User login
Esketamine nasal spray brings fast relief of depressive symptoms
Esketamine nasal spray, combined with standard-of-care treatment, quickly improved depression symptoms and suicidal ideation, according to results of a phase 2 study published April 16 in the American Journal of Psychiatry.
In a study of 68 patients randomly assigned to either esketamine or placebo with standard-of-care treatment, patients in the treatment group had a significantly greater improvement in scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) at 4 hours’ and 24 hours’ follow-up after the first dose, reported Carla M. Canuso, MD, and her coauthors.
Esketamine, a more potent sibling of ketamine, is an N-methyl-D-aspartate receptor antagonist that modulates glutamatergic transmission. It is “being developed as an intranasal formulation for treatment-resistant depression and for rapid reduction of symptoms of major depressive disorder, including suicidal ideation, in patients at imminent risk for suicide,” Dr. Canuso and her coauthors reported.
In the study, participants were assigned randomly to twice-weekly treatment with either placebo or 84 mg of intranasal esketamine (or a reduced dose of 56 mg in the event of intolerance). The study included 4 weeks of double-blind treatment followed by 8 weeks of follow-up.
Depressive and suicidal symptoms were evaluated using MADRS criteria 4 hours and 24 hours after the initial dose, on day 25, and all visits during posttreatment follow-up. Severity of suicide risk was evaluated using the Suicide Ideation and Behavior Assessment Tool.
MADRS scores were significantly improved in the esketamine group, compared with the placebo group, both 4 hours and 24 hours after initial dosing, but not at 25 days, Dr. Canuso and her colleagues reported. The esketamine group also had significantly greater improvement on the MADRS suicidal thoughts item 4 hours after first dose (P = .002), but not 24 hours after the first dose (P = .129) or at day 25 (P = .143).
The analysis also showed that, 4 hours after the first dose, 21.2% of participants in the esketamine group achieved resolution of suicide risk, compared with 9.7% for placebo patients. At 24 hours’ follow-up, 40% of esketamine patients and 6.5% of placebo patients had achieved resolution of suicide risk.
Serious adverse events (including suicidal ideation and suicide attempts) occurred in four participants in the esketamine group during the double-blind study phase. During the follow-up period, serious adverse events occurred in one patient in the treatment group and five patients in the placebo group. Other adverse events included nausea, dizziness, dysgeusia, and dissociation.
The results “may reflect a promising breakthrough in the clinical management of a potentially lethal condition for which there are no approved pharmacotherapies,” Dr. Canuso and her colleagues wrote. However, future research still is needed to evaluate the risk of dependence, they cautioned.
“Further investigation is needed to determine the rapid effect of esketamine on measures of suicidal ideation as well as the benefit of repeated esketamine dosing on symptoms of depression in this acutely ill patient population,” the authors concluded.
The investigators reported that, in addition to Dr. Canuso, several of the other authors are employed by Janssen Research and Development – and hold stock and/or stock options in Johnson & Johnson. The study was funded by Janssen Research and Development.
SOURCE: Canuso CM et al. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.17060720.
Before ketamine is offered as a treatment option for depression or suicidal ideation, the potential risks for dependence and abuse need to be carefully evaluated, wrote Robert Freedman, MD, professor and former chair of the department of psychiatry at the University of Colorado at Denver, Aurora, and his coauthors.
“In order to obtain FDA [Food and Drug Administration] approval for marketing, phase 3 trials need to include rigorous monitoring of patients’ craving after ketamine administration and urine monitoring before each subsequent administration to detect evidence of drug seeking from other sources,” he and his coauthors wrote.
Physicians have a responsibility to try to prevent epidemics such as the opioid crisis, and preemptive research into ketamine’s addictive properties may be one way to avoid another such crisis, he added.
“It would be wise for physicians, regulatory agencies, and the pharmaceutical industry to work together preemptively to establish a suitable framework for its therapeutic use,” he wrote. “Education of the public and physicians needs to balance both potential benefits and the risk of abuse.”
Dr. Freedman did not report any relevant disclosures.
Robert Freedman, MD, and his coauthors are members of the American Journal of Psychiatry’s editorial board. Their comments came in an editorial accompanying the study (Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18030290).
Before ketamine is offered as a treatment option for depression or suicidal ideation, the potential risks for dependence and abuse need to be carefully evaluated, wrote Robert Freedman, MD, professor and former chair of the department of psychiatry at the University of Colorado at Denver, Aurora, and his coauthors.
“In order to obtain FDA [Food and Drug Administration] approval for marketing, phase 3 trials need to include rigorous monitoring of patients’ craving after ketamine administration and urine monitoring before each subsequent administration to detect evidence of drug seeking from other sources,” he and his coauthors wrote.
Physicians have a responsibility to try to prevent epidemics such as the opioid crisis, and preemptive research into ketamine’s addictive properties may be one way to avoid another such crisis, he added.
“It would be wise for physicians, regulatory agencies, and the pharmaceutical industry to work together preemptively to establish a suitable framework for its therapeutic use,” he wrote. “Education of the public and physicians needs to balance both potential benefits and the risk of abuse.”
Dr. Freedman did not report any relevant disclosures.
Robert Freedman, MD, and his coauthors are members of the American Journal of Psychiatry’s editorial board. Their comments came in an editorial accompanying the study (Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18030290).
Before ketamine is offered as a treatment option for depression or suicidal ideation, the potential risks for dependence and abuse need to be carefully evaluated, wrote Robert Freedman, MD, professor and former chair of the department of psychiatry at the University of Colorado at Denver, Aurora, and his coauthors.
“In order to obtain FDA [Food and Drug Administration] approval for marketing, phase 3 trials need to include rigorous monitoring of patients’ craving after ketamine administration and urine monitoring before each subsequent administration to detect evidence of drug seeking from other sources,” he and his coauthors wrote.
Physicians have a responsibility to try to prevent epidemics such as the opioid crisis, and preemptive research into ketamine’s addictive properties may be one way to avoid another such crisis, he added.
“It would be wise for physicians, regulatory agencies, and the pharmaceutical industry to work together preemptively to establish a suitable framework for its therapeutic use,” he wrote. “Education of the public and physicians needs to balance both potential benefits and the risk of abuse.”
Dr. Freedman did not report any relevant disclosures.
Robert Freedman, MD, and his coauthors are members of the American Journal of Psychiatry’s editorial board. Their comments came in an editorial accompanying the study (Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.18030290).
Esketamine nasal spray, combined with standard-of-care treatment, quickly improved depression symptoms and suicidal ideation, according to results of a phase 2 study published April 16 in the American Journal of Psychiatry.
In a study of 68 patients randomly assigned to either esketamine or placebo with standard-of-care treatment, patients in the treatment group had a significantly greater improvement in scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) at 4 hours’ and 24 hours’ follow-up after the first dose, reported Carla M. Canuso, MD, and her coauthors.
Esketamine, a more potent sibling of ketamine, is an N-methyl-D-aspartate receptor antagonist that modulates glutamatergic transmission. It is “being developed as an intranasal formulation for treatment-resistant depression and for rapid reduction of symptoms of major depressive disorder, including suicidal ideation, in patients at imminent risk for suicide,” Dr. Canuso and her coauthors reported.
In the study, participants were assigned randomly to twice-weekly treatment with either placebo or 84 mg of intranasal esketamine (or a reduced dose of 56 mg in the event of intolerance). The study included 4 weeks of double-blind treatment followed by 8 weeks of follow-up.
Depressive and suicidal symptoms were evaluated using MADRS criteria 4 hours and 24 hours after the initial dose, on day 25, and all visits during posttreatment follow-up. Severity of suicide risk was evaluated using the Suicide Ideation and Behavior Assessment Tool.
