User login
Worsening dyspnea
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
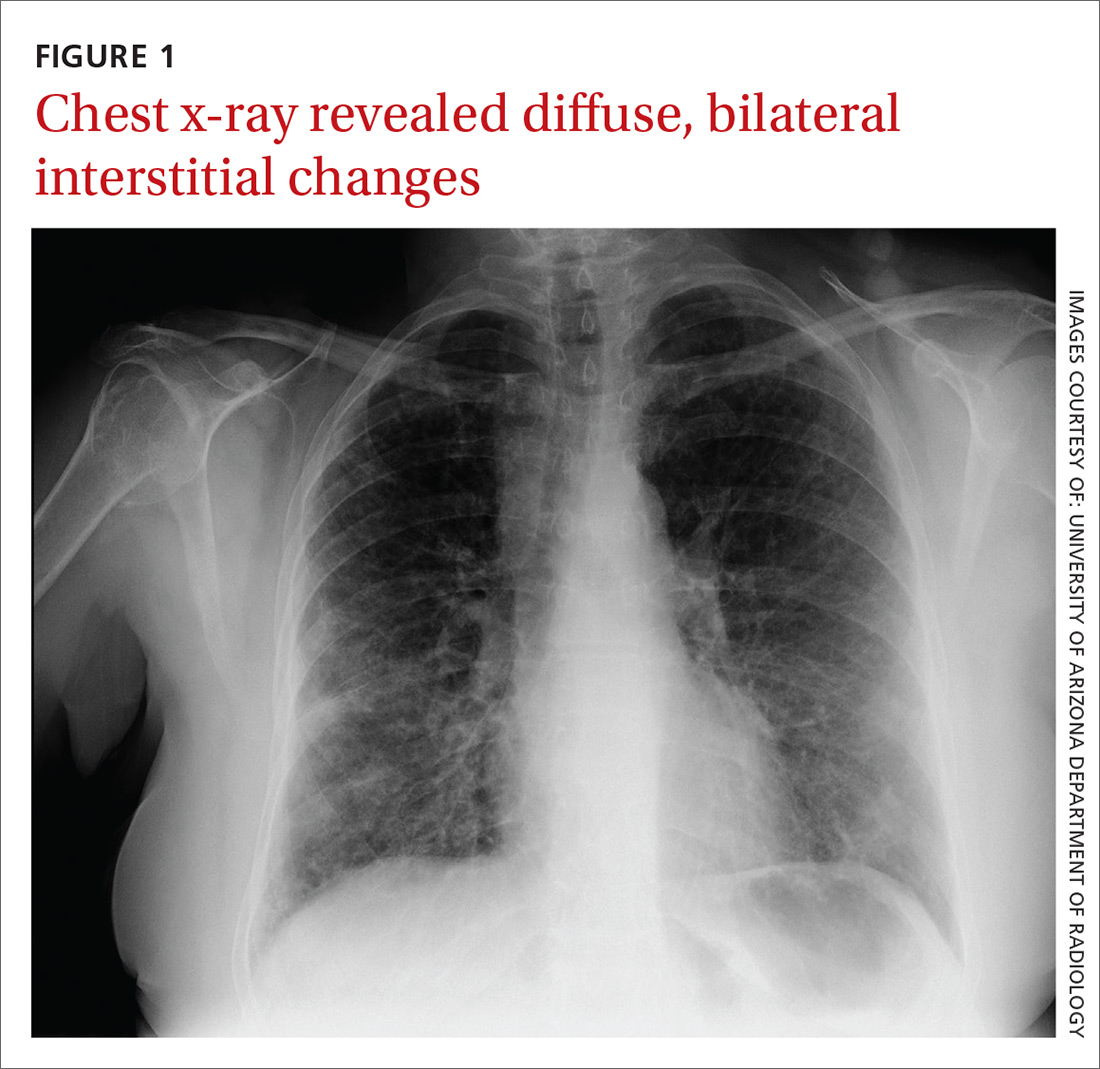
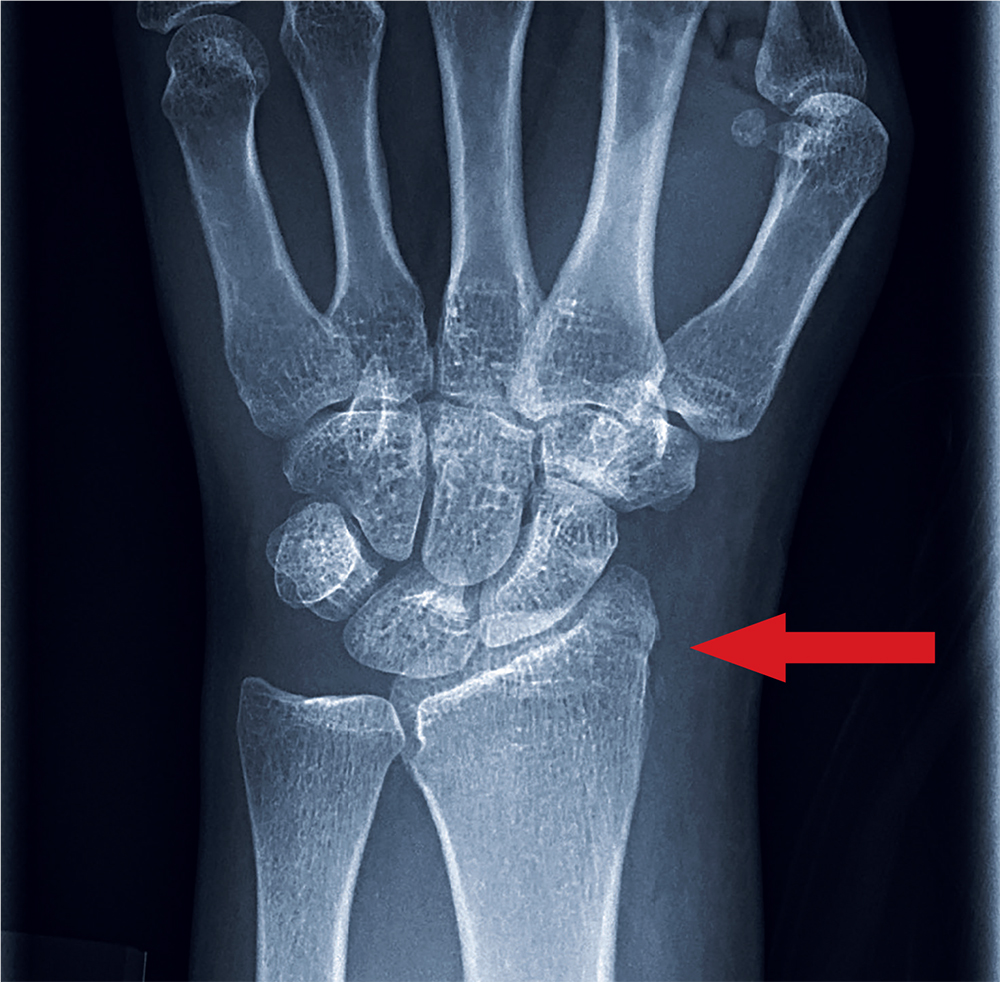
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
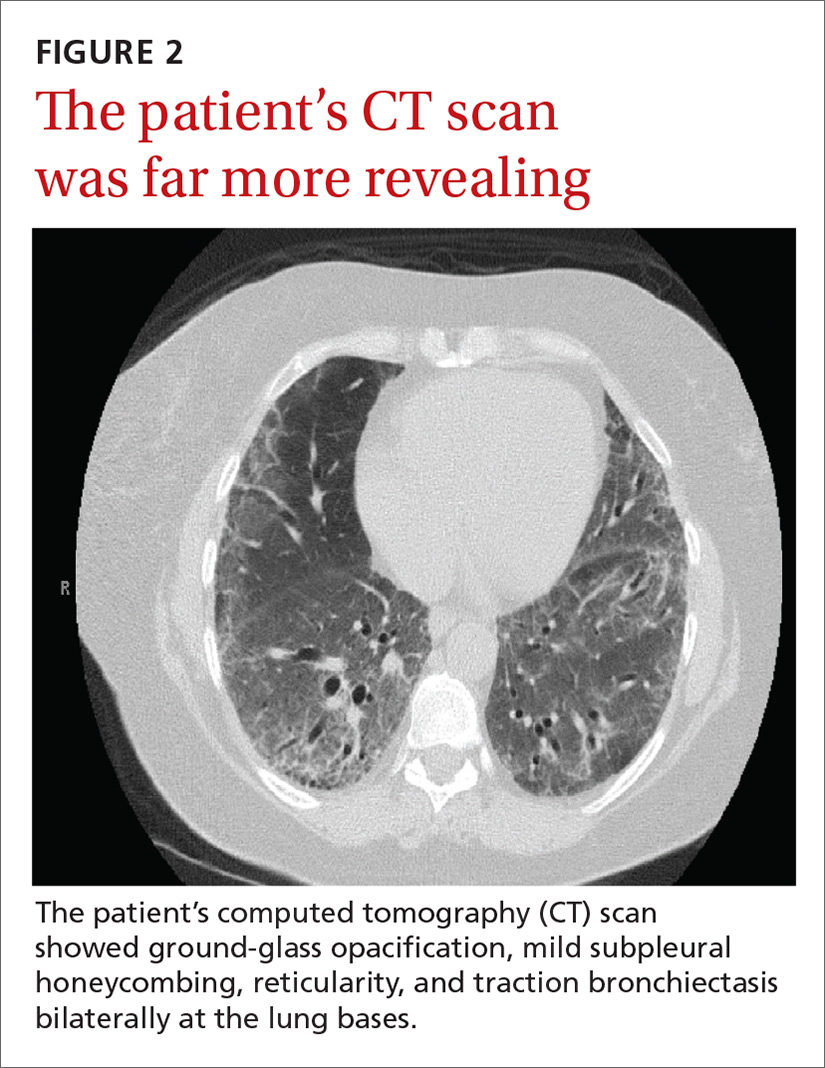
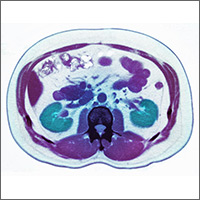
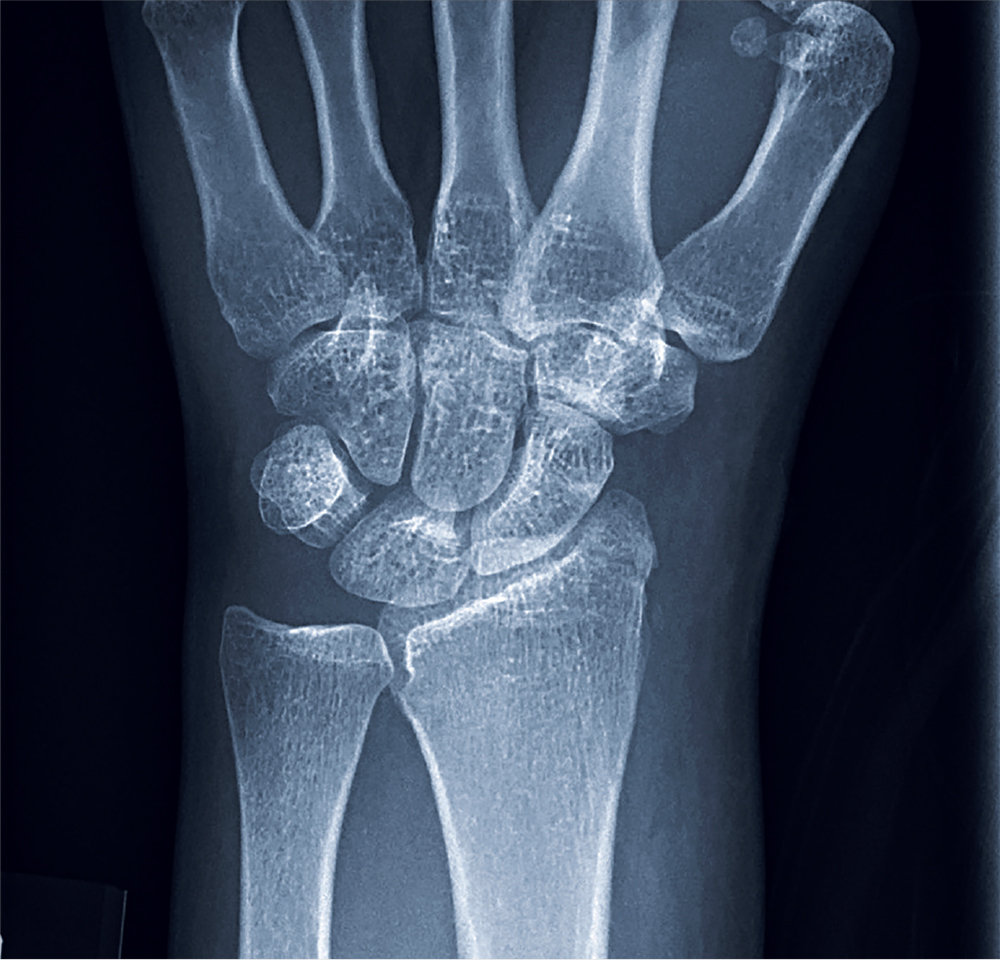
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
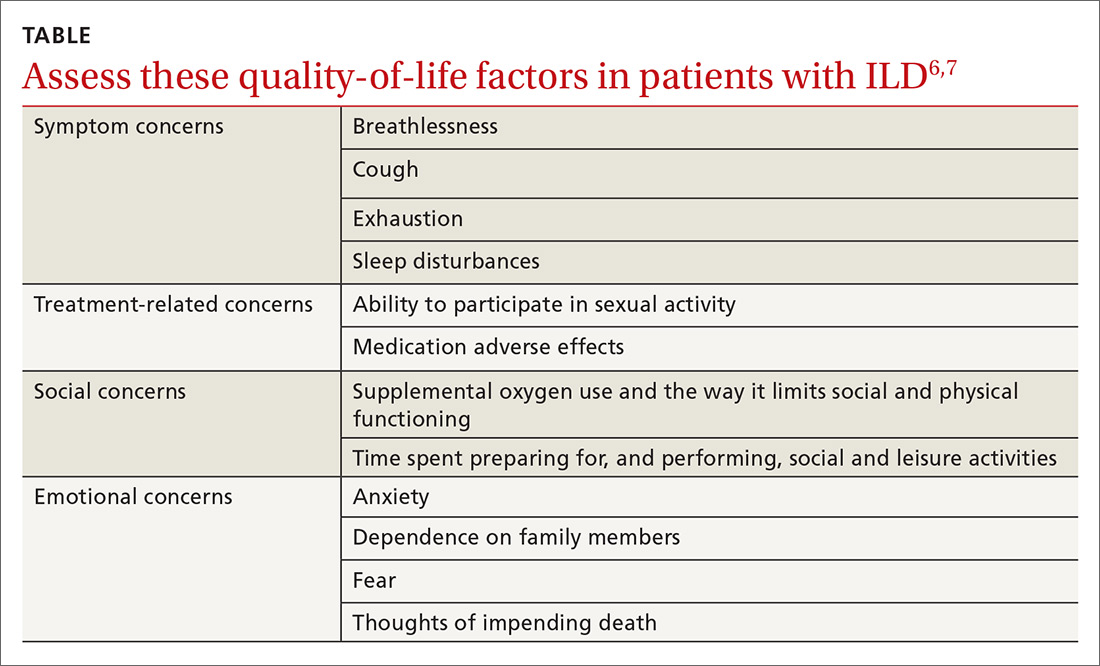
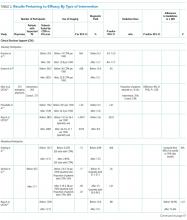
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
A 62-year-old woman presented with a 2- to 3-week history of fatigue, nonproductive cough, dyspnea on exertion, and intermittent fever/chills. Her past medical history was significant for rheumatoid arthritis (RA) that had been treated with methotrexate and prednisone for the past 6 years. The patient was currently smoking half a pack a day with a 40-pack year history. The patient was a lifelong resident of Arizona and had previously worked in a stone mine.
On physical examination she appeared comfortable without any increased work of breathing. Her vital signs included a temperature of 36.6° C, a blood pressure of 110/54 mm Hg, a pulse of 90 beats/min, respirations of 16/min, and room-air oxygen saturation of 87%. Pulmonary examination revealed scattered wheezes with fine bibasilar crackles. The remainder of her physical exam was normal. Because she was hypoxic, she was admitted to the hospital.
At the hospital, a chest x-ray showed diffuse, bilateral interstitial changes (FIGURE 1). Laboratory tests revealed a white blood cell count of 13,800/mcL (normal: 4500-10,500/mcL) with 73% neutrophils (normal: 40%-60%), 3% bands (normal: 0-3%), 14% monocytes (normal: 2%-8%), 6% eosinophils (normal: 1%-4%), and 3% lymphocytes (normal: 20%-30%). Community-acquired pneumonia was suspected, and the patient was started on levofloxacin. Over the next 2 days, her dyspnea worsened. She became tachycardic, and her oxygen requirement increased to 15 L/min via a non-rebreather mask. She was transferred to the intensive care unit.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Interstitial lung disease
Given the patient’s worsening respiratory status, a computed tomography (CT) scan was ordered (FIGURE 2). Review of the CT scan showed ground-glass opacification, mild subpleural honeycombing, reticularity, and traction bronchiectasis bilaterally at the lung bases. Bronchoscopy with lavage was performed to rule out infectious etiologies and was negative. These findings, along with the patient’s medical history of RA and use of methotrexate, led us to diagnose interstitial lung disease (ILD) in this patient.
ILD refers to a group of disorders that primarily affects the pulmonary interstitium, rather than the alveolar spaces or pleura.1 The most common causes of ILD seen in primary care are idiopathic pulmonary fibrosis, connective tissue disease, and hypersensitivity pneumonitis secondary to drugs (such as methotrexate, citalopram, fluoxetine, nitrofurantoin, and cephalosporins), radiation, or occupational exposures. (Textile, metal, and plastic workers are at a heightened risk, as are painters and individuals who work with animals.)1 In 2010, idiopathic pulmonary fibrosis had a prevalence of 18.2 cases per 100,000 people.2 Determining the underlying cause of ILD is important, as it may influence prognosis and treatment decisions.
The most common presenting symptoms of ILD are exertional dyspnea, cough with insidious onset, fatigue, and weakness.1,3 Bear in mind, however, that patients with ILD associated with a connective tissue disease may have more subtle manifestations of exertional dyspnea, such as a change in activity level or low resting oxygen saturations. The pulmonary exam can be normal or can reveal fine end-inspiratory crackles, and may include high-pitched, inspiratory rhonchi, or “squeaks.”1
When a diagnosis of ILD is suspected, investigation should begin with high-resolution CT (HRCT).1.3-5 In patients for whom a potential cause of ILD is not identified or who have more than one potential cause, specific patterns seen on the HRCT can help determine the most likely etiology.5 Chest x-ray has low sensitivity and specificity for ILD and can frequently be misinterpreted, as occurred with our patient.1
Rule out other causes of dyspnea
The differential diagnosis for dyspnea includes:
Heart failure. Congestive heart failure can present with acutely worsening dyspnea and cough, but is also commonly associated with orthopnea and/or paroxysmal nocturnal dyspnea. On physical examination, findings of volume overload such as pulmonary crackles, lower extremity edema, and elevated jugular venous pressure are additional signs that heart failure is present.
