User login
Early referral recommended for high-risk port-wine stain cases
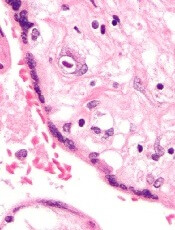
In high-risk port-wine stain phenotypes – forehead, hemifacial, and median – early referral to a pediatric neurologist is the best way to enable early symptom recognition of Sturge-Weber syndrome (SWS), according to results of a literature review.
If imaging is desired, electroencephalography (EEG) is cheaper and more minimally invasive than MRI (Pediatr Dermatol. 2017 Oct 16. doi: 10.1111/pde.13304).
Of the 34 studies analyzed, none examined the correlation between early detection of presymptomatic SWS and earlier seizure detection. There were also no data to verify improved outcomes with early detection, Michaela Zallmann, MD, of the department of dermatology at Eastern Health, Monash University, Box Hill, Australia, and her coauthors reported. While this indicates a need for further studies, it also shows a need for improved education and clinical monitoring as standard practice.
Additionally, negative imaging results do not obviate the possibility of SWS diagnosis, the investigators reported. In the studies that recorded false negatives, MRI was negative in 3%-6% of infants who later became symptomatic. Of the seven infants with false-negative results, four were less than 6 months old when the initial imaging was conducted. The investigators noted that it is not known at what age a negative MRI can reliably exclude SWS.
When imaging was used for early detection, EEG was shown to be safer and less expensive than MRI, according to the review. Of children who were not anesthetized for MRI, 30%-50% showed considerable distress, while EEG was shown to be minimally invasive. Findings from a retrospective chart review show that 14 MRIs were performed to detect one asymptomatic case of SWS, costing $11,768. In comparison, EEG costs $87 for a routine outpatient study.
“Demonstrating brain involvement on MRI in infants with high-risk PWS may facilitate more stringent counseling and monitoring, but … a negative MRI does not obviate the need for neurologic counseling and monitoring,” the investigators wrote. “Allaying anxiety about diagnostic uncertainty is not achieved using a scan but through detailed education, appropriate clinical monitoring, and nuanced reassurance.”
The investigators did not report any financial disclosures.
In high-risk port-wine stain phenotypes – forehead, hemifacial, and median – early referral to a pediatric neurologist is the best way to enable early symptom recognition of Sturge-Weber syndrome (SWS), according to results of a literature review.
If imaging is desired, electroencephalography (EEG) is cheaper and more minimally invasive than MRI (Pediatr Dermatol. 2017 Oct 16. doi: 10.1111/pde.13304).
Of the 34 studies analyzed, none examined the correlation between early detection of presymptomatic SWS and earlier seizure detection. There were also no data to verify improved outcomes with early detection, Michaela Zallmann, MD, of the department of dermatology at Eastern Health, Monash University, Box Hill, Australia, and her coauthors reported. While this indicates a need for further studies, it also shows a need for improved education and clinical monitoring as standard practice.
Additionally, negative imaging results do not obviate the possibility of SWS diagnosis, the investigators reported. In the studies that recorded false negatives, MRI was negative in 3%-6% of infants who later became symptomatic. Of the seven infants with false-negative results, four were less than 6 months old when the initial imaging was conducted. The investigators noted that it is not known at what age a negative MRI can reliably exclude SWS.
When imaging was used for early detection, EEG was shown to be safer and less expensive than MRI, according to the review. Of children who were not anesthetized for MRI, 30%-50% showed considerable distress, while EEG was shown to be minimally invasive. Findings from a retrospective chart review show that 14 MRIs were performed to detect one asymptomatic case of SWS, costing $11,768. In comparison, EEG costs $87 for a routine outpatient study.
“Demonstrating brain involvement on MRI in infants with high-risk PWS may facilitate more stringent counseling and monitoring, but … a negative MRI does not obviate the need for neurologic counseling and monitoring,” the investigators wrote. “Allaying anxiety about diagnostic uncertainty is not achieved using a scan but through detailed education, appropriate clinical monitoring, and nuanced reassurance.”
The investigators did not report any financial disclosures.
In high-risk port-wine stain phenotypes – forehead, hemifacial, and median – early referral to a pediatric neurologist is the best way to enable early symptom recognition of Sturge-Weber syndrome (SWS), according to results of a literature review.
If imaging is desired, electroencephalography (EEG) is cheaper and more minimally invasive than MRI (Pediatr Dermatol. 2017 Oct 16. doi: 10.1111/pde.13304).
Of the 34 studies analyzed, none examined the correlation between early detection of presymptomatic SWS and earlier seizure detection. There were also no data to verify improved outcomes with early detection, Michaela Zallmann, MD, of the department of dermatology at Eastern Health, Monash University, Box Hill, Australia, and her coauthors reported. While this indicates a need for further studies, it also shows a need for improved education and clinical monitoring as standard practice.
Additionally, negative imaging results do not obviate the possibility of SWS diagnosis, the investigators reported. In the studies that recorded false negatives, MRI was negative in 3%-6% of infants who later became symptomatic. Of the seven infants with false-negative results, four were less than 6 months old when the initial imaging was conducted. The investigators noted that it is not known at what age a negative MRI can reliably exclude SWS.
