User login
New NIH-Supported HIV Vaccine Efficacy Study Begins
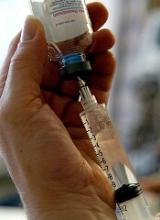
A historic trial to test safety and efficacy of an experimental HIV vaccine is under way at 15 sites in South Africa, where more than 1,000 people become infected with HIV every day, says the NIH.
The Phase2b/3 study, HVTN 702, is the first HIV vaccine efficacy study in 8 years. The regimen involves a new version of the only HIV vaccine candidate ever shown to provide some protection against the virus. That vaccine, tested in the 2009 RV144 clinical trial in Thailand, led by the U.S. military HIV Research program and the Thai Ministry of Health, delivered “landmark results.”
RV144 found for the first time that a vaccine could prevent HIV infection, “albeit modestly.” The vaccine was 31.2% effective at preventing infection over the nearly 4-year follow-up. HVTN 702, researchers hope, will provide more sustained protection; the components of the RV144 regimen have been modified to try to increase the magnitude and duration of immune responses. Recently, interim results were reported for
Researchers aim to enroll 5,400 men and women in HVTN 702, which will make it the largest and most advanced HIV vaccine clinical trial to take place in South Africa. “If an HIV vaccine were found to work in South Africa, it could dramatically alter the course of the pandemic,” said HVTN 702 Protocol Chair Glenda Gray, MBBCH, FC Paed (SA).
“[A] safe and effective vaccine could be the final nail in the coffin for HIV,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, a cofunder of the study. Results from
Source:
First new HIV vaccine efficacy study in seven years has begun [news release]. National Institute of Allergy and Infectious Disease; November 27, 2016. https://www.niaid.nih.gov/news-events/first-new-hiv-vaccine-efficacy-study-seven-years-has-begun. Accessed August 23, 2017.
A historic trial to test safety and efficacy of an experimental HIV vaccine is under way at 15 sites in South Africa, where more than 1,000 people become infected with HIV every day, says the NIH.
The Phase2b/3 study, HVTN 702, is the first HIV vaccine efficacy study in 8 years. The regimen involves a new version of the only HIV vaccine candidate ever shown to provide some protection against the virus. That vaccine, tested in the 2009 RV144 clinical trial in Thailand, led by the U.S. military HIV Research program and the Thai Ministry of Health, delivered “landmark results.”
RV144 found for the first time that a vaccine could prevent HIV infection, “albeit modestly.” The vaccine was 31.2% effective at preventing infection over the nearly 4-year follow-up. HVTN 702, researchers hope, will provide more sustained protection; the components of the RV144 regimen have been modified to try to increase the magnitude and duration of immune responses. Recently, interim results were reported for
Researchers aim to enroll 5,400 men and women in HVTN 702, which will make it the largest and most advanced HIV vaccine clinical trial to take place in South Africa. “If an HIV vaccine were found to work in South Africa, it could dramatically alter the course of the pandemic,” said HVTN 702 Protocol Chair Glenda Gray, MBBCH, FC Paed (SA).
“[A] safe and effective vaccine could be the final nail in the coffin for HIV,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, a cofunder of the study. Results from
Source:
First new HIV vaccine efficacy study in seven years has begun [news release]. National Institute of Allergy and Infectious Disease; November 27, 2016. https://www.niaid.nih.gov/news-events/first-new-hiv-vaccine-efficacy-study-seven-years-has-begun. Accessed August 23, 2017.
A historic trial to test safety and efficacy of an experimental HIV vaccine is under way at 15 sites in South Africa, where more than 1,000 people become infected with HIV every day, says the NIH.
The Phase2b/3 study, HVTN 702, is the first HIV vaccine efficacy study in 8 years. The regimen involves a new version of the only HIV vaccine candidate ever shown to provide some protection against the virus. That vaccine, tested in the 2009 RV144 clinical trial in Thailand, led by the U.S. military HIV Research program and the Thai Ministry of Health, delivered “landmark results.”
RV144 found for the first time that a vaccine could prevent HIV infection, “albeit modestly.” The vaccine was 31.2% effective at preventing infection over the nearly 4-year follow-up. HVTN 702, researchers hope, will provide more sustained protection; the components of the RV144 regimen have been modified to try to increase the magnitude and duration of immune responses. Recently, interim results were reported for
Researchers aim to enroll 5,400 men and women in HVTN 702, which will make it the largest and most advanced HIV vaccine clinical trial to take place in South Africa. “If an HIV vaccine were found to work in South Africa, it could dramatically alter the course of the pandemic,” said HVTN 702 Protocol Chair Glenda Gray, MBBCH, FC Paed (SA).
“[A] safe and effective vaccine could be the final nail in the coffin for HIV,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, a cofunder of the study. Results from
Source:
First new HIV vaccine efficacy study in seven years has begun [news release]. National Institute of Allergy and Infectious Disease; November 27, 2016. https://www.niaid.nih.gov/news-events/first-new-hiv-vaccine-efficacy-study-seven-years-has-begun. Accessed August 23, 2017.
Researchers estimate risk of death from BIA-ALCL
The risk of death from breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is less than 1 in a million, according to a study published in Aesthetic Surgery Journal.
Researchers analyzed data on breast implants and estimated the risk of death from BIA-ALCL to be 0.4 micromorts for a woman with bilateral, textured implants.
One micromort means a person’s risk of dying is 1 in a million. For context, a person who drives a car for 1 hour per day is said to have a micromort of 2, which is 5 times the BIA-ALCL micromort.
The researchers noted that there are no documented cases of BIA-ALCL in patients who have only received smooth-surface breast implants. Therefore, the risk of death from BIA-ALCL in a woman with smooth breast implants is “essentially 0.”
“We conducted this micromort study to bring real-life perspective for all existing and potential breast augmentation patients who might have reservations about implants based on the recent media coverage indicating that breast implants can be fatal—a sensationalized take on a very rare and very treatable condition,” said study author William P. Adams, Jr, MD, a professor in the Department of Plastic Surgery at the University of Texas Southwestern in Dallas.
“This analysis resonates with patients. They get it when you explain to a patient that their micromort risk from skiing for 1 day is 2 times higher than the micromort risk of having a textured breast implant for their lifetime—or that traveling 8 hours by car carries a 40-times higher micromort risk than having 2 textured breast implants for their lifetime.”
Dr Adams and his co-author analyzed data from the International Society of Aesthetic Plastic Surgery, the American Society of Plastic Surgeons, the American Society for Aesthetic Plastic Surgery, and the Austrian Breast Implant Register, as well as studies by Allergan and Sientra.
This led to a “conservative estimate” that approximately 30 million patients have textured breast implants worldwide (not including breast reconstructions).
This figure and the report of 12 deaths from BIA-ALCL worldwide suggest the risk of death from BIA-ALCL is 0.4 micromorts per patient (with 2 textured implants) or 0.2 micromorts per textured implant.
“The findings of this study are very important for patient education,” said study author David A. Sieber, MD, a plastic surgeon in private practice in San Francisco, California.
“The clear lymphoproliferative nature of BIA-ALCL, along with the calculated risks associated with its diagnosis, should be used for discussion during new consultations or at the time of presentation for evaluation of delayed-onset seromas.” ![]()
The risk of death from breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is less than 1 in a million, according to a study published in Aesthetic Surgery Journal.
Researchers analyzed data on breast implants and estimated the risk of death from BIA-ALCL to be 0.4 micromorts for a woman with bilateral, textured implants.
One micromort means a person’s risk of dying is 1 in a million. For context, a person who drives a car for 1 hour per day is said to have a micromort of 2, which is 5 times the BIA-ALCL micromort.
The researchers noted that there are no documented cases of BIA-ALCL in patients who have only received smooth-surface breast implants. Therefore, the risk of death from BIA-ALCL in a woman with smooth breast implants is “essentially 0.”
“We conducted this micromort study to bring real-life perspective for all existing and potential breast augmentation patients who might have reservations about implants based on the recent media coverage indicating that breast implants can be fatal—a sensationalized take on a very rare and very treatable condition,” said study author William P. Adams, Jr, MD, a professor in the Department of Plastic Surgery at the University of Texas Southwestern in Dallas.
“This analysis resonates with patients. They get it when you explain to a patient that their micromort risk from skiing for 1 day is 2 times higher than the micromort risk of having a textured breast implant for their lifetime—or that traveling 8 hours by car carries a 40-times higher micromort risk than having 2 textured breast implants for their lifetime.”
Dr Adams and his co-author analyzed data from the International Society of Aesthetic Plastic Surgery, the American Society of Plastic Surgeons, the American Society for Aesthetic Plastic Surgery, and the Austrian Breast Implant Register, as well as studies by Allergan and Sientra.
