User login
Psychological screening integration improves quality of care for children with abdominal pain
according to results from a recent report published in Pediatrics.
Natoshia R. Cunningham, PhD, from the divisions of behavioral medicine and clinical psychology and the department of pediatrics at the University of Cincinnati and her colleagues tested their screening process with a pilot study in a single clinic between August 2015 and October 2016. Researchers assessed patients with FAPD using the Screen for Child Anxiety Related Disorders (SCARED)–Child Report and Functional Disability Inventory (FDI)–Child Version. Clinically significant anxiety was defined as a score of at least 25 (range, 0-82) for the SCARED; clinical cutoffs for minimal, moderate, and severe disability in the child version of the FDI were defined as scores of 0-12, 13-29, and greater than 30, respectively. After fine-tuning the screening process in the pilot, the researchers scaled the effort to six different clinics within a large gastroenterology division at a Midwest urban medical center.
“Children with FAPD who are at the greatest risk for persistent functional disability (i.e., those with clinical elevations in all three risk areas) are now being immediately identified and managed as part of routine care,” Dr. Cunningham and colleagues wrote in their study.
Of 6,744 eligible children (mean age, 13.34 years; 58% female; 87.6% non-Hispanic white), 5,221 children completed the screening, with 1,291 of 1,369 children completing both the screening process and reporting abdominal pain as a presenting complaint. Researchers found 43.1% of children showed clinically significant anxiety under SCARED, with a mean SCARED score of 24.3. Children had a mean FDI score of 13.7, and nearly half of the children had functional disability that was moderate (34.2%) or severe (10.8%), with 61.5% overall reporting a pain level of least 4 out of 10 during the week.
There were 21.1% of children with “clinical elevations” in pain, disability, and anxiety, with researchers noting that, compared with patients without clinical anxiety, those with anxiety had significantly higher FDI scores (mean, 16.29 vs. 11.54) and higher pain levels (mean, 4.63 vs. 3.72).
Among those referred to psychological services, the number of referrals after implementing psychological screening nearly doubled to 15.2 patients per 1,000 per month between March 2017 and September 2017, compared with baseline referrals the previous year (8.3 per 1,000, March 2016 to September 2016).
The researchers suggested future work in psychological screening for children with FAPD should consider shortening screening time by using different outcome measures to lessen the burden on clinical staff; they noted it should also consider applying psychological screening in a telehealth, primary care, or school setting to increase access to care.
“Even in patients with all three risk factors, the decision to refer to psychological providers remains an individualized process driven by clinical judgement,” Dr. Cunningham and her colleagues wrote. “Many factors, including provider practice patterns and other considerations (e.g., patient and family interest, provider availability, distance to care, and insurance coverage) guide these decisions.”
The authors report no relevant financial disclosures.
SOURCE: Cunningham NR et al. Pediatrics. 2018 Jul 25. doi: 10.1542/peds.2017-2876.
according to results from a recent report published in Pediatrics.
Natoshia R. Cunningham, PhD, from the divisions of behavioral medicine and clinical psychology and the department of pediatrics at the University of Cincinnati and her colleagues tested their screening process with a pilot study in a single clinic between August 2015 and October 2016. Researchers assessed patients with FAPD using the Screen for Child Anxiety Related Disorders (SCARED)–Child Report and Functional Disability Inventory (FDI)–Child Version. Clinically significant anxiety was defined as a score of at least 25 (range, 0-82) for the SCARED; clinical cutoffs for minimal, moderate, and severe disability in the child version of the FDI were defined as scores of 0-12, 13-29, and greater than 30, respectively. After fine-tuning the screening process in the pilot, the researchers scaled the effort to six different clinics within a large gastroenterology division at a Midwest urban medical center.
“Children with FAPD who are at the greatest risk for persistent functional disability (i.e., those with clinical elevations in all three risk areas) are now being immediately identified and managed as part of routine care,” Dr. Cunningham and colleagues wrote in their study.
Of 6,744 eligible children (mean age, 13.34 years; 58% female; 87.6% non-Hispanic white), 5,221 children completed the screening, with 1,291 of 1,369 children completing both the screening process and reporting abdominal pain as a presenting complaint. Researchers found 43.1% of children showed clinically significant anxiety under SCARED, with a mean SCARED score of 24.3. Children had a mean FDI score of 13.7, and nearly half of the children had functional disability that was moderate (34.2%) or severe (10.8%), with 61.5% overall reporting a pain level of least 4 out of 10 during the week.
There were 21.1% of children with “clinical elevations” in pain, disability, and anxiety, with researchers noting that, compared with patients without clinical anxiety, those with anxiety had significantly higher FDI scores (mean, 16.29 vs. 11.54) and higher pain levels (mean, 4.63 vs. 3.72).
Among those referred to psychological services, the number of referrals after implementing psychological screening nearly doubled to 15.2 patients per 1,000 per month between March 2017 and September 2017, compared with baseline referrals the previous year (8.3 per 1,000, March 2016 to September 2016).
The researchers suggested future work in psychological screening for children with FAPD should consider shortening screening time by using different outcome measures to lessen the burden on clinical staff; they noted it should also consider applying psychological screening in a telehealth, primary care, or school setting to increase access to care.
“Even in patients with all three risk factors, the decision to refer to psychological providers remains an individualized process driven by clinical judgement,” Dr. Cunningham and her colleagues wrote. “Many factors, including provider practice patterns and other considerations (e.g., patient and family interest, provider availability, distance to care, and insurance coverage) guide these decisions.”
The authors report no relevant financial disclosures.
SOURCE: Cunningham NR et al. Pediatrics. 2018 Jul 25. doi: 10.1542/peds.2017-2876.
according to results from a recent report published in Pediatrics.
Natoshia R. Cunningham, PhD, from the divisions of behavioral medicine and clinical psychology and the department of pediatrics at the University of Cincinnati and her colleagues tested their screening process with a pilot study in a single clinic between August 2015 and October 2016. Researchers assessed patients with FAPD using the Screen for Child Anxiety Related Disorders (SCARED)–Child Report and Functional Disability Inventory (FDI)–Child Version. Clinically significant anxiety was defined as a score of at least 25 (range, 0-82) for the SCARED; clinical cutoffs for minimal, moderate, and severe disability in the child version of the FDI were defined as scores of 0-12, 13-29, and greater than 30, respectively. After fine-tuning the screening process in the pilot, the researchers scaled the effort to six different clinics within a large gastroenterology division at a Midwest urban medical center.
“Children with FAPD who are at the greatest risk for persistent functional disability (i.e., those with clinical elevations in all three risk areas) are now being immediately identified and managed as part of routine care,” Dr. Cunningham and colleagues wrote in their study.
Of 6,744 eligible children (mean age, 13.34 years; 58% female; 87.6% non-Hispanic white), 5,221 children completed the screening, with 1,291 of 1,369 children completing both the screening process and reporting abdominal pain as a presenting complaint. Researchers found 43.1% of children showed clinically significant anxiety under SCARED, with a mean SCARED score of 24.3. Children had a mean FDI score of 13.7, and nearly half of the children had functional disability that was moderate (34.2%) or severe (10.8%), with 61.5% overall reporting a pain level of least 4 out of 10 during the week.
There were 21.1% of children with “clinical elevations” in pain, disability, and anxiety, with researchers noting that, compared with patients without clinical anxiety, those with anxiety had significantly higher FDI scores (mean, 16.29 vs. 11.54) and higher pain levels (mean, 4.63 vs. 3.72).
Among those referred to psychological services, the number of referrals after implementing psychological screening nearly doubled to 15.2 patients per 1,000 per month between March 2017 and September 2017, compared with baseline referrals the previous year (8.3 per 1,000, March 2016 to September 2016).
The researchers suggested future work in psychological screening for children with FAPD should consider shortening screening time by using different outcome measures to lessen the burden on clinical staff; they noted it should also consider applying psychological screening in a telehealth, primary care, or school setting to increase access to care.
“Even in patients with all three risk factors, the decision to refer to psychological providers remains an individualized process driven by clinical judgement,” Dr. Cunningham and her colleagues wrote. “Many factors, including provider practice patterns and other considerations (e.g., patient and family interest, provider availability, distance to care, and insurance coverage) guide these decisions.”
The authors report no relevant financial disclosures.
SOURCE: Cunningham NR et al. Pediatrics. 2018 Jul 25. doi: 10.1542/peds.2017-2876.
FROM PEDIATRICS
Key clinical point: Implementing a screening system in clinical centers for pediatric patients with abdominal pain led to identification of anxiety and disability symptoms, additional tailored care, and psychological referrals for some patients with clinical anxiety.
Major finding: Of those who completed screening, 43.1% of children met criteria for clinically significant anxiety, 34.2% reported moderate disability, 10.8% reported severe functional disability, and 61.5% reported a pain level of at least 4 out of 10.
Study details: A report of 1,291 pediatric patients with abdominal pain screened at six clinics within a large gastroenterology division at a Midwest urban medical center.
Disclosures: The authors reported no relevant financial disclosures.
Source: Cunningham NR et al. Pediatrics. 2018 Jul 25. doi: 10.1542/peds.2017-2876.
Acute care prescriptions can be cut to minimize opioid exposure
ORLANDO – By cutting the number of pills prescribed after a surgical procedure, exposure to opioids can be minimized in a largely opioid-naive patient population at risk of new, persistent use, according to Michael J. Englesbe, MD, FACS, professor of surgery at the University of Michigan, Ann Arbor, who is leading a Michigan initiative to tailor acute care prescribing.
About 90% of surgically patients are opioid-naive, and of those, studies suggest about 6% may become new, persistent opioid users, according to Dr. Englesbe, codirector of the Michigan Opioid Prescribing and Engagement Network (Michigan-OPEN), a state-wide effort to transform acute pain prescribing across all surgical specialties.
“This is a very vulnerable population where their operation can lead to life-changing events way beyond their surgical outcomes,” Dr. Englesbe said in a presentation at the American College of Surgeons Quality and Safety Conference.
“We have to really worry about them,” he added. “It’s hard to identify who they are, and I think minimizing exposure to opioids is the best we have at this point.”
By following evidence-based prescribing guidelines after laparoscopic cholecystectomy, Dr. Englesbe and his colleagues were able to reduce prescription size by 63% with no increase in refills and no change in pain score, according to a research letter recently published in JAMA Surgery.
After adopting the guidelines, median postoperative opioid use dropped from 30 mg to 20 mg (P = .04), they reported.
Laparoscopic cholecystectomy patients could be prescribed as few as 10 5-mg tablets of oxycodone, according to recommendations developed by Michigan-OPEN that are published on opioidprescribing.info. Dr. Englesbe called the website figures “precise prescribing recommendations” that are still relatively generous, meeting or exceeding self-reported use for 75% of patients.
“I think this is an important template for change,” he said. “We’ve found the surgeons in the state very receptive, but more importantly, we’ve been able to partner with other really important stakeholders.” For example, one insurer in the state now aligns some hospital incentive reimbursement based on some of these prescribing methods, he added.
Dr. Englesbe reported no commercial disclosures related to his presentation.
ORLANDO – By cutting the number of pills prescribed after a surgical procedure, exposure to opioids can be minimized in a largely opioid-naive patient population at risk of new, persistent use, according to Michael J. Englesbe, MD, FACS, professor of surgery at the University of Michigan, Ann Arbor, who is leading a Michigan initiative to tailor acute care prescribing.
About 90% of surgically patients are opioid-naive, and of those, studies suggest about 6% may become new, persistent opioid users, according to Dr. Englesbe, codirector of the Michigan Opioid Prescribing and Engagement Network (Michigan-OPEN), a state-wide effort to transform acute pain prescribing across all surgical specialties.
“This is a very vulnerable population where their operation can lead to life-changing events way beyond their surgical outcomes,” Dr. Englesbe said in a presentation at the American College of Surgeons Quality and Safety Conference.
“We have to really worry about them,” he added. “It’s hard to identify who they are, and I think minimizing exposure to opioids is the best we have at this point.”
By following evidence-based prescribing guidelines after laparoscopic cholecystectomy, Dr. Englesbe and his colleagues were able to reduce prescription size by 63% with no increase in refills and no change in pain score, according to a research letter recently published in JAMA Surgery.
After adopting the guidelines, median postoperative opioid use dropped from 30 mg to 20 mg (P = .04), they reported.
Laparoscopic cholecystectomy patients could be prescribed as few as 10 5-mg tablets of oxycodone, according to recommendations developed by Michigan-OPEN that are published on opioidprescribing.info. Dr. Englesbe called the website figures “precise prescribing recommendations” that are still relatively generous, meeting or exceeding self-reported use for 75% of patients.
“I think this is an important template for change,” he said. “We’ve found the surgeons in the state very receptive, but more importantly, we’ve been able to partner with other really important stakeholders.” For example, one insurer in the state now aligns some hospital incentive reimbursement based on some of these prescribing methods, he added.
Dr. Englesbe reported no commercial disclosures related to his presentation.
ORLANDO – By cutting the number of pills prescribed after a surgical procedure, exposure to opioids can be minimized in a largely opioid-naive patient population at risk of new, persistent use, according to Michael J. Englesbe, MD, FACS, professor of surgery at the University of Michigan, Ann Arbor, who is leading a Michigan initiative to tailor acute care prescribing.
About 90% of surgically patients are opioid-naive, and of those, studies suggest about 6% may become new, persistent opioid users, according to Dr. Englesbe, codirector of the Michigan Opioid Prescribing and Engagement Network (Michigan-OPEN), a state-wide effort to transform acute pain prescribing across all surgical specialties.
“This is a very vulnerable population where their operation can lead to life-changing events way beyond their surgical outcomes,” Dr. Englesbe said in a presentation at the American College of Surgeons Quality and Safety Conference.
“We have to really worry about them,” he added. “It’s hard to identify who they are, and I think minimizing exposure to opioids is the best we have at this point.”
By following evidence-based prescribing guidelines after laparoscopic cholecystectomy, Dr. Englesbe and his colleagues were able to reduce prescription size by 63% with no increase in refills and no change in pain score, according to a research letter recently published in JAMA Surgery.