When imaging was used for early detection, EEG was shown to be safer and less expensive than MRI, according to the review. Of children who were not anesthetized for MRI, 30%-50% showed considerable distress, while EEG was shown to be minimally invasive. Findings from a retrospective chart review show that 14 MRIs were performed to detect one asymptomatic case of SWS, costing $11,768. In comparison, EEG costs $87 for a routine outpatient study.
“Demonstrating brain involvement on MRI in infants with high-risk PWS may facilitate more stringent counseling and monitoring, but … a negative MRI does not obviate the need for neurologic counseling and monitoring,” the investigators wrote. “Allaying anxiety about diagnostic uncertainty is not achieved using a scan but through detailed education, appropriate clinical monitoring, and nuanced reassurance.”
The investigators did not report any financial disclosures.
Key clinical point:
Major finding: To identify one case of Sturge-Weber syndrome, MRI cost $11,768, compared with $87 for a routine outpatient EEG.
Data source: A literature review of 34 articles.
Disclosures: The investigators did not report any financial disclosures.
Lumateperone shows broad phase 3 potential for psychiatric disorders
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
PARIS – including Alzheimer’s disease, on the strength of strong performances in phase 2 studies, Cedric O’Gorman, MD, reported at the annual congress of the European College of Neuropsychopharmacology.
The drug acts synergistically through the serotonergic, dopaminergic, and glutamatergic systems, providing a unique mechanism of action well-suited for treatment of a range of neuropsychiatric disorders, observed Dr. O’Gorman, who was vice president of medical affairs at the drug’s developer, Intra-Cellular Therapies during the ECNP congress and is now senior vice president for clinical development and medical affairs at Axsome Therapeutics in New York.
At lower doses, lumateperone is a potent serotonin 5-HT2A receptor antagonist that shows promise as a treatment for primary insomnia as well as agitation and aggression in elderly patients with dementia. As the dose increases, lumateperone also serves as a mesolimbic and mesocortical dopamine receptor phosphoprotein modulator with dual properties, acting at the level of the dopamine D2 receptor both as a presynaptic partial agonist and a postsynaptic antagonist.
The drug also enhances NMDA- and AMPA-induced currents in medial prefrontal cortex pyramidal neurons via activation of the D1 receptor. These attributes provide antipsychotic and antidepressant efficacy.
Positron emission tomography studies in patients with schizophrenia have shown that lumateperone at 60 mg once daily effectively reduced psychosis symptoms at roughly 40% striatal D2 receptor occupancy, which is much lower than the occupancy level required by approved antipsychotic drugs. This attribute, coupled with the drug’s potent effects on serotonin 5-HT2A receptors, serotonin transporters, and D1 receptors, probably accounts for lumateperone’s antipsychotic efficacy and accompanying improved psychosocial function and minimal motor disturbances as demonstrated in the clinical trials, according to Dr. O’Gorman.
The two randomized, double-blind, multicenter, placebo-controlled phase 3 trials in schizophrenia totaled 1,146 patients. The trials included an arm in which patients were randomized to risperidone (Risperdal) at 4 mg/day. The lumateperone and risperidone groups showed similar improvement as measured by the Positive and Negative Syndrome Scale score.
However, lumateperone had a better safety profile, with significantly less weight gain than in the risperidone group. Moreover, lumateperone-treated patients didn’t experience the adverse changes in blood glucose, triglycerides, total cholesterol, and prolactin observed in the risperidone group.
The safety profile of lumateperone as documented in the various clinical trials to date is similar to placebo, Dr. O’Gorman reported.
EXPERT ANALYSIS FROM THE ECNP CONGRESS
Beyond the Kegel: the who, why, and how of pelvic floor PT
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – When a woman is referred for pelvic floor physical therapy, what’s involved? Is there evidence behind the treatments, and what exactly does pelvic floor therapy look like?
Denise Hartzell Leggin, a physical therapist who specializes in pelvic floor dysfunction, reviewed how the female pelvic floor can change with age, and provided the rationale for pelvic floor physical therapy (PT) at the annual meeting of the North American Menopause Society.
“Physical therapists treat musculoskeletal and neuromuscular dysfunctions,” said Ms. Hartzell Leggin. So, when a physician suspects a musculoskeletal cause for pelvic floor dysfunction, a PT referral may be appropriate, she said.
Why refer for PT?
As part of the aging process, pelvic floor dysfunction can coexist with the genitourinary syndrome of menopause (GSM), said Ms. Hartzell. Though the pathophysiology is not always clear, aging does have some effect on the pelvic floor musculature and, together with GSM, can contribute to women’s urogenital symptoms in later life.
These symptoms, she said, can be the harbingers of “a host of clinical conditions,” including urinary incontinence and fecal incontinence, constipation, and bladder-emptying problems. Also, changes in the pelvic musculature from childbirth, surgeries, and hypotonicity or hypertonicity can contribute to sexual dysfunction in later life, said Ms. Hartzell Leggin, who is affiliated with Good Shepherd Penn Partners and in private practice in the Philadelphia area.
