User login
Science Has Spoken: Undetectable Equals Untransmittable
The consensus is in: Undetectable is Untransmittable (U = U). That is, scientific experts are finally willing to say that the concept of “Undetectable is Untransmittable” for HIV treatment is now “firmly established.” Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), writing in JAMA, says an “overwhelming” body of clinical evidence provides a firm basis for accepting the concept as scientifically sound.
In the JAMA commentary, Fauci and colleagues review the results of clinical trials validating U = U. One landmark study, for instance, showed that no linked HIV transmissions occurred among HIV serodifferent heterosexual couples when the partner living with HIV had a durably suppressed viral load. Subsequent studies confirmed the findings and extended them to male-male couples.
The key, the researchers all agree, is to be absolutely adherent to antiretroviral therapy (ART). Viral suppression measured at 6 months after starting therapy is required for U = U. Stopping ART represents a “significant challenge” to successful implementation of U = U. According to the clinical trials, when ART is stopped, viral rebound usually occurs within 2 to 3 weeks. In 2 studies, stopping ART caused viral rebound to levels that would have been associated with increased risk of HIV transmission.
The NIH experts say this consensus has a variety of implications. It gives incentive to people living with HIV to start and adhere to treatment, removes the sense of fear and guilt they may have about harming others, and reduces the risk of legal penalties arising from putting virus-free partners at risk. And because “prevention as control” is a critical tool, the U = U concept can support worldwide efforts to control—or even eliminate—the pandemic.
Source:
Eisinger RW, Dieffenbach CW, Fauci AS. JAMA. 2019.
doi: 10.1001/jama.2018.21167. [Epub ahead of print.]
The consensus is in: Undetectable is Untransmittable (U = U). That is, scientific experts are finally willing to say that the concept of “Undetectable is Untransmittable” for HIV treatment is now “firmly established.” Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), writing in JAMA, says an “overwhelming” body of clinical evidence provides a firm basis for accepting the concept as scientifically sound.
In the JAMA commentary, Fauci and colleagues review the results of clinical trials validating U = U. One landmark study, for instance, showed that no linked HIV transmissions occurred among HIV serodifferent heterosexual couples when the partner living with HIV had a durably suppressed viral load. Subsequent studies confirmed the findings and extended them to male-male couples.
The key, the researchers all agree, is to be absolutely adherent to antiretroviral therapy (ART). Viral suppression measured at 6 months after starting therapy is required for U = U. Stopping ART represents a “significant challenge” to successful implementation of U = U. According to the clinical trials, when ART is stopped, viral rebound usually occurs within 2 to 3 weeks. In 2 studies, stopping ART caused viral rebound to levels that would have been associated with increased risk of HIV transmission.
The NIH experts say this consensus has a variety of implications. It gives incentive to people living with HIV to start and adhere to treatment, removes the sense of fear and guilt they may have about harming others, and reduces the risk of legal penalties arising from putting virus-free partners at risk. And because “prevention as control” is a critical tool, the U = U concept can support worldwide efforts to control—or even eliminate—the pandemic.
Source:
Eisinger RW, Dieffenbach CW, Fauci AS. JAMA. 2019.
doi: 10.1001/jama.2018.21167. [Epub ahead of print.]
The consensus is in: Undetectable is Untransmittable (U = U). That is, scientific experts are finally willing to say that the concept of “Undetectable is Untransmittable” for HIV treatment is now “firmly established.” Anthony Fauci, director of the National Institute of Allergy and Infectious Diseases (NIAID), writing in JAMA, says an “overwhelming” body of clinical evidence provides a firm basis for accepting the concept as scientifically sound.
In the JAMA commentary, Fauci and colleagues review the results of clinical trials validating U = U. One landmark study, for instance, showed that no linked HIV transmissions occurred among HIV serodifferent heterosexual couples when the partner living with HIV had a durably suppressed viral load. Subsequent studies confirmed the findings and extended them to male-male couples.
The key, the researchers all agree, is to be absolutely adherent to antiretroviral therapy (ART). Viral suppression measured at 6 months after starting therapy is required for U = U. Stopping ART represents a “significant challenge” to successful implementation of U = U. According to the clinical trials, when ART is stopped, viral rebound usually occurs within 2 to 3 weeks. In 2 studies, stopping ART caused viral rebound to levels that would have been associated with increased risk of HIV transmission.
The NIH experts say this consensus has a variety of implications. It gives incentive to people living with HIV to start and adhere to treatment, removes the sense of fear and guilt they may have about harming others, and reduces the risk of legal penalties arising from putting virus-free partners at risk. And because “prevention as control” is a critical tool, the U = U concept can support worldwide efforts to control—or even eliminate—the pandemic.
Source:
Eisinger RW, Dieffenbach CW, Fauci AS. JAMA. 2019.
doi: 10.1001/jama.2018.21167. [Epub ahead of print.]
