other specialties; it’s specific to rheumatology, our patients, our work flow, and what we think is valuable,” said Dr. Worthing, a practicing rheumatologist in the Washington area.
To participate in an advanced APM, clinicians will need to have 25% of payments for Part B fall under professional services in the advanced APM or have 20% of their patients receive Part B professional services through the advanced APM. However, Dr. Worthing advised keeping an eye out for new thresholds for participating in the APM track because “it will probably be hard to get 25% of your Medicare reimbursements or 20% of your patients in the first year you’re in it.”
The RA APM’s treatment pathway
Undergirding the whole model is a treatment pathway that takes a standardized approach to RA care, based on ACR 2015 guidelines, and will be updated regularly by the ACR, Dr. Huston said. Following the guidelines gives an opportunity to lower spending but increase the percentage that’s going to rheumatologists by reducing the variability in initiation of expensive medications. “Currently, we get about 2.5 cents on the dollar for every dollar that’s spent on rheumatoid arthritis care, and we want to increase the part that’s going to the rheumatologist to provide more services but decrease total spending,” he said.
The pathway requires the use of methotrexate and/or another disease-modifying antirheumatic drug (DMARD) before targeted therapy. However, the model also allows for treating unique patients by requiring only 75% adherence to the guidelines. Deviation from the guidelines is allowed if a patient has a contraindication, intolerance, or inadequate response to a DMARD, or if there are barriers outside of the rheumatologist’s control, such as insurance coverage. The ACR guidelines also specify the frequency and type of monitoring that’s needed for treatment.
Because the ACR guidelines will be followed, the model asks that payers make patients eligible for lower out-of-pocket costs for medications. Following the guidelines should also reduce the need for prior authorizations. Providers in the advanced APM would attest to 75% adherence to the pathway, which would be subject to audit. “We want to reduce the reporting burden. In MIPS, it’s very complicated. It’s hard to know how to report all of this. In the APM pathway, we’re trying to simplify reporting. You only have to report two things; one of them is following the treatment pathway 75% of the time” and the other is an outcome measure, he said.
The payments made under this RA treatment pathway are divided into four areas: diagnosis and treatment planning, support for primary care physicians in diagnosing joint symptoms, the initial treatment of RA patients, and continued care for RA.
Diagnosis and treatment planning
This step offers a one-time payment to support all the costs of evaluation, testing, diagnosis, and treatment planning for a patient who has symptoms that potentially indicate RA, has not been previously treated or diagnosed with RA, or has been treated unsuccessfully for RA by other physicians. It is not dependent on the number of visits.
This phase also covers basic lab testing and imaging, which if not done by the rheumatology practice, would then have a standardized amount deducted from the payment and be paid separately. Lab tests and imaging performed for other conditions would be paid separately as well.
The payment covers communication with other physicians, spending more time with patients in a shared decision-making process regarding treatment options, and developing a RA treatment plan.
“If you don’t end up diagnosing RA, you still get the payment. But there will be two different payments; one is a little lower if they don’t have RA. If they do have RA, then you spend more time with them developing this treatment plan, so that would be a higher payment,” Dr. Huston said.
Support for primary care physicians in diagnosing joint symptoms
This payment goes to a rheumatologist or a nurse practitioner or physician assistant who is working under the supervision of a rheumatologist for a patient who is under the care of a primary care physician who has an agreement to work collaboratively with the rheumatology practice. The payment, which is limited to one bill for one patient in a 1-year period, is for communication between the rheumatologist and the primary care physician about patients with symptoms that might indicate RA to determine the need for referral.
“This communication could be a phone call, an email, face-to-face, or some other form of communication ... to discuss how fast the patient may need to be seen or if there are other tests that need to be done before expediting referrals for patients who are higher risk,” Dr. Huston explained.
The payment would still be made if the patient does not require referral to a rheumatologist.
Initial treatment of RA patients
Payment for initial treatment can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or a team comprising the rheumatologist and a primary care physician who have a formal arrangement to support the early treatment of RA.
The latter scenario is intended for rural areas and other areas where there is a shortage of rheumatologists. The formal arrangement would specify how payments are shared and who is responsible for each of the accountability requirements and for treatment pathway, “but there is a lot of flexibility, and this can vary quite a bit, so what happens in rural Alaska where the primary care doctors might be more involved is not going to be the same as in a big city where the primary care doctors may not want to be involved at all. So there is no requirement for primary care doctors to be involved, but it just provides the resources in areas where that might make sense,” Dr. Huston noted.
The initial treatment payment would be made monthly for 6 months, replacing evaluation and management billing for office visits related to RA. It pays for typical lab tests and imaging and allows flexibility for non–face-to-face communications, and enhanced services to patients who need them. This payment is also stratified to adjust for sicker patients who have more comorbidities, he said.
Continued care for RA
This component of the payment structure also can be made to a rheumatologist, a nurse practitioner or physician assistant under the supervision of a rheumatologist, or the rheumatologist–primary care physician team. Continued care payments are made monthly and, just as with initial treatment payments, they are meant to replace E&M billing for office visits and pay for the same kinds of resources used in initial treatment, including stratified payments to adjust for patient characteristics.
Patients who come to a rheumatologist with established RA would enter this treatment pathway under this kind of payment.
RA APM’s accountability requirements
Participants in this model would be required to see a patient face-to-face at least every 6 months and to document their disease activity using a validated scale approved by the ACR for use in the RA APM, such as the RAPID-3 (Routine Assessment of Patient Index Data–3), the CDAI (Clinical Disease Activity Index), the SDAI (Simple Disease Activity Index), or the DAS28 (28-joint Disease Activity Score). Payment would also require keeping a written treatment plan that’s consistent with the ACR’s approved treatment pathway.