The musculature of the pelvic floor functions as more than a bowl for carrying the pelvic organs, Ms. Hartzell Leggin said. The collective muscles and fascia form a sling that fills in the pelvic ring and functions as an integrated system with constant resting tone. But the musculature is also active and interactive.
“The diaphragm and the pelvic floor move in symmetry during respiration,” and pelvic floor tone tightens in anticipation of increased intra-abdominal pressure from a cough, a sneeze, or even a laugh. “These are active structures – the brain can talk to the pelvic floor and make it do something,” she said.
Who’s a good candidate?
Looking at risk factors for pelvic organ prolapse alone, Ms. Hartzell Leggin said these can include age, body mass index, a history of occupational or recreational heavy lifting, chronic cough, and even genetics.
However, one of the most significant risk factors for prolapse of pelvic organs is simply having had a vaginal delivery. Up to 50% of women who have delivered a child vaginally may eventually have some degree of pelvic organ prolapse, though not all women will be symptomatic, Ms. Hartzell Leggin said.
Since postsurgical pelvic organ prolapse rates may top 30% within 2 years, an initial referral for pelvic floor PT is a rational conservative approach, she said. And even if a patient progresses to surgery, PT may be a useful adjunct.
Pelvic floor dysfunction may also be considered if a diastasis recti is discovered on physical exam, or if the patient reports a linear abdominal bulge. Patients with diastasis recti are more likely to have pelvic floor dysfunction than the general population, she said, so it’s worth asking about any related symptoms.
For voiding issues, “conservative treatment is first-line,” said Ms. Hartzell Leggin, so a PT referral for pelvic floor therapy and, in some cases, some behavioral retraining can help with issues of urinary frequency and urgency. These are options that may be considered before prescribing anticholinergic medication, she said.
How does pelvic floor PT work?
When a physician refers a patient for pelvic floor PT, what’s the process? The physical therapy evaluation will begin with history taking, including the chief complaint, past medical and surgical history, and an obstetric/gynecologic/sexual history, said Ms. Hartzell Leggin. Medications are also reviewed.
The physical therapist’s examination should encompass a thorough orthopedic examination, with attention to the lumbar spine and hips, and posture and gait. An external and internal examination of the pelvic floor will look for muscle tone at rest and with strain, and for any defects or prolapse.
Pelvic floor strength is assessed according to ability to contract, with some assessment of strength available through palpation. More quantitative means may include manometry, dynamometry, or the use of progressive weighted vaginal cones.
There’s no single standardized measurement tool to assess pelvic floor strength. Palpation is a valuable tool for an experienced clinician, and it also can provide real-time feedback to the patient as she becomes more aware of her pelvic floor. The discipline is moving toward more standardized terminology, with several reporting scales now available to report pelvic floor strength, said Ms. Hartzell Leggin.
The Pelvic Floor Distress Inventory is a validated tool that captures information about the impact of pelvic floor dysfunction on a patient’s daily functioning. “I think I capture a lot when my patient comes in and completes that form,” said Ms. Hartzell Leggin. The Genitourinary Pain Index is another validated tool that measures urinary symptoms, pain, and associated quality of life impacts. Patients may be asked to keep a home therapy and symptom or voiding diary for additional information.
The pelvic floor PT treatment algorithm will vary, depending on whether there’s underlying hypertonicity or hypotonicity, but will involve pelvic floor exercises, soft tissue mobilization, and consideration of a variety of modalities including electrical stimulation and ultrasound. For hypertonicity, vaginal dilators may be used, while weighted vaginal cones may be used for hypotonicity.
Physical therapists should know when to refer a patient back to a physician and should always work as part of an interdisciplinary team, she said.
Ms. Hartzell Leggin reported that she is the president of Elite Rehabilitation Services in Audubon, Pa.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
Therapy receives rare pediatric disease designation
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()
The US Food and Drug Administration (FDA) has granted rare pediatric disease designation to ATA230 for the treatment of congenital cytomegalovirus (CMV) infection.
ATA230 is an allogeneic T-cell immunotherapy targeting antigens expressed by CMV.
Rare pediatric disease designation is granted to drugs that show promise to treat orphan diseases affecting fewer than 200,000 patients in the US, primarily patients age 18 and younger.
The designation provides incentives to advance the development of drugs for rare disease, including access to the FDA’s expedited review and approval programs.
Under the FDA’s Rare Pediatric Disease Priority Review Voucher Program, a drug developer with rare pediatric disease designation that receives an approval of a new drug application is eligible for a voucher that can be redeemed to obtain priority review for any subsequent marketing application.
The company developing ATA230 is Atara Biotherapeutics, Inc. The company also received orphan designation for ATA230.
ATA230 trials
ATA230 has been investigated in phase 1 and 2 studies of immunocompromised patients with CMV viremia or disease who are refractory or resistant to antiviral drug treatment in the post-transplant setting.
Researchers reported phase 2 results with ATA230 at the 2016 ASH Annual Meeting.
The data encompassed 15 patients with documented CMV mutations conferring resistance to antiviral therapies. The patients had received a median of 3 prior therapies.
Eleven of the 15 patients (73.3%) responded to ATA230, 6 with complete responses and 5 with partial responses.