A Lesion Hits Its Growth Spurt
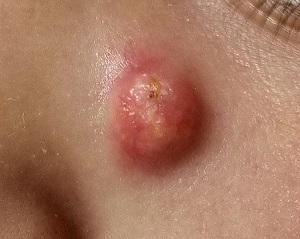
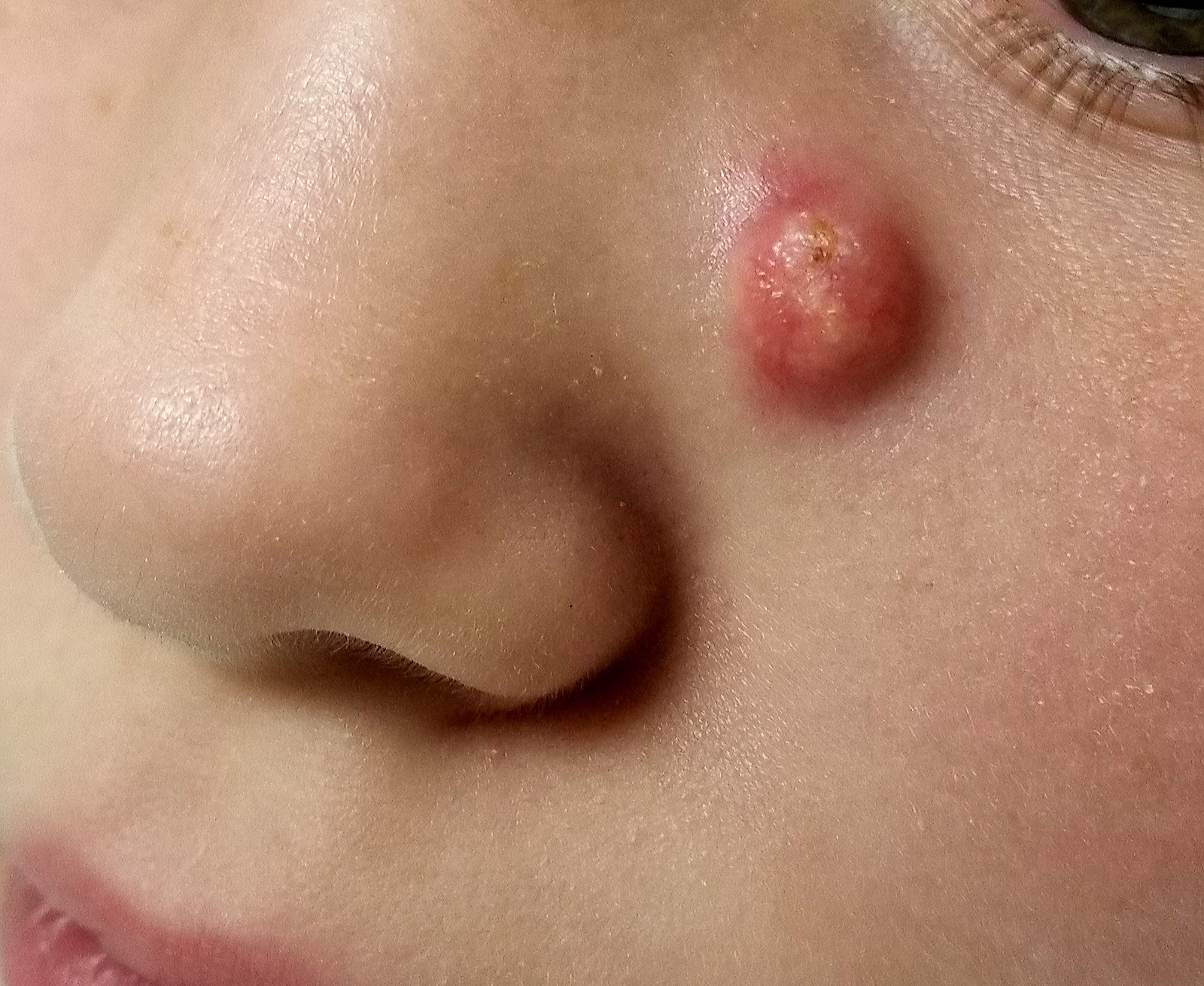
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.
EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.

EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
When she was 3 years old, a lesion appeared on this child’s face. It was small and caused little to no concern for several years. The child is now 9, and about a year ago, the lesion began to enlarge, ultimately reaching its present size.
First thought to be a pimple, the lesion was later deemed to be “cystic in nature” by another provider. By that point, however, the lesion was quite prominent—to the extent that it intrudes into the patient’s visual field. Perhaps more significantly for someone her age, it has prompted looks and comments that make her uncomfortable.
Fortunately, the lesion causes no pain or physical discomfort, and no other lesions have manifested. The child’s health is generally excellent.

EXAMINATION
A firm nodule, measuring 1.0 by 0.8 cm, is located on the patient’s left upper nasal sidewall. It stands out on an otherwise pristine face free of other blemishes. The lesion is predominantly red, with faint epidermal disturbance in the center. No punctum is appreciated. The lesion is quite firm on palpation, with just a hint of fluctuance but no tenderness or increased warmth.
Excision is clearly indicated; however, the wait for an appointment with a plastic surgeon is currently weeks to months. So an attempt is made to reduce the prominence of the lesion through incision and drainage, which also offers an opportunity to visualize its contents and possibly confirm a diagnosis. The lesion is opened with a #11 blade, and copious amounts of whitish, grainy material is digitally extruded.
What’s the diagnosis?
DISCUSSION
The contents are consistent with those of a somewhat unusual lesion, commonly called pilomatricoma. It is also known as calcifying epithelioma of Malherbe and pilomatrixoma.
This type of cyst is derived from the hair matrix and is commonly seen on the face, neck, scalp, and arms of children and young adults. This patient’s lesion was atypical in its prominence and erythema, at odds with the firm bluish intradermal papule or nodule usually seen in these cases. But the unique contents established the diagnosis with considerable certainty.
All that remained was the excision—which, given the patient’s age and the cosmetic concerns, would require above-average surgical skills. Once removed, the sample will be sent for pathologic examination, which should show anucleate squamous cells (“ghost cells”), benign viable squamous cells with a lining consisting of basaloid cells. Calcifications with foreign body giant cells account for the pathognomic white flecks seen in the extruded material.
Pilomatricoma’s cause is debatable, but it appears to involve increased levels of beta catenin caused by mutations of the APC gene. This effectively inhibits apoptosis, leading to focal increases in cell growth.
The differential for this type of lesion includes simple acne cyst (unlikely in such a young child), carbuncle (which would have been quite painful and full of pus), or even squamous cell carcinoma.
TAKE-HOME LEARNING POINTS
- Pilomatricomas are benign cysts usually seen on the face, neck, scalp, and arms of children and young adults.
- The typical pilomatricoma (sometimes called calcifying epithelioma of Malherbe) is an intradermal papule or nodule, often displaying a faintly bluish color, that is relatively firm on palpation.
- The contents of a pilomatricoma usually consist of whitish curds or flecks of material that represent calcified tissue mixed with foreign body giant cells.
- Pilomatricoma has little or no malignant potential but is often cosmetically significant.
