User login
David Henry's JCSO podcast, March-April 2018
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
In his bimonthly podcast, Dr David Henry, the JCSO Editor-in-Chief, discusses the approval of the biosimilars, bevacizumab-awwb and trastuzumab-dkst, and new therapies for virally associated cancers. Also in the line-up are an interview with Dr Daniel Haller on the latest advances in treating gastrointestinal cancers, and an article on hands-on advice on integrating survivorship care planning in a radiation oncology workflow. Research topics incude how to improve communication between oncology care providers and patient caregivers during hospice; the impact of patient education on enrollment in clinical trials; and organizational barriers to optimal lung cancer care in the community setting. A series of Case Reports that highlight some of the clincial challenges in treating patients with cancer round out the issue.
Listen to the podcast below
Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD
Click here to read the supplement
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD
Click here to read the supplement
Click here to read the supplement
CME: Opioid Use Disorder: Challenges and Solutions to a Rising Epidemic
Earn 1.25 CME Credits.
- Introduction and 2 Case Studies by Genie L. Bailey, MD
- Opioid Use Disorder: The Epidemic is Real by Kevin P. Hill, MD, MHS
- Managing the Opioid Use Disorder Crisis by Richard N. Rosenthal, MD
Click here to read the supplement
DDSEP® 8 Quick Quiz - April 2018 Question 2
Q2. Correct Answer: C
Rationale
The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patients fever, jaundice, and right upper quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns KE, Ashley SW, Hunter JG, Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointestinal Surgery. 2008;12(4):629-33.
Q2. Correct Answer: C
Rationale
The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patients fever, jaundice, and right upper quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns KE, Ashley SW, Hunter JG, Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointestinal Surgery. 2008;12(4):629-33.
Q2. Correct Answer: C
Rationale
The patient presents with acute gallstone pancreatitis. In patients with gallstone pancreatitis and evidence of cholangitis, ERCP with sphincterotomy and stone extraction should be performed. The patients fever, jaundice, and right upper quadrant pain are sufficient to make the diagnosis of cholangitis. It is too early in the course of the disease to evaluate for pancreatic necrosis. Typically, triglyceride levels above 1,000 mg/dL are required to induce pancreatitis. Finally, while the patient has cholelithiasis, there is no evidence of cholecystitis. Therefore, a HIDA scan is not warranted.
Reference
1. Behrns KE, Ashley SW, Hunter JG, Carr-Locke D. Early ERCP for gallstone pancreatitis: for whom and when? J Gastrointestinal Surgery. 2008;12(4):629-33.
A 50-year-old woman with no past medical history presents to the emergency department with the acute onset of severe epigastric pain and vomiting. She is afebrile with a blood pressure of 100/50 mm Hg, and pulse of 110 bpm. Physical exam shows right upper quadrant and epigastric tenderness to palpation without rebound. Labs demonstrate a white blood cell count of 17,000/mm3, hemoglobin of 16 g/dL, creatinine of 1.4 mg/dL, alanine aminotransferase of 215 U/L, aspartate aminotransferase of 190 U/L, a total bilirubin of 2.1 mg/dL, and triglycerides of 492 mg/dL. Right upper quadrant ultrasound reveals gallstones and a 1.2-cm common bile duct. The following day, despite being hydrated aggressively, the patient develops a fever and becomes jaundiced with worsening abdominal pain.
What would be the next step in the patient's management?
DDSEP® 8 Quick Quiz - April 2018 Question 1
Q1. Correct Answer: C
Rationale
The CagA strain of H. pylori has been found to be associated with an increased risk of gastric adenocarcinoma and MALT lymphoma. CagA-producing H. pylori infection also cause more severe mucosal inflammation and is associated with higher incidences of gastric and duodenal ulcers. A protective effect of CagA+ H. pylori against gastroesophageal reflux disease, reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma has been suggested, as some epidemiologic studies have shown a decreased prevalence of these disorders. Although further studies are needed to verify these relationships, no studies to date have demonstrated an increased risk of esophageal carcinoma associated with H. pylori. CagA-producing H. pylori has not been associated with gastric carcinoid tumor.
References
1. Fallone CA, Barkun AN, Göttke MU, et al. Association of Helicobacter pylori genotype with gastroesophageal reflux disease and other upper gastrointestinal diseases. Am J Gastroenterol. 2000;95(3):659-69.
2. Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003;125(6):1636-44.
3. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res. 2008;1:329-38.
Q1. Correct Answer: C
Rationale
The CagA strain of H. pylori has been found to be associated with an increased risk of gastric adenocarcinoma and MALT lymphoma. CagA-producing H. pylori infection also cause more severe mucosal inflammation and is associated with higher incidences of gastric and duodenal ulcers. A protective effect of CagA+ H. pylori against gastroesophageal reflux disease, reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma has been suggested, as some epidemiologic studies have shown a decreased prevalence of these disorders. Although further studies are needed to verify these relationships, no studies to date have demonstrated an increased risk of esophageal carcinoma associated with H. pylori. CagA-producing H. pylori has not been associated with gastric carcinoid tumor.
References
1. Fallone CA, Barkun AN, Göttke MU, et al. Association of Helicobacter pylori genotype with gastroesophageal reflux disease and other upper gastrointestinal diseases. Am J Gastroenterol. 2000;95(3):659-69.
2. Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003;125(6):1636-44.
3. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res. 2008;1:329-38.
Q1. Correct Answer: C
Rationale
The CagA strain of H. pylori has been found to be associated with an increased risk of gastric adenocarcinoma and MALT lymphoma. CagA-producing H. pylori infection also cause more severe mucosal inflammation and is associated with higher incidences of gastric and duodenal ulcers. A protective effect of CagA+ H. pylori against gastroesophageal reflux disease, reflux esophagitis, Barrett's esophagus, and esophageal adenocarcinoma has been suggested, as some epidemiologic studies have shown a decreased prevalence of these disorders. Although further studies are needed to verify these relationships, no studies to date have demonstrated an increased risk of esophageal carcinoma associated with H. pylori. CagA-producing H. pylori has not been associated with gastric carcinoid tumor.
References
1. Fallone CA, Barkun AN, Göttke MU, et al. Association of Helicobacter pylori genotype with gastroesophageal reflux disease and other upper gastrointestinal diseases. Am J Gastroenterol. 2000;95(3):659-69.
2. Huang JQ, Zheng GF, Sumanac K, et al. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology 2003;125(6):1636-44.
3. Islami F, Kamangar F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev Res. 2008;1:329-38.
The CagA strain of Helicobacter pylori is associated with which of the following?
The role of defensins in treating skin aging
Most skin-aging treatments work by prodding old fibroblasts and keratinocytes to accelerate the production of important cellular components. For example, retinoids act on retinoic acid receptors to activate collagen genes and deactivate collagenase genes. Glycolic acid, ascorbic acid, and certain growth factors stimulate synthesis of collagen by fibroblasts. Older fibroblasts and keratinocytes are sluggish for many reasons; they do not “hear” signals as well as younger cells do. Glycosaminoglycans such as heparan sulfate can help cells hear these signals. Heparan sulfate, for example, assists in the delivery of growth factors to cells, stabilizes them, and presents them to the receptors on the keratinocytes and fibroblasts, and amplifies cellular response to these factors.
A new angle in antiaging skin care is to create new keratinocytes rather than to stimulate old cells. For the last decade, personal care companies have touted the benefit of putting stem cells in cosmeceuticals, claiming that these cells would rejuvenate skin. However, this proved to be unsubstantiated marketing hype because the stem cells were plant derived (often from apples), had poor shelf life, and could not intercalate between the native skin cells and work with them to have any effect. Stems cells in cosmeceuticals became a point of disdain for savvy scientists.
Stem cells
Wounding the skin stimulates LGR6+ stem cells. This occurs when neutrophils in the immune system release defensins in response to injury, and, in turn, defensins activate LGR6+ stem cells. Situated above the follicular bulge, these cells are reported to have the capacity to synthesize all cutaneous cell lineages, including sebaceous gland and interfollicular epidermal cells.1,2 There are no specific studies that show that the LGR6+ cells generate new fibroblasts, but it seems likely. Transplantation of LGR6+ stem cells into the skin results in increased wound healing, hair follicle genesis, and angiogenesis.3 LGR6+ stem cells repopulate the epidermis by creating new basal stem cells. In regards to skin rejuvenation, it is clear that activated LGR6+ stems cells produce new, younger-acting keratinocytes in the epidermis.
Peptides
Defensin is a peptide. Peptides are short amino acid chains. These important substances are challenging to incorporate into topical formulations for various reasons, including stabilization difficulty, interaction with other molecules, and poor penetration (greater than 500 Dalton molecular weight). For these reasons, many peptide-containing formulations do not have efficacy. Attempts are underway to better develop or modify peptide products to enhance solubility, achieve better penetration, and target increased receptor activity. Defensins are peptides, which makes them difficult to formulate in a topical product. Special steps must be taken in the formulation process to stabilize defensin and allow penetration into the hair follicle where the LGR6+ cells reside. Fortunately, it is easier for a peptide to target the hair follicle because it can traverse through the “pore” – than it is to get a peptide to reach the fibroblasts in the dermis.