At 6 months, the overall survival was 72.7% in responders and 25% in non-responders.
Within the 6 months of follow-up, 1 of the 11 responders died of CMV, and 3 of the 4 non-responders died of CMV.
Adverse events occurred in 6 patients. One grade 3 event and 1 grade 4 event were considered possibly related to ATA230. ![]()
ACOG advises against vaginal seeding
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
The practice of vaginal seeding should not be performed outside of an approved research protocol until adequate data on safety and potential benefits are available, according to a new policy statement from the American College of Obstetricians and Gynecologists.
Vaginal seeding is “the practice of inoculating a cotton gauze or a cotton swab with vaginal fluids to transfer the vaginal flora to the mouth, nose, or skin of a newborn infant,” according to ACOG.
Data from several studies have suggested babies delivered by cesarean may lack the immunologic and metabolic benefits of vaginally delivered babies because of the unique properties of vaginal fluid, and a proof-of-concept study showed changes in newborns’ microbiome profiles when they received transfers of vaginal fluid soon after a cesarean delivery. However, the impact of the fluid transfer (vaginal seeding) remains unknown, according to the ACOG committee opinion (Obstet Gynecol. 2017;130:e274-8).
Additional safety concerns include the potential transfer of pathogens from mother to neonate from undiagnosed maternal conditions such as gonorrhea, human papillomavirus, group A streptococci, and others, the committee noted.
Women who wish to perform neonatal seeding themselves should be educated about the risks and tested for infectious diseases and pathogenic bacteria, the committee emphasized. Additionally, ACOG urged ob.gyns. to document the discussion in the medical record. The infant’s physician should also be made aware of the procedure because of the potential for neonatal infection.
The research on vaginal seeding currently consists of one pilot study, with an outcome measure of neonatal microbiota. No studies of other clinical outcomes have been completed.
“The paucity of data on this subject supports the need for additional research on the safety and benefit of vaginal seeding,” the ACOG Committee on Obstetric Practice wrote.
In the meantime, ACOG recommends exclusive breastfeeding in the first 6 months, noting that there are mixed data on associations between breastfeeding and the development of asthma and atopic disease in childhood.
FROM OBSTETRICS & GYNECOLOGY
Morphology index guides adnexal mass workup in postmenopausal women
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
PHILADELPHIA – The report provides guidelines for risk stratification and diagnostic evaluation when an ovarian mass is found.
Accurate and thorough evaluation of an adnexal mass in a menopausal woman must respect cancer prevalence data, Frederick Ueland, MD, one of the report’s coauthors, said at the annual meeting of the North American Menopause Society. In premenopausal women, there are “many tumors, but few cancers,” he said. Only about 15% of ovarian tumors are malignant when found before menopause.
But after menopause, there are “few tumors, but many cancers,” Dr. Ueland said. Up to 50% of tumors in postmenopausal women are malignant, with epithelial ovarian cancer, metastatic cancer, and granulosa cell tumors predominating.
Multiple clinical trials have taught physicians that “tumor morphology helps stratify cancer risk,” he noted.
Ultrasound is the best imaging modality to evaluate adnexal masses, he said. At his institution, the use of a morphology index to guide management of adnexal masses has reduced the number of surgeries performed to remove one cancer over the years, said Dr. Ueland, chief of the division of gynecologic oncology at the University of Kentucky, Lexington.
During the 1990s, when the Morphology Index was first used at the University of Kentucky, surgeons performed 12.5 surgeries per cancer. In the 2000s, that number fell to 5.2, and during the present decade, one cancer is detected in every 4 surgeries, he reported.
Limiting subjectivity is a key to accurate cancer detection when evaluating adnexal masses, so that the dual goals of accurate cancer detection and avoidance of unnecessary surgeries can be met, Dr. Ueland said. To address these dual needs, the first international consensus report on adnexal masses was issued in May 2017 (J Ultrasound Med. 2017 May;36[5]:849-863).
The report noted the sharp discrepancy between surgery rates in the United States and Europe. “In the United States, there are approximately 9.1 surgeries per malignancy, compared with the European International Ovarian Tumor Analysis center trials, with only 2.3 (oncology centers) and 5.9 (other centers) reported surgeries per malignancy, suggesting that there is room to improve our preoperative assessments,” the investigators wrote.
In reviewing management guidelines, Dr. Ueland said that, when the risk of malignancy is low, as with smooth-walled, unilocular or septate cysts, the mass can be monitored without surgery, with ultrasound reevaluation at the 6-month mark. If there are no concerning changes, the mass can then be imaged annually for 5 years. No further follow-up is needed at the 5-year mark, barring growth or other changes of the mass.
If the ultrasound evaluation of the mass shows intermediate risk, then secondary testing is needed. Masses that show as partly solid or that have small, irregular wall abnormalities, or atypical nonpapillary projections on ultrasound fall into this category. Secondary testing may be accomplished either by serial ultrasound or by using biomarker testing.
Commercially available triage biomarker tests such as OVA1, ROMA, and Overa may offer higher detection rates than cancer antigen 125 (CA 125) testing alone, Dr. Ueland said. For instance, OVA1, a multivariate index assay, detected 76% of malignancies missed by CA 125 (Am J Obstet Gynecol. 2016 Jul;215[1]:82.e1-11).
