User login
Hire Hard, Manage Easy
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
The socio-adaptive (or “nontechnical”) aspects of healthcare including leadership, followership, mentorship, culture, teamwork, and communication are not formally taught in medical training. Yet, they are critical to our daily lives as Hospitalists. The LPD series features brief “pearls of wisdom” that highlight these important lessons.
“If you can hire people whose passion intersects with the job, they won’t require any supervision at all. They will manage themselves better than anyone could ever manage them. Their fire comes from within, not from without.”
—Stephen Covey
When you initiate a quality or performance improvement project, you want to find someone who can help you do the necessary work and find that someone quickly. But be warned: leaders must learn to go slow when hiring for their team. Do not settle on whoever has available time or interest—they may have time to give or be eager for a reason.
We see this unfold in several ways. For example, individuals are sometimes “offered” up for a role: “This person has experience reviewing charts and abstracting data—and they have some time available. Would you like to hire them?” Similarly, eager students or faculty may be willing to jump on a project with you—“I am looking to join a project,” or “Yes, I can help with that,” are all too often heard in this context. Both scenarios share in common one truth: easy availability and willingness to help make it tempting to say, “Sure.”
While some of these individuals might be ideal, many are not. When hiring, you have to think hard about the role and an individual’s skill set that makes them well suited for it. Based on experience, we can tell you that once you go “soft” by selecting a suboptimal candidate, you are in trouble for at least three reasons. First, hiring the right people is the key to achieving success for your initiative. And success in your project reflects directly on you. People will make inferences about you based on the people you surround yourself with: if they are terrific, the assumption—right or wrong—is that you are as well. Second, we tend to compensate for underperforming employees, often at great cost to ourselves or others. When data collection for a project does not go well, we have found ourselves behind the screen filling in various portions of a data collection form. For example, a colleague once told us, “I hired this person to help, but they ended up needing so much assistance that it was often easier for me and others to do the work. The environment quickly became toxic.”
Third, it is often difficult to remove an underperforming employee or have them change positions. Health organizations (especially universities or other public institutions) can be rigid that way. An infection prevention leader told us of waiting a whole year to fill a crucial vacancy before she found the right person. It was ultimately the right decision, she said, adding, “My life is so much better.”
How can you be sure you have found the right person? Regardless of whether you are hiring for a permanent or temporary position, staff or faculty member, we recommend the following:
- Ensure recruits meet with several people. The more eyes on a candidate, the better. Often, someone will catch something you may not—and having many people involved helps get the team invested in the success of your hire.
- Standardize and solicit feedback. For example, we use a standardized template to garner feedback on administrative recruits, project managers, and faculty. This way, we all are evaluating potential colleagues through the same structured approach.
- Ensure skills match the role. For example, an ethnographic study would benefit from someone skilled in qualitative methods. Similarly, a project manager experienced in clinical trials would be best suited for patient recruitment and managing investigators at several sites. Identifying what is clearly needed in the role is a key step in hiring.
Management guru Jim Collins writes: “The moment you feel the need to tightly manage someone, you’ve made a hiring mistake. The best people don’t need to be managed. Guided, taught, led—yes. But not tightly managed.”1 True in management, and true in the world of healthcare. Hire Hard. In the long run, you will be able to manage easy.
Disclosures
Drs. Chopra and Saint are co-authors of the upcoming book, “Thirty Rules for Healthcare Leaders,” from which this article is adapted. Both authors have no other relevant conflicts of interest.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
1. Collins J. Recruitment and Selection. In: Garner E, ed. The Art of Managing People. 550 quotes on how to get the best out of others. Eric Garner & Ventus Publishing ApS. 2012;39. https://bookboon.com/en/the-art-of-managing-people-ebook. Accessed January 7, 2019.
© 2019 Society of Hospital Medicine
Introducing Leadership & Professional Development: A New Series in JHM
“I cannot say whether things will get better if we change; what I can say is they must change if they are to get better.”
—Georg C. Lichtenburg
Leading change is never easy. Many a physician has joined a committee, hired a promising project manager, assumed responsibility for an operational or clinical task—only to have it painfully falter or agonizingly fail. Unfortunately, some of us become disillusioned with the process, donning our white coats to return to the safe ensconce of clinical work rather than take on another perilous change or leadership task. But ask those that have tried and failed and those that have succeeded and they will tell you this: the lessons learned in the journey were invaluable.
Academic medical centers and healthcare organizations are increasingly turning to hospitalists to assume a myriad of leadership roles. With very little formal training, many of us jump in to improve organizational culture, financial accountability, and patient safety, literally building the bridge as we walk on it. The practical knowledge and know-how gleaned in efforts during these endeavors are perhaps just as important as evidence-based medicine. And yet, few venues to share and disseminate these insights currently exist.
This void represents the motivation behind the new Journal series entitled, “Leadership & Professional Development” or “LPD.” In these brief excerpts, lessons on leadership/followership, mentorship/menteeship, leading change and professional development will be shared using a conversational and pragmatic tone. Like a clinical case, pearls to help you navigate development and organizational challenges will be shared. The goal is simple: read an LPD and walk away with an “a-ha,” a new tool, or a strategy that you can use ASAP. For example, in the debut LPD—Hire Hard1—we emphasize a cardinal rule for hiring: wait for the right person. Waiting is not easy, but it is well worth it in the long run—the right person will make your job that much better. Remember the aphorism: A’s hire A’s while B’s hire C’s.
Many other nuggets of wisdom can fit an LPD model. For example, when it comes to stress, a technique that brings mindfulness to your day—one you can practice with every patient encounter—might be the ticket.2 Interested in mentoring? You’ll need to know the Six Golden Rules.3 And don’t forget about emotional intelligence, tight-loose-tight management or the tree-climbing monkey! Don’t know what these are? Time to read an LPD or two to find out!
As you might have guessed—some of these pieces are already written. They come from a book that my colleague, Sanjay Saint and I have been busy writing for over a year. The book distills much of what we have learned as clinicians, researchers and administrators into a collection we call, “Thirty Leadership Rules for Healthcare Providers.” But LPD is not an advert for the book; rather, our contributions will only account for some of the series. We hope this venue will become a platform in where readers like you can offer “pearls” to the broader community. The rules are simple: coin a rule/pearl, open with an illustrative quote, frame it in 650 words with no more than five references, and write it so that a reader can apply it to their work tomorrow. And don’t worry—we on the editorial team will help you craft th
Disclosures
Dr. Chopra has nothing to disclose.
1. Chopra V, Saint S. Hire Hard. Manage Easy. J Hosp Med. 2019;14(2):74. doi: 10.12788/jhm.3158.
2. Gilmartin H, Saint S, Rogers M, et al. Pilot randomised controlled trial to improve hand hygiene through mindful moments. BMJ Qual Saf. 2018;27(10):799-806. PubMed
3. Chopra V, Saint S. What Mentors Wish Their Mentees Knew. Harvard Business Review. 2017. https://hbr.org/2017/11/what-mentors-wish-their-mentees-knew. Accessed December 17, 2018. PubMed
“I cannot say whether things will get better if we change; what I can say is they must change if they are to get better.”
—Georg C. Lichtenburg
Leading change is never easy. Many a physician has joined a committee, hired a promising project manager, assumed responsibility for an operational or clinical task—only to have it painfully falter or agonizingly fail. Unfortunately, some of us become disillusioned with the process, donning our white coats to return to the safe ensconce of clinical work rather than take on another perilous change or leadership task. But ask those that have tried and failed and those that have succeeded and they will tell you this: the lessons learned in the journey were invaluable.
Academic medical centers and healthcare organizations are increasingly turning to hospitalists to assume a myriad of leadership roles. With very little formal training, many of us jump in to improve organizational culture, financial accountability, and patient safety, literally building the bridge as we walk on it. The practical knowledge and know-how gleaned in efforts during these endeavors are perhaps just as important as evidence-based medicine. And yet, few venues to share and disseminate these insights currently exist.
This void represents the motivation behind the new Journal series entitled, “Leadership & Professional Development” or “LPD.” In these brief excerpts, lessons on leadership/followership, mentorship/menteeship, leading change and professional development will be shared using a conversational and pragmatic tone. Like a clinical case, pearls to help you navigate development and organizational challenges will be shared. The goal is simple: read an LPD and walk away with an “a-ha,” a new tool, or a strategy that you can use ASAP. For example, in the debut LPD—Hire Hard1—we emphasize a cardinal rule for hiring: wait for the right person. Waiting is not easy, but it is well worth it in the long run—the right person will make your job that much better. Remember the aphorism: A’s hire A’s while B’s hire C’s.
Many other nuggets of wisdom can fit an LPD model. For example, when it comes to stress, a technique that brings mindfulness to your day—one you can practice with every patient encounter—might be the ticket.2 Interested in mentoring? You’ll need to know the Six Golden Rules.3 And don’t forget about emotional intelligence, tight-loose-tight management or the tree-climbing monkey! Don’t know what these are? Time to read an LPD or two to find out!
As you might have guessed—some of these pieces are already written. They come from a book that my colleague, Sanjay Saint and I have been busy writing for over a year. The book distills much of what we have learned as clinicians, researchers and administrators into a collection we call, “Thirty Leadership Rules for Healthcare Providers.” But LPD is not an advert for the book; rather, our contributions will only account for some of the series. We hope this venue will become a platform in where readers like you can offer “pearls” to the broader community. The rules are simple: coin a rule/pearl, open with an illustrative quote, frame it in 650 words with no more than five references, and write it so that a reader can apply it to their work tomorrow. And don’t worry—we on the editorial team will help you craft th
Disclosures
Dr. Chopra has nothing to disclose.
“I cannot say whether things will get better if we change; what I can say is they must change if they are to get better.”
—Georg C. Lichtenburg
Leading change is never easy. Many a physician has joined a committee, hired a promising project manager, assumed responsibility for an operational or clinical task—only to have it painfully falter or agonizingly fail. Unfortunately, some of us become disillusioned with the process, donning our white coats to return to the safe ensconce of clinical work rather than take on another perilous change or leadership task. But ask those that have tried and failed and those that have succeeded and they will tell you this: the lessons learned in the journey were invaluable.
Academic medical centers and healthcare organizations are increasingly turning to hospitalists to assume a myriad of leadership roles. With very little formal training, many of us jump in to improve organizational culture, financial accountability, and patient safety, literally building the bridge as we walk on it. The practical knowledge and know-how gleaned in efforts during these endeavors are perhaps just as important as evidence-based medicine. And yet, few venues to share and disseminate these insights currently exist.
This void represents the motivation behind the new Journal series entitled, “Leadership & Professional Development” or “LPD.” In these brief excerpts, lessons on leadership/followership, mentorship/menteeship, leading change and professional development will be shared using a conversational and pragmatic tone. Like a clinical case, pearls to help you navigate development and organizational challenges will be shared. The goal is simple: read an LPD and walk away with an “a-ha,” a new tool, or a strategy that you can use ASAP. For example, in the debut LPD—Hire Hard1—we emphasize a cardinal rule for hiring: wait for the right person. Waiting is not easy, but it is well worth it in the long run—the right person will make your job that much better. Remember the aphorism: A’s hire A’s while B’s hire C’s.
Many other nuggets of wisdom can fit an LPD model. For example, when it comes to stress, a technique that brings mindfulness to your day—one you can practice with every patient encounter—might be the ticket.2 Interested in mentoring? You’ll need to know the Six Golden Rules.3 And don’t forget about emotional intelligence, tight-loose-tight management or the tree-climbing monkey! Don’t know what these are? Time to read an LPD or two to find out!
As you might have guessed—some of these pieces are already written. They come from a book that my colleague, Sanjay Saint and I have been busy writing for over a year. The book distills much of what we have learned as clinicians, researchers and administrators into a collection we call, “Thirty Leadership Rules for Healthcare Providers.” But LPD is not an advert for the book; rather, our contributions will only account for some of the series. We hope this venue will become a platform in where readers like you can offer “pearls” to the broader community. The rules are simple: coin a rule/pearl, open with an illustrative quote, frame it in 650 words with no more than five references, and write it so that a reader can apply it to their work tomorrow. And don’t worry—we on the editorial team will help you craft th
Disclosures
Dr. Chopra has nothing to disclose.
