User login
Focus on preventing comorbidities in MS, physician urges
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
NASHVILLE, TENN. – Some patients use multiple sclerosis as an excuse to make poor health choices, but Allen C. Bowling, MD, PhD, of the Colorado Neurological Institute has seen another kind of story unfold. Fifteen to 20 years ago, Dr. Bowling said, he treated patients who took the development of MS in their 20s as a sign they needed to take better care of themselves. “They said MS was the best thing that happened to them ‘because it motivated me to make these healthy lifestyle changes I wouldn’t have made otherwise.’ ”
These patients have maintained their lifestyle changes, he said, lowering their risk of comorbidities and – perhaps – changing the course of their MS for the better.
“It’s all one machine, and sometimes we lose sight of that in our sub-sub-specialized world of treating MS ... You’re caring for a whole person. If you start thinking about that, it does make you think differently about how you treat the person, how you try to prevent disease in terms of certain pathways,” Dr. Bowling said in an interview at the annual meeting of the Consortium of Multiple Sclerosis Centers, where he spoke to colleagues about the importance of helping patients to adopt lifestyle changes.
According to Dr. Bowling, there’s evidence linking lifestyle-related comorbidities, poorer food quality, and tobacco use to higher levels of overall MS risk, relapses, disability, and symptoms.
Researchers have also linked other life factors to higher MS risks: obesity (linked to overall MS risk, disability, symptoms); lack of physical activity (linked to relapses, disability, symptoms); emotional factors (relapses, symptoms); and alcohol overuse (linked to overall risk, disability, symptoms).
“Data is mild to moderate to strong in all those areas for lifestyle approaches like diet, physical activity, emotional health, alcohol in moderation or less, and no tobacco smoking,” Dr. Bowling said.
He said he believes physical activity leads to “much higher and earlier success than diet” in MS patients, although there’s no confirmed “best exercise.”
As for nutrition, he said vitamins D and B12 are possibly beneficial. But he cautioned against the potential for harm from supplements and added that there’s no proven best diet for MS.
As for finding time to address these issues in clinic, Dr. Bowling recommended mentioning various lifestyle issues over multiple office visits.
“Some of the effort should be switched to the primary care doctor,” he said, “but you can use a strong collection of words to convey to the person with MS that this is serious: ‘It’s not MS, but it’s a serious issue, and you must see your primary care doctor.’ ”
He believes that this approach can have a significant impact, “especially for those aged 20-40, because the doctor they pay the most attention to may be their MS clinician.”
Dr. Bowling said that he receives royalties from a book he authored, “Optimal Health With Multiple Sclerosis.”
REPORTING FROM THE CMSC ANNUAL MEETING
Pregnancy may be ideal time to consider switching MS drugs
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN – Women with multiple sclerosis who fare poorly on specific medications before pregnancy don’t tend to do any better afterward, a new study finds. This suggests that pregnancy – a period when many women with MS stop taking their medication – should trigger discussions about switching from drugs that aren’t doing the job, the study’s lead author said.
“It’s a good time to consider the therapy that the individual is on, whether it’s one that’s effective for them, and whether it’s one they should return to when they start up therapy post-partum. It’s likely it will affect them the same way” after pregnancy as before, Caila Vaughn, MPH, PhD, of the University of Buffalo, said in an interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
From 2012-2017, the study authors sent surveys to 1,651 women in the New York State Multiple Sclerosis Consortium as part of an effort to understand how pregnancy affects women with MS, especially when relapses return in the post-partum period.
Of the 1,651 women, 635 (38% of the total) agreed to answer questions about their reproductive history.
Pregnancy data was available for 627 patients of whom 490 (78%) had been pregnant. Of those, 109 said they became pregnant after their MS diagnosis.
Fifty-three (49%) reported relapses in the 2 years prior to pregnancy and 46% reported them in the 2 subsequent years. Just 12% reported relapses during pregnancy, and 16% said they took disease-modifying drugs during pregnancy (60% had taken them before pregnancy).
Why does MS become less severe during pregnancy? “We believe the dormancy of the disease is related to an immune system that is naturally decreased and depressed during pregnancy,” Dr. Vaughn said. Afterward, she said, “the relapses are related to the recovery of the immune system post-partum.”
The researchers didn’t find any links between the use of disease-modifying drugs and relapses before, during, or after pregnancy.
Those who had relapses prior to pregnancy were more likely (P = 0.011) to have them afterward too. But researchers didn’t find a statistically significant link between relapses that occurred during and after pregnancy.
More than three-quarters of those who took disease-modifying drugs before pregnancy returned to using them afterward, in most cases within 3 months.
The study findings suggest that pregnancy is a helpful decision point when patients should take a closer look at the effects of their medications, Dr. Vaughn said. “In conjunction with a physician, they should decide if it’s a good one they should return to.”
Reflecting the findings of other research that suggests pregnancy is safe in women with MS, the study shows no sign that pregnancy – either before or after diagnosis of MS – boosts the risk that MS will get worse.
As for the possible effects of disease-modifying drugs on new mothers who breast-feed, the researchers found no evidence of adverse outcomes in 5 patients who took the medications while breast-feeding.
The study was funded by Teva. Dr. Vaughn reported no relevant disclosures. Several other study authors report various disclosures, including relationships with Teva.
SOURCE: Vaughn C. et al. Abstract FC04, 2018 annual meeting, Consortium of Multiple Sclerosis Centers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN – Women with multiple sclerosis who fare poorly on specific medications before pregnancy don’t tend to do any better afterward, a new study finds. This suggests that pregnancy – a period when many women with MS stop taking their medication – should trigger discussions about switching from drugs that aren’t doing the job, the study’s lead author said.
“It’s a good time to consider the therapy that the individual is on, whether it’s one that’s effective for them, and whether it’s one they should return to when they start up therapy post-partum. It’s likely it will affect them the same way” after pregnancy as before, Caila Vaughn, MPH, PhD, of the University of Buffalo, said in an interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
From 2012-2017, the study authors sent surveys to 1,651 women in the New York State Multiple Sclerosis Consortium as part of an effort to understand how pregnancy affects women with MS, especially when relapses return in the post-partum period.
Of the 1,651 women, 635 (38% of the total) agreed to answer questions about their reproductive history.
Pregnancy data was available for 627 patients of whom 490 (78%) had been pregnant. Of those, 109 said they became pregnant after their MS diagnosis.
Fifty-three (49%) reported relapses in the 2 years prior to pregnancy and 46% reported them in the 2 subsequent years. Just 12% reported relapses during pregnancy, and 16% said they took disease-modifying drugs during pregnancy (60% had taken them before pregnancy).
Why does MS become less severe during pregnancy? “We believe the dormancy of the disease is related to an immune system that is naturally decreased and depressed during pregnancy,” Dr. Vaughn said. Afterward, she said, “the relapses are related to the recovery of the immune system post-partum.”
The researchers didn’t find any links between the use of disease-modifying drugs and relapses before, during, or after pregnancy.
Those who had relapses prior to pregnancy were more likely (P = 0.011) to have them afterward too. But researchers didn’t find a statistically significant link between relapses that occurred during and after pregnancy.
