User login
Reducing Rates of Perioperative Deep Vein Thrombosis and Pulmonary Emboli in Hip and Knee Arthroplasty Patients: A Quality Improvement Project
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
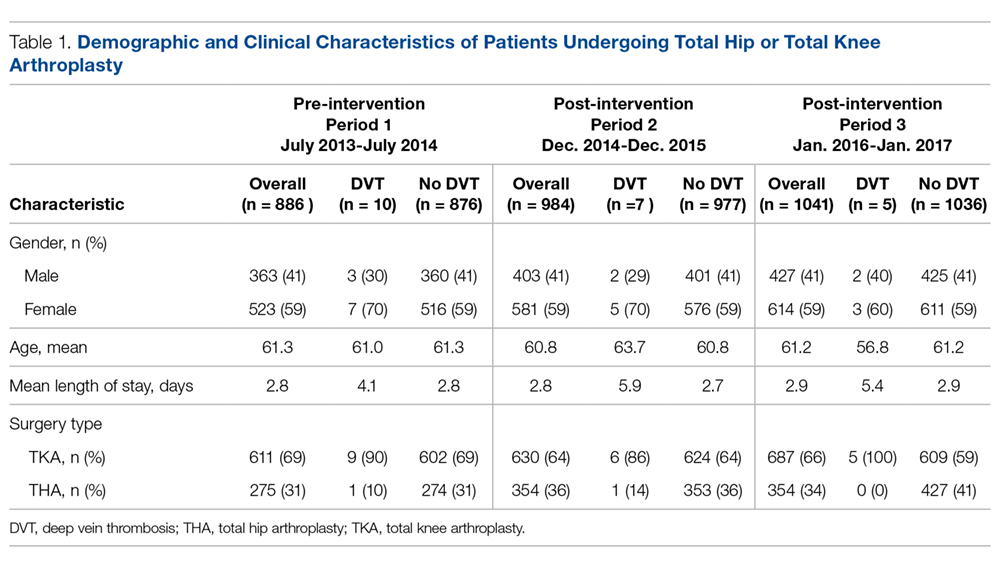
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
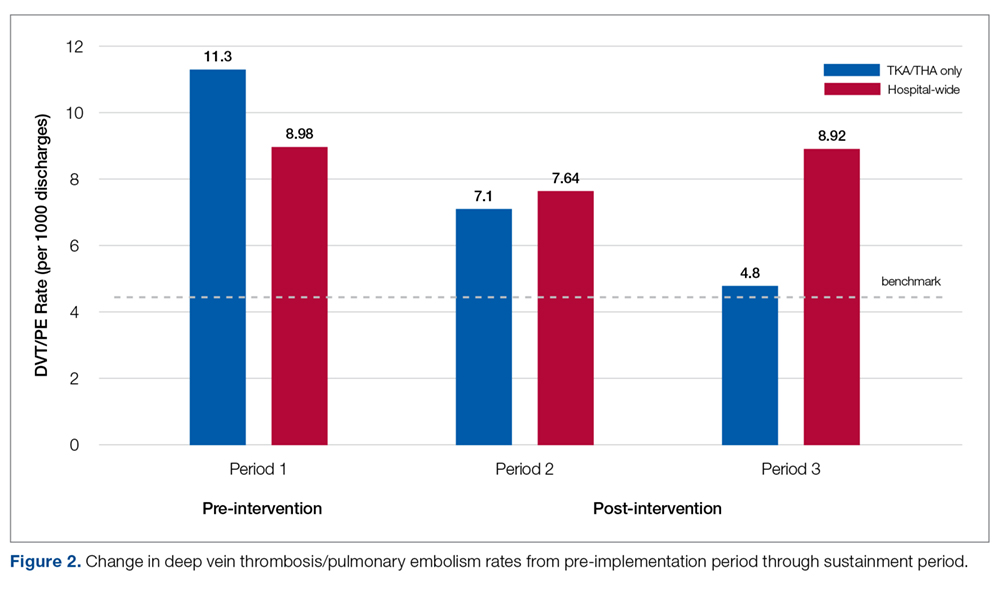
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
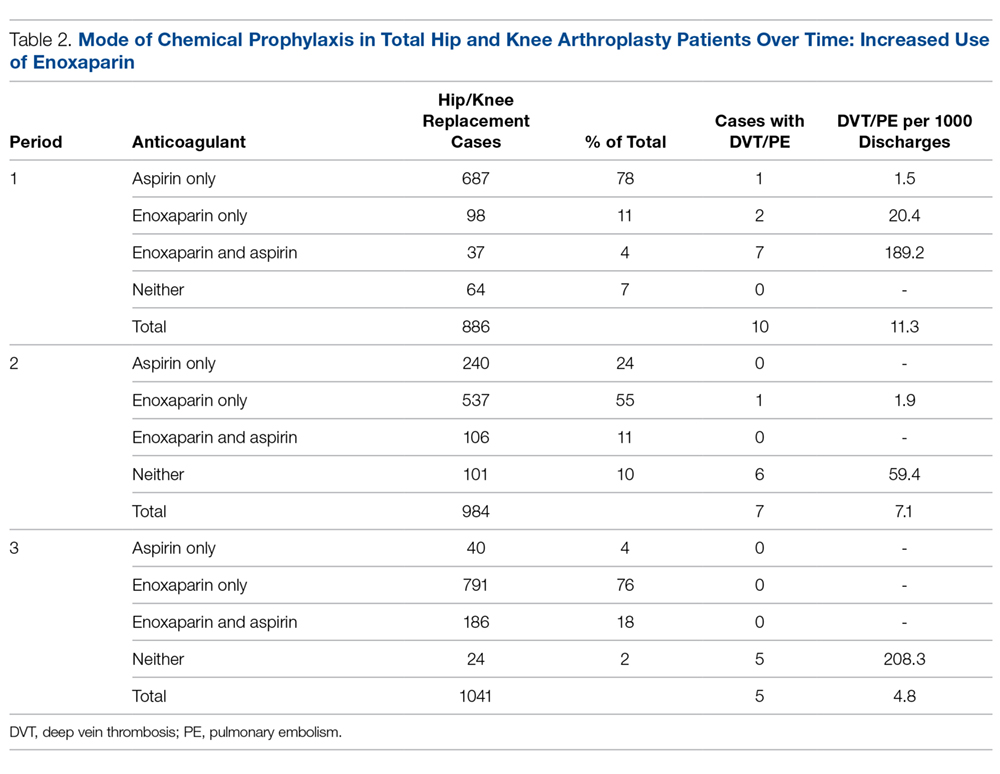
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
From Grant Medical Center (Dr. Fada, Ms. Lucki, and Dr. Polonia) and the OhioHealth Group (Ms. Long and Dr. Gascon), Columbus, OH; and the Indiana University School of Medicine, Indianapolis, IN (Dr. Hartwell).
- Objective: To decrease the rates of venous thromboembolism (VTE) associated with total knee arthroplasty (TKA) and total hip arthroplasty (THA), evaluate the effectiveness of the current practice of deep vein thrombosis (DVT) and pulmonary embolism (PE) prophylaxis, and improve patient care and recovery following surgery.
- Methods: A multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed to study trends in care, review best practices, identify root causes of suboptimal performance, and implement improvements.
- Results: DVT/PE rates associated with TKA/THA decreased nearly 60% over 2 years to a rate of 4.8 per 1000 discharges. Enoxaparin dosing has been sustained at 94% of patients, and 88% of patients experience early mobilization.
- Conclusion: Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practices, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Keywords: joint replacement; thrombosis; surgery; patient safety; prophylaxis.
Venous thromboembolism (VTE) in the form of deep vein thrombosis (DVT) and pulmonary embolism (PE) affects nearly 600,000 Americans annually, and is directly or indirectly responsible for at least 100,000 deaths per year.1 VTE has historically been viewed as a complication of major surgery (ie, abdominal or thoracic operations that require general anesthesia lasting ≥ 30 minutes),2,3 although it can occur outside of such settings. Risk factors for VTE include age, obesity, a history of VTE, cancer, bed rest of 5 or more days, major surgery, congestive heart failure, varicose veins, fracture (hip or leg), estrogen treatment, stroke, multiple trauma, childbirth, and myocardial infarction.4 VTE is a disease with long-term complications that can affect patients for several years, and can lead to an avoidable death.5 VTEs are of particular concern following total joint replacements.
The incidence of joint replacement procedures in the United States is high, with more than 1 million total hip and total knee replacement procedures performed each year. With the aging of the population, higher rates of diagnosis and treatment of advanced arthritis, and growing demand for improved mobility and quality of life, the annual procedure volumes are projected to increase considerably in the future, making joint replacements the most common elective surgical procedures in the coming decades.6 The Centers for Medicare & Medicaid Services (CMS) are introducing new payment models that incorpoarate total cost of care with improved quality outcomes that must take into account complications of major surgical procedures.7 Hospital-acquired perioperative DVT/PE rates are now publicly reported and may affect reimbursement rates from CMS for patients undergoing total hip arthroplasty (THA) or total knee arthroplasty (TKA).
Methods
Setting
OhioHealth Grant Medical Center (GMC), an American College of Surgeons verified Level 1 trauma center, was established in 1900 in downtown Columbus, Ohio, as the second member hospital of OhioHealth, a not-for-profit, faith-based health care system. The Bone and Joint Center at GMC performs approximately 1000 total joint procedures per year, with an overall orthopedic surgical case volume of approximately 6000 cases per year. In 2013 it was noted that the unadjusted DVT/PE rate of 11.3 per 1000 TKA/THA discharges was higher than the benchmark patient safety indicator of 4.51/1000 surgical patient discharges published by the Agency for Healthcare Research and Quality (AHRQ).
Intervention
In an effort to reduce DVT/PE rates for patients undergoing THA/TKA, a multidisciplinary quality improvement project was initiated. The purpose of this project was (1) to determine care opportunities within the surgical patient population to decrease the overall rates of DVT/PE, and (2) to determine if a multidisciplinary team could impact change. This initiative was led by 2 outcomes managers, a surgical outcomes manager and an orthopedic outcomes manager, due to the service line that these individuals supported. This multidisciplinary team’s goal was to promote increased collaboration among all team members in order to provide higher quality care to our hip and knee patient population and improve patient outcomes.
The use of multidisciplinary in-hospital teams limits adverse events, improves outcomes, and adds to patient and employee satisfaction. Acting like components of a machine, multidisciplinary in-hospital teams include staff from different levels of the treatment pyramid (eg, staff including nurses’ aides, surgical technicians, nurses, anesthesiologists, attending physicians, and others). Their teamwork counters the silo effect by enhancing communication between the different levels of health care workers, thus reducing adverse events.8
In August 2014, a multidisciplinary team of surgeons, intensivists, cardiologists, nurses, pharmacists, physical therapists, hospital quality and safety directors, and senior hospital administration was formed at GMC. The outcomes managers were tasked as the team leads to review the hospital’s rate of DVT/PE, reported as AHRQ’s Patient Safety Indicator (PSI) 12.9 The goals of this multidisciplinary quality improvement project were to decrease the rates of DVT/PE, evaluate the effectiveness of the current practice of DVT/PE prophylaxis, and improve patient care for patients undergoing THA/TKA. The team performed monthly case reviews to identify trends in care. Based on these reviews, several opportunities for improvement were identified, including (1) poor clinician understanding of the risk of DVT/PE; (2) lack of standardized use of mechanical prophylaxis in the operating room; (3) inconsistent use and under-dosing of enoxaparin; (4) delayed initiation of enoxaparin; (5) minimized exclusions for VTE prophylaxis utilizing trauma exclusions; and (6) delayed early mobilization.
The quality improvement committee reviewed evidence-based best practices, including American College of Chest Physicians recommendations10 and guidelines previously implemented at OhioHealth Grant Medical Center Trauma Center. This Level 1 trauma center had well-defined guidelines for DVT/PE prevention (Figure 1) and corresponding DVT/PE rates that were lower than Trauma Quality Improvement Program benchmarks. The collection and reporting of this data was deemed exempt from Institutional Review Board review at OhioHealth GMC.
From August through November 2014, the quality improvement team reviewed DVT/PE data on a monthly basis and issued evidence-based recommendations designed to address the identified areas of improvement, including screening for DVT/PE when clinically indicated, but not routine screening; maximum utilization of mechanical prophylaxis prior to induction of anesthesia; standardization of chemical prophylaxis postoperatively, including the use of enoxaparin over aspirin alone and dosing of enoxaparin according to the patient’s body mass index; emphasis on early mobility; and utilization of data to drive performance.
To determine the cumulative effectiveness of the guidelines in a specific orthopedic population, we compared DVT/PE rates in patients undergoing THA/TKA, the use of chemical prophylaxis, and adherence to early mobilization after surgery between the pre-implementation (July 2013-July 2014) period and post-implementation period (December 2014-December 2015). In order to assess continued compliance with best practices, DVT/PE rates were also calculated for a sustainment period (January 2016-January 2017).
Analysis
Descriptive statistics for continuous variables were reported as mean, standard deviations (SD), median, and range, and for dichotomous or categorical variables as frequencies and percentages. Efficacy of the revised guidelines was assessed in relationship to national and hospital benchmarks due to the small sample size of this study, as there was insufficient power for statistical analysis of DVT/PE rates.
Results
During the pre-implementation period, 886 THA/TKA procedures were performed. The number of surgeries increased slightly during the post-implementation period, with 984 THA/TKA procedures performed post-implementation and 1041 THA/TKA procedures performed during the sustainment period. Demographic and clinical characteristics of patients during the pre- and post-implementation periods are shown in Table 1.
Pre-implementation, 10 patients out of 886 patients who underwent TKA/THA surgeries were diagnosed with DVT/PE. This rate (11.3 per 1000 TKA/THA discharges) was more than 25% higher than the overall hospital rate (8.98 per 1000 surgical discharges) and 150% higher than the national benchmark (4.51). Post-implementation, 7 patients out of 984 who underwent THA/TKA surgeries were diagnosed with DVT/PE. This new rate (7.1 per 1000 TKA/THA discharges) was in line with the overall hospital rate (7.64 per 1000 surgical discharges), although both the overall hospital and TKA/THA rates remained above the national benchmark (4.51 per 1000 surgical discharges). However, the DVT/PE rate reduction has continued to decline, with 5 patients out of 1041 who underwent THA/TKA surgeries being diagnosed with DVT/PE (a rate of 4.8 per 1000 TKA/THA discharges) for the sustainment (third) period, bringing the current rate in line with the national benchmark. The change in DVT/PE rates over time is shown in Figure 2.
Prior to this quality improvement project, there were no standardized guidelines for enoxaparin dosing for patients undergoing TKA/THA, and enoxaparin dosing occurred for only 15% of TKA/THA patients (Table 2). Following implementation of the quality improvement committee recommendations for chemical prophylaxis, the rate of use of enoxaparin in TKA/THA patients increased to 66%; enoxaparin dosing increased further, with 94% of TKA/THA patients receiving enoxaparin during the sustainment (third) period.
Orthopedic best practice for out of bed day of surgery with physical therapy increased from 84% (745 patients mobilized/886 THA/TKA patients) pre-implementation to 88% (868 patients mobilized/984 THA/TKA patients) post-implementation. Early mobilization efforts remained increased through the sustainment period (917 patients mobilized/1041 THA/TKA patients; 88%).
Discussion
An outcomes manager–led multidisciplinary team was assembled in response to higher than expected rates of DVT/PE, particularly in patients undergoing elective THA/TKA. The intent of the quality improvement project was to identify all areas where care could be improved. Through the implementation of evidence-based best practices, the DVT/PE rate in patients undergoing TKA/THA was reduced from 1.13% to 0.48%, bringing DVT/PE rates in line with the AHRQ benchmark (0.451%). This project was successful because all parties were willing to examine current practices, identify opportunities for improvement, and actively engage in a collaborative effort to improve patient outcomes. The data presented here demonstrate that when interprofessional process improvements are utilized, improved efficiency can be achieved.
It was noted that there was an “implementation gap” between knowing the risk factors for DVT/PE and executing the recommended measures.11 While clinicians could articulate the risk of DVT/PE in their patient population, they underestimated the severity risk. As internists provided preoperative evaluation for many elective orthopedic patients, the quality improvement team focused education on the internists in regard to DVT/PE risk and prevention.
Based on recommendations from the American College of Physicians, the committee recommended the use of enoxaparin over the use of aspirin for DVT/PE prophylaxis.11 While this project was not designed to examine the correlation between this practice change and the decrease in the DVT/PE rate, it can be concluded that presenting evidence to clinicians does change ordering behavior, as enoxaparin dosing increased to 94% of patients following guideline implementation, compared to 15% of patients prior to guideline implementation.
Furthermore, THA/TKA patients with a body mass index (BMI) greater than 40 were dosed with enoxaparin 40 mg twice daily, instead of 30 mg twice daily used in patients with a BMI less than 40.12-14 Many clinicians were unaware of the option to increase the dose of enoxaparin. One orthopedic surgeon member on the quality improvement team became the champion for enoxaparin use in that population, and his leadership led to an increase in the use of guideline-based chemical prophylaxis. Bedside clinical pharmacists were instrumental in reviewing the enoxaparin orders and recommending increased dosing. Ongoing auditing of patient care helped to inform the team of compliance with VTE prophylaxis and understand barriers to the implementation of the standard work.
The root cause of poor compliance with the use of mechanical prophylaxis in the operating room was a knowledge gap regarding the importance of initiation prior to induction of anesthesia.15 This was corrected with targeted education of staff. Also, several nurses pointed out that, while they were aware of the best practice, sequential compression devices were physically unavailable for patients in the preoperative and postoperative areas. This was corrected by working with the vendor and hospital supply chain to increase periodic automatic replenishment levels.
It is intuitive that a reduction in the DVT/PE rate will translate into costs savings for the health care system and the patient, although this study was not powered or designed to study actual costs of treating DVT/PE. Costs associated with treating a DVT/PE are variable, but have been estimated to range from $9805 to $14,722.16 Taking these estimates and applying them to the current study, reducing the DVT/PE rate from 11.4 to 7.1 from pre-implementation to post-implementation, the total cost savings may be up to $4118 per TKA/THA discharge. Beyond cost considerations, the reduction of DVT/PE leads to improved patient outcomes and a reduction in morbidity and mortality.
Conclusion
Multidisciplinary teams are capable of effecting sustained improvements in patient care and outcomes when paired with lean management practices and a commitment to quality improvement. Collective efforts towards education, removal of barriers to carry out best practice, and having physicians champion the prevention of DVT/PE led to a clinically significant and sustained improvement in patient outcomes.
Corresponding author
Financial disclosures: None.
Acknowledgment: The authors thank Vijendra Mohan, MD, for his internal medicine expertise given on behalf of this effort.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
1. Office of the Surgeon General, U.S. The Surgeon General’s call to action to prevent deep vein thrombosis and pulmonary embolism. (2008).
2. Clagett GP, Reisch JS. Prevention of venous thromboembolism in general surgical patients. Results of meta-analysis. Ann Surg. 1988;208:227-240.
3. Collins R, Scrimgeour A, Yusuf S, et al. Reduction in fatal pulmonary embolism and venous thrombosis by perioperative administration of subcutaneous heparin. Overview of results of randomized trials in general, orthopedic, and urologic surgery. N Engl J Med. 1988;318:1162-1173.
4. Anderson FAA, Spencer FA Jr. Risk factors for venous thromboembolism. Circulation. 2003;107(23 Suppl 1):19-16.
5. Bosque J, Coleman SI, Di Cesare P. Relationship between deep vein thrombosis and pulmonary embolism following THA and TKA. Orthopedics. 2012;35:228-233.
6. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780-785.
7. McLawhorn AS, Buller LT. Bundled payments in total joint replacement: keeping our care affordable and high in quality. Curr Rev Musculoskelet Med. 2017;10:370-377.
8. Epstein NE. Multidisciplinary in-hospital teams improve patient outcomes: A review. Surg Neurol Int. 2014;5(Suppl 7):S295-303.
9. Agency for Healthcare Research and Quality (AHRQ). U.S. Department of Health and Human Services Patient Safety Indicator v4.5 Benchmark Data Tables. May, 2013.
10. Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S-e325S.
11. Maynard G. Preventing hospital associated venous thromboembolism: a guide for effective quality improvement. 2015. AHRQ Publication No. 16-0001-EF. Accessed online June 2, 2016. www.ahrq.gov/sites/default/files/wysiwyg/professionals/quality-patient-safety/patient-safety-resources/resources/vtguide/vteguide.pdf
12. Borkgren-Okonek MJ, Hart RW, Pantano JE, et al. Enoxaparin thromboprophylaxis in gastric bypass patients: extended duration, dose stratification, and antifactor Xa activity. Surg Obes Relat Dis. 2008;4:625-631.
13. Kothari SN, Lambert PJ, Mathiason MA. A comparison of thromboembolic and bleeding events following laparoscopic gastric bypass in patients treated with prophylactic regimens of unfractionated heparin or enoxaparin. Am J Surg. 2007;194:709-711.
14. Scholten DJ, Hoedema RM, Scholten SE. A comparison of two different prophylactic dose regimens of low molecular weight heparin in bariatric surgery. Obes Surg. 2002;12:19-24.
15. Association of Perioperative Registered Nurses (AORN). AORN guideline for prevention of venous stasis. AORN J. 2007;85:607-624.
16. Spyropoulos AC, Lin J. Direct medical costs of venous thromboembolism and subsequent hospital readmission rates: An administrative claims analysis from 30 managed care organizations. J Manag Care Pharm. 2007;13:475-486.
Procalcitonin, Will It Guide Us?
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
Study Overview
Objective. To assess whether procalcitonin-guided antibiotic usage results in less exposure to antibiotics than usual care, without a significantly higher rate of adverse events.
Design. Multi-center 1:1 randomized trial.
Setting and participants. This study was conducted at 14 academic hospitals in the United States between 2014 and 2017 in which procalcitonin assay was not routinely used. All adult patients in the emergency department with an initial diagnosis of acute lower respiratory tract infection without a decision to give or withhold antibiotics because of uncertainty regarding the need for antibiotics were included in the study. Patients were excluded if antibiotics were unlikely to be held in their case, such as if there was a need for mechanical ventilation or known severe immunosuppression, and if procalcitonin could be falsely elevated (chronic dialysis, metastatic cancer, surgery in the past 7 days).
Intervention. Patients were randomly assigned to receive guideline-based care using procalcitonin (procalcitonin group) or usual care (usual-care group). In the procalcitonin group, the procalcitonin assay results, and the procalcitonin treatment guidelines were provided to the treating physician. The guideline used previously established cutoffs (procalcitonin level of < 0.1 µg/L, antibiotics were strongly discouraged; 0.1 to 0.25 µg/L, antibiotics were discouraged; 0.25 to 0.5 µg/L, antibiotics were recommended; and > 0.5 µg/L, antibiotics were strongly recommended). Procalcitonin was measured initially in the emergency department. If the patient was hospitalized, procalcitonin was again measured 6 to 24 hours later, and on hospital days 3, 5, and 7. To implement this intervention, a multifaceted approach was used, which included sending letters to local primary care providers describing the trial, ensuring rapid delivery of procalcitonin results by tracking and coordinating blood samples with routine morning draws, and embedding the procalcitonin results and guidelines into the sites’ electronic health records. In the usual-care group, procalcitonin levels at enrollment were measured but not disclosed to clinicians. In both treatment groups, clinicians retained autonomy regarding care decisions.
