User login
PANACEA: pembrolizumab overcomes trastuzumab resistance for some
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab overcomes trastuzumab resistance in HER2-positive advanced breast cancer provided that the tumor expresses programmed death ligand 1 (PD-L1), a trial reported at the San Antonio Breast Cancer Symposium suggests. But presence of immune cells in the tumor is a major additional determinant of benefit.
The single-arm phase 1b/2 trial, called PANACEA (also KEYNOTE-014), enrolled 58 patients with HER2-positive advanced breast cancer that had progressed on trastuzumab (Herceptin) or trastuzumab emtansine (Kadcyla). All were given pembrolizumab (Keytruda), which unleashes antitumor immunity by targeting the programmed death-1 receptor on immune cells, in combination with trastuzumab.
With a median follow-up of 13.6 months, the cohort of patients having tumors positive for PD-L1 achieved an overall response rate of 15.2% and a disease control rate of 24%,” Dr. Loi reported in a press briefing and session, on behalf of the International Breast Cancer Study Group and Breast International Group. In contrast, there were no responses in the PD-L1–negative cohort.
Within the PD-L1–positive cohort, stromal levels of TILs in the metastatic lesion – which were low overall – influenced likelihood of benefit. The response rate was almost eight times higher in patients who had at least 5% of the stromal area densely infiltrated with TILs.
“The PANACEA study met its primary endpoint in the PD-L1–positive cohort. For responders, this combination offers durable control without chemotherapy,” Dr. Loi summarized.
“Metastatic HER2-positive breast cancer in this [heavily pretreated] setting is poorly immunogenic, as evidenced by the majority of patients having low TILs in their metastatic lesions. Saying that, however, we did observe a higher response rate in this study as compared to the equivalent triple-negative breast cancer studied in KEYNOTE-086,” she noted. “Future directions in this disease space should focus on combinations with effective anti-HER2 therapy, particularly in low-TIL patients.”
Predicting benefit
The trial is noteworthy for its efforts to identify the subset of patients most likely to benefit from immune checkpoint inhibition, according to press briefing moderator Virginia Kaklamani, MD, a professor of medicine in the division of hematology/oncology at the University of Texas Health Science Center, San Antonio, and a leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center.
In similar studies among patients with HER2-negative breast cancer, PD-L1 did not pan out as a strong predictive biomarker. “What do you think the difference is between that subset and the HER2-positive subset?” Dr. Kaklamani asked.
“First off, I think that there are technical issues with the PD-L1 assay. And we find that patients with high TILs or immune infiltration usually have high levels of PD-L1 expression on their TILs,” Dr. Loi replied. “So I think that PD-L1 can be expressed on the tumor as well as the TIL, and it certainly seems to be the TIL infiltrate that probably enriches for responders to a PD-L1 checkpoint inhibitor on its own or in this case with trastuzumab.”
Study details
In the PANACEA trial (additionally known as IBCSG 45-13 and BIG 4-13), the most common adverse event of any grade and type with the pembrolizumab-trastuzumab combination was fatigue, seen in 21% of patients, Dr. Loi reported. For immune-related adverse events specifically, 19.0% of patients experienced an event, 10.3% experienced an event of grade 3 or worse, and 6.9% stopped treatment because of these events.
“These frequencies are consistent with what has been reported in other solid tumor types with pembrolizumab,” she commented. There were no cardiac events reported.
Efficacy analyses were restricted largely to the PD-L1–positive cohort, given the lack of any response in the negative cohort.
Median duration of response in the positive cohort was 3.5 months, and median duration of disease control was 11.1 months. Five patients (10.8%) remain on treatment with no progression; three of them have completed 2 years of pembrolizumab.
Median progression-free and overall survival were 2.7 and 16.1 months, respectively; corresponding 12-month rates were 13% and 65%. “There is a tantalizing suggestion of a tail on the curve. ... Obviously, this requires further follow-up, and the numbers are small,” Dr. Loi commented.
The median baseline stromal TIL level in metastatic lesions was just 1%. “This is 20 times less than what we observe in primary HER2-positive breast cancers,” she pointed out.
Compared with the PD-L1–negative cohort, the PD-L1–positive cohort had higher TIL levels. Additionally, within that latter cohort, TIL level was higher among patients achieving response versus not (P = .006) and patients achieving disease control versus not (P = .0006).
“We then went on to try to identify a TIL cutoff that could enrich the population for responders. This has been done in other solid tumor types,” Dr. Loi explained.
Analyses in the PD-L1-positive cohort showed that TIL levels down to 5% predicted benefit. The 41% of patients having 5% or more TILs were dramatically more likely to have a response (39% vs. 5%) and disease control (47% vs. 5%).
TIL levels varied widely according to site of the metastasis, with higher levels seen in metastases from lung and lymph nodes, and lower levels seen in those from liver and skin.
“At this stage, we are not sure which is the chicken and the egg: Patients could have disease in their lung and their lymph nodes because their immune system is better controlling their disease,” Dr. Loi commented. “How we treat these patients is still an open question. In patients with liver metastases, perhaps we need to be more aggressive with the primary or tumor-control anti-HER2 therapy.”
Improving efficacy
Going forward, one strategy for improving pembrolizumab efficacy in this patient population might be priming the immune response, according to Dr. Loi.
“In HER2 disease, it’s very clear that oncogenic signaling is the driver, so targeting HER2 potently also will help relieve tumor-mediated immune suppression,” she elaborated. “In this particular context, targeting HER2 well is the key. Whether you need the addition of a little bit of chemo or some radiation, all this needs to be studied.”
Another strategy for improving pembrolizumab efficacy might be moving the drug to earlier disease settings, Dr. Loi proposed.
“By the time you get to advanced stage and have had multiple treatments, you actually have low levels of T-cell infiltration in your metastatic lesion, for whatever reasons – tumor burden, immunosuppression, multiple lines of treatment. That all reduces your chance of responding to pembrolizumab, for example, as monotherapy,” she elaborated. “We don’t know yet if chemotherapy in addition to pembrolizumab could change that tumor microenvironment. But still, I think the earlier in lines you go, the more chance you are going to have of preexisting effective antitumor immunity that can be reactivated with the addition of pembrolizumab.”
Dr. Loi disclosed that her institution receives research funding from Novartis, Pfizer, Merck, Genentech/Roche, and Puma. Merck provided study drug and support for PANACEA.
SOURCE: Loi S et al. SABCS 2017 Abstract GS2-06.
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab overcomes trastuzumab resistance in HER2-positive advanced breast cancer provided that the tumor expresses programmed death ligand 1 (PD-L1), a trial reported at the San Antonio Breast Cancer Symposium suggests. But presence of immune cells in the tumor is a major additional determinant of benefit.
The single-arm phase 1b/2 trial, called PANACEA (also KEYNOTE-014), enrolled 58 patients with HER2-positive advanced breast cancer that had progressed on trastuzumab (Herceptin) or trastuzumab emtansine (Kadcyla). All were given pembrolizumab (Keytruda), which unleashes antitumor immunity by targeting the programmed death-1 receptor on immune cells, in combination with trastuzumab.
With a median follow-up of 13.6 months, the cohort of patients having tumors positive for PD-L1 achieved an overall response rate of 15.2% and a disease control rate of 24%,” Dr. Loi reported in a press briefing and session, on behalf of the International Breast Cancer Study Group and Breast International Group. In contrast, there were no responses in the PD-L1–negative cohort.
Within the PD-L1–positive cohort, stromal levels of TILs in the metastatic lesion – which were low overall – influenced likelihood of benefit. The response rate was almost eight times higher in patients who had at least 5% of the stromal area densely infiltrated with TILs.
“The PANACEA study met its primary endpoint in the PD-L1–positive cohort. For responders, this combination offers durable control without chemotherapy,” Dr. Loi summarized.
“Metastatic HER2-positive breast cancer in this [heavily pretreated] setting is poorly immunogenic, as evidenced by the majority of patients having low TILs in their metastatic lesions. Saying that, however, we did observe a higher response rate in this study as compared to the equivalent triple-negative breast cancer studied in KEYNOTE-086,” she noted. “Future directions in this disease space should focus on combinations with effective anti-HER2 therapy, particularly in low-TIL patients.”
Predicting benefit
The trial is noteworthy for its efforts to identify the subset of patients most likely to benefit from immune checkpoint inhibition, according to press briefing moderator Virginia Kaklamani, MD, a professor of medicine in the division of hematology/oncology at the University of Texas Health Science Center, San Antonio, and a leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center.
In similar studies among patients with HER2-negative breast cancer, PD-L1 did not pan out as a strong predictive biomarker. “What do you think the difference is between that subset and the HER2-positive subset?” Dr. Kaklamani asked.
“First off, I think that there are technical issues with the PD-L1 assay. And we find that patients with high TILs or immune infiltration usually have high levels of PD-L1 expression on their TILs,” Dr. Loi replied. “So I think that PD-L1 can be expressed on the tumor as well as the TIL, and it certainly seems to be the TIL infiltrate that probably enriches for responders to a PD-L1 checkpoint inhibitor on its own or in this case with trastuzumab.”
Study details
In the PANACEA trial (additionally known as IBCSG 45-13 and BIG 4-13), the most common adverse event of any grade and type with the pembrolizumab-trastuzumab combination was fatigue, seen in 21% of patients, Dr. Loi reported. For immune-related adverse events specifically, 19.0% of patients experienced an event, 10.3% experienced an event of grade 3 or worse, and 6.9% stopped treatment because of these events.
“These frequencies are consistent with what has been reported in other solid tumor types with pembrolizumab,” she commented. There were no cardiac events reported.
Efficacy analyses were restricted largely to the PD-L1–positive cohort, given the lack of any response in the negative cohort.
Median duration of response in the positive cohort was 3.5 months, and median duration of disease control was 11.1 months. Five patients (10.8%) remain on treatment with no progression; three of them have completed 2 years of pembrolizumab.
Median progression-free and overall survival were 2.7 and 16.1 months, respectively; corresponding 12-month rates were 13% and 65%. “There is a tantalizing suggestion of a tail on the curve. ... Obviously, this requires further follow-up, and the numbers are small,” Dr. Loi commented.
The median baseline stromal TIL level in metastatic lesions was just 1%. “This is 20 times less than what we observe in primary HER2-positive breast cancers,” she pointed out.
Compared with the PD-L1–negative cohort, the PD-L1–positive cohort had higher TIL levels. Additionally, within that latter cohort, TIL level was higher among patients achieving response versus not (P = .006) and patients achieving disease control versus not (P = .0006).
“We then went on to try to identify a TIL cutoff that could enrich the population for responders. This has been done in other solid tumor types,” Dr. Loi explained.
Analyses in the PD-L1-positive cohort showed that TIL levels down to 5% predicted benefit. The 41% of patients having 5% or more TILs were dramatically more likely to have a response (39% vs. 5%) and disease control (47% vs. 5%).
TIL levels varied widely according to site of the metastasis, with higher levels seen in metastases from lung and lymph nodes, and lower levels seen in those from liver and skin.
“At this stage, we are not sure which is the chicken and the egg: Patients could have disease in their lung and their lymph nodes because their immune system is better controlling their disease,” Dr. Loi commented. “How we treat these patients is still an open question. In patients with liver metastases, perhaps we need to be more aggressive with the primary or tumor-control anti-HER2 therapy.”
Improving efficacy
Going forward, one strategy for improving pembrolizumab efficacy in this patient population might be priming the immune response, according to Dr. Loi.
“In HER2 disease, it’s very clear that oncogenic signaling is the driver, so targeting HER2 potently also will help relieve tumor-mediated immune suppression,” she elaborated. “In this particular context, targeting HER2 well is the key. Whether you need the addition of a little bit of chemo or some radiation, all this needs to be studied.”
Another strategy for improving pembrolizumab efficacy might be moving the drug to earlier disease settings, Dr. Loi proposed.
“By the time you get to advanced stage and have had multiple treatments, you actually have low levels of T-cell infiltration in your metastatic lesion, for whatever reasons – tumor burden, immunosuppression, multiple lines of treatment. That all reduces your chance of responding to pembrolizumab, for example, as monotherapy,” she elaborated. “We don’t know yet if chemotherapy in addition to pembrolizumab could change that tumor microenvironment. But still, I think the earlier in lines you go, the more chance you are going to have of preexisting effective antitumor immunity that can be reactivated with the addition of pembrolizumab.”
Dr. Loi disclosed that her institution receives research funding from Novartis, Pfizer, Merck, Genentech/Roche, and Puma. Merck provided study drug and support for PANACEA.