MADRS scores were significantly improved in the esketamine group, compared with the placebo group, both 4 hours and 24 hours after initial dosing, but not at 25 days, Dr. Canuso and her colleagues reported. The esketamine group also had significantly greater improvement on the MADRS suicidal thoughts item 4 hours after first dose (P = .002), but not 24 hours after the first dose (P = .129) or at day 25 (P = .143).
The analysis also showed that, 4 hours after the first dose, 21.2% of participants in the esketamine group achieved resolution of suicide risk, compared with 9.7% for placebo patients. At 24 hours’ follow-up, 40% of esketamine patients and 6.5% of placebo patients had achieved resolution of suicide risk.
Serious adverse events (including suicidal ideation and suicide attempts) occurred in four participants in the esketamine group during the double-blind study phase. During the follow-up period, serious adverse events occurred in one patient in the treatment group and five patients in the placebo group. Other adverse events included nausea, dizziness, dysgeusia, and dissociation.
The results “may reflect a promising breakthrough in the clinical management of a potentially lethal condition for which there are no approved pharmacotherapies,” Dr. Canuso and her colleagues wrote. However, future research still is needed to evaluate the risk of dependence, they cautioned.
“Further investigation is needed to determine the rapid effect of esketamine on measures of suicidal ideation as well as the benefit of repeated esketamine dosing on symptoms of depression in this acutely ill patient population,” the authors concluded.
The investigators reported that, in addition to Dr. Canuso, several of the other authors are employed by Janssen Research and Development – and hold stock and/or stock options in Johnson & Johnson. The study was funded by Janssen Research and Development.
SOURCE: Canuso CM et al. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.17060720.
Esketamine nasal spray, combined with standard-of-care treatment, quickly improved depression symptoms and suicidal ideation, according to results of a phase 2 study published April 16 in the American Journal of Psychiatry.
In a study of 68 patients randomly assigned to either esketamine or placebo with standard-of-care treatment, patients in the treatment group had a significantly greater improvement in scores on the Montgomery-Åsberg Depression Rating Scale (MADRS) at 4 hours’ and 24 hours’ follow-up after the first dose, reported Carla M. Canuso, MD, and her coauthors.
Esketamine, a more potent sibling of ketamine, is an N-methyl-D-aspartate receptor antagonist that modulates glutamatergic transmission. It is “being developed as an intranasal formulation for treatment-resistant depression and for rapid reduction of symptoms of major depressive disorder, including suicidal ideation, in patients at imminent risk for suicide,” Dr. Canuso and her coauthors reported.
In the study, participants were assigned randomly to twice-weekly treatment with either placebo or 84 mg of intranasal esketamine (or a reduced dose of 56 mg in the event of intolerance). The study included 4 weeks of double-blind treatment followed by 8 weeks of follow-up.
Depressive and suicidal symptoms were evaluated using MADRS criteria 4 hours and 24 hours after the initial dose, on day 25, and all visits during posttreatment follow-up. Severity of suicide risk was evaluated using the Suicide Ideation and Behavior Assessment Tool.
MADRS scores were significantly improved in the esketamine group, compared with the placebo group, both 4 hours and 24 hours after initial dosing, but not at 25 days, Dr. Canuso and her colleagues reported. The esketamine group also had significantly greater improvement on the MADRS suicidal thoughts item 4 hours after first dose (P = .002), but not 24 hours after the first dose (P = .129) or at day 25 (P = .143).
The analysis also showed that, 4 hours after the first dose, 21.2% of participants in the esketamine group achieved resolution of suicide risk, compared with 9.7% for placebo patients. At 24 hours’ follow-up, 40% of esketamine patients and 6.5% of placebo patients had achieved resolution of suicide risk.
Serious adverse events (including suicidal ideation and suicide attempts) occurred in four participants in the esketamine group during the double-blind study phase. During the follow-up period, serious adverse events occurred in one patient in the treatment group and five patients in the placebo group. Other adverse events included nausea, dizziness, dysgeusia, and dissociation.
The results “may reflect a promising breakthrough in the clinical management of a potentially lethal condition for which there are no approved pharmacotherapies,” Dr. Canuso and her colleagues wrote. However, future research still is needed to evaluate the risk of dependence, they cautioned.
“Further investigation is needed to determine the rapid effect of esketamine on measures of suicidal ideation as well as the benefit of repeated esketamine dosing on symptoms of depression in this acutely ill patient population,” the authors concluded.
The investigators reported that, in addition to Dr. Canuso, several of the other authors are employed by Janssen Research and Development – and hold stock and/or stock options in Johnson & Johnson. The study was funded by Janssen Research and Development.
SOURCE: Canuso CM et al. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.17060720.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Key clinical point: Esketamine nasal spray, combined with standard-of-care treatment, quickly improved depression symptoms and suicidal ideation.
Major finding: Patients in the esketamine treatment group had a significantly greater improvement in scores on MADRS at 4 hours and 24 hours follow-up.
Study details: A double-blind, proof-of-concept, phase 2 study of 68 patients with major depressive disorder.
Disclosures: The study was funded by Janssen Research and Development.
Source: Canuso CM et al. Am J Psychiatry. 2018. doi: 10.1176/appi.ajp.2018.17060720.
Short Takes
Giving iron supplements every other day may be superior to daily divided doses
Serum hepcidin levels and iron absorption were compared in women given daily dosing of ferrous sulfate, women given alternate-day dosing, and women given two divided doses daily. Women on the alternate-day regimen and the single-day regimens had higher iron absorption and lower hepcidin levels than did the women on the split-dosing regimen; these findings need to be confirmed in patients with iron-deficiency anemia.
Immediate percutaneous coronary intervention (PCI) of the culprit lesion only in patients presenting with acute myocardial infarction and cardiogenic shock may lead to better outcomes, even in those with multivessel disease
A total of 706 patients with multivessel coronary artery disease who presented with acute MI and cardiogenic shock were randomized to either PCI of the culprit lesion only (followed by optional staged revascularization of nonculprit lesions) or to immediate multivessel PCI. Patients who received PCI of the culprit lesion only had a lower 30-day risk of death or severe renal failure leading to renal-replacement therapy than did those who underwent immediate multivessel PCI.
Citation: Thiele H et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017 Oct. doi: 10.1056/NEJMoa1710261 (epub ahead of print).
Giving iron supplements every other day may be superior to daily divided doses
Serum hepcidin levels and iron absorption were compared in women given daily dosing of ferrous sulfate, women given alternate-day dosing, and women given two divided doses daily. Women on the alternate-day regimen and the single-day regimens had higher iron absorption and lower hepcidin levels than did the women on the split-dosing regimen; these findings need to be confirmed in patients with iron-deficiency anemia.
Immediate percutaneous coronary intervention (PCI) of the culprit lesion only in patients presenting with acute myocardial infarction and cardiogenic shock may lead to better outcomes, even in those with multivessel disease
A total of 706 patients with multivessel coronary artery disease who presented with acute MI and cardiogenic shock were randomized to either PCI of the culprit lesion only (followed by optional staged revascularization of nonculprit lesions) or to immediate multivessel PCI. Patients who received PCI of the culprit lesion only had a lower 30-day risk of death or severe renal failure leading to renal-replacement therapy than did those who underwent immediate multivessel PCI.
Citation: Thiele H et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017 Oct. doi: 10.1056/NEJMoa1710261 (epub ahead of print).
Giving iron supplements every other day may be superior to daily divided doses
Serum hepcidin levels and iron absorption were compared in women given daily dosing of ferrous sulfate, women given alternate-day dosing, and women given two divided doses daily. Women on the alternate-day regimen and the single-day regimens had higher iron absorption and lower hepcidin levels than did the women on the split-dosing regimen; these findings need to be confirmed in patients with iron-deficiency anemia.