What is your diagnosis? - February 2019
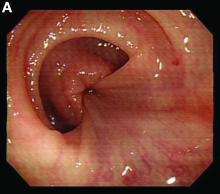
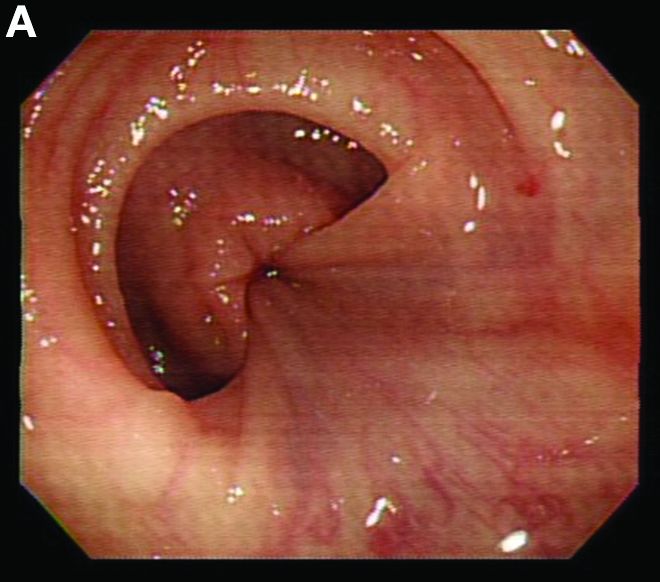
Retrograde colocolic intussusception induced by colonic adenocarcinoma
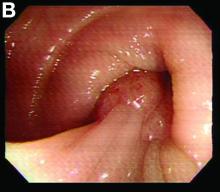
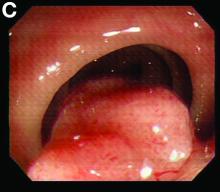
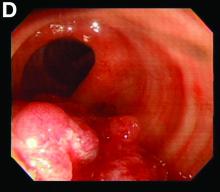
We used biopsy forceps for slow retraction (Figure B) and complete reduction (Figure C). The colonoscope was further inserted up to the cecum and no other lesions were found. A very large polyp was excised partially using a snare for the prevention of repeated intussusception and for histologic examination (Figure D). The pathology revealed adenocarcinoma and the patient underwent surgery. Recovery was uneventful and the patient was discharged 1 week later.
Intussusception is defined as the invagination of a segment of the bowel and its mesentery into the adjacent bowel lumen. It is a common cause of intestinal obstruction in children, but rare in adults. Adult intussusception accounts for 5% of all causes of bowel obstruction and 5%-10% of all intussusception, and usually has a lead point.1 Retrograde colocolic intussusception is especially rare, with only 26 cases reported up to 2014.2 Altered peristalsis in focal areas of the bowel wall can lead to dysrhythmic contractions and cause retrograde intussusception.
Adult colonic intussusception has atypical, nonspecific, intermittent, and vague symptoms and signs, resulting in a diagnostic challenge. Approximately one-half of patients present with symptoms of colonic obstruction with a duration of more than 1 month, as in our case. Many cases involve acute intestinal obstruction and are managed through emergency operation. Ultrasound imaging and computed tomography scans are the most sensitive and most commonly used preoperative diagnostic modalities. Colonoscopy is a useful tool for evaluating intussusception in colocolic intussusception,3 but there is no reported diagnosis of retrograde colocolic intussusception and reduction, as in this case.
Treatment of adult intussusception is more frequently surgical compared with that in children, and leads to resection of the involved bowel segment without reduction before resection.3 In our case, intussusception was reduced easily with biopsy forceps under a direct colonoscopic view and was cured through elective laparoscopic left hemicolectomy after histologic proof was obtained.
References
1. Joseph T, Desai AL. Retrograde intussusception of sigmoid colon. J R Soc Med. 2004;7:127-8.
2.Baba M, Higaki N, Ishida M, et al. A case of retrograde intussusception due to semipedunculated polypiform adenocarcinoma in tubular adenoma of the sigmoid colon in an adult. Jpn J Gastroenterol Surg. 2001;34:282-6.
3. Kamble MA, Thawait AP, Kamble AT. Left side reverse colocolic intussusception secondary to malignant polypoidal growth: a rare clinical entity. Int Surg J. 2014;1:39-42.
Retrograde colocolic intussusception induced by colonic adenocarcinoma
We used biopsy forceps for slow retraction (Figure B) and complete reduction (Figure C). The colonoscope was further inserted up to the cecum and no other lesions were found. A very large polyp was excised partially using a snare for the prevention of repeated intussusception and for histologic examination (Figure D). The pathology revealed adenocarcinoma and the patient underwent surgery. Recovery was uneventful and the patient was discharged 1 week later.
Intussusception is defined as the invagination of a segment of the bowel and its mesentery into the adjacent bowel lumen. It is a common cause of intestinal obstruction in children, but rare in adults. Adult intussusception accounts for 5% of all causes of bowel obstruction and 5%-10% of all intussusception, and usually has a lead point.1 Retrograde colocolic intussusception is especially rare, with only 26 cases reported up to 2014.2 Altered peristalsis in focal areas of the bowel wall can lead to dysrhythmic contractions and cause retrograde intussusception.
Adult colonic intussusception has atypical, nonspecific, intermittent, and vague symptoms and signs, resulting in a diagnostic challenge. Approximately one-half of patients present with symptoms of colonic obstruction with a duration of more than 1 month, as in our case. Many cases involve acute intestinal obstruction and are managed through emergency operation. Ultrasound imaging and computed tomography scans are the most sensitive and most commonly used preoperative diagnostic modalities. Colonoscopy is a useful tool for evaluating intussusception in colocolic intussusception,3 but there is no reported diagnosis of retrograde colocolic intussusception and reduction, as in this case.
Treatment of adult intussusception is more frequently surgical compared with that in children, and leads to resection of the involved bowel segment without reduction before resection.3 In our case, intussusception was reduced easily with biopsy forceps under a direct colonoscopic view and was cured through elective laparoscopic left hemicolectomy after histologic proof was obtained.
References
1. Joseph T, Desai AL. Retrograde intussusception of sigmoid colon. J R Soc Med. 2004;7:127-8.
2.Baba M, Higaki N, Ishida M, et al. A case of retrograde intussusception due to semipedunculated polypiform adenocarcinoma in tubular adenoma of the sigmoid colon in an adult. Jpn J Gastroenterol Surg. 2001;34:282-6.
3. Kamble MA, Thawait AP, Kamble AT. Left side reverse colocolic intussusception secondary to malignant polypoidal growth: a rare clinical entity. Int Surg J. 2014;1:39-42.
Retrograde colocolic intussusception induced by colonic adenocarcinoma
We used biopsy forceps for slow retraction (Figure B) and complete reduction (Figure C). The colonoscope was further inserted up to the cecum and no other lesions were found. A very large polyp was excised partially using a snare for the prevention of repeated intussusception and for histologic examination (Figure D). The pathology revealed adenocarcinoma and the patient underwent surgery. Recovery was uneventful and the patient was discharged 1 week later.