More than three-quarters of those who took disease-modifying drugs before pregnancy returned to using them afterward, in most cases within 3 months.
The study findings suggest that pregnancy is a helpful decision point when patients should take a closer look at the effects of their medications, Dr. Vaughn said. “In conjunction with a physician, they should decide if it’s a good one they should return to.”
Reflecting the findings of other research that suggests pregnancy is safe in women with MS, the study shows no sign that pregnancy – either before or after diagnosis of MS – boosts the risk that MS will get worse.
As for the possible effects of disease-modifying drugs on new mothers who breast-feed, the researchers found no evidence of adverse outcomes in 5 patients who took the medications while breast-feeding.
The study was funded by Teva. Dr. Vaughn reported no relevant disclosures. Several other study authors report various disclosures, including relationships with Teva.
SOURCE: Vaughn C. et al. Abstract FC04, 2018 annual meeting, Consortium of Multiple Sclerosis Centers.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN – Women with multiple sclerosis who fare poorly on specific medications before pregnancy don’t tend to do any better afterward, a new study finds. This suggests that pregnancy – a period when many women with MS stop taking their medication – should trigger discussions about switching from drugs that aren’t doing the job, the study’s lead author said.
“It’s a good time to consider the therapy that the individual is on, whether it’s one that’s effective for them, and whether it’s one they should return to when they start up therapy post-partum. It’s likely it will affect them the same way” after pregnancy as before, Caila Vaughn, MPH, PhD, of the University of Buffalo, said in an interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
From 2012-2017, the study authors sent surveys to 1,651 women in the New York State Multiple Sclerosis Consortium as part of an effort to understand how pregnancy affects women with MS, especially when relapses return in the post-partum period.
Of the 1,651 women, 635 (38% of the total) agreed to answer questions about their reproductive history.
Pregnancy data was available for 627 patients of whom 490 (78%) had been pregnant. Of those, 109 said they became pregnant after their MS diagnosis.
Fifty-three (49%) reported relapses in the 2 years prior to pregnancy and 46% reported them in the 2 subsequent years. Just 12% reported relapses during pregnancy, and 16% said they took disease-modifying drugs during pregnancy (60% had taken them before pregnancy).
Why does MS become less severe during pregnancy? “We believe the dormancy of the disease is related to an immune system that is naturally decreased and depressed during pregnancy,” Dr. Vaughn said. Afterward, she said, “the relapses are related to the recovery of the immune system post-partum.”
The researchers didn’t find any links between the use of disease-modifying drugs and relapses before, during, or after pregnancy.
Those who had relapses prior to pregnancy were more likely (P = 0.011) to have them afterward too. But researchers didn’t find a statistically significant link between relapses that occurred during and after pregnancy.
More than three-quarters of those who took disease-modifying drugs before pregnancy returned to using them afterward, in most cases within 3 months.
The study findings suggest that pregnancy is a helpful decision point when patients should take a closer look at the effects of their medications, Dr. Vaughn said. “In conjunction with a physician, they should decide if it’s a good one they should return to.”
Reflecting the findings of other research that suggests pregnancy is safe in women with MS, the study shows no sign that pregnancy – either before or after diagnosis of MS – boosts the risk that MS will get worse.
As for the possible effects of disease-modifying drugs on new mothers who breast-feed, the researchers found no evidence of adverse outcomes in 5 patients who took the medications while breast-feeding.
The study was funded by Teva. Dr. Vaughn reported no relevant disclosures. Several other study authors report various disclosures, including relationships with Teva.
SOURCE: Vaughn C. et al. Abstract FC04, 2018 annual meeting, Consortium of Multiple Sclerosis Centers.
REPORTING FROM THE CMSC ANNUAL MEETING
Key clinical point: Multiple sclerosis relapse rates are similar before and after pregnancy, suggesting it may be a good time to consider switching medications if feasible.
Major finding: 49% of women who were pregnant after MS diagnosis reported relapses in the 2 years prior to pregnancy and 46% reported them in the 2 subsequent years. Those who had relapses prior to pregnancy were more likely to have them afterward, too.
Study details: Survey of 109 women who became pregnant after MS diagnosis.
Disclosures: Teva funded the study. Several study authors report various disclosures, including relationships with Teva.
Source: Vaughn C. et al. Abstract FC04, 2018 annual meeting, Consortium of Multiple Sclerosis Centers.
MS clinic thrives by making regular care a ‘loss leader’
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Care for MS patients is expensive, and even non-profit treatment centers can’t survive on reimbursements alone. The solution, according to Terry Smith, CEO of the Multiple Sclerosis Center of Atlanta, is to transform regular care into a “loss leader” and embrace other revenue sources.
“The reimbursements for that 20- minute or 30-minute follow-up just really don’t cover all the resources necessary for comprehensive care,” Mr. Smith said in a video interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
Mr. Smith said his involvement in the MS community was sparked about 2 decades ago when his wife developed the condition. “I have seen what the center gets reimbursed for her office visit, and then what her neurologist gets reimbursed.”
The reimbursement for an MS patient’s follow-up, 25-minute appointment with a physician is $104.25, according to Mr. Smith. Yet these MS visits are “the cornerstone of treatment ... set the tone for how successful the care is.”
To make make up for losses, the Atlanta center has begun offering its own ancillary services. “Our doctors are at the forefront of telling patients we have a group of neurologists that handle both emergent as well as non-emergent neurology,” he said. “That offers a revenue stream beyond the patient encounter.”
Other sources include imaging and an infusion clinic managed for a local hospital through a professional service agreement. The Atlanta center also has created its own specialty pharmacy focused on MS. “We buy disease-modifying drugs, develop personal contact with patients on a regular basis, then develop an ongoing compliance-monitoring program,” he said.
Mr. Smith discloses a consulting fee from Novartis.
Watch the interview to learn more about the center’s efforts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Care for MS patients is expensive, and even non-profit treatment centers can’t survive on reimbursements alone. The solution, according to Terry Smith, CEO of the Multiple Sclerosis Center of Atlanta, is to transform regular care into a “loss leader” and embrace other revenue sources.
“The reimbursements for that 20- minute or 30-minute follow-up just really don’t cover all the resources necessary for comprehensive care,” Mr. Smith said in a video interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
Mr. Smith said his involvement in the MS community was sparked about 2 decades ago when his wife developed the condition. “I have seen what the center gets reimbursed for her office visit, and then what her neurologist gets reimbursed.”
The reimbursement for an MS patient’s follow-up, 25-minute appointment with a physician is $104.25, according to Mr. Smith. Yet these MS visits are “the cornerstone of treatment ... set the tone for how successful the care is.”
To make make up for losses, the Atlanta center has begun offering its own ancillary services. “Our doctors are at the forefront of telling patients we have a group of neurologists that handle both emergent as well as non-emergent neurology,” he said. “That offers a revenue stream beyond the patient encounter.”