Main outcome measures. The primary outcome was total antibiotic exposure, defined as the total number of antibiotic-days within 30 days after enrollment. The primary safety outcome was any adverse effects that could be attributable to withholding antibiotics in lower respiratory tract infections, within 30 days after enrollment. Secondary outcomes included admission to the intensive care unit (ICU), subsequent emergency department visits by day 30, and quality of life as assessed with the Airway Questionnaire 20.
Main results. 8360 patients with acute lower respiratory tract infection who presented to the emergency department were screened for eligibility; of these, 1664 patients underwent randomization. Ultimately, 1656 patients were included in the final analysis cohort (826 in the procalcitonin group and 830 in the usual-care group), because 8 patients withdrew. Of the cohort, 1345 (81.2%) patients completed the full 30-day follow up. Baseline characteristics were similar between the treatment groups. In the procalcitonin group, clinicians received the procalcitonin results for 95.9% of the patients. As a result of clinical care, 2.2% of the patients in the usual-care group also had procalcitonin testing. Clinicians adhered to the procalcitonin guideline recommendations for 64.8% of the procalcitonin group.
There was no significant difference in the intention-treat-treat analysis between the procalcitonin group and the usual-care group in antibiotic days during the first 30 days (mean antibiotic days, 4.2 and 4.3 days, respectively [95% confidence interval {CI}, –0.6 to 0.5; P = 0.87]). Within 30 days there was no significant difference in the proportion of patients with adverse outcomes in the procalcitonin group and usual-care group (11.7% and 13.1%, respectively [95% CI, –4.6 to 1.7]; P < 0.01 for noninferiority). There was no significant difference between the procalcitonin and usual-care groups for any of the secondary outcomes.
Conclusion. A procalcitonin-directed antibiotic administration guideline did not result in fewer antibiotic days than did usual-care among patients with suspected lower respiratory tract infection.
Commentary
Procalcitonin is a serum biomarker synthesized in thyroid neuroendocrine cells and is the precursor to calcitonin.1 It is undetectable in healthy human serum, but in the setting of systemic inflammation caused by bacterial infection, procalcitonin synthesis is induced in many tissues. Since its discovery in 1970, procalcitonin’s potential utility has been sought in various settings, such as guiding the initiation and/or discontinuation of antibiotics.2
In a prospective randomized trial in patients with an acute chronic obstructive pulmonary disease (COPD) exacerbation, treatment success was not better with antibiotics than placebo in patients with a procalcitonin level < 0.1 µg/L.3 Others replicated these results in COPD patients with acute exacerbation of COPD.4 Another small randomized trial showed that using procalcitonin in intensive care patients reduced antibiotic duration.5 Another small study found similar results in their critical care setting.6 Procalcitonin-guided antibiotic treatment produced similar results in patients with aspiration pneumonia.7 In summary, previously published studies nearly uniformly report reduced antibiotic duration or initiation using procalcitonin cutoffs without increasing adverse events.
In the current study, Huang and colleagues conducted a multi-center randomized trial in 14 academic US hospitals, while simultaneously attempting quality improvement methods for implementing and maximizing compliance with procalcitonin guidelines for local physicians. This study was able to achieve approximately 65% compliance with the guideline, which is relatively lower than in previously reported studies using procalcitonin guidelines. This study was larger and involved more hospitals than the other studies. Interestingly, this study did not find statistically significant differences in antibiotic usage or duration between the procalcitonin group compared to the usual-care group. While this result can be partially explained by the low rate of compliance with the guideline, the result may actually reflect the real-life pattern of procalcitonin guideline usage in clinicians. These results suggest that procalcitonin-based guidelines attempting to reduce antibiotic usage and exposure may be of low value, contrasting with findings from previous studies.
The Huang et al study is well-designed, had a low rate of follow-up loss and withdrawal, was conducted mostly at urban academic hospitals that had a high level of adherence to Joint Commission pneumonia core measures, and had appropriate statistical analyses; however, several factors should be considered when applying the results of this study to clinical practice. First, the large majority (80.1%) of the study cohort had final diagnoses of a COPD exacerbation, asthma exacerbation, or acute bronchitis. These patients had a moderate degree of disease (required hospitalization in 59% of patients with a mean hospital length of stay of 5 days), but their symptoms were severe enough for the patients to present to the emergency department. Patients with a suspected nonrespiratory infection or a milder degree of infection, especially in the ambulatory care setting, may have different antibiotic prescribing patterns. Also, patients in the ambulatory care setting likely have different causal organisms of their diagnosis. Second, this study excluded patients with severe disease who required ICU admission with either septic shock or respiratory failure, patients with pre-existing diseases that placed them at high risk (eg, immunosuppressed patients), and/or patients who had complications of their infection with either a lung abscess or empyema. This pattern of exclusion was widely similar to the other previous procalcitonin studies, which shows that procalcitonin guidelines should not be applied blindly in critically ill patients, even those not requiring ICU admission. Third, patients were excluded from the study if they were on chronic dialysis, had metastatic cancer, or had a recent surgery because of possible elevation of procalcitonin levels without a bacterial infection.
In conclusion, the current study did not find any difference in antibiotic exposure throughout the course of care (including discharge or hospitalization) of patients with a lower respiratory tract infection who presented to the emergency department when a procalcitonin guideline was implemented. The results of the current study raise questions regarding the new trend of widely accepting procalcitonin-based antibiotic usage.
Applications for Clinical Practice
Procalcitonin is a relatively new marker that is released during a systemic bacterial infection. While prior studies have supported systematic use of procalcitonin-based guidelines to initiate and discontinue antibiotics in order to limit antibiotic exposure, clinicians should be mindful that a procalcitonin antibiotic guideline may be useful in specific patients and should only be used in combination with usual clinical judgment. Clinicians must also recognize the medical conditions that may falsely elevate the procalcitonin level. Most important, the procalcitonin level should not be used as the sole indication to withhold antibiotics in an otherwise appropriately indicated clinical scenario.
—Minkyung Kwon, MD, Scott A. Helgeson, MD, and Vichaya Arunthari, MD
Pulmonary and Critical Care Medicine, Mayo Clinic Florida, Jacksonville, FL
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
1. Maruna P, Nedelnikova K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49:S57-S61.
2. Deftos LJ, Roos BA, Bronzert D, Parthemore JG. Immunochemical heterogeneity of calcitonin in plasma. J Clin Endocr Metab. 1975;40:409-412.
3. Wang JX, Zhang SM, Li XH, et al. Acute exacerbations of chronic obstructive pulmonary disease with low serum procalcitonin values do not benefit from antibiotic treatment: a prospective randomized controlled trial. Int J Infect Dis. 2016;48:40-45.
4. Corti C, Fally M, Fabricius-Bjerre A, et al. Point-of-care procalcitonin test to reduce antibiotic exposure in patients hospitalized with acute exacerbation of COPD. Int J Chron Obstruct Pulmon Dis. 2016;11:1381-1389.
5. Deliberato RO, Marra AR, Sanches PR, et al. Clinical and economic impact of procalcitonin to shorten antimicrobial therapy in septic patients with proven bacterial infection in an intensive care setting. Diagn Microbiol Infect Dis. 2013;76:266-271.
6. Najafi A, Khodadadian A, Sanatkar M, et al. The comparison of procalcitonin guidance administer antibiotics with empiric antibiotic therapy in critically ill patients admitted in intensive care unit. Acta Med Iran. 2015;53:562-567.
7. Tanaka K, Ogasawara T, Aoshima Y, et al. Procalcitonin-guided algorithm in nursing and healthcare-associated pneumonia. Respirology. 2014;19:220-220.
Hip Fracture in Nursing Home Residents with Advanced Dementia: An Opportunity for Palliative Care
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
Study Overview
Objective. To compare clinical outcomes (mortality, pain, physical restraint use, pressure ulcer, antipsychotic drug use) in long-term care nursing home (NH) residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management.
Design. A retrospective cohort study utilizing nationwide Medicare (Parts A, B, D and hospice) claims data linked with Centers for Medicare & Medicaid Services Minimum Data Set (MDS version 2.0) assessments.
Setting and participants. Long-stay NH residents older than 65 years with advanced dementia (defined as being assigned to Cognitive Performance Scale category 5 or 6 and a diagnosis of dementia or Alzheimer disease) and without a do not hospitalize (DNH) directive before hip fracture were identified by using MDS assessments completed from January 1, 2008 to December 31, 2013. Medicare (Part A – inpatient, or Part B – outpatient) claims data was then used to identify those residents who experienced a hip fracture within 2 years of the full MDS assessment using the International Classification of Diseases, Ninth Revision diagnostic codes. Procedure codes were used to determine whether a resident who experienced hip fracture underwent surgical repair.
Main outcome measures. The main outcome measure was all-cause mortality after hip fracture ascertained by the Medicare Enrollment File through 2013. The secondary outcome measures were documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status in NH residents who survived 6 months after hip fracture. These outcome measures were captured from the first MDS assessment completed between 120 and 240 days following the fracture or Medicare Part D claims. Documented pain was determined using a validated MDS 2.0 nursing assessment pain instrument within 7 days preceding MDS assessment. Physical restraint use was defined by the use of trunk, limb, or chair restraint within 7 days prior to MDS assessment. Pressure ulcer was defined as any stage 2 to 4 pressure ulcer. Antipsychotic drug use of any medication subclass was determined using Medicare Part D claims data and affirmative if drug was administered 180 days after hip fracture. Ambulatory status between 120 and 240 days following the fracture was determined in a subset of NH residents who were ambulatory before the hip fracture. The utilization of comfort-focused care after hip fracture was determined in NH residents who had a Medicare hospice claim or a new DNH directive in the 180 days after hip fracture.
The differences in survival among NH residents with advanced dementia and hip fracture were described by Kaplan-Meier curves. The association between surgical repair and survival was determined using multivariable Cox proportional hazards for all NH residents and stratified by pre-fracture ambulatory status. In those who survived 6 months after hip fracture, the associations between surgical repair and outcomes including documented pain, physical restraint use, pressure ulcers, antipsychotic drug use, and ambulatory status were determined using multivariable logistic regression models. Adjustment for differences in characteristics before hip fracture was performed using inverse probability of treatment weighting (IPTW) models.
Main results. 3083 long-stay NH residents with advanced dementia and hip fracture were included in the study. The cohort’s mean age was 84.2 ± 7.1 years, 79.2% were female (n = 2441), and 28.5% were ambulatory before hip fracture (n = 879). Of these NH residents, 84.8% (n = 2615) underwent surgical repair and 15.2% (n = 468) received nonsurgical management. At 6 months after hip fracture, mortality was 31.5% in the surgical group compared to 53.8% in the nonsurgical group. The greatest mortality difference between groups occurred in the first 30 days after hip fracture (11.5% in surgical group versus 30.6% in nonsurgical group). Surgical repair was associated with a decreased risk of death (Cox proportional hazard ratio) in the unadjusted (hazard ratio [HR], 0.55 [95% confidence interval {CI}, 0.49-0.61), multivariable adjusted (adjusted HR, 0.56 [95% CI, 0.49-0.63]), and IPTW (adjusted HR, 0.88 [95% CI, 0.79-0.98]) models. Similarly, surgically treated NH residents were less likely to die than those managed non-surgically when mortality was stratified by pre-fracture ambulatory status.
Among NH residents who survived 6 months after hip fracture, those who underwent surgical repair compared with those who received nonsurgical management had less documented pain (29.0% versus 30.9%), fewer pressure ulcers (11.2% versus 19.0%), greater physical restraint use (13.0% versus 11.1%), and greater antipsychotic drug use (29.5% versus 20.4%). In the adjusted IPTW models, surgical repair was associated with less pain (adjusted HR, 0.78 [95% CI, 0.61-0.99]) and fewer pressure ulcers (adjusted HR, 0.64 [95% CI, 0.47-0.86]).
Overall, 21.5% of NH residents utilized comfort-focused care within 6 months after hip fracture, with a mean time to utilization of hospice care of 56 ± 49 days. In those who were managed surgically, 19.3% utilized hospice care, as compared with 33.8% in those who did not receive surgical intervention. In NH residents who survived 6 months after hip fracture, only 1.1% in both groups acquired a DNH directive.
Conclusion. In older long-stay NH residents with advanced dementia and hip fracture, surgical repair was associated with lower all-cause mortality, less documented pain, and fewer pressure ulcers compared to nonsurgical management. However, adverse clinical outcomes such as pain, physical restraint use, pressure ulcers, and antipsychotic drug use were common regardless of treatment modality. The high incidence of these adverse outcomes and hazardous interventions, coupled with low utilization of comfort-focused care and DNH directive, highlight an opportunity to improve the quality of care in this vulnerable population.
Commentary
Hip fracture is very common in NH residents, with an overall incident rate of 2.3 per 100 person years and is associated with a high mortality rate of 36.2% by 6 months after fracture.1,2 Moreover, Neuman and colleagues have recently reported that among NH residents who have some degree of functional independence in locomotion prior to hip fracture, 54% either die or develop new total dependence in locomotion within 6 months of fracture and that severe cognitive impairment is a risk factor highly associated with these adverse outcomes.3 Despite this emerging knowledge, surgical repair of hip fracture remains the mainstay treatment in many NH residents in the hope of alleviating pain and improving mobility, and palliative care is considered only when patients are imminently dying or have deteriorated past the point of meaningful recovery. In cases of NH residents with advanced dementia whose life expectancy is limited and whose care goals may favor maintaining comfort, the health care proxies are frequently challenged with a difficult choice of either pursuing or foregoing surgical management—a complex medical decision to be made in the absence of sufficient evidence in this uniquely frail patient population.
The study reported by Berry and colleagues provides an important and timely investigation in examining associations of adverse clinical outcomes (mortality, pain, pressure ulcer) and hazardous interventions (physical restraint and antipsychotic drug use) in long-stay NH residents with advanced dementia and hip fracture who underwent surgical repair or nonsurgical management. The authors reported a 6-month mortality rate of 31.5% in NH residents who underwent surgical repair, an event rate similar to that reported by Neuman and colleagues. While surgical repair after hip fracture was associated with a decreased risk of death compared to nonsurgical management, high incidences of pain (29.0% to 30.9%) and pressure ulcers (11.2% to 19.0%), and frequent physical restraint use (11.1% to 13.0%) and antipsychotic drug use (20.4% to 29.5%) were noted in NH residents who survived 6 months after fracture regardless of treatment modality. These findings are consistent with the high rate of post-hip fracture functional disability previously reported by Neuman and colleagues, and highlight the trajectory of decline, frequent distressing symptoms, and prevalent use of pharmacologic and nonpharmacologic restraints in long-stay NH residents after hip fracture. Taken together, the low utilization of comfort-focused care (21.5%) and DNH directive (1.1%) in NH residents who survived 6 months suggest a missed opportunity to integrate palliative care in a patient population that stands to benefit from this intervention.
This study is the first to report the associations between hip fracture surgery and a reduction in adverse outcomes such as pain and pressure ulcer that commonly affect vulnerable NH residents with advanced dementia. This study was well designed and leveraged strengths of Medicare claims data linked with MDS assessments to capture outcome measures including pain, pressure ulcer, and restraint use that would otherwise be difficult to ascertain. However, as in all retrospective cohort design, there were limitations in this study. For instance, secondary outcomes were determined from a single time point (ie, first MDS assessment completed between 120 to 240 days following hip fracture) and thus data capture may be incomplete. Additionally, other conditions important to complex decision making in the care of frail older adults including postoperative complications (eg, delirium, infections, cardiac complications) and in-hospital mortality were not examined. Despite these limitations, this study has enhanced our understanding of the clinical course of long-term care NH residents with advanced dementia who endured hip fracture.
Applications for Clinical Practice
Patients’ goals of care should guide medical decision making in the management of hip fracture in NH residents with advanced dementia. The increased survival benefit of surgical repair of hip fracture in this patient population should be considered in the medical decision-making process if life-prolongation is preferred. However, palliative and hospice care need to be an important facet of discussion given the high rates of mortality, pain, pressure ulcer, and restraint use in this vulnerable subset of older adults.
—Fred Ko, MD, MS
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
1. Berry SD, Lee Y, Zullo AR, et al. Incidence of hip fracture in U.S. nursing homes. J Gerontol A Biol Sci Med Sci. 2016;71:1230-1234.
2. Neuman MD, Silber JH, Magaziner JS, et al. Survival and functional outcomes after hip fracture among nursing home residents. JAMA Intern Med. 2014;174:1273-1280.
3. Berry SD, Rothbaum RR, Kiel DP, et al. Association of clinical outcomes with surgical repair of hip fractures vs nonsurgical management in nursing home residents with advanced dementia. JAMA Intern Med. 2018;178:774-780.
Can the Use of Siri, Alexa, and Google Assistant for Medical Information Result in Patient Harm?
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
Study Overview
Objective. To determine the prevalence and nature of the harm that could result from patients or consumers using conversational assistants for medical information.
Design. Observational study.
Settings and participants. Participants were recruited from an online job posting site and were eligible if they were aged ≥ 21 years and were native speakers of English. There were no other eligibility requirements. Participants contacted a research assistant by phone or email, and eligibility was confirmed before scheduling the study visit and again after arrival. However, data from 4 participants was excluded after the participants disclosed that they were not native English speakers at the end of their study sessions. Participants were compensated for their time.
Each participant took part in a single 60-minute usability session. Following informed consent and administration of baseline questionnaires, each was assigned a random selection of 2 medication tasks and 1 emergency task (provided as written scenarios) to perform with each conversational assistant—Siri, Alexa, and Google Assistant—with the order of assistants and tasks counterbalanced. Before the participants completed their first task with each conversational assistant, the research assistant demonstrated how to activate the conversational assistant using a standard weather-related question, after which the participant was asked to think of a health-related question and given 5 minutes to practice interacting with the conversational assistant with their question. Participants were then asked to complete the 3 tasks in sequence, querying the conversational assistant in their own words. Tasks were considered completed either when participants stated that they had found an answer to the question or when 5 minutes had elapsed. At task completion, the research assistant asked the participant what they would do next given the information obtained during the interaction with the conversational assistant. After the participant completed the third task with a given conversational assistant, the research assistant administered the satisfaction questionnaire. After a participant finished interacting with all 3 conversational assistants, they were interviewed about their experience.
Measures and analysis. Interactions with conversational assistants were video recorded, with the audio transcribed for analysis. Since each task typically took multiple attempts before resolution or the participant gave up, usability metrics were coded at both the task and attempt level, including time, outcomes, and error analysis. Participant-reported actions for each medical task were rated for patient harm by 2 judges (an internist and a pharmacist) using a scale adapted from those used by the Agency for Healthcare Research and Quality and the US Food and Drug Administration. Scoring was based on the following values: 0 for no harm; 1 for mild harm, resulting in bodily or psychological injury; 2 for moderate harm, resulting in bodily or psychological injury adversely affecting the functional ability or quality of life; 3 for severe harm, resulting in bodily or psychological injury, including pain or disfigurement, that interferes substantially with functional ability or quality of life; and 4 was awarded in the event of death. The 2 judges first assigned ratings independently, then met to reach consensus on cases where they disagreed. Every harmful outcome was then analyzed to determine the type of error and cause of the outcome (user error, system error, or both). The satisfaction questionnaire included 6 self-report items with response values on a 7-point scale ranging from “Not at all” to “Very satisfied.”
Main results. 54 participants completed the study, with a mean age of 42 years (SD 18) and a higher representation of individuals in the 21- to 24-year-old category than the general US adult population (30% compared to 14%). Twenty-nine (54%) were female, 31 (57%) Caucasian, and 26 (50%) college educated. Most (52 [96%]) had high levels of health literacy. Only 8 (15%) reported using a conversational assistant regularly, while 22 (41%) had never used one, and 24 (44%) had tried one “a few times.” Forty-four (82%) used computers regularly.
Of the 168 tasks completed with reported actions, 49 (29.2%) could have resulted in some degree of harm, including 27 (16.1%) that could have resulted in death. An analysis of 44 cases that potentially resulted in harm yielded several recurring error scenarios, with blame attributed solely to the conversational assistant in 13 (30%) cases, to the user in 20 (46%) cases, and to both the user and the conversational assistant in the remaining 11 (25%) cases. The most common harm scenario (9 cases, (21%) is one where the participant fails to provide all the information in the task description, and the conversational assistant responds correctly to the partial query, which the user then accepts as the recommended action to take. The next most common type of harm scenario occurs when the participant provides a complete and correct utterance describing the problem and the conversational assistant responds with partial information (7 cases, 16%). Overall self-reported satisfaction with conversational assistants was neutral, with a median rating of 4 (IQR 1-6).
Outcomes by conversational assistant were significantly different (X24 = 132.2, P < 0.001). Alexa failed for most tasks (125/394 [91.9%]), resulting in significantly more attempts made but significantly fewer instances in which responses could lead to harm. Siri had the highest task completion rate (365 [77.6%]), in part because it typically displayed a list of web pages in its response that provided at least some information to the participant. However, because of this, it had the highest likelihood of causing harm for the tasks tested (27 [20.9%]). Median user satisfaction with the 3 conversational assistants was neutral, but with significant differences among them. Participants were least satisfied with Alexa and most satisfied with Siri, and stated they were most likely to follow the recommendations provided by Siri.
Qualitatively, most participants said they would use conversational assistants for medical information, but many felt they were not quite up to the task yet. When asked about their trust in the results provided by the conversational assistants, participants said they trusted Siri the most because it provided links to multiple websites in response to their queries, allowing them to choose the response that most closely matched their assumptions. They also appreciated that Siri provided a display of its speech recognition results, giving them more confidence in its responses, and allowing them to modify their query if needed. Many participants expressed frustration with the systems, but particularly Alexa.
Conclusion. Reliance on conversational assistants for actionable medical information represents a safety risk for patients and consumers. Patients should be cautioned to not use these technologies for answers to medical questions they intend to act on without further consultation from a health care provider.
Commentary
Roughly 9 in 10 American adults use the Internet,1 with the ability to easily access information through a variety of devices including smartphones, tablets, and laptop computers. This ease of access to information has played an important role in shifting how individuals access health information and interact with their health care provider.2,3 Online health information can increase patients’ knowledge of, competence with, and engagement in health care decision-making strategies. Online health information seeking can also complement and be used in synergy with provider-patient interactions. However, online health information is difficult to regulate, complicated further by the wide range of health information literacy among patients. Inaccurate or misleading health information can lead patients to make detrimental or even dangerous health decisions. These benefits and concerns similarly apply to conversational assistants like Siri (Apple), Alexa (Amazon), and Google Assistant, which are increasingly being used by patients and consumers to access medical- and health-related information. As these technologies are voice-activated, they appear to address some health literacy limitations. However, they still pose important limitations and safety risks,4 especially as conversational assistants are being perceived as a trustworthy parallel to clinical assessment and counseling systems.5
There has been little systematic research to explore potential risks of these platforms, as well as systematically characterize error types and error rates. This study aimed to determine the capabilities of widely used, general-purpose conversational assistants in responding to a broad range of medical questions when asked by laypersons in their own words and sought to conduct a systematic evaluation of the potential harm that could result from patients or consumers acting on the resulting recommendations. The study authors found that when asked questions about situations that require medical expertise, conversational assistants failed more than half of the time and led study participants to report that they would take actions that could have resulted in harm or death. Further, the authors characterized several failure modes, including errors due to misrecognition of study participant queries, study participant misunderstanding of tasks and responses by the conversation assistant, and limited understanding of the capabilities of the assistants to understand user queries. This misalignment of expectations by users that assistants can follow conversations/discourse led to frustrating experiences by some study participants.
