User login
Under his IDH2: Epigenetic drivers of clonal hematopoiesis
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
The world has surely changed in recent years, leading television and Hollywood to recreate many former works of postapocalyptic fiction. Watching the recent Hulu drama “The Handmaid’s Tale” leads many to make modern-day comparisons, but to this hematologist the connection is bone deep.
Hematopoietic stem cells face a century of carefully regulated symmetric division. They are tasked with the mission to generate the heterogeneous and environmentally responsive progenitor populations that will undergo coordinated differentiation and maturation.1
Within this epic battle of Gilead, much like hematopoiesis, there is a hierarchy within the Sons of Jacob. At the top there are few who drive leukemogenicity and require no other co-conspirators. MLL fusions and core binding factor are the Commanders that can overthrow the regulated governance of normal marrow homeostasis. Other myeloid disease alleles are soldiers of Gilead (i.e. FLT3-ITD, IDH1, IDH2, NPM1c, Spliceosome/Cohesin). Their individual characteristics can influence the natural history of the disease and define its strengths and weaknesses, particularly as new targeted therapies are developed.
Recently, Jongen-Lavrencic et al. reported that all disease alleles that persist as minimal residual disease after induction have a negative influence on outcome, except for three – DNMT3A, TET2, and ASXL1.2 What do we make of the “DTA” mutations? Are they the “Eye” – spies embedded in the polyclonal background of the marrow – intrinsically wired with malevolent intent? Are they the innocent, abused Handmaids – reprogrammed by inflammatory “Aunts” at the marrow’s Red Center and forced to clonally procreate? Previous works have suggested the latter, that persistence of mutations in genes found in clonal hematopoiesis (CH) do not portend an equivalent risk of relapse.3,4
A more encompassing question remains as to the equivalency of CH in the absence of a hematopoietic tumor versus CH postleukemia therapy. Comparisons to small cell lymphomas after successful treatment of transformed disease are disingenuous; CH is not itself a malignant state.5 Forty months of median follow-up for post-AML CH is simply not enough time. Work presented at the 2017 annual meeting of the American Society of Hematology by Jaiswal et al. showed that the approximately 1% risk of transformation per year was consistent over a 20-year period of follow up in the Swedish Nurse’s Study.
The recent work in the New England Journal of Medicine adds to the growing understanding of CH, but requires the test of time to know if these cells are truly innocent Handmaids or the Eye in a red cloak. I’ll be exploring these issues and taking your questions during a live Twitter Q&A on June 14 at noon ET. Follow me at @TheDoctorIsVin and @HematologyNews1 for more details. Blessed be the fruit.
Dr. Viny is with the Memorial Sloan-Kettering Cancer Center, N.Y., where he is a clinical instructor, is on the staff of the leukemia service, and is a clinical researcher in the Ross Levine Lab.
References
1. Orkin SH and Zon L. Cell. 2008 Feb 22;132(4):631-44.
2. Jongen-Lavrencic M et al. N Engl J Med 2018; 378:1189-99.
3. Bhatnagar B et al. Br J Haematol. 2016 Oct;175(2):226-36.
4. Shlush LI et al. Nature. 2017;547:104-8.
5. Bowman RL et al. Cell Stem Cell. 2018 Feb 1;22(2):157-70.
Advances in Hematology and Oncology (May 2018)
Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA
Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA

Click here to access May 2018 Advances In Hematology and Oncology Digital Edition.
Table of Contents
- Risk of Cancer-Associated Thrombosis and Bleeding in Veterans With Malignancy Who Are Receiving Direct Oral Anticoagulants
- Using Dermoscopy to Identify Melanoma and Improve Diagnostic Discrimination
- Prevalence of Suspicious Ultrasound Features in Hot Thyroid Nodules
- The Effect of Immunonutrition on Veterans Undergoing Major Surgery for Gastrointestinal Cancer
- Protons and Prostate Cancer
- The Use of Immuno-Oncology Therapies in the VHA

VF incidence increases significantly in kids after ALL treatment
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.
A 6-year prospective study from the Canadian STOPP investigators revealed that following treatment for acute lymphoblastic leukemia (ALL), approximately 1 out of 3 children experiences vertebral fractures (VF) and 1 out of 5 children shows non-VF.
Glucocorticoid use and vertebral fractures at diagnosis emerged as significant predictive factors.