This led to a “conservative estimate” that approximately 30 million patients have textured breast implants worldwide (not including breast reconstructions).
This figure and the report of 12 deaths from BIA-ALCL worldwide suggest the risk of death from BIA-ALCL is 0.4 micromorts per patient (with 2 textured implants) or 0.2 micromorts per textured implant.
“The findings of this study are very important for patient education,” said study author David A. Sieber, MD, a plastic surgeon in private practice in San Francisco, California.
“The clear lymphoproliferative nature of BIA-ALCL, along with the calculated risks associated with its diagnosis, should be used for discussion during new consultations or at the time of presentation for evaluation of delayed-onset seromas.” ![]()
The risk of death from breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is less than 1 in a million, according to a study published in Aesthetic Surgery Journal.
Researchers analyzed data on breast implants and estimated the risk of death from BIA-ALCL to be 0.4 micromorts for a woman with bilateral, textured implants.
One micromort means a person’s risk of dying is 1 in a million. For context, a person who drives a car for 1 hour per day is said to have a micromort of 2, which is 5 times the BIA-ALCL micromort.
The researchers noted that there are no documented cases of BIA-ALCL in patients who have only received smooth-surface breast implants. Therefore, the risk of death from BIA-ALCL in a woman with smooth breast implants is “essentially 0.”
“We conducted this micromort study to bring real-life perspective for all existing and potential breast augmentation patients who might have reservations about implants based on the recent media coverage indicating that breast implants can be fatal—a sensationalized take on a very rare and very treatable condition,” said study author William P. Adams, Jr, MD, a professor in the Department of Plastic Surgery at the University of Texas Southwestern in Dallas.
“This analysis resonates with patients. They get it when you explain to a patient that their micromort risk from skiing for 1 day is 2 times higher than the micromort risk of having a textured breast implant for their lifetime—or that traveling 8 hours by car carries a 40-times higher micromort risk than having 2 textured breast implants for their lifetime.”
Dr Adams and his co-author analyzed data from the International Society of Aesthetic Plastic Surgery, the American Society of Plastic Surgeons, the American Society for Aesthetic Plastic Surgery, and the Austrian Breast Implant Register, as well as studies by Allergan and Sientra.
This led to a “conservative estimate” that approximately 30 million patients have textured breast implants worldwide (not including breast reconstructions).
This figure and the report of 12 deaths from BIA-ALCL worldwide suggest the risk of death from BIA-ALCL is 0.4 micromorts per patient (with 2 textured implants) or 0.2 micromorts per textured implant.
“The findings of this study are very important for patient education,” said study author David A. Sieber, MD, a plastic surgeon in private practice in San Francisco, California.
“The clear lymphoproliferative nature of BIA-ALCL, along with the calculated risks associated with its diagnosis, should be used for discussion during new consultations or at the time of presentation for evaluation of delayed-onset seromas.” ![]()
Watching von Willebrand factor in action
Researchers have gained “important mechanistic insights” into how von Willebrand factor (VWF) controls bleeding, according to an article published in Nature Communications.
Fluorescence imaging and microfluidic tools allowed the researchers to capture images of individual VWF molecules on camera while manipulating the molecules with life-like mechanical forces emulating natural blood flow.
This revealed that VWF undergoes a 2-step, shape-shifting transformation to activate blood clotting.
This transformation is triggered when VWF senses certain changes in blood flow that are indicative of injury.
“Under normal circumstances, VWF molecules are compact and globular in shape,” said study author Hongxia Fu, PhD, of Boston Children’s Hospital in Massachusetts.
“But we found that, when blood flow rate increases, VWF rapidly elongates, stretching out more and more in response to higher shear stress.”
However, elongating is not sufficient to activate blood clotting. It’s only when the tensile forces generated in the elongated VWF hit critical levels that the shape-shifter’s transformation becomes complete.
The tensile forces activate “sticky” sites along VWF, allowing it to adhere to circulating platelets.
Normally, the rush of blood needed to reach these critically high tensile forces can only occur at sites of injury inside blood vessels. This specificity enables VWF to sense blood loss and activate rapidly and locally, without activating elsewhere in the body.
“This experiment really represents a new platform for seeing and measuring what’s happening in the blood on a molecular level,” said study author Wesley P. Wong, PhD, of Boston Children’s Hospital.
“Through the use of novel microfluidic technologies that allow us to mimic the body’s vasculature in combination with single-molecule imaging techniques, we are finally able to capture striking images that uncover the mystery of nature’s forces at work in our bodies.”
Yan Jiang, PhD, of Boston Children’s Hospital, said the new findings could inspire smart drugs that are designed to treat obstructive clotting, like deep vein thrombosis, at only diseased areas of the body.
“When you’re putting a generic drug into the circulatory system, it’s taking effect everywhere, even in places that can cause detriment,” Dr Jiang said. “But what if we could design a smart drug that can mimic the 2-step shape-shifting of VWF and only takes effect in areas where clotting is likely to occur?” ![]()
Researchers have gained “important mechanistic insights” into how von Willebrand factor (VWF) controls bleeding, according to an article published in Nature Communications.
Fluorescence imaging and microfluidic tools allowed the researchers to capture images of individual VWF molecules on camera while manipulating the molecules with life-like mechanical forces emulating natural blood flow.
This revealed that VWF undergoes a 2-step, shape-shifting transformation to activate blood clotting.
This transformation is triggered when VWF senses certain changes in blood flow that are indicative of injury.
“Under normal circumstances, VWF molecules are compact and globular in shape,” said study author Hongxia Fu, PhD, of Boston Children’s Hospital in Massachusetts.
“But we found that, when blood flow rate increases, VWF rapidly elongates, stretching out more and more in response to higher shear stress.”
However, elongating is not sufficient to activate blood clotting. It’s only when the tensile forces generated in the elongated VWF hit critical levels that the shape-shifter’s transformation becomes complete.
The tensile forces activate “sticky” sites along VWF, allowing it to adhere to circulating platelets.
Normally, the rush of blood needed to reach these critically high tensile forces can only occur at sites of injury inside blood vessels. This specificity enables VWF to sense blood loss and activate rapidly and locally, without activating elsewhere in the body.
“This experiment really represents a new platform for seeing and measuring what’s happening in the blood on a molecular level,” said study author Wesley P. Wong, PhD, of Boston Children’s Hospital.
“Through the use of novel microfluidic technologies that allow us to mimic the body’s vasculature in combination with single-molecule imaging techniques, we are finally able to capture striking images that uncover the mystery of nature’s forces at work in our bodies.”
Yan Jiang, PhD, of Boston Children’s Hospital, said the new findings could inspire smart drugs that are designed to treat obstructive clotting, like deep vein thrombosis, at only diseased areas of the body.
“When you’re putting a generic drug into the circulatory system, it’s taking effect everywhere, even in places that can cause detriment,” Dr Jiang said. “But what if we could design a smart drug that can mimic the 2-step shape-shifting of VWF and only takes effect in areas where clotting is likely to occur?” ![]()
Researchers have gained “important mechanistic insights” into how von Willebrand factor (VWF) controls bleeding, according to an article published in Nature Communications.
Fluorescence imaging and microfluidic tools allowed the researchers to capture images of individual VWF molecules on camera while manipulating the molecules with life-like mechanical forces emulating natural blood flow.
This revealed that VWF undergoes a 2-step, shape-shifting transformation to activate blood clotting.
This transformation is triggered when VWF senses certain changes in blood flow that are indicative of injury.
“Under normal circumstances, VWF molecules are compact and globular in shape,” said study author Hongxia Fu, PhD, of Boston Children’s Hospital in Massachusetts.
“But we found that, when blood flow rate increases, VWF rapidly elongates, stretching out more and more in response to higher shear stress.”
However, elongating is not sufficient to activate blood clotting. It’s only when the tensile forces generated in the elongated VWF hit critical levels that the shape-shifter’s transformation becomes complete.
The tensile forces activate “sticky” sites along VWF, allowing it to adhere to circulating platelets.
Normally, the rush of blood needed to reach these critically high tensile forces can only occur at sites of injury inside blood vessels. This specificity enables VWF to sense blood loss and activate rapidly and locally, without activating elsewhere in the body.
“This experiment really represents a new platform for seeing and measuring what’s happening in the blood on a molecular level,” said study author Wesley P. Wong, PhD, of Boston Children’s Hospital.
“Through the use of novel microfluidic technologies that allow us to mimic the body’s vasculature in combination with single-molecule imaging techniques, we are finally able to capture striking images that uncover the mystery of nature’s forces at work in our bodies.”