Pulmonary embolism (PE). Patients with PE commonly present with acute dyspnea, chest pain, and may also have a cough. Additional risk factors for PE (prolonged immobility, fracture, recent hospitalization) may also be present. A Wells score and a D-dimer test can be used to determine the probability of a patient having PE.
Asthma/chronic obstructive pulmonary disease. COPD exacerbations commonly present with a productive cough and worsening dyspnea. Pulmonary exam findings include wheezing, tachypnea, increased respiratory effort, and poor air movement.
Infection (including coccidioidomycosis in the desert southwest, where this patient lived). Our patient was initially treated for pneumonia because she had reported fevers associated with dyspnea and cough along with an elevated white blood cell count. Chest x-ray findings in patients with pneumonia can reveal either lobar consolidation or interstitial infiltrates.
Failure to respond to treatment of the more common causes of dyspnea, as occurred with our patient, should prompt consideration of ILD, particularly in those who have a history of connective tissue disease. Once a diagnosis of ILD is made, referral to a pulmonary specialist is advised.1,3
A poor prognosis and a focus on quality of life
Immunosuppressive therapy is currently the standard treatment for ILD, although there is little evidence to support this practice.1,3,4 Therapy usually includes corticosteroids with or without the addition of a second immunosuppressive agent such as azathioprine, mycophenolate mofetil, or cyclophosphamide.1,4
In addition to drug therapy, the American College of Chest Physicians recommends routine assessment of quality-of-life (QOL) concerns in patients with ILD (TABLE).6,7 Additional QOL tools available to physicians include the Medical Outcomes Study Short-Form 36-Item Instrument8 and the St. George’s Respiratory Questionnaire.9
The prognosis is poor, even with treatment. Patients with ILD have a life expectancy that averages 2 to 4 years from diagnosis.6 Patients with ILD are frequently distressed about worsening control of dyspnea and becoming a burden to family members; they also have anxiety about dying.6 It’s important to allocate sufficient time for end-of-life discussions, as studies have shown that patients would like their physicians to address the issue more thoroughly.10
Our patient was started on high-flow oxygen and high-dose steroids. Azathioprine was later added. The patient’s methotrexate was stopped, in light of its association with ILD. Unfortunately, the treatments were not successful and the patient’s respiratory status continued to deteriorate. A family meeting was held with the patient to discuss end-of-life wishes, and the patient expressed a preference for hospice care. She died a few days after hospice enrollment.
CORRESPONDENCE
Karyn B. Kolman, MD, University of Arizona College of Medicine at South Campus Family Medicine Residency, 2800 E Ajo Way, Room 3006, Tucson, AZ 85713; karyn.kolman@bannerhealth.com.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
1. Wallis A, Spinks K. The diagnosis and management of interstial lung disease. BMJ. 2015;350:h2072.
2. Raghu G, Chen SY, Hou Q, et al. Incidence and prevalence of idiopathic pulmonary fibrosis in US adults 18-64 years old. Eur Respir J. 2016;48:179-186.
3. Yunt ZX, Solomon JJ. Lung disease in rheumatoid arthritis. Rheum Dis Clin North Am. 2015;41:225-236.
4. Vij R, Strek ME. Diagnosis and treatment of connective tissue disease-associated interstitial lung disease. Chest. 2013;143:814-824.
5. Nair A, Walsh SL, Desai SR. Imaging of pulmonary involvement in rheumatic disease. Rheum Dis Clin North Am. 2015;41:167-196.
6. Gilbert CR, Smith CM. Advanced parenchymal lung disease: quality of life and palliative care. Mt Sinai J Med. 2009;76:63-70.
7. Swigris JJ, Stewart AL, Gould MK, et al. Patients’ perspectives on how idiopathic pulmonary fibrosis affects the quality of their lives. Health Qual Life Outcomes. 2005;3:61.
8. RAND. Medical Outcomes Study 36-Item Short Form Survey (SF-36). Available at: http://www.rand.org/health/surveys_tools/mos/mos_core_36item.html. Accessed May 27, 2016.
9. St George’s Respiratory Questionnaire. Available at: http://www.healthstatus.sgul.ac.uk/. Accessed May 27, 2016.
10. Bajwah S, Koffman J, Higginson IJ, et. al. ‘I wish I knew more…’ the end-of-life planning and information needs for end-stage fibrotic interstitial lung disease: views of patients, carers, and health professionals. BMJ Support Palliat Care. 2013;3;84-90.
Tamsulosin for patients with ureteral stones?
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
ILLUSTRATIVE CASE
A 54-year-old man presents to the emergency department (ED) with acute onset left flank pain that radiates to the groin. A computed tomography (CT) scan of the abdomen/pelvis without contrast reveals a 7-mm distal ureteral stone. He is deemed appropriate for outpatient management. In addition to pain medications, should you prescribe tamsulosin?
According to the most recent National Health and Nutrition Examination Survey, the population prevalence of kidney stones is 8.8% with a self-reported prevalence in men of 10.6% and a self-reported prevalence in women of 7.1%.2 Most ureteral stones can be treated in the outpatient setting with oral hydration, antiemetics, and pain control with nonsteroidal anti-inflammatory medications as first-line treatment and opioids as a second-line option.3 In addition, alpha-blockers are used for medical expulsive therapy (MET). In fact, the European Association of Urology guideline on urolithiasis states that MET may accelerate passage of ureteral stones.3
Recently, however, uncertainty has surrounded the effectiveness of the alpha-blocker tamsulosin. Two systematic reviews, limited by heterogeneity because some of the studies lacked a placebo control and blinding, concluded that alpha-blockers increased stone passage within one to 6 weeks when compared with placebo or no additional therapy.4,5 However, a recent large multicenter, randomized controlled trial (RCT) revealed no difference between tamsulosin and nifedipine or either one compared with placebo at decreasing the need for further treatment to achieve stone passage within 4 weeks.6
[polldaddy:9906038]
STUDY SUMMARY
New meta-analysis breaks down results by stone size
This meta-analysis by Wang et al, consisting of 8 randomized, double-blind, placebo-controlled trials of adult patients (N=1384), examined the effect of oral tamsulosin 0.4 mg/d (average of a 28-day course) on distal ureteral stone passage.1 A subgroup analysis comparing stone size (<5 mm and 5-10 mm) was also conducted to determine if stone size modified the effect of tamsulosin.
Although the initial search included studies published between 1966 and 2015, the 8 that were eventually analyzed were published between 2009 and 2015, were conducted in multiple countries (and included regardless of language), and were conducted in ED and outpatient urology settings. The main outcome measure was the risk difference in stone passage between the tamsulosin group and placebo group after follow-up imaging at 3 weeks with CT or plain film radiographs.
Tamsulosin helps some, but not all. The pooled risk of stone passage was higher in the tamsulosin group than in the placebo group (85% vs 66%; risk difference [RD]=17%; 95% confidence interval [CI], 6%-27%), but significant heterogeneity existed across the trials (I2=80.2%). After subgroup analysis by stone size, the researchers found that tamsulosin was beneficial for larger stones, 5 to 10 mm in size (6 trials, N=514; RD=22%; 95% CI, 12%-33%; number needed to treat=5), compared with placebo, but not for smaller stones, <5 mm in size (4 trials, N=533; RD=-0.3%; 95% CI, -4% to 3%). The measure of heterogeneity in the 5- to 10-mm subgroup demonstrated a less heterogeneous population of studies (I2=33%) than that for the <5-mm subgroup (I2=0%).
In terms of adverse events, tamsulosin did not increase the risk of dizziness (RD=.2%; 95% CI, -2.1% to 2.5%) or postural hypotension (RD=.1%; 95% CI, -0.4% to 0.5%) compared with placebo.
WHAT’S NEW
Passage of larger stones increases with tamsulosin
This meta-analysis included only randomized, double-blind, placebo-controlled trials. Prior meta-analyses did not. Also, this review included the SUSPEND (Spontaneous Urinary Stone Passage Enabled by Drugs) trial, an RCT discussed in a previous PURL (Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.) that recommended against the alpha-blockers tamsulosin and nifedipine for ureteral stones measuring <10 mm.6,7
But the subgroup analysis in this more recent review went one step further in the investigation of tamsulosin’s effect by examining passage rates by stone size (<5 mm vs 5-10 mm) and revealing that passage of larger stones (5-10 mm) increased with tamsulosin. The different results based on stone size may explain the recent uncertainty as to whether tamsulosin improves the rate of stone passage.
CAVEATS
Study doesn’t address proximal, or extra-large stones
Only distal stones were included in 7 of the 8 trials. Thus, this meta-analysis was unable to determine the effect on more proximal stones. Also, it’s unclear if the drug provides any benefit with stones >10 mm in size.
CHALLENGES TO IMPLEMENTATION
None worth mentioning
We see no challenges to implementation of this recommendation.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
1. Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
2. Scales CD Jr, Smith AC, Hanley JM, et al. Prevalence of kidney stones in the United States. Eur Urol. 2012;62:160-165.
3. Türk C, Petrik A, Sarica K, et al. EAU guidelines on diagnosis and conservative management of urolithiasis. Eur Urol. 2016;69:468-474.
4. Hollingsworth JM, Canales BK, Rogers MAM, et al. Alpha blockers for treatment of ureteric stones: systematic review and meta-analysis. BMJ. 2016;355:i6112.
5. Campschroer T, Zhu Y, Duijvesz D, et al. Alpha-blockers as medical expulsive therapy for ureteral stones. Cochrane Database Syst Rev. 2014:CD008509.
6. Pickard R, Starr K, MacLennan G, et al. Medical expulsion therapy in adults with ureteric colic: a multicentre, randomized, placebo-controlled trial. Lancet. 2015;386:341-349.
7. Slattengren AH, Prasad S, Jarrett JB. Kidney stones? It’s time to rethink those meds. J Fam Pract. 2016;65:118-120.
Copyright © 2018. The Family Physicians Inquiries Network. All rights reserved.
PRACTICE CHANGER
Prescribe tamsulosin for stone expulsion in patients with distal ureteral stones 5 to 10 mm in size.1
STRENGTH OF RECOMMENDATION
A: Based on a meta-analysis of randomized controlled trials.
Wang RC, Smith-Bindman R, Whitaker E, et al. Effect of tamsulosin on stone passage for ureteral stones: a systematic review and meta-analysis. Ann Emerg Med. 2017;69:353-361.
CDC provides advice on recent hepatitis A outbreaks
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
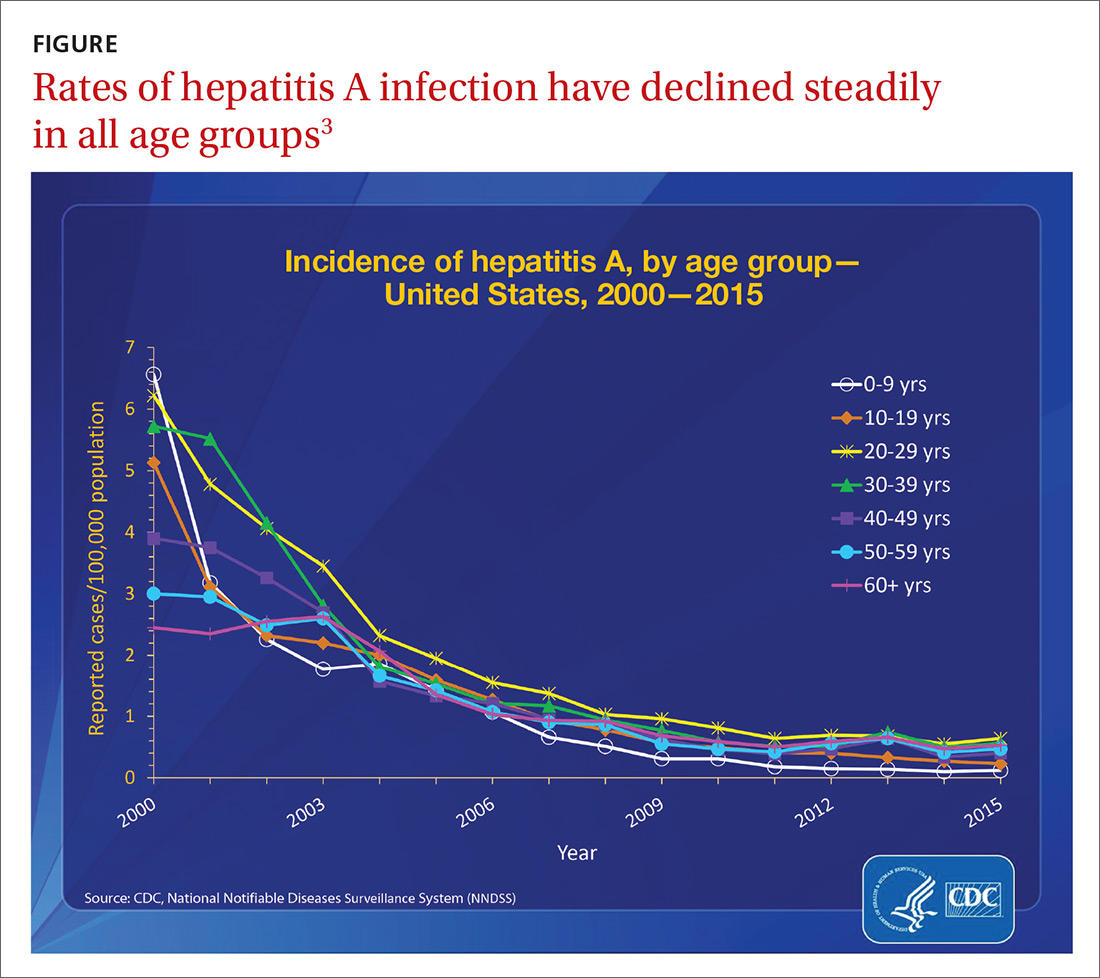
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
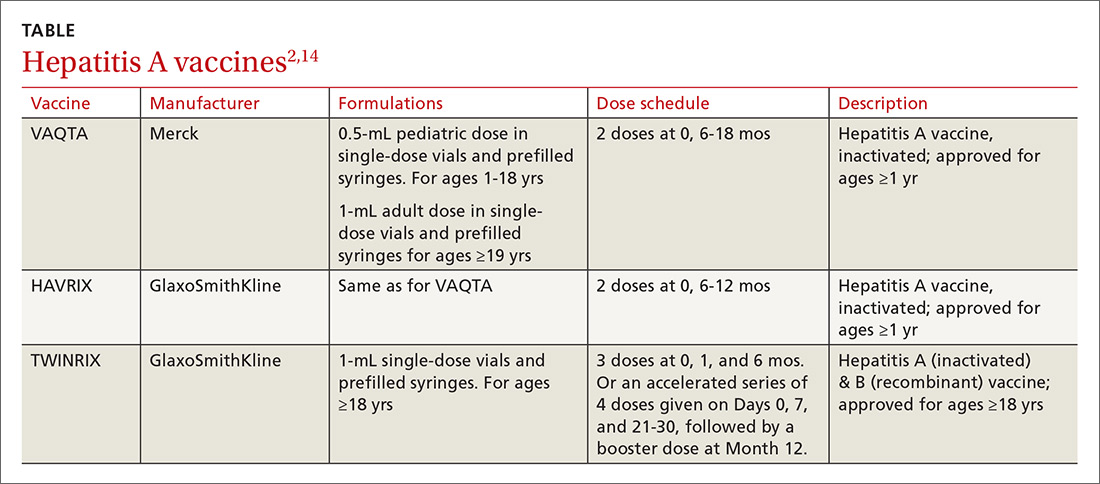
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
The epidemiology of hepatitis A virus (HAV) disease has changed. Since July 2016, there have been 5 large outbreaks of infection involving more than 1600 cases,1 with affected states requiring assistance from the Centers for Disease Control and Prevention (CDC). Two of these outbreaks were foodborne, and 3 involved person-to-person transmission.1
Before 2016, the number of outbreaks had been very low, and were predominantly associated with contaminated food, infected food handlers, and other food service-related exposures. Total annual cases of HAV infection had been declining steadily in all age groups since 1995 when HAV vaccine became available, from an estimated 271,000 cases resulting in 100 deaths2 to an estimated 2800 cases (with 1390 reported) resulting in 67 deaths in 2015 (FIGURE).3
Extent of the outbreaks
The largest hepatitis A outbreak involving person-to-person transmission in the United States in the past 20 years is occurring now in California. Predominantly affected are the homeless and users of illicit drugs, whose risk of infection is compounded by exposure to fecally-contaminated environments. As of December 1, the largest number of cases were recorded in San Diego (567), Santa Cruz (76), and Los Angeles (11).4 Adding 18 cases from other locations, the total has reached 672, resulting in 430 hospitalizations (64%) and 21 deaths (3%).4 In San Diego, 20% of those infected also had chronic hepatitis C and 5% had chronic hepatitis B.1
In southeastern Michigan, 555 cases have been reported, with 457 hospitalizations (82%) and 20 deaths (4%).5 In Utah, 91 cases and 53 hospitalizations (58%) have been documented.6 In these regions, the predominant risk factors have been homelessness and illicit drug use. And many of those infected have had chronic hepatitis C (27.5%), hepatitis B (13.2%), or both (9.9%).6 In 2 of the 3 states just described, the outbreaks have involved HAV genotype 1B.1
In New York City, an outbreak starting in January 2017 resulted in 51 cases. The epidemiology of this outbreak has been different from the others, involving men who have sex with men (MSM) and the HAV genotype 1A that matches a strain circulating among MSM in Europe.7
Low adult immunity is behind the outbreaks
These outbreaks have occurred in an adult US population that has low levels of immunity to HAV. In 2012 only 12.2% of adults ages 19 to 49 years had received 2 doses of HAV vaccine8 and only 24.2% of adults had antibodies to HAV,9 showing that most adults had never been infected with the virus or vaccinated. The reduction in HAV incidence previously described is due to the introduction of targeted, and then universal, child HAV vaccination recommendations by the Advisory Committee on Immunization Practices.
As the incidence of HAV disease declined, fewer individuals became infected as children, leading later to a susceptible pool of adults who had not been infected as children and who did not receive the vaccine in adulthood. Most of these adults will not be exposed to HAV due to decreased rates of infection in children, which, historically, has been the predominant means of adult exposure. The high hospitalization and death rates encountered in the recent and ongoing large outbreaks are explained by the multiple comorbidities of those infected.