Changes in medication require communication with the patient within 2 weeks to help improve treatment adherence. Quality measures will still need to be recorded, such as a functional assessment, tuberculosis screening prior to starting biologics, and having a plan for steroid use, but they are not required to be reported. “You attest to that,” he said.
However, participants will need to report that they are following the treatment pathway for patients and an outcome measure for continued care of RA. These are necessary, Dr. Huston said, because “we are asking that we increase the money that’s going to the rheumatologist for managing patients with RA, so we have to show that we’re accountable and doing good with that money and that we’re taking care of our patients. ... and if we have an outcome measure, then we don’t have to report all those process measures that we do in MIPS.”
The outcome measure would be reporting:
• At least (some %) of patients with low disease activity remained in low disease activity.
• At least (some %) of patients with moderate disease activity stayed in the same or a lower disease activity category.
• At least (some %) of patients with a high disease activity had a lower disease activity category.
It’s unknown yet what the threshold percentage for each disease activity level would be, but it will be obtained from the ACR’s Rheumatology Informatics System for Effectiveness (RISE) Registry and will be refined over time from there. The outcome measure is not validated yet – none exists for RA for use in clinical practice – because it’s not yet known how to risk stratify patients in these disease activity levels for their comorbidities and socioeconomic factors. “Those are all things we need to learn over time,” Dr. Huston said, “but this is our good-faith effort at developing an outcome measure which we think will become more robust as we gather more and more data.”
Performing all of these requirements would likely require more staff, and so the model will be built to account for these higher costs, Dr. Huston said.
Advantages of the RA APM
The RA APM’s advantages, according to Dr. Huston, stem from its payment for high-value services; the avoidance of the penalties and reporting burdens imposed by MIPS; a reduction in documentation requirements, allowing clinicians to take notes on history of present illness and review of systems however they want; a reduction in prior authorizations; and more control over performance measures.
Another big advantage of the RA APM is that participants “are not responsible for the price of drugs, whereas in MIPS you are responsible. When [the Centers for Medicare & Medicaid Services] calculates your cost category [in MIPS], that includes Part B drugs, and when the MIPS adjustment factor is applied to your revenue, that includes revenue from Part B drugs,” Dr. Huston said.
In addition, he noted that it will be possible for a rheumatologist to be a participant in just one or two APMs and still have the benefit of being out of MIPS.
Next steps
The next steps for the development of the RA APM include refining the treatment pathway, analyzing RISE data outcome thresholds, and modeling the financial impact on practices by running data from three to five practices across the country through the model to determine what the payment levels should be. Once those steps are completed, the RA APM can be submitted to the Physician-Focused Payment Model Technical Advisory Committee for approval, which will then send it to the CMS to run it through its Innovation Center to test the model in several practices to gather more data and refine the payment rates until it is ready to be expanded and implemented.
Dr. Huston, Dr. Worthing, and Dr. Laing had no relevant conflicts of interest to disclose.
AT ACR 2017
Injectable agent found to improve knee function in OA patients
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
SAN DIEGO – Among patients with mild to moderate patellofemoral osteoarthritis, intra-articular administration of the novel agent TPX-100 was safe and associated with functional benefits up to 1 year, a proof-of-concept study showed.
“We don’t yet have a disease-modifying drug for osteoarthritis [OA]; that’s sort of the holy grail for researchers,” lead study author Dawn McGuire, MD, said in an interview at the annual meeting of the American College of Rheumatology. “All of the patient-reported outcome and patient function indices that we studied moved in the same direction, showing a benefit of TPX-100. I think this is very promising.”
The study consisted of two parts. In Part A, four dose cohorts ranging from 20 mg to 200 mg per injection were enrolled. There were no dose-limiting toxicities or safety concerns at any dose, and the 200-mg dose was selected dose for Part B of the study.
The median age of the 118 patients was 60 years and their median body mass index was 29.2 kg/m2. No drug-related serious adverse events and no dose-limiting toxicities occurred across doses ranging from 20 mg to 200 mg per injection. The incidence of common adverse events such as knee pain was similar between placebo- and TPX-100-treated knees.
Quantitative MRI showed no measurable between-knee differences in cartilage thickness or volume at 6 or 12 months. However, statistically significant and clinically meaningful differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, including KOOS activities of daily living (P = .008 at 6 and 12 months), KOOS knee-related quality of life (P = .21 at 6 months and P = .03 at 12 months), and a significant reduction in pain going up or down stairs (P = .004 at 12 months). Subjects’ use of nonsteroidal anti-inflammatory medications declined by 63% during the study.
“In this study, we see some terrific, long-term results in knee-related core activities, which certainly were disease modifying from the patients’ perspective,” said Dr. McGuire, who noted that a phase 3 study is being planned. “In addition, patient’s pain going up and down stairs improved significantly, with a marked reduction in analgesic use. Any of us who are experienced in these tough areas of medicine know that early results can look extremely promising, but we have to do larger confirmatory studies.”
AT ACR 2017
Key clinical point:
Major finding: Statistically significant differences in knee function were observed in favor of TPX-100-treated knees, compared with controls, using Knee Injury and Osteoarthritis Outcome Score activities of daily living (P = .008 at 6 and 12 months) and a significant reduction in pain going up or down stairs (P = .004 at 12 months).
Study details: A randomized, proof-of-concept study involving 118 patients with patellofemoral knee OA.
Disclosures: OrthoTrophix sponsored the study. Dr. McGuire is chief medical officer and a cofounder of the company.






