Not only do these findings make important contributions to the literature of health information–seeking behaviors and limitations via conversational assistants, the study design highlights relevant approaches to evaluating interactions between users and conversational assistants and other voice-activated platforms. The authors designed a range of everyday task scenarios that real-life users may be experiencing and that can lead to querying home or smartphone devices to seek health- or medical-related information. These scenarios were also written with a level of real-life complexity that incorporated multiple facts to be considered for a successful resolution and the potential of harmful consequences should the correct course of action not be taken. In addition, they allowed study participants to interpret these task scenarios and query the conversational assistants in their own words, which further aligned with how users would typically interact with their devices.
However, this study also had some limitations, which the authors highlighted. Eligibility was limited to only English-speakers and the study sample was skewed towards younger, more educated individuals with high health literacy. Combined with the small convenience sample used, findings may not be generalizable to other/broader populations and further studies are needed, especially to highlight potential differences in population subgroups (eg, race/ethnicity, age, health literacy).
Applications for Clinical Practice
Because of the increased prevalence of online health-information–seeking behaviors by patients, clinicians must be prepared to adequately address, and in some cases, educate patients on the accuracy or relevance of medical/health information they find. Conversational assistants pose an important risk in health care as they incorporate natural language interfaces that can simulate and be misinterpreted as counseling systems by patients. As the authors highlight, laypersons cannot know what the full, detailed capabilities of conversational assistants are, either concerning their medical expertise or the aspects of natural language dialogue the conversational assistants can handle. Therefore, it is critical that clinicians and other providers emphasize the limitations of these technologies to patients and that any medical recommendations should be confirmed with health care professionals before they are acted on.
— Katrina F. Mateo, MPH
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
1. Pew Research Center. Demographics of Internet and Home Broadband Usage in the United States [online]. Accessed at: http://www.pewinternet.org/fact-sheet/internet-broadband/.
2. Tonsaker T, Bartlett G, Trpkov C. Health information on the Internet: gold mine or minefield? Can Fam Physician. 2014;60:407-408.
3. Tan SS-L, Goonawardene N. Internet health information seeking and the patient-physician relationship: a systematic review. J Med Internet Res. 2017;19:e9.
4. Chung H, Iorga M, Voas J, Lee S. Alexa, can I trust you? Computer (Long Beach Calif). 2017;50:100-104.
5. Miner AS, Milstein A, Hancock JT. Talking to machines about personal mental health problems. JAMA. 2017;318:1217.
Can taming inflammation help reduce aggression?
Several psychiatric disorders, including depression, schizophrenia, bipolar disorder, Alzheimer’s disease, traumatic brain injury, autism, and posttraumatic stress disorder, are associated with a dysregulated immune response and elevated levels of inflammatory biomarkers. Inflammation has long been associated with an increased risk of aggressive behavior.1,2 By taming immune system dysregulation, we might be able to more effectively reduce inflammation, and thus reduce aggression, in patients with psychiatric illness.
Inflammation and psychiatric symptoms
An overactivated immune response has been empirically correlated to the development of psychiatric symptoms. Inducing systemic inflammation has adverse effects on cognition and behavior, whereas suppressing inflammation can dramatically improve sensorium and mood. Brain regions involved in arousal and alarm are particularly susceptible to inflammation. Subcortical areas, such as the basal ganglia, and cortical circuits, such as the amygdala and anterior insula, are affected by neuroinflammation. Several modifiable factors, including a diet rich in high glycemic food, improper sleep hygiene, tobacco use, a sedentary lifestyle, obesity, and excess psychosocial stressors, can contribute to systemic inflammation and the development of psychiatric symptoms. Oral diseases, such as tooth decay, periodontitis, and gingivitis, also contribute significantly to overall inflammation.
Anti-inflammatory agents
Using nonsteroidal anti-inflammatory drugs as augmentation to standard treatments has shown promise in several psychiatric illnesses. For example, low-dose aspirin, 81 mg/d, has demonstrated reliable results as an adjunctive treatment for depression.3 Research also has shown that the use of ibuprofen may reduce the chances of individuals seeking psychiatric care.3
Individuals who are at high risk for psychosis and schizophrenia have measurable increases in inflammatory microglial activity.4 The severity of psychotic symptoms corresponds to the magnitude of the immune response; this suggests that neuroinflammation is a risk factor for psychosis, and that anti-inflammatory treatments might help prevent or ameliorate psychosis.
In a double-blind, placebo-controlled study, 70 patients diagnosed with schizophrenia who were taking an antipsychotic were randomized to adjunctive aspirin, 1,000 mg/d, or placebo.5 Participants who received aspirin had significant improvement as measured by changes in Positive and Negative Syndrome Scale total score.5
Targeting C-reactive protein
Inflammation has long been associated with impulsive aggression. C-reactive protein (CRP) is a biomarker produced in the liver in response to inflammatory triggers. In a study of 213 inpatients with schizophrenia, researchers compared 57 patients with higher levels of CRP (>1 mg/dL) with 156 patients with normal levels (<1 mg/dL).2 Compared with patients with normal CRP levels, those with higher levels displayed increased aggressive behavior. Researchers found that the chance of being physically restrained during hospitalization was almost 2.5 times greater for patients with elevated CRP levels on admission compared with those with normal CRP levels.
Statins have long been used to reduce C-reactive peptides in patients with cardiovascular conditions. The use of simvastatin has been shown to significantly reduce negative symptoms in patients with schizophrenia.6
Continue to: Vitamin C also can effectively...
Vitamin C also can effectively lower CRP levels. In a 2-month study, 396 participants with elevated CRP levels received vitamin C, 1,000 mg/d, vitamin E, 800 IU/d, or placebo.7 Although vitamin E didn’t reduce CRP levels, vitamin C reduced CRP by 25.3% compared with placebo. Vitamin C is as effective as statins in controlling this biomarker.
Several nonpharmacologic measures also can help reduce the immune system’s activation of CRP, including increased physical activity, increased intake of low glycemic food and supplemental omega-3 fatty acids, improved dental hygiene, and enhanced sleep.
Using a relatively simple and inexpensive laboratory test for measuring CRP might help predict or stratify the risk of aggressive behavior among psychiatric inpatients. For psychiatric patients with elevated inflammatory markers, the interventions described here may be useful as adjunctive treatments to help reduce aggression and injury in an inpatient setting.
1. Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158-165.
2. Barzilay R, Lobel T, Krivoy A, et al. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8-12.
3. Köhler O, Peterson L, Mors O, et al. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and Behavior. 2015;5(8):e00338. doi: 10.1002/brb3.338.
4. Bloomfield PS, Selvaraj S, Veronese M, et al. M icroglial activity in people at ultra high risk of psychosis and in schizophrenia; an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44-52.
5. Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520-527.
6. Tajik-Esmaeeli S, Moazen-Zadeh E, Abbasi N, et al. Simvastatin adjunct therapy for negative symptoms of schizophrenia: a randomized double-blind placebo-controlled trial. Int Clin Psychopharmacol. 2017;32(2):87-94.
7. Block G, Jensen CD, Dalvi TB, et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 2009;46(1):70-77.
Several psychiatric disorders, including depression, schizophrenia, bipolar disorder, Alzheimer’s disease, traumatic brain injury, autism, and posttraumatic stress disorder, are associated with a dysregulated immune response and elevated levels of inflammatory biomarkers. Inflammation has long been associated with an increased risk of aggressive behavior.1,2 By taming immune system dysregulation, we might be able to more effectively reduce inflammation, and thus reduce aggression, in patients with psychiatric illness.
Inflammation and psychiatric symptoms
An overactivated immune response has been empirically correlated to the development of psychiatric symptoms. Inducing systemic inflammation has adverse effects on cognition and behavior, whereas suppressing inflammation can dramatically improve sensorium and mood. Brain regions involved in arousal and alarm are particularly susceptible to inflammation. Subcortical areas, such as the basal ganglia, and cortical circuits, such as the amygdala and anterior insula, are affected by neuroinflammation. Several modifiable factors, including a diet rich in high glycemic food, improper sleep hygiene, tobacco use, a sedentary lifestyle, obesity, and excess psychosocial stressors, can contribute to systemic inflammation and the development of psychiatric symptoms. Oral diseases, such as tooth decay, periodontitis, and gingivitis, also contribute significantly to overall inflammation.
Anti-inflammatory agents
Using nonsteroidal anti-inflammatory drugs as augmentation to standard treatments has shown promise in several psychiatric illnesses. For example, low-dose aspirin, 81 mg/d, has demonstrated reliable results as an adjunctive treatment for depression.3 Research also has shown that the use of ibuprofen may reduce the chances of individuals seeking psychiatric care.3
Individuals who are at high risk for psychosis and schizophrenia have measurable increases in inflammatory microglial activity.4 The severity of psychotic symptoms corresponds to the magnitude of the immune response; this suggests that neuroinflammation is a risk factor for psychosis, and that anti-inflammatory treatments might help prevent or ameliorate psychosis.
In a double-blind, placebo-controlled study, 70 patients diagnosed with schizophrenia who were taking an antipsychotic were randomized to adjunctive aspirin, 1,000 mg/d, or placebo.5 Participants who received aspirin had significant improvement as measured by changes in Positive and Negative Syndrome Scale total score.5
Targeting C-reactive protein
Inflammation has long been associated with impulsive aggression. C-reactive protein (CRP) is a biomarker produced in the liver in response to inflammatory triggers. In a study of 213 inpatients with schizophrenia, researchers compared 57 patients with higher levels of CRP (>1 mg/dL) with 156 patients with normal levels (<1 mg/dL).2 Compared with patients with normal CRP levels, those with higher levels displayed increased aggressive behavior. Researchers found that the chance of being physically restrained during hospitalization was almost 2.5 times greater for patients with elevated CRP levels on admission compared with those with normal CRP levels.
Statins have long been used to reduce C-reactive peptides in patients with cardiovascular conditions. The use of simvastatin has been shown to significantly reduce negative symptoms in patients with schizophrenia.6
Continue to: Vitamin C also can effectively...
Vitamin C also can effectively lower CRP levels. In a 2-month study, 396 participants with elevated CRP levels received vitamin C, 1,000 mg/d, vitamin E, 800 IU/d, or placebo.7 Although vitamin E didn’t reduce CRP levels, vitamin C reduced CRP by 25.3% compared with placebo. Vitamin C is as effective as statins in controlling this biomarker.
Several nonpharmacologic measures also can help reduce the immune system’s activation of CRP, including increased physical activity, increased intake of low glycemic food and supplemental omega-3 fatty acids, improved dental hygiene, and enhanced sleep.
Using a relatively simple and inexpensive laboratory test for measuring CRP might help predict or stratify the risk of aggressive behavior among psychiatric inpatients. For psychiatric patients with elevated inflammatory markers, the interventions described here may be useful as adjunctive treatments to help reduce aggression and injury in an inpatient setting.
Several psychiatric disorders, including depression, schizophrenia, bipolar disorder, Alzheimer’s disease, traumatic brain injury, autism, and posttraumatic stress disorder, are associated with a dysregulated immune response and elevated levels of inflammatory biomarkers. Inflammation has long been associated with an increased risk of aggressive behavior.1,2 By taming immune system dysregulation, we might be able to more effectively reduce inflammation, and thus reduce aggression, in patients with psychiatric illness.
Inflammation and psychiatric symptoms
An overactivated immune response has been empirically correlated to the development of psychiatric symptoms. Inducing systemic inflammation has adverse effects on cognition and behavior, whereas suppressing inflammation can dramatically improve sensorium and mood. Brain regions involved in arousal and alarm are particularly susceptible to inflammation. Subcortical areas, such as the basal ganglia, and cortical circuits, such as the amygdala and anterior insula, are affected by neuroinflammation. Several modifiable factors, including a diet rich in high glycemic food, improper sleep hygiene, tobacco use, a sedentary lifestyle, obesity, and excess psychosocial stressors, can contribute to systemic inflammation and the development of psychiatric symptoms. Oral diseases, such as tooth decay, periodontitis, and gingivitis, also contribute significantly to overall inflammation.
Anti-inflammatory agents
Using nonsteroidal anti-inflammatory drugs as augmentation to standard treatments has shown promise in several psychiatric illnesses. For example, low-dose aspirin, 81 mg/d, has demonstrated reliable results as an adjunctive treatment for depression.3 Research also has shown that the use of ibuprofen may reduce the chances of individuals seeking psychiatric care.3
Individuals who are at high risk for psychosis and schizophrenia have measurable increases in inflammatory microglial activity.4 The severity of psychotic symptoms corresponds to the magnitude of the immune response; this suggests that neuroinflammation is a risk factor for psychosis, and that anti-inflammatory treatments might help prevent or ameliorate psychosis.
In a double-blind, placebo-controlled study, 70 patients diagnosed with schizophrenia who were taking an antipsychotic were randomized to adjunctive aspirin, 1,000 mg/d, or placebo.5 Participants who received aspirin had significant improvement as measured by changes in Positive and Negative Syndrome Scale total score.5
Targeting C-reactive protein
Inflammation has long been associated with impulsive aggression. C-reactive protein (CRP) is a biomarker produced in the liver in response to inflammatory triggers. In a study of 213 inpatients with schizophrenia, researchers compared 57 patients with higher levels of CRP (>1 mg/dL) with 156 patients with normal levels (<1 mg/dL).2 Compared with patients with normal CRP levels, those with higher levels displayed increased aggressive behavior. Researchers found that the chance of being physically restrained during hospitalization was almost 2.5 times greater for patients with elevated CRP levels on admission compared with those with normal CRP levels.
Statins have long been used to reduce C-reactive peptides in patients with cardiovascular conditions. The use of simvastatin has been shown to significantly reduce negative symptoms in patients with schizophrenia.6
Continue to: Vitamin C also can effectively...
Vitamin C also can effectively lower CRP levels. In a 2-month study, 396 participants with elevated CRP levels received vitamin C, 1,000 mg/d, vitamin E, 800 IU/d, or placebo.7 Although vitamin E didn’t reduce CRP levels, vitamin C reduced CRP by 25.3% compared with placebo. Vitamin C is as effective as statins in controlling this biomarker.
Several nonpharmacologic measures also can help reduce the immune system’s activation of CRP, including increased physical activity, increased intake of low glycemic food and supplemental omega-3 fatty acids, improved dental hygiene, and enhanced sleep.
Using a relatively simple and inexpensive laboratory test for measuring CRP might help predict or stratify the risk of aggressive behavior among psychiatric inpatients. For psychiatric patients with elevated inflammatory markers, the interventions described here may be useful as adjunctive treatments to help reduce aggression and injury in an inpatient setting.
1. Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158-165.
2. Barzilay R, Lobel T, Krivoy A, et al. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8-12.
3. Köhler O, Peterson L, Mors O, et al. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and Behavior. 2015;5(8):e00338. doi: 10.1002/brb3.338.
4. Bloomfield PS, Selvaraj S, Veronese M, et al. M icroglial activity in people at ultra high risk of psychosis and in schizophrenia; an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44-52.
5. Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520-527.
6. Tajik-Esmaeeli S, Moazen-Zadeh E, Abbasi N, et al. Simvastatin adjunct therapy for negative symptoms of schizophrenia: a randomized double-blind placebo-controlled trial. Int Clin Psychopharmacol. 2017;32(2):87-94.
7. Block G, Jensen CD, Dalvi TB, et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 2009;46(1):70-77.
1. Coccaro EF, Lee R, Coussons-Read M. Elevated plasma inflammatory markers in individuals with intermittent explosive disorder and correlation with aggression in humans. JAMA Psychiatry. 2014;71(2):158-165.
2. Barzilay R, Lobel T, Krivoy A, et al. Elevated C-reactive protein levels in schizophrenia inpatients is associated with aggressive behavior. Eur Psychiatry. 2016;31:8-12.
3. Köhler O, Peterson L, Mors O, et al. Inflammation and depression: combined use of selective serotonin reuptake inhibitors and NSAIDs or paracetamol and psychiatric outcomes. Brain and Behavior. 2015;5(8):e00338. doi: 10.1002/brb3.338.
4. Bloomfield PS, Selvaraj S, Veronese M, et al. M icroglial activity in people at ultra high risk of psychosis and in schizophrenia; an [11C]PBR28 PET brain imaging study. Am J Psychiatry. 2016;173(1):44-52.
5. Laan W, Grobbee DE, Selten JP, et al. Adjuvant aspirin therapy reduces symptoms of schizophrenia spectrum disorders: results from a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2010;71(5):520-527.
6. Tajik-Esmaeeli S, Moazen-Zadeh E, Abbasi N, et al. Simvastatin adjunct therapy for negative symptoms of schizophrenia: a randomized double-blind placebo-controlled trial. Int Clin Psychopharmacol. 2017;32(2):87-94.
7. Block G, Jensen CD, Dalvi TB, et al. Vitamin C treatment reduces elevated C-reactive protein. Free Radic Biol Med. 2009;46(1):70-77.
Fever, tachycardia, and tachypnea during a psychotic exacerbation
CASE Posing a threat to his family
Mr. C, age 23, who was diagnosed with schizophrenia with daily auditory hallucinations 4 years earlier, is transferred from an outside psychiatric hospital to our emergency department (ED) after developing fever, tachycardia, headache, and nasal congestion for the past day. He had been admitted to the psychiatric hospital 3 weeks ago due to concerns he was experiencing increased hallucinations and delusions and posed a threat to his sister and her children, with whom he had been living.
Mr. C tells us that while at the psychiatric hospital, he had been started on clozapine, 250 mg/d. He said that prior to clozapine, he had been taking risperidone. We are unable to confirm past treatment information with the psychiatric hospital, including exactly when the clozapine had been started or how fast it had been titrated. We also were not able to obtain information on his prior medication regimen.
In the ED, Mr. C is febrile (39.4°C; 102.9°F), tachycardic (160 beats per minute; reference range 60 to 100), and tachypneic (24 breaths per minute; reference range 12 to 20). His blood pressure is 130/68 mm Hg, and his lactate level is 2.3 mmol/L (reference range <1.9 mmol/L). After he receives 3 liters of fluid, Mr. C’s heart rate decreases to 117 and his lactate level to 1.1 mmol/L. His white blood cell count is 10.6 × 103/mm3 (reference range 4.0 to 10.0 × 103/mm3); a differential can be found in the Table. His electrocardiogram (ECG) demonstrates sinus tachycardia and a QTc of 510 ms (reference range <430 ms), but is otherwise unremarkable. His creatinine kinase (CK) level is within normal limits at 76 U/L (reference range 52 to 336 U/L). A C-reactive protein (CRP) level was not drawn at this time. Other than marijuana and cocaine use, Mr. C’s medical history is unremarkable.
Mr. C is admitted to the hospital and is started on treatment for sepsis. On the evening of Day 1, Mr. C experiences worsening tachycardia (140 beats per minute) and tachypnea (≥40 breaths per minute). His temperature increases to 103.3°F, and his blood pressure drops to 97/55 mm Hg. His troponin level is 19.0 ng/mL (reference range <0.01 ng/mL) and CK level is 491 U/L.
As Mr. C continues to deteriorate, a rapid response is called and he is placed on non-rebreather oxygen and transferred to the medical intensive care unit (MICU).
[polldaddy:10226034]
The authors’ observations
With Mr. C’s presenting symptoms, multiple conditions were included in the differential diagnosis. The initial concern was for sepsis. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Organ dysfunction is defined by a quick Sepsis-Related Organ Failure Assessment (qSOFA) score ≥2 and is associated with an increased probability of mortality (>10%). Although no infection had been identified in Mr. C, the combination of fever, altered vital signs, and elevated lactate level in the setting of a qSOFA score of 2 (for respiratory rate and blood pressure) raised suspicion enough to start empiric treatment.
With Mr. C’s subsequent deterioration on the evening of Day 1, we considered cardiopulmonary etiologies. His symptoms of dyspnea, hypotension, tachycardia, tachypnea, and fever were nonspecific and thus required consideration of multiple life-threatening etiologies. Thygesen et al2 published an expert consensus of the definition of myocardial infarction, which was of concern given our patient’s elevated troponin level. Because there was already concern for sepsis, the addition of cardiac symptoms required us to consider infectious endocarditis.3 Sudden onset of dyspnea and a drop in blood pressure were concerning for pulmonary embolism, although our patient did not have the usual risk factors (cancer, immobilization, recent surgery, etc.).4 Additionally, in light of Mr. C’s psychiatric history and recent stressors of being moved from his sister’s house and admitted to a psychiatric hospital, coupled with dyspnea and hypotension, we included Takotsubo cardiomyopathy in the differential.5,6 This disease often occurs in response to an emotional or physical stressor and is characterized by transient systolic dysfunction in the setting of ventricular wall-motion abnormalities reaching beyond the distribution of a single coronary artery. Acute ECG and biomarker findings mimic those of myocardial infarction.6
Continue to: Finally, we needed to consider...
Finally, we needed to consider the potential adverse effects of clozapine. Clozapine is a second-generation antipsychotic (SGA) used to treat patients with schizophrenia for whom other antipsychotic medications are ineffective. Clozapine has been shown to be more effective than first-generation antipsychotics (FGA) in reducing symptoms of schizophrenia.7 It has also been shown to be more effective than several SGAs, including quetiapine, risperidone, and olanzapine.7 In fact, in patients with an insufficient therapeutic response to an SGA, clozapine proves to be more effective than switching to a different SGA. As a result of more than 20 years of research, clozapine is the gold-standard for treatment-resistant schizophrenia.7 Yet despite this strong evidence supporting its use in patients with treatment-resistant schizophrenia, the medication continues to be underutilized, especially in patients at risk for suicide.7
It appears that clozapine remains a third-choice medication in the treatment of schizophrenia largely due to its serious adverse effect profile.7 The medication includes several black-box warnings, including severe neutropenia, orthostatic hypotension, bradycardia, syncope, seizures, myocarditis, cardiomyopathy, and mitral valve incompetence.8 Tachycardia, bradycardia, and orthostatic hypotension are all clozapine-related adverse effects associated with autonomic dysfunction, which can result in serious long-term cardiac complications.9 With regards to the drug’s neutropenia risk, the establishment of the Clozapine Risk Evaluation and Mitigation Strategy (REMS) program has allowed for safer use of clozapine and reduced deaths due to clozapine-induced agranulocytosis. Clinicians and pharmacists must be certified in order to prescribe clozapine, and patients must be registered and undergo frequent absolute neutrophil count (ANC) monitoring.
Clozapine-induced myocarditis, a condition observed in up to 3% of patients started on the medication,9 is more likely to develop early on during treatment, with a median time of detection of 16 days following drug initiation.10 Myocarditis often presents with nonspecific signs and symptoms that include chest pain, tachycardia, palpitations, dyspnea, fever, flu-like symptoms, and/or hypotension.
[polldaddy:10226036]
The authors’ observations
Initial workup in the MICU for Mr. C included an ABG analysis, ECG, and cardiology consult. The ABG analysis demonstrated metabolic alkalosis; his ECG demonstrated sinus tachycardia and nonspecific ST elevation in the lateral leads (Figure). The cardiology consult team started Mr. C on treatment for a non-ST-elevation myocardial infarction (NSTEMI), which it believed to be most likely due to myocarditis with secondary demand ischemia, and less likely acute coronary syndrome. The cardiology consult team also recommended performing a workup for pulmonary emboli and infectious endocarditis if Mr. C’s symptoms persist or the infectious source could not be identified.
