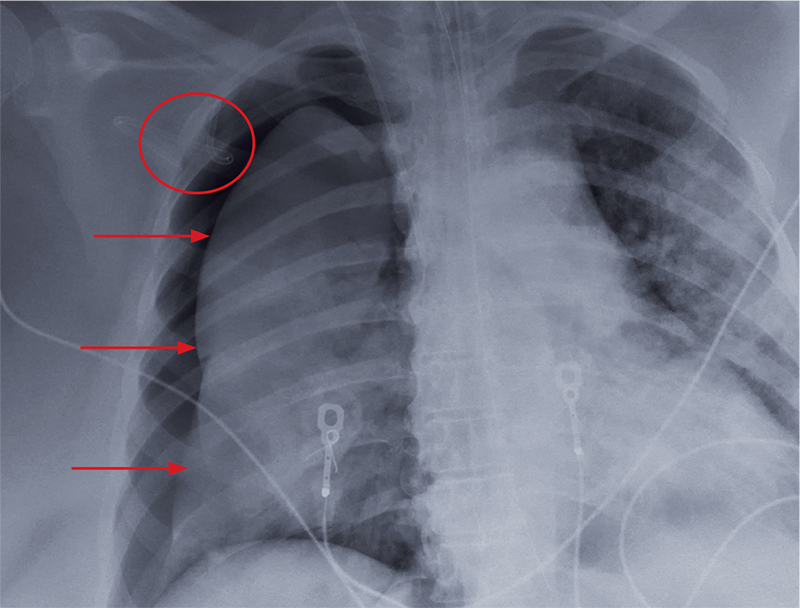
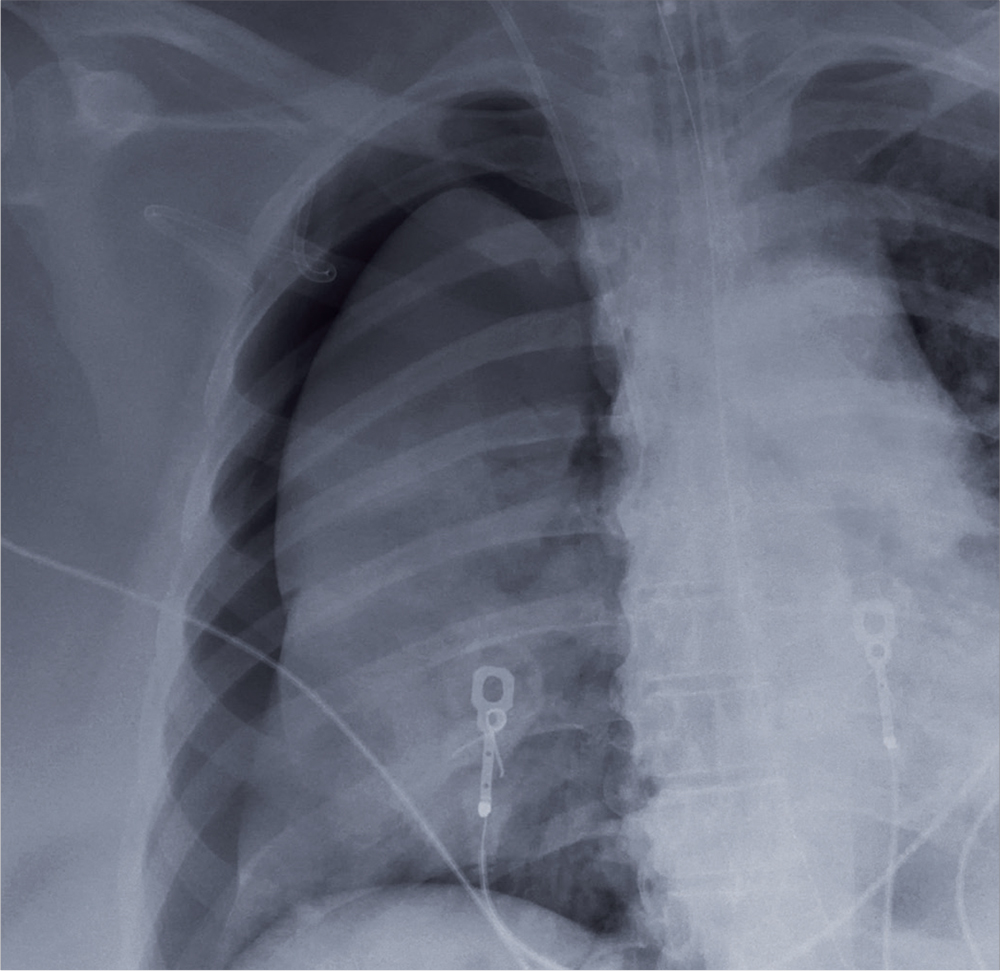
User login
Gene Therapy May Benefit Patients With Cerebral ALD
KANSAS CITY, MO—Lentiviral gene therapy halts inflammation and demyelination in patients with cerebral adrenoleukodystrophy (ALD), according to research presented at the 46th Annual Meeting of the Child Neurology Society and published in the New England Journal of Medicine. The treatment appears to stabilize neurologic function and to have an acceptable safety profile.
ALD is an X-linked genetic disease caused by a defect in ABCD1, which encodes the peroxisomal ABC half-transporter ALD protein. The defect results in abnormal breakdown of very-long-chain fatty acids, which build up and affect tissues in the nervous system. The most common phenotype of ALD is adrenomyeloneuropathy, which involves axonal degeneration in the spinal cord. A more severe phenotype is childhood cerebral adenopathy, which manifests as an inflammatory demyelination in the white matter.
Allogeneic bone marrow transplantation is the only other treatment with proven efficacy in childhood cerebral ALD. It halts disease progression and improves survival in presymptomatic patients. Finding a donor with identical human leukocyte antigen often is difficult, however. In addition, the treatment entails risks of graft failure and graft-versus-host disease, and the rate of treatment-related mortality is greater than 10%. Gene therapy “may be an alternative to allogeneic bone marrow transplantation, particularly for patients who do not have a matched sibling donor who are at risk for graft failure and graft-versus-host [disease],” said Florian Eichler, MD, Director of the Leukodystrophy Service at Massachusetts General Hospital for Children in Boston.
Researchers Transduced Stem Cells Ex Vivo
In the first trial of its kind, Dr. Eichler and colleagues investigated the safety and efficacy of gene therapy with autologous hematopoietic stem cells for cerebral ALD. The enrollment criteria for the phase II–III, single-arm, open-label study were identical to those for conventional bone marrow transplantation, said Dr. Eichler. Eligible participants were age 17 or younger, had a defect in ABCD1, and had evidence of an active, inflammatory lesion on brain MRI. Children with sibling donors for bone marrow transplantation were excluded from the study.
The investigators obtained CD34+ cells from participants through apheresis and transduced them ex vivo with a lentiviral vector containing normal ABCD1. After undergoing conditioning with busulfan and cyclophosphamide, the patients received an infusion of the transduced CD34+ cells. Patients were assessed regularly for graft-versus-host disease, death, and major functional disabilities. Dr. Eichler and colleagues also monitored participants for changes in neurologic function and increases in MRI lesions. At the end of a two-year follow-up period, patients were offered enrollment in a 13-year long-term follow-up study. The main study’s primary end point was being alive and having no major functional disability at 24 months.
Lesion Progression Stabilized in Most Patients
Dr. Eichler and colleagues treated 17 patients with a mean age at enrollment of 6. Participants’ median Loes score at baseline was 2.0, which indicated a low level of neurologic progression. All patients had an ALD neurologic function scale score of 0 at baseline, indicating an absence of clinical signs of cerebral disease.