SOURCE: Loi S et al. SABCS 2017 Abstract GS2-06.
SAN ANTONIO – The immune checkpoint inhibitor pembrolizumab overcomes trastuzumab resistance in HER2-positive advanced breast cancer provided that the tumor expresses programmed death ligand 1 (PD-L1), a trial reported at the San Antonio Breast Cancer Symposium suggests. But presence of immune cells in the tumor is a major additional determinant of benefit.
The single-arm phase 1b/2 trial, called PANACEA (also KEYNOTE-014), enrolled 58 patients with HER2-positive advanced breast cancer that had progressed on trastuzumab (Herceptin) or trastuzumab emtansine (Kadcyla). All were given pembrolizumab (Keytruda), which unleashes antitumor immunity by targeting the programmed death-1 receptor on immune cells, in combination with trastuzumab.
With a median follow-up of 13.6 months, the cohort of patients having tumors positive for PD-L1 achieved an overall response rate of 15.2% and a disease control rate of 24%,” Dr. Loi reported in a press briefing and session, on behalf of the International Breast Cancer Study Group and Breast International Group. In contrast, there were no responses in the PD-L1–negative cohort.
Within the PD-L1–positive cohort, stromal levels of TILs in the metastatic lesion – which were low overall – influenced likelihood of benefit. The response rate was almost eight times higher in patients who had at least 5% of the stromal area densely infiltrated with TILs.
“The PANACEA study met its primary endpoint in the PD-L1–positive cohort. For responders, this combination offers durable control without chemotherapy,” Dr. Loi summarized.
“Metastatic HER2-positive breast cancer in this [heavily pretreated] setting is poorly immunogenic, as evidenced by the majority of patients having low TILs in their metastatic lesions. Saying that, however, we did observe a higher response rate in this study as compared to the equivalent triple-negative breast cancer studied in KEYNOTE-086,” she noted. “Future directions in this disease space should focus on combinations with effective anti-HER2 therapy, particularly in low-TIL patients.”
Predicting benefit
The trial is noteworthy for its efforts to identify the subset of patients most likely to benefit from immune checkpoint inhibition, according to press briefing moderator Virginia Kaklamani, MD, a professor of medicine in the division of hematology/oncology at the University of Texas Health Science Center, San Antonio, and a leader of the Breast Cancer Program at the UT Health San Antonio Cancer Center.
In similar studies among patients with HER2-negative breast cancer, PD-L1 did not pan out as a strong predictive biomarker. “What do you think the difference is between that subset and the HER2-positive subset?” Dr. Kaklamani asked.
“First off, I think that there are technical issues with the PD-L1 assay. And we find that patients with high TILs or immune infiltration usually have high levels of PD-L1 expression on their TILs,” Dr. Loi replied. “So I think that PD-L1 can be expressed on the tumor as well as the TIL, and it certainly seems to be the TIL infiltrate that probably enriches for responders to a PD-L1 checkpoint inhibitor on its own or in this case with trastuzumab.”
Study details
In the PANACEA trial (additionally known as IBCSG 45-13 and BIG 4-13), the most common adverse event of any grade and type with the pembrolizumab-trastuzumab combination was fatigue, seen in 21% of patients, Dr. Loi reported. For immune-related adverse events specifically, 19.0% of patients experienced an event, 10.3% experienced an event of grade 3 or worse, and 6.9% stopped treatment because of these events.
“These frequencies are consistent with what has been reported in other solid tumor types with pembrolizumab,” she commented. There were no cardiac events reported.
Efficacy analyses were restricted largely to the PD-L1–positive cohort, given the lack of any response in the negative cohort.
Median duration of response in the positive cohort was 3.5 months, and median duration of disease control was 11.1 months. Five patients (10.8%) remain on treatment with no progression; three of them have completed 2 years of pembrolizumab.
Median progression-free and overall survival were 2.7 and 16.1 months, respectively; corresponding 12-month rates were 13% and 65%. “There is a tantalizing suggestion of a tail on the curve. ... Obviously, this requires further follow-up, and the numbers are small,” Dr. Loi commented.
The median baseline stromal TIL level in metastatic lesions was just 1%. “This is 20 times less than what we observe in primary HER2-positive breast cancers,” she pointed out.
Compared with the PD-L1–negative cohort, the PD-L1–positive cohort had higher TIL levels. Additionally, within that latter cohort, TIL level was higher among patients achieving response versus not (P = .006) and patients achieving disease control versus not (P = .0006).
“We then went on to try to identify a TIL cutoff that could enrich the population for responders. This has been done in other solid tumor types,” Dr. Loi explained.
Analyses in the PD-L1-positive cohort showed that TIL levels down to 5% predicted benefit. The 41% of patients having 5% or more TILs were dramatically more likely to have a response (39% vs. 5%) and disease control (47% vs. 5%).
TIL levels varied widely according to site of the metastasis, with higher levels seen in metastases from lung and lymph nodes, and lower levels seen in those from liver and skin.
“At this stage, we are not sure which is the chicken and the egg: Patients could have disease in their lung and their lymph nodes because their immune system is better controlling their disease,” Dr. Loi commented. “How we treat these patients is still an open question. In patients with liver metastases, perhaps we need to be more aggressive with the primary or tumor-control anti-HER2 therapy.”
Improving efficacy
Going forward, one strategy for improving pembrolizumab efficacy in this patient population might be priming the immune response, according to Dr. Loi.
“In HER2 disease, it’s very clear that oncogenic signaling is the driver, so targeting HER2 potently also will help relieve tumor-mediated immune suppression,” she elaborated. “In this particular context, targeting HER2 well is the key. Whether you need the addition of a little bit of chemo or some radiation, all this needs to be studied.”
Another strategy for improving pembrolizumab efficacy might be moving the drug to earlier disease settings, Dr. Loi proposed.
“By the time you get to advanced stage and have had multiple treatments, you actually have low levels of T-cell infiltration in your metastatic lesion, for whatever reasons – tumor burden, immunosuppression, multiple lines of treatment. That all reduces your chance of responding to pembrolizumab, for example, as monotherapy,” she elaborated. “We don’t know yet if chemotherapy in addition to pembrolizumab could change that tumor microenvironment. But still, I think the earlier in lines you go, the more chance you are going to have of preexisting effective antitumor immunity that can be reactivated with the addition of pembrolizumab.”
Dr. Loi disclosed that her institution receives research funding from Novartis, Pfizer, Merck, Genentech/Roche, and Puma. Merck provided study drug and support for PANACEA.
SOURCE: Loi S et al. SABCS 2017 Abstract GS2-06.
REPORTING FROM SABCS 2017
Key clinical point:
Major finding: The PD-L1–positive cohort had an overall response rate of 15.2% and a disease control rate of 24%.
Data source: A single-arm phase 1b/2 trial among 58 women with trastuzumab-resistant HER2-positive advanced breast cancer (PANACEA study).
Disclosures: Dr. Loi disclosed that her institution receives research funding from Novartis, Pfizer, Merck, Genentech/Roche, and Puma. Merck provided study drug and support.
Source: Loi S et al. SABCS 2017 Abstract GS2-06.
Flu vaccine did not protect children with acute leukemia
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
said April Sykes of St. Jude Children’s Research Hospital in Carmel, Ind., and her associates.
Patients aged 1-21 years being treated for acute leukemia during three successive influenza seasons (2011-2012, 2012-2013, and 2013-2014) were identified by a retrospective review of EHRs; of those patients, 354 (71%) patients received TIV, and 98 (20%) received a booster dose of flu vaccine.
Also, whether the children and youth received one or two doses of flu vaccine made no difference in the rates of influenza (0.60 vs. 1.02; P = .107), the investigators reported.
These data suggest “that influenza vaccine may be ineffective in children receiving therapy for acute leukemia and that routine administration of TIV may not reflect high-value care,” the researchers said. “Until more immunogenic and protective vaccines are developed, efforts to prevent influenza in high-risk populations should focus on more general strategies, such as avoiding ill persons and practicing good respiratory hygiene in households and health care facilities.”
Read more in the Journal of Pediatrics (2017 Nov 21. doi: 10.1016/j.jpeds.2017.08.071).
FROM THE JOURNAL OF PEDIATRICS
Novel PARP inhibitor boosts PFS in HER2- breast cancer with BRCA mutations
SAN ANTONIO – In women with advanced HER2-negative breast cancer with germline BRCA mutations, an investigational oral PARP inhibitor talazoparib was associated with a near doubling in progression-free survival (PFS) when compared with single-agent chemotherapy, results of the phase 3 EMBRACA trial show.
After a median follow-up of 11.2 months, the median PFS by blinded central review – the primary endpoint – was 8.6 months for patients assigned to receive talazoparib, compared with 5.6 months for patients randomized to receive the physician’s choice of either capecitabine, eribulin, gemcitabine, or vinorelbine, reported Jennifer K. Litton, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Patients who were assigned to talazoparib had an improvement in their global health status versus patients who had deterioration when randomized.”
Talazoparib is an oral inhibitor of poly ADP-ribose polymerase (PARP) with a dual mechanism of action: It both inhibits the PARP enzyme directly and traps PARP on single-stranded DNA breaks, preventing repair of DNA damage and leading to the death of malignant cells.
In the phase 2 ABRAZO trial, the PARP inhibitor showed “encouraging” efficacy and safety in patients with germline BRCA1/BRCA2 mutations who had received platinum-based chemotherapy or at least three prior cytotoxic regimens.
Dr. Litton reported results of the EMBRACA trial, a phase 3 study in patients with locally advanced or metastatic HER2 negative breast cancer a germline BRCA1 or BRCA2 mutation. Patients were stratified by number of prior chemotherapy regimens, by having triple-negative breast cancer or hormone receptor-positive breast cancer, and by having a history of either central nervous system metastases or no CNS metastases; they were then randomized on a 2:1 basis to either oral talazoparib 1 mg daily (287 patients) or to the physician’s choice of therapy with one of the agents noted before.
The patient characteristics were generally well balanced, although there was a higher percentage of patients aged younger than 50 years in the talazoparib group than in the group treated with other agents (63.4% vs. 46.5%, respectively), slightly more CNS metastases (15% vs. 13.9%), and a higher percentage of patients with a disease-free interval (time from initial diagnosis to advanced breast cancer) shorter than 12 months (37.6% vs. 29.2%).
The primary endpoint of PFS by blinded central review showed the aforementioned significant benefit of talazoparib. A PFS by subgroup analysis showed that talazoparib was significantly better in all parameters except for patients who had previously received platinum-based therapy.
The trial was also powered to show overall survival as a secondary endpoint, but the data are not mature, Dr. Litton said. An interim OS analysis showed an apparent trend favoring the PARP inhibitor, with a median of 22.3 months, compared with 19.5 months with physician’s choice of treatment.
The 24- and 36-month probabilities of survival were 45% and 34% respectively for patients treated with talazoparib, compared with 37% and 0% for patients treated with other agents.
The objective response rate by investigator rating was 62.6% with talazoparib, compared with 27.2% for other drugs (odds ratio, 4.99; P less than .0001).
Anemia was the most common hematologic adverse event, with grade 3 or greater occurring in 39.2% of patients on the PARP inhibitor, compared with 4.8% of patients treated with other agents.
Talazoparib, unlike other PARP inhibitors, was also associated with grade 1 or 2 alopecia, which occurred in 25.2% of those patients, compared with 27.8% of those receiving the physician’s choice of treatment.
Grade 3 or 4 serious adverse events occurred in about 25.5% of patients in each study arm. Events leading to permanent drug discontinuation were more common with physician’s choice agents at 9.5%, compared with 7.7% of patients treated with talazoparib.
Kent Osborne, MD, the director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston, who moderated a briefing where Dr. Litton presented the data, commented that patients may not be as enthusiastic about the results as investigators seem to be.
“I’ve heard doctors like you and I say ‘This is really great, we’ve got some activity from a PARP inhibitor;’ patients look at it and say ‘Gee, a few more responses and a 3-month prolongation on average of my time to progression is not a very big advantage,’ ” he said to Dr. Litton.
“So what’s the next step in the development of these drugs? Are they going to be used in combinations? Are we going to come up with a mechanism of resistance that we can then overcome to extend the duration of their benefit?” he asked.
Dr. Litton replied that she was encouraged by fact that the tails of the survival curves appear to be separating and that some patients have complete responses and some have relatively durable responses.