EVALUATION Gradual improvement
Mr. C demonstrates gradual improvement as his workup continues, and clozapine is held on the recommendation of the cardiac consult team. By Day 2, he stops complaining of auditory hallucinations, and does not report their return during the rest of his stay. His troponin level decreases to 8.6 ng/mL and lactate level to 1.4 mmol/L; trending is stopped for both. The erythrocyte sedimentation rate (ESR) is elevated at 59 mm/hr (reference range 0 to 22 mm/hr), along with a CRP level of 21 mg/L (reference range <8.0 mg/L). An echocardiogram demonstrates a 40% ejection fraction (reference range 55% to 75%) and moderate global hypokinesis. The cardiology consult team is concerned for Takotsubo cardiomyopathy with sepsis as a source of adrenergic surge vs myopericarditis of viral etiology. The cardiology team also suggests continued stoppage of clozapine, because the medication can cause hypotension and tachycardia.
Continue to: On Day 3...
On Day 3, Mr. C’s ST elevation resolves on ECG, and his CK level decreases to 70 U/L, at which point trending is stopped. On Day 5, Mr. C undergoes MRI, which demonstrates an ejection fraction of 55% and confirms myocarditis. No infectious source is identified.
By Day 6, with all other sources ruled out, clozapine is confirmed as the source of myocarditis for Mr. C.
The authors’ observations
Close cardiovascular monitoring should occur during the first 4 weeks after starting clozapine because 80% of cases of clozapine-induced myocarditis occur within 4 weeks of clozapine initiation.10 Baseline CRP, troponin I/T, and vital signs should be obtained before starting clozapine.11 Vital signs must be monitored to assess for fever, tachycardia, and deviations from baseline blood pressures.11 Although eosinophil counts and percentages can also be considered in addition to a baseline CRP value, they have not proven to be sensitive or specific for clozapine-induced myocarditis.12 A baseline echocardiogram can also be obtained, but is not necessary, especially given that it may not be readily available in all clinics, and could therefore delay initiation of clozapine and limit its use. C-reactive protein and troponin levels should be assessed weekly during the first 6 weeks of clozapine therapy.11 For symptomatic patients presenting with concern for clozapine-induced myocarditis, a CRP level >100 mg/L has 100% sensitivity in detecting clozapine-induced myocarditis.13 Clozapine should also be stopped if troponins levels reach twice the upper limit of normal. More mild elevations of CRP and troponins in the setting of persistent tachycardia or signs of an infectious process should be followed by daily CRP and troponins levels until these features resolve.11
Mr. C’s case highlights clinical features that clinicians should consider when screening for myocarditis. The development of myocarditis is associated with quick titrations of clozapine during Days 1 to 9. In this case, Mr. C had recently been titrated at an outside hospital, and the time frame during which this titration occurred was unknown. Given this lack of information, the potential for a rapid titration should alert the clinician to the risk of developing myocarditis. Increased age is also associated with an increased risk of myocarditis, with a 31% increase for each decade. Further, the concomitant use of valproate sodium during the titration period also increases the risk of myocarditis 2.5-fold.14
When evaluating a patient such as Mr. C, an important clinical sign that must not be overlooked is that an elevation of body temperature of 1°C is expected to give rise to a 10-beats-per-minute increase in heart rate when the fever is the result of an infection.15 During Day 1 of his hospitalization, Mr. C was tachycardic to 160 beats per minute, with a fever of 39.4°C. Thus, his heart rate was elevated well beyond what would be expected from a fever secondary to an infectious process. This further illustrates the need to consider adverse effects caused by medication, such as clozapine-induced tachycardia.
Continue to: While clozapine had already been stopped...
While clozapine had already been stopped in Mr. C, it is conceivable that other patients would potentially continue receiving it because of the medication’s demonstrated efficacy in reducing hallucinations; however, this would result in worsening and potentially serious cardiac symptoms.
[polldaddy:10226037]
The authors’ observations
A diagnosis of clozapine-induced myocarditis should be followed by a prompt discontinuation of clozapine. Discontinuation of the drug should lead to spontaneous resolution of the myocarditis, with significantly improved left ventricular function observed within 5 days.13 Historically, rechallenging a patient with clozapine was not recommended, due to fear of recurrence of myocarditis. However, recent case studies indicate that myocarditis need not be an absolute contraindication to restarting clozapine.16 Rather, the risks must be balanced against demonstrated efficacy in patients who had a limited response to other antipsychotics, as was the case with Mr. C. For these patients, the decision to rechallenge should be made with the patient’s informed consent and involve slow dose titration and increased monitoring.17 Should this rechallenge fail, another antipsychotic plus augmentation with a mood stabilizer or ECT may be more efficacious than an antipsychotic alone.18,19
OUTCOME Return to the psychiatric hospital
On Day 8, Mr. C is medically cleared; he had not reported auditory hallucinations since Day 2. He is discharged back to the psychiatric hospital for additional medication management of his schizophrenia.
Bottom Line
Clozapine-induced myocarditis should be included in the differential diagnosis for patients who present with nonspecific complaints and have an incomplete history pertaining to clozapine use. After discontinuing clozapine, and after myocarditis symptoms resolve, consider restarting clozapine in patients who have limited response to other treatments. If rechallenging fails, another antipsychotic plus augmentation with a mood stabilizer or electroconvulsive therapy may be more efficacious than an antipsychotic alone.
Related Resources
- Clozapine Risk Evaluation and Mitigation Strategy [REMS] Program. What is the Clozapine REMS Program? https://www.clozapinerems.com.
- Keating D, McWilliams S, Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7(1):e013881.
- Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7):68.
Drug Brand Names
Clozapine • Clozaril
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate • Depacon
1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567.
3. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893.
4. Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(3):598-603.
5. Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol. 2010;55(7):700-701.
6. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
7. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
8. Clozaril [package insert]. Rosemont, PA: HLS Therapeutics (USA), Inc.; 2016.
9. Ronaldson KJ. Cardiovascular disease in clozapine-treated Patients: evidence, mechanisms and management. CNS Drugs. 2017;31(9):777-795.
10. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007;30(1):47-57.
11. Goldsmith DR, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Prim Care Companion CNS Disord. 2017;19(4): doi: 10.4088/PCC.16l02083.
12. Ronaldson KJ, Fitzgerald PB, McNeil JJ. Evolution of troponin, C-reactive protein and eosinophil count with the onset of clozapine-induced myocarditis. Aust N Z J Psychiatry. 2015;49(5):486-487.
13. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
14. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012;141(2-3):173-178.
15. Davies P, Maconochie I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg Med J. 2009;26(9):641-643.
16. Cook SC, Ferguson BA, Cotes RO, et al. Clozapine-induced myocarditis: prevention and considerations in rechallenge. Psychosomatics. 2015;56(6):685-690.
17. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry. 2012;73(2):252-254.
18. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
19. Wenzheng W, Chengcheng PU, Jiangling Jiang, et al. Efficacy and safety of treating patients with refractory schizophrenia with antipsychotic medication and adjunctive electroconvulsive therapy: a systematic review and meta-analysis. Shanghai Arch Psychiatry. 2015;27(4):206-219.
CASE Posing a threat to his family
Mr. C, age 23, who was diagnosed with schizophrenia with daily auditory hallucinations 4 years earlier, is transferred from an outside psychiatric hospital to our emergency department (ED) after developing fever, tachycardia, headache, and nasal congestion for the past day. He had been admitted to the psychiatric hospital 3 weeks ago due to concerns he was experiencing increased hallucinations and delusions and posed a threat to his sister and her children, with whom he had been living.
Mr. C tells us that while at the psychiatric hospital, he had been started on clozapine, 250 mg/d. He said that prior to clozapine, he had been taking risperidone. We are unable to confirm past treatment information with the psychiatric hospital, including exactly when the clozapine had been started or how fast it had been titrated. We also were not able to obtain information on his prior medication regimen.
In the ED, Mr. C is febrile (39.4°C; 102.9°F), tachycardic (160 beats per minute; reference range 60 to 100), and tachypneic (24 breaths per minute; reference range 12 to 20). His blood pressure is 130/68 mm Hg, and his lactate level is 2.3 mmol/L (reference range <1.9 mmol/L). After he receives 3 liters of fluid, Mr. C’s heart rate decreases to 117 and his lactate level to 1.1 mmol/L. His white blood cell count is 10.6 × 103/mm3 (reference range 4.0 to 10.0 × 103/mm3); a differential can be found in the Table. His electrocardiogram (ECG) demonstrates sinus tachycardia and a QTc of 510 ms (reference range <430 ms), but is otherwise unremarkable. His creatinine kinase (CK) level is within normal limits at 76 U/L (reference range 52 to 336 U/L). A C-reactive protein (CRP) level was not drawn at this time. Other than marijuana and cocaine use, Mr. C’s medical history is unremarkable.
Mr. C is admitted to the hospital and is started on treatment for sepsis. On the evening of Day 1, Mr. C experiences worsening tachycardia (140 beats per minute) and tachypnea (≥40 breaths per minute). His temperature increases to 103.3°F, and his blood pressure drops to 97/55 mm Hg. His troponin level is 19.0 ng/mL (reference range <0.01 ng/mL) and CK level is 491 U/L.
As Mr. C continues to deteriorate, a rapid response is called and he is placed on non-rebreather oxygen and transferred to the medical intensive care unit (MICU).
[polldaddy:10226034]
The authors’ observations
With Mr. C’s presenting symptoms, multiple conditions were included in the differential diagnosis. The initial concern was for sepsis. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Organ dysfunction is defined by a quick Sepsis-Related Organ Failure Assessment (qSOFA) score ≥2 and is associated with an increased probability of mortality (>10%). Although no infection had been identified in Mr. C, the combination of fever, altered vital signs, and elevated lactate level in the setting of a qSOFA score of 2 (for respiratory rate and blood pressure) raised suspicion enough to start empiric treatment.
With Mr. C’s subsequent deterioration on the evening of Day 1, we considered cardiopulmonary etiologies. His symptoms of dyspnea, hypotension, tachycardia, tachypnea, and fever were nonspecific and thus required consideration of multiple life-threatening etiologies. Thygesen et al2 published an expert consensus of the definition of myocardial infarction, which was of concern given our patient’s elevated troponin level. Because there was already concern for sepsis, the addition of cardiac symptoms required us to consider infectious endocarditis.3 Sudden onset of dyspnea and a drop in blood pressure were concerning for pulmonary embolism, although our patient did not have the usual risk factors (cancer, immobilization, recent surgery, etc.).4 Additionally, in light of Mr. C’s psychiatric history and recent stressors of being moved from his sister’s house and admitted to a psychiatric hospital, coupled with dyspnea and hypotension, we included Takotsubo cardiomyopathy in the differential.5,6 This disease often occurs in response to an emotional or physical stressor and is characterized by transient systolic dysfunction in the setting of ventricular wall-motion abnormalities reaching beyond the distribution of a single coronary artery. Acute ECG and biomarker findings mimic those of myocardial infarction.6
Continue to: Finally, we needed to consider...
Finally, we needed to consider the potential adverse effects of clozapine. Clozapine is a second-generation antipsychotic (SGA) used to treat patients with schizophrenia for whom other antipsychotic medications are ineffective. Clozapine has been shown to be more effective than first-generation antipsychotics (FGA) in reducing symptoms of schizophrenia.7 It has also been shown to be more effective than several SGAs, including quetiapine, risperidone, and olanzapine.7 In fact, in patients with an insufficient therapeutic response to an SGA, clozapine proves to be more effective than switching to a different SGA. As a result of more than 20 years of research, clozapine is the gold-standard for treatment-resistant schizophrenia.7 Yet despite this strong evidence supporting its use in patients with treatment-resistant schizophrenia, the medication continues to be underutilized, especially in patients at risk for suicide.7
It appears that clozapine remains a third-choice medication in the treatment of schizophrenia largely due to its serious adverse effect profile.7 The medication includes several black-box warnings, including severe neutropenia, orthostatic hypotension, bradycardia, syncope, seizures, myocarditis, cardiomyopathy, and mitral valve incompetence.8 Tachycardia, bradycardia, and orthostatic hypotension are all clozapine-related adverse effects associated with autonomic dysfunction, which can result in serious long-term cardiac complications.9 With regards to the drug’s neutropenia risk, the establishment of the Clozapine Risk Evaluation and Mitigation Strategy (REMS) program has allowed for safer use of clozapine and reduced deaths due to clozapine-induced agranulocytosis. Clinicians and pharmacists must be certified in order to prescribe clozapine, and patients must be registered and undergo frequent absolute neutrophil count (ANC) monitoring.
Clozapine-induced myocarditis, a condition observed in up to 3% of patients started on the medication,9 is more likely to develop early on during treatment, with a median time of detection of 16 days following drug initiation.10 Myocarditis often presents with nonspecific signs and symptoms that include chest pain, tachycardia, palpitations, dyspnea, fever, flu-like symptoms, and/or hypotension.
[polldaddy:10226036]
The authors’ observations
Initial workup in the MICU for Mr. C included an ABG analysis, ECG, and cardiology consult. The ABG analysis demonstrated metabolic alkalosis; his ECG demonstrated sinus tachycardia and nonspecific ST elevation in the lateral leads (Figure). The cardiology consult team started Mr. C on treatment for a non-ST-elevation myocardial infarction (NSTEMI), which it believed to be most likely due to myocarditis with secondary demand ischemia, and less likely acute coronary syndrome. The cardiology consult team also recommended performing a workup for pulmonary emboli and infectious endocarditis if Mr. C’s symptoms persist or the infectious source could not be identified.
EVALUATION Gradual improvement
Mr. C demonstrates gradual improvement as his workup continues, and clozapine is held on the recommendation of the cardiac consult team. By Day 2, he stops complaining of auditory hallucinations, and does not report their return during the rest of his stay. His troponin level decreases to 8.6 ng/mL and lactate level to 1.4 mmol/L; trending is stopped for both. The erythrocyte sedimentation rate (ESR) is elevated at 59 mm/hr (reference range 0 to 22 mm/hr), along with a CRP level of 21 mg/L (reference range <8.0 mg/L). An echocardiogram demonstrates a 40% ejection fraction (reference range 55% to 75%) and moderate global hypokinesis. The cardiology consult team is concerned for Takotsubo cardiomyopathy with sepsis as a source of adrenergic surge vs myopericarditis of viral etiology. The cardiology team also suggests continued stoppage of clozapine, because the medication can cause hypotension and tachycardia.
Continue to: On Day 3...
On Day 3, Mr. C’s ST elevation resolves on ECG, and his CK level decreases to 70 U/L, at which point trending is stopped. On Day 5, Mr. C undergoes MRI, which demonstrates an ejection fraction of 55% and confirms myocarditis. No infectious source is identified.
By Day 6, with all other sources ruled out, clozapine is confirmed as the source of myocarditis for Mr. C.
The authors’ observations
Close cardiovascular monitoring should occur during the first 4 weeks after starting clozapine because 80% of cases of clozapine-induced myocarditis occur within 4 weeks of clozapine initiation.10 Baseline CRP, troponin I/T, and vital signs should be obtained before starting clozapine.11 Vital signs must be monitored to assess for fever, tachycardia, and deviations from baseline blood pressures.11 Although eosinophil counts and percentages can also be considered in addition to a baseline CRP value, they have not proven to be sensitive or specific for clozapine-induced myocarditis.12 A baseline echocardiogram can also be obtained, but is not necessary, especially given that it may not be readily available in all clinics, and could therefore delay initiation of clozapine and limit its use. C-reactive protein and troponin levels should be assessed weekly during the first 6 weeks of clozapine therapy.11 For symptomatic patients presenting with concern for clozapine-induced myocarditis, a CRP level >100 mg/L has 100% sensitivity in detecting clozapine-induced myocarditis.13 Clozapine should also be stopped if troponins levels reach twice the upper limit of normal. More mild elevations of CRP and troponins in the setting of persistent tachycardia or signs of an infectious process should be followed by daily CRP and troponins levels until these features resolve.11
Mr. C’s case highlights clinical features that clinicians should consider when screening for myocarditis. The development of myocarditis is associated with quick titrations of clozapine during Days 1 to 9. In this case, Mr. C had recently been titrated at an outside hospital, and the time frame during which this titration occurred was unknown. Given this lack of information, the potential for a rapid titration should alert the clinician to the risk of developing myocarditis. Increased age is also associated with an increased risk of myocarditis, with a 31% increase for each decade. Further, the concomitant use of valproate sodium during the titration period also increases the risk of myocarditis 2.5-fold.14
When evaluating a patient such as Mr. C, an important clinical sign that must not be overlooked is that an elevation of body temperature of 1°C is expected to give rise to a 10-beats-per-minute increase in heart rate when the fever is the result of an infection.15 During Day 1 of his hospitalization, Mr. C was tachycardic to 160 beats per minute, with a fever of 39.4°C. Thus, his heart rate was elevated well beyond what would be expected from a fever secondary to an infectious process. This further illustrates the need to consider adverse effects caused by medication, such as clozapine-induced tachycardia.
Continue to: While clozapine had already been stopped...
While clozapine had already been stopped in Mr. C, it is conceivable that other patients would potentially continue receiving it because of the medication’s demonstrated efficacy in reducing hallucinations; however, this would result in worsening and potentially serious cardiac symptoms.
[polldaddy:10226037]
The authors’ observations
A diagnosis of clozapine-induced myocarditis should be followed by a prompt discontinuation of clozapine. Discontinuation of the drug should lead to spontaneous resolution of the myocarditis, with significantly improved left ventricular function observed within 5 days.13 Historically, rechallenging a patient with clozapine was not recommended, due to fear of recurrence of myocarditis. However, recent case studies indicate that myocarditis need not be an absolute contraindication to restarting clozapine.16 Rather, the risks must be balanced against demonstrated efficacy in patients who had a limited response to other antipsychotics, as was the case with Mr. C. For these patients, the decision to rechallenge should be made with the patient’s informed consent and involve slow dose titration and increased monitoring.17 Should this rechallenge fail, another antipsychotic plus augmentation with a mood stabilizer or ECT may be more efficacious than an antipsychotic alone.18,19
OUTCOME Return to the psychiatric hospital
On Day 8, Mr. C is medically cleared; he had not reported auditory hallucinations since Day 2. He is discharged back to the psychiatric hospital for additional medication management of his schizophrenia.
Bottom Line
Clozapine-induced myocarditis should be included in the differential diagnosis for patients who present with nonspecific complaints and have an incomplete history pertaining to clozapine use. After discontinuing clozapine, and after myocarditis symptoms resolve, consider restarting clozapine in patients who have limited response to other treatments. If rechallenging fails, another antipsychotic plus augmentation with a mood stabilizer or electroconvulsive therapy may be more efficacious than an antipsychotic alone.
Related Resources
- Clozapine Risk Evaluation and Mitigation Strategy [REMS] Program. What is the Clozapine REMS Program? https://www.clozapinerems.com.
- Keating D, McWilliams S, Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7(1):e013881.
- Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7):68.
Drug Brand Names
Clozapine • Clozaril
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate • Depacon
CASE Posing a threat to his family
Mr. C, age 23, who was diagnosed with schizophrenia with daily auditory hallucinations 4 years earlier, is transferred from an outside psychiatric hospital to our emergency department (ED) after developing fever, tachycardia, headache, and nasal congestion for the past day. He had been admitted to the psychiatric hospital 3 weeks ago due to concerns he was experiencing increased hallucinations and delusions and posed a threat to his sister and her children, with whom he had been living.
Mr. C tells us that while at the psychiatric hospital, he had been started on clozapine, 250 mg/d. He said that prior to clozapine, he had been taking risperidone. We are unable to confirm past treatment information with the psychiatric hospital, including exactly when the clozapine had been started or how fast it had been titrated. We also were not able to obtain information on his prior medication regimen.
In the ED, Mr. C is febrile (39.4°C; 102.9°F), tachycardic (160 beats per minute; reference range 60 to 100), and tachypneic (24 breaths per minute; reference range 12 to 20). His blood pressure is 130/68 mm Hg, and his lactate level is 2.3 mmol/L (reference range <1.9 mmol/L). After he receives 3 liters of fluid, Mr. C’s heart rate decreases to 117 and his lactate level to 1.1 mmol/L. His white blood cell count is 10.6 × 103/mm3 (reference range 4.0 to 10.0 × 103/mm3); a differential can be found in the Table. His electrocardiogram (ECG) demonstrates sinus tachycardia and a QTc of 510 ms (reference range <430 ms), but is otherwise unremarkable. His creatinine kinase (CK) level is within normal limits at 76 U/L (reference range 52 to 336 U/L). A C-reactive protein (CRP) level was not drawn at this time. Other than marijuana and cocaine use, Mr. C’s medical history is unremarkable.
Mr. C is admitted to the hospital and is started on treatment for sepsis. On the evening of Day 1, Mr. C experiences worsening tachycardia (140 beats per minute) and tachypnea (≥40 breaths per minute). His temperature increases to 103.3°F, and his blood pressure drops to 97/55 mm Hg. His troponin level is 19.0 ng/mL (reference range <0.01 ng/mL) and CK level is 491 U/L.
As Mr. C continues to deteriorate, a rapid response is called and he is placed on non-rebreather oxygen and transferred to the medical intensive care unit (MICU).
[polldaddy:10226034]
The authors’ observations
With Mr. C’s presenting symptoms, multiple conditions were included in the differential diagnosis. The initial concern was for sepsis. Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Organ dysfunction is defined by a quick Sepsis-Related Organ Failure Assessment (qSOFA) score ≥2 and is associated with an increased probability of mortality (>10%). Although no infection had been identified in Mr. C, the combination of fever, altered vital signs, and elevated lactate level in the setting of a qSOFA score of 2 (for respiratory rate and blood pressure) raised suspicion enough to start empiric treatment.
With Mr. C’s subsequent deterioration on the evening of Day 1, we considered cardiopulmonary etiologies. His symptoms of dyspnea, hypotension, tachycardia, tachypnea, and fever were nonspecific and thus required consideration of multiple life-threatening etiologies. Thygesen et al2 published an expert consensus of the definition of myocardial infarction, which was of concern given our patient’s elevated troponin level. Because there was already concern for sepsis, the addition of cardiac symptoms required us to consider infectious endocarditis.3 Sudden onset of dyspnea and a drop in blood pressure were concerning for pulmonary embolism, although our patient did not have the usual risk factors (cancer, immobilization, recent surgery, etc.).4 Additionally, in light of Mr. C’s psychiatric history and recent stressors of being moved from his sister’s house and admitted to a psychiatric hospital, coupled with dyspnea and hypotension, we included Takotsubo cardiomyopathy in the differential.5,6 This disease often occurs in response to an emotional or physical stressor and is characterized by transient systolic dysfunction in the setting of ventricular wall-motion abnormalities reaching beyond the distribution of a single coronary artery. Acute ECG and biomarker findings mimic those of myocardial infarction.6
Continue to: Finally, we needed to consider...