Investigators reported a cumulative incidence of 32.5% for vertebral fractures (VF) following treatment and 23.0% for non-VF after 6 years.
Thirty-nine percent of children with VF were asymptomatic.
“The osteotoxicity of leukemia and its treatment are underscored by the observation that the non-VF incidence was more than 2.5 times higher than the general pediatric population,” the study authors wrote, “whereas the VF incidence was increased more than 2000 times.”
The STOPP investigators—the Steroid-Associated Osteoporosis in the Pediatric Population Consortium—published their findings in the Journal of Bone and Mineral Research.
To document the incidence and predictors of fractures and profile who is at greatest risk, the STOPP investigators enrolled 186 children who were diagnosed and treated at 10 children’s hospitals across Canada between 2005 and 2007.
Median age at diagnosis was 5.3 years, median duration of follow-up was 6 years, and 38.1% were boys.
Approximately 4 out of 5 children were treated with Children’s Oncology Group protocols and 1 out of 5 was treated with the Dana-Farber protocols.
In girls, glucocorticoid exposure was 7.0 g/m2 and in boys it was 8.9 g/m2.
The entire cohort received 54.3% of the total cumulative glucocorticoid dose in the first year and 83.1% in the second year.
For VF, incidence at baseline was 15.1%. Skeletal fractures at any site were reported in 36% of the children over the 6-year period, 71% occurring during the first 2 years. No VF was reported in the sixth year.
Of 38 children with VF, 32 sustained at least 1 VF in the first 2 years and 77% occurred while on therapy or within 6 months of the last glucocorticoid dose. None of the children had disease relapse.
Non-VF were reported in 1.6% of children at baseline; incidence peaked at years 2 (5.4%) and 5 (4.8%) and none occurred in the sixth year.
Thirty-one non-VFs were reported in 26 children; 18 occurred in the first 2 years and 23 occurred on therapy or within 6 months of the last glucocorticoid dose.
The STOPP investigators showed that glucocorticoid exposure, VF, and low spine bone mineral density Z-scores at baseline were significant predictors for risk of VF.
“[This] observation suggests the importance of baseline spine phenotype (both VF and low lumbar spine bone mineral density) in signaling the potential for future bone morbidity,” the STOPP investigators note.
“In revealing that vertebral fractures are frequent in children with ALL on chemotherapy, and that older children and those with more severe collapse are at risk for residual vertebral deformities, strategies to prevent vertebral fractures in those at greatest risk for permanent sequelae now merit further study,” said lead author Leanne Ward, MD, of the University of Ottawa, Canada, in a statement.
The study was primarily supported by an operating grant from the Canadian Institutes for Health Research (CIHR) with additional funding from a CIHR New Investigator Award, a Canadian Child Health Clinician Scientist Career Enhancement Award, a University of Ottawa Research Chair Award, a Children’s Hospital of Eastern Ontario (CHEO) Research Institute Capacity Building Award, and the CHEO Departments of Pediatrics and Surgery. This work was also supported by the University of Alberta Women and Children’s Health Research Institute.
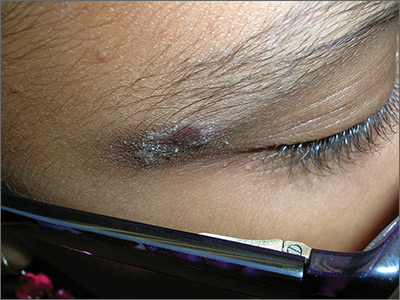
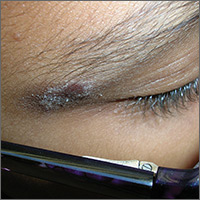
Rash on eyebrows
The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.
The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.
The FP recognized that this was a case of allergic contact dermatitis (ACD), based on the clinical presentation. The distribution of the erythema, scale, and post-inflammatory hyperpigmentation was highly suggestive of an ACD to nickel. In this case, the nickel in the patient’s eyeglasses and the snaps on her pants were the culprit. The plaque near the patient’s eye was actually in the shape of the metal on the inside of her glasses.
ACD is a delayed-type hypersensitivity reaction in which a foreign substance comes into contact with the skin and is linked to skin proteins; this forms an antigen complex, leading to sensitization. When the epidermis is re-exposed to the antigen, the sensitized T cells initiate an inflammatory cascade, leading to the skin changes seen in ACD.