Yan Jiang, PhD, of Boston Children’s Hospital, said the new findings could inspire smart drugs that are designed to treat obstructive clotting, like deep vein thrombosis, at only diseased areas of the body.
“When you’re putting a generic drug into the circulatory system, it’s taking effect everywhere, even in places that can cause detriment,” Dr Jiang said. “But what if we could design a smart drug that can mimic the 2-step shape-shifting of VWF and only takes effect in areas where clotting is likely to occur?” ![]()
FDA grants priority review to BLA for emicizumab
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for emicizumab.
The BLA is for emicizumab as once-weekly prophylaxis for adults, adolescents, and children with hemophilia A and factor VIII inhibitors.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the BLA for emicizumab by February 23, 2018.
About emicizumab
Emicizumab (formerly ACE910) is an investigational, bispecific monoclonal antibody designed to bring together factors IXa and X, proteins required to activate the natural coagulation cascade and restore the blood clotting process.
The drug is administered by subcutaneous injection of a ready-to-use solution. It was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The BLA for emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
HAVEN 1 is a randomized, phase 3 study in which researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis compared to on-demand bypassing agents (BPAs; no prophylaxis) in adults and adolescents (12 years of age and older) with hemophilia A and inhibitors to factor VIII.
The study included 109 patients who were previously treated with BPAs on-demand or as prophylaxis.
There was a significant reduction in treated bleeds of 87% (risk rate=0.13, P<0.0001) with emicizumab compared with no prophylaxis.
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of the BPA activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
HAVEN 2 is a single-arm, phase 3 study in which researchers are evaluating the efficacy, safety, and pharmacokinetics of once-weekly emicizumab in children (younger than 12 years of age) with hemophilia A and inhibitors to factor VIII who require treatment with BPAs.
The interim analysis included 19 children. After a median observation time of 12 weeks, 1 of the 19 children had a treated bleed. There were no reported joint or muscle bleeds.
The most common adverse events were mild injection site reactions and nasopharyngitis. No TEs or TMA events were observed. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for emicizumab.
The BLA is for emicizumab as once-weekly prophylaxis for adults, adolescents, and children with hemophilia A and factor VIII inhibitors.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the BLA for emicizumab by February 23, 2018.
About emicizumab
Emicizumab (formerly ACE910) is an investigational, bispecific monoclonal antibody designed to bring together factors IXa and X, proteins required to activate the natural coagulation cascade and restore the blood clotting process.
The drug is administered by subcutaneous injection of a ready-to-use solution. It was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The BLA for emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
HAVEN 1 is a randomized, phase 3 study in which researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis compared to on-demand bypassing agents (BPAs; no prophylaxis) in adults and adolescents (12 years of age and older) with hemophilia A and inhibitors to factor VIII.
The study included 109 patients who were previously treated with BPAs on-demand or as prophylaxis.
There was a significant reduction in treated bleeds of 87% (risk rate=0.13, P<0.0001) with emicizumab compared with no prophylaxis.
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of the BPA activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
HAVEN 2 is a single-arm, phase 3 study in which researchers are evaluating the efficacy, safety, and pharmacokinetics of once-weekly emicizumab in children (younger than 12 years of age) with hemophilia A and inhibitors to factor VIII who require treatment with BPAs.
The interim analysis included 19 children. After a median observation time of 12 weeks, 1 of the 19 children had a treated bleed. There were no reported joint or muscle bleeds.
The most common adverse events were mild injection site reactions and nasopharyngitis. No TEs or TMA events were observed. ![]()
The US Food and Drug Administration (FDA) has accepted for priority review the biologics license application (BLA) for emicizumab.
The BLA is for emicizumab as once-weekly prophylaxis for adults, adolescents, and children with hemophilia A and factor VIII inhibitors.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA plans to make a decision on the BLA for emicizumab by February 23, 2018.
About emicizumab
Emicizumab (formerly ACE910) is an investigational, bispecific monoclonal antibody designed to bring together factors IXa and X, proteins required to activate the natural coagulation cascade and restore the blood clotting process.
The drug is administered by subcutaneous injection of a ready-to-use solution. It was created by Chugai Pharmaceutical Co., Ltd. and is being co-developed by Chugai, Roche, and Genentech.
The BLA for emicizumab is based on results from a pair of phase 3 studies—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July. Interim results from HAVEN 2 were presented at ISTH as well.
HAVEN 1
HAVEN 1 is a randomized, phase 3 study in which researchers evaluated the efficacy, safety, and pharmacokinetics of emicizumab prophylaxis compared to on-demand bypassing agents (BPAs; no prophylaxis) in adults and adolescents (12 years of age and older) with hemophilia A and inhibitors to factor VIII.
The study included 109 patients who were previously treated with BPAs on-demand or as prophylaxis.
There was a significant reduction in treated bleeds of 87% (risk rate=0.13, P<0.0001) with emicizumab compared with no prophylaxis.
Adverse events occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of the BPA activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
HAVEN 2 is a single-arm, phase 3 study in which researchers are evaluating the efficacy, safety, and pharmacokinetics of once-weekly emicizumab in children (younger than 12 years of age) with hemophilia A and inhibitors to factor VIII who require treatment with BPAs.
The interim analysis included 19 children. After a median observation time of 12 weeks, 1 of the 19 children had a treated bleed. There were no reported joint or muscle bleeds.
The most common adverse events were mild injection site reactions and nasopharyngitis. No TEs or TMA events were observed. ![]()
Even short-term steroids can be problematic
Clinical Question: What is the frequency of short-term corticosteroid prescriptions and adverse events associated with their use?
Background: Long-term corticosteroid use is usually avoided given risks of complications. Less is known about the risk and frequency of short-term corticosteroid use.
Study Design: Retrospective cohort study and self-controlled case series.
Setting: National U.S. dataset of private insurance claims.
Synopsis: Data from 1,548,945 adults (aged 18-64 years) showed that 21.1% of adults received a prescription for short-term corticosteroids. Within 30 days of filling corticosteroids, incident rate ratios (IRR) were increased for sepsis (5.3; 95% confidence interval, 3.8-7.4), venous thromboembolism (3.3; 95% CI, 2.78-3.99), and fracture (1.87; 95% CI, 1.69-2.07).
Short-term corticosteroids were frequently prescribed for indications with little evidence of benefit, such as upper respiratory conditions, spinal conditions, and allergies. For these conditions, patients should be educated about the risks of short-term corticosteroid use and alternative treatments should be considered. This study only evaluated for these three adverse reactions and excluded the elderly, so these findings likely underestimate the adverse effects of short-term corticosteroids.
Bottom Line: Corticosteroids are frequently prescribed for short courses and were associated with increased rates of sepsis, venous thromboembolism, and fracture.
Citation: Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ. 2017;357:j1415.
Dr. Gray is assistant professor in the University of Kentucky division of hospital medicine and the Lexington VA Medical Center.
Clinical Question: What is the frequency of short-term corticosteroid prescriptions and adverse events associated with their use?
Background: Long-term corticosteroid use is usually avoided given risks of complications. Less is known about the risk and frequency of short-term corticosteroid use.
Study Design: Retrospective cohort study and self-controlled case series.
Setting: National U.S. dataset of private insurance claims.
Synopsis: Data from 1,548,945 adults (aged 18-64 years) showed that 21.1% of adults received a prescription for short-term corticosteroids. Within 30 days of filling corticosteroids, incident rate ratios (IRR) were increased for sepsis (5.3; 95% confidence interval, 3.8-7.4), venous thromboembolism (3.3; 95% CI, 2.78-3.99), and fracture (1.87; 95% CI, 1.69-2.07).
Short-term corticosteroids were frequently prescribed for indications with little evidence of benefit, such as upper respiratory conditions, spinal conditions, and allergies. For these conditions, patients should be educated about the risks of short-term corticosteroid use and alternative treatments should be considered. This study only evaluated for these three adverse reactions and excluded the elderly, so these findings likely underestimate the adverse effects of short-term corticosteroids.
Bottom Line: Corticosteroids are frequently prescribed for short courses and were associated with increased rates of sepsis, venous thromboembolism, and fracture.
Citation: Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ. 2017;357:j1415.
Dr. Gray is assistant professor in the University of Kentucky division of hospital medicine and the Lexington VA Medical Center.
Clinical Question: What is the frequency of short-term corticosteroid prescriptions and adverse events associated with their use?
Background: Long-term corticosteroid use is usually avoided given risks of complications. Less is known about the risk and frequency of short-term corticosteroid use.