Immediate percutaneous coronary intervention (PCI) of the culprit lesion only in patients presenting with acute myocardial infarction and cardiogenic shock may lead to better outcomes, even in those with multivessel disease
A total of 706 patients with multivessel coronary artery disease who presented with acute MI and cardiogenic shock were randomized to either PCI of the culprit lesion only (followed by optional staged revascularization of nonculprit lesions) or to immediate multivessel PCI. Patients who received PCI of the culprit lesion only had a lower 30-day risk of death or severe renal failure leading to renal-replacement therapy than did those who underwent immediate multivessel PCI.
Citation: Thiele H et al. PCI strategies in patients with acute myocardial infarction and cardiogenic shock. N Engl J Med. 2017 Oct. doi: 10.1056/NEJMoa1710261 (epub ahead of print).
Robotic approach falls short for sleeve gastrectomy
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
SEATTLE – according to a review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database.
“Robotic sleeve gastrectomy increases [use of] hospital resources. ... These findings may explain the low utilization rate of the robotic approach to sleeve gastrectomy,” said lead investigator Reza Alizadeh, MD, a surgery resident at the University of California, Irvine.
Sleeve gastrectomy has eclipsed gastric bypass as the most common weight loss surgery in United States. While most are done laparoscopically, the use of robots is becoming more common, so the investigators wanted to compare outcomes in a large number of cases. They turned to the metabolic and bariatric surgery database, which is jointly maintained by the American College of Surgeons and the American Society for Metabolic and Bariatric Surgery. Emergent, converted, and revision cases were excluded from the analysis to avoid confounding.
Almost 94% of the cases were done laparoscopically, with the rest done robotically. Mean operative time was 101 min in the robotic arm, and 1.5% of patients developed anastomotic leaks. Mean operative time in the laparoscopic group was 74 minutes, and 0.5% of patients developed leaks. After adjustment for potential confounders, leaks were 3.4 times more likely with the robotic approach (95% confidence interval, 2.47-4.0; P less than .01). It wasn’t possible to determine whether there were any differences in the type of stapling done in the two groups.
Meanwhile, 0.8% of robotic surgery patients developed surgical site infections versus 0.6% of the laparoscopic cases. After adjustment, infections were 38% more likely with the robot (95% CI, 1.01-1.89; P = 0.03). Dr. Alizadeh noted that the database only goes out to 30 days, so “the true complication rates may be underestimated.”
The findings are consistent with previous investigations. It’s unclear whether there’s something inherently riskier about robotic sleeve gastrectomy itself or whether surgeons haven’t quite got the knack of it yet. The higher leak rate with robotic surgery, “I believe, is mostly related to the small number of [robotic] cases being done. We are still in the beginning stages of utilizing the robotic approach. Maybe there’s a learning curve, and we need more experience and more practice,” Dr. Alizadeh said at the World Congress of Endoscopic Surgery hosted by SAGES & CAGS.
Indeed, others have reported that it takes more than two dozen cases to become proficient in another procedure, robotic esophagectomy.
The mean length of stay in the study was slightly, but not statistically significantly, longer in the robotic arm (1.8 vs. 1.7 days; P = 0.17). There was no statistically significant difference in in-hospital mortality.
The laparoscopic group had more men than did the robotic group (21.4% vs. 19.7%, respectively) and more chronic steroid use (1.7% vs. 1.3%), plus more patients were dependent on oxygen (0.7% vs. 0.3%). The robotic group had more obstructive sleep apnea than did the laparoscopic group (37.3% vs. 36% of cases) and a higher incidence of hypoalbuminemia (8.4% vs. 7%). The analysis adjusted for the differences.
The findings were pretty much the same when the team repeated their analysis with the 2016 database numbers, which were released while the SAGES presentation was being prepared. The only big difference was an increase in the number of robotic cases, up from 6.1% in 2015 to 6.6% of cases in 2016.
The was no external funding for the work, and the investigators had no relevant disclosures.
SOURCE: Alizadeh RF et al. SAGES 2018, Abstract S024.
REPORTING FROM SAGES 2018
Key clinical point: Operative times are longer, and leaks and surgical site infections more common, when surgeons opt for robotic instead of laparoscopic sleeve gastrectomy.
Major finding: Anastomotic leaks were 3.4 times more likely with the robotic approach (95% CI 2.47-4.0; P less than .01).
Study details: Review of 86,953 cases in the 2015 Metabolic and Bariatric Surgery Accreditation and Quality Improvement Program database
Disclosures: The was no external funding for the project, and the investigators had no relevant disclosures.
Source: Alizadeh RF et al. SAGES 2018, Abstract S024
SLE: Specialized lupus clinics may offer superior quality of care
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Patients with systemic lupus erythematosus (SLE) more often received care consistent with quality measures when they were seen in a specialized lupus clinic than they did in a general rheumatology clinic, according to results of a recent single-center, retrospective study.
Compared with a general rheumatology clinic, the lupus clinic had superior quality measure performance overall and for specific measures related to testing, treatment, and counseling, study authors reported in Arthritis Care & Research.
“Providing quality care in SLE is challenging as patients require ongoing, time consuming, multidisciplinary care,” wrote Shilpa Arora, MD, of John H. Stroger Hospital in Chicago, and her coauthors.
Lupus clinics and other disease-focused clinics at academic centers may provide better patient care by leveraging subspecialists’ current knowledge, experience, care processes, and multidisciplinary networks, according to Dr. Arora and her colleagues.
To assess whether a lupus clinic makes any difference in quality of care, Dr. Arora and her coinvestigators conducted a cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago.
Of that group of patients, 77 had received care in a subspecialty lupus clinic, and 73 received care at the general rheumatology clinic, according to the report.
Looking at validated quality measures for SLE testing, treatment, and counseling, Dr. Arora and her colleagues found performance was significantly greater overall for the lupus clinic than it was for the general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
In particular, patients treated at the lupus clinic were significantly more likely to get antiphospholipid antibody testing and bone mineral density testing, they said.
Lupus clinic patients were also more likely to be offered preventive measures, such as sunscreen counseling, cardiovascular disease risk assessment, and prescription of an angiotensin converting enzyme inhibitor when appropriate, they added.
To see whether there was any relationship between patient volume and quality measures, the researchers also looked at the number of SLE patients seen by each rheumatologist.
Dr. Arora and her associates did find a moderate correlation between the number of patients seen per rheumatologist and quality measure performance (rho, 0.48; P less than 0.001).
While this small study suggests subspecialty clinics provide high quality care, the authors said multicenter, prospective studies are needed to evaluate whether they also improve patient outcomes.
“It would be pertinent to see if these efficiencies translate over time to measurable gains in health status and health resource utilizations,” Dr. Arora and her colleagues concluded in their report.
Dr. Arora and her associates said there were no relevant financial disclosures or funding.
SOURCE: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
FROM ARTHRITIS CARE & RESEARCH
Key clinical point:
Major finding: Quality measure performance in the lupus clinic was superior to that in a general rheumatology clinic (85.8% vs. 70.2%; P = 0.001).
Study details: A cross-sectional, retrospective chart review including 150 consecutive patients who received care at Rush University in Chicago in either the subspecialty lupus clinic or the general rheumatology clinic.
Disclosures: Dr. Arora and her associates said there were no relevant disclosures or funding.
Source: Arora S et al. Arthritis Care Res (Hoboken). 2018 Apr 2. doi: 10.1002/acr.23569.
Healthy lifestyle linked to better colon cancer survival
Having a normal body mass index, being physically active, and eating abundant vegetables, fruits, and whole grains was linked to a significantly reduced risk of death during a prospective cohort study of 992 patients with stage III colon cancer.