Defensins
Defensins, or human beta-defensins, are host defense peptides that exhibit antimicrobial activities against numerous bacteria.4 LGR6+ stem cells, which are dormant until they are activated to respond to damage, are stimulated by defensins. Defensins have been shown to stimulate keratinocyte proliferation, migration, and wound healing. (3) **Human alpha-defensin 5 peptide has also been shown to enhance wound healing, increasing LGR5+ and LGR6+ stem cell migration in the wound bed.(1)***
When formulated in a manner that allows for stability and penetration into the hair follicle where the LGR6+ stem cells reside, defensin formulations can be applied topically. A product sold as DefenAge uses a patented formulation that uses albumin, a large and stable protein, to stabilize defensin and act as a carrier molecule while helping the defensin maintain its integrity and extend shelf life in the serum base. The albumin/defensin complex is incorporated into liposomes to prevent other ingredients in the cosmetic base from interacting with the peptide and to enhance delivery to the LGR6+ target cell.
The role of defensins in treating skin aging
- Old fibroblast and keratinocytes are sluggish and lazy.
- Old cells do not “hear” signals as well as younger cells.
- LGR6+ stem cells repopulate the epidermis with new, young keratinocytes.
- Defensin stimulates LGR6+ stem cells.
- The defensin/LGR6+ pathway plays a role in keratinization.
- Using topical defensin can improve the skin’s appearance.
Studying DefenAge
At this time, there is only one small multicenter, double-blind, placebo-controlled clinical study completed at three locations by investigators who are stockholders in the company and an independent dermatologic histopathologist who has no relation with the company; results have been reported in aesthetic dermatology industry newsletters. Each site had 15 patients for a total of 45 patients; all were women, aged 41-70 years (average age, 60 years), with little or no history of “quality” skin care. The study regimen used a system that contained alpha- and beta-defensins developed by Progenitor Biologics. Thirty patients used the three products in the DefenAge line: the 2-Minute Reveal Masque Exfoliator, 24/7 Barrier Balance Cream, and 8-in-1 BioSerum. The remaining patients received a three-part placebo system. Baseline biopsies were obtained to evaluate underlying conditions in the patients’ skin, and their skin was evaluated at 6 and 12 weeks, when additional biopsies were taken. Data analysis indicated that patients using DefenAge experienced significant improvement in coarse and fine wrinkles, pigmentation, pore prominence, epidermal thickness, as well as skin texture and evenness.
My personal opinion
I have never been a fan of formulations containing stem cells or peptides for the reasons listed above. DefenAge is unique in the way it has been stabilized, by penetrating the hair follicle rather than through the dermis and because defensin has very well-documented effects on the important LGR6+ stem cells. The effects of defensin on LGR6+ stem cells intrigue me. I do not intend to stop recommending retinoids for antiaging, but rather will add DefenAge to the antiaging regimen. In the past year, I have used DefenAge on many patients and have had many observations. I do not recommend starting retinoids and DefenAge at the same time because I have seen increased retinoid dermatitis. I suggest starting one the first month and then introducing the other product during the second month. Although no studies have been performed on this, my impression is that the DefenAge gives a quick result that helps improve patient compliance with the entire skin care regimen, but the effects reach a point at which no further improvement is seen. Combining DefenAge with a skin care regimen (targeted specifically to their Baumann Skin Type of course!) that includes a retinoid will increase efficacy. For wrinkle-prone skin types, I combine DefenAge with a retinoid, vitamin C, and heparan sulfate. After cleansing in the morning, I have them apply vitamin C followed by the DefenAge and an SPF. In the evening after cleansing, I have them apply a retinoid followed by a heparan sulfate analogue.
Conclusion
DefenAge offers a new approach to skin aging. At this time, there is much basic science research about the benefits of LGR6+ and that uses defensin to stimulate these stem cells; however, only one small clinical trial using defensin topically for antiaging has been published. It is doubtful that many studies will be performed because cosmetic companies are not allowed to make biologic claims so they have little incentive to demonstrate biologic changes. For this reason, we have to rely upon anecdotal reports from physicians such as the information that I have shared here.
Conflict of interest note: I have no financial relationship (no honorarium, stocks, or research funding) with Progenitor Biologics. I was asked to lecture in a DefenAge Symposium at the Vegas Cosmetic Surgery meeting but received no compensation. DefenAge products are sold through doctors, with my company, Skin Type Solutions Franchise Systems, as are heparan sulfate analogues, multiple brands of retinol, and 40 other product brands.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
2. Snippert HJ et al. Science. 2010 Mar 12;327(5971):1385-9.
3. Lough DM et al. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
4. Kiatsurayanon C et al. J Invest Dermatol. 2014 Aug;134(8):2163-73.
Most skin-aging treatments work by prodding old fibroblasts and keratinocytes to accelerate the production of important cellular components. For example, retinoids act on retinoic acid receptors to activate collagen genes and deactivate collagenase genes. Glycolic acid, ascorbic acid, and certain growth factors stimulate synthesis of collagen by fibroblasts. Older fibroblasts and keratinocytes are sluggish for many reasons; they do not “hear” signals as well as younger cells do. Glycosaminoglycans such as heparan sulfate can help cells hear these signals. Heparan sulfate, for example, assists in the delivery of growth factors to cells, stabilizes them, and presents them to the receptors on the keratinocytes and fibroblasts, and amplifies cellular response to these factors.
A new angle in antiaging skin care is to create new keratinocytes rather than to stimulate old cells. For the last decade, personal care companies have touted the benefit of putting stem cells in cosmeceuticals, claiming that these cells would rejuvenate skin. However, this proved to be unsubstantiated marketing hype because the stem cells were plant derived (often from apples), had poor shelf life, and could not intercalate between the native skin cells and work with them to have any effect. Stems cells in cosmeceuticals became a point of disdain for savvy scientists.
Stem cells
Wounding the skin stimulates LGR6+ stem cells. This occurs when neutrophils in the immune system release defensins in response to injury, and, in turn, defensins activate LGR6+ stem cells. Situated above the follicular bulge, these cells are reported to have the capacity to synthesize all cutaneous cell lineages, including sebaceous gland and interfollicular epidermal cells.1,2 There are no specific studies that show that the LGR6+ cells generate new fibroblasts, but it seems likely. Transplantation of LGR6+ stem cells into the skin results in increased wound healing, hair follicle genesis, and angiogenesis.3 LGR6+ stem cells repopulate the epidermis by creating new basal stem cells. In regards to skin rejuvenation, it is clear that activated LGR6+ stems cells produce new, younger-acting keratinocytes in the epidermis.
Peptides
Defensin is a peptide. Peptides are short amino acid chains. These important substances are challenging to incorporate into topical formulations for various reasons, including stabilization difficulty, interaction with other molecules, and poor penetration (greater than 500 Dalton molecular weight). For these reasons, many peptide-containing formulations do not have efficacy. Attempts are underway to better develop or modify peptide products to enhance solubility, achieve better penetration, and target increased receptor activity. Defensins are peptides, which makes them difficult to formulate in a topical product. Special steps must be taken in the formulation process to stabilize defensin and allow penetration into the hair follicle where the LGR6+ cells reside. Fortunately, it is easier for a peptide to target the hair follicle because it can traverse through the “pore” – than it is to get a peptide to reach the fibroblasts in the dermis.
Defensins
Defensins, or human beta-defensins, are host defense peptides that exhibit antimicrobial activities against numerous bacteria.4 LGR6+ stem cells, which are dormant until they are activated to respond to damage, are stimulated by defensins. Defensins have been shown to stimulate keratinocyte proliferation, migration, and wound healing. (3) **Human alpha-defensin 5 peptide has also been shown to enhance wound healing, increasing LGR5+ and LGR6+ stem cell migration in the wound bed.(1)***
When formulated in a manner that allows for stability and penetration into the hair follicle where the LGR6+ stem cells reside, defensin formulations can be applied topically. A product sold as DefenAge uses a patented formulation that uses albumin, a large and stable protein, to stabilize defensin and act as a carrier molecule while helping the defensin maintain its integrity and extend shelf life in the serum base. The albumin/defensin complex is incorporated into liposomes to prevent other ingredients in the cosmetic base from interacting with the peptide and to enhance delivery to the LGR6+ target cell.
The role of defensins in treating skin aging
- Old fibroblast and keratinocytes are sluggish and lazy.
- Old cells do not “hear” signals as well as younger cells.
- LGR6+ stem cells repopulate the epidermis with new, young keratinocytes.
- Defensin stimulates LGR6+ stem cells.
- The defensin/LGR6+ pathway plays a role in keratinization.
- Using topical defensin can improve the skin’s appearance.
Studying DefenAge
At this time, there is only one small multicenter, double-blind, placebo-controlled clinical study completed at three locations by investigators who are stockholders in the company and an independent dermatologic histopathologist who has no relation with the company; results have been reported in aesthetic dermatology industry newsletters. Each site had 15 patients for a total of 45 patients; all were women, aged 41-70 years (average age, 60 years), with little or no history of “quality” skin care. The study regimen used a system that contained alpha- and beta-defensins developed by Progenitor Biologics. Thirty patients used the three products in the DefenAge line: the 2-Minute Reveal Masque Exfoliator, 24/7 Barrier Balance Cream, and 8-in-1 BioSerum. The remaining patients received a three-part placebo system. Baseline biopsies were obtained to evaluate underlying conditions in the patients’ skin, and their skin was evaluated at 6 and 12 weeks, when additional biopsies were taken. Data analysis indicated that patients using DefenAge experienced significant improvement in coarse and fine wrinkles, pigmentation, pore prominence, epidermal thickness, as well as skin texture and evenness.
