User login
Weakness on one side of the body
FHM is a rare phenotype of migraine with aura with a characteristic presentation of motor aura. Motor aura presents as unilateral muscle weakness that tends to be felt first in the hands or arm and may spread to the face. To date, three distinct types have been identified by mutations in one of three genes. Type 1 is the most common and is associated with mutations in the gene CACNA1A. Mutations in ATP1A2 underlie type 2 FHM, and mutations in SCN1A underlie type 3 FHM.
FHM is distinguished from other hemiplegic migraine by family history of one or more affected first- or second-degree relatives. Genetic studies have shown FHM to have autosomal dominant inheritance. From half to three quarters of patients with FHM will have one of the more than 30 identified mutations on CACNA1A that diagnose type 1 FHM. These mutations affect transmission of glutamate in the neurons and neuronal reactions, increasing the susceptibility to cortical spreading depression associated with migraine. Mutations in ATP1A2 are found in about 20% of patients with FHM (type 2). More than 80 individual mutations have been identified, which alter sodium-potassium metabolism in neurons. About 5% of patients have type 3 FHM, associated with mutations in SCN1A that create gain of function or loss of function in neuronal voltage-gated sodium channels. Studies of other possible genes and mutations in relation to FHM, including PRRT2, are ongoing, but to date the associations are not clearly established.
Patients with FHM may also report sensory symptoms, visual disturbances, or aphasia. FHM generally affects people in their teens and twenties (women more than men) and has an estimated prevalence of 0.003% of the population. On average, patients report having two to three attacks per year, and some patients go for extended periods without a recurrent attack. Motor aura may occur on the same or opposite side of the body as headache and may alternate affected sides with each attack. Differential diagnoses that should be ruled out include transient ischemic attacks, infections (eg, meningitis, encephalitis), tumors, seizures, other inherited disorders, and metabolic issues.
Like other forms of migraine with aura, FHM is treated with abortive and/or preventive medications. Given the rarity of FHM, there are few studies specifically in families with this phenotype. Patients should be counseled on trigger avoidance to limit exposure. Acute treatment includes nonsteroidal anti-inflammatory drugs, acetaminophen, and other nonopioid pain relievers. The class of calcitonin gene-related peptide (CGRP) antagonists (rimegepant, ubrogepant, zavegepant) may be considered. However, with FHM, medications associated with ischemia must be avoided. As such, triptans and ergotamines are generally contraindicated, as are beta-blockers. Patients with FHM and more frequent or severe attacks may be considered for preventive treatment to improve function and quality of life and avoid reliance on acute therapies. Options include CGRP monoclonal antibodies (mAbs), administered subcutaneously or by intravenous infusion, and onabotulinumtoxinA injection. Current CGRP mAbs include eptinezumab, erenumab, fremanezumab, and galcanezumab. Combined CGRP mAb therapy with onabotulinumtoxinA may be an effective alternative for patients with resistant FHM.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
FHM is a rare phenotype of migraine with aura with a characteristic presentation of motor aura. Motor aura presents as unilateral muscle weakness that tends to be felt first in the hands or arm and may spread to the face. To date, three distinct types have been identified by mutations in one of three genes. Type 1 is the most common and is associated with mutations in the gene CACNA1A. Mutations in ATP1A2 underlie type 2 FHM, and mutations in SCN1A underlie type 3 FHM.
FHM is distinguished from other hemiplegic migraine by family history of one or more affected first- or second-degree relatives. Genetic studies have shown FHM to have autosomal dominant inheritance. From half to three quarters of patients with FHM will have one of the more than 30 identified mutations on CACNA1A that diagnose type 1 FHM. These mutations affect transmission of glutamate in the neurons and neuronal reactions, increasing the susceptibility to cortical spreading depression associated with migraine. Mutations in ATP1A2 are found in about 20% of patients with FHM (type 2). More than 80 individual mutations have been identified, which alter sodium-potassium metabolism in neurons. About 5% of patients have type 3 FHM, associated with mutations in SCN1A that create gain of function or loss of function in neuronal voltage-gated sodium channels. Studies of other possible genes and mutations in relation to FHM, including PRRT2, are ongoing, but to date the associations are not clearly established.
Patients with FHM may also report sensory symptoms, visual disturbances, or aphasia. FHM generally affects people in their teens and twenties (women more than men) and has an estimated prevalence of 0.003% of the population. On average, patients report having two to three attacks per year, and some patients go for extended periods without a recurrent attack. Motor aura may occur on the same or opposite side of the body as headache and may alternate affected sides with each attack. Differential diagnoses that should be ruled out include transient ischemic attacks, infections (eg, meningitis, encephalitis), tumors, seizures, other inherited disorders, and metabolic issues.
Like other forms of migraine with aura, FHM is treated with abortive and/or preventive medications. Given the rarity of FHM, there are few studies specifically in families with this phenotype. Patients should be counseled on trigger avoidance to limit exposure. Acute treatment includes nonsteroidal anti-inflammatory drugs, acetaminophen, and other nonopioid pain relievers. The class of calcitonin gene-related peptide (CGRP) antagonists (rimegepant, ubrogepant, zavegepant) may be considered. However, with FHM, medications associated with ischemia must be avoided. As such, triptans and ergotamines are generally contraindicated, as are beta-blockers. Patients with FHM and more frequent or severe attacks may be considered for preventive treatment to improve function and quality of life and avoid reliance on acute therapies. Options include CGRP monoclonal antibodies (mAbs), administered subcutaneously or by intravenous infusion, and onabotulinumtoxinA injection. Current CGRP mAbs include eptinezumab, erenumab, fremanezumab, and galcanezumab. Combined CGRP mAb therapy with onabotulinumtoxinA may be an effective alternative for patients with resistant FHM.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
FHM is a rare phenotype of migraine with aura with a characteristic presentation of motor aura. Motor aura presents as unilateral muscle weakness that tends to be felt first in the hands or arm and may spread to the face. To date, three distinct types have been identified by mutations in one of three genes. Type 1 is the most common and is associated with mutations in the gene CACNA1A. Mutations in ATP1A2 underlie type 2 FHM, and mutations in SCN1A underlie type 3 FHM.
FHM is distinguished from other hemiplegic migraine by family history of one or more affected first- or second-degree relatives. Genetic studies have shown FHM to have autosomal dominant inheritance. From half to three quarters of patients with FHM will have one of the more than 30 identified mutations on CACNA1A that diagnose type 1 FHM. These mutations affect transmission of glutamate in the neurons and neuronal reactions, increasing the susceptibility to cortical spreading depression associated with migraine. Mutations in ATP1A2 are found in about 20% of patients with FHM (type 2). More than 80 individual mutations have been identified, which alter sodium-potassium metabolism in neurons. About 5% of patients have type 3 FHM, associated with mutations in SCN1A that create gain of function or loss of function in neuronal voltage-gated sodium channels. Studies of other possible genes and mutations in relation to FHM, including PRRT2, are ongoing, but to date the associations are not clearly established.
Patients with FHM may also report sensory symptoms, visual disturbances, or aphasia. FHM generally affects people in their teens and twenties (women more than men) and has an estimated prevalence of 0.003% of the population. On average, patients report having two to three attacks per year, and some patients go for extended periods without a recurrent attack. Motor aura may occur on the same or opposite side of the body as headache and may alternate affected sides with each attack. Differential diagnoses that should be ruled out include transient ischemic attacks, infections (eg, meningitis, encephalitis), tumors, seizures, other inherited disorders, and metabolic issues.
Like other forms of migraine with aura, FHM is treated with abortive and/or preventive medications. Given the rarity of FHM, there are few studies specifically in families with this phenotype. Patients should be counseled on trigger avoidance to limit exposure. Acute treatment includes nonsteroidal anti-inflammatory drugs, acetaminophen, and other nonopioid pain relievers. The class of calcitonin gene-related peptide (CGRP) antagonists (rimegepant, ubrogepant, zavegepant) may be considered. However, with FHM, medications associated with ischemia must be avoided. As such, triptans and ergotamines are generally contraindicated, as are beta-blockers. Patients with FHM and more frequent or severe attacks may be considered for preventive treatment to improve function and quality of life and avoid reliance on acute therapies. Options include CGRP monoclonal antibodies (mAbs), administered subcutaneously or by intravenous infusion, and onabotulinumtoxinA injection. Current CGRP mAbs include eptinezumab, erenumab, fremanezumab, and galcanezumab. Combined CGRP mAb therapy with onabotulinumtoxinA may be an effective alternative for patients with resistant FHM.