1. Chopra V, Saint S. Hire Hard. Manage Easy. J Hosp Med. 2019;14(2):74. doi: 10.12788/jhm.3158.
2. Gilmartin H, Saint S, Rogers M, et al. Pilot randomised controlled trial to improve hand hygiene through mindful moments. BMJ Qual Saf. 2018;27(10):799-806. PubMed
3. Chopra V, Saint S. What Mentors Wish Their Mentees Knew. Harvard Business Review. 2017. https://hbr.org/2017/11/what-mentors-wish-their-mentees-knew. Accessed December 17, 2018. PubMed
1. Chopra V, Saint S. Hire Hard. Manage Easy. J Hosp Med. 2019;14(2):74. doi: 10.12788/jhm.3158.
2. Gilmartin H, Saint S, Rogers M, et al. Pilot randomised controlled trial to improve hand hygiene through mindful moments. BMJ Qual Saf. 2018;27(10):799-806. PubMed
3. Chopra V, Saint S. What Mentors Wish Their Mentees Knew. Harvard Business Review. 2017. https://hbr.org/2017/11/what-mentors-wish-their-mentees-knew. Accessed December 17, 2018. PubMed
© 2019 Society of Hospital Medicine
A Protean Protein
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
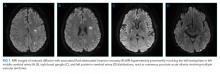
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
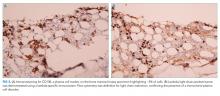
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
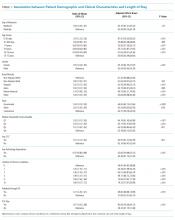
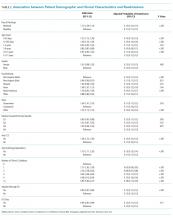
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
A 39-year-old man presented to a neurologist with three weeks of progressive leg weakness associated with numbness in his feet and fingertips. His medical history included hypertriglyceridemia, hypogonadism, and gout. He was taking fenofibrate and colchicine as needed. There was no family history of neurologic issues. He did not smoke or drink alcohol.
The patient appeared well with a heart rate of 76 beats per minute, blood pressure 133/72 mm Hg, temperature 36.6°C, respiratory rate 16 breaths per minute, and oxygen saturation 100% on room air. His cardiopulmonary and abdominal examinations were normal. His skin was warm and dry without rashes. On neurologic examination, upper extremity strength and sensation was normal. Bilateral hip flexion, knee flexion, and knee extension strength was 4/5; bilateral ankle dorsiflexion and plantar flexion strength was 3/5. Reflexes were trace in the arms and absent at the patellae and ankles. He had symmetric, length-dependent reduction in vibration, pinprick, and light touch sensation in his legs.
Peripheral neuropathy presenting with ascending symmetric motor and sensory deficits progressing over three weeks raises the suspicion of an acquired inflammatory demyelinating polyneuropathy (AIDP), a variant of Guillain-Barre Syndrome. Alternative causes of acute polyneuropathy include thiamine (B1) deficiency, vasculitis, sarcoidosis, or malignancy, particularly lymphoma and multiple myeloma. Further evaluation should include electromyography, nerve conduction studies, lumbar puncture with cerebrospinal fluid (CSF) protein, glucose, and cell count differential. Follow-up laboratory testing based on results of the above may include serum protein electrophoresis (SPEP), serum free light chains (sFLC), vitamin B12, human immunodeficiency virus (HIV), hepatitis B and C testing, antinuclear antibody, and erythrocyte sedimentation rate.
Electromyography and nerve conduction studies revealed a sensorimotor mixed axonal/demyelinating polyneuropathy in all extremities. CSF analysis found one white cell per mm3, glucose of 93 mg/dL, and protein of 313 mg/dL. Magnetic resonance imaging (MRI) of the spine without contrast showed normal cord parenchyma. The vitamin B12 level was 441 pg/mL (normal >200 pg/mL). Antibodies to HIV-1, HIV-2, hepatitis C virus, and Borrelia burgdorferi were negative. Serum protein electrophoresis (SPEP) and immunofixation were normal.
The patient received two courses of intravenous immunoglobulin (IVIG) for suspected AIDP. His weakness progressed over the next several weeks to the point that he required a wheelchair.
Progression of symptoms beyond three weeks and lack of response to IVIG are atypical for AIDP. Alternate diagnoses for a sensorimotor polyneuropathy should be considered. Causes of subacute or chronic demyelinating polyneuropathy include inflammatory conditions (chronic inflammatory demyelinating polyneuropathy [CIDP], connective-tissue disorders), paraprotein disorders (myeloma, amyloidosis, lymphoplasmacytic lymphoma), paraneoplastic syndromes, infectious diseases (HIV, Lyme disease), infiltrative disorders (sarcoidosis), medications or toxins, and hereditary disorders. Of these etiologies, the first three seem the most likely given the history and clinical course, the negative HIV and Lyme testing, and the absence of exposures and family history. Normal SPEP and immunofixation make paraprotein disorders less likely, but sFLC testing should be sent to evaluate for a light chain-only paraprotein. A paraneoplastic antibody panel and a CT of the chest, abdomen, and pelvis should be ordered to evaluate for sarcoidosis, lymphoma, or other malignancies. Although a peripheral nerve biopsy would further classify the polyneuropathy, it is of low diagnostic yield in patients with subacute and chronic distal symmetric polyneuropathies and is associated with significant morbidity. In the absence of history or physical exam findings to narrow the differential diagnosis for polyneuropathy, testing for paraneoplastic antibodies and imaging is appropriate.
The patient tested negative for antiganglioside GM1 and antimyelin-associated glycoprotein antibodies. Urine arsenic, lead, and mercury levels were normal. Tests for serum antinuclear antibody, rapid plasmin reagin, and a paraneoplastic neuropathy panel including amphiphysin antibody, CV2 antibody, and Hu auto-antibody were negative. Repeat electrodiagnostic testing was consistent with CIDP. The patient received prednisone 60 mg daily for six weeks and was then tapered to 30 mg daily over six weeks. Concurrently, he underwent twelve cycles of plasma exchange. His strength improved, and he could walk with a cane; however, weakness recurred when steroids were further tapered.
He was maintained on prednisone 50 mg daily. Over the next year, the patient’s lower extremities became flaccid and severely atrophied. He developed hyperpigmented patches on his trunk, severe gastroesophageal reflux disease (GERD), dysphonia, and gynecomastia. He had lost 60 pounds since symptom onset. He was prescribed levothyroxine for subclinical hypothyroidism (thyroid stimulating hormone 12.63 µIU/mL [normal 0.10-5.50 µIU/mL], free thyroxine 0.8 ng/dL [0.8-1.7 ng/dL]).
At this point, the diagnosis of CIDP should be questioned, and additional investigation is warranted. Although improvement was initially observed with plasma exchange and steroids, subsequent progression of symptoms despite prednisone suggests a nonimmune-mediated etiology, such as a neoplastic or infiltrative process. Conversely, negative serologic testing for paraneoplastic antibodies may be due to an antibody that has not been well characterized.
While prednisone could explain GERD and gynecomastia, the weight loss, dysphonia, and subclinical hypothyroidism may offer clues to the diagnosis underlying the neurological symptoms. Weight loss raises suspicion of a hypercatabolic process such as cancer, cachexia, systemic inflammation, heart failure, or chronic obstructive pulmonary disease. Causes of dysphonia relevant to this presentation include neurologic dysfunction related to malignant invasion of the vagus nerve or demyelinating disease. Subclinical hypothyroidism due to chronic autoimmune thyroiditis seems most likely in the absence of a medication effect or thyroid injury, yet infiltrative disorders of the thyroid (eg, amyloidosis, sarcoidosis, lymphoma) should also be considered. A diagnosis that unifies the neurologic and nonneurologic findings would be desirable; lymphoma with paraneoplastic peripheral neuropathy manifesting as CIDP seems most likely. As of yet, CT of the chest, abdomen, and pelvis or an 18-Fluoro-deoxyglucose positron emission tomography (FDG-PET) scan have not been obtained and would be helpful to evaluate for underlying malignancy. Further evaluation for a paraprotein disorder that includes sFLC is also still indicated to rule out a paraneoplastic disorder that may be associated with polyneuropathy.
Repeat SPEP and serum immunofixation were normal. sFLC assay showed elevated levels of both kappa and lambda light chains with a ratio of 0.61 (reference range: 0.26-1.25). Urine protein electrophoresis (UPEP) from a 24-hour specimen showed a homogenous band in the gamma region, but urine immunofixation demonstrated polyclonal light chains. The plasma vascular endothelial growth factor (VEGF) level was 612 pg/mL (reference range, 31-86 pg/mL).
CT imaging of the chest, abdomen, and pelvis with contrast demonstrated an enlarged liver and spleen and possible splenic infarcts. A skeletal survey and whole-body FGD-PET scan were normal. The patient declined bone marrow biopsy.
Polyneuropathy secondary to a monoclonal protein was previously considered, and an SPEP was normal. Full evaluation for a monoclonal protein additionally requires sFLC testing. If clinical suspicion remains high after a negative result, 24-hour UPEP and urine immunofixation should be obtained. Normal results in this case argue against the presence of a monoclonal protein.
The presence of a monoclonal protein and polyneuropathy are mandatory diagnostic criteria for POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal protein, and skin changes), a plasma cell proliferative disorder. Major diagnostic criteria include osteosclerotic bone lesions, Castleman’s disease, and markedly elevated VEGF levels. Castleman’s disease is a lymphoproliferative disorder characterized by angiofollicular lymphoid hyperplasia that results in lymphadenopathy in one or multiple lymph node regions. Imaging studies reveal organomegaly, one of many minor criteria, but not bone lesions or lymphadenopathy. A diagnosis of POEMS syndrome requires the presence of both mandatory, one major, and one minor criteria. Since only one of two of the mandatory criteria are met at this point, a diagnosis of POEMS syndrome cannot be made.
Eighteen months after symptom onset, the patient presented to the emergency department with dyspnea, orthopnea, and lower extremity edema. B-type natriuretic peptide was 1564 pg/mL. Transthoracic echocardiography showed a severely dilated and hypertrophied left ventricle. Left ventricular ejection fraction was 20%. A furosemide infusion was initiated. Angiography of the coronary vessels was not performed. Congo red stain of an abdominal adipose biopsy was negative for amyloid.
On hospital day five, he developed gangrenous changes in his right first toe. CT angiography of the abdomen and lower extremities demonstrated patent three vessel runoff to the foot with an infrarenal aortic thrombus. Heparin infusion was started. On hospital day 10, the patient developed expressive aphasia and somnolence, prompting intubation for airway protection. MRI and MR angiography (MRA) of the brain and cerebral vessels revealed multiple bilateral acute ischemic strokes (Figure 1) without flow limiting stenosis in cerebral vessels.
These clinical developments lead to an important opportunity to rethink this patient’s working diagnosis. The new diagnosis of heart failure in this young patient with polyneuropathy raises suspicion for an infiltrative cardiomyopathy such as amyloidosis, sarcoidosis, or Fabry disease. Of these, Fabry disease is the least likely because it is typically characterized by a painful burning sensation in response to specific triggers. Although polyneuropathy and heart failure may be concurrently observed with both sarcoidosis and amyloidosis, the absence of an apparent arrhythmia make amyloidosis the more likely of these two diagnoses. The development of an arterial thrombus and multiple strokes may represent emboli from a cardiac thrombus.
Cardiac imaging and tissue biopsy of the heart or other affected organs would distinguish between these diagnostic possibilities. An abdominal adipose biopsy negative for amyloid does not rule out amyloidosis, as the test is approximately 80% sensitive when cardiac amyloidosis is present and varies depending on the etiology of the amyloid protein (ie, light chain vs transthyretin). Evaluation of cardiac amyloid in the setting of peripheral neuropathy should include echocardiography (as was performed here) and repeat testing for a monoclonal protein.
If clinical suspicion of a paraprotein-associated disorder remains high and both SPEP and sFLC are normal, it is important to obtain a 24-hour UPEP and immunofixation. A monoclonal protein can be overlooked by SPEP and serum immunofixation if the monoclonal protein is composed only of a light chain or if the monoclonal protein is IgD or IgE. In these rare circumstances, sFLC analysis or 24-hour UPEP and immunofixation should mitigate the potential for a falsely negative SPEP/IFE. These studies are normal in this case, which argues against the presence of a monoclonal protein.