After adopting the guidelines, median postoperative opioid use dropped from 30 mg to 20 mg (P = .04), they reported.
Laparoscopic cholecystectomy patients could be prescribed as few as 10 5-mg tablets of oxycodone, according to recommendations developed by Michigan-OPEN that are published on opioidprescribing.info. Dr. Englesbe called the website figures “precise prescribing recommendations” that are still relatively generous, meeting or exceeding self-reported use for 75% of patients.
“I think this is an important template for change,” he said. “We’ve found the surgeons in the state very receptive, but more importantly, we’ve been able to partner with other really important stakeholders.” For example, one insurer in the state now aligns some hospital incentive reimbursement based on some of these prescribing methods, he added.
Dr. Englesbe reported no commercial disclosures related to his presentation.
EXPERT ANALYSIS FROM ACSQSC 2018
Hospitals gear up for new diagnosis: human trafficking
The woman arrived at the emergency department at Huntington Hospital on New York’s Long Island after she was hit by her boyfriend during an argument. Her situation raised concerns among the medical staff, which had recently been trained to be on the lookout for signs of sex trafficking.
An undocumented immigrant from El Salvador, she worked at a local “cantina” frequented by immigrants. Her job was to get patrons drinks and to dance with them, but many workers in those jobs are expected to offer sex, too. Her boyfriend didn’t want her to work there and that led to the fight, one doctor recalled.
As part of the intake process, the emergency staff asked the 36-year-old woman a series of questions about whether she’d ever had sex for money or whether she had to give someone else part of what she earned, among other things. The screening questions were part of a new program at Northwell Health, a 23-hospital system in the New York metro area that includes Huntington Hospital, to train staff and provide them with tools to identify and support victims of human trafficking.
There are no hard figures for how many people are involved in human trafficking, the term used when individuals are forced to work or have sex for someone else’s commercial benefit. Polaris, a Washington-based nonprofit that advocates for these people and runs help lines for them, said calls and texts to its national hotlines have steadily ticked up in recent years, increasing the number of cases 13% between 2016 and 2017, to 8,759.
But health care providers frequently fail to recognize these patients’ situation. According to a 2014 survey of about 100 survivors of sex trafficking, 88 percent said that while they were being trafficked they had contact with a health care provider, typically someone in an emergency department.
“When trafficking victims come through the health care system but we don’t identify them, it’s a big missed opportunity,” said Santhosh K. Paulus, MD, a family physician who is the site director of the Huntington Hospital’s family medicine residency program and who started the program at Northwell.
Northwell is one of a growing number of hospitals and health care systems that are putting such programs in place. They want to alert staff to be on the lookout for trafficking, much as they watch for signs of child abuse, domestic violence, and elder abuse.
Since last spring, nearly 300 staff members at Huntington Hospital and a family clinic have received training in how to spot trafficking victims and how to help them.
Training is given not only to doctors and nurses but also to registration and reception staff, social workers and security guards. Restore NYC, an organization that assists people caught up in sex trafficking, provided the initial training to key staff, and a hospital task force trains the others. During the next few years, similar efforts will be rolled out at all of Northwell’s 23 hospitals, Paulus said.
Identifying victims of trafficking is not unlike identifying victims of other forms of violence, said Wendy L. Macias-Konstantopoulos, MD, director of the human trafficking initiative at Massachusetts General Hospital in Boston.
One of the big red flags is when people delay coming in for medical care, such as waiting weeks to come in to get an injured ankle or sexually transmitted infection checked out, Dr. Macias-Konstantopoulos said. Or it may be a pattern of injuries that don’t make sense. Sometimes people are reluctant to explain their injury, or they come in with someone who seems overbearing.
“Having a high index of suspicion is the first step,” she said. “If we’re not asking about it, we’re just not going to see it.”
Starting in October, health care providers can also use new diagnosis codes in their records that differentiate trafficking from other types of abuse. This will help track the number of victims and provide appropriate treatment.
Asking may not be enough, however. Depending on what’s going on in their lives, these patients may not be willing or ready to acknowledge that they need help, said Holly Gibbs, human trafficking response program director for Dignity Health, a health care system with nearly 40 hospitals in California, Nevada and Arizona.
Ms. Gibbs knows the issue well. She was forced briefly into prostitution in Atlantic City, N.J., after meeting a man at a shopping mall as a 14-year-old and running away with him. The man persuaded Ms. Gibbs to go with him with promises of a new, glamorous life as a musician or model. At the time, Ms. Gibbs said, she thought that what happened to her was her own fault, a result of choices she made. No health care or law enforcement professional connected her to social services that could have helped her understand otherwise. She was reunited with her family by law enforcement personnel, who arrested the man. He was later convicted.
Dignity Health has implemented a human trafficking response program in the emergency departments and labor and delivery areas of each of its hospitals. Now it’s rolling out the program at clinics and physicians’ offices as well.
A key priority is to help clinicians know how to talk to patients about any violence they may be facing and to connect the patients with outside sources of help.
But in the end, if these patients don’t want assistance, “you respect their wishes,” Ms. Gibbs said. “They may not be ready to accept help now, but you may plant seeds so they’ll be able to accept it later on.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The woman arrived at the emergency department at Huntington Hospital on New York’s Long Island after she was hit by her boyfriend during an argument. Her situation raised concerns among the medical staff, which had recently been trained to be on the lookout for signs of sex trafficking.
An undocumented immigrant from El Salvador, she worked at a local “cantina” frequented by immigrants. Her job was to get patrons drinks and to dance with them, but many workers in those jobs are expected to offer sex, too. Her boyfriend didn’t want her to work there and that led to the fight, one doctor recalled.
As part of the intake process, the emergency staff asked the 36-year-old woman a series of questions about whether she’d ever had sex for money or whether she had to give someone else part of what she earned, among other things. The screening questions were part of a new program at Northwell Health, a 23-hospital system in the New York metro area that includes Huntington Hospital, to train staff and provide them with tools to identify and support victims of human trafficking.
There are no hard figures for how many people are involved in human trafficking, the term used when individuals are forced to work or have sex for someone else’s commercial benefit. Polaris, a Washington-based nonprofit that advocates for these people and runs help lines for them, said calls and texts to its national hotlines have steadily ticked up in recent years, increasing the number of cases 13% between 2016 and 2017, to 8,759.
But health care providers frequently fail to recognize these patients’ situation. According to a 2014 survey of about 100 survivors of sex trafficking, 88 percent said that while they were being trafficked they had contact with a health care provider, typically someone in an emergency department.
“When trafficking victims come through the health care system but we don’t identify them, it’s a big missed opportunity,” said Santhosh K. Paulus, MD, a family physician who is the site director of the Huntington Hospital’s family medicine residency program and who started the program at Northwell.
Northwell is one of a growing number of hospitals and health care systems that are putting such programs in place. They want to alert staff to be on the lookout for trafficking, much as they watch for signs of child abuse, domestic violence, and elder abuse.
Since last spring, nearly 300 staff members at Huntington Hospital and a family clinic have received training in how to spot trafficking victims and how to help them.
Training is given not only to doctors and nurses but also to registration and reception staff, social workers and security guards. Restore NYC, an organization that assists people caught up in sex trafficking, provided the initial training to key staff, and a hospital task force trains the others. During the next few years, similar efforts will be rolled out at all of Northwell’s 23 hospitals, Paulus said.
Identifying victims of trafficking is not unlike identifying victims of other forms of violence, said Wendy L. Macias-Konstantopoulos, MD, director of the human trafficking initiative at Massachusetts General Hospital in Boston.
One of the big red flags is when people delay coming in for medical care, such as waiting weeks to come in to get an injured ankle or sexually transmitted infection checked out, Dr. Macias-Konstantopoulos said. Or it may be a pattern of injuries that don’t make sense. Sometimes people are reluctant to explain their injury, or they come in with someone who seems overbearing.
“Having a high index of suspicion is the first step,” she said. “If we’re not asking about it, we’re just not going to see it.”
Starting in October, health care providers can also use new diagnosis codes in their records that differentiate trafficking from other types of abuse. This will help track the number of victims and provide appropriate treatment.
Asking may not be enough, however. Depending on what’s going on in their lives, these patients may not be willing or ready to acknowledge that they need help, said Holly Gibbs, human trafficking response program director for Dignity Health, a health care system with nearly 40 hospitals in California, Nevada and Arizona.
Ms. Gibbs knows the issue well. She was forced briefly into prostitution in Atlantic City, N.J., after meeting a man at a shopping mall as a 14-year-old and running away with him. The man persuaded Ms. Gibbs to go with him with promises of a new, glamorous life as a musician or model. At the time, Ms. Gibbs said, she thought that what happened to her was her own fault, a result of choices she made. No health care or law enforcement professional connected her to social services that could have helped her understand otherwise. She was reunited with her family by law enforcement personnel, who arrested the man. He was later convicted.
Dignity Health has implemented a human trafficking response program in the emergency departments and labor and delivery areas of each of its hospitals. Now it’s rolling out the program at clinics and physicians’ offices as well.
A key priority is to help clinicians know how to talk to patients about any violence they may be facing and to connect the patients with outside sources of help.
But in the end, if these patients don’t want assistance, “you respect their wishes,” Ms. Gibbs said. “They may not be ready to accept help now, but you may plant seeds so they’ll be able to accept it later on.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
The woman arrived at the emergency department at Huntington Hospital on New York’s Long Island after she was hit by her boyfriend during an argument. Her situation raised concerns among the medical staff, which had recently been trained to be on the lookout for signs of sex trafficking.
An undocumented immigrant from El Salvador, she worked at a local “cantina” frequented by immigrants. Her job was to get patrons drinks and to dance with them, but many workers in those jobs are expected to offer sex, too. Her boyfriend didn’t want her to work there and that led to the fight, one doctor recalled.
As part of the intake process, the emergency staff asked the 36-year-old woman a series of questions about whether she’d ever had sex for money or whether she had to give someone else part of what she earned, among other things. The screening questions were part of a new program at Northwell Health, a 23-hospital system in the New York metro area that includes Huntington Hospital, to train staff and provide them with tools to identify and support victims of human trafficking.
There are no hard figures for how many people are involved in human trafficking, the term used when individuals are forced to work or have sex for someone else’s commercial benefit. Polaris, a Washington-based nonprofit that advocates for these people and runs help lines for them, said calls and texts to its national hotlines have steadily ticked up in recent years, increasing the number of cases 13% between 2016 and 2017, to 8,759.
But health care providers frequently fail to recognize these patients’ situation. According to a 2014 survey of about 100 survivors of sex trafficking, 88 percent said that while they were being trafficked they had contact with a health care provider, typically someone in an emergency department.
“When trafficking victims come through the health care system but we don’t identify them, it’s a big missed opportunity,” said Santhosh K. Paulus, MD, a family physician who is the site director of the Huntington Hospital’s family medicine residency program and who started the program at Northwell.
Northwell is one of a growing number of hospitals and health care systems that are putting such programs in place. They want to alert staff to be on the lookout for trafficking, much as they watch for signs of child abuse, domestic violence, and elder abuse.
Since last spring, nearly 300 staff members at Huntington Hospital and a family clinic have received training in how to spot trafficking victims and how to help them.
Training is given not only to doctors and nurses but also to registration and reception staff, social workers and security guards. Restore NYC, an organization that assists people caught up in sex trafficking, provided the initial training to key staff, and a hospital task force trains the others. During the next few years, similar efforts will be rolled out at all of Northwell’s 23 hospitals, Paulus said.
Identifying victims of trafficking is not unlike identifying victims of other forms of violence, said Wendy L. Macias-Konstantopoulos, MD, director of the human trafficking initiative at Massachusetts General Hospital in Boston.
One of the big red flags is when people delay coming in for medical care, such as waiting weeks to come in to get an injured ankle or sexually transmitted infection checked out, Dr. Macias-Konstantopoulos said. Or it may be a pattern of injuries that don’t make sense. Sometimes people are reluctant to explain their injury, or they come in with someone who seems overbearing.
“Having a high index of suspicion is the first step,” she said. “If we’re not asking about it, we’re just not going to see it.”
Starting in October, health care providers can also use new diagnosis codes in their records that differentiate trafficking from other types of abuse. This will help track the number of victims and provide appropriate treatment.
Asking may not be enough, however. Depending on what’s going on in their lives, these patients may not be willing or ready to acknowledge that they need help, said Holly Gibbs, human trafficking response program director for Dignity Health, a health care system with nearly 40 hospitals in California, Nevada and Arizona.
Ms. Gibbs knows the issue well. She was forced briefly into prostitution in Atlantic City, N.J., after meeting a man at a shopping mall as a 14-year-old and running away with him. The man persuaded Ms. Gibbs to go with him with promises of a new, glamorous life as a musician or model. At the time, Ms. Gibbs said, she thought that what happened to her was her own fault, a result of choices she made. No health care or law enforcement professional connected her to social services that could have helped her understand otherwise. She was reunited with her family by law enforcement personnel, who arrested the man. He was later convicted.
Dignity Health has implemented a human trafficking response program in the emergency departments and labor and delivery areas of each of its hospitals. Now it’s rolling out the program at clinics and physicians’ offices as well.
A key priority is to help clinicians know how to talk to patients about any violence they may be facing and to connect the patients with outside sources of help.
But in the end, if these patients don’t want assistance, “you respect their wishes,” Ms. Gibbs said. “They may not be ready to accept help now, but you may plant seeds so they’ll be able to accept it later on.”
Kaiser Health News is a nonprofit national health policy news service. It is an editorially independent program of the Henry J. Kaiser Family Foundation that is not affiliated with Kaiser Permanente.
Questions abound about availability, legality of new cannabis-derived epilepsy drug
The first-ever federal approval of a marijuana-derived drug, Epidiolex (cannabidiol), comes with a cloud of complications.
Epidiolex is an oral solution of purified cannabidiol (CBD), a component of the marijuana plant that does not make people high.