Other sources include imaging and an infusion clinic managed for a local hospital through a professional service agreement. The Atlanta center also has created its own specialty pharmacy focused on MS. “We buy disease-modifying drugs, develop personal contact with patients on a regular basis, then develop an ongoing compliance-monitoring program,” he said.
Mr. Smith discloses a consulting fee from Novartis.
Watch the interview to learn more about the center’s efforts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NASHVILLE, TENN. – Care for MS patients is expensive, and even non-profit treatment centers can’t survive on reimbursements alone. The solution, according to Terry Smith, CEO of the Multiple Sclerosis Center of Atlanta, is to transform regular care into a “loss leader” and embrace other revenue sources.
“The reimbursements for that 20- minute or 30-minute follow-up just really don’t cover all the resources necessary for comprehensive care,” Mr. Smith said in a video interview at the 2018 annual meeting of the Consortium of Multiple Sclerosis Clinics.
Mr. Smith said his involvement in the MS community was sparked about 2 decades ago when his wife developed the condition. “I have seen what the center gets reimbursed for her office visit, and then what her neurologist gets reimbursed.”
The reimbursement for an MS patient’s follow-up, 25-minute appointment with a physician is $104.25, according to Mr. Smith. Yet these MS visits are “the cornerstone of treatment ... set the tone for how successful the care is.”
To make make up for losses, the Atlanta center has begun offering its own ancillary services. “Our doctors are at the forefront of telling patients we have a group of neurologists that handle both emergent as well as non-emergent neurology,” he said. “That offers a revenue stream beyond the patient encounter.”
Other sources include imaging and an infusion clinic managed for a local hospital through a professional service agreement. The Atlanta center also has created its own specialty pharmacy focused on MS. “We buy disease-modifying drugs, develop personal contact with patients on a regular basis, then develop an ongoing compliance-monitoring program,” he said.
Mr. Smith discloses a consulting fee from Novartis.
Watch the interview to learn more about the center’s efforts.
REPORTING FROM THE CMSC ANNUAL MEETING
Metastatic colorectal cancer chemo costs double in Washington vs. British Columbia
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First-line systemic therapy for metastatic colorectal cancer costs twice as much in western Washington state as it does just across the border in British Columbia, Canada, but the more costly therapy does not net better survival, finds a cohort study reported at the annual meeting of the American Society of Clinical Oncology.
Differences between the United States and Canada in health care systems are well established, with a multipayer mix of private and public insurers in the former, and a single-payer, universal, public system in the latter, lead study author Todd Yezefski, MD, a senior fellow at the Fred Hutchinson Cancer Research Center in Seattle and the University of Washington School of Medicine, noted in a press briefing.
“Several studies have shown that overall health care utilization and costs in the U.S. are higher than in Canada. However, outcomes are generally similar, if not worse, in the U.S.,” he commented. “There have really been few studies, though, that have looked at treatment patterns, costs, and outcomes associated with a specific disease such as colorectal cancer.”
Results of the study of 2,197 patients with newly diagnosed metastatic colorectal cancer showed that the monthly cost for first-line systemic therapy exceeded $12,000 in western Washington state (excluding Medicare beneficiaries), compared with about $6,000 in British Columbia, even though the leading regimen in the latter region contained a targeted therapy. At the same time, median overall survival for patients given systemic therapy was essentially the same, approaching 2 years.
“Despite significantly higher costs, patients in western Washington didn’t do any better than those in British Columbia. Another way of saying this is they got the same bang for more buck,” Dr. Yezefski summarized. “Drug prices in Canada are generally set by the government. In the United States, we believe that if Medicare is allowed to negotiate drug prices with pharmaceutical companies, drug prices can be lower, and private insurers will oftentimes follow suit.”
In future work, the investigators plan to repeat analyses after including Medicare patients in the western Washington cohort (likely rendering the two groups more comparable) and to assess other aspects of health care utilization, such as total duration of chemotherapy, hospital use, radiation therapy, and surgery.
“The United States is probably the only country in the world where we actually have no real way of constraining the cost of health care. Certainly, that pertains to the cost of drugs,” commented Richard L. Schilsky, MD, FACP, FASCO, chief medical officer of ASCO and moderator of the press briefing. The U.S. Food and Drug Administration considers only the safety and efficacy of drugs when deciding whether they should be allowed on the market, he noted. “Once they are on the market, Medicare is generally required by law to pay for the cost of drugs, and the private insurers typically follow suit. So there really is no way to put any brakes on the system, which is not the case in most other health care systems in the world.” Other countries generally have a second agency or appointed body that performs some type of value assessment to determine whether the health care system can actually afford to offer the drug to the population. “That, of course, is a hypothesized reason for why, in Canada, you can get what would generally be considered a very expensive treatment regimen in the U.S. at half the cost of what it takes to deliver a similar regimen in the U.S. and still get equivalent outcomes,” Dr. Schilsky said.
For the study, Dr. Yezefski and colleagues identified patients with metastatic colorectal cancer newly diagnosed in 2010 or later in the regional database linking western Washington Surveillance, Epidemiology and End Results (SEER) data to claims from two large commercial insurers, and in the BC Cancer Agency database. Analyses were based on data from 575 patients in western Washington and 1,622 similar patients in British Columbia. Median age was 60 years in the former group and 66 years in the latter.
The rate of receipt of first-line systemic therapy was higher among the western Washington group than among the British Columbia group (79% vs. 68%, P less than.01), possibly because they were younger, Dr. Yezefski speculated. The most common regimen given to the former was FOLFOX (oxaliplatin, 5-fluorouracil, and folinic acid) chemotherapy (39%), whereas the most common given to the latter was FOLFIRI (irinotecan, 5-fluorouracil, and folinic acid) chemotherapy with bevacizumab (Avastin) (32%).
The mean monthly per-patient cost of first-line systemic therapy was $12,345 in western Washington, roughly double the $6,195 in British Columbia (P less than .01). Mean lifetime monthly systemic therapy costs were also higher in the former region ($7,883 vs. $4,830, P less than .01). However, median overall survival between the two regions was essentially the same, both among patients who received systemic therapy (21.4 vs. 22.1 months) and among all patients (17.4 vs. 16.9 months). Dr. Yezefski disclosed that he had no relevant conflicts of interest.
The study received funding from the Fred Hutchinson Cancer Research Center and BC Cancer Agency.
SOURCE: Yezefski et al., abstract LBA3579, https://am.asco.org/abstracts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First-line systemic therapy for metastatic colorectal cancer costs twice as much in western Washington state as it does just across the border in British Columbia, Canada, but the more costly therapy does not net better survival, finds a cohort study reported at the annual meeting of the American Society of Clinical Oncology.
Differences between the United States and Canada in health care systems are well established, with a multipayer mix of private and public insurers in the former, and a single-payer, universal, public system in the latter, lead study author Todd Yezefski, MD, a senior fellow at the Fred Hutchinson Cancer Research Center in Seattle and the University of Washington School of Medicine, noted in a press briefing.
“Several studies have shown that overall health care utilization and costs in the U.S. are higher than in Canada. However, outcomes are generally similar, if not worse, in the U.S.,” he commented. “There have really been few studies, though, that have looked at treatment patterns, costs, and outcomes associated with a specific disease such as colorectal cancer.”