Transesophageal echocardiography showed grade IV atheromatous plaque within the descending thoracic aorta with mobile elements suggesting a superimposed thrombus; there was no intracardiac shunt or thrombus. MRA of the neck and great vessels was normal.
Testing for heparin-induced thrombocytopenia (HIT) was sent due to thrombocytopenia and the presence of thrombosis. An immunoassay for antiheparin-platelet factor 4 (anti-PF4) antibodies was substantially positive (optical density 2.178); however, functional testing with a washed platelet heparin-induced platelet activation assay was negative. Anticoagulation was changed to argatroban due to concern for HIT. Dry gangrenous changes developed in all distal toes on the right foot and three toes on the left foot. A right radial artery thrombus formed at the site of a prior arterial line.
Thrombocytopenia that develops between the fifth and tenth day following heparin exposure in a patient with new thromboses is consistent with HIT. However, the patient’s infrarenal aortic thrombus preceded the initiation of heparin, and negative functional testing undermines the diagnosis of HIT in this case. Therefore, the arterial thromboses may be related to an underlying unifying diagnosis.
A third SPEP showed a 0.1 g/dL M-spike in the gamma region, but standard immunofixation did not reveal a monoclonal protein (Figure 2). However, a specific request for immunofixation testing using IgD antisera detected an IgD heavy chain. A lambda chain comprising 3% of urine protein was detected on 24-hour urine immunofixation but was not detectable by serum immunofixation. Bone marrow biopsy demonstrated plasma cells comprising 5% of bone marrow cellularity (Figure 3); flow cytometry of the aspirate demonstrated an abnormal lambda-restricted plasma cell population.
When a monoclonal protein is identified but does not react with standard antisera to detect IgG, IgM, and IgA, immunofixation with IgD and IgE antisera are necessary to rule out a monoclonal IgD or IgE protein. The underlying IgD isotype coupled with its low abundance made detection of this monoclonal protein especially challenging. With the discovery of a monoclonal protein in the context of polyneuropathy, the mandatory criteria of POEMS syndrome are met. The elevated VEGF level and hypothyroidism meet major and minor criteria, respectively. Arterial thromboses and heart failure are other features that may be observed in cases of POEMS syndrome.
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M protein, and skin changes) was diagnosed. Prednisone was continued, and weekly cyclophosphamide was initiated. After six weeks, the VEGF level remained elevated, and a neurologic examination showed minimal improvement. Due to poor respiratory muscle strength and difficulty managing secretions, he underwent percutaneous tracheostomy and gastrostomy tube placement. Unfortunately, his condition further deteriorated and he subsequently died of sepsis from pneumonia.
An autopsy revealed acute bronchopneumonia and multiple acute and subacute cerebral infarctions. There was extensive peripheral mixed axonal/demyelinating neuropathy, hepatosplenomegaly, atrophy of the thyroid and adrenal glands, hyperpigmented patches and thickened integument, and severe aortic and coronary atherosclerotic disease with a healed myocardial infarction.
DISCUSSION
POEMS syndrome1 is a rare constellation of clinical and laboratory findings resulting from an underlying plasma cell proliferative disorder. This paraneoplastic syndrome is characterized by the chronic overproduction of proinflammatory and proangiogenic cytokines, including VEGF, which are postulated to drive its manifestations,2 though the exact pathogenesis is not understood. Some of the disease’s most common features are summarized by its name: polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma cell disorder, and skin changes.3
The International Myeloma Working Group (IMWG) diagnostic criteria1 (Table) require the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell proliferation), plus at least one major and one minor criterion. Delayed diagnosis or misdiagnosis of this protean disorder is often driven by its rarity and clinical overlap with other paraprotein-associated polyneuropathies. These include amyloidosis, cryoglobulinemia, and monoclonal gammopathy of undetermined significance (MGUS), which can all produce antibodies directed against neural antigens. In addition, polyneuropathy is often the first and most striking manifestation of POEMS syndrome, fostering confusion with CIDP as both disorders are subacute, symmetric, motor-dominant, mixed axonal/demyelinating polyneuropathies.4
IgD and IgE monoclonal gammopathies are extremely rare. IgD myeloma, for instance, accounts for 2% of multiple myeloma cases, and IgE myeloma has been reported fewer than 50 times.5 IgD is secreted only in very small amounts, ordinarily representing 0.25% of the immunoglobulins in serum, while the majority is found in the plasma membranes of mature B-cells.6 These monoclonal gammopathies often escape detection for two reasons: (1) the very low paraprotein concentration produces undetectable or small M-protein levels on electrophoresis,5 and (2) immunofixation is routinely performed without antisera against IgD and IgE heavy chains.7
While this case depicts a rare manifestation of a rare disease, the principles underlying its elusive diagnosis are routinely encountered. Recognition of the specific limitations of the SPEP, UPEP, sFLC, and immunofixation tests, outlined below, can assist the hospitalist when suspicion for paraproteinemia is high.
First, low levels of monoclonal proteins may be associated with a normal SPEP. Accordingly, suspicion of a plasma cell dyscrasia should prompt serum immunofixation, even when the electrophoretic pattern appears normal.8
Second, laboratories routinely perform immunofixation with antisera against IgG, IgA, and IgM heavy chains and kappa and lambda light chains, whereas testing with IgD or IgE antisera must be specifically requested. Thus, clinicians should screen for the presence of IgD and IgE in patients with an apparently free monoclonal immunoglobulin light chain in the serum or with a monoclonal serum protein and negative immunofixation. In this case, the paraprotein was not detected on the first two serum electrophoreses, likely due to a low serum concentration, then missed on immunofixation due to a lack of IgD antiserum. On admission to the hospital, this patient had a very low paraprotein concentration (0.1 g/dL) on SPEP, and the lab initially reported negative immunofixation. When asked to test specifically for IgD and IgE, the lab ran a more comprehensive immunofixation revealing IgD heavy chain paraprotein.
Third, this case illustrates the limitations of the sFLC assay. IMWG guidelines specify that sFLC assay in combination with SPEP and serum immunofixation is sufficient to screen for monoclonal plasma cell proliferative disorders other than light chain amyloidosis (which requires all the serum tests as well as 24-hour urine immunofixation).9 Though the sFLC assay has been demonstrated to be more sensitive than urine analysis for detecting monoclonal free light chains,10 it is still subject to false negatives. Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal sFLC,11 the latter of which likely explains why the sFLC ratio was repeatedly normal in this case. In these circumstances, monoclonal free light chains can be identified by urine studies.11 In this case, 24-hour urine immunofixation detected the excess light chain that was not evident on the sFLC assay. Even with these pitfalls in mind, there is still no evident explanation as to why the 24-hour urine studies done prior to the patient’s hospital admission did not reveal a monoclonal light chain.
This case also highlights the thrombotic diathesis in POEMS syndrome. Although the patient was treated with argatroban for suspected HIT, it is likely that the HIT antibody result was a false positive, and his thrombi were better explained by POEMS syndrome in and of itself. Coronary, limb, and cerebral artery thromboses have been linked to POEMS syndrome,12,13 all of which were present in this case. Laboratory testing for HIT involves an immunoassay to detect circulating HIT antibody and a functional assay to measure platelet activity in the presence of patient serum and heparin. The immunoassay binds anti-PF4/heparin complex irrespective of its ability to activate platelets. The presence of nonspecific antibodies may lead to cross-reactions with the immunoassay test components, which has been demonstrated in cases of MGUS.14 In this case, elevated production of monoclonal antibodies by plasma cells may have led to false-positive results. With moderate to high clinical suspicion of HIT, the combination of a positive immunoassay and negative functional assay (as in this case) make the diagnosis of HIT indeterminate.15
TEACHING POINTS
- If a monoclonal protein is suggested by SPEP but cannot be identified by standard immunofixation, request immunofixation for IgD or IgE. Screen patients for IgD and IgE paraproteins before making a diagnosis of light chain multiple myeloma.
- Polyclonal gammopathy or reduced renal clearance with accumulation of free light chains in the serum may mask the presence of low levels of monoclonal FLC and result in a normal sFLC ratio.
- Thrombosis is a less-recognized but documented feature of POEMS syndrome which may be mediated by the overproduction of proinflammatory and proangiogenic cytokines, though the precise pathogenesis is unknown.
Acknowledgment
The authors thank Dr. Theodore Kurtz and Dr. Anne Deucher from the department of laboratory medicine at the University of California, San Francisco for providing their respective expertise in clinical chemistry and hematopathology.
Disclosures
The authors have no conflicts of interests to disclose.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5.
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
1. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538-ee548. doi: 10.1016/S1470-2045(14)70442-5. PubMed
2. Watanabe O, Arimura K, Kitajima I, Osame M, Maruyama I. Greatly raised vascular endothelial growth factor (VEGF) in POEMS syndrome. Lancet. 1996;347(9002):702. doi: 10.1016/S0140-6736(96)91261-1. PubMed
3. Dispenzieri A. How I treat POEMS syndrome. Blood. 2012;119(24):5650-5658. doi: 10.1182/blood-2012-03-378992. PubMed
4. Nasu S, Misawa S, Sekiguchi Y, et al. Different neurological and physiological profiles in POEMS syndrome and chronic inflammatory demyelinating polyneuropathy. J Neurol Neurosurg Psychiatry. 2012;83(5):476-479. doi: 10.1136/jnnp-2011-301706. PubMed
5. Pandey S, Kyle RA. Unusual myelomas: a review of IgD and IgE variants. Oncology. 2013;27(8):798-803. PubMed
6. Vladutiu AO. Immunoglobulin D: properties, measurement, and clinical relevance. Clin Diagn Lab Immunol. 2000;7(2):131-140. doi: 10.1128/CDLI.7.2.131-140.2000. PubMed
7. Sinclair D, Cranfield T. IgD myeloma: A potential missed diagnosis. Ann Clin Biochem. 2001;38(5):564-565. doi: 10.1177/000456320103800517. PubMed
8. Dimopoulos M, Kyle R, Fermand JP, et al. Consensus recommendations for standard investigative workup: report of the International Myeloma Workshop Consensus Panel 3. Blood. 2011;117(18):4701-4705. doi: 10.1182/blood-2010-10-299529. PubMed
9. Dispenzieri A, Kyle R, Merlini G, et al. International Myeloma Working Group. International Myeloma Working Group guidelines for serum-free light chain analysis in multiple myeloma and related disorders. Leukemia. 2009;23(2):215-224. doi: 10.1038/leu.2008.307. PubMed
10. Dejoie T, Attal M, Moreau P, Harousseau JL, Avet-Loiseau H. Comparison of serum free light chain and urine electrophoresis for the detection of the light chain component of monoclonal immunoglobulins in light chain and intact immunoglobulin multiple myeloma. Haematologica. 2016;101(3):356-362. doi: 10.3324/haematol.2015.126797. PubMed
11. Levinson SS. Polyclonal free light chain of Ig may interfere with interpretation of monoclonal free light chain κ/λ ratio. Ann Clin Lab Sci. 2010;40(4):348-353. PubMed
12. Dispenzieri A, Kyle RA, Lacy MQ, et al. POEMS syndrome: definitions and long-term outcome. Blood. 2003;101(7):2496-2506. doi: 10.1182/blood-2002-07-2299. PubMed
13. Dupont SA, Dispenzieri A, Mauermann ML, Rabinstein AA, Brown RD. Cerebral infarction in POEMS syndrome: incidence, risk factors, and imaging characteristics. Neurology. 2009;73(16):1308-1312. doi: 10.1212/WNL.0b013e3181bd136b. PubMed
14. Markovic I, Debeljak Z, Bosnjak B, Marijanovic M. False positive immunoassay for heparin-induced thrombocytopenia in the presence of monoclonal gammopathy: a case report. Biochemia Medica. 2017;27(3):030801. doi: 10.11613/BM.2017.030801. PubMed
15. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
© 2019 Society of Hospital Medicine
Ethical Considerations in the Care of Hospitalized Patients with Opioid Use and Injection Drug Use Disorders
“Lord have mercy on me, was the kneeling drunkard’s plea.”