My personal opinion
I have never been a fan of formulations containing stem cells or peptides for the reasons listed above. DefenAge is unique in the way it has been stabilized, by penetrating the hair follicle rather than through the dermis and because defensin has very well-documented effects on the important LGR6+ stem cells. The effects of defensin on LGR6+ stem cells intrigue me. I do not intend to stop recommending retinoids for antiaging, but rather will add DefenAge to the antiaging regimen. In the past year, I have used DefenAge on many patients and have had many observations. I do not recommend starting retinoids and DefenAge at the same time because I have seen increased retinoid dermatitis. I suggest starting one the first month and then introducing the other product during the second month. Although no studies have been performed on this, my impression is that the DefenAge gives a quick result that helps improve patient compliance with the entire skin care regimen, but the effects reach a point at which no further improvement is seen. Combining DefenAge with a skin care regimen (targeted specifically to their Baumann Skin Type of course!) that includes a retinoid will increase efficacy. For wrinkle-prone skin types, I combine DefenAge with a retinoid, vitamin C, and heparan sulfate. After cleansing in the morning, I have them apply vitamin C followed by the DefenAge and an SPF. In the evening after cleansing, I have them apply a retinoid followed by a heparan sulfate analogue.
Conclusion
DefenAge offers a new approach to skin aging. At this time, there is much basic science research about the benefits of LGR6+ and that uses defensin to stimulate these stem cells; however, only one small clinical trial using defensin topically for antiaging has been published. It is doubtful that many studies will be performed because cosmetic companies are not allowed to make biologic claims so they have little incentive to demonstrate biologic changes. For this reason, we have to rely upon anecdotal reports from physicians such as the information that I have shared here.
Conflict of interest note: I have no financial relationship (no honorarium, stocks, or research funding) with Progenitor Biologics. I was asked to lecture in a DefenAge Symposium at the Vegas Cosmetic Surgery meeting but received no compensation. DefenAge products are sold through doctors, with my company, Skin Type Solutions Franchise Systems, as are heparan sulfate analogues, multiple brands of retinol, and 40 other product brands.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
2. Snippert HJ et al. Science. 2010 Mar 12;327(5971):1385-9.
3. Lough DM et al. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
4. Kiatsurayanon C et al. J Invest Dermatol. 2014 Aug;134(8):2163-73.
Most skin-aging treatments work by prodding old fibroblasts and keratinocytes to accelerate the production of important cellular components. For example, retinoids act on retinoic acid receptors to activate collagen genes and deactivate collagenase genes. Glycolic acid, ascorbic acid, and certain growth factors stimulate synthesis of collagen by fibroblasts. Older fibroblasts and keratinocytes are sluggish for many reasons; they do not “hear” signals as well as younger cells do. Glycosaminoglycans such as heparan sulfate can help cells hear these signals. Heparan sulfate, for example, assists in the delivery of growth factors to cells, stabilizes them, and presents them to the receptors on the keratinocytes and fibroblasts, and amplifies cellular response to these factors.
A new angle in antiaging skin care is to create new keratinocytes rather than to stimulate old cells. For the last decade, personal care companies have touted the benefit of putting stem cells in cosmeceuticals, claiming that these cells would rejuvenate skin. However, this proved to be unsubstantiated marketing hype because the stem cells were plant derived (often from apples), had poor shelf life, and could not intercalate between the native skin cells and work with them to have any effect. Stems cells in cosmeceuticals became a point of disdain for savvy scientists.
Stem cells
Wounding the skin stimulates LGR6+ stem cells. This occurs when neutrophils in the immune system release defensins in response to injury, and, in turn, defensins activate LGR6+ stem cells. Situated above the follicular bulge, these cells are reported to have the capacity to synthesize all cutaneous cell lineages, including sebaceous gland and interfollicular epidermal cells.1,2 There are no specific studies that show that the LGR6+ cells generate new fibroblasts, but it seems likely. Transplantation of LGR6+ stem cells into the skin results in increased wound healing, hair follicle genesis, and angiogenesis.3 LGR6+ stem cells repopulate the epidermis by creating new basal stem cells. In regards to skin rejuvenation, it is clear that activated LGR6+ stems cells produce new, younger-acting keratinocytes in the epidermis.
Peptides
Defensin is a peptide. Peptides are short amino acid chains. These important substances are challenging to incorporate into topical formulations for various reasons, including stabilization difficulty, interaction with other molecules, and poor penetration (greater than 500 Dalton molecular weight). For these reasons, many peptide-containing formulations do not have efficacy. Attempts are underway to better develop or modify peptide products to enhance solubility, achieve better penetration, and target increased receptor activity. Defensins are peptides, which makes them difficult to formulate in a topical product. Special steps must be taken in the formulation process to stabilize defensin and allow penetration into the hair follicle where the LGR6+ cells reside. Fortunately, it is easier for a peptide to target the hair follicle because it can traverse through the “pore” – than it is to get a peptide to reach the fibroblasts in the dermis.
Defensins
Defensins, or human beta-defensins, are host defense peptides that exhibit antimicrobial activities against numerous bacteria.4 LGR6+ stem cells, which are dormant until they are activated to respond to damage, are stimulated by defensins. Defensins have been shown to stimulate keratinocyte proliferation, migration, and wound healing. (3) **Human alpha-defensin 5 peptide has also been shown to enhance wound healing, increasing LGR5+ and LGR6+ stem cell migration in the wound bed.(1)***
When formulated in a manner that allows for stability and penetration into the hair follicle where the LGR6+ stem cells reside, defensin formulations can be applied topically. A product sold as DefenAge uses a patented formulation that uses albumin, a large and stable protein, to stabilize defensin and act as a carrier molecule while helping the defensin maintain its integrity and extend shelf life in the serum base. The albumin/defensin complex is incorporated into liposomes to prevent other ingredients in the cosmetic base from interacting with the peptide and to enhance delivery to the LGR6+ target cell.
The role of defensins in treating skin aging
- Old fibroblast and keratinocytes are sluggish and lazy.
- Old cells do not “hear” signals as well as younger cells.
- LGR6+ stem cells repopulate the epidermis with new, young keratinocytes.
- Defensin stimulates LGR6+ stem cells.
- The defensin/LGR6+ pathway plays a role in keratinization.
- Using topical defensin can improve the skin’s appearance.
Studying DefenAge
At this time, there is only one small multicenter, double-blind, placebo-controlled clinical study completed at three locations by investigators who are stockholders in the company and an independent dermatologic histopathologist who has no relation with the company; results have been reported in aesthetic dermatology industry newsletters. Each site had 15 patients for a total of 45 patients; all were women, aged 41-70 years (average age, 60 years), with little or no history of “quality” skin care. The study regimen used a system that contained alpha- and beta-defensins developed by Progenitor Biologics. Thirty patients used the three products in the DefenAge line: the 2-Minute Reveal Masque Exfoliator, 24/7 Barrier Balance Cream, and 8-in-1 BioSerum. The remaining patients received a three-part placebo system. Baseline biopsies were obtained to evaluate underlying conditions in the patients’ skin, and their skin was evaluated at 6 and 12 weeks, when additional biopsies were taken. Data analysis indicated that patients using DefenAge experienced significant improvement in coarse and fine wrinkles, pigmentation, pore prominence, epidermal thickness, as well as skin texture and evenness.
My personal opinion
I have never been a fan of formulations containing stem cells or peptides for the reasons listed above. DefenAge is unique in the way it has been stabilized, by penetrating the hair follicle rather than through the dermis and because defensin has very well-documented effects on the important LGR6+ stem cells. The effects of defensin on LGR6+ stem cells intrigue me. I do not intend to stop recommending retinoids for antiaging, but rather will add DefenAge to the antiaging regimen. In the past year, I have used DefenAge on many patients and have had many observations. I do not recommend starting retinoids and DefenAge at the same time because I have seen increased retinoid dermatitis. I suggest starting one the first month and then introducing the other product during the second month. Although no studies have been performed on this, my impression is that the DefenAge gives a quick result that helps improve patient compliance with the entire skin care regimen, but the effects reach a point at which no further improvement is seen. Combining DefenAge with a skin care regimen (targeted specifically to their Baumann Skin Type of course!) that includes a retinoid will increase efficacy. For wrinkle-prone skin types, I combine DefenAge with a retinoid, vitamin C, and heparan sulfate. After cleansing in the morning, I have them apply vitamin C followed by the DefenAge and an SPF. In the evening after cleansing, I have them apply a retinoid followed by a heparan sulfate analogue.
Conclusion
DefenAge offers a new approach to skin aging. At this time, there is much basic science research about the benefits of LGR6+ and that uses defensin to stimulate these stem cells; however, only one small clinical trial using defensin topically for antiaging has been published. It is doubtful that many studies will be performed because cosmetic companies are not allowed to make biologic claims so they have little incentive to demonstrate biologic changes. For this reason, we have to rely upon anecdotal reports from physicians such as the information that I have shared here.
Conflict of interest note: I have no financial relationship (no honorarium, stocks, or research funding) with Progenitor Biologics. I was asked to lecture in a DefenAge Symposium at the Vegas Cosmetic Surgery meeting but received no compensation. DefenAge products are sold through doctors, with my company, Skin Type Solutions Franchise Systems, as are heparan sulfate analogues, multiple brands of retinol, and 40 other product brands.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
References
1. Lough D et al. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
2. Snippert HJ et al. Science. 2010 Mar 12;327(5971):1385-9.
3. Lough DM et al. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
4. Kiatsurayanon C et al. J Invest Dermatol. 2014 Aug;134(8):2163-73.
April 2018 Digital Edition
Click here to access the April 2018 Digital Edition.
Table of Contents
- Understanding, Assessing, and Conceptualizing Suicide Risk Among Veterans With PTSD
- Reducing the Expenditures and Workload Associated With VA Partial-Fill Prescription Processing
- Acute Exertional Upper-Extremity Rhabdomyolysis in 3 Female Trainees
- A Practical Guide to Urine Drug Monitoring
- Synovial Chondromatosis: An Unusual Case of Knee Pain and Swelling
- Complementary and Integrative Health Therapies for Opioid Overuse

Click here to access the April 2018 Digital Edition.
Table of Contents
- Understanding, Assessing, and Conceptualizing Suicide Risk Among Veterans With PTSD
- Reducing the Expenditures and Workload Associated With VA Partial-Fill Prescription Processing
- Acute Exertional Upper-Extremity Rhabdomyolysis in 3 Female Trainees
- A Practical Guide to Urine Drug Monitoring
- Synovial Chondromatosis: An Unusual Case of Knee Pain and Swelling
- Complementary and Integrative Health Therapies for Opioid Overuse

Click here to access the April 2018 Digital Edition.
Table of Contents
- Understanding, Assessing, and Conceptualizing Suicide Risk Among Veterans With PTSD
- Reducing the Expenditures and Workload Associated With VA Partial-Fill Prescription Processing
- Acute Exertional Upper-Extremity Rhabdomyolysis in 3 Female Trainees
- A Practical Guide to Urine Drug Monitoring
- Synovial Chondromatosis: An Unusual Case of Knee Pain and Swelling
- Complementary and Integrative Health Therapies for Opioid Overuse

Neurologic and Spinal Cord Disorders in Federal Health Care System (April 2018)
Click here to access Neurologic and Spinal Cord Disorders in Federal Health Care System Digital Edition.
Table of Contents
- Neurology Research News
- Self-Management in Epilepsy Care: Untapped Opportunities
- Restless Legs Syndrome Among Veterans With Spinal Cord Lesions
- A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury

Click here to access Neurologic and Spinal Cord Disorders in Federal Health Care System Digital Edition.
Table of Contents
- Neurology Research News
- Self-Management in Epilepsy Care: Untapped Opportunities
- Restless Legs Syndrome Among Veterans With Spinal Cord Lesions
- A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury

Click here to access Neurologic and Spinal Cord Disorders in Federal Health Care System Digital Edition.
Table of Contents
- Neurology Research News
- Self-Management in Epilepsy Care: Untapped Opportunities
- Restless Legs Syndrome Among Veterans With Spinal Cord Lesions
- A Robotic Hand Device Safety Study for People With Cervical Spinal Cord Injury

Hyponatremia After Traumatic Brain Injury
Hyponatremia is a dangerous complication of major head trauma, and timely diagnosis and treatment can be fraught with “confounding factors” and complexity, say clinicians from the University of Newcastle and John Hunter Hospital in Australia. They reported a case of hyponatremia that required some clinical tightrope walking.
The patient, a 20-year-old university student, had fractured his skull in a skateboard fall while intoxicated. He was started on dexamethasone to reduce the risk of worsening cerebral edema. On day 3, he developed hypo-osmolar hyponatremia, which was worse on day 4, despite treatment, including IV fluid therapy, fluid restriction, and oral salt tablets. Although cognitively the patient was deteriorating, he seemed clinically euvolemic. However, the patient was in negative fluid balance, suggesting renal salt wasting (RSW). After a trial of isotonic normal saline, the patient’s serum sodium level fell further. The patient was then treated for suspected syndrome of inappropriate antidiuretic hormone (SIADH) with a hypertonic saline infusion. The rise in sodium was carefully controlled to avoid rapid overcorrection, which can lead to irreversible neurologic symptoms. Finally, the patient’s sodium level and neurologic status improved.
The clinicians say the case demonstrates the complexity of differentiating between the causes of hyponatremia after head injury. Volume status may be an indicator, they say, but current clinical and laboratory markers of volume status are often limited in accuracy. The hallmark of RSW is volume depletion, whereas diagnosis of SIADH depends on a coexisting euvolemic state (as with the patient).
As many as 10% of victims of traumatic brain injury develop hyponatremia, and it is associated with a worse prognosis, even in mild cases, the clinicians note. Making the right diagnosis is critical—the treatment chosen can easily compromise the outcome. Patients with neurosurgical conditions are often treated with considerable volumes of saline-containing fluid, with consequent dynamic changes in blood and extracellular volumes. Moreover, the patients have elevated levels of adrenergic hormones with their own confounding effects.
In the long term, the patient experienced significant neurologic sequelae, including prolonged posttraumatic amnesia. After extensive rehabilitation he was able to return to the university.
Hyponatremia is a dangerous complication of major head trauma, and timely diagnosis and treatment can be fraught with “confounding factors” and complexity, say clinicians from the University of Newcastle and John Hunter Hospital in Australia. They reported a case of hyponatremia that required some clinical tightrope walking.
The patient, a 20-year-old university student, had fractured his skull in a skateboard fall while intoxicated. He was started on dexamethasone to reduce the risk of worsening cerebral edema. On day 3, he developed hypo-osmolar hyponatremia, which was worse on day 4, despite treatment, including IV fluid therapy, fluid restriction, and oral salt tablets. Although cognitively the patient was deteriorating, he seemed clinically euvolemic. However, the patient was in negative fluid balance, suggesting renal salt wasting (RSW). After a trial of isotonic normal saline, the patient’s serum sodium level fell further. The patient was then treated for suspected syndrome of inappropriate antidiuretic hormone (SIADH) with a hypertonic saline infusion. The rise in sodium was carefully controlled to avoid rapid overcorrection, which can lead to irreversible neurologic symptoms. Finally, the patient’s sodium level and neurologic status improved.
The clinicians say the case demonstrates the complexity of differentiating between the causes of hyponatremia after head injury. Volume status may be an indicator, they say, but current clinical and laboratory markers of volume status are often limited in accuracy. The hallmark of RSW is volume depletion, whereas diagnosis of SIADH depends on a coexisting euvolemic state (as with the patient).
As many as 10% of victims of traumatic brain injury develop hyponatremia, and it is associated with a worse prognosis, even in mild cases, the clinicians note. Making the right diagnosis is critical—the treatment chosen can easily compromise the outcome. Patients with neurosurgical conditions are often treated with considerable volumes of saline-containing fluid, with consequent dynamic changes in blood and extracellular volumes. Moreover, the patients have elevated levels of adrenergic hormones with their own confounding effects.
In the long term, the patient experienced significant neurologic sequelae, including prolonged posttraumatic amnesia. After extensive rehabilitation he was able to return to the university.
Hyponatremia is a dangerous complication of major head trauma, and timely diagnosis and treatment can be fraught with “confounding factors” and complexity, say clinicians from the University of Newcastle and John Hunter Hospital in Australia. They reported a case of hyponatremia that required some clinical tightrope walking.
The patient, a 20-year-old university student, had fractured his skull in a skateboard fall while intoxicated. He was started on dexamethasone to reduce the risk of worsening cerebral edema. On day 3, he developed hypo-osmolar hyponatremia, which was worse on day 4, despite treatment, including IV fluid therapy, fluid restriction, and oral salt tablets. Although cognitively the patient was deteriorating, he seemed clinically euvolemic. However, the patient was in negative fluid balance, suggesting renal salt wasting (RSW). After a trial of isotonic normal saline, the patient’s serum sodium level fell further. The patient was then treated for suspected syndrome of inappropriate antidiuretic hormone (SIADH) with a hypertonic saline infusion. The rise in sodium was carefully controlled to avoid rapid overcorrection, which can lead to irreversible neurologic symptoms. Finally, the patient’s sodium level and neurologic status improved.
The clinicians say the case demonstrates the complexity of differentiating between the causes of hyponatremia after head injury. Volume status may be an indicator, they say, but current clinical and laboratory markers of volume status are often limited in accuracy. The hallmark of RSW is volume depletion, whereas diagnosis of SIADH depends on a coexisting euvolemic state (as with the patient).
As many as 10% of victims of traumatic brain injury develop hyponatremia, and it is associated with a worse prognosis, even in mild cases, the clinicians note. Making the right diagnosis is critical—the treatment chosen can easily compromise the outcome. Patients with neurosurgical conditions are often treated with considerable volumes of saline-containing fluid, with consequent dynamic changes in blood and extracellular volumes. Moreover, the patients have elevated levels of adrenergic hormones with their own confounding effects.
In the long term, the patient experienced significant neurologic sequelae, including prolonged posttraumatic amnesia. After extensive rehabilitation he was able to return to the university.
Hope, hepatology, and social determinants of health
Welcome to the April edition of GI & Hepatology News. April has always been a month where we have a sense of renewal and hope. For those of us living in northern climates, the distinct change in daylight and the melting of the snow (finally) both lifts us from the doldrums of winter darkness. In just over a month, we will gather in Washington for Digestive Disease Week® (DDW). I have seen a preview of AGA plenary sessions (basic science and clinical). They will be terrific. We will hear about advances in areas such as the microbiome, IBD-related inflammatory pathways, new insights into functional bowel disorders, and myriad new therapeutics (both medical and device) for us to share with our patients.
Substantial work is being done to better define an IBD severity index. These metrics are of critical importance for clinical researchers to use as we investigate the efficacy and effectiveness of new IBD drugs. You can also read about incorporating psychological care in the management of chronic diseases – a topic becoming more important as we expand our focus beyond just the biology of disease and into social determinants of health as we continue our transition to value-based reimbursement. Another topic included this month (and to which several DDW sessions are dedicated) is the devastating impact of opiates on our patients.
We have included a number of hepatology articles this month, such as the front-page story on NASH and its relationship with hepatocellular cancer. Pioglitazone benefits NASH patients with and without type 2 diabetes and biomarkers may predict liver transplant failures. There are selected articles about Barrett’s esophagus progression and risk stratification for colorectal cancer.
From Washington, we have received some good news. Please see the AGA commentary on the proposed budget. We were reminded last month about how Federal politics can impact U.S. medicine. With the (very late) reauthorization of the Children’s Health Insurance Plan (CHIP), we saw how political dysfunction can impact millions of American family’s lives. Changes in 340-B funding, continued transition from commercial to government payers, a tightening labor market, relentless increases in overhead expenses, all combine to reduce financial margins of both academic and nonacademic health systems. Economic pressures are leading to massive consolidations within the health care delivery system. Vertical integrations now have supplanted horizontal integrations as the industry trend. This situation that will impact many of our independent gastroenterology practices as demand-side management by large national corporations increases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Welcome to the April edition of GI & Hepatology News. April has always been a month where we have a sense of renewal and hope. For those of us living in northern climates, the distinct change in daylight and the melting of the snow (finally) both lifts us from the doldrums of winter darkness. In just over a month, we will gather in Washington for Digestive Disease Week® (DDW). I have seen a preview of AGA plenary sessions (basic science and clinical). They will be terrific. We will hear about advances in areas such as the microbiome, IBD-related inflammatory pathways, new insights into functional bowel disorders, and myriad new therapeutics (both medical and device) for us to share with our patients.
Substantial work is being done to better define an IBD severity index. These metrics are of critical importance for clinical researchers to use as we investigate the efficacy and effectiveness of new IBD drugs. You can also read about incorporating psychological care in the management of chronic diseases – a topic becoming more important as we expand our focus beyond just the biology of disease and into social determinants of health as we continue our transition to value-based reimbursement. Another topic included this month (and to which several DDW sessions are dedicated) is the devastating impact of opiates on our patients.
We have included a number of hepatology articles this month, such as the front-page story on NASH and its relationship with hepatocellular cancer. Pioglitazone benefits NASH patients with and without type 2 diabetes and biomarkers may predict liver transplant failures. There are selected articles about Barrett’s esophagus progression and risk stratification for colorectal cancer.
From Washington, we have received some good news. Please see the AGA commentary on the proposed budget. We were reminded last month about how Federal politics can impact U.S. medicine. With the (very late) reauthorization of the Children’s Health Insurance Plan (CHIP), we saw how political dysfunction can impact millions of American family’s lives. Changes in 340-B funding, continued transition from commercial to government payers, a tightening labor market, relentless increases in overhead expenses, all combine to reduce financial margins of both academic and nonacademic health systems. Economic pressures are leading to massive consolidations within the health care delivery system. Vertical integrations now have supplanted horizontal integrations as the industry trend. This situation that will impact many of our independent gastroenterology practices as demand-side management by large national corporations increases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Welcome to the April edition of GI & Hepatology News. April has always been a month where we have a sense of renewal and hope. For those of us living in northern climates, the distinct change in daylight and the melting of the snow (finally) both lifts us from the doldrums of winter darkness. In just over a month, we will gather in Washington for Digestive Disease Week® (DDW). I have seen a preview of AGA plenary sessions (basic science and clinical). They will be terrific. We will hear about advances in areas such as the microbiome, IBD-related inflammatory pathways, new insights into functional bowel disorders, and myriad new therapeutics (both medical and device) for us to share with our patients.
Substantial work is being done to better define an IBD severity index. These metrics are of critical importance for clinical researchers to use as we investigate the efficacy and effectiveness of new IBD drugs. You can also read about incorporating psychological care in the management of chronic diseases – a topic becoming more important as we expand our focus beyond just the biology of disease and into social determinants of health as we continue our transition to value-based reimbursement. Another topic included this month (and to which several DDW sessions are dedicated) is the devastating impact of opiates on our patients.
We have included a number of hepatology articles this month, such as the front-page story on NASH and its relationship with hepatocellular cancer. Pioglitazone benefits NASH patients with and without type 2 diabetes and biomarkers may predict liver transplant failures. There are selected articles about Barrett’s esophagus progression and risk stratification for colorectal cancer.
From Washington, we have received some good news. Please see the AGA commentary on the proposed budget. We were reminded last month about how Federal politics can impact U.S. medicine. With the (very late) reauthorization of the Children’s Health Insurance Plan (CHIP), we saw how political dysfunction can impact millions of American family’s lives. Changes in 340-B funding, continued transition from commercial to government payers, a tightening labor market, relentless increases in overhead expenses, all combine to reduce financial margins of both academic and nonacademic health systems. Economic pressures are leading to massive consolidations within the health care delivery system. Vertical integrations now have supplanted horizontal integrations as the industry trend. This situation that will impact many of our independent gastroenterology practices as demand-side management by large national corporations increases.
John I. Allen, MD, MBA, AGAF
Editor in Chief
Pain Management in an Opioid Epidemic What’s Appropriate, What’s Safe
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
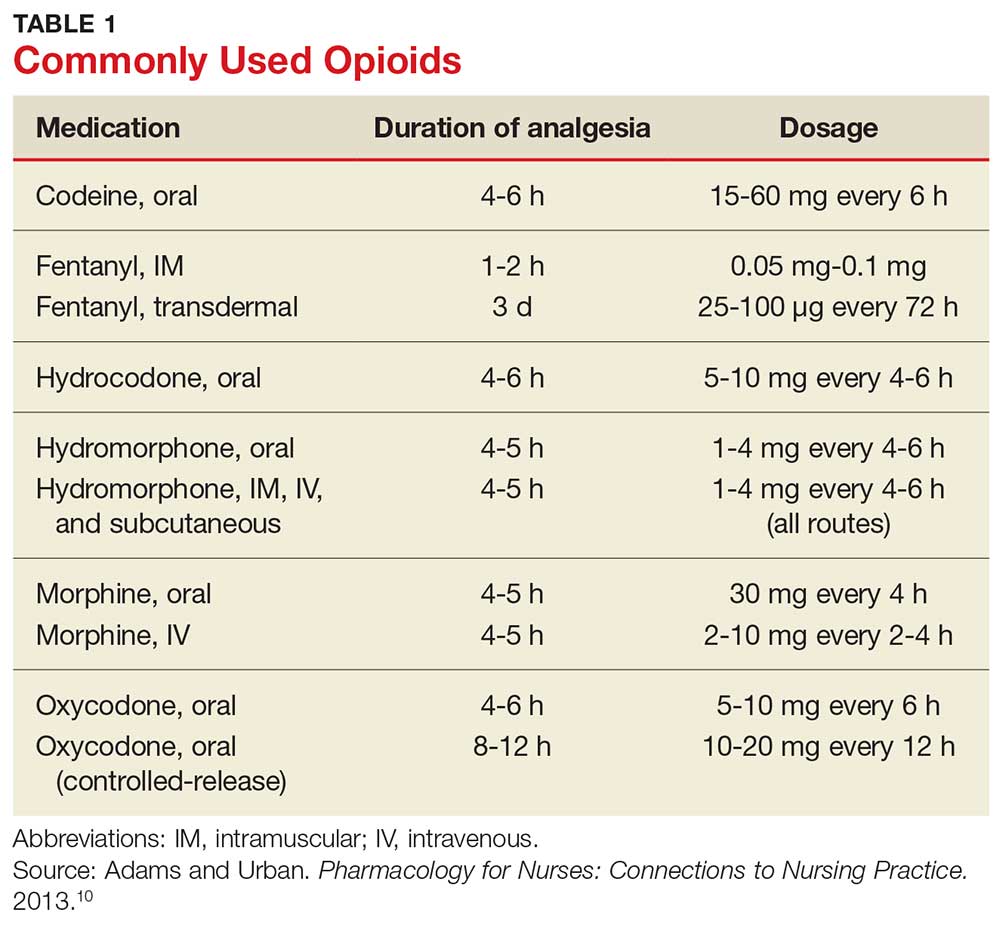
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10
Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
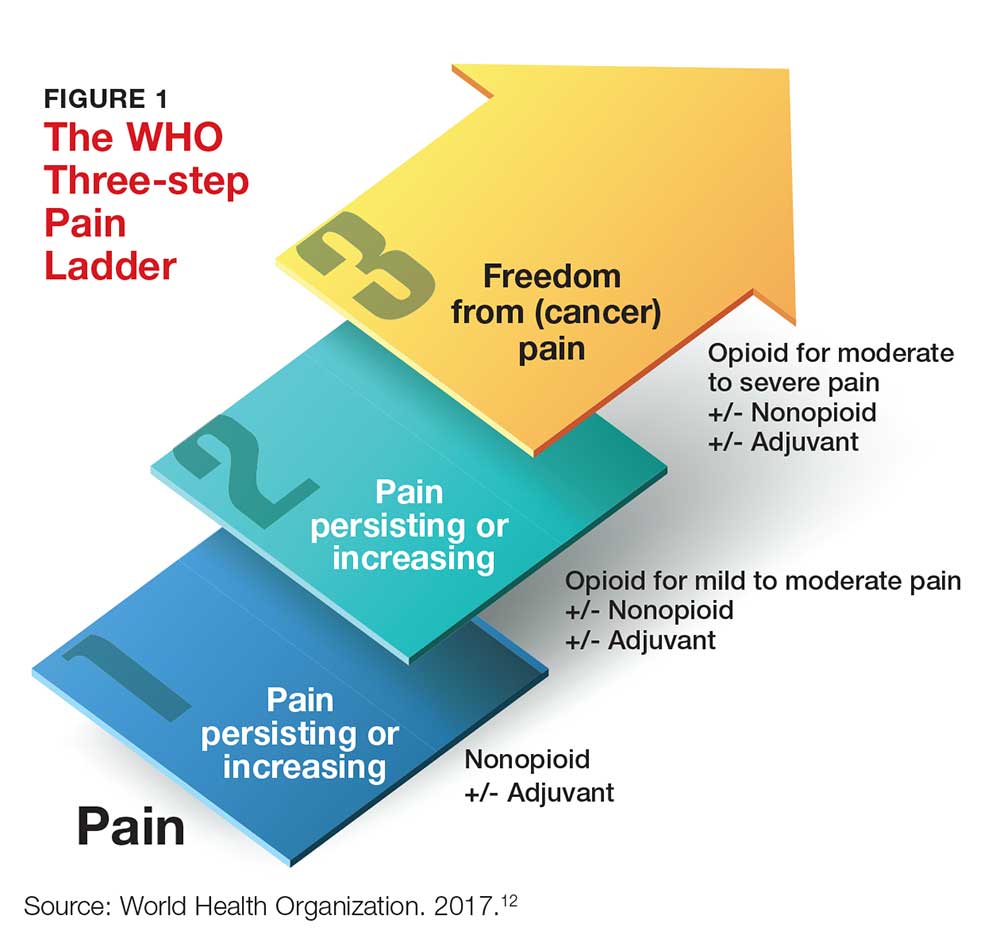
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.
Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
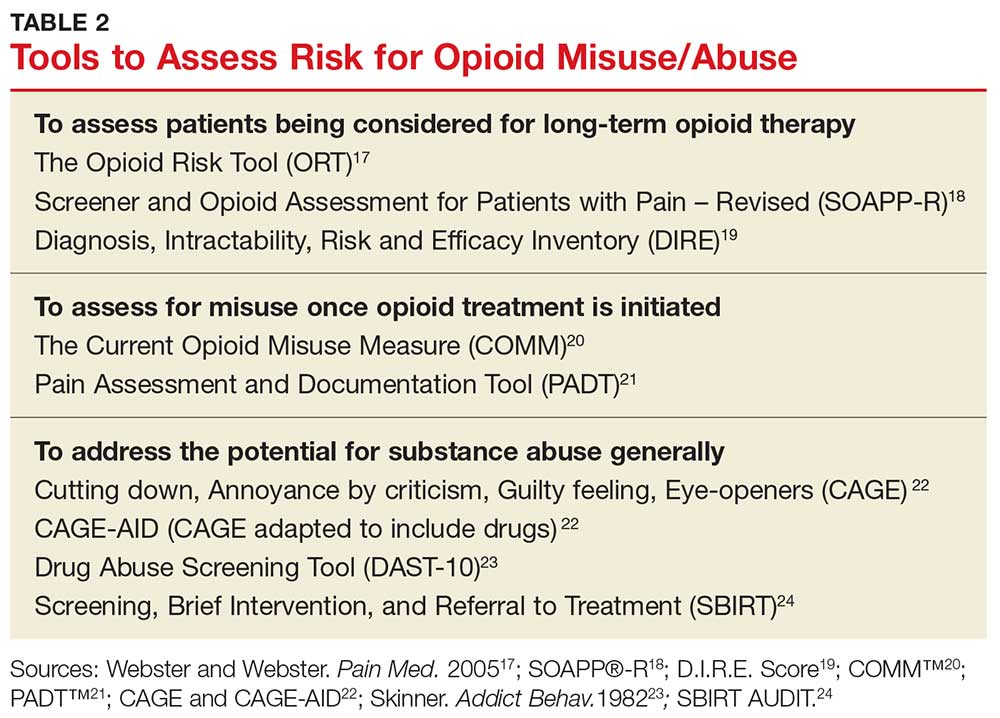
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25
Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30
CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10
Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.
Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25
Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30
CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
CE/CME No: CR-1804
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Understand the basic pharmacology of opioid medications and how they affect pain.
• Apply a stepwise approach to pain management, based on the World Health Organization's "pain ladder."
• Communicate to patients the key educational points on the risks of opioid use.
• Identify strategies to deter or detect opioid misuse or abubse.
FACULTY
Deborah Salani is an Associate Professor of Clinical and Director of the Accelerated BSN Program, Nichole A. Crenshaw is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Acute Care Nurse Practitioner Program, Brenda Owusu is an Assistant Professor of Clinical and Program Director for the Adult Gerontology Primary Care Nurse Practitioner Program, and Juan M. Gonzalez is an Assistant Professor of Clinical and Program Director for the Family Nurse Practitioner Program, at the University of Miami School of Nursing and Health Studies in Coral Gables, Florida.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid through March 31, 2019.
Article begins on next page >>
Abuse of prescribed controlled substances—particularly opioid analgesics—and associated morbidity and mortality are a serious public health problem. The response to this crisis must include prevention, early identification, and appropriate treatment of addiction. Prescribing NPs and PAs must understand how to manage acute and chronic pain while also being attentive to signs of drug seeking and opioid misuse and abuse. The information and tools outlined in this article can equip providers to combat the opioid epidemic.
Controlled prescription drug abuse and its associated morbidity and mortality are a serious public health problem globally. In 2015, more than 29 million people worldwide misused and abused drugs, according to the United Nations Office on Drugs and Crime.1 Opioid use disorders account for approximately 70% of that estimate.
In the US, the mortality associated with this abuse has been devastating. Between 1999 and 2014, drug overdose deaths nearly tripled; in 2014 alone, there were 47,055 such fatalities, 61% of which involved opioids.2,3 Since 2000, unintentional overdose deaths from opioids have increased by 200%.3 Overdose deaths associated with natural and semisynthetic opioids (the most commonly prescribed pain relievers) increased 9% from 2013 to 2014, while those associated with synthetic opioids (fentanyl and tramadol) nearly doubled in the same period.3
Further contributing to the problem, a person addicted to prescription opioid drugs is 40 times more likely to be addicted to heroin, compared to someone who is not addicted to opioids.4 Deaths related to heroin overdose continue to dramatically increase.3
A call to action
In August 2016, former US Surgeon General Vivek Murthy, MD, sent a personal letter to more than 2.3 million health care providers, seeking their assistance in addressing the prescription opioid crisis.5 Murthy acknowledged the challenges providers face when attempting to strike a balance between treating a patient’s pain and reducing the risk for opioid addiction. He explained that clinicians are uniquely situated to end this crisis, and he asked providers to pledge to “turn the tide” by taking three actions
- Become more educated about treating pain safely and effectively.
- Screen patients for opioid use disorder and make the appropriate evidence-based treatment referrals.
- Discuss and treat addiction as a chronic disorder.5
To help stem the epidemic of controlled prescription drug abuse, NPs and PAs must be knowledgeable about patient safety issues, including how to identify patients at risk for opioid misuse and recognize signs of misuse or abuse. This article aims to educate providers who have prescriptive authority about the pharmacology of opioids; safe and effective prescribing of these drugs; and how to identify and manage misuse and abuse.
Continue to: OVERVIEW OF PAIN
OVERVIEW OF PAIN
Pain, considered the fifth vital sign, is one of the more common reasons that people seek treatment from a health care provider. Pain is a personal, individual, subjective experience: It is whatever the patient says it is and exists whenever the patient says it does. Pain is an unpleasant sensory and emotional experience associated with actual or potential tissue damage.
Pain is classified as acute or chronic. Acute pain is a sudden but temporary, self-limiting response to some type of bodily injury; it generally lasts less than six months. Chronic pain is often associated with prolonged diseases such as cancer, fibromyalgia, and osteoarthritis; it persists for six months or longer.
Pain can be separated into two categories: nociceptive and neuropathic. Nociceptive pain originates from peripheral or visceral nociceptors as a result of injury and comprises somatic and visceral pain. Somatic pain is caused by injury to soft tissue, connective tissue, and bone; the classic description is a sharp, well-localized discomfort. Visceral pain originates from an organ or deeper structure; it is commonly described as dull, poorly localized, and sensitive to stretch, ischemia, and inflammation.
Neuropathic pain is an abnormal processing of pain stimuli by the peripheral nervous system or central nervous system (CNS) and can result from injury or inflammation to a nerve. Neuropathic pain is usually described by patients as electric, burning, and/or shooting. Examples include pain associated with cancer, diabetic neuropathy, and phantom-limb sensation (following amputation).
The physiologic experience of pain follows a defined set of phases. First is transduction, which occurs at the moment of injury or trauma; sensory nerve endings convert the noxious stimulus into a nerve impulse. Second is transmission of the pain impulse to the spinal column by means of chemical messengers known as neurotransmitters.
After the pain impulse reaches the spinal tract, it continues to the brain, at which point there is perception, the third step in the process. This leads to modulation (also known as anti-nociception). In this fourth step, neurons that originate in the brainstem are activated, releasing neurotransmitters that inhibit transmission of pain. Modulation occurs in several areas of the CNS and involves the neurotransmitters serotonin, norepinephrine, and endogenous opioids (eg, ß-endorphin).6 During modulation, the limbic nervous system provokes a response to the painful stimulus, triggering endogenous opioids to bind to opioid receptors.7
Continue to: ROLE OF OPIOIDS IN PAIN MANAGEMENT
ROLE OF OPIOIDS IN PAIN MANAGEMENT
Opioids have been used to control pain for centuries. They are extracted from the opium poppy plant, Papaver somniferum. From the substance extracted, roughly 9% to 14% is morphine and 0.8% to 2.5% is codeine.7 Opioids are used to treat many symptoms and ailments, including diarrhea, moderate to severe pain, and persistent cough.
Opioids work through receptors in the CNS, including mu, kappa, and delta opioid receptors and the opioid-like receptor nociceptin.7 The principal receptors associated with pain physiology and inhibition are mu and kappa. (Morphine, the gold standard for treating severe pain, is an opioid agonist that binds to mu and kappa receptors.)
Most opioids that are used clinically bind to mu receptors; these drugs provide analgesia but also present the risk for adverse effects, such as decreased respiratory drive, miosis, and decreased motor function of the gastrointestinal (GI) tract, which can lead to constipation.8 Because mu receptors are located mainly in the brain and spinal cord (as well as the GI tract), opioids also produce a feeling of euphoria that can lead to dependence.
Kappa receptors, in contrast, are located mainly in the limbic system, diencephalic area, and spinal cord. When these receptors are activated, they can produce spinal analgesia, dyspnea, dependence, and dysphoria.
Delta receptors also play a role in pain management and are associated with emotional and affective components of the experience of pain.7 They are largely located in the brain; when activated, they can lead to spinal and supraspinal anesthesia, as well as decreased gastric motility.8 Delta receptors have not been studied as much as mu and kappa receptors, but it has been suggested that they play a role in psychologic dependency.
Depending on the effect that a drug has on these receptors, it can be considered a full (or pure) opioid agonist, a partial agonist, or a mixed agonist–antagonist. By binding to opioid receptors, opioid agonists provide pain relief. Health care providers often prescribe a full agonist, such as morphine, hydrocodone, codeine, or oxycodone, to treat pain. Partial agonists, such as buprenorphine and butorphanol, often decrease activity at mu receptor sites. Mixed agonist–antagonists either block or bind opioids at receptor sites.9 Medications that block mu and kappa receptors are considered opioid antagonists, which are used not to treat pain but rather to reverse the effect of opioids (eg, naloxone).6
Table 1 lists commonly used opioids, their analgesic duration, and the standard approved dosages.10
Continue to: A STEPWISE APPROACH TO PAIN MANAGEMENT
A STEPWISE APPROACH TO PAIN MANAGEMENT
On January 1, 2018, The Joint Commission (JNC) implemented new and revised standards to ensure that all patients receive appropriate assessment and management of their pain. While these standards apply to accredited hospitals, they provide a solid framework for assessing and treating pain in any patient. JNC now requires that patients be included in the development of treatment plans, which should encompass realistic expectations and reasonable goals, and that providers promote safe opioid use by identifying and monitoring high-risk patients.11
One valuable tool that can help clinicians fulfill the obligation to provide safe and effective pain management is the World Health Organization’s “pain ladder” (see Figure 1).12 Originally released in 1986 to address cancer pain in the pediatric population, this tool has proven validity. It has since been expanded to guide treatment of pain in other patient populations. In addition, the steps of the “pain ladder” provide useful information on the clinical examination and documentation of pain, principles of pharmacotherapeutic management, and considerations when using different analgesics.
Pain is assessed on a scale of 1 to 10, with 1 representing the least pain. Medication recommendations are as follows
- For mild pain (ie, a score of 1-3): acetaminophen, NSAIDs, or other nonopioids.
- For moderate pain (pain score, 4-6): an opioid (eg, hydrocodone), with or without an adjunct medication.
- For severe pain (pain score, 7-10) or pain that has not responded to previous therapies: a stronger opioid (eg, morphine, hydromorphone, fentanyl), with or without an adjuvant drug.12
In all cases, patients should be informed about both pharmacotherapeutic and nonpharmacotherapeutic options. The latter include hypnosis, relaxation techniques, acupuncture, physical therapy, application of heat and cold, and electro-analgesia.
Pharmacologic options at any “step” of the ladder carry the risk for adverse effects. Thus, NPs and PAs who prescribe these medications need to apprise patients of the potential harms associated with their treatment.
Acetaminophen. Patients should be instructed on the safe use of acetaminophen, particularly with regard to dosing, since liver damage can occur. Patients should not take more than 4,000 mg in a 24-hour period, and each dose should not exceed 1,000 mg.
NSAIDs. These drugs are often used for short-term management of mild and moderate pain. Patients should be instructed to take these agents with food to decrease GI upset. Other common adverse effects include GI bleed or perforation and renal insufficiency or failure.
Opioids. Depending on which class of receptors an opioid medication targets, patients may develop any of the following: constipation, decreased GI motility, nausea, hypotension, urinary retention, euphoria, pruritus, miosis, dependence, respiratory depression, and sedation. It is important for NPs and PAs who prescribe these medications to remain vigilant for adverse effects and complications from opioid use and to educate the patient and his/her family about possible complications.6
Patients must be instructed not to drink alcohol or take other CNS depressants while taking an opioid. They should be advised about the dangers of operating heavy equipment or engaging in other activities that require mental and physical alertness, since opioids can cause drowsiness. Among the GI effects of some opioids (nausea, vomiting) is constipation—so patients should also be educated on the need to increase fluid intake and include high-fiber foods in their diet.9
But most important of all, patients taking an opioid should be informed that there is the potential for physical dependency and abuse with these agents, and these agents should be used only for acute, severe pain.
Continue to: DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
DETECTING & MANAGING PRESCRIPTION DRUG MISUSE & ABUSE
Every patient has a right to adequate and safe pain control—but NPs and PAs must be aware of the potential for some patients to misuse opioids by taking them in a different way than intended, in a different quantity than prescribed, or without a prescription.13 Having prescriptive authority confers an obligation for NPs and PAs to recognize the prevalence of drug misuse and its impact on patients, families, and society.
Regrettably, there is lack of clarity in the literature about specific characteristics and demographic data that can help determine who is at risk for opioid misuse.14 For example, risk factors that have been associated with drug misuse include a personal or family history of substance abuse; younger age; and an ongoing psychiatric condition.
In contrast, Kennedy and colleagues determined that patients seeking prescription opioids for misuse or abuse tend to be older; be of Caucasian background; have a history of overdose; be receiving methadone maintenance therapy; and have been incarcerated.15 In addition, several characteristics—having moderate or extreme pain, disability, or a history of being refused pain medication—were also associated with a history of seeking prescription opioids to abuse.15
This diverse set of variables underscores the importance of obtaining and documenting a complete history from patients who are experiencing (and seeking relief of) pain; performing a thorough physical exam; and asking specific questions about the patient’s level of pain and the potential for misuse of pain medication.
Gathering this information may help identify patients at risk for opioid misuse or abuse. Furthermore, it ensures that a patient’s chronic pain is not being undertreated and that he/she is not being undeservedly labeled or judged as a drug seeker or abuser.
Continue to: Tools and strategies for appropriate use of opioids
Tools and strategies for appropriate use of opioids
There are tools and strategies available to ensure proper use of opioids for managing chronic noncancer pain. Urine drug testing, screening tools for opioid abuse, prescription drug monitoring programs, and opioid treatment agreements should be considered for patients who require prescription opioids to treat pain.15
Urine drug testing. The CDC recommends that prescribing clinicians perform urine drug testing before initiating opioid therapy and at least annually afterward. It can be used to assess for prescription medications generally, controlled prescription drugs specifically, and substances of abuse.16 Urine drug testing can mitigate the risk for misuse or overdose of opioids, as well as identify patients who were prescribed an opioid but are not taking it. The prescribing provider is responsible for explaining to the patient why urine testing is being done, performing confirmatory testing, and discussing results with the patient.