Study Design: Retrospective cohort study and self-controlled case series.
Setting: National U.S. dataset of private insurance claims.
Synopsis: Data from 1,548,945 adults (aged 18-64 years) showed that 21.1% of adults received a prescription for short-term corticosteroids. Within 30 days of filling corticosteroids, incident rate ratios (IRR) were increased for sepsis (5.3; 95% confidence interval, 3.8-7.4), venous thromboembolism (3.3; 95% CI, 2.78-3.99), and fracture (1.87; 95% CI, 1.69-2.07).
Short-term corticosteroids were frequently prescribed for indications with little evidence of benefit, such as upper respiratory conditions, spinal conditions, and allergies. For these conditions, patients should be educated about the risks of short-term corticosteroid use and alternative treatments should be considered. This study only evaluated for these three adverse reactions and excluded the elderly, so these findings likely underestimate the adverse effects of short-term corticosteroids.
Bottom Line: Corticosteroids are frequently prescribed for short courses and were associated with increased rates of sepsis, venous thromboembolism, and fracture.
Citation: Waljee AK, Rogers MA, Lin P, et al. Short term use of oral corticosteroids and related harms among adults in the United States: Population based cohort study. BMJ. 2017;357:j1415.
Dr. Gray is assistant professor in the University of Kentucky division of hospital medicine and the Lexington VA Medical Center.
Axillary thermometry is the best choice for newborns
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
NASHVILLE, TENN. – Axillary thermometry outperformed both rectal and temporal artery thermometry in 205 newborns aged 12-72 hours in a study performed at the University of North Carolina at Chapel Hill.
The infants had two temperatures taken by each method over a period of 15 minutes, for a total of six readings per child and 1,230 measurements overall. Axillary thermometry proved both accurate and reliable. Rectal thermometry was accurate but less reliable, and temporal thermometry was reliable but less accurate.
Lead investigator Ketan Nadkarni, MD, a 3rd-year pediatrics resident, and his colleagues wanted to compare the three methods head-to-head to make sure axillary thermometers were okay to use in the nursery, and to see if it really was necessary to tell parents to use rectal thermometers; many are reluctant to use them. Plus, “there’s been a lot of controversy” in pediatrics “over the best way to measure temperature,” Dr. Nadkarni said at the Pediatric Hospital Medicine annual meeting.
“With our data, we think axillary is what we should continue to use in the newborn nursery,” he said. Some attending physicians still are hesitant to recommend axillary thermometers to new parents, but “all of the nurses are aware of” the study findings “and a lot of the residents are, too, so I think we are starting to move” in that direction.
The study had some unexpected findings as well: “The biggest surprise was how wide the distribution of rectal temperatures was. The distribution” around the mean “was way larger than we had thought, so [rectal thermometry was] not very reliable at all. Our study surprisingly exhibited suboptimal performance in terms of reliability,” for rectal thermometry, he said at the meeting, which was sponsored by the Society of Hospital Medicine, the American Academy of Pediatrics, and the Academic Pediatric Association.
Specifically, the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F. The second rectal temperature in the study sometimes varied a half a degree or more from the first taken shortly before, in the same infant.
The average distance of an axillary temperature from the axillary mean of 98.32º F was 0.32º F; for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Another surprise was that temporal thermometry overestimated temperature by an average of about a quarter of a degree, compared with rectal readings. Even small overestimates could lead to unnecessary sepsis work-ups; “the last thing we want is to hospitalize these kids when they don’t need to be,” Dr. Nadkarni said.
The mean axillary and rectal temperatures, meanwhile, were only 0.02º F apart, which was not statistically significant. “Axillary was absolutely interchangeable with rectal in terms of accuracy,” he said.
The children were born at 37 weeks’ gestation or later, and were excluded if they had a temperature of 100.4º F or higher by any method. Rectal and axillary temperatures were taken with a Welch Allyn SureTemp Plus 690. Temple temperatures were taken with an Exergen TAT-2000c.
The investigators plan to run a similar trial in the ED with children up to 3 months old.
There was no external funding for the work, and Dr. Nadkarni had no relevant financial disclosures.
AT PHM 2017
Key clinical point:
Major finding: The average distance of an axillary temperature from the axillary mean of 98.32º F was only 0.32º F, while the average distance of any given rectal measurement from the mean rectal temperature of 98.3º F was 0.45º F, and for temporal thermometry it was 0.34º F from a mean of 98.55º F.
Data source: Head-to-head thermometry study in more than 200 infants aged 12-72 hours.
Disclosures: There was no outside funding, and Dr. Nadkarni had no relevant financial disclosures.
All guts and glory – esophagus, stomach, small intestine
Prakash Gyawali, MD, led off the session with a lecture on functional heartburn, which he defined as burning retrosternal discomfort not relieved by antisecretory therapy, in a patient for whom endoscopy, esophageal pH monitoring, and manometry have revealed no evidence of gastroesophageal reflux disease (GERD), eosinophilic esophagitis (EoE), or major esophageal motility disorders. When performing pH monitoring to assess for functional heartburn, Dr. Gyawali advised that the test be done with patients off of proton pump inhibitors (PPIs). Despite similarity to the heartburn sensation of GERD, Dr. Gyawali pointed out how functional heartburn has features resembling irritable bowel syndrome, such as its association with anxiety and depression, and its response to neuromodulators (e.g., antidepressants). He discussed how new impedance-based esophageal metrics such as the postswallow-induced peristaltic wave index and measurement of mean nocturnal baseline impedance might be used to confirm a diagnosis of functional heartburn. Although neuromodulators remain the mainstay of management for functional heartburn, Dr. Gyawali discussed encouraging preliminary results of studies on psychotherapy, hypnotherapy, and alternative therapies such as acupuncture.
Rhonda Souza, MD, AGAF, discussed EoE, focusing especially on the controversial condition of PPI-responsive esophageal eosinophilia (PPI-REE) in which patients have typical EoE symptoms and histology, with no evidence of GERD by endoscopy or esophageal pH monitoring, yet they respond to PPI therapy. She discussed two possible explanations for PPI-REE: 1) the patients have subclinical GERD that responds to PPI antisecretory effects, or 2) the patients have EoE that responds to PPI anti-inflammatory effects. Dr. Souza reviewed data from her laboratory showing that omeprazole can block the secretion of eotaxin-3, a potent eosinophil chemoattractant in esophageal epithelial cells stimulated with allergic (Th2) cytokines in vitro. This potential anti-inflammatory PPI effect is entirely independent of any effect on gastric acid inhibition. After reviewing recent clinical and esophageal transcriptome data, Dr. Souza concluded that PPI-REE is probably just a subset of EoE, not an independent disorder.
John Inadomi, MD, AGAF, discussed Barrett’s esophagus and esophageal adenocarcinoma. He pointed out the inadequacy of current screening programs, noting studies showing that less than 10% of patients found to have esophageal adenocarcinoma had a prior diagnosis of Barrett’s esophagus. He estimated the annual incidence of adenocarcinoma at 0.12%-0.5% for patients with nondysplastic Barrett’s metaplasia. He discussed how current American College of Gastroenterology guidelines call for screening men who have chronic GERD symptoms with at least two other Barrett’s cancer risk factors (age greater than 50 years, white race, central obesity, cigarette smoking, family history of Barrett’s esophagus), and noted how the very low risk of esophageal adenocarcinoma in women (similar to the risk of breast cancer in men) supports the ACG recommendation not to screen women routinely for Barrett’s esophagus. Dr. Inadomi recommended endoscopic eradication as the preferred treatment for patients with confirmed dysplasia of any grade. He also noted that the removal of nodular lesions by endoscopic mucosal resection or endoscopic submucosal dissection is a crucial part of endoscopic eradication therapy.
In a lecture titled “The truth about PPIs,” Byron Cryer, MD, reviewed a number of potential adverse effects of PPIs, including Clostridium difficile–associated diarrhea, bone fractures, kidney disease, dementia, myocardial infarction, and interactions with clopidogrel. He pointed out that most of these putative adverse effects have been identified as modest increases in risks noted in observational studies, and the quality of this evidence is considered low or very low. In contrast, the benefits of PPIs for patients with complicated GERD and for patients at risk for NSAID complications have been established in high-quality, randomized controlled trials. Dr. Cryer concluded that, when PPIs are prescribed appropriately, their benefits likely outweigh their risks. However, he also noted that PPIs frequently are prescribed inappropriately, in which case they have no benefit and their potential for risk assumes greater importance.