—Johnny Cash
The Diagnostic and Statistical Manual of the American Psychiatric Association defines opioid-use disorder (OUD) as a problematic pattern of prescription and/or illicit opioid medication use leading to clinically significant impairment or distress.1 Compared with their non-OUD counterparts, patients with OUD have poorer overall health and worse health service outcomes, including higher rates of morbidity, mortality, HIV and HCV transmission, and 30-day readmissions.2 With the rate of fatal overdoses from opioids at crisis levels, leading scientific and professional organizations have declared OUD to be a public health emergency in the United States.3
The opioid epidemic affects hospitalists through the rising incidence of hospitalization, not only as a result of OUD’s indirect complications, but also its direct effects of intoxication and withdrawal.4 In caring for patients with OUD, hospitalists are often presented with many ethical dilemmas. Whether the dilemma involves timing and circumstances of discharge or the permission to leave the hospital floor, they often involve elements of mutual mistrust. In qualitative ethnographic studies, patients with OUD report not trusting that the medical staff will take their concerns of inadequately treated pain and other needs seriously. Providers may mistrust the patient’s report of pain and withhold treatment for OUD for nonclinical reasons.5 Here, we examine two ethical dilemmas specific to OUD in hospitalized patients. Our aim in describing these dilemmas is to help hospitalists recognize that targeting issues of mistrust may assist them to deliver better care to hospitalized patients with OUD.
DISCHARGING HOSPITALIZED PATIENTS WITH OUD
In the inpatient setting, ethical dilemmas surrounding discharge are common among people who inject drugs (PWID). These patients have disproportionately high rates of soft tissue and systemic infections, such as endocarditis and osteomyelitis, and subsequently often require long-term, outpatient parenteral antibiotic therapy (OPAT).6 From both the clinical and ethical perspectives, discharging PWID requiring OPAT to an unsupervised setting or continuing inpatient hospitalization to prevent a potential adverse event are equally imperfect solutions.
These patients may be clinically stable, suitable for discharge, and prefer to be discharged, but the practitioner’s concerns regarding untoward complications frequently override the patient’s wishes. Valid reasons for this exercise of what could be considered soft-paternalism are considered when physicians unilaterally decide what is best for patients, including refusal of community agencies to provide OPAT to PWID, inadequate social support and/or health literacy to administer the therapy, or varying degrees of homelessness that can affect timely follow-up. However, surveys of both hospitalists and infectious disease specialists also indicate that they may avoid discharge because of concerns the PWID will tamper with the intravenous (IV) catheter to inject drugs.7 This reluctance to discharge otherwise socially and medically suitable patients increases length of stay,7 decreases patient satisfaction, and could lead to misuse of limited hospital resources.
Both patient mistrust and stigmatization may contribute to this dilemma. Healthcare professionals have been shown to share and reflect a long-standing bias in their attitudes toward patients with substance-use disorders and OUD, in particular.8 Studies of providers’ attitudes are limited but suggest that legal concerns over liability and professional sanctions,9 reluctance to contribute to the development or relapse of addiction,10 and a strong psychological investment in not being deceived by the patient11 may influence physicians’ decisions about care.
Closely supervising IV antibiotic therapy for all PWID may not reflect current medical knowledge and may imply a moral assessment of patients’ culpability and lack of will power to resist using drugs.12 No evidence is available to suggest that inpatient parenteral antibiotic treatment offers superior adherence, and emerging evidence showing that carefully selected patients with an injection drug-use history can be safely and effectively treated as outpatients has been obtained.13,14 Ho et al. found high rates of treatment success in patients with adequate housing, a reliable guardian, and willingness to comply with appropriate IV catheter use.13 Although the study by Buehrle et al. found higher rates of OPAT failure among PWIDs, 25% of these failures were due to adverse drug reactions and only 2% were due to documented line manipulations.14 This research suggests that disposition to alternative settings for OPAT in PWID may be feasible, reasonable, and deserving of further study. Rather than treating PWIDs as a homogenous group of increased risk, contextualizing care based on individual risk stratification promotes more patient-centered care that is medically appropriate and potentially more cost efficient. A
Patient-centered approaches that respond to the individual needs of patients have altered the care delivery model in order to improve health services outcomes. In developing an alternative care model to inpatient treatment in PWID who required OPAT, Jafari et al.15 evaluated a community model of care that provided a home-like residence as an alternative to hospitalization where patients could receive OPAT in a medically and socially supportive environment. This environment, which included RN and mental health staff for substance-use counseling, wound care, medication management, and IV therapy, demonstrated lower rates of against medical advice (AMA) discharge and higher patient satisfaction compared with hospitalization.15
MOBILITY OFF OF THE HOSPITAL FLOOR FOR HOSPITALIZED PATIENTS WITH OUD
Ethical dilemmas may also arise when patients with OUD desire greater mobility in the hospital. Although some inpatients may be permitted to leave the floor, some treatment teams may believe that patients with OUD leave the floor to use drugs and that the patient’s IV will facilitate such behavior. Nursing and medical staff may also believe that, if they agree to a request to leave the floor, they are complicit in any potential drug use or harmful consequences resulting from this use. For their part, patients may have a desire for more mobility because of the sometimes unpleasant constraints of hospitalization, which are not unique to these patients16 or to distract them from their cravings. Patients, unable to tolerate the restriction emotionally or believing they are being treated unfairly, even punitively, may leave AMA rather than complete needed medical care. Once more, distrust of the patient and fear of liability may lead hospital staff to respond in counterproductive ways.
Addressing this dilemma depends, in part on creating an environment where PWID and patients with OUD are treated fairly and appropriately for their underlying illness. Such treatment includes ensuring withdrawal symptoms and pain are adequately treated, building trust by empathically addressing patients’ needs and preferences,17 and having a systematic (ie, policy-based) approach for requests to leave the floor. Th
Efforts to adequately treat withdrawal symptoms in the hospital setting have shown promise in maintaining patient engagement, reducing the rate of AMA discharges, and improving follow up with outpatient medical and substance-use treatment.18 Because physicians consistently cite the lack of advanced training in addiction medicine as a treatment limitation,12 training may go a long way in closing this knowledge and skill gap. Furthermore, systematic efforts to better educate and train hospitalists in the care of patients with addiction can improve both knowledge and attitudes about caring for this vulnerable population,19 thereby enhancing therapeutic relationships and patient centeredness. Finally, institutional policies promoting fair, systematic, and transparent guidance are needed for front-line practitioners to manage the legal, clinical, and ethical ambiguities involved when PWID wish to leave the hospital floor.
ENHANCING CARE DELIVERY TO PATIENTS WITH OUD
In addressing the mistrust some staff may have toward the patients described in the preceding ethical dilemmas, the use of universal precautions is an ethical and efficacious approach that balances reliance on patients’ veracity with due diligence in objective clinical assessments.20 These universal precautions, which are grounded in mutual respect and responsibility between physician and patient, include a set of strategies originally established in infectious disease practice and adapted to the management of chronic pain particularly when opioids are used.21 They are based on the recognition that identifying which patients prescribed opioids will develop an OUD or misuse opioids is difficult. Hence, the safest and least-stigmatizing approach is to treat all patients as individuals who could potentially be at risk. This is an ethically strong approach that seeks to balance the competing values of patent safety and patient centeredness, and involves taking a substance-use history from all patients admitted to the hospital and routinely checking state prescription-drug monitoring programs among other steps. Although self-reporting, at least of prescription-drug misuse, is fairly reliable,22 establishing expectations for mutual respect when working with patients with OUD and other addictive disorders is more likely to garner valid reports and a positive alliance. Once this relationship is established, the practitioner can respond to problematic behaviors with clear, compassionate limit setting.
From a broader perspective, a hospital system and culture that is unable to promote trust and adequately treat pain and withdrawal can create a “risk environment” for PWID.23 When providers are inadequately trained in the management of pain and addiction, or there is a shortage of addiction specialists, or inadequate policy guidance for managing the care of these patients, this can result in AMA discharges and reduced willingness to seek future care. Viewing this problem more expansively may persuade healthcare professionals that patients alone are not entirely responsible for the outcomes related to their illness but that modifying practices and structure at the hospital level has the potential to mitigate harm to this vulnerable population.
As inpatient team leaders, hospitalists have the unique opportunity to address the opioid crisis by enhancing the quality of care provided to hospitalized patients with OUD. This enhancement can be accomplished by destigmatizing substance-use disorders, establishing relationships of trust, and promoting remedies to structural deficiencies in the healthcare system that contribute to the problem. These approaches have the potential to enhance not only the care of patients with OUD but also the satisfaction of the treatment team caring for these patients.24 Su
Disclosures
The authors have no conflicts of interest to disclose, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
1. Hasin DS, O’Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834-851. doi:10.1176/appi.ajp.2013.12060782. PubMed
2. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
3. National Institute on Drug Abuse. Opioid Overdose Crisis 2018. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Last updated March 2018. Accessed July 1, 2018.
4. Kerr T, Wood E, Grafstein E, et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health. (Oxf). 2005;27(1):62-66. doi:10.1093/pubmed/fdh189. PubMed
5. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. doi:10.1007/s11606-002-0034-5. PubMed
6. DP Levine PB. Infections in Injection Drug Users. In: Mandell GL BJ, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005.
7. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. doi:10.1002/jhm.2582. PubMed
8. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. doi:10.1016/j.drugalcdep.2013.02.018. PubMed
9. Fishman SM. Risk of the view through the keyhole: there is much more to physician reactions to the DEA than the number of formal actions. Pain Med. 2006;7(4):360-362; discussion 365-366. doi:10.1111/j.1526-4637.2006.00194.x. PubMed
10. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375-382. doi:10.5055/jom.2014.0234. PubMed
11. Beach SR, Taylor JB, Kontos N. Teaching psychiatric trainees to “think dirty”: uncovering hidden motivations and deception. Psychosomatics. 2017;58(5):474-482. doi:10.1016/j.psym.2017.04.005. PubMed
12. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus. 2016;37(4):635-641. doi:10.1080/08897077.2016.1187240. PubMed
13. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641-2644. doi:10.1093/jac/dkq355. PubMed
14. Buehrle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis. 2017;4(3):ofx102. doi:10.1093/ofid/ofx102. PubMed
15. Jafari S, Joe R, Elliot D, Nagji A, Hayden S, Marsh DC. A community care model of intravenous antibiotic therapy for injection drug users with deep tissue infection for “reduce leaving against medical advice.” Int J Ment Health Addict. 2015;13:49-58. doi:10.1007/s11469-014-9511-4. PubMed
16. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. doi:10.1001/jama.2014.3695. PubMed
17. Joosten EA, De Jong CA, de Weert-van Oene GH, Sensky T, van der Staak CP. Shared decision-making: increases autonomy in substance-dependent patients. Subst Use Misuse. 2011;46(8):1037-1038. doi:10.3109/10826084.2011.552931. PubMed
18. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. doi:10.1097/00126334-200401010-00008. PubMed
19. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11) 752-758. doi:10.12788/jhm.2993. PubMed
20. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2S):S111-S133. PubMed
21. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112. doi: 10.1111/j.1526-4637.2005.05031.x. PubMed
22. Smith M, Rosenblum A, Parrino M, Fong C, Colucci S. Validity of self-reported misuse of prescription opioid analgesics. Subst Use Misuse. 2010;45(10):1509-1524. doi:10.3109/10826081003682107. PubMed
23. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
24. Sullivan MD, Leigh J, Gaster B. Brief report: Training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med. 2006;21(4):360-362. doi:10.1111/j.1525-1497.2006.00352.x. PubMed
“Lord have mercy on me, was the kneeling drunkard’s plea.”