According to the results of a phase 3 trial in patients with Lennox-Gastaut syndrome published earlier this year, median monthly drop seizures fell by 43.9% in a Epidiolex group compared with 21.8% in a placebo group (Lancet. 2018;391[10125]:1085-96). A 2017 study found that patients with Dravet syndrome who took the drug had fewer median convulsive seizures per month, dropping from 12.4 to 5.9, while there was barely a difference for the placebo group (N Engl J Med. 2017;376:2011-20). In both trials, patients received Epidiolex as an add-on treatment.
“There definitely are side effects such as sleepiness, abnormal liver function values, and other things that people will have to watch out for, such as interactions with other drugs,” Jacqueline A. French, MD, professor of neurology at New York University and chief scientific officer of the Epilepsy Foundation, said in an interview. “But it will definitely be a great benefit to some and a benefit to many.”
It’s not clear how the drug works, she said.
Greenwich Biosciences, the U.S. subsidiary of British drug maker GW Pharmaceuticals, plans to market Epidiolex this fall, spokesman Steve Schultz said in an interview. First, however, the U.S. Drug Enforcement Agency must reschedule the drug so it doesn’t fall under federal antimarijuana laws. It has 90 days after the FDA approval to do so, and its rescheduling is expected.
Then the focus will turn to the states, which have a crazy quilt of laws about marijuana and its medical use. “We’ve worked with the state legislators to enact laws or to modify laws to allow for our medicine to be made available,” Mr. Schultz said. “There may be one or two that we’re still working on.”
At issue: States and the District of Columbia have a wide variety of laws that appear to affect the sale of Epidiolex.
According to the website procon.org, 30 states and the District of Columbia allow medical marijuana, although the laws vary regarding what is allowed and do not address cannabis-derived pharmaceuticals.
Another 17 states have laws that address CBD specifically, often with language that allows its use to treat epilepsy in all or specific cases, according to procon.org.
In Oklahoma, an anticannabis law excludes “from the definition of marijuana ‘any federal Food and Drug Administration–approved cannabidiol drug or substance.’ ”
North Carolina attorney Rod Kight, who represents businesses in the cannabis industry, predicts that “once the federal government [via the DEA] changes the law, the states will fall in line. The only thing that would prevent them would be a lack of understanding of what’s going on.”
Meanwhile, many sellers of CBD-based products continue to promote their use as treatments for epilepsy and many other conditions.
Legal exceptions made by states for FDA-approved CBD-based drugs could affect access to unapproved CBD-based products. It’s also possible that Epidiolex could hurt sellers of cannabis products in other ways. “They’ll now have to compete with a drug made to a specific strength and purity, and that’s probably going to have some impact on their business,” William J. McNichol, adjunct professor at Rutgers Law School, Camden, N.J., said in an interview. “If you have a choice, are you going to choose aspirin I made myself in my artisan aspirin factory that the FDA never saw? Or aspirin made by Bayer?”
What’s next? For one, the FDA’s approval of Epidiolex seems likely to open the door for more cannabis-based medications. “The FDA is quite open to evaluating cannabinoid-based medicines as long as they go through their process,” said Mr. Schultz, the Greenwich Biosciences spokesman.
As for the legal front, Mr. Kight said potential changes in federal law could expand the legality of cannabis-based products by allowing them when they’re derived from “industrial hemp.” This could mean that patients with epilepsy will have more legal ways to buy CBD products.
Regardless of the legal situation, Mr. Kight said, “a physician can explain to patients across the board about how CBD might benefit them. The physician can say there are extract products that are out there and available over the counter.”
As for the maker of Epidiolex, GW Pharmaceuticals is recruiting for a phase 3 trial of a cannabinoid treatment for tuberous sclerosis complex, Mr. Schultz said. The results are due in the first half of 2019.
The company is also conducting cannabinoid research in the areas of autism and Rett syndrome, Mr. Schultz said, adding: “We also have done work in schizophrenia and in oncology and glioblastoma.”
Mr. Kight disclosed that he represents companies in the cannabis industry. Dr. French is president of the Epilepsy Study Consortium and disclosed working with multiple drug makers in the epilepsy field, including GW Pharmaceuticals. Dr. French receives fixed compensation by the consortium and is not paid by any pharmaceutical company. Mr. McNichol reported no relevant disclosures.
The first-ever federal approval of a marijuana-derived drug, Epidiolex (cannabidiol), comes with a cloud of complications.
Epidiolex is an oral solution of purified cannabidiol (CBD), a component of the marijuana plant that does not make people high.
According to the results of a phase 3 trial in patients with Lennox-Gastaut syndrome published earlier this year, median monthly drop seizures fell by 43.9% in a Epidiolex group compared with 21.8% in a placebo group (Lancet. 2018;391[10125]:1085-96). A 2017 study found that patients with Dravet syndrome who took the drug had fewer median convulsive seizures per month, dropping from 12.4 to 5.9, while there was barely a difference for the placebo group (N Engl J Med. 2017;376:2011-20). In both trials, patients received Epidiolex as an add-on treatment.
“There definitely are side effects such as sleepiness, abnormal liver function values, and other things that people will have to watch out for, such as interactions with other drugs,” Jacqueline A. French, MD, professor of neurology at New York University and chief scientific officer of the Epilepsy Foundation, said in an interview. “But it will definitely be a great benefit to some and a benefit to many.”
It’s not clear how the drug works, she said.
Greenwich Biosciences, the U.S. subsidiary of British drug maker GW Pharmaceuticals, plans to market Epidiolex this fall, spokesman Steve Schultz said in an interview. First, however, the U.S. Drug Enforcement Agency must reschedule the drug so it doesn’t fall under federal antimarijuana laws. It has 90 days after the FDA approval to do so, and its rescheduling is expected.
Then the focus will turn to the states, which have a crazy quilt of laws about marijuana and its medical use. “We’ve worked with the state legislators to enact laws or to modify laws to allow for our medicine to be made available,” Mr. Schultz said. “There may be one or two that we’re still working on.”
At issue: States and the District of Columbia have a wide variety of laws that appear to affect the sale of Epidiolex.
According to the website procon.org, 30 states and the District of Columbia allow medical marijuana, although the laws vary regarding what is allowed and do not address cannabis-derived pharmaceuticals.
Another 17 states have laws that address CBD specifically, often with language that allows its use to treat epilepsy in all or specific cases, according to procon.org.
In Oklahoma, an anticannabis law excludes “from the definition of marijuana ‘any federal Food and Drug Administration–approved cannabidiol drug or substance.’ ”
North Carolina attorney Rod Kight, who represents businesses in the cannabis industry, predicts that “once the federal government [via the DEA] changes the law, the states will fall in line. The only thing that would prevent them would be a lack of understanding of what’s going on.”
Meanwhile, many sellers of CBD-based products continue to promote their use as treatments for epilepsy and many other conditions.
Legal exceptions made by states for FDA-approved CBD-based drugs could affect access to unapproved CBD-based products. It’s also possible that Epidiolex could hurt sellers of cannabis products in other ways. “They’ll now have to compete with a drug made to a specific strength and purity, and that’s probably going to have some impact on their business,” William J. McNichol, adjunct professor at Rutgers Law School, Camden, N.J., said in an interview. “If you have a choice, are you going to choose aspirin I made myself in my artisan aspirin factory that the FDA never saw? Or aspirin made by Bayer?”
What’s next? For one, the FDA’s approval of Epidiolex seems likely to open the door for more cannabis-based medications. “The FDA is quite open to evaluating cannabinoid-based medicines as long as they go through their process,” said Mr. Schultz, the Greenwich Biosciences spokesman.
As for the legal front, Mr. Kight said potential changes in federal law could expand the legality of cannabis-based products by allowing them when they’re derived from “industrial hemp.” This could mean that patients with epilepsy will have more legal ways to buy CBD products.
Regardless of the legal situation, Mr. Kight said, “a physician can explain to patients across the board about how CBD might benefit them. The physician can say there are extract products that are out there and available over the counter.”
As for the maker of Epidiolex, GW Pharmaceuticals is recruiting for a phase 3 trial of a cannabinoid treatment for tuberous sclerosis complex, Mr. Schultz said. The results are due in the first half of 2019.
The company is also conducting cannabinoid research in the areas of autism and Rett syndrome, Mr. Schultz said, adding: “We also have done work in schizophrenia and in oncology and glioblastoma.”
Mr. Kight disclosed that he represents companies in the cannabis industry. Dr. French is president of the Epilepsy Study Consortium and disclosed working with multiple drug makers in the epilepsy field, including GW Pharmaceuticals. Dr. French receives fixed compensation by the consortium and is not paid by any pharmaceutical company. Mr. McNichol reported no relevant disclosures.
The first-ever federal approval of a marijuana-derived drug, Epidiolex (cannabidiol), comes with a cloud of complications.
Epidiolex is an oral solution of purified cannabidiol (CBD), a component of the marijuana plant that does not make people high.
According to the results of a phase 3 trial in patients with Lennox-Gastaut syndrome published earlier this year, median monthly drop seizures fell by 43.9% in a Epidiolex group compared with 21.8% in a placebo group (Lancet. 2018;391[10125]:1085-96). A 2017 study found that patients with Dravet syndrome who took the drug had fewer median convulsive seizures per month, dropping from 12.4 to 5.9, while there was barely a difference for the placebo group (N Engl J Med. 2017;376:2011-20). In both trials, patients received Epidiolex as an add-on treatment.
“There definitely are side effects such as sleepiness, abnormal liver function values, and other things that people will have to watch out for, such as interactions with other drugs,” Jacqueline A. French, MD, professor of neurology at New York University and chief scientific officer of the Epilepsy Foundation, said in an interview. “But it will definitely be a great benefit to some and a benefit to many.”
It’s not clear how the drug works, she said.
Greenwich Biosciences, the U.S. subsidiary of British drug maker GW Pharmaceuticals, plans to market Epidiolex this fall, spokesman Steve Schultz said in an interview. First, however, the U.S. Drug Enforcement Agency must reschedule the drug so it doesn’t fall under federal antimarijuana laws. It has 90 days after the FDA approval to do so, and its rescheduling is expected.
Then the focus will turn to the states, which have a crazy quilt of laws about marijuana and its medical use. “We’ve worked with the state legislators to enact laws or to modify laws to allow for our medicine to be made available,” Mr. Schultz said. “There may be one or two that we’re still working on.”
At issue: States and the District of Columbia have a wide variety of laws that appear to affect the sale of Epidiolex.
According to the website procon.org, 30 states and the District of Columbia allow medical marijuana, although the laws vary regarding what is allowed and do not address cannabis-derived pharmaceuticals.
Another 17 states have laws that address CBD specifically, often with language that allows its use to treat epilepsy in all or specific cases, according to procon.org.
In Oklahoma, an anticannabis law excludes “from the definition of marijuana ‘any federal Food and Drug Administration–approved cannabidiol drug or substance.’ ”
North Carolina attorney Rod Kight, who represents businesses in the cannabis industry, predicts that “once the federal government [via the DEA] changes the law, the states will fall in line. The only thing that would prevent them would be a lack of understanding of what’s going on.”
Meanwhile, many sellers of CBD-based products continue to promote their use as treatments for epilepsy and many other conditions.
Legal exceptions made by states for FDA-approved CBD-based drugs could affect access to unapproved CBD-based products. It’s also possible that Epidiolex could hurt sellers of cannabis products in other ways. “They’ll now have to compete with a drug made to a specific strength and purity, and that’s probably going to have some impact on their business,” William J. McNichol, adjunct professor at Rutgers Law School, Camden, N.J., said in an interview. “If you have a choice, are you going to choose aspirin I made myself in my artisan aspirin factory that the FDA never saw? Or aspirin made by Bayer?”
What’s next? For one, the FDA’s approval of Epidiolex seems likely to open the door for more cannabis-based medications. “The FDA is quite open to evaluating cannabinoid-based medicines as long as they go through their process,” said Mr. Schultz, the Greenwich Biosciences spokesman.
As for the legal front, Mr. Kight said potential changes in federal law could expand the legality of cannabis-based products by allowing them when they’re derived from “industrial hemp.” This could mean that patients with epilepsy will have more legal ways to buy CBD products.
Regardless of the legal situation, Mr. Kight said, “a physician can explain to patients across the board about how CBD might benefit them. The physician can say there are extract products that are out there and available over the counter.”
As for the maker of Epidiolex, GW Pharmaceuticals is recruiting for a phase 3 trial of a cannabinoid treatment for tuberous sclerosis complex, Mr. Schultz said. The results are due in the first half of 2019.
The company is also conducting cannabinoid research in the areas of autism and Rett syndrome, Mr. Schultz said, adding: “We also have done work in schizophrenia and in oncology and glioblastoma.”
Mr. Kight disclosed that he represents companies in the cannabis industry. Dr. French is president of the Epilepsy Study Consortium and disclosed working with multiple drug makers in the epilepsy field, including GW Pharmaceuticals. Dr. French receives fixed compensation by the consortium and is not paid by any pharmaceutical company. Mr. McNichol reported no relevant disclosures.
Early escitalopram for post-ACS depression improves MACE, 8 years on
Even years after their cardiac events, acute coronary syndrome patients whose depression was treated with 6 months of an antidepressant had fewer cardiovascular events, according to a randomized, controlled trial.
Compared with patients who were given placebo for depression after experiencing acute coronary syndrome (ACS), patients who received escitalopram experienced a lower incidence of a composite outcome of major adverse cardiovascular events (MACE), in this extension study of a 6-month trial reported previously (J Clin Psychiatry. 2015 Jan;76[1]:62-8).
After a median of 8.1 years, MACE, the study’s primary outcome, was experienced by 40.9% of patients taking escitalopram, compared with 53.6% of those taking placebo (hazard ratio 0.69, 95% confidence interval 0.49-0.96, P = .03). The investigators also tracked the individual MACE components (all-cause mortality, myocardial infarction, and percutaneous coronary intervention), finding that MI was also significantly less common in the escitalopram group compared with those who took placebo (8.7% vs. 15.2%, HR 0.54, 95% CI, 0.27-0.96, P = .04).