“One of the things that we’re going to be looking at are the correlatives, trying to identify who these extraordinary responders are and the mechanisms of resistance as best we can,” she said.
This study was funded by Pfizer, which developed the inhibitor. Dr. Litton has disclosed research funding with EMD Serono, AstraZeneca, Pfizer, Genentech, and GlaxoSmithKline, and serves on advisory boards for Pfizer and AstraZeneca, all uncompensated.
SOURCE: Litton et al. SABCS 2017 Abstract GS6-07.
SAN ANTONIO – In women with advanced HER2-negative breast cancer with germline BRCA mutations, an investigational oral PARP inhibitor talazoparib was associated with a near doubling in progression-free survival (PFS) when compared with single-agent chemotherapy, results of the phase 3 EMBRACA trial show.
After a median follow-up of 11.2 months, the median PFS by blinded central review – the primary endpoint – was 8.6 months for patients assigned to receive talazoparib, compared with 5.6 months for patients randomized to receive the physician’s choice of either capecitabine, eribulin, gemcitabine, or vinorelbine, reported Jennifer K. Litton, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Patients who were assigned to talazoparib had an improvement in their global health status versus patients who had deterioration when randomized.”
Talazoparib is an oral inhibitor of poly ADP-ribose polymerase (PARP) with a dual mechanism of action: It both inhibits the PARP enzyme directly and traps PARP on single-stranded DNA breaks, preventing repair of DNA damage and leading to the death of malignant cells.
In the phase 2 ABRAZO trial, the PARP inhibitor showed “encouraging” efficacy and safety in patients with germline BRCA1/BRCA2 mutations who had received platinum-based chemotherapy or at least three prior cytotoxic regimens.
Dr. Litton reported results of the EMBRACA trial, a phase 3 study in patients with locally advanced or metastatic HER2 negative breast cancer a germline BRCA1 or BRCA2 mutation. Patients were stratified by number of prior chemotherapy regimens, by having triple-negative breast cancer or hormone receptor-positive breast cancer, and by having a history of either central nervous system metastases or no CNS metastases; they were then randomized on a 2:1 basis to either oral talazoparib 1 mg daily (287 patients) or to the physician’s choice of therapy with one of the agents noted before.
The patient characteristics were generally well balanced, although there was a higher percentage of patients aged younger than 50 years in the talazoparib group than in the group treated with other agents (63.4% vs. 46.5%, respectively), slightly more CNS metastases (15% vs. 13.9%), and a higher percentage of patients with a disease-free interval (time from initial diagnosis to advanced breast cancer) shorter than 12 months (37.6% vs. 29.2%).
The primary endpoint of PFS by blinded central review showed the aforementioned significant benefit of talazoparib. A PFS by subgroup analysis showed that talazoparib was significantly better in all parameters except for patients who had previously received platinum-based therapy.
The trial was also powered to show overall survival as a secondary endpoint, but the data are not mature, Dr. Litton said. An interim OS analysis showed an apparent trend favoring the PARP inhibitor, with a median of 22.3 months, compared with 19.5 months with physician’s choice of treatment.
The 24- and 36-month probabilities of survival were 45% and 34% respectively for patients treated with talazoparib, compared with 37% and 0% for patients treated with other agents.
The objective response rate by investigator rating was 62.6% with talazoparib, compared with 27.2% for other drugs (odds ratio, 4.99; P less than .0001).
Anemia was the most common hematologic adverse event, with grade 3 or greater occurring in 39.2% of patients on the PARP inhibitor, compared with 4.8% of patients treated with other agents.
Talazoparib, unlike other PARP inhibitors, was also associated with grade 1 or 2 alopecia, which occurred in 25.2% of those patients, compared with 27.8% of those receiving the physician’s choice of treatment.
Grade 3 or 4 serious adverse events occurred in about 25.5% of patients in each study arm. Events leading to permanent drug discontinuation were more common with physician’s choice agents at 9.5%, compared with 7.7% of patients treated with talazoparib.
Kent Osborne, MD, the director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston, who moderated a briefing where Dr. Litton presented the data, commented that patients may not be as enthusiastic about the results as investigators seem to be.
“I’ve heard doctors like you and I say ‘This is really great, we’ve got some activity from a PARP inhibitor;’ patients look at it and say ‘Gee, a few more responses and a 3-month prolongation on average of my time to progression is not a very big advantage,’ ” he said to Dr. Litton.
“So what’s the next step in the development of these drugs? Are they going to be used in combinations? Are we going to come up with a mechanism of resistance that we can then overcome to extend the duration of their benefit?” he asked.
Dr. Litton replied that she was encouraged by fact that the tails of the survival curves appear to be separating and that some patients have complete responses and some have relatively durable responses.
“One of the things that we’re going to be looking at are the correlatives, trying to identify who these extraordinary responders are and the mechanisms of resistance as best we can,” she said.
This study was funded by Pfizer, which developed the inhibitor. Dr. Litton has disclosed research funding with EMD Serono, AstraZeneca, Pfizer, Genentech, and GlaxoSmithKline, and serves on advisory boards for Pfizer and AstraZeneca, all uncompensated.
SOURCE: Litton et al. SABCS 2017 Abstract GS6-07.
SAN ANTONIO – In women with advanced HER2-negative breast cancer with germline BRCA mutations, an investigational oral PARP inhibitor talazoparib was associated with a near doubling in progression-free survival (PFS) when compared with single-agent chemotherapy, results of the phase 3 EMBRACA trial show.
After a median follow-up of 11.2 months, the median PFS by blinded central review – the primary endpoint – was 8.6 months for patients assigned to receive talazoparib, compared with 5.6 months for patients randomized to receive the physician’s choice of either capecitabine, eribulin, gemcitabine, or vinorelbine, reported Jennifer K. Litton, MD, from the University of Texas MD Anderson Cancer Center in Houston.
“Patients who were assigned to talazoparib had an improvement in their global health status versus patients who had deterioration when randomized.”
Talazoparib is an oral inhibitor of poly ADP-ribose polymerase (PARP) with a dual mechanism of action: It both inhibits the PARP enzyme directly and traps PARP on single-stranded DNA breaks, preventing repair of DNA damage and leading to the death of malignant cells.
In the phase 2 ABRAZO trial, the PARP inhibitor showed “encouraging” efficacy and safety in patients with germline BRCA1/BRCA2 mutations who had received platinum-based chemotherapy or at least three prior cytotoxic regimens.
Dr. Litton reported results of the EMBRACA trial, a phase 3 study in patients with locally advanced or metastatic HER2 negative breast cancer a germline BRCA1 or BRCA2 mutation. Patients were stratified by number of prior chemotherapy regimens, by having triple-negative breast cancer or hormone receptor-positive breast cancer, and by having a history of either central nervous system metastases or no CNS metastases; they were then randomized on a 2:1 basis to either oral talazoparib 1 mg daily (287 patients) or to the physician’s choice of therapy with one of the agents noted before.
The patient characteristics were generally well balanced, although there was a higher percentage of patients aged younger than 50 years in the talazoparib group than in the group treated with other agents (63.4% vs. 46.5%, respectively), slightly more CNS metastases (15% vs. 13.9%), and a higher percentage of patients with a disease-free interval (time from initial diagnosis to advanced breast cancer) shorter than 12 months (37.6% vs. 29.2%).
The primary endpoint of PFS by blinded central review showed the aforementioned significant benefit of talazoparib. A PFS by subgroup analysis showed that talazoparib was significantly better in all parameters except for patients who had previously received platinum-based therapy.
The trial was also powered to show overall survival as a secondary endpoint, but the data are not mature, Dr. Litton said. An interim OS analysis showed an apparent trend favoring the PARP inhibitor, with a median of 22.3 months, compared with 19.5 months with physician’s choice of treatment.
The 24- and 36-month probabilities of survival were 45% and 34% respectively for patients treated with talazoparib, compared with 37% and 0% for patients treated with other agents.
The objective response rate by investigator rating was 62.6% with talazoparib, compared with 27.2% for other drugs (odds ratio, 4.99; P less than .0001).
Anemia was the most common hematologic adverse event, with grade 3 or greater occurring in 39.2% of patients on the PARP inhibitor, compared with 4.8% of patients treated with other agents.
Talazoparib, unlike other PARP inhibitors, was also associated with grade 1 or 2 alopecia, which occurred in 25.2% of those patients, compared with 27.8% of those receiving the physician’s choice of treatment.
Grade 3 or 4 serious adverse events occurred in about 25.5% of patients in each study arm. Events leading to permanent drug discontinuation were more common with physician’s choice agents at 9.5%, compared with 7.7% of patients treated with talazoparib.
Kent Osborne, MD, the director of the Dan L. Duncan Cancer Center at Baylor College of Medicine, Houston, who moderated a briefing where Dr. Litton presented the data, commented that patients may not be as enthusiastic about the results as investigators seem to be.
“I’ve heard doctors like you and I say ‘This is really great, we’ve got some activity from a PARP inhibitor;’ patients look at it and say ‘Gee, a few more responses and a 3-month prolongation on average of my time to progression is not a very big advantage,’ ” he said to Dr. Litton.
“So what’s the next step in the development of these drugs? Are they going to be used in combinations? Are we going to come up with a mechanism of resistance that we can then overcome to extend the duration of their benefit?” he asked.
Dr. Litton replied that she was encouraged by fact that the tails of the survival curves appear to be separating and that some patients have complete responses and some have relatively durable responses.
“One of the things that we’re going to be looking at are the correlatives, trying to identify who these extraordinary responders are and the mechanisms of resistance as best we can,” she said.
This study was funded by Pfizer, which developed the inhibitor. Dr. Litton has disclosed research funding with EMD Serono, AstraZeneca, Pfizer, Genentech, and GlaxoSmithKline, and serves on advisory boards for Pfizer and AstraZeneca, all uncompensated.
SOURCE: Litton et al. SABCS 2017 Abstract GS6-07.
REPORTING FROM SABCS 2017
Key clinical point: The investigational PARP inhibitor talazoparib extended progression-free survival of advanced HER2-negative breast cancer with germline BRCA mutations.
Major finding: Talazoparib was associated with a 46% reduction in risk for progression when compared with standard single agent therapies.
Data source: Randomized clinical trial in 431 patients with advanced, previously treated breast cancer with germline BRCA1 and BRCA2 mutations.
Disclosures: This study was funded by Pfizer, which developed the inhibitor. Dr. Litton disclosed that she has received research funding from EMD Serono, AstraZeneca, Pfizer, Genentech, and GlaxoSmithKline and that she serves on advisory boards for Pfizer and AstraZeneca, all uncompensated.
Source: Litton J et al. SABCS 2017 Abstract GS6-07.
Extra years of adjuvant bisphosphonate not needed in early breast cancer
SAN ANTONIO – When it comes to adjuvant bisphosphonate therapy following adjuvant chemotherapy for high-risk early breast cancer, more is not better than less, phase 3 data from the randomized SUCCESS A study suggest.
Among 3,421 patients randomized to adjuvant bisphosphonate therapy following chemotherapy, there was barely a speck of difference in either disease-free survival (DFS) or overall survival (OS) between patients randomized to either 2 years or 5 years of adjuvant bisphosphonate therapy with zoledronate, reported Wolfgang Janni, MD, from University Hospital Ulm (Germany).
“We conclude 5 years of adjuvant zoledronate treatment should not be considered currently in these patients in the absence of decreased bone density,” he said at the San Antonio Breast Cancer Symposium.
Adjuvant bisphosphonate therapy in patients with early breast cancer is associated with improved breast cancer–specific survival and reduced rates of breast cancer recurrence in bone, especially for postmenopausal patients, as shown in a meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, Dr. Janni noted.
German breast cancer guidelines state that postmenopausal women should be offered bisphosphonates as part of their adjuvant systemic therapy, but the optimal duration of therapy is uncertain, prompting the investigators to examine the issue in a randomized trial.
SUCCESS A was a multicenter, phase 3, randomized trial with a multifactorial 2 x 2 design, in patients with high-risk node-negative and node-positive disease. Patients were randomized to FEC100 chemotherapy followed by docetaxel with or without gemcitabine. Chemotherapy was followed by endocrine therapy with 2 years of tamoxifen followed by 3 years of anastrozole (Arimidex). At the start of endocrine therapy, patients were further randomized to receive either 2 or 5 years of adjuvant zoledronate, 4 mg intravenously every 3 months for 2 years, or the same schedule over 2 years, followed by 4 mg every 6 months for 3 years.