After 7 years of median follow-up, patients who most closely followed American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors had a 5-year survival probability of 85%, compared with 76% for patients who were least adherent (absolute risk reduction, 9%). After adjustment for multiple potential confounders, high guideline concordance was associated with a 42% lower risk of death during follow-up, compared with low guideline concordance (hazard ratio, 0.58; 95% confidence interval, 0.34-0.99; P = .01).
The cohort study included individuals with stage 3 colon cancer enrolled in the Cancer and Leukemia Group B (CALGB) 89803 randomized adjuvant chemotherapy trial, which ran from 1999 through 2001. Dr. Van Blarigan and her coinvestigators surveyed and scored each patient according to the ACS guidelines for cancer survivors. Scores ranged from 0 to 6 and increased with healthier behavior. The survival analysis compared individuals scoring 5 or 6 (highest guideline concordance) with those scoring 0 or 1 (lowest guideline concordance).
The 91 patients with the highest guideline concordance typically had a BMI of 23 kg/m2 or less, exercised more than 30 metabolic equivalent task hours per week, consumed more than three daily servings of fruits and vegetables, and ate mostly whole (versus refined) grains. In contrast, the 262 patients with the lowest guideline concordance had a median BMI of 33 kg/m2, exercised a median of 2 metabolic equivalent task hours per week, consumed less than two daily servings of fruits and vegetables, and ate mostly refined grains.
A closer look at individual factors linked survival with BMI between 23 kg/m2 and 29.9 kg/m2, with engaging in at least 150 minutes of moderate exercise per week, with consuming at least five daily servings of fruits and vegetables, and with choosing whole grains over refined grains. Although the ACS recommends limiting red or processed meat, this behavior did not show a protective effect, which mirrors findings from a prior study (J Clin Oncol. 2013 Aug 1;31[22]:2773-82). “Higher protein intake may be beneficial for cancer survivors,” the investigators noted.
They also examined alcohol consumption, which the ACS guidelines did not address. Women who consumed more than one alcoholic drink per day and men who consumed more than two drinks per day had a nonsignificantly higher risk of death than abstainers (HR, 1.28; 95% CI, 0.81-2.01). Compared with abstention, low to moderate alcohol consumption was tied to a lower risk of death, but this link also did not reach significance (HR, 0.87; 95% CI, 0.66-1.14).
The National Cancer Institute funded the study. Pharmacia and Upjohn Company (now Pfizer Oncology) provided partial funding for the CALGB 89803/Alliance trial. Dr. Van Blarigan and several of the other investigators were supported by National Cancer Institute awards. No other disclosures were reported.
SOURCE: Van Blarigan EL et al. JAMA Oncol. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0126.
“If you previously gave [colorectal cancer survivors] vague recommendations about diet and exercise, now you can be more precise and recommend five to six servings per day of fruits and vegetables and 150 minutes per week of exercise,” Michael J. Fisch, MD, MPH; Lorna H. McNeill, PhD, MPH; and Karen M. Basen-Engquist, PhD, MPH, wrote in an accompanying editorial in JAMA Oncology.
Although this was an observational study, the size of the association between survival and high adherence to American Cancer Society Nutrition and Physical Activity Guidelines was “certainly striking,” they wrote.
However, few study patients were younger than 50 years, were nonwhite, or had poor performance status, they noted. Additionally, contemporary adjuvant regimens (FOLFOX and CAPEOX) include oxaliplatin, which can cause chronic neurotoxicities that undermine physical activity.
Nonetheless, the data “strengthen the call to focus on lifestyle changes to extend and improve the lives of cancer survivors,” the editorialists concluded. Although making such changes is “notoriously difficult,” clues may come from six ongoing trials of weight control and physical activity in cancer survivors.
Dr. Fisch, Dr. McNeill, and Dr. Basen-Engquist all are at the University of Texas MD Anderson Cancer Center, Houston. Dr. Fisch also is with AIM Specialty Health, Chicago, Ill.; AIM is a subsidiary of Anthem. No other disclosures were reported. This editorial accompanied the article by Van Blanigan et al. (JAMA Oncology. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0124 ).
“If you previously gave [colorectal cancer survivors] vague recommendations about diet and exercise, now you can be more precise and recommend five to six servings per day of fruits and vegetables and 150 minutes per week of exercise,” Michael J. Fisch, MD, MPH; Lorna H. McNeill, PhD, MPH; and Karen M. Basen-Engquist, PhD, MPH, wrote in an accompanying editorial in JAMA Oncology.
Although this was an observational study, the size of the association between survival and high adherence to American Cancer Society Nutrition and Physical Activity Guidelines was “certainly striking,” they wrote.
However, few study patients were younger than 50 years, were nonwhite, or had poor performance status, they noted. Additionally, contemporary adjuvant regimens (FOLFOX and CAPEOX) include oxaliplatin, which can cause chronic neurotoxicities that undermine physical activity.
Nonetheless, the data “strengthen the call to focus on lifestyle changes to extend and improve the lives of cancer survivors,” the editorialists concluded. Although making such changes is “notoriously difficult,” clues may come from six ongoing trials of weight control and physical activity in cancer survivors.
Dr. Fisch, Dr. McNeill, and Dr. Basen-Engquist all are at the University of Texas MD Anderson Cancer Center, Houston. Dr. Fisch also is with AIM Specialty Health, Chicago, Ill.; AIM is a subsidiary of Anthem. No other disclosures were reported. This editorial accompanied the article by Van Blanigan et al. (JAMA Oncology. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0124 ).
“If you previously gave [colorectal cancer survivors] vague recommendations about diet and exercise, now you can be more precise and recommend five to six servings per day of fruits and vegetables and 150 minutes per week of exercise,” Michael J. Fisch, MD, MPH; Lorna H. McNeill, PhD, MPH; and Karen M. Basen-Engquist, PhD, MPH, wrote in an accompanying editorial in JAMA Oncology.
Although this was an observational study, the size of the association between survival and high adherence to American Cancer Society Nutrition and Physical Activity Guidelines was “certainly striking,” they wrote.
However, few study patients were younger than 50 years, were nonwhite, or had poor performance status, they noted. Additionally, contemporary adjuvant regimens (FOLFOX and CAPEOX) include oxaliplatin, which can cause chronic neurotoxicities that undermine physical activity.
Nonetheless, the data “strengthen the call to focus on lifestyle changes to extend and improve the lives of cancer survivors,” the editorialists concluded. Although making such changes is “notoriously difficult,” clues may come from six ongoing trials of weight control and physical activity in cancer survivors.
Dr. Fisch, Dr. McNeill, and Dr. Basen-Engquist all are at the University of Texas MD Anderson Cancer Center, Houston. Dr. Fisch also is with AIM Specialty Health, Chicago, Ill.; AIM is a subsidiary of Anthem. No other disclosures were reported. This editorial accompanied the article by Van Blanigan et al. (JAMA Oncology. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0124 ).
Having a normal body mass index, being physically active, and eating abundant vegetables, fruits, and whole grains was linked to a significantly reduced risk of death during a prospective cohort study of 992 patients with stage III colon cancer.
After 7 years of median follow-up, patients who most closely followed American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors had a 5-year survival probability of 85%, compared with 76% for patients who were least adherent (absolute risk reduction, 9%). After adjustment for multiple potential confounders, high guideline concordance was associated with a 42% lower risk of death during follow-up, compared with low guideline concordance (hazard ratio, 0.58; 95% confidence interval, 0.34-0.99; P = .01).