Who should be vaccinated against HAV
The CDC recommends giving HAV vaccine to all children at age one year, and to the following groups:2,10,11
- residents of a community that has a high rate of hepatitis A infection
- household members or other close personal contacts (eg, regular babysitters) of adopted children newly arrived from countries with high or intermediate hepatitis A endemicity
- men who have sex with other men
- users of illicit injection and noninjection drugs
- workers in, or travelers to, countries with high rates of hepatitis A infection
- individuals with chronic liver disease
- individuals who work with HAV-infected animals or with HAV in a research setting.
Outbreak-specific vaccine recommendations
The CDC has additionally recommended that, during outbreaks, health care providers should consider taking the following 4 steps:12,13
- Increase the availability of HAV vaccine to the homeless and to those who use illicit drugs; to anyone who has ongoing, close contact with people who are homeless or who use injection and non-injection drugs; and as post-exposure prophylaxis for unvaccinated people who have been exposed to HAV in the previous 2 weeks.
- Defer the second dose of HAV vaccine if it is in short supply.
- Perform pre-vaccination serologic testing to identify those who are immune, thereby preserving vaccine and reducing costs.
- Use TWINRIX if other HAV vaccines are unavailable, keeping in mind that a single dose of TWINRIX achieves 94% protection against HAV but only 31% against hepatitis B virus (HBV). Three doses of TWINRIX are needed for full protection against HBV.
Available vaccines
Three vaccines are available for protection against HAV (TABLE2,14). Post-exposure prevention of HAV can be achieved with HAV vaccine or immune globulin.15 Vaccine is preferred for individuals up to age 40 years and can be used for older individuals if immune globulin is unavailable.
The CDC reports that the supply of adult HAV vaccine is being strained by these large outbreaks.16 Physicians will need to stay in touch with their local public health departments regarding vaccine availability in the community and any local recommendations being made regarding vaccine administration, as well as to the status of any local HAV outbreaks.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
1. Nelson N. Hepatitis A outbreaks. Presented at: Advisory Committee on Immunization Practices; October 25, 2017; Atlanta, GA. Available at: https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2017-10/hepatitis-04-nelson.pdf. Accessed December 5, 2017.
2. CDC. Prevention of hepatitis A through passive or active immunization. Recommendations of the Advisory Committee on Immunization Practices. Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/rr5507a1.htm. Accessed November 28, 2017.
3. CDC. Viral hepatitis surveillance—United States, 2015. Available at: https://www.cdc.gov/hepatitis/statistics/2015surveillance/pdfs/2015HepSurveillanceRpt.pdf. Accessed November 28, 2017.
4. California Department of Public Health. Hepatitis A outbreak in California. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/Immunization/Hepatitis-A-Outbreak.aspx. Accessed November 28, 2017.
5. Michigan Department of Health & Human Services. Hepatitis A southeast Michigan outbreak. Available at: http://www.michigan.gov/mdhhs/0,5885,7-339-71550_2955_2976_82305_82310-447907--,00.html. Accessed November 28, 2017.
6. Utah Department of Health. Hepatitis A outbreak. Available at: http://health.utah.gov/epi/diseases/hepatitisA/HAVoutbreak_2017. Accessed November 28, 2017.
7. Latash J, Dorsinville M, Del Rosso P, et al. Notes from the field: increase in reported hepatitis A infections among men who have sex with men–New York City, January-August 2017. MMWR Morb Mortal Wkly Rep. 2017;66:999-1000.
8. CDC. Murphy TV, Denniston MM, Hill HA, et al. Progress toward eliminating hepatitis A disease in the United States. MMWR Morb Mortal Wkly Rep. 2016;65:29-41.
9. Klevens RM, Denniston MM, Jiles-Chapman RB, et al. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999-2012. Vaccine. 2015;33:6192-6198.
10. CDC. Vaccines and preventable diseases. Hepatitis A in-short. Available at: https://www.cdc.gov/vaccines/vpd/hepa/public/in-short-adult.html#who. Accessed November 20, 2017.
11. CDC. Updated recommendations from the Advisory Committee on Immunization Practices (ACIP) for use of hepatitis A vaccine in close contacts of newly arriving international adoptees. MMWR Morb Mortal Wkly Rep. 2009;58:1006-1007.
12. CDC. Interim outbreak-specific guidance on hepatitis A vaccine administration. Available at: https://www.cdc.gov/hepatitis/outbreaks/InterimOutbreakGuidance-HAV-VaccineAdmin.htm. Accessed November 20, 2017.
13. CDC. 2017–Outbreaks of hepatitis A in multiple states among people who are homeless and people who use drugs. Available at: https://www.cdc.gov/hepatitis/outbreaks/2017March-HepatitisA.htm. Accessed December 11, 2017.
14. CDC. Notice to readers: FDA approval of an alternate dosing schedule for a combined hepatitis A and B vaccine (Twinrix). Available at: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5640a5.htm. Accessed December 8, 2017.
15. CDC. Update: prevention of hepatitis A after exposure to hepatitis A virus and in International Travelers. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep. 2007;56:1080-1084.
16. CDC. Current vaccine shortages and delays. Available at: https://www.cdc.gov/vaccines/hcp/clinical-resources/shortages.html. Accessed November 28, 2017.
The evidence for herbal and botanical remedies, Part 1
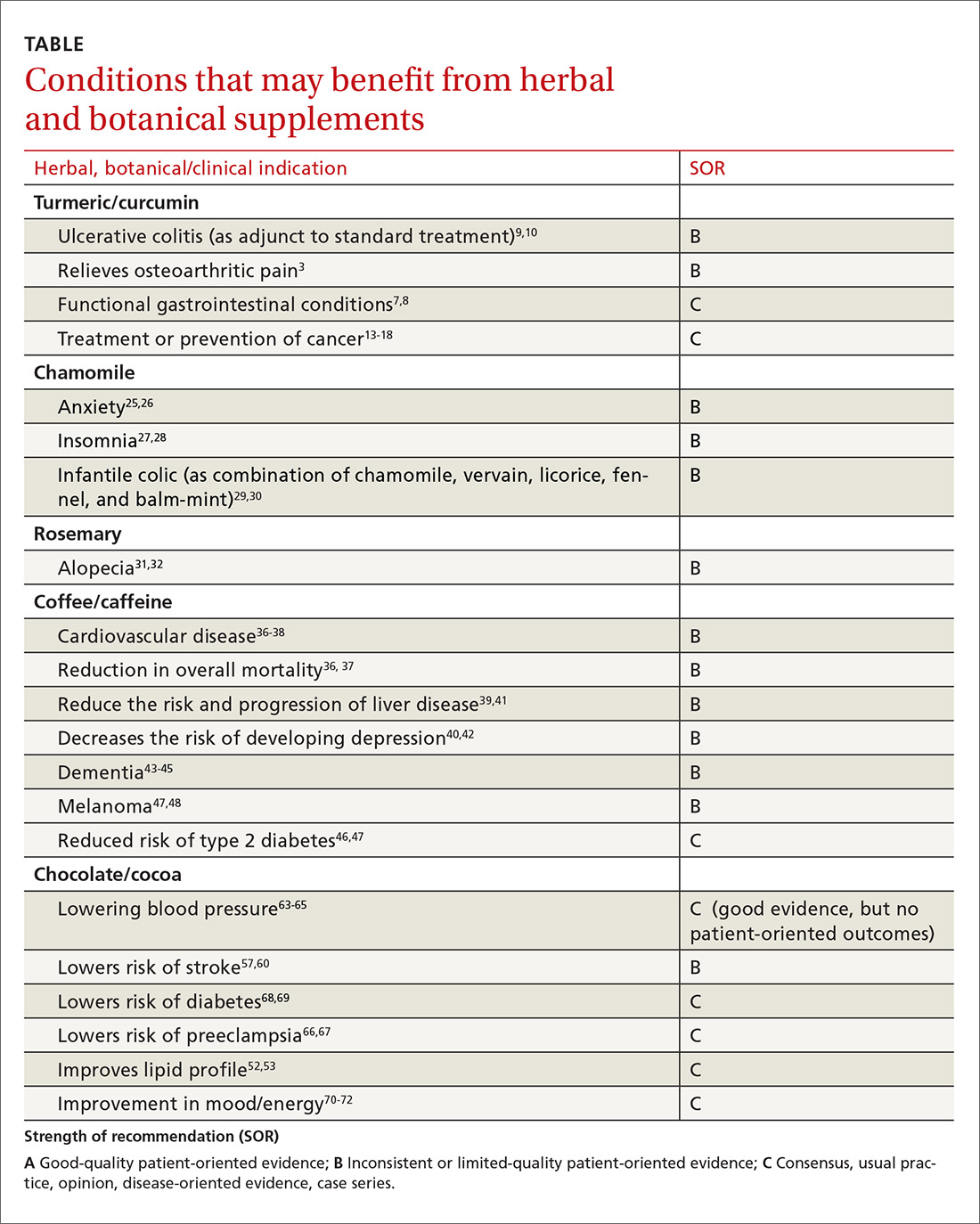
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)
In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)
In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)
In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
PRACTICE RECOMMENDATIONS
› Consider capsaicin as an alternative to oral and topical nonsteroidal anti-inflammatory drugs to treat musculoskeletal pain in patients who don't respond to the latter. B
› Consider ordering liver function monitoring for patients using butterbur because of the risk of toxicity. C
› Recommend that patients consider drinking green tea as part of a healthy diet. B
› Recommend peppermint to patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Brace for Impact
ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.
ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.
ANSWER
The radiograph shows an oblique fracture through the radial styloid process. The patient was placed in a splint and referred to outpatient orthopedics for follow-up.
A 35-year-old woman arrives at the emergency department following a motor vehicle accident. She was a restrained driver who was crossing an intersection when another vehicle pulled out in front of her. She recalls gripping the steering wheel in anticipation of impact. No air bags deployed. She complains of wrist pain, but denies any other ailment.
Medical history is unremarkable. Vital signs are normal. Physical examination of the patient’s left wrist shows no obvious deformity. There is mild soft-tissue swelling, decreased range of motion, and moderate point tenderness along the radial aspect of the wrist. The nailbeds have good capillary refill. Strong pulses are present, as well.
Triage has already obtained a radiograph of the left wrist (shown). What is your impression?
The evidence for herbal and botanical remedies, Part 2
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; malm0001@hotmail.com.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
PRACTICE RECOMMENDATIONS
› Inform patients that curcumin appears to be a safe and effective adjunctive therapy for ulcerative colitis when used along with mesalamine or sulfasalazine. B
› Recommend chamomile extract to patients experiencing mild to moderate generalized anxiety disorder. B
› Tell patients that coffee is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression (in patients with end-stage liver disease). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The Frontier of Transition Medicine: A Unique Inpatient Model for Transitions of Care
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
The transition of care from pediatric to adult providers has drawn increased national attention to the survival of patients with chronic childhood conditions into adulthood.ttps://www.ncbi.nlm.nih.gov/books/NBK11432/ While survival outcomes have improved due to advances in care, many of these patients experience gaps in medical care when they move from pediatric to adult healthcare systems, resulting in age-inappropriate and fragmented care in adulthood.4 Many youth with chronic childhood conditions are not prepared to move into adult healthcare, and this lack of transition preparation is associated with poorer health outcomes, including elevated glycosylated hemoglobin and loss of transplanted organs.5-7 National transition efforts have largely focused on the outpatient setting and there remains a paucity of literature on inpatient transitions of care.8,9 Although transition-age patients represent a small percentage of patients at children’s hospitals, they accumulate more hospital days and have higher resource utilization compared to their pediatric cohorts.10 In this issue, Coller et al.11 characterize the current state of pediatric to adult inpatient transitions of care among general pediatric services at US children’s hospitals. Over 50% of children’s hospitals did not have a specific adult-oriented hospital identified to receive transitioning patients. Fewer than half of hospitals (38%) had an explicit inpatient transition policy. Notably only 2% of hospitals could track patient outcomes through transitions; however, 41% had systems in place to address insurance issues. Institutions with combined internal medicine-pediatric (Med-Peds) providers more frequently had inpatient transition initiatives (P = .04). It is clear from Coller et al.11 that the adoption of transition initiatives has been delayed since its introduction at the US Surgeon’s conference in 1989, and much work is needed to bridge this gap.12
Coller et al.11 spearhead establishing standardized transition programs using the multidisciplinary Six Core Elements framework and highlight effective techniques from existing inpatient transition processes.13 While we encourage providers to utilize existing partnerships in the outpatient community to bridge the gap for this at-risk population, shifting to adult care continues to be disorganized in the face of some key barriers including challenges in addressing psychosocial needs, gaps in insurance, and poor care coordination between pediatric and adult healthcare systems.4
We propose several inpatient activities to improve transitions. First, we suggest the development of an inpatient transition or Med-Peds consult service across all hospitals. The Med-Peds consult service would implement the Six Core Elements, including transition readiness, transition planning, and providing insurance and referral resources. A Med-Peds consult service has been well received at our institution as it identifies clear leaders with expertise in transition. Coller et al.11 report only 11% of children’s hospitals surveyed had transition policies that referenced inpatient transitions of care. For those institutions without Med-Peds providers, we recommend establishing a hospital-wide transition policy, and identifying hospitalists trained in transitions, with multidisciplinary approaches to staff their transition consult service.
Tracking and monitoring youth in the inpatient transition process occurred in only 2% of hospitals surveyed. We urge for automatic consults to the transition service for adult aged patients admitted to children’s hospitals. With current electronic health records (EHRs), admission order sets with built-in transition consults for adolescents and young adults would improve the identification and tracking of youths. Assuming care of a pediatric patient with multiple comorbidities can be overwhelming for providers.14 The transition consult service could alleviate some of this anxiety with clear and concise documentation using standardized, readily available transition templates. These templates would summarize the patient’s past medical history and outline current medical problems, necessary subspecialty referrals, insurance status, limitations in activities of daily living, ancillary services (including physical therapy, occupational therapy, speech therapy, transportation services), and current level of readiness and independence.
In summary, the transition of care from pediatric to adult providers is a particularly vulnerable time for young adults with chronic medical conditions, and efforts focused on inpatient transitions of medical care have overall been limited. Crucial barriers include addressing psychosocial needs, gaps in insurance, and poor communication between pediatric and adult providers.4 Coller et al.11 have identified several gaps in inpatient transitions of care as well as multiple areas of focus to improve the patient experience. Based on the findings of this study, we urge children’s hospitals caring for adult patients to identify transition leaders, partner with an adult hospital to foster effective transitions, and to protocolize inpatient and outpatient models of transition. Perhaps the most concerning finding of this study was the widespread inability to track transition outcomes. Our group’s experience has led us to believe that coupling an inpatient transition consult team with EHR-based interventions to identify patients and follow outcomes has the most potential to improve inpatient transitions of care from pediatric to adult providers.
Disclosure
The authors have no conflicts of interests or financial disclosures.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
1. Elborn JS, Shale DJ, Britton JR. Cystic fibrosis: current survival and population estimates to the year 2000. Thorax. 1991;46(12):881-885.
2. Reid GJ, Webb GD, Barzel M, McCrindle BW, Irvine MJ, Siu SC. Estimates of life expectancy by adolescents and young adults with congenital heart disease. J Am Coll Cardiol. 2006;48(2):349-355. doi:10.1016/j.jacc.2006.03.041.
3. Ferris ME, Gipson DS, Kimmel PL, Eggers PW. Trends in treatment and outcomes of survival of adolescents initiating end-stage renal disease care in the United States of America. Pediatr Nephrol. 2006;21(7):1020-1026. doi:10.1007/s00467-006-0059-9.
4. Sharma N, O’Hare K, Antonelli RC, Sawicki GS. Transition care: future directions in education, health policy, and outcomes research. Acad Pediatr. 2014;14(2):120-127. doi:10.1016/j.acap.2013.11.007.
5. Harden PN, Walsh G, Bandler N, et al. Bridging the gap: an integrated paediatric to adult clinical service for young adults with kidney failure. BMJ. 2012;344:e3718. doi:10.1136/bmj.e3718.
6. Watson AR. Non-compliance and transfer from paediatric to adult transplant unit. Pediatr Nephrol. 2000;14(6):469-472.
7. Lotstein DS, Seid M, Klingensmith G, et al. Transition from pediatric to adult care for youth diagnosed with type 1 diabetes in adolescence. Pediatrics. 2013;131(4):e1062-1070. doi:10.1542/peds.2012-1450.
8. Scal P. Transition for youth with chronic conditions: primary care physicians’ approaches. Pediatrics. 2002;110(6 Pt 2):1315-1321.
9. Kelly AM, Kratz B, Bielski M, Rinehart PM. Implementing transitions for youth with complex chronic conditions using the medical home model. Pediatrics. 2002;110(6 Pt 2):1322-1327.
10. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13. doi:10.1542/peds.2010-2037.
11. Coller RJ, Ahrens S, Ehlenbach M, et al. Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals. J Hosp Med. 2018;13(1):13-20.
12. Olson D. Health Care Transitions for Young People. In Field MJ, Jette AM, Institute of Medicine (US) Committee on Disability in America, editors. The Future of Disability in America. Washington, DC: National Academy Press; 2007. https://www.ncbi.nlm.nih.gov/books/NBK11432/.
13. GotTransition.org. http://www.gottransition.org/. Accessed September 15, 2017.
14. Okumura MJ, Kerr EA, Cabana MD, Davis MM, Demonner S, Heisler M. Physician views on barriers to primary care for young adults with childhood-onset chronic disease. Pediatrics. 2010;125(4):e748-754. doi:10.1542/peds.2008-3451.
© 2018 Society of Hospital Medicine
Too Much of a Good Thing: Appropriate CTPA Use in the Diagnosis of PE
There is abundant evidence that the use of computed tomography pulmonary angiography (CTPA) is increasing in emergency departments and more patients are being diagnosed with pulmonary embolism (PE).1,2 The increasing availability and resolution of CTPA technology since the late 1990s has led some to suggest that PE is now being overdiagnosed, which is supported by decreasing PE case–fatality rates and the detection of small, subsegmental clots that do not result in any meaningful right-ventricular dysfunction.3,4 Indeed, recent guidelines allow that not all small PEs require anticoagulation therapy.5 Beyond overdiagnosis, there are potential patient-level harms associated with the liberal use of CTPA imaging, including the consequences of radiation and intravenous contrast exposure.4,6 At the societal level, excess CTPA use contributes to the growing costs of healthcare.2,7
Despite the above concerns, CTPA remains the diagnostic test of choice for PE. There are multiple approaches that are suggested to appropriately use CTPA in the workup of suspected PE, the most common of which is endorsed by best practice publications and combines a clinical score (eg, Well’s score) with D-dimer testing, reserving CTPA for those patients with high clinical risk and/or positive D-dimer.8,9 Despite the professional recommendation, studies have shown that the use of PE diagnostic algorithms in clinical practice is suboptimal, resulting in much practice variation and contributing to the overuse of CTPA.10,11 In this issue, as a means of clarifying what measures improve adherence with recommended best practices, Deblois and colleagues12 perform a systematic review of the published interventions that have attempted to reduce CTPA imaging in the diagnosis of PE.
Deblois and colleagues are to be commended for summarizing what is unfortunately a very heterogeneous literature, the limitations of which precluded a formal meta-analysis. The authors report that most of the 17 reviewed studies incorporated either electronic clinical decision support (CDS; usually imbedded into a computerized physician order entry) tools or educational interventions in a retrospective, before-and-after design; only 3 studies were experimental and included a control group. Most of the studies included efficacy, with a few evaluating safety. There was little available evidence regarding cost-effectiveness or barriers to implementation. The most studied approach, CDS, was associated with a decrease in the use of CTPA of between 8.3% and 25.4% along with an increase in PE diagnostic yield of between 3.3% and 4.4%. Likewise, the appropriate use of CTPA (consistent with best practice recommendations) increased with CDS intervention f
As discussed by the authors, CDS was the most studied and most effective intervention to improve appropriate CTPA use, albeit modest in its impact. The lack of contextual details about what factors made CDS effective or not effective makes it difficult to make general recommendations. One cited study did include physician reasons for not embracing CDS, which are not surprising in nature and reflect concerns about impaired efficiency and preference for native clinical judgement over that of electronic tools.
Moving forward, CDS, perhaps coupled with performance feedback, seems to offer the best hope of reducing inappropriate CTPA use. The growing use of electronic medical records, which is accelerated in the United States by the meaningful use provisions of the Health Information Technology for Economic and Clinical Health Act of 2009, implies that CDS tools are going to be implemented across the spectrum of diagnoses, including that of PE.13 The goals of CDS interventions, namely improved patient safety, quality, and cost-effectiveness, are more likely to be achieved if those studying and designing these electronic tools understand the day-to-day practice of clinical medicine. As summarized by Bates and colleagues14 in the “Ten Commandments for Effective Clinical Decision Support,” CDS interventions will be successful in changing physician behavior and promoting the right test or treatment only if they seamlessly fit into the clinical workflow, have no impact on (or improve upon) physician efficiency, and minimize the need for additional information from the user. As suggested by Deblois et al.,12 future studies of CDS interventions that aim to align CTPA use with recommended best practices should incorporate more rigorous methodological quality, include safety and cost-effectiveness outcomes, and, perhaps most importantly, attempt to understand the environmental and organizational factors that contribute to CDS tool effectiveness.
Disclosure
The authors have declared no conflicts of interest.
1. Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58(5):452-462. PubMed
2. Smith SB, Geske JB, Kathuria P, et al. Analysis of National Trends in Admissions for Pulmonary Embolism. Chest. 2016;150(1):35-45. PubMed
3. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. PubMed
4. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. PubMed
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. Sarma A, Heilbrun ME, Conner KE, Stevens SM, Woller SC, Elliott CG. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142(3):750-760. PubMed
7. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126(2):127-132. PubMed
8. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. PubMed
9. Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine: a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509-515. PubMed
10. Alhassan S, Sayf AA, Arsene C, Krayem H. Suboptimal implementation of diagnostic algorithms and overuse of computed tomography-pulmonary angiography in patients with suspected pulmonary embolism. Ann Thorac Med. 2016;11(4):254-260. PubMed
11. Crichlow A, Cuker A, Mills AM. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med. 2012;19(11):1219-1226. PubMed
12. Deblois S, Chartrand-Lefebvre C, Toporwicz K, Zhongyi C, Lepanto L. Interventions to reduce the overuse of imaging for pulmonary embolism: a systematic review. J Hosp Med. 2018;13(1):52-61. PubMed
13. Murphy EV. Clinical decision support: effectiveness in improving quality processes and clinical outcomes and factors that may influence success. Yale J Biol Med. 2014;87(2):187-197. PubMed
14. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530. PubMed
There is abundant evidence that the use of computed tomography pulmonary angiography (CTPA) is increasing in emergency departments and more patients are being diagnosed with pulmonary embolism (PE).1,2 The increasing availability and resolution of CTPA technology since the late 1990s has led some to suggest that PE is now being overdiagnosed, which is supported by decreasing PE case–fatality rates and the detection of small, subsegmental clots that do not result in any meaningful right-ventricular dysfunction.3,4 Indeed, recent guidelines allow that not all small PEs require anticoagulation therapy.5 Beyond overdiagnosis, there are potential patient-level harms associated with the liberal use of CTPA imaging, including the consequences of radiation and intravenous contrast exposure.4,6 At the societal level, excess CTPA use contributes to the growing costs of healthcare.2,7
Despite the above concerns, CTPA remains the diagnostic test of choice for PE. There are multiple approaches that are suggested to appropriately use CTPA in the workup of suspected PE, the most common of which is endorsed by best practice publications and combines a clinical score (eg, Well’s score) with D-dimer testing, reserving CTPA for those patients with high clinical risk and/or positive D-dimer.8,9 Despite the professional recommendation, studies have shown that the use of PE diagnostic algorithms in clinical practice is suboptimal, resulting in much practice variation and contributing to the overuse of CTPA.10,11 In this issue, as a means of clarifying what measures improve adherence with recommended best practices, Deblois and colleagues12 perform a systematic review of the published interventions that have attempted to reduce CTPA imaging in the diagnosis of PE.
Deblois and colleagues are to be commended for summarizing what is unfortunately a very heterogeneous literature, the limitations of which precluded a formal meta-analysis. The authors report that most of the 17 reviewed studies incorporated either electronic clinical decision support (CDS; usually imbedded into a computerized physician order entry) tools or educational interventions in a retrospective, before-and-after design; only 3 studies were experimental and included a control group. Most of the studies included efficacy, with a few evaluating safety. There was little available evidence regarding cost-effectiveness or barriers to implementation. The most studied approach, CDS, was associated with a decrease in the use of CTPA of between 8.3% and 25.4% along with an increase in PE diagnostic yield of between 3.3% and 4.4%. Likewise, the appropriate use of CTPA (consistent with best practice recommendations) increased with CDS intervention f
As discussed by the authors, CDS was the most studied and most effective intervention to improve appropriate CTPA use, albeit modest in its impact. The lack of contextual details about what factors made CDS effective or not effective makes it difficult to make general recommendations. One cited study did include physician reasons for not embracing CDS, which are not surprising in nature and reflect concerns about impaired efficiency and preference for native clinical judgement over that of electronic tools.
Moving forward, CDS, perhaps coupled with performance feedback, seems to offer the best hope of reducing inappropriate CTPA use. The growing use of electronic medical records, which is accelerated in the United States by the meaningful use provisions of the Health Information Technology for Economic and Clinical Health Act of 2009, implies that CDS tools are going to be implemented across the spectrum of diagnoses, including that of PE.13 The goals of CDS interventions, namely improved patient safety, quality, and cost-effectiveness, are more likely to be achieved if those studying and designing these electronic tools understand the day-to-day practice of clinical medicine. As summarized by Bates and colleagues14 in the “Ten Commandments for Effective Clinical Decision Support,” CDS interventions will be successful in changing physician behavior and promoting the right test or treatment only if they seamlessly fit into the clinical workflow, have no impact on (or improve upon) physician efficiency, and minimize the need for additional information from the user. As suggested by Deblois et al.,12 future studies of CDS interventions that aim to align CTPA use with recommended best practices should incorporate more rigorous methodological quality, include safety and cost-effectiveness outcomes, and, perhaps most importantly, attempt to understand the environmental and organizational factors that contribute to CDS tool effectiveness.
Disclosure
The authors have declared no conflicts of interest.
There is abundant evidence that the use of computed tomography pulmonary angiography (CTPA) is increasing in emergency departments and more patients are being diagnosed with pulmonary embolism (PE).1,2 The increasing availability and resolution of CTPA technology since the late 1990s has led some to suggest that PE is now being overdiagnosed, which is supported by decreasing PE case–fatality rates and the detection of small, subsegmental clots that do not result in any meaningful right-ventricular dysfunction.3,4 Indeed, recent guidelines allow that not all small PEs require anticoagulation therapy.5 Beyond overdiagnosis, there are potential patient-level harms associated with the liberal use of CTPA imaging, including the consequences of radiation and intravenous contrast exposure.4,6 At the societal level, excess CTPA use contributes to the growing costs of healthcare.2,7
Despite the above concerns, CTPA remains the diagnostic test of choice for PE. There are multiple approaches that are suggested to appropriately use CTPA in the workup of suspected PE, the most common of which is endorsed by best practice publications and combines a clinical score (eg, Well’s score) with D-dimer testing, reserving CTPA for those patients with high clinical risk and/or positive D-dimer.8,9 Despite the professional recommendation, studies have shown that the use of PE diagnostic algorithms in clinical practice is suboptimal, resulting in much practice variation and contributing to the overuse of CTPA.10,11 In this issue, as a means of clarifying what measures improve adherence with recommended best practices, Deblois and colleagues12 perform a systematic review of the published interventions that have attempted to reduce CTPA imaging in the diagnosis of PE.
Deblois and colleagues are to be commended for summarizing what is unfortunately a very heterogeneous literature, the limitations of which precluded a formal meta-analysis. The authors report that most of the 17 reviewed studies incorporated either electronic clinical decision support (CDS; usually imbedded into a computerized physician order entry) tools or educational interventions in a retrospective, before-and-after design; only 3 studies were experimental and included a control group. Most of the studies included efficacy, with a few evaluating safety. There was little available evidence regarding cost-effectiveness or barriers to implementation. The most studied approach, CDS, was associated with a decrease in the use of CTPA of between 8.3% and 25.4% along with an increase in PE diagnostic yield of between 3.3% and 4.4%. Likewise, the appropriate use of CTPA (consistent with best practice recommendations) increased with CDS intervention f
As discussed by the authors, CDS was the most studied and most effective intervention to improve appropriate CTPA use, albeit modest in its impact. The lack of contextual details about what factors made CDS effective or not effective makes it difficult to make general recommendations. One cited study did include physician reasons for not embracing CDS, which are not surprising in nature and reflect concerns about impaired efficiency and preference for native clinical judgement over that of electronic tools.
Moving forward, CDS, perhaps coupled with performance feedback, seems to offer the best hope of reducing inappropriate CTPA use. The growing use of electronic medical records, which is accelerated in the United States by the meaningful use provisions of the Health Information Technology for Economic and Clinical Health Act of 2009, implies that CDS tools are going to be implemented across the spectrum of diagnoses, including that of PE.13 The goals of CDS interventions, namely improved patient safety, quality, and cost-effectiveness, are more likely to be achieved if those studying and designing these electronic tools understand the day-to-day practice of clinical medicine. As summarized by Bates and colleagues14 in the “Ten Commandments for Effective Clinical Decision Support,” CDS interventions will be successful in changing physician behavior and promoting the right test or treatment only if they seamlessly fit into the clinical workflow, have no impact on (or improve upon) physician efficiency, and minimize the need for additional information from the user. As suggested by Deblois et al.,12 future studies of CDS interventions that aim to align CTPA use with recommended best practices should incorporate more rigorous methodological quality, include safety and cost-effectiveness outcomes, and, perhaps most importantly, attempt to understand the environmental and organizational factors that contribute to CDS tool effectiveness.
Disclosure
The authors have declared no conflicts of interest.
1. Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58(5):452-462. PubMed
2. Smith SB, Geske JB, Kathuria P, et al. Analysis of National Trends in Admissions for Pulmonary Embolism. Chest. 2016;150(1):35-45. PubMed
3. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. PubMed
4. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. PubMed
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. Sarma A, Heilbrun ME, Conner KE, Stevens SM, Woller SC, Elliott CG. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142(3):750-760. PubMed
7. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126(2):127-132. PubMed
8. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. PubMed
9. Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine: a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509-515. PubMed
10. Alhassan S, Sayf AA, Arsene C, Krayem H. Suboptimal implementation of diagnostic algorithms and overuse of computed tomography-pulmonary angiography in patients with suspected pulmonary embolism. Ann Thorac Med. 2016;11(4):254-260. PubMed
11. Crichlow A, Cuker A, Mills AM. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med. 2012;19(11):1219-1226. PubMed
12. Deblois S, Chartrand-Lefebvre C, Toporwicz K, Zhongyi C, Lepanto L. Interventions to reduce the overuse of imaging for pulmonary embolism: a systematic review. J Hosp Med. 2018;13(1):52-61. PubMed
13. Murphy EV. Clinical decision support: effectiveness in improving quality processes and clinical outcomes and factors that may influence success. Yale J Biol Med. 2014;87(2):187-197. PubMed
14. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530. PubMed
1. Kocher KE, Meurer WJ, Fazel R, Scott PA, Krumholz HM, Nallamothu BK. National trends in use of computed tomography in the emergency department. Ann Emerg Med. 2011;58(5):452-462. PubMed
2. Smith SB, Geske JB, Kathuria P, et al. Analysis of National Trends in Admissions for Pulmonary Embolism. Chest. 2016;150(1):35-45. PubMed
3. Wiener RS, Schwartz LM, Woloshin S. Time trends in pulmonary embolism in the United States: evidence of overdiagnosis. Arch Intern Med. 2011;171(9):831-837. PubMed
4. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. PubMed
5. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic Therapy for VTE Disease: CHEST Guideline and Expert Panel Report. Chest. 2016;149(2):315-352. PubMed
6. Sarma A, Heilbrun ME, Conner KE, Stevens SM, Woller SC, Elliott CG. Radiation and chest CT scan examinations: what do we know? Chest. 2012;142(3):750-760. PubMed
7. Fanikos J, Rao A, Seger AC, Carter D, Piazza G, Goldhaber SZ. Hospital costs of acute pulmonary embolism. Am J Med. 2013;126(2):127-132. PubMed
8. Raja AS, Greenberg JO, Qaseem A, et al. Evaluation of Patients With Suspected Acute Pulmonary Embolism: Best Practice Advice From the Clinical Guidelines Committee of the American College of Physicians. Ann Intern Med. 2015;163(9):701-711. PubMed
9. Schuur JD, Carney DP, Lyn ET, et al. A top-five list for emergency medicine: a pilot project to improve the value of emergency care. JAMA Intern Med. 2014;174(4):509-515. PubMed
10. Alhassan S, Sayf AA, Arsene C, Krayem H. Suboptimal implementation of diagnostic algorithms and overuse of computed tomography-pulmonary angiography in patients with suspected pulmonary embolism. Ann Thorac Med. 2016;11(4):254-260. PubMed
11. Crichlow A, Cuker A, Mills AM. Overuse of computed tomography pulmonary angiography in the evaluation of patients with suspected pulmonary embolism in the emergency department. Acad Emerg Med. 2012;19(11):1219-1226. PubMed
12. Deblois S, Chartrand-Lefebvre C, Toporwicz K, Zhongyi C, Lepanto L. Interventions to reduce the overuse of imaging for pulmonary embolism: a systematic review. J Hosp Med. 2018;13(1):52-61. PubMed
13. Murphy EV. Clinical decision support: effectiveness in improving quality processes and clinical outcomes and factors that may influence success. Yale J Biol Med. 2014;87(2):187-197. PubMed
14. Bates DW, Kuperman GJ, Wang S, et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J Am Med Inform Assoc. 2003;10(6):523-530. PubMed
Interventions to Reduce the Overuse of Imaging for Pulmonary Embolism: A Systematic Review
The last 2 decades have seen a dramatic rise in the use of medical imaging in general,1,2 as well as in the diagnostic workup of pulmonary embolism (PE) more specifically, since the introduction of multidetector row computed tomography pulmonary angiography (CTPA) in 1998.3 From 1999 to 2010, the proportions of emergency department (ED) visits associated with a diagnosis of PE and admissions for PE have increased markedly in the United States, where the situation has been well documented.4,5 A 14-fold increase in the use of CTPA was observed in health maintenance organizations from 2001 to 2008.3 A significant increase in the probability of having a diagnosis of PE in the ED was reported, likely because of increased access to CTPA, from 2001 to 2010.4 With a prevalence of 2% or less in the ED, diagnostic yields as low as 5% suggest a significant problem of overuse.6,7
Strategies have been proposed to improve the appropriateness of imaging in the detection of PE, and these rely on the use of a validated clinical decision rule (CDR) to assess the pretest probability of the diagnosis. The purpose of this systematic review is to summarize the evidence associated with interventions aimed at reducing the overuse of imaging in the diagnostic workup of PE in the ED and hospital wards. Specifically, the types of interventions, their clinical effectiveness, as well as possible harms will be assessed. A secondary objective is to appraise the impact of these interventions on healthcare costs as well as the facilitators and barriers to their implementation.
METHODS
Inclusion Criteria
Targeted settings were EDs and inpatient services of adult tertiary and quaternary care hospitals. The search addressed interventions aimed at reducing the overuse of imaging in the diagnostic workup for PE. The comparators were usual care or another type of related intervention. The main outcomes considered were the use of imaging, diagnostic yield, radiation dose, adherence to guidelines to a quality measure, safety, and costs; both experimental and observational studies were included.
Literature Search
A systematic literature search in the following electronic databases was performed: PubMed, MEDLINE, Embase, and EBM Reviews (Cochrane, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Cochrane Health Technology Assessment, and the NHS Economic Evaluation Database). The reference period was from 1998 to March 28, 2017, and publications in English and French were searched. The detailed search strategy, adapted to each of the databases, appears in supplemental Appendix 1.
Study Selection and Data Extraction
One author (SD) reviewed the titles of the selected articles and excluded those that obviously did not satisfy the inclusion criteria. Then, 2 authors (SD and LL) independently reviewed the titles and abstracts of the remaining articles. They reviewed the full manuscript of potentially relevant articles for inclusion. Disagreements that could not be resolved by discussion would have been arbitrated by a third author (CCL); however, no such disagreement occurred.
Quality and Risk of Bias Assessment
For experimental or quasiexperimental studies that involved an intervention group and a control group, the criteria proposed by the Cochrane collaborative for the evaluation of bias were used.8 For studies using a before and after design, the following main biases associated with such designs were assessed: history effect, maturation bias, testing bias, regression to the mean, and conditioning bias.9
Data Extraction and Synthesis
Data pertaining to efficacy, safety, costs, and facilitators and barriers to the implementation of interventions were extracted from the studies. The research process adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist.10 In view of the heterogeneity of the studies, a narrative synthesis was produced in accordance with the methodology proposed by Popay et al.11 The review protocol was registered in PROSPERO (this registry can be consulted at the following URL address: http://www.crd.york.ac.uk/PROSPERO/).
RESULTS
The search screened 2814 records after the removal of duplicates and studies published before 1998. The figure illustrates the literature selection process.12 Seventeen studies were included in the review following appraisal. Most of the studies (15/17) evaluated interventions in the ED,7,13-26 while the remaining studies (2/17) were conducted in clinical wards of acute care hospitals.27,28 Thirteen studies were conducted in the United States, 3 in Australia, and 1 in Europe. Four types of interventions were identified in the selected studies: electronic clinical decision support (CDS) (8/17), educational interventions (7/17), performance feedback reports (PFRs) (1/17), and an institutional clinical pretest policy (1/17). In 10 of the studies, the proposed intervention was mandatory.
One systematic review and meta-analysis pertaining to the impact of CDRs on CTPA use and yield was identified.29 Five of the studies it included were also included in the present review.13,16,21-23 However, its focus is different than the present one, which aims at assessing the evidence associated with the interventions being implemented to promote the use of the CDRs.29
The list of included studies appears in supplemental Appendix 2. The list of potentially relevant studies that were finally excluded is provided in supplemental Appendix 3.
Most studies (14/17) presented a before-after design, with data collection corresponding to periods preceding and following a specific intervention. Most of them are retrospective and assessed the efficacy and safety results. They were deemed of generally poor quality and were subject to many of the biases mentioned above as well as to an interaction between the intervention and its implementation context. The remaining 3 studies were experimental in design with a comparative control group.13,14,27 In 2 of these studies, a comparison was made with traditional clinical practice (no intervention).13,27 In the third, the intervention was compared with CDS only.14 The control group studies were of intermediate to very good quality and were subject to biases of performance, detection, selection, and attrition.
Table 1 summarizes the study characteristics of the included studies. The detailed methodological quality appraisal of the control group studies appears in supplemental Appendix 4.
Efficacy
CDS and PFRs
Eight of the studies appraised CDS interventions.13,16,17,19,21,22,24,28 They consisted of computer-based applications imbedded into the computerized physician order entry of the setting (ED or clinical ward of an acute care hospital), which are prompted when a physician orders an imaging exam or D-dimer test.
Educational Interventions and Policy
Seven of the interventions assessed in the included studies were educational in their essence, involving training sessions aimed at strengthening physician use of CDRs for the diagnosis of PE.15,18,20,23,25-27 Three studies observed a statistically significant impact on the
The impact of a policy fostering the use of a CDR and D-dimer was appraised in 1 study.7 This intervention translated into a significant reduction of CTPA use and a significant increase of CTPA diagnostic yield. However, only 4% of patient charts reported a clinical probability of PE, and in most cases, the type of CDR used was not mentioned.7
Safety
A minority of studies evaluated the safety of the interventions.13,18,19,23,25,27 Only 2 of these
The 2 studies involving a control group did not find significant differences between the intervention and the control groups with respect to mortality, complications because of thromboembolic and bleeding events, or any other adverse event during the 3-months’ follow-up.13,27
Jiménez et al.19 reported less than 1% mortality following the implementation of a CDS (0.7%; 95% CI, 0.2%-1.1%). In their study assessing the impact of an educational intervention, Kline et al.23 (2004) observed that none of the patients discharged with a fully negative Charlotte rule died suddenly and unexpectedly at 90-day follow-up. However, another educational intervention aimed at reducing ED patients’ radiation exposure observed a significant increase in the 90-day all-cause mortality of patients with negative CTPA, which was associated with a decline in the 90-day mortality of patients with negative ventilation/perfusion (V/Q) scanning.25
Jiménez et al.19 observed an absolute decrease of 2.5% in the incidence of symptomatic VTE events after the intervention (95% CI, 0.9%-4.6%; P < .01). The occurrence of VTE events, including PE, reached 1% in Goergen et al.18 and 3.9% in Kline et al.23 (2004) during follow-up.
Economic Aspects
Kline et al.13 (2014) found a significant decrease in charges and estimated costs for medical care within 90 days of initial ED presentation in the patients who were investigated with CTPA in the intervention group. The median costs of medical care within 30 days of the initial ED presentation were US $1274 in the control group and US $934 in the intervention group (P = .018).13 The median charges of medical care within 30 days of the initial ED presentation were US $7595 in the control group and US $6281 in the intervention group (P = .004).13
Facilitators and Barriers
Only 1 study appraised the reasons given by emergency physicians for not adhering to CDS recommendations.16 The reason most often given was the time needed to access and use the application, which was perceived as having a negative impact on productivity as well as a preference for intuitive clinical judgment.16 Though not the result of specific evaluation or data collection, some authors commented on the factors that may facilitate or impede the implementation of interventions to diminish the inappropriate use.14,20 Kanaan et al.20 proposed that factors other than the knowledge of current clinical guidelines may explain CTPA use. Booker and Johnson26 suggested that the demand for rapid turnover in the ED may lead to “so-called ‘blanket ordering’, which attempts to reach diagnosis as quickly as possible despite cost and patient safety.” Raja et al.14 (2015) suggested that the unambiguous representation of guidelines based on validated, high-quality evidence in the CDS may have improved physician adoption in their study.
DISCUSSION
Efficacy
Baseline values for the use of imaging and diagnostic yield show important variation, especially when compared with the study performed in Europe.19 In general, only a modest impact is measured with regard to a decrease in the use of imaging, an increase in diagnostic use, and adherence to validated CDRs.
Among the interventions appraised, CDS was evaluated in the largest number of included studies, and its
The impact of CDS on diagnostic yield was mixed because 3 studies observed an increase in diagnostic yield postintervention,16,21,22 and 3 others monitored no significant impact.19,24,28 Adherence to guidelines or a quality measure was assessed in 2 studies, which reported a significant increase in appropriate ordering.17,24 Raja et al.24 (2014) observed an 18.7% increase in appropriate ordering after the implementation of a CDS from 56.9% to 75.6% (P < .01). Geeting et al.17 observed a similar increase, with appropriate ordering increasing from 58% to 76% over the duration of the intervention. However, this increase in appropriate use was not associated with a variation in CTPA use or diagnostic yield, which leads the investigators to posit that the physicians gradually inflated the Wells score they keyed into the CDS despite that no threshold Wells score was required to perform a CTPA.17
Raja et al.14 (2015) demonstrated that the implementation of performance feedback reporting, in addition to a CDS, can significantly increase adherence to CDR for the evaluation of PE in the ED. Additional studies would help to better understand the potential impact of such reports on CTPA use in the diagnostic workup of PE. However, it suggests that a combination of interventions, including the implementation of a CDS, performance feedback reporting, and well-designed and specific educational interventions, may have a more significant impact than any of these types of interventions taken separately.
The impact of the educational interventions appraised in this review on the expected results is mixed, though it is difficult to compare the observed results and draw conclusive remarks, as the characteristics of the interventions and study designs are different from each other.
Safety
There is limited evidence on the safety of appraised interventions. Only 6 studies appraised venous thrombolic events or mortality.13,18,19,23,25,27 However, no adverse events were noted in those studies evaluating possible complications or missed diagnoses. Additional research is needed to confirm the safety of the interventions appraised in this systematic review.
Facilitators and Barriers
There are significant limitations with respect to the analysis of the factors that favor or impede the implementation of the interventions appraised in this review. However, 2 studies that did not meet the inclusion criteria appraised physicians’ perceptions and attitudes toward prescribing imaging tests in the diagnostic workup of PE.31,32 One is Swiss31 and the other is Canadian.32 Both were conducted in the ED of academic hospitals. Rohacek et al.31 observed that defensive behaviors, such as “fear of missing PE,” were frequent and associated with a lower probability of a positive CTPA (OR = 0.36; 95% CI, 0.14-0.92). Ahn et al.32 concluded that, although ED physicians who participated in their survey possessed limited knowledge of radiation doses of CTPA and V/Q scans, they opted for V/Q scans that emit lower radiation doses in younger patients, especially females, which may reflect efforts done in the study setting to reduce patients’ radiation exposure.
There is not enough data to conclude on safety and the impact on healthcare costs.
Implications for Future Research
Future controlled studies of high methodological quality would help to better understand the effects associated with the implementation of the interventions aimed at reducing the inappropriate use of imaging in the diagnostic workup of PE. Efficacy results show that the success of the implementation of the various types of interventions is variable. This variation may be at least partly attributable to contextual factors, such as the external environment, the organizational leadership and culture, or the microsystem, such as differences in care patterns.33-35 The impact of context factors on the effectiveness of the interventions should be assessed further with appropriate tools.33,34,36
CONCLUSION
The joint use of CDS and PFRs appears more effective than the other types of intervention in reducing the inappropriate use of CTPA. However, an approach combining these with well-designed educational interventions as well as policies may be even more effective.
Future studies of high methodological quality would strengthen the evidence concerning the relative efficacy and safety of the interventions appraised, especially when various types are combined. Future research should also aim at bringing answers to the knowledge gaps related to the factors of success and barriers associated with the implementation of the interventions.
Disclosure
The authors report no conflict of interest.
1. Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. PubMed
2. Canadian Institute for Health Information (CIHI). Medical Imaging in Canada 2012. https://www.cihi.ca/en/mit_summary_2012_en.pdf. Accessed December 14, 2016.
3. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. doi:10.1136/bmj.f3368. PubMed
4. Schissler AJ, Rozenshtein A, Schluger NW, Einstein AJ. National trends in emergency room diagnosis of pulmonary embolism, 2001-2010: a cross-sectional study. Respir Res. 2015;16:44-50. PubMed
5. Minges KE, Bikdeli B, Wang Y, et al. National Trends in Pulmonary Embolism Hospitalization Rates and Outcomes for Adults Aged >/=65 Years in the United States (1999 to 2010). Am J Cardiol. 2015;116(9):1436-1442. PubMed
6. Duriseti RS, Brandeau ML. Cost-effectiveness of strategies for diagnosing pulmonary embolism among emergency department patients presenting with undifferentiated symptoms. Ann Emerg Med. 2010;56(4):321-332.e310. PubMed
7. Char S, Yoon HC. Improving appropriate use of pulmonary computed tomography angiography by increasing the serum D-dimer threshold and assessing clinical probability. Perm J. 2014;18(4):10-15. PubMed
8. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928 PubMed
9. Champagne F, Brousselle A, Contendriopoulos AP, Hartz Z. L’analyse des effets. In: Brousselle A, Champagne F, Contandriopoulos AP, Hartz Z, editors. L’évaluation: Concepts et Méthodes 2e Edition. Montréal: Les Presses de l’Université de Montréal; 2011: 173-198.
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. PubMed
11. Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Manchester, UK: ESRC Methods Programme; 2006.
12. Velasco M, Perleth M, Drummond M, et al. Best practice in undertaking and reporting health technology assessments. Working group 4 report. Int J Technol Assess Health Care. 2002;18(2):361-422. PubMed
13. Kline JA, Jones AE, Shapiro NI, et al. Multicenter, randomized trial of quantitative pretest probability to reduce unnecessary medical radiation exposure in emergency department patients with chest pain and dyspnea. Circ Cardiovasc Imaging. 2014;7(1):66-73. PubMed
14. Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of Performance Feedback Reports on Adherence to Evidence-Based Guidelines in Use of CT for Evaluation of Pulmonary Embolism in the Emergency Department: A Randomized Trial. AJR Am J Roentgenol. 2015;205(5):936-940. PubMed
15. Agarwal A, Persaud J, Grabinski R, Rabinowitz D, Bremner A, Mendelson R. Pulmonary embolism: are we there yet? J Med Imaging Radiat Oncol. 2012;56(3):270-281. PubMed
16. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57(6):613-621. PubMed
17. Geeting GK, Beck M, Bruno MA, et al. Mandatory Assignment of Modified Wells Score Before CT Angiography for Pulmonary Embolism Fails to Improve Utilization or Percentage of Positive Cases. AJR Am J Roentgenol. 2016;207(2):442-449. PubMed
18. Goergen SK, Chan T, de Campo JF, et al. Reducing the use of diagnostic imaging in patients with suspected pulmonary embolism: validation of a risk assessment strategy. Emerg Med Australas. 2005;17(1):16-23. PubMed
19. Jiménez D, Resano S, Otero R, et al. Computerised clinical decision support for suspected PE. Thorax. 2015;70(9):909-911. PubMed
20. Kanaan Y, Knoepp UD, Kelly AM. The influence of education on appropriateness rates for CT pulmonary angiography in emergency department patients. Acad Radiol. 2013;20(9):1107-1114. PubMed
21. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
22. Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262(2):468-474. PubMed
23. Kline JA, Webb WB, Jones AE, Hernandez-Nino J. Impact of a rapid rule-out protocol for pulmonary embolism on the rate of screening, missed cases, and pulmonary vascular imaging in an urban US emergency department. Ann Emerg Med. 2004;44(5):490-502. PubMed
24. Raja AS, Gupta A, Ip IK, Mills AM, Khorasani R. The use of decision support to measure documented adherence to a national imaging quality measure. Acad Radiol. 2014;21(3):378-383. PubMed
25. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am J Roentgenol. 2010;194(2):392-397. PubMed
26. Booker MT, Johnson JO. Optimizing CT Pulmonary Angiogram Utilization in a Community Emergency Department: A Pre- and Postintervention Study. J Am Coll Radiol. 2017;14(1):65-71. PubMed
27. Goldstein NM, Kollef MH, Ward S, Gage BF. The impact of the introduction of a rapid D-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. Arch Intern Med. 2001;161(4):567-571. PubMed
28. Dunne RM, Ip IK, Abbett S, et al. Effect of Evidence-based Clinical Decision Support on the Use and Yield of CT Pulmonary Angiographic Imaging in Hospitalized Patients. Radiology. 2015;276(1):167-174. PubMed
29. Wang RC, Bent S, Weber E, Neilson J, Smith-Bindman R, Fahimi J. The Impact of Clinical Decision Rules on Computed Tomography Use and Yield for Pulmonary Embolism: A Systematic Review and Meta-analysis. Ann Emerg Med. 2016;67(6):693-701. PubMed
30. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
31. Rohacek M, Buatsi J, Szucs-Farkas Z, et al. Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med. 2012;38(8):1345-1351. PubMed
32. Ahn JS, Edmonds ML, McLeod SL, Dreyer JF. Familiarity with radiation exposure dose from diagnostic imaging for acute pulmonary embolism and current patterns of practice. CJEM. 2014;16(5):393-404. PubMed
33. Kringos DS, Sunol R, Wagner C, et al. The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. BMC Health Serv Res. 2015;15(277):015-0906. PubMed
34. Kaplan HC, Brady PW, Dritz MC, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q. 2010;88(4):500-559. PubMed
35. Pernod G, Caterino J, Maignan M, Tissier C, Kassis J, Lazarchick J. D-dimer use and pulmonary embolism diagnosis in emergency units: Why is there such a difference in pulmonary embolism prevalence between the United States of America and countries outside USA? PLoS ONE. 2017;12(1):e0169268. doi:10.1371/journal.pone.0169268 PubMed
36. Saillour-Glenisson F, Domecq S, Kret M, Sibe M, Dumond JP, Michel P. Design and validation of a questionnaire to assess organizational culture in French hospital wards. BMC Health Serv Res. 2016;16:491-503. PubMed
37. Kline JA, Nelson RD, Jackson RE, Courtney DM. Criteria for the safe use of D-dimer testing in emergency department patients with suspected pulmonary embolism: a multicenter US study. Ann Emerg Med. 2002;39(2):144-152. PubMed
38. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. New Engl J Med. 2006;354(22):2317-2327. PubMed
39. Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055. PubMed
40. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276-2315. PubMed
The last 2 decades have seen a dramatic rise in the use of medical imaging in general,1,2 as well as in the diagnostic workup of pulmonary embolism (PE) more specifically, since the introduction of multidetector row computed tomography pulmonary angiography (CTPA) in 1998.3 From 1999 to 2010, the proportions of emergency department (ED) visits associated with a diagnosis of PE and admissions for PE have increased markedly in the United States, where the situation has been well documented.4,5 A 14-fold increase in the use of CTPA was observed in health maintenance organizations from 2001 to 2008.3 A significant increase in the probability of having a diagnosis of PE in the ED was reported, likely because of increased access to CTPA, from 2001 to 2010.4 With a prevalence of 2% or less in the ED, diagnostic yields as low as 5% suggest a significant problem of overuse.6,7
Strategies have been proposed to improve the appropriateness of imaging in the detection of PE, and these rely on the use of a validated clinical decision rule (CDR) to assess the pretest probability of the diagnosis. The purpose of this systematic review is to summarize the evidence associated with interventions aimed at reducing the overuse of imaging in the diagnostic workup of PE in the ED and hospital wards. Specifically, the types of interventions, their clinical effectiveness, as well as possible harms will be assessed. A secondary objective is to appraise the impact of these interventions on healthcare costs as well as the facilitators and barriers to their implementation.
METHODS
Inclusion Criteria
Targeted settings were EDs and inpatient services of adult tertiary and quaternary care hospitals. The search addressed interventions aimed at reducing the overuse of imaging in the diagnostic workup for PE. The comparators were usual care or another type of related intervention. The main outcomes considered were the use of imaging, diagnostic yield, radiation dose, adherence to guidelines to a quality measure, safety, and costs; both experimental and observational studies were included.
Literature Search
A systematic literature search in the following electronic databases was performed: PubMed, MEDLINE, Embase, and EBM Reviews (Cochrane, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Cochrane Health Technology Assessment, and the NHS Economic Evaluation Database). The reference period was from 1998 to March 28, 2017, and publications in English and French were searched. The detailed search strategy, adapted to each of the databases, appears in supplemental Appendix 1.
Study Selection and Data Extraction
One author (SD) reviewed the titles of the selected articles and excluded those that obviously did not satisfy the inclusion criteria. Then, 2 authors (SD and LL) independently reviewed the titles and abstracts of the remaining articles. They reviewed the full manuscript of potentially relevant articles for inclusion. Disagreements that could not be resolved by discussion would have been arbitrated by a third author (CCL); however, no such disagreement occurred.
Quality and Risk of Bias Assessment
For experimental or quasiexperimental studies that involved an intervention group and a control group, the criteria proposed by the Cochrane collaborative for the evaluation of bias were used.8 For studies using a before and after design, the following main biases associated with such designs were assessed: history effect, maturation bias, testing bias, regression to the mean, and conditioning bias.9
Data Extraction and Synthesis
Data pertaining to efficacy, safety, costs, and facilitators and barriers to the implementation of interventions were extracted from the studies. The research process adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist.10 In view of the heterogeneity of the studies, a narrative synthesis was produced in accordance with the methodology proposed by Popay et al.11 The review protocol was registered in PROSPERO (this registry can be consulted at the following URL address: http://www.crd.york.ac.uk/PROSPERO/).
RESULTS
The search screened 2814 records after the removal of duplicates and studies published before 1998. The figure illustrates the literature selection process.12 Seventeen studies were included in the review following appraisal. Most of the studies (15/17) evaluated interventions in the ED,7,13-26 while the remaining studies (2/17) were conducted in clinical wards of acute care hospitals.27,28 Thirteen studies were conducted in the United States, 3 in Australia, and 1 in Europe. Four types of interventions were identified in the selected studies: electronic clinical decision support (CDS) (8/17), educational interventions (7/17), performance feedback reports (PFRs) (1/17), and an institutional clinical pretest policy (1/17). In 10 of the studies, the proposed intervention was mandatory.
One systematic review and meta-analysis pertaining to the impact of CDRs on CTPA use and yield was identified.29 Five of the studies it included were also included in the present review.13,16,21-23 However, its focus is different than the present one, which aims at assessing the evidence associated with the interventions being implemented to promote the use of the CDRs.29
The list of included studies appears in supplemental Appendix 2. The list of potentially relevant studies that were finally excluded is provided in supplemental Appendix 3.
Most studies (14/17) presented a before-after design, with data collection corresponding to periods preceding and following a specific intervention. Most of them are retrospective and assessed the efficacy and safety results. They were deemed of generally poor quality and were subject to many of the biases mentioned above as well as to an interaction between the intervention and its implementation context. The remaining 3 studies were experimental in design with a comparative control group.13,14,27 In 2 of these studies, a comparison was made with traditional clinical practice (no intervention).13,27 In the third, the intervention was compared with CDS only.14 The control group studies were of intermediate to very good quality and were subject to biases of performance, detection, selection, and attrition.
Table 1 summarizes the study characteristics of the included studies. The detailed methodological quality appraisal of the control group studies appears in supplemental Appendix 4.
Efficacy
CDS and PFRs
Eight of the studies appraised CDS interventions.13,16,17,19,21,22,24,28 They consisted of computer-based applications imbedded into the computerized physician order entry of the setting (ED or clinical ward of an acute care hospital), which are prompted when a physician orders an imaging exam or D-dimer test.
Educational Interventions and Policy
Seven of the interventions assessed in the included studies were educational in their essence, involving training sessions aimed at strengthening physician use of CDRs for the diagnosis of PE.15,18,20,23,25-27 Three studies observed a statistically significant impact on the
The impact of a policy fostering the use of a CDR and D-dimer was appraised in 1 study.7 This intervention translated into a significant reduction of CTPA use and a significant increase of CTPA diagnostic yield. However, only 4% of patient charts reported a clinical probability of PE, and in most cases, the type of CDR used was not mentioned.7
Safety
A minority of studies evaluated the safety of the interventions.13,18,19,23,25,27 Only 2 of these
The 2 studies involving a control group did not find significant differences between the intervention and the control groups with respect to mortality, complications because of thromboembolic and bleeding events, or any other adverse event during the 3-months’ follow-up.13,27
Jiménez et al.19 reported less than 1% mortality following the implementation of a CDS (0.7%; 95% CI, 0.2%-1.1%). In their study assessing the impact of an educational intervention, Kline et al.23 (2004) observed that none of the patients discharged with a fully negative Charlotte rule died suddenly and unexpectedly at 90-day follow-up. However, another educational intervention aimed at reducing ED patients’ radiation exposure observed a significant increase in the 90-day all-cause mortality of patients with negative CTPA, which was associated with a decline in the 90-day mortality of patients with negative ventilation/perfusion (V/Q) scanning.25
Jiménez et al.19 observed an absolute decrease of 2.5% in the incidence of symptomatic VTE events after the intervention (95% CI, 0.9%-4.6%; P < .01). The occurrence of VTE events, including PE, reached 1% in Goergen et al.18 and 3.9% in Kline et al.23 (2004) during follow-up.
Economic Aspects
Kline et al.13 (2014) found a significant decrease in charges and estimated costs for medical care within 90 days of initial ED presentation in the patients who were investigated with CTPA in the intervention group. The median costs of medical care within 30 days of the initial ED presentation were US $1274 in the control group and US $934 in the intervention group (P = .018).13 The median charges of medical care within 30 days of the initial ED presentation were US $7595 in the control group and US $6281 in the intervention group (P = .004).13
Facilitators and Barriers
Only 1 study appraised the reasons given by emergency physicians for not adhering to CDS recommendations.16 The reason most often given was the time needed to access and use the application, which was perceived as having a negative impact on productivity as well as a preference for intuitive clinical judgment.16 Though not the result of specific evaluation or data collection, some authors commented on the factors that may facilitate or impede the implementation of interventions to diminish the inappropriate use.14,20 Kanaan et al.20 proposed that factors other than the knowledge of current clinical guidelines may explain CTPA use. Booker and Johnson26 suggested that the demand for rapid turnover in the ED may lead to “so-called ‘blanket ordering’, which attempts to reach diagnosis as quickly as possible despite cost and patient safety.” Raja et al.14 (2015) suggested that the unambiguous representation of guidelines based on validated, high-quality evidence in the CDS may have improved physician adoption in their study.
DISCUSSION
Efficacy
Baseline values for the use of imaging and diagnostic yield show important variation, especially when compared with the study performed in Europe.19 In general, only a modest impact is measured with regard to a decrease in the use of imaging, an increase in diagnostic use, and adherence to validated CDRs.
Among the interventions appraised, CDS was evaluated in the largest number of included studies, and its
The impact of CDS on diagnostic yield was mixed because 3 studies observed an increase in diagnostic yield postintervention,16,21,22 and 3 others monitored no significant impact.19,24,28 Adherence to guidelines or a quality measure was assessed in 2 studies, which reported a significant increase in appropriate ordering.17,24 Raja et al.24 (2014) observed an 18.7% increase in appropriate ordering after the implementation of a CDS from 56.9% to 75.6% (P < .01). Geeting et al.17 observed a similar increase, with appropriate ordering increasing from 58% to 76% over the duration of the intervention. However, this increase in appropriate use was not associated with a variation in CTPA use or diagnostic yield, which leads the investigators to posit that the physicians gradually inflated the Wells score they keyed into the CDS despite that no threshold Wells score was required to perform a CTPA.17
Raja et al.14 (2015) demonstrated that the implementation of performance feedback reporting, in addition to a CDS, can significantly increase adherence to CDR for the evaluation of PE in the ED. Additional studies would help to better understand the potential impact of such reports on CTPA use in the diagnostic workup of PE. However, it suggests that a combination of interventions, including the implementation of a CDS, performance feedback reporting, and well-designed and specific educational interventions, may have a more significant impact than any of these types of interventions taken separately.
The impact of the educational interventions appraised in this review on the expected results is mixed, though it is difficult to compare the observed results and draw conclusive remarks, as the characteristics of the interventions and study designs are different from each other.
Safety
There is limited evidence on the safety of appraised interventions. Only 6 studies appraised venous thrombolic events or mortality.13,18,19,23,25,27 However, no adverse events were noted in those studies evaluating possible complications or missed diagnoses. Additional research is needed to confirm the safety of the interventions appraised in this systematic review.
Facilitators and Barriers
There are significant limitations with respect to the analysis of the factors that favor or impede the implementation of the interventions appraised in this review. However, 2 studies that did not meet the inclusion criteria appraised physicians’ perceptions and attitudes toward prescribing imaging tests in the diagnostic workup of PE.31,32 One is Swiss31 and the other is Canadian.32 Both were conducted in the ED of academic hospitals. Rohacek et al.31 observed that defensive behaviors, such as “fear of missing PE,” were frequent and associated with a lower probability of a positive CTPA (OR = 0.36; 95% CI, 0.14-0.92). Ahn et al.32 concluded that, although ED physicians who participated in their survey possessed limited knowledge of radiation doses of CTPA and V/Q scans, they opted for V/Q scans that emit lower radiation doses in younger patients, especially females, which may reflect efforts done in the study setting to reduce patients’ radiation exposure.
There is not enough data to conclude on safety and the impact on healthcare costs.
Implications for Future Research
Future controlled studies of high methodological quality would help to better understand the effects associated with the implementation of the interventions aimed at reducing the inappropriate use of imaging in the diagnostic workup of PE. Efficacy results show that the success of the implementation of the various types of interventions is variable. This variation may be at least partly attributable to contextual factors, such as the external environment, the organizational leadership and culture, or the microsystem, such as differences in care patterns.33-35 The impact of context factors on the effectiveness of the interventions should be assessed further with appropriate tools.33,34,36
CONCLUSION
The joint use of CDS and PFRs appears more effective than the other types of intervention in reducing the inappropriate use of CTPA. However, an approach combining these with well-designed educational interventions as well as policies may be even more effective.
Future studies of high methodological quality would strengthen the evidence concerning the relative efficacy and safety of the interventions appraised, especially when various types are combined. Future research should also aim at bringing answers to the knowledge gaps related to the factors of success and barriers associated with the implementation of the interventions.
Disclosure
The authors report no conflict of interest.
The last 2 decades have seen a dramatic rise in the use of medical imaging in general,1,2 as well as in the diagnostic workup of pulmonary embolism (PE) more specifically, since the introduction of multidetector row computed tomography pulmonary angiography (CTPA) in 1998.3 From 1999 to 2010, the proportions of emergency department (ED) visits associated with a diagnosis of PE and admissions for PE have increased markedly in the United States, where the situation has been well documented.4,5 A 14-fold increase in the use of CTPA was observed in health maintenance organizations from 2001 to 2008.3 A significant increase in the probability of having a diagnosis of PE in the ED was reported, likely because of increased access to CTPA, from 2001 to 2010.4 With a prevalence of 2% or less in the ED, diagnostic yields as low as 5% suggest a significant problem of overuse.6,7
Strategies have been proposed to improve the appropriateness of imaging in the detection of PE, and these rely on the use of a validated clinical decision rule (CDR) to assess the pretest probability of the diagnosis. The purpose of this systematic review is to summarize the evidence associated with interventions aimed at reducing the overuse of imaging in the diagnostic workup of PE in the ED and hospital wards. Specifically, the types of interventions, their clinical effectiveness, as well as possible harms will be assessed. A secondary objective is to appraise the impact of these interventions on healthcare costs as well as the facilitators and barriers to their implementation.
METHODS
Inclusion Criteria
Targeted settings were EDs and inpatient services of adult tertiary and quaternary care hospitals. The search addressed interventions aimed at reducing the overuse of imaging in the diagnostic workup for PE. The comparators were usual care or another type of related intervention. The main outcomes considered were the use of imaging, diagnostic yield, radiation dose, adherence to guidelines to a quality measure, safety, and costs; both experimental and observational studies were included.
Literature Search
A systematic literature search in the following electronic databases was performed: PubMed, MEDLINE, Embase, and EBM Reviews (Cochrane, ACP Journal Club, Database of Abstracts of Reviews of Effects, Cochrane Central Register of Controlled Trials, Cochrane Methodology Register, Cochrane Health Technology Assessment, and the NHS Economic Evaluation Database). The reference period was from 1998 to March 28, 2017, and publications in English and French were searched. The detailed search strategy, adapted to each of the databases, appears in supplemental Appendix 1.
Study Selection and Data Extraction
One author (SD) reviewed the titles of the selected articles and excluded those that obviously did not satisfy the inclusion criteria. Then, 2 authors (SD and LL) independently reviewed the titles and abstracts of the remaining articles. They reviewed the full manuscript of potentially relevant articles for inclusion. Disagreements that could not be resolved by discussion would have been arbitrated by a third author (CCL); however, no such disagreement occurred.
Quality and Risk of Bias Assessment
For experimental or quasiexperimental studies that involved an intervention group and a control group, the criteria proposed by the Cochrane collaborative for the evaluation of bias were used.8 For studies using a before and after design, the following main biases associated with such designs were assessed: history effect, maturation bias, testing bias, regression to the mean, and conditioning bias.9
Data Extraction and Synthesis
Data pertaining to efficacy, safety, costs, and facilitators and barriers to the implementation of interventions were extracted from the studies. The research process adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2009 checklist.10 In view of the heterogeneity of the studies, a narrative synthesis was produced in accordance with the methodology proposed by Popay et al.11 The review protocol was registered in PROSPERO (this registry can be consulted at the following URL address: http://www.crd.york.ac.uk/PROSPERO/).
RESULTS
The search screened 2814 records after the removal of duplicates and studies published before 1998. The figure illustrates the literature selection process.12 Seventeen studies were included in the review following appraisal. Most of the studies (15/17) evaluated interventions in the ED,7,13-26 while the remaining studies (2/17) were conducted in clinical wards of acute care hospitals.27,28 Thirteen studies were conducted in the United States, 3 in Australia, and 1 in Europe. Four types of interventions were identified in the selected studies: electronic clinical decision support (CDS) (8/17), educational interventions (7/17), performance feedback reports (PFRs) (1/17), and an institutional clinical pretest policy (1/17). In 10 of the studies, the proposed intervention was mandatory.
One systematic review and meta-analysis pertaining to the impact of CDRs on CTPA use and yield was identified.29 Five of the studies it included were also included in the present review.13,16,21-23 However, its focus is different than the present one, which aims at assessing the evidence associated with the interventions being implemented to promote the use of the CDRs.29
The list of included studies appears in supplemental Appendix 2. The list of potentially relevant studies that were finally excluded is provided in supplemental Appendix 3.
Most studies (14/17) presented a before-after design, with data collection corresponding to periods preceding and following a specific intervention. Most of them are retrospective and assessed the efficacy and safety results. They were deemed of generally poor quality and were subject to many of the biases mentioned above as well as to an interaction between the intervention and its implementation context. The remaining 3 studies were experimental in design with a comparative control group.13,14,27 In 2 of these studies, a comparison was made with traditional clinical practice (no intervention).13,27 In the third, the intervention was compared with CDS only.14 The control group studies were of intermediate to very good quality and were subject to biases of performance, detection, selection, and attrition.
Table 1 summarizes the study characteristics of the included studies. The detailed methodological quality appraisal of the control group studies appears in supplemental Appendix 4.
Efficacy
CDS and PFRs
Eight of the studies appraised CDS interventions.13,16,17,19,21,22,24,28 They consisted of computer-based applications imbedded into the computerized physician order entry of the setting (ED or clinical ward of an acute care hospital), which are prompted when a physician orders an imaging exam or D-dimer test.
Educational Interventions and Policy
Seven of the interventions assessed in the included studies were educational in their essence, involving training sessions aimed at strengthening physician use of CDRs for the diagnosis of PE.15,18,20,23,25-27 Three studies observed a statistically significant impact on the
The impact of a policy fostering the use of a CDR and D-dimer was appraised in 1 study.7 This intervention translated into a significant reduction of CTPA use and a significant increase of CTPA diagnostic yield. However, only 4% of patient charts reported a clinical probability of PE, and in most cases, the type of CDR used was not mentioned.7
Safety
A minority of studies evaluated the safety of the interventions.13,18,19,23,25,27 Only 2 of these
The 2 studies involving a control group did not find significant differences between the intervention and the control groups with respect to mortality, complications because of thromboembolic and bleeding events, or any other adverse event during the 3-months’ follow-up.13,27
Jiménez et al.19 reported less than 1% mortality following the implementation of a CDS (0.7%; 95% CI, 0.2%-1.1%). In their study assessing the impact of an educational intervention, Kline et al.23 (2004) observed that none of the patients discharged with a fully negative Charlotte rule died suddenly and unexpectedly at 90-day follow-up. However, another educational intervention aimed at reducing ED patients’ radiation exposure observed a significant increase in the 90-day all-cause mortality of patients with negative CTPA, which was associated with a decline in the 90-day mortality of patients with negative ventilation/perfusion (V/Q) scanning.25
Jiménez et al.19 observed an absolute decrease of 2.5% in the incidence of symptomatic VTE events after the intervention (95% CI, 0.9%-4.6%; P < .01). The occurrence of VTE events, including PE, reached 1% in Goergen et al.18 and 3.9% in Kline et al.23 (2004) during follow-up.
Economic Aspects
Kline et al.13 (2014) found a significant decrease in charges and estimated costs for medical care within 90 days of initial ED presentation in the patients who were investigated with CTPA in the intervention group. The median costs of medical care within 30 days of the initial ED presentation were US $1274 in the control group and US $934 in the intervention group (P = .018).13 The median charges of medical care within 30 days of the initial ED presentation were US $7595 in the control group and US $6281 in the intervention group (P = .004).13
Facilitators and Barriers
Only 1 study appraised the reasons given by emergency physicians for not adhering to CDS recommendations.16 The reason most often given was the time needed to access and use the application, which was perceived as having a negative impact on productivity as well as a preference for intuitive clinical judgment.16 Though not the result of specific evaluation or data collection, some authors commented on the factors that may facilitate or impede the implementation of interventions to diminish the inappropriate use.14,20 Kanaan et al.20 proposed that factors other than the knowledge of current clinical guidelines may explain CTPA use. Booker and Johnson26 suggested that the demand for rapid turnover in the ED may lead to “so-called ‘blanket ordering’, which attempts to reach diagnosis as quickly as possible despite cost and patient safety.” Raja et al.14 (2015) suggested that the unambiguous representation of guidelines based on validated, high-quality evidence in the CDS may have improved physician adoption in their study.
DISCUSSION
Efficacy
Baseline values for the use of imaging and diagnostic yield show important variation, especially when compared with the study performed in Europe.19 In general, only a modest impact is measured with regard to a decrease in the use of imaging, an increase in diagnostic use, and adherence to validated CDRs.
Among the interventions appraised, CDS was evaluated in the largest number of included studies, and its
The impact of CDS on diagnostic yield was mixed because 3 studies observed an increase in diagnostic yield postintervention,16,21,22 and 3 others monitored no significant impact.19,24,28 Adherence to guidelines or a quality measure was assessed in 2 studies, which reported a significant increase in appropriate ordering.17,24 Raja et al.24 (2014) observed an 18.7% increase in appropriate ordering after the implementation of a CDS from 56.9% to 75.6% (P < .01). Geeting et al.17 observed a similar increase, with appropriate ordering increasing from 58% to 76% over the duration of the intervention. However, this increase in appropriate use was not associated with a variation in CTPA use or diagnostic yield, which leads the investigators to posit that the physicians gradually inflated the Wells score they keyed into the CDS despite that no threshold Wells score was required to perform a CTPA.17
Raja et al.14 (2015) demonstrated that the implementation of performance feedback reporting, in addition to a CDS, can significantly increase adherence to CDR for the evaluation of PE in the ED. Additional studies would help to better understand the potential impact of such reports on CTPA use in the diagnostic workup of PE. However, it suggests that a combination of interventions, including the implementation of a CDS, performance feedback reporting, and well-designed and specific educational interventions, may have a more significant impact than any of these types of interventions taken separately.
The impact of the educational interventions appraised in this review on the expected results is mixed, though it is difficult to compare the observed results and draw conclusive remarks, as the characteristics of the interventions and study designs are different from each other.
Safety
There is limited evidence on the safety of appraised interventions. Only 6 studies appraised venous thrombolic events or mortality.13,18,19,23,25,27 However, no adverse events were noted in those studies evaluating possible complications or missed diagnoses. Additional research is needed to confirm the safety of the interventions appraised in this systematic review.
Facilitators and Barriers
There are significant limitations with respect to the analysis of the factors that favor or impede the implementation of the interventions appraised in this review. However, 2 studies that did not meet the inclusion criteria appraised physicians’ perceptions and attitudes toward prescribing imaging tests in the diagnostic workup of PE.31,32 One is Swiss31 and the other is Canadian.32 Both were conducted in the ED of academic hospitals. Rohacek et al.31 observed that defensive behaviors, such as “fear of missing PE,” were frequent and associated with a lower probability of a positive CTPA (OR = 0.36; 95% CI, 0.14-0.92). Ahn et al.32 concluded that, although ED physicians who participated in their survey possessed limited knowledge of radiation doses of CTPA and V/Q scans, they opted for V/Q scans that emit lower radiation doses in younger patients, especially females, which may reflect efforts done in the study setting to reduce patients’ radiation exposure.
There is not enough data to conclude on safety and the impact on healthcare costs.
Implications for Future Research
Future controlled studies of high methodological quality would help to better understand the effects associated with the implementation of the interventions aimed at reducing the inappropriate use of imaging in the diagnostic workup of PE. Efficacy results show that the success of the implementation of the various types of interventions is variable. This variation may be at least partly attributable to contextual factors, such as the external environment, the organizational leadership and culture, or the microsystem, such as differences in care patterns.33-35 The impact of context factors on the effectiveness of the interventions should be assessed further with appropriate tools.33,34,36
CONCLUSION
The joint use of CDS and PFRs appears more effective than the other types of intervention in reducing the inappropriate use of CTPA. However, an approach combining these with well-designed educational interventions as well as policies may be even more effective.
Future studies of high methodological quality would strengthen the evidence concerning the relative efficacy and safety of the interventions appraised, especially when various types are combined. Future research should also aim at bringing answers to the knowledge gaps related to the factors of success and barriers associated with the implementation of the interventions.
Disclosure
The authors report no conflict of interest.
1. Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. PubMed
2. Canadian Institute for Health Information (CIHI). Medical Imaging in Canada 2012. https://www.cihi.ca/en/mit_summary_2012_en.pdf. Accessed December 14, 2016.
3. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. doi:10.1136/bmj.f3368. PubMed
4. Schissler AJ, Rozenshtein A, Schluger NW, Einstein AJ. National trends in emergency room diagnosis of pulmonary embolism, 2001-2010: a cross-sectional study. Respir Res. 2015;16:44-50. PubMed
5. Minges KE, Bikdeli B, Wang Y, et al. National Trends in Pulmonary Embolism Hospitalization Rates and Outcomes for Adults Aged >/=65 Years in the United States (1999 to 2010). Am J Cardiol. 2015;116(9):1436-1442. PubMed
6. Duriseti RS, Brandeau ML. Cost-effectiveness of strategies for diagnosing pulmonary embolism among emergency department patients presenting with undifferentiated symptoms. Ann Emerg Med. 2010;56(4):321-332.e310. PubMed
7. Char S, Yoon HC. Improving appropriate use of pulmonary computed tomography angiography by increasing the serum D-dimer threshold and assessing clinical probability. Perm J. 2014;18(4):10-15. PubMed
8. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928 PubMed
9. Champagne F, Brousselle A, Contendriopoulos AP, Hartz Z. L’analyse des effets. In: Brousselle A, Champagne F, Contandriopoulos AP, Hartz Z, editors. L’évaluation: Concepts et Méthodes 2e Edition. Montréal: Les Presses de l’Université de Montréal; 2011: 173-198.
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. PubMed
11. Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Manchester, UK: ESRC Methods Programme; 2006.
12. Velasco M, Perleth M, Drummond M, et al. Best practice in undertaking and reporting health technology assessments. Working group 4 report. Int J Technol Assess Health Care. 2002;18(2):361-422. PubMed
13. Kline JA, Jones AE, Shapiro NI, et al. Multicenter, randomized trial of quantitative pretest probability to reduce unnecessary medical radiation exposure in emergency department patients with chest pain and dyspnea. Circ Cardiovasc Imaging. 2014;7(1):66-73. PubMed
14. Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of Performance Feedback Reports on Adherence to Evidence-Based Guidelines in Use of CT for Evaluation of Pulmonary Embolism in the Emergency Department: A Randomized Trial. AJR Am J Roentgenol. 2015;205(5):936-940. PubMed
15. Agarwal A, Persaud J, Grabinski R, Rabinowitz D, Bremner A, Mendelson R. Pulmonary embolism: are we there yet? J Med Imaging Radiat Oncol. 2012;56(3):270-281. PubMed
16. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57(6):613-621. PubMed
17. Geeting GK, Beck M, Bruno MA, et al. Mandatory Assignment of Modified Wells Score Before CT Angiography for Pulmonary Embolism Fails to Improve Utilization or Percentage of Positive Cases. AJR Am J Roentgenol. 2016;207(2):442-449. PubMed
18. Goergen SK, Chan T, de Campo JF, et al. Reducing the use of diagnostic imaging in patients with suspected pulmonary embolism: validation of a risk assessment strategy. Emerg Med Australas. 2005;17(1):16-23. PubMed
19. Jiménez D, Resano S, Otero R, et al. Computerised clinical decision support for suspected PE. Thorax. 2015;70(9):909-911. PubMed
20. Kanaan Y, Knoepp UD, Kelly AM. The influence of education on appropriateness rates for CT pulmonary angiography in emergency department patients. Acad Radiol. 2013;20(9):1107-1114. PubMed
21. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
22. Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262(2):468-474. PubMed
23. Kline JA, Webb WB, Jones AE, Hernandez-Nino J. Impact of a rapid rule-out protocol for pulmonary embolism on the rate of screening, missed cases, and pulmonary vascular imaging in an urban US emergency department. Ann Emerg Med. 2004;44(5):490-502. PubMed
24. Raja AS, Gupta A, Ip IK, Mills AM, Khorasani R. The use of decision support to measure documented adherence to a national imaging quality measure. Acad Radiol. 2014;21(3):378-383. PubMed
25. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am J Roentgenol. 2010;194(2):392-397. PubMed
26. Booker MT, Johnson JO. Optimizing CT Pulmonary Angiogram Utilization in a Community Emergency Department: A Pre- and Postintervention Study. J Am Coll Radiol. 2017;14(1):65-71. PubMed
27. Goldstein NM, Kollef MH, Ward S, Gage BF. The impact of the introduction of a rapid D-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. Arch Intern Med. 2001;161(4):567-571. PubMed
28. Dunne RM, Ip IK, Abbett S, et al. Effect of Evidence-based Clinical Decision Support on the Use and Yield of CT Pulmonary Angiographic Imaging in Hospitalized Patients. Radiology. 2015;276(1):167-174. PubMed
29. Wang RC, Bent S, Weber E, Neilson J, Smith-Bindman R, Fahimi J. The Impact of Clinical Decision Rules on Computed Tomography Use and Yield for Pulmonary Embolism: A Systematic Review and Meta-analysis. Ann Emerg Med. 2016;67(6):693-701. PubMed
30. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
31. Rohacek M, Buatsi J, Szucs-Farkas Z, et al. Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med. 2012;38(8):1345-1351. PubMed
32. Ahn JS, Edmonds ML, McLeod SL, Dreyer JF. Familiarity with radiation exposure dose from diagnostic imaging for acute pulmonary embolism and current patterns of practice. CJEM. 2014;16(5):393-404. PubMed
33. Kringos DS, Sunol R, Wagner C, et al. The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. BMC Health Serv Res. 2015;15(277):015-0906. PubMed
34. Kaplan HC, Brady PW, Dritz MC, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q. 2010;88(4):500-559. PubMed
35. Pernod G, Caterino J, Maignan M, Tissier C, Kassis J, Lazarchick J. D-dimer use and pulmonary embolism diagnosis in emergency units: Why is there such a difference in pulmonary embolism prevalence between the United States of America and countries outside USA? PLoS ONE. 2017;12(1):e0169268. doi:10.1371/journal.pone.0169268 PubMed
36. Saillour-Glenisson F, Domecq S, Kret M, Sibe M, Dumond JP, Michel P. Design and validation of a questionnaire to assess organizational culture in French hospital wards. BMC Health Serv Res. 2016;16:491-503. PubMed
37. Kline JA, Nelson RD, Jackson RE, Courtney DM. Criteria for the safe use of D-dimer testing in emergency department patients with suspected pulmonary embolism: a multicenter US study. Ann Emerg Med. 2002;39(2):144-152. PubMed
38. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. New Engl J Med. 2006;354(22):2317-2327. PubMed
39. Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055. PubMed
40. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276-2315. PubMed
1. Smith-Bindman R, Miglioretti DL, Johnson E, et al. Use of diagnostic imaging studies and associated radiation exposure for patients enrolled in large integrated health care systems, 1996-2010. JAMA. 2012;307(22):2400-2409. PubMed
2. Canadian Institute for Health Information (CIHI). Medical Imaging in Canada 2012. https://www.cihi.ca/en/mit_summary_2012_en.pdf. Accessed December 14, 2016.
3. Wiener RS, Schwartz LM, Woloshin S. When a test is too good: how CT pulmonary angiograms find pulmonary emboli that do not need to be found. BMJ. 2013;347:f3368. doi:10.1136/bmj.f3368. PubMed
4. Schissler AJ, Rozenshtein A, Schluger NW, Einstein AJ. National trends in emergency room diagnosis of pulmonary embolism, 2001-2010: a cross-sectional study. Respir Res. 2015;16:44-50. PubMed
5. Minges KE, Bikdeli B, Wang Y, et al. National Trends in Pulmonary Embolism Hospitalization Rates and Outcomes for Adults Aged >/=65 Years in the United States (1999 to 2010). Am J Cardiol. 2015;116(9):1436-1442. PubMed
6. Duriseti RS, Brandeau ML. Cost-effectiveness of strategies for diagnosing pulmonary embolism among emergency department patients presenting with undifferentiated symptoms. Ann Emerg Med. 2010;56(4):321-332.e310. PubMed
7. Char S, Yoon HC. Improving appropriate use of pulmonary computed tomography angiography by increasing the serum D-dimer threshold and assessing clinical probability. Perm J. 2014;18(4):10-15. PubMed
8. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928 PubMed
9. Champagne F, Brousselle A, Contendriopoulos AP, Hartz Z. L’analyse des effets. In: Brousselle A, Champagne F, Contandriopoulos AP, Hartz Z, editors. L’évaluation: Concepts et Méthodes 2e Edition. Montréal: Les Presses de l’Université de Montréal; 2011: 173-198.
10. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006-1012. PubMed
11. Popay J, Roberts H, Sowden A, et al. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. Manchester, UK: ESRC Methods Programme; 2006.
12. Velasco M, Perleth M, Drummond M, et al. Best practice in undertaking and reporting health technology assessments. Working group 4 report. Int J Technol Assess Health Care. 2002;18(2):361-422. PubMed
13. Kline JA, Jones AE, Shapiro NI, et al. Multicenter, randomized trial of quantitative pretest probability to reduce unnecessary medical radiation exposure in emergency department patients with chest pain and dyspnea. Circ Cardiovasc Imaging. 2014;7(1):66-73. PubMed
14. Raja AS, Ip IK, Dunne RM, Schuur JD, Mills AM, Khorasani R. Effects of Performance Feedback Reports on Adherence to Evidence-Based Guidelines in Use of CT for Evaluation of Pulmonary Embolism in the Emergency Department: A Randomized Trial. AJR Am J Roentgenol. 2015;205(5):936-940. PubMed
15. Agarwal A, Persaud J, Grabinski R, Rabinowitz D, Bremner A, Mendelson R. Pulmonary embolism: are we there yet? J Med Imaging Radiat Oncol. 2012;56(3):270-281. PubMed
16. Drescher FS, Chandrika S, Weir ID, et al. Effectiveness and acceptability of a computerized decision support system using modified Wells criteria for evaluation of suspected pulmonary embolism. Ann Emerg Med. 2011;57(6):613-621. PubMed
17. Geeting GK, Beck M, Bruno MA, et al. Mandatory Assignment of Modified Wells Score Before CT Angiography for Pulmonary Embolism Fails to Improve Utilization or Percentage of Positive Cases. AJR Am J Roentgenol. 2016;207(2):442-449. PubMed
18. Goergen SK, Chan T, de Campo JF, et al. Reducing the use of diagnostic imaging in patients with suspected pulmonary embolism: validation of a risk assessment strategy. Emerg Med Australas. 2005;17(1):16-23. PubMed
19. Jiménez D, Resano S, Otero R, et al. Computerised clinical decision support for suspected PE. Thorax. 2015;70(9):909-911. PubMed
20. Kanaan Y, Knoepp UD, Kelly AM. The influence of education on appropriateness rates for CT pulmonary angiography in emergency department patients. Acad Radiol. 2013;20(9):1107-1114. PubMed
21. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
22. Raja AS, Ip IK, Prevedello LM, et al. Effect of computerized clinical decision support on the use and yield of CT pulmonary angiography in the emergency department. Radiology. 2012;262(2):468-474. PubMed
23. Kline JA, Webb WB, Jones AE, Hernandez-Nino J. Impact of a rapid rule-out protocol for pulmonary embolism on the rate of screening, missed cases, and pulmonary vascular imaging in an urban US emergency department. Ann Emerg Med. 2004;44(5):490-502. PubMed
24. Raja AS, Gupta A, Ip IK, Mills AM, Khorasani R. The use of decision support to measure documented adherence to a national imaging quality measure. Acad Radiol. 2014;21(3):378-383. PubMed
25. Stein EG, Haramati LB, Chamarthy M, Sprayregen S, Davitt MM, Freeman LM. Success of a safe and simple algorithm to reduce use of CT pulmonary angiography in the emergency department. AJR Am J Roentgenol. 2010;194(2):392-397. PubMed
26. Booker MT, Johnson JO. Optimizing CT Pulmonary Angiogram Utilization in a Community Emergency Department: A Pre- and Postintervention Study. J Am Coll Radiol. 2017;14(1):65-71. PubMed
27. Goldstein NM, Kollef MH, Ward S, Gage BF. The impact of the introduction of a rapid D-dimer assay on the diagnostic evaluation of suspected pulmonary embolism. Arch Intern Med. 2001;161(4):567-571. PubMed
28. Dunne RM, Ip IK, Abbett S, et al. Effect of Evidence-based Clinical Decision Support on the Use and Yield of CT Pulmonary Angiographic Imaging in Hospitalized Patients. Radiology. 2015;276(1):167-174. PubMed
29. Wang RC, Bent S, Weber E, Neilson J, Smith-Bindman R, Fahimi J. The Impact of Clinical Decision Rules on Computed Tomography Use and Yield for Pulmonary Embolism: A Systematic Review and Meta-analysis. Ann Emerg Med. 2016;67(6):693-701. PubMed
30. Prevedello LM, Raja AS, Ip IK, Sodickson A, Khorasani R. Does clinical decision support reduce unwarranted variation in yield of CT pulmonary angiogram? Am J Med. 2013;126(11):975-981. PubMed
31. Rohacek M, Buatsi J, Szucs-Farkas Z, et al. Ordering CT pulmonary angiography to exclude pulmonary embolism: defense versus evidence in the emergency room. Intensive Care Med. 2012;38(8):1345-1351. PubMed
32. Ahn JS, Edmonds ML, McLeod SL, Dreyer JF. Familiarity with radiation exposure dose from diagnostic imaging for acute pulmonary embolism and current patterns of practice. CJEM. 2014;16(5):393-404. PubMed
33. Kringos DS, Sunol R, Wagner C, et al. The influence of context on the effectiveness of hospital quality improvement strategies: a review of systematic reviews. BMC Health Serv Res. 2015;15(277):015-0906. PubMed
34. Kaplan HC, Brady PW, Dritz MC, et al. The influence of context on quality improvement success in health care: a systematic review of the literature. Milbank Q. 2010;88(4):500-559. PubMed
35. Pernod G, Caterino J, Maignan M, Tissier C, Kassis J, Lazarchick J. D-dimer use and pulmonary embolism diagnosis in emergency units: Why is there such a difference in pulmonary embolism prevalence between the United States of America and countries outside USA? PLoS ONE. 2017;12(1):e0169268. doi:10.1371/journal.pone.0169268 PubMed
36. Saillour-Glenisson F, Domecq S, Kret M, Sibe M, Dumond JP, Michel P. Design and validation of a questionnaire to assess organizational culture in French hospital wards. BMC Health Serv Res. 2016;16:491-503. PubMed
37. Kline JA, Nelson RD, Jackson RE, Courtney DM. Criteria for the safe use of D-dimer testing in emergency department patients with suspected pulmonary embolism: a multicenter US study. Ann Emerg Med. 2002;39(2):144-152. PubMed
38. Stein PD, Fowler SE, Goodman LR, et al. Multidetector computed tomography for acute pulmonary embolism. New Engl J Med. 2006;354(22):2317-2327. PubMed
39. Stein PD, Woodard PK, Weg JG, et al. Diagnostic pathways in acute pulmonary embolism: recommendations of the PIOPED II investigators. Am J Med. 2006;119(12):1048-1055. PubMed
40. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29(18):2276-2315. PubMed
© 2018 Society of Hospital Medicine
Transitioning from General Pediatric to Adult-Oriented Inpatient Care: National Survey of US Children’s Hospitals
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
Over 90% of children with chronic diseases now survive into adulthood.1,2 Clinical advances overcoming diseases previously fatal in childhood create new challenges for health systems with limited capacity to manage young adults with complicated and unfamiliar childhood-onset conditions. Consequently, improving the transition from pediatric to adult-oriented care has become a national priority.
Although major pediatric-adult transition initiatives—such as the Six Core Elements Framework,3 a technical brief from the Agency for Healthcare Research and Quality,4 and joint statements from major medical societies5,6—outline key transition recommendations generally and for outpatients, they contain limited or no guidance specifically devoted to transitioning inpatient hospital care from pediatric to adult-oriented settings. Key unknowns include whether, when, and how to transition inpatient care from children’s to nonchildren’s hospitals and how this can be integrated into comprehensive youth-adult transition care.
Nevertheless, the number of discharges of 18- to 21-year-old patients with chronic conditions admitted to children’s hospitals is increasing at a faster rate than discharges of other age groups,7 suggesting both that the population is growing in size and that there are important barriers to transitioning these patients into nonchildren’s hospital settings. Spending on adult patients 18 years or older admitted to children’s hospitals has grown to $1 billion annually.8 Hospitalizations are a commonly proposed outcome measure of pediatric-adult transition work.1,9,10 For example, higher rates of avoidable hospitalizations during early adulthood have been observed for 15- to 22-year-olds with kidney failure cared for exclusively in adult-oriented facilities and during the years immediately after transfer to adult care.11
While research is beginning to describe outcomes of adult-aged patients with childhood-onset chronic conditions admitted to children’s hospitals,7,12,13 there has been no comprehensive description of efforts within children’s hospitals to transition such patients into adult-oriented inpatient settings. This information is necessary to outline institutional needs, delineate opportunities for improvement, and help clinicians strategically organize services for patients requiring this transition.
We sought to characterize the current state of the transition from pediatric- to adult-oriented inpatient care across general pediatric inpatient services at US children’s hospitals. We hypothesized that only a limited and inconsistent set of activities would be practiced. We also hypothesized that institutions having formal outpatient transition processes or providers with specialization to care for this age group, such as dual-trained internal medicine–pediatrics (med–peds) physicians, would report performing more activities.