A total of 2,987 of the 3,421 patients randomized to a zoledronate schedule were available for inclusion in the analysis.
As noted, adapted DFS and OS, measured starting from 2 years after the start of zoledronate with a maximum observation time of 48 months, were virtually identical between the two treatment groups, with respective P values of .827 and .713. Similarly, in a multivariate regression analysis model adjusted for age, body mass index, menopausal status, tumor size, nodal stage, histological grade and type, hormone receptor status, HER2 status, surgery type, and chemotherapy regimen, the hazard ratio for 5 vs. 2 years was 0.97 for DFS and 0.98 for OS. Neither endpoint was significantly different between the groups.
Similarly, there was no significant differences in the number of bone recurrences as first distant recurrences or in premenopausal vs. postmenopausal women.
Adverse events of any grade were significantly higher with 5 years of bisphosphonate therapy (46.2% vs. 27.2%, P less than .001), including significantly higher grade 3 or greater adverse events (7.6% vs. 5.1%, P = .006).
Following presentation of the data in an oral session, moderator Sibylle Loibl, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, questioned whether the follow-up was long enough to detect a clinically meaningful difference.
“The negative result of this study might be due to the small observation time,” Dr. Janni conceded.”We have a quite intensive drug regimen for the first 2 years, so this might also be a contributing factor [as to why] we did not see any difference.”
The SUCCESS A study was supported by AstraZeneca, Chugai, Janssen Diagnostics, Lilly, Novartis, and Sanofi-Aventis. Dr. Janni has reported financial relationships with AstraZeneca, Chugai, Janssen, Lilly, Novartis, and Sanofi.
SOURCE: Janni et al. SABCS 2017 Abstract GS1-06
SAN ANTONIO – When it comes to adjuvant bisphosphonate therapy following adjuvant chemotherapy for high-risk early breast cancer, more is not better than less, phase 3 data from the randomized SUCCESS A study suggest.
Among 3,421 patients randomized to adjuvant bisphosphonate therapy following chemotherapy, there was barely a speck of difference in either disease-free survival (DFS) or overall survival (OS) between patients randomized to either 2 years or 5 years of adjuvant bisphosphonate therapy with zoledronate, reported Wolfgang Janni, MD, from University Hospital Ulm (Germany).
“We conclude 5 years of adjuvant zoledronate treatment should not be considered currently in these patients in the absence of decreased bone density,” he said at the San Antonio Breast Cancer Symposium.
Adjuvant bisphosphonate therapy in patients with early breast cancer is associated with improved breast cancer–specific survival and reduced rates of breast cancer recurrence in bone, especially for postmenopausal patients, as shown in a meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, Dr. Janni noted.
German breast cancer guidelines state that postmenopausal women should be offered bisphosphonates as part of their adjuvant systemic therapy, but the optimal duration of therapy is uncertain, prompting the investigators to examine the issue in a randomized trial.
SUCCESS A was a multicenter, phase 3, randomized trial with a multifactorial 2 x 2 design, in patients with high-risk node-negative and node-positive disease. Patients were randomized to FEC100 chemotherapy followed by docetaxel with or without gemcitabine. Chemotherapy was followed by endocrine therapy with 2 years of tamoxifen followed by 3 years of anastrozole (Arimidex). At the start of endocrine therapy, patients were further randomized to receive either 2 or 5 years of adjuvant zoledronate, 4 mg intravenously every 3 months for 2 years, or the same schedule over 2 years, followed by 4 mg every 6 months for 3 years.
A total of 2,987 of the 3,421 patients randomized to a zoledronate schedule were available for inclusion in the analysis.
As noted, adapted DFS and OS, measured starting from 2 years after the start of zoledronate with a maximum observation time of 48 months, were virtually identical between the two treatment groups, with respective P values of .827 and .713. Similarly, in a multivariate regression analysis model adjusted for age, body mass index, menopausal status, tumor size, nodal stage, histological grade and type, hormone receptor status, HER2 status, surgery type, and chemotherapy regimen, the hazard ratio for 5 vs. 2 years was 0.97 for DFS and 0.98 for OS. Neither endpoint was significantly different between the groups.
Similarly, there was no significant differences in the number of bone recurrences as first distant recurrences or in premenopausal vs. postmenopausal women.
Adverse events of any grade were significantly higher with 5 years of bisphosphonate therapy (46.2% vs. 27.2%, P less than .001), including significantly higher grade 3 or greater adverse events (7.6% vs. 5.1%, P = .006).
Following presentation of the data in an oral session, moderator Sibylle Loibl, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, questioned whether the follow-up was long enough to detect a clinically meaningful difference.
“The negative result of this study might be due to the small observation time,” Dr. Janni conceded.”We have a quite intensive drug regimen for the first 2 years, so this might also be a contributing factor [as to why] we did not see any difference.”
The SUCCESS A study was supported by AstraZeneca, Chugai, Janssen Diagnostics, Lilly, Novartis, and Sanofi-Aventis. Dr. Janni has reported financial relationships with AstraZeneca, Chugai, Janssen, Lilly, Novartis, and Sanofi.
SOURCE: Janni et al. SABCS 2017 Abstract GS1-06
SAN ANTONIO – When it comes to adjuvant bisphosphonate therapy following adjuvant chemotherapy for high-risk early breast cancer, more is not better than less, phase 3 data from the randomized SUCCESS A study suggest.
Among 3,421 patients randomized to adjuvant bisphosphonate therapy following chemotherapy, there was barely a speck of difference in either disease-free survival (DFS) or overall survival (OS) between patients randomized to either 2 years or 5 years of adjuvant bisphosphonate therapy with zoledronate, reported Wolfgang Janni, MD, from University Hospital Ulm (Germany).
“We conclude 5 years of adjuvant zoledronate treatment should not be considered currently in these patients in the absence of decreased bone density,” he said at the San Antonio Breast Cancer Symposium.
Adjuvant bisphosphonate therapy in patients with early breast cancer is associated with improved breast cancer–specific survival and reduced rates of breast cancer recurrence in bone, especially for postmenopausal patients, as shown in a meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group, Dr. Janni noted.
German breast cancer guidelines state that postmenopausal women should be offered bisphosphonates as part of their adjuvant systemic therapy, but the optimal duration of therapy is uncertain, prompting the investigators to examine the issue in a randomized trial.
SUCCESS A was a multicenter, phase 3, randomized trial with a multifactorial 2 x 2 design, in patients with high-risk node-negative and node-positive disease. Patients were randomized to FEC100 chemotherapy followed by docetaxel with or without gemcitabine. Chemotherapy was followed by endocrine therapy with 2 years of tamoxifen followed by 3 years of anastrozole (Arimidex). At the start of endocrine therapy, patients were further randomized to receive either 2 or 5 years of adjuvant zoledronate, 4 mg intravenously every 3 months for 2 years, or the same schedule over 2 years, followed by 4 mg every 6 months for 3 years.
A total of 2,987 of the 3,421 patients randomized to a zoledronate schedule were available for inclusion in the analysis.
As noted, adapted DFS and OS, measured starting from 2 years after the start of zoledronate with a maximum observation time of 48 months, were virtually identical between the two treatment groups, with respective P values of .827 and .713. Similarly, in a multivariate regression analysis model adjusted for age, body mass index, menopausal status, tumor size, nodal stage, histological grade and type, hormone receptor status, HER2 status, surgery type, and chemotherapy regimen, the hazard ratio for 5 vs. 2 years was 0.97 for DFS and 0.98 for OS. Neither endpoint was significantly different between the groups.
Similarly, there was no significant differences in the number of bone recurrences as first distant recurrences or in premenopausal vs. postmenopausal women.
Adverse events of any grade were significantly higher with 5 years of bisphosphonate therapy (46.2% vs. 27.2%, P less than .001), including significantly higher grade 3 or greater adverse events (7.6% vs. 5.1%, P = .006).
Following presentation of the data in an oral session, moderator Sibylle Loibl, MD, PhD, of the German Breast Group in Neu-Isenburg, Germany, questioned whether the follow-up was long enough to detect a clinically meaningful difference.
“The negative result of this study might be due to the small observation time,” Dr. Janni conceded.”We have a quite intensive drug regimen for the first 2 years, so this might also be a contributing factor [as to why] we did not see any difference.”
The SUCCESS A study was supported by AstraZeneca, Chugai, Janssen Diagnostics, Lilly, Novartis, and Sanofi-Aventis. Dr. Janni has reported financial relationships with AstraZeneca, Chugai, Janssen, Lilly, Novartis, and Sanofi.
SOURCE: Janni et al. SABCS 2017 Abstract GS1-06
REPORTING FROM SABCS 2017
Key clinical point: Five years of adjuvant bisphosphonate therapy offered no survival advantages over 2 years of therapy for women with early breast cancers.
Major finding: Neither adapted disease-free survival nor overall survival were significantly better with 3 extra years of zoledronate therapy.
Data source: Randomized phase 3 trial.
Disclosures: The SUCCESS A study was supported by AstraZeneca, Chugai, Janssen Diagnostics, Lilly, Novartis, and Sanofi-Aventis. Dr. Janni has reported financial relationships with AstraZeneca, Chugai, Janssen, Lilly, Novartis, and Sanofi.
Source: Janni et al., SABCS 2017 abstract GS1-06
Make the diagnosis - January 2018
Cutaneous lupus erythematosus can be classified into acute, subacute, and chronic lesions. Chronic cutaneous lupus, or discoid lupus erythematosus (DLE), may occur independently of or in combination with systemic lupus erythematosus (SLE). They are one of the more common skin presentations seen in lupus. Young adults are typically affected, with a female-to-male ratio of 2:1. Progression from DLE to SLE is uncommon. However, patients with SLE will frequently develop discoid lesions.
The differential diagnosis includes: subacute cutaneous lupus, lichen planus, seborrheic dermatitis, Jessner’s lymphocytic infiltrate, polymorphous light eruption, rosacea, granuloma faciale, and sarcoidosis. Histology of DLE may reveal hyperkeratosis, a thin epidermis with effacement of the rete ridges, a lichenoid and vacuolar interface dermatitis, and follicular plugging. Damaged keratinocytes called colloid bodies may be present. Increased mucin and thickening of the basement membrane are commonly seen. Active lesions will exhibit more of an inflammatory infiltrate. Direct immunofluorescence of lesional skin is positive in more than 75% of cases.
This case and the photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Cutaneous lupus erythematosus can be classified into acute, subacute, and chronic lesions. Chronic cutaneous lupus, or discoid lupus erythematosus (DLE), may occur independently of or in combination with systemic lupus erythematosus (SLE). They are one of the more common skin presentations seen in lupus. Young adults are typically affected, with a female-to-male ratio of 2:1. Progression from DLE to SLE is uncommon. However, patients with SLE will frequently develop discoid lesions.
The differential diagnosis includes: subacute cutaneous lupus, lichen planus, seborrheic dermatitis, Jessner’s lymphocytic infiltrate, polymorphous light eruption, rosacea, granuloma faciale, and sarcoidosis. Histology of DLE may reveal hyperkeratosis, a thin epidermis with effacement of the rete ridges, a lichenoid and vacuolar interface dermatitis, and follicular plugging. Damaged keratinocytes called colloid bodies may be present. Increased mucin and thickening of the basement membrane are commonly seen. Active lesions will exhibit more of an inflammatory infiltrate. Direct immunofluorescence of lesional skin is positive in more than 75% of cases.
This case and the photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
Cutaneous lupus erythematosus can be classified into acute, subacute, and chronic lesions. Chronic cutaneous lupus, or discoid lupus erythematosus (DLE), may occur independently of or in combination with systemic lupus erythematosus (SLE). They are one of the more common skin presentations seen in lupus. Young adults are typically affected, with a female-to-male ratio of 2:1. Progression from DLE to SLE is uncommon. However, patients with SLE will frequently develop discoid lesions.
The differential diagnosis includes: subacute cutaneous lupus, lichen planus, seborrheic dermatitis, Jessner’s lymphocytic infiltrate, polymorphous light eruption, rosacea, granuloma faciale, and sarcoidosis. Histology of DLE may reveal hyperkeratosis, a thin epidermis with effacement of the rete ridges, a lichenoid and vacuolar interface dermatitis, and follicular plugging. Damaged keratinocytes called colloid bodies may be present. Increased mucin and thickening of the basement membrane are commonly seen. Active lesions will exhibit more of an inflammatory infiltrate. Direct immunofluorescence of lesional skin is positive in more than 75% of cases.