Heidi Moawad, MD, Clinical Assistant Professor, Department of Medical Education, Case Western Reserve University School of Medicine, Cleveland, Ohio.
Heidi Moawad, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
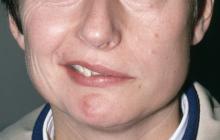
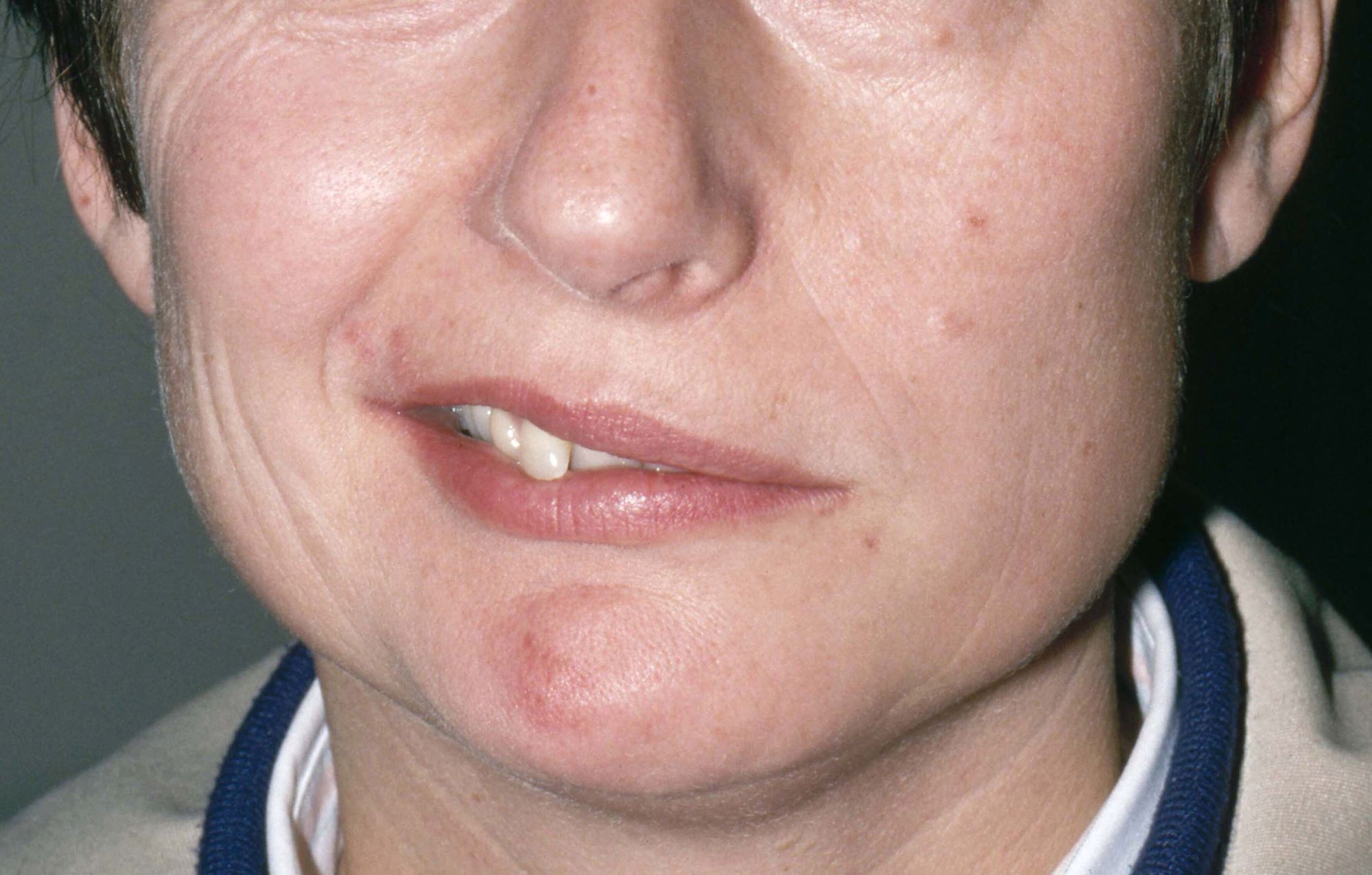
The patient is 35-year-old woman presenting for recurrent, unilateral headaches associated with weakness in the hand, arm, or face on one side of the body. The patient says this weakness sometimes occurs on the right side and other times on the left, often with a tingling sensation in the affected side, and is followed by an intense headache lasting for several hours.
She notes that the headaches started after recovery from a mild case of COVID. Over the past 2 years, five attacks have occurred, all following a similar pattern. With each attack, the motor weakness fully resolved with resolution of the headache. Two of the headaches were preceded by visual disturbances that resolved with headache onset.
Physical exam reveals an apparently healthy woman without fever or respiratory symptoms. Weight, blood pressure, and heart rate are within healthy ranges. All lab work is within normal ranges. Her facial appearance is normal at presentation, but she shows a photo taken during her last attack, in which she shows left side facial paralysis. Family history includes her mother with hemiplegic migraine and father with type 2 diabetes. You suspect familial hemiplegic migraine (FHM) and order genetic testing.
Announcing Our 2024 AGA Council Chair and Section Leaders
Meet Our New Chair
Douglas J. Robertson, MD, MPH, AGAFAGA Institute Council Chair
VA Medical Center, White River Junction, Vermont
Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
Dr. Robertson will serve as council chair for 3 years (May 2024-May 2027; DDW 2025, 2026 and 2027).
Section Leadership
, the driving force behind AGA’s programming at Digestive Disease Week (DDW). We welcome 8 members into their new roles as section vice chairs, joining the existing 17 Council members. Each new vice chair will serve a 2-year term that began immediately following this year’s DDW meeting and extends through DDW 2026. Following their term as vice chair, they will move into the role of section chair for an additional 2 years through DDW 2028. 
We are also pleased to announce the members joining nominating committees during the 2026 nomination/election cycle. The chairs of the nominating committee will be the immediate past section chairs, whom we also recognize and thank for their service and dedication to the section and the council.
Basic & Clinical Intestinal Disorders (BCID)
Uma Sundaram, MDVice chair
Marshall University School of Medicine, Huntington, West Virginia
Nominating committee members
- Colleen Renee Kelly, MD, AGAF, Chair
- Amy C. Engevik, PhD, Medical University of South Carolina
- Ravinder Gill, PhD, University of Illinois at Chicago
- Madhusudan Grover, MD, Mayo Clinic, Rochester, Minnesota
- Lisa L. Strate, MD, Harborview Medical Center, Seattle
Clinical Practice (CP)
Linda Anh Nguyen, MDVice Chair
Stanford (Calif.) University School of Medicine
Nominating committee members
- Gary W. Falk, MD, MS, AGAF, Chair
- Megan Adams, MD, JD, MSc, VA Ann Arbor Healthcare System Endoscopy Unit
- Mohammad Bilal, MD, Minneapolis VA Health Care System
- Carolyn Newberry, MD, Weill Cornell Medical Center, New York
- Adam Weizman, MD, MSc, Mount Sinai Hospital, Toronto
Endoscopy, Technology & Imaging (ETI)
Vivek Kaul, MD, AGAFVice Chair
University of Rochester (N.Y.) Medical Center
Nominating committee members
- Irving Waxman, MD, Chair
- Sushovan Guha, MD, PhD, University of Texas at Houston
- Pichamol Jirapinyo, MD, MPH, Brigham and Women’s Hospital, Boston
- Vladimir Kushnir, MD, Washington University St. Louis Barnes–Jewish West County Hospital
- Andrew C. Storm, MD, Mayo Clinic, Rochester, Minnesota
Immunology, Microbiology & Inflammatory Bowel Diseases (IMIBD)
Florian Rieder, MDVice Chair
Cleveland Clinic Foundation
Nominating committee members
- Fernando S. Velayos, MD, AGAF, Chair
- Brigid S. Boland, MD, University of California, San Diego
- Karen L. Edelblum, PhD, Icahn School of Medicine at Mount Sinai, New York
- Michael Kattah, MD, PhD, UCSF Gastroenterology
- Andres J. Yarur, MD, Cedars Sinai Medical Center. Los Angeles
Liver & Biliary (LB)
Don Rockey, MDVice Chair
Medical University of South Carolina, Charleston
Nominating committee members
- Gyongyi Szabo, MD, PhD, AGAF, Chair
- Brett Fortune, MD, MSc, Montefiore Medical Center
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine, Houston
- Cynthia Ann Moylan, MD, MHS, MS, Duke University, Durham, North Carolina
- Douglas A. Simonetto, MD, Mayo Clinic, Rochester, Minnesota
Microbiome & Microbial Therapy (MMT)
Jessica Allegretti, MD, MPHVice Chair
Brigham and Women’s Hospital, Boston
Nominating committee members
- Purna C. Kashyap, MBBS, AGAF, Chair
- Melinda Engevik, PhD, Medical University of South Carolina
- Christian Jobin, PhD, University of Florida
- Vanessa Leone, PhD, The University of Wisconsin–Madison
- Jun Yu, MD, PhD, The Chinese University of Hong Kong
Obesity, Metabolism & Nutrition (OMN)
Berkeley M. Limketkai, MD, PhDVice Chair
University of California Los Angeles
Nominating committee members
- Andres Jose Acosta, MD, PhD, Chair
- Barham K. Abu Dayyeh, MD, MPH, Mayo Clinic, Rochester, Minnesota
- Alan L. Buchman, MD, MSPH, University of Illinois at Chicago
- Octavia Pickett-Blakely, MD, MHS, Hospital of the University of Pennsylvania
- Robert Shulman, MD, Texas Children’s Hospital, Baylor College of Medicine
Pediatric Gastroenterology & Developmental Biology (PGDB)
Kelli L. VanDussen, PhDVice Chair
Cincinnati Children’s Hospital Medical Center
Meet Our New Chair
Douglas J. Robertson, MD, MPH, AGAFAGA Institute Council Chair
VA Medical Center, White River Junction, Vermont
Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
Dr. Robertson will serve as council chair for 3 years (May 2024-May 2027; DDW 2025, 2026 and 2027).