The vector copy number in peripheral blood ranged from 0.10 to 1.55 in the 14 patients assessed at month 24. At month 36, the number ranged from 0.36 to 1.83 in the three patients assessed. The number had generally stabilized by two months after infusion. The investigators did not observe preferential integration in or near genes that have previously been associated with serious adverse events related to gene therapy (eg, MDS1, EVI1, and LMO2). All participants had expression of ALD protein in peripheral-blood leukocytes at the most recent follow-up (median follow-up time was 29.4 months). The median percentage of CD14+ cells that expressed ALD protein was 19% at 24 months.
Of the 17 patients, 15 were alive at 24 months and maintained an ALD neurologic function scale score of 0 or 1. In untreated patients, ALD neurologic function scale score usually increases significantly after the first symptoms appear. One patient in the study was progressing rapidly at the time of enrollment and subsequently died of disease progression. Another patient with disease progression on MRI withdrew from the study after the infusion and subsequently died from the complications of an allogeneic transplantation
An exploratory analysis indicated that participants with higher vector copy number in their peripheral blood tended to have better neurologic outcomes, while participants with lower vector copy number tended to have poor neurologic outcomes.
Lesion progression, as measured with the Loes score, had stabilized in 12 of the 17 patients (71%) at the time of the interim analysis. “Half the patients stabilized immediately in their Loes scores after treatment. The other half tended to progress within the first 12 months and then stabilize over time,” said Dr. Eichler.
Gadolinium enhancement resolved in 16 of 17 patients by six months. It reappeared in some patients after treatment, although it was much fainter and more poorly circumscribed, compared with the time of screening, said Dr. Eichler.
The investigators did not observe graft failure or graft-versus-host disease in the population. Most adverse events associated with the treatment were consistent with those associated with myeloablative chemotherapy and occurred during conditioning or during the first two weeks after the infusion. One serious adverse event that possibly was related to treatment was hemorrhagic cystitis associated with BK virus, which resolved with conservative measures.
The results have implications for leukodystrophies in general, according to Dr. Eichler. “Specific phenotypes, even within the same individual leukodystrophy, require different approaches” such as ex vivo or in vivo gene therapy, he added. Overall, the safety data are reassuring, and gene therapy appears to have encouraging efficacy in ALD, he concluded.
—Erik Greb
Suggested Reading
Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630-1638.
Engelen M. Optimizing treatment for cerebral adrenoleukodystrophy in the era of gene therapy. N Engl J Med. 2017;377(17):1682-1684.
KANSAS CITY, MO—Lentiviral gene therapy halts inflammation and demyelination in patients with cerebral adrenoleukodystrophy (ALD), according to research presented at the 46th Annual Meeting of the Child Neurology Society and published in the New England Journal of Medicine. The treatment appears to stabilize neurologic function and to have an acceptable safety profile.
ALD is an X-linked genetic disease caused by a defect in ABCD1, which encodes the peroxisomal ABC half-transporter ALD protein. The defect results in abnormal breakdown of very-long-chain fatty acids, which build up and affect tissues in the nervous system. The most common phenotype of ALD is adrenomyeloneuropathy, which involves axonal degeneration in the spinal cord. A more severe phenotype is childhood cerebral adenopathy, which manifests as an inflammatory demyelination in the white matter.
Allogeneic bone marrow transplantation is the only other treatment with proven efficacy in childhood cerebral ALD. It halts disease progression and improves survival in presymptomatic patients. Finding a donor with identical human leukocyte antigen often is difficult, however. In addition, the treatment entails risks of graft failure and graft-versus-host disease, and the rate of treatment-related mortality is greater than 10%. Gene therapy “may be an alternative to allogeneic bone marrow transplantation, particularly for patients who do not have a matched sibling donor who are at risk for graft failure and graft-versus-host [disease],” said Florian Eichler, MD, Director of the Leukodystrophy Service at Massachusetts General Hospital for Children in Boston.
Researchers Transduced Stem Cells Ex Vivo
In the first trial of its kind, Dr. Eichler and colleagues investigated the safety and efficacy of gene therapy with autologous hematopoietic stem cells for cerebral ALD. The enrollment criteria for the phase II–III, single-arm, open-label study were identical to those for conventional bone marrow transplantation, said Dr. Eichler. Eligible participants were age 17 or younger, had a defect in ABCD1, and had evidence of an active, inflammatory lesion on brain MRI. Children with sibling donors for bone marrow transplantation were excluded from the study.
The investigators obtained CD34+ cells from participants through apheresis and transduced them ex vivo with a lentiviral vector containing normal ABCD1. After undergoing conditioning with busulfan and cyclophosphamide, the patients received an infusion of the transduced CD34+ cells. Patients were assessed regularly for graft-versus-host disease, death, and major functional disabilities. Dr. Eichler and colleagues also monitored participants for changes in neurologic function and increases in MRI lesions. At the end of a two-year follow-up period, patients were offered enrollment in a 13-year long-term follow-up study. The main study’s primary end point was being alive and having no major functional disability at 24 months.
Lesion Progression Stabilized in Most Patients
Dr. Eichler and colleagues treated 17 patients with a mean age at enrollment of 6. Participants’ median Loes score at baseline was 2.0, which indicated a low level of neurologic progression. All patients had an ALD neurologic function scale score of 0 at baseline, indicating an absence of clinical signs of cerebral disease.
The vector copy number in peripheral blood ranged from 0.10 to 1.55 in the 14 patients assessed at month 24. At month 36, the number ranged from 0.36 to 1.83 in the three patients assessed. The number had generally stabilized by two months after infusion. The investigators did not observe preferential integration in or near genes that have previously been associated with serious adverse events related to gene therapy (eg, MDS1, EVI1, and LMO2). All participants had expression of ALD protein in peripheral-blood leukocytes at the most recent follow-up (median follow-up time was 29.4 months). The median percentage of CD14+ cells that expressed ALD protein was 19% at 24 months.
Of the 17 patients, 15 were alive at 24 months and maintained an ALD neurologic function scale score of 0 or 1. In untreated patients, ALD neurologic function scale score usually increases significantly after the first symptoms appear. One patient in the study was progressing rapidly at the time of enrollment and subsequently died of disease progression. Another patient with disease progression on MRI withdrew from the study after the infusion and subsequently died from the complications of an allogeneic transplantation
An exploratory analysis indicated that participants with higher vector copy number in their peripheral blood tended to have better neurologic outcomes, while participants with lower vector copy number tended to have poor neurologic outcomes.
Lesion progression, as measured with the Loes score, had stabilized in 12 of the 17 patients (71%) at the time of the interim analysis. “Half the patients stabilized immediately in their Loes scores after treatment. The other half tended to progress within the first 12 months and then stabilize over time,” said Dr. Eichler.
Gadolinium enhancement resolved in 16 of 17 patients by six months. It reappeared in some patients after treatment, although it was much fainter and more poorly circumscribed, compared with the time of screening, said Dr. Eichler.
The investigators did not observe graft failure or graft-versus-host disease in the population. Most adverse events associated with the treatment were consistent with those associated with myeloablative chemotherapy and occurred during conditioning or during the first two weeks after the infusion. One serious adverse event that possibly was related to treatment was hemorrhagic cystitis associated with BK virus, which resolved with conservative measures.
The results have implications for leukodystrophies in general, according to Dr. Eichler. “Specific phenotypes, even within the same individual leukodystrophy, require different approaches” such as ex vivo or in vivo gene therapy, he added. Overall, the safety data are reassuring, and gene therapy appears to have encouraging efficacy in ALD, he concluded.