This case and the photo were submitted by Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at edermatologynews.com. To submit a case for possible publication, send an email to dermnews@frontlinemedcom.com.
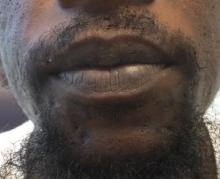
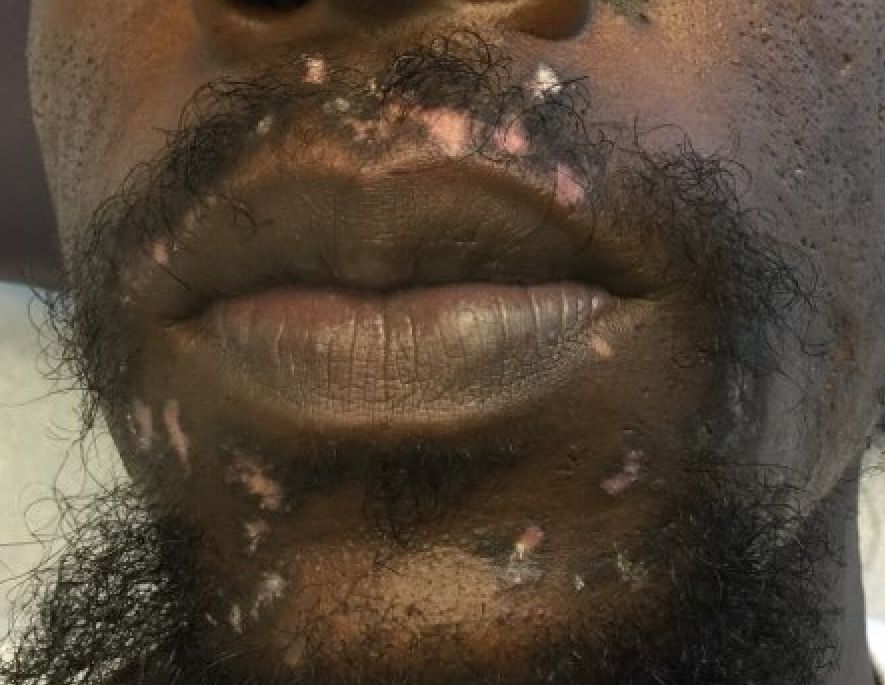
A 32-year-old male with no significant past medical history presented with a 2-year history of asymptomatic perioral lesions. On physical examination, multiple erythematous to hypopigmented atrophic plaques with peripheral hyperpigmentation were present.
High-intensity treadmill workouts preserved motor function in early-stage Parkinson’s
Six months of high-intensity treadmill walking largely preserved motor function in patients with recently diagnosed Parkinson’s disease, a phase 2 study has determined.
Patients randomized to the high-intensity exercise arm stayed very close to their baseline motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS), while the scores of those randomized to a wait list increased a mean of 3 points over 24 weeks. The scores of patients randomized to an intermediate-intensity treadmill workout increased 2 points over the study, which was not significantly different than the increase seen in the control group, Margaret Schenkman, PhD, and her colleagues wrote Dec. 11 in JAMA Neurology.
“A larger efficacy trial is warranted to determine whether exercising at 80%-85% maximum heart rate produces meaningful clinical benefits in de novo Parkinson disease,” wrote Dr. Schenkman and her colleagues. “Meanwhile, clinicians may safely prescribe exercise at this intensity level for this population.”
The Study in Parkinson Disease of Exercise (SPARX) randomized 128 patients with newly diagnosed Parkinson’s to 30-minute treadmill workouts, four times weekly, at either 80%-85% or 60%-65% maximum heart rate. They were compared against a control group of wait-listed patients.
Subjects were a mean of 64 years old and had been diagnosed for about 3-4 months before enrolling. Their mean total UPDRS score was 23.
Both active arms hit the gym a mean of 3 days/week and were able to exercise at their target heart rates, confirming the feasibility of treadmill workouts for this patient population.
The mean change in UPDRS motor score in the high-intensity group was an increase of 0.3 at 24 weeks, compared with an increase of 3.2 in the usual care group – a statistically significant difference. The mean change in the moderate-intensity group was an increase of 2.0, a nonsignificant difference.
Adverse events consisted largely of falls and musculoskeletal pain. There were 6 falls in the high-intensity group, 5 in the moderate-intensity group, and 11 in the control group. Of these falls, one in the high-intensity and one in the moderate-intensity group were considered serious.
SPARX was largely funded by a grant from the National Institute of Neurological Disorders and Stroke. None of the investigators reported having any financial conflicts.
SOURCE: Schenkman M et al. JAMA Neurol. 2017 Dec 11. doi: 10.1001/jamaneurol.2017.3517
Six months of high-intensity treadmill walking largely preserved motor function in patients with recently diagnosed Parkinson’s disease, a phase 2 study has determined.
Patients randomized to the high-intensity exercise arm stayed very close to their baseline motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS), while the scores of those randomized to a wait list increased a mean of 3 points over 24 weeks. The scores of patients randomized to an intermediate-intensity treadmill workout increased 2 points over the study, which was not significantly different than the increase seen in the control group, Margaret Schenkman, PhD, and her colleagues wrote Dec. 11 in JAMA Neurology.
“A larger efficacy trial is warranted to determine whether exercising at 80%-85% maximum heart rate produces meaningful clinical benefits in de novo Parkinson disease,” wrote Dr. Schenkman and her colleagues. “Meanwhile, clinicians may safely prescribe exercise at this intensity level for this population.”
The Study in Parkinson Disease of Exercise (SPARX) randomized 128 patients with newly diagnosed Parkinson’s to 30-minute treadmill workouts, four times weekly, at either 80%-85% or 60%-65% maximum heart rate. They were compared against a control group of wait-listed patients.
Subjects were a mean of 64 years old and had been diagnosed for about 3-4 months before enrolling. Their mean total UPDRS score was 23.
Both active arms hit the gym a mean of 3 days/week and were able to exercise at their target heart rates, confirming the feasibility of treadmill workouts for this patient population.
The mean change in UPDRS motor score in the high-intensity group was an increase of 0.3 at 24 weeks, compared with an increase of 3.2 in the usual care group – a statistically significant difference. The mean change in the moderate-intensity group was an increase of 2.0, a nonsignificant difference.
Adverse events consisted largely of falls and musculoskeletal pain. There were 6 falls in the high-intensity group, 5 in the moderate-intensity group, and 11 in the control group. Of these falls, one in the high-intensity and one in the moderate-intensity group were considered serious.
SPARX was largely funded by a grant from the National Institute of Neurological Disorders and Stroke. None of the investigators reported having any financial conflicts.
SOURCE: Schenkman M et al. JAMA Neurol. 2017 Dec 11. doi: 10.1001/jamaneurol.2017.3517
Six months of high-intensity treadmill walking largely preserved motor function in patients with recently diagnosed Parkinson’s disease, a phase 2 study has determined.
Patients randomized to the high-intensity exercise arm stayed very close to their baseline motor scores on the Unified Parkinson’s Disease Rating Scale (UPDRS), while the scores of those randomized to a wait list increased a mean of 3 points over 24 weeks. The scores of patients randomized to an intermediate-intensity treadmill workout increased 2 points over the study, which was not significantly different than the increase seen in the control group, Margaret Schenkman, PhD, and her colleagues wrote Dec. 11 in JAMA Neurology.
“A larger efficacy trial is warranted to determine whether exercising at 80%-85% maximum heart rate produces meaningful clinical benefits in de novo Parkinson disease,” wrote Dr. Schenkman and her colleagues. “Meanwhile, clinicians may safely prescribe exercise at this intensity level for this population.”
The Study in Parkinson Disease of Exercise (SPARX) randomized 128 patients with newly diagnosed Parkinson’s to 30-minute treadmill workouts, four times weekly, at either 80%-85% or 60%-65% maximum heart rate. They were compared against a control group of wait-listed patients.
Subjects were a mean of 64 years old and had been diagnosed for about 3-4 months before enrolling. Their mean total UPDRS score was 23.
Both active arms hit the gym a mean of 3 days/week and were able to exercise at their target heart rates, confirming the feasibility of treadmill workouts for this patient population.
The mean change in UPDRS motor score in the high-intensity group was an increase of 0.3 at 24 weeks, compared with an increase of 3.2 in the usual care group – a statistically significant difference. The mean change in the moderate-intensity group was an increase of 2.0, a nonsignificant difference.
Adverse events consisted largely of falls and musculoskeletal pain. There were 6 falls in the high-intensity group, 5 in the moderate-intensity group, and 11 in the control group. Of these falls, one in the high-intensity and one in the moderate-intensity group were considered serious.
SPARX was largely funded by a grant from the National Institute of Neurological Disorders and Stroke. None of the investigators reported having any financial conflicts.
SOURCE: Schenkman M et al. JAMA Neurol. 2017 Dec 11. doi: 10.1001/jamaneurol.2017.3517
FROM JAMA NEUROLOGY
Key clinical point:
Major finding: The high-intensity exercisers had a mean increase of 0.3 points on the UPDRS motor scale, compared with a 3.2-point increase in the control group.
Study details: The study randomized 128 patients to high-intensity or moderate-intensity treadmill exercise or to a wait list.
Disclosures: SPARX was largely funded by a grant from the National Institute of Neurological Disorders and Stroke. None of the investigators reported having any financial conflicts.
Source: Schenkman M et al. JAMA Neurol. 2017 Dec 11. doi: 10.1001/jamaneurol.2017.3517
Interleukin-23 inhibition for psoriasis shows ‘wow’ factor
GENEVA – The merits of addressing interleukin-23 as a novel therapeutic target in moderate to severe plaque psoriasis were abundantly displayed in 2-year outcomes data for two anti–IL-23 monoclonal antibodies – guselkumab and tildrakizumab – in studies presented back to back at the annual congress of the European Academy of Dermatology and Venereology.
These long-term, open-label extensions of previously reported phase 3, randomized, double-blind clinical trials provided evidence of multiple advantages for IL-23 inhibition. The story was similar for both agents: After 2 years of use in the extension studies, the two biologics demonstrated stellar treatment response rates that would have been unimaginable only a few years ago, maintenance of efficacy without drop-off over time, exceedingly low dropout rates, and a safety picture that remains reassuring as experience accumulates. Also, the subcutaneously administered IL-23 inhibitors are attractive from a patient convenience standpoint in that maintenance guselkumab is dosed at 100 mg once every 8 weeks, and tildrakizumab is given once every 12 weeks.
Still, there are differences between the two drugs, most notably in apparent effectiveness. While more than half of guselkumab-treated patients had a Psoriasis Area Severity Index (PASI) 100 response – that is, totally clear skin – at 2 years, that was the case for only one-quarter to one-third of patients on tildrakizumab.
Guselkumab (Tremfya) was approved by the Food and Drug Administration in July 2017 for treatment of adults with moderate to severe plaque psoriasis. Tildrakizumab remains investigational.
Guselkumab
The 2-year, open-label extension of the phase 3 VOYAGE 1 trial included 735 patients who were either on guselkumab continuously, crossed from adalimumab (Humira) to guselkumab after 48 weeks, or switched from placebo after 16 weeks.
PASI 100 rates at 2 years were 49%-55% in the three patient groups. Of the patients in these groups, 54%-59% achieved an Investigator’s Global Assessment (IGA) score of 0. IGA scores of 0 or 1, meaning clear or almost clear skin, were present in 82%-85% of patients at 2 years.
“Dropout rate is an important consideration in long-term studies,” observed Dr. Blauvelt, a dermatologist and president of Oregon Medical Research Center in Portland. “For patients on continuous guselkumab there was a 6% dropout rate in the first year and 6% in the second year, so 88% of patients that started guselkumab were still on guselkumab 2 years later. That’s impressive. In the other two groups, the dropout rate was 2% per year.”
A Dermatology Life Quality Index (DLQI) score of 0 or 1, meaning no disease effects on quality of life, was recorded in 62.5% of the continuous guselkumab group at 48 weeks and 71.1% at 2 years.
“The interesting thing here is that, even though the efficacy numbers are fairly constant between year 1 and year 2, the DLQI goes up and up. Surprising? Maybe not. I think it shows patients are getting happier and happier over time with their disease control,” Dr. Blauvelt continued.
Rates of serious adverse events remained low and stable, with no negative surprises during year 2. The serious infection rate was 1.02 cases/100 patient-years in year 1 and 0.84 cases/100 patient-years in year 2. No cases of tuberculosis, opportunistic infections, or serious hypersensitivity reactions occurred during 2 years of treatment.