If the mass has high-risk characteristics, then a prompt surgical referral to a gynecologic oncologist is a must. Included in this category are mostly solid masses and those with papillary projections, as well as those associated with any ascites. No secondary testing or watchful waiting is recommended in these cases, said Dr. Ueland, since they carry a greater than 25% risk of malignancy.
Dr. Ueland is currently enrolling patients in a clinical trial to assess whether serial transvaginal ultrasonography with Morphology Index can reduce false-positive results by more accurately distinguishing benign from malignant ovarian tumors. He reported having no financial disclosures.
koakes@frontlinemedcom.com
On Twitter @karioakes
EXPERT ANALYSIS FROM NAMS 2017
eConsult gastroenterology model could improve access
ORLANDO – A review of gastroenterology electronic consultations, or eConsults, at a tertiary care academic medical center suggests that such referrals could improve timely access to specialist care, while cutting costs.
The findings underscore the need for careful study of this burgeoning care delivery model, which is a form of telemedicine, Jennifer Wang, MD, said at the World Congress of Gastroenterology at ACG 2017.
The review of 130 eConsults conducted between Jan. 1, 2015, and May 8, 2017, looked at questions asked, gastroenterology content, eConsult response time, change in referral plans, and indirect cost savings through avoided referrals and travel, according to Dr. Wang of the University of Virginia, Charlottesville, which is one of five centers that are part of an eConsult model project.
Of the 130 eConsults, 68 (52%) were resolved without face-to-face consultation with a gastroenterologist; the patients followed up with a primary care physician. The remaining 62 cases led to a face-to-face visit in the GI clinic.
The mean response time to eConsult was 54 hours, compared with a greater than 30-day wait time for an initial consultation in the ambulatory GI clinic, she said.
The most frequently queried subjects were etiology of chronic diarrhea (14%), colon cancer screening modality (12%), and chronic abdominal pain management (9%). The most common type of question asked pertained to diagnosis (70%).
The total mileage saved between patients’ homes and the GI clinic was estimated to be 1,583 miles. “You can also imagine the cost saved by not having to miss a day of work,” Dr. Wang said.
The model is not only cost effective, but can potentially be life saving, she added.
In one case, a 40-year-old woman with a 6-month history of abdominal pain was diagnosed with lymphoma during an eConsult and underwent biopsy and chemotherapy immediately, whereas the 30-day wait for a face-to-face visit would have delayed her diagnosis, Dr. Wang explained.
The eConsult model is being tested as a means of providing primary care physicians with direct, efficient, and timely access to specialist expertise in the management of their patients and potentially avoiding the need for face-to-face referrals, Dr. Wang said.
Increased demand for eConsult is anticipated, and therefore its financial and medical-legal implications should be further studied, she said. One question is how specialists can be incentivized to provide eConsults.
“I think the key would be to come up with a sustainable payment model and reimbursement strategy, and to have protected time for specialists to review eConsults,” she said.
Dr. Wang reported having no financial disclosures.
ORLANDO – A review of gastroenterology electronic consultations, or eConsults, at a tertiary care academic medical center suggests that such referrals could improve timely access to specialist care, while cutting costs.
The findings underscore the need for careful study of this burgeoning care delivery model, which is a form of telemedicine, Jennifer Wang, MD, said at the World Congress of Gastroenterology at ACG 2017.
The review of 130 eConsults conducted between Jan. 1, 2015, and May 8, 2017, looked at questions asked, gastroenterology content, eConsult response time, change in referral plans, and indirect cost savings through avoided referrals and travel, according to Dr. Wang of the University of Virginia, Charlottesville, which is one of five centers that are part of an eConsult model project.
Of the 130 eConsults, 68 (52%) were resolved without face-to-face consultation with a gastroenterologist; the patients followed up with a primary care physician. The remaining 62 cases led to a face-to-face visit in the GI clinic.
The mean response time to eConsult was 54 hours, compared with a greater than 30-day wait time for an initial consultation in the ambulatory GI clinic, she said.
The most frequently queried subjects were etiology of chronic diarrhea (14%), colon cancer screening modality (12%), and chronic abdominal pain management (9%). The most common type of question asked pertained to diagnosis (70%).
The total mileage saved between patients’ homes and the GI clinic was estimated to be 1,583 miles. “You can also imagine the cost saved by not having to miss a day of work,” Dr. Wang said.
The model is not only cost effective, but can potentially be life saving, she added.
In one case, a 40-year-old woman with a 6-month history of abdominal pain was diagnosed with lymphoma during an eConsult and underwent biopsy and chemotherapy immediately, whereas the 30-day wait for a face-to-face visit would have delayed her diagnosis, Dr. Wang explained.
The eConsult model is being tested as a means of providing primary care physicians with direct, efficient, and timely access to specialist expertise in the management of their patients and potentially avoiding the need for face-to-face referrals, Dr. Wang said.
Increased demand for eConsult is anticipated, and therefore its financial and medical-legal implications should be further studied, she said. One question is how specialists can be incentivized to provide eConsults.