Risk-assessment tools. A number of web-based tools help the prescribing provider assess a patient’s risk for misuse or abuse of opioids and other substances. They fall into three general categories of use: assessing patients being considered for long-term opioid therapy; assessing for misuse once opioid treatment is initiated; and addressing the potential for substance abuse generally.17-24 Table 2 lists examples. Although screening tools are not 100% accurate at identifying who is a substance abuser, they do alert the provider that a potential problem exists and needs to be explored. As such, they should be considered one component of comprehensive risk assessment, monitoring, and mitigation.25
Prescription drug monitoring programs (PDMPs). NPs and PAs must also be aware of “doctor shopping,” in which a person seeks prescriptions from multiple providers (often under false pretenses) and has them filled at multiple pharmacies. PDMPs are designed to monitor for suspected abuse, diversion, or inappropriate prescribing. These state-run electronic databases track the amount of controlled substances prescribed, dispensed, and refilled for a given patient.26 This information can assist providers in identifying high-risk patients who may benefit from an early intervention program.27 Once a patient is identified as having an opioid use disorder, NPs and PAs must provide appropriate referral to an evidence-based practice for treatment of abuse. It is essential to recognize that an opioid use disorder is a chronic illness and that relapses occur.
Opioid treatment agreements. These have been presented as a strategy to prevent prescription drug abuse; however, there is little evidence to support their effectiveness in preventing medication misuse, abuse, or diversion of opioids. In fact, research has shown that such agreements can put the patient–provider therapeutic relationship at risk for disruption, since patients may feel mistrusted or stigmatized by the suggestion that they might behave inappropriately.28 The position of the American Pain Society and the American Academy of Pain Management is that patients and clinicians should have ongoing discussions about chronic opioid therapy that include goals, expectations, risks, and alternatives to opioids.29 If a written agreement is used, it needs to address the patient’s and the clinician’s responsibilities and expectations in managing chronic pain.28
Continue to: Additional resources for providers
Additional resources for providers
Many other resources are available for prescribers of controlled substances. For example, the CDC has published guidelines for prescribing opioids to patients with chronic pain, with a goal of increasing patient–provider communication.16 Additional goals include improving the safety of opioid use, maintaining the effectiveness of treatment, and reducing the necessity and practice of long-term therapy.
The FDA has also published a blueprint on how opioid analgesics can be formulated to deter abuse and, thus, be safer.30 Although directed at the pharmaceutical industry—the FDA encourages manufacturers to develop abuse-deterrent mechanisms, such as physical and chemical barriers, aversion technology, and new delivery systems—the guidance may enlighten providers on how abusers can alter or manipulate oral opioids to achieve the desired effects.30
CONCLUSION
Because NPs and PAs are authorized to prescribe Schedule II-V drugs in their scope of practice, they must have knowledge of drug-seeking behaviors and drug misuse before they prescribe opioids for pain relief. They must be attentive to patients’ pain-control needs and consider how to avoid or reduce the potential for misuse and abuse. Understanding the experience of pain and how opioids modulate it, as well as using available risk-assessment strategies, will help providers offer safe, effective treatment to their patients.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.
1. United Nations Office on Drugs and Crime. World drug report 2015. www.unodc.org/documents/wdr2015/World_Drug_Report_2015.pdf. Accessed March 21, 2018.
2. Rudd RA, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010-2015. MMWR Morb Mortal Wkly Rep. 2016;65(5051): 1445-1452.
3. Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(5051):1378-1382.
4. Jones CM, Logan J, Gladden RM, Bohm MK. Vital signs: demographic and substance use trends among heroin users—United States, 2002-2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719-725.
5. US Department of Health and Human Services. United States Surgeon General. Letter from the Surgeon General. 2016. https://turnthetiderx.org/#. Accessed March 21, 2018.
6. Arcangelo VP, Peterson AM, Wilbur V, Reinhold JA. Pharmacotherapeutics for Advanced Practice. 4th ed. Philadelphia, PA: Wolters Kluwer; 2017:1-23.
7. Adams MP, Holland N, Urban CQ. Pharmacology for Nurses: A Pathophysiologic Approach. 5th ed. Upper Saddle River, NJ: Pearson Education; 2016:239-252.
8. Grossman S, Porth CM. Somatosensory function, pain, and headache. In: Porth’s Pathophysiology: Concepts of Altered Health States. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014:441.
9. Woo TM, Robinson MV. Pain management: Acute and chronic pain. In: Pharmacotherapeutics for Advanced Practice Nurse Prescribers. 4th ed. Philadelphia, PA: FA Davis; 2015:1361.
10. Adams MP, Urban CQ. Pharmacology for Nurses: Connections to Nursing Practice. 2nd ed. Upper Saddle River, NJ: Pearson Education; 2013:437.
11. Joint Commission enhances pain assessment and management requirements for accredited hospitals. The Joint Commission Perspectives. 2017;37(7):1-4. www.jointcommission.org/assets/1/18/Joint_Commission_Enhances_Pain_Assessment_and_Management_Requirements_for_Accredited_Hospitals1.PDF. Accessed March 21, 2018.
12. World Health Organization. WHO’s cancer pain ladder for adults. 2017. www.who.int/cancer/palliative/painladder/en/. Accessed March 21, 2018.
13. National Institutes of Health. National Institute on Drug Abuse. Opioids: brief description. www.drugabuse.gov/drugs-abuse/opioids. Accessed March 21, 2018.
14. Hudspeth RS. Safe opioid prescribing for adults by nurse practitioners: Part 1. Patient history and assessment standards and techniques. J Nurse Pract. 2016;12(3):141-148.
15. Kennedy MC, Kerr T, DeBeck K, et al. Seeking prescription opioids from physicians for nonmedical use among people who inject drugs in a Canadian setting. Am J Addict. 2016;25(4):275-282.
16. US Department of Health and Human Services. CDC. CDC guideline for prescribing opioids for chronic pain—United States, 2016. www.cdc.gov/mmwr/volumes/65/rr/rr6501e1.htm. Accessed March 21, 2018.
17. Webster LR, Webster R. Predicting aberrant behaviors in opioid‐treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6(6):432. [Tool available at www.drugabuse.gov/sites/default/files/files/OpioidRiskTool.pdf.] Accessed March 21, 2018.
18. Screener and Opioid Assessment for Patients with Pain—Revised (SOAPP®-R). http://nationalpaincentre.mcmaster.ca/documents/soapp_r_sample_watermark.pdf. Accessed March 21, 2018.
19. D.I.R.E. Score: Patient Selection for Chronic Opioid Analgesia. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/D.I.R.E.%20Score.pdf. Accessed March 21, 2018.
20. Current Opioid Misuse Measure (COMM)™. www.opioidprescribing.com/documents/09-comm-inflexxion.pdf. Accessed March 21, 2018.
21. Pain Assessment and Documentation Tool (PADT™). www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/Pain%20Assess ment%20Documentation%20Tool%20%28PADT%29.pdf. Accessed March 21, 2018.
22. The CAGE and CAGE-AID Questionnaires. www.ucdenver.edu/academics/colleges/PublicHealth/research/centers/CHWE/Documents/CAGE-AID.pdf. Accessed March 21, 2018.
23. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363-371. [DAST-10 available at https://cde.drugabuse.gov/sites/nida_cde/files/DrugAbuseScreeningTest_2014Mar24.pdf.] Accessed March 21, 2018.
24. SBIRT AUDIT forms (English and Spanish). www.communitycarenc.org/media/tool-resource-files/sbirt-audit-forms.pdf. Accessed March 21, 2018.
25. Cheattle MD. Risk assessment: safe opioid prescribing tools. 2017. https://www.practicalpainmanagement.com/resource-centers/opioid-prescribing-monitoring/risk-assessment-safe-opioid-prescribing-tools. Accessed March 21, 2018.
26. Ali MM, Dowd WN, Classen T, et al. Prescription drug monitoring programs, nonmedical use of prescription drugs, and heroin use: evidence from the National Survey of Drug Use and Health. Addict Behav. 2017;69:65-77.
27. US Department of Health and Human Services. CDC. Drug overdose deaths hit record numbers in 2014. www.cdc.gov/media/releases/2015/p1218-drug-overdose.html. Accessed March 21, 2018.
28. McGee S, Silverman RD. Treatment agreements, informed consent, and the role of state medical boards in opioid prescribing. Pain Med. 2015;16(1):25-29.
29. Chou R, Fanciullo GJ, Fine PG, et al; for the American Pain Society–American Academy of Pain Medicine Opioids Guidelines Panel. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113-130.
30. US Department of Health and Human Services. FDA Center for Drug Evaluation and Research. Abuse-deterrent opioids—evaluation and labeling guidance for industry. 2015. www.fda.gov/downloads/Drugs/Guid ances/UCM334743.pdf. Accessed March 21, 2018.