Sheila Crowe, MD, AGAF, delivered the last lecture of the session, discussing celiac disease and gluten sensitivity. She reviewed recent data suggesting a role for infection with reovirus in triggering the development of celiac disease, and she noted that tissue transglutaminase IgA remains the best test to screen for the condition. She discussed the controversial topic of nonceliac gluten sensitivity, in which patients report symptoms or health alterations that they perceive to be the result of gluten ingestion. She pointed out difficulties in establishing an unequivocal diagnosis of nonceliac gluten sensitivity and, for patients without celiac disease, she highlighted a number of potential drawbacks of a gluten-free diet, including its expense, higher fat and sugar content, and increased levels of toxic metals such as arsenic and mercury.
Dr. Spechler is chief of the division of gastroenterology and co-director of the Center for Esophageal Diseases, Baylor University Medical Center at Dallas; he is an investigator/professor and co-director of the Center for Esophageal Research, Baylor Scott and White Research Institute, Dallas. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
Prakash Gyawali, MD, led off the session with a lecture on functional heartburn, which he defined as burning retrosternal discomfort not relieved by antisecretory therapy, in a patient for whom endoscopy, esophageal pH monitoring, and manometry have revealed no evidence of gastroesophageal reflux disease (GERD), eosinophilic esophagitis (EoE), or major esophageal motility disorders. When performing pH monitoring to assess for functional heartburn, Dr. Gyawali advised that the test be done with patients off of proton pump inhibitors (PPIs). Despite similarity to the heartburn sensation of GERD, Dr. Gyawali pointed out how functional heartburn has features resembling irritable bowel syndrome, such as its association with anxiety and depression, and its response to neuromodulators (e.g., antidepressants). He discussed how new impedance-based esophageal metrics such as the postswallow-induced peristaltic wave index and measurement of mean nocturnal baseline impedance might be used to confirm a diagnosis of functional heartburn. Although neuromodulators remain the mainstay of management for functional heartburn, Dr. Gyawali discussed encouraging preliminary results of studies on psychotherapy, hypnotherapy, and alternative therapies such as acupuncture.
Rhonda Souza, MD, AGAF, discussed EoE, focusing especially on the controversial condition of PPI-responsive esophageal eosinophilia (PPI-REE) in which patients have typical EoE symptoms and histology, with no evidence of GERD by endoscopy or esophageal pH monitoring, yet they respond to PPI therapy. She discussed two possible explanations for PPI-REE: 1) the patients have subclinical GERD that responds to PPI antisecretory effects, or 2) the patients have EoE that responds to PPI anti-inflammatory effects. Dr. Souza reviewed data from her laboratory showing that omeprazole can block the secretion of eotaxin-3, a potent eosinophil chemoattractant in esophageal epithelial cells stimulated with allergic (Th2) cytokines in vitro. This potential anti-inflammatory PPI effect is entirely independent of any effect on gastric acid inhibition. After reviewing recent clinical and esophageal transcriptome data, Dr. Souza concluded that PPI-REE is probably just a subset of EoE, not an independent disorder.
John Inadomi, MD, AGAF, discussed Barrett’s esophagus and esophageal adenocarcinoma. He pointed out the inadequacy of current screening programs, noting studies showing that less than 10% of patients found to have esophageal adenocarcinoma had a prior diagnosis of Barrett’s esophagus. He estimated the annual incidence of adenocarcinoma at 0.12%-0.5% for patients with nondysplastic Barrett’s metaplasia. He discussed how current American College of Gastroenterology guidelines call for screening men who have chronic GERD symptoms with at least two other Barrett’s cancer risk factors (age greater than 50 years, white race, central obesity, cigarette smoking, family history of Barrett’s esophagus), and noted how the very low risk of esophageal adenocarcinoma in women (similar to the risk of breast cancer in men) supports the ACG recommendation not to screen women routinely for Barrett’s esophagus. Dr. Inadomi recommended endoscopic eradication as the preferred treatment for patients with confirmed dysplasia of any grade. He also noted that the removal of nodular lesions by endoscopic mucosal resection or endoscopic submucosal dissection is a crucial part of endoscopic eradication therapy.
In a lecture titled “The truth about PPIs,” Byron Cryer, MD, reviewed a number of potential adverse effects of PPIs, including Clostridium difficile–associated diarrhea, bone fractures, kidney disease, dementia, myocardial infarction, and interactions with clopidogrel. He pointed out that most of these putative adverse effects have been identified as modest increases in risks noted in observational studies, and the quality of this evidence is considered low or very low. In contrast, the benefits of PPIs for patients with complicated GERD and for patients at risk for NSAID complications have been established in high-quality, randomized controlled trials. Dr. Cryer concluded that, when PPIs are prescribed appropriately, their benefits likely outweigh their risks. However, he also noted that PPIs frequently are prescribed inappropriately, in which case they have no benefit and their potential for risk assumes greater importance.
Sheila Crowe, MD, AGAF, delivered the last lecture of the session, discussing celiac disease and gluten sensitivity. She reviewed recent data suggesting a role for infection with reovirus in triggering the development of celiac disease, and she noted that tissue transglutaminase IgA remains the best test to screen for the condition. She discussed the controversial topic of nonceliac gluten sensitivity, in which patients report symptoms or health alterations that they perceive to be the result of gluten ingestion. She pointed out difficulties in establishing an unequivocal diagnosis of nonceliac gluten sensitivity and, for patients without celiac disease, she highlighted a number of potential drawbacks of a gluten-free diet, including its expense, higher fat and sugar content, and increased levels of toxic metals such as arsenic and mercury.
Dr. Spechler is chief of the division of gastroenterology and co-director of the Center for Esophageal Diseases, Baylor University Medical Center at Dallas; he is an investigator/professor and co-director of the Center for Esophageal Research, Baylor Scott and White Research Institute, Dallas. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
Prakash Gyawali, MD, led off the session with a lecture on functional heartburn, which he defined as burning retrosternal discomfort not relieved by antisecretory therapy, in a patient for whom endoscopy, esophageal pH monitoring, and manometry have revealed no evidence of gastroesophageal reflux disease (GERD), eosinophilic esophagitis (EoE), or major esophageal motility disorders. When performing pH monitoring to assess for functional heartburn, Dr. Gyawali advised that the test be done with patients off of proton pump inhibitors (PPIs). Despite similarity to the heartburn sensation of GERD, Dr. Gyawali pointed out how functional heartburn has features resembling irritable bowel syndrome, such as its association with anxiety and depression, and its response to neuromodulators (e.g., antidepressants). He discussed how new impedance-based esophageal metrics such as the postswallow-induced peristaltic wave index and measurement of mean nocturnal baseline impedance might be used to confirm a diagnosis of functional heartburn. Although neuromodulators remain the mainstay of management for functional heartburn, Dr. Gyawali discussed encouraging preliminary results of studies on psychotherapy, hypnotherapy, and alternative therapies such as acupuncture.
Rhonda Souza, MD, AGAF, discussed EoE, focusing especially on the controversial condition of PPI-responsive esophageal eosinophilia (PPI-REE) in which patients have typical EoE symptoms and histology, with no evidence of GERD by endoscopy or esophageal pH monitoring, yet they respond to PPI therapy. She discussed two possible explanations for PPI-REE: 1) the patients have subclinical GERD that responds to PPI antisecretory effects, or 2) the patients have EoE that responds to PPI anti-inflammatory effects. Dr. Souza reviewed data from her laboratory showing that omeprazole can block the secretion of eotaxin-3, a potent eosinophil chemoattractant in esophageal epithelial cells stimulated with allergic (Th2) cytokines in vitro. This potential anti-inflammatory PPI effect is entirely independent of any effect on gastric acid inhibition. After reviewing recent clinical and esophageal transcriptome data, Dr. Souza concluded that PPI-REE is probably just a subset of EoE, not an independent disorder.
John Inadomi, MD, AGAF, discussed Barrett’s esophagus and esophageal adenocarcinoma. He pointed out the inadequacy of current screening programs, noting studies showing that less than 10% of patients found to have esophageal adenocarcinoma had a prior diagnosis of Barrett’s esophagus. He estimated the annual incidence of adenocarcinoma at 0.12%-0.5% for patients with nondysplastic Barrett’s metaplasia. He discussed how current American College of Gastroenterology guidelines call for screening men who have chronic GERD symptoms with at least two other Barrett’s cancer risk factors (age greater than 50 years, white race, central obesity, cigarette smoking, family history of Barrett’s esophagus), and noted how the very low risk of esophageal adenocarcinoma in women (similar to the risk of breast cancer in men) supports the ACG recommendation not to screen women routinely for Barrett’s esophagus. Dr. Inadomi recommended endoscopic eradication as the preferred treatment for patients with confirmed dysplasia of any grade. He also noted that the removal of nodular lesions by endoscopic mucosal resection or endoscopic submucosal dissection is a crucial part of endoscopic eradication therapy.