Intussusception is defined as the invagination of a segment of the bowel and its mesentery into the adjacent bowel lumen. It is a common cause of intestinal obstruction in children, but rare in adults. Adult intussusception accounts for 5% of all causes of bowel obstruction and 5%-10% of all intussusception, and usually has a lead point.1 Retrograde colocolic intussusception is especially rare, with only 26 cases reported up to 2014.2 Altered peristalsis in focal areas of the bowel wall can lead to dysrhythmic contractions and cause retrograde intussusception.
Adult colonic intussusception has atypical, nonspecific, intermittent, and vague symptoms and signs, resulting in a diagnostic challenge. Approximately one-half of patients present with symptoms of colonic obstruction with a duration of more than 1 month, as in our case. Many cases involve acute intestinal obstruction and are managed through emergency operation. Ultrasound imaging and computed tomography scans are the most sensitive and most commonly used preoperative diagnostic modalities. Colonoscopy is a useful tool for evaluating intussusception in colocolic intussusception,3 but there is no reported diagnosis of retrograde colocolic intussusception and reduction, as in this case.
Treatment of adult intussusception is more frequently surgical compared with that in children, and leads to resection of the involved bowel segment without reduction before resection.3 In our case, intussusception was reduced easily with biopsy forceps under a direct colonoscopic view and was cured through elective laparoscopic left hemicolectomy after histologic proof was obtained.
References
1. Joseph T, Desai AL. Retrograde intussusception of sigmoid colon. J R Soc Med. 2004;7:127-8.
2.Baba M, Higaki N, Ishida M, et al. A case of retrograde intussusception due to semipedunculated polypiform adenocarcinoma in tubular adenoma of the sigmoid colon in an adult. Jpn J Gastroenterol Surg. 2001;34:282-6.
3. Kamble MA, Thawait AP, Kamble AT. Left side reverse colocolic intussusception secondary to malignant polypoidal growth: a rare clinical entity. Int Surg J. 2014;1:39-42.
Preventing postpartum depression: Start with women at greatest risk
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
The last decade has brought appropriate attention to the high prevalence of postpartum mood and anxiety disorders, with postpartum depression (PPD) constituting the most common complication in modern obstetrics.
There have been very substantial efforts in more than 40 states in the United States to enhance screening for PPD and to increase support groups for women with postpartum depressive or anxiety symptoms. However, less focus has been paid to the outcomes of these screening initiatives.
A question that comes to mind is whether patients who are screened actually get referred for treatment, and if they do receive treatment, whether they recover and become well. One study referenced previously in this column noted that even in settings where women are screened for PPD, the vast majority of women are not referred, and of those who are referred, even fewer of those are treated or become well.1
It is noteworthy, then, that the U.S. Preventive Services Task Force has recommended screening for perinatal depression (just before and after birth) and issued draft recommendations regarding prevention of perinatal depression where it is suggested that patients at risk for perinatal depression be referred for appropriate “counseling interventions” – specifically, either cognitive-behavioral therapy (CBT) or interpersonal psychotherapy (IPT).2
The recommendation is a striking one because of the volume of patients who would be included. For example, the USPSTF recommends patients with histories of depression, depression during pregnancy, a history of child abuse, or even a family history of depression should receive preventive interventions with CBT or IPT. The recommendation is puzzling because of the data on risk for perinatal depression in those populations and the lack of available resources for patients who would be deemed “at risk.” Women with histories of depression are at a threefold increased risk for PPD (25%-30%). Depression during pregnancy is the strongest predictor of PPD and risk for PPD among these patients is as high as 75%.
So, there are a vast number of women who may be “at risk” for perinatal depression. But even with some data suggesting that IPT and CBT may be able to prevent perinatal depression, the suggestion that resources be made available to patients who are at risk is naive, because counseling interventions such as IPT or CBT, or even simply referrals to psychiatrists are not available even to patients who screen in for perinatal depression in real time during pregnancy and the postpartum period. I have previously written that the follow-up of women post partum who suffer from PPD is still far from meeting the needs who suffer from the disorder, and early detection and referrals to appropriate clinicians who are facile with both pharmacologic and nonpharmacologic interventions seem the most effective way to manage these patients and to see that they receive treatment.
The question then becomes: If the numbers or scale of the prevention initiative suggested in this draft recommendation from the USPSTF is an overreach, is there a group of patients for whom a preventive intervention could be pursued? The patients at highest risk for PPD include those with a history of PPD (50%), bipolar disorder (50%-60%), or postpartum psychosis (80%). And while there is not substantial literature for specifically using IPT, CBT, or other counseling interventions to mitigate risk for recurrence in women with histories of PPD, bipolar disorder, or postpartum psychosis, there are ways of identifying this population at risk and following them closely to mitigate the risk for recurrence.
To make this recommendation feasible, an infrastructure needs to be in place in both low resource settings and in all communities so that these patients can be referred and effectively treated. If we move to prevention, we ought to start with the populations that we already know are at greatest risk and that we can inquire about, and there are very easy-to-use screens that screen for bipolar disorder or that screen for past history of depression with which these women can be identified.
In committee opinion 757, the American College of Obstetricians and Gynecologists recommends women be screened at least once during the perinatal period for depression and anxiety symptoms and highlighted several validated tools, such as the Edinburgh Postnatal Depression Scale.3 We also need a better system of early detection and early intervention so that women at less-considerable risk for perinatal depression would have the opportunity for early identification, treatment, and referral, which we do not have at the current time.
An update of the ACOG committee opinion also states, “It is recommended that all obstetrician-gynecologists and other obstetric care providers complete a full assessment of mood and emotional well-being (including screening for PPD and anxiety with a validated instrument) during the comprehensive postpartum visit for each patient.” This is recommended in addition to any screening for depression and anxiety during the pregnancy.
It is exciting that after decades of failing to attend to such a common complication of modern obstetrics, particularly now that we understand the adverse effects of PPD as it affects child development, family functioning, and risk for later childhood psychopathology. But in addition to recognizing the problem, we must come up with methods to carefully identify a navigable route for the women suffering from PPD to get their needs met. The route includes publicly identifying the illness, understanding which treatments are most effective and can be scaled for delivery to large numbers of women, and then, most critically, configuring social systems to absorb, effectively manage, and monitor the women we identify as needing treatment.
Dr. Cohen is the director of the Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital in Boston, which provides information resources and conducts clinical care and research in reproductive mental health. He has been a consultant to manufacturers of psychiatric medications. Email him at obnews@mdedge.com.
References
1. J Clin Psychiatry. 2016 Sep;77[9]:1189-200.
2. Draft Recommendation Statement: Perinatal Depression: Preventive Interventions. U.S. Preventive Services Task Force. Aug 2018.