—Erik Greb
Suggested Reading
Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630-1638.
Engelen M. Optimizing treatment for cerebral adrenoleukodystrophy in the era of gene therapy. N Engl J Med. 2017;377(17):1682-1684.
KANSAS CITY, MO—Lentiviral gene therapy halts inflammation and demyelination in patients with cerebral adrenoleukodystrophy (ALD), according to research presented at the 46th Annual Meeting of the Child Neurology Society and published in the New England Journal of Medicine. The treatment appears to stabilize neurologic function and to have an acceptable safety profile.
ALD is an X-linked genetic disease caused by a defect in ABCD1, which encodes the peroxisomal ABC half-transporter ALD protein. The defect results in abnormal breakdown of very-long-chain fatty acids, which build up and affect tissues in the nervous system. The most common phenotype of ALD is adrenomyeloneuropathy, which involves axonal degeneration in the spinal cord. A more severe phenotype is childhood cerebral adenopathy, which manifests as an inflammatory demyelination in the white matter.
Allogeneic bone marrow transplantation is the only other treatment with proven efficacy in childhood cerebral ALD. It halts disease progression and improves survival in presymptomatic patients. Finding a donor with identical human leukocyte antigen often is difficult, however. In addition, the treatment entails risks of graft failure and graft-versus-host disease, and the rate of treatment-related mortality is greater than 10%. Gene therapy “may be an alternative to allogeneic bone marrow transplantation, particularly for patients who do not have a matched sibling donor who are at risk for graft failure and graft-versus-host [disease],” said Florian Eichler, MD, Director of the Leukodystrophy Service at Massachusetts General Hospital for Children in Boston.
Researchers Transduced Stem Cells Ex Vivo
In the first trial of its kind, Dr. Eichler and colleagues investigated the safety and efficacy of gene therapy with autologous hematopoietic stem cells for cerebral ALD. The enrollment criteria for the phase II–III, single-arm, open-label study were identical to those for conventional bone marrow transplantation, said Dr. Eichler. Eligible participants were age 17 or younger, had a defect in ABCD1, and had evidence of an active, inflammatory lesion on brain MRI. Children with sibling donors for bone marrow transplantation were excluded from the study.
The investigators obtained CD34+ cells from participants through apheresis and transduced them ex vivo with a lentiviral vector containing normal ABCD1. After undergoing conditioning with busulfan and cyclophosphamide, the patients received an infusion of the transduced CD34+ cells. Patients were assessed regularly for graft-versus-host disease, death, and major functional disabilities. Dr. Eichler and colleagues also monitored participants for changes in neurologic function and increases in MRI lesions. At the end of a two-year follow-up period, patients were offered enrollment in a 13-year long-term follow-up study. The main study’s primary end point was being alive and having no major functional disability at 24 months.
Lesion Progression Stabilized in Most Patients
Dr. Eichler and colleagues treated 17 patients with a mean age at enrollment of 6. Participants’ median Loes score at baseline was 2.0, which indicated a low level of neurologic progression. All patients had an ALD neurologic function scale score of 0 at baseline, indicating an absence of clinical signs of cerebral disease.
The vector copy number in peripheral blood ranged from 0.10 to 1.55 in the 14 patients assessed at month 24. At month 36, the number ranged from 0.36 to 1.83 in the three patients assessed. The number had generally stabilized by two months after infusion. The investigators did not observe preferential integration in or near genes that have previously been associated with serious adverse events related to gene therapy (eg, MDS1, EVI1, and LMO2). All participants had expression of ALD protein in peripheral-blood leukocytes at the most recent follow-up (median follow-up time was 29.4 months). The median percentage of CD14+ cells that expressed ALD protein was 19% at 24 months.
Of the 17 patients, 15 were alive at 24 months and maintained an ALD neurologic function scale score of 0 or 1. In untreated patients, ALD neurologic function scale score usually increases significantly after the first symptoms appear. One patient in the study was progressing rapidly at the time of enrollment and subsequently died of disease progression. Another patient with disease progression on MRI withdrew from the study after the infusion and subsequently died from the complications of an allogeneic transplantation
An exploratory analysis indicated that participants with higher vector copy number in their peripheral blood tended to have better neurologic outcomes, while participants with lower vector copy number tended to have poor neurologic outcomes.
Lesion progression, as measured with the Loes score, had stabilized in 12 of the 17 patients (71%) at the time of the interim analysis. “Half the patients stabilized immediately in their Loes scores after treatment. The other half tended to progress within the first 12 months and then stabilize over time,” said Dr. Eichler.
Gadolinium enhancement resolved in 16 of 17 patients by six months. It reappeared in some patients after treatment, although it was much fainter and more poorly circumscribed, compared with the time of screening, said Dr. Eichler.
The investigators did not observe graft failure or graft-versus-host disease in the population. Most adverse events associated with the treatment were consistent with those associated with myeloablative chemotherapy and occurred during conditioning or during the first two weeks after the infusion. One serious adverse event that possibly was related to treatment was hemorrhagic cystitis associated with BK virus, which resolved with conservative measures.
The results have implications for leukodystrophies in general, according to Dr. Eichler. “Specific phenotypes, even within the same individual leukodystrophy, require different approaches” such as ex vivo or in vivo gene therapy, he added. Overall, the safety data are reassuring, and gene therapy appears to have encouraging efficacy in ALD, he concluded.
—Erik Greb
Suggested Reading
Eichler F, Duncan C, Musolino PL, et al. Hematopoietic stem-cell gene therapy for cerebral adrenoleukodystrophy. N Engl J Med. 2017;377(17):1630-1638.
Engelen M. Optimizing treatment for cerebral adrenoleukodystrophy in the era of gene therapy. N Engl J Med. 2017;377(17):1682-1684.
CLL drug combinations induce MRD negativity
Atlanta – The potential of two- and three-drug combinations to induce deep, minimal residual disease (MRD)–negative responses in patients with chronic lymphocytic leukemia (CLL) was demonstrated in multiple studies presented at the annual meeting of the American Society of Hematology.
Previous studies have suggested that, in contrast to single-agent kinase inhibitor treatment, combinations of treatments, namely kinase or B-cell lyphoma 2 inhibitors combined with anti-CD20 antibodies, can induce MRD negativity at a high rate. For example, in a report on the CLL14 trial, 11 of 12 previously untreated patients were MRD negative after treatment with the BCL-2 inhibitor venetoclax and anti-CD20 antibody obinutuzumab (Blood. 2017;129:2702-5. doi:10.1182/blood-2017-01-761973).
This new batch of studies presented at ASH 2017 provided additional evidence for the venetoclax and obinutuzumab combination, as well as other combinations that appear to provide favorable rates of MRD-negative CLL, including obinutuzumab and venetoclax plus ibrutinib and, notably, a combination that included venetoclax and ibrutinib but no anti-CD20 antibody.
A phase II trial presented by Nitin Jain, MD, of MD Anderson Cancer Center, Houston, looked at the combination of venetoclax and ibrutinib for patients with previously untreated high-risk CLL or relapsed/refractory CLL. The regimen under evaluation starts with daily ibrutinib monotherapy for 3 months before venetoclax is added.
For the first line cohort of 36 patients, the bone marrow MRD-negativity proportion was 0% after 3 months of ibrutinib and 21% after 3 months of ibrutinib plus venetoclax, then 45%, 80%, and 100%, respectively, after 6, 9, and 12 months of combination therapy, Dr. Jain reported. In the relapsed/refractory cohort, MRD negativity was 8% after 3 months of combination therapy and 40% after 12 months. “Responses continue to improve with time, with many patients achieving bone marrow MRD-negative remission,” Dr. Jain said.