Results of the study of 2,197 patients with newly diagnosed metastatic colorectal cancer showed that the monthly cost for first-line systemic therapy exceeded $12,000 in western Washington state (excluding Medicare beneficiaries), compared with about $6,000 in British Columbia, even though the leading regimen in the latter region contained a targeted therapy. At the same time, median overall survival for patients given systemic therapy was essentially the same, approaching 2 years.
“Despite significantly higher costs, patients in western Washington didn’t do any better than those in British Columbia. Another way of saying this is they got the same bang for more buck,” Dr. Yezefski summarized. “Drug prices in Canada are generally set by the government. In the United States, we believe that if Medicare is allowed to negotiate drug prices with pharmaceutical companies, drug prices can be lower, and private insurers will oftentimes follow suit.”
In future work, the investigators plan to repeat analyses after including Medicare patients in the western Washington cohort (likely rendering the two groups more comparable) and to assess other aspects of health care utilization, such as total duration of chemotherapy, hospital use, radiation therapy, and surgery.
“The United States is probably the only country in the world where we actually have no real way of constraining the cost of health care. Certainly, that pertains to the cost of drugs,” commented Richard L. Schilsky, MD, FACP, FASCO, chief medical officer of ASCO and moderator of the press briefing. The U.S. Food and Drug Administration considers only the safety and efficacy of drugs when deciding whether they should be allowed on the market, he noted. “Once they are on the market, Medicare is generally required by law to pay for the cost of drugs, and the private insurers typically follow suit. So there really is no way to put any brakes on the system, which is not the case in most other health care systems in the world.” Other countries generally have a second agency or appointed body that performs some type of value assessment to determine whether the health care system can actually afford to offer the drug to the population. “That, of course, is a hypothesized reason for why, in Canada, you can get what would generally be considered a very expensive treatment regimen in the U.S. at half the cost of what it takes to deliver a similar regimen in the U.S. and still get equivalent outcomes,” Dr. Schilsky said.
For the study, Dr. Yezefski and colleagues identified patients with metastatic colorectal cancer newly diagnosed in 2010 or later in the regional database linking western Washington Surveillance, Epidemiology and End Results (SEER) data to claims from two large commercial insurers, and in the BC Cancer Agency database. Analyses were based on data from 575 patients in western Washington and 1,622 similar patients in British Columbia. Median age was 60 years in the former group and 66 years in the latter.
The rate of receipt of first-line systemic therapy was higher among the western Washington group than among the British Columbia group (79% vs. 68%, P less than.01), possibly because they were younger, Dr. Yezefski speculated. The most common regimen given to the former was FOLFOX (oxaliplatin, 5-fluorouracil, and folinic acid) chemotherapy (39%), whereas the most common given to the latter was FOLFIRI (irinotecan, 5-fluorouracil, and folinic acid) chemotherapy with bevacizumab (Avastin) (32%).
The mean monthly per-patient cost of first-line systemic therapy was $12,345 in western Washington, roughly double the $6,195 in British Columbia (P less than .01). Mean lifetime monthly systemic therapy costs were also higher in the former region ($7,883 vs. $4,830, P less than .01). However, median overall survival between the two regions was essentially the same, both among patients who received systemic therapy (21.4 vs. 22.1 months) and among all patients (17.4 vs. 16.9 months). Dr. Yezefski disclosed that he had no relevant conflicts of interest.
The study received funding from the Fred Hutchinson Cancer Research Center and BC Cancer Agency.
SOURCE: Yezefski et al., abstract LBA3579, https://am.asco.org/abstracts.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
First-line systemic therapy for metastatic colorectal cancer costs twice as much in western Washington state as it does just across the border in British Columbia, Canada, but the more costly therapy does not net better survival, finds a cohort study reported at the annual meeting of the American Society of Clinical Oncology.
Differences between the United States and Canada in health care systems are well established, with a multipayer mix of private and public insurers in the former, and a single-payer, universal, public system in the latter, lead study author Todd Yezefski, MD, a senior fellow at the Fred Hutchinson Cancer Research Center in Seattle and the University of Washington School of Medicine, noted in a press briefing.
“Several studies have shown that overall health care utilization and costs in the U.S. are higher than in Canada. However, outcomes are generally similar, if not worse, in the U.S.,” he commented. “There have really been few studies, though, that have looked at treatment patterns, costs, and outcomes associated with a specific disease such as colorectal cancer.”
Results of the study of 2,197 patients with newly diagnosed metastatic colorectal cancer showed that the monthly cost for first-line systemic therapy exceeded $12,000 in western Washington state (excluding Medicare beneficiaries), compared with about $6,000 in British Columbia, even though the leading regimen in the latter region contained a targeted therapy. At the same time, median overall survival for patients given systemic therapy was essentially the same, approaching 2 years.
“Despite significantly higher costs, patients in western Washington didn’t do any better than those in British Columbia. Another way of saying this is they got the same bang for more buck,” Dr. Yezefski summarized. “Drug prices in Canada are generally set by the government. In the United States, we believe that if Medicare is allowed to negotiate drug prices with pharmaceutical companies, drug prices can be lower, and private insurers will oftentimes follow suit.”
In future work, the investigators plan to repeat analyses after including Medicare patients in the western Washington cohort (likely rendering the two groups more comparable) and to assess other aspects of health care utilization, such as total duration of chemotherapy, hospital use, radiation therapy, and surgery.
“The United States is probably the only country in the world where we actually have no real way of constraining the cost of health care. Certainly, that pertains to the cost of drugs,” commented Richard L. Schilsky, MD, FACP, FASCO, chief medical officer of ASCO and moderator of the press briefing. The U.S. Food and Drug Administration considers only the safety and efficacy of drugs when deciding whether they should be allowed on the market, he noted. “Once they are on the market, Medicare is generally required by law to pay for the cost of drugs, and the private insurers typically follow suit. So there really is no way to put any brakes on the system, which is not the case in most other health care systems in the world.” Other countries generally have a second agency or appointed body that performs some type of value assessment to determine whether the health care system can actually afford to offer the drug to the population. “That, of course, is a hypothesized reason for why, in Canada, you can get what would generally be considered a very expensive treatment regimen in the U.S. at half the cost of what it takes to deliver a similar regimen in the U.S. and still get equivalent outcomes,” Dr. Schilsky said.
For the study, Dr. Yezefski and colleagues identified patients with metastatic colorectal cancer newly diagnosed in 2010 or later in the regional database linking western Washington Surveillance, Epidemiology and End Results (SEER) data to claims from two large commercial insurers, and in the BC Cancer Agency database. Analyses were based on data from 575 patients in western Washington and 1,622 similar patients in British Columbia. Median age was 60 years in the former group and 66 years in the latter.
The rate of receipt of first-line systemic therapy was higher among the western Washington group than among the British Columbia group (79% vs. 68%, P less than.01), possibly because they were younger, Dr. Yezefski speculated. The most common regimen given to the former was FOLFOX (oxaliplatin, 5-fluorouracil, and folinic acid) chemotherapy (39%), whereas the most common given to the latter was FOLFIRI (irinotecan, 5-fluorouracil, and folinic acid) chemotherapy with bevacizumab (Avastin) (32%).