In a lecture titled “The truth about PPIs,” Byron Cryer, MD, reviewed a number of potential adverse effects of PPIs, including Clostridium difficile–associated diarrhea, bone fractures, kidney disease, dementia, myocardial infarction, and interactions with clopidogrel. He pointed out that most of these putative adverse effects have been identified as modest increases in risks noted in observational studies, and the quality of this evidence is considered low or very low. In contrast, the benefits of PPIs for patients with complicated GERD and for patients at risk for NSAID complications have been established in high-quality, randomized controlled trials. Dr. Cryer concluded that, when PPIs are prescribed appropriately, their benefits likely outweigh their risks. However, he also noted that PPIs frequently are prescribed inappropriately, in which case they have no benefit and their potential for risk assumes greater importance.
Sheila Crowe, MD, AGAF, delivered the last lecture of the session, discussing celiac disease and gluten sensitivity. She reviewed recent data suggesting a role for infection with reovirus in triggering the development of celiac disease, and she noted that tissue transglutaminase IgA remains the best test to screen for the condition. She discussed the controversial topic of nonceliac gluten sensitivity, in which patients report symptoms or health alterations that they perceive to be the result of gluten ingestion. She pointed out difficulties in establishing an unequivocal diagnosis of nonceliac gluten sensitivity and, for patients without celiac disease, she highlighted a number of potential drawbacks of a gluten-free diet, including its expense, higher fat and sugar content, and increased levels of toxic metals such as arsenic and mercury.
Dr. Spechler is chief of the division of gastroenterology and co-director of the Center for Esophageal Diseases, Baylor University Medical Center at Dallas; he is an investigator/professor and co-director of the Center for Esophageal Research, Baylor Scott and White Research Institute, Dallas. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
Hot topics in 2017
The 2017 Postgraduate Course started out with four hot topics that dominated the year – opioid dependence, a cure for hepatitis C, and understanding and then manipulating the microbiome. From David Dickerson, MD, we learned that abdominal pain is complex and with an evolving classification scheme. Ignoring the biopsychosocial aspects and origins of pain is a sure way to lead to addiction and “pain behavior.” He reviewed the opioid guidelines that involve a comprehensive approach to therapy – setting functional goals, assessing the risks and benefits, and using the lowest necessary doses of short-acting agents for a defined period of time and then reassessing. In patients with chronic pain and opioid dependence, the gastroenterologist should seek the help of a chronic pain specialist. We should also refer for nonpharmacologic therapy such as cognitive behavioral therapy and biofeedback.
Norah Terrault, MD, MPH, discussed management of chronic hepatitis C (HCV) after the cure, achievable in more than 95% of patients, which has resulted in a sharp decline in listings for liver transplant. A cure is defined as undetectable HCV RNA 12 weeks after completion of therapy. So what happens next? If the patient is at risk for reinfection, they should have HCV RNA testing annually or if their liver enzymes increase. Otherwise, if their pretreatment fibrosis is low stage, no further monitoring is needed and they can follow up with their primary care provider. Intermediate-stage fibrosis should be monitored for progression. Advanced-stage fibrosis needs long-term follow-up for hepatocellular carcinoma and variceal surveillance. Modifiable risk factors, i.e., metabolic fatty liver and alcohol abuse, should be identified with appropriate counseling provided.
We went back to the microbiome for our last talk: Larry Brandt, MD, AGAF, discussed FMT for Clostridium difficile infection (CDI). Patients should be considered for FMT if they have more than 3 recurrences of mild to moderate CDI and failure to respond to standard therapy; more than 2 episodes of CDI resulting in hospitalization and significant morbidity; moderate CDI with no response after 1 week of standard therapy; and severe CDI with no response to standard therapy within 48 hours. Serious adverse events associated with FMT include infections and perhaps new-onset immune-mediated disease such as Sjogren’s, rheumatoid arthritis, and idiopathic thrombocytopenic purpura. It is hoped that the NIH-sponsored AGA national registry for FMT will help better define outcomes and adverse events over the next 10 years.
Dr. Mahadevan is professor of clinical medicine at UCSF Medical Center, San Francisco. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
The 2017 Postgraduate Course started out with four hot topics that dominated the year – opioid dependence, a cure for hepatitis C, and understanding and then manipulating the microbiome. From David Dickerson, MD, we learned that abdominal pain is complex and with an evolving classification scheme. Ignoring the biopsychosocial aspects and origins of pain is a sure way to lead to addiction and “pain behavior.” He reviewed the opioid guidelines that involve a comprehensive approach to therapy – setting functional goals, assessing the risks and benefits, and using the lowest necessary doses of short-acting agents for a defined period of time and then reassessing. In patients with chronic pain and opioid dependence, the gastroenterologist should seek the help of a chronic pain specialist. We should also refer for nonpharmacologic therapy such as cognitive behavioral therapy and biofeedback.
Norah Terrault, MD, MPH, discussed management of chronic hepatitis C (HCV) after the cure, achievable in more than 95% of patients, which has resulted in a sharp decline in listings for liver transplant. A cure is defined as undetectable HCV RNA 12 weeks after completion of therapy. So what happens next? If the patient is at risk for reinfection, they should have HCV RNA testing annually or if their liver enzymes increase. Otherwise, if their pretreatment fibrosis is low stage, no further monitoring is needed and they can follow up with their primary care provider. Intermediate-stage fibrosis should be monitored for progression. Advanced-stage fibrosis needs long-term follow-up for hepatocellular carcinoma and variceal surveillance. Modifiable risk factors, i.e., metabolic fatty liver and alcohol abuse, should be identified with appropriate counseling provided.
We went back to the microbiome for our last talk: Larry Brandt, MD, AGAF, discussed FMT for Clostridium difficile infection (CDI). Patients should be considered for FMT if they have more than 3 recurrences of mild to moderate CDI and failure to respond to standard therapy; more than 2 episodes of CDI resulting in hospitalization and significant morbidity; moderate CDI with no response after 1 week of standard therapy; and severe CDI with no response to standard therapy within 48 hours. Serious adverse events associated with FMT include infections and perhaps new-onset immune-mediated disease such as Sjogren’s, rheumatoid arthritis, and idiopathic thrombocytopenic purpura. It is hoped that the NIH-sponsored AGA national registry for FMT will help better define outcomes and adverse events over the next 10 years.
Dr. Mahadevan is professor of clinical medicine at UCSF Medical Center, San Francisco. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
The 2017 Postgraduate Course started out with four hot topics that dominated the year – opioid dependence, a cure for hepatitis C, and understanding and then manipulating the microbiome. From David Dickerson, MD, we learned that abdominal pain is complex and with an evolving classification scheme. Ignoring the biopsychosocial aspects and origins of pain is a sure way to lead to addiction and “pain behavior.” He reviewed the opioid guidelines that involve a comprehensive approach to therapy – setting functional goals, assessing the risks and benefits, and using the lowest necessary doses of short-acting agents for a defined period of time and then reassessing. In patients with chronic pain and opioid dependence, the gastroenterologist should seek the help of a chronic pain specialist. We should also refer for nonpharmacologic therapy such as cognitive behavioral therapy and biofeedback.
Norah Terrault, MD, MPH, discussed management of chronic hepatitis C (HCV) after the cure, achievable in more than 95% of patients, which has resulted in a sharp decline in listings for liver transplant. A cure is defined as undetectable HCV RNA 12 weeks after completion of therapy. So what happens next? If the patient is at risk for reinfection, they should have HCV RNA testing annually or if their liver enzymes increase. Otherwise, if their pretreatment fibrosis is low stage, no further monitoring is needed and they can follow up with their primary care provider. Intermediate-stage fibrosis should be monitored for progression. Advanced-stage fibrosis needs long-term follow-up for hepatocellular carcinoma and variceal surveillance. Modifiable risk factors, i.e., metabolic fatty liver and alcohol abuse, should be identified with appropriate counseling provided.
We went back to the microbiome for our last talk: Larry Brandt, MD, AGAF, discussed FMT for Clostridium difficile infection (CDI). Patients should be considered for FMT if they have more than 3 recurrences of mild to moderate CDI and failure to respond to standard therapy; more than 2 episodes of CDI resulting in hospitalization and significant morbidity; moderate CDI with no response after 1 week of standard therapy; and severe CDI with no response to standard therapy within 48 hours. Serious adverse events associated with FMT include infections and perhaps new-onset immune-mediated disease such as Sjogren’s, rheumatoid arthritis, and idiopathic thrombocytopenic purpura. It is hoped that the NIH-sponsored AGA national registry for FMT will help better define outcomes and adverse events over the next 10 years.