Contact dermatitis can sometimes be diagnosed clinically with a good history and physical exam. However, there are many cases in which patch testing is needed to find the offending allergens or confirm the suspicion regarding a specific allergen. The differential diagnosis includes cutaneous candidiasis, impetigo, plaque psoriasis, and seborrheic dermatitis.
Patients with ACD should avoid the allergen that is causing the reaction. In cases of nickel ACD, the patient may cover the metal tab of his or her jeans with an iron-on patch or a few coats of clear nail polish. Fortunately, some jeans manufacturers now make nickel-free metal tabs (eg, Levis’s).
Cool compresses can soothe the symptoms of acute cases of ACD. Localized acute ACD lesions respond best to mid-potency (eg, 0.1% triamcinolone) to high-potency (eg, 0.05% clobetasol) topical steroids. On areas of thinner skin, lower-potency steroids such as desonide ointment can minimize the risk of skin atrophy.
In this case, the FP recommended that the patient get glasses that were nickel free and explained how to avoid the nickel that still exists in some pants. She was also given desonide 0.05% cream to apply to the affected area for symptomatic relief.
Adapted from: Usatine RP, Jacob SE. Rash on eyebrows and periumbilical region. J Fam Pract. 2017;66:45-47.
FDA expands Xeljanz approval to certain adults with ulcerative colitis
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
In two 8-week placebo-controlled trials, 10 mg of Xeljanz given twice daily induced remissions in 17%-18% of patients. In a placebo-controlled trial among the patients who responded by week 8, Xeljanz, at a 5-mg or 10-mg dose given twice daily, was effective in inducing remission by week 52 in 34% and 41% of patients, respectively. Additionally, 35% and 47% of those patients sustained corticosteroid-free remissions when treated with 5-mg and 10-mg doses, respectively.
“New treatments are needed for patients with moderately to severely active ulcerative colitis,” said Julie Beitz, MD, director of the Office of Drug Evaluation III in FDA’s Center for Drug Evaluation and Research in a press release. “Today’s approval provides an alternative therapy for a debilitating disease with limited treatment options.”
Xeljanz is the first oral medication approved for chronic use in moderately to severely active UC. The FDA states that other FDA-approved treatments for the chronic treatment of moderately to severely active ulcerative colitis must be administered through an intravenous infusion or subcutaneous injection.
Xeljanz, made by Pfizer Labs, was previously approved in 2012 for rheumatoid arthritis and in 2017 for psoriatic arthritis.
Find the full press release on the FDA’s website.
FDA approves estradiol vaginal inserts for dyspareunia*
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
dwatson@mdedge.com
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
dwatson@mdedge.com
, in a 4-mcg and 10-mcg dose. The 4-mcg dose is a lower dose than any currently available.
The hormone treatment is intended for dyspareunia resulting from menopausal vulvar and vaginal atrophy. The patient places a soft gel capsule in the lower part of the vagina, daily for 2 weeks, then at a reduced rate of twice per week. The capsule dissolves and reintroduces estrogen to the tissue.
The manufacturer of the product, TherapeuticsMD, has committed to conduct a postapproval observational study, as a condition of approval.
Estradiol comes with a boxed warning of risks of endometrial cancer, stroke, deep vein thrombosis, pulmonary embolism, myocardial infarction, breast cancer, and “probable dementia.” The most common adverse reaction to the estradiol vaginal inserts was headache.
Editor's Note: This article has been corrected to state that the 4-mcg dose showed significant improvement in severity of dyspareunia.
dwatson@mdedge.com
iPad app puts cognitive screening in the hands of MS patients
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
NASHVILLE, TENN. – A new computer tablet application puts cognitive screening literally in the hands of patients with multiple sclerosis.
The Multiple Sclerosis Performance Test (MSPT), created specifically for iPad, presents patients with four assessments that they can complete in a short time before any clinic visit, according to Stephen M. Rao, PhD, who helped develop the tool. After patients complete the test battery, the program translates their results into adjusted normative data and feeds them directly into the individual electronic medical record. When the clinic visit begins, everything is ready for the physician and patient to review together. The program not only provides a solid baseline assessment, but can also, over time, create a longitudinal profile of a patient’s cognitive status, and help to guide management decisions, Dr. Rao said at the annual meeting of the Consortium of Multiple Sclerosis Centers.