The mean monthly per-patient cost of first-line systemic therapy was $12,345 in western Washington, roughly double the $6,195 in British Columbia (P less than .01). Mean lifetime monthly systemic therapy costs were also higher in the former region ($7,883 vs. $4,830, P less than .01). However, median overall survival between the two regions was essentially the same, both among patients who received systemic therapy (21.4 vs. 22.1 months) and among all patients (17.4 vs. 16.9 months). Dr. Yezefski disclosed that he had no relevant conflicts of interest.
The study received funding from the Fred Hutchinson Cancer Research Center and BC Cancer Agency.
SOURCE: Yezefski et al., abstract LBA3579, https://am.asco.org/abstracts.
REPORTING FROM ASCO 2018
Key clinical point: The higher cost for metastatic CRC systemic therapy in the United States versus Canada does not translate to better survival.
Major finding: Monthly cost of first-line systemic therapy was $12,345 in western Washington vs. $6,195 in British Columbia (P less than .01), but median overall survival was statistically indistinguishable (21.4 vs. 22.1 months).
Study details: A cohort study of 2,197 patients with newly diagnosed metastatic CRC from a regional database linking western Washington SEER to claims from two large commercial insurers and from the BC (British Columbia) Cancer Agency database.
Disclosures: Dr. Yezefski disclosed that he had no relevant conflicts of interest. The study received funding from the Fred Hutchinson Cancer Research Center and the BC Cancer Agency.
Source: Yezefski et al. Abstract LBA3579.
Geriatric assessments enhance patient care in advanced cancer
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – and boosted patient satisfaction, results of a randomized trial show.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Supriya Gupta Mohile, MD, MS, from the University of Rochester, New York, discusses how a standardized written questionnaire and objective tests for physical performance and cognition can enhance the doctor-patient relationship and lead to specific recommendations for interventions, compared with usual care.
Dr. Mohile had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – and boosted patient satisfaction, results of a randomized trial show.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Supriya Gupta Mohile, MD, MS, from the University of Rochester, New York, discusses how a standardized written questionnaire and objective tests for physical performance and cognition can enhance the doctor-patient relationship and lead to specific recommendations for interventions, compared with usual care.
Dr. Mohile had no relevant financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – and boosted patient satisfaction, results of a randomized trial show.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Supriya Gupta Mohile, MD, MS, from the University of Rochester, New York, discusses how a standardized written questionnaire and objective tests for physical performance and cognition can enhance the doctor-patient relationship and lead to specific recommendations for interventions, compared with usual care.
Dr. Mohile had no relevant financial disclosures.
REPORTING FROM ASCO 2018
Stronger abiraterone response in mCRPC seen in black men
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results of a prospective clinical trial in 100 men with metastatic castration-resistant prostate cancer (mCRPC) showed that black men were more likely to have a decline in prostate-specific antigen (PSA) and a longer median time to PSA rise in response to treatment with abiraterone (Zytiga) than white men receiving the same treatment (16.8 vs. 11.5 months).
The findings support earlier evidence indicating a stronger response to abiraterone among African Americans compared with Caucasians and suggest that at least some of the observed racial disparities in prostate cancer outcomes could be explained by genetic differences, according to lead study author Daniel George, MD, from Duke University in Durham, N.C.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Dr. George discusses the study findings, as well as issues surrounding the problems of recruiting African Americans for clinical trials and ensuring access to the standard of advanced prostate cancer care for all patients.
Dr. George disclosed consulting or advisory roles and research funding from numerous pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results of a prospective clinical trial in 100 men with metastatic castration-resistant prostate cancer (mCRPC) showed that black men were more likely to have a decline in prostate-specific antigen (PSA) and a longer median time to PSA rise in response to treatment with abiraterone (Zytiga) than white men receiving the same treatment (16.8 vs. 11.5 months).
The findings support earlier evidence indicating a stronger response to abiraterone among African Americans compared with Caucasians and suggest that at least some of the observed racial disparities in prostate cancer outcomes could be explained by genetic differences, according to lead study author Daniel George, MD, from Duke University in Durham, N.C.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Dr. George discusses the study findings, as well as issues surrounding the problems of recruiting African Americans for clinical trials and ensuring access to the standard of advanced prostate cancer care for all patients.
Dr. George disclosed consulting or advisory roles and research funding from numerous pharmaceutical companies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Results of a prospective clinical trial in 100 men with metastatic castration-resistant prostate cancer (mCRPC) showed that black men were more likely to have a decline in prostate-specific antigen (PSA) and a longer median time to PSA rise in response to treatment with abiraterone (Zytiga) than white men receiving the same treatment (16.8 vs. 11.5 months).
The findings support earlier evidence indicating a stronger response to abiraterone among African Americans compared with Caucasians and suggest that at least some of the observed racial disparities in prostate cancer outcomes could be explained by genetic differences, according to lead study author Daniel George, MD, from Duke University in Durham, N.C.
In this video interview from the annual meeting of the American Society of Clinical Oncology, Dr. George discusses the study findings, as well as issues surrounding the problems of recruiting African Americans for clinical trials and ensuring access to the standard of advanced prostate cancer care for all patients.
Dr. George disclosed consulting or advisory roles and research funding from numerous pharmaceutical companies.
REPORTING FROM ASCO 2018
Head and neck cancers: Women less commonly receive intensive chemo
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
CHICAGO – Women with head and neck cancer less commonly receive intensive chemotherapy (35% vs. 46%) and radiation therapy (60% vs. 70%) than do their male counterparts, finds an analysis of 223 female patients and 661 male patients with stage II-IVB disease treated at Kaiser Permanente Northern California. And this apparent undertreatment may be compromising survival for women, as their ratio of cancer deaths to other deaths is nearly twice that of men (adjusted relative hazard ratio, 1.92; 95% CI, 1.07-3.43).
In this video interview from the annual meeting of the American Society of Clinical Oncology, senior study author Jed A. Katzel, MD, of Kaiser Permanente in Santa Clara, Calif., described the new statistical approach used to assess outcomes and discussed ongoing research to pin down the reasons for the apparent treatment disparities, including patient preferences and the influences of tumor site and HPV status.
Dr. Katzel reported no financial disclosures.
REPORTING FROM ASCO 2018
A Path to Safer Opioids?
NIH researchers tested the common wisdom that opioids act only on the same surface receptors as endogenous opioids. They discovered that opioids, such as morphine and oxycodone, bind to receptors inside neurons—which are not the targets of naturally occurring opioids. The difference in the actions could help guide the design of pain relievers that do not produce addiction or other adverse opioid-related effects.
The researchers used a new type of antibody biosensor—a nanobody—that generates a fluorescent signal when certain proteins are activated. The signal allowed researchers to track chemicals as they move through cells and responded to stimuli.