Aspirin for primary cardiovascular prevention, RIP
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
SNOWMASS, COLO. – during the space of a few short weeks in autumn 2018.
“Is aspirin safe and effective for primary prevention? The short answer here is no,” Patrick T. O’Gara, MD, declared at the annual Cardiovascular Conference at Snowmass sponsored by the American College of Cardiology.
“Think of all those decades of aspirin therapy in the hopes of making ourselves healthier,” added Dr. O’Gara, professor of medicine at Harvard Medical School, Boston, and a past president of the American College of Cardiology.
He cited the results of three placebo-controlled randomized trials totaling more than 47,000 patients without known cardiovascular disease: ARRIVE, published in late September 2018, followed in October by ASPREE and ASCEND.
• ARRIVE. This double-blind study conducted in seven countries included 12,546 patients deemed at moderate cardiovascular risk, with an estimated 10-year cardiovascular event risk of 17%. Eligibility was restricted to men aged 55 and up and women aged 60 or older. After a median follow-up of 5 years, there was no difference between patients assigned to enteric-coated aspirin at 100 mg/day versus placebo in the incidence of major adverse cardiovascular events, with a hazard ratio of 0.96. However, GI bleeding events were 2.1-fold more common in the aspirin group (Lancet. 2018 Sep 22;392[10152]:1036-46).
• ASPREE. This double-blind trial, conducted in Australia and the United States, included 19,114 community-dwelling participants aged 70 years or older, or 65 years or older for Hispanics and blacks in the United States. After a median 4.7 years of follow-up, there was no difference in major adverse cardiovascular events between subjects randomized to 100 mg/day of enteric-coated aspirin and those on placebo. So, as in ARRIVE, no benefit. However, the rate of major hemorrhage was 38% greater in the aspirin group (N Engl J Med. 2018 Oct 18;379[16]:1509-18).
Moreover, the rate of all-cause mortality was 14% greater in the aspirin group, a statistically significant difference, compared with controls. Drilling down, the investigators showed that the major contributor to this excess mortality in the aspirin group was their 31% greater rate of cancer-related death (N Engl J Med. 2018 Oct 18;379[16]:1519-28).
“Remember, we used to think that taking aspirin reduced the incidence of GI cancer, and, in particular, colon adenocarcinoma? Well, here’s a very startling observation in 19,114 healthy elderly patients showing an increase in cancer-associated death with the use of aspirin,” commented Dr. O’Gara.
• ASCEND. This study randomized 15,480 subjects with diabetes but no known cardiovascular disease to 100 mg/day of aspirin or placebo and followed them for a mean of 7.4 years. There was a significant 12% relative risk reduction in the composite endpoint of serious vascular events in the aspirin group; however, the aspirin-treated patients also had a 29% greater rate of major bleeding events (N Engl J Med. 2018 Oct 18;379[16]:1529-39).
“So in dealing with our diabetic patients, we could perhaps say there is a small reduction in the risk of cardiovascular outcomes that is overwhelmed by more than a factor of two with regard to an increase in the risk of bleeding,” the cardiologist observed.
How did physicians get the aspirin story for primary prevention so wrong for so long? Dr. O’Gara pointed to the Physicians’ Health Study, conducted mainly back in the 1970s, as one of the benchmark studies that led to the widespread use of aspirin in this way.
“I think the aspirin story has now been put into sharp focus just within the course of the last 6 months and should force all of us to reassess what it is that we advise patients,” he concluded.
Dr. O’Gara’s presentation was the talk of the meeting, as many attendees hadn’t yet caught up with the latest aspirin data.
During an Q&A session, Robert A. Vogel, MD, a preventive cardiology authority at the University of Colorado, Denver, was asked, given the new emphasis placed upon coronary artery calcium as a supplemental risk assessment tool in the latest guidelines, at what magnitude of coronary artery calcium score in a patient with no history of coronary disease he would give aspirin for secondary prevention.
“I know I don’t know the answer to that question,” Dr. Vogel replied. “I no longer reflexively give aspirin to, say, a 60-year-old with a calcium score of 200. I will give a statin. Statins in my book are so effective and safe that my threshold for giving a statin in a 60-year-old is virtually nothing. But with a calcium score of 2,000 or 5,000, I worry just like you worry.”
He noted that the primary prevention patients in the three recent major trials were mostly 60-70 years of age or older. It’s safe to assume that by that point in life many of them had silent atherosclerosis and would have had a non-zero coronary artery calcium score, had they been tested. And yet, aspirin didn’t provide any net benefit in those groups, unlike the drug’s rock-solid proven value in patients who have actually experienced a cardiovascular event.
Dr. O’Gara reported receiving funding from the National Heart, Lung and Blood Institute, the National Institute of Dental and Craniofacial Research, from Medtronic in conjunction with the ongoing pivotal APOLLO transcatheter mitral valve replacement trial, and from Edwards Lifesciences for the ongoing EARLY TAVR trial.
This article was updated 1/31/19.
REPORTING FROM ACC SNOWMASS 2019
Experts cite different approaches to try for methotrexate-related nausea, fatigue
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
Methotrexate-related nausea and fatigue are an issue for many rheumatology patients who otherwise benefit from the treatment, but
Subcutaneous dosing is one option suggested by panel members Christopher Ritchlin, MD, of the University of Rochester (N.Y.) and Douglas Veale, MD, of St. Vincent’s University Hospital, Dublin, who along with several other colleagues fielded questions during a discussion session at the Winter Rheumatology Symposium sponsored by the American College of Rheumatology.
“I like sub-Q,” agreed discussion moderator Michael E. Weinblatt, MD, of Brigham and Women’s Hospital, Boston. “But I actually would lower the dose sub-Q before I gave it because you actually may get a higher serum level,” he said, explaining that that may negate the benefits with respect to nausea.
His preferred approach, he said, is to either split dosing – for example, splitting a 20-mg dose into two 10-mg doses followed 10 hours later with Leucovorin (folinic acid) – or to give the full methotrexate dose at bedtime followed 10 hours later with Leucovorin.
“I use a lot of Leucovorin – a lot,” he noted.
For those patients who get nauseated at the mere mention of methotrexate, though, it’s probably best to try a different drug, he said.
Another approach, suggested by Vivian Bykerk, MD, of the Hospital for Special Surgery in New York, is to give Zofran (ondansetron) with methotrexate. Other suggestions included lowering the methotrexate dose and injecting the drug.