The other MACE components were seen less frequently in the escitalopram group, but the differences from those taking placebo were not statistically significant. The study did not show a mortality benefit for those who took escitalopram, noted first author Jae-Min Kim, MD, of the department of psychiatry at Chonnam University Medical School, Gwangju, South Korea, and his coauthors.
The randomized, double-blind, placebo-controlled trial comprised 300 patients who recently had ACS and also met DSM-IV criteria for major or minor depression.
Over a 5-year period at a single site, patients were randomized to receive escitalopram at an initial dose of 10 mg daily (149 patients), or placebo (151), for 24 weeks after their ACS event. Escitalopram doses could be adjusted according to clinician judgment from 5 to 20 mg daily.
Patients who consented to participate were enrolled and screened for depression within 2 weeks of their ACS event, and any who scored higher than 10 on the Beck Depression Inventory at the initial screen or at follow-up appointments up to 12 weeks post enrollment went on for further screening.
Demographic data were collected with an eye toward identifying factors that could affect cardiac outcomes, said Dr. Kim and his colleagues. So in addition to age, sex, and educational level, the investigators tracked participants’ living situations and marital and employment status.
Patients were a mean of 60 years old, and about 60% were male. The median Beck Depression Inventory score was 16-17.
The study differed from others, said the investigators, because it followed patients for a longer period of time. In previous studies, “[E]ffects on cardiac outcomes have not generally been found within the treatment period,” they wrote.
Though the study’s generalizability might be limited by the fact that it was conducted at a single site and all participants were Asian, the single-site design and South Korea’s comprehensive patient data registries also allowed for complete data collection over a very long follow-up period, noted Dr. Kim and his colleagues.
Adherence was high, at 93% for those taking escitalopram and 95% for those taking placebo. Other work published from the study has shown that those taking 24 weeks of escitalopram had less depression than did those taking placebo, they added.
One coauthor reported receiving financial support from Janssen and Roche. The study was supported by the National Research Foundation of Korea.
SOURCE: Kim J-M et al. JAMA. 2018;320(4):350-8.
Even years after their cardiac events, acute coronary syndrome patients whose depression was treated with 6 months of an antidepressant had fewer cardiovascular events, according to a randomized, controlled trial.
Compared with patients who were given placebo for depression after experiencing acute coronary syndrome (ACS), patients who received escitalopram experienced a lower incidence of a composite outcome of major adverse cardiovascular events (MACE), in this extension study of a 6-month trial reported previously (J Clin Psychiatry. 2015 Jan;76[1]:62-8).
After a median of 8.1 years, MACE, the study’s primary outcome, was experienced by 40.9% of patients taking escitalopram, compared with 53.6% of those taking placebo (hazard ratio 0.69, 95% confidence interval 0.49-0.96, P = .03). The investigators also tracked the individual MACE components (all-cause mortality, myocardial infarction, and percutaneous coronary intervention), finding that MI was also significantly less common in the escitalopram group compared with those who took placebo (8.7% vs. 15.2%, HR 0.54, 95% CI, 0.27-0.96, P = .04).
The other MACE components were seen less frequently in the escitalopram group, but the differences from those taking placebo were not statistically significant. The study did not show a mortality benefit for those who took escitalopram, noted first author Jae-Min Kim, MD, of the department of psychiatry at Chonnam University Medical School, Gwangju, South Korea, and his coauthors.
The randomized, double-blind, placebo-controlled trial comprised 300 patients who recently had ACS and also met DSM-IV criteria for major or minor depression.
Over a 5-year period at a single site, patients were randomized to receive escitalopram at an initial dose of 10 mg daily (149 patients), or placebo (151), for 24 weeks after their ACS event. Escitalopram doses could be adjusted according to clinician judgment from 5 to 20 mg daily.
Patients who consented to participate were enrolled and screened for depression within 2 weeks of their ACS event, and any who scored higher than 10 on the Beck Depression Inventory at the initial screen or at follow-up appointments up to 12 weeks post enrollment went on for further screening.
Demographic data were collected with an eye toward identifying factors that could affect cardiac outcomes, said Dr. Kim and his colleagues. So in addition to age, sex, and educational level, the investigators tracked participants’ living situations and marital and employment status.
Patients were a mean of 60 years old, and about 60% were male. The median Beck Depression Inventory score was 16-17.
The study differed from others, said the investigators, because it followed patients for a longer period of time. In previous studies, “[E]ffects on cardiac outcomes have not generally been found within the treatment period,” they wrote.
Though the study’s generalizability might be limited by the fact that it was conducted at a single site and all participants were Asian, the single-site design and South Korea’s comprehensive patient data registries also allowed for complete data collection over a very long follow-up period, noted Dr. Kim and his colleagues.
Adherence was high, at 93% for those taking escitalopram and 95% for those taking placebo. Other work published from the study has shown that those taking 24 weeks of escitalopram had less depression than did those taking placebo, they added.
One coauthor reported receiving financial support from Janssen and Roche. The study was supported by the National Research Foundation of Korea.
SOURCE: Kim J-M et al. JAMA. 2018;320(4):350-8.
Even years after their cardiac events, acute coronary syndrome patients whose depression was treated with 6 months of an antidepressant had fewer cardiovascular events, according to a randomized, controlled trial.
Compared with patients who were given placebo for depression after experiencing acute coronary syndrome (ACS), patients who received escitalopram experienced a lower incidence of a composite outcome of major adverse cardiovascular events (MACE), in this extension study of a 6-month trial reported previously (J Clin Psychiatry. 2015 Jan;76[1]:62-8).
After a median of 8.1 years, MACE, the study’s primary outcome, was experienced by 40.9% of patients taking escitalopram, compared with 53.6% of those taking placebo (hazard ratio 0.69, 95% confidence interval 0.49-0.96, P = .03). The investigators also tracked the individual MACE components (all-cause mortality, myocardial infarction, and percutaneous coronary intervention), finding that MI was also significantly less common in the escitalopram group compared with those who took placebo (8.7% vs. 15.2%, HR 0.54, 95% CI, 0.27-0.96, P = .04).
The other MACE components were seen less frequently in the escitalopram group, but the differences from those taking placebo were not statistically significant. The study did not show a mortality benefit for those who took escitalopram, noted first author Jae-Min Kim, MD, of the department of psychiatry at Chonnam University Medical School, Gwangju, South Korea, and his coauthors.
The randomized, double-blind, placebo-controlled trial comprised 300 patients who recently had ACS and also met DSM-IV criteria for major or minor depression.
Over a 5-year period at a single site, patients were randomized to receive escitalopram at an initial dose of 10 mg daily (149 patients), or placebo (151), for 24 weeks after their ACS event. Escitalopram doses could be adjusted according to clinician judgment from 5 to 20 mg daily.
Patients who consented to participate were enrolled and screened for depression within 2 weeks of their ACS event, and any who scored higher than 10 on the Beck Depression Inventory at the initial screen or at follow-up appointments up to 12 weeks post enrollment went on for further screening.
Demographic data were collected with an eye toward identifying factors that could affect cardiac outcomes, said Dr. Kim and his colleagues. So in addition to age, sex, and educational level, the investigators tracked participants’ living situations and marital and employment status.
Patients were a mean of 60 years old, and about 60% were male. The median Beck Depression Inventory score was 16-17.
The study differed from others, said the investigators, because it followed patients for a longer period of time. In previous studies, “[E]ffects on cardiac outcomes have not generally been found within the treatment period,” they wrote.
Though the study’s generalizability might be limited by the fact that it was conducted at a single site and all participants were Asian, the single-site design and South Korea’s comprehensive patient data registries also allowed for complete data collection over a very long follow-up period, noted Dr. Kim and his colleagues.
Adherence was high, at 93% for those taking escitalopram and 95% for those taking placebo. Other work published from the study has shown that those taking 24 weeks of escitalopram had less depression than did those taking placebo, they added.
One coauthor reported receiving financial support from Janssen and Roche. The study was supported by the National Research Foundation of Korea.
SOURCE: Kim J-M et al. JAMA. 2018;320(4):350-8.
FROM JAMA
Key clinical point: .
Major finding: The hazard ratio was 0.69 for MACE in the escitalopram group compared with placebo (P = .03).
Study details: Randomized, double-blind, placebo-controlled trial of 300 patients with ACS given escitalopram or placebo.
Disclosures: The National Research Council of Korea sponsored the study. One author reported funding from Janssen and Roche.
Source: Kim J-M et al. JAMA. 2018;320(4):350-8.
How to bring behavioral care into your office
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
There is consensus within both the medical and public health communities that an integrated model of health care, in which behavioral health is integrated into primary care settings, is the optimal way to improve the health of a population (not just treat disease) while managing costs and improving the patient’s experience of care. Such a model is especially compelling for pediatric care.
There are 74 million children under 18 years in the United States and the prevalence of psychiatric disorders in youth is 20%, or 15 million; after vaccinations and following development, managing psychiatric symptoms is the most common issue in pediatric primary care.
While some psychiatric illnesses can be well managed by primary care clinicians alone, some illnesses require specialized therapy or more complex pharmacologic treatment. Untreated or inadequately treated childhood mental illness can lead to a longer and worse course of illness, academic difficulties, emergence of associated illnesses (such as substance use disorders), and legal problems. For those children with chronic medical conditions, emotional disorders cause distress, and affect adherence and family functioning. We will discuss some practical strategies to begin to bring behavioral health care into the pediatric primary care setting.
Start by implementing behavioral health screening into annual and sick visits. Broad instruments, such as the Pediatric Symptom Checklist (PSC, 35 items) or the Child Behavior Check List (CBCL, 113 items) can be filled out by caregivers in the waiting room or online before a visit, and can suggest specific disorders or simply the need for a full psychiatric assessment. Electronic medical records may have publicly available questionnaires such as PSC built into their software, facilitating use of a tablet or home computer, and may ease scoring and downloading of results. Depending on the structure of your practice, you could have one clinician in charge of managing screening. You may become comfortable diagnosing certain disorders, such as ADHD, a major depressive episode, or an anxiety disorder, and you may begin medication treatment when appropriate. You can use instruments developed for specific disease entities (such as ADHD, obsessive compulsive disorder [OCD], anxiety, or depression) to monitor your patient’s treatment response, and they may be done virtually to minimize unnecessary visits.
Treatment algorithms for most psychiatric illnesses are available through the American Academy of Pediatrics and the American Academy of Child and Adolescent Psychiatry, and can guide you through the early stages of treatment. Psychotherapy is the first-line treatment for mild to moderate anxiety and mood disorders, and it is critical to the treatment of more severe disorders. Difficulty in finding a therapist who is skilled in a specific treatment, is a good fit, and accepts insurance can be a significant barrier to care. Establishing a coordinated relationship with a team of therapists can facilitate referrals. Some states have programs in which primary care physicians can have telephone consultations with mental health clinicians or to access referral services for therapy, such as the Massachusetts Child Psychiatry Access Project.
If you have a busy enough practice, consider bringing a social worker or psychologist to work with you. Such a clinician could perform diagnostic assessments, ongoing therapy, parent guidance, family work, or care coordination. Consider how to make it cost-effective for this clinician and your group, whether by inviting that person to sublet one of your offices, or having that person directly employed by you and benefiting from your office staff and patient flow. Many states now reimburse for screening and these funds could contribute to the expense of a social worker. This approach would bring you from coordination to true colocation, which greatly improves the likelihood of compliance with therapy, enhances coordination of a patient’s care, creates opportunities for ongoing education between disciplines, and diminishes stigma of acknowledging a mental illness. Anxiety disorders are the most common illnesses of youth, with mood disorders emerging in adolescence, and substance use disorders in later adolescence. Consider this in seeking a clinician with a specific interest or skill set (such as cognitive behavioral therapy for anxiety or mood problems, dialectical behavior therapy for chronic suicidality, or motivational interviewing for substance abuse).
Beyond diagnosing and treating psychiatric illness in your patients, a primary care pediatric setting with integrated behavioral health would improve the health of our young patients by investing in prevention and parental support. Universal prevention efforts are a hallmark of good pediatric care, from vaccines to educating parents and children about injury prevention (bike helmets, smoke detectors, and car seats) and risky behaviors (smoking). Educate your patients and their parents about best practices to promote good mental health, from good sleep hygiene to regular exercise and healthy stress management techniques. You could use posters and pamphlets, videos and smartphone apps, or screening instruments and discussion.
If you invest in a colocated mental health clinician, you can expand your prevention efforts beyond the universal. Screen for a family history of anxiety, mood, and substance use disorders, and screen for adverse childhood experiences scores. Chronic stress and a family history of specific psychiatric illnesses significantly increase the risk of specific illnesses in your patients. There are evidence-based interventions that can be used to prevent the emergence of many disorders in young people at specific risk. For example, parents who have struggled with anxiety can learn specific strategies for managing their children’s anxiety, significantly lowering the risk of anxiety disorders in their children. These skills can be taught individually or in groups, depending on the prevalence in your practice. Those insurers who reimburse for therapy have a reimbursement schedule for work with parents as well.
There may be funds available to support your investment in integrated care. Under the Affordable Care Act, Medicaid enhanced funding for Health Homes for enrolled children. There have been federal grants for primary care offices to engage in different levels of integration and measure outcomes (Project LAUNCH – Linking Actions for Unmet Needs in Children’s Health). There may be funding at the state level or from private foundations dedicated to public health research and initiatives. Even if you do not invest in procuring outside funding, you should consider how to measure patient outcomes once you are making any efforts at integrating behavioral health care into your practice. Outcome measures include questionnaire scores, treatment adherence, number of school absences, number of office or ED visits, or global measurements, such as the Child Global Assessment Scale (CGAS). Such data can inform you about how to adjust your approach, and could contribute to the larger effort to understand what strategies are most effective and feasible. Addressing the behavioral health needs of your patients could meaningfully contribute to the efforts to make the vision of integrated care – that which truly promotes health in our young people – a reality.