—Johnny Cash
The Diagnostic and Statistical Manual of the American Psychiatric Association defines opioid-use disorder (OUD) as a problematic pattern of prescription and/or illicit opioid medication use leading to clinically significant impairment or distress.1 Compared with their non-OUD counterparts, patients with OUD have poorer overall health and worse health service outcomes, including higher rates of morbidity, mortality, HIV and HCV transmission, and 30-day readmissions.2 With the rate of fatal overdoses from opioids at crisis levels, leading scientific and professional organizations have declared OUD to be a public health emergency in the United States.3
The opioid epidemic affects hospitalists through the rising incidence of hospitalization, not only as a result of OUD’s indirect complications, but also its direct effects of intoxication and withdrawal.4 In caring for patients with OUD, hospitalists are often presented with many ethical dilemmas. Whether the dilemma involves timing and circumstances of discharge or the permission to leave the hospital floor, they often involve elements of mutual mistrust. In qualitative ethnographic studies, patients with OUD report not trusting that the medical staff will take their concerns of inadequately treated pain and other needs seriously. Providers may mistrust the patient’s report of pain and withhold treatment for OUD for nonclinical reasons.5 Here, we examine two ethical dilemmas specific to OUD in hospitalized patients. Our aim in describing these dilemmas is to help hospitalists recognize that targeting issues of mistrust may assist them to deliver better care to hospitalized patients with OUD.
DISCHARGING HOSPITALIZED PATIENTS WITH OUD
In the inpatient setting, ethical dilemmas surrounding discharge are common among people who inject drugs (PWID). These patients have disproportionately high rates of soft tissue and systemic infections, such as endocarditis and osteomyelitis, and subsequently often require long-term, outpatient parenteral antibiotic therapy (OPAT).6 From both the clinical and ethical perspectives, discharging PWID requiring OPAT to an unsupervised setting or continuing inpatient hospitalization to prevent a potential adverse event are equally imperfect solutions.
These patients may be clinically stable, suitable for discharge, and prefer to be discharged, but the practitioner’s concerns regarding untoward complications frequently override the patient’s wishes. Valid reasons for this exercise of what could be considered soft-paternalism are considered when physicians unilaterally decide what is best for patients, including refusal of community agencies to provide OPAT to PWID, inadequate social support and/or health literacy to administer the therapy, or varying degrees of homelessness that can affect timely follow-up. However, surveys of both hospitalists and infectious disease specialists also indicate that they may avoid discharge because of concerns the PWID will tamper with the intravenous (IV) catheter to inject drugs.7 This reluctance to discharge otherwise socially and medically suitable patients increases length of stay,7 decreases patient satisfaction, and could lead to misuse of limited hospital resources.
Both patient mistrust and stigmatization may contribute to this dilemma. Healthcare professionals have been shown to share and reflect a long-standing bias in their attitudes toward patients with substance-use disorders and OUD, in particular.8 Studies of providers’ attitudes are limited but suggest that legal concerns over liability and professional sanctions,9 reluctance to contribute to the development or relapse of addiction,10 and a strong psychological investment in not being deceived by the patient11 may influence physicians’ decisions about care.
Closely supervising IV antibiotic therapy for all PWID may not reflect current medical knowledge and may imply a moral assessment of patients’ culpability and lack of will power to resist using drugs.12 No evidence is available to suggest that inpatient parenteral antibiotic treatment offers superior adherence, and emerging evidence showing that carefully selected patients with an injection drug-use history can be safely and effectively treated as outpatients has been obtained.13,14 Ho et al. found high rates of treatment success in patients with adequate housing, a reliable guardian, and willingness to comply with appropriate IV catheter use.13 Although the study by Buehrle et al. found higher rates of OPAT failure among PWIDs, 25% of these failures were due to adverse drug reactions and only 2% were due to documented line manipulations.14 This research suggests that disposition to alternative settings for OPAT in PWID may be feasible, reasonable, and deserving of further study. Rather than treating PWIDs as a homogenous group of increased risk, contextualizing care based on individual risk stratification promotes more patient-centered care that is medically appropriate and potentially more cost efficient. A
Patient-centered approaches that respond to the individual needs of patients have altered the care delivery model in order to improve health services outcomes. In developing an alternative care model to inpatient treatment in PWID who required OPAT, Jafari et al.15 evaluated a community model of care that provided a home-like residence as an alternative to hospitalization where patients could receive OPAT in a medically and socially supportive environment. This environment, which included RN and mental health staff for substance-use counseling, wound care, medication management, and IV therapy, demonstrated lower rates of against medical advice (AMA) discharge and higher patient satisfaction compared with hospitalization.15
MOBILITY OFF OF THE HOSPITAL FLOOR FOR HOSPITALIZED PATIENTS WITH OUD
Ethical dilemmas may also arise when patients with OUD desire greater mobility in the hospital. Although some inpatients may be permitted to leave the floor, some treatment teams may believe that patients with OUD leave the floor to use drugs and that the patient’s IV will facilitate such behavior. Nursing and medical staff may also believe that, if they agree to a request to leave the floor, they are complicit in any potential drug use or harmful consequences resulting from this use. For their part, patients may have a desire for more mobility because of the sometimes unpleasant constraints of hospitalization, which are not unique to these patients16 or to distract them from their cravings. Patients, unable to tolerate the restriction emotionally or believing they are being treated unfairly, even punitively, may leave AMA rather than complete needed medical care. Once more, distrust of the patient and fear of liability may lead hospital staff to respond in counterproductive ways.
Addressing this dilemma depends, in part on creating an environment where PWID and patients with OUD are treated fairly and appropriately for their underlying illness. Such treatment includes ensuring withdrawal symptoms and pain are adequately treated, building trust by empathically addressing patients’ needs and preferences,17 and having a systematic (ie, policy-based) approach for requests to leave the floor. Th
Efforts to adequately treat withdrawal symptoms in the hospital setting have shown promise in maintaining patient engagement, reducing the rate of AMA discharges, and improving follow up with outpatient medical and substance-use treatment.18 Because physicians consistently cite the lack of advanced training in addiction medicine as a treatment limitation,12 training may go a long way in closing this knowledge and skill gap. Furthermore, systematic efforts to better educate and train hospitalists in the care of patients with addiction can improve both knowledge and attitudes about caring for this vulnerable population,19 thereby enhancing therapeutic relationships and patient centeredness. Finally, institutional policies promoting fair, systematic, and transparent guidance are needed for front-line practitioners to manage the legal, clinical, and ethical ambiguities involved when PWID wish to leave the hospital floor.
ENHANCING CARE DELIVERY TO PATIENTS WITH OUD
In addressing the mistrust some staff may have toward the patients described in the preceding ethical dilemmas, the use of universal precautions is an ethical and efficacious approach that balances reliance on patients’ veracity with due diligence in objective clinical assessments.20 These universal precautions, which are grounded in mutual respect and responsibility between physician and patient, include a set of strategies originally established in infectious disease practice and adapted to the management of chronic pain particularly when opioids are used.21 They are based on the recognition that identifying which patients prescribed opioids will develop an OUD or misuse opioids is difficult. Hence, the safest and least-stigmatizing approach is to treat all patients as individuals who could potentially be at risk. This is an ethically strong approach that seeks to balance the competing values of patent safety and patient centeredness, and involves taking a substance-use history from all patients admitted to the hospital and routinely checking state prescription-drug monitoring programs among other steps. Although self-reporting, at least of prescription-drug misuse, is fairly reliable,22 establishing expectations for mutual respect when working with patients with OUD and other addictive disorders is more likely to garner valid reports and a positive alliance. Once this relationship is established, the practitioner can respond to problematic behaviors with clear, compassionate limit setting.
From a broader perspective, a hospital system and culture that is unable to promote trust and adequately treat pain and withdrawal can create a “risk environment” for PWID.23 When providers are inadequately trained in the management of pain and addiction, or there is a shortage of addiction specialists, or inadequate policy guidance for managing the care of these patients, this can result in AMA discharges and reduced willingness to seek future care. Viewing this problem more expansively may persuade healthcare professionals that patients alone are not entirely responsible for the outcomes related to their illness but that modifying practices and structure at the hospital level has the potential to mitigate harm to this vulnerable population.
As inpatient team leaders, hospitalists have the unique opportunity to address the opioid crisis by enhancing the quality of care provided to hospitalized patients with OUD. This enhancement can be accomplished by destigmatizing substance-use disorders, establishing relationships of trust, and promoting remedies to structural deficiencies in the healthcare system that contribute to the problem. These approaches have the potential to enhance not only the care of patients with OUD but also the satisfaction of the treatment team caring for these patients.24 Su
Disclosures
The authors have no conflicts of interest to disclose, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
“Lord have mercy on me, was the kneeling drunkard’s plea.”
—Johnny Cash
The Diagnostic and Statistical Manual of the American Psychiatric Association defines opioid-use disorder (OUD) as a problematic pattern of prescription and/or illicit opioid medication use leading to clinically significant impairment or distress.1 Compared with their non-OUD counterparts, patients with OUD have poorer overall health and worse health service outcomes, including higher rates of morbidity, mortality, HIV and HCV transmission, and 30-day readmissions.2 With the rate of fatal overdoses from opioids at crisis levels, leading scientific and professional organizations have declared OUD to be a public health emergency in the United States.3
The opioid epidemic affects hospitalists through the rising incidence of hospitalization, not only as a result of OUD’s indirect complications, but also its direct effects of intoxication and withdrawal.4 In caring for patients with OUD, hospitalists are often presented with many ethical dilemmas. Whether the dilemma involves timing and circumstances of discharge or the permission to leave the hospital floor, they often involve elements of mutual mistrust. In qualitative ethnographic studies, patients with OUD report not trusting that the medical staff will take their concerns of inadequately treated pain and other needs seriously. Providers may mistrust the patient’s report of pain and withhold treatment for OUD for nonclinical reasons.5 Here, we examine two ethical dilemmas specific to OUD in hospitalized patients. Our aim in describing these dilemmas is to help hospitalists recognize that targeting issues of mistrust may assist them to deliver better care to hospitalized patients with OUD.
DISCHARGING HOSPITALIZED PATIENTS WITH OUD
In the inpatient setting, ethical dilemmas surrounding discharge are common among people who inject drugs (PWID). These patients have disproportionately high rates of soft tissue and systemic infections, such as endocarditis and osteomyelitis, and subsequently often require long-term, outpatient parenteral antibiotic therapy (OPAT).6 From both the clinical and ethical perspectives, discharging PWID requiring OPAT to an unsupervised setting or continuing inpatient hospitalization to prevent a potential adverse event are equally imperfect solutions.
These patients may be clinically stable, suitable for discharge, and prefer to be discharged, but the practitioner’s concerns regarding untoward complications frequently override the patient’s wishes. Valid reasons for this exercise of what could be considered soft-paternalism are considered when physicians unilaterally decide what is best for patients, including refusal of community agencies to provide OPAT to PWID, inadequate social support and/or health literacy to administer the therapy, or varying degrees of homelessness that can affect timely follow-up. However, surveys of both hospitalists and infectious disease specialists also indicate that they may avoid discharge because of concerns the PWID will tamper with the intravenous (IV) catheter to inject drugs.7 This reluctance to discharge otherwise socially and medically suitable patients increases length of stay,7 decreases patient satisfaction, and could lead to misuse of limited hospital resources.
Both patient mistrust and stigmatization may contribute to this dilemma. Healthcare professionals have been shown to share and reflect a long-standing bias in their attitudes toward patients with substance-use disorders and OUD, in particular.8 Studies of providers’ attitudes are limited but suggest that legal concerns over liability and professional sanctions,9 reluctance to contribute to the development or relapse of addiction,10 and a strong psychological investment in not being deceived by the patient11 may influence physicians’ decisions about care.