For fatigue, there has been some suggestion of a benefit with B-12 supplementation, panel members said. Dr. Bykerk noted some ongoing work that is demonstrating a benefit with sublingual B-12 for methotrexate intolerance in general, and it appears to allow for much higher methotrexate dosing, she said.
Early reports regarding those as-yet-unpublished data are, indeed, promising, Dr. Weinblatt said, noting that in his practice he has been using subcutaneous B-12 given at about 1,000 mcg 2 days before methotrexate and then on the day of methotrexate for patients with fatigue who are “failing Leucovorin, failing caffeine.”
Addressing fatigue and nausea in patients on methotrexate is important because, in his experience in appropriately monitored patients, it’s not serious adverse events, but rather fatigue and nausea, that most often lead to stopping methotrexate.
Dr. Weinblatt reported financial relationships with nearly 20 pharmaceutical companies. Dr. Bykerk reported financial relationships with Amgen, Brainstorm, Bristol-Myers Squibb, Pfizer, and Regeneron. Dr. Veale reported financial relationships with AbbVie, Janssen, Pfizer, Roche, and UCB. Dr. Ritchlin reported financial relationships with AbbVie, Amgen, Janssen, Lilly, Novartis, Pfizer, and UCB.
EXPERT ANALYSIS FROM THE WINTER RHEUMATOLOGY SYMPOSIUM
Impact of Migraine on Health Care in Obese Adults
In a population of obese adults in the United States, migraineurs showed greater total health care utilization and expenses than non-migraineurs, a recent study found. Therefore, treatment plans that address risk factors associated with migraine and comorbidities may help reduce the utilization of health care services and costs. This 7-year retrospective study used longitudinal panel data from 2006 to 2013 from the Household Component of the Medical Expenditure Panel Survey to identify obese adults reporting migraines. Outcomes compared in migraineurs versus non-migraineurs were as follows: annualized per-person medical care, prescription drug, and total health expenses. Researchers found:
- In 23,596 obese adults, 4.7% reported migraine (n=1025) approximating 3 million civilian non-institutionalized individuals in the United States.
- Logistic regression showed that the following sociodemographic characteristics increased migraine risk: age (18–45 years), females, white race, poor perceived health status, and greater Charlson comorbidity index.
- Migraineurs showed $1401, $813, and $2213 greater annual medical, prescription drug, and total health expenses than non-migraineurs, respectively.
- After adjustment, total health expenses increased by 31.6% in migraineurs versus non-migraineurs.
Wu J, Davis-Ajami ML, Lu ZK. Impact of migraine on health care utilization and expenses in obese adults: A US population-based study. [Published online ahead of print December 31, 2018]. Clinicoecon Outcomes Res. doi:10.2147/CEOR.S189699.
In a population of obese adults in the United States, migraineurs showed greater total health care utilization and expenses than non-migraineurs, a recent study found. Therefore, treatment plans that address risk factors associated with migraine and comorbidities may help reduce the utilization of health care services and costs. This 7-year retrospective study used longitudinal panel data from 2006 to 2013 from the Household Component of the Medical Expenditure Panel Survey to identify obese adults reporting migraines. Outcomes compared in migraineurs versus non-migraineurs were as follows: annualized per-person medical care, prescription drug, and total health expenses. Researchers found:
- In 23,596 obese adults, 4.7% reported migraine (n=1025) approximating 3 million civilian non-institutionalized individuals in the United States.
- Logistic regression showed that the following sociodemographic characteristics increased migraine risk: age (18–45 years), females, white race, poor perceived health status, and greater Charlson comorbidity index.
- Migraineurs showed $1401, $813, and $2213 greater annual medical, prescription drug, and total health expenses than non-migraineurs, respectively.
- After adjustment, total health expenses increased by 31.6% in migraineurs versus non-migraineurs.
Wu J, Davis-Ajami ML, Lu ZK. Impact of migraine on health care utilization and expenses in obese adults: A US population-based study. [Published online ahead of print December 31, 2018]. Clinicoecon Outcomes Res. doi:10.2147/CEOR.S189699.
In a population of obese adults in the United States, migraineurs showed greater total health care utilization and expenses than non-migraineurs, a recent study found. Therefore, treatment plans that address risk factors associated with migraine and comorbidities may help reduce the utilization of health care services and costs. This 7-year retrospective study used longitudinal panel data from 2006 to 2013 from the Household Component of the Medical Expenditure Panel Survey to identify obese adults reporting migraines. Outcomes compared in migraineurs versus non-migraineurs were as follows: annualized per-person medical care, prescription drug, and total health expenses. Researchers found:
- In 23,596 obese adults, 4.7% reported migraine (n=1025) approximating 3 million civilian non-institutionalized individuals in the United States.
- Logistic regression showed that the following sociodemographic characteristics increased migraine risk: age (18–45 years), females, white race, poor perceived health status, and greater Charlson comorbidity index.
- Migraineurs showed $1401, $813, and $2213 greater annual medical, prescription drug, and total health expenses than non-migraineurs, respectively.
- After adjustment, total health expenses increased by 31.6% in migraineurs versus non-migraineurs.
Wu J, Davis-Ajami ML, Lu ZK. Impact of migraine on health care utilization and expenses in obese adults: A US population-based study. [Published online ahead of print December 31, 2018]. Clinicoecon Outcomes Res. doi:10.2147/CEOR.S189699.
Are People with Migraine More Pessimistic?
Optimism and pessimism are associated with migraine and migraine‐related disability. This according to a recent study that found that people with migraine were less optimistic and more pessimistic than controls and endorsed higher levels of anxious and depressive symptoms. The sample population was selected through a stratified, multi‐stage area probability sample of households. A validated questionnaire eliciting data on demographics, headache features, migraine‐related disability, depression (PHQ‐9), anxiety (GAD‐7), optimism, and pessimism was administered to people with migraine and headache‐free control participants via trained interviewers. The odds for having migraine/no headache diagnosis were calculated by binary logistic regression, and ordinal regression was performed to check associations between migraine‐related disability and optimism. Researchers found:
- Out of 600 individuals, 302 met inclusion criteria and were included (140 controls [with no history of headache disorders] and 162 people meeting criteria for migraine [29 with chronic migraine, that is, ≥15 headache days/month]).
- Pessimism and anxiety were predictors of meeting criteria for migraine, while optimism was inversely associated with migraine‐related disability.
Peres MFP, Belitardo A, Mercante JP, et al. Optimism, pessimism, and migraine: A cross‐sectional, population‐based study. [Published online ahead of print January 19, 2019]. Headache. doi:10.1111/head.13471.