Dr. Swick is an attending psychiatrist in the division of child psychiatry at Massachusetts General Hospital, Boston, and director of the Parenting at a Challenging Time (PACT) Program at the Vernon Cancer Center at Newton Wellesley Hospital, also in Boston. Dr. Jellinek is professor emeritus of psychiatry and pediatrics at Harvard Medical School, Boston. Email them at pdnews@mdedge.com.
Real-World Evidence for Safety and Effectiveness of Repeated Courses of Hyaluronic Acid Injections on the Time to Knee Replacement Surgery
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.
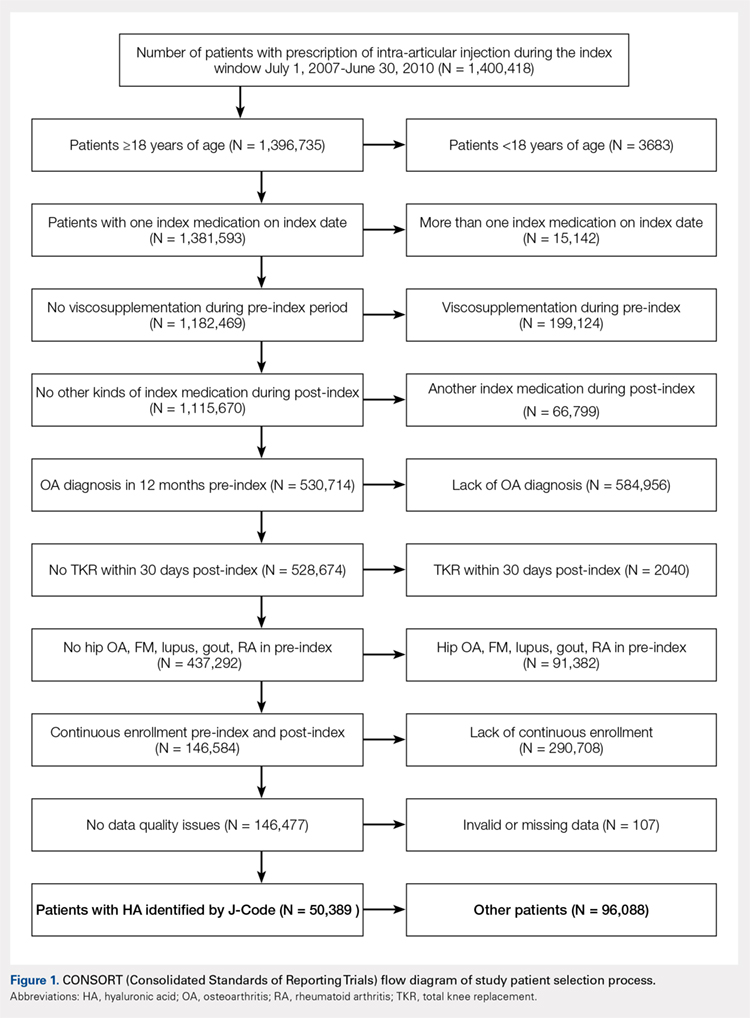
Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.
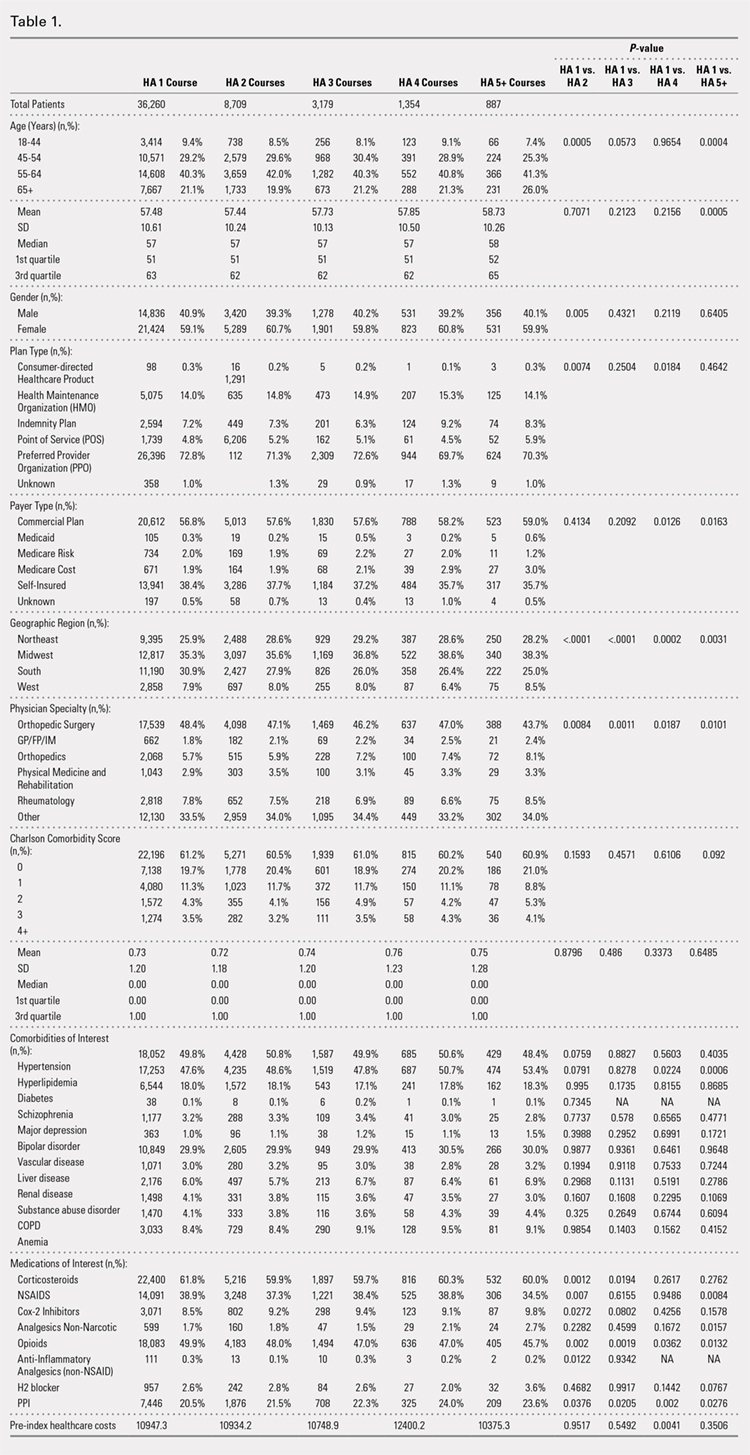
PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).
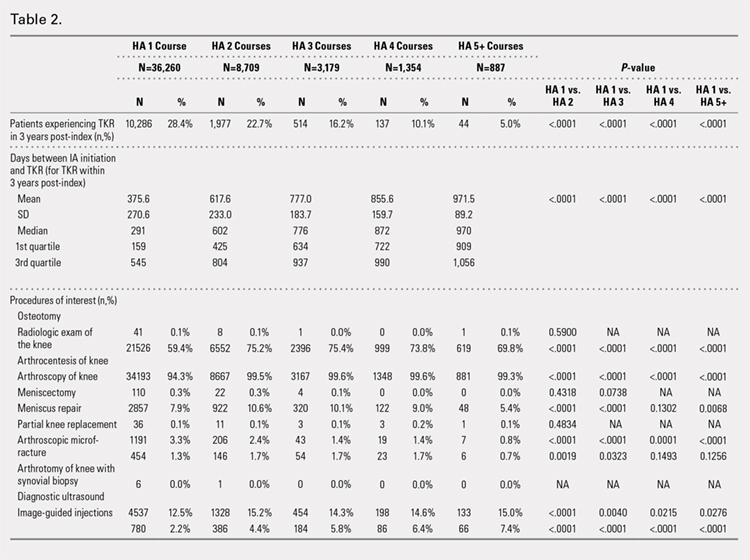
ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.
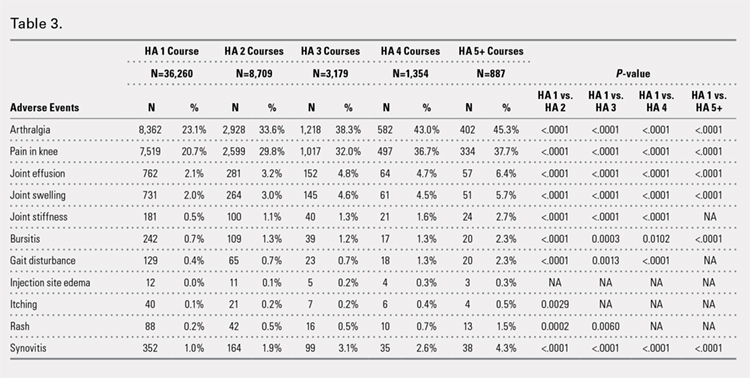
TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).
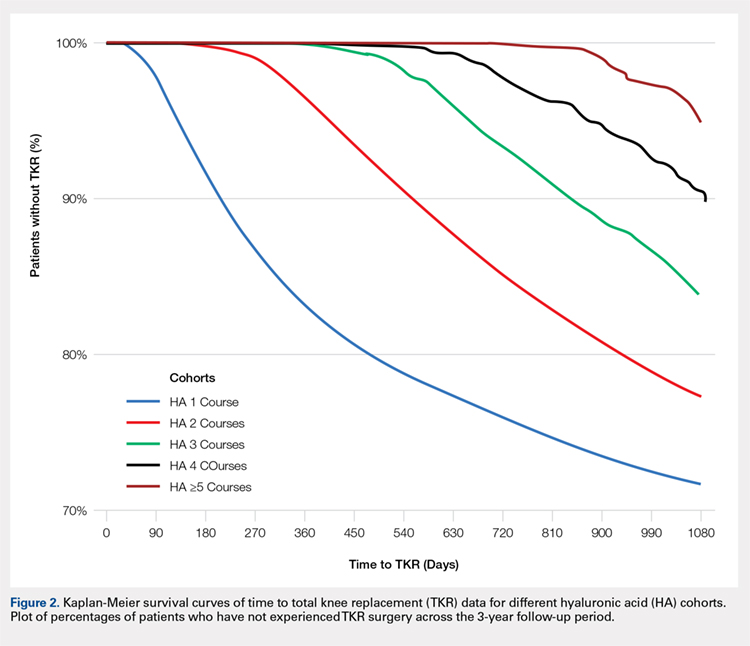
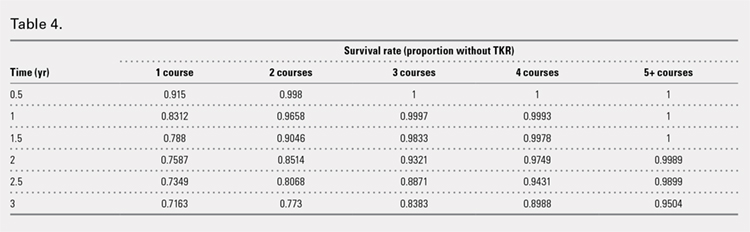
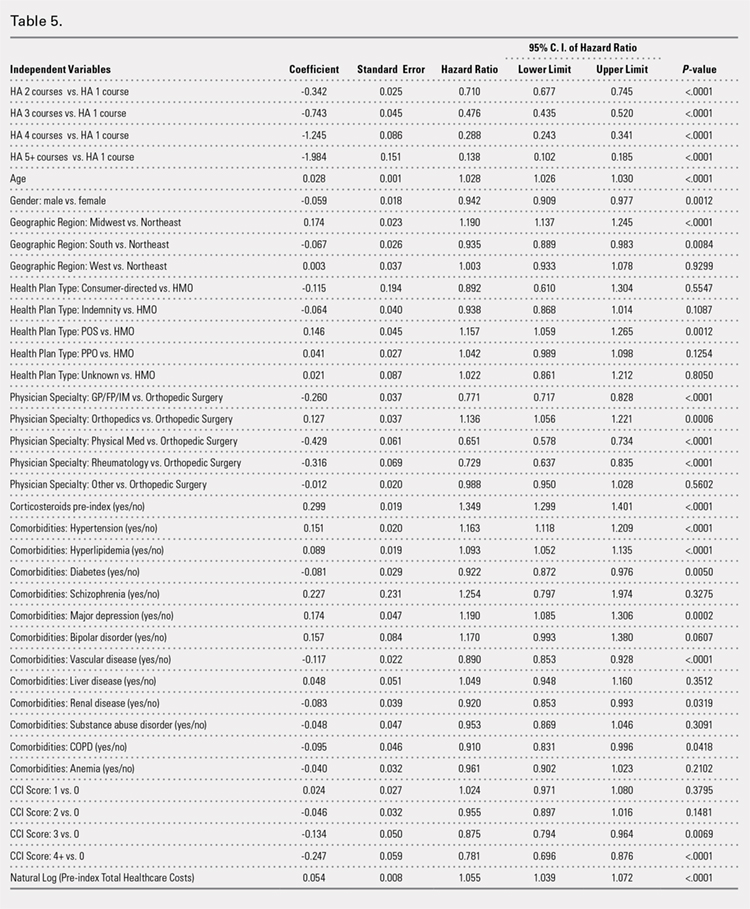
Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
ABSTRACT
Osteoarthritis (OA) of the knee is a top cause of disability among the elderly. Total knee replacement (TKR) has been available as an effective and definite surgical method to treat severe OA of the knee. However, TKR is a significant procedure with potential risk for serious complications and high costs. Alternative lower risk therapies that can delay or obviate TKR are valuable to those who are poor candidates for surgery or wish to avoid TKR as long as possible. Given the chondroprotective effects of hyaluronic acid (HA) injections, they are a safe and effective treatment to improve pain, function, and longevity of the knee. Thus, HA features the potential to delay or obviate TKR.
We aim to study the safety and effectiveness of repeated courses of HA on the time to TKR over a 3-year period using data from a large US health plan administrative claims database.
Retrospective analyses were conducted by identifying knee OA patients during the selection period (2007-2010). The follow-up period was 36 months, post-index date of initial HA injection. Procedural outcomes and adverse events of interest were tabulated and analyzed. A Cox proportional hazards model was used to model the risk of TKR.