“I think the key would be to come up with a sustainable payment model and reimbursement strategy, and to have protected time for specialists to review eConsults,” she said.
Dr. Wang reported having no financial disclosures.
ORLANDO – A review of gastroenterology electronic consultations, or eConsults, at a tertiary care academic medical center suggests that such referrals could improve timely access to specialist care, while cutting costs.
The findings underscore the need for careful study of this burgeoning care delivery model, which is a form of telemedicine, Jennifer Wang, MD, said at the World Congress of Gastroenterology at ACG 2017.
The review of 130 eConsults conducted between Jan. 1, 2015, and May 8, 2017, looked at questions asked, gastroenterology content, eConsult response time, change in referral plans, and indirect cost savings through avoided referrals and travel, according to Dr. Wang of the University of Virginia, Charlottesville, which is one of five centers that are part of an eConsult model project.
Of the 130 eConsults, 68 (52%) were resolved without face-to-face consultation with a gastroenterologist; the patients followed up with a primary care physician. The remaining 62 cases led to a face-to-face visit in the GI clinic.
The mean response time to eConsult was 54 hours, compared with a greater than 30-day wait time for an initial consultation in the ambulatory GI clinic, she said.
The most frequently queried subjects were etiology of chronic diarrhea (14%), colon cancer screening modality (12%), and chronic abdominal pain management (9%). The most common type of question asked pertained to diagnosis (70%).
The total mileage saved between patients’ homes and the GI clinic was estimated to be 1,583 miles. “You can also imagine the cost saved by not having to miss a day of work,” Dr. Wang said.
The model is not only cost effective, but can potentially be life saving, she added.
In one case, a 40-year-old woman with a 6-month history of abdominal pain was diagnosed with lymphoma during an eConsult and underwent biopsy and chemotherapy immediately, whereas the 30-day wait for a face-to-face visit would have delayed her diagnosis, Dr. Wang explained.
The eConsult model is being tested as a means of providing primary care physicians with direct, efficient, and timely access to specialist expertise in the management of their patients and potentially avoiding the need for face-to-face referrals, Dr. Wang said.
Increased demand for eConsult is anticipated, and therefore its financial and medical-legal implications should be further studied, she said. One question is how specialists can be incentivized to provide eConsults.
“I think the key would be to come up with a sustainable payment model and reimbursement strategy, and to have protected time for specialists to review eConsults,” she said.
Dr. Wang reported having no financial disclosures.
AT THE WORLD CONGRESS OF GASTROENTEROLOGY
Key clinical point:
Major finding: The mean response time to eConsult was 54 hours, compared with a greater than 30-day wait time for an initial consultation in the ambulatory GI clinic.
Data source: A review of 130 eConsults.
Disclosures: Dr. Wang reported having no financial disclosures.
Listen carefully
The widespread use of fetal ultrasound has dramatically decreased the number of delivery room surprises. In this country, most infants with a cardiac anomaly detected in utero probably are delivered at tertiary medical centers, leapfrogging over the maternity units at their local community hospitals. But infants with critical cardiac conditions continue to arrive unheralded at every hospital, both large and small. And many of these babies are asymptomatic without tachypnea or cyanosis. A study reported in the October 2017 Pediatrics by Hu et al. suggests a strategy for detecting these little masters of disguise before their lesions get them into serious trouble (doi: 10.1542/peds.2017-1154).
Pulse oximetry has been widely debated as a method for detecting congenital heart disease, because of course it misses the cases that are acyanotic. In a series of more than 150,000 asymptomatic neonates, and 92% for major congenital heart disease. Their false-positive rate for both categories was just a bit over 1%.
This may be another case in which the training of the examiner and the environment are critical to a positive result. As I think back to the conditions in which I examined newborns, I wonder how careful I was that my auscultating was being done in a quiet environment. If I was in the nursery, there may have been other babies crying, a radio playing, or nurses conversing with one another. I may have been deluding myself that, over the years in practice, I had developed the ability to cancel out those auditory distractions. It was probably dumb luck that I didn’t miss any critical murmurs.
And then there is the timing. The investigators don’t describe how soon after birth the auscultation was performed. Is there an optimum time in relation to the neonate’s transition from his/her fetal circulation? How long did the examiners listen? Were they in a rush to get back to their offices and busy waiting room or were they hospitalists?
I found this to be an interesting study, and if repeated by other investigators, it provides an example of how technology advancement doesn’t always render our old examining skills obsolete. In fact, it may demand we sharpen them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
The widespread use of fetal ultrasound has dramatically decreased the number of delivery room surprises. In this country, most infants with a cardiac anomaly detected in utero probably are delivered at tertiary medical centers, leapfrogging over the maternity units at their local community hospitals. But infants with critical cardiac conditions continue to arrive unheralded at every hospital, both large and small. And many of these babies are asymptomatic without tachypnea or cyanosis. A study reported in the October 2017 Pediatrics by Hu et al. suggests a strategy for detecting these little masters of disguise before their lesions get them into serious trouble (doi: 10.1542/peds.2017-1154).