Finally, we needed to consider the potential adverse effects of clozapine. Clozapine is a second-generation antipsychotic (SGA) used to treat patients with schizophrenia for whom other antipsychotic medications are ineffective. Clozapine has been shown to be more effective than first-generation antipsychotics (FGA) in reducing symptoms of schizophrenia.7 It has also been shown to be more effective than several SGAs, including quetiapine, risperidone, and olanzapine.7 In fact, in patients with an insufficient therapeutic response to an SGA, clozapine proves to be more effective than switching to a different SGA. As a result of more than 20 years of research, clozapine is the gold-standard for treatment-resistant schizophrenia.7 Yet despite this strong evidence supporting its use in patients with treatment-resistant schizophrenia, the medication continues to be underutilized, especially in patients at risk for suicide.7
It appears that clozapine remains a third-choice medication in the treatment of schizophrenia largely due to its serious adverse effect profile.7 The medication includes several black-box warnings, including severe neutropenia, orthostatic hypotension, bradycardia, syncope, seizures, myocarditis, cardiomyopathy, and mitral valve incompetence.8 Tachycardia, bradycardia, and orthostatic hypotension are all clozapine-related adverse effects associated with autonomic dysfunction, which can result in serious long-term cardiac complications.9 With regards to the drug’s neutropenia risk, the establishment of the Clozapine Risk Evaluation and Mitigation Strategy (REMS) program has allowed for safer use of clozapine and reduced deaths due to clozapine-induced agranulocytosis. Clinicians and pharmacists must be certified in order to prescribe clozapine, and patients must be registered and undergo frequent absolute neutrophil count (ANC) monitoring.
Clozapine-induced myocarditis, a condition observed in up to 3% of patients started on the medication,9 is more likely to develop early on during treatment, with a median time of detection of 16 days following drug initiation.10 Myocarditis often presents with nonspecific signs and symptoms that include chest pain, tachycardia, palpitations, dyspnea, fever, flu-like symptoms, and/or hypotension.
[polldaddy:10226036]
The authors’ observations
Initial workup in the MICU for Mr. C included an ABG analysis, ECG, and cardiology consult. The ABG analysis demonstrated metabolic alkalosis; his ECG demonstrated sinus tachycardia and nonspecific ST elevation in the lateral leads (Figure). The cardiology consult team started Mr. C on treatment for a non-ST-elevation myocardial infarction (NSTEMI), which it believed to be most likely due to myocarditis with secondary demand ischemia, and less likely acute coronary syndrome. The cardiology consult team also recommended performing a workup for pulmonary emboli and infectious endocarditis if Mr. C’s symptoms persist or the infectious source could not be identified.
EVALUATION Gradual improvement
Mr. C demonstrates gradual improvement as his workup continues, and clozapine is held on the recommendation of the cardiac consult team. By Day 2, he stops complaining of auditory hallucinations, and does not report their return during the rest of his stay. His troponin level decreases to 8.6 ng/mL and lactate level to 1.4 mmol/L; trending is stopped for both. The erythrocyte sedimentation rate (ESR) is elevated at 59 mm/hr (reference range 0 to 22 mm/hr), along with a CRP level of 21 mg/L (reference range <8.0 mg/L). An echocardiogram demonstrates a 40% ejection fraction (reference range 55% to 75%) and moderate global hypokinesis. The cardiology consult team is concerned for Takotsubo cardiomyopathy with sepsis as a source of adrenergic surge vs myopericarditis of viral etiology. The cardiology team also suggests continued stoppage of clozapine, because the medication can cause hypotension and tachycardia.
Continue to: On Day 3...
On Day 3, Mr. C’s ST elevation resolves on ECG, and his CK level decreases to 70 U/L, at which point trending is stopped. On Day 5, Mr. C undergoes MRI, which demonstrates an ejection fraction of 55% and confirms myocarditis. No infectious source is identified.
By Day 6, with all other sources ruled out, clozapine is confirmed as the source of myocarditis for Mr. C.
The authors’ observations
Close cardiovascular monitoring should occur during the first 4 weeks after starting clozapine because 80% of cases of clozapine-induced myocarditis occur within 4 weeks of clozapine initiation.10 Baseline CRP, troponin I/T, and vital signs should be obtained before starting clozapine.11 Vital signs must be monitored to assess for fever, tachycardia, and deviations from baseline blood pressures.11 Although eosinophil counts and percentages can also be considered in addition to a baseline CRP value, they have not proven to be sensitive or specific for clozapine-induced myocarditis.12 A baseline echocardiogram can also be obtained, but is not necessary, especially given that it may not be readily available in all clinics, and could therefore delay initiation of clozapine and limit its use. C-reactive protein and troponin levels should be assessed weekly during the first 6 weeks of clozapine therapy.11 For symptomatic patients presenting with concern for clozapine-induced myocarditis, a CRP level >100 mg/L has 100% sensitivity in detecting clozapine-induced myocarditis.13 Clozapine should also be stopped if troponins levels reach twice the upper limit of normal. More mild elevations of CRP and troponins in the setting of persistent tachycardia or signs of an infectious process should be followed by daily CRP and troponins levels until these features resolve.11
Mr. C’s case highlights clinical features that clinicians should consider when screening for myocarditis. The development of myocarditis is associated with quick titrations of clozapine during Days 1 to 9. In this case, Mr. C had recently been titrated at an outside hospital, and the time frame during which this titration occurred was unknown. Given this lack of information, the potential for a rapid titration should alert the clinician to the risk of developing myocarditis. Increased age is also associated with an increased risk of myocarditis, with a 31% increase for each decade. Further, the concomitant use of valproate sodium during the titration period also increases the risk of myocarditis 2.5-fold.14
When evaluating a patient such as Mr. C, an important clinical sign that must not be overlooked is that an elevation of body temperature of 1°C is expected to give rise to a 10-beats-per-minute increase in heart rate when the fever is the result of an infection.15 During Day 1 of his hospitalization, Mr. C was tachycardic to 160 beats per minute, with a fever of 39.4°C. Thus, his heart rate was elevated well beyond what would be expected from a fever secondary to an infectious process. This further illustrates the need to consider adverse effects caused by medication, such as clozapine-induced tachycardia.
Continue to: While clozapine had already been stopped...
While clozapine had already been stopped in Mr. C, it is conceivable that other patients would potentially continue receiving it because of the medication’s demonstrated efficacy in reducing hallucinations; however, this would result in worsening and potentially serious cardiac symptoms.
[polldaddy:10226037]
The authors’ observations
A diagnosis of clozapine-induced myocarditis should be followed by a prompt discontinuation of clozapine. Discontinuation of the drug should lead to spontaneous resolution of the myocarditis, with significantly improved left ventricular function observed within 5 days.13 Historically, rechallenging a patient with clozapine was not recommended, due to fear of recurrence of myocarditis. However, recent case studies indicate that myocarditis need not be an absolute contraindication to restarting clozapine.16 Rather, the risks must be balanced against demonstrated efficacy in patients who had a limited response to other antipsychotics, as was the case with Mr. C. For these patients, the decision to rechallenge should be made with the patient’s informed consent and involve slow dose titration and increased monitoring.17 Should this rechallenge fail, another antipsychotic plus augmentation with a mood stabilizer or ECT may be more efficacious than an antipsychotic alone.18,19
OUTCOME Return to the psychiatric hospital
On Day 8, Mr. C is medically cleared; he had not reported auditory hallucinations since Day 2. He is discharged back to the psychiatric hospital for additional medication management of his schizophrenia.
Bottom Line
Clozapine-induced myocarditis should be included in the differential diagnosis for patients who present with nonspecific complaints and have an incomplete history pertaining to clozapine use. After discontinuing clozapine, and after myocarditis symptoms resolve, consider restarting clozapine in patients who have limited response to other treatments. If rechallenging fails, another antipsychotic plus augmentation with a mood stabilizer or electroconvulsive therapy may be more efficacious than an antipsychotic alone.
Related Resources
- Clozapine Risk Evaluation and Mitigation Strategy [REMS] Program. What is the Clozapine REMS Program? https://www.clozapinerems.com.
- Keating D, McWilliams S, Schneider I, et al. Pharmacological guidelines for schizophrenia: a systematic review and comparison of recommendations for the first episode. BMJ Open. 2017;7(1):e013881.
- Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7):68.
Drug Brand Names
Clozapine • Clozaril
Olanzapine • Zyprexa
Quetiapine • Seroquel
Risperidone • Risperdal
Valproate • Depacon
1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567.
3. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893.
4. Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(3):598-603.
5. Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol. 2010;55(7):700-701.
6. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
7. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
8. Clozaril [package insert]. Rosemont, PA: HLS Therapeutics (USA), Inc.; 2016.
9. Ronaldson KJ. Cardiovascular disease in clozapine-treated Patients: evidence, mechanisms and management. CNS Drugs. 2017;31(9):777-795.
10. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007;30(1):47-57.
11. Goldsmith DR, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Prim Care Companion CNS Disord. 2017;19(4): doi: 10.4088/PCC.16l02083.
12. Ronaldson KJ, Fitzgerald PB, McNeil JJ. Evolution of troponin, C-reactive protein and eosinophil count with the onset of clozapine-induced myocarditis. Aust N Z J Psychiatry. 2015;49(5):486-487.
13. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
14. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012;141(2-3):173-178.
15. Davies P, Maconochie I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg Med J. 2009;26(9):641-643.
16. Cook SC, Ferguson BA, Cotes RO, et al. Clozapine-induced myocarditis: prevention and considerations in rechallenge. Psychosomatics. 2015;56(6):685-690.
17. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry. 2012;73(2):252-254.
18. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
19. Wenzheng W, Chengcheng PU, Jiangling Jiang, et al. Efficacy and safety of treating patients with refractory schizophrenia with antipsychotic medication and adjunctive electroconvulsive therapy: a systematic review and meta-analysis. Shanghai Arch Psychiatry. 2015;27(4):206-219.
1. Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810.
2. Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551-2567.
3. Cahill TJ, Prendergast BD. Infective endocarditis. Lancet. 2016;387(10021):882-893.
4. Stein PD, Terrin ML, Hales CA, et al. Clinical, laboratory, roentgenographic, and electrocardiographic findings in patients with acute pulmonary embolism and no pre-existing cardiac or pulmonary disease. Chest. 1991;100(3):598-603.
5. Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (tako-tsubo/stress-induced cardiomyopathy): potential pre-disposing factors? J Am Coll Cardiol. 2010;55(7):700-701.
6. Templin C, Ghadri JR, Diekmann J, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(10):929-938.
7. Warnez S, Alessi-Severini S. Clozapine: a review of clinical practice guidelines and prescribing trends. BMC Psychiatry. 2014;14:102.
8. Clozaril [package insert]. Rosemont, PA: HLS Therapeutics (USA), Inc.; 2016.
9. Ronaldson KJ. Cardiovascular disease in clozapine-treated Patients: evidence, mechanisms and management. CNS Drugs. 2017;31(9):777-795.
10. Haas SJ, Hill R, Krum H, et al. Clozapine-associated myocarditis: a review of 116 cases of suspected myocarditis associated with the use of clozapine in Australia during 1993-2003. Drug Saf. 2007;30(1):47-57.
11. Goldsmith DR, Cotes RO. An unmet need: a clozapine-induced myocarditis screening protocol. Prim Care Companion CNS Disord. 2017;19(4): doi: 10.4088/PCC.16l02083.
12. Ronaldson KJ, Fitzgerald PB, McNeil JJ. Evolution of troponin, C-reactive protein and eosinophil count with the onset of clozapine-induced myocarditis. Aust N Z J Psychiatry. 2015;49(5):486-487.
13. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. A new monitoring protocol for clozapine-induced myocarditis based on an analysis of 75 cases and 94 controls. Aust N Z J Psychiatry. 2011;45(6):458-465.
14. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Rapid clozapine dose titration and concomitant sodium valproate increase the risk of myocarditis with clozapine: a case-control study. Schizophr Res. 2012;141(2-3):173-178.
15. Davies P, Maconochie I. The relationship between body temperature, heart rate and respiratory rate in children. Emerg Med J. 2009;26(9):641-643.
16. Cook SC, Ferguson BA, Cotes RO, et al. Clozapine-induced myocarditis: prevention and considerations in rechallenge. Psychosomatics. 2015;56(6):685-690.
17. Ronaldson KJ, Fitzgerald PB, Taylor AJ, et al. Observations from 8 cases of clozapine rechallenge after development of myocarditis. J Clin Psychiatry. 2012;73(2):252-254.
18. Singh SP, Singh V, Kar N, et al. Efficacy of antidepressants in treating the negative symptoms of chronic schizophrenia: meta-analysis. Br J Psychiatry. 2010;197(3):174-179.
19. Wenzheng W, Chengcheng PU, Jiangling Jiang, et al. Efficacy and safety of treating patients with refractory schizophrenia with antipsychotic medication and adjunctive electroconvulsive therapy: a systematic review and meta-analysis. Shanghai Arch Psychiatry. 2015;27(4):206-219.
Psychotropic-induced hyponatremia
Hyponatremia is a common, multifactorial clinical condition. Hyponatremia is usually defined as a plasma sodium level <135 mmol/L; however, some studies define it as a level <130 mmol/L. Hyponatremia results from the inability of the kidney to excrete a sufficient amount of fluid, or is due to excessive fluid intake. Increases in osmolality stimulate thirst and result in increased fluid intake. This increase in osmolality is recognized by the osmoreceptors located in the hypothalamus, which release antidiuretic hormone (ADH). Antidiuretic hormone works on the collecting ducts within the kidneys, triggering increased fluid reabsorption resulting in decreased fluid loss and a reduction in thirst.
The syndrome of inappropriate antidiuretic hormone (SIADH) occurs when there is persistent ADH stimulation resulting in hyponatremia. SIADH commonly presents as euvolemic hyponatremia. Common diagnostic criteria for SIADH are listed in Table 1.1
Medications are a major cause of SIADH, and psychotropics are a primary offender. Most of the data for drug-induced SIADH come from case reports and small case series, such as those described in Table 2.2-4 The extent to which each psychotropic class causes SIADH remains unknown. In this article, we focus on 3 classes of psychotropics, and their role in causing SIADH.
Antidepressants
There is a fair amount of data associating antidepressants with SIADH. The incidence of SIADH with selective serotonin reuptake inhibitors (SSRIs) varies greatly among studies, from .06% to 40%.5-12 This wide variation is due to the way each study defined hyponatremia. A higher incidence was found when hyponatremia was defined as <135 mmol/L as opposed to <130 mmol/L. A large cohort study of SSRIs found that there was an increased risk with fluoxetine, escitalopram, and citalopram (.078% to .085%) vs paroxetine and sertraline (.033% to .053%).13 Studies comparing the incidence of SIADH with SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) found that the rates were equal or slightly higher with the SNRI venlafaxine.13 SNRIs as a group have an estimated incidence of .08% to 4%, based on studies that defined hyponatremia as <130 mmol/L.13,14 Tricyclic antidepressants have an estimated incidence of .005% to 16.7%, based on a retrospective study that reviewed 15 studies and 100 case reports.15 Mirtazapine and bupropion do not have enough evidence to obtain a true definition of incidence; case reports for these drugs suggest a causal link for hyponatremia. Table 37,9,12-15 provides an overview of the incidence rate of hyponatremia for select antidepressants. It is clear that a more stringent cutoff for hyponatremia (<130 mmol/L) reduces the incidence rates. More evidence is needed to identify the true incidence and prevalence of SIADH with these agents.
Antipsychotics
Compared with antidepressants, there’s less evidence linking SIADH with antipsychotics; this data come mainly from case reports and observational studies. Serrano et al16 reported on a cross-sectional study that included 88 patients receiving clozapine, 61 patients receiving other atypical antipsychotics, 23 patients receiving typical antipsychotics, and 11 patients receiving both typical and atypical antipsychotics. They reported incidence rates of 3.4% for clozapine, 4.9% for atypical antipsychotics, 26.1% for typical antipsychotics, and 9.1% for the group receiving both typical and atypical antipsychotics.16 The primary theory for the decreased incidence of SIADH with use of atypical antipsychotics is related to decreased rates of psychogenic polydipsia leading to lower incidence of hyponatremia.
Mood stabilizers
Several studies have associated carbamazepine/oxcarbazepine, valproic acid, and lamotrigine with SIADH.17-23 Studies show incidence rates ranging from 4.8% to 41.5% for these medications. Carbamazepine appears to have the highest incidence of SIADH. A limitation of these studies is the small sample sizes, which ranged from 12 to 60 participants.
Pathophysiology
The kidneys are responsible for maintaining homeostasis between bodily fluids and serum sodium levels. ADH, which is produced by the hypothalamus, plays a significant role in fluid balance, thirst, and fluid retention. Inappropriate and continuous secretion of ADH, despite normal or high fluid status, results in hyposmolality and hyponatremia. The specific mechanisms by which psychotropic medications cause SIADH are listed in Table 4.24
Diagnosis
Diagnosis of SIADH can be complex because there are many clinical reasons a patient may have hyponatremia. For example, SIADH and psychogenic polydipsia both result in hyponatremia, and sometimes the 2 conditions can be difficult to distinguish. Hyponatremia is typically discovered by routine blood testing if the patient is asymptomatic. Table 525 highlights the major laboratory markers that distinguish SIADH and psychogenic polydipsia.
Continue to: Treatment
Treatment
The primary treatment for SIADH is cessation of the offending agent. Based on the patient’s clinical presentation, free water restriction (.5 to 1 L/d) can be implemented to increase serum sodium levels. If the patient is having neurologic complications due to the severity of hyponatremia, correction with hypertonic saline is indicated. Upon resolution, the recommended course of action is to switch to a medication in a different class. Re-challenging the patient with the same medication is not recommended unless there is no other alternative class of medication.24 Table 626 highlights other causes of hyponatremia, what laboratory markers to assess, and how to treat high-risk individuals.
Hyponatremia is a complex medical complication that can be life-threatening. Psychotropics are a relatively common cause of hyponatremia, specifically SIADH. Older adults appear to be at highest risk, as most case reports are in patients age ≥65. Patients who are prescribed psychotropics should be treated with the lowest effective dose and monitored for signs and symptoms of hyponatremia throughout therapy.
Related Resources
- Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guidelines on diagnosis and treatment of hyponatremia. Eur J Endocrinol. 2014;170(3):G1-G47.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-S42.
Drug Brand Names
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Fluoxetine • Prozac
Haloperidol • Haldol
Lamotrigine • Lamictal
Levathyroxine • Levothroid
Mirtazapine • Remeron
Oxcarbazepine • Trileptal
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
Venlafaxine • Effexor
1. Sahay M, Sahay R. Hyponatremia: a practical approach. Indian J Endocrinol Metab. 2014;18(6):760-771.
2. Kenes MT, Hamblin S, Tumuluri SS, et al. Syndrome of inappropriate antidiuretic hormone in a patient receiving high-dose haloperidol and quetiapine therapy. J Neuropsychiatry Clin Neurosci. 2016;28(2):e29-e30. doi: 10.1176/appi.neuropsych.15110392.
3. Twardowschy CA, Bertolucci CB, Gracia Cde M, et al. Severe hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) associated with fluoxetine: case report. Arq Neuropsiquiatr. 2006;64(1):142-145.
4. Patel KR, Meesala A, Stanilla JK. Sodium valproate–induced hyponatremia: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(5):PCC.09100941. doi: 10.4088/PCC.09100941.
5. Pillans PI, Coulter DM. Fluoxetine and hyponatraemia—a potential hazard in the elderly. N Z Med J. 1994;107(973):85‑86.
6. Strachan J, Shepherd J. Hyponatraemia associated with the use of selective serotonin reuptake inhibitors. Aust N Z J Psychiatry. 1998;32(2):295‑298.
7. Bouman WP, Pinner G, Johnson H. Incidence of selective serotonin reuptake inhibitor (SSRI) induced hyponatraemia due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion in the elderly. Int J Geriatr Psychiatry. 1998;13(1):12‑15.
8. Wilkinson TJ, Begg EJ, Winter AC, et al. Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br J Clin Pharmacol. 1999;47(2):211‑217.
9. Kirby D, Harrigan S, Ames D. Hyponatraemia in elderly psychiatric patients treated with selective serotonin reuptake inhibitors and venlafaxine: a retrospective controlled study in an inpatient unit. Int J Geriatr Psychiatry. 2002;17(3):231‑237.
10. Wee R, Lim WK. Selective serotonin re‑uptake inhibitors (SSRIs) and hyponatraemia in the elderly. Int J Geriatr Psychiatry. 2004;19(6):590‑591.
11. Jung YE, Jun TY, Kim KS, et al. Hyponatremia associated with selective serotonin reuptake inhibitors, mirtazapine, and venlafaxine in Korean patients with major depressive disorder. Int J Clin Pharmacol Ther. 2011;49(7):437‑443.
12. Letmaier M, Painold A, Holl AK, et al. Hyponatremia during psychopharmacological treatment: Results of a drug surveillance program. Int J Neuropsychopharmacol. 2012;15(6):739‑748.
13. Coupland CA, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15(28):1‑202, iii‑iv.
14. Leah-Møller KB, Hansen AH, Torstensson M, et al. Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. 2016;6(5):e011200. doi: 10.1136/bmjopen-2016-011200.
15. De Picker LD, Van Den Eede F, Dumont G, et al. Antidepressants and the risk of hyponatremia: a class by class review of literature. Psychosomatics. 2014;55(6):536-547.
16. Serrano A, Rangel N, Carrizo E, et al. Safety of long-term clozapine administration. Frequency of cardiomyopathy and hyponatraemia: two cross-sectional, naturalistic studies. Aust N Z J Psychiatry. 2014;48(2):183‑192.
17. Uhde TW, Post RM. Effects of carbamazepine on serum electrolytes: clinical and theoretical implications. J Clin Psychopharmacol. 1983;3(2):103‑106.
18. Lahr MB. Hyponatremia during carbamazepine therapy. Clin Pharmacol Ther. 1985;37(6):693‑696.
19. Joffe RT, Post RM, Uhde TW. Effects of carbamazepine on serum electrolytes in affectively ill patients. Psychol Med. 1986;16(2):331‑335.
20. Vieweg V, Glick JL, Herring S, et al. Absence of carbamazepine‑induced hyponatremia among patients also given lithium. Am J Psychiatry. 1987;144(7):943‑947.
21. Yassa R, Iskandar H, Nastase C, et al. Carbamazepine and hyponatremia in patients with affective disorder. Am J Psychiatry. 1988;145(3):339‑342.
22. Kastner T, Friedman DL, Pond WS. Carbamazepine‑induced hyponatremia in patients with mental retardation. Am J Ment Retard. 1992;96(5):536‑540.
23. Kelly BD, Hillery J. Hyponatremia during carbamazepine therapy in patients with intellectual disability. J Intellect Disabil Res. 2001;45(Pt 2):152‑156.
24. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
25. Siragy HM. Hyponatremia, fluid-electrolyte disorders and the syndrome of inappropriate antidiuretic hormone secretion: diagnosis and treatment options. Endocr Pract. 2006;12(4):446-457.
26. Braun M, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
Hyponatremia is a common, multifactorial clinical condition. Hyponatremia is usually defined as a plasma sodium level <135 mmol/L; however, some studies define it as a level <130 mmol/L. Hyponatremia results from the inability of the kidney to excrete a sufficient amount of fluid, or is due to excessive fluid intake. Increases in osmolality stimulate thirst and result in increased fluid intake. This increase in osmolality is recognized by the osmoreceptors located in the hypothalamus, which release antidiuretic hormone (ADH). Antidiuretic hormone works on the collecting ducts within the kidneys, triggering increased fluid reabsorption resulting in decreased fluid loss and a reduction in thirst.