Tildrakizumab
Two-year results from the ongoing 5-year extension of the phase 3 reSURFACE 1 and reSURFACE 2 trials were presented by Kim A. Papp, MD, PhD, president of Probity Medical Research, Waterloo, Ont. This presentation of 2-year outcomes for 1,237 study participants was a feat, considering that the 12-week results of the trials had been published less than 3 months earlier (Lancet. 2017 Jul 15;390[10091]:276-88).
“I think these data are very compelling that the loss of response over time is minimal,” according to the dermatologist. “We’ve also seen that safety over 2 years has no surprises; in fact, it’s remarkably quiet. The rate of severe infections, which is important to look at for any treatment suppressing the immune system, is low and occurs almost independent of dose, which is very hopeful. It’s a promising sign.”
Indeed, the serious infection rate was 0.8 cases/100 patient-years regardless of whether subjects were on tildrakizumab at 100 mg or 200 mg.
Controversy over how to report long-term outcomes
A hot topic among clinical trialists in dermatology concerns how to report study results. The traditional method in studies funded by pharmaceutical companies is known as the “last observation carried forward” analysis. It casts the study drug results in the most favorable possible light because, when a subject drops out of a trial for any reason, their last measured value for response to treatment is carried forward as though the patient completed the study. Thus, psoriasis patients who drop out because they couldn’t tolerate a therapy or developed a serious side effect dictating discontinuation will be scored on the basis of their last PASI response, creating a bias in favor of active treatment.
A more conservative analytic method is known as the “nonresponder imputation” analysis. By this method, a patient who drops out of a trial is automatically categorized as a treatment failure, even if the reason was that the patient moved and could no longer make visits to the study center.
The prespecified guselkumab analysis presented by Dr. Blauvelt involved nonresponder imputation through year 1 and imputation based on the reason for discontinuation in the second year. In contrast, the 2-year tildrakizumab analysis presented by Dr. Papp used the far more common last observation carried forward method.
To help the audience appreciate the importance of looking at the analytic methods used in a studies and help them understand the clinical significance of the results, Dr. Blauvelt provided a reanalysis of the 2-year guselkumab data using the last observation carried forward method. Across the board, the numbers became more favorable. For example, the PASI 75 rate of 95.7% using the prespecified nonresponder imputation analysis crept up to 96.8% under the last observation carried forward method; for comparison, the PASI 75 rates were 81%-84% in the tildrakizumab analysis.
“If you wanted to compare apples to apples with some other drugs, you would use these numbers – the as-observed analysis numbers used by most other companies with other drugs. If you wanted to determine what the true-life numbers are, they’d probably be something between the nonresponder imputation and as-observed numbers,” said to Dr. Blauvelt.
Dr. Papp was untroubled by the use of the last observation carried forward method in the particular case of the tildrakizumab long-term extension study.
“There is reason to believe the as-observed analysis doesn’t affect the integrity of the data because the dropout rate is extraordinarily low,” he said.
The guselkumab analysis was sponsored by Janssen Pharmaceutica; the tildrakizumab analysis was sponsored by Merck and by Sun Pharma. Dr. Blauvelt and Dr. Papp were paid investigators in both studies and serve as scientific advisers to virtually all companies invested in the psoriasis therapy developmental pipeline.
GENEVA – The merits of addressing interleukin-23 as a novel therapeutic target in moderate to severe plaque psoriasis were abundantly displayed in 2-year outcomes data for two anti–IL-23 monoclonal antibodies – guselkumab and tildrakizumab – in studies presented back to back at the annual congress of the European Academy of Dermatology and Venereology.
These long-term, open-label extensions of previously reported phase 3, randomized, double-blind clinical trials provided evidence of multiple advantages for IL-23 inhibition. The story was similar for both agents: After 2 years of use in the extension studies, the two biologics demonstrated stellar treatment response rates that would have been unimaginable only a few years ago, maintenance of efficacy without drop-off over time, exceedingly low dropout rates, and a safety picture that remains reassuring as experience accumulates. Also, the subcutaneously administered IL-23 inhibitors are attractive from a patient convenience standpoint in that maintenance guselkumab is dosed at 100 mg once every 8 weeks, and tildrakizumab is given once every 12 weeks.
Still, there are differences between the two drugs, most notably in apparent effectiveness. While more than half of guselkumab-treated patients had a Psoriasis Area Severity Index (PASI) 100 response – that is, totally clear skin – at 2 years, that was the case for only one-quarter to one-third of patients on tildrakizumab.
Guselkumab (Tremfya) was approved by the Food and Drug Administration in July 2017 for treatment of adults with moderate to severe plaque psoriasis. Tildrakizumab remains investigational.
Guselkumab
The 2-year, open-label extension of the phase 3 VOYAGE 1 trial included 735 patients who were either on guselkumab continuously, crossed from adalimumab (Humira) to guselkumab after 48 weeks, or switched from placebo after 16 weeks.
PASI 100 rates at 2 years were 49%-55% in the three patient groups. Of the patients in these groups, 54%-59% achieved an Investigator’s Global Assessment (IGA) score of 0. IGA scores of 0 or 1, meaning clear or almost clear skin, were present in 82%-85% of patients at 2 years.
“Dropout rate is an important consideration in long-term studies,” observed Dr. Blauvelt, a dermatologist and president of Oregon Medical Research Center in Portland. “For patients on continuous guselkumab there was a 6% dropout rate in the first year and 6% in the second year, so 88% of patients that started guselkumab were still on guselkumab 2 years later. That’s impressive. In the other two groups, the dropout rate was 2% per year.”
A Dermatology Life Quality Index (DLQI) score of 0 or 1, meaning no disease effects on quality of life, was recorded in 62.5% of the continuous guselkumab group at 48 weeks and 71.1% at 2 years.
“The interesting thing here is that, even though the efficacy numbers are fairly constant between year 1 and year 2, the DLQI goes up and up. Surprising? Maybe not. I think it shows patients are getting happier and happier over time with their disease control,” Dr. Blauvelt continued.
Rates of serious adverse events remained low and stable, with no negative surprises during year 2. The serious infection rate was 1.02 cases/100 patient-years in year 1 and 0.84 cases/100 patient-years in year 2. No cases of tuberculosis, opportunistic infections, or serious hypersensitivity reactions occurred during 2 years of treatment.
Tildrakizumab
Two-year results from the ongoing 5-year extension of the phase 3 reSURFACE 1 and reSURFACE 2 trials were presented by Kim A. Papp, MD, PhD, president of Probity Medical Research, Waterloo, Ont. This presentation of 2-year outcomes for 1,237 study participants was a feat, considering that the 12-week results of the trials had been published less than 3 months earlier (Lancet. 2017 Jul 15;390[10091]:276-88).
“I think these data are very compelling that the loss of response over time is minimal,” according to the dermatologist. “We’ve also seen that safety over 2 years has no surprises; in fact, it’s remarkably quiet. The rate of severe infections, which is important to look at for any treatment suppressing the immune system, is low and occurs almost independent of dose, which is very hopeful. It’s a promising sign.”
Indeed, the serious infection rate was 0.8 cases/100 patient-years regardless of whether subjects were on tildrakizumab at 100 mg or 200 mg.
Controversy over how to report long-term outcomes
A hot topic among clinical trialists in dermatology concerns how to report study results. The traditional method in studies funded by pharmaceutical companies is known as the “last observation carried forward” analysis. It casts the study drug results in the most favorable possible light because, when a subject drops out of a trial for any reason, their last measured value for response to treatment is carried forward as though the patient completed the study. Thus, psoriasis patients who drop out because they couldn’t tolerate a therapy or developed a serious side effect dictating discontinuation will be scored on the basis of their last PASI response, creating a bias in favor of active treatment.
A more conservative analytic method is known as the “nonresponder imputation” analysis. By this method, a patient who drops out of a trial is automatically categorized as a treatment failure, even if the reason was that the patient moved and could no longer make visits to the study center.
The prespecified guselkumab analysis presented by Dr. Blauvelt involved nonresponder imputation through year 1 and imputation based on the reason for discontinuation in the second year. In contrast, the 2-year tildrakizumab analysis presented by Dr. Papp used the far more common last observation carried forward method.
To help the audience appreciate the importance of looking at the analytic methods used in a studies and help them understand the clinical significance of the results, Dr. Blauvelt provided a reanalysis of the 2-year guselkumab data using the last observation carried forward method. Across the board, the numbers became more favorable. For example, the PASI 75 rate of 95.7% using the prespecified nonresponder imputation analysis crept up to 96.8% under the last observation carried forward method; for comparison, the PASI 75 rates were 81%-84% in the tildrakizumab analysis.
“If you wanted to compare apples to apples with some other drugs, you would use these numbers – the as-observed analysis numbers used by most other companies with other drugs. If you wanted to determine what the true-life numbers are, they’d probably be something between the nonresponder imputation and as-observed numbers,” said to Dr. Blauvelt.
Dr. Papp was untroubled by the use of the last observation carried forward method in the particular case of the tildrakizumab long-term extension study.
“There is reason to believe the as-observed analysis doesn’t affect the integrity of the data because the dropout rate is extraordinarily low,” he said.
The guselkumab analysis was sponsored by Janssen Pharmaceutica; the tildrakizumab analysis was sponsored by Merck and by Sun Pharma. Dr. Blauvelt and Dr. Papp were paid investigators in both studies and serve as scientific advisers to virtually all companies invested in the psoriasis therapy developmental pipeline.
GENEVA – The merits of addressing interleukin-23 as a novel therapeutic target in moderate to severe plaque psoriasis were abundantly displayed in 2-year outcomes data for two anti–IL-23 monoclonal antibodies – guselkumab and tildrakizumab – in studies presented back to back at the annual congress of the European Academy of Dermatology and Venereology.
These long-term, open-label extensions of previously reported phase 3, randomized, double-blind clinical trials provided evidence of multiple advantages for IL-23 inhibition. The story was similar for both agents: After 2 years of use in the extension studies, the two biologics demonstrated stellar treatment response rates that would have been unimaginable only a few years ago, maintenance of efficacy without drop-off over time, exceedingly low dropout rates, and a safety picture that remains reassuring as experience accumulates. Also, the subcutaneously administered IL-23 inhibitors are attractive from a patient convenience standpoint in that maintenance guselkumab is dosed at 100 mg once every 8 weeks, and tildrakizumab is given once every 12 weeks.
Still, there are differences between the two drugs, most notably in apparent effectiveness. While more than half of guselkumab-treated patients had a Psoriasis Area Severity Index (PASI) 100 response – that is, totally clear skin – at 2 years, that was the case for only one-quarter to one-third of patients on tildrakizumab.
Guselkumab (Tremfya) was approved by the Food and Drug Administration in July 2017 for treatment of adults with moderate to severe plaque psoriasis. Tildrakizumab remains investigational.
Guselkumab
The 2-year, open-label extension of the phase 3 VOYAGE 1 trial included 735 patients who were either on guselkumab continuously, crossed from adalimumab (Humira) to guselkumab after 48 weeks, or switched from placebo after 16 weeks.
PASI 100 rates at 2 years were 49%-55% in the three patient groups. Of the patients in these groups, 54%-59% achieved an Investigator’s Global Assessment (IGA) score of 0. IGA scores of 0 or 1, meaning clear or almost clear skin, were present in 82%-85% of patients at 2 years.
“Dropout rate is an important consideration in long-term studies,” observed Dr. Blauvelt, a dermatologist and president of Oregon Medical Research Center in Portland. “For patients on continuous guselkumab there was a 6% dropout rate in the first year and 6% in the second year, so 88% of patients that started guselkumab were still on guselkumab 2 years later. That’s impressive. In the other two groups, the dropout rate was 2% per year.”
A Dermatology Life Quality Index (DLQI) score of 0 or 1, meaning no disease effects on quality of life, was recorded in 62.5% of the continuous guselkumab group at 48 weeks and 71.1% at 2 years.
“The interesting thing here is that, even though the efficacy numbers are fairly constant between year 1 and year 2, the DLQI goes up and up. Surprising? Maybe not. I think it shows patients are getting happier and happier over time with their disease control,” Dr. Blauvelt continued.
Rates of serious adverse events remained low and stable, with no negative surprises during year 2. The serious infection rate was 1.02 cases/100 patient-years in year 1 and 0.84 cases/100 patient-years in year 2. No cases of tuberculosis, opportunistic infections, or serious hypersensitivity reactions occurred during 2 years of treatment.