A total of 50,389 patients who received HA for treatment of knee OA and met the study inclusion criteria were analyzed. Successive courses of HA showed a good safety profile and led to high proportions of patients without TKR 3 years after treatment initiation. Multivariate statistical modeling showed that multiple courses of HA injections significantly decreased the rates of TKR (95.0% without TKR for ≥5 courses vs 71.6% without TKR for 1 course; hazard ratio, 0.138; P < .0001).
Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effect of repeated HA courses on delaying TKR beyond a 3-year time horizon.
Continue to: Osteoarthritis (OA) of the knee...
Osteoarthritis (OA) of the knee has emerged as one of the main causes of disability in the United States. Although no currently known cure of OA can reverse the progression of the disease, total knee replacement (TKR) is an effective and definitive treatment. However, TKR is an invasive procedure with potential risk for serious complications, and it has imposed high costs on the US healthcare system, with expenses accounting for hospital expenditures of TKR estimated at $28.5 billion in 2009.1Alternative low-risk therapies that can delay or obviate TKR are valuable to a number of patients, especially the poor candidates for surgery or those who wish to avoid TKR.
Intra-articular (IA) hyaluronic acid (HA) injections have been available as a safe and effective treatment option to alleviate pain and to improve joint functions.2 Results of randomized double-blind controlled clinical trials have demonstrated the pain-relieving effect of IA HA injections.3-5 Furthermore, a recent network meta-analysis comparing various pharmacologic interventions for knee OA has confirmed the efficacy of IA HA injections, which outperformed other interventions when compared with oral placebos.6,7 IA therapies are more effective than oral therapies for knee OA pain, with IA HA injections demonstrating the most pain reduction, potentially due to the benefit associated with needle injection and aspiration. Recent experimental studies have also suggested that IA HA may provide cartilage protection, reduce inflammation, and boost the viscosity of synovial fluid;8 IA HA may also exert therapeutic effects by inhibiting bone formation in OA patients.9,10 HA possesses the potential to delay or obviate TKR. Previous research with a case series review of patients in an orthopedic specialty practice reported that the use of IA HA injections in patients with grade IV OA delayed TKR substantially.11 One study analyzed retrospective medical claims data from a single private insurer and discovered potential evidence for the modest benefit of IA HA injections in delaying TKR.12
More detailed research work on a large sample of patients with knee OA and the requirement of TKR as a condition for inclusion using US administrative claims data has demonstrated the TKR-delaying effects of IA HA injections in comparison with a control group without claims for IA HA injections.13,14 This study also uses real-world US administrative data but utilizes a different approach by starting with a sample of patients with knee OA and evidence of IA HA injections and then assessing the effect of repeated courses of HA treatment on the delay of TKR, without TKR as a mandatory condition for inclusion. All patients with knee OA within the time window were included, regardless of the need for TKR compared with previous studies which only considered patients who ultimately received TKR. Safety information and effectiveness information were examined to achieve a balanced risk-benefit assessment. We also analyzed how multiple courses of HA treatment and other potentially relevant covariates at baseline affected the risk of receiving TKR in a multivariate survival model. We aimed to achieve a realistic assessment of the clinical utility of HA injections in delaying TKR in a real-world setting using both safety and effectiveness data.
METHODS
DATA SOURCE
A retrospective cohort observational study using IMS Health’s PharMetrics Plus Health Plan Claims Database was conducted by identifying knee OA patients with claims indicating initiation of HA injection at an index date during the selection period (July 1, 2007 to June 30, 2010). All common HA agents in the US market during this period (Euflexxa, Hyalgan, Orthovisc, Supartz, and Synvisc) were selected via the corresponding J-codes and pooled for investigation of HA class effects. The follow-up period was 36 months, post-index date of the initial HA injection. Outcomes were measured, and adverse events were identified during this period. The time window for identification of adverse events was within 2 weeks from any injection during the course of therapy (evidence of an emergency room visit and/or physician office visit with requisite code). The data during the 12-month pre-index baseline period from the claims database was used to obtain information about baseline patient characteristics, such as age, gender, type of coverage, physician specialty, Charlson Comorbidity Index (CCI), major comorbidities, and major medications of interest commonly used among patients with knee OA.
STUDY SAMPLE SELECTION
The eligible patients required an outpatient claim indicating the initiation of HA injection. The date of the first claim for the patient within the selection window was defined as their index date. Patients had to be ≥18 years of age in the year of their index date. They had to present at least 1 clinical knee OA diagnosis at any point in the 12-month pre-index period (including the index date), and only patients who were continuously enrolled from 12 months pre-index to 36 months post-index date were evaluated. Among these patients (approximately 1.4 million), the following were excluded to minimize complications in data analysis and interpretation: patients with evidence of any HA use in the pre-index period; patients with evidence of a different kind of HA index medication in the post-index period; patients with evidence of TKR within 30 days of the index event during the post-index period; patients with evidence of 2 different kinds of HA index medications on the index date; and patients with evidence of diagnosis of hip OA, fibromyalgia, rheumatoid arthritis, lupus, or gout during the pre-index period.
Five patient cohorts were defined according to the number of courses of IA HA injections over the entire post-index period.
Continue to: Statistical analysis...
STATISTICAL ANALYSIS
All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc.). Descriptive statistics such as means, standard deviations, medians, and 25% and 75% percentiles (Q1 and Q3, respectively) were provided for the continuous variables. Numbers and percentages were provided for the categorical variables. For statistical testing, Student’s t-tests were applied for the continuous variables and chi-square tests for the categorical variables. All the statistical tests were two-tailed. The sample sizes in this database study are remarkably large, such that differences that are not clinically important could still be statistically significant at the conventional alpha level of 0.05. Thus, we applied a more stringent requirement of the alpha level of 0.0001 to identify highly statistically significant results. The number and percentage of patients within each cohort with at least 1 instance of an adverse event of interest (those adverse events commonly expected for patients who receive IA injections for knee OA) were assessed. Times to TKR during the 36-month post-index period were analyzed and compared among different cohorts. Any patients who had not undergone TKR by the end of the post-index period were considered censored at 36 months. The Kaplan-Meier method was employed to model survival curves with time to TKR data, and log-rank tests were used to compare survival curves among different cohorts. A Cox proportional hazards model (PHM) was used to model the risk of TKR with a pre-specified set of covariates adjusted for baseline attributes, such as age, gender, comorbidities, and pre-index healthcare costs. Hazard ratios with 95% confidence intervals were used to examine the measures of event risk.
RESULTS
PATIENT CHARACTERISTICS
Applying study selection criteria to the claims database yielded 50,389 patients (Figure 1), providing an ample sample size for the statistical analysis. Only patients with evidence of knee OA and use of HA injections (the index medication of interest) were selected, regardless of whether they received TKR during the post-index period. The requirement for a knee OA diagnosis during the 12-month pre-index period resulted in the significant attrition of patients, with 584,956 patients being excluded. Among the 50,389 patients who received HA for treatment of knee OA, 36,260 (72.0%) received a single course of treatment, 8709 (17.3%) received 2 courses, 3179 (6.3%) received 3 courses, 1354 (2.7%) received 4 courses, and 887 (1.8%) received ≥5 courses of treatment.

Comparison of baseline characteristics among the 5 IA HA cohorts showed the fairly similar baseline characteristics of all cohorts (Table 1). Geographic region, physician specialty, and opioid use showed differences among the cohorts. Cohorts with ≥5 HA courses presented lower proportions of patients from Southern US states, patients seeing orthopedic surgeons, and patients using opioids than cohorts with fewer HA courses.

PROCEDURES OF INTEREST
An analysis of the procedures patients received after HA treatment initiation showed that higher numbers of HA treatment courses resulted in lower proportions of patients receiving TKR within 3 years after HA treatment initiation (Table 2). With an increasing number of HA treatment courses, the proportion of patients with TKR within 3 years post-index consistently decreased from 28.4% (for 1 HA course) to 5.0% (for ≥5 HA courses), with all differences being highly statistically significant (P < .0001). Similarly, partial knee replacement exhibited a similar trend, with the proportion of patients decreasing from 3.3% (for 1 HA course) to 0.8% (for ≥5 HA courses; P < .0001). Among the patients with TKR within 3 years post-index, increasing numbers of treatment courses correlated with increasing time to TKR, with a mean of 375.6 days (for 1 HA course) rising to a mean of 971.5 days (for ≥5 HA courses; P < .0001). On the other hand, patients with multiple courses of HA treatment were more likely to undergo radiologic examinations of the knee, arthrocenteses, and image-guided injections than patients with only a single course of HA treatment (P < .0001).

ADVERSE EVENTS
Arthralgia and joint pain in the knee were the most commonly recorded adverse events (Table 3). More courses of HA treatment were associated with higher rates of adverse events. Overall, the reported adverse events profile of repeated courses of HA treatment consisted of mostly common and mild adverse events and displayed no safety concern for patients with knee OA that was followed-up for 3 years. The causality of these adverse events directly related to HA injections vs a specific disease state cannot be determined from an administrative claims data set.

TIME TO TKR
Successive courses of HA led to high proportions of patients without TKR 3 years after HA treatment initiation. This result is evident in the Kaplan-Meier survival curves of time to TKR for different HA cohorts (Figure 2), with log-rank tests of multiple courses vs a single course of HA (P < .0001) showing highly statistically significance. Tabulation of proportions of patients without TKR by various time points showed that increasing numbers of HA treatment courses correlated with higher proportions of patients without TKR at almost all time points (Table 4); within 3 years post-index, 71.6% of patients in the 1 HA course cohort exhibited no TKR, whereas 95.0% of patients in ≥5 HA courses cohort presented no TKR. We also performed a multivariate Cox PHM (Table 5) to account for baseline characteristics of different HA cohorts with covariates when estimating the risks of receiving TKR. The results of the Cox PHM showed that multiple courses of HA treatment significantly decreased the risk of TKR (hazard ratio, 0.138 for ≥5 HA courses vs 1 HA course; P < .0001). Inspection of other highly significant covariates showed that being older, living in the Midwest region of the US (vs the Northeast), receiving pre-index corticosteroids, having an orthopedic surgeon as a treating physician (vs a general practitioner, a rheumatologist, or a physical medicine and rehabilitation specialist), experiencing hypertension or hyperlipidemia, and higher pre-index total healthcare costs were associated with an increased risk of TKR (all P < .0001). Vascular disease and high CCI scores were associated with a decreased risk of TKR (P < .0001).



Continue to: Discussion...
DISCUSSION
This study demonstrated that multiple courses of HA treatment can delay the need for surgery for up to 3 years, with risk for both TKR and partial knee replacement decreasing in a dose-dependent manner. The potentially confounding effect of differences in baseline characteristics that could influence patients’ propensity to receive TKR in a database study was controlled by performing a multivariate analysis with covariate adjustment. The TKR-delaying effect of HA injection was more prominent in cohorts with a high number of HA treatment courses: 19 out of 20 patients in the cohort of ≥5 HA courses were free of TKR at the end of the 3-year post-index period. Such a high proportion of patients avoiding TKR with repeated courses of HA suggests that some patients may be able to successfully delay TKR well beyond the 3-year time span. This finding is counter-evidence to the frequently made assumption15 that all patients with knee OA will eventually progress to a state of disability, making TKR inevitable. The patients with end-stage radiographic knee OA can also benefit from IA HA injections for an extended period of time;16 the latest evidence indicates that nonoperative management can improve symptoms irrespective of radiographic disease severity, implying that TKR needs not to be the only therapeutic option for patients with end-stage radiographic knee OA.17 This finding suggests that HA treatment should be considered an important clinical treatment option for patients with knee OA.
Although the incidence rates of certain adverse events, such as arthralgia/joint pain, are sizable, these temporary adverse events commonly occur among patients who receive IA injections for knee OA; most of these events may simply include symptoms of the remaining underlying knee OA. These results are consistent with those of previous literature reporting the safety of repeated treatment with IA HA injections in a prospective clinical trial18 and demonstrating that repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
Multivariate modeling outcomes of factors influencing risk of receiving TKR are broadly consistent with the generally accepted notions that different levels of disease severity and patients’ willingness to consider TKR at baseline influence the likelihood and timing of receiving TKR.19,20 Age and obesity are common risk factors for progression of OA. Orthopedic surgeons are more likely to recommend surgery than non-surgeons. The pre-index use of corticosteroids and high pre-index healthcare costs could be associated with more severe symptoms at baseline. Patients with vascular disease or severe comorbidities, as evidenced by high CCI scores, make poor candidates for major elective surgeries such as TKR. These results are intuitive and validate the clinical insights of this study. Moreover, inclusion of these covariates in the analysis model allows for indirect adjustment of the most important prognostic factors for TKR at baseline, permitting proper statistical comparison of the results for different cohort groups.
Recently, the efficacy of HA injections for OA patients has become the subject of debate when the American Academy of Orthopaedic Surgeons (AAOS) revised its clinical practice guideline, recommending against the use of HA.21 The AAOS’ findings differ from those of other clinical societies, such as the American College of Rheumatology22 and the European League Against Rheumatism,23 which provide no strong recommendation against the use of HA injections. The announcement of the new guideline by AAOS caused concern among clinicians and payers who had valued IA HA injections as a means to control knee OA pain before patients progress to TKR;24 on the other hand, the demand for nonoperative treatment of knee OA remains high. Utilization rates of TKR have increased dramatically, and surgeries are now performed on younger patients with increasing burden on the healthcare system,25,26 in spite of the fact that as high as a third of TKR surgeries may have been performed in inappropriate patients.27 Part of the confusion surrounding clinical utility of HA stems from the fact that up until recently, relatively little research looked into the practical benefits of HA in actual clinical practice. Analyses of databases such as registries are now gaining attention to overcome that problem. Examination of large administrative databases maintained by commercial payers offers the benefit of probing realistically the safety and efficacy of treatments in actual clinical environments in a very large number of patients with heterogeneous backgrounds. Recently, the Agency for Healthcare Research and Quality’s Technology Assessment Program in the US called for such studies to determine whether HA injections can delay progression to TKR.28 The results of this study and several others11,13,14,16 suggest that use of HA to treat OA of the knee is associated with the delay of TKR, supporting the utility of HA in clinical practice and the healthcare system. Potential clinical benefits of delaying TKR may include the reduced risk of aseptic loosening if younger patients can wait for TKR or more time to allow the modification of risk factors in patients who will ultimately undergo TKR.