“About half of people with MS do have cognitive problems, which, above and beyond the physical problems, can result in major challenges with work, the ability to engage in social activities, and the need for personal assistance,” said Dr. Rao, who is the Ralph and Luci Schey Chair and director of the Schey Center for Cognitive Neuroimaging at the Cleveland Clinic. “But despite that, even comprehensive MS care centers rarely screen for cognitive dysfunction using objective neuropsychological tests.”
Time is the issue for most clinics, he said. Although the paper-and-pencil screening tools out there take only 10 minutes or so, most centers don’t have the luxury of carving out those extra moments or dedicating a staff member to administer the test and handle the data.
The MSPT attempts to sidestep the problem of time and manpower. In Dr. Rao’s center and the other 10 in the United States and Europe that now use the tool, patients simply arrive a bit early for their appointment and complete the three components: a structured patient history; the Neurological Quality of Life assessment; and an electronic adaptation of the MS Functional Composite.
It assesses cognition with a processing speed test based on the Symbol Digit Modalities Test, which has long been validated for MS patients. A contrast sensitivity test assesses visual acuity. A simple manual-dexterity test, in which patients move peg symbols into “holes,” tests upper extremity function, and a video-recorded walking speed test assesses lower extremity function.
The system was validated in 165 patients with MS and 217 healthy controls. It correlated well with the paper-and-pencil Symbol Digits Modalities Test, and correlated more highly than that test with cerebral T2 lesion load (MS Journal. 2017;23:1929-37).
The MSPT is part of a Biogen-sponsored project that Dr. Rao and colleagues unveiled at the American Academy of Neurology annual meeting in April, called the Multiple Sclerosis Partners Advancing Technology and Health Solutions (MS PATHS). It will gather longitudinal data on 11,000 patients using the MSPT program, and correlate it to multiple clinical and socioeconomic outcomes, Dr. Rao said.
The processing speed test portion of MS PATHS isn’t only available to PATHS centers, he added. Any clinician can obtain it by simply registering with Biogen and downloading the standalone version, which is called CogEval.
After downloading, the clinician must register with Biogen, which then will email a code to unlock the program. CogEval can be used on any iPad system that runs iOS 11 or higher. Results don’t get uploaded automatically into an EHR, but they can be entered manually or printed.
Dr. Rao disclosed that he received financial support from Biogen for the research and development of the MSPT program.
SOURCE: Rao SM et al. CMSC 2018. doi: 10.1177/1352458516688955
REPORTING FROM THE CMSC ANNUAL MEETING
Tinea Incognito in a Tattoo
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
To the Editor:
Tinea incognito occurs when superficial fungal infections fail to demonstrate typical clinical features in the setting of immune suppression caused by topical or systemic steroids.1,2 A case of tinea corporis obscured by an allergic tattoo reaction is presented.
A 52-year-old man presented for evaluation of a rash overlying a tattoo on the right calf of 3 weeks’ duration (Figure, A). The tattoo was placed 4 years prior to presentation. Within 6 months of the tattoo’s placement, pruritus, scaling, and edema developed in a 2-mm rim around the outer border and in the eyes of the elephant tattoo but not in the lettering portion of the tattoo, which was added by a different tattoo artist with a different red dye. A diagnosis of red dye tattoo allergic reaction was made. Daily treatment with tacrolimus ointment 0.1% and halobetasol propionate cream 0.05% under occlusion for 18 months provided only partial relief of incessant pruritus. Three months prior to presentation the tattoo reaction appeared to become worse with more pruritus and extension outside the bounds of the original tattoo.

Physical examination revealed the red rim of the tattoo was erythematous, edematous, and crusted. In addition, a 5×4-cm well-demarcated, erythematous, scaling patch was present overlying the elephant tattoo on the right calf and extending superiorly and laterally away from the tattoo. Scaling and maceration also were present in the web spaces between the fourth and fifth toes, and the toenails were yellowed, thickened, and dystrophic with signs of distal onycholysis. A potassium hydroxide preparation performed from the plaque on the right calf demonstrated septate fungal hyphae.
The diagnosis of tinea corporis secondary to tinea pedis overlying a red dye tattoo allergic reaction was made. Tacrolimus and halobetasol propionate were discontinued and treatment with ketoconazole cream 2% twice daily and oral terbinafine 250 mg once daily was started. The erythematous patch beyond the borders of the tattoo cleared within weeks, but the patient reported worsening of cracking, itching, and swelling overlying the red dye in the rim of the tattoo following discontinuation of topical anti-inflammatory drugs (Figure, B).
A potassium hydroxide preparation demonstrated that the expansible rash was tinea corporis disguised in its character by the coloration of the tattoo; the erythematous, edematous, pruritic tattoo allergic reaction at its rim; and suppression of the normal inflammatory response by daily use of a topical steroid and a calcineurin inhibitor. The latter effect (an immunocompromised district) impacts the classic exaggerated scaling, inflamed rim, and central clearing of tinea corporis present in individuals with a normal inflammatory response.2 Although tinea incognito is classically described on the ankles and lower legs of patients with stasis dermatitis chronically treated with topical steroids, it could occur anywhere in the setting of immunosuppression.3
An analysis of this case using Occam’s razor suggests that the association of this tattoo and tinea was not a coincidence. This guiding principle (heuristic) suggests that economy and succinctness in the logic of science is most likely to produce a correct medical diagnosis (eg, associated findings can be explained by identifying one underlying cause).4 The topical anti-inflammatory drugs increase the likelihood that the patient’s interdigital tinea would spread to this precise location symmetrically expanding in the outline of the tattoo.2
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
- Gathings RM, Abide JM, Brodell RT. An unusual inflammatory rash. JAMA Pediatr. 2014;168:185-186.
- Ruocco V, Brunetti G, Puca RV, et al. The immunocompromised district: a unifying concept for lymphoedematous, herpes-infected and otherwise damaged sites. J Eur Acad Dermatol Venereol. 2009;23:1364-1373.
- Romano C, Maritati E, Gianni C. Tinea incognito in Italy: a 15-year survey. Mycoses. 2006;49:383-387.
- Jefferys WH, Berger JO. Ockham’s razor and Bayesian analysis. American Scientist. 1992;80:64-72.
Practice Points
- Health care providers should have a low threshold to perform a potassium hydroxide preparation when the possibility of a superficial fungal infection is considered.
- Tinea incognito occurs when a superficial fungal infection has unusual clinical features in the setting of local immune suppression.
Dr Jame Abraham's top ASCO selections in breast cancer
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
Jame Abraham, MD, FACP, an Editor on The Journal of Community and Supportive Oncology, shares his top selections in breast cancer from this year's annual meeting of the American Society of Clinical Oncology in Chicago.
1001 Efficacy of sacituzumab govitecan (anti-Trop-2-SN-38 antibody-drug conjugate) for treatment-refractory hormone-receptor positive (HR+)/HER2- metastatic breast cancer (mBC) (Aditya Bardia et al). The study drug was well tolerated and produced objective responses in this heavily pretreated population, with an overall response rate of 31% at 6 months and a clinical benefit rate of 48%.
LBA1 TAILORx: Phase III trial of chemoendocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2-negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score (Joseph A Sparano et al)
506 PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): randomised phase 3 non-inferiority trial with definitive 4-year (yr) disease-free survival (DFS) results (Helena Margaret Earl et al). Six months of trastuzumab was found to be noninferior to 12 months, although cardiac events were reduced in the 6-month group compared with the 12-month group (4% vs 8% of patients, respectively, ended treatment because of cardiotoxicity).
In addition, Dr David Henry, the Editor-in-Chief of JCSO, also selected:
500 Adjuvant denosumab in early breast cancer: disease-free survival analysis of postmenopausal patients in the ABCSG-18 trial (Michael Gnant et al). In this double-blind placebo controlled trial, disease-free survival in the denosumab group was 89% at 5 years and 80% at 8 years, compared with 87% and 77%, respectively, for placebo.
AAN Publishes Practice Guideline on DMTs for MS
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.
LOS ANGELES—Clinicians must engage in an ongoing dialogue with patients with multiple sclerosis (MS) regarding treatment decisions throughout the disease course, according to a new practice guideline from the American Academy of Ne
When considering therapy for a patient with MS, clinicians must take into account the patient’s preferences regarding safety, route of administration, lifestyle, cost, efficacy, common adverse events, and tolerability, the guideline says.
The guideline—which includes 30 recommendations related to starting, switching, and stopping DMTs—“emphasizes that you need to have that discussion with patients, but it does not say for an individual which drug you should take,” said guideline author Ruth Ann Marrie, MD, PhD, Professor of Neurology and Community Health Sciences at the University of Manitoba in Winnipeg. “It provides information about the available therapies so that each clinician–patient dyad can use information specific to that patient to make the best decision for that particular scenario.”
The guideline was presented at the 70th Annual Meeting of the AAN and published in the April 24 issue of Neurology. It was endorsed by the MS Association of America and the National MS Society.
Therapeutic Advances
Neurologists have “little evidence to decide which drug to use at what time,” said lead guideline author Alexander Rae-Grant, MD, Professor of Neurology at the Cleveland Clinic.
The previous AAN clinical practice guideline on DMTs in MS, published in 2002, reviewed the injectable medications that were approved for MS at the time, such as interferon beta-1b, interferon beta-1a, and glatiramer acetate. Since then, “the treatment landscape has changed considerably … with more than 17 medications currently approved and widely prescribed for treating MS in the United States,” the authors said. “As a result, clinicians and people with MS may now choose from several medications with differing mechanisms of action, risk profiles, and monitoring requirements.” Clinicians must balance the potential therapeutic benefits and risks of a medication and the long-term risk of MS-related morbidity.
Since the 2002 guideline was published, studies have evaluated the treatment of patients with a first episode of demyelination, Dr. Rae-Grant said. “We now know that treating earlier is better, in terms of reducing the number of new relapses,” which reduces the risk of further brain injury, he said.
For people with highly active MS, clinicians should prescribe alemtuzumab, fingolimod, or natalizumab (Level B recommendation), according to the guideline. In addition, the guideline discusses pregnancy planning, setting realistic treatment expectations, and managing the risk of progressive multifocal leukoencephalopathy (PML). It mentions off-label options and support programs for people who are unable to afford approved DMTs.
Methods and Limitations
To assess evidence for starting, switching, and stopping DMTs for MS and develop relevant recommendations, the guideline authors surveyed peer-reviewed research articles, systematic reviews, and abstracts that they identified searching MEDLINE, CENTRAL, and EMBASE through November 2016. They selected 20 Cochrane reviews and 73 articles for data extraction and rated the studies using the AAN therapeutic classification of evidence scheme. The authors made recommendations using a modified Delphi process.
The guideline largely is based on trial data, which may limit the generalizability of the results to real-world populations, the authors noted. In addition, they anticipate that new research in the rapidly changing field of MS treatment will necessitate updates to the recommendations.
Future studies of DMTs for MS should address gaps in knowledge about long-term outcomes, safety in patients with comorbidities, predictive markers for patient response to treatment, treatment strategies for the initial management of MS, DMT use in pregnant women, and outcomes after stopping DMTs, according to the guideline. Neurologists also need evidence about the effect of DMTs on measures that are important to patients, but are not standard trial outcomes, such as cognition, fatigue, urinary urgency, pain, and visual function.
Navigating a Complex Landscape
The recommendations and systematic review of the evidence “reflect the complexity of MS management in the current treatment era,” said Tanuja Chitnis, MD, of Partners MS Center at Brigham and Women’s Hospital in Boston, and colleagues, in an accompanying editorial. “These statements serve as guidelines for MS patient care; however, they do not replace the clinician–patient relationship on which the most informed decision rests.”
The summary of more than 50 trials “provides a useful scale that can inform clinical decisions,” Dr. Chitnis and colleagues said. Certain drugs that were found to have limited evidence in the systematic review nevertheless “are in common clinical use … based on scientific principles and clinical acumen,” they said. In addition, the guideline reviews the evidence for combination therapies that rarely are used in clinical practice, which “may confuse the casual reader,” the editorialists noted.
“The experienced MS clinician … is needed to help guide patients through this complex landscape,” Dr. Chitnis and colleagues said. “The revised AAN guidelines are a starting point for the use of the multiple treatments now available for MS; however, further work is needed to further refine the choices appropriate for the individual patient.”
—Jake Remaly
Suggested Reading
Chitnis T, Giovannoni G, Trojano M. Complexity of MS management in the current treatment era. Neurology. 2018;90(17):761-762.
Corboy JR, Weinshenker BG, Wingerchuk DM. Comment on 2018 American Academy of Neurology guidelines on disease-modifying therapies in MS. Neurology. 2018 Apr 23 [Epub ahead of print].
Rae-Grant A, Day GS, Marrie RA, et al. Comprehensive systematic review summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):789-800.
Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis. Neurology. 2018;90(17):777-788.