Pulse oximetry has been widely debated as a method for detecting congenital heart disease, because of course it misses the cases that are acyanotic. In a series of more than 150,000 asymptomatic neonates, and 92% for major congenital heart disease. Their false-positive rate for both categories was just a bit over 1%.
This may be another case in which the training of the examiner and the environment are critical to a positive result. As I think back to the conditions in which I examined newborns, I wonder how careful I was that my auscultating was being done in a quiet environment. If I was in the nursery, there may have been other babies crying, a radio playing, or nurses conversing with one another. I may have been deluding myself that, over the years in practice, I had developed the ability to cancel out those auditory distractions. It was probably dumb luck that I didn’t miss any critical murmurs.
And then there is the timing. The investigators don’t describe how soon after birth the auscultation was performed. Is there an optimum time in relation to the neonate’s transition from his/her fetal circulation? How long did the examiners listen? Were they in a rush to get back to their offices and busy waiting room or were they hospitalists?
I found this to be an interesting study, and if repeated by other investigators, it provides an example of how technology advancement doesn’t always render our old examining skills obsolete. In fact, it may demand we sharpen them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
The widespread use of fetal ultrasound has dramatically decreased the number of delivery room surprises. In this country, most infants with a cardiac anomaly detected in utero probably are delivered at tertiary medical centers, leapfrogging over the maternity units at their local community hospitals. But infants with critical cardiac conditions continue to arrive unheralded at every hospital, both large and small. And many of these babies are asymptomatic without tachypnea or cyanosis. A study reported in the October 2017 Pediatrics by Hu et al. suggests a strategy for detecting these little masters of disguise before their lesions get them into serious trouble (doi: 10.1542/peds.2017-1154).
Pulse oximetry has been widely debated as a method for detecting congenital heart disease, because of course it misses the cases that are acyanotic. In a series of more than 150,000 asymptomatic neonates, and 92% for major congenital heart disease. Their false-positive rate for both categories was just a bit over 1%.
This may be another case in which the training of the examiner and the environment are critical to a positive result. As I think back to the conditions in which I examined newborns, I wonder how careful I was that my auscultating was being done in a quiet environment. If I was in the nursery, there may have been other babies crying, a radio playing, or nurses conversing with one another. I may have been deluding myself that, over the years in practice, I had developed the ability to cancel out those auditory distractions. It was probably dumb luck that I didn’t miss any critical murmurs.
And then there is the timing. The investigators don’t describe how soon after birth the auscultation was performed. Is there an optimum time in relation to the neonate’s transition from his/her fetal circulation? How long did the examiners listen? Were they in a rush to get back to their offices and busy waiting room or were they hospitalists?
I found this to be an interesting study, and if repeated by other investigators, it provides an example of how technology advancement doesn’t always render our old examining skills obsolete. In fact, it may demand we sharpen them.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at pdnews@frontlinemedcom.com.
Idelalisib efficacy against CLL tarnished by toxicity
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
NEW YORK – PI3K inhibitors are highly active against B-cell malignancies, but this class of drugs, led by
Idelalisib is a potent inhibitor of the delta isoform of phosphatidylinositol 3-kinase (PI3K) that in a phase 1 trial was associated at higher dose levels with a median progression-free survival (PFS) of 32 months in patients with CLL who had received a median of five prior lines of therapy, noted Jennifer R. Brown, MD, PhD, director of the CLL center at the Dana-Farber Cancer Institute in Boston.
“This is really a very effective drug. So what’s happened? Why aren’t we using it more?” she asked rhetorically at an international congress on hematologic malignancies.
“This relates to a pattern of toxicities that has becoming increasingly familiar to us,” she added.
There is increasing evidence to suggest that the toxicities associated with idelalisib are immune mediated, indicating both the need for caution among clinicians who think about prescribing the drug, and a potential future use for this and other PI3K inhibitors as immunomodulatory agents, Dr. Brown said.
Registration trial toxicities
Among 760 patients enrolled in trials for the idelalisib registration programs, grade 3 or greater diarrhea and/or colitis and transaminitis each occurred in 14% of patients, rash occurred in 6%, and pneumonitis of any grade was seen in 3%.
Among patients with relapsed disease, transaminitis was often self-limiting and usually resolved when the drug was withheld, and about 75% of patients were successfully restarted on idelalisib at the same or lower dose, Dr. Brown noted.
Rashes, which can occur any time with therapy, were also successfully managed by withholding drug and then rechallenging, with the addition of corticosteroids as necessary.
Patients who developed drug-related pneumonitis were less likely than those with other toxicities to be rechallenged, and most required steroids until the infections resolved.
“The steroid responsiveness of many of these side effects suggested that they were autoimmune,” Dr. Brown said.
Drugs only work when you take them
The toxicities seen with idelalisib have had a marked effect on the use of the drug. In registration trials for idelalisib in combination with rituximab or ofatumumab (Arzerra), each of which had at least 2 years of follow-up, only 22.5% of 369 patients remained on idelalisib, primarily because of toxicities rather than disease progression. The combined 2-year progression in these trials was 13.3% In contrast, 40.7% of patients discontinued idelalisib because of adverse events.
Out to about 7 months, survival rates for patients who discontinued idelalisib because of disease progression or adverse events were roughly similar, but survival for the patients who stopped because of side effects began to plateau out to 2 years, Dr. Brown noted.