Optimism and pessimism are associated with migraine and migraine‐related disability. This according to a recent study that found that people with migraine were less optimistic and more pessimistic than controls and endorsed higher levels of anxious and depressive symptoms. The sample population was selected through a stratified, multi‐stage area probability sample of households. A validated questionnaire eliciting data on demographics, headache features, migraine‐related disability, depression (PHQ‐9), anxiety (GAD‐7), optimism, and pessimism was administered to people with migraine and headache‐free control participants via trained interviewers. The odds for having migraine/no headache diagnosis were calculated by binary logistic regression, and ordinal regression was performed to check associations between migraine‐related disability and optimism. Researchers found:
- Out of 600 individuals, 302 met inclusion criteria and were included (140 controls [with no history of headache disorders] and 162 people meeting criteria for migraine [29 with chronic migraine, that is, ≥15 headache days/month]).
- Pessimism and anxiety were predictors of meeting criteria for migraine, while optimism was inversely associated with migraine‐related disability.
Peres MFP, Belitardo A, Mercante JP, et al. Optimism, pessimism, and migraine: A cross‐sectional, population‐based study. [Published online ahead of print January 19, 2019]. Headache. doi:10.1111/head.13471.
Optimism and pessimism are associated with migraine and migraine‐related disability. This according to a recent study that found that people with migraine were less optimistic and more pessimistic than controls and endorsed higher levels of anxious and depressive symptoms. The sample population was selected through a stratified, multi‐stage area probability sample of households. A validated questionnaire eliciting data on demographics, headache features, migraine‐related disability, depression (PHQ‐9), anxiety (GAD‐7), optimism, and pessimism was administered to people with migraine and headache‐free control participants via trained interviewers. The odds for having migraine/no headache diagnosis were calculated by binary logistic regression, and ordinal regression was performed to check associations between migraine‐related disability and optimism. Researchers found:
- Out of 600 individuals, 302 met inclusion criteria and were included (140 controls [with no history of headache disorders] and 162 people meeting criteria for migraine [29 with chronic migraine, that is, ≥15 headache days/month]).
- Pessimism and anxiety were predictors of meeting criteria for migraine, while optimism was inversely associated with migraine‐related disability.
Peres MFP, Belitardo A, Mercante JP, et al. Optimism, pessimism, and migraine: A cross‐sectional, population‐based study. [Published online ahead of print January 19, 2019]. Headache. doi:10.1111/head.13471.
Migraine Age of Onset and Ischemic Stroke Risk
Increased stroke risk in late life was observed in participants with late onset of migraine with aura (MA), as compared to participants with no headache in a recent ongoing, prospective, longitudinal, community‐based cohort study. Longer cumulative exposure to migraine with visual aura, however, was not associated with increased risk of ischemic stroke in late life. Participants were interviewed to ascertain migraine history at the third visit (1993–1995) and followed for ischemic stroke incidence over 20 years. Researchers performed a post hoc analysis to evaluate the association between the age of migraine onset and ischemic stroke. They found:
- There were 447 migraineurs with MA and 1128 migraineurs without aura (MO) identified among 11,592 black and white participants.
- There was an association between the age of MA onset at age ≥50 years (average duration=4.75 years) and ischemic stroke when compared to the no headache group (multivariable adjusted HR=2.17).
- MA onset at <50 years (average duration=28.17 years) was not associated with stroke (multivariable adjusted HR=1.31).
- MO was not associated with increased stroke regardless of the age of onset.
Androulakis XM, Sen S, Kodumuri N, et al. Migraine age of onset and association with ischemic stroke in late life: 20 years follow‐up in ARIC. [Published online ahead of print January 21, 2019]. Headache. doi:10.1111/head.13468.
Increased stroke risk in late life was observed in participants with late onset of migraine with aura (MA), as compared to participants with no headache in a recent ongoing, prospective, longitudinal, community‐based cohort study. Longer cumulative exposure to migraine with visual aura, however, was not associated with increased risk of ischemic stroke in late life. Participants were interviewed to ascertain migraine history at the third visit (1993–1995) and followed for ischemic stroke incidence over 20 years. Researchers performed a post hoc analysis to evaluate the association between the age of migraine onset and ischemic stroke. They found:
- There were 447 migraineurs with MA and 1128 migraineurs without aura (MO) identified among 11,592 black and white participants.
- There was an association between the age of MA onset at age ≥50 years (average duration=4.75 years) and ischemic stroke when compared to the no headache group (multivariable adjusted HR=2.17).
- MA onset at <50 years (average duration=28.17 years) was not associated with stroke (multivariable adjusted HR=1.31).
- MO was not associated with increased stroke regardless of the age of onset.
Androulakis XM, Sen S, Kodumuri N, et al. Migraine age of onset and association with ischemic stroke in late life: 20 years follow‐up in ARIC. [Published online ahead of print January 21, 2019]. Headache. doi:10.1111/head.13468.
Increased stroke risk in late life was observed in participants with late onset of migraine with aura (MA), as compared to participants with no headache in a recent ongoing, prospective, longitudinal, community‐based cohort study. Longer cumulative exposure to migraine with visual aura, however, was not associated with increased risk of ischemic stroke in late life. Participants were interviewed to ascertain migraine history at the third visit (1993–1995) and followed for ischemic stroke incidence over 20 years. Researchers performed a post hoc analysis to evaluate the association between the age of migraine onset and ischemic stroke. They found:
- There were 447 migraineurs with MA and 1128 migraineurs without aura (MO) identified among 11,592 black and white participants.
- There was an association between the age of MA onset at age ≥50 years (average duration=4.75 years) and ischemic stroke when compared to the no headache group (multivariable adjusted HR=2.17).
- MA onset at <50 years (average duration=28.17 years) was not associated with stroke (multivariable adjusted HR=1.31).
- MO was not associated with increased stroke regardless of the age of onset.
Androulakis XM, Sen S, Kodumuri N, et al. Migraine age of onset and association with ischemic stroke in late life: 20 years follow‐up in ARIC. [Published online ahead of print January 21, 2019]. Headache. doi:10.1111/head.13468.
Hospitalist PA and health system leader: Emilie Thornhill Davis
Building a collaborative practice
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.
Building a collaborative practice
Building a collaborative practice
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.