Closely supervising IV antibiotic therapy for all PWID may not reflect current medical knowledge and may imply a moral assessment of patients’ culpability and lack of will power to resist using drugs.12 No evidence is available to suggest that inpatient parenteral antibiotic treatment offers superior adherence, and emerging evidence showing that carefully selected patients with an injection drug-use history can be safely and effectively treated as outpatients has been obtained.13,14 Ho et al. found high rates of treatment success in patients with adequate housing, a reliable guardian, and willingness to comply with appropriate IV catheter use.13 Although the study by Buehrle et al. found higher rates of OPAT failure among PWIDs, 25% of these failures were due to adverse drug reactions and only 2% were due to documented line manipulations.14 This research suggests that disposition to alternative settings for OPAT in PWID may be feasible, reasonable, and deserving of further study. Rather than treating PWIDs as a homogenous group of increased risk, contextualizing care based on individual risk stratification promotes more patient-centered care that is medically appropriate and potentially more cost efficient. A
Patient-centered approaches that respond to the individual needs of patients have altered the care delivery model in order to improve health services outcomes. In developing an alternative care model to inpatient treatment in PWID who required OPAT, Jafari et al.15 evaluated a community model of care that provided a home-like residence as an alternative to hospitalization where patients could receive OPAT in a medically and socially supportive environment. This environment, which included RN and mental health staff for substance-use counseling, wound care, medication management, and IV therapy, demonstrated lower rates of against medical advice (AMA) discharge and higher patient satisfaction compared with hospitalization.15
MOBILITY OFF OF THE HOSPITAL FLOOR FOR HOSPITALIZED PATIENTS WITH OUD
Ethical dilemmas may also arise when patients with OUD desire greater mobility in the hospital. Although some inpatients may be permitted to leave the floor, some treatment teams may believe that patients with OUD leave the floor to use drugs and that the patient’s IV will facilitate such behavior. Nursing and medical staff may also believe that, if they agree to a request to leave the floor, they are complicit in any potential drug use or harmful consequences resulting from this use. For their part, patients may have a desire for more mobility because of the sometimes unpleasant constraints of hospitalization, which are not unique to these patients16 or to distract them from their cravings. Patients, unable to tolerate the restriction emotionally or believing they are being treated unfairly, even punitively, may leave AMA rather than complete needed medical care. Once more, distrust of the patient and fear of liability may lead hospital staff to respond in counterproductive ways.
Addressing this dilemma depends, in part on creating an environment where PWID and patients with OUD are treated fairly and appropriately for their underlying illness. Such treatment includes ensuring withdrawal symptoms and pain are adequately treated, building trust by empathically addressing patients’ needs and preferences,17 and having a systematic (ie, policy-based) approach for requests to leave the floor. Th
Efforts to adequately treat withdrawal symptoms in the hospital setting have shown promise in maintaining patient engagement, reducing the rate of AMA discharges, and improving follow up with outpatient medical and substance-use treatment.18 Because physicians consistently cite the lack of advanced training in addiction medicine as a treatment limitation,12 training may go a long way in closing this knowledge and skill gap. Furthermore, systematic efforts to better educate and train hospitalists in the care of patients with addiction can improve both knowledge and attitudes about caring for this vulnerable population,19 thereby enhancing therapeutic relationships and patient centeredness. Finally, institutional policies promoting fair, systematic, and transparent guidance are needed for front-line practitioners to manage the legal, clinical, and ethical ambiguities involved when PWID wish to leave the hospital floor.
ENHANCING CARE DELIVERY TO PATIENTS WITH OUD
In addressing the mistrust some staff may have toward the patients described in the preceding ethical dilemmas, the use of universal precautions is an ethical and efficacious approach that balances reliance on patients’ veracity with due diligence in objective clinical assessments.20 These universal precautions, which are grounded in mutual respect and responsibility between physician and patient, include a set of strategies originally established in infectious disease practice and adapted to the management of chronic pain particularly when opioids are used.21 They are based on the recognition that identifying which patients prescribed opioids will develop an OUD or misuse opioids is difficult. Hence, the safest and least-stigmatizing approach is to treat all patients as individuals who could potentially be at risk. This is an ethically strong approach that seeks to balance the competing values of patent safety and patient centeredness, and involves taking a substance-use history from all patients admitted to the hospital and routinely checking state prescription-drug monitoring programs among other steps. Although self-reporting, at least of prescription-drug misuse, is fairly reliable,22 establishing expectations for mutual respect when working with patients with OUD and other addictive disorders is more likely to garner valid reports and a positive alliance. Once this relationship is established, the practitioner can respond to problematic behaviors with clear, compassionate limit setting.
From a broader perspective, a hospital system and culture that is unable to promote trust and adequately treat pain and withdrawal can create a “risk environment” for PWID.23 When providers are inadequately trained in the management of pain and addiction, or there is a shortage of addiction specialists, or inadequate policy guidance for managing the care of these patients, this can result in AMA discharges and reduced willingness to seek future care. Viewing this problem more expansively may persuade healthcare professionals that patients alone are not entirely responsible for the outcomes related to their illness but that modifying practices and structure at the hospital level has the potential to mitigate harm to this vulnerable population.
As inpatient team leaders, hospitalists have the unique opportunity to address the opioid crisis by enhancing the quality of care provided to hospitalized patients with OUD. This enhancement can be accomplished by destigmatizing substance-use disorders, establishing relationships of trust, and promoting remedies to structural deficiencies in the healthcare system that contribute to the problem. These approaches have the potential to enhance not only the care of patients with OUD but also the satisfaction of the treatment team caring for these patients.24 Su
Disclosures
The authors have no conflicts of interest to disclose, financial or otherwise. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the U.S. Department of Veterans Affairs, the U.S. Government, or the VA National Center for Ethics in Health Care.
1. Hasin DS, O’Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834-851. doi:10.1176/appi.ajp.2013.12060782. PubMed
2. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
3. National Institute on Drug Abuse. Opioid Overdose Crisis 2018. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Last updated March 2018. Accessed July 1, 2018.
4. Kerr T, Wood E, Grafstein E, et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health. (Oxf). 2005;27(1):62-66. doi:10.1093/pubmed/fdh189. PubMed
5. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. doi:10.1007/s11606-002-0034-5. PubMed
6. DP Levine PB. Infections in Injection Drug Users. In: Mandell GL BJ, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005.
7. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. doi:10.1002/jhm.2582. PubMed
8. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. doi:10.1016/j.drugalcdep.2013.02.018. PubMed
9. Fishman SM. Risk of the view through the keyhole: there is much more to physician reactions to the DEA than the number of formal actions. Pain Med. 2006;7(4):360-362; discussion 365-366. doi:10.1111/j.1526-4637.2006.00194.x. PubMed
10. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375-382. doi:10.5055/jom.2014.0234. PubMed
11. Beach SR, Taylor JB, Kontos N. Teaching psychiatric trainees to “think dirty”: uncovering hidden motivations and deception. Psychosomatics. 2017;58(5):474-482. doi:10.1016/j.psym.2017.04.005. PubMed
12. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus. 2016;37(4):635-641. doi:10.1080/08897077.2016.1187240. PubMed
13. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641-2644. doi:10.1093/jac/dkq355. PubMed
14. Buehrle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis. 2017;4(3):ofx102. doi:10.1093/ofid/ofx102. PubMed
15. Jafari S, Joe R, Elliot D, Nagji A, Hayden S, Marsh DC. A community care model of intravenous antibiotic therapy for injection drug users with deep tissue infection for “reduce leaving against medical advice.” Int J Ment Health Addict. 2015;13:49-58. doi:10.1007/s11469-014-9511-4. PubMed
16. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. doi:10.1001/jama.2014.3695. PubMed
17. Joosten EA, De Jong CA, de Weert-van Oene GH, Sensky T, van der Staak CP. Shared decision-making: increases autonomy in substance-dependent patients. Subst Use Misuse. 2011;46(8):1037-1038. doi:10.3109/10826084.2011.552931. PubMed
18. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. doi:10.1097/00126334-200401010-00008. PubMed
19. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11) 752-758. doi:10.12788/jhm.2993. PubMed
20. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2S):S111-S133. PubMed
21. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112. doi: 10.1111/j.1526-4637.2005.05031.x. PubMed
22. Smith M, Rosenblum A, Parrino M, Fong C, Colucci S. Validity of self-reported misuse of prescription opioid analgesics. Subst Use Misuse. 2010;45(10):1509-1524. doi:10.3109/10826081003682107. PubMed
23. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
24. Sullivan MD, Leigh J, Gaster B. Brief report: Training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med. 2006;21(4):360-362. doi:10.1111/j.1525-1497.2006.00352.x. PubMed
1. Hasin DS, O’Brien CP, Auriacombe M, et al. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry. 2013;170(8):834-851. doi:10.1176/appi.ajp.2013.12060782. PubMed
2. Donroe JH, Holt SR, Tetrault JM. Caring for patients with opioid use disorder in the hospital. CMAJ. 2016;188(17-18):1232-1239. doi:10.1503/cmaj.160290. PubMed
3. National Institute on Drug Abuse. Opioid Overdose Crisis 2018. https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis. Last updated March 2018. Accessed July 1, 2018.
4. Kerr T, Wood E, Grafstein E, et al. High rates of primary care and emergency department use among injection drug users in Vancouver. J Public Health. (Oxf). 2005;27(1):62-66. doi:10.1093/pubmed/fdh189. PubMed
5. Merrill JO, Rhodes LA, Deyo RA, Marlatt GA, Bradley KA. Mutual mistrust in the medical care of drug users: the keys to the “narc” cabinet. J Gen Intern Med. 2002;17(5):327-333. doi:10.1007/s11606-002-0034-5. PubMed
6. DP Levine PB. Infections in Injection Drug Users. In: Mandell GL BJ, Dolin R, eds. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases. 6th ed. Philadelphia: Churchill Livingstone; 2005.
7. Fanucchi L, Leedy N, Li J, Thornton AC. Perceptions and practices of physicians regarding outpatient parenteral antibiotic therapy in persons who inject drugs. J Hosp Med. 2016;11(8):581-582. doi:10.1002/jhm.2582. PubMed
8. van Boekel LC, Brouwers EP, van Weeghel J, Garretsen HF. Stigma among health professionals towards patients with substance use disorders and its consequences for healthcare delivery: systematic review. Drug Alcohol Depend. 2013;131(1-2):23-35. doi:10.1016/j.drugalcdep.2013.02.018. PubMed
9. Fishman SM. Risk of the view through the keyhole: there is much more to physician reactions to the DEA than the number of formal actions. Pain Med. 2006;7(4):360-362; discussion 365-366. doi:10.1111/j.1526-4637.2006.00194.x. PubMed
10. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. 2014;10(6):375-382. doi:10.5055/jom.2014.0234. PubMed
11. Beach SR, Taylor JB, Kontos N. Teaching psychiatric trainees to “think dirty”: uncovering hidden motivations and deception. Psychosomatics. 2017;58(5):474-482. doi:10.1016/j.psym.2017.04.005. PubMed
12. Wakeman SE, Pham-Kanter G, Donelan K. Attitudes, practices, and preparedness to care for patients with substance use disorder: results from a survey of general internists. Subst Abus. 2016;37(4):635-641. doi:10.1080/08897077.2016.1187240. PubMed
13. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65(12):2641-2644. doi:10.1093/jac/dkq355. PubMed
14. Buehrle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis. 2017;4(3):ofx102. doi:10.1093/ofid/ofx102. PubMed
15. Jafari S, Joe R, Elliot D, Nagji A, Hayden S, Marsh DC. A community care model of intravenous antibiotic therapy for injection drug users with deep tissue infection for “reduce leaving against medical advice.” Int J Ment Health Addict. 2015;13:49-58. doi:10.1007/s11469-014-9511-4. PubMed
16. Detsky AS, Krumholz HM. Reducing the trauma of hospitalization. JAMA. 2014;311(21):2169-2170. doi:10.1001/jama.2014.3695. PubMed
17. Joosten EA, De Jong CA, de Weert-van Oene GH, Sensky T, van der Staak CP. Shared decision-making: increases autonomy in substance-dependent patients. Subst Use Misuse. 2011;46(8):1037-1038. doi:10.3109/10826084.2011.552931. PubMed
18. Chan AC, Palepu A, Guh DP, et al. HIV-positive injection drug users who leave the hospital against medical advice: the mitigating role of methadone and social support. J Acquir Immune Defic Syndr. 2004;35(1):56-59. doi:10.1097/00126334-200401010-00008. PubMed
19. Englander H, Collins D, Perry SP, Rabinowitz M, Phoutrides E, Nicolaidis C. “We’ve learned it’s a medical illness, not a moral choice”: qualitative study of the effects of a multicomponent addiction intervention on hospital providers’ attitudes and experiences. J Hosp Med. 2018;13(11) 752-758. doi:10.12788/jhm.2993. PubMed
20. Kaye AD, Jones MR, Kaye AM, et al. Prescription opioid abuse in chronic pain: an updated review of opioid abuse predictors and strategies to curb opioid abuse (part 2). Pain Physician. 2017;20(2S):S111-S133. PubMed
21. Gourlay DL, Heit HA, Almahrezi A. Universal precautions in pain medicine: a rational approach to the treatment of chronic pain. Pain Med. 2005;6(2):107-112. doi: 10.1111/j.1526-4637.2005.05031.x. PubMed
22. Smith M, Rosenblum A, Parrino M, Fong C, Colucci S. Validity of self-reported misuse of prescription opioid analgesics. Subst Use Misuse. 2010;45(10):1509-1524. doi:10.3109/10826081003682107. PubMed
23. McNeil R, Small W, Wood E, Kerr T. Hospitals as a ‘risk environment’: an ethno-epidemiological study of voluntary and involuntary discharge from hospital against medical advice among people who inject drugs. Soc Sci Med. 2014;105:59-66. doi:10.1016/j.socscimed.2014.01.010. PubMed
24. Sullivan MD, Leigh J, Gaster B. Brief report: Training internists in shared decision making about chronic opioid treatment for noncancer pain. J Gen Intern Med. 2006;21(4):360-362. doi:10.1111/j.1525-1497.2006.00352.x. PubMed
© 2019 Society of Hospital Medicine
Association of Weekend Admission and Weekend Discharge with Length of Stay and 30-Day Readmission in Children’s Hospitals
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
Increasingly, metrics such as length of stay (LOS) and readmissions are being utilized in the United States to assess quality of healthcare because these factors may represent opportunities to reduce cost and improve healthcare delivery.1-8 However, the relatively low rate of pediatric readmissions,9 coupled with limited data regarding recommended LOS or best practices to prevent readmissions in children, challenges the ability of hospitals to safely reduce LOS and readmission rates for children.10–12
In adults, weekend admission is associated with prolonged LOS, increased readmission rates, and increased risk of mortality.13-21 This association is referred to as the “weekend effect.” While the weekend effect has been examined in children, the results of these studies have been variable, with some studies supporting this association and others refuting it.22-31 In contrast to patient demographic and clinical characteristics that are known to affect LOS and readmissions,32 the weekend effect represents a potentially modifiable aspect of a hospitalization that could be targeted to improve healthcare delivery.