The syndrome of inappropriate antidiuretic hormone (SIADH) occurs when there is persistent ADH stimulation resulting in hyponatremia. SIADH commonly presents as euvolemic hyponatremia. Common diagnostic criteria for SIADH are listed in Table 1.1
Medications are a major cause of SIADH, and psychotropics are a primary offender. Most of the data for drug-induced SIADH come from case reports and small case series, such as those described in Table 2.2-4 The extent to which each psychotropic class causes SIADH remains unknown. In this article, we focus on 3 classes of psychotropics, and their role in causing SIADH.
Antidepressants
There is a fair amount of data associating antidepressants with SIADH. The incidence of SIADH with selective serotonin reuptake inhibitors (SSRIs) varies greatly among studies, from .06% to 40%.5-12 This wide variation is due to the way each study defined hyponatremia. A higher incidence was found when hyponatremia was defined as <135 mmol/L as opposed to <130 mmol/L. A large cohort study of SSRIs found that there was an increased risk with fluoxetine, escitalopram, and citalopram (.078% to .085%) vs paroxetine and sertraline (.033% to .053%).13 Studies comparing the incidence of SIADH with SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) found that the rates were equal or slightly higher with the SNRI venlafaxine.13 SNRIs as a group have an estimated incidence of .08% to 4%, based on studies that defined hyponatremia as <130 mmol/L.13,14 Tricyclic antidepressants have an estimated incidence of .005% to 16.7%, based on a retrospective study that reviewed 15 studies and 100 case reports.15 Mirtazapine and bupropion do not have enough evidence to obtain a true definition of incidence; case reports for these drugs suggest a causal link for hyponatremia. Table 37,9,12-15 provides an overview of the incidence rate of hyponatremia for select antidepressants. It is clear that a more stringent cutoff for hyponatremia (<130 mmol/L) reduces the incidence rates. More evidence is needed to identify the true incidence and prevalence of SIADH with these agents.
Antipsychotics
Compared with antidepressants, there’s less evidence linking SIADH with antipsychotics; this data come mainly from case reports and observational studies. Serrano et al16 reported on a cross-sectional study that included 88 patients receiving clozapine, 61 patients receiving other atypical antipsychotics, 23 patients receiving typical antipsychotics, and 11 patients receiving both typical and atypical antipsychotics. They reported incidence rates of 3.4% for clozapine, 4.9% for atypical antipsychotics, 26.1% for typical antipsychotics, and 9.1% for the group receiving both typical and atypical antipsychotics.16 The primary theory for the decreased incidence of SIADH with use of atypical antipsychotics is related to decreased rates of psychogenic polydipsia leading to lower incidence of hyponatremia.
Mood stabilizers
Several studies have associated carbamazepine/oxcarbazepine, valproic acid, and lamotrigine with SIADH.17-23 Studies show incidence rates ranging from 4.8% to 41.5% for these medications. Carbamazepine appears to have the highest incidence of SIADH. A limitation of these studies is the small sample sizes, which ranged from 12 to 60 participants.
Pathophysiology
The kidneys are responsible for maintaining homeostasis between bodily fluids and serum sodium levels. ADH, which is produced by the hypothalamus, plays a significant role in fluid balance, thirst, and fluid retention. Inappropriate and continuous secretion of ADH, despite normal or high fluid status, results in hyposmolality and hyponatremia. The specific mechanisms by which psychotropic medications cause SIADH are listed in Table 4.24
Diagnosis
Diagnosis of SIADH can be complex because there are many clinical reasons a patient may have hyponatremia. For example, SIADH and psychogenic polydipsia both result in hyponatremia, and sometimes the 2 conditions can be difficult to distinguish. Hyponatremia is typically discovered by routine blood testing if the patient is asymptomatic. Table 525 highlights the major laboratory markers that distinguish SIADH and psychogenic polydipsia.
Continue to: Treatment
Treatment
The primary treatment for SIADH is cessation of the offending agent. Based on the patient’s clinical presentation, free water restriction (.5 to 1 L/d) can be implemented to increase serum sodium levels. If the patient is having neurologic complications due to the severity of hyponatremia, correction with hypertonic saline is indicated. Upon resolution, the recommended course of action is to switch to a medication in a different class. Re-challenging the patient with the same medication is not recommended unless there is no other alternative class of medication.24 Table 626 highlights other causes of hyponatremia, what laboratory markers to assess, and how to treat high-risk individuals.
Hyponatremia is a complex medical complication that can be life-threatening. Psychotropics are a relatively common cause of hyponatremia, specifically SIADH. Older adults appear to be at highest risk, as most case reports are in patients age ≥65. Patients who are prescribed psychotropics should be treated with the lowest effective dose and monitored for signs and symptoms of hyponatremia throughout therapy.
Related Resources
- Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guidelines on diagnosis and treatment of hyponatremia. Eur J Endocrinol. 2014;170(3):G1-G47.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-S42.
Drug Brand Names
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Fluoxetine • Prozac
Haloperidol • Haldol
Lamotrigine • Lamictal
Levathyroxine • Levothroid
Mirtazapine • Remeron
Oxcarbazepine • Trileptal
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
Venlafaxine • Effexor
Hyponatremia is a common, multifactorial clinical condition. Hyponatremia is usually defined as a plasma sodium level <135 mmol/L; however, some studies define it as a level <130 mmol/L. Hyponatremia results from the inability of the kidney to excrete a sufficient amount of fluid, or is due to excessive fluid intake. Increases in osmolality stimulate thirst and result in increased fluid intake. This increase in osmolality is recognized by the osmoreceptors located in the hypothalamus, which release antidiuretic hormone (ADH). Antidiuretic hormone works on the collecting ducts within the kidneys, triggering increased fluid reabsorption resulting in decreased fluid loss and a reduction in thirst.
The syndrome of inappropriate antidiuretic hormone (SIADH) occurs when there is persistent ADH stimulation resulting in hyponatremia. SIADH commonly presents as euvolemic hyponatremia. Common diagnostic criteria for SIADH are listed in Table 1.1
Medications are a major cause of SIADH, and psychotropics are a primary offender. Most of the data for drug-induced SIADH come from case reports and small case series, such as those described in Table 2.2-4 The extent to which each psychotropic class causes SIADH remains unknown. In this article, we focus on 3 classes of psychotropics, and their role in causing SIADH.
Antidepressants
There is a fair amount of data associating antidepressants with SIADH. The incidence of SIADH with selective serotonin reuptake inhibitors (SSRIs) varies greatly among studies, from .06% to 40%.5-12 This wide variation is due to the way each study defined hyponatremia. A higher incidence was found when hyponatremia was defined as <135 mmol/L as opposed to <130 mmol/L. A large cohort study of SSRIs found that there was an increased risk with fluoxetine, escitalopram, and citalopram (.078% to .085%) vs paroxetine and sertraline (.033% to .053%).13 Studies comparing the incidence of SIADH with SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) found that the rates were equal or slightly higher with the SNRI venlafaxine.13 SNRIs as a group have an estimated incidence of .08% to 4%, based on studies that defined hyponatremia as <130 mmol/L.13,14 Tricyclic antidepressants have an estimated incidence of .005% to 16.7%, based on a retrospective study that reviewed 15 studies and 100 case reports.15 Mirtazapine and bupropion do not have enough evidence to obtain a true definition of incidence; case reports for these drugs suggest a causal link for hyponatremia. Table 37,9,12-15 provides an overview of the incidence rate of hyponatremia for select antidepressants. It is clear that a more stringent cutoff for hyponatremia (<130 mmol/L) reduces the incidence rates. More evidence is needed to identify the true incidence and prevalence of SIADH with these agents.
Antipsychotics
Compared with antidepressants, there’s less evidence linking SIADH with antipsychotics; this data come mainly from case reports and observational studies. Serrano et al16 reported on a cross-sectional study that included 88 patients receiving clozapine, 61 patients receiving other atypical antipsychotics, 23 patients receiving typical antipsychotics, and 11 patients receiving both typical and atypical antipsychotics. They reported incidence rates of 3.4% for clozapine, 4.9% for atypical antipsychotics, 26.1% for typical antipsychotics, and 9.1% for the group receiving both typical and atypical antipsychotics.16 The primary theory for the decreased incidence of SIADH with use of atypical antipsychotics is related to decreased rates of psychogenic polydipsia leading to lower incidence of hyponatremia.
Mood stabilizers
Several studies have associated carbamazepine/oxcarbazepine, valproic acid, and lamotrigine with SIADH.17-23 Studies show incidence rates ranging from 4.8% to 41.5% for these medications. Carbamazepine appears to have the highest incidence of SIADH. A limitation of these studies is the small sample sizes, which ranged from 12 to 60 participants.
Pathophysiology
The kidneys are responsible for maintaining homeostasis between bodily fluids and serum sodium levels. ADH, which is produced by the hypothalamus, plays a significant role in fluid balance, thirst, and fluid retention. Inappropriate and continuous secretion of ADH, despite normal or high fluid status, results in hyposmolality and hyponatremia. The specific mechanisms by which psychotropic medications cause SIADH are listed in Table 4.24
Diagnosis
Diagnosis of SIADH can be complex because there are many clinical reasons a patient may have hyponatremia. For example, SIADH and psychogenic polydipsia both result in hyponatremia, and sometimes the 2 conditions can be difficult to distinguish. Hyponatremia is typically discovered by routine blood testing if the patient is asymptomatic. Table 525 highlights the major laboratory markers that distinguish SIADH and psychogenic polydipsia.
Continue to: Treatment
Treatment
The primary treatment for SIADH is cessation of the offending agent. Based on the patient’s clinical presentation, free water restriction (.5 to 1 L/d) can be implemented to increase serum sodium levels. If the patient is having neurologic complications due to the severity of hyponatremia, correction with hypertonic saline is indicated. Upon resolution, the recommended course of action is to switch to a medication in a different class. Re-challenging the patient with the same medication is not recommended unless there is no other alternative class of medication.24 Table 626 highlights other causes of hyponatremia, what laboratory markers to assess, and how to treat high-risk individuals.
Hyponatremia is a complex medical complication that can be life-threatening. Psychotropics are a relatively common cause of hyponatremia, specifically SIADH. Older adults appear to be at highest risk, as most case reports are in patients age ≥65. Patients who are prescribed psychotropics should be treated with the lowest effective dose and monitored for signs and symptoms of hyponatremia throughout therapy.
Related Resources
- Spasovski G, Vanholder R, Allolio B, et al. Clinical practice guidelines on diagnosis and treatment of hyponatremia. Eur J Endocrinol. 2014;170(3):G1-G47.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-S42.
Drug Brand Names
Bupropion • Wellbutrin
Carbamazepine • Tegretol
Citalopram • Celexa
Clozapine • Clozaril
Escitalopram • Lexapro
Fluoxetine • Prozac
Haloperidol • Haldol
Lamotrigine • Lamictal
Levathyroxine • Levothroid
Mirtazapine • Remeron
Oxcarbazepine • Trileptal
Paroxetine • Paxil
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
Venlafaxine • Effexor
1. Sahay M, Sahay R. Hyponatremia: a practical approach. Indian J Endocrinol Metab. 2014;18(6):760-771.
2. Kenes MT, Hamblin S, Tumuluri SS, et al. Syndrome of inappropriate antidiuretic hormone in a patient receiving high-dose haloperidol and quetiapine therapy. J Neuropsychiatry Clin Neurosci. 2016;28(2):e29-e30. doi: 10.1176/appi.neuropsych.15110392.
3. Twardowschy CA, Bertolucci CB, Gracia Cde M, et al. Severe hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) associated with fluoxetine: case report. Arq Neuropsiquiatr. 2006;64(1):142-145.
4. Patel KR, Meesala A, Stanilla JK. Sodium valproate–induced hyponatremia: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(5):PCC.09100941. doi: 10.4088/PCC.09100941.
5. Pillans PI, Coulter DM. Fluoxetine and hyponatraemia—a potential hazard in the elderly. N Z Med J. 1994;107(973):85‑86.
6. Strachan J, Shepherd J. Hyponatraemia associated with the use of selective serotonin reuptake inhibitors. Aust N Z J Psychiatry. 1998;32(2):295‑298.
7. Bouman WP, Pinner G, Johnson H. Incidence of selective serotonin reuptake inhibitor (SSRI) induced hyponatraemia due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion in the elderly. Int J Geriatr Psychiatry. 1998;13(1):12‑15.
8. Wilkinson TJ, Begg EJ, Winter AC, et al. Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br J Clin Pharmacol. 1999;47(2):211‑217.
9. Kirby D, Harrigan S, Ames D. Hyponatraemia in elderly psychiatric patients treated with selective serotonin reuptake inhibitors and venlafaxine: a retrospective controlled study in an inpatient unit. Int J Geriatr Psychiatry. 2002;17(3):231‑237.
10. Wee R, Lim WK. Selective serotonin re‑uptake inhibitors (SSRIs) and hyponatraemia in the elderly. Int J Geriatr Psychiatry. 2004;19(6):590‑591.
11. Jung YE, Jun TY, Kim KS, et al. Hyponatremia associated with selective serotonin reuptake inhibitors, mirtazapine, and venlafaxine in Korean patients with major depressive disorder. Int J Clin Pharmacol Ther. 2011;49(7):437‑443.
12. Letmaier M, Painold A, Holl AK, et al. Hyponatremia during psychopharmacological treatment: Results of a drug surveillance program. Int J Neuropsychopharmacol. 2012;15(6):739‑748.
13. Coupland CA, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15(28):1‑202, iii‑iv.
14. Leah-Møller KB, Hansen AH, Torstensson M, et al. Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. 2016;6(5):e011200. doi: 10.1136/bmjopen-2016-011200.
15. De Picker LD, Van Den Eede F, Dumont G, et al. Antidepressants and the risk of hyponatremia: a class by class review of literature. Psychosomatics. 2014;55(6):536-547.
16. Serrano A, Rangel N, Carrizo E, et al. Safety of long-term clozapine administration. Frequency of cardiomyopathy and hyponatraemia: two cross-sectional, naturalistic studies. Aust N Z J Psychiatry. 2014;48(2):183‑192.
17. Uhde TW, Post RM. Effects of carbamazepine on serum electrolytes: clinical and theoretical implications. J Clin Psychopharmacol. 1983;3(2):103‑106.
18. Lahr MB. Hyponatremia during carbamazepine therapy. Clin Pharmacol Ther. 1985;37(6):693‑696.
19. Joffe RT, Post RM, Uhde TW. Effects of carbamazepine on serum electrolytes in affectively ill patients. Psychol Med. 1986;16(2):331‑335.
20. Vieweg V, Glick JL, Herring S, et al. Absence of carbamazepine‑induced hyponatremia among patients also given lithium. Am J Psychiatry. 1987;144(7):943‑947.
21. Yassa R, Iskandar H, Nastase C, et al. Carbamazepine and hyponatremia in patients with affective disorder. Am J Psychiatry. 1988;145(3):339‑342.
22. Kastner T, Friedman DL, Pond WS. Carbamazepine‑induced hyponatremia in patients with mental retardation. Am J Ment Retard. 1992;96(5):536‑540.
23. Kelly BD, Hillery J. Hyponatremia during carbamazepine therapy in patients with intellectual disability. J Intellect Disabil Res. 2001;45(Pt 2):152‑156.
24. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
25. Siragy HM. Hyponatremia, fluid-electrolyte disorders and the syndrome of inappropriate antidiuretic hormone secretion: diagnosis and treatment options. Endocr Pract. 2006;12(4):446-457.
26. Braun M, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
1. Sahay M, Sahay R. Hyponatremia: a practical approach. Indian J Endocrinol Metab. 2014;18(6):760-771.
2. Kenes MT, Hamblin S, Tumuluri SS, et al. Syndrome of inappropriate antidiuretic hormone in a patient receiving high-dose haloperidol and quetiapine therapy. J Neuropsychiatry Clin Neurosci. 2016;28(2):e29-e30. doi: 10.1176/appi.neuropsych.15110392.
3. Twardowschy CA, Bertolucci CB, Gracia Cde M, et al. Severe hyponatremia and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH) associated with fluoxetine: case report. Arq Neuropsiquiatr. 2006;64(1):142-145.
4. Patel KR, Meesala A, Stanilla JK. Sodium valproate–induced hyponatremia: a case report. Prim Care Companion J Clin Psychiatry. 2010;12(5):PCC.09100941. doi: 10.4088/PCC.09100941.
5. Pillans PI, Coulter DM. Fluoxetine and hyponatraemia—a potential hazard in the elderly. N Z Med J. 1994;107(973):85‑86.
6. Strachan J, Shepherd J. Hyponatraemia associated with the use of selective serotonin reuptake inhibitors. Aust N Z J Psychiatry. 1998;32(2):295‑298.
7. Bouman WP, Pinner G, Johnson H. Incidence of selective serotonin reuptake inhibitor (SSRI) induced hyponatraemia due to the syndrome of inappropriate antidiuretic hormone (SIADH) secretion in the elderly. Int J Geriatr Psychiatry. 1998;13(1):12‑15.
8. Wilkinson TJ, Begg EJ, Winter AC, et al. Incidence and risk factors for hyponatraemia following treatment with fluoxetine or paroxetine in elderly people. Br J Clin Pharmacol. 1999;47(2):211‑217.
9. Kirby D, Harrigan S, Ames D. Hyponatraemia in elderly psychiatric patients treated with selective serotonin reuptake inhibitors and venlafaxine: a retrospective controlled study in an inpatient unit. Int J Geriatr Psychiatry. 2002;17(3):231‑237.
10. Wee R, Lim WK. Selective serotonin re‑uptake inhibitors (SSRIs) and hyponatraemia in the elderly. Int J Geriatr Psychiatry. 2004;19(6):590‑591.
11. Jung YE, Jun TY, Kim KS, et al. Hyponatremia associated with selective serotonin reuptake inhibitors, mirtazapine, and venlafaxine in Korean patients with major depressive disorder. Int J Clin Pharmacol Ther. 2011;49(7):437‑443.
12. Letmaier M, Painold A, Holl AK, et al. Hyponatremia during psychopharmacological treatment: Results of a drug surveillance program. Int J Neuropsychopharmacol. 2012;15(6):739‑748.
13. Coupland CA, Dhiman P, Barton G, et al. A study of the safety and harms of antidepressant drugs for older people: a cohort study using a large primary care database. Health Technol Assess. 2011;15(28):1‑202, iii‑iv.
14. Leah-Møller KB, Hansen AH, Torstensson M, et al. Antidepressants and the risk of hyponatremia: a Danish register-based population study. BMJ Open. 2016;6(5):e011200. doi: 10.1136/bmjopen-2016-011200.
15. De Picker LD, Van Den Eede F, Dumont G, et al. Antidepressants and the risk of hyponatremia: a class by class review of literature. Psychosomatics. 2014;55(6):536-547.
16. Serrano A, Rangel N, Carrizo E, et al. Safety of long-term clozapine administration. Frequency of cardiomyopathy and hyponatraemia: two cross-sectional, naturalistic studies. Aust N Z J Psychiatry. 2014;48(2):183‑192.
17. Uhde TW, Post RM. Effects of carbamazepine on serum electrolytes: clinical and theoretical implications. J Clin Psychopharmacol. 1983;3(2):103‑106.
18. Lahr MB. Hyponatremia during carbamazepine therapy. Clin Pharmacol Ther. 1985;37(6):693‑696.
19. Joffe RT, Post RM, Uhde TW. Effects of carbamazepine on serum electrolytes in affectively ill patients. Psychol Med. 1986;16(2):331‑335.
20. Vieweg V, Glick JL, Herring S, et al. Absence of carbamazepine‑induced hyponatremia among patients also given lithium. Am J Psychiatry. 1987;144(7):943‑947.
21. Yassa R, Iskandar H, Nastase C, et al. Carbamazepine and hyponatremia in patients with affective disorder. Am J Psychiatry. 1988;145(3):339‑342.
22. Kastner T, Friedman DL, Pond WS. Carbamazepine‑induced hyponatremia in patients with mental retardation. Am J Ment Retard. 1992;96(5):536‑540.
23. Kelly BD, Hillery J. Hyponatremia during carbamazepine therapy in patients with intellectual disability. J Intellect Disabil Res. 2001;45(Pt 2):152‑156.
24. Sahoo S, Grover S. Hyponatremia and psychotropics. J Geriatr Ment Health. 2016;3(2):108-122.
25. Siragy HM. Hyponatremia, fluid-electrolyte disorders and the syndrome of inappropriate antidiuretic hormone secretion: diagnosis and treatment options. Endocr Pract. 2006;12(4):446-457.
26. Braun M, Barstow CH, Pyzocha NJ. Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. Am Fam Physician. 2015;91(5):299-307.
Psychiatry’s social impact: Pervasive and multifaceted
Psychiatry has an enormous swath of effects on the social structure of society, perhaps more than any other medical specialty. Its ramifications can be observed and experienced across medical, scientific, legal, financial, political, sexual, religious, cultural, sociological, and artistic aspects of the aggregate of humans living together that we call society.
And yet, despite its pervasive and significant consequences at multiple levels of human communities, psychiatry remains inadequately appreciated or understood. In fact, it is sometimes maligned in a manner that no other medical discipline ever has to face.
I will expound on what may sound like a sweeping statement, and let you decide if society is indeed influenced in myriad ways by the wide array of psychiatric brain disorders that impact various core components of society.
Consider the following major societal repercussions of psychiatric disorders:
- Twenty-five percent of the population suffers from a psychiatric disorder per the landmark Epidemiological Catchment Area (ECA) study,1,2 funded by the National Institutes of Health. This translates to 85 million children, adolescents, adults, and older adults. No other medical specialty comes close to affecting this massive number of individuals in society.
- According to the World Health Organization (WHO), 4 of the top 10 causes of disability across all medical conditions are psychiatric disorders (Table3). Depression, alcoholism, schizophrenia, and bipolar disorder account for the greatest proportion of individuals with disabilities. Obviously, the impact of psychiatry in society is more significant than any other medical specialty as far as functional disability is concerned.
- The jails and prisons of the country are brimming with psychiatric patients who are arrested, incarcerated, and criminalized because their brain disorder disrupts their behavior. This is one of the most serious (and frankly outrageous) legal problems in our society. It occurred after our society decided to shutter state-supported hospitals (asylums) where psychiatric patients used to be treated as medically ill persons by health care professionals such as physicians, nurses, psychologists, and social workers, not prison guards. Remember that in the 1960s, 50% of all hospital beds in the United States were occupied by psychiatric patients, which is another historical indication of the societal impact of psychiatry.
- Alcohol and drug abuse are undoubtedly one of society’s most intractable problems. They are not only psychiatric disorders, but are often associated with multiple other psychiatric comorbidities and can lead to a host of general medical and surgical consequences. They are not only costly in financial terms, but they also lead to an increase in crime and forensic problems. Premature death is a heavy toll for society due to alcohol and substance use, as the opioid epidemic clearly has demonstrated over the past few years.
- Homelessness is an endemic sociological cancer in the body of society and is very often driven by psychiatric disorders and addictions. Countless numbers of severely mentally ill patients became homeless when asylums were closed and they were “freed” from restrictive institutional settings. Homelessness and imprisonment became the heavy and shameful price of “freedom” for persons with disabling psychiatric disorders in our “advanced” society.
- Suicide, both completed and attempted, is intimately associated with psychiatric disorders. Approximately 47,000 deaths from suicide were reported in the United States in 2017.4 Given that more than 30 million Americans suffer from mood disorders, millions of suicide attempts take place, crowding the emergency rooms of the country with individuals who need to receive emergent health care. The tragic toll of suicide and the heavy medical care costs of suicide attempts are incalculable, and unfortunately have been growing steadily over the past 20 years.