The study showed “very impressive MRD rates,” commented Ian W. Flinn, MD, of Sarah Cannon Research Institute, Nashville, Tenn. “I think it’s still up for further discussion and research to find which is the best combination.”
Dr. Flinn presented results from a phase Ib GP28331 study of venetoclax and obinutuzumab in patients with previously untreated CLL in which MRD was assessed in peripheral blood and bone marrow.
All 32 patients achieved peripheral blood negativity at some point on study, Dr. Flinn said, and 75% achieved bone marrow negativity. The complete response rate was 72% (23/32), but notably, high rates of undetectable bone marrow MRD were seen irrespective of response status, Dr. Flinn said.
All patients had at least one adverse event, with grade 3-4 neutropenia, febrile neutropenia, and thrombocytopenia reported most commonly.
Preliminary progression-free survival data (PFS) suggested “durable clinical outcomes” for the combination, with an estimated 18-month PFS of 90.5%, he said.
A third study assessed the combination of obinutuzumab, ibrutinib, and venetoclax, finding that the combination induced MRD negativity in 14 of 24 (58%) treatment-naive CLL patients, according to Kerry A. Rogers, MD, of Ohio State University, Columbus.
The combination had a 96% response rate and is “extremely effective at eliminating detectable CLL,” Dr. Rogers noted.
Most adverse events were hematologic, and high-grade adverse events were rare, she said.
Results for the study’s primary endpoint, rate of MRD-negative complete remission, are expected by May 2018, Dr. Rogers said, adding that further follow-up will be needed to determine PFS for the combination.
Session attendees asked presenters whether they felt there was scientific justification for inclusion of the anti-CD20 antibody rituximab in future trials designed in part to assess MRD negativity.
“I think that obinutuzumab has greater efficacy in patients with CLL, compared to rituximab, and so our hypothesis is that it is a superior antibody to combine with venetoclax,” Dr. Flinn said.
AbbVie provided funding for the study on venetoclax and ibrutinib. Dr. Jain reported disclosures from venetoclax makers AbbVie and Genentech, ibrutinib makers Janssen and Pharmacyclics, and others.
Genentech and AbbVie provided support for the study on venetoclax and obinutuzumab. Dr. Flinn reported disclosures related to both companies and others.
Dr. Rogers reported no conflicts related to the study on obinutuzumab, ibrutinib, and venetoclax. One of her associates reported disclosures related to ibrutinib makers Novartis and Pharmacylics, among others.
SOURCE: Jain N et al. ASH 2017 Abstract 429; Flinn I et al. ASH 2017 Abstract 430; Rogers K et al. ASH 2017 Abstract 431.
Atlanta – The potential of two- and three-drug combinations to induce deep, minimal residual disease (MRD)–negative responses in patients with chronic lymphocytic leukemia (CLL) was demonstrated in multiple studies presented at the annual meeting of the American Society of Hematology.
Previous studies have suggested that, in contrast to single-agent kinase inhibitor treatment, combinations of treatments, namely kinase or B-cell lyphoma 2 inhibitors combined with anti-CD20 antibodies, can induce MRD negativity at a high rate. For example, in a report on the CLL14 trial, 11 of 12 previously untreated patients were MRD negative after treatment with the BCL-2 inhibitor venetoclax and anti-CD20 antibody obinutuzumab (Blood. 2017;129:2702-5. doi:10.1182/blood-2017-01-761973).
This new batch of studies presented at ASH 2017 provided additional evidence for the venetoclax and obinutuzumab combination, as well as other combinations that appear to provide favorable rates of MRD-negative CLL, including obinutuzumab and venetoclax plus ibrutinib and, notably, a combination that included venetoclax and ibrutinib but no anti-CD20 antibody.
A phase II trial presented by Nitin Jain, MD, of MD Anderson Cancer Center, Houston, looked at the combination of venetoclax and ibrutinib for patients with previously untreated high-risk CLL or relapsed/refractory CLL. The regimen under evaluation starts with daily ibrutinib monotherapy for 3 months before venetoclax is added.
For the first line cohort of 36 patients, the bone marrow MRD-negativity proportion was 0% after 3 months of ibrutinib and 21% after 3 months of ibrutinib plus venetoclax, then 45%, 80%, and 100%, respectively, after 6, 9, and 12 months of combination therapy, Dr. Jain reported. In the relapsed/refractory cohort, MRD negativity was 8% after 3 months of combination therapy and 40% after 12 months. “Responses continue to improve with time, with many patients achieving bone marrow MRD-negative remission,” Dr. Jain said.
The study showed “very impressive MRD rates,” commented Ian W. Flinn, MD, of Sarah Cannon Research Institute, Nashville, Tenn. “I think it’s still up for further discussion and research to find which is the best combination.”
Dr. Flinn presented results from a phase Ib GP28331 study of venetoclax and obinutuzumab in patients with previously untreated CLL in which MRD was assessed in peripheral blood and bone marrow.
All 32 patients achieved peripheral blood negativity at some point on study, Dr. Flinn said, and 75% achieved bone marrow negativity. The complete response rate was 72% (23/32), but notably, high rates of undetectable bone marrow MRD were seen irrespective of response status, Dr. Flinn said.
All patients had at least one adverse event, with grade 3-4 neutropenia, febrile neutropenia, and thrombocytopenia reported most commonly.
Preliminary progression-free survival data (PFS) suggested “durable clinical outcomes” for the combination, with an estimated 18-month PFS of 90.5%, he said.
A third study assessed the combination of obinutuzumab, ibrutinib, and venetoclax, finding that the combination induced MRD negativity in 14 of 24 (58%) treatment-naive CLL patients, according to Kerry A. Rogers, MD, of Ohio State University, Columbus.
The combination had a 96% response rate and is “extremely effective at eliminating detectable CLL,” Dr. Rogers noted.
Most adverse events were hematologic, and high-grade adverse events were rare, she said.
Results for the study’s primary endpoint, rate of MRD-negative complete remission, are expected by May 2018, Dr. Rogers said, adding that further follow-up will be needed to determine PFS for the combination.
Session attendees asked presenters whether they felt there was scientific justification for inclusion of the anti-CD20 antibody rituximab in future trials designed in part to assess MRD negativity.
“I think that obinutuzumab has greater efficacy in patients with CLL, compared to rituximab, and so our hypothesis is that it is a superior antibody to combine with venetoclax,” Dr. Flinn said.
AbbVie provided funding for the study on venetoclax and ibrutinib. Dr. Jain reported disclosures from venetoclax makers AbbVie and Genentech, ibrutinib makers Janssen and Pharmacyclics, and others.
Genentech and AbbVie provided support for the study on venetoclax and obinutuzumab. Dr. Flinn reported disclosures related to both companies and others.
Dr. Rogers reported no conflicts related to the study on obinutuzumab, ibrutinib, and venetoclax. One of her associates reported disclosures related to ibrutinib makers Novartis and Pharmacylics, among others.
SOURCE: Jain N et al. ASH 2017 Abstract 429; Flinn I et al. ASH 2017 Abstract 430; Rogers K et al. ASH 2017 Abstract 431.
Atlanta – The potential of two- and three-drug combinations to induce deep, minimal residual disease (MRD)–negative responses in patients with chronic lymphocytic leukemia (CLL) was demonstrated in multiple studies presented at the annual meeting of the American Society of Hematology.