As of March 2016, 23.2% of patients who received idelalisib in clinical trials in combination with other agents as second- or third-line therapy had died, compared with 31% of controls, indicating a clear survival benefit with the drug.
“This is probably because the benefit of disease control in that setting overwhelmed the adverse event or infections problem,” she said.
Many of the deaths in registration trials were related to opportunistic infections, including Pneumocystis jiroveci pneumonia, fungal infection, and cytomegalovirus.
“Idelalisib, I think, is a prototypical delta inhibitor with a pattern of immune-mediated toxicity that remains unpredictable and can be severe. We now have pretty good data, based on the Gilead [sponsor] trials, that younger age and less prior therapy predispose to this toxicity,” Dr. Brown said.
Evidence is less robust, but growing, that mutated IGHV and a decrease in regulatory T cells may be also be risk factors for immune-mediated toxicities with idelalisib. Immune modulation with the drug may also account for associated neutropenia, sepsis, and opportunistic infections seen with idelalisib therapy, she added.
So how to use it?
Currently, the best uses for idelalisib and other PI3K inhibitors in CLL appear to be in single-agent therapy in patients with relapsed disease who cannot tolerate a Bruton’s tyrosine kinase (BTK) inhibitor such as ibrutinib (Imbruvica) or in patients whose disease has progressed on a BTK inhibitor.
“Where I think about this drug is in older, more heavily pretreated patients, who are generally at less risk for toxicities, and if they have significant comorbidities that may impact BTK-inhibitor tolerability, usually cardiac,” Dr. Brown said.
Future expansion of PI3K inhibitors in B-cell malignancies may require identifying a biomarker for tolerance, alternative dosing schedules, or identification of an idelalisib/drug X combination that might mitigate the toxicity, she said.
The immune-activation properties of PI3K-delta inhibitors suggests that they might also play a role as antitumor immunomodulatory agents in treatment of both hematologic malignancies and solid tumors, Dr. Brown concluded.
Idelalisib trials were sponsored by Gilead Sciences. Dr. Brown disclosed serving as a consultant for Gilead and other companies.
EXPERT ANALYSIS FROM LYMPHOMA & MYELOMA
Precision medicine’s future in rheumatic diseases outlined at ACR 2017
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
The treatment and prevention of rheumatic diseases through a precision medicine approach that takes into account individual variability in genes, environment, and lifestyle has not yet become a reality in clinical practice, but researchers in the field hope to demonstrate its potential at a course before and a session during the annual meeting of the American College of Rheumatology in San Diego.
In a 2-day premeeting course that begins on Friday, Nov. 3, a multidisciplinary faculty will discuss the technologies involved in precision medicine research; the complexities and challenges of identifying optimal drug treatments for rheumatic diseases; past, ongoing, and future research initiatives in precision medicine affecting rheumatic diseases; and how large data-set analysis and collaborative networks of researchers can shape its future.
Later, during the meeting on the afternoon of Monday, Nov. 6, an ACR session will look at how precision medicine research in patients with systemic lupus erythematosus (SLE) has laid groundwork to improve clinical trial design and the implementation of more tailored treatment for SLE and other complex autoimmune diseases.
The first day of the 2-day premeeting course will cover current approaches to studying familial and pediatric rheumatic diseases as well as an outline of how precision medicine could affect treatment approaches to SLE. Later in the day, presentations will focus on examples of personalized medicine approaches that have been derived from studies of cancer genomics and familial syndromes and the genetic underpinning of different responses to medications. The impact of direct-to-consumer genetic testing will also be discussed. Abstract presentations at the end of the day will focus on new genetic biomarkers that differentiate active disease from remission in granulomatosis with polyangiitis and mutations related to cancer-associated myositis.
The second day of the course on Saturday, Nov. 4, will focus on the technology behind precision medicine, followed by data analysis and integration into clinical practice. Technology presentations aim to demonstrate how transcriptional analysis of single immune cells and epigenetics in small numbers of cells can provide information about disease activity and prognosis. Examples of how multiple types of data can be integrated to form a picture of the pathogenesis of diseases such as rheumatoid arthritis will be discussed. Two abstract presentations will serve to show how analysis of tissue-specific serum biomarkers can identify rheumatoid arthritis patients with structural progression and how the presence of a specific antibody can predict better response to anti–tumor necrosis factor treatment and better disease control over time. In the afternoon, sessions will focus on analyzing single-cell data in key cell subpopulations in rheumatic disease and specifically address immune cell interactions across systems in SLE.
On Monday at 1:00 p.m., Maria Virginia Pascual, PhD, director of the Drukier Institute for Children’s Health and the Ronay A. Menschel Professor of Pediatrics at Cornell University in New York will speak in the ACR session, “Precision Medicine for Rheumatic Disease: Closer Than You Think?” Dr. Pascual will provide an overview of her lab’s recent work in transcriptional profiling of pediatric SLE patients, which demonstrates the potential value of immunomonitoring in order to stratify patients into discrete molecular groups to make clinical trial design better and offer more targeted therapies.
FROM ACR 2017