Section Leadership
, the driving force behind AGA’s programming at Digestive Disease Week (DDW). We welcome 8 members into their new roles as section vice chairs, joining the existing 17 Council members. Each new vice chair will serve a 2-year term that began immediately following this year’s DDW meeting and extends through DDW 2026. Following their term as vice chair, they will move into the role of section chair for an additional 2 years through DDW 2028. 
We are also pleased to announce the members joining nominating committees during the 2026 nomination/election cycle. The chairs of the nominating committee will be the immediate past section chairs, whom we also recognize and thank for their service and dedication to the section and the council.
Basic & Clinical Intestinal Disorders (BCID)
Uma Sundaram, MDVice chair
Marshall University School of Medicine, Huntington, West Virginia
Nominating committee members
- Colleen Renee Kelly, MD, AGAF, Chair
- Amy C. Engevik, PhD, Medical University of South Carolina
- Ravinder Gill, PhD, University of Illinois at Chicago
- Madhusudan Grover, MD, Mayo Clinic, Rochester, Minnesota
- Lisa L. Strate, MD, Harborview Medical Center, Seattle
Clinical Practice (CP)
Linda Anh Nguyen, MDVice Chair
Stanford (Calif.) University School of Medicine
Nominating committee members
- Gary W. Falk, MD, MS, AGAF, Chair
- Megan Adams, MD, JD, MSc, VA Ann Arbor Healthcare System Endoscopy Unit
- Mohammad Bilal, MD, Minneapolis VA Health Care System
- Carolyn Newberry, MD, Weill Cornell Medical Center, New York
- Adam Weizman, MD, MSc, Mount Sinai Hospital, Toronto
Endoscopy, Technology & Imaging (ETI)
Vivek Kaul, MD, AGAFVice Chair
University of Rochester (N.Y.) Medical Center
Nominating committee members
- Irving Waxman, MD, Chair
- Sushovan Guha, MD, PhD, University of Texas at Houston
- Pichamol Jirapinyo, MD, MPH, Brigham and Women’s Hospital, Boston
- Vladimir Kushnir, MD, Washington University St. Louis Barnes–Jewish West County Hospital
- Andrew C. Storm, MD, Mayo Clinic, Rochester, Minnesota
Immunology, Microbiology & Inflammatory Bowel Diseases (IMIBD)
Florian Rieder, MDVice Chair
Cleveland Clinic Foundation
Nominating committee members
- Fernando S. Velayos, MD, AGAF, Chair
- Brigid S. Boland, MD, University of California, San Diego
- Karen L. Edelblum, PhD, Icahn School of Medicine at Mount Sinai, New York
- Michael Kattah, MD, PhD, UCSF Gastroenterology
- Andres J. Yarur, MD, Cedars Sinai Medical Center. Los Angeles
Liver & Biliary (LB)
Don Rockey, MDVice Chair
Medical University of South Carolina, Charleston
Nominating committee members
- Gyongyi Szabo, MD, PhD, AGAF, Chair
- Brett Fortune, MD, MSc, Montefiore Medical Center
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine, Houston
- Cynthia Ann Moylan, MD, MHS, MS, Duke University, Durham, North Carolina
- Douglas A. Simonetto, MD, Mayo Clinic, Rochester, Minnesota
Microbiome & Microbial Therapy (MMT)
Jessica Allegretti, MD, MPHVice Chair
Brigham and Women’s Hospital, Boston
Nominating committee members
- Purna C. Kashyap, MBBS, AGAF, Chair
- Melinda Engevik, PhD, Medical University of South Carolina
- Christian Jobin, PhD, University of Florida
- Vanessa Leone, PhD, The University of Wisconsin–Madison
- Jun Yu, MD, PhD, The Chinese University of Hong Kong
Obesity, Metabolism & Nutrition (OMN)
Berkeley M. Limketkai, MD, PhDVice Chair
University of California Los Angeles
Nominating committee members
- Andres Jose Acosta, MD, PhD, Chair
- Barham K. Abu Dayyeh, MD, MPH, Mayo Clinic, Rochester, Minnesota
- Alan L. Buchman, MD, MSPH, University of Illinois at Chicago
- Octavia Pickett-Blakely, MD, MHS, Hospital of the University of Pennsylvania
- Robert Shulman, MD, Texas Children’s Hospital, Baylor College of Medicine
Pediatric Gastroenterology & Developmental Biology (PGDB)
Kelli L. VanDussen, PhDVice Chair
Cincinnati Children’s Hospital Medical Center
Meet Our New Chair
Douglas J. Robertson, MD, MPH, AGAFAGA Institute Council Chair
VA Medical Center, White River Junction, Vermont
Geisel School of Medicine at Dartmouth, Hanover, New Hampshire
Dr. Robertson will serve as council chair for 3 years (May 2024-May 2027; DDW 2025, 2026 and 2027).
Section Leadership
, the driving force behind AGA’s programming at Digestive Disease Week (DDW). We welcome 8 members into their new roles as section vice chairs, joining the existing 17 Council members. Each new vice chair will serve a 2-year term that began immediately following this year’s DDW meeting and extends through DDW 2026. Following their term as vice chair, they will move into the role of section chair for an additional 2 years through DDW 2028. 
We are also pleased to announce the members joining nominating committees during the 2026 nomination/election cycle. The chairs of the nominating committee will be the immediate past section chairs, whom we also recognize and thank for their service and dedication to the section and the council.