Previous studies have suggested that, in contrast to single-agent kinase inhibitor treatment, combinations of treatments, namely kinase or B-cell lyphoma 2 inhibitors combined with anti-CD20 antibodies, can induce MRD negativity at a high rate. For example, in a report on the CLL14 trial, 11 of 12 previously untreated patients were MRD negative after treatment with the BCL-2 inhibitor venetoclax and anti-CD20 antibody obinutuzumab (Blood. 2017;129:2702-5. doi:10.1182/blood-2017-01-761973).
This new batch of studies presented at ASH 2017 provided additional evidence for the venetoclax and obinutuzumab combination, as well as other combinations that appear to provide favorable rates of MRD-negative CLL, including obinutuzumab and venetoclax plus ibrutinib and, notably, a combination that included venetoclax and ibrutinib but no anti-CD20 antibody.
A phase II trial presented by Nitin Jain, MD, of MD Anderson Cancer Center, Houston, looked at the combination of venetoclax and ibrutinib for patients with previously untreated high-risk CLL or relapsed/refractory CLL. The regimen under evaluation starts with daily ibrutinib monotherapy for 3 months before venetoclax is added.
For the first line cohort of 36 patients, the bone marrow MRD-negativity proportion was 0% after 3 months of ibrutinib and 21% after 3 months of ibrutinib plus venetoclax, then 45%, 80%, and 100%, respectively, after 6, 9, and 12 months of combination therapy, Dr. Jain reported. In the relapsed/refractory cohort, MRD negativity was 8% after 3 months of combination therapy and 40% after 12 months. “Responses continue to improve with time, with many patients achieving bone marrow MRD-negative remission,” Dr. Jain said.
The study showed “very impressive MRD rates,” commented Ian W. Flinn, MD, of Sarah Cannon Research Institute, Nashville, Tenn. “I think it’s still up for further discussion and research to find which is the best combination.”
Dr. Flinn presented results from a phase Ib GP28331 study of venetoclax and obinutuzumab in patients with previously untreated CLL in which MRD was assessed in peripheral blood and bone marrow.
All 32 patients achieved peripheral blood negativity at some point on study, Dr. Flinn said, and 75% achieved bone marrow negativity. The complete response rate was 72% (23/32), but notably, high rates of undetectable bone marrow MRD were seen irrespective of response status, Dr. Flinn said.
All patients had at least one adverse event, with grade 3-4 neutropenia, febrile neutropenia, and thrombocytopenia reported most commonly.
Preliminary progression-free survival data (PFS) suggested “durable clinical outcomes” for the combination, with an estimated 18-month PFS of 90.5%, he said.
A third study assessed the combination of obinutuzumab, ibrutinib, and venetoclax, finding that the combination induced MRD negativity in 14 of 24 (58%) treatment-naive CLL patients, according to Kerry A. Rogers, MD, of Ohio State University, Columbus.
The combination had a 96% response rate and is “extremely effective at eliminating detectable CLL,” Dr. Rogers noted.
Most adverse events were hematologic, and high-grade adverse events were rare, she said.
Results for the study’s primary endpoint, rate of MRD-negative complete remission, are expected by May 2018, Dr. Rogers said, adding that further follow-up will be needed to determine PFS for the combination.
Session attendees asked presenters whether they felt there was scientific justification for inclusion of the anti-CD20 antibody rituximab in future trials designed in part to assess MRD negativity.
“I think that obinutuzumab has greater efficacy in patients with CLL, compared to rituximab, and so our hypothesis is that it is a superior antibody to combine with venetoclax,” Dr. Flinn said.
AbbVie provided funding for the study on venetoclax and ibrutinib. Dr. Jain reported disclosures from venetoclax makers AbbVie and Genentech, ibrutinib makers Janssen and Pharmacyclics, and others.
Genentech and AbbVie provided support for the study on venetoclax and obinutuzumab. Dr. Flinn reported disclosures related to both companies and others.
Dr. Rogers reported no conflicts related to the study on obinutuzumab, ibrutinib, and venetoclax. One of her associates reported disclosures related to ibrutinib makers Novartis and Pharmacylics, among others.
SOURCE: Jain N et al. ASH 2017 Abstract 429; Flinn I et al. ASH 2017 Abstract 430; Rogers K et al. ASH 2017 Abstract 431.
REPORTING FROM ASH 2017
Evidence builds for long-term ineffectiveness of steroid shots for knee OA
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
SAN DIEGO – Real-world, nontrial research confirms the findings of a high-profile study released earlier in 2017: Corticosteroid shots are ineffective in the long term for knee osteoarthritis. In fact, researchers found a greater likelihood of a worsening condition in knees treated with the injections.
“Our findings are consistent with the latest randomized, controlled trial,” said study coauthor Jie Wei, MD, of Central South University in Changsha, China. She spoke in a plenary presentation about the study findings at the annual meeting of the American College of Rheumatology.
The use of corticosteroids for knee OA is a controversial topic. As Dr. Wei noted, there has been wide disagreement among medical societies about whether the treatment is useful in the long term for patients with pain flare-ups.
For the randomized, controlled study released in 2017, researchers tracked 140 patients aged 45 and older with inflammation of the synovial membrane. They were randomly assigned to injections of intra-articular triamcinolone or a placebo.
After 2 years of injections every 12 weeks, there was no difference in reported pain between the intervention and control groups. Also, those who received injections lost more cartilage (JAMA. 2017 May 16;317[19]:1967-75).
Researchers launched the new study to seek insight through a real-life cohort. They examined findings from the Osteoarthritis Initiative, a longitudinal study of 4,796 patients aged 45-79 at four U.S. clinics with knee OA or high risk of knee OA. Patients underwent annual examinations at baseline and annually for 4 years.
In an adjusted marginal structural analysis, knee replacement or worsening of Kellgren Lawrence grade at the tibial femoral joint was more likely in 149 injection knees than 2,191 noninjection knees (odds ratio, 5.74; 95% confidence interval, 2.01-16.42).
Knee replacement or joint space width worsening at the tibial femoral joint was also more likely in 120 injection knees than 2,112 noninjection knees (OR, 1.64; 95% CI, 0.91-2.93).
In another analysis, researchers tracked 134 injection knees (58 whose OA progressed) and 498 noninjection knees (132 whose OA progressed) for up to 8 years. After adjustment, the injection knees were more likely to have progressed (hazard ratio, 1.60; 95% CI, 1.21-2.12,).
“Several explanations may account for our study findings,” Dr. Wei said. One possibility, she said, is that corticosteroids may hurt chondrocytes by, among other things, inducing apoptosis and synovial membrane inflammation.
It’s also possible, she said, that patients may feel pain relief after injections and subsequently boost the risk of OA progression by increasing their physical activity.
“We need to know what types of physical activities may increase the OA progression,” she said. “Did patients who received steroid injection indeed increase this type of physical activity compared to subjects without steroid injection?”
Dr. Wei noted the study’s limitations, including the fact that patients who received injections had more pain at baseline, potentially indicating they had worse structural lesions that are more susceptible to progression.
The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
REPORTING FROM ACR 2017
Key clinical point:
Major finding: In adjusted analysis of 134 injection knees and 498 noninjection knees tracked for up to 8 years, OA in injection knees was more likely to have progressed (HR, 1.60; 95% CI, 1.21-2.12).
Study details: Cohort analysis of data from the Osteoarthritis Initiative, which tracked patients with (or at high risk of) knee OA at four U.S. clinics.
Disclosures: The study authors reported no relevant disclosures. The National Natural Science Foundation of China funded the study. The Osteoarthritis Initiative is a partnership between the National Institutes of Health and Merck, Novartis, GlaxoSmithKline, and Pfizer.