- Homicide is sometimes committed by persons with a psychiatric disorder, most commonly antisocial personality disorder. The rate of homicide often is used as a measure of a city’s quality of life, and urban areas where access to psychiatric care is limited tend to have high homicide rates.
- School problems, whether due to attention-deficit/hyperactivity disorder, below-average intellectual abilities, conduct disorder, bullying, impulsive behavior, substance use, broken homes, or dysfunctional families (often due to addictive or psychiatric disorders), are a major societal problem. Whether the problem is truancy, school fights, or dropping out before getting a high school diploma, psychiatric illness is frequently the underlying reason.
- Sexual controversies, such as expanding and evolving gender identity issues and discrimination against non-cisgender individuals, have instigated both positive and negative initiatives in society. Sexual abuse of children and its grave psychiatric implications in adulthood continues to happen despite public outrage and law enforcement efforts, and is often driven by individuals with serious psychopathology. In addition, sexual addiction (and its many biopsychosocial complications) is often associated with neuropsychiatric disorders.
- Poverty and the perpetual underclass are often a result of psychiatric disorders, and represent an ongoing societal challenge that has proven impossible to fix just by throwing money at it. Whether the affected individuals are seriously mentally ill, addicted, cognitively impaired or challenged, or unmotivated because of a neuropsychiatric disorder, poverty is practically impossible to eliminate.
- One positive impact of psychiatry in society is that artistic abilities, writing talent, musical creativity, entrepreneurship, and high productivity are often associated with certain psychiatric conditions, such as bipolar disorder, autism, obsessive-compulsive disorder, and psychosis spectrum disorders. Society is enriched by the creative energy and out-of-the-box thinking of persons with mild to moderate neuropsychiatric disorders.
- The financial impact of psychiatry is massive. The direct and indirect costs of psychiatric and addictive disorders are estimated to be more than $400 billion/year. Even a single serious psychiatric disorder, such as schizophrenia, costs society approximately $70 billion/year. The same holds true for bipolar disorder and depression. Thus, psychiatry accounts for a substantial portion of the financial expenditures in society.
- And last but certainly not least are the impediments to psychiatric treatment for tens of millions of individuals in our society who need treatment the most: the lack of health insurance parity; the stigma of seeking psychiatric help; the serious shortage of psychiatrists, especially in inner-city areas and rural regions; the poor public understanding about psychiatric illness; and the fact that the success rate of psychiatric treatment is very similar to (and sometimes better than) that of serious cardiac, pulmonary, hepatic, or renal diseases. There are also many flawed religious, cultural, or philosophical belief systems that fail to accept that the mind is a product of brain biology and function and that psychiatric disorders are brain disorders that affect thought, mood impulses, cognition, and behavior, just as other brain disorders cause muscle weakness, epileptic seizures, or stroke. The public must understand that depression can be caused by stroke or multiple sclerosis, that Parkinson’s disease can cause hallucinations and delusions, and that brain tumors can cause personality changes.
Continue to: So, what should society do to address...
So, what should society do to address the multiple impacts of psychiatry on its structure and function? I have a brief answer: intensive research. If society would embark on a massive research effort to discover preventions and cures for psychiatric disorders, the return on investment would be tremendous in human and financial terms. Currently, only a miniscule amount of money (<0.5% of the annual cost of psychiatric disorders) is invested in psychiatric brain research. Society should embark on a BHAG (pronounced Bee Hag), an acronym for “Big Hairy Audacious Goal,” a term coined by Jim Collins and Jerry Poras, who authored the seminal book Built to Last: Successful Habits of Visionary Companies. The BHAG is an ambitious and visionary goal that steers a company (or in this case, society) to a much brighter future. It would be on the scale of the Manhattan Project in the 1940s, which developed the nuclear bomb that put an end to World War II. When it comes to psychiatry, society should do no less.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area program. Historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934-941.
2. Robins LN, Regier DA (eds). Psychiatric disorders in America: The Epidemiological Catchment Area Study. New York, NY: The Free Press; 1992.
3. World Health Organization. Global Burden of Disease (GBD) 2000 estimates. https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000/en/. Accessed January 17, 2019.
4. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Accessed January 18, 2019.
Psychiatry has an enormous swath of effects on the social structure of society, perhaps more than any other medical specialty. Its ramifications can be observed and experienced across medical, scientific, legal, financial, political, sexual, religious, cultural, sociological, and artistic aspects of the aggregate of humans living together that we call society.
And yet, despite its pervasive and significant consequences at multiple levels of human communities, psychiatry remains inadequately appreciated or understood. In fact, it is sometimes maligned in a manner that no other medical discipline ever has to face.
I will expound on what may sound like a sweeping statement, and let you decide if society is indeed influenced in myriad ways by the wide array of psychiatric brain disorders that impact various core components of society.
Consider the following major societal repercussions of psychiatric disorders:
- Twenty-five percent of the population suffers from a psychiatric disorder per the landmark Epidemiological Catchment Area (ECA) study,1,2 funded by the National Institutes of Health. This translates to 85 million children, adolescents, adults, and older adults. No other medical specialty comes close to affecting this massive number of individuals in society.
- According to the World Health Organization (WHO), 4 of the top 10 causes of disability across all medical conditions are psychiatric disorders (Table3). Depression, alcoholism, schizophrenia, and bipolar disorder account for the greatest proportion of individuals with disabilities. Obviously, the impact of psychiatry in society is more significant than any other medical specialty as far as functional disability is concerned.
- The jails and prisons of the country are brimming with psychiatric patients who are arrested, incarcerated, and criminalized because their brain disorder disrupts their behavior. This is one of the most serious (and frankly outrageous) legal problems in our society. It occurred after our society decided to shutter state-supported hospitals (asylums) where psychiatric patients used to be treated as medically ill persons by health care professionals such as physicians, nurses, psychologists, and social workers, not prison guards. Remember that in the 1960s, 50% of all hospital beds in the United States were occupied by psychiatric patients, which is another historical indication of the societal impact of psychiatry.
- Alcohol and drug abuse are undoubtedly one of society’s most intractable problems. They are not only psychiatric disorders, but are often associated with multiple other psychiatric comorbidities and can lead to a host of general medical and surgical consequences. They are not only costly in financial terms, but they also lead to an increase in crime and forensic problems. Premature death is a heavy toll for society due to alcohol and substance use, as the opioid epidemic clearly has demonstrated over the past few years.
- Homelessness is an endemic sociological cancer in the body of society and is very often driven by psychiatric disorders and addictions. Countless numbers of severely mentally ill patients became homeless when asylums were closed and they were “freed” from restrictive institutional settings. Homelessness and imprisonment became the heavy and shameful price of “freedom” for persons with disabling psychiatric disorders in our “advanced” society.
- Suicide, both completed and attempted, is intimately associated with psychiatric disorders. Approximately 47,000 deaths from suicide were reported in the United States in 2017.4 Given that more than 30 million Americans suffer from mood disorders, millions of suicide attempts take place, crowding the emergency rooms of the country with individuals who need to receive emergent health care. The tragic toll of suicide and the heavy medical care costs of suicide attempts are incalculable, and unfortunately have been growing steadily over the past 20 years.
- Homicide is sometimes committed by persons with a psychiatric disorder, most commonly antisocial personality disorder. The rate of homicide often is used as a measure of a city’s quality of life, and urban areas where access to psychiatric care is limited tend to have high homicide rates.
- School problems, whether due to attention-deficit/hyperactivity disorder, below-average intellectual abilities, conduct disorder, bullying, impulsive behavior, substance use, broken homes, or dysfunctional families (often due to addictive or psychiatric disorders), are a major societal problem. Whether the problem is truancy, school fights, or dropping out before getting a high school diploma, psychiatric illness is frequently the underlying reason.
- Sexual controversies, such as expanding and evolving gender identity issues and discrimination against non-cisgender individuals, have instigated both positive and negative initiatives in society. Sexual abuse of children and its grave psychiatric implications in adulthood continues to happen despite public outrage and law enforcement efforts, and is often driven by individuals with serious psychopathology. In addition, sexual addiction (and its many biopsychosocial complications) is often associated with neuropsychiatric disorders.
- Poverty and the perpetual underclass are often a result of psychiatric disorders, and represent an ongoing societal challenge that has proven impossible to fix just by throwing money at it. Whether the affected individuals are seriously mentally ill, addicted, cognitively impaired or challenged, or unmotivated because of a neuropsychiatric disorder, poverty is practically impossible to eliminate.
- One positive impact of psychiatry in society is that artistic abilities, writing talent, musical creativity, entrepreneurship, and high productivity are often associated with certain psychiatric conditions, such as bipolar disorder, autism, obsessive-compulsive disorder, and psychosis spectrum disorders. Society is enriched by the creative energy and out-of-the-box thinking of persons with mild to moderate neuropsychiatric disorders.
- The financial impact of psychiatry is massive. The direct and indirect costs of psychiatric and addictive disorders are estimated to be more than $400 billion/year. Even a single serious psychiatric disorder, such as schizophrenia, costs society approximately $70 billion/year. The same holds true for bipolar disorder and depression. Thus, psychiatry accounts for a substantial portion of the financial expenditures in society.
- And last but certainly not least are the impediments to psychiatric treatment for tens of millions of individuals in our society who need treatment the most: the lack of health insurance parity; the stigma of seeking psychiatric help; the serious shortage of psychiatrists, especially in inner-city areas and rural regions; the poor public understanding about psychiatric illness; and the fact that the success rate of psychiatric treatment is very similar to (and sometimes better than) that of serious cardiac, pulmonary, hepatic, or renal diseases. There are also many flawed religious, cultural, or philosophical belief systems that fail to accept that the mind is a product of brain biology and function and that psychiatric disorders are brain disorders that affect thought, mood impulses, cognition, and behavior, just as other brain disorders cause muscle weakness, epileptic seizures, or stroke. The public must understand that depression can be caused by stroke or multiple sclerosis, that Parkinson’s disease can cause hallucinations and delusions, and that brain tumors can cause personality changes.
Continue to: So, what should society do to address...
So, what should society do to address the multiple impacts of psychiatry on its structure and function? I have a brief answer: intensive research. If society would embark on a massive research effort to discover preventions and cures for psychiatric disorders, the return on investment would be tremendous in human and financial terms. Currently, only a miniscule amount of money (<0.5% of the annual cost of psychiatric disorders) is invested in psychiatric brain research. Society should embark on a BHAG (pronounced Bee Hag), an acronym for “Big Hairy Audacious Goal,” a term coined by Jim Collins and Jerry Poras, who authored the seminal book Built to Last: Successful Habits of Visionary Companies. The BHAG is an ambitious and visionary goal that steers a company (or in this case, society) to a much brighter future. It would be on the scale of the Manhattan Project in the 1940s, which developed the nuclear bomb that put an end to World War II. When it comes to psychiatry, society should do no less.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
Psychiatry has an enormous swath of effects on the social structure of society, perhaps more than any other medical specialty. Its ramifications can be observed and experienced across medical, scientific, legal, financial, political, sexual, religious, cultural, sociological, and artistic aspects of the aggregate of humans living together that we call society.
And yet, despite its pervasive and significant consequences at multiple levels of human communities, psychiatry remains inadequately appreciated or understood. In fact, it is sometimes maligned in a manner that no other medical discipline ever has to face.
I will expound on what may sound like a sweeping statement, and let you decide if society is indeed influenced in myriad ways by the wide array of psychiatric brain disorders that impact various core components of society.
Consider the following major societal repercussions of psychiatric disorders:
- Twenty-five percent of the population suffers from a psychiatric disorder per the landmark Epidemiological Catchment Area (ECA) study,1,2 funded by the National Institutes of Health. This translates to 85 million children, adolescents, adults, and older adults. No other medical specialty comes close to affecting this massive number of individuals in society.
- According to the World Health Organization (WHO), 4 of the top 10 causes of disability across all medical conditions are psychiatric disorders (Table3). Depression, alcoholism, schizophrenia, and bipolar disorder account for the greatest proportion of individuals with disabilities. Obviously, the impact of psychiatry in society is more significant than any other medical specialty as far as functional disability is concerned.
- The jails and prisons of the country are brimming with psychiatric patients who are arrested, incarcerated, and criminalized because their brain disorder disrupts their behavior. This is one of the most serious (and frankly outrageous) legal problems in our society. It occurred after our society decided to shutter state-supported hospitals (asylums) where psychiatric patients used to be treated as medically ill persons by health care professionals such as physicians, nurses, psychologists, and social workers, not prison guards. Remember that in the 1960s, 50% of all hospital beds in the United States were occupied by psychiatric patients, which is another historical indication of the societal impact of psychiatry.
- Alcohol and drug abuse are undoubtedly one of society’s most intractable problems. They are not only psychiatric disorders, but are often associated with multiple other psychiatric comorbidities and can lead to a host of general medical and surgical consequences. They are not only costly in financial terms, but they also lead to an increase in crime and forensic problems. Premature death is a heavy toll for society due to alcohol and substance use, as the opioid epidemic clearly has demonstrated over the past few years.
- Homelessness is an endemic sociological cancer in the body of society and is very often driven by psychiatric disorders and addictions. Countless numbers of severely mentally ill patients became homeless when asylums were closed and they were “freed” from restrictive institutional settings. Homelessness and imprisonment became the heavy and shameful price of “freedom” for persons with disabling psychiatric disorders in our “advanced” society.
- Suicide, both completed and attempted, is intimately associated with psychiatric disorders. Approximately 47,000 deaths from suicide were reported in the United States in 2017.4 Given that more than 30 million Americans suffer from mood disorders, millions of suicide attempts take place, crowding the emergency rooms of the country with individuals who need to receive emergent health care. The tragic toll of suicide and the heavy medical care costs of suicide attempts are incalculable, and unfortunately have been growing steadily over the past 20 years.
- Homicide is sometimes committed by persons with a psychiatric disorder, most commonly antisocial personality disorder. The rate of homicide often is used as a measure of a city’s quality of life, and urban areas where access to psychiatric care is limited tend to have high homicide rates.
- School problems, whether due to attention-deficit/hyperactivity disorder, below-average intellectual abilities, conduct disorder, bullying, impulsive behavior, substance use, broken homes, or dysfunctional families (often due to addictive or psychiatric disorders), are a major societal problem. Whether the problem is truancy, school fights, or dropping out before getting a high school diploma, psychiatric illness is frequently the underlying reason.
- Sexual controversies, such as expanding and evolving gender identity issues and discrimination against non-cisgender individuals, have instigated both positive and negative initiatives in society. Sexual abuse of children and its grave psychiatric implications in adulthood continues to happen despite public outrage and law enforcement efforts, and is often driven by individuals with serious psychopathology. In addition, sexual addiction (and its many biopsychosocial complications) is often associated with neuropsychiatric disorders.
- Poverty and the perpetual underclass are often a result of psychiatric disorders, and represent an ongoing societal challenge that has proven impossible to fix just by throwing money at it. Whether the affected individuals are seriously mentally ill, addicted, cognitively impaired or challenged, or unmotivated because of a neuropsychiatric disorder, poverty is practically impossible to eliminate.
- One positive impact of psychiatry in society is that artistic abilities, writing talent, musical creativity, entrepreneurship, and high productivity are often associated with certain psychiatric conditions, such as bipolar disorder, autism, obsessive-compulsive disorder, and psychosis spectrum disorders. Society is enriched by the creative energy and out-of-the-box thinking of persons with mild to moderate neuropsychiatric disorders.
- The financial impact of psychiatry is massive. The direct and indirect costs of psychiatric and addictive disorders are estimated to be more than $400 billion/year. Even a single serious psychiatric disorder, such as schizophrenia, costs society approximately $70 billion/year. The same holds true for bipolar disorder and depression. Thus, psychiatry accounts for a substantial portion of the financial expenditures in society.
- And last but certainly not least are the impediments to psychiatric treatment for tens of millions of individuals in our society who need treatment the most: the lack of health insurance parity; the stigma of seeking psychiatric help; the serious shortage of psychiatrists, especially in inner-city areas and rural regions; the poor public understanding about psychiatric illness; and the fact that the success rate of psychiatric treatment is very similar to (and sometimes better than) that of serious cardiac, pulmonary, hepatic, or renal diseases. There are also many flawed religious, cultural, or philosophical belief systems that fail to accept that the mind is a product of brain biology and function and that psychiatric disorders are brain disorders that affect thought, mood impulses, cognition, and behavior, just as other brain disorders cause muscle weakness, epileptic seizures, or stroke. The public must understand that depression can be caused by stroke or multiple sclerosis, that Parkinson’s disease can cause hallucinations and delusions, and that brain tumors can cause personality changes.
Continue to: So, what should society do to address...
So, what should society do to address the multiple impacts of psychiatry on its structure and function? I have a brief answer: intensive research. If society would embark on a massive research effort to discover preventions and cures for psychiatric disorders, the return on investment would be tremendous in human and financial terms. Currently, only a miniscule amount of money (<0.5% of the annual cost of psychiatric disorders) is invested in psychiatric brain research. Society should embark on a BHAG (pronounced Bee Hag), an acronym for “Big Hairy Audacious Goal,” a term coined by Jim Collins and Jerry Poras, who authored the seminal book Built to Last: Successful Habits of Visionary Companies. The BHAG is an ambitious and visionary goal that steers a company (or in this case, society) to a much brighter future. It would be on the scale of the Manhattan Project in the 1940s, which developed the nuclear bomb that put an end to World War II. When it comes to psychiatry, society should do no less.
To comment on this editorial or other topics of interest: henry.nasrallah@currentpsychiatry.com.
1. Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area program. Historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934-941.
2. Robins LN, Regier DA (eds). Psychiatric disorders in America: The Epidemiological Catchment Area Study. New York, NY: The Free Press; 1992.
3. World Health Organization. Global Burden of Disease (GBD) 2000 estimates. https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000/en/. Accessed January 17, 2019.
4. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Accessed January 18, 2019.
1. Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area program. Historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41(10):934-941.
2. Robins LN, Regier DA (eds). Psychiatric disorders in America: The Epidemiological Catchment Area Study. New York, NY: The Free Press; 1992.
3. World Health Organization. Global Burden of Disease (GBD) 2000 estimates. https://www.who.int/healthinfo/global_burden_disease/estimates_regional_2000/en/. Accessed January 17, 2019.
4. American Foundation for Suicide Prevention. Suicide statistics. https://afsp.org/about-suicide/suicide-statistics. Accessed January 18, 2019.
The effect of collateral information on involuntary psychiatric commitment
Collateral information is a key component obtained during the psych
Here I describe a case in which collateral information obtained about a patient was a primary factor in that patient’s involuntary commitment. However, the patient’s subsequent behavior observed on an inpatient psychiatric unit was entirely inconsistent with those behaviors described by the collateral informant to be “continuous and dangerous.”
CASE
Mr. M, age 18, presented to an emergency psychiatric center for evaluation of dangerous and aggressive behavior. He had a history of autism spectrum disorder (ASD), which was well managed with oral risperidone. He was petitioned for an involuntary psychiatric admission by his foster mother, who reported that Mr. M was aggressive and dangerous, often punching holes in the walls of their home, and that he threatened to assault his foster siblings on several occasions. She detailed a progressively declining history for Mr. M and said that he was “constantly talking to voices in his head that absolutely consume him,” to the extent that Mr. M could not pay attention to his daily tasks. The admitting psychiatrist upheld the petition for involuntary admission, citing that based on the foster’s mother collateral information, Mr. M was deemed to be a danger to others and therefore fulfilled criteria for involuntary psychiatric admission.
Once admitted to the inpatient psychiatric unit, Mr. M was observed to be pleasant, cooperative, and fully engaged in the milieu. At no point during his 7-day admission was he observed to be internally preoccupied or remotely disorganized. Mr. M was switched from oral risperidone to oral haloperidol because he developed acute gynecomastia, and was discharged home.
Does collateral information lead to unfair bias?
The importance of collateral information on the psychiatric admission process must not be understated. It is an opportunity to hear a first-hand account of behaviors consistent with an acute psychiatric disturbance, and guides us in formulating a clinically appropriate assessment and plan. But what happens when our patients’ close contacts or informants provide misleading or unintentionally suboptimal collateral information? How must we reconcile the ethical and legal obligation we have to balance patient autonomy with beneficence?
Studies examining patients’ attitudes toward involuntary admissions have routinely found that patients are less likely than clinical staff to view the involuntary admission as clinically justified.2 Consistent with these findings, Mr. M did not view his admission as necessary. At first, he seemed to lack insight regarding the events precipitating his involuntary admission, describing himself not as responding to internal stimuli, but rather, “imaginative because I have autism.” As time went on, though, it was clear that his account of his behavior was in fact correct.
Mr. M’s diagnosis of ASD further complicated the over-reliance on misleading collateral information provided by his foster mother, because the admitting psychiatrist invariably perceived Mr. M as a poor historian. A study examining how subjective histories described by patients with neurologic or psychiatric disorders are perceived by clinicians found physicians had a tendency for negative stereotyping and placed less credence on those patients’ subjective histories.3 Other literature has similarly concluded that there is an urgent need to carefully weigh information supplied to us by collateral informants because the first-hand accounts of perceivably dangerous behavior often are incomplete or misleading.4-5
Continue to: Ideas for improvement...
Ideas for improvement: respecting patient autonomy
These issues underscore the need for a more thorough review of collateral information to ensure that patient autonomy is not unjustly violated. How do we implement these necessary ideas without creating further undue burden during the admission process? Certainly, I am not suggesting that we evaluate the collateral informant to the degree that we evaluate the patient. However, I have outlined some suggestions for ensuring we act in our patients’ best interest when processing collateral information during an admission:
- Until proven otherwise, the patient’s story is true. If our patient maintains descriptions of his behavior that exist in stark opposition to the collateral information we obtain, we should only not believe the patient if his presentation suggests he may be acutely impaired or a poor historian (such as profound disorganization, overt psychosis, or failing to have capacity).
- Treat symptoms, not diagnoses. In this case, Mr. M was described by his foster mother to be psychotic in addition to having ASD, and an inexperienced psychiatrist may have initiated a titration to a higher antipsychotic dose. However, in the absence of any observable signs of aggression or psychosis, there was simply no indication for further titration of his antipsychotic.
- Document, document, document. When collateral information is supplied to us, it is crucial that we maintain a detailed account of this information. If we have a reason to believe that a patient poses an immediate danger to himself or others, we should carefully document our reasoning so that changes in behavior (if any) can be observed on a day-to-day basis.
1. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgmont). 2010;7(10):30-40.
2. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
3. Crichton P, Carel H, Kidd IJ. Epistemic injustice in psychiatry. BJPsych Bull. 2017;41(2):65-70.
4. Marett C, Mossman D. What is your liability for involuntary commitment based on fault information? Current Psychiatry. 2017;16(3):21-25,33.
5. Lincoln AL, Allen M. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
Collateral information is a key component obtained during the psych
Here I describe a case in which collateral information obtained about a patient was a primary factor in that patient’s involuntary commitment. However, the patient’s subsequent behavior observed on an inpatient psychiatric unit was entirely inconsistent with those behaviors described by the collateral informant to be “continuous and dangerous.”