Basic & Clinical Intestinal Disorders (BCID)
Uma Sundaram, MDVice chair
Marshall University School of Medicine, Huntington, West Virginia
Nominating committee members
- Colleen Renee Kelly, MD, AGAF, Chair
- Amy C. Engevik, PhD, Medical University of South Carolina
- Ravinder Gill, PhD, University of Illinois at Chicago
- Madhusudan Grover, MD, Mayo Clinic, Rochester, Minnesota
- Lisa L. Strate, MD, Harborview Medical Center, Seattle
Clinical Practice (CP)
Linda Anh Nguyen, MDVice Chair
Stanford (Calif.) University School of Medicine
Nominating committee members
- Gary W. Falk, MD, MS, AGAF, Chair
- Megan Adams, MD, JD, MSc, VA Ann Arbor Healthcare System Endoscopy Unit
- Mohammad Bilal, MD, Minneapolis VA Health Care System
- Carolyn Newberry, MD, Weill Cornell Medical Center, New York
- Adam Weizman, MD, MSc, Mount Sinai Hospital, Toronto
Endoscopy, Technology & Imaging (ETI)
Vivek Kaul, MD, AGAFVice Chair
University of Rochester (N.Y.) Medical Center
Nominating committee members
- Irving Waxman, MD, Chair
- Sushovan Guha, MD, PhD, University of Texas at Houston
- Pichamol Jirapinyo, MD, MPH, Brigham and Women’s Hospital, Boston
- Vladimir Kushnir, MD, Washington University St. Louis Barnes–Jewish West County Hospital
- Andrew C. Storm, MD, Mayo Clinic, Rochester, Minnesota
Immunology, Microbiology & Inflammatory Bowel Diseases (IMIBD)
Florian Rieder, MDVice Chair
Cleveland Clinic Foundation
Nominating committee members
- Fernando S. Velayos, MD, AGAF, Chair
- Brigid S. Boland, MD, University of California, San Diego
- Karen L. Edelblum, PhD, Icahn School of Medicine at Mount Sinai, New York
- Michael Kattah, MD, PhD, UCSF Gastroenterology
- Andres J. Yarur, MD, Cedars Sinai Medical Center. Los Angeles
Liver & Biliary (LB)
Don Rockey, MDVice Chair
Medical University of South Carolina, Charleston
Nominating committee members
- Gyongyi Szabo, MD, PhD, AGAF, Chair
- Brett Fortune, MD, MSc, Montefiore Medical Center
- Ruben Hernaez, MD, MPH, PhD, Baylor College of Medicine, Houston
- Cynthia Ann Moylan, MD, MHS, MS, Duke University, Durham, North Carolina
- Douglas A. Simonetto, MD, Mayo Clinic, Rochester, Minnesota
Microbiome & Microbial Therapy (MMT)
Jessica Allegretti, MD, MPHVice Chair
Brigham and Women’s Hospital, Boston
Nominating committee members
- Purna C. Kashyap, MBBS, AGAF, Chair
- Melinda Engevik, PhD, Medical University of South Carolina
- Christian Jobin, PhD, University of Florida
- Vanessa Leone, PhD, The University of Wisconsin–Madison
- Jun Yu, MD, PhD, The Chinese University of Hong Kong
Obesity, Metabolism & Nutrition (OMN)
Berkeley M. Limketkai, MD, PhDVice Chair
University of California Los Angeles
Nominating committee members
- Andres Jose Acosta, MD, PhD, Chair
- Barham K. Abu Dayyeh, MD, MPH, Mayo Clinic, Rochester, Minnesota
- Alan L. Buchman, MD, MSPH, University of Illinois at Chicago
- Octavia Pickett-Blakely, MD, MHS, Hospital of the University of Pennsylvania
- Robert Shulman, MD, Texas Children’s Hospital, Baylor College of Medicine
Pediatric Gastroenterology & Developmental Biology (PGDB)
Kelli L. VanDussen, PhDVice Chair
Cincinnati Children’s Hospital Medical Center
Dupilumab Safe, Effective Over 5 Years in Moderate to Severe Atopic Dermatitis
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
Over 5 years, dupilumab demonstrated acceptable safety and sustained efficacy, with significant improvements in the signs and symptoms of AD, in the treatment of moderate to severe atopic dermatitis (AD).
METHODOLOGY:
- The phase 3 multinational LIBERTY AD open-label extension study evaluated the safety and efficacy of dupilumab in 2677 adults with moderate to severe AD who had previously participated in dupilumab trials over 5 years; 334 patients (12.5%) completed treatment up to 5 years.
- Patients started with subcutaneous dupilumab, initially dosed weekly after a loading dose, then every 2 weeks in 2019.
- The primary outcomes were the incidence and rate of treatment-emergent adverse events (TEAEs).
TAKEAWAY:
- Overall, 14,717 TEAEs were reported over 5 years. The exposure-adjusted incidence rate decreased over time and was 252.48 events per 100 patient-years.
- The most common TEAEs were nasopharyngitis (28.9%), worsening AD (16.7%), upper respiratory tract infection (13.6%), conjunctivitis (10.3%), allergic conjunctivitis (9%), headache (8.1%), oral herpes (7.5%), and injection-site reactions (5.2%).
- Serious and severe TEAE rates were 10.6% and 10.0%, respectively. Exposure-adjusted incidence rates were 6.66 and 6.71 events per 100 patient-years, respectively.
- At week 260, 67.5% of patients had achieved clear or almost clear skin according to the Investigator’s Global Assessment, and 88.9% experienced a 75% or greater improvement in the Eczema Area and Severity Index.
IN PRACTICE:
“Safety and efficacy results from up to 5 years of dupilumab treatment in the LIBERTY AD open-label extension study support dupilumab as a continuous long-term treatment for adults with moderate to severe AD,” the authors concluded.
SOURCE:
The study was led by Lisa A. Beck, MD, University of Rochester, Rochester, New York, and was published online in JAMA Dermatology.
LIMITATIONS:
Study limitations included the absence of a placebo arm and treatment interruptions stemming from protocol changes. The number of patients who received biweekly doses was small. The early conclusion of the trial by the sponsor because of regulatory approval also resulted in a lower number of patients at later stages.
DISCLOSURES:
This study was funded by dupilumab manufacturers Sanofi and Regeneron Pharmaceuticals. Several authors declared ties with various pharmaceutical companies including Sanofi and Regeneron, and several authors were employees of Sanofi or Regeneron. No disclosures were reported by other authors.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
Eosinophilic Esophagitis Often Persists Despite Treatment
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
In a large, retrospective, real-world cohort study, investigators examined the patient journey in 613 child, adolescent, and adult patients with eosinophilic esophagitis (EoE) via healthcare claims database and electronic medical record data. As we enter into an exciting era in novel biologic therapies in EoE, the article provides comprehensive and reliable information in several critical and actionable areas with respect to EoE diagnosis and management.
The study highlights the heterogeneity of the patient experience in EoE and suggests that improvements in the reliability and precision of EoE care models will impact healthcare utilization. In particular, the findings support the need for structured and systematic mechanisms for appropriate follow-up after the index diagnosis and increased use and continued development of novel therapies.
Anand Jain, MD, is assistant professor in the Division of Digestive Diseases at Emory University School of Medicine, Atlanta, Georgia. Ravinder Mittal, MD, AGAF, is professor in the Division of Gastroenterology at the University of California, San Diego, and staff physician at the San Diego VA Hospital. They report no conflicts of interest.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
based on a recent retrospective study.
Challenging patient journeys were common across age groups, with a range of ongoing symptoms and histological abnormalities supporting high unmet need among patients with EoE, lead author Olulade Ayodele, MBBS, MPH, of Takeda Development Center Americas and colleagues reported.
“Recent studies have found that patients with EoE experience a complicated journey to diagnosis and a substantial disease burden, which requires significant healthcare resource utilization,” the investigators wrote in Gastro Hep Advances . “Reasons for this may include delays in diagnosis owing to nonspecific symptoms, adaptive behaviors, progression of silent disease, lack of adequate follow-up or referral, or suboptimal treatment after diagnosis.”Two medications are currently Food and Drug administration approved for EoE: dupilumab, a biologic for patients aged 1 year and older, and budesonide oral suspension, a topical corticosteroid for patients aged 11 years and older.
The investigators noted that “biologic therapies may not always be selected as first-line treatment, and are often associated with high costs”; however, the effects of real-world treatment decisions like these are poorly documented, prompting the present study.
The final dataset comprised 613 patients with newly diagnosed EoE treated in a rural integrated healthcare system, all of whom had at least 12 months of data before and after a predetermined index date. Individuals were stratified by age, including 182 children, 146 adolescents, 244 adults, and 41 older adults.
Signs and symptoms of EoE frequently worsened after the index date, including dysphagia (34.6% before, 49.9% after), abdominal pain (33.0% before, 48.1% after), and nausea/vomiting (20.1% before, 31.5% after).
At baseline, 80.5% of endoscopies were abnormal and 87.9% of patients had more than 15 eosinophils/high-power field. These parameters improved post index; however, 3 years later, 62.3% of patients still had abnormal endoscopic appearance and 51.2% had abnormal histologic activity.
Before and after index, the most prescribed treatments were corticosteroids (47.3% before, 87.9% after) and proton pump inhibitors (51.1% before, 96.1% after).