Source: Lei G et al. ACR 2017 Abstract 1788.
VIDEO: CTCs may identify asymptomatic late breast cancer recurrences
SAN ANTONIO – Although the initial ardor over the use of circulating tumor cells (CTCs) in cancer diagnosis has cooled, new research suggests that they may play a role in identifying late breast cancer recurrences in otherwise asymptomatic patients, according to members of the ECOG-ACRIN cancer research group.
In this video interview from the the San Antonio Breast Cancer Symposium, Joseph A. Sparano, MD, of Montefiore Medical Center and Albert Einstein College of Medicine in New York, describes ECOG-ACRIN’s experiments showing that patients with hormone receptor–positive disease and HER2-negative breast cancer have a significantly elevated risk for recurrence, supporting CTCs as prognostic biomarkers for late recurrences.
If the findings can be replicated in prospective clinical trials, CTC assay results could help clinicians choose treatments for patients who are at risk for late recurrence.
ECOG-ACRIN received funding for this study from the Breast Cancer Research Foundation, Susan G. Komen, and the National Cancer Institute. Dr. Sparano declared no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Although the initial ardor over the use of circulating tumor cells (CTCs) in cancer diagnosis has cooled, new research suggests that they may play a role in identifying late breast cancer recurrences in otherwise asymptomatic patients, according to members of the ECOG-ACRIN cancer research group.
In this video interview from the the San Antonio Breast Cancer Symposium, Joseph A. Sparano, MD, of Montefiore Medical Center and Albert Einstein College of Medicine in New York, describes ECOG-ACRIN’s experiments showing that patients with hormone receptor–positive disease and HER2-negative breast cancer have a significantly elevated risk for recurrence, supporting CTCs as prognostic biomarkers for late recurrences.
If the findings can be replicated in prospective clinical trials, CTC assay results could help clinicians choose treatments for patients who are at risk for late recurrence.
ECOG-ACRIN received funding for this study from the Breast Cancer Research Foundation, Susan G. Komen, and the National Cancer Institute. Dr. Sparano declared no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
SAN ANTONIO – Although the initial ardor over the use of circulating tumor cells (CTCs) in cancer diagnosis has cooled, new research suggests that they may play a role in identifying late breast cancer recurrences in otherwise asymptomatic patients, according to members of the ECOG-ACRIN cancer research group.
In this video interview from the the San Antonio Breast Cancer Symposium, Joseph A. Sparano, MD, of Montefiore Medical Center and Albert Einstein College of Medicine in New York, describes ECOG-ACRIN’s experiments showing that patients with hormone receptor–positive disease and HER2-negative breast cancer have a significantly elevated risk for recurrence, supporting CTCs as prognostic biomarkers for late recurrences.
If the findings can be replicated in prospective clinical trials, CTC assay results could help clinicians choose treatments for patients who are at risk for late recurrence.
ECOG-ACRIN received funding for this study from the Breast Cancer Research Foundation, Susan G. Komen, and the National Cancer Institute. Dr. Sparano declared no conflicts of interest.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM SABCS 2017
Virtual reality enters the rheumatology realm
SAN DIEGO – When pain strikes arthritis patients, a beach trip may be the last thing on their minds. But a Los Angeles rheumatologist says a virtual voyage to the shore – or the fjords of Iceland or a Cirque du Soleil performance – may be just what they need to find relief without leaving their chairs.
Swamy Venuturupalli, MD, has been testing virtual reality (VR) software on patients as part of a clinical feasibility study into its use to treat rheumatologic pain. It appears to be the first VR study in rheumatology.
Dr. Venuturupalli is working with gastroenterologist Brennan Spiegel, MD, a VR researcher who talked about the power of the treatment in a presentation at the annual meeting of the American College of Rheumatology.
“Like a drug, we have to understand when and how to use this. It can be very powerful,” Dr. Spiegel, professor of medicine and public health at UCLA and director of Health Services Research at Cedars-Sinai Health System, said in his presentation.
“In recent years, VR technology has become increasingly affordable, immersive, flexible, and portable, enabling its use in a broad range of environments, including the inpatient medical setting,” wrote researchers in a 2017 systematic review of 11 randomized, controlled VR trials. “The capacity of VR to modulate subjective experience makes it a compelling intervention in inpatient medical settings, where VR may offer respite from the confining nature of medical wards, or where it may augment or replace analgesics in pain management” (Innov Clin Neurosci. 2017 Jan-Feb;14[1-2]:14-21).
In his ACR presentation, Dr. Spiegel said VR allows patients to escape the “psychosocial jail cell” of a hospital room. “We can put them on a helicopter and fly them over Icelandic fjords or let them sit on stage during a Cirque du Soleil performance.”
In terms of pain, he pointed to a 2017 study that he conducted with colleagues at Cedars-Sinai Medical Center. In 100 inpatients with pain scores of 3 or more on a 10-point scale, researchers compared a onetime 3-dimensional VR immersion via headset to exposure to a 2-dimensional nature video. Both interventions lasted 15 minutes.
Those in the VR group (n = 50) had greater pain improvement than did those in the 2-D group (–1.3 vs –0.6 points; P = .008), and the percentage of patients whose pain diminished was higher (65% vs. 40%; P = .01; number needed to treat = 4) (JMIR Ment Health. 2017 Jan-Mar;4[1]:e9).
Researchers reported no adverse events in the study. However, Dr. Spiegel said patients who undergo VR treatments may feel vertigo, especially if the technology is subpar, and there is a theoretical risk of seizure.
He also noted that VR can cause other negative effects. One patient had a panic attack during an immersive experience of simply throwing balls at cartoon teddy bears in a virtual environment. In this patient, “any concept of violence was enough to trigger a panic attack,” Dr. Spiegel said.
But another immersive virtual experience, putting her on a stage at Cirque du Soleil performance, was a success. “It empowered her, it made her feel brave,” Dr. Spiegel said.
He cautioned about limits of VR: Not every patient is eligible, will want to use it, or will benefit. And while the headsets used in VR have improved, he said, there’s still a way to go to make them more comfortable.
As for cost, VR equipment can be pricey. Hunter Hoffman, director of a virtual reality research center at the University of Washington, Seattle, told MIT Technology Review that the equipment for a VR pain study cost $35,000.
Dr. Venuturupalli, the rheumatologist who’s working with Dr. Spiegel, said his clinic is testing VR technology in patients with chronic pain syndromes. They’re immersed in environments such as one that simulates swimming with dolphins. “Some of my patients try to reach out and touch the dolphin in that state,” he said.
The feasibility study, now in progress, aims to enroll 20 patients and be completed by next March, Dr. Venuturupalli said. So far, the time span of improvement in patients has been variable, he said.
“Our goal is to have a full-fledged virtual reality clinic,” he said. “Like we prescribe physical therapy, we might have a VR therapy prescription that might include certain experiences. And as this technology gets cheaper and cheaper, our patients can have it at their own homes.”
Dr. Spiegel and Dr. Venuturupalli reported no relevant disclosures. Dr. Venuturupalli reports that his study is using donated VR technology from AppliedVR. The firm has created a partnership with Cedars-Sinai Medical Center.
SAN DIEGO – When pain strikes arthritis patients, a beach trip may be the last thing on their minds. But a Los Angeles rheumatologist says a virtual voyage to the shore – or the fjords of Iceland or a Cirque du Soleil performance – may be just what they need to find relief without leaving their chairs.
Swamy Venuturupalli, MD, has been testing virtual reality (VR) software on patients as part of a clinical feasibility study into its use to treat rheumatologic pain. It appears to be the first VR study in rheumatology.