Tildrakizumab
Two-year results from the ongoing 5-year extension of the phase 3 reSURFACE 1 and reSURFACE 2 trials were presented by Kim A. Papp, MD, PhD, president of Probity Medical Research, Waterloo, Ont. This presentation of 2-year outcomes for 1,237 study participants was a feat, considering that the 12-week results of the trials had been published less than 3 months earlier (Lancet. 2017 Jul 15;390[10091]:276-88).
“I think these data are very compelling that the loss of response over time is minimal,” according to the dermatologist. “We’ve also seen that safety over 2 years has no surprises; in fact, it’s remarkably quiet. The rate of severe infections, which is important to look at for any treatment suppressing the immune system, is low and occurs almost independent of dose, which is very hopeful. It’s a promising sign.”
Indeed, the serious infection rate was 0.8 cases/100 patient-years regardless of whether subjects were on tildrakizumab at 100 mg or 200 mg.
Controversy over how to report long-term outcomes
A hot topic among clinical trialists in dermatology concerns how to report study results. The traditional method in studies funded by pharmaceutical companies is known as the “last observation carried forward” analysis. It casts the study drug results in the most favorable possible light because, when a subject drops out of a trial for any reason, their last measured value for response to treatment is carried forward as though the patient completed the study. Thus, psoriasis patients who drop out because they couldn’t tolerate a therapy or developed a serious side effect dictating discontinuation will be scored on the basis of their last PASI response, creating a bias in favor of active treatment.
A more conservative analytic method is known as the “nonresponder imputation” analysis. By this method, a patient who drops out of a trial is automatically categorized as a treatment failure, even if the reason was that the patient moved and could no longer make visits to the study center.
The prespecified guselkumab analysis presented by Dr. Blauvelt involved nonresponder imputation through year 1 and imputation based on the reason for discontinuation in the second year. In contrast, the 2-year tildrakizumab analysis presented by Dr. Papp used the far more common last observation carried forward method.
To help the audience appreciate the importance of looking at the analytic methods used in a studies and help them understand the clinical significance of the results, Dr. Blauvelt provided a reanalysis of the 2-year guselkumab data using the last observation carried forward method. Across the board, the numbers became more favorable. For example, the PASI 75 rate of 95.7% using the prespecified nonresponder imputation analysis crept up to 96.8% under the last observation carried forward method; for comparison, the PASI 75 rates were 81%-84% in the tildrakizumab analysis.
“If you wanted to compare apples to apples with some other drugs, you would use these numbers – the as-observed analysis numbers used by most other companies with other drugs. If you wanted to determine what the true-life numbers are, they’d probably be something between the nonresponder imputation and as-observed numbers,” said to Dr. Blauvelt.
Dr. Papp was untroubled by the use of the last observation carried forward method in the particular case of the tildrakizumab long-term extension study.
“There is reason to believe the as-observed analysis doesn’t affect the integrity of the data because the dropout rate is extraordinarily low,” he said.
The guselkumab analysis was sponsored by Janssen Pharmaceutica; the tildrakizumab analysis was sponsored by Merck and by Sun Pharma. Dr. Blauvelt and Dr. Papp were paid investigators in both studies and serve as scientific advisers to virtually all companies invested in the psoriasis therapy developmental pipeline.
expert analysis from tHE EADV CONGRESS
VIDEO: CAR T cell axi-cel drives B-cell lymphomas into remission
ATLANTA – In the ZUMA-1 trial, more than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta; axi-cel) had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion.
Updated combined phase 1 and 2 results in 108 patients with diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma showed an objective response rate of 82% of patients – including 58% showing complete responses – after a median follow-up of 15.4 months.
In a video interview at the annual meeting of the American Society of Hematology, Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston discusses the use of CAR T cells directed against the CD19 antigen in patients with relapsed/refractory B-cell lymphomas and describes efforts to improve responses while managing adverse events common to CAR T-cell therapies, notably cytokine release syndrome.
ZUMA-1 is supported by Kite Pharma, which developed axicabtagene ciloleucel, and the Leukemia & Lymphoma Society’s Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – In the ZUMA-1 trial, more than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta; axi-cel) had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion.
Updated combined phase 1 and 2 results in 108 patients with diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma showed an objective response rate of 82% of patients – including 58% showing complete responses – after a median follow-up of 15.4 months.
In a video interview at the annual meeting of the American Society of Hematology, Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston discusses the use of CAR T cells directed against the CD19 antigen in patients with relapsed/refractory B-cell lymphomas and describes efforts to improve responses while managing adverse events common to CAR T-cell therapies, notably cytokine release syndrome.
ZUMA-1 is supported by Kite Pharma, which developed axicabtagene ciloleucel, and the Leukemia & Lymphoma Society’s Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
ATLANTA – In the ZUMA-1 trial, more than one-third of patients with refractory large B-cell lymphomas treated with the chimeric antigen receptor (CAR) T-cell product axicabtagene ciloleucel (Yescarta; axi-cel) had durable responses, with some patients having complete responses lasting more than 1 year after a single infusion.
Updated combined phase 1 and 2 results in 108 patients with diffuse large B-cell lymphoma, primary mediastinal B-cell lymphoma, or transformed follicular lymphoma showed an objective response rate of 82% of patients – including 58% showing complete responses – after a median follow-up of 15.4 months.
In a video interview at the annual meeting of the American Society of Hematology, Sattva S. Neelapu, MD, from the University of Texas MD Anderson Cancer Center in Houston discusses the use of CAR T cells directed against the CD19 antigen in patients with relapsed/refractory B-cell lymphomas and describes efforts to improve responses while managing adverse events common to CAR T-cell therapies, notably cytokine release syndrome.
ZUMA-1 is supported by Kite Pharma, which developed axicabtagene ciloleucel, and the Leukemia & Lymphoma Society’s Therapy Acceleration Program. Dr. Neelapu reported receiving advisory board fees from the company.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
REPORTING FROM ASH 2017
Consider ‘impactibility’ to prevent hospital readmissions
With the goal of reducing 28-day or 30-day readmissions, some health care teams are turning to predictive models to identify patients at high risk for readmission and to efficiently focus resource-intensive prevention strategies. Recently, there’s been a rapid multiplying of these models.
Many of these models do accurately predict readmission risk, according to a recent BMJ editorial. “Among the 14 published models that target all unplanned readmissions (rather than readmissions for specific patient groups), the ‘C statistic’ ranges from 0.55 to 0.80, meaning that, when presented with two patients, these models correctly identify the higher risk individual between 55% and 80% of the time,” the authors wrote.
But, the authors suggested, the real value is not in simply making predictions but in using predictive models in ways that improve outcomes for patients.
“This will require linking predictive models to actionable opportunities for improving care,” they wrote. “Such linkages will most likely be identified through close collaboration between analytical teams, health care practitioners, and patients.” Being at high risk of readmission is not the only consideration; the patient must also be able to benefit from interventions being considered – they must be “impactible.”
“The distinction between predictive risk and impactibility might explain why practitioners tend to identify quite different patients for intervention than predictive risk models,” the authors wrote.
But together, predictive models and clinicians might produce more effective decisions than either does alone. “One of the strengths of predictive models is that they produce objective and consistent judgments regarding readmission risk, whereas clinical judgment can be affected by personal attitudes or attentiveness. Predictive risk models can also be operationalised across whole populations, and might therefore identify needs that would otherwise be missed by clinical teams (e.g., among more socioeconomically deprived neighbourhoods or groups with inadequate primary care). On the other hand, clinicians have access to a much wider range of information regarding patients than predictive risk models, which is essential to judge impactibility.”
The authors conclude, “The predictive modelling enterprise would benefit enormously from such collaboration because the real goal of this activity lies not in predicting the risk of readmission but in identifying patients at risk for preventable readmissions and ‘impactible’ by available interventions.”
Reference
Steventon A et al. Preventing hospital readmissions: The importance of considering ‘impactibility,’ not just predicted risk. BMJ Qual Saf. 2017 Oct;26(10):782-5. Accessed Oct. 9, 2017.
With the goal of reducing 28-day or 30-day readmissions, some health care teams are turning to predictive models to identify patients at high risk for readmission and to efficiently focus resource-intensive prevention strategies. Recently, there’s been a rapid multiplying of these models.
Many of these models do accurately predict readmission risk, according to a recent BMJ editorial. “Among the 14 published models that target all unplanned readmissions (rather than readmissions for specific patient groups), the ‘C statistic’ ranges from 0.55 to 0.80, meaning that, when presented with two patients, these models correctly identify the higher risk individual between 55% and 80% of the time,” the authors wrote.
But, the authors suggested, the real value is not in simply making predictions but in using predictive models in ways that improve outcomes for patients.
“This will require linking predictive models to actionable opportunities for improving care,” they wrote. “Such linkages will most likely be identified through close collaboration between analytical teams, health care practitioners, and patients.” Being at high risk of readmission is not the only consideration; the patient must also be able to benefit from interventions being considered – they must be “impactible.”
“The distinction between predictive risk and impactibility might explain why practitioners tend to identify quite different patients for intervention than predictive risk models,” the authors wrote.
But together, predictive models and clinicians might produce more effective decisions than either does alone. “One of the strengths of predictive models is that they produce objective and consistent judgments regarding readmission risk, whereas clinical judgment can be affected by personal attitudes or attentiveness. Predictive risk models can also be operationalised across whole populations, and might therefore identify needs that would otherwise be missed by clinical teams (e.g., among more socioeconomically deprived neighbourhoods or groups with inadequate primary care). On the other hand, clinicians have access to a much wider range of information regarding patients than predictive risk models, which is essential to judge impactibility.”
The authors conclude, “The predictive modelling enterprise would benefit enormously from such collaboration because the real goal of this activity lies not in predicting the risk of readmission but in identifying patients at risk for preventable readmissions and ‘impactible’ by available interventions.”
Reference
Steventon A et al. Preventing hospital readmissions: The importance of considering ‘impactibility,’ not just predicted risk. BMJ Qual Saf. 2017 Oct;26(10):782-5. Accessed Oct. 9, 2017.
With the goal of reducing 28-day or 30-day readmissions, some health care teams are turning to predictive models to identify patients at high risk for readmission and to efficiently focus resource-intensive prevention strategies. Recently, there’s been a rapid multiplying of these models.
Many of these models do accurately predict readmission risk, according to a recent BMJ editorial. “Among the 14 published models that target all unplanned readmissions (rather than readmissions for specific patient groups), the ‘C statistic’ ranges from 0.55 to 0.80, meaning that, when presented with two patients, these models correctly identify the higher risk individual between 55% and 80% of the time,” the authors wrote.
But, the authors suggested, the real value is not in simply making predictions but in using predictive models in ways that improve outcomes for patients.
“This will require linking predictive models to actionable opportunities for improving care,” they wrote. “Such linkages will most likely be identified through close collaboration between analytical teams, health care practitioners, and patients.” Being at high risk of readmission is not the only consideration; the patient must also be able to benefit from interventions being considered – they must be “impactible.”
“The distinction between predictive risk and impactibility might explain why practitioners tend to identify quite different patients for intervention than predictive risk models,” the authors wrote.
But together, predictive models and clinicians might produce more effective decisions than either does alone. “One of the strengths of predictive models is that they produce objective and consistent judgments regarding readmission risk, whereas clinical judgment can be affected by personal attitudes or attentiveness. Predictive risk models can also be operationalised across whole populations, and might therefore identify needs that would otherwise be missed by clinical teams (e.g., among more socioeconomically deprived neighbourhoods or groups with inadequate primary care). On the other hand, clinicians have access to a much wider range of information regarding patients than predictive risk models, which is essential to judge impactibility.”
The authors conclude, “The predictive modelling enterprise would benefit enormously from such collaboration because the real goal of this activity lies not in predicting the risk of readmission but in identifying patients at risk for preventable readmissions and ‘impactible’ by available interventions.”
Reference
Steventon A et al. Preventing hospital readmissions: The importance of considering ‘impactibility,’ not just predicted risk. BMJ Qual Saf. 2017 Oct;26(10):782-5. Accessed Oct. 9, 2017.
Three-month response to CAR T-cells looks durable in DLBCL
ATLANTA – Responses 3 months after chimeric antigen receptor (CAR) T-cell therapy look durable in adults with transplant-ineligible relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to updated results from the single-arm, global, phase 2 JULIET trial.