The first discovery was that the opioid receptors were activated not only on the surface, but also in the endosome, where the mu-receptor remained activated over several minutes. The researchers also found that there are large differences across a range of opioid drugs in how strongly they induce receptor activation in the endosome. Yet another discovery was that the opioid drugs uniquely induce rapid nanobody signaling, within tens of seconds, in the Golgi apparatus. Therapeutic opioids also uniquely activated mu-opioid receptors in Golgi outposts, in the long, branched structures of neurons.
Based on those findings, the researchers hypothesize that current medically used opioids distort the normal time and spatial sequence of mu-opioid receptor activating and signaling. That distortion may provide the mechanistic link, they say, that explains the undesired adverse effects of opioid medicines.
NIH researchers tested the common wisdom that opioids act only on the same surface receptors as endogenous opioids. They discovered that opioids, such as morphine and oxycodone, bind to receptors inside neurons—which are not the targets of naturally occurring opioids. The difference in the actions could help guide the design of pain relievers that do not produce addiction or other adverse opioid-related effects.
The researchers used a new type of antibody biosensor—a nanobody—that generates a fluorescent signal when certain proteins are activated. The signal allowed researchers to track chemicals as they move through cells and responded to stimuli.
The first discovery was that the opioid receptors were activated not only on the surface, but also in the endosome, where the mu-receptor remained activated over several minutes. The researchers also found that there are large differences across a range of opioid drugs in how strongly they induce receptor activation in the endosome. Yet another discovery was that the opioid drugs uniquely induce rapid nanobody signaling, within tens of seconds, in the Golgi apparatus. Therapeutic opioids also uniquely activated mu-opioid receptors in Golgi outposts, in the long, branched structures of neurons.
Based on those findings, the researchers hypothesize that current medically used opioids distort the normal time and spatial sequence of mu-opioid receptor activating and signaling. That distortion may provide the mechanistic link, they say, that explains the undesired adverse effects of opioid medicines.
NIH researchers tested the common wisdom that opioids act only on the same surface receptors as endogenous opioids. They discovered that opioids, such as morphine and oxycodone, bind to receptors inside neurons—which are not the targets of naturally occurring opioids. The difference in the actions could help guide the design of pain relievers that do not produce addiction or other adverse opioid-related effects.
The researchers used a new type of antibody biosensor—a nanobody—that generates a fluorescent signal when certain proteins are activated. The signal allowed researchers to track chemicals as they move through cells and responded to stimuli.
The first discovery was that the opioid receptors were activated not only on the surface, but also in the endosome, where the mu-receptor remained activated over several minutes. The researchers also found that there are large differences across a range of opioid drugs in how strongly they induce receptor activation in the endosome. Yet another discovery was that the opioid drugs uniquely induce rapid nanobody signaling, within tens of seconds, in the Golgi apparatus. Therapeutic opioids also uniquely activated mu-opioid receptors in Golgi outposts, in the long, branched structures of neurons.
Based on those findings, the researchers hypothesize that current medically used opioids distort the normal time and spatial sequence of mu-opioid receptor activating and signaling. That distortion may provide the mechanistic link, they say, that explains the undesired adverse effects of opioid medicines.
Cytotect®CP found to be safe and effective after allo-HCT
A small retrospective study of 23 transplant patients has confirmed that CMV hyperimmune globulin (Cytotect®CP) is a safe and effective salvage therapy for patients with cytomegalovirus (CMV) infection after allogeneic hematopoietic cell transplant (allo-HCT).
Cytotect®CP used as salvage therapy resulted in a 78% overall response rate and 70% of all patients cleared CMV infection, according to investigators.
They observed no clinically significant adverse events.
CMV is a major factor contributing to high mortality rates in allo-HCT patients.
And because Cytotect®CP is less toxic than commonly used treatments for CMV infection, investigators suggested that it be used prophylactically in patients known to have a predisposition to CMV infection.
They reported their findings in the journal Bone Marrow Transplantation.
Patient characteristics and methods
All 23 patients transplanted at 8 centers in France were CMV seropositive at the time of transplant, and 70% received the transplant from a CMV serostatus negative donor.
Recipient positivity and donor negativity, the investigators indicated, is a risk factor for developing recurrent CMV infection after allo-HCT.
The patients’ median age was 53, 11 were male and 12 female.
Five patients (22%) received a haploidentical transplant and 14 (61%) from an unrelated donor, which the investigators pointed out is also a known risk factor for developing recurrent CMV infection.
Thirteen (57%) were in complete remission from their underlying disease, 4 (17%) in partial remission, 1 (4%) had stable disease, and 5 (22%) were in relapse or had progressive disease.
Most (83%) had a peripheral blood transplant, 15 (65%) had a reduced intensity conditioning regimen, and 16 (70%) had antithymocyte globulin as part of their conditioning regimen.
All patients received valacyclovir as antiviral prophylaxis prior to receiving Cytotect®CP. The investigators mentioned in the paper that valacyclovir has not been proven to be effective in treating CMV.
They noted that other CMV treatments, such as ganciclovir, foscavir, and dicofovir, cause high levels of toxicity, frequently leading to treatment discontinuation.
Seventeen patients (74%) had a history of graft-versus-host disease (GVHD), and 11 (49%) had active GVHD at the time CMV hyperimmune globulin was administered.
Investigators used quantitative polymerase chain reaction (PCR) to quantify CMV viral load in the blood.
Treatment could begin when patients’ viral load was greater than 3-3.5 log UI/mL, according to the Francophone Society of Bone Marrow Transplantation and Cellular therapy.
Three patients received Cytotect®CP at a prophylaxis dose (200 U/kg/week) to prevent CMV recurrences and 20 as preemptive therapy (400 U/kg on days 1, 4, 8 then 200 U/kg on days 12and 16).
Seven patients (30%) received Cytotect®CP as monotherapy, 5 (22%) in combination with ganciclovir, 5 (22%) in combination with foscavir, 2 (9%) in combination with ganciclovir and foscavir, and 4 (17%) with some other combination.
Investigators restricted their analysis to 100-day overall survival (OS), starting at the beginning of Cytotect®CP treatment to death within 100 days, regardless of the cause of death.
Results
Eighteen patients (78%) responded to Cytotect®CP, and 16 of the responders converted to CMV-PCR negative.
Median time to achieve CMV-PCR response was 15 days (range, 3-51).
Four patients did not respond to therapy, and 1 patient had a non-evaluable response. The latter patient died 13 days after the introduction of Cytotect®CP due to another infection.
Eight patients died within 100 days after initiation of Cytotect®CP. Two deaths were related to CMV and 6 were unrelated.
Four patients who responded to Cytotect®CP experienced CMV relapse between 9 and 49 days after their best response to therapy.
Five responders died within 100 days due to the following causes: GVHD (n = 2), other infection (n = 1), underlying disease (n = 1), and CMV-related causes (n = 1).
Two of the 4 nonresponders died of other infection (n = 1) and GVHD (n = 1).
Investigators estimated the 100-day OS from the start of Cytotect®CP to be 69.6%. They observed no statistical difference (P=0.258) between those who responded (73.7%) and those who didn’t (50.0%).