The cohort study included individuals with stage 3 colon cancer enrolled in the Cancer and Leukemia Group B (CALGB) 89803 randomized adjuvant chemotherapy trial, which ran from 1999 through 2001. Dr. Van Blarigan and her coinvestigators surveyed and scored each patient according to the ACS guidelines for cancer survivors. Scores ranged from 0 to 6 and increased with healthier behavior. The survival analysis compared individuals scoring 5 or 6 (highest guideline concordance) with those scoring 0 or 1 (lowest guideline concordance).
The 91 patients with the highest guideline concordance typically had a BMI of 23 kg/m2 or less, exercised more than 30 metabolic equivalent task hours per week, consumed more than three daily servings of fruits and vegetables, and ate mostly whole (versus refined) grains. In contrast, the 262 patients with the lowest guideline concordance had a median BMI of 33 kg/m2, exercised a median of 2 metabolic equivalent task hours per week, consumed less than two daily servings of fruits and vegetables, and ate mostly refined grains.
A closer look at individual factors linked survival with BMI between 23 kg/m2 and 29.9 kg/m2, with engaging in at least 150 minutes of moderate exercise per week, with consuming at least five daily servings of fruits and vegetables, and with choosing whole grains over refined grains. Although the ACS recommends limiting red or processed meat, this behavior did not show a protective effect, which mirrors findings from a prior study (J Clin Oncol. 2013 Aug 1;31[22]:2773-82). “Higher protein intake may be beneficial for cancer survivors,” the investigators noted.
They also examined alcohol consumption, which the ACS guidelines did not address. Women who consumed more than one alcoholic drink per day and men who consumed more than two drinks per day had a nonsignificantly higher risk of death than abstainers (HR, 1.28; 95% CI, 0.81-2.01). Compared with abstention, low to moderate alcohol consumption was tied to a lower risk of death, but this link also did not reach significance (HR, 0.87; 95% CI, 0.66-1.14).
The National Cancer Institute funded the study. Pharmacia and Upjohn Company (now Pfizer Oncology) provided partial funding for the CALGB 89803/Alliance trial. Dr. Van Blarigan and several of the other investigators were supported by National Cancer Institute awards. No other disclosures were reported.
SOURCE: Van Blarigan EL et al. JAMA Oncol. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0126.
Having a normal body mass index, being physically active, and eating abundant vegetables, fruits, and whole grains was linked to a significantly reduced risk of death during a prospective cohort study of 992 patients with stage III colon cancer.
After 7 years of median follow-up, patients who most closely followed American Cancer Society Nutrition and Physical Activity Guidelines for Cancer Survivors had a 5-year survival probability of 85%, compared with 76% for patients who were least adherent (absolute risk reduction, 9%). After adjustment for multiple potential confounders, high guideline concordance was associated with a 42% lower risk of death during follow-up, compared with low guideline concordance (hazard ratio, 0.58; 95% confidence interval, 0.34-0.99; P = .01).
The cohort study included individuals with stage 3 colon cancer enrolled in the Cancer and Leukemia Group B (CALGB) 89803 randomized adjuvant chemotherapy trial, which ran from 1999 through 2001. Dr. Van Blarigan and her coinvestigators surveyed and scored each patient according to the ACS guidelines for cancer survivors. Scores ranged from 0 to 6 and increased with healthier behavior. The survival analysis compared individuals scoring 5 or 6 (highest guideline concordance) with those scoring 0 or 1 (lowest guideline concordance).
The 91 patients with the highest guideline concordance typically had a BMI of 23 kg/m2 or less, exercised more than 30 metabolic equivalent task hours per week, consumed more than three daily servings of fruits and vegetables, and ate mostly whole (versus refined) grains. In contrast, the 262 patients with the lowest guideline concordance had a median BMI of 33 kg/m2, exercised a median of 2 metabolic equivalent task hours per week, consumed less than two daily servings of fruits and vegetables, and ate mostly refined grains.
A closer look at individual factors linked survival with BMI between 23 kg/m2 and 29.9 kg/m2, with engaging in at least 150 minutes of moderate exercise per week, with consuming at least five daily servings of fruits and vegetables, and with choosing whole grains over refined grains. Although the ACS recommends limiting red or processed meat, this behavior did not show a protective effect, which mirrors findings from a prior study (J Clin Oncol. 2013 Aug 1;31[22]:2773-82). “Higher protein intake may be beneficial for cancer survivors,” the investigators noted.
They also examined alcohol consumption, which the ACS guidelines did not address. Women who consumed more than one alcoholic drink per day and men who consumed more than two drinks per day had a nonsignificantly higher risk of death than abstainers (HR, 1.28; 95% CI, 0.81-2.01). Compared with abstention, low to moderate alcohol consumption was tied to a lower risk of death, but this link also did not reach significance (HR, 0.87; 95% CI, 0.66-1.14).
The National Cancer Institute funded the study. Pharmacia and Upjohn Company (now Pfizer Oncology) provided partial funding for the CALGB 89803/Alliance trial. Dr. Van Blarigan and several of the other investigators were supported by National Cancer Institute awards. No other disclosures were reported.
SOURCE: Van Blarigan EL et al. JAMA Oncol. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0126.
FROM JAMA ONCOLOGY
Key clinical point:
Major finding: Five-year survival probability was 85% for highly guideline-adherent patients and 76% for patients with low adherence (absolute risk reduction, 9%).
Study details: Prospective cohort study of 992 patients with stage III colon cancer.
Disclosures: The National Cancer Institute funded the study. Pharmacia and Upjohn Company (now Pfizer Oncology) partially funded the CALGB 89803/Alliance trial. Dr. Van Blarigan and several of the other investigators were supported by National Cancer Institute awards. No other disclosures were reported.
Source: Van Blarigan EL et al. JAMA Oncol. 2018 Apr 12. doi: 10.1001/jamaoncol.2018.0126.
Advanced practice nurses and physician assistants are not the same
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at pdnews@mdedge.com
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at pdnews@mdedge.com
Looking across a hospital ward, emergency department, or primary care clinic aligned side by side, you may not see any differences between an advanced practice nurse (APN) or physician assistant (PA). However, if you took a closer look at their education programs and credentialing, you would find considerable differences.
Although both professions hold advanced degrees, the approach to patient care differs, as well as the training they receive, including different models of practice. The APN is trained according to the nursing model, while the PA attends programs that are more in line with the medical model. The APN has a patient-centered model, while the PA adheres to a disease-centered model. Consequently, their approach to caring for the same patient population differs in viewpoint and philosophy.
Entry into the APN programs requires a nursing degree or related field from an accredited college or university. The curriculum includes coursework in health care policy, advocacy, outcomes, advanced assessment, diagnosis, and practice skills as well as, pharmacology, pathophysiology, and a final capstone project.
There are six specialty APN tracks including pediatrics, women’s/gender health, family practice, adult-gerontology, psychiatric, and neonatal. Additionally, there are three additional advanced practice registered nurses tracks: certified nurse anesthesia, certified nurse midwife, and clinical nurse leader. In addition to academic hours, there is a minimum of 1,000 supervised, direct patient care clinical hours in a variety of locations covering all populations specific to the identified specialty.
The Bureau of Labor Statistics defines the role of physician assistant as follows: “Physician assistants practice medicine under the supervision of physicians and surgeons. PAs are formally trained to provide diagnostic, therapeutic, and preventive health care services, as delegated by a physician.” The physician assistant program is a master’s prepared education.
School requirements include completing 2 years of pre-physician assistant undergraduate studies prior to applying to the School of Biomedical Sciences. Many programs have a 200-hour health care experience requirement, which can be either paid or unpaid. However, unlike the APN program, this is not required by all PA programs, but it is strongly encouraged.
Accredited PA programs require completing a 3-year graduate program that includes clinical rotations and results in a Master of Science in Physician Assistant Studies. Physician assistant programs typically involve 1,000 classroom hours and 2,000 or more hours in a clinical setting. The course work focuses on biochemistry, pathology, anatomy and physiology, ethics, and biology.