Dr. Mahadevan is professor of clinical medicine at UCSF Medical Center, San Francisco. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
Latest U.S. alcohol use data critiqued
The findings of a recent study in JAMA Psychiatry suggest dramatic increases – approaching 50% – in the prevalence of alcohol use disorders in the United States. But the study’s methodology has come under scrutiny: The data sets that the researchers used might be too different for reliable comparison.
The JAMA Psychiatry research, led by Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism, analyzed data from two waves of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a cross-sectional, federal survey administered during 2001-2002, known as Wave 1, and during 2012-2013, known as Wave 3 (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161). The findings were stark: In addition to a sharp increase in the rate of 12-month alcohol use and high-risk drinking, Dr. Grant and her associates found that the rate of DSM-IV alcohol use disorder climbed from 8.5% of the population during the first wave to 12.7% of the population during the third.
“I would urge caution in drawing conclusions based on only two time points,” Dr. Grucza said. Comparing those two waves of data is inherently problematic, he explained.
“The NESARC made important changes between 2001-2002 and 2012-2013 – and several of these changes could be expected to lead to higher rates of disclosure,” said Dr. Grucza, a professor of psychiatry at Washington University in St. Louis. “This would lead to apparent increases in a variety of things – for example, they saw a 100% increase in prevalence of marijuana use among adults. So, the problem isn’t with the 2012-2013 NESARC, per se, but in the comparison between the 2012-2013 NESARC and the 2001-2002 NESARC.”
The changes include differences in the way each wave of the data was collected. The 2001-2002 survey was conducted using U.S. Census Bureau employees and didn’t offer participants incentives for survey completion. The 2012-2013 NESARC, however, was conducted through a private contractor; in addition, participants provided biological samples and received modest cash incentives for completing the survey.
“We can only speculate, but [the collection of DNA through samples of saliva] might make the participants think that their drug use would be known – and that they might therefore disclose it, anyway,” Dr. Grucza said. “I would guess that they would have been assured that this wasn’t the case during the informed consent process, but that process tends to be long, and people don’t pay attention to the whole informed consent document.”
The NESARC findings differed substantially from those found by the National Survey on Drug Use and Health (NSDUH), a federal survey of people aged 12 or older released annually by the Substance Abuse and Mental Health Services Administration. The NSDUH gathers data first in a screening phase and then in an interview phase; data are gathered in the interview phase through computer-assisted, self-administered interviews. Dr. Grucza said countless studies “suggest people respond more faithfully when disclosing to a computer as opposed to a live interviewer.”
According to the NSDUH, the rate of past-month use of alcohol fell slightly in 2015 from the previous year’s rate and was comparable to the estimates in 2005-2013.
However, Dr. Grant pointed out that substantial changes made to the questions on the 2015 NSDUH also make comparisons to previous years problematic. As a result, she said, the “NSDUH is not able to estimate the trends during the time period analyzed in the NESARC,” Dr. Grant said. “We stand by the reliability and validity of our survey data.”
For his part, Robert L. DuPont, MD, said it’s important to look at the data broadly. “The bigger picture is that alcohol is the most commonly used addictive substance by Americans, and there is evidence of increased problems resulting from drinking. For example, alcohol liver disease and cirrhosis of the liver are rising in adults and have been described as one of a group of ‘diseases of despair,’ along with suicide and drug overdose deaths,” said Dr. DuPont, the first director of the National Institute on Drug Abuse and the president of the Institute for Behavior and Health in Rockville, Md. “The fundamental message is that alcohol (and drug use) patterns show complexity that has significant public health importance. Alcohol use and related problems are more complex than a bumper sticker stating that they are either ‘up’ or ‘down.’ ”
Dr. Grucza, Dr. Grant, and Dr. DuPont had no conflicts to disclose.
The findings of a recent study in JAMA Psychiatry suggest dramatic increases – approaching 50% – in the prevalence of alcohol use disorders in the United States. But the study’s methodology has come under scrutiny: The data sets that the researchers used might be too different for reliable comparison.
The JAMA Psychiatry research, led by Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism, analyzed data from two waves of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a cross-sectional, federal survey administered during 2001-2002, known as Wave 1, and during 2012-2013, known as Wave 3 (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161). The findings were stark: In addition to a sharp increase in the rate of 12-month alcohol use and high-risk drinking, Dr. Grant and her associates found that the rate of DSM-IV alcohol use disorder climbed from 8.5% of the population during the first wave to 12.7% of the population during the third.
“I would urge caution in drawing conclusions based on only two time points,” Dr. Grucza said. Comparing those two waves of data is inherently problematic, he explained.
“The NESARC made important changes between 2001-2002 and 2012-2013 – and several of these changes could be expected to lead to higher rates of disclosure,” said Dr. Grucza, a professor of psychiatry at Washington University in St. Louis. “This would lead to apparent increases in a variety of things – for example, they saw a 100% increase in prevalence of marijuana use among adults. So, the problem isn’t with the 2012-2013 NESARC, per se, but in the comparison between the 2012-2013 NESARC and the 2001-2002 NESARC.”
The changes include differences in the way each wave of the data was collected. The 2001-2002 survey was conducted using U.S. Census Bureau employees and didn’t offer participants incentives for survey completion. The 2012-2013 NESARC, however, was conducted through a private contractor; in addition, participants provided biological samples and received modest cash incentives for completing the survey.
“We can only speculate, but [the collection of DNA through samples of saliva] might make the participants think that their drug use would be known – and that they might therefore disclose it, anyway,” Dr. Grucza said. “I would guess that they would have been assured that this wasn’t the case during the informed consent process, but that process tends to be long, and people don’t pay attention to the whole informed consent document.”
The NESARC findings differed substantially from those found by the National Survey on Drug Use and Health (NSDUH), a federal survey of people aged 12 or older released annually by the Substance Abuse and Mental Health Services Administration. The NSDUH gathers data first in a screening phase and then in an interview phase; data are gathered in the interview phase through computer-assisted, self-administered interviews. Dr. Grucza said countless studies “suggest people respond more faithfully when disclosing to a computer as opposed to a live interviewer.”
According to the NSDUH, the rate of past-month use of alcohol fell slightly in 2015 from the previous year’s rate and was comparable to the estimates in 2005-2013.
However, Dr. Grant pointed out that substantial changes made to the questions on the 2015 NSDUH also make comparisons to previous years problematic. As a result, she said, the “NSDUH is not able to estimate the trends during the time period analyzed in the NESARC,” Dr. Grant said. “We stand by the reliability and validity of our survey data.”
For his part, Robert L. DuPont, MD, said it’s important to look at the data broadly. “The bigger picture is that alcohol is the most commonly used addictive substance by Americans, and there is evidence of increased problems resulting from drinking. For example, alcohol liver disease and cirrhosis of the liver are rising in adults and have been described as one of a group of ‘diseases of despair,’ along with suicide and drug overdose deaths,” said Dr. DuPont, the first director of the National Institute on Drug Abuse and the president of the Institute for Behavior and Health in Rockville, Md. “The fundamental message is that alcohol (and drug use) patterns show complexity that has significant public health importance. Alcohol use and related problems are more complex than a bumper sticker stating that they are either ‘up’ or ‘down.’ ”
Dr. Grucza, Dr. Grant, and Dr. DuPont had no conflicts to disclose.
The findings of a recent study in JAMA Psychiatry suggest dramatic increases – approaching 50% – in the prevalence of alcohol use disorders in the United States. But the study’s methodology has come under scrutiny: The data sets that the researchers used might be too different for reliable comparison.
The JAMA Psychiatry research, led by Bridget F. Grant, PhD, of the National Institute on Alcohol Abuse and Alcoholism, analyzed data from two waves of the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a cross-sectional, federal survey administered during 2001-2002, known as Wave 1, and during 2012-2013, known as Wave 3 (JAMA Psychiatry. 2017 Aug 9. doi: 10.1001/jamapsychiatry.2017.2161). The findings were stark: In addition to a sharp increase in the rate of 12-month alcohol use and high-risk drinking, Dr. Grant and her associates found that the rate of DSM-IV alcohol use disorder climbed from 8.5% of the population during the first wave to 12.7% of the population during the third.
“I would urge caution in drawing conclusions based on only two time points,” Dr. Grucza said. Comparing those two waves of data is inherently problematic, he explained.