The investigators believe that Cytotect®CP is an alternative option for treatment of CMV infection because it avoids renal and bone marrow impairment and should be considered as prophylaxis in select patients.
They recommend a large prospective study be conducted to confirm safety and efficacy results of CMV hyperimmune globulin.
Cytotect®CP is authorized in more than 15 countries for the prophylaxis of CMV infection in patients receiving immunosuppressive treatment, particularly transplant recipients.
In French transplant centers, according to the study authors, use of Cytotect®CP is limited to the salvage setting for recurrent or refractory CMV infections and sometimes in combination for CMV pneumonia.
Biotest, the commercializer of Cytotect®CP, provided a grant for this study.
A small retrospective study of 23 transplant patients has confirmed that CMV hyperimmune globulin (Cytotect®CP) is a safe and effective salvage therapy for patients with cytomegalovirus (CMV) infection after allogeneic hematopoietic cell transplant (allo-HCT).
Cytotect®CP used as salvage therapy resulted in a 78% overall response rate and 70% of all patients cleared CMV infection, according to investigators.
They observed no clinically significant adverse events.
CMV is a major factor contributing to high mortality rates in allo-HCT patients.
And because Cytotect®CP is less toxic than commonly used treatments for CMV infection, investigators suggested that it be used prophylactically in patients known to have a predisposition to CMV infection.
They reported their findings in the journal Bone Marrow Transplantation.
Patient characteristics and methods
All 23 patients transplanted at 8 centers in France were CMV seropositive at the time of transplant, and 70% received the transplant from a CMV serostatus negative donor.
Recipient positivity and donor negativity, the investigators indicated, is a risk factor for developing recurrent CMV infection after allo-HCT.
The patients’ median age was 53, 11 were male and 12 female.
Five patients (22%) received a haploidentical transplant and 14 (61%) from an unrelated donor, which the investigators pointed out is also a known risk factor for developing recurrent CMV infection.
Thirteen (57%) were in complete remission from their underlying disease, 4 (17%) in partial remission, 1 (4%) had stable disease, and 5 (22%) were in relapse or had progressive disease.
Most (83%) had a peripheral blood transplant, 15 (65%) had a reduced intensity conditioning regimen, and 16 (70%) had antithymocyte globulin as part of their conditioning regimen.
All patients received valacyclovir as antiviral prophylaxis prior to receiving Cytotect®CP. The investigators mentioned in the paper that valacyclovir has not been proven to be effective in treating CMV.
They noted that other CMV treatments, such as ganciclovir, foscavir, and dicofovir, cause high levels of toxicity, frequently leading to treatment discontinuation.
Seventeen patients (74%) had a history of graft-versus-host disease (GVHD), and 11 (49%) had active GVHD at the time CMV hyperimmune globulin was administered.
Investigators used quantitative polymerase chain reaction (PCR) to quantify CMV viral load in the blood.
Treatment could begin when patients’ viral load was greater than 3-3.5 log UI/mL, according to the Francophone Society of Bone Marrow Transplantation and Cellular therapy.
Three patients received Cytotect®CP at a prophylaxis dose (200 U/kg/week) to prevent CMV recurrences and 20 as preemptive therapy (400 U/kg on days 1, 4, 8 then 200 U/kg on days 12and 16).
Seven patients (30%) received Cytotect®CP as monotherapy, 5 (22%) in combination with ganciclovir, 5 (22%) in combination with foscavir, 2 (9%) in combination with ganciclovir and foscavir, and 4 (17%) with some other combination.
Investigators restricted their analysis to 100-day overall survival (OS), starting at the beginning of Cytotect®CP treatment to death within 100 days, regardless of the cause of death.
Results
Eighteen patients (78%) responded to Cytotect®CP, and 16 of the responders converted to CMV-PCR negative.
Median time to achieve CMV-PCR response was 15 days (range, 3-51).
Four patients did not respond to therapy, and 1 patient had a non-evaluable response. The latter patient died 13 days after the introduction of Cytotect®CP due to another infection.
Eight patients died within 100 days after initiation of Cytotect®CP. Two deaths were related to CMV and 6 were unrelated.
Four patients who responded to Cytotect®CP experienced CMV relapse between 9 and 49 days after their best response to therapy.
Five responders died within 100 days due to the following causes: GVHD (n = 2), other infection (n = 1), underlying disease (n = 1), and CMV-related causes (n = 1).
Two of the 4 nonresponders died of other infection (n = 1) and GVHD (n = 1).
Investigators estimated the 100-day OS from the start of Cytotect®CP to be 69.6%. They observed no statistical difference (P=0.258) between those who responded (73.7%) and those who didn’t (50.0%).
The investigators believe that Cytotect®CP is an alternative option for treatment of CMV infection because it avoids renal and bone marrow impairment and should be considered as prophylaxis in select patients.
They recommend a large prospective study be conducted to confirm safety and efficacy results of CMV hyperimmune globulin.
Cytotect®CP is authorized in more than 15 countries for the prophylaxis of CMV infection in patients receiving immunosuppressive treatment, particularly transplant recipients.
In French transplant centers, according to the study authors, use of Cytotect®CP is limited to the salvage setting for recurrent or refractory CMV infections and sometimes in combination for CMV pneumonia.
Biotest, the commercializer of Cytotect®CP, provided a grant for this study.
A small retrospective study of 23 transplant patients has confirmed that CMV hyperimmune globulin (Cytotect®CP) is a safe and effective salvage therapy for patients with cytomegalovirus (CMV) infection after allogeneic hematopoietic cell transplant (allo-HCT).
Cytotect®CP used as salvage therapy resulted in a 78% overall response rate and 70% of all patients cleared CMV infection, according to investigators.
They observed no clinically significant adverse events.
CMV is a major factor contributing to high mortality rates in allo-HCT patients.
And because Cytotect®CP is less toxic than commonly used treatments for CMV infection, investigators suggested that it be used prophylactically in patients known to have a predisposition to CMV infection.
They reported their findings in the journal Bone Marrow Transplantation.
Patient characteristics and methods
All 23 patients transplanted at 8 centers in France were CMV seropositive at the time of transplant, and 70% received the transplant from a CMV serostatus negative donor.
Recipient positivity and donor negativity, the investigators indicated, is a risk factor for developing recurrent CMV infection after allo-HCT.
The patients’ median age was 53, 11 were male and 12 female.
Five patients (22%) received a haploidentical transplant and 14 (61%) from an unrelated donor, which the investigators pointed out is also a known risk factor for developing recurrent CMV infection.
Thirteen (57%) were in complete remission from their underlying disease, 4 (17%) in partial remission, 1 (4%) had stable disease, and 5 (22%) were in relapse or had progressive disease.
Most (83%) had a peripheral blood transplant, 15 (65%) had a reduced intensity conditioning regimen, and 16 (70%) had antithymocyte globulin as part of their conditioning regimen.
All patients received valacyclovir as antiviral prophylaxis prior to receiving Cytotect®CP. The investigators mentioned in the paper that valacyclovir has not been proven to be effective in treating CMV.