Both the APN and PA practices are regulated by the state through licensure laws and policy that determine the scope of practice and allow prescriptive authority.
Both programs began in 1965 in response to a shortage of primary care physicians, yet each program took a different route to address this need. According to the May 2017 Bureau of Labor Statistics, there were more than 109,000 physician assistants and more than 166,000 nurse practitioners practicing in the United States.
With the enactment of the Affordable Care Act in 2010, the mandate for APN’s and PA’s to lead patient-centered medical homes continued to grow to meet the demand. Both roles provide direct patient care under the sponsorship of a physician, yet both roles have gained a greater level of independence as state and federal requirements have relaxed restrictive physician collaboration and oversight rules, which has allowed both roles to practice at the highest level of their training. These relaxed restrictions come at a time when a growing physician shortage is met by increased demands placed on the health care system.
Ms. Thew is a certified family nurse practitioner in the division of adolescent medicine at the Medical College of Wisconsin, Milwaukee. Email her at pdnews@mdedge.com
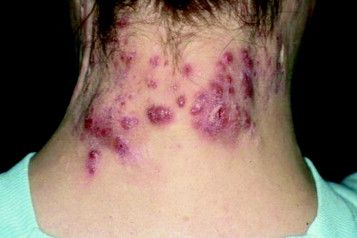
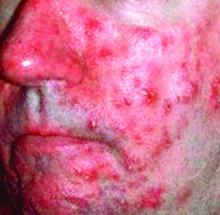
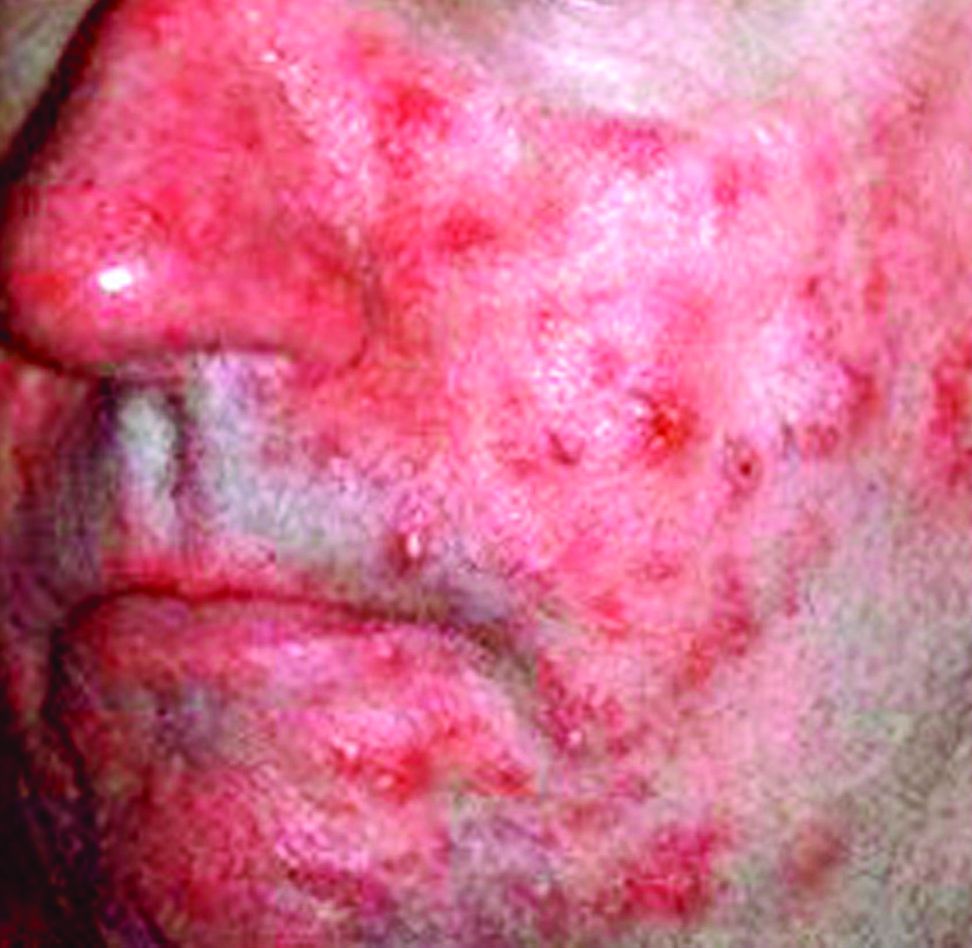
Surgical excision essential in severe hidradenitis suppurativa
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Medical therapy alone is never sufficient in Hurley stage III hidradenitis suppurativa (HS), Iltefat H. Hamzavi, MD, observed at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
“Even with the advances in biologics and antibiotic therapy, you still have to excise once you’re in full-blown Hurley stage III disease. Surgery has to be part of your protocol,” according to Dr. Hamzavi, a dermatologist at Henry Ford Hospital in Detroit, which runs one of the nation’s largest hidradenitis suppurativa clinics, with roughly 1,600 patients.
“Of course we’re biased. But until the data can set us free, you’re stuck with me,” the dermatologist quipped.
A core principle of the Henry Ford algorithm is this: “Medical therapy [for patients with advanced HS] stabilizes them and reduces their draining and pain, then you try to bring them back to a lower stage with surgical options,” he explained.
Although other HS staging systems exist, Dr. Hamzavi and his colleagues rely on the Hurley staging system to guide their treatment. Basically, Hurley stage I consists of follicular nodules and abscesses. When the nodules connect to form sinus tracts with scarring, that’s stage II. And if the sinus tracts interconnect throughout an entire area, that’s stage III.
Hurley stage I
First-line treatment of localized Hurley stage I disease at Henry Ford is a 10% topical benzoyl peroxide wash left on for 5 minutes before bathing, followed by postbathing topical clindamycin 1% lotion or solution applied to the nodules. If this maintenance regimen isn’t sufficient to prevent formation of new and worsening nodules, Dr. Hamzavi supplements it with up to three once-monthly 1064-nm Nd:YAG laser sessions aimed at follicular ablation. It’s a laser application he and his colleagues pioneered (Dermatol Surg. 2009 Aug;35[8]:1188-98). They subsequently documented the histopathologic basis of the procedure’s efficacy, which entails selective thermolysis of follicles, destruction of inflammatory lesions in the superficial to mid-dermis, followed by fibrosis and scarring (Arch Dermatol. 2011 Jan;147[1]:21-8).
In generalized Hurley stage I HS, the Henry Ford approach is to supplement the topical regimen and laser sessions with oral doxycycline at 100-150 mg daily for 1-6 months.
“The theory here is this is a dysbiotic event. The antibiotics reduce commensal bacteria, which ultimately reduces the reactive inflammatory response. But when you stop the antibiotics, the inflammatory response returns. So antibiotics can help stabilize the disease state but really can’t reverse the disease state. For that we have to turn to ablative treatment options: laser, surgery,” the dermatologist continued.
Hurley stage II
“At this point you’re looking at procedures,” according to Dr. Hamzavi. “Once you have sinus tracts it’s critical to remove them.”
The treatment backbone in stage II disease is 8-10 weeks of oral clindamycin and rifampin, both at 300 mg twice daily.
“This is one of the fundamental building blocks of HS clinics throughout the world,” he noted.
Clostridium difficile infection is exceedingly rare in HS patients on this regimen, for reasons still unclear.
If this dual-antibiotic regimen doesn’t dramatically reduce drainage and pain, he adds levofloxacin at 500 mg twice daily for up to 2 weeks in an effort to calm down unstable, decompensating disease.