Emilie Thornhill Davis, PA-C, is the assistant vice president for advanced practice providers at Ochsner Health System in New Orleans. She is the former chair of SHM’s Nurse Practitioner and Physician Assistant (NP/PA) Committee, and has spoken multiple times at the SHM Annual Conference.
In honor of the inaugural National Hospitalist Day, to be held on Thursday, March 7, 2019, The Hospitalist spoke with Ms. Davis about the unique contributions of NP and PA hospitalists to the specialty of hospital medicine.
Where did you get your education?
I got my undergraduate degree from Mercer University and then went on to get my prerequisites for PA school, and worked clinically for a year prior to starting graduate school in Savannah, Georgia, at South University.
Was your intention always to be a PA?
During my sophomore year of college, Mercer was starting a PA program. Having been taken care of by PAs for most of my life, I realized that this was a profession I was very interested in. I shadowed a lot of PAs and found that they had extremely high levels of satisfaction. I saw the versatility to do so many types of medicine as a PA.
How did you become interested in hospital medicine?
When I was in PA school, we had small groups that were led by a PA who practiced clinically. The PA who was my small group leader was a hospitalist and was a fantastic role model. I did a clinical rotation with her team, and then went on to do my elective with her team in hospital medicine. When I graduated, I got my first job with the hospital medicine group that I had done those clinical rotations with in Savannah. And then in 2013, after about a year and a half, life brought me to New Orleans and I started working at Ochsner in the department of hospital medicine. I was one of the first two PAs that this group had employed.
What is your current role and title at Ochsner?
From 2013 to 2018, I worked in the department of hospital medicine, and for the last 2 years I functioned as the system lead for advanced practice providers in the department of hospital medicine. In September of 2018, I accepted the role of assistant vice president of advanced practice providers for Ochsner Health System.
What are your areas of interest or research?
I’ve had the opportunity to speak at the annual Society of Hospital Medicine Conference for 3 years in a row on innovative models of care, and nurse practitioner and PA utilization in hospital medicine. I was the chair for the NP/PA committee, and during that time we developed a toolkit aimed at providing a resource to hospital medicine groups around nurse practitioner and PA integration to practice in full utilization.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Nurse practitioners and PAs are perfectly set up to integrate into practice in hospital medicine. Training for PAs specifically is based on the medicine model, where you have a year of didactic and a year of clinical work in all the major disciplines of medicine. And so in a clinical year as a PA, I would rotate through primary care, internal medicine, general surgery, ob.gyn., psychiatry, emergency medicine, pediatrics. When I come out of school, I’m generalist trained, and depending on where your emphasis was during clinical rotations, that could include a lot of inpatient experience.
I transitioned very smoothly into my first role in hospital medicine as a PA, because I had gained that experience while I was a student on clinical rotations. PAs and nurse practitioners are – when they’re utilized appropriately and at the top of their experience and training – able to provide services to patients that can improve quality outcomes, enhance throughput, decrease length of stay, and improve all the different areas that we focus on as hospitalists.
What roles can a PA occupy in relation to physicians and nurse practitioners in hospital medicine?
When you’re looking at a PA versus a nurse practitioner in hospital medicine, you’ll notice that there are differences in the way that PAs and nurse practitioners are trained. All PAs are trained on a medical model and have a very similar kind of generalist background, whereas a nurse practitioner is typically schooled with nursing training that includes bedside experience that you can’t always guarantee with PAs. But once we enter into practice, our scope and the way that we take care of patients over time becomes very similar. So a PA and a nurse practitioner for the most part can function in very similar capacities in hospital medicine.
The only thing that creates a difference for PAs and NPs are federal and state rules and regulations, as well as hospital policies that might create “scope of practice” barriers. For instance, when I first moved to Louisiana, PAs were not able to prescribe Schedule II medications. That created a barrier whenever I was discharging patients who needed prescriptions for Schedule II. That has since changed in the state of Louisiana; now both PAs and NPs have full prescriptive authority in the state.
I would compare the work of PAs and NPs to that of physicians like this: Once you have NPs or PAs who are trained and have experience in the specialty that they are working in, they are able to provide services that would otherwise be provided by physicians.
How does a hospitalist PA work differently from a PA in other care settings?
The scope of practice for a PA is defined by the physician they’re working with. So my day-to-day work as a PA in hospital medicine looked very similar to a physician’s day-to-day work in hospital medicine. In cardiology, for example, the same would likely hold true, but with tasks unique to that specialty.
How does SHM support hospitalist PAs?
SHM is the home where you have physicians, nurse practitioners, and PAs all represented by one society, which I think is really important whenever we’re talking about a membership organization that reflects what things truly look like in practice. When I am a member of SHM, and the physician I work with is a member of SHM, we are getting the same journals and are both familiar with the changes that occur nationally in our specialty; this really helps us to align ourselves clinically, and to understand what’s going on across the country.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
I think the first thing we have to do is make sure that we’re getting the nomenclature right, that we’re referring to nurse practitioners and physician assistants by their appropriate names and recognizing their role in hospital medicine. Every year that I spoke at the SHM Annual Conference, I had many hospital medicine leaders come up to me and say they needed help with incorporating NPs and PAs, not only clinically, but also making sure they were represented within their hospital system. That’s why we developed the toolkit, which provides resources for integrating NPs and PAs into practice.
There is an investment early on when you bring PAs into your group to train them. We often use the SHM core competencies when we are referring to a training guide for PAs or NPs as a way to categorize the different materials that they would need to know to practice efficiently, but I do think those could be expanded upon.
What’s on the horizon for NPs and PAs in hospital medicine?
One national trend I see is an increase in the number of NPs and PAs entering hospital medicine. The other big trend is the formal development of postgraduate fellowships for PAs in hospital medicine. As the complexity of our health systems continues to grow, the feeling is that to get a nurse practitioner and PA the training they need, there are benefits to having a protected postgrad year to learn.
One unique thing about nurse practitioners and PAs is their versatility and their ability to move among the various medical specialties. As a PA or a nurse practitioner, if I’m working in hospital medicine and I have a really strong foundation, there’s nothing to say that I couldn’t then accept a job in CV surgery or cardiology and bring those skills with me from hospital medicine.
But this is kind of a double-edged sword, because it also means that you may have a PA or NP leave your HM group after 1-2 years. That kind of turnover is a difficult thing to address, because it means dealing with issues such as workplace culture and compensation. But that shows why training and engagement is important early on in that first year – to make sure that NPs and PAs feel fully supported to meet the demands that hospital medicine requires. All of those things really factor into whether an NP or PA will choose to continue in the field.