CASE
Mr. M, age 18, presented to an emergency psychiatric center for evaluation of dangerous and aggressive behavior. He had a history of autism spectrum disorder (ASD), which was well managed with oral risperidone. He was petitioned for an involuntary psychiatric admission by his foster mother, who reported that Mr. M was aggressive and dangerous, often punching holes in the walls of their home, and that he threatened to assault his foster siblings on several occasions. She detailed a progressively declining history for Mr. M and said that he was “constantly talking to voices in his head that absolutely consume him,” to the extent that Mr. M could not pay attention to his daily tasks. The admitting psychiatrist upheld the petition for involuntary admission, citing that based on the foster’s mother collateral information, Mr. M was deemed to be a danger to others and therefore fulfilled criteria for involuntary psychiatric admission.
Once admitted to the inpatient psychiatric unit, Mr. M was observed to be pleasant, cooperative, and fully engaged in the milieu. At no point during his 7-day admission was he observed to be internally preoccupied or remotely disorganized. Mr. M was switched from oral risperidone to oral haloperidol because he developed acute gynecomastia, and was discharged home.
Does collateral information lead to unfair bias?
The importance of collateral information on the psychiatric admission process must not be understated. It is an opportunity to hear a first-hand account of behaviors consistent with an acute psychiatric disturbance, and guides us in formulating a clinically appropriate assessment and plan. But what happens when our patients’ close contacts or informants provide misleading or unintentionally suboptimal collateral information? How must we reconcile the ethical and legal obligation we have to balance patient autonomy with beneficence?
Studies examining patients’ attitudes toward involuntary admissions have routinely found that patients are less likely than clinical staff to view the involuntary admission as clinically justified.2 Consistent with these findings, Mr. M did not view his admission as necessary. At first, he seemed to lack insight regarding the events precipitating his involuntary admission, describing himself not as responding to internal stimuli, but rather, “imaginative because I have autism.” As time went on, though, it was clear that his account of his behavior was in fact correct.
Mr. M’s diagnosis of ASD further complicated the over-reliance on misleading collateral information provided by his foster mother, because the admitting psychiatrist invariably perceived Mr. M as a poor historian. A study examining how subjective histories described by patients with neurologic or psychiatric disorders are perceived by clinicians found physicians had a tendency for negative stereotyping and placed less credence on those patients’ subjective histories.3 Other literature has similarly concluded that there is an urgent need to carefully weigh information supplied to us by collateral informants because the first-hand accounts of perceivably dangerous behavior often are incomplete or misleading.4-5
Continue to: Ideas for improvement...
Ideas for improvement: respecting patient autonomy
These issues underscore the need for a more thorough review of collateral information to ensure that patient autonomy is not unjustly violated. How do we implement these necessary ideas without creating further undue burden during the admission process? Certainly, I am not suggesting that we evaluate the collateral informant to the degree that we evaluate the patient. However, I have outlined some suggestions for ensuring we act in our patients’ best interest when processing collateral information during an admission:
- Until proven otherwise, the patient’s story is true. If our patient maintains descriptions of his behavior that exist in stark opposition to the collateral information we obtain, we should only not believe the patient if his presentation suggests he may be acutely impaired or a poor historian (such as profound disorganization, overt psychosis, or failing to have capacity).
- Treat symptoms, not diagnoses. In this case, Mr. M was described by his foster mother to be psychotic in addition to having ASD, and an inexperienced psychiatrist may have initiated a titration to a higher antipsychotic dose. However, in the absence of any observable signs of aggression or psychosis, there was simply no indication for further titration of his antipsychotic.
- Document, document, document. When collateral information is supplied to us, it is crucial that we maintain a detailed account of this information. If we have a reason to believe that a patient poses an immediate danger to himself or others, we should carefully document our reasoning so that changes in behavior (if any) can be observed on a day-to-day basis.
Collateral information is a key component obtained during the psych
Here I describe a case in which collateral information obtained about a patient was a primary factor in that patient’s involuntary commitment. However, the patient’s subsequent behavior observed on an inpatient psychiatric unit was entirely inconsistent with those behaviors described by the collateral informant to be “continuous and dangerous.”
CASE
Mr. M, age 18, presented to an emergency psychiatric center for evaluation of dangerous and aggressive behavior. He had a history of autism spectrum disorder (ASD), which was well managed with oral risperidone. He was petitioned for an involuntary psychiatric admission by his foster mother, who reported that Mr. M was aggressive and dangerous, often punching holes in the walls of their home, and that he threatened to assault his foster siblings on several occasions. She detailed a progressively declining history for Mr. M and said that he was “constantly talking to voices in his head that absolutely consume him,” to the extent that Mr. M could not pay attention to his daily tasks. The admitting psychiatrist upheld the petition for involuntary admission, citing that based on the foster’s mother collateral information, Mr. M was deemed to be a danger to others and therefore fulfilled criteria for involuntary psychiatric admission.
Once admitted to the inpatient psychiatric unit, Mr. M was observed to be pleasant, cooperative, and fully engaged in the milieu. At no point during his 7-day admission was he observed to be internally preoccupied or remotely disorganized. Mr. M was switched from oral risperidone to oral haloperidol because he developed acute gynecomastia, and was discharged home.
Does collateral information lead to unfair bias?
The importance of collateral information on the psychiatric admission process must not be understated. It is an opportunity to hear a first-hand account of behaviors consistent with an acute psychiatric disturbance, and guides us in formulating a clinically appropriate assessment and plan. But what happens when our patients’ close contacts or informants provide misleading or unintentionally suboptimal collateral information? How must we reconcile the ethical and legal obligation we have to balance patient autonomy with beneficence?
Studies examining patients’ attitudes toward involuntary admissions have routinely found that patients are less likely than clinical staff to view the involuntary admission as clinically justified.2 Consistent with these findings, Mr. M did not view his admission as necessary. At first, he seemed to lack insight regarding the events precipitating his involuntary admission, describing himself not as responding to internal stimuli, but rather, “imaginative because I have autism.” As time went on, though, it was clear that his account of his behavior was in fact correct.
Mr. M’s diagnosis of ASD further complicated the over-reliance on misleading collateral information provided by his foster mother, because the admitting psychiatrist invariably perceived Mr. M as a poor historian. A study examining how subjective histories described by patients with neurologic or psychiatric disorders are perceived by clinicians found physicians had a tendency for negative stereotyping and placed less credence on those patients’ subjective histories.3 Other literature has similarly concluded that there is an urgent need to carefully weigh information supplied to us by collateral informants because the first-hand accounts of perceivably dangerous behavior often are incomplete or misleading.4-5
Continue to: Ideas for improvement...
Ideas for improvement: respecting patient autonomy
These issues underscore the need for a more thorough review of collateral information to ensure that patient autonomy is not unjustly violated. How do we implement these necessary ideas without creating further undue burden during the admission process? Certainly, I am not suggesting that we evaluate the collateral informant to the degree that we evaluate the patient. However, I have outlined some suggestions for ensuring we act in our patients’ best interest when processing collateral information during an admission:
- Until proven otherwise, the patient’s story is true. If our patient maintains descriptions of his behavior that exist in stark opposition to the collateral information we obtain, we should only not believe the patient if his presentation suggests he may be acutely impaired or a poor historian (such as profound disorganization, overt psychosis, or failing to have capacity).
- Treat symptoms, not diagnoses. In this case, Mr. M was described by his foster mother to be psychotic in addition to having ASD, and an inexperienced psychiatrist may have initiated a titration to a higher antipsychotic dose. However, in the absence of any observable signs of aggression or psychosis, there was simply no indication for further titration of his antipsychotic.
- Document, document, document. When collateral information is supplied to us, it is crucial that we maintain a detailed account of this information. If we have a reason to believe that a patient poses an immediate danger to himself or others, we should carefully document our reasoning so that changes in behavior (if any) can be observed on a day-to-day basis.
1. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgmont). 2010;7(10):30-40.
2. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
3. Crichton P, Carel H, Kidd IJ. Epistemic injustice in psychiatry. BJPsych Bull. 2017;41(2):65-70.
4. Marett C, Mossman D. What is your liability for involuntary commitment based on fault information? Current Psychiatry. 2017;16(3):21-25,33.
5. Lincoln AL, Allen M. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
1. Testa M, West SG. Civil commitment in the United States. Psychiatry (Edgmont). 2010;7(10):30-40.
2. Roe D, Weishut DJ, Jaglom M, et al. Patients’ and staff members’ attitudes about the rights of hospitalized psychiatric patients. Psychiatr Serv. 2002;53(1):87-91.
3. Crichton P, Carel H, Kidd IJ. Epistemic injustice in psychiatry. BJPsych Bull. 2017;41(2):65-70.
4. Marett C, Mossman D. What is your liability for involuntary commitment based on fault information? Current Psychiatry. 2017;16(3):21-25,33.
5. Lincoln AL, Allen M. The influence of collateral information on access to inpatient psychiatric services. International Journal of Psychosocial Rehabilitation. 2002;6:99-108.
Differentiating serotonin syndrome and neuroleptic malignant syndrome
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
Serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) are each rare psychiatric emergencies that can lead to fatal outcomes. Their clinical presentations can overlap, which can make it difficult to differentiate between the 2 syndromes; however, their treatments are distinct, and it is imperative to know how to identify symptoms and accurately diagnose each of them to provide appropriate intervention. This article summarizes the 2 syndromes and their treatments, with a focus on how clinicians can distinguish them, provide prompt intervention, and prevent occurrence.
Serotonin syndrome
Mechanism. The decarboxylation and hydroxylation of tryptophan forms serotonin, also known as 5-hydroxytryptamine (5-HT), which can then be metabolized by monoamine oxidase-A (MAO-A) into 5-hydroxyindoleacetic acid (5-HIAA).1Medications can disrupt this pathway of serotonin production or its metabolism, and result in excessive levels of serotonin, which subsequently leads to an overactivation of central and peripheral serotonin receptors.1 Increased receptor activation leads to further upregulation, and ultimately more serotonin transmission. This can be caused by monotherapy or use of multiple serotonergic agents, polypharmacy with a combination of medication classes, drug interactions, or overdose. The wide variety of medications often prescribed by different clinicians can make identification of excessive serotonergic activity difficult, especially because mood stabilizers such as lithium,2 and non-psychiatric medications such as
- inhibition of serotonin uptake (seen with selective serotonin reuptake inhibitors [SSRIs], serotonin-norepinephrine reuptake inhibitors [SNRIs], and tricyclic antidepressants [TCAs])
- inhibition of serotonin metabolism (seen with monoamine oxidase inhibitors [MAOIs])
- increased serotonin synthesis (seen with stimulants)
- increased serotonin release (seen with stimulants and opiates)
- activation of serotonin receptors (seen with lithium)
- inhibition of certain cytochrome P450 (CYP450) enzymes (seen with ciprofloxacin, fluconazole, etc.).
It is important to recognize that various serotonergic agents are involved in the CYP450 system. Inhibition of the CYP450 pathway by common antibiotics such as ciprofloxacin, or antifungals such as fluconazole, may result in an accumulation of serotonergic agents and place patients at increased risk for developing SS.
Clinical presentation. The clinical presentation of SS can range from mild to fatal. There is no specific laboratory test for diagnosis, although an elevation of the total creatine kinase (CK) and leukocyte count, as well as increased transaminase levels or lower bicarbonate levels, have been reported in the literature.4
Symptoms of SS generally present within 24 hours of starting/changing therapy and include a triad of mental status changes (altered mental status [AMS]), autonomic instability, and abnormalities of neuromuscular tone. Examples of AMS include agitation, anxiety, disorientation, and restlessness. Symptoms of autonomic instability include hypertension, tachycardia, tachypnea, hyperthermia, diaphoresis, flushed skin, vomiting, diarrhea, and arrhythmias. Symptoms stemming from changes in neuromuscular tone include tremors, clonus, hyperreflexia, and muscle rigidity.1 The multiple possible clinical presentations, as well as symptoms that overlap with those of other syndromes, can make SS difficult to recognize quickly in a clinical setting.
Diagnostic criteria. Sternbach’s diagnostic criteria for SS are defined as the presence of 3 or more of the 10 most common clinical features (Table 25). Due to concerns that Sternbach’s diagnostic criteria overemphasized an abnormal mental state (leading to possible confusion of SS with other AMS syndromes), the Hunter serotonin toxicity criteria6 (Figure6) were developed in 2003, and were found to be more sensitive and specific than Sternbach’s criteria. Both tools are often used in clinical practice.
Treatment. Treatment of SS begins with prompt discontinuation of all serotonergic agents. The intensity of treatment depends on the severity of the symptoms. Mild symptoms can be managed with supportive care,3 and in such cases, the syndrome generally resolves within 24 hours.7 Clinicians may use supportive care to normalize vital signs (oxygenation to maintain SpO2 >94%, IV fluids for volume depletion, cooling agents, antihypertensives, benzodiazepines for sedation or control of agitation, etc.). Patients who are more ill may require more aggressive treatment, such as the use of a serotonergic antagonist (ie, cyproheptadine) and those who are severely hyperthermic (temperature >41.1ºC) may require neuromuscular sedation, paralysis, and possibly endotracheal intubation.3
Continue to: Management pitfalls include...
Management pitfalls include misdiagnosis of SS, failure to recognize its rapid rate of progression, and adverse effects of pharmacologic therapy.3 The most effective treatment for SS is prevention. SS can be prevented by astute pharmacologic understanding, avoidance of polypharmacy, and physician education.3
Neuroleptic malignant syndrome
Possible mechanisms. Neuromuscular malignant syndrome is thought to result from dopamine receptor antagonism leading to a hypodopaminergic state in the striatum and hypothalamus.8 The pathophysiology behind NMS has not fully been elucidated; however, several hypotheses attempt to explain this life-threatening reaction. The first focuses on dopamine D2 receptor antagonism, because many of the neuroleptic (antipsychotic) medications that can precipitate NMS are involved in dopamine blockade. In this theory, blocking dopamine D2 receptors in the anterior hypothalamus explains the hyperthermia seen in NMS, while blockade in the corpus striatum is believed to lead to muscle rigidity.9
The second hypothesis suggests that neuroleptics may have a direct toxic effect to muscle cells. Neuroleptics influence calcium transport across the sarcoplasmic reticulum and can lead to increased calcium release, which may contribute to the muscle rigidity and hyperthermia seen in NMS.9
The third hypothesis involves hyperactivity of the sympathetic nervous system; it is thought that psychologic stressors alter frontal lobe function, with neuroleptics disrupting the inhibitory pathways of the sympathetic nervous system. The autonomic nervous system innervates multiple organ systems, so this excessively dysregulated sympathetic nervous system may be responsible for multiple NMS symptoms (hyperthermia, muscle rigidity, hypertension, diaphoresis, tachycardia, elevated CK.10
NMS can be caused by neuroleptic agents (both first- and second-generation antipsychotics) as well as antiemetics (Table 31). The time between use of these medications and onset of symptoms is highly variable. NMS can occur after a single dose, after a dose adjustment, or possibly after years of treatment with the same medication. It is not dose-dependent.11 In certain individuals, NMS may occur at therapeutic doses.
Continue to: Clinical presentation
Clinical presentation. Patients with NMS typically present with a tetrad of symptoms: mental status changes, muscular rigidity, hyperthermia, and autonomic instability.12 Mental status changes can include confusion and agitation, as well as catatonic signs and mutism. The muscular rigidity of NMS is characterized by “lead pipe rigidity” and may be accompanied by tremor, dystonia, or dyskinesias. Laboratory findings include elevated serum CK (from severe rigidity), often >1,000 U/L, although normal levels can be observed if rigidity has not yet developed.13
Treatment. The first step for treatment is to discontinue the causative medication.14 Initiate supportive therapy immediately to restrict the progression of symptoms. Interventions include cooling blankets, fluid resuscitation, and antihypertensives to maintain autonomic stability15 or benzodiazepines to control agitation. In severe cases, muscular rigidity may extend to the airways and intubation may be required. The severity of these symptoms may warrant admission to the ICU for close monitoring. Pharmacologic treatment with
Differentiating between SS and NMS
Differentiating between these 2 syndromes (Table 417) is critical to direct appropriate intervention. Table 517 outlines the treatment overview for SS and NMS.
Detailed history. A detailed history is imperative in making accurate diagnoses. Useful components of the history include a patient’s duration of symptoms and medication history (prescription medications as well as over-the-counter medications, supplements, and illicit drugs). Also assess for medical comorbidities, because certain medical diagnoses may alert the clinician that it is likely the patient had been prescribed serotonergic agents or neuroleptics, and renal or liver impairment may alert the clinician of decreased metabolism rates. Medication history is arguably the most useful piece of the interview, because serotonergic agents can cause SS, whereas dopamine blockers cause NMS. It should be noted that excess serotonin acts as a true toxidrome and is concentration-dependent in causing SS, whereas NMS is an idiosyncratic reaction to a drug.
Physical exam. Although there are many overlapping clinical manifestations, SS produces neuromuscular hyperactivity (ie, clonus, hyperreflexia), whereas NMS is characterized by more sluggish responses (ie, rigidity, bradyreflexia).18
Continue to: Laboratory findings
Laboratory findings. Overlap between NMS and SS also occurs with lab findings; both syndromes can result in leukocytosis, elevated CK from muscle damage, and low serum iron levels. However, these findings are more commonly associated with NMS and are seen in 75% of cases.17,19
Course of illness. Duration of symptoms can also help differentiate the 2 syndromes. SS typically develops within 24 hours of starting/changing therapy, whereas NMS symptoms can be present for days to weeks. Resolution of symptoms may also be helpful in differentiation because SS typically resolves within a few days of initiating treatment, whereas NMS resolves within 9 to 14 days of starting treatment.19
Bottom Line
The clinical presentations of serotonin syndrome (SS) and neuroleptic malignant syndrome (NMS) overlap, which can make them difficult to differentiate; however, they each have distinct approaches to treatment. Features in SS that are distinct from NMS include a history of serotonergic agents, rapid onset of symptoms, hyperreflexia, and clonus. NMS is slower in onset and can be found in patients who are prescribed dopamine antagonists, with distinct symptoms of rigidity and hyporeflexia.
Related Resources
- Kimmel R. Serotonin syndrome or NMS? Clues to diagnosis. Current Psychiatry. 2010;9(2):92.
- Strawn JR, Keck Jr PE, Caroff SN. Neuroleptic malignant syndrome: Answers to 6 tough questions. Current Psychiatry. 2008;7(1):95-101.
Drug Brand Names
Amantadine • Symmetrel
Amitriptyline • Elavil, Endep
Aripiprazole • Abilify
Bromocriptine • Cycloset, Parlodel
Bupropion • Wellbutrin, Zyban
Buspirone • BuSpar
Carbamazepine • Carbatrol, Tegretol
Chlorpromazine • Thorazine
Ciprofloxacin • Cipro
Citalopram • Celexa
Clomipramine • Anafranil
Clozapine • Clozaril
Cyclobenzaprine • Amrix, Flexeril
Cyproheptadine • Periactin
Dantrolene • Dantrium
Desipramine • Norpramin
Desvenlafaxine • Pristiq
Dextromethorphan • Benylin, Dexalone
Dolasetron • Anzemet
Doxepin • Silenor
Droperidol • Inapsine
Duloxetine • Cymbalt
Escitalopram • Lexapro
Fentanyl • Actiq, Duragesic
Fluconazole • Diflucan
Fluoxetine • Prozac
Fluphenazine • Prolixin
Fluvoxamine • Luvox
Granisetron • Kytril
Haloperidol • Haldol
Isocarboxazid • Marplan
Levomilnacipran • Fetzima
Linezolid • Zyvox
Lithium • Eskalith, Lithobid
Meperidone • Demerol
Metoclopramide • Reglan
Milnacipran • Savella
Nefazodone • Serzone
Olanzapine • Zyprexa
Ondansetron • Zofran
Paliperidone • Invega
Palonosetron • Aloxi
Paroxetine • Paxil
Pentazocine • Talwin, Talacen
Perphenazine • Trilafon
Phenelzine • Nardil
Procarbazine • Matulane
Prochlorperazine • Compazine
Promethazine • Phenergan
Quetiapine • Seroquel
Rasagiline • Azilect
Risperidone • Risperdal
Safinamide • Xadago
Selegiline • Eldepryl, Zelapar
Sertraline • Zoloft
Sibutramine • Meridia
Tedizolid • Sivextro
Thioridazine • Mellaril
Tranylcypromine • Parnate
Tramadol • Ultram
Trazodone • Desyrel, Oleptro
Venlafaxine • Effexor
Vilazodone • Viibryd
Vortioxetine • Trintellix
Valproate • Depacon
Ziprasidone • Geodon
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.
1. Volpi-Abadie J, Kaye AM, Kaye AD. Serotonin syndrome. Ochsner J. 2013;13(4):533-540.
2. Werneke U, Jamshidi F, Taylor D, et al. Conundrums in neurology: diagnosing serotonin syndrome – a meta-analysis of cases. BMC Neurol. 2016;16:97.
3. Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11):1112-1120.
4. Birmes P, Coppin D, Schmitt L, et al. Serotonin syndrome: a brief review. CMAJ. 2003;168(11):1439-1442.
5. Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148:705-713.
6. Dunkley EJ, Isbister GK, Sibbritt D, et al. The Hunter serotonin toxicity criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003; 96(9):635-642.
7. Lappin RI, Auchincloss EL. Treatment of the serotonin syndrome with cyproheptadine. N Engl J Med. 1994;331(15):1021-1022.
8. Nisijima K. Serotonin syndrome overlapping with neuroleptic malignant syndrome: A case report and approaches for differentially diagnosing the two syndromes. Asian J Psychiatr. 2015;18:100-101.
9. Adnet P, Lestavel P, Krivosic-Horber R. Neuroleptic malignant syndrome. Br J Anaesth. 2000;85(1):129-135.
10. Gurrera R. Sympathoadrenal hyperactivity and the etiology of neuroleptic malignant syndrome. Am J Psychiatry. 1999;156:169-180.
11. Pope HG Jr, Aizley HG, Keck PE Jr, et al. Neuroleptic malignant syndrome: long-term follow-up of 20 cases. J Clin Psychiatry. 1991;52(5):208-212.
12. Velamoor VR, Norman RM, Caroff SN, et al. Progression of symptoms in neuroleptic malignant syndrome. J Nerv Ment Dis. 1994;182(3):168-173.
13. Caroff SN, Mann SC. Neuroleptic malignant syndrome. Med Clin North Am. 1993;77(1):185-202.
14. Pileggi DJ, Cook AM. Neuroleptic malignant syndrome. Ann Pharmacother. 2016;50(11):973-981.
15. San Gabriel MC, Eddula-Changala B, Tan Y, et al. Electroconvulsive in a schizophrenic patient with neuroleptic malignant syndrome and rhabdomyolysis. J ECT. 2015;31(3):197-200.
16. Buggenhout S, Vandenberghe J, Sienaert P. Electroconvulsion therapy for neuroleptic malignant syndrome. Tijdschr Psychiatr. 2014;56(9):612-615.
17. Perry PJ, Wilborn CA. Serotonin syndrome vs neuroleptic malignant syndrome: a contrast of causes, diagnoses, and management. Ann Clin Psychiatry. 2012;24(2):155-162.
18. Mills KC. Serotonin syndrome. A clinical update. Crit Care Clin. 1997;13(4):763-783.
19. Dosi R, Ambaliya A, Joshi H, et al. Serotonin syndrome versus neuroleptic malignant syndrome: a challenge clinical quandary. BMJ Case Rep. 2014;2014:bcr201404154. doi:10.1136/bcr-2014-204154.