Dapsone at 50-150 mg/day for up to 12 weeks is an additional option. It’s most useful in patients with nodules that are disproportionately painful, in Dr. Hamzavi’s experience.
Deroofing is a simple procedure that should be considered for all sinus tracts. It entails numbing the area with a ring block then introducing a curette or surgical probe into the sinus tract to open it up and get rid of the gelatinous material within. Dutch investigators have detailed the technique (J Am Acad Dermatol. 2010 Sep;63[3]:475-80).
Tumor necrosis factor–inhibitor therapy has been a major advance in Hurley stage II and III disease. “It doesn’t work in everybody, but a lot of patients can be stabilized,” Dr. Hamzavi observed.
Efficacy has been amply demonstrated for adalimumab (Humira) and infliximab (Remicade). In Dr. Hamzavi’s experience infliximab works better, probably because it offers more dosing options.
Assuming medical therapy has resulted in disease stabilization, CO2 laser excision of sinus tracts under local anesthesia can then be employed as an office procedure to turn back the clock and return to an earlier stage of disease. Dermatologists at the Cleveland Clinic have described the technique in detail (Dermatol Surg. 2010 Feb;36[2]:208-13).
Hurley stage III
If biologic therapy doesn’t bring disease stabilization, the patient is likely headed for surgical excision using the CO2 laser. The Henry Ford team favors a specific regimen of surgical preparation using wide-spectrum antibiotics. The program begins with 6 weeks of IV ertapenem at 1 g/day delivered by a peripherally inserted central catheter managed by infectious disease colleagues.
“IV ertapenem is a drug you may not know much about. We find it works really well as a great way to bridge patients towards surgery,” the dermatologist explained.
The IV ertapenem is followed by 6 weeks of oral triple therapy with rifampin, moxifloxacin, and metronidazole then another 6 weeks of rifampin plus moxifloxacin. Next it’s surgical excision time.
Lifestyle modification
Lifestyle modification deserves to be a major priority in all HS patients, regardless of Hurley stage. Smoking cessation results in significantly greater likelihood of favorable response to first-line therapy. In obese patients, greater than 15% weight loss has been associated with significant reduction in disease severity. A sartorial shift to loose-fitting clothing can quiet down skin lesions through decreased friction and pressure. And proper utilization of warm compression will rapidly decrease acute lesional pain.
Dr. Hamzavi and his coinvestigators have described the Henry Ford Hospital treatment algorithm in a review of HS published in an open-access journal meant to serve as a resource for patients and physicians alike (F1000Res. 2017 Jul 28;6:1272. doi: 10.12688/f1000research.11337.1. eCollection 2017).
He reported serving as a consultant to AbbVie, Incyte, and UCB.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
How to avoid severe diarrhea from apremilast
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
KAUAI, HAWAII – Physicians have become much more cognizant of severe diarrhea and nausea as potential side effects of apremilast since the Food and Drug Administration–approved change in the warnings and precautions section of the drug’s labeling in June 2017. Jashin J. Wu, MD, director of the psoriasis clinic at Kaiser Permanente Los Angeles Medical Center, has a tip for avoiding these problems: Delay up-titrating.
“In my opinion, that may be too quick of an up-titration. I tell patients that, if they feel the GI issues are still a problem for them on day 6, they should take 30 mg just once a day for the first 1-2 months. After that we’ll see how they’re doing, and if they feel they can make the jump to twice a day, then they can go for it. Of course, I also tell them that maybe their psoriasis will not clear as well as if they’d been on apremilast twice a day right from day 6, but if they’re able to tolerate it and can continue to take it, they can improve while they’re on it,” the dermatologist said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Dr. Wu presented an update on recent developments regarding the newest oral drugs for psoriasis and one of the oldest: apremilast and methotrexate, respectively.
Apremilast
The revised warning label highlighting the risks of severe diarrhea and nausea associated with the oral phosphodiesterase-4 inhibitor says that most such events have occurred within the first few weeks of therapy. The guidance also notes that patients who reduced the dosage or discontinued treatment outright generally improved rapidly.
“I see this in a lot of my patients. They have to go to the bathroom pretty often. It’s actually unusual for me for a patient not to have any GI issues at all,” according to Dr. Wu.
He shared a number of other fresh insights into apremilast’s safety and efficacy derived from recent studies.
Efficacy appears to increase at least out to 1 year
A report from the phase 3b, randomized, placebo-controlled, 250-patient LIBERATE trial showed that the week 16 PASI 75 response rate was 39.8% with apremilast, 48.2% with etanercept (Enbrel), and 11.9% with placebo. After week 16, everyone switched to apremilast. The PASI 75 rate in patients on apremilast all along climbed from 39.8% at week 16 to 52.7% at week 52. That result was in the same ballpark as the 57% rate in those switched from etanercept to apremilast and the 53.4% PASI 75 rate at week 52 in patients switched from placebo to apremilast (J Eur Acad Dermatol Venereol. 2017 Mar;31[3]:507-17).
“It was interesting to see that, as the study continued on for 1 year, the PASI 75 rate continued to improve. That’s worth noting: In general, I tell patients you have to be on a drug for about 3 months before we’re going to say if it worked or not, and that’s true even with drugs for other conditions, like doxycycline for acne. But this study seems to indicate that you have much better improvement at the 1-year point, and that’s not so much true for the biologics,” the dermatologist observed.
Safety to 3 years looks reassuringly good: 3-year follow-up of the 1,184-patient, phase 3, randomized, controlled ESTEEM 1 and 2 trials provided by far the longest look to date at apremilast’s safety. There were no surprises, no serious opportunistic infections, and no significant changes in laboratory values (J Am Acad Dermatol. 2017 Aug;77[2]:310-17.e1).
Of note, 21.9% of patients on apremilast lost more than 5% of their baseline body weight. Most of the weight loss occurred during year 1 of treatment and mostly in patients with a higher baseline body mass index.
“It seems like apremilast is definitely a good option if patients can tolerate the GI upset,” Dr. Wu said.
Apremilast can safely and effectively be combined with other psoriasis therapies: Dermatologists at the University of Toronto reported on a retrospective analysis of 81 biologic-naive psoriasis patients treated with apremilast in combination with methotrexate, acitretin (Soriatane), cyclosporine, narrowband UVB, etanercept, infliximab (Remicade), adalimumab (Humira), and/or ustekinumab (Stelara). Of these patients, 81% achieved a PASI 75 response at week 12 (J Cutan Med Surg. 2016 Jul;20[4]:313-6).
“That’s pretty good. It’s certainly better than apremilast by itself. So if you can get the payer to cover a combination of apremilast and something else, it may help get to PASI 75,” Dr. Wu noted.
Session chair Craig L. Leonardi, MD, said he hasn’t had any luck in going that route.
“The insurance industry just won’t give me apremilast in combination with a biologic drug. Even though it makes complete sense to use it in place of methotrexate with a biologic, I just can’t get it,” according to Dr. Leonardi, a dermatologist at Saint Louis University.
“I don’t have those limitations at Kaiser,” according to Dr. Wu. “I personally have only used apremilast and methotrexate and apremilast and acitretin in combination. I just want to be kind to Kaiser and not give two branded medications to a patient, but I certainly think it’s a feasible option.”
Methotrexate
A simple response prediction rule: Kenneth B. Gordon, MD, professor and chair of dermatology at the Medical College of Wisconsin in Milwaukee, and his coinvestigators at AbbVie developed a methotrexate response/nonresponse prediction rule using data on 110 participants in the phase 3 CHAMPION trial. Then they validated the rule in the phase 3 M10-255 trial. They found that a PASI 25 response to methotrexate at week 4 was associated with an 8.9-fold increased likelihood of a week-16 PASI 75 response. Patients with a predicted response probability of less than 30% were asked to discontinue the drug; their week 16 PASI 75 rate was only 21.1%, compared with a 65.8% response rate in patients with a prediction rating (J Am Acad Dermatol. 2017 Dec;77[6]:1030-7).