With increasing national attention toward improving quality of care and reducing LOS and healthcare costs, more definitive evidence of the weekend effect is necessary to prioritize resource use at both the local and national levels. Therefore, we sought to determine the association of weekend admission and weekend discharge on LOS and 30-day readmissions, respectively, among a national cohort of children. We hypothesized that children admitted on the weekend would have longer LOS, whereas those discharged on the weekend would have higher readmission rates.
METHODS
Study Design and Data Source
We conducted a multicenter, retrospective, cross-sectional study. Data were obtained from the Pediatric Health Information System (PHIS), an administrative and billing database of 46 free-standing tertiary care pediatric hospitals affiliated with the Children’s Hospital Association (Lenexa, Kansas). Patient data are de-identified within PHIS; however, encrypted patient identifiers allow individual patients to be followed across visits. This study was not considered human subjects research by the policies of the Cincinnati Children’s Hospital Institutional Review Board.
Participants
We included hospitalizations to a PHIS-participating hospital for children aged 0-17 years between October 1, 2014 and September 30, 2015. We excluded children who were transferred from/to another institution, left against medical advice, or died in the hospital because these indications may result in incomplete LOS information and would not consistently contribute to readmission rates. We also excluded birth hospitalizations and children admitted for planned procedures. Birth hospitalizations were defined as hospitalizations that began on the day of birth.
Main Exposures
No standard definition of weekend admission or discharge was identified in the literature.33 Thus, we defined a weekend admission as an admission between 3:00
Main Outcomes
Our outcomes included LOS for weekend admission and 30-day readmissions for weekend discharge. LOS, measured in hours, was defined using the reported admission and discharge times. Readmissions were defined as a return to the same hospital within the subsequent 30 days following discharge.
Patient Demographics and Other Study Variables
Patient demographics included age, gender, race/ethnicity, payer, and median household income quartile based on the patient’s home ZIP code. Other study variables included presence of a complex chronic condition (CCC),34 technology dependence,34 number of chronic conditions of any complexity, admission through the emergency department, intensive care unit (ICU) admission, and case mix index. ICU admission and case mix index were chosen as markers for severity of illness. ICU admission was defined as any child who incurred ICU charges at any time following admission. Case mix index in PHIS is a relative weight assigned to each discharge based on the All-Patient Refined Diagnostic Group (APR-DRG; 3M) assignment and APR-DRG severity of illness, which ranges from 1 (minor) to 4 (extreme). The weights are derived by the Children’s Hospital Association from the HCUP KID 2012 database as the ratio of the average cost for discharges within a specific APR-DRG severity of illness combination to the average cost for all discharges in the database.
Statistical Analysis
Continuous variables were summarized with medians and interquartile ranges, while categorical variables were summarized with frequencies and percentages. Differences in admission and discharge characteristics between weekend and weekday were assessed using Wilcoxon rank sum tests for continuous variables and chi-square tests of association for categorical variables. We used generalized linear mixed modeling (GLMM) techniques to assess the impact of weekend admission on LOS and weekend discharge on readmission, adjusting for important patient demographic and clinical characteristics. Furthermore, we used GLMM point estimates to describe the variation across hospitals of the impact of weekday versus weekend care on LOS and readmissions. We assumed an underlying log-normal distribution for LOS and an underlying binomial distribution for 30-day readmission. All GLMMs included a random intercept for each hospital to account for patient clustering within a hospital. All statistical analyses were performed using SAS v.9.4 (SAS Institute, Cary, North Carolina), and P values <.05 were considered statistically significant.
RESULTS
We identified 390,745 hospitalizations that met inclusion criteria (Supplementary Figure 1). The median LOS among our cohort was 41 hours (interquartile range [IQR] 24-71) and the median 30-day readmission rate was 8.2% (IQR 7.2-9.4).
Admission Demographics for Weekends and Weekdays
Among the included hospitalizations, 92,266 (23.6%) admissions occurred on a weekend (Supplementary Table 1). Overall, a higher percentage of children <5 years of age were admitted on a weekend compared with those admitted on a weekday (53.3% vs 49.1%, P < .001). We observed a small but statistically significant difference in the proportion of weekend versus weekday admissions according to gender, race/ethnicity, payer, and median household income quartile. Children with medical complexity and those with technology dependence were admitted less frequently on a weekend. A higher proportion of children were admitted through the emergency department on a weekend and a higher frequency of ICU utilization was observed for children admitted on a weekend compared with those admitted on a weekday.
Association Between Study Variables and Length of Stay
In comparing adjusted LOS for weekend versus weekday admissions across 43 hospitals, not only did LOS vary across hospitals (P < .001), but the association between LOS and weekend versus weekday care also varied across hospitals (P < .001) (Figure 1). Weekend admission was associated with a significantly longer LOS at eight (18.6%) hospitals and a significantly shorter LOS at four (9.3%) hospitals with nonstatistically significant differences at the remaining hospitals.
In adjusted analyses, we observed that infants ≤30 days of age, on average, had an adjusted LOS that was 24% longer than that of 15- to 17-year-olds, while children aged 1-14 years had an adjusted LOS that was 6%-18% shorter (Table 1). ICU utilization, admission through the emergency department, and number of chronic conditions had the greatest association with LOS. As the number of chronic conditions increased, the LOS increased. No association was found between weekend versus weekday admission and LOS (adjusted LOS [95% CI]: weekend 63.70 [61.01-66.52] hours versus weekday 63.40 [60.73-66.19] hours, P = .112).
Discharge Demographics for Weekends and Weekdays
Of the included hospitalizations, 127,421 (32.6%) discharges occurred on a weekend (Supplementary Table 2). Overall, a greater percentage of weekend discharges comprised children <5 years of age compared with the percentage of weekday discharges for children <5 years of age (51.5% vs 49.5%, P < .001). No statistically significant differences were found in gender, payer, or median household income quartile between those children discharged on a weekend versus those discharged on a weekday. We found small, statistically significant differences in the proportion of weekend versus weekday discharges according to race/ethnicity, with fewer non-Hispanic white children being discharged on the weekend versus weekday. Children with medical complexity, technology dependence, and patients with ICU utilization were less frequently discharged on a weekend compared with those discharged on a weekday.
Association Between Study Variables and Readmissions
In comparing the adjusted odds of readmissions for weekend versus weekday discharges across 43 PHIS hospitals, we observed significant variation (P < .001) in readmission rates from hospital to hospital (Figure 2). However, the direction of impact of weekend care on readmissions was similar (P = .314) across hospitals (ie, for 37 of 43 hospitals, the readmission rate was greater for weekend discharges compared with that for weekday discharges). For 17 (39.5%) of 43 hospitals, weekend discharge was associated with a significantly higher readmission rate, while the differences between weekday and weekend discharge were not statistically significant for the remaining hospitals.
In adjusted analyses, we observed that infants <1 year were more likely to be readmitted compared with 15- to 17-year-olds, while children 5-14 years of age were less likely to be readmitted (Table 2). Medical complexity and the number of chronic conditions had the greatest association with readmissions, with increased likelihood of readmission observed as the number of chronic conditions increased. Weekend discharge was associated with increased probability of readmission compared with weekday discharge (adjusted probability of readmission [95% CI]: weekend 0.13 [0.12-0.13] vs weekday 0.11 [0.11-0.12], P < .001).
DISCUSSION
While the reasons for the weekend effect are unclear, data supporting this difference have been observed across many diverse patient groups and health systems both nationally and internationally.13-27,31 Weekend care is thought to differ from weekday care because of differences in physician and nurse staffing, availability of ancillary services, access to diagnostic testing and therapeutic interventions, ability to arrange outpatient follow-up, and individual patient clinical factors, including acuity of illness. Few studies have assessed the effect of weekend discharges on patient or system outcomes. Among children within a single health system, readmission risk was associated with weekend admission but not with weekend discharge.22 This observation suggests that if differential care exists, then it occurs during initial clinical management rather than during discharge planning. Consequently, understanding the interaction of weekend admission and LOS is important. In addition, the relative paucity of pediatric data examining a weekend discharge effect limits the ability to generalize these findings across other hospitals or health systems.
In contrast to prior work, we observed a modest increased risk for readmission among those discharged on the weekend in a large sample of children. Auger and Davis reported a lack of association between weekend discharge and readmissions at one tertiary care children’s hospital, citing reduced discharge volumes on the weekend, especially among children with medical complexity, as a possible driver for their observation.22 The inclusion of a much larger population across 43 hospitals in our study may explain our different findings compared with previous research. In addition, the inclusion/exclusion criteria differed between the two studies; we excluded index admissions for planned procedures in this study (which are more likely to occur during the week), which may have contributed to the differing conclusions. Although Auger and Davis suggest that differences in initial clinical management may be responsible for the weekend effect,22 our observations suggest that discharge planning practices may also contribute to readmission risk. For example, a family’s inability to access compounded medications at a local pharmacy or to access primary care following discharge could reasonably contribute to treatment failure and increased readmission risk. Attention to improving and standardizing discharge practices may alleviate differences in readmission risk among children discharged on a weekend.
Individual patient characteristics greatly influence LOS and readmission risk. Congruent with prior studies, medical complexity and technology dependence were among the factors in our study that had the strongest association with LOS and readmission risk.32 As with prior studies22, we observed that children with medical complexity and technology dependence were less frequently admitted and discharged on a weekend than on a weekday, which suggests that physicians may avoid complicated discharges on the weekend. Children with medical complexity present a unique challenge to physicians when assessing discharge readiness, given that these children frequently require careful coordination of durable medical equipment, obtainment of special medication preparations, and possibly the resumption or establishment of home health services. Notably, we cannot discern from our data what proportion of discharges may be delayed over the weekend secondary to challenges involved in coordinating care for children with medical complexity. Future investigations aimed at assessing physician decision making and discharge readiness in relation to discharge timing among children with medical complexity may establish this relationship more clearly.
We observed substantial variation in LOS and readmission risk across 43 tertiary care children’s hospitals. Since the 1970s, numerous studies have reported worse outcomes among patients admitted on the weekend. While the majority of studies support the weekend effect, several recent studies suggest that patients admitted on the weekend are at no greater risk of adverse outcomes than those admitted during the week.35-37 Our work builds on the existing literature, demonstrating a complex and variable relationship between weekend admission/discharge, LOS, and readmission risk across hospitals. Notably, while many hospitals in our study experienced a significant weekend effect in LOS or readmission risk, only four hospitals experienced a statistically significant weekend effect for both LOS and readmission risk (three hospitals experienced increased risk for both, while one hospital experienced increased readmission risk but decreased LOS). Future investigations of the weekend effect should focus on exploring the differences in admission/discharge practices and staffing patterns of hospitals that did or did not experience a weekend effect.