LIMITATIONS
Follow-up period was limited to 3 years post-index date because longer follow-up data were not available at the time of the study design. If an incorrect adverse event or OA diagnosis was listed in the medical record, or if the medical record was incomplete, then patients might have been misclassified, resulting in selection bias. The claims dataset includes no uninsured and Medicare patients, as the population in the database consisted primarily of commercially-insured patients in the US. Therefore, the results are most generalizable to other commercially-insured patients in the US. Generalizability to other populations may not be assured if they differ in their accessibility to physician services or prescriptions from the patients in this study. Other treatments such as the nonsteroidal anti-inflammatory drugs used by patients were not included within the pre-specified statistical model because their potential effects were assumed to be short-lived and much less than those of corticosteroid. Including these treatments would overload the statistical model with too many covariates, leading to potential computational instability. The database used provides no information on systemic factors, including plan limits on medication use, that could affect care. Given the large and diverse nature of the healthcare plans in the database. However, these factors should not have materially affected our study results. The claims database also lacks direct indicators of OA disease severity, such as Kellgren-Lawrence scores or patient-reported outcomes, including pain and function questionnaire scores. Our multivariate analysis indirectly makes up for this deficiency by considering other baseline characteristics or clinical indicators that may be correlated with information unavailable in a claims database. Patients who opt to undergo repeated courses of HA treatment may be more inclined to avoid surgery or may naturally experience OA disease progression more slowly, making them potentially different from patients who select to undergo surgery earlier without repeated courses of HA treatment. This condition may introduce a bias that causes difficulty in proving the causality between repeated HA use and delay of TKR.
CONCLUSION
Analysis of the knee OA patient data from a real-world database showed that repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years. Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
- Murphy L, Helmick CG. The impact of osteoarthritis in the United States: a population-health perspective. Am J Nurs. 2012;112(3 Suppl 1):S13-S19.
- Arnold W, Fullerton DS, Holder S, May CS. Viscosupplementation: managed care issues for osteoarthritis of the knee. J Manag Care Pharm. 2007;13(4 Suppl):S3-S19.
- Strand V, Conaghan PG, Lohmander LS, et al. An integrated analysis of five double-blind, randomized controlled trials evaluating the safety and efficacy of a hyaluronan product for intra-articular injection in osteoarthritis of the knee. Osteoarthritis Cartilage. 2006;14(9):859-866.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. A multicenter, randomized controlled trial comparing a single intra-articular injection of Gel-200, a new cross-linked formulation of hyaluronic acid, to phosphate buffered saline for treatment of osteoarthritis of the knee. Osteoarthritis Cartilage. 2012;20(5):350-356.
- Strand V, McIntyre LF, Beach WR, Miller LE, Block JE. Safety and efficacy of US-approved viscosupplements for knee osteoarthritis: a systematic review and meta-analysis of randomized, saline-controlled trials. J Pain Res. 2015;8:217-228.
- Bannuru RR, Schmid CH, Kent DM, Vaysbrott EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46-54.
- Mandl LA, Losina E. Relative efficacy of knee osteoarthritis treatments: are all placebos created equal? Ann Intern Med. 2015;162(1):71-72.
- Kusayama Y, Akamatsu Y, Kumagai K, Kobayashi H, Aratake M, Saito T. Changes in synovial fluid biomarkers and clinical efficacy of intra-articular injections of hyaluronic acid for patients with knee osteoarthritis. J Exp Orthop. 2014;1(1):16. doi:10.1186/s40634-014-0016-7.
- Kaneko K, Higuchi C, Kunugiza Y, et al. Hyaluronan inhibits BMP-induced osteoblast differentiation. FEBS Lett. 2015;589(4):447-454. doi:10.1016/j.febslet.2014.
- Altman RD, Manjoo A, Fierlinger A, Niazi F, Nicholls M. The mechanism of action for hyaluronic acid treatment in the osteoarthritic knee: a systematic review. BMC Musculoskelet Disord. 2015;16:321. doi:10.1186/s12891-015-0775-z.
- Waddell DD, Bricker DC. Total knee replacement delayed with hylan G-F 20 use in patients with grade IV osteoarthritis. J Manag Care Pharm. 2007;13(2):113-121.
- Khan T, Nanchanatt G, Farber K, Jan S. Analysis of the effectiveness of hyaluronic acid in prevention of total knee replacement in osteoarthritis patients. J Manag Care Pharm. 2014;20:S49.
- Abbott T, Altman RD, Dimeff R, et al. Do hyaluronic acid injections delay total knee replacement surgery? Arthritis Rheum. 2013;65(Suppl 10):2139.
- Altman R, Lim S, Steen R, Dasa V. Intra-articular hyaluronic acid delays total knee replacement in patients with knee osteoarthritis: evidence from a large U.S. health claims database. Osteoarthritis Cartilage. 2015;23(Suppl 2):A403-A404.
- Mather RC 3rd, Hug KT, Orlando LA, et al. Economic evaluation of access to musculoskeletal care: the case of waiting for total knee arthroplasty. BMC Musculoskelet Disord. 2014;15:22. doi:10.1186/1471-2474-15-22.
- Waddell DD, Joseph B. Delayed total knee replacement with Hylan G-F 20. J Knee Surg. 2016;29(2):159-168. doi:10.1055/s-0034-1395281.
- Atukorala I, Makovey J, Williams M, Ochoa Albiztegui E, Eyles JP, Hunter DJ. If you have end-stage radiographic knee osteoarthritis can you respond to non-surgical management? Osteoarthritis Cartilage. 2015;23(Suppl 2):A329.
- Strand V, Baraf HS, Lavin PT, Lim S, Hosokawa H. Effectiveness and safety of a multicenter extension and retreatment trial of Gel-200 in patients with knee osteoarthritis. Cartilage. 2012;3(4):297-304. doi:10.1177/1947603512451024.
- Riddle DL, Kong X, Jiranek WA. Two-year incidence and predictors of future knee arthroplasty in persons with symptomatic knee osteoarthritis: preliminary analysis of longitudinal data from the osteoarthritis initiative. Knee. 2009;16(6):494-500.
- Hawker GA, Guan J, Croxford R, et al. A prospective population-based study of the predictors of undergoing total joint arthroplasty. Arthritis Rheum. 2006;54(10):3212-3220.
- Jevsevar DS. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571-576. doi:10.5435/JAAOS-21-09-571.
- Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-474.
- Jordan KM, Arden NK, Doherty M, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a task force of the standing committee for international clinical studies including therapeutic trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-1155.
- Bannuru RR, Vaysbrot EE, McIntyre LF. Did the American Academy of Orthopaedic Surgeons osteoarthritis guidelines miss the mark? Arthroscopy. 2014;30(1):86-89. doi:10.1016/j.arthro.2013.10.007.
- Losina E, Thornhill TS, Rome BN, Wright J, Katz JN. The dramatic increase in total knee replacement utilization rates in the United States cannot be fully explained by growth in population size and the obesity epidemic. J Bone Joint Surg Am. 2012;94(3):201-207. doi:10.2106/JBJS.J.01958.
- Weinstein AM, Rome BN, Reichmann WM, et al. Estimating the burden of total knee replacement in the United States. J Bone Joint Surg Am. 2013;95(5):385-392. doi:10.2106/JBJS.L.00206.
- Riddle DL, Jiranek WA, Hayes CW. Use of a validated algorithm to judge the appropriateness of total knee arthroplasty in the United States: a multicenter longitudinal cohort study. Arthritis Rheumatol. 2014;66(8):2134-2143. doi:10.1002/art.38685.
- NewBerry SJ, Fitzgerald JD, Maglione MA, et al. Agency for Healthcare Research and Quality Web site. Systematic Review for Effectiveness of Hyaluronic Acid in the Treatment of Severe Degenerative Joint Disease (DJD) of the Knee: Technology Assessment Report. http://www.ahrq.gov/research/findings/ta/call-for-public-review.html. Published July 23, 2015. Accessed December 22, 2014.
TAKE-HOME POINTS
- Repeated courses of treatment with HA are safe and are associated with the delay of TKR for up to 3 years.
- HA treatment should be considered an important clinical treatment option for patients with knee OA.
- Repeated courses of treatment with HA are safe.
- Repeated courses of HA treatment pose no greater safety risk than a single course of HA treatment.
- Additional research is needed to evaluate the effects of repeated HA courses on delaying TKR beyond a 3-year period.
How to prescribe effectively for opioid use disorder
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
NEW ORLEANS – Physicians committed to fighting the national opioid epidemic really need to take the 8-hour training course on addiction treatment required to obtain a Drug Enforcement Administration ‘X’ number, because it will enable them to prescribe buprenorphine, a drug with unique advantages for many affected patients, Ellie Grossman, MD, asserted at the annual meeting of the American College of Physicians.
Buprenorphine (Subutex) is one of the three medications approved for treatment of opioid use disorder (OUD), along with methadone and naltrexone (Revia). And for certain patients, it’s clearly the best choice, according to Dr. Grossman, a general internist at Harvard Medical School, Boston, and the primary care lead for behavioral health integration at the Cambridge (Mass.) Health Alliance.
The DEA X number certification process, which entails obtaining a waiver through SAMHSA – the Substance Abuse and Mental Health Services Administration – is bureaucratic. It’s unpopular with many physicians. But it’s well worth 8 hours of an internist’s time to get the waiver and gain the ability to prescribe buprenorphine.
“The requirement is admittedly clunky, and many people have strong feelings about whether this is a regulation that should exist,” according to Dr. Grossman. “I myself didn’t need to have special training to prescribe methadone, a full opioid agonist that my patients could easily die from. But I did have to undergo an 8-hour training to prescribe buprenorphine, and it’s much harder to die from that drug.”
She addressed which of the three medications for OUD is the best fit in a given patient, the appropriate treatment duration, and the role of adjunctive counseling, which – spoiler alert – has been cast into question by the results of a major government-funded randomized trial.
Dr. Grossman’s overriding message: “You are saving lives by getting people on medication.”
Indeed, studies have shown that patients with OUD who receive no treatment have a sixfold increase in the standardized mortality ratio, compared with the general population. Contrast that with the less than 2-fold increased risk with medication-assisted treatment and roughly a 2.5-fold increased risk when medication is given short term to cover withdrawal and then tapered and discontinued.
Other documented benefits of long-term medication-assisted treatment of patients with OUD as described in a 2014 Cochrane review include reductions in injection drug use, crime days, HIV-related risk behaviors and seroconversion, and improved health and social functioning.
Of note, those well-documented benefits apply only to methadone, a full opioid agonist, and buprenorphine, a partial agonist, because those two drugs have been around long enough to generate long-term outcome data. Naltrexone, which has a completely different mechanism of action – it’s a full opioid antagonist – has not as of yet.
Individualizing medical therapy for OUD
Physicians can’t write a prescription for methadone. The drug must be administered at a certified opioid treatment program, or OTP, otherwise known as a methadone clinic. Those clinics are highly regulated at both the federal and state levels, with lots of minutia involved. Patient counseling and drug screening are required.
In contrast, a physician with a DEA X number can write a prescription for buprenorphine and have a patient fill it at a pharmacy. There is inherently less structure surrounding buprenorphine therapy than that of methadone, Dr. Grossman noted. There are no hard and fast rules about how often a physician has to see the patient or do drug screens or counseling. Buprenorphine is available as once-daily oral sublingual therapy and, more recently, in long-acting injectable and implantable formulations, although Dr. Grossman believes the jury is still out about how these nonoral agents are best utilized.
“I’m often asked, ‘Which is better, methadone or buprenorphine?’ Really, the answer is they’re both pretty darn good,” according to Dr. Grossman.
The Cochrane review concluded that, in the studies that have used real-world dosing – that is, higher doses than in the initial studies – high-dose buprenorphine and high-dose methadone have similar rates of retention in treatment.
“What I tell patients is that a lot hinges on the structure of the treatment delivery system,” Dr. Grossman said. “If it’s methadone, they’re going to the OTP every day. Some people need more structure; they need a set of eyes on them every day. Or if they are at high risk for medication diversion – for example, someone else in their household might want to steal their medications – going to a methadone program gets around that. Also, when somebody has been on methadone in the past and did well on it and wants to go on it again, I’m likely to say, ‘That sounds like a good fit.’”
Buprenorphine is a good option for patients who don’t require close, structured supervision. It has fewer drug interactions than does methadone and is less prone to cause QTc prolongation. Also, it’s a more realistic option for patients who live so far from an OTP that daily attendance is impractical. And ob.gyns increasingly favor buprenorphine, because the problem of neonatal abstinence syndrome is less severe than when mothers are on methadone.
As for extended-release naltrexone (Vivitrol), the pivotal double-blind Russian trial that won FDA approval for treatment of OUD showed a dramatic improvement in opioid-free weeks (Lancet. 2011 Apr 30;377[9776]:1506-13).
More recently, the 24-week, multicenter, open-label X:BOT trial randomized 570 U.S. patients with OUD to once-monthly extended-release naltrexone or daily sublingual buprenorphine-naloxone (Suboxone). The dropout rate was higher in the extended-release naltrexone arm because patients had to be opioid free for 2 weeks before starting on the opioid antagonist. As a practical matter, that can be difficult to achieve unless a patient has just been released from jail or prison. But the per-protocol relapse rates were similar (Lancet. 2018 Jan 27;391[10118]:309-18).