“Four weeks of methotrexate may be sufficient to determine the long-term response. It may not be necessary to put them on the drug for 3 months,” Dr. Wu commented.
Subcutaneous methotrexate: European investigators demonstrated that an intensified dosing schedule of subcutaneous methotrexate was safe and effective for treatment of moderate to severe plaque psoriasis in the 52-week, phase 3, randomized, 16-center, double-blind, 120-patient, placebo-controlled METOP study.
The intensified subcutaneous regimen consisted of 17.5 mg/week initially, escalated to 22.5 mg/week after 8 weeks if a patient hadn’t achieved at least a PASI 50 response at that point. The primary outcome, the PASI 75 response at week 16, was 41% in the subcutaneous methotrexate group and 10% in controls, with a maximum PASI 75 rate of 51% seen beginning at week 24. The week 4 and 8 PASI 50 rates were 50% and 58%, respectively, with methotrexate versus 3% and 17% in placebo-treated controls. The subcutaneous regimen was generally well tolerated, with no serious infections or malignancies arising during 52 weeks (Lancet. 2017 Feb 4;389[10068]:528-37).
Dr. Wu reported receiving research funding from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, and Regeneron.
The Global Academy for Medical Education/SDEF and this news organization are owned by the same parent company.
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR
2 in 1: Rosacea-like demodicosis, papulopustular rosacea may be phenotypes of same disease
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
according to the authors of a retrospective study.
Currently, there is a lack of consensus among physicians and health care organizations on how Demodex mites affect the development of rosacea. Many experts separate rosacea into two camps: PPR not caused by Demodex and rosacea-like demodicosis caused by Demodex.
During each evaluation session, each patient underwent two consecutive standardized skin surface biopsies (SSSBs), a small 1 square centimeter sample of the horny skin layer and the follicular content, on each cheek. The first sample, SSSB1, was a superficial sample, and SSSB2, the second sample, was a deep sample. The sum of the two samples, SSSB1+2, also was noted.
During the same session, patients had Demodex densities (Dds) measured. To avoid any confounding factors that could affect facial skin symptoms, Dr. Forton and Dr. de Maertelaer evaluated a subgroup of 132 patients who had not been treated in the previous 3 months and had no other facial dermatoses, such as acne vulgaris and seborrheic dermatitis.
The study revealed that, among the 242 patients in the primary analysis group, those with persistent erythema had higher Dds than did those without and the differences were statistically significant when comparing SSSB2 (208 D/cm2 vs. 130 D/cm2; P = 0.031) and SSB1+2 (298 D/cm2 vs. 191 D/cm2; P = 0.025), respectively.
This pattern of greater Dds density among patients with persistent erythema, compared with patients without, also held true among the subgroup of 132 patients who had not received any recent dermatological treatments, but the difference was not statistically significant. Nonetheless, patients with follicular scales did have greater SSSBs than did those without follicular scales.
As part of the study, Dr. Forton and Dr. de Maertelaer analyzed the findings of a case report of a 19-year-old woman who had a facial papulopustular eruption that had been present for 1 year. She had two SSSBs taken on each cheek: the right had follicular scales and papulopustules, and the left was clinically normal. This revealed that she had much higher Dds on the affected cheek than on the clinically normal one (108 and 216 D/cm2 vs. 12 and 20 D/cm2). She was subsequently diagnosed with rosacea-like demodicosis.
After treating the areas with acaricidal ointment, the symptoms improved. But 27 months after stopping maintenance treatments, the facial eruptions reappeared, and the papulopustules were larger than during her original consultation, leading to the diagnosis of PPR. This time, the Dds was high on both cheeks but responded to acaricidal treatment, which indicates that her eruptions were caused Demodex mites.
“All our observations, therefore, highlight the nosological confusion that persists between PPR and rosacea-like demodicosis and the need to update the consensus concerning the definition and classification of rosacea. Moreover, they suggest that PPR and rosacea-like demodicosis may be phenotypes of the same disease,” wrote Dr. Forton and Dr. de Maertelaer. “This concept is supported by our case report, with many features indicating that the second presentation was an evolution of the first.”
They cautioned that their research is not definitive but should provide strong evidence that PPR and rosacea-like demodicosis are the same disease.
“While our observations do not prove a causative role of Demodex in rosacea, they nevertheless support the idea that PPR and rosacea-like demodicosis should no longer be considered as two separate entities but, rather, as two phenotypes of the same disease,” they wrote. “As such, the definition of rosacea subtype II (PPR) should be reconsidered and simplified to include all patients with central face papulopustules – with or without persistent erythema – and thus also patients with ‘rosacea-like demodicosis,’ which is a term that should therefore disappear.”
This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer had no conflicts of interest to declare.
SOURCE: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
FROM THE JOURNAL OF THE EUROPEAN ACADEMY OF DERMATOLOGY AND VENEREOLOGY
Key clinical point: Demodex densities were greater in patients with persistent erythema than in those without.
Major finding: Deep tissue samples showed higher mite densities in patients with persistent erythema than in those without (SSSB2: 208 D/cm2 and 130 D/cm2; P = 0.031).
Study details: A retrospective, observational, case-control study of 242 patients with central face papulopustules.
Disclosures: This study received no external funding. Dr. Forton works for Galderma as a consultant. Dr. de Maertelaer has no conflicts of interest to declare.
Source: Forton F et al. J Eur Acad Dermatol Venereol. 2018 Feb 25. doi: 10.1111/jdv.14885.
Hormonal contraception boosts breast cancer risk
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
KAUAI, HAWAII – Current or recent use of hormonal contraception is associated with an increased risk of breast cancer, although in response to the Danish study findings.
“The data looks clear. It’s not a big risk, but it’s clearly a higher risk,” Lawrence F. Eichenfield, MD, said at the Hawaii Dermatology Seminar provided by the Global Academy for Medical Education/Skin Disease Education Foundation.
Investigators at the University of Copenhagen conducted a prospective cohort study among all 1.8 million Danish women aged 15-49 years who had not had cancer, venous thromboembolism, or treatment for infertility. Comprehensive nationwide registries were used to follow the women for an average of 10.9 years, which represents 19.6 million person-years of follow-up.
During that time, 11,517 cases of invasive breast cancer occurred in the study population. The relative risk was 1.2-fold greater in current or recent users of hormonal contraception, compared with never users of oral estrogen/progestin contraceptives or progestin-containing intrauterine devices. The risk rose with duration of use: from 1.09-fold with less than 1 year of exposure to 1.38-fold with more than 10 years of hormonal contraception.
From a population standpoint, there were 13 additional breast cancers diagnosed per 100,000 person-years of hormonal contraception use. That worked out to one extra breast cancer for every 7,690 women using hormonal contraception for 1 year (N Engl J Med. 2017 Dec 7;377[23]:2228-39).
Dr. Eichenfield pointed out that among Danish patients who used an oral contraceptive for less than 5 years, the excess risk of breast cancer went away within a year of stopping the medication. If patients used hormonal contraception for more than 5 years, the excess breast cancer risk lasted for 5 years after stopping.
He noted that the antiandrogen spironolactone, widely used off-label to treat adult female acne, has been shown to be free of any increased breast cancer risk.
He reported serving as a consultant to and/or recipient of research grants from more than a dozen pharmaceutical companies.
SDEF/Global Academy for Medical Education and this news organization are owned by the same parent company..
EXPERT ANALYSIS FROM SDEF HAWAII DERMATOLOGY SEMINAR