They noted that other CMV treatments, such as ganciclovir, foscavir, and dicofovir, cause high levels of toxicity, frequently leading to treatment discontinuation.
Seventeen patients (74%) had a history of graft-versus-host disease (GVHD), and 11 (49%) had active GVHD at the time CMV hyperimmune globulin was administered.
Investigators used quantitative polymerase chain reaction (PCR) to quantify CMV viral load in the blood.
Treatment could begin when patients’ viral load was greater than 3-3.5 log UI/mL, according to the Francophone Society of Bone Marrow Transplantation and Cellular therapy.
Three patients received Cytotect®CP at a prophylaxis dose (200 U/kg/week) to prevent CMV recurrences and 20 as preemptive therapy (400 U/kg on days 1, 4, 8 then 200 U/kg on days 12and 16).
Seven patients (30%) received Cytotect®CP as monotherapy, 5 (22%) in combination with ganciclovir, 5 (22%) in combination with foscavir, 2 (9%) in combination with ganciclovir and foscavir, and 4 (17%) with some other combination.
Investigators restricted their analysis to 100-day overall survival (OS), starting at the beginning of Cytotect®CP treatment to death within 100 days, regardless of the cause of death.
Results
Eighteen patients (78%) responded to Cytotect®CP, and 16 of the responders converted to CMV-PCR negative.
Median time to achieve CMV-PCR response was 15 days (range, 3-51).
Four patients did not respond to therapy, and 1 patient had a non-evaluable response. The latter patient died 13 days after the introduction of Cytotect®CP due to another infection.
Eight patients died within 100 days after initiation of Cytotect®CP. Two deaths were related to CMV and 6 were unrelated.
Four patients who responded to Cytotect®CP experienced CMV relapse between 9 and 49 days after their best response to therapy.
Five responders died within 100 days due to the following causes: GVHD (n = 2), other infection (n = 1), underlying disease (n = 1), and CMV-related causes (n = 1).
Two of the 4 nonresponders died of other infection (n = 1) and GVHD (n = 1).
Investigators estimated the 100-day OS from the start of Cytotect®CP to be 69.6%. They observed no statistical difference (P=0.258) between those who responded (73.7%) and those who didn’t (50.0%).
The investigators believe that Cytotect®CP is an alternative option for treatment of CMV infection because it avoids renal and bone marrow impairment and should be considered as prophylaxis in select patients.
They recommend a large prospective study be conducted to confirm safety and efficacy results of CMV hyperimmune globulin.
Cytotect®CP is authorized in more than 15 countries for the prophylaxis of CMV infection in patients receiving immunosuppressive treatment, particularly transplant recipients.
In French transplant centers, according to the study authors, use of Cytotect®CP is limited to the salvage setting for recurrent or refractory CMV infections and sometimes in combination for CMV pneumonia.
Biotest, the commercializer of Cytotect®CP, provided a grant for this study.
The Evidence for Herbal and Botanical Remedies, Part 2
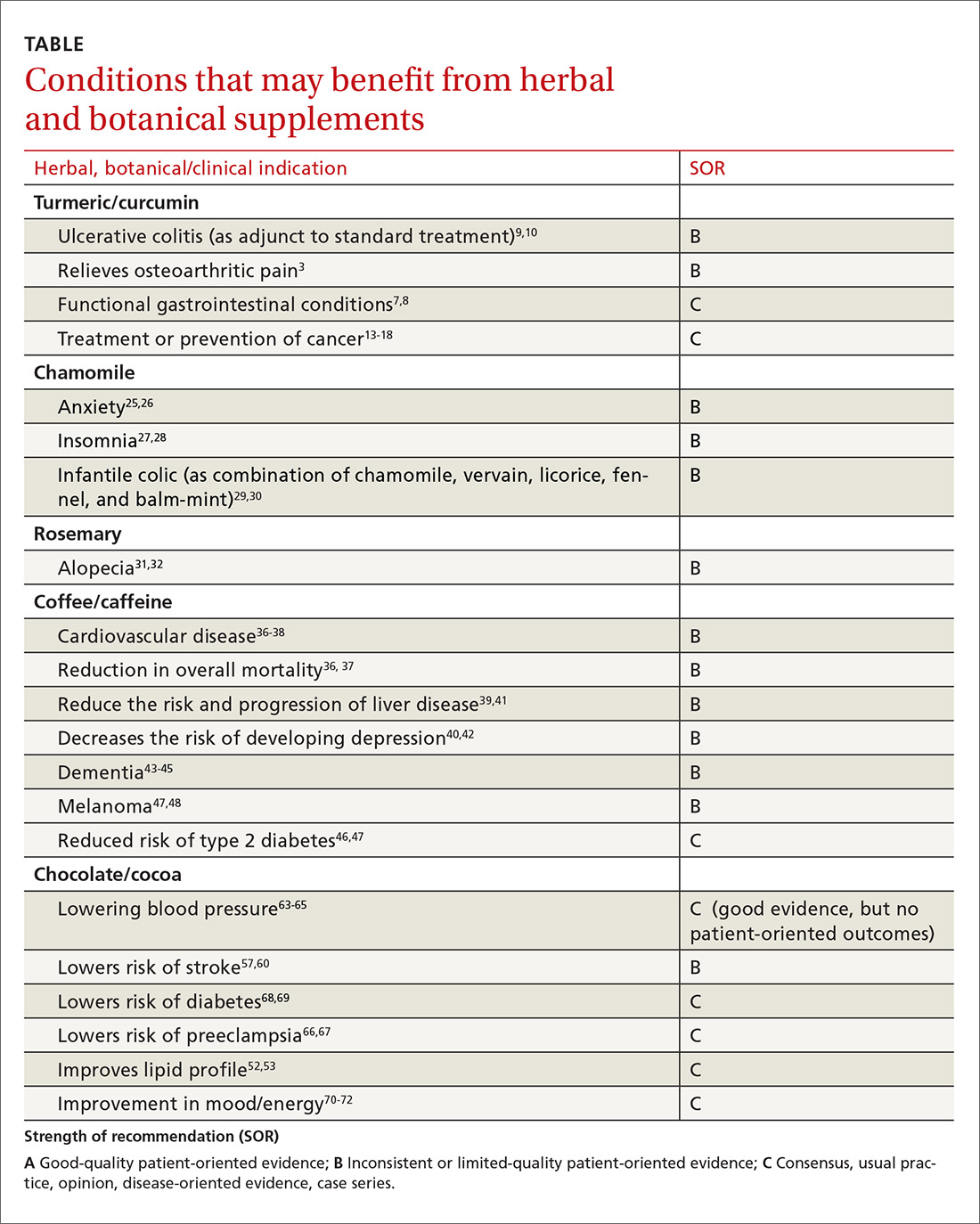
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
PRACTICE RECOMMENDATIONS
› Inform patients that curcumin appears to be a safe and effective adjunctive therapy for ulcerative colitis when used along with mesalamine or sulfasalazine. B
› Recommend chamomile extract to patients experiencing mild to moderate generalized anxiety disorder. B
› Tell patients that coffee is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression (in patients with end-stage liver disease). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series