After index, 44.0% of patients discontinued their first-line treatment, and 13.9% experienced disease progression.
“We found that a substantial portion of patients with EoE received variable medical treatments, and did not report undergoing follow-up care, consulting with specialists, or routinely undergoing endoscopy with biopsy after diagnosis; the reasons for this are unknown, but experiences do not appear to be consistent with current guideline recommendations,” Dr. Ayodele and colleagues wrote.
They also noted substantial healthcare resource utilization; more than half of the patients visited emergency departments, and nearly one in five were admitted as inpatients.
“Our findings outline the persistent disease activity and difficult therapeutic journeys faced by patients with EoE irrespective of their age, as well as the substantial disease burden,” the investigators concluded. “These data highlight the potential unmet medical need of patients with EoE in the United States.”The study was funded by Shire Human Genetic Therapies, a member of the Takeda group of companies. The investigators disclosed additional relationships with RTI Health Solutions and Receptos/Celgene.
FROM GASTRO HEP ADVANCES
Eribulin Similar to Taxane When Paired With Dual HER2 Blockade in BC
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
The results of this multicenter, randomized, open-label, parallel-group, phase 3 Japanese trial suggest that patients who cannot tolerate the standard taxane-based regimen have a new option for treatment.
“Our study is the first to show the non-inferiority of eribulin to a taxane, when used in combination with dual HER2 blockade as first-line treatment for this population,” lead author Toshinari Yamashita, MD, PhD, from the Kanagawa Cancer Center, in Kanagawa, Japan, said at the annual meeting of the American Society of Clinical Oncology.
“To our knowledge, noninferiority of eribulin to a taxane when used in combination with dual HER2 blockade has not been investigated,” Dr. Yamashita said.
“The combination of trastuzumab, pertuzumab, and taxane is a current standard first-line therapy for recurrent or metastatic HER2-positive breast cancer,” explained Dr. Yamashita. “However, because of taxane-induced toxicity, the development of less toxic but equally effective alternatives are needed.
“Because its efficacy is comparable to that of the current standard regimen, the combination of eribulin, trastuzumab, and pertuzumab is one of the options for first-line treatment of how to fight locally advanced or metastatic breast cancer,” he continued.
Study Results and Methods
The trial enrolled 446 patients, mean age 56 years, all of whom had locally advanced or metastatic breast cancer and no prior use of chemotherapy, excluding T-DM1. Patients who had received hormonal or HER2 therapy alone or the combination, as treatment for recurrence, were also eligible.
They were randomized 1:1 to receive a 21-day chemotherapy cycle of either (i) eribulin (1.4 mg/m2 on days 1 and 8), or (ii) a taxane (docetaxel 75 mg/m2 on day 1 or paclitaxel 80 mg/m2 on days 1, 8 and 15), each being administered in combination with a dual HER2 blockade of trastuzumab plus pertuzumab.
Baseline characteristics of both groups were well balanced, with 257 (57.6%) having ER-positive disease, 292 (65.5%) visceral metastasis, and 263 (59%) with de novo stage 4 disease, explained Dr. Yamashita.
For the primary endpoint, the median progression-free survival (PFS) was 14 versus 12.9 months in the eribulin and taxane group, respectively (hazard ratio [HR] 0.95, P = .6817), confirming non-inferiority of the study regimen, he reported.
The clinical benefit rate was similar between the two groups, with an objective response rate of 76.8% in the eribulin group and 75.2% in the taxane group.
Median OS was 65.3 months in the taxane group, but has not been reached in the study group (HR 1.09).
In terms of side-effects, the incidence of treatment-emergent adverse events was similar between the eribulin and taxane groups (58.9% vs 59.2%, respectively, for grade 3 or higher).
“Skin-related adverse events (62.4% vs 40.6%), diarrhea (54.1% vs 36.6%), and edema (42.2% vs 8.5%) tend to be more common with taxane, whereas neutropenia (61.6% vs 30.7%) and peripheral neuropathy (61.2% vs 52.8%) tend to be more common with eribulin use,” he said.
Overall, “these results suggest that eribulin is less toxic chemotherapeutic partner for dual HER2 blockade and can be used for a longer,” he said.
Findings Are a ‘Clinical Pearl’
Harold Burstein, MD, PhD, a breast cancer expert at Dana-Farber Cancer Institute and professor at Harvard Medical School in Boston, described the findings as “a nice clinical pearl,” because some patients do not tolerate taxane therapy. “In such cases, you can substitute eribulin, which is usually tolerated without allergic hypersensitivity issues,” he said in an interview.
Eribulin has specific properties that “could make it a perfect candidate” as an adjunct to standard treatment regimens across different breast cancer subtypes, observed Wynne Wijaya, MD an oncology researcher at the University of Oxford, England, and Universitas Gadjah Mada, in Yogyakarta, Indonesia, in a recent review (World J Exp Med. 2024;14[2]:92558).
Dr. Wijaya, who was not involved in this study, said in an interview that the findings have important implications.
“This encouraging result adds eribulin as another option in the first line treatment regimen for patients with HER2-positive, locally advanced or metastatic breast cancer, especially in terms of side effects/toxicities,” she said. “As clinicians, we can offer to tailor the choice of therapy between eribulin versus taxane in the regimen based on [which side effects patients are better able to tolerate]. It would also be interesting and worthwhile to conduct similar trials in different types of populations to provide more robust evidence.”
Eisai Co. funded the research. Dr. Yamashita disclosed ties with AstraZeneca, Chugai Pharma, Daiichi Sankyo, Eisai, Kyowa Hakko Kiri, Lilly, MSD, Pfizer, Taiho, Gilead Sciences, Nihonkayaku, Ono Yakuhin, and Seagen. Dr. Burstein disclosed a research grant from National Cancer Institute. Dr. Wijaya had no relevant disclosures.
FROM ASCO 2024
Gut Biomarkers Accurately Flag Autism Spectrum Disorder
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
, new research shows.
The findings could form the basis for development of a noninvasive diagnostic test for ASD and also provide novel therapeutic targets, wrote investigators, led by Siew C. Ng, MBBS, PhD, with the Microbiota I-Center (MagIC), the Chinese University of Hong Kong.
Their study was published online in Nature Microbiology.
Beyond Bacteria
The gut microbiome has been shown to play a central role in modulating the gut-brain axis, potentially influencing the development of ASD.
However, most studies in ASD have focused on the bacterial component of the microbiome. Whether nonbacterial microorganisms (such as gut archaea, fungi, and viruses) or function of the gut microbiome are altered in ASD remains unclear.
To investigate, the researchers performed metagenomic sequencing on fecal samples from 1627 boys and girls aged 1-13 years with and without ASD from five cohorts in China.
After controlling for diet, medication, and comorbidity, they identified 14 archaea, 51 bacteria, 7 fungi, 18 viruses, 27 microbial genes, and 12 metabolic pathways that were altered in children with ASD.
Machine-learning models using single-kingdom panels (archaea, bacteria, fungi, viruses) achieved area under the curve (AUC) values ranging from 0.68 to 0.87 in differentiating children with ASD from neurotypical control children.
A model based on a panel of 31 multikingdom and functional markers achieved “high predictive value” for ASD, achieving an AUC of 0.91, with comparable performance among boys and girls.
“The reproducible performance of the models across ages, sexes, and cohorts highlights their potential as promising diagnostic tools for ASD,” the investigators wrote.
They also noted that the accuracy of the model was largely driven by the biosynthesis pathways of ubiquinol-7 and thiamine diphosphate, which were less abundant in children with ASD, and may serve as therapeutic targets.
‘Exciting’ Possibilities
“This study broadens our understanding by including fungi, archaea, and viruses, where previous studies have largely focused on the role of gut bacteria in autism,” Bhismadev Chakrabarti, PhD, research director of the Centre for Autism at the University of Reading, United Kingdom, said in a statement from the nonprofit UK Science Media Centre.
“The results are broadly in line with previous studies that show reduced microbial diversity in autistic individuals. It also examines one of the largest samples seen in a study like this, which further strengthens the results,” Dr. Chakrabarti added.
He said this research may provide “new ways of detecting autism, if microbial markers turn out to strengthen the ability of genetic and behavioral tests to detect autism. A future platform that can combine genetic, microbial, and simple behavioral assessments could help address the detection gap.
“One limitation of this data is that it cannot assess any causal role for the microbiota in the development of autism,” Dr. Chakrabarti noted.