“Many people interpret this study as saying, with the right patient who can get into an opioid-free state or, if you inherit an opioid-free state, the choice between extended-release naltrexone and buprenorphine-naloxone may be a bit of a wash in terms of clinical effectiveness, as best we can detect,” Dr. Grossman explained. “That said, they’re very different experiences: One is a shot in your butt once a month, the other is something you put in your mouth once a day. Patients typically have a strong point of view regarding what they’re up for.”
Extended-release naltrexone doesn’t require a DEA waiver or attendance at an OTP. But it costs roughly $800 per injection, although many insurers do cover it after additional paperwork is completed. While Dr. Grossman does use extended-release naltrexone in her own practice, it comes with some baggage. The drug comes in a powder, which is mixed with a diluent in the office, creating a thick, frothy substance that’s slow to inject. It has to be kept refrigerated, then warmed up in time for the patient visit.
“If you live somewhere where there’s no OTP and you don’t have a DEA X number, and you have a patient with OUD who’s interested in extended-release naltrexone, it’s not crazy to think about,” she noted.
Duration of medical therapy
Study after study demonstrates that, when treatment stops, the risk of relapse goes up.
“We as health care providers are used to the mentality of chronic diseases, like diabetes, where you’re probably on medicine for the rest of your life,” Dr. Grossman said. “OUD is another chronic disease where you might have a patient on medication for the rest of their life, although you may not want to drum that into their head right up front. It’s kind of scary. I don’t usually talk that way with my diabetic patients when I give them their diagnosis. So, I don’t push it.
“But the reality is, to give them the best chance of health, they should be on medication for a good long time,” she added. “And that’s true for all of the OUD medications.”
The role of counseling
The best evidence of the utility of adjunctive counseling in the treatment of OUD comes from the landmark Prescription Opioid Addiction Treatment Study (POATS), a 653-patient multicenter trial conducted by the National Drug Abuse Treatment Network and funded by the National Institute on Drug Abuse. Participants were randomized to standard medical management including medication and a meeting with a physician every 1-2 weeks, or to standard therapy plus individual counseling with a trained substance use counselor.
To the surprise of many, given that SAMHSA guidance strongly recommends counseling and other forms of behavioral therapy, there was no difference in outcomes between the two groups (Drug Alcohol Depend. 2015 May 1;150:112-9).
Subsequent parsing of the POATS data showed that the subgroup of people who were using heroin rather than prescription pills and who actually attended at least 60% of their counseling sessions did better than if they were randomized to no counseling.
“There’s still room for quibbling about the study, but many people would say, ‘You know, it’s not a slam dunk that everybody needs counseling,’ ” the internist commented.
“So, how do we pick the right treatment for our patients with OUD? It’s what feels right for them,” Dr. Grossman cautioned. “This gets back to what we do every day in managing chronic diseases: We nudge, we encourage, we use our motivational interviewing skills to help people figure out how they can change their lives and get healthier. There’s a long list of things going on in our patients’ lives that are going to help guide that decision.
“The message here: Medication is better than no medication, but it’s not a slam dunk which medication or how,” she concluded.
REPORTING FROM ACP INTERNAL MEDICINE
FDA approves Orilissa for endometriosis pain
The Food and Drug Administration has approved elagolix (Orilissa) for oral treatment of moderate to severe pain associated with endometriosis, announced AbbVie and Neurocrine Biosciences; this approval makes it the first such treatment in more than a decade. It is expected to be available in the United States in early August.
Elagolix is a gonadotropin-releasing hormone (GnRH) antagonist and the first and only one developed specifically for managing this kind of pain.
The approval is based on two 6-month, randomized, double-blind, placebo-controlled phase 3 trials that compared a total of 952 adult women treated with either elagolix with 734 treated with placebo. All of the women experienced moderate to severe endometriosis pain; their ages ranged from 18 to 49 years.
Of the women in the treatment group, 475 were treated with a 150-mg daily dose, and 477 were treated with a 200-mg twice-daily dose. Both treatment groups showed significantly greater mean reductions in pain – both daily menstrual and nonmenstrual pelvic pain – at 6 months. Furthermore, women in the 200-mg twice-daily group also showed statistically significant greater reductions in pain with sex at 3 months, compared with placebo. Altogether, these represent the three most common kinds of endometriosis pain.
The most concerning adverse event associated with elagolix is dose-dependent decreases in bone mineral density; this effect limits treatment to either 150 mg daily for up to 24 months or 200 mg twice daily for up to 6 months. Bone mineral density loss might not be completely reversible, even with treatment cessation. Common adverse events (occurring in at least 5%) included hot flush/night sweats, headache, and nausea. Elagolix is not recommended for women who are or may be pregnant, have osteoporosis, have severe liver disease, or take strong OATP1B1 inhibitors.
Full prescribing information, as well as further details on the approval, can be found on the AbbVie website.
The Food and Drug Administration has approved elagolix (Orilissa) for oral treatment of moderate to severe pain associated with endometriosis, announced AbbVie and Neurocrine Biosciences; this approval makes it the first such treatment in more than a decade. It is expected to be available in the United States in early August.
Elagolix is a gonadotropin-releasing hormone (GnRH) antagonist and the first and only one developed specifically for managing this kind of pain.
The approval is based on two 6-month, randomized, double-blind, placebo-controlled phase 3 trials that compared a total of 952 adult women treated with either elagolix with 734 treated with placebo. All of the women experienced moderate to severe endometriosis pain; their ages ranged from 18 to 49 years.
Of the women in the treatment group, 475 were treated with a 150-mg daily dose, and 477 were treated with a 200-mg twice-daily dose. Both treatment groups showed significantly greater mean reductions in pain – both daily menstrual and nonmenstrual pelvic pain – at 6 months. Furthermore, women in the 200-mg twice-daily group also showed statistically significant greater reductions in pain with sex at 3 months, compared with placebo. Altogether, these represent the three most common kinds of endometriosis pain.
The most concerning adverse event associated with elagolix is dose-dependent decreases in bone mineral density; this effect limits treatment to either 150 mg daily for up to 24 months or 200 mg twice daily for up to 6 months. Bone mineral density loss might not be completely reversible, even with treatment cessation. Common adverse events (occurring in at least 5%) included hot flush/night sweats, headache, and nausea. Elagolix is not recommended for women who are or may be pregnant, have osteoporosis, have severe liver disease, or take strong OATP1B1 inhibitors.
Full prescribing information, as well as further details on the approval, can be found on the AbbVie website.
The Food and Drug Administration has approved elagolix (Orilissa) for oral treatment of moderate to severe pain associated with endometriosis, announced AbbVie and Neurocrine Biosciences; this approval makes it the first such treatment in more than a decade. It is expected to be available in the United States in early August.
Elagolix is a gonadotropin-releasing hormone (GnRH) antagonist and the first and only one developed specifically for managing this kind of pain.
The approval is based on two 6-month, randomized, double-blind, placebo-controlled phase 3 trials that compared a total of 952 adult women treated with either elagolix with 734 treated with placebo. All of the women experienced moderate to severe endometriosis pain; their ages ranged from 18 to 49 years.
Of the women in the treatment group, 475 were treated with a 150-mg daily dose, and 477 were treated with a 200-mg twice-daily dose. Both treatment groups showed significantly greater mean reductions in pain – both daily menstrual and nonmenstrual pelvic pain – at 6 months. Furthermore, women in the 200-mg twice-daily group also showed statistically significant greater reductions in pain with sex at 3 months, compared with placebo. Altogether, these represent the three most common kinds of endometriosis pain.
The most concerning adverse event associated with elagolix is dose-dependent decreases in bone mineral density; this effect limits treatment to either 150 mg daily for up to 24 months or 200 mg twice daily for up to 6 months. Bone mineral density loss might not be completely reversible, even with treatment cessation. Common adverse events (occurring in at least 5%) included hot flush/night sweats, headache, and nausea. Elagolix is not recommended for women who are or may be pregnant, have osteoporosis, have severe liver disease, or take strong OATP1B1 inhibitors.
Full prescribing information, as well as further details on the approval, can be found on the AbbVie website.
Fathers and mothers report similar rates of depression at well-child visit
Prevalence of depression in fathers was similar to that of mothers screened at well-child visits, according to a research letter published in JAMA Pediatrics.
An analysis of data obtained from the Child Health Improvement through Computer Automation (CHICA) pediatric health surveillance system found that 4.4% of fathers who attended the well-child visit and completed a prescreening form scored positive for depression, comparable with the rate in mothers (5%). Overall, fathers composed 11.7% of parents who screened positive for depression, reported Erika R. Cheng, PhD, of Indiana University, Indianapolis, and her colleagues.
Dr. Cheng and her colleagues estimated prevalence of paternal depression using CHICA data from parents of children aged 15 months and younger from five community health centers in Indianapolis between August 1, 2016, and December 31, 2017. CHICA is a 20-question prescreen of pediatric health conditions based on the patient’s existing data.
Maternal postpartum depression was assessed using a modified version of the Edinburgh Postnatal Depression Scale, administered every 90 days during the child’s first 15 months of life, Dr. Cheng and her colleagues wrote.
Fathers were present at 30.8% of well-child visits (2,946 of 9,572) and completed the prescreening at 806 visits (8.4% of total visits). A total of 36 (4.4%) fathers screened positive for depression, compared with 273 (5%) mothers. However, since CHICA assesses depression for only one parent, some cases of paternal depression may have been missed, the investigators added.
“Pediatric clinics are thus promising settings in which to address depression in both parents as part of a family-centered approach to care,” Dr. Cheng and her coauthors concluded. “Addressing these gaps could improve detection and treatment rates of postnatal depression in both mothers and fathers.”
Two of the authors coinvented the CHICA system, and one is a co-owner of Digital Health Solutions, which licensed the system. No other disclosures were reported.
SOURCE: Cheng ER et al. JAMA Pediatr. 2018 Jul 23. doi:10.1001/jamapediatrics.2018.1505.
Prevalence of depression in fathers was similar to that of mothers screened at well-child visits, according to a research letter published in JAMA Pediatrics.
An analysis of data obtained from the Child Health Improvement through Computer Automation (CHICA) pediatric health surveillance system found that 4.4% of fathers who attended the well-child visit and completed a prescreening form scored positive for depression, comparable with the rate in mothers (5%). Overall, fathers composed 11.7% of parents who screened positive for depression, reported Erika R. Cheng, PhD, of Indiana University, Indianapolis, and her colleagues.
Dr. Cheng and her colleagues estimated prevalence of paternal depression using CHICA data from parents of children aged 15 months and younger from five community health centers in Indianapolis between August 1, 2016, and December 31, 2017. CHICA is a 20-question prescreen of pediatric health conditions based on the patient’s existing data.
Maternal postpartum depression was assessed using a modified version of the Edinburgh Postnatal Depression Scale, administered every 90 days during the child’s first 15 months of life, Dr. Cheng and her colleagues wrote.
Fathers were present at 30.8% of well-child visits (2,946 of 9,572) and completed the prescreening at 806 visits (8.4% of total visits). A total of 36 (4.4%) fathers screened positive for depression, compared with 273 (5%) mothers. However, since CHICA assesses depression for only one parent, some cases of paternal depression may have been missed, the investigators added.
“Pediatric clinics are thus promising settings in which to address depression in both parents as part of a family-centered approach to care,” Dr. Cheng and her coauthors concluded. “Addressing these gaps could improve detection and treatment rates of postnatal depression in both mothers and fathers.”
Two of the authors coinvented the CHICA system, and one is a co-owner of Digital Health Solutions, which licensed the system. No other disclosures were reported.
SOURCE: Cheng ER et al. JAMA Pediatr. 2018 Jul 23. doi:10.1001/jamapediatrics.2018.1505.
Prevalence of depression in fathers was similar to that of mothers screened at well-child visits, according to a research letter published in JAMA Pediatrics.
An analysis of data obtained from the Child Health Improvement through Computer Automation (CHICA) pediatric health surveillance system found that 4.4% of fathers who attended the well-child visit and completed a prescreening form scored positive for depression, comparable with the rate in mothers (5%). Overall, fathers composed 11.7% of parents who screened positive for depression, reported Erika R. Cheng, PhD, of Indiana University, Indianapolis, and her colleagues.
Dr. Cheng and her colleagues estimated prevalence of paternal depression using CHICA data from parents of children aged 15 months and younger from five community health centers in Indianapolis between August 1, 2016, and December 31, 2017. CHICA is a 20-question prescreen of pediatric health conditions based on the patient’s existing data.
Maternal postpartum depression was assessed using a modified version of the Edinburgh Postnatal Depression Scale, administered every 90 days during the child’s first 15 months of life, Dr. Cheng and her colleagues wrote.
Fathers were present at 30.8% of well-child visits (2,946 of 9,572) and completed the prescreening at 806 visits (8.4% of total visits). A total of 36 (4.4%) fathers screened positive for depression, compared with 273 (5%) mothers. However, since CHICA assesses depression for only one parent, some cases of paternal depression may have been missed, the investigators added.
“Pediatric clinics are thus promising settings in which to address depression in both parents as part of a family-centered approach to care,” Dr. Cheng and her coauthors concluded. “Addressing these gaps could improve detection and treatment rates of postnatal depression in both mothers and fathers.”
Two of the authors coinvented the CHICA system, and one is a co-owner of Digital Health Solutions, which licensed the system. No other disclosures were reported.
SOURCE: Cheng ER et al. JAMA Pediatr. 2018 Jul 23. doi:10.1001/jamapediatrics.2018.1505.
FROM JAMA PEDIATRICS
Key clinical point: Depression prevalence during pediatric well-child care visits was similar in fathers and mothers.
Major finding: Fathers screened positive for depression at a rate of 4.4%, compared with 5% of mothers.
Study details: An analysis of parent responses from 9,572 clinic visits between August 2016 and December 2017.
Disclosures: Two of the authors coinvented the CHICA system, and one is a co-owner of Digital Health Solutions, which licensed the system. No other disclosures were reported.
Source: Cheng ER et al. JAMA Pediatr. 2018 Jul 23. doi:10.1001/jamapediatrics.2018.1505.