Dr. Venuturupalli is working with gastroenterologist Brennan Spiegel, MD, a VR researcher who talked about the power of the treatment in a presentation at the annual meeting of the American College of Rheumatology.
“Like a drug, we have to understand when and how to use this. It can be very powerful,” Dr. Spiegel, professor of medicine and public health at UCLA and director of Health Services Research at Cedars-Sinai Health System, said in his presentation.
“In recent years, VR technology has become increasingly affordable, immersive, flexible, and portable, enabling its use in a broad range of environments, including the inpatient medical setting,” wrote researchers in a 2017 systematic review of 11 randomized, controlled VR trials. “The capacity of VR to modulate subjective experience makes it a compelling intervention in inpatient medical settings, where VR may offer respite from the confining nature of medical wards, or where it may augment or replace analgesics in pain management” (Innov Clin Neurosci. 2017 Jan-Feb;14[1-2]:14-21).
In his ACR presentation, Dr. Spiegel said VR allows patients to escape the “psychosocial jail cell” of a hospital room. “We can put them on a helicopter and fly them over Icelandic fjords or let them sit on stage during a Cirque du Soleil performance.”
In terms of pain, he pointed to a 2017 study that he conducted with colleagues at Cedars-Sinai Medical Center. In 100 inpatients with pain scores of 3 or more on a 10-point scale, researchers compared a onetime 3-dimensional VR immersion via headset to exposure to a 2-dimensional nature video. Both interventions lasted 15 minutes.
Those in the VR group (n = 50) had greater pain improvement than did those in the 2-D group (–1.3 vs –0.6 points; P = .008), and the percentage of patients whose pain diminished was higher (65% vs. 40%; P = .01; number needed to treat = 4) (JMIR Ment Health. 2017 Jan-Mar;4[1]:e9).
Researchers reported no adverse events in the study. However, Dr. Spiegel said patients who undergo VR treatments may feel vertigo, especially if the technology is subpar, and there is a theoretical risk of seizure.
He also noted that VR can cause other negative effects. One patient had a panic attack during an immersive experience of simply throwing balls at cartoon teddy bears in a virtual environment. In this patient, “any concept of violence was enough to trigger a panic attack,” Dr. Spiegel said.
But another immersive virtual experience, putting her on a stage at Cirque du Soleil performance, was a success. “It empowered her, it made her feel brave,” Dr. Spiegel said.
He cautioned about limits of VR: Not every patient is eligible, will want to use it, or will benefit. And while the headsets used in VR have improved, he said, there’s still a way to go to make them more comfortable.
As for cost, VR equipment can be pricey. Hunter Hoffman, director of a virtual reality research center at the University of Washington, Seattle, told MIT Technology Review that the equipment for a VR pain study cost $35,000.
Dr. Venuturupalli, the rheumatologist who’s working with Dr. Spiegel, said his clinic is testing VR technology in patients with chronic pain syndromes. They’re immersed in environments such as one that simulates swimming with dolphins. “Some of my patients try to reach out and touch the dolphin in that state,” he said.
The feasibility study, now in progress, aims to enroll 20 patients and be completed by next March, Dr. Venuturupalli said. So far, the time span of improvement in patients has been variable, he said.
“Our goal is to have a full-fledged virtual reality clinic,” he said. “Like we prescribe physical therapy, we might have a VR therapy prescription that might include certain experiences. And as this technology gets cheaper and cheaper, our patients can have it at their own homes.”
Dr. Spiegel and Dr. Venuturupalli reported no relevant disclosures. Dr. Venuturupalli reports that his study is using donated VR technology from AppliedVR. The firm has created a partnership with Cedars-Sinai Medical Center.
SAN DIEGO – When pain strikes arthritis patients, a beach trip may be the last thing on their minds. But a Los Angeles rheumatologist says a virtual voyage to the shore – or the fjords of Iceland or a Cirque du Soleil performance – may be just what they need to find relief without leaving their chairs.
Swamy Venuturupalli, MD, has been testing virtual reality (VR) software on patients as part of a clinical feasibility study into its use to treat rheumatologic pain. It appears to be the first VR study in rheumatology.
Dr. Venuturupalli is working with gastroenterologist Brennan Spiegel, MD, a VR researcher who talked about the power of the treatment in a presentation at the annual meeting of the American College of Rheumatology.
“Like a drug, we have to understand when and how to use this. It can be very powerful,” Dr. Spiegel, professor of medicine and public health at UCLA and director of Health Services Research at Cedars-Sinai Health System, said in his presentation.
“In recent years, VR technology has become increasingly affordable, immersive, flexible, and portable, enabling its use in a broad range of environments, including the inpatient medical setting,” wrote researchers in a 2017 systematic review of 11 randomized, controlled VR trials. “The capacity of VR to modulate subjective experience makes it a compelling intervention in inpatient medical settings, where VR may offer respite from the confining nature of medical wards, or where it may augment or replace analgesics in pain management” (Innov Clin Neurosci. 2017 Jan-Feb;14[1-2]:14-21).
In his ACR presentation, Dr. Spiegel said VR allows patients to escape the “psychosocial jail cell” of a hospital room. “We can put them on a helicopter and fly them over Icelandic fjords or let them sit on stage during a Cirque du Soleil performance.”
In terms of pain, he pointed to a 2017 study that he conducted with colleagues at Cedars-Sinai Medical Center. In 100 inpatients with pain scores of 3 or more on a 10-point scale, researchers compared a onetime 3-dimensional VR immersion via headset to exposure to a 2-dimensional nature video. Both interventions lasted 15 minutes.
Those in the VR group (n = 50) had greater pain improvement than did those in the 2-D group (–1.3 vs –0.6 points; P = .008), and the percentage of patients whose pain diminished was higher (65% vs. 40%; P = .01; number needed to treat = 4) (JMIR Ment Health. 2017 Jan-Mar;4[1]:e9).
Researchers reported no adverse events in the study. However, Dr. Spiegel said patients who undergo VR treatments may feel vertigo, especially if the technology is subpar, and there is a theoretical risk of seizure.
He also noted that VR can cause other negative effects. One patient had a panic attack during an immersive experience of simply throwing balls at cartoon teddy bears in a virtual environment. In this patient, “any concept of violence was enough to trigger a panic attack,” Dr. Spiegel said.
But another immersive virtual experience, putting her on a stage at Cirque du Soleil performance, was a success. “It empowered her, it made her feel brave,” Dr. Spiegel said.
He cautioned about limits of VR: Not every patient is eligible, will want to use it, or will benefit. And while the headsets used in VR have improved, he said, there’s still a way to go to make them more comfortable.
As for cost, VR equipment can be pricey. Hunter Hoffman, director of a virtual reality research center at the University of Washington, Seattle, told MIT Technology Review that the equipment for a VR pain study cost $35,000.
Dr. Venuturupalli, the rheumatologist who’s working with Dr. Spiegel, said his clinic is testing VR technology in patients with chronic pain syndromes. They’re immersed in environments such as one that simulates swimming with dolphins. “Some of my patients try to reach out and touch the dolphin in that state,” he said.
The feasibility study, now in progress, aims to enroll 20 patients and be completed by next March, Dr. Venuturupalli said. So far, the time span of improvement in patients has been variable, he said.
“Our goal is to have a full-fledged virtual reality clinic,” he said. “Like we prescribe physical therapy, we might have a VR therapy prescription that might include certain experiences. And as this technology gets cheaper and cheaper, our patients can have it at their own homes.”