This study has several limitations
CONCLUSION
In a study of 43 children’s hospitals, children discharged on the weekend had a slightly increased readmission risk compared with children discharged on a weekday. Wide variation in the weekend effect on LOS and readmission risk was evident across hospitals. Individual patient characteristics had a greater impact on LOS and readmission risk than the weekend effect. Future investigations aimed at understanding which factors contribute most strongly to a weekend effect within individual hospitals (eg, differences in institutional admission/discharge practices) may help alleviate the weekend effect and improve healthcare quality.
Acknowledgments
This manuscript resulted from “Paper in a Day,” a Pediatric Research in Inpatient Settings (PRIS) Network-sponsored workshop presented at the Pediatric Hospital Medicine 2017 annual meeting. Workshop participants learned how to ask and answer a health services research question and efficiently prepare a manuscript for publication. The following are the members of the PRIS Network who contributed to this work: Jessica L. Bettenhausen, MD; Rebecca M. Cantu, MD, MPH; Jillian M Cotter, MD; Megan Deisz, MD; Teresa Frazer, MD; Pratichi Goenka, MD; Ashley Jenkins, MD; Kathryn E. Kyler, MD; Janet T. Lau, MD; Brian E. Lee, MD; Christiane Lenzen, MD; Trisha Marshall, MD; John M. Morrison MD, PhD; Lauren Nassetta, MD; Raymond Parlar-Chun, MD; Sonya Tang Girdwood MD, PhD; Tony R Tarchichi, MD; Irina G. Trifonova, MD; Jacqueline M. Walker, MD, MHPE; and Susan C. Walley, MD. See appendix for contact information for members of the PRIS Network
Funding
The authors have no financial relationships relevant to this article to disclose.
Disclosures
The authors have no conflicts of interest to disclose.
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
1. Crossing the Quality Chasm: The IOM Health Care Quality Initiative : Health and Medicine Division. http://www.nationalacademies.org/hmd/Global/News%20Announcements/Crossing-the-Quality-Chasm-The-IOM-Health-Care-Quality-Initiative.aspx. Accessed November 20, 2017.
2. Institute for Healthcare Improvement: IHI Home Page. http://www.ihi.org:80/Pages/default.aspx. Accessed November 20, 2017.
3. Berry JG, Zaslavsky AM, Toomey SL, et al. Recognizing differences in hospital quality performance for pediatric inpatient care. Pediatrics. 2015;136(2):251-262. doi:10.1542/peds.2014-3131
4. NQF: All-Cause Admissions and Readmissions Measures - Final Report. http://www.qualityforum.org/Publications/2015/04/All-Cause_Admissions_and_Readmissions_Measures_-_Final_Report.aspx. Accessed March 24, 2018.
5. Hospital Inpatient Potentially Preventable Readmissions Information and Reports. https://www.illinois.gov/hfs/MedicalProviders/hospitals/PPRReports/Pages/default.aspx. Accessed November 6, 2016.
6. Potentially Preventable Readmissions in Texas Medicaid and CHIP Programs - Fiscal Year 2013 | Texas Health and Human Services. https://hhs.texas.gov/reports/2016/08/potentially-preventable-readmissions-texas-medicaid-and-chip-programs-fiscal-year. Accessed November 6, 2016.
7. Statewide Planning and Research Cooperative System. http://www.health.ny.gov/statistics/sparcs/sb/. Accessed November 6, 2016.
8. HCA Implements Potentially Preventable Readmission (PPR) Adjustments. Wash State Hosp Assoc. http://www.wsha.org/articles/hca-implements-potentially-preventable-readmission-ppr-adjustments/. Accessed November 8, 2016.
9. Berry JG, Toomey SL, Zaslavsky AM, et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309(4):372-380. doi:10.1001/jama.2012.188351 PubMed
10. Bardach NS, Vittinghoff E, Asteria-Peñaloza R, et al. Measuring hospital quality using pediatric readmission and revisit rates. Pediatrics. 2013;132(3):429-436. doi:10.1542/peds.2012-3527 PubMed
11. Berry JG, Blaine K, Rogers J, et al. A framework of pediatric hospital discharge care informed by legislation, research, and practice. JAMA Pediatr. 2014;168(10):955-962; quiz 965-966. doi:10.1001/jamapediatrics.2014.891 PubMed
12. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: seamless transitions and (Re)admissions network. Pediatrics. 2015;135(1):164. doi:10.1542/peds.2014-1887 PubMed
13. Freemantle N, Ray D, McNulty D, et al. Increased mortality associated with weekend hospital admission: a case for expanded seven day services? BMJ. 2015;351:h4596. doi:10.1136/bmj.h4596 PubMed
14. Schilling PL, Campbell DA, Englesbe MJ, Davis MM. A comparison of in-hospital mortality risk conferred by high hospital occupancy, differences in nurse staffing levels, weekend admission, and seasonal influenza. Med Care. 2010;48(3):224-232. doi:10.1097/MLR.0b013e3181c162c0 PubMed
15. Cram P, Hillis SL, Barnett M, Rosenthal GE. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am J Med. 2004;117(3):151-157. doi:10.1016/j.amjmed.2004.02.035 PubMed
16. Zapf MAC, Kothari AN, Markossian T, et al. The “weekend effect” in urgent general operative procedures. Surgery. 2015;158(2):508-514. doi:10.1016/j.surg.2015.02.024 PubMed
17. Freemantle N, Richardson M, Wood J, et al. Weekend hospitalization and additional risk of death: an analysis of inpatient data. J R Soc Med. 2012;105(2):74-84. doi:10.1258/jrsm.2012.120009 PubMed
18. Bell CM, Redelmeier DA. Mortality among patients admitted to hospitals on weekends as compared with weekdays. N Engl J Med. 2001;345(9):663-668. doi:10.1056/NEJMsa003376 PubMed
19. Coiera E, Wang Y, Magrabi F, Concha OP, Gallego B, Runciman W. Predicting the cumulative risk of death during hospitalization by modeling weekend, weekday and diurnal mortality risks. BMC Health Serv Res. 2014;14:226. doi:10.1186/1472-6963-14-226 PubMed
20. Powell ES, Khare RK, Courtney DM, Feinglass J. The weekend effect for patients with sepsis presenting to the emergency department. J Emerg Med. 2013;45(5):641-648. doi:10.1016/j.jemermed.2013.04.042 PubMed
21. Ananthakrishnan AN, McGinley EL, Saeian K. Outcomes of weekend admissions for upper gastrointestinal hemorrhage: a nationwide analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2009;7(3):296-302e1. doi:10.1016/j.cgh.2008.08.013 PubMed
22. Auger KA, Davis MM. Pediatric weekend admission and increased unplanned readmission rates. J Hosp Med. 2015;10(11):743-745. doi:10.1002/jhm.2426 PubMed
23. Goldstein SD, Papandria DJ, Aboagye J, et al. The “weekend effect” in pediatric surgery - increased mortality for children undergoing urgent surgery during the weekend. J Pediatr Surg. 2014;49(7):1087-1091. doi:10.1016/j.jpedsurg.2014.01.001 PubMed
24. Adil MM, Vidal G, Beslow LA. Weekend effect in children with stroke in the nationwide inpatient sample. Stroke. 2016;47(6):1436-1443. doi:10.1161/STROKEAHA.116.013453 PubMed
25. McCrory MC, Spaeder MC, Gower EW, et al. Time of admission to the PICU and mortality. Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2017;18(10):915-923. doi:10.1097/PCC.0000000000001268 PubMed
26. Mangold WD. Neonatal mortality by the day of the week in the 1974-75 Arkansas live birth cohort. Am J Public Health. 1981;71(6):601-605. PubMed
27. MacFarlane A. Variations in number of births and perinatal mortality by day of week in England and Wales. Br Med J. 1978;2(6153):1670-1673. PubMed
28. McShane P, Draper ES, McKinney PA, McFadzean J, Parslow RC, Paediatric intensive care audit network (PICANet). Effects of out-of-hours and winter admissions and number of patients per unit on mortality in pediatric intensive care. J Pediatr. 2013;163(4):1039-1044.e5. doi:10.1016/j.jpeds.2013.03.061 PubMed
29. Hixson ED, Davis S, Morris S, Harrison AM. Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med J Soc Crit Care Med World Fed Pediatr Intensive Crit Care Soc. 2005;6(5):523-530. PubMed
30. Gonzalez KW, Dalton BGA, Weaver KL, Sherman AK, St Peter SD, Snyder CL. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr Surg Int. 2016;32(7):665-669. doi:10.1007/s00383-016-3901-6 PubMed
31. Desai V, Gonda D, Ryan SL, et al. The effect of weekend and after-hours surgery on morbidity and mortality rates in pediatric neurosurgery patients. J Neurosurg Pediatr. 2015;16(6):726-731. doi:10.3171/2015.6.PEDS15184 PubMed
32. Berry JG, Hall DE, Kuo DZ, et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children’s hospitals. JAMA. 2011;305(7):682-690. doi:10.1001/jama.2011.122 PubMed
33. Hoshijima H, Takeuchi R, Mihara T, et al. Weekend versus weekday admission and short-term mortality: A meta-analysis of 88 cohort studies including 56,934,649 participants. Medicine (Baltimore). 2017;96(17):e6685. doi:10.1097/MD.0000000000006685 PubMed
34. Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi:10.1186/1471-2431-14-199 PubMed
35. Li L, Rothwell PM, Oxford Vascular Study. Biases in detection of apparent “weekend effect” on outcome with administrative coding data: population based study of stroke. BMJ. 2016;353:i2648. doi: https://doi.org/10.1136/bmj.i2648 PubMed
36. Bray BD, Cloud GC, James MA, et al. Weekly variation in health-care quality by day and time of admission: a nationwide, registry-based, prospective cohort study of acute stroke care. The Lancet. 2016;388(10040):170-177. doi:10.1016/S0140-6736(16)30443-3 PubMed
37. Ko SQ, Strom JB, Shen C, Yeh RW. Mortality, Length of Stay, and Cost of Weekend Admissions. J Hosp Med. 2018. doi:10.12788/jhm.2906 PubMed
38. Tubbs-Cooley HL, Cimiotti JP, Silber JH, Sloane DM, Aiken LH. An observational study of nurse staffing ratios and hospital readmission among children admitted for common conditions. BMJ Qual Saf. 2013;22(9):735-742. doi:10.1136/bmjqs-2012-001610 PubMed
39. Ong M, Bostrom A, Vidyarthi A, McCulloch C, Auerbach A. House staff team workload and organization effects on patient outcomes in an academic general internal medicine inpatient service. Arch Intern Med. 2007;167(1):47-52. doi:10.1001/archinte.167.1.47 PubMed
© 2018 Society of Hospital Medicine
Identifying Observation Stays in Medicare Data: Policy Implications of a Definition
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
© 2019 Society of Hospital Medicine
SPRINT MIND with extension planned
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
Also today, medical ethics and economics, PTSD after traumatic brain injury may be predicted by race and ethnicity, and aspirin for primary cardiovascular prevention.
Amazon Alexa
Apple Podcasts
Google Podcasts
Spotify
February 2019 Prostate Cancer
Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

Click here to access February 2019 Prostate Cancer
Table of Contents
- Prostate Cancer Surveillance After Radiation Therapy in a National Delivery System
- Skeletal-Related Events in Patients With Multiple Myeloma and Prostate Cancer Who Receive Standard vs Extended-Interval Bisphosphonate Dosing
- Primary Urethral Carcinoma With Nodal Metastasis
- Presentation of a Rare Malignancy: Leiomyosarcoma of the Prostate
- Research News: Prostate Cancer
- Management of Patients With Treatment-Resistant Metastatic Prostate Cancer

2019 Directory of VA and DoD Facilities
AGA Clinical Practice Update: Functional gastrointestinal symptoms in patients with inflammatory bowel disease
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY