User login
Cancer clinics continue to close and consolidate
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
and increased costs in the process, according to the Community Oncology Alliance.
From 2008 to 2018, a total of 1,653 oncology clinics and/or practices were affected in the following ways: 423 clinics closed, 658 practices were acquired by hospitals, 168 practices merged or were acquired by a corporate entity, 359 practices reported that they were struggling financially, and 45 practices sent patients elsewhere for chemotherapy, the COA said its 2018 Practice Impact Report.
“The shifting and shrinking community cancer care system reduces access to cancer care, inflates spending at the more expensive hospital setting, and is a disservice to patients, their caregivers, and support networks,” Jeff Vacirca, MD, president of COA said in a written statement.
Crunching the numbers another way shows that 13.8 practices a month have closed, been acquired by hospitals, or undergone mergers since 2008. The number of community clinics that have closed increased by 11.3% since the 2016 Practice Impact Report, and consolidations with hospitals rose by 8%. Practices with financial struggles were down by 7.9% from 2016, “which is proportional to the number of practices that have been acquired or moved into the hospital setting,” the COA said in the 2018 report.
“No one can look at [recent] trends and say that there hasn’t been a clear and negative dismantling of our cancer care system over the last decade,” Ted Okon, executive director of COA, said in the statement accompanying the report. “This situation is the direct result of the misguided 340B [drug discount program] and [the 2013] sequester cut allowed to take place by our elected officials in Washington. The pressures of these misguided public policies have been a one-two punch, pushing and pulling community oncology practices to close, consolidate, or be acquired by hospitals, all at the expense of patients.”
More Older Patients Should be Included in Epilepsy Drug Trials
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
Too few older adults are included in antiepileptic drug trials according to a systematic review and meta-analysis that included 184 studies.
- In 1991 to 1992, the mean age of patients included in clinical trials was 27 years, which had increased to about 42 years in 2015 to 2016.
- In 83 studies (44%), inclusion criteria included a maximum age limit.
- The requirement for limiting a participant’s maximum age did not decline significantly over time between the two time periods (r = 0.072, P=.816).
- The only disease-related exclusion criteria for entry into a clinical trial for antiepileptic drugs that was linked to a drop in the average age of enrolled patients were neurological conditions other than epilepsy.
Desmarais P, Miville C, Milán-Tomás A, et al. Age representation in antiepileptic drug trials: a systematic review and meta-analysis. Epilepsy Res. 2018;142:9-15.
New Definitions for NORSE and FIRES
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
An international team of experts has published standardized definitions of several seizure-related disorders, including new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related disorders.
- NORSE is described as a clinical presentation rather than a specific diagnosis.
- NORSE occurs in a patient who is not experiencing active epilepsy or any other preexisting neurological disorder.
- In NORSE, the patient does not have a clear acute or active structural, toxic or metabolic cause of their condition.
- The expert group defined FIRES as a subtype of NORSE that involves a prior febrile infection that started between 2 weeks and 24 hours before the refractory status epilepticus began.
- The experts also offered standardized definitions for infantile hemiconvulsion-hemiplegia and epilepsy syndrome (IHHE) and for prolonged, refractory and super-refractory status epilepticus.
Hirsch LJ, Gaspard N, van Baalen A, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59(4):739-744.
Understanding and Managing Sunflower Syndrome
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.
Sunflower syndrome, a type of reflex seizure usually accompanied by eyelid myoclonia, can be managed with valproate or polypharmacy suggests a recent study of affected patients managed by the Department of Neurology, Division of Child Neurology, Stanford University School of Medicine.
- Sunflower syndrome, a photosensitive epilepsy, is triggered when patients wave their hands in front of the sun.
- A review of 13 cases, occurring mostly in girls with an average age of onset of 5.5 years, found that many of these patients had intellectual, attentional, or academic problems.
- Most patients had eyelid myoclonia, with or without absence seizures, while six also had spontaneous seizures.
- Nine children received valproate, of which 66% experienced significant improvement or stopped having seizures.
- No patients taking levetiracetam or lamotrigine monotherapy saw their seizures resolve, but 3 patients did improve on polypharmacy.
Baumer FM, Porter BE. Clinical and electrographic features of sunflower syndrome. Epilepsy Res. 2018;142:58-63.
Take action to prevent maternal mortality
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.
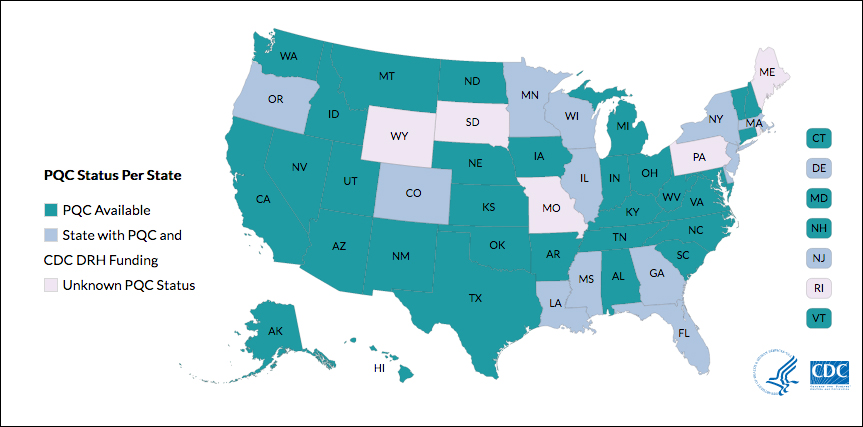
Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.

Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
The facts
While other industrialized nations are seeing a decrease in their maternal mortality rates, the United States has noted a 26% increase over a 15-year period. This is especially true for women of color: black women are nearly 4 times as likely to die from pregnancy related causes as compared to non-Hispanic white women. Postpartum hemorrhage and preeclampsia are often the leading causes of maternal death; however, suicide and overdoses are becoming increasingly more common. This information is highlighted in the March 2018 OBG Management article “Factors critical to reducing US maternal mortality and morbidity,” by Lucia DiVenere, MA, Government and Political Affairs, at the American College of Obstetricians and Gynecologists (ACOG).1
Although there are efforts to improve these outcomes, programs vary by state. One initiative is the perinatal quality collaboratives (PQCs), state or multistate networks of teams working to improve the quality of care for mothers and babies (see “Has your state established a perinatal quality collaborative?”).
Currently, only 33 states have a maternal mortality review committee (MMRC) comprised of an interdisciplinary team of ObGyns, nurses, and other stakeholders. The MMRC reviews each maternal death in their state and provides recommendations and policy changes to help prevent further loss of life.

Many states currently have active collaboratives, and others are in development. The CDC’s Division of Reproductive Health (DRH) currently provides support for state-based PQCs in Colorado, Delaware, Florida, Georgia, Illinois, Louisiana, Massachusetts, Minnesota, Mississippi, New Jersey, New York, Oregon, and Wisconsin. The status of PQCs in Maine, Rhode Island, Pennsylvania, Missouri, South Dakota, and Wyoming is unknown.1
The CDC can help people establish a collaborative. Visit: https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html.
Reference
- Centers for Disease Control and Prevention. Reproductive health: State Perinatal Quality Collaboratives. CDC website. https://www.cdc.gov/reproductivehealth/maternalinfanthealth/pqc-states.html Updated October 17, 2017. Accessed April 4, 2018.
The bill
Preventing Maternal Deaths Act/Maternal Health Accountability Act (H.R. 1318/S. 1112) is a bipartisan, bicameral effort to reduce maternal mortality and reduce health care disparities.
The bills authorize the Centers for Disease Control and Prevention (CDC) to help states create or expand state MMRCs through annual grant funding of $7 million through fiscal year 2022. Through the MMRCs, the CDC would have the ability to gather data on maternal mortality and health care disparities, allowing the agency to better understand leading causes of maternal death as well as a state’s successes and pitfalls in interventions.
Currently the House bill (H.R. 1318) has 102 cosponsors (https://cqrcengage.com/acog/app/bill/903056?0) and the Senate bill (S. 1112) has 17 cosponsors (https://cqrcengage.com/acog/app/bill/943204?1). Click these links to see if your representative is a cosponsor.
Not sure who your representative is? Click here to find out: http://cqrcengage.com/acog/app/lookup?1&m=29525.
Take action
Both the Senate and House bills have been referred to health committees. However, no advances have been made since March 2017. In order for the bills to move forward, your representatives need to hear from you.
If your representative is a cosponsor of the bill, thank them for their support, but also ask what we can do to ensure this bill becomes law.
If your representative is not a cosponsor, follow this link to email your representative: http://cqrcengage.com/acog/app/onestep-write-a-letter?0&engagementId=306574. You also can call your representative’s office and speak directly to a staff member.
When calling or emailing, highlight the following:
- I am an ObGyn and I am asking [your Representative/Senator] to support H.R. 1318 or S. 1112.
- While maternal mortality rates are decreasing in other parts of the world, they are increasing in the United States. We have the highest maternal mortality rate in the developing world.
- This bill gives all states the opportunity to have a maternal mortality review committee, allowing health care leaders to review each maternal death and analyze how further deaths can be prevented.
- Congress has invested in programs addressing infant mortality, birth defects, and preterm birth. It is time we put the same investment into saving our nation’s mothers.
- As an ObGyn, I urge you to support this bill.
More from ACOG
Want to know what other advocacy opportunities are available? Check out ACOG action at http://cqrcengage.com/acog/home?3.
Share your thoughts! Send your Letter to the Editor to rbarbieri@mdedge.com. Please include your name and the city and state in which you practice.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
- DiVenere L. Factors critical to reducing US maternal mortality and morbidity. OBG Manag. 2018;30(3):30−33.
Combined Cholesterol and Blood Pressure Lowering Treatment Reduces First Stroke Risk
LOS ANGELES—Among adults with an intermediate risk of cardiovascular disease, a combination of blood pressure and cholesterol lowering treatments significantly reduces the risk of a first stroke, compared with placebo, according to a study presented at the International Stroke Conference 2018. “Use of these well-tolerated and simple-to-implement therapies has the potential to reduce stroke by 44%,” said Jackie Bosch, PhD, Associate Professor at the School of Rehabilitation Science at McMaster University in Hamilton, Ontario.
“These results indicate that to prevent stroke in those at moderate risk, blood pressure lowering plus lipid lowering should be considered in those with elevated blood pressure, and lipid lowering should be considered by all,” said Dr. Bosch.
Seventy-five percent of strokes are first strokes, which often result in permanent disability or death, said Dr. Bosch. Blood pressure and cholesterol account for about two-thirds of stroke risk. Treatment for high blood pressure and high cholesterol is recommended for high-risk patients. Data for patients who are at moderate risk are lacking, however, she said.
The HOPE-3 Trial
To examine the role of antihypertensive therapy and statins for primary stroke prevention, Dr. Bosch and colleagues analyzed data from the Heart Outcomes Prevention Evaluation-3 (HOPE-3) trial. They sought to investigate whether a combination of cholesterol lowering and blood pressure lowering drugs safely reduces major cardiovascular events in individuals at intermediate risk who have had no previous vascular events.
Researchers recruited an intermediate stroke risk population with an estimated 1% risk of major cardiovascular events per year. The researchers included women age 65 and older and men age 55 and older with at least one additional risk factor (ie, increased waist-to-hip ratio, current or recent tobacco use, low HDL cholesterol, dysglycemia, mild renal dysfunction, or a family history of coronary heart disease). Patients with cardiovascular disease or an indication or contraindication for any of the medicines studied were excluded.
Researchers randomized 12,705 participants from 21 countries to receive either candesartan (16 mg) plus hydrochlorothiazide (12.5 mg) daily or placebo, as well as rosuvastatin (10 mg) daily or placebo.
The coprimary study outcomes were the composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke and the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, resuscitated cardiac arrest, heart failure, and arterial revascularization. The primary outcomes were published in 2016. The present analysis focused on stroke outcomes.
Combined Therapies Significantly Reduced Stroke Risk
The mean age of participants was 66, and 46% of participants were women. The mean baseline blood pressure was 138/82 mm Hg. In all, 166 strokes occurred during a median follow-up of 5.6 years.
Candesartan plus hydrochlorothiazide reduced stroke by 20%, compared with placebo, although this effect was not statistically significant. Rosuvastatin significantly reduced stroke by 30%, compared with placebo. In a prespecified subgroup analysis of participants in the upper third of systolic blood pressure (> 143.5 mm Hg), candesartan plus hydrochlorothiazide reduced stroke by 42%, versus placebo, said Dr. Bosch. The combination of cholesterol and blood pressure lowering treatments (rosuvastatin and candesartan plus hydrochlorothiazide) reduced stroke risk by 44% and reduced disabling stroke risk by 45%, compared with placebo. The number needed to treat with combination therapy for one year to prevent one stroke was 714.
Rates of permanent discontinuation did not differ significantly between active and placebo-assigned patients, said the researchers.
—Erica Tricarico
LOS ANGELES—Among adults with an intermediate risk of cardiovascular disease, a combination of blood pressure and cholesterol lowering treatments significantly reduces the risk of a first stroke, compared with placebo, according to a study presented at the International Stroke Conference 2018. “Use of these well-tolerated and simple-to-implement therapies has the potential to reduce stroke by 44%,” said Jackie Bosch, PhD, Associate Professor at the School of Rehabilitation Science at McMaster University in Hamilton, Ontario.
“These results indicate that to prevent stroke in those at moderate risk, blood pressure lowering plus lipid lowering should be considered in those with elevated blood pressure, and lipid lowering should be considered by all,” said Dr. Bosch.
Seventy-five percent of strokes are first strokes, which often result in permanent disability or death, said Dr. Bosch. Blood pressure and cholesterol account for about two-thirds of stroke risk. Treatment for high blood pressure and high cholesterol is recommended for high-risk patients. Data for patients who are at moderate risk are lacking, however, she said.
The HOPE-3 Trial
To examine the role of antihypertensive therapy and statins for primary stroke prevention, Dr. Bosch and colleagues analyzed data from the Heart Outcomes Prevention Evaluation-3 (HOPE-3) trial. They sought to investigate whether a combination of cholesterol lowering and blood pressure lowering drugs safely reduces major cardiovascular events in individuals at intermediate risk who have had no previous vascular events.
Researchers recruited an intermediate stroke risk population with an estimated 1% risk of major cardiovascular events per year. The researchers included women age 65 and older and men age 55 and older with at least one additional risk factor (ie, increased waist-to-hip ratio, current or recent tobacco use, low HDL cholesterol, dysglycemia, mild renal dysfunction, or a family history of coronary heart disease). Patients with cardiovascular disease or an indication or contraindication for any of the medicines studied were excluded.
Researchers randomized 12,705 participants from 21 countries to receive either candesartan (16 mg) plus hydrochlorothiazide (12.5 mg) daily or placebo, as well as rosuvastatin (10 mg) daily or placebo.
The coprimary study outcomes were the composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke and the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, resuscitated cardiac arrest, heart failure, and arterial revascularization. The primary outcomes were published in 2016. The present analysis focused on stroke outcomes.
Combined Therapies Significantly Reduced Stroke Risk
The mean age of participants was 66, and 46% of participants were women. The mean baseline blood pressure was 138/82 mm Hg. In all, 166 strokes occurred during a median follow-up of 5.6 years.
Candesartan plus hydrochlorothiazide reduced stroke by 20%, compared with placebo, although this effect was not statistically significant. Rosuvastatin significantly reduced stroke by 30%, compared with placebo. In a prespecified subgroup analysis of participants in the upper third of systolic blood pressure (> 143.5 mm Hg), candesartan plus hydrochlorothiazide reduced stroke by 42%, versus placebo, said Dr. Bosch. The combination of cholesterol and blood pressure lowering treatments (rosuvastatin and candesartan plus hydrochlorothiazide) reduced stroke risk by 44% and reduced disabling stroke risk by 45%, compared with placebo. The number needed to treat with combination therapy for one year to prevent one stroke was 714.
Rates of permanent discontinuation did not differ significantly between active and placebo-assigned patients, said the researchers.
—Erica Tricarico
LOS ANGELES—Among adults with an intermediate risk of cardiovascular disease, a combination of blood pressure and cholesterol lowering treatments significantly reduces the risk of a first stroke, compared with placebo, according to a study presented at the International Stroke Conference 2018. “Use of these well-tolerated and simple-to-implement therapies has the potential to reduce stroke by 44%,” said Jackie Bosch, PhD, Associate Professor at the School of Rehabilitation Science at McMaster University in Hamilton, Ontario.
“These results indicate that to prevent stroke in those at moderate risk, blood pressure lowering plus lipid lowering should be considered in those with elevated blood pressure, and lipid lowering should be considered by all,” said Dr. Bosch.
Seventy-five percent of strokes are first strokes, which often result in permanent disability or death, said Dr. Bosch. Blood pressure and cholesterol account for about two-thirds of stroke risk. Treatment for high blood pressure and high cholesterol is recommended for high-risk patients. Data for patients who are at moderate risk are lacking, however, she said.
The HOPE-3 Trial
To examine the role of antihypertensive therapy and statins for primary stroke prevention, Dr. Bosch and colleagues analyzed data from the Heart Outcomes Prevention Evaluation-3 (HOPE-3) trial. They sought to investigate whether a combination of cholesterol lowering and blood pressure lowering drugs safely reduces major cardiovascular events in individuals at intermediate risk who have had no previous vascular events.
Researchers recruited an intermediate stroke risk population with an estimated 1% risk of major cardiovascular events per year. The researchers included women age 65 and older and men age 55 and older with at least one additional risk factor (ie, increased waist-to-hip ratio, current or recent tobacco use, low HDL cholesterol, dysglycemia, mild renal dysfunction, or a family history of coronary heart disease). Patients with cardiovascular disease or an indication or contraindication for any of the medicines studied were excluded.
Researchers randomized 12,705 participants from 21 countries to receive either candesartan (16 mg) plus hydrochlorothiazide (12.5 mg) daily or placebo, as well as rosuvastatin (10 mg) daily or placebo.
The coprimary study outcomes were the composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke and the composite of cardiovascular death, nonfatal myocardial infarction, nonfatal stroke, resuscitated cardiac arrest, heart failure, and arterial revascularization. The primary outcomes were published in 2016. The present analysis focused on stroke outcomes.
Combined Therapies Significantly Reduced Stroke Risk
The mean age of participants was 66, and 46% of participants were women. The mean baseline blood pressure was 138/82 mm Hg. In all, 166 strokes occurred during a median follow-up of 5.6 years.
Candesartan plus hydrochlorothiazide reduced stroke by 20%, compared with placebo, although this effect was not statistically significant. Rosuvastatin significantly reduced stroke by 30%, compared with placebo. In a prespecified subgroup analysis of participants in the upper third of systolic blood pressure (> 143.5 mm Hg), candesartan plus hydrochlorothiazide reduced stroke by 42%, versus placebo, said Dr. Bosch. The combination of cholesterol and blood pressure lowering treatments (rosuvastatin and candesartan plus hydrochlorothiazide) reduced stroke risk by 44% and reduced disabling stroke risk by 45%, compared with placebo. The number needed to treat with combination therapy for one year to prevent one stroke was 714.
Rates of permanent discontinuation did not differ significantly between active and placebo-assigned patients, said the researchers.
—Erica Tricarico
OlympiAD: No statistically significant boost in OS with olaparib in HER2-negative mBC
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
CHICAGO – Median overall survival (OS) in patients with HER2-negative metastatic breast cancer (mBC) and germline BRCA mutation (gBRCAm), although not statistically significant, was 2.2 months longer with olaparib versus physician’s choice chemotherapy (TPC), according to the final analysis of the OlympiAD study.
The results suggested the possibility of greater benefit among chemotherapy naive patients for metastatic breast cancer, with no cumulative toxicity reported with extended exposure, Mark E. Robson, MD, said at the annual meeting of the American Association for Cancer Research.
OlympiAD was a randomized, controlled, open-label, multicenter, phase 3 study of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared TPC monotherapy (capecitabine, vinorelbine, or eribulin). Patients were stratified by prior chemotherapy, prior platinum, and receptor status (ER+ and/or PR+ vs. TNBC). Of 302 randomized patients, 205 received olaparib and 91 received TPC (6 TPC patients declined treatment). Eligible patients had HER2-negative mBC and a germline BRCA mutation. In addition, patients should have received less than or equal to two chemotherapy lines in the metastatic setting, with prior anthracycline and taxane treatment either as (neo)adjuvant therapy or in the metastatic setting.
The data presented at AACR was a follow-up on the primary progression-free survival (PFS) analysis, which demonstrated significant benefit in olaparib over standard chemotherapy TPC (7.0 vs 4.2 months, HR 0.58, 95% confidence interval, 0.43-0.80, P less than .001). Overall response rate (ORR) in the olaparib arm was double of that observed on the TPC arm in measurable disease patients (59.9% vs. 28.8%).
At the final OS analysis with 192 deaths, HR for OS in the olaparib vs TPC group was 0.90 (95% CI, 0.66-1.23; P = .513), reported Dr. Robson of Memorial Sloan Kettering Cancer Center, New York.
“The preplanned subgroup analyses according to the stratification factors were not powered to detect survival advantages, and were considered only hypothesis generating,” he said.
In patients who had not received chemotherapy in the metastatic setting, there was a median difference in OS of 7.9 months with olaparib (HR 0.51, 95% CI, 0.29-0.90; nominal P = .02; median 22.6 vs. 14.7 months).
Median follow-up for OS was 18.9 months for olaparib vs. 15.5 months in the TPC group.
No differences were observed between patients that were ER and/or PgR positive vs. TNBC, or whether patients received prior platinum, Dr. Robson said.
Grade 3 adverse events were similar to those in the primary analysis with no cumulative toxicity with extended exposure, he said.
SOURCE: Robson ME et al. AACR Annual Meeting Abstract CT038.
FROM THE AACR ANNUAL MEETING
Key clinical point: Median overall survival was not significantly different with olaparib versus chemotherapy in patients with BRCA-mutated, HER2-negative metastatic breast cancer.
Major finding: Median overall survival in patients with HER2-negative metastatic breast cancer and a germline BRCA mutation was 19.3 months versus 17.1 months for olaparib versus chemotherapy (HR 0.90 95% CI 0.66, 1.23; P = .513).
Study details: Randomized, controlled, open-label, phase 3 trial (OlympiAD) of olaparib tablet monotherapy (300 mg, twice daily) compared with predeclared physician’s choice chemotherapy (capecitabine, vinorelbine, or eribulin).
Disclosures: Dr. Robson disclosed relationships with AstraZeneca, AbbVie, McKesson, Myriad Genetics, and Medivation.
Source: Robson ME et al. AACR Annual Meeting Abstract CT038.
Distrust
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
The odds are that you are an employee. In 2016, for the first time ever, fewer than half of physicians in this country owned their own practice. There are numerous explanations for this shift away from independent ownership. But the bottom line is that more physicians are employees than owners (“For the first time, physician practice owners are not the majority,” By Brendan Murphy, AMA Wire, May 31, 2017). The transition to employee status doesn’t always go well.
While an increasing number of physicians are uninterested in or maybe even intimidated by the challenges of practice ownership, they seem to be even less interested in accepting the uncomfortable realities that can be associated with being an employee.
Practice ownership comes with a host of worries including cash flow, staffing, and overhead. On the other hand, an employee has only one critical concern: Can she trust her employer? You may not have considered your relationship with your employer in terms of trust. But I urge you to look at a recent commentary in Clinician Reviews by Randy D. Danielson, PhD, PA, DAAPA, titled, “Do You Trust Your Employer? (2018 Apr;28[4]:6-8). Dr. Danielson relates the experiences of a colleague who complains that the organization for which he worked completely lacked transparency of its goals and failed to provide accurate financial data. This combination of deficiencies prevented “providers from making a positive impact on cost containment.” The colleague added that the organization’s complex compensation formulas did “not account for the vagaries and complexities of health care.”
Do any of these complaints sound familiar to you? Do you share the same lack of trust in your employer that this provider has voiced? The remainder of Dr. Danielson’s commentary is a discussion of the concept of organizational trust and includes this unsurprising observation: “Lack of trust, particularly between management and employers, creates a hostile work environment in which stress levels are high and productivity is reduced.” It makes one wonder how much of the burnout epidemic among physicians and other providers might be the result of organizational distrust.
At what point in your career did you begin to lose trust in your employer? In retrospect, should you have been more diligent in researching its financial history? How did its acquisitions and reorganizations affect its employees? Did they reflect a pattern that is consistent with your philosophy about how and to whom health care should be delivered?
How carefully did you interview the organization’s employees? Did you sense any distrust? This kind of information doesn’t usually seep out in a 1-day visit and meetings with handpicked employees. Did employees feel that there was sufficient transparency? It is likely that they sat on committees. But did those committees have a voice that was heard and acted upon?
If you were going to purchase a practice you would have done hours, days, and weeks of due diligence before signing a purchase and sales agreement. Deciding whether or not to sign a contract with an employer demands an equivalent amount of research and investigation. You already may have discovered that being trapped by a noncompete clause with an organization you don’t trust can put you on the fast track to burnout.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@mdedge.com.
ERAS reduced opioid use, improved same-day discharge after gyn surgery
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
The ERAS pathway described by Dr. Carter-Brooks embraces the core tenets of enhanced recovery, including standardized patient education, multimodal analgesia, and predefined postoperative metrics, according to invited discussant Mark Walters, MD.
In fact, systematic culture change requires the involvement of surgeons, nurses, anesthesiologists, and administrative staff, Dr. Walters added.
“Additionally, such significant behavioral changes inevitably result in unintended consequences that must be carefully documented to learn how to mitigate harm in future patients,” he said.
Dr. Walters is professor and vice chair of gynecology in the Center of Urogynecology and Reconstructive Pelvic Surgery, department of obstetrics and gynecology at the Cleveland Clinic. He is a consultant and teacher for Coloplast.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
ORLANDO – The implementation of enhanced recovery after surgery (ERAS) pathways increased same-day discharge rates, but also was associated with a slight increase in readmissions within 30 days, according to a retrospective review of urogynecology cases at a single institution.
ERAS implementation also decreased total opioid use and pain scores, increased preemptive antiemetic use, and reduced rescue antiemetic needs in the postanesthesia care unit, Charelle M. Carter-Brooks, MD, reported at the annual scientific meeting of the Society of Gynecologic Surgeons.
In a separate study at an urban safety-net hospital, ERAS implementation was feasible and rapidly accomplished, and resulted in a number of improved outcomes among gynecologic surgery patients, including reduced intraoperative opioid and intravenous fluid use, reduced postoperative intravenous opioid use, and shorter Foley catheter duration – all without an increase in total adverse events.
In the first study, same-day discharge rates were 91.7% in 137 women who underwent urogynecologic surgery after ERAS implementation vs. 25.9% in 121 patients who underwent surgery before ERAS implementation, and average length of admission decreased by 17.4 hours (27.7 vs. 10.3 hours), Dr. Carter-Brooks of Magee-Womens Hospital, University of Pittsburgh Medical Center, reported in an oral paper presentation.
Operative time and postsurgical recovery room times were similar before and after ERAS implementation, but earlier discharge in the ERAS group was associated with about a 5% increase in readmission rates within 30 days (readmission rates of 1.5% and 6.7% before and after implementation), she noted.
Other outcomes, including postoperative complications, urinary tract infections, emergency room visits, unanticipated office visits, and returns to the operating room were similar in the two groups, she said.
After adjusting for age, body mass index, comorbidities, and operative time, length of stay decreased by 13.6 hours after ERAS implementation; after adjusting for age and operative time, same-day discharge was 32 times more likely after ERAS implementation; and after adjusting for age, operative time, and prolapse surgery type, readmission was 5.7 times more likely after ERAS implementation, she said.
In a survey of 77 post-ERAS implementation patients conducted during postoperative nursing calls, 86.7%, 89.6%, and 93.5% reported very good or excellent pain control, surgery preparedness, and overall surgical experience, respectively, and 90% said they did not recall experiencing postoperative nausea during recovery, she added.
In a poster presented at the meeting, Dr. Carter-Brooks further noted that there was a 69% reduction in overall opioid use in the patients who underwent surgery after ERAS implementation, as well as a doubling in the median number of preemptive antiemetic doses (4 vs. 2) and a significant reduction in the percentage of patients receiving a rescue antiemetic after implementation (21.6% vs. 13.6%).
Patients included in the study were women with a mean age of 65.5 years and mean body mass index of 28.2 kg/m2. The most common preoperative diagnosis (in 93.8% of patients) was prolapse. Apical suspension procedures performed were transvaginal in 58 cases, laparoscopic or robotic in 112, and obliterative in 61. Most patients had a hysterectomy, including 83 laparoscopic or robotic, 64 transvaginal, and 1 combined procedure. Demographic and surgical procedures did not differ significantly in the pre- and post-ERAS groups, Dr. Carter-Brooks noted.
Surgeries were performed by seven different surgeons either before ERAS implementation (Jan. 1 to June 30, 2016) or after implementation (Feb. 2 to July 31, 2017).
ERAS – a multidisciplinary approach to patient perioperative care – involves implementation of evidence-based interventions to improve early discharge and length of stay in patients undergoing major elective surgery.
ERAS pathways, which are commonly used in colorectal surgery, were developed to hasten postoperative recovery and are now being increasingly adopted for gynecologic procedures, but data focusing on outcomes with ERAS in the prolapse repair setting are limited, Dr. Carter-Brooks noted.
The ERAS pathway in her study involved a preoperative optimization phase that included counseling about tobacco and alcohol cessation, education about ERAS pathway expectations, and recommendations regarding diet and exercise 1-2 weeks prior to surgery. On the day of surgery, the pathway involved a multimodal pain regimen and postoperative nausea and vomiting prevention.
In response to discussion questions about which interventions contributed most to improvements in same-day discharge rates and patient satisfaction, which interventions were most difficult to implement, and whether additional interventions could prevent readmissions, Dr. Carter-Brooks said that, in her experience the multimodal focus on pain and nausea/vomiting prevention has been particularly helpful, as has the emphasis on educating patients about the interventions and expectations.
“For the preoperative appointment we really spend about 15-30 minutes on education and expectations and prepare the patient to go home. We also encourage them to be advocates and stakeholders in their own recovery, and ... we think that has significantly improved our patients wanting to go home the day of surgery,” she said.
The most difficult aspect of implementation was changing the culture in the hospital, she added.
Support of leadership team members who advocated for change was key to achieving that. Regular audits to review outcomes and make changes as needed to achieve the intended benefit were also important, she noted.
As for readmissions, the numbers overall were small, and their relation to ERAS is questionable and something that is still being tracked and assessed, she said.
In the second study, early outcomes after ERAS implementation were encouraging. Compared with 96 patients who underwent gynecologic surgery between June 1 and Aug. 31, 2015 (before ERAS implementation), 65 who underwent surgery afterward (between February and April 2017) had decreased intraoperative opioid use in open surgery (95 mg vs. 115 mg) and in minimally invasive surgery (75 mg vs. 95 mg), as well as decreased intravenous opioid use postoperatively for open surgery (44% vs. 71%), Mary Louise Fowler, a 4th-year medical student at Boston University, reported at the conference.
The ERAS patients also had shorter Foley catheter duration for minimally invasive surgery (16 vs. 2.3 hours), and they had a trend toward decreased intraoperative fluids for minimally invasive surgery (3.3 vs. 4.2 mL/kg per hour), Ms. Fowler said.
“We also found that there was no significant difference in the length of stay and postdischarge 3-day adverse outcomes,” she said.
The multidisciplinary consensus-based ERAS pathway developed at her institution was implemented beginning Feb. 1, 2017, in response to the national call to reduce opioid use, she explained, noting that a predetermined 4-month time line facilitated implementation by the target date.
Eligible patients included those undergoing benign or oncologic gynecologic surgery with a planned overnight stay.
“Preliminary positive outcomes have been found [with ERAS] at our urban safety-net hospital, specifically in looking at decreased opioid use without a resultant total adverse event increase. It is important for us to continue to monitor ERAS in terms of long-term care to ensure adherence, safety, and effectiveness,” she said, adding that tracking of outcomes will continue, and a future goal is to assess impacts on cost.
Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
SOURCES: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
REPORTING FROM SGS 2018
Key clinical point: ERAS pathways improve same-day discharge rates and reduce opioid use in gynecologic surgery.
Major finding: Same-day discharge rates before and after ERAS were 25.9% and 91.7%, respectively.
Study details: A retrospective review of 258 patients; a study of 161 patients.
Disclosures: Dr. Carter-Brooks’s study was supported by a National Institutes of Health grant. She and Ms. Fowler each reported having no disclosures.
Sources: Carter-Brooks C et al.; Fowler M et al. SGS 2018, Oral presentation 2; Oral posters 1 and 16.
Apixaban prevails in study of 163,000 DOAC users
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
ORLANDO – Apixaban outperformed both rivaroxaban and dabigatran in a retrospective, observational study of real-world prescribing of direct oral anticoagulants in nearly 163,000 U.S. patients with nonvalvular atrial fibrillation, Steven B. Deitelzweig, MD, reported at the annual meeting of the American College of Cardiology.
This ongoing study, known as ARISTOPHANES (Anticoagulants for Reduction in Stroke: Observational Pooled Analysis on Health Outcomes and Experience of Patients), is the largest real-world analysis of direct oral anticoagulants (DOACs) to date. Unlike most of the previous observational studies of DOACs, which used a single insurance claims database, ARISTOPHANES pools data from Medicare and four large U.S. commercial insurance claims databases that collectively cover more than 180 million Americans.
This was a study of real-world prescribing. Unlike in randomized trials, where everyone is on a standard-dose DOAC, lower-dose therapy was common. It was prescribed for 21% of patients on apixaban, 15% on dabigatran, and 24% on rivaroxaban.
ARISTOPHANES results
The biggest difference in outcome was between patients on apixaban and those on dabigatran. The incidence of stroke/systemic embolism in 27,096 patients on apixaban (Eliquis) was 1.01% in 360 days, for a statistically significant 31% reduction in risk relative to the 1.42% incidence rate in 27,096 extensively matched patients on dabigatran (Pradaxa). The 360-day incidence of major bleeding was 2.7% in the apixaban group and 3.3% in those on dabigatran, for a 23% relative risk reduction. In the apixaban/rivaroxaban comparison, which included 62,619 patients in each group, the incidence of stroke/systemic embolism was 1.21% with apixaban and 1.42% with rivaroxaban, for a 27% relative risk reduction. Major bleeding occurred in 3.1% of the apixaban group and 5.3% of those on rivaroxaban, for a 46% reduction in risk favoring apixaban.
In a comparison of 27,538 patients on dabigatran and an equal number of rivaroxaban, the 360-day cumulative incidence of stroke/systemic embolism was 1.40% with dabigatran versus 1.23% with rivaroxaban, while the major bleeding rate was 3.28% in the dabigatran group and 4.76% with rivaroxaban.
Dr. Deitelzweig was quick to acknowledge the major limitation of ARISTOPHANES.
“Only associations can be drawn from a nonrandomized, retrospective, observational study, not conclusions regarding causality, even though the cohorts were matched using propensity scoring,” he emphasized.
The critique
Session cochair Jeanne E. Poole, MD, professor of medicine and director of the clinical cardiac electrophysiology program at the University of Washington, Seattle, commented, “The problem, of course, with using these large databases is that you may not be able to find out important information, such as whether rivaroxaban was being taken appropriately with meals, which is frequently not the case. If it wasn’t, that decreases absorption and efficacy by 40%. That’s a limitation.”
Audience member James A. Reiffel, MD, rose to add that, in his view, another significant limitation of all real-world, observational analyses using claims data is that it’s not possible to know why physicians selected a given drug for a given patient. He used as an example a patient with atrial fibrillation and gastroesophageal reflux disease (GERD).
“People with GERD may be less likely to get dabigatran, and if they’re less likely to get dabigatran with GERD, maybe they’re less likely to get a GI bleed. I don’t know,” said Dr. Reiffel, professor of clinical medicine and director of the electrocardiography laboratory at Columbia University Medical Center, New York.
“We have to take all the real-world analyses with a little grain of salt,” he added.
Dr. Deitelzweig replied, “This study is not meant to be the be-all and end-all.”
That being said, he noted that although randomized trials have shown that DOACs are at least as effective and safe as warfarin for stroke prevention in atrial fibrillation, there have been no randomized, head-to-head clinical trials comparing them, nor are any such studies likely to be done for the foreseeable future. Yet physicians and their patients are hungry for comparative effectiveness data, even if it doesn’t rise to the status of level I evidence.
ARISTOPHANES is sponsored by Bristol-Myers Squibb and Pfizer. Dr. Deitelzweig reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
SOURCE: Deitelzweig SB. ACC 2018.
REPORTING FROM ACC 2018
Key clinical point:
Major finding: The risk of stroke/systemic embolism in apixaban-treated patients was 31% lower than in those on dabigatran and 27% lower than with rivaroxaban.
Study details: This retrospective, observational study based upon claims data included 162,707 propensity score-matched patients with atrial fibrillation on a direct oral anticoagulant for stroke prevention.
Disclosures: The ongoing ARISTOPHANES study is sponsored by Bristol-Myers Squibb and Pfizer. The presenter reported serving as a consultant to Pfizer and receiving research grants from Bristol-Myers Squibb and Portola.
Source: Deitelzweig SB. ACC 2018.