Dr. Spiegel and Dr. Venuturupalli reported no relevant disclosures. Dr. Venuturupalli reports that his study is using donated VR technology from AppliedVR. The firm has created a partnership with Cedars-Sinai Medical Center.
REPORTING FROM ACR 2017
Developmental disabilities up significantly since 2014
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.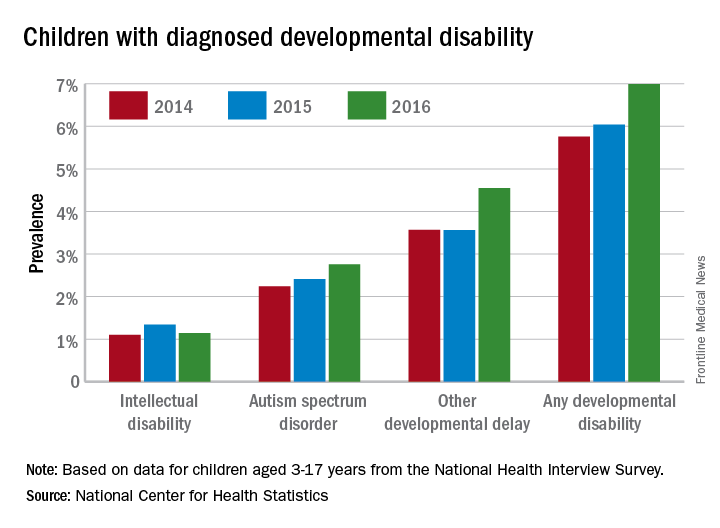
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
In 2016, the prevalence of any diagnosed developmental disability in children aged 3-17 years was 6.99% – a statistically significant increase of 21% over the 5.76% recorded in 2014, the NCHS said in a recent Data Brief.
Autism spectrum disorder was up by a similar amount: 23% from 2014, when prevalence was 2.24%, to 2016, when the prevalence was 2.76% among children aged 3-17 years. Intellectual disability rose in 2015 but dropped in 2016, so the overall increase in prevalence was just 3.6%. The prevalence of other developmental delays, on the other hand, held steady from 2014 to 2015 and then took a big jump, 27.5%, in 2016, the NCHS investigators reported.
The estimates are based on reports by parents or guardians of ever receiving a diagnosis of each developmental disability from a physician or other medical professional.
Activating the Immune System to Treat Multiple Myeloma
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
Sagar Lonial, MD
Chief Medical Officer, Winship Cancer Institute of Emory University
Chair, Dept. of Hematology and Medical Oncology, Emory School of Medicine
Faculty/Faculty Disclosure
Dr. Lonial reports that he is a compensated consultant for Bristol-Myers Squibb; Celgene Corporation; Janssen Pharmaceuticals, Inc.; Merck & Co., Inc.; Millennium Pharmaceuticals, Inc.; Novartis Pharmaceuticals Corporation; and Onyx Pharmaceuticals, Inc.
MACRA Monday: BMI screening and follow-up
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
If you haven’t started reporting quality data for the Merit-Based Incentive Payment System (MIPS), there’s still time to avoid a 4% cut to your Medicare payments.
Under the Pick Your Pace approach being offered this year, the Centers for Medicare & Medicaid Services allows clinicians to test the system by reporting on one quality measure for one patient through paper-based claims. Be sure to append a Quality Data Code (QDC) to the claim form for care provided up to Dec. 31, 2017, in order to avoid a penalty in payment year 2019.
Consider this measure:
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Measure #128: Preventive Care and Screening: Body Mass Index Screening and Follow-Up Plan
This measure is aimed at capturing the percentage of patients aged 18 years and older who have had their body mass index (BMI) calculated and documented in the last 6 months and a follow-up plan developed if the BMI was too high or too low.
What you need to do: Assess the patient’s BMI during the visit or document that it was done in the last 6 months. Patient-reported height and weight values cannot be used. If the BMI is outside of normal parameters (18.5 kg/m2 to 25 kg/m2), develop a follow-up plan or document that one was made in the last 6 months.
Eligible cases include patients who were aged 18 years or older on the date of the encounter and a patient encounter during the performance period. Applicable codes include (CPT or HCPCS): 90791, 90792, 90832, 90834, 90837, 96150, 96151, 96152, 97161, 97162, 97163, 97165, 97166, 97167, 97802, 97803, 98960, 99201, 99202, 99203, 99204, 99205, 99212, 99213, 99214, 99215, D7140, D7210, G0101, G0108, G0270, G0271, G0402, G0438, G0439, G0447 without telehealth modifiers GQ or GT.
To get credit under MIPS, be sure to include a QDC that shows that you successfully performed the measure or had a good reason for not doing so. For instance, G8420 indicates that BMI has been documented within normal parameters and no follow-up plan is required, while G8417 and G8418 indicate BMI above and below normal parameters, respectively, with a documented follow-up plan.
Use exclusion code G8938 if the BMI has been documented as being outside of normal limits, but a follow-up plan is not documented because the patient is not eligible. For example, patients are considered not eligible if they are 65 years or older and weight reduction or gain would complicate an underlying health condition.
CMS has a full list measures available for claims-based reporting at qpp.cms.gov. The American Medical Association has also created a step-by-step guide for reporting on one quality measure.
Certain clinicians are exempt from reporting and do not face a penalty under MIPS:
- Those who enrolled in Medicare for the first time during a performance period.
- Those who have Medicare Part B allowed charges of $30,000 or less.
- Those who have 100 or fewer Medicare Part B patients.
- Those who are significantly participating in an Advanced Alternative Payment Model (APM).
A Medication Tracker That Patients Swallow
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
Making sure some patients are taking their medication correctly may be a little easier now. The FDA has approved Abilify MyCite (apiprazole), which has an ingestible sensor.
Abilify MyCite is approved for treatment of schizophrenia, acute treatment of manic and mixed episodes associated with bipolar 1 disorder, and as an add-on treatment for depression in adults.
The sensor, embedded in the pill, records when the medicine was taken, and sends a message to a wearable patch, which then transmits information to a mobile application. Patients can track the ingestion of the medication on their smartphone and can allow their caregivers and physicians to access information through a web-based portal.
However, the FDA notes that Abilify MyCite’s prescribing information includes a caution that the product has not been shown to improve patient adherence with a treatment regimen. Moreover, Ability MyCite should not be used to track drug ingestion in real time or during an emergency, because detection may be delayed or may not occur. Before prescribing it for a patient, health care professionals should make sure the patient is capable and willing to use the drug, patch, and app.
When the Fix Fails
ANSWER
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.
ANSWER
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.
ANSWER
The radiograph shows an adequately positioned endotracheal tube. It also shows bilateral infiltrates, greater in the right than in the left lung.
A pigtail catheter is present, up high near the apex; despite this, a pneumothorax of moderate size remains on the right. The likely explanation is that the catheter is either not properly positioned or is kinked.
Prompt surgical consultation for new chest tube placement was obtained.
A 60-year-old woman is transferred to your facility from an outside hospital for tertiary care. She was reportedly at home with family when she suddenly collapsed and became unresponsive. She was taken to a nearby hospital, where she was resuscitated, stabilized, and urgently sent to your facility for possible cardiac intervention.
You assess the patient immediately upon arrival;with no family present, history is limited to the chart. You note an intubated female on mild sedation. Her vital signs include a temperature of 37.0°C; blood pressure, 130/80 mm Hg; heart rate, 70 beats/min; and O2 saturation, 98% on 100% FiO2.
The heart rate monitor shows sinus rhythm. A right chest tube is in place. Auscultation reveals bilateral rhonchi.
Portable chest radiograph is obtained (shown). What is your impression?