This study was supported by InnoHK, the Government of Hong Kong, Special Administrative Region of the People’s Republic of China, The D. H. Chen Foundation, and the New Cornerstone Science Foundation through the New Cornerstone Investigator Program. Dr. Ng has served as an advisory board member for Pfizer, Ferring, Janssen, and AbbVie; has received honoraria as a speaker for Ferring, Tillotts, Menarini, Janssen, AbbVie, and Takeda; is a scientific cofounder and shareholder of GenieBiome; receives patent royalties through her affiliated institutions; and is named as a co-inventor of patent applications that cover the therapeutic and diagnostic use of microbiome. Dr. Chakrabarti has no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MICROBIOLOGY
Combat Exposure Increases Chronic Pain Among Women in the US Military
TOPLINE:
Combat exposure is strongly associated with chronic pain in active-duty servicewomen and female civilian dependents of military personnel on active duty; a lower socioeconomic status and mental health conditions further increased the likelihood of chronic pain.
METHODOLOGY:
- Researchers analyzed claims data from the Military Health System to identify chronic pain diagnoses among active-duty servicewomen and civilian dependents of individuals on active duty.
- A total of 3,473,401 individuals (median age, 29 years) were included in the study, with 644,478 active-duty servicewomen and 2,828,923 civilian dependents.
- The study compared the incidence of chronic pain during 2006-2013, a period of heightened deployment intensity, with 2014-2020, a period of reduced deployment intensity.
- The primary outcome was the diagnosis of chronic pain.
TAKEAWAY:
- Active-duty servicewomen in the years 2006-2013 had a 53% increase in the odds of reporting chronic pain compared with those in the period between 2014 and 2020 (odds ratio [OR], 1.53; 95% CI, 1.48-1.58).
- Civilian dependents in the years 2006-2013 had a 96% increase in the odds of chronic pain compared with those in the later interval (OR, 1.96; 95% CI, 1.93-1.99).
- In 2006-2013, junior enlisted active-duty servicewomen had nearly a twofold increase in the odds of chronic pain (OR, 1.95; 95% CI, 1.83-2.09), while junior enlisted dependents had more than a threefold increase in the odds of chronic pain (OR, 3.05; 95% CI, 2.87-3.25) compared with senior officers.
- Comorbid mental conditions also were associated with an increased odds of reporting chronic pain (OR, 1.67; 95% CI, 1.65-1.69).
IN PRACTICE:
“The potential for higher rates of chronic pain in women veterans has been theorized to result from differences in support structures, family conflict, coping strategies, stress regulation, and exposure to military sexual trauma,” the authors wrote. “Our results suggest that these contributing factors may carry over to the women dependents of combat veterans in addition, indicating a line of research that requires urgent further exploration.”
SOURCE:
The study was led by Andrew J. Schoenfeld, MD, MSc, of the Center for Surgery and Public Health, Department of Orthopaedic Surgery at Brigham and Women’s Hospital and Harvard Medical School, in Boston. It was published online on July 5, 2024, in JAMA Network Open.
LIMITATIONS:
This study relied on claims-based data, which may have issues with coding accuracy and limited clinical granularity. The population size reduced over time owing to military downsizing, which could impact the findings. The prevalence of chronic pain in the population was likely underestimated because individuals who did not report symptoms or were diagnosed after separation from service were not identified.
DISCLOSURES:
This study was funded by the US Department of Defense. The lead author reported receiving grants and personal fees, serving as the editor-in-chief for Spine, acting as a consultant, and having other ties with various sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Combat exposure is strongly associated with chronic pain in active-duty servicewomen and female civilian dependents of military personnel on active duty; a lower socioeconomic status and mental health conditions further increased the likelihood of chronic pain.
METHODOLOGY:
- Researchers analyzed claims data from the Military Health System to identify chronic pain diagnoses among active-duty servicewomen and civilian dependents of individuals on active duty.
- A total of 3,473,401 individuals (median age, 29 years) were included in the study, with 644,478 active-duty servicewomen and 2,828,923 civilian dependents.
- The study compared the incidence of chronic pain during 2006-2013, a period of heightened deployment intensity, with 2014-2020, a period of reduced deployment intensity.
- The primary outcome was the diagnosis of chronic pain.
TAKEAWAY:
- Active-duty servicewomen in the years 2006-2013 had a 53% increase in the odds of reporting chronic pain compared with those in the period between 2014 and 2020 (odds ratio [OR], 1.53; 95% CI, 1.48-1.58).
- Civilian dependents in the years 2006-2013 had a 96% increase in the odds of chronic pain compared with those in the later interval (OR, 1.96; 95% CI, 1.93-1.99).
- In 2006-2013, junior enlisted active-duty servicewomen had nearly a twofold increase in the odds of chronic pain (OR, 1.95; 95% CI, 1.83-2.09), while junior enlisted dependents had more than a threefold increase in the odds of chronic pain (OR, 3.05; 95% CI, 2.87-3.25) compared with senior officers.
- Comorbid mental conditions also were associated with an increased odds of reporting chronic pain (OR, 1.67; 95% CI, 1.65-1.69).
IN PRACTICE:
“The potential for higher rates of chronic pain in women veterans has been theorized to result from differences in support structures, family conflict, coping strategies, stress regulation, and exposure to military sexual trauma,” the authors wrote. “Our results suggest that these contributing factors may carry over to the women dependents of combat veterans in addition, indicating a line of research that requires urgent further exploration.”
SOURCE:
The study was led by Andrew J. Schoenfeld, MD, MSc, of the Center for Surgery and Public Health, Department of Orthopaedic Surgery at Brigham and Women’s Hospital and Harvard Medical School, in Boston. It was published online on July 5, 2024, in JAMA Network Open.
LIMITATIONS:
This study relied on claims-based data, which may have issues with coding accuracy and limited clinical granularity. The population size reduced over time owing to military downsizing, which could impact the findings. The prevalence of chronic pain in the population was likely underestimated because individuals who did not report symptoms or were diagnosed after separation from service were not identified.
DISCLOSURES:
This study was funded by the US Department of Defense. The lead author reported receiving grants and personal fees, serving as the editor-in-chief for Spine, acting as a consultant, and having other ties with various sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
TOPLINE:
Combat exposure is strongly associated with chronic pain in active-duty servicewomen and female civilian dependents of military personnel on active duty; a lower socioeconomic status and mental health conditions further increased the likelihood of chronic pain.
METHODOLOGY:
- Researchers analyzed claims data from the Military Health System to identify chronic pain diagnoses among active-duty servicewomen and civilian dependents of individuals on active duty.
- A total of 3,473,401 individuals (median age, 29 years) were included in the study, with 644,478 active-duty servicewomen and 2,828,923 civilian dependents.
- The study compared the incidence of chronic pain during 2006-2013, a period of heightened deployment intensity, with 2014-2020, a period of reduced deployment intensity.
- The primary outcome was the diagnosis of chronic pain.
TAKEAWAY:
- Active-duty servicewomen in the years 2006-2013 had a 53% increase in the odds of reporting chronic pain compared with those in the period between 2014 and 2020 (odds ratio [OR], 1.53; 95% CI, 1.48-1.58).
- Civilian dependents in the years 2006-2013 had a 96% increase in the odds of chronic pain compared with those in the later interval (OR, 1.96; 95% CI, 1.93-1.99).
- In 2006-2013, junior enlisted active-duty servicewomen had nearly a twofold increase in the odds of chronic pain (OR, 1.95; 95% CI, 1.83-2.09), while junior enlisted dependents had more than a threefold increase in the odds of chronic pain (OR, 3.05; 95% CI, 2.87-3.25) compared with senior officers.
- Comorbid mental conditions also were associated with an increased odds of reporting chronic pain (OR, 1.67; 95% CI, 1.65-1.69).
IN PRACTICE:
“The potential for higher rates of chronic pain in women veterans has been theorized to result from differences in support structures, family conflict, coping strategies, stress regulation, and exposure to military sexual trauma,” the authors wrote. “Our results suggest that these contributing factors may carry over to the women dependents of combat veterans in addition, indicating a line of research that requires urgent further exploration.”
SOURCE:
The study was led by Andrew J. Schoenfeld, MD, MSc, of the Center for Surgery and Public Health, Department of Orthopaedic Surgery at Brigham and Women’s Hospital and Harvard Medical School, in Boston. It was published online on July 5, 2024, in JAMA Network Open.