Fully 95% of patients who had a complete response to CTL019 (tisagenlecleucel; Kymriah) at 3 months maintained that complete response at 6 months, Stephen J. Schuster, MD, said at the annual meeting of the American Society of Hematology.
Patients with relapsed/refractory DLBCL tend to face a very poor prognosis, noted Dr. Schuster of Perelman School of Medicine and Abramson Cancer Center, University of Pennsylvania, Philadelphia. High-dose chemotherapy followed by autologous stem cell transplantation “is capable of long-term survival, but in very few patients,” he said. A dismal 8% of patients completely respond to salvage treatment and only about one in five partially respond. Both levels of response are short-lived, with a median survival of about 4 months.
Meanwhile, CTL019 therapy has produced durable complete remissions in children with lymphoblastic leukemia and in adults with chronic lymphocytic leukemia, Dr. Schuster and his associates wrote in an article simultaneously published in the New England Journal of Medicine (2017 Dec 10. doi: 10.1056/NEJMoa1708566).
To test the CAR T-cell therapy in relapsed/refractory DLBCL, they enrolled affected adults who had received at least two prior lines of antineoplastic treatment and who were not candidates for autologous stem cell transplantation.
Treatment consisted of a single CTL019 infusion (median dose, 3.1 × 108 cells; range, 0.1 × 108 to 6.0 × 108 cells), usually after lymphodepleting chemotherapy. Previously, patients had received a median of three lines of therapy, and about half had undergone autologous stem cell transplantation.
Median time from infusion to data cutoff in March 2017 was 5.6 months. Among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53% and 40% had a complete response. Overall response rates were 38% at 3 months and 37% at 6 months. Rates of complete response as confirmed by 18F-fluorodeoxyglucose–positron-emission tomography (PET) were 32% at 3 months and 30% at 6 months.These findings highlight the predictive power of 3-month response to CTL019 therapy in relapsed/refractory DLBCL, Dr. Schuster said. Among all responders, 74% remained relapse free at 6 months, meaning that median duration of response and median overall survival were not reached at data cutoff.
Dr. Schuster also reported that 26% of patients were infused as outpatients, which he called “easy to do” and appropriate as long as patients who become febrile are admitted and monitored for cytokine release syndrome. Three-quarters of patients who were infused as outpatients were able to remain home for at least 3 days afterward, he said.
Adverse events typified those of CAR T-cell therapy, including cytokine release syndrome (all grades: 58%; grade 3-4: 23%) and neurological toxicities (all grades: 21%; grade 3-4: 12%). The current labeling for CTL019 in children and young adults with acute lymphoblastic leukemia also includes a boxed warning for these toxicities.Tisagenlecleucel, the first-ever approved CAR T-cell therapy, is made by using a lentiviral vector to genetically engineer a patient’s own T-cells to express a CAR for the pan-B-cell CD19 antigen. These anti-CD19 CAR T-cells are then expanded in the laboratory, frozen for shipping purposes, and infused back into patients. In October 2017, Novartis submitted a biologics license application to the Food and Drug Administration to expand the label for CTL019 to include transplant-ineligible relapsed/refractory DLBCL.
Novartis Pharmaceuticals anticipates large-scale production in 2018, Dr. Schuster said. Manufacturing time has been cut to 22 days from the 30-day turnaround used in the trial, he reported.
Dr. Schuster also said that he sees no point in retreating patients whose relapsed/refractory DLBCL doesn’t respond to tisagenlecleucel, and that JULIET did not test this approach. “If someone fails therapy and you retreat, you don’t see success, in my experience,” he said. “If patients respond and then fail later, then you retreat and you may succeed.”
Novartis Pharmaceuticals sponsored JULIET. Dr. Schuster disclosed consultancy and research funding from Novartis and ties to Celgene, Gilead, Genentech, and several other pharmaceutical companies.
SOURCE: Schuster S et al. ASH 2017 Abstract 577.
ATLANTA – Responses 3 months after chimeric antigen receptor (CAR) T-cell therapy look durable in adults with transplant-ineligible relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to updated results from the single-arm, global, phase 2 JULIET trial.
Fully 95% of patients who had a complete response to CTL019 (tisagenlecleucel; Kymriah) at 3 months maintained that complete response at 6 months, Stephen J. Schuster, MD, said at the annual meeting of the American Society of Hematology.
Patients with relapsed/refractory DLBCL tend to face a very poor prognosis, noted Dr. Schuster of Perelman School of Medicine and Abramson Cancer Center, University of Pennsylvania, Philadelphia. High-dose chemotherapy followed by autologous stem cell transplantation “is capable of long-term survival, but in very few patients,” he said. A dismal 8% of patients completely respond to salvage treatment and only about one in five partially respond. Both levels of response are short-lived, with a median survival of about 4 months.
Meanwhile, CTL019 therapy has produced durable complete remissions in children with lymphoblastic leukemia and in adults with chronic lymphocytic leukemia, Dr. Schuster and his associates wrote in an article simultaneously published in the New England Journal of Medicine (2017 Dec 10. doi: 10.1056/NEJMoa1708566).
To test the CAR T-cell therapy in relapsed/refractory DLBCL, they enrolled affected adults who had received at least two prior lines of antineoplastic treatment and who were not candidates for autologous stem cell transplantation.
Treatment consisted of a single CTL019 infusion (median dose, 3.1 × 108 cells; range, 0.1 × 108 to 6.0 × 108 cells), usually after lymphodepleting chemotherapy. Previously, patients had received a median of three lines of therapy, and about half had undergone autologous stem cell transplantation.
Median time from infusion to data cutoff in March 2017 was 5.6 months. Among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53% and 40% had a complete response. Overall response rates were 38% at 3 months and 37% at 6 months. Rates of complete response as confirmed by 18F-fluorodeoxyglucose–positron-emission tomography (PET) were 32% at 3 months and 30% at 6 months.These findings highlight the predictive power of 3-month response to CTL019 therapy in relapsed/refractory DLBCL, Dr. Schuster said. Among all responders, 74% remained relapse free at 6 months, meaning that median duration of response and median overall survival were not reached at data cutoff.
Dr. Schuster also reported that 26% of patients were infused as outpatients, which he called “easy to do” and appropriate as long as patients who become febrile are admitted and monitored for cytokine release syndrome. Three-quarters of patients who were infused as outpatients were able to remain home for at least 3 days afterward, he said.
Adverse events typified those of CAR T-cell therapy, including cytokine release syndrome (all grades: 58%; grade 3-4: 23%) and neurological toxicities (all grades: 21%; grade 3-4: 12%). The current labeling for CTL019 in children and young adults with acute lymphoblastic leukemia also includes a boxed warning for these toxicities.Tisagenlecleucel, the first-ever approved CAR T-cell therapy, is made by using a lentiviral vector to genetically engineer a patient’s own T-cells to express a CAR for the pan-B-cell CD19 antigen. These anti-CD19 CAR T-cells are then expanded in the laboratory, frozen for shipping purposes, and infused back into patients. In October 2017, Novartis submitted a biologics license application to the Food and Drug Administration to expand the label for CTL019 to include transplant-ineligible relapsed/refractory DLBCL.
Novartis Pharmaceuticals anticipates large-scale production in 2018, Dr. Schuster said. Manufacturing time has been cut to 22 days from the 30-day turnaround used in the trial, he reported.
Dr. Schuster also said that he sees no point in retreating patients whose relapsed/refractory DLBCL doesn’t respond to tisagenlecleucel, and that JULIET did not test this approach. “If someone fails therapy and you retreat, you don’t see success, in my experience,” he said. “If patients respond and then fail later, then you retreat and you may succeed.”
Novartis Pharmaceuticals sponsored JULIET. Dr. Schuster disclosed consultancy and research funding from Novartis and ties to Celgene, Gilead, Genentech, and several other pharmaceutical companies.
SOURCE: Schuster S et al. ASH 2017 Abstract 577.
ATLANTA – Responses 3 months after chimeric antigen receptor (CAR) T-cell therapy look durable in adults with transplant-ineligible relapsed/refractory diffuse large B-cell lymphoma (DLBCL), according to updated results from the single-arm, global, phase 2 JULIET trial.
Fully 95% of patients who had a complete response to CTL019 (tisagenlecleucel; Kymriah) at 3 months maintained that complete response at 6 months, Stephen J. Schuster, MD, said at the annual meeting of the American Society of Hematology.
Patients with relapsed/refractory DLBCL tend to face a very poor prognosis, noted Dr. Schuster of Perelman School of Medicine and Abramson Cancer Center, University of Pennsylvania, Philadelphia. High-dose chemotherapy followed by autologous stem cell transplantation “is capable of long-term survival, but in very few patients,” he said. A dismal 8% of patients completely respond to salvage treatment and only about one in five partially respond. Both levels of response are short-lived, with a median survival of about 4 months.
Meanwhile, CTL019 therapy has produced durable complete remissions in children with lymphoblastic leukemia and in adults with chronic lymphocytic leukemia, Dr. Schuster and his associates wrote in an article simultaneously published in the New England Journal of Medicine (2017 Dec 10. doi: 10.1056/NEJMoa1708566).
To test the CAR T-cell therapy in relapsed/refractory DLBCL, they enrolled affected adults who had received at least two prior lines of antineoplastic treatment and who were not candidates for autologous stem cell transplantation.
Treatment consisted of a single CTL019 infusion (median dose, 3.1 × 108 cells; range, 0.1 × 108 to 6.0 × 108 cells), usually after lymphodepleting chemotherapy. Previously, patients had received a median of three lines of therapy, and about half had undergone autologous stem cell transplantation.
Median time from infusion to data cutoff in March 2017 was 5.6 months. Among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53% and 40% had a complete response. Overall response rates were 38% at 3 months and 37% at 6 months. Rates of complete response as confirmed by 18F-fluorodeoxyglucose–positron-emission tomography (PET) were 32% at 3 months and 30% at 6 months.These findings highlight the predictive power of 3-month response to CTL019 therapy in relapsed/refractory DLBCL, Dr. Schuster said. Among all responders, 74% remained relapse free at 6 months, meaning that median duration of response and median overall survival were not reached at data cutoff.
Dr. Schuster also reported that 26% of patients were infused as outpatients, which he called “easy to do” and appropriate as long as patients who become febrile are admitted and monitored for cytokine release syndrome. Three-quarters of patients who were infused as outpatients were able to remain home for at least 3 days afterward, he said.
Adverse events typified those of CAR T-cell therapy, including cytokine release syndrome (all grades: 58%; grade 3-4: 23%) and neurological toxicities (all grades: 21%; grade 3-4: 12%). The current labeling for CTL019 in children and young adults with acute lymphoblastic leukemia also includes a boxed warning for these toxicities.Tisagenlecleucel, the first-ever approved CAR T-cell therapy, is made by using a lentiviral vector to genetically engineer a patient’s own T-cells to express a CAR for the pan-B-cell CD19 antigen. These anti-CD19 CAR T-cells are then expanded in the laboratory, frozen for shipping purposes, and infused back into patients. In October 2017, Novartis submitted a biologics license application to the Food and Drug Administration to expand the label for CTL019 to include transplant-ineligible relapsed/refractory DLBCL.
Novartis Pharmaceuticals anticipates large-scale production in 2018, Dr. Schuster said. Manufacturing time has been cut to 22 days from the 30-day turnaround used in the trial, he reported.
Dr. Schuster also said that he sees no point in retreating patients whose relapsed/refractory DLBCL doesn’t respond to tisagenlecleucel, and that JULIET did not test this approach. “If someone fails therapy and you retreat, you don’t see success, in my experience,” he said. “If patients respond and then fail later, then you retreat and you may succeed.”
Novartis Pharmaceuticals sponsored JULIET. Dr. Schuster disclosed consultancy and research funding from Novartis and ties to Celgene, Gilead, Genentech, and several other pharmaceutical companies.
SOURCE: Schuster S et al. ASH 2017 Abstract 577.
REPORTING FROM ASH 2017
Key clinical point:
Major finding: Among 81 patients with at least 3 months of follow-up, best overall response rate was 53% (95% CI, 42%-64%; P less than .0001) and rates of complete response were 32% at 3 months and 30% at 6 months.
Study details: JULIET is an international, single-arm, phase 2 study of adults with relapsed/refractory DLBCL.
Disclosures: Novartis Pharmaceuticals sponsored JULIET. Dr. Schuster reported consultancy and research funding from Novartis and ties to Celgene, Gilead, Genentech, and several other pharmaceutical companies.
Source: Schuster S et al. ASH 2017 Abstract 577.