METHODS
Study Design, Setting, Participants
We conducted a national survey of leaders of inpatient general pediatrics services at US children’s hospitals from January 2016 to July 2016. Hospitals were identified using the online Children’s Hospital Association directory. Hospitals without inpatient general pediatrics services (eg, rehabilitation or subspecialty-only facilities) were excluded.
We identified a single respondent from each of the 195 remaining children’s hospitals using a structured protocol. Phone numbers and e-mail addresses of potential respondents were gathered from hospital or medical school directories. Following a standard script, study team members contacted potential respondents to describe the purpose of the study and to confirm their contact information. Hospitals were also allowed to designate a different individual with more specific expertise to participate, when relevant (eg, specific faculty member leading a related quality improvement initiative). The goal was to identify a leader of inpatient care with the most knowledge of institutional practices related to the transition to adult inpatient care. Examples of respondent roles included director of inpatient pediatrics, chief of hospital medicine or general pediatrics, medical director, and similar titles.
Survey Elements
As part of a larger quality improvement initiative at our institution, a multidisciplinary team of pediatric and internal medicine healthcare providers (physicians, nurse practitioners, nurses, case managers, social workers, child life specialists), as well as parents and patients, developed an “ideal state” with this transition and a consensus-based conceptual framework of key patient and institutional determinants of a formal inpatient transition initiative for children with chronic conditions within a children’s hospital (Figure).
Institutional Context and Factors Influencing Inpatient Transitions
The following hospital characteristics were assessed: administrative structure (free-standing, hospital-within-hospital, or “free-leaning,” ie, separate physical structure but same administrative structure as a general hospital), urban versus rural, academic versus nonacademic, presence of an inpatient adolescent unit, presence of subspecialty admitting services, and providers with med–peds or family medicine training. The following provider group characteristics were assessed: number of full-time equivalents (FTEs), scope of practice (inpatient only, combination inpatient/outpatient), proportion of providers at a “senior” level (ie, at least 7 years posttraining or at an associate professor rank), estimated number of discharges per week, and proportion of patients cared for without resident physicians.
Inpatient Transition Initiative
Each institution was categorized as having or not having an inpatient transition initiative by whether they indicated having either (1) an institutional leader of the transition from pediatric to adult-oriented inpatient settings or (2) an inpatient transition process, for which “process” was defined as “a standard, organized, and predictable set of transition activities that may or may not be documented, but the steps are generally agreed upon.”
Specific Inpatient Transition Activities
Respondents indicated whether 22 activities occurred consistently, defined as at least 50% of the time. To facilitate description, activities were grouped into categories using the labels from the Six Core Elements framework3 (Table 1): Policy, Tracking and Monitoring, Readiness, Planning, Transfer of Care, and Transfer Completion. Respondents were also asked whether outpatient pediatric-adult transition activities existed at their institution and whether they were linked to inpatient transition activities.
Data Collection
After verifying contact information, respondents received an advanced priming phone call followed by a mailed request to participate with a printed uniform resource locator (URL) to the web survey. Two email reminders containing the URL were sent to nonresponders at 5 and 10 days after the initial mailing. Remaining nonresponders then received a reminder phone call, followed by a mailed paper copy of the survey questionnaire to be completed by hand approximately 2 weeks after the last emailed request. The survey was administered using the Qualtrics web survey platform (www.qualtrics.com). Data collection occurred between January 2016 and July 2016. Participants received a $20 incentive.
Statistical Analysis
Descriptive statistics summarized the current state of inpatient transition at general pediatrics services across US children’s hospitals. Exploratory factor analysis assessed whether individual activities were sufficiently correlated to allow grouping items and constructing scales. Differences in institutional or respondent characteristics between hospitals that did and did not report having an inpatient initiative were compared using t tests for continuous data. Fisher’s exact test was used for categorical data because some cell sizes were ≤5. Bivariate logistic regression quantified associations between presence versus absence of specific transition activities and presence versus absence of an inpatient transition initiative. Analyses were completed in STATA (SE version 14.0; StataCorp, College Station, Texas). The institutional review board at our institution approved this study.
RESULTS
Responses were received from 96 of 195 children’s hospitals (49.2% response rate). Responding institution characteristics are summarized in Table 2. Free-standing children’s hospitals made up just over one-third of the sample (36%), while the remaining were free-leaning (22%) or hospital-within-hospital (43%). Most children’s hospitals (58%) did not have a specific adult-oriented hospital identified to receive transitioning patients. Slightly more than 10% had an inpatient adolescent unit. The majority of institutions were academic medical centers (78%) in urban locations (88%). Respondents represented small (<5 FTE, 21%), medium (6-10 FTE, 36%), and large provider groups (11+ FTE, 44%). Although 70% of respondents described their groups as “hospitalist only,” meaning providers only practiced inpatient general pediatrics, nearly 30% had providers practicing inpatient and outpatient general pediatrics. Just over 40% of respondents reported having med–peds providers. Pediatric-adult transition processes for outpatient care were present at 45% of institutions.
Transition Activities
Thirty-eight percent of children’s hospitals had an inpatient transition initiative using our study definition—31% by having a set of generally agreed upon activities, 19% by having a leader, and 11% having both. Inpatient transition leaders included pediatric hospitalists (43%), pediatric subspecialists and primary care providers (14% each), med–peds providers (11%), or case managers (7%). Respondent and institutional characteristics were similar at institutions that did and did not have an inpatient transition initiative (Table 2); however, children’s hospitals with inpatient transition initiatives more often had med–peds providers (P = .04). Institutions with pediatric-adult outpatient care transition processes more often had an inpatient initiative (71% and 29%, respectively; P = .001).
Exploratory factor analysis identified 2 groups of well-correlated items, which we grouped into “preparation” and “transfer initiation” scales (supplementary Appendix). The preparation scale was composed of the following 5 items (Cronbach α = 0.84): proactive identification of patients anticipated to need transition, proactive identification of patients overdue for transition, readiness formally assessed, timing discussed with family, and patient and/or family informed that the next stay would be at the adult facility. The transfer initiation scale comprised the following 6 items (Cronbach α = 0.72): transition education provided to families, primary care–subspecialist agreement on timing, subspecialist–subspecialist agreement on timing, patient decision-making ability established, adult facility tour, and standardized handoff communication between healthcare providers. While these items were analyzed only in this scale, other activities were analyzed as independent variables. In this analysis, 40.9% of institutions had a preparation scale score of 0 (no items performed), while 13% had all 5 items performed. Transfer initiation scale scores ranged from 0 (47%) to 6 (2%).
Specific activities varied widely across institutions, and none of the activities occurred at a majority of children’s hospitals (Table 3). Only 11% of children’s hospital transition policies referenced transitions of inpatient care. The activity most commonly reported across children’s hospitals was addressing potential insurance problems (41%). The least common inpatient transition activities were having child life consult during the first adult hospital stay (6%) or having a system to track and monitor youth in the inpatient transition process (2%). Transition processes and policies were relatively new among institutions that had them—average years an inpatient transition process had been in place was 1.2 (SD 0.4), and average years with a transition policy, including inpatient care, was 1.3 (SD 0.4).
Transition Activities at Hospitals With and Without an Inpatient Transition Initiative
Most activities assessed in this study (both scales plus 5 of 11 individual activities) were significantly more common in children’s hospitals with an inpatient transition initiative (Table 3). The most common activity was addressing potential insurance problems (46%), and the least common activity was having a system to track and monitor youth in the inpatient transition process (3%). The majority of institutions without an inpatient transition initiative (53%) performed 0 transfer initiation scale items. Large effect sizes between hospitals with and without a transition initiative were observed for use of a checklist to complete tasks (odds ratio [OR] 9.6, P = .04) and creation of a transition care plan (OR 9.0, P = .008). Of the 6 activities performed at similarly low frequencies at institutions with and without an initiative, half involved transition planning, the essential step after readiness but before actual transfer of care.
DISCUSSION
We conducted the first national survey describing the policies and procedures of the transition of general inpatient care from children’s to adult-oriented hospitals for youth and young adults with chronic conditions. Our main findings demonstrate that a relatively small number of general inpatient services at children’s hospitals have leaders or dedicated processes to shepherd this transition, and a minority have a specific adult hospital identified to receive their patients. Even among institutions with inpatient transition initiatives, there is wide variability in the performance of activities to facilitate transitioning out of US children’s hospitals. In these institutions, performance seems to be more lacking in later links of the transition chain. Results from this work can serve as a baseline and identify organizational needs and opportunities for future work.
Children’s hospital general services with and without an inpatient pediatric-adult transition initiative had largely similar characteristics; however, the limited sample size may lack power to detect some differences. Perhaps not surprisingly, having med–peds providers and outpatient transition processes were the characteristics most associated with having an inpatient pediatric-adult transition initiative. The observation that over 70% of hospitals with an outpatient process had an inpatient transition leader or dedicated process makes us optimistic that as general transition efforts expand, more robust inpatient transition activities may be achievable.
We appreciate that the most appropriate location to care for hospitalized young adults with childhood-onset chronic conditions is neither known nor answered with this study. Both options face challenges—adult-oriented hospitals may not be equipped to care for adult manifestations of childhood-onset conditions,14,15 while children’s hospitals may lack the resources and expertise to provide comprehensive care to adults.7 Although hospital charges and lengths of stay may be greater when adults with childhood-onset chronic conditions are admitted to children’s compared with adult hospitals,12,13,16 important confounders such as severity of illness could explain why adult-aged patients may both remain in children’s hospitals at older ages and simultaneously have worse outcomes than peers. Regardless, at some point, transitioning care into an adult-oriented hospital may be in patients’ best interests. If so, families and providers need guidance on (1) the important aspects of this transition and (2) how to effectively implement the transition.
Because the most important inpatient transition care activities are not empirically known, we designed our survey to assess a broad set of desirable activities emerging from our multidisciplinary quality improvement work. We mapped these activities to the categories used by the Six Core Elements framework.3 Addressing insurance issues was one of the most commonly reported activities, although still fewer than 50% of hospitals reported addressing these problems. It was notable that the majority of institutions without a transition initiative performed none of the transfer initiation scale items. In addition, 2 features of transition efforts highlighted by advocates nationally—use of a checklist and creation of a transition care plan— were 9 times more likely when sites had transition initiatives. Such findings may be motivating for institutions that are considering establishing a transition initiative. Overall, we were not surprised with hospitals’ relatively low performance across most transition activities because only about 40% of US families of children with special healthcare needs report receiving the general services they need to transition to adult healthcare.17
We suspect that a number of the studied inpatient transition activities may be uncommon for structural reasons. For example, having child life consultation during an initial adult stay was rare. In fact, we observed post hoc that it occurred only in hospital-within-hospital systems, an expected finding because adult-only facilities are unlikely to have child life personnel. Other barriers, however, are less obviously structural. Almost no respondents indicated providing a tour of an adult facility, which was true whether the children’s hospital was free-standing or hospital-within-hospital. Given that hospitals with med–peds providers more often had inpatient transition initiatives, it would be interesting to examine whether institutions with med–peds training programs are able to overcome more of these barriers because of the bridges inherently created between departments even when at physically separated sites.
Having a system to track and/or monitor youth going through the transition process was also uncommon. This presumably valuable activity is one of the Six Core Elements3 and is reminiscent of population management strategies increasingly common in primary care.18 Pediatric hospitalists might benefit from adopting a similar philosophy for certain patient populations. Determining whether this activity would be most appropriately managed by inpatient providers versus being integrated into a comprehensive tracking and/or monitoring strategy (ie, inpatient care plus primary care, subspecialty care, school, employment, insurance, etc.) is worth continued consideration.
Although the activities we studied spanned many important dimensions, the most important transition activities in any given context may differ based on institutional resources and those of nearby adult healthcare providers.16 For example, an activity may be absent at a children’s hospital because it is already readily handled in primary care within that health system. Understanding how local resources and patient needs influence the relationship between transition activities and outcomes is an important next step in this line of work. Such research could inform how institutions adapt effective transition activities (eg, developing care plans) to most efficiently meet the needs of their patients and families.
Our findings align with and advance the limited work published on this aspect of transition. A systematic literature review of general healthcare transition interventions found that meeting adult providers prior to transitioning out of the pediatric system was associated with less concern about admission to the adult hospital floor.9 Formally recognizing inpatient care as a part of a comprehensive approach to transition may help adults with childhood-onset chronic conditions progress into adult-oriented hospitals. Inpatient and outpatient providers can educate one another on critical aspects of transition that span across settings. The Cystic Fibrosis (CF) Foundation has established a set of processes to facilitate the transition to adult care and specifically articulates the transfer to adult inpatient settings.19,20 Perhaps as a result, CF is also one of few conditions with fewer adult patients being admitted to children’s hospitals7 despite the increasing number of adults living with the condition.19 Adapting the CF Foundation approach to other chronic conditions may be an effective approach.
Our study has important limitations. Most pertinently, the list of transition activities was developed at a single institution. Although drawing on accepted national guidelines and a diverse local quality improvement group, our listed activities could not be exhaustive. Care plan development and posttransition follow-up activities may benefit from ongoing development in subsequent work. Continuing to identify and integrate approaches taken at other children’s hospitals will also be informative. For example, some children’s hospitals have introduced adult medicine consultative services to focus on transition, attending children’s hospital safety rounds, and sharing standard care protocols for adult patients still cared for in pediatric settings (eg, stroke and myocardial infarction).16
In addition, our findings are limited to generalist teams at children’s hospitals and may not be applicable to inpatient subspecialty services. We could not compare differences in respondents versus nonrespondents to determine whether important selection bias exists. Respondent answers could not be verified. Despite our attempt to identify the most informed respondent at each hospital, responses may have differed with other hospital respondents. We used a novel instrument with unknown psychometric properties. Our data provide only the children’s hospital perspective, and perspectives of others (eg, families, primary care pediatricians or internists, subspecialists, etc.) will be valuable to explore in subsequent research. Subsequent research should investigate the relative importance and feasibility of specific inpatient transition activities, ideal timing, as well as the expected outcomes of high-quality inpatient transition. An important question for future work is to identify which patients are most likely to benefit by having inpatient care as part of their transition plan.
CONCLUSIONS
Nevertheless, the clinical and health services implications of this facet of transition appear to be substantial.16 To meet the Maternal and Child Health Bureau (MCHB) core outcome for children with special healthcare needs to receive “the services necessary to make transitions to adult healthcare,”21 development, validation, and implementation of effective inpatient-specific transition activities and a set of measurable processes and outcomes are needed. A key direction for the healthcare transitions field, with respect to inpatient care, is to determine the activities most effective at improving relevant patient and family outcomes. Ultimately, we advocate that the transition of inpatient care be integrated into comprehensive approaches to transitional care.
Disclosure: The project described was supported in part by the Clinical and Translational Science Award (CTSA) program, through the National Institutes of Health (NIH) National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project was also supported by the University of Wisconsin Departments of Pediatrics and Medicine. The authors have no financial or other relationships relevant to this article to disclose.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
1. Vaks Y, Bensen R, Steidtmann D, et al. Better health, less spending: Redesigning the transition from pediatric to adult healthcare for youth with chronic illness. Healthc (Amst). 2016;4(1):57-68.
2. Bensen R, Steidtmann D, Vaks Y. A Triple Aim Approach to Transition from Pediatric to Adult Health Care for Youth with Special Health Care Needs. Palo Alto, CA: Lucile Packard Foundation for Children’s Health; 2014.
3. Got Transition. Center for Health Care Transition Improvement 2016; http://www.gottransition.org/. Accessed April 4, 2016.
4. McPheeters M, Davis AM, Taylor JL, Brown RF, Potter SA, Epstein RA. Transition Care for Children with Special Health Needs. Technical Brief No. 15. Rockville, MD: Agency for Healthcare Research and Quality; 2014.
5. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians, Transitions Clinical Report Authoring Group, Cooley WC, Sagerman PJ. Supporting the health care transition from adolescence to adulthood in the medical home. Pediatrics. 2011;128(1):182-200.
6. American Academy of Pediatrics, American Academy of Family Physicians, American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics. 2002;110(6 Pt 2):1304-1306.
7. Goodman DM, Hall M, Levin A, et al. Adults with chronic health conditions originating in childhood: inpatient experience in children’s hospitals. Pediatrics. 2011;128(1):5-13.
8. Goodman DM, Mendez E, Throop C, Ogata ES. Adult survivors of pediatric illness: the impact on pediatric hospitals. Pediatrics. 2002;110(3):583-589.
9. Bloom SR, Kuhlthau K, Van Cleave J, Knapp AA, Newacheck P, Perrin JM. Health care transition for youth with special health care needs. J Adolesc Health. 2012;51(3):213-219.
10. Fair C, Cuttance J, Sharma N, et al. International and Interdisciplinary Identification of Health Care Transition Outcomes. JAMA Pediatr. 2016;170(3):205-211.
11. Samuel SM, Nettel-Aguirre A, Soo A, Hemmelgarn B, Tonelli M, Foster B. Avoidable hospitalizations in youth with kidney failure after transfer to or with only adult care. Pediatrics. 2014;133(4):e993-e1000.
12. Okumura MJ, Campbell AD, Nasr SZ, Davis MM. Inpatient health care use among adult survivors of chronic childhood illnesses in the United States. Arch Pediatr Adolesc Med. 2006;160(10):1054-1060.
13. Edwards JD, Houtrow AJ, Vasilevskis EE, Dudley RA, Okumura MJ. Multi-institutional profile of adults admitted to pediatric intensive care units. JAMA Pediatr. 2013;167(5):436-443.
14. Peter NG, Forke CM, Ginsburg KR, Schwarz DF. Transition from pediatric to adult care: internists’ perspectives. Pediatrics. 2009;123(2):417-423.
15. Okumura MJ, Heisler M, Davis MM, Cabana MD, Demonner S, Kerr EA. Comfort of general internists and general pediatricians in providing care for young adults with chronic illnesses of childhood. J Gen Intern Med. 2008;23(10):1621-1627.
16. Kinnear B, O’Toole JK. Care of Adults in Children’s Hospitals: Acknowledging the Aging Elephant in the Room. JAMA Pediatr. 2015;169(12):1081-1082.
17. McManus MA, Pollack LR, Cooley WC, et al. Current status of transition preparation among youth with special needs in the United States. Pediatrics. 2013;131(6):1090-1097.
18. Kelleher KJ, Cooper J, Deans K, et al. Cost saving and quality of care in a pediatric accountable care organization. Pediatrics. 2015;135(3):e582-e589.
19. Tuchman LK, Schwartz LA, Sawicki GS, Britto MT. Cystic fibrosis and transition to adult medical care. Pediatrics. 2010;125(3):566-573.
20. Yankaskas JR, Marshall BC, Sufian B, Simon RH, Rodman D. Cystic fibrosis adult care: consensus conference report. Chest. 2004;125(1 Suppl):1S-39S.
21. CSHCN Core System Outcomes: Goals for a System of Care. The National Survey of Children with Special Health Care Needs Chartbook 2009-2010. http://mchb.hrsa.gov/cshcn0910/core/co.html Accessed November 30, 2016.
© 2018 Society of Hospital Medicine