LIMITATIONS:
This study relied on claims-based data, which may have issues with coding accuracy and limited clinical granularity. The population size reduced over time owing to military downsizing, which could impact the findings. The prevalence of chronic pain in the population was likely underestimated because individuals who did not report symptoms or were diagnosed after separation from service were not identified.
DISCLOSURES:
This study was funded by the US Department of Defense. The lead author reported receiving grants and personal fees, serving as the editor-in-chief for Spine, acting as a consultant, and having other ties with various sources outside the submitted work.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article appeared on Medscape.com.
Survey: Ob.Gyn.s Cite Burnout as Major Practice Issue
Nearly half (46%) of ob.gyn.s, and physicians generally, said in a new survey their employers were not paying enough attention to the extent of burnout among their physicians.
Findings of the survey were reported in Medscape’s Ob/Gyn Practice Issues Report 2024.
“It’s a big problem,” said Nigel Girgrah, MD, PhD, chief wellness officer at Ochsner Health in New Orleans, Louisiana. But he added he expects that 46% level to drop in the next few years.
“There’s an increasing awareness among executives in healthcare that well-being is a leading quality indicator, if not the indicator, of favorable outcomes in patient care, staff turnover, and other vital areas,” he said.
However, there are structural barriers that threaten work-life balance, says Eve Espey, MD, MPH, chair of the Department of Obstetrics and Gynecology at the University of New Mexico in Albuquerque. In a specialty made up mostly of women, she notes, there is no federal policy on paid medical leave, leading to women ob.gyn.s pulling double duty at work and at home.
“To me, the most important way to engage on burnout is not offering yoga lessons; it’s dealing with these big structural issues,” she said.
What’s Driving the Burnout
Ob.gyn. survey respondents said the top two reasons for burnout are too many bureaucratic tasks, such as paperwork and charting, (cited by 62%) and too many hours at work (cited by 53%).
More than half (55%) said the paperwork burden had increased in the past year; 34% said it stayed the same; and 11% said it had decreased.
“I think the regulatory burden has gotten higher for most of my colleagues,” Dr. Espey, said. “Coding and billing rules are pretty opaque to most ob.gyn.s and a lot of the tasks that used to be done by medical assistants are now done by physicians.”
‘Big Hope’ Is AI
“The big hope on the horizon is AI and programs that do basically what scribes do,” Dr. Espey said. “People feel like that will be a game-changer when it comes to paperwork.”
She said the root problem is that EHRs are used mostly for billing, not for communication. Two thirds of the respondents said they sometimes or always copy and paste from charts, which can lead to note bloat.
“You can’t write concise notes in EHRs,” Dr. Espey said. “I think this is an area where the promise of AI would be very helpful.”
Nearly 40% of ob.gyn.s were enthusiastic about the potential for AI in the survey; 43% were neutral on the subject; and the remainder were apprehensive. Asked about how their practices have most commonly used AI so far, 23% said it was used for researching conditions. The next highest usage was for help in diagnosing conditions (mentioned by 13%).
More than half (53%) of the ob.gyn.s said they thought AI would free up more time for patient conversations and care while only 38% of doctors generally thought it would.
Hard to Spend More Time With One Patient
Nearly three in 10 ob.gyn.s said they have a patient quota at their workplace, compared with one in four for physicians generally.
“Clearly, there can be thresholds (for ob.gyn.s) in the office and ambulatory care settings,” said Catherine Cansino, MD, MPH, a Sacramento ob.gyn. affiliated with University of California–Davis Medical Center. She added that on the inpatient side, patient quotas come from the allotted time an ob.gyn. can spend with a patient. “There are definitely hoops to jump through when we want to spend more time with a patient, and it can be tough for them to get a follow-up appointment.”
Most ob.gyn.s (62%) in the survey would give their employer good ratings for offering an engaging and welcoming atmosphere. But nearly 40% said their employer emphasizes patient quotas and CPT codes more than care quality.
Data for this report were drawn from several Medscape surveys performed between March and October of 2023.
Nearly half (46%) of ob.gyn.s, and physicians generally, said in a new survey their employers were not paying enough attention to the extent of burnout among their physicians.
Findings of the survey were reported in Medscape’s Ob/Gyn Practice Issues Report 2024.
“It’s a big problem,” said Nigel Girgrah, MD, PhD, chief wellness officer at Ochsner Health in New Orleans, Louisiana. But he added he expects that 46% level to drop in the next few years.
“There’s an increasing awareness among executives in healthcare that well-being is a leading quality indicator, if not the indicator, of favorable outcomes in patient care, staff turnover, and other vital areas,” he said.
However, there are structural barriers that threaten work-life balance, says Eve Espey, MD, MPH, chair of the Department of Obstetrics and Gynecology at the University of New Mexico in Albuquerque. In a specialty made up mostly of women, she notes, there is no federal policy on paid medical leave, leading to women ob.gyn.s pulling double duty at work and at home.
“To me, the most important way to engage on burnout is not offering yoga lessons; it’s dealing with these big structural issues,” she said.
What’s Driving the Burnout
Ob.gyn. survey respondents said the top two reasons for burnout are too many bureaucratic tasks, such as paperwork and charting, (cited by 62%) and too many hours at work (cited by 53%).
More than half (55%) said the paperwork burden had increased in the past year; 34% said it stayed the same; and 11% said it had decreased.
“I think the regulatory burden has gotten higher for most of my colleagues,” Dr. Espey, said. “Coding and billing rules are pretty opaque to most ob.gyn.s and a lot of the tasks that used to be done by medical assistants are now done by physicians.”
‘Big Hope’ Is AI
“The big hope on the horizon is AI and programs that do basically what scribes do,” Dr. Espey said. “People feel like that will be a game-changer when it comes to paperwork.”
She said the root problem is that EHRs are used mostly for billing, not for communication. Two thirds of the respondents said they sometimes or always copy and paste from charts, which can lead to note bloat.
“You can’t write concise notes in EHRs,” Dr. Espey said. “I think this is an area where the promise of AI would be very helpful.”
Nearly 40% of ob.gyn.s were enthusiastic about the potential for AI in the survey; 43% were neutral on the subject; and the remainder were apprehensive. Asked about how their practices have most commonly used AI so far, 23% said it was used for researching conditions. The next highest usage was for help in diagnosing conditions (mentioned by 13%).
More than half (53%) of the ob.gyn.s said they thought AI would free up more time for patient conversations and care while only 38% of doctors generally thought it would.
Hard to Spend More Time With One Patient
Nearly three in 10 ob.gyn.s said they have a patient quota at their workplace, compared with one in four for physicians generally.
“Clearly, there can be thresholds (for ob.gyn.s) in the office and ambulatory care settings,” said Catherine Cansino, MD, MPH, a Sacramento ob.gyn. affiliated with University of California–Davis Medical Center. She added that on the inpatient side, patient quotas come from the allotted time an ob.gyn. can spend with a patient. “There are definitely hoops to jump through when we want to spend more time with a patient, and it can be tough for them to get a follow-up appointment.”
Most ob.gyn.s (62%) in the survey would give their employer good ratings for offering an engaging and welcoming atmosphere. But nearly 40% said their employer emphasizes patient quotas and CPT codes more than care quality.
Data for this report were drawn from several Medscape surveys performed between March and October of 2023.
Nearly half (46%) of ob.gyn.s, and physicians generally, said in a new survey their employers were not paying enough attention to the extent of burnout among their physicians.
Findings of the survey were reported in Medscape’s Ob/Gyn Practice Issues Report 2024.
“It’s a big problem,” said Nigel Girgrah, MD, PhD, chief wellness officer at Ochsner Health in New Orleans, Louisiana. But he added he expects that 46% level to drop in the next few years.
“There’s an increasing awareness among executives in healthcare that well-being is a leading quality indicator, if not the indicator, of favorable outcomes in patient care, staff turnover, and other vital areas,” he said.
However, there are structural barriers that threaten work-life balance, says Eve Espey, MD, MPH, chair of the Department of Obstetrics and Gynecology at the University of New Mexico in Albuquerque. In a specialty made up mostly of women, she notes, there is no federal policy on paid medical leave, leading to women ob.gyn.s pulling double duty at work and at home.
“To me, the most important way to engage on burnout is not offering yoga lessons; it’s dealing with these big structural issues,” she said.