“The NESARC made important changes between 2001-2002 and 2012-2013 – and several of these changes could be expected to lead to higher rates of disclosure,” said Dr. Grucza, a professor of psychiatry at Washington University in St. Louis. “This would lead to apparent increases in a variety of things – for example, they saw a 100% increase in prevalence of marijuana use among adults. So, the problem isn’t with the 2012-2013 NESARC, per se, but in the comparison between the 2012-2013 NESARC and the 2001-2002 NESARC.”
The changes include differences in the way each wave of the data was collected. The 2001-2002 survey was conducted using U.S. Census Bureau employees and didn’t offer participants incentives for survey completion. The 2012-2013 NESARC, however, was conducted through a private contractor; in addition, participants provided biological samples and received modest cash incentives for completing the survey.
“We can only speculate, but [the collection of DNA through samples of saliva] might make the participants think that their drug use would be known – and that they might therefore disclose it, anyway,” Dr. Grucza said. “I would guess that they would have been assured that this wasn’t the case during the informed consent process, but that process tends to be long, and people don’t pay attention to the whole informed consent document.”
The NESARC findings differed substantially from those found by the National Survey on Drug Use and Health (NSDUH), a federal survey of people aged 12 or older released annually by the Substance Abuse and Mental Health Services Administration. The NSDUH gathers data first in a screening phase and then in an interview phase; data are gathered in the interview phase through computer-assisted, self-administered interviews. Dr. Grucza said countless studies “suggest people respond more faithfully when disclosing to a computer as opposed to a live interviewer.”
According to the NSDUH, the rate of past-month use of alcohol fell slightly in 2015 from the previous year’s rate and was comparable to the estimates in 2005-2013.
However, Dr. Grant pointed out that substantial changes made to the questions on the 2015 NSDUH also make comparisons to previous years problematic. As a result, she said, the “NSDUH is not able to estimate the trends during the time period analyzed in the NESARC,” Dr. Grant said. “We stand by the reliability and validity of our survey data.”
For his part, Robert L. DuPont, MD, said it’s important to look at the data broadly. “The bigger picture is that alcohol is the most commonly used addictive substance by Americans, and there is evidence of increased problems resulting from drinking. For example, alcohol liver disease and cirrhosis of the liver are rising in adults and have been described as one of a group of ‘diseases of despair,’ along with suicide and drug overdose deaths,” said Dr. DuPont, the first director of the National Institute on Drug Abuse and the president of the Institute for Behavior and Health in Rockville, Md. “The fundamental message is that alcohol (and drug use) patterns show complexity that has significant public health importance. Alcohol use and related problems are more complex than a bumper sticker stating that they are either ‘up’ or ‘down.’ ”
Dr. Grucza, Dr. Grant, and Dr. DuPont had no conflicts to disclose.
The full scope of GI advances
What distinguished this year’s course offering was the overall approach and philosophy to utilize educational processes and educational theory resulting in an educational program that adhered to the AGA’s commitment to high-quality, evidence-based, and theory-driven programming.
As a first step in planning the course, we performed a needs assessment. By identifying what learners need to know, we endeavored to develop the ideal course. Our course directors, supported by the AGA staff, reviewed past course evaluations, and in particular, the comments related to suggestions for future programs. We also reviewed and discussed with experts the emerging trend topics and need-to-know areas in GI and hepatology. In doing so, an outline of topics was created, which was subsequently approved by AGA Institute’s Education and Training Committee.
Objectives
At the completion of this course the attendee will be able to:
1. Identify new strategies in the evaluation and management of GI and hepatobiliary problems
2. Recognize medical, surgical, and technological advances in the field of GI and hepatology
3. Apply new strategies for evaluation, therapeutic options, and technology to the optimal care of patients
It is challenging to craft large audience educational experiences so that they also address adult learning principles. We know that adult learners benefit from experiences that are relevant, are problem-centered (rather than content oriented), promote active learning, and provide feedback to the learner. We therefore requested that each session begin with a brief case. Having clinical examples helps learners frame the disease process, and can help demonstrate the importance of learning the material. Finally, all participants were given the opportunity to review each session, and the course in its entirety, to help us improve future programming.
Lunch sessions promoted active learning with the opportunity for interaction, and we also included case-based breakout sessions. Not only was CME accreditation provided, but Maintenance of Certification (MOC) credit was also available.
This educational offering provided a setting to hear from leaders in GI and hepatology, and for learners to gain new insights to take home and apply to the care of patients. The sections that follow provide brief summaries of the sessions from the course written by the moderators.
Please visit http://pgcourse.gastro.org/home to access the content from DDW.
Dr. Rose is a professor of medicine, the Senior Associate Dean for Education, University of Connecticut School of Medicine, Farmington, and the 2017 AGA Postgraduate Course Director. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
What distinguished this year’s course offering was the overall approach and philosophy to utilize educational processes and educational theory resulting in an educational program that adhered to the AGA’s commitment to high-quality, evidence-based, and theory-driven programming.
As a first step in planning the course, we performed a needs assessment. By identifying what learners need to know, we endeavored to develop the ideal course. Our course directors, supported by the AGA staff, reviewed past course evaluations, and in particular, the comments related to suggestions for future programs. We also reviewed and discussed with experts the emerging trend topics and need-to-know areas in GI and hepatology. In doing so, an outline of topics was created, which was subsequently approved by AGA Institute’s Education and Training Committee.
Objectives
At the completion of this course the attendee will be able to:
1. Identify new strategies in the evaluation and management of GI and hepatobiliary problems
2. Recognize medical, surgical, and technological advances in the field of GI and hepatology
3. Apply new strategies for evaluation, therapeutic options, and technology to the optimal care of patients
It is challenging to craft large audience educational experiences so that they also address adult learning principles. We know that adult learners benefit from experiences that are relevant, are problem-centered (rather than content oriented), promote active learning, and provide feedback to the learner. We therefore requested that each session begin with a brief case. Having clinical examples helps learners frame the disease process, and can help demonstrate the importance of learning the material. Finally, all participants were given the opportunity to review each session, and the course in its entirety, to help us improve future programming.
Lunch sessions promoted active learning with the opportunity for interaction, and we also included case-based breakout sessions. Not only was CME accreditation provided, but Maintenance of Certification (MOC) credit was also available.
This educational offering provided a setting to hear from leaders in GI and hepatology, and for learners to gain new insights to take home and apply to the care of patients. The sections that follow provide brief summaries of the sessions from the course written by the moderators.
Please visit http://pgcourse.gastro.org/home to access the content from DDW.
Dr. Rose is a professor of medicine, the Senior Associate Dean for Education, University of Connecticut School of Medicine, Farmington, and the 2017 AGA Postgraduate Course Director. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.
What distinguished this year’s course offering was the overall approach and philosophy to utilize educational processes and educational theory resulting in an educational program that adhered to the AGA’s commitment to high-quality, evidence-based, and theory-driven programming.
As a first step in planning the course, we performed a needs assessment. By identifying what learners need to know, we endeavored to develop the ideal course. Our course directors, supported by the AGA staff, reviewed past course evaluations, and in particular, the comments related to suggestions for future programs. We also reviewed and discussed with experts the emerging trend topics and need-to-know areas in GI and hepatology. In doing so, an outline of topics was created, which was subsequently approved by AGA Institute’s Education and Training Committee.
Objectives
At the completion of this course the attendee will be able to:
1. Identify new strategies in the evaluation and management of GI and hepatobiliary problems
2. Recognize medical, surgical, and technological advances in the field of GI and hepatology
3. Apply new strategies for evaluation, therapeutic options, and technology to the optimal care of patients
It is challenging to craft large audience educational experiences so that they also address adult learning principles. We know that adult learners benefit from experiences that are relevant, are problem-centered (rather than content oriented), promote active learning, and provide feedback to the learner. We therefore requested that each session begin with a brief case. Having clinical examples helps learners frame the disease process, and can help demonstrate the importance of learning the material. Finally, all participants were given the opportunity to review each session, and the course in its entirety, to help us improve future programming.
Lunch sessions promoted active learning with the opportunity for interaction, and we also included case-based breakout sessions. Not only was CME accreditation provided, but Maintenance of Certification (MOC) credit was also available.
This educational offering provided a setting to hear from leaders in GI and hepatology, and for learners to gain new insights to take home and apply to the care of patients. The sections that follow provide brief summaries of the sessions from the course written by the moderators.
Please visit http://pgcourse.gastro.org/home to access the content from DDW.
Dr. Rose is a professor of medicine, the Senior Associate Dean for Education, University of Connecticut School of Medicine, Farmington, and the 2017 AGA Postgraduate Course Director. This is a summary provided by the moderator of one of the AGA Postgraduate Courses held at DDW 2017.