What’s Driving the Burnout
Ob.gyn. survey respondents said the top two reasons for burnout are too many bureaucratic tasks, such as paperwork and charting, (cited by 62%) and too many hours at work (cited by 53%).
More than half (55%) said the paperwork burden had increased in the past year; 34% said it stayed the same; and 11% said it had decreased.
“I think the regulatory burden has gotten higher for most of my colleagues,” Dr. Espey, said. “Coding and billing rules are pretty opaque to most ob.gyn.s and a lot of the tasks that used to be done by medical assistants are now done by physicians.”
‘Big Hope’ Is AI
“The big hope on the horizon is AI and programs that do basically what scribes do,” Dr. Espey said. “People feel like that will be a game-changer when it comes to paperwork.”
She said the root problem is that EHRs are used mostly for billing, not for communication. Two thirds of the respondents said they sometimes or always copy and paste from charts, which can lead to note bloat.
“You can’t write concise notes in EHRs,” Dr. Espey said. “I think this is an area where the promise of AI would be very helpful.”
Nearly 40% of ob.gyn.s were enthusiastic about the potential for AI in the survey; 43% were neutral on the subject; and the remainder were apprehensive. Asked about how their practices have most commonly used AI so far, 23% said it was used for researching conditions. The next highest usage was for help in diagnosing conditions (mentioned by 13%).
More than half (53%) of the ob.gyn.s said they thought AI would free up more time for patient conversations and care while only 38% of doctors generally thought it would.
Hard to Spend More Time With One Patient
Nearly three in 10 ob.gyn.s said they have a patient quota at their workplace, compared with one in four for physicians generally.
“Clearly, there can be thresholds (for ob.gyn.s) in the office and ambulatory care settings,” said Catherine Cansino, MD, MPH, a Sacramento ob.gyn. affiliated with University of California–Davis Medical Center. She added that on the inpatient side, patient quotas come from the allotted time an ob.gyn. can spend with a patient. “There are definitely hoops to jump through when we want to spend more time with a patient, and it can be tough for them to get a follow-up appointment.”
Most ob.gyn.s (62%) in the survey would give their employer good ratings for offering an engaging and welcoming atmosphere. But nearly 40% said their employer emphasizes patient quotas and CPT codes more than care quality.
Data for this report were drawn from several Medscape surveys performed between March and October of 2023.
Women’s Risk for Lupus Rises With Greater Intake of Ultraprocessed Foods
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
TOPLINE:
A higher intake of ultraprocessed foods increases the risk for systemic lupus erythematosus (SLE) by over 50% in women. The risk doubled in those with anti–double-stranded DNA antibodies.
METHODOLOGY:
- Researchers assessed 204,175 women from two Nurses’ Health Study cohorts from 1984 to 2016.
- Participants completed semiquantitative food frequency questionnaires every 4 years for the assessment of dietary intake.
- Incident SLE cases were self-reported and confirmed using medical records, with 212 cases identified.
TAKEAWAY:
- A higher cumulative average daily intake of ultraprocessed foods was associated with a 56% increased risk for SLE (95% confidence interval [CI], 1.04-2.32).
- The risk for anti–double-stranded DNA antibody-positive SLE was more than doubled (hazard ratio, 2.05; 95% CI, 1.15-3.65).
- Sugar or artificially sweetened beverages were associated with a 45% increased risk for SLE (95% CI, 1.01-2.09).
- No significant interactions with body mass index were observed in the association between ultraprocessed food intake and SLE.
IN PRACTICE:
This study is too preliminary to have practical application.
SOURCE:
The study was led by Sinara Rossato, PhD, Harvard T.H. Chan School of Public Health, Boston. It was published online in Arthritis Care & Research.
LIMITATIONS:
The study’s generalizability is limited due to the predominantly White female population of registered nurses. The relatively high baseline age of participants may not fully capture the peak incidence age range for SLE. The observational nature of the study cannot establish causality between ultraprocessed food intake and SLE risk.
DISCLOSURES:
The study was supported by the National Institutes of Health. The authors did not declare any competing interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication.
A version of this article first appeared on Medscape.com.
Plastic Surgeon to Pay $5 Million for Restriction of Negative Reviews, Directing Fake Reviews
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.
A Seattle plastic surgeon who illegally restricted patients from posting negative reviews about his practice and directed his staff to post fake positive reviews will pay $5 million for violating Washington state’s consumer protection law.
According to a July 1 consent decree, Javad Sajan, MD, and his practice Allure Esthetic must pay $1.5 million in restitution to 21,000 patients and $3.5 million to the state for manipulation of patient ratings.
In an April decision, US District Judge Ricardo S. Martinez sided with the state, ruling that Allure Esthetic’s actions violated the federal Consumer Review Fairness Act (CRFA).
“Writing a truthful review about a business should not subject you to threats or intimidation,” Mr. Ferguson said in a July 2 statement. “Consumers rely on reviews when determining who to trust, especially services that affect their health and safety. This resolution holds Allure accountable for brazenly violating that trust — and the law — and ensures the clinic stops its harmful conduct.”
In court documents, Dr. Sajan’s attorneys had argued that the agreements did not violate CRFA because patients had the opportunity to modify the language or decline signing them.
The surgeon’s practice is “pleased to have resolved its case with the Attorney General’s Office,” according to a statement provided by Dr. Sajan’s attorney. “The cooperative settlement, while not admitting fault and resolving claims asserted by both sides, allows Allure Esthetic to continue to focus on its core mission of providing compassionate care to patients and serving the community. The decision to settle was not an easy one, but it was necessary to allocate time and resources where they matter most — the patients.”
The dispute stemmed from NDAs that Dr. Sajan’s practice required patients to sign starting in 2017, according to Mr. Ferguson’s complaint. The terms instructed patients to contact the business directly if they had concerns rather than post a negative review.
If patients posted negative reviews, the clinic, in some cases, threatened litigation, according to the lawsuit. In other cases, patients were allegedly offered money and free services in exchange for taking the reviews down. Patients who accepted cash or services were required to sign a second agreement forbidding them from posting future negative reviews and imposing a $250,000 penalty for failure to comply, according to court documents.
In addition, Mr. Ferguson accused Dr. Sajan of creating fake positive accounts of patient experiences and buying fake followers on social media. State investigators found Dr. Sajan directed Allure Esthetic’s employees to create fake Gmail accounts to post the false reviews, many of which are still online today, according to the state’s complaint.
Mr. Ferguson also claimed Dr. Sajan and his practice manipulated social media to appear more popular by purchasing followers through an online vendor. The practice also allegedly used a social media bot tool to buy thousands of fake likes on Instagram, YouTube, and other social media.
After filing the lawsuit, Mr. Ferguson’s office uncovered further evidence of Dr. Sajan’s efforts to influence his professional reputation through fabrication, according to the July 2 release. Allure Esthetic “rigged” “best doctor” competitions hosted by local media outlets by paying staff and contractors to vote for Dr. Sajan as “best plastic surgeon” in the region, according to the release. The staff cast as many votes as websites allowed, despite not being patients of Allure Esthetic.
The practice also allegedly edited before-and-after photos of patients to make their results appear better and kept tens of thousands of dollars in rebates intended for its patients.
In addition to paying $5 million, the consent decree requires Dr. Sajan and his practice also:
- Stop posting or influencing consumer reviews; perform a full audit of all public reviews on Google, Yelp, and other third-party review platforms; and request removal of every review Allure Esthetic was involved in creating, posting, or shaping in any manner.
- Remove all misleading “before-and-after” photographs of plastic surgery procedures from its website and social media and stop altering photographs of future procedures.
- Cease use of and attempts to enforce all illegal NDAs and notify patients who previously signed them that they are released from the terms of those NDAs.
- Pay a third-party forensic accounting firm to perform a full, independent audit of Allure Esthetic’s consumer rebate program to identify consumers who are owed rebates that were unlawfully claimed by Allure Esthetic.
Additionally, the attorney general’s office will continue to monitor Allure Esthetic, and upon request, the practice must provide information that demonstrates its compliance with the consent decree for the next 10 years.
The practice must also develop internal policies and implement a training program to educate staff about nondeceptive advertising and compliance with consumer protection laws.
Dr. Sajan and his practice agreed to the terms of the consent decree, and the settlement is not considered an admission of liability.
A version of this article first appeared on Medscape.com.